User login
FDA: Endocrinologic and Metabolic Drugs Advisory Committee
CV outcomes data needed to expand approval of TG-lowering agent
SILVER SPRING, MD. – An approved formulation of an omega-3 fatty acid – icosapent ethyl – was effective in reducing triglycerides when added to a statin in adults with mixed dyslipidemia, at a high risk of coronary heart disease, but it should not be approved for treating such patients until the results of a cardiovascular outcomes trial become available, an expert panel has advised the Food and Drug Administration.
Icosapent ethyl is a purified formulation of ethyl eicosapentaenoic acid (EPA), derived from fish oil, which was approved by the FDA in 2012 as monotherapy for treatment of severe hypertriglyceridemia (500 mg/dL or higher), based on the premise that lowering triglyceride levels reduces the risk of pancreatitis, not that it affects cardiovascular outcomes, according to the FDA. It comes in a capsule formulation and is marketed as Vascepa by Amarin Pharmaceuticals Ireland Ltd.
On the basis of the results of the ANCHOR study, the manufacturer has proposed that Vascepa be approved as an adjunct to diet in combination with a statin to reduce triglycerides (TG), non-HDL cholesterol (non-HDL-C), apolipoprotein B (Apo B), LDL cholesterol (LDL-C), total cholesterol (TC) and very-LDL cholesterol (VLDL-C) "in adults with mixed dyslipidemia and coronary heart disease or a CHD risk equivalent."
However, at its meeting on Oct. 16, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee voted 9-2 that the favorable effects of treatment with 4 g/day of Vascepa on triglycerides and other lipid and lipoprotein parameters over placebo after 12 weeks in the ANCHOR study were not sufficient to recommend approval. Those who voted against approval recommended waiting for the results of the REDUCE-IT study, a large CV outcomes trial of Vascepa currently underway, to determine if these effects translate into improved cardiovascular outcomes, before approving the expanded indication.
The ANCHOR study showed that Vascepa "effectively lowers triglycerides in the proposed population," but until the CV outcomes study is completed, "I just don’t know if it will reduce CV risk," said Dr. Ellen Seely, professor of medicine at Harvard University and an endocrinologist at Brigham and Women’s Hospital, Boston.
Another panel member, Dr. Brendan Everett, director of the general cardiology inpatient service at Brigham and Women’s, said that he was optimistic about the data and that treatment with Vascepa "could very well offer potential substantial benefits for patients with coronary heart disease or coronary heart disease risk equivalents." However, it’s not clear that reducing triglycerides will have an impact on that risk and "I am wary ... of approving a drug that has a potential market of tens of millions of people without any hard efficacy data," he added.
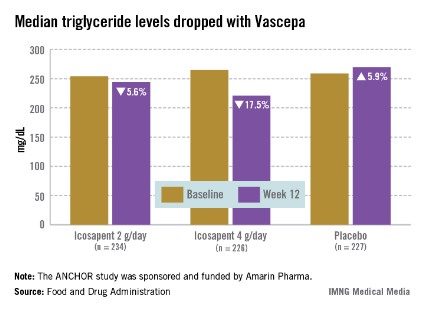
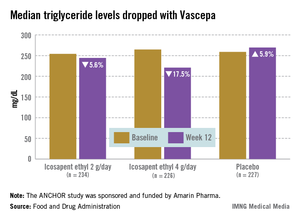
The ANCHOR study compared two doses of Vascepa to placebo in about 700 mostly white, male patients with mixed dyslipidemia and at high cardiovascular risk, who were taking a statin. Their mean age was 61 years and 73% had diabetes. After 12 weeks, the fasting TG value increased by a median of 5.9% from baseline among those on placebo, compared with a median 17.5% reduction among those on 4 g of Vascepa daily, the proposed dose – a 21.5% treatment difference that was highly statistically significant. Other changes among those on placebo went in the adverse direction, with the exception of HDL-C, which increased by a median of 4.8% vs. a median reduction of 1% among those on Vascepa.
Overall, Vascepa was well tolerated, with no new safety issues identified, according to the company. The most common adverse events associated with treatment were diarrhea, urinary tract infections, upper respiratory tract infections, and nausea, and the withdrawal rate due to adverse events was low (2% among those on 4 g/day). The FDA reviewers agreed with the company’s assessment of safety, although several panelists pointed out that the safety data are from a relatively small number of patients studied, and expressed some concerns about bleeding events (2.6% of those on 4 g/day vs. 1.7% of those on placebo).
