User login
Path of Least Resistance: Guidance for Antibiotic Stewardship in Acne
Path of Least Resistance: Guidance for Antibiotic Stewardship in Acne
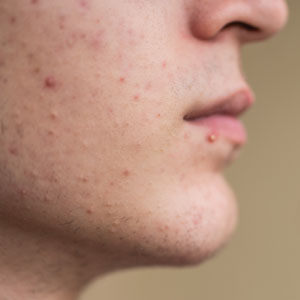
Dermatologists have long relied on oral antibiotics to manage moderate to severe acne1-4; however, it is critical to reassess how these medications are used in clinical practice as concerns about antibiotic resistance grow.5 The question is not whether antibiotics are effective for acne treatment—we know they are—but how to optimize their use to balance clinical benefit with responsible prescribing. Resistance in Cutibacterium acnes has been well documented in laboratory settings, but clinical treatment failure due to resistance remains rare and difficult to quantify.6,7 Still, minimizing unnecessary exposure is good clinical practice. Whether antibiotic resistance ultimately proves to drive clinical failure or remains largely theoretical, stewardship safeguards future treatment options.
In this article, we present a practical, expert-based framework aligned with American Academy of Dermatology (AAD) guidelines to support responsible antibiotic use in acne management. Seven prescribing principles are outlined to help clinicians maintain efficacy while minimizing resistance risk. Mechanisms of resistance in C acnes and broader microbiome impacts also are discussed.
MECHANISMS OF RESISTANCE IN ACNE THERAPY
Antibiotic resistance in acne primarily involves C acnes and arises through selective pressure from prolonged or subtherapeutic antibiotic exposure. Resistance mechanisms include point mutations in ribosomal binding sites, leading to decreased binding affinity for tetracyclines and macrolides as well as efflux pump activation and biofilm formation.8,9 Over time, resistant strains may proliferate and outcompete susceptible populations, potentially contributing to reduced clinical efficacy. Importantly, the use of broad-spectrum antibiotics may disrupt the skin and gut microbiota, promoting resistance among nontarget organisms.5 These concerns underscore the importance of limiting antibiotic use to appropriate indications, combining antibiotics with adjunctive nonantibiotic therapies, and avoiding monotherapy.
PRESCRIBING PRINCIPLES FOR RESPONSIBLE ORAL ANTIBIOTIC USE IN ACNE
The following principles are derived from our clinical experience and are aligned with AAD guidelines on acne treatment.10 This practical framework supports safe, effective, and streamlined prescribing.
Reserve Oral Antibiotics for Appropriate Cases
Oral antibiotics should be considered for patients with moderate to severe inflammatory acne when rapid anti-inflammatory control is needed. They are not indicated for comedonal or mild papulopustular acne. Before initiating treatment, clinicians should weigh the potential benefits against the risks associated with antibiotic exposure, including resistance and microbiome disruption.
Combine Oral Antibiotics With Topical Retinoids
Oral antibiotics should not be used as monotherapy. Topical retinoids should be initiated concurrently with oral antibiotics to maximize anti-inflammatory benefit, support transition to maintenance therapy, and reduce risk for resistance.
Consider Adding an Adjunctive Topical Antimicrobial Agent
Adjunctive topical antimicrobials can help reduce bacterial load. Benzoyl peroxide remains a first-line option due to its bactericidal activity and lack of resistance induction; however, recent product recalls involving benzene contamination may have raised safety concerns among some clinicians and patients.11,12 While no definitive harm has been established, alternative topical agents approved by the US Food and Drug Administration (eg, azelaic acid) may be used based on shared decision-making, tolerability, cost, access, and patient preference. Use of topical antibiotics (eg, clindamycin, erythromycin) as monotherapy is discouraged due to their higher resistance potential, which is consistent with AAD guidance.
Limit Treatment Duration to 12 Weeks or Less
Antibiotic use should be time limited, with discontinuation ideally within 8 to 12 weeks as clinical improvement is demonstrated. Repeated or prolonged courses should be avoided to minimize risk for resistance.
Simplify Treatment Regimens to Enhance Adherence
Regimen simplicity improves adherence, especially in adolescents. A two-agent regimen of an oral antibiotic and a topical retinoid typically is sufficient during the induction phase.13,14
Select Narrower-Spectrum Antibiotics When Feasible
Using a narrower-spectrum antibiotic may help minimize disruption to nontarget microbiota.15,16 Sarecycline has shown narrower in vitro activity within the tetracycline class,17,18 though clinical decisions should be informed by access, availability, and cost. Regardless of the agent used (eg, doxycycline, minocycline, or sarecycline), all antibiotics should be used judiciously and for the shortest effective duration.
Use Systemic Nonantibiotic Therapies When Appropriate
If there is inadequate response to oral antibiotic therapy, consider switching to systemic nonantibiotic options. Hormonal therapy may be appropriate for select female patients. Oral isotretinoin should be considered for patients with severe, recalcitrant, or scarring acne. Cycling between antibiotic classes without clear benefit is discouraged.
FINAL THOUGHTS
Oral antibiotics remain a foundational component in the management of moderate to severe acne; however, their use must be intentional, time limited, and guided by best practices to minimize the emergence of antimicrobial resistance. By adhering to the prescribing principles we have outlined here, which are rooted in clinical expertise and consistent with AAD guidelines, dermatologists can preserve antibiotic efficacy, optimize patient outcomes, and reduce long-term microbiologic risks. Stewardship is not about withholding treatment; it is about optimizing care today to protect treatment options for tomorrow.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
- Grada A, Armstrong A, Bunick C, et al. Trends in oral antibiotic use for acne treatment: a retrospective, population-based study in the United States, 2014 to 2016. J Drugs Dermatol. 2023;22:265-270.
- Perche PO, Peck GM, Robinson L, et al. Prescribing trends for acne vulgaris visits in the United States. Antibiotics. 2023;12:269.
- Karadag A, Aslan Kayıran M, Wu CY, et al. Antibiotic resistance in acne: changes, consequences and concerns. J Eur Acad Dermatol Venereol. 2021;35:73-78.
- Eady AE, Cove JH, Layton AM. Is antibiotic resistance in cutaneous propionibacteria clinically relevant? implications of resistance for acne patients and prescribers. Am J Clin Dermatol. 2003;4:813-831.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J Dermatol. 1989;121:51-57.
- Grossman TH. Tetracycline antibiotics and resistance. Cold Spring Harb Perspect Med. 2016;6:a025387.
- Kayiran M AS, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006-1035.
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:037702.
- Kucera K, Zenzola N, Hudspeth A, et al. Evaluation of benzene presence and formation in benzoyl peroxide drug products. J Invest Dermatol. 2025;145:1147-1154.E11.
- Grada A, Perche P, Feldman S. Adherence and persistence to acne medications: a population-based claims database analysis. J Drugs Dermatol. 2022;21:758-764.<.li>
- Anderson KL, Dothard EH, Huang KE, et al. Frequency of primary nonadherence to acne treatment. JAMA Dermatol. 2015;151:623-626.
- Grada A, Bunick CG. Spectrum of antibiotic activity and its relevance to the microbiome. JAMA Netw Open. 2021;4:E215357-E215357.
- Francino M. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:164577.
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911.
- Zhanel G, Critchley I, Lin L-Y, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2019;63:1297-1318.
Dermatologists have long relied on oral antibiotics to manage moderate to severe acne1-4; however, it is critical to reassess how these medications are used in clinical practice as concerns about antibiotic resistance grow.5 The question is not whether antibiotics are effective for acne treatment—we know they are—but how to optimize their use to balance clinical benefit with responsible prescribing. Resistance in Cutibacterium acnes has been well documented in laboratory settings, but clinical treatment failure due to resistance remains rare and difficult to quantify.6,7 Still, minimizing unnecessary exposure is good clinical practice. Whether antibiotic resistance ultimately proves to drive clinical failure or remains largely theoretical, stewardship safeguards future treatment options.
In this article, we present a practical, expert-based framework aligned with American Academy of Dermatology (AAD) guidelines to support responsible antibiotic use in acne management. Seven prescribing principles are outlined to help clinicians maintain efficacy while minimizing resistance risk. Mechanisms of resistance in C acnes and broader microbiome impacts also are discussed.
MECHANISMS OF RESISTANCE IN ACNE THERAPY
Antibiotic resistance in acne primarily involves C acnes and arises through selective pressure from prolonged or subtherapeutic antibiotic exposure. Resistance mechanisms include point mutations in ribosomal binding sites, leading to decreased binding affinity for tetracyclines and macrolides as well as efflux pump activation and biofilm formation.8,9 Over time, resistant strains may proliferate and outcompete susceptible populations, potentially contributing to reduced clinical efficacy. Importantly, the use of broad-spectrum antibiotics may disrupt the skin and gut microbiota, promoting resistance among nontarget organisms.5 These concerns underscore the importance of limiting antibiotic use to appropriate indications, combining antibiotics with adjunctive nonantibiotic therapies, and avoiding monotherapy.
PRESCRIBING PRINCIPLES FOR RESPONSIBLE ORAL ANTIBIOTIC USE IN ACNE
The following principles are derived from our clinical experience and are aligned with AAD guidelines on acne treatment.10 This practical framework supports safe, effective, and streamlined prescribing.
Reserve Oral Antibiotics for Appropriate Cases
Oral antibiotics should be considered for patients with moderate to severe inflammatory acne when rapid anti-inflammatory control is needed. They are not indicated for comedonal or mild papulopustular acne. Before initiating treatment, clinicians should weigh the potential benefits against the risks associated with antibiotic exposure, including resistance and microbiome disruption.
Combine Oral Antibiotics With Topical Retinoids
Oral antibiotics should not be used as monotherapy. Topical retinoids should be initiated concurrently with oral antibiotics to maximize anti-inflammatory benefit, support transition to maintenance therapy, and reduce risk for resistance.
Consider Adding an Adjunctive Topical Antimicrobial Agent
Adjunctive topical antimicrobials can help reduce bacterial load. Benzoyl peroxide remains a first-line option due to its bactericidal activity and lack of resistance induction; however, recent product recalls involving benzene contamination may have raised safety concerns among some clinicians and patients.11,12 While no definitive harm has been established, alternative topical agents approved by the US Food and Drug Administration (eg, azelaic acid) may be used based on shared decision-making, tolerability, cost, access, and patient preference. Use of topical antibiotics (eg, clindamycin, erythromycin) as monotherapy is discouraged due to their higher resistance potential, which is consistent with AAD guidance.
Limit Treatment Duration to 12 Weeks or Less
Antibiotic use should be time limited, with discontinuation ideally within 8 to 12 weeks as clinical improvement is demonstrated. Repeated or prolonged courses should be avoided to minimize risk for resistance.
Simplify Treatment Regimens to Enhance Adherence
Regimen simplicity improves adherence, especially in adolescents. A two-agent regimen of an oral antibiotic and a topical retinoid typically is sufficient during the induction phase.13,14
Select Narrower-Spectrum Antibiotics When Feasible
Using a narrower-spectrum antibiotic may help minimize disruption to nontarget microbiota.15,16 Sarecycline has shown narrower in vitro activity within the tetracycline class,17,18 though clinical decisions should be informed by access, availability, and cost. Regardless of the agent used (eg, doxycycline, minocycline, or sarecycline), all antibiotics should be used judiciously and for the shortest effective duration.
Use Systemic Nonantibiotic Therapies When Appropriate
If there is inadequate response to oral antibiotic therapy, consider switching to systemic nonantibiotic options. Hormonal therapy may be appropriate for select female patients. Oral isotretinoin should be considered for patients with severe, recalcitrant, or scarring acne. Cycling between antibiotic classes without clear benefit is discouraged.
FINAL THOUGHTS
Oral antibiotics remain a foundational component in the management of moderate to severe acne; however, their use must be intentional, time limited, and guided by best practices to minimize the emergence of antimicrobial resistance. By adhering to the prescribing principles we have outlined here, which are rooted in clinical expertise and consistent with AAD guidelines, dermatologists can preserve antibiotic efficacy, optimize patient outcomes, and reduce long-term microbiologic risks. Stewardship is not about withholding treatment; it is about optimizing care today to protect treatment options for tomorrow.
Dermatologists have long relied on oral antibiotics to manage moderate to severe acne1-4; however, it is critical to reassess how these medications are used in clinical practice as concerns about antibiotic resistance grow.5 The question is not whether antibiotics are effective for acne treatment—we know they are—but how to optimize their use to balance clinical benefit with responsible prescribing. Resistance in Cutibacterium acnes has been well documented in laboratory settings, but clinical treatment failure due to resistance remains rare and difficult to quantify.6,7 Still, minimizing unnecessary exposure is good clinical practice. Whether antibiotic resistance ultimately proves to drive clinical failure or remains largely theoretical, stewardship safeguards future treatment options.
In this article, we present a practical, expert-based framework aligned with American Academy of Dermatology (AAD) guidelines to support responsible antibiotic use in acne management. Seven prescribing principles are outlined to help clinicians maintain efficacy while minimizing resistance risk. Mechanisms of resistance in C acnes and broader microbiome impacts also are discussed.
MECHANISMS OF RESISTANCE IN ACNE THERAPY
Antibiotic resistance in acne primarily involves C acnes and arises through selective pressure from prolonged or subtherapeutic antibiotic exposure. Resistance mechanisms include point mutations in ribosomal binding sites, leading to decreased binding affinity for tetracyclines and macrolides as well as efflux pump activation and biofilm formation.8,9 Over time, resistant strains may proliferate and outcompete susceptible populations, potentially contributing to reduced clinical efficacy. Importantly, the use of broad-spectrum antibiotics may disrupt the skin and gut microbiota, promoting resistance among nontarget organisms.5 These concerns underscore the importance of limiting antibiotic use to appropriate indications, combining antibiotics with adjunctive nonantibiotic therapies, and avoiding monotherapy.
PRESCRIBING PRINCIPLES FOR RESPONSIBLE ORAL ANTIBIOTIC USE IN ACNE
The following principles are derived from our clinical experience and are aligned with AAD guidelines on acne treatment.10 This practical framework supports safe, effective, and streamlined prescribing.
Reserve Oral Antibiotics for Appropriate Cases
Oral antibiotics should be considered for patients with moderate to severe inflammatory acne when rapid anti-inflammatory control is needed. They are not indicated for comedonal or mild papulopustular acne. Before initiating treatment, clinicians should weigh the potential benefits against the risks associated with antibiotic exposure, including resistance and microbiome disruption.
Combine Oral Antibiotics With Topical Retinoids
Oral antibiotics should not be used as monotherapy. Topical retinoids should be initiated concurrently with oral antibiotics to maximize anti-inflammatory benefit, support transition to maintenance therapy, and reduce risk for resistance.
Consider Adding an Adjunctive Topical Antimicrobial Agent
Adjunctive topical antimicrobials can help reduce bacterial load. Benzoyl peroxide remains a first-line option due to its bactericidal activity and lack of resistance induction; however, recent product recalls involving benzene contamination may have raised safety concerns among some clinicians and patients.11,12 While no definitive harm has been established, alternative topical agents approved by the US Food and Drug Administration (eg, azelaic acid) may be used based on shared decision-making, tolerability, cost, access, and patient preference. Use of topical antibiotics (eg, clindamycin, erythromycin) as monotherapy is discouraged due to their higher resistance potential, which is consistent with AAD guidance.
Limit Treatment Duration to 12 Weeks or Less
Antibiotic use should be time limited, with discontinuation ideally within 8 to 12 weeks as clinical improvement is demonstrated. Repeated or prolonged courses should be avoided to minimize risk for resistance.
Simplify Treatment Regimens to Enhance Adherence
Regimen simplicity improves adherence, especially in adolescents. A two-agent regimen of an oral antibiotic and a topical retinoid typically is sufficient during the induction phase.13,14
Select Narrower-Spectrum Antibiotics When Feasible
Using a narrower-spectrum antibiotic may help minimize disruption to nontarget microbiota.15,16 Sarecycline has shown narrower in vitro activity within the tetracycline class,17,18 though clinical decisions should be informed by access, availability, and cost. Regardless of the agent used (eg, doxycycline, minocycline, or sarecycline), all antibiotics should be used judiciously and for the shortest effective duration.
Use Systemic Nonantibiotic Therapies When Appropriate
If there is inadequate response to oral antibiotic therapy, consider switching to systemic nonantibiotic options. Hormonal therapy may be appropriate for select female patients. Oral isotretinoin should be considered for patients with severe, recalcitrant, or scarring acne. Cycling between antibiotic classes without clear benefit is discouraged.
FINAL THOUGHTS
Oral antibiotics remain a foundational component in the management of moderate to severe acne; however, their use must be intentional, time limited, and guided by best practices to minimize the emergence of antimicrobial resistance. By adhering to the prescribing principles we have outlined here, which are rooted in clinical expertise and consistent with AAD guidelines, dermatologists can preserve antibiotic efficacy, optimize patient outcomes, and reduce long-term microbiologic risks. Stewardship is not about withholding treatment; it is about optimizing care today to protect treatment options for tomorrow.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
- Grada A, Armstrong A, Bunick C, et al. Trends in oral antibiotic use for acne treatment: a retrospective, population-based study in the United States, 2014 to 2016. J Drugs Dermatol. 2023;22:265-270.
- Perche PO, Peck GM, Robinson L, et al. Prescribing trends for acne vulgaris visits in the United States. Antibiotics. 2023;12:269.
- Karadag A, Aslan Kayıran M, Wu CY, et al. Antibiotic resistance in acne: changes, consequences and concerns. J Eur Acad Dermatol Venereol. 2021;35:73-78.
- Eady AE, Cove JH, Layton AM. Is antibiotic resistance in cutaneous propionibacteria clinically relevant? implications of resistance for acne patients and prescribers. Am J Clin Dermatol. 2003;4:813-831.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J Dermatol. 1989;121:51-57.
- Grossman TH. Tetracycline antibiotics and resistance. Cold Spring Harb Perspect Med. 2016;6:a025387.
- Kayiran M AS, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006-1035.
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:037702.
- Kucera K, Zenzola N, Hudspeth A, et al. Evaluation of benzene presence and formation in benzoyl peroxide drug products. J Invest Dermatol. 2025;145:1147-1154.E11.
- Grada A, Perche P, Feldman S. Adherence and persistence to acne medications: a population-based claims database analysis. J Drugs Dermatol. 2022;21:758-764.<.li>
- Anderson KL, Dothard EH, Huang KE, et al. Frequency of primary nonadherence to acne treatment. JAMA Dermatol. 2015;151:623-626.
- Grada A, Bunick CG. Spectrum of antibiotic activity and its relevance to the microbiome. JAMA Netw Open. 2021;4:E215357-E215357.
- Francino M. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:164577.
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911.
- Zhanel G, Critchley I, Lin L-Y, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2019;63:1297-1318.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
- Grada A, Armstrong A, Bunick C, et al. Trends in oral antibiotic use for acne treatment: a retrospective, population-based study in the United States, 2014 to 2016. J Drugs Dermatol. 2023;22:265-270.
- Perche PO, Peck GM, Robinson L, et al. Prescribing trends for acne vulgaris visits in the United States. Antibiotics. 2023;12:269.
- Karadag A, Aslan Kayıran M, Wu CY, et al. Antibiotic resistance in acne: changes, consequences and concerns. J Eur Acad Dermatol Venereol. 2021;35:73-78.
- Eady AE, Cove JH, Layton AM. Is antibiotic resistance in cutaneous propionibacteria clinically relevant? implications of resistance for acne patients and prescribers. Am J Clin Dermatol. 2003;4:813-831.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J Dermatol. 1989;121:51-57.
- Grossman TH. Tetracycline antibiotics and resistance. Cold Spring Harb Perspect Med. 2016;6:a025387.
- Kayiran M AS, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006-1035.
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:037702.
- Kucera K, Zenzola N, Hudspeth A, et al. Evaluation of benzene presence and formation in benzoyl peroxide drug products. J Invest Dermatol. 2025;145:1147-1154.E11.
- Grada A, Perche P, Feldman S. Adherence and persistence to acne medications: a population-based claims database analysis. J Drugs Dermatol. 2022;21:758-764.<.li>
- Anderson KL, Dothard EH, Huang KE, et al. Frequency of primary nonadherence to acne treatment. JAMA Dermatol. 2015;151:623-626.
- Grada A, Bunick CG. Spectrum of antibiotic activity and its relevance to the microbiome. JAMA Netw Open. 2021;4:E215357-E215357.
- Francino M. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:164577.
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911.
- Zhanel G, Critchley I, Lin L-Y, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2019;63:1297-1318.
Path of Least Resistance: Guidance for Antibiotic Stewardship in Acne
Path of Least Resistance: Guidance for Antibiotic Stewardship in Acne
Practice Point
- Oral antibiotics remain a cornerstone in the treatment of moderate to severe acne, but growing concerns about antibiotic resistance necessitate more intentional prescribing.
Poly-L-Lactic Acid Reconstitution Technique to Reduce Needle Obstruction
Poly-L-Lactic Acid Reconstitution Technique to Reduce Needle Obstruction
Practice Gap
Poly-L-lactic acid is approved by the US Food and Drug Administration for addressing fat loss due to HAART in patients with HIV.2,3 When used as a dermal filler for correction of facial lipoatrophy, PLLA is well tolerated and has been shown to improve quality of life.2,3 Poly-L-lactic acid is available for clinical use as microparticles of lyophilized alpha hydroxy acid polymers. Once injected (after the carrier substance is absorbed), PLLA induces an inflammatory response that ultimately leads to the production of new collagen.3 Unfortunately, PLLA microparticles often obstruct needles and make the product difficult to use, potentially hindering effective injection; thus, it is in the best interest of the patient to mitigate needle obstruction during this procedure. In this article, we describe a simple and effective way to mitigate this problem by utilizing a water bath to warm the filler prior to injection.
Technique
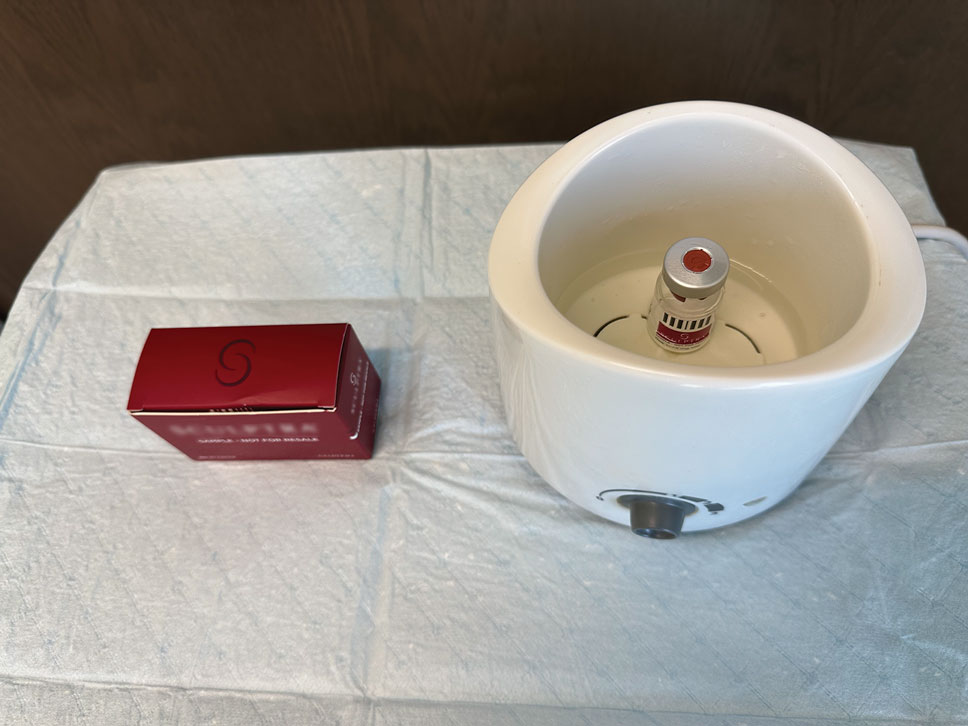
The required supplies include a thermostatic water bath, reconstituted PLLA, a syringe, and a 26-gauge injection needle. Because laboratory-grade heated water baths typically cost between $300 and $3000,4 we recommend using a more affordable, commercially available thermostatic water bath (eg, baby bottle warmer)(Figure 1) to warm the filler prior to injection, as the optimal temperature for this technique can still be achieved while remaining cost effective. Vials of PLLA reconstituted with 7 mL of sterile water and 2 mL lidocaine hydrochloride 1% should be labeled with the date of reconstitution and manually agitated for 30 seconds. The reconstituted product should be stored for 24 hours to ensure even suspension and powder saturation.5 On the day of the procedure, the vial should be placed into the water bath (heated to 100 °C) for 10 minutes prior to injection (Figure 2) and agitated again immediately before withdrawal into the syringe. The clinician then should sterilize the rubber top and draw the product from the warmed vial using the same size needle that will be used for injection. Although a larger gauge needle may make drawing up the product easier in typical practice, drawing and injecting with the same gauge needle helps prevent larger particles from clogging a smaller injection needle. Using a 26-gauge injection needle for withdrawal further reduces clogging by serving as a filter to prevent larger product particles from entering the injection syringe. The vials of PLLA can be kept in the water bath throughout the procedure between uses to keep the filler at a consistent temperature.

Practice Implications
Although many clinicians reduce needle obstructions by warming PLLA before injection, a published protocol currently is not available. One consideration when utilizing this technique is the limited data on the clinical stability and efficacy of PLLA at varying temperatures. Two studies recommend bringing the reconstituted vial to room temperature prior to injection, while others have documented an endothermic melting point in the range of 120 °C to 180
- James J, Carruthers A, Carruthers J. HIV-associated facial lipoatrophy. Dermatol Surg. 2002;28:979-986. doi:10.1046/j.1524-4725.2002.02099.x
- Duracinsky M, Leclercq P, Herrmann S, et al. Safety of poly-L-lactic acid (New-Fill®) in the treatment of facial lipoatrophy: a large observational study among HIV-positive patients. BMC Infect Dis. 2014;14:474. doi:10.1186/1471-2334-14-474
- Sickles CK, Nassereddin A, Patel P, et al. Poly-L-lactic acid. StatPearls [Internet]. Updated February 28, 2024. Accessed October 31, 2025. https://www.ncbi.nlm.nih.gov/books/NBK507871/
- Laboratory equipment: Water bath. Global Lab Supply. (n.d.). http://www.globallabsupply.com/Water-Bath-s/2122.htm
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Vleggaar D, Fitzgerald R, Lorenc ZP, et al. Consensus recommendations on the use of injectable poly-L-lactic acid for facial and nonfacial volumization. J Drugs Dermatol. 2014;13:s44-51.
- Sedush NG, Kalinin KT, Azarkevich PN, et al. Physicochemical characteristics and hydrolytic degradation of polylactic acid dermal fillers: a comparative study. Cosmetics. 2023;10:110. doi:10.3390/cosmetics10040110
Practice Gap
Poly-L-lactic acid is approved by the US Food and Drug Administration for addressing fat loss due to HAART in patients with HIV.2,3 When used as a dermal filler for correction of facial lipoatrophy, PLLA is well tolerated and has been shown to improve quality of life.2,3 Poly-L-lactic acid is available for clinical use as microparticles of lyophilized alpha hydroxy acid polymers. Once injected (after the carrier substance is absorbed), PLLA induces an inflammatory response that ultimately leads to the production of new collagen.3 Unfortunately, PLLA microparticles often obstruct needles and make the product difficult to use, potentially hindering effective injection; thus, it is in the best interest of the patient to mitigate needle obstruction during this procedure. In this article, we describe a simple and effective way to mitigate this problem by utilizing a water bath to warm the filler prior to injection.
Technique
The required supplies include a thermostatic water bath, reconstituted PLLA, a syringe, and a 26-gauge injection needle. Because laboratory-grade heated water baths typically cost between $300 and $3000,4 we recommend using a more affordable, commercially available thermostatic water bath (eg, baby bottle warmer)(Figure 1) to warm the filler prior to injection, as the optimal temperature for this technique can still be achieved while remaining cost effective. Vials of PLLA reconstituted with 7 mL of sterile water and 2 mL lidocaine hydrochloride 1% should be labeled with the date of reconstitution and manually agitated for 30 seconds. The reconstituted product should be stored for 24 hours to ensure even suspension and powder saturation.5 On the day of the procedure, the vial should be placed into the water bath (heated to 100 °C) for 10 minutes prior to injection (Figure 2) and agitated again immediately before withdrawal into the syringe. The clinician then should sterilize the rubber top and draw the product from the warmed vial using the same size needle that will be used for injection. Although a larger gauge needle may make drawing up the product easier in typical practice, drawing and injecting with the same gauge needle helps prevent larger particles from clogging a smaller injection needle. Using a 26-gauge injection needle for withdrawal further reduces clogging by serving as a filter to prevent larger product particles from entering the injection syringe. The vials of PLLA can be kept in the water bath throughout the procedure between uses to keep the filler at a consistent temperature.


Practice Implications
Although many clinicians reduce needle obstructions by warming PLLA before injection, a published protocol currently is not available. One consideration when utilizing this technique is the limited data on the clinical stability and efficacy of PLLA at varying temperatures. Two studies recommend bringing the reconstituted vial to room temperature prior to injection, while others have documented an endothermic melting point in the range of 120 °C to 180
Practice Gap
Poly-L-lactic acid is approved by the US Food and Drug Administration for addressing fat loss due to HAART in patients with HIV.2,3 When used as a dermal filler for correction of facial lipoatrophy, PLLA is well tolerated and has been shown to improve quality of life.2,3 Poly-L-lactic acid is available for clinical use as microparticles of lyophilized alpha hydroxy acid polymers. Once injected (after the carrier substance is absorbed), PLLA induces an inflammatory response that ultimately leads to the production of new collagen.3 Unfortunately, PLLA microparticles often obstruct needles and make the product difficult to use, potentially hindering effective injection; thus, it is in the best interest of the patient to mitigate needle obstruction during this procedure. In this article, we describe a simple and effective way to mitigate this problem by utilizing a water bath to warm the filler prior to injection.
Technique
The required supplies include a thermostatic water bath, reconstituted PLLA, a syringe, and a 26-gauge injection needle. Because laboratory-grade heated water baths typically cost between $300 and $3000,4 we recommend using a more affordable, commercially available thermostatic water bath (eg, baby bottle warmer)(Figure 1) to warm the filler prior to injection, as the optimal temperature for this technique can still be achieved while remaining cost effective. Vials of PLLA reconstituted with 7 mL of sterile water and 2 mL lidocaine hydrochloride 1% should be labeled with the date of reconstitution and manually agitated for 30 seconds. The reconstituted product should be stored for 24 hours to ensure even suspension and powder saturation.5 On the day of the procedure, the vial should be placed into the water bath (heated to 100 °C) for 10 minutes prior to injection (Figure 2) and agitated again immediately before withdrawal into the syringe. The clinician then should sterilize the rubber top and draw the product from the warmed vial using the same size needle that will be used for injection. Although a larger gauge needle may make drawing up the product easier in typical practice, drawing and injecting with the same gauge needle helps prevent larger particles from clogging a smaller injection needle. Using a 26-gauge injection needle for withdrawal further reduces clogging by serving as a filter to prevent larger product particles from entering the injection syringe. The vials of PLLA can be kept in the water bath throughout the procedure between uses to keep the filler at a consistent temperature.


