User login
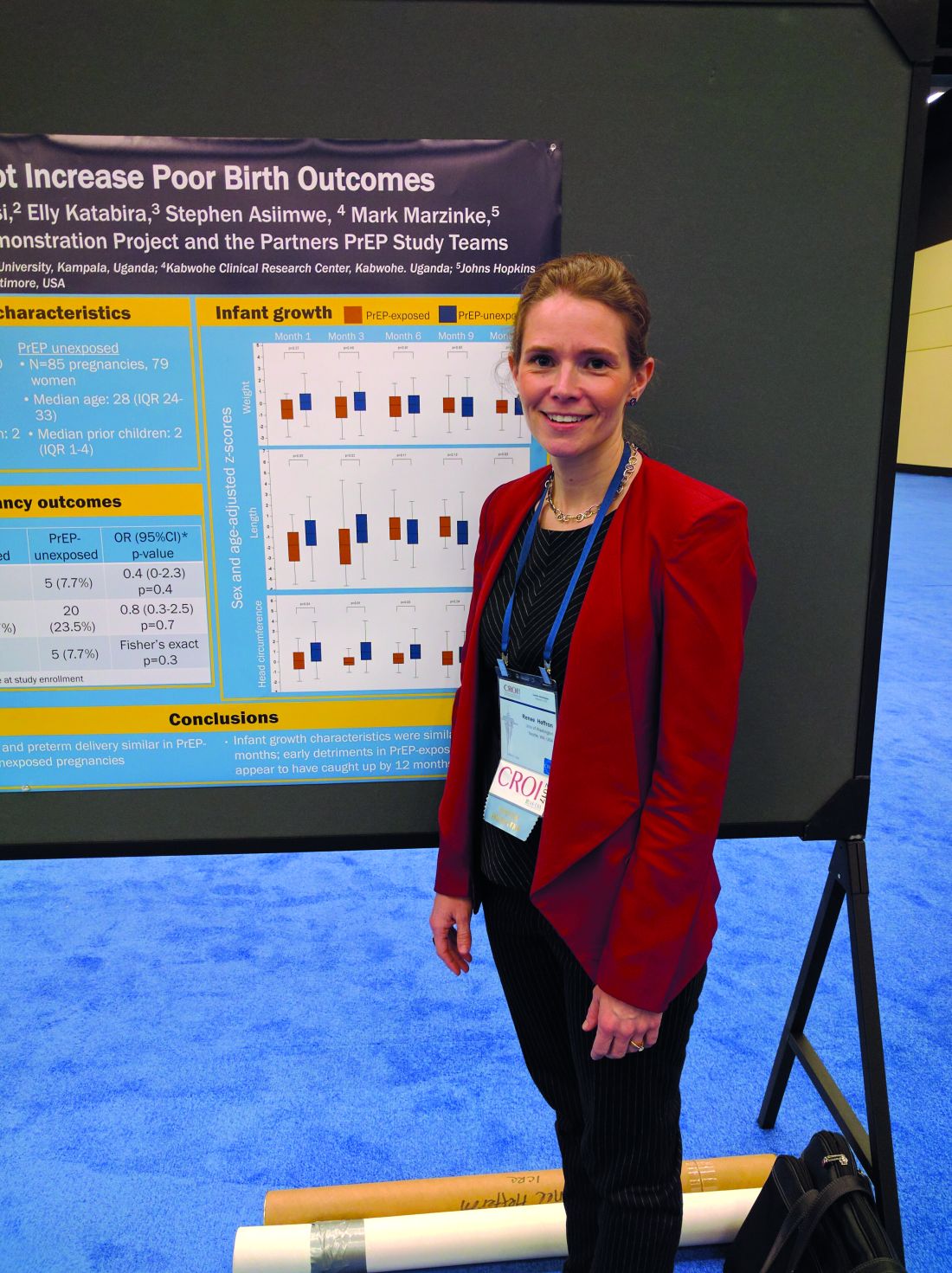
PrEP appears to be safe in pregnancy
SEATTLE – Pre-exposure prophylaxis therapy combined with antiretroviral therapy (ART) appears to be safe in pregnant women, according to an open-label study of high-risk women in Kenya and Uganda who were part of HIV-serodiscordant couples.
The safety profile of the drugs has not been well studied in pregnant women because, in the registration trials of Truvada (emtricitabine and tenofovir disoproxil fumarate; Gilead), women were instructed to stop taking the drugs when they became pregnant. Current guidelines offer counseling and the choice to continue PrEP after a woman becomes pregnant.
“We’ve been trying to gather as much data as we can. This is a small study, but I believe it’s the first study of women who used PrEP throughout their pregnancy,” said Dr Heffron.
The researchers analyzed data among women participating in a PrEP/ART study. Those who became pregnant during the study were counseled and offered the choice to continue PrEP, and the researchers tracked pregnancy and development outcomes in offspring out to 1 year.
The researchers studied 34 women who became pregnant during the Partners Demonstration Project, which evaluated HIV-prevention preference and adherence among more than 1,000 HIV-serodiscordant couples; 30 of the women (88%) opted to continue PrEP. The researchers compared their outcomes (30 women, 30 pregnancies) to the outcomes of the placebo arm of the Partners PrEP Study (79 women unexposed to PrEP, 88 pregnancies).
The researchers measured medication adherence by recording pill bottle openings via medication event monitoring system caps, which use microcircuits to record the date and time when a bottle is opened. The women opened a pill bottle on a median of 71% of days. A total of 74% of plasma samples showed detectable levels of tenofovir, and 35% had concentrations higher than 40 ng/mL.
The rate of pregnancy loss was similar between the two groups at 16.7% PrEP-exposed patients versus 23.5% PrEP-unexposed patients (adjusted odds ratio, 0.8; P = .7). The frequency of preterm delivery also was similar at 0% PrEP-exposed patients versus 7.7% PrEP unexposed patients (aOR, 0.4; P = .4). There were no congenital anomalies seen among PrEP-exposed babies.
The researchers also looked at growth outcomes out to 1 year, including standardized measures of head circumferences, height, and weight. In early measurements, PrEP-exposed babies were slightly smaller on average than were unexposed babies, but by 12 months, the two groups were indistinguishable. Dr. Heffron suspects the unexposed population may have been slightly larger than average.
The study was funded by the Bill & Melinda Gates Foundation, the National Institute of Mental Health, and the United States Agency for International Development. Dr Heffron reported having no financial disclosures.
SEATTLE – Pre-exposure prophylaxis therapy combined with antiretroviral therapy (ART) appears to be safe in pregnant women, according to an open-label study of high-risk women in Kenya and Uganda who were part of HIV-serodiscordant couples.
The safety profile of the drugs has not been well studied in pregnant women because, in the registration trials of Truvada (emtricitabine and tenofovir disoproxil fumarate; Gilead), women were instructed to stop taking the drugs when they became pregnant. Current guidelines offer counseling and the choice to continue PrEP after a woman becomes pregnant.
“We’ve been trying to gather as much data as we can. This is a small study, but I believe it’s the first study of women who used PrEP throughout their pregnancy,” said Dr Heffron.
The researchers analyzed data among women participating in a PrEP/ART study. Those who became pregnant during the study were counseled and offered the choice to continue PrEP, and the researchers tracked pregnancy and development outcomes in offspring out to 1 year.
The researchers studied 34 women who became pregnant during the Partners Demonstration Project, which evaluated HIV-prevention preference and adherence among more than 1,000 HIV-serodiscordant couples; 30 of the women (88%) opted to continue PrEP. The researchers compared their outcomes (30 women, 30 pregnancies) to the outcomes of the placebo arm of the Partners PrEP Study (79 women unexposed to PrEP, 88 pregnancies).
The researchers measured medication adherence by recording pill bottle openings via medication event monitoring system caps, which use microcircuits to record the date and time when a bottle is opened. The women opened a pill bottle on a median of 71% of days. A total of 74% of plasma samples showed detectable levels of tenofovir, and 35% had concentrations higher than 40 ng/mL.
