User login
Case
A 76-year-old woman with a history of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and atrial fibrillation presents with shortness of breath. She is tachypneic, her pulse is 105 beats per minute, and her blood pressure is 105/60 mm/Hg. She is obese and has an immeasurable venous pressure with decreased breath sounds in both lung bases, and irregular and distant heart sounds. What is the role of brain (or B-type) natriuretic peptide (BNP) in the diagnosis and management of this patient?
Overview
Each year, more than 1 million patients are admitted to hospitals with acutely decompensated heart failure (ADHF). Although many of these patients carry a pre-admission diagnosis of CHF, their common presenting symptoms are not specific for ADHF, which leads to delays in diagnosis and therapy initiation, and increased diagnostic costs and potentially worse outcomes. Clinical risk scores from NHANES and the Framingham heart study have limited sensitivity, missing nearly 20% of patients.1,2 Moreover, these scores are underused by clinicians who depend heavily on clinical gestalt.3
Once ADHF is diagnosed, ongoing bedside assessment of volume status is a difficult and inexact science. The physiologic goal is achievement of normal left ventricular end diastolic volume; however, surrogate measures of this status, including weight change, venous pressure, and pulmonary and cardiac auscultatory findings, have significant limitations. After discharge, patients have high and heterogeneous risks of readmission, death, and other adverse events. Identifying patients with the highest risk might allow for intensive strategies to improve outcomes.
BNP is a neurohormone released from the ventricular cells in response to increased cardiac filling pressures. Plasma measurements of BNP have been shown to reflect volume status, to predict risk at admission and discharge, and to serve as a treatment guide in a variety of clinical settings.4 This simple laboratory test increasingly has been used to diagnose and manage ADHF; its utility and limitations deserve critical review.
Review of the Data
CHF diagnosis. Since introduction of the rapid BNP assay, several trials have evaluated its clinical utility in determining whether ADHF is the cause of a patient’s dyspnea. The largest of these trials, the Breathing Not Properly Multinational Study, conducted by McCullough et al, enrolled nearly 1,600 patients who presented with the primary complaint of dyspnea.5 After reviewing conventional clinical information, ED physicians were asked to determine the likelihood that ADHF was the etiology of a patient’s dyspnea. These likelihoods were classified as low (<20%), intermediate (20%-80%), or high (>80%). The admission BNP was recorded but was not available for the ED physician decisions.
The “gold standard” was the opinion of two adjudicating cardiologists who reviewed the cases retrospectively and determined whether the dyspnea resulted from ADHF. They were blinded to both the ED physician’s opinion and the BNP results. The accuracy of the ED physician’s initial assessment and the impact of the BNP results were compared with this gold standard.
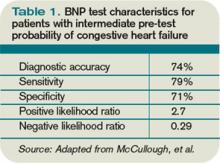
For the entire cohort, the use of BNP (with a cutoff point of 100 pg/mL) would have improved the ED physician’s assessment from 74% diagnostic accuracy to 81%, which is statistically significant. Most important, in those patients initially given an intermediate likelihood of CHF, BNP results correctly classified 75% of these patients and rarely missed ADHF cases (<10%).
Atrial fibrillation. Since the original trials that established a BNP cutoff of 100 pg/mL for determining the presence of ADHF, several adjustments have been suggested. The presence of atrial fibrillation has been shown to increase BNP values independent of cardiac filling pressures. Breidthardt et al examined patients with atrial fibrillation presenting with dyspnea.4 In their analysis, using a cutoff of 100 pg/mL remained robust in identifying patients without ADHF. However, in the 100 pg/mL-500 pg/mL range, the test was not able to discriminate between atrial fibrillation and ADHF. Values greater than 500 pg/mL proved accurate in supporting the diagnosis of ADHF.
Renal failure. Renal dysfunction also elevates BNP levels independent of filling pressures. McCullough et al re-examined data from their Breathing Not Properly Multinational Study and found that the glomerular filtration rate (GFR) was inversely related to BNP levels.5 They recommend using a cutoff point of 200 pg/mL when the GFR is below 60 mg/dL. Other authors recommend not using BNP levels to diagnose ADHF when the GFR is less than 60 mg/dL due to the lack of data supporting this approach. Until clarified, clinicians should be cautious of interpreting BNP elevations in the setting of kidney disease.
