User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
FDA grants accelerated approval to nivolumab for Hodgkin lymphoma
The Food and Drug Administration has granted accelerated approval to nivolumab for the treatment of patients with classical Hodgkin lymphoma (cHL) that has relapsed or progressed after autologous hematopoietic stem cell transplantation (HSCT) and posttransplantation brentuximab vedotin.
Approval was based on a 65% objective response rate in 95 patients treated with nivolumab following autologous HSCT and posttransplantation brentuximab vedotin. All patients in the single-arm, multicenter trial had relapsed or refractory cHL and were enrolled regardless of PD-L1 expression status. Patients received a median of 17 doses of nivolumab, the FDA said in a written statement.
The median time to response was 2.1 months (range, 0.7-5.7 months). The estimated median duration of response was 8.7 months.
The FDA also issued a warning for complications of allogeneic HSCT after nivolumab, reporting that transplant-related deaths have occurred. Health care professionals should follow patients closely for early evidence of transplant-related complications, such as hyperacute graft-versus-host disease (GVHD), severe acute GVHD, steroid-requiring febrile syndrome, hepatic veno-occlusive disease, and other immune-mediated adverse reactions, they said.
The most common adverse reactions in a second single-arm study used to evaluate safety (n = 263) were upper respiratory tract infection, cough, pyrexia, and diarrhea. Other immune-mediated adverse reactions, occurring in 1%-5% of patients, included rash, pneumonitis, hepatitis, hyperthyroidism, and colitis. The most common serious adverse reactions, which were reported in 1%-3% of patients, were pneumonia, pleural effusion, pneumonitis, pyrexia, infusion-related reaction, and rash.
Nivolumab is marketed as Opdivo by Bristol-Myers Squibb and has been previously approved to treat advanced renal cell carcinoma, lung cancer, and melanoma.
On Twitter @NikolaidesLaura
The Food and Drug Administration has granted accelerated approval to nivolumab for the treatment of patients with classical Hodgkin lymphoma (cHL) that has relapsed or progressed after autologous hematopoietic stem cell transplantation (HSCT) and posttransplantation brentuximab vedotin.
Approval was based on a 65% objective response rate in 95 patients treated with nivolumab following autologous HSCT and posttransplantation brentuximab vedotin. All patients in the single-arm, multicenter trial had relapsed or refractory cHL and were enrolled regardless of PD-L1 expression status. Patients received a median of 17 doses of nivolumab, the FDA said in a written statement.
The median time to response was 2.1 months (range, 0.7-5.7 months). The estimated median duration of response was 8.7 months.
The FDA also issued a warning for complications of allogeneic HSCT after nivolumab, reporting that transplant-related deaths have occurred. Health care professionals should follow patients closely for early evidence of transplant-related complications, such as hyperacute graft-versus-host disease (GVHD), severe acute GVHD, steroid-requiring febrile syndrome, hepatic veno-occlusive disease, and other immune-mediated adverse reactions, they said.
The most common adverse reactions in a second single-arm study used to evaluate safety (n = 263) were upper respiratory tract infection, cough, pyrexia, and diarrhea. Other immune-mediated adverse reactions, occurring in 1%-5% of patients, included rash, pneumonitis, hepatitis, hyperthyroidism, and colitis. The most common serious adverse reactions, which were reported in 1%-3% of patients, were pneumonia, pleural effusion, pneumonitis, pyrexia, infusion-related reaction, and rash.
Nivolumab is marketed as Opdivo by Bristol-Myers Squibb and has been previously approved to treat advanced renal cell carcinoma, lung cancer, and melanoma.
On Twitter @NikolaidesLaura
The Food and Drug Administration has granted accelerated approval to nivolumab for the treatment of patients with classical Hodgkin lymphoma (cHL) that has relapsed or progressed after autologous hematopoietic stem cell transplantation (HSCT) and posttransplantation brentuximab vedotin.
Approval was based on a 65% objective response rate in 95 patients treated with nivolumab following autologous HSCT and posttransplantation brentuximab vedotin. All patients in the single-arm, multicenter trial had relapsed or refractory cHL and were enrolled regardless of PD-L1 expression status. Patients received a median of 17 doses of nivolumab, the FDA said in a written statement.
The median time to response was 2.1 months (range, 0.7-5.7 months). The estimated median duration of response was 8.7 months.
The FDA also issued a warning for complications of allogeneic HSCT after nivolumab, reporting that transplant-related deaths have occurred. Health care professionals should follow patients closely for early evidence of transplant-related complications, such as hyperacute graft-versus-host disease (GVHD), severe acute GVHD, steroid-requiring febrile syndrome, hepatic veno-occlusive disease, and other immune-mediated adverse reactions, they said.
The most common adverse reactions in a second single-arm study used to evaluate safety (n = 263) were upper respiratory tract infection, cough, pyrexia, and diarrhea. Other immune-mediated adverse reactions, occurring in 1%-5% of patients, included rash, pneumonitis, hepatitis, hyperthyroidism, and colitis. The most common serious adverse reactions, which were reported in 1%-3% of patients, were pneumonia, pleural effusion, pneumonitis, pyrexia, infusion-related reaction, and rash.
Nivolumab is marketed as Opdivo by Bristol-Myers Squibb and has been previously approved to treat advanced renal cell carcinoma, lung cancer, and melanoma.
On Twitter @NikolaidesLaura
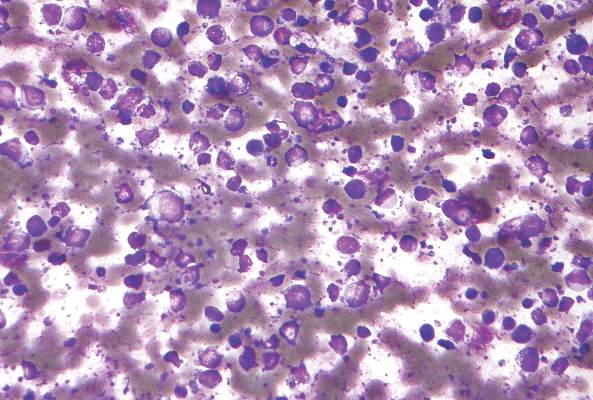
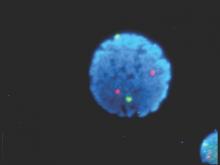
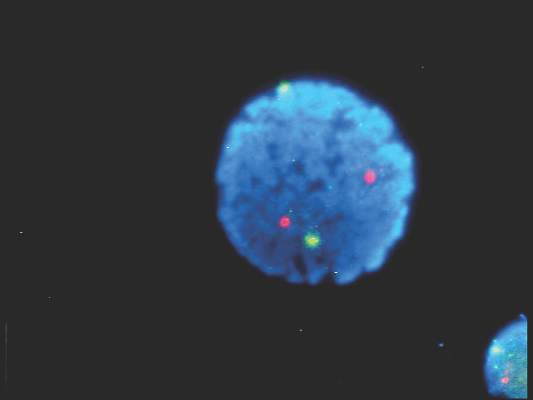
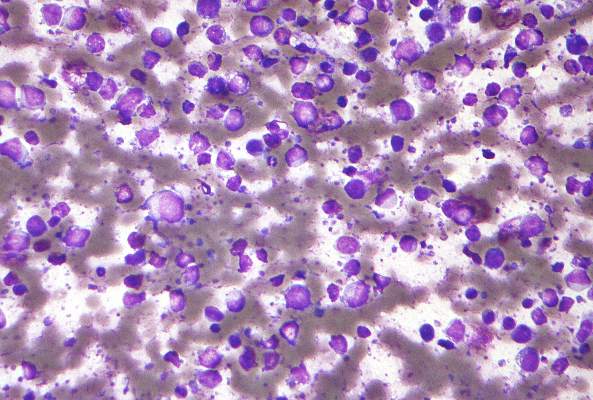
Epstein-Barr virus DNA in plasma reliably detects EBV-positive lymphoproliferative disorders
Detection of Epstein-Barr virus DNA in plasma reliably signaled “a broad range” of EBV+ diseases, according to investigators.
In contrast, the presence of EBV DNA in peripheral blood mononuclear cells did not reliably predict EBV diseases, said Dr. Jennifer A. Kanakry and her associates at Johns Hopkins University, Baltimore. Patients without EBV diseases can have EBV DNA in their PBMCs, particularly if they are immunocompromised, the researchers observed.
Latent EBV infection is associated with lymphomas, lymphoproliferative disorders, hemophagocytic lymphohistiocytosis, solid tumors, and other diseases. To characterize the relationship between these diseases and EBV DNA, the researchers studied viral quantitative real-time polymerase chain reaction assays of plasma and PBMCs from 2,146 patients tested at Johns Hopkins over 5 years. Patients were usually immunocompromised and hospitalized, the investigators noted (Blood 2016;127:2007-17).
A total of 535 patients (25%) had EBV detected in plasma or PBMCs. Notably, 69% of patients who did not have EBV diseases had EBV in PBMCs, but not in plasma. Among 105 patients with active systemic EBV+ diseases, 99% had EBV DNA in plasma, but only 54% had EBV in PBMCs. Furthermore, the number of copies of EBV DNA distinguished untreated EBV+ lymphoma, remitted EBV+ lymphoma, and EBV- lymphoma, and also distinguished untreated, EBV+ post-transplantation lymphoproliferative disorder (PTLD), EBV+ PTLD in remission, and EBV– PTLD.
“Cell-free (plasma) EBV DNA performs better than cellular EBV DNA as a marker of a broad range of EBV+ diseases,” the investigators concluded. “Within a largely immunocompromised and hospitalized cohort, detection of EBV DNA in plasma is uncommon in the absence of EBV+ disease.”
