User login
Platelet-rich plasma (PRP) injections have become a popular treatment option in a variety of specialties including sports medicine, maxillofacial surgery, dermatology, cosmetology, and reproductive medicine.1 PRP is an autologous blood product derived from whole blood, using a centrifuge to isolate a concentrated layer of platelets. The a-granules in platelets release transforming growth factor b 1, vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor 1, and other mediators that enhance the natural healing process.2
When patients ask. Familiarity with the use of PRP to treat specific musculoskeletal (MSK) conditions is essential for family physicians who frequently are asked by patients about whether PRP is right for them. These patients may have experienced failure of medication therapy or declined surgical intervention, or may not be surgical candidates. This review details the evidence surrounding common intra-articular and extra-articular applications of PRP. But first, a word about how PRP is prepared, its contraindications, and costs.
Preparation and types of PRP
Although there are many commercial systems for preparing PRP, there is no consensus on the optimal formulation.2 Other terms for PRP, such as autologous concentrated platelets and super-concentrated platelets, are based on concentration of red blood cells, leukocytes, and fibrin.3 PRP therapies usually are categorized as leukocyte-rich PRP (LR-PRP) or leukocyte-poor PRP (LP-PRP), based on neutrophil concentrations that are above and below baseline.2 Leukocyte concentration is one of the most debated topics in PRP therapy.4
Common commercially available preparation systems produce platelet concentrations between 3 to 6 times the baseline platelet count.5 Although there is no universally agreed upon PRP formulation, studies have shown 2 centrifugation cycles (“double-spun” or “dual centrifugation”) that yield platelet concentrations between 1.8 to 1.9 times the baseline values significantly improve MSK conditions.6-8
For MSK purposes, PRP may be injected into intratendinous, peritendinous, and intra-articular spaces. Currently, there is no consensus regarding injection frequency. Many studies have incorporated single-injection protocols, while some have used 2 to 3 injections repeated over several weeks to months. PRP commonly is injected at point-of-care without requiring storage.
Contraindications. PRP has been shown to be safe, with most adverse effects attributed to local injection site pain, bleeding, swelling, and bruising.9
Contraindications to PRP include active malignancy or recent remission from malignancy with the exception of nonmetastatic skin tumors.10 PRP is not recommended for patients with an allergy to manufacturing components (eg, dimethyl sulfoxide), thrombocytopenia, nonsteroidal anti-inflammatory drug use within 2 weeks, active infection causing fever, and local infection at the injection site.10 Since local anesthetics may impair platelet function, they should not be given at the same injection site as PRP.10
Continue to: Cost
Cost. PRP is not covered by most insurance plans.11,12 The cost for PRP may range from $500 to $2500 for a single injection.12
Evidence-based summary by condition
Knee osteoarthritis
❯❯❯ Consider using PRP
Knee osteoarthritis (OA) is a common cause of pain and disability. Treatment options include physical therapy, pharmacotherapy, and surgery. PRP has gained popularity as a nonsurgical option. A recent meta-analysis by Costa et al13 of 40 studies with 3035 participants comparing intra-articular PRP with hyaluronic acid (HA), corticosteroid, and saline injections, found that PRP appears to be more effective or as effective as other nonsurgical modalities. However, due to study heterogeneity and high risk for bias, the authors could not recommend PRP for knee OA in clinical practice.13
Despite Costa et al’s findings, reproducible data have demonstrated the superiority of PRP over other nonsurgical treatment options for knee OA. A 2021 systematic review and meta-analysis of 18 randomized controlled trials (RCTs; N = 811) by Belk et al6 comparing PRP to HA injections showed a higher mean improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the PRP group compared to the HA group (44.7% vs 12.6%, respectively; P < .01).6 Six of 11 studies using the visual analog scale (VAS) for pain reported significantly less pain in the PRP group compared to the HA group (P < .05).6 The mean follow-up time was 11.1 months.6 Three of 6 studies reported improved subjective International Knee Documentation Committee (IKDC) scores (range from 0-100, with higher scores representing higher levels of function and lower levels of symptoms) in the PRP group compared to the HA group: 75.7 ± 15.1 vs 65.6 ± 16.9 (P = .004); 65.5 ± 3.6 vs 55.8 ± 3.8 (P = .01); and 60.8 ± 9.8 vs 48.4 ± 6.2 (P < .05).6 There was concern for moderate-to-high heterogeneity.6
Other systematic reviews and meta-analyses found similar efficacy of PRP for knee OA, including improved WOMAC scores and patient-reported outcomes (eg, pain, physical function, stiffness) compared to other injectable options.14,15 A systematic review of 14 RCTs (N = 1423) by Shen et al15 showed improved WOMAC scores at 3 months (mean differences [MD] = –14.53; 95% CI, –29.97 to –7.09; P < .001), 6 months
Despite a lack of consensus regarding the optimal preparation of PRP for knee OA, another recent RCT (N = 192) found significant improvement in mean subjective IKDC scores in the LR-PRP group (45.5 ± 15.5 to 60.7 ± 21.1; P < .0005) and the LP-PRP group (46.8 ± 15.8 to 62.9 ± 19.9; P < .0005), indicating efficacy regardless of PRP type.4
Continue to: Ankle osteoarthritis
Ankle osteoarthritis
❯ ❯ ❯ Additional research is needed
Ankle OA affects 3.4% of all adults and is more common in the younger population than knee or hip OA.16 An RCT (N = 100) investigating PRP vs placebo (saline) injections showed no statistically significant difference in American Orthopedic Foot and Ankle Society scores evaluating pain and function over 26 weeks (–2 points; 95% CI, –5 to 1; P = .16).16 Limitations to this study include its small sample size and the PRP formulation used. (The intervention group received 2 injections of 2 mL of PRP, and the platelet concentration was not reported.)16
Hip osteoarthritis
❯ ❯ ❯ Additional research is needed
Symptomatic hip OA occurs in 40% of adults older than 65 years, with a higher prevalence in women.18 Currently, corticosteroid injections are the only intra-articular therapy recommended by international guidelines for hip OA.19 A systematic review and meta-analysis comparing PRP to HA injections that included 4 RCTs (N = 303) showed a statistically significant reduction in VAS scores at 2 months in the PRP group compared to the HA group (weighted mean difference [WMD] = –0.376; 95% CI, –0.614 to –0.138; P = .002).18 However, there were no significant differences in VAS scores between the PRP and HA groups at 6 months (WMD = –0.141; 95% CI, –0.401 to 0.119; P = .289) and 12 months (WMD = –0.083; 95% CI, –0.343 to 0.117; P = .534). Likewise, no significant differences were found in WOMAC scores at 6 months (WMD = –2.841; 95% CI, –6.248 to 0.565; P = .102) and 12 months (WMD = –3.134; 95% CI, –6.624 to 0.356; P = .078) and Harris Hip Scores (HHS) at 6 months (WMD = 2.782; 95% CI, –6.639 to 12.203; P =.563) and 12 months (WMD = 0.706; 95% CI, –6.333 to 7.745; P = .844).18
A systematic review of 6 RCTs (N = 408) by Belk et al20 comparing PRP to HA for hip OA found similar short-term improvements in WOMAC scores (standardized mean differences [SMD] = 0.27; 95% CI, –0.05 to 0.59; P = .09), VAS scores (MD = 0.59; 95% CI, –0.741 to 1.92; P = .39), and HHS (MD = -0.81; 95% CI, –10.06 to 8.43; P = .93). The average follow-up time was 12.2 and 11.9 months for the PRP and HA groups, respectively.20
LR-PRP, which was used in 1 of the 6 RCTs, showed improvement in VAS scores and HHS from baseline, but no significant difference compared to HA at the latest follow-up.20 A pooled subanalysis of the 3 studies that used LP-PRP found no difference in WOMAC scores between the PRP and HA groups (SMD = 0.42; 95% CI, –0.01 to 0.86; P = .06).20 Future studies comparing the efficacy of intra-articular steroid vs PRP for hip OA would be beneficial.18
Continue to: Rotator cuff tendinopathy
Rotator cuff tendinopathy
❯ ❯ ❯ Consider PRP for short-term pain relief
Painful conditions of the rotator cuff include impingement syndrome, tendonitis, and partial and complete tears. A 2021 RCT (N = 58) by Dadgostar et al21 comparing PRP injection to corticosteroid therapy (methylprednisolone and lidocaine) for the treatment of rotator cuff tendinopathy showed significant improvement in VAS scores at 3 months in the PRP group compared to the corticosteroid group (6.66
Another RCT (N = 99) by Kwong et al22 comparing PRP to corticosteroids found similar short-term advantages of LP-PRP with an improved VAS score (–13.6 vs 0.4; P = .03), American Shoulder and Elbow Surgeons score (13.0 vs 2.9; P = .02), and Western Ontario Rotator Cuff Index score (16.8 vs 5.8; P = .03). However, there was no long-term benefit of PRP over corticosteroids found at 12 months.22
A 2021 systematic review and meta-analysis by Hamid et al23 that included 8 RCTs (N = 976) favored PRP over control (no injection, saline injections, and/or shoulder rehabilitation) with improved VAS scores at 12 months (SMD = –0.5; 95% CI, –0.7 to –0.2; P < .001). The evidence on functional outcome was mixed. Data pooled from 2 studies (n = 228) found better Shoulder Pain and Disability Index (SPADI) scores compared to controls at 3- and 6-month follow-ups. However, there were no significant differences in Disabilities of the Arm, Shoulder and Hand (DASH) scores between the 2 groups.23
Patellar tendinopathy
❯ ❯ ❯ Consider using PRP for return to sport
Patellar tendinopathy, a common MSK condition encountered in the primary care setting, has an overall prevalence of 22% in elite athletes at some point in their career.24 Nonsurgical management options include rest, ice, eccentric and isometric exercises, anti-inflammatory drugs, extracorporeal shock wave therapy (ESWT), and dry needling (DN).
