User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
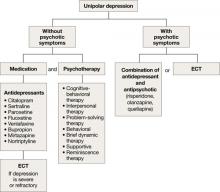
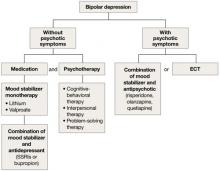
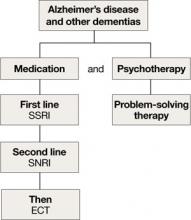
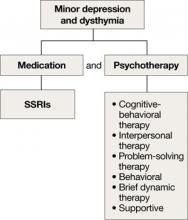
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Sharing a patient’s care: Secrets for success
Split treatment occurs when a psychiatrist and psychotherapist share a patient’s care. These relationships create opportunities and challenges for both clinicians and patients. Some patients may experience positive transference, such as nurturing and idealization, whereas others may have negative transference, including narcissistic wounds and devaluation of 1 or both clinicians.1 This article will help you navigate split treatment relationships.
Types of relationships
Duplicative services involve a patient receiving psychotherapy or psychopharmacology from ≥2 clinicians; the latter is more common. Duplicative psychopharmacology can occur inadvertently when drugs prescribed by a nonpsychiatrist overlap with psychotropics in their effects.
Duplicative opinions. When sought with the knowledge of all parties, second opinions can strengthen the doctor-patient relationship and improve outcomes. Duplicative opinions may be pathologic when patients do not disclose their opinion-seeking to both clinicians. This form of “doctor shopping” is common among patients with personality disorders, especially borderline personality disorder, and those who seek drugs from multiple providers to abuse, stockpile, or sell them.
Strategies for split treatment
During the first session, discuss the patient’s expectations. Establish a treatment plan, setting forth what you are and are not responsible for managing. If the patient is transferring treatment to you, ask if he or she has terminated treatment with the prior clinician.
If psychotropics are indicated, ask the patient to agree that only you will manage medications for mood, attention, cognition, energy, and sleep, except in an emergency such as an allergic reaction. Inform your patient that having multiple physicians managing these symptoms puts him or her at risk for drug-drug interactions and ineffective treatment. Ask your patient to notify you about any new medications.
A patient’s psychodynamic issues with medications can complicate pharmacotherapy. For example, a patient may experience transference reactions to medications, such as issues relating to control of the treatment process or psychogenic side effects. Ideally, the psychotherapist and psychiatrist work together to determine which clinician could better address these issues.
If a patient reports that another clinician devalued your treatment, don’t react immediately. Explore with your patient how the discussion with the other clinician came about. Patients can misinterpret what other clinicians say or lie in an attempt to create conflict between you and your colleagues. Give the other clinician the benefit of the doubt and if possible, speak to him or her before commenting about the alleged statements. Look at these situations as an opportunity to set limits with patients.
1. Bradley SS. Non-physician psychotherapist-physician pharmacotherapists: a new model for concurrent treatment. Psychiatr Clin North Am. 1990;13(2):307-322.
Split treatment occurs when a psychiatrist and psychotherapist share a patient’s care. These relationships create opportunities and challenges for both clinicians and patients. Some patients may experience positive transference, such as nurturing and idealization, whereas others may have negative transference, including narcissistic wounds and devaluation of 1 or both clinicians.1 This article will help you navigate split treatment relationships.
Types of relationships
Duplicative services involve a patient receiving psychotherapy or psychopharmacology from ≥2 clinicians; the latter is more common. Duplicative psychopharmacology can occur inadvertently when drugs prescribed by a nonpsychiatrist overlap with psychotropics in their effects.
Duplicative opinions. When sought with the knowledge of all parties, second opinions can strengthen the doctor-patient relationship and improve outcomes. Duplicative opinions may be pathologic when patients do not disclose their opinion-seeking to both clinicians. This form of “doctor shopping” is common among patients with personality disorders, especially borderline personality disorder, and those who seek drugs from multiple providers to abuse, stockpile, or sell them.
Strategies for split treatment
During the first session, discuss the patient’s expectations. Establish a treatment plan, setting forth what you are and are not responsible for managing. If the patient is transferring treatment to you, ask if he or she has terminated treatment with the prior clinician.
If psychotropics are indicated, ask the patient to agree that only you will manage medications for mood, attention, cognition, energy, and sleep, except in an emergency such as an allergic reaction. Inform your patient that having multiple physicians managing these symptoms puts him or her at risk for drug-drug interactions and ineffective treatment. Ask your patient to notify you about any new medications.
A patient’s psychodynamic issues with medications can complicate pharmacotherapy. For example, a patient may experience transference reactions to medications, such as issues relating to control of the treatment process or psychogenic side effects. Ideally, the psychotherapist and psychiatrist work together to determine which clinician could better address these issues.
If a patient reports that another clinician devalued your treatment, don’t react immediately. Explore with your patient how the discussion with the other clinician came about. Patients can misinterpret what other clinicians say or lie in an attempt to create conflict between you and your colleagues. Give the other clinician the benefit of the doubt and if possible, speak to him or her before commenting about the alleged statements. Look at these situations as an opportunity to set limits with patients.
Split treatment occurs when a psychiatrist and psychotherapist share a patient’s care. These relationships create opportunities and challenges for both clinicians and patients. Some patients may experience positive transference, such as nurturing and idealization, whereas others may have negative transference, including narcissistic wounds and devaluation of 1 or both clinicians.1 This article will help you navigate split treatment relationships.
Types of relationships
Duplicative services involve a patient receiving psychotherapy or psychopharmacology from ≥2 clinicians; the latter is more common. Duplicative psychopharmacology can occur inadvertently when drugs prescribed by a nonpsychiatrist overlap with psychotropics in their effects.
Duplicative opinions. When sought with the knowledge of all parties, second opinions can strengthen the doctor-patient relationship and improve outcomes. Duplicative opinions may be pathologic when patients do not disclose their opinion-seeking to both clinicians. This form of “doctor shopping” is common among patients with personality disorders, especially borderline personality disorder, and those who seek drugs from multiple providers to abuse, stockpile, or sell them.
Strategies for split treatment
During the first session, discuss the patient’s expectations. Establish a treatment plan, setting forth what you are and are not responsible for managing. If the patient is transferring treatment to you, ask if he or she has terminated treatment with the prior clinician.
If psychotropics are indicated, ask the patient to agree that only you will manage medications for mood, attention, cognition, energy, and sleep, except in an emergency such as an allergic reaction. Inform your patient that having multiple physicians managing these symptoms puts him or her at risk for drug-drug interactions and ineffective treatment. Ask your patient to notify you about any new medications.
A patient’s psychodynamic issues with medications can complicate pharmacotherapy. For example, a patient may experience transference reactions to medications, such as issues relating to control of the treatment process or psychogenic side effects. Ideally, the psychotherapist and psychiatrist work together to determine which clinician could better address these issues.
If a patient reports that another clinician devalued your treatment, don’t react immediately. Explore with your patient how the discussion with the other clinician came about. Patients can misinterpret what other clinicians say or lie in an attempt to create conflict between you and your colleagues. Give the other clinician the benefit of the doubt and if possible, speak to him or her before commenting about the alleged statements. Look at these situations as an opportunity to set limits with patients.
1. Bradley SS. Non-physician psychotherapist-physician pharmacotherapists: a new model for concurrent treatment. Psychiatr Clin North Am. 1990;13(2):307-322.
1. Bradley SS. Non-physician psychotherapist-physician pharmacotherapists: a new model for concurrent treatment. Psychiatr Clin North Am. 1990;13(2):307-322.
Treat the patient, not the disease: Practicing psychiatry in the era of guidelines, protocols, and algorithms
Personalized care is at the heart of good medical care. It is an indispensable ingredient for optimal clinical outcomes because each patient is unique, as an individual and as a patient, and requires customized treatment.
If 10 patients with depression walk into a psychiatrist’s office on any given day, each will be different and should be treated accordingly. Their symptoms may be similar thematically but they differ widely in presentation and content. Their medical and psychiatric histories and social, educational, religious, ethnic, socioeconomic, and attitudinal diversity can be stunning in complexity and disparity. Just as patients’ symptoms can be similar yet different, so can their response to a specific antidepressant or psychotherapy. Their clinical and functional outcomes will vary widely in degree and valence. Every psychiatrist expects (and enjoys) the richness of patient backgrounds and manages each individually.
Given these individual differences among our psychiatric patients, why are practitioners being barraged by various entities to abandon the traditional medical approach to their patients? Why is there a push to transform personalized clinical care to an assembly-line system, where patients are defined by their disease and are managed like “human widgets” as though they can be “processed” in an identical, protocolized, mechanical manner? This is completely antithetical to the magnificent personal approach inherent in the classic and highly effective doctor-patient relationship.
There is nothing wrong with treating patients based on up-to-date practice guidelines and evidence-based principles of clinical effectiveness. The issue is whether clinical decisions should be made by the physician, one patient at a time, rather than imposing the dreaded “cookie-cutter” approach of protocols or algorithms on a population of patients whose only commonality is a DSM-IV-TR diagnosis. The not-so-hidden agenda of the business-oriented managed care systems is to lower costs, not to provide the best personalized medical care. Who came up with the absurd notion that there is such a thing as “an average patient” who would respond to a prepackaged, economically efficient “average treatment”? That is a serious disservice to the spectrum of patients suffering from psychiatric illnesses and an insult to skilled, compassionate psychiatrists who can provide customized care to each patient.
It is certainly paradoxical that at a time when personalized medicine is advocated as “best practice” in medical care, managed care health systems are propagating and implementing a contrarian movement of homogenizing treatment into rigid protocols with a preset, algorithmic approach. These competing messages create a confusing state of cognitive dissonance, especially for trainees, as to how clinicians should deliver medical care for their patients.
It is well known that a large proportion of psychiatric disorders (>80%) have no evidence-based, FDA-approved treatments, and no practice guidelines, protocols, or standards of care.1 This is where psychiatrists have to use more art than science—including the necessary, but often maligned, off-label treatments—to help reduce their patients’ suffering. In these situations, the physician-patient relationship simply cannot be superseded by any prepackaged protocol, and physicians should decide what is best for their patient.
So let physicians unite behind what makes medicine such a noble profession: combining the best available scientific knowledge with experience and well-honed clinical judgment to deliver customized care, one patient at a time. We must treat our patients exactly as we want to be treated when we inevitably suffer from an illness.
1. Devulapalli K, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian Journal of Psychiatry. 2009;2:29-36.
Personalized care is at the heart of good medical care. It is an indispensable ingredient for optimal clinical outcomes because each patient is unique, as an individual and as a patient, and requires customized treatment.
If 10 patients with depression walk into a psychiatrist’s office on any given day, each will be different and should be treated accordingly. Their symptoms may be similar thematically but they differ widely in presentation and content. Their medical and psychiatric histories and social, educational, religious, ethnic, socioeconomic, and attitudinal diversity can be stunning in complexity and disparity. Just as patients’ symptoms can be similar yet different, so can their response to a specific antidepressant or psychotherapy. Their clinical and functional outcomes will vary widely in degree and valence. Every psychiatrist expects (and enjoys) the richness of patient backgrounds and manages each individually.
Given these individual differences among our psychiatric patients, why are practitioners being barraged by various entities to abandon the traditional medical approach to their patients? Why is there a push to transform personalized clinical care to an assembly-line system, where patients are defined by their disease and are managed like “human widgets” as though they can be “processed” in an identical, protocolized, mechanical manner? This is completely antithetical to the magnificent personal approach inherent in the classic and highly effective doctor-patient relationship.
There is nothing wrong with treating patients based on up-to-date practice guidelines and evidence-based principles of clinical effectiveness. The issue is whether clinical decisions should be made by the physician, one patient at a time, rather than imposing the dreaded “cookie-cutter” approach of protocols or algorithms on a population of patients whose only commonality is a DSM-IV-TR diagnosis. The not-so-hidden agenda of the business-oriented managed care systems is to lower costs, not to provide the best personalized medical care. Who came up with the absurd notion that there is such a thing as “an average patient” who would respond to a prepackaged, economically efficient “average treatment”? That is a serious disservice to the spectrum of patients suffering from psychiatric illnesses and an insult to skilled, compassionate psychiatrists who can provide customized care to each patient.
It is certainly paradoxical that at a time when personalized medicine is advocated as “best practice” in medical care, managed care health systems are propagating and implementing a contrarian movement of homogenizing treatment into rigid protocols with a preset, algorithmic approach. These competing messages create a confusing state of cognitive dissonance, especially for trainees, as to how clinicians should deliver medical care for their patients.
It is well known that a large proportion of psychiatric disorders (>80%) have no evidence-based, FDA-approved treatments, and no practice guidelines, protocols, or standards of care.1 This is where psychiatrists have to use more art than science—including the necessary, but often maligned, off-label treatments—to help reduce their patients’ suffering. In these situations, the physician-patient relationship simply cannot be superseded by any prepackaged protocol, and physicians should decide what is best for their patient.
So let physicians unite behind what makes medicine such a noble profession: combining the best available scientific knowledge with experience and well-honed clinical judgment to deliver customized care, one patient at a time. We must treat our patients exactly as we want to be treated when we inevitably suffer from an illness.
Personalized care is at the heart of good medical care. It is an indispensable ingredient for optimal clinical outcomes because each patient is unique, as an individual and as a patient, and requires customized treatment.
If 10 patients with depression walk into a psychiatrist’s office on any given day, each will be different and should be treated accordingly. Their symptoms may be similar thematically but they differ widely in presentation and content. Their medical and psychiatric histories and social, educational, religious, ethnic, socioeconomic, and attitudinal diversity can be stunning in complexity and disparity. Just as patients’ symptoms can be similar yet different, so can their response to a specific antidepressant or psychotherapy. Their clinical and functional outcomes will vary widely in degree and valence. Every psychiatrist expects (and enjoys) the richness of patient backgrounds and manages each individually.
Given these individual differences among our psychiatric patients, why are practitioners being barraged by various entities to abandon the traditional medical approach to their patients? Why is there a push to transform personalized clinical care to an assembly-line system, where patients are defined by their disease and are managed like “human widgets” as though they can be “processed” in an identical, protocolized, mechanical manner? This is completely antithetical to the magnificent personal approach inherent in the classic and highly effective doctor-patient relationship.
There is nothing wrong with treating patients based on up-to-date practice guidelines and evidence-based principles of clinical effectiveness. The issue is whether clinical decisions should be made by the physician, one patient at a time, rather than imposing the dreaded “cookie-cutter” approach of protocols or algorithms on a population of patients whose only commonality is a DSM-IV-TR diagnosis. The not-so-hidden agenda of the business-oriented managed care systems is to lower costs, not to provide the best personalized medical care. Who came up with the absurd notion that there is such a thing as “an average patient” who would respond to a prepackaged, economically efficient “average treatment”? That is a serious disservice to the spectrum of patients suffering from psychiatric illnesses and an insult to skilled, compassionate psychiatrists who can provide customized care to each patient.
It is certainly paradoxical that at a time when personalized medicine is advocated as “best practice” in medical care, managed care health systems are propagating and implementing a contrarian movement of homogenizing treatment into rigid protocols with a preset, algorithmic approach. These competing messages create a confusing state of cognitive dissonance, especially for trainees, as to how clinicians should deliver medical care for their patients.
It is well known that a large proportion of psychiatric disorders (>80%) have no evidence-based, FDA-approved treatments, and no practice guidelines, protocols, or standards of care.1 This is where psychiatrists have to use more art than science—including the necessary, but often maligned, off-label treatments—to help reduce their patients’ suffering. In these situations, the physician-patient relationship simply cannot be superseded by any prepackaged protocol, and physicians should decide what is best for their patient.
So let physicians unite behind what makes medicine such a noble profession: combining the best available scientific knowledge with experience and well-honed clinical judgment to deliver customized care, one patient at a time. We must treat our patients exactly as we want to be treated when we inevitably suffer from an illness.
1. Devulapalli K, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian Journal of Psychiatry. 2009;2:29-36.
1. Devulapalli K, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian Journal of Psychiatry. 2009;2:29-36.
Adolescents who self-harm: How to protect them from themselves
Discuss this article at http://currentpsychiatry.blogspot.com/2010/08/adolescents-who-self-harm.html#comments
Josh, age 16, gets poor grades in school and occasionally smokes marijuana and abuses inhalants. After his girlfriend breaks up with him, he cuts his wrist with a hunting knife. While bleeding profusely, Josh calls his mother at work, who calls 911. The cut is deep and requires sutures. Josh says he did not try to kill himself; he only wanted to carve his girlfriend’s initials into his wrist to show his love for her.
When treating teenagers with self-harming thoughts and behavior, it may be difficult to distinguish suicide attempts from self-injury without intent to die. Understanding adolescent self-harm, suicide risk assessment, and treatment options guides clinicians to appropriate interventions. Recognizing the need for aggressive treatment—including psychiatric hospitalization—is essential to keeping self-harming teenagers safe.
Suicidal vs nonsuicidal self-harm
Suicidal behavior involves intent to end one’s life and includes ideation (thoughts) and actions (non fatal or fatal attempts).1 Nonsuicidal self-injury (NSSI) involves socially unacceptable, self-inflicted harm to one’s body without intent to die.2
Suicide is the third leading cause of death among youths age 12 to 19, claiming almost 2,000 lives each year.3 Nearly 1 in 5 (17%) U.S. high school students has suicidal thoughts each year, and almost 1 in 10 (8%) attempts suicide.4
Studies report a 13% to 23% lifetime prevalence of NSSI.5 These behaviors often begin between age 13 to 15.6 Cutting and hitting are the most common forms of NSSI; other methods include burning, scratching, and interfering with wound healing. Most teens who harm themselves without suicidal intent report that they feel little or no pain during the act.5 Unlike suicide attempts, NSSI can be viewed as a means to stay alive. Many adolescents injure themselves to cope with overwhelming feelings that can produce suicidal thoughts. Self-injury may distract the adolescent from painful emotions, reduce tensions, or penetrate numbness.7
Teenagers who hurt themselves but do not intend to die are at high risk for suicide and suicide attempts. Adolescents who engage in NSSI are more likely to experience suicidal behaviors, and vice versa.8 In a large study, 70% of adolescents who engaged in NSSI had made at least 1 suicide attempt and 55% made multiple suicide attempts.2 Current suicidal ideation is a risk factor for suicide, and a past suicide attempt is the strongest predictor of future suicidal behavior.9
Risk factors for suicidal behavior and NSSI overlap (Table 1)2,5,6,10,11 and include:
- depression
- substance use
- anxiety
- impulsive aggression
- history of childhood trauma.
Many teens who engage in NSSI report depression.2 A history of psychiatric illness—especially depression—increases the likelihood of adolescent suicide.8 A study comparing adolescents who engaged in NSSI with those who attempted suicide found that both groups reported similar levels of suicidal ideation and depressive symptoms.6 However, adolescents with a history of NSSI and attempted suicide reported higher levels of suicidal ideation and fewer reasons for living than those who attempted suicide but have no history of NSSI.12
Factors that protect against suicidal behavior include:
- a good parent-child relationship
- strong cultural or religious values
- an intact family
- a sense of connection with peer group and community.13
No studies have determined protective factors for NSSI.
Table 1
Characteristics of teens who harm themselves
| Factor | Comments |
|---|---|
| Older age | Both suicide attempts and NSSI are more common in mid-adolescence (age 13 to 15) |
| Sex | Males complete suicide more often (4:1) but more females make attempts. Sex differences have not been consistently identified for NSSI |
| Psychiatric illness | Diagnoses associated with adolescent suicide include major depression, substance abuse, and conduct disorder |
| Psychosocial and situational risks (usually combined with psychiatric illness) | Recent loss or rejection, living alone (eg, running away or homeless), poor social supports, family conflicts, family suicidal behavior, poor communication with parents, availability of firearms, exposure to suicide in the community or media, academic difficulties, legal problems, gender identity conflicts, history of maltreatment, being bullied, and risky behaviors |
| NSSI: nonsuicidal self-injury | |
| Source: References 2,5,6,10,11 | |
CASE CONTINUED: ‘No point in living’
As Josh becomes less guarded, he says that he sees “no point in living” without his girlfriend. He thought the only way to feel better was to “get high,” but this left him feeling even more despondent and anxious. He wrote a suicide note, but after cutting himself he was unsure he wanted to die. Josh says that when he feels depressed he can’t talk to his parents because “they wouldn’t understand and don’t care.”
Assessing self-harming adolescents
Distinguishing between suicidal behaviors and NSSI can be challenging (Table 2). Identifying risk factors for adolescent suicidal behavior must be coupled with a thorough psychiatric evaluation. If possible, interview the adolescent alone and obtain collateral information from parents, family members, teachers, caseworkers, probation officers, and others as needed. Also examine family interactions because conflicts and communication problems could undermine the teenager’s safety.
Consider using standardized measures of suicidal intentions such as the Scale for Suicidal Ideation (SSI).14 Although the SSI was developed for adults, a large case-control study validated the scale’s use in adolescent psychiatric outpatients and students.15 In addition to assessing subjective reports of suicidal intent, the SSI also takes into account objective indicators of increased risk, such as planning an attempt, hiding details from others, and making preparations for death.
Questions to ask. After a self-harm incident, it may be helpful to begin the psychiatric interview with a general question such as, “What happened that led you to hurt yourself?” This does not categorize the act as suicidal and allows the adolescent to describe it in his or her own words. Shea16 suggests normalizing the act by assuming that self-harm occurred, rather than making the patient admit to it. For example, an interviewer might say “Many people I know who are hurting inside also try to hurt their body; how often do you do that?”
Inquire about suicidal intent in a few ways. For example, first ask, “Do you ever wish you were dead?” and follow up with, “Would you ever do anything to try to make yourself dead?” Asking about suicidal thoughts does not increase suicidal thoughts or behavior.10,17
Reviewing thoughts and feelings leading up to a self-harm act can help identify triggers, coping difficulties, and issues to address in treatment. This behavioral analysis can be completed using the mnemonic ABC:
- Antecedents (situations or stressors leading to self-harming thoughts or actions)
- Behavior characteristics (frequency, intensity, and duration of self-harming)
- Consequences (eg, emotional relief, care and attention from others).
The results of this analysis could suggest treatment strategies, such as cognitive restructuring or techniques for decreasing feelings of distress.
The Risk of Suicide Questionnaire, which is designed for adolescents, asks:18
- Are you here because you tried to hurt yourself?
- In the past week, have you been having thoughts about killing yourself?
- Have you ever tried to hurt yourself in the past?
- Has something very stressful happened to you in the past few weeks?
Research has yet to determine whether this simple, rapid screen accurately identifies the need for psychiatric hospitalization or risk for suicidal outcomes. Although clinician- and self-administered suicide questionnaires may be useful for screening large populations, they are not a substitute for a thorough clinical assessment.
Inpatient or outpatient? When evaluating self-harming adolescents, first determine if they are in imminent danger of suicide and if more intensive services, such as hospitalization, are needed to maintain safety. Inpatient psychiatric services are appropriate for adolescents with suicidal thoughts or self-harm behaviors in addition to acute psychiatric disorders, significant substance abuse, serious medical issues, poor social supports, or inability to be managed safely as an outpatient.19 See the Table for a list of additional factors to consider.
Consent for treatment may be required because many self-harming adolescents do not present with life-threatening symptoms. Laws regarding consent vary among states. In some jurisdictions, patients age ≥15 can consent to mental health treatment without parents’ knowledge or consent. If an adolescent is in imminent danger and cannot voluntarily consent to treatment, physicians can initiate mental health “holds.” Some states allow registered nurses, psychologists, licensed social workers, and others to initiate mental health holds.
Table 2
Strategies for assessing adolescent self-harm
| Complete a thorough psychiatric evaluation |
| Interview the adolescent separately from parents |
| Obtain collateral information from parents and family, teachers, caseworkers, and others as needed |
| Use an empathic, nonjudgmental manner |
| Note appearance and presence of scarring and bruises, and patient’s clothing style |
Ask about current and past self-harming thoughts and behavior:
|
| Ask about acute stressors (eg, break-up, loss or rejection, conflict with parents) |
| Inquire about thoughts, feelings, and events leading up to the self-harm episode |
| Assess for psychosis and ask about homicidal thoughts. If yes, assess whether there is a duty to warn others |
| Ask about drug/alcohol use and consider a urine toxicology screen to help clarify whether substance abuse problems may be contributing to self-harm |
| Assess family interaction and communication style, noting conflicts that might impact safety |
| Consider using a standardized measure, such as the Scale for Suicidal Ideation14,15 |
CASE CONTINUED: Inpatient treatment
After the interview Josh says he still feels that “there is no point in living” and he cannot develop an adequate safety plan with his family. He is hospitalized to maintain safety, improve his coping skills and communication with his family, and mobilize safety plans, social supports, and follow-up care.
Maintaining safety
Psychosocial treatments for suicidal behaviors and NSSI are similar because with both, the priority is to help the patient maintain safety. This may include:
- developing a collaborative safety plan with family
- increasing monitoring
- removing access to firearms or other lethal means
- helping the adolescent to develop alternate, safer coping methods.
Many clinicians rely on no-harm contracts or agreements; however, there is no evidence that they are effective.20 The American Psychiatric Association recommends against using no-harm contracts with patients who are new, in an emergency setting, using substances, agitated, psychotic, or impulsive.21 Instead, clinicians, adolescents, and families can discuss specific steps the patient can take to remain safe. This collaborative plan should identify situations likely to trigger self-harming impulses; adaptive ways the teenager can cope, such as taking a nap or jogging; methods for communicating distress to family members and other helpers; and places to go for help, such as an emergency room. These safety plans should draw on the patient’s internal and external resources.
CASE CONTINUED: Strengthening relationships
While in the hospital, Josh finds it helpful to use a 0-to-10 scale to measure his distress and let his family know the intensity of his feelings. He identifies situations when he felt like hurting himself, such as being humiliated in math class. Josh learns about cognitive distortions—such as “they don’t care” and “there is no point in living”—and discusses methods for managing his feelings if he encounters further disappointments. His parents become more attentive when Josh explains his feelings, which allows the family to develop a collaborative safety plan. Josh decides to strengthen friendships he had been neglecting and agrees to attend a substance abuse treatment program.
Psychosocial treatment
In addition to maintaining safety, treatment goals for self-harming adolescents include:
- managing underlying psychiatric disorders
- identifying triggers for self-injurious acts
- improving family relationships
- developing better communication and coping skills.
Improving affective language skills, acquiring frustration tolerance, and learning alternatives to self-injury are key to strengthening coping abilities. Address problem-solving skills because self-harming adolescents often lack these abilities.22
Treatment of self-harming adolescents often consists of cognitive-behavioral therapy (CBT)23 or dialectical behavior therapy (DBT).24 CBT involves examining cognitive distortions or otherwise unhealthy beliefs about oneself, others, and life in general, focusing specifically on thoughts the patient has immediately before engaging in self-harm. DBT also integrates emotion regulation training and mindfulness. A review of 28 studies found these therapies effectively reduced self-harm behaviors in adults.25 However, few studies have examined these therapies’ efficacy in self-harming adolescents.
Pharmacotherapy
Psychopharmacology should focus on treating underlying psychiatric disorders. No medications are specifically effective for treating suicidal thoughts, suicidal behaviors, or NSSI. Some evidence suggests that antidepressants may trigger suicidal thoughts in a small proportion of youth,26 but the benefits of antidepressants outweigh the risk of suicidal thoughts.27 When prescribing antidepressants, inform patients and their parents of possible adverse reactions and monitor the patient regularly.28
Take precautions when prescribing medication for self-harming adolescents. For example, benzodiazepines may cause disinhibition, and larger quantities of medication could be lethal in an overdose. If possible, arrange for parents or guardians to monitor medication use.
What to document
Good documentation is especially helpful when an adolescent requires involuntary commitment or is discharged home. Involuntary commitment is based on legal interpretation of 3 circumstances—danger to self, danger to others, or gravely disabled—in which safety concerns may override an individual’s civil rights. If involuntary commitment is needed, a physician must clarify how the youth meets ≥1 of these criteria. If the adolescent is discharged, document that the patient is not an imminent danger to self or others and why you made this determination. Also note that follow-up services and a safety plan are in place, a parent will monitor safety issues and remove firearms and other lethal means from the home, and acute conflicts have been resolved. Other details, such as using the patient’s words to describe reasons for living, can be helpful.
Related Resources
- National Alliance for the Mentally Ill. National Helpline. 800-950-6264.
- American Foundation for Suicide Prevention. www.afsp.org.
- American Association of Suicidology. www.suicidology.org.
- National Suicide Prevention Lifeline. 800-273-TALK (8255).
Acknowledgements
The authors wish to thank students Scott Schubert from Regis University, Denver, CO, and Emily Peterson from Beloit College, Beloit, WI, for their help in preparing the manuscript.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table
Hospitalization or home? Acute crisis planning for self-harming youth
Hospitalization is more appropriate when ≥1 of the following is present with suicidal or self-injurious thoughts:
|
Home is more appropriate when:
|
1. O’Carroll PW, Berman AL, Maris RW, et al. Beyond the Tower of Babel: a nomenclature for suicidology. Suicide Life Threat Behav. 1996;26:237-252.
2. Nock MK, Joiner TE, Jr, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
3. Centers for Disease Control and Prevention. Injury prevention and control: data and statistics (WISQARS). Available at: http://www.cdc.gov/injury/wisqars. Accessed June 22, 2010.
4. National Center for Health Statistics. Health, United States, 2006. Available at: http://www.cdc.gov/nchs/data/hus/hus06.pdf#062. Accessed May 28, 2010.
5. Jacobson CM, Gould M. The epidemiology and phenomenology of non-suicidal self-injurious behavior among adolescents: a critical review of the literature. Arch Suicide Res. 2007;11(2):129-147.
6. Muehlenkamp JJ, Gutierrez PM. An investigation of differences between self-injurious behavior and suicide attempts in a sample of adolescents. Suicide Life Threat Behav. 2004;34:12-23.
7. Nixon MK, Cloutier PF, Aggarwal S. Affect regulation and addictive aspects of repetitive self-injury in hospitalized adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41:1333-1341.
8. Whitlock J, Knox KL. The relationship between self-injurious behavior and suicide in a young adult population. Arch Pediatr Adolesc Med. 2007;161:634-640.
9. Bridge JA, Goldstein TR, Brent DA. Adolescent suicide and suicidal behavior. J Child Psychol Psychiatry. 2006;47:372-394.
10. American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(suppl 7):24S-51S.
11. Laye-Gindhu A, Schonert-Reichl KA. Nonsuicidal self-harm among community adolescents: understanding the “whats” and “whys” of self-harm. J Youth Adolesc. 2005;34:447-457.
12. Muehlenkamp JJ, Gutierrez PM. Risk for suicide attempts among adolescents who engage in non-suicidal self-injury. Arch Suicide Res. 2007;11:69-82.
13. Gould MS, Greenberg T, Velting DM, et al. Youth suicide risk and preventive interventions: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2003;42:386-405.
14. Beck AT, Kovacs M, Weissmann A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;46:343-352.
15. Holi MM, Pelkonen M, Karlsson L, et al. Psychometric properties and clinical utility of the Scale for Suicidal Ideation (SSI) in adolescents. BMC Psychiatry. 2005;5:8.-
16. Shea SC. The practical art of suicide assessment: a guide for mental health professionals and substance abuse counselors. New York, NY: John Wiley; 1999.
17. Gould MS, Marrocco FA, Kleinman M, et al. Evaluating iatrogenic risk of youth suicide screening programs. JAMA. 2005;293:1635-1643.
18. Horowitz LM, Wang PS, Koocher GP, et al. Detecting suicide risk in a pediatric emergency department: development of a brief screening tool. Pediatrics. 2001;107:1133-1137.
19. Kennedy SP, Baraff LJ, Suddath RL, et al. Emergency department management of suicidal adolescents. Ann Emerg Med. 2004;43:452-460.
20. Lewis LM. No-harm contracts: a review of what we know. Suicide Life Threat Behav. 2007;37:50-57.
21. American Psychiatric Association. Practice guideline for the assessment and treatment of patients with suicidal behavior. Am J Psychiatry. 2003;160(suppl 11):1-60.
22. Speckens AE, Hawton K. Social problem solving in adolescents with suicidal behavior: a systematic review. Suicide Life Threat Behav. 2005;35:365-387.
23. Henriques G, Beck AT, Brown GK. Cognitive therapy for adolescent and young adult suicide attempters. American Behavioral Scientist. 2003;46:1258-1268.
24. Rathus JH, Miller AL. Dialectical behavior therapy adapted for suicidal adolescents. Suicide Life Threat Behav. 2002;32:146-157.
25. Tarrier N, Taylor K, Gooding P. Cognitive-behavioral interventions to reduce suicide behavior: a systematic review and meta-analysis. Behav Modif. 2008;32:77-108.
26. Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292:338-343.
27. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
28. Simon GE. The antidepressant quandary—considering suicide risk when treating adolescent depression. N Engl J Med. 2006;355:2722-2723.
Discuss this article at http://currentpsychiatry.blogspot.com/2010/08/adolescents-who-self-harm.html#comments
Josh, age 16, gets poor grades in school and occasionally smokes marijuana and abuses inhalants. After his girlfriend breaks up with him, he cuts his wrist with a hunting knife. While bleeding profusely, Josh calls his mother at work, who calls 911. The cut is deep and requires sutures. Josh says he did not try to kill himself; he only wanted to carve his girlfriend’s initials into his wrist to show his love for her.
When treating teenagers with self-harming thoughts and behavior, it may be difficult to distinguish suicide attempts from self-injury without intent to die. Understanding adolescent self-harm, suicide risk assessment, and treatment options guides clinicians to appropriate interventions. Recognizing the need for aggressive treatment—including psychiatric hospitalization—is essential to keeping self-harming teenagers safe.
Suicidal vs nonsuicidal self-harm
Suicidal behavior involves intent to end one’s life and includes ideation (thoughts) and actions (non fatal or fatal attempts).1 Nonsuicidal self-injury (NSSI) involves socially unacceptable, self-inflicted harm to one’s body without intent to die.2
Suicide is the third leading cause of death among youths age 12 to 19, claiming almost 2,000 lives each year.3 Nearly 1 in 5 (17%) U.S. high school students has suicidal thoughts each year, and almost 1 in 10 (8%) attempts suicide.4
Studies report a 13% to 23% lifetime prevalence of NSSI.5 These behaviors often begin between age 13 to 15.6 Cutting and hitting are the most common forms of NSSI; other methods include burning, scratching, and interfering with wound healing. Most teens who harm themselves without suicidal intent report that they feel little or no pain during the act.5 Unlike suicide attempts, NSSI can be viewed as a means to stay alive. Many adolescents injure themselves to cope with overwhelming feelings that can produce suicidal thoughts. Self-injury may distract the adolescent from painful emotions, reduce tensions, or penetrate numbness.7
Teenagers who hurt themselves but do not intend to die are at high risk for suicide and suicide attempts. Adolescents who engage in NSSI are more likely to experience suicidal behaviors, and vice versa.8 In a large study, 70% of adolescents who engaged in NSSI had made at least 1 suicide attempt and 55% made multiple suicide attempts.2 Current suicidal ideation is a risk factor for suicide, and a past suicide attempt is the strongest predictor of future suicidal behavior.9
Risk factors for suicidal behavior and NSSI overlap (Table 1)2,5,6,10,11 and include:
- depression
- substance use
- anxiety
- impulsive aggression
- history of childhood trauma.
Many teens who engage in NSSI report depression.2 A history of psychiatric illness—especially depression—increases the likelihood of adolescent suicide.8 A study comparing adolescents who engaged in NSSI with those who attempted suicide found that both groups reported similar levels of suicidal ideation and depressive symptoms.6 However, adolescents with a history of NSSI and attempted suicide reported higher levels of suicidal ideation and fewer reasons for living than those who attempted suicide but have no history of NSSI.12
Factors that protect against suicidal behavior include:
- a good parent-child relationship
- strong cultural or religious values
- an intact family
- a sense of connection with peer group and community.13
No studies have determined protective factors for NSSI.
Table 1
Characteristics of teens who harm themselves
| Factor | Comments |
|---|---|
| Older age | Both suicide attempts and NSSI are more common in mid-adolescence (age 13 to 15) |
| Sex | Males complete suicide more often (4:1) but more females make attempts. Sex differences have not been consistently identified for NSSI |
| Psychiatric illness | Diagnoses associated with adolescent suicide include major depression, substance abuse, and conduct disorder |
| Psychosocial and situational risks (usually combined with psychiatric illness) | Recent loss or rejection, living alone (eg, running away or homeless), poor social supports, family conflicts, family suicidal behavior, poor communication with parents, availability of firearms, exposure to suicide in the community or media, academic difficulties, legal problems, gender identity conflicts, history of maltreatment, being bullied, and risky behaviors |
| NSSI: nonsuicidal self-injury | |
| Source: References 2,5,6,10,11 | |
CASE CONTINUED: ‘No point in living’
As Josh becomes less guarded, he says that he sees “no point in living” without his girlfriend. He thought the only way to feel better was to “get high,” but this left him feeling even more despondent and anxious. He wrote a suicide note, but after cutting himself he was unsure he wanted to die. Josh says that when he feels depressed he can’t talk to his parents because “they wouldn’t understand and don’t care.”
Assessing self-harming adolescents
Distinguishing between suicidal behaviors and NSSI can be challenging (Table 2). Identifying risk factors for adolescent suicidal behavior must be coupled with a thorough psychiatric evaluation. If possible, interview the adolescent alone and obtain collateral information from parents, family members, teachers, caseworkers, probation officers, and others as needed. Also examine family interactions because conflicts and communication problems could undermine the teenager’s safety.
Consider using standardized measures of suicidal intentions such as the Scale for Suicidal Ideation (SSI).14 Although the SSI was developed for adults, a large case-control study validated the scale’s use in adolescent psychiatric outpatients and students.15 In addition to assessing subjective reports of suicidal intent, the SSI also takes into account objective indicators of increased risk, such as planning an attempt, hiding details from others, and making preparations for death.
Questions to ask. After a self-harm incident, it may be helpful to begin the psychiatric interview with a general question such as, “What happened that led you to hurt yourself?” This does not categorize the act as suicidal and allows the adolescent to describe it in his or her own words. Shea16 suggests normalizing the act by assuming that self-harm occurred, rather than making the patient admit to it. For example, an interviewer might say “Many people I know who are hurting inside also try to hurt their body; how often do you do that?”
Inquire about suicidal intent in a few ways. For example, first ask, “Do you ever wish you were dead?” and follow up with, “Would you ever do anything to try to make yourself dead?” Asking about suicidal thoughts does not increase suicidal thoughts or behavior.10,17
Reviewing thoughts and feelings leading up to a self-harm act can help identify triggers, coping difficulties, and issues to address in treatment. This behavioral analysis can be completed using the mnemonic ABC:
- Antecedents (situations or stressors leading to self-harming thoughts or actions)
- Behavior characteristics (frequency, intensity, and duration of self-harming)
- Consequences (eg, emotional relief, care and attention from others).
The results of this analysis could suggest treatment strategies, such as cognitive restructuring or techniques for decreasing feelings of distress.
The Risk of Suicide Questionnaire, which is designed for adolescents, asks:18
- Are you here because you tried to hurt yourself?
- In the past week, have you been having thoughts about killing yourself?
- Have you ever tried to hurt yourself in the past?
- Has something very stressful happened to you in the past few weeks?
Research has yet to determine whether this simple, rapid screen accurately identifies the need for psychiatric hospitalization or risk for suicidal outcomes. Although clinician- and self-administered suicide questionnaires may be useful for screening large populations, they are not a substitute for a thorough clinical assessment.
Inpatient or outpatient? When evaluating self-harming adolescents, first determine if they are in imminent danger of suicide and if more intensive services, such as hospitalization, are needed to maintain safety. Inpatient psychiatric services are appropriate for adolescents with suicidal thoughts or self-harm behaviors in addition to acute psychiatric disorders, significant substance abuse, serious medical issues, poor social supports, or inability to be managed safely as an outpatient.19 See the Table for a list of additional factors to consider.
Consent for treatment may be required because many self-harming adolescents do not present with life-threatening symptoms. Laws regarding consent vary among states. In some jurisdictions, patients age ≥15 can consent to mental health treatment without parents’ knowledge or consent. If an adolescent is in imminent danger and cannot voluntarily consent to treatment, physicians can initiate mental health “holds.” Some states allow registered nurses, psychologists, licensed social workers, and others to initiate mental health holds.
Table 2
Strategies for assessing adolescent self-harm
| Complete a thorough psychiatric evaluation |
| Interview the adolescent separately from parents |
| Obtain collateral information from parents and family, teachers, caseworkers, and others as needed |
| Use an empathic, nonjudgmental manner |
| Note appearance and presence of scarring and bruises, and patient’s clothing style |
Ask about current and past self-harming thoughts and behavior:
|
| Ask about acute stressors (eg, break-up, loss or rejection, conflict with parents) |
| Inquire about thoughts, feelings, and events leading up to the self-harm episode |
| Assess for psychosis and ask about homicidal thoughts. If yes, assess whether there is a duty to warn others |
| Ask about drug/alcohol use and consider a urine toxicology screen to help clarify whether substance abuse problems may be contributing to self-harm |
| Assess family interaction and communication style, noting conflicts that might impact safety |
| Consider using a standardized measure, such as the Scale for Suicidal Ideation14,15 |
CASE CONTINUED: Inpatient treatment
After the interview Josh says he still feels that “there is no point in living” and he cannot develop an adequate safety plan with his family. He is hospitalized to maintain safety, improve his coping skills and communication with his family, and mobilize safety plans, social supports, and follow-up care.
Maintaining safety
Psychosocial treatments for suicidal behaviors and NSSI are similar because with both, the priority is to help the patient maintain safety. This may include:
- developing a collaborative safety plan with family
- increasing monitoring
- removing access to firearms or other lethal means
- helping the adolescent to develop alternate, safer coping methods.
Many clinicians rely on no-harm contracts or agreements; however, there is no evidence that they are effective.20 The American Psychiatric Association recommends against using no-harm contracts with patients who are new, in an emergency setting, using substances, agitated, psychotic, or impulsive.21 Instead, clinicians, adolescents, and families can discuss specific steps the patient can take to remain safe. This collaborative plan should identify situations likely to trigger self-harming impulses; adaptive ways the teenager can cope, such as taking a nap or jogging; methods for communicating distress to family members and other helpers; and places to go for help, such as an emergency room. These safety plans should draw on the patient’s internal and external resources.
CASE CONTINUED: Strengthening relationships
While in the hospital, Josh finds it helpful to use a 0-to-10 scale to measure his distress and let his family know the intensity of his feelings. He identifies situations when he felt like hurting himself, such as being humiliated in math class. Josh learns about cognitive distortions—such as “they don’t care” and “there is no point in living”—and discusses methods for managing his feelings if he encounters further disappointments. His parents become more attentive when Josh explains his feelings, which allows the family to develop a collaborative safety plan. Josh decides to strengthen friendships he had been neglecting and agrees to attend a substance abuse treatment program.
Psychosocial treatment
In addition to maintaining safety, treatment goals for self-harming adolescents include:
- managing underlying psychiatric disorders
- identifying triggers for self-injurious acts
- improving family relationships
- developing better communication and coping skills.
Improving affective language skills, acquiring frustration tolerance, and learning alternatives to self-injury are key to strengthening coping abilities. Address problem-solving skills because self-harming adolescents often lack these abilities.22
Treatment of self-harming adolescents often consists of cognitive-behavioral therapy (CBT)23 or dialectical behavior therapy (DBT).24 CBT involves examining cognitive distortions or otherwise unhealthy beliefs about oneself, others, and life in general, focusing specifically on thoughts the patient has immediately before engaging in self-harm. DBT also integrates emotion regulation training and mindfulness. A review of 28 studies found these therapies effectively reduced self-harm behaviors in adults.25 However, few studies have examined these therapies’ efficacy in self-harming adolescents.