The National Cancer Institute, National Institutes of Health, and Center for AIDS Research funded the study. The researchers had no disclosures.
Detection of Epstein-Barr virus DNA in plasma reliably signaled “a broad range” of EBV+ diseases, according to investigators.
In contrast, the presence of EBV DNA in peripheral blood mononuclear cells did not reliably predict EBV diseases, said Dr. Jennifer A. Kanakry and her associates at Johns Hopkins University, Baltimore. Patients without EBV diseases can have EBV DNA in their PBMCs, particularly if they are immunocompromised, the researchers observed.
Latent EBV infection is associated with lymphomas, lymphoproliferative disorders, hemophagocytic lymphohistiocytosis, solid tumors, and other diseases. To characterize the relationship between these diseases and EBV DNA, the researchers studied viral quantitative real-time polymerase chain reaction assays of plasma and PBMCs from 2,146 patients tested at Johns Hopkins over 5 years. Patients were usually immunocompromised and hospitalized, the investigators noted (Blood 2016;127:2007-17).
A total of 535 patients (25%) had EBV detected in plasma or PBMCs. Notably, 69% of patients who did not have EBV diseases had EBV in PBMCs, but not in plasma. Among 105 patients with active systemic EBV+ diseases, 99% had EBV DNA in plasma, but only 54% had EBV in PBMCs. Furthermore, the number of copies of EBV DNA distinguished untreated EBV+ lymphoma, remitted EBV+ lymphoma, and EBV- lymphoma, and also distinguished untreated, EBV+ post-transplantation lymphoproliferative disorder (PTLD), EBV+ PTLD in remission, and EBV– PTLD.
“Cell-free (plasma) EBV DNA performs better than cellular EBV DNA as a marker of a broad range of EBV+ diseases,” the investigators concluded. “Within a largely immunocompromised and hospitalized cohort, detection of EBV DNA in plasma is uncommon in the absence of EBV+ disease.”
The National Cancer Institute, National Institutes of Health, and Center for AIDS Research funded the study. The researchers had no disclosures.
Detection of Epstein-Barr virus DNA in plasma reliably signaled “a broad range” of EBV+ diseases, according to investigators.
In contrast, the presence of EBV DNA in peripheral blood mononuclear cells did not reliably predict EBV diseases, said Dr. Jennifer A. Kanakry and her associates at Johns Hopkins University, Baltimore. Patients without EBV diseases can have EBV DNA in their PBMCs, particularly if they are immunocompromised, the researchers observed.
Latent EBV infection is associated with lymphomas, lymphoproliferative disorders, hemophagocytic lymphohistiocytosis, solid tumors, and other diseases. To characterize the relationship between these diseases and EBV DNA, the researchers studied viral quantitative real-time polymerase chain reaction assays of plasma and PBMCs from 2,146 patients tested at Johns Hopkins over 5 years. Patients were usually immunocompromised and hospitalized, the investigators noted (Blood 2016;127:2007-17).
A total of 535 patients (25%) had EBV detected in plasma or PBMCs. Notably, 69% of patients who did not have EBV diseases had EBV in PBMCs, but not in plasma. Among 105 patients with active systemic EBV+ diseases, 99% had EBV DNA in plasma, but only 54% had EBV in PBMCs. Furthermore, the number of copies of EBV DNA distinguished untreated EBV+ lymphoma, remitted EBV+ lymphoma, and EBV- lymphoma, and also distinguished untreated, EBV+ post-transplantation lymphoproliferative disorder (PTLD), EBV+ PTLD in remission, and EBV– PTLD.
“Cell-free (plasma) EBV DNA performs better than cellular EBV DNA as a marker of a broad range of EBV+ diseases,” the investigators concluded. “Within a largely immunocompromised and hospitalized cohort, detection of EBV DNA in plasma is uncommon in the absence of EBV+ disease.”
The National Cancer Institute, National Institutes of Health, and Center for AIDS Research funded the study. The researchers had no disclosures.
FROM BLOOD
Key clinical point: Epstein-Barr virus DNA was a more specific indicator of EBV+ diseases when detected in plasma, as opposed to peripheral blood mononuclear cells.
Major finding: Among 105 patients with active systemic EBV+ diseases, 99% had EBV DNA in plasma, but 54% had EBV in PBMCs.
Data source: Viral quantitative real-time polymerase chain reaction assays of plasma and PBMCs from 2,146 patients.
Disclosures: The National Cancer Institute, National Institutes of Health, and Center for AIDS Research funded the study. The researchers had no disclosures.
Low transformation rate in nodular lymphocyte–predominant Hodgkin lymphoma
Fewer than 8% of cases of nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) transformed to diffuse large B-cell lymphoma (DLBCL), based on a large prospective single-center study with long-term follow-up.
This rate was lower than the risk of transformation reported for transformed follicular lymphoma or chronic lymphocytic leukemia, according to Dr. Saad Kenderian and his associates at the Mayo Clinic, Rochester, Minn. Transformation was significantly associated with splenic involvement at presentation and with prior chemotherapy exposure, but did not worsen overall survival, they added.
“To our knowledge, this cohort represents the largest analysis to date of consecutive patients with NLPHL,” they said.
The study comprised 222 patients with newly diagnosed NLPHL who were treated at Mayo Clinic between 1970 and 2011. Median age at diagnosis was 40 years, and two-thirds of patients were men. The median follow-up period was 16 years (Blood 2016;12:1960-6. doi: 10.1182/blood-2015-08-665505).
During follow up, 17 cases (7.6%) transformed to DLBCL, for a transformation rate of 0.74 cases for every 100 patient-years, the investigators said. Median time to transformation was 35 months (range, 6-268 months). Predictors of transformation included any prior chemotherapy exposure (P = .04) and splenic involvement (P = .03). The rates of 40-year freedom from transformation were 87% when there was no splenic involvement and 21% when the spleen was involved, and were 87% if radiation therapy was used as a single modality compared with 77% in patients treated with prior chemotherapy or chemoradiation.
Five-year overall survival was 76% in patients with transformed disease, which was similar to overall survival among patients whose disease did not transform to DLBCL, the researchers noted.
Other studies of NLPHL have reported anywhere from a 2% to a 17% transformation rate, but those studies had smaller sample sizes, shorter follow-up periods, and less rigorous enrollment criteria and methods to confirm transformation, the investigators noted. “The finding of splenic involvement as a risk factor for transformation was reported by previous investigators. Interestingly, the association between exposure to prior chemotherapy and reduced freedom from transformation has not been reported in the past, but it has been observed in other low-grade lymphoma studies,” they added. “In contrast to follicular lymphoma, transformed NLPHL is not associated with an adverse impact on OS, suggesting a possibly different biology of transformation.”
The research was partially supported by Lymphoma SPORE and the Predolin Foundation. The investigators had no disclosures.
Kenderian et al. report a lower rate of transformation (7.6%) to diffuse large B-cell lymphoma for patients with nodular lymphocyte–predominant Hodgkin lymphoma compared with other series and found that transformation did not have a negative impact on overall survival. Reassuringly, even if transformation occurs, it is generally at a low rate. Also, these patients do well with additional treatment and do not have worse overall survival. At the MD Anderson Cancer Center, we have used a regimen based on R-CHOP and have not seen transformations. But only through large cooperative clinical trials can we determine whether R-CHOP or other more novel regimens are actually superior to ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or rituximab (R)-ABVD for patients at high risk of transformation.
Dr. Michelle Fanale is at the University of Texas MD Anderson Cancer Center, Houston. She had no disclosures. These comments are from her editorial (Blood 2016;1927:1946-7 doi: 10.1182/blood-2016-03-699108).
Kenderian et al. report a lower rate of transformation (7.6%) to diffuse large B-cell lymphoma for patients with nodular lymphocyte–predominant Hodgkin lymphoma compared with other series and found that transformation did not have a negative impact on overall survival. Reassuringly, even if transformation occurs, it is generally at a low rate. Also, these patients do well with additional treatment and do not have worse overall survival. At the MD Anderson Cancer Center, we have used a regimen based on R-CHOP and have not seen transformations. But only through large cooperative clinical trials can we determine whether R-CHOP or other more novel regimens are actually superior to ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or rituximab (R)-ABVD for patients at high risk of transformation.
Dr. Michelle Fanale is at the University of Texas MD Anderson Cancer Center, Houston. She had no disclosures. These comments are from her editorial (Blood 2016;1927:1946-7 doi: 10.1182/blood-2016-03-699108).
Kenderian et al. report a lower rate of transformation (7.6%) to diffuse large B-cell lymphoma for patients with nodular lymphocyte–predominant Hodgkin lymphoma compared with other series and found that transformation did not have a negative impact on overall survival. Reassuringly, even if transformation occurs, it is generally at a low rate. Also, these patients do well with additional treatment and do not have worse overall survival. At the MD Anderson Cancer Center, we have used a regimen based on R-CHOP and have not seen transformations. But only through large cooperative clinical trials can we determine whether R-CHOP or other more novel regimens are actually superior to ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) or rituximab (R)-ABVD for patients at high risk of transformation.
Dr. Michelle Fanale is at the University of Texas MD Anderson Cancer Center, Houston. She had no disclosures. These comments are from her editorial (Blood 2016;1927:1946-7 doi: 10.1182/blood-2016-03-699108).
Fewer than 8% of cases of nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) transformed to diffuse large B-cell lymphoma (DLBCL), based on a large prospective single-center study with long-term follow-up.
This rate was lower than the risk of transformation reported for transformed follicular lymphoma or chronic lymphocytic leukemia, according to Dr. Saad Kenderian and his associates at the Mayo Clinic, Rochester, Minn. Transformation was significantly associated with splenic involvement at presentation and with prior chemotherapy exposure, but did not worsen overall survival, they added.