The rate of pregnancy loss was similar between the two groups at 16.7% PrEP-exposed patients versus 23.5% PrEP-unexposed patients (adjusted odds ratio, 0.8; P = .7). The frequency of preterm delivery also was similar at 0% PrEP-exposed patients versus 7.7% PrEP unexposed patients (aOR, 0.4; P = .4). There were no congenital anomalies seen among PrEP-exposed babies.
The researchers also looked at growth outcomes out to 1 year, including standardized measures of head circumferences, height, and weight. In early measurements, PrEP-exposed babies were slightly smaller on average than were unexposed babies, but by 12 months, the two groups were indistinguishable. Dr. Heffron suspects the unexposed population may have been slightly larger than average.
The study was funded by the Bill & Melinda Gates Foundation, the National Institute of Mental Health, and the United States Agency for International Development. Dr Heffron reported having no financial disclosures.
SEATTLE – Pre-exposure prophylaxis therapy combined with antiretroviral therapy (ART) appears to be safe in pregnant women, according to an open-label study of high-risk women in Kenya and Uganda who were part of HIV-serodiscordant couples.
The safety profile of the drugs has not been well studied in pregnant women because, in the registration trials of Truvada (emtricitabine and tenofovir disoproxil fumarate; Gilead), women were instructed to stop taking the drugs when they became pregnant. Current guidelines offer counseling and the choice to continue PrEP after a woman becomes pregnant.
“We’ve been trying to gather as much data as we can. This is a small study, but I believe it’s the first study of women who used PrEP throughout their pregnancy,” said Dr Heffron.
The researchers analyzed data among women participating in a PrEP/ART study. Those who became pregnant during the study were counseled and offered the choice to continue PrEP, and the researchers tracked pregnancy and development outcomes in offspring out to 1 year.
The researchers studied 34 women who became pregnant during the Partners Demonstration Project, which evaluated HIV-prevention preference and adherence among more than 1,000 HIV-serodiscordant couples; 30 of the women (88%) opted to continue PrEP. The researchers compared their outcomes (30 women, 30 pregnancies) to the outcomes of the placebo arm of the Partners PrEP Study (79 women unexposed to PrEP, 88 pregnancies).
The researchers measured medication adherence by recording pill bottle openings via medication event monitoring system caps, which use microcircuits to record the date and time when a bottle is opened. The women opened a pill bottle on a median of 71% of days. A total of 74% of plasma samples showed detectable levels of tenofovir, and 35% had concentrations higher than 40 ng/mL.
The rate of pregnancy loss was similar between the two groups at 16.7% PrEP-exposed patients versus 23.5% PrEP-unexposed patients (adjusted odds ratio, 0.8; P = .7). The frequency of preterm delivery also was similar at 0% PrEP-exposed patients versus 7.7% PrEP unexposed patients (aOR, 0.4; P = .4). There were no congenital anomalies seen among PrEP-exposed babies.
The researchers also looked at growth outcomes out to 1 year, including standardized measures of head circumferences, height, and weight. In early measurements, PrEP-exposed babies were slightly smaller on average than were unexposed babies, but by 12 months, the two groups were indistinguishable. Dr. Heffron suspects the unexposed population may have been slightly larger than average.
The study was funded by the Bill & Melinda Gates Foundation, the National Institute of Mental Health, and the United States Agency for International Development. Dr Heffron reported having no financial disclosures.
Key clinical point: The study is the first to confirm safety of PrEP in pregnancy.
Major finding: In this study, 16.7% of PrEP-exposed women experienced pregnancy loss versus 23.5% of unexposed.
Data source: Open-label, case-controlled study of 30 PrEP-exposed women and 79 controls.
Disclosures: The study was funded by the Bill & Melinda Gates Foundation, the National Institute of Mental Health, and the United States Agency for International Development. Dr Heffron reported having no financial disclosures.
Antibody could replace conventional antiviral therapy in HIV
SEATTLE – In a phase II trial, an antibody that targets domain 1 of the CD4 receptor maintained viral suppression among patients who had been taking combination antiretroviral therapy (cART). The study lasted up to 16 weeks, and no viral rebound was seen.
In vitro studies showed that UB-421 can neutralize more than 850 strains of HIV-1, and it binds to the CD4 receptor with an affinity about 100 times greater than that of the gp120, essentially outcompeting the virus for access to T cells.
Most broadly neutralizing antibodies, which target the gp120 protein on the HIV virus, tend to allow viral breakthrough from the development of resistance. “Based on previous studies, in every viral isolate, we get 100% neutralization,” said Dr Liao.
The study included 29 males who had successfully suppressed viral loads on cART. They were assigned to cohort 1 (10 mg/kg weekly, 8-week interruption) or cohort 2 (25 mg/kg weekly, biweekly, 16-week interruption). 27 patients completed all doses.
After the interruption period, 22 of 27 patients who completed treatment restarted cART. There were no viral rebounds during the treatment periods or during the follow-up period among those who restarted cART. The five patients who discontinued cART experienced viral rebound, but all then restarted cART and achieved viral suppression.
During the periods of UB-421 therapy, proviral DNA count dropped (P = .014), suggesting that the antibody may deplete the HIV-1 reservoir and has potential to be curative.
There was no difference in CD4+ T-cell counts before treatment and after the study ended. That’s important, says Dr. Liao, because some researchers expressed concern that blocking CD4 could lead to immunosuppression.
Regulatory T-cell numbers dropped during UB-421 therapy (interquartile range 1.7-3.1; P less than .01), potentially boosting immunity. “Besides being an entry inhibitor, there is also a lot of immunomodulatory activity,” said Dr Liao.
She also believes that the injecting the drug is more convenient than taking daily oral medications, and in fact, some patients refused to go back to cART at the end of the trial, requesting ongoing therapy with UB-421 instead. The company also is working on intramuscular and subcutaneous formulations that could be dosed monthly.
The company is planning a phase III trial in Taiwan and plans to file an IND in the United States soon, she added.
SEATTLE – In a phase II trial, an antibody that targets domain 1 of the CD4 receptor maintained viral suppression among patients who had been taking combination antiretroviral therapy (cART). The study lasted up to 16 weeks, and no viral rebound was seen.
In vitro studies showed that UB-421 can neutralize more than 850 strains of HIV-1, and it binds to the CD4 receptor with an affinity about 100 times greater than that of the gp120, essentially outcompeting the virus for access to T cells.
Most broadly neutralizing antibodies, which target the gp120 protein on the HIV virus, tend to allow viral breakthrough from the development of resistance. “Based on previous studies, in every viral isolate, we get 100% neutralization,” said Dr Liao.
The study included 29 males who had successfully suppressed viral loads on cART. They were assigned to cohort 1 (10 mg/kg weekly, 8-week interruption) or cohort 2 (25 mg/kg weekly, biweekly, 16-week interruption). 27 patients completed all doses.
After the interruption period, 22 of 27 patients who completed treatment restarted cART. There were no viral rebounds during the treatment periods or during the follow-up period among those who restarted cART. The five patients who discontinued cART experienced viral rebound, but all then restarted cART and achieved viral suppression.
During the periods of UB-421 therapy, proviral DNA count dropped (P = .014), suggesting that the antibody may deplete the HIV-1 reservoir and has potential to be curative.
There was no difference in CD4+ T-cell counts before treatment and after the study ended. That’s important, says Dr. Liao, because some researchers expressed concern that blocking CD4 could lead to immunosuppression.
Regulatory T-cell numbers dropped during UB-421 therapy (interquartile range 1.7-3.1; P less than .01), potentially boosting immunity. “Besides being an entry inhibitor, there is also a lot of immunomodulatory activity,” said Dr Liao.
