User login
Case Report
A 44-year-old woman presented with a localized patch of hair loss on the frontal scalp of several month’s duration. She had been bitten by a tick at this site during the summer. Two months later
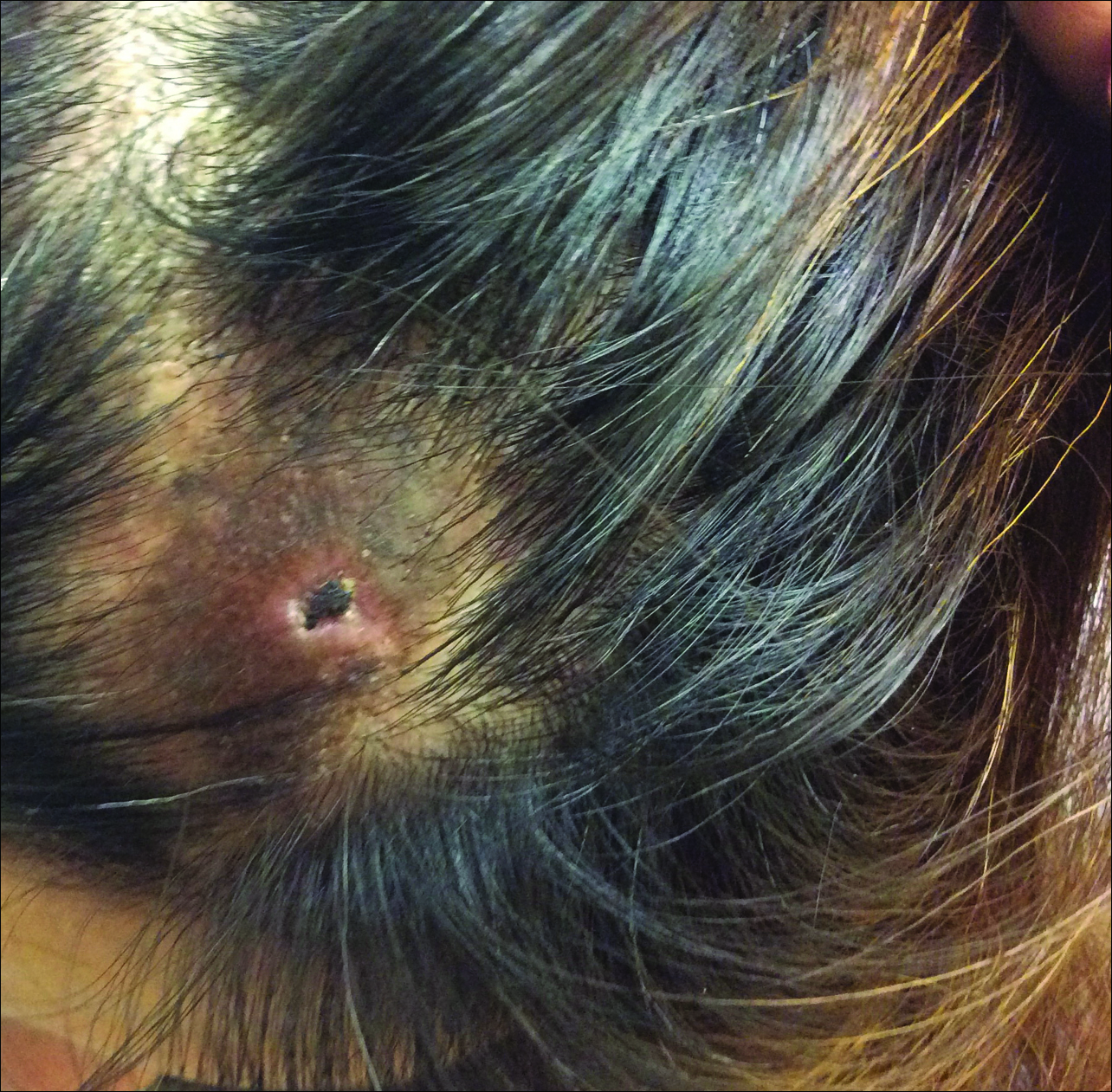
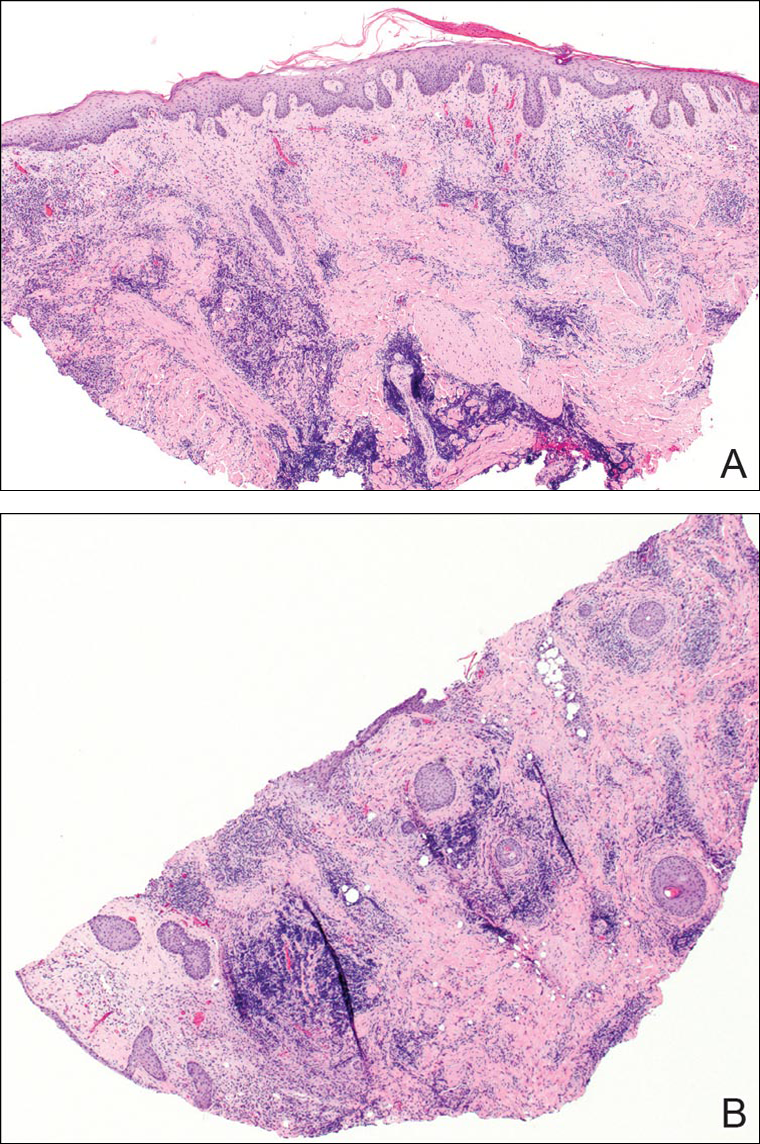
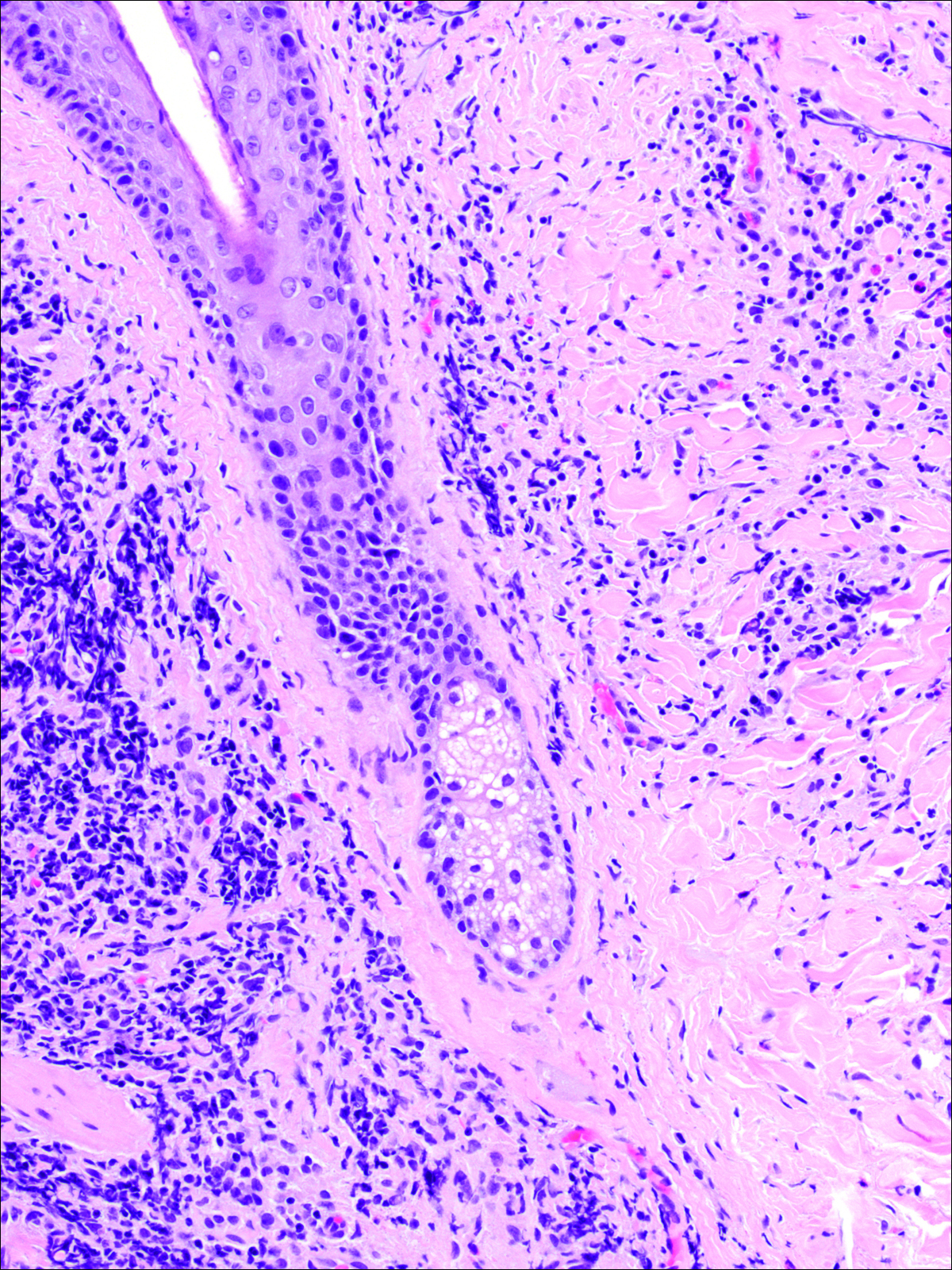
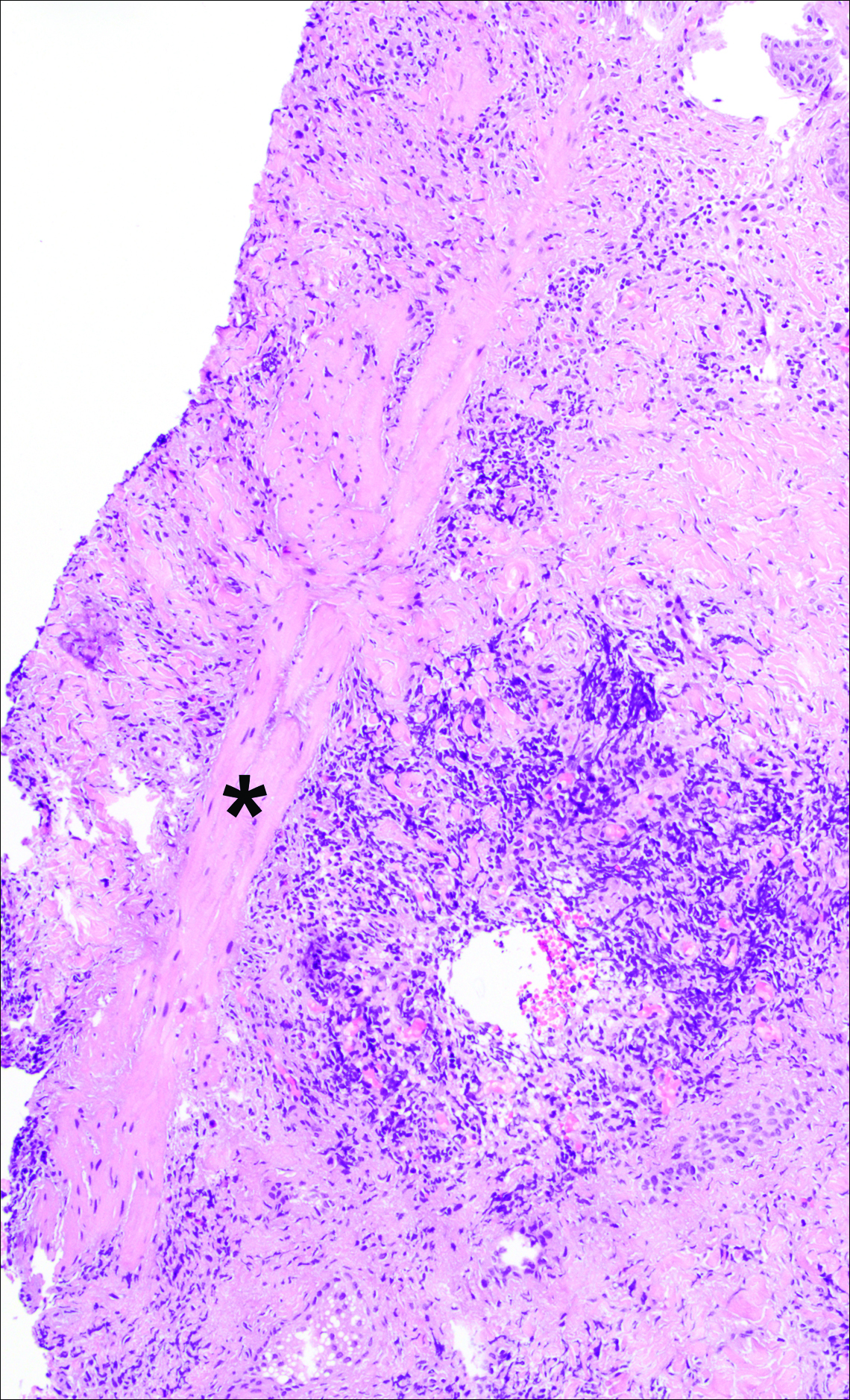