“To our knowledge, this cohort represents the largest analysis to date of consecutive patients with NLPHL,” they said.
The study comprised 222 patients with newly diagnosed NLPHL who were treated at Mayo Clinic between 1970 and 2011. Median age at diagnosis was 40 years, and two-thirds of patients were men. The median follow-up period was 16 years (Blood 2016;12:1960-6. doi: 10.1182/blood-2015-08-665505).
During follow up, 17 cases (7.6%) transformed to DLBCL, for a transformation rate of 0.74 cases for every 100 patient-years, the investigators said. Median time to transformation was 35 months (range, 6-268 months). Predictors of transformation included any prior chemotherapy exposure (P = .04) and splenic involvement (P = .03). The rates of 40-year freedom from transformation were 87% when there was no splenic involvement and 21% when the spleen was involved, and were 87% if radiation therapy was used as a single modality compared with 77% in patients treated with prior chemotherapy or chemoradiation.
Five-year overall survival was 76% in patients with transformed disease, which was similar to overall survival among patients whose disease did not transform to DLBCL, the researchers noted.
Other studies of NLPHL have reported anywhere from a 2% to a 17% transformation rate, but those studies had smaller sample sizes, shorter follow-up periods, and less rigorous enrollment criteria and methods to confirm transformation, the investigators noted. “The finding of splenic involvement as a risk factor for transformation was reported by previous investigators. Interestingly, the association between exposure to prior chemotherapy and reduced freedom from transformation has not been reported in the past, but it has been observed in other low-grade lymphoma studies,” they added. “In contrast to follicular lymphoma, transformed NLPHL is not associated with an adverse impact on OS, suggesting a possibly different biology of transformation.”
The research was partially supported by Lymphoma SPORE and the Predolin Foundation. The investigators had no disclosures.
Fewer than 8% of cases of nodular lymphocyte–predominant Hodgkin lymphoma (NLPHL) transformed to diffuse large B-cell lymphoma (DLBCL), based on a large prospective single-center study with long-term follow-up.
This rate was lower than the risk of transformation reported for transformed follicular lymphoma or chronic lymphocytic leukemia, according to Dr. Saad Kenderian and his associates at the Mayo Clinic, Rochester, Minn. Transformation was significantly associated with splenic involvement at presentation and with prior chemotherapy exposure, but did not worsen overall survival, they added.
“To our knowledge, this cohort represents the largest analysis to date of consecutive patients with NLPHL,” they said.
The study comprised 222 patients with newly diagnosed NLPHL who were treated at Mayo Clinic between 1970 and 2011. Median age at diagnosis was 40 years, and two-thirds of patients were men. The median follow-up period was 16 years (Blood 2016;12:1960-6. doi: 10.1182/blood-2015-08-665505).
During follow up, 17 cases (7.6%) transformed to DLBCL, for a transformation rate of 0.74 cases for every 100 patient-years, the investigators said. Median time to transformation was 35 months (range, 6-268 months). Predictors of transformation included any prior chemotherapy exposure (P = .04) and splenic involvement (P = .03). The rates of 40-year freedom from transformation were 87% when there was no splenic involvement and 21% when the spleen was involved, and were 87% if radiation therapy was used as a single modality compared with 77% in patients treated with prior chemotherapy or chemoradiation.
Five-year overall survival was 76% in patients with transformed disease, which was similar to overall survival among patients whose disease did not transform to DLBCL, the researchers noted.
Other studies of NLPHL have reported anywhere from a 2% to a 17% transformation rate, but those studies had smaller sample sizes, shorter follow-up periods, and less rigorous enrollment criteria and methods to confirm transformation, the investigators noted. “The finding of splenic involvement as a risk factor for transformation was reported by previous investigators. Interestingly, the association between exposure to prior chemotherapy and reduced freedom from transformation has not been reported in the past, but it has been observed in other low-grade lymphoma studies,” they added. “In contrast to follicular lymphoma, transformed NLPHL is not associated with an adverse impact on OS, suggesting a possibly different biology of transformation.”
The research was partially supported by Lymphoma SPORE and the Predolin Foundation. The investigators had no disclosures.
FROM BLOOD
Key clinical point: The risk of transformation to diffuse large B-cell lymphoma is low in patients with nodular lymphocyte–predominant Hodgkin lymphoma.
Major finding: Only 7.6% of cases transformed over a median of 16 years of follow-up, and transformation did not worsen overall survival.
Data source: A prospective single-center study of 222 consecutive adults with NLPHL.
Disclosures: The research was partially supported by Lymphoma SPORE and the Predolin Foundation. The investigators had no disclosures.
New single-tube assay detects one CLL cell in 1 million leukocytes
Chronic lymphocytic leukemia cells can be identified at levels as low as 0.0010% with a newly developed and validated single-tube assay, Dr. Andy C. Rawstro of St. James’s Institute of Oncology, Leeds, England, and his colleagues said in a European Research Initiative on CLL (ERIC) project report in Leukemia.
The high-throughput sequencing assay consists of a core panel of six markers – CD19, CD20, CD5, CD43, CD79b and CD81 – with a component specification independent of instrument and reagents. The new assay eliminates the need to distribute the blood sample across multiple tubes, which can impair sensitivity in cases with poor cellularity. The assay can be locally revalidated using normal peripheral blood and can be used to investigate new markers.
The new assay was validated in a multicenter study of 128 samples from 108 patients with CLL or monoclonal B-cell lymphocytosis, studied either at diagnosis or after FCR-based (fludarabine, cyclophosphamide, rituximab) treatment and compared with peripheral blood samples from healthy young women. In a parallel analysis, the assay compared with flow cytometry results at the 0.010% level, the minimal residual disease threshold defined in the 2008 International Workshop on CLL guidelines. The new assay, however, also was able to detect disease at the 0.0010% (one CLL cell in 1 million leukocytes) level.
The ability to detect disease below the levels that can be assessed by flow cytometry may prove to be a valuable resource to improve quantification of minimal residual disease and evaluate treatment response in CLL. Using minimal residual disease as a surrogate measure for treatment effectiveness would avoid the need for prolonged observation times in assessing new therapies, the researchers said (Leukemia 2016;30:929-36).
Dr. Rawstro disclosed receiving honoraria from Celgene, Abbvie, Gilead, Roche, and GSK. He receives royalty payments from BD Biosciences (Intrasure reagent) and study reagents. He is a consultant for Gilead and Biogen Idec.
On Twitter @maryjodales
Chronic lymphocytic leukemia cells can be identified at levels as low as 0.0010% with a newly developed and validated single-tube assay, Dr. Andy C. Rawstro of St. James’s Institute of Oncology, Leeds, England, and his colleagues said in a European Research Initiative on CLL (ERIC) project report in Leukemia.
The high-throughput sequencing assay consists of a core panel of six markers – CD19, CD20, CD5, CD43, CD79b and CD81 – with a component specification independent of instrument and reagents. The new assay eliminates the need to distribute the blood sample across multiple tubes, which can impair sensitivity in cases with poor cellularity. The assay can be locally revalidated using normal peripheral blood and can be used to investigate new markers.
The new assay was validated in a multicenter study of 128 samples from 108 patients with CLL or monoclonal B-cell lymphocytosis, studied either at diagnosis or after FCR-based (fludarabine, cyclophosphamide, rituximab) treatment and compared with peripheral blood samples from healthy young women. In a parallel analysis, the assay compared with flow cytometry results at the 0.010% level, the minimal residual disease threshold defined in the 2008 International Workshop on CLL guidelines. The new assay, however, also was able to detect disease at the 0.0010% (one CLL cell in 1 million leukocytes) level.
The ability to detect disease below the levels that can be assessed by flow cytometry may prove to be a valuable resource to improve quantification of minimal residual disease and evaluate treatment response in CLL. Using minimal residual disease as a surrogate measure for treatment effectiveness would avoid the need for prolonged observation times in assessing new therapies, the researchers said (Leukemia 2016;30:929-36).
Dr. Rawstro disclosed receiving honoraria from Celgene, Abbvie, Gilead, Roche, and GSK. He receives royalty payments from BD Biosciences (Intrasure reagent) and study reagents. He is a consultant for Gilead and Biogen Idec.
On Twitter @maryjodales
Chronic lymphocytic leukemia cells can be identified at levels as low as 0.0010% with a newly developed and validated single-tube assay, Dr. Andy C. Rawstro of St. James’s Institute of Oncology, Leeds, England, and his colleagues said in a European Research Initiative on CLL (ERIC) project report in Leukemia.
The high-throughput sequencing assay consists of a core panel of six markers – CD19, CD20, CD5, CD43, CD79b and CD81 – with a component specification independent of instrument and reagents. The new assay eliminates the need to distribute the blood sample across multiple tubes, which can impair sensitivity in cases with poor cellularity. The assay can be locally revalidated using normal peripheral blood and can be used to investigate new markers.
The new assay was validated in a multicenter study of 128 samples from 108 patients with CLL or monoclonal B-cell lymphocytosis, studied either at diagnosis or after FCR-based (fludarabine, cyclophosphamide, rituximab) treatment and compared with peripheral blood samples from healthy young women. In a parallel analysis, the assay compared with flow cytometry results at the 0.010% level, the minimal residual disease threshold defined in the 2008 International Workshop on CLL guidelines. The new assay, however, also was able to detect disease at the 0.0010% (one CLL cell in 1 million leukocytes) level.
The ability to detect disease below the levels that can be assessed by flow cytometry may prove to be a valuable resource to improve quantification of minimal residual disease and evaluate treatment response in CLL. Using minimal residual disease as a surrogate measure for treatment effectiveness would avoid the need for prolonged observation times in assessing new therapies, the researchers said (Leukemia 2016;30:929-36).