Pharmacotherapy
Psychopharmacology should focus on treating underlying psychiatric disorders. No medications are specifically effective for treating suicidal thoughts, suicidal behaviors, or NSSI. Some evidence suggests that antidepressants may trigger suicidal thoughts in a small proportion of youth,26 but the benefits of antidepressants outweigh the risk of suicidal thoughts.27 When prescribing antidepressants, inform patients and their parents of possible adverse reactions and monitor the patient regularly.28
Take precautions when prescribing medication for self-harming adolescents. For example, benzodiazepines may cause disinhibition, and larger quantities of medication could be lethal in an overdose. If possible, arrange for parents or guardians to monitor medication use.
What to document
Good documentation is especially helpful when an adolescent requires involuntary commitment or is discharged home. Involuntary commitment is based on legal interpretation of 3 circumstances—danger to self, danger to others, or gravely disabled—in which safety concerns may override an individual’s civil rights. If involuntary commitment is needed, a physician must clarify how the youth meets ≥1 of these criteria. If the adolescent is discharged, document that the patient is not an imminent danger to self or others and why you made this determination. Also note that follow-up services and a safety plan are in place, a parent will monitor safety issues and remove firearms and other lethal means from the home, and acute conflicts have been resolved. Other details, such as using the patient’s words to describe reasons for living, can be helpful.
Related Resources
- National Alliance for the Mentally Ill. National Helpline. 800-950-6264.
- American Foundation for Suicide Prevention. www.afsp.org.
- American Association of Suicidology. www.suicidology.org.
- National Suicide Prevention Lifeline. 800-273-TALK (8255).
Acknowledgements
The authors wish to thank students Scott Schubert from Regis University, Denver, CO, and Emily Peterson from Beloit College, Beloit, WI, for their help in preparing the manuscript.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table
Hospitalization or home? Acute crisis planning for self-harming youth
Hospitalization is more appropriate when ≥1 of the following is present with suicidal or self-injurious thoughts:
|
Home is more appropriate when:
|
Discuss this article at http://currentpsychiatry.blogspot.com/2010/08/adolescents-who-self-harm.html#comments
Josh, age 16, gets poor grades in school and occasionally smokes marijuana and abuses inhalants. After his girlfriend breaks up with him, he cuts his wrist with a hunting knife. While bleeding profusely, Josh calls his mother at work, who calls 911. The cut is deep and requires sutures. Josh says he did not try to kill himself; he only wanted to carve his girlfriend’s initials into his wrist to show his love for her.
When treating teenagers with self-harming thoughts and behavior, it may be difficult to distinguish suicide attempts from self-injury without intent to die. Understanding adolescent self-harm, suicide risk assessment, and treatment options guides clinicians to appropriate interventions. Recognizing the need for aggressive treatment—including psychiatric hospitalization—is essential to keeping self-harming teenagers safe.
Suicidal vs nonsuicidal self-harm
Suicidal behavior involves intent to end one’s life and includes ideation (thoughts) and actions (non fatal or fatal attempts).1 Nonsuicidal self-injury (NSSI) involves socially unacceptable, self-inflicted harm to one’s body without intent to die.2
Suicide is the third leading cause of death among youths age 12 to 19, claiming almost 2,000 lives each year.3 Nearly 1 in 5 (17%) U.S. high school students has suicidal thoughts each year, and almost 1 in 10 (8%) attempts suicide.4
Studies report a 13% to 23% lifetime prevalence of NSSI.5 These behaviors often begin between age 13 to 15.6 Cutting and hitting are the most common forms of NSSI; other methods include burning, scratching, and interfering with wound healing. Most teens who harm themselves without suicidal intent report that they feel little or no pain during the act.5 Unlike suicide attempts, NSSI can be viewed as a means to stay alive. Many adolescents injure themselves to cope with overwhelming feelings that can produce suicidal thoughts. Self-injury may distract the adolescent from painful emotions, reduce tensions, or penetrate numbness.7
Teenagers who hurt themselves but do not intend to die are at high risk for suicide and suicide attempts. Adolescents who engage in NSSI are more likely to experience suicidal behaviors, and vice versa.8 In a large study, 70% of adolescents who engaged in NSSI had made at least 1 suicide attempt and 55% made multiple suicide attempts.2 Current suicidal ideation is a risk factor for suicide, and a past suicide attempt is the strongest predictor of future suicidal behavior.9
Risk factors for suicidal behavior and NSSI overlap (Table 1)2,5,6,10,11 and include:
- depression
- substance use
- anxiety
- impulsive aggression
- history of childhood trauma.
Many teens who engage in NSSI report depression.2 A history of psychiatric illness—especially depression—increases the likelihood of adolescent suicide.8 A study comparing adolescents who engaged in NSSI with those who attempted suicide found that both groups reported similar levels of suicidal ideation and depressive symptoms.6 However, adolescents with a history of NSSI and attempted suicide reported higher levels of suicidal ideation and fewer reasons for living than those who attempted suicide but have no history of NSSI.12
Factors that protect against suicidal behavior include:
- a good parent-child relationship
- strong cultural or religious values
- an intact family
- a sense of connection with peer group and community.13
No studies have determined protective factors for NSSI.
Table 1
Characteristics of teens who harm themselves
| Factor | Comments |
|---|---|
| Older age | Both suicide attempts and NSSI are more common in mid-adolescence (age 13 to 15) |
| Sex | Males complete suicide more often (4:1) but more females make attempts. Sex differences have not been consistently identified for NSSI |
| Psychiatric illness | Diagnoses associated with adolescent suicide include major depression, substance abuse, and conduct disorder |
| Psychosocial and situational risks (usually combined with psychiatric illness) | Recent loss or rejection, living alone (eg, running away or homeless), poor social supports, family conflicts, family suicidal behavior, poor communication with parents, availability of firearms, exposure to suicide in the community or media, academic difficulties, legal problems, gender identity conflicts, history of maltreatment, being bullied, and risky behaviors |
| NSSI: nonsuicidal self-injury | |
| Source: References 2,5,6,10,11 | |
CASE CONTINUED: ‘No point in living’
As Josh becomes less guarded, he says that he sees “no point in living” without his girlfriend. He thought the only way to feel better was to “get high,” but this left him feeling even more despondent and anxious. He wrote a suicide note, but after cutting himself he was unsure he wanted to die. Josh says that when he feels depressed he can’t talk to his parents because “they wouldn’t understand and don’t care.”
Assessing self-harming adolescents
Distinguishing between suicidal behaviors and NSSI can be challenging (Table 2). Identifying risk factors for adolescent suicidal behavior must be coupled with a thorough psychiatric evaluation. If possible, interview the adolescent alone and obtain collateral information from parents, family members, teachers, caseworkers, probation officers, and others as needed. Also examine family interactions because conflicts and communication problems could undermine the teenager’s safety.
Consider using standardized measures of suicidal intentions such as the Scale for Suicidal Ideation (SSI).14 Although the SSI was developed for adults, a large case-control study validated the scale’s use in adolescent psychiatric outpatients and students.15 In addition to assessing subjective reports of suicidal intent, the SSI also takes into account objective indicators of increased risk, such as planning an attempt, hiding details from others, and making preparations for death.
Questions to ask. After a self-harm incident, it may be helpful to begin the psychiatric interview with a general question such as, “What happened that led you to hurt yourself?” This does not categorize the act as suicidal and allows the adolescent to describe it in his or her own words. Shea16 suggests normalizing the act by assuming that self-harm occurred, rather than making the patient admit to it. For example, an interviewer might say “Many people I know who are hurting inside also try to hurt their body; how often do you do that?”
Inquire about suicidal intent in a few ways. For example, first ask, “Do you ever wish you were dead?” and follow up with, “Would you ever do anything to try to make yourself dead?” Asking about suicidal thoughts does not increase suicidal thoughts or behavior.10,17
Reviewing thoughts and feelings leading up to a self-harm act can help identify triggers, coping difficulties, and issues to address in treatment. This behavioral analysis can be completed using the mnemonic ABC:
- Antecedents (situations or stressors leading to self-harming thoughts or actions)
- Behavior characteristics (frequency, intensity, and duration of self-harming)
- Consequences (eg, emotional relief, care and attention from others).
The results of this analysis could suggest treatment strategies, such as cognitive restructuring or techniques for decreasing feelings of distress.
The Risk of Suicide Questionnaire, which is designed for adolescents, asks:18
- Are you here because you tried to hurt yourself?
- In the past week, have you been having thoughts about killing yourself?
- Have you ever tried to hurt yourself in the past?
- Has something very stressful happened to you in the past few weeks?
Research has yet to determine whether this simple, rapid screen accurately identifies the need for psychiatric hospitalization or risk for suicidal outcomes. Although clinician- and self-administered suicide questionnaires may be useful for screening large populations, they are not a substitute for a thorough clinical assessment.
Inpatient or outpatient? When evaluating self-harming adolescents, first determine if they are in imminent danger of suicide and if more intensive services, such as hospitalization, are needed to maintain safety. Inpatient psychiatric services are appropriate for adolescents with suicidal thoughts or self-harm behaviors in addition to acute psychiatric disorders, significant substance abuse, serious medical issues, poor social supports, or inability to be managed safely as an outpatient.19 See the Table for a list of additional factors to consider.
Consent for treatment may be required because many self-harming adolescents do not present with life-threatening symptoms. Laws regarding consent vary among states. In some jurisdictions, patients age ≥15 can consent to mental health treatment without parents’ knowledge or consent. If an adolescent is in imminent danger and cannot voluntarily consent to treatment, physicians can initiate mental health “holds.” Some states allow registered nurses, psychologists, licensed social workers, and others to initiate mental health holds.
Table 2
Strategies for assessing adolescent self-harm
| Complete a thorough psychiatric evaluation |
| Interview the adolescent separately from parents |
| Obtain collateral information from parents and family, teachers, caseworkers, and others as needed |
| Use an empathic, nonjudgmental manner |
| Note appearance and presence of scarring and bruises, and patient’s clothing style |
Ask about current and past self-harming thoughts and behavior:
|
| Ask about acute stressors (eg, break-up, loss or rejection, conflict with parents) |
| Inquire about thoughts, feelings, and events leading up to the self-harm episode |
| Assess for psychosis and ask about homicidal thoughts. If yes, assess whether there is a duty to warn others |
| Ask about drug/alcohol use and consider a urine toxicology screen to help clarify whether substance abuse problems may be contributing to self-harm |
| Assess family interaction and communication style, noting conflicts that might impact safety |
| Consider using a standardized measure, such as the Scale for Suicidal Ideation14,15 |
CASE CONTINUED: Inpatient treatment
After the interview Josh says he still feels that “there is no point in living” and he cannot develop an adequate safety plan with his family. He is hospitalized to maintain safety, improve his coping skills and communication with his family, and mobilize safety plans, social supports, and follow-up care.
Maintaining safety
Psychosocial treatments for suicidal behaviors and NSSI are similar because with both, the priority is to help the patient maintain safety. This may include:
- developing a collaborative safety plan with family
- increasing monitoring
- removing access to firearms or other lethal means
- helping the adolescent to develop alternate, safer coping methods.
Many clinicians rely on no-harm contracts or agreements; however, there is no evidence that they are effective.20 The American Psychiatric Association recommends against using no-harm contracts with patients who are new, in an emergency setting, using substances, agitated, psychotic, or impulsive.21 Instead, clinicians, adolescents, and families can discuss specific steps the patient can take to remain safe. This collaborative plan should identify situations likely to trigger self-harming impulses; adaptive ways the teenager can cope, such as taking a nap or jogging; methods for communicating distress to family members and other helpers; and places to go for help, such as an emergency room. These safety plans should draw on the patient’s internal and external resources.
CASE CONTINUED: Strengthening relationships
While in the hospital, Josh finds it helpful to use a 0-to-10 scale to measure his distress and let his family know the intensity of his feelings. He identifies situations when he felt like hurting himself, such as being humiliated in math class. Josh learns about cognitive distortions—such as “they don’t care” and “there is no point in living”—and discusses methods for managing his feelings if he encounters further disappointments. His parents become more attentive when Josh explains his feelings, which allows the family to develop a collaborative safety plan. Josh decides to strengthen friendships he had been neglecting and agrees to attend a substance abuse treatment program.
Psychosocial treatment
In addition to maintaining safety, treatment goals for self-harming adolescents include:
- managing underlying psychiatric disorders
- identifying triggers for self-injurious acts
- improving family relationships
- developing better communication and coping skills.
Improving affective language skills, acquiring frustration tolerance, and learning alternatives to self-injury are key to strengthening coping abilities. Address problem-solving skills because self-harming adolescents often lack these abilities.22
Treatment of self-harming adolescents often consists of cognitive-behavioral therapy (CBT)23 or dialectical behavior therapy (DBT).24 CBT involves examining cognitive distortions or otherwise unhealthy beliefs about oneself, others, and life in general, focusing specifically on thoughts the patient has immediately before engaging in self-harm. DBT also integrates emotion regulation training and mindfulness. A review of 28 studies found these therapies effectively reduced self-harm behaviors in adults.25 However, few studies have examined these therapies’ efficacy in self-harming adolescents.
Pharmacotherapy
Psychopharmacology should focus on treating underlying psychiatric disorders. No medications are specifically effective for treating suicidal thoughts, suicidal behaviors, or NSSI. Some evidence suggests that antidepressants may trigger suicidal thoughts in a small proportion of youth,26 but the benefits of antidepressants outweigh the risk of suicidal thoughts.27 When prescribing antidepressants, inform patients and their parents of possible adverse reactions and monitor the patient regularly.28
Take precautions when prescribing medication for self-harming adolescents. For example, benzodiazepines may cause disinhibition, and larger quantities of medication could be lethal in an overdose. If possible, arrange for parents or guardians to monitor medication use.
What to document
Good documentation is especially helpful when an adolescent requires involuntary commitment or is discharged home. Involuntary commitment is based on legal interpretation of 3 circumstances—danger to self, danger to others, or gravely disabled—in which safety concerns may override an individual’s civil rights. If involuntary commitment is needed, a physician must clarify how the youth meets ≥1 of these criteria. If the adolescent is discharged, document that the patient is not an imminent danger to self or others and why you made this determination. Also note that follow-up services and a safety plan are in place, a parent will monitor safety issues and remove firearms and other lethal means from the home, and acute conflicts have been resolved. Other details, such as using the patient’s words to describe reasons for living, can be helpful.
Related Resources
- National Alliance for the Mentally Ill. National Helpline. 800-950-6264.
- American Foundation for Suicide Prevention. www.afsp.org.
- American Association of Suicidology. www.suicidology.org.
- National Suicide Prevention Lifeline. 800-273-TALK (8255).
Acknowledgements
The authors wish to thank students Scott Schubert from Regis University, Denver, CO, and Emily Peterson from Beloit College, Beloit, WI, for their help in preparing the manuscript.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table
Hospitalization or home? Acute crisis planning for self-harming youth
Hospitalization is more appropriate when ≥1 of the following is present with suicidal or self-injurious thoughts:
|
Home is more appropriate when:
|
1. O’Carroll PW, Berman AL, Maris RW, et al. Beyond the Tower of Babel: a nomenclature for suicidology. Suicide Life Threat Behav. 1996;26:237-252.
2. Nock MK, Joiner TE, Jr, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
3. Centers for Disease Control and Prevention. Injury prevention and control: data and statistics (WISQARS). Available at: http://www.cdc.gov/injury/wisqars. Accessed June 22, 2010.
4. National Center for Health Statistics. Health, United States, 2006. Available at: http://www.cdc.gov/nchs/data/hus/hus06.pdf#062. Accessed May 28, 2010.
5. Jacobson CM, Gould M. The epidemiology and phenomenology of non-suicidal self-injurious behavior among adolescents: a critical review of the literature. Arch Suicide Res. 2007;11(2):129-147.
6. Muehlenkamp JJ, Gutierrez PM. An investigation of differences between self-injurious behavior and suicide attempts in a sample of adolescents. Suicide Life Threat Behav. 2004;34:12-23.
7. Nixon MK, Cloutier PF, Aggarwal S. Affect regulation and addictive aspects of repetitive self-injury in hospitalized adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41:1333-1341.
8. Whitlock J, Knox KL. The relationship between self-injurious behavior and suicide in a young adult population. Arch Pediatr Adolesc Med. 2007;161:634-640.
9. Bridge JA, Goldstein TR, Brent DA. Adolescent suicide and suicidal behavior. J Child Psychol Psychiatry. 2006;47:372-394.
10. American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(suppl 7):24S-51S.
11. Laye-Gindhu A, Schonert-Reichl KA. Nonsuicidal self-harm among community adolescents: understanding the “whats” and “whys” of self-harm. J Youth Adolesc. 2005;34:447-457.
12. Muehlenkamp JJ, Gutierrez PM. Risk for suicide attempts among adolescents who engage in non-suicidal self-injury. Arch Suicide Res. 2007;11:69-82.
13. Gould MS, Greenberg T, Velting DM, et al. Youth suicide risk and preventive interventions: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2003;42:386-405.
14. Beck AT, Kovacs M, Weissmann A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;46:343-352.
15. Holi MM, Pelkonen M, Karlsson L, et al. Psychometric properties and clinical utility of the Scale for Suicidal Ideation (SSI) in adolescents. BMC Psychiatry. 2005;5:8.-
16. Shea SC. The practical art of suicide assessment: a guide for mental health professionals and substance abuse counselors. New York, NY: John Wiley; 1999.
17. Gould MS, Marrocco FA, Kleinman M, et al. Evaluating iatrogenic risk of youth suicide screening programs. JAMA. 2005;293:1635-1643.
18. Horowitz LM, Wang PS, Koocher GP, et al. Detecting suicide risk in a pediatric emergency department: development of a brief screening tool. Pediatrics. 2001;107:1133-1137.
19. Kennedy SP, Baraff LJ, Suddath RL, et al. Emergency department management of suicidal adolescents. Ann Emerg Med. 2004;43:452-460.
20. Lewis LM. No-harm contracts: a review of what we know. Suicide Life Threat Behav. 2007;37:50-57.
21. American Psychiatric Association. Practice guideline for the assessment and treatment of patients with suicidal behavior. Am J Psychiatry. 2003;160(suppl 11):1-60.
22. Speckens AE, Hawton K. Social problem solving in adolescents with suicidal behavior: a systematic review. Suicide Life Threat Behav. 2005;35:365-387.
23. Henriques G, Beck AT, Brown GK. Cognitive therapy for adolescent and young adult suicide attempters. American Behavioral Scientist. 2003;46:1258-1268.
24. Rathus JH, Miller AL. Dialectical behavior therapy adapted for suicidal adolescents. Suicide Life Threat Behav. 2002;32:146-157.
25. Tarrier N, Taylor K, Gooding P. Cognitive-behavioral interventions to reduce suicide behavior: a systematic review and meta-analysis. Behav Modif. 2008;32:77-108.
26. Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292:338-343.
27. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
28. Simon GE. The antidepressant quandary—considering suicide risk when treating adolescent depression. N Engl J Med. 2006;355:2722-2723.
1. O’Carroll PW, Berman AL, Maris RW, et al. Beyond the Tower of Babel: a nomenclature for suicidology. Suicide Life Threat Behav. 1996;26:237-252.
2. Nock MK, Joiner TE, Jr, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144(1):65-72.
3. Centers for Disease Control and Prevention. Injury prevention and control: data and statistics (WISQARS). Available at: http://www.cdc.gov/injury/wisqars. Accessed June 22, 2010.
4. National Center for Health Statistics. Health, United States, 2006. Available at: http://www.cdc.gov/nchs/data/hus/hus06.pdf#062. Accessed May 28, 2010.
5. Jacobson CM, Gould M. The epidemiology and phenomenology of non-suicidal self-injurious behavior among adolescents: a critical review of the literature. Arch Suicide Res. 2007;11(2):129-147.
6. Muehlenkamp JJ, Gutierrez PM. An investigation of differences between self-injurious behavior and suicide attempts in a sample of adolescents. Suicide Life Threat Behav. 2004;34:12-23.
7. Nixon MK, Cloutier PF, Aggarwal S. Affect regulation and addictive aspects of repetitive self-injury in hospitalized adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41:1333-1341.
8. Whitlock J, Knox KL. The relationship between self-injurious behavior and suicide in a young adult population. Arch Pediatr Adolesc Med. 2007;161:634-640.
9. Bridge JA, Goldstein TR, Brent DA. Adolescent suicide and suicidal behavior. J Child Psychol Psychiatry. 2006;47:372-394.
10. American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment and treatment of children and adolescents with suicidal behavior. J Am Acad Child Adolesc Psychiatry. 2001;40(suppl 7):24S-51S.
11. Laye-Gindhu A, Schonert-Reichl KA. Nonsuicidal self-harm among community adolescents: understanding the “whats” and “whys” of self-harm. J Youth Adolesc. 2005;34:447-457.
12. Muehlenkamp JJ, Gutierrez PM. Risk for suicide attempts among adolescents who engage in non-suicidal self-injury. Arch Suicide Res. 2007;11:69-82.
13. Gould MS, Greenberg T, Velting DM, et al. Youth suicide risk and preventive interventions: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2003;42:386-405.
14. Beck AT, Kovacs M, Weissmann A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;46:343-352.
15. Holi MM, Pelkonen M, Karlsson L, et al. Psychometric properties and clinical utility of the Scale for Suicidal Ideation (SSI) in adolescents. BMC Psychiatry. 2005;5:8.-
16. Shea SC. The practical art of suicide assessment: a guide for mental health professionals and substance abuse counselors. New York, NY: John Wiley; 1999.
17. Gould MS, Marrocco FA, Kleinman M, et al. Evaluating iatrogenic risk of youth suicide screening programs. JAMA. 2005;293:1635-1643.
18. Horowitz LM, Wang PS, Koocher GP, et al. Detecting suicide risk in a pediatric emergency department: development of a brief screening tool. Pediatrics. 2001;107:1133-1137.
19. Kennedy SP, Baraff LJ, Suddath RL, et al. Emergency department management of suicidal adolescents. Ann Emerg Med. 2004;43:452-460.
20. Lewis LM. No-harm contracts: a review of what we know. Suicide Life Threat Behav. 2007;37:50-57.
21. American Psychiatric Association. Practice guideline for the assessment and treatment of patients with suicidal behavior. Am J Psychiatry. 2003;160(suppl 11):1-60.
22. Speckens AE, Hawton K. Social problem solving in adolescents with suicidal behavior: a systematic review. Suicide Life Threat Behav. 2005;35:365-387.
23. Henriques G, Beck AT, Brown GK. Cognitive therapy for adolescent and young adult suicide attempters. American Behavioral Scientist. 2003;46:1258-1268.
24. Rathus JH, Miller AL. Dialectical behavior therapy adapted for suicidal adolescents. Suicide Life Threat Behav. 2002;32:146-157.
25. Tarrier N, Taylor K, Gooding P. Cognitive-behavioral interventions to reduce suicide behavior: a systematic review and meta-analysis. Behav Modif. 2008;32:77-108.
26. Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292:338-343.
27. Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683-1696.
28. Simon GE. The antidepressant quandary—considering suicide risk when treating adolescent depression. N Engl J Med. 2006;355:2722-2723.
Bipolar disorder and substance abuse: Overcome the challenges of ‘dual diagnosis’ patients
After testing positive for cocaine on a recent court-mandated urine drug screen, Mr. M, age 49, is referred by his parole officer for psychiatric and substance abuse treatment. Mr. M has bipolar I disorder and alcohol, cocaine, and opioid dependence. He says he has been hospitalized or incarcerated at least once each year for the past 22 years. Mr. M has seen numerous psychiatrists as an outpatient, but rarely for more than 2 to 3 months and has not taken any psychotropics for more than 5 months.
Approximately 1 week before his recent urine drug screen, Mr. M became euphoric, stopped sleeping for several days, and spent $2,000 on cocaine and “escorts.” He reports that each day he smokes 30 cigarettes and drinks 1 pint of liquor and prefers to use cocaine and opiates by IV injection. Several years ago Mr. M was diagnosed with hepatitis C virus (HCV) but received no further workup or treatment. Although he denies manic or psychotic symptoms, Mr. M is observed speaking to unseen others in the waiting room and has difficulty remaining still during his interview. His chief concern is insomnia, stating, “Doc, I just need something to help me sleep.”
The high prevalence of substance use disorders (SUDs) in persons with bipolar disorder (BD) is well documented.1,2 Up to 60% of bipolar patients develop an SUD at some point in their lives.3-5 Alcohol use disorders are particularly common among BD patients, with a lifetime prevalence of roughly 50%.2-5 Recent epidemiologic data indicate that 38% of persons with bipolar I disorder and 19% of those with bipolar II disorder meet criteria for alcohol dependence.5 Comorbid SUDs in patients with BD are associated with:
- poor treatment compliance
- longer and more frequent mood episodes
- more mixed episodes
- more hospitalizations
- more frequent suicide attempts.1,2
The impact of co-occurring SUDs on suicidality is particularly high among those with bipolar I disorder.6
Frequently referred to as “dual diagnosis” conditions, co-occurring BD and SUDs may be more accurately envisioned as multi-morbid, rather than comorbid, illnesses. Data from the Stanley Foundation Bipolar Network suggest that 42% of BD patients have a lifetime history of ≥2 comorbid axis I disorders.7 Rates of generalized anxiety disorder, panic disorder, and posttraumatic stress disorder are particularly high in BD patients with co-occurring SUDs.8,9 In addition, the presence of 1 SUD may mark the presence of other SUDs; for example, alcohol dependence is strongly associated with polysubstance abuse, especially in females with BD.8 Furthermore, medical comorbidities that impact treatment decisions also are highly prevalent in BD patients with comorbid SUDs.10 In particular, HCV rates are higher in persons with BD compared with the general population,11 and are >5 times as likely in bipolar patients with co-occurring SUDs.12
Unfortunately, limited treatment research guides clinical management of comorbid BD and SUDs.2,13,14 Clinical trials of medications for BD traditionally have excluded patients with SUDs, and persons with serious mental illness usually are ineligible for SUD treatment studies.13 Furthermore, the few randomized controlled trials (RCTs) conducted in persons with both illnesses have been constrained by relatively small sample sizes and low retention rates. In the absence of a definitive consensus for optimal treatment, this article outlines general clinical considerations and an integrated approach to assessing and managing this complex patient population.
Birds of a feather
Multiple hypotheses try to account for the high rate of SUDs in patients with BD (Table 1), but none fully explain the complex interaction observed clinically.14,15 Substance dependence and BD are chronic remitting/relapsing disorders with heterogeneous presentations and highly variable natural histories. As with SUDs, BD may go undiagnosed and untreated for years, coming to clinical attention only after substantial disease progression.16
The fluctuating illness course in BD and SUDs makes diagnosis and treatment difficult. Symptomatic periods often are interrupted by spontaneous remissions and longer—although usually temporary—periods of perceived control. Both BD and SUDs may be associated with profound mood instability, increased impulsivity, altered responsiveness to reward, and impaired executive function.17 Finally, the high degree of heritability in BD18 and many SUDs19 may make treatment engagement more difficult if either disorder is present in multiple family members because of:
- potential for greater clinical severity
- reduced psychosocial resources
- altered familial behavioral norms that may impede the patient’s recognition of illness.
Denial of illness is a critical symptom that may fluctuate with disease course in both disorders. Persons with BD or SUDs may be least likely to recognize that they are ill during periods of highest symptom severity. Accordingly, treatment adherence in patients with either disorder may be limited at baseline and decline further when the 2 illnesses co-occur.20
Involvement in the criminal justice system and medical comorbidities, particularly HCV, also complicate diagnosis and treatment of BD patients with SUDs. For more information about these topics, see Box1 and Box2.
Table 1
Why is substance abuse so prevalent among bipolar patients?
| Proposed hypothesis | Selected limitations of this hypothesis |
|---|---|
| Self-medication: substance abuse occurs as an attempt to regulate mood | High rates of substance use during euthymia; high prevalence of alcohol/depressant use during depressive phase, stimulant use during manic phase |
| Common neurobiologic or genetic risk factors | Specific evidence from linkage/association studies currently is lacking |
| Substance use occurs as a symptom of bipolar disorder | High percentage of patients with bipolar disorder do not have SUDs; there is a poor correlation of onset, course of bipolar, and SUD symptoms |
| Substance use unmasks bipolar disorder or a bipolar diathesis | Emergence of mania before SUD is common and predictive of more severe course of bipolar disorder |
| High comorbidity rates are an artifact of misdiagnosis based on overlapping symptoms and poor diagnostic boundaries | Very high prevalence of SUDs also is observed in longitudinal studies of patients initially hospitalized for mania |
| SUDs: substance use disorders | |
| Source: References 14,15 | |
Integrated clinical management
Assessment. Although not intended to be comprehensive, suggestions for routine assessment of patients with suspected SUDs and/or BD are listed in Table 2. Because clinicians may encounter dual diagnosis patients in general psychiatric clinics or specialty (addiction or mood disorder) clinics, it is useful to obtain a thorough substance use history in all patients with known or suspected BD as well as a thorough history of hypomania/mania and depression in all patients with addictive disorders. BD diagnoses by self-report or chart history in patients with SUDs should be considered cautiously because BD often is overdiagnosed in persons engaged in active substance abuse or experiencing withdrawal.21 If past or present mood symptoms and substance use have co-occurred, further focused assessment of mood symptoms before alcohol and drug use or during extended periods of abstinence are necessary to make the diagnosis of bipolar disorder with confidence. Family history of SUDs and/or BD are neither necessary nor sufficient for either diagnosis; however, collateral information from family or significant others could help make the diagnosis and may identify aids and obstacles to treatment planning and engagement.
When a patient’s clinical history strongly supports the diagnoses of BD and co-occurring SUDs, more detailed inquiry is warranted. Determining the patient’s age at onset of each disorder may have prognostic value because onset of mania before SUDs developed, especially in adolescence, may predict a more severe course of both illnesses.22 A complete alcohol use history should include routine questioning about past withdrawal. Previous withdrawal seizures in an actively drinking BD patient may tip the balance toward adding an anticonvulsant for mood stabilization. A thorough SUD history should elicit information about polysubstance abuse or dependence and include screening for injection drug use and other risk factors for HCV and human immunodeficiency virus (HIV), such as hypersexuality during manic or hypomanic episodes. Document the date of the last screening for HCV/ HIV in BD patients at high risk of infection. The U.S. Centers for Disease Control and Prevention recommends that all patients at high risk for HIV consider voluntary screening at least annually.23
Assess your patient’s historical and ongoing alcohol and other drug abuse at the initial visit, and continue to monitor substance use at all subsequent visits, especially in patients with HCV. When feasible, order urine drug screening and laboratory testing for alcohol use biomarkers such as carbohydrate-deficient transferrin and gamma-glutamyltransferase to supplement self-report data, especially in patients with poor insight or low motivation. Assess suicidal ideation and any changes in suicide risk factors at every visit.
Treatment. No biologic therapies have been FDA-approved for treating patients with co-occurring BD and SUDs. Comorbid SUDs in BD patients—as well as rapid cycling and mixed mood episodes, both of which are more common in patients with comorbid SUDs—predict poor response to lithium.17 However, the evidence base for optimal pharmacotherapy remains extremely limited. Published double-blind, placebo-controlled RCTs in persons with BD and co-morbid SUDs are limited to only 1 trial each of lithium, carbamazepine, quetiapine, and naltrexone, and 2 comparisons of lithium plus divalproex vs lithium alone (Table 3).24-29
Salloum et al24 reported that bipolar I disorder patients with alcohol dependence who received divalproex plus lithium as maintenance treatment had fewer heavy drinking days and fewer drinks per heavy drinking day than those receiving lithium plus placebo. However, the addition of divalproex did not improve manic or depressive symptoms, and depression remission rates remained relatively low in both groups. A recent 6-month study comparing lithium vs lithium plus divalproex in patients with SUDs and rapid-cycling BD found no additional benefit of divalproex over lithium monotherapy in retention, mood, or substance use outcomes.28 However, modest evidence that anticonvulsants such as valproic acid and carbamazepine may help treat acute alcohol withdrawal2 could support their preferential use as mood stabilizers over lithium in actively drinking BD patients.
Research underscores the difficulty in keeping dual diagnosis patients in treatment. Salloum et al24 reported that only one-third of randomized subjects completed the 24-week study. In the Kemp study,28 79% of 149 recruited subjects failed to complete the lead-in stabilization phase. Of the 31 remaining subjects who were randomized to lithium or lithium/divalproex combination, only 8 (26% of those randomized, 5% of those recruited) completed the 6-month trial.
Substance abuse is associated with significantly decreased treatment adherence in persons with BD20 and may affect medication choice. For example, caution may be warranted in the use of lamotrigine in substance-abusing patients with poor adherence because re-titration from the starting dose is recommended if the medicine has been missed for a consecutive period exceeding 5 half-lives of the drug.30
Notable progress has been made in developing psychosocial treatments for comorbid SUDs and BD. Integrated group therapy has been designed to address the 2 disorders simultaneously by emphasizing the relationship between the disorders and highlighting similarities in cognitive and behavioral change that promote recovery in both.31 In a recent well-controlled RCT, this approach reduced alcohol and other drug use to approximately one-half the levels observed in those who received only group drug counseling.31
Research suggests that an integrated approach that encompasses psychiatric, medical, psychosocial, and legal dimensions simultaneously may be most effective. For patients such as Mr. M, this would include aggressively treating mood symptoms while employing motivational interviewing techniques to improve engagement in substance dependence treatment. If possible, involving family members, parole officials, housing agencies, and other public assistance workers in the treatment plan may increase treatment adherence and reduce loss of contact during illness exacerbations. Stabilization of substance use and psychiatric morbidity should be accompanied by timely evaluation of HCV and other medical comorbidities in order to improve long-term prognosis.
Table 2
Assessing patients you suspect have comorbid BD and SUDs
| Initial assessment |
|---|
Thorough substance use history in all patients with known or suspected bipolar disorder:
|
Thorough evaluation of any history of hypomania/mania and depression in all patients with known or suspected SUDs:
|
| Assess risk factors, screening status for hepatitis C, HIV |
| Obtain collateral information from family and significant others if feasible and appropriate |
| Detailed assessment of suicide risk |
| Follow-up assessments |
| Substance use since last visit by self-report |
| Consider UDS, CDT, GGT |
| Medication adherence |
| Detailed assessment of suicide risk |
| BD: bipolar disorder; CDT: carbohydrate-deficient transferrin; GGT: gamma-glutamyltransferase; HIV: human immunodeficiency virus; SUDs: substance use disorders; UDS: urine drug screen |
Table 3
Pharmacotherapy for bipolar disorder and co-occurring SUDs
| Study | Diagnoses/N* | Medications | Outcome |
|---|---|---|---|
| Salloum et al, 200524 | BD I, alcohol dependence. N=59 [20] | Divalproex + lithium vs lithium, 24 weeks | Decreased number of heavy drinking days, fewer drinks per heavy drinking day |
| Geller et al, 199825 | BD I, BP II, MDD (adolescents); alcohol, cannabis abuse. N=25 [21] | Lithium, 6 weeks | Decreased cannabis-positive urine drug screen (lithium > placebo) |
| Brady et al, 200226 | BD I, BD II, cyclothymia; cocaine dependence. N=57 | Carbamazepine, 12 weeks | Trend toward longer time to cocaine use |
| Brown et al, 200827 | BD I, BD II; N=102 | Quetiapine, 12 weeks | Decreased HAM-D scores (quetiapine > placebo) |
| Kemp et al, 200928 | BD I, BD II, rapid cycling; alcohol, cannabis, cocaine abuse or dependence. N=31 [8] | Divalproex + lithium vs lithium, 6 months | No group differences |
| Brown et al, 200929 | BD I, BD II; alcohol dependence. N=50 [26] | Naltrexone, 12 weeks | Trend toward increased probability of no drinking days |
| * N=number of subjects randomized to double-blind treatment. Numbers in brackets indicate the number of subjects who completed all study visits (when reported) | |||
| BD: bipolar disorder; HAM-D: 17-item Hamilton Rating Scale for Depression; MDD: major depressive disorder; SUDs: substance use disorders | |||
Related Resources
- International Society for Bipolar Disorders. www.isbd.org.
- National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
- National Institute on Drug Abuse. www.nida.nih.gov.
Drug Brand Names
- Carbamazepine • Tegretol
- Divalproex/valproic acid • Depakote
- Lamotrigine • Lamictal
- Lithium • Lithobid
- Naltrexone • ReVia
- Quetiapine • Seroquel
Disclosure
Dr. Tolliver receives research grant funding from Forest Laboratories and the National Institute on Alcohol Abuse and Alcoholism. Neither source influenced the content or submission of this manuscript.
A recent analysis of data from >65,000 veterans found bipolar patients with comorbid substance use disorders (SUDs) were 7 times more likely to have hepatitis C virus (HCV) than patients with no serious mental illness.a Matthews and colleagues found that 29.6% of persons diagnosed with bipolar disorder (BD) and SUDs tested positive for HCV—roughly 5 times the relative risk of patients without either diagnosis.b The high prevalence of HCV in patients with comorbid BD and SUDs may be the result of injection drug use, increased risky sexual behavior while manic or intoxicated, or both.
HCV has multiple treatment implications for these patients. Alcohol abuse and dependence are the most common SUDs that co-occur with BD,c-e and patients with HCV who drink alcohol excessively have more severe hepatic fibrosis, accelerated disease progression, and higher rates of cirrhosis and hepatocellular carcinoma than HCV patients who do not drink.f Medications commonly used to treat BD or alcohol dependence may have adverse effects on the liver and require more careful monitoring in the presence of HCV infection. For example, valproic acid has been reported to improve drinking outcomes in alcohol-dependent patients with BDg but has been associated with higher rates of marked hepatic transaminase elevation in patients with HCV infection compared with those without HCV.h Marked elevation of hepatic transaminases may be observed in HCV-infected individuals treated with other medications such as lithium or antidepressants,h and valproic acid use is not an absolute contraindication in HCV patients. Nevertheless, the effects of valproic acid in HCV-infected BD patients who drink alcohol are unknown and therefore cautious and frequent monitoring of hepatic enzymes are warranted in this population.
Finally, both SUDs and BD complicate HCV treatment. In a database review of >113,000 veterans with HCV infection, Butt and colleagues found that individuals with BD accounted for 10.4% of the HCV-infected sample but only 5% of those who received HCV treatment.i Similarly, patients with alcohol abuse or dependence made up 44.3% of the HCV-infected sample but only 28.9% of those who received HCV treatment.
Because the rate of liver biopsy in untreated patients was low, the decision not to treat appeared to be based more often on other criteria. This is not surprising; pegylated interferon-alfa—the most effective treatment for chronic HCV—has been associated with multiple neuropsychiatric symptoms observed in BD, including depression, mania, psychosis, and suicidal ideation.j Emergence of severe psychiatric complications usually results in permanent discontinuation of interferon treatment. Likewise, the presence of alcohol abuse or other SUDs is a strong negative predictor of interferon treatment response and retention and generally has been considered a relative contraindication for interferon initiation.f
References
a. Himelhoch S, McCarthy JF, Ganoczy D, et al. Understanding associations between serious mental illness and hepatitis C virus among veterans: a national multivariate analysis. Psychosomatics. 2009;50:30-37.
b. Matthews AM, Huckans MS, Blackwell AD, et al. Hepatitis C testing and infection rates in bipolar patients with and without comorbid substance use disorders. Bipolar Disord. 2008;10:266-270.
c. Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807-816.
d. Hasin DS, Stinson FS, Ogburn E, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830-842.
e. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2007;64:543-552.
f. Bhattacharya R, Shuhart M. Hepatitis C and alcohol. J Clin Gastroenterol. 2003;36:242-252.
g. Salloum IM, Cornelius JR, Daley DC, et al. Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2005;62:37-45.
h. Felker BL, Sloan KL, Dominitz JA, et al. The safety of valproic acid use for patients with hepatitis C infection. Am J Psychiatry. 2003;160:174-178.
i. Butt AA, Justice AC, Skanderson M, et al. Rate and predictors of treatment prescription for hepatitis C. Gut. 2006;56:385-389.
j. Onyike CU, Bonner JO, Lyketsos CG, et al. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry. 2004;161:429-435.
Long recognized to be more prevalent in forensic populations, bipolar disorder (BD) is especially overrepresented among those with repeat arrests and incarcerations. In a recent study of >79,000 inmates incarcerated in Texas in 2006 and 2007, those with BD were 3.3 times more likely to have had ≥4 previous incarcerations.k
Comorbid substance use disorder (SUD) is a significant risk factor for criminal arrest. For example, in a Los Angeles County (CA) sample of inmates with BD, 75.8% had co-occurring SUDs, compared with 18.5% in a comparison group of hospitalized BD patients.l The association of SUDs with arrest is especially high in females with BD. In the Los Angeles sample mentioned above, incarcerated bipolar women were >38 times more likely to have a SUD than a comparison group of female patients treated in the community.m
References
k. Baillargeon J, Binswanger IA, Penn JV, et al. Psychiatric disorders and repeat incarcerations: the revolving prison door. Am J Psychiatry. 2009;166:103-109.
l. Quanbeck CD, Stone DC, Scott CL, et al. Clinical and legal correlates of inmates with bipolar disorder at time of criminal arrest. J Clin Psychiatry. 2004;65:198-203.
m. McDermott BE, Quanbeck CD, Frye MA. Comorbid substance use disorder in women with bipolar disorder is associated with criminal arrest. Bipolar Disord. 2007;9(5):536-540.
1. Levin FR, Hennessey G. Bipolar disorder and substance abuse. Biol Psychiatry. 2004;56:738-748.
2. Frye MA, Salloum IM. Bipolar disorder and comorbid alcoholism: prevalence rate and treatment considerations. Bipolar Disord. 2006;8:677-685.
3. Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807-816.
4. Hasin DS, Stinson FS, Ogburn E, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830-842.
5. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2007;64:543-552.
6. Sublette EM, Carballo JJ, Moreno C, et al. Substance use disorders and suicide attempts in bipolar subtypes. J Psychiatric Res. 2009;43:230-238.
7. McElroy SL, Altshuler LL, Suppes T, et al. Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry. 2001;158:420-426.
8. Frye MA, Altshuler LL, McElroy SL, et al. Gender differences in prevalence, risk, and clinical correlates of alcoholism comorbidity in bipolar disorder. Am J Psychiatry. 2003;160:883-889.
9. Simon NM, Otto MW, Wisniewski SR, et al. Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Am J Psychiatry. 2004;161:2222-2229.
10. Perron BE, Howard MO, Nienhuis JK, et al. Prevalence and burden of general medical conditions among adults with bipolar I disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2009;70:1407-1415.
11. Himelhoch S, McCarthy JF, Ganoczy D, et al. Understanding associations between serious mental illness and hepatitis C virus among veterans: a national multivariate analysis. Psychosomatics. 2009;50:30-37.
12. Matthews AM, Huckans MS, Blackwell AD, et al. Hepatitis C testing and infection rates in bipolar patients with and without comorbid substance use disorders. Bipolar Disord. 2008;10:266-270.
13. Singh JB, Zarate CA. Pharmacological treatment of psychiatric comorbidity in bipolar disorder: a review of controlled trials. Bipolar Disord. 2006;8:696-709.
14. Vornik LA, Brown ES. Management of comorbid bipolar disorder and substance abuse. J Clin Psychiatry. 2006;67(suppl 7):24-30.
15. Strakowski SM, DelBello MP. The co-occurrence of bipolar and substance use disorders. Clin Psych Rev. 2000;20:191-206.
16. Berk M, Dodd S, Callaly P, et al. History of illness prior to a diagnosis of bipolar disorder or schizoaffective disorder. J Affect Disord. 2007;103:181-186.
17. Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorders and recurrent depression. 2nd ed. New York, NY: Oxford University Press; 2007.
18. McGuffin P, Rijsdijk F, Andrew M, et al. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497-502.
19. Kendler KS, Prescott CA, Myers J, et al. The structure of genetic and environmental risk factors for common psychiatric and substance abuse disorders in men and women. Arch Gen Psychiatry. 2003;60:929-937.
