User login
Cancer Therapy & Research Center (CTRC)/ American Association for Cancer Research (AACR): San Antonio Breast Cancer Symposium (SABCS)
New insights into aromatase inhibitor therapy nonpersistence
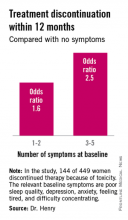
SAN ANTONIO – Discontinuation of aromatase inhibitor therapy because of toxicity is significantly more likely to occur in breast cancer patients having a greater burden of specific symptoms even before starting on the endocrine agent.
The baseline predictive symptoms identified in the randomized prospective Exemestane and Letrozole Pharmacogenetics (ELPh) trial were self-reported depression, anxiety, poor sleep quality, difficulty concentrating, and a tired feeling. Patients with three to five of these symptoms before going on an aromatase inhibitor (AI) were 2.5-fold more likely to stop treatment because of toxicity within the first 12 months than were those with none of the symptoms, Dr. Norah L. Henry reported at the San Antonio Breast Cancer Symposium.
Early discontinuation of AI therapy because of toxicity is a big problem, occurring in 20%-30% of patients who start on treatment. In an earlier study by Dr. Henry and her colleagues, discontinuation was most often from arthralgias or other musculoskeletal complaints (J. Clin. Oncol. 2012;30:936-42).
The ELPh trial included 449 evaluable postmenopausal women with early-stage estrogen receptor–positive breast cancer who were randomized to open-label exemestane or letrozole for 2 years.
To test the study hypothesis that certain baseline patient-reported symptoms increased the likelihood of early treatment discontinuation, participants were evaluated at baseline and again 1, 3, 6, 12, and 24 months after starting on an AI. At each time point, depression was self-rated using the Center for Epidemiologic Studies Depression Scale, sleep disturbance by the Pittsburgh Sleep Quality Index, and anxiety by the Hospital Anxiety and Depression Scale. Other baseline self-reported symptoms included in the prospective evaluation were joint pain, vaginal dryness, forgetfulness, difficulty concentrating, and feeling tired, explained Dr. Henry, a medical oncologist at the University of Michigan, Ann Arbor.
One hundred forty-four of the 449 women (31.2%) discontinued AI therapy because of toxicity by 12 months. In a multivariate logistic regression analysis, two of the baseline symptoms turned out to be independently associated with a significantly increased rate of treatment discontinuation: poor sleep quality as defined by a PSQI score greater than 5, reported by 45% of subjects at baseline, was associated with a 1.8-fold increased risk; and moderate or severe difficulty in concentrating was associated with a 2.6-fold increased likelihood of treatment discontinuation.
While the other symptoms under study were not individually associated with an increased risk of treatment discontinuation, the collective burden imposed by having a greater number of baseline symptoms was associated with an increased risk.
Several earlier studies by other investigators had identified prior chemotherapy, older age, and greater body mass index as being predictive of nonpersistence with AI therapy. Interestingly, neither prior chemotherapy nor greater body mass index was associated with early treatment discontinuation in the ELPh study, Dr. Henry noted.
Patients assigned to exemestane were 63% more likely to halt treatment within 12 months than were those randomized to letrozole.
The clinical relevance of these ELPh study findings is that early identification of patients with a greater burden of baseline symptoms predictive of nonadherence might improve persistence on AI therapy.
"Up-front management of these symptoms rather than waiting until symptoms become particularly problematic may improve persistence with AI therapy," she said.
Possible interventions might include preferential use of letrozole or tamoxifen in such patients, adoption of an exercise program or behavioral intervention, or pharmacologic therapy with an SSRI or a serotonin–norepinephrine reuptake inhibitors, a strategy now under study in the ongoing SWOG S1202 trial, in which patients are randomized to duloxetine or placebo.
Audience member Steven E. Vogl called the ELPh results important information.
"It recalls the history of chemotherapy-induced cognitive impairment, which in the latest couple of analyses seems to exist before the chemotherapy," observed Dr. Vogl of Bronx, N.Y.
The ELPh study was conducted by the Consortium on Breast Cancer Pharmacogenomics and funded chiefly by the Damon Runyon Cancer Research Foundation. Dr. Henry reported having received research grants from Astra Zeneca, Eli Lilly, and Sanofi Aventis.
SAN ANTONIO – Discontinuation of aromatase inhibitor therapy because of toxicity is significantly more likely to occur in breast cancer patients having a greater burden of specific symptoms even before starting on the endocrine agent.
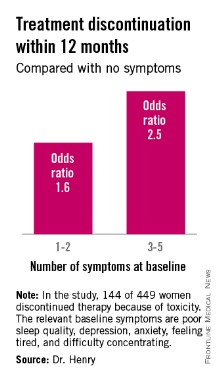
The baseline predictive symptoms identified in the randomized prospective Exemestane and Letrozole Pharmacogenetics (ELPh) trial were self-reported depression, anxiety, poor sleep quality, difficulty concentrating, and a tired feeling. Patients with three to five of these symptoms before going on an aromatase inhibitor (AI) were 2.5-fold more likely to stop treatment because of toxicity within the first 12 months than were those with none of the symptoms, Dr. Norah L. Henry reported at the San Antonio Breast Cancer Symposium.
Early discontinuation of AI therapy because of toxicity is a big problem, occurring in 20%-30% of patients who start on treatment. In an earlier study by Dr. Henry and her colleagues, discontinuation was most often from arthralgias or other musculoskeletal complaints (J. Clin. Oncol. 2012;30:936-42).
The ELPh trial included 449 evaluable postmenopausal women with early-stage estrogen receptor–positive breast cancer who were randomized to open-label exemestane or letrozole for 2 years.
To test the study hypothesis that certain baseline patient-reported symptoms increased the likelihood of early treatment discontinuation, participants were evaluated at baseline and again 1, 3, 6, 12, and 24 months after starting on an AI. At each time point, depression was self-rated using the Center for Epidemiologic Studies Depression Scale, sleep disturbance by the Pittsburgh Sleep Quality Index, and anxiety by the Hospital Anxiety and Depression Scale. Other baseline self-reported symptoms included in the prospective evaluation were joint pain, vaginal dryness, forgetfulness, difficulty concentrating, and feeling tired, explained Dr. Henry, a medical oncologist at the University of Michigan, Ann Arbor.
One hundred forty-four of the 449 women (31.2%) discontinued AI therapy because of toxicity by 12 months. In a multivariate logistic regression analysis, two of the baseline symptoms turned out to be independently associated with a significantly increased rate of treatment discontinuation: poor sleep quality as defined by a PSQI score greater than 5, reported by 45% of subjects at baseline, was associated with a 1.8-fold increased risk; and moderate or severe difficulty in concentrating was associated with a 2.6-fold increased likelihood of treatment discontinuation.
While the other symptoms under study were not individually associated with an increased risk of treatment discontinuation, the collective burden imposed by having a greater number of baseline symptoms was associated with an increased risk.
Several earlier studies by other investigators had identified prior chemotherapy, older age, and greater body mass index as being predictive of nonpersistence with AI therapy. Interestingly, neither prior chemotherapy nor greater body mass index was associated with early treatment discontinuation in the ELPh study, Dr. Henry noted.
Patients assigned to exemestane were 63% more likely to halt treatment within 12 months than were those randomized to letrozole.
The clinical relevance of these ELPh study findings is that early identification of patients with a greater burden of baseline symptoms predictive of nonadherence might improve persistence on AI therapy.
"Up-front management of these symptoms rather than waiting until symptoms become particularly problematic may improve persistence with AI therapy," she said.
Possible interventions might include preferential use of letrozole or tamoxifen in such patients, adoption of an exercise program or behavioral intervention, or pharmacologic therapy with an SSRI or a serotonin–norepinephrine reuptake inhibitors, a strategy now under study in the ongoing SWOG S1202 trial, in which patients are randomized to duloxetine or placebo.
Audience member Steven E. Vogl called the ELPh results important information.
"It recalls the history of chemotherapy-induced cognitive impairment, which in the latest couple of analyses seems to exist before the chemotherapy," observed Dr. Vogl of Bronx, N.Y.
The ELPh study was conducted by the Consortium on Breast Cancer Pharmacogenomics and funded chiefly by the Damon Runyon Cancer Research Foundation. Dr. Henry reported having received research grants from Astra Zeneca, Eli Lilly, and Sanofi Aventis.
SAN ANTONIO – Discontinuation of aromatase inhibitor therapy because of toxicity is significantly more likely to occur in breast cancer patients having a greater burden of specific symptoms even before starting on the endocrine agent.
The baseline predictive symptoms identified in the randomized prospective Exemestane and Letrozole Pharmacogenetics (ELPh) trial were self-reported depression, anxiety, poor sleep quality, difficulty concentrating, and a tired feeling. Patients with three to five of these symptoms before going on an aromatase inhibitor (AI) were 2.5-fold more likely to stop treatment because of toxicity within the first 12 months than were those with none of the symptoms, Dr. Norah L. Henry reported at the San Antonio Breast Cancer Symposium.
Early discontinuation of AI therapy because of toxicity is a big problem, occurring in 20%-30% of patients who start on treatment. In an earlier study by Dr. Henry and her colleagues, discontinuation was most often from arthralgias or other musculoskeletal complaints (J. Clin. Oncol. 2012;30:936-42).
The ELPh trial included 449 evaluable postmenopausal women with early-stage estrogen receptor–positive breast cancer who were randomized to open-label exemestane or letrozole for 2 years.
To test the study hypothesis that certain baseline patient-reported symptoms increased the likelihood of early treatment discontinuation, participants were evaluated at baseline and again 1, 3, 6, 12, and 24 months after starting on an AI. At each time point, depression was self-rated using the Center for Epidemiologic Studies Depression Scale, sleep disturbance by the Pittsburgh Sleep Quality Index, and anxiety by the Hospital Anxiety and Depression Scale. Other baseline self-reported symptoms included in the prospective evaluation were joint pain, vaginal dryness, forgetfulness, difficulty concentrating, and feeling tired, explained Dr. Henry, a medical oncologist at the University of Michigan, Ann Arbor.
One hundred forty-four of the 449 women (31.2%) discontinued AI therapy because of toxicity by 12 months. In a multivariate logistic regression analysis, two of the baseline symptoms turned out to be independently associated with a significantly increased rate of treatment discontinuation: poor sleep quality as defined by a PSQI score greater than 5, reported by 45% of subjects at baseline, was associated with a 1.8-fold increased risk; and moderate or severe difficulty in concentrating was associated with a 2.6-fold increased likelihood of treatment discontinuation.
While the other symptoms under study were not individually associated with an increased risk of treatment discontinuation, the collective burden imposed by having a greater number of baseline symptoms was associated with an increased risk.
Several earlier studies by other investigators had identified prior chemotherapy, older age, and greater body mass index as being predictive of nonpersistence with AI therapy. Interestingly, neither prior chemotherapy nor greater body mass index was associated with early treatment discontinuation in the ELPh study, Dr. Henry noted.
Patients assigned to exemestane were 63% more likely to halt treatment within 12 months than were those randomized to letrozole.
The clinical relevance of these ELPh study findings is that early identification of patients with a greater burden of baseline symptoms predictive of nonadherence might improve persistence on AI therapy.
"Up-front management of these symptoms rather than waiting until symptoms become particularly problematic may improve persistence with AI therapy," she said.
Possible interventions might include preferential use of letrozole or tamoxifen in such patients, adoption of an exercise program or behavioral intervention, or pharmacologic therapy with an SSRI or a serotonin–norepinephrine reuptake inhibitors, a strategy now under study in the ongoing SWOG S1202 trial, in which patients are randomized to duloxetine or placebo.
Audience member Steven E. Vogl called the ELPh results important information.
"It recalls the history of chemotherapy-induced cognitive impairment, which in the latest couple of analyses seems to exist before the chemotherapy," observed Dr. Vogl of Bronx, N.Y.
The ELPh study was conducted by the Consortium on Breast Cancer Pharmacogenomics and funded chiefly by the Damon Runyon Cancer Research Foundation. Dr. Henry reported having received research grants from Astra Zeneca, Eli Lilly, and Sanofi Aventis.
AT SABCS 2013
Major finding: Breast cancer patients with at least three of five self-reported symptoms prior to going on adjuvant aromatase inhibitor therapy were 2.5-fold more likely to discontinue treatment within the first 12 months because of toxicity.
Data source: The Exemestane and Letrozole Pharmacogenetics trial included 449 evaluable women with early-stage estrogen receptor–positive breast cancer who were prospectively evaluated for the relationship between a variety of baseline patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy.
Disclosures: The study was funded by the Damon Runyon Cancer Research Foundation. Dr. Norah L. Henry reported having received research grants from Astra Zeneca, Eli Lilly, and Sanofi Aventis.
No benefit seen for investigational antiangiogenic agent in metastatic breast cancer
SAN ANTONIO – Adding the investigational antiangiogenesis agent ramucirumab to first-line chemotherapy did not significantly benefit metastatic breast cancer patients in a large phase III trial, Dr. John R. Mackey said at the San Antonio Breast Cancer Symposium.
The ROSE/TRIO-12 trial included 1,144 metastatic breast cancer patients in 25 countries who were randomized 2:1 to first-line docetaxel plus ramucirumab, a recombinant human IgG1 monoclonal antibody that binds to vascular endothelial growth factor receptor-2, or to docetaxel and placebo. After a median follow-up of 16.2 months, investigator-assessed progression-free survival was 9.5 months in the ramucirumab group and was not significantly different at 8.2 months in controls. No clinically defined subgroup showed a benefit with combination therapy, reported Dr. Mackey, professor of oncology at the University of Alberta, Edmonton, and director of Translational Research in Oncology (TRIO), which conducted the trial.