Dr. Rawstro disclosed receiving honoraria from Celgene, Abbvie, Gilead, Roche, and GSK. He receives royalty payments from BD Biosciences (Intrasure reagent) and study reagents. He is a consultant for Gilead and Biogen Idec.
On Twitter @maryjodales
FROM LEUKEMIA
Key clinical point: Chronic lymphocytic leukemia cells can be identified at levels as low as 0.0010% with a newly developed and validated single-tube assay.
Major finding: In a parallel analysis, the assay compared with flow cytometry results at the 0.010% level, and also was able to detect disease at the 0.0010% (one CLL cell in 1 million leukocytes) level.
Data source: The new assay was validated in a multicenter study of 128 samples from 108 patients with CLL or monoclonal B-cell lymphocytosis.
Disclosures: Dr. Rawstro disclosed receiving honoraria from Celgene, Abbvie, Gilead, Roche, and GSK. He receives royalty payments from BD Biosciences (Intrasure reagent) and study reagents. He is a consultant for Gilead and Biogen Idec.
Dose-escalated blinatumomab plus dexamethasone induced responses in relapsed/refractory DLBCL
Blinatumomab, administered in a stepwise, dose-escalated regimen with prophylactic dexamethasone, induced responses in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL), according to a report published in Blood.
The phase II study included 25 patients who were refractory to their last treatment, had relapsed after autologous hematopoietic stem cell transplant (HSCT), or had relapsed disease and were ineligible for autologous HSCT.
In a previous phase I study that did not use a dose-escalated regimen, neurological adverse events were dose limiting at a maximum tolerated blinatumomab dose of 60 mcg/d. The current study used a 14-day stepwise dose escalation and corticosteroid premedication to minimize adverse events: blinatumomab was given at 9 mcg/d in the first week, 28 mcg/d in the second week, and 112 mcg/d thereafter.
Patients had received a median of three prior therapies. In total, 17 of 25 patients ended in cycle 1 (induction) because of disease progression, adverse events, or physician decision; 7 ended in cycle 2 (consolidation), and 1 ended in retreatment.
Among the 21 evaluable patients who received at least 1 week of blinatumomab at the target dose of 112 mcg/day or who discontinued treatment earlier because of progressive disease, the overall response rate was 43% (9/21) and the complete response rate was 19% (4/21). “At least 1 week of treatment at the target dose of 112 mcg/d appears to be required for efficacy,” wrote Dr. Andreas Viardot of the department of internal medicine III, University Hospital Ulm (Germany) and colleagues (Blood. 2016;127(11):1410-16).
The most common adverse events of grade 3 or higher were thrombocytopenia (17%) and leukopenia (17%). Grade 3 neurologic events included encephalopathy (9%) and aphasia (9%); no grade 4 or 5 neurologic events were reported. Cytokine release syndrome or cytokine storm were not reported.
Blinatumomab (Blincyto) is a bispecific T-cell engager antibody approved for the treatment of Philadelphia chromosome-negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Blinatumomab transiently links CD3-positive T cells to CD19-positive B cells, which activates T cells and leads to T cell–mediated lysis of tumor cells and concomitant T-cell proliferation.
The study was supported by Amgen, the maker of Blincyto. Dr. Viardot reported having financial ties with Amgen and several other drug companies.
Blinatumomab, administered in a stepwise, dose-escalated regimen with prophylactic dexamethasone, induced responses in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL), according to a report published in Blood.
The phase II study included 25 patients who were refractory to their last treatment, had relapsed after autologous hematopoietic stem cell transplant (HSCT), or had relapsed disease and were ineligible for autologous HSCT.
In a previous phase I study that did not use a dose-escalated regimen, neurological adverse events were dose limiting at a maximum tolerated blinatumomab dose of 60 mcg/d. The current study used a 14-day stepwise dose escalation and corticosteroid premedication to minimize adverse events: blinatumomab was given at 9 mcg/d in the first week, 28 mcg/d in the second week, and 112 mcg/d thereafter.
Patients had received a median of three prior therapies. In total, 17 of 25 patients ended in cycle 1 (induction) because of disease progression, adverse events, or physician decision; 7 ended in cycle 2 (consolidation), and 1 ended in retreatment.
Among the 21 evaluable patients who received at least 1 week of blinatumomab at the target dose of 112 mcg/day or who discontinued treatment earlier because of progressive disease, the overall response rate was 43% (9/21) and the complete response rate was 19% (4/21). “At least 1 week of treatment at the target dose of 112 mcg/d appears to be required for efficacy,” wrote Dr. Andreas Viardot of the department of internal medicine III, University Hospital Ulm (Germany) and colleagues (Blood. 2016;127(11):1410-16).
The most common adverse events of grade 3 or higher were thrombocytopenia (17%) and leukopenia (17%). Grade 3 neurologic events included encephalopathy (9%) and aphasia (9%); no grade 4 or 5 neurologic events were reported. Cytokine release syndrome or cytokine storm were not reported.
Blinatumomab (Blincyto) is a bispecific T-cell engager antibody approved for the treatment of Philadelphia chromosome-negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Blinatumomab transiently links CD3-positive T cells to CD19-positive B cells, which activates T cells and leads to T cell–mediated lysis of tumor cells and concomitant T-cell proliferation.
The study was supported by Amgen, the maker of Blincyto. Dr. Viardot reported having financial ties with Amgen and several other drug companies.
Blinatumomab, administered in a stepwise, dose-escalated regimen with prophylactic dexamethasone, induced responses in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL), according to a report published in Blood.
The phase II study included 25 patients who were refractory to their last treatment, had relapsed after autologous hematopoietic stem cell transplant (HSCT), or had relapsed disease and were ineligible for autologous HSCT.
In a previous phase I study that did not use a dose-escalated regimen, neurological adverse events were dose limiting at a maximum tolerated blinatumomab dose of 60 mcg/d. The current study used a 14-day stepwise dose escalation and corticosteroid premedication to minimize adverse events: blinatumomab was given at 9 mcg/d in the first week, 28 mcg/d in the second week, and 112 mcg/d thereafter.
Patients had received a median of three prior therapies. In total, 17 of 25 patients ended in cycle 1 (induction) because of disease progression, adverse events, or physician decision; 7 ended in cycle 2 (consolidation), and 1 ended in retreatment.
Among the 21 evaluable patients who received at least 1 week of blinatumomab at the target dose of 112 mcg/day or who discontinued treatment earlier because of progressive disease, the overall response rate was 43% (9/21) and the complete response rate was 19% (4/21). “At least 1 week of treatment at the target dose of 112 mcg/d appears to be required for efficacy,” wrote Dr. Andreas Viardot of the department of internal medicine III, University Hospital Ulm (Germany) and colleagues (Blood. 2016;127(11):1410-16).
The most common adverse events of grade 3 or higher were thrombocytopenia (17%) and leukopenia (17%). Grade 3 neurologic events included encephalopathy (9%) and aphasia (9%); no grade 4 or 5 neurologic events were reported. Cytokine release syndrome or cytokine storm were not reported.
Blinatumomab (Blincyto) is a bispecific T-cell engager antibody approved for the treatment of Philadelphia chromosome-negative relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Blinatumomab transiently links CD3-positive T cells to CD19-positive B cells, which activates T cells and leads to T cell–mediated lysis of tumor cells and concomitant T-cell proliferation.
The study was supported by Amgen, the maker of Blincyto. Dr. Viardot reported having financial ties with Amgen and several other drug companies.
FROM BLOOD
Key clinical point: Stepwise dose-escalated administration of blinatumomab plus dexamethasone may be effective in patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL).
Major finding: The overall response rate in 21 evaluable patients was 43% and the complete response rate was 19%.
Data sources: Phase II study of 25 patients who had received a median of three prior therapies.
Disclosures: The study was supported by Amgen, the maker of blinatumomab. Dr. Viardot reported having financial ties with Amgen and several other drug makers.
VP Biden to AACR: Help me help you
Stronger teamwork among researchers, sharing data, and realignment of incentives for scientific breakthroughs, in addition to more funding, are key steps needed to advance cancer research, Vice President Joe Biden said during the annual meeting of the American Association for Cancer Research (AACR).
During a plenary speech to close the meeting, Vice President Biden praised the dedication of current cancer researchers and pledged to break down the walls that prevent them from achieving more progress in the field.
“I made a commitment that I will – as I gain this information and knowledge – I will eliminate the barriers that get in your way, get in the way of science and research and development,” he said. “I had to ... learn from all of you how we can proceed, how we can break down silos, how we can accommodate more rapidly the efforts you’re making.”
Vice President Biden, who is leading a new $1 billion initiative to eliminate cancer called “Moonshot,” outlined the top obstacles to cancer research he has garnered from recent visits with renowned cancer scientists and research leaders around the world. This includes a lack of unity among researchers, poor rewards for novel research, and limited data sharing, he said.
“The way the system now is set up, researchers are not incentivized to share their data,” Vice President Biden said, acknowledging that some medical experts are against the idea. “But every expert I’ve spoken to said you need to share these data to move this process rapidly.”
Involving patients earlier in clinical trials design is also a primary focus, he said. Patients should understand more about trials and be more open to signing up.
He noted the “incredible” research currently being conducted by various entities, such as AACR’s Project Genie, Orion Foundation, and The Parker Institute. Mr. Biden stressed however, that such efforts are too isolated.
“It raises [the] question: ‘Why is all this being done separately?’ ” Vice President Biden said. “Why is so much money being spent when if it’s aggregated, everyone acknowledges, the answers would come more quickly?”
