User login
American Society of Clinical Oncology (ASCO): Breast Cancer Symposium
Genetic alterations may predict everolimus efficacy
SAN FRANCISCO – Among postmenopausal women with advanced hormone receptor–positive breast cancer, everolimus appeared to be more effective in the patients with one or no alterations in four key genes.
In a secondary analysis of data from the Breast Cancer Trials of Oral Everolimus–2 (BOLERO-2) study, one or no alterations in the PIK3CA; CCND1; FGFR1 and FGFR2 genes were seen in 76% of 227 tumor samples from women who took everolimus. These patients had significantly better progression-free survival than did patients with two or more alterations in these genes, Dr. Hope S. Rugo reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The number of patients with multiple gene alterations was admittedly small, but "potentially we have identified a population of patients who are less likely to benefit from everolimus," Dr. Rugo said.
She and her associates performed next-generation sequencing on DNA extracted tumor samples taken at diagnosis in BOLERO-2. Point mutations, short insertions or deletions, copy number alterations, or gene rearrangements were assessed in 219 tumors. Each tumor averaged 4 somatic mutations, with a range of 0-15 somatic mutations. Of the 182 genes sequenced, 104 had at least one known somatic mutation.
In the primary data analysis, all patients on everolimus had a 55% improvement in progression-free survival. In the secondary analysis of patients with minimal or no genetic alterations, 73% had an improvement in progression-free survival, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
The genetic analysis found alterations in PIK3CA in 48% of samples, altered CCND1 in 31% of samples, altered P53 in 23%, and altered FGFR1 in 18%, so the researchers used these common alterations to seek predictors of treatment response. The four most commonly altered genes and the overall "tumor genetic landscape" in the current analysis were similar to those seen in previous large analyses such as The Cancer Genome Atlas Network (Nature 2012;490:61-70).
There was a hint that patients with alterations of FGFR1 or FGFR2 might be less likely to benefit from everolimus therapy. These numbers were small, however, and so the finding is only a hypothesis-generating observation, she said.
The sample group comprised approximately one-third of the cohort in BOLERO-2, the trial data that was the basis for the approval of everolimus. Baseline characteristics and outcomes were similar between the subgroup in the secondary analysis and the overall cohort.
The BOLERO-2 study comprised 724 postmenopausal women with advanced hormone receptor–positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer that had recurred or progressed despite nonsteroidal aromatase inhibitor therapy. The women were randomized to treatment with exemestane plus everolimus or placebo. At 18 months of follow-up, median progression-free survival was 11 months in the patients in the exemestane-plus-everolimus group, significantly longer than the 4-month median progression-free survival seen in the exemestane-plus-placebo group (N. Engl. J. Med. 2012;366:520-29).
As in other recent trials, most of the tumor samples for the genetic analysis were archived from the time of initial diagnosis rather than after metastasis. "Although the impact on the results shown here is unknown, we do know that additional mutations in these pathways are acquired with metastatic progression," Dr. Rugo said. "I think it’s important for all of us to try to obtain metastatic tumor tissue for these analyses moving forward."
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Novartis Pharmaceuticals Corp., which markets everolimus (Afinitor), funded the study. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Among postmenopausal women with advanced hormone receptor–positive breast cancer, everolimus appeared to be more effective in the patients with one or no alterations in four key genes.
In a secondary analysis of data from the Breast Cancer Trials of Oral Everolimus–2 (BOLERO-2) study, one or no alterations in the PIK3CA; CCND1; FGFR1 and FGFR2 genes were seen in 76% of 227 tumor samples from women who took everolimus. These patients had significantly better progression-free survival than did patients with two or more alterations in these genes, Dr. Hope S. Rugo reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The number of patients with multiple gene alterations was admittedly small, but "potentially we have identified a population of patients who are less likely to benefit from everolimus," Dr. Rugo said.
She and her associates performed next-generation sequencing on DNA extracted tumor samples taken at diagnosis in BOLERO-2. Point mutations, short insertions or deletions, copy number alterations, or gene rearrangements were assessed in 219 tumors. Each tumor averaged 4 somatic mutations, with a range of 0-15 somatic mutations. Of the 182 genes sequenced, 104 had at least one known somatic mutation.
In the primary data analysis, all patients on everolimus had a 55% improvement in progression-free survival. In the secondary analysis of patients with minimal or no genetic alterations, 73% had an improvement in progression-free survival, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
The genetic analysis found alterations in PIK3CA in 48% of samples, altered CCND1 in 31% of samples, altered P53 in 23%, and altered FGFR1 in 18%, so the researchers used these common alterations to seek predictors of treatment response. The four most commonly altered genes and the overall "tumor genetic landscape" in the current analysis were similar to those seen in previous large analyses such as The Cancer Genome Atlas Network (Nature 2012;490:61-70).
There was a hint that patients with alterations of FGFR1 or FGFR2 might be less likely to benefit from everolimus therapy. These numbers were small, however, and so the finding is only a hypothesis-generating observation, she said.
The sample group comprised approximately one-third of the cohort in BOLERO-2, the trial data that was the basis for the approval of everolimus. Baseline characteristics and outcomes were similar between the subgroup in the secondary analysis and the overall cohort.
The BOLERO-2 study comprised 724 postmenopausal women with advanced hormone receptor–positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer that had recurred or progressed despite nonsteroidal aromatase inhibitor therapy. The women were randomized to treatment with exemestane plus everolimus or placebo. At 18 months of follow-up, median progression-free survival was 11 months in the patients in the exemestane-plus-everolimus group, significantly longer than the 4-month median progression-free survival seen in the exemestane-plus-placebo group (N. Engl. J. Med. 2012;366:520-29).
As in other recent trials, most of the tumor samples for the genetic analysis were archived from the time of initial diagnosis rather than after metastasis. "Although the impact on the results shown here is unknown, we do know that additional mutations in these pathways are acquired with metastatic progression," Dr. Rugo said. "I think it’s important for all of us to try to obtain metastatic tumor tissue for these analyses moving forward."
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Novartis Pharmaceuticals Corp., which markets everolimus (Afinitor), funded the study. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Among postmenopausal women with advanced hormone receptor–positive breast cancer, everolimus appeared to be more effective in the patients with one or no alterations in four key genes.
In a secondary analysis of data from the Breast Cancer Trials of Oral Everolimus–2 (BOLERO-2) study, one or no alterations in the PIK3CA; CCND1; FGFR1 and FGFR2 genes were seen in 76% of 227 tumor samples from women who took everolimus. These patients had significantly better progression-free survival than did patients with two or more alterations in these genes, Dr. Hope S. Rugo reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The number of patients with multiple gene alterations was admittedly small, but "potentially we have identified a population of patients who are less likely to benefit from everolimus," Dr. Rugo said.
She and her associates performed next-generation sequencing on DNA extracted tumor samples taken at diagnosis in BOLERO-2. Point mutations, short insertions or deletions, copy number alterations, or gene rearrangements were assessed in 219 tumors. Each tumor averaged 4 somatic mutations, with a range of 0-15 somatic mutations. Of the 182 genes sequenced, 104 had at least one known somatic mutation.
In the primary data analysis, all patients on everolimus had a 55% improvement in progression-free survival. In the secondary analysis of patients with minimal or no genetic alterations, 73% had an improvement in progression-free survival, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
The genetic analysis found alterations in PIK3CA in 48% of samples, altered CCND1 in 31% of samples, altered P53 in 23%, and altered FGFR1 in 18%, so the researchers used these common alterations to seek predictors of treatment response. The four most commonly altered genes and the overall "tumor genetic landscape" in the current analysis were similar to those seen in previous large analyses such as The Cancer Genome Atlas Network (Nature 2012;490:61-70).
There was a hint that patients with alterations of FGFR1 or FGFR2 might be less likely to benefit from everolimus therapy. These numbers were small, however, and so the finding is only a hypothesis-generating observation, she said.
The sample group comprised approximately one-third of the cohort in BOLERO-2, the trial data that was the basis for the approval of everolimus. Baseline characteristics and outcomes were similar between the subgroup in the secondary analysis and the overall cohort.
The BOLERO-2 study comprised 724 postmenopausal women with advanced hormone receptor–positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer that had recurred or progressed despite nonsteroidal aromatase inhibitor therapy. The women were randomized to treatment with exemestane plus everolimus or placebo. At 18 months of follow-up, median progression-free survival was 11 months in the patients in the exemestane-plus-everolimus group, significantly longer than the 4-month median progression-free survival seen in the exemestane-plus-placebo group (N. Engl. J. Med. 2012;366:520-29).
As in other recent trials, most of the tumor samples for the genetic analysis were archived from the time of initial diagnosis rather than after metastasis. "Although the impact on the results shown here is unknown, we do know that additional mutations in these pathways are acquired with metastatic progression," Dr. Rugo said. "I think it’s important for all of us to try to obtain metastatic tumor tissue for these analyses moving forward."
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Novartis Pharmaceuticals Corp., which markets everolimus (Afinitor), funded the study. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: In a secondary analysis, patients with one or no alterations in four genes had a 73% improvement in progression-free survival with everolimus therapy, compared with the 55% improvement in the general study population.
Data source: A retrospective, secondary analysis of genetic alterations in 227 tumor samples from women with advanced hormone receptor–positive breast cancer in the BOLERO-2 trial.
