User login
American Society of Clinical Oncology (ASCO): Breast Cancer Symposium
Axillary treatment unnecessary after some total mastectomies
SAN FRANCISCO – Regional recurrence and recurrence-free survival rates were statistically similar in subgroups of 730 patients who had sentinel lymph node–positive, invasive breast cancer and underwent total mastectomy.
The outcomes were comparable regardless of whether or not patients had a subsequent completion axillary lymph node dissection and radiation therapy. A marginally higher 10-year regional recurrence rate of 4.9% in patients who did not have completion lymphadenectomy was not significantly different from rates seen in patients who had completion lymphadenectomy but not radiation therapy (1.4% of whom had a regional recurrence) or in patients who had completion lymphadenectomy plus radiation therapy (3.1% had a regional recurrence), in the retrospective institutional study.
Recurrence-free survival rates also did not differ between groups, reported Dr. Elizabeth FitzSullivan and her associates.
Using the M.D. Anderson Cancer Center nomogram to predict whether lymph nodes other than the sentinel node will have cancer, the investigators predicted a significantly lower probability of additional lymph node positivity in the 98 patients who did not undergo completion lymphadenectomy (a median 10% probability), compared with the 632 patients who had a completion lymphadenectomy (23% probability), reported Dr. FitzSullivan of the University of Texas M.D. Anderson Cancer Center, Houston.
"In select patients with early-stage breast cancer treated with mastectomy with a positive sentinel lymph node biopsy, completion lymphadenectomy may be avoided without adversely affecting recurrence or recurrence-free survival," the investigators concluded in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology. The study won a Merit Award at the meeting.
The retrospective analysis of data from patients treated at the M.D. Anderson Cancer Center during 1994-2010 defined completion lymphadenectomy as removal of 10 or more lymph nodes. Median follow-up was 66 months.
In general, patients with early-stage breast cancer who are treated with conserving therapy and who have minimal axillary disease on sentinel lymph node biopsy often don’t undergo completion lymphadenectomy. The increasing rate of total mastectomies heightens the need to identify patients who undergo total mastectomy who may not benefit from completion lymphadenectomy and axillary radiation therapy, the researchers noted.
There were several baseline characteristics that differed significantly between patients who did or did not undergo completion lymphadenectomy in the study. Compared with patients who did have completion lymphadenectomy, those who did not undergo the additional axillary surgery were older (57 years vs. 53 years, respectively); had smaller tumors (a median of 2 cm vs. 2.3 cm); and were less likely to have stage T3 disease (8% vs. 17%), lymphovascular invasion (25% vs. 41%), or extranodal extension of disease (4% vs. 24%). The median size of sentinel node metastasis was smaller in patients who did not have completion lymphadenectomy (1.1 mm) compared with those who did (4 mm).
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. FitzSullivan reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Regional recurrence and recurrence-free survival rates were statistically similar in subgroups of 730 patients who had sentinel lymph node–positive, invasive breast cancer and underwent total mastectomy.
The outcomes were comparable regardless of whether or not patients had a subsequent completion axillary lymph node dissection and radiation therapy. A marginally higher 10-year regional recurrence rate of 4.9% in patients who did not have completion lymphadenectomy was not significantly different from rates seen in patients who had completion lymphadenectomy but not radiation therapy (1.4% of whom had a regional recurrence) or in patients who had completion lymphadenectomy plus radiation therapy (3.1% had a regional recurrence), in the retrospective institutional study.
Recurrence-free survival rates also did not differ between groups, reported Dr. Elizabeth FitzSullivan and her associates.
Using the M.D. Anderson Cancer Center nomogram to predict whether lymph nodes other than the sentinel node will have cancer, the investigators predicted a significantly lower probability of additional lymph node positivity in the 98 patients who did not undergo completion lymphadenectomy (a median 10% probability), compared with the 632 patients who had a completion lymphadenectomy (23% probability), reported Dr. FitzSullivan of the University of Texas M.D. Anderson Cancer Center, Houston.
"In select patients with early-stage breast cancer treated with mastectomy with a positive sentinel lymph node biopsy, completion lymphadenectomy may be avoided without adversely affecting recurrence or recurrence-free survival," the investigators concluded in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology. The study won a Merit Award at the meeting.
The retrospective analysis of data from patients treated at the M.D. Anderson Cancer Center during 1994-2010 defined completion lymphadenectomy as removal of 10 or more lymph nodes. Median follow-up was 66 months.
In general, patients with early-stage breast cancer who are treated with conserving therapy and who have minimal axillary disease on sentinel lymph node biopsy often don’t undergo completion lymphadenectomy. The increasing rate of total mastectomies heightens the need to identify patients who undergo total mastectomy who may not benefit from completion lymphadenectomy and axillary radiation therapy, the researchers noted.
There were several baseline characteristics that differed significantly between patients who did or did not undergo completion lymphadenectomy in the study. Compared with patients who did have completion lymphadenectomy, those who did not undergo the additional axillary surgery were older (57 years vs. 53 years, respectively); had smaller tumors (a median of 2 cm vs. 2.3 cm); and were less likely to have stage T3 disease (8% vs. 17%), lymphovascular invasion (25% vs. 41%), or extranodal extension of disease (4% vs. 24%). The median size of sentinel node metastasis was smaller in patients who did not have completion lymphadenectomy (1.1 mm) compared with those who did (4 mm).
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. FitzSullivan reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Regional recurrence and recurrence-free survival rates were statistically similar in subgroups of 730 patients who had sentinel lymph node–positive, invasive breast cancer and underwent total mastectomy.
The outcomes were comparable regardless of whether or not patients had a subsequent completion axillary lymph node dissection and radiation therapy. A marginally higher 10-year regional recurrence rate of 4.9% in patients who did not have completion lymphadenectomy was not significantly different from rates seen in patients who had completion lymphadenectomy but not radiation therapy (1.4% of whom had a regional recurrence) or in patients who had completion lymphadenectomy plus radiation therapy (3.1% had a regional recurrence), in the retrospective institutional study.
Recurrence-free survival rates also did not differ between groups, reported Dr. Elizabeth FitzSullivan and her associates.
Using the M.D. Anderson Cancer Center nomogram to predict whether lymph nodes other than the sentinel node will have cancer, the investigators predicted a significantly lower probability of additional lymph node positivity in the 98 patients who did not undergo completion lymphadenectomy (a median 10% probability), compared with the 632 patients who had a completion lymphadenectomy (23% probability), reported Dr. FitzSullivan of the University of Texas M.D. Anderson Cancer Center, Houston.
"In select patients with early-stage breast cancer treated with mastectomy with a positive sentinel lymph node biopsy, completion lymphadenectomy may be avoided without adversely affecting recurrence or recurrence-free survival," the investigators concluded in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology. The study won a Merit Award at the meeting.
The retrospective analysis of data from patients treated at the M.D. Anderson Cancer Center during 1994-2010 defined completion lymphadenectomy as removal of 10 or more lymph nodes. Median follow-up was 66 months.
In general, patients with early-stage breast cancer who are treated with conserving therapy and who have minimal axillary disease on sentinel lymph node biopsy often don’t undergo completion lymphadenectomy. The increasing rate of total mastectomies heightens the need to identify patients who undergo total mastectomy who may not benefit from completion lymphadenectomy and axillary radiation therapy, the researchers noted.
There were several baseline characteristics that differed significantly between patients who did or did not undergo completion lymphadenectomy in the study. Compared with patients who did have completion lymphadenectomy, those who did not undergo the additional axillary surgery were older (57 years vs. 53 years, respectively); had smaller tumors (a median of 2 cm vs. 2.3 cm); and were less likely to have stage T3 disease (8% vs. 17%), lymphovascular invasion (25% vs. 41%), or extranodal extension of disease (4% vs. 24%). The median size of sentinel node metastasis was smaller in patients who did not have completion lymphadenectomy (1.1 mm) compared with those who did (4 mm).
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. FitzSullivan reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: Median 10-year regional recurrence rates were 4.9% in patients who did not undergo completion lymphadenectomy, 3.1% for patients who had completion lymphadenectomy plus radiation therapy, and 1.4% for patients with completion lymphadenectomy but not radiation therapy.
Data source: A retrospective study of 730 patients with invasive breast cancer and a positive sentinel lymph node biopsy, treated with total mastectomy, between 1994 and 2010 at the University of Texas M.D. Anderson Cancer Center, Houston.
Disclosures: Dr. FitzSullivan reported having no financial disclosures.
No greater risk of lymph node involvement in triple-negative breast cancer
SAN FRANCISCO – Patients with triple-negative breast cancer had no higher risk for metastases in the lymph nodes as compared with patients whose breast cancer was not triple-negative, a review of 2,957 cases found.
The study included patients with invasive breast cancer treated surgically between January 2000 and May 2012. Immunohistochemical identification of markers showed that 2,201 (74%) had luminal A subtype breast cancer (estrogen receptor– and progesterone receptor–positive and HER2 negative), 344 (12%) had luminal B subtype (all three markers positive), 144 (5%) were HER2 positive but estrogen and progesterone receptors–negative, and 278 (9%) were negative for all three markers (the triple-negative group). The study excluded men, patients treated with neoadjuvant therapy, patients with distant metastases, and those who did not undergo nodal sampling.
At least one positive lymph node was found in 35% of patients, and four or more positive nodes were found in 10% of patients.
Patients in the triple-negative group were significantly younger at diagnosis and significantly more likely to have higher-grade tumors, compared with patients with other subtypes of breast cancer. Grade 3 cancer was seen in 87% of the triple-negative group and in 27% of the luminal A group, 51% of the luminal B group, and 80% of the HER2-positive group, Dr. Alexandra Gangi and her associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In multivariant analyses, younger age, higher tumor grade, larger tumor size, and lymphovascular invasion predicted increased likelihood of lymph node metastases. The triple-negative phenotype predicted neither a higher risk for lymph node positivity nor a greater likelihood of having four or more positive lymph nodes, which was found in 9% of the triple-negative group, 9% of the luminal A group, 14% of the luminal B group, and 19% of the HER2-positive group, reported Dr. Gangi of Cedars-Sinai Hospital, Los Angeles.
The likelihood of lymph node metastases was 50% higher in patients younger than 50 years as compared with older patients, 70% higher with grade 2 cancer as compared with grade 1 cancer, and 90% higher with grade 3 cancer as compared with grade 1 cancer. Lymphovascular invasion increased the likelihood of lymph node involvement four-fold. The risk of lymph node metastases was three times higher in patients with stage T2 breast cancer and 11 times higher in patients with stage T3 disease as compared with those with stage T1 breast cancer. All of these differences were statistically significant.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Gangi reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Patients with triple-negative breast cancer had no higher risk for metastases in the lymph nodes as compared with patients whose breast cancer was not triple-negative, a review of 2,957 cases found.
The study included patients with invasive breast cancer treated surgically between January 2000 and May 2012. Immunohistochemical identification of markers showed that 2,201 (74%) had luminal A subtype breast cancer (estrogen receptor– and progesterone receptor–positive and HER2 negative), 344 (12%) had luminal B subtype (all three markers positive), 144 (5%) were HER2 positive but estrogen and progesterone receptors–negative, and 278 (9%) were negative for all three markers (the triple-negative group). The study excluded men, patients treated with neoadjuvant therapy, patients with distant metastases, and those who did not undergo nodal sampling.
At least one positive lymph node was found in 35% of patients, and four or more positive nodes were found in 10% of patients.
Patients in the triple-negative group were significantly younger at diagnosis and significantly more likely to have higher-grade tumors, compared with patients with other subtypes of breast cancer. Grade 3 cancer was seen in 87% of the triple-negative group and in 27% of the luminal A group, 51% of the luminal B group, and 80% of the HER2-positive group, Dr. Alexandra Gangi and her associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In multivariant analyses, younger age, higher tumor grade, larger tumor size, and lymphovascular invasion predicted increased likelihood of lymph node metastases. The triple-negative phenotype predicted neither a higher risk for lymph node positivity nor a greater likelihood of having four or more positive lymph nodes, which was found in 9% of the triple-negative group, 9% of the luminal A group, 14% of the luminal B group, and 19% of the HER2-positive group, reported Dr. Gangi of Cedars-Sinai Hospital, Los Angeles.
The likelihood of lymph node metastases was 50% higher in patients younger than 50 years as compared with older patients, 70% higher with grade 2 cancer as compared with grade 1 cancer, and 90% higher with grade 3 cancer as compared with grade 1 cancer. Lymphovascular invasion increased the likelihood of lymph node involvement four-fold. The risk of lymph node metastases was three times higher in patients with stage T2 breast cancer and 11 times higher in patients with stage T3 disease as compared with those with stage T1 breast cancer. All of these differences were statistically significant.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Gangi reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Patients with triple-negative breast cancer had no higher risk for metastases in the lymph nodes as compared with patients whose breast cancer was not triple-negative, a review of 2,957 cases found.
The study included patients with invasive breast cancer treated surgically between January 2000 and May 2012. Immunohistochemical identification of markers showed that 2,201 (74%) had luminal A subtype breast cancer (estrogen receptor– and progesterone receptor–positive and HER2 negative), 344 (12%) had luminal B subtype (all three markers positive), 144 (5%) were HER2 positive but estrogen and progesterone receptors–negative, and 278 (9%) were negative for all three markers (the triple-negative group). The study excluded men, patients treated with neoadjuvant therapy, patients with distant metastases, and those who did not undergo nodal sampling.
At least one positive lymph node was found in 35% of patients, and four or more positive nodes were found in 10% of patients.
Patients in the triple-negative group were significantly younger at diagnosis and significantly more likely to have higher-grade tumors, compared with patients with other subtypes of breast cancer. Grade 3 cancer was seen in 87% of the triple-negative group and in 27% of the luminal A group, 51% of the luminal B group, and 80% of the HER2-positive group, Dr. Alexandra Gangi and her associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In multivariant analyses, younger age, higher tumor grade, larger tumor size, and lymphovascular invasion predicted increased likelihood of lymph node metastases. The triple-negative phenotype predicted neither a higher risk for lymph node positivity nor a greater likelihood of having four or more positive lymph nodes, which was found in 9% of the triple-negative group, 9% of the luminal A group, 14% of the luminal B group, and 19% of the HER2-positive group, reported Dr. Gangi of Cedars-Sinai Hospital, Los Angeles.
The likelihood of lymph node metastases was 50% higher in patients younger than 50 years as compared with older patients, 70% higher with grade 2 cancer as compared with grade 1 cancer, and 90% higher with grade 3 cancer as compared with grade 1 cancer. Lymphovascular invasion increased the likelihood of lymph node involvement four-fold. The risk of lymph node metastases was three times higher in patients with stage T2 breast cancer and 11 times higher in patients with stage T3 disease as compared with those with stage T1 breast cancer. All of these differences were statistically significant.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Gangi reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: The likelihood of lymph node metastases was 50% higher in patients younger than 50 years as compared with older patients, 70% higher with grade 2 cancer as compared with grade 1 cancer, and 90% higher with grade 3 cancer as compared with grade 1 cancer.
Data source: A prospect review of 2,957 women treated surgically for breast cancer between January 2000 and May 2012.
Disclosures: Dr. Gangi reported having no financial disclosures.
Women choose mastectomy to gain control
SAN FRANCISCO – Fear and a desire for control over breast cancer may drive women to choose mastectomy over less aggressive management, a qualitative study of 30 patients has shown.
She interviewed 15 women who were candidates for breast-conserving surgery but chose unilateral mastectomy and 15 average-risk women who were candidates for surgery in one breast but also chose prophylactic contralateral mastectomy.
Fear led patients to overestimate their risk of local recurrence and contralateral cancer and to misunderstand their odds of dying of breast cancer. The fear combined with wanting to eliminate and control the risk of cancer resulted in the patient choosing mastectomy or bilateral mastectomy, factors that probably are contributing to increasing rates of mastectomy for early-stage breast cancer, Dr. Andrea M. Covelli reported in a poster at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
When deciding on treatment, the women sought out multiple sources of information but gave greatest weight to the experiences of people they’d known with breast cancer and information from breast cancer survivors. It was the patients, not clinicians, who raised the topic of contralateral prophylactic mastectomy, which some patients chose in an attempt to eliminate any risk of contralateral breast cancer.
"More surgery is seen as more control," reported Dr. Covelli of the University of Toronto. A better understanding of patients’ decision process may help physicians be better able to discuss issues important to their patients in making treatment decisions.
Dr. Covelli conducted semistructured one-on-one interviews with patients with early-stage breast cancer chosen from five hospitals in the greater Toronto area to represent a variety of ages and ethnicities. Twelve were treated at academic cancer centers, 6 were treated at academic noncancer centers, and 12 were treated at community medical centers.
She identified several themes in the results. The diagnosis brought shock and fear. Patients discussed both breast-conserving surgery and unilateral mastectomy during their surgical consultation, during which the physician discouraged contralateral prophylactic mastectomy. Patients relied on multiple sources of information in their decision making, but the greatest impact came from the experiences of others with breast cancer, she reported.
Women who chose unilateral mastectomy did so most often out of fear of recurrence and the misguided notion that it would give them a survival advantage. Occasionally, they chose unilateral mastectomy to avoid radiation therapy.
Women who also chose contralateral prophylactic mastectomy initiated discussions about it, which their surgeons then discouraged. These patients chose it anyway because they overestimated the risk of contralateral cancer and mistakenly believed it would improve their chance of survival. Occasionally they added contralateral prophylactic mastectomy for body symmetry.
In essence, patients chose these more aggressive surgeries because they were actively trying to control their cancer outcomes and ensure that they "never have to go through this again," Dr. Covelli said.
The study cohort had a mean age of 55 years, with ages ranging from 36 to 84 years.
Dr. Covelli has received funding from Roche Canada and the Canadian Breast Cancer Foundation Physician Fellowship Award.
On Twitter @sherryboschert
This study asks the question, What motivates a woman with early-stage breast cancer to choose mastectomy, whether that be a unilateral mastectomy when she’s a candidate for breast conservation or the addition of a contralateral prophylactic mastectomy in the absence of an indication?

