User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Better trials, more cooperation needed to improve continuous glucose monitoring devices
When continuous glucose monitoring (CGM) devices were first introduced, many thought they would revolutionize intensive insulin therapy for patients with diabetes. More than 15 years later, uptake is increasing but still remains low.
An expert working group convened by the (Diabetes Care. 2017 Oct 25. doi: 10.2337/dci17-0043).
“The aim is to improve safety and efficacy in order to support the advancement of the technology in achieving its potential to improve quality of life and health outcomes for more people with diabetes,” John R. Petrie, MD, of the University of Glasgow, Scotland, and members of the working group wrote in the joint scientific statement.
Barriers to the uptake of CGM devices include cost and “human factors” issues such as the need for more audible alarms and easier-to-read displays, according to the joint statement. A lack of standardization in displaying results and uncertainty over the best way to use CGM data are also obstacles to widespread adoption.
The working group called for a systematic premarketing and postapproval evaluation of CGM system performance, as well as greater investment in clinical trials, including head-to-head comparisons and large independent registry studies. Other recommendations include the need for standardization of glucose data reporting, improved consistency and accessibility of postmarketing safety reports, and increased communication and cooperation between the various stakeholder groups.
The most important recommendation from the working group is the call for stakeholders to cooperate and communicate regularly, said A. Jay Cohen, MD, medical director of the Endocrine Clinic in Memphis, Tenn.
“That’s going to drive the momentum of change that’s going to help patients, and it’s also going to drive innovation,” he said.
After reviewing the joint scientific statement, Dr. Cohen said he would strongly encourage collaboration between stakeholders in government, insurance, clinical practice, industry, and patients to advance the use of CGM systems.
“The CGM systems have been not evolutionary, but revolutionary, in improvement in care for persons with diabetes and their families,” Dr. Cohen said. “They are a game changer, and besides … this unequivocally saves employers and insurers money, so it’s very cost effective in a very intuitive way.”
The joint scientific statement is based on review of more than 50 data sources, including recent clinical trials, research abstracts, regulatory authority databases, manufacturing company documents, and the clinical experience of the committee members.
“The guidelines set forth by this scientific statement will greatly inform providers and further advance the standardization, accuracy, and safety of CGM systems,” William T. Cefalu, MD, chief scientific, medical, and mission officer of the ADA, said in a statement.
Most members of the working group have relationships with industry, but industry “is considered to have had no impact on the manuscript or its content by reviewers from the ADA and EASD.”
When continuous glucose monitoring (CGM) devices were first introduced, many thought they would revolutionize intensive insulin therapy for patients with diabetes. More than 15 years later, uptake is increasing but still remains low.
An expert working group convened by the (Diabetes Care. 2017 Oct 25. doi: 10.2337/dci17-0043).
“The aim is to improve safety and efficacy in order to support the advancement of the technology in achieving its potential to improve quality of life and health outcomes for more people with diabetes,” John R. Petrie, MD, of the University of Glasgow, Scotland, and members of the working group wrote in the joint scientific statement.
Barriers to the uptake of CGM devices include cost and “human factors” issues such as the need for more audible alarms and easier-to-read displays, according to the joint statement. A lack of standardization in displaying results and uncertainty over the best way to use CGM data are also obstacles to widespread adoption.
The working group called for a systematic premarketing and postapproval evaluation of CGM system performance, as well as greater investment in clinical trials, including head-to-head comparisons and large independent registry studies. Other recommendations include the need for standardization of glucose data reporting, improved consistency and accessibility of postmarketing safety reports, and increased communication and cooperation between the various stakeholder groups.
The most important recommendation from the working group is the call for stakeholders to cooperate and communicate regularly, said A. Jay Cohen, MD, medical director of the Endocrine Clinic in Memphis, Tenn.
“That’s going to drive the momentum of change that’s going to help patients, and it’s also going to drive innovation,” he said.
After reviewing the joint scientific statement, Dr. Cohen said he would strongly encourage collaboration between stakeholders in government, insurance, clinical practice, industry, and patients to advance the use of CGM systems.
“The CGM systems have been not evolutionary, but revolutionary, in improvement in care for persons with diabetes and their families,” Dr. Cohen said. “They are a game changer, and besides … this unequivocally saves employers and insurers money, so it’s very cost effective in a very intuitive way.”
The joint scientific statement is based on review of more than 50 data sources, including recent clinical trials, research abstracts, regulatory authority databases, manufacturing company documents, and the clinical experience of the committee members.
“The guidelines set forth by this scientific statement will greatly inform providers and further advance the standardization, accuracy, and safety of CGM systems,” William T. Cefalu, MD, chief scientific, medical, and mission officer of the ADA, said in a statement.
Most members of the working group have relationships with industry, but industry “is considered to have had no impact on the manuscript or its content by reviewers from the ADA and EASD.”
When continuous glucose monitoring (CGM) devices were first introduced, many thought they would revolutionize intensive insulin therapy for patients with diabetes. More than 15 years later, uptake is increasing but still remains low.
An expert working group convened by the (Diabetes Care. 2017 Oct 25. doi: 10.2337/dci17-0043).
“The aim is to improve safety and efficacy in order to support the advancement of the technology in achieving its potential to improve quality of life and health outcomes for more people with diabetes,” John R. Petrie, MD, of the University of Glasgow, Scotland, and members of the working group wrote in the joint scientific statement.
Barriers to the uptake of CGM devices include cost and “human factors” issues such as the need for more audible alarms and easier-to-read displays, according to the joint statement. A lack of standardization in displaying results and uncertainty over the best way to use CGM data are also obstacles to widespread adoption.
The working group called for a systematic premarketing and postapproval evaluation of CGM system performance, as well as greater investment in clinical trials, including head-to-head comparisons and large independent registry studies. Other recommendations include the need for standardization of glucose data reporting, improved consistency and accessibility of postmarketing safety reports, and increased communication and cooperation between the various stakeholder groups.
The most important recommendation from the working group is the call for stakeholders to cooperate and communicate regularly, said A. Jay Cohen, MD, medical director of the Endocrine Clinic in Memphis, Tenn.
“That’s going to drive the momentum of change that’s going to help patients, and it’s also going to drive innovation,” he said.
After reviewing the joint scientific statement, Dr. Cohen said he would strongly encourage collaboration between stakeholders in government, insurance, clinical practice, industry, and patients to advance the use of CGM systems.
“The CGM systems have been not evolutionary, but revolutionary, in improvement in care for persons with diabetes and their families,” Dr. Cohen said. “They are a game changer, and besides … this unequivocally saves employers and insurers money, so it’s very cost effective in a very intuitive way.”
The joint scientific statement is based on review of more than 50 data sources, including recent clinical trials, research abstracts, regulatory authority databases, manufacturing company documents, and the clinical experience of the committee members.
“The guidelines set forth by this scientific statement will greatly inform providers and further advance the standardization, accuracy, and safety of CGM systems,” William T. Cefalu, MD, chief scientific, medical, and mission officer of the ADA, said in a statement.
Most members of the working group have relationships with industry, but industry “is considered to have had no impact on the manuscript or its content by reviewers from the ADA and EASD.”
FROM DIABETES CARE
DiaRem score predicts remission of type 2 diabetes after sleeve gastrectomy
SAN DIEGO – The DiaRem score was effective in predicting remission of type 2 diabetes following laparoscopic sleeve gastrectomy, results from a single-center study showed.
Developed by clinicians at Geisinger Clinic, the DiaRem is a simple score that helps predict remission of type 2 diabetes in severely obese subjects with metabolic syndrome who undergo Roux-en-Y gastric bypass surgery (Lancet Diabetes Endocrinol 2014;2[1]:38-45). The DiaRem score spans from 0 to 22 and is divided into five groups corresponding to five probability ranges for type 2 diabetes remission: 0-2 (88%-99%), 3-7 (64%-88%), 8-12 (23%-49%), 13-17 (11%-33%), 18-22 (2%-16%). In an effort to assess the feasibility of using the DiaRem score to predict remission of type 2 diabetes after laparoscopic sleeve gastrectomy, Raul J. Rosenthal, MD, FACS, and his associates conducted a 4-year retrospective review of 162 patients at the Cleveland Clinic Florida, Weston. “This is the first report that uses the DiaRem score for similar subjects that underwent sleeve gastrectomy instead,” Dr. Rosenthal said in an interview in advance of the annual clinical congress of the American College of Surgeons.
The mean age of the 162 patients was 55 years, 61% were women, 74% were non-Hispanic, their mean body mass index was 43.2 kg/m2, 33% had a preoperative hemoglobin A1c level between 7% and 8.9%, and 22% had an HbA1c of 9%. All had a minimum follow-up of 1 year after their laparoscopic sleeve gastrectomy and 67% had follow-up of 3 years or more, said Dr. Rosenthal, professor and chairman of the department of general surgery at Cleveland Clinic Florida.
Based on results of the DiaRem scores, 58% of patients achieved complete remission of type 2 diabetes, 6% achieved partial remission, and 36% had no remission. Specifically, 96% had DiaRem scores between 0 and 2; 92% had scores between 3 and 7; 50% had scores between 8 and 12, 20% had scores between 13 and 17, and 24% had scores between 18 and 22. “We were pleased to find out that 58% of patients that underwent sleeve gastrectomy achieved complete remission of type 2 diabetes mellitus,” said Dr. Rosenthal, who also directs the clinic’s bariatric and metabolic institute. “This compares favorably to previous reports in which patients achieved 33% of complete remission after gastric bypass.” The researchers also found that 84% of patients achieved remission in 12 months and the rest in 3 years. They observed medication reduction in 93% of the patients.
“Sleeve gastrectomy is a valid bariatric-metabolic procedure in patients with type 2 diabetes,” Dr. Rosenthal concluded. “The main limitation of this study is that is it a retrospective one, and we do not have a control group of patients that underwent gastric bypass or medical treatment to compare.”
The findings were presented at the meeting by Emanuele Lo Menzo, MD. Dr. Rosenthal disclosed that he is a consultant for Medtronic. Dr. Lo Menzo reported having no financial disclosures.
SAN DIEGO – The DiaRem score was effective in predicting remission of type 2 diabetes following laparoscopic sleeve gastrectomy, results from a single-center study showed.