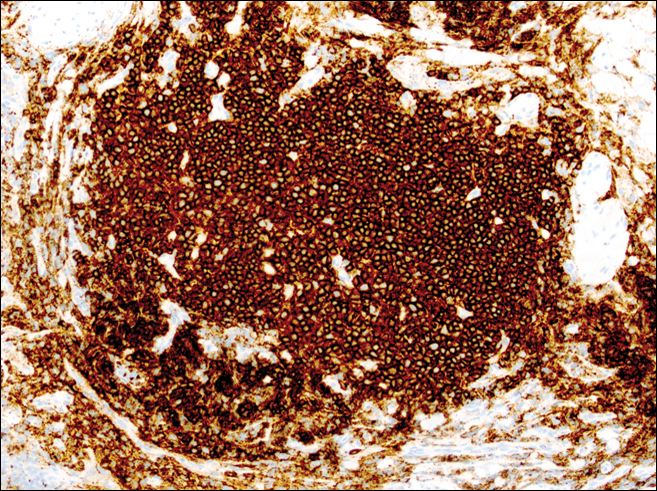
A punch biopsy was obtained from an indurated area of hyperpigmentation adjacent to the eschar. Both vertical and horizontal sections were obtained, revealing a relatively normal epidermis, a marked decrease in follicular structures with loss of sebaceous glands, and dense perifollicular lymphocytic inflammation with a few scattered eosinophils (Figures 2 and 3).