A 2014 RCT (N = 23) evaluating DN vs PRP for patellar tendinopathy favored PRP with improved VAS scores (mean ± SD = 25.4 ± 23.2 points; P = .01 vs 5.2 ± 12.5 points; P = .20) at 12 weeks (P = .02). However, at ≥ 26 weeks, the improvement in pain and function scores was similar between the DN and PRP groups (33.2 ± 14.0 points; P = .001 vs 28.9 ± 25.2 points; P = .01). Notably, there was significantly more improvement in the PRP group at 12 weeks (P = .02) but not at 26 weeks (P = .66).25
Continue to: Another perspective study...
Another prospective study (N = 31) comparing PRP to physiotherapy showed a greater improvement in sport activity level reflected by the Tegner score in the PRP group (percentage improvement, 39 ± 22%) compared to control (20 ± 27%; P = .048) at 6 months.7
A recent RCT (N = 20) revealed improved VAS scores at 6 months with rehabilitation paired with either bone marrow mesenchymal stem cells (BM-MSC) or LP-PRP when compared with baseline (BM-MSC group: 4.23 ± 2.13 to 2.52 ± 2.37; P = .0621; LP-PRP group: 3.10 ± 1.20 to 1.13 ± 1.25; P = .0083). Pain was significantly reduced during sport play in both groups at 6 months when compared with baseline (BM-MSC group: 6.91 ± 1.11 to 3.06 ± 2.89, P = .0049; PRP group: 7.03 ± 1.42 to 1.94 ± 1.24, P = .0001).26
A 2019 systematic review and meta-analysis (N = 2530) demonstrated greater improvements in Victorian Institute of Sport Assessment scale for patellar tendinopathy (VISA-P) with multiple injections of PRP (38.7 points; 95% CI, 26.3-51.2 points) compared to single injections of PRP (24.3 points; 95% CI, 18.2-30.5 points), eccentric exercise (28.3 points; 95% CI, 18.9-37.8 points) and ESWT (27.4 points; 95% CI, 10.0-39.8 points) after 6 months.27 In contrast, an RCT (n = 57) comparing a single injection of LR-PRP or LP-PRP was no more effective than a single injection of saline for improvement in mean VISA-P scores (P > .05) at 1 year.28
Lateral epicondylitis
❯ ❯ ❯ Consider using PRP
Lateral epicondylitis (“tennis elbow”) is caused by overuse of the elbow extensors at the site of the lateral epicondyle. Chronic lateral epicondylosis involves tissue degeneration and microtrauma. Most cases of epicondylar tendinopathies are treated nonoperatively, with corticosteroid injections being a mainstay of treatment despite their short-term benefit29 and potential to deteriorate connective tissue over time. Recent studies suggest PRP therapy for epicondylitis and epicondylosis may increase long-term pain relief and improve function.
A 2017 systematic review and meta-analysis of 16 RCTs (N = 1018) concluded PRP was more efficacious than control injections (bupivacaine) for pain reduction in tendinopathies (effect size = 0.47; 95% CI, 0.22-0.72).30 In the review, lateral epicondylitis was evaluated in 12 studies and was most responsive to PRP (effect size = 0.57) when compared to control injection.30 In another systematic review (5 RCTs; 250 patients), corticosteroid injections improved pain within the first 6 weeks of treatment. However, PRP outperformed corticosteroid in VAS scores (21.3 ± 28.1 vs 42.4 ± 26.8) and DASH scores (17.6 ± 24.0 vs 36.5 ± 23.8) (P < .001) at 2 years.31
Continue to: A 2022 systematic review...
A 2022 systematic review and meta-analysis (26 studies; N = 1040) comparing scores at baseline vs 2 years post-PRP showed improvement in VAS scores (7.4 ± 1.30 vs 3.71 ± 2.35; P < .001), DASH scores (60.8 ± 12.5 vs 13.0 ± 18.5; P < .001), Patient-Rated Tennis Elbow Evaluation (55.6 ± 14.7 vs 48.8 ± 4.1; P < .001), and Mayo Clinic Performance Index (55.5 ± 6.1 vs 93.0 ± 6.7; P < .001).32
Regarding the therapeutic effects of different PRP types in lateral epicondylitis, a 2022 systematic review of 33 studies (N = 2420) found improved function and pain relief with LR-PRP and LP-PRP with no significant differences.33 Pretreatment VAS scores in the LR-PRP group, which ranged from 6.1 to 8.0, improved to 1.5 to 4.0 at 3 months and 0.6 to 3.3 after 1 year.33 Similarly, pretreatment VAS scores in the LP-PRP group, which ranged from 4.2 to 8.4, improved to 1.6 to 5.9 at 3 months and 0.7 to 2.7 after 1 year.34 DASH scores also improved in the LR-PRP and LP-PRP groups, with pretreatment scores (LR-PRP, 47.0 to 54.3; LP-PRP, 30.0 to 67.7) improving to 20.0 to 22.0 and 5.5 to 19.0, respectively, at 1 year.33
Achilles tendinopathy
❯ ❯ ❯ Do not use PRP; evidence is lacking
Achilles tendinopathy, caused by chronic overuse and overload resulting in microtrauma and poor tissue healing, typically occurs in the most poorly vascularized portion of the tendon and is common in runners. First-line treatments for Achilles tendinopathy include eccentric strength training and anti-inflammatory drugs.34,35 Corticosteroid injections are not recommended, given concern for degraded tendon tissue over time and worse function.34
A 2020 systematic review of 11 randomized and nonrandomized clinical trials (N = 406) found PRP improved Victorian Institute of Sports Assessment—Achilles (VISA-A) scores at 24 weeks compared to other nonsurgical treatment options (41.2 vs 70.12; P < .018).34 However, a higher-quality 2021 systematic review and meta-analysis of 4 RCTs (N = 170) comparing PRP injections with placebo showed no significant difference in VISA-A scores at 3 months (0.23; 95% CI, –0.45 to 0.91), 6 months (0.83; 95% CI, –0.26 to 1.92), and 12 months (0.83; 95% CI, –0.77 to 2.44).36 Therefore, further studies are warranted to evaluate the benefit of PRP injections for Achilles tendinopathy.
Conclusions
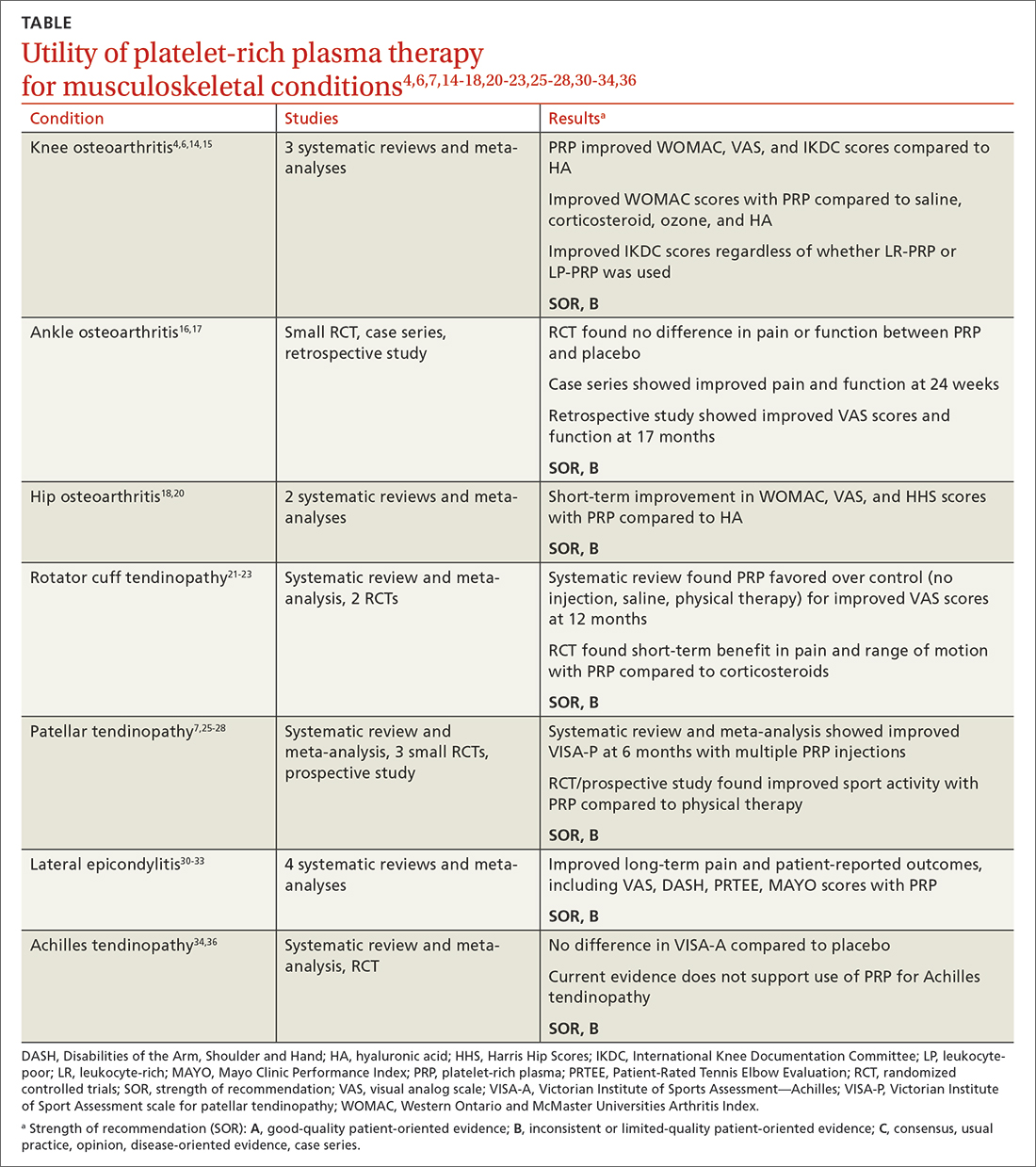
While high-quality studies support the use of PRP for knee OA and lateral epicondylitis, they have a moderate-to-high risk for bias. Several RCTs show that PRP provides superior short-term pain relief and range of motion compared to corticosteroids for rotator cuff tendinopathy. Multiple injections of PRP for patellar tendinopathy may accelerate return to sport and improve symptoms over the long term. However, current evidence does not support PRP therapy for Achilles tendinopathy. Given variability in PRP preparation, an accurate interpretation of the literature regarding its use in MSK conditions is recommended (TABLE4,6,7,14-18,20-23,25-28,30-34,36).
