User login
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
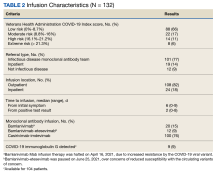
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
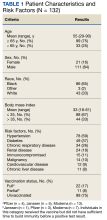
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
Early in the COVID-19 pandemic, monoclonal antibody (Mab) therapy was the only outpatient therapy for patients with COVID-19 experiencing mild-to-moderate symptoms. The Blocking Viral Attachment and Cell Entry with SARS-CoV-2 Neutralizing Antibodies (BLAZE-1) and the REGN-COV2 (Regeneron) clinical trials found participants treated with Mab had a shorter duration of symptoms and fewer hospitalizations compared with those receiving placebo.1,2 Mab therapy was most efficacious early in the disease course, and the initial US Food and Drug Administration (FDA) Emergency Use Authorization (EUA) of Mab therapies required use within 10 days of symptom onset.3
The impact of the COVID-19 pandemic has been felt disproportionately among marginalized racial and ethnic groups in the US. The COVID-19 Associated Hospitalization Surveillance Network found that non-Hispanic Black persons have significantly higher rates of hospitalization and death by COVID-19 compared with White persons.4-7 However, marginalized groups are underrepresented in the receipt of therapeutic agents for COVID-19. From March 2020 through August 2021, the mean monthly Mab use among Black patients (2.8%) was lower compared with White patients (4.0%), and Black patients received Mab 22.4% less often than White patients.7
The Mab clinical trials BLAZE-1 and REGN-COV2 study populations consisted of > 80% White participants.1,2 Receipt of COVID-19 outpatient treatments may not align with the disease burden in marginalized racial and ethnic groups, leading to health disparities. Although not exhaustive, reasons for these disparities include patient, health care practitioner, and systems-level issues: patient awareness, trust, and engagement with the health care system; health care practitioner awareness and advocacy to pursue COVID-19 treatment for the patient; and health care capacity to provide the medication and service.7
Here, we describe a novel, quality improvement initiative at the Atlanta Veterans Affairs Health Care System (AVAHCS) in Georgia that paired a proactive laboratory-based surveillance strategy to identify and engage veterans for Mab. By centralizing the surveillance and outreach process, we sought to reduce barriers to the Mab referral process and optimize access to life-saving medication.
Implementation
AVAHCS serves a diverse population of more than 129,000 (50.8% non-Hispanic Black veterans, 37.5% White veterans, and 11.7% of other races) at a main medical campus and 18 surrounding community-based outpatient clinics. From December 28, 2020, to August 31, 2021, veterans with a positive COVID-19 nasopharyngeal polymerase chain reaction (PCR) test at AVAHCS were screened daily. A central Mab team consisting of infectious disease (ID) clinical pharmacists and physicians reviewed daily lists of positive laboratory results and identified high-risk individuals for Mab eligibility, using the FDA EUA inclusion criteria. Eligible patients were called by a Mab team member to discuss Mab treatment, provide anticipatory guidance, obtain verbal consent, and schedule the infusion. Conventional referrals from non-Mab team members (eg, primary care physicians) were also accepted into the screening process and underwent the same procedures and risk prioritization strategy as those identified by the Mab team.
Clinic resources allowed for 1 to 2 patients per day to be given Mab, increasing to a maximum of 5 patients per day during the COVID-19 Delta variant surge. We followed our best clinical judgment in prioritizing patient selection, and we aligned our practice with the standards of our affiliated partner, Emory University. In circumstances where patients who were Mab-eligible outnumbered infusion availability, patients were prioritized using the Veterans Health Administration (VHA) COVID-19 (VACO) Index for 30-day COVID-19 mortality.8 As COVID-19 variants developed resistance to the recommended Mab infusions, bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab, local protocols adapted to EUA revisions. The Mab team also adopted FDA eligibility criteria revisions as they were available.9,10
We describe the outcomes of our centralized screening process for Mab therapy, as measured by screening, uptake, and time to receipt of Mab from screening. We also describe the demographic and clinical characteristics of Mab recipients. Clinical outcomes include postinfusion adverse events (AEs) at day 1 and day 7, emergency department (ED) visits, inpatient hospitalization, and death.
Results
The Mab team screened 2028 veterans who were COVID-19 positive between December 28, 2020, and August 31, 2021, and identified 289 veterans (14%) who met the EUA criteria. One hundred thirty-two veterans (46%) completed Mab infusion, and of the remaining 145 veterans, 124 (86%) declined treatment, and 21 (14%) veterans did not complete Mab infusion largely due to not keeping the appointment. The Mab team active surveillance strategy identified 101 of 132 infusion candidates (77%); 82% had outpatient Mab infusion.
The mean age of veterans who received Mab was 55 years (range, 29-90), and 75% of veterans were aged ≥ 65 years; most were male (84%) and 86 (65%) identified as non-Hispanic Black individuals (Table 1).
Postinfusion AEs reported at day 1 and day 7 occurred for 38 veterans (29%) and 11 veterans (8%), respectively. Sixteen patients (12%) had postinfusion ED visit, and 12 patients (9%) required hospitalization. Eleven of the 12 hospitalized patients (92%) had worsening respiratory symptoms. No deaths occurred in the 132 patients who received Mab.