Historical Perspective
Tick bite alopecia was first described in the French literature in 19211 and in the English-language literature in 1955.2 A few additional cases were subsequently reported.3-5 In 2008, Castelli et al6 described the histologic and immunohistochemical features of 25 tick bite cases, a few of which resulted in alopecia. Other than these reports, little original information has been written about tick bite alopecia.
Clinical and Histologic Presentation
Tick bite alopecia is well described in the veterinary literature.7-9 It is possible that the condition is underreported in humans because the cause is often obvious or the alopecia is never discovered. The typical presentation is a roughly oval zone of alopecia that develops 1 to 2 weeks after the removal of a tick from the scalp. Often there is a small central eschar representing the site of tick attachment and the surrounding scalp may appear scaly. In one report of 2 siblings, multiple oval zones of alopecia resembling the moth-eaten alopecia of syphilis were noted in both patients, but only a single attached tick was found.2 In some reported cases, hair loss was only temporary, and at least partial if not complete regrowth of hair occurred.3,4 Follow-up on most cases is not provided, but to our knowledge permanent alopecia has not been described.
Information about the histologic findings of tick bite alopecia is particularly limited. In a report by Heyl,3 biopsies were conducted in 2 patients, but the areas selected for biopsy were the sites of tick attachment. Centrally dense, acute, and chronic inflammation was seen, as well as marked tissue necrosis of the connective tissue and hair follicles. Peripheral to the attachment zone, tissue necrosis was not found, but telogen hairs with “crumpled up hair shafts” were present.3 The histologic findings presented by Castelli et al6 were based on a single case of tick bite alopecia; however, the specimen was a generous excisional biopsy, allowing for a panoramic histologic view of the lesion. In the center of the specimen, hair follicles were absent, but residual follicular streamers and follicular remnants were surrounded by lymphocytic inflammation. Sebaceous glands were conspicuously absent, but foci with naked hairs, fibrosis, and granulomatous inflammation were seen. Peripherally, the hair follicles were thinned and miniaturized with an increased number of catagen/telogen hairs. Some follicles showed lamellar fibroplasia and perifollicular chronic inflammation. The inflammatory infiltrate consisted predominantly of helper T cells with a smaller population of B lymphocytes and a few plasma cells.6 In 2016, Lynch et al5 described a single case of tick bite alopecia and noted pseudolymphomatous inflammation with germinal center formation associated with hair miniaturization and an elevated catagen/telogen count; focal follicular mucinosis also was noted.Our histologic findings are similar to those of Castelli et al,6 except that the inflammatory infiltrate was clearly B-cell dominant, with a suggestion of germinal center formation, as noted by Lynch et al.5 This inflammatory pattern often can be encountered in a chronic tick bite lesion. Destruction of follicles and associated sebaceous glands and their replacement by follicular scars indicate that at least in the central portion of the lesion some permanent hair loss occurs. The presence of catagen/telogen hairs and miniaturized follicles indicates the potential for at least partial regrowth.
Similar to other investigators who have described tick bite alopecia, we can only speculate as to the mechanism by which clinical alopecia occurs. Given the density of the inflammatory infiltrate and perifollicular inflammation, it seems reasonable to assume that inflammation either destroys hair follicles or precipitates the catagen/telogen phase, resulting in temporary hair loss. The inflammation itself may be due to the presence of tick parts or the antigens in their saliva (or both). The delay between tick attachment and the onset of alopecia can be attributed to the time it takes follicles to cycle into the catagen/telogen phase and shed the hair shaft.
- Sauphar L. Alopecie peladoide consecutive a une piqure de tique. Bull Soc Fr Dermatol Syphiligr. 1921;28:442.
