User login
Heart Rhythm Society (HRS): Heart Rhythm 2013
Watchman device hits home run in PROTECT AF trial
DENVER – The investigational Watchman left atrial appendage closure device for stroke prevention in patients with atrial fibrillation proved markedly superior to warfarin in the 4-year follow-up of the randomized PROTECT AF trial.
Watchman recipients demonstrated a 40% reduction in the primary efficacy endpoint – a composite of stroke, systemic embolism, or cardiovascular death – compared with the warfarin-treated controls (95% confidence interval, 0.38-0.97; P = .03).
The Watchman group also showed a statistically significant 34% reduction in risk of all-cause mortality, compared with the 4.8% incidence in the warfarin arm (95% CI, 0.45-0.98; P = .04).
"This is something that’s really extraordinary. I wasn’t expecting to see a statistically significant decrease in all-cause mortality," Dr. Vivek Reddy said in presenting the new PROTECT AF data at the annual meeting of the Heart Rhythm Society.
The reduced all-cause mortality rate in the Watchman group resulted from fewer deaths due to cardiovascular-, pulmonary disease–, and hemorrhagic stroke–related causes.
In an earlier interim study analysis with 2.3 years and roughly 1,600 person-years of follow-up, the Watchman was merely statistically noninferior to warfarin in terms of efficacy (Circulation 2013;127:720-9). But in the updated analysis, with a mean follow-up of 45 months and 2,621 person-years, the device therapy has evolved in statistical parlance from noninferior to superior.
"The amount of benefit hasn’t changed. It’s been about a 40% difference throughout most of the trial. What’s changed is that with more follow-up the confidence intervals have narrowed, increasing the degree of certainty that the number is really true," according to Dr. Reddy of Mount Sinai School of Medicine, New York.
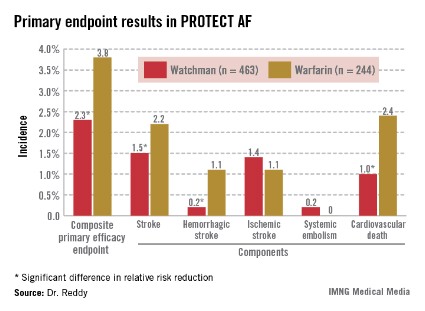
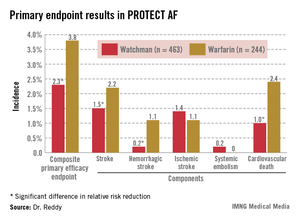
The Watchman’s superiority in terms of the primary efficacy endpoint was driven by an 85% reduction in the risk of hemorrhagic stroke and a 60% decrease in cardiovascular death compared with the warfarin group (see graphic). Importantly, the Watchman’s efficacy advantage was preserved in all prespecified patient subgroups, including the participants at greatest stroke risk: the roughly 20% with a baseline prior stroke or transient ischemic attack (TIA).
The Watchman is designed as a replacement for warfarin, a stroke prophylaxis drug whose numerous shortcomings are well known. The device is implanted in the left atrial appendage in a catheter procedure which entails an atrial puncture. Upon deployment, the Watchman excludes the left atrial appendage from the systemic circulation. The benefits seen in PROTECT AF and other Watchman studies confirm the notion that the left atrial appendage is critical to the pathogenesis of stroke in atrial fibrillation (AF), Dr. Reddy observed.
The 707 PROTECT AF participants at 59 centers were split fairly evenly between those with paroxysmal, persistent, and permanent AF. Subjects were randomized two-to-one to the Watchman or conventional warfarin therapy. Subjects in the warfarin group spent an average of 70% of their time with drug levels in therapeutic range, a considerably higher figure than studies would suggest is the norm in everyday clinical practice.
The composite primary safety endpoint occurred in a similar proportion of Watchman recipients and controls. However, the nature and timing of the adverse events differed in the two groups.
The most common adverse event in the Watchman group was serious pericardial effusion, which occurred in 4.8% of patients. Two-thirds of affected patients were managed via percutaneous intervention; the rest underwent surgery. The incidence of pericardial effusion decreased with greater operator experience: The rate was 6.3% in the first half of the study and 3.7% afterwards. Of note, in more recent studies begun since procedural refinements were introduced, the pericardial effusion rate has been lower than in PROTECT AF.
The chief adverse event in the warfarin group was, not surprisingly, major bleeding, which occurred in 4.1% of subjects, compared with a 0.6% rate in Watchman recipients. Unlike adverse events in the Watchman group, which were mostly procedure related, complications in the warfarin group were spread out over the full course of follow-up.
Dr. Reddy considers the PROTECT AF results broadly applicable even in this era of new oral anticoagulants.
"Despite the availability of novel anticoagulants, warfarin is still the most widely prescribed agent for stroke prevention," he noted.
Asked if he thought the Food and Drug Administration is likely to approve the Watchman based upon the new evidence from PROTECT AF as well as other studies, Dr. Reddy said it’s difficult to predict the agency’s actions but he certainly considers the device approvable.
If and when the Watchman is approved, he added, the first population where it is likely to see widespread use is in AF patients for whom warfarin is contraindicated. It would also be logical to consider the device in patients with a relative contraindication to warfarin, such as those on dialysis, at increased risk for falls, and elderly patients on polypharmacy; indeed, those are patient groups in which any anticoagulant is problematic. Head-to-head comparative studies of the Watchman and the novel anticoagulants would be welcome but are unlikely to occur anytime soon, Dr. Reddy said.
A fifth and final year of follow-up is scheduled for the PROTECT AF study.
The trial is sponsored by Boston Scientific. Dr. Reddy reported serving as a consultant to or receiving research grant support from Boston Scientific, Coherex Medical, and St. Jude Medical.
DENVER – The investigational Watchman left atrial appendage closure device for stroke prevention in patients with atrial fibrillation proved markedly superior to warfarin in the 4-year follow-up of the randomized PROTECT AF trial.
Watchman recipients demonstrated a 40% reduction in the primary efficacy endpoint – a composite of stroke, systemic embolism, or cardiovascular death – compared with the warfarin-treated controls (95% confidence interval, 0.38-0.97; P = .03).

The Watchman group also showed a statistically significant 34% reduction in risk of all-cause mortality, compared with the 4.8% incidence in the warfarin arm (95% CI, 0.45-0.98; P = .04).
"This is something that’s really extraordinary. I wasn’t expecting to see a statistically significant decrease in all-cause mortality," Dr. Vivek Reddy said in presenting the new PROTECT AF data at the annual meeting of the Heart Rhythm Society.
The reduced all-cause mortality rate in the Watchman group resulted from fewer deaths due to cardiovascular-, pulmonary disease–, and hemorrhagic stroke–related causes.
In an earlier interim study analysis with 2.3 years and roughly 1,600 person-years of follow-up, the Watchman was merely statistically noninferior to warfarin in terms of efficacy (Circulation 2013;127:720-9). But in the updated analysis, with a mean follow-up of 45 months and 2,621 person-years, the device therapy has evolved in statistical parlance from noninferior to superior.
"The amount of benefit hasn’t changed. It’s been about a 40% difference throughout most of the trial. What’s changed is that with more follow-up the confidence intervals have narrowed, increasing the degree of certainty that the number is really true," according to Dr. Reddy of Mount Sinai School of Medicine, New York.
The Watchman’s superiority in terms of the primary efficacy endpoint was driven by an 85% reduction in the risk of hemorrhagic stroke and a 60% decrease in cardiovascular death compared with the warfarin group (see graphic). Importantly, the Watchman’s efficacy advantage was preserved in all prespecified patient subgroups, including the participants at greatest stroke risk: the roughly 20% with a baseline prior stroke or transient ischemic attack (TIA).
The Watchman is designed as a replacement for warfarin, a stroke prophylaxis drug whose numerous shortcomings are well known. The device is implanted in the left atrial appendage in a catheter procedure which entails an atrial puncture. Upon deployment, the Watchman excludes the left atrial appendage from the systemic circulation. The benefits seen in PROTECT AF and other Watchman studies confirm the notion that the left atrial appendage is critical to the pathogenesis of stroke in atrial fibrillation (AF), Dr. Reddy observed.
The 707 PROTECT AF participants at 59 centers were split fairly evenly between those with paroxysmal, persistent, and permanent AF. Subjects were randomized two-to-one to the Watchman or conventional warfarin therapy. Subjects in the warfarin group spent an average of 70% of their time with drug levels in therapeutic range, a considerably higher figure than studies would suggest is the norm in everyday clinical practice.
The composite primary safety endpoint occurred in a similar proportion of Watchman recipients and controls. However, the nature and timing of the adverse events differed in the two groups.
The most common adverse event in the Watchman group was serious pericardial effusion, which occurred in 4.8% of patients. Two-thirds of affected patients were managed via percutaneous intervention; the rest underwent surgery. The incidence of pericardial effusion decreased with greater operator experience: The rate was 6.3% in the first half of the study and 3.7% afterwards. Of note, in more recent studies begun since procedural refinements were introduced, the pericardial effusion rate has been lower than in PROTECT AF.
The chief adverse event in the warfarin group was, not surprisingly, major bleeding, which occurred in 4.1% of subjects, compared with a 0.6% rate in Watchman recipients. Unlike adverse events in the Watchman group, which were mostly procedure related, complications in the warfarin group were spread out over the full course of follow-up.
Dr. Reddy considers the PROTECT AF results broadly applicable even in this era of new oral anticoagulants.
"Despite the availability of novel anticoagulants, warfarin is still the most widely prescribed agent for stroke prevention," he noted.
Asked if he thought the Food and Drug Administration is likely to approve the Watchman based upon the new evidence from PROTECT AF as well as other studies, Dr. Reddy said it’s difficult to predict the agency’s actions but he certainly considers the device approvable.
If and when the Watchman is approved, he added, the first population where it is likely to see widespread use is in AF patients for whom warfarin is contraindicated. It would also be logical to consider the device in patients with a relative contraindication to warfarin, such as those on dialysis, at increased risk for falls, and elderly patients on polypharmacy; indeed, those are patient groups in which any anticoagulant is problematic. Head-to-head comparative studies of the Watchman and the novel anticoagulants would be welcome but are unlikely to occur anytime soon, Dr. Reddy said.
A fifth and final year of follow-up is scheduled for the PROTECT AF study.
The trial is sponsored by Boston Scientific. Dr. Reddy reported serving as a consultant to or receiving research grant support from Boston Scientific, Coherex Medical, and St. Jude Medical.
DENVER – The investigational Watchman left atrial appendage closure device for stroke prevention in patients with atrial fibrillation proved markedly superior to warfarin in the 4-year follow-up of the randomized PROTECT AF trial.
Watchman recipients demonstrated a 40% reduction in the primary efficacy endpoint – a composite of stroke, systemic embolism, or cardiovascular death – compared with the warfarin-treated controls (95% confidence interval, 0.38-0.97; P = .03).

The Watchman group also showed a statistically significant 34% reduction in risk of all-cause mortality, compared with the 4.8% incidence in the warfarin arm (95% CI, 0.45-0.98; P = .04).
"This is something that’s really extraordinary. I wasn’t expecting to see a statistically significant decrease in all-cause mortality," Dr. Vivek Reddy said in presenting the new PROTECT AF data at the annual meeting of the Heart Rhythm Society.
The reduced all-cause mortality rate in the Watchman group resulted from fewer deaths due to cardiovascular-, pulmonary disease–, and hemorrhagic stroke–related causes.
In an earlier interim study analysis with 2.3 years and roughly 1,600 person-years of follow-up, the Watchman was merely statistically noninferior to warfarin in terms of efficacy (Circulation 2013;127:720-9). But in the updated analysis, with a mean follow-up of 45 months and 2,621 person-years, the device therapy has evolved in statistical parlance from noninferior to superior.
"The amount of benefit hasn’t changed. It’s been about a 40% difference throughout most of the trial. What’s changed is that with more follow-up the confidence intervals have narrowed, increasing the degree of certainty that the number is really true," according to Dr. Reddy of Mount Sinai School of Medicine, New York.
The Watchman’s superiority in terms of the primary efficacy endpoint was driven by an 85% reduction in the risk of hemorrhagic stroke and a 60% decrease in cardiovascular death compared with the warfarin group (see graphic). Importantly, the Watchman’s efficacy advantage was preserved in all prespecified patient subgroups, including the participants at greatest stroke risk: the roughly 20% with a baseline prior stroke or transient ischemic attack (TIA).
The Watchman is designed as a replacement for warfarin, a stroke prophylaxis drug whose numerous shortcomings are well known. The device is implanted in the left atrial appendage in a catheter procedure which entails an atrial puncture. Upon deployment, the Watchman excludes the left atrial appendage from the systemic circulation. The benefits seen in PROTECT AF and other Watchman studies confirm the notion that the left atrial appendage is critical to the pathogenesis of stroke in atrial fibrillation (AF), Dr. Reddy observed.