20. Weiss RD, Greenfield SF, Najavits LM, et al. Medication compliance among patients with bipolar disorder and substance use disorder. J Clin Psychiatry. 1998;59:172-174.
21. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder inpatients with mood instability. J Clin Psychiatry. 2008;69:1751-1757.
22. Winokur G, Coryell W, Akiskal HS, et al. Alcoholism in manic-depressive (bipolar) illness: familial illness, course of illness, and the primary-secondary distinction. Am J Psychiatry. 1995;152:365-372.
23. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR. 2006;55(RR14):1-17.
24. Salloum IM, Cornelius JR, Daley DC, et al. Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2005;62:37-45.
25. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry. 1998;37(2):171-178.
26. Brady KT, Sonne SC, Malcolm RJ, et al. Carbamazepine in the treatment of cocaine dependence: subtyping by affective disorder. Exp Clin Psychopharmacol. 2002;10:276-285.
27. Brown ES, Garza M, Carmody TJ. A randomized, double-blind, placebo-controlled add-on trial of quetiapine in outpatients with bipolar disorder and alcohol use disorders. J Clin Psychiatry. 2008;69:701-705.
28. Kemp DE, Gao K, Ganocy S, et al. A 6-month, double-blind, maintenance trial of lithium monotherapy versus the combination of lithium and divalproex for rapid-cycling bipolar disorder and co-occurring substance abuse or dependence. J Clin Psychiatry. 2009;70:113-121.
29. Brown ES, Carmody TJ, Schmitz JM, et al. A randomized, double-blind, placebo-controlled pilot study of naltrexone in outpatients with bipolar disorder and alcohol dependence. Alcohol Clin Exp Res. 2009;3:1863-1869.
30. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
31. Weiss RD, Griffin ML, Kolodziej MR, et al. A randomized trial of integrated group therapy versus group drug counseling for patients with bipolar disorder and substance dependence. Am J Psychiatry. 2007;164:100-107.
After testing positive for cocaine on a recent court-mandated urine drug screen, Mr. M, age 49, is referred by his parole officer for psychiatric and substance abuse treatment. Mr. M has bipolar I disorder and alcohol, cocaine, and opioid dependence. He says he has been hospitalized or incarcerated at least once each year for the past 22 years. Mr. M has seen numerous psychiatrists as an outpatient, but rarely for more than 2 to 3 months and has not taken any psychotropics for more than 5 months.
Approximately 1 week before his recent urine drug screen, Mr. M became euphoric, stopped sleeping for several days, and spent $2,000 on cocaine and “escorts.” He reports that each day he smokes 30 cigarettes and drinks 1 pint of liquor and prefers to use cocaine and opiates by IV injection. Several years ago Mr. M was diagnosed with hepatitis C virus (HCV) but received no further workup or treatment. Although he denies manic or psychotic symptoms, Mr. M is observed speaking to unseen others in the waiting room and has difficulty remaining still during his interview. His chief concern is insomnia, stating, “Doc, I just need something to help me sleep.”
The high prevalence of substance use disorders (SUDs) in persons with bipolar disorder (BD) is well documented.1,2 Up to 60% of bipolar patients develop an SUD at some point in their lives.3-5 Alcohol use disorders are particularly common among BD patients, with a lifetime prevalence of roughly 50%.2-5 Recent epidemiologic data indicate that 38% of persons with bipolar I disorder and 19% of those with bipolar II disorder meet criteria for alcohol dependence.5 Comorbid SUDs in patients with BD are associated with:
- poor treatment compliance
- longer and more frequent mood episodes
- more mixed episodes
- more hospitalizations
- more frequent suicide attempts.1,2
The impact of co-occurring SUDs on suicidality is particularly high among those with bipolar I disorder.6
Frequently referred to as “dual diagnosis” conditions, co-occurring BD and SUDs may be more accurately envisioned as multi-morbid, rather than comorbid, illnesses. Data from the Stanley Foundation Bipolar Network suggest that 42% of BD patients have a lifetime history of ≥2 comorbid axis I disorders.7 Rates of generalized anxiety disorder, panic disorder, and posttraumatic stress disorder are particularly high in BD patients with co-occurring SUDs.8,9 In addition, the presence of 1 SUD may mark the presence of other SUDs; for example, alcohol dependence is strongly associated with polysubstance abuse, especially in females with BD.8 Furthermore, medical comorbidities that impact treatment decisions also are highly prevalent in BD patients with comorbid SUDs.10 In particular, HCV rates are higher in persons with BD compared with the general population,11 and are >5 times as likely in bipolar patients with co-occurring SUDs.12
Unfortunately, limited treatment research guides clinical management of comorbid BD and SUDs.2,13,14 Clinical trials of medications for BD traditionally have excluded patients with SUDs, and persons with serious mental illness usually are ineligible for SUD treatment studies.13 Furthermore, the few randomized controlled trials (RCTs) conducted in persons with both illnesses have been constrained by relatively small sample sizes and low retention rates. In the absence of a definitive consensus for optimal treatment, this article outlines general clinical considerations and an integrated approach to assessing and managing this complex patient population.
Birds of a feather
Multiple hypotheses try to account for the high rate of SUDs in patients with BD (Table 1), but none fully explain the complex interaction observed clinically.14,15 Substance dependence and BD are chronic remitting/relapsing disorders with heterogeneous presentations and highly variable natural histories. As with SUDs, BD may go undiagnosed and untreated for years, coming to clinical attention only after substantial disease progression.16
The fluctuating illness course in BD and SUDs makes diagnosis and treatment difficult. Symptomatic periods often are interrupted by spontaneous remissions and longer—although usually temporary—periods of perceived control. Both BD and SUDs may be associated with profound mood instability, increased impulsivity, altered responsiveness to reward, and impaired executive function.17 Finally, the high degree of heritability in BD18 and many SUDs19 may make treatment engagement more difficult if either disorder is present in multiple family members because of:
- potential for greater clinical severity
- reduced psychosocial resources
- altered familial behavioral norms that may impede the patient’s recognition of illness.
Denial of illness is a critical symptom that may fluctuate with disease course in both disorders. Persons with BD or SUDs may be least likely to recognize that they are ill during periods of highest symptom severity. Accordingly, treatment adherence in patients with either disorder may be limited at baseline and decline further when the 2 illnesses co-occur.20
Involvement in the criminal justice system and medical comorbidities, particularly HCV, also complicate diagnosis and treatment of BD patients with SUDs. For more information about these topics, see Box1 and Box2.
Table 1
Why is substance abuse so prevalent among bipolar patients?
| Proposed hypothesis | Selected limitations of this hypothesis |
|---|---|
| Self-medication: substance abuse occurs as an attempt to regulate mood | High rates of substance use during euthymia; high prevalence of alcohol/depressant use during depressive phase, stimulant use during manic phase |
| Common neurobiologic or genetic risk factors | Specific evidence from linkage/association studies currently is lacking |
| Substance use occurs as a symptom of bipolar disorder | High percentage of patients with bipolar disorder do not have SUDs; there is a poor correlation of onset, course of bipolar, and SUD symptoms |
| Substance use unmasks bipolar disorder or a bipolar diathesis | Emergence of mania before SUD is common and predictive of more severe course of bipolar disorder |
| High comorbidity rates are an artifact of misdiagnosis based on overlapping symptoms and poor diagnostic boundaries | Very high prevalence of SUDs also is observed in longitudinal studies of patients initially hospitalized for mania |
| SUDs: substance use disorders | |
| Source: References 14,15 | |
Integrated clinical management
Assessment. Although not intended to be comprehensive, suggestions for routine assessment of patients with suspected SUDs and/or BD are listed in Table 2. Because clinicians may encounter dual diagnosis patients in general psychiatric clinics or specialty (addiction or mood disorder) clinics, it is useful to obtain a thorough substance use history in all patients with known or suspected BD as well as a thorough history of hypomania/mania and depression in all patients with addictive disorders. BD diagnoses by self-report or chart history in patients with SUDs should be considered cautiously because BD often is overdiagnosed in persons engaged in active substance abuse or experiencing withdrawal.21 If past or present mood symptoms and substance use have co-occurred, further focused assessment of mood symptoms before alcohol and drug use or during extended periods of abstinence are necessary to make the diagnosis of bipolar disorder with confidence. Family history of SUDs and/or BD are neither necessary nor sufficient for either diagnosis; however, collateral information from family or significant others could help make the diagnosis and may identify aids and obstacles to treatment planning and engagement.
When a patient’s clinical history strongly supports the diagnoses of BD and co-occurring SUDs, more detailed inquiry is warranted. Determining the patient’s age at onset of each disorder may have prognostic value because onset of mania before SUDs developed, especially in adolescence, may predict a more severe course of both illnesses.22 A complete alcohol use history should include routine questioning about past withdrawal. Previous withdrawal seizures in an actively drinking BD patient may tip the balance toward adding an anticonvulsant for mood stabilization. A thorough SUD history should elicit information about polysubstance abuse or dependence and include screening for injection drug use and other risk factors for HCV and human immunodeficiency virus (HIV), such as hypersexuality during manic or hypomanic episodes. Document the date of the last screening for HCV/ HIV in BD patients at high risk of infection. The U.S. Centers for Disease Control and Prevention recommends that all patients at high risk for HIV consider voluntary screening at least annually.23
Assess your patient’s historical and ongoing alcohol and other drug abuse at the initial visit, and continue to monitor substance use at all subsequent visits, especially in patients with HCV. When feasible, order urine drug screening and laboratory testing for alcohol use biomarkers such as carbohydrate-deficient transferrin and gamma-glutamyltransferase to supplement self-report data, especially in patients with poor insight or low motivation. Assess suicidal ideation and any changes in suicide risk factors at every visit.
Treatment. No biologic therapies have been FDA-approved for treating patients with co-occurring BD and SUDs. Comorbid SUDs in BD patients—as well as rapid cycling and mixed mood episodes, both of which are more common in patients with comorbid SUDs—predict poor response to lithium.17 However, the evidence base for optimal pharmacotherapy remains extremely limited. Published double-blind, placebo-controlled RCTs in persons with BD and co-morbid SUDs are limited to only 1 trial each of lithium, carbamazepine, quetiapine, and naltrexone, and 2 comparisons of lithium plus divalproex vs lithium alone (Table 3).24-29
Salloum et al24 reported that bipolar I disorder patients with alcohol dependence who received divalproex plus lithium as maintenance treatment had fewer heavy drinking days and fewer drinks per heavy drinking day than those receiving lithium plus placebo. However, the addition of divalproex did not improve manic or depressive symptoms, and depression remission rates remained relatively low in both groups. A recent 6-month study comparing lithium vs lithium plus divalproex in patients with SUDs and rapid-cycling BD found no additional benefit of divalproex over lithium monotherapy in retention, mood, or substance use outcomes.28 However, modest evidence that anticonvulsants such as valproic acid and carbamazepine may help treat acute alcohol withdrawal2 could support their preferential use as mood stabilizers over lithium in actively drinking BD patients.
Research underscores the difficulty in keeping dual diagnosis patients in treatment. Salloum et al24 reported that only one-third of randomized subjects completed the 24-week study. In the Kemp study,28 79% of 149 recruited subjects failed to complete the lead-in stabilization phase. Of the 31 remaining subjects who were randomized to lithium or lithium/divalproex combination, only 8 (26% of those randomized, 5% of those recruited) completed the 6-month trial.
Substance abuse is associated with significantly decreased treatment adherence in persons with BD20 and may affect medication choice. For example, caution may be warranted in the use of lamotrigine in substance-abusing patients with poor adherence because re-titration from the starting dose is recommended if the medicine has been missed for a consecutive period exceeding 5 half-lives of the drug.30
Notable progress has been made in developing psychosocial treatments for comorbid SUDs and BD. Integrated group therapy has been designed to address the 2 disorders simultaneously by emphasizing the relationship between the disorders and highlighting similarities in cognitive and behavioral change that promote recovery in both.31 In a recent well-controlled RCT, this approach reduced alcohol and other drug use to approximately one-half the levels observed in those who received only group drug counseling.31
Research suggests that an integrated approach that encompasses psychiatric, medical, psychosocial, and legal dimensions simultaneously may be most effective. For patients such as Mr. M, this would include aggressively treating mood symptoms while employing motivational interviewing techniques to improve engagement in substance dependence treatment. If possible, involving family members, parole officials, housing agencies, and other public assistance workers in the treatment plan may increase treatment adherence and reduce loss of contact during illness exacerbations. Stabilization of substance use and psychiatric morbidity should be accompanied by timely evaluation of HCV and other medical comorbidities in order to improve long-term prognosis.
Table 2
Assessing patients you suspect have comorbid BD and SUDs
| Initial assessment |
|---|
Thorough substance use history in all patients with known or suspected bipolar disorder:
|
Thorough evaluation of any history of hypomania/mania and depression in all patients with known or suspected SUDs:
|
| Assess risk factors, screening status for hepatitis C, HIV |
| Obtain collateral information from family and significant others if feasible and appropriate |
| Detailed assessment of suicide risk |
| Follow-up assessments |
| Substance use since last visit by self-report |
| Consider UDS, CDT, GGT |
| Medication adherence |
| Detailed assessment of suicide risk |
| BD: bipolar disorder; CDT: carbohydrate-deficient transferrin; GGT: gamma-glutamyltransferase; HIV: human immunodeficiency virus; SUDs: substance use disorders; UDS: urine drug screen |
Table 3
Pharmacotherapy for bipolar disorder and co-occurring SUDs
| Study | Diagnoses/N* | Medications | Outcome |
|---|---|---|---|
| Salloum et al, 200524 | BD I, alcohol dependence. N=59 [20] | Divalproex + lithium vs lithium, 24 weeks | Decreased number of heavy drinking days, fewer drinks per heavy drinking day |
| Geller et al, 199825 | BD I, BP II, MDD (adolescents); alcohol, cannabis abuse. N=25 [21] | Lithium, 6 weeks | Decreased cannabis-positive urine drug screen (lithium > placebo) |
| Brady et al, 200226 | BD I, BD II, cyclothymia; cocaine dependence. N=57 | Carbamazepine, 12 weeks | Trend toward longer time to cocaine use |
| Brown et al, 200827 | BD I, BD II; N=102 | Quetiapine, 12 weeks | Decreased HAM-D scores (quetiapine > placebo) |
| Kemp et al, 200928 | BD I, BD II, rapid cycling; alcohol, cannabis, cocaine abuse or dependence. N=31 [8] | Divalproex + lithium vs lithium, 6 months | No group differences |
| Brown et al, 200929 | BD I, BD II; alcohol dependence. N=50 [26] | Naltrexone, 12 weeks | Trend toward increased probability of no drinking days |
| * N=number of subjects randomized to double-blind treatment. Numbers in brackets indicate the number of subjects who completed all study visits (when reported) | |||
| BD: bipolar disorder; HAM-D: 17-item Hamilton Rating Scale for Depression; MDD: major depressive disorder; SUDs: substance use disorders | |||
Related Resources
- International Society for Bipolar Disorders. www.isbd.org.
- National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
- National Institute on Drug Abuse. www.nida.nih.gov.
Drug Brand Names
- Carbamazepine • Tegretol
- Divalproex/valproic acid • Depakote
- Lamotrigine • Lamictal
- Lithium • Lithobid
- Naltrexone • ReVia
- Quetiapine • Seroquel
Disclosure
Dr. Tolliver receives research grant funding from Forest Laboratories and the National Institute on Alcohol Abuse and Alcoholism. Neither source influenced the content or submission of this manuscript.
A recent analysis of data from >65,000 veterans found bipolar patients with comorbid substance use disorders (SUDs) were 7 times more likely to have hepatitis C virus (HCV) than patients with no serious mental illness.a Matthews and colleagues found that 29.6% of persons diagnosed with bipolar disorder (BD) and SUDs tested positive for HCV—roughly 5 times the relative risk of patients without either diagnosis.b The high prevalence of HCV in patients with comorbid BD and SUDs may be the result of injection drug use, increased risky sexual behavior while manic or intoxicated, or both.
HCV has multiple treatment implications for these patients. Alcohol abuse and dependence are the most common SUDs that co-occur with BD,c-e and patients with HCV who drink alcohol excessively have more severe hepatic fibrosis, accelerated disease progression, and higher rates of cirrhosis and hepatocellular carcinoma than HCV patients who do not drink.f Medications commonly used to treat BD or alcohol dependence may have adverse effects on the liver and require more careful monitoring in the presence of HCV infection. For example, valproic acid has been reported to improve drinking outcomes in alcohol-dependent patients with BDg but has been associated with higher rates of marked hepatic transaminase elevation in patients with HCV infection compared with those without HCV.h Marked elevation of hepatic transaminases may be observed in HCV-infected individuals treated with other medications such as lithium or antidepressants,h and valproic acid use is not an absolute contraindication in HCV patients. Nevertheless, the effects of valproic acid in HCV-infected BD patients who drink alcohol are unknown and therefore cautious and frequent monitoring of hepatic enzymes are warranted in this population.
Finally, both SUDs and BD complicate HCV treatment. In a database review of >113,000 veterans with HCV infection, Butt and colleagues found that individuals with BD accounted for 10.4% of the HCV-infected sample but only 5% of those who received HCV treatment.i Similarly, patients with alcohol abuse or dependence made up 44.3% of the HCV-infected sample but only 28.9% of those who received HCV treatment.
Because the rate of liver biopsy in untreated patients was low, the decision not to treat appeared to be based more often on other criteria. This is not surprising; pegylated interferon-alfa—the most effective treatment for chronic HCV—has been associated with multiple neuropsychiatric symptoms observed in BD, including depression, mania, psychosis, and suicidal ideation.j Emergence of severe psychiatric complications usually results in permanent discontinuation of interferon treatment. Likewise, the presence of alcohol abuse or other SUDs is a strong negative predictor of interferon treatment response and retention and generally has been considered a relative contraindication for interferon initiation.f
References
a. Himelhoch S, McCarthy JF, Ganoczy D, et al. Understanding associations between serious mental illness and hepatitis C virus among veterans: a national multivariate analysis. Psychosomatics. 2009;50:30-37.
b. Matthews AM, Huckans MS, Blackwell AD, et al. Hepatitis C testing and infection rates in bipolar patients with and without comorbid substance use disorders. Bipolar Disord. 2008;10:266-270.
c. Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807-816.
d. Hasin DS, Stinson FS, Ogburn E, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830-842.
e. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2007;64:543-552.
f. Bhattacharya R, Shuhart M. Hepatitis C and alcohol. J Clin Gastroenterol. 2003;36:242-252.
g. Salloum IM, Cornelius JR, Daley DC, et al. Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2005;62:37-45.
h. Felker BL, Sloan KL, Dominitz JA, et al. The safety of valproic acid use for patients with hepatitis C infection. Am J Psychiatry. 2003;160:174-178.
i. Butt AA, Justice AC, Skanderson M, et al. Rate and predictors of treatment prescription for hepatitis C. Gut. 2006;56:385-389.
j. Onyike CU, Bonner JO, Lyketsos CG, et al. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry. 2004;161:429-435.
Long recognized to be more prevalent in forensic populations, bipolar disorder (BD) is especially overrepresented among those with repeat arrests and incarcerations. In a recent study of >79,000 inmates incarcerated in Texas in 2006 and 2007, those with BD were 3.3 times more likely to have had ≥4 previous incarcerations.k
Comorbid substance use disorder (SUD) is a significant risk factor for criminal arrest. For example, in a Los Angeles County (CA) sample of inmates with BD, 75.8% had co-occurring SUDs, compared with 18.5% in a comparison group of hospitalized BD patients.l The association of SUDs with arrest is especially high in females with BD. In the Los Angeles sample mentioned above, incarcerated bipolar women were >38 times more likely to have a SUD than a comparison group of female patients treated in the community.m
References
k. Baillargeon J, Binswanger IA, Penn JV, et al. Psychiatric disorders and repeat incarcerations: the revolving prison door. Am J Psychiatry. 2009;166:103-109.
l. Quanbeck CD, Stone DC, Scott CL, et al. Clinical and legal correlates of inmates with bipolar disorder at time of criminal arrest. J Clin Psychiatry. 2004;65:198-203.
m. McDermott BE, Quanbeck CD, Frye MA. Comorbid substance use disorder in women with bipolar disorder is associated with criminal arrest. Bipolar Disord. 2007;9(5):536-540.
After testing positive for cocaine on a recent court-mandated urine drug screen, Mr. M, age 49, is referred by his parole officer for psychiatric and substance abuse treatment. Mr. M has bipolar I disorder and alcohol, cocaine, and opioid dependence. He says he has been hospitalized or incarcerated at least once each year for the past 22 years. Mr. M has seen numerous psychiatrists as an outpatient, but rarely for more than 2 to 3 months and has not taken any psychotropics for more than 5 months.
Approximately 1 week before his recent urine drug screen, Mr. M became euphoric, stopped sleeping for several days, and spent $2,000 on cocaine and “escorts.” He reports that each day he smokes 30 cigarettes and drinks 1 pint of liquor and prefers to use cocaine and opiates by IV injection. Several years ago Mr. M was diagnosed with hepatitis C virus (HCV) but received no further workup or treatment. Although he denies manic or psychotic symptoms, Mr. M is observed speaking to unseen others in the waiting room and has difficulty remaining still during his interview. His chief concern is insomnia, stating, “Doc, I just need something to help me sleep.”
The high prevalence of substance use disorders (SUDs) in persons with bipolar disorder (BD) is well documented.1,2 Up to 60% of bipolar patients develop an SUD at some point in their lives.3-5 Alcohol use disorders are particularly common among BD patients, with a lifetime prevalence of roughly 50%.2-5 Recent epidemiologic data indicate that 38% of persons with bipolar I disorder and 19% of those with bipolar II disorder meet criteria for alcohol dependence.5 Comorbid SUDs in patients with BD are associated with:
- poor treatment compliance
- longer and more frequent mood episodes
- more mixed episodes
- more hospitalizations
- more frequent suicide attempts.1,2
The impact of co-occurring SUDs on suicidality is particularly high among those with bipolar I disorder.6
Frequently referred to as “dual diagnosis” conditions, co-occurring BD and SUDs may be more accurately envisioned as multi-morbid, rather than comorbid, illnesses. Data from the Stanley Foundation Bipolar Network suggest that 42% of BD patients have a lifetime history of ≥2 comorbid axis I disorders.7 Rates of generalized anxiety disorder, panic disorder, and posttraumatic stress disorder are particularly high in BD patients with co-occurring SUDs.8,9 In addition, the presence of 1 SUD may mark the presence of other SUDs; for example, alcohol dependence is strongly associated with polysubstance abuse, especially in females with BD.8 Furthermore, medical comorbidities that impact treatment decisions also are highly prevalent in BD patients with comorbid SUDs.10 In particular, HCV rates are higher in persons with BD compared with the general population,11 and are >5 times as likely in bipolar patients with co-occurring SUDs.12
Unfortunately, limited treatment research guides clinical management of comorbid BD and SUDs.2,13,14 Clinical trials of medications for BD traditionally have excluded patients with SUDs, and persons with serious mental illness usually are ineligible for SUD treatment studies.13 Furthermore, the few randomized controlled trials (RCTs) conducted in persons with both illnesses have been constrained by relatively small sample sizes and low retention rates. In the absence of a definitive consensus for optimal treatment, this article outlines general clinical considerations and an integrated approach to assessing and managing this complex patient population.
Birds of a feather
Multiple hypotheses try to account for the high rate of SUDs in patients with BD (Table 1), but none fully explain the complex interaction observed clinically.14,15 Substance dependence and BD are chronic remitting/relapsing disorders with heterogeneous presentations and highly variable natural histories. As with SUDs, BD may go undiagnosed and untreated for years, coming to clinical attention only after substantial disease progression.16
The fluctuating illness course in BD and SUDs makes diagnosis and treatment difficult. Symptomatic periods often are interrupted by spontaneous remissions and longer—although usually temporary—periods of perceived control. Both BD and SUDs may be associated with profound mood instability, increased impulsivity, altered responsiveness to reward, and impaired executive function.17 Finally, the high degree of heritability in BD18 and many SUDs19 may make treatment engagement more difficult if either disorder is present in multiple family members because of:
- potential for greater clinical severity
- reduced psychosocial resources
- altered familial behavioral norms that may impede the patient’s recognition of illness.
Denial of illness is a critical symptom that may fluctuate with disease course in both disorders. Persons with BD or SUDs may be least likely to recognize that they are ill during periods of highest symptom severity. Accordingly, treatment adherence in patients with either disorder may be limited at baseline and decline further when the 2 illnesses co-occur.20
Involvement in the criminal justice system and medical comorbidities, particularly HCV, also complicate diagnosis and treatment of BD patients with SUDs. For more information about these topics, see Box1 and Box2.
Table 1
Why is substance abuse so prevalent among bipolar patients?
| Proposed hypothesis | Selected limitations of this hypothesis |
|---|---|
| Self-medication: substance abuse occurs as an attempt to regulate mood | High rates of substance use during euthymia; high prevalence of alcohol/depressant use during depressive phase, stimulant use during manic phase |
| Common neurobiologic or genetic risk factors | Specific evidence from linkage/association studies currently is lacking |
| Substance use occurs as a symptom of bipolar disorder | High percentage of patients with bipolar disorder do not have SUDs; there is a poor correlation of onset, course of bipolar, and SUD symptoms |
| Substance use unmasks bipolar disorder or a bipolar diathesis | Emergence of mania before SUD is common and predictive of more severe course of bipolar disorder |
| High comorbidity rates are an artifact of misdiagnosis based on overlapping symptoms and poor diagnostic boundaries | Very high prevalence of SUDs also is observed in longitudinal studies of patients initially hospitalized for mania |
| SUDs: substance use disorders | |
| Source: References 14,15 | |
Integrated clinical management
Assessment. Although not intended to be comprehensive, suggestions for routine assessment of patients with suspected SUDs and/or BD are listed in Table 2. Because clinicians may encounter dual diagnosis patients in general psychiatric clinics or specialty (addiction or mood disorder) clinics, it is useful to obtain a thorough substance use history in all patients with known or suspected BD as well as a thorough history of hypomania/mania and depression in all patients with addictive disorders. BD diagnoses by self-report or chart history in patients with SUDs should be considered cautiously because BD often is overdiagnosed in persons engaged in active substance abuse or experiencing withdrawal.21 If past or present mood symptoms and substance use have co-occurred, further focused assessment of mood symptoms before alcohol and drug use or during extended periods of abstinence are necessary to make the diagnosis of bipolar disorder with confidence. Family history of SUDs and/or BD are neither necessary nor sufficient for either diagnosis; however, collateral information from family or significant others could help make the diagnosis and may identify aids and obstacles to treatment planning and engagement.
When a patient’s clinical history strongly supports the diagnoses of BD and co-occurring SUDs, more detailed inquiry is warranted. Determining the patient’s age at onset of each disorder may have prognostic value because onset of mania before SUDs developed, especially in adolescence, may predict a more severe course of both illnesses.22 A complete alcohol use history should include routine questioning about past withdrawal. Previous withdrawal seizures in an actively drinking BD patient may tip the balance toward adding an anticonvulsant for mood stabilization. A thorough SUD history should elicit information about polysubstance abuse or dependence and include screening for injection drug use and other risk factors for HCV and human immunodeficiency virus (HIV), such as hypersexuality during manic or hypomanic episodes. Document the date of the last screening for HCV/ HIV in BD patients at high risk of infection. The U.S. Centers for Disease Control and Prevention recommends that all patients at high risk for HIV consider voluntary screening at least annually.23
Assess your patient’s historical and ongoing alcohol and other drug abuse at the initial visit, and continue to monitor substance use at all subsequent visits, especially in patients with HCV. When feasible, order urine drug screening and laboratory testing for alcohol use biomarkers such as carbohydrate-deficient transferrin and gamma-glutamyltransferase to supplement self-report data, especially in patients with poor insight or low motivation. Assess suicidal ideation and any changes in suicide risk factors at every visit.
Treatment. No biologic therapies have been FDA-approved for treating patients with co-occurring BD and SUDs. Comorbid SUDs in BD patients—as well as rapid cycling and mixed mood episodes, both of which are more common in patients with comorbid SUDs—predict poor response to lithium.17 However, the evidence base for optimal pharmacotherapy remains extremely limited. Published double-blind, placebo-controlled RCTs in persons with BD and co-morbid SUDs are limited to only 1 trial each of lithium, carbamazepine, quetiapine, and naltrexone, and 2 comparisons of lithium plus divalproex vs lithium alone (Table 3).24-29
Salloum et al24 reported that bipolar I disorder patients with alcohol dependence who received divalproex plus lithium as maintenance treatment had fewer heavy drinking days and fewer drinks per heavy drinking day than those receiving lithium plus placebo. However, the addition of divalproex did not improve manic or depressive symptoms, and depression remission rates remained relatively low in both groups. A recent 6-month study comparing lithium vs lithium plus divalproex in patients with SUDs and rapid-cycling BD found no additional benefit of divalproex over lithium monotherapy in retention, mood, or substance use outcomes.28 However, modest evidence that anticonvulsants such as valproic acid and carbamazepine may help treat acute alcohol withdrawal2 could support their preferential use as mood stabilizers over lithium in actively drinking BD patients.
Research underscores the difficulty in keeping dual diagnosis patients in treatment. Salloum et al24 reported that only one-third of randomized subjects completed the 24-week study. In the Kemp study,28 79% of 149 recruited subjects failed to complete the lead-in stabilization phase. Of the 31 remaining subjects who were randomized to lithium or lithium/divalproex combination, only 8 (26% of those randomized, 5% of those recruited) completed the 6-month trial.
Substance abuse is associated with significantly decreased treatment adherence in persons with BD20 and may affect medication choice. For example, caution may be warranted in the use of lamotrigine in substance-abusing patients with poor adherence because re-titration from the starting dose is recommended if the medicine has been missed for a consecutive period exceeding 5 half-lives of the drug.30
Notable progress has been made in developing psychosocial treatments for comorbid SUDs and BD. Integrated group therapy has been designed to address the 2 disorders simultaneously by emphasizing the relationship between the disorders and highlighting similarities in cognitive and behavioral change that promote recovery in both.31 In a recent well-controlled RCT, this approach reduced alcohol and other drug use to approximately one-half the levels observed in those who received only group drug counseling.31
Research suggests that an integrated approach that encompasses psychiatric, medical, psychosocial, and legal dimensions simultaneously may be most effective. For patients such as Mr. M, this would include aggressively treating mood symptoms while employing motivational interviewing techniques to improve engagement in substance dependence treatment. If possible, involving family members, parole officials, housing agencies, and other public assistance workers in the treatment plan may increase treatment adherence and reduce loss of contact during illness exacerbations. Stabilization of substance use and psychiatric morbidity should be accompanied by timely evaluation of HCV and other medical comorbidities in order to improve long-term prognosis.
Table 2
Assessing patients you suspect have comorbid BD and SUDs
| Initial assessment |
|---|
Thorough substance use history in all patients with known or suspected bipolar disorder:
|
Thorough evaluation of any history of hypomania/mania and depression in all patients with known or suspected SUDs:
|
| Assess risk factors, screening status for hepatitis C, HIV |
| Obtain collateral information from family and significant others if feasible and appropriate |
| Detailed assessment of suicide risk |
| Follow-up assessments |
| Substance use since last visit by self-report |
| Consider UDS, CDT, GGT |
| Medication adherence |
| Detailed assessment of suicide risk |
| BD: bipolar disorder; CDT: carbohydrate-deficient transferrin; GGT: gamma-glutamyltransferase; HIV: human immunodeficiency virus; SUDs: substance use disorders; UDS: urine drug screen |
Table 3
Pharmacotherapy for bipolar disorder and co-occurring SUDs
| Study | Diagnoses/N* | Medications | Outcome |
|---|---|---|---|
| Salloum et al, 200524 | BD I, alcohol dependence. N=59 [20] | Divalproex + lithium vs lithium, 24 weeks | Decreased number of heavy drinking days, fewer drinks per heavy drinking day |
| Geller et al, 199825 | BD I, BP II, MDD (adolescents); alcohol, cannabis abuse. N=25 [21] | Lithium, 6 weeks | Decreased cannabis-positive urine drug screen (lithium > placebo) |
| Brady et al, 200226 | BD I, BD II, cyclothymia; cocaine dependence. N=57 | Carbamazepine, 12 weeks | Trend toward longer time to cocaine use |
| Brown et al, 200827 | BD I, BD II; N=102 | Quetiapine, 12 weeks | Decreased HAM-D scores (quetiapine > placebo) |
| Kemp et al, 200928 | BD I, BD II, rapid cycling; alcohol, cannabis, cocaine abuse or dependence. N=31 [8] | Divalproex + lithium vs lithium, 6 months | No group differences |
| Brown et al, 200929 | BD I, BD II; alcohol dependence. N=50 [26] | Naltrexone, 12 weeks | Trend toward increased probability of no drinking days |
| * N=number of subjects randomized to double-blind treatment. Numbers in brackets indicate the number of subjects who completed all study visits (when reported) | |||
| BD: bipolar disorder; HAM-D: 17-item Hamilton Rating Scale for Depression; MDD: major depressive disorder; SUDs: substance use disorders | |||
Related Resources
- International Society for Bipolar Disorders. www.isbd.org.
- National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
- National Institute on Drug Abuse. www.nida.nih.gov.
Drug Brand Names
- Carbamazepine • Tegretol
- Divalproex/valproic acid • Depakote
- Lamotrigine • Lamictal
- Lithium • Lithobid
- Naltrexone • ReVia
- Quetiapine • Seroquel
Disclosure
Dr. Tolliver receives research grant funding from Forest Laboratories and the National Institute on Alcohol Abuse and Alcoholism. Neither source influenced the content or submission of this manuscript.
A recent analysis of data from >65,000 veterans found bipolar patients with comorbid substance use disorders (SUDs) were 7 times more likely to have hepatitis C virus (HCV) than patients with no serious mental illness.a Matthews and colleagues found that 29.6% of persons diagnosed with bipolar disorder (BD) and SUDs tested positive for HCV—roughly 5 times the relative risk of patients without either diagnosis.b The high prevalence of HCV in patients with comorbid BD and SUDs may be the result of injection drug use, increased risky sexual behavior while manic or intoxicated, or both.
HCV has multiple treatment implications for these patients. Alcohol abuse and dependence are the most common SUDs that co-occur with BD,c-e and patients with HCV who drink alcohol excessively have more severe hepatic fibrosis, accelerated disease progression, and higher rates of cirrhosis and hepatocellular carcinoma than HCV patients who do not drink.f Medications commonly used to treat BD or alcohol dependence may have adverse effects on the liver and require more careful monitoring in the presence of HCV infection. For example, valproic acid has been reported to improve drinking outcomes in alcohol-dependent patients with BDg but has been associated with higher rates of marked hepatic transaminase elevation in patients with HCV infection compared with those without HCV.h Marked elevation of hepatic transaminases may be observed in HCV-infected individuals treated with other medications such as lithium or antidepressants,h and valproic acid use is not an absolute contraindication in HCV patients. Nevertheless, the effects of valproic acid in HCV-infected BD patients who drink alcohol are unknown and therefore cautious and frequent monitoring of hepatic enzymes are warranted in this population.
Finally, both SUDs and BD complicate HCV treatment. In a database review of >113,000 veterans with HCV infection, Butt and colleagues found that individuals with BD accounted for 10.4% of the HCV-infected sample but only 5% of those who received HCV treatment.i Similarly, patients with alcohol abuse or dependence made up 44.3% of the HCV-infected sample but only 28.9% of those who received HCV treatment.
Because the rate of liver biopsy in untreated patients was low, the decision not to treat appeared to be based more often on other criteria. This is not surprising; pegylated interferon-alfa—the most effective treatment for chronic HCV—has been associated with multiple neuropsychiatric symptoms observed in BD, including depression, mania, psychosis, and suicidal ideation.j Emergence of severe psychiatric complications usually results in permanent discontinuation of interferon treatment. Likewise, the presence of alcohol abuse or other SUDs is a strong negative predictor of interferon treatment response and retention and generally has been considered a relative contraindication for interferon initiation.f
References
a. Himelhoch S, McCarthy JF, Ganoczy D, et al. Understanding associations between serious mental illness and hepatitis C virus among veterans: a national multivariate analysis. Psychosomatics. 2009;50:30-37.
b. Matthews AM, Huckans MS, Blackwell AD, et al. Hepatitis C testing and infection rates in bipolar patients with and without comorbid substance use disorders. Bipolar Disord. 2008;10:266-270.
c. Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807-816.
d. Hasin DS, Stinson FS, Ogburn E, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830-842.
e. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2007;64:543-552.
f. Bhattacharya R, Shuhart M. Hepatitis C and alcohol. J Clin Gastroenterol. 2003;36:242-252.
g. Salloum IM, Cornelius JR, Daley DC, et al. Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2005;62:37-45.
h. Felker BL, Sloan KL, Dominitz JA, et al. The safety of valproic acid use for patients with hepatitis C infection. Am J Psychiatry. 2003;160:174-178.
i. Butt AA, Justice AC, Skanderson M, et al. Rate and predictors of treatment prescription for hepatitis C. Gut. 2006;56:385-389.
j. Onyike CU, Bonner JO, Lyketsos CG, et al. Mania during treatment of chronic hepatitis C with pegylated interferon and ribavirin. Am J Psychiatry. 2004;161:429-435.
Long recognized to be more prevalent in forensic populations, bipolar disorder (BD) is especially overrepresented among those with repeat arrests and incarcerations. In a recent study of >79,000 inmates incarcerated in Texas in 2006 and 2007, those with BD were 3.3 times more likely to have had ≥4 previous incarcerations.k
Comorbid substance use disorder (SUD) is a significant risk factor for criminal arrest. For example, in a Los Angeles County (CA) sample of inmates with BD, 75.8% had co-occurring SUDs, compared with 18.5% in a comparison group of hospitalized BD patients.l The association of SUDs with arrest is especially high in females with BD. In the Los Angeles sample mentioned above, incarcerated bipolar women were >38 times more likely to have a SUD than a comparison group of female patients treated in the community.m
References
k. Baillargeon J, Binswanger IA, Penn JV, et al. Psychiatric disorders and repeat incarcerations: the revolving prison door. Am J Psychiatry. 2009;166:103-109.
l. Quanbeck CD, Stone DC, Scott CL, et al. Clinical and legal correlates of inmates with bipolar disorder at time of criminal arrest. J Clin Psychiatry. 2004;65:198-203.
m. McDermott BE, Quanbeck CD, Frye MA. Comorbid substance use disorder in women with bipolar disorder is associated with criminal arrest. Bipolar Disord. 2007;9(5):536-540.
1. Levin FR, Hennessey G. Bipolar disorder and substance abuse. Biol Psychiatry. 2004;56:738-748.
2. Frye MA, Salloum IM. Bipolar disorder and comorbid alcoholism: prevalence rate and treatment considerations. Bipolar Disord. 2006;8:677-685.
3. Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807-816.
4. Hasin DS, Stinson FS, Ogburn E, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830-842.
5. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2007;64:543-552.
6. Sublette EM, Carballo JJ, Moreno C, et al. Substance use disorders and suicide attempts in bipolar subtypes. J Psychiatric Res. 2009;43:230-238.
7. McElroy SL, Altshuler LL, Suppes T, et al. Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry. 2001;158:420-426.
8. Frye MA, Altshuler LL, McElroy SL, et al. Gender differences in prevalence, risk, and clinical correlates of alcoholism comorbidity in bipolar disorder. Am J Psychiatry. 2003;160:883-889.
9. Simon NM, Otto MW, Wisniewski SR, et al. Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Am J Psychiatry. 2004;161:2222-2229.
10. Perron BE, Howard MO, Nienhuis JK, et al. Prevalence and burden of general medical conditions among adults with bipolar I disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2009;70:1407-1415.
11. Himelhoch S, McCarthy JF, Ganoczy D, et al. Understanding associations between serious mental illness and hepatitis C virus among veterans: a national multivariate analysis. Psychosomatics. 2009;50:30-37.
12. Matthews AM, Huckans MS, Blackwell AD, et al. Hepatitis C testing and infection rates in bipolar patients with and without comorbid substance use disorders. Bipolar Disord. 2008;10:266-270.
13. Singh JB, Zarate CA. Pharmacological treatment of psychiatric comorbidity in bipolar disorder: a review of controlled trials. Bipolar Disord. 2006;8:696-709.
14. Vornik LA, Brown ES. Management of comorbid bipolar disorder and substance abuse. J Clin Psychiatry. 2006;67(suppl 7):24-30.
15. Strakowski SM, DelBello MP. The co-occurrence of bipolar and substance use disorders. Clin Psych Rev. 2000;20:191-206.
16. Berk M, Dodd S, Callaly P, et al. History of illness prior to a diagnosis of bipolar disorder or schizoaffective disorder. J Affect Disord. 2007;103:181-186.
17. Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorders and recurrent depression. 2nd ed. New York, NY: Oxford University Press; 2007.
18. McGuffin P, Rijsdijk F, Andrew M, et al. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497-502.
19. Kendler KS, Prescott CA, Myers J, et al. The structure of genetic and environmental risk factors for common psychiatric and substance abuse disorders in men and women. Arch Gen Psychiatry. 2003;60:929-937.
20. Weiss RD, Greenfield SF, Najavits LM, et al. Medication compliance among patients with bipolar disorder and substance use disorder. J Clin Psychiatry. 1998;59:172-174.
21. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder inpatients with mood instability. J Clin Psychiatry. 2008;69:1751-1757.
22. Winokur G, Coryell W, Akiskal HS, et al. Alcoholism in manic-depressive (bipolar) illness: familial illness, course of illness, and the primary-secondary distinction. Am J Psychiatry. 1995;152:365-372.
23. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR. 2006;55(RR14):1-17.
24. Salloum IM, Cornelius JR, Daley DC, et al. Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2005;62:37-45.
25. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry. 1998;37(2):171-178.
26. Brady KT, Sonne SC, Malcolm RJ, et al. Carbamazepine in the treatment of cocaine dependence: subtyping by affective disorder. Exp Clin Psychopharmacol. 2002;10:276-285.
27. Brown ES, Garza M, Carmody TJ. A randomized, double-blind, placebo-controlled add-on trial of quetiapine in outpatients with bipolar disorder and alcohol use disorders. J Clin Psychiatry. 2008;69:701-705.
28. Kemp DE, Gao K, Ganocy S, et al. A 6-month, double-blind, maintenance trial of lithium monotherapy versus the combination of lithium and divalproex for rapid-cycling bipolar disorder and co-occurring substance abuse or dependence. J Clin Psychiatry. 2009;70:113-121.
29. Brown ES, Carmody TJ, Schmitz JM, et al. A randomized, double-blind, placebo-controlled pilot study of naltrexone in outpatients with bipolar disorder and alcohol dependence. Alcohol Clin Exp Res. 2009;3:1863-1869.
30. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
31. Weiss RD, Griffin ML, Kolodziej MR, et al. A randomized trial of integrated group therapy versus group drug counseling for patients with bipolar disorder and substance dependence. Am J Psychiatry. 2007;164:100-107.
1. Levin FR, Hennessey G. Bipolar disorder and substance abuse. Biol Psychiatry. 2004;56:738-748.
2. Frye MA, Salloum IM. Bipolar disorder and comorbid alcoholism: prevalence rate and treatment considerations. Bipolar Disord. 2006;8:677-685.
3. Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807-816.
4. Hasin DS, Stinson FS, Ogburn E, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830-842.
5. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2007;64:543-552.
6. Sublette EM, Carballo JJ, Moreno C, et al. Substance use disorders and suicide attempts in bipolar subtypes. J Psychiatric Res. 2009;43:230-238.
7. McElroy SL, Altshuler LL, Suppes T, et al. Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry. 2001;158:420-426.
8. Frye MA, Altshuler LL, McElroy SL, et al. Gender differences in prevalence, risk, and clinical correlates of alcoholism comorbidity in bipolar disorder. Am J Psychiatry. 2003;160:883-889.