Disclosures: Novartis Pharmaceuticals Corp., which markets everolimus (Afinitor), funded the study. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
Breast cancer receptor change may predict outcomes
SAN FRANCISCO – A change in the status of any breast cancer tumor biomarker after neoadjuvant therapy was independently associated with a 37% decreased likelihood of recurrence in 5 years, a multivariate analysis showed.
The subtyping of breast cancer by receptor status changed in 41% of 398 samples between the initial tumor and residual disease after neoadjuvant chemotherapy.
In patients with any change in the status of estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2), after neoadjuvant chemotherapy, 63% were relapse free 5 years later as compared with 48% of patients with no receptor change, a significant difference.
Overall survival at 5 years, however, did not differ significantly based on receptor status change, Dr. Napa Parinyanitikul and her associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. At 5 years, 73% of patients with a receptor status change and 63% with no change were alive.
The study analyzed data on patients treated at her institution in 1992-2012 who had tumor biomarker results from pretreatment biopsies and from samples of residual disease after neoadjuvant chemotherapy. Most patients in the study were white, with clinical stage II or III disease.
Of the tumors, 49% were hormone receptor–positive and HER2 negative; 18% were HER2 positive, and 33% were negative for all three receptors (triple-negative breast cancer). Neoadjuvant chemotherapy consisted of a taxane-based regimen in 10%, an anthracycline-based regimen in 19%, an anthracycline/taxane-based regimen in 71%, and trastuzumab-based chemotherapy in 49%. The 88% of patients with hormone receptor-positive disease received adjuvant endocrine therapy with tamoxifen or an aromatase inhibitor.
After a median of 40 months of follow-up, 32% of women had died and 42% had a recurrence of breast cancer, reported Dr. Parinyanitikul, a fellow at the University of Texas MD Anderson Cancer Center, Houston.
The likelihood of receptor status changes differed significantly by the subtype of breast cancer. Of hormone receptor–positive cancers, 51% had a change in receptor status, compared with 27% of HER2-positive cancers and 22% of triple-negative breast cancers.
Other factors did not significantly affect the likelihood of receptor status change, including patient age or race, histology, tumor stage or grade, the presence of lymphovascular invasion, or the class of neoadjuvant chemotherapeutic agents.
Of ER-positive tumors, 11% changed to ER negative after neoadjuvant chemotherapy; of ER-negative tumors, 21% changed to ER positive. Of the PR-positive tumors, 35% changed to PR-negative; of PR-negative tumors, 12% changed to PR positive. Of HER2-positive tumors, 40% changed to HER2 negative; of HER2-negative tumors, 3% changed to HER2 positive. Among 35 patients treated with trastuzumab, the HER2 status changed in 16 (46%).
Patients who had tumors with an ER-negative status that did not change had the worst 5-year rates for overall survival (47%) and relapse-free survival (40%), compared with patients whose tumors had an ER-positive status did not change (81% overall survival and 63% relapse-free survival), patients whose ER status changed from positive to negative (67% and 66% survival rates, respectively), and patients whose ER status changed from negative to positive (75% and 59%, respectively).
Similarly, patients who had PR-negative tumors whose status did not change had significantly lower rates of overall survival (51%) and relapse-free survival (41%), compared with patients whose PR-positive tumor status did not change (87% and 67%, respectively), those who changed from PR positive to PR negative (78% and 73%, respectively), and patients whose PR-negative status changed to PR positive (69% and 55%, respectively).
Changes or lack of change in HER2 subgroups were not significantly associated with varying outcomes.
The investigators also looked at the absolute percent changes in ER or PR status using cutoffs in 10% increments from 10% to 50%. The 5-year overall survival rate was significantly greater in ER-positive patients who had at least a 20% change in ER status, compared with those with less than a 20% change (87% vs. 73%, respectively). The 5-year relapse-free survival rate also was significantly greater if the ER status changed by at least 20% (71%), compared with smaller ER changes (59%).
Of cancers that were hormone receptor–positive and HER2 negative before neoadjuvant chemotherapy, 20% changed to HER2-positive or triple-negative breast cancer after treatment, 12% of tumors that had been HER2 positive changed to triple negative tumors, and 2% of triple negative breast cancers changed to HER2-positive after treatment. These changes produced only one significant effect on outcomes: 5-year overall survival rates were significantly lower in patients who changed from HER2-positive to triple-negative breast cancer (23%), compared with those whose HER2-positive cancer did not change to triple negative (71%).
Larger prospective studies are needed to confirm the findings and to determine any impact of biomarker changes on long-term survival, she said.
Previous studies have reported discordance in hormone receptor status in up to 51% of breast cancers between the primary tumor and residual disease and changes in HER2 status in up to 43% of cases. Conclusions about associations between changes in receptor status and clinical outcomes have been inconsistent.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Parinyanitikul reported having no financial disclosures.
On Twitter @sherryboschert
Technical artifacts when testing receptor status in breast cancer complicate assessments of their potential ramifications on outcomes.

|
|
ER and PR status can change if you repeat the testing on the same specimen without any change in the actual marker status. In one "classic" study, the HER2 or ER status changed in 23% of samples after they were air-mailed from one center to another. You can’t conclude that it was the airplane flight that changed receptor status.
Similarly, in the current study, you can’t conclude that it was the chemotherapy that changed the receptor status, because part of this discordance is from the technical imprecision of the test. If a test is 90% accurate, for example, 10% of results on first testing will be "noise" and approximately 20% of results on repeat testing will be discordant.
The study also did not report the proportion of cases in which initial receptor status tests were conducted in one laboratory and the second test was conducted at a different center, as is often the case in the real world. That also introduces a very large, obvious cause for receptor discordance independent of therapy.
Dr. Lajos Pusztai is a professor of medicine and director of the Breast Medical Oncology Section at Yale University, New Haven, Conn. He made his remarks during a question-and-answer session at the meeting. Dr. Pusztai reported financial associations with BiPar Sciences/Sanofi, Bristol-Myers Squibb, Pfizer, AstraZeneca, Roche/Genentech, and Foundation Medicine.
Technical artifacts when testing receptor status in breast cancer complicate assessments of their potential ramifications on outcomes.

|
|
ER and PR status can change if you repeat the testing on the same specimen without any change in the actual marker status. In one "classic" study, the HER2 or ER status changed in 23% of samples after they were air-mailed from one center to another. You can’t conclude that it was the airplane flight that changed receptor status.
Similarly, in the current study, you can’t conclude that it was the chemotherapy that changed the receptor status, because part of this discordance is from the technical imprecision of the test. If a test is 90% accurate, for example, 10% of results on first testing will be "noise" and approximately 20% of results on repeat testing will be discordant.
The study also did not report the proportion of cases in which initial receptor status tests were conducted in one laboratory and the second test was conducted at a different center, as is often the case in the real world. That also introduces a very large, obvious cause for receptor discordance independent of therapy.
Dr. Lajos Pusztai is a professor of medicine and director of the Breast Medical Oncology Section at Yale University, New Haven, Conn. He made his remarks during a question-and-answer session at the meeting. Dr. Pusztai reported financial associations with BiPar Sciences/Sanofi, Bristol-Myers Squibb, Pfizer, AstraZeneca, Roche/Genentech, and Foundation Medicine.
Technical artifacts when testing receptor status in breast cancer complicate assessments of their potential ramifications on outcomes.

|
|
ER and PR status can change if you repeat the testing on the same specimen without any change in the actual marker status. In one "classic" study, the HER2 or ER status changed in 23% of samples after they were air-mailed from one center to another. You can’t conclude that it was the airplane flight that changed receptor status.
Similarly, in the current study, you can’t conclude that it was the chemotherapy that changed the receptor status, because part of this discordance is from the technical imprecision of the test. If a test is 90% accurate, for example, 10% of results on first testing will be "noise" and approximately 20% of results on repeat testing will be discordant.
The study also did not report the proportion of cases in which initial receptor status tests were conducted in one laboratory and the second test was conducted at a different center, as is often the case in the real world. That also introduces a very large, obvious cause for receptor discordance independent of therapy.
Dr. Lajos Pusztai is a professor of medicine and director of the Breast Medical Oncology Section at Yale University, New Haven, Conn. He made his remarks during a question-and-answer session at the meeting. Dr. Pusztai reported financial associations with BiPar Sciences/Sanofi, Bristol-Myers Squibb, Pfizer, AstraZeneca, Roche/Genentech, and Foundation Medicine.
SAN FRANCISCO – A change in the status of any breast cancer tumor biomarker after neoadjuvant therapy was independently associated with a 37% decreased likelihood of recurrence in 5 years, a multivariate analysis showed.
The subtyping of breast cancer by receptor status changed in 41% of 398 samples between the initial tumor and residual disease after neoadjuvant chemotherapy.
In patients with any change in the status of estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2), after neoadjuvant chemotherapy, 63% were relapse free 5 years later as compared with 48% of patients with no receptor change, a significant difference.
Overall survival at 5 years, however, did not differ significantly based on receptor status change, Dr. Napa Parinyanitikul and her associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. At 5 years, 73% of patients with a receptor status change and 63% with no change were alive.
The study analyzed data on patients treated at her institution in 1992-2012 who had tumor biomarker results from pretreatment biopsies and from samples of residual disease after neoadjuvant chemotherapy. Most patients in the study were white, with clinical stage II or III disease.