Median overall survival was 27.3 months with ramucirumab and 27.2 months in controls. However, the overall response rate, disease control rate, and time to progression were greater in the combined therapy group.
"We’re hopeful that we can go back to the tissue samples and find predictive biomarkers because there is a signal here. We are seeing improvements in several endpoints and some patients clearly are benefiting, but we do not understand the biology sufficiently well to be able to pick them out of the general population," he said.
Dr. Mackey noted that ramucirumab is the focus of a broad phase III clinical trial program. Definitive phase III studies of the antiangiogenesis agent in lung and colon cancer are underway. And two phase III trials in gastric cancer – REGARD and RAINBOW – have been completed with positive results for overall survival. "I think we can say that at least in another disease setting, this agent has activity."
Despite the negative results in ROSE/TRIO-12 plus the negative outcomes for trials of bevacizumab (Avastin), this is not the end of the line for antiangiogenesis agents in breast cancer, said conference codirector Dr. Peter Ravdin of the University of Texas, San Antonio. "Breast cancer is a really diverse disease. Drugs that may on average have little value in breast cancer may be very effective in selected populations."
The ROSE study was funded by Eli Lilly. Dr. Mackey declared having no financial conflicts of interest.
SAN ANTONIO – Adding the investigational antiangiogenesis agent ramucirumab to first-line chemotherapy did not significantly benefit metastatic breast cancer patients in a large phase III trial, Dr. John R. Mackey said at the San Antonio Breast Cancer Symposium.
The ROSE/TRIO-12 trial included 1,144 metastatic breast cancer patients in 25 countries who were randomized 2:1 to first-line docetaxel plus ramucirumab, a recombinant human IgG1 monoclonal antibody that binds to vascular endothelial growth factor receptor-2, or to docetaxel and placebo. After a median follow-up of 16.2 months, investigator-assessed progression-free survival was 9.5 months in the ramucirumab group and was not significantly different at 8.2 months in controls. No clinically defined subgroup showed a benefit with combination therapy, reported Dr. Mackey, professor of oncology at the University of Alberta, Edmonton, and director of Translational Research in Oncology (TRIO), which conducted the trial.
Median overall survival was 27.3 months with ramucirumab and 27.2 months in controls. However, the overall response rate, disease control rate, and time to progression were greater in the combined therapy group.
"We’re hopeful that we can go back to the tissue samples and find predictive biomarkers because there is a signal here. We are seeing improvements in several endpoints and some patients clearly are benefiting, but we do not understand the biology sufficiently well to be able to pick them out of the general population," he said.
Dr. Mackey noted that ramucirumab is the focus of a broad phase III clinical trial program. Definitive phase III studies of the antiangiogenesis agent in lung and colon cancer are underway. And two phase III trials in gastric cancer – REGARD and RAINBOW – have been completed with positive results for overall survival. "I think we can say that at least in another disease setting, this agent has activity."
Despite the negative results in ROSE/TRIO-12 plus the negative outcomes for trials of bevacizumab (Avastin), this is not the end of the line for antiangiogenesis agents in breast cancer, said conference codirector Dr. Peter Ravdin of the University of Texas, San Antonio. "Breast cancer is a really diverse disease. Drugs that may on average have little value in breast cancer may be very effective in selected populations."
The ROSE study was funded by Eli Lilly. Dr. Mackey declared having no financial conflicts of interest.
SAN ANTONIO – Adding the investigational antiangiogenesis agent ramucirumab to first-line chemotherapy did not significantly benefit metastatic breast cancer patients in a large phase III trial, Dr. John R. Mackey said at the San Antonio Breast Cancer Symposium.
The ROSE/TRIO-12 trial included 1,144 metastatic breast cancer patients in 25 countries who were randomized 2:1 to first-line docetaxel plus ramucirumab, a recombinant human IgG1 monoclonal antibody that binds to vascular endothelial growth factor receptor-2, or to docetaxel and placebo. After a median follow-up of 16.2 months, investigator-assessed progression-free survival was 9.5 months in the ramucirumab group and was not significantly different at 8.2 months in controls. No clinically defined subgroup showed a benefit with combination therapy, reported Dr. Mackey, professor of oncology at the University of Alberta, Edmonton, and director of Translational Research in Oncology (TRIO), which conducted the trial.
Median overall survival was 27.3 months with ramucirumab and 27.2 months in controls. However, the overall response rate, disease control rate, and time to progression were greater in the combined therapy group.
"We’re hopeful that we can go back to the tissue samples and find predictive biomarkers because there is a signal here. We are seeing improvements in several endpoints and some patients clearly are benefiting, but we do not understand the biology sufficiently well to be able to pick them out of the general population," he said.
Dr. Mackey noted that ramucirumab is the focus of a broad phase III clinical trial program. Definitive phase III studies of the antiangiogenesis agent in lung and colon cancer are underway. And two phase III trials in gastric cancer – REGARD and RAINBOW – have been completed with positive results for overall survival. "I think we can say that at least in another disease setting, this agent has activity."
Despite the negative results in ROSE/TRIO-12 plus the negative outcomes for trials of bevacizumab (Avastin), this is not the end of the line for antiangiogenesis agents in breast cancer, said conference codirector Dr. Peter Ravdin of the University of Texas, San Antonio. "Breast cancer is a really diverse disease. Drugs that may on average have little value in breast cancer may be very effective in selected populations."
The ROSE study was funded by Eli Lilly. Dr. Mackey declared having no financial conflicts of interest.
AT SABCS 2013
Major finding: Metastatic breast cancer patients randomized to docetaxel plus ramucirumab had a median progression-free survival of 9.5 months, not significantly better than the 8.2 months with docetaxel plus placebo.
Data source: The ROSE/TRIO-12 trial included 1,444 patients with metastatic breast cancer who were randomized 2:1 to first-line docetaxel plus ramucirumab or placebo.
Disclosures: The ROSE trial was funded by Eli Lilly. The presenter reported having no financial conflicts.
Extracorporeal shock wave therapy promising for lymphedema
SAN ANTONIO – Extracorporeal shock wave therapy shows early promise for limiting the often-vexing problem of lymphedema arising after axillary lymphadenectomy.
In the first 10 affected patients treated in an ongoing randomized, sham-controlled clinical trial, lymphedema, as measured by median whole-arm water displacement, dropped from 4,200 mL at baseline by 192.5 mL after 10 once-weekly extracorporeal shock wave (ECSW) sessions. Patients who received sham sessions had a 12.5-mL decline from baseline measures, Dr. Sara Imboden reported at the San Antonio Breast Cancer Symposium.
Quantitative CT measures indicated the mean total cross-sectional area of the most swollen part of the arm decreased by 3% in the ECSW group, compared with 1.4% in the control group, added Dr. Imboden of the University of Bern (Switzerland).
A mean of 22 axillary lymph nodes had been removed from the breast cancer patients in the study. The ECSW therapy was performed over the length of the edematous arm at an energy density of 0.25-0.69 mJ/mm2 once per week for 10 weeks. The control group followed the same treatment schedule, but the shock waves were contained inside the probe during their sessions.
None of the patients had to interrupt ECSW therapy due to complications.
The researchers plan to expand the randomized trial to include 30 patients, and to augment the results with patient reports of symptoms based on questionnaires assessing body image and with extended follow-up to evaluate functioning and recurrences.
The likely mechanism of benefit in patients with lymphedema involves ECSW-induced stimulation of angiogenesis and lymphatic vessel regeneration. These results have been demonstrated in animal studies.
No medications have been shown effective in treating lymphedema. Conventional therapy entails repeated manual lymph drainage and compression bandages.
Dr. Imboden reported having no financial conflicts of interest with regard to this university-funded study.
SAN ANTONIO – Extracorporeal shock wave therapy shows early promise for limiting the often-vexing problem of lymphedema arising after axillary lymphadenectomy.
In the first 10 affected patients treated in an ongoing randomized, sham-controlled clinical trial, lymphedema, as measured by median whole-arm water displacement, dropped from 4,200 mL at baseline by 192.5 mL after 10 once-weekly extracorporeal shock wave (ECSW) sessions. Patients who received sham sessions had a 12.5-mL decline from baseline measures, Dr. Sara Imboden reported at the San Antonio Breast Cancer Symposium.
Quantitative CT measures indicated the mean total cross-sectional area of the most swollen part of the arm decreased by 3% in the ECSW group, compared with 1.4% in the control group, added Dr. Imboden of the University of Bern (Switzerland).
A mean of 22 axillary lymph nodes had been removed from the breast cancer patients in the study. The ECSW therapy was performed over the length of the edematous arm at an energy density of 0.25-0.69 mJ/mm2 once per week for 10 weeks. The control group followed the same treatment schedule, but the shock waves were contained inside the probe during their sessions.
None of the patients had to interrupt ECSW therapy due to complications.
The researchers plan to expand the randomized trial to include 30 patients, and to augment the results with patient reports of symptoms based on questionnaires assessing body image and with extended follow-up to evaluate functioning and recurrences.
The likely mechanism of benefit in patients with lymphedema involves ECSW-induced stimulation of angiogenesis and lymphatic vessel regeneration. These results have been demonstrated in animal studies.
No medications have been shown effective in treating lymphedema. Conventional therapy entails repeated manual lymph drainage and compression bandages.
Dr. Imboden reported having no financial conflicts of interest with regard to this university-funded study.
SAN ANTONIO – Extracorporeal shock wave therapy shows early promise for limiting the often-vexing problem of lymphedema arising after axillary lymphadenectomy.
In the first 10 affected patients treated in an ongoing randomized, sham-controlled clinical trial, lymphedema, as measured by median whole-arm water displacement, dropped from 4,200 mL at baseline by 192.5 mL after 10 once-weekly extracorporeal shock wave (ECSW) sessions. Patients who received sham sessions had a 12.5-mL decline from baseline measures, Dr. Sara Imboden reported at the San Antonio Breast Cancer Symposium.
Quantitative CT measures indicated the mean total cross-sectional area of the most swollen part of the arm decreased by 3% in the ECSW group, compared with 1.4% in the control group, added Dr. Imboden of the University of Bern (Switzerland).
A mean of 22 axillary lymph nodes had been removed from the breast cancer patients in the study. The ECSW therapy was performed over the length of the edematous arm at an energy density of 0.25-0.69 mJ/mm2 once per week for 10 weeks. The control group followed the same treatment schedule, but the shock waves were contained inside the probe during their sessions.
None of the patients had to interrupt ECSW therapy due to complications.
The researchers plan to expand the randomized trial to include 30 patients, and to augment the results with patient reports of symptoms based on questionnaires assessing body image and with extended follow-up to evaluate functioning and recurrences.
The likely mechanism of benefit in patients with lymphedema involves ECSW-induced stimulation of angiogenesis and lymphatic vessel regeneration. These results have been demonstrated in animal studies.
No medications have been shown effective in treating lymphedema. Conventional therapy entails repeated manual lymph drainage and compression bandages.
Dr. Imboden reported having no financial conflicts of interest with regard to this university-funded study.
AT SABCS 2013
Major finding: Women with lymphedema following axillary lymphadenectomy had a median 192.5-mL reduction in whole-arm water volume displacement following 10 weekly sessions of extracorporeal shock wave therapy. Controls who underwent sham therapy had a modest 12.5-mL decrease.
Data source: This is an interim report on the first 10 patients in a planned 30-patient, randomized, sham-controlled trial.
Disclosures: Dr. Imboden reported having no financial conflicts of interest with regard to this university-funded study.
Survival no better after primary tumor removal in metastatic breast cancer
SAN ANTONIO – Surgical removal of the primary tumor and affected lymph nodes afforded no overall survival benefit in women who had metastatic breast cancer at initial presentation, according to a pair of randomized trials presented at the San Antonio Breast Cancer Symposium.
In a first trial, conducted among 350 women in India who had responded to initial chemotherapy, about 20% of the women were still alive at 5 years regardless of whether they had surgery or just received more systemic therapy, first author Dr. Rajendra Badwe, director of the Tata Memorial Hospital in Mumbai, India, reported in a session and at a press briefing.
Superior locoregional control conferred by surgery was canceled out by a higher risk of progression in distant sites, lending support to more than 20-year-old preclinical data by Dr. Bernard Fisher and his colleagues suggesting that an intact primary suppresses growth of distant metastases (Cancer Res. 1989;49:1996-2001).
"Locoregional treatment of the primary tumor in women presenting with metastatic breast cancer did not result in any overall survival benefit and hence should not be offered as a routine practice," Dr. Badwe commented.
"The biological fallout of this study is that surgical removal of the primary tumor in these women appears to confer a growth advantage on distant metastases. ... This is the first time that we have evidence in human studies that locoregional treatment has a great kinetic effect on distant metastases," he added.
In a second trial, conducted among 293 women in Turkey with untreated de novo metastatic breast cancer, median estimated survival was statistically indistinguishable, at about 3.5 years, regardless of whether women had up-front surgery or simply systemic therapy, first author Dr. Atilla Soran reported in a session on behalf of the Turkish Federation of Societies for Breast Diseases.
Subgroup analyses suggested that surgery conferred a survival advantage among women with solitary bone metastases but a survival disadvantage among women with multiple liver or lung metastases.
The rate of locoregional progression was one-fifth as high with surgery as with systemic therapy.
"There was no statistically significant difference in overall survival at early follow-up, but we need longer follow-up," Dr. Soran commented. "There were potentially important subgroup differences."