The 707 PROTECT AF participants at 59 centers were split fairly evenly between those with paroxysmal, persistent, and permanent AF. Subjects were randomized two-to-one to the Watchman or conventional warfarin therapy. Subjects in the warfarin group spent an average of 70% of their time with drug levels in therapeutic range, a considerably higher figure than studies would suggest is the norm in everyday clinical practice.
The composite primary safety endpoint occurred in a similar proportion of Watchman recipients and controls. However, the nature and timing of the adverse events differed in the two groups.
The most common adverse event in the Watchman group was serious pericardial effusion, which occurred in 4.8% of patients. Two-thirds of affected patients were managed via percutaneous intervention; the rest underwent surgery. The incidence of pericardial effusion decreased with greater operator experience: The rate was 6.3% in the first half of the study and 3.7% afterwards. Of note, in more recent studies begun since procedural refinements were introduced, the pericardial effusion rate has been lower than in PROTECT AF.
The chief adverse event in the warfarin group was, not surprisingly, major bleeding, which occurred in 4.1% of subjects, compared with a 0.6% rate in Watchman recipients. Unlike adverse events in the Watchman group, which were mostly procedure related, complications in the warfarin group were spread out over the full course of follow-up.
Dr. Reddy considers the PROTECT AF results broadly applicable even in this era of new oral anticoagulants.
"Despite the availability of novel anticoagulants, warfarin is still the most widely prescribed agent for stroke prevention," he noted.
Asked if he thought the Food and Drug Administration is likely to approve the Watchman based upon the new evidence from PROTECT AF as well as other studies, Dr. Reddy said it’s difficult to predict the agency’s actions but he certainly considers the device approvable.
If and when the Watchman is approved, he added, the first population where it is likely to see widespread use is in AF patients for whom warfarin is contraindicated. It would also be logical to consider the device in patients with a relative contraindication to warfarin, such as those on dialysis, at increased risk for falls, and elderly patients on polypharmacy; indeed, those are patient groups in which any anticoagulant is problematic. Head-to-head comparative studies of the Watchman and the novel anticoagulants would be welcome but are unlikely to occur anytime soon, Dr. Reddy said.
A fifth and final year of follow-up is scheduled for the PROTECT AF study.
The trial is sponsored by Boston Scientific. Dr. Reddy reported serving as a consultant to or receiving research grant support from Boston Scientific, Coherex Medical, and St. Jude Medical.
AT HEART RHYTHM 2013
Major finding: Patients with atrial fibrillation who received the Watchman left atrial appendage closure device had a 40% lower risk of the combined endpoint of stroke, systemic embolism, or cardiovascular death than those randomized to warfarin. They also had a 34% lower risk of all-cause mortality.
Data source: This analysis of the prospective, multicenter PROTECT AF trial included 707 randomized patients followed for a mean of 45 months.
Disclosures: PROTECT AF is sponsored by Boston Scientific. Dr. Reddy reported serving as a consultant to or receiving research grant support from Boston Scientific, Coherex Medical, and St. Jude Medical.
Renal dysfunction improved after AF ablation
DENVER – Catheter ablation of atrial fibrillation in patients with comorbid moderate chronic kidney disease may have a side benefit: improved renal function, according to Dr. Nazem W. Akoum.
Among 81 patients with stage 3 or 4 chronic kidney disease (CKD) who underwent successful AF ablation, the mean estimated glomerular filtration rate (eGFR) improved significantly, from 46.7 mL/min per 1.73 m2 at baseline to 53 mL/min per 1.73 m2 at follow-up 3 months post procedure, Dr. Akoum reported at the annual meeting of the Heart Rhythm Society.
In contrast, the eGFR in 382 patients with stage 1 or 2 CKD who underwent AF ablation didn’t change significantly, from a baseline mean of 103.6 mL/min per 1.73 m2, added Dr. Akoum, an electrophysiologist at the University of Utah, Salt Lake City.
Patients with AF and stage 5 or 6 CKD were excluded from participation in this study. Given the requirement for preablation quantification of the extent of atrial fibrosis via delayed-enhancement MRI with gadolinium contrast, there were safety concerns regarding exposing patients with advanced CKD to gadolinium.
Patients with stage 1 or 2 CKD averaged 15.8% left atrial fibrosis, while stage 3 or 4 patients averaged 19.1%.
The improvement in eGFR noted in the group with stage 3 or 4 CKD following restoration of sinus rhythm appeared to be independent of left ventricular systolic performance or medication effects. The postablation regularization of ventricular activation and the resultant improvement in endothelial function may have contributed to the welcome renal side benefit, Dr. Akoum said.
He reported having no relevant financial conflicts.
DENVER – Catheter ablation of atrial fibrillation in patients with comorbid moderate chronic kidney disease may have a side benefit: improved renal function, according to Dr. Nazem W. Akoum.
Among 81 patients with stage 3 or 4 chronic kidney disease (CKD) who underwent successful AF ablation, the mean estimated glomerular filtration rate (eGFR) improved significantly, from 46.7 mL/min per 1.73 m2 at baseline to 53 mL/min per 1.73 m2 at follow-up 3 months post procedure, Dr. Akoum reported at the annual meeting of the Heart Rhythm Society.
In contrast, the eGFR in 382 patients with stage 1 or 2 CKD who underwent AF ablation didn’t change significantly, from a baseline mean of 103.6 mL/min per 1.73 m2, added Dr. Akoum, an electrophysiologist at the University of Utah, Salt Lake City.
Patients with AF and stage 5 or 6 CKD were excluded from participation in this study. Given the requirement for preablation quantification of the extent of atrial fibrosis via delayed-enhancement MRI with gadolinium contrast, there were safety concerns regarding exposing patients with advanced CKD to gadolinium.
Patients with stage 1 or 2 CKD averaged 15.8% left atrial fibrosis, while stage 3 or 4 patients averaged 19.1%.
The improvement in eGFR noted in the group with stage 3 or 4 CKD following restoration of sinus rhythm appeared to be independent of left ventricular systolic performance or medication effects. The postablation regularization of ventricular activation and the resultant improvement in endothelial function may have contributed to the welcome renal side benefit, Dr. Akoum said.
He reported having no relevant financial conflicts.
DENVER – Catheter ablation of atrial fibrillation in patients with comorbid moderate chronic kidney disease may have a side benefit: improved renal function, according to Dr. Nazem W. Akoum.
Among 81 patients with stage 3 or 4 chronic kidney disease (CKD) who underwent successful AF ablation, the mean estimated glomerular filtration rate (eGFR) improved significantly, from 46.7 mL/min per 1.73 m2 at baseline to 53 mL/min per 1.73 m2 at follow-up 3 months post procedure, Dr. Akoum reported at the annual meeting of the Heart Rhythm Society.
In contrast, the eGFR in 382 patients with stage 1 or 2 CKD who underwent AF ablation didn’t change significantly, from a baseline mean of 103.6 mL/min per 1.73 m2, added Dr. Akoum, an electrophysiologist at the University of Utah, Salt Lake City.
Patients with AF and stage 5 or 6 CKD were excluded from participation in this study. Given the requirement for preablation quantification of the extent of atrial fibrosis via delayed-enhancement MRI with gadolinium contrast, there were safety concerns regarding exposing patients with advanced CKD to gadolinium.
Patients with stage 1 or 2 CKD averaged 15.8% left atrial fibrosis, while stage 3 or 4 patients averaged 19.1%.
The improvement in eGFR noted in the group with stage 3 or 4 CKD following restoration of sinus rhythm appeared to be independent of left ventricular systolic performance or medication effects. The postablation regularization of ventricular activation and the resultant improvement in endothelial function may have contributed to the welcome renal side benefit, Dr. Akoum said.
He reported having no relevant financial conflicts.
AT HEART RHYTHM 2013
Major finding: Patients with stage 3 or 4 chronic kidney disease who underwent catheter ablation of atrial fibrillation showed significant improvement in renal function 3 months post procedure, with their estimated glomerular filtration rate improving from a baseline mean of 46.7 mL/min per 1.73 m2 to 53 mL/min per 1.73 m2.
Data source: A prospective study of 463 patients with stage 1-4 chronic kidney disease undergoing AF ablation.
Disclosures: Dr. Nazem W. Akoum reported having no relevant financial conflicts.
Antipsychotics linked to sudden cardiac death risk
DENVER – Both the second-generation as well as the first-generation antipsychotic agents proved independently associated with greater than threefold increased risks of sudden cardiac death, according to results from a large, population-based study.
However, schizophrenia per se was not linked to an increased risk, Dr. Audrey Uy-Evanado reported at the annual meeting of the Heart Rhythm Society.
She presented data from the ongoing landmark Oregon Sudden Unexplained Death Study involving 1,544 documented sudden cardiac deaths (SCDs) and 774 matched controls.
The prevalence of diagnosed schizophrenia among patients with SCD was 2.5%, significantly greater than the 0.5% figure in controls. At the time of SCD, 8.2% of subjects were on an antipsychotic agent, compared with 1.9% of controls.
Second-generation, or "atypical," antipsychotic agents such as risperidone (Risperdal) and olanzapine (Zyprexa) were being used by 6.3% of the SCD cohort and 1.3% of controls. First-generation antipsychotic agents such as haloperidol, fluphenazine, and chlorpromazine were used by 2.6% of the SCD group, compared with 0.5% of controls. All of these between-group differences were highly significant.
Third-generation antipsychotic agents such as aripiprazole (Abilify) were so rarely used – only 0.1% of patients were on a third-generation agent – that it wasn’t possible to draw any conclusions about SCD risk, according to Dr. Uy-Evanado of Cedars-Sinai Medical Center in Los Angeles.
In terms of cardiovascular risk factors, hypertension and obesity were significantly more prevalent in the control group – but the SCD cohort had increased rates of diabetes, chronic kidney disease, and severe left ventricular dysfunction.
Aside from schizophrenia, rates of other psychiatric disorders didn’t differ between the two groups.
In a multivariate logistic regression analysis adjusted for age, gender, and comorbidities, having schizophrenia was not associated with an increased risk of SCD. However, patients on a first-generation antipsychotic agent had a 3.76-fold increased risk of SCD (P = .0002), and those on a second-generation antipsychotic drug had a 3.3-fold increased risk.
This study was undertaken as a follow-up to an earlier report from the Oregon Sudden Unexplained Death Study, which broadly identified antipsychotic drug therapy as being associated with SCD. That observation raised the question as to whether the risk is limited to specific classes of antipsychotic agents. The answer appears to be no.
The Oregon Sudden Unexplained Death Study is funded by Oregon Health and Science University and the Centers for Disease Control and Prevention. Dr. Uy-Evanado reported having no conflicts of interest.
DENVER – Both the second-generation as well as the first-generation antipsychotic agents proved independently associated with greater than threefold increased risks of sudden cardiac death, according to results from a large, population-based study.
However, schizophrenia per se was not linked to an increased risk, Dr. Audrey Uy-Evanado reported at the annual meeting of the Heart Rhythm Society.
She presented data from the ongoing landmark Oregon Sudden Unexplained Death Study involving 1,544 documented sudden cardiac deaths (SCDs) and 774 matched controls.
The prevalence of diagnosed schizophrenia among patients with SCD was 2.5%, significantly greater than the 0.5% figure in controls. At the time of SCD, 8.2% of subjects were on an antipsychotic agent, compared with 1.9% of controls.
Second-generation, or "atypical," antipsychotic agents such as risperidone (Risperdal) and olanzapine (Zyprexa) were being used by 6.3% of the SCD cohort and 1.3% of controls. First-generation antipsychotic agents such as haloperidol, fluphenazine, and chlorpromazine were used by 2.6% of the SCD group, compared with 0.5% of controls. All of these between-group differences were highly significant.
Third-generation antipsychotic agents such as aripiprazole (Abilify) were so rarely used – only 0.1% of patients were on a third-generation agent – that it wasn’t possible to draw any conclusions about SCD risk, according to Dr. Uy-Evanado of Cedars-Sinai Medical Center in Los Angeles.
In terms of cardiovascular risk factors, hypertension and obesity were significantly more prevalent in the control group – but the SCD cohort had increased rates of diabetes, chronic kidney disease, and severe left ventricular dysfunction.
Aside from schizophrenia, rates of other psychiatric disorders didn’t differ between the two groups.
In a multivariate logistic regression analysis adjusted for age, gender, and comorbidities, having schizophrenia was not associated with an increased risk of SCD. However, patients on a first-generation antipsychotic agent had a 3.76-fold increased risk of SCD (P = .0002), and those on a second-generation antipsychotic drug had a 3.3-fold increased risk.
This study was undertaken as a follow-up to an earlier report from the Oregon Sudden Unexplained Death Study, which broadly identified antipsychotic drug therapy as being associated with SCD. That observation raised the question as to whether the risk is limited to specific classes of antipsychotic agents. The answer appears to be no.