Of the tumors, 49% were hormone receptor–positive and HER2 negative; 18% were HER2 positive, and 33% were negative for all three receptors (triple-negative breast cancer). Neoadjuvant chemotherapy consisted of a taxane-based regimen in 10%, an anthracycline-based regimen in 19%, an anthracycline/taxane-based regimen in 71%, and trastuzumab-based chemotherapy in 49%. The 88% of patients with hormone receptor-positive disease received adjuvant endocrine therapy with tamoxifen or an aromatase inhibitor.
After a median of 40 months of follow-up, 32% of women had died and 42% had a recurrence of breast cancer, reported Dr. Parinyanitikul, a fellow at the University of Texas MD Anderson Cancer Center, Houston.
The likelihood of receptor status changes differed significantly by the subtype of breast cancer. Of hormone receptor–positive cancers, 51% had a change in receptor status, compared with 27% of HER2-positive cancers and 22% of triple-negative breast cancers.
Other factors did not significantly affect the likelihood of receptor status change, including patient age or race, histology, tumor stage or grade, the presence of lymphovascular invasion, or the class of neoadjuvant chemotherapeutic agents.
Of ER-positive tumors, 11% changed to ER negative after neoadjuvant chemotherapy; of ER-negative tumors, 21% changed to ER positive. Of the PR-positive tumors, 35% changed to PR-negative; of PR-negative tumors, 12% changed to PR positive. Of HER2-positive tumors, 40% changed to HER2 negative; of HER2-negative tumors, 3% changed to HER2 positive. Among 35 patients treated with trastuzumab, the HER2 status changed in 16 (46%).
Patients who had tumors with an ER-negative status that did not change had the worst 5-year rates for overall survival (47%) and relapse-free survival (40%), compared with patients whose tumors had an ER-positive status did not change (81% overall survival and 63% relapse-free survival), patients whose ER status changed from positive to negative (67% and 66% survival rates, respectively), and patients whose ER status changed from negative to positive (75% and 59%, respectively).
Similarly, patients who had PR-negative tumors whose status did not change had significantly lower rates of overall survival (51%) and relapse-free survival (41%), compared with patients whose PR-positive tumor status did not change (87% and 67%, respectively), those who changed from PR positive to PR negative (78% and 73%, respectively), and patients whose PR-negative status changed to PR positive (69% and 55%, respectively).
Changes or lack of change in HER2 subgroups were not significantly associated with varying outcomes.
The investigators also looked at the absolute percent changes in ER or PR status using cutoffs in 10% increments from 10% to 50%. The 5-year overall survival rate was significantly greater in ER-positive patients who had at least a 20% change in ER status, compared with those with less than a 20% change (87% vs. 73%, respectively). The 5-year relapse-free survival rate also was significantly greater if the ER status changed by at least 20% (71%), compared with smaller ER changes (59%).
Of cancers that were hormone receptor–positive and HER2 negative before neoadjuvant chemotherapy, 20% changed to HER2-positive or triple-negative breast cancer after treatment, 12% of tumors that had been HER2 positive changed to triple negative tumors, and 2% of triple negative breast cancers changed to HER2-positive after treatment. These changes produced only one significant effect on outcomes: 5-year overall survival rates were significantly lower in patients who changed from HER2-positive to triple-negative breast cancer (23%), compared with those whose HER2-positive cancer did not change to triple negative (71%).
Larger prospective studies are needed to confirm the findings and to determine any impact of biomarker changes on long-term survival, she said.
Previous studies have reported discordance in hormone receptor status in up to 51% of breast cancers between the primary tumor and residual disease and changes in HER2 status in up to 43% of cases. Conclusions about associations between changes in receptor status and clinical outcomes have been inconsistent.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Parinyanitikul reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – A change in the status of any breast cancer tumor biomarker after neoadjuvant therapy was independently associated with a 37% decreased likelihood of recurrence in 5 years, a multivariate analysis showed.
The subtyping of breast cancer by receptor status changed in 41% of 398 samples between the initial tumor and residual disease after neoadjuvant chemotherapy.
In patients with any change in the status of estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2), after neoadjuvant chemotherapy, 63% were relapse free 5 years later as compared with 48% of patients with no receptor change, a significant difference.
Overall survival at 5 years, however, did not differ significantly based on receptor status change, Dr. Napa Parinyanitikul and her associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. At 5 years, 73% of patients with a receptor status change and 63% with no change were alive.
The study analyzed data on patients treated at her institution in 1992-2012 who had tumor biomarker results from pretreatment biopsies and from samples of residual disease after neoadjuvant chemotherapy. Most patients in the study were white, with clinical stage II or III disease.
Of the tumors, 49% were hormone receptor–positive and HER2 negative; 18% were HER2 positive, and 33% were negative for all three receptors (triple-negative breast cancer). Neoadjuvant chemotherapy consisted of a taxane-based regimen in 10%, an anthracycline-based regimen in 19%, an anthracycline/taxane-based regimen in 71%, and trastuzumab-based chemotherapy in 49%. The 88% of patients with hormone receptor-positive disease received adjuvant endocrine therapy with tamoxifen or an aromatase inhibitor.
After a median of 40 months of follow-up, 32% of women had died and 42% had a recurrence of breast cancer, reported Dr. Parinyanitikul, a fellow at the University of Texas MD Anderson Cancer Center, Houston.
The likelihood of receptor status changes differed significantly by the subtype of breast cancer. Of hormone receptor–positive cancers, 51% had a change in receptor status, compared with 27% of HER2-positive cancers and 22% of triple-negative breast cancers.
Other factors did not significantly affect the likelihood of receptor status change, including patient age or race, histology, tumor stage or grade, the presence of lymphovascular invasion, or the class of neoadjuvant chemotherapeutic agents.
Of ER-positive tumors, 11% changed to ER negative after neoadjuvant chemotherapy; of ER-negative tumors, 21% changed to ER positive. Of the PR-positive tumors, 35% changed to PR-negative; of PR-negative tumors, 12% changed to PR positive. Of HER2-positive tumors, 40% changed to HER2 negative; of HER2-negative tumors, 3% changed to HER2 positive. Among 35 patients treated with trastuzumab, the HER2 status changed in 16 (46%).
Patients who had tumors with an ER-negative status that did not change had the worst 5-year rates for overall survival (47%) and relapse-free survival (40%), compared with patients whose tumors had an ER-positive status did not change (81% overall survival and 63% relapse-free survival), patients whose ER status changed from positive to negative (67% and 66% survival rates, respectively), and patients whose ER status changed from negative to positive (75% and 59%, respectively).
Similarly, patients who had PR-negative tumors whose status did not change had significantly lower rates of overall survival (51%) and relapse-free survival (41%), compared with patients whose PR-positive tumor status did not change (87% and 67%, respectively), those who changed from PR positive to PR negative (78% and 73%, respectively), and patients whose PR-negative status changed to PR positive (69% and 55%, respectively).
Changes or lack of change in HER2 subgroups were not significantly associated with varying outcomes.
The investigators also looked at the absolute percent changes in ER or PR status using cutoffs in 10% increments from 10% to 50%. The 5-year overall survival rate was significantly greater in ER-positive patients who had at least a 20% change in ER status, compared with those with less than a 20% change (87% vs. 73%, respectively). The 5-year relapse-free survival rate also was significantly greater if the ER status changed by at least 20% (71%), compared with smaller ER changes (59%).
Of cancers that were hormone receptor–positive and HER2 negative before neoadjuvant chemotherapy, 20% changed to HER2-positive or triple-negative breast cancer after treatment, 12% of tumors that had been HER2 positive changed to triple negative tumors, and 2% of triple negative breast cancers changed to HER2-positive after treatment. These changes produced only one significant effect on outcomes: 5-year overall survival rates were significantly lower in patients who changed from HER2-positive to triple-negative breast cancer (23%), compared with those whose HER2-positive cancer did not change to triple negative (71%).
Larger prospective studies are needed to confirm the findings and to determine any impact of biomarker changes on long-term survival, she said.
Previous studies have reported discordance in hormone receptor status in up to 51% of breast cancers between the primary tumor and residual disease and changes in HER2 status in up to 43% of cases. Conclusions about associations between changes in receptor status and clinical outcomes have been inconsistent.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Parinyanitikul reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: Receptor status changed in 41% of breast cancers after neoadjuvant chemotherapy. Relapse within 5 years was significantly less likely in patients with a receptor change (63%) than in those with no receptor change (48%).
Data source: A retrospective study of 398 women with data on ER, PR, and HER2 status in the primary tumor and in residual disease after neoadjuvant chemotherapy.
Disclosures: Dr. Parinyanitikul reported having no financial disclosures.