Obesity. Obesity has a negative effect on BNP levels, decreasing the sensitivity of the test in these patients.6 Although no study defines how to adjust for body mass index (BMI), clinicians should be cautious about using a low BNP to rule out ADHF in a dyspneic obese patient.
Historical BNP values. If historical BNP values are available, studies of biological variation have shown that an increase to 123% from 66% from baseline is representative of a clinically meaningful increase in cardiac filling pressures. Less significant changes could merely represent biological variation and should be cautiously interpreted.7
Cost effectiveness. The cost effectiveness of using BNP measurements in dyspneic ED patients has been examined as well. Mueller et al found in a Swiss hospital that BNP testing was associated with a 25% decrease in treatment cost, length of stay (LOS), and ICU usage.8 However, LOS is significantly longer in Switzerland compared with the U.S., and given that much of the cost savings was attributed to reducing LOS, it is not possible to extrapolate these data to the U.S. health system. More evidence is needed to truly evaluate the cost effectiveness of BNP testing.
Serial BNP testing. Once a patient has been diagnosed with ADHF and admitted to the hospital, diuretics are indicated with the goal of achieving euvolemia. The bedside assessment of volume status remains a difficult and inexact science, and failure to appropriately remove fluid is associated with readmissions. Conversely, overdiuresis with a concomitant rise in creatinine has been associated with increased morbidity and mortality.
Several studies have shown that the reduction of volume associated with diuretic administration is coupled with a rapid decrease in BNP levels. Therefore, serial BNP measurement has been evaluated as a tool to guide the daily assessment of volume status in patients admitted with ADHF. Unfortunately, frequent measurements of BNP reveal that a great deal of variance, or “noise,” is present in these repeat measurements. Data do not clearly show how to incorporate serial BNP measurements into daily diuretic management.9
Mortality prediction. Nearly 3.5% of admitted heart failure patients will die during their hospitalization. For perspective, the rate of hospital mortality with acute myocardial infarction is 7%. BNP serves as a powerful and independent predictor of inpatient mortality. The ADHERE (Acute Decompensated Heart Failure National Registry) study showed that when divided into BNP quartiles of <430 pg/mL, 430 pg/mL to 839 pg/mL, 840 pg/mL to 1,729 pg/mL, and >1,730 pg/mL, patients’ risk of inpatient death was accurately predicted as 1.9%, 2.8%, 3.8%, and 6.0%, respectively.10 Even when adjusted for other risk factors, BNP remained a powerful predictor; the mortality rate more than doubled from the lowest to highest quartile.
Different strategies have been proposed to improve the outcomes in these highest-risk patients; however, to date, no evidence-based strategy offers a meaningful way to reduce inpatient mortality beyond the current standard of care.
Readmission and 30-day mortality. The 30-day readmission rate after discharge for ADHF is more than than 25%. A study of Medicare patients showed that more than $17 billion (more than 15% of all Medicare payments to hospitals) was associated with unplanned rehospitalizations.11 As bundling payment trends develop, hospitals have an enormous incentive to identify CHF patients with the highest risk of readmission and attempt to mitigate that risk.
From a patient-centered view, upon hospital discharge a patient with ADHF also realizes a 1 in 10 chance of dying within the first 30 days.
At discharge, BNP serves as a powerful and independent marker of increased risk of readmission, morbidity, and mortality. O’Connor et al developed a discharge risk model in patients with severe left ventricular dysfunction; the ESCAPE risk model and discharge score showed elevated BNP was the single most powerful predictor of six-month mortality.12 For every doubling of the BNP, the odds of death at six months increased by 1.4 times.
After combining discharge BNP with other factors, the ESCAPE discharge score was fairly successful at discriminating between patients who would and would not survive to six months. By identifying these outpatients, intensive management strategies could be focused on individuals with the highest risk. The data support the idea that readmission reductions are significant when outpatients obtain early follow-up. Many healthcare centers struggle to schedule early follow-up for all heart failure patients.