|
|
The bottom line is that the diagnosis is met with fear and shock. When we present options in a very neutral, evidence-based way, we are talking to our patients about breast-conserving surgery and mastectomy. And we are, and we should be, talking about whether there’s a role for contralateral prophylactic surgery.
Bearing witness is something that needs to be understood here. A lot of the discussion that you have with your patients is going to be interpreted based on what those patients are hearing, seeing, and witnessing in their own lives. So, if Aunt Judy had breast-conserving surgery and died 5 years later, you’ve got to take that into account.
There is a desire for control. There is a need to reduce risks so it never happens to them again, and there is a desire to increase their odds of surviving. That comes down to patients’ decision-making experience, their reasons for choosing a surgery that is far more aggressive than it possibly should be, and their goal to control cancer. We’re seeing an increasing mastectomy rate – more surgery, more control.
There is a process of deliberation where we take many factors into consideration before determining what we want to do. The implication based on this abstract, I think, is whether clinicians need to identify the deliberations that are leading to a decision before acting surgically.
Dr. Covelli’s preliminary work suggests that decisions around surgery are incredibly complex, and ensuring decisions are informed is complicated. Learning how patients deliberate is important – what experiences and what social networks are informing that decision? (And I don’t mean Twitter and Facebook, I mean in patients’ own real, face-to-face lives.) What is the role of the preoperative work-up in influencing those deliberations? How is that preoperative MRI influencing these decisions, based on anything that potentially might be happening?
I think this is very provocative work that can go further. The take-home points for me: Engage cognitively, engage with evidence, engage with data, but you have to engage practically. You need to know: Where is that person hearing information that I’m not telling her? How is she processing that information with information that I have just given? Engage cognitively; engage affectively.
Dr. Don S. Dizon is a medical gynecologic oncologist and director of the oncology sexual health clinic at Massachusetts General Hospital, Boston. These are excerpts of his remarks as the discussant of Dr. Covelli’s study at the meeting. He disclosed that he has been employed by UpToDate.
This study asks the question, What motivates a woman with early-stage breast cancer to choose mastectomy, whether that be a unilateral mastectomy when she’s a candidate for breast conservation or the addition of a contralateral prophylactic mastectomy in the absence of an indication?

|
|
The bottom line is that the diagnosis is met with fear and shock. When we present options in a very neutral, evidence-based way, we are talking to our patients about breast-conserving surgery and mastectomy. And we are, and we should be, talking about whether there’s a role for contralateral prophylactic surgery.
Bearing witness is something that needs to be understood here. A lot of the discussion that you have with your patients is going to be interpreted based on what those patients are hearing, seeing, and witnessing in their own lives. So, if Aunt Judy had breast-conserving surgery and died 5 years later, you’ve got to take that into account.
There is a desire for control. There is a need to reduce risks so it never happens to them again, and there is a desire to increase their odds of surviving. That comes down to patients’ decision-making experience, their reasons for choosing a surgery that is far more aggressive than it possibly should be, and their goal to control cancer. We’re seeing an increasing mastectomy rate – more surgery, more control.
There is a process of deliberation where we take many factors into consideration before determining what we want to do. The implication based on this abstract, I think, is whether clinicians need to identify the deliberations that are leading to a decision before acting surgically.
Dr. Covelli’s preliminary work suggests that decisions around surgery are incredibly complex, and ensuring decisions are informed is complicated. Learning how patients deliberate is important – what experiences and what social networks are informing that decision? (And I don’t mean Twitter and Facebook, I mean in patients’ own real, face-to-face lives.) What is the role of the preoperative work-up in influencing those deliberations? How is that preoperative MRI influencing these decisions, based on anything that potentially might be happening?
I think this is very provocative work that can go further. The take-home points for me: Engage cognitively, engage with evidence, engage with data, but you have to engage practically. You need to know: Where is that person hearing information that I’m not telling her? How is she processing that information with information that I have just given? Engage cognitively; engage affectively.
Dr. Don S. Dizon is a medical gynecologic oncologist and director of the oncology sexual health clinic at Massachusetts General Hospital, Boston. These are excerpts of his remarks as the discussant of Dr. Covelli’s study at the meeting. He disclosed that he has been employed by UpToDate.
This study asks the question, What motivates a woman with early-stage breast cancer to choose mastectomy, whether that be a unilateral mastectomy when she’s a candidate for breast conservation or the addition of a contralateral prophylactic mastectomy in the absence of an indication?

|
|
The bottom line is that the diagnosis is met with fear and shock. When we present options in a very neutral, evidence-based way, we are talking to our patients about breast-conserving surgery and mastectomy. And we are, and we should be, talking about whether there’s a role for contralateral prophylactic surgery.
Bearing witness is something that needs to be understood here. A lot of the discussion that you have with your patients is going to be interpreted based on what those patients are hearing, seeing, and witnessing in their own lives. So, if Aunt Judy had breast-conserving surgery and died 5 years later, you’ve got to take that into account.
There is a desire for control. There is a need to reduce risks so it never happens to them again, and there is a desire to increase their odds of surviving. That comes down to patients’ decision-making experience, their reasons for choosing a surgery that is far more aggressive than it possibly should be, and their goal to control cancer. We’re seeing an increasing mastectomy rate – more surgery, more control.
There is a process of deliberation where we take many factors into consideration before determining what we want to do. The implication based on this abstract, I think, is whether clinicians need to identify the deliberations that are leading to a decision before acting surgically.
Dr. Covelli’s preliminary work suggests that decisions around surgery are incredibly complex, and ensuring decisions are informed is complicated. Learning how patients deliberate is important – what experiences and what social networks are informing that decision? (And I don’t mean Twitter and Facebook, I mean in patients’ own real, face-to-face lives.) What is the role of the preoperative work-up in influencing those deliberations? How is that preoperative MRI influencing these decisions, based on anything that potentially might be happening?
I think this is very provocative work that can go further. The take-home points for me: Engage cognitively, engage with evidence, engage with data, but you have to engage practically. You need to know: Where is that person hearing information that I’m not telling her? How is she processing that information with information that I have just given? Engage cognitively; engage affectively.
Dr. Don S. Dizon is a medical gynecologic oncologist and director of the oncology sexual health clinic at Massachusetts General Hospital, Boston. These are excerpts of his remarks as the discussant of Dr. Covelli’s study at the meeting. He disclosed that he has been employed by UpToDate.
SAN FRANCISCO – Fear and a desire for control over breast cancer may drive women to choose mastectomy over less aggressive management, a qualitative study of 30 patients has shown.
She interviewed 15 women who were candidates for breast-conserving surgery but chose unilateral mastectomy and 15 average-risk women who were candidates for surgery in one breast but also chose prophylactic contralateral mastectomy.
Fear led patients to overestimate their risk of local recurrence and contralateral cancer and to misunderstand their odds of dying of breast cancer. The fear combined with wanting to eliminate and control the risk of cancer resulted in the patient choosing mastectomy or bilateral mastectomy, factors that probably are contributing to increasing rates of mastectomy for early-stage breast cancer, Dr. Andrea M. Covelli reported in a poster at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
When deciding on treatment, the women sought out multiple sources of information but gave greatest weight to the experiences of people they’d known with breast cancer and information from breast cancer survivors. It was the patients, not clinicians, who raised the topic of contralateral prophylactic mastectomy, which some patients chose in an attempt to eliminate any risk of contralateral breast cancer.
"More surgery is seen as more control," reported Dr. Covelli of the University of Toronto. A better understanding of patients’ decision process may help physicians be better able to discuss issues important to their patients in making treatment decisions.
Dr. Covelli conducted semistructured one-on-one interviews with patients with early-stage breast cancer chosen from five hospitals in the greater Toronto area to represent a variety of ages and ethnicities. Twelve were treated at academic cancer centers, 6 were treated at academic noncancer centers, and 12 were treated at community medical centers.
She identified several themes in the results. The diagnosis brought shock and fear. Patients discussed both breast-conserving surgery and unilateral mastectomy during their surgical consultation, during which the physician discouraged contralateral prophylactic mastectomy. Patients relied on multiple sources of information in their decision making, but the greatest impact came from the experiences of others with breast cancer, she reported.
Women who chose unilateral mastectomy did so most often out of fear of recurrence and the misguided notion that it would give them a survival advantage. Occasionally, they chose unilateral mastectomy to avoid radiation therapy.
Women who also chose contralateral prophylactic mastectomy initiated discussions about it, which their surgeons then discouraged. These patients chose it anyway because they overestimated the risk of contralateral cancer and mistakenly believed it would improve their chance of survival. Occasionally they added contralateral prophylactic mastectomy for body symmetry.
In essence, patients chose these more aggressive surgeries because they were actively trying to control their cancer outcomes and ensure that they "never have to go through this again," Dr. Covelli said.
The study cohort had a mean age of 55 years, with ages ranging from 36 to 84 years.
Dr. Covelli has received funding from Roche Canada and the Canadian Breast Cancer Foundation Physician Fellowship Award.
On Twitter @sherryboschert
SAN FRANCISCO – Fear and a desire for control over breast cancer may drive women to choose mastectomy over less aggressive management, a qualitative study of 30 patients has shown.
She interviewed 15 women who were candidates for breast-conserving surgery but chose unilateral mastectomy and 15 average-risk women who were candidates for surgery in one breast but also chose prophylactic contralateral mastectomy.
Fear led patients to overestimate their risk of local recurrence and contralateral cancer and to misunderstand their odds of dying of breast cancer. The fear combined with wanting to eliminate and control the risk of cancer resulted in the patient choosing mastectomy or bilateral mastectomy, factors that probably are contributing to increasing rates of mastectomy for early-stage breast cancer, Dr. Andrea M. Covelli reported in a poster at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
When deciding on treatment, the women sought out multiple sources of information but gave greatest weight to the experiences of people they’d known with breast cancer and information from breast cancer survivors. It was the patients, not clinicians, who raised the topic of contralateral prophylactic mastectomy, which some patients chose in an attempt to eliminate any risk of contralateral breast cancer.
"More surgery is seen as more control," reported Dr. Covelli of the University of Toronto. A better understanding of patients’ decision process may help physicians be better able to discuss issues important to their patients in making treatment decisions.
Dr. Covelli conducted semistructured one-on-one interviews with patients with early-stage breast cancer chosen from five hospitals in the greater Toronto area to represent a variety of ages and ethnicities. Twelve were treated at academic cancer centers, 6 were treated at academic noncancer centers, and 12 were treated at community medical centers.
She identified several themes in the results. The diagnosis brought shock and fear. Patients discussed both breast-conserving surgery and unilateral mastectomy during their surgical consultation, during which the physician discouraged contralateral prophylactic mastectomy. Patients relied on multiple sources of information in their decision making, but the greatest impact came from the experiences of others with breast cancer, she reported.
Women who chose unilateral mastectomy did so most often out of fear of recurrence and the misguided notion that it would give them a survival advantage. Occasionally, they chose unilateral mastectomy to avoid radiation therapy.
Women who also chose contralateral prophylactic mastectomy initiated discussions about it, which their surgeons then discouraged. These patients chose it anyway because they overestimated the risk of contralateral cancer and mistakenly believed it would improve their chance of survival. Occasionally they added contralateral prophylactic mastectomy for body symmetry.
In essence, patients chose these more aggressive surgeries because they were actively trying to control their cancer outcomes and ensure that they "never have to go through this again," Dr. Covelli said.
The study cohort had a mean age of 55 years, with ages ranging from 36 to 84 years.
Dr. Covelli has received funding from Roche Canada and the Canadian Breast Cancer Foundation Physician Fellowship Award.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: Fear and a desire for control over cancer drove women to choose mastectomy.
Data source: A qualitative study of 30 women with early-stage breast cancer who were candidates for breast-conserving surgery but chose unilateral mastectomy with or without contralateral prophylactic mastectomy.
Disclosures: Dr. Covelli has received funding from Roche Canada and the Canadian Breast Cancer Foundation Physician Fellowship Award.
Cardiovascular risk factors common with breast cancer
SAN FRANCISCO – Breast cancer survivors shared several body composition characteristics associated with increased cardiac risk and were referred for cardiac consultation most commonly for a high body mass index, an elevated LDL* level, insufficient exercise, and exposure to anthracycline, two separate studies found.
In the first study, kinesiologists measured various characteristics in 3,674 nononcology female patients and compared them with measurements in 740 women in a breast cancer survivorship clinic who were stratified into 8 groups according to the type of treatment they received. All breast cancer patients underwent surgery: 41 women had surgery alone, 13 also underwent chemotherapy, 51 had surgery and radiotherapy, and 48 had surgery and hormone therapy. Most cancer survivors underwent multiple therapies: surgery, chemotherapy, radiation, and hormone therapy in 244; surgery, radiation, and hormone therapy in 207; surgery, chemotherapy, and radiation in 83; and surgery, chemotherapy, and hormone therapy in 30, David H. Jones and his associates found.
Statistically significant differences were seen between the control group and five of the eight treatment groups. Compared with the control group, patients who underwent surgery, chemotherapy, radiation, and hormone therapy had significantly higher mean diastolic blood pressure (77 vs. 74 mm Hg), mean systolic blood pressure (128 vs. 123 mm Hg), mean arterial pressure (94 vs. 90 mm Hg), A faster mean heart rate (77 vs. 73 beats per minute), a higher percentage of body fat (37% vs. 34%), and a greater waist circumference (88 vs. 85 cm), Mr. Jones reported in a poster at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Patients who underwent surgery, radiation, and hormone therapy also had a higher mean systolic blood pressure compared with controls (130 mm Hg), a higher mean arterial pressure (94 mm Hg), and greater body fat (37%). Patients who underwent surgery, chemotherapy, and radiotherapy had a higher mean systolic blood pressure (129 mm Hg), mean arterial pressure (94 mm Hg), heart rate (79 bpm), and body fat (38%) compared with controls, reported Mr. Jones of Ville-Marie Medical Center, Montreal.
Patients treated with surgery alone had a higher body mass index (29 vs. 26 kg/mm2), body fat percentage (39%), and waist circumference (90 cm) than did controls. Patients who underwent surgery and chemotherapy had significantly less mean muscle mass compared with controls (9.6 vs. 10.1 kg).
Previous studies have associated these body composition characteristics with increased risk for cardiovascular diseases and metabolic problems, Mr. Jones noted.
In the second study of 365 women with nonmetastatic breast cancer seen at a survivorship center in 2006-2012, 13% already were being followed by a cardiologist, 21% were referred to cardio-oncology after their initial visit to the survivorship center, and 66% were not referred, Jennifer R. Klemp, Ph.D., and her associates found.
Patients who were not referred had an average of four risk factors for cardiovascular disease, significantly fewer than the average of six cardiovascular risk factors in patients referred to cardio-oncology and those already seeing a cardiologist, reported Dr. Klemp, director of cancer survivorship at the University of Kansas, Westwood.
The risk factors considered in the study included exposure to cardiotoxic breast cancer treatment as well as traditional risk factors: a BMI greater than 25, diabetes; hypertension, an elevated HDL level, a history of smoking, a family history of an MI before age 60 years, and exercising fewer than 150 min/wk. Breast cancer treatment–related risk factors included an ejection fraction less than 50%; anti-hormone therapy; use of tamoxifen, anthracycline, or Herceptin (trastuzumab); and left chest wall radiation.
Among patients referred to cardio-oncology, 92% showed up. Most often they received additional diagnostic tests, changes in medications, or return visits for follow-up.
"These findings demonstrate the need to determine how to include treatment-related risk factors along with traditional cardiovascular risk factors in assessing and managing cardiovascular risk in breast cancer survivors," Dr. Klemp said.
Mr. Jones and Dr. Klemp reported having no relevant financial disclosures. Most of Dr. Jones’ associates were employees or leaders of Ville-Marie Medical Center.
On Twitter @sherryboschert
*Correction, 11/20/2013: A previous version of this article misstated one of the symptoms for both increased cardiac risk and increased risk for cardio-oncology referrals.
The study by Mr. Jones and his coinvestigators compared anthropometric baseline and vital sign measurements in 3,674 nononcology female patients with measurements in 740 cancer survivors. They evaluated the patients post treatment by eight different treatment regimens. Compared with a nononcology group, the patients undergoing breast cancer surgery alone had significantly higher blood pressure values, greater amounts of body fat, and larger waist circumferences. Adding two additional therapies such as hormone therapy, radiotherapy, or chemotherapy tended to show worsening parameters. The more therapy the patient had, the more likely she was to have one of these worse parameters.
The investigators concluded that several unfavorable body composition characteristics seemed to be associated with women who had completed treatment for breast cancer. And many of these changes in body composition led to an increased risk of developing cardiovascular diseases and different types of metabolic problems.