The two trials have implications – both for other ongoing trials and for patient care – according to invited discussant Dr. Seema A. Khan, a professor of surgery and Bluhm Family Professor of Cancer Research at Northwestern University, Chicago, and a physician at the university’s Lynn Sage Breast Center.
"A large benefit of primary site local therapy seems unlikely based on the data we saw today," she maintained. "The assumptions we have used in our ongoing trial designs will need to be reassessed. Preplanned pooled analyses may yield sufficient power to detect smaller differences."
As for patient care, "it is pretty clear that at this point in time, we have to make sure that our patients understand that there is really no proven survival advantage to primary site local therapy," Dr. Khan said. "So I don’t think this treatment should be offered to patients with asymptomatic tumors unless they are participating in a clinical trial. There may be a local control advantage; we need to see more data on that."
Indian trial
Participants in the Indian trial, conducted between 2005 and 2013, were women with de novo metastatic breast cancer who had had a complete or partial response to anthracycline chemotherapy alone or with a taxane.
They were randomized evenly to receive locoregional therapy (lumpectomy or mastectomy with axillary lymph node dissection, plus radiation therapy to the chest wall or breast and lymph nodes) or no locoregional therapy as a control. Both groups received hormonal therapy if indicated.
In the control group, about 10% of women underwent a palliative mastectomy because of impending fungation or pain in the breast, as permitted by study protocol, according to Dr. Badwe.
The patients thus received contemporary therapy, he said, except that the 16% with HER2-positive disease did not receive the targeted agent trastuzumab (Herceptin).
Results showed that median overall survival was about 18 months, and the 5-year rate was 19.2% with locoregional therapy and 20.5% without it, a nonsignificant difference. "Uniformly, there was no difference at all in any subsets," Dr. Badwe reported.
The study had a one-sided superiority design, he noted. "We wouldn’t have been able to see a 2.5- or 3-month difference, or a 4% detriment" in overall survival.
The surgery group had a lower risk of local progression-free survival events (hazard ratio, 0.16; P = .00), but a higher risk of distant progression-free survival events (HR, 1.42; P = .01).
Several theories might explain why removal of the primary would accelerate growth of metastases, according to Dr. Badwe.
"The first and the foremost is that the act of surgery itself might elaborate some growth factors which might allow metastatic disease to grow. The second possibility, which was suggested by Dr. Fisher (Cancer Res. 1989;49:1996-2001) is that the primary tumor, which predates the onset of distant metastases, elaborates some inhibitory factors, and they are not there once the primary tumor is removed, bestowing autonomy of growth on the distant metastases," he explained. "And the third possibility is the act of surgery might induce some more metastatic processes by dissemination and create new disease."
Session attendee Dr. Steven Vogl, an oncologist in the Bronx, N.Y., said, "The ascertainment of disease progression requires disease that you can follow. A woman with bone-only metastases with her cancer in place, you can tell when her cancer is getting worse because the primary site or the axillary nodes are getting bigger. It’s much more difficult if you’ve taken those off and irradiated the chest wall. Have you looked at your data to see if that’s what was going on, why you had more distant metastases, because they couldn’t progress locally? This is a medical trial explanation that contradicts Fisher’s biologic hypothesis."
"There was a fixed time duration at which systemic investigations were performed to assess whether the distant metastases progressed," Dr. Badwe replied. If anything, the patients who did not have surgery had more assessments of their distant metastases, he said.
Dr. Tari A. King, a session attendee from the Memorial Sloan-Kettering Cancer Center, New York, noted that a lack of HER2 therapy in the trial may have had a large effect.
"We do have prospective registry data here [abstract 18-09, presented in a poster session] from a trial that we completed in the United States sponsored by the Translational Breast Cancer Research Consortium, and the patients in our study, whether they received surgery or not, their 2-year overall survival is far superior to what you’ve just showed us," she commented. "So I’m not sure that we can really apply your data to the modern targeted therapy regimens that we see in the United States."
Turkish trial
The Turkish trial, known as the MF07-01 trial, was conduced between 2008 and 2012 among treatment-naïve patients.
They were randomized evenly to receive either systemic therapy alone or surgery for the primary tumor (with or without axillary dissection) followed by radiation therapy if indicated, plus systemic therapy.
All patients received hormonal therapy as needed, and those with HER2-positive disease received trastuzumab.
With a median follow-up of 18 months, the median overall survival was 46 months with initial surgery and 42 months with initial systemic therapy, a nonsignificant difference, reported Dr. Soran, who disclosed no relevant conflicts of interest.
In unplanned subgroup analyses, the findings were similar for most subgroups of patients. However, surgery yielded superior survival in patients with solitary bone metastases (not reached vs. 42 months, P = .02) and inferior survival in patients having multiple liver or pulmonary metastases (16 months vs. not reached, P = .02).
The rate of locoregional progression was much lower with initial surgery than with initial systemic therapy (0.7% vs. 3.6%).
Dr. Soran emphasized that the trial’s planned median follow-up is 36 months, so the presented results are only preliminary. Quality of life and morbidity analyses are ongoing.
Dr. Badwe and Dr. Soran disclosed no relevant conflicts of interest.
SAN ANTONIO – Surgical removal of the primary tumor and affected lymph nodes afforded no overall survival benefit in women who had metastatic breast cancer at initial presentation, according to a pair of randomized trials presented at the San Antonio Breast Cancer Symposium.
In a first trial, conducted among 350 women in India who had responded to initial chemotherapy, about 20% of the women were still alive at 5 years regardless of whether they had surgery or just received more systemic therapy, first author Dr. Rajendra Badwe, director of the Tata Memorial Hospital in Mumbai, India, reported in a session and at a press briefing.
Superior locoregional control conferred by surgery was canceled out by a higher risk of progression in distant sites, lending support to more than 20-year-old preclinical data by Dr. Bernard Fisher and his colleagues suggesting that an intact primary suppresses growth of distant metastases (Cancer Res. 1989;49:1996-2001).
"Locoregional treatment of the primary tumor in women presenting with metastatic breast cancer did not result in any overall survival benefit and hence should not be offered as a routine practice," Dr. Badwe commented.
"The biological fallout of this study is that surgical removal of the primary tumor in these women appears to confer a growth advantage on distant metastases. ... This is the first time that we have evidence in human studies that locoregional treatment has a great kinetic effect on distant metastases," he added.
In a second trial, conducted among 293 women in Turkey with untreated de novo metastatic breast cancer, median estimated survival was statistically indistinguishable, at about 3.5 years, regardless of whether women had up-front surgery or simply systemic therapy, first author Dr. Atilla Soran reported in a session on behalf of the Turkish Federation of Societies for Breast Diseases.
Subgroup analyses suggested that surgery conferred a survival advantage among women with solitary bone metastases but a survival disadvantage among women with multiple liver or lung metastases.
The rate of locoregional progression was one-fifth as high with surgery as with systemic therapy.
"There was no statistically significant difference in overall survival at early follow-up, but we need longer follow-up," Dr. Soran commented. "There were potentially important subgroup differences."
The two trials have implications – both for other ongoing trials and for patient care – according to invited discussant Dr. Seema A. Khan, a professor of surgery and Bluhm Family Professor of Cancer Research at Northwestern University, Chicago, and a physician at the university’s Lynn Sage Breast Center.
"A large benefit of primary site local therapy seems unlikely based on the data we saw today," she maintained. "The assumptions we have used in our ongoing trial designs will need to be reassessed. Preplanned pooled analyses may yield sufficient power to detect smaller differences."
As for patient care, "it is pretty clear that at this point in time, we have to make sure that our patients understand that there is really no proven survival advantage to primary site local therapy," Dr. Khan said. "So I don’t think this treatment should be offered to patients with asymptomatic tumors unless they are participating in a clinical trial. There may be a local control advantage; we need to see more data on that."
Indian trial
Participants in the Indian trial, conducted between 2005 and 2013, were women with de novo metastatic breast cancer who had had a complete or partial response to anthracycline chemotherapy alone or with a taxane.
They were randomized evenly to receive locoregional therapy (lumpectomy or mastectomy with axillary lymph node dissection, plus radiation therapy to the chest wall or breast and lymph nodes) or no locoregional therapy as a control. Both groups received hormonal therapy if indicated.
In the control group, about 10% of women underwent a palliative mastectomy because of impending fungation or pain in the breast, as permitted by study protocol, according to Dr. Badwe.
The patients thus received contemporary therapy, he said, except that the 16% with HER2-positive disease did not receive the targeted agent trastuzumab (Herceptin).
Results showed that median overall survival was about 18 months, and the 5-year rate was 19.2% with locoregional therapy and 20.5% without it, a nonsignificant difference. "Uniformly, there was no difference at all in any subsets," Dr. Badwe reported.
The study had a one-sided superiority design, he noted. "We wouldn’t have been able to see a 2.5- or 3-month difference, or a 4% detriment" in overall survival.
The surgery group had a lower risk of local progression-free survival events (hazard ratio, 0.16; P = .00), but a higher risk of distant progression-free survival events (HR, 1.42; P = .01).
Several theories might explain why removal of the primary would accelerate growth of metastases, according to Dr. Badwe.
"The first and the foremost is that the act of surgery itself might elaborate some growth factors which might allow metastatic disease to grow. The second possibility, which was suggested by Dr. Fisher (Cancer Res. 1989;49:1996-2001) is that the primary tumor, which predates the onset of distant metastases, elaborates some inhibitory factors, and they are not there once the primary tumor is removed, bestowing autonomy of growth on the distant metastases," he explained. "And the third possibility is the act of surgery might induce some more metastatic processes by dissemination and create new disease."
Session attendee Dr. Steven Vogl, an oncologist in the Bronx, N.Y., said, "The ascertainment of disease progression requires disease that you can follow. A woman with bone-only metastases with her cancer in place, you can tell when her cancer is getting worse because the primary site or the axillary nodes are getting bigger. It’s much more difficult if you’ve taken those off and irradiated the chest wall. Have you looked at your data to see if that’s what was going on, why you had more distant metastases, because they couldn’t progress locally? This is a medical trial explanation that contradicts Fisher’s biologic hypothesis."
"There was a fixed time duration at which systemic investigations were performed to assess whether the distant metastases progressed," Dr. Badwe replied. If anything, the patients who did not have surgery had more assessments of their distant metastases, he said.
Dr. Tari A. King, a session attendee from the Memorial Sloan-Kettering Cancer Center, New York, noted that a lack of HER2 therapy in the trial may have had a large effect.
"We do have prospective registry data here [abstract 18-09, presented in a poster session] from a trial that we completed in the United States sponsored by the Translational Breast Cancer Research Consortium, and the patients in our study, whether they received surgery or not, their 2-year overall survival is far superior to what you’ve just showed us," she commented. "So I’m not sure that we can really apply your data to the modern targeted therapy regimens that we see in the United States."
Turkish trial
The Turkish trial, known as the MF07-01 trial, was conduced between 2008 and 2012 among treatment-naïve patients.
They were randomized evenly to receive either systemic therapy alone or surgery for the primary tumor (with or without axillary dissection) followed by radiation therapy if indicated, plus systemic therapy.
All patients received hormonal therapy as needed, and those with HER2-positive disease received trastuzumab.
With a median follow-up of 18 months, the median overall survival was 46 months with initial surgery and 42 months with initial systemic therapy, a nonsignificant difference, reported Dr. Soran, who disclosed no relevant conflicts of interest.
In unplanned subgroup analyses, the findings were similar for most subgroups of patients. However, surgery yielded superior survival in patients with solitary bone metastases (not reached vs. 42 months, P = .02) and inferior survival in patients having multiple liver or pulmonary metastases (16 months vs. not reached, P = .02).
The rate of locoregional progression was much lower with initial surgery than with initial systemic therapy (0.7% vs. 3.6%).
Dr. Soran emphasized that the trial’s planned median follow-up is 36 months, so the presented results are only preliminary. Quality of life and morbidity analyses are ongoing.
Dr. Badwe and Dr. Soran disclosed no relevant conflicts of interest.
SAN ANTONIO – Surgical removal of the primary tumor and affected lymph nodes afforded no overall survival benefit in women who had metastatic breast cancer at initial presentation, according to a pair of randomized trials presented at the San Antonio Breast Cancer Symposium.
In a first trial, conducted among 350 women in India who had responded to initial chemotherapy, about 20% of the women were still alive at 5 years regardless of whether they had surgery or just received more systemic therapy, first author Dr. Rajendra Badwe, director of the Tata Memorial Hospital in Mumbai, India, reported in a session and at a press briefing.
Superior locoregional control conferred by surgery was canceled out by a higher risk of progression in distant sites, lending support to more than 20-year-old preclinical data by Dr. Bernard Fisher and his colleagues suggesting that an intact primary suppresses growth of distant metastases (Cancer Res. 1989;49:1996-2001).
"Locoregional treatment of the primary tumor in women presenting with metastatic breast cancer did not result in any overall survival benefit and hence should not be offered as a routine practice," Dr. Badwe commented.
"The biological fallout of this study is that surgical removal of the primary tumor in these women appears to confer a growth advantage on distant metastases. ... This is the first time that we have evidence in human studies that locoregional treatment has a great kinetic effect on distant metastases," he added.
In a second trial, conducted among 293 women in Turkey with untreated de novo metastatic breast cancer, median estimated survival was statistically indistinguishable, at about 3.5 years, regardless of whether women had up-front surgery or simply systemic therapy, first author Dr. Atilla Soran reported in a session on behalf of the Turkish Federation of Societies for Breast Diseases.
Subgroup analyses suggested that surgery conferred a survival advantage among women with solitary bone metastases but a survival disadvantage among women with multiple liver or lung metastases.
The rate of locoregional progression was one-fifth as high with surgery as with systemic therapy.
