User login
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.
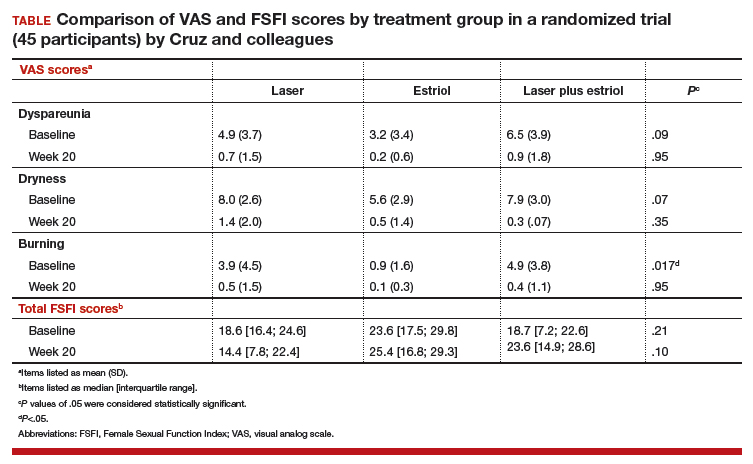
While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.

While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
GSM encompasses a constellation of symptoms involving the vulva, vagina, urethra, and bladder, and it can affect quality of life in more than half of women by 3 years past menopause.1,2 Local estrogen creams, tablets, and rings are considered the gold standard treatment for GSM.3 The rising cost of many of these pharmacologic treatments has created headlines and concerns over price gouging for drugs used to treat female sexual dysfunction.4 Recent alternatives to local estrogens include vaginal moisturizers and lubricants, vaginal dehydroepiandrosterone (DHEA) suppositories, oral ospemifene, and vaginal laser therapy.
Laser treatment (with fractionated CO2, erbium, and hybrid lasers) activates heat shock proteins and tissue growth factors to stimulateneocollagenesis and neovascularization within the vaginal epithelium,but it is expensive and not covered by insurance because it is considered a cosmetic procedure.5Most evidence on laser therapy for GSM comes from prospective case series with small numbers and short-term follow-up with no comparison arms.6,7 A recent trial by Cruz and colleagues, however, is notable because it is one of the first published studies that compared vaginal laser with vaginal estrogen alone and with a combination laser plus estrogen arm. We need level 1 comparative data from studies such as this to help us counsel the millions of US women with GSM.
Details of the study
In this single-site randomized, double-blind, placebo-controlled trial conducted in Brazil, postmenopausal women were assigned to 1 of 3 treatment groups (15 per group):
- CO2 laser (MonaLisa Touch, SmartXide 2 system; DEKA Laser; Florence, Italy): 2 treatments total, 1 month apart, plus placebo cream (laser arm)
- estriol cream (1 mg estriol 3 times per week for 20 weeks) plus sham laser (estriol arm)
- CO2 laser plus estriol cream 3 times per week (laser plus estriol combination arm).
The primary outcome included a change in visual analog scale (VAS) score for symptoms related to vulvovaginal atrophy (VVA), including dyspareunia, dryness, and burning (0–10 scale with 0 = no symptoms and 10 = most severe symptoms), and change in the objective Vaginal Health Index (VHI). Assessments were made at baseline and at 8 and 20 weeks. Participants were included if they were menopausal for at least 2 years and had at least 1 moderately bothersome VVA symptom (based on a VAS score of 4 or greater).
Secondary outcomes included the objective FSFI questionnaire evaluating desire, arousal, lubrication, orgasm, satisfaction, and pain. FSFI scores can range from 2 (severe dysfunction) to 36 (no dysfunction). A total FSFI score less than 26 was deemed equivalent to dysfunction. Cytologic smear evaluation using a vaginal maturation index was included in all 3 treatment arms. Sample size calculation of 45 patients (15 per arm) for this trial was based on a 3-point difference in the VHI.
The baseline characteristics for participants in each treatment arm were similar, except that participants in the vaginal estriol group were less symptomatic at baseline. This group had less burning at baseline based on the FSFI and less dyspareunia based on the VAS.
On July 30, 2018, the US Food and Drug Administration (FDA) issued a safety warning against the use of energy-based devices for vaginal "rejuvenation"1 and sent warning letters to 7 companies--Alma Lasers; BTL Aesthetics; BTL Industries, Inc; Cynosure, Inc; InMode MD; Sciton, Inc; and Thermigen, Inc.2 The concern relates to marketing claims made on many of these companies' websites on the use of radiofrequency and laser technology for such specific conditions as vaginal laxity, vaginal dryness, urinary incontinence, and sexual function and response. These devices are neither cleared nor approved by the FDA for these specific indications; they are rather approved for general gynecologic conditions, such as the treatment of genital warts and precancerous conditions.