Incentives for new research and the way in which funding is alloted must also be redesigned, he stressed. Today, it takes too long for researchers to get projects approved by the government and funding dispersed. He acknowledged the difficulty researchers face in obtaining grants and the fact that those who think “outside the box” are less likely to receive funding.
“It seems to me that we slow down our best minds by making them spend years in the lab before they can get their own grants and, when they do, they spend a third of their time writing a grant that takes months to be approved and awarded,” he said. “It’s like asking Derek Jeter to take several years off to sell bonds to build Yankee stadium.”
The Vice President did not purport to have all the answers, and asked those at the AARC meeting to provide feedback on his suggestions.
“The question I’d ask you to contemplate, because I’d like you to communicate with us, is, ‘Does it require realigning incentives; changing behaviors to take advantage of this inflection point? Does it require sharing more knowledge, treatment, and understanding? Or does that slow the process up?’ ”
He added,“I hope you all know it, but you’re one of the most valuable resources that our great country has, those of you sitting in this room. So ask your institutions, your colleagues, your mentors, your administrators: How can we move your ideas faster together in the interest of patients?”
The Vice President’s Moonshot initiative was announced during President Obama’s 2016 State of the Union Address. The effort includes a new Cancer Moonshot Task Force that will focus on federal investments, targeted incentives, private sector efforts from industry and philanthropy, patient engagement initiatives, and other mechanisms to support cancer research and enable progress in treatment and care, according to the White House. As part of the plan, the President’s fiscal 2017 budget proposes $755 million in mandatory funds for new cancer-related research activities at the National Institutes of Health and the Food and Drug Administration. The initiative also includes increased investments by the Department of Defense and the Department of Veterans Affairs in cancer research, including through funding centers of excellence focused on specific cancers and conducting longitudinal studies to determine risk factors and enhance treatment.
On Twitter @legal_med
Stronger teamwork among researchers, sharing data, and realignment of incentives for scientific breakthroughs, in addition to more funding, are key steps needed to advance cancer research, Vice President Joe Biden said during the annual meeting of the American Association for Cancer Research (AACR).
During a plenary speech to close the meeting, Vice President Biden praised the dedication of current cancer researchers and pledged to break down the walls that prevent them from achieving more progress in the field.
“I made a commitment that I will – as I gain this information and knowledge – I will eliminate the barriers that get in your way, get in the way of science and research and development,” he said. “I had to ... learn from all of you how we can proceed, how we can break down silos, how we can accommodate more rapidly the efforts you’re making.”
Vice President Biden, who is leading a new $1 billion initiative to eliminate cancer called “Moonshot,” outlined the top obstacles to cancer research he has garnered from recent visits with renowned cancer scientists and research leaders around the world. This includes a lack of unity among researchers, poor rewards for novel research, and limited data sharing, he said.
“The way the system now is set up, researchers are not incentivized to share their data,” Vice President Biden said, acknowledging that some medical experts are against the idea. “But every expert I’ve spoken to said you need to share these data to move this process rapidly.”
Involving patients earlier in clinical trials design is also a primary focus, he said. Patients should understand more about trials and be more open to signing up.
He noted the “incredible” research currently being conducted by various entities, such as AACR’s Project Genie, Orion Foundation, and The Parker Institute. Mr. Biden stressed however, that such efforts are too isolated.
“It raises [the] question: ‘Why is all this being done separately?’ ” Vice President Biden said. “Why is so much money being spent when if it’s aggregated, everyone acknowledges, the answers would come more quickly?”
Incentives for new research and the way in which funding is alloted must also be redesigned, he stressed. Today, it takes too long for researchers to get projects approved by the government and funding dispersed. He acknowledged the difficulty researchers face in obtaining grants and the fact that those who think “outside the box” are less likely to receive funding.
“It seems to me that we slow down our best minds by making them spend years in the lab before they can get their own grants and, when they do, they spend a third of their time writing a grant that takes months to be approved and awarded,” he said. “It’s like asking Derek Jeter to take several years off to sell bonds to build Yankee stadium.”
The Vice President did not purport to have all the answers, and asked those at the AARC meeting to provide feedback on his suggestions.
“The question I’d ask you to contemplate, because I’d like you to communicate with us, is, ‘Does it require realigning incentives; changing behaviors to take advantage of this inflection point? Does it require sharing more knowledge, treatment, and understanding? Or does that slow the process up?’ ”
He added,“I hope you all know it, but you’re one of the most valuable resources that our great country has, those of you sitting in this room. So ask your institutions, your colleagues, your mentors, your administrators: How can we move your ideas faster together in the interest of patients?”
The Vice President’s Moonshot initiative was announced during President Obama’s 2016 State of the Union Address. The effort includes a new Cancer Moonshot Task Force that will focus on federal investments, targeted incentives, private sector efforts from industry and philanthropy, patient engagement initiatives, and other mechanisms to support cancer research and enable progress in treatment and care, according to the White House. As part of the plan, the President’s fiscal 2017 budget proposes $755 million in mandatory funds for new cancer-related research activities at the National Institutes of Health and the Food and Drug Administration. The initiative also includes increased investments by the Department of Defense and the Department of Veterans Affairs in cancer research, including through funding centers of excellence focused on specific cancers and conducting longitudinal studies to determine risk factors and enhance treatment.
On Twitter @legal_med
Stronger teamwork among researchers, sharing data, and realignment of incentives for scientific breakthroughs, in addition to more funding, are key steps needed to advance cancer research, Vice President Joe Biden said during the annual meeting of the American Association for Cancer Research (AACR).
During a plenary speech to close the meeting, Vice President Biden praised the dedication of current cancer researchers and pledged to break down the walls that prevent them from achieving more progress in the field.
“I made a commitment that I will – as I gain this information and knowledge – I will eliminate the barriers that get in your way, get in the way of science and research and development,” he said. “I had to ... learn from all of you how we can proceed, how we can break down silos, how we can accommodate more rapidly the efforts you’re making.”
Vice President Biden, who is leading a new $1 billion initiative to eliminate cancer called “Moonshot,” outlined the top obstacles to cancer research he has garnered from recent visits with renowned cancer scientists and research leaders around the world. This includes a lack of unity among researchers, poor rewards for novel research, and limited data sharing, he said.
“The way the system now is set up, researchers are not incentivized to share their data,” Vice President Biden said, acknowledging that some medical experts are against the idea. “But every expert I’ve spoken to said you need to share these data to move this process rapidly.”
Involving patients earlier in clinical trials design is also a primary focus, he said. Patients should understand more about trials and be more open to signing up.
He noted the “incredible” research currently being conducted by various entities, such as AACR’s Project Genie, Orion Foundation, and The Parker Institute. Mr. Biden stressed however, that such efforts are too isolated.
“It raises [the] question: ‘Why is all this being done separately?’ ” Vice President Biden said. “Why is so much money being spent when if it’s aggregated, everyone acknowledges, the answers would come more quickly?”
Incentives for new research and the way in which funding is alloted must also be redesigned, he stressed. Today, it takes too long for researchers to get projects approved by the government and funding dispersed. He acknowledged the difficulty researchers face in obtaining grants and the fact that those who think “outside the box” are less likely to receive funding.
“It seems to me that we slow down our best minds by making them spend years in the lab before they can get their own grants and, when they do, they spend a third of their time writing a grant that takes months to be approved and awarded,” he said. “It’s like asking Derek Jeter to take several years off to sell bonds to build Yankee stadium.”
The Vice President did not purport to have all the answers, and asked those at the AARC meeting to provide feedback on his suggestions.
“The question I’d ask you to contemplate, because I’d like you to communicate with us, is, ‘Does it require realigning incentives; changing behaviors to take advantage of this inflection point? Does it require sharing more knowledge, treatment, and understanding? Or does that slow the process up?’ ”
He added,“I hope you all know it, but you’re one of the most valuable resources that our great country has, those of you sitting in this room. So ask your institutions, your colleagues, your mentors, your administrators: How can we move your ideas faster together in the interest of patients?”
The Vice President’s Moonshot initiative was announced during President Obama’s 2016 State of the Union Address. The effort includes a new Cancer Moonshot Task Force that will focus on federal investments, targeted incentives, private sector efforts from industry and philanthropy, patient engagement initiatives, and other mechanisms to support cancer research and enable progress in treatment and care, according to the White House. As part of the plan, the President’s fiscal 2017 budget proposes $755 million in mandatory funds for new cancer-related research activities at the National Institutes of Health and the Food and Drug Administration. The initiative also includes increased investments by the Department of Defense and the Department of Veterans Affairs in cancer research, including through funding centers of excellence focused on specific cancers and conducting longitudinal studies to determine risk factors and enhance treatment.
On Twitter @legal_med
FROM THE AACR ANNUAL MEETING
In newly diagnosed CLL, mutation tests are advised
Patients with newly diagnosed chronic lymphocytic leukemia should standardly undergo immunoglobulin heavy-chain variable region gene (IGHV) mutation status and interphase fluorescence in situ hybridization (FISH) tests, based on the results of a meta-analysis published in Blood.
“This change will help define the minimal standard initial prognostic evaluation for patients with CLL and help facilitate use of the powerful, recently developed, integrated prognostic indices, all of which are dependent on these 2 variables,” wrote Dr. Sameer A. Parikh of Mayo Clinic, Rochester, Minn., and associates.
IGHV and FISH have prognostic value independent of clinical stage in patients with newly diagnosed and previously untreated CLL, they said (Blood. 2016;127[14]:1752-60). Better understanding of the patient’s risk of disease progression at diagnosis can guide counseling and follow-up intervals, and could potentially influence the decision to treat high-risk patients on early intervention protocols.
