User login
Cancer Therapy & Research Center (CTRC)/ American Association for Cancer Research (AACR): San Antonio Breast Cancer Symposium (SABCS)
Veliparib-carboplatin combo is first 'graduate' of I-SPY2 trial
SAN ANTONIO – The combination of veliparib and carboplatin is the first "graduate" of the I-SPY 2 trial, investigators reported at the San Antonio Breast Cancer Symposium.
The phase 2 trial uses an adaptive design to screen novel agents and regimens by assessing whether they improve response when added to neoadjuvant chemotherapy, with the aim of matching them to breast cancer biomarker signatures most likely to respond.
The novel agents or regimens graduate, or meet the bar for further testing, if there is a 85% probability or better that they will be superior to chemotherapy alone for at least one signature in a modestly sized phase 3 neoadjuvant trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
First results from 115 patients, reported in a session and press briefing by lead author Dr. Hope S. Rugo, showed that based on the estimated pathologic complete responses (pCRs) seen, there was a 90% probability that the combination of veliparib—an oral investigational inhibitor of poly-ADP-ribose, or PARP—and carboplatin added to chemotherapy would be superior to chemotherapy alone among patients with triple-negative disease.
In contrast, the probability in all patients with HER2-negative disease was only slightly better than a coin toss. And the probability in the subset with hormone receptor–positive, HER2-negative disease was less than one in 10.
"Veliparib and carboplatin has graduated with the triple-negative signature, the subset recommended for this drug’s subsequent development," said Dr. Rugo, who is a professor of medicine and director of breast oncology and clinical trials education at the UCSF Helen Diller Family Comprehensive Cancer Center in San Francisco.
"We identified a biomarker signature–drug pair for veliparib and carboplatin on the basis of a modest number of patients...This trial design will accelerate the process of identifying drugs that are effective for specific breast cancer subtypes and thereby reduce the cost, time, and numbers of patients needed to get effective drugs to market," she noted.
The trial was not designed to assess the contribution of each agent—veliparib (ABT-888, manufactured by AbbVie) and carboplatin—individually, Dr. Rugo acknowledged; regardless, their combination had a large effect size.
"I-SPY 2 is a biomarker-rich trial. Additional response predictors are under evaluation," such as BRCA status, she further noted.
"A lot of work has been done with the FDA [Food and Drug Administration] recently evaluating drugs for accelerated approval based on their response in the neoadjuvant setting. How this plays out in the future for other drugs [besides pertuzumab] remains to be seen," Dr. Rugo commented. "Clearly there needs to be a plan for outcome data that can correspond to the pCR rates in order for these drugs to obtain final approval. But there is the hope that this will be a mechanism for accelerated approval."
The trial has six other arms testing investigational regimens. Results for the arm that is testing neratinib (PB272, Puma Biotechnology), a tyrosine kinase inhibitor, are expected shortly.
Women are eligible for I-SPY 2 (Investigation of Serial Studies to Predict Your Therapeutic Response With Imaging And moLecular Analysis 2) if they have tumors measuring at least 2.5 cm. Those whose tumors have all of three favorable characteristics (hormone receptor positive, HER2 negative, and Low MammaPrint result) are excluded.
Eight biomarker signatures are created according to hormone receptor status, HER2 status, and MammaPrint result (High or Ultra High).
The trial uses adaptive randomization, whereby each patient’s MRI and pCR data are fed into an algorithm, affecting how the next patient is randomized. "This allows us to learn and adapt from each patient as we go along, and each new patient benefits from information obtained from the prior patient. In addition, this allows us to add and drop agents as we go along based on success of each agent or regimen," explained Dr. Rugo, who disclosed no conflicts of interest related to the trial.
Patients are randomized to receive weekly paclitaxel plus a novel agent/regimen or weekly paclitaxel alone—each followed by standard doxorubicin and cyclophosphamide (AC) chemotherapy and then surgery. The endpoint of pCR is defined as no residual cancer in the breast or lymph nodes at the time of surgery.