Developed by clinicians at Geisinger Clinic, the DiaRem is a simple score that helps predict remission of type 2 diabetes in severely obese subjects with metabolic syndrome who undergo Roux-en-Y gastric bypass surgery (Lancet Diabetes Endocrinol 2014;2[1]:38-45). The DiaRem score spans from 0 to 22 and is divided into five groups corresponding to five probability ranges for type 2 diabetes remission: 0-2 (88%-99%), 3-7 (64%-88%), 8-12 (23%-49%), 13-17 (11%-33%), 18-22 (2%-16%). In an effort to assess the feasibility of using the DiaRem score to predict remission of type 2 diabetes after laparoscopic sleeve gastrectomy, Raul J. Rosenthal, MD, FACS, and his associates conducted a 4-year retrospective review of 162 patients at the Cleveland Clinic Florida, Weston. “This is the first report that uses the DiaRem score for similar subjects that underwent sleeve gastrectomy instead,” Dr. Rosenthal said in an interview in advance of the annual clinical congress of the American College of Surgeons.
The mean age of the 162 patients was 55 years, 61% were women, 74% were non-Hispanic, their mean body mass index was 43.2 kg/m2, 33% had a preoperative hemoglobin A1c level between 7% and 8.9%, and 22% had an HbA1c of 9%. All had a minimum follow-up of 1 year after their laparoscopic sleeve gastrectomy and 67% had follow-up of 3 years or more, said Dr. Rosenthal, professor and chairman of the department of general surgery at Cleveland Clinic Florida.
Based on results of the DiaRem scores, 58% of patients achieved complete remission of type 2 diabetes, 6% achieved partial remission, and 36% had no remission. Specifically, 96% had DiaRem scores between 0 and 2; 92% had scores between 3 and 7; 50% had scores between 8 and 12, 20% had scores between 13 and 17, and 24% had scores between 18 and 22. “We were pleased to find out that 58% of patients that underwent sleeve gastrectomy achieved complete remission of type 2 diabetes mellitus,” said Dr. Rosenthal, who also directs the clinic’s bariatric and metabolic institute. “This compares favorably to previous reports in which patients achieved 33% of complete remission after gastric bypass.” The researchers also found that 84% of patients achieved remission in 12 months and the rest in 3 years. They observed medication reduction in 93% of the patients.
“Sleeve gastrectomy is a valid bariatric-metabolic procedure in patients with type 2 diabetes,” Dr. Rosenthal concluded. “The main limitation of this study is that is it a retrospective one, and we do not have a control group of patients that underwent gastric bypass or medical treatment to compare.”
The findings were presented at the meeting by Emanuele Lo Menzo, MD. Dr. Rosenthal disclosed that he is a consultant for Medtronic. Dr. Lo Menzo reported having no financial disclosures.
SAN DIEGO – The DiaRem score was effective in predicting remission of type 2 diabetes following laparoscopic sleeve gastrectomy, results from a single-center study showed.
Developed by clinicians at Geisinger Clinic, the DiaRem is a simple score that helps predict remission of type 2 diabetes in severely obese subjects with metabolic syndrome who undergo Roux-en-Y gastric bypass surgery (Lancet Diabetes Endocrinol 2014;2[1]:38-45). The DiaRem score spans from 0 to 22 and is divided into five groups corresponding to five probability ranges for type 2 diabetes remission: 0-2 (88%-99%), 3-7 (64%-88%), 8-12 (23%-49%), 13-17 (11%-33%), 18-22 (2%-16%). In an effort to assess the feasibility of using the DiaRem score to predict remission of type 2 diabetes after laparoscopic sleeve gastrectomy, Raul J. Rosenthal, MD, FACS, and his associates conducted a 4-year retrospective review of 162 patients at the Cleveland Clinic Florida, Weston. “This is the first report that uses the DiaRem score for similar subjects that underwent sleeve gastrectomy instead,” Dr. Rosenthal said in an interview in advance of the annual clinical congress of the American College of Surgeons.
The mean age of the 162 patients was 55 years, 61% were women, 74% were non-Hispanic, their mean body mass index was 43.2 kg/m2, 33% had a preoperative hemoglobin A1c level between 7% and 8.9%, and 22% had an HbA1c of 9%. All had a minimum follow-up of 1 year after their laparoscopic sleeve gastrectomy and 67% had follow-up of 3 years or more, said Dr. Rosenthal, professor and chairman of the department of general surgery at Cleveland Clinic Florida.
Based on results of the DiaRem scores, 58% of patients achieved complete remission of type 2 diabetes, 6% achieved partial remission, and 36% had no remission. Specifically, 96% had DiaRem scores between 0 and 2; 92% had scores between 3 and 7; 50% had scores between 8 and 12, 20% had scores between 13 and 17, and 24% had scores between 18 and 22. “We were pleased to find out that 58% of patients that underwent sleeve gastrectomy achieved complete remission of type 2 diabetes mellitus,” said Dr. Rosenthal, who also directs the clinic’s bariatric and metabolic institute. “This compares favorably to previous reports in which patients achieved 33% of complete remission after gastric bypass.” The researchers also found that 84% of patients achieved remission in 12 months and the rest in 3 years. They observed medication reduction in 93% of the patients.
“Sleeve gastrectomy is a valid bariatric-metabolic procedure in patients with type 2 diabetes,” Dr. Rosenthal concluded. “The main limitation of this study is that is it a retrospective one, and we do not have a control group of patients that underwent gastric bypass or medical treatment to compare.”
The findings were presented at the meeting by Emanuele Lo Menzo, MD. Dr. Rosenthal disclosed that he is a consultant for Medtronic. Dr. Lo Menzo reported having no financial disclosures.
AT THE ACS CLINICAL CONGRESS
Key clinical point: The DiaRem score is useful in predicting remission of type 2 diabetes following laparoscopic sleeve gastrectomy.
Major finding: Results of the DiaRem scores indicated that 58% of patients achieved complete remission of type 2 diabetes.
Study details: A retrospective analysis of 162 patients who underwent laparoscopic sleeve gastrectomy.
Disclosures: Dr. Rosenthal disclosed that he is a consultant for Medtronic. Dr. Lo Menzo reported having no financial disclosures.
Low risk of bariatric surgery complications in IBD
Bariatric surgery is safe and feasible in patients with inflammatory bowel disease (IBD), with a low risk of postoperative complications vs. controls, according to results of a recent cohort study.
Besides a significantly higher risk of perioperative small-bowel obstruction and a 1-day increase in hospital stay, outcomes were comparable between patients with IBD and controls (Obes Surg. 2017 Oct 10. doi: 10.1007/s11695-017-2955-4).
Limitations of the retrospective study, according to the authors, included a potential underestimation of short-term postoperative complications, since the data set used in the study was limited to in-hospital stays and would not include events occurring after discharge.
Nevertheless, “our data show that it is reasonable to carefully proceed with bariatric interventions in obese IBD patients, especially those who are at higher risk of cardiovascular mortality and drastic need for weight reduction, to accrue benefits of weight loss,” wrote Fateh Bazerbachi, MD, of the Mayo Clinic, Rochester, Minn. and his coauthors.
Bariatric surgery is the “most effective solution” for obesity, and “appropriate candidates should not be deprived of this important, potentially life-saving procedure, if the intervention is deemed acceptably safe,” Dr. Bazerbachi and his colleagues noted.
Their cohort study included data for 314,864 adult patients in the Nationwide Inpatient Sample who underwent bariatric surgery between 2011 and 2013. Of that group, 790 patients had underlying IBD (459 Crohn’s disease, 331 ulcerative colitis). Remaining patients made up the comparator group.
The primary outcomes evaluated in the study included risks of systemic and technical complications. Risk of perioperative small-bowel obstruction was significantly higher in the IBD group (adjusted odds ratio, 4.0; 95% confidence interval, 2.2-7.4). However, the rates of other complications were similar between the two groups, data show.
Secondary outcomes in the study included length of hospital stay and mortality. Mean length of hospital stay was 3.4 days for IBD patients, vs. 2.5 days for the comparison group (P = .01), according to the report. Mortality was 0.25% for controls, while no deaths were reported in the IBD group.
In the future, bariatric surgeons may face increasing demand to treat IBD patients, given the increasing prevalence of obesity in the IBD patient population, Dr. Bazerbachi and his colleagues said.
Some surgeons may believe that bariatric intervention is more challenging in IBD patients, in part because of the underlying inflammatory state that might interfere with healing of wounds and recovery of bowel motility, they said. Bariatric surgery, however, can reduce body mass index, which in turn might make future IBD surgeries less challenging.
Another potential advantage is reduction in cardiovascular risk, which is elevated in IBD patients both due to obesity as well as the IBD condition, they added.
“Further studies are certainly needed to examine long-term outcomes of bariatric surgery on IBD and to determine whether cardiovascular mortality is reduced from these interventions in this susceptible cohort of obese IBD patients,” Dr. Bazerbachi and his colleagues wrote.
The authors declared that they had no conflicts of interest.
Bariatric surgery is safe and feasible in patients with inflammatory bowel disease (IBD), with a low risk of postoperative complications vs. controls, according to results of a recent cohort study.
Besides a significantly higher risk of perioperative small-bowel obstruction and a 1-day increase in hospital stay, outcomes were comparable between patients with IBD and controls (Obes Surg. 2017 Oct 10. doi: 10.1007/s11695-017-2955-4).
Limitations of the retrospective study, according to the authors, included a potential underestimation of short-term postoperative complications, since the data set used in the study was limited to in-hospital stays and would not include events occurring after discharge.
Nevertheless, “our data show that it is reasonable to carefully proceed with bariatric interventions in obese IBD patients, especially those who are at higher risk of cardiovascular mortality and drastic need for weight reduction, to accrue benefits of weight loss,” wrote Fateh Bazerbachi, MD, of the Mayo Clinic, Rochester, Minn. and his coauthors.
Bariatric surgery is the “most effective solution” for obesity, and “appropriate candidates should not be deprived of this important, potentially life-saving procedure, if the intervention is deemed acceptably safe,” Dr. Bazerbachi and his colleagues noted.
Their cohort study included data for 314,864 adult patients in the Nationwide Inpatient Sample who underwent bariatric surgery between 2011 and 2013. Of that group, 790 patients had underlying IBD (459 Crohn’s disease, 331 ulcerative colitis). Remaining patients made up the comparator group.
The primary outcomes evaluated in the study included risks of systemic and technical complications. Risk of perioperative small-bowel obstruction was significantly higher in the IBD group (adjusted odds ratio, 4.0; 95% confidence interval, 2.2-7.4). However, the rates of other complications were similar between the two groups, data show.
Secondary outcomes in the study included length of hospital stay and mortality. Mean length of hospital stay was 3.4 days for IBD patients, vs. 2.5 days for the comparison group (P = .01), according to the report. Mortality was 0.25% for controls, while no deaths were reported in the IBD group.
In the future, bariatric surgeons may face increasing demand to treat IBD patients, given the increasing prevalence of obesity in the IBD patient population, Dr. Bazerbachi and his colleagues said.