|
|
These findings corroborate those from previous studies. For example, these patients have been found to have more high-risk features after they complete their breast cancer therapy. One small study that looked at physical activity in the year after breast cancer was treated found that total activity was reduced after breast cancer treatment – including household, sports, and occupational activities. Across the board, patients remained less active 1 year post treatment, with implications for their cardiovascular health (Breast Cancer Res. Treat. 2010;123:417-25).
This becomes very important when you think about the increased number of risk factors following therapy, and the fact that patients got less exercise the year after treatment. When you put that in perspective, the lifetime risk of death from cardiovascular disease among women aged 55 and older increases dramatically with additional risk factors (N. Engl. J. Med. 2012;366:321-9).
The study by Dr. Klemp and his associates looked at cardiovascular risk factors among 365 breast cancer survivors and the outcomes of cardio-oncology referrals at the university’s survivorship center between 2006 and 2012. The investigators evaluated the number of patients with three or more cardiovascular risk factors, both preexisting and treatment related. The cardiac risk factors were pretty similar to those in the general population. The cardiology referrals accounted for 21% of patients. The most common risk factors associated with cardio-oncology referrals were a high body mass index, an elevated LDL* level, less exercise exposure, and anthracycline exposure. The most common outcomes for those seen by cardio-oncology were additional diagnostic tests and medication changes. Interventions by cardiology referrals correlated with a higher number of risk factors. Patients with the highest number of risk factors were the most likely to continue with the cardiologist, receive medication, and undergo diagnostic tests.
These two studies together demonstrate the important message that cardiovascular disease is a significant risk for breast cancer survivors. With time, as the risk of breast cancer death decreases, the risk of cardiovascular death increases.
The best method for improved outcomes from breast cancer may be the addition of an exercise machine in our waiting rooms.
Dr. Julia White is the director of breast radiation oncology at Ohio State University, Columbus. These are excerpts of her remarks as the discussant of these papers at the meeting. She reported having no financial relevant financial disclosures.
The study by Mr. Jones and his coinvestigators compared anthropometric baseline and vital sign measurements in 3,674 nononcology female patients with measurements in 740 cancer survivors. They evaluated the patients post treatment by eight different treatment regimens. Compared with a nononcology group, the patients undergoing breast cancer surgery alone had significantly higher blood pressure values, greater amounts of body fat, and larger waist circumferences. Adding two additional therapies such as hormone therapy, radiotherapy, or chemotherapy tended to show worsening parameters. The more therapy the patient had, the more likely she was to have one of these worse parameters.
The investigators concluded that several unfavorable body composition characteristics seemed to be associated with women who had completed treatment for breast cancer. And many of these changes in body composition led to an increased risk of developing cardiovascular diseases and different types of metabolic problems.

|
|
These findings corroborate those from previous studies. For example, these patients have been found to have more high-risk features after they complete their breast cancer therapy. One small study that looked at physical activity in the year after breast cancer was treated found that total activity was reduced after breast cancer treatment – including household, sports, and occupational activities. Across the board, patients remained less active 1 year post treatment, with implications for their cardiovascular health (Breast Cancer Res. Treat. 2010;123:417-25).
This becomes very important when you think about the increased number of risk factors following therapy, and the fact that patients got less exercise the year after treatment. When you put that in perspective, the lifetime risk of death from cardiovascular disease among women aged 55 and older increases dramatically with additional risk factors (N. Engl. J. Med. 2012;366:321-9).
The study by Dr. Klemp and his associates looked at cardiovascular risk factors among 365 breast cancer survivors and the outcomes of cardio-oncology referrals at the university’s survivorship center between 2006 and 2012. The investigators evaluated the number of patients with three or more cardiovascular risk factors, both preexisting and treatment related. The cardiac risk factors were pretty similar to those in the general population. The cardiology referrals accounted for 21% of patients. The most common risk factors associated with cardio-oncology referrals were a high body mass index, an elevated LDL* level, less exercise exposure, and anthracycline exposure. The most common outcomes for those seen by cardio-oncology were additional diagnostic tests and medication changes. Interventions by cardiology referrals correlated with a higher number of risk factors. Patients with the highest number of risk factors were the most likely to continue with the cardiologist, receive medication, and undergo diagnostic tests.
These two studies together demonstrate the important message that cardiovascular disease is a significant risk for breast cancer survivors. With time, as the risk of breast cancer death decreases, the risk of cardiovascular death increases.
The best method for improved outcomes from breast cancer may be the addition of an exercise machine in our waiting rooms.
Dr. Julia White is the director of breast radiation oncology at Ohio State University, Columbus. These are excerpts of her remarks as the discussant of these papers at the meeting. She reported having no financial relevant financial disclosures.
The study by Mr. Jones and his coinvestigators compared anthropometric baseline and vital sign measurements in 3,674 nononcology female patients with measurements in 740 cancer survivors. They evaluated the patients post treatment by eight different treatment regimens. Compared with a nononcology group, the patients undergoing breast cancer surgery alone had significantly higher blood pressure values, greater amounts of body fat, and larger waist circumferences. Adding two additional therapies such as hormone therapy, radiotherapy, or chemotherapy tended to show worsening parameters. The more therapy the patient had, the more likely she was to have one of these worse parameters.
The investigators concluded that several unfavorable body composition characteristics seemed to be associated with women who had completed treatment for breast cancer. And many of these changes in body composition led to an increased risk of developing cardiovascular diseases and different types of metabolic problems.