As such, the ability to target individuals with the highest discharge scores for intensive follow-up might improve outcomes. These patients could undergo early evaluation for such advanced therapies as resynchronization, left ventricular assist device implantation, or listing for transplantation. Currently, this strategy is not proven. It also is possible that these high-risk patients might have such advanced diseases that their risk cannot be modified by our current medications and advanced therapies.
Back to the Case
This patient has symptoms and signs that could be caused by ADHF or COPD. Her presentation is consistent with an intermediate probability of ADHF. A rapid BNP reveals a level of 950 pg/mL.
Even considering the higher cutoff required because of her coexistent atrial fibrillation, her BNP is consistent with ADHF. Additionally, her obesity likely has decreased the true value of her BNP. A previous BNP drawn when the patient was not in ADHF was 250 ng/mL, meaning that at least a 70% increase is present.
She was admitted and treated with intravenous diuretics with improvement in her congestion and relief of her symptoms. Daily BNPs were not drawn and her diuretics were titrated based on bedside clinical assessments. Her admission BNP elevation would predict a moderately high risk of short- and intermediate term of morbidity and mortality.
At discharge, a repeat BNP also could add to her risk stratification, though it would not be clear what do with this prognostic information beyond the standard of care.
Bottom Line
BNP measurement in specific situations can complement conventional clinical information in determining the presence of ADHF and also can enhance clinicians’ ability to risk-stratify patients during and after hospitalization. TH
Dr. Wolfe is a hospitalist and assistant professor of medicine at the University of Colorado Denver.
References
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20(2):301-306.
- McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Eng J Med. 1971;285(26):1441-1446.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944-1956.
- Breidthardt T, Noveanu M, Cayir S, et al. The use of B-type natriuretic peptide in the management of patients with atrial fibrillation and dyspnea. Int J Cardiol. 2009;136(2):193-199.
- McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
- Iwanaga Y, Hihara Y, Nizuma S, et al. BNP in overweight and obese patients with heart failure: an analysis based on the BNP-LV diastolic wall stress relationship. J Card Fail. 2007;13(8):663-667.
- O’Hanlon R, O’Shea P, Ledwidge M. The biologic variability of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide in stable heart failure patients. J Card Fail. 2007;13(1):50-55.
- Mueller C, Laule-Kilian K, Schindler C, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Arch Intern Med. 2006;166(1):1081-1087.
- Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J. 2006;152(5):828-834.
- Fonarow GC, Peacock WF, Phillips CO, et al. ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;48 (19):1943-1950.
- Jencks SF, Williams MC, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
- O’Connor CM, Hasselblad V, Mehta RH, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55(9):872-878.
Case
A 76-year-old woman with a history of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and atrial fibrillation presents with shortness of breath. She is tachypneic, her pulse is 105 beats per minute, and her blood pressure is 105/60 mm/Hg. She is obese and has an immeasurable venous pressure with decreased breath sounds in both lung bases, and irregular and distant heart sounds. What is the role of brain (or B-type) natriuretic peptide (BNP) in the diagnosis and management of this patient?
Overview
Each year, more than 1 million patients are admitted to hospitals with acutely decompensated heart failure (ADHF). Although many of these patients carry a pre-admission diagnosis of CHF, their common presenting symptoms are not specific for ADHF, which leads to delays in diagnosis and therapy initiation, and increased diagnostic costs and potentially worse outcomes. Clinical risk scores from NHANES and the Framingham heart study have limited sensitivity, missing nearly 20% of patients.1,2 Moreover, these scores are underused by clinicians who depend heavily on clinical gestalt.3
Once ADHF is diagnosed, ongoing bedside assessment of volume status is a difficult and inexact science. The physiologic goal is achievement of normal left ventricular end diastolic volume; however, surrogate measures of this status, including weight change, venous pressure, and pulmonary and cardiac auscultatory findings, have significant limitations. After discharge, patients have high and heterogeneous risks of readmission, death, and other adverse events. Identifying patients with the highest risk might allow for intensive strategies to improve outcomes.