Some surgeons may believe that bariatric intervention is more challenging in IBD patients, in part because of the underlying inflammatory state that might interfere with healing of wounds and recovery of bowel motility, they said. Bariatric surgery, however, can reduce body mass index, which in turn might make future IBD surgeries less challenging.
Another potential advantage is reduction in cardiovascular risk, which is elevated in IBD patients both due to obesity as well as the IBD condition, they added.
“Further studies are certainly needed to examine long-term outcomes of bariatric surgery on IBD and to determine whether cardiovascular mortality is reduced from these interventions in this susceptible cohort of obese IBD patients,” Dr. Bazerbachi and his colleagues wrote.
The authors declared that they had no conflicts of interest.
Bariatric surgery is safe and feasible in patients with inflammatory bowel disease (IBD), with a low risk of postoperative complications vs. controls, according to results of a recent cohort study.
Besides a significantly higher risk of perioperative small-bowel obstruction and a 1-day increase in hospital stay, outcomes were comparable between patients with IBD and controls (Obes Surg. 2017 Oct 10. doi: 10.1007/s11695-017-2955-4).
Limitations of the retrospective study, according to the authors, included a potential underestimation of short-term postoperative complications, since the data set used in the study was limited to in-hospital stays and would not include events occurring after discharge.
Nevertheless, “our data show that it is reasonable to carefully proceed with bariatric interventions in obese IBD patients, especially those who are at higher risk of cardiovascular mortality and drastic need for weight reduction, to accrue benefits of weight loss,” wrote Fateh Bazerbachi, MD, of the Mayo Clinic, Rochester, Minn. and his coauthors.
Bariatric surgery is the “most effective solution” for obesity, and “appropriate candidates should not be deprived of this important, potentially life-saving procedure, if the intervention is deemed acceptably safe,” Dr. Bazerbachi and his colleagues noted.
Their cohort study included data for 314,864 adult patients in the Nationwide Inpatient Sample who underwent bariatric surgery between 2011 and 2013. Of that group, 790 patients had underlying IBD (459 Crohn’s disease, 331 ulcerative colitis). Remaining patients made up the comparator group.
The primary outcomes evaluated in the study included risks of systemic and technical complications. Risk of perioperative small-bowel obstruction was significantly higher in the IBD group (adjusted odds ratio, 4.0; 95% confidence interval, 2.2-7.4). However, the rates of other complications were similar between the two groups, data show.
Secondary outcomes in the study included length of hospital stay and mortality. Mean length of hospital stay was 3.4 days for IBD patients, vs. 2.5 days for the comparison group (P = .01), according to the report. Mortality was 0.25% for controls, while no deaths were reported in the IBD group.
In the future, bariatric surgeons may face increasing demand to treat IBD patients, given the increasing prevalence of obesity in the IBD patient population, Dr. Bazerbachi and his colleagues said.
Some surgeons may believe that bariatric intervention is more challenging in IBD patients, in part because of the underlying inflammatory state that might interfere with healing of wounds and recovery of bowel motility, they said. Bariatric surgery, however, can reduce body mass index, which in turn might make future IBD surgeries less challenging.
Another potential advantage is reduction in cardiovascular risk, which is elevated in IBD patients both due to obesity as well as the IBD condition, they added.
“Further studies are certainly needed to examine long-term outcomes of bariatric surgery on IBD and to determine whether cardiovascular mortality is reduced from these interventions in this susceptible cohort of obese IBD patients,” Dr. Bazerbachi and his colleagues wrote.
The authors declared that they had no conflicts of interest.
FROM OBESITY SURGERY
Key clinical point: Watch for perioperative small-bowel obstruction in IBD patients undergoing bariatric surgery.
Major finding: IBD patients had a higher risk of perioperative small bowel obstruction (adjusted odds ratio, 4.0; 95% confidence interval, 2.2-7.4) and a 1-day increase in hospital stay (P = .01), compared with controls.
Data source: Retrospective cohort study of Nationwide Inpatient Sample data including 790 patients with underlying IBD.
Disclosures: The authors declared that they had no conflicts of interest.
Many women have unprotected sex in year after bariatric surgery
More than 40% of reproductive-age women reported having unprotected sex in the year after undergoing bariatric surgery, despite recommendations to avoid pregnancy for at least a year, a new study finds. Another 4% of women reported trying to conceive in the 12 months after surgery.
“We were surprised to find such a large percentage of women were not using contraception, and we were also surprised to find so many were actively trying to conceive,” the study’s lead author Marie N. Menke, MD, MPH, of the University of Pittsburgh, said in an interview. “Reproductive-age women who are considering bariatric surgery should be counseled that they should plan to use contraceptives after surgery for about 12-18 months.”
Pregnancy isn’t recommended over that period mainly because of the risks to the fetus, Dr. Menke said. “These risks are different from obesity in general. We don’t know exactly why surgery causes these risks, but ideally, patients would be weight-stable prior to conception.”
In 2013, the American Association of Clinical Endocrinologists, the Obesity Society, and the American Society for Metabolic & Bariatric Surgery provided a Grade D recommendation for a 12-18 month delay in conception (Obesity [Silver Spring] 2013;21[suppl 1]:S1-27). A recent study provided more insight into the potential risks of pregnancy soon after weight-loss procedures. It reported that, compared with those who gave birth more than 4 years later, women who gave birth within 2 years of bariatric surgery had higher risks of premature birth, admission to neonatal intensive care units, and small-for-gestational-age infants (JAMA Surg. 2017 Feb 1;152[2]:1-8).
The current prospective cohort study included women who underwent bariatric surgery for the first time during 2005-2009 at 10 U.S. hospitals. The women, all aged 18-44 years, answered questions for as long as 7 years, until January 2015 (Obstet Gynecol. 2017 Oct 6. doi: 10.1097/AOG.0000000000002323).
The analysis included 710 women who provided conception data. The median body mass index of the women was 46.3 kg/m2. Most patients underwent Roux-en-Y gastric bypass (73%), followed by laparoscopic adjustable gastric banding (23%).
Researchers found that 4.3% of the women tried to conceive in the first year after surgery (95% confidence interval, 2.4-6.3), and 13.1% did so in the second year (95% CI, 9.3-17.0; P less than .001).
During the first year after surgery, 12.7% of women had no intercourse (95% CI, 9.4-16.0), 40.5% had only protected intercourse (95% CI, 35.6-45.4), and 41.5% (95% CI, 36.4-46.6) had unprotected intercourse while not trying to conceive.
Why are the unprotected sex numbers so high? “We wonder if some women simply feel that they cannot get pregnant,” Dr. Menke said. “Our group has previously reported that 42% of women who had attempted to conceive prior to bariatric surgery had a history of infertility. Some of these women went on to deliver a live birth, but many did not.”
The study reports that 183 of the 710 women did ultimately conceive and that the number may be as high as 237 because data were missing for 54 women who may have not wanted to report a nonlive birth. Of the women who reported conceiving, 68.9% had live births, 1.1% had stillbirths, 1.1% had ectopic pregnancies, 21.9% miscarried and 7.1% had abortions.
“We’d really like this research to be a reason to consider presurgical contraceptive counseling as one of the steps prior to bariatric surgery,” Dr. Menke said. “This may include a referral by the bariatric surgeon to a physician would who provide counseling, prescribe, or both.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Menke reported having no relevant disclosures. Her coauthors reported financial relationships with pharmaceutical and medical device companies with products to treat metabolic diseases.
More than 40% of reproductive-age women reported having unprotected sex in the year after undergoing bariatric surgery, despite recommendations to avoid pregnancy for at least a year, a new study finds. Another 4% of women reported trying to conceive in the 12 months after surgery.
“We were surprised to find such a large percentage of women were not using contraception, and we were also surprised to find so many were actively trying to conceive,” the study’s lead author Marie N. Menke, MD, MPH, of the University of Pittsburgh, said in an interview. “Reproductive-age women who are considering bariatric surgery should be counseled that they should plan to use contraceptives after surgery for about 12-18 months.”
Pregnancy isn’t recommended over that period mainly because of the risks to the fetus, Dr. Menke said. “These risks are different from obesity in general. We don’t know exactly why surgery causes these risks, but ideally, patients would be weight-stable prior to conception.”
In 2013, the American Association of Clinical Endocrinologists, the Obesity Society, and the American Society for Metabolic & Bariatric Surgery provided a Grade D recommendation for a 12-18 month delay in conception (Obesity [Silver Spring] 2013;21[suppl 1]:S1-27). A recent study provided more insight into the potential risks of pregnancy soon after weight-loss procedures. It reported that, compared with those who gave birth more than 4 years later, women who gave birth within 2 years of bariatric surgery had higher risks of premature birth, admission to neonatal intensive care units, and small-for-gestational-age infants (JAMA Surg. 2017 Feb 1;152[2]:1-8).
The current prospective cohort study included women who underwent bariatric surgery for the first time during 2005-2009 at 10 U.S. hospitals. The women, all aged 18-44 years, answered questions for as long as 7 years, until January 2015 (Obstet Gynecol. 2017 Oct 6. doi: 10.1097/AOG.0000000000002323).
The analysis included 710 women who provided conception data. The median body mass index of the women was 46.3 kg/m2. Most patients underwent Roux-en-Y gastric bypass (73%), followed by laparoscopic adjustable gastric banding (23%).
Researchers found that 4.3% of the women tried to conceive in the first year after surgery (95% confidence interval, 2.4-6.3), and 13.1% did so in the second year (95% CI, 9.3-17.0; P less than .001).
During the first year after surgery, 12.7% of women had no intercourse (95% CI, 9.4-16.0), 40.5% had only protected intercourse (95% CI, 35.6-45.4), and 41.5% (95% CI, 36.4-46.6) had unprotected intercourse while not trying to conceive.
Why are the unprotected sex numbers so high? “We wonder if some women simply feel that they cannot get pregnant,” Dr. Menke said. “Our group has previously reported that 42% of women who had attempted to conceive prior to bariatric surgery had a history of infertility. Some of these women went on to deliver a live birth, but many did not.”
The study reports that 183 of the 710 women did ultimately conceive and that the number may be as high as 237 because data were missing for 54 women who may have not wanted to report a nonlive birth. Of the women who reported conceiving, 68.9% had live births, 1.1% had stillbirths, 1.1% had ectopic pregnancies, 21.9% miscarried and 7.1% had abortions.
“We’d really like this research to be a reason to consider presurgical contraceptive counseling as one of the steps prior to bariatric surgery,” Dr. Menke said. “This may include a referral by the bariatric surgeon to a physician would who provide counseling, prescribe, or both.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Menke reported having no relevant disclosures. Her coauthors reported financial relationships with pharmaceutical and medical device companies with products to treat metabolic diseases.
