User login
HCV Hub
AbbVie
acid
addicted
addiction
adolescent
adult sites
Advocacy
advocacy
agitated states
AJO, postsurgical analgesic, knee, replacement, surgery
alcohol
amphetamine
androgen
antibody
apple cider vinegar
assistance
Assistance
association
at home
attorney
audit
ayurvedic
baby
ban
baricitinib
bed bugs
best
bible
bisexual
black
bleach
blog
bulimia nervosa
buy
cannabis
certificate
certification
certified
cervical cancer, concurrent chemoradiotherapy, intravoxel incoherent motion magnetic resonance imaging, MRI, IVIM, diffusion-weighted MRI, DWI
charlie sheen
cheap
cheapest
child
childhood
childlike
children
chronic fatigue syndrome
Cladribine Tablets
cocaine
cock
combination therapies, synergistic antitumor efficacy, pertuzumab, trastuzumab, ipilimumab, nivolumab, palbociclib, letrozole, lapatinib, docetaxel, trametinib, dabrafenib, carflzomib, lenalidomide
contagious
Cortical Lesions
cream
creams
crime
criminal
cure
dangerous
dangers
dasabuvir
Dasabuvir
dead
deadly
death
dementia
dependence
dependent
depression
dermatillomania
die
diet
direct-acting antivirals
Disability
Discount
discount
dog
drink
drug abuse
drug-induced
dying
eastern medicine
eat
ect
eczema
electroconvulsive therapy
electromagnetic therapy
electrotherapy
epa
epilepsy
erectile dysfunction
explosive disorder
fake
Fake-ovir
fatal
fatalities
fatality
fibromyalgia
financial
Financial
fish oil
food
foods
foundation
free
Gabriel Pardo
gaston
general hospital
genetic
geriatric
Giancarlo Comi
gilead
Gilead
glaucoma
Glenn S. Williams
Glenn Williams
Gloria Dalla Costa
gonorrhea
Greedy
greedy
guns
hallucinations
harvoni
Harvoni
herbal
herbs
heroin
herpes
Hidradenitis Suppurativa,
holistic
home
home remedies
home remedy
homeopathic
homeopathy
hydrocortisone
ice
image
images
job
kid
kids
kill
killer
laser
lawsuit
lawyer
ledipasvir
Ledipasvir
lesbian
lesions
lights
liver
lupus
marijuana
melancholic
memory loss
menopausal
mental retardation
military
milk
moisturizers
monoamine oxidase inhibitor drugs
MRI
MS
murder
national
natural
natural cure
natural cures
natural medications
natural medicine
natural medicines
natural remedies
natural remedy
natural treatment
natural treatments
naturally
Needy
needy
Neurology Reviews
neuropathic
nightclub massacre
nightclub shooting
nude
nudity
nutraceuticals
OASIS
oasis
off label
ombitasvir
Ombitasvir
ombitasvir/paritaprevir/ritonavir with dasabuvir
orlando shooting
overactive thyroid gland
overdose
overdosed
Paolo Preziosa
paritaprevir
Paritaprevir
pediatric
pedophile
photo
photos
picture
post partum
postnatal
pregnancy
pregnant
prenatal
prepartum
prison
program
Program
Protest
protest
psychedelics
pulse nightclub
puppy
purchase
purchasing
rape
recall
recreational drug
Rehabilitation
Retinal Measurements
retrograde ejaculation
risperdal
ritonavir
Ritonavir
ritonavir with dasabuvir
robin williams
sales
sasquatch
schizophrenia
seizure
seizures
sex
sexual
sexy
shock treatment
silver
sleep disorders
smoking
sociopath
sofosbuvir
Sofosbuvir
sovaldi
ssri
store
sue
suicidal
suicide
supplements
support
Support
Support Path
teen
teenage
teenagers
Telerehabilitation
testosterone
Th17
Th17:FoxP3+Treg cell ratio
Th22
toxic
toxin
tragedy
treatment resistant
V Pak
vagina
velpatasvir
Viekira Pa
Viekira Pak
viekira pak
violence
virgin
vitamin
VPak
weight loss
withdrawal
wrinkles
xxx
young adult
young adults
zoloft
financial
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
All-oral simeprevir-sofosbuvir beat interferon-based regimen for HCV with compensated cirrhosis
Patients with compensated cirrhosis and chronic genotype 1 hepatitis C virus infections were significantly more likely to clear their infections and had fewer adverse effects when treated with simeprevir and sofosbuvir instead of peginterferon, ribavirin, and sofosbuvir, investigators reported in the April issue of Gastroenterology. “Patients given the interferon-containing regimen had a significantly greater rate of virologic relapse than patients given simeprevir and sofosbuvir ... and reported worse outcomes and more side effects,” said Dr. Brian L. Pearlman and his associates of Atlanta Medical Center and Emory University, Atlanta.
Liver disease associated with chronic hepatitis C virus (HCV) infection causes at least 350,000 deaths per year worldwide. Genotype 1 (GT-1) HCV is the most common HCV strain and the hardest to treat, especially when patients have cirrhosis. Treatments for chronic GT-1 HCV infections historically included interferon, but achieved only moderate rates of sustained virologic response (SVR) and caused adverse somatic and psychiatric effects. All-oral, interferon-free regimens are now in widespread use, but many of the pivotal trials were industry sponsored and had strict enrollment criteria, potentially limiting their generalizability in community settings, the researchers reported (Gastroenterology 2015 April [doi:10.1053/j.gastro.2014.12.027]).Their open-label trial included 82 patients with HCV GT-1a infection and Child’s grade A cirrhosis recruited from two clinical practices in Atlanta. Thirty-two of the patients were treatment naive, while 50 had previously not responded to peginterferon and ribavirin treatment. Patients were randomized to either 12 weeks of all-oral simeprevir (150 mg/day) and sofosbuvir (400 mg/day), or peginterferon alfa 2b (1.5 mcg/kg per week), ribavirin (1000-1200 mg/day), and sofosbuvir (400 mg/day).Fully 54 of 58 (93%) patients given the simeprevir-sofosbuvir regimen had undetectable levels of HCV RNA at 12 weeks (SVR12), compared with 18 of 24 (75%) patients who received the interferon-containing regimen (P = .02), the researchers reported. Rates of SVR12 among prior nonresponders were 92% and 64%, and were 95% and 80% for treatment-naive patients, although P-values did not reach statistical significance. The interferon group also had a higher rate of virologic relapse than did patients given simeprevir and sofosbuvir (P = .009), and had worse self-reported outcomes and more side effects, the researchers said. Patients in both groups who achieved SVR12 had higher quality-of-life scores than those who did not. Notably, almost half of patients were African American, a group that has historically had lower cure rates compared with other racial groups, said the investigators.
“Given that several new regimens for genotype 1 infection, including the sofosbuvir-ledipasvir combination pill, already have been approved, we believe that this study is very timely,” Dr. Pearlman and his associates said. “Although these are not head-to-head comparisons, the 12-week simeprevir and sofosbuvir regimen in this trial may compare favorably with the SVR rates for 12 weeks of sofosbuvir/ledipasvir and 12 weeks of the regimen, paritaprevir/ritonavir, ombitasvir and dasabuvir plus ribavirin for patients with cirrhosis, particularly for prior nonresponders to older therapies, with the caveat that many more patients were studied in the registration trials.”While industry-sponsored trials have had psychiatric exclusion criteria, several patients in the open-label trial had serious psychiatric conditions, said the researchers. A patient with stable bipolar disorder received the interferon-containing regimen, and two patients with stable schizophrenia received the all-oral regimen. None reported worsening psychiatric symptoms on treatment.
Dr. Pearlman reported having contracted research for Johnson & Johnson, Gilead, Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, and Merck; and having served on speaker and advisory boards for Johnson & Johnson, Gilead, and Abbvie. He also was an investigator and author in the COSMOS trial of simeprevir and sofosbuvir in patients with HCV/HIV coinfection. The other authors reported no conflicts of interest.
At the time of sofosbuvir (SOF) and simeprevir (SMV) approval, efficacy results of the combinations assessed in this study were still limited, especially in patients with cirrhosis, GT-1a, and/or previous nonresponse. The recent study by Dr. Pearlman and his associates aims to give light to some of the questions

|
| Dr. Xavier Forns |
raised. In this open-label trial, 82 patients with GT-1a cirrhosis (61% null responders) were recruited. Patients were randomized 2:1 to either 12 weeks of all-oral SMV/SOF or to peginterferon (PEG)/ribavirin (RBV)/SOF. Fully 93% of patients given the all-oral regimen achieved SVR12, compared with 75% who received the interferon-containing regimen. SVR12 rates were higher in the SMV/SOF arm than in the PEG/RBV/SOF arm, independent of previous response (92% vs. 64% in null responders and 95% vs. 80% in naive patients). Moreover, virologic relapse was lower in the all-oral arm (12.5% vs. 8%; P = .009). These results, however, are based on a limited number of patients, particularly in the interferon-containing arm. In addition, the PEG used in this study was alfa-2b, which has not been studied in combination with SOF in clinical trials (its potential impact is thus unknown). One important point of the study is the lack of prognostic value of early viral kinetics: Rapid virologic response was not related to SVR12, which has been a hallmark in previous interferon-based regimens.
Regarding safety and tolerance, there were poorer self-reported outcomes and more side effects in the interferon group. The latter seems obvious because of

|
| Dr. Sabela Lens |
the well-known interferon profile, but RBV has been associated with higher rates of anemia in patients with cirrhosis undergoing interferon-free regimens. The use of SMV/SOF without RBV is certainly a crucial point of the study.
With the inherent limitations of the small number of patients, the study by Dr. Pearlman and his associates supports a greater efficacy and a better tolerance for a regimen combining SMV/SOF as compared to PEG/RBV/SOF in patients with GT-1a cirrhosis.
Dr. Sabela Lens and Dr. Xavier Forns are in the Liver Unit, Hospital Clínic Barcelona, IDIBAPS and CIBERehd, University of Barcelona. Dr. Lens has acted as an adviser for Janssen, MSD, and Gilead, and Dr. Forns has acted as an adviser for Janssen, Abbvie, and Gilead and has received unrestricted grant support from Janssen and MSD.
At the time of sofosbuvir (SOF) and simeprevir (SMV) approval, efficacy results of the combinations assessed in this study were still limited, especially in patients with cirrhosis, GT-1a, and/or previous nonresponse. The recent study by Dr. Pearlman and his associates aims to give light to some of the questions

|
| Dr. Xavier Forns |
raised. In this open-label trial, 82 patients with GT-1a cirrhosis (61% null responders) were recruited. Patients were randomized 2:1 to either 12 weeks of all-oral SMV/SOF or to peginterferon (PEG)/ribavirin (RBV)/SOF. Fully 93% of patients given the all-oral regimen achieved SVR12, compared with 75% who received the interferon-containing regimen. SVR12 rates were higher in the SMV/SOF arm than in the PEG/RBV/SOF arm, independent of previous response (92% vs. 64% in null responders and 95% vs. 80% in naive patients). Moreover, virologic relapse was lower in the all-oral arm (12.5% vs. 8%; P = .009). These results, however, are based on a limited number of patients, particularly in the interferon-containing arm. In addition, the PEG used in this study was alfa-2b, which has not been studied in combination with SOF in clinical trials (its potential impact is thus unknown). One important point of the study is the lack of prognostic value of early viral kinetics: Rapid virologic response was not related to SVR12, which has been a hallmark in previous interferon-based regimens.
Regarding safety and tolerance, there were poorer self-reported outcomes and more side effects in the interferon group. The latter seems obvious because of

|
| Dr. Sabela Lens |
the well-known interferon profile, but RBV has been associated with higher rates of anemia in patients with cirrhosis undergoing interferon-free regimens. The use of SMV/SOF without RBV is certainly a crucial point of the study.
With the inherent limitations of the small number of patients, the study by Dr. Pearlman and his associates supports a greater efficacy and a better tolerance for a regimen combining SMV/SOF as compared to PEG/RBV/SOF in patients with GT-1a cirrhosis.
Dr. Sabela Lens and Dr. Xavier Forns are in the Liver Unit, Hospital Clínic Barcelona, IDIBAPS and CIBERehd, University of Barcelona. Dr. Lens has acted as an adviser for Janssen, MSD, and Gilead, and Dr. Forns has acted as an adviser for Janssen, Abbvie, and Gilead and has received unrestricted grant support from Janssen and MSD.
