User login
HCV Hub
AbbVie
acid
addicted
addiction
adolescent
adult sites
Advocacy
advocacy
agitated states
AJO, postsurgical analgesic, knee, replacement, surgery
alcohol
amphetamine
androgen
antibody
apple cider vinegar
assistance
Assistance
association
at home
attorney
audit
ayurvedic
baby
ban
baricitinib
bed bugs
best
bible
bisexual
black
bleach
blog
bulimia nervosa
buy
cannabis
certificate
certification
certified
cervical cancer, concurrent chemoradiotherapy, intravoxel incoherent motion magnetic resonance imaging, MRI, IVIM, diffusion-weighted MRI, DWI
charlie sheen
cheap
cheapest
child
childhood
childlike
children
chronic fatigue syndrome
Cladribine Tablets
cocaine
cock
combination therapies, synergistic antitumor efficacy, pertuzumab, trastuzumab, ipilimumab, nivolumab, palbociclib, letrozole, lapatinib, docetaxel, trametinib, dabrafenib, carflzomib, lenalidomide
contagious
Cortical Lesions
cream
creams
crime
criminal
cure
dangerous
dangers
dasabuvir
Dasabuvir
dead
deadly
death
dementia
dependence
dependent
depression
dermatillomania
die
diet
direct-acting antivirals
Disability
Discount
discount
dog
drink
drug abuse
drug-induced
dying
eastern medicine
eat
ect
eczema
electroconvulsive therapy
electromagnetic therapy
electrotherapy
epa
epilepsy
erectile dysfunction
explosive disorder
fake
Fake-ovir
fatal
fatalities
fatality
fibromyalgia
financial
Financial
fish oil
food
foods
foundation
free
Gabriel Pardo
gaston
general hospital
genetic
geriatric
Giancarlo Comi
gilead
Gilead
glaucoma
Glenn S. Williams
Glenn Williams
Gloria Dalla Costa
gonorrhea
Greedy
greedy
guns
hallucinations
harvoni
Harvoni
herbal
herbs
heroin
herpes
Hidradenitis Suppurativa,
holistic
home
home remedies
home remedy
homeopathic
homeopathy
hydrocortisone
ice
image
images
job
kid
kids
kill
killer
laser
lawsuit
lawyer
ledipasvir
Ledipasvir
lesbian
lesions
lights
liver
lupus
marijuana
melancholic
memory loss
menopausal
mental retardation
military
milk
moisturizers
monoamine oxidase inhibitor drugs
MRI
MS
murder
national
natural
natural cure
natural cures
natural medications
natural medicine
natural medicines
natural remedies
natural remedy
natural treatment
natural treatments
naturally
Needy
needy
Neurology Reviews
neuropathic
nightclub massacre
nightclub shooting
nude
nudity
nutraceuticals
OASIS
oasis
off label
ombitasvir
Ombitasvir
ombitasvir/paritaprevir/ritonavir with dasabuvir
orlando shooting
overactive thyroid gland
overdose
overdosed
Paolo Preziosa
paritaprevir
Paritaprevir
pediatric
pedophile
photo
photos
picture
post partum
postnatal
pregnancy
pregnant
prenatal
prepartum
prison
program
Program
Protest
protest
psychedelics
pulse nightclub
puppy
purchase
purchasing
rape
recall
recreational drug
Rehabilitation
Retinal Measurements
retrograde ejaculation
risperdal
ritonavir
Ritonavir
ritonavir with dasabuvir
robin williams
sales
sasquatch
schizophrenia
seizure
seizures
sex
sexual
sexy
shock treatment
silver
sleep disorders
smoking
sociopath
sofosbuvir
Sofosbuvir
sovaldi
ssri
store
sue
suicidal
suicide
supplements
support
Support
Support Path
teen
teenage
teenagers
Telerehabilitation
testosterone
Th17
Th17:FoxP3+Treg cell ratio
Th22
toxic
toxin
tragedy
treatment resistant
V Pak
vagina
velpatasvir
Viekira Pa
Viekira Pak
viekira pak
violence
virgin
vitamin
VPak
weight loss
withdrawal
wrinkles
xxx
young adult
young adults
zoloft
financial
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
DDW: VIDEO: What we don’t know in the management of liver disease and coagulopathy
WASHINGTON – Your patient has cirrhosis, platelets 60,000 mm3, an INR of 2.0, serum creatinine of 1.2 mg/dL, and requires an endoscopic retrograde cholangiopancreatography with sphincterotomy.
What do you do next?
Management of a patient such as this is challenging, but not just because of the long-perceived risk for bleeding, Dr. Patrick S. Kamath of the Mayo Clinic in Rochester, Minn., said during a clinical symposium at the annual Digestive Disease Week.
Several other factors must be considered, including the clotting risk in patients with liver disease and the fact that procedure-related bleeding risk cannot be adequately determined preprocedure. Transfusions also carry their own dangers in this patient population and should be approached with caution, he said.
To hear more from this world-renowned liver expert, check out our interview as we sat down with Dr. Kamath at this year’s DDW.
Dr. Kamath reported no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
WASHINGTON – Your patient has cirrhosis, platelets 60,000 mm3, an INR of 2.0, serum creatinine of 1.2 mg/dL, and requires an endoscopic retrograde cholangiopancreatography with sphincterotomy.
What do you do next?
Management of a patient such as this is challenging, but not just because of the long-perceived risk for bleeding, Dr. Patrick S. Kamath of the Mayo Clinic in Rochester, Minn., said during a clinical symposium at the annual Digestive Disease Week.
Several other factors must be considered, including the clotting risk in patients with liver disease and the fact that procedure-related bleeding risk cannot be adequately determined preprocedure. Transfusions also carry their own dangers in this patient population and should be approached with caution, he said.
To hear more from this world-renowned liver expert, check out our interview as we sat down with Dr. Kamath at this year’s DDW.
Dr. Kamath reported no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
WASHINGTON – Your patient has cirrhosis, platelets 60,000 mm3, an INR of 2.0, serum creatinine of 1.2 mg/dL, and requires an endoscopic retrograde cholangiopancreatography with sphincterotomy.
What do you do next?
Management of a patient such as this is challenging, but not just because of the long-perceived risk for bleeding, Dr. Patrick S. Kamath of the Mayo Clinic in Rochester, Minn., said during a clinical symposium at the annual Digestive Disease Week.
Several other factors must be considered, including the clotting risk in patients with liver disease and the fact that procedure-related bleeding risk cannot be adequately determined preprocedure. Transfusions also carry their own dangers in this patient population and should be approached with caution, he said.
To hear more from this world-renowned liver expert, check out our interview as we sat down with Dr. Kamath at this year’s DDW.
Dr. Kamath reported no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @pwendl
AT DDW 2015
DDW: Barrett’s ‘indefinite for dysplasia’ may be cancer harbinger
WASHINGTON – A diagnosis of Barrett’s esophagus “indefinite for dysplasia” doesn’t mean that the patient is home free; it may, in fact, be an indicator of increased risk for progression to high-grade dysplasia or esophageal adenocarcinoma, investigators suggest.
Among 87 patients who had follow-up endoscopies and biopsies within a year of a diagnosis of Barrett’s indefinite for dysplasia (IND), 7 had progression of disease, including 5 who developed high-grade dysplasia or adenocarcinoma, and 2 who developed low-grade disease.
Of these seven patients, four had disease progression with 6 months of a diagnosis of Barrett’s IND, Dr, Michelle Ma reported at the annual Digestive Disease Week.
“Indefinite for dysplasia is an important diagnostic category that is associated with an increased risk for prevalent dysplasia, particularly within the first 6 months after diagnosis. After 12 months, the risk for progressing to advanced neoplasia is low, and similar to other studies,” said Dr. Ma of the University of Pennsylvania, Philadelphia.
Although there is fairly strong evidence to guide the management of patients with low-grade and high-grade dysplasia, it’s less clear whether Barrett’s IND is risk marker for progression. Dr. Ma said, citing a somewhat fuzzy definition of the term: “Epithelial abnormalities insufficient to diagnose dysplasia, or epithelial abnormalities unclear due to inflammation or sampling.”
Uncertainty about the natural history of IND is reflected in practice guidelines, which either don’t address it, call for repeat endoscopy in 6 months, or call for an expert gastrointestinal pathology review with repeat endoscopy to clarify dysplasia status if the evidence is of low quality, Dr. Ma said.
To determine the rate of, and risk factors for, neoplastic progression of IND, the authors took a retrospective look at patients in their center’s pathology database and Barrett’s esophagus register with a histopathologic diagnosis of IND from 2000 through 2014. They excluded patients with frank dysplasia or carcinoma on diagnosis, and those who did not have follow-up endoscopies.
The investigatoes factored demographic variables into their analysis, including age, gender, body-mass index, smoking history, use of proton-pump inhibitors (PPI), nonsteroidal anti-inflammatory drugs, and family history of Barrett’s or esophageal adenocarcinoma. They also considered endoscopic/pathologic characteristics such as hiatal hernia size, Barrett’s segment length, muscosal nodularity, and multifocal IND.
A total of 106 patients with IND were eligible for the analysis, 87 of whom had follow-up endoscopy and biopsy within a year of diagnosis. As noted before, 7 of these patients had prevalent disease, with prevalence rates of 2.3% for low-grade dysplasia, 4.6% for high-grade dysplasia, and 1.1% for esophageal adenocarcinoma.
To determine the incidence of dysplasia, the authors first excluded the 7 patients with prevealent dysplasia and 33 patients who did not have a surveillance endoscopy in the first year following a diagnosis of IND, leaving 66 patients for analysis. Of this group, 3 developed incident dysplasia or adenocarcinoma, yielding an incidence of 4.5% over a median of 32 months of follow-up.
An analysis of risk factors for prevalent dysplasia showed that none of the variables was significant, although Barrett’s segment length approached statistical significance. Looking at risk factors for incident dysplasia, both smoking status (P = .0207) and segment length (P = .0253) were significant predictors.
A pathologist who was not involved in the study pointed out that a diagnosis of IND can mean many things to many people.
“Indefinite for dysplasia is really not an entity, it’s about 5,000 things that we choose to put into that category. It’s a horribly non-reproducible diagnosis,” commented Dr. Henry D. Appelman, a professor of anatomic pathology at the University of Michigan in Ann Arbor, during a aquestion-and-answer period.
He questioned whether the diagnoses of IND were subject to review at her center.
Dr. Ma noted that Barrett’s specimens at her institution are routinely reviewed by pathologists specializing in Barrett’s, who will also review slides of patients referred to the center from other institutions.
The study was internally funded. The authors reported no conflicts of interest.
WASHINGTON – A diagnosis of Barrett’s esophagus “indefinite for dysplasia” doesn’t mean that the patient is home free; it may, in fact, be an indicator of increased risk for progression to high-grade dysplasia or esophageal adenocarcinoma, investigators suggest.
Among 87 patients who had follow-up endoscopies and biopsies within a year of a diagnosis of Barrett’s indefinite for dysplasia (IND), 7 had progression of disease, including 5 who developed high-grade dysplasia or adenocarcinoma, and 2 who developed low-grade disease.
Of these seven patients, four had disease progression with 6 months of a diagnosis of Barrett’s IND, Dr, Michelle Ma reported at the annual Digestive Disease Week.