IGHV and FISH also appear to provide additional information on progression-free and overall survival.
The researchers cautioned, however, that the results of these tests should not be used to initiate CLL-specific therapy. Only patients who meet indications for therapy based on the 2008 International Workshop on Chronic Lymphocytic Leukemia guidelines should receive treatment.
Further, they noted, the median age of patients included in studies that they analyzed was 64 years; the median age of patients with CLL is 72 years. The prognostic abilities of IGHV mutation and FISH may differ in these older individuals with CLL.
The researchers analyzed 31 studies that met the criteria for inclusion – full-length publications that included at least 200 patients and reported on the prognostic value of IGHV and/or FISH for predicting progression-free or overall survival in patients with newly diagnosed CLL.
They found that the median progression-free survival (range, about 1-5 years) was significantly shorter for patients with unmutated IGHV genes, than was the median progression-free survival (range, about 9-19 years) for those with mutated IGHV genes. Similarly, the median overall survival was significantly shorter for patients with unmutated IGHV (range, about 3-10 years) than for those with mutated IGHV (range, about 18-26 years).
For patients with high-risk FISH (including del17p13 and del11q23), the median progression-free survival was significantly shorter (range, about 0.1-5 years) than for those with low/intermediate-risk FISH (including del13q, normal, and trisomy 12; range, about 1.5-22 years). Median overall survival also significantly differed, ranging from about 3-10 years for patients with high-risk FISH and from about 7.5-20.5 years for those with low/intermediate-risk FISH.
In multivariable analyses, the hazard ratio for high-risk FISH ranged from 1.3 to 4.7 for progression-free survival and from 0.9 to 8.2 for overall survival. In studies reporting the results of multivariable analysis, high-risk FISH remained an independent predictor of progression-free survival in 8 of 17 studies and of overall survival in 10 of 14 studies, including in 10 of 13 studies adjusting for the prognostic impact of IGHV.
In multivariable analyses, IGHV remained an independent predictor of progression-free survival in 15 of 18 studies, including 12 of 15 studies adjusting for the prognostic impact of FISH. IGHV remained an independent predictor of overall survival in 11 of 15 studies reporting the results of multivariable analysis, including 10 of 14 studies adjusting for the prognostic impact of FISH.
Patients with newly diagnosed chronic lymphocytic leukemia should standardly undergo immunoglobulin heavy-chain variable region gene (IGHV) mutation status and interphase fluorescence in situ hybridization (FISH) tests, based on the results of a meta-analysis published in Blood.
“This change will help define the minimal standard initial prognostic evaluation for patients with CLL and help facilitate use of the powerful, recently developed, integrated prognostic indices, all of which are dependent on these 2 variables,” wrote Dr. Sameer A. Parikh of Mayo Clinic, Rochester, Minn., and associates.
IGHV and FISH have prognostic value independent of clinical stage in patients with newly diagnosed and previously untreated CLL, they said (Blood. 2016;127[14]:1752-60). Better understanding of the patient’s risk of disease progression at diagnosis can guide counseling and follow-up intervals, and could potentially influence the decision to treat high-risk patients on early intervention protocols.
IGHV and FISH also appear to provide additional information on progression-free and overall survival.
The researchers cautioned, however, that the results of these tests should not be used to initiate CLL-specific therapy. Only patients who meet indications for therapy based on the 2008 International Workshop on Chronic Lymphocytic Leukemia guidelines should receive treatment.
Further, they noted, the median age of patients included in studies that they analyzed was 64 years; the median age of patients with CLL is 72 years. The prognostic abilities of IGHV mutation and FISH may differ in these older individuals with CLL.
The researchers analyzed 31 studies that met the criteria for inclusion – full-length publications that included at least 200 patients and reported on the prognostic value of IGHV and/or FISH for predicting progression-free or overall survival in patients with newly diagnosed CLL.
They found that the median progression-free survival (range, about 1-5 years) was significantly shorter for patients with unmutated IGHV genes, than was the median progression-free survival (range, about 9-19 years) for those with mutated IGHV genes. Similarly, the median overall survival was significantly shorter for patients with unmutated IGHV (range, about 3-10 years) than for those with mutated IGHV (range, about 18-26 years).
For patients with high-risk FISH (including del17p13 and del11q23), the median progression-free survival was significantly shorter (range, about 0.1-5 years) than for those with low/intermediate-risk FISH (including del13q, normal, and trisomy 12; range, about 1.5-22 years). Median overall survival also significantly differed, ranging from about 3-10 years for patients with high-risk FISH and from about 7.5-20.5 years for those with low/intermediate-risk FISH.
In multivariable analyses, the hazard ratio for high-risk FISH ranged from 1.3 to 4.7 for progression-free survival and from 0.9 to 8.2 for overall survival. In studies reporting the results of multivariable analysis, high-risk FISH remained an independent predictor of progression-free survival in 8 of 17 studies and of overall survival in 10 of 14 studies, including in 10 of 13 studies adjusting for the prognostic impact of IGHV.
In multivariable analyses, IGHV remained an independent predictor of progression-free survival in 15 of 18 studies, including 12 of 15 studies adjusting for the prognostic impact of FISH. IGHV remained an independent predictor of overall survival in 11 of 15 studies reporting the results of multivariable analysis, including 10 of 14 studies adjusting for the prognostic impact of FISH.
Patients with newly diagnosed chronic lymphocytic leukemia should standardly undergo immunoglobulin heavy-chain variable region gene (IGHV) mutation status and interphase fluorescence in situ hybridization (FISH) tests, based on the results of a meta-analysis published in Blood.
“This change will help define the minimal standard initial prognostic evaluation for patients with CLL and help facilitate use of the powerful, recently developed, integrated prognostic indices, all of which are dependent on these 2 variables,” wrote Dr. Sameer A. Parikh of Mayo Clinic, Rochester, Minn., and associates.
IGHV and FISH have prognostic value independent of clinical stage in patients with newly diagnosed and previously untreated CLL, they said (Blood. 2016;127[14]:1752-60). Better understanding of the patient’s risk of disease progression at diagnosis can guide counseling and follow-up intervals, and could potentially influence the decision to treat high-risk patients on early intervention protocols.
IGHV and FISH also appear to provide additional information on progression-free and overall survival.
The researchers cautioned, however, that the results of these tests should not be used to initiate CLL-specific therapy. Only patients who meet indications for therapy based on the 2008 International Workshop on Chronic Lymphocytic Leukemia guidelines should receive treatment.
Further, they noted, the median age of patients included in studies that they analyzed was 64 years; the median age of patients with CLL is 72 years. The prognostic abilities of IGHV mutation and FISH may differ in these older individuals with CLL.
The researchers analyzed 31 studies that met the criteria for inclusion – full-length publications that included at least 200 patients and reported on the prognostic value of IGHV and/or FISH for predicting progression-free or overall survival in patients with newly diagnosed CLL.
They found that the median progression-free survival (range, about 1-5 years) was significantly shorter for patients with unmutated IGHV genes, than was the median progression-free survival (range, about 9-19 years) for those with mutated IGHV genes. Similarly, the median overall survival was significantly shorter for patients with unmutated IGHV (range, about 3-10 years) than for those with mutated IGHV (range, about 18-26 years).
For patients with high-risk FISH (including del17p13 and del11q23), the median progression-free survival was significantly shorter (range, about 0.1-5 years) than for those with low/intermediate-risk FISH (including del13q, normal, and trisomy 12; range, about 1.5-22 years). Median overall survival also significantly differed, ranging from about 3-10 years for patients with high-risk FISH and from about 7.5-20.5 years for those with low/intermediate-risk FISH.
In multivariable analyses, the hazard ratio for high-risk FISH ranged from 1.3 to 4.7 for progression-free survival and from 0.9 to 8.2 for overall survival. In studies reporting the results of multivariable analysis, high-risk FISH remained an independent predictor of progression-free survival in 8 of 17 studies and of overall survival in 10 of 14 studies, including in 10 of 13 studies adjusting for the prognostic impact of IGHV.
In multivariable analyses, IGHV remained an independent predictor of progression-free survival in 15 of 18 studies, including 12 of 15 studies adjusting for the prognostic impact of FISH. IGHV remained an independent predictor of overall survival in 11 of 15 studies reporting the results of multivariable analysis, including 10 of 14 studies adjusting for the prognostic impact of FISH.
FROM BLOOD
FDG-PET guides need for eBEACOPP in advanced Hodgkin’s
Using FDG-PET (fluorodeoxyglucose positron emission tomography) imaging to gauge treatment response after the first two rounds of ABVD therapy helps to determine which patients with advanced Hodgkin’s lymphoma should be switched to a more aggressive eBEACOPP regimen, according to the results of the Southwest Oncology Group (SWOG) S0816 study.
In this large U.S. trial of PET scanning to guide treatment approach in people with high-risk stage II or stage III-IV Hodgkin’s lymphoma, progression-free survival at 2 years for those with early interim positive PET scans was 64%, which is much higher than the expected progression-free survival of 15%-30%, according to Dr. Oliver Press, a SWOG member at Fred Hutchinson Cancer Research Center and the lead author of study, which was published ahead of print in the Journal of Clinical Oncology (2016 April 11. doi: 10.1200/JCO.2015.63.1119).
In addition, just 20% of patients in the trial were exposed to eBEACOPP, which usually results in infertility, can cause sustained heart or lung damage, and increases the risk of secondary cancers.
Researchers recruited 358 Hodgkin’s patients to the trial and were able to evaluate 331 of them. All trial volunteers were given two rounds of standard ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) chemotherapy, followed by a PET scan. If the scan was negative, patients received four more cycles of ABVD. If the scan was positive, with a Deauville score of 4-5, they were advised to switch to eBEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone), a seven-drug combination used in Europe. Of 60 patients with positive interim PET scans, 11 patients declined to switch, and 49 switched as planned to six cycles of eBEACOPP.