"There was no statistically significant difference in overall survival at early follow-up, but we need longer follow-up," Dr. Soran commented. "There were potentially important subgroup differences."
The two trials have implications – both for other ongoing trials and for patient care – according to invited discussant Dr. Seema A. Khan, a professor of surgery and Bluhm Family Professor of Cancer Research at Northwestern University, Chicago, and a physician at the university’s Lynn Sage Breast Center.
"A large benefit of primary site local therapy seems unlikely based on the data we saw today," she maintained. "The assumptions we have used in our ongoing trial designs will need to be reassessed. Preplanned pooled analyses may yield sufficient power to detect smaller differences."
As for patient care, "it is pretty clear that at this point in time, we have to make sure that our patients understand that there is really no proven survival advantage to primary site local therapy," Dr. Khan said. "So I don’t think this treatment should be offered to patients with asymptomatic tumors unless they are participating in a clinical trial. There may be a local control advantage; we need to see more data on that."
Indian trial
Participants in the Indian trial, conducted between 2005 and 2013, were women with de novo metastatic breast cancer who had had a complete or partial response to anthracycline chemotherapy alone or with a taxane.
They were randomized evenly to receive locoregional therapy (lumpectomy or mastectomy with axillary lymph node dissection, plus radiation therapy to the chest wall or breast and lymph nodes) or no locoregional therapy as a control. Both groups received hormonal therapy if indicated.
In the control group, about 10% of women underwent a palliative mastectomy because of impending fungation or pain in the breast, as permitted by study protocol, according to Dr. Badwe.
The patients thus received contemporary therapy, he said, except that the 16% with HER2-positive disease did not receive the targeted agent trastuzumab (Herceptin).
Results showed that median overall survival was about 18 months, and the 5-year rate was 19.2% with locoregional therapy and 20.5% without it, a nonsignificant difference. "Uniformly, there was no difference at all in any subsets," Dr. Badwe reported.
The study had a one-sided superiority design, he noted. "We wouldn’t have been able to see a 2.5- or 3-month difference, or a 4% detriment" in overall survival.
The surgery group had a lower risk of local progression-free survival events (hazard ratio, 0.16; P = .00), but a higher risk of distant progression-free survival events (HR, 1.42; P = .01).
Several theories might explain why removal of the primary would accelerate growth of metastases, according to Dr. Badwe.
"The first and the foremost is that the act of surgery itself might elaborate some growth factors which might allow metastatic disease to grow. The second possibility, which was suggested by Dr. Fisher (Cancer Res. 1989;49:1996-2001) is that the primary tumor, which predates the onset of distant metastases, elaborates some inhibitory factors, and they are not there once the primary tumor is removed, bestowing autonomy of growth on the distant metastases," he explained. "And the third possibility is the act of surgery might induce some more metastatic processes by dissemination and create new disease."
Session attendee Dr. Steven Vogl, an oncologist in the Bronx, N.Y., said, "The ascertainment of disease progression requires disease that you can follow. A woman with bone-only metastases with her cancer in place, you can tell when her cancer is getting worse because the primary site or the axillary nodes are getting bigger. It’s much more difficult if you’ve taken those off and irradiated the chest wall. Have you looked at your data to see if that’s what was going on, why you had more distant metastases, because they couldn’t progress locally? This is a medical trial explanation that contradicts Fisher’s biologic hypothesis."
"There was a fixed time duration at which systemic investigations were performed to assess whether the distant metastases progressed," Dr. Badwe replied. If anything, the patients who did not have surgery had more assessments of their distant metastases, he said.
Dr. Tari A. King, a session attendee from the Memorial Sloan-Kettering Cancer Center, New York, noted that a lack of HER2 therapy in the trial may have had a large effect.
"We do have prospective registry data here [abstract 18-09, presented in a poster session] from a trial that we completed in the United States sponsored by the Translational Breast Cancer Research Consortium, and the patients in our study, whether they received surgery or not, their 2-year overall survival is far superior to what you’ve just showed us," she commented. "So I’m not sure that we can really apply your data to the modern targeted therapy regimens that we see in the United States."
Turkish trial
The Turkish trial, known as the MF07-01 trial, was conduced between 2008 and 2012 among treatment-naïve patients.
They were randomized evenly to receive either systemic therapy alone or surgery for the primary tumor (with or without axillary dissection) followed by radiation therapy if indicated, plus systemic therapy.
All patients received hormonal therapy as needed, and those with HER2-positive disease received trastuzumab.
With a median follow-up of 18 months, the median overall survival was 46 months with initial surgery and 42 months with initial systemic therapy, a nonsignificant difference, reported Dr. Soran, who disclosed no relevant conflicts of interest.
In unplanned subgroup analyses, the findings were similar for most subgroups of patients. However, surgery yielded superior survival in patients with solitary bone metastases (not reached vs. 42 months, P = .02) and inferior survival in patients having multiple liver or pulmonary metastases (16 months vs. not reached, P = .02).
The rate of locoregional progression was much lower with initial surgery than with initial systemic therapy (0.7% vs. 3.6%).
Dr. Soran emphasized that the trial’s planned median follow-up is 36 months, so the presented results are only preliminary. Quality of life and morbidity analyses are ongoing.
Dr. Badwe and Dr. Soran disclosed no relevant conflicts of interest.
AT SABCS 2013
Major finding: There was no significant difference in overall survival with surgery vs. systemic therapy in either the Indian trial (5-year rate, 19.2% vs. 20.5%) or the Turkish trial (median, 46 vs. 42 months).
Data source: A randomized trial among 350 women in India with de novo metastatic breast cancer who had had a response to chemotherapy, and a randomized trial in 293 treatment-naïve patients in Turkey with untreated de novo metastatic breast cancer.
Disclosures: Dr. Badwe and Dr. Soran disclosed no relevant conflicts of interest.
No overall benefit seen with bisphosphonate treatment in chemoresistant breast cancer
SAN ANTONIO – Adjuvant zoledronate failed to improve outcomes in breast cancer patients with residual tumor following neoadjuvant chemotherapy in a large, randomized, phase-3 trial, Dr. Gunter von Minckwitz reported at the San Antonio Breast Cancer Symposium.
There was, however, a bright spot buried within the results of the Neo-Adjuvant Trial Add-On (NATAN) trial: the subset of participants over age 55 years showed a 17% improvement in disease-free survival compared with controls. Although this difference didn’t reach statistical significance because of limited patient numbers, it was closely similar to the benefit seen for adjuvant bisphosphonate therapy in the Early Breast Cancer Trialists Collaborative Group’s practice-changing meta-analysis presented earlier at the San Antonio symposium.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That meta-analysis, presented by Dr. Robert Coleman of the University of Sheffield (England), included more than 17,000 participants in randomized trials. The conclusion was that adjuvant bisphosphonate therapy was of significant benefit in postmenopausal breast cancer patients, with a 17% reduction in the risk of mortality and a 34% decrease in the risk of bone metastases, compared with controls. Premenopausal patients didn’t benefit from adjuvant bisphosphonate therapy.
"We had discussed doing another bisphosphonate trial, this one to be limited to postmenopausal patients without a pathologic complete response after neoadjuvant chemotherapy, but having seen Robert Coleman’s presentation yesterday we are not very much in favor of that any longer. We have our practice guideline meeting for Germany in January, and I expect that we will give a recommendation to use bisphosphonates in postmenopausal patients, so it makes no sense to do another prospective trial," said Dr. von Minckwitz, chairman of the German Breast Group, Neu-Isenburg, and a gynecologist at the University of Frankfurt.
NATAN was carried out because patients with residual disease after neoadjuvant chemotherapy have a worse prognosis than those with a pathologic complete response, and they have few adjuvant treatment options. The study included 654 patients with residual disease after at least four cycles of neoadjuvant anthracycline/taxane-based chemotherapy. Patients were randomized to a planned 5 years of postsurgical intravenous zoledronate or observation, plus adjuvant endocrine therapy and/or trastuzumab as indicated.
The study was halted early due to futility after a median follow-up of 48 months because of virtually identical event-free survival rates in the two study arms. Women over age 55 years, who comprised one-third of the study population, were the only subgroup with a strong, albeit nonsignificant, trend toward benefit for zoledronate.
There is a clear need for new treatment options for women with residual tumor after neoadjuvant chemotherapy, particularly those who aren’t postmenopausal. Several novel agents are now in clinical trials in patients with chemoresistant breast cancer, including rucaparib, an oral small-molecule inhibitor of PARP (poly ADP-ribose polymerase), for triple-negative breast cancer; palbociclib, an oral and selective inhibitor of cyclin dependent kinases 4 and 6, in women with hormone receptor–positive/HER2-negative disease; and trastuzumab emtansine, a conjugate of trastuzumab and the cytotoxic agent mertansine, in patients with chemoresistant HER2-positive breast cancer, Dr. von Minckwitz said.
NATAN was funded by Novartis. Dr. von Minckwitz reported having received research grants from, and serving as a speaker and consultant for, Novartis and Roche.
SAN ANTONIO – Adjuvant zoledronate failed to improve outcomes in breast cancer patients with residual tumor following neoadjuvant chemotherapy in a large, randomized, phase-3 trial, Dr. Gunter von Minckwitz reported at the San Antonio Breast Cancer Symposium.
There was, however, a bright spot buried within the results of the Neo-Adjuvant Trial Add-On (NATAN) trial: the subset of participants over age 55 years showed a 17% improvement in disease-free survival compared with controls. Although this difference didn’t reach statistical significance because of limited patient numbers, it was closely similar to the benefit seen for adjuvant bisphosphonate therapy in the Early Breast Cancer Trialists Collaborative Group’s practice-changing meta-analysis presented earlier at the San Antonio symposium.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That meta-analysis, presented by Dr. Robert Coleman of the University of Sheffield (England), included more than 17,000 participants in randomized trials. The conclusion was that adjuvant bisphosphonate therapy was of significant benefit in postmenopausal breast cancer patients, with a 17% reduction in the risk of mortality and a 34% decrease in the risk of bone metastases, compared with controls. Premenopausal patients didn’t benefit from adjuvant bisphosphonate therapy.
"We had discussed doing another bisphosphonate trial, this one to be limited to postmenopausal patients without a pathologic complete response after neoadjuvant chemotherapy, but having seen Robert Coleman’s presentation yesterday we are not very much in favor of that any longer. We have our practice guideline meeting for Germany in January, and I expect that we will give a recommendation to use bisphosphonates in postmenopausal patients, so it makes no sense to do another prospective trial," said Dr. von Minckwitz, chairman of the German Breast Group, Neu-Isenburg, and a gynecologist at the University of Frankfurt.
NATAN was carried out because patients with residual disease after neoadjuvant chemotherapy have a worse prognosis than those with a pathologic complete response, and they have few adjuvant treatment options. The study included 654 patients with residual disease after at least four cycles of neoadjuvant anthracycline/taxane-based chemotherapy. Patients were randomized to a planned 5 years of postsurgical intravenous zoledronate or observation, plus adjuvant endocrine therapy and/or trastuzumab as indicated.
The study was halted early due to futility after a median follow-up of 48 months because of virtually identical event-free survival rates in the two study arms. Women over age 55 years, who comprised one-third of the study population, were the only subgroup with a strong, albeit nonsignificant, trend toward benefit for zoledronate.
There is a clear need for new treatment options for women with residual tumor after neoadjuvant chemotherapy, particularly those who aren’t postmenopausal. Several novel agents are now in clinical trials in patients with chemoresistant breast cancer, including rucaparib, an oral small-molecule inhibitor of PARP (poly ADP-ribose polymerase), for triple-negative breast cancer; palbociclib, an oral and selective inhibitor of cyclin dependent kinases 4 and 6, in women with hormone receptor–positive/HER2-negative disease; and trastuzumab emtansine, a conjugate of trastuzumab and the cytotoxic agent mertansine, in patients with chemoresistant HER2-positive breast cancer, Dr. von Minckwitz said.
NATAN was funded by Novartis. Dr. von Minckwitz reported having received research grants from, and serving as a speaker and consultant for, Novartis and Roche.
SAN ANTONIO – Adjuvant zoledronate failed to improve outcomes in breast cancer patients with residual tumor following neoadjuvant chemotherapy in a large, randomized, phase-3 trial, Dr. Gunter von Minckwitz reported at the San Antonio Breast Cancer Symposium.
There was, however, a bright spot buried within the results of the Neo-Adjuvant Trial Add-On (NATAN) trial: the subset of participants over age 55 years showed a 17% improvement in disease-free survival compared with controls. Although this difference didn’t reach statistical significance because of limited patient numbers, it was closely similar to the benefit seen for adjuvant bisphosphonate therapy in the Early Breast Cancer Trialists Collaborative Group’s practice-changing meta-analysis presented earlier at the San Antonio symposium.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
That meta-analysis, presented by Dr. Robert Coleman of the University of Sheffield (England), included more than 17,000 participants in randomized trials. The conclusion was that adjuvant bisphosphonate therapy was of significant benefit in postmenopausal breast cancer patients, with a 17% reduction in the risk of mortality and a 34% decrease in the risk of bone metastases, compared with controls. Premenopausal patients didn’t benefit from adjuvant bisphosphonate therapy.
"We had discussed doing another bisphosphonate trial, this one to be limited to postmenopausal patients without a pathologic complete response after neoadjuvant chemotherapy, but having seen Robert Coleman’s presentation yesterday we are not very much in favor of that any longer. We have our practice guideline meeting for Germany in January, and I expect that we will give a recommendation to use bisphosphonates in postmenopausal patients, so it makes no sense to do another prospective trial," said Dr. von Minckwitz, chairman of the German Breast Group, Neu-Isenburg, and a gynecologist at the University of Frankfurt.