BNP is a neurohormone released from the ventricular cells in response to increased cardiac filling pressures. Plasma measurements of BNP have been shown to reflect volume status, to predict risk at admission and discharge, and to serve as a treatment guide in a variety of clinical settings.4 This simple laboratory test increasingly has been used to diagnose and manage ADHF; its utility and limitations deserve critical review.
Review of the Data
CHF diagnosis. Since introduction of the rapid BNP assay, several trials have evaluated its clinical utility in determining whether ADHF is the cause of a patient’s dyspnea. The largest of these trials, the Breathing Not Properly Multinational Study, conducted by McCullough et al, enrolled nearly 1,600 patients who presented with the primary complaint of dyspnea.5 After reviewing conventional clinical information, ED physicians were asked to determine the likelihood that ADHF was the etiology of a patient’s dyspnea. These likelihoods were classified as low (<20%), intermediate (20%-80%), or high (>80%). The admission BNP was recorded but was not available for the ED physician decisions.
The “gold standard” was the opinion of two adjudicating cardiologists who reviewed the cases retrospectively and determined whether the dyspnea resulted from ADHF. They were blinded to both the ED physician’s opinion and the BNP results. The accuracy of the ED physician’s initial assessment and the impact of the BNP results were compared with this gold standard.
For the entire cohort, the use of BNP (with a cutoff point of 100 pg/mL) would have improved the ED physician’s assessment from 74% diagnostic accuracy to 81%, which is statistically significant. Most important, in those patients initially given an intermediate likelihood of CHF, BNP results correctly classified 75% of these patients and rarely missed ADHF cases (<10%).
Atrial fibrillation. Since the original trials that established a BNP cutoff of 100 pg/mL for determining the presence of ADHF, several adjustments have been suggested. The presence of atrial fibrillation has been shown to increase BNP values independent of cardiac filling pressures. Breidthardt et al examined patients with atrial fibrillation presenting with dyspnea.4 In their analysis, using a cutoff of 100 pg/mL remained robust in identifying patients without ADHF. However, in the 100 pg/mL-500 pg/mL range, the test was not able to discriminate between atrial fibrillation and ADHF. Values greater than 500 pg/mL proved accurate in supporting the diagnosis of ADHF.
Renal failure. Renal dysfunction also elevates BNP levels independent of filling pressures. McCullough et al re-examined data from their Breathing Not Properly Multinational Study and found that the glomerular filtration rate (GFR) was inversely related to BNP levels.5 They recommend using a cutoff point of 200 pg/mL when the GFR is below 60 mg/dL. Other authors recommend not using BNP levels to diagnose ADHF when the GFR is less than 60 mg/dL due to the lack of data supporting this approach. Until clarified, clinicians should be cautious of interpreting BNP elevations in the setting of kidney disease.
Obesity. Obesity has a negative effect on BNP levels, decreasing the sensitivity of the test in these patients.6 Although no study defines how to adjust for body mass index (BMI), clinicians should be cautious about using a low BNP to rule out ADHF in a dyspneic obese patient.
Historical BNP values. If historical BNP values are available, studies of biological variation have shown that an increase to 123% from 66% from baseline is representative of a clinically meaningful increase in cardiac filling pressures. Less significant changes could merely represent biological variation and should be cautiously interpreted.7
Cost effectiveness. The cost effectiveness of using BNP measurements in dyspneic ED patients has been examined as well. Mueller et al found in a Swiss hospital that BNP testing was associated with a 25% decrease in treatment cost, length of stay (LOS), and ICU usage.8 However, LOS is significantly longer in Switzerland compared with the U.S., and given that much of the cost savings was attributed to reducing LOS, it is not possible to extrapolate these data to the U.S. health system. More evidence is needed to truly evaluate the cost effectiveness of BNP testing.