- Ross MS, Friede H. Alopecia due to tick bite. AMA Arch Derm. 1955;71:524-525.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7:537-542.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40:555-556.
- Lynch MC, Milchak MA, Parnes H, et al. Tick bite alopecia: a report and review [published online April 19, 2016]. Am J Dermatopathol. doi:10.1097/DAD.0000000000000598.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Nemeth NM, Ruder MG, Gerhold RW, et al. Demodectic mange, dermatophilosis, and other parasitic and bacterial dermatologic diseases in free-ranging white-tailed deer (Odocoileus virginianus) in the United States from 1975 to 2012. Vet Pathol. 2014;51:633-640.
- Welch DA, Samuel WM, Hudson RJ. Bioenergetic consequences of alopecia induced by Dermacentor albipictus (Acari: Ixodidae) on moose. J Med Entomol. 1990;27:656-660.
- Samuel WM. Locations of moose in northwestern Canada with hair loss probably caused by the winter tick, Dermacentor albipictus (Acari: Ixodidae). J Wildl Dis. 1989;25:436-439.
Case Report
A 44-year-old woman presented with a localized patch of hair loss on the frontal scalp of several month’s duration. She had been bitten by a tick at this site during the summer. Two months later

A punch biopsy was obtained from an indurated area of hyperpigmentation adjacent to the eschar. Both vertical and horizontal sections were obtained, revealing a relatively normal epidermis, a marked decrease in follicular structures with loss of sebaceous glands, and dense perifollicular lymphocytic inflammation with a few scattered eosinophils (Figures 2 and 3).




Historical Perspective
Tick bite alopecia was first described in the French literature in 19211 and in the English-language literature in 1955.2 A few additional cases were subsequently reported.3-5 In 2008, Castelli et al6 described the histologic and immunohistochemical features of 25 tick bite cases, a few of which resulted in alopecia. Other than these reports, little original information has been written about tick bite alopecia.
Clinical and Histologic Presentation
Tick bite alopecia is well described in the veterinary literature.7-9 It is possible that the condition is underreported in humans because the cause is often obvious or the alopecia is never discovered. The typical presentation is a roughly oval zone of alopecia that develops 1 to 2 weeks after the removal of a tick from the scalp. Often there is a small central eschar representing the site of tick attachment and the surrounding scalp may appear scaly. In one report of 2 siblings, multiple oval zones of alopecia resembling the moth-eaten alopecia of syphilis were noted in both patients, but only a single attached tick was found.2 In some reported cases, hair loss was only temporary, and at least partial if not complete regrowth of hair occurred.3,4 Follow-up on most cases is not provided, but to our knowledge permanent alopecia has not been described.
Information about the histologic findings of tick bite alopecia is particularly limited. In a report by Heyl,3 biopsies were conducted in 2 patients, but the areas selected for biopsy were the sites of tick attachment. Centrally dense, acute, and chronic inflammation was seen, as well as marked tissue necrosis of the connective tissue and hair follicles. Peripheral to the attachment zone, tissue necrosis was not found, but telogen hairs with “crumpled up hair shafts” were present.3 The histologic findings presented by Castelli et al6 were based on a single case of tick bite alopecia; however, the specimen was a generous excisional biopsy, allowing for a panoramic histologic view of the lesion. In the center of the specimen, hair follicles were absent, but residual follicular streamers and follicular remnants were surrounded by lymphocytic inflammation. Sebaceous glands were conspicuously absent, but foci with naked hairs, fibrosis, and granulomatous inflammation were seen. Peripherally, the hair follicles were thinned and miniaturized with an increased number of catagen/telogen hairs. Some follicles showed lamellar fibroplasia and perifollicular chronic inflammation. The inflammatory infiltrate consisted predominantly of helper T cells with a smaller population of B lymphocytes and a few plasma cells.6 In 2016, Lynch et al5 described a single case of tick bite alopecia and noted pseudolymphomatous inflammation with germinal center formation associated with hair miniaturization and an elevated catagen/telogen count; focal follicular mucinosis also was noted.Our histologic findings are similar to those of Castelli et al,6 except that the inflammatory infiltrate was clearly B-cell dominant, with a suggestion of germinal center formation, as noted by Lynch et al.5 This inflammatory pattern often can be encountered in a chronic tick bite lesion. Destruction of follicles and associated sebaceous glands and their replacement by follicular scars indicate that at least in the central portion of the lesion some permanent hair loss occurs. The presence of catagen/telogen hairs and miniaturized follicles indicates the potential for at least partial regrowth.