9. Simon NM, Otto MW, Wisniewski SR, et al. Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Am J Psychiatry. 2004;161:2222-2229.
10. Perron BE, Howard MO, Nienhuis JK, et al. Prevalence and burden of general medical conditions among adults with bipolar I disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2009;70:1407-1415.
11. Himelhoch S, McCarthy JF, Ganoczy D, et al. Understanding associations between serious mental illness and hepatitis C virus among veterans: a national multivariate analysis. Psychosomatics. 2009;50:30-37.
12. Matthews AM, Huckans MS, Blackwell AD, et al. Hepatitis C testing and infection rates in bipolar patients with and without comorbid substance use disorders. Bipolar Disord. 2008;10:266-270.
13. Singh JB, Zarate CA. Pharmacological treatment of psychiatric comorbidity in bipolar disorder: a review of controlled trials. Bipolar Disord. 2006;8:696-709.
14. Vornik LA, Brown ES. Management of comorbid bipolar disorder and substance abuse. J Clin Psychiatry. 2006;67(suppl 7):24-30.
15. Strakowski SM, DelBello MP. The co-occurrence of bipolar and substance use disorders. Clin Psych Rev. 2000;20:191-206.
16. Berk M, Dodd S, Callaly P, et al. History of illness prior to a diagnosis of bipolar disorder or schizoaffective disorder. J Affect Disord. 2007;103:181-186.
17. Goodwin FK, Jamison KR. Manic-depressive illness: bipolar disorders and recurrent depression. 2nd ed. New York, NY: Oxford University Press; 2007.
18. McGuffin P, Rijsdijk F, Andrew M, et al. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497-502.
19. Kendler KS, Prescott CA, Myers J, et al. The structure of genetic and environmental risk factors for common psychiatric and substance abuse disorders in men and women. Arch Gen Psychiatry. 2003;60:929-937.
20. Weiss RD, Greenfield SF, Najavits LM, et al. Medication compliance among patients with bipolar disorder and substance use disorder. J Clin Psychiatry. 1998;59:172-174.
21. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder inpatients with mood instability. J Clin Psychiatry. 2008;69:1751-1757.
22. Winokur G, Coryell W, Akiskal HS, et al. Alcoholism in manic-depressive (bipolar) illness: familial illness, course of illness, and the primary-secondary distinction. Am J Psychiatry. 1995;152:365-372.
23. Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR. 2006;55(RR14):1-17.
24. Salloum IM, Cornelius JR, Daley DC, et al. Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2005;62:37-45.
25. Geller B, Cooper TB, Sun K, et al. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry. 1998;37(2):171-178.
26. Brady KT, Sonne SC, Malcolm RJ, et al. Carbamazepine in the treatment of cocaine dependence: subtyping by affective disorder. Exp Clin Psychopharmacol. 2002;10:276-285.
27. Brown ES, Garza M, Carmody TJ. A randomized, double-blind, placebo-controlled add-on trial of quetiapine in outpatients with bipolar disorder and alcohol use disorders. J Clin Psychiatry. 2008;69:701-705.
28. Kemp DE, Gao K, Ganocy S, et al. A 6-month, double-blind, maintenance trial of lithium monotherapy versus the combination of lithium and divalproex for rapid-cycling bipolar disorder and co-occurring substance abuse or dependence. J Clin Psychiatry. 2009;70:113-121.
29. Brown ES, Carmody TJ, Schmitz JM, et al. A randomized, double-blind, placebo-controlled pilot study of naltrexone in outpatients with bipolar disorder and alcohol dependence. Alcohol Clin Exp Res. 2009;3:1863-1869.
30. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
31. Weiss RD, Griffin ML, Kolodziej MR, et al. A randomized trial of integrated group therapy versus group drug counseling for patients with bipolar disorder and substance dependence. Am J Psychiatry. 2007;164:100-107.
Depression in older adults: How to treat its distinct clinical manifestations
Discuss this article at http://currentpsychiatry.blogspot.com/2010/08/depression-in-older-adults.html#comments
Depression in older adults (age ≥65) can devastate their quality of life and increase the likelihood of institutionalization because of behavioral problems.1 Depression is a primary risk factor for suicide, and suicide rates are highest among those age ≥65, especially among white males.2 The burden of geriatric depression can extend to caregivers.1 Prompt recognition and treatment of depression could help minimize morbidity and reduce suffering in older adults and their caregivers.
Although geriatric depression varies in severity and presentation, common categories include:
- major depressive disorder (MDD)
- vascular depression
- dysthymia
- depression in the context of dementias, psychosis, bipolar disorder, and executive dysfunction.
Diagnoses in this population generally correspond with DSM-IV-TR criteria, but geriatric depression has distinct clinical manifestations.1,2 Compared with younger depressed patients, older adults are less likely to endorse depressed mood and more likely to report a lack of emotions.1,2 Older patients report feelings of irritability and fearfulness more often than sadness.1,2 Mood symptoms tend to be transient, reoccur frequently, and display either a diurnal pattern or multiple fluctuations in a single day.1,2 Other common presentations include loss of interest in usual activities, lack of motivation, social withdrawal, and decline in activities of daily living.1,2
Summary of recommendations
Age-specific recommendations for assessing and treating geriatric depression can be generated in part from evidence-based reviews, meta-analyses,3 and geriatric expert consensus guidelines.4 Such guidelines and recommendations often do not take into account the marked heterogeneity of medical, cognitive, and overall functioning in patients age ≥65, however, because they are based on studies of younger populations and patients with complicated issues often are excluded from studies. The recommendations in this article are based largely on findings from a National Institutes of Health (NIH)-sponsored project by Alexopoulos et al to develop consensus guidelines for managing geriatric depression and expert opinion from clinicians who treat geriatric patients.4
During your initial clinical evaluation, confirm the diagnosis and type, duration, and severity of depression. Seek to understand the biopsychosocial context of each patient’s presentation. Carefully consider your patient’s suicide risk. Hospitalization may be required if he or she is at high risk for suicide or has complex medical and social circumstances that cannot be managed adequately in an outpatient setting.5
Unipolar major depression
For unipolar, nonpsychotic geriatric depression, the NIH-Alexopoulos et al guidelines emphasize a combination of antidepressants and psychotherapy (Algorithm 1).4 Selective serotonin reuptake inhibitors (SSRIs) and venlafaxine are first-line options.4,6,7 Tricyclic antidepressants (TCAs), bupropion, and mirtazapine are alternatives.4 Among SSRIs, citalopram, escitalopram, and sertraline are preferred initial antidepressants. Fluoxetine is used less frequently.4 Paroxetine also is less commonly used because of its anticholinergic effects and because the drug inhibits cytochrome P4502D6,2 which metabolizes several medications commonly prescribed for older adults. Among TCAs, nortriptyline is preferred.4 Studies have shown that duloxetine improves depression and is safe and well-tolerated in older adults with recurrent MDD.8 Electroconvulsive therapy (ECT) is an option for treating severe or treatment-resistant unipolar major depression.9
For unipolar depression with psychotic symptoms, guidelines recommend a combination of an antidepressant and an antipsychotic or ECT.4 Atypical antipsychotics are preferred over typical antipsychotics4; risperidone, olanzapine, and quetiapine are most frequently used.4 Clinical data on aripiprazole and ziprasidone in older adults are limited. Many geriatric experts recommend continuing an antipsychotic for 6 months after symptom remission, then gradually tapering the dose.4
During acute illness, administer an anti-depressant for 6 to 12 weeks at the individually determined dose required to achieve symptom remission.6 For an older adult experiencing a first lifetime episode of major depression, continue antidepressant treatment for 1 year after remission.4 If your patient has had 2 lifetime episodes of major depression, continue the antidepressant at the same dose used to achieve remission for at least 3 years. For patients who have had ≥3 episodes of depression or whose index episode was particularly severe or involved significant suicidal thoughts or behaviors, continue maintenance treatment indefinitely.
Algorithm 1: Treatment for unipolar depression in geriatric patients
ECT: electroconvulsive therapy
Bipolar depression
Mood stabilizers such as lithium or valproate—as monotherapy or in combination with an antidepressant—are recommended to treat bipolar depression without psychotic symptoms in older adults (Algorithm 2).10 For bipolar depression with psychotic symptoms, a combination of a mood stabilizer and an atypical antipsychotic or ECT is recommended.10
Older adults’ increased sensitivity to side effects and reduced ability to tolerate lithium may limit its use and may prompt you to consider atypical antipsychotics as alternatives to other mood stabilizers. Although quetiapine and fluoxetineolanzapine combination are well studied in younger patients,11,12 there is a lack of data to support their clinical effectiveness and tolerability in older adults. Among antidepressants, SSRIs or bupropion are preferred over TCAs to prevent a switch to mania.10 Lamotrigine is an effective maintenance treatment for bipolar depressive episodes in older adults.13
Although optimal mood stabilizer and antidepressant dosing for this population has not been adequately assessed, pharmacotherapy that has been effective generally should be continued without modification for at least 6 to 12 months.10 After the patient achieves remission, gradually discontinue antidepressants while maintaining the mood stabilizer.10
Algorithm 2: Bipolar depression: Options for combination therapy
ECT: electroconvulsive therapy; SSRIs: selective serotonin reuptake inhibitors
Depression in dementia
Managing depression in dementia patients is similar to treatment in older adults without dementia,5,14 although pharmacologic agents must be carefully selected because of increased risk of side effects (Algorithm 3). American Psychiatric Association practice guidelines recommend considering antidepressants for depressed patients with dementia even if their mood disturbances do not meet DSM-IV-TR criteria for MDD.5
SSRIs’ lower side effect profile make them the preferred treatment; the selective serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine is a second-line option.4,14 Avoid TCAs and other agents with anticholinergic side effects because of potential cardiovascular complications and cognitive side effects, unless SSRIs or SNRIs are ineffective or contraindicated.14 Recently clinicians have been reluctant to use antipsychotics in patients with dementia, because of the FDA’s “black-box” warning regarding the increased mortality risk associated with their use in this population.
When using ECT to treat depression in patients with dementia, the treatment protocol often is modified to twice-a-week, unilateral stimulus because of these patients’ increased risk of delirium.14 The safety of ECT to treat depression in patients with dementia has not been adequately assessed.14
Algorithm 3: Treating comorbid depression and dementia
ECT: electroconvulsive therapy; SNRI: selective serotoninnorepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor
Vascular depression
The “vascular depression hypothesis” proposes that accumulation of subcortical white matter hyperintensities can disrupt frontostriatal pathways, resulting in depressive symptoms.15 This hypothesis is supported by the confluence of depression and vascular risk factors.15 Sertraline, citalopram, nortriptyline,16 and trazodone15 have been shown to reduce depressive symptoms after a stroke.
Minor depression and dysthymia
Although the efficacy of antidepressants in minor depression—depression that does not meet criteria for MDD—is not well established, expert consensus guidelines recommend SSRIs and psychotherapy, separately or in combination, for minor depression and dysthymia in older adults (Algorithm 4).4 Depression in executive dysfunction responds poorly to SSRI treatment2; however, behaviorally oriented psychotherapeutic interventions such as problem-solving therapy (PST) show promise.2
Algorithm 4: Minor depression: SSRIs plus psychotherapy
SSRIs: selective serotonin reuptake inhibitors
Comorbid medical conditions
When an older adult has a medical problem that likely contributes to depression—such as hypothyroidism—treat the condition and prescribe antidepressants simultaneously.2 However, if the medical problem likely causes depression—such as substance withdrawal—treat the condition first and prescribe antidepressants only if mood symptoms persist.2
Refractory depression
If your patient does not respond to an antidepressant trial of adequate dosage and duration, first make sure he or she is taking it correctly (Algorithm 5). After ruling out poor adherence, screen for comorbid psychiatric or medical conditions or psychosocial stressors and reassess the principal diagnosis.5
If these steps don’t address your patient’s depressive symptoms, expert consensus guidelines suggest switching to a different antidepressant:4
- If you first prescribed an SSRI, consider venlafaxine XR or bupropion SR.4,17
- If your patient initially received a TCA or bupropion, an SSRI or venlafaxine XR would be appropriate.4
- If venlafaxine XR was the first antidepressant, a SSRI is recommended.4
If your patient experienced a partial response but not full remission with the initial antidepressant, consider adding a second antidepressant or an augmenting agent:4
- If your patient first received an SSRI, adding bupropion, lithium, or nortriptyline is recommended.
- If the initial antidepressant was a TCA or bupropion, consider adding lithium or an SSRI.
- Augmenting venlafaxine XR with lithium is recommended.4
The National Institutes of Mental Health-sponsored Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study of treatment-resistant depression in mixed-age groups reported that patients who do not attain remission with an initial SSRI may respond to switching to bupropion SR or venlafaxine XR.17 Augmenting an SSRI with bupropion SR has been shown to be effective.18 In addition, consider mirtazapine augmentation,19 especially if your patient experiences insomnia or anorexia. A combination of mirtazapine and venlafaxine have better efficacy and tolerability compared with the monoamine oxidase inhibitor tranylcypromine.19 Some studies have shown augmenting SSRIs with buspirone in patients with severe depression is efficacious and safe in younger adults,20 but this practice is not well studied in older patients.
Algorithm 5: Treatment-resistant geriatric depression: Partial vs no response
SNRI: selective serotonin-norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant
Nonpharmacologic treatments
ECT is an important therapeutic intervention because of its safety, efficacy, and faster clinical response.6,7,9,21 Consider ECT for older adults with severe or psychotic major depression, acute suicidality, catatonia, or severe malnutrition caused by refusal to eat. Patients who remain significantly symptomatic after multiple medication trials, do not tolerate medications well, or have comorbid medical conditions that preclude antidepressant use also are potential candidates for ECT.5,22
ECT can be administered to many older depressed adults with relatively low complication rates. Pretreatment clinical and laboratory evaluations and consultation with medical colleagues may minimize the risk of adverse effects, including cardiovascular instability, delirium, and falls.9 Anterograde memory loss—a common concern for clinicians and patients—usually is temporary and can be reduced by modifying the ECT administration parameters, such as switching from bilateral to unilateral stimulus and spacing treatments.9 Use caution when considering ECT for patients with cardiovascular or neurologic conditions—such as myocardial infarction or cerebrovascular accident within 6 months of treatment—that may increase the risk of adverse effects. Some pharmacologic agents, such as benzodiazepines and anticonvulsant mood stabilizers, may decrease ECT’s efficacy by inhibiting seizure.22
Depressive relapse after ECT is a major clinical concern.21 Continuation ECT— within the first 6 months of remission— aims to prevent relapse of the same episode, whereas maintenance ECT—beyond the first 6 months—helps avert occurrence of new episodes.4,21 Relapse and recurrence also can be prevented with continuation or maintenance pharmacotherapy,4,21 which should be initiated immediately after the index course of ECT.21 Typically, ECT continuation/maintenance treatments are provided weekly, then gradually spaced out to once a month based on the minimum frequency that is effective for an individual patient.21
Psychotherapy for geriatric depression generally is effective.23 One-half of older patients prefer psychotherapy over pharmacotherapy.24 Efficacious psychotherapies include behavioral therapy, cognitive-behavioral therapy (CBT), PST, brief dynamic therapy, interpersonal therapy, supportive therapy, and reminiscence therapy.23 CBT has the most empiric support for treating geriatric depression.5,6
Psychotherapy alone is appropriate for mild-to-moderate depression, although severe depression requires adding medication.25 The combination of pharmacotherapy and psychotherapy appears to be more effective than either intervention alone in preventing recurrent major depression, especially when a specific psychosocial stressor has been identified.5,6 CBT, interpersonal therapy, and family-focused therapy enhance pharmacotherapy outcomes in bipolar disorder.13
The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) study found that in mixed-age patients, pharmacotherapy plus psychotherapy is more beneficial than medication alone in stabilizing bipolar depression.26 For older adults with executive dysfunction, research suggests that PST is more effective than other psychotherapies.27 Psychosocial interventions—such as psychoeducation for the family and caregivers, family counseling, and participation in senior citizen centers and services—are strongly recommended for many patients.4
Related Resources
- Blazer DG, Steffens DC, Koenig HG. Mood disorders. In: Blazer DG, Steffens DC, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:275-300.
- American Association for Geriatric Psychiatry. www.aagponline.org.
Drug Brand Names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Buspirone • Buspar
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluoxetine-olanzapine • Symbyax
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Tranylcypromine • Parnate
- Trazodone • Desyrel
- Valproate • Depakote
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with the manufacturer of any product mentioned in this article or with manufacturers of competing products.
1. Lyketsos CG, Lee HB. Diagnosis and treatment of depression in Alzheimer’s disease. A practical update for the clinician. Dement Geriatr Cogn Disord. 2004;17(1-2):55-64.
2. Alexopoulos G. Late-life mood disorders. In: Sadavoy J, Jarvik LF, Grossberg GT, et al, eds. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York, NY: W.W. Norton and Company; 2004:609-653.
3. Shanmugham B, Karp J, Drayer R, et al. Evidence-based pharmacologic interventions of geriatric depression. Psychiatr Clin North Am. 2005;28(4):821-835,viii.
4. Alexopoulos GS, Katz IR, Reynolds CF, III, et al. The expert consensus guidelines series. Pharmacotherapy of depressive disorders in older patients. Postgrad Med. 2001; Spect No Pharmacolotherapy:1–86.
5. American Psychiatric Association practice guidelines for the treatment of psychiatric disorders. Arlington, VA: American Psychiatric Association; 2006:793–794.
6. Bartels SJ, Dums AR, Oxman TE, et al. Evidence-based practice in geriatric mental health care. Psychiatr Serv. 2002;53(11):1419-1431.
7. Bartels SJ, Dums AR, Oxman TE, et al. Evidence-based practices in geriatric mental health care: an overview of systematic reviews and meta-analyses. Psychiatr Clin North Am. 2003;26(4):971-990,x–xi.
8. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6):900-909.
9. Alexopoulos GS, Young RC, Abrams RC. ECT in the high-risk geriatric patient. Convuls Ther. 1989;5(1):75-87.
10. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12:342-357.
11. Vieta E, Calabrese JR, Goikolea JM, et al. Quetiapine monotherapy in the treatment of patients with bipolar I or II depression and a rapid-cycling disease course: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2007;9(4):413-425.
12. Corya SA, Perlis RH, Keck PE, Jr, et al. A 24-week open-label extension study of olanzapine-fluoxetine combination and olanzapine monotherapy in the treatment of bipolar depression. J Clin Psychiatry. 2006;67(5):798-806.
13. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
14. Lyketsos CG, Olin J. Depression in Alzheimer’s disease: overview and treatment. Biol Psychiatry. 2002;52(3):243-252.
15. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54(10):915-922.
16. Starkstein SE, Mizrahi R, Power BD. Antidepressant therapy in post-stroke depression. Expert Opin Pharmacother. 2008;9(8):1291-1298.
17. Rush AJ, Trivedi MH, Wisniewski SR, et al. and STAR*D Study Team. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
18. Trivedi MH, Fava M, Wisniewski SR, et al. and the STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252.
19. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1541.
20. Appelberg BG, Syvälahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001;62(6):448-452.
21. Greenberg RM, Kellner CH. Electroconvulsive therapy: a selected review. Am J Geriatric Psychiatry. 2005;13(4):268-281.
22. Kaplan HI, Sadock BJ. Electroconvulsive therapy. In: Kaplan and Sadock’s synopsis of psychiatry. 8th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 1998:1138–1143.
23. Gum A, Areán P. Current status of psychotherapy for mental disorders in the elderly. Curr Psychiatry Rep. 2004;6:32-38.
24. Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in primary care settings: a randomized controlled trial. JAMA. 2002;288:2836-2845.
25. Niederehe G, Schneider LS. Treatments for depression and anxiety in the aged. In: Nathan PE, Gorman JM, eds. A guide to treatments that work. New York, NY: Oxford University Press; 1998:270–287.
26. Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry. 2007;64:419-426.
27. Alexopoulos GS, Raue P, Areán P. Problem-solving therapy versus supportive therapy in geriatric major depression with executive dysfunction. Am J Geriatr Psychiatry. 2003;11:46-52.
Discuss this article at http://currentpsychiatry.blogspot.com/2010/08/depression-in-older-adults.html#comments
Depression in older adults (age ≥65) can devastate their quality of life and increase the likelihood of institutionalization because of behavioral problems.1 Depression is a primary risk factor for suicide, and suicide rates are highest among those age ≥65, especially among white males.2 The burden of geriatric depression can extend to caregivers.1 Prompt recognition and treatment of depression could help minimize morbidity and reduce suffering in older adults and their caregivers.
Although geriatric depression varies in severity and presentation, common categories include:
- major depressive disorder (MDD)
- vascular depression
- dysthymia
- depression in the context of dementias, psychosis, bipolar disorder, and executive dysfunction.
Diagnoses in this population generally correspond with DSM-IV-TR criteria, but geriatric depression has distinct clinical manifestations.1,2 Compared with younger depressed patients, older adults are less likely to endorse depressed mood and more likely to report a lack of emotions.1,2 Older patients report feelings of irritability and fearfulness more often than sadness.1,2 Mood symptoms tend to be transient, reoccur frequently, and display either a diurnal pattern or multiple fluctuations in a single day.1,2 Other common presentations include loss of interest in usual activities, lack of motivation, social withdrawal, and decline in activities of daily living.1,2
Summary of recommendations
Age-specific recommendations for assessing and treating geriatric depression can be generated in part from evidence-based reviews, meta-analyses,3 and geriatric expert consensus guidelines.4 Such guidelines and recommendations often do not take into account the marked heterogeneity of medical, cognitive, and overall functioning in patients age ≥65, however, because they are based on studies of younger populations and patients with complicated issues often are excluded from studies. The recommendations in this article are based largely on findings from a National Institutes of Health (NIH)-sponsored project by Alexopoulos et al to develop consensus guidelines for managing geriatric depression and expert opinion from clinicians who treat geriatric patients.4
During your initial clinical evaluation, confirm the diagnosis and type, duration, and severity of depression. Seek to understand the biopsychosocial context of each patient’s presentation. Carefully consider your patient’s suicide risk. Hospitalization may be required if he or she is at high risk for suicide or has complex medical and social circumstances that cannot be managed adequately in an outpatient setting.5
Unipolar major depression
For unipolar, nonpsychotic geriatric depression, the NIH-Alexopoulos et al guidelines emphasize a combination of antidepressants and psychotherapy (Algorithm 1).4 Selective serotonin reuptake inhibitors (SSRIs) and venlafaxine are first-line options.4,6,7 Tricyclic antidepressants (TCAs), bupropion, and mirtazapine are alternatives.4 Among SSRIs, citalopram, escitalopram, and sertraline are preferred initial antidepressants. Fluoxetine is used less frequently.4 Paroxetine also is less commonly used because of its anticholinergic effects and because the drug inhibits cytochrome P4502D6,2 which metabolizes several medications commonly prescribed for older adults. Among TCAs, nortriptyline is preferred.4 Studies have shown that duloxetine improves depression and is safe and well-tolerated in older adults with recurrent MDD.8 Electroconvulsive therapy (ECT) is an option for treating severe or treatment-resistant unipolar major depression.9
For unipolar depression with psychotic symptoms, guidelines recommend a combination of an antidepressant and an antipsychotic or ECT.4 Atypical antipsychotics are preferred over typical antipsychotics4; risperidone, olanzapine, and quetiapine are most frequently used.4 Clinical data on aripiprazole and ziprasidone in older adults are limited. Many geriatric experts recommend continuing an antipsychotic for 6 months after symptom remission, then gradually tapering the dose.4
During acute illness, administer an anti-depressant for 6 to 12 weeks at the individually determined dose required to achieve symptom remission.6 For an older adult experiencing a first lifetime episode of major depression, continue antidepressant treatment for 1 year after remission.4 If your patient has had 2 lifetime episodes of major depression, continue the antidepressant at the same dose used to achieve remission for at least 3 years. For patients who have had ≥3 episodes of depression or whose index episode was particularly severe or involved significant suicidal thoughts or behaviors, continue maintenance treatment indefinitely.
Algorithm 1: Treatment for unipolar depression in geriatric patients
ECT: electroconvulsive therapy
Bipolar depression
Mood stabilizers such as lithium or valproate—as monotherapy or in combination with an antidepressant—are recommended to treat bipolar depression without psychotic symptoms in older adults (Algorithm 2).10 For bipolar depression with psychotic symptoms, a combination of a mood stabilizer and an atypical antipsychotic or ECT is recommended.10
Older adults’ increased sensitivity to side effects and reduced ability to tolerate lithium may limit its use and may prompt you to consider atypical antipsychotics as alternatives to other mood stabilizers. Although quetiapine and fluoxetineolanzapine combination are well studied in younger patients,11,12 there is a lack of data to support their clinical effectiveness and tolerability in older adults. Among antidepressants, SSRIs or bupropion are preferred over TCAs to prevent a switch to mania.10 Lamotrigine is an effective maintenance treatment for bipolar depressive episodes in older adults.13
Although optimal mood stabilizer and antidepressant dosing for this population has not been adequately assessed, pharmacotherapy that has been effective generally should be continued without modification for at least 6 to 12 months.10 After the patient achieves remission, gradually discontinue antidepressants while maintaining the mood stabilizer.10
Algorithm 2: Bipolar depression: Options for combination therapy
ECT: electroconvulsive therapy; SSRIs: selective serotonin reuptake inhibitors
Depression in dementia
Managing depression in dementia patients is similar to treatment in older adults without dementia,5,14 although pharmacologic agents must be carefully selected because of increased risk of side effects (Algorithm 3). American Psychiatric Association practice guidelines recommend considering antidepressants for depressed patients with dementia even if their mood disturbances do not meet DSM-IV-TR criteria for MDD.5
SSRIs’ lower side effect profile make them the preferred treatment; the selective serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine is a second-line option.4,14 Avoid TCAs and other agents with anticholinergic side effects because of potential cardiovascular complications and cognitive side effects, unless SSRIs or SNRIs are ineffective or contraindicated.14 Recently clinicians have been reluctant to use antipsychotics in patients with dementia, because of the FDA’s “black-box” warning regarding the increased mortality risk associated with their use in this population.
When using ECT to treat depression in patients with dementia, the treatment protocol often is modified to twice-a-week, unilateral stimulus because of these patients’ increased risk of delirium.14 The safety of ECT to treat depression in patients with dementia has not been adequately assessed.14
Algorithm 3: Treating comorbid depression and dementia
ECT: electroconvulsive therapy; SNRI: selective serotoninnorepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor
Vascular depression
The “vascular depression hypothesis” proposes that accumulation of subcortical white matter hyperintensities can disrupt frontostriatal pathways, resulting in depressive symptoms.15 This hypothesis is supported by the confluence of depression and vascular risk factors.15 Sertraline, citalopram, nortriptyline,16 and trazodone15 have been shown to reduce depressive symptoms after a stroke.
Minor depression and dysthymia
Although the efficacy of antidepressants in minor depression—depression that does not meet criteria for MDD—is not well established, expert consensus guidelines recommend SSRIs and psychotherapy, separately or in combination, for minor depression and dysthymia in older adults (Algorithm 4).4 Depression in executive dysfunction responds poorly to SSRI treatment2; however, behaviorally oriented psychotherapeutic interventions such as problem-solving therapy (PST) show promise.2
Algorithm 4: Minor depression: SSRIs plus psychotherapy
SSRIs: selective serotonin reuptake inhibitors
Comorbid medical conditions
When an older adult has a medical problem that likely contributes to depression—such as hypothyroidism—treat the condition and prescribe antidepressants simultaneously.2 However, if the medical problem likely causes depression—such as substance withdrawal—treat the condition first and prescribe antidepressants only if mood symptoms persist.2
Refractory depression
If your patient does not respond to an antidepressant trial of adequate dosage and duration, first make sure he or she is taking it correctly (Algorithm 5). After ruling out poor adherence, screen for comorbid psychiatric or medical conditions or psychosocial stressors and reassess the principal diagnosis.5
If these steps don’t address your patient’s depressive symptoms, expert consensus guidelines suggest switching to a different antidepressant:4
- If you first prescribed an SSRI, consider venlafaxine XR or bupropion SR.4,17
- If your patient initially received a TCA or bupropion, an SSRI or venlafaxine XR would be appropriate.4
- If venlafaxine XR was the first antidepressant, a SSRI is recommended.4
If your patient experienced a partial response but not full remission with the initial antidepressant, consider adding a second antidepressant or an augmenting agent:4
- If your patient first received an SSRI, adding bupropion, lithium, or nortriptyline is recommended.
- If the initial antidepressant was a TCA or bupropion, consider adding lithium or an SSRI.
- Augmenting venlafaxine XR with lithium is recommended.4
The National Institutes of Mental Health-sponsored Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study of treatment-resistant depression in mixed-age groups reported that patients who do not attain remission with an initial SSRI may respond to switching to bupropion SR or venlafaxine XR.17 Augmenting an SSRI with bupropion SR has been shown to be effective.18 In addition, consider mirtazapine augmentation,19 especially if your patient experiences insomnia or anorexia. A combination of mirtazapine and venlafaxine have better efficacy and tolerability compared with the monoamine oxidase inhibitor tranylcypromine.19 Some studies have shown augmenting SSRIs with buspirone in patients with severe depression is efficacious and safe in younger adults,20 but this practice is not well studied in older patients.
Algorithm 5: Treatment-resistant geriatric depression: Partial vs no response
SNRI: selective serotonin-norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant
Nonpharmacologic treatments
ECT is an important therapeutic intervention because of its safety, efficacy, and faster clinical response.6,7,9,21 Consider ECT for older adults with severe or psychotic major depression, acute suicidality, catatonia, or severe malnutrition caused by refusal to eat. Patients who remain significantly symptomatic after multiple medication trials, do not tolerate medications well, or have comorbid medical conditions that preclude antidepressant use also are potential candidates for ECT.5,22
ECT can be administered to many older depressed adults with relatively low complication rates. Pretreatment clinical and laboratory evaluations and consultation with medical colleagues may minimize the risk of adverse effects, including cardiovascular instability, delirium, and falls.9 Anterograde memory loss—a common concern for clinicians and patients—usually is temporary and can be reduced by modifying the ECT administration parameters, such as switching from bilateral to unilateral stimulus and spacing treatments.9 Use caution when considering ECT for patients with cardiovascular or neurologic conditions—such as myocardial infarction or cerebrovascular accident within 6 months of treatment—that may increase the risk of adverse effects. Some pharmacologic agents, such as benzodiazepines and anticonvulsant mood stabilizers, may decrease ECT’s efficacy by inhibiting seizure.22
Depressive relapse after ECT is a major clinical concern.21 Continuation ECT— within the first 6 months of remission— aims to prevent relapse of the same episode, whereas maintenance ECT—beyond the first 6 months—helps avert occurrence of new episodes.4,21 Relapse and recurrence also can be prevented with continuation or maintenance pharmacotherapy,4,21 which should be initiated immediately after the index course of ECT.21 Typically, ECT continuation/maintenance treatments are provided weekly, then gradually spaced out to once a month based on the minimum frequency that is effective for an individual patient.21
Psychotherapy for geriatric depression generally is effective.23 One-half of older patients prefer psychotherapy over pharmacotherapy.24 Efficacious psychotherapies include behavioral therapy, cognitive-behavioral therapy (CBT), PST, brief dynamic therapy, interpersonal therapy, supportive therapy, and reminiscence therapy.23 CBT has the most empiric support for treating geriatric depression.5,6
Psychotherapy alone is appropriate for mild-to-moderate depression, although severe depression requires adding medication.25 The combination of pharmacotherapy and psychotherapy appears to be more effective than either intervention alone in preventing recurrent major depression, especially when a specific psychosocial stressor has been identified.5,6 CBT, interpersonal therapy, and family-focused therapy enhance pharmacotherapy outcomes in bipolar disorder.13
The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) study found that in mixed-age patients, pharmacotherapy plus psychotherapy is more beneficial than medication alone in stabilizing bipolar depression.26 For older adults with executive dysfunction, research suggests that PST is more effective than other psychotherapies.27 Psychosocial interventions—such as psychoeducation for the family and caregivers, family counseling, and participation in senior citizen centers and services—are strongly recommended for many patients.4
Related Resources
- Blazer DG, Steffens DC, Koenig HG. Mood disorders. In: Blazer DG, Steffens DC, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:275-300.
- American Association for Geriatric Psychiatry. www.aagponline.org.
Drug Brand Names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Buspirone • Buspar
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluoxetine-olanzapine • Symbyax
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Tranylcypromine • Parnate
- Trazodone • Desyrel
- Valproate • Depakote
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with the manufacturer of any product mentioned in this article or with manufacturers of competing products.
Discuss this article at http://currentpsychiatry.blogspot.com/2010/08/depression-in-older-adults.html#comments
Depression in older adults (age ≥65) can devastate their quality of life and increase the likelihood of institutionalization because of behavioral problems.1 Depression is a primary risk factor for suicide, and suicide rates are highest among those age ≥65, especially among white males.2 The burden of geriatric depression can extend to caregivers.1 Prompt recognition and treatment of depression could help minimize morbidity and reduce suffering in older adults and their caregivers.
Although geriatric depression varies in severity and presentation, common categories include:
- major depressive disorder (MDD)
- vascular depression
- dysthymia
- depression in the context of dementias, psychosis, bipolar disorder, and executive dysfunction.
Diagnoses in this population generally correspond with DSM-IV-TR criteria, but geriatric depression has distinct clinical manifestations.1,2 Compared with younger depressed patients, older adults are less likely to endorse depressed mood and more likely to report a lack of emotions.1,2 Older patients report feelings of irritability and fearfulness more often than sadness.1,2 Mood symptoms tend to be transient, reoccur frequently, and display either a diurnal pattern or multiple fluctuations in a single day.1,2 Other common presentations include loss of interest in usual activities, lack of motivation, social withdrawal, and decline in activities of daily living.1,2
Summary of recommendations
Age-specific recommendations for assessing and treating geriatric depression can be generated in part from evidence-based reviews, meta-analyses,3 and geriatric expert consensus guidelines.4 Such guidelines and recommendations often do not take into account the marked heterogeneity of medical, cognitive, and overall functioning in patients age ≥65, however, because they are based on studies of younger populations and patients with complicated issues often are excluded from studies. The recommendations in this article are based largely on findings from a National Institutes of Health (NIH)-sponsored project by Alexopoulos et al to develop consensus guidelines for managing geriatric depression and expert opinion from clinicians who treat geriatric patients.4
During your initial clinical evaluation, confirm the diagnosis and type, duration, and severity of depression. Seek to understand the biopsychosocial context of each patient’s presentation. Carefully consider your patient’s suicide risk. Hospitalization may be required if he or she is at high risk for suicide or has complex medical and social circumstances that cannot be managed adequately in an outpatient setting.5
Unipolar major depression
For unipolar, nonpsychotic geriatric depression, the NIH-Alexopoulos et al guidelines emphasize a combination of antidepressants and psychotherapy (Algorithm 1).4 Selective serotonin reuptake inhibitors (SSRIs) and venlafaxine are first-line options.4,6,7 Tricyclic antidepressants (TCAs), bupropion, and mirtazapine are alternatives.4 Among SSRIs, citalopram, escitalopram, and sertraline are preferred initial antidepressants. Fluoxetine is used less frequently.4 Paroxetine also is less commonly used because of its anticholinergic effects and because the drug inhibits cytochrome P4502D6,2 which metabolizes several medications commonly prescribed for older adults. Among TCAs, nortriptyline is preferred.4 Studies have shown that duloxetine improves depression and is safe and well-tolerated in older adults with recurrent MDD.8 Electroconvulsive therapy (ECT) is an option for treating severe or treatment-resistant unipolar major depression.9
For unipolar depression with psychotic symptoms, guidelines recommend a combination of an antidepressant and an antipsychotic or ECT.4 Atypical antipsychotics are preferred over typical antipsychotics4; risperidone, olanzapine, and quetiapine are most frequently used.4 Clinical data on aripiprazole and ziprasidone in older adults are limited. Many geriatric experts recommend continuing an antipsychotic for 6 months after symptom remission, then gradually tapering the dose.4
During acute illness, administer an anti-depressant for 6 to 12 weeks at the individually determined dose required to achieve symptom remission.6 For an older adult experiencing a first lifetime episode of major depression, continue antidepressant treatment for 1 year after remission.4 If your patient has had 2 lifetime episodes of major depression, continue the antidepressant at the same dose used to achieve remission for at least 3 years. For patients who have had ≥3 episodes of depression or whose index episode was particularly severe or involved significant suicidal thoughts or behaviors, continue maintenance treatment indefinitely.
Algorithm 1: Treatment for unipolar depression in geriatric patients
ECT: electroconvulsive therapy
Bipolar depression
Mood stabilizers such as lithium or valproate—as monotherapy or in combination with an antidepressant—are recommended to treat bipolar depression without psychotic symptoms in older adults (Algorithm 2).10 For bipolar depression with psychotic symptoms, a combination of a mood stabilizer and an atypical antipsychotic or ECT is recommended.10
Older adults’ increased sensitivity to side effects and reduced ability to tolerate lithium may limit its use and may prompt you to consider atypical antipsychotics as alternatives to other mood stabilizers. Although quetiapine and fluoxetineolanzapine combination are well studied in younger patients,11,12 there is a lack of data to support their clinical effectiveness and tolerability in older adults. Among antidepressants, SSRIs or bupropion are preferred over TCAs to prevent a switch to mania.10 Lamotrigine is an effective maintenance treatment for bipolar depressive episodes in older adults.13
Although optimal mood stabilizer and antidepressant dosing for this population has not been adequately assessed, pharmacotherapy that has been effective generally should be continued without modification for at least 6 to 12 months.10 After the patient achieves remission, gradually discontinue antidepressants while maintaining the mood stabilizer.10
Algorithm 2: Bipolar depression: Options for combination therapy
ECT: electroconvulsive therapy; SSRIs: selective serotonin reuptake inhibitors
Depression in dementia
Managing depression in dementia patients is similar to treatment in older adults without dementia,5,14 although pharmacologic agents must be carefully selected because of increased risk of side effects (Algorithm 3). American Psychiatric Association practice guidelines recommend considering antidepressants for depressed patients with dementia even if their mood disturbances do not meet DSM-IV-TR criteria for MDD.5
SSRIs’ lower side effect profile make them the preferred treatment; the selective serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine is a second-line option.4,14 Avoid TCAs and other agents with anticholinergic side effects because of potential cardiovascular complications and cognitive side effects, unless SSRIs or SNRIs are ineffective or contraindicated.14 Recently clinicians have been reluctant to use antipsychotics in patients with dementia, because of the FDA’s “black-box” warning regarding the increased mortality risk associated with their use in this population.
When using ECT to treat depression in patients with dementia, the treatment protocol often is modified to twice-a-week, unilateral stimulus because of these patients’ increased risk of delirium.14 The safety of ECT to treat depression in patients with dementia has not been adequately assessed.14
Algorithm 3: Treating comorbid depression and dementia
ECT: electroconvulsive therapy; SNRI: selective serotoninnorepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor
Vascular depression
The “vascular depression hypothesis” proposes that accumulation of subcortical white matter hyperintensities can disrupt frontostriatal pathways, resulting in depressive symptoms.15 This hypothesis is supported by the confluence of depression and vascular risk factors.15 Sertraline, citalopram, nortriptyline,16 and trazodone15 have been shown to reduce depressive symptoms after a stroke.
Minor depression and dysthymia
Although the efficacy of antidepressants in minor depression—depression that does not meet criteria for MDD—is not well established, expert consensus guidelines recommend SSRIs and psychotherapy, separately or in combination, for minor depression and dysthymia in older adults (Algorithm 4).4 Depression in executive dysfunction responds poorly to SSRI treatment2; however, behaviorally oriented psychotherapeutic interventions such as problem-solving therapy (PST) show promise.2
Algorithm 4: Minor depression: SSRIs plus psychotherapy
SSRIs: selective serotonin reuptake inhibitors
Comorbid medical conditions
When an older adult has a medical problem that likely contributes to depression—such as hypothyroidism—treat the condition and prescribe antidepressants simultaneously.2 However, if the medical problem likely causes depression—such as substance withdrawal—treat the condition first and prescribe antidepressants only if mood symptoms persist.2
Refractory depression
If your patient does not respond to an antidepressant trial of adequate dosage and duration, first make sure he or she is taking it correctly (Algorithm 5). After ruling out poor adherence, screen for comorbid psychiatric or medical conditions or psychosocial stressors and reassess the principal diagnosis.5
If these steps don’t address your patient’s depressive symptoms, expert consensus guidelines suggest switching to a different antidepressant:4
- If you first prescribed an SSRI, consider venlafaxine XR or bupropion SR.4,17
- If your patient initially received a TCA or bupropion, an SSRI or venlafaxine XR would be appropriate.4
- If venlafaxine XR was the first antidepressant, a SSRI is recommended.4
If your patient experienced a partial response but not full remission with the initial antidepressant, consider adding a second antidepressant or an augmenting agent:4
- If your patient first received an SSRI, adding bupropion, lithium, or nortriptyline is recommended.
- If the initial antidepressant was a TCA or bupropion, consider adding lithium or an SSRI.
- Augmenting venlafaxine XR with lithium is recommended.4
The National Institutes of Mental Health-sponsored Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study of treatment-resistant depression in mixed-age groups reported that patients who do not attain remission with an initial SSRI may respond to switching to bupropion SR or venlafaxine XR.17 Augmenting an SSRI with bupropion SR has been shown to be effective.18 In addition, consider mirtazapine augmentation,19 especially if your patient experiences insomnia or anorexia. A combination of mirtazapine and venlafaxine have better efficacy and tolerability compared with the monoamine oxidase inhibitor tranylcypromine.19 Some studies have shown augmenting SSRIs with buspirone in patients with severe depression is efficacious and safe in younger adults,20 but this practice is not well studied in older patients.
Algorithm 5: Treatment-resistant geriatric depression: Partial vs no response
SNRI: selective serotonin-norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant
Nonpharmacologic treatments
ECT is an important therapeutic intervention because of its safety, efficacy, and faster clinical response.6,7,9,21 Consider ECT for older adults with severe or psychotic major depression, acute suicidality, catatonia, or severe malnutrition caused by refusal to eat. Patients who remain significantly symptomatic after multiple medication trials, do not tolerate medications well, or have comorbid medical conditions that preclude antidepressant use also are potential candidates for ECT.5,22
ECT can be administered to many older depressed adults with relatively low complication rates. Pretreatment clinical and laboratory evaluations and consultation with medical colleagues may minimize the risk of adverse effects, including cardiovascular instability, delirium, and falls.9 Anterograde memory loss—a common concern for clinicians and patients—usually is temporary and can be reduced by modifying the ECT administration parameters, such as switching from bilateral to unilateral stimulus and spacing treatments.9 Use caution when considering ECT for patients with cardiovascular or neurologic conditions—such as myocardial infarction or cerebrovascular accident within 6 months of treatment—that may increase the risk of adverse effects. Some pharmacologic agents, such as benzodiazepines and anticonvulsant mood stabilizers, may decrease ECT’s efficacy by inhibiting seizure.22
Depressive relapse after ECT is a major clinical concern.21 Continuation ECT— within the first 6 months of remission— aims to prevent relapse of the same episode, whereas maintenance ECT—beyond the first 6 months—helps avert occurrence of new episodes.4,21 Relapse and recurrence also can be prevented with continuation or maintenance pharmacotherapy,4,21 which should be initiated immediately after the index course of ECT.21 Typically, ECT continuation/maintenance treatments are provided weekly, then gradually spaced out to once a month based on the minimum frequency that is effective for an individual patient.21
Psychotherapy for geriatric depression generally is effective.23 One-half of older patients prefer psychotherapy over pharmacotherapy.24 Efficacious psychotherapies include behavioral therapy, cognitive-behavioral therapy (CBT), PST, brief dynamic therapy, interpersonal therapy, supportive therapy, and reminiscence therapy.23 CBT has the most empiric support for treating geriatric depression.5,6
Psychotherapy alone is appropriate for mild-to-moderate depression, although severe depression requires adding medication.25 The combination of pharmacotherapy and psychotherapy appears to be more effective than either intervention alone in preventing recurrent major depression, especially when a specific psychosocial stressor has been identified.5,6 CBT, interpersonal therapy, and family-focused therapy enhance pharmacotherapy outcomes in bipolar disorder.13
The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) study found that in mixed-age patients, pharmacotherapy plus psychotherapy is more beneficial than medication alone in stabilizing bipolar depression.26 For older adults with executive dysfunction, research suggests that PST is more effective than other psychotherapies.27 Psychosocial interventions—such as psychoeducation for the family and caregivers, family counseling, and participation in senior citizen centers and services—are strongly recommended for many patients.4
Related Resources
- Blazer DG, Steffens DC, Koenig HG. Mood disorders. In: Blazer DG, Steffens DC, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009:275-300.