The Oregon Sudden Unexplained Death Study is funded by Oregon Health and Science University and the Centers for Disease Control and Prevention. Dr. Uy-Evanado reported having no conflicts of interest.
DENVER – Both the second-generation as well as the first-generation antipsychotic agents proved independently associated with greater than threefold increased risks of sudden cardiac death, according to results from a large, population-based study.
However, schizophrenia per se was not linked to an increased risk, Dr. Audrey Uy-Evanado reported at the annual meeting of the Heart Rhythm Society.
She presented data from the ongoing landmark Oregon Sudden Unexplained Death Study involving 1,544 documented sudden cardiac deaths (SCDs) and 774 matched controls.
The prevalence of diagnosed schizophrenia among patients with SCD was 2.5%, significantly greater than the 0.5% figure in controls. At the time of SCD, 8.2% of subjects were on an antipsychotic agent, compared with 1.9% of controls.
Second-generation, or "atypical," antipsychotic agents such as risperidone (Risperdal) and olanzapine (Zyprexa) were being used by 6.3% of the SCD cohort and 1.3% of controls. First-generation antipsychotic agents such as haloperidol, fluphenazine, and chlorpromazine were used by 2.6% of the SCD group, compared with 0.5% of controls. All of these between-group differences were highly significant.
Third-generation antipsychotic agents such as aripiprazole (Abilify) were so rarely used – only 0.1% of patients were on a third-generation agent – that it wasn’t possible to draw any conclusions about SCD risk, according to Dr. Uy-Evanado of Cedars-Sinai Medical Center in Los Angeles.
In terms of cardiovascular risk factors, hypertension and obesity were significantly more prevalent in the control group – but the SCD cohort had increased rates of diabetes, chronic kidney disease, and severe left ventricular dysfunction.
Aside from schizophrenia, rates of other psychiatric disorders didn’t differ between the two groups.
In a multivariate logistic regression analysis adjusted for age, gender, and comorbidities, having schizophrenia was not associated with an increased risk of SCD. However, patients on a first-generation antipsychotic agent had a 3.76-fold increased risk of SCD (P = .0002), and those on a second-generation antipsychotic drug had a 3.3-fold increased risk.
This study was undertaken as a follow-up to an earlier report from the Oregon Sudden Unexplained Death Study, which broadly identified antipsychotic drug therapy as being associated with SCD. That observation raised the question as to whether the risk is limited to specific classes of antipsychotic agents. The answer appears to be no.
The Oregon Sudden Unexplained Death Study is funded by Oregon Health and Science University and the Centers for Disease Control and Prevention. Dr. Uy-Evanado reported having no conflicts of interest.
AT HEART RHYTHM 2013
Major finding: Use of a first-generation antipsychotic agent was independently associated with a 3.76-fold increased risk of sudden cardiac death in a large, ongoing community-based study. Use of a second-generation antipsychotic drug was associated with a 3.31-fold increased risk.
Data source: This analysis from the Oregon Sudden Unexplained Death Study involved 1,544 documented cases of sudden cardiac death and 774 controls.
Disclosures: The Oregon Sudden Unexplained Death Study is sponsored by Oregon Health and Science University and the Centers for Disease Control and Prevention. The presenter reported having no conflicts of interest.
DECAAF points way to improved AF ablation
DENVER – Preprocedural quantification of atrial fibrosis via delayed-enhancement MRI offers a potent means of boosting atrial fibrillation ablation success rates by weeding out likely nonresponders, a study has shown.
Results of the double-blind, multinational prospective DECAAF trial (Delayed-Enhancement MRI Determinant of Successful Catheter Ablation for Atrial Fibrillation) show that the extent of atrial fibrosis is a strong predictor of patient outcome. The more fibrosis present when measured preprocedurally by delayed-enhancement MRI (DE-MRI) with gadolinium contrast, the less likely a patient would remain in sinus rhythm at follow-up more than 1 year following ablation, Dr. Nassir Marrouche reported at the annual meeting of the Heart Rhythm Society.
"Hopefully this will help us better individualize the therapy. We have to accept at some point that we can’t cure everybody with atrial fibrillation. This modality helps us to better define which patients should be eligible," according to Dr. Marrouche, director of the comprehensive arrhythmia research and management center at the University of Utah, Salt Lake City.
One attractive feature of this management strategy is that patients who are candidates for AF ablation already routinely undergo a preprocedural 3-D scan with CT or MRI. It’s a straightforward matter to train an MRI technician to measure atrial fibrosis, as DECAAF showed.
This is a practice-changing development, he said: "We recommend that in every single ablation patient, before you touch their heart you really should know how extensive their disease is."
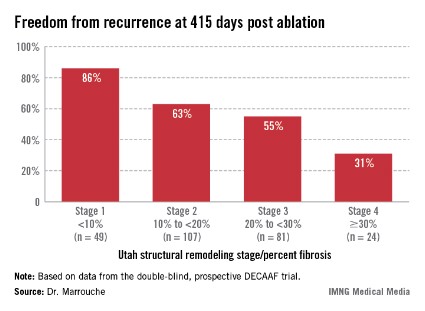
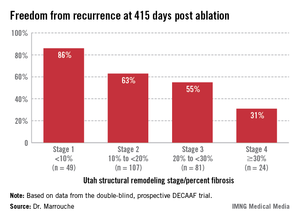
Patients undergoing a first ablation procedure for paroxysmal AF typically have about a 60% success rate, meaning they remain free of atrial arrhythmia at 1 year. In contrast, DECAAF participants with less than 10% fibrosis in their left atrium had a success rate of 86% at 415 days of follow-up.
Patients with less than 10% preprocedural atrial fibrosis are categorized as Utah stage 1 disease using a left atrial structural remodeling classification system previously described by Dr. Marrouche and his coworkers . At the other end of the spectrum, DECAAF subjects who were Utah stage 4, with at least 30% fibrosis, had a dismal 31% freedom of recurrence 415 days after the end of the standard 90-day postprocedural blanking period. Patients with stage 2 or 3 atrial fibrosis had intermediate long-term outcomes (see box).
"We sit down with a stage 4 patient and say, ‘Listen, your chance of being cured of this disease at long-term follow-up is not very high,’" the electrophysiologist explained. "We put our expectations on the table. We now have 5-year follow-up data showing a 5%-10% success in Utah 4 patients. We in Utah today are very reluctant to take such patients into the lab and ablate them. Instead, we try every option to rate-control them. But Utah stage 1 is straightforward. I make that decision for the patient: ‘You should be ablated early on.’ "
DECAAF included 261 patients with AF who underwent a first ablation procedure at 15 centers in the United States, Europe, and Australia. But first they all underwent DE-MRI, the results of which were sent to a core lab for blinded quantification of atrial fibrosis. The interventionalists remained blinded to the fibrosis findings.
Most DECAAF participants had paroxysmal AF. The ablation procedure consisted of pulmonary vein isolation alone in 68% of patients and pulmonary vein isolation plus other ablation maneuvers in the rest.
The only predictor of atrial fibrosis on DE-MRI was a history of hypertension. Duration of AF, patient age, AF type, and comorbid conditions were not associated with the extent of fibrosis.
In a multivariate analysis controlled for numerous potential confounders, the only significant predictor of AF recurrence was atrial fibrosis. For each 1% increase in fibrosis, the risk of recurrence climbed by 6.3%. Among the factors adjusted for in this multivariate analysis were age, sex, hypertension, heart failure, mitral valve disease, diabetes, AF type, left atrial volume, and procedure type.
Dr. Richard I. Fogel, chair of the scientific sessions program committee and HRS president-elect, commented that he found it surprising and counterintuitive that AF duration was not a predictor of the extent of atrial fibrosis in DECAAF.
"I thought that the theory was ‘atrial fibrillation begets atrial fibrillation,’ " said Dr. Fogel, a cardiologist and electrophysiologist in group practice in Indianapolis.
Dr. Marrouche replied that large gaps remain in understanding the pathophysiology of AF in humans.
"We’ve known for a long time from animal models that AF begets AF. With MRI, for the first time we’re opening the heart in humans with AF, and it doesn’t look like the animal models at all. The fibrosis in humans isn’t diffuse; it’s patchy, and it’s localized," according to Dr. Marrouche.
Every single participant in DECAAF had atrial fibrosis on the posterior left atrial wall; indeed, posterior wall fibrosis accounted for 57% of the total burden of fibrosis. But some patients with, say, a 15-year history of symptomatic AF, had minimal fibrosis, while some middle-aged marathon runners with recent-onset AF had 50% fibrosis indicative of advanced disease.
Why some patients with AF are relatively protected against tissue fibrosis while others aren’t is an important question for further study. Possible factors worthy of investigation include genetics and inflammation, he said.
Dr. Marrouche reported having no relevant financial conflicts.
DENVER – Preprocedural quantification of atrial fibrosis via delayed-enhancement MRI offers a potent means of boosting atrial fibrillation ablation success rates by weeding out likely nonresponders, a study has shown.
Results of the double-blind, multinational prospective DECAAF trial (Delayed-Enhancement MRI Determinant of Successful Catheter Ablation for Atrial Fibrillation) show that the extent of atrial fibrosis is a strong predictor of patient outcome. The more fibrosis present when measured preprocedurally by delayed-enhancement MRI (DE-MRI) with gadolinium contrast, the less likely a patient would remain in sinus rhythm at follow-up more than 1 year following ablation, Dr. Nassir Marrouche reported at the annual meeting of the Heart Rhythm Society.

"Hopefully this will help us better individualize the therapy. We have to accept at some point that we can’t cure everybody with atrial fibrillation. This modality helps us to better define which patients should be eligible," according to Dr. Marrouche, director of the comprehensive arrhythmia research and management center at the University of Utah, Salt Lake City.
One attractive feature of this management strategy is that patients who are candidates for AF ablation already routinely undergo a preprocedural 3-D scan with CT or MRI. It’s a straightforward matter to train an MRI technician to measure atrial fibrosis, as DECAAF showed.
This is a practice-changing development, he said: "We recommend that in every single ablation patient, before you touch their heart you really should know how extensive their disease is."
Patients undergoing a first ablation procedure for paroxysmal AF typically have about a 60% success rate, meaning they remain free of atrial arrhythmia at 1 year. In contrast, DECAAF participants with less than 10% fibrosis in their left atrium had a success rate of 86% at 415 days of follow-up.
Patients with less than 10% preprocedural atrial fibrosis are categorized as Utah stage 1 disease using a left atrial structural remodeling classification system previously described by Dr. Marrouche and his coworkers . At the other end of the spectrum, DECAAF subjects who were Utah stage 4, with at least 30% fibrosis, had a dismal 31% freedom of recurrence 415 days after the end of the standard 90-day postprocedural blanking period. Patients with stage 2 or 3 atrial fibrosis had intermediate long-term outcomes (see box).
"We sit down with a stage 4 patient and say, ‘Listen, your chance of being cured of this disease at long-term follow-up is not very high,’" the electrophysiologist explained. "We put our expectations on the table. We now have 5-year follow-up data showing a 5%-10% success in Utah 4 patients. We in Utah today are very reluctant to take such patients into the lab and ablate them. Instead, we try every option to rate-control them. But Utah stage 1 is straightforward. I make that decision for the patient: ‘You should be ablated early on.’ "
DECAAF included 261 patients with AF who underwent a first ablation procedure at 15 centers in the United States, Europe, and Australia. But first they all underwent DE-MRI, the results of which were sent to a core lab for blinded quantification of atrial fibrosis. The interventionalists remained blinded to the fibrosis findings.
Most DECAAF participants had paroxysmal AF. The ablation procedure consisted of pulmonary vein isolation alone in 68% of patients and pulmonary vein isolation plus other ablation maneuvers in the rest.
The only predictor of atrial fibrosis on DE-MRI was a history of hypertension. Duration of AF, patient age, AF type, and comorbid conditions were not associated with the extent of fibrosis.
In a multivariate analysis controlled for numerous potential confounders, the only significant predictor of AF recurrence was atrial fibrosis. For each 1% increase in fibrosis, the risk of recurrence climbed by 6.3%. Among the factors adjusted for in this multivariate analysis were age, sex, hypertension, heart failure, mitral valve disease, diabetes, AF type, left atrial volume, and procedure type.
Dr. Richard I. Fogel, chair of the scientific sessions program committee and HRS president-elect, commented that he found it surprising and counterintuitive that AF duration was not a predictor of the extent of atrial fibrosis in DECAAF.
"I thought that the theory was ‘atrial fibrillation begets atrial fibrillation,’ " said Dr. Fogel, a cardiologist and electrophysiologist in group practice in Indianapolis.
Dr. Marrouche replied that large gaps remain in understanding the pathophysiology of AF in humans.