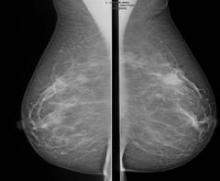
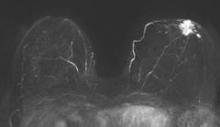
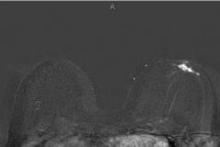
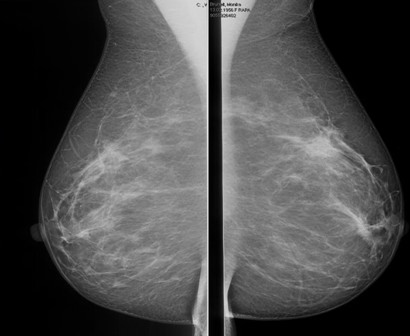
Abbreviated MRI breast cancer screening protocol accurate
SAN FRANCISCO – A 3-minute MRI allowed a radiologist to rule out breast cancer with 99% accuracy in a prospective study of 443 women at a slightly increased or intermediate lifetime risk of breast cancer.
Extending the expert’s reading time to no more than 30 seconds to also interpret the first postcontrast subtracted (FAST) images provided the same sensitivity and specificity as a full diagnostic MRI protocol that had patients on the MRI table for 21 minutes on average, Dr. Christiane K. Kuhl reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Conventional breast MRI protocols are time consuming and more expensive, because they are designed for diagnosis, not screening, said Dr. Kuhl, director of the department of diagnostic and interventional radiology at Rheinisch-Westfälische Technische Hochschule Aachen (Germany) University. The study aimed to trade some of the high sensitivity of MRI in detecting breast cancer for faster image acquisition and interpretation, perhaps eventually leading to lower cost and greater accessibility to MRI.
For the study, experienced breast radiologists were asked to review the maximum intensity projection (MIP) of the FAST images to determine whether there was significant enhancement. They then examined the FAST images for possible further categorization of any enhancement seen on the MIP, and analyzed the MRI under the full diagnostic protocol.
The prospective proof-of-concept study included patients who had imaging done between January 2009 and June 2010 and who were followed for 2 years to validate negative diagnoses. These were asymptomatic women with an intermediate risk with less than a 25% estimated lifetime risk of breast cancer. All of the women had a normal or benign mammogram and, if they had dense breasts, a normal or benign ultrasound result.
Acquiring the MIP and FAST images took less than 3 minutes with the patient on the MRI table. Reading the maximum intensity projection averaged 3 seconds, and reading the MIP plus FAST image averaged 28 seconds.
MRI screening identified 11 cancers, for an additional yield of 18 cancers per 1,000 examinations beyond what could be detected by mammography. Reading the MIP alone had a negative predictive value of 99%; the maximum intensity projection plus FAST readings and the full MRI protocol had negative predictive values just under 100%, Dr. Kuhl reported. The sensitivity of the MIP was close to 95% compared with 100% for MIP plus FAST or the full protocol. The specificity and positive predictive value could not be calculated for the MIP because the MIP reading detected only the presence or absence of significant enhancement. Evaluating the MIP plus FAST or the full MRI protocol produced similar specificities (in the mid-90% range) and positive predictive values (in the low 20% range).
All 11 breast cancers were intermediate- or high-grade cancers; 4 were ductal carcinoma in situ (DCIS) and 7 were invasive cancers. The median tumor size was 8 mm, and the mean age of the women with cancer was 51 years.
Conventional breast cancer screening using mammography picks up cancers that may be prognostically irrelevant, which add to potential overdiagnosis of breast cancer, she noted. In 11 studies that compared screening with breast MRI and mammography, MRI detected two to four times as many cancers as did mammography or ultrasound. Finding more DCIS and invasive cancers via MRI doesn’t necessarily add to the problem of overdiagnosis, she said, because the issue is as much underdiagnosis of prognostically relevant disease as it is overdiagnosis of relevant disease.
The technology of mammography favors detection of slowly growing cancers because of its focus on architectural distortions, spiculations, and calcifications that reflect regressive changes, while MRI technology detects angiogenic and protease activity that is biased toward biologically active disease, she said.
Previous data from her institution show that the sensitivity of MRI for detecting DCIS increases from 80% with low-grade DCIS to 98% with high-grade DCIS, but the sensitivity of mammography decreases as DCIS grade increases, dropping from 61% with low-grade DCIS to 35% with high-grade DCIS without necrosis*.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Kuhl has been a consultant to Bayer. She reported no other relevant financial disclosures.
*This story was updated 10/16/2013.
On Twitter @sherryboschert
SAN FRANCISCO – A 3-minute MRI allowed a radiologist to rule out breast cancer with 99% accuracy in a prospective study of 443 women at a slightly increased or intermediate lifetime risk of breast cancer.
Extending the expert’s reading time to no more than 30 seconds to also interpret the first postcontrast subtracted (FAST) images provided the same sensitivity and specificity as a full diagnostic MRI protocol that had patients on the MRI table for 21 minutes on average, Dr. Christiane K. Kuhl reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Conventional breast MRI protocols are time consuming and more expensive, because they are designed for diagnosis, not screening, said Dr. Kuhl, director of the department of diagnostic and interventional radiology at Rheinisch-Westfälische Technische Hochschule Aachen (Germany) University. The study aimed to trade some of the high sensitivity of MRI in detecting breast cancer for faster image acquisition and interpretation, perhaps eventually leading to lower cost and greater accessibility to MRI.
For the study, experienced breast radiologists were asked to review the maximum intensity projection (MIP) of the FAST images to determine whether there was significant enhancement. They then examined the FAST images for possible further categorization of any enhancement seen on the MIP, and analyzed the MRI under the full diagnostic protocol.
The prospective proof-of-concept study included patients who had imaging done between January 2009 and June 2010 and who were followed for 2 years to validate negative diagnoses. These were asymptomatic women with an intermediate risk with less than a 25% estimated lifetime risk of breast cancer. All of the women had a normal or benign mammogram and, if they had dense breasts, a normal or benign ultrasound result.
Acquiring the MIP and FAST images took less than 3 minutes with the patient on the MRI table. Reading the maximum intensity projection averaged 3 seconds, and reading the MIP plus FAST image averaged 28 seconds.
MRI screening identified 11 cancers, for an additional yield of 18 cancers per 1,000 examinations beyond what could be detected by mammography. Reading the MIP alone had a negative predictive value of 99%; the maximum intensity projection plus FAST readings and the full MRI protocol had negative predictive values just under 100%, Dr. Kuhl reported. The sensitivity of the MIP was close to 95% compared with 100% for MIP plus FAST or the full protocol. The specificity and positive predictive value could not be calculated for the MIP because the MIP reading detected only the presence or absence of significant enhancement. Evaluating the MIP plus FAST or the full MRI protocol produced similar specificities (in the mid-90% range) and positive predictive values (in the low 20% range).
All 11 breast cancers were intermediate- or high-grade cancers; 4 were ductal carcinoma in situ (DCIS) and 7 were invasive cancers. The median tumor size was 8 mm, and the mean age of the women with cancer was 51 years.
Conventional breast cancer screening using mammography picks up cancers that may be prognostically irrelevant, which add to potential overdiagnosis of breast cancer, she noted. In 11 studies that compared screening with breast MRI and mammography, MRI detected two to four times as many cancers as did mammography or ultrasound. Finding more DCIS and invasive cancers via MRI doesn’t necessarily add to the problem of overdiagnosis, she said, because the issue is as much underdiagnosis of prognostically relevant disease as it is overdiagnosis of relevant disease.
The technology of mammography favors detection of slowly growing cancers because of its focus on architectural distortions, spiculations, and calcifications that reflect regressive changes, while MRI technology detects angiogenic and protease activity that is biased toward biologically active disease, she said.
Previous data from her institution show that the sensitivity of MRI for detecting DCIS increases from 80% with low-grade DCIS to 98% with high-grade DCIS, but the sensitivity of mammography decreases as DCIS grade increases, dropping from 61% with low-grade DCIS to 35% with high-grade DCIS without necrosis*.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Kuhl has been a consultant to Bayer. She reported no other relevant financial disclosures.
*This story was updated 10/16/2013.
On Twitter @sherryboschert
SAN FRANCISCO – A 3-minute MRI allowed a radiologist to rule out breast cancer with 99% accuracy in a prospective study of 443 women at a slightly increased or intermediate lifetime risk of breast cancer.
Extending the expert’s reading time to no more than 30 seconds to also interpret the first postcontrast subtracted (FAST) images provided the same sensitivity and specificity as a full diagnostic MRI protocol that had patients on the MRI table for 21 minutes on average, Dr. Christiane K. Kuhl reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Conventional breast MRI protocols are time consuming and more expensive, because they are designed for diagnosis, not screening, said Dr. Kuhl, director of the department of diagnostic and interventional radiology at Rheinisch-Westfälische Technische Hochschule Aachen (Germany) University. The study aimed to trade some of the high sensitivity of MRI in detecting breast cancer for faster image acquisition and interpretation, perhaps eventually leading to lower cost and greater accessibility to MRI.
For the study, experienced breast radiologists were asked to review the maximum intensity projection (MIP) of the FAST images to determine whether there was significant enhancement. They then examined the FAST images for possible further categorization of any enhancement seen on the MIP, and analyzed the MRI under the full diagnostic protocol.
The prospective proof-of-concept study included patients who had imaging done between January 2009 and June 2010 and who were followed for 2 years to validate negative diagnoses. These were asymptomatic women with an intermediate risk with less than a 25% estimated lifetime risk of breast cancer. All of the women had a normal or benign mammogram and, if they had dense breasts, a normal or benign ultrasound result.