Similar to other investigators who have described tick bite alopecia, we can only speculate as to the mechanism by which clinical alopecia occurs. Given the density of the inflammatory infiltrate and perifollicular inflammation, it seems reasonable to assume that inflammation either destroys hair follicles or precipitates the catagen/telogen phase, resulting in temporary hair loss. The inflammation itself may be due to the presence of tick parts or the antigens in their saliva (or both). The delay between tick attachment and the onset of alopecia can be attributed to the time it takes follicles to cycle into the catagen/telogen phase and shed the hair shaft.
Case Report
A 44-year-old woman presented with a localized patch of hair loss on the frontal scalp of several month’s duration. She had been bitten by a tick at this site during the summer. Two months later

A punch biopsy was obtained from an indurated area of hyperpigmentation adjacent to the eschar. Both vertical and horizontal sections were obtained, revealing a relatively normal epidermis, a marked decrease in follicular structures with loss of sebaceous glands, and dense perifollicular lymphocytic inflammation with a few scattered eosinophils (Figures 2 and 3).




Historical Perspective
Tick bite alopecia was first described in the French literature in 19211 and in the English-language literature in 1955.2 A few additional cases were subsequently reported.3-5 In 2008, Castelli et al6 described the histologic and immunohistochemical features of 25 tick bite cases, a few of which resulted in alopecia. Other than these reports, little original information has been written about tick bite alopecia.
Clinical and Histologic Presentation
Tick bite alopecia is well described in the veterinary literature.7-9 It is possible that the condition is underreported in humans because the cause is often obvious or the alopecia is never discovered. The typical presentation is a roughly oval zone of alopecia that develops 1 to 2 weeks after the removal of a tick from the scalp. Often there is a small central eschar representing the site of tick attachment and the surrounding scalp may appear scaly. In one report of 2 siblings, multiple oval zones of alopecia resembling the moth-eaten alopecia of syphilis were noted in both patients, but only a single attached tick was found.2 In some reported cases, hair loss was only temporary, and at least partial if not complete regrowth of hair occurred.3,4 Follow-up on most cases is not provided, but to our knowledge permanent alopecia has not been described.
Information about the histologic findings of tick bite alopecia is particularly limited. In a report by Heyl,3 biopsies were conducted in 2 patients, but the areas selected for biopsy were the sites of tick attachment. Centrally dense, acute, and chronic inflammation was seen, as well as marked tissue necrosis of the connective tissue and hair follicles. Peripheral to the attachment zone, tissue necrosis was not found, but telogen hairs with “crumpled up hair shafts” were present.3 The histologic findings presented by Castelli et al6 were based on a single case of tick bite alopecia; however, the specimen was a generous excisional biopsy, allowing for a panoramic histologic view of the lesion. In the center of the specimen, hair follicles were absent, but residual follicular streamers and follicular remnants were surrounded by lymphocytic inflammation. Sebaceous glands were conspicuously absent, but foci with naked hairs, fibrosis, and granulomatous inflammation were seen. Peripherally, the hair follicles were thinned and miniaturized with an increased number of catagen/telogen hairs. Some follicles showed lamellar fibroplasia and perifollicular chronic inflammation. The inflammatory infiltrate consisted predominantly of helper T cells with a smaller population of B lymphocytes and a few plasma cells.6 In 2016, Lynch et al5 described a single case of tick bite alopecia and noted pseudolymphomatous inflammation with germinal center formation associated with hair miniaturization and an elevated catagen/telogen count; focal follicular mucinosis also was noted.Our histologic findings are similar to those of Castelli et al,6 except that the inflammatory infiltrate was clearly B-cell dominant, with a suggestion of germinal center formation, as noted by Lynch et al.5 This inflammatory pattern often can be encountered in a chronic tick bite lesion. Destruction of follicles and associated sebaceous glands and their replacement by follicular scars indicate that at least in the central portion of the lesion some permanent hair loss occurs. The presence of catagen/telogen hairs and miniaturized follicles indicates the potential for at least partial regrowth.