The results are used to predict the probability of superiority in a phase 3 neoadjuvant trial having just 300 patients and using pCR as its endpoint.
The findings that Dr. Rugo presented were based on 115 patients with HER2-negative disease: 71 randomized to veliparib-carboplatin plus chemotherapy and 44 randomized to chemotherapy alone.
Among patients with triple-negative disease, the estimated rate of pCR was 52% with veliparib-carboplatin and 26% without it. There was a 90% predictive probability that the combination with chemotherapy would be superior to chemotherapy alone in phase 3 testing in this subset.
In contrast, the difference in estimated pCR rates with addition of veliparib-carboplatin was much smaller among all patients with HER2-negative disease (33% vs. 22%) and in the opposite direction among the subset with hormone receptor–positive, HER2-negative disease (14% vs. 19%). The predictive probabilities that the combination with chemotherapy would be superior in phase 3 testing in these groups were just 55% and 9%, respectively.
"The toxicity was moderately increased as expected, but it was well managed with dose reduction and delay," Dr. Rugo reported.
Dr. Rugo disclosed no relevant conflicts of interest.
I would like to congratulate the first graduate from the I-SPY 2 trial.
The efficacy results with neoadjuvant carboplatin in the Cancer and Leukemia Group B (CALGB) 40603 trial, also presented at the symposium, are consistent with the I-SPY 2 prediction, with carboplatin really increasing pCR rates in triple-negative disease although direct cross-trial comparison is limited.
The rate of pCR was very similar, about 50%, in both trials with carboplatin-containing chemotherapy even though the former trial did not use veliparib. This suggests the contribution of veliparib is modest if any in this particular patient population.
What further complicates the interpretation is that the activity of veliparib is likely influenced by germline BRCA status and may also be dependent on the extent of somatic homologous DNA recombination defects in the tumor.
Two ongoing randomized trials will be especially important to clarifying the role of neoadjuvant veliparib in triple-negative breast cancer among BRCA carriers. Until these results are available, the value of veliparib to increase pCR rate in triple-negative disease remains uncertain.
Dr. Lajos Pusztai is with the Yale Cancer Center and Smilow Cancer Hospital in New Haven, Connecticut. He was the official discussant to the paper at the meeting.
I would like to congratulate the first graduate from the I-SPY 2 trial.
The efficacy results with neoadjuvant carboplatin in the Cancer and Leukemia Group B (CALGB) 40603 trial, also presented at the symposium, are consistent with the I-SPY 2 prediction, with carboplatin really increasing pCR rates in triple-negative disease although direct cross-trial comparison is limited.
The rate of pCR was very similar, about 50%, in both trials with carboplatin-containing chemotherapy even though the former trial did not use veliparib. This suggests the contribution of veliparib is modest if any in this particular patient population.
What further complicates the interpretation is that the activity of veliparib is likely influenced by germline BRCA status and may also be dependent on the extent of somatic homologous DNA recombination defects in the tumor.
Two ongoing randomized trials will be especially important to clarifying the role of neoadjuvant veliparib in triple-negative breast cancer among BRCA carriers. Until these results are available, the value of veliparib to increase pCR rate in triple-negative disease remains uncertain.
Dr. Lajos Pusztai is with the Yale Cancer Center and Smilow Cancer Hospital in New Haven, Connecticut. He was the official discussant to the paper at the meeting.
I would like to congratulate the first graduate from the I-SPY 2 trial.
The efficacy results with neoadjuvant carboplatin in the Cancer and Leukemia Group B (CALGB) 40603 trial, also presented at the symposium, are consistent with the I-SPY 2 prediction, with carboplatin really increasing pCR rates in triple-negative disease although direct cross-trial comparison is limited.
The rate of pCR was very similar, about 50%, in both trials with carboplatin-containing chemotherapy even though the former trial did not use veliparib. This suggests the contribution of veliparib is modest if any in this particular patient population.
What further complicates the interpretation is that the activity of veliparib is likely influenced by germline BRCA status and may also be dependent on the extent of somatic homologous DNA recombination defects in the tumor.