She also believes that the injecting the drug is more convenient than taking daily oral medications, and in fact, some patients refused to go back to cART at the end of the trial, requesting ongoing therapy with UB-421 instead. The company also is working on intramuscular and subcutaneous formulations that could be dosed monthly.
The company is planning a phase III trial in Taiwan and plans to file an IND in the United States soon, she added.
SEATTLE – In a phase II trial, an antibody that targets domain 1 of the CD4 receptor maintained viral suppression among patients who had been taking combination antiretroviral therapy (cART). The study lasted up to 16 weeks, and no viral rebound was seen.
In vitro studies showed that UB-421 can neutralize more than 850 strains of HIV-1, and it binds to the CD4 receptor with an affinity about 100 times greater than that of the gp120, essentially outcompeting the virus for access to T cells.
Most broadly neutralizing antibodies, which target the gp120 protein on the HIV virus, tend to allow viral breakthrough from the development of resistance. “Based on previous studies, in every viral isolate, we get 100% neutralization,” said Dr Liao.
The study included 29 males who had successfully suppressed viral loads on cART. They were assigned to cohort 1 (10 mg/kg weekly, 8-week interruption) or cohort 2 (25 mg/kg weekly, biweekly, 16-week interruption). 27 patients completed all doses.
After the interruption period, 22 of 27 patients who completed treatment restarted cART. There were no viral rebounds during the treatment periods or during the follow-up period among those who restarted cART. The five patients who discontinued cART experienced viral rebound, but all then restarted cART and achieved viral suppression.
During the periods of UB-421 therapy, proviral DNA count dropped (P = .014), suggesting that the antibody may deplete the HIV-1 reservoir and has potential to be curative.
There was no difference in CD4+ T-cell counts before treatment and after the study ended. That’s important, says Dr. Liao, because some researchers expressed concern that blocking CD4 could lead to immunosuppression.
Regulatory T-cell numbers dropped during UB-421 therapy (interquartile range 1.7-3.1; P less than .01), potentially boosting immunity. “Besides being an entry inhibitor, there is also a lot of immunomodulatory activity,” said Dr Liao.
She also believes that the injecting the drug is more convenient than taking daily oral medications, and in fact, some patients refused to go back to cART at the end of the trial, requesting ongoing therapy with UB-421 instead. The company also is working on intramuscular and subcutaneous formulations that could be dosed monthly.
The company is planning a phase III trial in Taiwan and plans to file an IND in the United States soon, she added.
Key clinical point: Antibody substitution for antiretroviral therapy maintained viral suppression for 16 weeks.
Major finding: No viral breakthroughs occurred during the antibody treatment period.
Data source: Phase II, open-label, randomized study in 29 patients.
Disclosures: United BioPharma sponsored the study. Dr. Liao is an employee of United BioPharma.
Ibalizumab suppressed HIV-1 in multidrug-resistant patients
Seattle – The antibody ibalizumab combined with an optimized background regimen maintained viral suppression out to 24 weeks, in an open-label extension study of patients with multidrug-resistant HIV-1 infections.
The researchers had previously shown that the drug achieved viral suppression after 7 days in many patients.
Ibalizumab works by blocking an epitope on the second extracellular domain of the CD4 receptor, preventing the HIV virus from entering the cell. The 40 patients in the study had failed on at least one drug in three different classes, though they had to have sensitivity to at least one antiretroviral drug, which was used to construct the optimized background regimen (OBR).
“These patients are the most vulnerable and most at risk in terms of needing a new class of drugs. This is the first new class of drug that will go for approval in a decade,” said Brinda Emu, MD, of the deparment of internal medicine at Yale University, New Haven, Conn., who presented the study in a poster session at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
The average duration of HIV infection among patients was 21 years. Forty-three percent had to take the investigational agent fostemsavir as part of their OBR. After a 7-day monitoring period, patients received a 2,000-mg IV dose of ibalizumab. Seven days later, at day 14, 83% had achieved at least a 0.5 log10 reduction in viral load, compared with 3% during the monitoring period (P less than .0001); 60% achieved at least a 1 log10 reduction, compared with 0% during the monitoring period (P less than .0001).
The OBR was then started at week 14, and patients received an injection of 800 mg ibalizumab every 2 weeks beginning at day 21 and continuing until week 24.
The current research reports the results of the extension study. At week 24, the mean viral load had decreased 1.6 log10, compared with baseline – 55% of patients had a decrease of at least 1 log10, and 48% had a reduction of at least 2 log10; 43% of patients had undetectable levels of virus, and 50% had fewer than 200 copies/mL.
Nine patients reported a total of 17 serious adverse events, 1 of which led to drug discontinuation. Overall. there were nine discontinuations due to four deaths, three consent withdrawals, and two losses to follow-up.
“In an indication with very resistant virus and limited options, combining ibalizumab with at least one other active agent can provide a way to decrease viral load and increase CD4+ T cells. What I want to know is, What if we started this a little bit earlier as we do with many of our other drugs?” asked Dr. Emu.
She said that some providers had had concerns that adherence may be low with an injectable drug, but the results were reassuring. “I will say anecdotally that I’ve seen the complete opposite. One of the things we noticed is that seeing these patients every 2 weeks to give them their IV infusions has made them more adherent to the rest of their regimen. Perhaps it’s that ability to check in, and the relationship that builds up over time with your providers. Despite it being an infusional agent, or perhaps because of that, adherence has been pretty good.”
The study was funded by TaiMed Biologics. Dr. Emu has served on TaiMed’s advisory board.
Seattle – The antibody ibalizumab combined with an optimized background regimen maintained viral suppression out to 24 weeks, in an open-label extension study of patients with multidrug-resistant HIV-1 infections.
The researchers had previously shown that the drug achieved viral suppression after 7 days in many patients.
Ibalizumab works by blocking an epitope on the second extracellular domain of the CD4 receptor, preventing the HIV virus from entering the cell. The 40 patients in the study had failed on at least one drug in three different classes, though they had to have sensitivity to at least one antiretroviral drug, which was used to construct the optimized background regimen (OBR).
“These patients are the most vulnerable and most at risk in terms of needing a new class of drugs. This is the first new class of drug that will go for approval in a decade,” said Brinda Emu, MD, of the deparment of internal medicine at Yale University, New Haven, Conn., who presented the study in a poster session at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
The average duration of HIV infection among patients was 21 years. Forty-three percent had to take the investigational agent fostemsavir as part of their OBR. After a 7-day monitoring period, patients received a 2,000-mg IV dose of ibalizumab. Seven days later, at day 14, 83% had achieved at least a 0.5 log10 reduction in viral load, compared with 3% during the monitoring period (P less than .0001); 60% achieved at least a 1 log10 reduction, compared with 0% during the monitoring period (P less than .0001).
The OBR was then started at week 14, and patients received an injection of 800 mg ibalizumab every 2 weeks beginning at day 21 and continuing until week 24.
The current research reports the results of the extension study. At week 24, the mean viral load had decreased 1.6 log10, compared with baseline – 55% of patients had a decrease of at least 1 log10, and 48% had a reduction of at least 2 log10; 43% of patients had undetectable levels of virus, and 50% had fewer than 200 copies/mL.
Nine patients reported a total of 17 serious adverse events, 1 of which led to drug discontinuation. Overall. there were nine discontinuations due to four deaths, three consent withdrawals, and two losses to follow-up.
“In an indication with very resistant virus and limited options, combining ibalizumab with at least one other active agent can provide a way to decrease viral load and increase CD4+ T cells. What I want to know is, What if we started this a little bit earlier as we do with many of our other drugs?” asked Dr. Emu.
She said that some providers had had concerns that adherence may be low with an injectable drug, but the results were reassuring. “I will say anecdotally that I’ve seen the complete opposite. One of the things we noticed is that seeing these patients every 2 weeks to give them their IV infusions has made them more adherent to the rest of their regimen. Perhaps it’s that ability to check in, and the relationship that builds up over time with your providers. Despite it being an infusional agent, or perhaps because of that, adherence has been pretty good.”