More than 40% of reproductive-age women reported having unprotected sex in the year after undergoing bariatric surgery, despite recommendations to avoid pregnancy for at least a year, a new study finds. Another 4% of women reported trying to conceive in the 12 months after surgery.
“We were surprised to find such a large percentage of women were not using contraception, and we were also surprised to find so many were actively trying to conceive,” the study’s lead author Marie N. Menke, MD, MPH, of the University of Pittsburgh, said in an interview. “Reproductive-age women who are considering bariatric surgery should be counseled that they should plan to use contraceptives after surgery for about 12-18 months.”
Pregnancy isn’t recommended over that period mainly because of the risks to the fetus, Dr. Menke said. “These risks are different from obesity in general. We don’t know exactly why surgery causes these risks, but ideally, patients would be weight-stable prior to conception.”
In 2013, the American Association of Clinical Endocrinologists, the Obesity Society, and the American Society for Metabolic & Bariatric Surgery provided a Grade D recommendation for a 12-18 month delay in conception (Obesity [Silver Spring] 2013;21[suppl 1]:S1-27). A recent study provided more insight into the potential risks of pregnancy soon after weight-loss procedures. It reported that, compared with those who gave birth more than 4 years later, women who gave birth within 2 years of bariatric surgery had higher risks of premature birth, admission to neonatal intensive care units, and small-for-gestational-age infants (JAMA Surg. 2017 Feb 1;152[2]:1-8).
The current prospective cohort study included women who underwent bariatric surgery for the first time during 2005-2009 at 10 U.S. hospitals. The women, all aged 18-44 years, answered questions for as long as 7 years, until January 2015 (Obstet Gynecol. 2017 Oct 6. doi: 10.1097/AOG.0000000000002323).
The analysis included 710 women who provided conception data. The median body mass index of the women was 46.3 kg/m2. Most patients underwent Roux-en-Y gastric bypass (73%), followed by laparoscopic adjustable gastric banding (23%).
Researchers found that 4.3% of the women tried to conceive in the first year after surgery (95% confidence interval, 2.4-6.3), and 13.1% did so in the second year (95% CI, 9.3-17.0; P less than .001).
During the first year after surgery, 12.7% of women had no intercourse (95% CI, 9.4-16.0), 40.5% had only protected intercourse (95% CI, 35.6-45.4), and 41.5% (95% CI, 36.4-46.6) had unprotected intercourse while not trying to conceive.
Why are the unprotected sex numbers so high? “We wonder if some women simply feel that they cannot get pregnant,” Dr. Menke said. “Our group has previously reported that 42% of women who had attempted to conceive prior to bariatric surgery had a history of infertility. Some of these women went on to deliver a live birth, but many did not.”
The study reports that 183 of the 710 women did ultimately conceive and that the number may be as high as 237 because data were missing for 54 women who may have not wanted to report a nonlive birth. Of the women who reported conceiving, 68.9% had live births, 1.1% had stillbirths, 1.1% had ectopic pregnancies, 21.9% miscarried and 7.1% had abortions.
“We’d really like this research to be a reason to consider presurgical contraceptive counseling as one of the steps prior to bariatric surgery,” Dr. Menke said. “This may include a referral by the bariatric surgeon to a physician would who provide counseling, prescribe, or both.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Menke reported having no relevant disclosures. Her coauthors reported financial relationships with pharmaceutical and medical device companies with products to treat metabolic diseases.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: In the first year after surgery, 4.3% of women surveyed tried to conceive (95% CI, 2.4-6.3), and another 41.5% had unprotected intercourse (95% CI, 36.4-46.6).
Data source: Prospective cohort study of 710 women, aged 18-44 years, who underwent bariatric surgery for the first time during 2005-2009 at 10 U.S. hospitals.
Disclosures: The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Menke reported having no relevant disclosures. Her coauthors reported financial relationships with pharmaceutical and medical device companies with products to treat metabolic diseases.
FDA panel advises approval of semaglutide to lower HbA1c in patients with type 2 diabetes
The Food and drug Administration’s Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) recommended the approval of a once-weekly semaglutide injection for adults with type 2 diabetes mellitus. The vote was 16-0 in favor of approval, with one committee member abstaining.
The committee met on Oct. 18 and discussed the safety and efficacy of new drug application (NDA) 209637 for semaglutide injection when used to help glycemic control in addition to diet and exercise. Semaglutide, manufactured and submitted for approval by Novo Nordisk, is described by the company as “an investigational analog of native human glucagonlike peptide–1,” with a half-life of approximately 1 week, making the agent appropriate for weekly dosing.
The researchers found some increase in diabetic retinopathy in the SUSTAIN trials, but a post-hoc analysis found that “To the extent that the data suggest a signal that there was progression of diabetic retinopathy in patients with significant decreases in HbA1c, these events should be expected because they are consistent with treatments that decrease HbA1c. While this decrease may result in an initial increase in retinopathy, ocular health is ultimately benefited by decreasing HbA1c.”
Novo Nordisk submitted the application for semaglutide in December 2016; the drug also is being reviewed in Europe and Japan.
The FDA is not obligated to follow the committee’s recommendation but considers it as part of the review process of new drug applications.
The Food and drug Administration’s Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) recommended the approval of a once-weekly semaglutide injection for adults with type 2 diabetes mellitus. The vote was 16-0 in favor of approval, with one committee member abstaining.
The committee met on Oct. 18 and discussed the safety and efficacy of new drug application (NDA) 209637 for semaglutide injection when used to help glycemic control in addition to diet and exercise. Semaglutide, manufactured and submitted for approval by Novo Nordisk, is described by the company as “an investigational analog of native human glucagonlike peptide–1,” with a half-life of approximately 1 week, making the agent appropriate for weekly dosing.
The researchers found some increase in diabetic retinopathy in the SUSTAIN trials, but a post-hoc analysis found that “To the extent that the data suggest a signal that there was progression of diabetic retinopathy in patients with significant decreases in HbA1c, these events should be expected because they are consistent with treatments that decrease HbA1c. While this decrease may result in an initial increase in retinopathy, ocular health is ultimately benefited by decreasing HbA1c.”
Novo Nordisk submitted the application for semaglutide in December 2016; the drug also is being reviewed in Europe and Japan.
The FDA is not obligated to follow the committee’s recommendation but considers it as part of the review process of new drug applications.
The Food and drug Administration’s Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) recommended the approval of a once-weekly semaglutide injection for adults with type 2 diabetes mellitus. The vote was 16-0 in favor of approval, with one committee member abstaining.
The committee met on Oct. 18 and discussed the safety and efficacy of new drug application (NDA) 209637 for semaglutide injection when used to help glycemic control in addition to diet and exercise. Semaglutide, manufactured and submitted for approval by Novo Nordisk, is described by the company as “an investigational analog of native human glucagonlike peptide–1,” with a half-life of approximately 1 week, making the agent appropriate for weekly dosing.
The researchers found some increase in diabetic retinopathy in the SUSTAIN trials, but a post-hoc analysis found that “To the extent that the data suggest a signal that there was progression of diabetic retinopathy in patients with significant decreases in HbA1c, these events should be expected because they are consistent with treatments that decrease HbA1c. While this decrease may result in an initial increase in retinopathy, ocular health is ultimately benefited by decreasing HbA1c.”
Novo Nordisk submitted the application for semaglutide in December 2016; the drug also is being reviewed in Europe and Japan.
The FDA is not obligated to follow the committee’s recommendation but considers it as part of the review process of new drug applications.
AT AN FDA ADVISORY COMMITTEE MEETING
SGLT inhibition back on track in phase 3 type T1DM trial
LISBON – Despite some earlier concerns over safety, sodium-glucose cotransporter (SGLT) inhibitors appear to be back on track for adjunctive use in people with type 1 diabetes (T1DM), according to phase 3 study findings presented at the annual meeting of the European Association for the Study of Diabetes.
Results of the DEPICT-1 study, showed improved glycemic control, greater weight loss, and no increased risk for either hypoglycemia or ketoacidosis in people treated with the SGLT2 inhibitor dapagliflozin (Farxiga) versus those treated with placebo.

When researchers compared the two dapagliflozin doses with placebo, the mean difference in body weight from baseline to week 24 was –2.96% and –3.72% (both P less than .0001).
Rates of any hypoglycemia in the placebo, 5-mg dapagliflozin, and 10-mg dapagliflozin groups were a respective 79.6%, 79.4%, and 79.4%, and rates of severe hypoglycemia were 7.3%, 7.6%, and 6.4%.
Diabetic ketoacidosis occurred in a respective 1.2%, 1.4%, and 1.7% of patients treated with placebo, dapagliflozin 5 mg, and dapagliflozin 10 mg, respectively.
“Based on this 24-week study, dapagliflozin may be considered as a good candidate as an adjunct to insulin to improve glycemic control in type 1 diabetes,” said the lead investigator for the DEPICT-1 trial, Paresh Dandona, MD, PhD, at the EASD 2017 meeting.
“There may also be other potential long-term cardiovascular and renal benefits, which have recently been demonstrated in type 2 diabetes,” suggested Dr. Dandona, who is a distinguished professor of medicine and chief of the division of endocrinology at the State University of New York at Buffalo. “Of course, that’s an open question, which needs to be investigated and determined in future.”
DEPICT-1 is the first phase 3, randomized, blinded, clinical trial of a selective SGLT2 inhibitor in T1DM, the study’s investigators said in an early online publication (Lancet Diabetes Endocrinol. 2017 Sep 13. doi: 10.1016/S2213-8587[17]30308-X). The study was conducted at 143 sites in 17 centers and involved 833 people with T1DM who were not achieving optimal blood glucose control.
Adults with an HbA1c of 7.7% or higher who had been taking insulin for at least 12 months were recruited and underwent an 8-week lead-in period to optimize their diabetes management before being randomized to take placebo or dapagliflozin 5 mg or 10 mg for 52 weeks. The primary endpoint was the change in HbA1c at Week 24.
DEPICT-1 “provides encouraging short-term data for the efficacy of adjunct SGLT2 inhibition in type 1 diabetes but might also provide insights into how the risk of ketoacidosis can be minimized,” John Petrie, MD, commented in an editorial that accompanied the published findings.
Dr. Petrie, professor of diabetic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow, observed, however, that the results needed to be considered in the context of similar, and also recently presented, findings from the inTandem3 trial (N Engl J Med. 2017 Sep 13. doi: 10.1056/NEJMoa1708337) with the investigational dual SGLT1/SGLT2 inhibitor sotagliflozin. Other trials are expected to report soon with another SGLT2 inhibitor, empagliflozin.