Continue to: Concerning the effectiveness of PRP...
Concerning the effectiveness of PRP, it is important to consider early publication bias. Although recent studies have shown its benefits,6,14,15,37 additional studies comparing PRP to placebo will help demonstrate its efficacy. Interestingly, a literature search by Bar-Or et al38 found intra-articular saline may have a therapeutic effect on knee OA and confound findings when used as a placebo.
Recognizing the presence or lack of clinically significant improvement in the literature is important. For example, while some recent studies have shown PRP exceeds the minimal clinically significant difference for knee OA and lateral epicondylitis, others have not.32,37 A 2021 systematic review of 11 clinical practice guidelines for the use of PRP in knee OA found that 9 were “uncertain or unable to make a recommendation” and 2 recommended against it.39
In its 2021 position statement for the responsible use of regenerative medicine, the American Medical Society for Sports Medicine includes guidance on integrating orthobiologics into clinical practice. The guideline emphasizes informed consent and provides an evidence-based rationale for using PRP in certain patient populations (lateral epicondylitis and younger patients with mild-to-moderate knee OA), recommending its use only after exhausting other conservative options.40 Patients should be referred to physicians with experience using PRP and image-guided procedures.
CORRESPONDENCE
Gregory D. Bentz Jr, MD, 3640 High Street Suite 3B, Portsmouth, VA 23707; [email protected]
1. Cecerska-Heryć E, Goszka M, Serwin N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. doi: 10.1016/j.cytogfr.2021.11.003
2. Le ADK, Enweze L, DeBaun MR, et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. doi: 10.1007/s12178-018-9527-7
3. Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. doi: 10.3390/ijms21207794
4. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50:609-617. doi: 10.1177/03635465211064303
5. Mariani E, Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21:1328. doi: 10.3390/ijms21041328
6. Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49:249-260. doi: 10.1177/0363546520909397
7. Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909-915. doi: 10.1007/s00264-009-0845-7
8. Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40:598-603. doi: 10.1016/j.injury.2008.11.026
9. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1665-1677. doi: 10.1007/s00167-015-3784-4
10. Cook J, Young M. Biologic therapies for tendon and muscle injury. UpToDate. Updated August 11, 2022. Accessed May 23, 2023. www.uptodate.com/contents/biologic-therapies-for-tendon-and-muscle-injury
11. Bendich I, Rubenstein WJ, Cole BJ, et al. What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from Level I randomized controlled trials. Arthroscopy. 2020;36:1983-1991.e1. doi: 10.1016/j.arthro.2020.02.004
12. Jones IA, Togashi RC, Thomas Vangsness C Jr. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11:558-565. doi: 10.1007/s12178-018-9514-z
13. Costa LAV, Lenza M, Irrgang JJ, et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2023;51:1074-1086 doi: 10.1177/03635465211062243
14. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505. doi: 10.1016/j.arthro.2015.08.005
15. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3
16. Paget LDA, Reurink G, de Vos RJ, et al; PRIMA Study Group. Effect of platelet-rich plasma injections vs. placebo on ankle symptoms and function in patients with ankle osteoarthritis: a randomized clinical trial. JAMA. 2021;326:1595-1605. doi: 10.1001/jama.2021.16602
17. Evans A, Ibrahim M, Pope R, et al. Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma. J Orthop. 2020;18:226-236. doi: 10.1016/j.jor.2020.01.037
18. Ye Y, Zhou X, Mao S, et al. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279-287. doi: 10.1016/j.ijsu.2018.03.078.
19. Berney M, McCarroll P, Glynn L, et al. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci. 2021;190:1021-1025. doi: 10.1007/s11845-020-02388-z
20. Belk JW, Houck DA, Littlefield CP, et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of Level I and II randomized controlled trials. Arthroscopy. 2022;38:2035-2046. doi: 10.1016/j.arthro.2021.11.005
21. Dadgostar H, Fahimipour F, Pahlevan Sabagh A, et al. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res. 2021;16:333. doi: 10.1186/s13018-021-02470-x
22. Kwong CA, Woodmass JM, Gusnowski EM, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37:510-517. doi: 10.1016/j.arthro.2020.10.037
23. A Hamid MS, Sazlina SG. Platelet-rich plasma for rotator cuff tendinopathy: a systematic review and meta-analysis. PLoS One. 2021;16:e0251111. doi: 10.1371/journal.pone.0251111
24. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561-567. doi: 10.1177/0363546504270454
25. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416.
26. Rodas G, Soler-Rich R, Rius-Tarruella J, et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49:1492-1504. doi: 10.1177/0363546521998725
27. Andriolo L, Altamura SA, Reale D, et al. Nonsurgical treatments of patellar tendinopathy: multiple injections of platelet-rich plasma are a suitable option: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1001-1018. doi: 10.1177/0363546518759674
28. Scott A, LaPrade RF, Harmon KG, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
29. Kemp JA, Olson MA, Tao MA, et al. Platelet-rich plasma versus corticosteroid injection for the treatment of lateral epicondylitis: a systematic review of systematic reviews. Int J Sports Phys Ther. 2021;16:597-605. doi: 10.26603/001c.24148
30. Miller LE, Parrish WR, Roides B, et al. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017- 000237
31. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J. 2018;4:11.
32. Niemiec P, Szyluk K, Jarosz A, et al. Effectiveness of platelet-rich plasma for lateral epicondylitis: a systematic review and meta-analysis based on achievement of minimal clinically important difference. Orthop J Sports Med. 2022;10:23259671221086920. doi: 10.1177/23259671221086920
33. Li S, Yang G, Zhang H, et al. A systematic review on the efficacy of different types of platelet-rich plasma in the management of lateral epicondylitis. J Shoulder Elbow Surg. 2022;311533-1544. doi: 10.1016/j.jse.2022.02.017.
34. Madhi MI, Yausep OE, Khamdan K, et al. The use of PRP in treatment of Achilles tendinopathy: a systematic review of literature. Study design: systematic review of literature. Ann Med Surg (Lond). 2020;55:320-326. doi: 10.1016/j.amsu.2020.04.042
35. Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2012;1:134-137.
36. Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: a systematic review with meta-analysis. Foot Ankle Surg. 2021;27:486-495. doi: 10.1016/j.fas.2020.07.009
37. Dai WL, Zhou AG, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33:659-670.e1. doi: 10.1016/j.arthro.2016.09.024
38. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16-22. doi: 10.2174/1874312901711010016
39. Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9:23259671211030272. doi: 10.1177/23259671211030272
40. Finnoff JT, Awan TM, Borg-Stein J, et al. American Medical Society for Sports Medicine position statement: principles for the responsible use of regenerative medicine in sports medicine. Clin J Sport Med. 2021;31:530-541. doi: 10.1097/JSM.0000000000000973
Platelet-rich plasma (PRP) injections have become a popular treatment option in a variety of specialties including sports medicine, maxillofacial surgery, dermatology, cosmetology, and reproductive medicine.1 PRP is an autologous blood product derived from whole blood, using a centrifuge to isolate a concentrated layer of platelets. The a-granules in platelets release transforming growth factor b 1, vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor 1, and other mediators that enhance the natural healing process.2

When patients ask. Familiarity with the use of PRP to treat specific musculoskeletal (MSK) conditions is essential for family physicians who frequently are asked by patients about whether PRP is right for them. These patients may have experienced failure of medication therapy or declined surgical intervention, or may not be surgical candidates. This review details the evidence surrounding common intra-articular and extra-articular applications of PRP. But first, a word about how PRP is prepared, its contraindications, and costs.