“Indefinite for dysplasia is an important diagnostic category that is associated with an increased risk for prevalent dysplasia, particularly within the first 6 months after diagnosis. After 12 months, the risk for progressing to advanced neoplasia is low, and similar to other studies,” said Dr. Ma of the University of Pennsylvania, Philadelphia.
Although there is fairly strong evidence to guide the management of patients with low-grade and high-grade dysplasia, it’s less clear whether Barrett’s IND is risk marker for progression. Dr. Ma said, citing a somewhat fuzzy definition of the term: “Epithelial abnormalities insufficient to diagnose dysplasia, or epithelial abnormalities unclear due to inflammation or sampling.”
Uncertainty about the natural history of IND is reflected in practice guidelines, which either don’t address it, call for repeat endoscopy in 6 months, or call for an expert gastrointestinal pathology review with repeat endoscopy to clarify dysplasia status if the evidence is of low quality, Dr. Ma said.
To determine the rate of, and risk factors for, neoplastic progression of IND, the authors took a retrospective look at patients in their center’s pathology database and Barrett’s esophagus register with a histopathologic diagnosis of IND from 2000 through 2014. They excluded patients with frank dysplasia or carcinoma on diagnosis, and those who did not have follow-up endoscopies.
The investigatoes factored demographic variables into their analysis, including age, gender, body-mass index, smoking history, use of proton-pump inhibitors (PPI), nonsteroidal anti-inflammatory drugs, and family history of Barrett’s or esophageal adenocarcinoma. They also considered endoscopic/pathologic characteristics such as hiatal hernia size, Barrett’s segment length, muscosal nodularity, and multifocal IND.
A total of 106 patients with IND were eligible for the analysis, 87 of whom had follow-up endoscopy and biopsy within a year of diagnosis. As noted before, 7 of these patients had prevalent disease, with prevalence rates of 2.3% for low-grade dysplasia, 4.6% for high-grade dysplasia, and 1.1% for esophageal adenocarcinoma.
To determine the incidence of dysplasia, the authors first excluded the 7 patients with prevealent dysplasia and 33 patients who did not have a surveillance endoscopy in the first year following a diagnosis of IND, leaving 66 patients for analysis. Of this group, 3 developed incident dysplasia or adenocarcinoma, yielding an incidence of 4.5% over a median of 32 months of follow-up.
An analysis of risk factors for prevalent dysplasia showed that none of the variables was significant, although Barrett’s segment length approached statistical significance. Looking at risk factors for incident dysplasia, both smoking status (P = .0207) and segment length (P = .0253) were significant predictors.
A pathologist who was not involved in the study pointed out that a diagnosis of IND can mean many things to many people.
“Indefinite for dysplasia is really not an entity, it’s about 5,000 things that we choose to put into that category. It’s a horribly non-reproducible diagnosis,” commented Dr. Henry D. Appelman, a professor of anatomic pathology at the University of Michigan in Ann Arbor, during a aquestion-and-answer period.
He questioned whether the diagnoses of IND were subject to review at her center.
Dr. Ma noted that Barrett’s specimens at her institution are routinely reviewed by pathologists specializing in Barrett’s, who will also review slides of patients referred to the center from other institutions.
The study was internally funded. The authors reported no conflicts of interest.
WASHINGTON – A diagnosis of Barrett’s esophagus “indefinite for dysplasia” doesn’t mean that the patient is home free; it may, in fact, be an indicator of increased risk for progression to high-grade dysplasia or esophageal adenocarcinoma, investigators suggest.
Among 87 patients who had follow-up endoscopies and biopsies within a year of a diagnosis of Barrett’s indefinite for dysplasia (IND), 7 had progression of disease, including 5 who developed high-grade dysplasia or adenocarcinoma, and 2 who developed low-grade disease.
Of these seven patients, four had disease progression with 6 months of a diagnosis of Barrett’s IND, Dr, Michelle Ma reported at the annual Digestive Disease Week.
“Indefinite for dysplasia is an important diagnostic category that is associated with an increased risk for prevalent dysplasia, particularly within the first 6 months after diagnosis. After 12 months, the risk for progressing to advanced neoplasia is low, and similar to other studies,” said Dr. Ma of the University of Pennsylvania, Philadelphia.
Although there is fairly strong evidence to guide the management of patients with low-grade and high-grade dysplasia, it’s less clear whether Barrett’s IND is risk marker for progression. Dr. Ma said, citing a somewhat fuzzy definition of the term: “Epithelial abnormalities insufficient to diagnose dysplasia, or epithelial abnormalities unclear due to inflammation or sampling.”
Uncertainty about the natural history of IND is reflected in practice guidelines, which either don’t address it, call for repeat endoscopy in 6 months, or call for an expert gastrointestinal pathology review with repeat endoscopy to clarify dysplasia status if the evidence is of low quality, Dr. Ma said.
To determine the rate of, and risk factors for, neoplastic progression of IND, the authors took a retrospective look at patients in their center’s pathology database and Barrett’s esophagus register with a histopathologic diagnosis of IND from 2000 through 2014. They excluded patients with frank dysplasia or carcinoma on diagnosis, and those who did not have follow-up endoscopies.
The investigatoes factored demographic variables into their analysis, including age, gender, body-mass index, smoking history, use of proton-pump inhibitors (PPI), nonsteroidal anti-inflammatory drugs, and family history of Barrett’s or esophageal adenocarcinoma. They also considered endoscopic/pathologic characteristics such as hiatal hernia size, Barrett’s segment length, muscosal nodularity, and multifocal IND.
A total of 106 patients with IND were eligible for the analysis, 87 of whom had follow-up endoscopy and biopsy within a year of diagnosis. As noted before, 7 of these patients had prevalent disease, with prevalence rates of 2.3% for low-grade dysplasia, 4.6% for high-grade dysplasia, and 1.1% for esophageal adenocarcinoma.
To determine the incidence of dysplasia, the authors first excluded the 7 patients with prevealent dysplasia and 33 patients who did not have a surveillance endoscopy in the first year following a diagnosis of IND, leaving 66 patients for analysis. Of this group, 3 developed incident dysplasia or adenocarcinoma, yielding an incidence of 4.5% over a median of 32 months of follow-up.
An analysis of risk factors for prevalent dysplasia showed that none of the variables was significant, although Barrett’s segment length approached statistical significance. Looking at risk factors for incident dysplasia, both smoking status (P = .0207) and segment length (P = .0253) were significant predictors.
A pathologist who was not involved in the study pointed out that a diagnosis of IND can mean many things to many people.
“Indefinite for dysplasia is really not an entity, it’s about 5,000 things that we choose to put into that category. It’s a horribly non-reproducible diagnosis,” commented Dr. Henry D. Appelman, a professor of anatomic pathology at the University of Michigan in Ann Arbor, during a aquestion-and-answer period.
He questioned whether the diagnoses of IND were subject to review at her center.
Dr. Ma noted that Barrett’s specimens at her institution are routinely reviewed by pathologists specializing in Barrett’s, who will also review slides of patients referred to the center from other institutions.
The study was internally funded. The authors reported no conflicts of interest.
AT DDW 2015
Key clinical point: Barrett’s esophagus indefinite for dysplasia may progress to dysplasia or adenocarcinoma within a year of diagnosis.
Major finding: Seven of 87 patients with a diagnosis of Barrett’s indefinite for dysplasia (IND) had disease progression within 1 year.
Data source: Retrospective case series of 87 patients who had follow-up endoscopies and biopsies within a year of a diagnosis of Barrett’s IND.
Disclosures: The study was internally funded. The authors reported no conflicts of interest.
DDW: Significant worker productivity gains with newer hepatitis C drugs
Achieving a cure in hepatitis C infection could result in significant economic gains, with a study estimating that the beneficial effects in terms of improved worker productivity could total around $3.23 billion per year for the United States alone.
Researchers used data on work productivity and activity scores from patients enrolled in clinical trials of the all-oral sofosbuvir and lepidasvir combo to estimate the impact of achieving sustained virologic response at 12 weeks (SVR-12) on workers’ productivity.
They calculated an average work productivity loss of $4,954 for each employed patient with chronic hepatitis C infection per year in the United States and $1,129 per year for the five European Union countries included in the mix.
“These new all-oral combinations such as lepidasvir and sofosbuvir have cure rates between 95% and 99% with minimum side effects, [so] treating patients with these combinations results in improved work productivity, improved quality of life, and patient-reported outcomes that can translate into economic benefit,” Dr. Zobair M. Younossi of the Inova Fairfax Medical Campus, Falls Church, Va., said at the annual Digestive Disease Week.
No conflicts of interest were disclosed.
Achieving a cure in hepatitis C infection could result in significant economic gains, with a study estimating that the beneficial effects in terms of improved worker productivity could total around $3.23 billion per year for the United States alone.
Researchers used data on work productivity and activity scores from patients enrolled in clinical trials of the all-oral sofosbuvir and lepidasvir combo to estimate the impact of achieving sustained virologic response at 12 weeks (SVR-12) on workers’ productivity.
They calculated an average work productivity loss of $4,954 for each employed patient with chronic hepatitis C infection per year in the United States and $1,129 per year for the five European Union countries included in the mix.
“These new all-oral combinations such as lepidasvir and sofosbuvir have cure rates between 95% and 99% with minimum side effects, [so] treating patients with these combinations results in improved work productivity, improved quality of life, and patient-reported outcomes that can translate into economic benefit,” Dr. Zobair M. Younossi of the Inova Fairfax Medical Campus, Falls Church, Va., said at the annual Digestive Disease Week.
No conflicts of interest were disclosed.
Achieving a cure in hepatitis C infection could result in significant economic gains, with a study estimating that the beneficial effects in terms of improved worker productivity could total around $3.23 billion per year for the United States alone.
Researchers used data on work productivity and activity scores from patients enrolled in clinical trials of the all-oral sofosbuvir and lepidasvir combo to estimate the impact of achieving sustained virologic response at 12 weeks (SVR-12) on workers’ productivity.
They calculated an average work productivity loss of $4,954 for each employed patient with chronic hepatitis C infection per year in the United States and $1,129 per year for the five European Union countries included in the mix.
“These new all-oral combinations such as lepidasvir and sofosbuvir have cure rates between 95% and 99% with minimum side effects, [so] treating patients with these combinations results in improved work productivity, improved quality of life, and patient-reported outcomes that can translate into economic benefit,” Dr. Zobair M. Younossi of the Inova Fairfax Medical Campus, Falls Church, Va., said at the annual Digestive Disease Week.
No conflicts of interest were disclosed.
FROM DDW 2015
Key clinical point: Achieving a cure in hepatitis C infection could result in significant economic gains from improved worker productivity.
Major finding: The beneficial effects in terms of improved worker productivity could total around $3.23 billion per year for the United States alone.
Data source: Economic model using data from hepatitis C clinical trials in the United States and Europe.
Disclosures: No conflicts of interest were disclosed.
DDW: Biologic agents improve Crohn’s disease picture
WASHINGTON – The course of Crohn’s disease has changed for the better since the introduction of biological therapies and other treatments in the late 1990s.
“In an era of novel treatment options and strategies for Crohn’s disease, we are seeing that the hospitalization and surgery rates have declined, but progression to a complicated phenotype is unfortunately still common nowadays,” said Dr. Steven Jeuring from Maastricht (the Netherlands) University Medical Center.