"We’ve known for a long time from animal models that AF begets AF. With MRI, for the first time we’re opening the heart in humans with AF, and it doesn’t look like the animal models at all. The fibrosis in humans isn’t diffuse; it’s patchy, and it’s localized," according to Dr. Marrouche.
Every single participant in DECAAF had atrial fibrosis on the posterior left atrial wall; indeed, posterior wall fibrosis accounted for 57% of the total burden of fibrosis. But some patients with, say, a 15-year history of symptomatic AF, had minimal fibrosis, while some middle-aged marathon runners with recent-onset AF had 50% fibrosis indicative of advanced disease.
Why some patients with AF are relatively protected against tissue fibrosis while others aren’t is an important question for further study. Possible factors worthy of investigation include genetics and inflammation, he said.
Dr. Marrouche reported having no relevant financial conflicts.
DENVER – Preprocedural quantification of atrial fibrosis via delayed-enhancement MRI offers a potent means of boosting atrial fibrillation ablation success rates by weeding out likely nonresponders, a study has shown.
Results of the double-blind, multinational prospective DECAAF trial (Delayed-Enhancement MRI Determinant of Successful Catheter Ablation for Atrial Fibrillation) show that the extent of atrial fibrosis is a strong predictor of patient outcome. The more fibrosis present when measured preprocedurally by delayed-enhancement MRI (DE-MRI) with gadolinium contrast, the less likely a patient would remain in sinus rhythm at follow-up more than 1 year following ablation, Dr. Nassir Marrouche reported at the annual meeting of the Heart Rhythm Society.

"Hopefully this will help us better individualize the therapy. We have to accept at some point that we can’t cure everybody with atrial fibrillation. This modality helps us to better define which patients should be eligible," according to Dr. Marrouche, director of the comprehensive arrhythmia research and management center at the University of Utah, Salt Lake City.
One attractive feature of this management strategy is that patients who are candidates for AF ablation already routinely undergo a preprocedural 3-D scan with CT or MRI. It’s a straightforward matter to train an MRI technician to measure atrial fibrosis, as DECAAF showed.
This is a practice-changing development, he said: "We recommend that in every single ablation patient, before you touch their heart you really should know how extensive their disease is."
Patients undergoing a first ablation procedure for paroxysmal AF typically have about a 60% success rate, meaning they remain free of atrial arrhythmia at 1 year. In contrast, DECAAF participants with less than 10% fibrosis in their left atrium had a success rate of 86% at 415 days of follow-up.
Patients with less than 10% preprocedural atrial fibrosis are categorized as Utah stage 1 disease using a left atrial structural remodeling classification system previously described by Dr. Marrouche and his coworkers . At the other end of the spectrum, DECAAF subjects who were Utah stage 4, with at least 30% fibrosis, had a dismal 31% freedom of recurrence 415 days after the end of the standard 90-day postprocedural blanking period. Patients with stage 2 or 3 atrial fibrosis had intermediate long-term outcomes (see box).
"We sit down with a stage 4 patient and say, ‘Listen, your chance of being cured of this disease at long-term follow-up is not very high,’" the electrophysiologist explained. "We put our expectations on the table. We now have 5-year follow-up data showing a 5%-10% success in Utah 4 patients. We in Utah today are very reluctant to take such patients into the lab and ablate them. Instead, we try every option to rate-control them. But Utah stage 1 is straightforward. I make that decision for the patient: ‘You should be ablated early on.’ "
DECAAF included 261 patients with AF who underwent a first ablation procedure at 15 centers in the United States, Europe, and Australia. But first they all underwent DE-MRI, the results of which were sent to a core lab for blinded quantification of atrial fibrosis. The interventionalists remained blinded to the fibrosis findings.
Most DECAAF participants had paroxysmal AF. The ablation procedure consisted of pulmonary vein isolation alone in 68% of patients and pulmonary vein isolation plus other ablation maneuvers in the rest.
The only predictor of atrial fibrosis on DE-MRI was a history of hypertension. Duration of AF, patient age, AF type, and comorbid conditions were not associated with the extent of fibrosis.
In a multivariate analysis controlled for numerous potential confounders, the only significant predictor of AF recurrence was atrial fibrosis. For each 1% increase in fibrosis, the risk of recurrence climbed by 6.3%. Among the factors adjusted for in this multivariate analysis were age, sex, hypertension, heart failure, mitral valve disease, diabetes, AF type, left atrial volume, and procedure type.
Dr. Richard I. Fogel, chair of the scientific sessions program committee and HRS president-elect, commented that he found it surprising and counterintuitive that AF duration was not a predictor of the extent of atrial fibrosis in DECAAF.
"I thought that the theory was ‘atrial fibrillation begets atrial fibrillation,’ " said Dr. Fogel, a cardiologist and electrophysiologist in group practice in Indianapolis.
Dr. Marrouche replied that large gaps remain in understanding the pathophysiology of AF in humans.
"We’ve known for a long time from animal models that AF begets AF. With MRI, for the first time we’re opening the heart in humans with AF, and it doesn’t look like the animal models at all. The fibrosis in humans isn’t diffuse; it’s patchy, and it’s localized," according to Dr. Marrouche.
Every single participant in DECAAF had atrial fibrosis on the posterior left atrial wall; indeed, posterior wall fibrosis accounted for 57% of the total burden of fibrosis. But some patients with, say, a 15-year history of symptomatic AF, had minimal fibrosis, while some middle-aged marathon runners with recent-onset AF had 50% fibrosis indicative of advanced disease.
Why some patients with AF are relatively protected against tissue fibrosis while others aren’t is an important question for further study. Possible factors worthy of investigation include genetics and inflammation, he said.
Dr. Marrouche reported having no relevant financial conflicts.
AT HEART RHYTHM 2013
Major Finding: Patients undergoing a first ablation procedure for atrial fibrillation had an 86% long-term rate of maintenance of sinus rhythm if a preprocedural delayed-enhancement MRI showed minimal left atrial fibrosis. For every 1% of additional fibrosis present on MRI, the risk of atrial arrhythmia recurrence rose by 6.3%.
Data Source: A double-blind, prospective, multinational trial in 261 AF patients who underwent delayed enhancement MRI prior to catheter ablation with long-term follow-up.
Disclosures: The presenter reported having no relevant financial conflicts.
Upcoming guidelines on inherited arrhythmias contain surprises
DENVER – Major new guidelines on the diagnosis and management of patients with inherited primary arrhythmia syndromes have been jointly issued by the Heart Rhythm Society and its European and Asian counterparts.
The guidelines were sorely needed, according to Dr. Silvia G. Priori, cochair of the expert consensus panel writing group.
The field of inherited arrhythmias is rapidly evolving, with new pathogenic genetic mutations being found all the time. Much has changed in the 7 years since issuance of the last major guidelines: the American College of Cardiology/American Heart Association/European Society of Cardiology guidelines on prevention of sudden cardiac death (Circulation 2006;114:e385-484), noted Dr. Priori of the University of Pavia (Italy).
Her cochair, Dr. Arthur A. Wilde of the University of Amsterdam pointed out that the new 69-page report is the first major document to address some of the newer inherited arrhythmia syndromes, including catecholaminergic polymorphic ventricular tachycardia (CPVT), short QT syndrome, early repolarization, and progressive cardiac conduction disease (PCCD). In addition, the new report proposes major changes in the diagnostic criteria for the two most common primary arrhythmia syndromes: long QT syndrome (LQTS) and Brugada syndrome.
"Many of our colleagues in the field will be surprised," Dr. Priori predicted.
Here are the highlights:
• LQTS: With an estimated prevalence of roughly 1 per 2,000 live births worldwide, this is the most common of the inherited arrhythmia syndromes. What is likely to come as a surprise to many physicians is the expert consensus panel’s recommendation that the diagnosis of LQTS requires either the finding of an unequivocally causative mutation in one of the LQTS genes or, in the absence of such a defect, either a QTc interval of 480-499 ms in repeated 12-lead ECGs in a patient with unexplained syncope or a QTc of 500 ms in repeated ECGs in the absence of a secondary cause for QT prolongation in a nonsyncopal patient.
"A single ECG reading 10 ms above the upper limit of normal is not enough to establish the diagnosis. That’s quite different from what’s being done in common practice. Many of the patients who are referred to the centers of expertise on inherited arrhythmias are borderline patients in whom maybe one ECG was abnormal, and yet because of that they’ve been labeled as being affected by a genetic disease even if the genetic studies were negative. A single abnormal QTc measurement in a patient with negative genetic testing is not enough," Dr. Priori declared.
Dr. Wilde said the new guidelines loosen up the guidance on participation in competitive sports. The blanket prohibition of the past has been replaced by a case-by-case approach, with a Class I recommendation for routine referral to a clinical expert for evaluation of the risk posed by athletic activity. For example, although swimming is a very-high-risk activity for patients with the LQTS1 genotype, that’s not true for those who have LQTS2 or -3.
"It’s clear that if a patient with long QT syndrome has exercise-related syncopal events, that patient should not participate in competitive sports. But if the patient is asymptomatic and has minor QT prolongation, there’s probably not much reason for concern," he said.
"This is a sharp departure," Dr. Priori observed. "In several European countries, if you have the diagnosis of long QT syndrome, sports participation is not permitted, even if your physician clears you. So we hope with this document to slowly, carefully, begin to allow patients with this condition to do sports safely. We wanted to lift the ban so that a physician who feels a specific patient would have a low risk in the proper environment could make that recommendation."
• Brugada syndrome: Far more common in Asia than the western world, Brugada syndrome is 8- to 10-fold more frequent in males than females. The big change in the new guidelines is that the diagnosis no longer requires specific ECG changes plus clinical manifestations. Now, Brugada syndrome, like LQTS, is a pure ECG diagnosis. It is made definitively when a type 1 ST-segment elevation is noted either spontaneously or after administration of an intravenous sodium channel-blocking agent; the ST finding has to be observed in at least one right precordial lead placed in a standard or superior position up to the second intercostal space.
An implantable cardioverter-defibrillator is clearly indicated in a Brugada syndrome patient with a prior cardiac arrest or documented ventricular arrhythmias. The controversy lies in how to manage the asymptomatic patient. The guidelines give ICD implantation a weak Class IIb recommendation – meaning it "may be considered" – when such patients exhibit inducible ventricular arrhythmias during programmed electrical stimulation in the electrophysiology lab.
• Catecholaminergic polymorphic ventricular tachycardia: The prevalence of CPVT is unclear, but it has been estimated at 1 per 5,000 live births, according to Dr. Wilde. This highly malignant condition is diagnosed in patients with a known pathogenic mutation, or in the presence of a structurally normal heart, a normal resting ECG, and unexplained exercise- or catecholamine-induced bidirectional VT of polymorphic ventricular premature beats or VT before age 40 years. First-line therapy is a long-acting beta-blocker such as nadolol, coupled with exercise restriction. ICD therapy is problematic because the inevitable inappropriate shocks increase sympathetic tone, triggering true shockable arrhythmias in a vicious cycle.
• Short QT syndrome: This is a rare channelopathy. It is diagnosed on the basis of a QTc of 330 ms or less, or a QTc of less than 360 ms in the presence of a pathogenic mutation, family history of sudden death before age 40 years, cardiac arrest in the absence of structural heart disease, or a family history of short QT syndrome.
• Progressive cardiac conduction disease: Still incompletely understood, PCCD is diagnosed in individuals under age 50 years who have unexplained progressive conduction abnormalities and a structurally normal heart with no skeletal myopathies. Pacemaker implantation is the most useful therapy.
• Early repolarization: The first report linking this extremely common ECG finding to sudden death came less than 5 years ago. Early repolarization, as characterized by J-point and ST-segment elevation in two or more contiguous leads, is present in up to 10% of normal individuals. In preparticipation athletic screening programs, it can be found in up to 15%-20% of subjects.
"There’s no reason for concern if that’s the only thing you find. It’s something you shouldn’t even communicate if there is no other issue. If a patient with early repolarization has no symptoms and no family history of premature sudden death, just leave it," Dr. Wilde advised.
On the other hand, if a patient with the early repolarization ECG pattern in two or more contiguous inferior and/or lateral leads experiences exercise-induced syncopal symptoms, further evaluation is warranted. Given how common and generally benign the early repolarization ECG pattern is, the expert panel recommended a conservative approach to diagnosis, urging that the formal diagnosis of early repolarization syndrome be restricted largely to those with the characteristic ECG findings who in addition have been resuscitated from unexplained ventricular fibrillation or polymorphic VT.
Dr. Priori emphasized that the guidelines have as a Class I recommendation that patients with a diagnosed or suspected inherited arrhythmia syndrome that can result in sudden cardiac death – and their first-degree relatives, as well – should be evaluated in a specialized multidisciplinary inherited arrhythmia clinic. Such clinics are more common in Europe than the United States; however, thought leaders in American electrophysiology now recognize that the increasing complexity of the field requires that more of these dedicated clinics be created in the United States, she said.