Acquiring the MIP and FAST images took less than 3 minutes with the patient on the MRI table. Reading the maximum intensity projection averaged 3 seconds, and reading the MIP plus FAST image averaged 28 seconds.
MRI screening identified 11 cancers, for an additional yield of 18 cancers per 1,000 examinations beyond what could be detected by mammography. Reading the MIP alone had a negative predictive value of 99%; the maximum intensity projection plus FAST readings and the full MRI protocol had negative predictive values just under 100%, Dr. Kuhl reported. The sensitivity of the MIP was close to 95% compared with 100% for MIP plus FAST or the full protocol. The specificity and positive predictive value could not be calculated for the MIP because the MIP reading detected only the presence or absence of significant enhancement. Evaluating the MIP plus FAST or the full MRI protocol produced similar specificities (in the mid-90% range) and positive predictive values (in the low 20% range).
All 11 breast cancers were intermediate- or high-grade cancers; 4 were ductal carcinoma in situ (DCIS) and 7 were invasive cancers. The median tumor size was 8 mm, and the mean age of the women with cancer was 51 years.
Conventional breast cancer screening using mammography picks up cancers that may be prognostically irrelevant, which add to potential overdiagnosis of breast cancer, she noted. In 11 studies that compared screening with breast MRI and mammography, MRI detected two to four times as many cancers as did mammography or ultrasound. Finding more DCIS and invasive cancers via MRI doesn’t necessarily add to the problem of overdiagnosis, she said, because the issue is as much underdiagnosis of prognostically relevant disease as it is overdiagnosis of relevant disease.
The technology of mammography favors detection of slowly growing cancers because of its focus on architectural distortions, spiculations, and calcifications that reflect regressive changes, while MRI technology detects angiogenic and protease activity that is biased toward biologically active disease, she said.
Previous data from her institution show that the sensitivity of MRI for detecting DCIS increases from 80% with low-grade DCIS to 98% with high-grade DCIS, but the sensitivity of mammography decreases as DCIS grade increases, dropping from 61% with low-grade DCIS to 35% with high-grade DCIS without necrosis*.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Kuhl has been a consultant to Bayer. She reported no other relevant financial disclosures.
*This story was updated 10/16/2013.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: MRI screening identified 11 cancers, for an additional yield of 18 cancers per 1,000 examinations beyond what could be detected by mammography.
Data source: A prospective proof-of-concept study of MRI screening in 443 women with slightly increased or intermediate risk for breast cancer and negative mammograms.
Disclosures: Dr. Kuhl has been a consultant to Bayer. She reported no other relevant financial disclosures.
Perioperative MRI fails to reduce recurrence risk in women with ductal carcinoma in situ
Routine use of magnetic resonance imaging does not improve outcomes in women undergoing lumpectomy for ductal carcinoma in situ, according to the results of a retrospective cohort study conducted at the Memorial Sloan-Kettering Cancer Center in New York.
A team led by Dr. Melissa L. Pilewskie studied 2,321 patients who underwent lumpectomy for ductal carcinoma in situ (DCIS) between 1997 and 2010. A quarter had breast MRI before or immediately after their surgery, in addition to conventional imaging with mammography and/or ultrasound.
Study results, being reported in full later this week at the breast cancer symposium sponsored by the American Society of Clinical Oncology, showed that the 5-year rate of locoregional recurrence was about 8%, with no significant difference between the groups who did and did not have MRI.
The findings were the same after adjustment for potential confounders and also when analyses were restricted to the subset of patients who did not receive radiation therapy, according to Dr. Pilewskie, a breast surgeon at Sloan-Kettering.
In addition, perioperative MRI did not reduce the rate of contralateral breast cancer, which stood at about 4% in each group.
"In the absence of evidence that MRI is improving our surgical management or – as we showed here – long-term outcomes, the routine use of this test for DCIS should be questioned," Dr. Pilewskie commented in a related press briefing.
She outlined circumstances that may justify this additional testing. "I think that MRI can be a useful adjunct if there are discrepancies or still clinical questions when someone comes in, between the imaging that they have on their mammogram or ultrasound and their physical exam or their presentation," she said. "The majority of women who present with DCIS have a normal exam and calcifications or changes on a mammogram. So when there are differences, and someone has a palpable mass or nipple discharge or a different presentation that wasn’t answered by their imaging, I think MRI can help give additional information. But that again is not the routine woman who presents with DCIS."
With a median 59-month follow-up, the groups who did and did not receive MRI were statistically indistinguishable in terms of the actuarial rate of locoregional recurrence at 5 years (8.5% vs. 7.2%) and at 8 years (14.6% vs. 10.2%).
"When comparing the MRI and no-MRI groups, there were some differences between them in that the women who had an MRI had more high-risk features," Dr. Pilewskie noted, and those features might have influenced the decision to obtain this additional imaging and outcomes.
However, MRI was not a significant predictor of locoregional recurrence in a multivariate analysis that adjusted for these and other potential confounders: age, family history, mode of presentation, tamoxifen or other hormonal therapy, margin status, number of excisions, and year of surgery.
On the other hand, receipt of radiation therapy, receipt of endocrine therapy, and negative margins were all significantly associated with a lower risk of locoregional recurrence.
In a subset analysis, MRI also failed to reduce the risk of locoregional recurrence in the roughly one-third of patients who did not receive radiation therapy.
Similarly, patients who did and did not receive MRI were statistically indistinguishable in terms of the actuarial rate of contralateral breast cancer at 5 years (3.5% vs. 3.5%) and at 8 years (3.5% vs. 5.1%). Again, results were essentially the same in the subset who did not receive radiation therapy.
Dr. Steven O’Day, director of clinical research at the Beverly Hills Cancer Institute in California and moderator of the press briefing, commented, "There has been a tremendous increase in the use of MRI perioperatively and postoperatively in invasive as well as noninvasive breast cancer. And this [study] I think just grounds us to continue to – as new technologies, new imaging is used – be sure that we are actually" improving outcomes.
He concurred that the added sensitivity of MRI may be helpful in challenging cases. "But its routine use certainly in DCIS from this large retrospective study has not been shown as an independent predictor to improve locoregional or contralateral breast outcomes," he said. "So I think this is an important study" and leads us to want "to study MRI further prospectively both in DCIS and invasive cancer."
Giving some background to the research, Dr. Pilewskie noted that current guidelines do not address when MRI should be used in the work-up of patients with DCIS.
"However, about 30% of physicians currently obtain a perioperative MRI to look for areas of additional disease in patients with DCIS, and theoretically, treating this additional disease found by MRI could result in a lower risk of local recurrence or contralateral breast cancer down the road," she said. "And, again theoretically, this effect could be most pronounced in women treated with excision alone, meaning just having lumpectomy and no radiation therapy."
Overall, 26% of the patients studied had breast MRI near the time of surgery, most commonly preoperatively, to assess disease extent.
Dr. Pilewskie and Dr. O’Day disclosed no relevant conflicts of interest.
Routine use of magnetic resonance imaging does not improve outcomes in women undergoing lumpectomy for ductal carcinoma in situ, according to the results of a retrospective cohort study conducted at the Memorial Sloan-Kettering Cancer Center in New York.
A team led by Dr. Melissa L. Pilewskie studied 2,321 patients who underwent lumpectomy for ductal carcinoma in situ (DCIS) between 1997 and 2010. A quarter had breast MRI before or immediately after their surgery, in addition to conventional imaging with mammography and/or ultrasound.
Study results, being reported in full later this week at the breast cancer symposium sponsored by the American Society of Clinical Oncology, showed that the 5-year rate of locoregional recurrence was about 8%, with no significant difference between the groups who did and did not have MRI.
The findings were the same after adjustment for potential confounders and also when analyses were restricted to the subset of patients who did not receive radiation therapy, according to Dr. Pilewskie, a breast surgeon at Sloan-Kettering.
In addition, perioperative MRI did not reduce the rate of contralateral breast cancer, which stood at about 4% in each group.
"In the absence of evidence that MRI is improving our surgical management or – as we showed here – long-term outcomes, the routine use of this test for DCIS should be questioned," Dr. Pilewskie commented in a related press briefing.
She outlined circumstances that may justify this additional testing. "I think that MRI can be a useful adjunct if there are discrepancies or still clinical questions when someone comes in, between the imaging that they have on their mammogram or ultrasound and their physical exam or their presentation," she said. "The majority of women who present with DCIS have a normal exam and calcifications or changes on a mammogram. So when there are differences, and someone has a palpable mass or nipple discharge or a different presentation that wasn’t answered by their imaging, I think MRI can help give additional information. But that again is not the routine woman who presents with DCIS."
With a median 59-month follow-up, the groups who did and did not receive MRI were statistically indistinguishable in terms of the actuarial rate of locoregional recurrence at 5 years (8.5% vs. 7.2%) and at 8 years (14.6% vs. 10.2%).
"When comparing the MRI and no-MRI groups, there were some differences between them in that the women who had an MRI had more high-risk features," Dr. Pilewskie noted, and those features might have influenced the decision to obtain this additional imaging and outcomes.
However, MRI was not a significant predictor of locoregional recurrence in a multivariate analysis that adjusted for these and other potential confounders: age, family history, mode of presentation, tamoxifen or other hormonal therapy, margin status, number of excisions, and year of surgery.