|
|
These findings corroborate those from previous studies. For example, these patients have been found to have more high-risk features after they complete their breast cancer therapy. One small study that looked at physical activity in the year after breast cancer was treated found that total activity was reduced after breast cancer treatment – including household, sports, and occupational activities. Across the board, patients remained less active 1 year post treatment, with implications for their cardiovascular health (Breast Cancer Res. Treat. 2010;123:417-25).
This becomes very important when you think about the increased number of risk factors following therapy, and the fact that patients got less exercise the year after treatment. When you put that in perspective, the lifetime risk of death from cardiovascular disease among women aged 55 and older increases dramatically with additional risk factors (N. Engl. J. Med. 2012;366:321-9).
The study by Dr. Klemp and his associates looked at cardiovascular risk factors among 365 breast cancer survivors and the outcomes of cardio-oncology referrals at the university’s survivorship center between 2006 and 2012. The investigators evaluated the number of patients with three or more cardiovascular risk factors, both preexisting and treatment related. The cardiac risk factors were pretty similar to those in the general population. The cardiology referrals accounted for 21% of patients. The most common risk factors associated with cardio-oncology referrals were a high body mass index, an elevated LDL* level, less exercise exposure, and anthracycline exposure. The most common outcomes for those seen by cardio-oncology were additional diagnostic tests and medication changes. Interventions by cardiology referrals correlated with a higher number of risk factors. Patients with the highest number of risk factors were the most likely to continue with the cardiologist, receive medication, and undergo diagnostic tests.
These two studies together demonstrate the important message that cardiovascular disease is a significant risk for breast cancer survivors. With time, as the risk of breast cancer death decreases, the risk of cardiovascular death increases.
The best method for improved outcomes from breast cancer may be the addition of an exercise machine in our waiting rooms.
Dr. Julia White is the director of breast radiation oncology at Ohio State University, Columbus. These are excerpts of her remarks as the discussant of these papers at the meeting. She reported having no financial relevant financial disclosures.
SAN FRANCISCO – Breast cancer survivors shared several body composition characteristics associated with increased cardiac risk and were referred for cardiac consultation most commonly for a high body mass index, an elevated LDL* level, insufficient exercise, and exposure to anthracycline, two separate studies found.
In the first study, kinesiologists measured various characteristics in 3,674 nononcology female patients and compared them with measurements in 740 women in a breast cancer survivorship clinic who were stratified into 8 groups according to the type of treatment they received. All breast cancer patients underwent surgery: 41 women had surgery alone, 13 also underwent chemotherapy, 51 had surgery and radiotherapy, and 48 had surgery and hormone therapy. Most cancer survivors underwent multiple therapies: surgery, chemotherapy, radiation, and hormone therapy in 244; surgery, radiation, and hormone therapy in 207; surgery, chemotherapy, and radiation in 83; and surgery, chemotherapy, and hormone therapy in 30, David H. Jones and his associates found.
Statistically significant differences were seen between the control group and five of the eight treatment groups. Compared with the control group, patients who underwent surgery, chemotherapy, radiation, and hormone therapy had significantly higher mean diastolic blood pressure (77 vs. 74 mm Hg), mean systolic blood pressure (128 vs. 123 mm Hg), mean arterial pressure (94 vs. 90 mm Hg), A faster mean heart rate (77 vs. 73 beats per minute), a higher percentage of body fat (37% vs. 34%), and a greater waist circumference (88 vs. 85 cm), Mr. Jones reported in a poster at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Patients who underwent surgery, radiation, and hormone therapy also had a higher mean systolic blood pressure compared with controls (130 mm Hg), a higher mean arterial pressure (94 mm Hg), and greater body fat (37%). Patients who underwent surgery, chemotherapy, and radiotherapy had a higher mean systolic blood pressure (129 mm Hg), mean arterial pressure (94 mm Hg), heart rate (79 bpm), and body fat (38%) compared with controls, reported Mr. Jones of Ville-Marie Medical Center, Montreal.
Patients treated with surgery alone had a higher body mass index (29 vs. 26 kg/mm2), body fat percentage (39%), and waist circumference (90 cm) than did controls. Patients who underwent surgery and chemotherapy had significantly less mean muscle mass compared with controls (9.6 vs. 10.1 kg).
Previous studies have associated these body composition characteristics with increased risk for cardiovascular diseases and metabolic problems, Mr. Jones noted.
In the second study of 365 women with nonmetastatic breast cancer seen at a survivorship center in 2006-2012, 13% already were being followed by a cardiologist, 21% were referred to cardio-oncology after their initial visit to the survivorship center, and 66% were not referred, Jennifer R. Klemp, Ph.D., and her associates found.
Patients who were not referred had an average of four risk factors for cardiovascular disease, significantly fewer than the average of six cardiovascular risk factors in patients referred to cardio-oncology and those already seeing a cardiologist, reported Dr. Klemp, director of cancer survivorship at the University of Kansas, Westwood.
The risk factors considered in the study included exposure to cardiotoxic breast cancer treatment as well as traditional risk factors: a BMI greater than 25, diabetes; hypertension, an elevated HDL level, a history of smoking, a family history of an MI before age 60 years, and exercising fewer than 150 min/wk. Breast cancer treatment–related risk factors included an ejection fraction less than 50%; anti-hormone therapy; use of tamoxifen, anthracycline, or Herceptin (trastuzumab); and left chest wall radiation.
Among patients referred to cardio-oncology, 92% showed up. Most often they received additional diagnostic tests, changes in medications, or return visits for follow-up.
"These findings demonstrate the need to determine how to include treatment-related risk factors along with traditional cardiovascular risk factors in assessing and managing cardiovascular risk in breast cancer survivors," Dr. Klemp said.
Mr. Jones and Dr. Klemp reported having no relevant financial disclosures. Most of Dr. Jones’ associates were employees or leaders of Ville-Marie Medical Center.
On Twitter @sherryboschert
*Correction, 11/20/2013: A previous version of this article misstated one of the symptoms for both increased cardiac risk and increased risk for cardio-oncology referrals.
SAN FRANCISCO – Breast cancer survivors shared several body composition characteristics associated with increased cardiac risk and were referred for cardiac consultation most commonly for a high body mass index, an elevated LDL* level, insufficient exercise, and exposure to anthracycline, two separate studies found.
In the first study, kinesiologists measured various characteristics in 3,674 nononcology female patients and compared them with measurements in 740 women in a breast cancer survivorship clinic who were stratified into 8 groups according to the type of treatment they received. All breast cancer patients underwent surgery: 41 women had surgery alone, 13 also underwent chemotherapy, 51 had surgery and radiotherapy, and 48 had surgery and hormone therapy. Most cancer survivors underwent multiple therapies: surgery, chemotherapy, radiation, and hormone therapy in 244; surgery, radiation, and hormone therapy in 207; surgery, chemotherapy, and radiation in 83; and surgery, chemotherapy, and hormone therapy in 30, David H. Jones and his associates found.
Statistically significant differences were seen between the control group and five of the eight treatment groups. Compared with the control group, patients who underwent surgery, chemotherapy, radiation, and hormone therapy had significantly higher mean diastolic blood pressure (77 vs. 74 mm Hg), mean systolic blood pressure (128 vs. 123 mm Hg), mean arterial pressure (94 vs. 90 mm Hg), A faster mean heart rate (77 vs. 73 beats per minute), a higher percentage of body fat (37% vs. 34%), and a greater waist circumference (88 vs. 85 cm), Mr. Jones reported in a poster at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Patients who underwent surgery, radiation, and hormone therapy also had a higher mean systolic blood pressure compared with controls (130 mm Hg), a higher mean arterial pressure (94 mm Hg), and greater body fat (37%). Patients who underwent surgery, chemotherapy, and radiotherapy had a higher mean systolic blood pressure (129 mm Hg), mean arterial pressure (94 mm Hg), heart rate (79 bpm), and body fat (38%) compared with controls, reported Mr. Jones of Ville-Marie Medical Center, Montreal.
Patients treated with surgery alone had a higher body mass index (29 vs. 26 kg/mm2), body fat percentage (39%), and waist circumference (90 cm) than did controls. Patients who underwent surgery and chemotherapy had significantly less mean muscle mass compared with controls (9.6 vs. 10.1 kg).
Previous studies have associated these body composition characteristics with increased risk for cardiovascular diseases and metabolic problems, Mr. Jones noted.
In the second study of 365 women with nonmetastatic breast cancer seen at a survivorship center in 2006-2012, 13% already were being followed by a cardiologist, 21% were referred to cardio-oncology after their initial visit to the survivorship center, and 66% were not referred, Jennifer R. Klemp, Ph.D., and her associates found.
Patients who were not referred had an average of four risk factors for cardiovascular disease, significantly fewer than the average of six cardiovascular risk factors in patients referred to cardio-oncology and those already seeing a cardiologist, reported Dr. Klemp, director of cancer survivorship at the University of Kansas, Westwood.
The risk factors considered in the study included exposure to cardiotoxic breast cancer treatment as well as traditional risk factors: a BMI greater than 25, diabetes; hypertension, an elevated HDL level, a history of smoking, a family history of an MI before age 60 years, and exercising fewer than 150 min/wk. Breast cancer treatment–related risk factors included an ejection fraction less than 50%; anti-hormone therapy; use of tamoxifen, anthracycline, or Herceptin (trastuzumab); and left chest wall radiation.
Among patients referred to cardio-oncology, 92% showed up. Most often they received additional diagnostic tests, changes in medications, or return visits for follow-up.
"These findings demonstrate the need to determine how to include treatment-related risk factors along with traditional cardiovascular risk factors in assessing and managing cardiovascular risk in breast cancer survivors," Dr. Klemp said.
Mr. Jones and Dr. Klemp reported having no relevant financial disclosures. Most of Dr. Jones’ associates were employees or leaders of Ville-Marie Medical Center.
On Twitter @sherryboschert
*Correction, 11/20/2013: A previous version of this article misstated one of the symptoms for both increased cardiac risk and increased risk for cardio-oncology referrals.
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: Mean blood pressures were 128/77 mm Hg in patients treated with surgery, chemotherapy, radiation, and hormone therapy compared with 123/74 mm Hg in controls, in one study. In a second study, 13% of patients at a survivorship center were seeing a cardiologist and 21% were referred to one.
Data source: A prospective study comparing 740 breast cancer survivors with 3,674 nononcology patients, and a separate retrospective study of 365 women at a survivorship center.
Disclosures: Mr. Jones and Dr. Klemp reported having no relevant financial disclosures. Most of Mr. Jones’ associates were employees or leaders of Ville-Marie Medical Center.
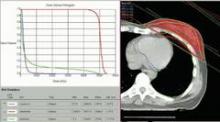
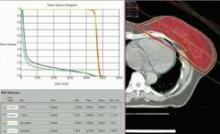
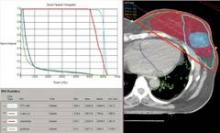
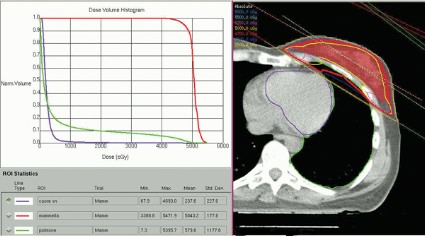
Partial, whole breast irradiation 10-year outcomes similar
SAN FRANCISCO – Ten years of follow-up showed no significant difference in breast cancer locoregional recurrence, distant metastasis, or survival rates in 274 patients treated with accelerated partial breast irradiation compared with 274 matched patients treated with whole breast irradiation.
The data came from records on 3,009 patients with early-stage breast cancer who were treated with breast-conserving therapy at one institution between 1980 and 2012.
Four percent in each group developed local recurrence, 1% in each group had a regional recurrence, and 6% had distant metastases after partial breast irradiation and 3%, after whole breast irradiation. There was a nonsignificant statistical trend toward a higher rate of contralateral breast failure in the whole breast irradiation group (9%) compared with the partial breast irradiation group (3%, P = .06), Dr. Jessica Wobb reported in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Rates of disease-free survival were 91% in the partial breast irradiation group and 93% in the whole breast irradiation group. Cause-specific survival rates were 93% and 94%, respectively, and overall survival rates were 75% and 82%, reported Dr. Wobb of the Beaumont Cancer Institute, Royal Oak, Mich. None of these differences reached statistical significance.
This is one of the first reports on prolonged follow-up after accelerated partial breast irradiation, she noted. Mean follow-up was 7.8 years after partial breast irradiation and 8.1 years after whole breast irradiation, a difference that was statistically significant, but amounted to less than 4 months. All patients were followed for at least 1 year.
Patients in the cohorts were matched by age (within 3 years); T stage (Tis, T1, or T2); and estrogen receptor (ER) status. The mean age was 63 years of age in both groups. Eighty-eight percent in both groups had ER-positive tumors. The stage distribution in both groups consisted of 18% with stage Tis tumors, 71% with T1 tumors, and 11% with T2 tumors.
Significantly fewer patients in the partial breast irradiation group received adjuvant hormonal therapy (54%) compared with those in the whole breast irradiation group (68%). There was a trend toward smaller tumors in patients undergoing partial breast irradiation than in those receiving whole breast irradiation, with mean tumor sizes of 11.4 mm and 13 mm (P = .06).
Other characteristics were similar between the groups, including the proportion with negative lymph nodes (91% of patients undergoing partial breast irradiation and 86% of those who got whole breast irradiation), the proportion with negative final margins (94% and 95%, respectively), and the proportion who received adjuvant chemotherapy (15% and 18%).
Close tumor margins increased the risk for ipsilateral breast tumor recurrence in both groups, and positive margins increased the recurrence risk in the whole breast irradiation group, a univariate analysis found.
Dr. Wobb reported having no relevant financial disclosures.
On Twitter @sherryboschert
In the absence of prospective, randomized trial data on accelerated partial breast irradiation to guide us, we are left with the accumulation of institutional data. The institution that, in my opinion, has contributed most to our knowledge base is the group at William Beaumont Hospital. We’re fortunate to have an update of their experience in that they’ve performed an updated a matched-pair analysis looking at their partial breast irradiation patients (using interstitial catheter or balloon-based brachytherapy two different techniques), compared with their whole breast irradiation patients.
In this matched-pair comparison, the investigators saw no difference in local failure, regional failure, distant metastases, or overall survival.

|
|
Of course, we have to ask, in a matched pair, how good is the match? We do notice that in their group it’s a pretty good match, but we see that for whole breast irradiation, there are slightly larger tumors in that cohort and slightly more positive-node patients. Perhaps the most unsettling aspect is that there is more hormonal therapy in the whole breast irradiation group. This could reflect two things: One is an imbalance in prognostic factors between the two cohorts; the other is an impact of hormonal therapy on local and regional control outcomes.
When we look at their results related to clinical variables and outcome, not surprisingly we find that a negative margin is always better irrespective of whether the patient is getting whole breast irradiation or partial breast irradiation. Interestingly, in the partial breast irradiation group, younger age was associated with a higher risk of local failure.
What’s missing from this analysis? Again, this is not a fault of the investigators; just by virtue of this being a retrospective collection of data, it’s sometimes hard to get all this data. The questions that I think are pertinent in 2013 relate to grade, triple-negative phenotype versus other phenotypes, human epidermal growth factor receptor 2 status, and lymphatic vascular invasion. Unfortunately, none of that information is present in this analysis.
Dr. David E. Wazer is a professor of radiation oncology at Brown University, Providence, R.I. These are excerpts of his remarks as the discussant of Dr. Wobb’s study at the meeting. Dr. Wazer reported financial associations with the American Brachytherapy Society, Advanced Radiation Therapy, and American Journal of Clinical Oncology.
In the absence of prospective, randomized trial data on accelerated partial breast irradiation to guide us, we are left with the accumulation of institutional data. The institution that, in my opinion, has contributed most to our knowledge base is the group at William Beaumont Hospital. We’re fortunate to have an update of their experience in that they’ve performed an updated a matched-pair analysis looking at their partial breast irradiation patients (using interstitial catheter or balloon-based brachytherapy two different techniques), compared with their whole breast irradiation patients.
In this matched-pair comparison, the investigators saw no difference in local failure, regional failure, distant metastases, or overall survival.

|
|
Of course, we have to ask, in a matched pair, how good is the match? We do notice that in their group it’s a pretty good match, but we see that for whole breast irradiation, there are slightly larger tumors in that cohort and slightly more positive-node patients. Perhaps the most unsettling aspect is that there is more hormonal therapy in the whole breast irradiation group. This could reflect two things: One is an imbalance in prognostic factors between the two cohorts; the other is an impact of hormonal therapy on local and regional control outcomes.
When we look at their results related to clinical variables and outcome, not surprisingly we find that a negative margin is always better irrespective of whether the patient is getting whole breast irradiation or partial breast irradiation. Interestingly, in the partial breast irradiation group, younger age was associated with a higher risk of local failure.
What’s missing from this analysis? Again, this is not a fault of the investigators; just by virtue of this being a retrospective collection of data, it’s sometimes hard to get all this data. The questions that I think are pertinent in 2013 relate to grade, triple-negative phenotype versus other phenotypes, human epidermal growth factor receptor 2 status, and lymphatic vascular invasion. Unfortunately, none of that information is present in this analysis.
Dr. David E. Wazer is a professor of radiation oncology at Brown University, Providence, R.I. These are excerpts of his remarks as the discussant of Dr. Wobb’s study at the meeting. Dr. Wazer reported financial associations with the American Brachytherapy Society, Advanced Radiation Therapy, and American Journal of Clinical Oncology.
In the absence of prospective, randomized trial data on accelerated partial breast irradiation to guide us, we are left with the accumulation of institutional data. The institution that, in my opinion, has contributed most to our knowledge base is the group at William Beaumont Hospital. We’re fortunate to have an update of their experience in that they’ve performed an updated a matched-pair analysis looking at their partial breast irradiation patients (using interstitial catheter or balloon-based brachytherapy two different techniques), compared with their whole breast irradiation patients.
In this matched-pair comparison, the investigators saw no difference in local failure, regional failure, distant metastases, or overall survival.