The study was funded by TaiMed Biologics. Dr. Emu has served on TaiMed’s advisory board.
Seattle – The antibody ibalizumab combined with an optimized background regimen maintained viral suppression out to 24 weeks, in an open-label extension study of patients with multidrug-resistant HIV-1 infections.
The researchers had previously shown that the drug achieved viral suppression after 7 days in many patients.
Ibalizumab works by blocking an epitope on the second extracellular domain of the CD4 receptor, preventing the HIV virus from entering the cell. The 40 patients in the study had failed on at least one drug in three different classes, though they had to have sensitivity to at least one antiretroviral drug, which was used to construct the optimized background regimen (OBR).
“These patients are the most vulnerable and most at risk in terms of needing a new class of drugs. This is the first new class of drug that will go for approval in a decade,” said Brinda Emu, MD, of the deparment of internal medicine at Yale University, New Haven, Conn., who presented the study in a poster session at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
The average duration of HIV infection among patients was 21 years. Forty-three percent had to take the investigational agent fostemsavir as part of their OBR. After a 7-day monitoring period, patients received a 2,000-mg IV dose of ibalizumab. Seven days later, at day 14, 83% had achieved at least a 0.5 log10 reduction in viral load, compared with 3% during the monitoring period (P less than .0001); 60% achieved at least a 1 log10 reduction, compared with 0% during the monitoring period (P less than .0001).
The OBR was then started at week 14, and patients received an injection of 800 mg ibalizumab every 2 weeks beginning at day 21 and continuing until week 24.
The current research reports the results of the extension study. At week 24, the mean viral load had decreased 1.6 log10, compared with baseline – 55% of patients had a decrease of at least 1 log10, and 48% had a reduction of at least 2 log10; 43% of patients had undetectable levels of virus, and 50% had fewer than 200 copies/mL.
Nine patients reported a total of 17 serious adverse events, 1 of which led to drug discontinuation. Overall. there were nine discontinuations due to four deaths, three consent withdrawals, and two losses to follow-up.
“In an indication with very resistant virus and limited options, combining ibalizumab with at least one other active agent can provide a way to decrease viral load and increase CD4+ T cells. What I want to know is, What if we started this a little bit earlier as we do with many of our other drugs?” asked Dr. Emu.
She said that some providers had had concerns that adherence may be low with an injectable drug, but the results were reassuring. “I will say anecdotally that I’ve seen the complete opposite. One of the things we noticed is that seeing these patients every 2 weeks to give them their IV infusions has made them more adherent to the rest of their regimen. Perhaps it’s that ability to check in, and the relationship that builds up over time with your providers. Despite it being an infusional agent, or perhaps because of that, adherence has been pretty good.”
The study was funded by TaiMed Biologics. Dr. Emu has served on TaiMed’s advisory board.
Key clinical point: An extension study showed viral load suppression out to 24 weeks in multidrug-resistant HIV patients.
Major finding: 55% of patients with resistant HIV had viral reduction at week 24.
Data source: A single-arm, open-label study of 40 patients.
Disclosures: The study was funded by TaiMed Biologics. Dr. Emu has served on TaiMed’s advisory board.
Small study: Drug combo achieves negative bacterial culture in all TB patients
SEATTLE – An all-oral drug combination achieved negative bacterial culture in 100% of patients with extensively drug resistant (XDR) or multidrug resistant (MDR) tuberculosis at 4 months, according to a study.
The drugs used were bedaquiline (400 mg once daily for 2 weeks followed by 200 mg three times per week), pretomanid (200 mg once daily), and linezolid (600 mg twice daily). The study, Nix-TB, was an open-label, two-site trial that examined a simplified and shortened all-oral regimen. Pretomanid is an experimental drug, while bedaquiline and linezolid are both approved medications.
The mortality rate among study participants was less than 6%.
“I was surprised at how successful this study was. These are patients who are generally very ill, with a very poor prognosis,” noted Francesca Conradie, MD, deputy director of the clinical HIV unit at the University of Witwatersrand (Johannesburg, South Africa), who presented the results at a poster session at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
To date, the trial has enrolled 72 subjects (51% HIV positive, 65% XDR-TB, 35% MDR-TB). HIV-infected subjects had to have CD4 counts of at least 50 cell/mcL. The researchers evaluated clinical, laboratory, and sputum liquid cultures at baseline and at weeks 1, 2, 4, 6, and 8, and then every 4-6 weeks throughout the 6-month treatment period.
Forty patients have finished 6 months of therapy and 31 have completed 6-months of posttherapy follow-up.
Four patients died during the first 8 weeks of therapy. Of the survivors, 74% were culture negative at 8 weeks, and all were culture negative at 4 months. Two patients experienced relapses or reinfections at 6 months following therapy.
Twenty-seven percent of patients experienced serious adverse events, but no patients withdrew from the trials for clinical adverse events or laboratory abnormalities.
Linezolid-associated peripheral neuropathy and myelosuppression occurred, with 71% of patients having experienced at least one dose interruption as a result. Seven patients experienced grade 3 or 4 transaminitis, but all such cases resolved and those patients continued the study regimen.
Some hepatic enzyme changes were seen among patients. A total of 14.1% developed alanine transaminase levels greater than 3 times the upper limit of normal (ULN), and 7.0% had levels greater than 5 x ULN. A total of 14.9% had aspartate transaminase (AST) enzymes at greater than 3 x ULN, and 2.8% had AST levels greater than 5 x ULN. A total of 4.2% had alkaline phosphatase levels reaching greater than 3 x ULN. In all cases, the values returned to normal with a pause in therapy.
Dr. Conradie characterized these results as reassuring, in light of the fact that the STAND study of pretomanid in combination with moxifloxacin and pyrazinamide was ended prematurely because of liver safety concerns.
The linezolid side effect profile is concerning, and the study will continue with modified linezolid doses, Dr. Conradie acknowledged. “We’re looking to see if we could do a study with a lower dose” of linezolid or a study that doesn’t involve giving linezolid for the entire period of the treatment, she noted.
Dr Conradie has served on advisory boards for ViiV, Janssen, Merck, GSK, Mylan, and Sanofi Aventis. The study was funded by the TB Foundation.
SEATTLE – An all-oral drug combination achieved negative bacterial culture in 100% of patients with extensively drug resistant (XDR) or multidrug resistant (MDR) tuberculosis at 4 months, according to a study.
The drugs used were bedaquiline (400 mg once daily for 2 weeks followed by 200 mg three times per week), pretomanid (200 mg once daily), and linezolid (600 mg twice daily). The study, Nix-TB, was an open-label, two-site trial that examined a simplified and shortened all-oral regimen. Pretomanid is an experimental drug, while bedaquiline and linezolid are both approved medications.
The mortality rate among study participants was less than 6%.
“I was surprised at how successful this study was. These are patients who are generally very ill, with a very poor prognosis,” noted Francesca Conradie, MD, deputy director of the clinical HIV unit at the University of Witwatersrand (Johannesburg, South Africa), who presented the results at a poster session at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
To date, the trial has enrolled 72 subjects (51% HIV positive, 65% XDR-TB, 35% MDR-TB). HIV-infected subjects had to have CD4 counts of at least 50 cell/mcL. The researchers evaluated clinical, laboratory, and sputum liquid cultures at baseline and at weeks 1, 2, 4, 6, and 8, and then every 4-6 weeks throughout the 6-month treatment period.
Forty patients have finished 6 months of therapy and 31 have completed 6-months of posttherapy follow-up.
Four patients died during the first 8 weeks of therapy. Of the survivors, 74% were culture negative at 8 weeks, and all were culture negative at 4 months. Two patients experienced relapses or reinfections at 6 months following therapy.
