User login
WASHINGTON – Use of a vaginal bowel control device currently under review by the Food and Drug Administration resulted in a significant reduction of fecal incontinence episodes and significant improvement in quality of life, with only mild and transient adverse events, a pivotal study has shown.
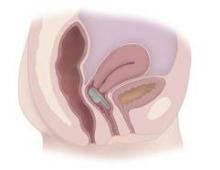
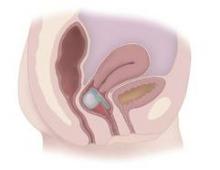
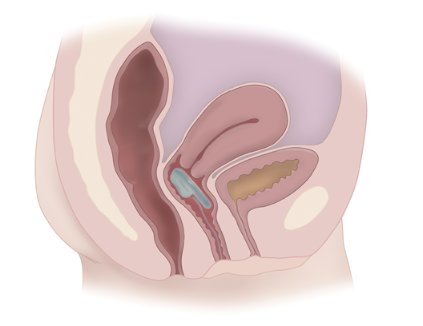
The device – the Vaginal Bowel Control (VBC) System made by Pelvalon (Sunnyvale, Calif.) – consists of a silicone base and a posteriorly directed balloon. The patient controls the inflation pump, inflating the balloon to deflect the rectovaginal septum and interrupt stool passage, and deflating it to allow bowel movements.
"It provides [the patient with] dynamic control of the rectum," said Holly E. Richter, Ph.D., M.D., who served as the national principal investigator of the study. "It’s a new paradigm for treating patients with fecal incontinence."
At the 1-month primary endpoint, 79% of the 61 patients in the study’s intent-to-treat cohort – and 86% of patients in the per-protocol cohort – had experienced treatment success, which was defined as a 50% or greater reduction in fecal incontinence episodes, Dr. Richter reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association.
Mean weekly fecal incontinence episodes were reduced from 5.9 per week at baseline to 1.1 at 1 month.
After the 1-month treatment period, women were given the option of continuing with an additional 2-month study period. The rate of treatment success held steady among the 44 women who continued, with 86% seeing a 50% or greater reduction in fecal incontinence at 3 months, said Dr. Richter, director of the division of urogynecology and pelvic reconstructive surgery at the University of Alabama at Birmingham.
Total continence was achieved in approximately 40% of women in the 1-month and 3-month per-protocol cohorts, she noted.
Scores on the Fecal Incontinence Quality of Life survey and the Modified Manchester Health Questionnaire improved significantly across all subscales. At 1 month, 86% reported their fecal incontinence to be "very much better" (57%) or "much better" (29%). Almost all – 96% – reported their insert to be comfortable, and half said they could not feel it.
To qualify for the six-center study, women had to have a history of fecal incontinence of at least 6 months, with 4 or more incontinence episodes recorded during a 2-week baseline bowel diary.
Study participants had a mean age of 61 years and a mean of 5.9 weekly fecal incontinence episodes (or 12 in their diaries).Thirty percent were obese. Nearly 50% had prior hysterectomy, 8% had prior pelvic prolapse surgery, and 15% had prior urinary incontinence surgery.
There were no device-related serious adverse events. The most common complaint was cramping or discomfort, which most often occurred during the fitting period. There were approximately a half-dozen reported changes in urinary incontinence or overactive bladder symptoms.
"We believe that the Vaginal Bowel Control System can be tried early in the algorithm of treatment for fecal incontinence," Dr. Richter said.
It won’t be a good option for everyone, however. After being fitted with the device (it is made with three base sizes and two balloon sizes), women in the study were instructed to try it for a week before entering the 1-month treatment period. Approximately 40 women who were screened and fitted did not continue. This rate of discomfort or nonacceptance is similar to that associated with pessaries, Dr. Richter noted.
The initial fitting procedure for the new bowel control device "requires a little more provider and patient training, beyond [what is needed] for use of a traditional vaginal pessary," she said.