At the time of sofosbuvir (SOF) and simeprevir (SMV) approval, efficacy results of the combinations assessed in this study were still limited, especially in patients with cirrhosis, GT-1a, and/or previous nonresponse. The recent study by Dr. Pearlman and his associates aims to give light to some of the questions

|
| Dr. Xavier Forns |
raised. In this open-label trial, 82 patients with GT-1a cirrhosis (61% null responders) were recruited. Patients were randomized 2:1 to either 12 weeks of all-oral SMV/SOF or to peginterferon (PEG)/ribavirin (RBV)/SOF. Fully 93% of patients given the all-oral regimen achieved SVR12, compared with 75% who received the interferon-containing regimen. SVR12 rates were higher in the SMV/SOF arm than in the PEG/RBV/SOF arm, independent of previous response (92% vs. 64% in null responders and 95% vs. 80% in naive patients). Moreover, virologic relapse was lower in the all-oral arm (12.5% vs. 8%; P = .009). These results, however, are based on a limited number of patients, particularly in the interferon-containing arm. In addition, the PEG used in this study was alfa-2b, which has not been studied in combination with SOF in clinical trials (its potential impact is thus unknown). One important point of the study is the lack of prognostic value of early viral kinetics: Rapid virologic response was not related to SVR12, which has been a hallmark in previous interferon-based regimens.
Regarding safety and tolerance, there were poorer self-reported outcomes and more side effects in the interferon group. The latter seems obvious because of

|
| Dr. Sabela Lens |
the well-known interferon profile, but RBV has been associated with higher rates of anemia in patients with cirrhosis undergoing interferon-free regimens. The use of SMV/SOF without RBV is certainly a crucial point of the study.
With the inherent limitations of the small number of patients, the study by Dr. Pearlman and his associates supports a greater efficacy and a better tolerance for a regimen combining SMV/SOF as compared to PEG/RBV/SOF in patients with GT-1a cirrhosis.
Dr. Sabela Lens and Dr. Xavier Forns are in the Liver Unit, Hospital Clínic Barcelona, IDIBAPS and CIBERehd, University of Barcelona. Dr. Lens has acted as an adviser for Janssen, MSD, and Gilead, and Dr. Forns has acted as an adviser for Janssen, Abbvie, and Gilead and has received unrestricted grant support from Janssen and MSD.
Patients with compensated cirrhosis and chronic genotype 1 hepatitis C virus infections were significantly more likely to clear their infections and had fewer adverse effects when treated with simeprevir and sofosbuvir instead of peginterferon, ribavirin, and sofosbuvir, investigators reported in the April issue of Gastroenterology. “Patients given the interferon-containing regimen had a significantly greater rate of virologic relapse than patients given simeprevir and sofosbuvir ... and reported worse outcomes and more side effects,” said Dr. Brian L. Pearlman and his associates of Atlanta Medical Center and Emory University, Atlanta.
Liver disease associated with chronic hepatitis C virus (HCV) infection causes at least 350,000 deaths per year worldwide. Genotype 1 (GT-1) HCV is the most common HCV strain and the hardest to treat, especially when patients have cirrhosis. Treatments for chronic GT-1 HCV infections historically included interferon, but achieved only moderate rates of sustained virologic response (SVR) and caused adverse somatic and psychiatric effects. All-oral, interferon-free regimens are now in widespread use, but many of the pivotal trials were industry sponsored and had strict enrollment criteria, potentially limiting their generalizability in community settings, the researchers reported (Gastroenterology 2015 April [doi:10.1053/j.gastro.2014.12.027]).Their open-label trial included 82 patients with HCV GT-1a infection and Child’s grade A cirrhosis recruited from two clinical practices in Atlanta. Thirty-two of the patients were treatment naive, while 50 had previously not responded to peginterferon and ribavirin treatment. Patients were randomized to either 12 weeks of all-oral simeprevir (150 mg/day) and sofosbuvir (400 mg/day), or peginterferon alfa 2b (1.5 mcg/kg per week), ribavirin (1000-1200 mg/day), and sofosbuvir (400 mg/day).Fully 54 of 58 (93%) patients given the simeprevir-sofosbuvir regimen had undetectable levels of HCV RNA at 12 weeks (SVR12), compared with 18 of 24 (75%) patients who received the interferon-containing regimen (P = .02), the researchers reported. Rates of SVR12 among prior nonresponders were 92% and 64%, and were 95% and 80% for treatment-naive patients, although P-values did not reach statistical significance. The interferon group also had a higher rate of virologic relapse than did patients given simeprevir and sofosbuvir (P = .009), and had worse self-reported outcomes and more side effects, the researchers said. Patients in both groups who achieved SVR12 had higher quality-of-life scores than those who did not. Notably, almost half of patients were African American, a group that has historically had lower cure rates compared with other racial groups, said the investigators.
“Given that several new regimens for genotype 1 infection, including the sofosbuvir-ledipasvir combination pill, already have been approved, we believe that this study is very timely,” Dr. Pearlman and his associates said. “Although these are not head-to-head comparisons, the 12-week simeprevir and sofosbuvir regimen in this trial may compare favorably with the SVR rates for 12 weeks of sofosbuvir/ledipasvir and 12 weeks of the regimen, paritaprevir/ritonavir, ombitasvir and dasabuvir plus ribavirin for patients with cirrhosis, particularly for prior nonresponders to older therapies, with the caveat that many more patients were studied in the registration trials.”While industry-sponsored trials have had psychiatric exclusion criteria, several patients in the open-label trial had serious psychiatric conditions, said the researchers. A patient with stable bipolar disorder received the interferon-containing regimen, and two patients with stable schizophrenia received the all-oral regimen. None reported worsening psychiatric symptoms on treatment.
Dr. Pearlman reported having contracted research for Johnson & Johnson, Gilead, Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, and Merck; and having served on speaker and advisory boards for Johnson & Johnson, Gilead, and Abbvie. He also was an investigator and author in the COSMOS trial of simeprevir and sofosbuvir in patients with HCV/HIV coinfection. The other authors reported no conflicts of interest.
Patients with compensated cirrhosis and chronic genotype 1 hepatitis C virus infections were significantly more likely to clear their infections and had fewer adverse effects when treated with simeprevir and sofosbuvir instead of peginterferon, ribavirin, and sofosbuvir, investigators reported in the April issue of Gastroenterology. “Patients given the interferon-containing regimen had a significantly greater rate of virologic relapse than patients given simeprevir and sofosbuvir ... and reported worse outcomes and more side effects,” said Dr. Brian L. Pearlman and his associates of Atlanta Medical Center and Emory University, Atlanta.
Liver disease associated with chronic hepatitis C virus (HCV) infection causes at least 350,000 deaths per year worldwide. Genotype 1 (GT-1) HCV is the most common HCV strain and the hardest to treat, especially when patients have cirrhosis. Treatments for chronic GT-1 HCV infections historically included interferon, but achieved only moderate rates of sustained virologic response (SVR) and caused adverse somatic and psychiatric effects. All-oral, interferon-free regimens are now in widespread use, but many of the pivotal trials were industry sponsored and had strict enrollment criteria, potentially limiting their generalizability in community settings, the researchers reported (Gastroenterology 2015 April [doi:10.1053/j.gastro.2014.12.027]).Their open-label trial included 82 patients with HCV GT-1a infection and Child’s grade A cirrhosis recruited from two clinical practices in Atlanta. Thirty-two of the patients were treatment naive, while 50 had previously not responded to peginterferon and ribavirin treatment. Patients were randomized to either 12 weeks of all-oral simeprevir (150 mg/day) and sofosbuvir (400 mg/day), or peginterferon alfa 2b (1.5 mcg/kg per week), ribavirin (1000-1200 mg/day), and sofosbuvir (400 mg/day).Fully 54 of 58 (93%) patients given the simeprevir-sofosbuvir regimen had undetectable levels of HCV RNA at 12 weeks (SVR12), compared with 18 of 24 (75%) patients who received the interferon-containing regimen (P = .02), the researchers reported. Rates of SVR12 among prior nonresponders were 92% and 64%, and were 95% and 80% for treatment-naive patients, although P-values did not reach statistical significance. The interferon group also had a higher rate of virologic relapse than did patients given simeprevir and sofosbuvir (P = .009), and had worse self-reported outcomes and more side effects, the researchers said. Patients in both groups who achieved SVR12 had higher quality-of-life scores than those who did not. Notably, almost half of patients were African American, a group that has historically had lower cure rates compared with other racial groups, said the investigators.
“Given that several new regimens for genotype 1 infection, including the sofosbuvir-ledipasvir combination pill, already have been approved, we believe that this study is very timely,” Dr. Pearlman and his associates said. “Although these are not head-to-head comparisons, the 12-week simeprevir and sofosbuvir regimen in this trial may compare favorably with the SVR rates for 12 weeks of sofosbuvir/ledipasvir and 12 weeks of the regimen, paritaprevir/ritonavir, ombitasvir and dasabuvir plus ribavirin for patients with cirrhosis, particularly for prior nonresponders to older therapies, with the caveat that many more patients were studied in the registration trials.”While industry-sponsored trials have had psychiatric exclusion criteria, several patients in the open-label trial had serious psychiatric conditions, said the researchers. A patient with stable bipolar disorder received the interferon-containing regimen, and two patients with stable schizophrenia received the all-oral regimen. None reported worsening psychiatric symptoms on treatment.
Dr. Pearlman reported having contracted research for Johnson & Johnson, Gilead, Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, and Merck; and having served on speaker and advisory boards for Johnson & Johnson, Gilead, and Abbvie. He also was an investigator and author in the COSMOS trial of simeprevir and sofosbuvir in patients with HCV/HIV coinfection. The other authors reported no conflicts of interest.
Key clinical point: An all-oral simeprevir-sofosbuvir combination outperformed peginterferon/ribavirin/sofosbuvir in patients with genotype 1a HCV infection and compensated cirrhosis.
Major finding: Rates of sustained virologic response at 12 weeks were 93% for the simeprevir-sofosbuvir regimen and 75% for the interferon-containing regimen (P = .02).
Data source: Prospective open-label study of 82 treatment-naive and treatment-experienced patients with HCV infection and Child’s grade A cirrhosis.
Disclosures: Dr. Pearlman reported having contracted research for Johnson & Johnson, Gilead, Abbvie, Bristol-Myers Squibb, Boehringer Ingelheim, and Merck; and having served on speaker and advisory boards for Johnson & Johnson, Gilead, and Abbvie. Dr. Pearlman also reported having been an investigator and author in the COSMOS trial of sofosbuvir and simeprevir. The other authors reported no conflicts of interest.
New hepatitis treatment cost effective for some patient types
For individuals infected with hepatitis C virus (HCV), new regimens are highly effective, but also very expensive, at approximately $28,000 for 4 weeks of treatment. However, the treatment is cost effective for HCV patients with cirrhosis and for those without cirrhosis who have failed other treatments, according to a new study.
In an analysis of the cost effectiveness of sofosbuvir-based treatments for patients with HCV genotypes 2 and 3, Dr. Benjamin Linas of Boston Medical Center reported that when compared with pegylated interferon and ribavirin therapy, treatment regimens based on the new direct-acting antiviral are only cost effective for select groups (Ann. Intern Med, [doi:10.7326/M15-0674]).
Cure rates with sofosbuvir are higher than are rates with the previous standard of care, and sustained virologic response (SVR) “is associated with a greatly reduced lifetime risk for liver-related morbidity and mortality,” noted Dr. Linas and his colleagues.
Using sophisticated statistical modeling to compare HCV treatment with pegylated interferon and ribavirin – the previous standard of care – to the newly Food and Drug Administration–approved sofosbuvir-ribavirin regimen, Dr. Linas explored clinical outcomes and costs for several different patient groups, including treatment-naive and treatment-experienced HCV genotype 2 and 3 patients with and without cirrhosis.
Clinical outcomes, modeled from clinical trial and observational cohort data, were expressed as life expectancy in quality-adjusted life-years (QALYs) and lifetime medical costs. Investigators then calculated the incremental cost-effectiveness ratio (ICER) for each treatment strategy, dividing any additional cost for a more expensive treatment by the QALYs gained from this regimen.
Using the commonly accepted ICER threshold of $100,000 per QALY, Dr. Linas and colleagues concluded that sofosbuvir-based HCV therapy for treatment-naive patients without cirrhosis was not cost effective, with ICERs of $238,000-$266,000. Interferon-based treatments, they noted, still achieve an approximately 80% rate of cure in this population.