Twenty-seven percent of patients experienced serious adverse events, but no patients withdrew from the trials for clinical adverse events or laboratory abnormalities.
Linezolid-associated peripheral neuropathy and myelosuppression occurred, with 71% of patients having experienced at least one dose interruption as a result. Seven patients experienced grade 3 or 4 transaminitis, but all such cases resolved and those patients continued the study regimen.
Some hepatic enzyme changes were seen among patients. A total of 14.1% developed alanine transaminase levels greater than 3 times the upper limit of normal (ULN), and 7.0% had levels greater than 5 x ULN. A total of 14.9% had aspartate transaminase (AST) enzymes at greater than 3 x ULN, and 2.8% had AST levels greater than 5 x ULN. A total of 4.2% had alkaline phosphatase levels reaching greater than 3 x ULN. In all cases, the values returned to normal with a pause in therapy.
Dr. Conradie characterized these results as reassuring, in light of the fact that the STAND study of pretomanid in combination with moxifloxacin and pyrazinamide was ended prematurely because of liver safety concerns.
The linezolid side effect profile is concerning, and the study will continue with modified linezolid doses, Dr. Conradie acknowledged. “We’re looking to see if we could do a study with a lower dose” of linezolid or a study that doesn’t involve giving linezolid for the entire period of the treatment, she noted.
Dr Conradie has served on advisory boards for ViiV, Janssen, Merck, GSK, Mylan, and Sanofi Aventis. The study was funded by the TB Foundation.
SEATTLE – An all-oral drug combination achieved negative bacterial culture in 100% of patients with extensively drug resistant (XDR) or multidrug resistant (MDR) tuberculosis at 4 months, according to a study.
The drugs used were bedaquiline (400 mg once daily for 2 weeks followed by 200 mg three times per week), pretomanid (200 mg once daily), and linezolid (600 mg twice daily). The study, Nix-TB, was an open-label, two-site trial that examined a simplified and shortened all-oral regimen. Pretomanid is an experimental drug, while bedaquiline and linezolid are both approved medications.
The mortality rate among study participants was less than 6%.
“I was surprised at how successful this study was. These are patients who are generally very ill, with a very poor prognosis,” noted Francesca Conradie, MD, deputy director of the clinical HIV unit at the University of Witwatersrand (Johannesburg, South Africa), who presented the results at a poster session at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
To date, the trial has enrolled 72 subjects (51% HIV positive, 65% XDR-TB, 35% MDR-TB). HIV-infected subjects had to have CD4 counts of at least 50 cell/mcL. The researchers evaluated clinical, laboratory, and sputum liquid cultures at baseline and at weeks 1, 2, 4, 6, and 8, and then every 4-6 weeks throughout the 6-month treatment period.
Forty patients have finished 6 months of therapy and 31 have completed 6-months of posttherapy follow-up.
Four patients died during the first 8 weeks of therapy. Of the survivors, 74% were culture negative at 8 weeks, and all were culture negative at 4 months. Two patients experienced relapses or reinfections at 6 months following therapy.
Twenty-seven percent of patients experienced serious adverse events, but no patients withdrew from the trials for clinical adverse events or laboratory abnormalities.
Linezolid-associated peripheral neuropathy and myelosuppression occurred, with 71% of patients having experienced at least one dose interruption as a result. Seven patients experienced grade 3 or 4 transaminitis, but all such cases resolved and those patients continued the study regimen.
Some hepatic enzyme changes were seen among patients. A total of 14.1% developed alanine transaminase levels greater than 3 times the upper limit of normal (ULN), and 7.0% had levels greater than 5 x ULN. A total of 14.9% had aspartate transaminase (AST) enzymes at greater than 3 x ULN, and 2.8% had AST levels greater than 5 x ULN. A total of 4.2% had alkaline phosphatase levels reaching greater than 3 x ULN. In all cases, the values returned to normal with a pause in therapy.
Dr. Conradie characterized these results as reassuring, in light of the fact that the STAND study of pretomanid in combination with moxifloxacin and pyrazinamide was ended prematurely because of liver safety concerns.
The linezolid side effect profile is concerning, and the study will continue with modified linezolid doses, Dr. Conradie acknowledged. “We’re looking to see if we could do a study with a lower dose” of linezolid or a study that doesn’t involve giving linezolid for the entire period of the treatment, she noted.
Dr Conradie has served on advisory boards for ViiV, Janssen, Merck, GSK, Mylan, and Sanofi Aventis. The study was funded by the TB Foundation.
AT CROI
Key clinical point: An oral, three-drug combination led to undetectable bacteria levels.
Major finding: All of the patients in the study were culture negative at 4 months.
Data source: Open-label trial of 72 patients at two centers.
Disclosures: Dr. Conradie has served on advisory boards for ViiV, Janssen, Merck, GSK, Mylan, and Sanofi Aventis. The study was funded by the TB Foundation.
Two-drug combo looks good for HIV maintenance therapy
SEATTLE – In HIV patients with suppressed virus and no history of virologic failure, a two-drug combination of dolutegravir and rilpivirine (DTG+RPV) proved noninferior to three- and four-drug regimens in maintaining viral RNA counts at less than 50 copies/mL.
The results come from an integrated analysis of two open-label, multicenter phase III clinical trials – SWORD 1 and SWORD 2 – the results of which were presented at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
The combined studies included 1,024 HIV-1 infected adults who had successful viral suppression on a three- or four-drug current antiretroviral (CAR) therapy. They were randomized to continue CAR or switch to the two-drug regimen.
At week 48, a pooled analysis showed that the percentage of patients with HIV-1 RNA less than 50 copies/mL was 95% in CAR patients and 96% in DTG+RPV patients (difference, –0.6%; 95% confidence interval, –4.3% to 3.0%) in SWORD 1. In SWORD 2, 94% of patients achieved those levels in both arms (difference, –0.2%; 95% CI, –3.9% to 4.2%).
Adverse events were reported in 77% of patients in the DTG+RPV group and 71% of patients in the CAR group, with the most common being nasopharyngitis, headache, upper respiratory tract infection, diarrhea, and back pain. Five percent of patients in the DTG+RPV group had serious adverse events, compared with 4% in the CAR group.
The rate of adverse events leading to study withdrawal was higher in the DTG+RPV group (4%) than the CAR group (less than 1%).
The switch to DTG+RPV had no significant effect on lipid levels, but was associated with improvements in bone biomarkers, including bone-specific alkaline phosphatase (baseline 15.9 mcg/L to 12.9 mcg/L in DTG+RPV versus 16.2 to 17.1 in CAR; P less than .001), osteocalcin (23.8 mcg/L to 19.0 mcg/L in DTG+RPV versus 24.0 to 23.1 in CAR; P less than .001), and procollagen 1 N-terminal propeptide (53.0 mcg/L to 45.6 mcg/L in DTG+RPV versus 55.3 to 54.7 in CAR; P less than .001).
ViiV and Janssen, who sponsored the two studies, have formed a partnership to formulate the two drugs as a single pill, according to Kimberly Smith, MD, MPH, vice president and head of global research and medical strategy at ViiV. “We’re hoping to have the tablet available to people by this time next year,” she said.
The two drugs were chosen for their potency and safety. “Dolutegravir has shown itself to be the most potent integrase inhibitor, it’s well tolerated, and it has a high barrier to resistance. That’s why we thought it would be a good lead drug for a two-drug regimen. We added rilpivirine to it because it’s also a well-tolerated drug. The two have small doses, so we thought we could make a very small pill,” said Dr. Smith.
Joseph Eron, MD, director of Clinical Core at the University of North Carolina, Chapel Hill, who chaired a press conference that discussed the results, noted that the regimen has the potential to have fewer drug interactions. “There are potential distinct advantages to this particular combination.”
SEATTLE – In HIV patients with suppressed virus and no history of virologic failure, a two-drug combination of dolutegravir and rilpivirine (DTG+RPV) proved noninferior to three- and four-drug regimens in maintaining viral RNA counts at less than 50 copies/mL.