"Patients in the study could put the device in and take it out as they wished. Most patients used it all day long, and some took it out at night," she said. Patients were instructed to "take it out once or twice a week to wash it."
Dr. Richter is a paid consultant for Pelvalon. Her coinvestigators reported consulting for Pelvalon and other companies.
Fecal incontinence is one of the most emotionally devastating of all nonfatal medical conditions, and there is a need for new and effective strategies when medical therapy fails. The vaginal bowel control device appears to hold promise for some women with fecal incontinence by mechanically impeding stool passage to the anorectum by means of an inflatable balloon inserted into the vagina. The premise is that it can be used during times when the patient is not near to toileting facilities, can be inserted and removed at will, and is not a safety concern.
It is important to emphasize that this was not a randomized, placebo-controlled trial but rather a proof-of-concept study. The 40% complete continence response is 1) similar to that observed with the sacral nerve stimulation trial that resulted in Food and Drug Administration approval in the absence of a double-blind, controlled trial (Ann. Surg. 2010;1251:441), and 2) superior to the injectable bulking agent (6% complete continence), which was approved on the basis of a randomized, double-blind, sham-controlled trial (Lancet 2011;377:997).
However, complete continence is not necessary to improve quality of life in patients with incontinence. All three of these techniques require future studies to determine which incontinent patients are likely to benefit from each of these modalities. It must be recognized that there are many causes of incontinence, which often require different therapeutic approaches.
I look forward to reading a peer-reviewed paper on this new technique and to future randomized, controlled studies on this innovative approach to a condition with a large unmet need.
Dr. Arnold Wald is professor of medicine at the University of Wisconsin School of Medicine and Public Health, Madison.
Fecal incontinence is one of the most emotionally devastating of all nonfatal medical conditions, and there is a need for new and effective strategies when medical therapy fails. The vaginal bowel control device appears to hold promise for some women with fecal incontinence by mechanically impeding stool passage to the anorectum by means of an inflatable balloon inserted into the vagina. The premise is that it can be used during times when the patient is not near to toileting facilities, can be inserted and removed at will, and is not a safety concern.
It is important to emphasize that this was not a randomized, placebo-controlled trial but rather a proof-of-concept study. The 40% complete continence response is 1) similar to that observed with the sacral nerve stimulation trial that resulted in Food and Drug Administration approval in the absence of a double-blind, controlled trial (Ann. Surg. 2010;1251:441), and 2) superior to the injectable bulking agent (6% complete continence), which was approved on the basis of a randomized, double-blind, sham-controlled trial (Lancet 2011;377:997).
However, complete continence is not necessary to improve quality of life in patients with incontinence. All three of these techniques require future studies to determine which incontinent patients are likely to benefit from each of these modalities. It must be recognized that there are many causes of incontinence, which often require different therapeutic approaches.
I look forward to reading a peer-reviewed paper on this new technique and to future randomized, controlled studies on this innovative approach to a condition with a large unmet need.
Dr. Arnold Wald is professor of medicine at the University of Wisconsin School of Medicine and Public Health, Madison.
Fecal incontinence is one of the most emotionally devastating of all nonfatal medical conditions, and there is a need for new and effective strategies when medical therapy fails. The vaginal bowel control device appears to hold promise for some women with fecal incontinence by mechanically impeding stool passage to the anorectum by means of an inflatable balloon inserted into the vagina. The premise is that it can be used during times when the patient is not near to toileting facilities, can be inserted and removed at will, and is not a safety concern.
It is important to emphasize that this was not a randomized, placebo-controlled trial but rather a proof-of-concept study. The 40% complete continence response is 1) similar to that observed with the sacral nerve stimulation trial that resulted in Food and Drug Administration approval in the absence of a double-blind, controlled trial (Ann. Surg. 2010;1251:441), and 2) superior to the injectable bulking agent (6% complete continence), which was approved on the basis of a randomized, double-blind, sham-controlled trial (Lancet 2011;377:997).
