User login
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
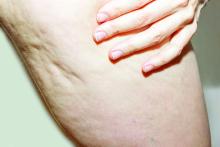
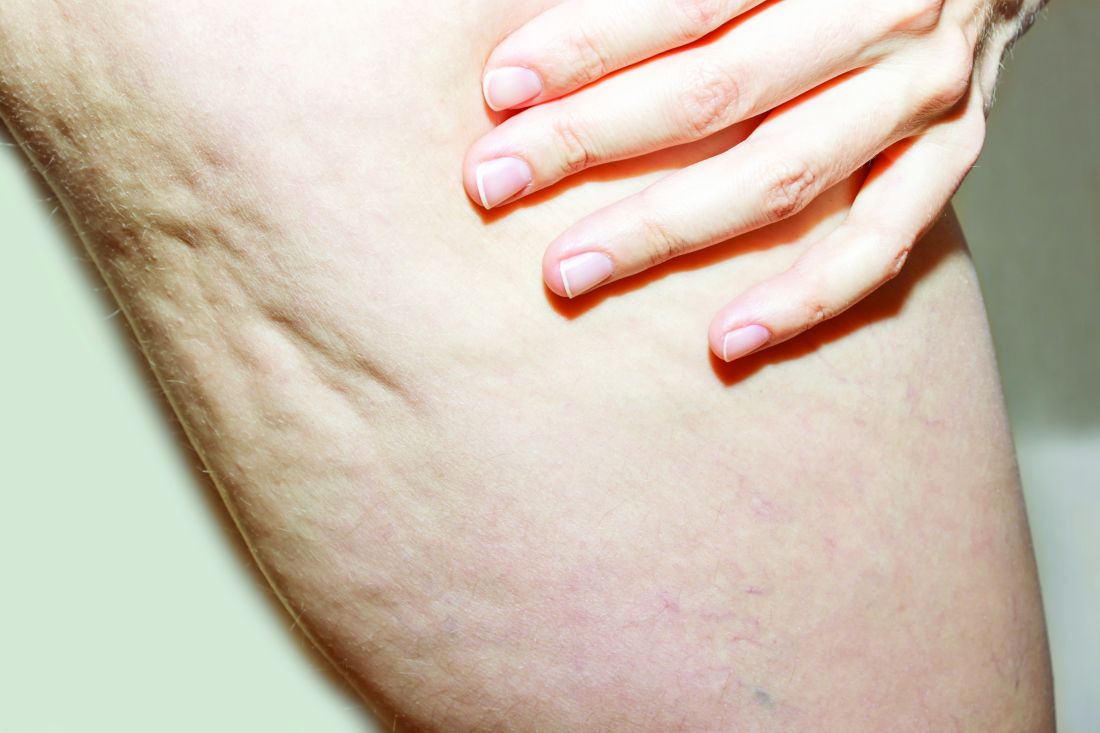
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
.
The findings build on results from a 12-week study of the device, marketed as Resonic. In that trial of 56 women with moderate to severe cellulite, a single treatment provided a roughly 1.01-point reduction in the five-point Cellulite Severity Scale (CSS) at 12 weeks, which corresponds to a roughly 29.5% reduction in cellulite from baseline.
The device, which is indicated for long-term improvement in the appearance of cellulite, emits rapid acoustic pulses and shock waves at 50 Hz that are transmitted through the skin. The device “induces physical shearing of fibrous septa through rapid acoustic pulses,” investigators led by Elizabeth Tanzi, MD, who practices cosmetic dermatology in Chevy Chase, Md., wrote in the follow-up study, which was published in Dermatologic Surgery in February “In contrast to current treatment options, the device requires no anesthesia or downtime and was well-tolerated based on an average pain score of 2.4 (on a scale 0–10) during treatment” in the 12-week study, they noted.
To evaluate the long-term efficacy of the acoustic subcision device, Dr. Tanzi and her coauthors at four centers prospectively followed 42 patients who participated in the 12-week trial. The study involved four visits: screening, a single treatment visit, and a follow-up visit 12 weeks after treatment and another after 52 weeks. Because of lockdowns and other reasons related to the COVID-19 pandemic, several participants were unable to make it to follow-up visits and had follow-up visits beyond the 52-week time-point, the authors explained.
Blinded board-certified dermatologists assessed efficacy by correctly identifying post-treatment photographs, from the visit after 52 weeks, and using a 6-point simplified CSS. They also assessed safety and collected data on participant satisfaction. The mean age of the women was 45.5 years, and their mean BMI was 23.9 kg/m2. The blinded reviewers correctly identified post-treatment photographs at the visit after 52 weeks at a rate of 95.2%.
In addition, 70.4% of the study participants had at least a 1-point change in their CSS score from baseline. Overall, their mean reduction in CSS score from baseline was 1.09 at the visit after 52 weeks, and a mean 34.1% reduction in cellulite at that visit, the authors reported.
In other findings, 41 of the 42 study participants (97.6%) rated their cellulite improvement as good and 33 (78.6%) agreed that the treatment was relatively pain free. Immediately following treatment, 85.7% reported an expected adverse event attributable to the device or treatment, which included mild to moderate erythema (76.7%), mild contusion/bruise (5.3%), mild pain (1.7%) and mild heat (1.7%). All adverse events resolved without intervention.
The study authors acknowledged certain limitations of the study, including the lack of a control group and the inability to differentiate effectiveness of the treatment on the buttocks versus the thighs.
“Cellulite is a common complaint among those presenting to cosmetic dermatology clinics, and prior treatment options have been somewhat disappointing in terms of invasiveness, side effects, or lack of improvement,” said Patricia M. Richey, MD, director of Mohs surgery at Boston Medical Center, who also conducts research for the Wellman Center for Photomedicine and the Dermatology Laser and Cosmetic Center at Massachusetts General Hospital, Boston.
Acoustic subcision “would potentially be a very attractive and unparalleled option given tolerability and sustained clinical improvement after only one treatment,” she told this news organization. “I agree with the authors that a possible limitation is the lack of comparison between response in different body areas,” namely, the buttocks versus the thighs, she said. “This information would be helpful to set patient expectations, and I suspect future studies will address this.”
Also asked to comment on the study, Pooja Sodha, MD, director of the Center for Laser and Cosmetic Dermatology at George Washington University, Washington, said in an interview that while the results were modest after a single treatment, “there is room for further experimentation to see how modifications of settings, treatment numbers, treatment intervals, and location-specific treatment regimens based on tissue depth and tissue band size/dimple size may enhance results.”
She added that cost of treatment and correlation with clinical improvement “will become a more real-world matter when it comes to bringing this more broadly to the clinic settings.”
Soliton sponsored the trial prior to its acquisition by AbbVie. Dr. Tanzi reported having no relevant financial disclosures. Four coauthors reported being employees, consultants, or advisory board members, or having stock options in AbbVie. Dr. Richey and Dr. Sodha were not involved with the study and reported having no disclosures.
FROM DERMATOLOGIC SURGERY