The results come from an integrated analysis of two open-label, multicenter phase III clinical trials – SWORD 1 and SWORD 2 – the results of which were presented at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
The combined studies included 1,024 HIV-1 infected adults who had successful viral suppression on a three- or four-drug current antiretroviral (CAR) therapy. They were randomized to continue CAR or switch to the two-drug regimen.
At week 48, a pooled analysis showed that the percentage of patients with HIV-1 RNA less than 50 copies/mL was 95% in CAR patients and 96% in DTG+RPV patients (difference, –0.6%; 95% confidence interval, –4.3% to 3.0%) in SWORD 1. In SWORD 2, 94% of patients achieved those levels in both arms (difference, –0.2%; 95% CI, –3.9% to 4.2%).
Adverse events were reported in 77% of patients in the DTG+RPV group and 71% of patients in the CAR group, with the most common being nasopharyngitis, headache, upper respiratory tract infection, diarrhea, and back pain. Five percent of patients in the DTG+RPV group had serious adverse events, compared with 4% in the CAR group.
The rate of adverse events leading to study withdrawal was higher in the DTG+RPV group (4%) than the CAR group (less than 1%).
The switch to DTG+RPV had no significant effect on lipid levels, but was associated with improvements in bone biomarkers, including bone-specific alkaline phosphatase (baseline 15.9 mcg/L to 12.9 mcg/L in DTG+RPV versus 16.2 to 17.1 in CAR; P less than .001), osteocalcin (23.8 mcg/L to 19.0 mcg/L in DTG+RPV versus 24.0 to 23.1 in CAR; P less than .001), and procollagen 1 N-terminal propeptide (53.0 mcg/L to 45.6 mcg/L in DTG+RPV versus 55.3 to 54.7 in CAR; P less than .001).
ViiV and Janssen, who sponsored the two studies, have formed a partnership to formulate the two drugs as a single pill, according to Kimberly Smith, MD, MPH, vice president and head of global research and medical strategy at ViiV. “We’re hoping to have the tablet available to people by this time next year,” she said.
The two drugs were chosen for their potency and safety. “Dolutegravir has shown itself to be the most potent integrase inhibitor, it’s well tolerated, and it has a high barrier to resistance. That’s why we thought it would be a good lead drug for a two-drug regimen. We added rilpivirine to it because it’s also a well-tolerated drug. The two have small doses, so we thought we could make a very small pill,” said Dr. Smith.
Joseph Eron, MD, director of Clinical Core at the University of North Carolina, Chapel Hill, who chaired a press conference that discussed the results, noted that the regimen has the potential to have fewer drug interactions. “There are potential distinct advantages to this particular combination.”
SEATTLE – In HIV patients with suppressed virus and no history of virologic failure, a two-drug combination of dolutegravir and rilpivirine (DTG+RPV) proved noninferior to three- and four-drug regimens in maintaining viral RNA counts at less than 50 copies/mL.
The results come from an integrated analysis of two open-label, multicenter phase III clinical trials – SWORD 1 and SWORD 2 – the results of which were presented at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
The combined studies included 1,024 HIV-1 infected adults who had successful viral suppression on a three- or four-drug current antiretroviral (CAR) therapy. They were randomized to continue CAR or switch to the two-drug regimen.
At week 48, a pooled analysis showed that the percentage of patients with HIV-1 RNA less than 50 copies/mL was 95% in CAR patients and 96% in DTG+RPV patients (difference, –0.6%; 95% confidence interval, –4.3% to 3.0%) in SWORD 1. In SWORD 2, 94% of patients achieved those levels in both arms (difference, –0.2%; 95% CI, –3.9% to 4.2%).
Adverse events were reported in 77% of patients in the DTG+RPV group and 71% of patients in the CAR group, with the most common being nasopharyngitis, headache, upper respiratory tract infection, diarrhea, and back pain. Five percent of patients in the DTG+RPV group had serious adverse events, compared with 4% in the CAR group.
The rate of adverse events leading to study withdrawal was higher in the DTG+RPV group (4%) than the CAR group (less than 1%).
The switch to DTG+RPV had no significant effect on lipid levels, but was associated with improvements in bone biomarkers, including bone-specific alkaline phosphatase (baseline 15.9 mcg/L to 12.9 mcg/L in DTG+RPV versus 16.2 to 17.1 in CAR; P less than .001), osteocalcin (23.8 mcg/L to 19.0 mcg/L in DTG+RPV versus 24.0 to 23.1 in CAR; P less than .001), and procollagen 1 N-terminal propeptide (53.0 mcg/L to 45.6 mcg/L in DTG+RPV versus 55.3 to 54.7 in CAR; P less than .001).
ViiV and Janssen, who sponsored the two studies, have formed a partnership to formulate the two drugs as a single pill, according to Kimberly Smith, MD, MPH, vice president and head of global research and medical strategy at ViiV. “We’re hoping to have the tablet available to people by this time next year,” she said.
The two drugs were chosen for their potency and safety. “Dolutegravir has shown itself to be the most potent integrase inhibitor, it’s well tolerated, and it has a high barrier to resistance. That’s why we thought it would be a good lead drug for a two-drug regimen. We added rilpivirine to it because it’s also a well-tolerated drug. The two have small doses, so we thought we could make a very small pill,” said Dr. Smith.
Joseph Eron, MD, director of Clinical Core at the University of North Carolina, Chapel Hill, who chaired a press conference that discussed the results, noted that the regimen has the potential to have fewer drug interactions. “There are potential distinct advantages to this particular combination.”
Key clinical point: A two-drug combination could reduce cumulative drug exposure in HIV maintenance.
Major finding: Both two-drug and CAR regimens achieved viral suppression in about 95% of patients at week 48.
Data source: An integrated analysis of two open-label, multicenter phase III clinical trials, SWORD 1 and SWORD 2.
Disclosures: The study was sponsored by ViiV Healthcare and Janssen. Dr. Smith is an employee of Viiv. Dr. Llibre has consulted for ViiV and Janssen. Dr. Eron has received consulting fees from AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV, Merck, Tibotec/Janssen, and Tobira.
Prednisone reduces TB-IRIS risk
SEATTLE – In patients coinfected with HIV and tuberculosis, addition of prednisone to an antiretroviral therapy (ART)/TB regimen significantly reduced the incidence of tuberculosis-immune reconstitution inflammatory syndrome (TB-IRIS).
The study showed a reduction of incidence of TB-IRIS – a worsening of the inflammatory elements of tuberculosis that often occurs within a few weeks of starting ART – in the prednisone group, with no sign of adverse events associated with immunosuppression. That safety profile “gives us the reassurance that this is something that could be scaled up. It’s effective but it’s also safe,” said Graeme Meintjes, MD, PhD, professor of medicine at the University of Cape Town, South Africa.
TB-IRIS occurs in 18% of patients undergoing ART/TB regimens, and 25% of these patients wind up in the hospital (Future Microbiol. 2015;10[6]:1077-99).
Ironically, TB-IRIS is of increasing concern because of improved treatment strategies. Clinical trials have shown that, in patients with low CD4 counts, TB mortality is decreased by about 20% if ART is started within 2 weeks of initiation of TB treatment. But when ART is started so soon after TB therapy, patients with low CD4 counts are at about a twofold increased risk of TB-IRIS (Ann Intern Med. 2015;163[1]:32-9).
“That brought into focus the need for an intervention to prevent this complication,” said Dr. Meintjes, who presented the study at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
There were concerns that prednisone could put HIV patients at greater risk of opportunistic infections such as Kaposi’s sarcoma, although Dr. Meintjes noted that those risks were seen in a context of patients who were not taking ART. His team also showed in a previous study (AIDS. 2010;24:2381-90) that prednisone reduced duration of symptoms and hospitalization in TB-IRIS, with no increase in serious infections.