With a median follow-up of nearly 40 months, the Kaplan-Meier estimate for 2-year overall survival was 98%, and the 2-year estimate for progression-free survival was 79%. In the subset of patients who had positive PET scans after two cycles of ABVD, the 2-year estimate for progression-free survival was 64%, more than double the expected remission rate.
At least seven phase II and III cooperative group studies are underway testing this approach in advanced-stage Hodgkin’s lymphoma, the researchers wrote. “We hope that in the future, molecular biomarker studies at initial diagnosis, or the combination of biomarkers and molecular imaging, may define patients who require more intense therapy with eBEACOPP or other novel targeted drugs with greater accuracy than is achievable with current technology.”
The study was funded by the National Cancer Institute, the David and Patricia Giuliani Family Foundation, the Lymphoma Foundation, the Adam Spector Fund for Hodgkin Research, and the Ernest & Jeanette Dicker Charitable Foundation.
On Twitter @maryjodales
Using FDG-PET (fluorodeoxyglucose positron emission tomography) imaging to gauge treatment response after the first two rounds of ABVD therapy helps to determine which patients with advanced Hodgkin’s lymphoma should be switched to a more aggressive eBEACOPP regimen, according to the results of the Southwest Oncology Group (SWOG) S0816 study.
In this large U.S. trial of PET scanning to guide treatment approach in people with high-risk stage II or stage III-IV Hodgkin’s lymphoma, progression-free survival at 2 years for those with early interim positive PET scans was 64%, which is much higher than the expected progression-free survival of 15%-30%, according to Dr. Oliver Press, a SWOG member at Fred Hutchinson Cancer Research Center and the lead author of study, which was published ahead of print in the Journal of Clinical Oncology (2016 April 11. doi: 10.1200/JCO.2015.63.1119).
In addition, just 20% of patients in the trial were exposed to eBEACOPP, which usually results in infertility, can cause sustained heart or lung damage, and increases the risk of secondary cancers.
Researchers recruited 358 Hodgkin’s patients to the trial and were able to evaluate 331 of them. All trial volunteers were given two rounds of standard ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) chemotherapy, followed by a PET scan. If the scan was negative, patients received four more cycles of ABVD. If the scan was positive, with a Deauville score of 4-5, they were advised to switch to eBEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone), a seven-drug combination used in Europe. Of 60 patients with positive interim PET scans, 11 patients declined to switch, and 49 switched as planned to six cycles of eBEACOPP.
With a median follow-up of nearly 40 months, the Kaplan-Meier estimate for 2-year overall survival was 98%, and the 2-year estimate for progression-free survival was 79%. In the subset of patients who had positive PET scans after two cycles of ABVD, the 2-year estimate for progression-free survival was 64%, more than double the expected remission rate.
At least seven phase II and III cooperative group studies are underway testing this approach in advanced-stage Hodgkin’s lymphoma, the researchers wrote. “We hope that in the future, molecular biomarker studies at initial diagnosis, or the combination of biomarkers and molecular imaging, may define patients who require more intense therapy with eBEACOPP or other novel targeted drugs with greater accuracy than is achievable with current technology.”
The study was funded by the National Cancer Institute, the David and Patricia Giuliani Family Foundation, the Lymphoma Foundation, the Adam Spector Fund for Hodgkin Research, and the Ernest & Jeanette Dicker Charitable Foundation.
On Twitter @maryjodales
Using FDG-PET (fluorodeoxyglucose positron emission tomography) imaging to gauge treatment response after the first two rounds of ABVD therapy helps to determine which patients with advanced Hodgkin’s lymphoma should be switched to a more aggressive eBEACOPP regimen, according to the results of the Southwest Oncology Group (SWOG) S0816 study.
In this large U.S. trial of PET scanning to guide treatment approach in people with high-risk stage II or stage III-IV Hodgkin’s lymphoma, progression-free survival at 2 years for those with early interim positive PET scans was 64%, which is much higher than the expected progression-free survival of 15%-30%, according to Dr. Oliver Press, a SWOG member at Fred Hutchinson Cancer Research Center and the lead author of study, which was published ahead of print in the Journal of Clinical Oncology (2016 April 11. doi: 10.1200/JCO.2015.63.1119).
In addition, just 20% of patients in the trial were exposed to eBEACOPP, which usually results in infertility, can cause sustained heart or lung damage, and increases the risk of secondary cancers.
Researchers recruited 358 Hodgkin’s patients to the trial and were able to evaluate 331 of them. All trial volunteers were given two rounds of standard ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) chemotherapy, followed by a PET scan. If the scan was negative, patients received four more cycles of ABVD. If the scan was positive, with a Deauville score of 4-5, they were advised to switch to eBEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone), a seven-drug combination used in Europe. Of 60 patients with positive interim PET scans, 11 patients declined to switch, and 49 switched as planned to six cycles of eBEACOPP.
With a median follow-up of nearly 40 months, the Kaplan-Meier estimate for 2-year overall survival was 98%, and the 2-year estimate for progression-free survival was 79%. In the subset of patients who had positive PET scans after two cycles of ABVD, the 2-year estimate for progression-free survival was 64%, more than double the expected remission rate.
At least seven phase II and III cooperative group studies are underway testing this approach in advanced-stage Hodgkin’s lymphoma, the researchers wrote. “We hope that in the future, molecular biomarker studies at initial diagnosis, or the combination of biomarkers and molecular imaging, may define patients who require more intense therapy with eBEACOPP or other novel targeted drugs with greater accuracy than is achievable with current technology.”
The study was funded by the National Cancer Institute, the David and Patricia Giuliani Family Foundation, the Lymphoma Foundation, the Adam Spector Fund for Hodgkin Research, and the Ernest & Jeanette Dicker Charitable Foundation.
On Twitter @maryjodales
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Using FDG-PET imaging to gauge treatment response after the first two rounds of ABVD therapy helps to determine which patients with advanced Hodgkin’s lymphoma should be switched to the eBEACOPP regimen.
Major finding: Progression-free survival at 2 years for those with early interim positive PET scans was 64%; the historical progression-free survival for this group is 15%-30%.
Data source: Evaluations of 331 patients in the Southwest Oncology Group S0816 study.
Disclosures: The study was funded by the National Cancer Institute, the David and Patricia Giuliani Family Foundation, the Lymphoma Foundation, the Adam Spector Fund for Hodgkin Research, and the Ernest & Jeanette Dicker Charitable Foundation.
CUDC-907 enters phase II for relapsed or refractory lymphoma and multiple myeloma
Another oral, small-molecule therapy called CUDC-907 is emerging from phase I testing as a treatment option for patients with relapsed or refractory lymphoma and multiple myeloma.
The CUDC-907 dose to be used in phase II studies will be 60 mg on a 5-days-on/2-days-off dosing schedule, according to Dr. Anas Younes of Memorial Sloan Kettering Cancer Center, New York, and his colleagues. A dose-expansion trial of this dose is ongoing, and the drug appears to be useful in particular for patients with refractory and relapsed diffuse large B-cell lymphoma.
The researchers tested CUDC-907, which is designed to inhibit histone deacetylase and PI3K enzyme pathways, for overall safety and response in 44 patients at four cancer centers. All participants had lymphoma or multiple myeloma and were refractory to treatment or had relapsed after two or more previous regimens.
The 44 participants were sequentially assigned to 21-day cycles of CUDC-907: 10 to once daily, 12 to twice weekly, 15 to three times weekly, and 7 to daily for 5 days followed by a 2-day break. The maximum tolerated doses were 60 mg for the once-daily schedule, 150 mg for the twice-weekly schedule, 150 mg for the three-times-weekly schedule, and 60 mg for the 5-on/2-off schedule. At data cutoff, 37 of the 44 patients had discontinued CUDC-907 because of disease progression, Dr. Younes and his associates reported in a study published online (Lancet Oncol. 2016 Mar 31. doi: 10.1016/S1470-2045(15)00584-7).
Four dose-limiting toxicities occurred in 3 of 40 evaluable patients. Grade 3 or worse adverse events occurred in 19 of 44 patients: 9 had thrombocytopenia, 3 had neutropenia, 3 had hyperglycemia. Adverse events led to dose reductions in six patients and treatment discontinuation in seven.
Of 37 response-evaluable patients, two had complete responses and three had partial responses. All five were seen in the subgroup of nine patients with diffuse large B-cell lymphoma, and three occurred in the five patients with transformed follicular disease. The 21 patients with stable disease included those with diffuse large B-cell lymphoma, Hodgkin’s lymphoma, and multiple myeloma. This ongoing trial is registered at ClinicalTrials.gov as NCT01742988.
The study was sponsored by Curis, the maker of CUDC-907, and the Leukemia and Lymphoma Society. Five of the 15 investigators are employees of Curis.
On Twitter @maryjodales
Another oral, small-molecule therapy called CUDC-907 is emerging from phase I testing as a treatment option for patients with relapsed or refractory lymphoma and multiple myeloma.
The CUDC-907 dose to be used in phase II studies will be 60 mg on a 5-days-on/2-days-off dosing schedule, according to Dr. Anas Younes of Memorial Sloan Kettering Cancer Center, New York, and his colleagues. A dose-expansion trial of this dose is ongoing, and the drug appears to be useful in particular for patients with refractory and relapsed diffuse large B-cell lymphoma.
The researchers tested CUDC-907, which is designed to inhibit histone deacetylase and PI3K enzyme pathways, for overall safety and response in 44 patients at four cancer centers. All participants had lymphoma or multiple myeloma and were refractory to treatment or had relapsed after two or more previous regimens.