A community-based study comparing outcomes for patients with Crohn’s Disease (CD) before and after the introduction of infliximab (Remicade) in the Netherlands in 1999 showed the risk of hospitalization was 55% lower for patients treated after 1999, and the risk for hospitalization during the course of the disease was 35% lower. Similarly, the risk for requiring surgery was 77% lower for patients treated in the modern era, and the risk for surgery at any time in the disease course was 54% lower, Dr. Jeuring said at the annual Digestive Disease Week.
The prevalence of a complicated CD phenotype, marked by the presence of bowel stricturing and/or penetration, was also 48% lower at the time of diagnosis among patients treated within the last two decades, but the rate of progression from an inflammatory to complicated phenotype remained unchanged from the pre-biologics era, Dr. Jeuring said.
He and his colleagues conducted a retrospective study with a population-based cohort of adults with incident inflammatory bowel disease diagnosed from 1991 through 1998 (342 patients) and from 1999 through 2011 (820 patients). The cohort represented 93% of all patients in the IBD registry of the South Limburg region of the Netherlands.
They found that the distribution of disease phenotypes was significantly different between the two time periods, with 45% of patients having complicated disease in the pre-biologics era, compared with 37% during the biologics era, translating into a 23% reduction over time. However, in both cohorts, a fairly large proportion of patients already had complicated disease at the time of diagnosis, Dr. Jeuring noted.
When the investigators looked at the risk of developing stricturing or penetrating disease during 8 years of follow-up, however, they found that there was no difference between the groups, with 30% of patients in the earlier cohort having disease progression, compared with 28% of patients in the later cohort, translating into a non-significant hazard ratio (HR) of 0.95.
Dr. Jeuring said that this finding was “very, very disappointing.”
More encouraging, however was the finding that hospitalization rates in the modern era were significantly lower than in the pre-biologics period. For example, the likelihood of being hospitalized at any time during 8 years of follow-up was 72% in the earlier cohort, compared with 52% in the later cohort.
The HR for being hospitalized at the time of diagnosis among the later vs. earlier cohorts was 0.45, and was statistically significant. Similarly, the HR for being hospitalized at any time over 8 years was 0.65 for patients in the modern era (also significant, with a 95% confidence interval that did not cross 1).
Even more dramatically, more modern therapies were associated with a significant reduction in the risk of being rehospitalized, with a significant HR of 0.29.
The risk for surgical resection either at diagnosis or during the 8-year follow-up period was 52% among patients diagnosed and started on treatment in the 1990s, compared with 25% for patients treated in the new millennium. For the latter cohort, the HRs for surgery at the time of diagnosis and for surgery during follow-up were 0.23 and 0.46, respectively. Both were statistically significant.
The lower risk for surgery for patients in the modern era is primarily driven by decreased risk for surgery for inflammatory disease, Dr, Jeuring said.
The study was supported by Maastricht University Medical Center. Dr. Jeuring reported having no conflicts of interest.
WASHINGTON – The course of Crohn’s disease has changed for the better since the introduction of biological therapies and other treatments in the late 1990s.
“In an era of novel treatment options and strategies for Crohn’s disease, we are seeing that the hospitalization and surgery rates have declined, but progression to a complicated phenotype is unfortunately still common nowadays,” said Dr. Steven Jeuring from Maastricht (the Netherlands) University Medical Center.
A community-based study comparing outcomes for patients with Crohn’s Disease (CD) before and after the introduction of infliximab (Remicade) in the Netherlands in 1999 showed the risk of hospitalization was 55% lower for patients treated after 1999, and the risk for hospitalization during the course of the disease was 35% lower. Similarly, the risk for requiring surgery was 77% lower for patients treated in the modern era, and the risk for surgery at any time in the disease course was 54% lower, Dr. Jeuring said at the annual Digestive Disease Week.
The prevalence of a complicated CD phenotype, marked by the presence of bowel stricturing and/or penetration, was also 48% lower at the time of diagnosis among patients treated within the last two decades, but the rate of progression from an inflammatory to complicated phenotype remained unchanged from the pre-biologics era, Dr. Jeuring said.
He and his colleagues conducted a retrospective study with a population-based cohort of adults with incident inflammatory bowel disease diagnosed from 1991 through 1998 (342 patients) and from 1999 through 2011 (820 patients). The cohort represented 93% of all patients in the IBD registry of the South Limburg region of the Netherlands.
They found that the distribution of disease phenotypes was significantly different between the two time periods, with 45% of patients having complicated disease in the pre-biologics era, compared with 37% during the biologics era, translating into a 23% reduction over time. However, in both cohorts, a fairly large proportion of patients already had complicated disease at the time of diagnosis, Dr. Jeuring noted.
When the investigators looked at the risk of developing stricturing or penetrating disease during 8 years of follow-up, however, they found that there was no difference between the groups, with 30% of patients in the earlier cohort having disease progression, compared with 28% of patients in the later cohort, translating into a non-significant hazard ratio (HR) of 0.95.
Dr. Jeuring said that this finding was “very, very disappointing.”
More encouraging, however was the finding that hospitalization rates in the modern era were significantly lower than in the pre-biologics period. For example, the likelihood of being hospitalized at any time during 8 years of follow-up was 72% in the earlier cohort, compared with 52% in the later cohort.
The HR for being hospitalized at the time of diagnosis among the later vs. earlier cohorts was 0.45, and was statistically significant. Similarly, the HR for being hospitalized at any time over 8 years was 0.65 for patients in the modern era (also significant, with a 95% confidence interval that did not cross 1).
Even more dramatically, more modern therapies were associated with a significant reduction in the risk of being rehospitalized, with a significant HR of 0.29.
The risk for surgical resection either at diagnosis or during the 8-year follow-up period was 52% among patients diagnosed and started on treatment in the 1990s, compared with 25% for patients treated in the new millennium. For the latter cohort, the HRs for surgery at the time of diagnosis and for surgery during follow-up were 0.23 and 0.46, respectively. Both were statistically significant.
The lower risk for surgery for patients in the modern era is primarily driven by decreased risk for surgery for inflammatory disease, Dr, Jeuring said.
The study was supported by Maastricht University Medical Center. Dr. Jeuring reported having no conflicts of interest.
WASHINGTON – The course of Crohn’s disease has changed for the better since the introduction of biological therapies and other treatments in the late 1990s.
“In an era of novel treatment options and strategies for Crohn’s disease, we are seeing that the hospitalization and surgery rates have declined, but progression to a complicated phenotype is unfortunately still common nowadays,” said Dr. Steven Jeuring from Maastricht (the Netherlands) University Medical Center.
A community-based study comparing outcomes for patients with Crohn’s Disease (CD) before and after the introduction of infliximab (Remicade) in the Netherlands in 1999 showed the risk of hospitalization was 55% lower for patients treated after 1999, and the risk for hospitalization during the course of the disease was 35% lower. Similarly, the risk for requiring surgery was 77% lower for patients treated in the modern era, and the risk for surgery at any time in the disease course was 54% lower, Dr. Jeuring said at the annual Digestive Disease Week.
The prevalence of a complicated CD phenotype, marked by the presence of bowel stricturing and/or penetration, was also 48% lower at the time of diagnosis among patients treated within the last two decades, but the rate of progression from an inflammatory to complicated phenotype remained unchanged from the pre-biologics era, Dr. Jeuring said.
He and his colleagues conducted a retrospective study with a population-based cohort of adults with incident inflammatory bowel disease diagnosed from 1991 through 1998 (342 patients) and from 1999 through 2011 (820 patients). The cohort represented 93% of all patients in the IBD registry of the South Limburg region of the Netherlands.
They found that the distribution of disease phenotypes was significantly different between the two time periods, with 45% of patients having complicated disease in the pre-biologics era, compared with 37% during the biologics era, translating into a 23% reduction over time. However, in both cohorts, a fairly large proportion of patients already had complicated disease at the time of diagnosis, Dr. Jeuring noted.
When the investigators looked at the risk of developing stricturing or penetrating disease during 8 years of follow-up, however, they found that there was no difference between the groups, with 30% of patients in the earlier cohort having disease progression, compared with 28% of patients in the later cohort, translating into a non-significant hazard ratio (HR) of 0.95.
Dr. Jeuring said that this finding was “very, very disappointing.”
More encouraging, however was the finding that hospitalization rates in the modern era were significantly lower than in the pre-biologics period. For example, the likelihood of being hospitalized at any time during 8 years of follow-up was 72% in the earlier cohort, compared with 52% in the later cohort.
The HR for being hospitalized at the time of diagnosis among the later vs. earlier cohorts was 0.45, and was statistically significant. Similarly, the HR for being hospitalized at any time over 8 years was 0.65 for patients in the modern era (also significant, with a 95% confidence interval that did not cross 1).
Even more dramatically, more modern therapies were associated with a significant reduction in the risk of being rehospitalized, with a significant HR of 0.29.
The risk for surgical resection either at diagnosis or during the 8-year follow-up period was 52% among patients diagnosed and started on treatment in the 1990s, compared with 25% for patients treated in the new millennium. For the latter cohort, the HRs for surgery at the time of diagnosis and for surgery during follow-up were 0.23 and 0.46, respectively. Both were statistically significant.
The lower risk for surgery for patients in the modern era is primarily driven by decreased risk for surgery for inflammatory disease, Dr, Jeuring said.
The study was supported by Maastricht University Medical Center. Dr. Jeuring reported having no conflicts of interest.
AT DDW 2015
Key clinical point: The advent of biologic agents has improved hospitalization and surgery rates for patients with Crohn’s disease.
Major finding: The risk of hospitalization for patients treated after 1999 or for hospitalization during the course of the disease was 55% and 35% lower, respectively. and the risks for requiring surgery in the modern era or at any time in the disease course was 77% and 54% lower.
Data source: Population-based cohort study of 1,162 patients with inflammatory bowel disease in the South Limburg region of the Netherlands.
Disclosures: The study was supported by Maastricht University Medical Center. Dr. Jeuring reported having no conflicts of interest.
DDW: Statin use associated with significantly lower risk of new-onset IBD
WASHINGTON – A prescription for a statin was associated with about a 40% lower risk of new-onset inflammatory bowel disease in a study that evaluated data from a large U.S. health claims database over a 5-year period, Dr. Ryan Ungaro said at the annual Digestive Disease Week.
The protective effect was seen with different statins and was not associated with the intensity of statin treatment, said Dr. Ungaro, a gastroenterologist at Mount Sinai Hospital, New York.
He and his associates conducted a case-control study by using a national medical claims and pharmacy database, identifying 87,579 patients aged 18 and older with an ICD-9 code for a diagnosis of ulcerative colitis (UC) or Crohn’s disease (CD) from January 2008 through December 2012, and 189,526 controls (each case was matched with up to 10 controls, matched for age, gender, race, and state of residence). The median age of cases and controls was about 51 years, and about 41% were male. About 47% were diagnosed with CD, and about 44% were diagnosed with UC. A smaller group of patients who were new-onset cases were included who had at least 1 year with no IBD-related diagnostic code or prescription before the index diagnosis.
Statin use was associated with about a 40% reduced risk of new-onset IBD (odds ratio, 0.59), with similar trends for UC and CD, Dr. Ungaro reported. Separately, the risks of new-onset CD (OR, 0.55) and new-onset UC (OR, 0.62) associated with having a prescription for a statin were also significantly lower.
This association was maintained “regardless of which specific statin a patient was exposed to,” he noted. There was no significant difference in risk of IBD based on the intensity of statin treatment, according to American Heart Association guidelines for low-, moderate-, and high-intensity treatment.