The researchers randomized 240 patients to receive prednisone (40 mg/day for 2 weeks, then 20 mg/day for 2 weeks) within 48 hours of starting ART. All patients started ART within 30 days of starting TB treatment, and had a CD4 count of 100 or fewer cells/microL.
Excluded patients included those with rifampicin resistance, neurobiological tuberculosis, Kaposi’s sarcoma, or hepatitis B, and those who weren’t on the standard first line TB treatment because they couldn’t tolerate it. Patients were also excluded if they had a poor clinical response to TB treatment.
Most endpoints were followed for 12 weeks, but HIV-related cancers were monitored out to 1 year.
Forty-seven percent of patients in the placebo group experienced TB-IRIS within 12 weeks, compared with 33% of patients in the prednisone group (risk ratio, 0.70; 95% confidence interval 0.51-0.96). In an open-label extension study, the researchers noted that 28% of patients who started out in the placebo arm eventually received corticosteroids to treat TB-IRIS, compared with 13% of those who started out in the prednisone arm (RR 0.47, 95% CI 0.27-0.83).
Subjects in the prednisone arm had a lower rate of grade 3 adverse events (29% vs. 45%, P = .01).
There was a similar mortality rate in both arms, and no difference in the incidence of Kaposi’s sarcoma or new-onset AIDS-defining infections.
Dr. Meintjes pointed out that patient selection is important. The trial patients were seen in the clinical setting, not sicker, hospitalized patients, and they had to be improving with TB treatment; significant comorbidities were excluded. “In those patients, prednisone was safe.”
Dr. Meintjes reported having no relevant financial disclosures.
SEATTLE – In patients coinfected with HIV and tuberculosis, addition of prednisone to an antiretroviral therapy (ART)/TB regimen significantly reduced the incidence of tuberculosis-immune reconstitution inflammatory syndrome (TB-IRIS).
The study showed a reduction of incidence of TB-IRIS – a worsening of the inflammatory elements of tuberculosis that often occurs within a few weeks of starting ART – in the prednisone group, with no sign of adverse events associated with immunosuppression. That safety profile “gives us the reassurance that this is something that could be scaled up. It’s effective but it’s also safe,” said Graeme Meintjes, MD, PhD, professor of medicine at the University of Cape Town, South Africa.
TB-IRIS occurs in 18% of patients undergoing ART/TB regimens, and 25% of these patients wind up in the hospital (Future Microbiol. 2015;10[6]:1077-99).
Ironically, TB-IRIS is of increasing concern because of improved treatment strategies. Clinical trials have shown that, in patients with low CD4 counts, TB mortality is decreased by about 20% if ART is started within 2 weeks of initiation of TB treatment. But when ART is started so soon after TB therapy, patients with low CD4 counts are at about a twofold increased risk of TB-IRIS (Ann Intern Med. 2015;163[1]:32-9).
“That brought into focus the need for an intervention to prevent this complication,” said Dr. Meintjes, who presented the study at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
There were concerns that prednisone could put HIV patients at greater risk of opportunistic infections such as Kaposi’s sarcoma, although Dr. Meintjes noted that those risks were seen in a context of patients who were not taking ART. His team also showed in a previous study (AIDS. 2010;24:2381-90) that prednisone reduced duration of symptoms and hospitalization in TB-IRIS, with no increase in serious infections.
The researchers randomized 240 patients to receive prednisone (40 mg/day for 2 weeks, then 20 mg/day for 2 weeks) within 48 hours of starting ART. All patients started ART within 30 days of starting TB treatment, and had a CD4 count of 100 or fewer cells/microL.
Excluded patients included those with rifampicin resistance, neurobiological tuberculosis, Kaposi’s sarcoma, or hepatitis B, and those who weren’t on the standard first line TB treatment because they couldn’t tolerate it. Patients were also excluded if they had a poor clinical response to TB treatment.
Most endpoints were followed for 12 weeks, but HIV-related cancers were monitored out to 1 year.
Forty-seven percent of patients in the placebo group experienced TB-IRIS within 12 weeks, compared with 33% of patients in the prednisone group (risk ratio, 0.70; 95% confidence interval 0.51-0.96). In an open-label extension study, the researchers noted that 28% of patients who started out in the placebo arm eventually received corticosteroids to treat TB-IRIS, compared with 13% of those who started out in the prednisone arm (RR 0.47, 95% CI 0.27-0.83).
Subjects in the prednisone arm had a lower rate of grade 3 adverse events (29% vs. 45%, P = .01).
There was a similar mortality rate in both arms, and no difference in the incidence of Kaposi’s sarcoma or new-onset AIDS-defining infections.
Dr. Meintjes pointed out that patient selection is important. The trial patients were seen in the clinical setting, not sicker, hospitalized patients, and they had to be improving with TB treatment; significant comorbidities were excluded. “In those patients, prednisone was safe.”
Dr. Meintjes reported having no relevant financial disclosures.
SEATTLE – In patients coinfected with HIV and tuberculosis, addition of prednisone to an antiretroviral therapy (ART)/TB regimen significantly reduced the incidence of tuberculosis-immune reconstitution inflammatory syndrome (TB-IRIS).
The study showed a reduction of incidence of TB-IRIS – a worsening of the inflammatory elements of tuberculosis that often occurs within a few weeks of starting ART – in the prednisone group, with no sign of adverse events associated with immunosuppression. That safety profile “gives us the reassurance that this is something that could be scaled up. It’s effective but it’s also safe,” said Graeme Meintjes, MD, PhD, professor of medicine at the University of Cape Town, South Africa.
TB-IRIS occurs in 18% of patients undergoing ART/TB regimens, and 25% of these patients wind up in the hospital (Future Microbiol. 2015;10[6]:1077-99).
Ironically, TB-IRIS is of increasing concern because of improved treatment strategies. Clinical trials have shown that, in patients with low CD4 counts, TB mortality is decreased by about 20% if ART is started within 2 weeks of initiation of TB treatment. But when ART is started so soon after TB therapy, patients with low CD4 counts are at about a twofold increased risk of TB-IRIS (Ann Intern Med. 2015;163[1]:32-9).
“That brought into focus the need for an intervention to prevent this complication,” said Dr. Meintjes, who presented the study at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
There were concerns that prednisone could put HIV patients at greater risk of opportunistic infections such as Kaposi’s sarcoma, although Dr. Meintjes noted that those risks were seen in a context of patients who were not taking ART. His team also showed in a previous study (AIDS. 2010;24:2381-90) that prednisone reduced duration of symptoms and hospitalization in TB-IRIS, with no increase in serious infections.
The researchers randomized 240 patients to receive prednisone (40 mg/day for 2 weeks, then 20 mg/day for 2 weeks) within 48 hours of starting ART. All patients started ART within 30 days of starting TB treatment, and had a CD4 count of 100 or fewer cells/microL.
Excluded patients included those with rifampicin resistance, neurobiological tuberculosis, Kaposi’s sarcoma, or hepatitis B, and those who weren’t on the standard first line TB treatment because they couldn’t tolerate it. Patients were also excluded if they had a poor clinical response to TB treatment.
Most endpoints were followed for 12 weeks, but HIV-related cancers were monitored out to 1 year.
Forty-seven percent of patients in the placebo group experienced TB-IRIS within 12 weeks, compared with 33% of patients in the prednisone group (risk ratio, 0.70; 95% confidence interval 0.51-0.96). In an open-label extension study, the researchers noted that 28% of patients who started out in the placebo arm eventually received corticosteroids to treat TB-IRIS, compared with 13% of those who started out in the prednisone arm (RR 0.47, 95% CI 0.27-0.83).
Subjects in the prednisone arm had a lower rate of grade 3 adverse events (29% vs. 45%, P = .01).
There was a similar mortality rate in both arms, and no difference in the incidence of Kaposi’s sarcoma or new-onset AIDS-defining infections.