In the constrained resources of the real world, Dr. Linas and his associates noted that this analysis is important. “Treatment strategies that do not use limited resources where they are likely to have the greatest impact may result in unequal access to interferon-free regimens, thereby limiting the population-level benefits of new HCV treatments,” they said.
In an editorial accompanying Dr. Linas’ study, Dr. Etzion and Dr. Ghany placed this analysis of the cost-effectiveness of sofosbuvir in the context of payer and clinician priorities. They noted that “from the patient and physician perspective, the benefits of new treatment are evident.” For payers, however, resources are constrained and tough decisions will have to be made.
This cost-effectiveness analysis is needed to inform resource allocation decisions, since the cost of using direct-acting antivirals to treat all those infected in the United States alone would exceed $300 billion. In that context, the editorial noted, quality-of-life assessments become important. HCV infection causes relatively little diminution of quality of life until the stage of cirrhosis is reached; in this analysis, therefore, interferon-based regimens are still a reasonable choice for treatment-naive HCV patients without cirrhosis.
Though Dr. Linas’ study also models incremental cost-effectiveness ratios for various lower price points for sofosbuvir, the editorial authors point out that the price point for the public’s willingness to eradicate HCV has not been established.
Dr. Ohad Etzion and Dr. Marc G. Ghany are at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md. Dr. Ghany reported nonfinancial support from Bristol Meyers Squibb.
In an editorial accompanying Dr. Linas’ study, Dr. Etzion and Dr. Ghany placed this analysis of the cost-effectiveness of sofosbuvir in the context of payer and clinician priorities. They noted that “from the patient and physician perspective, the benefits of new treatment are evident.” For payers, however, resources are constrained and tough decisions will have to be made.
This cost-effectiveness analysis is needed to inform resource allocation decisions, since the cost of using direct-acting antivirals to treat all those infected in the United States alone would exceed $300 billion. In that context, the editorial noted, quality-of-life assessments become important. HCV infection causes relatively little diminution of quality of life until the stage of cirrhosis is reached; in this analysis, therefore, interferon-based regimens are still a reasonable choice for treatment-naive HCV patients without cirrhosis.
Though Dr. Linas’ study also models incremental cost-effectiveness ratios for various lower price points for sofosbuvir, the editorial authors point out that the price point for the public’s willingness to eradicate HCV has not been established.
Dr. Ohad Etzion and Dr. Marc G. Ghany are at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md. Dr. Ghany reported nonfinancial support from Bristol Meyers Squibb.
In an editorial accompanying Dr. Linas’ study, Dr. Etzion and Dr. Ghany placed this analysis of the cost-effectiveness of sofosbuvir in the context of payer and clinician priorities. They noted that “from the patient and physician perspective, the benefits of new treatment are evident.” For payers, however, resources are constrained and tough decisions will have to be made.
This cost-effectiveness analysis is needed to inform resource allocation decisions, since the cost of using direct-acting antivirals to treat all those infected in the United States alone would exceed $300 billion. In that context, the editorial noted, quality-of-life assessments become important. HCV infection causes relatively little diminution of quality of life until the stage of cirrhosis is reached; in this analysis, therefore, interferon-based regimens are still a reasonable choice for treatment-naive HCV patients without cirrhosis.
Though Dr. Linas’ study also models incremental cost-effectiveness ratios for various lower price points for sofosbuvir, the editorial authors point out that the price point for the public’s willingness to eradicate HCV has not been established.
Dr. Ohad Etzion and Dr. Marc G. Ghany are at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Md. Dr. Ghany reported nonfinancial support from Bristol Meyers Squibb.
For individuals infected with hepatitis C virus (HCV), new regimens are highly effective, but also very expensive, at approximately $28,000 for 4 weeks of treatment. However, the treatment is cost effective for HCV patients with cirrhosis and for those without cirrhosis who have failed other treatments, according to a new study.
In an analysis of the cost effectiveness of sofosbuvir-based treatments for patients with HCV genotypes 2 and 3, Dr. Benjamin Linas of Boston Medical Center reported that when compared with pegylated interferon and ribavirin therapy, treatment regimens based on the new direct-acting antiviral are only cost effective for select groups (Ann. Intern Med, [doi:10.7326/M15-0674]).
Cure rates with sofosbuvir are higher than are rates with the previous standard of care, and sustained virologic response (SVR) “is associated with a greatly reduced lifetime risk for liver-related morbidity and mortality,” noted Dr. Linas and his colleagues.
Using sophisticated statistical modeling to compare HCV treatment with pegylated interferon and ribavirin – the previous standard of care – to the newly Food and Drug Administration–approved sofosbuvir-ribavirin regimen, Dr. Linas explored clinical outcomes and costs for several different patient groups, including treatment-naive and treatment-experienced HCV genotype 2 and 3 patients with and without cirrhosis.
Clinical outcomes, modeled from clinical trial and observational cohort data, were expressed as life expectancy in quality-adjusted life-years (QALYs) and lifetime medical costs. Investigators then calculated the incremental cost-effectiveness ratio (ICER) for each treatment strategy, dividing any additional cost for a more expensive treatment by the QALYs gained from this regimen.
Using the commonly accepted ICER threshold of $100,000 per QALY, Dr. Linas and colleagues concluded that sofosbuvir-based HCV therapy for treatment-naive patients without cirrhosis was not cost effective, with ICERs of $238,000-$266,000. Interferon-based treatments, they noted, still achieve an approximately 80% rate of cure in this population.
In the constrained resources of the real world, Dr. Linas and his associates noted that this analysis is important. “Treatment strategies that do not use limited resources where they are likely to have the greatest impact may result in unequal access to interferon-free regimens, thereby limiting the population-level benefits of new HCV treatments,” they said.
For individuals infected with hepatitis C virus (HCV), new regimens are highly effective, but also very expensive, at approximately $28,000 for 4 weeks of treatment. However, the treatment is cost effective for HCV patients with cirrhosis and for those without cirrhosis who have failed other treatments, according to a new study.
In an analysis of the cost effectiveness of sofosbuvir-based treatments for patients with HCV genotypes 2 and 3, Dr. Benjamin Linas of Boston Medical Center reported that when compared with pegylated interferon and ribavirin therapy, treatment regimens based on the new direct-acting antiviral are only cost effective for select groups (Ann. Intern Med, [doi:10.7326/M15-0674]).
Cure rates with sofosbuvir are higher than are rates with the previous standard of care, and sustained virologic response (SVR) “is associated with a greatly reduced lifetime risk for liver-related morbidity and mortality,” noted Dr. Linas and his colleagues.
Using sophisticated statistical modeling to compare HCV treatment with pegylated interferon and ribavirin – the previous standard of care – to the newly Food and Drug Administration–approved sofosbuvir-ribavirin regimen, Dr. Linas explored clinical outcomes and costs for several different patient groups, including treatment-naive and treatment-experienced HCV genotype 2 and 3 patients with and without cirrhosis.
Clinical outcomes, modeled from clinical trial and observational cohort data, were expressed as life expectancy in quality-adjusted life-years (QALYs) and lifetime medical costs. Investigators then calculated the incremental cost-effectiveness ratio (ICER) for each treatment strategy, dividing any additional cost for a more expensive treatment by the QALYs gained from this regimen.
Using the commonly accepted ICER threshold of $100,000 per QALY, Dr. Linas and colleagues concluded that sofosbuvir-based HCV therapy for treatment-naive patients without cirrhosis was not cost effective, with ICERs of $238,000-$266,000. Interferon-based treatments, they noted, still achieve an approximately 80% rate of cure in this population.
In the constrained resources of the real world, Dr. Linas and his associates noted that this analysis is important. “Treatment strategies that do not use limited resources where they are likely to have the greatest impact may result in unequal access to interferon-free regimens, thereby limiting the population-level benefits of new HCV treatments,” they said.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: New treatment for hepatitis C virus (HCV) is cost effective for patients with cirrhosis and for those without cirrhosis who have failed other treatments.
Major finding: Incremental cost-effectiveness ratios for sofosbuvir-based HCV treatment ranged from $238,000 to $266,000 for treatment-naive noncirrhotic patients, exceeding the $100,000 per quality-adjusted life-year cost-effectiveness threshold.
Data source: Application of the Hepatitis C Cost-Effectiveness model to clinical trial and observational cohort data.
Disclosures: The National Institute on Drug Abuse and the National Institute of Allergy and Infectious Disease provided funding. Dr. Weinstein reported receiving personal compensation from OptumInsight; Dr. Kim reported receiving grants and personal compensation from Gilead Sciences, AbbVie Pharmaceuticals, and Bristol-Myers Squibb. The remaining authors reported no relevant disclosures.
FDA: Avoid using amiodarone with some hepatitis C antivirals
Taking the antiarrythmic drug amiodarone with the hepatitis C antiviral drugs ledipasvir and sofosbuvir, or with sofosbuvir plus another direct-acting antiviral drug, has been associated with cases of symptomatic bradycardia – including a fatal cardiac arrest – according to the Food and Drug Administration.
Because of the reports, the antiviral drugs’ labels now recommend against using amiodarone with those hepatitis C drugs.
An FDA statement issued March 24 described the bradycardia cases as “serious and life-threatening.” Gilead Sciences markets the ledipasvir and sofosbuvir combination as Harvoni and markets sofosbuvir as Sovaldi to treat chronic hepatitis C virus (HCV) infection.
Gilead issued a “Dear Health Care Provider” letter that provides further details of the cases. There have been nine postmarketing reports of symptomatic bradycardia in patients who were taking amiodarone with Harvoni; amiodarone with Sovaldi plus another hepatitis C antiviral drug, simeprevir (Olysio); or amiodarone with an investigational hepatitis C antiviral drug, daclatasvir.
Of those cases, six occurred with in the first 24 hours of starting treatment with the antivirals, and three cases occurred within the first 2-12 days after antiviral therapy was started. A pacemaker was needed in three cases, and one case was a fatal cardiac arrest.
In three cases, a “rechallenge with HCV treatment in the setting of continued amiodarone therapy resulted in recurrence of symptomatic bradycardia,” according to the Gilead letter.
The effect of coadministration on the blood levels of the antiviral drugs is not known, nor is the mechanism behind the cardiac effect.
The labeling of the fixed-dose combination of ledipasvir and sofosbuvir (Harvoni) now includes a section on “serious symptomatic bradycardia” when coadministered with amiodarone, and says that coadministration is not recommended. The label adds that if a patient on amiodarone or Harvoni has no other alternative than to take that combination, patients should be counseled about the bradycardia risk.
Cardiac monitoring is recommended for inpatients during the first 48 hours the patient is taking the drugs, “after which outpatient or self-monitoring of the heart rate should occur on a daily basis through at least the first 2 weeks of treatment.”
The label notes that amiodarone has a long half-life, so cardiac monitoring is still necessary if the patient discontinues amiodarone just before starting treatment with Harvoni. Similar labeling changes have been made to the Sovaldi label.
Adverse events associated with Harvoni or Sovaldi should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/.
Taking the antiarrythmic drug amiodarone with the hepatitis C antiviral drugs ledipasvir and sofosbuvir, or with sofosbuvir plus another direct-acting antiviral drug, has been associated with cases of symptomatic bradycardia – including a fatal cardiac arrest – according to the Food and Drug Administration.
Because of the reports, the antiviral drugs’ labels now recommend against using amiodarone with those hepatitis C drugs.
An FDA statement issued March 24 described the bradycardia cases as “serious and life-threatening.” Gilead Sciences markets the ledipasvir and sofosbuvir combination as Harvoni and markets sofosbuvir as Sovaldi to treat chronic hepatitis C virus (HCV) infection.
Gilead issued a “Dear Health Care Provider” letter that provides further details of the cases. There have been nine postmarketing reports of symptomatic bradycardia in patients who were taking amiodarone with Harvoni; amiodarone with Sovaldi plus another hepatitis C antiviral drug, simeprevir (Olysio); or amiodarone with an investigational hepatitis C antiviral drug, daclatasvir.
Of those cases, six occurred with in the first 24 hours of starting treatment with the antivirals, and three cases occurred within the first 2-12 days after antiviral therapy was started. A pacemaker was needed in three cases, and one case was a fatal cardiac arrest.
In three cases, a “rechallenge with HCV treatment in the setting of continued amiodarone therapy resulted in recurrence of symptomatic bradycardia,” according to the Gilead letter.