Similar to other investigators who have described tick bite alopecia, we can only speculate as to the mechanism by which clinical alopecia occurs. Given the density of the inflammatory infiltrate and perifollicular inflammation, it seems reasonable to assume that inflammation either destroys hair follicles or precipitates the catagen/telogen phase, resulting in temporary hair loss. The inflammation itself may be due to the presence of tick parts or the antigens in their saliva (or both). The delay between tick attachment and the onset of alopecia can be attributed to the time it takes follicles to cycle into the catagen/telogen phase and shed the hair shaft.
- Sauphar L. Alopecie peladoide consecutive a une piqure de tique. Bull Soc Fr Dermatol Syphiligr. 1921;28:442.
- Ross MS, Friede H. Alopecia due to tick bite. AMA Arch Derm. 1955;71:524-525.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7:537-542.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40:555-556.
- Lynch MC, Milchak MA, Parnes H, et al. Tick bite alopecia: a report and review [published online April 19, 2016]. Am J Dermatopathol. doi:10.1097/DAD.0000000000000598.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Nemeth NM, Ruder MG, Gerhold RW, et al. Demodectic mange, dermatophilosis, and other parasitic and bacterial dermatologic diseases in free-ranging white-tailed deer (Odocoileus virginianus) in the United States from 1975 to 2012. Vet Pathol. 2014;51:633-640.
- Welch DA, Samuel WM, Hudson RJ. Bioenergetic consequences of alopecia induced by Dermacentor albipictus (Acari: Ixodidae) on moose. J Med Entomol. 1990;27:656-660.
- Samuel WM. Locations of moose in northwestern Canada with hair loss probably caused by the winter tick, Dermacentor albipictus (Acari: Ixodidae). J Wildl Dis. 1989;25:436-439.
- Sauphar L. Alopecie peladoide consecutive a une piqure de tique. Bull Soc Fr Dermatol Syphiligr. 1921;28:442.
- Ross MS, Friede H. Alopecia due to tick bite. AMA Arch Derm. 1955;71:524-525.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7:537-542.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40:555-556.
- Lynch MC, Milchak MA, Parnes H, et al. Tick bite alopecia: a report and review [published online April 19, 2016]. Am J Dermatopathol. doi:10.1097/DAD.0000000000000598.
- Castelli E, Caputo V, Morello V, et al. Local reactions to tick bites. Am J Dermatopathol. 2008;30:241-248.
- Nemeth NM, Ruder MG, Gerhold RW, et al. Demodectic mange, dermatophilosis, and other parasitic and bacterial dermatologic diseases in free-ranging white-tailed deer (Odocoileus virginianus) in the United States from 1975 to 2012. Vet Pathol. 2014;51:633-640.
- Welch DA, Samuel WM, Hudson RJ. Bioenergetic consequences of alopecia induced by Dermacentor albipictus (Acari: Ixodidae) on moose. J Med Entomol. 1990;27:656-660.
- Samuel WM. Locations of moose in northwestern Canada with hair loss probably caused by the winter tick, Dermacentor albipictus (Acari: Ixodidae). J Wildl Dis. 1989;25:436-439.
Practice Points
- Tick bite alopecia should be included in the differential diagnosis of both solitary and moth-eaten lesions of localized hair loss.
- In most cases, hair regrowth can be expected in a lesion of tick bite alopecia.