Discussion
This novel initiative to optimize access to outpatient COVID-19 treatment demonstrated how the Mab team proactively screened and reached out to eligible veterans with COVID-19 promptly. This approach removed layers in the traditional referral process that could be barriers to accessing care. More than three-quarters of patients who received Mab were identified through this strategy, and the uptake was high at 46%. Conventional passive referrals were suboptimal for identifying candidates, which was also the case at a neighboring institution.
In an Emory University study, referrals to the Mab clinic were made through a traditional, decentralized referral system and resulted in a lower uptake of Mab treatment (4.6%).11 One of the key advantages of the AVAHCS program was that we were able to provide individual education about COVID-19 and counsel on the benefits and risks of therapy. Having a structured, telehealth follow-up plan provided additional reassurance and support to the patient. These personalized patient connections likely helped increase acceptance of the Mab therapy.
Our surveillance and outreach strategy had high uptake among Black patients (65%), which exceeded the proportion of AVAHCS Black veterans (54%).12 In the Emory study, just 30% of the participants were Black patients.11 In a study of bamlanivimab use in Chicago, Black individuals represented just 11% of the study population. White patients were more likely to receive bamlanivimab compared with others races, and the likelihood of receiving bamlanivimab was significantly worse for Black patients (odds ratio, 0.28) compared with White patients.13 These studies highlight the disparity in COVID-19 outpatient treatment that does not reflect the racial and minority group representation of the community at large.
Limitations
The VHA medication allocation system at times created a significant mismatch in supply and demand, which significantly limited the AVAHCS Mab program. VHA facilities nationwide with Mab programs received discrete allocations through the US Department of Health and Human Services via VHA pharmacy benefits management services. Despite our large catchment, AVAHCS was allocated 6 or fewer doses of Mab per week during the evaluated period.
Without formal national guidance in the early period of Mab, the AVAHCS Mab team conferred with Emory University Mab clinicians as well as at other VHA facilities in the country to develop an optimal approach to resource allocation. The Mab team considered all EUA criteria to be as inclusive as possible. However, during times of high demand, our utilitarian approach tried to identify the highest-risk patients who would benefit the most from Mab. The VACO index was validated in early 2021, which facilitated decision making when demand was greater than supply. One limitation of the VACO index is its exclusion of several original Mab EUA criteria, including weight, hypertension, and nonmalignancy-related immunosuppression, into its algorithm.3,8
Conclusions
Through proactive screening and direct outreach to patients, the AVAHCS was able to achieve timely administration of Mab infusion that was well within the initial EUA time frame of 10 days and comparable with the time frame in the REGN-COV2 and BLAZE-1 trials. Improving access to resources by changing the referral structure helped engage veterans who may have otherwise missed the time frame for Mab therapy. The experience of the Mab infusion program at the AVAHCS provided valuable insight into how a health care system could effectively screen a large population and distribute the limited resource of Mab therapy in a timely and proportionate fashion among its represented demographic groups.
Acknowledgments
The authors acknowledge the Veterans Health Administration VISN 7 Clinical Resource Hub and Tele Primary Care group for their support.
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305
1. Chen P, Nirula A, Heller B, et al; BLAZE-1 Investigators. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
2. Weinreich DM, Sivapalasingam S, Norton T, et al; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
3. US Food and Drug Administration. Fact sheet for health care providers, emergency use authorization (EUA) of bamlanivimab and etesevimab. Accessed August 6, 2023. https://www.fda.gov/media/145802/download
4. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-net). Updated March 24, 2023. Accessed August 6, 2023. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html
5. Centers for Disease Control and Prevention, National Center for Health Statistics. Provisional COVID-19 deaths: distribution of deaths by race and Hispanic origin. Updated July 26, 2023. Accessed August 8, 2023. https://data.cdc.gov/NCHS/Provisional-COVID-19-Deaths-Distribution-of-Deaths/pj7m-y5uh
6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med. 2020;382(26):2534-2543. doi:10.1056NEJMsa2011686
7. Wiltz JL, Feehan AK, Mollinari AM, et al. Racial and ethnic disparities in receipt of medications for treatment of COVID-19 - United States, March 2020-August 2021. MMWR Morb Mortal Wkly Rep. 2022;71(3):96-102. doi:10.15585/mmwr.mm7103e1
8. King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: the Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi:10.1371/journal.pone.0241825
9. US Food and Drug Administration, Office of Media Affairs. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. Accessed August 8, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
10. National Institutes of Health. Information on COVID-19 treatment, prevention and research. Accessed August 8, 2023. https://www.covid19treatmentguidelines.nih.gov
11. Anderson B, Smith Z, Edupuganti S, Yan X, Masi CM, Wu HM. Effect of monoclonal antibody treatment on clinical outcomes in ambulatory patients with coronavirus disease 2019. Open Forum Infect Dis. 2021;8(7):ofab315. Published 2021 Jun 12. doi:10.1093/ofid/ofab315
12. United States Census Bureau. Quick facts: DeKalb County, Georgia. Updated July 1, 2022. Accessed August 8, 2023. www.census.gov/quickfacts/dekalbcountygeorgia
13. Kumar R, Wu EL, Stosor V, et al. Real-world experience of bamlanivimab for coronavirus disease 2019 (COVID-19): a case-control study. Clin Infect Dis. 2022;74(1):24-31. doi:10.1093/cid/ciab305