The effect of coadministration on the blood levels of the antiviral drugs is not known, nor is the mechanism behind the cardiac effect.
The labeling of the fixed-dose combination of ledipasvir and sofosbuvir (Harvoni) now includes a section on “serious symptomatic bradycardia” when coadministered with amiodarone, and says that coadministration is not recommended. The label adds that if a patient on amiodarone or Harvoni has no other alternative than to take that combination, patients should be counseled about the bradycardia risk.
Cardiac monitoring is recommended for inpatients during the first 48 hours the patient is taking the drugs, “after which outpatient or self-monitoring of the heart rate should occur on a daily basis through at least the first 2 weeks of treatment.”
The label notes that amiodarone has a long half-life, so cardiac monitoring is still necessary if the patient discontinues amiodarone just before starting treatment with Harvoni. Similar labeling changes have been made to the Sovaldi label.
Adverse events associated with Harvoni or Sovaldi should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/.
Taking the antiarrythmic drug amiodarone with the hepatitis C antiviral drugs ledipasvir and sofosbuvir, or with sofosbuvir plus another direct-acting antiviral drug, has been associated with cases of symptomatic bradycardia – including a fatal cardiac arrest – according to the Food and Drug Administration.
Because of the reports, the antiviral drugs’ labels now recommend against using amiodarone with those hepatitis C drugs.
An FDA statement issued March 24 described the bradycardia cases as “serious and life-threatening.” Gilead Sciences markets the ledipasvir and sofosbuvir combination as Harvoni and markets sofosbuvir as Sovaldi to treat chronic hepatitis C virus (HCV) infection.
Gilead issued a “Dear Health Care Provider” letter that provides further details of the cases. There have been nine postmarketing reports of symptomatic bradycardia in patients who were taking amiodarone with Harvoni; amiodarone with Sovaldi plus another hepatitis C antiviral drug, simeprevir (Olysio); or amiodarone with an investigational hepatitis C antiviral drug, daclatasvir.
Of those cases, six occurred with in the first 24 hours of starting treatment with the antivirals, and three cases occurred within the first 2-12 days after antiviral therapy was started. A pacemaker was needed in three cases, and one case was a fatal cardiac arrest.
In three cases, a “rechallenge with HCV treatment in the setting of continued amiodarone therapy resulted in recurrence of symptomatic bradycardia,” according to the Gilead letter.
The effect of coadministration on the blood levels of the antiviral drugs is not known, nor is the mechanism behind the cardiac effect.
The labeling of the fixed-dose combination of ledipasvir and sofosbuvir (Harvoni) now includes a section on “serious symptomatic bradycardia” when coadministered with amiodarone, and says that coadministration is not recommended. The label adds that if a patient on amiodarone or Harvoni has no other alternative than to take that combination, patients should be counseled about the bradycardia risk.
Cardiac monitoring is recommended for inpatients during the first 48 hours the patient is taking the drugs, “after which outpatient or self-monitoring of the heart rate should occur on a daily basis through at least the first 2 weeks of treatment.”
The label notes that amiodarone has a long half-life, so cardiac monitoring is still necessary if the patient discontinues amiodarone just before starting treatment with Harvoni. Similar labeling changes have been made to the Sovaldi label.
Adverse events associated with Harvoni or Sovaldi should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/.
Novel HCV therapies found cost effective, with caveats
Two different statistical models found that novel therapies for chronic HCV infection, particularly the combination of sofosbuvir and ledipasvir, are cost effective in most patients, according to separate reports published online March 17 in Annals of Internal Medicine.
However, both groups of researchers cautioned that if these expensive agents are made available to the millions of eligible patients across the country, it would have an immense impact on health care costs for both public and private payers.
The novel therapies, which typically contain sofosbuvir in combination with ledipasvir, simeprevir, or daclatasvir, substantially reduce the length of treatment, achieve much higher rates of sustained viral response (SVR), and offer interferon-free alternatives for patients who can’t tolerate or don’t respond to standard interferon-based treatments. But it is unclear whether these benefits justify their profound expense, compared with current care. Both statistical models were developed to examine this issue, but from different perspectives.
In one study, funded primarily by the National Institutes of Health, investigators constructed a model that simulated 120 possible clinical courses of HCV-infected adults based on different ages and sexes, treatment histories, HCV genotypes, fibrosis scores, and interferon tolerances. For each of these patient profiles, they ran simulations in which patients received either “the old standard of care” (peginterferon and ribavirin, either with or without boceprevir and telaprevir) or sofosbuvir plus ledipasvir.
The average per-patient cost of standard care ranged from $15,000 to $71,000, depending on the patient profile, while that of sofosbuvir-ledipasvir ranged from $66,000 to $154,000, said Jagpreet Chhatwal, Ph.D., of the University of Texas MD Anderson Cancer Center, Houston, and his associates.
Compared with standard care, treating 10,000 patients with sofosbuvir-ledipasvir was projected to prevent 600 cases of decompensated cirrhosis, 310 cases of hepatocellular carcinoma (HCC), 60 liver transplantations, and 550 liver-related deaths, which would result in substantial cost savings. Also, compared with standard care, the incremental cost-effectiveness ratio of sofosbuvir-ledipasvir was $55,400 per additional quality-of-life-year (QALY) gained, which falls well within the accepted range for therapies for other medical conditions. Thus, the new therapy proved to be cost-effective for most HCV patients (Ann. Intern. Med. 2015 March 17 [doi:10.7326/M14-1336]). But there was an important caveat: Many more patients would be eligible for the novel therapies than for the standard care, because the novel therapies are much more easily tolerated. With the addition of so many eligible patients, the resources needed to treat them “could be immense and unsustainable.” Compared with standard care, giving these novel HCV therapies to all eligible patients “would cost an additional $65 billion in the next 5 years,” which would not be counterbalanced by the estimated $16 billion saved by preventing cirrhosis, HCC, and transplantations.
Therefore, “despite the cost-effectiveness of [novel] HCV treatments, our analysis shows that it is unaffordable at the current price,” Dr. Chhatwal and his associates said.
In the other study, funded primarily by CVS Health, researchers developed a discrete-event simulation model of the natural history and progression of liver disease in treatment-naive patients, categorized by whether they were infected with HCV genotype 1, 2, or 3. Several possible treatment regimens were considered for each genotype, and the SVR rates they were projected to attain were derived from those reported in clinical trials, said Mehdi Najafzadeh, Ph.D., of the division of pharmacoepidemiology and pharmacoeconomics, Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
“From a societal perspective, the newly approved PEG-free regimen of sofosbuvir-ledipasvir for 12 weeks could be very cost-effective relative to usual care (costing $12,825/QALY gained) for patients with HCV genotype 1.” This treatment proved to be the optimal strategy in the greatest number of simulations involving genotype 1.
Similarly, for genotype 3 the combination of sofosbuvir plus ledipasvir plus ribavirin for 12 weeks cost $73,000/QALY gained, compared with usual care. This also represents “relatively good value.” However, for genotype 2 the most cost-effective novel therapy, sofosbuvir-ribavirin, was $110,168/QALY gained, which is not considered cost-effective, Dr. Najafzadeh and his associates said (Ann. Intern. Med. 2015 March 17 [doi:10.7326;M14-1152]). Again, an important caveat to these findings was that, at their current prices, the cost of these drugs were not outweighed by the savings that accrued from preventing the complications of HCV. And “regardless of the cost-effectiveness of novel HCV treatments, there is considerable concern that their very high prices could substantially increase short-term overall drug spending for many public and private payers,” the investigators noted.
However, the fact that these regimens don’t reduce health care costs “is an exceptionally high bar” to hold them to – one that “is generally not expected when evaluating whether a new strategy represents good value for the money,” they added.
The recent development and widespread use of well-tolerated and highly efficacious direct-acting antiviral agents (DAAs) represent a paradigm shift in which the retail cost of treatment is now the most significant barrier to hepatitis C virus (HCV) eradication. While we have begun to learn the medical value of curing HCV in the context of the staggering burden of chronic liver disease, much less is known about the economic value of the cost of therapy. There has been swift public outcry over the $1,000 per pill price tag of sofosbuvir, and demand for the medications remains high as nearly all HCV-infected patients are now treatment eligible. Despite the high cost, these two studies collectively demonstrate a favorable incremental cost-effectiveness ratio per adjusted life-year relative to interferon-containing regimens in most patients with HCV: genotype 1, treatment-experienced, and cirrhotics.
These studies illustrate the paradox at the crux of the issue: How can the novel HCV therapies be both cost effective for most HCV patients but simultaneously unaffordable for payers? Although the price of achieving a sustained virologic response (SVR) is reduced with the DAA regimens, the cost of treating all infected patients in the United States could exceed $300 billion, which greatly outweighs the short-term cost of the annual HCV-related burden (approximately $6.5 billion [Hepatology 2013;57:2164-70]).
Treatment of other chronic illness such as HIV may incur greater costs but are distributed over a lifetime. Additionally, the current payers may not be the recipients of the downstream financial benefits of prevented liver-related outcomes. Ultimately, value depends on perspective; payers may balk at the price for the same cure that our patients consider invaluable.
Dr. J.P. Norvell is assistant professor of medicine, Emory University, Atlanta. He has been a consultant to Gilead Sciences.
The recent development and widespread use of well-tolerated and highly efficacious direct-acting antiviral agents (DAAs) represent a paradigm shift in which the retail cost of treatment is now the most significant barrier to hepatitis C virus (HCV) eradication. While we have begun to learn the medical value of curing HCV in the context of the staggering burden of chronic liver disease, much less is known about the economic value of the cost of therapy. There has been swift public outcry over the $1,000 per pill price tag of sofosbuvir, and demand for the medications remains high as nearly all HCV-infected patients are now treatment eligible. Despite the high cost, these two studies collectively demonstrate a favorable incremental cost-effectiveness ratio per adjusted life-year relative to interferon-containing regimens in most patients with HCV: genotype 1, treatment-experienced, and cirrhotics.
These studies illustrate the paradox at the crux of the issue: How can the novel HCV therapies be both cost effective for most HCV patients but simultaneously unaffordable for payers? Although the price of achieving a sustained virologic response (SVR) is reduced with the DAA regimens, the cost of treating all infected patients in the United States could exceed $300 billion, which greatly outweighs the short-term cost of the annual HCV-related burden (approximately $6.5 billion [Hepatology 2013;57:2164-70]).
Treatment of other chronic illness such as HIV may incur greater costs but are distributed over a lifetime. Additionally, the current payers may not be the recipients of the downstream financial benefits of prevented liver-related outcomes. Ultimately, value depends on perspective; payers may balk at the price for the same cure that our patients consider invaluable.
Dr. J.P. Norvell is assistant professor of medicine, Emory University, Atlanta. He has been a consultant to Gilead Sciences.
The recent development and widespread use of well-tolerated and highly efficacious direct-acting antiviral agents (DAAs) represent a paradigm shift in which the retail cost of treatment is now the most significant barrier to hepatitis C virus (HCV) eradication. While we have begun to learn the medical value of curing HCV in the context of the staggering burden of chronic liver disease, much less is known about the economic value of the cost of therapy. There has been swift public outcry over the $1,000 per pill price tag of sofosbuvir, and demand for the medications remains high as nearly all HCV-infected patients are now treatment eligible. Despite the high cost, these two studies collectively demonstrate a favorable incremental cost-effectiveness ratio per adjusted life-year relative to interferon-containing regimens in most patients with HCV: genotype 1, treatment-experienced, and cirrhotics.
These studies illustrate the paradox at the crux of the issue: How can the novel HCV therapies be both cost effective for most HCV patients but simultaneously unaffordable for payers? Although the price of achieving a sustained virologic response (SVR) is reduced with the DAA regimens, the cost of treating all infected patients in the United States could exceed $300 billion, which greatly outweighs the short-term cost of the annual HCV-related burden (approximately $6.5 billion [Hepatology 2013;57:2164-70]).
Treatment of other chronic illness such as HIV may incur greater costs but are distributed over a lifetime. Additionally, the current payers may not be the recipients of the downstream financial benefits of prevented liver-related outcomes. Ultimately, value depends on perspective; payers may balk at the price for the same cure that our patients consider invaluable.
Dr. J.P. Norvell is assistant professor of medicine, Emory University, Atlanta. He has been a consultant to Gilead Sciences.
Two different statistical models found that novel therapies for chronic HCV infection, particularly the combination of sofosbuvir and ledipasvir, are cost effective in most patients, according to separate reports published online March 17 in Annals of Internal Medicine.