In inTandem3, sotagliflozin helped more people who had T1DM and were on stable insulin to achieve an HbA1c level of 7% or lower at week 24 with no episodes of severe hypoglycemia or diabetic ketoacidosis, compared with those who took insulin alone (28.6% vs. 15.2%; P less than .001). However, the overall rates of ketoacidosis were higher in the patients treated with sotagliflozin than in those treated with placebo, at 3% versus 0.6%, respectively.
In both inTandem3 and DEPICT-1, strategies were in place to help carefully monitor and manage ketoacidosis. Dr. Petrie observed that patients in the latter trial were given a meter that measured both their blood glucose and ketones and were seen in a clinic regularly to assess the risk of ketoacidosis. These frequent visits, which occurred every 2 weeks, were shown to have an independent effect on HbA1c, he pointed out.
“Nevertheless, the investigators kept real-world clinical practice in mind by providing a very simple rule that insulin doses should be reduced by no more than 20% when study medication was started and that they should subsequently be titrated back towards the initial dose,” Dr. Petrie said. “This rule seemed to be quite effective in mitigating ketoacidosis and is feasible to implement in modern clinical practice since meters that measure ketones are increasingly available.
So does this mean a license for SGLT-targeting agents in T1DM could be coming soon? Not yet, Dr. Petrie suggests. “Regulators are likely to await at least the results of the 12-month follow-up, and data from other ongoing trials, before considering an indication for SGLT2 inhibitors in type 1 diabetes,” he said.
Adjunct treatment with these drugs also may require that individuals have “a good understanding of the early-warning symptoms of ketoacidosis” and be prepared to undertake regular home monitoring of both their blood glucose and ketones, as well as having a high level of communication with their diabetes health care providers.
AstraZeneca and Bristol-Myers Squibb funded the study.
Dr. Dandona received research support from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, and AbbVie and was a consultant to AstraZeneca, Novo Nordisk, Sanofi-Aventis, and others.
Dr. Petrie disclosed receiving personal fees and travel expenses from Novo Nordisk; grants and personal fees from Sanofi-Aventis, Quintiles, and Janssen; nonfinancial support from Merck and Itamar Medical; and personal fees from Lilly, ACI Clinical, and Pfizer.
LISBON – Despite some earlier concerns over safety, sodium-glucose cotransporter (SGLT) inhibitors appear to be back on track for adjunctive use in people with type 1 diabetes (T1DM), according to phase 3 study findings presented at the annual meeting of the European Association for the Study of Diabetes.
Results of the DEPICT-1 study, showed improved glycemic control, greater weight loss, and no increased risk for either hypoglycemia or ketoacidosis in people treated with the SGLT2 inhibitor dapagliflozin (Farxiga) versus those treated with placebo.

When researchers compared the two dapagliflozin doses with placebo, the mean difference in body weight from baseline to week 24 was –2.96% and –3.72% (both P less than .0001).
Rates of any hypoglycemia in the placebo, 5-mg dapagliflozin, and 10-mg dapagliflozin groups were a respective 79.6%, 79.4%, and 79.4%, and rates of severe hypoglycemia were 7.3%, 7.6%, and 6.4%.
Diabetic ketoacidosis occurred in a respective 1.2%, 1.4%, and 1.7% of patients treated with placebo, dapagliflozin 5 mg, and dapagliflozin 10 mg, respectively.
“Based on this 24-week study, dapagliflozin may be considered as a good candidate as an adjunct to insulin to improve glycemic control in type 1 diabetes,” said the lead investigator for the DEPICT-1 trial, Paresh Dandona, MD, PhD, at the EASD 2017 meeting.
“There may also be other potential long-term cardiovascular and renal benefits, which have recently been demonstrated in type 2 diabetes,” suggested Dr. Dandona, who is a distinguished professor of medicine and chief of the division of endocrinology at the State University of New York at Buffalo. “Of course, that’s an open question, which needs to be investigated and determined in future.”
DEPICT-1 is the first phase 3, randomized, blinded, clinical trial of a selective SGLT2 inhibitor in T1DM, the study’s investigators said in an early online publication (Lancet Diabetes Endocrinol. 2017 Sep 13. doi: 10.1016/S2213-8587[17]30308-X). The study was conducted at 143 sites in 17 centers and involved 833 people with T1DM who were not achieving optimal blood glucose control.
Adults with an HbA1c of 7.7% or higher who had been taking insulin for at least 12 months were recruited and underwent an 8-week lead-in period to optimize their diabetes management before being randomized to take placebo or dapagliflozin 5 mg or 10 mg for 52 weeks. The primary endpoint was the change in HbA1c at Week 24.
DEPICT-1 “provides encouraging short-term data for the efficacy of adjunct SGLT2 inhibition in type 1 diabetes but might also provide insights into how the risk of ketoacidosis can be minimized,” John Petrie, MD, commented in an editorial that accompanied the published findings.
Dr. Petrie, professor of diabetic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow, observed, however, that the results needed to be considered in the context of similar, and also recently presented, findings from the inTandem3 trial (N Engl J Med. 2017 Sep 13. doi: 10.1056/NEJMoa1708337) with the investigational dual SGLT1/SGLT2 inhibitor sotagliflozin. Other trials are expected to report soon with another SGLT2 inhibitor, empagliflozin.
In inTandem3, sotagliflozin helped more people who had T1DM and were on stable insulin to achieve an HbA1c level of 7% or lower at week 24 with no episodes of severe hypoglycemia or diabetic ketoacidosis, compared with those who took insulin alone (28.6% vs. 15.2%; P less than .001). However, the overall rates of ketoacidosis were higher in the patients treated with sotagliflozin than in those treated with placebo, at 3% versus 0.6%, respectively.
In both inTandem3 and DEPICT-1, strategies were in place to help carefully monitor and manage ketoacidosis. Dr. Petrie observed that patients in the latter trial were given a meter that measured both their blood glucose and ketones and were seen in a clinic regularly to assess the risk of ketoacidosis. These frequent visits, which occurred every 2 weeks, were shown to have an independent effect on HbA1c, he pointed out.
“Nevertheless, the investigators kept real-world clinical practice in mind by providing a very simple rule that insulin doses should be reduced by no more than 20% when study medication was started and that they should subsequently be titrated back towards the initial dose,” Dr. Petrie said. “This rule seemed to be quite effective in mitigating ketoacidosis and is feasible to implement in modern clinical practice since meters that measure ketones are increasingly available.
So does this mean a license for SGLT-targeting agents in T1DM could be coming soon? Not yet, Dr. Petrie suggests. “Regulators are likely to await at least the results of the 12-month follow-up, and data from other ongoing trials, before considering an indication for SGLT2 inhibitors in type 1 diabetes,” he said.
Adjunct treatment with these drugs also may require that individuals have “a good understanding of the early-warning symptoms of ketoacidosis” and be prepared to undertake regular home monitoring of both their blood glucose and ketones, as well as having a high level of communication with their diabetes health care providers.
AstraZeneca and Bristol-Myers Squibb funded the study.
Dr. Dandona received research support from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, and AbbVie and was a consultant to AstraZeneca, Novo Nordisk, Sanofi-Aventis, and others.
Dr. Petrie disclosed receiving personal fees and travel expenses from Novo Nordisk; grants and personal fees from Sanofi-Aventis, Quintiles, and Janssen; nonfinancial support from Merck and Itamar Medical; and personal fees from Lilly, ACI Clinical, and Pfizer.
LISBON – Despite some earlier concerns over safety, sodium-glucose cotransporter (SGLT) inhibitors appear to be back on track for adjunctive use in people with type 1 diabetes (T1DM), according to phase 3 study findings presented at the annual meeting of the European Association for the Study of Diabetes.
Results of the DEPICT-1 study, showed improved glycemic control, greater weight loss, and no increased risk for either hypoglycemia or ketoacidosis in people treated with the SGLT2 inhibitor dapagliflozin (Farxiga) versus those treated with placebo.

When researchers compared the two dapagliflozin doses with placebo, the mean difference in body weight from baseline to week 24 was –2.96% and –3.72% (both P less than .0001).
Rates of any hypoglycemia in the placebo, 5-mg dapagliflozin, and 10-mg dapagliflozin groups were a respective 79.6%, 79.4%, and 79.4%, and rates of severe hypoglycemia were 7.3%, 7.6%, and 6.4%.
Diabetic ketoacidosis occurred in a respective 1.2%, 1.4%, and 1.7% of patients treated with placebo, dapagliflozin 5 mg, and dapagliflozin 10 mg, respectively.
“Based on this 24-week study, dapagliflozin may be considered as a good candidate as an adjunct to insulin to improve glycemic control in type 1 diabetes,” said the lead investigator for the DEPICT-1 trial, Paresh Dandona, MD, PhD, at the EASD 2017 meeting.
“There may also be other potential long-term cardiovascular and renal benefits, which have recently been demonstrated in type 2 diabetes,” suggested Dr. Dandona, who is a distinguished professor of medicine and chief of the division of endocrinology at the State University of New York at Buffalo. “Of course, that’s an open question, which needs to be investigated and determined in future.”
DEPICT-1 is the first phase 3, randomized, blinded, clinical trial of a selective SGLT2 inhibitor in T1DM, the study’s investigators said in an early online publication (Lancet Diabetes Endocrinol. 2017 Sep 13. doi: 10.1016/S2213-8587[17]30308-X). The study was conducted at 143 sites in 17 centers and involved 833 people with T1DM who were not achieving optimal blood glucose control.
Adults with an HbA1c of 7.7% or higher who had been taking insulin for at least 12 months were recruited and underwent an 8-week lead-in period to optimize their diabetes management before being randomized to take placebo or dapagliflozin 5 mg or 10 mg for 52 weeks. The primary endpoint was the change in HbA1c at Week 24.
DEPICT-1 “provides encouraging short-term data for the efficacy of adjunct SGLT2 inhibition in type 1 diabetes but might also provide insights into how the risk of ketoacidosis can be minimized,” John Petrie, MD, commented in an editorial that accompanied the published findings.
Dr. Petrie, professor of diabetic medicine at the Institute of Cardiovascular and Medical Sciences at the University of Glasgow, observed, however, that the results needed to be considered in the context of similar, and also recently presented, findings from the inTandem3 trial (N Engl J Med. 2017 Sep 13. doi: 10.1056/NEJMoa1708337) with the investigational dual SGLT1/SGLT2 inhibitor sotagliflozin. Other trials are expected to report soon with another SGLT2 inhibitor, empagliflozin.
In inTandem3, sotagliflozin helped more people who had T1DM and were on stable insulin to achieve an HbA1c level of 7% or lower at week 24 with no episodes of severe hypoglycemia or diabetic ketoacidosis, compared with those who took insulin alone (28.6% vs. 15.2%; P less than .001). However, the overall rates of ketoacidosis were higher in the patients treated with sotagliflozin than in those treated with placebo, at 3% versus 0.6%, respectively.