Another finding was that the strongest protective effect was seen in older people, with an OR of 0.55 among those aged 60 years and older. There was no significant effect among those aged 18-30 years, but there were a limited number of statin prescriptions for younger patients, Dr. Ungaro noted.
Limitations of the study include the retrospective design, the inability to directly validate cases, and the reliance on a prescription as a surrogate for the patient actually taking the medication, he said.
Based on the results, “we think that future studies should confirm this protective effect, as well as continue to investigate statins in established IBD,” he concluded.
He referred to recent research demonstrating that statins have immunomodulatory effects “and may actually potentially be beneficial in established IBD.” In addition, a few clinical studies that have looked at the effects of statins in people with established IBD have found that statins are associated with a decreased need for oral steroids and may be associated with improvements in clinical indices and disease and inflammatory markers. Basic science studies indicate that statins are associated with amelioration of disease in mouse models of IBD, may decrease mediators of inflammation, such as tumor necrosis factor-alpha, and may also suppress antigen presentation and T-cell proliferation, he noted.
Dr. Ungaro and his coauthors had no relevant financial disclosures.
WASHINGTON – A prescription for a statin was associated with about a 40% lower risk of new-onset inflammatory bowel disease in a study that evaluated data from a large U.S. health claims database over a 5-year period, Dr. Ryan Ungaro said at the annual Digestive Disease Week.
The protective effect was seen with different statins and was not associated with the intensity of statin treatment, said Dr. Ungaro, a gastroenterologist at Mount Sinai Hospital, New York.
He and his associates conducted a case-control study by using a national medical claims and pharmacy database, identifying 87,579 patients aged 18 and older with an ICD-9 code for a diagnosis of ulcerative colitis (UC) or Crohn’s disease (CD) from January 2008 through December 2012, and 189,526 controls (each case was matched with up to 10 controls, matched for age, gender, race, and state of residence). The median age of cases and controls was about 51 years, and about 41% were male. About 47% were diagnosed with CD, and about 44% were diagnosed with UC. A smaller group of patients who were new-onset cases were included who had at least 1 year with no IBD-related diagnostic code or prescription before the index diagnosis.
Statin use was associated with about a 40% reduced risk of new-onset IBD (odds ratio, 0.59), with similar trends for UC and CD, Dr. Ungaro reported. Separately, the risks of new-onset CD (OR, 0.55) and new-onset UC (OR, 0.62) associated with having a prescription for a statin were also significantly lower.
This association was maintained “regardless of which specific statin a patient was exposed to,” he noted. There was no significant difference in risk of IBD based on the intensity of statin treatment, according to American Heart Association guidelines for low-, moderate-, and high-intensity treatment.
Another finding was that the strongest protective effect was seen in older people, with an OR of 0.55 among those aged 60 years and older. There was no significant effect among those aged 18-30 years, but there were a limited number of statin prescriptions for younger patients, Dr. Ungaro noted.
Limitations of the study include the retrospective design, the inability to directly validate cases, and the reliance on a prescription as a surrogate for the patient actually taking the medication, he said.
Based on the results, “we think that future studies should confirm this protective effect, as well as continue to investigate statins in established IBD,” he concluded.
He referred to recent research demonstrating that statins have immunomodulatory effects “and may actually potentially be beneficial in established IBD.” In addition, a few clinical studies that have looked at the effects of statins in people with established IBD have found that statins are associated with a decreased need for oral steroids and may be associated with improvements in clinical indices and disease and inflammatory markers. Basic science studies indicate that statins are associated with amelioration of disease in mouse models of IBD, may decrease mediators of inflammation, such as tumor necrosis factor-alpha, and may also suppress antigen presentation and T-cell proliferation, he noted.
Dr. Ungaro and his coauthors had no relevant financial disclosures.
WASHINGTON – A prescription for a statin was associated with about a 40% lower risk of new-onset inflammatory bowel disease in a study that evaluated data from a large U.S. health claims database over a 5-year period, Dr. Ryan Ungaro said at the annual Digestive Disease Week.
The protective effect was seen with different statins and was not associated with the intensity of statin treatment, said Dr. Ungaro, a gastroenterologist at Mount Sinai Hospital, New York.
He and his associates conducted a case-control study by using a national medical claims and pharmacy database, identifying 87,579 patients aged 18 and older with an ICD-9 code for a diagnosis of ulcerative colitis (UC) or Crohn’s disease (CD) from January 2008 through December 2012, and 189,526 controls (each case was matched with up to 10 controls, matched for age, gender, race, and state of residence). The median age of cases and controls was about 51 years, and about 41% were male. About 47% were diagnosed with CD, and about 44% were diagnosed with UC. A smaller group of patients who were new-onset cases were included who had at least 1 year with no IBD-related diagnostic code or prescription before the index diagnosis.
Statin use was associated with about a 40% reduced risk of new-onset IBD (odds ratio, 0.59), with similar trends for UC and CD, Dr. Ungaro reported. Separately, the risks of new-onset CD (OR, 0.55) and new-onset UC (OR, 0.62) associated with having a prescription for a statin were also significantly lower.
This association was maintained “regardless of which specific statin a patient was exposed to,” he noted. There was no significant difference in risk of IBD based on the intensity of statin treatment, according to American Heart Association guidelines for low-, moderate-, and high-intensity treatment.
Another finding was that the strongest protective effect was seen in older people, with an OR of 0.55 among those aged 60 years and older. There was no significant effect among those aged 18-30 years, but there were a limited number of statin prescriptions for younger patients, Dr. Ungaro noted.
Limitations of the study include the retrospective design, the inability to directly validate cases, and the reliance on a prescription as a surrogate for the patient actually taking the medication, he said.
Based on the results, “we think that future studies should confirm this protective effect, as well as continue to investigate statins in established IBD,” he concluded.
He referred to recent research demonstrating that statins have immunomodulatory effects “and may actually potentially be beneficial in established IBD.” In addition, a few clinical studies that have looked at the effects of statins in people with established IBD have found that statins are associated with a decreased need for oral steroids and may be associated with improvements in clinical indices and disease and inflammatory markers. Basic science studies indicate that statins are associated with amelioration of disease in mouse models of IBD, may decrease mediators of inflammation, such as tumor necrosis factor-alpha, and may also suppress antigen presentation and T-cell proliferation, he noted.
Dr. Ungaro and his coauthors had no relevant financial disclosures.
AT DDW 2015
Key clinical point: A reduced risk of new onset inflammatory bowel disease (IBD) may be another benefit of statin therapy.
Major finding: A prescription for a statin was associated with about a 40% lower risk of new-onset IBD, an effect that was seen with different statins and only in older people, in a large case-control study.
Data source: The retrospective study evaluated the risk of new-onset Crohn’s disease (CD) and new-onset ulcerative colitis (UC), in about 87,500 patients with a diagnostic code for UC or CD, and controls using a US national medical claims and pharmacy database from 2008-2012.
Disclosures: Dr. Ungaro and his coauthors had no relevant disclosures.
Shortening all-oral HCV therapy feasible
VIENNA – All-oral HCV therapy with grazoprevir and elbasvir plus sofosbuvir achieved high, sustained virologic response (SVR) rates at 12 weeks after only 6-8 weeks of therapy in a proof-of-concept phase II study.
“HCV therapy is evolving toward a once-daily, short duration, pan-genotypic, highly effective and safe regimen,” said Dr. Fred Poordad of the Texas Liver Institute, San Antonio, who reported the findings of the C-SWIFT study at the meeting sponsored by the European Association for the Study of the Liver.
“The aim of this study was to combine three highly potent direct-acting antivirals (DAAs) grazoprevir, elbasvir and sofosbuvir, each with a different mechanisms of action, to see if we could shorten therapy in two of the most difficult-to-cure populations, genotype 1 [GT1] and genotype 3 [GT3] with and without cirrhosis,” he said.
For inclusion, patients had to be treatment naive and older than 18 years of age. Cirrhosis had to be present, assessed either by liver biopsy or via noninvasive testing, and the minimum hemoglobin at enrollment needed to be 9.5 g/dL. Patients also had to be negative for HIV and the hepatitis B virus, and have liver enzyme below 350 IU/L.
Treatment consisted of a fixed-dose, once daily combination of grazoprevir 100 mg and elbasvir 50 mg plus additional sofosbuvir 400 mg, once daily. The duration of therapy depended on genotype and the presence or absence of cirrhosis.
A total of 102 patients with GT1 were enrolled, of whom 61 had no cirrhosis and were treated with the three-drug combination for a total of 4 (n = 31) or 6 (n = 30) weeks. Patients with GT1 and cirrhosis were treated with the regimen for 6 (n = 20) or 8 (n = 21) weeks.
There were 41 GT3 patients enrolled, of whom 29 had no cirrhosis and were treated for 8 (n = 15) or 12 weeks (n = 14). The 12 GT3 cirrhotic patients received 12 weeks of the regimen.
Dr. Poordad noted that the majority of patients studied were male, in their mid-50s, and of white ethnicity. The majority of the GT1 patients were subtype 1a.
Results showed that 33% of GT1 patients without cirrhosis achieved SVR12 with 4 weeks of treatment, and 87% with 6 weeks therapy. SVR12 was achieved in 80% and 94% of patients with GT1 and cirrhosis after 6 and 8 weeks of therapy, respectively.
No patient experienced breakthrough. Twenty of the noncirrhotic GT1 patients treated for 4 weeks relapsed, as did four who were treated for 6 weeks. Four relapses also occurred in cirrhotic GT1 patients treated for 6 weeks and one patient with cirrhosis relapsed after 8 weeks of treatment.
Dr. Poordad noted that patients relapsed most commonly if they had either wild-type virus or resistant-associated variants (RAV) already present at baseline.
In the GT3 arms, SVR12 was achieved by 93% and 100% of the noncirrhotic patients treated for 8 weeks and 12 weeks, respectively, and by 91% of those with cirrhosis who had 12 weeks of treatment. One patient discontinued treatment early for a reason other than virologic failure and two patients relapsed – one patient without cirrhosis in the 8-week treatment group and one in the cirrhotic group.
“All of the patients became ‘target not detected’ at the end of treatment,” Dr. Poordad observed. “The patient in the 8-week arm relapsed somewhere between SVR4 and SVR12, while the cirrhotic patient relapsed in the first 4 weeks of follow-up” he said.
Of the two GT3 patients with biologic failures, one did not have baseline RAVS and also had wild-type virus at relapse. The other patient had RAVs at baseline in NS3 and in NS5A at relapse.
There were “very few adverse events,” Dr. Poordad said, noting that there were no significant declines in hemoglobin or elevations in liver enzymes or bilirubin. The most common adverse events were headache, fatigue, and nausea, all of which occurred in fairly low numbers.
“This novel regimen of grazoprevir/elbasvir with sofosbuvir was able to shorten therapy duration to 8 weeks or less among patients who were cirrhotic and noncirrhotic with genotype 1,” Dr. Poordad said in summary.
Even GT3 patients were able to achieve high SVR12 rates with 8-12 weeks of therapy, including those who had cirrhosis.
“The concept of shortening therapy, even in the cirrhotic patients, has been demonstrated by this study,” he concluded.
He noted that retreatment of virologic failures was ongoing.
The study was sponsored by Merck & Co. Dr. Poordad has received grant/research support from Merck & Co. and numerous other pharmaceutical companies.