|
|
Of course, we have to ask, in a matched pair, how good is the match? We do notice that in their group it’s a pretty good match, but we see that for whole breast irradiation, there are slightly larger tumors in that cohort and slightly more positive-node patients. Perhaps the most unsettling aspect is that there is more hormonal therapy in the whole breast irradiation group. This could reflect two things: One is an imbalance in prognostic factors between the two cohorts; the other is an impact of hormonal therapy on local and regional control outcomes.
When we look at their results related to clinical variables and outcome, not surprisingly we find that a negative margin is always better irrespective of whether the patient is getting whole breast irradiation or partial breast irradiation. Interestingly, in the partial breast irradiation group, younger age was associated with a higher risk of local failure.
What’s missing from this analysis? Again, this is not a fault of the investigators; just by virtue of this being a retrospective collection of data, it’s sometimes hard to get all this data. The questions that I think are pertinent in 2013 relate to grade, triple-negative phenotype versus other phenotypes, human epidermal growth factor receptor 2 status, and lymphatic vascular invasion. Unfortunately, none of that information is present in this analysis.
Dr. David E. Wazer is a professor of radiation oncology at Brown University, Providence, R.I. These are excerpts of his remarks as the discussant of Dr. Wobb’s study at the meeting. Dr. Wazer reported financial associations with the American Brachytherapy Society, Advanced Radiation Therapy, and American Journal of Clinical Oncology.
SAN FRANCISCO – Ten years of follow-up showed no significant difference in breast cancer locoregional recurrence, distant metastasis, or survival rates in 274 patients treated with accelerated partial breast irradiation compared with 274 matched patients treated with whole breast irradiation.
The data came from records on 3,009 patients with early-stage breast cancer who were treated with breast-conserving therapy at one institution between 1980 and 2012.
Four percent in each group developed local recurrence, 1% in each group had a regional recurrence, and 6% had distant metastases after partial breast irradiation and 3%, after whole breast irradiation. There was a nonsignificant statistical trend toward a higher rate of contralateral breast failure in the whole breast irradiation group (9%) compared with the partial breast irradiation group (3%, P = .06), Dr. Jessica Wobb reported in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Rates of disease-free survival were 91% in the partial breast irradiation group and 93% in the whole breast irradiation group. Cause-specific survival rates were 93% and 94%, respectively, and overall survival rates were 75% and 82%, reported Dr. Wobb of the Beaumont Cancer Institute, Royal Oak, Mich. None of these differences reached statistical significance.
This is one of the first reports on prolonged follow-up after accelerated partial breast irradiation, she noted. Mean follow-up was 7.8 years after partial breast irradiation and 8.1 years after whole breast irradiation, a difference that was statistically significant, but amounted to less than 4 months. All patients were followed for at least 1 year.
Patients in the cohorts were matched by age (within 3 years); T stage (Tis, T1, or T2); and estrogen receptor (ER) status. The mean age was 63 years of age in both groups. Eighty-eight percent in both groups had ER-positive tumors. The stage distribution in both groups consisted of 18% with stage Tis tumors, 71% with T1 tumors, and 11% with T2 tumors.
Significantly fewer patients in the partial breast irradiation group received adjuvant hormonal therapy (54%) compared with those in the whole breast irradiation group (68%). There was a trend toward smaller tumors in patients undergoing partial breast irradiation than in those receiving whole breast irradiation, with mean tumor sizes of 11.4 mm and 13 mm (P = .06).
Other characteristics were similar between the groups, including the proportion with negative lymph nodes (91% of patients undergoing partial breast irradiation and 86% of those who got whole breast irradiation), the proportion with negative final margins (94% and 95%, respectively), and the proportion who received adjuvant chemotherapy (15% and 18%).
Close tumor margins increased the risk for ipsilateral breast tumor recurrence in both groups, and positive margins increased the recurrence risk in the whole breast irradiation group, a univariate analysis found.
Dr. Wobb reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Ten years of follow-up showed no significant difference in breast cancer locoregional recurrence, distant metastasis, or survival rates in 274 patients treated with accelerated partial breast irradiation compared with 274 matched patients treated with whole breast irradiation.
The data came from records on 3,009 patients with early-stage breast cancer who were treated with breast-conserving therapy at one institution between 1980 and 2012.
Four percent in each group developed local recurrence, 1% in each group had a regional recurrence, and 6% had distant metastases after partial breast irradiation and 3%, after whole breast irradiation. There was a nonsignificant statistical trend toward a higher rate of contralateral breast failure in the whole breast irradiation group (9%) compared with the partial breast irradiation group (3%, P = .06), Dr. Jessica Wobb reported in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
Rates of disease-free survival were 91% in the partial breast irradiation group and 93% in the whole breast irradiation group. Cause-specific survival rates were 93% and 94%, respectively, and overall survival rates were 75% and 82%, reported Dr. Wobb of the Beaumont Cancer Institute, Royal Oak, Mich. None of these differences reached statistical significance.
This is one of the first reports on prolonged follow-up after accelerated partial breast irradiation, she noted. Mean follow-up was 7.8 years after partial breast irradiation and 8.1 years after whole breast irradiation, a difference that was statistically significant, but amounted to less than 4 months. All patients were followed for at least 1 year.
Patients in the cohorts were matched by age (within 3 years); T stage (Tis, T1, or T2); and estrogen receptor (ER) status. The mean age was 63 years of age in both groups. Eighty-eight percent in both groups had ER-positive tumors. The stage distribution in both groups consisted of 18% with stage Tis tumors, 71% with T1 tumors, and 11% with T2 tumors.
Significantly fewer patients in the partial breast irradiation group received adjuvant hormonal therapy (54%) compared with those in the whole breast irradiation group (68%). There was a trend toward smaller tumors in patients undergoing partial breast irradiation than in those receiving whole breast irradiation, with mean tumor sizes of 11.4 mm and 13 mm (P = .06).
Other characteristics were similar between the groups, including the proportion with negative lymph nodes (91% of patients undergoing partial breast irradiation and 86% of those who got whole breast irradiation), the proportion with negative final margins (94% and 95%, respectively), and the proportion who received adjuvant chemotherapy (15% and 18%).
Close tumor margins increased the risk for ipsilateral breast tumor recurrence in both groups, and positive margins increased the recurrence risk in the whole breast irradiation group, a univariate analysis found.
Dr. Wobb reported having no relevant financial disclosures.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: Four percent developed local recurrence and 1% had regional recurrence in both partial and whole breast irradiation groups. For the partial vs. whole breast irradiation groups, distant metastases developed in 6% and 3%, disease-free survival rates were 91% and 93%, and overall survival rates were 75% and 82.
Data source: A retrospective study of 274 matched pairs of patients with early-stage breast cancer treated with breast-conserving therapy at one institution.
Disclosures: Dr. Wobb reported having no relevant financial disclosures.
Heart irradiation is lower with contemporary breast radiotherapy
SAN FRANCISCO – The hearts of 100 consecutive patients who underwent adjuvant radiotherapy for left-sided breast cancer in 2011 received an average of 2.9 Gray of radiation, considerably less than the mean cardiac exposure of 4.9-Gy reported in a recent review of 2,168 patients treated from 1958 to 2001 in Sweden and Denmark.
The findings confirm that three-dimensional conformal radiation therapy (3D-CRT) reduces cardiac exposure to radiation, Dr. Federico Lonardi and his associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. But certain areas of the heart still receive high doses when patients have adverse anatomic conditions that are not well suited to 3D-CRT. Because heart structures may differ in radiosensitivity, higher doses to small volumes of the heart, such as the coronary artery, might be associated with more risk, the researchers cautioned.
Most patients received a mean cardiac dose of 2-3 Gy (32%), 21% of patients were exposed to 1.15-1.99 Gy, and 1% got 0.8 Gy in a study of a consecutive series of breast cancer patients treated at Mater Salutis Hospital in Legnago, Italy. Only 17% of patients received a mean cardiac dose of more than 5 Gy, and 13% received 4.16-4.83 Gy.
The cardiac dose ranged from 0.8 to 13.05 Gy in Dr. Lonardi’s study, compared with a range of 0.03 to 27.72 Gy in the recently published Scandinavian study (N. Engl. J. Med. 2013;368:987-998). In the published study, the longitudinal risk for major cardiac events increased in a linear fashion, with a 7% increase for cardiac events with every 1 Gy increase in radiation to the heart.
In the Italian study, the median volumes of heart exposed to higher doses of radiation were "consistently low" with 4% of heart volumes exposed to 5 Gy or more, 3% exposed to 10Gy or more, 2% exposed to 15 Gy or more, and 0.7% exposed to 25 Gy or more Dr. Lonardi reported.
These patients received full-breast 3D-CRT with two to four customized tangential fields after mastectomy (10% of patients) or quadrantectomy (90%). The whole breast (or chest wall) received 50 Gy/25 fractions in 66 patients and 45 Gy/18 fractions in 34 patients. Boost to surgical bed (10 Gy/4-5 fractions) was delivered by photons in 10 patients. Median number of tangential fields was two (range, two to four). Patients were treated while supine on a breast board, without immobilization devices or instructions to hold their breath. They were freely breathing but were asked to minimize respiratory motion during the CT scan used to plan radiation delivery and the treatment itself. No dose constraints were specified for heart structures; a mean heart dose lower than 5 Gy was recommended at the time of treatment.
A preliminary assessment of radiation delivered to the left anterior descending coronary arteries in this series suggests that they received 9-25 Gy, Dr. Lonardi reported.
Based on estimates using previous models, the probability of death from cardiac causes within 15 years after standard fractionated radiotherapy may be less than 1% if less than 10% of the heart is exposed to 25Gy or more, he noted. "In this perspective, our results appear very favorable, though they confirm that the heart may receive high doses to limited volumes despite the use of standard 3D techniques. In such cases, high-conformal, intensity-modulated techniques are helpful" to further reduce the exposure of critical heart structures to radiation.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Lonardi reported having no financial disclosures.
On Twitter @sherryboschert
This was an interesting study. I really give the authors a lot of credit. They basically looked at 100 consecutive cases of patients treated with adjuvant radiotherapy for left-sided breast cancer and brought forward what they were doing without trying to optimize or minimize the presentation or cardiac dose in the patients they were treating.

|
| Dr. Julia White |
Their mean doses were lower than those reported by Darby et al. in a study that looked at 2,168 patients treated during 1958-2001 in both Sweden and Denmark. The researchers reviewed individual radiotherapy charts, and then did 20 consecutive individual CT-based three-dimensional planning scans to model what the type of radiotherapy looked like many years ago (N. Engl. J. Med. 2013;368:987-98).
This was an interesting study. I really give the authors a lot of credit. They basically looked at 100 consecutive cases of patients treated with adjuvant radiotherapy for left-sided breast cancer and brought forward what they were doing without trying to optimize or minimize the presentation or cardiac dose in the patients they were treating.

|
| Dr. Julia White |
Their mean doses were lower than those reported by Darby et al. in a study that looked at 2,168 patients treated during 1958-2001 in both Sweden and Denmark. The researchers reviewed individual radiotherapy charts, and then did 20 consecutive individual CT-based three-dimensional planning scans to model what the type of radiotherapy looked like many years ago (N. Engl. J. Med. 2013;368:987-98).
This was an interesting study. I really give the authors a lot of credit. They basically looked at 100 consecutive cases of patients treated with adjuvant radiotherapy for left-sided breast cancer and brought forward what they were doing without trying to optimize or minimize the presentation or cardiac dose in the patients they were treating.

|
| Dr. Julia White |
Their mean doses were lower than those reported by Darby et al. in a study that looked at 2,168 patients treated during 1958-2001 in both Sweden and Denmark. The researchers reviewed individual radiotherapy charts, and then did 20 consecutive individual CT-based three-dimensional planning scans to model what the type of radiotherapy looked like many years ago (N. Engl. J. Med. 2013;368:987-98).
SAN FRANCISCO – The hearts of 100 consecutive patients who underwent adjuvant radiotherapy for left-sided breast cancer in 2011 received an average of 2.9 Gray of radiation, considerably less than the mean cardiac exposure of 4.9-Gy reported in a recent review of 2,168 patients treated from 1958 to 2001 in Sweden and Denmark.
The findings confirm that three-dimensional conformal radiation therapy (3D-CRT) reduces cardiac exposure to radiation, Dr. Federico Lonardi and his associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. But certain areas of the heart still receive high doses when patients have adverse anatomic conditions that are not well suited to 3D-CRT. Because heart structures may differ in radiosensitivity, higher doses to small volumes of the heart, such as the coronary artery, might be associated with more risk, the researchers cautioned.
Most patients received a mean cardiac dose of 2-3 Gy (32%), 21% of patients were exposed to 1.15-1.99 Gy, and 1% got 0.8 Gy in a study of a consecutive series of breast cancer patients treated at Mater Salutis Hospital in Legnago, Italy. Only 17% of patients received a mean cardiac dose of more than 5 Gy, and 13% received 4.16-4.83 Gy.
The cardiac dose ranged from 0.8 to 13.05 Gy in Dr. Lonardi’s study, compared with a range of 0.03 to 27.72 Gy in the recently published Scandinavian study (N. Engl. J. Med. 2013;368:987-998). In the published study, the longitudinal risk for major cardiac events increased in a linear fashion, with a 7% increase for cardiac events with every 1 Gy increase in radiation to the heart.
In the Italian study, the median volumes of heart exposed to higher doses of radiation were "consistently low" with 4% of heart volumes exposed to 5 Gy or more, 3% exposed to 10Gy or more, 2% exposed to 15 Gy or more, and 0.7% exposed to 25 Gy or more Dr. Lonardi reported.
These patients received full-breast 3D-CRT with two to four customized tangential fields after mastectomy (10% of patients) or quadrantectomy (90%). The whole breast (or chest wall) received 50 Gy/25 fractions in 66 patients and 45 Gy/18 fractions in 34 patients. Boost to surgical bed (10 Gy/4-5 fractions) was delivered by photons in 10 patients. Median number of tangential fields was two (range, two to four). Patients were treated while supine on a breast board, without immobilization devices or instructions to hold their breath. They were freely breathing but were asked to minimize respiratory motion during the CT scan used to plan radiation delivery and the treatment itself. No dose constraints were specified for heart structures; a mean heart dose lower than 5 Gy was recommended at the time of treatment.
A preliminary assessment of radiation delivered to the left anterior descending coronary arteries in this series suggests that they received 9-25 Gy, Dr. Lonardi reported.
Based on estimates using previous models, the probability of death from cardiac causes within 15 years after standard fractionated radiotherapy may be less than 1% if less than 10% of the heart is exposed to 25Gy or more, he noted. "In this perspective, our results appear very favorable, though they confirm that the heart may receive high doses to limited volumes despite the use of standard 3D techniques. In such cases, high-conformal, intensity-modulated techniques are helpful" to further reduce the exposure of critical heart structures to radiation.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Lonardi reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – The hearts of 100 consecutive patients who underwent adjuvant radiotherapy for left-sided breast cancer in 2011 received an average of 2.9 Gray of radiation, considerably less than the mean cardiac exposure of 4.9-Gy reported in a recent review of 2,168 patients treated from 1958 to 2001 in Sweden and Denmark.
The findings confirm that three-dimensional conformal radiation therapy (3D-CRT) reduces cardiac exposure to radiation, Dr. Federico Lonardi and his associates reported at a breast cancer symposium sponsored by the American Society of Clinical Oncology. But certain areas of the heart still receive high doses when patients have adverse anatomic conditions that are not well suited to 3D-CRT. Because heart structures may differ in radiosensitivity, higher doses to small volumes of the heart, such as the coronary artery, might be associated with more risk, the researchers cautioned.
Most patients received a mean cardiac dose of 2-3 Gy (32%), 21% of patients were exposed to 1.15-1.99 Gy, and 1% got 0.8 Gy in a study of a consecutive series of breast cancer patients treated at Mater Salutis Hospital in Legnago, Italy. Only 17% of patients received a mean cardiac dose of more than 5 Gy, and 13% received 4.16-4.83 Gy.
The cardiac dose ranged from 0.8 to 13.05 Gy in Dr. Lonardi’s study, compared with a range of 0.03 to 27.72 Gy in the recently published Scandinavian study (N. Engl. J. Med. 2013;368:987-998). In the published study, the longitudinal risk for major cardiac events increased in a linear fashion, with a 7% increase for cardiac events with every 1 Gy increase in radiation to the heart.
In the Italian study, the median volumes of heart exposed to higher doses of radiation were "consistently low" with 4% of heart volumes exposed to 5 Gy or more, 3% exposed to 10Gy or more, 2% exposed to 15 Gy or more, and 0.7% exposed to 25 Gy or more Dr. Lonardi reported.
These patients received full-breast 3D-CRT with two to four customized tangential fields after mastectomy (10% of patients) or quadrantectomy (90%). The whole breast (or chest wall) received 50 Gy/25 fractions in 66 patients and 45 Gy/18 fractions in 34 patients. Boost to surgical bed (10 Gy/4-5 fractions) was delivered by photons in 10 patients. Median number of tangential fields was two (range, two to four). Patients were treated while supine on a breast board, without immobilization devices or instructions to hold their breath. They were freely breathing but were asked to minimize respiratory motion during the CT scan used to plan radiation delivery and the treatment itself. No dose constraints were specified for heart structures; a mean heart dose lower than 5 Gy was recommended at the time of treatment.
A preliminary assessment of radiation delivered to the left anterior descending coronary arteries in this series suggests that they received 9-25 Gy, Dr. Lonardi reported.
Based on estimates using previous models, the probability of death from cardiac causes within 15 years after standard fractionated radiotherapy may be less than 1% if less than 10% of the heart is exposed to 25Gy or more, he noted. "In this perspective, our results appear very favorable, though they confirm that the heart may receive high doses to limited volumes despite the use of standard 3D techniques. In such cases, high-conformal, intensity-modulated techniques are helpful" to further reduce the exposure of critical heart structures to radiation.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Lonardi reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: In an Italian study, the median volumes of heart exposed to higher doses of radiation were "consistently low" with 4% of heart volumes exposed to 5 Gy or more, 3% exposed to 10Gy or more, 2% exposed to 15 Gy or more, and 0.7% exposed to 25 Gy or more.
Data source: Retrospective review of 100 consecutive patients treated with radiotherapy for left-sided breast cancer at one institution in 2011.
Disclosures: Dr. Lonardi reported having no financial disclosures.
Breast cancer hormone therapy may affect cognitive function
SAN FRANCISCO – Patients with breast cancer who received hormone therapy were over seven times more likely to show cognitive decline as were untreated patients after controlling for other factors, based on a prospective study of 81 patients.
Further, objective results on neuropsychological testing tended to back up patients’ complaints of cognitive difficulties.
Hormone therapy may be a risk factor for cognitive deficits, and interventional studies should be designed to focus on this group of patients, Dr. Hope S. Rugo and her associates recommended in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The study collected neuropsychological test results and patient reports before treatment and at several points after starting hormone therapy (22 patients), chemotherapy (14), or chemotherapy followed by hormone therapy (33), and in a control group of 12 untreated patients.
Compared with baseline results, nearly 25% of patients had cognitive decline on neuropsychological testing after 5 months. (Among treated patients, this occurred 1 month after ending chemotherapy or 5 months after starting hormone therapy.) Nearly 35% had cognitive declines at 9 months of follow-up, and 30% had cognitive declines after 18 months.
"Decline in cognitive function is common in patients receiving adjuvant therapy for early-stage breast cancer," concluded Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco. "Ongoing hormone therapy appears to be a risk factor for worse cognitive function."
Other factors that did not predict cognitive decline in the multivariate analysis included age, education level, average estradiol level over time, and estimated verbal IQ at baseline.
Separate univariate conditional logistic regression analyses found that hormone therapy predicted cognitive decline with an odds ratio of 5, but chemotherapy, radiation therapy, average fatigue over time, and average depression over time were not predictive of cognitive decline at any point.
The study enrolled women aged 35-80 years with early-stage breast cancer. Those who underwent adjuvant therapy received 3-4 months of chemotherapy alone, 5 years of hormone therapy alone, or both.
A separate analysis in the same study looked at how well subjective patient reports correlated with objective measures on neuropsychological tests. Dr. Lara Heflin and her associates found significant cross-section correlations between patient-reported cognitive problems and psychological distress and fatigue.
After researchers controlled for the influence of depression and fatigue, however, significant relationships remained between patients’ perceived cognitive functioning and measurable cognitive decline from baseline (pretreatment) to the first follow-up. Patients whose scores indicated memory decline were more likely to perceive memory problems, and patients whose scores on letter fluency declined were more likely to perceive problems with verbal fluency.
"Patients who self-report cognitive problems may indeed be experiencing cognitive decline, and their self-report should not simply be attributed to fatigue or to psychological factors such as anxiety and depression," concluded Dr. Heflin, a visiting professor of psychology at New Mexico Highlands University, Las Vegas.
Patients in the study had no prior chemotherapy or central nervous system radiation and no history of major psychiatric illness, serious head injury, neurologic disease, drug or alcohol abuse, or significant medical illness. The median age was 54 years, and 78% of patients were white. Patient characteristics were similar between groups.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
The National Institutes of Health funded the study. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
On Twitter @sherryboschert
While the relationship between chemotherapy and neurocognitive changes is increasingly well described, cognitive changes associated with hormone therapy for breast cancer has proven to be somewhat complex. Chemotherapy can confound this. There are questions about different reports of outcomes for different drugs, for selective estrogen-receptor modulators and aromatase inhibitors, as well as how menopausal status, prior oophorectomy, and hormone therapy all affect cognitive changes in women.