However, both groups of researchers cautioned that if these expensive agents are made available to the millions of eligible patients across the country, it would have an immense impact on health care costs for both public and private payers.
The novel therapies, which typically contain sofosbuvir in combination with ledipasvir, simeprevir, or daclatasvir, substantially reduce the length of treatment, achieve much higher rates of sustained viral response (SVR), and offer interferon-free alternatives for patients who can’t tolerate or don’t respond to standard interferon-based treatments. But it is unclear whether these benefits justify their profound expense, compared with current care. Both statistical models were developed to examine this issue, but from different perspectives.
In one study, funded primarily by the National Institutes of Health, investigators constructed a model that simulated 120 possible clinical courses of HCV-infected adults based on different ages and sexes, treatment histories, HCV genotypes, fibrosis scores, and interferon tolerances. For each of these patient profiles, they ran simulations in which patients received either “the old standard of care” (peginterferon and ribavirin, either with or without boceprevir and telaprevir) or sofosbuvir plus ledipasvir.
The average per-patient cost of standard care ranged from $15,000 to $71,000, depending on the patient profile, while that of sofosbuvir-ledipasvir ranged from $66,000 to $154,000, said Jagpreet Chhatwal, Ph.D., of the University of Texas MD Anderson Cancer Center, Houston, and his associates.
Compared with standard care, treating 10,000 patients with sofosbuvir-ledipasvir was projected to prevent 600 cases of decompensated cirrhosis, 310 cases of hepatocellular carcinoma (HCC), 60 liver transplantations, and 550 liver-related deaths, which would result in substantial cost savings. Also, compared with standard care, the incremental cost-effectiveness ratio of sofosbuvir-ledipasvir was $55,400 per additional quality-of-life-year (QALY) gained, which falls well within the accepted range for therapies for other medical conditions. Thus, the new therapy proved to be cost-effective for most HCV patients (Ann. Intern. Med. 2015 March 17 [doi:10.7326/M14-1336]). But there was an important caveat: Many more patients would be eligible for the novel therapies than for the standard care, because the novel therapies are much more easily tolerated. With the addition of so many eligible patients, the resources needed to treat them “could be immense and unsustainable.” Compared with standard care, giving these novel HCV therapies to all eligible patients “would cost an additional $65 billion in the next 5 years,” which would not be counterbalanced by the estimated $16 billion saved by preventing cirrhosis, HCC, and transplantations.
Therefore, “despite the cost-effectiveness of [novel] HCV treatments, our analysis shows that it is unaffordable at the current price,” Dr. Chhatwal and his associates said.
In the other study, funded primarily by CVS Health, researchers developed a discrete-event simulation model of the natural history and progression of liver disease in treatment-naive patients, categorized by whether they were infected with HCV genotype 1, 2, or 3. Several possible treatment regimens were considered for each genotype, and the SVR rates they were projected to attain were derived from those reported in clinical trials, said Mehdi Najafzadeh, Ph.D., of the division of pharmacoepidemiology and pharmacoeconomics, Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
“From a societal perspective, the newly approved PEG-free regimen of sofosbuvir-ledipasvir for 12 weeks could be very cost-effective relative to usual care (costing $12,825/QALY gained) for patients with HCV genotype 1.” This treatment proved to be the optimal strategy in the greatest number of simulations involving genotype 1.
Similarly, for genotype 3 the combination of sofosbuvir plus ledipasvir plus ribavirin for 12 weeks cost $73,000/QALY gained, compared with usual care. This also represents “relatively good value.” However, for genotype 2 the most cost-effective novel therapy, sofosbuvir-ribavirin, was $110,168/QALY gained, which is not considered cost-effective, Dr. Najafzadeh and his associates said (Ann. Intern. Med. 2015 March 17 [doi:10.7326;M14-1152]). Again, an important caveat to these findings was that, at their current prices, the cost of these drugs were not outweighed by the savings that accrued from preventing the complications of HCV. And “regardless of the cost-effectiveness of novel HCV treatments, there is considerable concern that their very high prices could substantially increase short-term overall drug spending for many public and private payers,” the investigators noted.
However, the fact that these regimens don’t reduce health care costs “is an exceptionally high bar” to hold them to – one that “is generally not expected when evaluating whether a new strategy represents good value for the money,” they added.
Two different statistical models found that novel therapies for chronic HCV infection, particularly the combination of sofosbuvir and ledipasvir, are cost effective in most patients, according to separate reports published online March 17 in Annals of Internal Medicine.
However, both groups of researchers cautioned that if these expensive agents are made available to the millions of eligible patients across the country, it would have an immense impact on health care costs for both public and private payers.
The novel therapies, which typically contain sofosbuvir in combination with ledipasvir, simeprevir, or daclatasvir, substantially reduce the length of treatment, achieve much higher rates of sustained viral response (SVR), and offer interferon-free alternatives for patients who can’t tolerate or don’t respond to standard interferon-based treatments. But it is unclear whether these benefits justify their profound expense, compared with current care. Both statistical models were developed to examine this issue, but from different perspectives.
In one study, funded primarily by the National Institutes of Health, investigators constructed a model that simulated 120 possible clinical courses of HCV-infected adults based on different ages and sexes, treatment histories, HCV genotypes, fibrosis scores, and interferon tolerances. For each of these patient profiles, they ran simulations in which patients received either “the old standard of care” (peginterferon and ribavirin, either with or without boceprevir and telaprevir) or sofosbuvir plus ledipasvir.
The average per-patient cost of standard care ranged from $15,000 to $71,000, depending on the patient profile, while that of sofosbuvir-ledipasvir ranged from $66,000 to $154,000, said Jagpreet Chhatwal, Ph.D., of the University of Texas MD Anderson Cancer Center, Houston, and his associates.
Compared with standard care, treating 10,000 patients with sofosbuvir-ledipasvir was projected to prevent 600 cases of decompensated cirrhosis, 310 cases of hepatocellular carcinoma (HCC), 60 liver transplantations, and 550 liver-related deaths, which would result in substantial cost savings. Also, compared with standard care, the incremental cost-effectiveness ratio of sofosbuvir-ledipasvir was $55,400 per additional quality-of-life-year (QALY) gained, which falls well within the accepted range for therapies for other medical conditions. Thus, the new therapy proved to be cost-effective for most HCV patients (Ann. Intern. Med. 2015 March 17 [doi:10.7326/M14-1336]). But there was an important caveat: Many more patients would be eligible for the novel therapies than for the standard care, because the novel therapies are much more easily tolerated. With the addition of so many eligible patients, the resources needed to treat them “could be immense and unsustainable.” Compared with standard care, giving these novel HCV therapies to all eligible patients “would cost an additional $65 billion in the next 5 years,” which would not be counterbalanced by the estimated $16 billion saved by preventing cirrhosis, HCC, and transplantations.
Therefore, “despite the cost-effectiveness of [novel] HCV treatments, our analysis shows that it is unaffordable at the current price,” Dr. Chhatwal and his associates said.
In the other study, funded primarily by CVS Health, researchers developed a discrete-event simulation model of the natural history and progression of liver disease in treatment-naive patients, categorized by whether they were infected with HCV genotype 1, 2, or 3. Several possible treatment regimens were considered for each genotype, and the SVR rates they were projected to attain were derived from those reported in clinical trials, said Mehdi Najafzadeh, Ph.D., of the division of pharmacoepidemiology and pharmacoeconomics, Brigham and Women’s Hospital and Harvard Medical School, Boston, and his associates.
“From a societal perspective, the newly approved PEG-free regimen of sofosbuvir-ledipasvir for 12 weeks could be very cost-effective relative to usual care (costing $12,825/QALY gained) for patients with HCV genotype 1.” This treatment proved to be the optimal strategy in the greatest number of simulations involving genotype 1.
Similarly, for genotype 3 the combination of sofosbuvir plus ledipasvir plus ribavirin for 12 weeks cost $73,000/QALY gained, compared with usual care. This also represents “relatively good value.” However, for genotype 2 the most cost-effective novel therapy, sofosbuvir-ribavirin, was $110,168/QALY gained, which is not considered cost-effective, Dr. Najafzadeh and his associates said (Ann. Intern. Med. 2015 March 17 [doi:10.7326;M14-1152]). Again, an important caveat to these findings was that, at their current prices, the cost of these drugs were not outweighed by the savings that accrued from preventing the complications of HCV. And “regardless of the cost-effectiveness of novel HCV treatments, there is considerable concern that their very high prices could substantially increase short-term overall drug spending for many public and private payers,” the investigators noted.
However, the fact that these regimens don’t reduce health care costs “is an exceptionally high bar” to hold them to – one that “is generally not expected when evaluating whether a new strategy represents good value for the money,” they added.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Two separate computerized statistical models found that new HCV therapies are cost effective in most cases, with important caveats.
Major finding: Compared with standard care, treating 10,000 patients with sofosbuvir-ledipasvir was projected to prevent 600 cases of decompensated cirrhosis, 310 cases of hepatocellular carcinoma, 60 liver transplantations, and 550 liver-related deaths.
Data source: A microsimulation model of the cost-effectiveness of various HCV therapies, and a discrete-event simulation model of cost-effectiveness from a societal standpoint.
Disclosures: Dr. Chhatwal’s study was supported by the National Center for Advancing Translational Sciences and the Veterans Affairs Health Services Research and Development Center for Innovations in Quality, Effectiveness, and Safety. Dr. Najafzadeh’s study was supported by an unrestricted grant from CVS Health to Brigham and Women’s Hospital and by a Canadian Institutes of Health Research fellowship. Both research groups’ financial disclosures are available at www.annals.org.
ASCEND trial to test HCV treatment in primary care setting
The new ASCEND trial will examine whether primary care physicians can use a new antiviral therapy to treat hepatitis C virus as effectively as specialists do, according to a press release from the National Institutes of Health.
While past treatments for HCV were long-term, complex, and generally required a specialist physician, new drug developments may not require such in-depth treatment and could be handled by a primary care physician. The ASCEND study will involve 600 patients in the Washington, D.C. area, with 350 patients continuing their normal treatment with a specialist, and 250 patients receiving their treatment at a primary care location. Both groups will receive the same medication.
“The recent advent of direct-acting antiviral medications has offered promising new treatment options for people who are chronically infected with hepatitis C,” Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Disease, said. “The ASCEND study will help determine whether these medications are similarly effective when administered in an urban, community-based setting.”
Find the full press release on the NIH website.
The new ASCEND trial will examine whether primary care physicians can use a new antiviral therapy to treat hepatitis C virus as effectively as specialists do, according to a press release from the National Institutes of Health.
While past treatments for HCV were long-term, complex, and generally required a specialist physician, new drug developments may not require such in-depth treatment and could be handled by a primary care physician. The ASCEND study will involve 600 patients in the Washington, D.C. area, with 350 patients continuing their normal treatment with a specialist, and 250 patients receiving their treatment at a primary care location. Both groups will receive the same medication.
“The recent advent of direct-acting antiviral medications has offered promising new treatment options for people who are chronically infected with hepatitis C,” Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Disease, said. “The ASCEND study will help determine whether these medications are similarly effective when administered in an urban, community-based setting.”
Find the full press release on the NIH website.
The new ASCEND trial will examine whether primary care physicians can use a new antiviral therapy to treat hepatitis C virus as effectively as specialists do, according to a press release from the National Institutes of Health.
While past treatments for HCV were long-term, complex, and generally required a specialist physician, new drug developments may not require such in-depth treatment and could be handled by a primary care physician. The ASCEND study will involve 600 patients in the Washington, D.C. area, with 350 patients continuing their normal treatment with a specialist, and 250 patients receiving their treatment at a primary care location. Both groups will receive the same medication.
“The recent advent of direct-acting antiviral medications has offered promising new treatment options for people who are chronically infected with hepatitis C,” Dr. Anthony S. Fauci, director of the National Institute of Allergy and Infectious Disease, said. “The ASCEND study will help determine whether these medications are similarly effective when administered in an urban, community-based setting.”
Find the full press release on the NIH website.
Case studies highlight HCV health care transmission risk
Hospitals should carefully monitor equipment for hepatitis C virus contamination and the possibility of health care transmission, according to a Feb. 27 report from the Centers for Disease Control and Prevention.
The CDC report focuses on two specific cases of health care–transmitted HCV. In 2010, a patient without HCV in a New Jersey hospital was treated by an anesthesiologist who had immediately beforehand treated someone with HCV. Despite the single commonality between the two patients, patient A contracted HCV.