In both inTandem3 and DEPICT-1, strategies were in place to help carefully monitor and manage ketoacidosis. Dr. Petrie observed that patients in the latter trial were given a meter that measured both their blood glucose and ketones and were seen in a clinic regularly to assess the risk of ketoacidosis. These frequent visits, which occurred every 2 weeks, were shown to have an independent effect on HbA1c, he pointed out.
“Nevertheless, the investigators kept real-world clinical practice in mind by providing a very simple rule that insulin doses should be reduced by no more than 20% when study medication was started and that they should subsequently be titrated back towards the initial dose,” Dr. Petrie said. “This rule seemed to be quite effective in mitigating ketoacidosis and is feasible to implement in modern clinical practice since meters that measure ketones are increasingly available.
So does this mean a license for SGLT-targeting agents in T1DM could be coming soon? Not yet, Dr. Petrie suggests. “Regulators are likely to await at least the results of the 12-month follow-up, and data from other ongoing trials, before considering an indication for SGLT2 inhibitors in type 1 diabetes,” he said.
Adjunct treatment with these drugs also may require that individuals have “a good understanding of the early-warning symptoms of ketoacidosis” and be prepared to undertake regular home monitoring of both their blood glucose and ketones, as well as having a high level of communication with their diabetes health care providers.
AstraZeneca and Bristol-Myers Squibb funded the study.
Dr. Dandona received research support from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, and AbbVie and was a consultant to AstraZeneca, Novo Nordisk, Sanofi-Aventis, and others.
Dr. Petrie disclosed receiving personal fees and travel expenses from Novo Nordisk; grants and personal fees from Sanofi-Aventis, Quintiles, and Janssen; nonfinancial support from Merck and Itamar Medical; and personal fees from Lilly, ACI Clinical, and Pfizer.
AT EASD 2017
Key clinical point:
Major finding: Dapagliflozin 5 mg and 10 mg reduced HbA1c from Week 0 to Week 24 more (0.42% and 0.45%, respectively) than did placebo (both P less than .0001).
Data source: A phase 3 randomized, double-blind, parallel-controlled, multicenter trial conducted in 833 people with type 1 diabetes.
Disclosures: AstraZeneca and Bristol-Myers Squibb funded the study. Dr. Dandona disclosed acting as a consultant to AstraZeneca, Novo Nordisk, Sanofi-Aventis, Merck, Intarcia, and AbbVie, as well as receiving research support from AstraZeneca, Novo Nordisk, Boehringer Ingelheim, and AbbVie. Dr. Petrie disclosed receiving personal fees and travel expenses from Novo Nordisk; grants and personal fees from Sanofi-Aventis, Quintiles, and Janssen; nonfinancial support from Merck and Itamar Medical; and personal fees from Lilly, ACI Clinical, and Pfizer.
Vitality predicts T2DM major cardiovascular event risk
LISBON – according to the results of a primary care study.
Seldom feeling “full of pep” or not having “a lot of energy” was associated with an increased risk of a major cardiovascular event (MACE) in middle-aged (55-66 years) adults with T2DM, Marta Vergara, MD, reported at the annual meeting of the European Association for the Study of Diabetes.
“It’s well known that patients with type 2 diabetes have a high risk for developing cardiovascular disease, and it is the main cause of death,” said Dr. Vergara of Linköping (Sweden) University.
While several risk factors for cardiovascular disease are known and widely monitored for in clinical practice worldwide, including psychological aspects such as mental stress, ways to identify patients earlier are needed.
“We need more clinically useful and easy-to-manage measurement instruments,” Dr. Vergara said.
Using data from the ongoing Cardiovascular Risk Factors in Patients With Diabetes – a Prospective Study in Primary Care (CARDIPP), Dr. Vergara and colleagues identified two questions used in the 36-Item Short Form (SF-36) that might fit the bill:
- ”How much time during the past 4 weeks did you feel full of pep?”
- “How much time during the past 4 weeks did you have a lot of energy?”
Patients had answered these questions using a 6-point scale ranging from 1, all of the time, to 6 none of the time.
In addition to completing the full SF-36 at recruitment, all patients enrolled in CARDIPP underwent thorough assessment of baseline cardiovascular risk. This included asking about their duration of diabetes and smoking history, taking glycemic and anthropometric readings, and recording their systolic blood pressure. Pulse wave velocity was also used to assess patients’ carotid-femoral artery stiffness.
Over a follow-up period of 7 years, 59 (7.8%) of 761 men and women aged 55-66 years developed ischemic heart disease or had a stroke and needed hospital treatment or died.
“Our data support the use of questions about sense of vitality in order to add prognostic information about subsequent risk of MACE independently of traditional risk markers, such as diabetes duration, age, HbA1c, gender, smoking, systolic blood pressure, and carotid-femoral pulse wave velocity and sagittal abdominal diameter,” Dr. Vergara said.
“We suggest that not feeling full of pep and not having a lot of energy could be used as risk markers for MACE in patients with type 2 diabetes.” These are two easy questions that could be asked as part of the risk assessment process in primary care, she suggested. “Other questions from the SF-36, such as feeling tired or worn out, were not independent markers of MACE,” Dr. Vergara said.
She said she had no relevant financial disclosures.
LISBON – according to the results of a primary care study.
Seldom feeling “full of pep” or not having “a lot of energy” was associated with an increased risk of a major cardiovascular event (MACE) in middle-aged (55-66 years) adults with T2DM, Marta Vergara, MD, reported at the annual meeting of the European Association for the Study of Diabetes.
“It’s well known that patients with type 2 diabetes have a high risk for developing cardiovascular disease, and it is the main cause of death,” said Dr. Vergara of Linköping (Sweden) University.
While several risk factors for cardiovascular disease are known and widely monitored for in clinical practice worldwide, including psychological aspects such as mental stress, ways to identify patients earlier are needed.
“We need more clinically useful and easy-to-manage measurement instruments,” Dr. Vergara said.
Using data from the ongoing Cardiovascular Risk Factors in Patients With Diabetes – a Prospective Study in Primary Care (CARDIPP), Dr. Vergara and colleagues identified two questions used in the 36-Item Short Form (SF-36) that might fit the bill:
- ”How much time during the past 4 weeks did you feel full of pep?”
- “How much time during the past 4 weeks did you have a lot of energy?”
Patients had answered these questions using a 6-point scale ranging from 1, all of the time, to 6 none of the time.
In addition to completing the full SF-36 at recruitment, all patients enrolled in CARDIPP underwent thorough assessment of baseline cardiovascular risk. This included asking about their duration of diabetes and smoking history, taking glycemic and anthropometric readings, and recording their systolic blood pressure. Pulse wave velocity was also used to assess patients’ carotid-femoral artery stiffness.
Over a follow-up period of 7 years, 59 (7.8%) of 761 men and women aged 55-66 years developed ischemic heart disease or had a stroke and needed hospital treatment or died.
“Our data support the use of questions about sense of vitality in order to add prognostic information about subsequent risk of MACE independently of traditional risk markers, such as diabetes duration, age, HbA1c, gender, smoking, systolic blood pressure, and carotid-femoral pulse wave velocity and sagittal abdominal diameter,” Dr. Vergara said.
“We suggest that not feeling full of pep and not having a lot of energy could be used as risk markers for MACE in patients with type 2 diabetes.” These are two easy questions that could be asked as part of the risk assessment process in primary care, she suggested. “Other questions from the SF-36, such as feeling tired or worn out, were not independent markers of MACE,” Dr. Vergara said.
She said she had no relevant financial disclosures.
LISBON – according to the results of a primary care study.
Seldom feeling “full of pep” or not having “a lot of energy” was associated with an increased risk of a major cardiovascular event (MACE) in middle-aged (55-66 years) adults with T2DM, Marta Vergara, MD, reported at the annual meeting of the European Association for the Study of Diabetes.
“It’s well known that patients with type 2 diabetes have a high risk for developing cardiovascular disease, and it is the main cause of death,” said Dr. Vergara of Linköping (Sweden) University.
While several risk factors for cardiovascular disease are known and widely monitored for in clinical practice worldwide, including psychological aspects such as mental stress, ways to identify patients earlier are needed.
“We need more clinically useful and easy-to-manage measurement instruments,” Dr. Vergara said.
Using data from the ongoing Cardiovascular Risk Factors in Patients With Diabetes – a Prospective Study in Primary Care (CARDIPP), Dr. Vergara and colleagues identified two questions used in the 36-Item Short Form (SF-36) that might fit the bill:
- ”How much time during the past 4 weeks did you feel full of pep?”
- “How much time during the past 4 weeks did you have a lot of energy?”
Patients had answered these questions using a 6-point scale ranging from 1, all of the time, to 6 none of the time.
In addition to completing the full SF-36 at recruitment, all patients enrolled in CARDIPP underwent thorough assessment of baseline cardiovascular risk. This included asking about their duration of diabetes and smoking history, taking glycemic and anthropometric readings, and recording their systolic blood pressure. Pulse wave velocity was also used to assess patients’ carotid-femoral artery stiffness.
Over a follow-up period of 7 years, 59 (7.8%) of 761 men and women aged 55-66 years developed ischemic heart disease or had a stroke and needed hospital treatment or died.
“Our data support the use of questions about sense of vitality in order to add prognostic information about subsequent risk of MACE independently of traditional risk markers, such as diabetes duration, age, HbA1c, gender, smoking, systolic blood pressure, and carotid-femoral pulse wave velocity and sagittal abdominal diameter,” Dr. Vergara said.
“We suggest that not feeling full of pep and not having a lot of energy could be used as risk markers for MACE in patients with type 2 diabetes.” These are two easy questions that could be asked as part of the risk assessment process in primary care, she suggested. “Other questions from the SF-36, such as feeling tired or worn out, were not independent markers of MACE,” Dr. Vergara said.
She said she had no relevant financial disclosures.
AT EASD 2017
Key clinical point: Two simple questions about vitality could help assess the risk of major cardiovascular events in patients with type 2 diabetes mellitus.
Major finding: The hazard ratios for seldom feeling “full of pep” and seldom having “lots of energy” and MACE were a respective 1.31 (P = .003) and 1.44 (P less than .0001).
Data source: A prospective, observational primary care study of 761 patients with type 2 diabetes mellitus.
Disclosures: The presenting author had no relevant financial disclosures.
Oral semaglutide achieves outcomes similar to those of subcutaneous semaglutide
, according to a phase 2, placebo-controlled trial published in the Oct. 17 edition of JAMA.