On the other hand, receipt of radiation therapy, receipt of endocrine therapy, and negative margins were all significantly associated with a lower risk of locoregional recurrence.
In a subset analysis, MRI also failed to reduce the risk of locoregional recurrence in the roughly one-third of patients who did not receive radiation therapy.
Similarly, patients who did and did not receive MRI were statistically indistinguishable in terms of the actuarial rate of contralateral breast cancer at 5 years (3.5% vs. 3.5%) and at 8 years (3.5% vs. 5.1%). Again, results were essentially the same in the subset who did not receive radiation therapy.
Dr. Steven O’Day, director of clinical research at the Beverly Hills Cancer Institute in California and moderator of the press briefing, commented, "There has been a tremendous increase in the use of MRI perioperatively and postoperatively in invasive as well as noninvasive breast cancer. And this [study] I think just grounds us to continue to – as new technologies, new imaging is used – be sure that we are actually" improving outcomes.
He concurred that the added sensitivity of MRI may be helpful in challenging cases. "But its routine use certainly in DCIS from this large retrospective study has not been shown as an independent predictor to improve locoregional or contralateral breast outcomes," he said. "So I think this is an important study" and leads us to want "to study MRI further prospectively both in DCIS and invasive cancer."
Giving some background to the research, Dr. Pilewskie noted that current guidelines do not address when MRI should be used in the work-up of patients with DCIS.
"However, about 30% of physicians currently obtain a perioperative MRI to look for areas of additional disease in patients with DCIS, and theoretically, treating this additional disease found by MRI could result in a lower risk of local recurrence or contralateral breast cancer down the road," she said. "And, again theoretically, this effect could be most pronounced in women treated with excision alone, meaning just having lumpectomy and no radiation therapy."
Overall, 26% of the patients studied had breast MRI near the time of surgery, most commonly preoperatively, to assess disease extent.
Dr. Pilewskie and Dr. O’Day disclosed no relevant conflicts of interest.
Routine use of magnetic resonance imaging does not improve outcomes in women undergoing lumpectomy for ductal carcinoma in situ, according to the results of a retrospective cohort study conducted at the Memorial Sloan-Kettering Cancer Center in New York.
A team led by Dr. Melissa L. Pilewskie studied 2,321 patients who underwent lumpectomy for ductal carcinoma in situ (DCIS) between 1997 and 2010. A quarter had breast MRI before or immediately after their surgery, in addition to conventional imaging with mammography and/or ultrasound.
Study results, being reported in full later this week at the breast cancer symposium sponsored by the American Society of Clinical Oncology, showed that the 5-year rate of locoregional recurrence was about 8%, with no significant difference between the groups who did and did not have MRI.
The findings were the same after adjustment for potential confounders and also when analyses were restricted to the subset of patients who did not receive radiation therapy, according to Dr. Pilewskie, a breast surgeon at Sloan-Kettering.
In addition, perioperative MRI did not reduce the rate of contralateral breast cancer, which stood at about 4% in each group.
"In the absence of evidence that MRI is improving our surgical management or – as we showed here – long-term outcomes, the routine use of this test for DCIS should be questioned," Dr. Pilewskie commented in a related press briefing.
She outlined circumstances that may justify this additional testing. "I think that MRI can be a useful adjunct if there are discrepancies or still clinical questions when someone comes in, between the imaging that they have on their mammogram or ultrasound and their physical exam or their presentation," she said. "The majority of women who present with DCIS have a normal exam and calcifications or changes on a mammogram. So when there are differences, and someone has a palpable mass or nipple discharge or a different presentation that wasn’t answered by their imaging, I think MRI can help give additional information. But that again is not the routine woman who presents with DCIS."
With a median 59-month follow-up, the groups who did and did not receive MRI were statistically indistinguishable in terms of the actuarial rate of locoregional recurrence at 5 years (8.5% vs. 7.2%) and at 8 years (14.6% vs. 10.2%).
"When comparing the MRI and no-MRI groups, there were some differences between them in that the women who had an MRI had more high-risk features," Dr. Pilewskie noted, and those features might have influenced the decision to obtain this additional imaging and outcomes.
However, MRI was not a significant predictor of locoregional recurrence in a multivariate analysis that adjusted for these and other potential confounders: age, family history, mode of presentation, tamoxifen or other hormonal therapy, margin status, number of excisions, and year of surgery.
On the other hand, receipt of radiation therapy, receipt of endocrine therapy, and negative margins were all significantly associated with a lower risk of locoregional recurrence.
In a subset analysis, MRI also failed to reduce the risk of locoregional recurrence in the roughly one-third of patients who did not receive radiation therapy.
Similarly, patients who did and did not receive MRI were statistically indistinguishable in terms of the actuarial rate of contralateral breast cancer at 5 years (3.5% vs. 3.5%) and at 8 years (3.5% vs. 5.1%). Again, results were essentially the same in the subset who did not receive radiation therapy.
Dr. Steven O’Day, director of clinical research at the Beverly Hills Cancer Institute in California and moderator of the press briefing, commented, "There has been a tremendous increase in the use of MRI perioperatively and postoperatively in invasive as well as noninvasive breast cancer. And this [study] I think just grounds us to continue to – as new technologies, new imaging is used – be sure that we are actually" improving outcomes.
He concurred that the added sensitivity of MRI may be helpful in challenging cases. "But its routine use certainly in DCIS from this large retrospective study has not been shown as an independent predictor to improve locoregional or contralateral breast outcomes," he said. "So I think this is an important study" and leads us to want "to study MRI further prospectively both in DCIS and invasive cancer."
Giving some background to the research, Dr. Pilewskie noted that current guidelines do not address when MRI should be used in the work-up of patients with DCIS.
"However, about 30% of physicians currently obtain a perioperative MRI to look for areas of additional disease in patients with DCIS, and theoretically, treating this additional disease found by MRI could result in a lower risk of local recurrence or contralateral breast cancer down the road," she said. "And, again theoretically, this effect could be most pronounced in women treated with excision alone, meaning just having lumpectomy and no radiation therapy."
Overall, 26% of the patients studied had breast MRI near the time of surgery, most commonly preoperatively, to assess disease extent.
Dr. Pilewskie and Dr. O’Day disclosed no relevant conflicts of interest.
FROM THE ASCO BREAST CANCER SYMPOSIUM
Major finding: Women who received MRI in addition to conventional imaging did not have a lower 5-year rate of locoregional recurrence when compared with women who received conventional imaging alone (8.5% vs. 7.2%).
Data source: A retrospective cohort study of 2,321 patients who underwent lumpectomy for DCIS.
Disclosures: Dr. Pilewskie and Dr. O’Day disclosed no relevant conflicts of interest.
Most women misestimate their breast cancer risk
Most women do not have an accurate understanding of their breast cancer risk – a finding that has important implications for prevention and early detection, as well as psychological well-being, according to a survey of nearly 10,000 women undergoing mammography screening.
When asked to estimate their lifetime personal breast cancer risk, just 9.4% of the women gave a value that was within 10% of their actual calculated risk, according to data reported in a press briefing held in advance of the breast cancer symposium sponsored by the American Society of Clinical Oncology, where the study will be presented in full.
"Despite all the ongoing media attention, awareness campaigns, pink ribbons, breast cancer walks, and breast cancer month, most women lack accurate knowledge of their own breast cancer risk," maintained first author Dr. Jonathan D. Herman, an ob.gyn. at Hofstra University, New Hyde Park, N.Y. This tells us that "our education messaging is far off and we should change the way breast cancer awareness is presented."
"We began to think: What happens to women when they underestimate their risk of breast cancer? Well, they probably don’t get the necessary or most accurate treatment," he said. In particular, this group could benefit from a tailored plan of chemoprevention and early detection. On the other hand, "we think that women who overestimate their risk are worrying about getting breast cancer more than they really have to."
In the study, the investigators surveyed 9,873 women aged 35-70 years who were about to undergo screening at 21 Long Island mammography centers. The anonymous questionnaire included many questions adapted from the National Cancer Institute’s Breast Cancer Risk Assessment Tool, which is available online and typically used by physicians.
The women’s subjective estimate of risk was compared with their risk as calculated with the tool. Their estimate was considered inaccurate if it differed from their calculated risk by more than 10%.
Most of the women were at average calculated risk, with 35% having a 5%-10% lifetime risk and 40% having a 10%-15% lifetime risk.
Just 9.4% of the women, however, accurately estimated their risk, while 46% overestimated their risk and 45% underestimated their risk.
The predominant direction of estimation error varied by race/ethnicity. Of the white women, 10% accurately estimated their risk, 39% underestimated, and 51% overestimated their risk. Women of other ethnicities were more likely to underestimate their breast cancer risks. Just 9% of African American women were in line with their risk, with 58% underestimating and 34% overestimating. Asian women had similar assessments. Hispanic women’s inaccurate assessments were more balanced, with 50% underestimating and 41% overestimating risk. Although these differences were statistically significant, it is more important to note that the overall level of understanding was very low, Dr. Herman said.
Ideally, patients should learn of their breast cancer risk from their physician, he said, but the study data told another story. "All of these women were about to have mammography, so they obviously had some interest in their breast health," but when asked when they last spoke to their doctor about their personal breast cancer risk, "we were shocked to find that 40% of women said they never ever had a conversation with a health care provider," he reported.