The expert consensus statement was a joint project of the Heart Rhythm Society, the European Heart Rhythm Association, and the Asia Pacific Heart Rhythm Society. The document is available at the HRS website and will be published this fall in Heart Rhythm, EP Europace, and the Journal of Arrhythmias.
Dr. Priori reported serving as a consultant to Medtronic, Boston Scientific, Biotronic, and Transgenomic. Dr. Wilde disclosed serving as a consultant to Sorin.
inherited primary arrhythmia, Dr. Silvia G. Priori, arrythmia guidelines, Arthur Wilde
DENVER – Major new guidelines on the diagnosis and management of patients with inherited primary arrhythmia syndromes have been jointly issued by the Heart Rhythm Society and its European and Asian counterparts.
The guidelines were sorely needed, according to Dr. Silvia G. Priori, cochair of the expert consensus panel writing group.
The field of inherited arrhythmias is rapidly evolving, with new pathogenic genetic mutations being found all the time. Much has changed in the 7 years since issuance of the last major guidelines: the American College of Cardiology/American Heart Association/European Society of Cardiology guidelines on prevention of sudden cardiac death (Circulation 2006;114:e385-484), noted Dr. Priori of the University of Pavia (Italy).
Her cochair, Dr. Arthur A. Wilde of the University of Amsterdam pointed out that the new 69-page report is the first major document to address some of the newer inherited arrhythmia syndromes, including catecholaminergic polymorphic ventricular tachycardia (CPVT), short QT syndrome, early repolarization, and progressive cardiac conduction disease (PCCD). In addition, the new report proposes major changes in the diagnostic criteria for the two most common primary arrhythmia syndromes: long QT syndrome (LQTS) and Brugada syndrome.
"Many of our colleagues in the field will be surprised," Dr. Priori predicted.
Here are the highlights:
• LQTS: With an estimated prevalence of roughly 1 per 2,000 live births worldwide, this is the most common of the inherited arrhythmia syndromes. What is likely to come as a surprise to many physicians is the expert consensus panel’s recommendation that the diagnosis of LQTS requires either the finding of an unequivocally causative mutation in one of the LQTS genes or, in the absence of such a defect, either a QTc interval of 480-499 ms in repeated 12-lead ECGs in a patient with unexplained syncope or a QTc of 500 ms in repeated ECGs in the absence of a secondary cause for QT prolongation in a nonsyncopal patient.
"A single ECG reading 10 ms above the upper limit of normal is not enough to establish the diagnosis. That’s quite different from what’s being done in common practice. Many of the patients who are referred to the centers of expertise on inherited arrhythmias are borderline patients in whom maybe one ECG was abnormal, and yet because of that they’ve been labeled as being affected by a genetic disease even if the genetic studies were negative. A single abnormal QTc measurement in a patient with negative genetic testing is not enough," Dr. Priori declared.
Dr. Wilde said the new guidelines loosen up the guidance on participation in competitive sports. The blanket prohibition of the past has been replaced by a case-by-case approach, with a Class I recommendation for routine referral to a clinical expert for evaluation of the risk posed by athletic activity. For example, although swimming is a very-high-risk activity for patients with the LQTS1 genotype, that’s not true for those who have LQTS2 or -3.
"It’s clear that if a patient with long QT syndrome has exercise-related syncopal events, that patient should not participate in competitive sports. But if the patient is asymptomatic and has minor QT prolongation, there’s probably not much reason for concern," he said.
"This is a sharp departure," Dr. Priori observed. "In several European countries, if you have the diagnosis of long QT syndrome, sports participation is not permitted, even if your physician clears you. So we hope with this document to slowly, carefully, begin to allow patients with this condition to do sports safely. We wanted to lift the ban so that a physician who feels a specific patient would have a low risk in the proper environment could make that recommendation."
• Brugada syndrome: Far more common in Asia than the western world, Brugada syndrome is 8- to 10-fold more frequent in males than females. The big change in the new guidelines is that the diagnosis no longer requires specific ECG changes plus clinical manifestations. Now, Brugada syndrome, like LQTS, is a pure ECG diagnosis. It is made definitively when a type 1 ST-segment elevation is noted either spontaneously or after administration of an intravenous sodium channel-blocking agent; the ST finding has to be observed in at least one right precordial lead placed in a standard or superior position up to the second intercostal space.
An implantable cardioverter-defibrillator is clearly indicated in a Brugada syndrome patient with a prior cardiac arrest or documented ventricular arrhythmias. The controversy lies in how to manage the asymptomatic patient. The guidelines give ICD implantation a weak Class IIb recommendation – meaning it "may be considered" – when such patients exhibit inducible ventricular arrhythmias during programmed electrical stimulation in the electrophysiology lab.
• Catecholaminergic polymorphic ventricular tachycardia: The prevalence of CPVT is unclear, but it has been estimated at 1 per 5,000 live births, according to Dr. Wilde. This highly malignant condition is diagnosed in patients with a known pathogenic mutation, or in the presence of a structurally normal heart, a normal resting ECG, and unexplained exercise- or catecholamine-induced bidirectional VT of polymorphic ventricular premature beats or VT before age 40 years. First-line therapy is a long-acting beta-blocker such as nadolol, coupled with exercise restriction. ICD therapy is problematic because the inevitable inappropriate shocks increase sympathetic tone, triggering true shockable arrhythmias in a vicious cycle.
• Short QT syndrome: This is a rare channelopathy. It is diagnosed on the basis of a QTc of 330 ms or less, or a QTc of less than 360 ms in the presence of a pathogenic mutation, family history of sudden death before age 40 years, cardiac arrest in the absence of structural heart disease, or a family history of short QT syndrome.
• Progressive cardiac conduction disease: Still incompletely understood, PCCD is diagnosed in individuals under age 50 years who have unexplained progressive conduction abnormalities and a structurally normal heart with no skeletal myopathies. Pacemaker implantation is the most useful therapy.
• Early repolarization: The first report linking this extremely common ECG finding to sudden death came less than 5 years ago. Early repolarization, as characterized by J-point and ST-segment elevation in two or more contiguous leads, is present in up to 10% of normal individuals. In preparticipation athletic screening programs, it can be found in up to 15%-20% of subjects.
"There’s no reason for concern if that’s the only thing you find. It’s something you shouldn’t even communicate if there is no other issue. If a patient with early repolarization has no symptoms and no family history of premature sudden death, just leave it," Dr. Wilde advised.
On the other hand, if a patient with the early repolarization ECG pattern in two or more contiguous inferior and/or lateral leads experiences exercise-induced syncopal symptoms, further evaluation is warranted. Given how common and generally benign the early repolarization ECG pattern is, the expert panel recommended a conservative approach to diagnosis, urging that the formal diagnosis of early repolarization syndrome be restricted largely to those with the characteristic ECG findings who in addition have been resuscitated from unexplained ventricular fibrillation or polymorphic VT.
Dr. Priori emphasized that the guidelines have as a Class I recommendation that patients with a diagnosed or suspected inherited arrhythmia syndrome that can result in sudden cardiac death – and their first-degree relatives, as well – should be evaluated in a specialized multidisciplinary inherited arrhythmia clinic. Such clinics are more common in Europe than the United States; however, thought leaders in American electrophysiology now recognize that the increasing complexity of the field requires that more of these dedicated clinics be created in the United States, she said.
The expert consensus statement was a joint project of the Heart Rhythm Society, the European Heart Rhythm Association, and the Asia Pacific Heart Rhythm Society. The document is available at the HRS website and will be published this fall in Heart Rhythm, EP Europace, and the Journal of Arrhythmias.
Dr. Priori reported serving as a consultant to Medtronic, Boston Scientific, Biotronic, and Transgenomic. Dr. Wilde disclosed serving as a consultant to Sorin.
DENVER – Major new guidelines on the diagnosis and management of patients with inherited primary arrhythmia syndromes have been jointly issued by the Heart Rhythm Society and its European and Asian counterparts.
The guidelines were sorely needed, according to Dr. Silvia G. Priori, cochair of the expert consensus panel writing group.
The field of inherited arrhythmias is rapidly evolving, with new pathogenic genetic mutations being found all the time. Much has changed in the 7 years since issuance of the last major guidelines: the American College of Cardiology/American Heart Association/European Society of Cardiology guidelines on prevention of sudden cardiac death (Circulation 2006;114:e385-484), noted Dr. Priori of the University of Pavia (Italy).
Her cochair, Dr. Arthur A. Wilde of the University of Amsterdam pointed out that the new 69-page report is the first major document to address some of the newer inherited arrhythmia syndromes, including catecholaminergic polymorphic ventricular tachycardia (CPVT), short QT syndrome, early repolarization, and progressive cardiac conduction disease (PCCD). In addition, the new report proposes major changes in the diagnostic criteria for the two most common primary arrhythmia syndromes: long QT syndrome (LQTS) and Brugada syndrome.
"Many of our colleagues in the field will be surprised," Dr. Priori predicted.
Here are the highlights:
• LQTS: With an estimated prevalence of roughly 1 per 2,000 live births worldwide, this is the most common of the inherited arrhythmia syndromes. What is likely to come as a surprise to many physicians is the expert consensus panel’s recommendation that the diagnosis of LQTS requires either the finding of an unequivocally causative mutation in one of the LQTS genes or, in the absence of such a defect, either a QTc interval of 480-499 ms in repeated 12-lead ECGs in a patient with unexplained syncope or a QTc of 500 ms in repeated ECGs in the absence of a secondary cause for QT prolongation in a nonsyncopal patient.
"A single ECG reading 10 ms above the upper limit of normal is not enough to establish the diagnosis. That’s quite different from what’s being done in common practice. Many of the patients who are referred to the centers of expertise on inherited arrhythmias are borderline patients in whom maybe one ECG was abnormal, and yet because of that they’ve been labeled as being affected by a genetic disease even if the genetic studies were negative. A single abnormal QTc measurement in a patient with negative genetic testing is not enough," Dr. Priori declared.
Dr. Wilde said the new guidelines loosen up the guidance on participation in competitive sports. The blanket prohibition of the past has been replaced by a case-by-case approach, with a Class I recommendation for routine referral to a clinical expert for evaluation of the risk posed by athletic activity. For example, although swimming is a very-high-risk activity for patients with the LQTS1 genotype, that’s not true for those who have LQTS2 or -3.
"It’s clear that if a patient with long QT syndrome has exercise-related syncopal events, that patient should not participate in competitive sports. But if the patient is asymptomatic and has minor QT prolongation, there’s probably not much reason for concern," he said.
"This is a sharp departure," Dr. Priori observed. "In several European countries, if you have the diagnosis of long QT syndrome, sports participation is not permitted, even if your physician clears you. So we hope with this document to slowly, carefully, begin to allow patients with this condition to do sports safely. We wanted to lift the ban so that a physician who feels a specific patient would have a low risk in the proper environment could make that recommendation."
• Brugada syndrome: Far more common in Asia than the western world, Brugada syndrome is 8- to 10-fold more frequent in males than females. The big change in the new guidelines is that the diagnosis no longer requires specific ECG changes plus clinical manifestations. Now, Brugada syndrome, like LQTS, is a pure ECG diagnosis. It is made definitively when a type 1 ST-segment elevation is noted either spontaneously or after administration of an intravenous sodium channel-blocking agent; the ST finding has to be observed in at least one right precordial lead placed in a standard or superior position up to the second intercostal space.
An implantable cardioverter-defibrillator is clearly indicated in a Brugada syndrome patient with a prior cardiac arrest or documented ventricular arrhythmias. The controversy lies in how to manage the asymptomatic patient. The guidelines give ICD implantation a weak Class IIb recommendation – meaning it "may be considered" – when such patients exhibit inducible ventricular arrhythmias during programmed electrical stimulation in the electrophysiology lab.
• Catecholaminergic polymorphic ventricular tachycardia: The prevalence of CPVT is unclear, but it has been estimated at 1 per 5,000 live births, according to Dr. Wilde. This highly malignant condition is diagnosed in patients with a known pathogenic mutation, or in the presence of a structurally normal heart, a normal resting ECG, and unexplained exercise- or catecholamine-induced bidirectional VT of polymorphic ventricular premature beats or VT before age 40 years. First-line therapy is a long-acting beta-blocker such as nadolol, coupled with exercise restriction. ICD therapy is problematic because the inevitable inappropriate shocks increase sympathetic tone, triggering true shockable arrhythmias in a vicious cycle.
• Short QT syndrome: This is a rare channelopathy. It is diagnosed on the basis of a QTc of 330 ms or less, or a QTc of less than 360 ms in the presence of a pathogenic mutation, family history of sudden death before age 40 years, cardiac arrest in the absence of structural heart disease, or a family history of short QT syndrome.
• Progressive cardiac conduction disease: Still incompletely understood, PCCD is diagnosed in individuals under age 50 years who have unexplained progressive conduction abnormalities and a structurally normal heart with no skeletal myopathies. Pacemaker implantation is the most useful therapy.