In 2011, a patient in Wisconsin with a history of diabetes and chronic renal disease was diagnosed with HCV-4, a subtype of the disease that is rare in that part of the world. The infection occurred in 2009, when this patient had a kidney transplant at the same time as a patient with HCV-4 also was having a kidney transplant. The two transplants shared a surgeon, but the source of infection was likely a perfusion machine where an HCV-positive kidney and an HCV-negative kidney were both stored without cleaning the machine.
“These cases illustrate the importance of partnerships and communication between public health and health care professionals to ensure that basic infection control and injection safety practices are optimized wherever health care is delivered,” the CDC investigators concluded.
Read the full report in the MMWR (2015;64:165-70).
Hospitals should carefully monitor equipment for hepatitis C virus contamination and the possibility of health care transmission, according to a Feb. 27 report from the Centers for Disease Control and Prevention.
The CDC report focuses on two specific cases of health care–transmitted HCV. In 2010, a patient without HCV in a New Jersey hospital was treated by an anesthesiologist who had immediately beforehand treated someone with HCV. Despite the single commonality between the two patients, patient A contracted HCV.
In 2011, a patient in Wisconsin with a history of diabetes and chronic renal disease was diagnosed with HCV-4, a subtype of the disease that is rare in that part of the world. The infection occurred in 2009, when this patient had a kidney transplant at the same time as a patient with HCV-4 also was having a kidney transplant. The two transplants shared a surgeon, but the source of infection was likely a perfusion machine where an HCV-positive kidney and an HCV-negative kidney were both stored without cleaning the machine.
“These cases illustrate the importance of partnerships and communication between public health and health care professionals to ensure that basic infection control and injection safety practices are optimized wherever health care is delivered,” the CDC investigators concluded.
Read the full report in the MMWR (2015;64:165-70).
Hospitals should carefully monitor equipment for hepatitis C virus contamination and the possibility of health care transmission, according to a Feb. 27 report from the Centers for Disease Control and Prevention.
The CDC report focuses on two specific cases of health care–transmitted HCV. In 2010, a patient without HCV in a New Jersey hospital was treated by an anesthesiologist who had immediately beforehand treated someone with HCV. Despite the single commonality between the two patients, patient A contracted HCV.
In 2011, a patient in Wisconsin with a history of diabetes and chronic renal disease was diagnosed with HCV-4, a subtype of the disease that is rare in that part of the world. The infection occurred in 2009, when this patient had a kidney transplant at the same time as a patient with HCV-4 also was having a kidney transplant. The two transplants shared a surgeon, but the source of infection was likely a perfusion machine where an HCV-positive kidney and an HCV-negative kidney were both stored without cleaning the machine.
“These cases illustrate the importance of partnerships and communication between public health and health care professionals to ensure that basic infection control and injection safety practices are optimized wherever health care is delivered,” the CDC investigators concluded.
Read the full report in the MMWR (2015;64:165-70).
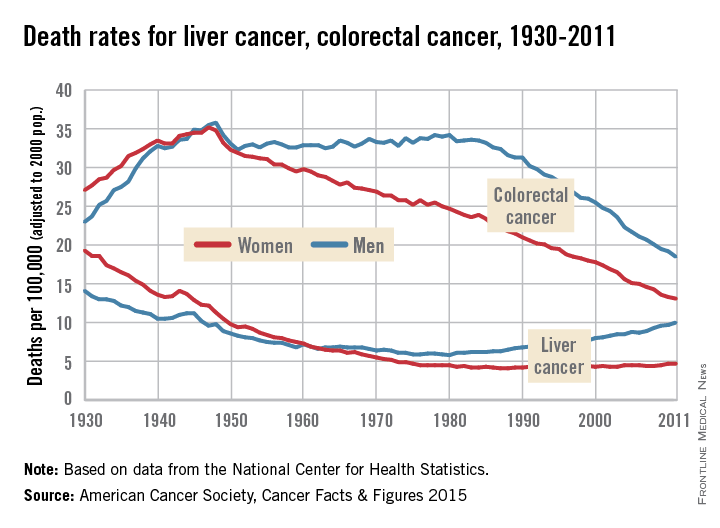
Death rates rising for liver cancer, falling for colorectal cancer
Death rates for liver cancer have been rising for some time among both men and women, while rates of colon and rectal cancer continue to decline from historic highs in the 1940s, the American Cancer Society reported.
In 2011, the death rate for liver cancer was 10/100,000 population (age-adjusted to the 2000 U.S. standard population) for men and 4.7/100,000 for women. The death rate for men reached its all-time low in 1980, when it was 5.8; for women, the low was 4.1 in 1987-1988. From 2007 to 2011, the overall death rate rose by 2.5% per year, according to the ACS.
“Screening for liver cancer has not been shown to reduce mortality,” the report said. “Nonetheless, many doctors in the U.S. screen individuals at high risk for the disease (e.g., those with cirrhosis) with ultrasound or blood tests.”
Death rates for colorectal cancer went the opposite way over that recent 4-year period – dropping by 2.5% a year overall from 2007 to 2011. The longer-term trend has seen rates for women declining since reaching a high of 35.2/100,000 in 1947, while the rate for men has been dropping since 1980, when it was 34.2. In 2011, the death rates for colorectal cancer were 13.1 in women and 18.5 in men, the ACS reported.
“This trend reflects declining incidence rates and improvements in early detection and treatment,” the ACS said in its report, which used data from the National Center for Health Statistics.
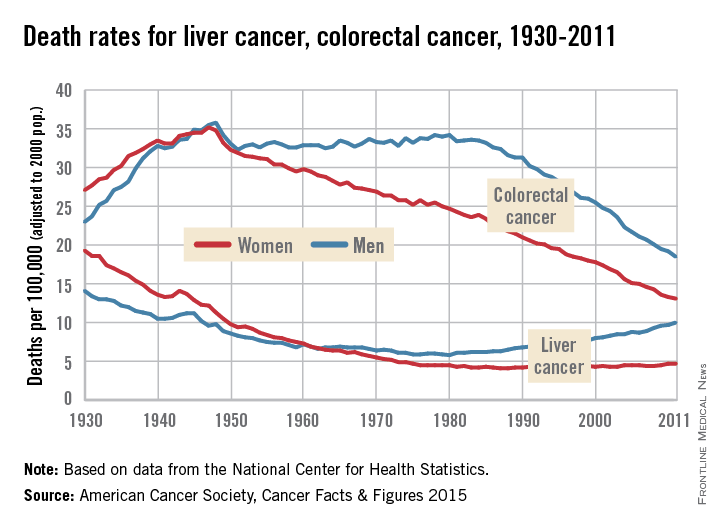
Death rates for liver cancer have been rising for some time among both men and women, while rates of colon and rectal cancer continue to decline from historic highs in the 1940s, the American Cancer Society reported.
In 2011, the death rate for liver cancer was 10/100,000 population (age-adjusted to the 2000 U.S. standard population) for men and 4.7/100,000 for women. The death rate for men reached its all-time low in 1980, when it was 5.8; for women, the low was 4.1 in 1987-1988. From 2007 to 2011, the overall death rate rose by 2.5% per year, according to the ACS.
“Screening for liver cancer has not been shown to reduce mortality,” the report said. “Nonetheless, many doctors in the U.S. screen individuals at high risk for the disease (e.g., those with cirrhosis) with ultrasound or blood tests.”
Death rates for colorectal cancer went the opposite way over that recent 4-year period – dropping by 2.5% a year overall from 2007 to 2011. The longer-term trend has seen rates for women declining since reaching a high of 35.2/100,000 in 1947, while the rate for men has been dropping since 1980, when it was 34.2. In 2011, the death rates for colorectal cancer were 13.1 in women and 18.5 in men, the ACS reported.
“This trend reflects declining incidence rates and improvements in early detection and treatment,” the ACS said in its report, which used data from the National Center for Health Statistics.

Death rates for liver cancer have been rising for some time among both men and women, while rates of colon and rectal cancer continue to decline from historic highs in the 1940s, the American Cancer Society reported.
In 2011, the death rate for liver cancer was 10/100,000 population (age-adjusted to the 2000 U.S. standard population) for men and 4.7/100,000 for women. The death rate for men reached its all-time low in 1980, when it was 5.8; for women, the low was 4.1 in 1987-1988. From 2007 to 2011, the overall death rate rose by 2.5% per year, according to the ACS.
“Screening for liver cancer has not been shown to reduce mortality,” the report said. “Nonetheless, many doctors in the U.S. screen individuals at high risk for the disease (e.g., those with cirrhosis) with ultrasound or blood tests.”
Death rates for colorectal cancer went the opposite way over that recent 4-year period – dropping by 2.5% a year overall from 2007 to 2011. The longer-term trend has seen rates for women declining since reaching a high of 35.2/100,000 in 1947, while the rate for men has been dropping since 1980, when it was 34.2. In 2011, the death rates for colorectal cancer were 13.1 in women and 18.5 in men, the ACS reported.
“This trend reflects declining incidence rates and improvements in early detection and treatment,” the ACS said in its report, which used data from the National Center for Health Statistics.

Two regimens achieve high response in HCV/HIV coinfection
Patients coinfected with HIV and hepatitis C genotype 1 show high response rates to a three-drug regimen of ombitasvir, paritaprevir codosed with ritonavir, and dasabuvir, plus ribavirin, according to an open-label, uncontrolled pilot study published in JAMA.
Among the 63 patients – who were either HCV treatment-naive or had previously experienced treatment failure with peginteferon plus ribavirin – treated with the all-oral ombitasvir, paritaprevir codosed with ritonavir, and dasabuvir (known as 3D) plus ribavirin regimen, 94% of those treated for 12 weeks and 91% of those treated for 24 weeks achieved a sustained virologic response 12 weeks (SVR12) after treatment.
There were no confirmed HIV breakthroughs or serious adverse events – even though the study group included some patients with compensated cirrhosis – although around half of patients experienced treatment-related fatigue, 19% experienced insomnia, 18% experienced nausea, and 16% experienced headache (JAMA 2015 Feb. 23 [doi:10.1001/jama.2015.1328]).
“The efficacy and safety of the 3D-plus-ribavirin regimen for patients co-infected with HCV/HIV-1 need to be further investigated in larger studies; however, response rates reported here are similar to those in studies of HCV-monoinfected patients, which suggest that difficult-to-cure patients, such as prior [pegylated interferon] null responders with cirrhosis and HCV genotype 1a, may benefit from longer treatment duration,” wrote Dr. Mark S. Sulkowski, director of the viral hepatitis center and professor of medicine at Johns Hopkins University, Baltimore, and his colleagues.
In another study, researchers found that a 12-week course of a fixed-dose combination of ledipasvir and sofosbuvir in treatment-naive patients with hepatitis C genotype 1 and HIV coinfection achieved sustained virologic response rates of 98% at 12 weeks after treatment.
The open-label, phase 2b study in 50 patients recorded one case of relapse at week 4, which may have been linked to resistance-associated mutation, but no discontinuations or serious adverse events attributable to the study drug (JAMA 2015 Feb. 23 [doi:10.1001/jama.2015.1373]).
The study excluded individuals with cirrhosis, but many participants had poor prognostic factors, including African American race, male sex, IL28B non-CC genotype, and genotype 1a infection.
“These results show for the first time, to our knowledge, that an interferon- and ribavirin-free therapy is associated with high SVR rates in patients co-infected with HCV and HIV,” wrote Dr. Anu Osinusi of the University of Maryland, Baltimore, and her colleagues.
Dr. Sulkowski’s study was funded by AbbVie. Some of his coauthors declared a range of funding and consultancies from pharmaceutical companies including AbbVie and several coauthors are employees of AbbVie.
Dr. Osinusi’s study was funded by the National Institutes of Health, the German Research Foundation, and Gilead Sciences. Four coauthors reported speaking engagements, consultancies, fees, grants, stocks and other support from pharmaceutical companies including Gilead.
Treatment of hepatitis C and HIV coinfection is complicated by HIV clinicians’ reluctance to use interferon alfa and hepatologists’ hesitation in treating patients with HIV; however, these two studies show the potential of new treatment regimens of all-oral direct antiviral agents.
With liver disease the second-leading cause of death in people with HIV, the high virologic response rates observed in these studies suggest that future barriers to prevention of unnecessary deaths caused by hepatitis C may be related to failures of the health care system.