The findings suggest a need to improve communication about risk by primary care providers, especially as the U.S. Preventive Services Task Force is now putting greater emphasis on informed decision making, Dr. Herman acknowledged.
But patients could be spurred to action as well, by moving beyond the pink ribbons and asking their physician, "What are my breast cancer numbers? I need to know that," he proposed.
Dr. Steven O’Day, director of clinical research at the Beverly Hills (Calif.) Cancer Institute and moderator of the press briefing, noted that the study has important implications for making use of guidelines for women at elevated risk. "To make decisions about chemoprevention, an accurate understanding of prognosis and risk both from the patient’s perspective and the physician’s is going to be essential to making good decisions in terms of surveillance and chemoprevention," he said.
A follow-up study planned by Dr. Herman will be important for informing efforts to improve risk communication, according to Dr. O’Day. "It’s a huge hurdle, yet how we are going to implement this [in primary care], as well as the tertiary-care oncology setting?" he commented. "If we don’t, the implications are huge; interventions by increased surveillance or chemoprevention are not trivial in terms of cost as well as potential side effects and morbidity. And these are difficult decisions even with accurate information. And without the information, you really can’t make any decision, in my mind."
Dr. Herman disclosed no relevant conflicts of interest. Dr. O’Day disclosed no relevant conflicts of interest.
Most women do not have an accurate understanding of their breast cancer risk – a finding that has important implications for prevention and early detection, as well as psychological well-being, according to a survey of nearly 10,000 women undergoing mammography screening.
When asked to estimate their lifetime personal breast cancer risk, just 9.4% of the women gave a value that was within 10% of their actual calculated risk, according to data reported in a press briefing held in advance of the breast cancer symposium sponsored by the American Society of Clinical Oncology, where the study will be presented in full.
"Despite all the ongoing media attention, awareness campaigns, pink ribbons, breast cancer walks, and breast cancer month, most women lack accurate knowledge of their own breast cancer risk," maintained first author Dr. Jonathan D. Herman, an ob.gyn. at Hofstra University, New Hyde Park, N.Y. This tells us that "our education messaging is far off and we should change the way breast cancer awareness is presented."
"We began to think: What happens to women when they underestimate their risk of breast cancer? Well, they probably don’t get the necessary or most accurate treatment," he said. In particular, this group could benefit from a tailored plan of chemoprevention and early detection. On the other hand, "we think that women who overestimate their risk are worrying about getting breast cancer more than they really have to."
In the study, the investigators surveyed 9,873 women aged 35-70 years who were about to undergo screening at 21 Long Island mammography centers. The anonymous questionnaire included many questions adapted from the National Cancer Institute’s Breast Cancer Risk Assessment Tool, which is available online and typically used by physicians.
The women’s subjective estimate of risk was compared with their risk as calculated with the tool. Their estimate was considered inaccurate if it differed from their calculated risk by more than 10%.
Most of the women were at average calculated risk, with 35% having a 5%-10% lifetime risk and 40% having a 10%-15% lifetime risk.
Just 9.4% of the women, however, accurately estimated their risk, while 46% overestimated their risk and 45% underestimated their risk.
The predominant direction of estimation error varied by race/ethnicity. Of the white women, 10% accurately estimated their risk, 39% underestimated, and 51% overestimated their risk. Women of other ethnicities were more likely to underestimate their breast cancer risks. Just 9% of African American women were in line with their risk, with 58% underestimating and 34% overestimating. Asian women had similar assessments. Hispanic women’s inaccurate assessments were more balanced, with 50% underestimating and 41% overestimating risk. Although these differences were statistically significant, it is more important to note that the overall level of understanding was very low, Dr. Herman said.
Ideally, patients should learn of their breast cancer risk from their physician, he said, but the study data told another story. "All of these women were about to have mammography, so they obviously had some interest in their breast health," but when asked when they last spoke to their doctor about their personal breast cancer risk, "we were shocked to find that 40% of women said they never ever had a conversation with a health care provider," he reported.
The findings suggest a need to improve communication about risk by primary care providers, especially as the U.S. Preventive Services Task Force is now putting greater emphasis on informed decision making, Dr. Herman acknowledged.
But patients could be spurred to action as well, by moving beyond the pink ribbons and asking their physician, "What are my breast cancer numbers? I need to know that," he proposed.
Dr. Steven O’Day, director of clinical research at the Beverly Hills (Calif.) Cancer Institute and moderator of the press briefing, noted that the study has important implications for making use of guidelines for women at elevated risk. "To make decisions about chemoprevention, an accurate understanding of prognosis and risk both from the patient’s perspective and the physician’s is going to be essential to making good decisions in terms of surveillance and chemoprevention," he said.
A follow-up study planned by Dr. Herman will be important for informing efforts to improve risk communication, according to Dr. O’Day. "It’s a huge hurdle, yet how we are going to implement this [in primary care], as well as the tertiary-care oncology setting?" he commented. "If we don’t, the implications are huge; interventions by increased surveillance or chemoprevention are not trivial in terms of cost as well as potential side effects and morbidity. And these are difficult decisions even with accurate information. And without the information, you really can’t make any decision, in my mind."
Dr. Herman disclosed no relevant conflicts of interest. Dr. O’Day disclosed no relevant conflicts of interest.
Most women do not have an accurate understanding of their breast cancer risk – a finding that has important implications for prevention and early detection, as well as psychological well-being, according to a survey of nearly 10,000 women undergoing mammography screening.
When asked to estimate their lifetime personal breast cancer risk, just 9.4% of the women gave a value that was within 10% of their actual calculated risk, according to data reported in a press briefing held in advance of the breast cancer symposium sponsored by the American Society of Clinical Oncology, where the study will be presented in full.
"Despite all the ongoing media attention, awareness campaigns, pink ribbons, breast cancer walks, and breast cancer month, most women lack accurate knowledge of their own breast cancer risk," maintained first author Dr. Jonathan D. Herman, an ob.gyn. at Hofstra University, New Hyde Park, N.Y. This tells us that "our education messaging is far off and we should change the way breast cancer awareness is presented."
"We began to think: What happens to women when they underestimate their risk of breast cancer? Well, they probably don’t get the necessary or most accurate treatment," he said. In particular, this group could benefit from a tailored plan of chemoprevention and early detection. On the other hand, "we think that women who overestimate their risk are worrying about getting breast cancer more than they really have to."
In the study, the investigators surveyed 9,873 women aged 35-70 years who were about to undergo screening at 21 Long Island mammography centers. The anonymous questionnaire included many questions adapted from the National Cancer Institute’s Breast Cancer Risk Assessment Tool, which is available online and typically used by physicians.
The women’s subjective estimate of risk was compared with their risk as calculated with the tool. Their estimate was considered inaccurate if it differed from their calculated risk by more than 10%.
Most of the women were at average calculated risk, with 35% having a 5%-10% lifetime risk and 40% having a 10%-15% lifetime risk.
Just 9.4% of the women, however, accurately estimated their risk, while 46% overestimated their risk and 45% underestimated their risk.
The predominant direction of estimation error varied by race/ethnicity. Of the white women, 10% accurately estimated their risk, 39% underestimated, and 51% overestimated their risk. Women of other ethnicities were more likely to underestimate their breast cancer risks. Just 9% of African American women were in line with their risk, with 58% underestimating and 34% overestimating. Asian women had similar assessments. Hispanic women’s inaccurate assessments were more balanced, with 50% underestimating and 41% overestimating risk. Although these differences were statistically significant, it is more important to note that the overall level of understanding was very low, Dr. Herman said.
Ideally, patients should learn of their breast cancer risk from their physician, he said, but the study data told another story. "All of these women were about to have mammography, so they obviously had some interest in their breast health," but when asked when they last spoke to their doctor about their personal breast cancer risk, "we were shocked to find that 40% of women said they never ever had a conversation with a health care provider," he reported.
The findings suggest a need to improve communication about risk by primary care providers, especially as the U.S. Preventive Services Task Force is now putting greater emphasis on informed decision making, Dr. Herman acknowledged.
But patients could be spurred to action as well, by moving beyond the pink ribbons and asking their physician, "What are my breast cancer numbers? I need to know that," he proposed.
Dr. Steven O’Day, director of clinical research at the Beverly Hills (Calif.) Cancer Institute and moderator of the press briefing, noted that the study has important implications for making use of guidelines for women at elevated risk. "To make decisions about chemoprevention, an accurate understanding of prognosis and risk both from the patient’s perspective and the physician’s is going to be essential to making good decisions in terms of surveillance and chemoprevention," he said.
A follow-up study planned by Dr. Herman will be important for informing efforts to improve risk communication, according to Dr. O’Day. "It’s a huge hurdle, yet how we are going to implement this [in primary care], as well as the tertiary-care oncology setting?" he commented. "If we don’t, the implications are huge; interventions by increased surveillance or chemoprevention are not trivial in terms of cost as well as potential side effects and morbidity. And these are difficult decisions even with accurate information. And without the information, you really can’t make any decision, in my mind."
Dr. Herman disclosed no relevant conflicts of interest. Dr. O’Day disclosed no relevant conflicts of interest.
Major Finding: Overall, 44.7% of women underestimated their risk and 45.9% overestimated their risk, while just 9.4% gave an accurate estimate.
Data Source: A survey of 9,873 women undergoing breast cancer screening at Long Island mammography centers.