However, complete continence is not necessary to improve quality of life in patients with incontinence. All three of these techniques require future studies to determine which incontinent patients are likely to benefit from each of these modalities. It must be recognized that there are many causes of incontinence, which often require different therapeutic approaches.
I look forward to reading a peer-reviewed paper on this new technique and to future randomized, controlled studies on this innovative approach to a condition with a large unmet need.
Dr. Arnold Wald is professor of medicine at the University of Wisconsin School of Medicine and Public Health, Madison.
WASHINGTON – Use of a vaginal bowel control device currently under review by the Food and Drug Administration resulted in a significant reduction of fecal incontinence episodes and significant improvement in quality of life, with only mild and transient adverse events, a pivotal study has shown.
The device – the Vaginal Bowel Control (VBC) System made by Pelvalon (Sunnyvale, Calif.) – consists of a silicone base and a posteriorly directed balloon. The patient controls the inflation pump, inflating the balloon to deflect the rectovaginal septum and interrupt stool passage, and deflating it to allow bowel movements.
"It provides [the patient with] dynamic control of the rectum," said Holly E. Richter, Ph.D., M.D., who served as the national principal investigator of the study. "It’s a new paradigm for treating patients with fecal incontinence."
At the 1-month primary endpoint, 79% of the 61 patients in the study’s intent-to-treat cohort – and 86% of patients in the per-protocol cohort – had experienced treatment success, which was defined as a 50% or greater reduction in fecal incontinence episodes, Dr. Richter reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association.
Mean weekly fecal incontinence episodes were reduced from 5.9 per week at baseline to 1.1 at 1 month.
After the 1-month treatment period, women were given the option of continuing with an additional 2-month study period. The rate of treatment success held steady among the 44 women who continued, with 86% seeing a 50% or greater reduction in fecal incontinence at 3 months, said Dr. Richter, director of the division of urogynecology and pelvic reconstructive surgery at the University of Alabama at Birmingham.
Total continence was achieved in approximately 40% of women in the 1-month and 3-month per-protocol cohorts, she noted.
Scores on the Fecal Incontinence Quality of Life survey and the Modified Manchester Health Questionnaire improved significantly across all subscales. At 1 month, 86% reported their fecal incontinence to be "very much better" (57%) or "much better" (29%). Almost all – 96% – reported their insert to be comfortable, and half said they could not feel it.
To qualify for the six-center study, women had to have a history of fecal incontinence of at least 6 months, with 4 or more incontinence episodes recorded during a 2-week baseline bowel diary.
Study participants had a mean age of 61 years and a mean of 5.9 weekly fecal incontinence episodes (or 12 in their diaries).Thirty percent were obese. Nearly 50% had prior hysterectomy, 8% had prior pelvic prolapse surgery, and 15% had prior urinary incontinence surgery.
There were no device-related serious adverse events. The most common complaint was cramping or discomfort, which most often occurred during the fitting period. There were approximately a half-dozen reported changes in urinary incontinence or overactive bladder symptoms.
"We believe that the Vaginal Bowel Control System can be tried early in the algorithm of treatment for fecal incontinence," Dr. Richter said.
It won’t be a good option for everyone, however. After being fitted with the device (it is made with three base sizes and two balloon sizes), women in the study were instructed to try it for a week before entering the 1-month treatment period. Approximately 40 women who were screened and fitted did not continue. This rate of discomfort or nonacceptance is similar to that associated with pessaries, Dr. Richter noted.
The initial fitting procedure for the new bowel control device "requires a little more provider and patient training, beyond [what is needed] for use of a traditional vaginal pessary," she said.
"Patients in the study could put the device in and take it out as they wished. Most patients used it all day long, and some took it out at night," she said. Patients were instructed to "take it out once or twice a week to wash it."
Dr. Richter is a paid consultant for Pelvalon. Her coinvestigators reported consulting for Pelvalon and other companies.