NATAN was carried out because patients with residual disease after neoadjuvant chemotherapy have a worse prognosis than those with a pathologic complete response, and they have few adjuvant treatment options. The study included 654 patients with residual disease after at least four cycles of neoadjuvant anthracycline/taxane-based chemotherapy. Patients were randomized to a planned 5 years of postsurgical intravenous zoledronate or observation, plus adjuvant endocrine therapy and/or trastuzumab as indicated.
The study was halted early due to futility after a median follow-up of 48 months because of virtually identical event-free survival rates in the two study arms. Women over age 55 years, who comprised one-third of the study population, were the only subgroup with a strong, albeit nonsignificant, trend toward benefit for zoledronate.
There is a clear need for new treatment options for women with residual tumor after neoadjuvant chemotherapy, particularly those who aren’t postmenopausal. Several novel agents are now in clinical trials in patients with chemoresistant breast cancer, including rucaparib, an oral small-molecule inhibitor of PARP (poly ADP-ribose polymerase), for triple-negative breast cancer; palbociclib, an oral and selective inhibitor of cyclin dependent kinases 4 and 6, in women with hormone receptor–positive/HER2-negative disease; and trastuzumab emtansine, a conjugate of trastuzumab and the cytotoxic agent mertansine, in patients with chemoresistant HER2-positive breast cancer, Dr. von Minckwitz said.
NATAN was funded by Novartis. Dr. von Minckwitz reported having received research grants from, and serving as a speaker and consultant for, Novartis and Roche.
AT SABCS 2013
Major finding: Women with residual tumor after neoadjuvant chemotherapy who were randomized to adjuvant zoledronate didn’t have a lower risk of recurrent disease or death during a median 4 years of follow-up than those who didn’t get the bisphosphonate, although there was a strong trend for benefit in the subgroup over age 55.
Data source: NATAN was a randomized, phase-3 clinical trial including 654 patients with post-neoadjuvant chemoresistant breast cancer.
Disclosures: The trial was sponsored by Novartis. Dr. von Minckwitz reported having received research grants from, and serving as a speaker and consultant for, Novartis and Roche.
Novel treatment promising for chronic neuropathic postmastectomy pain
SAN ANTONIO – Perineural injection of bupivacaine and dexamethasone was a simple and effective treatment for chronic neuropathic pain following mastectomy, based on results of a pilot study.
The effectiveness of this novel therapy strongly suggests the source of this common pain syndrome is damage to the T4 and T5 sensory nerves during surgery, rather than damage to the intercostobrachial nerve, as traditionally thought, according to Dr. Cathy J. Tang of the University of California, San Francisco.
The T4 and T5 sensory nerves come off the chest wall and enter the breast accompanied by a blood vessel. When these nerves are cut and cauterized during mastectomy, the resultant nerve damage can manifest as neuroma formation and neuropathic pain along the two dermatomes, she said at the San Antonio Breast Cancer Symposium.
Chronic postmastectomy breast pain is commonly referred to as postmastectomy pain syndrome. Published estimates of its incidence after mastectomy range from 20% to 68%. The pain can start in the immediate postoperative period, or onset can be delayed up to 6 months or more post mastectomy. The pain is typically experienced as a shooting or burning pain, with point tenderness. It persists well after the expected healing period.
The intervention involves identifying a patient’s points of maximum pain or tenderness, usually located laterally along the midaxillary line or at the inframammary fold directly below the nipple. These points are injected at the level of the chest wall. Each injection consists of 2 mL of an equal ratio of 0.5% bupivacaine plus 4 mg/mL of dexamethasone followed by a minute or two of massage to enhance infiltration of the area.
Dr. Tang reported on 19 patients who developed postmastectomy pain syndrome after either partial mastectomy, total mastectomy with immediate reconstruction, or lateral core biopsy in one case. A total of 29 points of maximum tenderness were identified and treated. All patients had pain relief within minutes, with point pain scores on a 0-10 scale falling from 8-9 to 0-1. Long-term pain relief was experienced after 17 of the 29 initial injections (59%) in 11 patients. Pain was resolved at another nine sites after a second injection. A third injection at one recalcitrant site led to long-term pain relief. Thus, perineural injections alleviated pain at 27 of 29 treated sites, or 93%, at a mean of 10.7 months of follow-up.
In light of how simple and safe this treatment is, Dr. Tang urged routine inquiry about postmastectomy neuropathic pain. Patients with postmastectomy pain often report an inability to lie on the affected side or to wear a bra.
The study also indicates the importance of careful dissection of the T4 and T5 sensory nerves during mastectomy in order to minimize the risk of postoperative neuroma formation.
Dr. Tang reported having no financial conflicts regarding this unfunded study.
SAN ANTONIO – Perineural injection of bupivacaine and dexamethasone was a simple and effective treatment for chronic neuropathic pain following mastectomy, based on results of a pilot study.
The effectiveness of this novel therapy strongly suggests the source of this common pain syndrome is damage to the T4 and T5 sensory nerves during surgery, rather than damage to the intercostobrachial nerve, as traditionally thought, according to Dr. Cathy J. Tang of the University of California, San Francisco.
The T4 and T5 sensory nerves come off the chest wall and enter the breast accompanied by a blood vessel. When these nerves are cut and cauterized during mastectomy, the resultant nerve damage can manifest as neuroma formation and neuropathic pain along the two dermatomes, she said at the San Antonio Breast Cancer Symposium.
Chronic postmastectomy breast pain is commonly referred to as postmastectomy pain syndrome. Published estimates of its incidence after mastectomy range from 20% to 68%. The pain can start in the immediate postoperative period, or onset can be delayed up to 6 months or more post mastectomy. The pain is typically experienced as a shooting or burning pain, with point tenderness. It persists well after the expected healing period.
The intervention involves identifying a patient’s points of maximum pain or tenderness, usually located laterally along the midaxillary line or at the inframammary fold directly below the nipple. These points are injected at the level of the chest wall. Each injection consists of 2 mL of an equal ratio of 0.5% bupivacaine plus 4 mg/mL of dexamethasone followed by a minute or two of massage to enhance infiltration of the area.
Dr. Tang reported on 19 patients who developed postmastectomy pain syndrome after either partial mastectomy, total mastectomy with immediate reconstruction, or lateral core biopsy in one case. A total of 29 points of maximum tenderness were identified and treated. All patients had pain relief within minutes, with point pain scores on a 0-10 scale falling from 8-9 to 0-1. Long-term pain relief was experienced after 17 of the 29 initial injections (59%) in 11 patients. Pain was resolved at another nine sites after a second injection. A third injection at one recalcitrant site led to long-term pain relief. Thus, perineural injections alleviated pain at 27 of 29 treated sites, or 93%, at a mean of 10.7 months of follow-up.
In light of how simple and safe this treatment is, Dr. Tang urged routine inquiry about postmastectomy neuropathic pain. Patients with postmastectomy pain often report an inability to lie on the affected side or to wear a bra.
The study also indicates the importance of careful dissection of the T4 and T5 sensory nerves during mastectomy in order to minimize the risk of postoperative neuroma formation.
Dr. Tang reported having no financial conflicts regarding this unfunded study.
SAN ANTONIO – Perineural injection of bupivacaine and dexamethasone was a simple and effective treatment for chronic neuropathic pain following mastectomy, based on results of a pilot study.
The effectiveness of this novel therapy strongly suggests the source of this common pain syndrome is damage to the T4 and T5 sensory nerves during surgery, rather than damage to the intercostobrachial nerve, as traditionally thought, according to Dr. Cathy J. Tang of the University of California, San Francisco.
The T4 and T5 sensory nerves come off the chest wall and enter the breast accompanied by a blood vessel. When these nerves are cut and cauterized during mastectomy, the resultant nerve damage can manifest as neuroma formation and neuropathic pain along the two dermatomes, she said at the San Antonio Breast Cancer Symposium.
Chronic postmastectomy breast pain is commonly referred to as postmastectomy pain syndrome. Published estimates of its incidence after mastectomy range from 20% to 68%. The pain can start in the immediate postoperative period, or onset can be delayed up to 6 months or more post mastectomy. The pain is typically experienced as a shooting or burning pain, with point tenderness. It persists well after the expected healing period.
The intervention involves identifying a patient’s points of maximum pain or tenderness, usually located laterally along the midaxillary line or at the inframammary fold directly below the nipple. These points are injected at the level of the chest wall. Each injection consists of 2 mL of an equal ratio of 0.5% bupivacaine plus 4 mg/mL of dexamethasone followed by a minute or two of massage to enhance infiltration of the area.
Dr. Tang reported on 19 patients who developed postmastectomy pain syndrome after either partial mastectomy, total mastectomy with immediate reconstruction, or lateral core biopsy in one case. A total of 29 points of maximum tenderness were identified and treated. All patients had pain relief within minutes, with point pain scores on a 0-10 scale falling from 8-9 to 0-1. Long-term pain relief was experienced after 17 of the 29 initial injections (59%) in 11 patients. Pain was resolved at another nine sites after a second injection. A third injection at one recalcitrant site led to long-term pain relief. Thus, perineural injections alleviated pain at 27 of 29 treated sites, or 93%, at a mean of 10.7 months of follow-up.
In light of how simple and safe this treatment is, Dr. Tang urged routine inquiry about postmastectomy neuropathic pain. Patients with postmastectomy pain often report an inability to lie on the affected side or to wear a bra.
The study also indicates the importance of careful dissection of the T4 and T5 sensory nerves during mastectomy in order to minimize the risk of postoperative neuroma formation.
Dr. Tang reported having no financial conflicts regarding this unfunded study.
AT SABCS 2013
Major finding: Injection of a combination of bupivacaine and dexamethasone at well-defined sites of maximum pain and tenderness resolved pain at 93% of treated sites in patients with chronic neuropathic postmastectomy pain.
Data source: A prospective case series involving 19 patients with postmastectomy pain syndrome. A total of 29 sites of maximum pain and tenderness were treated.
Disclosures: The study was conducted free of commercial support. The presenter reported having no relevant financial conflicts.
Exercise protects black women against ER-negative breast cancer
SAN ANTONIO – African-American women had half the risk of developing estrogen receptor–negative breast cancer if they exercised vigorously 3 hours per week, according to data from the Black Women’s Health Study.
The findings are based on nearly 20 years of follow-up on 44,704 women in the prospective, observational Black Women's Health Study. During this period, 1,377 subjects were diagnosed with invasive breast cancer, including 327 with ER-negative disease.
Black women who reported having engaged in vigorous exercise from high school onwards for a lifetime average of 3 hours or more per week had a 47% reduction in the risk of ER-negative breast cancer, compared with women exercising vigorously for less than 1 hour per week after adjusting for age, education, parity, and dietary pattern, Lucile Adams-Campbell, Ph.D., said at the San Antonio Breast Cancer Symposium.
Exercise proved unrelated to the risk of developing the generally more indolent ER-positive breast cancers in this population, said Dr. Adams-Campbell, professor of oncology at the Georgetown University Lombardi Comprehensive Cancer Center in Washington, D.C.
The inverse relationship between vigorous exercise and ER-negative breast cancer risk was statistically significant only among postmenopausal black women. In that population, the adjusted risk was reduced by 59% in participants with a lifetime average of at least 3 hours of exercise per week, compared with less than 1 hour.
The Centers for Disease Control and Prevention defines vigorous exercise as more than 6.0 metabolic equivalents (more than 7 kcal/min). Examples of activities through which this is readily accomplished include jogging, bicycling at more than 10 mph, or briskly walking uphill.
Dr. Adams-Campbell is a coleader of the ongoing Black Women’s Health Study, which aims to shed light on the elevated risks of hypertension, stroke, diabetes, lupus, and other diseases present in African-American women. The study is funded by the National Cancer Institute. Dr. Adams-Campbell reported having no relevant financial interests.
SAN ANTONIO – African-American women had half the risk of developing estrogen receptor–negative breast cancer if they exercised vigorously 3 hours per week, according to data from the Black Women’s Health Study.
The findings are based on nearly 20 years of follow-up on 44,704 women in the prospective, observational Black Women's Health Study. During this period, 1,377 subjects were diagnosed with invasive breast cancer, including 327 with ER-negative disease.
Black women who reported having engaged in vigorous exercise from high school onwards for a lifetime average of 3 hours or more per week had a 47% reduction in the risk of ER-negative breast cancer, compared with women exercising vigorously for less than 1 hour per week after adjusting for age, education, parity, and dietary pattern, Lucile Adams-Campbell, Ph.D., said at the San Antonio Breast Cancer Symposium.
Exercise proved unrelated to the risk of developing the generally more indolent ER-positive breast cancers in this population, said Dr. Adams-Campbell, professor of oncology at the Georgetown University Lombardi Comprehensive Cancer Center in Washington, D.C.
The inverse relationship between vigorous exercise and ER-negative breast cancer risk was statistically significant only among postmenopausal black women. In that population, the adjusted risk was reduced by 59% in participants with a lifetime average of at least 3 hours of exercise per week, compared with less than 1 hour.
The Centers for Disease Control and Prevention defines vigorous exercise as more than 6.0 metabolic equivalents (more than 7 kcal/min). Examples of activities through which this is readily accomplished include jogging, bicycling at more than 10 mph, or briskly walking uphill.
Dr. Adams-Campbell is a coleader of the ongoing Black Women’s Health Study, which aims to shed light on the elevated risks of hypertension, stroke, diabetes, lupus, and other diseases present in African-American women. The study is funded by the National Cancer Institute. Dr. Adams-Campbell reported having no relevant financial interests.