Two ongoing randomized trials will be especially important to clarifying the role of neoadjuvant veliparib in triple-negative breast cancer among BRCA carriers. Until these results are available, the value of veliparib to increase pCR rate in triple-negative disease remains uncertain.
Dr. Lajos Pusztai is with the Yale Cancer Center and Smilow Cancer Hospital in New Haven, Connecticut. He was the official discussant to the paper at the meeting.
SAN ANTONIO – The combination of veliparib and carboplatin is the first "graduate" of the I-SPY 2 trial, investigators reported at the San Antonio Breast Cancer Symposium.
The phase 2 trial uses an adaptive design to screen novel agents and regimens by assessing whether they improve response when added to neoadjuvant chemotherapy, with the aim of matching them to breast cancer biomarker signatures most likely to respond.
The novel agents or regimens graduate, or meet the bar for further testing, if there is a 85% probability or better that they will be superior to chemotherapy alone for at least one signature in a modestly sized phase 3 neoadjuvant trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
First results from 115 patients, reported in a session and press briefing by lead author Dr. Hope S. Rugo, showed that based on the estimated pathologic complete responses (pCRs) seen, there was a 90% probability that the combination of veliparib—an oral investigational inhibitor of poly-ADP-ribose, or PARP—and carboplatin added to chemotherapy would be superior to chemotherapy alone among patients with triple-negative disease.
In contrast, the probability in all patients with HER2-negative disease was only slightly better than a coin toss. And the probability in the subset with hormone receptor–positive, HER2-negative disease was less than one in 10.
"Veliparib and carboplatin has graduated with the triple-negative signature, the subset recommended for this drug’s subsequent development," said Dr. Rugo, who is a professor of medicine and director of breast oncology and clinical trials education at the UCSF Helen Diller Family Comprehensive Cancer Center in San Francisco.
"We identified a biomarker signature–drug pair for veliparib and carboplatin on the basis of a modest number of patients...This trial design will accelerate the process of identifying drugs that are effective for specific breast cancer subtypes and thereby reduce the cost, time, and numbers of patients needed to get effective drugs to market," she noted.
The trial was not designed to assess the contribution of each agent—veliparib (ABT-888, manufactured by AbbVie) and carboplatin—individually, Dr. Rugo acknowledged; regardless, their combination had a large effect size.
"I-SPY 2 is a biomarker-rich trial. Additional response predictors are under evaluation," such as BRCA status, she further noted.
"A lot of work has been done with the FDA [Food and Drug Administration] recently evaluating drugs for accelerated approval based on their response in the neoadjuvant setting. How this plays out in the future for other drugs [besides pertuzumab] remains to be seen," Dr. Rugo commented. "Clearly there needs to be a plan for outcome data that can correspond to the pCR rates in order for these drugs to obtain final approval. But there is the hope that this will be a mechanism for accelerated approval."
The trial has six other arms testing investigational regimens. Results for the arm that is testing neratinib (PB272, Puma Biotechnology), a tyrosine kinase inhibitor, are expected shortly.
Women are eligible for I-SPY 2 (Investigation of Serial Studies to Predict Your Therapeutic Response With Imaging And moLecular Analysis 2) if they have tumors measuring at least 2.5 cm. Those whose tumors have all of three favorable characteristics (hormone receptor positive, HER2 negative, and Low MammaPrint result) are excluded.
Eight biomarker signatures are created according to hormone receptor status, HER2 status, and MammaPrint result (High or Ultra High).
The trial uses adaptive randomization, whereby each patient’s MRI and pCR data are fed into an algorithm, affecting how the next patient is randomized. "This allows us to learn and adapt from each patient as we go along, and each new patient benefits from information obtained from the prior patient. In addition, this allows us to add and drop agents as we go along based on success of each agent or regimen," explained Dr. Rugo, who disclosed no conflicts of interest related to the trial.