The main issue discussed was the clinical significance of the effects of treatment with Vascepa, when administered with a statin, on triglycerides, and other changes in lipid and lipoproteins observed in the ANCHOR study, whether these changes would translate into a meaningful reduction ion CV risk, in the target population. The panelists agreed that Vascepa was effective in lowering TGs, but they were concerned about the possibility that the effects on TGs could be overstated because Vascepa was compared with placebo in the study, and they agreed that the impact on CV outcomes was uncertain, based on the data.
During the FDA review of the data, Dr. Mary Dunne Roberts, a clinical reviewer in the FDA’s Division of Metabolism and Endocrinology Products, referred to the ongoing debate over whether drug-induced modulation of TGs results in improved CV outcomes. Results of recent CV outcome trials with fenofibrate and niacin have raised questions about whether targeting lipids and lipoproteins other than LDL-cholesterol results in incremental cardiovascular benefits in patients on optimal statin therapy and LDL-C control, she pointed out.
In 2011, Amarin launched REDUCE-IT, which is comparing the impact of treatment with 4 g of Vascepa per day to placebo added to statin therapy in 8,000 patients with CV disease or CV risk, on a composite endpoint of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, and unstable angina requiring hospitalization. At the beginning of October, about 6,000 patients with a median TG level of 202 mg/dL had been enrolled. The company expects the study to be completed in 2016 or 2017.
Panelists agreed that REDUCE-IT was a well-designed, important study, with an appropriate group of patients, which should provide answers about whether CV outcomes will be improved with Vascepa, and they were encouraged that the study was underway.
Panelist Dr. Peter Wilson, professor of medicine in the cardiology division of Emory University, Atlanta, said that there was no need to prescribe more agents "because we think they work," and he expressed concern about overprescribing. "There is already overuse of some omega-3s without proven benefit. We need the trial results in hand to guide decisions."
Vascepa is one of two omega-3 fatty acid formulations approved by the FDA; the other is Lovaza, a combination of EPA and docosahexaenoic acid (DHA). Many omega- 3 fatty acid products are available over the counter, with variable combinations and strengths.
The FDA usually follows the recommendations of its advisory panels. Members have been cleared of potential conflicts; occasionally, a panelist is given a waiver for a potential conflict, but not at this meeting.
SILVER SPRING, MD. – An approved formulation of an omega-3 fatty acid – icosapent ethyl – was effective in reducing triglycerides when added to a statin in adults with mixed dyslipidemia, at a high risk of coronary heart disease, but it should not be approved for treating such patients until the results of a cardiovascular outcomes trial become available, an expert panel has advised the Food and Drug Administration.
Icosapent ethyl is a purified formulation of ethyl eicosapentaenoic acid (EPA), derived from fish oil, which was approved by the FDA in 2012 as monotherapy for treatment of severe hypertriglyceridemia (500 mg/dL or higher), based on the premise that lowering triglyceride levels reduces the risk of pancreatitis, not that it affects cardiovascular outcomes, according to the FDA. It comes in a capsule formulation and is marketed as Vascepa by Amarin Pharmaceuticals Ireland Ltd.

On the basis of the results of the ANCHOR study, the manufacturer has proposed that Vascepa be approved as an adjunct to diet in combination with a statin to reduce triglycerides (TG), non-HDL cholesterol (non-HDL-C), apolipoprotein B (Apo B), LDL cholesterol (LDL-C), total cholesterol (TC) and very-LDL cholesterol (VLDL-C) "in adults with mixed dyslipidemia and coronary heart disease or a CHD risk equivalent."
However, at its meeting on Oct. 16, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee voted 9-2 that the favorable effects of treatment with 4 g/day of Vascepa on triglycerides and other lipid and lipoprotein parameters over placebo after 12 weeks in the ANCHOR study were not sufficient to recommend approval. Those who voted against approval recommended waiting for the results of the REDUCE-IT study, a large CV outcomes trial of Vascepa currently underway, to determine if these effects translate into improved cardiovascular outcomes, before approving the expanded indication.
The ANCHOR study showed that Vascepa "effectively lowers triglycerides in the proposed population," but until the CV outcomes study is completed, "I just don’t know if it will reduce CV risk," said Dr. Ellen Seely, professor of medicine at Harvard University and an endocrinologist at Brigham and Women’s Hospital, Boston.
Another panel member, Dr. Brendan Everett, director of the general cardiology inpatient service at Brigham and Women’s, said that he was optimistic about the data and that treatment with Vascepa "could very well offer potential substantial benefits for patients with coronary heart disease or coronary heart disease risk equivalents." However, it’s not clear that reducing triglycerides will have an impact on that risk and "I am wary ... of approving a drug that has a potential market of tens of millions of people without any hard efficacy data," he added.