SAN ANTONIO – African-American women had half the risk of developing estrogen receptor–negative breast cancer if they exercised vigorously 3 hours per week, according to data from the Black Women’s Health Study.
The findings are based on nearly 20 years of follow-up on 44,704 women in the prospective, observational Black Women's Health Study. During this period, 1,377 subjects were diagnosed with invasive breast cancer, including 327 with ER-negative disease.
Black women who reported having engaged in vigorous exercise from high school onwards for a lifetime average of 3 hours or more per week had a 47% reduction in the risk of ER-negative breast cancer, compared with women exercising vigorously for less than 1 hour per week after adjusting for age, education, parity, and dietary pattern, Lucile Adams-Campbell, Ph.D., said at the San Antonio Breast Cancer Symposium.
Exercise proved unrelated to the risk of developing the generally more indolent ER-positive breast cancers in this population, said Dr. Adams-Campbell, professor of oncology at the Georgetown University Lombardi Comprehensive Cancer Center in Washington, D.C.
The inverse relationship between vigorous exercise and ER-negative breast cancer risk was statistically significant only among postmenopausal black women. In that population, the adjusted risk was reduced by 59% in participants with a lifetime average of at least 3 hours of exercise per week, compared with less than 1 hour.
The Centers for Disease Control and Prevention defines vigorous exercise as more than 6.0 metabolic equivalents (more than 7 kcal/min). Examples of activities through which this is readily accomplished include jogging, bicycling at more than 10 mph, or briskly walking uphill.
Dr. Adams-Campbell is a coleader of the ongoing Black Women’s Health Study, which aims to shed light on the elevated risks of hypertension, stroke, diabetes, lupus, and other diseases present in African-American women. The study is funded by the National Cancer Institute. Dr. Adams-Campbell reported having no relevant financial interests.
AT SABCS 2013
Major finding: Black women who reported engaging in an average of least 3 hours of vigorous exercise per week over their lifetime had a 47% reduction in the risk of developing estrogen receptor–negative breast cancer, compared with those averaging less than 1 hour per week of vigorous exercise.
Data source: This analysis from the ongoing Black Women’s Health Study included nearly 45,000 women aged 30 years or older at enrollment, with close to 20 years of prospective follow-up.
Disclosures: The study is funded by the National Cancer Institute. The presenter reported having no financial conflicts.
Findings set stage for immunomodulatory approaches in breast cancer
SAN ANTONIO – Tumor-infiltrating lymphocyte levels at breast cancer diagnosis predict response to therapies for both HER2-positive and triple-negative breast cancers, according to studies presented at the San Antonio Breast Cancer Symposium.
Tumor-infiltrating lymphocytes (TILs) appeared to be a useful biomarker of response to trastuzumab (Herceptin) as well as to chemotherapy. By extension, breast cancers appear to be immunogenic, contrary to traditional thinking. Trastuzumab and chemotherapy appear to act not only on the tumor, but to enhance antitumor immunity as well.
The findings could result in a paradigm shift in treatment approaches. Clinical outcomes could potentially be improved in women with HER2-positive or triple-negative breast cancers by targeting the immune system with immunomodulatory agents in addition to providing standard therapies, according to Dr. Sherene Loi, a medical oncologist and head of the translational breast cancer genomics laboratory at the Peter MacCallum Cancer Center in Melbourne.
Dr. Loi presented an analysis of breast cancer samples from 156 women with operable or locally advanced HER2-positive breast cancer who participated in the previously reported GeparQuattro trial. All of the women received neoadjuvant trastuzumab and cytotoxic chemotherapy. In a multivariate analysis, for every 10% increase in the level of stromal TILs there was a 16% increase in the pathologic complete response rate, meaning no residual invasive cancer in the breast or lymph nodes at surgery.
"These data reinforced for us that there is a relationship between the immune system and responses to trastuzumab and chemotherapy," she said.
In another study presented at the meeting, Dr. Sylvia Adams demonstrated the prognostic value of TILs in patients with triple-negative breast cancer. Tumors were analyzed from 481 participants in Eastern Cooperative Oncology Group 2197 and 1199, two previously reported phase III randomized, prospective trials of adjuvant chemotherapy in triple-negative breast cancer.
At least 10% lymphocytic infiltration in the tumor stroma was seen in 80% of tumors, meaning that 10% or more of all cells in the stroma were TILs. For each 10% increment in stromal TILs at the time of breast cancer diagnosis, there was an 18% reduction in the risk of distant recurrence and a "very impressive" 19% reduction in the risk of mortality at a median followup of 10.6 years, reported Dr. Adams of New York University.
She noted that this new analysis confirms an earlier study led by Dr. Loi, the prospective, randomized BIG 02-98 trial. In that study, TILs were associated with improved prognosis in patients with operable triple-negative breast cancer. The new validation study, Dr. Adams noted, raises to Level I the evidence that supports measuring stromal TILs as a biomarker in triple-negative breast cancer.
"The data provide strong evidence for the incorporation of this feature into the AJCC (American Joint Committee on Cancer) staging for triple-negative breast cancer and for utilization of stromal TILs as a stratifier in clinical trials," Dr. Adams said. "And most important, I would like to say – because some may ask ‘Is this just another prognostic factor for breast cancer?’ – is that these data really help provide additional evidence that some breast cancers can be immunogenic and that the endogenous immune response to cancer is predictive of survival in triple-negative breast cancer. So this actually opens up the area for introducing immunotherapies in triple-negative breast cancer. There is a hope that by targeting and harnessing the immune system we can improve cure rates."
Dr. Loi provided a glimpse into the possible way forward. In an effort to learn how trastuzumab affects antitumor immunity, she and her colleagues performed gene expression analyses on HER2-positive breast tumor specimens from 202 participants in the phase III randomized FinHER clinical trial of adjuvant trastuzumab or no trastuzumab along with postoperative chemotherapy. The greatest clinical response to trastuzumab was seen in patients whose tumors highly expressed the immunosuppressive gene known as programmed cell death 1 (PD-1).
This led to studies conducted in a mouse model of HER2-positive breast cancer. Dr. Loi and her colleagues showed that combining trastuzumab with a T-cell checkpoint inhibitor – an anti-PD-1 agent resulted in greater reductions in tumor size than with trastuzumab alone.
More than half a dozen T-cell checkpoint inhibitors are in the developmental pipeline for treatment of a range of malignancies, including the anti-PD-1 agents nivolumab, lambrolizumab, and pidilizumab. Clinical trials testing the combination of trastuzumab and a PD-1 inhibitor in HER2-positive breast cancer patients are being planned.
Dr. Loi’s work was funded by the European Union RESPONSIFY project, the Breast Cancer Research Foundation, and the Fonds J.C. Heuson. She and Dr. Adams reported having no relevant financial conflicts of interest.
SAN ANTONIO – Tumor-infiltrating lymphocyte levels at breast cancer diagnosis predict response to therapies for both HER2-positive and triple-negative breast cancers, according to studies presented at the San Antonio Breast Cancer Symposium.
Tumor-infiltrating lymphocytes (TILs) appeared to be a useful biomarker of response to trastuzumab (Herceptin) as well as to chemotherapy. By extension, breast cancers appear to be immunogenic, contrary to traditional thinking. Trastuzumab and chemotherapy appear to act not only on the tumor, but to enhance antitumor immunity as well.
The findings could result in a paradigm shift in treatment approaches. Clinical outcomes could potentially be improved in women with HER2-positive or triple-negative breast cancers by targeting the immune system with immunomodulatory agents in addition to providing standard therapies, according to Dr. Sherene Loi, a medical oncologist and head of the translational breast cancer genomics laboratory at the Peter MacCallum Cancer Center in Melbourne.
Dr. Loi presented an analysis of breast cancer samples from 156 women with operable or locally advanced HER2-positive breast cancer who participated in the previously reported GeparQuattro trial. All of the women received neoadjuvant trastuzumab and cytotoxic chemotherapy. In a multivariate analysis, for every 10% increase in the level of stromal TILs there was a 16% increase in the pathologic complete response rate, meaning no residual invasive cancer in the breast or lymph nodes at surgery.
"These data reinforced for us that there is a relationship between the immune system and responses to trastuzumab and chemotherapy," she said.
In another study presented at the meeting, Dr. Sylvia Adams demonstrated the prognostic value of TILs in patients with triple-negative breast cancer. Tumors were analyzed from 481 participants in Eastern Cooperative Oncology Group 2197 and 1199, two previously reported phase III randomized, prospective trials of adjuvant chemotherapy in triple-negative breast cancer.
At least 10% lymphocytic infiltration in the tumor stroma was seen in 80% of tumors, meaning that 10% or more of all cells in the stroma were TILs. For each 10% increment in stromal TILs at the time of breast cancer diagnosis, there was an 18% reduction in the risk of distant recurrence and a "very impressive" 19% reduction in the risk of mortality at a median followup of 10.6 years, reported Dr. Adams of New York University.
She noted that this new analysis confirms an earlier study led by Dr. Loi, the prospective, randomized BIG 02-98 trial. In that study, TILs were associated with improved prognosis in patients with operable triple-negative breast cancer. The new validation study, Dr. Adams noted, raises to Level I the evidence that supports measuring stromal TILs as a biomarker in triple-negative breast cancer.
"The data provide strong evidence for the incorporation of this feature into the AJCC (American Joint Committee on Cancer) staging for triple-negative breast cancer and for utilization of stromal TILs as a stratifier in clinical trials," Dr. Adams said. "And most important, I would like to say – because some may ask ‘Is this just another prognostic factor for breast cancer?’ – is that these data really help provide additional evidence that some breast cancers can be immunogenic and that the endogenous immune response to cancer is predictive of survival in triple-negative breast cancer. So this actually opens up the area for introducing immunotherapies in triple-negative breast cancer. There is a hope that by targeting and harnessing the immune system we can improve cure rates."
Dr. Loi provided a glimpse into the possible way forward. In an effort to learn how trastuzumab affects antitumor immunity, she and her colleagues performed gene expression analyses on HER2-positive breast tumor specimens from 202 participants in the phase III randomized FinHER clinical trial of adjuvant trastuzumab or no trastuzumab along with postoperative chemotherapy. The greatest clinical response to trastuzumab was seen in patients whose tumors highly expressed the immunosuppressive gene known as programmed cell death 1 (PD-1).
This led to studies conducted in a mouse model of HER2-positive breast cancer. Dr. Loi and her colleagues showed that combining trastuzumab with a T-cell checkpoint inhibitor – an anti-PD-1 agent resulted in greater reductions in tumor size than with trastuzumab alone.
More than half a dozen T-cell checkpoint inhibitors are in the developmental pipeline for treatment of a range of malignancies, including the anti-PD-1 agents nivolumab, lambrolizumab, and pidilizumab. Clinical trials testing the combination of trastuzumab and a PD-1 inhibitor in HER2-positive breast cancer patients are being planned.
Dr. Loi’s work was funded by the European Union RESPONSIFY project, the Breast Cancer Research Foundation, and the Fonds J.C. Heuson. She and Dr. Adams reported having no relevant financial conflicts of interest.
SAN ANTONIO – Tumor-infiltrating lymphocyte levels at breast cancer diagnosis predict response to therapies for both HER2-positive and triple-negative breast cancers, according to studies presented at the San Antonio Breast Cancer Symposium.
Tumor-infiltrating lymphocytes (TILs) appeared to be a useful biomarker of response to trastuzumab (Herceptin) as well as to chemotherapy. By extension, breast cancers appear to be immunogenic, contrary to traditional thinking. Trastuzumab and chemotherapy appear to act not only on the tumor, but to enhance antitumor immunity as well.
The findings could result in a paradigm shift in treatment approaches. Clinical outcomes could potentially be improved in women with HER2-positive or triple-negative breast cancers by targeting the immune system with immunomodulatory agents in addition to providing standard therapies, according to Dr. Sherene Loi, a medical oncologist and head of the translational breast cancer genomics laboratory at the Peter MacCallum Cancer Center in Melbourne.
Dr. Loi presented an analysis of breast cancer samples from 156 women with operable or locally advanced HER2-positive breast cancer who participated in the previously reported GeparQuattro trial. All of the women received neoadjuvant trastuzumab and cytotoxic chemotherapy. In a multivariate analysis, for every 10% increase in the level of stromal TILs there was a 16% increase in the pathologic complete response rate, meaning no residual invasive cancer in the breast or lymph nodes at surgery.
"These data reinforced for us that there is a relationship between the immune system and responses to trastuzumab and chemotherapy," she said.
In another study presented at the meeting, Dr. Sylvia Adams demonstrated the prognostic value of TILs in patients with triple-negative breast cancer. Tumors were analyzed from 481 participants in Eastern Cooperative Oncology Group 2197 and 1199, two previously reported phase III randomized, prospective trials of adjuvant chemotherapy in triple-negative breast cancer.
At least 10% lymphocytic infiltration in the tumor stroma was seen in 80% of tumors, meaning that 10% or more of all cells in the stroma were TILs. For each 10% increment in stromal TILs at the time of breast cancer diagnosis, there was an 18% reduction in the risk of distant recurrence and a "very impressive" 19% reduction in the risk of mortality at a median followup of 10.6 years, reported Dr. Adams of New York University.
She noted that this new analysis confirms an earlier study led by Dr. Loi, the prospective, randomized BIG 02-98 trial. In that study, TILs were associated with improved prognosis in patients with operable triple-negative breast cancer. The new validation study, Dr. Adams noted, raises to Level I the evidence that supports measuring stromal TILs as a biomarker in triple-negative breast cancer.