Dr. Camilla S. Graham is with the viral hepatitis center at Beth Israel Deaconess Medical Center, Boston. These comments are taken from an accompanying editorial (JAMA 2015 Feb. 23 [doi:10.1001/jama.2015.1111]). There were no conflicts of interest declared.
Treatment of hepatitis C and HIV coinfection is complicated by HIV clinicians’ reluctance to use interferon alfa and hepatologists’ hesitation in treating patients with HIV; however, these two studies show the potential of new treatment regimens of all-oral direct antiviral agents.
With liver disease the second-leading cause of death in people with HIV, the high virologic response rates observed in these studies suggest that future barriers to prevention of unnecessary deaths caused by hepatitis C may be related to failures of the health care system.
Dr. Camilla S. Graham is with the viral hepatitis center at Beth Israel Deaconess Medical Center, Boston. These comments are taken from an accompanying editorial (JAMA 2015 Feb. 23 [doi:10.1001/jama.2015.1111]). There were no conflicts of interest declared.
Treatment of hepatitis C and HIV coinfection is complicated by HIV clinicians’ reluctance to use interferon alfa and hepatologists’ hesitation in treating patients with HIV; however, these two studies show the potential of new treatment regimens of all-oral direct antiviral agents.
With liver disease the second-leading cause of death in people with HIV, the high virologic response rates observed in these studies suggest that future barriers to prevention of unnecessary deaths caused by hepatitis C may be related to failures of the health care system.
Dr. Camilla S. Graham is with the viral hepatitis center at Beth Israel Deaconess Medical Center, Boston. These comments are taken from an accompanying editorial (JAMA 2015 Feb. 23 [doi:10.1001/jama.2015.1111]). There were no conflicts of interest declared.
Patients coinfected with HIV and hepatitis C genotype 1 show high response rates to a three-drug regimen of ombitasvir, paritaprevir codosed with ritonavir, and dasabuvir, plus ribavirin, according to an open-label, uncontrolled pilot study published in JAMA.
Among the 63 patients – who were either HCV treatment-naive or had previously experienced treatment failure with peginteferon plus ribavirin – treated with the all-oral ombitasvir, paritaprevir codosed with ritonavir, and dasabuvir (known as 3D) plus ribavirin regimen, 94% of those treated for 12 weeks and 91% of those treated for 24 weeks achieved a sustained virologic response 12 weeks (SVR12) after treatment.
There were no confirmed HIV breakthroughs or serious adverse events – even though the study group included some patients with compensated cirrhosis – although around half of patients experienced treatment-related fatigue, 19% experienced insomnia, 18% experienced nausea, and 16% experienced headache (JAMA 2015 Feb. 23 [doi:10.1001/jama.2015.1328]).
“The efficacy and safety of the 3D-plus-ribavirin regimen for patients co-infected with HCV/HIV-1 need to be further investigated in larger studies; however, response rates reported here are similar to those in studies of HCV-monoinfected patients, which suggest that difficult-to-cure patients, such as prior [pegylated interferon] null responders with cirrhosis and HCV genotype 1a, may benefit from longer treatment duration,” wrote Dr. Mark S. Sulkowski, director of the viral hepatitis center and professor of medicine at Johns Hopkins University, Baltimore, and his colleagues.
In another study, researchers found that a 12-week course of a fixed-dose combination of ledipasvir and sofosbuvir in treatment-naive patients with hepatitis C genotype 1 and HIV coinfection achieved sustained virologic response rates of 98% at 12 weeks after treatment.
The open-label, phase 2b study in 50 patients recorded one case of relapse at week 4, which may have been linked to resistance-associated mutation, but no discontinuations or serious adverse events attributable to the study drug (JAMA 2015 Feb. 23 [doi:10.1001/jama.2015.1373]).
The study excluded individuals with cirrhosis, but many participants had poor prognostic factors, including African American race, male sex, IL28B non-CC genotype, and genotype 1a infection.
“These results show for the first time, to our knowledge, that an interferon- and ribavirin-free therapy is associated with high SVR rates in patients co-infected with HCV and HIV,” wrote Dr. Anu Osinusi of the University of Maryland, Baltimore, and her colleagues.
Dr. Sulkowski’s study was funded by AbbVie. Some of his coauthors declared a range of funding and consultancies from pharmaceutical companies including AbbVie and several coauthors are employees of AbbVie.
Dr. Osinusi’s study was funded by the National Institutes of Health, the German Research Foundation, and Gilead Sciences. Four coauthors reported speaking engagements, consultancies, fees, grants, stocks and other support from pharmaceutical companies including Gilead.
Patients coinfected with HIV and hepatitis C genotype 1 show high response rates to a three-drug regimen of ombitasvir, paritaprevir codosed with ritonavir, and dasabuvir, plus ribavirin, according to an open-label, uncontrolled pilot study published in JAMA.
Among the 63 patients – who were either HCV treatment-naive or had previously experienced treatment failure with peginteferon plus ribavirin – treated with the all-oral ombitasvir, paritaprevir codosed with ritonavir, and dasabuvir (known as 3D) plus ribavirin regimen, 94% of those treated for 12 weeks and 91% of those treated for 24 weeks achieved a sustained virologic response 12 weeks (SVR12) after treatment.
There were no confirmed HIV breakthroughs or serious adverse events – even though the study group included some patients with compensated cirrhosis – although around half of patients experienced treatment-related fatigue, 19% experienced insomnia, 18% experienced nausea, and 16% experienced headache (JAMA 2015 Feb. 23 [doi:10.1001/jama.2015.1328]).
“The efficacy and safety of the 3D-plus-ribavirin regimen for patients co-infected with HCV/HIV-1 need to be further investigated in larger studies; however, response rates reported here are similar to those in studies of HCV-monoinfected patients, which suggest that difficult-to-cure patients, such as prior [pegylated interferon] null responders with cirrhosis and HCV genotype 1a, may benefit from longer treatment duration,” wrote Dr. Mark S. Sulkowski, director of the viral hepatitis center and professor of medicine at Johns Hopkins University, Baltimore, and his colleagues.
In another study, researchers found that a 12-week course of a fixed-dose combination of ledipasvir and sofosbuvir in treatment-naive patients with hepatitis C genotype 1 and HIV coinfection achieved sustained virologic response rates of 98% at 12 weeks after treatment.
The open-label, phase 2b study in 50 patients recorded one case of relapse at week 4, which may have been linked to resistance-associated mutation, but no discontinuations or serious adverse events attributable to the study drug (JAMA 2015 Feb. 23 [doi:10.1001/jama.2015.1373]).
The study excluded individuals with cirrhosis, but many participants had poor prognostic factors, including African American race, male sex, IL28B non-CC genotype, and genotype 1a infection.
“These results show for the first time, to our knowledge, that an interferon- and ribavirin-free therapy is associated with high SVR rates in patients co-infected with HCV and HIV,” wrote Dr. Anu Osinusi of the University of Maryland, Baltimore, and her colleagues.
Dr. Sulkowski’s study was funded by AbbVie. Some of his coauthors declared a range of funding and consultancies from pharmaceutical companies including AbbVie and several coauthors are employees of AbbVie.
Dr. Osinusi’s study was funded by the National Institutes of Health, the German Research Foundation, and Gilead Sciences. Four coauthors reported speaking engagements, consultancies, fees, grants, stocks and other support from pharmaceutical companies including Gilead.
FROM JAMA
Threefold increase in cirrhosis risk with HCV
Individuals with hepatitis C infection are three times more likely to develop cirrhosis than are those who are hepatitis C negative, and fibrosis progression is pronounced within the first 5 years after infection, a retrospective cohort study has found.
Analysis of 10-year follow-up data from 1,840 individuals in a national database of hepatitis C (HCV) infected veterans, and 1,840 uninfected controls, found 18.4% of HCV+ individuals developed cirrhosis, compared with 6.1% of uninfected individuals, and they had a significantly higher rate of hepatic decompensation events (1.79% vs. 0.33%).
Increasing age, white race, hypertension, anemia, and a history of alcohol abuse were associated with a higher risk of cirrhosis among HCV+ individuals, but baseline or recent history of alcohol abuse or dependence did not significantly impact risk, according to a paper published online in JAMA Internal Medicine (2015;175:178-85 [doi:10.1001/jamainternmed.2014.6502]).
“Our study shows that fibrosis progression after HCV infection starts early and that a substantial proportion of HCV-infected persons develop significant fibrosis or cirrhosis within the first 5-10 years of infection [but] on the other hand, progression of cirrhosis to hepatic decompensation is uncommon in the first 9 years after cirrhosis,” wrote Dr. Adeel A. Butt of the University of Pittsburgh, and colleagues.
The National Institutes of Health and the VA Pittsburgh Healthcare System supported the study. Two authors declared receiving grants and fees from private industry.
Individuals with hepatitis C infection are three times more likely to develop cirrhosis than are those who are hepatitis C negative, and fibrosis progression is pronounced within the first 5 years after infection, a retrospective cohort study has found.
Analysis of 10-year follow-up data from 1,840 individuals in a national database of hepatitis C (HCV) infected veterans, and 1,840 uninfected controls, found 18.4% of HCV+ individuals developed cirrhosis, compared with 6.1% of uninfected individuals, and they had a significantly higher rate of hepatic decompensation events (1.79% vs. 0.33%).
Increasing age, white race, hypertension, anemia, and a history of alcohol abuse were associated with a higher risk of cirrhosis among HCV+ individuals, but baseline or recent history of alcohol abuse or dependence did not significantly impact risk, according to a paper published online in JAMA Internal Medicine (2015;175:178-85 [doi:10.1001/jamainternmed.2014.6502]).
“Our study shows that fibrosis progression after HCV infection starts early and that a substantial proportion of HCV-infected persons develop significant fibrosis or cirrhosis within the first 5-10 years of infection [but] on the other hand, progression of cirrhosis to hepatic decompensation is uncommon in the first 9 years after cirrhosis,” wrote Dr. Adeel A. Butt of the University of Pittsburgh, and colleagues.
The National Institutes of Health and the VA Pittsburgh Healthcare System supported the study. Two authors declared receiving grants and fees from private industry.
Individuals with hepatitis C infection are three times more likely to develop cirrhosis than are those who are hepatitis C negative, and fibrosis progression is pronounced within the first 5 years after infection, a retrospective cohort study has found.
Analysis of 10-year follow-up data from 1,840 individuals in a national database of hepatitis C (HCV) infected veterans, and 1,840 uninfected controls, found 18.4% of HCV+ individuals developed cirrhosis, compared with 6.1% of uninfected individuals, and they had a significantly higher rate of hepatic decompensation events (1.79% vs. 0.33%).
Increasing age, white race, hypertension, anemia, and a history of alcohol abuse were associated with a higher risk of cirrhosis among HCV+ individuals, but baseline or recent history of alcohol abuse or dependence did not significantly impact risk, according to a paper published online in JAMA Internal Medicine (2015;175:178-85 [doi:10.1001/jamainternmed.2014.6502]).
“Our study shows that fibrosis progression after HCV infection starts early and that a substantial proportion of HCV-infected persons develop significant fibrosis or cirrhosis within the first 5-10 years of infection [but] on the other hand, progression of cirrhosis to hepatic decompensation is uncommon in the first 9 years after cirrhosis,” wrote Dr. Adeel A. Butt of the University of Pittsburgh, and colleagues.
The National Institutes of Health and the VA Pittsburgh Healthcare System supported the study. Two authors declared receiving grants and fees from private industry.
Key clinical point: Individuals with hepatitis C infection are three times more likely to develop cirrhosis than are those who are hepatitis C negative.
Major finding: The incidence of fibrosis in HCV+ individuals was 18.4% compared to 6.1% in uninfected individuals.
Data source: Retrospective cohort study of 1,840 HCV+ individuals.
Disclosures: The National Institutes of Health and the VA Pittsburgh Healthcare System supported the study. Two authors declared receiving grants and fees from private industry.
Human liver cells can induce antiviral reaction against hepatitis C
Immunity against the hepatitis C virus can be induced by cultured primary human liver sinusoidal endothelial cells, according to a study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.040).
“We found HLSECs [primary human LSECs] express many of the receptors implicated in HCV [hepatitis C virus] attachment and entry and HLSEC-to-hepatocyte contact was dispensable for uptake,” wrote lead author Dr. Silvia M. Giugliano of the University of Colorado, Denver, and her associates.