Practice Implications
Although many clinicians reduce needle obstructions by warming PLLA before injection, a published protocol currently is not available. One consideration when utilizing this technique is the limited data on the clinical stability and efficacy of PLLA at varying temperatures. Two studies recommend bringing the reconstituted vial to room temperature prior to injection, while others have documented an endothermic melting point in the range of 120 °C to 180
- James J, Carruthers A, Carruthers J. HIV-associated facial lipoatrophy. Dermatol Surg. 2002;28:979-986. doi:10.1046/j.1524-4725.2002.02099.x
- Duracinsky M, Leclercq P, Herrmann S, et al. Safety of poly-L-lactic acid (New-Fill®) in the treatment of facial lipoatrophy: a large observational study among HIV-positive patients. BMC Infect Dis. 2014;14:474. doi:10.1186/1471-2334-14-474
- Sickles CK, Nassereddin A, Patel P, et al. Poly-L-lactic acid. StatPearls [Internet]. Updated February 28, 2024. Accessed October 31, 2025. https://www.ncbi.nlm.nih.gov/books/NBK507871/
- Laboratory equipment: Water bath. Global Lab Supply. (n.d.). http://www.globallabsupply.com/Water-Bath-s/2122.htm
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Vleggaar D, Fitzgerald R, Lorenc ZP, et al. Consensus recommendations on the use of injectable poly-L-lactic acid for facial and nonfacial volumization. J Drugs Dermatol. 2014;13:s44-51.
- Sedush NG, Kalinin KT, Azarkevich PN, et al. Physicochemical characteristics and hydrolytic degradation of polylactic acid dermal fillers: a comparative study. Cosmetics. 2023;10:110. doi:10.3390/cosmetics10040110
- James J, Carruthers A, Carruthers J. HIV-associated facial lipoatrophy. Dermatol Surg. 2002;28:979-986. doi:10.1046/j.1524-4725.2002.02099.x
- Duracinsky M, Leclercq P, Herrmann S, et al. Safety of poly-L-lactic acid (New-Fill®) in the treatment of facial lipoatrophy: a large observational study among HIV-positive patients. BMC Infect Dis. 2014;14:474. doi:10.1186/1471-2334-14-474
- Sickles CK, Nassereddin A, Patel P, et al. Poly-L-lactic acid. StatPearls [Internet]. Updated February 28, 2024. Accessed October 31, 2025. https://www.ncbi.nlm.nih.gov/books/NBK507871/
- Laboratory equipment: Water bath. Global Lab Supply. (n.d.). http://www.globallabsupply.com/Water-Bath-s/2122.htm
- Lin MJ, Dubin DP, Goldberg DJ, et al. Practices in the usage and reconstitution of poly-L-lactic acid. J Drugs Dermatol. 2019;18:880-886.
- Vleggaar D, Fitzgerald R, Lorenc ZP, et al. Consensus recommendations on the use of injectable poly-L-lactic acid for facial and nonfacial volumization. J Drugs Dermatol. 2014;13:s44-51.
- Sedush NG, Kalinin KT, Azarkevich PN, et al. Physicochemical characteristics and hydrolytic degradation of polylactic acid dermal fillers: a comparative study. Cosmetics. 2023;10:110. doi:10.3390/cosmetics10040110
Poly-L-Lactic Acid Reconstitution Technique to Reduce Needle Obstruction
Poly-L-Lactic Acid Reconstitution Technique to Reduce Needle Obstruction
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
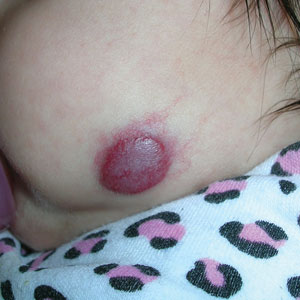
Infantile hemangioma (IH) is the most common vascular tumor of infancy, appearing within the first few weeks of life and typically reaching peak size by age 3 to 5 months.1 It classically manifests as a raised or flat bright-red lesion in the upper dermis of the skin and/or subcutaneous tissue and can vary in number, size, shape, and location.2 It is characterized by a rapid proliferative phase, especially between 5 and 8 weeks of age, followed by gradual spontaneous regression over 1 to 10 years.1-3
Infantile hemangiomas are categorized based on depth (superficial, deep, or mixed) and distribution pattern (focal, multifocal, segmental, or indeterminate).4 In most cases, complete regression occurs by age 4 years, but there can be residual telangiectasia, fibrofatty tissue, and/or scarring.1,4 About 10% to 15% of IHs result in complications that require medical intervention (eg, visual, airway, or auditory compromise; ulceration; disfigurement); ideally, these patients should be referred to a specialist by 5 weeks of age.4 Prompt assessment of IH severity is essential to prevent or mitigate potential complications and ultimately improve outcomes.3 Social drivers of health contribute to delayed diagnosis and management of hemangiomas, leading to increased complications in some patient populations.5-7
Epidemiology
Infantile hemangiomas are estimated to manifest in 4.5% of infants in the United States.1 The most common type is superficial IH, typically found on the head or neck.5 Risk factors in infants include female sex, White race, premature birth, and low birth weight (<1000 g).1,3 Maternal risk factors include advanced gestational age (ie, >35 years), multiple gestations, family history of IH, tobacco use, use of progesterone therapy during pregnancy, and pre-eclampsia.1,3
Focal IH typically manifests as a single localized lesion that can occur anywhere on the body.2,3 In contrast, segmental IH manifests in a linear pattern and/or is distributed on a large anatomic area, most commonly on the face and less frequently the extremities and trunk.
Key Clinical Features
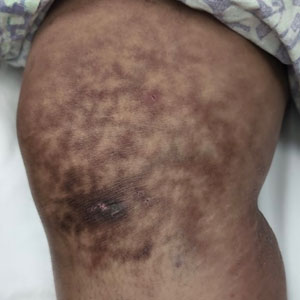
Superficial IH in patients with darker skin tones may appear as a dark-red or violaceous papule or plaque compared to bright red in lighter skin tones.5 Deep IH may appear as a soft, round, flesh-colored or blue-hued subcutaneous mass, the color of which may be harder to appreciate in those with darker skin tones.5
Worth Noting
Complications from IH may require imaging, close follow-up, systemic therapy, multidisciplinary care, and advanced health literacy and patient/family navigation. Multifocal IHs (≥5 lesions) are more likely to be associated with infantile hepatic hemangiomas.2,3 Large (>5 cm) segmental IHs on the face and lumbosacral area require further evaluation for PHACES (posterior fossa malformation, hemangiomas, arterial anomalies, cardiac defects, eye anomalies, and sternal raphe/cleft defects) and LUMBAR (lower-body segmental IH; urogenital anomalies and ulceration; myelopathy; bony deformities; anorectal malformations and arterial anomalies; and renal anomalies) syndromes, which are more common in patients of Hispanic ethnicity.2,3
The Infantile Hemangioma Referral Score is a recently validated tool that can assist primary care physicians in timely referral of IHs requiring early specialist intervention.4,9 It takes into account the location, number, and size of the lesions and the age of the patient; these factors help to determine which IHs may be managed conservatively vs those that may require treatment to prevent life-threatening complications.1-3
Systemic corticosteroids historically have been the primary treatment for IH; however, in the past decade, propranolol oral solution (4.28 mg/mL) has become the first-line therapy for most infants requiring systemic management.10 It is the only medication approved by the US Food and Drug Administration for proliferating IH, with treatment initiation as young as 5 weeks corrected age.11 As a nonselective beta-blocker, propranolol is believed to reduce IHs through vasoconstriction or by inhibition of angiogenesis.1,4,10
For small superficial IHs, treatment options include timolol maleate ophthalmic solution 0.5% (one drop applied twice daily to the IH) or pulsed dye laser therapy.4,10 Surgical excision typically is avoided during infancy due to concerns about anesthetic risks and potential blood loss.4,10 Surgery is reserved for cases involving residual fibrofatty tissue, postinvolution scarring, obstruction of vital structures, or lesions in aesthetically sensitive areas as well as when propranolol is contraindicated.4,10
Health Disparity Highlight
Infants with skin of color and those of lower socioeconomic status (SES) face a heightened risk for delayed diagnosis and more advanced disease at the initial evaluation for IH.5,7 Access barriers such as geographic limitations to specialty services, lack of insurance, underinsurance, and language differences impact timely diagnosis and treatment.5,6 Implementation of telemedicine services in areas with limited access to specialists can facilitate early evaluation and risk stratification for IH.12
A retrospective cohort study of 804 children seen at a large academic hospital found that those of lower SES were more likely to seek care after 3 months of age than their higher-SES counterparts.6 Those who presented after 6 months of age also had higher IH severity scores compared to their counterparts with higher SES.6 Delayed access to care may cause children to miss the critical treatment window during the rapid proliferative growth phase.6,12 However, children insured through Medicaid or the Children’s Health Insurance Program who participated in institutional care management programs (which assist in scheduling specialty care appointments within the institution) sought treatment earlier regardless of their SES, suggesting that such programs may help reduce disparities in timely access for children of lower SES.6
An epidemiologic study analyzing the demographics of children hospitalized across the United States demonstrated that Black infants with IH were more likely to belong to the lowest income quartile compared with White infants or those of other races. They also were 2 times older on average at initial presentation (1.8 vs 1.0 years), experienced longer hospitalizations (16.4 vs 13.8 days), and underwent more IH-related procedures than White infants and infants of other races (2.4, 1.9, and 2.1, respectively).7
These and other factors may contribute to missed windows of opportunity for timely treatment of high-risk IHs in patients with darker skin tones and/or those facing challenges stemming from social drivers of health.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Mitra R, Fitzsimons HL, Hale T, et al. Recent advances in understanding the molecular basis of infantile haemangioma development. Br J Dermatol. 2024;191:661-669.
- Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392.
- Sebaratnam DF, Rodríguez Bandera AL, Wong LCF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85:1395-1404.
- Taye ME, Shah J, Seiverling EV, et al. Diagnosis of vascular anomalies in patients with skin of color. J Clin Aesthet Dermatol. 2024;17:54-62.
- Lie E, Psoter KJ, Püttgen KB. Lower socioeconomic status is associated with delayed access to care for infantile hemangioma: a cohort study. J Am Acad Dermatol. 2023;88:E221-E230.
- Kumar KD, Desai AD, Shah VP, et al. Racial discrepancies in presentation of hospitalized infantile hemangioma cases using the Kids’ Inpatient Database. Health Sci Rep. 2023;6:E1092.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567.
- Léauté-Labrèze C, Baselga Torres E, Weibel L, et al. The infantile hemangioma referral score: a validated tool for physicians. Pediatrics. 2020;145:E20191628.
- Macca L, Altavilla D, Di Bartolomeo L, et al. Update on treatment of infantile hemangiomas: what’s new in the last five years? Front Pharmacol. 2022;13:879602.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475.
- Frieden IJ, Püttgen KB, Drolet BA, et al. Management of infantile hemangiomas during the COVID pandemic. Pediatr Dermatol. 2020;37:412-418.
Infantile hemangioma (IH) is the most common vascular tumor of infancy, appearing within the first few weeks of life and typically reaching peak size by age 3 to 5 months.1 It classically manifests as a raised or flat bright-red lesion in the upper dermis of the skin and/or subcutaneous tissue and can vary in number, size, shape, and location.2 It is characterized by a rapid proliferative phase, especially between 5 and 8 weeks of age, followed by gradual spontaneous regression over 1 to 10 years.1-3
Infantile hemangiomas are categorized based on depth (superficial, deep, or mixed) and distribution pattern (focal, multifocal, segmental, or indeterminate).4 In most cases, complete regression occurs by age 4 years, but there can be residual telangiectasia, fibrofatty tissue, and/or scarring.1,4 About 10% to 15% of IHs result in complications that require medical intervention (eg, visual, airway, or auditory compromise; ulceration; disfigurement); ideally, these patients should be referred to a specialist by 5 weeks of age.4 Prompt assessment of IH severity is essential to prevent or mitigate potential complications and ultimately improve outcomes.3 Social drivers of health contribute to delayed diagnosis and management of hemangiomas, leading to increased complications in some patient populations.5-7
Epidemiology
Infantile hemangiomas are estimated to manifest in 4.5% of infants in the United States.1 The most common type is superficial IH, typically found on the head or neck.5 Risk factors in infants include female sex, White race, premature birth, and low birth weight (<1000 g).1,3 Maternal risk factors include advanced gestational age (ie, >35 years), multiple gestations, family history of IH, tobacco use, use of progesterone therapy during pregnancy, and pre-eclampsia.1,3
Focal IH typically manifests as a single localized lesion that can occur anywhere on the body.2,3 In contrast, segmental IH manifests in a linear pattern and/or is distributed on a large anatomic area, most commonly on the face and less frequently the extremities and trunk.
Key Clinical Features
Superficial IH in patients with darker skin tones may appear as a dark-red or violaceous papule or plaque compared to bright red in lighter skin tones.5 Deep IH may appear as a soft, round, flesh-colored or blue-hued subcutaneous mass, the color of which may be harder to appreciate in those with darker skin tones.5
Worth Noting
Complications from IH may require imaging, close follow-up, systemic therapy, multidisciplinary care, and advanced health literacy and patient/family navigation. Multifocal IHs (≥5 lesions) are more likely to be associated with infantile hepatic hemangiomas.2,3 Large (>5 cm) segmental IHs on the face and lumbosacral area require further evaluation for PHACES (posterior fossa malformation, hemangiomas, arterial anomalies, cardiac defects, eye anomalies, and sternal raphe/cleft defects) and LUMBAR (lower-body segmental IH; urogenital anomalies and ulceration; myelopathy; bony deformities; anorectal malformations and arterial anomalies; and renal anomalies) syndromes, which are more common in patients of Hispanic ethnicity.2,3
The Infantile Hemangioma Referral Score is a recently validated tool that can assist primary care physicians in timely referral of IHs requiring early specialist intervention.4,9 It takes into account the location, number, and size of the lesions and the age of the patient; these factors help to determine which IHs may be managed conservatively vs those that may require treatment to prevent life-threatening complications.1-3
Systemic corticosteroids historically have been the primary treatment for IH; however, in the past decade, propranolol oral solution (4.28 mg/mL) has become the first-line therapy for most infants requiring systemic management.10 It is the only medication approved by the US Food and Drug Administration for proliferating IH, with treatment initiation as young as 5 weeks corrected age.11 As a nonselective beta-blocker, propranolol is believed to reduce IHs through vasoconstriction or by inhibition of angiogenesis.1,4,10
For small superficial IHs, treatment options include timolol maleate ophthalmic solution 0.5% (one drop applied twice daily to the IH) or pulsed dye laser therapy.4,10 Surgical excision typically is avoided during infancy due to concerns about anesthetic risks and potential blood loss.4,10 Surgery is reserved for cases involving residual fibrofatty tissue, postinvolution scarring, obstruction of vital structures, or lesions in aesthetically sensitive areas as well as when propranolol is contraindicated.4,10
Health Disparity Highlight
Infants with skin of color and those of lower socioeconomic status (SES) face a heightened risk for delayed diagnosis and more advanced disease at the initial evaluation for IH.5,7 Access barriers such as geographic limitations to specialty services, lack of insurance, underinsurance, and language differences impact timely diagnosis and treatment.5,6 Implementation of telemedicine services in areas with limited access to specialists can facilitate early evaluation and risk stratification for IH.12
A retrospective cohort study of 804 children seen at a large academic hospital found that those of lower SES were more likely to seek care after 3 months of age than their higher-SES counterparts.6 Those who presented after 6 months of age also had higher IH severity scores compared to their counterparts with higher SES.6 Delayed access to care may cause children to miss the critical treatment window during the rapid proliferative growth phase.6,12 However, children insured through Medicaid or the Children’s Health Insurance Program who participated in institutional care management programs (which assist in scheduling specialty care appointments within the institution) sought treatment earlier regardless of their SES, suggesting that such programs may help reduce disparities in timely access for children of lower SES.6
An epidemiologic study analyzing the demographics of children hospitalized across the United States demonstrated that Black infants with IH were more likely to belong to the lowest income quartile compared with White infants or those of other races. They also were 2 times older on average at initial presentation (1.8 vs 1.0 years), experienced longer hospitalizations (16.4 vs 13.8 days), and underwent more IH-related procedures than White infants and infants of other races (2.4, 1.9, and 2.1, respectively).7
These and other factors may contribute to missed windows of opportunity for timely treatment of high-risk IHs in patients with darker skin tones and/or those facing challenges stemming from social drivers of health.
Infantile hemangioma (IH) is the most common vascular tumor of infancy, appearing within the first few weeks of life and typically reaching peak size by age 3 to 5 months.1 It classically manifests as a raised or flat bright-red lesion in the upper dermis of the skin and/or subcutaneous tissue and can vary in number, size, shape, and location.2 It is characterized by a rapid proliferative phase, especially between 5 and 8 weeks of age, followed by gradual spontaneous regression over 1 to 10 years.1-3
Infantile hemangiomas are categorized based on depth (superficial, deep, or mixed) and distribution pattern (focal, multifocal, segmental, or indeterminate).4 In most cases, complete regression occurs by age 4 years, but there can be residual telangiectasia, fibrofatty tissue, and/or scarring.1,4 About 10% to 15% of IHs result in complications that require medical intervention (eg, visual, airway, or auditory compromise; ulceration; disfigurement); ideally, these patients should be referred to a specialist by 5 weeks of age.4 Prompt assessment of IH severity is essential to prevent or mitigate potential complications and ultimately improve outcomes.3 Social drivers of health contribute to delayed diagnosis and management of hemangiomas, leading to increased complications in some patient populations.5-7
Epidemiology
Infantile hemangiomas are estimated to manifest in 4.5% of infants in the United States.1 The most common type is superficial IH, typically found on the head or neck.5 Risk factors in infants include female sex, White race, premature birth, and low birth weight (<1000 g).1,3 Maternal risk factors include advanced gestational age (ie, >35 years), multiple gestations, family history of IH, tobacco use, use of progesterone therapy during pregnancy, and pre-eclampsia.1,3
Focal IH typically manifests as a single localized lesion that can occur anywhere on the body.2,3 In contrast, segmental IH manifests in a linear pattern and/or is distributed on a large anatomic area, most commonly on the face and less frequently the extremities and trunk.
Key Clinical Features
Superficial IH in patients with darker skin tones may appear as a dark-red or violaceous papule or plaque compared to bright red in lighter skin tones.5 Deep IH may appear as a soft, round, flesh-colored or blue-hued subcutaneous mass, the color of which may be harder to appreciate in those with darker skin tones.5
Worth Noting
Complications from IH may require imaging, close follow-up, systemic therapy, multidisciplinary care, and advanced health literacy and patient/family navigation. Multifocal IHs (≥5 lesions) are more likely to be associated with infantile hepatic hemangiomas.2,3 Large (>5 cm) segmental IHs on the face and lumbosacral area require further evaluation for PHACES (posterior fossa malformation, hemangiomas, arterial anomalies, cardiac defects, eye anomalies, and sternal raphe/cleft defects) and LUMBAR (lower-body segmental IH; urogenital anomalies and ulceration; myelopathy; bony deformities; anorectal malformations and arterial anomalies; and renal anomalies) syndromes, which are more common in patients of Hispanic ethnicity.2,3
The Infantile Hemangioma Referral Score is a recently validated tool that can assist primary care physicians in timely referral of IHs requiring early specialist intervention.4,9 It takes into account the location, number, and size of the lesions and the age of the patient; these factors help to determine which IHs may be managed conservatively vs those that may require treatment to prevent life-threatening complications.1-3
Systemic corticosteroids historically have been the primary treatment for IH; however, in the past decade, propranolol oral solution (4.28 mg/mL) has become the first-line therapy for most infants requiring systemic management.10 It is the only medication approved by the US Food and Drug Administration for proliferating IH, with treatment initiation as young as 5 weeks corrected age.11 As a nonselective beta-blocker, propranolol is believed to reduce IHs through vasoconstriction or by inhibition of angiogenesis.1,4,10
For small superficial IHs, treatment options include timolol maleate ophthalmic solution 0.5% (one drop applied twice daily to the IH) or pulsed dye laser therapy.4,10 Surgical excision typically is avoided during infancy due to concerns about anesthetic risks and potential blood loss.4,10 Surgery is reserved for cases involving residual fibrofatty tissue, postinvolution scarring, obstruction of vital structures, or lesions in aesthetically sensitive areas as well as when propranolol is contraindicated.4,10
Health Disparity Highlight
Infants with skin of color and those of lower socioeconomic status (SES) face a heightened risk for delayed diagnosis and more advanced disease at the initial evaluation for IH.5,7 Access barriers such as geographic limitations to specialty services, lack of insurance, underinsurance, and language differences impact timely diagnosis and treatment.5,6 Implementation of telemedicine services in areas with limited access to specialists can facilitate early evaluation and risk stratification for IH.12
A retrospective cohort study of 804 children seen at a large academic hospital found that those of lower SES were more likely to seek care after 3 months of age than their higher-SES counterparts.6 Those who presented after 6 months of age also had higher IH severity scores compared to their counterparts with higher SES.6 Delayed access to care may cause children to miss the critical treatment window during the rapid proliferative growth phase.6,12 However, children insured through Medicaid or the Children’s Health Insurance Program who participated in institutional care management programs (which assist in scheduling specialty care appointments within the institution) sought treatment earlier regardless of their SES, suggesting that such programs may help reduce disparities in timely access for children of lower SES.6
An epidemiologic study analyzing the demographics of children hospitalized across the United States demonstrated that Black infants with IH were more likely to belong to the lowest income quartile compared with White infants or those of other races. They also were 2 times older on average at initial presentation (1.8 vs 1.0 years), experienced longer hospitalizations (16.4 vs 13.8 days), and underwent more IH-related procedures than White infants and infants of other races (2.4, 1.9, and 2.1, respectively).7
These and other factors may contribute to missed windows of opportunity for timely treatment of high-risk IHs in patients with darker skin tones and/or those facing challenges stemming from social drivers of health.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Mitra R, Fitzsimons HL, Hale T, et al. Recent advances in understanding the molecular basis of infantile haemangioma development. Br J Dermatol. 2024;191:661-669.
- Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392.
- Sebaratnam DF, Rodríguez Bandera AL, Wong LCF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85:1395-1404.
- Taye ME, Shah J, Seiverling EV, et al. Diagnosis of vascular anomalies in patients with skin of color. J Clin Aesthet Dermatol. 2024;17:54-62.
- Lie E, Psoter KJ, Püttgen KB. Lower socioeconomic status is associated with delayed access to care for infantile hemangioma: a cohort study. J Am Acad Dermatol. 2023;88:E221-E230.
- Kumar KD, Desai AD, Shah VP, et al. Racial discrepancies in presentation of hospitalized infantile hemangioma cases using the Kids’ Inpatient Database. Health Sci Rep. 2023;6:E1092.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567.
- Léauté-Labrèze C, Baselga Torres E, Weibel L, et al. The infantile hemangioma referral score: a validated tool for physicians. Pediatrics. 2020;145:E20191628.
- Macca L, Altavilla D, Di Bartolomeo L, et al. Update on treatment of infantile hemangiomas: what’s new in the last five years? Front Pharmacol. 2022;13:879602.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475.
- Frieden IJ, Püttgen KB, Drolet BA, et al. Management of infantile hemangiomas during the COVID pandemic. Pediatr Dermatol. 2020;37:412-418.
- Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85-94.
- Mitra R, Fitzsimons HL, Hale T, et al. Recent advances in understanding the molecular basis of infantile haemangioma development. Br J Dermatol. 2024;191:661-669.
- Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392.
- Sebaratnam DF, Rodríguez Bandera AL, Wong LCF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85:1395-1404.
- Taye ME, Shah J, Seiverling EV, et al. Diagnosis of vascular anomalies in patients with skin of color. J Clin Aesthet Dermatol. 2024;17:54-62.
- Lie E, Psoter KJ, Püttgen KB. Lower socioeconomic status is associated with delayed access to care for infantile hemangioma: a cohort study. J Am Acad Dermatol. 2023;88:E221-E230.
- Kumar KD, Desai AD, Shah VP, et al. Racial discrepancies in presentation of hospitalized infantile hemangioma cases using the Kids’ Inpatient Database. Health Sci Rep. 2023;6:E1092.
- Chiller KG, Passaro D, Frieden IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol. 2002;138:1567.
- Léauté-Labrèze C, Baselga Torres E, Weibel L, et al. The infantile hemangioma referral score: a validated tool for physicians. Pediatrics. 2020;145:E20191628.
- Macca L, Altavilla D, Di Bartolomeo L, et al. Update on treatment of infantile hemangiomas: what’s new in the last five years? Front Pharmacol. 2022;13:879602.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475.
- Frieden IJ, Püttgen KB, Drolet BA, et al. Management of infantile hemangiomas during the COVID pandemic. Pediatr Dermatol. 2020;37:412-418.
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Early Infantile Hemangioma Diagnosis Is Key in Skin of Color
Cost Analysis of Dermatology Residency Applications From 2021 to 2024 Using the Texas Seeking Transparency in Application to Residency Database
Cost Analysis of Dermatology Residency Applications From 2021 to 2024 Using the Texas Seeking Transparency in Application to Residency Database
To the Editor:
Residency applicants, especially in competitive specialties such as dermatology, face major financial barriers due to the high costs of applications, interviews, and away rotations.1 While several studies have examined application costs of other specialties, few have analyzed expenses associated with dermatology applications.1,2 There are no data examining costs following the start of the COVID-19 pandemic in 2020; thus, our study evaluated dermatology application cost trends from 2021 to 2024 and compared them to other specialties to identify strategies to reduce the financial burden on applicants.
Self-reported total application costs, application fees, interview expenses, and away rotation costs from 2021 to 2024 were collected from the Texas Seeking Transparency in Application to Residency (STAR) database powered by the UT Southwestern Medical Center (Dallas, Texas).3 The mean total application expenses per year were compared among specialties, and an analysis of variance was used to determine if the differences were statistically significant.
The number of applicants who recorded information in the Texas STAR database was 110 in 2021, 163 in 2022, 136 in 2023, and 129 in 2024.3 The total dermatology application expenses increased from $2805 in 2021 to $6231 in 2024; interview costs increased from $404 in 2021 to $911 in 2024; and away rotation costs increased from $850 in 2021 to $3812 in 2024 (all P<.05)(Table). There was no significant change in application fees during the study period ($2176 in 2021 to $2125 in 2024 [P=.58]). Dermatology had the fourth highest average total cost over the study period compared to all other specialties, increasing from $2250 in 2021 to $5250 in 2024, following orthopedic surgery ($2250 in 2021 to $6750 in 2024), plastic surgery ($2250 in 2021 to $9750 in 2024), and neurosurgery ($1750 in 2021 to $11,250 in 2024).

Our study found that dermatology residency application costs have increased significantly from 2021 to 2024, primarily driven by rising interview and away rotation expenses (both P<.05). This trend places dermatology among the most expensive fields to apply to for residency. A cross-sectional survey of dermatology residency program directors identified away rotations as one of the top 5 selection criteria, underscoring their importance in the matching process.4 In addition, a cross-sectional analysis of 345 dermatology residents found that 26.2% matched at institutions where they had mentors, including those they connected with through away rotations.5,6 Overall, the high cost of away rotations partially may reflect the competitive nature of the specialty, as building connections at programs may enhance the chances of matching. These costs also can vary based on geography, as rotating in high-cost urban centers can be more expensive than in rural areas; however, rural rotations may be less common due to limited program availability and applicant preferences. For example, nearly 50% of 2024 Electronic Residency Application Service applicants indicated a preference for urban settings, while fewer than 5% selected rural settings.7 Additionally, the high costs associated with applying to residency programs and completing away rotations can disproportionately impact students from rural backgrounds and underrepresented minorities, who may have fewer financial resources.
In our study, the lower application-related expenses in 2021 (during the pandemic) compared to those of 2024 (postpandemic) likely stem from the Association of American Medical Colleges’ recommendation to conduct virtual interviews during the pandemic.8 In 2024, some dermatology programs returned to in-person interviews, with some applicants consequently incurring higher costs related to travel, lodging, and other associated expenses.8 A cost-analysis study of 4153 dermatology applicants from 2016 to 2021 found that the average application costs were $1759 per applicant during the pandemic, when virtual interviews replaced in-person ones, whereas costs were $8476 per applicant during periods with in-person interviews and no COVID-19 restrictions.2 However, we did not observe a significant change in application fees over our study period, likely because the pandemic did not affect application numbers. A cross-sectional analysis of dermatology applicants during the pandemic similarly reported reductions in application-related expenses during the period when interviews were conducted virtually,9 supporting the trend observed in our study. Overall, our findings taken together with other studies highlight the pandemic’s role in reducing expenses and underscore the potential for exploring additional cost-saving measures.
Implementing strategies to reduce these financial burdens—including virtual interviews, increasing student funding for away rotations, and limiting the number of applications individual students can submit—could help alleviate socioeconomic disparities. The new signaling system for residency programs aims to reduce the number of applications submitted, as applicants typically receive interviews only from the limited number of programs they signal, reducing overall application costs. However, our data from the Texas STAR database suggest that application numbers remained relatively stable from 2021 to 2024, indicating that, despite signaling, many applicants still may apply broadly in hopes of improving their chances in an increasingly competitive field. Although a definitive solution to reducing the financial burden on dermatology applicants remains elusive, these strategies can raise awareness and encourage important dialogues.
Limitations of our study include the voluntary nature of the Texas STAR survey, leading to potential voluntary response bias, as well as the small sample size. Students who choose to submit cost data may differ systematically from those who do not; for example, students who match may be more likely to report their outcomes, while those who do not match may be less likely to participate, potentially introducing selection bias. In addition, general awareness of the Texas STAR survey may vary across institutions and among students, further limiting the number of students who participate. Additionally, 2021 was the only presignaling year included, making it difficult to assess longer-term trends. Despite these limitations, the Texas STAR database remains a valuable resource for analyzing general residency application expenses and trends, as it offers comprehensive data from more than 100 medical schools and includes many variables.3
In conclusion, our study found that total dermatology residency application costs have increased significantly from 2021 to 2024 (all P<.05), making dermatology among the most expensive specialties for applying. This study sets the foundation for future survey-based research for applicants and program directors on strategies to alleviate financial burdens.
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Gorgy M, Shah S, Arbuiso S, et al. Comparison of cost changes due to the COVID-19 pandemic for dermatology residency applications in the USA. Clin Exp Dermatol. 2022;47:600-602. doi:10.1111/ced.15001<.li>
- UT Southwestern. Texas STAR. 2024. Accessed November 5, 2025. https://www.utsouthwestern.edu/education/medical-school/about-the-school/student-affairs/texas-star.html
- Baldwin K, Weidner Z, Ahn J, et al. Are away rotations critical for a successful match in orthopaedic surgery? Clin Orthop Relat Res. 2009;467:3340-3345. doi:10.1007/s11999-009-0920-9
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439. doi:10.1016/j.jaad.2021.06.861
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760. doi:10.1155/2014/692760
- Association of American Medical Colleges. Decoding geographic and setting preferences in residency selection. January 18, 2024. Accessed October 27, 2025. https://www.aamc.org/services/eras-institutions/geographic-preferences
- Association of American Medical Colleges. Virtual interviews: tips for program directors. Updated May 14, 2020. https://med.stanford.edu/content/dam/sm/gme/program_portal/pd/pd_meet/2019-2020/8-6-20-Virtual_Interview_Tips_for_Program_Directors_05142020.pdf
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
To the Editor:
Residency applicants, especially in competitive specialties such as dermatology, face major financial barriers due to the high costs of applications, interviews, and away rotations.1 While several studies have examined application costs of other specialties, few have analyzed expenses associated with dermatology applications.1,2 There are no data examining costs following the start of the COVID-19 pandemic in 2020; thus, our study evaluated dermatology application cost trends from 2021 to 2024 and compared them to other specialties to identify strategies to reduce the financial burden on applicants.
Self-reported total application costs, application fees, interview expenses, and away rotation costs from 2021 to 2024 were collected from the Texas Seeking Transparency in Application to Residency (STAR) database powered by the UT Southwestern Medical Center (Dallas, Texas).3 The mean total application expenses per year were compared among specialties, and an analysis of variance was used to determine if the differences were statistically significant.
The number of applicants who recorded information in the Texas STAR database was 110 in 2021, 163 in 2022, 136 in 2023, and 129 in 2024.3 The total dermatology application expenses increased from $2805 in 2021 to $6231 in 2024; interview costs increased from $404 in 2021 to $911 in 2024; and away rotation costs increased from $850 in 2021 to $3812 in 2024 (all P<.05)(Table). There was no significant change in application fees during the study period ($2176 in 2021 to $2125 in 2024 [P=.58]). Dermatology had the fourth highest average total cost over the study period compared to all other specialties, increasing from $2250 in 2021 to $5250 in 2024, following orthopedic surgery ($2250 in 2021 to $6750 in 2024), plastic surgery ($2250 in 2021 to $9750 in 2024), and neurosurgery ($1750 in 2021 to $11,250 in 2024).

Our study found that dermatology residency application costs have increased significantly from 2021 to 2024, primarily driven by rising interview and away rotation expenses (both P<.05). This trend places dermatology among the most expensive fields to apply to for residency. A cross-sectional survey of dermatology residency program directors identified away rotations as one of the top 5 selection criteria, underscoring their importance in the matching process.4 In addition, a cross-sectional analysis of 345 dermatology residents found that 26.2% matched at institutions where they had mentors, including those they connected with through away rotations.5,6 Overall, the high cost of away rotations partially may reflect the competitive nature of the specialty, as building connections at programs may enhance the chances of matching. These costs also can vary based on geography, as rotating in high-cost urban centers can be more expensive than in rural areas; however, rural rotations may be less common due to limited program availability and applicant preferences. For example, nearly 50% of 2024 Electronic Residency Application Service applicants indicated a preference for urban settings, while fewer than 5% selected rural settings.7 Additionally, the high costs associated with applying to residency programs and completing away rotations can disproportionately impact students from rural backgrounds and underrepresented minorities, who may have fewer financial resources.
In our study, the lower application-related expenses in 2021 (during the pandemic) compared to those of 2024 (postpandemic) likely stem from the Association of American Medical Colleges’ recommendation to conduct virtual interviews during the pandemic.8 In 2024, some dermatology programs returned to in-person interviews, with some applicants consequently incurring higher costs related to travel, lodging, and other associated expenses.8 A cost-analysis study of 4153 dermatology applicants from 2016 to 2021 found that the average application costs were $1759 per applicant during the pandemic, when virtual interviews replaced in-person ones, whereas costs were $8476 per applicant during periods with in-person interviews and no COVID-19 restrictions.2 However, we did not observe a significant change in application fees over our study period, likely because the pandemic did not affect application numbers. A cross-sectional analysis of dermatology applicants during the pandemic similarly reported reductions in application-related expenses during the period when interviews were conducted virtually,9 supporting the trend observed in our study. Overall, our findings taken together with other studies highlight the pandemic’s role in reducing expenses and underscore the potential for exploring additional cost-saving measures.
Implementing strategies to reduce these financial burdens—including virtual interviews, increasing student funding for away rotations, and limiting the number of applications individual students can submit—could help alleviate socioeconomic disparities. The new signaling system for residency programs aims to reduce the number of applications submitted, as applicants typically receive interviews only from the limited number of programs they signal, reducing overall application costs. However, our data from the Texas STAR database suggest that application numbers remained relatively stable from 2021 to 2024, indicating that, despite signaling, many applicants still may apply broadly in hopes of improving their chances in an increasingly competitive field. Although a definitive solution to reducing the financial burden on dermatology applicants remains elusive, these strategies can raise awareness and encourage important dialogues.
Limitations of our study include the voluntary nature of the Texas STAR survey, leading to potential voluntary response bias, as well as the small sample size. Students who choose to submit cost data may differ systematically from those who do not; for example, students who match may be more likely to report their outcomes, while those who do not match may be less likely to participate, potentially introducing selection bias. In addition, general awareness of the Texas STAR survey may vary across institutions and among students, further limiting the number of students who participate. Additionally, 2021 was the only presignaling year included, making it difficult to assess longer-term trends. Despite these limitations, the Texas STAR database remains a valuable resource for analyzing general residency application expenses and trends, as it offers comprehensive data from more than 100 medical schools and includes many variables.3
In conclusion, our study found that total dermatology residency application costs have increased significantly from 2021 to 2024 (all P<.05), making dermatology among the most expensive specialties for applying. This study sets the foundation for future survey-based research for applicants and program directors on strategies to alleviate financial burdens.
To the Editor:
Residency applicants, especially in competitive specialties such as dermatology, face major financial barriers due to the high costs of applications, interviews, and away rotations.1 While several studies have examined application costs of other specialties, few have analyzed expenses associated with dermatology applications.1,2 There are no data examining costs following the start of the COVID-19 pandemic in 2020; thus, our study evaluated dermatology application cost trends from 2021 to 2024 and compared them to other specialties to identify strategies to reduce the financial burden on applicants.
Self-reported total application costs, application fees, interview expenses, and away rotation costs from 2021 to 2024 were collected from the Texas Seeking Transparency in Application to Residency (STAR) database powered by the UT Southwestern Medical Center (Dallas, Texas).3 The mean total application expenses per year were compared among specialties, and an analysis of variance was used to determine if the differences were statistically significant.
The number of applicants who recorded information in the Texas STAR database was 110 in 2021, 163 in 2022, 136 in 2023, and 129 in 2024.3 The total dermatology application expenses increased from $2805 in 2021 to $6231 in 2024; interview costs increased from $404 in 2021 to $911 in 2024; and away rotation costs increased from $850 in 2021 to $3812 in 2024 (all P<.05)(Table). There was no significant change in application fees during the study period ($2176 in 2021 to $2125 in 2024 [P=.58]). Dermatology had the fourth highest average total cost over the study period compared to all other specialties, increasing from $2250 in 2021 to $5250 in 2024, following orthopedic surgery ($2250 in 2021 to $6750 in 2024), plastic surgery ($2250 in 2021 to $9750 in 2024), and neurosurgery ($1750 in 2021 to $11,250 in 2024).