• Early repolarization: The first report linking this extremely common ECG finding to sudden death came less than 5 years ago. Early repolarization, as characterized by J-point and ST-segment elevation in two or more contiguous leads, is present in up to 10% of normal individuals. In preparticipation athletic screening programs, it can be found in up to 15%-20% of subjects.
"There’s no reason for concern if that’s the only thing you find. It’s something you shouldn’t even communicate if there is no other issue. If a patient with early repolarization has no symptoms and no family history of premature sudden death, just leave it," Dr. Wilde advised.
On the other hand, if a patient with the early repolarization ECG pattern in two or more contiguous inferior and/or lateral leads experiences exercise-induced syncopal symptoms, further evaluation is warranted. Given how common and generally benign the early repolarization ECG pattern is, the expert panel recommended a conservative approach to diagnosis, urging that the formal diagnosis of early repolarization syndrome be restricted largely to those with the characteristic ECG findings who in addition have been resuscitated from unexplained ventricular fibrillation or polymorphic VT.
Dr. Priori emphasized that the guidelines have as a Class I recommendation that patients with a diagnosed or suspected inherited arrhythmia syndrome that can result in sudden cardiac death – and their first-degree relatives, as well – should be evaluated in a specialized multidisciplinary inherited arrhythmia clinic. Such clinics are more common in Europe than the United States; however, thought leaders in American electrophysiology now recognize that the increasing complexity of the field requires that more of these dedicated clinics be created in the United States, she said.
The expert consensus statement was a joint project of the Heart Rhythm Society, the European Heart Rhythm Association, and the Asia Pacific Heart Rhythm Society. The document is available at the HRS website and will be published this fall in Heart Rhythm, EP Europace, and the Journal of Arrhythmias.
Dr. Priori reported serving as a consultant to Medtronic, Boston Scientific, Biotronic, and Transgenomic. Dr. Wilde disclosed serving as a consultant to Sorin.
inherited primary arrhythmia, Dr. Silvia G. Priori, arrythmia guidelines, Arthur Wilde
inherited primary arrhythmia, Dr. Silvia G. Priori, arrythmia guidelines, Arthur Wilde
AT HEART RHYTHM 2013
BRUISE CONTROL: Continue warfarin during cardiac device surgery
DENVER – Uninterrupted warfarin therapy during pacemaker or implantable cardioverter-defibrillator surgery in patients at high thromboembolic risk proved superior to the guideline-recommended practice of discontinuing warfarin and bridging with heparin, according to a large, multicenter, randomized clinical trial.
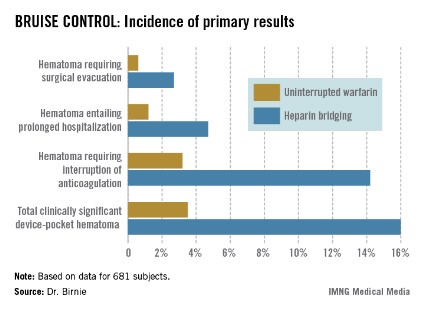
The primary outcome in the 17-center, 681-patient BRUISE CONTROL (Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial) trial was the incidence of clinically significant device-pocket hematoma. The rate was 16% in patients randomized to heparin bridging, compared with 3.5% with uninterrupted warfarin, Dr. David H. Birnie reported at the annual meeting of the Heart Rhythm Society.
These results are clearly practice changing. Heparin bridging has been the standard of care. It is recommended in this common clinical scenario in all of the major guidelines, but that’s bound to change as a result of BRUISE CONTROL, predicted Dr. Birnie of the University of Ottawa Heart Institute.
"This trial was a home run. It was unequivocally positive," he commented. "For sure, our clinical practice changed as soon as we saw those results."
Device-pocket hematoma is a "very nasty" complication of cardiac device surgery, Dr. Birnie noted. It is quite painful, can cause device infection, and is difficult to treat. Clinically significant device-pocket hematoma was defined in this trial as a hematoma resulting in prolonged hospitalization for an additional day or more, or interruption of oral anticoagulation for at least 24 hours, and/or requiring additional surgery. All three components of the primary endpoint were significantly less frequent in the uninterrupted warfarin group (see chart).
Performing device surgery in patients on uninterrupted warfarin with a median international normalized ratio (INR) of 2.3 was not associated with any increase in major perioperative bleeding or other surgical or thromboembolic complications. And patient satisfaction surveys indicated subjects greatly preferred having their procedure without stopping their warfarin.
BRUISE CONTROL was planned as a definitive 1,000-patient clinical trial. However, the Data and Safety Monitoring Board halted the study after an interim analysis involving the first 681 subjects.
Of note, this was a study restricted to patients at high stroke risk – greater than 5% annually – as defined by the presence of atrial fibrillation and a CHADS2 score of 3 or more or the presence of a mechanical heart valve.
Dr. Birnie said that although it seems counterintuitive to have less bleeding when cardiac device surgery is performed on a fully anticoagulated patient, as occurred in BRUISE CONTROL, he and his coinvestigators have an explanatory hypothesis: "When bleeding occurs in a fully anticoagulated patient, the operator can readily see it and address it. On the contrary, with bridging you get hemostasis at the time of surgery, but you may have missed a tiny little thing, and then 24 hours later when you start up your heparin bridging again, that’s when the bleeding occurs. It’s a physiologically plausible explanation," he said.
In a multivariate analysis, neither the use of pressure dressings, nor the use of sandbags, nor injection of antibleeding agents into the device pocket before closure had any significant impact on the incidence of clinically significant hematomas. Only three factors did: uninterrupted warfarin therapy, which reduced the risk by 84%; aspirin therapy, which doubled the risk; and the presence of diabetes mellitus, which was inexplicably associated with a 52% reduction in hematoma risk.
Each year roughly 1.6 million people worldwide undergo pacemaker or implantable cardioverter-defibrillator (ICD) implantation. Up to one-third of them are on long-term oral anticoagulation, most commonly with warfarin.
Dr. Birnie stressed that the BRUISE CONTROL findings have no relevance to patients on one of the new oral anticoagulants.
"The whole risk/benefit ratio of the new agents is completely different from warfarin. Their onset and offset of action is hours as opposed to the 5 days for warfarin," he noted.
With that point in mind, he and his coinvestigators have just started the BRUISE CONTROL-2 trial, which is examining whether it’s better to stop the new agents around the time of device surgery or continue the medication uninterrupted.
Simultaneous with Dr. Birnie’s presentation of the BRUISE CONTROL data in Denver, the study results were published online (N. Engl. J. Med. 2013 May 9 [doi: 10.1056/NEJMoa1302946]).
BRUISE CONTROL was funded by the Canadian Institutes of Health Research and the Ministry of Health and Long-Term Care of Ontario. Dr. Birnie reported having no conflicts of interest.
DENVER – Uninterrupted warfarin therapy during pacemaker or implantable cardioverter-defibrillator surgery in patients at high thromboembolic risk proved superior to the guideline-recommended practice of discontinuing warfarin and bridging with heparin, according to a large, multicenter, randomized clinical trial.
The primary outcome in the 17-center, 681-patient BRUISE CONTROL (Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial) trial was the incidence of clinically significant device-pocket hematoma. The rate was 16% in patients randomized to heparin bridging, compared with 3.5% with uninterrupted warfarin, Dr. David H. Birnie reported at the annual meeting of the Heart Rhythm Society.
These results are clearly practice changing. Heparin bridging has been the standard of care. It is recommended in this common clinical scenario in all of the major guidelines, but that’s bound to change as a result of BRUISE CONTROL, predicted Dr. Birnie of the University of Ottawa Heart Institute.
"This trial was a home run. It was unequivocally positive," he commented. "For sure, our clinical practice changed as soon as we saw those results."
Device-pocket hematoma is a "very nasty" complication of cardiac device surgery, Dr. Birnie noted. It is quite painful, can cause device infection, and is difficult to treat. Clinically significant device-pocket hematoma was defined in this trial as a hematoma resulting in prolonged hospitalization for an additional day or more, or interruption of oral anticoagulation for at least 24 hours, and/or requiring additional surgery. All three components of the primary endpoint were significantly less frequent in the uninterrupted warfarin group (see chart).
Performing device surgery in patients on uninterrupted warfarin with a median international normalized ratio (INR) of 2.3 was not associated with any increase in major perioperative bleeding or other surgical or thromboembolic complications. And patient satisfaction surveys indicated subjects greatly preferred having their procedure without stopping their warfarin.
BRUISE CONTROL was planned as a definitive 1,000-patient clinical trial. However, the Data and Safety Monitoring Board halted the study after an interim analysis involving the first 681 subjects.
Of note, this was a study restricted to patients at high stroke risk – greater than 5% annually – as defined by the presence of atrial fibrillation and a CHADS2 score of 3 or more or the presence of a mechanical heart valve.
Dr. Birnie said that although it seems counterintuitive to have less bleeding when cardiac device surgery is performed on a fully anticoagulated patient, as occurred in BRUISE CONTROL, he and his coinvestigators have an explanatory hypothesis: "When bleeding occurs in a fully anticoagulated patient, the operator can readily see it and address it. On the contrary, with bridging you get hemostasis at the time of surgery, but you may have missed a tiny little thing, and then 24 hours later when you start up your heparin bridging again, that’s when the bleeding occurs. It’s a physiologically plausible explanation," he said.
In a multivariate analysis, neither the use of pressure dressings, nor the use of sandbags, nor injection of antibleeding agents into the device pocket before closure had any significant impact on the incidence of clinically significant hematomas. Only three factors did: uninterrupted warfarin therapy, which reduced the risk by 84%; aspirin therapy, which doubled the risk; and the presence of diabetes mellitus, which was inexplicably associated with a 52% reduction in hematoma risk.
Each year roughly 1.6 million people worldwide undergo pacemaker or implantable cardioverter-defibrillator (ICD) implantation. Up to one-third of them are on long-term oral anticoagulation, most commonly with warfarin.
Dr. Birnie stressed that the BRUISE CONTROL findings have no relevance to patients on one of the new oral anticoagulants.
"The whole risk/benefit ratio of the new agents is completely different from warfarin. Their onset and offset of action is hours as opposed to the 5 days for warfarin," he noted.
With that point in mind, he and his coinvestigators have just started the BRUISE CONTROL-2 trial, which is examining whether it’s better to stop the new agents around the time of device surgery or continue the medication uninterrupted.
Simultaneous with Dr. Birnie’s presentation of the BRUISE CONTROL data in Denver, the study results were published online (N. Engl. J. Med. 2013 May 9 [doi: 10.1056/NEJMoa1302946]).
BRUISE CONTROL was funded by the Canadian Institutes of Health Research and the Ministry of Health and Long-Term Care of Ontario. Dr. Birnie reported having no conflicts of interest.
DENVER – Uninterrupted warfarin therapy during pacemaker or implantable cardioverter-defibrillator surgery in patients at high thromboembolic risk proved superior to the guideline-recommended practice of discontinuing warfarin and bridging with heparin, according to a large, multicenter, randomized clinical trial.
The primary outcome in the 17-center, 681-patient BRUISE CONTROL (Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial) trial was the incidence of clinically significant device-pocket hematoma. The rate was 16% in patients randomized to heparin bridging, compared with 3.5% with uninterrupted warfarin, Dr. David H. Birnie reported at the annual meeting of the Heart Rhythm Society.
These results are clearly practice changing. Heparin bridging has been the standard of care. It is recommended in this common clinical scenario in all of the major guidelines, but that’s bound to change as a result of BRUISE CONTROL, predicted Dr. Birnie of the University of Ottawa Heart Institute.
"This trial was a home run. It was unequivocally positive," he commented. "For sure, our clinical practice changed as soon as we saw those results."
Device-pocket hematoma is a "very nasty" complication of cardiac device surgery, Dr. Birnie noted. It is quite painful, can cause device infection, and is difficult to treat. Clinically significant device-pocket hematoma was defined in this trial as a hematoma resulting in prolonged hospitalization for an additional day or more, or interruption of oral anticoagulation for at least 24 hours, and/or requiring additional surgery. All three components of the primary endpoint were significantly less frequent in the uninterrupted warfarin group (see chart).
Performing device surgery in patients on uninterrupted warfarin with a median international normalized ratio (INR) of 2.3 was not associated with any increase in major perioperative bleeding or other surgical or thromboembolic complications. And patient satisfaction surveys indicated subjects greatly preferred having their procedure without stopping their warfarin.
BRUISE CONTROL was planned as a definitive 1,000-patient clinical trial. However, the Data and Safety Monitoring Board halted the study after an interim analysis involving the first 681 subjects.
Of note, this was a study restricted to patients at high stroke risk – greater than 5% annually – as defined by the presence of atrial fibrillation and a CHADS2 score of 3 or more or the presence of a mechanical heart valve.