In a cohort study, investigators exposed HLSECs and immortalized liver endothelial cells (TMNK-1) to several variants of HCV: full-length transmitted/founder virus, sucrose-purified Japanese fulminant hepatitis–1 (JFH-1), a virus encoding a luciferase reporter, and the HCV-specific pathogen-associated molecular pattern molecules (PAMP, substrate for RIG-I). Cells were analyzed by using polymerase chain reaction (PCR), immunofluorescence, and immunohistochemical assays.
Results showed that HLSECs, regardless of contact between cells, were able to internalize HCV when exposed to it and replicate, though not translate HCV RNA. The HCV RNA “induced consistent and broad transcription of multiple interferons (IFNs) [via] pattern recognition receptors (TLR7 and retinoic acid-inducible gene 1).” Furthermore, supernatants from primary HLSECs transfected with HCV-specific PAMP molecules had larger inductions of IFNs and IFN-stimulated genes in HLSECs.
“Conditioned media from IFN-stimulated HLSECs induced expression of antiviral genes by uninfected primary human hepatocytes,” wrote Dr. Giugliano and her coauthors. “Exosomes, derived from HLSECs following stimulation with either type I or type III IFNs, controlled HCV replication in a dose-dependent manner.”
However, the investigators say that more study is required to understand why HCV is able to present so persistently if the means to combat the virus are so apparent in the immune system.
“These results raise a number of intriguing questions, for example, how the use of exogenous IFN to treat viral hepatitis could be expected to induce additional, previously unrecognized antiviral mechanisms involving HLSECs,” says the study. “Further work is warranted to understand why despite these innate immune responses, HCV is able to establish persistence and fail eradication with IFN-based antiviral therapy.”
The study supported by grants R21-AI103361 and U19 AI 1066328; Dr. Rosen received a Merit Review grant and Dr. Shaw received grant R21-AI 106000. The authors reported no financial conflicts of interest.
Liver sinusoidal endothelial cells (LSECs) are a large component of the liver immune system but are not very well characterized for their role in diseases like chronic hepatitis C virus (HCV) infection. Dr. Giugliano and her associates tested the antiviral activity of primary human LSECs and an immortalized LSEC cell line (TMNK-1), following exposure to several viral variants. These included naturally

|
| Dr. Arash Grakoui |
occurring founder genotype 1a virus, a JFH-1 infectious clone (cloned originally from a patient with fulminant hepatitis), a modified cell culture of adaptive JFH-1 virus expressing luciferase reporter, and HCV-specific pathogen-associated molecular pattern poly UC RNA that is a substrate for RIG-I. They found LSECs were able to acquire HCV in an LSEC-to-hepatocyte–contact independent manner but that these cells were not permissive for productive infection (replication). Importantly, viral RNA through HCV uptake induced robust transcription of type I and III interferon (IFN) by LSECs via TLR7 and RIG-I pathways. LSEC-derived IFN had antiviral effects on HCV-infected human hepatoma cell line, Huh7.5 cells in vitro, and this effect was mediated through the production of exosomes. The authors concluded that LSECs likely have an important antiviral role in HCV infection through bystander induction of an antiviral state. This study highlights the idea that these cells can function similarly to other innate/phagocytic cells like monocytes/macrophages and furthers the idea that innate activation of LSECs may have protective effects on the antiviral state of hepatocytes. Mechanisms of antiviral immunity by LSECs, whether it occurs through exosomes or IFN, will have a significant impact in the field as the answers will have relevance to numerous facets of liver immunology, including gene transfer, organ transplant, and response to other hepatotropic infections.
Dr. Arash Grakoui is an associate professor jointly appointed in the departments of medicine, division of infectious diseases and Yerkes National Primate Research Center, department of microbiology and immunology at Emory University, Atlanta. He has no conflicts of interest.
Liver sinusoidal endothelial cells (LSECs) are a large component of the liver immune system but are not very well characterized for their role in diseases like chronic hepatitis C virus (HCV) infection. Dr. Giugliano and her associates tested the antiviral activity of primary human LSECs and an immortalized LSEC cell line (TMNK-1), following exposure to several viral variants. These included naturally

|
| Dr. Arash Grakoui |
occurring founder genotype 1a virus, a JFH-1 infectious clone (cloned originally from a patient with fulminant hepatitis), a modified cell culture of adaptive JFH-1 virus expressing luciferase reporter, and HCV-specific pathogen-associated molecular pattern poly UC RNA that is a substrate for RIG-I. They found LSECs were able to acquire HCV in an LSEC-to-hepatocyte–contact independent manner but that these cells were not permissive for productive infection (replication). Importantly, viral RNA through HCV uptake induced robust transcription of type I and III interferon (IFN) by LSECs via TLR7 and RIG-I pathways. LSEC-derived IFN had antiviral effects on HCV-infected human hepatoma cell line, Huh7.5 cells in vitro, and this effect was mediated through the production of exosomes. The authors concluded that LSECs likely have an important antiviral role in HCV infection through bystander induction of an antiviral state. This study highlights the idea that these cells can function similarly to other innate/phagocytic cells like monocytes/macrophages and furthers the idea that innate activation of LSECs may have protective effects on the antiviral state of hepatocytes. Mechanisms of antiviral immunity by LSECs, whether it occurs through exosomes or IFN, will have a significant impact in the field as the answers will have relevance to numerous facets of liver immunology, including gene transfer, organ transplant, and response to other hepatotropic infections.
Dr. Arash Grakoui is an associate professor jointly appointed in the departments of medicine, division of infectious diseases and Yerkes National Primate Research Center, department of microbiology and immunology at Emory University, Atlanta. He has no conflicts of interest.
Liver sinusoidal endothelial cells (LSECs) are a large component of the liver immune system but are not very well characterized for their role in diseases like chronic hepatitis C virus (HCV) infection. Dr. Giugliano and her associates tested the antiviral activity of primary human LSECs and an immortalized LSEC cell line (TMNK-1), following exposure to several viral variants. These included naturally

|
| Dr. Arash Grakoui |
occurring founder genotype 1a virus, a JFH-1 infectious clone (cloned originally from a patient with fulminant hepatitis), a modified cell culture of adaptive JFH-1 virus expressing luciferase reporter, and HCV-specific pathogen-associated molecular pattern poly UC RNA that is a substrate for RIG-I. They found LSECs were able to acquire HCV in an LSEC-to-hepatocyte–contact independent manner but that these cells were not permissive for productive infection (replication). Importantly, viral RNA through HCV uptake induced robust transcription of type I and III interferon (IFN) by LSECs via TLR7 and RIG-I pathways. LSEC-derived IFN had antiviral effects on HCV-infected human hepatoma cell line, Huh7.5 cells in vitro, and this effect was mediated through the production of exosomes. The authors concluded that LSECs likely have an important antiviral role in HCV infection through bystander induction of an antiviral state. This study highlights the idea that these cells can function similarly to other innate/phagocytic cells like monocytes/macrophages and furthers the idea that innate activation of LSECs may have protective effects on the antiviral state of hepatocytes. Mechanisms of antiviral immunity by LSECs, whether it occurs through exosomes or IFN, will have a significant impact in the field as the answers will have relevance to numerous facets of liver immunology, including gene transfer, organ transplant, and response to other hepatotropic infections.
Dr. Arash Grakoui is an associate professor jointly appointed in the departments of medicine, division of infectious diseases and Yerkes National Primate Research Center, department of microbiology and immunology at Emory University, Atlanta. He has no conflicts of interest.
Immunity against the hepatitis C virus can be induced by cultured primary human liver sinusoidal endothelial cells, according to a study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.040).
“We found HLSECs [primary human LSECs] express many of the receptors implicated in HCV [hepatitis C virus] attachment and entry and HLSEC-to-hepatocyte contact was dispensable for uptake,” wrote lead author Dr. Silvia M. Giugliano of the University of Colorado, Denver, and her associates.
In a cohort study, investigators exposed HLSECs and immortalized liver endothelial cells (TMNK-1) to several variants of HCV: full-length transmitted/founder virus, sucrose-purified Japanese fulminant hepatitis–1 (JFH-1), a virus encoding a luciferase reporter, and the HCV-specific pathogen-associated molecular pattern molecules (PAMP, substrate for RIG-I). Cells were analyzed by using polymerase chain reaction (PCR), immunofluorescence, and immunohistochemical assays.
Results showed that HLSECs, regardless of contact between cells, were able to internalize HCV when exposed to it and replicate, though not translate HCV RNA. The HCV RNA “induced consistent and broad transcription of multiple interferons (IFNs) [via] pattern recognition receptors (TLR7 and retinoic acid-inducible gene 1).” Furthermore, supernatants from primary HLSECs transfected with HCV-specific PAMP molecules had larger inductions of IFNs and IFN-stimulated genes in HLSECs.
“Conditioned media from IFN-stimulated HLSECs induced expression of antiviral genes by uninfected primary human hepatocytes,” wrote Dr. Giugliano and her coauthors. “Exosomes, derived from HLSECs following stimulation with either type I or type III IFNs, controlled HCV replication in a dose-dependent manner.”
However, the investigators say that more study is required to understand why HCV is able to present so persistently if the means to combat the virus are so apparent in the immune system.
“These results raise a number of intriguing questions, for example, how the use of exogenous IFN to treat viral hepatitis could be expected to induce additional, previously unrecognized antiviral mechanisms involving HLSECs,” says the study. “Further work is warranted to understand why despite these innate immune responses, HCV is able to establish persistence and fail eradication with IFN-based antiviral therapy.”
The study supported by grants R21-AI103361 and U19 AI 1066328; Dr. Rosen received a Merit Review grant and Dr. Shaw received grant R21-AI 106000. The authors reported no financial conflicts of interest.
Immunity against the hepatitis C virus can be induced by cultured primary human liver sinusoidal endothelial cells, according to a study published in the February issue of Gastroenterology (doi:10.1053/j.gastro.2014.10.040).
“We found HLSECs [primary human LSECs] express many of the receptors implicated in HCV [hepatitis C virus] attachment and entry and HLSEC-to-hepatocyte contact was dispensable for uptake,” wrote lead author Dr. Silvia M. Giugliano of the University of Colorado, Denver, and her associates.
In a cohort study, investigators exposed HLSECs and immortalized liver endothelial cells (TMNK-1) to several variants of HCV: full-length transmitted/founder virus, sucrose-purified Japanese fulminant hepatitis–1 (JFH-1), a virus encoding a luciferase reporter, and the HCV-specific pathogen-associated molecular pattern molecules (PAMP, substrate for RIG-I). Cells were analyzed by using polymerase chain reaction (PCR), immunofluorescence, and immunohistochemical assays.
Results showed that HLSECs, regardless of contact between cells, were able to internalize HCV when exposed to it and replicate, though not translate HCV RNA. The HCV RNA “induced consistent and broad transcription of multiple interferons (IFNs) [via] pattern recognition receptors (TLR7 and retinoic acid-inducible gene 1).” Furthermore, supernatants from primary HLSECs transfected with HCV-specific PAMP molecules had larger inductions of IFNs and IFN-stimulated genes in HLSECs.
“Conditioned media from IFN-stimulated HLSECs induced expression of antiviral genes by uninfected primary human hepatocytes,” wrote Dr. Giugliano and her coauthors. “Exosomes, derived from HLSECs following stimulation with either type I or type III IFNs, controlled HCV replication in a dose-dependent manner.”
However, the investigators say that more study is required to understand why HCV is able to present so persistently if the means to combat the virus are so apparent in the immune system.
“These results raise a number of intriguing questions, for example, how the use of exogenous IFN to treat viral hepatitis could be expected to induce additional, previously unrecognized antiviral mechanisms involving HLSECs,” says the study. “Further work is warranted to understand why despite these innate immune responses, HCV is able to establish persistence and fail eradication with IFN-based antiviral therapy.”
The study supported by grants R21-AI103361 and U19 AI 1066328; Dr. Rosen received a Merit Review grant and Dr. Shaw received grant R21-AI 106000. The authors reported no financial conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Cultured primary human liver sinusoidal endothelial cells (HLSECs) induce self-amplifying interferon-mediated responses and release of exosomes with antiviral activity, thus allowing it to mediate immunity against the hepatitis C virus (HCV).
Major finding: HLSECs and CV-specific PAMP molecules induced IFNs and replicated HCV RNA when exposed to various forms of HCV.
Data source: Cohort study
Disclosures: Study supported by grants R21-AI103361 and U19 AI 1066328; Dr. Rosen received a Merit Review grant and Dr. Shaw received grant R21-AI 106000. The authors reported no financial conflicts of interest.