- American Association for Geriatric Psychiatry. www.aagponline.org.
Drug Brand Names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin, Zyban
- Buspirone • Buspar
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluoxetine-olanzapine • Symbyax
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Nortriptyline • Aventyl, Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Tranylcypromine • Parnate
- Trazodone • Desyrel
- Valproate • Depakote
- Venlafaxine • Effexor
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with the manufacturer of any product mentioned in this article or with manufacturers of competing products.
1. Lyketsos CG, Lee HB. Diagnosis and treatment of depression in Alzheimer’s disease. A practical update for the clinician. Dement Geriatr Cogn Disord. 2004;17(1-2):55-64.
2. Alexopoulos G. Late-life mood disorders. In: Sadavoy J, Jarvik LF, Grossberg GT, et al, eds. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York, NY: W.W. Norton and Company; 2004:609-653.
3. Shanmugham B, Karp J, Drayer R, et al. Evidence-based pharmacologic interventions of geriatric depression. Psychiatr Clin North Am. 2005;28(4):821-835,viii.
4. Alexopoulos GS, Katz IR, Reynolds CF, III, et al. The expert consensus guidelines series. Pharmacotherapy of depressive disorders in older patients. Postgrad Med. 2001; Spect No Pharmacolotherapy:1–86.
5. American Psychiatric Association practice guidelines for the treatment of psychiatric disorders. Arlington, VA: American Psychiatric Association; 2006:793–794.
6. Bartels SJ, Dums AR, Oxman TE, et al. Evidence-based practice in geriatric mental health care. Psychiatr Serv. 2002;53(11):1419-1431.
7. Bartels SJ, Dums AR, Oxman TE, et al. Evidence-based practices in geriatric mental health care: an overview of systematic reviews and meta-analyses. Psychiatr Clin North Am. 2003;26(4):971-990,x–xi.
8. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6):900-909.
9. Alexopoulos GS, Young RC, Abrams RC. ECT in the high-risk geriatric patient. Convuls Ther. 1989;5(1):75-87.
10. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12:342-357.
11. Vieta E, Calabrese JR, Goikolea JM, et al. Quetiapine monotherapy in the treatment of patients with bipolar I or II depression and a rapid-cycling disease course: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2007;9(4):413-425.
12. Corya SA, Perlis RH, Keck PE, Jr, et al. A 24-week open-label extension study of olanzapine-fluoxetine combination and olanzapine monotherapy in the treatment of bipolar depression. J Clin Psychiatry. 2006;67(5):798-806.
13. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
14. Lyketsos CG, Olin J. Depression in Alzheimer’s disease: overview and treatment. Biol Psychiatry. 2002;52(3):243-252.
15. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54(10):915-922.
16. Starkstein SE, Mizrahi R, Power BD. Antidepressant therapy in post-stroke depression. Expert Opin Pharmacother. 2008;9(8):1291-1298.
17. Rush AJ, Trivedi MH, Wisniewski SR, et al. and STAR*D Study Team. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
18. Trivedi MH, Fava M, Wisniewski SR, et al. and the STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252.
19. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1541.
20. Appelberg BG, Syvälahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001;62(6):448-452.
21. Greenberg RM, Kellner CH. Electroconvulsive therapy: a selected review. Am J Geriatric Psychiatry. 2005;13(4):268-281.
22. Kaplan HI, Sadock BJ. Electroconvulsive therapy. In: Kaplan and Sadock’s synopsis of psychiatry. 8th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 1998:1138–1143.
23. Gum A, Areán P. Current status of psychotherapy for mental disorders in the elderly. Curr Psychiatry Rep. 2004;6:32-38.
24. Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in primary care settings: a randomized controlled trial. JAMA. 2002;288:2836-2845.
25. Niederehe G, Schneider LS. Treatments for depression and anxiety in the aged. In: Nathan PE, Gorman JM, eds. A guide to treatments that work. New York, NY: Oxford University Press; 1998:270–287.
26. Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry. 2007;64:419-426.
27. Alexopoulos GS, Raue P, Areán P. Problem-solving therapy versus supportive therapy in geriatric major depression with executive dysfunction. Am J Geriatr Psychiatry. 2003;11:46-52.
1. Lyketsos CG, Lee HB. Diagnosis and treatment of depression in Alzheimer’s disease. A practical update for the clinician. Dement Geriatr Cogn Disord. 2004;17(1-2):55-64.
2. Alexopoulos G. Late-life mood disorders. In: Sadavoy J, Jarvik LF, Grossberg GT, et al, eds. Comprehensive textbook of geriatric psychiatry. 3rd ed. New York, NY: W.W. Norton and Company; 2004:609-653.
3. Shanmugham B, Karp J, Drayer R, et al. Evidence-based pharmacologic interventions of geriatric depression. Psychiatr Clin North Am. 2005;28(4):821-835,viii.
4. Alexopoulos GS, Katz IR, Reynolds CF, III, et al. The expert consensus guidelines series. Pharmacotherapy of depressive disorders in older patients. Postgrad Med. 2001; Spect No Pharmacolotherapy:1–86.
5. American Psychiatric Association practice guidelines for the treatment of psychiatric disorders. Arlington, VA: American Psychiatric Association; 2006:793–794.
6. Bartels SJ, Dums AR, Oxman TE, et al. Evidence-based practice in geriatric mental health care. Psychiatr Serv. 2002;53(11):1419-1431.
7. Bartels SJ, Dums AR, Oxman TE, et al. Evidence-based practices in geriatric mental health care: an overview of systematic reviews and meta-analyses. Psychiatr Clin North Am. 2003;26(4):971-990,x–xi.
8. Raskin J, Wiltse CG, Siegal A, et al. Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. Am J Psychiatry. 2007;164(6):900-909.
9. Alexopoulos GS, Young RC, Abrams RC. ECT in the high-risk geriatric patient. Convuls Ther. 1989;5(1):75-87.
10. Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12:342-357.
11. Vieta E, Calabrese JR, Goikolea JM, et al. Quetiapine monotherapy in the treatment of patients with bipolar I or II depression and a rapid-cycling disease course: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2007;9(4):413-425.
12. Corya SA, Perlis RH, Keck PE, Jr, et al. A 24-week open-label extension study of olanzapine-fluoxetine combination and olanzapine monotherapy in the treatment of bipolar depression. J Clin Psychiatry. 2006;67(5):798-806.
13. Sajatovic M, Gyulai L, Calabrese JR, et al. Maintenance treatment outcomes in older patients with bipolar I disorder. Am J Geriatr Psychiatry. 2005;13(4):305-311.
14. Lyketsos CG, Olin J. Depression in Alzheimer’s disease: overview and treatment. Biol Psychiatry. 2002;52(3):243-252.
15. Alexopoulos GS, Meyers BS, Young RC, et al. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54(10):915-922.
16. Starkstein SE, Mizrahi R, Power BD. Antidepressant therapy in post-stroke depression. Expert Opin Pharmacother. 2008;9(8):1291-1298.
17. Rush AJ, Trivedi MH, Wisniewski SR, et al. and STAR*D Study Team. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
18. Trivedi MH, Fava M, Wisniewski SR, et al. and the STAR*D Study Team. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354(12):1243-1252.
19. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531-1541.
20. Appelberg BG, Syvälahti EK, Koskinen TE, et al. Patients with severe depression may benefit from buspirone augmentation of selective serotonin reuptake inhibitors: results from a placebo-controlled, randomized, double-blind, placebo wash-in study. J Clin Psychiatry. 2001;62(6):448-452.
21. Greenberg RM, Kellner CH. Electroconvulsive therapy: a selected review. Am J Geriatric Psychiatry. 2005;13(4):268-281.
22. Kaplan HI, Sadock BJ. Electroconvulsive therapy. In: Kaplan and Sadock’s synopsis of psychiatry. 8th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 1998:1138–1143.
23. Gum A, Areán P. Current status of psychotherapy for mental disorders in the elderly. Curr Psychiatry Rep. 2004;6:32-38.
24. Unützer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in primary care settings: a randomized controlled trial. JAMA. 2002;288:2836-2845.
25. Niederehe G, Schneider LS. Treatments for depression and anxiety in the aged. In: Nathan PE, Gorman JM, eds. A guide to treatments that work. New York, NY: Oxford University Press; 1998:270–287.
26. Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry. 2007;64:419-426.
27. Alexopoulos GS, Raue P, Areán P. Problem-solving therapy versus supportive therapy in geriatric major depression with executive dysfunction. Am J Geriatr Psychiatry. 2003;11:46-52.
Excess mortality in patients with mood disorders
How to manage your patient’s dementia by discontinuing medications
Mrs. J, age 75, has moderate Alzheimer’s dementia and lives at home with her husband. Since her Alzheimer’s disease (AD) diagnosis 2 years ago, Mrs. J generally has been cooperative and not physically aggressive, but has experienced occasional depressive symptoms. However, Mr. J reports that recently his wife is becoming increasingly confused and agitated and wanders the house at night. His efforts to calm and coax her back to bed often lead to increased agitation and yelling. On 1 occasion Mrs. J pushed her husband. Mr. J is concerned that if these behaviors continue he may not be able to care for her at home. Mr. J read online that antipsychotics might reduce aggressive behavior, but is concerned about the increased risk of mortality and stroke with these medications.
Mrs. J receives donepezil, 10 mg/d, sertra-line, 50 mg/d, and extended-release oxybutynin, 10 mg/d. Her over-the-counter (OTC) medications include acetaminophen, 650 mg as needed for pain, ranitidine, 150 mg/d, and docusate sodium, 100 mg/d. Several nights last week, Mr. J gave his wife an unknown OTC sleep medication, hoping it would stop her nighttime wandering, but it did not help. Physical examination, laboratory testing, and urine culture are all normal.
- During the initial evaluation of cognitive complaints, look to discontinue medications that may cause cognitive impairment, including anticholinergics.
- In addition to worsening cognitive impairment, anticholinergic medications may contribute to behavioral disturbances, psychosis, and delirium in patients with dementia.
- Side effects of acetylcholinesterase inhibitors can prompt anticholinergic use, which is likely to negate the beneficial effects of the acetylcholinesterase inhibitor.
- Nonpsychiatric medications, including over-the-counter drugs, can have anticholinergic properties. Consult with nonpsychiatric clinicians to discontinue inessential medications that may be harmful for dementia patients.
Most dementia patients experience neuropsychiatric disturbances, especially at later stages, that often lead to caregiver distress and nursing home placement. Although these symptoms may signal progressing dementia, environmental factors, medical conditions, and medications may worsen functioning and should be considered in the assessment.1
Mrs. J has no medical problems that were identified as possible triggers for her behavior. Mr. J’s interference with his wife’s wandering could have increased her agitation, but he is gentle toward her and she has become agitated with no apparent trigger. “Sundowning” and poor sleep also may be involved, as sleep deprivation can lead to delirium and worsen cognitive deficits and behavioral problems.1 Depression also should be considered.1 Finally, Mrs. J is taking several medications with anticholinergic properties—oxybutynin, ranitidine, and an unknown OTC sleep medication, which likely contains diphenhydramine or doxylamine—that might contribute to her agitation.
Patients with dementia are highly sensitive to the cognitive and psychiatric adverse effects of anticholinergic medications. In studies of patients with mild or moderate Alzheimer’s dementia who received the potent anticholinergic scopolamine, adverse effects included:
- memory impairment
- restlessness
- disjointed speech
- motor incoordination
- drowsiness
- euphoria
- agitation
- hallucinations
- hostility.
Many of these effects worsened with increasing doses.2,3 Age-matched controls experienced less severe memory impairment and no behavioral symptoms, which suggests that dementia-related damage to the cholinergic system leads to increased sensitivity to anticholinergics.
A cross-sectional study of 230 patients with AD identified anticholinergic use as a risk factor for psychosis (odds ratio 2.13, 95% confidence interval, 1.03 to 4.43), after adjusting for age and cognition.4 Among patients receiving 2 or 3 anticholinergics, 69% had psychotic symptoms compared with 48% of those receiving 1 anticholinergic and 32% of those receiving no anticholinergics.4 Anticholinergic overdoses can cause psychotic symptoms and delirium. A subtle presentation of delirium from prescribed anticholinergics may be confused with worsening dementia.1 The sum of the evidence suggests that drugs with anticholinergic effects can contribute to agitation and psychosis in dementia.
When to discontinue
When diagnosing dementia it is important to address other potential causes of cognitive impairment, including medications. Approximately one-third of patients with dementia receive anticholinergic drugs, which suggests that providers often do not recognize the potential for harm with these medications.5 After patients receive acetylcholinesterase inhibitors (AChEIs)—which are used to enhance cognition in dementia patients—increased anticholinergic use may follow, often to treat adverse effects of AChEIs.5 This may negate the benefits of AChEIs and pose risk of further harm from the anticholinergics.1,5 Although any time is a good time to discontinue an inessential anticholinergic in a patient with dementia, providers might consider screening for these drugs at the initial diagnosis, after initiating a cholinesterase inhibitor or increasing a dose, or if the patient develops psychotic or behavioral symptoms.
For Mrs. J, ranitidine and oxybutynin likely were used to treat gastrointestinal complaints and urinary frequency, which are known adverse effects of AChEIs. Many OTC preparations for insomnia, respiratory symptoms, and allergies contain older, anticholinergic antihistamines. Advise caregivers of dementia patients about possible adverse effects of OTC medications to prevent anticholinergic exposure. The Table provides a partial list of medications thought to have clinically significant anticholinergic effects.
‘Pharmacologic debridement’ refers to tapering and discontinuing medications that are no longer necessary or appropriate. Prescribers often are hesitant to discontinue medications prescribed by other clinicians and may assume that a medication used long term has been tolerated and helpful. However, as patients age—particularly if they develop dementia—their ability to tolerate a medication can change. Patients with dementia also may have difficulty attributing adverse experiences to medications and communicating these effects to providers. Some medical providers may not recognize adverse psychiatric and cognitive effects of the nonpsychiatric medications they prescribe because they do not have sufficient dementia expertise. Consulting with these providers may help determine the risk-benefit considerations of these medications.
Generally, anticholinergics should be discontinued if they are not essential to a patient’s health or if safer non-anticholinergic alternatives are available.5 Tapering may be necessary to prevent adverse effects from cholinergic rebound if a potent anticholinergic has been used chronically.5 The first step in addressing Mrs. J’s agitation is to discontinue the anticholinergic medications and monitor her symptoms. This pharmacologic debridement may avert the use of antipsychotics, which carry serious risks for dementia patients.1
Table
Drugs with clinically significant anticholinergic effects*
| Drug class | Medication(s) |
|---|---|
| Anticonvulsants | Carbamazepine |
| Antidepressants | Amitriptyline, clomipramine, desipramine, doxepin, imipramine, nortriptyline, paroxetine, protriptyline, trimipramine |
| Antihistamines | Azelastine nasal spray, brompheniramine, carbinoxamine, chlorpheniramine, clemastine, cyproheptadine, dexbrompheniramine, dexchlorpheniramine, diphenhydramine, hydroxyzine, mepyramine olopatadine nasal spray, phenyltoloxamine, promethazine, triprolidine |
| Antiparkinsonian agents | Benztropine, procyclidine, trihexyphenidyl |
| Antipsychotics | Chlorpromazine, clozapine, loxapine, molindone, olanzapine, pimozide, promethazine, quetiapine, thioridazine |
| Asthma and chronic obstructive pulmonary disease medication | Glycopyrrolate, ipratropium,† tiotropium† |
| Bladder antispasmodics | Darifenacin, flavoxate, oxybutynin, solifenacin, tolterodine, trospium |
| Gastrointestinal antispasmodics | Atropine, belladonna alkaloids, clidinium, dicyclomine, hyoscyamine, methscopolamine, propantheline |
| Insomnia medications | Diphenhydramine, doxylamine |
| Motion sickness/dizziness/nausea medications | Dimenhydrinate, meclizine, prochlorperazine, promethazine, scopolamine, trimethobenzamide |
| Muscle relaxants and pain medications | Cyclobenzaprine, meperidine, orphenadrine, phenyltoloxamine |
| Ulcer and acid reflux agents | Cimetidine, glycopyrrolate, ranitidine |
| *Not a comprehensive list †Unknown whether CNS effects are important Source: Reference 5 | |
- Cancelli I, Beltrame M, D’Anna L, et al. Drugs with anticholinergic properties: a potential risk factor for psychosis onset in Alzheimer’s disease? Expert Opin Drug Saf. 2009;8(5):549-557.
- Meeks TW, Jeste DV. Beyond the black box: what is the role for antipsychotics in dementia? Current Psychiatry. 2008;7(6): 50-65.
- Centers for Education and Research on Therapeutics. Anticholinergic pocket reference card. www.chainonline.org/home/content_images/Anticholinergic%20Pocket%20Card%20CLR%203_12_10.pdf.
Drug Brand Names
- Amitriptyline • Elavil
- Atropine • Sal-Tropine
- Azelastine nasal spray • Astelin
- Belladonna alkaloids • Donnatal
- Benztropine • Cogentin
- Brompheniramine • Dimetane
- Carbamazepine • Carbatrol, Tegretol, others
- Carbinoxamine • Palgic
- Chlorpheniramine • Chlor-Trimeton
- Chlorpromazine • Thorazine
- Cimetidine • Tagamet
- Clemastine • Tavist
- Clidinium • Quarzan
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Cyclobenzaprine • Flexeril
- Cyproheptadine • Periactin
- Darifenacin • Enablex
- Desipramine • Norpramin
- Dexbrompheniramine • Drixoral
- Dexchlorpheniramine • Polaramine
- Dicyclomine • Bentyl
- Dimenhydrinate • Dramamine
- Diphenhydramine • Benadryl, Sominex, others
- Docusate Sodium • Colace
- Donepezil • Aricept
- Doxepin • Adapin
- Doxylamine • Aldex, Unisom, others
- Flavoxate • Urispas
- Glycopyrrolate • Robinul
- Hydroxyzine • Atarax
- Hyoscyamine • Cystospaz, Levbid
- Imipramine • Tofranil
- Ipratropium • Atrovent
- Loxapine • Loxitane
- Meclizine • Antivert
- Meperidine • Demerol
- Mepyramine • Anthisan
- Methscopolamine • Pamine
- Molindone • Moban
- Nortriptyline • Aventyl
- Olanzapine • Zyprexa
- Olopatadine nasal spray • Patanase
- Orphenadrine • Norflex
- Oxybutynin extended-release • Ditropan XL
- Paroxetine • Paxil
- Phenyltoloxamine • Dologesic, Durayin, others
- Pimozide • Orap
- Prochlorperazine • Compazine
- Procyclidine • Kemadrin
- Promethazine • Phenergan
- Propanthelin • Pro-Banthine
- Protriptyline • Vivactil
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Scopolamine • Scopace
- Sertraline • Zoloft
- Solifenacin • VESIcare
- Thioridazine • Mellaril
- Tiotropium • Spiriva
- Tolterodine • Detrol
- Trihexyphenidyl • Artane
- Trimethobenzamide • Tigan
- Trimipramine • Surmontil
- Triprolidine • Actifed
- Trospium • Sanctura
Acknowledgements
This work was supported by an Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement #5 U18 HSO16094.
Disclosure
Dr. Carnahan receives grant/research support from the Agency for Healthcare Research and Quality.
1. Swanson KA, Carnahan RM. Dementia and comorbidities: an overview of diagnosis and management. J Pharm Pract. 2007;20:296-317.
2. Sunderland T, Tariot P, Murphy DL, et al. Scopolamine challenges in Alzheimer’s disease. Psychopharmacology (Berl). 1985;87(2):247-249.
3. Sunderland T, Tariot PN, Cohen RM, et al. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose response study. Arch Gen Psychiatry. 1987;44(5):418-426.
4. Cancelli I, Valentinis L, Merlino G, et al. Drugs with anticholinergic properties as a risk factor for psychosis in patients affected by Alzheimer’s disease. Clin Pharmacol Ther. 2008;84(1):63-68.
5. Carnahan RM, Lund BC, Perry PJ, et al. The concurrent use of anticholinergics and cholinesterase inhibitors: rare event or common practice? J Am Geriatr Soc. 2004;52:2082-2087.
Mrs. J, age 75, has moderate Alzheimer’s dementia and lives at home with her husband. Since her Alzheimer’s disease (AD) diagnosis 2 years ago, Mrs. J generally has been cooperative and not physically aggressive, but has experienced occasional depressive symptoms. However, Mr. J reports that recently his wife is becoming increasingly confused and agitated and wanders the house at night. His efforts to calm and coax her back to bed often lead to increased agitation and yelling. On 1 occasion Mrs. J pushed her husband. Mr. J is concerned that if these behaviors continue he may not be able to care for her at home. Mr. J read online that antipsychotics might reduce aggressive behavior, but is concerned about the increased risk of mortality and stroke with these medications.
Mrs. J receives donepezil, 10 mg/d, sertra-line, 50 mg/d, and extended-release oxybutynin, 10 mg/d. Her over-the-counter (OTC) medications include acetaminophen, 650 mg as needed for pain, ranitidine, 150 mg/d, and docusate sodium, 100 mg/d. Several nights last week, Mr. J gave his wife an unknown OTC sleep medication, hoping it would stop her nighttime wandering, but it did not help. Physical examination, laboratory testing, and urine culture are all normal.
- During the initial evaluation of cognitive complaints, look to discontinue medications that may cause cognitive impairment, including anticholinergics.
- In addition to worsening cognitive impairment, anticholinergic medications may contribute to behavioral disturbances, psychosis, and delirium in patients with dementia.
- Side effects of acetylcholinesterase inhibitors can prompt anticholinergic use, which is likely to negate the beneficial effects of the acetylcholinesterase inhibitor.
- Nonpsychiatric medications, including over-the-counter drugs, can have anticholinergic properties. Consult with nonpsychiatric clinicians to discontinue inessential medications that may be harmful for dementia patients.
Most dementia patients experience neuropsychiatric disturbances, especially at later stages, that often lead to caregiver distress and nursing home placement. Although these symptoms may signal progressing dementia, environmental factors, medical conditions, and medications may worsen functioning and should be considered in the assessment.1
Mrs. J has no medical problems that were identified as possible triggers for her behavior. Mr. J’s interference with his wife’s wandering could have increased her agitation, but he is gentle toward her and she has become agitated with no apparent trigger. “Sundowning” and poor sleep also may be involved, as sleep deprivation can lead to delirium and worsen cognitive deficits and behavioral problems.1 Depression also should be considered.1 Finally, Mrs. J is taking several medications with anticholinergic properties—oxybutynin, ranitidine, and an unknown OTC sleep medication, which likely contains diphenhydramine or doxylamine—that might contribute to her agitation.
Patients with dementia are highly sensitive to the cognitive and psychiatric adverse effects of anticholinergic medications. In studies of patients with mild or moderate Alzheimer’s dementia who received the potent anticholinergic scopolamine, adverse effects included:
- memory impairment
- restlessness
- disjointed speech
- motor incoordination
- drowsiness
- euphoria
- agitation
- hallucinations
- hostility.
Many of these effects worsened with increasing doses.2,3 Age-matched controls experienced less severe memory impairment and no behavioral symptoms, which suggests that dementia-related damage to the cholinergic system leads to increased sensitivity to anticholinergics.
A cross-sectional study of 230 patients with AD identified anticholinergic use as a risk factor for psychosis (odds ratio 2.13, 95% confidence interval, 1.03 to 4.43), after adjusting for age and cognition.4 Among patients receiving 2 or 3 anticholinergics, 69% had psychotic symptoms compared with 48% of those receiving 1 anticholinergic and 32% of those receiving no anticholinergics.4 Anticholinergic overdoses can cause psychotic symptoms and delirium. A subtle presentation of delirium from prescribed anticholinergics may be confused with worsening dementia.1 The sum of the evidence suggests that drugs with anticholinergic effects can contribute to agitation and psychosis in dementia.
When to discontinue
When diagnosing dementia it is important to address other potential causes of cognitive impairment, including medications. Approximately one-third of patients with dementia receive anticholinergic drugs, which suggests that providers often do not recognize the potential for harm with these medications.5 After patients receive acetylcholinesterase inhibitors (AChEIs)—which are used to enhance cognition in dementia patients—increased anticholinergic use may follow, often to treat adverse effects of AChEIs.5 This may negate the benefits of AChEIs and pose risk of further harm from the anticholinergics.1,5 Although any time is a good time to discontinue an inessential anticholinergic in a patient with dementia, providers might consider screening for these drugs at the initial diagnosis, after initiating a cholinesterase inhibitor or increasing a dose, or if the patient develops psychotic or behavioral symptoms.
For Mrs. J, ranitidine and oxybutynin likely were used to treat gastrointestinal complaints and urinary frequency, which are known adverse effects of AChEIs. Many OTC preparations for insomnia, respiratory symptoms, and allergies contain older, anticholinergic antihistamines. Advise caregivers of dementia patients about possible adverse effects of OTC medications to prevent anticholinergic exposure. The Table provides a partial list of medications thought to have clinically significant anticholinergic effects.
‘Pharmacologic debridement’ refers to tapering and discontinuing medications that are no longer necessary or appropriate. Prescribers often are hesitant to discontinue medications prescribed by other clinicians and may assume that a medication used long term has been tolerated and helpful. However, as patients age—particularly if they develop dementia—their ability to tolerate a medication can change. Patients with dementia also may have difficulty attributing adverse experiences to medications and communicating these effects to providers. Some medical providers may not recognize adverse psychiatric and cognitive effects of the nonpsychiatric medications they prescribe because they do not have sufficient dementia expertise. Consulting with these providers may help determine the risk-benefit considerations of these medications.
Generally, anticholinergics should be discontinued if they are not essential to a patient’s health or if safer non-anticholinergic alternatives are available.5 Tapering may be necessary to prevent adverse effects from cholinergic rebound if a potent anticholinergic has been used chronically.5 The first step in addressing Mrs. J’s agitation is to discontinue the anticholinergic medications and monitor her symptoms. This pharmacologic debridement may avert the use of antipsychotics, which carry serious risks for dementia patients.1
Table
Drugs with clinically significant anticholinergic effects*
| Drug class | Medication(s) |
|---|---|
| Anticonvulsants | Carbamazepine |
| Antidepressants | Amitriptyline, clomipramine, desipramine, doxepin, imipramine, nortriptyline, paroxetine, protriptyline, trimipramine |
| Antihistamines | Azelastine nasal spray, brompheniramine, carbinoxamine, chlorpheniramine, clemastine, cyproheptadine, dexbrompheniramine, dexchlorpheniramine, diphenhydramine, hydroxyzine, mepyramine olopatadine nasal spray, phenyltoloxamine, promethazine, triprolidine |
| Antiparkinsonian agents | Benztropine, procyclidine, trihexyphenidyl |
| Antipsychotics | Chlorpromazine, clozapine, loxapine, molindone, olanzapine, pimozide, promethazine, quetiapine, thioridazine |
| Asthma and chronic obstructive pulmonary disease medication | Glycopyrrolate, ipratropium,† tiotropium† |
| Bladder antispasmodics | Darifenacin, flavoxate, oxybutynin, solifenacin, tolterodine, trospium |
| Gastrointestinal antispasmodics | Atropine, belladonna alkaloids, clidinium, dicyclomine, hyoscyamine, methscopolamine, propantheline |
| Insomnia medications | Diphenhydramine, doxylamine |
| Motion sickness/dizziness/nausea medications | Dimenhydrinate, meclizine, prochlorperazine, promethazine, scopolamine, trimethobenzamide |
| Muscle relaxants and pain medications | Cyclobenzaprine, meperidine, orphenadrine, phenyltoloxamine |
| Ulcer and acid reflux agents | Cimetidine, glycopyrrolate, ranitidine |
| *Not a comprehensive list †Unknown whether CNS effects are important Source: Reference 5 | |
- Cancelli I, Beltrame M, D’Anna L, et al. Drugs with anticholinergic properties: a potential risk factor for psychosis onset in Alzheimer’s disease? Expert Opin Drug Saf. 2009;8(5):549-557.
- Meeks TW, Jeste DV. Beyond the black box: what is the role for antipsychotics in dementia? Current Psychiatry. 2008;7(6): 50-65.
- Centers for Education and Research on Therapeutics. Anticholinergic pocket reference card. www.chainonline.org/home/content_images/Anticholinergic%20Pocket%20Card%20CLR%203_12_10.pdf.
Drug Brand Names
- Amitriptyline • Elavil
- Atropine • Sal-Tropine
- Azelastine nasal spray • Astelin
- Belladonna alkaloids • Donnatal
- Benztropine • Cogentin
- Brompheniramine • Dimetane
- Carbamazepine • Carbatrol, Tegretol, others
- Carbinoxamine • Palgic
- Chlorpheniramine • Chlor-Trimeton
- Chlorpromazine • Thorazine
- Cimetidine • Tagamet
- Clemastine • Tavist
- Clidinium • Quarzan
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Cyclobenzaprine • Flexeril
- Cyproheptadine • Periactin
- Darifenacin • Enablex
- Desipramine • Norpramin
- Dexbrompheniramine • Drixoral
- Dexchlorpheniramine • Polaramine
- Dicyclomine • Bentyl
- Dimenhydrinate • Dramamine
- Diphenhydramine • Benadryl, Sominex, others
- Docusate Sodium • Colace
- Donepezil • Aricept
- Doxepin • Adapin
- Doxylamine • Aldex, Unisom, others
- Flavoxate • Urispas
- Glycopyrrolate • Robinul
- Hydroxyzine • Atarax
- Hyoscyamine • Cystospaz, Levbid
- Imipramine • Tofranil
- Ipratropium • Atrovent
- Loxapine • Loxitane
- Meclizine • Antivert
- Meperidine • Demerol
- Mepyramine • Anthisan
- Methscopolamine • Pamine
- Molindone • Moban
- Nortriptyline • Aventyl
- Olanzapine • Zyprexa
- Olopatadine nasal spray • Patanase
- Orphenadrine • Norflex
- Oxybutynin extended-release • Ditropan XL
- Paroxetine • Paxil
- Phenyltoloxamine • Dologesic, Durayin, others
- Pimozide • Orap
- Prochlorperazine • Compazine
- Procyclidine • Kemadrin
- Promethazine • Phenergan
- Propanthelin • Pro-Banthine
- Protriptyline • Vivactil
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Scopolamine • Scopace
- Sertraline • Zoloft
- Solifenacin • VESIcare
- Thioridazine • Mellaril
- Tiotropium • Spiriva
- Tolterodine • Detrol
- Trihexyphenidyl • Artane
- Trimethobenzamide • Tigan
- Trimipramine • Surmontil
- Triprolidine • Actifed
- Trospium • Sanctura
Acknowledgements
This work was supported by an Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement #5 U18 HSO16094.
Disclosure
Dr. Carnahan receives grant/research support from the Agency for Healthcare Research and Quality.
Mrs. J, age 75, has moderate Alzheimer’s dementia and lives at home with her husband. Since her Alzheimer’s disease (AD) diagnosis 2 years ago, Mrs. J generally has been cooperative and not physically aggressive, but has experienced occasional depressive symptoms. However, Mr. J reports that recently his wife is becoming increasingly confused and agitated and wanders the house at night. His efforts to calm and coax her back to bed often lead to increased agitation and yelling. On 1 occasion Mrs. J pushed her husband. Mr. J is concerned that if these behaviors continue he may not be able to care for her at home. Mr. J read online that antipsychotics might reduce aggressive behavior, but is concerned about the increased risk of mortality and stroke with these medications.
Mrs. J receives donepezil, 10 mg/d, sertra-line, 50 mg/d, and extended-release oxybutynin, 10 mg/d. Her over-the-counter (OTC) medications include acetaminophen, 650 mg as needed for pain, ranitidine, 150 mg/d, and docusate sodium, 100 mg/d. Several nights last week, Mr. J gave his wife an unknown OTC sleep medication, hoping it would stop her nighttime wandering, but it did not help. Physical examination, laboratory testing, and urine culture are all normal.
- During the initial evaluation of cognitive complaints, look to discontinue medications that may cause cognitive impairment, including anticholinergics.
- In addition to worsening cognitive impairment, anticholinergic medications may contribute to behavioral disturbances, psychosis, and delirium in patients with dementia.
- Side effects of acetylcholinesterase inhibitors can prompt anticholinergic use, which is likely to negate the beneficial effects of the acetylcholinesterase inhibitor.
- Nonpsychiatric medications, including over-the-counter drugs, can have anticholinergic properties. Consult with nonpsychiatric clinicians to discontinue inessential medications that may be harmful for dementia patients.
Most dementia patients experience neuropsychiatric disturbances, especially at later stages, that often lead to caregiver distress and nursing home placement. Although these symptoms may signal progressing dementia, environmental factors, medical conditions, and medications may worsen functioning and should be considered in the assessment.1
Mrs. J has no medical problems that were identified as possible triggers for her behavior. Mr. J’s interference with his wife’s wandering could have increased her agitation, but he is gentle toward her and she has become agitated with no apparent trigger. “Sundowning” and poor sleep also may be involved, as sleep deprivation can lead to delirium and worsen cognitive deficits and behavioral problems.1 Depression also should be considered.1 Finally, Mrs. J is taking several medications with anticholinergic properties—oxybutynin, ranitidine, and an unknown OTC sleep medication, which likely contains diphenhydramine or doxylamine—that might contribute to her agitation.
Patients with dementia are highly sensitive to the cognitive and psychiatric adverse effects of anticholinergic medications. In studies of patients with mild or moderate Alzheimer’s dementia who received the potent anticholinergic scopolamine, adverse effects included:
- memory impairment
- restlessness
- disjointed speech
- motor incoordination
- drowsiness
- euphoria
- agitation
- hallucinations
- hostility.
Many of these effects worsened with increasing doses.2,3 Age-matched controls experienced less severe memory impairment and no behavioral symptoms, which suggests that dementia-related damage to the cholinergic system leads to increased sensitivity to anticholinergics.
A cross-sectional study of 230 patients with AD identified anticholinergic use as a risk factor for psychosis (odds ratio 2.13, 95% confidence interval, 1.03 to 4.43), after adjusting for age and cognition.4 Among patients receiving 2 or 3 anticholinergics, 69% had psychotic symptoms compared with 48% of those receiving 1 anticholinergic and 32% of those receiving no anticholinergics.4 Anticholinergic overdoses can cause psychotic symptoms and delirium. A subtle presentation of delirium from prescribed anticholinergics may be confused with worsening dementia.1 The sum of the evidence suggests that drugs with anticholinergic effects can contribute to agitation and psychosis in dementia.
When to discontinue
When diagnosing dementia it is important to address other potential causes of cognitive impairment, including medications. Approximately one-third of patients with dementia receive anticholinergic drugs, which suggests that providers often do not recognize the potential for harm with these medications.5 After patients receive acetylcholinesterase inhibitors (AChEIs)—which are used to enhance cognition in dementia patients—increased anticholinergic use may follow, often to treat adverse effects of AChEIs.5 This may negate the benefits of AChEIs and pose risk of further harm from the anticholinergics.1,5 Although any time is a good time to discontinue an inessential anticholinergic in a patient with dementia, providers might consider screening for these drugs at the initial diagnosis, after initiating a cholinesterase inhibitor or increasing a dose, or if the patient develops psychotic or behavioral symptoms.
For Mrs. J, ranitidine and oxybutynin likely were used to treat gastrointestinal complaints and urinary frequency, which are known adverse effects of AChEIs. Many OTC preparations for insomnia, respiratory symptoms, and allergies contain older, anticholinergic antihistamines. Advise caregivers of dementia patients about possible adverse effects of OTC medications to prevent anticholinergic exposure. The Table provides a partial list of medications thought to have clinically significant anticholinergic effects.
‘Pharmacologic debridement’ refers to tapering and discontinuing medications that are no longer necessary or appropriate. Prescribers often are hesitant to discontinue medications prescribed by other clinicians and may assume that a medication used long term has been tolerated and helpful. However, as patients age—particularly if they develop dementia—their ability to tolerate a medication can change. Patients with dementia also may have difficulty attributing adverse experiences to medications and communicating these effects to providers. Some medical providers may not recognize adverse psychiatric and cognitive effects of the nonpsychiatric medications they prescribe because they do not have sufficient dementia expertise. Consulting with these providers may help determine the risk-benefit considerations of these medications.
Generally, anticholinergics should be discontinued if they are not essential to a patient’s health or if safer non-anticholinergic alternatives are available.5 Tapering may be necessary to prevent adverse effects from cholinergic rebound if a potent anticholinergic has been used chronically.5 The first step in addressing Mrs. J’s agitation is to discontinue the anticholinergic medications and monitor her symptoms. This pharmacologic debridement may avert the use of antipsychotics, which carry serious risks for dementia patients.1
Table
Drugs with clinically significant anticholinergic effects*
| Drug class | Medication(s) |
|---|---|
| Anticonvulsants | Carbamazepine |
| Antidepressants | Amitriptyline, clomipramine, desipramine, doxepin, imipramine, nortriptyline, paroxetine, protriptyline, trimipramine |
| Antihistamines | Azelastine nasal spray, brompheniramine, carbinoxamine, chlorpheniramine, clemastine, cyproheptadine, dexbrompheniramine, dexchlorpheniramine, diphenhydramine, hydroxyzine, mepyramine olopatadine nasal spray, phenyltoloxamine, promethazine, triprolidine |
| Antiparkinsonian agents | Benztropine, procyclidine, trihexyphenidyl |
| Antipsychotics | Chlorpromazine, clozapine, loxapine, molindone, olanzapine, pimozide, promethazine, quetiapine, thioridazine |
| Asthma and chronic obstructive pulmonary disease medication | Glycopyrrolate, ipratropium,† tiotropium† |
| Bladder antispasmodics | Darifenacin, flavoxate, oxybutynin, solifenacin, tolterodine, trospium |
| Gastrointestinal antispasmodics | Atropine, belladonna alkaloids, clidinium, dicyclomine, hyoscyamine, methscopolamine, propantheline |
| Insomnia medications | Diphenhydramine, doxylamine |
| Motion sickness/dizziness/nausea medications | Dimenhydrinate, meclizine, prochlorperazine, promethazine, scopolamine, trimethobenzamide |
| Muscle relaxants and pain medications | Cyclobenzaprine, meperidine, orphenadrine, phenyltoloxamine |
| Ulcer and acid reflux agents | Cimetidine, glycopyrrolate, ranitidine |
| *Not a comprehensive list †Unknown whether CNS effects are important Source: Reference 5 | |
- Cancelli I, Beltrame M, D’Anna L, et al. Drugs with anticholinergic properties: a potential risk factor for psychosis onset in Alzheimer’s disease? Expert Opin Drug Saf. 2009;8(5):549-557.
- Meeks TW, Jeste DV. Beyond the black box: what is the role for antipsychotics in dementia? Current Psychiatry. 2008;7(6): 50-65.
- Centers for Education and Research on Therapeutics. Anticholinergic pocket reference card. www.chainonline.org/home/content_images/Anticholinergic%20Pocket%20Card%20CLR%203_12_10.pdf.
Drug Brand Names
- Amitriptyline • Elavil
- Atropine • Sal-Tropine
- Azelastine nasal spray • Astelin
- Belladonna alkaloids • Donnatal
- Benztropine • Cogentin
- Brompheniramine • Dimetane
- Carbamazepine • Carbatrol, Tegretol, others
- Carbinoxamine • Palgic
- Chlorpheniramine • Chlor-Trimeton
- Chlorpromazine • Thorazine
- Cimetidine • Tagamet
- Clemastine • Tavist
- Clidinium • Quarzan
- Clomipramine • Anafranil
- Clozapine • Clozaril
- Cyclobenzaprine • Flexeril
- Cyproheptadine • Periactin
- Darifenacin • Enablex
- Desipramine • Norpramin
- Dexbrompheniramine • Drixoral
- Dexchlorpheniramine • Polaramine
- Dicyclomine • Bentyl
- Dimenhydrinate • Dramamine
- Diphenhydramine • Benadryl, Sominex, others
- Docusate Sodium • Colace
- Donepezil • Aricept
- Doxepin • Adapin
- Doxylamine • Aldex, Unisom, others
- Flavoxate • Urispas
- Glycopyrrolate • Robinul
- Hydroxyzine • Atarax
- Hyoscyamine • Cystospaz, Levbid
- Imipramine • Tofranil
- Ipratropium • Atrovent
- Loxapine • Loxitane
- Meclizine • Antivert
- Meperidine • Demerol
- Mepyramine • Anthisan
- Methscopolamine • Pamine
- Molindone • Moban
- Nortriptyline • Aventyl
- Olanzapine • Zyprexa
- Olopatadine nasal spray • Patanase
- Orphenadrine • Norflex
- Oxybutynin extended-release • Ditropan XL
- Paroxetine • Paxil
- Phenyltoloxamine • Dologesic, Durayin, others
- Pimozide • Orap
- Prochlorperazine • Compazine
- Procyclidine • Kemadrin
- Promethazine • Phenergan
- Propanthelin • Pro-Banthine
- Protriptyline • Vivactil
- Quetiapine • Seroquel
- Ranitidine • Zantac
- Scopolamine • Scopace
- Sertraline • Zoloft
- Solifenacin • VESIcare
- Thioridazine • Mellaril
- Tiotropium • Spiriva
- Tolterodine • Detrol
- Trihexyphenidyl • Artane
- Trimethobenzamide • Tigan
- Trimipramine • Surmontil
- Triprolidine • Actifed
- Trospium • Sanctura
Acknowledgements
This work was supported by an Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement #5 U18 HSO16094.
Disclosure
Dr. Carnahan receives grant/research support from the Agency for Healthcare Research and Quality.
1. Swanson KA, Carnahan RM. Dementia and comorbidities: an overview of diagnosis and management. J Pharm Pract. 2007;20:296-317.
2. Sunderland T, Tariot P, Murphy DL, et al. Scopolamine challenges in Alzheimer’s disease. Psychopharmacology (Berl). 1985;87(2):247-249.
3. Sunderland T, Tariot PN, Cohen RM, et al. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose response study. Arch Gen Psychiatry. 1987;44(5):418-426.
4. Cancelli I, Valentinis L, Merlino G, et al. Drugs with anticholinergic properties as a risk factor for psychosis in patients affected by Alzheimer’s disease. Clin Pharmacol Ther. 2008;84(1):63-68.
5. Carnahan RM, Lund BC, Perry PJ, et al. The concurrent use of anticholinergics and cholinesterase inhibitors: rare event or common practice? J Am Geriatr Soc. 2004;52:2082-2087.
1. Swanson KA, Carnahan RM. Dementia and comorbidities: an overview of diagnosis and management. J Pharm Pract. 2007;20:296-317.
2. Sunderland T, Tariot P, Murphy DL, et al. Scopolamine challenges in Alzheimer’s disease. Psychopharmacology (Berl). 1985;87(2):247-249.
3. Sunderland T, Tariot PN, Cohen RM, et al. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose response study. Arch Gen Psychiatry. 1987;44(5):418-426.
4. Cancelli I, Valentinis L, Merlino G, et al. Drugs with anticholinergic properties as a risk factor for psychosis in patients affected by Alzheimer’s disease. Clin Pharmacol Ther. 2008;84(1):63-68.