Dr. Birnie said that although it seems counterintuitive to have less bleeding when cardiac device surgery is performed on a fully anticoagulated patient, as occurred in BRUISE CONTROL, he and his coinvestigators have an explanatory hypothesis: "When bleeding occurs in a fully anticoagulated patient, the operator can readily see it and address it. On the contrary, with bridging you get hemostasis at the time of surgery, but you may have missed a tiny little thing, and then 24 hours later when you start up your heparin bridging again, that’s when the bleeding occurs. It’s a physiologically plausible explanation," he said.
In a multivariate analysis, neither the use of pressure dressings, nor the use of sandbags, nor injection of antibleeding agents into the device pocket before closure had any significant impact on the incidence of clinically significant hematomas. Only three factors did: uninterrupted warfarin therapy, which reduced the risk by 84%; aspirin therapy, which doubled the risk; and the presence of diabetes mellitus, which was inexplicably associated with a 52% reduction in hematoma risk.
Each year roughly 1.6 million people worldwide undergo pacemaker or implantable cardioverter-defibrillator (ICD) implantation. Up to one-third of them are on long-term oral anticoagulation, most commonly with warfarin.
Dr. Birnie stressed that the BRUISE CONTROL findings have no relevance to patients on one of the new oral anticoagulants.
"The whole risk/benefit ratio of the new agents is completely different from warfarin. Their onset and offset of action is hours as opposed to the 5 days for warfarin," he noted.
With that point in mind, he and his coinvestigators have just started the BRUISE CONTROL-2 trial, which is examining whether it’s better to stop the new agents around the time of device surgery or continue the medication uninterrupted.
Simultaneous with Dr. Birnie’s presentation of the BRUISE CONTROL data in Denver, the study results were published online (N. Engl. J. Med. 2013 May 9 [doi: 10.1056/NEJMoa1302946]).
BRUISE CONTROL was funded by the Canadian Institutes of Health Research and the Ministry of Health and Long-Term Care of Ontario. Dr. Birnie reported having no conflicts of interest.
AT HEART RHYTHM 2013
Major finding: The incidence of clinically significant device-pocket hematoma in patients at high thromboembolic risk who underwent pacemaker or implantable cardioverter-defibrillator surgery was 3.5% if they remained on full-dose warfarin, compared with 16% with the guideline-recommended, standard-of-care practice of interrupting warfarin in favor of heparin bridging.
Data source: BRUISE CONTROL was a 681-patient, 17-center randomized clinical trial.
Disclosures: BRUISE CONTROL was funded by the Canadian Institutes of Health Research and the Ministry of Health and Long-Term Care of Ontario. Dr. Birnie reported having no conflicts of interest.
Biventricular pacing bests conventional tx in BLOCK HF trial
DENVER – Biventricular pacing rather than the right ventricular pacing recommended in current guidelines results in significantly better quality of life and symptom status, an analysis of secondary outcomes from the BLOCK HF trial have shown.
In a previous report of the trial’s primary outcome (N. Engl. J. Med. 2013;368:1585-93), the biventricular (BiV) pacing group showed a highly significant 26% reduction in risk of a composite of all-cause mortality, an urgent care visit for heart failure requiring intravenous therapy, or a 15% or greater increase in the left ventricular end-systolic volume index during an average of 37 months of follow-up, compared with patients who had right ventricular (RV) pacing.
The new report on prespecified secondary endpoints including quality of life and symptom status was undertaken because of researchers’ and regulatory agencies’ growing appreciation of the importance of such outcomes in patients with a chronic progressive disease such as heart failure, principal investigator Dr. Anne B. Curtis reported at the annual meeting of the Heart Rhythm Society.
BLOCK HF (Biventricular versus Right Ventricular Pacing in Patients with Left Ventricular Dysfunction and Atrioventricular Block) was a randomized, double-blind, prospective, 60-center clinical trial involving 691 patients in the United States and Canada. All had AV block warranting pacemaker therapy as well as New York Heart Association(NYHA) class I-III heart failure with a left ventricular ejection fraction of 50% or less. All participants got a cardiac resynchronization therapy device featuring BiV pacing. Patients were assigned in double-blind fashion for their device to run in BiV or conventional RV pacing mode, explained Dr. Curtis, professor and chair of the department of medicine at the University of Buffalo (N.Y.).
The new secondary analysis tabulated changes in the Packer clinical composite score, NYHA functional class, and quality of life, as measured by the Minnesota Living With Heart Failure Questionnaire, at 6, 12, 18, and 24 months.
The Packer clinical composite score classifies patients as improved, worsened, or unchanged based on patient global assessment, heart failure hospitalization, change in symptoms, and other factors. At all four time points through 24 months, significantly more patients in the BiV group were rated as improved and significantly fewer as worsened than in the RV group. For example, at 6 months 53% of the BiV group were categorized as improved and 23% as worsened, compared with 39% and 28% in the RV group, Dr. Curtis reported.
From a mean baseline quality of life score of 25, the BiV group improved by an average of 5 points at the 6-month assessment compared with a 0.3-point gain in the RV group. At 12 months, the BiV group still showed a 3.9-point improvement, significantly better than the average 0.9-point gain in the RV patients. At 18 and 24 months, however, there was no longer a significant difference between the two groups in terms of quality of life scores. Dr. Curtis attributed this drop-off to the fact that 86 patients in the RV arm crossed over to BiV pacing because of deteriorating heart failure, compared with 15 crossovers in the BiV group. In the intention-to-treat analysis employed in BLOCK HF, those crossovers to BiV pacing are still counted as being part of the RV group.
"If you could mandate that patients stayed in the same arm, I think you’d continue to see differences over time, but you can’t do that," she said.
An analysis of changes in NYHA functional class showed the BiV group had better outcomes at 12 months, but at not the other time points.
The BLOCK HF trial was undertaken in response to evidence suggesting that sustained RV apical pacing can degrade ventricular function, especially in patients with preexisting systolic dysfunction.
At present, cardiac resynchronization therapy devices aren’t approved by the Food and Drug Administration for patients with AV block with left ventricular dysfunction. Medtronic officials have indicated they plan to seek an expanded indication based on the BLOCK HF data.
One audience member, noting that the study outcomes were better with BiV pacing in patients across the full spectrum of depressed ejection fractions, asked Dr. Curtis if she expects an expansion of cardiac resynchronization therapy indications to include patients with heart block and a normal ejection fraction.
"I would anticipate that the guidelines will change for the type of patients studied here. But we didn’t study patients with a normal ejection fraction because the more normal the patient, the larger the sample size and longer the follow-up you’d need to show a difference. So I doubt that this study will change guidelines for patients with normal ejection fractions," she said.
Dr. Curtis reported that she serves as a consultant to Medtronic, which sponsored the BLOCK HF trial,
DENVER – Biventricular pacing rather than the right ventricular pacing recommended in current guidelines results in significantly better quality of life and symptom status, an analysis of secondary outcomes from the BLOCK HF trial have shown.
In a previous report of the trial’s primary outcome (N. Engl. J. Med. 2013;368:1585-93), the biventricular (BiV) pacing group showed a highly significant 26% reduction in risk of a composite of all-cause mortality, an urgent care visit for heart failure requiring intravenous therapy, or a 15% or greater increase in the left ventricular end-systolic volume index during an average of 37 months of follow-up, compared with patients who had right ventricular (RV) pacing.
The new report on prespecified secondary endpoints including quality of life and symptom status was undertaken because of researchers’ and regulatory agencies’ growing appreciation of the importance of such outcomes in patients with a chronic progressive disease such as heart failure, principal investigator Dr. Anne B. Curtis reported at the annual meeting of the Heart Rhythm Society.
BLOCK HF (Biventricular versus Right Ventricular Pacing in Patients with Left Ventricular Dysfunction and Atrioventricular Block) was a randomized, double-blind, prospective, 60-center clinical trial involving 691 patients in the United States and Canada. All had AV block warranting pacemaker therapy as well as New York Heart Association(NYHA) class I-III heart failure with a left ventricular ejection fraction of 50% or less. All participants got a cardiac resynchronization therapy device featuring BiV pacing. Patients were assigned in double-blind fashion for their device to run in BiV or conventional RV pacing mode, explained Dr. Curtis, professor and chair of the department of medicine at the University of Buffalo (N.Y.).
The new secondary analysis tabulated changes in the Packer clinical composite score, NYHA functional class, and quality of life, as measured by the Minnesota Living With Heart Failure Questionnaire, at 6, 12, 18, and 24 months.
The Packer clinical composite score classifies patients as improved, worsened, or unchanged based on patient global assessment, heart failure hospitalization, change in symptoms, and other factors. At all four time points through 24 months, significantly more patients in the BiV group were rated as improved and significantly fewer as worsened than in the RV group. For example, at 6 months 53% of the BiV group were categorized as improved and 23% as worsened, compared with 39% and 28% in the RV group, Dr. Curtis reported.
From a mean baseline quality of life score of 25, the BiV group improved by an average of 5 points at the 6-month assessment compared with a 0.3-point gain in the RV group. At 12 months, the BiV group still showed a 3.9-point improvement, significantly better than the average 0.9-point gain in the RV patients. At 18 and 24 months, however, there was no longer a significant difference between the two groups in terms of quality of life scores. Dr. Curtis attributed this drop-off to the fact that 86 patients in the RV arm crossed over to BiV pacing because of deteriorating heart failure, compared with 15 crossovers in the BiV group. In the intention-to-treat analysis employed in BLOCK HF, those crossovers to BiV pacing are still counted as being part of the RV group.
"If you could mandate that patients stayed in the same arm, I think you’d continue to see differences over time, but you can’t do that," she said.
An analysis of changes in NYHA functional class showed the BiV group had better outcomes at 12 months, but at not the other time points.
The BLOCK HF trial was undertaken in response to evidence suggesting that sustained RV apical pacing can degrade ventricular function, especially in patients with preexisting systolic dysfunction.
At present, cardiac resynchronization therapy devices aren’t approved by the Food and Drug Administration for patients with AV block with left ventricular dysfunction. Medtronic officials have indicated they plan to seek an expanded indication based on the BLOCK HF data.
One audience member, noting that the study outcomes were better with BiV pacing in patients across the full spectrum of depressed ejection fractions, asked Dr. Curtis if she expects an expansion of cardiac resynchronization therapy indications to include patients with heart block and a normal ejection fraction.
"I would anticipate that the guidelines will change for the type of patients studied here. But we didn’t study patients with a normal ejection fraction because the more normal the patient, the larger the sample size and longer the follow-up you’d need to show a difference. So I doubt that this study will change guidelines for patients with normal ejection fractions," she said.
Dr. Curtis reported that she serves as a consultant to Medtronic, which sponsored the BLOCK HF trial,
DENVER – Biventricular pacing rather than the right ventricular pacing recommended in current guidelines results in significantly better quality of life and symptom status, an analysis of secondary outcomes from the BLOCK HF trial have shown.
In a previous report of the trial’s primary outcome (N. Engl. J. Med. 2013;368:1585-93), the biventricular (BiV) pacing group showed a highly significant 26% reduction in risk of a composite of all-cause mortality, an urgent care visit for heart failure requiring intravenous therapy, or a 15% or greater increase in the left ventricular end-systolic volume index during an average of 37 months of follow-up, compared with patients who had right ventricular (RV) pacing.
The new report on prespecified secondary endpoints including quality of life and symptom status was undertaken because of researchers’ and regulatory agencies’ growing appreciation of the importance of such outcomes in patients with a chronic progressive disease such as heart failure, principal investigator Dr. Anne B. Curtis reported at the annual meeting of the Heart Rhythm Society.
BLOCK HF (Biventricular versus Right Ventricular Pacing in Patients with Left Ventricular Dysfunction and Atrioventricular Block) was a randomized, double-blind, prospective, 60-center clinical trial involving 691 patients in the United States and Canada. All had AV block warranting pacemaker therapy as well as New York Heart Association(NYHA) class I-III heart failure with a left ventricular ejection fraction of 50% or less. All participants got a cardiac resynchronization therapy device featuring BiV pacing. Patients were assigned in double-blind fashion for their device to run in BiV or conventional RV pacing mode, explained Dr. Curtis, professor and chair of the department of medicine at the University of Buffalo (N.Y.).
The new secondary analysis tabulated changes in the Packer clinical composite score, NYHA functional class, and quality of life, as measured by the Minnesota Living With Heart Failure Questionnaire, at 6, 12, 18, and 24 months.
The Packer clinical composite score classifies patients as improved, worsened, or unchanged based on patient global assessment, heart failure hospitalization, change in symptoms, and other factors. At all four time points through 24 months, significantly more patients in the BiV group were rated as improved and significantly fewer as worsened than in the RV group. For example, at 6 months 53% of the BiV group were categorized as improved and 23% as worsened, compared with 39% and 28% in the RV group, Dr. Curtis reported.