Our study found that dermatology residency application costs have increased significantly from 2021 to 2024, primarily driven by rising interview and away rotation expenses (both P<.05). This trend places dermatology among the most expensive fields to apply to for residency. A cross-sectional survey of dermatology residency program directors identified away rotations as one of the top 5 selection criteria, underscoring their importance in the matching process.4 In addition, a cross-sectional analysis of 345 dermatology residents found that 26.2% matched at institutions where they had mentors, including those they connected with through away rotations.5,6 Overall, the high cost of away rotations partially may reflect the competitive nature of the specialty, as building connections at programs may enhance the chances of matching. These costs also can vary based on geography, as rotating in high-cost urban centers can be more expensive than in rural areas; however, rural rotations may be less common due to limited program availability and applicant preferences. For example, nearly 50% of 2024 Electronic Residency Application Service applicants indicated a preference for urban settings, while fewer than 5% selected rural settings.7 Additionally, the high costs associated with applying to residency programs and completing away rotations can disproportionately impact students from rural backgrounds and underrepresented minorities, who may have fewer financial resources.
In our study, the lower application-related expenses in 2021 (during the pandemic) compared to those of 2024 (postpandemic) likely stem from the Association of American Medical Colleges’ recommendation to conduct virtual interviews during the pandemic.8 In 2024, some dermatology programs returned to in-person interviews, with some applicants consequently incurring higher costs related to travel, lodging, and other associated expenses.8 A cost-analysis study of 4153 dermatology applicants from 2016 to 2021 found that the average application costs were $1759 per applicant during the pandemic, when virtual interviews replaced in-person ones, whereas costs were $8476 per applicant during periods with in-person interviews and no COVID-19 restrictions.2 However, we did not observe a significant change in application fees over our study period, likely because the pandemic did not affect application numbers. A cross-sectional analysis of dermatology applicants during the pandemic similarly reported reductions in application-related expenses during the period when interviews were conducted virtually,9 supporting the trend observed in our study. Overall, our findings taken together with other studies highlight the pandemic’s role in reducing expenses and underscore the potential for exploring additional cost-saving measures.
Implementing strategies to reduce these financial burdens—including virtual interviews, increasing student funding for away rotations, and limiting the number of applications individual students can submit—could help alleviate socioeconomic disparities. The new signaling system for residency programs aims to reduce the number of applications submitted, as applicants typically receive interviews only from the limited number of programs they signal, reducing overall application costs. However, our data from the Texas STAR database suggest that application numbers remained relatively stable from 2021 to 2024, indicating that, despite signaling, many applicants still may apply broadly in hopes of improving their chances in an increasingly competitive field. Although a definitive solution to reducing the financial burden on dermatology applicants remains elusive, these strategies can raise awareness and encourage important dialogues.
Limitations of our study include the voluntary nature of the Texas STAR survey, leading to potential voluntary response bias, as well as the small sample size. Students who choose to submit cost data may differ systematically from those who do not; for example, students who match may be more likely to report their outcomes, while those who do not match may be less likely to participate, potentially introducing selection bias. In addition, general awareness of the Texas STAR survey may vary across institutions and among students, further limiting the number of students who participate. Additionally, 2021 was the only presignaling year included, making it difficult to assess longer-term trends. Despite these limitations, the Texas STAR database remains a valuable resource for analyzing general residency application expenses and trends, as it offers comprehensive data from more than 100 medical schools and includes many variables.3
In conclusion, our study found that total dermatology residency application costs have increased significantly from 2021 to 2024 (all P<.05), making dermatology among the most expensive specialties for applying. This study sets the foundation for future survey-based research for applicants and program directors on strategies to alleviate financial burdens.
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Gorgy M, Shah S, Arbuiso S, et al. Comparison of cost changes due to the COVID-19 pandemic for dermatology residency applications in the USA. Clin Exp Dermatol. 2022;47:600-602. doi:10.1111/ced.15001<.li>
- UT Southwestern. Texas STAR. 2024. Accessed November 5, 2025. https://www.utsouthwestern.edu/education/medical-school/about-the-school/student-affairs/texas-star.html
- Baldwin K, Weidner Z, Ahn J, et al. Are away rotations critical for a successful match in orthopaedic surgery? Clin Orthop Relat Res. 2009;467:3340-3345. doi:10.1007/s11999-009-0920-9
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439. doi:10.1016/j.jaad.2021.06.861
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760. doi:10.1155/2014/692760
- Association of American Medical Colleges. Decoding geographic and setting preferences in residency selection. January 18, 2024. Accessed October 27, 2025. https://www.aamc.org/services/eras-institutions/geographic-preferences
- Association of American Medical Colleges. Virtual interviews: tips for program directors. Updated May 14, 2020. https://med.stanford.edu/content/dam/sm/gme/program_portal/pd/pd_meet/2019-2020/8-6-20-Virtual_Interview_Tips_for_Program_Directors_05142020.pdf
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Gorgy M, Shah S, Arbuiso S, et al. Comparison of cost changes due to the COVID-19 pandemic for dermatology residency applications in the USA. Clin Exp Dermatol. 2022;47:600-602. doi:10.1111/ced.15001<.li>
- UT Southwestern. Texas STAR. 2024. Accessed November 5, 2025. https://www.utsouthwestern.edu/education/medical-school/about-the-school/student-affairs/texas-star.html
- Baldwin K, Weidner Z, Ahn J, et al. Are away rotations critical for a successful match in orthopaedic surgery? Clin Orthop Relat Res. 2009;467:3340-3345. doi:10.1007/s11999-009-0920-9
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439. doi:10.1016/j.jaad.2021.06.861
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760. doi:10.1155/2014/692760
- Association of American Medical Colleges. Decoding geographic and setting preferences in residency selection. January 18, 2024. Accessed October 27, 2025. https://www.aamc.org/services/eras-institutions/geographic-preferences
- Association of American Medical Colleges. Virtual interviews: tips for program directors. Updated May 14, 2020. https://med.stanford.edu/content/dam/sm/gme/program_portal/pd/pd_meet/2019-2020/8-6-20-Virtual_Interview_Tips_for_Program_Directors_05142020.pdf
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
Cost Analysis of Dermatology Residency Applications From 2021 to 2024 Using the Texas Seeking Transparency in Application to Residency Database
Cost Analysis of Dermatology Residency Applications From 2021 to 2024 Using the Texas Seeking Transparency in Application to Residency Database
PRACTICE POINTS
- Dermatology application costs increased from 2021 to 2024, largely due to expenses related to away rotations and, in some cases, a return to in-person interviews.
- Away rotations play a critical role in the dermatology match; however, they also contribute substantially to financial burden.
- The cost-saving impact of virtual interviews during the COVID-19 pandemic highlights a meaningful opportunity for future cost reduction.
- Further interventions are needed to meaningfully reduce financial burden and promote equity.
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Pediatric alopecia areata (AA) is a chronic autoimmune disease of the hair follicles characterized by nonscarring hair loss. Its incidence in children in the United States ranges from 13.6 to 33.5 per 100,000 person-years, with a prevalence of 0.04% to 0.11%.1 Alopecia areata has important effects on quality of life, particularly in children. Hair loss at an early age can decrease participation in school, sports, and extracurricular activities2 and is associated with increased rates of comorbid anxiety and depression.3 Families also experience psychosocial stress, often comparable to other chronic pediatric illnesses.4 Thus, management requires not only medical therapy but also psychosocial support and school-based accommodations.
Systemic therapies for treatment of AA in adolescents and adults are increasingly available, including US Food and Drug Administration (FDA)–approved Janus kinase (JAK) inhibitors such as baricitinib, deuruxolitinib (for adults), and ritlecitinib (for adolescents and adults); however, no systemic therapies have been approved by the FDA for children younger than 12 years. The therapeutic gap is most acute for those aged 6 to 11 years, for whom the psychosocial burden is high but treatment options are limited.3
This article highlights options and strategies for managing AA in children aged 6 to 11 years, emphasizing supportive and psychosocial care (including camouflage techniques), topical therapies, and off-label systemic approaches.
Supportive and Psychosocial Care
Treatment of AA in children extends beyond the affected child to include parents, caregivers, and even school staff (eg, teachers, principals, nurses).4 Disease-specific organizations such as the National Alopecia Areata Foundation (naaf.org) and the Children’s Alopecia Project (childrensalopeciaproject.org) provide education, support groups, and advocacy resources. These organizations assist families in navigating school accommodations, including Section 504 plans that may allow children with AA to wear hats in school to mitigate stigma. Additional resources include handouts for teachers and school nurses developed by the Society for Pediatric Dermatology.5
Psychological support for these patients is critical. Many children benefit from seeing a psychologist, particularly if anxiety, depression, and/or bullying is present.3 In clinics without embedded psychology services, dermatologists should maintain referral lists or encourage families to seek guidance from their pediatrician.
Camouflage techniques can help children cope with visible hair loss. Wigs and hairpieces are available free of charge through charitable organizations for patients younger than 17; however, young children often find adhesives uncomfortable, and they will not wear nonadherent wigs for long periods of time. Alternatives include soft hats, bonnets, scarves, and beanies. For partial hair loss, root concealers, scalp powders, or hair mascara can be useful. Temporary eyebrow tattoos are a good cosmetic approach, whereas microblading generally is not advised in children younger than 12 due to procedural risks including pain.
Topical Therapies
Topical agents remain the mainstay of treatment for AA in children aged 6 to 11 years. Potent class 1 or class 2 topical corticosteroids commonly are used, sometimes in combination with calcineurin inhibitors or topical minoxidil. Off-label compounded topical JAK inhibitors also have been tried in this population and may be helpful for eyebrow hair loss,6 though data on their efficacy for scalp AA are mixed.7 Intralesional corticosteroid injections, effective in adolescents and adults, generally are poorly tolerated by younger children and may cause considerable distress. Contact immunotherapy with squaric acid dibutyl ester or anthralin can be considered, but these agents are designed to elicit irritation, which may be intolerable for young children.8 Shared decision-making with families is essential to balance efficacy, tolerability, and treatment burden.
Systemic Therapies
Systemic therapy generally is reserved for children with extensive or refractory AA. Low-dose oral minoxidil is emerging as an off-label option. One systematic review reported that low-dose oral minoxidil was well tolerated in pediatric patients with minimal adverse effects.9 Doses of 0.01 to 0.02 mg/kg/d are reasonable starting points, achieved by cutting tablets or compounding oral solutions.10
In children with AA and concurrent atopic dermatitis, dupilumab may offer dual benefit. A real-world observational study demonstrated hair regrowth in pediatric patients with AA treated with dupilumab.11 Immunosuppressive options such as low-dose methotrexate or pulse corticosteroids (dexamethasone or prednisolone) also may be considered, although use of these agents requires careful monitoring due to increased risk for infection, clinically significant blood count and liver enzyme changes, and metabolic adverse effects related to long-term use of corticosteroids.
Clinical trials of JAK inhibitors in children aged 6 to 11 years are anticipated to begin in late 2025. Until then, off-label use of ritlecitinib, baricitinib, tofacitinib, or other JAK inhibitors may be considered in select cases with considerable disease burden and quality-of-life impairment following thorough discussion with the patient and their caregivers. Currently available pediatric data show few serious adverse events in children—the most common included upper respiratory infections (nasopharyngitis), acne, and headaches—but long-term risks remain unknown. Dosing challenges also exist for children who cannot swallow pills; currently ritlecitinib is available only as a capsule that cannot be opened while other JAK inhibitors are available in more accessible forms (baricitinib can be crushed and dissolved, and tofacitinib is available in liquid formulation for other pediatric indications). Insurance coverage is a major barrier, as these therapies are not FDA approved for AA in this age group.
Final Thoughts
Alopecia areata in children aged 6 to 11 years presents unique therapeutic challenges. While highly effective systemic therapies exist for older patients, younger children have limited options. For the 6-to-11 age group, management strategies should prioritize psychosocial support, topical therapy, and low-burden systemic alternatives such as low-dose oral minoxidil. Family education, school-based accommodations, and access to camouflage techniques are integral to holistic care. The commencement of pediatric clinical trials for JAK inhibitors offers hope for more robust treatment strategies in the near future. In the meantime, clinicians must engage in shared decision-making, tailoring therapy to the child’s disease severity, emotional well-being, and family priorities.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42(suppl 1):12-23. doi:10.1111/pde.15803
- Paller AS, Rangel SM, Chamlin SL, et al; Pediatric Dermatology Research Alliance. Stigmatization and mental health impact of chronic pediatric skin disorders. JAMA Dermatol. 2024;160:621-630.
- van Dalen M, Muller KS, Kasperkovitz-Oosterloo JM, et al. Anxiety, depression, and quality of life in children and adults with alopecia areata: systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1054898.
- Yücesoy SN, Uzunçakmak TK, Selçukog?lu Ö, et al. Evaluation of quality of life scores and family impact scales in pediatric patients with alopecia areata: a cross-sectional cohort study. Int J Dermatol. 2024;63:1414-1420.
- Alopecia areata. Society for Pediatric Dermatology. Accessed November 17, 2025. https://pedsderm.net/site/assets/files/18580/spd_school_handout_1_alopecia.pdf
- Liu LY, King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019;80:1778-1779.
- Bokhari L, Sinclair R. Treatment of alopecia universalis with topical Janus kinase inhibitors—a double blind, placebo, and active controlled pilot study. Int J Dermatol. 2018;57:1464-1470.
- Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33:300-304.
- Williams KN, Olukoga CTY, Tosti A. Evaluation of the safety and effectiveness of oral minoxidil in children: a systematic review. Dermatol Ther (Heidelb). 2024;14:1709-1727.
- Lemes LR, Melo DF, de Oliveira DS, et al. Topical and oral minoxidil for hair disorders in pediatric patients: what do we know so far? Dermatol Ther. 2020;33:E13950.
- David E, Shokrian N, Del Duca E, et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res. 2024;316:487.
Pediatric alopecia areata (AA) is a chronic autoimmune disease of the hair follicles characterized by nonscarring hair loss. Its incidence in children in the United States ranges from 13.6 to 33.5 per 100,000 person-years, with a prevalence of 0.04% to 0.11%.1 Alopecia areata has important effects on quality of life, particularly in children. Hair loss at an early age can decrease participation in school, sports, and extracurricular activities2 and is associated with increased rates of comorbid anxiety and depression.3 Families also experience psychosocial stress, often comparable to other chronic pediatric illnesses.4 Thus, management requires not only medical therapy but also psychosocial support and school-based accommodations.
Systemic therapies for treatment of AA in adolescents and adults are increasingly available, including US Food and Drug Administration (FDA)–approved Janus kinase (JAK) inhibitors such as baricitinib, deuruxolitinib (for adults), and ritlecitinib (for adolescents and adults); however, no systemic therapies have been approved by the FDA for children younger than 12 years. The therapeutic gap is most acute for those aged 6 to 11 years, for whom the psychosocial burden is high but treatment options are limited.3
This article highlights options and strategies for managing AA in children aged 6 to 11 years, emphasizing supportive and psychosocial care (including camouflage techniques), topical therapies, and off-label systemic approaches.
Supportive and Psychosocial Care
Treatment of AA in children extends beyond the affected child to include parents, caregivers, and even school staff (eg, teachers, principals, nurses).4 Disease-specific organizations such as the National Alopecia Areata Foundation (naaf.org) and the Children’s Alopecia Project (childrensalopeciaproject.org) provide education, support groups, and advocacy resources. These organizations assist families in navigating school accommodations, including Section 504 plans that may allow children with AA to wear hats in school to mitigate stigma. Additional resources include handouts for teachers and school nurses developed by the Society for Pediatric Dermatology.5
Psychological support for these patients is critical. Many children benefit from seeing a psychologist, particularly if anxiety, depression, and/or bullying is present.3 In clinics without embedded psychology services, dermatologists should maintain referral lists or encourage families to seek guidance from their pediatrician.
Camouflage techniques can help children cope with visible hair loss. Wigs and hairpieces are available free of charge through charitable organizations for patients younger than 17; however, young children often find adhesives uncomfortable, and they will not wear nonadherent wigs for long periods of time. Alternatives include soft hats, bonnets, scarves, and beanies. For partial hair loss, root concealers, scalp powders, or hair mascara can be useful. Temporary eyebrow tattoos are a good cosmetic approach, whereas microblading generally is not advised in children younger than 12 due to procedural risks including pain.
Topical Therapies
Topical agents remain the mainstay of treatment for AA in children aged 6 to 11 years. Potent class 1 or class 2 topical corticosteroids commonly are used, sometimes in combination with calcineurin inhibitors or topical minoxidil. Off-label compounded topical JAK inhibitors also have been tried in this population and may be helpful for eyebrow hair loss,6 though data on their efficacy for scalp AA are mixed.7 Intralesional corticosteroid injections, effective in adolescents and adults, generally are poorly tolerated by younger children and may cause considerable distress. Contact immunotherapy with squaric acid dibutyl ester or anthralin can be considered, but these agents are designed to elicit irritation, which may be intolerable for young children.8 Shared decision-making with families is essential to balance efficacy, tolerability, and treatment burden.
Systemic Therapies
Systemic therapy generally is reserved for children with extensive or refractory AA. Low-dose oral minoxidil is emerging as an off-label option. One systematic review reported that low-dose oral minoxidil was well tolerated in pediatric patients with minimal adverse effects.9 Doses of 0.01 to 0.02 mg/kg/d are reasonable starting points, achieved by cutting tablets or compounding oral solutions.10
In children with AA and concurrent atopic dermatitis, dupilumab may offer dual benefit. A real-world observational study demonstrated hair regrowth in pediatric patients with AA treated with dupilumab.11 Immunosuppressive options such as low-dose methotrexate or pulse corticosteroids (dexamethasone or prednisolone) also may be considered, although use of these agents requires careful monitoring due to increased risk for infection, clinically significant blood count and liver enzyme changes, and metabolic adverse effects related to long-term use of corticosteroids.
Clinical trials of JAK inhibitors in children aged 6 to 11 years are anticipated to begin in late 2025. Until then, off-label use of ritlecitinib, baricitinib, tofacitinib, or other JAK inhibitors may be considered in select cases with considerable disease burden and quality-of-life impairment following thorough discussion with the patient and their caregivers. Currently available pediatric data show few serious adverse events in children—the most common included upper respiratory infections (nasopharyngitis), acne, and headaches—but long-term risks remain unknown. Dosing challenges also exist for children who cannot swallow pills; currently ritlecitinib is available only as a capsule that cannot be opened while other JAK inhibitors are available in more accessible forms (baricitinib can be crushed and dissolved, and tofacitinib is available in liquid formulation for other pediatric indications). Insurance coverage is a major barrier, as these therapies are not FDA approved for AA in this age group.
Final Thoughts
Alopecia areata in children aged 6 to 11 years presents unique therapeutic challenges. While highly effective systemic therapies exist for older patients, younger children have limited options. For the 6-to-11 age group, management strategies should prioritize psychosocial support, topical therapy, and low-burden systemic alternatives such as low-dose oral minoxidil. Family education, school-based accommodations, and access to camouflage techniques are integral to holistic care. The commencement of pediatric clinical trials for JAK inhibitors offers hope for more robust treatment strategies in the near future. In the meantime, clinicians must engage in shared decision-making, tailoring therapy to the child’s disease severity, emotional well-being, and family priorities.
Pediatric alopecia areata (AA) is a chronic autoimmune disease of the hair follicles characterized by nonscarring hair loss. Its incidence in children in the United States ranges from 13.6 to 33.5 per 100,000 person-years, with a prevalence of 0.04% to 0.11%.1 Alopecia areata has important effects on quality of life, particularly in children. Hair loss at an early age can decrease participation in school, sports, and extracurricular activities2 and is associated with increased rates of comorbid anxiety and depression.3 Families also experience psychosocial stress, often comparable to other chronic pediatric illnesses.4 Thus, management requires not only medical therapy but also psychosocial support and school-based accommodations.
Systemic therapies for treatment of AA in adolescents and adults are increasingly available, including US Food and Drug Administration (FDA)–approved Janus kinase (JAK) inhibitors such as baricitinib, deuruxolitinib (for adults), and ritlecitinib (for adolescents and adults); however, no systemic therapies have been approved by the FDA for children younger than 12 years. The therapeutic gap is most acute for those aged 6 to 11 years, for whom the psychosocial burden is high but treatment options are limited.3
This article highlights options and strategies for managing AA in children aged 6 to 11 years, emphasizing supportive and psychosocial care (including camouflage techniques), topical therapies, and off-label systemic approaches.
Supportive and Psychosocial Care
Treatment of AA in children extends beyond the affected child to include parents, caregivers, and even school staff (eg, teachers, principals, nurses).4 Disease-specific organizations such as the National Alopecia Areata Foundation (naaf.org) and the Children’s Alopecia Project (childrensalopeciaproject.org) provide education, support groups, and advocacy resources. These organizations assist families in navigating school accommodations, including Section 504 plans that may allow children with AA to wear hats in school to mitigate stigma. Additional resources include handouts for teachers and school nurses developed by the Society for Pediatric Dermatology.5
Psychological support for these patients is critical. Many children benefit from seeing a psychologist, particularly if anxiety, depression, and/or bullying is present.3 In clinics without embedded psychology services, dermatologists should maintain referral lists or encourage families to seek guidance from their pediatrician.
Camouflage techniques can help children cope with visible hair loss. Wigs and hairpieces are available free of charge through charitable organizations for patients younger than 17; however, young children often find adhesives uncomfortable, and they will not wear nonadherent wigs for long periods of time. Alternatives include soft hats, bonnets, scarves, and beanies. For partial hair loss, root concealers, scalp powders, or hair mascara can be useful. Temporary eyebrow tattoos are a good cosmetic approach, whereas microblading generally is not advised in children younger than 12 due to procedural risks including pain.
Topical Therapies
Topical agents remain the mainstay of treatment for AA in children aged 6 to 11 years. Potent class 1 or class 2 topical corticosteroids commonly are used, sometimes in combination with calcineurin inhibitors or topical minoxidil. Off-label compounded topical JAK inhibitors also have been tried in this population and may be helpful for eyebrow hair loss,6 though data on their efficacy for scalp AA are mixed.7 Intralesional corticosteroid injections, effective in adolescents and adults, generally are poorly tolerated by younger children and may cause considerable distress. Contact immunotherapy with squaric acid dibutyl ester or anthralin can be considered, but these agents are designed to elicit irritation, which may be intolerable for young children.8 Shared decision-making with families is essential to balance efficacy, tolerability, and treatment burden.
Systemic Therapies
Systemic therapy generally is reserved for children with extensive or refractory AA. Low-dose oral minoxidil is emerging as an off-label option. One systematic review reported that low-dose oral minoxidil was well tolerated in pediatric patients with minimal adverse effects.9 Doses of 0.01 to 0.02 mg/kg/d are reasonable starting points, achieved by cutting tablets or compounding oral solutions.10
In children with AA and concurrent atopic dermatitis, dupilumab may offer dual benefit. A real-world observational study demonstrated hair regrowth in pediatric patients with AA treated with dupilumab.11 Immunosuppressive options such as low-dose methotrexate or pulse corticosteroids (dexamethasone or prednisolone) also may be considered, although use of these agents requires careful monitoring due to increased risk for infection, clinically significant blood count and liver enzyme changes, and metabolic adverse effects related to long-term use of corticosteroids.
Clinical trials of JAK inhibitors in children aged 6 to 11 years are anticipated to begin in late 2025. Until then, off-label use of ritlecitinib, baricitinib, tofacitinib, or other JAK inhibitors may be considered in select cases with considerable disease burden and quality-of-life impairment following thorough discussion with the patient and their caregivers. Currently available pediatric data show few serious adverse events in children—the most common included upper respiratory infections (nasopharyngitis), acne, and headaches—but long-term risks remain unknown. Dosing challenges also exist for children who cannot swallow pills; currently ritlecitinib is available only as a capsule that cannot be opened while other JAK inhibitors are available in more accessible forms (baricitinib can be crushed and dissolved, and tofacitinib is available in liquid formulation for other pediatric indications). Insurance coverage is a major barrier, as these therapies are not FDA approved for AA in this age group.
Final Thoughts
Alopecia areata in children aged 6 to 11 years presents unique therapeutic challenges. While highly effective systemic therapies exist for older patients, younger children have limited options. For the 6-to-11 age group, management strategies should prioritize psychosocial support, topical therapy, and low-burden systemic alternatives such as low-dose oral minoxidil. Family education, school-based accommodations, and access to camouflage techniques are integral to holistic care. The commencement of pediatric clinical trials for JAK inhibitors offers hope for more robust treatment strategies in the near future. In the meantime, clinicians must engage in shared decision-making, tailoring therapy to the child’s disease severity, emotional well-being, and family priorities.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42(suppl 1):12-23. doi:10.1111/pde.15803
- Paller AS, Rangel SM, Chamlin SL, et al; Pediatric Dermatology Research Alliance. Stigmatization and mental health impact of chronic pediatric skin disorders. JAMA Dermatol. 2024;160:621-630.
- van Dalen M, Muller KS, Kasperkovitz-Oosterloo JM, et al. Anxiety, depression, and quality of life in children and adults with alopecia areata: systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1054898.
- Yücesoy SN, Uzunçakmak TK, Selçukog?lu Ö, et al. Evaluation of quality of life scores and family impact scales in pediatric patients with alopecia areata: a cross-sectional cohort study. Int J Dermatol. 2024;63:1414-1420.
- Alopecia areata. Society for Pediatric Dermatology. Accessed November 17, 2025. https://pedsderm.net/site/assets/files/18580/spd_school_handout_1_alopecia.pdf
- Liu LY, King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019;80:1778-1779.
- Bokhari L, Sinclair R. Treatment of alopecia universalis with topical Janus kinase inhibitors—a double blind, placebo, and active controlled pilot study. Int J Dermatol. 2018;57:1464-1470.
- Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33:300-304.
- Williams KN, Olukoga CTY, Tosti A. Evaluation of the safety and effectiveness of oral minoxidil in children: a systematic review. Dermatol Ther (Heidelb). 2024;14:1709-1727.
- Lemes LR, Melo DF, de Oliveira DS, et al. Topical and oral minoxidil for hair disorders in pediatric patients: what do we know so far? Dermatol Ther. 2020;33:E13950.
- David E, Shokrian N, Del Duca E, et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res. 2024;316:487.
- Adhanom R, Ansbro B, Castelo-Soccio L. Epidemiology of pediatric alopecia areata. Pediatr Dermatol. 2025;42(suppl 1):12-23. doi:10.1111/pde.15803
- Paller AS, Rangel SM, Chamlin SL, et al; Pediatric Dermatology Research Alliance. Stigmatization and mental health impact of chronic pediatric skin disorders. JAMA Dermatol. 2024;160:621-630.
- van Dalen M, Muller KS, Kasperkovitz-Oosterloo JM, et al. Anxiety, depression, and quality of life in children and adults with alopecia areata: systematic review and meta-analysis. Front Med (Lausanne). 2022;9:1054898.
- Yücesoy SN, Uzunçakmak TK, Selçukog?lu Ö, et al. Evaluation of quality of life scores and family impact scales in pediatric patients with alopecia areata: a cross-sectional cohort study. Int J Dermatol. 2024;63:1414-1420.
- Alopecia areata. Society for Pediatric Dermatology. Accessed November 17, 2025. https://pedsderm.net/site/assets/files/18580/spd_school_handout_1_alopecia.pdf
- Liu LY, King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. 2019;80:1778-1779.
- Bokhari L, Sinclair R. Treatment of alopecia universalis with topical Janus kinase inhibitors—a double blind, placebo, and active controlled pilot study. Int J Dermatol. 2018;57:1464-1470.
- Hill ND, Bunata K, Hebert AA. Treatment of alopecia areata with squaric acid dibutylester. Clin Dermatol. 2015;33:300-304.
- Williams KN, Olukoga CTY, Tosti A. Evaluation of the safety and effectiveness of oral minoxidil in children: a systematic review. Dermatol Ther (Heidelb). 2024;14:1709-1727.
- Lemes LR, Melo DF, de Oliveira DS, et al. Topical and oral minoxidil for hair disorders in pediatric patients: what do we know so far? Dermatol Ther. 2020;33:E13950.
- David E, Shokrian N, Del Duca E, et al. Dupilumab induces hair regrowth in pediatric alopecia areata: a real-world, single-center observational study. Arch Dermatol Res. 2024;316:487.
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Therapeutic Approaches for Alopecia Areata in Children Aged 6 to 11 Years
Solitary Yellow Papule on the Upper Back in an Infant
The Diagnosis: Juvenile Xanthogranuloma
Given the patient’s age, clinical features of the lesion, and characteristic setting-sun pattern on dermoscopy, a diagnosis of juvenile xanthogranuloma (JXG) was made. The patient showed no other signs of neurofibromatosis type 1 (NF1) or systemic disease and was managed conservatively with observation and routine follow-up. Minimal growth of the lesion was noted at 1-year follow-up, and he was meeting all age-appropriate developmental milestones and showed no other symptoms consistent with NF1.
Juvenile xanthogranuloma is the most common childhood non–Langerhans cell histiocytosis. While it typically manifests as an isolated condition, JXG also can be associated with NF1 as well as juvenile myelomonocytic leukemia.1-3 Neurofibromatosis type 1 is a multisystem disorder with variable clinical manifestations that commonly is associated with skin findings such as café au lait macules, intertriginous freckling, and neurofibromas, in addition to JXG.2,3 Diagnosis of JXG should prompt noninvasive evaluation for further signs and symptoms of NF1, including thorough patient and family history and physical examination to identify other characteristic cutaneous findings, and can include consideration of slit lamp eye examination and radiography for identification of osseous findings.
The pathogenesis of JXG is not fully known, though there is evidence that it may be associated with a mutation in the mitogen-activated protein kinase pathway.1 The majority of cases appear in the first year of life.4 Clinically, JXG can manifest with extracutaneous lesions, including on the eyes and lungs.5-7 Juvenile xanthogranuloma can be noninvasively diagnosed with dermoscopy. As seen in our patient, dermoscopic findings include a red-yellow or yellow-orange background with an erythematous border, typically described as a setting-sun pattern.4,8 Biopsy can confirm the diagnosis; however, given the usually benign course, this often is unnecessary. Most pediatric patients with cutaneous manifestations have a self-limited course with regression over several months to years. Generally, no treatment is required for cutaneous manifestations alone; however, lesions can be removed for aesthetic concerns. For those with systemic involvement, a range of other treatments have been used, including chemotherapy, radiotherapy, systemic corticosteroids, and cyclosporine.6,7
The differential diagnosis for JXG includes Brooke-Spiegler syndrome, Fabry disease, solitary cutaneous mastocytoma, and tuberous sclerosis complex. Brooke-Spiegler syndrome is an autosomal-dominant condition characterized by the growth of adnexal neoplasms, including trichoepitheliomas, cylindromas, and spiradenomas. These lesions usually manifest on the face but can include other areas such as the trunk.9 Fabry disease is an X-linked recessive lysosomal storage disorder with cutaneous manifestations such as angiokeratoma corporis diffusum and hypohidrosis. Patients also may present with systemic symptoms including hypertension and renal and cardiovascular disease.10 Mastocytosis encompasses several clinical disorders defined by mast cell hyperplasia and accumulation in various organ systems, and solitary cutaneous mastocytoma is the most common manifestation in children.11,12 Cutaneous mastocytoma can manifest as a single red-brown or yellow papule, usually located on the arms or legs.13 Solitary cutaneous mastocytomas in pediatric patients typically are diagnosed based on clinical appearance and the formation of a wheal upon firm palpation (Darier sign).11-13 Our patient did not demonstrate the Darier sign, and the lesion was asymptomatic. Tuberous sclerosis complex is an autosomal-dominant neurocutaneous disorder with neurologic and skin findings that occur early in the disease course and include facial angiofibromas, hypomelanotic macules, shagreen patches, and café-au-lait macules.14
- Durham BH, Lopez Rodrigo E, Picarsic J, et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med. 2019;25:1839-1842.
- Friedman JM. Neurofibromatosis 1. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1998.
- Miraglia E, Laghi A, Moramarco A, et al. Juvenile xanthogranuloma in neurofibromatosis type 1. Prevalence and possible correlation with lymphoproliferative diseases: experience of a single center and review of the literature. Clin Ther. 2022;173:353-355.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 8, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526103/
- Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
- Freyer DR, Kennedy R, Bostrom BC, et al. Juvenile xanthogranuloma: forms of systemic disease and their clinical implications. J Pediatr. 1996;129:227-237.
- Murphy JT, Soeken T, Megison S, et al. Juvenile xanthogranuloma: diverse presentations of noncutaneous disease. J Pediatr Hematol Oncol.2014;36:641-645.
- Xu J, Ma L. Dermoscopic patterns in juvenile xanthogranuloma based on the histological classification. Front Med (Lausanne). 2021;7:618946.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bokhari SRA, Zulfiqar H, Hariz A. Fabry disease. StatPearls [Internet]. StatPearls Publishing; 2025. Update July 4, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK435996/
- Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35-45.
- Klaiber N, Kumar S, Irani AM. Mastocytosis in children. Curr Allergy Asthma Rep. 2017;17:80.
- Sławin´ ska M, Kaszuba A, Lange M, et al. Dermoscopic features of different forms of cutaneous mastocytosis: a systematic review. J Clin Med. 2022;11:4649.
- Teng JM, Cowen EW, Wataya-Kaneda M, et al. Dermatologic and dental aspects of the 2012 International Tuberous Sclerosis Complex Consensus Statements. JAMA Dermatol. 2014;150:1095-1101.
The Diagnosis: Juvenile Xanthogranuloma
Given the patient’s age, clinical features of the lesion, and characteristic setting-sun pattern on dermoscopy, a diagnosis of juvenile xanthogranuloma (JXG) was made. The patient showed no other signs of neurofibromatosis type 1 (NF1) or systemic disease and was managed conservatively with observation and routine follow-up. Minimal growth of the lesion was noted at 1-year follow-up, and he was meeting all age-appropriate developmental milestones and showed no other symptoms consistent with NF1.
Juvenile xanthogranuloma is the most common childhood non–Langerhans cell histiocytosis. While it typically manifests as an isolated condition, JXG also can be associated with NF1 as well as juvenile myelomonocytic leukemia.1-3 Neurofibromatosis type 1 is a multisystem disorder with variable clinical manifestations that commonly is associated with skin findings such as café au lait macules, intertriginous freckling, and neurofibromas, in addition to JXG.2,3 Diagnosis of JXG should prompt noninvasive evaluation for further signs and symptoms of NF1, including thorough patient and family history and physical examination to identify other characteristic cutaneous findings, and can include consideration of slit lamp eye examination and radiography for identification of osseous findings.
The pathogenesis of JXG is not fully known, though there is evidence that it may be associated with a mutation in the mitogen-activated protein kinase pathway.1 The majority of cases appear in the first year of life.4 Clinically, JXG can manifest with extracutaneous lesions, including on the eyes and lungs.5-7 Juvenile xanthogranuloma can be noninvasively diagnosed with dermoscopy. As seen in our patient, dermoscopic findings include a red-yellow or yellow-orange background with an erythematous border, typically described as a setting-sun pattern.4,8 Biopsy can confirm the diagnosis; however, given the usually benign course, this often is unnecessary. Most pediatric patients with cutaneous manifestations have a self-limited course with regression over several months to years. Generally, no treatment is required for cutaneous manifestations alone; however, lesions can be removed for aesthetic concerns. For those with systemic involvement, a range of other treatments have been used, including chemotherapy, radiotherapy, systemic corticosteroids, and cyclosporine.6,7
The differential diagnosis for JXG includes Brooke-Spiegler syndrome, Fabry disease, solitary cutaneous mastocytoma, and tuberous sclerosis complex. Brooke-Spiegler syndrome is an autosomal-dominant condition characterized by the growth of adnexal neoplasms, including trichoepitheliomas, cylindromas, and spiradenomas. These lesions usually manifest on the face but can include other areas such as the trunk.9 Fabry disease is an X-linked recessive lysosomal storage disorder with cutaneous manifestations such as angiokeratoma corporis diffusum and hypohidrosis. Patients also may present with systemic symptoms including hypertension and renal and cardiovascular disease.10 Mastocytosis encompasses several clinical disorders defined by mast cell hyperplasia and accumulation in various organ systems, and solitary cutaneous mastocytoma is the most common manifestation in children.11,12 Cutaneous mastocytoma can manifest as a single red-brown or yellow papule, usually located on the arms or legs.13 Solitary cutaneous mastocytomas in pediatric patients typically are diagnosed based on clinical appearance and the formation of a wheal upon firm palpation (Darier sign).11-13 Our patient did not demonstrate the Darier sign, and the lesion was asymptomatic. Tuberous sclerosis complex is an autosomal-dominant neurocutaneous disorder with neurologic and skin findings that occur early in the disease course and include facial angiofibromas, hypomelanotic macules, shagreen patches, and café-au-lait macules.14
The Diagnosis: Juvenile Xanthogranuloma
Given the patient’s age, clinical features of the lesion, and characteristic setting-sun pattern on dermoscopy, a diagnosis of juvenile xanthogranuloma (JXG) was made. The patient showed no other signs of neurofibromatosis type 1 (NF1) or systemic disease and was managed conservatively with observation and routine follow-up. Minimal growth of the lesion was noted at 1-year follow-up, and he was meeting all age-appropriate developmental milestones and showed no other symptoms consistent with NF1.
Juvenile xanthogranuloma is the most common childhood non–Langerhans cell histiocytosis. While it typically manifests as an isolated condition, JXG also can be associated with NF1 as well as juvenile myelomonocytic leukemia.1-3 Neurofibromatosis type 1 is a multisystem disorder with variable clinical manifestations that commonly is associated with skin findings such as café au lait macules, intertriginous freckling, and neurofibromas, in addition to JXG.2,3 Diagnosis of JXG should prompt noninvasive evaluation for further signs and symptoms of NF1, including thorough patient and family history and physical examination to identify other characteristic cutaneous findings, and can include consideration of slit lamp eye examination and radiography for identification of osseous findings.
The pathogenesis of JXG is not fully known, though there is evidence that it may be associated with a mutation in the mitogen-activated protein kinase pathway.1 The majority of cases appear in the first year of life.4 Clinically, JXG can manifest with extracutaneous lesions, including on the eyes and lungs.5-7 Juvenile xanthogranuloma can be noninvasively diagnosed with dermoscopy. As seen in our patient, dermoscopic findings include a red-yellow or yellow-orange background with an erythematous border, typically described as a setting-sun pattern.4,8 Biopsy can confirm the diagnosis; however, given the usually benign course, this often is unnecessary. Most pediatric patients with cutaneous manifestations have a self-limited course with regression over several months to years. Generally, no treatment is required for cutaneous manifestations alone; however, lesions can be removed for aesthetic concerns. For those with systemic involvement, a range of other treatments have been used, including chemotherapy, radiotherapy, systemic corticosteroids, and cyclosporine.6,7
The differential diagnosis for JXG includes Brooke-Spiegler syndrome, Fabry disease, solitary cutaneous mastocytoma, and tuberous sclerosis complex. Brooke-Spiegler syndrome is an autosomal-dominant condition characterized by the growth of adnexal neoplasms, including trichoepitheliomas, cylindromas, and spiradenomas. These lesions usually manifest on the face but can include other areas such as the trunk.9 Fabry disease is an X-linked recessive lysosomal storage disorder with cutaneous manifestations such as angiokeratoma corporis diffusum and hypohidrosis. Patients also may present with systemic symptoms including hypertension and renal and cardiovascular disease.10 Mastocytosis encompasses several clinical disorders defined by mast cell hyperplasia and accumulation in various organ systems, and solitary cutaneous mastocytoma is the most common manifestation in children.11,12 Cutaneous mastocytoma can manifest as a single red-brown or yellow papule, usually located on the arms or legs.13 Solitary cutaneous mastocytomas in pediatric patients typically are diagnosed based on clinical appearance and the formation of a wheal upon firm palpation (Darier sign).11-13 Our patient did not demonstrate the Darier sign, and the lesion was asymptomatic. Tuberous sclerosis complex is an autosomal-dominant neurocutaneous disorder with neurologic and skin findings that occur early in the disease course and include facial angiofibromas, hypomelanotic macules, shagreen patches, and café-au-lait macules.14
- Durham BH, Lopez Rodrigo E, Picarsic J, et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med. 2019;25:1839-1842.
- Friedman JM. Neurofibromatosis 1. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1998.
- Miraglia E, Laghi A, Moramarco A, et al. Juvenile xanthogranuloma in neurofibromatosis type 1. Prevalence and possible correlation with lymphoproliferative diseases: experience of a single center and review of the literature. Clin Ther. 2022;173:353-355.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 8, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526103/
- Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
- Freyer DR, Kennedy R, Bostrom BC, et al. Juvenile xanthogranuloma: forms of systemic disease and their clinical implications. J Pediatr. 1996;129:227-237.
- Murphy JT, Soeken T, Megison S, et al. Juvenile xanthogranuloma: diverse presentations of noncutaneous disease. J Pediatr Hematol Oncol.2014;36:641-645.
- Xu J, Ma L. Dermoscopic patterns in juvenile xanthogranuloma based on the histological classification. Front Med (Lausanne). 2021;7:618946.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bokhari SRA, Zulfiqar H, Hariz A. Fabry disease. StatPearls [Internet]. StatPearls Publishing; 2025. Update July 4, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK435996/
- Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35-45.
- Klaiber N, Kumar S, Irani AM. Mastocytosis in children. Curr Allergy Asthma Rep. 2017;17:80.
- Sławin´ ska M, Kaszuba A, Lange M, et al. Dermoscopic features of different forms of cutaneous mastocytosis: a systematic review. J Clin Med. 2022;11:4649.
- Teng JM, Cowen EW, Wataya-Kaneda M, et al. Dermatologic and dental aspects of the 2012 International Tuberous Sclerosis Complex Consensus Statements. JAMA Dermatol. 2014;150:1095-1101.
- Durham BH, Lopez Rodrigo E, Picarsic J, et al. Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med. 2019;25:1839-1842.
- Friedman JM. Neurofibromatosis 1. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1998.
- Miraglia E, Laghi A, Moramarco A, et al. Juvenile xanthogranuloma in neurofibromatosis type 1. Prevalence and possible correlation with lymphoproliferative diseases: experience of a single center and review of the literature. Clin Ther. 2022;173:353-355.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 8, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526103/
- Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
- Freyer DR, Kennedy R, Bostrom BC, et al. Juvenile xanthogranuloma: forms of systemic disease and their clinical implications. J Pediatr. 1996;129:227-237.
- Murphy JT, Soeken T, Megison S, et al. Juvenile xanthogranuloma: diverse presentations of noncutaneous disease. J Pediatr Hematol Oncol.2014;36:641-645.
- Xu J, Ma L. Dermoscopic patterns in juvenile xanthogranuloma based on the histological classification. Front Med (Lausanne). 2021;7:618946.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bokhari SRA, Zulfiqar H, Hariz A. Fabry disease. StatPearls [Internet]. StatPearls Publishing; 2025. Update July 4, 2023. Accessed November 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK435996/
- Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35-45.
- Klaiber N, Kumar S, Irani AM. Mastocytosis in children. Curr Allergy Asthma Rep. 2017;17:80.
- Sławin´ ska M, Kaszuba A, Lange M, et al. Dermoscopic features of different forms of cutaneous mastocytosis: a systematic review. J Clin Med. 2022;11:4649.
- Teng JM, Cowen EW, Wataya-Kaneda M, et al. Dermatologic and dental aspects of the 2012 International Tuberous Sclerosis Complex Consensus Statements. JAMA Dermatol. 2014;150:1095-1101.
A 6-month-old male infant with a history of cradle cap and an infantile hemangioma on the left shoulder presented to the dermatology clinic for evaluation of a slow-growing yellow papule on the upper back of 3 months’ duration. The lesion initially was noted 2 months prior to the current presentation by the patient’s pediatrician, who recommended follow-up with dermatology after an unsuccessful attempt at incision and drainage. Physical examination revealed a 7-mm, yellow, dome-shaped papule with a red collarette on the right upper back. No axillary freckling, ocular findings, or other skin findings were found. The patient was born at term with no complications, and his mother reported that he was otherwise healthy. There were no developmental concerns or known allergies, and his family history was negative for any similar lesions. Dermoscopic examination of the lesion revealed a well-circumscribed, circular, yellow-orange papule with an erythematous border and setting-sun appearance.
Staff Perspectives on the VISN 20 Tele-Neuropsychology Program
Staff Perspectives on the VISN 20 Tele-Neuropsychology Program
There are 2.7 million (48%) rural veterans enrolled in the Veterans Health Administration (VHA).1 Many VHA-enrolled rural veterans are aged ≥ 65 years (54%), a medically complex population that requires more extensive health care.1 These veterans may live far from US Department of Veterans Affairs (VA) medical centers (VAMCs) and often receive most of their care at rural community-based outpatient clinics (CBOCs). In addition to face-to-face (F2F) services provided at these clinics, many patient care needs may be met using telehealth technology, which can connect veterans at CBOCs with remote health care practitioners (HCPs).
This technology is used across medical specialties throughout the VA and has expanded into neuropsychology services to improve access amid the shortage of rural neuropsychologists. Prior research suggests that access to neuropsychology services improves the functional outcomes of people with diverse medical conditions, including dementia, brain injury, and epilepsy, and reduces emergency department visits, hospitalization duration, and health care costs.2-6 Given that veterans unable to access neuropsychology services may be at risk for poorer outcomes, identifying ways to improve access is a priority. Tele-neuropsychology (teleNP) has been used to expand access for rural veterans in need of these services.7,8
TeleNP is the application of audiovisual technologies to enable remote clinical encounters for neuropsychological assessments.9 TeleNP has been shown to be generally equivalent to F2F care, without significant differences compared with in-person visits.10-13 TeleNP was increasingly implemented following the COVID-19 pandemic and remains an enduring and expanding feature of neuropsychology care delivery.8,14-18 TeleNP services can increase access to care, especially for rural veterans and those with limited transportation.
Research in non-VA samples suggests a high level of clinician satisfaction with teleNP.16 In VA samples, research has found high levels of patient satisfaction with teleNP both within Veterans Integrated Services Network (VISN) 20 and in a VA health care system outside VISN 20.7,19 Investigating staff perceptions of these services and their utility compared with non-VA F2F visits is pertinent to the overall feasibility and effectiveness of teleNP.
TELE-NEUROPSYCHOLOGY PROGRAM
A clinical resource hub (CRH) is a VISN-governed program that provides veteran health care when local VHA facilities have service gaps.20,21 CRH 20 serves several Pacific Northwest VISN 20 health care systems and began providing teleNP in 2015. The CRH 20 teleNP service serves older adults in rural settings with > 570 teleNP evaluations completed over a recent 12-month period (May 2023 to May 2024). In the CRH 20 teleNP program, veterans are offered services by CRH 20 neuropsychologists via telehealth to a patient’s local VAMC, larger health care clinic, CBOC, or via Veterans Video Connect to the home.
Referral pathways to the CRH 20 teleNP program differ across sites. For VISN 20 sites that do not have any in-house neuropsychology services, referrals are initiated by HCPs from any discipline. At 2 sites with in-house neuropsychology programs, CRH 20 teleNP referrals typically are forwarded from the inhouse service whenever the veteran prefers to be seen at an outlying clinic. All sites, including the CBOCs, are equipped fully for testing, and the HCP encounters veterans in a private office via video-based telehealth technology after a telehealth technician orients them to the space. The private office minimizes environmental disruptions and uses standardized technology to ensure valid results. A limited number of evaluations are offered at home (< 5% of the evaluations) if the veteran is unable to come to a VHA facility, has access to reliable internet, and a minimally distracting home setting.
In VISN 20, teleNP is a routine practice for delivering services to rural sites, most of which lack neuropsychologists. However, there is limited information about the extent to which the referral sources find the service useful. This quality improvement (QI) project aimed to better understand how well-established teleNP services were received by referral sources/stakeholders and how services could be improved. Prior to the advent of the CRH 20 teleNP program, staff had the option of referring for F2F evaluations in the local community (outside the VA) at some sites, an option that remains. This QI project examined staff perspectives on the usefulness of CRH 20 teleNP services compared with non-VA F2F services. We administered an anonymous, confidential survey examining these factors to VISN 20 staff within 4 VA health care systems.
METHODS
This QI project used a mixed quantitative and qualitative descriptive survey design to elicit feedback. The authors (3 neuropsychologists, 1 geropsychologist, and 1 research coordinator) developed the survey questions. The 13-question survey was voluntary, anonymous, and confidential, and respondents were given an opportunity to ask questions, with the first author serving as the point of contact.
The survey ascertained information about respondents and their work setting (ie, facility type, specific work setting and location, profession, and rurality of patients). First respondents were asked whether they have referred patients to neuropsychology services in the past year. Those who had not referred patients during the past year were asked about reasons for nonreferral with an option to provide an open-ended response. Respondents who did refer were asked how they refer for neuropsychology services and about the usefulness and timeliness of both teleNP and non-VA F2F services. Respondents were asked to respond with their preference for teleNP vs non-VA F2F with an open-ended prompt. Finally, respondents were invited to share any feedback for improvement regarding teleNP services.
A link to the survey, hosted on the VA Research Electronic Data Capture system, was emailed to facility and service line leaders at the 4 VISN 20 health care systems for distribution to the staff. All staff were included because in many of the facilities, particularly those that are highly rural with low staffing, it is not uncommon for technicians, nurses, and other support staff to assist with placing consults. In particular, VISN 20 nurses often have an optimal understanding of referral pathways to care for patients and are positioned to give and receive feedback about the utility of neuropsychological evaluations. The Research and Development Committee at the Boise VA Medical Center determined this project to be QI and exempt from institutional review board oversight. The VISN 20 employee labor relations HR supervisor approved this survey, with union awareness. Responses were anonymous.
Data were imported into Microsoft Excel and IBM SPSS Statistics for further analysis. Data were summarized using descriptive statistics, frequencies, and percentages. Nonparametric χ2 and Wilcoxon signed-rank tests were used to test for differences. An inductive approach to develop codes was used for the 3 open-ended questions. Two authors (CC, CEG) independently coded the responses and reviewed discrepancies. Final code applications were based on consensus.
RESULTS
The survey was deployed for 1 month between February 7, 2024, and June 15, 2024, at each of the 4 health care systems. Thirty-three staff members responded; of these, 1 person did not respond to an item on whether they referred for neuropsychology services. Eighteen of 33 respondents reported referring patients to teleNP or F2F neuropsychology services in the past year. Fourteen of the 33 respondents stated they did not refer; of these, 2 were unfamiliar with the teleNP service and 12 provided other reasons (eg, new to VA, not in their professional scope to order consults, did not have patients needing services).
The analysis focused on the 18 respondents who referred for neuropsychology services. Thirteen were within health care system A, and 5 were within health care system B (which had no nearby non-VA contracted neuropsychology services) and none were in the other 2 health care systems. Ten of 18 respondents (56%) stated they practiced primarily in a rural setting. Five respondents worked in a CBOC, 12 in a main VA facility, 9 in a primary care setting, 8 in a mental health setting, and 3 in other settings (eg, domiciliary). Participants could select > 1 setting. The 18 respondents who referred to neuropsychology services included 7 psychologists, 1 nurse, 2 social workers, 1 social services assistant, 4 nurse practitioners, 2 physicians, and 1 unknown HCP.
When asked to categorize the usefulness of services, more respondents characterized teleNP as very much so (1 on a 5-point scale) than F2F referrals (Figure). The mean (SD) of 1.5 (0.8) for teleNP usefulness fell between very much so and mostly and 1 respondent indicated not applicable. Similarly, the mean (SD) for non-VA F2F usefulness was 1.7 (0.9); 9 respondents rated this item as not applicable. A Wilcoxon signed-rank test of related samples indicated no significant differences between the pairs of ratings (Z = 1.50; P = .41).
Respondents with rural patients were more likely to refer them to teleNP services compared with respondents with nonrural patients (χ2 = 5.7; P = .02). However, ratings of teleNP usefulness did not significantly differ for those serving rural vs with nonrural patients (χ2 = 1.4; P = .49). Mean (SD) rating of teleNP usefulness was 1.3 (0.7) for the 9 rural subgroup respondents (between very much so and mostly) vs 1.8 (0.9) for the 8 nonrural subgroup respondents (between very much so and mostly). The mean (SD) rating for non-VA F2F usefulness was 1.8 (1.0) for the 4 rural subgroup respondents and 1.6 (0.8) for the 5 nonrural subgroup, between very much so and mostly for both groups.
Most respondents had no preference between teleNP or F2F. Notably, the responses underlying this group were multifaceted and corresponded to multiple codes (ie, access, preference for in-person services, technology, space and logistics, and service boundaries and requirements). According to 1 respondent, “the logistics of scheduling/room availability, technological challenges, and client behavioral issues that are likely to occur could possibly be more easily addressed via in-person sessions for some clients and providers.”
Six of 18 respondents preferred teleNP, citing timeliness, ease of access, and evaluation quality. One respondent noted that the “majority of my veterans live in extremely remote areas” and may need to take a plane for their visit. The 3 respondents who preferred in-person neuropsychology services cited veterans’ preference for in-person services.
Open-Ended Feedback
Thirteen respondents offered feedback on what is working well with teleNP services. Reasons mentioned were related to the service (ie, timeliness, access, quality) and the neuropsychologist (ie, communication and HCP skills). One respondent described the service and neuropsychologists positively, stating that they were “responsive, notes are readily available, clear assessments and recommendations, being available by [Microsoft] Teams/email.”
Ten respondents provided suggestions for improvement. Suggestions focused on expanding services, such as to “all veterans with cognitive/memory concerns that desire testing,” individuals with attention-deficit/hyperactivity disorder and co-occurring mental health concerns, and those in residential programs. Suggestions included hiring psychology technicians or more staff and providing education at local clinics.
DISCUSSION
This QI project examines VA staff perspectives on the usefulness of CRH 20 teleNP services and non-VA F2F services. While the small sample size limits generalizability, this preliminary study suggests that VA teleNP evaluations were similar to those conducted F2F in non-VA settings. While ratings of teleNP usefulness did not differ significantly for those serving rural vs nonrural veterans, respondents serving rural patients were more likely to refer patients to teleNP, suggesting that teleNP may increase access in rural settings, consistent with other studies.7,8,13 This article also presents qualitative suggestions for improving teleNP delivery within the VHA. This is the first known initiative to report on VHA staff satisfaction with a teleNP service and expands the limited literature to date on satisfaction with teleNP services. The findings provide initial support for continued use and, potentially, expansion of teleNP services within this CRH remote hub-and-spoke model.
Limitations
A significant limitation of the current work is the small sample size of survey respondents. In particular, while teleNP turnaround time was perceived as faster than non-VA F2F care, only 8 respondents reported on timeliness of F2F evaluation results, which renders it difficult to draw conclusions. Interestingly, not all respondents reported referring to neuropsychology services within the previous year; the most common reasons reflect the perception that referral to neuropsychology was outside of that staff member’s role or not clinically indicated.
One additional possible explanation for the absence of reporting on utility of teleNP specifically is that respondents did not track whether their patient was seen by teleNP or F2F services, based on how the referral process varies at each health care system. For example, in health care system C, a large number of referrals are forwarded to the service by local VA F2F neuropsychologists. This may speak to the seamlessness of the teleNP process, such that local staff and/or referring HCPs are unaware of the modality over which neuropsychology is being conducted. It is plausible that the reason behind this smaller response rate in health care systems B and C relates to how neuropsychology consults are processed at these local VAMCs. We suspect that in these settings, the HCPs referring for neuropsychological evaluations (eg, primary care, mental health) may be unaware that their referrals are being triaged to neuropsychologists in a different program (CRH 20 teleNP). Therefore, they would not necessarily know that they used teleNP and didn’t complete the survey.
The referral process for these 2 sites contrasts with the process for other VISN 20 sites where there is no local neuropsychology program triaging. In these settings, referrals from local HCPs come directly to teleNP; thus, it is more likely that these HCPs are aware of teleNP services. There were only 2 physicians who completed the survey, which may relate to their workload and a workflow where other staff have been increasingly requested to order the consults for the physician. This type of workflow results in an increase in the number of VHA staff involved in patient care. Ratings of usefulness were highest in health care system B, which does not have neuropsychology services at the facility or in the community; this may relate to elevated teleNP satisfaction ratings.
Further work may help identify which aspects of a teleNP service make it more useful than F2F care for this population or determine whether there were HCPor setting-specific factors that influenced the ratings (ie, preference for VA care or comparison of favorability ratings for the HCPs who conduct teleNP and F2F within the same system). The latter comparisons could not be drawn in the current systems due to the absence of HCPs who provide both teleNP and F2F modalities within VISN 20. Another consideration for future work would be to use a previously published/validated survey measure and piloting of questions with a naive sample before implementation.
CONCLUSIONS
This analysis provides initial support for feasibility and acceptability of teleNP as an alternative to traditional in-person neuropsychological evaluations. The small number of survey respondents may reflect the multiple pathways through which consults are forwarded to CRH 20, which includes both direct HCP referrals and forwarded consults from local neuropsychology services. CRH 20 has completed > 570 teleNP evaluations within 1 year, suggesting that lack of awareness may not be hindering veteran access to the service. Replication with a larger sample that is more broadly representative of key stakeholders in veteran care, identification of populations that would benefit most from teleNP services, and dissemination studies of the expansion of teleNP services are all important directions for future work. The robustness and longevity of the VISN 20 teleNP program, coupled with the preliminary positive findings from this project, demonstrate support for further assessment of the potential impact of telehealth on neuropsychological care within the VHA and show that barriers associated with access to health care services in remote settings may be mitigated through teleNP service delivery.
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated March 10, 2025. Accessed July 7, 2025. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Braun M, Tupper D, Kaufmann P, et al. Neuropsychological assessment: a valuable tool in the diagnosis and management of neurological, neurodevelopmental, medical, and psychiatric disorders. Cogn Behav Neurol. 2011;24(3):107-114. doi:10.1097/wnn.0b013e3182351289
- Donders J. The incremental value of neuropsychological assessment: a critical review. Clin Neuropsychol. 2020;34(1):56-87. doi:10.1080/13854046.2019.1575471
- Williams MW, Rapport LJ, Hanks RA, et al. Incremental value of neuropsychological evaluations to computed tomography in predicting long-term outcomes after traumatic brain injury. Clin Neuropsychol. 2013;27(3):356-375. doi:10.1080/13854046.2013.765507
- Sieg E, Mai Q, Mosti C, Brook M. The utility of neuropsychological consultation in identifying medical inpatients with suspected cognitive impairment at risk for greater hospital utilization. Clin Neuropsychol. 2019;33(1):75-89. doi:10.1080/13854046.2018.1465124
- Vankirk KM, Horner MD, Turner TH, et al. CE hospital service utilization is reduced following neuropsychological evaluation in a sample of U.S. veterans. Clin Neuropsychol. 2013;27(5):750-761. doi:10.1080/13854046.2013.783122
- Appleman ER, O’Connor MK, Boucher SJ, et al. Teleneuropsychology clinic development and patient satisfaction. Clin Neuropsychol. 2021;35(4):819-837. doi:10.1080/13854046.2020.1871515
- Stelmokas J, Ratcliffe LN, Lengu K, et al. Evaluation of teleneuropsychology services in veterans during COVID-19. Psychol Serv. 2024;21(1):65-72. doi:10.1037/ser0000810
- Bilder R Postal KS, Barisa M, et al. Inter Organizational Practice Committee recommendations/guidance for teleneuropsychology in response to the COVID-19 pandemic. Arch Clin Neuropsychol. 2020;35(6):647-659. doi:10.1093/arclin/acaa046
- Brearly TW, Shura RD, Martindale SL, et al. Neuropsychological test administration by videoconference: a systematic review and meta-analysis. Neuropsychol Rev. 2017;27(2):174-186. doi:10.1007/s11065-017-9349-1
- Brown AD, Kelso W, Eratne D, et al. Investigating equivalence of in-person and telehealth-based neuropsychological assessment performance for individuals being investigated for younger onset dementia. Arch Clin Neuropsychol. 2024;39(5):594-607. doi:10.1093/arclin/acad108
- Chapman JE, Ponsford J, Bagot KL, et al. The use of videoconferencing in clinical neuropsychology practice: a mixed methods evaluation of neuropsychologists’ experiences and views. Aust Psychol. 2020;55(6):618-633. doi:10.1111/ap.12471
- Marra DE, Hamlet KM, Bauer RM, et al. Validity of teleneuropsychology for older adults in response to COVID-19: a systematic and critical review. Clin Neuropsychol. 2020;34:1411-1452. doi:10.1080/13854046.2020.1769192
- Hammers DB, Stolwyk R, Harder L, et al. A survey of international clinical teleneuropsychology service provision prior to COVID-19. Clin Neuropsychol. 2020;34(7-8):1267- 1283. doi:10.1080/13854046.2020.1810323
- Marra DE, Hoelzle JB, Davis JJ, et al. Initial changes in neuropsychologists’ clinical practice during the COVID-19 pandemic: a survey study. Clin Neuropsychol. 2020;34(7- 8):1251-1266. doi:10.1080/13854046.2020.1800098
- Parsons MW, Gardner MM, Sherman, JC et al. Feasibility and acceptance of direct-to-home teleneuropsychology services during the COVID-19 pandemic. J Int Neuropsychol Soc. 2022;28(2):210-215. doi:10.1017/s1355617721000436
- Rochette AD, Rahman-Filipiak A, Spencer RJ, et al. Teleneuropsychology practice survey during COVID-19 within the United States. Appl Neuropsychol Adult. 2022;29(6):1312- 1322. doi:10.1080/23279095.2021.1872576
- Messler AC, Hargrave DD, Trittschuh EH, et al. National survey of telehealth neuropsychology practices: current attitudes, practices, and relevance of tele-neuropsychology three years after the onset of COVID-19. Clin Neuropsychol. 2023;39:1017-1036. doi:10.1080/13854046.2023.2192422
- Rautman L, Sordahl JA. Veteran satisfaction with tele-neuropsychology services. Clin Neuropsychol. 2018;32:1453949. doi:10.1080/13854046.2018.1453949
- US Department of Veterans Affairs. Patient care services: clinical resource hubs. Updated March 20, 2024. Accessed August 4, 2025. https://www.patientcare .va.gov/primarycare/CRH.asp
- Burnett K, Stockdale SE, Yoon J, et al. The Clinical Resource Hub initiative: first-year implementation of the Veterans Health Administration regional telehealth contingency staffing program. Ambul Care Manage. 2023;46(3):228-239. doi:10.1097/JAC.0000000000000468
There are 2.7 million (48%) rural veterans enrolled in the Veterans Health Administration (VHA).1 Many VHA-enrolled rural veterans are aged ≥ 65 years (54%), a medically complex population that requires more extensive health care.1 These veterans may live far from US Department of Veterans Affairs (VA) medical centers (VAMCs) and often receive most of their care at rural community-based outpatient clinics (CBOCs). In addition to face-to-face (F2F) services provided at these clinics, many patient care needs may be met using telehealth technology, which can connect veterans at CBOCs with remote health care practitioners (HCPs).
This technology is used across medical specialties throughout the VA and has expanded into neuropsychology services to improve access amid the shortage of rural neuropsychologists. Prior research suggests that access to neuropsychology services improves the functional outcomes of people with diverse medical conditions, including dementia, brain injury, and epilepsy, and reduces emergency department visits, hospitalization duration, and health care costs.2-6 Given that veterans unable to access neuropsychology services may be at risk for poorer outcomes, identifying ways to improve access is a priority. Tele-neuropsychology (teleNP) has been used to expand access for rural veterans in need of these services.7,8
TeleNP is the application of audiovisual technologies to enable remote clinical encounters for neuropsychological assessments.9 TeleNP has been shown to be generally equivalent to F2F care, without significant differences compared with in-person visits.10-13 TeleNP was increasingly implemented following the COVID-19 pandemic and remains an enduring and expanding feature of neuropsychology care delivery.8,14-18 TeleNP services can increase access to care, especially for rural veterans and those with limited transportation.
Research in non-VA samples suggests a high level of clinician satisfaction with teleNP.16 In VA samples, research has found high levels of patient satisfaction with teleNP both within Veterans Integrated Services Network (VISN) 20 and in a VA health care system outside VISN 20.7,19 Investigating staff perceptions of these services and their utility compared with non-VA F2F visits is pertinent to the overall feasibility and effectiveness of teleNP.
TELE-NEUROPSYCHOLOGY PROGRAM
A clinical resource hub (CRH) is a VISN-governed program that provides veteran health care when local VHA facilities have service gaps.20,21 CRH 20 serves several Pacific Northwest VISN 20 health care systems and began providing teleNP in 2015. The CRH 20 teleNP service serves older adults in rural settings with > 570 teleNP evaluations completed over a recent 12-month period (May 2023 to May 2024). In the CRH 20 teleNP program, veterans are offered services by CRH 20 neuropsychologists via telehealth to a patient’s local VAMC, larger health care clinic, CBOC, or via Veterans Video Connect to the home.