5. Carnahan RM, Lund BC, Perry PJ, et al. The concurrent use of anticholinergics and cholinesterase inhibitors: rare event or common practice? J Am Geriatr Soc. 2004;52:2082-2087.
The woman who saw the light
HISTORY: Visual disturbances
Ms. G, age 30, has schizoaffective disorder and presents with worsening auditory hallucinations, paranoid delusions, and thought broadcasting and insertion, which is suspected to be related to a change from olanzapine to aripiprazole. She complains of visual disturbances—a hallucination of flashing lights—that she describes as occurring “all the time, like salt-and-pepper dancing, and sometimes splotches.” Her visual symptoms are worse when she closes her eyes and in dimly lit environments, but occur in daylight as well. Her vision is otherwise unimpaired.
The visual disturbances started with Ms. G’s first psychotic break at age 23 and have persisted continuously. She reports that at first, the spots were more intense, like an incessant strobe light. These symptoms are aggravated by sleep deprivation and anger and improve only with topiramate, 100 mg/d, which was prescribed a year earlier after a neurologic consultation but discontinued because of cognitive side effects.
Ms. G also complains of dull, aching, continuous headaches in the center of her forehead, which do not wake her, are not worse in the morning, and do not vary with the intensity of her visual symptoms. The headaches are incorporated into her system of delusions; she believes they “drain the electricity from her head” and make her feel better. She denies a history of migraines. Ms. G has a history of experiencing well-formed visual hallucinations, such as the devil, but has not had them for several years.
She had 5 to 10 episodes over the last 2 years that she describes as seizures—“an earthquake, a huge whooshing noise,” accompanied by heart palpitations that occur with stress, in public, in bright lights, or whenever she feels vulnerable. These episodes are not associated with loss of consciousness, incontinence, amnesia, or post-ictal confusion and she can stop them with relaxation techniques such as deep breathing and reminding herself to calm down. The last episode was 2 months ago and seemed unrelated to her visual phenomena. She also complains of occasional paresthesias in her extremities but denies dizziness, presyncope, or blurry or double vision.
Ms. G is obese and has no history of head injury or birth defects. Her family history is negative for epilepsy; however, her mother has bipolar disorder and father has schizophrenia. Her medications are phentermine, 37.5 mg/d for short-term management of obesity, oxcarbazepine, 600 mg/d for supposed partial seizures, sertraline, 100 mg/d for depression, aripiprazole, 10 mg/d, and nizatidine, 150 mg/d, for gastroesophageal reflux disease. She does not use alcohol, cigarettes, or illicit drugs. Ms. G obtained a master’s degree in health communications and works as a research assistant at a local medical school.
The authors’ observations
Visual hallucinations are not distortions of reality but original false perceptions that co-occur with real perceptions.1 They vary from simple, elemental hallucinations involving flashes of light or geometric figures to more complex elaborations such as seeing crawling insects or a flock of angels.2 Hallucinations can originate anywhere in the visual system from the lens to the visual cortex and can be vascular, toxic, electrophysiologic, structural, or neurochemical manifestations of neurologic, psychiatric, or ophthalmologic disorders. Depending on the hallucination’s etiology, treatment varies from emergent ophthalmologic examination and retinal surgery to antipsychotic therapy to simple observation. Photopsia is a perception of flashing lights, and its etiology varies; causes can be categorized as emergent or nonemergent.
Emergent causes of photopsia typically have a sudden onset, often can be diagnosed from associated symptoms or medical history, and must be recognized quickly to prevent permanent vision loss. Posterior vitreous detachment and retinal tearing can manifest as flashes of light, floaters, or blurred vision in the peripheral visual field that are worse in dim illumination and localizable by the patient. Retinal detachment can cause photopsia, floaters, or a “curtain” or “shadow” moving over the visual field, which may be accompanied by central or peripheral vision loss.3 Severe myopia is a risk factor for retinal detachment.
New-onset photopsia can suggest cytomegaloviral retinitis in an immune-compromised patient, and ocular-vascular crisis in a patient with sickle cell disease. New-onset or recurrent photopsia with bilateral blurred vision, ataxia, vertigo, dysarthria, or drop attacks may be caused by vertebrobasilar artery insufficiency (VBAI). Photopsia has been reported as the presenting symptom in carotid artery dissection.4 Photopsia correlated with eye movement implicates the retina or optic nerve. All of these etiologies except for VBAI should be referred to an ophthalmologist for indirect ophthalmoscopy and evaluation.
Nonemergent causes of photopsia include:
- drug toxicity
- migraine headache
- thyroid ophthalmopathy
- arteriovenous malformations
- seizures
- psychiatric disorders.
Digoxin, cocaine, paclitaxel, and clomiphene may have ocular side effects, including photopsia. The first symptoms of digitalis toxicity often are visual and include photopsia, yellow or green discoloration of the visual field, halos, and the appearance of frost over objects.5 Signs of cocaine intoxication can include visual hallucinations such as photopsia, shadows, moving objects, and insects crawling and co-occurring euphoria, hypervigilance, impaired judgment, and autonomic changes such as tachycardia, pupillary dilation, hypertension, and nausea.6 High-dose paclitaxel infusion has been reported to cause photopsia.7 Clomiphene can cause flickering lights and shimmering that persists after discontinuing the medication.8
Migraine with aura, which includes photopsia 39% of the time,9 often involves an expanding central visual disturbance or scotoma, and usually is confined to 1 visual field but may involve both. The aura typically lasts 10 to 20 minutes and often is followed by headache. Patients often report a history of episodic stereotyped auras and headache. Ocular examination is normal.
Ophthalmic migraine, also known as ocular or retinal migraine, is thought to be caused by transient vasospasm of the choroidal or retinal arteries and can be precipitated by postural changes, exercise, and oral contraceptives. It manifests as a gradual visual disturbance in a mosaic scotomata pattern that enlarges, producing total unilateral visual loss lasting from minutes to an hour, and—misleadingly—can be associated with minimal or no headache. Ocular examination is normal, and a personal or family history of migraine confirms the diagnosis.
Seizure activity in the occipital cortex and adjacent association areas can produce static light and stars. In a prospective study of 18 patients with occipital epilepsy, visual seizures lasted from a few seconds to 3 minutes—although rarely 20 to 150 minutes—and occurred in multiple clusters a day or week.10 All but 2 patients had secondary generalized tonic-clonic convulsions. Occipital seizures often are misdiagnosed as the visual aura of migraine, particularly when elementary visual hallucinations are followed by post-ictal headache and vomiting.11 Lights with a color or a spherical shape suggest occipital epilepsy. Consider ordering an electroencephalogram (EEG) if the clinical diagnosis is unclear.
EXAMINATION: No obvious cause
During a mental status exam, Ms. G is pleasant, cooperative, alert, and oriented to person, place, and time. Her speech is fluent, her affect is full, and she describes her mood as a “sensitive, overwhelmed state.” Her thoughts occasionally are tangential, and she perseverates on guilty, embarrassed, and paranoid thoughts. She has auditory hallucinations and experiences photopsia during the interview, but denies other visual hallucinations. She shows no deficits in attention, concentration, memory, language, calculations, visuospatial abilities, or general fund of knowledge.
Her physical exam shows pupils that are equal, round, and reactive to light with normal extraocular movements, intact visual fields, 20/20 visual acuity in both eyes with glasses, and sharp optic disc margins with no retinal abnormalities apparent on funduscopic exam. The rest of her physical and neurologic exam is normal. Her complete blood count, electrolytes, kidney, liver function tests, urinalysis, and serum toxicology screens are normal. She has no history of immunodeficiency.
An MRI performed a year earlier showed mild diffuse congestion of a left maxillary sinus but no other intracranial abnormalities, and a waking EEG with sphenoidal electrodes was normal.
The authors’ observations
Evaluation of patients with photopsia includes taking a history and performing an eye examination including visual acuity testing, visual field testing, and funduscopic examination. The appearance, location, and duration of photopsia may help narrow the differential diagnosis and identify emergent cases (Table 1 and Table 2). Seizure activity associated with flashing lights suggests occipital epilepsy. A history of hypertension, diabetes, or polymyalgia rheumatica points to vascular causes, and hyperthyroidism suggests that flashing lights may be phosphenes of Graves’ disease. Myopia or eye trauma could indicate vitreous traction. Prescription medications and illicit drug use may point to a toxic etiology.12
Table 1
Diagnoses suggested by photopsia phenomena
| Feature | Suggested diagnosis |
|---|---|
| Appearance | |
| Twinkling lights with grey spots | Emboli |
| Zigzag lines | Occipital epilepsy |
| White flashes | Vitreous traction on the retina |
| Flickering lights and shimmering | Clomiphene use |
| Temporally located flashes of light, floaters, or blurred vision that is worse in dim illumination | Posterior vitreous detachment and retinal tearing |
| Location | |
| Temporal | Migraine or retinal pathology |
| Central | Occipital lesion or embolus |
| Monocular | Retinal pathology |
| Binocular | Cerebral cause |
| Duration | |
| Lightning quick | Vitreous detachment |
| Lasts up to 30 minutes | Migraine or occipital epilepsy |
Table 2
Photopsia differential diagnosis: Look for co-occurring symptoms
| Co-occurring symptoms | Suggests |
|---|---|
| Seizure activity associated with flashing lights | Occipital epilepsy |
| Yellow or green discoloration to the visual field, halos, and appearance of frost over objects | Digitalis toxicity |
| Shadows, moving objects, and insects crawling with co-occurring euphoria, hypervigilance, impaired judgment, and autonomic changes such as tachycardia, pupillary dilation, hypertension, and nausea | Cocaine intoxication |
| Floaters, or a ‘curtain’ or ‘shadow’ moving over the visual field, which may be accompanied by central or peripheral vision loss | Retinal detachment |
| Bilateral blurred vision, ataxia, vertigo, dysarthria, or drop attacks | Vertebrobasilar artery insufficiency |
OUTCOME: Diagnosis of exclusion
Ms. G’s visual disturbance resembles vitreous detachment or classic migraine; however, the onset is not sudden, her funduscopic exam is normal, and she has no discrete episodes with concomitant headache. Ms. G’s improvement with topiramate is inconsistent with an ophthalmic origin because such photopsia should not improve with medication. It is consistent with recurrent seizures or status epilepticus of the occipital cortex; however, she did not experience multiple discrete episodes a day or generalized seizures experienced by most occipital seizure patients, and her EEG and clinical history are not consistent with seizures. Because we cannot rule out occipital lobe epilepsy, we refer Ms. G for repeat EEG and routine ophthalmologic exam, both of which are normal.
Perceptual disturbances are common in schizoaffective disorder and schizophrenia,13-15 which we consider the most likely etiology of Ms. G’s photopsia, given her normal neurologic and ophthalmologic examinations. We switch her from aripiprazole to ziprasidone, 40 mg/d, discontinue phentermine, and taper and discontinue oxcarbazepine.
Related resources
- Ophthalmology Web. www.ophthalmologyweb.com.
- Aleman A, Larøi F. Hallucinations: the science of idiosyncratic perception. Washington, DC: American Psychological Association; 2008.
Drug Brand Names
- Aripiprazole • Abilify
- Clomiphene • Clomid, Serophene
- Digoxin • Lanoxin
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paclitaxel • Onxol, Taxol
- Phentermine • Adipex-P, Inoamin
- Sertraline • Zoloft
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jaspers K. General psychopathology. Manchester, United Kingdom: Manchester University Press; 1962.
2. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121:1819-1840.
3. Amos JF. Differential diagnosis of common etiologies of photopsia. J Am Optom Assoc. 1999;70(8):485-504.
4. Biousse V, Touboul PJ, D’Anglejan-Chatillon J, et al. Ophthalmologic manifestations of internal carotid artery dissection. Am J Ophthalmol. 1998;126(4):565-577.
5. Butler VP, Jr, Odel JG, Rath E, et al. Digitalis-induced visual disturbances with therapeutic serum digitalis concentrations. Ann Intern Med. 1995;123(9):676-680.
6. Mitchell J, Vierkant AD. Delusions and hallucinations of cocaine abusers and paranoid schizophrenics: a comparative study. J Psychol. 1991;125(3):301-310.
7. Seidman AD, Barrett S, Canezo S. Photopsia during 3-hour paclitaxel administration at doses > or = 250 mg/m2. J Clin Oncol. 1994;12(8):1741-1742.
8. Purvin VA. Visual disturbance secondary to clomiphene citrate. Arch Ophthalmol. 1995;113(4):482-484.
9. Queiroz LP, Rapoport AM, Weeks RE, et al. Characteristics of migraine visual aura. Headache. 1997;37(3):137-141.
10. Panayiotopoulos CP. Visual phenomena and headache in occipital epilepsy: a review, a systematic study and differentiation from migraine. Epileptic Disord. 1999;1(4):205-216.
11. Walker MC, Smith SJ, Sisodiya SM, et al. Case of simple partial status epilepticus in occipital lobe epilepsy misdiagnosed as migraine: clinical, electrophysiological, and magnetic resonance imaging characteristics. Epilepsia. 1995;36(12):1233-1236.
12. Lichter M. Flashing lights—a warning. The Canadian Journal of Diagnosis. 2002;19:31-35.
13. Parnas J, Handest P, Saebye D, et al. Anomalies of subjective experience in schizophrenia and psychotic bipolar illness. Acta Psychiatr Scand. 2003;108(2):126-133.
14. Phillipson OT, Harris JP. Perceptual changes in schizophrenia: a questionnaire survey. Psychol Med. 1985;15(4):859-866.
15. Cutting J, Dunne F. Subjective experience of schizophrenia. Schizophr Bull. 1989;15(2):217-231.
HISTORY: Visual disturbances
Ms. G, age 30, has schizoaffective disorder and presents with worsening auditory hallucinations, paranoid delusions, and thought broadcasting and insertion, which is suspected to be related to a change from olanzapine to aripiprazole. She complains of visual disturbances—a hallucination of flashing lights—that she describes as occurring “all the time, like salt-and-pepper dancing, and sometimes splotches.” Her visual symptoms are worse when she closes her eyes and in dimly lit environments, but occur in daylight as well. Her vision is otherwise unimpaired.
The visual disturbances started with Ms. G’s first psychotic break at age 23 and have persisted continuously. She reports that at first, the spots were more intense, like an incessant strobe light. These symptoms are aggravated by sleep deprivation and anger and improve only with topiramate, 100 mg/d, which was prescribed a year earlier after a neurologic consultation but discontinued because of cognitive side effects.
Ms. G also complains of dull, aching, continuous headaches in the center of her forehead, which do not wake her, are not worse in the morning, and do not vary with the intensity of her visual symptoms. The headaches are incorporated into her system of delusions; she believes they “drain the electricity from her head” and make her feel better. She denies a history of migraines. Ms. G has a history of experiencing well-formed visual hallucinations, such as the devil, but has not had them for several years.
She had 5 to 10 episodes over the last 2 years that she describes as seizures—“an earthquake, a huge whooshing noise,” accompanied by heart palpitations that occur with stress, in public, in bright lights, or whenever she feels vulnerable. These episodes are not associated with loss of consciousness, incontinence, amnesia, or post-ictal confusion and she can stop them with relaxation techniques such as deep breathing and reminding herself to calm down. The last episode was 2 months ago and seemed unrelated to her visual phenomena. She also complains of occasional paresthesias in her extremities but denies dizziness, presyncope, or blurry or double vision.
Ms. G is obese and has no history of head injury or birth defects. Her family history is negative for epilepsy; however, her mother has bipolar disorder and father has schizophrenia. Her medications are phentermine, 37.5 mg/d for short-term management of obesity, oxcarbazepine, 600 mg/d for supposed partial seizures, sertraline, 100 mg/d for depression, aripiprazole, 10 mg/d, and nizatidine, 150 mg/d, for gastroesophageal reflux disease. She does not use alcohol, cigarettes, or illicit drugs. Ms. G obtained a master’s degree in health communications and works as a research assistant at a local medical school.
The authors’ observations
Visual hallucinations are not distortions of reality but original false perceptions that co-occur with real perceptions.1 They vary from simple, elemental hallucinations involving flashes of light or geometric figures to more complex elaborations such as seeing crawling insects or a flock of angels.2 Hallucinations can originate anywhere in the visual system from the lens to the visual cortex and can be vascular, toxic, electrophysiologic, structural, or neurochemical manifestations of neurologic, psychiatric, or ophthalmologic disorders. Depending on the hallucination’s etiology, treatment varies from emergent ophthalmologic examination and retinal surgery to antipsychotic therapy to simple observation. Photopsia is a perception of flashing lights, and its etiology varies; causes can be categorized as emergent or nonemergent.
Emergent causes of photopsia typically have a sudden onset, often can be diagnosed from associated symptoms or medical history, and must be recognized quickly to prevent permanent vision loss. Posterior vitreous detachment and retinal tearing can manifest as flashes of light, floaters, or blurred vision in the peripheral visual field that are worse in dim illumination and localizable by the patient. Retinal detachment can cause photopsia, floaters, or a “curtain” or “shadow” moving over the visual field, which may be accompanied by central or peripheral vision loss.3 Severe myopia is a risk factor for retinal detachment.
New-onset photopsia can suggest cytomegaloviral retinitis in an immune-compromised patient, and ocular-vascular crisis in a patient with sickle cell disease. New-onset or recurrent photopsia with bilateral blurred vision, ataxia, vertigo, dysarthria, or drop attacks may be caused by vertebrobasilar artery insufficiency (VBAI). Photopsia has been reported as the presenting symptom in carotid artery dissection.4 Photopsia correlated with eye movement implicates the retina or optic nerve. All of these etiologies except for VBAI should be referred to an ophthalmologist for indirect ophthalmoscopy and evaluation.
Nonemergent causes of photopsia include:
- drug toxicity
- migraine headache
- thyroid ophthalmopathy
- arteriovenous malformations
- seizures
- psychiatric disorders.
Digoxin, cocaine, paclitaxel, and clomiphene may have ocular side effects, including photopsia. The first symptoms of digitalis toxicity often are visual and include photopsia, yellow or green discoloration of the visual field, halos, and the appearance of frost over objects.5 Signs of cocaine intoxication can include visual hallucinations such as photopsia, shadows, moving objects, and insects crawling and co-occurring euphoria, hypervigilance, impaired judgment, and autonomic changes such as tachycardia, pupillary dilation, hypertension, and nausea.6 High-dose paclitaxel infusion has been reported to cause photopsia.7 Clomiphene can cause flickering lights and shimmering that persists after discontinuing the medication.8
Migraine with aura, which includes photopsia 39% of the time,9 often involves an expanding central visual disturbance or scotoma, and usually is confined to 1 visual field but may involve both. The aura typically lasts 10 to 20 minutes and often is followed by headache. Patients often report a history of episodic stereotyped auras and headache. Ocular examination is normal.
Ophthalmic migraine, also known as ocular or retinal migraine, is thought to be caused by transient vasospasm of the choroidal or retinal arteries and can be precipitated by postural changes, exercise, and oral contraceptives. It manifests as a gradual visual disturbance in a mosaic scotomata pattern that enlarges, producing total unilateral visual loss lasting from minutes to an hour, and—misleadingly—can be associated with minimal or no headache. Ocular examination is normal, and a personal or family history of migraine confirms the diagnosis.
Seizure activity in the occipital cortex and adjacent association areas can produce static light and stars. In a prospective study of 18 patients with occipital epilepsy, visual seizures lasted from a few seconds to 3 minutes—although rarely 20 to 150 minutes—and occurred in multiple clusters a day or week.10 All but 2 patients had secondary generalized tonic-clonic convulsions. Occipital seizures often are misdiagnosed as the visual aura of migraine, particularly when elementary visual hallucinations are followed by post-ictal headache and vomiting.11 Lights with a color or a spherical shape suggest occipital epilepsy. Consider ordering an electroencephalogram (EEG) if the clinical diagnosis is unclear.
EXAMINATION: No obvious cause
During a mental status exam, Ms. G is pleasant, cooperative, alert, and oriented to person, place, and time. Her speech is fluent, her affect is full, and she describes her mood as a “sensitive, overwhelmed state.” Her thoughts occasionally are tangential, and she perseverates on guilty, embarrassed, and paranoid thoughts. She has auditory hallucinations and experiences photopsia during the interview, but denies other visual hallucinations. She shows no deficits in attention, concentration, memory, language, calculations, visuospatial abilities, or general fund of knowledge.
Her physical exam shows pupils that are equal, round, and reactive to light with normal extraocular movements, intact visual fields, 20/20 visual acuity in both eyes with glasses, and sharp optic disc margins with no retinal abnormalities apparent on funduscopic exam. The rest of her physical and neurologic exam is normal. Her complete blood count, electrolytes, kidney, liver function tests, urinalysis, and serum toxicology screens are normal. She has no history of immunodeficiency.
An MRI performed a year earlier showed mild diffuse congestion of a left maxillary sinus but no other intracranial abnormalities, and a waking EEG with sphenoidal electrodes was normal.
The authors’ observations
Evaluation of patients with photopsia includes taking a history and performing an eye examination including visual acuity testing, visual field testing, and funduscopic examination. The appearance, location, and duration of photopsia may help narrow the differential diagnosis and identify emergent cases (Table 1 and Table 2). Seizure activity associated with flashing lights suggests occipital epilepsy. A history of hypertension, diabetes, or polymyalgia rheumatica points to vascular causes, and hyperthyroidism suggests that flashing lights may be phosphenes of Graves’ disease. Myopia or eye trauma could indicate vitreous traction. Prescription medications and illicit drug use may point to a toxic etiology.12
Table 1
Diagnoses suggested by photopsia phenomena
| Feature | Suggested diagnosis |
|---|---|
| Appearance | |
| Twinkling lights with grey spots | Emboli |
| Zigzag lines | Occipital epilepsy |
| White flashes | Vitreous traction on the retina |
| Flickering lights and shimmering | Clomiphene use |
| Temporally located flashes of light, floaters, or blurred vision that is worse in dim illumination | Posterior vitreous detachment and retinal tearing |
| Location | |
| Temporal | Migraine or retinal pathology |
| Central | Occipital lesion or embolus |
| Monocular | Retinal pathology |
| Binocular | Cerebral cause |
| Duration | |
| Lightning quick | Vitreous detachment |
| Lasts up to 30 minutes | Migraine or occipital epilepsy |
Table 2
Photopsia differential diagnosis: Look for co-occurring symptoms
| Co-occurring symptoms | Suggests |
|---|---|
| Seizure activity associated with flashing lights | Occipital epilepsy |
| Yellow or green discoloration to the visual field, halos, and appearance of frost over objects | Digitalis toxicity |
| Shadows, moving objects, and insects crawling with co-occurring euphoria, hypervigilance, impaired judgment, and autonomic changes such as tachycardia, pupillary dilation, hypertension, and nausea | Cocaine intoxication |
| Floaters, or a ‘curtain’ or ‘shadow’ moving over the visual field, which may be accompanied by central or peripheral vision loss | Retinal detachment |
| Bilateral blurred vision, ataxia, vertigo, dysarthria, or drop attacks | Vertebrobasilar artery insufficiency |
OUTCOME: Diagnosis of exclusion
Ms. G’s visual disturbance resembles vitreous detachment or classic migraine; however, the onset is not sudden, her funduscopic exam is normal, and she has no discrete episodes with concomitant headache. Ms. G’s improvement with topiramate is inconsistent with an ophthalmic origin because such photopsia should not improve with medication. It is consistent with recurrent seizures or status epilepticus of the occipital cortex; however, she did not experience multiple discrete episodes a day or generalized seizures experienced by most occipital seizure patients, and her EEG and clinical history are not consistent with seizures. Because we cannot rule out occipital lobe epilepsy, we refer Ms. G for repeat EEG and routine ophthalmologic exam, both of which are normal.
Perceptual disturbances are common in schizoaffective disorder and schizophrenia,13-15 which we consider the most likely etiology of Ms. G’s photopsia, given her normal neurologic and ophthalmologic examinations. We switch her from aripiprazole to ziprasidone, 40 mg/d, discontinue phentermine, and taper and discontinue oxcarbazepine.
Related resources
- Ophthalmology Web. www.ophthalmologyweb.com.
- Aleman A, Larøi F. Hallucinations: the science of idiosyncratic perception. Washington, DC: American Psychological Association; 2008.
Drug Brand Names
- Aripiprazole • Abilify
- Clomiphene • Clomid, Serophene
- Digoxin • Lanoxin
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paclitaxel • Onxol, Taxol
- Phentermine • Adipex-P, Inoamin
- Sertraline • Zoloft
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
HISTORY: Visual disturbances
Ms. G, age 30, has schizoaffective disorder and presents with worsening auditory hallucinations, paranoid delusions, and thought broadcasting and insertion, which is suspected to be related to a change from olanzapine to aripiprazole. She complains of visual disturbances—a hallucination of flashing lights—that she describes as occurring “all the time, like salt-and-pepper dancing, and sometimes splotches.” Her visual symptoms are worse when she closes her eyes and in dimly lit environments, but occur in daylight as well. Her vision is otherwise unimpaired.
The visual disturbances started with Ms. G’s first psychotic break at age 23 and have persisted continuously. She reports that at first, the spots were more intense, like an incessant strobe light. These symptoms are aggravated by sleep deprivation and anger and improve only with topiramate, 100 mg/d, which was prescribed a year earlier after a neurologic consultation but discontinued because of cognitive side effects.
Ms. G also complains of dull, aching, continuous headaches in the center of her forehead, which do not wake her, are not worse in the morning, and do not vary with the intensity of her visual symptoms. The headaches are incorporated into her system of delusions; she believes they “drain the electricity from her head” and make her feel better. She denies a history of migraines. Ms. G has a history of experiencing well-formed visual hallucinations, such as the devil, but has not had them for several years.
She had 5 to 10 episodes over the last 2 years that she describes as seizures—“an earthquake, a huge whooshing noise,” accompanied by heart palpitations that occur with stress, in public, in bright lights, or whenever she feels vulnerable. These episodes are not associated with loss of consciousness, incontinence, amnesia, or post-ictal confusion and she can stop them with relaxation techniques such as deep breathing and reminding herself to calm down. The last episode was 2 months ago and seemed unrelated to her visual phenomena. She also complains of occasional paresthesias in her extremities but denies dizziness, presyncope, or blurry or double vision.
Ms. G is obese and has no history of head injury or birth defects. Her family history is negative for epilepsy; however, her mother has bipolar disorder and father has schizophrenia. Her medications are phentermine, 37.5 mg/d for short-term management of obesity, oxcarbazepine, 600 mg/d for supposed partial seizures, sertraline, 100 mg/d for depression, aripiprazole, 10 mg/d, and nizatidine, 150 mg/d, for gastroesophageal reflux disease. She does not use alcohol, cigarettes, or illicit drugs. Ms. G obtained a master’s degree in health communications and works as a research assistant at a local medical school.
The authors’ observations
Visual hallucinations are not distortions of reality but original false perceptions that co-occur with real perceptions.1 They vary from simple, elemental hallucinations involving flashes of light or geometric figures to more complex elaborations such as seeing crawling insects or a flock of angels.2 Hallucinations can originate anywhere in the visual system from the lens to the visual cortex and can be vascular, toxic, electrophysiologic, structural, or neurochemical manifestations of neurologic, psychiatric, or ophthalmologic disorders. Depending on the hallucination’s etiology, treatment varies from emergent ophthalmologic examination and retinal surgery to antipsychotic therapy to simple observation. Photopsia is a perception of flashing lights, and its etiology varies; causes can be categorized as emergent or nonemergent.
Emergent causes of photopsia typically have a sudden onset, often can be diagnosed from associated symptoms or medical history, and must be recognized quickly to prevent permanent vision loss. Posterior vitreous detachment and retinal tearing can manifest as flashes of light, floaters, or blurred vision in the peripheral visual field that are worse in dim illumination and localizable by the patient. Retinal detachment can cause photopsia, floaters, or a “curtain” or “shadow” moving over the visual field, which may be accompanied by central or peripheral vision loss.3 Severe myopia is a risk factor for retinal detachment.
New-onset photopsia can suggest cytomegaloviral retinitis in an immune-compromised patient, and ocular-vascular crisis in a patient with sickle cell disease. New-onset or recurrent photopsia with bilateral blurred vision, ataxia, vertigo, dysarthria, or drop attacks may be caused by vertebrobasilar artery insufficiency (VBAI). Photopsia has been reported as the presenting symptom in carotid artery dissection.4 Photopsia correlated with eye movement implicates the retina or optic nerve. All of these etiologies except for VBAI should be referred to an ophthalmologist for indirect ophthalmoscopy and evaluation.
Nonemergent causes of photopsia include:
- drug toxicity
- migraine headache
- thyroid ophthalmopathy
- arteriovenous malformations
- seizures
- psychiatric disorders.
Digoxin, cocaine, paclitaxel, and clomiphene may have ocular side effects, including photopsia. The first symptoms of digitalis toxicity often are visual and include photopsia, yellow or green discoloration of the visual field, halos, and the appearance of frost over objects.5 Signs of cocaine intoxication can include visual hallucinations such as photopsia, shadows, moving objects, and insects crawling and co-occurring euphoria, hypervigilance, impaired judgment, and autonomic changes such as tachycardia, pupillary dilation, hypertension, and nausea.6 High-dose paclitaxel infusion has been reported to cause photopsia.7 Clomiphene can cause flickering lights and shimmering that persists after discontinuing the medication.8
Migraine with aura, which includes photopsia 39% of the time,9 often involves an expanding central visual disturbance or scotoma, and usually is confined to 1 visual field but may involve both. The aura typically lasts 10 to 20 minutes and often is followed by headache. Patients often report a history of episodic stereotyped auras and headache. Ocular examination is normal.
Ophthalmic migraine, also known as ocular or retinal migraine, is thought to be caused by transient vasospasm of the choroidal or retinal arteries and can be precipitated by postural changes, exercise, and oral contraceptives. It manifests as a gradual visual disturbance in a mosaic scotomata pattern that enlarges, producing total unilateral visual loss lasting from minutes to an hour, and—misleadingly—can be associated with minimal or no headache. Ocular examination is normal, and a personal or family history of migraine confirms the diagnosis.
Seizure activity in the occipital cortex and adjacent association areas can produce static light and stars. In a prospective study of 18 patients with occipital epilepsy, visual seizures lasted from a few seconds to 3 minutes—although rarely 20 to 150 minutes—and occurred in multiple clusters a day or week.10 All but 2 patients had secondary generalized tonic-clonic convulsions. Occipital seizures often are misdiagnosed as the visual aura of migraine, particularly when elementary visual hallucinations are followed by post-ictal headache and vomiting.11 Lights with a color or a spherical shape suggest occipital epilepsy. Consider ordering an electroencephalogram (EEG) if the clinical diagnosis is unclear.
EXAMINATION: No obvious cause
During a mental status exam, Ms. G is pleasant, cooperative, alert, and oriented to person, place, and time. Her speech is fluent, her affect is full, and she describes her mood as a “sensitive, overwhelmed state.” Her thoughts occasionally are tangential, and she perseverates on guilty, embarrassed, and paranoid thoughts. She has auditory hallucinations and experiences photopsia during the interview, but denies other visual hallucinations. She shows no deficits in attention, concentration, memory, language, calculations, visuospatial abilities, or general fund of knowledge.
Her physical exam shows pupils that are equal, round, and reactive to light with normal extraocular movements, intact visual fields, 20/20 visual acuity in both eyes with glasses, and sharp optic disc margins with no retinal abnormalities apparent on funduscopic exam. The rest of her physical and neurologic exam is normal. Her complete blood count, electrolytes, kidney, liver function tests, urinalysis, and serum toxicology screens are normal. She has no history of immunodeficiency.
An MRI performed a year earlier showed mild diffuse congestion of a left maxillary sinus but no other intracranial abnormalities, and a waking EEG with sphenoidal electrodes was normal.
The authors’ observations
Evaluation of patients with photopsia includes taking a history and performing an eye examination including visual acuity testing, visual field testing, and funduscopic examination. The appearance, location, and duration of photopsia may help narrow the differential diagnosis and identify emergent cases (Table 1 and Table 2). Seizure activity associated with flashing lights suggests occipital epilepsy. A history of hypertension, diabetes, or polymyalgia rheumatica points to vascular causes, and hyperthyroidism suggests that flashing lights may be phosphenes of Graves’ disease. Myopia or eye trauma could indicate vitreous traction. Prescription medications and illicit drug use may point to a toxic etiology.12
Table 1
Diagnoses suggested by photopsia phenomena
| Feature | Suggested diagnosis |
|---|---|
| Appearance | |
| Twinkling lights with grey spots | Emboli |
| Zigzag lines | Occipital epilepsy |
| White flashes | Vitreous traction on the retina |
| Flickering lights and shimmering | Clomiphene use |
| Temporally located flashes of light, floaters, or blurred vision that is worse in dim illumination | Posterior vitreous detachment and retinal tearing |
| Location | |
| Temporal | Migraine or retinal pathology |
| Central | Occipital lesion or embolus |
| Monocular | Retinal pathology |
| Binocular | Cerebral cause |
| Duration | |
| Lightning quick | Vitreous detachment |
| Lasts up to 30 minutes | Migraine or occipital epilepsy |
Table 2
Photopsia differential diagnosis: Look for co-occurring symptoms
| Co-occurring symptoms | Suggests |
|---|---|
| Seizure activity associated with flashing lights | Occipital epilepsy |
| Yellow or green discoloration to the visual field, halos, and appearance of frost over objects | Digitalis toxicity |
| Shadows, moving objects, and insects crawling with co-occurring euphoria, hypervigilance, impaired judgment, and autonomic changes such as tachycardia, pupillary dilation, hypertension, and nausea | Cocaine intoxication |
| Floaters, or a ‘curtain’ or ‘shadow’ moving over the visual field, which may be accompanied by central or peripheral vision loss | Retinal detachment |
| Bilateral blurred vision, ataxia, vertigo, dysarthria, or drop attacks | Vertebrobasilar artery insufficiency |
OUTCOME: Diagnosis of exclusion
Ms. G’s visual disturbance resembles vitreous detachment or classic migraine; however, the onset is not sudden, her funduscopic exam is normal, and she has no discrete episodes with concomitant headache. Ms. G’s improvement with topiramate is inconsistent with an ophthalmic origin because such photopsia should not improve with medication. It is consistent with recurrent seizures or status epilepticus of the occipital cortex; however, she did not experience multiple discrete episodes a day or generalized seizures experienced by most occipital seizure patients, and her EEG and clinical history are not consistent with seizures. Because we cannot rule out occipital lobe epilepsy, we refer Ms. G for repeat EEG and routine ophthalmologic exam, both of which are normal.
Perceptual disturbances are common in schizoaffective disorder and schizophrenia,13-15 which we consider the most likely etiology of Ms. G’s photopsia, given her normal neurologic and ophthalmologic examinations. We switch her from aripiprazole to ziprasidone, 40 mg/d, discontinue phentermine, and taper and discontinue oxcarbazepine.
Related resources
- Ophthalmology Web. www.ophthalmologyweb.com.
- Aleman A, Larøi F. Hallucinations: the science of idiosyncratic perception. Washington, DC: American Psychological Association; 2008.
Drug Brand Names
- Aripiprazole • Abilify
- Clomiphene • Clomid, Serophene
- Digoxin • Lanoxin
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
- Paclitaxel • Onxol, Taxol
- Phentermine • Adipex-P, Inoamin
- Sertraline • Zoloft
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Jaspers K. General psychopathology. Manchester, United Kingdom: Manchester University Press; 1962.
2. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121:1819-1840.
3. Amos JF. Differential diagnosis of common etiologies of photopsia. J Am Optom Assoc. 1999;70(8):485-504.
4. Biousse V, Touboul PJ, D’Anglejan-Chatillon J, et al. Ophthalmologic manifestations of internal carotid artery dissection. Am J Ophthalmol. 1998;126(4):565-577.
5. Butler VP, Jr, Odel JG, Rath E, et al. Digitalis-induced visual disturbances with therapeutic serum digitalis concentrations. Ann Intern Med. 1995;123(9):676-680.
6. Mitchell J, Vierkant AD. Delusions and hallucinations of cocaine abusers and paranoid schizophrenics: a comparative study. J Psychol. 1991;125(3):301-310.
7. Seidman AD, Barrett S, Canezo S. Photopsia during 3-hour paclitaxel administration at doses > or = 250 mg/m2. J Clin Oncol. 1994;12(8):1741-1742.
8. Purvin VA. Visual disturbance secondary to clomiphene citrate. Arch Ophthalmol. 1995;113(4):482-484.
9. Queiroz LP, Rapoport AM, Weeks RE, et al. Characteristics of migraine visual aura. Headache. 1997;37(3):137-141.
10. Panayiotopoulos CP. Visual phenomena and headache in occipital epilepsy: a review, a systematic study and differentiation from migraine. Epileptic Disord. 1999;1(4):205-216.
11. Walker MC, Smith SJ, Sisodiya SM, et al. Case of simple partial status epilepticus in occipital lobe epilepsy misdiagnosed as migraine: clinical, electrophysiological, and magnetic resonance imaging characteristics. Epilepsia. 1995;36(12):1233-1236.
12. Lichter M. Flashing lights—a warning. The Canadian Journal of Diagnosis. 2002;19:31-35.
13. Parnas J, Handest P, Saebye D, et al. Anomalies of subjective experience in schizophrenia and psychotic bipolar illness. Acta Psychiatr Scand. 2003;108(2):126-133.
14. Phillipson OT, Harris JP. Perceptual changes in schizophrenia: a questionnaire survey. Psychol Med. 1985;15(4):859-866.
15. Cutting J, Dunne F. Subjective experience of schizophrenia. Schizophr Bull. 1989;15(2):217-231.
1. Jaspers K. General psychopathology. Manchester, United Kingdom: Manchester University Press; 1962.
2. Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121:1819-1840.
3. Amos JF. Differential diagnosis of common etiologies of photopsia. J Am Optom Assoc. 1999;70(8):485-504.
4. Biousse V, Touboul PJ, D’Anglejan-Chatillon J, et al. Ophthalmologic manifestations of internal carotid artery dissection. Am J Ophthalmol. 1998;126(4):565-577.
5. Butler VP, Jr, Odel JG, Rath E, et al. Digitalis-induced visual disturbances with therapeutic serum digitalis concentrations. Ann Intern Med. 1995;123(9):676-680.
6. Mitchell J, Vierkant AD. Delusions and hallucinations of cocaine abusers and paranoid schizophrenics: a comparative study. J Psychol. 1991;125(3):301-310.
7. Seidman AD, Barrett S, Canezo S. Photopsia during 3-hour paclitaxel administration at doses > or = 250 mg/m2. J Clin Oncol. 1994;12(8):1741-1742.
8. Purvin VA. Visual disturbance secondary to clomiphene citrate. Arch Ophthalmol. 1995;113(4):482-484.
9. Queiroz LP, Rapoport AM, Weeks RE, et al. Characteristics of migraine visual aura. Headache. 1997;37(3):137-141.
10. Panayiotopoulos CP. Visual phenomena and headache in occipital epilepsy: a review, a systematic study and differentiation from migraine. Epileptic Disord. 1999;1(4):205-216.
11. Walker MC, Smith SJ, Sisodiya SM, et al. Case of simple partial status epilepticus in occipital lobe epilepsy misdiagnosed as migraine: clinical, electrophysiological, and magnetic resonance imaging characteristics. Epilepsia. 1995;36(12):1233-1236.
12. Lichter M. Flashing lights—a warning. The Canadian Journal of Diagnosis. 2002;19:31-35.
13. Parnas J, Handest P, Saebye D, et al. Anomalies of subjective experience in schizophrenia and psychotic bipolar illness. Acta Psychiatr Scand. 2003;108(2):126-133.
14. Phillipson OT, Harris JP. Perceptual changes in schizophrenia: a questionnaire survey. Psychol Med. 1985;15(4):859-866.
15. Cutting J, Dunne F. Subjective experience of schizophrenia. Schizophr Bull. 1989;15(2):217-231.
Cholesterol, mood, and vascular health: Untangling the relationship
Discuss this article at http://currentpsychiatry.blogspot.com/2010/07/cholesterol-mood-and-vascular-health.html#comments
A growing body of literature examining the putative links among cholesterol, mood disorders, and suicide has produced inconsistent findings and unclear clinical implications that may leave psychiatrists unsure of how to interpret the data. Understanding cholesterol’s role in mood disorders may be relevant to the 2 primary causes of excess deaths in patients with mood disorders: suicide and vascular disease.1
Plausible links
In the early 1990s several studies suggested a link between low cholesterol (<160 mg/dL) and unnatural deaths, including suicide.2-4 Follow-up studies confirmed associations between low cholesterol and suicide attempts, especially violent ones.5 These associations are compelling given the neurobiologic effects of cholesterol, such as a net reduction of serotonergic function (Box 1). Low cholesterol may predispose an individual to aggression, impulsivity, and violence (Table 1).6 Many studies have found that patients with mood disorders have lower cholesterol levels;7 however, other research suggests they are at increased risk of hyperlipidemia, typically hypertriglyceridemia rather than hypercholesterolemia.8
Depression. Several studies have shown an association between low cholesterol and depressive symptoms, although this finding has not been replicated in Asian subjects.9,10 Patients with manic or mixed syndromes have been found to have lower serum cholesterol,11 and individuals with major depression and bipolar disorder have lower cholesterol levels in the brain compared with healthy controls.12 Some studies have observed higher total cholesterol levels after patients receive pharmacotherapy for major depressive symptoms.13 These findings have led to speculation that low serum cholesterol in patients with mood disorders is partially a state-dependent effect of depressive illness.
Suicide. Cohort, case-control, and cross-sectional studies have linked low cholesterol to an increased risk of suicide.2,5 Individuals who attempt suicide by violent means have lower cholesterol compared with those who use less violent methods.5,14 A meta-analysis found statistically significant correlations between low cholesterol and future or past suicidal behavior; however, low cholesterol explained <0.01% of suicidal behavior.15 Studies comparing cholesterol levels of individuals following violent vs nonviolent suicide attempts have demonstrated stronger associations.15
Assessing suicide risk. Current evidence does not support considering low serum cholesterol a risk factor for suicide. One study used cholesterol as a clinical predictor of suicide,16 but this model has not been prospectively validated. As a whole, the evidence does not suggest that cholesterol levels explain a substantial portion of suicidal behaviors.
The neurobiologic effects of low cholesterol—particularly those related to serotonergic hypofunction—are thought to be mediate impulsive, aggressive, and violent behaviors that may predispose an individual to suicide.a,b The CNS contains one-fourth of the body’s free cholesterol,c which is synthesized primarily in situ.
Cholesterol improves membrane stability, reduces permeability, and may influence serotonergic function. Cholesterol depletion may impair function of 5-HT1A and 5-HT7 receptorsd,e and serotonin transporter activity.f Reduced cholesterol after treatment with simvastatin—an HMG-CoA reductase inhibitor that readily crosses the blood-brain barrier—resulted in acute (1-month) increases in serotonin transporter activity followed by subacute (>2 months) decreases.g Lower cholesterol levels may further decrease expression of serotonin receptors and cause a net reduction in serotonergic activity.