Serial BNP testing. Once a patient has been diagnosed with ADHF and admitted to the hospital, diuretics are indicated with the goal of achieving euvolemia. The bedside assessment of volume status remains a difficult and inexact science, and failure to appropriately remove fluid is associated with readmissions. Conversely, overdiuresis with a concomitant rise in creatinine has been associated with increased morbidity and mortality.
Several studies have shown that the reduction of volume associated with diuretic administration is coupled with a rapid decrease in BNP levels. Therefore, serial BNP measurement has been evaluated as a tool to guide the daily assessment of volume status in patients admitted with ADHF. Unfortunately, frequent measurements of BNP reveal that a great deal of variance, or “noise,” is present in these repeat measurements. Data do not clearly show how to incorporate serial BNP measurements into daily diuretic management.9
Mortality prediction. Nearly 3.5% of admitted heart failure patients will die during their hospitalization. For perspective, the rate of hospital mortality with acute myocardial infarction is 7%. BNP serves as a powerful and independent predictor of inpatient mortality. The ADHERE (Acute Decompensated Heart Failure National Registry) study showed that when divided into BNP quartiles of <430 pg/mL, 430 pg/mL to 839 pg/mL, 840 pg/mL to 1,729 pg/mL, and >1,730 pg/mL, patients’ risk of inpatient death was accurately predicted as 1.9%, 2.8%, 3.8%, and 6.0%, respectively.10 Even when adjusted for other risk factors, BNP remained a powerful predictor; the mortality rate more than doubled from the lowest to highest quartile.
Different strategies have been proposed to improve the outcomes in these highest-risk patients; however, to date, no evidence-based strategy offers a meaningful way to reduce inpatient mortality beyond the current standard of care.
Readmission and 30-day mortality. The 30-day readmission rate after discharge for ADHF is more than than 25%. A study of Medicare patients showed that more than $17 billion (more than 15% of all Medicare payments to hospitals) was associated with unplanned rehospitalizations.11 As bundling payment trends develop, hospitals have an enormous incentive to identify CHF patients with the highest risk of readmission and attempt to mitigate that risk.
From a patient-centered view, upon hospital discharge a patient with ADHF also realizes a 1 in 10 chance of dying within the first 30 days.
At discharge, BNP serves as a powerful and independent marker of increased risk of readmission, morbidity, and mortality. O’Connor et al developed a discharge risk model in patients with severe left ventricular dysfunction; the ESCAPE risk model and discharge score showed elevated BNP was the single most powerful predictor of six-month mortality.12 For every doubling of the BNP, the odds of death at six months increased by 1.4 times.
After combining discharge BNP with other factors, the ESCAPE discharge score was fairly successful at discriminating between patients who would and would not survive to six months. By identifying these outpatients, intensive management strategies could be focused on individuals with the highest risk. The data support the idea that readmission reductions are significant when outpatients obtain early follow-up. Many healthcare centers struggle to schedule early follow-up for all heart failure patients.
As such, the ability to target individuals with the highest discharge scores for intensive follow-up might improve outcomes. These patients could undergo early evaluation for such advanced therapies as resynchronization, left ventricular assist device implantation, or listing for transplantation. Currently, this strategy is not proven. It also is possible that these high-risk patients might have such advanced diseases that their risk cannot be modified by our current medications and advanced therapies.
Back to the Case
This patient has symptoms and signs that could be caused by ADHF or COPD. Her presentation is consistent with an intermediate probability of ADHF. A rapid BNP reveals a level of 950 pg/mL.
Even considering the higher cutoff required because of her coexistent atrial fibrillation, her BNP is consistent with ADHF. Additionally, her obesity likely has decreased the true value of her BNP. A previous BNP drawn when the patient was not in ADHF was 250 ng/mL, meaning that at least a 70% increase is present.
She was admitted and treated with intravenous diuretics with improvement in her congestion and relief of her symptoms. Daily BNPs were not drawn and her diuretics were titrated based on bedside clinical assessments. Her admission BNP elevation would predict a moderately high risk of short- and intermediate term of morbidity and mortality.
At discharge, a repeat BNP also could add to her risk stratification, though it would not be clear what do with this prognostic information beyond the standard of care.