Preparation and types of PRP
Although there are many commercial systems for preparing PRP, there is no consensus on the optimal formulation.2 Other terms for PRP, such as autologous concentrated platelets and super-concentrated platelets, are based on concentration of red blood cells, leukocytes, and fibrin.3 PRP therapies usually are categorized as leukocyte-rich PRP (LR-PRP) or leukocyte-poor PRP (LP-PRP), based on neutrophil concentrations that are above and below baseline.2 Leukocyte concentration is one of the most debated topics in PRP therapy.4
Common commercially available preparation systems produce platelet concentrations between 3 to 6 times the baseline platelet count.5 Although there is no universally agreed upon PRP formulation, studies have shown 2 centrifugation cycles (“double-spun” or “dual centrifugation”) that yield platelet concentrations between 1.8 to 1.9 times the baseline values significantly improve MSK conditions.6-8
For MSK purposes, PRP may be injected into intratendinous, peritendinous, and intra-articular spaces. Currently, there is no consensus regarding injection frequency. Many studies have incorporated single-injection protocols, while some have used 2 to 3 injections repeated over several weeks to months. PRP commonly is injected at point-of-care without requiring storage.
Contraindications. PRP has been shown to be safe, with most adverse effects attributed to local injection site pain, bleeding, swelling, and bruising.9
Contraindications to PRP include active malignancy or recent remission from malignancy with the exception of nonmetastatic skin tumors.10 PRP is not recommended for patients with an allergy to manufacturing components (eg, dimethyl sulfoxide), thrombocytopenia, nonsteroidal anti-inflammatory drug use within 2 weeks, active infection causing fever, and local infection at the injection site.10 Since local anesthetics may impair platelet function, they should not be given at the same injection site as PRP.10
Continue to: Cost
Cost. PRP is not covered by most insurance plans.11,12 The cost for PRP may range from $500 to $2500 for a single injection.12
Evidence-based summary by condition
Knee osteoarthritis
❯❯❯ Consider using PRP
Knee osteoarthritis (OA) is a common cause of pain and disability. Treatment options include physical therapy, pharmacotherapy, and surgery. PRP has gained popularity as a nonsurgical option. A recent meta-analysis by Costa et al13 of 40 studies with 3035 participants comparing intra-articular PRP with hyaluronic acid (HA), corticosteroid, and saline injections, found that PRP appears to be more effective or as effective as other nonsurgical modalities. However, due to study heterogeneity and high risk for bias, the authors could not recommend PRP for knee OA in clinical practice.13
Despite Costa et al’s findings, reproducible data have demonstrated the superiority of PRP over other nonsurgical treatment options for knee OA. A 2021 systematic review and meta-analysis of 18 randomized controlled trials (RCTs; N = 811) by Belk et al6 comparing PRP to HA injections showed a higher mean improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the PRP group compared to the HA group (44.7% vs 12.6%, respectively; P < .01).6 Six of 11 studies using the visual analog scale (VAS) for pain reported significantly less pain in the PRP group compared to the HA group (P < .05).6 The mean follow-up time was 11.1 months.6 Three of 6 studies reported improved subjective International Knee Documentation Committee (IKDC) scores (range from 0-100, with higher scores representing higher levels of function and lower levels of symptoms) in the PRP group compared to the HA group: 75.7 ± 15.1 vs 65.6 ± 16.9 (P = .004); 65.5 ± 3.6 vs 55.8 ± 3.8 (P = .01); and 60.8 ± 9.8 vs 48.4 ± 6.2 (P < .05).6 There was concern for moderate-to-high heterogeneity.6
Other systematic reviews and meta-analyses found similar efficacy of PRP for knee OA, including improved WOMAC scores and patient-reported outcomes (eg, pain, physical function, stiffness) compared to other injectable options.14,15 A systematic review of 14 RCTs (N = 1423) by Shen et al15 showed improved WOMAC scores at 3 months (mean differences [MD] = –14.53; 95% CI, –29.97 to –7.09; P < .001), 6 months
Despite a lack of consensus regarding the optimal preparation of PRP for knee OA, another recent RCT (N = 192) found significant improvement in mean subjective IKDC scores in the LR-PRP group (45.5 ± 15.5 to 60.7 ± 21.1; P < .0005) and the LP-PRP group (46.8 ± 15.8 to 62.9 ± 19.9; P < .0005), indicating efficacy regardless of PRP type.4
Continue to: Ankle osteoarthritis
Ankle osteoarthritis
❯ ❯ ❯ Additional research is needed
Ankle OA affects 3.4% of all adults and is more common in the younger population than knee or hip OA.16 An RCT (N = 100) investigating PRP vs placebo (saline) injections showed no statistically significant difference in American Orthopedic Foot and Ankle Society scores evaluating pain and function over 26 weeks (–2 points; 95% CI, –5 to 1; P = .16).16 Limitations to this study include its small sample size and the PRP formulation used. (The intervention group received 2 injections of 2 mL of PRP, and the platelet concentration was not reported.)16
Hip osteoarthritis
❯ ❯ ❯ Additional research is needed
Symptomatic hip OA occurs in 40% of adults older than 65 years, with a higher prevalence in women.18 Currently, corticosteroid injections are the only intra-articular therapy recommended by international guidelines for hip OA.19 A systematic review and meta-analysis comparing PRP to HA injections that included 4 RCTs (N = 303) showed a statistically significant reduction in VAS scores at 2 months in the PRP group compared to the HA group (weighted mean difference [WMD] = –0.376; 95% CI, –0.614 to –0.138; P = .002).18 However, there were no significant differences in VAS scores between the PRP and HA groups at 6 months (WMD = –0.141; 95% CI, –0.401 to 0.119; P = .289) and 12 months (WMD = –0.083; 95% CI, –0.343 to 0.117; P = .534). Likewise, no significant differences were found in WOMAC scores at 6 months (WMD = –2.841; 95% CI, –6.248 to 0.565; P = .102) and 12 months (WMD = –3.134; 95% CI, –6.624 to 0.356; P = .078) and Harris Hip Scores (HHS) at 6 months (WMD = 2.782; 95% CI, –6.639 to 12.203; P =.563) and 12 months (WMD = 0.706; 95% CI, –6.333 to 7.745; P = .844).18
A systematic review of 6 RCTs (N = 408) by Belk et al20 comparing PRP to HA for hip OA found similar short-term improvements in WOMAC scores (standardized mean differences [SMD] = 0.27; 95% CI, –0.05 to 0.59; P = .09), VAS scores (MD = 0.59; 95% CI, –0.741 to 1.92; P = .39), and HHS (MD = -0.81; 95% CI, –10.06 to 8.43; P = .93). The average follow-up time was 12.2 and 11.9 months for the PRP and HA groups, respectively.20
LR-PRP, which was used in 1 of the 6 RCTs, showed improvement in VAS scores and HHS from baseline, but no significant difference compared to HA at the latest follow-up.20 A pooled subanalysis of the 3 studies that used LP-PRP found no difference in WOMAC scores between the PRP and HA groups (SMD = 0.42; 95% CI, –0.01 to 0.86; P = .06).20 Future studies comparing the efficacy of intra-articular steroid vs PRP for hip OA would be beneficial.18
Continue to: Rotator cuff tendinopathy
Rotator cuff tendinopathy
❯ ❯ ❯ Consider PRP for short-term pain relief
Painful conditions of the rotator cuff include impingement syndrome, tendonitis, and partial and complete tears. A 2021 RCT (N = 58) by Dadgostar et al21 comparing PRP injection to corticosteroid therapy (methylprednisolone and lidocaine) for the treatment of rotator cuff tendinopathy showed significant improvement in VAS scores at 3 months in the PRP group compared to the corticosteroid group (6.66
Another RCT (N = 99) by Kwong et al22 comparing PRP to corticosteroids found similar short-term advantages of LP-PRP with an improved VAS score (–13.6 vs 0.4; P = .03), American Shoulder and Elbow Surgeons score (13.0 vs 2.9; P = .02), and Western Ontario Rotator Cuff Index score (16.8 vs 5.8; P = .03). However, there was no long-term benefit of PRP over corticosteroids found at 12 months.22
A 2021 systematic review and meta-analysis by Hamid et al23 that included 8 RCTs (N = 976) favored PRP over control (no injection, saline injections, and/or shoulder rehabilitation) with improved VAS scores at 12 months (SMD = –0.5; 95% CI, –0.7 to –0.2; P < .001). The evidence on functional outcome was mixed. Data pooled from 2 studies (n = 228) found better Shoulder Pain and Disability Index (SPADI) scores compared to controls at 3- and 6-month follow-ups. However, there were no significant differences in Disabilities of the Arm, Shoulder and Hand (DASH) scores between the 2 groups.23
Patellar tendinopathy
❯ ❯ ❯ Consider using PRP for return to sport
Patellar tendinopathy, a common MSK condition encountered in the primary care setting, has an overall prevalence of 22% in elite athletes at some point in their career.24 Nonsurgical management options include rest, ice, eccentric and isometric exercises, anti-inflammatory drugs, extracorporeal shock wave therapy (ESWT), and dry needling (DN).