Dr. Meintjes pointed out that patient selection is important. The trial patients were seen in the clinical setting, not sicker, hospitalized patients, and they had to be improving with TB treatment; significant comorbidities were excluded. “In those patients, prednisone was safe.”
Dr. Meintjes reported having no relevant financial disclosures.
AT CROI
Key clinical point:
Major finding: Forty-seven percent of placebo patients experienced TB-IRIS within 12 weeks, compared with 33% of patients in the prednisone group.
Data source: Randomized, placebo-controlled trial of 240 patients.
Disclosures: Dr. Meintjes reported having no relevant financial disclosures.
LEEP tops cryotherapy for cervical dysplasia in women with HIV
SEATTLE – HIV-positive women treated with cryotherapy for cervical intraepithelial neoplasia (CIN) 2/3 were 52% more likely than women treated with loop excision to have a recurrence within 2 years, according to a randomized trial in Kenya.
The investigators called on the World Health Organization and other groups to support loop excision as the first-line option for HIV-positive women in sub-Saharan Africa and other low-resource settings, but the findings also support its use, when possible, in Western countries.
For the study, 200 HIV-positive women were randomized to cryotherapy and 200 to LEEP for treatment of CIN 2/3 at the Coptic Hope Center for Infectious Diseases in Nairobi, with follow-up Pap smears every 6 months afterwards for 2 years.
At 12 months, 36 women in the LEEP group (18%) had recurrent high-grade squamous intraepithelial lesions (HSIL), versus 54 women (27%) in the cryotherapy arm. At 24 months, HSIL was detected in 52 women who had LEEP (26%), versus 74 who had cryotherapy (37%).
Overall, the rate of recurrence of HSIL was 21.1 per 100 woman-years after cryotherapy and 14.0 per 100 woman-years after LEEP.
It’s unclear how those results would have played out in the United States, Ms. Green said, noting that LEEP failure rates are far lower among women who do not have HIV, but the success of LEEP in HIV-positive women in the United States has not been well studied.
The World Health Organization “recommends posttreatment follow-up at 12 months, regardless of HIV status. Our findings indicate that women should be screened at more frequent intervals, particularly following cryotherapy,” she said at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
Women were excluded from the study if they were pregnant. The median age was 37 years, and almost all the subjects were on antiretroviral therapy at the time of intervention, with a median CD4 count of 380 cells/mcL. Median follow-up was 2.1 years in both arms; most of the women completed all four follow-up visits. The majority in both groups had CIN 3; about 10 in each arm had carcinoma in situ.
“Cervical screening and treatment using visual inspection with acetic acid and cryotherapy is often implemented in resource-limited settings with high HIV-1 endemicity; however … HIV testing and referral for LEEP may be more effective” and cost effective if it prevents later hysterectomy, Ms. Greene said.
Ms. Greene had no disclosures. The work was funded by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and the Centers for Disease Control and Prevention.
SEATTLE – HIV-positive women treated with cryotherapy for cervical intraepithelial neoplasia (CIN) 2/3 were 52% more likely than women treated with loop excision to have a recurrence within 2 years, according to a randomized trial in Kenya.
The investigators called on the World Health Organization and other groups to support loop excision as the first-line option for HIV-positive women in sub-Saharan Africa and other low-resource settings, but the findings also support its use, when possible, in Western countries.
For the study, 200 HIV-positive women were randomized to cryotherapy and 200 to LEEP for treatment of CIN 2/3 at the Coptic Hope Center for Infectious Diseases in Nairobi, with follow-up Pap smears every 6 months afterwards for 2 years.
At 12 months, 36 women in the LEEP group (18%) had recurrent high-grade squamous intraepithelial lesions (HSIL), versus 54 women (27%) in the cryotherapy arm. At 24 months, HSIL was detected in 52 women who had LEEP (26%), versus 74 who had cryotherapy (37%).
Overall, the rate of recurrence of HSIL was 21.1 per 100 woman-years after cryotherapy and 14.0 per 100 woman-years after LEEP.
It’s unclear how those results would have played out in the United States, Ms. Green said, noting that LEEP failure rates are far lower among women who do not have HIV, but the success of LEEP in HIV-positive women in the United States has not been well studied.
The World Health Organization “recommends posttreatment follow-up at 12 months, regardless of HIV status. Our findings indicate that women should be screened at more frequent intervals, particularly following cryotherapy,” she said at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
Women were excluded from the study if they were pregnant. The median age was 37 years, and almost all the subjects were on antiretroviral therapy at the time of intervention, with a median CD4 count of 380 cells/mcL. Median follow-up was 2.1 years in both arms; most of the women completed all four follow-up visits. The majority in both groups had CIN 3; about 10 in each arm had carcinoma in situ.
“Cervical screening and treatment using visual inspection with acetic acid and cryotherapy is often implemented in resource-limited settings with high HIV-1 endemicity; however … HIV testing and referral for LEEP may be more effective” and cost effective if it prevents later hysterectomy, Ms. Greene said.
Ms. Greene had no disclosures. The work was funded by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and the Centers for Disease Control and Prevention.
SEATTLE – HIV-positive women treated with cryotherapy for cervical intraepithelial neoplasia (CIN) 2/3 were 52% more likely than women treated with loop excision to have a recurrence within 2 years, according to a randomized trial in Kenya.
The investigators called on the World Health Organization and other groups to support loop excision as the first-line option for HIV-positive women in sub-Saharan Africa and other low-resource settings, but the findings also support its use, when possible, in Western countries.
For the study, 200 HIV-positive women were randomized to cryotherapy and 200 to LEEP for treatment of CIN 2/3 at the Coptic Hope Center for Infectious Diseases in Nairobi, with follow-up Pap smears every 6 months afterwards for 2 years.
At 12 months, 36 women in the LEEP group (18%) had recurrent high-grade squamous intraepithelial lesions (HSIL), versus 54 women (27%) in the cryotherapy arm. At 24 months, HSIL was detected in 52 women who had LEEP (26%), versus 74 who had cryotherapy (37%).
Overall, the rate of recurrence of HSIL was 21.1 per 100 woman-years after cryotherapy and 14.0 per 100 woman-years after LEEP.
It’s unclear how those results would have played out in the United States, Ms. Green said, noting that LEEP failure rates are far lower among women who do not have HIV, but the success of LEEP in HIV-positive women in the United States has not been well studied.
The World Health Organization “recommends posttreatment follow-up at 12 months, regardless of HIV status. Our findings indicate that women should be screened at more frequent intervals, particularly following cryotherapy,” she said at the Conference on Retroviruses & Opportunistic Infections in partnership with the International Antiviral Society.
Women were excluded from the study if they were pregnant. The median age was 37 years, and almost all the subjects were on antiretroviral therapy at the time of intervention, with a median CD4 count of 380 cells/mcL. Median follow-up was 2.1 years in both arms; most of the women completed all four follow-up visits. The majority in both groups had CIN 3; about 10 in each arm had carcinoma in situ.
“Cervical screening and treatment using visual inspection with acetic acid and cryotherapy is often implemented in resource-limited settings with high HIV-1 endemicity; however … HIV testing and referral for LEEP may be more effective” and cost effective if it prevents later hysterectomy, Ms. Greene said.
Ms. Greene had no disclosures. The work was funded by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) and the Centers for Disease Control and Prevention.
AT CROI
Key clinical point:
Major finding: At 12 months, 36 women in the LEEP group (18%) had recurrent high-grade squamous intraepithelial lesions, versus 54 women (27%) in the cryotherapy group. At 24 months, HSIL was detected in 52 women in the LEEP arm (26%) versus 74 who had cryotherapy (37%).
Data source: Randomized trial of 400 women in Kenya.
Disclosures: The lead investigator had no disclosures. The work was funded by the U.S. President’s Emergency Plan for AIDS Relief and the Centers for Disease Control and Prevention.