In a parallel-group, dosage-finding 26-week trial, 632 patients with type 2 diabetes and poor glycemic control were randomized to either 2.5 mg, 5 mg, 10 mg, 20 mg, or 40 mg of once-daily oral semaglutide escalated over 4 weeks (standard escalation); 40 mg escalated over 8 weeks; 40 mg escalated over 2 weeks; once-weekly subcutaneous semaglutide (1 mg) for 26 weeks, or oral placebo.
The study found that all patients who had received oral or subcutaneous semaglutide showed significantly reduced mean hemoglobin A1c levels compared with placebo. Almost all patients treated with semaglutide – both oral and subcutaneous – showed a reduction in HbA1c levels while only 74% of patients in the placebo group did (JAMA 2017 Oct 17;318[15]:1460-70. doi: 10.1001/jama.2017.14752).
The estimated treatment difference compared with placebo at week 26 ranged from –0.4% for the 2.5-mg group to –1.6% for the 40-mg-over-4-weeks group, and –1.6% for the subcutaneous group.
Researchers also saw a dose-dependent decrease in mean body weight from baseline to 26 weeks, ranging from –0.9 kg in the 2.5-mg group to –5.7 kg in the 40-mg standard escalation group, compared with –1.2 kg in the placebo group. The difference between treatment and placebo was significant only for doses at or above 10 mg.
The most common adverse events reported were mild to moderate gastrointestinal problems, mostly associated with oral and subcutaneous semaglutide, which are a known side effect of GLP-1 receptor agonists. There was also a higher rate of premature discontinuation of treatment due to gastrointestinal effects in the semaglutide-treated patients.
However, the overall rate of hypoglycemic episodes was low, and was similar in both semaglutide groups and the placebo group.
There were three cases of acute pancreatitis in patients treated with semaglutide, and the treatment was also associated with a significant increase in heart rate, compared with placebo.
“A longer study duration may have demonstrated the maximum HbA1c level and weight reductions in the groups administered the higher doses of the medication,” wrote Melanie Davies, MD, professor of diabetes medicine at the University of Leicester, England, and her coauthors. “Future trials should assess the efficacy of oral semaglutide in patients with a high baseline HbA1c level to explore its potential in patients who are less well controlled, and in combination with other glucose-lowering agents.”
Semaglutide manufacturer Novo Nordisk provided editorial support. Three authors declared board membership and consultancy fees from the company, as well as institutional grants, lecture fees, and other funding from other pharmaceutical companies. Two authors declared shares in Novo Nordisk. One author declared a patent relating to semaglutide, and two authors declared funding from pharmaceutical companies including Novo Nordisk.
, according to a phase 2, placebo-controlled trial published in the Oct. 17 edition of JAMA.
In a parallel-group, dosage-finding 26-week trial, 632 patients with type 2 diabetes and poor glycemic control were randomized to either 2.5 mg, 5 mg, 10 mg, 20 mg, or 40 mg of once-daily oral semaglutide escalated over 4 weeks (standard escalation); 40 mg escalated over 8 weeks; 40 mg escalated over 2 weeks; once-weekly subcutaneous semaglutide (1 mg) for 26 weeks, or oral placebo.
The study found that all patients who had received oral or subcutaneous semaglutide showed significantly reduced mean hemoglobin A1c levels compared with placebo. Almost all patients treated with semaglutide – both oral and subcutaneous – showed a reduction in HbA1c levels while only 74% of patients in the placebo group did (JAMA 2017 Oct 17;318[15]:1460-70. doi: 10.1001/jama.2017.14752).
The estimated treatment difference compared with placebo at week 26 ranged from –0.4% for the 2.5-mg group to –1.6% for the 40-mg-over-4-weeks group, and –1.6% for the subcutaneous group.
Researchers also saw a dose-dependent decrease in mean body weight from baseline to 26 weeks, ranging from –0.9 kg in the 2.5-mg group to –5.7 kg in the 40-mg standard escalation group, compared with –1.2 kg in the placebo group. The difference between treatment and placebo was significant only for doses at or above 10 mg.
The most common adverse events reported were mild to moderate gastrointestinal problems, mostly associated with oral and subcutaneous semaglutide, which are a known side effect of GLP-1 receptor agonists. There was also a higher rate of premature discontinuation of treatment due to gastrointestinal effects in the semaglutide-treated patients.
However, the overall rate of hypoglycemic episodes was low, and was similar in both semaglutide groups and the placebo group.
There were three cases of acute pancreatitis in patients treated with semaglutide, and the treatment was also associated with a significant increase in heart rate, compared with placebo.
“A longer study duration may have demonstrated the maximum HbA1c level and weight reductions in the groups administered the higher doses of the medication,” wrote Melanie Davies, MD, professor of diabetes medicine at the University of Leicester, England, and her coauthors. “Future trials should assess the efficacy of oral semaglutide in patients with a high baseline HbA1c level to explore its potential in patients who are less well controlled, and in combination with other glucose-lowering agents.”
Semaglutide manufacturer Novo Nordisk provided editorial support. Three authors declared board membership and consultancy fees from the company, as well as institutional grants, lecture fees, and other funding from other pharmaceutical companies. Two authors declared shares in Novo Nordisk. One author declared a patent relating to semaglutide, and two authors declared funding from pharmaceutical companies including Novo Nordisk.
, according to a phase 2, placebo-controlled trial published in the Oct. 17 edition of JAMA.
In a parallel-group, dosage-finding 26-week trial, 632 patients with type 2 diabetes and poor glycemic control were randomized to either 2.5 mg, 5 mg, 10 mg, 20 mg, or 40 mg of once-daily oral semaglutide escalated over 4 weeks (standard escalation); 40 mg escalated over 8 weeks; 40 mg escalated over 2 weeks; once-weekly subcutaneous semaglutide (1 mg) for 26 weeks, or oral placebo.
The study found that all patients who had received oral or subcutaneous semaglutide showed significantly reduced mean hemoglobin A1c levels compared with placebo. Almost all patients treated with semaglutide – both oral and subcutaneous – showed a reduction in HbA1c levels while only 74% of patients in the placebo group did (JAMA 2017 Oct 17;318[15]:1460-70. doi: 10.1001/jama.2017.14752).
The estimated treatment difference compared with placebo at week 26 ranged from –0.4% for the 2.5-mg group to –1.6% for the 40-mg-over-4-weeks group, and –1.6% for the subcutaneous group.
Researchers also saw a dose-dependent decrease in mean body weight from baseline to 26 weeks, ranging from –0.9 kg in the 2.5-mg group to –5.7 kg in the 40-mg standard escalation group, compared with –1.2 kg in the placebo group. The difference between treatment and placebo was significant only for doses at or above 10 mg.
The most common adverse events reported were mild to moderate gastrointestinal problems, mostly associated with oral and subcutaneous semaglutide, which are a known side effect of GLP-1 receptor agonists. There was also a higher rate of premature discontinuation of treatment due to gastrointestinal effects in the semaglutide-treated patients.
However, the overall rate of hypoglycemic episodes was low, and was similar in both semaglutide groups and the placebo group.
There were three cases of acute pancreatitis in patients treated with semaglutide, and the treatment was also associated with a significant increase in heart rate, compared with placebo.
“A longer study duration may have demonstrated the maximum HbA1c level and weight reductions in the groups administered the higher doses of the medication,” wrote Melanie Davies, MD, professor of diabetes medicine at the University of Leicester, England, and her coauthors. “Future trials should assess the efficacy of oral semaglutide in patients with a high baseline HbA1c level to explore its potential in patients who are less well controlled, and in combination with other glucose-lowering agents.”
Semaglutide manufacturer Novo Nordisk provided editorial support. Three authors declared board membership and consultancy fees from the company, as well as institutional grants, lecture fees, and other funding from other pharmaceutical companies. Two authors declared shares in Novo Nordisk. One author declared a patent relating to semaglutide, and two authors declared funding from pharmaceutical companies including Novo Nordisk.
FROM JAMA
Key clinical point: Oral semaglutide achieves similar glucose control in patients with type 2 diabetes when compared with subcutaneous semaglutide.
Major finding: The estimated treatment difference in HbA1c compared with placebo ranged from –0.4% to –1.6% in the oral semaglutide group, and –1.6% for the subcutaneous group
Data source: A phase 2, randomized, parallel-group, dosage-finding trial of 632 patients with type 2 diabetes.
Disclosures: Semaglutide manufacturer Novo Nordisk provided editorial support. Three authors declared board membership and consultancy fees from the company, as well as institutional grants, lecture fees and other funding from other pharmaceutical companies. Two authors declared shares in Novo Nordisk. One author declared a patent relating to semaglutide, and two authors declared funding from pharmaceutical companies including Novo Nordisk.
Nearly 10% of patients with candidemia had CDI
SAN DIEGO – Nearly one in ten adults hospitalized with candidemia had Clostridium difficile coinfections in a large multistate study.
“,” Sharon Tsay, MD, said at an annual scientific meeting on infectious diseases. “In patients with CDI, one in 100 developed candidemia, but in patients with candidemia, nearly one in 10 had CDI,” she said. Patients with diabetes, hemodialysis, solid organ transplantation, or a prior recent hospital stay were significantly more likely to have a CDI coinfection even after the researchers controlled for potential confounders, she reported.
Both candidemia and CDI are serious health care–associated infections that disproportionately affect older, severely ill, and immunosuppressed patients, noted Dr. Tsay, an Epidemic Intelligence Service officer in the mycotic diseases branch at the Centers for Disease Control and Prevention, Atlanta. Every year in the United States, about 50,000 individuals are hospitalized with candidemia, and about 30% die within 30 days of diagnosis. The prevalence of CDI is about tenfold higher, and 30-day mortality rates range between about 1% and 9%.
To understand why candidemia and CDI occur together, consider the effects of oral vancomycin therapy, Dr. Tsay said. Antibiotic pressure disrupts normal gut flora, leading to decreased immunity and Candida colonization. Disrupting the gut microbiome also increases the risk of CDI, which can damage gut mucosa, especially in hypervirulent cases such as C. difficile ribotype 027. Vancomycin can also directly damage the gut mucosa, after which Candida can translocate into the bloodstream.
To better characterize CDI and candidemia coinfections in the United States, Dr. Tsay and her associates analyzed data from CDC’s Emerging Infections Program, which tracks infections of high public health significance in 10 states across the country. Among 2,129 patients with a positive blood culture for Candida from 2014 through 2016, 193 (9%) had a diagnosis of CDI within 90 days. Two-thirds of CDI cases preceded candidemia (median, 10 days) and one-third occurred afterward (median, 7 days). Rates of 30-day mortality rates were 25% in patients with and without CDI. For both groups, Candida albicans was the most commonly identified species, followed by C. glabrata and C. parapsilosis.