Referral pathways to the CRH 20 teleNP program differ across sites. For VISN 20 sites that do not have any in-house neuropsychology services, referrals are initiated by HCPs from any discipline. At 2 sites with in-house neuropsychology programs, CRH 20 teleNP referrals typically are forwarded from the inhouse service whenever the veteran prefers to be seen at an outlying clinic. All sites, including the CBOCs, are equipped fully for testing, and the HCP encounters veterans in a private office via video-based telehealth technology after a telehealth technician orients them to the space. The private office minimizes environmental disruptions and uses standardized technology to ensure valid results. A limited number of evaluations are offered at home (< 5% of the evaluations) if the veteran is unable to come to a VHA facility, has access to reliable internet, and a minimally distracting home setting.
In VISN 20, teleNP is a routine practice for delivering services to rural sites, most of which lack neuropsychologists. However, there is limited information about the extent to which the referral sources find the service useful. This quality improvement (QI) project aimed to better understand how well-established teleNP services were received by referral sources/stakeholders and how services could be improved. Prior to the advent of the CRH 20 teleNP program, staff had the option of referring for F2F evaluations in the local community (outside the VA) at some sites, an option that remains. This QI project examined staff perspectives on the usefulness of CRH 20 teleNP services compared with non-VA F2F services. We administered an anonymous, confidential survey examining these factors to VISN 20 staff within 4 VA health care systems.
METHODS
This QI project used a mixed quantitative and qualitative descriptive survey design to elicit feedback. The authors (3 neuropsychologists, 1 geropsychologist, and 1 research coordinator) developed the survey questions. The 13-question survey was voluntary, anonymous, and confidential, and respondents were given an opportunity to ask questions, with the first author serving as the point of contact.
The survey ascertained information about respondents and their work setting (ie, facility type, specific work setting and location, profession, and rurality of patients). First respondents were asked whether they have referred patients to neuropsychology services in the past year. Those who had not referred patients during the past year were asked about reasons for nonreferral with an option to provide an open-ended response. Respondents who did refer were asked how they refer for neuropsychology services and about the usefulness and timeliness of both teleNP and non-VA F2F services. Respondents were asked to respond with their preference for teleNP vs non-VA F2F with an open-ended prompt. Finally, respondents were invited to share any feedback for improvement regarding teleNP services.
A link to the survey, hosted on the VA Research Electronic Data Capture system, was emailed to facility and service line leaders at the 4 VISN 20 health care systems for distribution to the staff. All staff were included because in many of the facilities, particularly those that are highly rural with low staffing, it is not uncommon for technicians, nurses, and other support staff to assist with placing consults. In particular, VISN 20 nurses often have an optimal understanding of referral pathways to care for patients and are positioned to give and receive feedback about the utility of neuropsychological evaluations. The Research and Development Committee at the Boise VA Medical Center determined this project to be QI and exempt from institutional review board oversight. The VISN 20 employee labor relations HR supervisor approved this survey, with union awareness. Responses were anonymous.
Data were imported into Microsoft Excel and IBM SPSS Statistics for further analysis. Data were summarized using descriptive statistics, frequencies, and percentages. Nonparametric χ2 and Wilcoxon signed-rank tests were used to test for differences. An inductive approach to develop codes was used for the 3 open-ended questions. Two authors (CC, CEG) independently coded the responses and reviewed discrepancies. Final code applications were based on consensus.
RESULTS
The survey was deployed for 1 month between February 7, 2024, and June 15, 2024, at each of the 4 health care systems. Thirty-three staff members responded; of these, 1 person did not respond to an item on whether they referred for neuropsychology services. Eighteen of 33 respondents reported referring patients to teleNP or F2F neuropsychology services in the past year. Fourteen of the 33 respondents stated they did not refer; of these, 2 were unfamiliar with the teleNP service and 12 provided other reasons (eg, new to VA, not in their professional scope to order consults, did not have patients needing services).
The analysis focused on the 18 respondents who referred for neuropsychology services. Thirteen were within health care system A, and 5 were within health care system B (which had no nearby non-VA contracted neuropsychology services) and none were in the other 2 health care systems. Ten of 18 respondents (56%) stated they practiced primarily in a rural setting. Five respondents worked in a CBOC, 12 in a main VA facility, 9 in a primary care setting, 8 in a mental health setting, and 3 in other settings (eg, domiciliary). Participants could select > 1 setting. The 18 respondents who referred to neuropsychology services included 7 psychologists, 1 nurse, 2 social workers, 1 social services assistant, 4 nurse practitioners, 2 physicians, and 1 unknown HCP.
When asked to categorize the usefulness of services, more respondents characterized teleNP as very much so (1 on a 5-point scale) than F2F referrals (Figure). The mean (SD) of 1.5 (0.8) for teleNP usefulness fell between very much so and mostly and 1 respondent indicated not applicable. Similarly, the mean (SD) for non-VA F2F usefulness was 1.7 (0.9); 9 respondents rated this item as not applicable. A Wilcoxon signed-rank test of related samples indicated no significant differences between the pairs of ratings (Z = 1.50; P = .41).
Respondents with rural patients were more likely to refer them to teleNP services compared with respondents with nonrural patients (χ2 = 5.7; P = .02). However, ratings of teleNP usefulness did not significantly differ for those serving rural vs with nonrural patients (χ2 = 1.4; P = .49). Mean (SD) rating of teleNP usefulness was 1.3 (0.7) for the 9 rural subgroup respondents (between very much so and mostly) vs 1.8 (0.9) for the 8 nonrural subgroup respondents (between very much so and mostly). The mean (SD) rating for non-VA F2F usefulness was 1.8 (1.0) for the 4 rural subgroup respondents and 1.6 (0.8) for the 5 nonrural subgroup, between very much so and mostly for both groups.
Most respondents had no preference between teleNP or F2F. Notably, the responses underlying this group were multifaceted and corresponded to multiple codes (ie, access, preference for in-person services, technology, space and logistics, and service boundaries and requirements). According to 1 respondent, “the logistics of scheduling/room availability, technological challenges, and client behavioral issues that are likely to occur could possibly be more easily addressed via in-person sessions for some clients and providers.”
Six of 18 respondents preferred teleNP, citing timeliness, ease of access, and evaluation quality. One respondent noted that the “majority of my veterans live in extremely remote areas” and may need to take a plane for their visit. The 3 respondents who preferred in-person neuropsychology services cited veterans’ preference for in-person services.
Open-Ended Feedback
Thirteen respondents offered feedback on what is working well with teleNP services. Reasons mentioned were related to the service (ie, timeliness, access, quality) and the neuropsychologist (ie, communication and HCP skills). One respondent described the service and neuropsychologists positively, stating that they were “responsive, notes are readily available, clear assessments and recommendations, being available by [Microsoft] Teams/email.”
Ten respondents provided suggestions for improvement. Suggestions focused on expanding services, such as to “all veterans with cognitive/memory concerns that desire testing,” individuals with attention-deficit/hyperactivity disorder and co-occurring mental health concerns, and those in residential programs. Suggestions included hiring psychology technicians or more staff and providing education at local clinics.
DISCUSSION
This QI project examines VA staff perspectives on the usefulness of CRH 20 teleNP services and non-VA F2F services. While the small sample size limits generalizability, this preliminary study suggests that VA teleNP evaluations were similar to those conducted F2F in non-VA settings. While ratings of teleNP usefulness did not differ significantly for those serving rural vs nonrural veterans, respondents serving rural patients were more likely to refer patients to teleNP, suggesting that teleNP may increase access in rural settings, consistent with other studies.7,8,13 This article also presents qualitative suggestions for improving teleNP delivery within the VHA. This is the first known initiative to report on VHA staff satisfaction with a teleNP service and expands the limited literature to date on satisfaction with teleNP services. The findings provide initial support for continued use and, potentially, expansion of teleNP services within this CRH remote hub-and-spoke model.
Limitations
A significant limitation of the current work is the small sample size of survey respondents. In particular, while teleNP turnaround time was perceived as faster than non-VA F2F care, only 8 respondents reported on timeliness of F2F evaluation results, which renders it difficult to draw conclusions. Interestingly, not all respondents reported referring to neuropsychology services within the previous year; the most common reasons reflect the perception that referral to neuropsychology was outside of that staff member’s role or not clinically indicated.
One additional possible explanation for the absence of reporting on utility of teleNP specifically is that respondents did not track whether their patient was seen by teleNP or F2F services, based on how the referral process varies at each health care system. For example, in health care system C, a large number of referrals are forwarded to the service by local VA F2F neuropsychologists. This may speak to the seamlessness of the teleNP process, such that local staff and/or referring HCPs are unaware of the modality over which neuropsychology is being conducted. It is plausible that the reason behind this smaller response rate in health care systems B and C relates to how neuropsychology consults are processed at these local VAMCs. We suspect that in these settings, the HCPs referring for neuropsychological evaluations (eg, primary care, mental health) may be unaware that their referrals are being triaged to neuropsychologists in a different program (CRH 20 teleNP). Therefore, they would not necessarily know that they used teleNP and didn’t complete the survey.
The referral process for these 2 sites contrasts with the process for other VISN 20 sites where there is no local neuropsychology program triaging. In these settings, referrals from local HCPs come directly to teleNP; thus, it is more likely that these HCPs are aware of teleNP services. There were only 2 physicians who completed the survey, which may relate to their workload and a workflow where other staff have been increasingly requested to order the consults for the physician. This type of workflow results in an increase in the number of VHA staff involved in patient care. Ratings of usefulness were highest in health care system B, which does not have neuropsychology services at the facility or in the community; this may relate to elevated teleNP satisfaction ratings.
Further work may help identify which aspects of a teleNP service make it more useful than F2F care for this population or determine whether there were HCPor setting-specific factors that influenced the ratings (ie, preference for VA care or comparison of favorability ratings for the HCPs who conduct teleNP and F2F within the same system). The latter comparisons could not be drawn in the current systems due to the absence of HCPs who provide both teleNP and F2F modalities within VISN 20. Another consideration for future work would be to use a previously published/validated survey measure and piloting of questions with a naive sample before implementation.
CONCLUSIONS
This analysis provides initial support for feasibility and acceptability of teleNP as an alternative to traditional in-person neuropsychological evaluations. The small number of survey respondents may reflect the multiple pathways through which consults are forwarded to CRH 20, which includes both direct HCP referrals and forwarded consults from local neuropsychology services. CRH 20 has completed > 570 teleNP evaluations within 1 year, suggesting that lack of awareness may not be hindering veteran access to the service. Replication with a larger sample that is more broadly representative of key stakeholders in veteran care, identification of populations that would benefit most from teleNP services, and dissemination studies of the expansion of teleNP services are all important directions for future work. The robustness and longevity of the VISN 20 teleNP program, coupled with the preliminary positive findings from this project, demonstrate support for further assessment of the potential impact of telehealth on neuropsychological care within the VHA and show that barriers associated with access to health care services in remote settings may be mitigated through teleNP service delivery.
There are 2.7 million (48%) rural veterans enrolled in the Veterans Health Administration (VHA).1 Many VHA-enrolled rural veterans are aged ≥ 65 years (54%), a medically complex population that requires more extensive health care.1 These veterans may live far from US Department of Veterans Affairs (VA) medical centers (VAMCs) and often receive most of their care at rural community-based outpatient clinics (CBOCs). In addition to face-to-face (F2F) services provided at these clinics, many patient care needs may be met using telehealth technology, which can connect veterans at CBOCs with remote health care practitioners (HCPs).
This technology is used across medical specialties throughout the VA and has expanded into neuropsychology services to improve access amid the shortage of rural neuropsychologists. Prior research suggests that access to neuropsychology services improves the functional outcomes of people with diverse medical conditions, including dementia, brain injury, and epilepsy, and reduces emergency department visits, hospitalization duration, and health care costs.2-6 Given that veterans unable to access neuropsychology services may be at risk for poorer outcomes, identifying ways to improve access is a priority. Tele-neuropsychology (teleNP) has been used to expand access for rural veterans in need of these services.7,8
TeleNP is the application of audiovisual technologies to enable remote clinical encounters for neuropsychological assessments.9 TeleNP has been shown to be generally equivalent to F2F care, without significant differences compared with in-person visits.10-13 TeleNP was increasingly implemented following the COVID-19 pandemic and remains an enduring and expanding feature of neuropsychology care delivery.8,14-18 TeleNP services can increase access to care, especially for rural veterans and those with limited transportation.
Research in non-VA samples suggests a high level of clinician satisfaction with teleNP.16 In VA samples, research has found high levels of patient satisfaction with teleNP both within Veterans Integrated Services Network (VISN) 20 and in a VA health care system outside VISN 20.7,19 Investigating staff perceptions of these services and their utility compared with non-VA F2F visits is pertinent to the overall feasibility and effectiveness of teleNP.
TELE-NEUROPSYCHOLOGY PROGRAM
A clinical resource hub (CRH) is a VISN-governed program that provides veteran health care when local VHA facilities have service gaps.20,21 CRH 20 serves several Pacific Northwest VISN 20 health care systems and began providing teleNP in 2015. The CRH 20 teleNP service serves older adults in rural settings with > 570 teleNP evaluations completed over a recent 12-month period (May 2023 to May 2024). In the CRH 20 teleNP program, veterans are offered services by CRH 20 neuropsychologists via telehealth to a patient’s local VAMC, larger health care clinic, CBOC, or via Veterans Video Connect to the home.