A 2014 RCT (N = 23) evaluating DN vs PRP for patellar tendinopathy favored PRP with improved VAS scores (mean ± SD = 25.4 ± 23.2 points; P = .01 vs 5.2 ± 12.5 points; P = .20) at 12 weeks (P = .02). However, at ≥ 26 weeks, the improvement in pain and function scores was similar between the DN and PRP groups (33.2 ± 14.0 points; P = .001 vs 28.9 ± 25.2 points; P = .01). Notably, there was significantly more improvement in the PRP group at 12 weeks (P = .02) but not at 26 weeks (P = .66).25
Continue to: Another perspective study...
Another prospective study (N = 31) comparing PRP to physiotherapy showed a greater improvement in sport activity level reflected by the Tegner score in the PRP group (percentage improvement, 39 ± 22%) compared to control (20 ± 27%; P = .048) at 6 months.7
A recent RCT (N = 20) revealed improved VAS scores at 6 months with rehabilitation paired with either bone marrow mesenchymal stem cells (BM-MSC) or LP-PRP when compared with baseline (BM-MSC group: 4.23 ± 2.13 to 2.52 ± 2.37; P = .0621; LP-PRP group: 3.10 ± 1.20 to 1.13 ± 1.25; P = .0083). Pain was significantly reduced during sport play in both groups at 6 months when compared with baseline (BM-MSC group: 6.91 ± 1.11 to 3.06 ± 2.89, P = .0049; PRP group: 7.03 ± 1.42 to 1.94 ± 1.24, P = .0001).26
A 2019 systematic review and meta-analysis (N = 2530) demonstrated greater improvements in Victorian Institute of Sport Assessment scale for patellar tendinopathy (VISA-P) with multiple injections of PRP (38.7 points; 95% CI, 26.3-51.2 points) compared to single injections of PRP (24.3 points; 95% CI, 18.2-30.5 points), eccentric exercise (28.3 points; 95% CI, 18.9-37.8 points) and ESWT (27.4 points; 95% CI, 10.0-39.8 points) after 6 months.27 In contrast, an RCT (n = 57) comparing a single injection of LR-PRP or LP-PRP was no more effective than a single injection of saline for improvement in mean VISA-P scores (P > .05) at 1 year.28
Lateral epicondylitis
❯ ❯ ❯ Consider using PRP
Lateral epicondylitis (“tennis elbow”) is caused by overuse of the elbow extensors at the site of the lateral epicondyle. Chronic lateral epicondylosis involves tissue degeneration and microtrauma. Most cases of epicondylar tendinopathies are treated nonoperatively, with corticosteroid injections being a mainstay of treatment despite their short-term benefit29 and potential to deteriorate connective tissue over time. Recent studies suggest PRP therapy for epicondylitis and epicondylosis may increase long-term pain relief and improve function.
A 2017 systematic review and meta-analysis of 16 RCTs (N = 1018) concluded PRP was more efficacious than control injections (bupivacaine) for pain reduction in tendinopathies (effect size = 0.47; 95% CI, 0.22-0.72).30 In the review, lateral epicondylitis was evaluated in 12 studies and was most responsive to PRP (effect size = 0.57) when compared to control injection.30 In another systematic review (5 RCTs; 250 patients), corticosteroid injections improved pain within the first 6 weeks of treatment. However, PRP outperformed corticosteroid in VAS scores (21.3 ± 28.1 vs 42.4 ± 26.8) and DASH scores (17.6 ± 24.0 vs 36.5 ± 23.8) (P < .001) at 2 years.31
Continue to: A 2022 systematic review...
A 2022 systematic review and meta-analysis (26 studies; N = 1040) comparing scores at baseline vs 2 years post-PRP showed improvement in VAS scores (7.4 ± 1.30 vs 3.71 ± 2.35; P < .001), DASH scores (60.8 ± 12.5 vs 13.0 ± 18.5; P < .001), Patient-Rated Tennis Elbow Evaluation (55.6 ± 14.7 vs 48.8 ± 4.1; P < .001), and Mayo Clinic Performance Index (55.5 ± 6.1 vs 93.0 ± 6.7; P < .001).32
Regarding the therapeutic effects of different PRP types in lateral epicondylitis, a 2022 systematic review of 33 studies (N = 2420) found improved function and pain relief with LR-PRP and LP-PRP with no significant differences.33 Pretreatment VAS scores in the LR-PRP group, which ranged from 6.1 to 8.0, improved to 1.5 to 4.0 at 3 months and 0.6 to 3.3 after 1 year.33 Similarly, pretreatment VAS scores in the LP-PRP group, which ranged from 4.2 to 8.4, improved to 1.6 to 5.9 at 3 months and 0.7 to 2.7 after 1 year.34 DASH scores also improved in the LR-PRP and LP-PRP groups, with pretreatment scores (LR-PRP, 47.0 to 54.3; LP-PRP, 30.0 to 67.7) improving to 20.0 to 22.0 and 5.5 to 19.0, respectively, at 1 year.33
Achilles tendinopathy
❯ ❯ ❯ Do not use PRP; evidence is lacking
Achilles tendinopathy, caused by chronic overuse and overload resulting in microtrauma and poor tissue healing, typically occurs in the most poorly vascularized portion of the tendon and is common in runners. First-line treatments for Achilles tendinopathy include eccentric strength training and anti-inflammatory drugs.34,35 Corticosteroid injections are not recommended, given concern for degraded tendon tissue over time and worse function.34
A 2020 systematic review of 11 randomized and nonrandomized clinical trials (N = 406) found PRP improved Victorian Institute of Sports Assessment—Achilles (VISA-A) scores at 24 weeks compared to other nonsurgical treatment options (41.2 vs 70.12; P < .018).34 However, a higher-quality 2021 systematic review and meta-analysis of 4 RCTs (N = 170) comparing PRP injections with placebo showed no significant difference in VISA-A scores at 3 months (0.23; 95% CI, –0.45 to 0.91), 6 months (0.83; 95% CI, –0.26 to 1.92), and 12 months (0.83; 95% CI, –0.77 to 2.44).36 Therefore, further studies are warranted to evaluate the benefit of PRP injections for Achilles tendinopathy.
Conclusions
While high-quality studies support the use of PRP for knee OA and lateral epicondylitis, they have a moderate-to-high risk for bias. Several RCTs show that PRP provides superior short-term pain relief and range of motion compared to corticosteroids for rotator cuff tendinopathy. Multiple injections of PRP for patellar tendinopathy may accelerate return to sport and improve symptoms over the long term. However, current evidence does not support PRP therapy for Achilles tendinopathy. Given variability in PRP preparation, an accurate interpretation of the literature regarding its use in MSK conditions is recommended (TABLE4,6,7,14-18,20-23,25-28,30-34,36).

Continue to: Concerning the effectiveness of PRP...
Concerning the effectiveness of PRP, it is important to consider early publication bias. Although recent studies have shown its benefits,6,14,15,37 additional studies comparing PRP to placebo will help demonstrate its efficacy. Interestingly, a literature search by Bar-Or et al38 found intra-articular saline may have a therapeutic effect on knee OA and confound findings when used as a placebo.
Recognizing the presence or lack of clinically significant improvement in the literature is important. For example, while some recent studies have shown PRP exceeds the minimal clinically significant difference for knee OA and lateral epicondylitis, others have not.32,37 A 2021 systematic review of 11 clinical practice guidelines for the use of PRP in knee OA found that 9 were “uncertain or unable to make a recommendation” and 2 recommended against it.39
In its 2021 position statement for the responsible use of regenerative medicine, the American Medical Society for Sports Medicine includes guidance on integrating orthobiologics into clinical practice. The guideline emphasizes informed consent and provides an evidence-based rationale for using PRP in certain patient populations (lateral epicondylitis and younger patients with mild-to-moderate knee OA), recommending its use only after exhausting other conservative options.40 Patients should be referred to physicians with experience using PRP and image-guided procedures.
CORRESPONDENCE
Gregory D. Bentz Jr, MD, 3640 High Street Suite 3B, Portsmouth, VA 23707; [email protected]
Platelet-rich plasma (PRP) injections have become a popular treatment option in a variety of specialties including sports medicine, maxillofacial surgery, dermatology, cosmetology, and reproductive medicine.1 PRP is an autologous blood product derived from whole blood, using a centrifuge to isolate a concentrated layer of platelets. The a-granules in platelets release transforming growth factor b 1, vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor 1, and other mediators that enhance the natural healing process.2

When patients ask. Familiarity with the use of PRP to treat specific musculoskeletal (MSK) conditions is essential for family physicians who frequently are asked by patients about whether PRP is right for them. These patients may have experienced failure of medication therapy or declined surgical intervention, or may not be surgical candidates. This review details the evidence surrounding common intra-articular and extra-articular applications of PRP. But first, a word about how PRP is prepared, its contraindications, and costs.