In addition, cholesterol is necessary for synapse formation and myelin production. Cholesterol depletion may have more diffuse effects on neurotransmission, such as gamma-aminobutyric acid receptors,hN-methyl-D-aspartate receptors,i opioid signaling,j and excitatory amino acids transport.k
Impulsivity associated with low serotonergic function and low total cholesterol has been suggested as a potential pathway for suicide.l Low cholesterol is associated with self-report measures of impulsivity;m however, increased impulsivity associated with lipid-lowering therapy may be temporary,n which is similar to the time-limited changes in serotonin transporter activity.g Human and animal data have suggested that low cholesterol may be linked to violent behaviors, including suicide.o
Source:
a. Vevera J, Fisar Z, Kvasnicka T, et al. Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res. 2005;133(2-3):197-203.
b. Kaplan JR, Shively CA, Fontenot MB, et al. Demonstration of an association among dietary cholesterol, central serotonergic activity, and social behavior in monkeys. Psychosom Med. 1994;56(6):479-484.
c. Chattopadhyay A, Paila YD. Lipid-protein interactions, regulation and dysfunction of brain cholesterol. Biochem Biophys Res Commun. 2007;354(3):627-633.
d. Singh P, Paila YD, Chattopadhyay A. Differential effects of cholesterol and 7-dehydrocholesterol on the ligand binding activity of the hippocampal serotonin(1A) receptor: implications in SLOS. Biochem Biophys Res Commun. 2007;358(2):495-499.
e. Sjögren B, Hamblin MW, Svenningsson P. Cholesterol depletion reduces serotonin binding and signaling via human 5-HT(7(a)) receptors. Eur J Pharmacol. 2006;552(1-3):1-10.
f. Scanlon SM, Williams DC, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40(35):10507-10513.
g. Vevera J, Fisar Z, Kvasnicka T, et al. Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res. 2005;133(2-3):197-203.
h. Sooksawate T, Simmonds MA. Effects of membrane cholesterol on the sensitivity of the GABA(A) receptor to GABA in acutely dissociated rat hippocampal neurones. Neuropharmacology. 2001;40(2):178-184.
i. Abulrob A, Tauskela JS, Mealing G, et al. Protection by cholesterol-extracting cyclodextrins: a role for N-methyl-daspartate receptor redistribution. J Neurochem. 2005;92(6):1477-1486.
j. Huang P, Xu W, Yoon SI, et al. Cholesterol reduction by methyl-beta-cyclodextrin attenuates the delta opioid receptor-mediated signaling in neuronal cells but enhances it in non-neuronal cells. Biochem Pharmacol. 2007;73(4):534-549.
k. Butchbach ME, Tian G, Guo H, et al. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function. J Biol Chem. 2004;279(33):34388-34396.
l. Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Annals N Y Acad Sci. 1997;836:288-301.
m. Garland M, Hickey D, Corvin A, et al. Total serum cholesterol in relation to psychological correlates in parasuicide. Br J Psychiatry. 2000;177:77-83.
n. Ormiston T, Wolkowitz OM, Reus VI, et al. Behavioral implications of lowering cholesterol levels: a double-blind pilot study. Psychosomatics. 2003;44(5):412-414.
o. Golomb BA. Cholesterol and violence: is there a connection? Ann Intern Med. 1998;128(6):478-487.
Table 1
Psychiatric features associated with low cholesterol*
| Symptoms |
| Anxiety, depressed mood, emotional lability, euphoria, impulsivity, irritability, suicidal ideation, aggression |
| Syndromes |
| Anorexia nervosa, bipolar disorder, borderline personality disorder, major depressive disorder, seasonal affective disorder |
| Behaviors |
| Suicide and suicide attempts, violence |
| *Small studies have suggested possible relationships with dissociative and panic disorders |
Effects of lipid-lowering agents
If there is a causal relationship between low cholesterol and mood disorders, then it stands to reason that using cholesterol-lowering drugs would increase the risk of depression and suicide. However, the data do not support that conclusion.
Many case reports have documented adverse psychiatric reactions to statins, including depression, suicidality, emotional lability, agitation, irritability, anxiety, panic, and euphoria.17 In an early analysis of primary prevention trials, patients receiving cholesterol-lowering treatment—mainly non-statins—were estimated to have twice the risk of death by suicide or violence compared with controls.3 However, a more recent meta-analysis of larger clinical trials of lipid-lowering agents including statins and observational studies did not reveal an association between lipid-lowering medications and suicide.15,18
In a large case-control study, statin users had a lower risk of depression (adjusted odds ratio [OR] 0.4, 95% confidence interval [CI], 0.2 to 0.9) than patients taking non-statin lipid-lowering drugs (adjusted OR 1.0, 95% CI, 0.5 to 2.1).19 However, statins reduced cholesterol more (30% to 50%) than non-statin drugs (10% to 20%). A clinical trial of >1,000 patients with stable coronary artery disease treated with pravastatin—an HMG-CoA reductase inhibitor with low lipophilicity that is less likely than other statins to cross the blood-brain barrier—revealed no changes in self-reported anger, impulsiveness, anxiety, or depression.20
This study did not exclude patients with psychiatric illness—who are at greatest risk of suicide—but other trials of lipid-lowering drugs did.21 As a result, the effects of lipid-lowering medications on psychiatric patients are unclear. A clinical trial is underway to assess the effects of pravastatin (low lipophilicity), simvastatin (high lipophilicity), or placebo on mood, sleep, and aggression.21
Low cholesterol: State or trait?
Much of the research linking low cholesterol, mood disorders, and suicidality could be confounded by depressed mood leading to reduced serum cholesterol. There has been considerable debate about whether low cholesterol predisposes patients to suicide or if depression independently leads to poor nutrition and therefore low cholesterol and increased suicide risk.6,22
Some researchers have suggested that depression lowers cholesterol and increases risk of suicide,23 but study designs have limited the ability to discern the directionality of the relationship. Attempts to control for depression-related malnutrition and weight loss—which lowers total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C)24—suggest the association may be independent of these variables.25-27 These findings suggest that cholesterol may be considered a trait marker and is not entirely state-dependent. However, multiple, large, long-term randomized controlled trials have not shown increased depression and suicide with use of lipid-lowering agents in healthy populations.20
The Figure illustrates known epidemiologic associations of low cholesterol, low serotoninergic function, and suicide and contrasts conceptual models of cholesterol as a state and a trait marker. A case can be made for cholesterol as both a state and a trait marker, and these models could overlap, with depression-induced decreases in cholesterol further mediating changes in serotonergic function and related behavioral sequelae.
Figure
Cholesterol, depression, and suicide: How are they linked? 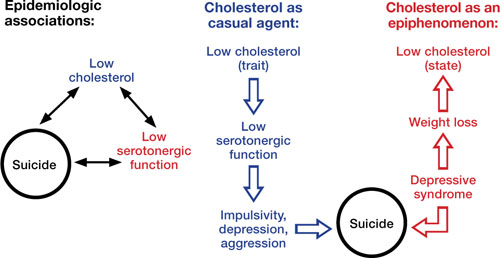
Low cholesterol may be considered a trait marker, predisposing patients to lower serotonergic function and placing them at greater risk for impulsivity, depression, aggression, and suicide. Other models suggest that lower cholesterol is a state-dependent consequence of depression, and not part of a causal chain toward suicide
Improving cardiac health
Limited epidemiologic studies suggest that patients with mood disorders may have lower levels of total cholesterol and LDL-C, but higher rates of hypertriglyceridemia compared with the general population.8 Unfortunately, psychiatric patients—who may be at increased risk of developing cardiovascular disease—may be less likely to be screened and appropriately treated for lipid abnormalities.28 To address this disparity, consider assuming an active role in assessing and managing hyperlipidemia in your patients with mood disorders. Be aware of your patients’ lipid profile and ensure that they follow monitoring recommendations.
The National Cholesterol Education Program recommends screening all adults age >20 for hyperlipidemia every 5 years using measures of total cholesterol, LDL-C, HDL-C, and triglycerides. If LDL-C or triglycerides exceed target values (Table 2), appropriate management includes recommending lifestyle changes and pharmacotherapy (Box 2).
Patients should receive a fasting lipid profile before and 12 weeks after starting any antipsychotic and semiannually thereafter.29 Consider closely monitoring lipids when patients gain weight with psychotropics. Refer patients with hyperlipidemia to a primary care physician, but in the absence of such a provider, mental health clinicians who are familiar with treatment guidelines can manage these patients.30
Closely monitor individuals with mood disorders for changes in behavior or mental status after starting a lipid-lowering agent. Consider discontinuing the drug if a patient develops an adverse reaction. If symptoms return after medication rechallenge, consider other management strategies such as an alternate lipid-lowering agent or re-emphasizing behavioral measures.
Table 2
National Cholesterol Education Program recommended LDL levels
| Risk category* | LDL goal | When to consider medications |
|---|---|---|
| CHD or CHD equivalent | <100 mg/dL | ≥130 mg/dL |
| ≥2 major risk factors | <130 mg/dL | ≥130 to 160 mg/dL (based on 10-year risk) |
| 0 or 1 risk factor | <160 mg/dL | ≥190 mg/dL |
| CHD: coronary heart disease; HDL: high-density lipoprotein; LDL: low-density lipoprotein | ||
| *Risk category is based on the presence of CHD or equivalent and major risk factors for CHD. CHD equivalents include symptomatic carotid artery disease, peripheral artery disease, and abdominal aortic aneurysm. Major risk factors include smoking, hypertension, low HDL, family history, and age. LDL levels to consider medications for those with ≥2 major risk factors vary by 10-year CHD risk | ||
| Source: National Cholesterol Education Program, Adult Treatment Panel III (ATP III) Quick Desk Reference. www.nhlbi.nih.gov/guidelines/cholesterol/atglance.htm | ||
National Cholesterol Education Program guidelines state that when a patient’s low-density lipoprotein cholesterol (LDL-C) exceeds targets (Table 2), first recommend lifestyle changes such as a diet low in saturated fat (<7% of calories) and cholesterol (<200 mg/d), weight management, and exercise. Increases in soluble fiber (10 to 25 g/d) and plant stanols/sterols also may be considered. If LDL-C levels are still too high, pharmacologic therapy such as an HMGCoA reductase inhibitor is suggested.
Treatment of elevated triglycerides (≥150 mg/dL) includes reaching the target LDL-C, intensifying a weight management program, and increasing exercise. Address quitting smoking and limiting alcohol when indicated. If triglyceride levels are ≥200 mg/dL after the LDL-C target is reached, set a secondary goal of reaching a target non-high-density lipoprotein cholesterol (HDL-C) (non-HDL-C; total cholesterol minus HDL-C) 30 mg/dL greater than the LDL goal. This can be achieved by adding an LDL-lowering drug such as a statin, nicotinic acid, or ezetimibe. When triglycerides are ≥500 mg/dL, more aggressive intervention, such as with a fibrate, omega-3 fatty acids, very low-fat diets, and exercise, is required to prevent pancreatitis.
Source: National Heart Lung and Blood Institute. National Cholesterol Education Program. www.nhlbi.nih.gov/guidelines/cholesterol/index.htm
Related Resources
- Fiedorowicz JG, Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. 2007;152(1):11-20.
- National Cholesterol Education Program, Adult Treatment Panel III (ATP III) Quick Desk Reference. www.nhlbi.nih.gov/guidelines/cholesterol/atglance.htm.
- Executive Summary of the third report of the national Cholesterol Education Program (nCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
Drug Brand Names
- Ezetimibe • Zetia
- Pravastatin • Pravachol
- Simvastatin • Zocor
Acknowledgements
Dr. Fiedorowicz thanks Lois Warren and Miriam Weiner for their editorial assistance.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fiedorowicz is supported by the national Institutes of Health (1K23MH083695-01A210), nARSAD, and the Institute for Clinical and Translational Science at the University of Iowa (3 UL1 RR024979-03S4). He has received support for participating in a colleague’s investigator-initiated project with Eli Lilly. Dr. Haynes’ research is supported by grants from the national Institutes of Health (nHLBI: HL58972 & HL14388; nCRR CTSA: 1UL1RR024979).
1. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844-850.
2. Lindberg G, Råstam L, Gullberg B, et al. Low serum cholesterol concentration and short term mortality from injuries in men and women. BMJ. 1992;305(6848):277-279.
3. Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301(6747):309-314.
4. Neaton JD, Blackburn H, Jacobs D, et al. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med. 1992;152(7):1490-1500.
5. Fiedorowicz JG, Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. 2007;152(1):11-20.
6. Golomb BA. Cholesterol and violence: is there a connection? Ann Intern Med. 1998;128(6):478-487.
7. Pae CU, Kim JJ, Lee SJ, et al. Aberration of cholesterol level in first-onset bipolar I patients. J Affect Disord. 2004;83(1):79-82.
8. Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, et al. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20(3):131-137.
9. Chung KH, Tsai SY, Lee HC. Mood symptoms and serum lipids in acute phase of bipolar disorder in Taiwan. Psychiatry Clin Neurosci. 2007;61(4):428-433.
10. Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord. 2006;90(1):21-27.
11. Sagud M, Mihaljevic-Peles A, Pivac N, et al. Platelet serotonin and serum lipids in psychotic mania. J Affect Disord. 2007;97(1-3):247-251.
12. Beasley CL, Honer WG, Bergmann K, et al. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord. 2005;7(5):449-455.
13. Gabriel A. Changes in plasma cholesterol in mood disorder patients: does treatment make a difference? J Affect Disord. 2007;99(1-3):273-278.
14. Lalovic A, Levy E, Luheshi G, et al. Cholesterol content in brains of suicide completers. Int J Neuropsychopharmacol. 2007;10(2):159-166.
15. Lester D. Serum cholesterol levels and suicide: a meta-analysis. Suicide Life Threat Behav. 2002;32(3):333-346.
16. Coryell W, Schlesser M. Combined biological tests for suicide prediction. Psychiatry Res. 2007;150(2):187-191.
17. Tatley M, Savage R. Psychiatric adverse reactions with statins, fibrates and ezetimibe: implications for the use of lipid-lowering agents. Drug Saf. 2007;30(3):195-201.
18. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
19. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
20. Stewart RA, Sharples KJ, North FM, et al. Long-term assessment of psychological well-being in a randomized placebo-controlled trial of cholesterol reduction with pravastatin. The LIPID Study Investigators. Arch Intern Med. 2000;160(20):3144-3152.
21. Golomb BA, Criqui MH, White HL, et al. The UCSD Statin Study: a randomized controlled trial assessing the impact of statins on selected noncardiac outcomes. Control Clin Trials. 2004;25(2):178-202.
22. Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Annals N Y Acad Sci. 1997;836:288-301.
23. Law MR, Thompson SG, Wald NJ. Assessing possible hazards of reducing serum cholesterol. BMJ. 1994;308(6925):373-379.
24. Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56(2):320-328.
25. Garland M, Hickey D, Corvin A, et al. Total serum cholesterol in relation to psychological correlates in parasuicide. Br J Psychiatry. 2000;177:77-83.
26. Golier JA, Marzuk PM, Leon AC, et al. Low serum cholesterol level and attempted suicide. Am J Psychiatry. 1995;152(3):419-423.
27. Kunugi H, Takei N, Aoki H, et al. Low serum cholesterol in suicide attempters. Biol Psychiatry. 1997;41(2):196-200.
28. Murray DP, Weiner M, Prabhakar M, et al. Mania and mortality: why the excess cardiovascular risk in bipolar disorder? Curr Psychiatry Rep. 2009;11(6):475-480.
29. Sernyak MJ. Implementation of monitoring and management guidelines for second-generation antipsychotics. J Clin Psychiatry. 2007;68(suppl 4):14-18.
30. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334-1349.
Discuss this article at http://currentpsychiatry.blogspot.com/2010/07/cholesterol-mood-and-vascular-health.html#comments
A growing body of literature examining the putative links among cholesterol, mood disorders, and suicide has produced inconsistent findings and unclear clinical implications that may leave psychiatrists unsure of how to interpret the data. Understanding cholesterol’s role in mood disorders may be relevant to the 2 primary causes of excess deaths in patients with mood disorders: suicide and vascular disease.1
Plausible links
In the early 1990s several studies suggested a link between low cholesterol (<160 mg/dL) and unnatural deaths, including suicide.2-4 Follow-up studies confirmed associations between low cholesterol and suicide attempts, especially violent ones.5 These associations are compelling given the neurobiologic effects of cholesterol, such as a net reduction of serotonergic function (Box 1). Low cholesterol may predispose an individual to aggression, impulsivity, and violence (Table 1).6 Many studies have found that patients with mood disorders have lower cholesterol levels;7 however, other research suggests they are at increased risk of hyperlipidemia, typically hypertriglyceridemia rather than hypercholesterolemia.8
Depression. Several studies have shown an association between low cholesterol and depressive symptoms, although this finding has not been replicated in Asian subjects.9,10 Patients with manic or mixed syndromes have been found to have lower serum cholesterol,11 and individuals with major depression and bipolar disorder have lower cholesterol levels in the brain compared with healthy controls.12 Some studies have observed higher total cholesterol levels after patients receive pharmacotherapy for major depressive symptoms.13 These findings have led to speculation that low serum cholesterol in patients with mood disorders is partially a state-dependent effect of depressive illness.
Suicide. Cohort, case-control, and cross-sectional studies have linked low cholesterol to an increased risk of suicide.2,5 Individuals who attempt suicide by violent means have lower cholesterol compared with those who use less violent methods.5,14 A meta-analysis found statistically significant correlations between low cholesterol and future or past suicidal behavior; however, low cholesterol explained <0.01% of suicidal behavior.15 Studies comparing cholesterol levels of individuals following violent vs nonviolent suicide attempts have demonstrated stronger associations.15
Assessing suicide risk. Current evidence does not support considering low serum cholesterol a risk factor for suicide. One study used cholesterol as a clinical predictor of suicide,16 but this model has not been prospectively validated. As a whole, the evidence does not suggest that cholesterol levels explain a substantial portion of suicidal behaviors.
The neurobiologic effects of low cholesterol—particularly those related to serotonergic hypofunction—are thought to be mediate impulsive, aggressive, and violent behaviors that may predispose an individual to suicide.a,b The CNS contains one-fourth of the body’s free cholesterol,c which is synthesized primarily in situ.
Cholesterol improves membrane stability, reduces permeability, and may influence serotonergic function. Cholesterol depletion may impair function of 5-HT1A and 5-HT7 receptorsd,e and serotonin transporter activity.f Reduced cholesterol after treatment with simvastatin—an HMG-CoA reductase inhibitor that readily crosses the blood-brain barrier—resulted in acute (1-month) increases in serotonin transporter activity followed by subacute (>2 months) decreases.g Lower cholesterol levels may further decrease expression of serotonin receptors and cause a net reduction in serotonergic activity.
In addition, cholesterol is necessary for synapse formation and myelin production. Cholesterol depletion may have more diffuse effects on neurotransmission, such as gamma-aminobutyric acid receptors,hN-methyl-D-aspartate receptors,i opioid signaling,j and excitatory amino acids transport.k
Impulsivity associated with low serotonergic function and low total cholesterol has been suggested as a potential pathway for suicide.l Low cholesterol is associated with self-report measures of impulsivity;m however, increased impulsivity associated with lipid-lowering therapy may be temporary,n which is similar to the time-limited changes in serotonin transporter activity.g Human and animal data have suggested that low cholesterol may be linked to violent behaviors, including suicide.o
Source:
a. Vevera J, Fisar Z, Kvasnicka T, et al. Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res. 2005;133(2-3):197-203.
b. Kaplan JR, Shively CA, Fontenot MB, et al. Demonstration of an association among dietary cholesterol, central serotonergic activity, and social behavior in monkeys. Psychosom Med. 1994;56(6):479-484.
c. Chattopadhyay A, Paila YD. Lipid-protein interactions, regulation and dysfunction of brain cholesterol. Biochem Biophys Res Commun. 2007;354(3):627-633.
d. Singh P, Paila YD, Chattopadhyay A. Differential effects of cholesterol and 7-dehydrocholesterol on the ligand binding activity of the hippocampal serotonin(1A) receptor: implications in SLOS. Biochem Biophys Res Commun. 2007;358(2):495-499.
e. Sjögren B, Hamblin MW, Svenningsson P. Cholesterol depletion reduces serotonin binding and signaling via human 5-HT(7(a)) receptors. Eur J Pharmacol. 2006;552(1-3):1-10.
f. Scanlon SM, Williams DC, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40(35):10507-10513.
g. Vevera J, Fisar Z, Kvasnicka T, et al. Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res. 2005;133(2-3):197-203.
h. Sooksawate T, Simmonds MA. Effects of membrane cholesterol on the sensitivity of the GABA(A) receptor to GABA in acutely dissociated rat hippocampal neurones. Neuropharmacology. 2001;40(2):178-184.
i. Abulrob A, Tauskela JS, Mealing G, et al. Protection by cholesterol-extracting cyclodextrins: a role for N-methyl-daspartate receptor redistribution. J Neurochem. 2005;92(6):1477-1486.
j. Huang P, Xu W, Yoon SI, et al. Cholesterol reduction by methyl-beta-cyclodextrin attenuates the delta opioid receptor-mediated signaling in neuronal cells but enhances it in non-neuronal cells. Biochem Pharmacol. 2007;73(4):534-549.
k. Butchbach ME, Tian G, Guo H, et al. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function. J Biol Chem. 2004;279(33):34388-34396.
l. Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Annals N Y Acad Sci. 1997;836:288-301.
m. Garland M, Hickey D, Corvin A, et al. Total serum cholesterol in relation to psychological correlates in parasuicide. Br J Psychiatry. 2000;177:77-83.
n. Ormiston T, Wolkowitz OM, Reus VI, et al. Behavioral implications of lowering cholesterol levels: a double-blind pilot study. Psychosomatics. 2003;44(5):412-414.
o. Golomb BA. Cholesterol and violence: is there a connection? Ann Intern Med. 1998;128(6):478-487.
Table 1
Psychiatric features associated with low cholesterol*
| Symptoms |
| Anxiety, depressed mood, emotional lability, euphoria, impulsivity, irritability, suicidal ideation, aggression |
| Syndromes |
| Anorexia nervosa, bipolar disorder, borderline personality disorder, major depressive disorder, seasonal affective disorder |
| Behaviors |
| Suicide and suicide attempts, violence |
| *Small studies have suggested possible relationships with dissociative and panic disorders |
Effects of lipid-lowering agents
If there is a causal relationship between low cholesterol and mood disorders, then it stands to reason that using cholesterol-lowering drugs would increase the risk of depression and suicide. However, the data do not support that conclusion.
Many case reports have documented adverse psychiatric reactions to statins, including depression, suicidality, emotional lability, agitation, irritability, anxiety, panic, and euphoria.17 In an early analysis of primary prevention trials, patients receiving cholesterol-lowering treatment—mainly non-statins—were estimated to have twice the risk of death by suicide or violence compared with controls.3 However, a more recent meta-analysis of larger clinical trials of lipid-lowering agents including statins and observational studies did not reveal an association between lipid-lowering medications and suicide.15,18
In a large case-control study, statin users had a lower risk of depression (adjusted odds ratio [OR] 0.4, 95% confidence interval [CI], 0.2 to 0.9) than patients taking non-statin lipid-lowering drugs (adjusted OR 1.0, 95% CI, 0.5 to 2.1).19 However, statins reduced cholesterol more (30% to 50%) than non-statin drugs (10% to 20%). A clinical trial of >1,000 patients with stable coronary artery disease treated with pravastatin—an HMG-CoA reductase inhibitor with low lipophilicity that is less likely than other statins to cross the blood-brain barrier—revealed no changes in self-reported anger, impulsiveness, anxiety, or depression.20
This study did not exclude patients with psychiatric illness—who are at greatest risk of suicide—but other trials of lipid-lowering drugs did.21 As a result, the effects of lipid-lowering medications on psychiatric patients are unclear. A clinical trial is underway to assess the effects of pravastatin (low lipophilicity), simvastatin (high lipophilicity), or placebo on mood, sleep, and aggression.21
Low cholesterol: State or trait?
Much of the research linking low cholesterol, mood disorders, and suicidality could be confounded by depressed mood leading to reduced serum cholesterol. There has been considerable debate about whether low cholesterol predisposes patients to suicide or if depression independently leads to poor nutrition and therefore low cholesterol and increased suicide risk.6,22
Some researchers have suggested that depression lowers cholesterol and increases risk of suicide,23 but study designs have limited the ability to discern the directionality of the relationship. Attempts to control for depression-related malnutrition and weight loss—which lowers total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C)24—suggest the association may be independent of these variables.25-27 These findings suggest that cholesterol may be considered a trait marker and is not entirely state-dependent. However, multiple, large, long-term randomized controlled trials have not shown increased depression and suicide with use of lipid-lowering agents in healthy populations.20
The Figure illustrates known epidemiologic associations of low cholesterol, low serotoninergic function, and suicide and contrasts conceptual models of cholesterol as a state and a trait marker. A case can be made for cholesterol as both a state and a trait marker, and these models could overlap, with depression-induced decreases in cholesterol further mediating changes in serotonergic function and related behavioral sequelae.
Figure
Cholesterol, depression, and suicide: How are they linked? 
Low cholesterol may be considered a trait marker, predisposing patients to lower serotonergic function and placing them at greater risk for impulsivity, depression, aggression, and suicide. Other models suggest that lower cholesterol is a state-dependent consequence of depression, and not part of a causal chain toward suicide
Improving cardiac health
Limited epidemiologic studies suggest that patients with mood disorders may have lower levels of total cholesterol and LDL-C, but higher rates of hypertriglyceridemia compared with the general population.8 Unfortunately, psychiatric patients—who may be at increased risk of developing cardiovascular disease—may be less likely to be screened and appropriately treated for lipid abnormalities.28 To address this disparity, consider assuming an active role in assessing and managing hyperlipidemia in your patients with mood disorders. Be aware of your patients’ lipid profile and ensure that they follow monitoring recommendations.
The National Cholesterol Education Program recommends screening all adults age >20 for hyperlipidemia every 5 years using measures of total cholesterol, LDL-C, HDL-C, and triglycerides. If LDL-C or triglycerides exceed target values (Table 2), appropriate management includes recommending lifestyle changes and pharmacotherapy (Box 2).
Patients should receive a fasting lipid profile before and 12 weeks after starting any antipsychotic and semiannually thereafter.29 Consider closely monitoring lipids when patients gain weight with psychotropics. Refer patients with hyperlipidemia to a primary care physician, but in the absence of such a provider, mental health clinicians who are familiar with treatment guidelines can manage these patients.30
Closely monitor individuals with mood disorders for changes in behavior or mental status after starting a lipid-lowering agent. Consider discontinuing the drug if a patient develops an adverse reaction. If symptoms return after medication rechallenge, consider other management strategies such as an alternate lipid-lowering agent or re-emphasizing behavioral measures.
Table 2
National Cholesterol Education Program recommended LDL levels
| Risk category* | LDL goal | When to consider medications |
|---|---|---|
| CHD or CHD equivalent | <100 mg/dL | ≥130 mg/dL |
| ≥2 major risk factors | <130 mg/dL | ≥130 to 160 mg/dL (based on 10-year risk) |
| 0 or 1 risk factor | <160 mg/dL | ≥190 mg/dL |
| CHD: coronary heart disease; HDL: high-density lipoprotein; LDL: low-density lipoprotein | ||
| *Risk category is based on the presence of CHD or equivalent and major risk factors for CHD. CHD equivalents include symptomatic carotid artery disease, peripheral artery disease, and abdominal aortic aneurysm. Major risk factors include smoking, hypertension, low HDL, family history, and age. LDL levels to consider medications for those with ≥2 major risk factors vary by 10-year CHD risk | ||
| Source: National Cholesterol Education Program, Adult Treatment Panel III (ATP III) Quick Desk Reference. www.nhlbi.nih.gov/guidelines/cholesterol/atglance.htm | ||
National Cholesterol Education Program guidelines state that when a patient’s low-density lipoprotein cholesterol (LDL-C) exceeds targets (Table 2), first recommend lifestyle changes such as a diet low in saturated fat (<7% of calories) and cholesterol (<200 mg/d), weight management, and exercise. Increases in soluble fiber (10 to 25 g/d) and plant stanols/sterols also may be considered. If LDL-C levels are still too high, pharmacologic therapy such as an HMGCoA reductase inhibitor is suggested.
Treatment of elevated triglycerides (≥150 mg/dL) includes reaching the target LDL-C, intensifying a weight management program, and increasing exercise. Address quitting smoking and limiting alcohol when indicated. If triglyceride levels are ≥200 mg/dL after the LDL-C target is reached, set a secondary goal of reaching a target non-high-density lipoprotein cholesterol (HDL-C) (non-HDL-C; total cholesterol minus HDL-C) 30 mg/dL greater than the LDL goal. This can be achieved by adding an LDL-lowering drug such as a statin, nicotinic acid, or ezetimibe. When triglycerides are ≥500 mg/dL, more aggressive intervention, such as with a fibrate, omega-3 fatty acids, very low-fat diets, and exercise, is required to prevent pancreatitis.
Source: National Heart Lung and Blood Institute. National Cholesterol Education Program. www.nhlbi.nih.gov/guidelines/cholesterol/index.htm
Related Resources
- Fiedorowicz JG, Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. 2007;152(1):11-20.
- National Cholesterol Education Program, Adult Treatment Panel III (ATP III) Quick Desk Reference. www.nhlbi.nih.gov/guidelines/cholesterol/atglance.htm.
- Executive Summary of the third report of the national Cholesterol Education Program (nCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
Drug Brand Names
- Ezetimibe • Zetia
- Pravastatin • Pravachol
- Simvastatin • Zocor
Acknowledgements
Dr. Fiedorowicz thanks Lois Warren and Miriam Weiner for their editorial assistance.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fiedorowicz is supported by the national Institutes of Health (1K23MH083695-01A210), nARSAD, and the Institute for Clinical and Translational Science at the University of Iowa (3 UL1 RR024979-03S4). He has received support for participating in a colleague’s investigator-initiated project with Eli Lilly. Dr. Haynes’ research is supported by grants from the national Institutes of Health (nHLBI: HL58972 & HL14388; nCRR CTSA: 1UL1RR024979).
Discuss this article at http://currentpsychiatry.blogspot.com/2010/07/cholesterol-mood-and-vascular-health.html#comments
A growing body of literature examining the putative links among cholesterol, mood disorders, and suicide has produced inconsistent findings and unclear clinical implications that may leave psychiatrists unsure of how to interpret the data. Understanding cholesterol’s role in mood disorders may be relevant to the 2 primary causes of excess deaths in patients with mood disorders: suicide and vascular disease.1
Plausible links
In the early 1990s several studies suggested a link between low cholesterol (<160 mg/dL) and unnatural deaths, including suicide.2-4 Follow-up studies confirmed associations between low cholesterol and suicide attempts, especially violent ones.5 These associations are compelling given the neurobiologic effects of cholesterol, such as a net reduction of serotonergic function (Box 1). Low cholesterol may predispose an individual to aggression, impulsivity, and violence (Table 1).6 Many studies have found that patients with mood disorders have lower cholesterol levels;7 however, other research suggests they are at increased risk of hyperlipidemia, typically hypertriglyceridemia rather than hypercholesterolemia.8
Depression. Several studies have shown an association between low cholesterol and depressive symptoms, although this finding has not been replicated in Asian subjects.9,10 Patients with manic or mixed syndromes have been found to have lower serum cholesterol,11 and individuals with major depression and bipolar disorder have lower cholesterol levels in the brain compared with healthy controls.12 Some studies have observed higher total cholesterol levels after patients receive pharmacotherapy for major depressive symptoms.13 These findings have led to speculation that low serum cholesterol in patients with mood disorders is partially a state-dependent effect of depressive illness.
Suicide. Cohort, case-control, and cross-sectional studies have linked low cholesterol to an increased risk of suicide.2,5 Individuals who attempt suicide by violent means have lower cholesterol compared with those who use less violent methods.5,14 A meta-analysis found statistically significant correlations between low cholesterol and future or past suicidal behavior; however, low cholesterol explained <0.01% of suicidal behavior.15 Studies comparing cholesterol levels of individuals following violent vs nonviolent suicide attempts have demonstrated stronger associations.15
Assessing suicide risk. Current evidence does not support considering low serum cholesterol a risk factor for suicide. One study used cholesterol as a clinical predictor of suicide,16 but this model has not been prospectively validated. As a whole, the evidence does not suggest that cholesterol levels explain a substantial portion of suicidal behaviors.
The neurobiologic effects of low cholesterol—particularly those related to serotonergic hypofunction—are thought to be mediate impulsive, aggressive, and violent behaviors that may predispose an individual to suicide.a,b The CNS contains one-fourth of the body’s free cholesterol,c which is synthesized primarily in situ.
Cholesterol improves membrane stability, reduces permeability, and may influence serotonergic function. Cholesterol depletion may impair function of 5-HT1A and 5-HT7 receptorsd,e and serotonin transporter activity.f Reduced cholesterol after treatment with simvastatin—an HMG-CoA reductase inhibitor that readily crosses the blood-brain barrier—resulted in acute (1-month) increases in serotonin transporter activity followed by subacute (>2 months) decreases.g Lower cholesterol levels may further decrease expression of serotonin receptors and cause a net reduction in serotonergic activity.
In addition, cholesterol is necessary for synapse formation and myelin production. Cholesterol depletion may have more diffuse effects on neurotransmission, such as gamma-aminobutyric acid receptors,hN-methyl-D-aspartate receptors,i opioid signaling,j and excitatory amino acids transport.k
Impulsivity associated with low serotonergic function and low total cholesterol has been suggested as a potential pathway for suicide.l Low cholesterol is associated with self-report measures of impulsivity;m however, increased impulsivity associated with lipid-lowering therapy may be temporary,n which is similar to the time-limited changes in serotonin transporter activity.g Human and animal data have suggested that low cholesterol may be linked to violent behaviors, including suicide.o
Source:
a. Vevera J, Fisar Z, Kvasnicka T, et al. Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res. 2005;133(2-3):197-203.
b. Kaplan JR, Shively CA, Fontenot MB, et al. Demonstration of an association among dietary cholesterol, central serotonergic activity, and social behavior in monkeys. Psychosom Med. 1994;56(6):479-484.
c. Chattopadhyay A, Paila YD. Lipid-protein interactions, regulation and dysfunction of brain cholesterol. Biochem Biophys Res Commun. 2007;354(3):627-633.
d. Singh P, Paila YD, Chattopadhyay A. Differential effects of cholesterol and 7-dehydrocholesterol on the ligand binding activity of the hippocampal serotonin(1A) receptor: implications in SLOS. Biochem Biophys Res Commun. 2007;358(2):495-499.
e. Sjögren B, Hamblin MW, Svenningsson P. Cholesterol depletion reduces serotonin binding and signaling via human 5-HT(7(a)) receptors. Eur J Pharmacol. 2006;552(1-3):1-10.
f. Scanlon SM, Williams DC, Schloss P. Membrane cholesterol modulates serotonin transporter activity. Biochemistry. 2001;40(35):10507-10513.
g. Vevera J, Fisar Z, Kvasnicka T, et al. Cholesterol-lowering therapy evokes time-limited changes in serotonergic transmission. Psychiatry Res. 2005;133(2-3):197-203.
h. Sooksawate T, Simmonds MA. Effects of membrane cholesterol on the sensitivity of the GABA(A) receptor to GABA in acutely dissociated rat hippocampal neurones. Neuropharmacology. 2001;40(2):178-184.
i. Abulrob A, Tauskela JS, Mealing G, et al. Protection by cholesterol-extracting cyclodextrins: a role for N-methyl-daspartate receptor redistribution. J Neurochem. 2005;92(6):1477-1486.
j. Huang P, Xu W, Yoon SI, et al. Cholesterol reduction by methyl-beta-cyclodextrin attenuates the delta opioid receptor-mediated signaling in neuronal cells but enhances it in non-neuronal cells. Biochem Pharmacol. 2007;73(4):534-549.
k. Butchbach ME, Tian G, Guo H, et al. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function. J Biol Chem. 2004;279(33):34388-34396.
l. Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Annals N Y Acad Sci. 1997;836:288-301.
m. Garland M, Hickey D, Corvin A, et al. Total serum cholesterol in relation to psychological correlates in parasuicide. Br J Psychiatry. 2000;177:77-83.
n. Ormiston T, Wolkowitz OM, Reus VI, et al. Behavioral implications of lowering cholesterol levels: a double-blind pilot study. Psychosomatics. 2003;44(5):412-414.
o. Golomb BA. Cholesterol and violence: is there a connection? Ann Intern Med. 1998;128(6):478-487.
Table 1
Psychiatric features associated with low cholesterol*
| Symptoms |
| Anxiety, depressed mood, emotional lability, euphoria, impulsivity, irritability, suicidal ideation, aggression |
| Syndromes |
| Anorexia nervosa, bipolar disorder, borderline personality disorder, major depressive disorder, seasonal affective disorder |
| Behaviors |
| Suicide and suicide attempts, violence |
| *Small studies have suggested possible relationships with dissociative and panic disorders |
Effects of lipid-lowering agents
If there is a causal relationship between low cholesterol and mood disorders, then it stands to reason that using cholesterol-lowering drugs would increase the risk of depression and suicide. However, the data do not support that conclusion.
Many case reports have documented adverse psychiatric reactions to statins, including depression, suicidality, emotional lability, agitation, irritability, anxiety, panic, and euphoria.17 In an early analysis of primary prevention trials, patients receiving cholesterol-lowering treatment—mainly non-statins—were estimated to have twice the risk of death by suicide or violence compared with controls.3 However, a more recent meta-analysis of larger clinical trials of lipid-lowering agents including statins and observational studies did not reveal an association between lipid-lowering medications and suicide.15,18
In a large case-control study, statin users had a lower risk of depression (adjusted odds ratio [OR] 0.4, 95% confidence interval [CI], 0.2 to 0.9) than patients taking non-statin lipid-lowering drugs (adjusted OR 1.0, 95% CI, 0.5 to 2.1).19 However, statins reduced cholesterol more (30% to 50%) than non-statin drugs (10% to 20%). A clinical trial of >1,000 patients with stable coronary artery disease treated with pravastatin—an HMG-CoA reductase inhibitor with low lipophilicity that is less likely than other statins to cross the blood-brain barrier—revealed no changes in self-reported anger, impulsiveness, anxiety, or depression.20
This study did not exclude patients with psychiatric illness—who are at greatest risk of suicide—but other trials of lipid-lowering drugs did.21 As a result, the effects of lipid-lowering medications on psychiatric patients are unclear. A clinical trial is underway to assess the effects of pravastatin (low lipophilicity), simvastatin (high lipophilicity), or placebo on mood, sleep, and aggression.21
Low cholesterol: State or trait?
Much of the research linking low cholesterol, mood disorders, and suicidality could be confounded by depressed mood leading to reduced serum cholesterol. There has been considerable debate about whether low cholesterol predisposes patients to suicide or if depression independently leads to poor nutrition and therefore low cholesterol and increased suicide risk.6,22
Some researchers have suggested that depression lowers cholesterol and increases risk of suicide,23 but study designs have limited the ability to discern the directionality of the relationship. Attempts to control for depression-related malnutrition and weight loss—which lowers total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C)24—suggest the association may be independent of these variables.25-27 These findings suggest that cholesterol may be considered a trait marker and is not entirely state-dependent. However, multiple, large, long-term randomized controlled trials have not shown increased depression and suicide with use of lipid-lowering agents in healthy populations.20
The Figure illustrates known epidemiologic associations of low cholesterol, low serotoninergic function, and suicide and contrasts conceptual models of cholesterol as a state and a trait marker. A case can be made for cholesterol as both a state and a trait marker, and these models could overlap, with depression-induced decreases in cholesterol further mediating changes in serotonergic function and related behavioral sequelae.
Figure
Cholesterol, depression, and suicide: How are they linked? 
Low cholesterol may be considered a trait marker, predisposing patients to lower serotonergic function and placing them at greater risk for impulsivity, depression, aggression, and suicide. Other models suggest that lower cholesterol is a state-dependent consequence of depression, and not part of a causal chain toward suicide
Improving cardiac health
Limited epidemiologic studies suggest that patients with mood disorders may have lower levels of total cholesterol and LDL-C, but higher rates of hypertriglyceridemia compared with the general population.8 Unfortunately, psychiatric patients—who may be at increased risk of developing cardiovascular disease—may be less likely to be screened and appropriately treated for lipid abnormalities.28 To address this disparity, consider assuming an active role in assessing and managing hyperlipidemia in your patients with mood disorders. Be aware of your patients’ lipid profile and ensure that they follow monitoring recommendations.
The National Cholesterol Education Program recommends screening all adults age >20 for hyperlipidemia every 5 years using measures of total cholesterol, LDL-C, HDL-C, and triglycerides. If LDL-C or triglycerides exceed target values (Table 2), appropriate management includes recommending lifestyle changes and pharmacotherapy (Box 2).
Patients should receive a fasting lipid profile before and 12 weeks after starting any antipsychotic and semiannually thereafter.29 Consider closely monitoring lipids when patients gain weight with psychotropics. Refer patients with hyperlipidemia to a primary care physician, but in the absence of such a provider, mental health clinicians who are familiar with treatment guidelines can manage these patients.30
Closely monitor individuals with mood disorders for changes in behavior or mental status after starting a lipid-lowering agent. Consider discontinuing the drug if a patient develops an adverse reaction. If symptoms return after medication rechallenge, consider other management strategies such as an alternate lipid-lowering agent or re-emphasizing behavioral measures.
Table 2
National Cholesterol Education Program recommended LDL levels
| Risk category* | LDL goal | When to consider medications |
|---|---|---|
| CHD or CHD equivalent | <100 mg/dL | ≥130 mg/dL |
| ≥2 major risk factors | <130 mg/dL | ≥130 to 160 mg/dL (based on 10-year risk) |
| 0 or 1 risk factor | <160 mg/dL | ≥190 mg/dL |
| CHD: coronary heart disease; HDL: high-density lipoprotein; LDL: low-density lipoprotein | ||
| *Risk category is based on the presence of CHD or equivalent and major risk factors for CHD. CHD equivalents include symptomatic carotid artery disease, peripheral artery disease, and abdominal aortic aneurysm. Major risk factors include smoking, hypertension, low HDL, family history, and age. LDL levels to consider medications for those with ≥2 major risk factors vary by 10-year CHD risk | ||
| Source: National Cholesterol Education Program, Adult Treatment Panel III (ATP III) Quick Desk Reference. www.nhlbi.nih.gov/guidelines/cholesterol/atglance.htm | ||
National Cholesterol Education Program guidelines state that when a patient’s low-density lipoprotein cholesterol (LDL-C) exceeds targets (Table 2), first recommend lifestyle changes such as a diet low in saturated fat (<7% of calories) and cholesterol (<200 mg/d), weight management, and exercise. Increases in soluble fiber (10 to 25 g/d) and plant stanols/sterols also may be considered. If LDL-C levels are still too high, pharmacologic therapy such as an HMGCoA reductase inhibitor is suggested.
Treatment of elevated triglycerides (≥150 mg/dL) includes reaching the target LDL-C, intensifying a weight management program, and increasing exercise. Address quitting smoking and limiting alcohol when indicated. If triglyceride levels are ≥200 mg/dL after the LDL-C target is reached, set a secondary goal of reaching a target non-high-density lipoprotein cholesterol (HDL-C) (non-HDL-C; total cholesterol minus HDL-C) 30 mg/dL greater than the LDL goal. This can be achieved by adding an LDL-lowering drug such as a statin, nicotinic acid, or ezetimibe. When triglycerides are ≥500 mg/dL, more aggressive intervention, such as with a fibrate, omega-3 fatty acids, very low-fat diets, and exercise, is required to prevent pancreatitis.
Source: National Heart Lung and Blood Institute. National Cholesterol Education Program. www.nhlbi.nih.gov/guidelines/cholesterol/index.htm
Related Resources
- Fiedorowicz JG, Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. 2007;152(1):11-20.
- National Cholesterol Education Program, Adult Treatment Panel III (ATP III) Quick Desk Reference. www.nhlbi.nih.gov/guidelines/cholesterol/atglance.htm.
- Executive Summary of the third report of the national Cholesterol Education Program (nCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-2497.
Drug Brand Names
- Ezetimibe • Zetia
- Pravastatin • Pravachol
- Simvastatin • Zocor
Acknowledgements
Dr. Fiedorowicz thanks Lois Warren and Miriam Weiner for their editorial assistance.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fiedorowicz is supported by the national Institutes of Health (1K23MH083695-01A210), nARSAD, and the Institute for Clinical and Translational Science at the University of Iowa (3 UL1 RR024979-03S4). He has received support for participating in a colleague’s investigator-initiated project with Eli Lilly. Dr. Haynes’ research is supported by grants from the national Institutes of Health (nHLBI: HL58972 & HL14388; nCRR CTSA: 1UL1RR024979).
1. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844-850.
2. Lindberg G, Råstam L, Gullberg B, et al. Low serum cholesterol concentration and short term mortality from injuries in men and women. BMJ. 1992;305(6848):277-279.
3. Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301(6747):309-314.
4. Neaton JD, Blackburn H, Jacobs D, et al. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med. 1992;152(7):1490-1500.
5. Fiedorowicz JG, Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. 2007;152(1):11-20.
6. Golomb BA. Cholesterol and violence: is there a connection? Ann Intern Med. 1998;128(6):478-487.
7. Pae CU, Kim JJ, Lee SJ, et al. Aberration of cholesterol level in first-onset bipolar I patients. J Affect Disord. 2004;83(1):79-82.
8. Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, et al. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20(3):131-137.
9. Chung KH, Tsai SY, Lee HC. Mood symptoms and serum lipids in acute phase of bipolar disorder in Taiwan. Psychiatry Clin Neurosci. 2007;61(4):428-433.
10. Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord. 2006;90(1):21-27.
11. Sagud M, Mihaljevic-Peles A, Pivac N, et al. Platelet serotonin and serum lipids in psychotic mania. J Affect Disord. 2007;97(1-3):247-251.
12. Beasley CL, Honer WG, Bergmann K, et al. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord. 2005;7(5):449-455.
13. Gabriel A. Changes in plasma cholesterol in mood disorder patients: does treatment make a difference? J Affect Disord. 2007;99(1-3):273-278.
14. Lalovic A, Levy E, Luheshi G, et al. Cholesterol content in brains of suicide completers. Int J Neuropsychopharmacol. 2007;10(2):159-166.
15. Lester D. Serum cholesterol levels and suicide: a meta-analysis. Suicide Life Threat Behav. 2002;32(3):333-346.
16. Coryell W, Schlesser M. Combined biological tests for suicide prediction. Psychiatry Res. 2007;150(2):187-191.
17. Tatley M, Savage R. Psychiatric adverse reactions with statins, fibrates and ezetimibe: implications for the use of lipid-lowering agents. Drug Saf. 2007;30(3):195-201.
18. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
19. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
20. Stewart RA, Sharples KJ, North FM, et al. Long-term assessment of psychological well-being in a randomized placebo-controlled trial of cholesterol reduction with pravastatin. The LIPID Study Investigators. Arch Intern Med. 2000;160(20):3144-3152.
21. Golomb BA, Criqui MH, White HL, et al. The UCSD Statin Study: a randomized controlled trial assessing the impact of statins on selected noncardiac outcomes. Control Clin Trials. 2004;25(2):178-202.
22. Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Annals N Y Acad Sci. 1997;836:288-301.
23. Law MR, Thompson SG, Wald NJ. Assessing possible hazards of reducing serum cholesterol. BMJ. 1994;308(6925):373-379.
24. Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56(2):320-328.
25. Garland M, Hickey D, Corvin A, et al. Total serum cholesterol in relation to psychological correlates in parasuicide. Br J Psychiatry. 2000;177:77-83.
26. Golier JA, Marzuk PM, Leon AC, et al. Low serum cholesterol level and attempted suicide. Am J Psychiatry. 1995;152(3):419-423.
27. Kunugi H, Takei N, Aoki H, et al. Low serum cholesterol in suicide attempters. Biol Psychiatry. 1997;41(2):196-200.
28. Murray DP, Weiner M, Prabhakar M, et al. Mania and mortality: why the excess cardiovascular risk in bipolar disorder? Curr Psychiatry Rep. 2009;11(6):475-480.
29. Sernyak MJ. Implementation of monitoring and management guidelines for second-generation antipsychotics. J Clin Psychiatry. 2007;68(suppl 4):14-18.
30. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334-1349.
1. Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844-850.
2. Lindberg G, Råstam L, Gullberg B, et al. Low serum cholesterol concentration and short term mortality from injuries in men and women. BMJ. 1992;305(6848):277-279.
3. Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. BMJ. 1990;301(6747):309-314.
4. Neaton JD, Blackburn H, Jacobs D, et al. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med. 1992;152(7):1490-1500.
5. Fiedorowicz JG, Coryell WH. Cholesterol and suicide attempts: a prospective study of depressed inpatients. Psychiatry Res. 2007;152(1):11-20.
6. Golomb BA. Cholesterol and violence: is there a connection? Ann Intern Med. 1998;128(6):478-487.
7. Pae CU, Kim JJ, Lee SJ, et al. Aberration of cholesterol level in first-onset bipolar I patients. J Affect Disord. 2004;83(1):79-82.
8. Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, et al. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20(3):131-137.
9. Chung KH, Tsai SY, Lee HC. Mood symptoms and serum lipids in acute phase of bipolar disorder in Taiwan. Psychiatry Clin Neurosci. 2007;61(4):428-433.
10. Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord. 2006;90(1):21-27.
11. Sagud M, Mihaljevic-Peles A, Pivac N, et al. Platelet serotonin and serum lipids in psychotic mania. J Affect Disord. 2007;97(1-3):247-251.
12. Beasley CL, Honer WG, Bergmann K, et al. Reductions in cholesterol and synaptic markers in association cortex in mood disorders. Bipolar Disord. 2005;7(5):449-455.
13. Gabriel A. Changes in plasma cholesterol in mood disorder patients: does treatment make a difference? J Affect Disord. 2007;99(1-3):273-278.
14. Lalovic A, Levy E, Luheshi G, et al. Cholesterol content in brains of suicide completers. Int J Neuropsychopharmacol. 2007;10(2):159-166.
15. Lester D. Serum cholesterol levels and suicide: a meta-analysis. Suicide Life Threat Behav. 2002;32(3):333-346.
16. Coryell W, Schlesser M. Combined biological tests for suicide prediction. Psychiatry Res. 2007;150(2):187-191.
17. Tatley M, Savage R. Psychiatric adverse reactions with statins, fibrates and ezetimibe: implications for the use of lipid-lowering agents. Drug Saf. 2007;30(3):195-201.
18. Callréus T, Agerskov Andersen U, Hallas J, et al. Cardiovascular drugs and the risk of suicide: a nested case-control study. Eur J Clin Pharmacol. 2007;63(6):591-596.
19. Yang CC, Jick SS, Jick H. Lipid-lowering drugs and the risk of depression and suicidal behavior. Arch Intern Med. 2003;163(16):1926-1932.
20. Stewart RA, Sharples KJ, North FM, et al. Long-term assessment of psychological well-being in a randomized placebo-controlled trial of cholesterol reduction with pravastatin. The LIPID Study Investigators. Arch Intern Med. 2000;160(20):3144-3152.
21. Golomb BA, Criqui MH, White HL, et al. The UCSD Statin Study: a randomized controlled trial assessing the impact of statins on selected noncardiac outcomes. Control Clin Trials. 2004;25(2):178-202.
22. Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Annals N Y Acad Sci. 1997;836:288-301.
23. Law MR, Thompson SG, Wald NJ. Assessing possible hazards of reducing serum cholesterol. BMJ. 1994;308(6925):373-379.
24. Dattilo AM, Kris-Etherton PM. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr. 1992;56(2):320-328.
25. Garland M, Hickey D, Corvin A, et al. Total serum cholesterol in relation to psychological correlates in parasuicide. Br J Psychiatry. 2000;177:77-83.
26. Golier JA, Marzuk PM, Leon AC, et al. Low serum cholesterol level and attempted suicide. Am J Psychiatry. 1995;152(3):419-423.
27. Kunugi H, Takei N, Aoki H, et al. Low serum cholesterol in suicide attempters. Biol Psychiatry. 1997;41(2):196-200.
28. Murray DP, Weiner M, Prabhakar M, et al. Mania and mortality: why the excess cardiovascular risk in bipolar disorder? Curr Psychiatry Rep. 2009;11(6):475-480.
29. Sernyak MJ. Implementation of monitoring and management guidelines for second-generation antipsychotics. J Clin Psychiatry. 2007;68(suppl 4):14-18.
30. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334-1349.
Treating insomnia across women’s life stages
Discuss this article at http://currentpsychiatry.blogspot.com/2010/07/treating-insomnia-in-women.html#comments
Ms. A, age 44, reports a 3-month history of forgetfulness, difficulty concentrating, and insomnia. She says she can fall asleep but wakes up multiple times during the night and feels tired during the day. She has no history of a mood or anxiety disorder or medications that might be responsible for her symptoms.
Before her current insomnia began, Ms. A could sleep for 7 to 8 hours at night. Her husband suffers from obstructive sleep apnea (OSA), and his snoring occasionally would awaken her, but she slept well overall. Ms. A cannot identify anything that could be causing her sleep complaints. She states “The weird thing is that sometimes I am not sure if I’m cold or hot” and “I sometimes wake up drenched in sweat.” She also reports recent changes in the timing of her otherwise regular menstrual flow.
Ms. A attributes her memory problems to her poor sleep. A recent audit at her company held her responsible for several accounting errors, and Ms. A is worried that she might lose her job. She denies symptoms that would suggest major depression. You are unable to elicit a history of limb movements or excessive snoring.
Compared with men, women have a 1.3- to 1.8-fold greater risk for developing insomnia.Improve sleep with group CBT for insomnia,” Current Psychiatry, April 2009.) Pharmacotherapy during pregnancy and for breast-feeding mothers is guided by evaluating the risk/benefit ratio and safety considerations.
Maintain a high index of suspicion for breathing-related sleep disorders, such as OSA,21 and RLS.22 Atypical presentations of OSA are common in pregnant or postpartum women; compared with men, women with OSA are more likely to report fatigue and less likely than to report sleepiness. Refer patients whom you think may have OSA for polysomnography.
If you suspect RLS, check for low ferritin and folate levels. Nutritional supplements may be necessary for women in high-risk groups, including those who are pregnant or have varicose veins, venous reflux, folate deficiency, uremia, diabetes, thyroid problems, peripheral neuropathy, Parkinson’s disease, or certain autoimmune disorders, such as Sjögren’s syndrome, celiac disease, and rheumatoid arthritis.23 Advise these patients to avoid caffeine.
Although indicated for treating RLS, ropinirole and pramipexole are FDA Pregnancy Category C, which means animal studies have shown adverse effects on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite risks. Opioids, carbamazepine, or gabapentin may be safer for pregnant patients.24
Insomnia during menopause
The prevalence of insomnia increases from 33% to 36% in premenopausal women to 44% to 61% in postmenopausal women.14 Hot flashes, comorbid mood disturbances, sleep-disordered breathing, and RLS contribute to increased insomnia risk in postmenopausal women (Table 3).4,14,25,26
Treatment strategy. Always inquire about sleep in perimenopausal/postmenopausal women, even when her presenting complaint is related to menstrual cycle changes or vasomotor symptoms such as hot flashes.16 Assess patients for OSA, RLS, and mood, anxiety, and cognitive symptoms.26 In addition to pharmacotherapy and behavioral therapy, treatment options include hormone replacement therapy (HRT) and herbal and dietary supplements (Table 4).27-32
Table 3
Sleep difficulties during menopause: Differential diagnoses
| Condition | Features | Findings | Other considerations |
|---|---|---|---|
| Hot flashes (prevalence: 75% to 85%)14 | Vasomotor phenomenon characterized by feelings such as ‘spreading warmth,’ diaphoresis, palpitations, nausea, and insomnia Mediated through the preoptic area of the anterior hypothalamus, which regulates temperature and sleep Increased brain norepinephrine metabolism | Discrepancies between objective (PSG) and subjective measures (surveys)4 Discrepancies between self-reported and laboratory reported sleep data might be explained by thermoregulatory differences between NREM and REM sleep24 | Nocturnal hot flashes trigger awakenings and insomnia14 Hot flashes can follow arousals and awakenings HRT is highly effective in treating hot flashes; however, data on its direct effects on sleep complaints are inconsistent |
| Primary menopausal insomnia25 | Menopausal symptoms (eg, hot flashes) trigger insomnia that persists secondary to behavioral conditioning | Increase in nocturnal skin temperature coincides with decrease in skin resistance and waking episodes in PSG | Behavioral insomnia therapies are useful adjuncts to treatment of menopause symptoms |
| Sleep-disordered breathing (OSA) | Menopause increases risk for OSA independent of body weight Redistribution of body fat with an increase in the waist-to-hip circumference ratio occurs in menopause Loss of ventilatory drive because of diminished progesterone levels | Sleep fragmentation and daytime sleepiness are common, as opposed to apneic episodes or oxygen desaturation in men | Maintain a high index of suspicion and promptly refer patients to a sleep center |
| Restless legs syndrome | Related to iron deficiency | Low ferritin and folate levels | Advise patients to avoid caffeine |
| HRT: hormone replacement therapy; NREM: non-rapid eye movement; OSA: obstructive sleep apnea; PSG: polysomnography; REM: rapid eye movement | |||
Table 4
Treating insomnia in menopausal women
| Therapy | Comments |
|---|---|
| Hormone replacement therapy (HRT) | Effective for hot flashes, insomnia,26-28 and sleep apnea29 Long-term safety is questionable4 |
| Behavioral therapy (cognitive-behavioral therapy,30 stimulus control therapy, sleep restriction therapy, sleep hygiene, hypnotherapy, biofeedback) | Limited data in menopausal women |
| Sedatives/hypnotics/antidepressants (eg, zolpidem, 10 mg; eszopiclone, 3 mg; trazodone, 75 mg; ramelteon, 8 mg; SSRIs and SNRIs) | Benzodiazepines may be useful, although not specifically evaluated in menopausal women. Risk of tolerance, dependence, and psychomotor slowing |
| Herbal and dietary supplements (Cimicifuga racemosa [Black cohosh],31 valerian | Popular alternatives to HRT; however, evidence of efficacy as treatment for insomnia is inconclusive |
| SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors | |
Comorbid psychiatric disorders
Women have a higher prevalence of psychiatric disorders such as major depressive disorder and anxiety disorders than men.1 Women have a 10% to 25% lifetime risk of developing major depression. Three quarters of depressed patients experience insomnia.1 Recent literature suggests insomnia is a risk factor for depression,33 which emphasizes the need to screen women who present with sleep problems for depression and anxiety.
Five percent to 20% of women experience postpartum depression. Depression and insomnia are correlated to the rapid decline in estrogen and progesterone after delivery.34
Treatment strategy. Insomnia is a common presenting symptom in patients with psychiatric conditions such as mood and anxiety disorders. Treating the underlying psychiatric disorder often alleviates sleeping difficulties. However, if the insomnia is disabling, treat the psychiatric disorder and insomnia concurrently.
CASE CONTINUED: Perimenopausal insomnia
Based on her history, you diagnose Ms. A with insomnia related to general medical condition (perimenopause). There are no indications to refer her for polysomnography. You educate Ms. A about sleep hygiene and recommend that she discuss her menstrual and physical complaints with her primary care physician or gynecologist. Ms. A is not interested in HRT because she has a strong family history of endometrial cancer. You reassure Ms. A and schedule a follow-up visit in 2 months to re-evaluate her insomnia.
Related resource
- Krahn LE. Perimenopausal depression? Ask how she’s sleeping. Current Psychiatry. 2005;4(6):39-53.
Drug brand names
- Carbamazepine • Carbatrol, Tegretol, others
- Escitalopram • Lexapro
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Gabapentin • Neurontin, Gabarone
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Ramelteon • Rozerem
- Ropinirole • Requip
- Sertraline • Zoloft
- Trazodone • Desyrel
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors thank Dr. Namita Dhiman and Darrel E. Willoughby for their assistance with this article.
1. Krystal AD. Depression and insomnia in women. Clin Cornerstone. 2004;6(suppl 1B):S19-S28.
2. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97-111.
3. Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12(6):383-389.
4. Soares CN, Murray BJ. Sleep disorders in women: clinical evidence and treatment strategies. Psychiatr Clin North Am. 2006;29(4):1095-1113.
5. Ohayon M. Epidemiological study on insomnia in the general population. Sleep. 1996;19(3 suppl):S7-S15.
6. Daley M, Morin CM, LeBlanc M, et al. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009;10(4):427-438.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
8. Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;2(suppl 2):S347-S353.
9. Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85-93.
10. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28(2):193-213.
11. Manber R, Bootzin RR. Sleep and the menstrual cycle. Health Psychol. 1997;16:209-214.
12. Ito M, Kohsaka M, Fukuda N, et al. Effects of menstrual cycle on plasma melatonin level and sleep characteristics. Jpn J Psychiatry Neurol. 1993;47:478-479.
13. Driver HS, Dijk DJ, Werth E, et al. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81:728-735.
14. Moline ML, Broch L, Zak R. Sleep in women across the life cycle from adulthood through menopause. Med Clin North Am. 2004;88(3):705-736.
15. Steiner M, Pearlstein T, Cohen LS, et al. Expert guidelines for the treatment of severe PMS, PMDD, and comorbidities: the role of SSRIs. J Womens Health (Larchmt). 2006;15(1):57-69.
16. Krystal AD. Insomnia in women. Clin Cornerstone. 2003;5(3):41-50.
17. Mindell JA, Jacobson BJ. Sleep disturbances during pregnancy. J Obstet Gynecol Neonatal Nurs. 2000;29(6):590-597.
18. Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95(1):14-18.
19. Brunner DP, Münch M, Biedermann K, et al. Changes in sleep and sleep electroencephalogram during pregnancy. Sleep. 1994;17(7):576-582.
20. Ross LE, Murray BJ, Steiner M. Sleep and perinatal mood disorders: a critical review. J Psychiatry Neurosci. 2005;30(4):247-256.
21. Edwards N, Middleton PG, Blyton DM, et al. Sleep disordered breathing and pregnancy. Thorax. 2002;57(6):555-558.
22. Manconi M, Govoni V, De Vito A, et al. Restless legs syndrome and pregnancy. Neurology. 2004;63(6):1065-1069.
23. Lee KA, Zaffke ME, Baratte-Beebe K. Restless legs syndrome and sleep disturbance during pregnancy: the role of folate and iron. J Womens Health Gend Based Med. 2001;10(4):335-341.
24. Djokanovic N, Garcia-Bournissen F, Koren G. Medications for restless legs syndrome in pregnancy. J Obstet Gynaecol Can. 2008;30(6):505-507.
25. Freedman RR, Roehrs TA. Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause. 2006;13(4):576-583.
26. Krystal AD, Edinger J, Wohlgemuth W, et al. Sleep in perimenopausal and postmenopausal women. Sleep Med Rev. 1998;2(4):243-253.
27. Polo-Kantola P, Erkkola R, Irjala K, et al. Effect of short-term transdermal estrogen replacement therapy on sleep: a randomized, double-blind crossover trial in postmenopausal women. Fertil Steril. 1999;71(5):873-880.
28. Watts NB, Notelovitz M, Timmons MC, et al. Comparison of oral estrogens and estrogens plus androgen on bone mineral density, menopausal symptoms, and lipid-lipoprotein profiles in surgical menopause. Obstet Gynecol. 1995;85(4):529-537.Erratum in: Obstet Gynecol 1995;85(5 Pt 1):668.
29. Boyle GJ, Murrihy R. A preliminary study of hormone replacement therapy and psychological mood states in perimenopausal women. Psychol Rep. 2001;88(1):160-170.
30. Cistulli PA, Barnes DJ, Grunstein RR, et al. Effect of short-term hormone replacement in the treatment of obstructive sleep apnoea in postmenopausal women. Thorax. 1994;49:699-702.
31. Yang CM, Spielman AJ, Glovinsky P. Nonpharmacologic strategies in the management of insomnia. Psychiatr Clin North Am. 2006;29(4):895-919.
32. Mahady GB. Black cohosh (Actaea/Cimicifuga racemosa): review of the clinical data for safety and efficacy in menopausal symptoms. Treat Endocrinol. 2005;4(3):177-184.
33. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
34. Burt VK, Stein K. Epidemiology of depression throughout the female life cycle. J Clin Psychiatry. 2002;63(suppl 7):9-15.
Discuss this article at http://currentpsychiatry.blogspot.com/2010/07/treating-insomnia-in-women.html#comments
Ms. A, age 44, reports a 3-month history of forgetfulness, difficulty concentrating, and insomnia. She says she can fall asleep but wakes up multiple times during the night and feels tired during the day. She has no history of a mood or anxiety disorder or medications that might be responsible for her symptoms.
Before her current insomnia began, Ms. A could sleep for 7 to 8 hours at night. Her husband suffers from obstructive sleep apnea (OSA), and his snoring occasionally would awaken her, but she slept well overall. Ms. A cannot identify anything that could be causing her sleep complaints. She states “The weird thing is that sometimes I am not sure if I’m cold or hot” and “I sometimes wake up drenched in sweat.” She also reports recent changes in the timing of her otherwise regular menstrual flow.
Ms. A attributes her memory problems to her poor sleep. A recent audit at her company held her responsible for several accounting errors, and Ms. A is worried that she might lose her job. She denies symptoms that would suggest major depression. You are unable to elicit a history of limb movements or excessive snoring.
Compared with men, women have a 1.3- to 1.8-fold greater risk for developing insomnia.Improve sleep with group CBT for insomnia,” Current Psychiatry, April 2009.) Pharmacotherapy during pregnancy and for breast-feeding mothers is guided by evaluating the risk/benefit ratio and safety considerations.
Maintain a high index of suspicion for breathing-related sleep disorders, such as OSA,21 and RLS.22 Atypical presentations of OSA are common in pregnant or postpartum women; compared with men, women with OSA are more likely to report fatigue and less likely than to report sleepiness. Refer patients whom you think may have OSA for polysomnography.
If you suspect RLS, check for low ferritin and folate levels. Nutritional supplements may be necessary for women in high-risk groups, including those who are pregnant or have varicose veins, venous reflux, folate deficiency, uremia, diabetes, thyroid problems, peripheral neuropathy, Parkinson’s disease, or certain autoimmune disorders, such as Sjögren’s syndrome, celiac disease, and rheumatoid arthritis.23 Advise these patients to avoid caffeine.
Although indicated for treating RLS, ropinirole and pramipexole are FDA Pregnancy Category C, which means animal studies have shown adverse effects on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite risks. Opioids, carbamazepine, or gabapentin may be safer for pregnant patients.24
Insomnia during menopause
The prevalence of insomnia increases from 33% to 36% in premenopausal women to 44% to 61% in postmenopausal women.14 Hot flashes, comorbid mood disturbances, sleep-disordered breathing, and RLS contribute to increased insomnia risk in postmenopausal women (Table 3).4,14,25,26
Treatment strategy. Always inquire about sleep in perimenopausal/postmenopausal women, even when her presenting complaint is related to menstrual cycle changes or vasomotor symptoms such as hot flashes.16 Assess patients for OSA, RLS, and mood, anxiety, and cognitive symptoms.26 In addition to pharmacotherapy and behavioral therapy, treatment options include hormone replacement therapy (HRT) and herbal and dietary supplements (Table 4).27-32
Table 3
Sleep difficulties during menopause: Differential diagnoses
| Condition | Features | Findings | Other considerations |
|---|---|---|---|
| Hot flashes (prevalence: 75% to 85%)14 | Vasomotor phenomenon characterized by feelings such as ‘spreading warmth,’ diaphoresis, palpitations, nausea, and insomnia Mediated through the preoptic area of the anterior hypothalamus, which regulates temperature and sleep Increased brain norepinephrine metabolism | Discrepancies between objective (PSG) and subjective measures (surveys)4 Discrepancies between self-reported and laboratory reported sleep data might be explained by thermoregulatory differences between NREM and REM sleep24 | Nocturnal hot flashes trigger awakenings and insomnia14 Hot flashes can follow arousals and awakenings HRT is highly effective in treating hot flashes; however, data on its direct effects on sleep complaints are inconsistent |
| Primary menopausal insomnia25 | Menopausal symptoms (eg, hot flashes) trigger insomnia that persists secondary to behavioral conditioning | Increase in nocturnal skin temperature coincides with decrease in skin resistance and waking episodes in PSG | Behavioral insomnia therapies are useful adjuncts to treatment of menopause symptoms |
| Sleep-disordered breathing (OSA) | Menopause increases risk for OSA independent of body weight Redistribution of body fat with an increase in the waist-to-hip circumference ratio occurs in menopause Loss of ventilatory drive because of diminished progesterone levels | Sleep fragmentation and daytime sleepiness are common, as opposed to apneic episodes or oxygen desaturation in men | Maintain a high index of suspicion and promptly refer patients to a sleep center |
| Restless legs syndrome | Related to iron deficiency | Low ferritin and folate levels | Advise patients to avoid caffeine |
| HRT: hormone replacement therapy; NREM: non-rapid eye movement; OSA: obstructive sleep apnea; PSG: polysomnography; REM: rapid eye movement | |||
Table 4
Treating insomnia in menopausal women
| Therapy | Comments |
|---|---|
| Hormone replacement therapy (HRT) | Effective for hot flashes, insomnia,26-28 and sleep apnea29 Long-term safety is questionable4 |
| Behavioral therapy (cognitive-behavioral therapy,30 stimulus control therapy, sleep restriction therapy, sleep hygiene, hypnotherapy, biofeedback) | Limited data in menopausal women |
| Sedatives/hypnotics/antidepressants (eg, zolpidem, 10 mg; eszopiclone, 3 mg; trazodone, 75 mg; ramelteon, 8 mg; SSRIs and SNRIs) | Benzodiazepines may be useful, although not specifically evaluated in menopausal women. Risk of tolerance, dependence, and psychomotor slowing |
| Herbal and dietary supplements (Cimicifuga racemosa [Black cohosh],31 valerian | Popular alternatives to HRT; however, evidence of efficacy as treatment for insomnia is inconclusive |
| SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors | |
Comorbid psychiatric disorders
Women have a higher prevalence of psychiatric disorders such as major depressive disorder and anxiety disorders than men.1 Women have a 10% to 25% lifetime risk of developing major depression. Three quarters of depressed patients experience insomnia.1 Recent literature suggests insomnia is a risk factor for depression,33 which emphasizes the need to screen women who present with sleep problems for depression and anxiety.
Five percent to 20% of women experience postpartum depression. Depression and insomnia are correlated to the rapid decline in estrogen and progesterone after delivery.34
Treatment strategy. Insomnia is a common presenting symptom in patients with psychiatric conditions such as mood and anxiety disorders. Treating the underlying psychiatric disorder often alleviates sleeping difficulties. However, if the insomnia is disabling, treat the psychiatric disorder and insomnia concurrently.
CASE CONTINUED: Perimenopausal insomnia
Based on her history, you diagnose Ms. A with insomnia related to general medical condition (perimenopause). There are no indications to refer her for polysomnography. You educate Ms. A about sleep hygiene and recommend that she discuss her menstrual and physical complaints with her primary care physician or gynecologist. Ms. A is not interested in HRT because she has a strong family history of endometrial cancer. You reassure Ms. A and schedule a follow-up visit in 2 months to re-evaluate her insomnia.
Related resource
- Krahn LE. Perimenopausal depression? Ask how she’s sleeping. Current Psychiatry. 2005;4(6):39-53.
Drug brand names
- Carbamazepine • Carbatrol, Tegretol, others
- Escitalopram • Lexapro
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Gabapentin • Neurontin, Gabarone
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Ramelteon • Rozerem
- Ropinirole • Requip
- Sertraline • Zoloft
- Trazodone • Desyrel
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors thank Dr. Namita Dhiman and Darrel E. Willoughby for their assistance with this article.
Discuss this article at http://currentpsychiatry.blogspot.com/2010/07/treating-insomnia-in-women.html#comments
Ms. A, age 44, reports a 3-month history of forgetfulness, difficulty concentrating, and insomnia. She says she can fall asleep but wakes up multiple times during the night and feels tired during the day. She has no history of a mood or anxiety disorder or medications that might be responsible for her symptoms.
Before her current insomnia began, Ms. A could sleep for 7 to 8 hours at night. Her husband suffers from obstructive sleep apnea (OSA), and his snoring occasionally would awaken her, but she slept well overall. Ms. A cannot identify anything that could be causing her sleep complaints. She states “The weird thing is that sometimes I am not sure if I’m cold or hot” and “I sometimes wake up drenched in sweat.” She also reports recent changes in the timing of her otherwise regular menstrual flow.
Ms. A attributes her memory problems to her poor sleep. A recent audit at her company held her responsible for several accounting errors, and Ms. A is worried that she might lose her job. She denies symptoms that would suggest major depression. You are unable to elicit a history of limb movements or excessive snoring.
Compared with men, women have a 1.3- to 1.8-fold greater risk for developing insomnia.Improve sleep with group CBT for insomnia,” Current Psychiatry, April 2009.) Pharmacotherapy during pregnancy and for breast-feeding mothers is guided by evaluating the risk/benefit ratio and safety considerations.
Maintain a high index of suspicion for breathing-related sleep disorders, such as OSA,21 and RLS.22 Atypical presentations of OSA are common in pregnant or postpartum women; compared with men, women with OSA are more likely to report fatigue and less likely than to report sleepiness. Refer patients whom you think may have OSA for polysomnography.
If you suspect RLS, check for low ferritin and folate levels. Nutritional supplements may be necessary for women in high-risk groups, including those who are pregnant or have varicose veins, venous reflux, folate deficiency, uremia, diabetes, thyroid problems, peripheral neuropathy, Parkinson’s disease, or certain autoimmune disorders, such as Sjögren’s syndrome, celiac disease, and rheumatoid arthritis.23 Advise these patients to avoid caffeine.
Although indicated for treating RLS, ropinirole and pramipexole are FDA Pregnancy Category C, which means animal studies have shown adverse effects on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite risks. Opioids, carbamazepine, or gabapentin may be safer for pregnant patients.24
Insomnia during menopause
The prevalence of insomnia increases from 33% to 36% in premenopausal women to 44% to 61% in postmenopausal women.14 Hot flashes, comorbid mood disturbances, sleep-disordered breathing, and RLS contribute to increased insomnia risk in postmenopausal women (Table 3).4,14,25,26
Treatment strategy. Always inquire about sleep in perimenopausal/postmenopausal women, even when her presenting complaint is related to menstrual cycle changes or vasomotor symptoms such as hot flashes.16 Assess patients for OSA, RLS, and mood, anxiety, and cognitive symptoms.26 In addition to pharmacotherapy and behavioral therapy, treatment options include hormone replacement therapy (HRT) and herbal and dietary supplements (Table 4).27-32
Table 3
Sleep difficulties during menopause: Differential diagnoses
| Condition | Features | Findings | Other considerations |
|---|---|---|---|
| Hot flashes (prevalence: 75% to 85%)14 | Vasomotor phenomenon characterized by feelings such as ‘spreading warmth,’ diaphoresis, palpitations, nausea, and insomnia Mediated through the preoptic area of the anterior hypothalamus, which regulates temperature and sleep Increased brain norepinephrine metabolism | Discrepancies between objective (PSG) and subjective measures (surveys)4 Discrepancies between self-reported and laboratory reported sleep data might be explained by thermoregulatory differences between NREM and REM sleep24 | Nocturnal hot flashes trigger awakenings and insomnia14 Hot flashes can follow arousals and awakenings HRT is highly effective in treating hot flashes; however, data on its direct effects on sleep complaints are inconsistent |
| Primary menopausal insomnia25 | Menopausal symptoms (eg, hot flashes) trigger insomnia that persists secondary to behavioral conditioning | Increase in nocturnal skin temperature coincides with decrease in skin resistance and waking episodes in PSG | Behavioral insomnia therapies are useful adjuncts to treatment of menopause symptoms |
| Sleep-disordered breathing (OSA) | Menopause increases risk for OSA independent of body weight Redistribution of body fat with an increase in the waist-to-hip circumference ratio occurs in menopause Loss of ventilatory drive because of diminished progesterone levels | Sleep fragmentation and daytime sleepiness are common, as opposed to apneic episodes or oxygen desaturation in men | Maintain a high index of suspicion and promptly refer patients to a sleep center |
| Restless legs syndrome | Related to iron deficiency | Low ferritin and folate levels | Advise patients to avoid caffeine |
| HRT: hormone replacement therapy; NREM: non-rapid eye movement; OSA: obstructive sleep apnea; PSG: polysomnography; REM: rapid eye movement | |||
Table 4
Treating insomnia in menopausal women
| Therapy | Comments |
|---|---|
| Hormone replacement therapy (HRT) | Effective for hot flashes, insomnia,26-28 and sleep apnea29 Long-term safety is questionable4 |
| Behavioral therapy (cognitive-behavioral therapy,30 stimulus control therapy, sleep restriction therapy, sleep hygiene, hypnotherapy, biofeedback) | Limited data in menopausal women |
| Sedatives/hypnotics/antidepressants (eg, zolpidem, 10 mg; eszopiclone, 3 mg; trazodone, 75 mg; ramelteon, 8 mg; SSRIs and SNRIs) | Benzodiazepines may be useful, although not specifically evaluated in menopausal women. Risk of tolerance, dependence, and psychomotor slowing |
| Herbal and dietary supplements (Cimicifuga racemosa [Black cohosh],31 valerian | Popular alternatives to HRT; however, evidence of efficacy as treatment for insomnia is inconclusive |
| SNRIs: serotonin-norepinephrine reuptake inhibitors; SSRIs: selective serotonin reuptake inhibitors | |
Comorbid psychiatric disorders
Women have a higher prevalence of psychiatric disorders such as major depressive disorder and anxiety disorders than men.1 Women have a 10% to 25% lifetime risk of developing major depression. Three quarters of depressed patients experience insomnia.1 Recent literature suggests insomnia is a risk factor for depression,33 which emphasizes the need to screen women who present with sleep problems for depression and anxiety.
Five percent to 20% of women experience postpartum depression. Depression and insomnia are correlated to the rapid decline in estrogen and progesterone after delivery.34
Treatment strategy. Insomnia is a common presenting symptom in patients with psychiatric conditions such as mood and anxiety disorders. Treating the underlying psychiatric disorder often alleviates sleeping difficulties. However, if the insomnia is disabling, treat the psychiatric disorder and insomnia concurrently.
CASE CONTINUED: Perimenopausal insomnia
Based on her history, you diagnose Ms. A with insomnia related to general medical condition (perimenopause). There are no indications to refer her for polysomnography. You educate Ms. A about sleep hygiene and recommend that she discuss her menstrual and physical complaints with her primary care physician or gynecologist. Ms. A is not interested in HRT because she has a strong family history of endometrial cancer. You reassure Ms. A and schedule a follow-up visit in 2 months to re-evaluate her insomnia.
Related resource
- Krahn LE. Perimenopausal depression? Ask how she’s sleeping. Current Psychiatry. 2005;4(6):39-53.
Drug brand names
- Carbamazepine • Carbatrol, Tegretol, others
- Escitalopram • Lexapro
- Eszopiclone • Lunesta
- Fluoxetine • Prozac
- Gabapentin • Neurontin, Gabarone
- Paroxetine • Paxil
- Pramipexole • Mirapex
- Ramelteon • Rozerem
- Ropinirole • Requip
- Sertraline • Zoloft
- Trazodone • Desyrel
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors thank Dr. Namita Dhiman and Darrel E. Willoughby for their assistance with this article.
1. Krystal AD. Depression and insomnia in women. Clin Cornerstone. 2004;6(suppl 1B):S19-S28.
2. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97-111.
3. Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12(6):383-389.
4. Soares CN, Murray BJ. Sleep disorders in women: clinical evidence and treatment strategies. Psychiatr Clin North Am. 2006;29(4):1095-1113.
5. Ohayon M. Epidemiological study on insomnia in the general population. Sleep. 1996;19(3 suppl):S7-S15.
6. Daley M, Morin CM, LeBlanc M, et al. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009;10(4):427-438.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
8. Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;2(suppl 2):S347-S353.
9. Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85-93.
10. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28(2):193-213.
11. Manber R, Bootzin RR. Sleep and the menstrual cycle. Health Psychol. 1997;16:209-214.
12. Ito M, Kohsaka M, Fukuda N, et al. Effects of menstrual cycle on plasma melatonin level and sleep characteristics. Jpn J Psychiatry Neurol. 1993;47:478-479.
13. Driver HS, Dijk DJ, Werth E, et al. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81:728-735.
14. Moline ML, Broch L, Zak R. Sleep in women across the life cycle from adulthood through menopause. Med Clin North Am. 2004;88(3):705-736.
15. Steiner M, Pearlstein T, Cohen LS, et al. Expert guidelines for the treatment of severe PMS, PMDD, and comorbidities: the role of SSRIs. J Womens Health (Larchmt). 2006;15(1):57-69.
16. Krystal AD. Insomnia in women. Clin Cornerstone. 2003;5(3):41-50.
17. Mindell JA, Jacobson BJ. Sleep disturbances during pregnancy. J Obstet Gynecol Neonatal Nurs. 2000;29(6):590-597.
18. Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95(1):14-18.
19. Brunner DP, Münch M, Biedermann K, et al. Changes in sleep and sleep electroencephalogram during pregnancy. Sleep. 1994;17(7):576-582.
20. Ross LE, Murray BJ, Steiner M. Sleep and perinatal mood disorders: a critical review. J Psychiatry Neurosci. 2005;30(4):247-256.
21. Edwards N, Middleton PG, Blyton DM, et al. Sleep disordered breathing and pregnancy. Thorax. 2002;57(6):555-558.
22. Manconi M, Govoni V, De Vito A, et al. Restless legs syndrome and pregnancy. Neurology. 2004;63(6):1065-1069.
23. Lee KA, Zaffke ME, Baratte-Beebe K. Restless legs syndrome and sleep disturbance during pregnancy: the role of folate and iron. J Womens Health Gend Based Med. 2001;10(4):335-341.
24. Djokanovic N, Garcia-Bournissen F, Koren G. Medications for restless legs syndrome in pregnancy. J Obstet Gynaecol Can. 2008;30(6):505-507.
25. Freedman RR, Roehrs TA. Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause. 2006;13(4):576-583.
26. Krystal AD, Edinger J, Wohlgemuth W, et al. Sleep in perimenopausal and postmenopausal women. Sleep Med Rev. 1998;2(4):243-253.
27. Polo-Kantola P, Erkkola R, Irjala K, et al. Effect of short-term transdermal estrogen replacement therapy on sleep: a randomized, double-blind crossover trial in postmenopausal women. Fertil Steril. 1999;71(5):873-880.
28. Watts NB, Notelovitz M, Timmons MC, et al. Comparison of oral estrogens and estrogens plus androgen on bone mineral density, menopausal symptoms, and lipid-lipoprotein profiles in surgical menopause. Obstet Gynecol. 1995;85(4):529-537.Erratum in: Obstet Gynecol 1995;85(5 Pt 1):668.
29. Boyle GJ, Murrihy R. A preliminary study of hormone replacement therapy and psychological mood states in perimenopausal women. Psychol Rep. 2001;88(1):160-170.
30. Cistulli PA, Barnes DJ, Grunstein RR, et al. Effect of short-term hormone replacement in the treatment of obstructive sleep apnoea in postmenopausal women. Thorax. 1994;49:699-702.
31. Yang CM, Spielman AJ, Glovinsky P. Nonpharmacologic strategies in the management of insomnia. Psychiatr Clin North Am. 2006;29(4):895-919.
32. Mahady GB. Black cohosh (Actaea/Cimicifuga racemosa): review of the clinical data for safety and efficacy in menopausal symptoms. Treat Endocrinol. 2005;4(3):177-184.
33. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
34. Burt VK, Stein K. Epidemiology of depression throughout the female life cycle. J Clin Psychiatry. 2002;63(suppl 7):9-15.
1. Krystal AD. Depression and insomnia in women. Clin Cornerstone. 2004;6(suppl 1B):S19-S28.
2. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97-111.
3. Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12(6):383-389.
4. Soares CN, Murray BJ. Sleep disorders in women: clinical evidence and treatment strategies. Psychiatr Clin North Am. 2006;29(4):1095-1113.
5. Ohayon M. Epidemiological study on insomnia in the general population. Sleep. 1996;19(3 suppl):S7-S15.
6. Daley M, Morin CM, LeBlanc M, et al. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009;10(4):427-438.
7. Diagnostic and statistical manual of mental disorders, 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000.
8. Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;2(suppl 2):S347-S353.
9. Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85-93.
10. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh Sleep Quality Index (PSQI): a new instrument for psychiatric research and practice. Psychiatry Res. 1989;28(2):193-213.
11. Manber R, Bootzin RR. Sleep and the menstrual cycle. Health Psychol. 1997;16:209-214.
12. Ito M, Kohsaka M, Fukuda N, et al. Effects of menstrual cycle on plasma melatonin level and sleep characteristics. Jpn J Psychiatry Neurol. 1993;47:478-479.
13. Driver HS, Dijk DJ, Werth E, et al. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81:728-735.
14. Moline ML, Broch L, Zak R. Sleep in women across the life cycle from adulthood through menopause. Med Clin North Am. 2004;88(3):705-736.
15. Steiner M, Pearlstein T, Cohen LS, et al. Expert guidelines for the treatment of severe PMS, PMDD, and comorbidities: the role of SSRIs. J Womens Health (Larchmt). 2006;15(1):57-69.
16. Krystal AD. Insomnia in women. Clin Cornerstone. 2003;5(3):41-50.
17. Mindell JA, Jacobson BJ. Sleep disturbances during pregnancy. J Obstet Gynecol Neonatal Nurs. 2000;29(6):590-597.
18. Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95(1):14-18.
19. Brunner DP, Münch M, Biedermann K, et al. Changes in sleep and sleep electroencephalogram during pregnancy. Sleep. 1994;17(7):576-582.
20. Ross LE, Murray BJ, Steiner M. Sleep and perinatal mood disorders: a critical review. J Psychiatry Neurosci. 2005;30(4):247-256.
21. Edwards N, Middleton PG, Blyton DM, et al. Sleep disordered breathing and pregnancy. Thorax. 2002;57(6):555-558.
22. Manconi M, Govoni V, De Vito A, et al. Restless legs syndrome and pregnancy. Neurology. 2004;63(6):1065-1069.
23. Lee KA, Zaffke ME, Baratte-Beebe K. Restless legs syndrome and sleep disturbance during pregnancy: the role of folate and iron. J Womens Health Gend Based Med. 2001;10(4):335-341.
24. Djokanovic N, Garcia-Bournissen F, Koren G. Medications for restless legs syndrome in pregnancy. J Obstet Gynaecol Can. 2008;30(6):505-507.
25. Freedman RR, Roehrs TA. Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause. 2006;13(4):576-583.
26. Krystal AD, Edinger J, Wohlgemuth W, et al. Sleep in perimenopausal and postmenopausal women. Sleep Med Rev. 1998;2(4):243-253.
27. Polo-Kantola P, Erkkola R, Irjala K, et al. Effect of short-term transdermal estrogen replacement therapy on sleep: a randomized, double-blind crossover trial in postmenopausal women. Fertil Steril. 1999;71(5):873-880.
28. Watts NB, Notelovitz M, Timmons MC, et al. Comparison of oral estrogens and estrogens plus androgen on bone mineral density, menopausal symptoms, and lipid-lipoprotein profiles in surgical menopause. Obstet Gynecol. 1995;85(4):529-537.Erratum in: Obstet Gynecol 1995;85(5 Pt 1):668.
29. Boyle GJ, Murrihy R. A preliminary study of hormone replacement therapy and psychological mood states in perimenopausal women. Psychol Rep. 2001;88(1):160-170.
30. Cistulli PA, Barnes DJ, Grunstein RR, et al. Effect of short-term hormone replacement in the treatment of obstructive sleep apnoea in postmenopausal women. Thorax. 1994;49:699-702.
31. Yang CM, Spielman AJ, Glovinsky P. Nonpharmacologic strategies in the management of insomnia. Psychiatr Clin North Am. 2006;29(4):895-919.
32. Mahady GB. Black cohosh (Actaea/Cimicifuga racemosa): review of the clinical data for safety and efficacy in menopausal symptoms. Treat Endocrinol. 2005;4(3):177-184.
33. Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
34. Burt VK, Stein K. Epidemiology of depression throughout the female life cycle. J Clin Psychiatry. 2002;63(suppl 7):9-15.