The ANCHOR study compared two doses of Vascepa to placebo in about 700 mostly white, male patients with mixed dyslipidemia and at high cardiovascular risk, who were taking a statin. Their mean age was 61 years and 73% had diabetes. After 12 weeks, the fasting TG value increased by a median of 5.9% from baseline among those on placebo, compared with a median 17.5% reduction among those on 4 g of Vascepa daily, the proposed dose – a 21.5% treatment difference that was highly statistically significant. Other changes among those on placebo went in the adverse direction, with the exception of HDL-C, which increased by a median of 4.8% vs. a median reduction of 1% among those on Vascepa.
Overall, Vascepa was well tolerated, with no new safety issues identified, according to the company. The most common adverse events associated with treatment were diarrhea, urinary tract infections, upper respiratory tract infections, and nausea, and the withdrawal rate due to adverse events was low (2% among those on 4 g/day). The FDA reviewers agreed with the company’s assessment of safety, although several panelists pointed out that the safety data are from a relatively small number of patients studied, and expressed some concerns about bleeding events (2.6% of those on 4 g/day vs. 1.7% of those on placebo).
The main issue discussed was the clinical significance of the effects of treatment with Vascepa, when administered with a statin, on triglycerides, and other changes in lipid and lipoproteins observed in the ANCHOR study, whether these changes would translate into a meaningful reduction ion CV risk, in the target population. The panelists agreed that Vascepa was effective in lowering TGs, but they were concerned about the possibility that the effects on TGs could be overstated because Vascepa was compared with placebo in the study, and they agreed that the impact on CV outcomes was uncertain, based on the data.
During the FDA review of the data, Dr. Mary Dunne Roberts, a clinical reviewer in the FDA’s Division of Metabolism and Endocrinology Products, referred to the ongoing debate over whether drug-induced modulation of TGs results in improved CV outcomes. Results of recent CV outcome trials with fenofibrate and niacin have raised questions about whether targeting lipids and lipoproteins other than LDL-cholesterol results in incremental cardiovascular benefits in patients on optimal statin therapy and LDL-C control, she pointed out.
In 2011, Amarin launched REDUCE-IT, which is comparing the impact of treatment with 4 g of Vascepa per day to placebo added to statin therapy in 8,000 patients with CV disease or CV risk, on a composite endpoint of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, and unstable angina requiring hospitalization. At the beginning of October, about 6,000 patients with a median TG level of 202 mg/dL had been enrolled. The company expects the study to be completed in 2016 or 2017.
Panelists agreed that REDUCE-IT was a well-designed, important study, with an appropriate group of patients, which should provide answers about whether CV outcomes will be improved with Vascepa, and they were encouraged that the study was underway.
Panelist Dr. Peter Wilson, professor of medicine in the cardiology division of Emory University, Atlanta, said that there was no need to prescribe more agents "because we think they work," and he expressed concern about overprescribing. "There is already overuse of some omega-3s without proven benefit. We need the trial results in hand to guide decisions."
Vascepa is one of two omega-3 fatty acid formulations approved by the FDA; the other is Lovaza, a combination of EPA and docosahexaenoic acid (DHA). Many omega- 3 fatty acid products are available over the counter, with variable combinations and strengths.
The FDA usually follows the recommendations of its advisory panels. Members have been cleared of potential conflicts; occasionally, a panelist is given a waiver for a potential conflict, but not at this meeting.
SILVER SPRING, MD. – An approved formulation of an omega-3 fatty acid – icosapent ethyl – was effective in reducing triglycerides when added to a statin in adults with mixed dyslipidemia, at a high risk of coronary heart disease, but it should not be approved for treating such patients until the results of a cardiovascular outcomes trial become available, an expert panel has advised the Food and Drug Administration.
Icosapent ethyl is a purified formulation of ethyl eicosapentaenoic acid (EPA), derived from fish oil, which was approved by the FDA in 2012 as monotherapy for treatment of severe hypertriglyceridemia (500 mg/dL or higher), based on the premise that lowering triglyceride levels reduces the risk of pancreatitis, not that it affects cardiovascular outcomes, according to the FDA. It comes in a capsule formulation and is marketed as Vascepa by Amarin Pharmaceuticals Ireland Ltd.

On the basis of the results of the ANCHOR study, the manufacturer has proposed that Vascepa be approved as an adjunct to diet in combination with a statin to reduce triglycerides (TG), non-HDL cholesterol (non-HDL-C), apolipoprotein B (Apo B), LDL cholesterol (LDL-C), total cholesterol (TC) and very-LDL cholesterol (VLDL-C) "in adults with mixed dyslipidemia and coronary heart disease or a CHD risk equivalent."