VIENNA – All-oral HCV therapy with grazoprevir and elbasvir plus sofosbuvir achieved high, sustained virologic response (SVR) rates at 12 weeks after only 6-8 weeks of therapy in a proof-of-concept phase II study.
“HCV therapy is evolving toward a once-daily, short duration, pan-genotypic, highly effective and safe regimen,” said Dr. Fred Poordad of the Texas Liver Institute, San Antonio, who reported the findings of the C-SWIFT study at the meeting sponsored by the European Association for the Study of the Liver.
“The aim of this study was to combine three highly potent direct-acting antivirals (DAAs) grazoprevir, elbasvir and sofosbuvir, each with a different mechanisms of action, to see if we could shorten therapy in two of the most difficult-to-cure populations, genotype 1 [GT1] and genotype 3 [GT3] with and without cirrhosis,” he said.
For inclusion, patients had to be treatment naive and older than 18 years of age. Cirrhosis had to be present, assessed either by liver biopsy or via noninvasive testing, and the minimum hemoglobin at enrollment needed to be 9.5 g/dL. Patients also had to be negative for HIV and the hepatitis B virus, and have liver enzyme below 350 IU/L.
Treatment consisted of a fixed-dose, once daily combination of grazoprevir 100 mg and elbasvir 50 mg plus additional sofosbuvir 400 mg, once daily. The duration of therapy depended on genotype and the presence or absence of cirrhosis.
A total of 102 patients with GT1 were enrolled, of whom 61 had no cirrhosis and were treated with the three-drug combination for a total of 4 (n = 31) or 6 (n = 30) weeks. Patients with GT1 and cirrhosis were treated with the regimen for 6 (n = 20) or 8 (n = 21) weeks.
There were 41 GT3 patients enrolled, of whom 29 had no cirrhosis and were treated for 8 (n = 15) or 12 weeks (n = 14). The 12 GT3 cirrhotic patients received 12 weeks of the regimen.
Dr. Poordad noted that the majority of patients studied were male, in their mid-50s, and of white ethnicity. The majority of the GT1 patients were subtype 1a.
Results showed that 33% of GT1 patients without cirrhosis achieved SVR12 with 4 weeks of treatment, and 87% with 6 weeks therapy. SVR12 was achieved in 80% and 94% of patients with GT1 and cirrhosis after 6 and 8 weeks of therapy, respectively.
No patient experienced breakthrough. Twenty of the noncirrhotic GT1 patients treated for 4 weeks relapsed, as did four who were treated for 6 weeks. Four relapses also occurred in cirrhotic GT1 patients treated for 6 weeks and one patient with cirrhosis relapsed after 8 weeks of treatment.
Dr. Poordad noted that patients relapsed most commonly if they had either wild-type virus or resistant-associated variants (RAV) already present at baseline.
In the GT3 arms, SVR12 was achieved by 93% and 100% of the noncirrhotic patients treated for 8 weeks and 12 weeks, respectively, and by 91% of those with cirrhosis who had 12 weeks of treatment. One patient discontinued treatment early for a reason other than virologic failure and two patients relapsed – one patient without cirrhosis in the 8-week treatment group and one in the cirrhotic group.
“All of the patients became ‘target not detected’ at the end of treatment,” Dr. Poordad observed. “The patient in the 8-week arm relapsed somewhere between SVR4 and SVR12, while the cirrhotic patient relapsed in the first 4 weeks of follow-up” he said.
Of the two GT3 patients with biologic failures, one did not have baseline RAVS and also had wild-type virus at relapse. The other patient had RAVs at baseline in NS3 and in NS5A at relapse.
There were “very few adverse events,” Dr. Poordad said, noting that there were no significant declines in hemoglobin or elevations in liver enzymes or bilirubin. The most common adverse events were headache, fatigue, and nausea, all of which occurred in fairly low numbers.
“This novel regimen of grazoprevir/elbasvir with sofosbuvir was able to shorten therapy duration to 8 weeks or less among patients who were cirrhotic and noncirrhotic with genotype 1,” Dr. Poordad said in summary.
Even GT3 patients were able to achieve high SVR12 rates with 8-12 weeks of therapy, including those who had cirrhosis.
“The concept of shortening therapy, even in the cirrhotic patients, has been demonstrated by this study,” he concluded.
He noted that retreatment of virologic failures was ongoing.
The study was sponsored by Merck & Co. Dr. Poordad has received grant/research support from Merck & Co. and numerous other pharmaceutical companies.
VIENNA – All-oral HCV therapy with grazoprevir and elbasvir plus sofosbuvir achieved high, sustained virologic response (SVR) rates at 12 weeks after only 6-8 weeks of therapy in a proof-of-concept phase II study.
“HCV therapy is evolving toward a once-daily, short duration, pan-genotypic, highly effective and safe regimen,” said Dr. Fred Poordad of the Texas Liver Institute, San Antonio, who reported the findings of the C-SWIFT study at the meeting sponsored by the European Association for the Study of the Liver.
“The aim of this study was to combine three highly potent direct-acting antivirals (DAAs) grazoprevir, elbasvir and sofosbuvir, each with a different mechanisms of action, to see if we could shorten therapy in two of the most difficult-to-cure populations, genotype 1 [GT1] and genotype 3 [GT3] with and without cirrhosis,” he said.
For inclusion, patients had to be treatment naive and older than 18 years of age. Cirrhosis had to be present, assessed either by liver biopsy or via noninvasive testing, and the minimum hemoglobin at enrollment needed to be 9.5 g/dL. Patients also had to be negative for HIV and the hepatitis B virus, and have liver enzyme below 350 IU/L.
Treatment consisted of a fixed-dose, once daily combination of grazoprevir 100 mg and elbasvir 50 mg plus additional sofosbuvir 400 mg, once daily. The duration of therapy depended on genotype and the presence or absence of cirrhosis.
A total of 102 patients with GT1 were enrolled, of whom 61 had no cirrhosis and were treated with the three-drug combination for a total of 4 (n = 31) or 6 (n = 30) weeks. Patients with GT1 and cirrhosis were treated with the regimen for 6 (n = 20) or 8 (n = 21) weeks.
There were 41 GT3 patients enrolled, of whom 29 had no cirrhosis and were treated for 8 (n = 15) or 12 weeks (n = 14). The 12 GT3 cirrhotic patients received 12 weeks of the regimen.
Dr. Poordad noted that the majority of patients studied were male, in their mid-50s, and of white ethnicity. The majority of the GT1 patients were subtype 1a.
Results showed that 33% of GT1 patients without cirrhosis achieved SVR12 with 4 weeks of treatment, and 87% with 6 weeks therapy. SVR12 was achieved in 80% and 94% of patients with GT1 and cirrhosis after 6 and 8 weeks of therapy, respectively.
No patient experienced breakthrough. Twenty of the noncirrhotic GT1 patients treated for 4 weeks relapsed, as did four who were treated for 6 weeks. Four relapses also occurred in cirrhotic GT1 patients treated for 6 weeks and one patient with cirrhosis relapsed after 8 weeks of treatment.
Dr. Poordad noted that patients relapsed most commonly if they had either wild-type virus or resistant-associated variants (RAV) already present at baseline.
In the GT3 arms, SVR12 was achieved by 93% and 100% of the noncirrhotic patients treated for 8 weeks and 12 weeks, respectively, and by 91% of those with cirrhosis who had 12 weeks of treatment. One patient discontinued treatment early for a reason other than virologic failure and two patients relapsed – one patient without cirrhosis in the 8-week treatment group and one in the cirrhotic group.
“All of the patients became ‘target not detected’ at the end of treatment,” Dr. Poordad observed. “The patient in the 8-week arm relapsed somewhere between SVR4 and SVR12, while the cirrhotic patient relapsed in the first 4 weeks of follow-up” he said.
Of the two GT3 patients with biologic failures, one did not have baseline RAVS and also had wild-type virus at relapse. The other patient had RAVs at baseline in NS3 and in NS5A at relapse.
There were “very few adverse events,” Dr. Poordad said, noting that there were no significant declines in hemoglobin or elevations in liver enzymes or bilirubin. The most common adverse events were headache, fatigue, and nausea, all of which occurred in fairly low numbers.
“This novel regimen of grazoprevir/elbasvir with sofosbuvir was able to shorten therapy duration to 8 weeks or less among patients who were cirrhotic and noncirrhotic with genotype 1,” Dr. Poordad said in summary.
Even GT3 patients were able to achieve high SVR12 rates with 8-12 weeks of therapy, including those who had cirrhosis.
“The concept of shortening therapy, even in the cirrhotic patients, has been demonstrated by this study,” he concluded.
He noted that retreatment of virologic failures was ongoing.
The study was sponsored by Merck & Co. Dr. Poordad has received grant/research support from Merck & Co. and numerous other pharmaceutical companies.
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: Shortening HCV therapy to 6-8 weeks is possible, even in cirrhotic patients.
Major finding: SVR12 was achieved by 87%-94% of GT1 patients treated for 6-8 weeks and by 91%-100% of GT3 patients treated for 8-12 weeks.
Data source: Phase II, open-label study of 143 treatment-naive patients with HCV genotype 1 or 3 with or without cirrhosis treated with a fixed-dose combination of grazoprevir (100 mg) and elbasvir (50 mg) plus sofosbuvir (400 mg) for 4, 6, 8, or 12 weeks.
Disclosures: The study was sponsored by Merck & Co. Dr. Poordad has received grant/research support from Merck & Co. and numerous other pharmaceutical companies.
ILC: Direct antivirals safely clear HCV despite ESRD
VIENNA – A 12-week, fixed-dose regimen safely and effectively eradicated chronic hepatitis C infection from the first 10 patients with advanced chronic kidney disease in a multicenter U.S. series.
The regimen of three direct antiviral agents is already on the U.S. market. So far, 20 HCV patients with advanced chronic kidney disease have been treated and there have been no cases of virologic failure. All 10 patients who have been followed for at least 4 weeks after completing treatment sustained their virologic response, Dr. Paul J. Pockros said at the meeting sponsored by the European Association for the Study of the Liver.
The series has exclusively enrolled patients with genotype 1 hepatitis C virus (HCV) infection. The seven enrolled patients infected with genotype 1b HCV received the three direct antiviral agents only, the combination of ombitasvir, paritaprevir boosted with ritonavir, and dasabuvir, which together are marketed as Viekira Pak. The 13 patients with a genotype 1a infection received treatment with both the three-drug regimen plus ribavirin, which was effective but resulted in significant hemoglobin reduction in eight patients that required dosage interruptions. All patients were then able to restart and continue treatment, said Dr. Pockros, a gastroenterologist at the Scripps Clinic in La Jolla, Calif.
The efficacy and safety of these three direct antivirals in patients with advanced chronic kidney disease – an estimated glomerular filtration rate (eGFR) of no greater than 30 mL/min/1.73 m2 – contrasts with the caution that exists for another major, direct antiviral agent for hepatitis C eradication, sofosbuvir, which is marketed as Sovaldi as an individual drug and as Harvoni when formulated with ledipavir. The labels for both forms of sofosbuvir say that the drug’s safety and efficacy “has not been established in patients with severe renal impairment (eGFR <30 mL/min/1.73m2) or end stage renal disease (ESRD) requiring hemodialysis. No dose recommendation can be given for patients with severe renal impairment or ESRD.”
A new phase of the trial starting soon will test whether patients infected with genotype 1a HCV can be effectively treated with the three direct antiviral agents alone without ribavirin, Dr. Pockros said. Another soon-to-start aspect of the trial will test the regimen in patients with cirrhosis. The current series has so far enrolled only treatment naive patients without cirrhosis.