Preparation and types of PRP
Although there are many commercial systems for preparing PRP, there is no consensus on the optimal formulation.2 Other terms for PRP, such as autologous concentrated platelets and super-concentrated platelets, are based on concentration of red blood cells, leukocytes, and fibrin.3 PRP therapies usually are categorized as leukocyte-rich PRP (LR-PRP) or leukocyte-poor PRP (LP-PRP), based on neutrophil concentrations that are above and below baseline.2 Leukocyte concentration is one of the most debated topics in PRP therapy.4
Common commercially available preparation systems produce platelet concentrations between 3 to 6 times the baseline platelet count.5 Although there is no universally agreed upon PRP formulation, studies have shown 2 centrifugation cycles (“double-spun” or “dual centrifugation”) that yield platelet concentrations between 1.8 to 1.9 times the baseline values significantly improve MSK conditions.6-8
For MSK purposes, PRP may be injected into intratendinous, peritendinous, and intra-articular spaces. Currently, there is no consensus regarding injection frequency. Many studies have incorporated single-injection protocols, while some have used 2 to 3 injections repeated over several weeks to months. PRP commonly is injected at point-of-care without requiring storage.
Contraindications. PRP has been shown to be safe, with most adverse effects attributed to local injection site pain, bleeding, swelling, and bruising.9
Contraindications to PRP include active malignancy or recent remission from malignancy with the exception of nonmetastatic skin tumors.10 PRP is not recommended for patients with an allergy to manufacturing components (eg, dimethyl sulfoxide), thrombocytopenia, nonsteroidal anti-inflammatory drug use within 2 weeks, active infection causing fever, and local infection at the injection site.10 Since local anesthetics may impair platelet function, they should not be given at the same injection site as PRP.10
Continue to: Cost
Cost. PRP is not covered by most insurance plans.11,12 The cost for PRP may range from $500 to $2500 for a single injection.12
Evidence-based summary by condition
Knee osteoarthritis
❯❯❯ Consider using PRP
Knee osteoarthritis (OA) is a common cause of pain and disability. Treatment options include physical therapy, pharmacotherapy, and surgery. PRP has gained popularity as a nonsurgical option. A recent meta-analysis by Costa et al13 of 40 studies with 3035 participants comparing intra-articular PRP with hyaluronic acid (HA), corticosteroid, and saline injections, found that PRP appears to be more effective or as effective as other nonsurgical modalities. However, due to study heterogeneity and high risk for bias, the authors could not recommend PRP for knee OA in clinical practice.13
Despite Costa et al’s findings, reproducible data have demonstrated the superiority of PRP over other nonsurgical treatment options for knee OA. A 2021 systematic review and meta-analysis of 18 randomized controlled trials (RCTs; N = 811) by Belk et al6 comparing PRP to HA injections showed a higher mean improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the PRP group compared to the HA group (44.7% vs 12.6%, respectively; P < .01).6 Six of 11 studies using the visual analog scale (VAS) for pain reported significantly less pain in the PRP group compared to the HA group (P < .05).6 The mean follow-up time was 11.1 months.6 Three of 6 studies reported improved subjective International Knee Documentation Committee (IKDC) scores (range from 0-100, with higher scores representing higher levels of function and lower levels of symptoms) in the PRP group compared to the HA group: 75.7 ± 15.1 vs 65.6 ± 16.9 (P = .004); 65.5 ± 3.6 vs 55.8 ± 3.8 (P = .01); and 60.8 ± 9.8 vs 48.4 ± 6.2 (P < .05).6 There was concern for moderate-to-high heterogeneity.6
Other systematic reviews and meta-analyses found similar efficacy of PRP for knee OA, including improved WOMAC scores and patient-reported outcomes (eg, pain, physical function, stiffness) compared to other injectable options.14,15 A systematic review of 14 RCTs (N = 1423) by Shen et al15 showed improved WOMAC scores at 3 months (mean differences [MD] = –14.53; 95% CI, –29.97 to –7.09; P < .001), 6 months
Despite a lack of consensus regarding the optimal preparation of PRP for knee OA, another recent RCT (N = 192) found significant improvement in mean subjective IKDC scores in the LR-PRP group (45.5 ± 15.5 to 60.7 ± 21.1; P < .0005) and the LP-PRP group (46.8 ± 15.8 to 62.9 ± 19.9; P < .0005), indicating efficacy regardless of PRP type.4
Continue to: Ankle osteoarthritis
Ankle osteoarthritis
❯ ❯ ❯ Additional research is needed
Ankle OA affects 3.4% of all adults and is more common in the younger population than knee or hip OA.16 An RCT (N = 100) investigating PRP vs placebo (saline) injections showed no statistically significant difference in American Orthopedic Foot and Ankle Society scores evaluating pain and function over 26 weeks (–2 points; 95% CI, –5 to 1; P = .16).16 Limitations to this study include its small sample size and the PRP formulation used. (The intervention group received 2 injections of 2 mL of PRP, and the platelet concentration was not reported.)16
Hip osteoarthritis
❯ ❯ ❯ Additional research is needed
Symptomatic hip OA occurs in 40% of adults older than 65 years, with a higher prevalence in women.18 Currently, corticosteroid injections are the only intra-articular therapy recommended by international guidelines for hip OA.19 A systematic review and meta-analysis comparing PRP to HA injections that included 4 RCTs (N = 303) showed a statistically significant reduction in VAS scores at 2 months in the PRP group compared to the HA group (weighted mean difference [WMD] = –0.376; 95% CI, –0.614 to –0.138; P = .002).18 However, there were no significant differences in VAS scores between the PRP and HA groups at 6 months (WMD = –0.141; 95% CI, –0.401 to 0.119; P = .289) and 12 months (WMD = –0.083; 95% CI, –0.343 to 0.117; P = .534). Likewise, no significant differences were found in WOMAC scores at 6 months (WMD = –2.841; 95% CI, –6.248 to 0.565; P = .102) and 12 months (WMD = –3.134; 95% CI, –6.624 to 0.356; P = .078) and Harris Hip Scores (HHS) at 6 months (WMD = 2.782; 95% CI, –6.639 to 12.203; P =.563) and 12 months (WMD = 0.706; 95% CI, –6.333 to 7.745; P = .844).18
A systematic review of 6 RCTs (N = 408) by Belk et al20 comparing PRP to HA for hip OA found similar short-term improvements in WOMAC scores (standardized mean differences [SMD] = 0.27; 95% CI, –0.05 to 0.59; P = .09), VAS scores (MD = 0.59; 95% CI, –0.741 to 1.92; P = .39), and HHS (MD = -0.81; 95% CI, –10.06 to 8.43; P = .93). The average follow-up time was 12.2 and 11.9 months for the PRP and HA groups, respectively.20
LR-PRP, which was used in 1 of the 6 RCTs, showed improvement in VAS scores and HHS from baseline, but no significant difference compared to HA at the latest follow-up.20 A pooled subanalysis of the 3 studies that used LP-PRP found no difference in WOMAC scores between the PRP and HA groups (SMD = 0.42; 95% CI, –0.01 to 0.86; P = .06).20 Future studies comparing the efficacy of intra-articular steroid vs PRP for hip OA would be beneficial.18
Continue to: Rotator cuff tendinopathy
Rotator cuff tendinopathy
❯ ❯ ❯ Consider PRP for short-term pain relief
Painful conditions of the rotator cuff include impingement syndrome, tendonitis, and partial and complete tears. A 2021 RCT (N = 58) by Dadgostar et al21 comparing PRP injection to corticosteroid therapy (methylprednisolone and lidocaine) for the treatment of rotator cuff tendinopathy showed significant improvement in VAS scores at 3 months in the PRP group compared to the corticosteroid group (6.66
Another RCT (N = 99) by Kwong et al22 comparing PRP to corticosteroids found similar short-term advantages of LP-PRP with an improved VAS score (–13.6 vs 0.4; P = .03), American Shoulder and Elbow Surgeons score (13.0 vs 2.9; P = .02), and Western Ontario Rotator Cuff Index score (16.8 vs 5.8; P = .03). However, there was no long-term benefit of PRP over corticosteroids found at 12 months.22
A 2021 systematic review and meta-analysis by Hamid et al23 that included 8 RCTs (N = 976) favored PRP over control (no injection, saline injections, and/or shoulder rehabilitation) with improved VAS scores at 12 months (SMD = –0.5; 95% CI, –0.7 to –0.2; P < .001). The evidence on functional outcome was mixed. Data pooled from 2 studies (n = 228) found better Shoulder Pain and Disability Index (SPADI) scores compared to controls at 3- and 6-month follow-ups. However, there were no significant differences in Disabilities of the Arm, Shoulder and Hand (DASH) scores between the 2 groups.23
Patellar tendinopathy
❯ ❯ ❯ Consider using PRP for return to sport
Patellar tendinopathy, a common MSK condition encountered in the primary care setting, has an overall prevalence of 22% in elite athletes at some point in their career.24 Nonsurgical management options include rest, ice, eccentric and isometric exercises, anti-inflammatory drugs, extracorporeal shock wave therapy (ESWT), and dry needling (DN).
A 2014 RCT (N = 23) evaluating DN vs PRP for patellar tendinopathy favored PRP with improved VAS scores (mean ± SD = 25.4 ± 23.2 points; P = .01 vs 5.2 ± 12.5 points; P = .20) at 12 weeks (P = .02). However, at ≥ 26 weeks, the improvement in pain and function scores was similar between the DN and PRP groups (33.2 ± 14.0 points; P = .001 vs 28.9 ± 25.2 points; P = .01). Notably, there was significantly more improvement in the PRP group at 12 weeks (P = .02) but not at 26 weeks (P = .66).25
Continue to: Another perspective study...