The FDA sent the safety warning related to energy-based vaginal therapies to patients and providers and have encouraged them to submit any adverse events to MedWatch, the FDA Safety Information and Adverse Event Reporting system.1 The "It has come to our attention letters" issued by the FDA to the above manufacturers request additional information and FDA clearance or approval numbers for claims made on their websites--specifically, referenced benefits of energy-based devices for vaginal, vulvar, and sexual health.2 This information is requested from manufacturers in writing by August 30, 2018 (30 days).
References
- FDA warns against use of energy-based devices to perform vaginal 'rejuvenation' or vaginal cosmetic procedures: FDA safety communication. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm615013.htm. Updated July 30, 2018. Accessed July 30, 2018.
- Letters to industry. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ResourcesforYou/Industry/ucm111104.htm. Updated July 30, 2018. Accessed July 30, 2018.
Laser treatment improved dryness, burning, and dyspareunia but caused more pain
All 3 treatment groups showed statistically significant improvement in vaginal dryness at 20 weeks, but only the laser-alone arm and the laser plus estriol arms showed improvement in dyspareunia and burning. The total FSFI scores improved significantly only in the laser plus estriol arm (TABLE). No difference in the vaginal maturation index was noted between groups; however, improved numbers of parabasal cells were found in participants in the laser treatment arms.

While participants in the laser treatment arms (alone and in combination with estriol) showed significant improvement in the VAS domains of dyspareunia and burning compared with those treated with estriol alone, there was a contradictory finding of more pain in both laser arms at 20 weeks compared with the estriol-alone group, based on the FSFI. The FSFI is a validated, objective quality-of-life questionnaire, and the finding of more pain with laser treatment is a concern.
Exercise caution when interpreting these study findings. While this preliminary study showed that fractionated CO2 laser treatment had favorable outcomes for dyspareunia, dryness, and burning, the propensity for increased vaginal pain with this treatment is a concern. This study was not adequately powered to analyze multiple comparisons in postmenopausal women with GSM symptoms. There were significant baseline differences, with less bothersome burning and sexual complaints based on the FSFI and VAS, in the vaginal estriol arm. The finding of more pain in the laser treatment arms at 20 weeks compared with that in the vaginal estriol arm is of concern and warrants further investigation.
-- Cheryl B. Iglesia, MD
Study strengths and weaknesses
This study is one of the first of its kind to compare laser therapy alone and in combination with local estriol to vaginal estriol alone for the treatment of GSM. The trial’s strength is in its design as a double-blind, placebo-controlled block randomized trial, which adds to the prospective cohort trials that generally show favorable outcomes for fractionated laser for the treatment of GSM.
The study’s weaknesses include its small sample size, single trial site, and short-term follow-up. Findings from this trial should be considered preliminary and not generalizable. Other weaknesses are the 3 of 45 participants lost to follow-up and the significant baseline differences among the women, with lower bothersome baseline VAS scores in the estriol arm.
Furthermore, this study was not powered for multiple comparisons, and conclusions favoring laser therapy cannot be overinflated. Lasers such as CO2 target the chromophore water, and indiscriminate use in severely dry vaginal epithelium may cause more pain or scarring. Longer-term follow-up is needed.
More research also is needed to develop guidelines related to pre-laser treatment to achieve optimal vaginal pH and ideal vaginal maturation, including, for example, vaginal priming with estrogen, DHEA, or other moisturizers.
This study also suggests the use of vaginal laser therapy as a drug delivery mechanism for combination therapy. Many vaginal estrogen treatments are expensive (despite prescription drug coverage), and laser treatments are very expensive (and not covered by insurance), so research to optimize outcomes and minimize patient expense is needed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013;10(7):1790–1799.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause. 2014;21(10):1063–1068.
- The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–753.
- Thomas K. Prices keep rising for drugs treating painful sex in women. New York Times. June 3, 2018. https://www.nytimes.com/2018/06/03/health/vagina-womens-health-drug-prices.html. Accessed July 15, 2018.
- Tadir Y, Gaspar A, Lev-Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of meno-pause: consensus and controversies. Lasers Surg Med. 2017;49(2):137–159.
- Athanasiou S, Pitsouni E, Antonopoulou S, et al. The effect of microablative fractional CO2 laser on vaginal flora of postmenopausal women. Climacteric. 2016;19(5):512–518.
- Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause. 2017;24(7):810–814.