The RUBY-1 trial has been run at nine U.S. centers, where researchers enrolled seven patients with stage 4 chronic kidney disease (an eGFR of 15-29 ml/min/ 1.73m2), and 13 patients on hemodialysis and with stage 5 chronic kidney disease, defined as an eGFR of less than 15 mL/min/1.73m2. Fourteen of the 20 enrolled patients (70%) were African American, and 15% were Hispanic, a demographic pattern that is “a fair representation” of U.S. patients with both hepatitis C infection and end-stage renal disease, Dr. Prockros said.
The three direct antivirals tested in the current study are all metabolized in the liver and require no dose modification when used in patients with renal dysfunction. Pharmacokinetic studies done as part of the study showed no differences in blood levels of these drugs in the patients with advanced chronic kidney disease compared with historical patients with better renal function. Several reports published in 2014 documented the efficacy of ombitasvir, paritaprevir plus ritonavir, and dasabuvir for eradicating chronic HCV infection in patients with more normal renal function (N. Engl. J. Med. 2014;370:1594-1603, 1604-14, 1973-82, 1983-92).
RUBY-1 was sponsored by AbbVie, which markets Viekira Pak. Dr. Pockros disclosed ties with AbbVie, Janssen, Bristol-Myers Squibb, Gilead, Merck, Conatus, and Roche Molecular.
On Twitter @mitchelzoler
VIENNA – A 12-week, fixed-dose regimen safely and effectively eradicated chronic hepatitis C infection from the first 10 patients with advanced chronic kidney disease in a multicenter U.S. series.
The regimen of three direct antiviral agents is already on the U.S. market. So far, 20 HCV patients with advanced chronic kidney disease have been treated and there have been no cases of virologic failure. All 10 patients who have been followed for at least 4 weeks after completing treatment sustained their virologic response, Dr. Paul J. Pockros said at the meeting sponsored by the European Association for the Study of the Liver.
The series has exclusively enrolled patients with genotype 1 hepatitis C virus (HCV) infection. The seven enrolled patients infected with genotype 1b HCV received the three direct antiviral agents only, the combination of ombitasvir, paritaprevir boosted with ritonavir, and dasabuvir, which together are marketed as Viekira Pak. The 13 patients with a genotype 1a infection received treatment with both the three-drug regimen plus ribavirin, which was effective but resulted in significant hemoglobin reduction in eight patients that required dosage interruptions. All patients were then able to restart and continue treatment, said Dr. Pockros, a gastroenterologist at the Scripps Clinic in La Jolla, Calif.
The efficacy and safety of these three direct antivirals in patients with advanced chronic kidney disease – an estimated glomerular filtration rate (eGFR) of no greater than 30 mL/min/1.73 m2 – contrasts with the caution that exists for another major, direct antiviral agent for hepatitis C eradication, sofosbuvir, which is marketed as Sovaldi as an individual drug and as Harvoni when formulated with ledipavir. The labels for both forms of sofosbuvir say that the drug’s safety and efficacy “has not been established in patients with severe renal impairment (eGFR <30 mL/min/1.73m2) or end stage renal disease (ESRD) requiring hemodialysis. No dose recommendation can be given for patients with severe renal impairment or ESRD.”
A new phase of the trial starting soon will test whether patients infected with genotype 1a HCV can be effectively treated with the three direct antiviral agents alone without ribavirin, Dr. Pockros said. Another soon-to-start aspect of the trial will test the regimen in patients with cirrhosis. The current series has so far enrolled only treatment naive patients without cirrhosis.
The RUBY-1 trial has been run at nine U.S. centers, where researchers enrolled seven patients with stage 4 chronic kidney disease (an eGFR of 15-29 ml/min/ 1.73m2), and 13 patients on hemodialysis and with stage 5 chronic kidney disease, defined as an eGFR of less than 15 mL/min/1.73m2. Fourteen of the 20 enrolled patients (70%) were African American, and 15% were Hispanic, a demographic pattern that is “a fair representation” of U.S. patients with both hepatitis C infection and end-stage renal disease, Dr. Prockros said.
The three direct antivirals tested in the current study are all metabolized in the liver and require no dose modification when used in patients with renal dysfunction. Pharmacokinetic studies done as part of the study showed no differences in blood levels of these drugs in the patients with advanced chronic kidney disease compared with historical patients with better renal function. Several reports published in 2014 documented the efficacy of ombitasvir, paritaprevir plus ritonavir, and dasabuvir for eradicating chronic HCV infection in patients with more normal renal function (N. Engl. J. Med. 2014;370:1594-1603, 1604-14, 1973-82, 1983-92).
RUBY-1 was sponsored by AbbVie, which markets Viekira Pak. Dr. Pockros disclosed ties with AbbVie, Janssen, Bristol-Myers Squibb, Gilead, Merck, Conatus, and Roche Molecular.
On Twitter @mitchelzoler
VIENNA – A 12-week, fixed-dose regimen safely and effectively eradicated chronic hepatitis C infection from the first 10 patients with advanced chronic kidney disease in a multicenter U.S. series.
The regimen of three direct antiviral agents is already on the U.S. market. So far, 20 HCV patients with advanced chronic kidney disease have been treated and there have been no cases of virologic failure. All 10 patients who have been followed for at least 4 weeks after completing treatment sustained their virologic response, Dr. Paul J. Pockros said at the meeting sponsored by the European Association for the Study of the Liver.
The series has exclusively enrolled patients with genotype 1 hepatitis C virus (HCV) infection. The seven enrolled patients infected with genotype 1b HCV received the three direct antiviral agents only, the combination of ombitasvir, paritaprevir boosted with ritonavir, and dasabuvir, which together are marketed as Viekira Pak. The 13 patients with a genotype 1a infection received treatment with both the three-drug regimen plus ribavirin, which was effective but resulted in significant hemoglobin reduction in eight patients that required dosage interruptions. All patients were then able to restart and continue treatment, said Dr. Pockros, a gastroenterologist at the Scripps Clinic in La Jolla, Calif.
The efficacy and safety of these three direct antivirals in patients with advanced chronic kidney disease – an estimated glomerular filtration rate (eGFR) of no greater than 30 mL/min/1.73 m2 – contrasts with the caution that exists for another major, direct antiviral agent for hepatitis C eradication, sofosbuvir, which is marketed as Sovaldi as an individual drug and as Harvoni when formulated with ledipavir. The labels for both forms of sofosbuvir say that the drug’s safety and efficacy “has not been established in patients with severe renal impairment (eGFR <30 mL/min/1.73m2) or end stage renal disease (ESRD) requiring hemodialysis. No dose recommendation can be given for patients with severe renal impairment or ESRD.”
A new phase of the trial starting soon will test whether patients infected with genotype 1a HCV can be effectively treated with the three direct antiviral agents alone without ribavirin, Dr. Pockros said. Another soon-to-start aspect of the trial will test the regimen in patients with cirrhosis. The current series has so far enrolled only treatment naive patients without cirrhosis.
The RUBY-1 trial has been run at nine U.S. centers, where researchers enrolled seven patients with stage 4 chronic kidney disease (an eGFR of 15-29 ml/min/ 1.73m2), and 13 patients on hemodialysis and with stage 5 chronic kidney disease, defined as an eGFR of less than 15 mL/min/1.73m2. Fourteen of the 20 enrolled patients (70%) were African American, and 15% were Hispanic, a demographic pattern that is “a fair representation” of U.S. patients with both hepatitis C infection and end-stage renal disease, Dr. Prockros said.
The three direct antivirals tested in the current study are all metabolized in the liver and require no dose modification when used in patients with renal dysfunction. Pharmacokinetic studies done as part of the study showed no differences in blood levels of these drugs in the patients with advanced chronic kidney disease compared with historical patients with better renal function. Several reports published in 2014 documented the efficacy of ombitasvir, paritaprevir plus ritonavir, and dasabuvir for eradicating chronic HCV infection in patients with more normal renal function (N. Engl. J. Med. 2014;370:1594-1603, 1604-14, 1973-82, 1983-92).
RUBY-1 was sponsored by AbbVie, which markets Viekira Pak. Dr. Pockros disclosed ties with AbbVie, Janssen, Bristol-Myers Squibb, Gilead, Merck, Conatus, and Roche Molecular.
On Twitter @mitchelzoler
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: The trio of direct antiviral agents marketed as Viekira Pak safely eradicated chronic genotype 1 hepatitis C infection in patients with chronic kidney disease.
Major finding: All 10 patients followed so far to at least 4 weeks after completing treatment maintained a sustained virologic response.
Data source: RUBY-1, an open-label series of 20 patients with chronic HCV and advanced chronic kidney disease.
Disclosures: RUBY-1 was sponsored by AbbVie, which markets Viekira Pak. Dr. Pockros disclosed ties with AbbVie, Janssen, Bristol-Myers Squibb, Gilead, Merck, Conatus, and Roche Molecular.
ILC: Europe issues hepatitis C treatment priority list
VIENNA – The new European recommendations for diagnosing and treating hepatitis C infection highlight the paradox gripping the field: Safe and potent antiviral drugs are available to cure most patients after 12 weeks of treatment, but cure is not broadly available because the agents are prohibitively expensive.
“Virtually everyone infected by hepatitis C virus [HCV] has the right to be treated,“ Dr. Jean-Michel Pawlotsky said during a session at the meeting sponsored by the European Association for the Study of the Liver (EASL) that introduced the association’s new hepatitis C treatment recommendations.
The recommendations, released online around the time of the meeting, say: “Because of the approval of highly efficacious new HCV treatment regimens, access to therapy must be broadened.” In addition, they call for expanded screening to find more of the many unidentified cases of chronic hepatitis C infection (J. Hepatology 2015 [doi:10.1016/j.jhep.2015.03.025]). “However, we also have to acknowledge the reality that these drugs are currently too expensive, and the huge number of patients with HCV infection makes it impossible that we could treat all infected patients over the next couple of years,” said Dr. Pawlotsky, head of the department of virology at the Henri Mondor University Hospital in Créteil, France.
The prioritization scheme the EASL panel published gives highest treatment priority to patients with F3 or F4 liver fibrosis on the Metavir scoring system, HIV- or hepatitis B virus–coinfected patients, liver transplant candidates or recent recipients, and patients with significant extrahepatic manifestations, debilitating fatigue, or a high risk for transmitting infection. The list puts patients with moderate liver fibrosis, with a Metavir score of F2, a step down in priority but still rates them candidates for treatment. But the prioritization table rates patients with F1 or F0 fibrosis and none of the other stated complications as reasonable for deferred treatment.
This approach, as well as the general scheme recommended for drug selection, is “remarkably similar” to the guidance first issued and then revised last year by experts assembled by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America, commented Dr. Donald M. Jensen, a hepatologist in Oak Park, Ill., who helped draft the U.S. guidance. “The more these two guidelines are consistent, the more powerful they are,” Dr. Jensen said as an invited discussant at the session.
Perhaps the biggest difference between the HCV treatment recommendations from the European and American groups is that the EASL document included daclatasvir (Daklinza), a direct-acting antiviral for HCV that became available for use in Europe in 2014, but which remains under Food and Drug Administration review for U.S. use as of May 2015.