Bottom Line
BNP measurement in specific situations can complement conventional clinical information in determining the presence of ADHF and also can enhance clinicians’ ability to risk-stratify patients during and after hospitalization. TH
Dr. Wolfe is a hospitalist and assistant professor of medicine at the University of Colorado Denver.
References
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20(2):301-306.
- McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Eng J Med. 1971;285(26):1441-1446.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944-1956.
- Breidthardt T, Noveanu M, Cayir S, et al. The use of B-type natriuretic peptide in the management of patients with atrial fibrillation and dyspnea. Int J Cardiol. 2009;136(2):193-199.
- McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
- Iwanaga Y, Hihara Y, Nizuma S, et al. BNP in overweight and obese patients with heart failure: an analysis based on the BNP-LV diastolic wall stress relationship. J Card Fail. 2007;13(8):663-667.
- O’Hanlon R, O’Shea P, Ledwidge M. The biologic variability of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide in stable heart failure patients. J Card Fail. 2007;13(1):50-55.
- Mueller C, Laule-Kilian K, Schindler C, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Arch Intern Med. 2006;166(1):1081-1087.
- Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J. 2006;152(5):828-834.
- Fonarow GC, Peacock WF, Phillips CO, et al. ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;48 (19):1943-1950.
- Jencks SF, Williams MC, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
- O’Connor CM, Hasselblad V, Mehta RH, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55(9):872-878.
Case
A 76-year-old woman with a history of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and atrial fibrillation presents with shortness of breath. She is tachypneic, her pulse is 105 beats per minute, and her blood pressure is 105/60 mm/Hg. She is obese and has an immeasurable venous pressure with decreased breath sounds in both lung bases, and irregular and distant heart sounds. What is the role of brain (or B-type) natriuretic peptide (BNP) in the diagnosis and management of this patient?
Overview
Each year, more than 1 million patients are admitted to hospitals with acutely decompensated heart failure (ADHF). Although many of these patients carry a pre-admission diagnosis of CHF, their common presenting symptoms are not specific for ADHF, which leads to delays in diagnosis and therapy initiation, and increased diagnostic costs and potentially worse outcomes. Clinical risk scores from NHANES and the Framingham heart study have limited sensitivity, missing nearly 20% of patients.1,2 Moreover, these scores are underused by clinicians who depend heavily on clinical gestalt.3
Once ADHF is diagnosed, ongoing bedside assessment of volume status is a difficult and inexact science. The physiologic goal is achievement of normal left ventricular end diastolic volume; however, surrogate measures of this status, including weight change, venous pressure, and pulmonary and cardiac auscultatory findings, have significant limitations. After discharge, patients have high and heterogeneous risks of readmission, death, and other adverse events. Identifying patients with the highest risk might allow for intensive strategies to improve outcomes.
BNP is a neurohormone released from the ventricular cells in response to increased cardiac filling pressures. Plasma measurements of BNP have been shown to reflect volume status, to predict risk at admission and discharge, and to serve as a treatment guide in a variety of clinical settings.4 This simple laboratory test increasingly has been used to diagnose and manage ADHF; its utility and limitations deserve critical review.
Review of the Data
CHF diagnosis. Since introduction of the rapid BNP assay, several trials have evaluated its clinical utility in determining whether ADHF is the cause of a patient’s dyspnea. The largest of these trials, the Breathing Not Properly Multinational Study, conducted by McCullough et al, enrolled nearly 1,600 patients who presented with the primary complaint of dyspnea.5 After reviewing conventional clinical information, ED physicians were asked to determine the likelihood that ADHF was the etiology of a patient’s dyspnea. These likelihoods were classified as low (<20%), intermediate (20%-80%), or high (>80%). The admission BNP was recorded but was not available for the ED physician decisions.
The “gold standard” was the opinion of two adjudicating cardiologists who reviewed the cases retrospectively and determined whether the dyspnea resulted from ADHF. They were blinded to both the ED physician’s opinion and the BNP results. The accuracy of the ED physician’s initial assessment and the impact of the BNP results were compared with this gold standard.