Referral pathways to the CRH 20 teleNP program differ across sites. For VISN 20 sites that do not have any in-house neuropsychology services, referrals are initiated by HCPs from any discipline. At 2 sites with in-house neuropsychology programs, CRH 20 teleNP referrals typically are forwarded from the inhouse service whenever the veteran prefers to be seen at an outlying clinic. All sites, including the CBOCs, are equipped fully for testing, and the HCP encounters veterans in a private office via video-based telehealth technology after a telehealth technician orients them to the space. The private office minimizes environmental disruptions and uses standardized technology to ensure valid results. A limited number of evaluations are offered at home (< 5% of the evaluations) if the veteran is unable to come to a VHA facility, has access to reliable internet, and a minimally distracting home setting.
In VISN 20, teleNP is a routine practice for delivering services to rural sites, most of which lack neuropsychologists. However, there is limited information about the extent to which the referral sources find the service useful. This quality improvement (QI) project aimed to better understand how well-established teleNP services were received by referral sources/stakeholders and how services could be improved. Prior to the advent of the CRH 20 teleNP program, staff had the option of referring for F2F evaluations in the local community (outside the VA) at some sites, an option that remains. This QI project examined staff perspectives on the usefulness of CRH 20 teleNP services compared with non-VA F2F services. We administered an anonymous, confidential survey examining these factors to VISN 20 staff within 4 VA health care systems.
METHODS
This QI project used a mixed quantitative and qualitative descriptive survey design to elicit feedback. The authors (3 neuropsychologists, 1 geropsychologist, and 1 research coordinator) developed the survey questions. The 13-question survey was voluntary, anonymous, and confidential, and respondents were given an opportunity to ask questions, with the first author serving as the point of contact.
The survey ascertained information about respondents and their work setting (ie, facility type, specific work setting and location, profession, and rurality of patients). First respondents were asked whether they have referred patients to neuropsychology services in the past year. Those who had not referred patients during the past year were asked about reasons for nonreferral with an option to provide an open-ended response. Respondents who did refer were asked how they refer for neuropsychology services and about the usefulness and timeliness of both teleNP and non-VA F2F services. Respondents were asked to respond with their preference for teleNP vs non-VA F2F with an open-ended prompt. Finally, respondents were invited to share any feedback for improvement regarding teleNP services.
A link to the survey, hosted on the VA Research Electronic Data Capture system, was emailed to facility and service line leaders at the 4 VISN 20 health care systems for distribution to the staff. All staff were included because in many of the facilities, particularly those that are highly rural with low staffing, it is not uncommon for technicians, nurses, and other support staff to assist with placing consults. In particular, VISN 20 nurses often have an optimal understanding of referral pathways to care for patients and are positioned to give and receive feedback about the utility of neuropsychological evaluations. The Research and Development Committee at the Boise VA Medical Center determined this project to be QI and exempt from institutional review board oversight. The VISN 20 employee labor relations HR supervisor approved this survey, with union awareness. Responses were anonymous.
Data were imported into Microsoft Excel and IBM SPSS Statistics for further analysis. Data were summarized using descriptive statistics, frequencies, and percentages. Nonparametric χ2 and Wilcoxon signed-rank tests were used to test for differences. An inductive approach to develop codes was used for the 3 open-ended questions. Two authors (CC, CEG) independently coded the responses and reviewed discrepancies. Final code applications were based on consensus.
RESULTS
The survey was deployed for 1 month between February 7, 2024, and June 15, 2024, at each of the 4 health care systems. Thirty-three staff members responded; of these, 1 person did not respond to an item on whether they referred for neuropsychology services. Eighteen of 33 respondents reported referring patients to teleNP or F2F neuropsychology services in the past year. Fourteen of the 33 respondents stated they did not refer; of these, 2 were unfamiliar with the teleNP service and 12 provided other reasons (eg, new to VA, not in their professional scope to order consults, did not have patients needing services).
The analysis focused on the 18 respondents who referred for neuropsychology services. Thirteen were within health care system A, and 5 were within health care system B (which had no nearby non-VA contracted neuropsychology services) and none were in the other 2 health care systems. Ten of 18 respondents (56%) stated they practiced primarily in a rural setting. Five respondents worked in a CBOC, 12 in a main VA facility, 9 in a primary care setting, 8 in a mental health setting, and 3 in other settings (eg, domiciliary). Participants could select > 1 setting. The 18 respondents who referred to neuropsychology services included 7 psychologists, 1 nurse, 2 social workers, 1 social services assistant, 4 nurse practitioners, 2 physicians, and 1 unknown HCP.
When asked to categorize the usefulness of services, more respondents characterized teleNP as very much so (1 on a 5-point scale) than F2F referrals (Figure). The mean (SD) of 1.5 (0.8) for teleNP usefulness fell between very much so and mostly and 1 respondent indicated not applicable. Similarly, the mean (SD) for non-VA F2F usefulness was 1.7 (0.9); 9 respondents rated this item as not applicable. A Wilcoxon signed-rank test of related samples indicated no significant differences between the pairs of ratings (Z = 1.50; P = .41).
Respondents with rural patients were more likely to refer them to teleNP services compared with respondents with nonrural patients (χ2 = 5.7; P = .02). However, ratings of teleNP usefulness did not significantly differ for those serving rural vs with nonrural patients (χ2 = 1.4; P = .49). Mean (SD) rating of teleNP usefulness was 1.3 (0.7) for the 9 rural subgroup respondents (between very much so and mostly) vs 1.8 (0.9) for the 8 nonrural subgroup respondents (between very much so and mostly). The mean (SD) rating for non-VA F2F usefulness was 1.8 (1.0) for the 4 rural subgroup respondents and 1.6 (0.8) for the 5 nonrural subgroup, between very much so and mostly for both groups.
Most respondents had no preference between teleNP or F2F. Notably, the responses underlying this group were multifaceted and corresponded to multiple codes (ie, access, preference for in-person services, technology, space and logistics, and service boundaries and requirements). According to 1 respondent, “the logistics of scheduling/room availability, technological challenges, and client behavioral issues that are likely to occur could possibly be more easily addressed via in-person sessions for some clients and providers.”
Six of 18 respondents preferred teleNP, citing timeliness, ease of access, and evaluation quality. One respondent noted that the “majority of my veterans live in extremely remote areas” and may need to take a plane for their visit. The 3 respondents who preferred in-person neuropsychology services cited veterans’ preference for in-person services.
Open-Ended Feedback
Thirteen respondents offered feedback on what is working well with teleNP services. Reasons mentioned were related to the service (ie, timeliness, access, quality) and the neuropsychologist (ie, communication and HCP skills). One respondent described the service and neuropsychologists positively, stating that they were “responsive, notes are readily available, clear assessments and recommendations, being available by [Microsoft] Teams/email.”
Ten respondents provided suggestions for improvement. Suggestions focused on expanding services, such as to “all veterans with cognitive/memory concerns that desire testing,” individuals with attention-deficit/hyperactivity disorder and co-occurring mental health concerns, and those in residential programs. Suggestions included hiring psychology technicians or more staff and providing education at local clinics.
DISCUSSION
This QI project examines VA staff perspectives on the usefulness of CRH 20 teleNP services and non-VA F2F services. While the small sample size limits generalizability, this preliminary study suggests that VA teleNP evaluations were similar to those conducted F2F in non-VA settings. While ratings of teleNP usefulness did not differ significantly for those serving rural vs nonrural veterans, respondents serving rural patients were more likely to refer patients to teleNP, suggesting that teleNP may increase access in rural settings, consistent with other studies.7,8,13 This article also presents qualitative suggestions for improving teleNP delivery within the VHA. This is the first known initiative to report on VHA staff satisfaction with a teleNP service and expands the limited literature to date on satisfaction with teleNP services. The findings provide initial support for continued use and, potentially, expansion of teleNP services within this CRH remote hub-and-spoke model.
Limitations
A significant limitation of the current work is the small sample size of survey respondents. In particular, while teleNP turnaround time was perceived as faster than non-VA F2F care, only 8 respondents reported on timeliness of F2F evaluation results, which renders it difficult to draw conclusions. Interestingly, not all respondents reported referring to neuropsychology services within the previous year; the most common reasons reflect the perception that referral to neuropsychology was outside of that staff member’s role or not clinically indicated.
One additional possible explanation for the absence of reporting on utility of teleNP specifically is that respondents did not track whether their patient was seen by teleNP or F2F services, based on how the referral process varies at each health care system. For example, in health care system C, a large number of referrals are forwarded to the service by local VA F2F neuropsychologists. This may speak to the seamlessness of the teleNP process, such that local staff and/or referring HCPs are unaware of the modality over which neuropsychology is being conducted. It is plausible that the reason behind this smaller response rate in health care systems B and C relates to how neuropsychology consults are processed at these local VAMCs. We suspect that in these settings, the HCPs referring for neuropsychological evaluations (eg, primary care, mental health) may be unaware that their referrals are being triaged to neuropsychologists in a different program (CRH 20 teleNP). Therefore, they would not necessarily know that they used teleNP and didn’t complete the survey.
The referral process for these 2 sites contrasts with the process for other VISN 20 sites where there is no local neuropsychology program triaging. In these settings, referrals from local HCPs come directly to teleNP; thus, it is more likely that these HCPs are aware of teleNP services. There were only 2 physicians who completed the survey, which may relate to their workload and a workflow where other staff have been increasingly requested to order the consults for the physician. This type of workflow results in an increase in the number of VHA staff involved in patient care. Ratings of usefulness were highest in health care system B, which does not have neuropsychology services at the facility or in the community; this may relate to elevated teleNP satisfaction ratings.
Further work may help identify which aspects of a teleNP service make it more useful than F2F care for this population or determine whether there were HCPor setting-specific factors that influenced the ratings (ie, preference for VA care or comparison of favorability ratings for the HCPs who conduct teleNP and F2F within the same system). The latter comparisons could not be drawn in the current systems due to the absence of HCPs who provide both teleNP and F2F modalities within VISN 20. Another consideration for future work would be to use a previously published/validated survey measure and piloting of questions with a naive sample before implementation.
CONCLUSIONS
This analysis provides initial support for feasibility and acceptability of teleNP as an alternative to traditional in-person neuropsychological evaluations. The small number of survey respondents may reflect the multiple pathways through which consults are forwarded to CRH 20, which includes both direct HCP referrals and forwarded consults from local neuropsychology services. CRH 20 has completed > 570 teleNP evaluations within 1 year, suggesting that lack of awareness may not be hindering veteran access to the service. Replication with a larger sample that is more broadly representative of key stakeholders in veteran care, identification of populations that would benefit most from teleNP services, and dissemination studies of the expansion of teleNP services are all important directions for future work. The robustness and longevity of the VISN 20 teleNP program, coupled with the preliminary positive findings from this project, demonstrate support for further assessment of the potential impact of telehealth on neuropsychological care within the VHA and show that barriers associated with access to health care services in remote settings may be mitigated through teleNP service delivery.
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated March 10, 2025. Accessed July 7, 2025. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Braun M, Tupper D, Kaufmann P, et al. Neuropsychological assessment: a valuable tool in the diagnosis and management of neurological, neurodevelopmental, medical, and psychiatric disorders. Cogn Behav Neurol. 2011;24(3):107-114. doi:10.1097/wnn.0b013e3182351289
- Donders J. The incremental value of neuropsychological assessment: a critical review. Clin Neuropsychol. 2020;34(1):56-87. doi:10.1080/13854046.2019.1575471
- Williams MW, Rapport LJ, Hanks RA, et al. Incremental value of neuropsychological evaluations to computed tomography in predicting long-term outcomes after traumatic brain injury. Clin Neuropsychol. 2013;27(3):356-375. doi:10.1080/13854046.2013.765507
- Sieg E, Mai Q, Mosti C, Brook M. The utility of neuropsychological consultation in identifying medical inpatients with suspected cognitive impairment at risk for greater hospital utilization. Clin Neuropsychol. 2019;33(1):75-89. doi:10.1080/13854046.2018.1465124
- Vankirk KM, Horner MD, Turner TH, et al. CE hospital service utilization is reduced following neuropsychological evaluation in a sample of U.S. veterans. Clin Neuropsychol. 2013;27(5):750-761. doi:10.1080/13854046.2013.783122
- Appleman ER, O’Connor MK, Boucher SJ, et al. Teleneuropsychology clinic development and patient satisfaction. Clin Neuropsychol. 2021;35(4):819-837. doi:10.1080/13854046.2020.1871515
- Stelmokas J, Ratcliffe LN, Lengu K, et al. Evaluation of teleneuropsychology services in veterans during COVID-19. Psychol Serv. 2024;21(1):65-72. doi:10.1037/ser0000810
- Bilder R Postal KS, Barisa M, et al. Inter Organizational Practice Committee recommendations/guidance for teleneuropsychology in response to the COVID-19 pandemic. Arch Clin Neuropsychol. 2020;35(6):647-659. doi:10.1093/arclin/acaa046
- Brearly TW, Shura RD, Martindale SL, et al. Neuropsychological test administration by videoconference: a systematic review and meta-analysis. Neuropsychol Rev. 2017;27(2):174-186. doi:10.1007/s11065-017-9349-1
- Brown AD, Kelso W, Eratne D, et al. Investigating equivalence of in-person and telehealth-based neuropsychological assessment performance for individuals being investigated for younger onset dementia. Arch Clin Neuropsychol. 2024;39(5):594-607. doi:10.1093/arclin/acad108
- Chapman JE, Ponsford J, Bagot KL, et al. The use of videoconferencing in clinical neuropsychology practice: a mixed methods evaluation of neuropsychologists’ experiences and views. Aust Psychol. 2020;55(6):618-633. doi:10.1111/ap.12471
- Marra DE, Hamlet KM, Bauer RM, et al. Validity of teleneuropsychology for older adults in response to COVID-19: a systematic and critical review. Clin Neuropsychol. 2020;34:1411-1452. doi:10.1080/13854046.2020.1769192
- Hammers DB, Stolwyk R, Harder L, et al. A survey of international clinical teleneuropsychology service provision prior to COVID-19. Clin Neuropsychol. 2020;34(7-8):1267- 1283. doi:10.1080/13854046.2020.1810323
- Marra DE, Hoelzle JB, Davis JJ, et al. Initial changes in neuropsychologists’ clinical practice during the COVID-19 pandemic: a survey study. Clin Neuropsychol. 2020;34(7- 8):1251-1266. doi:10.1080/13854046.2020.1800098
- Parsons MW, Gardner MM, Sherman, JC et al. Feasibility and acceptance of direct-to-home teleneuropsychology services during the COVID-19 pandemic. J Int Neuropsychol Soc. 2022;28(2):210-215. doi:10.1017/s1355617721000436
- Rochette AD, Rahman-Filipiak A, Spencer RJ, et al. Teleneuropsychology practice survey during COVID-19 within the United States. Appl Neuropsychol Adult. 2022;29(6):1312- 1322. doi:10.1080/23279095.2021.1872576
- Messler AC, Hargrave DD, Trittschuh EH, et al. National survey of telehealth neuropsychology practices: current attitudes, practices, and relevance of tele-neuropsychology three years after the onset of COVID-19. Clin Neuropsychol. 2023;39:1017-1036. doi:10.1080/13854046.2023.2192422
- Rautman L, Sordahl JA. Veteran satisfaction with tele-neuropsychology services. Clin Neuropsychol. 2018;32:1453949. doi:10.1080/13854046.2018.1453949
- US Department of Veterans Affairs. Patient care services: clinical resource hubs. Updated March 20, 2024. Accessed August 4, 2025. https://www.patientcare .va.gov/primarycare/CRH.asp
- Burnett K, Stockdale SE, Yoon J, et al. The Clinical Resource Hub initiative: first-year implementation of the Veterans Health Administration regional telehealth contingency staffing program. Ambul Care Manage. 2023;46(3):228-239. doi:10.1097/JAC.0000000000000468
- US Department of Veterans Affairs, Office of Rural Health. Rural veterans. Updated March 10, 2025. Accessed July 7, 2025. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Braun M, Tupper D, Kaufmann P, et al. Neuropsychological assessment: a valuable tool in the diagnosis and management of neurological, neurodevelopmental, medical, and psychiatric disorders. Cogn Behav Neurol. 2011;24(3):107-114. doi:10.1097/wnn.0b013e3182351289
- Donders J. The incremental value of neuropsychological assessment: a critical review. Clin Neuropsychol. 2020;34(1):56-87. doi:10.1080/13854046.2019.1575471
- Williams MW, Rapport LJ, Hanks RA, et al. Incremental value of neuropsychological evaluations to computed tomography in predicting long-term outcomes after traumatic brain injury. Clin Neuropsychol. 2013;27(3):356-375. doi:10.1080/13854046.2013.765507
- Sieg E, Mai Q, Mosti C, Brook M. The utility of neuropsychological consultation in identifying medical inpatients with suspected cognitive impairment at risk for greater hospital utilization. Clin Neuropsychol. 2019;33(1):75-89. doi:10.1080/13854046.2018.1465124
- Vankirk KM, Horner MD, Turner TH, et al. CE hospital service utilization is reduced following neuropsychological evaluation in a sample of U.S. veterans. Clin Neuropsychol. 2013;27(5):750-761. doi:10.1080/13854046.2013.783122
- Appleman ER, O’Connor MK, Boucher SJ, et al. Teleneuropsychology clinic development and patient satisfaction. Clin Neuropsychol. 2021;35(4):819-837. doi:10.1080/13854046.2020.1871515
- Stelmokas J, Ratcliffe LN, Lengu K, et al. Evaluation of teleneuropsychology services in veterans during COVID-19. Psychol Serv. 2024;21(1):65-72. doi:10.1037/ser0000810
- Bilder R Postal KS, Barisa M, et al. Inter Organizational Practice Committee recommendations/guidance for teleneuropsychology in response to the COVID-19 pandemic. Arch Clin Neuropsychol. 2020;35(6):647-659. doi:10.1093/arclin/acaa046
- Brearly TW, Shura RD, Martindale SL, et al. Neuropsychological test administration by videoconference: a systematic review and meta-analysis. Neuropsychol Rev. 2017;27(2):174-186. doi:10.1007/s11065-017-9349-1
- Brown AD, Kelso W, Eratne D, et al. Investigating equivalence of in-person and telehealth-based neuropsychological assessment performance for individuals being investigated for younger onset dementia. Arch Clin Neuropsychol. 2024;39(5):594-607. doi:10.1093/arclin/acad108
- Chapman JE, Ponsford J, Bagot KL, et al. The use of videoconferencing in clinical neuropsychology practice: a mixed methods evaluation of neuropsychologists’ experiences and views. Aust Psychol. 2020;55(6):618-633. doi:10.1111/ap.12471
- Marra DE, Hamlet KM, Bauer RM, et al. Validity of teleneuropsychology for older adults in response to COVID-19: a systematic and critical review. Clin Neuropsychol. 2020;34:1411-1452. doi:10.1080/13854046.2020.1769192
- Hammers DB, Stolwyk R, Harder L, et al. A survey of international clinical teleneuropsychology service provision prior to COVID-19. Clin Neuropsychol. 2020;34(7-8):1267- 1283. doi:10.1080/13854046.2020.1810323
- Marra DE, Hoelzle JB, Davis JJ, et al. Initial changes in neuropsychologists’ clinical practice during the COVID-19 pandemic: a survey study. Clin Neuropsychol. 2020;34(7- 8):1251-1266. doi:10.1080/13854046.2020.1800098
- Parsons MW, Gardner MM, Sherman, JC et al. Feasibility and acceptance of direct-to-home teleneuropsychology services during the COVID-19 pandemic. J Int Neuropsychol Soc. 2022;28(2):210-215. doi:10.1017/s1355617721000436
- Rochette AD, Rahman-Filipiak A, Spencer RJ, et al. Teleneuropsychology practice survey during COVID-19 within the United States. Appl Neuropsychol Adult. 2022;29(6):1312- 1322. doi:10.1080/23279095.2021.1872576
- Messler AC, Hargrave DD, Trittschuh EH, et al. National survey of telehealth neuropsychology practices: current attitudes, practices, and relevance of tele-neuropsychology three years after the onset of COVID-19. Clin Neuropsychol. 2023;39:1017-1036. doi:10.1080/13854046.2023.2192422
- Rautman L, Sordahl JA. Veteran satisfaction with tele-neuropsychology services. Clin Neuropsychol. 2018;32:1453949. doi:10.1080/13854046.2018.1453949
- US Department of Veterans Affairs. Patient care services: clinical resource hubs. Updated March 20, 2024. Accessed August 4, 2025. https://www.patientcare .va.gov/primarycare/CRH.asp
- Burnett K, Stockdale SE, Yoon J, et al. The Clinical Resource Hub initiative: first-year implementation of the Veterans Health Administration regional telehealth contingency staffing program. Ambul Care Manage. 2023;46(3):228-239. doi:10.1097/JAC.0000000000000468
Staff Perspectives on the VISN 20 Tele-Neuropsychology Program
Staff Perspectives on the VISN 20 Tele-Neuropsychology Program
Gadolinium Intermediate Elimination and Persistent Symptoms After Magnetic Resonance Imaging Contrast Agent Exposure
Gadolinium Intermediate Elimination and Persistent Symptoms After Magnetic Resonance Imaging Contrast Agent Exposure
Magnetic resonance image (MRI) contrast agents can induce profound complications, including gadolinium encephalopathy, kidney injury, gadolinium-associated plaques, and progressive systemic fibrosis, which can be fatal.1-10 About 50% of MRIs use gadolinium-based contrast (Gd3+), a toxic rare earth metal ion that enhances imaging but requires binding with pharmaceutical ligands to reduce toxicity and promote renal elimination (Figure 1). Despite these measures, Gd3+ can persist in the body, including the brain.11,12 Wastewater treatment fails to remove these agents, making Gd3+ a growing pollutant in water and the food chain.13-15 Because Gd3+ is a rare earth metal ion in the milieu intérieur, there is an urgent need to study its biological and long-term effects (Appendix 1).
Case Presentation
A 65-year-old Vietnam-era veteran presented to nephrology at the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, for evaluation of gadolinium-induced symptoms. His medical history included metabolic syndrome, hypertension, hyperlipidemia, hypogonadism, cervical spondylosis, and an elevated prostate-specific antigen, previously assessed with a contrast-enhanced MRI in 2019 (Gadobenic acid, 19 mL). Surgical history included cervical fusion and ankle hardware.
The patient had a scheduled MRI 25 days earlier, following an elevated prostate specific antigen test result, prompting urologic surveillance and concern for malignancy. In preparation for the contrast-enhanced MRI, his right arm was cannulated with a line primed with gadobenic acid contrast. Though the technician stated the infusion had not started, the patient’s symptoms began shortly after entry into the scanner, before any programmed pulse sequences. The patient experienced claustrophobia, diaphoresis, palpitations, xerostomia, dysgeusia, shortness of breath, and a sensation of heat in his groin, chest, “kidneys,” and lower back. The MRI was terminated prematurely in response to the patient’s acute symptomatology. The patient continued experiencing new symptoms intermittently during the following week, including lightheadedness, headaches, right clavicular pain, raspy voice, edema, and a sense of doom.
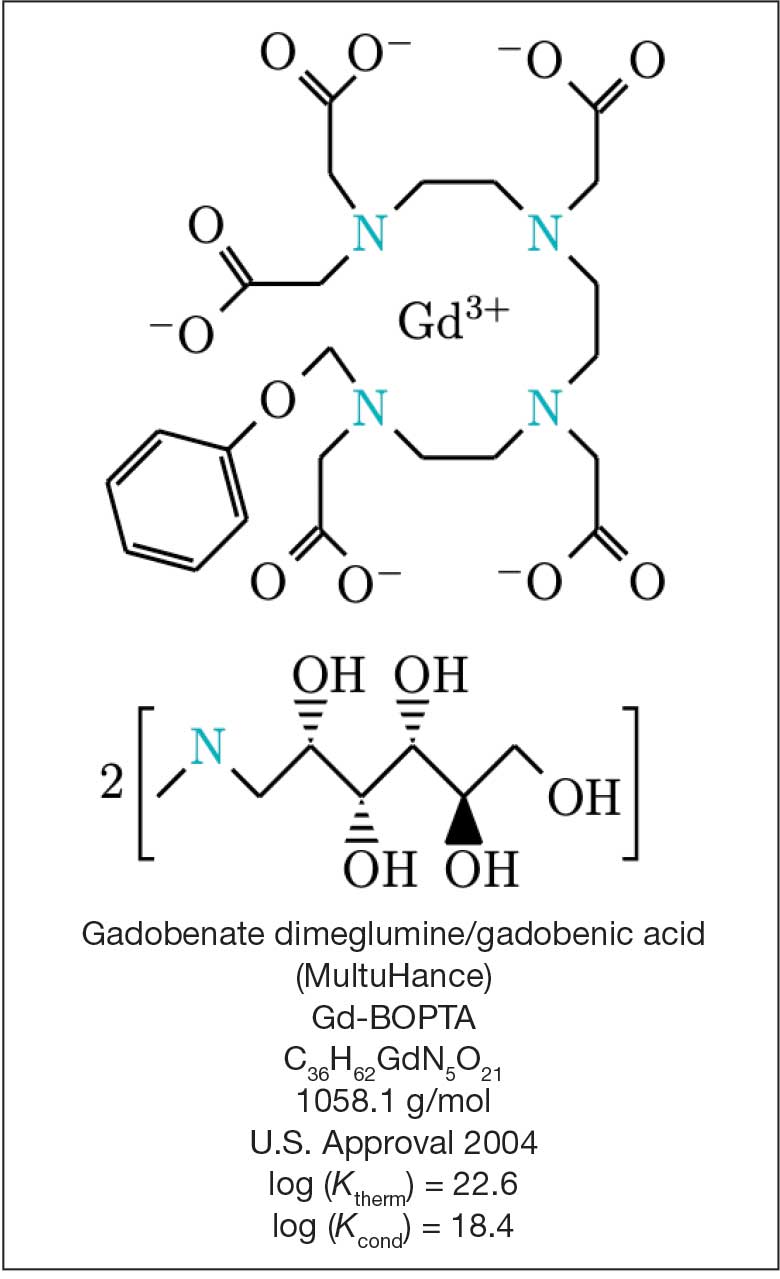
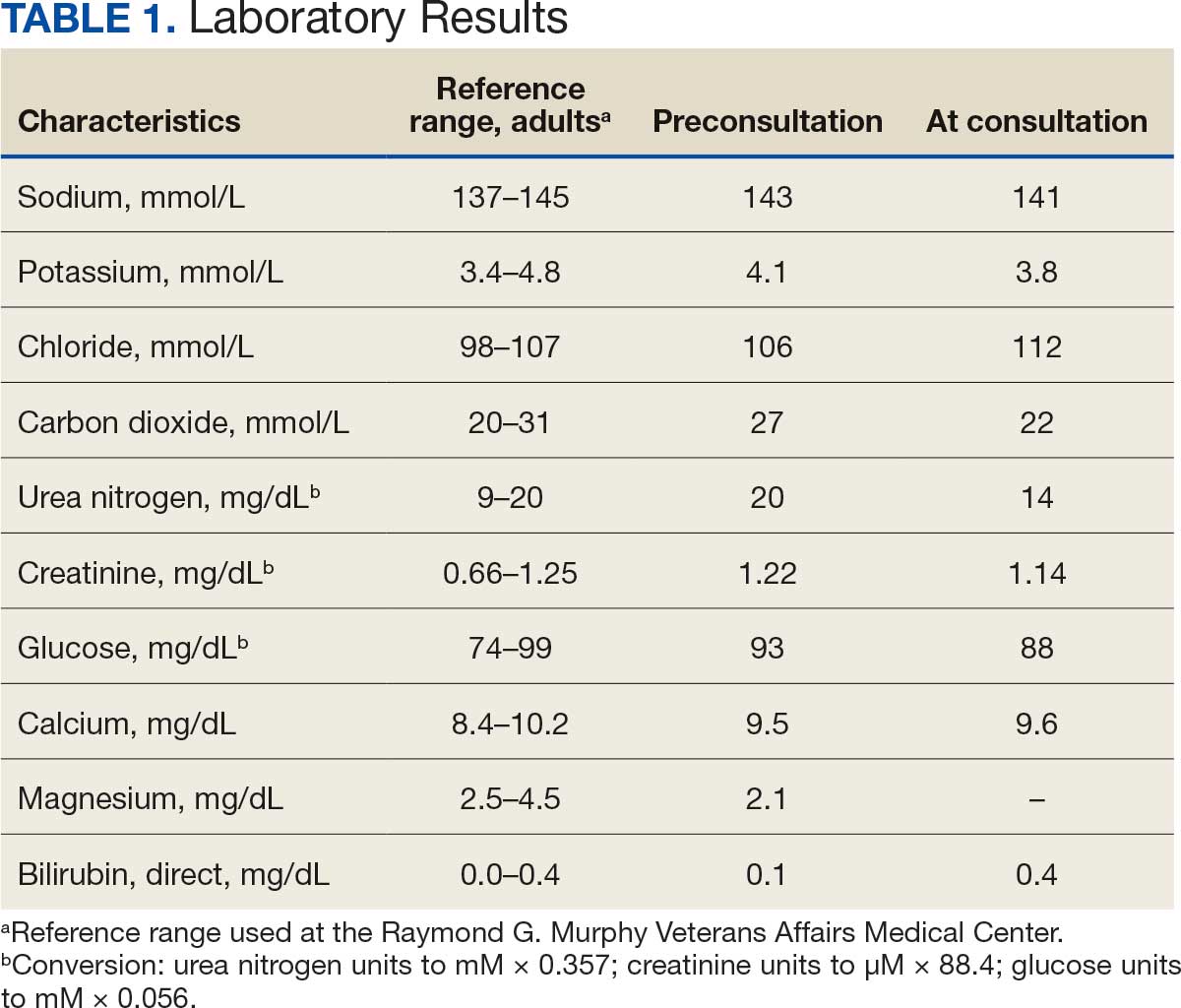
The patient presented to the RGMVAMC emergency department (ED) 8 days after the MRI with worsening symptoms and was hospitalized for 10 days. During this time, he was referred to nephrology for outpatient evaluation. While awaiting his nephrology appointment, the patient presented to the RGMVAMC ED 20 days after the initial episode with ongoing symptoms. “I thought I was dying,” he said. Laboratory results and a 12-lead electrocardiogram showed a finely static background, wide P waves (> 80 ms) with notching in lead II, sinusoidal P waves in V1, R transition in V2, RR’ in V2, ST flat in lead III, and sinus bradycardia (Table 1 and Appendix 2).
The patient’s medical and surgical histories were reviewed at the nephrology evaluation 25 days following the MRI. He reported that household water was sourced from a well and that he filtered his drinking water with a reverse osmosis system. He served in the US Army for 10 years as an engineer specializing in mechanical systems, power generation, and vehicles. Following Army retirement, the patient served in the US Air Force Reserves for 15 years, working as a crew chief in pneudraulics. The patient reported stopping tobacco use 1 year before and also reported regular use of a broad array of prescription medications and dietary supplements, including dexamethasone (4 mg twice daily), fluticasone nasal spray (50 mcg per nostril, twice daily), ibuprofen (400 mg twice daily, as needed), loratadine (10 mg daily), aspirin (81 mg daily), and metoprolol succinate (50 mg nightly). In addition, he reported consistent use of cholecalciferol (3000 IU daily), another supplemental vitamin D preparation, chelated magnesium glycinate (3 tablets daily for bone issues), turmeric (1 tablet daily), a multivitamin (Living Green Liquid Gel, daily), and a mega-B complex.
Physical examination revealed a well-nourished, tall man with hypertension (145/87 mmHg) and bilateral lower extremity edema. Oral examination showed poor dentition, including missing molars (#1-3, #14-16, #17-19, #30-31), with the anterior teeth replaced by bridges supported by dental implants. The review of systems was otherwise unremarkable, with nocturia noted before the consultation.
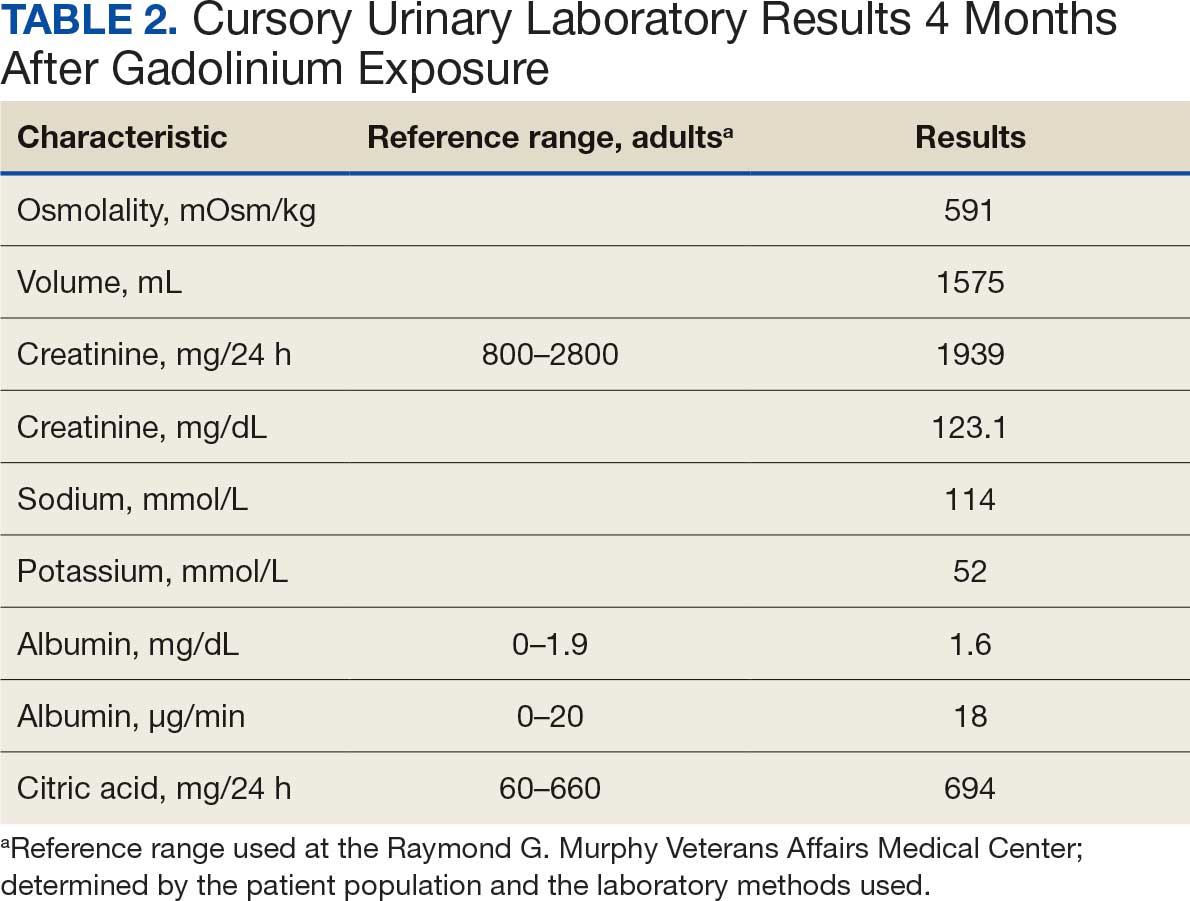
Serum and urine gadolinium testing, (Mayo Clinic Laboratories) revealed gadolinium levels of 0.3 mcg/24 h in the urine and 0.1 ng/mL in the serum. Nonzero values indicated detectable gadolinium, suggesting retention. The patient had a prior gadolinium exposure during a 2019 MRI (about 1340 days before) and suspected a repeat exposure on day 0, although the MRI technician stated that no contrast was administered. Given his elevated vitamin D levels, the patient was advised to minimize dietary supplements, particularly vitamin D, to avoid confounding symptoms. The plan included monitoring symptoms and a follow-up evaluation with repeat laboratory tests on day 116.
At the nephrology follow-up 4 months postexposure, the patient's symptoms had primarily abated, with a marked reduction in the previously noted metallic dysgeusia. Physical examination remained consistent with prior findings. He was afebrile (97.7 °F) with a blood pressure of 111/72 mmHg, a pulse of 63 beats per minute, and an oxygen saturation of 98% on ambient air. Laboratory analysis revealed serum and urine gadolinium levels below detectable thresholds (< 0.1 ng/mL and < 0.1 mcg/24 h). A 24-hour creatinine clearance, calculated from a urine volume of 1300 mL, measured at an optimal 106 mL/min, indicating preserved renal function (Tables 2 and 3). Of note, his 24-hour oxalate was above the reference range, with a urine pH below the reference range and a high supersaturation index for calcium oxalate.
Discussion
Use of enhanced MRI has increased in the Veterans Health Administration (Figure 2). A growing range of indications for enhanced procedures (eg, cardiac MRI) has contributed to this rise. The market has grown with new gadolinium-based contrast agents, such as gadopiclenol. However, reliance on untested assumptions about the safety of newer agents and need for robust clinical trials pose potential risks to patient safety.
Without prospective evidence, the American College of Radiology (ACR) classifies gadolinium-based contrast agents into 3 groups: Group 1, associated with the highest number of nephrogenic systemic fibrosis cases; Group 2, linked to few, if any, unconfounded cases; and Group 3, where data on nephrogenic systemic fibrosis risk have been limited. As of April 2024, the ACR reclassified Group 3 agents (Ablavar/Vasovist/Angiomark and Primovist/Eovist) into Group 2. Curiously, Vueway and Elucirem were approved in late 2022 and should clearly be categorized as Group 3 (Table 4).There were 19 cases of nephrogenic systemic fibrosis or similar manifestations, 8 of which were unconfounded by other factors. These patients had been exposed to gadobutrol, often combined with other agents. Gadobutrol—like other Group 2 agents—has been associated with nephrogenic systemic fibrosis.16,17 Despite US Food and Drug Administration (FDA) documentation of rising reports, many clinicians remain unaware that nephrogenic systemic fibrosis is increasingly linked to Group 2 agents classified by the ACR.18 While declines in reported cases of nephrogenic systemic fibrosis may suggest reduced incidence, this trend may reflect diminished clinical vigilance and underreporting, particularly given emerging evidence implicating even Group 2 gadolinium-based contrast agents in delayed and underrecognized presentations. This information has yet to permeate the medical community, particularly among nephrologists. Considering these cases, revisiting the ACR guidelines may be prudent.
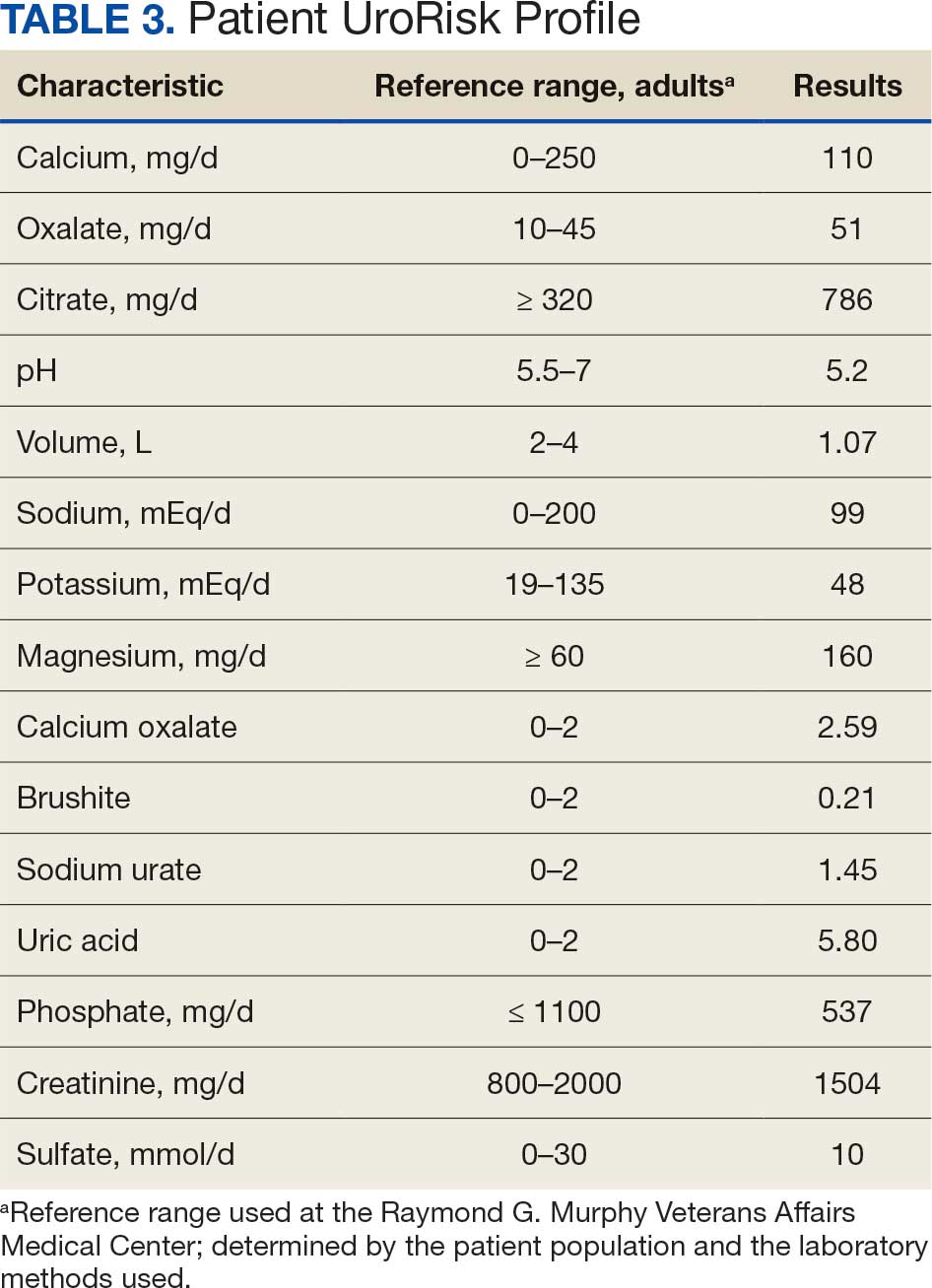
To address this growing concern, clinicians must adopt stricter vigilance and actively pursue updated information to mitigate patient risks tied to these contrast agents.
There exists an illusion of knowledge in disregarding the confounded exposures of MRI contrast agents. Ten distinct brands of contrast agents have been approved for clinical use. With repeated imaging, patients are often exposed to varying formulations of gadolinium-based agents. Yet investigators commonly discard these data points when assessing risk. By doing so, they assume—without evidence—that some formulations are inherently less likely to provoke adverse effects (AEs) than others. This untested presumption becomes perilous, especially given the limited understanding of the mechanisms underlying gadolinium-induced pathologies. As Aldous Huxley warned, “Facts do not cease to exist because they are ignored.”19
Gadolinium Persistence
Contrary to expectations, gadolinium persists in the body far longer than initially presumed. Symptoms associated with gadolinium exposure (SAGE) encapsulate the chronic, often enigmatic maladies tied to MRI contrast agents.20 The prolonged retention of this rare earth metal offers a compelling hypothesis for the etiology of SAGE. It has been hypothesized that Lewis base-rich metabolites increase susceptibility to gadolinium-based contrast agent complications.21
The blood and urine concentration elimination curves of gadolinium are exponential and categorized as fast, intermediate, and long-term.1 For urinary elimination, the function of the curves is exponential. The quantity of gadolinium in the urine at a time (t) after exposure (D[Gd](t)) is equal to the product of the amount of gadolinium in the sample (urine or blood) at the end of the fast elimination period (D[Gd](t0)) and the exponential decay with k being a rate constant.
To the authors’ knowledge, we are the only research team currently investigating the rate constant for the intermediate- and long-term phase gadolinium elimination. The Retention and Toxicity of Gadolinium-based Contrast Agents study was approved by the University of New Mexico Health Sciences Center Institutional Review Board on May 27, 2020 (IRB ID 19-660). The data for the patient in this case were compared with preliminary results for patients with exposure-to-measurement intervals < 100 days.
The patient in this case presented with detectable gadolinium levels in urine and serum shortly after an attempted contrast-enhanced MRI procedure (Figure 3). The presence of detectable gadolinium levels in the patient’s urine and serum suggests a likely exposure to a contrast agent about 27 days before his consultation. While the technician reported that no contrast was administered during the attempted MRI, it remains possible that a small amount was introduced during cannulation, potentially triggering the patient’s symptoms. Linear modeling of semilogarithmic plots for participants exposed to contrast agents within 100 days (urine: P = 1.8 × 10ˉ8, adjusted r² = 0.62; blood: P = .005, adjusted r² = 0.21) provided clearance rates (k values) for urine and blood. Extrapolating from these models to the presumed exposure date, the intercepts estimate that the patient received between 0.5% and 8% of a standard contrast dose.
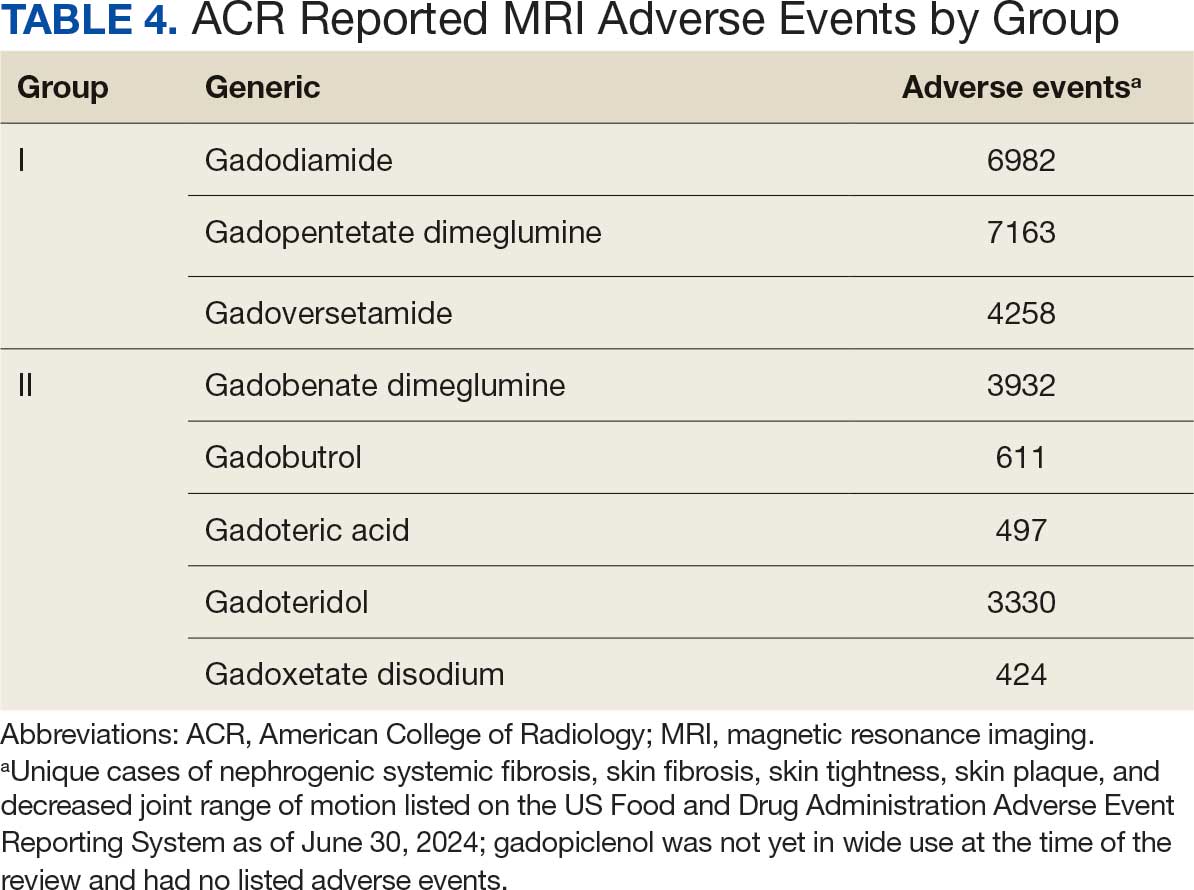
MRI contrast agents can cause skin disease. Systemic fibrosis is considered one of the most severe AEs. Skin pathophysiology involving myeloid cells is driven by elevated levels of monocyte chemoattractant protein-1, which recruits circulating fibroblasts via the C-C chemokine receptor 2.22,23 This occurs alongside activation of NADPH oxidase Nox4.4,24,25 Intracellular gadolinium-rich nanoparticles likely serve as catalysts for this reactive cascade.2,18,22,26,27 These particles assemble around intracellular lipid droplets and ferrule them in spiculated rare earth-rich shells that compromise cellular architecture.2,18,21,22,26,27 Frequently sequestered within endosomal compartments, they disrupt vesicular integrity and threaten cellular homeostasis. Interference with degradative systems such as the endolysosomal axis perturbs energy-recycling pathways—an insidious disturbance, particularly in cells with high metabolic demand. Skin-related symptoms are among the most frequently reported AEs, according to the FDA AE reporting system.18
Studies indicate repeated exposure to MRI contrast agents can lead to permanent gadolinium retention in the brain and other vital organs. Intravenous (IV) contrast agents cross the blood-brain barrier rapidly, while intrathecal administration has been linked to significant and lasting neurologic effects.18
Gadolinium is chemically bound to pharmaceutical ligands to enhance renal clearance and reduce toxicity. However, available data from human samples suggest potential ligand exchanges with undefined physiologic substances. This exchange may facilitate gadolinium precipitation and accumulation within cells into spiculated nanoparticles. Transmission electron microscopy reveals the formation of unilamellar bodies associated with mitochondriopathy and cellular damage, particularly in renal proximal tubules.2,18,22,26,27 It is proposed that intracellular nanoparticle formation represents a key mechanism driving the systemic symptoms observed in patients.1,2,18, 22,26,27
Any hypothesis based on free soluble gadolinium—or concept derived from it—should be discarded. The high affinity of pharmaceutical ligands for gadolinium suggests that the cationic rare earth metal remains predominantly in a ligand-bound, soluble form. It is hypothesized that gadolinium undergoes ligand exchange with physiologic substances, directly leading to nanoparticle formation. Current data demonstrate gadolinium precipitation according to the Le Chatelier’s principle. Since precipitated gadolinium does not readily re-equilibrate with pharmaceutical ligands, repeated administration of different contrast agent brands may contribute to nanoparticle growth.26
Meanwhile, a growing number of patients are turning to chelation therapy, a largely untested treatment. The premise of chelation therapy is rooted in several unproven assumptions.18,21 First, it assumes that clinically significant amounts of gadolinium persist in compartments such as the extracellular space, where they can be effectively chelated and cleared. Second, it presumes that free gadolinium is the primary driver of chronic symptoms, an assertion that remains scientifically unsubstantiated. Finally, chelation proponents overlook the potential harm caused by depleting essential physiological metals during the process, assuming without evidence that the scant removal of gadolinium outweighs the risk of physiological mineral depletion.
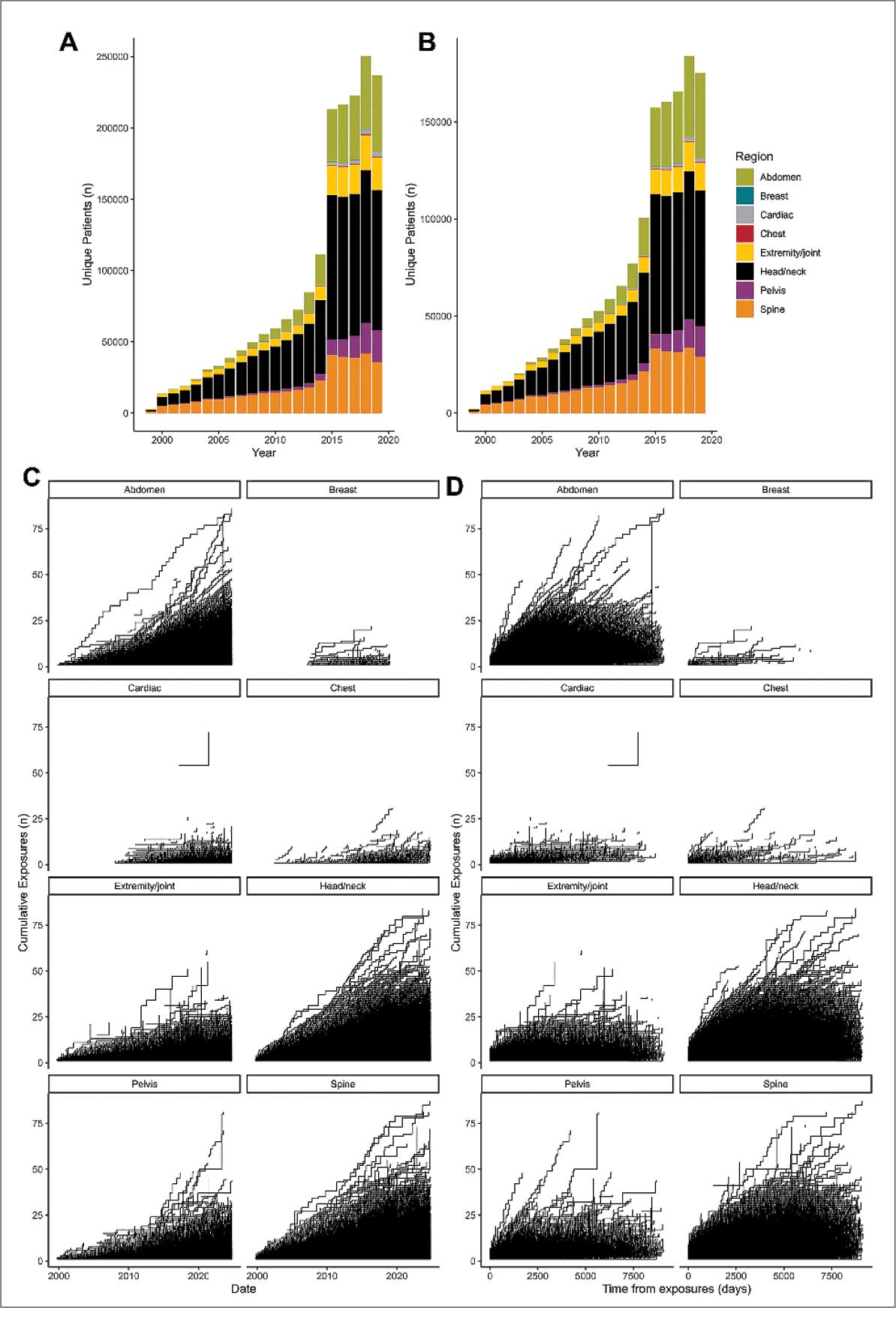
These assumptions underpin an unproven remedy that demands critical scrutiny. Recent findings reveal that gadolinium deposits in the skin and kidney often take the form of intracellular nanoparticles, directly challenging the foundation of chelation therapy. Chelation advocates must demonstrate that these intracellular gadolinium deposits neither trigger cellular toxicity nor initiate a cytokine cascade. Chelation supporters must prove that the systemic response to these foreign particles is unrelated to the symptoms reported by patients. Until then, the validity of chelation therapy remains highly questionable.
The causality of the symptoms, mainly whether IV gadolinium was administered, was examined. The null hypothesis stated that the patient was not exposed to gadolinium. However, this hypothesis was contradicted by the detection of gadolinium in the serum and urine 27 days after the potential exposure.
Two plausible explanations exist for the nonzero gadolinium levels detected in the serum and urine. The first possibility is that minute quantities of gadolinium were introduced during cannulation, with the amount being sufficient to persist in measurable concentrations 27 days postexposure. The second possibility is that the gadolinium originated from an MRI contrast agent administered 4 years earlier. In this scenario, gadolinium stored in organ reservoirs such as bone, liver, or kidneys may have been mobilized into the extracellular fluid compartment due to the administration of high-dose steroids 20 days after the recent contrast-enhanced MRI procedure attempt. Coyte et al reported elevated gadolinium levels in the serum, cord blood, breast milk, and placenta of pregnant women with prior exposure to MRI contrast agents.28 These findings suggest that gadolinium, stored in organs such as bone may be remobilized by variables affecting bone remodeling (eg, high-dose steroids).
Significantly, the patient exhibited elevated urinary oxalate levels. Previous research has found that oxalic acid reacts rapidly with MRI contrast agents, forming digadolinium trioxalate. While the gadolinium-rich nanoparticles identified in tissues such as the skin and kidney (including the human kidney) are amorphous, these in vitro findings establish a proof-of-concept: the intracellular environment facilitates gadolinium dissociation from pharmaceutical chelates.
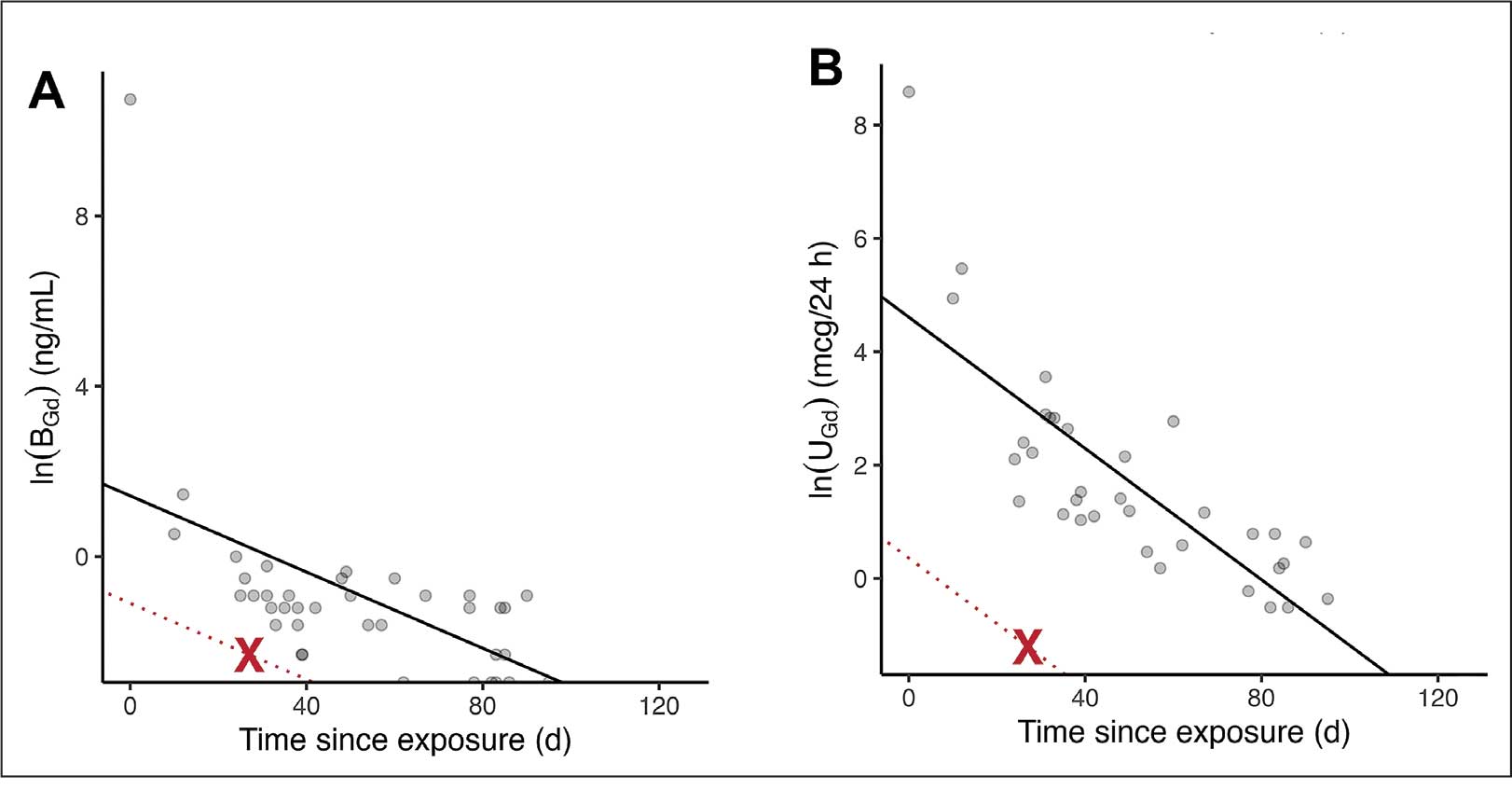
Furthermore, in vitro experiments show that proteins and lysosomal pH promote this dissociation, underscoring how human metabolic conditions—particularly oxalic acid concentration—may drive intracellular gadolinium deposition.
Patient Perspective
“They put something into my body that they cannot get out.” This stark realization underpins the patient’s profound concern about gadolinium-based contrast agents and their potential long-term effects. Reflecting on his experience, the patient expressed deep fears about the unknown future impacts: “I’m concerned about my kidneys, I’m concerned about my heart, and I’m concerned about my brain. I don’t know how this stuff is going to affect me in the future.”
He drew an unsettling parallel between gadolinium and heavy metals: “Heavy metal is poison. The body does not produce this kind of stuff on its own.” His reaction to the procedure left a lasting impression, prompting him to question the logic of using a substance that cannot be purged: “Why would you put something into someone’s body that you cannot extract? Nobody—nobody—should experience what I went through.”
The patient emphasized the lack of clear research on long-term outcomes, which compounds his anxiety: “If there was research that said, ‘Well, this is only going to affect these organs for this long,’ OK, I might be able to accept that. But there is no research like that. Nobody can tell me what’s going to happen in 5 years.”
Strengths and Limitations
A significant strength of this approach is the ability to track gadolinium elimination and symptom resolution over time, supported by unique access to intermediate and long-term clearance data from our ongoing research protocol. The investigators were equipped to back-extrapolate the exposure, which provided a rare opportunity to correlate gadolinium levels with clinical outcomes. The primary limitation is the lack of a defined clinical case definition for gadolinium toxicity and limited mechanistic understanding of SAGE, which hinders diagnosis and management.
Metabolites, proteins, and lipids rich in Lewis bases could initiate this process as substrates for intracellular gadolinium sedimentation. Future studies should investigate whether metabolic conditions such as oxalate burden or altered parathyroid hormone levels modulate gadolinium compartmentalization and tissue retention. If gadolinium-rich nanoparticle formation and accumulation disrupt cellular equilibrium, it underscores an urgent need to understand the implications of long-term gadolinium retention. The research team continues to gather evidence that the gadolinium cation remains chelated from the moment MRI contrast agents are administered through to the formation of intracellular nanoparticles. Retained gadolinium nanoparticles may act as a nidus, triggering cellular signaling cascades that lead to multisymptomatic illnesses. Intracellular and insoluble retained gadolinium challenges proponents of untested chelation therapies.
Conclusions
This case highlights emerging clinical and ethical concerns surrounding gadolinium-based contrast agent use. Clinicians may benefit from considering gadolinium retention as a contributor to persistent, unexplained symptoms—particularly in patients with recent imaging exposure. As contrast use continues to rise within federal health systems, regulatory and administrative stakeholders would do well to re-examine current safety frameworks. Informed consent should reflect what is known: gadolinium can remain in the body long after administration, potentially indefinitely. The long-term consequences of cumulative exposure remain poorly defined, but the presence of a lanthanide element in human tissue warrants greater attention from researchers and regulators alike. Interest in alternative imaging modalities and long-term safety monitoring would mark progress toward more transparent, accountable care.
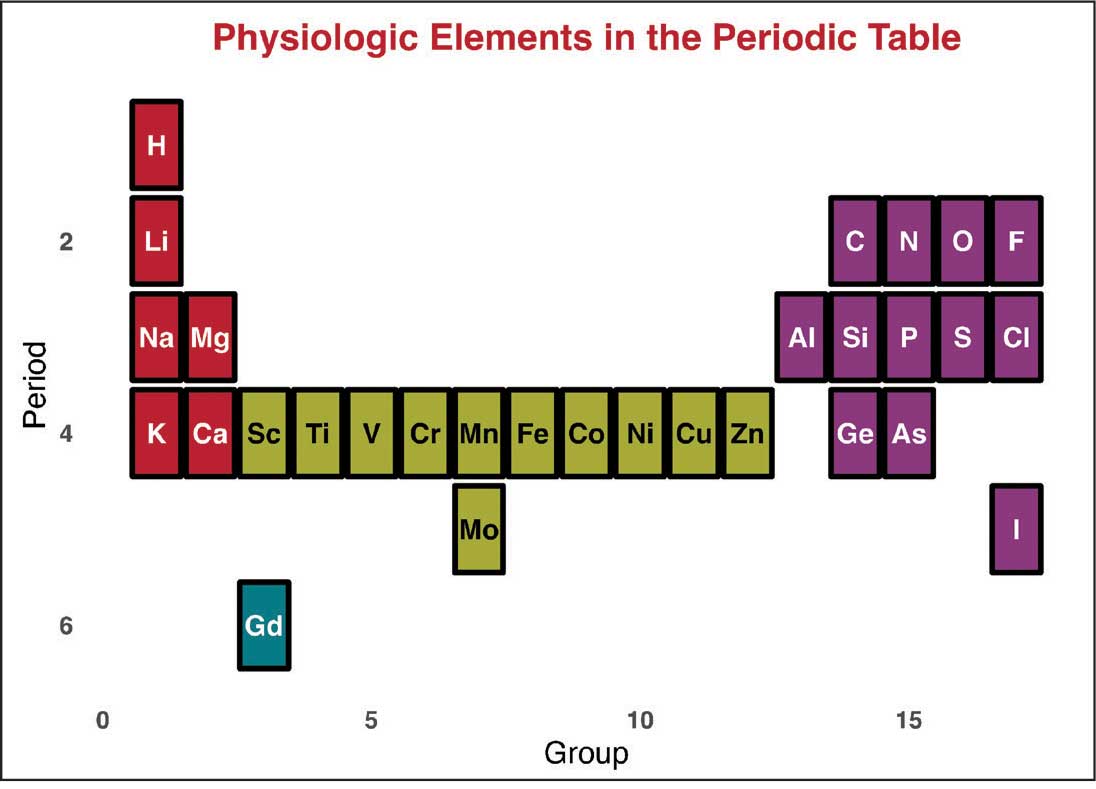
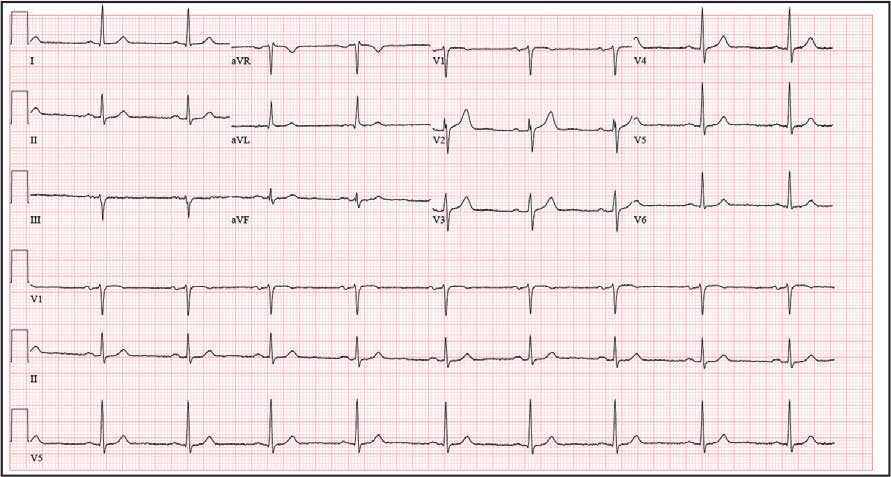
Jackson DB, MacIntyre T, Duarte-Miramontes V, et al. Gadolinium deposition disease: a case report and the prevalence of enhanced MRI procedures within the Veterans Health Administration. Fed Pract. 2022;39:218-225. doi:10.12788/fp.0258
Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1:561-568.doi:10.34067/kid.0000272019
Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28:154-162. doi:10.1097/MNH.0000000000000475
Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311:F1-F11. doi:10.1152/ajprenal.00166.2016
Maramattom BV, Manno EM, Wijdicks EF, et al. Gadolinium encephalopathy in a patient with renal failure. Neurology. 2005;64:1276-1278.doi:10.1212/01.WNL.0000156805.45547.6E
Sam AD II, Morasch MD, Collins J, et al. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg. 2003;38:313-318. doi:10.1016/s0741-5214(03)00315-x
Schenker MP, Solomon JA, Roberts DA. Gadolinium arteriography complicated by acute pancreatitis and acute renal failure. J Vasc Interv Radiol. 2001;12:393. doi:10.1016/s1051-0443(07)61925-3
Gemery J, Idelson B, Reid S, et al. Acute renal failure after arteriography with a gadolinium-based contrast agent. AJR Am J Roentgenol. 1998;171:1277-1278. doi:10.2214/ajr.171.5.9798860
Akgun H, Gonlusen G, Cartwright J Jr, et al. Are gadolinium-based contrast media nephrotoxic? A renal biopsy study. Arch Pathol Lab Med. 2006;130:1354-1357. doi:10.5858/2006-130-1354-AGCMNA
Gathings RM, Reddy R, Santa Cruz D, et al. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151:316-319. doi:10.1001/jamadermatol.2014.2660
McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834-841. doi:10.1148/radiol.13131669
Schmidt K, Bau M, Merschel G, et al. Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci Total Environ. 2019;687:1401-1408. doi:10.1016/j.scitotenv.2019.07.075
Kulaksız S, Bau M. Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl Geochem. 2011;26:1877-1885.
Brunjes R, Hofmann T. Anthropogenic gadolinium in freshwater and drinking water systems. Water Res. 2020;182:115966. doi:10.1016/j.watres.2020.115966
Endrikat J, Gutberlet M, Hoffmann KT, et al. Clinical safety of gadobutrol: review of over 25 years of use exceeding 100 million administrations. Invest Radiol. 2024;59(9):605-613. doi:10.1097/RLI.0000000000001072
Elmholdt TR, Jørgensen B, Ramsing M, et al. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287. doi:10.1093/ndtplus/sfq028
Cunningham A, Kirk M, Hong E, et al. The safety of magnetic resonance imaging contrast agents. Front Toxicol. 2024;6:1376587. doi:10.3389/ftox.2024.1376587
Huxley A. Complete Essays. Volume II, 1926-1929. Chicago; 2000:227.
McDonald RJ, Weinreb JC, Davenport MS. Symptoms associated with gadolinium exposure (SAGE): a suggested term. Radiology. 2022;302(2):270-273. doi:10.1148/radiol.2021211349
Henderson IM, Benevidez AD, Mowry CD, et al. Precipitation of gadolinium from magnetic resonance imaging contrast agents may be the Brass tacks of toxicity. Magn Reson Imaging. 2025;119:110383. doi:10.1016/j.mri.2025.110383
Do C, Drel V, Tan C, et al. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143. doi:10.1016/j.jid.2019.03.1145
Drel VR, Tan C, Barnes JL, et al. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH oxidase 4 for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
DeAguero J, Howard T, Kusewitt D, et al. The onset of rare earth metallosis begins with renal gadolinium-rich nanoparticles from magnetic resonance imaging contrast agent exposure. Sci Rep. 2023;13(1):2025. doi:10.1038/s41598-023-28666-1
Do C, Ford B, Lee DY, et al. Gadolinium-based contrast agents: Stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
Coyte RM, Darrah T, Olesik J, et al. Gadolinium during human pregnancy following administration of gadolinium chelate before pregnancy. Birth Defects Res. 2023;115(14):1264-1273. doi:10.1002/bdr2.2209
Magnetic resonance image (MRI) contrast agents can induce profound complications, including gadolinium encephalopathy, kidney injury, gadolinium-associated plaques, and progressive systemic fibrosis, which can be fatal.1-10 About 50% of MRIs use gadolinium-based contrast (Gd3+), a toxic rare earth metal ion that enhances imaging but requires binding with pharmaceutical ligands to reduce toxicity and promote renal elimination (Figure 1). Despite these measures, Gd3+ can persist in the body, including the brain.11,12 Wastewater treatment fails to remove these agents, making Gd3+ a growing pollutant in water and the food chain.13-15 Because Gd3+ is a rare earth metal ion in the milieu intérieur, there is an urgent need to study its biological and long-term effects (Appendix 1).
Case Presentation
A 65-year-old Vietnam-era veteran presented to nephrology at the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, for evaluation of gadolinium-induced symptoms. His medical history included metabolic syndrome, hypertension, hyperlipidemia, hypogonadism, cervical spondylosis, and an elevated prostate-specific antigen, previously assessed with a contrast-enhanced MRI in 2019 (Gadobenic acid, 19 mL). Surgical history included cervical fusion and ankle hardware.
The patient had a scheduled MRI 25 days earlier, following an elevated prostate specific antigen test result, prompting urologic surveillance and concern for malignancy. In preparation for the contrast-enhanced MRI, his right arm was cannulated with a line primed with gadobenic acid contrast. Though the technician stated the infusion had not started, the patient’s symptoms began shortly after entry into the scanner, before any programmed pulse sequences. The patient experienced claustrophobia, diaphoresis, palpitations, xerostomia, dysgeusia, shortness of breath, and a sensation of heat in his groin, chest, “kidneys,” and lower back. The MRI was terminated prematurely in response to the patient’s acute symptomatology. The patient continued experiencing new symptoms intermittently during the following week, including lightheadedness, headaches, right clavicular pain, raspy voice, edema, and a sense of doom.