Another prospective study (N = 31) comparing PRP to physiotherapy showed a greater improvement in sport activity level reflected by the Tegner score in the PRP group (percentage improvement, 39 ± 22%) compared to control (20 ± 27%; P = .048) at 6 months.7
A recent RCT (N = 20) revealed improved VAS scores at 6 months with rehabilitation paired with either bone marrow mesenchymal stem cells (BM-MSC) or LP-PRP when compared with baseline (BM-MSC group: 4.23 ± 2.13 to 2.52 ± 2.37; P = .0621; LP-PRP group: 3.10 ± 1.20 to 1.13 ± 1.25; P = .0083). Pain was significantly reduced during sport play in both groups at 6 months when compared with baseline (BM-MSC group: 6.91 ± 1.11 to 3.06 ± 2.89, P = .0049; PRP group: 7.03 ± 1.42 to 1.94 ± 1.24, P = .0001).26
A 2019 systematic review and meta-analysis (N = 2530) demonstrated greater improvements in Victorian Institute of Sport Assessment scale for patellar tendinopathy (VISA-P) with multiple injections of PRP (38.7 points; 95% CI, 26.3-51.2 points) compared to single injections of PRP (24.3 points; 95% CI, 18.2-30.5 points), eccentric exercise (28.3 points; 95% CI, 18.9-37.8 points) and ESWT (27.4 points; 95% CI, 10.0-39.8 points) after 6 months.27 In contrast, an RCT (n = 57) comparing a single injection of LR-PRP or LP-PRP was no more effective than a single injection of saline for improvement in mean VISA-P scores (P > .05) at 1 year.28
Lateral epicondylitis
❯ ❯ ❯ Consider using PRP
Lateral epicondylitis (“tennis elbow”) is caused by overuse of the elbow extensors at the site of the lateral epicondyle. Chronic lateral epicondylosis involves tissue degeneration and microtrauma. Most cases of epicondylar tendinopathies are treated nonoperatively, with corticosteroid injections being a mainstay of treatment despite their short-term benefit29 and potential to deteriorate connective tissue over time. Recent studies suggest PRP therapy for epicondylitis and epicondylosis may increase long-term pain relief and improve function.
A 2017 systematic review and meta-analysis of 16 RCTs (N = 1018) concluded PRP was more efficacious than control injections (bupivacaine) for pain reduction in tendinopathies (effect size = 0.47; 95% CI, 0.22-0.72).30 In the review, lateral epicondylitis was evaluated in 12 studies and was most responsive to PRP (effect size = 0.57) when compared to control injection.30 In another systematic review (5 RCTs; 250 patients), corticosteroid injections improved pain within the first 6 weeks of treatment. However, PRP outperformed corticosteroid in VAS scores (21.3 ± 28.1 vs 42.4 ± 26.8) and DASH scores (17.6 ± 24.0 vs 36.5 ± 23.8) (P < .001) at 2 years.31
Continue to: A 2022 systematic review...
A 2022 systematic review and meta-analysis (26 studies; N = 1040) comparing scores at baseline vs 2 years post-PRP showed improvement in VAS scores (7.4 ± 1.30 vs 3.71 ± 2.35; P < .001), DASH scores (60.8 ± 12.5 vs 13.0 ± 18.5; P < .001), Patient-Rated Tennis Elbow Evaluation (55.6 ± 14.7 vs 48.8 ± 4.1; P < .001), and Mayo Clinic Performance Index (55.5 ± 6.1 vs 93.0 ± 6.7; P < .001).32
Regarding the therapeutic effects of different PRP types in lateral epicondylitis, a 2022 systematic review of 33 studies (N = 2420) found improved function and pain relief with LR-PRP and LP-PRP with no significant differences.33 Pretreatment VAS scores in the LR-PRP group, which ranged from 6.1 to 8.0, improved to 1.5 to 4.0 at 3 months and 0.6 to 3.3 after 1 year.33 Similarly, pretreatment VAS scores in the LP-PRP group, which ranged from 4.2 to 8.4, improved to 1.6 to 5.9 at 3 months and 0.7 to 2.7 after 1 year.34 DASH scores also improved in the LR-PRP and LP-PRP groups, with pretreatment scores (LR-PRP, 47.0 to 54.3; LP-PRP, 30.0 to 67.7) improving to 20.0 to 22.0 and 5.5 to 19.0, respectively, at 1 year.33
Achilles tendinopathy
❯ ❯ ❯ Do not use PRP; evidence is lacking
Achilles tendinopathy, caused by chronic overuse and overload resulting in microtrauma and poor tissue healing, typically occurs in the most poorly vascularized portion of the tendon and is common in runners. First-line treatments for Achilles tendinopathy include eccentric strength training and anti-inflammatory drugs.34,35 Corticosteroid injections are not recommended, given concern for degraded tendon tissue over time and worse function.34
A 2020 systematic review of 11 randomized and nonrandomized clinical trials (N = 406) found PRP improved Victorian Institute of Sports Assessment—Achilles (VISA-A) scores at 24 weeks compared to other nonsurgical treatment options (41.2 vs 70.12; P < .018).34 However, a higher-quality 2021 systematic review and meta-analysis of 4 RCTs (N = 170) comparing PRP injections with placebo showed no significant difference in VISA-A scores at 3 months (0.23; 95% CI, –0.45 to 0.91), 6 months (0.83; 95% CI, –0.26 to 1.92), and 12 months (0.83; 95% CI, –0.77 to 2.44).36 Therefore, further studies are warranted to evaluate the benefit of PRP injections for Achilles tendinopathy.
Conclusions
While high-quality studies support the use of PRP for knee OA and lateral epicondylitis, they have a moderate-to-high risk for bias. Several RCTs show that PRP provides superior short-term pain relief and range of motion compared to corticosteroids for rotator cuff tendinopathy. Multiple injections of PRP for patellar tendinopathy may accelerate return to sport and improve symptoms over the long term. However, current evidence does not support PRP therapy for Achilles tendinopathy. Given variability in PRP preparation, an accurate interpretation of the literature regarding its use in MSK conditions is recommended (TABLE4,6,7,14-18,20-23,25-28,30-34,36).

Continue to: Concerning the effectiveness of PRP...
Concerning the effectiveness of PRP, it is important to consider early publication bias. Although recent studies have shown its benefits,6,14,15,37 additional studies comparing PRP to placebo will help demonstrate its efficacy. Interestingly, a literature search by Bar-Or et al38 found intra-articular saline may have a therapeutic effect on knee OA and confound findings when used as a placebo.
Recognizing the presence or lack of clinically significant improvement in the literature is important. For example, while some recent studies have shown PRP exceeds the minimal clinically significant difference for knee OA and lateral epicondylitis, others have not.32,37 A 2021 systematic review of 11 clinical practice guidelines for the use of PRP in knee OA found that 9 were “uncertain or unable to make a recommendation” and 2 recommended against it.39
In its 2021 position statement for the responsible use of regenerative medicine, the American Medical Society for Sports Medicine includes guidance on integrating orthobiologics into clinical practice. The guideline emphasizes informed consent and provides an evidence-based rationale for using PRP in certain patient populations (lateral epicondylitis and younger patients with mild-to-moderate knee OA), recommending its use only after exhausting other conservative options.40 Patients should be referred to physicians with experience using PRP and image-guided procedures.
CORRESPONDENCE
Gregory D. Bentz Jr, MD, 3640 High Street Suite 3B, Portsmouth, VA 23707; [email protected]
1. Cecerska-Heryć E, Goszka M, Serwin N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. doi: 10.1016/j.cytogfr.2021.11.003
2. Le ADK, Enweze L, DeBaun MR, et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. doi: 10.1007/s12178-018-9527-7
3. Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. doi: 10.3390/ijms21207794
4. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50:609-617. doi: 10.1177/03635465211064303
5. Mariani E, Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21:1328. doi: 10.3390/ijms21041328
6. Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49:249-260. doi: 10.1177/0363546520909397
7. Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909-915. doi: 10.1007/s00264-009-0845-7
8. Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40:598-603. doi: 10.1016/j.injury.2008.11.026
9. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1665-1677. doi: 10.1007/s00167-015-3784-4
10. Cook J, Young M. Biologic therapies for tendon and muscle injury. UpToDate. Updated August 11, 2022. Accessed May 23, 2023. www.uptodate.com/contents/biologic-therapies-for-tendon-and-muscle-injury
11. Bendich I, Rubenstein WJ, Cole BJ, et al. What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from Level I randomized controlled trials. Arthroscopy. 2020;36:1983-1991.e1. doi: 10.1016/j.arthro.2020.02.004
12. Jones IA, Togashi RC, Thomas Vangsness C Jr. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11:558-565. doi: 10.1007/s12178-018-9514-z
13. Costa LAV, Lenza M, Irrgang JJ, et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2023;51:1074-1086 doi: 10.1177/03635465211062243
14. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505. doi: 10.1016/j.arthro.2015.08.005
15. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3
16. Paget LDA, Reurink G, de Vos RJ, et al; PRIMA Study Group. Effect of platelet-rich plasma injections vs. placebo on ankle symptoms and function in patients with ankle osteoarthritis: a randomized clinical trial. JAMA. 2021;326:1595-1605. doi: 10.1001/jama.2021.16602
17. Evans A, Ibrahim M, Pope R, et al. Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma. J Orthop. 2020;18:226-236. doi: 10.1016/j.jor.2020.01.037
18. Ye Y, Zhou X, Mao S, et al. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279-287. doi: 10.1016/j.ijsu.2018.03.078.