Disclosures: Dr. Herman disclosed no relevant conflicts of interest. Dr. O’Day disclosed no relevant conflicts of interest.
No increase in cardiovascular risk seen after radiation therapy for DCIS
Women who receive radiation therapy as part of their treatment for very early breast cancer do not have an elevated risk of developing or dying from cardiovascular disease later in life, new data suggest.
In a population-based cohort study, researchers assessed cardiovascular outcomes among 10,468 women in the Netherlands given a diagnosis of ductal carcinoma in situ (DCIS) before the age of 75 years during 1989-2004.
Overall, 28% of the cohort received radiation therapy after their surgery (lumpectomy or mastectomy), lead investigator Naomi B. Boekel reported in a press briefing held in advance of the breast cancer symposium sponsored by the American Society of Clinical Oncology, where the findings will be presented in full.
During a median follow-up of 10 years, about 9% of the patients received a diagnosis of cardiovascular disease.
There was no significant difference in the rate between patients who did vs. did not receive radiation, or between patients who received left-sided radiation (in which the heart gets a low direct dose) vs. right-sided radiation (in which the heart gets only a scatter dose).
Specifically, the rate of cardiovascular disease diagnosis was 9% in patients who received surgery alone, compared with 8% in patients who received surgery plus radiation, a nonsignificant difference. The CVD rate was 7% in patients who received left-sided radiation therapy vs. 8% in their counterparts who received right-sided radiation therapy, another nonsignificant difference.
Furthermore, DCIS survivors as a whole had a 23% lower risk of dying from cardiovascular disease, compared with peers in the general Dutch population.
"This lower risk might be due to lifestyle adaption after DCIS diagnosis," proposed Ms. Boekel, a doctoral student at the Netherlands Cancer Institute in Amsterdam. "It could be due to conflicting risk factors between DCIS and cardiovascular disease, such as age at menopause. Or it could also be due to differences in health consciousness in that DCIS patients are probably more health conscious than the general population."
The risk of all-cause mortality was essentially the same between the DCIS cohort and the general population.
"We also looked at this for specific cardiovascular diseases and there were similar results, so there is no risk difference between these treatment groups," Ms. Boekel reported.
"Although these results are reassuring, longer follow-up is necessary before definitive conclusions can be drawn," she commented.
There is no established upper limit for such follow-up, she said. "But other studies have shown that the increased risk of cardiovascular disease starts after 5 to 10 years. ... We do have half of the cohort at more than 10 years, but [estimates] are less precise. So we need another 5 to 10 years before we are sure."
Strengths of the study, according to Dr. Steven O’Day, director of clinical research at the Beverly Hills (Calif.) Cancer Institute and moderator of the press briefing, included its occurrence in a time period in which more modern radiation therapy techniques were used. Also, the follow-up was fairly lengthy, although he agreed that more is needed.
"This is an important study that allows us to feel comfortable continuing our aggressive treatment of DCIS, with screening and trying to reduce overall mortality from breast cancer," Dr. O’Day maintained.
Ms. Boekel disclosed no relevant conflicts of interest. Dr. O’Day disclosed no relevant conflicts of interest.
Women who receive radiation therapy as part of their treatment for very early breast cancer do not have an elevated risk of developing or dying from cardiovascular disease later in life, new data suggest.
In a population-based cohort study, researchers assessed cardiovascular outcomes among 10,468 women in the Netherlands given a diagnosis of ductal carcinoma in situ (DCIS) before the age of 75 years during 1989-2004.
Overall, 28% of the cohort received radiation therapy after their surgery (lumpectomy or mastectomy), lead investigator Naomi B. Boekel reported in a press briefing held in advance of the breast cancer symposium sponsored by the American Society of Clinical Oncology, where the findings will be presented in full.
During a median follow-up of 10 years, about 9% of the patients received a diagnosis of cardiovascular disease.
There was no significant difference in the rate between patients who did vs. did not receive radiation, or between patients who received left-sided radiation (in which the heart gets a low direct dose) vs. right-sided radiation (in which the heart gets only a scatter dose).
Specifically, the rate of cardiovascular disease diagnosis was 9% in patients who received surgery alone, compared with 8% in patients who received surgery plus radiation, a nonsignificant difference. The CVD rate was 7% in patients who received left-sided radiation therapy vs. 8% in their counterparts who received right-sided radiation therapy, another nonsignificant difference.
Furthermore, DCIS survivors as a whole had a 23% lower risk of dying from cardiovascular disease, compared with peers in the general Dutch population.
"This lower risk might be due to lifestyle adaption after DCIS diagnosis," proposed Ms. Boekel, a doctoral student at the Netherlands Cancer Institute in Amsterdam. "It could be due to conflicting risk factors between DCIS and cardiovascular disease, such as age at menopause. Or it could also be due to differences in health consciousness in that DCIS patients are probably more health conscious than the general population."
The risk of all-cause mortality was essentially the same between the DCIS cohort and the general population.
"We also looked at this for specific cardiovascular diseases and there were similar results, so there is no risk difference between these treatment groups," Ms. Boekel reported.
"Although these results are reassuring, longer follow-up is necessary before definitive conclusions can be drawn," she commented.
There is no established upper limit for such follow-up, she said. "But other studies have shown that the increased risk of cardiovascular disease starts after 5 to 10 years. ... We do have half of the cohort at more than 10 years, but [estimates] are less precise. So we need another 5 to 10 years before we are sure."
Strengths of the study, according to Dr. Steven O’Day, director of clinical research at the Beverly Hills (Calif.) Cancer Institute and moderator of the press briefing, included its occurrence in a time period in which more modern radiation therapy techniques were used. Also, the follow-up was fairly lengthy, although he agreed that more is needed.
"This is an important study that allows us to feel comfortable continuing our aggressive treatment of DCIS, with screening and trying to reduce overall mortality from breast cancer," Dr. O’Day maintained.
Ms. Boekel disclosed no relevant conflicts of interest. Dr. O’Day disclosed no relevant conflicts of interest.
Women who receive radiation therapy as part of their treatment for very early breast cancer do not have an elevated risk of developing or dying from cardiovascular disease later in life, new data suggest.
In a population-based cohort study, researchers assessed cardiovascular outcomes among 10,468 women in the Netherlands given a diagnosis of ductal carcinoma in situ (DCIS) before the age of 75 years during 1989-2004.
Overall, 28% of the cohort received radiation therapy after their surgery (lumpectomy or mastectomy), lead investigator Naomi B. Boekel reported in a press briefing held in advance of the breast cancer symposium sponsored by the American Society of Clinical Oncology, where the findings will be presented in full.
During a median follow-up of 10 years, about 9% of the patients received a diagnosis of cardiovascular disease.
There was no significant difference in the rate between patients who did vs. did not receive radiation, or between patients who received left-sided radiation (in which the heart gets a low direct dose) vs. right-sided radiation (in which the heart gets only a scatter dose).
Specifically, the rate of cardiovascular disease diagnosis was 9% in patients who received surgery alone, compared with 8% in patients who received surgery plus radiation, a nonsignificant difference. The CVD rate was 7% in patients who received left-sided radiation therapy vs. 8% in their counterparts who received right-sided radiation therapy, another nonsignificant difference.
Furthermore, DCIS survivors as a whole had a 23% lower risk of dying from cardiovascular disease, compared with peers in the general Dutch population.
"This lower risk might be due to lifestyle adaption after DCIS diagnosis," proposed Ms. Boekel, a doctoral student at the Netherlands Cancer Institute in Amsterdam. "It could be due to conflicting risk factors between DCIS and cardiovascular disease, such as age at menopause. Or it could also be due to differences in health consciousness in that DCIS patients are probably more health conscious than the general population."
The risk of all-cause mortality was essentially the same between the DCIS cohort and the general population.
"We also looked at this for specific cardiovascular diseases and there were similar results, so there is no risk difference between these treatment groups," Ms. Boekel reported.
"Although these results are reassuring, longer follow-up is necessary before definitive conclusions can be drawn," she commented.
There is no established upper limit for such follow-up, she said. "But other studies have shown that the increased risk of cardiovascular disease starts after 5 to 10 years. ... We do have half of the cohort at more than 10 years, but [estimates] are less precise. So we need another 5 to 10 years before we are sure."
Strengths of the study, according to Dr. Steven O’Day, director of clinical research at the Beverly Hills (Calif.) Cancer Institute and moderator of the press briefing, included its occurrence in a time period in which more modern radiation therapy techniques were used. Also, the follow-up was fairly lengthy, although he agreed that more is needed.
"This is an important study that allows us to feel comfortable continuing our aggressive treatment of DCIS, with screening and trying to reduce overall mortality from breast cancer," Dr. O’Day maintained.
Ms. Boekel disclosed no relevant conflicts of interest. Dr. O’Day disclosed no relevant conflicts of interest.
FROM THE ASCO BREAST CANCER SYMPOSIUM
Major finding: The rate of CVD diagnosis did not differ between patients treated with surgery alone and patients treated with surgery plus radiation therapy (9% vs. 8%).
Data source: A population-based cohort study of 10,468 women in the Netherlands treated for DCIS
Disclosures: Ms. Boekel disclosed no relevant conflicts of interest. Dr. O’Day disclosed no relevant conflicts of interest.