|
|
The important points of this study are that there are measurements at baseline, 1 month, and 18 months for a longitudinal picture. In addition, both comprehensive neuropsychological testing and patient-reported outcomes are available at each of those time points.
Previous studies have assessed these relationships, including a small study of memory impairment with anastrozole vs. tamoxifen in 31 patients treated for a minimum of 3 months. There was no difference between groups in depression, anxiety and fatigue, but researchers found that those on anastrozole had significantly poorer performance on learning and memory measures than those taking tamoxifen (Menopause 2007;14:995-8).
Another small substudy compared 94 patients enrolled in the ATAC (Arimidex, Tamoxifen Alone or in Combination) study to 35 noncancer controls. There were no differences in working memory, attention and visual memory, but those on endocrine therapy had poorer performance on tests of verbal memory and processing speed compared with controls.
The IBIS II (International Breast Cancer Intervention II) prevention study comparing anastrozole vs. placebo showed no overall difference in cognitive function with the addition of anastrozole. At 6 months, those on anastrozole had poorer memory, but this finding resolved by 24 months.
In a subset of 179 patients in the TEAM (Tamoxifen Exemestane Adjuvant Multinational) phase III adjuvant study, comparing exemestane with tamoxifen then exemestane, there was little change from baseline to 1 year in all domains of cognitive function with the addition of exemestane.
A substudy done from the BIG 1-98 (Breast International Group 1-98) trial indicated significant improvement in cognitive function from year 5 of therapy to year 6 after cessation of hormone therapies. Interestingly, perceived cognition did not change in the patients.
This finding corroborates similar results from a prospective, longitudinal study of cognitive complaints and neuropsychological testing for verbal memory, psychomotor speed, and executive functioning in 189 patients who were beginning hormone therapy at the University of California, Los Angeles. (J. Natl. Cancer Inst. 2013;105:791-801).
While the relationship between chemotherapy and neurocognitive changes is increasingly well described, cognitive changes associated with hormone therapy for breast cancer has proven to be somewhat complex. Chemotherapy can confound this. There are questions about different reports of outcomes for different drugs, for selective estrogen-receptor modulators and aromatase inhibitors, as well as how menopausal status, prior oophorectomy, and hormone therapy all affect cognitive changes in women.

|
|
The important points of this study are that there are measurements at baseline, 1 month, and 18 months for a longitudinal picture. In addition, both comprehensive neuropsychological testing and patient-reported outcomes are available at each of those time points.
Previous studies have assessed these relationships, including a small study of memory impairment with anastrozole vs. tamoxifen in 31 patients treated for a minimum of 3 months. There was no difference between groups in depression, anxiety and fatigue, but researchers found that those on anastrozole had significantly poorer performance on learning and memory measures than those taking tamoxifen (Menopause 2007;14:995-8).
Another small substudy compared 94 patients enrolled in the ATAC (Arimidex, Tamoxifen Alone or in Combination) study to 35 noncancer controls. There were no differences in working memory, attention and visual memory, but those on endocrine therapy had poorer performance on tests of verbal memory and processing speed compared with controls.
The IBIS II (International Breast Cancer Intervention II) prevention study comparing anastrozole vs. placebo showed no overall difference in cognitive function with the addition of anastrozole. At 6 months, those on anastrozole had poorer memory, but this finding resolved by 24 months.
In a subset of 179 patients in the TEAM (Tamoxifen Exemestane Adjuvant Multinational) phase III adjuvant study, comparing exemestane with tamoxifen then exemestane, there was little change from baseline to 1 year in all domains of cognitive function with the addition of exemestane.
A substudy done from the BIG 1-98 (Breast International Group 1-98) trial indicated significant improvement in cognitive function from year 5 of therapy to year 6 after cessation of hormone therapies. Interestingly, perceived cognition did not change in the patients.
This finding corroborates similar results from a prospective, longitudinal study of cognitive complaints and neuropsychological testing for verbal memory, psychomotor speed, and executive functioning in 189 patients who were beginning hormone therapy at the University of California, Los Angeles. (J. Natl. Cancer Inst. 2013;105:791-801).
While the relationship between chemotherapy and neurocognitive changes is increasingly well described, cognitive changes associated with hormone therapy for breast cancer has proven to be somewhat complex. Chemotherapy can confound this. There are questions about different reports of outcomes for different drugs, for selective estrogen-receptor modulators and aromatase inhibitors, as well as how menopausal status, prior oophorectomy, and hormone therapy all affect cognitive changes in women.

|
|
The important points of this study are that there are measurements at baseline, 1 month, and 18 months for a longitudinal picture. In addition, both comprehensive neuropsychological testing and patient-reported outcomes are available at each of those time points.
Previous studies have assessed these relationships, including a small study of memory impairment with anastrozole vs. tamoxifen in 31 patients treated for a minimum of 3 months. There was no difference between groups in depression, anxiety and fatigue, but researchers found that those on anastrozole had significantly poorer performance on learning and memory measures than those taking tamoxifen (Menopause 2007;14:995-8).
Another small substudy compared 94 patients enrolled in the ATAC (Arimidex, Tamoxifen Alone or in Combination) study to 35 noncancer controls. There were no differences in working memory, attention and visual memory, but those on endocrine therapy had poorer performance on tests of verbal memory and processing speed compared with controls.
The IBIS II (International Breast Cancer Intervention II) prevention study comparing anastrozole vs. placebo showed no overall difference in cognitive function with the addition of anastrozole. At 6 months, those on anastrozole had poorer memory, but this finding resolved by 24 months.
In a subset of 179 patients in the TEAM (Tamoxifen Exemestane Adjuvant Multinational) phase III adjuvant study, comparing exemestane with tamoxifen then exemestane, there was little change from baseline to 1 year in all domains of cognitive function with the addition of exemestane.
A substudy done from the BIG 1-98 (Breast International Group 1-98) trial indicated significant improvement in cognitive function from year 5 of therapy to year 6 after cessation of hormone therapies. Interestingly, perceived cognition did not change in the patients.
This finding corroborates similar results from a prospective, longitudinal study of cognitive complaints and neuropsychological testing for verbal memory, psychomotor speed, and executive functioning in 189 patients who were beginning hormone therapy at the University of California, Los Angeles. (J. Natl. Cancer Inst. 2013;105:791-801).
SAN FRANCISCO – Patients with breast cancer who received hormone therapy were over seven times more likely to show cognitive decline as were untreated patients after controlling for other factors, based on a prospective study of 81 patients.
Further, objective results on neuropsychological testing tended to back up patients’ complaints of cognitive difficulties.
Hormone therapy may be a risk factor for cognitive deficits, and interventional studies should be designed to focus on this group of patients, Dr. Hope S. Rugo and her associates recommended in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The study collected neuropsychological test results and patient reports before treatment and at several points after starting hormone therapy (22 patients), chemotherapy (14), or chemotherapy followed by hormone therapy (33), and in a control group of 12 untreated patients.
Compared with baseline results, nearly 25% of patients had cognitive decline on neuropsychological testing after 5 months. (Among treated patients, this occurred 1 month after ending chemotherapy or 5 months after starting hormone therapy.) Nearly 35% had cognitive declines at 9 months of follow-up, and 30% had cognitive declines after 18 months.
"Decline in cognitive function is common in patients receiving adjuvant therapy for early-stage breast cancer," concluded Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco. "Ongoing hormone therapy appears to be a risk factor for worse cognitive function."
Other factors that did not predict cognitive decline in the multivariate analysis included age, education level, average estradiol level over time, and estimated verbal IQ at baseline.
Separate univariate conditional logistic regression analyses found that hormone therapy predicted cognitive decline with an odds ratio of 5, but chemotherapy, radiation therapy, average fatigue over time, and average depression over time were not predictive of cognitive decline at any point.
The study enrolled women aged 35-80 years with early-stage breast cancer. Those who underwent adjuvant therapy received 3-4 months of chemotherapy alone, 5 years of hormone therapy alone, or both.
A separate analysis in the same study looked at how well subjective patient reports correlated with objective measures on neuropsychological tests. Dr. Lara Heflin and her associates found significant cross-section correlations between patient-reported cognitive problems and psychological distress and fatigue.
After researchers controlled for the influence of depression and fatigue, however, significant relationships remained between patients’ perceived cognitive functioning and measurable cognitive decline from baseline (pretreatment) to the first follow-up. Patients whose scores indicated memory decline were more likely to perceive memory problems, and patients whose scores on letter fluency declined were more likely to perceive problems with verbal fluency.
"Patients who self-report cognitive problems may indeed be experiencing cognitive decline, and their self-report should not simply be attributed to fatigue or to psychological factors such as anxiety and depression," concluded Dr. Heflin, a visiting professor of psychology at New Mexico Highlands University, Las Vegas.
Patients in the study had no prior chemotherapy or central nervous system radiation and no history of major psychiatric illness, serious head injury, neurologic disease, drug or alcohol abuse, or significant medical illness. The median age was 54 years, and 78% of patients were white. Patient characteristics were similar between groups.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
The National Institutes of Health funded the study. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Patients with breast cancer who received hormone therapy were over seven times more likely to show cognitive decline as were untreated patients after controlling for other factors, based on a prospective study of 81 patients.
Further, objective results on neuropsychological testing tended to back up patients’ complaints of cognitive difficulties.
Hormone therapy may be a risk factor for cognitive deficits, and interventional studies should be designed to focus on this group of patients, Dr. Hope S. Rugo and her associates recommended in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The study collected neuropsychological test results and patient reports before treatment and at several points after starting hormone therapy (22 patients), chemotherapy (14), or chemotherapy followed by hormone therapy (33), and in a control group of 12 untreated patients.
Compared with baseline results, nearly 25% of patients had cognitive decline on neuropsychological testing after 5 months. (Among treated patients, this occurred 1 month after ending chemotherapy or 5 months after starting hormone therapy.) Nearly 35% had cognitive declines at 9 months of follow-up, and 30% had cognitive declines after 18 months.
"Decline in cognitive function is common in patients receiving adjuvant therapy for early-stage breast cancer," concluded Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco. "Ongoing hormone therapy appears to be a risk factor for worse cognitive function."
Other factors that did not predict cognitive decline in the multivariate analysis included age, education level, average estradiol level over time, and estimated verbal IQ at baseline.
Separate univariate conditional logistic regression analyses found that hormone therapy predicted cognitive decline with an odds ratio of 5, but chemotherapy, radiation therapy, average fatigue over time, and average depression over time were not predictive of cognitive decline at any point.
The study enrolled women aged 35-80 years with early-stage breast cancer. Those who underwent adjuvant therapy received 3-4 months of chemotherapy alone, 5 years of hormone therapy alone, or both.
A separate analysis in the same study looked at how well subjective patient reports correlated with objective measures on neuropsychological tests. Dr. Lara Heflin and her associates found significant cross-section correlations between patient-reported cognitive problems and psychological distress and fatigue.
After researchers controlled for the influence of depression and fatigue, however, significant relationships remained between patients’ perceived cognitive functioning and measurable cognitive decline from baseline (pretreatment) to the first follow-up. Patients whose scores indicated memory decline were more likely to perceive memory problems, and patients whose scores on letter fluency declined were more likely to perceive problems with verbal fluency.
"Patients who self-report cognitive problems may indeed be experiencing cognitive decline, and their self-report should not simply be attributed to fatigue or to psychological factors such as anxiety and depression," concluded Dr. Heflin, a visiting professor of psychology at New Mexico Highlands University, Las Vegas.
Patients in the study had no prior chemotherapy or central nervous system radiation and no history of major psychiatric illness, serious head injury, neurologic disease, drug or alcohol abuse, or significant medical illness. The median age was 54 years, and 78% of patients were white. Patient characteristics were similar between groups.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
The National Institutes of Health funded the study. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: The odds of any decline in cognitive function were significantly higher in patients on hormone therapy (OR, 7.7), compared with untreated patients in a multivariate conditional logistic regression analysis.
Data source: Prospective longitudinal study of 81 patients with breast cancer treated with hormone therapy (22 patients), chemotherapy (14), chemotherapy followed by hormone therapy (33), or no therapy (12).
Disclosures: NIH funded the study. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
Tumor distance from nipple boosts nomogram results
SAN FRANCISCO – The distance of a breast cancer from a woman’s nipple added to the ability of nomograms to predict sentinel lymph node positivity, based on a study of data from 401 breast cancers.
The data came from 395 patients who had clinical stage T1 (85% of the cohort) or T2 tumors and underwent prebiopsy ultrasounds at the Mayo Clinic, Rochester, Minn. The investigators performed a second review of the images to measure the tumors’ distance from the nipple.
Nomograms published online by the Memorial Sloan-Kettering Cancer Center and by the University of Texas M.D. Anderson Cancer Center do a reasonably good job of discriminating which newly diagnosed breast cancer patients who have not undergone neoadjuvant therapy are likely to have positive and negative sentinel nodes. Using those two nomograms alone on the patients in the study produced an area under the curve (AUC) of 0.71 for the Memorial Sloan-Kettering nomogram and 0.74 for the M.D. Anderson nomogram. Adding a tumor-to-nipple distance of 2 cm or less improved the AUCs to 0.73 and 0.76, respectively, Dr. Miraj Shah-Khan and her coinvestigators reported.
Sentinel lymph nodes were positive in 20% of the 401 tumors; 17 of 33 tumors within 2 cm of the nipple had positive sentinel lymph nodes (52%), compared with 17% of 368 tumors farther than 2 cm from the nipple, Dr. Shah-Khan, of the Medical College of Wisconsin, Milwaukee, and her associates reported in a poster presented at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In each subgroup of probability predicted by the nomograms, tumor-to-nipple distance helped to refine the probability of a positive node.
When the Memorial Sloan-Kettering nomogram predicted less than a 25% chance of node positivity, adding a tumor-to-nipple distance of 2 cm or less changed the probability to 35%, compared with 11% if the distance was greater than 2 cm. A probability of 25%-49% from the same nomogram changed to 62% if a tumor was within 2 cm of the nipple or 29% for tumors farther from the nipple. A probability of 50% or greater from the nomogram changed to 100% if a tumor was within 2 cm of the nipple or 32% if it was farther away.
When the M.D. Anderson nomogram predicted less than a 25% chance of node positivity, adding a tumor-to-nipple distance of 2 cm or less changed the probability to 42%, compared with 14% if the distance was greater than 2 cm. A probability of 25%-49% from the same nomogram changed to 83% if a tumor was within 2 cm of the nipple or 40% for tumors farther from the nipple. A probability of 50% or greater from the nomogram changed to 100% if a tumor was within 2 cm of the nipple or 67% if it was farther away, she reported.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Shah-Khan reported having no financial disclosures.
On Twitter @sherryboschert
The Mayo Clinic group has previously proposed that the tumor’s distance from the nipple is a predictive factor of whether there is tumor in the sentinel node. There are data showing that the distance from the nipple affects the positivity rate.