The patient presented to the RGMVAMC emergency department (ED) 8 days after the MRI with worsening symptoms and was hospitalized for 10 days. During this time, he was referred to nephrology for outpatient evaluation. While awaiting his nephrology appointment, the patient presented to the RGMVAMC ED 20 days after the initial episode with ongoing symptoms. “I thought I was dying,” he said. Laboratory results and a 12-lead electrocardiogram showed a finely static background, wide P waves (> 80 ms) with notching in lead II, sinusoidal P waves in V1, R transition in V2, RR’ in V2, ST flat in lead III, and sinus bradycardia (Table 1 and Appendix 2).
The patient’s medical and surgical histories were reviewed at the nephrology evaluation 25 days following the MRI. He reported that household water was sourced from a well and that he filtered his drinking water with a reverse osmosis system. He served in the US Army for 10 years as an engineer specializing in mechanical systems, power generation, and vehicles. Following Army retirement, the patient served in the US Air Force Reserves for 15 years, working as a crew chief in pneudraulics. The patient reported stopping tobacco use 1 year before and also reported regular use of a broad array of prescription medications and dietary supplements, including dexamethasone (4 mg twice daily), fluticasone nasal spray (50 mcg per nostril, twice daily), ibuprofen (400 mg twice daily, as needed), loratadine (10 mg daily), aspirin (81 mg daily), and metoprolol succinate (50 mg nightly). In addition, he reported consistent use of cholecalciferol (3000 IU daily), another supplemental vitamin D preparation, chelated magnesium glycinate (3 tablets daily for bone issues), turmeric (1 tablet daily), a multivitamin (Living Green Liquid Gel, daily), and a mega-B complex.
Physical examination revealed a well-nourished, tall man with hypertension (145/87 mmHg) and bilateral lower extremity edema. Oral examination showed poor dentition, including missing molars (#1-3, #14-16, #17-19, #30-31), with the anterior teeth replaced by bridges supported by dental implants. The review of systems was otherwise unremarkable, with nocturia noted before the consultation.