From a mean baseline quality of life score of 25, the BiV group improved by an average of 5 points at the 6-month assessment compared with a 0.3-point gain in the RV group. At 12 months, the BiV group still showed a 3.9-point improvement, significantly better than the average 0.9-point gain in the RV patients. At 18 and 24 months, however, there was no longer a significant difference between the two groups in terms of quality of life scores. Dr. Curtis attributed this drop-off to the fact that 86 patients in the RV arm crossed over to BiV pacing because of deteriorating heart failure, compared with 15 crossovers in the BiV group. In the intention-to-treat analysis employed in BLOCK HF, those crossovers to BiV pacing are still counted as being part of the RV group.
"If you could mandate that patients stayed in the same arm, I think you’d continue to see differences over time, but you can’t do that," she said.
An analysis of changes in NYHA functional class showed the BiV group had better outcomes at 12 months, but at not the other time points.
The BLOCK HF trial was undertaken in response to evidence suggesting that sustained RV apical pacing can degrade ventricular function, especially in patients with preexisting systolic dysfunction.
At present, cardiac resynchronization therapy devices aren’t approved by the Food and Drug Administration for patients with AV block with left ventricular dysfunction. Medtronic officials have indicated they plan to seek an expanded indication based on the BLOCK HF data.
One audience member, noting that the study outcomes were better with BiV pacing in patients across the full spectrum of depressed ejection fractions, asked Dr. Curtis if she expects an expansion of cardiac resynchronization therapy indications to include patients with heart block and a normal ejection fraction.
"I would anticipate that the guidelines will change for the type of patients studied here. But we didn’t study patients with a normal ejection fraction because the more normal the patient, the larger the sample size and longer the follow-up you’d need to show a difference. So I doubt that this study will change guidelines for patients with normal ejection fractions," she said.
Dr. Curtis reported that she serves as a consultant to Medtronic, which sponsored the BLOCK HF trial,
AT HEART RHYTHM 2013
Major finding: Fifty-three percent of patients with atrioventricular block and systolic dysfunction were rated as "improved" after 6 months of biventricular pacing, compared with just 39% of patients on conventional right ventricular pacing.
Data source: A prespecified secondary analysis of the BLOCK HF trial, a randomized, double-blind, prospective multicenter study involving 691 patients.
Disclosures: The BLOCK HF trial was sponsored by Medtronic. The presenter disclosed that she serves as a paid consultant to the device company.
Antitachycardia pacing in ICDs linked to higher mortality
DENVER – The use of antitachycardia pacing was associated with increased mortality in a proprietary registry of patients with an implantable cardioverter defibrillator or cardiac resynchronization therapy defibrillator.
The finding from the ALTITUDE study of Boston Scientific’s Latitude remote electronic monitoring system is an early warning signal of a potentially serious issue in cardiac device programming, Dr. Leslie A. Saxon said at the annual meeting of the Heart Rhythm Society. The database encompasses 225,000 device recipients who have received 195,000 shocks recorded during 21 million device transmissions.
Antitachycardia pacing (ATP) is an essentially painless means of terminating ventricular tachycardia (VT) without resorting to an unpleasant "full-on" shock. Moreover, major randomized trials including MADIT-II and SCD-HeFT as well as ALTITUDE data have consistently shown that mortality risk following first use of shock therapy is two- to threefold higher in implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy defibrillator (CRT-D) recipients.
ATP detection programming has burgeoned since 2008, when devices with two-zone ATP detection became available. In 2006, roughly 60% of patients in the Latitude registry had ATP programming switched on. By 2011, virtually all patients had "ATP on," which has become the device industry’s out-of-the-box device setting.
ALTITUDE shows that nearly one-quarter of device recipients now receive ATP therapy during the first year after device implantation. Unexpectedly, however, shock rates have remained essentially stable during this period of first-year ATP therapy. Thus, ATP has not resulted in the anticipated meaningful reduction in shock rates, said Dr. Saxon, professor of medicine and chief of the division of cardiovascular medicine at the University of Southern California, Los Angeles.
Moreover, mortality risk during an average 3.5 years of follow-up was lowest in device recipients who received no therapy, greater in those with shock-only or ATP-only therapy, and worst in those who received ATP that accelerated VT; indeed, the mortality risk in that subgroup was threefold higher than in patients who received no device therapy, she said.
Further, device VT detection programming set to a rate of greater than 170 bpm was independently associated with a significant 8% improvement in survival compared with a setting of 170 bpm or less. Programming the VT detection to more than 170 bpm resulted in a significantly lower incidence of shock (7.6% vs.10.4%) and ATP therapy (13.9% vs. 22.4%).
ALTITUDE is not a randomized study, she noted. The decisions for particular programming settings aren’t known. Only limited information on clinical comorbidities is available. Thus, the study findings should be considered hypothesis generating and nondefinitive, although they are concerning.
"It’s unclear if the need for ATP or the ATP itself is a marker of increased risk. However – given the frequency of ATP and the mortality association found in this study – strategies to explore reducing ATP therapies seem justified," Dr. Saxon said. "We’ve put so much emphasis on device shocks, and here we are with a fourfold greater number of patients getting ATP, which is associated with a mortality risk similar to shock. There’s no reason not to explore withholding ATP treatment to try to further improve device outcomes."
Audience member Dr. James G. Porterfield of the University of Tennessee, Memphis, asked: "Do you have enough confidence in this data to start programming ATP off yet?"
"I do," she replied. "But I think it’s very important that we individualize therapy because I also have scores of patients who have symptomatic VT terminated with two-zone therapy."
The current practice in her cardiology group is to turn on ATP only in those patients who have documented VT, and to test the ATP to make sure it’s effective, added Dr. Saxon, who chairs the ALTITUDE study physician panel.
She reported receiving consultant fees from Boston Scientific, which operates the Latitude registry.
DENVER – The use of antitachycardia pacing was associated with increased mortality in a proprietary registry of patients with an implantable cardioverter defibrillator or cardiac resynchronization therapy defibrillator.
The finding from the ALTITUDE study of Boston Scientific’s Latitude remote electronic monitoring system is an early warning signal of a potentially serious issue in cardiac device programming, Dr. Leslie A. Saxon said at the annual meeting of the Heart Rhythm Society. The database encompasses 225,000 device recipients who have received 195,000 shocks recorded during 21 million device transmissions.
Antitachycardia pacing (ATP) is an essentially painless means of terminating ventricular tachycardia (VT) without resorting to an unpleasant "full-on" shock. Moreover, major randomized trials including MADIT-II and SCD-HeFT as well as ALTITUDE data have consistently shown that mortality risk following first use of shock therapy is two- to threefold higher in implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy defibrillator (CRT-D) recipients.
ATP detection programming has burgeoned since 2008, when devices with two-zone ATP detection became available. In 2006, roughly 60% of patients in the Latitude registry had ATP programming switched on. By 2011, virtually all patients had "ATP on," which has become the device industry’s out-of-the-box device setting.
ALTITUDE shows that nearly one-quarter of device recipients now receive ATP therapy during the first year after device implantation. Unexpectedly, however, shock rates have remained essentially stable during this period of first-year ATP therapy. Thus, ATP has not resulted in the anticipated meaningful reduction in shock rates, said Dr. Saxon, professor of medicine and chief of the division of cardiovascular medicine at the University of Southern California, Los Angeles.
Moreover, mortality risk during an average 3.5 years of follow-up was lowest in device recipients who received no therapy, greater in those with shock-only or ATP-only therapy, and worst in those who received ATP that accelerated VT; indeed, the mortality risk in that subgroup was threefold higher than in patients who received no device therapy, she said.
Further, device VT detection programming set to a rate of greater than 170 bpm was independently associated with a significant 8% improvement in survival compared with a setting of 170 bpm or less. Programming the VT detection to more than 170 bpm resulted in a significantly lower incidence of shock (7.6% vs.10.4%) and ATP therapy (13.9% vs. 22.4%).
ALTITUDE is not a randomized study, she noted. The decisions for particular programming settings aren’t known. Only limited information on clinical comorbidities is available. Thus, the study findings should be considered hypothesis generating and nondefinitive, although they are concerning.
"It’s unclear if the need for ATP or the ATP itself is a marker of increased risk. However – given the frequency of ATP and the mortality association found in this study – strategies to explore reducing ATP therapies seem justified," Dr. Saxon said. "We’ve put so much emphasis on device shocks, and here we are with a fourfold greater number of patients getting ATP, which is associated with a mortality risk similar to shock. There’s no reason not to explore withholding ATP treatment to try to further improve device outcomes."
Audience member Dr. James G. Porterfield of the University of Tennessee, Memphis, asked: "Do you have enough confidence in this data to start programming ATP off yet?"
"I do," she replied. "But I think it’s very important that we individualize therapy because I also have scores of patients who have symptomatic VT terminated with two-zone therapy."
The current practice in her cardiology group is to turn on ATP only in those patients who have documented VT, and to test the ATP to make sure it’s effective, added Dr. Saxon, who chairs the ALTITUDE study physician panel.
She reported receiving consultant fees from Boston Scientific, which operates the Latitude registry.
DENVER – The use of antitachycardia pacing was associated with increased mortality in a proprietary registry of patients with an implantable cardioverter defibrillator or cardiac resynchronization therapy defibrillator.
The finding from the ALTITUDE study of Boston Scientific’s Latitude remote electronic monitoring system is an early warning signal of a potentially serious issue in cardiac device programming, Dr. Leslie A. Saxon said at the annual meeting of the Heart Rhythm Society. The database encompasses 225,000 device recipients who have received 195,000 shocks recorded during 21 million device transmissions.
Antitachycardia pacing (ATP) is an essentially painless means of terminating ventricular tachycardia (VT) without resorting to an unpleasant "full-on" shock. Moreover, major randomized trials including MADIT-II and SCD-HeFT as well as ALTITUDE data have consistently shown that mortality risk following first use of shock therapy is two- to threefold higher in implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy defibrillator (CRT-D) recipients.
ATP detection programming has burgeoned since 2008, when devices with two-zone ATP detection became available. In 2006, roughly 60% of patients in the Latitude registry had ATP programming switched on. By 2011, virtually all patients had "ATP on," which has become the device industry’s out-of-the-box device setting.
ALTITUDE shows that nearly one-quarter of device recipients now receive ATP therapy during the first year after device implantation. Unexpectedly, however, shock rates have remained essentially stable during this period of first-year ATP therapy. Thus, ATP has not resulted in the anticipated meaningful reduction in shock rates, said Dr. Saxon, professor of medicine and chief of the division of cardiovascular medicine at the University of Southern California, Los Angeles.
Moreover, mortality risk during an average 3.5 years of follow-up was lowest in device recipients who received no therapy, greater in those with shock-only or ATP-only therapy, and worst in those who received ATP that accelerated VT; indeed, the mortality risk in that subgroup was threefold higher than in patients who received no device therapy, she said.
Further, device VT detection programming set to a rate of greater than 170 bpm was independently associated with a significant 8% improvement in survival compared with a setting of 170 bpm or less. Programming the VT detection to more than 170 bpm resulted in a significantly lower incidence of shock (7.6% vs.10.4%) and ATP therapy (13.9% vs. 22.4%).
ALTITUDE is not a randomized study, she noted. The decisions for particular programming settings aren’t known. Only limited information on clinical comorbidities is available. Thus, the study findings should be considered hypothesis generating and nondefinitive, although they are concerning.
"It’s unclear if the need for ATP or the ATP itself is a marker of increased risk. However – given the frequency of ATP and the mortality association found in this study – strategies to explore reducing ATP therapies seem justified," Dr. Saxon said. "We’ve put so much emphasis on device shocks, and here we are with a fourfold greater number of patients getting ATP, which is associated with a mortality risk similar to shock. There’s no reason not to explore withholding ATP treatment to try to further improve device outcomes."
Audience member Dr. James G. Porterfield of the University of Tennessee, Memphis, asked: "Do you have enough confidence in this data to start programming ATP off yet?"
"I do," she replied. "But I think it’s very important that we individualize therapy because I also have scores of patients who have symptomatic VT terminated with two-zone therapy."
The current practice in her cardiology group is to turn on ATP only in those patients who have documented VT, and to test the ATP to make sure it’s effective, added Dr. Saxon, who chairs the ALTITUDE study physician panel.
She reported receiving consultant fees from Boston Scientific, which operates the Latitude registry.
AT HEART RHYTHM 2013
Major finding: Programming the device’s VT detection to more than 170 bpm resulted in a significantly lower incidence of shock (7.6% vs. 10.4%) and ATP therapy (13.9% vs. 22.4%).
Data source: The ALTITUDE study of data obtained from 225,000 cardiac device recipients in the proprietary Latitude network device remote monitoring system.
Disclosures: Boston Scientific sponsors the Latitude network and the ongoing ALTITUDE study. Dr. Saxon disclosed that she is a consultant to the company.