WASHINGTON – Use of a vaginal bowel control device currently under review by the Food and Drug Administration resulted in a significant reduction of fecal incontinence episodes and significant improvement in quality of life, with only mild and transient adverse events, a pivotal study has shown.
The device – the Vaginal Bowel Control (VBC) System made by Pelvalon (Sunnyvale, Calif.) – consists of a silicone base and a posteriorly directed balloon. The patient controls the inflation pump, inflating the balloon to deflect the rectovaginal septum and interrupt stool passage, and deflating it to allow bowel movements.
"It provides [the patient with] dynamic control of the rectum," said Holly E. Richter, Ph.D., M.D., who served as the national principal investigator of the study. "It’s a new paradigm for treating patients with fecal incontinence."
At the 1-month primary endpoint, 79% of the 61 patients in the study’s intent-to-treat cohort – and 86% of patients in the per-protocol cohort – had experienced treatment success, which was defined as a 50% or greater reduction in fecal incontinence episodes, Dr. Richter reported at the scientific meetings of the American Urogynecologic Society and the International Urogynecological Association.
Mean weekly fecal incontinence episodes were reduced from 5.9 per week at baseline to 1.1 at 1 month.
After the 1-month treatment period, women were given the option of continuing with an additional 2-month study period. The rate of treatment success held steady among the 44 women who continued, with 86% seeing a 50% or greater reduction in fecal incontinence at 3 months, said Dr. Richter, director of the division of urogynecology and pelvic reconstructive surgery at the University of Alabama at Birmingham.
Total continence was achieved in approximately 40% of women in the 1-month and 3-month per-protocol cohorts, she noted.
Scores on the Fecal Incontinence Quality of Life survey and the Modified Manchester Health Questionnaire improved significantly across all subscales. At 1 month, 86% reported their fecal incontinence to be "very much better" (57%) or "much better" (29%). Almost all – 96% – reported their insert to be comfortable, and half said they could not feel it.
To qualify for the six-center study, women had to have a history of fecal incontinence of at least 6 months, with 4 or more incontinence episodes recorded during a 2-week baseline bowel diary.
Study participants had a mean age of 61 years and a mean of 5.9 weekly fecal incontinence episodes (or 12 in their diaries).Thirty percent were obese. Nearly 50% had prior hysterectomy, 8% had prior pelvic prolapse surgery, and 15% had prior urinary incontinence surgery.
There were no device-related serious adverse events. The most common complaint was cramping or discomfort, which most often occurred during the fitting period. There were approximately a half-dozen reported changes in urinary incontinence or overactive bladder symptoms.
"We believe that the Vaginal Bowel Control System can be tried early in the algorithm of treatment for fecal incontinence," Dr. Richter said.
It won’t be a good option for everyone, however. After being fitted with the device (it is made with three base sizes and two balloon sizes), women in the study were instructed to try it for a week before entering the 1-month treatment period. Approximately 40 women who were screened and fitted did not continue. This rate of discomfort or nonacceptance is similar to that associated with pessaries, Dr. Richter noted.
The initial fitting procedure for the new bowel control device "requires a little more provider and patient training, beyond [what is needed] for use of a traditional vaginal pessary," she said.
"Patients in the study could put the device in and take it out as they wished. Most patients used it all day long, and some took it out at night," she said. Patients were instructed to "take it out once or twice a week to wash it."
Dr. Richter is a paid consultant for Pelvalon. Her coinvestigators reported consulting for Pelvalon and other companies.
AT AUGS/IUGA 2014
Key clinical finding: A controllable vaginal approach to bowel continence may be useful in the future.
Major finding: Almost 80% of 61 patients in an intent-to-treat cohort saw a 50% or greater reduction in fecal incontinence episodes with use of the Vaginal Bowel Control System, a device placed in the vagina to restore bowel control.
Data source: A multicenter, prospective, open-label study of women with fecal incontinence.
Disclosures: Dr. Richter is a paid consultant for Pelvalon. Her coinvestigators reported consulting for Pelvalon and other companies.