Serum and urine gadolinium testing, (Mayo Clinic Laboratories) revealed gadolinium levels of 0.3 mcg/24 h in the urine and 0.1 ng/mL in the serum. Nonzero values indicated detectable gadolinium, suggesting retention. The patient had a prior gadolinium exposure during a 2019 MRI (about 1340 days before) and suspected a repeat exposure on day 0, although the MRI technician stated that no contrast was administered. Given his elevated vitamin D levels, the patient was advised to minimize dietary supplements, particularly vitamin D, to avoid confounding symptoms. The plan included monitoring symptoms and a follow-up evaluation with repeat laboratory tests on day 116.
At the nephrology follow-up 4 months postexposure, the patient's symptoms had primarily abated, with a marked reduction in the previously noted metallic dysgeusia. Physical examination remained consistent with prior findings. He was afebrile (97.7 °F) with a blood pressure of 111/72 mmHg, a pulse of 63 beats per minute, and an oxygen saturation of 98% on ambient air. Laboratory analysis revealed serum and urine gadolinium levels below detectable thresholds (< 0.1 ng/mL and < 0.1 mcg/24 h). A 24-hour creatinine clearance, calculated from a urine volume of 1300 mL, measured at an optimal 106 mL/min, indicating preserved renal function (Tables 2 and 3). Of note, his 24-hour oxalate was above the reference range, with a urine pH below the reference range and a high supersaturation index for calcium oxalate.
Discussion
Use of enhanced MRI has increased in the Veterans Health Administration (Figure 2). A growing range of indications for enhanced procedures (eg, cardiac MRI) has contributed to this rise. The market has grown with new gadolinium-based contrast agents, such as gadopiclenol. However, reliance on untested assumptions about the safety of newer agents and need for robust clinical trials pose potential risks to patient safety.
Without prospective evidence, the American College of Radiology (ACR) classifies gadolinium-based contrast agents into 3 groups: Group 1, associated with the highest number of nephrogenic systemic fibrosis cases; Group 2, linked to few, if any, unconfounded cases; and Group 3, where data on nephrogenic systemic fibrosis risk have been limited. As of April 2024, the ACR reclassified Group 3 agents (Ablavar/Vasovist/Angiomark and Primovist/Eovist) into Group 2. Curiously, Vueway and Elucirem were approved in late 2022 and should clearly be categorized as Group 3 (Table 4).There were 19 cases of nephrogenic systemic fibrosis or similar manifestations, 8 of which were unconfounded by other factors. These patients had been exposed to gadobutrol, often combined with other agents. Gadobutrol—like other Group 2 agents—has been associated with nephrogenic systemic fibrosis.16,17 Despite US Food and Drug Administration (FDA) documentation of rising reports, many clinicians remain unaware that nephrogenic systemic fibrosis is increasingly linked to Group 2 agents classified by the ACR.18 While declines in reported cases of nephrogenic systemic fibrosis may suggest reduced incidence, this trend may reflect diminished clinical vigilance and underreporting, particularly given emerging evidence implicating even Group 2 gadolinium-based contrast agents in delayed and underrecognized presentations. This information has yet to permeate the medical community, particularly among nephrologists. Considering these cases, revisiting the ACR guidelines may be prudent.

To address this growing concern, clinicians must adopt stricter vigilance and actively pursue updated information to mitigate patient risks tied to these contrast agents.
There exists an illusion of knowledge in disregarding the confounded exposures of MRI contrast agents. Ten distinct brands of contrast agents have been approved for clinical use. With repeated imaging, patients are often exposed to varying formulations of gadolinium-based agents. Yet investigators commonly discard these data points when assessing risk. By doing so, they assume—without evidence—that some formulations are inherently less likely to provoke adverse effects (AEs) than others. This untested presumption becomes perilous, especially given the limited understanding of the mechanisms underlying gadolinium-induced pathologies. As Aldous Huxley warned, “Facts do not cease to exist because they are ignored.”19
Gadolinium Persistence
Contrary to expectations, gadolinium persists in the body far longer than initially presumed. Symptoms associated with gadolinium exposure (SAGE) encapsulate the chronic, often enigmatic maladies tied to MRI contrast agents.20 The prolonged retention of this rare earth metal offers a compelling hypothesis for the etiology of SAGE. It has been hypothesized that Lewis base-rich metabolites increase susceptibility to gadolinium-based contrast agent complications.21
The blood and urine concentration elimination curves of gadolinium are exponential and categorized as fast, intermediate, and long-term.1 For urinary elimination, the function of the curves is exponential. The quantity of gadolinium in the urine at a time (t) after exposure (D[Gd](t)) is equal to the product of the amount of gadolinium in the sample (urine or blood) at the end of the fast elimination period (D[Gd](t0)) and the exponential decay with k being a rate constant.
To the authors’ knowledge, we are the only research team currently investigating the rate constant for the intermediate- and long-term phase gadolinium elimination. The Retention and Toxicity of Gadolinium-based Contrast Agents study was approved by the University of New Mexico Health Sciences Center Institutional Review Board on May 27, 2020 (IRB ID 19-660). The data for the patient in this case were compared with preliminary results for patients with exposure-to-measurement intervals < 100 days.
The patient in this case presented with detectable gadolinium levels in urine and serum shortly after an attempted contrast-enhanced MRI procedure (Figure 3). The presence of detectable gadolinium levels in the patient’s urine and serum suggests a likely exposure to a contrast agent about 27 days before his consultation. While the technician reported that no contrast was administered during the attempted MRI, it remains possible that a small amount was introduced during cannulation, potentially triggering the patient’s symptoms. Linear modeling of semilogarithmic plots for participants exposed to contrast agents within 100 days (urine: P = 1.8 × 10ˉ8, adjusted r² = 0.62; blood: P = .005, adjusted r² = 0.21) provided clearance rates (k values) for urine and blood. Extrapolating from these models to the presumed exposure date, the intercepts estimate that the patient received between 0.5% and 8% of a standard contrast dose.

MRI contrast agents can cause skin disease. Systemic fibrosis is considered one of the most severe AEs. Skin pathophysiology involving myeloid cells is driven by elevated levels of monocyte chemoattractant protein-1, which recruits circulating fibroblasts via the C-C chemokine receptor 2.22,23 This occurs alongside activation of NADPH oxidase Nox4.4,24,25 Intracellular gadolinium-rich nanoparticles likely serve as catalysts for this reactive cascade.2,18,22,26,27 These particles assemble around intracellular lipid droplets and ferrule them in spiculated rare earth-rich shells that compromise cellular architecture.2,18,21,22,26,27 Frequently sequestered within endosomal compartments, they disrupt vesicular integrity and threaten cellular homeostasis. Interference with degradative systems such as the endolysosomal axis perturbs energy-recycling pathways—an insidious disturbance, particularly in cells with high metabolic demand. Skin-related symptoms are among the most frequently reported AEs, according to the FDA AE reporting system.18
Studies indicate repeated exposure to MRI contrast agents can lead to permanent gadolinium retention in the brain and other vital organs. Intravenous (IV) contrast agents cross the blood-brain barrier rapidly, while intrathecal administration has been linked to significant and lasting neurologic effects.18
Gadolinium is chemically bound to pharmaceutical ligands to enhance renal clearance and reduce toxicity. However, available data from human samples suggest potential ligand exchanges with undefined physiologic substances. This exchange may facilitate gadolinium precipitation and accumulation within cells into spiculated nanoparticles. Transmission electron microscopy reveals the formation of unilamellar bodies associated with mitochondriopathy and cellular damage, particularly in renal proximal tubules.2,18,22,26,27 It is proposed that intracellular nanoparticle formation represents a key mechanism driving the systemic symptoms observed in patients.1,2,18, 22,26,27
Any hypothesis based on free soluble gadolinium—or concept derived from it—should be discarded. The high affinity of pharmaceutical ligands for gadolinium suggests that the cationic rare earth metal remains predominantly in a ligand-bound, soluble form. It is hypothesized that gadolinium undergoes ligand exchange with physiologic substances, directly leading to nanoparticle formation. Current data demonstrate gadolinium precipitation according to the Le Chatelier’s principle. Since precipitated gadolinium does not readily re-equilibrate with pharmaceutical ligands, repeated administration of different contrast agent brands may contribute to nanoparticle growth.26
Meanwhile, a growing number of patients are turning to chelation therapy, a largely untested treatment. The premise of chelation therapy is rooted in several unproven assumptions.18,21 First, it assumes that clinically significant amounts of gadolinium persist in compartments such as the extracellular space, where they can be effectively chelated and cleared. Second, it presumes that free gadolinium is the primary driver of chronic symptoms, an assertion that remains scientifically unsubstantiated. Finally, chelation proponents overlook the potential harm caused by depleting essential physiological metals during the process, assuming without evidence that the scant removal of gadolinium outweighs the risk of physiological mineral depletion.

These assumptions underpin an unproven remedy that demands critical scrutiny. Recent findings reveal that gadolinium deposits in the skin and kidney often take the form of intracellular nanoparticles, directly challenging the foundation of chelation therapy. Chelation advocates must demonstrate that these intracellular gadolinium deposits neither trigger cellular toxicity nor initiate a cytokine cascade. Chelation supporters must prove that the systemic response to these foreign particles is unrelated to the symptoms reported by patients. Until then, the validity of chelation therapy remains highly questionable.
The causality of the symptoms, mainly whether IV gadolinium was administered, was examined. The null hypothesis stated that the patient was not exposed to gadolinium. However, this hypothesis was contradicted by the detection of gadolinium in the serum and urine 27 days after the potential exposure.
Two plausible explanations exist for the nonzero gadolinium levels detected in the serum and urine. The first possibility is that minute quantities of gadolinium were introduced during cannulation, with the amount being sufficient to persist in measurable concentrations 27 days postexposure. The second possibility is that the gadolinium originated from an MRI contrast agent administered 4 years earlier. In this scenario, gadolinium stored in organ reservoirs such as bone, liver, or kidneys may have been mobilized into the extracellular fluid compartment due to the administration of high-dose steroids 20 days after the recent contrast-enhanced MRI procedure attempt. Coyte et al reported elevated gadolinium levels in the serum, cord blood, breast milk, and placenta of pregnant women with prior exposure to MRI contrast agents.28 These findings suggest that gadolinium, stored in organs such as bone may be remobilized by variables affecting bone remodeling (eg, high-dose steroids).
Significantly, the patient exhibited elevated urinary oxalate levels. Previous research has found that oxalic acid reacts rapidly with MRI contrast agents, forming digadolinium trioxalate. While the gadolinium-rich nanoparticles identified in tissues such as the skin and kidney (including the human kidney) are amorphous, these in vitro findings establish a proof-of-concept: the intracellular environment facilitates gadolinium dissociation from pharmaceutical chelates.

Furthermore, in vitro experiments show that proteins and lysosomal pH promote this dissociation, underscoring how human metabolic conditions—particularly oxalic acid concentration—may drive intracellular gadolinium deposition.
Patient Perspective
“They put something into my body that they cannot get out.” This stark realization underpins the patient’s profound concern about gadolinium-based contrast agents and their potential long-term effects. Reflecting on his experience, the patient expressed deep fears about the unknown future impacts: “I’m concerned about my kidneys, I’m concerned about my heart, and I’m concerned about my brain. I don’t know how this stuff is going to affect me in the future.”
He drew an unsettling parallel between gadolinium and heavy metals: “Heavy metal is poison. The body does not produce this kind of stuff on its own.” His reaction to the procedure left a lasting impression, prompting him to question the logic of using a substance that cannot be purged: “Why would you put something into someone’s body that you cannot extract? Nobody—nobody—should experience what I went through.”
The patient emphasized the lack of clear research on long-term outcomes, which compounds his anxiety: “If there was research that said, ‘Well, this is only going to affect these organs for this long,’ OK, I might be able to accept that. But there is no research like that. Nobody can tell me what’s going to happen in 5 years.”
Strengths and Limitations
A significant strength of this approach is the ability to track gadolinium elimination and symptom resolution over time, supported by unique access to intermediate and long-term clearance data from our ongoing research protocol. The investigators were equipped to back-extrapolate the exposure, which provided a rare opportunity to correlate gadolinium levels with clinical outcomes. The primary limitation is the lack of a defined clinical case definition for gadolinium toxicity and limited mechanistic understanding of SAGE, which hinders diagnosis and management.
Metabolites, proteins, and lipids rich in Lewis bases could initiate this process as substrates for intracellular gadolinium sedimentation. Future studies should investigate whether metabolic conditions such as oxalate burden or altered parathyroid hormone levels modulate gadolinium compartmentalization and tissue retention. If gadolinium-rich nanoparticle formation and accumulation disrupt cellular equilibrium, it underscores an urgent need to understand the implications of long-term gadolinium retention. The research team continues to gather evidence that the gadolinium cation remains chelated from the moment MRI contrast agents are administered through to the formation of intracellular nanoparticles. Retained gadolinium nanoparticles may act as a nidus, triggering cellular signaling cascades that lead to multisymptomatic illnesses. Intracellular and insoluble retained gadolinium challenges proponents of untested chelation therapies.
Conclusions
This case highlights emerging clinical and ethical concerns surrounding gadolinium-based contrast agent use. Clinicians may benefit from considering gadolinium retention as a contributor to persistent, unexplained symptoms—particularly in patients with recent imaging exposure. As contrast use continues to rise within federal health systems, regulatory and administrative stakeholders would do well to re-examine current safety frameworks. Informed consent should reflect what is known: gadolinium can remain in the body long after administration, potentially indefinitely. The long-term consequences of cumulative exposure remain poorly defined, but the presence of a lanthanide element in human tissue warrants greater attention from researchers and regulators alike. Interest in alternative imaging modalities and long-term safety monitoring would mark progress toward more transparent, accountable care.


Magnetic resonance image (MRI) contrast agents can induce profound complications, including gadolinium encephalopathy, kidney injury, gadolinium-associated plaques, and progressive systemic fibrosis, which can be fatal.1-10 About 50% of MRIs use gadolinium-based contrast (Gd3+), a toxic rare earth metal ion that enhances imaging but requires binding with pharmaceutical ligands to reduce toxicity and promote renal elimination (Figure 1). Despite these measures, Gd3+ can persist in the body, including the brain.11,12 Wastewater treatment fails to remove these agents, making Gd3+ a growing pollutant in water and the food chain.13-15 Because Gd3+ is a rare earth metal ion in the milieu intérieur, there is an urgent need to study its biological and long-term effects (Appendix 1).
Case Presentation
A 65-year-old Vietnam-era veteran presented to nephrology at the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, for evaluation of gadolinium-induced symptoms. His medical history included metabolic syndrome, hypertension, hyperlipidemia, hypogonadism, cervical spondylosis, and an elevated prostate-specific antigen, previously assessed with a contrast-enhanced MRI in 2019 (Gadobenic acid, 19 mL). Surgical history included cervical fusion and ankle hardware.
The patient had a scheduled MRI 25 days earlier, following an elevated prostate specific antigen test result, prompting urologic surveillance and concern for malignancy. In preparation for the contrast-enhanced MRI, his right arm was cannulated with a line primed with gadobenic acid contrast. Though the technician stated the infusion had not started, the patient’s symptoms began shortly after entry into the scanner, before any programmed pulse sequences. The patient experienced claustrophobia, diaphoresis, palpitations, xerostomia, dysgeusia, shortness of breath, and a sensation of heat in his groin, chest, “kidneys,” and lower back. The MRI was terminated prematurely in response to the patient’s acute symptomatology. The patient continued experiencing new symptoms intermittently during the following week, including lightheadedness, headaches, right clavicular pain, raspy voice, edema, and a sense of doom.


The patient presented to the RGMVAMC emergency department (ED) 8 days after the MRI with worsening symptoms and was hospitalized for 10 days. During this time, he was referred to nephrology for outpatient evaluation. While awaiting his nephrology appointment, the patient presented to the RGMVAMC ED 20 days after the initial episode with ongoing symptoms. “I thought I was dying,” he said. Laboratory results and a 12-lead electrocardiogram showed a finely static background, wide P waves (> 80 ms) with notching in lead II, sinusoidal P waves in V1, R transition in V2, RR’ in V2, ST flat in lead III, and sinus bradycardia (Table 1 and Appendix 2).
The patient’s medical and surgical histories were reviewed at the nephrology evaluation 25 days following the MRI. He reported that household water was sourced from a well and that he filtered his drinking water with a reverse osmosis system. He served in the US Army for 10 years as an engineer specializing in mechanical systems, power generation, and vehicles. Following Army retirement, the patient served in the US Air Force Reserves for 15 years, working as a crew chief in pneudraulics. The patient reported stopping tobacco use 1 year before and also reported regular use of a broad array of prescription medications and dietary supplements, including dexamethasone (4 mg twice daily), fluticasone nasal spray (50 mcg per nostril, twice daily), ibuprofen (400 mg twice daily, as needed), loratadine (10 mg daily), aspirin (81 mg daily), and metoprolol succinate (50 mg nightly). In addition, he reported consistent use of cholecalciferol (3000 IU daily), another supplemental vitamin D preparation, chelated magnesium glycinate (3 tablets daily for bone issues), turmeric (1 tablet daily), a multivitamin (Living Green Liquid Gel, daily), and a mega-B complex.
Physical examination revealed a well-nourished, tall man with hypertension (145/87 mmHg) and bilateral lower extremity edema. Oral examination showed poor dentition, including missing molars (#1-3, #14-16, #17-19, #30-31), with the anterior teeth replaced by bridges supported by dental implants. The review of systems was otherwise unremarkable, with nocturia noted before the consultation.

Serum and urine gadolinium testing, (Mayo Clinic Laboratories) revealed gadolinium levels of 0.3 mcg/24 h in the urine and 0.1 ng/mL in the serum. Nonzero values indicated detectable gadolinium, suggesting retention. The patient had a prior gadolinium exposure during a 2019 MRI (about 1340 days before) and suspected a repeat exposure on day 0, although the MRI technician stated that no contrast was administered. Given his elevated vitamin D levels, the patient was advised to minimize dietary supplements, particularly vitamin D, to avoid confounding symptoms. The plan included monitoring symptoms and a follow-up evaluation with repeat laboratory tests on day 116.
At the nephrology follow-up 4 months postexposure, the patient's symptoms had primarily abated, with a marked reduction in the previously noted metallic dysgeusia. Physical examination remained consistent with prior findings. He was afebrile (97.7 °F) with a blood pressure of 111/72 mmHg, a pulse of 63 beats per minute, and an oxygen saturation of 98% on ambient air. Laboratory analysis revealed serum and urine gadolinium levels below detectable thresholds (< 0.1 ng/mL and < 0.1 mcg/24 h). A 24-hour creatinine clearance, calculated from a urine volume of 1300 mL, measured at an optimal 106 mL/min, indicating preserved renal function (Tables 2 and 3). Of note, his 24-hour oxalate was above the reference range, with a urine pH below the reference range and a high supersaturation index for calcium oxalate.
Discussion
Use of enhanced MRI has increased in the Veterans Health Administration (Figure 2). A growing range of indications for enhanced procedures (eg, cardiac MRI) has contributed to this rise. The market has grown with new gadolinium-based contrast agents, such as gadopiclenol. However, reliance on untested assumptions about the safety of newer agents and need for robust clinical trials pose potential risks to patient safety.
Without prospective evidence, the American College of Radiology (ACR) classifies gadolinium-based contrast agents into 3 groups: Group 1, associated with the highest number of nephrogenic systemic fibrosis cases; Group 2, linked to few, if any, unconfounded cases; and Group 3, where data on nephrogenic systemic fibrosis risk have been limited. As of April 2024, the ACR reclassified Group 3 agents (Ablavar/Vasovist/Angiomark and Primovist/Eovist) into Group 2. Curiously, Vueway and Elucirem were approved in late 2022 and should clearly be categorized as Group 3 (Table 4).There were 19 cases of nephrogenic systemic fibrosis or similar manifestations, 8 of which were unconfounded by other factors. These patients had been exposed to gadobutrol, often combined with other agents. Gadobutrol—like other Group 2 agents—has been associated with nephrogenic systemic fibrosis.16,17 Despite US Food and Drug Administration (FDA) documentation of rising reports, many clinicians remain unaware that nephrogenic systemic fibrosis is increasingly linked to Group 2 agents classified by the ACR.18 While declines in reported cases of nephrogenic systemic fibrosis may suggest reduced incidence, this trend may reflect diminished clinical vigilance and underreporting, particularly given emerging evidence implicating even Group 2 gadolinium-based contrast agents in delayed and underrecognized presentations. This information has yet to permeate the medical community, particularly among nephrologists. Considering these cases, revisiting the ACR guidelines may be prudent.

To address this growing concern, clinicians must adopt stricter vigilance and actively pursue updated information to mitigate patient risks tied to these contrast agents.
There exists an illusion of knowledge in disregarding the confounded exposures of MRI contrast agents. Ten distinct brands of contrast agents have been approved for clinical use. With repeated imaging, patients are often exposed to varying formulations of gadolinium-based agents. Yet investigators commonly discard these data points when assessing risk. By doing so, they assume—without evidence—that some formulations are inherently less likely to provoke adverse effects (AEs) than others. This untested presumption becomes perilous, especially given the limited understanding of the mechanisms underlying gadolinium-induced pathologies. As Aldous Huxley warned, “Facts do not cease to exist because they are ignored.”19
Gadolinium Persistence
Contrary to expectations, gadolinium persists in the body far longer than initially presumed. Symptoms associated with gadolinium exposure (SAGE) encapsulate the chronic, often enigmatic maladies tied to MRI contrast agents.20 The prolonged retention of this rare earth metal offers a compelling hypothesis for the etiology of SAGE. It has been hypothesized that Lewis base-rich metabolites increase susceptibility to gadolinium-based contrast agent complications.21
The blood and urine concentration elimination curves of gadolinium are exponential and categorized as fast, intermediate, and long-term.1 For urinary elimination, the function of the curves is exponential. The quantity of gadolinium in the urine at a time (t) after exposure (D[Gd](t)) is equal to the product of the amount of gadolinium in the sample (urine or blood) at the end of the fast elimination period (D[Gd](t0)) and the exponential decay with k being a rate constant.
To the authors’ knowledge, we are the only research team currently investigating the rate constant for the intermediate- and long-term phase gadolinium elimination. The Retention and Toxicity of Gadolinium-based Contrast Agents study was approved by the University of New Mexico Health Sciences Center Institutional Review Board on May 27, 2020 (IRB ID 19-660). The data for the patient in this case were compared with preliminary results for patients with exposure-to-measurement intervals < 100 days.
The patient in this case presented with detectable gadolinium levels in urine and serum shortly after an attempted contrast-enhanced MRI procedure (Figure 3). The presence of detectable gadolinium levels in the patient’s urine and serum suggests a likely exposure to a contrast agent about 27 days before his consultation. While the technician reported that no contrast was administered during the attempted MRI, it remains possible that a small amount was introduced during cannulation, potentially triggering the patient’s symptoms. Linear modeling of semilogarithmic plots for participants exposed to contrast agents within 100 days (urine: P = 1.8 × 10ˉ8, adjusted r² = 0.62; blood: P = .005, adjusted r² = 0.21) provided clearance rates (k values) for urine and blood. Extrapolating from these models to the presumed exposure date, the intercepts estimate that the patient received between 0.5% and 8% of a standard contrast dose.

MRI contrast agents can cause skin disease. Systemic fibrosis is considered one of the most severe AEs. Skin pathophysiology involving myeloid cells is driven by elevated levels of monocyte chemoattractant protein-1, which recruits circulating fibroblasts via the C-C chemokine receptor 2.22,23 This occurs alongside activation of NADPH oxidase Nox4.4,24,25 Intracellular gadolinium-rich nanoparticles likely serve as catalysts for this reactive cascade.2,18,22,26,27 These particles assemble around intracellular lipid droplets and ferrule them in spiculated rare earth-rich shells that compromise cellular architecture.2,18,21,22,26,27 Frequently sequestered within endosomal compartments, they disrupt vesicular integrity and threaten cellular homeostasis. Interference with degradative systems such as the endolysosomal axis perturbs energy-recycling pathways—an insidious disturbance, particularly in cells with high metabolic demand. Skin-related symptoms are among the most frequently reported AEs, according to the FDA AE reporting system.18
Studies indicate repeated exposure to MRI contrast agents can lead to permanent gadolinium retention in the brain and other vital organs. Intravenous (IV) contrast agents cross the blood-brain barrier rapidly, while intrathecal administration has been linked to significant and lasting neurologic effects.18
Gadolinium is chemically bound to pharmaceutical ligands to enhance renal clearance and reduce toxicity. However, available data from human samples suggest potential ligand exchanges with undefined physiologic substances. This exchange may facilitate gadolinium precipitation and accumulation within cells into spiculated nanoparticles. Transmission electron microscopy reveals the formation of unilamellar bodies associated with mitochondriopathy and cellular damage, particularly in renal proximal tubules.2,18,22,26,27 It is proposed that intracellular nanoparticle formation represents a key mechanism driving the systemic symptoms observed in patients.1,2,18, 22,26,27
Any hypothesis based on free soluble gadolinium—or concept derived from it—should be discarded. The high affinity of pharmaceutical ligands for gadolinium suggests that the cationic rare earth metal remains predominantly in a ligand-bound, soluble form. It is hypothesized that gadolinium undergoes ligand exchange with physiologic substances, directly leading to nanoparticle formation. Current data demonstrate gadolinium precipitation according to the Le Chatelier’s principle. Since precipitated gadolinium does not readily re-equilibrate with pharmaceutical ligands, repeated administration of different contrast agent brands may contribute to nanoparticle growth.26
Meanwhile, a growing number of patients are turning to chelation therapy, a largely untested treatment. The premise of chelation therapy is rooted in several unproven assumptions.18,21 First, it assumes that clinically significant amounts of gadolinium persist in compartments such as the extracellular space, where they can be effectively chelated and cleared. Second, it presumes that free gadolinium is the primary driver of chronic symptoms, an assertion that remains scientifically unsubstantiated. Finally, chelation proponents overlook the potential harm caused by depleting essential physiological metals during the process, assuming without evidence that the scant removal of gadolinium outweighs the risk of physiological mineral depletion.