The 44 participants were sequentially assigned to 21-day cycles of CUDC-907: 10 to once daily, 12 to twice weekly, 15 to three times weekly, and 7 to daily for 5 days followed by a 2-day break. The maximum tolerated doses were 60 mg for the once-daily schedule, 150 mg for the twice-weekly schedule, 150 mg for the three-times-weekly schedule, and 60 mg for the 5-on/2-off schedule. At data cutoff, 37 of the 44 patients had discontinued CUDC-907 because of disease progression, Dr. Younes and his associates reported in a study published online (Lancet Oncol. 2016 Mar 31. doi: 10.1016/S1470-2045(15)00584-7).
Four dose-limiting toxicities occurred in 3 of 40 evaluable patients. Grade 3 or worse adverse events occurred in 19 of 44 patients: 9 had thrombocytopenia, 3 had neutropenia, 3 had hyperglycemia. Adverse events led to dose reductions in six patients and treatment discontinuation in seven.
Of 37 response-evaluable patients, two had complete responses and three had partial responses. All five were seen in the subgroup of nine patients with diffuse large B-cell lymphoma, and three occurred in the five patients with transformed follicular disease. The 21 patients with stable disease included those with diffuse large B-cell lymphoma, Hodgkin’s lymphoma, and multiple myeloma. This ongoing trial is registered at ClinicalTrials.gov as NCT01742988.
The study was sponsored by Curis, the maker of CUDC-907, and the Leukemia and Lymphoma Society. Five of the 15 investigators are employees of Curis.
On Twitter @maryjodales
Another oral, small-molecule therapy called CUDC-907 is emerging from phase I testing as a treatment option for patients with relapsed or refractory lymphoma and multiple myeloma.
The CUDC-907 dose to be used in phase II studies will be 60 mg on a 5-days-on/2-days-off dosing schedule, according to Dr. Anas Younes of Memorial Sloan Kettering Cancer Center, New York, and his colleagues. A dose-expansion trial of this dose is ongoing, and the drug appears to be useful in particular for patients with refractory and relapsed diffuse large B-cell lymphoma.
The researchers tested CUDC-907, which is designed to inhibit histone deacetylase and PI3K enzyme pathways, for overall safety and response in 44 patients at four cancer centers. All participants had lymphoma or multiple myeloma and were refractory to treatment or had relapsed after two or more previous regimens.
The 44 participants were sequentially assigned to 21-day cycles of CUDC-907: 10 to once daily, 12 to twice weekly, 15 to three times weekly, and 7 to daily for 5 days followed by a 2-day break. The maximum tolerated doses were 60 mg for the once-daily schedule, 150 mg for the twice-weekly schedule, 150 mg for the three-times-weekly schedule, and 60 mg for the 5-on/2-off schedule. At data cutoff, 37 of the 44 patients had discontinued CUDC-907 because of disease progression, Dr. Younes and his associates reported in a study published online (Lancet Oncol. 2016 Mar 31. doi: 10.1016/S1470-2045(15)00584-7).
Four dose-limiting toxicities occurred in 3 of 40 evaluable patients. Grade 3 or worse adverse events occurred in 19 of 44 patients: 9 had thrombocytopenia, 3 had neutropenia, 3 had hyperglycemia. Adverse events led to dose reductions in six patients and treatment discontinuation in seven.
Of 37 response-evaluable patients, two had complete responses and three had partial responses. All five were seen in the subgroup of nine patients with diffuse large B-cell lymphoma, and three occurred in the five patients with transformed follicular disease. The 21 patients with stable disease included those with diffuse large B-cell lymphoma, Hodgkin’s lymphoma, and multiple myeloma. This ongoing trial is registered at ClinicalTrials.gov as NCT01742988.
The study was sponsored by Curis, the maker of CUDC-907, and the Leukemia and Lymphoma Society. Five of the 15 investigators are employees of Curis.
On Twitter @maryjodales
FROM THE LANCET ONCOLOGY
Key clinical point: The CUDC-907 dose to be used in phase II studies will be 60 mg on a 5-days-on/2-days-off dosing schedule.
Major finding: Two complete responses and three partial responses were seen in the subgroup of nine patients with diffuse large B-cell lymphoma; three occurred in the five patients with transformed follicular disease.
Data source: A dose-escalation study involving 44 patients at four cancer centers.
Disclosures: The study was sponsored by Curis, the maker of CUDC-907, and the Leukemia and Lymphoma Society. Five of the 15 investigators are employees of Curis.
FDA approves venetoclax for CLL with 17p deletion
Venetoclax has been approved for the treatment of patients with chronic lymphocytic leukemia (CLL) who have a 17p deletion and who have been treated with a least one prior therapy, the Food and Drug Administration has announced.
The drug will be marketed as Venclexta, and is indicated for daily use after detection of a 17p deletion is confirmed through the use of the FDA-approved companion diagnostic test, the Vysis CLL FISH probe kit. A 17p deletion occurs in about 10% of patients with untreated CLL and in about 20% of patients with relapsed CLL. Venetoclax targets the B-cell lymphoma 2 (BCL-2) protein, according to the FDA press release.
“Up to half of people whose CLL progressed have 17p deletion,” Dr. Sandra Horning, chief medical officer and head of Global Product Development for Genentech, said in a press release issued by the company. Venclexta will be marketed by AbbVie and Genentech USA. The Vysis CLL FISH probe kit is manufactured by Abbott Molecular.
“For certain patients with CLL who have not had favorable outcomes with other therapies, Venclexta may provide a new option,” Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a press release issued by the FDA.
The approval was based on a clinical trial of 106 patients who had CLL and 17p deletions and who had received at least one prior therapy. Trial participants took oral venetoclax daily, beginning with a 20 mg dose that was increased over a 5-week period to 400 mg. A complete or partial remission of CLL occurred in 80% of trial participants. Data on venetoclax also was presented at the annual meeting of the American Society of Hematology.
The most common side effects of venetoclax include neutropenia, diarrhea, nausea, anemia, upper respiratory tract infection, thrombocytopenia, and fatigue.
The FDA granted the Venclexta application breakthrough therapy designation, priority review status, and accelerated approval for this indication. Venclexta also received orphan drug designation.
On Twitter @maryjodales
Venetoclax has been approved for the treatment of patients with chronic lymphocytic leukemia (CLL) who have a 17p deletion and who have been treated with a least one prior therapy, the Food and Drug Administration has announced.
The drug will be marketed as Venclexta, and is indicated for daily use after detection of a 17p deletion is confirmed through the use of the FDA-approved companion diagnostic test, the Vysis CLL FISH probe kit. A 17p deletion occurs in about 10% of patients with untreated CLL and in about 20% of patients with relapsed CLL. Venetoclax targets the B-cell lymphoma 2 (BCL-2) protein, according to the FDA press release.
“Up to half of people whose CLL progressed have 17p deletion,” Dr. Sandra Horning, chief medical officer and head of Global Product Development for Genentech, said in a press release issued by the company. Venclexta will be marketed by AbbVie and Genentech USA. The Vysis CLL FISH probe kit is manufactured by Abbott Molecular.
“For certain patients with CLL who have not had favorable outcomes with other therapies, Venclexta may provide a new option,” Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a press release issued by the FDA.
The approval was based on a clinical trial of 106 patients who had CLL and 17p deletions and who had received at least one prior therapy. Trial participants took oral venetoclax daily, beginning with a 20 mg dose that was increased over a 5-week period to 400 mg. A complete or partial remission of CLL occurred in 80% of trial participants. Data on venetoclax also was presented at the annual meeting of the American Society of Hematology.
The most common side effects of venetoclax include neutropenia, diarrhea, nausea, anemia, upper respiratory tract infection, thrombocytopenia, and fatigue.
The FDA granted the Venclexta application breakthrough therapy designation, priority review status, and accelerated approval for this indication. Venclexta also received orphan drug designation.
On Twitter @maryjodales
Venetoclax has been approved for the treatment of patients with chronic lymphocytic leukemia (CLL) who have a 17p deletion and who have been treated with a least one prior therapy, the Food and Drug Administration has announced.
The drug will be marketed as Venclexta, and is indicated for daily use after detection of a 17p deletion is confirmed through the use of the FDA-approved companion diagnostic test, the Vysis CLL FISH probe kit. A 17p deletion occurs in about 10% of patients with untreated CLL and in about 20% of patients with relapsed CLL. Venetoclax targets the B-cell lymphoma 2 (BCL-2) protein, according to the FDA press release.
“Up to half of people whose CLL progressed have 17p deletion,” Dr. Sandra Horning, chief medical officer and head of Global Product Development for Genentech, said in a press release issued by the company. Venclexta will be marketed by AbbVie and Genentech USA. The Vysis CLL FISH probe kit is manufactured by Abbott Molecular.
“For certain patients with CLL who have not had favorable outcomes with other therapies, Venclexta may provide a new option,” Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in a press release issued by the FDA.
The approval was based on a clinical trial of 106 patients who had CLL and 17p deletions and who had received at least one prior therapy. Trial participants took oral venetoclax daily, beginning with a 20 mg dose that was increased over a 5-week period to 400 mg. A complete or partial remission of CLL occurred in 80% of trial participants. Data on venetoclax also was presented at the annual meeting of the American Society of Hematology.
The most common side effects of venetoclax include neutropenia, diarrhea, nausea, anemia, upper respiratory tract infection, thrombocytopenia, and fatigue.
The FDA granted the Venclexta application breakthrough therapy designation, priority review status, and accelerated approval for this indication. Venclexta also received orphan drug designation.
On Twitter @maryjodales