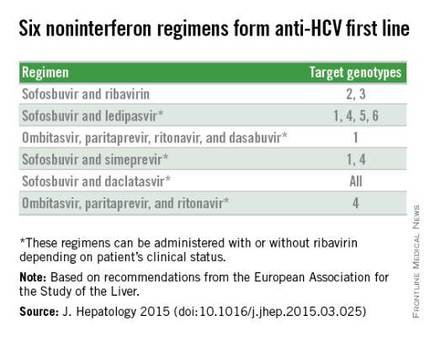
With daclatasvir as an option, the EASL panel highlighted six HCV regimens as first-line HCV options that all avoid treatment with interferon. The recommendations further fine-tune the options from this list of six regimens depending on the infecting HCV genotype, as well as special clinical situations such as compensated or decompensated cirrhosis, recent liver transplant, or end-stage renal disease. The EASL panel also firmly relegated interferon-containing regimens to second-line status.
Another significant issue in choosing among HCV treatments are drug-drug interactions. The EASL panel endorsed a website maintained by the University of Liverpool (England) as an excellent source for researching drug-interaction issues, Dr. Pawlotsky said.
On Twitter @mitchelzoler
VIENNA – The new European recommendations for diagnosing and treating hepatitis C infection highlight the paradox gripping the field: Safe and potent antiviral drugs are available to cure most patients after 12 weeks of treatment, but cure is not broadly available because the agents are prohibitively expensive.
“Virtually everyone infected by hepatitis C virus [HCV] has the right to be treated,“ Dr. Jean-Michel Pawlotsky said during a session at the meeting sponsored by the European Association for the Study of the Liver (EASL) that introduced the association’s new hepatitis C treatment recommendations.
The recommendations, released online around the time of the meeting, say: “Because of the approval of highly efficacious new HCV treatment regimens, access to therapy must be broadened.” In addition, they call for expanded screening to find more of the many unidentified cases of chronic hepatitis C infection (J. Hepatology 2015 [doi:10.1016/j.jhep.2015.03.025]). “However, we also have to acknowledge the reality that these drugs are currently too expensive, and the huge number of patients with HCV infection makes it impossible that we could treat all infected patients over the next couple of years,” said Dr. Pawlotsky, head of the department of virology at the Henri Mondor University Hospital in Créteil, France.
The prioritization scheme the EASL panel published gives highest treatment priority to patients with F3 or F4 liver fibrosis on the Metavir scoring system, HIV- or hepatitis B virus–coinfected patients, liver transplant candidates or recent recipients, and patients with significant extrahepatic manifestations, debilitating fatigue, or a high risk for transmitting infection. The list puts patients with moderate liver fibrosis, with a Metavir score of F2, a step down in priority but still rates them candidates for treatment. But the prioritization table rates patients with F1 or F0 fibrosis and none of the other stated complications as reasonable for deferred treatment.
This approach, as well as the general scheme recommended for drug selection, is “remarkably similar” to the guidance first issued and then revised last year by experts assembled by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America, commented Dr. Donald M. Jensen, a hepatologist in Oak Park, Ill., who helped draft the U.S. guidance. “The more these two guidelines are consistent, the more powerful they are,” Dr. Jensen said as an invited discussant at the session.
Perhaps the biggest difference between the HCV treatment recommendations from the European and American groups is that the EASL document included daclatasvir (Daklinza), a direct-acting antiviral for HCV that became available for use in Europe in 2014, but which remains under Food and Drug Administration review for U.S. use as of May 2015.

With daclatasvir as an option, the EASL panel highlighted six HCV regimens as first-line HCV options that all avoid treatment with interferon. The recommendations further fine-tune the options from this list of six regimens depending on the infecting HCV genotype, as well as special clinical situations such as compensated or decompensated cirrhosis, recent liver transplant, or end-stage renal disease. The EASL panel also firmly relegated interferon-containing regimens to second-line status.
Another significant issue in choosing among HCV treatments are drug-drug interactions. The EASL panel endorsed a website maintained by the University of Liverpool (England) as an excellent source for researching drug-interaction issues, Dr. Pawlotsky said.
On Twitter @mitchelzoler
VIENNA – The new European recommendations for diagnosing and treating hepatitis C infection highlight the paradox gripping the field: Safe and potent antiviral drugs are available to cure most patients after 12 weeks of treatment, but cure is not broadly available because the agents are prohibitively expensive.
“Virtually everyone infected by hepatitis C virus [HCV] has the right to be treated,“ Dr. Jean-Michel Pawlotsky said during a session at the meeting sponsored by the European Association for the Study of the Liver (EASL) that introduced the association’s new hepatitis C treatment recommendations.
The recommendations, released online around the time of the meeting, say: “Because of the approval of highly efficacious new HCV treatment regimens, access to therapy must be broadened.” In addition, they call for expanded screening to find more of the many unidentified cases of chronic hepatitis C infection (J. Hepatology 2015 [doi:10.1016/j.jhep.2015.03.025]). “However, we also have to acknowledge the reality that these drugs are currently too expensive, and the huge number of patients with HCV infection makes it impossible that we could treat all infected patients over the next couple of years,” said Dr. Pawlotsky, head of the department of virology at the Henri Mondor University Hospital in Créteil, France.
The prioritization scheme the EASL panel published gives highest treatment priority to patients with F3 or F4 liver fibrosis on the Metavir scoring system, HIV- or hepatitis B virus–coinfected patients, liver transplant candidates or recent recipients, and patients with significant extrahepatic manifestations, debilitating fatigue, or a high risk for transmitting infection. The list puts patients with moderate liver fibrosis, with a Metavir score of F2, a step down in priority but still rates them candidates for treatment. But the prioritization table rates patients with F1 or F0 fibrosis and none of the other stated complications as reasonable for deferred treatment.
This approach, as well as the general scheme recommended for drug selection, is “remarkably similar” to the guidance first issued and then revised last year by experts assembled by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America, commented Dr. Donald M. Jensen, a hepatologist in Oak Park, Ill., who helped draft the U.S. guidance. “The more these two guidelines are consistent, the more powerful they are,” Dr. Jensen said as an invited discussant at the session.
Perhaps the biggest difference between the HCV treatment recommendations from the European and American groups is that the EASL document included daclatasvir (Daklinza), a direct-acting antiviral for HCV that became available for use in Europe in 2014, but which remains under Food and Drug Administration review for U.S. use as of May 2015.

With daclatasvir as an option, the EASL panel highlighted six HCV regimens as first-line HCV options that all avoid treatment with interferon. The recommendations further fine-tune the options from this list of six regimens depending on the infecting HCV genotype, as well as special clinical situations such as compensated or decompensated cirrhosis, recent liver transplant, or end-stage renal disease. The EASL panel also firmly relegated interferon-containing regimens to second-line status.
Another significant issue in choosing among HCV treatments are drug-drug interactions. The EASL panel endorsed a website maintained by the University of Liverpool (England) as an excellent source for researching drug-interaction issues, Dr. Pawlotsky said.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE INTERNATIONAL LIVER CONGRESS 2015
ILC: HCV treatment delays dented efficacy in era before all-oral therapy
VIENNA – Delaying hepatitis C virus (HVC) treatment can have a detrimental effect on the outcomes of some patients, according to the results of a large 10-year retrospective analysis involving more than 187,000 U.S. veterans.
Indeed, waiting until a patient with HCV infection develops advanced cirrhosis, as measured by the Fibrosis-4 (FIB4) index, can reduce the chances that treatments will be effective and significantly increases morbidity and mortality. Health economist Jeffrey McCombs, Ph.D., of the University of Southern California, Los Angeles, presented data from a 10-year retrospective analysis of the Department of Veteran’s Affairs Veterans Health Administration HCV clinical registry involving more than 187,000 U.S. veterans with detectable HCV levels.
The caveat is that older treatments rather than newer all-oral direct-acting agents were used in the study population at the time of data analyses.
The analysis built on three previous analyses of data from the VA System, the first of which found that viral load suppression reduced the risk of liver events by around 27% and death by 45% (JAMA Intern. Med. 2014;174: 204-12). This analysis also found that relative to patients with genotype 1 (GT1), patients with GT2 were at lower risk and patients with GT3 were at higher risk of liver events and death.
The second analysis looked at treatment timing and found that treatment effectiveness was significantly reduced if treatment was started after common laboratory tests to detect liver changes became abnormal.
The third analysis looked at using the FIB4 index to monitor the risk of long-term morbidity and mortality and found that a FIB4 in excess of 3.4 was associated with a significantly increased risk for liver events and death, whereas a FIB4 of 1.45 was associated with moderate risk.
“This brought us to the last research question, looking at the relationship between treatment efficacy and exceeding certain FIB4 levels,” Dr. McCombs said at the meeting sponsored by the European Association for the Study of the Liver. The analysis compared treating early versus treating late, based on FIB4 levels – FIB4 of 1.0, FIB4 of 1.45, and FIB4 of 3.25.
Initiating treatment early before patients’ FIB4 exceeded 1.0 was associated with a 41% reduction in morbidity and a 36% reduction in mortality. Waiting until the FIB4 exceeded 1.0 resulted in a 30% reduction in liver events and 40% in mortality.
Treating patients early before exceeding a FIB4 of 1.45 was associated with morbidity and mortality reductions of 39% and 40% and waiting until the FIB4 exceeded this threshold was associated with reductions of 27% and 39%, respectively.
Using the FIB4 threshold of 3.25 showed even smaller reductions in morbidity and mortality. When treating early, the reductions were 36% and 45%; when treating late, the reductions were 11% and 25%.
“This is the kind of information you need when you talk with your payers,” Dr. McCombs said. “If you restrict access to treatment, you are not going to be as effective.”
Treating early or late appeared to have a similar effect on viral load and the number needed to treat (NNT) increased using the FIB4 3.5 threshold. Indeed, if treatment is initiated before the FIB4 rises above 3.25, the NNT is 180 patients to prevent one death, Dr. McCombs said, but if treatment is delayed, then the NNT jumps to 325 patients.
In patients with low FIB4 a ‘watch and wait strategy’ could potentially be feasible, he acknowledged in an interview. “The bottom line is that we can’t afford to treat everybody now,” he said.
“The FIB4 is a parameter we can probably watch, but it is unknown exactly how long we can wait,” Dr. McCombs said.
The data were derived from a study that was supported by a grant from Bristol-Myers Squibb. Dr. McCombs had no disclosures.
VIENNA – Delaying hepatitis C virus (HVC) treatment can have a detrimental effect on the outcomes of some patients, according to the results of a large 10-year retrospective analysis involving more than 187,000 U.S. veterans.
Indeed, waiting until a patient with HCV infection develops advanced cirrhosis, as measured by the Fibrosis-4 (FIB4) index, can reduce the chances that treatments will be effective and significantly increases morbidity and mortality. Health economist Jeffrey McCombs, Ph.D., of the University of Southern California, Los Angeles, presented data from a 10-year retrospective analysis of the Department of Veteran’s Affairs Veterans Health Administration HCV clinical registry involving more than 187,000 U.S. veterans with detectable HCV levels.
The caveat is that older treatments rather than newer all-oral direct-acting agents were used in the study population at the time of data analyses.
The analysis built on three previous analyses of data from the VA System, the first of which found that viral load suppression reduced the risk of liver events by around 27% and death by 45% (JAMA Intern. Med. 2014;174: 204-12). This analysis also found that relative to patients with genotype 1 (GT1), patients with GT2 were at lower risk and patients with GT3 were at higher risk of liver events and death.