For the entire cohort, the use of BNP (with a cutoff point of 100 pg/mL) would have improved the ED physician’s assessment from 74% diagnostic accuracy to 81%, which is statistically significant. Most important, in those patients initially given an intermediate likelihood of CHF, BNP results correctly classified 75% of these patients and rarely missed ADHF cases (<10%).
Atrial fibrillation. Since the original trials that established a BNP cutoff of 100 pg/mL for determining the presence of ADHF, several adjustments have been suggested. The presence of atrial fibrillation has been shown to increase BNP values independent of cardiac filling pressures. Breidthardt et al examined patients with atrial fibrillation presenting with dyspnea.4 In their analysis, using a cutoff of 100 pg/mL remained robust in identifying patients without ADHF. However, in the 100 pg/mL-500 pg/mL range, the test was not able to discriminate between atrial fibrillation and ADHF. Values greater than 500 pg/mL proved accurate in supporting the diagnosis of ADHF.
Renal failure. Renal dysfunction also elevates BNP levels independent of filling pressures. McCullough et al re-examined data from their Breathing Not Properly Multinational Study and found that the glomerular filtration rate (GFR) was inversely related to BNP levels.5 They recommend using a cutoff point of 200 pg/mL when the GFR is below 60 mg/dL. Other authors recommend not using BNP levels to diagnose ADHF when the GFR is less than 60 mg/dL due to the lack of data supporting this approach. Until clarified, clinicians should be cautious of interpreting BNP elevations in the setting of kidney disease.
Obesity. Obesity has a negative effect on BNP levels, decreasing the sensitivity of the test in these patients.6 Although no study defines how to adjust for body mass index (BMI), clinicians should be cautious about using a low BNP to rule out ADHF in a dyspneic obese patient.
Historical BNP values. If historical BNP values are available, studies of biological variation have shown that an increase to 123% from 66% from baseline is representative of a clinically meaningful increase in cardiac filling pressures. Less significant changes could merely represent biological variation and should be cautiously interpreted.7
Cost effectiveness. The cost effectiveness of using BNP measurements in dyspneic ED patients has been examined as well. Mueller et al found in a Swiss hospital that BNP testing was associated with a 25% decrease in treatment cost, length of stay (LOS), and ICU usage.8 However, LOS is significantly longer in Switzerland compared with the U.S., and given that much of the cost savings was attributed to reducing LOS, it is not possible to extrapolate these data to the U.S. health system. More evidence is needed to truly evaluate the cost effectiveness of BNP testing.
Serial BNP testing. Once a patient has been diagnosed with ADHF and admitted to the hospital, diuretics are indicated with the goal of achieving euvolemia. The bedside assessment of volume status remains a difficult and inexact science, and failure to appropriately remove fluid is associated with readmissions. Conversely, overdiuresis with a concomitant rise in creatinine has been associated with increased morbidity and mortality.
Several studies have shown that the reduction of volume associated with diuretic administration is coupled with a rapid decrease in BNP levels. Therefore, serial BNP measurement has been evaluated as a tool to guide the daily assessment of volume status in patients admitted with ADHF. Unfortunately, frequent measurements of BNP reveal that a great deal of variance, or “noise,” is present in these repeat measurements. Data do not clearly show how to incorporate serial BNP measurements into daily diuretic management.9
Mortality prediction. Nearly 3.5% of admitted heart failure patients will die during their hospitalization. For perspective, the rate of hospital mortality with acute myocardial infarction is 7%. BNP serves as a powerful and independent predictor of inpatient mortality. The ADHERE (Acute Decompensated Heart Failure National Registry) study showed that when divided into BNP quartiles of <430 pg/mL, 430 pg/mL to 839 pg/mL, 840 pg/mL to 1,729 pg/mL, and >1,730 pg/mL, patients’ risk of inpatient death was accurately predicted as 1.9%, 2.8%, 3.8%, and 6.0%, respectively.10 Even when adjusted for other risk factors, BNP remained a powerful predictor; the mortality rate more than doubled from the lowest to highest quartile.