A multivariate model identified four risk factors for CDI in patients with candidemia – solid organ transplantation (odds ratio, 3.0), hemodialysis (OR, 1.8), prior hospital stay (OR, 1.7), and diabetes (OR, 1.4). Data were limited to case report forms and did not include information about CDI severity or treatment, Dr. Tsay said.
Dr. Tsay and her associates reported having no conflicts of interest.
SAN DIEGO – Nearly one in ten adults hospitalized with candidemia had Clostridium difficile coinfections in a large multistate study.
“,” Sharon Tsay, MD, said at an annual scientific meeting on infectious diseases. “In patients with CDI, one in 100 developed candidemia, but in patients with candidemia, nearly one in 10 had CDI,” she said. Patients with diabetes, hemodialysis, solid organ transplantation, or a prior recent hospital stay were significantly more likely to have a CDI coinfection even after the researchers controlled for potential confounders, she reported.
Both candidemia and CDI are serious health care–associated infections that disproportionately affect older, severely ill, and immunosuppressed patients, noted Dr. Tsay, an Epidemic Intelligence Service officer in the mycotic diseases branch at the Centers for Disease Control and Prevention, Atlanta. Every year in the United States, about 50,000 individuals are hospitalized with candidemia, and about 30% die within 30 days of diagnosis. The prevalence of CDI is about tenfold higher, and 30-day mortality rates range between about 1% and 9%.
To understand why candidemia and CDI occur together, consider the effects of oral vancomycin therapy, Dr. Tsay said. Antibiotic pressure disrupts normal gut flora, leading to decreased immunity and Candida colonization. Disrupting the gut microbiome also increases the risk of CDI, which can damage gut mucosa, especially in hypervirulent cases such as C. difficile ribotype 027. Vancomycin can also directly damage the gut mucosa, after which Candida can translocate into the bloodstream.
To better characterize CDI and candidemia coinfections in the United States, Dr. Tsay and her associates analyzed data from CDC’s Emerging Infections Program, which tracks infections of high public health significance in 10 states across the country. Among 2,129 patients with a positive blood culture for Candida from 2014 through 2016, 193 (9%) had a diagnosis of CDI within 90 days. Two-thirds of CDI cases preceded candidemia (median, 10 days) and one-third occurred afterward (median, 7 days). Rates of 30-day mortality rates were 25% in patients with and without CDI. For both groups, Candida albicans was the most commonly identified species, followed by C. glabrata and C. parapsilosis.
A multivariate model identified four risk factors for CDI in patients with candidemia – solid organ transplantation (odds ratio, 3.0), hemodialysis (OR, 1.8), prior hospital stay (OR, 1.7), and diabetes (OR, 1.4). Data were limited to case report forms and did not include information about CDI severity or treatment, Dr. Tsay said.
Dr. Tsay and her associates reported having no conflicts of interest.
SAN DIEGO – Nearly one in ten adults hospitalized with candidemia had Clostridium difficile coinfections in a large multistate study.
“,” Sharon Tsay, MD, said at an annual scientific meeting on infectious diseases. “In patients with CDI, one in 100 developed candidemia, but in patients with candidemia, nearly one in 10 had CDI,” she said. Patients with diabetes, hemodialysis, solid organ transplantation, or a prior recent hospital stay were significantly more likely to have a CDI coinfection even after the researchers controlled for potential confounders, she reported.
Both candidemia and CDI are serious health care–associated infections that disproportionately affect older, severely ill, and immunosuppressed patients, noted Dr. Tsay, an Epidemic Intelligence Service officer in the mycotic diseases branch at the Centers for Disease Control and Prevention, Atlanta. Every year in the United States, about 50,000 individuals are hospitalized with candidemia, and about 30% die within 30 days of diagnosis. The prevalence of CDI is about tenfold higher, and 30-day mortality rates range between about 1% and 9%.
To understand why candidemia and CDI occur together, consider the effects of oral vancomycin therapy, Dr. Tsay said. Antibiotic pressure disrupts normal gut flora, leading to decreased immunity and Candida colonization. Disrupting the gut microbiome also increases the risk of CDI, which can damage gut mucosa, especially in hypervirulent cases such as C. difficile ribotype 027. Vancomycin can also directly damage the gut mucosa, after which Candida can translocate into the bloodstream.
To better characterize CDI and candidemia coinfections in the United States, Dr. Tsay and her associates analyzed data from CDC’s Emerging Infections Program, which tracks infections of high public health significance in 10 states across the country. Among 2,129 patients with a positive blood culture for Candida from 2014 through 2016, 193 (9%) had a diagnosis of CDI within 90 days. Two-thirds of CDI cases preceded candidemia (median, 10 days) and one-third occurred afterward (median, 7 days). Rates of 30-day mortality rates were 25% in patients with and without CDI. For both groups, Candida albicans was the most commonly identified species, followed by C. glabrata and C. parapsilosis.
A multivariate model identified four risk factors for CDI in patients with candidemia – solid organ transplantation (odds ratio, 3.0), hemodialysis (OR, 1.8), prior hospital stay (OR, 1.7), and diabetes (OR, 1.4). Data were limited to case report forms and did not include information about CDI severity or treatment, Dr. Tsay said.
Dr. Tsay and her associates reported having no conflicts of interest.
AT IDWEEK 2017
Key clinical point: Look for candidemia and Clostridium difficile infection occurring together.
Major finding: Among 2,129 patients with a positive blood culture for Candida, 193 (9%) had a diagnosis of CDI within 90 days. Risk factors for coinfection included solid organ transplant, hemodialysis, recent hospital stay, and diabetes.
Data source: A multistate analysis of data from the Centers for Disease Control’s Emerging Infections Program.
Disclosures: Dr. Tsay and her associates reported having no conflicts of interest.
Intensive exercise didn’t improve glycemic control for obese pregnant women
An intensive exercise intervention for obese pregnant women did not improve maternal glycemia, but did attenuate excessive gestational weight gain, according to the a single-center, randomized controlled trial published in Obstetrics & Gynecology.
The Healthy Eating, Exercise and Lifestyle Trial, conducted from November 2013 through April 2016 at the Coombe Women and Infants University Hospital in Dublin, randomized 88 pregnant women with a body mass index of 30 kg/m2 or greater at their first prenatal visit to either a medically supervised exercise program or to the control group. The intervention consisted of 50-60 minutes of exercise at least once per week, including resistance or weights and aerobic exercises (Obstet Gynecol. 2017;130:1001-10).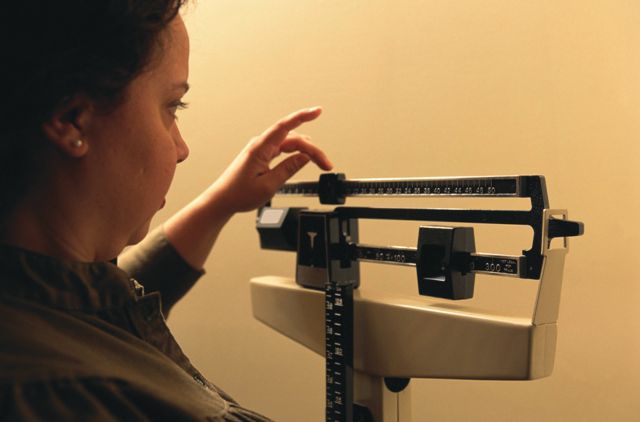
However, excessive gestational weight gain at 36 weeks’ gestation (greater than 9.1 kg) was lower in the exercise group at 22.2%, compared with 43.2% in the control group (P less than .05), Niamh Daly of Coombe Women and Infants University Hospital and her coauthors reported.
“Our study may have failed to improve maternal glycemia because the ideal time to begin such a program could be before pregnancy,” the researchers wrote. “Pregnant women who are obese, however, should be advised to exercise because it attenuates excessive gestational weight gain.”
The study was partially funded by Friends of the Coombe, a charity organization. The authors reported having no financial disclosures.
An intensive exercise intervention for obese pregnant women did not improve maternal glycemia, but did attenuate excessive gestational weight gain, according to the a single-center, randomized controlled trial published in Obstetrics & Gynecology.
The Healthy Eating, Exercise and Lifestyle Trial, conducted from November 2013 through April 2016 at the Coombe Women and Infants University Hospital in Dublin, randomized 88 pregnant women with a body mass index of 30 kg/m2 or greater at their first prenatal visit to either a medically supervised exercise program or to the control group. The intervention consisted of 50-60 minutes of exercise at least once per week, including resistance or weights and aerobic exercises (Obstet Gynecol. 2017;130:1001-10).
However, excessive gestational weight gain at 36 weeks’ gestation (greater than 9.1 kg) was lower in the exercise group at 22.2%, compared with 43.2% in the control group (P less than .05), Niamh Daly of Coombe Women and Infants University Hospital and her coauthors reported.
“Our study may have failed to improve maternal glycemia because the ideal time to begin such a program could be before pregnancy,” the researchers wrote. “Pregnant women who are obese, however, should be advised to exercise because it attenuates excessive gestational weight gain.”
The study was partially funded by Friends of the Coombe, a charity organization. The authors reported having no financial disclosures.
An intensive exercise intervention for obese pregnant women did not improve maternal glycemia, but did attenuate excessive gestational weight gain, according to the a single-center, randomized controlled trial published in Obstetrics & Gynecology.
The Healthy Eating, Exercise and Lifestyle Trial, conducted from November 2013 through April 2016 at the Coombe Women and Infants University Hospital in Dublin, randomized 88 pregnant women with a body mass index of 30 kg/m2 or greater at their first prenatal visit to either a medically supervised exercise program or to the control group. The intervention consisted of 50-60 minutes of exercise at least once per week, including resistance or weights and aerobic exercises (Obstet Gynecol. 2017;130:1001-10).
However, excessive gestational weight gain at 36 weeks’ gestation (greater than 9.1 kg) was lower in the exercise group at 22.2%, compared with 43.2% in the control group (P less than .05), Niamh Daly of Coombe Women and Infants University Hospital and her coauthors reported.
“Our study may have failed to improve maternal glycemia because the ideal time to begin such a program could be before pregnancy,” the researchers wrote. “Pregnant women who are obese, however, should be advised to exercise because it attenuates excessive gestational weight gain.”
The study was partially funded by Friends of the Coombe, a charity organization. The authors reported having no financial disclosures.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: There was no difference in mean fasting plasma glucose between the control group (90.0 plus or minus 9.0 mg/dL) and the exercise group (93.6 plus or minus 7.2 mg/dL) at 24-28 weeks’ gestation (P = .13).
Data source: A single-center, randomized controlled trial of 88 pregnant women with a BMI of 30 kg/m2 or greater.
Disclosures: The study was partially funded by Friends of the Coombe, a charity organization. The authors reported having no financial disclosures.