The second analysis looked at treatment timing and found that treatment effectiveness was significantly reduced if treatment was started after common laboratory tests to detect liver changes became abnormal.
The third analysis looked at using the FIB4 index to monitor the risk of long-term morbidity and mortality and found that a FIB4 in excess of 3.4 was associated with a significantly increased risk for liver events and death, whereas a FIB4 of 1.45 was associated with moderate risk.
“This brought us to the last research question, looking at the relationship between treatment efficacy and exceeding certain FIB4 levels,” Dr. McCombs said at the meeting sponsored by the European Association for the Study of the Liver. The analysis compared treating early versus treating late, based on FIB4 levels – FIB4 of 1.0, FIB4 of 1.45, and FIB4 of 3.25.
Initiating treatment early before patients’ FIB4 exceeded 1.0 was associated with a 41% reduction in morbidity and a 36% reduction in mortality. Waiting until the FIB4 exceeded 1.0 resulted in a 30% reduction in liver events and 40% in mortality.
Treating patients early before exceeding a FIB4 of 1.45 was associated with morbidity and mortality reductions of 39% and 40% and waiting until the FIB4 exceeded this threshold was associated with reductions of 27% and 39%, respectively.
Using the FIB4 threshold of 3.25 showed even smaller reductions in morbidity and mortality. When treating early, the reductions were 36% and 45%; when treating late, the reductions were 11% and 25%.
“This is the kind of information you need when you talk with your payers,” Dr. McCombs said. “If you restrict access to treatment, you are not going to be as effective.”
Treating early or late appeared to have a similar effect on viral load and the number needed to treat (NNT) increased using the FIB4 3.5 threshold. Indeed, if treatment is initiated before the FIB4 rises above 3.25, the NNT is 180 patients to prevent one death, Dr. McCombs said, but if treatment is delayed, then the NNT jumps to 325 patients.
In patients with low FIB4 a ‘watch and wait strategy’ could potentially be feasible, he acknowledged in an interview. “The bottom line is that we can’t afford to treat everybody now,” he said.
“The FIB4 is a parameter we can probably watch, but it is unknown exactly how long we can wait,” Dr. McCombs said.
The data were derived from a study that was supported by a grant from Bristol-Myers Squibb. Dr. McCombs had no disclosures.
VIENNA – Delaying hepatitis C virus (HVC) treatment can have a detrimental effect on the outcomes of some patients, according to the results of a large 10-year retrospective analysis involving more than 187,000 U.S. veterans.
Indeed, waiting until a patient with HCV infection develops advanced cirrhosis, as measured by the Fibrosis-4 (FIB4) index, can reduce the chances that treatments will be effective and significantly increases morbidity and mortality. Health economist Jeffrey McCombs, Ph.D., of the University of Southern California, Los Angeles, presented data from a 10-year retrospective analysis of the Department of Veteran’s Affairs Veterans Health Administration HCV clinical registry involving more than 187,000 U.S. veterans with detectable HCV levels.
The caveat is that older treatments rather than newer all-oral direct-acting agents were used in the study population at the time of data analyses.
The analysis built on three previous analyses of data from the VA System, the first of which found that viral load suppression reduced the risk of liver events by around 27% and death by 45% (JAMA Intern. Med. 2014;174: 204-12). This analysis also found that relative to patients with genotype 1 (GT1), patients with GT2 were at lower risk and patients with GT3 were at higher risk of liver events and death.
The second analysis looked at treatment timing and found that treatment effectiveness was significantly reduced if treatment was started after common laboratory tests to detect liver changes became abnormal.
The third analysis looked at using the FIB4 index to monitor the risk of long-term morbidity and mortality and found that a FIB4 in excess of 3.4 was associated with a significantly increased risk for liver events and death, whereas a FIB4 of 1.45 was associated with moderate risk.
“This brought us to the last research question, looking at the relationship between treatment efficacy and exceeding certain FIB4 levels,” Dr. McCombs said at the meeting sponsored by the European Association for the Study of the Liver. The analysis compared treating early versus treating late, based on FIB4 levels – FIB4 of 1.0, FIB4 of 1.45, and FIB4 of 3.25.
Initiating treatment early before patients’ FIB4 exceeded 1.0 was associated with a 41% reduction in morbidity and a 36% reduction in mortality. Waiting until the FIB4 exceeded 1.0 resulted in a 30% reduction in liver events and 40% in mortality.
Treating patients early before exceeding a FIB4 of 1.45 was associated with morbidity and mortality reductions of 39% and 40% and waiting until the FIB4 exceeded this threshold was associated with reductions of 27% and 39%, respectively.
Using the FIB4 threshold of 3.25 showed even smaller reductions in morbidity and mortality. When treating early, the reductions were 36% and 45%; when treating late, the reductions were 11% and 25%.
“This is the kind of information you need when you talk with your payers,” Dr. McCombs said. “If you restrict access to treatment, you are not going to be as effective.”
Treating early or late appeared to have a similar effect on viral load and the number needed to treat (NNT) increased using the FIB4 3.5 threshold. Indeed, if treatment is initiated before the FIB4 rises above 3.25, the NNT is 180 patients to prevent one death, Dr. McCombs said, but if treatment is delayed, then the NNT jumps to 325 patients.
In patients with low FIB4 a ‘watch and wait strategy’ could potentially be feasible, he acknowledged in an interview. “The bottom line is that we can’t afford to treat everybody now,” he said.
“The FIB4 is a parameter we can probably watch, but it is unknown exactly how long we can wait,” Dr. McCombs said.
The data were derived from a study that was supported by a grant from Bristol-Myers Squibb. Dr. McCombs had no disclosures.
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: Efficacy diminishes when HCV treatment is delayed, particularly in patients who progress to advanced cirrhosis.
Major finding: Delaying treatment until the FIB4 index threshold exceeded 3.25 was associated with an 11% reduction in morbidity and 25% reduction in mortality; when treatment was started earlier, the reductions were 36% and 45%, respectively.
Data source: A 10-year retrospective analysis of the Veterans Health Administration HCV clinical registry involving more than 187,000 U.S. veterans.
Disclosures: The data were derived from a study that was supported by a grant from Bristol-Myers Squibb. Dr. McCombs had no disclosures.
HCV spike in four Appalachian states tied to drug abuse
Acute hepatitis C virus infections more than tripled among young people in Kentucky, Tennessee, Virginia, and West Virginia between 2006 and 2012, investigators reported online May 8 in Morbidity and Mortality Weekly Report.
“The increase in acute HCV infections in central Appalachia is highly correlated with the region’s epidemic of prescription opioid abuse and facilitated by an upsurge in the number of persons who inject drugs,” said Dr. Jon Zibbell at the Centers for Disease Control and Prevention and his associates.
Nationally, acute HCV infections have risen most steeply in states east of the Mississippi. To further explore the trend, the researchers examined HCV case data from the National Notifiable Disease Surveillance System, and data on 217,789 admissions to substance abuse treatment centers related to opioid or injection drug abuse (MMWR 2015;64:453-8).
Confirmed HCV cases among individuals aged 30 years and younger rose by 364% in the four Appalachian states during 2006-2012, the investigators found. “The increasing incidence among nonurban residents was at least double that of urban residents each year,” they said. Among patients with known risk factors for HCV infection, 73% reported injection drug use.
During the same time, treatment admissions for opioid dependency among individuals aged 12-29 years rose by 21% in the four states, and self-reported injection drug use rose by more than 12%, the researchers said. “Evidence-based strategies as well as integrated-service provision are urgently needed in drug treatment programs to ensure patients are tested for HCV, and persons found to be HCV infected are linked to care and receive appropriate treatment,” they concluded. “These efforts will require further collaboration among federal partners and state and local health departments to better address the syndemic of opioid abuse and HCV infection.”
The investigators declared no funding sources or financial conflicts of interest.
Acute hepatitis C virus infections more than tripled among young people in Kentucky, Tennessee, Virginia, and West Virginia between 2006 and 2012, investigators reported online May 8 in Morbidity and Mortality Weekly Report.
“The increase in acute HCV infections in central Appalachia is highly correlated with the region’s epidemic of prescription opioid abuse and facilitated by an upsurge in the number of persons who inject drugs,” said Dr. Jon Zibbell at the Centers for Disease Control and Prevention and his associates.
Nationally, acute HCV infections have risen most steeply in states east of the Mississippi. To further explore the trend, the researchers examined HCV case data from the National Notifiable Disease Surveillance System, and data on 217,789 admissions to substance abuse treatment centers related to opioid or injection drug abuse (MMWR 2015;64:453-8).
Confirmed HCV cases among individuals aged 30 years and younger rose by 364% in the four Appalachian states during 2006-2012, the investigators found. “The increasing incidence among nonurban residents was at least double that of urban residents each year,” they said. Among patients with known risk factors for HCV infection, 73% reported injection drug use.
During the same time, treatment admissions for opioid dependency among individuals aged 12-29 years rose by 21% in the four states, and self-reported injection drug use rose by more than 12%, the researchers said. “Evidence-based strategies as well as integrated-service provision are urgently needed in drug treatment programs to ensure patients are tested for HCV, and persons found to be HCV infected are linked to care and receive appropriate treatment,” they concluded. “These efforts will require further collaboration among federal partners and state and local health departments to better address the syndemic of opioid abuse and HCV infection.”
The investigators declared no funding sources or financial conflicts of interest.
Acute hepatitis C virus infections more than tripled among young people in Kentucky, Tennessee, Virginia, and West Virginia between 2006 and 2012, investigators reported online May 8 in Morbidity and Mortality Weekly Report.
“The increase in acute HCV infections in central Appalachia is highly correlated with the region’s epidemic of prescription opioid abuse and facilitated by an upsurge in the number of persons who inject drugs,” said Dr. Jon Zibbell at the Centers for Disease Control and Prevention and his associates.
Nationally, acute HCV infections have risen most steeply in states east of the Mississippi. To further explore the trend, the researchers examined HCV case data from the National Notifiable Disease Surveillance System, and data on 217,789 admissions to substance abuse treatment centers related to opioid or injection drug abuse (MMWR 2015;64:453-8).
Confirmed HCV cases among individuals aged 30 years and younger rose by 364% in the four Appalachian states during 2006-2012, the investigators found. “The increasing incidence among nonurban residents was at least double that of urban residents each year,” they said. Among patients with known risk factors for HCV infection, 73% reported injection drug use.
During the same time, treatment admissions for opioid dependency among individuals aged 12-29 years rose by 21% in the four states, and self-reported injection drug use rose by more than 12%, the researchers said. “Evidence-based strategies as well as integrated-service provision are urgently needed in drug treatment programs to ensure patients are tested for HCV, and persons found to be HCV infected are linked to care and receive appropriate treatment,” they concluded. “These efforts will require further collaboration among federal partners and state and local health departments to better address the syndemic of opioid abuse and HCV infection.”
The investigators declared no funding sources or financial conflicts of interest.
Key clinical point: Hepatitis C virus infections more than tripled among young people in Kentucky, Tennessee, Virginia, and West Virginia, and were strongly tied to rises in opioid and injection drug abuse.
Major finding: From 2006 to 2012, the number of acute HCV infections increased by 364% among individuals aged 30 years or less.
Data source: Analysis of HCV case data from the National Notifiable Disease Surveillance System and of substance abuse admissions data from the Treatment Episode Data Set.
Disclosures: The investigators reported no funding sources or financial conflicts of interest.