|
|
This study factors in the M.D. Anderson and the Memorial Sloan-Kettering nomograms to create a more robust model for predicting sentinel node positivity. The distance from the nipple and the distance from the skin, when used in addition to the nomogram, increase the predictive ability.
If you are a user of nomograms or if you are a selective user of nomograms for some patients with invasive breast cancer who are still on the fence about whether or not they should undergo a sentinel node procedure, this additional measure gives you a bit more ability to predict the positivity rate with more certainty. I’m a very selective user of nomograms because the individual patient in front of me is not a statistic. For me, nomograms become useful only for those patients who are fence-sitters. It is not a routine part of my practice.
Dr. David W. Ollila is a professor of surgical oncology at the University of North Carolina, Chapel Hill. He reported having no financial disclosures.
The Mayo Clinic group has previously proposed that the tumor’s distance from the nipple is a predictive factor of whether there is tumor in the sentinel node. There are data showing that the distance from the nipple affects the positivity rate.

|
|
This study factors in the M.D. Anderson and the Memorial Sloan-Kettering nomograms to create a more robust model for predicting sentinel node positivity. The distance from the nipple and the distance from the skin, when used in addition to the nomogram, increase the predictive ability.
If you are a user of nomograms or if you are a selective user of nomograms for some patients with invasive breast cancer who are still on the fence about whether or not they should undergo a sentinel node procedure, this additional measure gives you a bit more ability to predict the positivity rate with more certainty. I’m a very selective user of nomograms because the individual patient in front of me is not a statistic. For me, nomograms become useful only for those patients who are fence-sitters. It is not a routine part of my practice.
Dr. David W. Ollila is a professor of surgical oncology at the University of North Carolina, Chapel Hill. He reported having no financial disclosures.
The Mayo Clinic group has previously proposed that the tumor’s distance from the nipple is a predictive factor of whether there is tumor in the sentinel node. There are data showing that the distance from the nipple affects the positivity rate.