"The data provide strong evidence for the incorporation of this feature into the AJCC (American Joint Committee on Cancer) staging for triple-negative breast cancer and for utilization of stromal TILs as a stratifier in clinical trials," Dr. Adams said. "And most important, I would like to say – because some may ask ‘Is this just another prognostic factor for breast cancer?’ – is that these data really help provide additional evidence that some breast cancers can be immunogenic and that the endogenous immune response to cancer is predictive of survival in triple-negative breast cancer. So this actually opens up the area for introducing immunotherapies in triple-negative breast cancer. There is a hope that by targeting and harnessing the immune system we can improve cure rates."
Dr. Loi provided a glimpse into the possible way forward. In an effort to learn how trastuzumab affects antitumor immunity, she and her colleagues performed gene expression analyses on HER2-positive breast tumor specimens from 202 participants in the phase III randomized FinHER clinical trial of adjuvant trastuzumab or no trastuzumab along with postoperative chemotherapy. The greatest clinical response to trastuzumab was seen in patients whose tumors highly expressed the immunosuppressive gene known as programmed cell death 1 (PD-1).
This led to studies conducted in a mouse model of HER2-positive breast cancer. Dr. Loi and her colleagues showed that combining trastuzumab with a T-cell checkpoint inhibitor – an anti-PD-1 agent resulted in greater reductions in tumor size than with trastuzumab alone.
More than half a dozen T-cell checkpoint inhibitors are in the developmental pipeline for treatment of a range of malignancies, including the anti-PD-1 agents nivolumab, lambrolizumab, and pidilizumab. Clinical trials testing the combination of trastuzumab and a PD-1 inhibitor in HER2-positive breast cancer patients are being planned.
Dr. Loi’s work was funded by the European Union RESPONSIFY project, the Breast Cancer Research Foundation, and the Fonds J.C. Heuson. She and Dr. Adams reported having no relevant financial conflicts of interest.
AT SABCS 2013
Major finding: For every 10% increment in stromal tumor-infiltrating lymphocytes present at the time of diagnosis of HER2-positive breast cancer, the likelihood of a pathologic complete response to neoadjuvant trastuzumab plus chemotherapy climbed by 16%. In a separate study, every 10% increment in stromal tumor-infiltrating lymphocytes present in triple-negative breast cancer specimens was associated with 19% reduction in the risk of mortality at almost 11 years of followup.
Data source: These translational studies entailed measurement of tumor-infiltrating lymphocyte levels in HER2-positive tumors from 156 participants in the randomized, prospective GeparQuattro trial and 481 triple-negative cancer specimens from participants in the phase III ECOG 2197 and 1199 randomized adjuvant chemotherapy trials.
Disclosures: The presenters reported having no financial conflicts of interest.
Breast MRI screening finds undetected cancers in 11 per 1,000 average-risk women
SAN ANTONIO – MRI screening of women who were at average risk of breast cancer and had a negative screening mammogram resulted in the diagnosis of 11 cases of cancer per 1,000 women screened, Dr. Simone Schrading said at the San Antonio Breast Cancer Symposium.
"The additional cancer detection rate is high, even in heavily prescreened women. In experienced hands, the positive predictive value of MRI screening in this average-risk cohort was comparable to that seen in mammographic screening programs or in MRI high-risk screening cohorts," said Dr. Schrading, of the University of Aachen, Germany.
While MRI is well established as a screening method for women at high familial risk of breast cancer, there have been no data to support its use in average-risk women.
Dr. Schrading reported on a prospective, single-center study evaluating the additional cancer yield and accuracy of breast MRI screening in 1,725 women at average risk for breast cancer. All had normal clinical breast examinations and digital screening mammograms. In addition, 89% of the women had undergone high-frequency breast ultrasound screening, which was normal in all cases. None of the subjects had a personal or family history of breast or ovarian cancer, and none had been diagnosed with breast proliferative changes or atypia. Their mean age was 55 years (range, 42-71 years).
MRI screening detected breast cancers in 18 of these patients, for a detection rate of 11 per 1,000 screened. Seven malignancies were ductal carcinoma in situ, and the other 11 were invasive breast cancer. The mean size of the invasive cancers was 11 mm. Five of the 7 ductal carcinomas in situ and 6 of 11 invasive cancers were high-grade cancers. On the other hand, the stage distribution of the invasive cancers was favorable: All were staged as pN0, M0.
Nearly 91% of screening MRIs were negative as defined by a BIRADS 1 or 2 rating. Another 5.9% were rated BIRADS 3; follow-up MRIs in this group of patients showed no breast cancers. Suspicious lesions – that is, BIRADS 4 or 5 – were noted in 54 patients, or 3.2% of the study group, and they were evaluated by MRI-guided biopsy. The lesions proved benign in 28 of 54 cases, high risk in 8, and malignant in 18.
Thus, the positive predictive value of breast MRI screening in this average-risk, extensively prescreened population was 33% if only BIRADS 4 and 5 cancers were defined as true positives, and 48% if BIRADS 4 and 5 high-risk lesions were also counted.
The age distribution of the patients with MRI-detected breast cancers was similar to that of the total study population. Mammographic breast density did not predict the likelihood of identifying breast cancer by MRI.
Dr. Laura J. Esserman rose from the audience to question the cost-benefit ratio of introducing MRI screening for the vast population of average-risk women.
The aggregate cost of breast cancer screening in the United States is already at least $8 billion annually. Utilizing MRI to screen average-risk women would push that figure up by an order of magnitude, observed Dr. Esserman, professor of surgery and of radiology and director of the Carol Franc Buck Breast Care Center at the University of California, San Francisco.
"MRI screening is still very expensive, it’s true," Dr. Schrading replied. "The main reason for the high cost of MRI is the long acquisition time and the long reading time. The long acquisition time is because the MRI protocols we have today are designed for diagnostic purposes, not for the purpose of screening. Our goal is to use a screening protocol for MRI, where the acquisition time is only 3 minutes and the reading time is less than 30 seconds. This might be a way to reduce the cost of MRI," she said.
The study was conducted free of commercial support. Dr. Schrading declared having no financial conflicts of interest.
SAN ANTONIO – MRI screening of women who were at average risk of breast cancer and had a negative screening mammogram resulted in the diagnosis of 11 cases of cancer per 1,000 women screened, Dr. Simone Schrading said at the San Antonio Breast Cancer Symposium.
"The additional cancer detection rate is high, even in heavily prescreened women. In experienced hands, the positive predictive value of MRI screening in this average-risk cohort was comparable to that seen in mammographic screening programs or in MRI high-risk screening cohorts," said Dr. Schrading, of the University of Aachen, Germany.
While MRI is well established as a screening method for women at high familial risk of breast cancer, there have been no data to support its use in average-risk women.
Dr. Schrading reported on a prospective, single-center study evaluating the additional cancer yield and accuracy of breast MRI screening in 1,725 women at average risk for breast cancer. All had normal clinical breast examinations and digital screening mammograms. In addition, 89% of the women had undergone high-frequency breast ultrasound screening, which was normal in all cases. None of the subjects had a personal or family history of breast or ovarian cancer, and none had been diagnosed with breast proliferative changes or atypia. Their mean age was 55 years (range, 42-71 years).
MRI screening detected breast cancers in 18 of these patients, for a detection rate of 11 per 1,000 screened. Seven malignancies were ductal carcinoma in situ, and the other 11 were invasive breast cancer. The mean size of the invasive cancers was 11 mm. Five of the 7 ductal carcinomas in situ and 6 of 11 invasive cancers were high-grade cancers. On the other hand, the stage distribution of the invasive cancers was favorable: All were staged as pN0, M0.
Nearly 91% of screening MRIs were negative as defined by a BIRADS 1 or 2 rating. Another 5.9% were rated BIRADS 3; follow-up MRIs in this group of patients showed no breast cancers. Suspicious lesions – that is, BIRADS 4 or 5 – were noted in 54 patients, or 3.2% of the study group, and they were evaluated by MRI-guided biopsy. The lesions proved benign in 28 of 54 cases, high risk in 8, and malignant in 18.
Thus, the positive predictive value of breast MRI screening in this average-risk, extensively prescreened population was 33% if only BIRADS 4 and 5 cancers were defined as true positives, and 48% if BIRADS 4 and 5 high-risk lesions were also counted.
The age distribution of the patients with MRI-detected breast cancers was similar to that of the total study population. Mammographic breast density did not predict the likelihood of identifying breast cancer by MRI.
Dr. Laura J. Esserman rose from the audience to question the cost-benefit ratio of introducing MRI screening for the vast population of average-risk women.
The aggregate cost of breast cancer screening in the United States is already at least $8 billion annually. Utilizing MRI to screen average-risk women would push that figure up by an order of magnitude, observed Dr. Esserman, professor of surgery and of radiology and director of the Carol Franc Buck Breast Care Center at the University of California, San Francisco.
"MRI screening is still very expensive, it’s true," Dr. Schrading replied. "The main reason for the high cost of MRI is the long acquisition time and the long reading time. The long acquisition time is because the MRI protocols we have today are designed for diagnostic purposes, not for the purpose of screening. Our goal is to use a screening protocol for MRI, where the acquisition time is only 3 minutes and the reading time is less than 30 seconds. This might be a way to reduce the cost of MRI," she said.
The study was conducted free of commercial support. Dr. Schrading declared having no financial conflicts of interest.
SAN ANTONIO – MRI screening of women who were at average risk of breast cancer and had a negative screening mammogram resulted in the diagnosis of 11 cases of cancer per 1,000 women screened, Dr. Simone Schrading said at the San Antonio Breast Cancer Symposium.
"The additional cancer detection rate is high, even in heavily prescreened women. In experienced hands, the positive predictive value of MRI screening in this average-risk cohort was comparable to that seen in mammographic screening programs or in MRI high-risk screening cohorts," said Dr. Schrading, of the University of Aachen, Germany.
While MRI is well established as a screening method for women at high familial risk of breast cancer, there have been no data to support its use in average-risk women.
Dr. Schrading reported on a prospective, single-center study evaluating the additional cancer yield and accuracy of breast MRI screening in 1,725 women at average risk for breast cancer. All had normal clinical breast examinations and digital screening mammograms. In addition, 89% of the women had undergone high-frequency breast ultrasound screening, which was normal in all cases. None of the subjects had a personal or family history of breast or ovarian cancer, and none had been diagnosed with breast proliferative changes or atypia. Their mean age was 55 years (range, 42-71 years).
MRI screening detected breast cancers in 18 of these patients, for a detection rate of 11 per 1,000 screened. Seven malignancies were ductal carcinoma in situ, and the other 11 were invasive breast cancer. The mean size of the invasive cancers was 11 mm. Five of the 7 ductal carcinomas in situ and 6 of 11 invasive cancers were high-grade cancers. On the other hand, the stage distribution of the invasive cancers was favorable: All were staged as pN0, M0.
Nearly 91% of screening MRIs were negative as defined by a BIRADS 1 or 2 rating. Another 5.9% were rated BIRADS 3; follow-up MRIs in this group of patients showed no breast cancers. Suspicious lesions – that is, BIRADS 4 or 5 – were noted in 54 patients, or 3.2% of the study group, and they were evaluated by MRI-guided biopsy. The lesions proved benign in 28 of 54 cases, high risk in 8, and malignant in 18.
Thus, the positive predictive value of breast MRI screening in this average-risk, extensively prescreened population was 33% if only BIRADS 4 and 5 cancers were defined as true positives, and 48% if BIRADS 4 and 5 high-risk lesions were also counted.
The age distribution of the patients with MRI-detected breast cancers was similar to that of the total study population. Mammographic breast density did not predict the likelihood of identifying breast cancer by MRI.
Dr. Laura J. Esserman rose from the audience to question the cost-benefit ratio of introducing MRI screening for the vast population of average-risk women.
The aggregate cost of breast cancer screening in the United States is already at least $8 billion annually. Utilizing MRI to screen average-risk women would push that figure up by an order of magnitude, observed Dr. Esserman, professor of surgery and of radiology and director of the Carol Franc Buck Breast Care Center at the University of California, San Francisco.
"MRI screening is still very expensive, it’s true," Dr. Schrading replied. "The main reason for the high cost of MRI is the long acquisition time and the long reading time. The long acquisition time is because the MRI protocols we have today are designed for diagnostic purposes, not for the purpose of screening. Our goal is to use a screening protocol for MRI, where the acquisition time is only 3 minutes and the reading time is less than 30 seconds. This might be a way to reduce the cost of MRI," she said.
The study was conducted free of commercial support. Dr. Schrading declared having no financial conflicts of interest.
AT SABCS 2013
Major finding: Breast MRI screening of women who were at average risk for breast cancer and already had a negative mammogram had a breast cancer detection rate of 11 cases per 1,000 women screened.
Data source: This was a prospective, uncontrolled, single-center study of breast MRI screening in 1,725 women with no personal or family history of breast or ovarian cancer, no diagnosis of proliferative changes, and a mean age of 55 years.
Disclosures: The study was conducted free of commercial support. Dr. Schrading declared having no financial conflicts of interest.
Add-on agents boost neoadjuvant chemo response in triple-negative disease
SAN ANTONIO – When added to standard neoadjuvant chemotherapy, carboplatin and bevacizumab each improve the odds of eradicating local disease in women with triple-negative breast cancer, but their impact on toxicity differs, based on findings from a randomized phase II trial.
Adding carboplatin to standard chemotherapy improved the odds of pathologic complete response (pCR) by 71%-76% with some increase in toxicity such as neutropenia and thrombocytopenia. Adding the antiangiogenic agent bevacizumab improved the odds of pCR by 58%, but with a considerable increase in toxicity such as hypertension and postoperative complications, based on results of the Cancer and Leukemia Group B (CALGB)/Alliance 40603 trial of 443 women with stage II or III triple-negative breast cancer. Dr. William M. Sikov reported the results in a session and at a press briefing at the San Antonio Breast Cancer Symposium.