These assumptions underpin an unproven remedy that demands critical scrutiny. Recent findings reveal that gadolinium deposits in the skin and kidney often take the form of intracellular nanoparticles, directly challenging the foundation of chelation therapy. Chelation advocates must demonstrate that these intracellular gadolinium deposits neither trigger cellular toxicity nor initiate a cytokine cascade. Chelation supporters must prove that the systemic response to these foreign particles is unrelated to the symptoms reported by patients. Until then, the validity of chelation therapy remains highly questionable.
The causality of the symptoms, mainly whether IV gadolinium was administered, was examined. The null hypothesis stated that the patient was not exposed to gadolinium. However, this hypothesis was contradicted by the detection of gadolinium in the serum and urine 27 days after the potential exposure.
Two plausible explanations exist for the nonzero gadolinium levels detected in the serum and urine. The first possibility is that minute quantities of gadolinium were introduced during cannulation, with the amount being sufficient to persist in measurable concentrations 27 days postexposure. The second possibility is that the gadolinium originated from an MRI contrast agent administered 4 years earlier. In this scenario, gadolinium stored in organ reservoirs such as bone, liver, or kidneys may have been mobilized into the extracellular fluid compartment due to the administration of high-dose steroids 20 days after the recent contrast-enhanced MRI procedure attempt. Coyte et al reported elevated gadolinium levels in the serum, cord blood, breast milk, and placenta of pregnant women with prior exposure to MRI contrast agents.28 These findings suggest that gadolinium, stored in organs such as bone may be remobilized by variables affecting bone remodeling (eg, high-dose steroids).
Significantly, the patient exhibited elevated urinary oxalate levels. Previous research has found that oxalic acid reacts rapidly with MRI contrast agents, forming digadolinium trioxalate. While the gadolinium-rich nanoparticles identified in tissues such as the skin and kidney (including the human kidney) are amorphous, these in vitro findings establish a proof-of-concept: the intracellular environment facilitates gadolinium dissociation from pharmaceutical chelates.

Furthermore, in vitro experiments show that proteins and lysosomal pH promote this dissociation, underscoring how human metabolic conditions—particularly oxalic acid concentration—may drive intracellular gadolinium deposition.
Patient Perspective
“They put something into my body that they cannot get out.” This stark realization underpins the patient’s profound concern about gadolinium-based contrast agents and their potential long-term effects. Reflecting on his experience, the patient expressed deep fears about the unknown future impacts: “I’m concerned about my kidneys, I’m concerned about my heart, and I’m concerned about my brain. I don’t know how this stuff is going to affect me in the future.”
He drew an unsettling parallel between gadolinium and heavy metals: “Heavy metal is poison. The body does not produce this kind of stuff on its own.” His reaction to the procedure left a lasting impression, prompting him to question the logic of using a substance that cannot be purged: “Why would you put something into someone’s body that you cannot extract? Nobody—nobody—should experience what I went through.”
The patient emphasized the lack of clear research on long-term outcomes, which compounds his anxiety: “If there was research that said, ‘Well, this is only going to affect these organs for this long,’ OK, I might be able to accept that. But there is no research like that. Nobody can tell me what’s going to happen in 5 years.”
Strengths and Limitations
A significant strength of this approach is the ability to track gadolinium elimination and symptom resolution over time, supported by unique access to intermediate and long-term clearance data from our ongoing research protocol. The investigators were equipped to back-extrapolate the exposure, which provided a rare opportunity to correlate gadolinium levels with clinical outcomes. The primary limitation is the lack of a defined clinical case definition for gadolinium toxicity and limited mechanistic understanding of SAGE, which hinders diagnosis and management.
Metabolites, proteins, and lipids rich in Lewis bases could initiate this process as substrates for intracellular gadolinium sedimentation. Future studies should investigate whether metabolic conditions such as oxalate burden or altered parathyroid hormone levels modulate gadolinium compartmentalization and tissue retention. If gadolinium-rich nanoparticle formation and accumulation disrupt cellular equilibrium, it underscores an urgent need to understand the implications of long-term gadolinium retention. The research team continues to gather evidence that the gadolinium cation remains chelated from the moment MRI contrast agents are administered through to the formation of intracellular nanoparticles. Retained gadolinium nanoparticles may act as a nidus, triggering cellular signaling cascades that lead to multisymptomatic illnesses. Intracellular and insoluble retained gadolinium challenges proponents of untested chelation therapies.
Conclusions
This case highlights emerging clinical and ethical concerns surrounding gadolinium-based contrast agent use. Clinicians may benefit from considering gadolinium retention as a contributor to persistent, unexplained symptoms—particularly in patients with recent imaging exposure. As contrast use continues to rise within federal health systems, regulatory and administrative stakeholders would do well to re-examine current safety frameworks. Informed consent should reflect what is known: gadolinium can remain in the body long after administration, potentially indefinitely. The long-term consequences of cumulative exposure remain poorly defined, but the presence of a lanthanide element in human tissue warrants greater attention from researchers and regulators alike. Interest in alternative imaging modalities and long-term safety monitoring would mark progress toward more transparent, accountable care.


Jackson DB, MacIntyre T, Duarte-Miramontes V, et al. Gadolinium deposition disease: a case report and the prevalence of enhanced MRI procedures within the Veterans Health Administration. Fed Pract. 2022;39:218-225. doi:10.12788/fp.0258
Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1:561-568.doi:10.34067/kid.0000272019
Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28:154-162. doi:10.1097/MNH.0000000000000475
Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311:F1-F11. doi:10.1152/ajprenal.00166.2016
Maramattom BV, Manno EM, Wijdicks EF, et al. Gadolinium encephalopathy in a patient with renal failure. Neurology. 2005;64:1276-1278.doi:10.1212/01.WNL.0000156805.45547.6E
Sam AD II, Morasch MD, Collins J, et al. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg. 2003;38:313-318. doi:10.1016/s0741-5214(03)00315-x
Schenker MP, Solomon JA, Roberts DA. Gadolinium arteriography complicated by acute pancreatitis and acute renal failure. J Vasc Interv Radiol. 2001;12:393. doi:10.1016/s1051-0443(07)61925-3
Gemery J, Idelson B, Reid S, et al. Acute renal failure after arteriography with a gadolinium-based contrast agent. AJR Am J Roentgenol. 1998;171:1277-1278. doi:10.2214/ajr.171.5.9798860
Akgun H, Gonlusen G, Cartwright J Jr, et al. Are gadolinium-based contrast media nephrotoxic? A renal biopsy study. Arch Pathol Lab Med. 2006;130:1354-1357. doi:10.5858/2006-130-1354-AGCMNA
Gathings RM, Reddy R, Santa Cruz D, et al. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151:316-319. doi:10.1001/jamadermatol.2014.2660
McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834-841. doi:10.1148/radiol.13131669
Schmidt K, Bau M, Merschel G, et al. Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci Total Environ. 2019;687:1401-1408. doi:10.1016/j.scitotenv.2019.07.075
Kulaksız S, Bau M. Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl Geochem. 2011;26:1877-1885.
Brunjes R, Hofmann T. Anthropogenic gadolinium in freshwater and drinking water systems. Water Res. 2020;182:115966. doi:10.1016/j.watres.2020.115966
Endrikat J, Gutberlet M, Hoffmann KT, et al. Clinical safety of gadobutrol: review of over 25 years of use exceeding 100 million administrations. Invest Radiol. 2024;59(9):605-613. doi:10.1097/RLI.0000000000001072
Elmholdt TR, Jørgensen B, Ramsing M, et al. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287. doi:10.1093/ndtplus/sfq028
Cunningham A, Kirk M, Hong E, et al. The safety of magnetic resonance imaging contrast agents. Front Toxicol. 2024;6:1376587. doi:10.3389/ftox.2024.1376587
Huxley A. Complete Essays. Volume II, 1926-1929. Chicago; 2000:227.
McDonald RJ, Weinreb JC, Davenport MS. Symptoms associated with gadolinium exposure (SAGE): a suggested term. Radiology. 2022;302(2):270-273. doi:10.1148/radiol.2021211349
Henderson IM, Benevidez AD, Mowry CD, et al. Precipitation of gadolinium from magnetic resonance imaging contrast agents may be the Brass tacks of toxicity. Magn Reson Imaging. 2025;119:110383. doi:10.1016/j.mri.2025.110383
Do C, Drel V, Tan C, et al. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143. doi:10.1016/j.jid.2019.03.1145
Drel VR, Tan C, Barnes JL, et al. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH oxidase 4 for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
DeAguero J, Howard T, Kusewitt D, et al. The onset of rare earth metallosis begins with renal gadolinium-rich nanoparticles from magnetic resonance imaging contrast agent exposure. Sci Rep. 2023;13(1):2025. doi:10.1038/s41598-023-28666-1
Do C, Ford B, Lee DY, et al. Gadolinium-based contrast agents: Stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
Coyte RM, Darrah T, Olesik J, et al. Gadolinium during human pregnancy following administration of gadolinium chelate before pregnancy. Birth Defects Res. 2023;115(14):1264-1273. doi:10.1002/bdr2.2209
Jackson DB, MacIntyre T, Duarte-Miramontes V, et al. Gadolinium deposition disease: a case report and the prevalence of enhanced MRI procedures within the Veterans Health Administration. Fed Pract. 2022;39:218-225. doi:10.12788/fp.0258
Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1:561-568.doi:10.34067/kid.0000272019
Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28:154-162. doi:10.1097/MNH.0000000000000475
Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311:F1-F11. doi:10.1152/ajprenal.00166.2016
Maramattom BV, Manno EM, Wijdicks EF, et al. Gadolinium encephalopathy in a patient with renal failure. Neurology. 2005;64:1276-1278.doi:10.1212/01.WNL.0000156805.45547.6E
Sam AD II, Morasch MD, Collins J, et al. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg. 2003;38:313-318. doi:10.1016/s0741-5214(03)00315-x
Schenker MP, Solomon JA, Roberts DA. Gadolinium arteriography complicated by acute pancreatitis and acute renal failure. J Vasc Interv Radiol. 2001;12:393. doi:10.1016/s1051-0443(07)61925-3
Gemery J, Idelson B, Reid S, et al. Acute renal failure after arteriography with a gadolinium-based contrast agent. AJR Am J Roentgenol. 1998;171:1277-1278. doi:10.2214/ajr.171.5.9798860
Akgun H, Gonlusen G, Cartwright J Jr, et al. Are gadolinium-based contrast media nephrotoxic? A renal biopsy study. Arch Pathol Lab Med. 2006;130:1354-1357. doi:10.5858/2006-130-1354-AGCMNA
Gathings RM, Reddy R, Santa Cruz D, et al. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151:316-319. doi:10.1001/jamadermatol.2014.2660
McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834-841. doi:10.1148/radiol.13131669
Schmidt K, Bau M, Merschel G, et al. Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci Total Environ. 2019;687:1401-1408. doi:10.1016/j.scitotenv.2019.07.075
Kulaksız S, Bau M. Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl Geochem. 2011;26:1877-1885.
Brunjes R, Hofmann T. Anthropogenic gadolinium in freshwater and drinking water systems. Water Res. 2020;182:115966. doi:10.1016/j.watres.2020.115966
Endrikat J, Gutberlet M, Hoffmann KT, et al. Clinical safety of gadobutrol: review of over 25 years of use exceeding 100 million administrations. Invest Radiol. 2024;59(9):605-613. doi:10.1097/RLI.0000000000001072
Elmholdt TR, Jørgensen B, Ramsing M, et al. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287. doi:10.1093/ndtplus/sfq028
Cunningham A, Kirk M, Hong E, et al. The safety of magnetic resonance imaging contrast agents. Front Toxicol. 2024;6:1376587. doi:10.3389/ftox.2024.1376587
Huxley A. Complete Essays. Volume II, 1926-1929. Chicago; 2000:227.
McDonald RJ, Weinreb JC, Davenport MS. Symptoms associated with gadolinium exposure (SAGE): a suggested term. Radiology. 2022;302(2):270-273. doi:10.1148/radiol.2021211349
Henderson IM, Benevidez AD, Mowry CD, et al. Precipitation of gadolinium from magnetic resonance imaging contrast agents may be the Brass tacks of toxicity. Magn Reson Imaging. 2025;119:110383. doi:10.1016/j.mri.2025.110383
Do C, Drel V, Tan C, et al. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143. doi:10.1016/j.jid.2019.03.1145
Drel VR, Tan C, Barnes JL, et al. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH oxidase 4 for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
DeAguero J, Howard T, Kusewitt D, et al. The onset of rare earth metallosis begins with renal gadolinium-rich nanoparticles from magnetic resonance imaging contrast agent exposure. Sci Rep. 2023;13(1):2025. doi:10.1038/s41598-023-28666-1
Do C, Ford B, Lee DY, et al. Gadolinium-based contrast agents: Stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
Coyte RM, Darrah T, Olesik J, et al. Gadolinium during human pregnancy following administration of gadolinium chelate before pregnancy. Birth Defects Res. 2023;115(14):1264-1273. doi:10.1002/bdr2.2209
Gadolinium Intermediate Elimination and Persistent Symptoms After Magnetic Resonance Imaging Contrast Agent Exposure
Gadolinium Intermediate Elimination and Persistent Symptoms After Magnetic Resonance Imaging Contrast Agent Exposure
Reticulated Hyperpigmentation on the Knee and Thigh
Reticulated Hyperpigmentation on the Knee and Thigh
The patient was diagnosed with erythema ab igne based on characteristic skin findings on physical examination along with a convincing history of chronic localized heat exposure. Erythema ab igne manifests as a persistent reticulated, erythematous, or hyperpigmented rash at sites of chronic heat exposure.1 Commonplace items that emit heat such as electric heaters, car heaters, heating pads, hot water bottles, and, in our case, laptops also emit infrared radiation, which can lead to changes in the skin with long-term exposure.2 Because exposure to these sources often is limited to one area of the body, erythema ab igne usually manifests locally, as exemplified in this case. Chronic heat exposure and infrared radiation from these sources are thought to induce hyperthermia below the threshold for a thermal burn, and the cutaneous findings correspond with the dermal venous plexus.3
Diagnosis of erythema ab igne primarily is made clinically based on characteristic skin findings and exposure history. Relevant history may include occupations with prolonged heat exposure, such as baking, silversmithing, or foundry work. Heat exposure also may result from cultural practices such as cupping with moxibustion.4 Additionally, repeated use of heating pads or hot water bottles for pain relief by patients diagnosed with chronic pain or an underlying illness may contribute to development of erythema ab igne.1,4
Biopsy was not needed for diagnosis of this patient, but if the presentation is equivocal and history of potential exposures is unclear, a biopsy may be taken. A hematoxylin and eosin stain would reveal dilation of small vascular channels in the superficial dermis, contributing to the classic reticulated appearance. Biopsy findings also would reveal either an interface dermatitis or pigment incontinence containing melanin-laden macrophages correlating to either the erythema or hyperpigmentation, respectively.4
The prognosis for erythema ab igne is excellent, especially if diagnosed early. Treatment involves removal of the inciting heat source.1 The discoloration may resolve within a few months to years or may persist. If the hyperpigmentation is persistent, patients may consider laser treatments or lightening agents such as topical hydroquinone or topical tretinoin.4 However, if undiagnosed, patients may be at risk for development of a cutaneous malignancy, such as squamous cell carcinoma, Merkel cell carcinoma, poorly differentiated carcinoma, or cutaneous marginal zone lymphoma.2,4 Malignant transformation has been reported to occur decades after the initial skin eruption, although the risk is rare5; however, due to this risk, patients with erythema ab igne should be followed regularly and screened for new lesions in the affected areas.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kesty K, Feldman SR. Erythema ab igne: evolving technology, evolving presentation. Dermatol Online J. 2014;20:13030.
- Harview CL, Krenitsky A. Erythema ab igne: a clinical review. Cutis. 2023;111:E33-E38. doi:10.12788/cutis.0771
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
The patient was diagnosed with erythema ab igne based on characteristic skin findings on physical examination along with a convincing history of chronic localized heat exposure. Erythema ab igne manifests as a persistent reticulated, erythematous, or hyperpigmented rash at sites of chronic heat exposure.1 Commonplace items that emit heat such as electric heaters, car heaters, heating pads, hot water bottles, and, in our case, laptops also emit infrared radiation, which can lead to changes in the skin with long-term exposure.2 Because exposure to these sources often is limited to one area of the body, erythema ab igne usually manifests locally, as exemplified in this case. Chronic heat exposure and infrared radiation from these sources are thought to induce hyperthermia below the threshold for a thermal burn, and the cutaneous findings correspond with the dermal venous plexus.3
Diagnosis of erythema ab igne primarily is made clinically based on characteristic skin findings and exposure history. Relevant history may include occupations with prolonged heat exposure, such as baking, silversmithing, or foundry work. Heat exposure also may result from cultural practices such as cupping with moxibustion.4 Additionally, repeated use of heating pads or hot water bottles for pain relief by patients diagnosed with chronic pain or an underlying illness may contribute to development of erythema ab igne.1,4
Biopsy was not needed for diagnosis of this patient, but if the presentation is equivocal and history of potential exposures is unclear, a biopsy may be taken. A hematoxylin and eosin stain would reveal dilation of small vascular channels in the superficial dermis, contributing to the classic reticulated appearance. Biopsy findings also would reveal either an interface dermatitis or pigment incontinence containing melanin-laden macrophages correlating to either the erythema or hyperpigmentation, respectively.4
The prognosis for erythema ab igne is excellent, especially if diagnosed early. Treatment involves removal of the inciting heat source.1 The discoloration may resolve within a few months to years or may persist. If the hyperpigmentation is persistent, patients may consider laser treatments or lightening agents such as topical hydroquinone or topical tretinoin.4 However, if undiagnosed, patients may be at risk for development of a cutaneous malignancy, such as squamous cell carcinoma, Merkel cell carcinoma, poorly differentiated carcinoma, or cutaneous marginal zone lymphoma.2,4 Malignant transformation has been reported to occur decades after the initial skin eruption, although the risk is rare5; however, due to this risk, patients with erythema ab igne should be followed regularly and screened for new lesions in the affected areas.
The patient was diagnosed with erythema ab igne based on characteristic skin findings on physical examination along with a convincing history of chronic localized heat exposure. Erythema ab igne manifests as a persistent reticulated, erythematous, or hyperpigmented rash at sites of chronic heat exposure.1 Commonplace items that emit heat such as electric heaters, car heaters, heating pads, hot water bottles, and, in our case, laptops also emit infrared radiation, which can lead to changes in the skin with long-term exposure.2 Because exposure to these sources often is limited to one area of the body, erythema ab igne usually manifests locally, as exemplified in this case. Chronic heat exposure and infrared radiation from these sources are thought to induce hyperthermia below the threshold for a thermal burn, and the cutaneous findings correspond with the dermal venous plexus.3
Diagnosis of erythema ab igne primarily is made clinically based on characteristic skin findings and exposure history. Relevant history may include occupations with prolonged heat exposure, such as baking, silversmithing, or foundry work. Heat exposure also may result from cultural practices such as cupping with moxibustion.4 Additionally, repeated use of heating pads or hot water bottles for pain relief by patients diagnosed with chronic pain or an underlying illness may contribute to development of erythema ab igne.1,4
Biopsy was not needed for diagnosis of this patient, but if the presentation is equivocal and history of potential exposures is unclear, a biopsy may be taken. A hematoxylin and eosin stain would reveal dilation of small vascular channels in the superficial dermis, contributing to the classic reticulated appearance. Biopsy findings also would reveal either an interface dermatitis or pigment incontinence containing melanin-laden macrophages correlating to either the erythema or hyperpigmentation, respectively.4
The prognosis for erythema ab igne is excellent, especially if diagnosed early. Treatment involves removal of the inciting heat source.1 The discoloration may resolve within a few months to years or may persist. If the hyperpigmentation is persistent, patients may consider laser treatments or lightening agents such as topical hydroquinone or topical tretinoin.4 However, if undiagnosed, patients may be at risk for development of a cutaneous malignancy, such as squamous cell carcinoma, Merkel cell carcinoma, poorly differentiated carcinoma, or cutaneous marginal zone lymphoma.2,4 Malignant transformation has been reported to occur decades after the initial skin eruption, although the risk is rare5; however, due to this risk, patients with erythema ab igne should be followed regularly and screened for new lesions in the affected areas.
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kesty K, Feldman SR. Erythema ab igne: evolving technology, evolving presentation. Dermatol Online J. 2014;20:13030.
- Harview CL, Krenitsky A. Erythema ab igne: a clinical review. Cutis. 2023;111:E33-E38. doi:10.12788/cutis.0771
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
- Tan S, Bertucci V. Erythema ab igne: an old condition new again. CMAJ. 2000;162:77-78.
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kesty K, Feldman SR. Erythema ab igne: evolving technology, evolving presentation. Dermatol Online J. 2014;20:13030.
- Harview CL, Krenitsky A. Erythema ab igne: a clinical review. Cutis. 2023;111:E33-E38. doi:10.12788/cutis.0771
- Wipf AJ, Brown MR. Malignant transformation of erythema ab igne. JAAD Case Rep. 2022;26:85-87. doi:10.1016/j.jdcr.2022.06.018
Reticulated Hyperpigmentation on the Knee and Thigh
Reticulated Hyperpigmentation on the Knee and Thigh
A 25-year-old woman with an unremarkable medical history presented to the dermatology clinic for evaluation of a persistent rash on the right knee and distal thigh of several months’ duration. The patient noted that the rash had been asymptomatic, and she denied any history of trauma to the area. She reported that she worked as a teacher and had repeatedly stayed up late using her laptop for months. Rather than use a desk, she often would work sitting with her laptop in her lap.
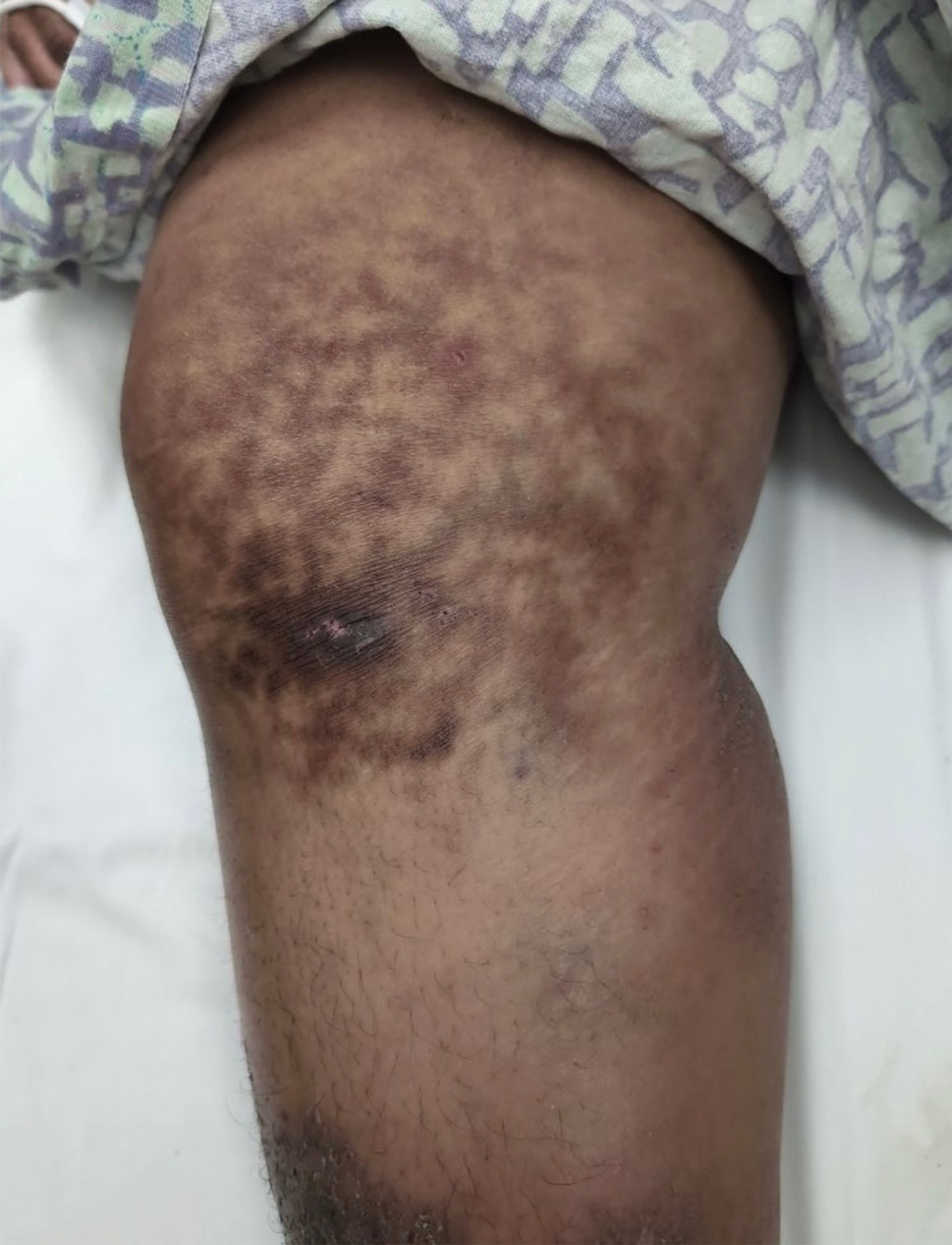
LLMs Show High Accuracy in Extracting CRC Data From VA Health Records
TOPLINE: Large Language Models (LLMs) achieve more than 95% accuracy in extracting colorectal cancer and dysplasia diagnoses from Veterans Health Administration (VHA) pathology reports, including patients with Million Veteran Program (MVP) genomic data. The validated approach using publicly available LLMs demonstrates excellent performance across both Inflammatory Bowel Disease (IBD) and non-IBD populations.
METHODOLOGY:
Researchers analyzed 116,373 pathology reports generated in the VHA between 1999 and 2024, utilizing search term filtering followed by simple yes/no question prompts for identifying colorectal dysplasia, high-grade dysplasia and/or colorectal adenocarcinoma, and invasive colorectal cancer.
Results were compared to blinded manual chart review of 200 to 300 pathology reports for each patient cohort and diagnostic task, totaling 3,816 reviewed reports, to validate the LLM approach.
Validation was performed independently in IBD and non-IBD populations using Gemma-2 and Llama-3 LLMs without any task-specific training or fine-tuning.
Performance metrics included F1 scores, positive predictive value, negative predictive value, sensitivity, specificity, and Matthew's correlation coefficient to evaluate accuracy across different tasks.
TAKEAWAY:
In patients with IBD in the MVP, the LLM achieved (F1-score, 96.9%; 95% confidence interval [CI], 94.0%-99.6%) for identifying dysplasia, (F1-score, 93.7%; 95% CI, 88.2%-98.4%) for identifying high-grade dysplasia/colorectal cancer, and (F1-score, 98%; 95% CI, 96.3%-99.4%) for identifying colorectal cancer.
In non-IBD MVP patients, the LLM demonstrated (F1-score, 99.2%; 95% CI, 98.2%-100%) for identifying colorectal dysplasia, (F1-score, 96.5%; 95% CI, 93.0%-99.2%) for high-grade dysplasia/colorectal cancer, and (F1-score, 95%; 95% CI, 92.8%-97.2%) for identifying colorectal cancer.
Agreement between reviewers was excellent across tasks, with (Cohen's kappa, 89%-97%) for main tasks, and (Cohen's kappa, 78.1%-93.1%) for indefinite for dysplasia in IBD cohort.
The LLM approach maintained high accuracy when applied to full pathology reports, with (F1-score, 97.1%; 95% CI, 93.5%-100%) for dysplasia detection in IBD patients.
IN PRACTICE: “We have shown that LLMs are powerful, potentially generalizable tools for accurately extracting important information from clinical semistructured and unstructured text and which require little human-led development.” the authors of the study wrote
SOURCE: The study was based on data from the Million Veteran Program and supported by the Office of Research and Development, Veterans Health Administration, and the US Department of Veterans Affairs Biomedical Laboratory. It was published online in BMJ Open Gastroenterology.
LIMITATIONS: According to the authors, this research may be specific to the VHA system and the LLM models used. The authors did not test larger models. The authors acknowledge that without long-term access to graphics processing units, they could not feasibly test larger models, which may overcome some of the shortcomings seen in smaller models. Additionally, the researchers could not rule out overlap between Million Veteran Program and Corporate Data Warehouse reports, though they state that results in either cohort alone are sufficient validation compared with previously published work.
DISCLOSURES: The study was supported by Merit Review Award from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service, AGA Research Foundation, National Institutes of Health grants, and the National Library of Medicine Training Grant. Kit Curtius reported receiving an investigator-led research grant from Phathom Pharmaceuticals. Shailja C Shah disclosed being a paid consultant for RedHill Biopharma and Phathom Pharmaceuticals, and an unpaid scientific advisory board member for Ilico Genetics, Inc.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Large Language Models (LLMs) achieve more than 95% accuracy in extracting colorectal cancer and dysplasia diagnoses from Veterans Health Administration (VHA) pathology reports, including patients with Million Veteran Program (MVP) genomic data. The validated approach using publicly available LLMs demonstrates excellent performance across both Inflammatory Bowel Disease (IBD) and non-IBD populations.
METHODOLOGY:
Researchers analyzed 116,373 pathology reports generated in the VHA between 1999 and 2024, utilizing search term filtering followed by simple yes/no question prompts for identifying colorectal dysplasia, high-grade dysplasia and/or colorectal adenocarcinoma, and invasive colorectal cancer.
Results were compared to blinded manual chart review of 200 to 300 pathology reports for each patient cohort and diagnostic task, totaling 3,816 reviewed reports, to validate the LLM approach.
Validation was performed independently in IBD and non-IBD populations using Gemma-2 and Llama-3 LLMs without any task-specific training or fine-tuning.
Performance metrics included F1 scores, positive predictive value, negative predictive value, sensitivity, specificity, and Matthew's correlation coefficient to evaluate accuracy across different tasks.
TAKEAWAY:
In patients with IBD in the MVP, the LLM achieved (F1-score, 96.9%; 95% confidence interval [CI], 94.0%-99.6%) for identifying dysplasia, (F1-score, 93.7%; 95% CI, 88.2%-98.4%) for identifying high-grade dysplasia/colorectal cancer, and (F1-score, 98%; 95% CI, 96.3%-99.4%) for identifying colorectal cancer.
In non-IBD MVP patients, the LLM demonstrated (F1-score, 99.2%; 95% CI, 98.2%-100%) for identifying colorectal dysplasia, (F1-score, 96.5%; 95% CI, 93.0%-99.2%) for high-grade dysplasia/colorectal cancer, and (F1-score, 95%; 95% CI, 92.8%-97.2%) for identifying colorectal cancer.
Agreement between reviewers was excellent across tasks, with (Cohen's kappa, 89%-97%) for main tasks, and (Cohen's kappa, 78.1%-93.1%) for indefinite for dysplasia in IBD cohort.
The LLM approach maintained high accuracy when applied to full pathology reports, with (F1-score, 97.1%; 95% CI, 93.5%-100%) for dysplasia detection in IBD patients.
IN PRACTICE: “We have shown that LLMs are powerful, potentially generalizable tools for accurately extracting important information from clinical semistructured and unstructured text and which require little human-led development.” the authors of the study wrote
SOURCE: The study was based on data from the Million Veteran Program and supported by the Office of Research and Development, Veterans Health Administration, and the US Department of Veterans Affairs Biomedical Laboratory. It was published online in BMJ Open Gastroenterology.
LIMITATIONS: According to the authors, this research may be specific to the VHA system and the LLM models used. The authors did not test larger models. The authors acknowledge that without long-term access to graphics processing units, they could not feasibly test larger models, which may overcome some of the shortcomings seen in smaller models. Additionally, the researchers could not rule out overlap between Million Veteran Program and Corporate Data Warehouse reports, though they state that results in either cohort alone are sufficient validation compared with previously published work.
DISCLOSURES: The study was supported by Merit Review Award from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service, AGA Research Foundation, National Institutes of Health grants, and the National Library of Medicine Training Grant. Kit Curtius reported receiving an investigator-led research grant from Phathom Pharmaceuticals. Shailja C Shah disclosed being a paid consultant for RedHill Biopharma and Phathom Pharmaceuticals, and an unpaid scientific advisory board member for Ilico Genetics, Inc.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
TOPLINE: Large Language Models (LLMs) achieve more than 95% accuracy in extracting colorectal cancer and dysplasia diagnoses from Veterans Health Administration (VHA) pathology reports, including patients with Million Veteran Program (MVP) genomic data. The validated approach using publicly available LLMs demonstrates excellent performance across both Inflammatory Bowel Disease (IBD) and non-IBD populations.
METHODOLOGY:
Researchers analyzed 116,373 pathology reports generated in the VHA between 1999 and 2024, utilizing search term filtering followed by simple yes/no question prompts for identifying colorectal dysplasia, high-grade dysplasia and/or colorectal adenocarcinoma, and invasive colorectal cancer.
Results were compared to blinded manual chart review of 200 to 300 pathology reports for each patient cohort and diagnostic task, totaling 3,816 reviewed reports, to validate the LLM approach.
Validation was performed independently in IBD and non-IBD populations using Gemma-2 and Llama-3 LLMs without any task-specific training or fine-tuning.
Performance metrics included F1 scores, positive predictive value, negative predictive value, sensitivity, specificity, and Matthew's correlation coefficient to evaluate accuracy across different tasks.
TAKEAWAY:
In patients with IBD in the MVP, the LLM achieved (F1-score, 96.9%; 95% confidence interval [CI], 94.0%-99.6%) for identifying dysplasia, (F1-score, 93.7%; 95% CI, 88.2%-98.4%) for identifying high-grade dysplasia/colorectal cancer, and (F1-score, 98%; 95% CI, 96.3%-99.4%) for identifying colorectal cancer.
In non-IBD MVP patients, the LLM demonstrated (F1-score, 99.2%; 95% CI, 98.2%-100%) for identifying colorectal dysplasia, (F1-score, 96.5%; 95% CI, 93.0%-99.2%) for high-grade dysplasia/colorectal cancer, and (F1-score, 95%; 95% CI, 92.8%-97.2%) for identifying colorectal cancer.
Agreement between reviewers was excellent across tasks, with (Cohen's kappa, 89%-97%) for main tasks, and (Cohen's kappa, 78.1%-93.1%) for indefinite for dysplasia in IBD cohort.
The LLM approach maintained high accuracy when applied to full pathology reports, with (F1-score, 97.1%; 95% CI, 93.5%-100%) for dysplasia detection in IBD patients.
IN PRACTICE: “We have shown that LLMs are powerful, potentially generalizable tools for accurately extracting important information from clinical semistructured and unstructured text and which require little human-led development.” the authors of the study wrote
SOURCE: The study was based on data from the Million Veteran Program and supported by the Office of Research and Development, Veterans Health Administration, and the US Department of Veterans Affairs Biomedical Laboratory. It was published online in BMJ Open Gastroenterology.
LIMITATIONS: According to the authors, this research may be specific to the VHA system and the LLM models used. The authors did not test larger models. The authors acknowledge that without long-term access to graphics processing units, they could not feasibly test larger models, which may overcome some of the shortcomings seen in smaller models. Additionally, the researchers could not rule out overlap between Million Veteran Program and Corporate Data Warehouse reports, though they state that results in either cohort alone are sufficient validation compared with previously published work.
DISCLOSURES: The study was supported by Merit Review Award from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service, AGA Research Foundation, National Institutes of Health grants, and the National Library of Medicine Training Grant. Kit Curtius reported receiving an investigator-led research grant from Phathom Pharmaceuticals. Shailja C Shah disclosed being a paid consultant for RedHill Biopharma and Phathom Pharmaceuticals, and an unpaid scientific advisory board member for Ilico Genetics, Inc.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.