|
|
This study factors in the M.D. Anderson and the Memorial Sloan-Kettering nomograms to create a more robust model for predicting sentinel node positivity. The distance from the nipple and the distance from the skin, when used in addition to the nomogram, increase the predictive ability.
If you are a user of nomograms or if you are a selective user of nomograms for some patients with invasive breast cancer who are still on the fence about whether or not they should undergo a sentinel node procedure, this additional measure gives you a bit more ability to predict the positivity rate with more certainty. I’m a very selective user of nomograms because the individual patient in front of me is not a statistic. For me, nomograms become useful only for those patients who are fence-sitters. It is not a routine part of my practice.
Dr. David W. Ollila is a professor of surgical oncology at the University of North Carolina, Chapel Hill. He reported having no financial disclosures.
SAN FRANCISCO – The distance of a breast cancer from a woman’s nipple added to the ability of nomograms to predict sentinel lymph node positivity, based on a study of data from 401 breast cancers.
The data came from 395 patients who had clinical stage T1 (85% of the cohort) or T2 tumors and underwent prebiopsy ultrasounds at the Mayo Clinic, Rochester, Minn. The investigators performed a second review of the images to measure the tumors’ distance from the nipple.
Nomograms published online by the Memorial Sloan-Kettering Cancer Center and by the University of Texas M.D. Anderson Cancer Center do a reasonably good job of discriminating which newly diagnosed breast cancer patients who have not undergone neoadjuvant therapy are likely to have positive and negative sentinel nodes. Using those two nomograms alone on the patients in the study produced an area under the curve (AUC) of 0.71 for the Memorial Sloan-Kettering nomogram and 0.74 for the M.D. Anderson nomogram. Adding a tumor-to-nipple distance of 2 cm or less improved the AUCs to 0.73 and 0.76, respectively, Dr. Miraj Shah-Khan and her coinvestigators reported.
Sentinel lymph nodes were positive in 20% of the 401 tumors; 17 of 33 tumors within 2 cm of the nipple had positive sentinel lymph nodes (52%), compared with 17% of 368 tumors farther than 2 cm from the nipple, Dr. Shah-Khan, of the Medical College of Wisconsin, Milwaukee, and her associates reported in a poster presented at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In each subgroup of probability predicted by the nomograms, tumor-to-nipple distance helped to refine the probability of a positive node.
When the Memorial Sloan-Kettering nomogram predicted less than a 25% chance of node positivity, adding a tumor-to-nipple distance of 2 cm or less changed the probability to 35%, compared with 11% if the distance was greater than 2 cm. A probability of 25%-49% from the same nomogram changed to 62% if a tumor was within 2 cm of the nipple or 29% for tumors farther from the nipple. A probability of 50% or greater from the nomogram changed to 100% if a tumor was within 2 cm of the nipple or 32% if it was farther away.
When the M.D. Anderson nomogram predicted less than a 25% chance of node positivity, adding a tumor-to-nipple distance of 2 cm or less changed the probability to 42%, compared with 14% if the distance was greater than 2 cm. A probability of 25%-49% from the same nomogram changed to 83% if a tumor was within 2 cm of the nipple or 40% for tumors farther from the nipple. A probability of 50% or greater from the nomogram changed to 100% if a tumor was within 2 cm of the nipple or 67% if it was farther away, she reported.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Shah-Khan reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – The distance of a breast cancer from a woman’s nipple added to the ability of nomograms to predict sentinel lymph node positivity, based on a study of data from 401 breast cancers.
The data came from 395 patients who had clinical stage T1 (85% of the cohort) or T2 tumors and underwent prebiopsy ultrasounds at the Mayo Clinic, Rochester, Minn. The investigators performed a second review of the images to measure the tumors’ distance from the nipple.
Nomograms published online by the Memorial Sloan-Kettering Cancer Center and by the University of Texas M.D. Anderson Cancer Center do a reasonably good job of discriminating which newly diagnosed breast cancer patients who have not undergone neoadjuvant therapy are likely to have positive and negative sentinel nodes. Using those two nomograms alone on the patients in the study produced an area under the curve (AUC) of 0.71 for the Memorial Sloan-Kettering nomogram and 0.74 for the M.D. Anderson nomogram. Adding a tumor-to-nipple distance of 2 cm or less improved the AUCs to 0.73 and 0.76, respectively, Dr. Miraj Shah-Khan and her coinvestigators reported.
Sentinel lymph nodes were positive in 20% of the 401 tumors; 17 of 33 tumors within 2 cm of the nipple had positive sentinel lymph nodes (52%), compared with 17% of 368 tumors farther than 2 cm from the nipple, Dr. Shah-Khan, of the Medical College of Wisconsin, Milwaukee, and her associates reported in a poster presented at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In each subgroup of probability predicted by the nomograms, tumor-to-nipple distance helped to refine the probability of a positive node.
When the Memorial Sloan-Kettering nomogram predicted less than a 25% chance of node positivity, adding a tumor-to-nipple distance of 2 cm or less changed the probability to 35%, compared with 11% if the distance was greater than 2 cm. A probability of 25%-49% from the same nomogram changed to 62% if a tumor was within 2 cm of the nipple or 29% for tumors farther from the nipple. A probability of 50% or greater from the nomogram changed to 100% if a tumor was within 2 cm of the nipple or 32% if it was farther away.
When the M.D. Anderson nomogram predicted less than a 25% chance of node positivity, adding a tumor-to-nipple distance of 2 cm or less changed the probability to 42%, compared with 14% if the distance was greater than 2 cm. A probability of 25%-49% from the same nomogram changed to 83% if a tumor was within 2 cm of the nipple or 40% for tumors farther from the nipple. A probability of 50% or greater from the nomogram changed to 100% if a tumor was within 2 cm of the nipple or 67% if it was farther away, she reported.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Dr. Shah-Khan reported having no financial disclosures.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: Positive sentinel nodes were seen in 17 of 33 (52%) tumors within 2 cm of the nipple, compared with 17% of 368 tumors more than 2 cm from the nipple.
Data source: A second review of prebiopsy ultrasound images of 401 clinical T1 or T2 breast cancer tumors in 395 patients and comparison with nomograms for predicting sentinel node positivity.
Disclosures: Dr. Shah-Khan reported having no financial disclosures.
Oral therapy for metastatic breast cancer grows in Medicare patients
SAN FRANCISCO – Injectable fulvestrant and oral anastrozole were the top picks for first-, second- and third-line treatment of metastatic breast cancer in women enrolled in Medicare Part D, based on a retrospective study of 681 women.
Total treatment costs averaged $102,000/patient, including costs for physicians, inpatient and outpatient care, hospice, skilled nursing, and durable medical equipment, reported Dr. Hope S. Rugo and her associates.
Their analysis updates a similar previous study that did not include data on oral medications. Thus, this study provides a more complete picture of treatment patterns and costs for patients with metastatic breast cancer. Oral medications now comprise three of the five most common first-, second- or third-line therapies in women who developed metastatic breast cancer, according to the researchers.
The investigators analyzed SEER (Surveillance Epidemiology and End Results) cancer registry data and Medicare data for 7,905 women who were diagnosed with breast cancer in 2001-2007 with concurrent or subsequent metastases and were enrolled in Medicare from 12 months prior to diagnosis through 2009 or death. Of these, 82% received first-line treatment. Data on oral therapies were available only for the 681 patients enrolled in Medicare Part D, the federal program that subsidizes the costs of prescription drugs for Medicare beneficiaries.
In the overall sample, 48% of the women went on to second-line therapy and 26% had third-line therapy. In the Part D subgroup, 63% went on to second-line therapy and 45% had third-line therapy, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
The mean total cost per patient in the cohort as a whole was $127,000. Fulvestrant was the top choice for first-line therapy, used in 19%. Injectable vinorelbine was the most common second-line chemotherapy, used in 18% of those who got second-line treatment and in 16% of those who got third-line therapy, she reported in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The findings were similar to those from a previous report presented by another group of investigators at the 2011 Breast Cancer Symposium. Those researchers used SEER data on women diagnosed in 2001-2005 and enrolled in Medicare until 2008 or death. Fulvestrant was most common as first-line therapy and vinorelbine was most common for second- and third-line treatment. The mean per-patient cost in that study was $110,000.
In the current analysis of the 681 patients enrolled in Medicare Part D, fulvestrant still was the most common first-line treatment given to 9.1% of patients, but oral anastrozole was a close second at 8.7%. The next most common choices were oral letrozole (7%), any taxane drug (5%), and oral tamoxifen (4%).
Of the 427 patients in the Part D subgroup who got second-line therapy, 19% received fulvestrant, 9% got anastrozole, 8% got letrozole, 7% received vinorelbine, and 4% took tamoxifen.
For third-line treatment of 309 patients in the Part D subgroup, anastrozole and fulvestrant tied for top choice (11% each), tamoxifen or letrozole was used in 7% each, and vinorelbine was used in 6%.
The total costs per drug were highest for vinorelbine: a mean of $155,000 in second-line treatment and $134,000 in third-line treatment. In comparison, mean costs for the other top second-line therapies were $111,000 for fulvestrant, $106,000 for anastrozole, $108,000 for letrozole, and $107,000 for tamoxifen. Mean costs for other third-line treatments were $110,000 for anastrozole, $100,000 for fulvestrant, $101,000 for tamoxifen, and $71,000 for letrozole.
The study excluded patients with a history of other cancers before a diagnosis of breast cancer, patients who were not eligible for Medicare Part A or Part B benefits, patients enrolled in health maintenance organizations, and those who were first diagnosed with metastatic breast cancer at the time of death or autopsy.
The data did not identify disease progression, so the investigators used a published algorithm to identify the date of metastases. They also created an algorithm to identify first-, second- and third-line treatments in the data, based on factors such as the length of time before administering a new agent. These methods may have misclassified some treatments. Other limitations of the study include using Medicare data from 2001-2007, which may not reflect recent advances in treatment. SEER data cover just 28% of the U.S. population, and 90% of the study population was 65 years in age or older, so the cohort may not be representative of all patients with metastatic breast cancer.
The symposium was co-sponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Eisai funded the study and one of the investigators was an employee of the company. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Injectable fulvestrant and oral anastrozole were the top picks for first-, second- and third-line treatment of metastatic breast cancer in women enrolled in Medicare Part D, based on a retrospective study of 681 women.
Total treatment costs averaged $102,000/patient, including costs for physicians, inpatient and outpatient care, hospice, skilled nursing, and durable medical equipment, reported Dr. Hope S. Rugo and her associates.
Their analysis updates a similar previous study that did not include data on oral medications. Thus, this study provides a more complete picture of treatment patterns and costs for patients with metastatic breast cancer. Oral medications now comprise three of the five most common first-, second- or third-line therapies in women who developed metastatic breast cancer, according to the researchers.
The investigators analyzed SEER (Surveillance Epidemiology and End Results) cancer registry data and Medicare data for 7,905 women who were diagnosed with breast cancer in 2001-2007 with concurrent or subsequent metastases and were enrolled in Medicare from 12 months prior to diagnosis through 2009 or death. Of these, 82% received first-line treatment. Data on oral therapies were available only for the 681 patients enrolled in Medicare Part D, the federal program that subsidizes the costs of prescription drugs for Medicare beneficiaries.
In the overall sample, 48% of the women went on to second-line therapy and 26% had third-line therapy. In the Part D subgroup, 63% went on to second-line therapy and 45% had third-line therapy, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
The mean total cost per patient in the cohort as a whole was $127,000. Fulvestrant was the top choice for first-line therapy, used in 19%. Injectable vinorelbine was the most common second-line chemotherapy, used in 18% of those who got second-line treatment and in 16% of those who got third-line therapy, she reported in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The findings were similar to those from a previous report presented by another group of investigators at the 2011 Breast Cancer Symposium. Those researchers used SEER data on women diagnosed in 2001-2005 and enrolled in Medicare until 2008 or death. Fulvestrant was most common as first-line therapy and vinorelbine was most common for second- and third-line treatment. The mean per-patient cost in that study was $110,000.
In the current analysis of the 681 patients enrolled in Medicare Part D, fulvestrant still was the most common first-line treatment given to 9.1% of patients, but oral anastrozole was a close second at 8.7%. The next most common choices were oral letrozole (7%), any taxane drug (5%), and oral tamoxifen (4%).
Of the 427 patients in the Part D subgroup who got second-line therapy, 19% received fulvestrant, 9% got anastrozole, 8% got letrozole, 7% received vinorelbine, and 4% took tamoxifen.
For third-line treatment of 309 patients in the Part D subgroup, anastrozole and fulvestrant tied for top choice (11% each), tamoxifen or letrozole was used in 7% each, and vinorelbine was used in 6%.
The total costs per drug were highest for vinorelbine: a mean of $155,000 in second-line treatment and $134,000 in third-line treatment. In comparison, mean costs for the other top second-line therapies were $111,000 for fulvestrant, $106,000 for anastrozole, $108,000 for letrozole, and $107,000 for tamoxifen. Mean costs for other third-line treatments were $110,000 for anastrozole, $100,000 for fulvestrant, $101,000 for tamoxifen, and $71,000 for letrozole.
The study excluded patients with a history of other cancers before a diagnosis of breast cancer, patients who were not eligible for Medicare Part A or Part B benefits, patients enrolled in health maintenance organizations, and those who were first diagnosed with metastatic breast cancer at the time of death or autopsy.
The data did not identify disease progression, so the investigators used a published algorithm to identify the date of metastases. They also created an algorithm to identify first-, second- and third-line treatments in the data, based on factors such as the length of time before administering a new agent. These methods may have misclassified some treatments. Other limitations of the study include using Medicare data from 2001-2007, which may not reflect recent advances in treatment. SEER data cover just 28% of the U.S. population, and 90% of the study population was 65 years in age or older, so the cohort may not be representative of all patients with metastatic breast cancer.
The symposium was co-sponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Eisai funded the study and one of the investigators was an employee of the company. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – Injectable fulvestrant and oral anastrozole were the top picks for first-, second- and third-line treatment of metastatic breast cancer in women enrolled in Medicare Part D, based on a retrospective study of 681 women.
Total treatment costs averaged $102,000/patient, including costs for physicians, inpatient and outpatient care, hospice, skilled nursing, and durable medical equipment, reported Dr. Hope S. Rugo and her associates.
Their analysis updates a similar previous study that did not include data on oral medications. Thus, this study provides a more complete picture of treatment patterns and costs for patients with metastatic breast cancer. Oral medications now comprise three of the five most common first-, second- or third-line therapies in women who developed metastatic breast cancer, according to the researchers.
The investigators analyzed SEER (Surveillance Epidemiology and End Results) cancer registry data and Medicare data for 7,905 women who were diagnosed with breast cancer in 2001-2007 with concurrent or subsequent metastases and were enrolled in Medicare from 12 months prior to diagnosis through 2009 or death. Of these, 82% received first-line treatment. Data on oral therapies were available only for the 681 patients enrolled in Medicare Part D, the federal program that subsidizes the costs of prescription drugs for Medicare beneficiaries.
In the overall sample, 48% of the women went on to second-line therapy and 26% had third-line therapy. In the Part D subgroup, 63% went on to second-line therapy and 45% had third-line therapy, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
The mean total cost per patient in the cohort as a whole was $127,000. Fulvestrant was the top choice for first-line therapy, used in 19%. Injectable vinorelbine was the most common second-line chemotherapy, used in 18% of those who got second-line treatment and in 16% of those who got third-line therapy, she reported in a poster presentation at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
The findings were similar to those from a previous report presented by another group of investigators at the 2011 Breast Cancer Symposium. Those researchers used SEER data on women diagnosed in 2001-2005 and enrolled in Medicare until 2008 or death. Fulvestrant was most common as first-line therapy and vinorelbine was most common for second- and third-line treatment. The mean per-patient cost in that study was $110,000.
In the current analysis of the 681 patients enrolled in Medicare Part D, fulvestrant still was the most common first-line treatment given to 9.1% of patients, but oral anastrozole was a close second at 8.7%. The next most common choices were oral letrozole (7%), any taxane drug (5%), and oral tamoxifen (4%).
Of the 427 patients in the Part D subgroup who got second-line therapy, 19% received fulvestrant, 9% got anastrozole, 8% got letrozole, 7% received vinorelbine, and 4% took tamoxifen.
For third-line treatment of 309 patients in the Part D subgroup, anastrozole and fulvestrant tied for top choice (11% each), tamoxifen or letrozole was used in 7% each, and vinorelbine was used in 6%.
The total costs per drug were highest for vinorelbine: a mean of $155,000 in second-line treatment and $134,000 in third-line treatment. In comparison, mean costs for the other top second-line therapies were $111,000 for fulvestrant, $106,000 for anastrozole, $108,000 for letrozole, and $107,000 for tamoxifen. Mean costs for other third-line treatments were $110,000 for anastrozole, $100,000 for fulvestrant, $101,000 for tamoxifen, and $71,000 for letrozole.
The study excluded patients with a history of other cancers before a diagnosis of breast cancer, patients who were not eligible for Medicare Part A or Part B benefits, patients enrolled in health maintenance organizations, and those who were first diagnosed with metastatic breast cancer at the time of death or autopsy.
The data did not identify disease progression, so the investigators used a published algorithm to identify the date of metastases. They also created an algorithm to identify first-, second- and third-line treatments in the data, based on factors such as the length of time before administering a new agent. These methods may have misclassified some treatments. Other limitations of the study include using Medicare data from 2001-2007, which may not reflect recent advances in treatment. SEER data cover just 28% of the U.S. population, and 90% of the study population was 65 years in age or older, so the cohort may not be representative of all patients with metastatic breast cancer.
The symposium was co-sponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Eisai funded the study and one of the investigators was an employee of the company. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: In patients enrolled in Medicare Part D, fulvestrant was the most common first-line treatment given to 9.1% of patients, but oral anastrozole was a close second at 8.7%. The next most common choices were oral letrozole (7%), any taxane drug (5%), and oral tamoxifen (4%).
Data source: A retrospective study of 681 women who were diagnosed in 2001-2007 with breast cancer, had concurrent or subsequent metastases, and were enrolled in Medicare Part D until 2009 or death.
Disclosures: Eisai funded the study and one of the investigators was an employee of the company. Dr. Rugo reported having financial associations with Merck, Novartis, and Pfizer.
Everolimus effective in women with early failure of adjuvant therapy
SAN FRANCISCO – In patients who had hormone receptor–positive, HER2-negative advanced breast cancer, everolimus plus exemestane was just as effective among women who had a recurrence during or within a year of adjuvant therapy.
The results were seen in a secondary, subgroup analysis of 137 patients in the BOLERO-2 (Breast Cancer Trials of Oral Everolimus 2) study. The subset that had recurrences during or within a year of adjuvant therapy comprised 19% of the patients in the study, and their outcomes were similar to the overall results of the trial, which support the use of everolimus plus exemestane as first-line therapy in postmenopausal patients with hormone receptor–positive advanced breast cancer that recurs after adjuvant therapy, Dr. Hope S. Rugo and her associates reported in a poster session at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In the women who had a recurrence during or within 12 months of adjuvant therapy, everolimus plus exemestane improved median progression-free survival to 11.5 months, compared with 4.1 months with placebo plus exemestane.
The multicenter, double-blind BOLERO-2 study randomized 724 postmenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer that had recurred or progressed despite nonsteroidal aromatase inhibitor therapy. Patients received open-label exemestane plus blinded therapy with either placebo or everolimus, which inhibits the mammalian target of rapamycin (mTOR) signaling pathway. At 18 months of follow-up, patients in the exemestane-plus-everolimus group had a significantly longer median progression-free survival of 11 months as compared to 4 months for the control group (N. Engl. J. Med. 2012;366:520-529).
Similar efficacy was found in various analyses of subgroups in the study, including patients with visceral metastases, patients with bone-only metastases, and patients whose disease recurred after adjuvant therapy.
In the subgroup analysis of patients who entered the study after a recurrence during or within a year of adjuvant therapy, 100 patients received everolimus plus exemestane and 37 received placebo and exemestane. Almost all of the 137 patients had received nonsteroidal aromatase inhibitor therapy as their last therapy before entering the BOLERO-2 trial.
The median progression-free survival rates of 11.5 months with the everolimus combination and 4.1 months with the placebo combination were based on investigators’ local radiologic assessments. Central radiologic assessment by an independent radiology committee confirmed these results, finding that median progression-free survival reached 15.2 months in the everolimus group, compared with 4.2 months in the placebo group. Hazard ratios were 0.39 under the local assessment and 0.32 under the central assessment, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
Rates of the most common grade 3 or 4 adverse events seen in the everolimus-plus-exemestane group in the subset analysis – hyperglycemia in 8%, stomatitis in 4%, diarrhea in 4%, and fatigue in 3% – were similar to rates seen in the overall BOLERO-2 population and were within the known safety profile of everolimus. Some patients in the everolimus group (but not the placebo group) developed pneumonitis or interstitial lung disease, but these were mostly low grade and manageable by conventional strategies.
Baseline characteristics were well balanced in the randomized groups in the subset analysis.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Novartis Pharmaceuticals, which markets everolimus, funded the study, and some of the investigators were company employees. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – In patients who had hormone receptor–positive, HER2-negative advanced breast cancer, everolimus plus exemestane was just as effective among women who had a recurrence during or within a year of adjuvant therapy.
The results were seen in a secondary, subgroup analysis of 137 patients in the BOLERO-2 (Breast Cancer Trials of Oral Everolimus 2) study. The subset that had recurrences during or within a year of adjuvant therapy comprised 19% of the patients in the study, and their outcomes were similar to the overall results of the trial, which support the use of everolimus plus exemestane as first-line therapy in postmenopausal patients with hormone receptor–positive advanced breast cancer that recurs after adjuvant therapy, Dr. Hope S. Rugo and her associates reported in a poster session at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In the women who had a recurrence during or within 12 months of adjuvant therapy, everolimus plus exemestane improved median progression-free survival to 11.5 months, compared with 4.1 months with placebo plus exemestane.
The multicenter, double-blind BOLERO-2 study randomized 724 postmenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer that had recurred or progressed despite nonsteroidal aromatase inhibitor therapy. Patients received open-label exemestane plus blinded therapy with either placebo or everolimus, which inhibits the mammalian target of rapamycin (mTOR) signaling pathway. At 18 months of follow-up, patients in the exemestane-plus-everolimus group had a significantly longer median progression-free survival of 11 months as compared to 4 months for the control group (N. Engl. J. Med. 2012;366:520-529).
Similar efficacy was found in various analyses of subgroups in the study, including patients with visceral metastases, patients with bone-only metastases, and patients whose disease recurred after adjuvant therapy.
In the subgroup analysis of patients who entered the study after a recurrence during or within a year of adjuvant therapy, 100 patients received everolimus plus exemestane and 37 received placebo and exemestane. Almost all of the 137 patients had received nonsteroidal aromatase inhibitor therapy as their last therapy before entering the BOLERO-2 trial.
The median progression-free survival rates of 11.5 months with the everolimus combination and 4.1 months with the placebo combination were based on investigators’ local radiologic assessments. Central radiologic assessment by an independent radiology committee confirmed these results, finding that median progression-free survival reached 15.2 months in the everolimus group, compared with 4.2 months in the placebo group. Hazard ratios were 0.39 under the local assessment and 0.32 under the central assessment, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
Rates of the most common grade 3 or 4 adverse events seen in the everolimus-plus-exemestane group in the subset analysis – hyperglycemia in 8%, stomatitis in 4%, diarrhea in 4%, and fatigue in 3% – were similar to rates seen in the overall BOLERO-2 population and were within the known safety profile of everolimus. Some patients in the everolimus group (but not the placebo group) developed pneumonitis or interstitial lung disease, but these were mostly low grade and manageable by conventional strategies.
Baseline characteristics were well balanced in the randomized groups in the subset analysis.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Novartis Pharmaceuticals, which markets everolimus, funded the study, and some of the investigators were company employees. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
On Twitter @sherryboschert
SAN FRANCISCO – In patients who had hormone receptor–positive, HER2-negative advanced breast cancer, everolimus plus exemestane was just as effective among women who had a recurrence during or within a year of adjuvant therapy.
The results were seen in a secondary, subgroup analysis of 137 patients in the BOLERO-2 (Breast Cancer Trials of Oral Everolimus 2) study. The subset that had recurrences during or within a year of adjuvant therapy comprised 19% of the patients in the study, and their outcomes were similar to the overall results of the trial, which support the use of everolimus plus exemestane as first-line therapy in postmenopausal patients with hormone receptor–positive advanced breast cancer that recurs after adjuvant therapy, Dr. Hope S. Rugo and her associates reported in a poster session at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
In the women who had a recurrence during or within 12 months of adjuvant therapy, everolimus plus exemestane improved median progression-free survival to 11.5 months, compared with 4.1 months with placebo plus exemestane.
The multicenter, double-blind BOLERO-2 study randomized 724 postmenopausal women with hormone receptor–positive, HER2-negative advanced breast cancer that had recurred or progressed despite nonsteroidal aromatase inhibitor therapy. Patients received open-label exemestane plus blinded therapy with either placebo or everolimus, which inhibits the mammalian target of rapamycin (mTOR) signaling pathway. At 18 months of follow-up, patients in the exemestane-plus-everolimus group had a significantly longer median progression-free survival of 11 months as compared to 4 months for the control group (N. Engl. J. Med. 2012;366:520-529).
Similar efficacy was found in various analyses of subgroups in the study, including patients with visceral metastases, patients with bone-only metastases, and patients whose disease recurred after adjuvant therapy.
In the subgroup analysis of patients who entered the study after a recurrence during or within a year of adjuvant therapy, 100 patients received everolimus plus exemestane and 37 received placebo and exemestane. Almost all of the 137 patients had received nonsteroidal aromatase inhibitor therapy as their last therapy before entering the BOLERO-2 trial.
The median progression-free survival rates of 11.5 months with the everolimus combination and 4.1 months with the placebo combination were based on investigators’ local radiologic assessments. Central radiologic assessment by an independent radiology committee confirmed these results, finding that median progression-free survival reached 15.2 months in the everolimus group, compared with 4.2 months in the placebo group. Hazard ratios were 0.39 under the local assessment and 0.32 under the central assessment, reported Dr. Rugo, director of the Breast Oncology Clinical Trials Program at the University of California, San Francisco.
Rates of the most common grade 3 or 4 adverse events seen in the everolimus-plus-exemestane group in the subset analysis – hyperglycemia in 8%, stomatitis in 4%, diarrhea in 4%, and fatigue in 3% – were similar to rates seen in the overall BOLERO-2 population and were within the known safety profile of everolimus. Some patients in the everolimus group (but not the placebo group) developed pneumonitis or interstitial lung disease, but these were mostly low grade and manageable by conventional strategies.
Baseline characteristics were well balanced in the randomized groups in the subset analysis.
The symposium was cosponsored by the American Society of Breast Disease, the American Society of Breast Surgeons, the National Consortium of Breast Centers, the Society of Surgical Oncology, and the American Society for Radiation Oncology.
Novartis Pharmaceuticals, which markets everolimus, funded the study, and some of the investigators were company employees. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.
On Twitter @sherryboschert
AT THE ASCO BREAST CANCER SYMPOSIUM
Major finding: Median progression-free survival was 11.5 months in the everolimus-plus-exemestane group and 4.1 months in the placebo-plus exemestane group.
Data source: A secondary analysis of a subset of 137 patients who had a recurrence of hormone receptor–positive, HER2-negative breast cancer during or within 12 months of adjuvant therapy before randomization in the BOLERO-2 trial.
Disclosures: Novartis Pharmaceuticals, which markets everolimus, funded the study, and some of the investigators were company employees. Dr. Rugo reported having financial associations with Novartis, Merck, and Pfizer.