19. Berney M, McCarroll P, Glynn L, et al. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci. 2021;190:1021-1025. doi: 10.1007/s11845-020-02388-z
20. Belk JW, Houck DA, Littlefield CP, et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of Level I and II randomized controlled trials. Arthroscopy. 2022;38:2035-2046. doi: 10.1016/j.arthro.2021.11.005
21. Dadgostar H, Fahimipour F, Pahlevan Sabagh A, et al. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res. 2021;16:333. doi: 10.1186/s13018-021-02470-x
22. Kwong CA, Woodmass JM, Gusnowski EM, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37:510-517. doi: 10.1016/j.arthro.2020.10.037
23. A Hamid MS, Sazlina SG. Platelet-rich plasma for rotator cuff tendinopathy: a systematic review and meta-analysis. PLoS One. 2021;16:e0251111. doi: 10.1371/journal.pone.0251111
24. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561-567. doi: 10.1177/0363546504270454
25. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416.
26. Rodas G, Soler-Rich R, Rius-Tarruella J, et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49:1492-1504. doi: 10.1177/0363546521998725
27. Andriolo L, Altamura SA, Reale D, et al. Nonsurgical treatments of patellar tendinopathy: multiple injections of platelet-rich plasma are a suitable option: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1001-1018. doi: 10.1177/0363546518759674
28. Scott A, LaPrade RF, Harmon KG, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
29. Kemp JA, Olson MA, Tao MA, et al. Platelet-rich plasma versus corticosteroid injection for the treatment of lateral epicondylitis: a systematic review of systematic reviews. Int J Sports Phys Ther. 2021;16:597-605. doi: 10.26603/001c.24148
30. Miller LE, Parrish WR, Roides B, et al. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017- 000237
31. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J. 2018;4:11.
32. Niemiec P, Szyluk K, Jarosz A, et al. Effectiveness of platelet-rich plasma for lateral epicondylitis: a systematic review and meta-analysis based on achievement of minimal clinically important difference. Orthop J Sports Med. 2022;10:23259671221086920. doi: 10.1177/23259671221086920
33. Li S, Yang G, Zhang H, et al. A systematic review on the efficacy of different types of platelet-rich plasma in the management of lateral epicondylitis. J Shoulder Elbow Surg. 2022;311533-1544. doi: 10.1016/j.jse.2022.02.017.
34. Madhi MI, Yausep OE, Khamdan K, et al. The use of PRP in treatment of Achilles tendinopathy: a systematic review of literature. Study design: systematic review of literature. Ann Med Surg (Lond). 2020;55:320-326. doi: 10.1016/j.amsu.2020.04.042
35. Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2012;1:134-137.
36. Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: a systematic review with meta-analysis. Foot Ankle Surg. 2021;27:486-495. doi: 10.1016/j.fas.2020.07.009
37. Dai WL, Zhou AG, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33:659-670.e1. doi: 10.1016/j.arthro.2016.09.024
38. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16-22. doi: 10.2174/1874312901711010016
39. Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9:23259671211030272. doi: 10.1177/23259671211030272
40. Finnoff JT, Awan TM, Borg-Stein J, et al. American Medical Society for Sports Medicine position statement: principles for the responsible use of regenerative medicine in sports medicine. Clin J Sport Med. 2021;31:530-541. doi: 10.1097/JSM.0000000000000973
1. Cecerska-Heryć E, Goszka M, Serwin N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. doi: 10.1016/j.cytogfr.2021.11.003
2. Le ADK, Enweze L, DeBaun MR, et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. doi: 10.1007/s12178-018-9527-7
3. Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. doi: 10.3390/ijms21207794
4. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50:609-617. doi: 10.1177/03635465211064303
5. Mariani E, Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21:1328. doi: 10.3390/ijms21041328
6. Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49:249-260. doi: 10.1177/0363546520909397
7. Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909-915. doi: 10.1007/s00264-009-0845-7
8. Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40:598-603. doi: 10.1016/j.injury.2008.11.026
9. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1665-1677. doi: 10.1007/s00167-015-3784-4
10. Cook J, Young M. Biologic therapies for tendon and muscle injury. UpToDate. Updated August 11, 2022. Accessed May 23, 2023. www.uptodate.com/contents/biologic-therapies-for-tendon-and-muscle-injury
11. Bendich I, Rubenstein WJ, Cole BJ, et al. What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from Level I randomized controlled trials. Arthroscopy. 2020;36:1983-1991.e1. doi: 10.1016/j.arthro.2020.02.004
12. Jones IA, Togashi RC, Thomas Vangsness C Jr. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11:558-565. doi: 10.1007/s12178-018-9514-z
13. Costa LAV, Lenza M, Irrgang JJ, et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2023;51:1074-1086 doi: 10.1177/03635465211062243
14. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505. doi: 10.1016/j.arthro.2015.08.005
15. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3
16. Paget LDA, Reurink G, de Vos RJ, et al; PRIMA Study Group. Effect of platelet-rich plasma injections vs. placebo on ankle symptoms and function in patients with ankle osteoarthritis: a randomized clinical trial. JAMA. 2021;326:1595-1605. doi: 10.1001/jama.2021.16602
17. Evans A, Ibrahim M, Pope R, et al. Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma. J Orthop. 2020;18:226-236. doi: 10.1016/j.jor.2020.01.037
18. Ye Y, Zhou X, Mao S, et al. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279-287. doi: 10.1016/j.ijsu.2018.03.078.
19. Berney M, McCarroll P, Glynn L, et al. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci. 2021;190:1021-1025. doi: 10.1007/s11845-020-02388-z
20. Belk JW, Houck DA, Littlefield CP, et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of Level I and II randomized controlled trials. Arthroscopy. 2022;38:2035-2046. doi: 10.1016/j.arthro.2021.11.005
21. Dadgostar H, Fahimipour F, Pahlevan Sabagh A, et al. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res. 2021;16:333. doi: 10.1186/s13018-021-02470-x
22. Kwong CA, Woodmass JM, Gusnowski EM, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37:510-517. doi: 10.1016/j.arthro.2020.10.037
23. A Hamid MS, Sazlina SG. Platelet-rich plasma for rotator cuff tendinopathy: a systematic review and meta-analysis. PLoS One. 2021;16:e0251111. doi: 10.1371/journal.pone.0251111
24. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561-567. doi: 10.1177/0363546504270454
25. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416.
26. Rodas G, Soler-Rich R, Rius-Tarruella J, et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49:1492-1504. doi: 10.1177/0363546521998725
27. Andriolo L, Altamura SA, Reale D, et al. Nonsurgical treatments of patellar tendinopathy: multiple injections of platelet-rich plasma are a suitable option: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1001-1018. doi: 10.1177/0363546518759674
28. Scott A, LaPrade RF, Harmon KG, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
29. Kemp JA, Olson MA, Tao MA, et al. Platelet-rich plasma versus corticosteroid injection for the treatment of lateral epicondylitis: a systematic review of systematic reviews. Int J Sports Phys Ther. 2021;16:597-605. doi: 10.26603/001c.24148
30. Miller LE, Parrish WR, Roides B, et al. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017- 000237
31. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J. 2018;4:11.
32. Niemiec P, Szyluk K, Jarosz A, et al. Effectiveness of platelet-rich plasma for lateral epicondylitis: a systematic review and meta-analysis based on achievement of minimal clinically important difference. Orthop J Sports Med. 2022;10:23259671221086920. doi: 10.1177/23259671221086920
33. Li S, Yang G, Zhang H, et al. A systematic review on the efficacy of different types of platelet-rich plasma in the management of lateral epicondylitis. J Shoulder Elbow Surg. 2022;311533-1544. doi: 10.1016/j.jse.2022.02.017.
34. Madhi MI, Yausep OE, Khamdan K, et al. The use of PRP in treatment of Achilles tendinopathy: a systematic review of literature. Study design: systematic review of literature. Ann Med Surg (Lond). 2020;55:320-326. doi: 10.1016/j.amsu.2020.04.042
35. Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2012;1:134-137.
36. Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: a systematic review with meta-analysis. Foot Ankle Surg. 2021;27:486-495. doi: 10.1016/j.fas.2020.07.009
37. Dai WL, Zhou AG, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33:659-670.e1. doi: 10.1016/j.arthro.2016.09.024
38. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16-22. doi: 10.2174/1874312901711010016
39. Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9:23259671211030272. doi: 10.1177/23259671211030272
40. Finnoff JT, Awan TM, Borg-Stein J, et al. American Medical Society for Sports Medicine position statement: principles for the responsible use of regenerative medicine in sports medicine. Clin J Sport Med. 2021;31:530-541. doi: 10.1097/JSM.0000000000000973
PRACTICE RECOMMENDATIONS
› Consider plateletrich plasma (PRP) for conservative management of knee osteoarthritis and lateral epicondylitis. B
› Consider giving multiple injections of PRP for longterm pain relief and expedited return to sport in patellar tendinopathy. B
› Do not use PRP for Achilles tendinopathy due to a lack of clinical evidence. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series