Dr. Sikov of Warren Alpert Medical School of Brown University, Providence, R.I., acknowledged that the trial was not powered to assess recurrence-free and overall survivals. While it remains unclear whether pCR predicts improved survival, data from other trials and a meta-analysis suggest that pCR is a good surrogate endpoint and have prompted the Food and Drug Administration to consider this endpoint for new drug approval pending long-term data.
Based on the results from this trial and those of the similar GeparSixto trial reported at the 2013 ASCO annual meeting, he said, "if you have decided a patient with triple-negative breast cancer should receive neoadjuvant chemotherapy based on the size of the tumor, based on the desire to improve the chance of breast conservation, then I think it makes sense to add carboplatin to your neoadjuvant regimen, and that you can comfortably do so with acceptable additional toxicities."
Alternatively, Dr. Sikov said that bevacizumab "increased the pCR rate, but at the cost of significant toxicities, and I don’t think that [bevacizumab] should be routinely added to neoadjuvant chemotherapy."
In the CALGB/Alliance 40603 trial, which was funded in part by Genentech, about three-fourths of the women were white, and roughly two-thirds had clinical stage II disease; 55% had clinically positive nodes.
All of the women were treated with weekly paclitaxel followed by dose-dense AC (doxorubicin and cyclophosphamide).
In a 2-by-2 factorial design, patients were randomized to additionally receive carboplatin or not, and to additionally receive bevacizumab (Avastin) or not.
Adding carboplatin increased the rate of pCR in the breast only (60% vs. 46%; odds ratio, 1.76; P = .002) and pCR in both the breast and axilla (54% vs. 41%; odds ratio, 1.71; P = .003).
Similarly, adding bevacizumab increased the rate of pCR in the breast only (59% vs. 48%; odds ratio, 1.58; P = .009) but conferred only a trend toward an increased rate of pCR in both the breast and axilla (52% vs. 44%; odds ratio, 1.36; P = .057).
There was no significant interaction between the treatment effect of carboplatin and the treatment effect of bevacizumab, according to Dr. Sikov, who disclosed no conflicts of interest related to the trial.
In terms of toxicity, adding carboplatin increased rates of grade 3 or worse neutropenia and thrombocytopenia. Adding bevacizumab increased rates of grade 3 or worse hypertension, bleeding, and thrombosis. And adding both drugs increased rates of both sets of adverse events, with a higher rate of grade 3 or worse febrile neutropenia.
Serious adverse events were more common with added bevacizumab and with added carboplatin-bevacizumab. For example, 15% and 16% of patients in these arms, respectively, developed febrile neutropenia during receipt of the AC part of chemotherapy.
Also, addition of bevacizumab was associated with a higher rate of postsurgical complications (9% vs. 5%), most often hematoma or seroma, and a higher rate of delayed surgical complications (4% vs. 1%), such as wound healing issues.
Three randomized neoadjuvant studies – CALGB 40603, GeparSixto, and I-SPY 2 – have now established that inclusion of carboplatin increases pCR rate in triple-negative disease. This provides a valuable new treatment option for patients with high-risk triple-negative disease.
The impact on survival may be modest but real, I believe. Patient-level benefits other than survival also exist that can be derived from a more effective neoadjuvant chemotherapy, such as the potential for lesser surgery and tailored adjuvant therapy.
The variable and inconsistent results from the three randomized neoadjuvant studies [testing bevacizumab], including the CALGB 40603, and the disappointing survival results in both metastatic and adjuvant trials, indicate no current role for bevacizumab in the routine practice in breast cancer. However, this also suggests that a small and elusive subset of patients do benefit from this therapy, but their identification remains a challenge.
Dr. Lajos Pusztai is with the Yale Cancer Center and Smilow Cancer Hospital in New Haven, Conn. He was the discussant of the study at the meeting and had no relevant financial disclosures.
Three randomized neoadjuvant studies – CALGB 40603, GeparSixto, and I-SPY 2 – have now established that inclusion of carboplatin increases pCR rate in triple-negative disease. This provides a valuable new treatment option for patients with high-risk triple-negative disease.
The impact on survival may be modest but real, I believe. Patient-level benefits other than survival also exist that can be derived from a more effective neoadjuvant chemotherapy, such as the potential for lesser surgery and tailored adjuvant therapy.
The variable and inconsistent results from the three randomized neoadjuvant studies [testing bevacizumab], including the CALGB 40603, and the disappointing survival results in both metastatic and adjuvant trials, indicate no current role for bevacizumab in the routine practice in breast cancer. However, this also suggests that a small and elusive subset of patients do benefit from this therapy, but their identification remains a challenge.
Dr. Lajos Pusztai is with the Yale Cancer Center and Smilow Cancer Hospital in New Haven, Conn. He was the discussant of the study at the meeting and had no relevant financial disclosures.
Three randomized neoadjuvant studies – CALGB 40603, GeparSixto, and I-SPY 2 – have now established that inclusion of carboplatin increases pCR rate in triple-negative disease. This provides a valuable new treatment option for patients with high-risk triple-negative disease.
The impact on survival may be modest but real, I believe. Patient-level benefits other than survival also exist that can be derived from a more effective neoadjuvant chemotherapy, such as the potential for lesser surgery and tailored adjuvant therapy.
The variable and inconsistent results from the three randomized neoadjuvant studies [testing bevacizumab], including the CALGB 40603, and the disappointing survival results in both metastatic and adjuvant trials, indicate no current role for bevacizumab in the routine practice in breast cancer. However, this also suggests that a small and elusive subset of patients do benefit from this therapy, but their identification remains a challenge.
Dr. Lajos Pusztai is with the Yale Cancer Center and Smilow Cancer Hospital in New Haven, Conn. He was the discussant of the study at the meeting and had no relevant financial disclosures.
SAN ANTONIO – When added to standard neoadjuvant chemotherapy, carboplatin and bevacizumab each improve the odds of eradicating local disease in women with triple-negative breast cancer, but their impact on toxicity differs, based on findings from a randomized phase II trial.
Adding carboplatin to standard chemotherapy improved the odds of pathologic complete response (pCR) by 71%-76% with some increase in toxicity such as neutropenia and thrombocytopenia. Adding the antiangiogenic agent bevacizumab improved the odds of pCR by 58%, but with a considerable increase in toxicity such as hypertension and postoperative complications, based on results of the Cancer and Leukemia Group B (CALGB)/Alliance 40603 trial of 443 women with stage II or III triple-negative breast cancer. Dr. William M. Sikov reported the results in a session and at a press briefing at the San Antonio Breast Cancer Symposium.
Dr. Sikov of Warren Alpert Medical School of Brown University, Providence, R.I., acknowledged that the trial was not powered to assess recurrence-free and overall survivals. While it remains unclear whether pCR predicts improved survival, data from other trials and a meta-analysis suggest that pCR is a good surrogate endpoint and have prompted the Food and Drug Administration to consider this endpoint for new drug approval pending long-term data.
Based on the results from this trial and those of the similar GeparSixto trial reported at the 2013 ASCO annual meeting, he said, "if you have decided a patient with triple-negative breast cancer should receive neoadjuvant chemotherapy based on the size of the tumor, based on the desire to improve the chance of breast conservation, then I think it makes sense to add carboplatin to your neoadjuvant regimen, and that you can comfortably do so with acceptable additional toxicities."
Alternatively, Dr. Sikov said that bevacizumab "increased the pCR rate, but at the cost of significant toxicities, and I don’t think that [bevacizumab] should be routinely added to neoadjuvant chemotherapy."
In the CALGB/Alliance 40603 trial, which was funded in part by Genentech, about three-fourths of the women were white, and roughly two-thirds had clinical stage II disease; 55% had clinically positive nodes.
All of the women were treated with weekly paclitaxel followed by dose-dense AC (doxorubicin and cyclophosphamide).
In a 2-by-2 factorial design, patients were randomized to additionally receive carboplatin or not, and to additionally receive bevacizumab (Avastin) or not.
Adding carboplatin increased the rate of pCR in the breast only (60% vs. 46%; odds ratio, 1.76; P = .002) and pCR in both the breast and axilla (54% vs. 41%; odds ratio, 1.71; P = .003).
Similarly, adding bevacizumab increased the rate of pCR in the breast only (59% vs. 48%; odds ratio, 1.58; P = .009) but conferred only a trend toward an increased rate of pCR in both the breast and axilla (52% vs. 44%; odds ratio, 1.36; P = .057).
There was no significant interaction between the treatment effect of carboplatin and the treatment effect of bevacizumab, according to Dr. Sikov, who disclosed no conflicts of interest related to the trial.
In terms of toxicity, adding carboplatin increased rates of grade 3 or worse neutropenia and thrombocytopenia. Adding bevacizumab increased rates of grade 3 or worse hypertension, bleeding, and thrombosis. And adding both drugs increased rates of both sets of adverse events, with a higher rate of grade 3 or worse febrile neutropenia.
Serious adverse events were more common with added bevacizumab and with added carboplatin-bevacizumab. For example, 15% and 16% of patients in these arms, respectively, developed febrile neutropenia during receipt of the AC part of chemotherapy.
Also, addition of bevacizumab was associated with a higher rate of postsurgical complications (9% vs. 5%), most often hematoma or seroma, and a higher rate of delayed surgical complications (4% vs. 1%), such as wound healing issues.
SAN ANTONIO – When added to standard neoadjuvant chemotherapy, carboplatin and bevacizumab each improve the odds of eradicating local disease in women with triple-negative breast cancer, but their impact on toxicity differs, based on findings from a randomized phase II trial.
Adding carboplatin to standard chemotherapy improved the odds of pathologic complete response (pCR) by 71%-76% with some increase in toxicity such as neutropenia and thrombocytopenia. Adding the antiangiogenic agent bevacizumab improved the odds of pCR by 58%, but with a considerable increase in toxicity such as hypertension and postoperative complications, based on results of the Cancer and Leukemia Group B (CALGB)/Alliance 40603 trial of 443 women with stage II or III triple-negative breast cancer. Dr. William M. Sikov reported the results in a session and at a press briefing at the San Antonio Breast Cancer Symposium.
Dr. Sikov of Warren Alpert Medical School of Brown University, Providence, R.I., acknowledged that the trial was not powered to assess recurrence-free and overall survivals. While it remains unclear whether pCR predicts improved survival, data from other trials and a meta-analysis suggest that pCR is a good surrogate endpoint and have prompted the Food and Drug Administration to consider this endpoint for new drug approval pending long-term data.
Based on the results from this trial and those of the similar GeparSixto trial reported at the 2013 ASCO annual meeting, he said, "if you have decided a patient with triple-negative breast cancer should receive neoadjuvant chemotherapy based on the size of the tumor, based on the desire to improve the chance of breast conservation, then I think it makes sense to add carboplatin to your neoadjuvant regimen, and that you can comfortably do so with acceptable additional toxicities."
Alternatively, Dr. Sikov said that bevacizumab "increased the pCR rate, but at the cost of significant toxicities, and I don’t think that [bevacizumab] should be routinely added to neoadjuvant chemotherapy."
In the CALGB/Alliance 40603 trial, which was funded in part by Genentech, about three-fourths of the women were white, and roughly two-thirds had clinical stage II disease; 55% had clinically positive nodes.
All of the women were treated with weekly paclitaxel followed by dose-dense AC (doxorubicin and cyclophosphamide).
In a 2-by-2 factorial design, patients were randomized to additionally receive carboplatin or not, and to additionally receive bevacizumab (Avastin) or not.
Adding carboplatin increased the rate of pCR in the breast only (60% vs. 46%; odds ratio, 1.76; P = .002) and pCR in both the breast and axilla (54% vs. 41%; odds ratio, 1.71; P = .003).
Similarly, adding bevacizumab increased the rate of pCR in the breast only (59% vs. 48%; odds ratio, 1.58; P = .009) but conferred only a trend toward an increased rate of pCR in both the breast and axilla (52% vs. 44%; odds ratio, 1.36; P = .057).
There was no significant interaction between the treatment effect of carboplatin and the treatment effect of bevacizumab, according to Dr. Sikov, who disclosed no conflicts of interest related to the trial.
In terms of toxicity, adding carboplatin increased rates of grade 3 or worse neutropenia and thrombocytopenia. Adding bevacizumab increased rates of grade 3 or worse hypertension, bleeding, and thrombosis. And adding both drugs increased rates of both sets of adverse events, with a higher rate of grade 3 or worse febrile neutropenia.
Serious adverse events were more common with added bevacizumab and with added carboplatin-bevacizumab. For example, 15% and 16% of patients in these arms, respectively, developed febrile neutropenia during receipt of the AC part of chemotherapy.
Also, addition of bevacizumab was associated with a higher rate of postsurgical complications (9% vs. 5%), most often hematoma or seroma, and a higher rate of delayed surgical complications (4% vs. 1%), such as wound healing issues.
AT SABCS 2013
Major finding: Carboplatin improved the odds of pCR by 71%-76% with some additional toxicity; bevacizumab improved the odds of pCR by 58% with considerable additional toxicity.
Data Source: A randomized phase II trial testing addition of carboplatin and/or bevacizumab to neoadjuvant chemotherapy in 443 women with stage II or III triple-negative breast cancer (the CALGB/Alliance 40603 trial).
Disclosures: Dr. Sikov disclosed no conflicts of interest. The trial was funded in part by Genentech.