However, at its meeting on Oct. 16, the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee voted 9-2 that the favorable effects of treatment with 4 g/day of Vascepa on triglycerides and other lipid and lipoprotein parameters over placebo after 12 weeks in the ANCHOR study were not sufficient to recommend approval. Those who voted against approval recommended waiting for the results of the REDUCE-IT study, a large CV outcomes trial of Vascepa currently underway, to determine if these effects translate into improved cardiovascular outcomes, before approving the expanded indication.
The ANCHOR study showed that Vascepa "effectively lowers triglycerides in the proposed population," but until the CV outcomes study is completed, "I just don’t know if it will reduce CV risk," said Dr. Ellen Seely, professor of medicine at Harvard University and an endocrinologist at Brigham and Women’s Hospital, Boston.
Another panel member, Dr. Brendan Everett, director of the general cardiology inpatient service at Brigham and Women’s, said that he was optimistic about the data and that treatment with Vascepa "could very well offer potential substantial benefits for patients with coronary heart disease or coronary heart disease risk equivalents." However, it’s not clear that reducing triglycerides will have an impact on that risk and "I am wary ... of approving a drug that has a potential market of tens of millions of people without any hard efficacy data," he added.
The ANCHOR study compared two doses of Vascepa to placebo in about 700 mostly white, male patients with mixed dyslipidemia and at high cardiovascular risk, who were taking a statin. Their mean age was 61 years and 73% had diabetes. After 12 weeks, the fasting TG value increased by a median of 5.9% from baseline among those on placebo, compared with a median 17.5% reduction among those on 4 g of Vascepa daily, the proposed dose – a 21.5% treatment difference that was highly statistically significant. Other changes among those on placebo went in the adverse direction, with the exception of HDL-C, which increased by a median of 4.8% vs. a median reduction of 1% among those on Vascepa.
Overall, Vascepa was well tolerated, with no new safety issues identified, according to the company. The most common adverse events associated with treatment were diarrhea, urinary tract infections, upper respiratory tract infections, and nausea, and the withdrawal rate due to adverse events was low (2% among those on 4 g/day). The FDA reviewers agreed with the company’s assessment of safety, although several panelists pointed out that the safety data are from a relatively small number of patients studied, and expressed some concerns about bleeding events (2.6% of those on 4 g/day vs. 1.7% of those on placebo).
The main issue discussed was the clinical significance of the effects of treatment with Vascepa, when administered with a statin, on triglycerides, and other changes in lipid and lipoproteins observed in the ANCHOR study, whether these changes would translate into a meaningful reduction ion CV risk, in the target population. The panelists agreed that Vascepa was effective in lowering TGs, but they were concerned about the possibility that the effects on TGs could be overstated because Vascepa was compared with placebo in the study, and they agreed that the impact on CV outcomes was uncertain, based on the data.
During the FDA review of the data, Dr. Mary Dunne Roberts, a clinical reviewer in the FDA’s Division of Metabolism and Endocrinology Products, referred to the ongoing debate over whether drug-induced modulation of TGs results in improved CV outcomes. Results of recent CV outcome trials with fenofibrate and niacin have raised questions about whether targeting lipids and lipoproteins other than LDL-cholesterol results in incremental cardiovascular benefits in patients on optimal statin therapy and LDL-C control, she pointed out.
In 2011, Amarin launched REDUCE-IT, which is comparing the impact of treatment with 4 g of Vascepa per day to placebo added to statin therapy in 8,000 patients with CV disease or CV risk, on a composite endpoint of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, and unstable angina requiring hospitalization. At the beginning of October, about 6,000 patients with a median TG level of 202 mg/dL had been enrolled. The company expects the study to be completed in 2016 or 2017.
Panelists agreed that REDUCE-IT was a well-designed, important study, with an appropriate group of patients, which should provide answers about whether CV outcomes will be improved with Vascepa, and they were encouraged that the study was underway.
Panelist Dr. Peter Wilson, professor of medicine in the cardiology division of Emory University, Atlanta, said that there was no need to prescribe more agents "because we think they work," and he expressed concern about overprescribing. "There is already overuse of some omega-3s without proven benefit. We need the trial results in hand to guide decisions."
Vascepa is one of two omega-3 fatty acid formulations approved by the FDA; the other is Lovaza, a combination of EPA and docosahexaenoic acid (DHA). Many omega- 3 fatty acid products are available over the counter, with variable combinations and strengths.
The FDA usually follows the recommendations of its advisory panels. Members have been cleared of potential conflicts; occasionally, a panelist is given a waiver for a potential conflict, but not at this meeting.
FROM AN FDA ADVISORY PANEL MEETING