Different strategies have been proposed to improve the outcomes in these highest-risk patients; however, to date, no evidence-based strategy offers a meaningful way to reduce inpatient mortality beyond the current standard of care.
Readmission and 30-day mortality. The 30-day readmission rate after discharge for ADHF is more than than 25%. A study of Medicare patients showed that more than $17 billion (more than 15% of all Medicare payments to hospitals) was associated with unplanned rehospitalizations.11 As bundling payment trends develop, hospitals have an enormous incentive to identify CHF patients with the highest risk of readmission and attempt to mitigate that risk.
From a patient-centered view, upon hospital discharge a patient with ADHF also realizes a 1 in 10 chance of dying within the first 30 days.
At discharge, BNP serves as a powerful and independent marker of increased risk of readmission, morbidity, and mortality. O’Connor et al developed a discharge risk model in patients with severe left ventricular dysfunction; the ESCAPE risk model and discharge score showed elevated BNP was the single most powerful predictor of six-month mortality.12 For every doubling of the BNP, the odds of death at six months increased by 1.4 times.
After combining discharge BNP with other factors, the ESCAPE discharge score was fairly successful at discriminating between patients who would and would not survive to six months. By identifying these outpatients, intensive management strategies could be focused on individuals with the highest risk. The data support the idea that readmission reductions are significant when outpatients obtain early follow-up. Many healthcare centers struggle to schedule early follow-up for all heart failure patients.
As such, the ability to target individuals with the highest discharge scores for intensive follow-up might improve outcomes. These patients could undergo early evaluation for such advanced therapies as resynchronization, left ventricular assist device implantation, or listing for transplantation. Currently, this strategy is not proven. It also is possible that these high-risk patients might have such advanced diseases that their risk cannot be modified by our current medications and advanced therapies.
Back to the Case
This patient has symptoms and signs that could be caused by ADHF or COPD. Her presentation is consistent with an intermediate probability of ADHF. A rapid BNP reveals a level of 950 pg/mL.
Even considering the higher cutoff required because of her coexistent atrial fibrillation, her BNP is consistent with ADHF. Additionally, her obesity likely has decreased the true value of her BNP. A previous BNP drawn when the patient was not in ADHF was 250 ng/mL, meaning that at least a 70% increase is present.
She was admitted and treated with intravenous diuretics with improvement in her congestion and relief of her symptoms. Daily BNPs were not drawn and her diuretics were titrated based on bedside clinical assessments. Her admission BNP elevation would predict a moderately high risk of short- and intermediate term of morbidity and mortality.
At discharge, a repeat BNP also could add to her risk stratification, though it would not be clear what do with this prognostic information beyond the standard of care.
Bottom Line
BNP measurement in specific situations can complement conventional clinical information in determining the presence of ADHF and also can enhance clinicians’ ability to risk-stratify patients during and after hospitalization. TH
Dr. Wolfe is a hospitalist and assistant professor of medicine at the University of Colorado Denver.
References
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20(2):301-306.
- McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Eng J Med. 1971;285(26):1441-1446.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944-1956.
- Breidthardt T, Noveanu M, Cayir S, et al. The use of B-type natriuretic peptide in the management of patients with atrial fibrillation and dyspnea. Int J Cardiol. 2009;136(2):193-199.
- McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
- Iwanaga Y, Hihara Y, Nizuma S, et al. BNP in overweight and obese patients with heart failure: an analysis based on the BNP-LV diastolic wall stress relationship. J Card Fail. 2007;13(8):663-667.
- O’Hanlon R, O’Shea P, Ledwidge M. The biologic variability of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide in stable heart failure patients. J Card Fail. 2007;13(1):50-55.
- Mueller C, Laule-Kilian K, Schindler C, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Arch Intern Med. 2006;166(1):1081-1087.
- Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J. 2006;152(5):828-834.
- Fonarow GC, Peacock WF, Phillips CO, et al. ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;48 (19):1943-1950.
- Jencks SF, Williams MC, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
- O’Connor CM, Hasselblad V, Mehta RH, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55(9):872-878.