Patients are randomized to receive weekly paclitaxel plus a novel agent/regimen or weekly paclitaxel alone—each followed by standard doxorubicin and cyclophosphamide (AC) chemotherapy and then surgery. The endpoint of pCR is defined as no residual cancer in the breast or lymph nodes at the time of surgery.
The results are used to predict the probability of superiority in a phase 3 neoadjuvant trial having just 300 patients and using pCR as its endpoint.
The findings that Dr. Rugo presented were based on 115 patients with HER2-negative disease: 71 randomized to veliparib-carboplatin plus chemotherapy and 44 randomized to chemotherapy alone.
Among patients with triple-negative disease, the estimated rate of pCR was 52% with veliparib-carboplatin and 26% without it. There was a 90% predictive probability that the combination with chemotherapy would be superior to chemotherapy alone in phase 3 testing in this subset.
In contrast, the difference in estimated pCR rates with addition of veliparib-carboplatin was much smaller among all patients with HER2-negative disease (33% vs. 22%) and in the opposite direction among the subset with hormone receptor–positive, HER2-negative disease (14% vs. 19%). The predictive probabilities that the combination with chemotherapy would be superior in phase 3 testing in these groups were just 55% and 9%, respectively.
"The toxicity was moderately increased as expected, but it was well managed with dose reduction and delay," Dr. Rugo reported.
Dr. Rugo disclosed no relevant conflicts of interest.
SAN ANTONIO – The combination of veliparib and carboplatin is the first "graduate" of the I-SPY 2 trial, investigators reported at the San Antonio Breast Cancer Symposium.
The phase 2 trial uses an adaptive design to screen novel agents and regimens by assessing whether they improve response when added to neoadjuvant chemotherapy, with the aim of matching them to breast cancer biomarker signatures most likely to respond.
The novel agents or regimens graduate, or meet the bar for further testing, if there is a 85% probability or better that they will be superior to chemotherapy alone for at least one signature in a modestly sized phase 3 neoadjuvant trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
First results from 115 patients, reported in a session and press briefing by lead author Dr. Hope S. Rugo, showed that based on the estimated pathologic complete responses (pCRs) seen, there was a 90% probability that the combination of veliparib—an oral investigational inhibitor of poly-ADP-ribose, or PARP—and carboplatin added to chemotherapy would be superior to chemotherapy alone among patients with triple-negative disease.
In contrast, the probability in all patients with HER2-negative disease was only slightly better than a coin toss. And the probability in the subset with hormone receptor–positive, HER2-negative disease was less than one in 10.
"Veliparib and carboplatin has graduated with the triple-negative signature, the subset recommended for this drug’s subsequent development," said Dr. Rugo, who is a professor of medicine and director of breast oncology and clinical trials education at the UCSF Helen Diller Family Comprehensive Cancer Center in San Francisco.
"We identified a biomarker signature–drug pair for veliparib and carboplatin on the basis of a modest number of patients...This trial design will accelerate the process of identifying drugs that are effective for specific breast cancer subtypes and thereby reduce the cost, time, and numbers of patients needed to get effective drugs to market," she noted.
The trial was not designed to assess the contribution of each agent—veliparib (ABT-888, manufactured by AbbVie) and carboplatin—individually, Dr. Rugo acknowledged; regardless, their combination had a large effect size.
"I-SPY 2 is a biomarker-rich trial. Additional response predictors are under evaluation," such as BRCA status, she further noted.
"A lot of work has been done with the FDA [Food and Drug Administration] recently evaluating drugs for accelerated approval based on their response in the neoadjuvant setting. How this plays out in the future for other drugs [besides pertuzumab] remains to be seen," Dr. Rugo commented. "Clearly there needs to be a plan for outcome data that can correspond to the pCR rates in order for these drugs to obtain final approval. But there is the hope that this will be a mechanism for accelerated approval."
The trial has six other arms testing investigational regimens. Results for the arm that is testing neratinib (PB272, Puma Biotechnology), a tyrosine kinase inhibitor, are expected shortly.
Women are eligible for I-SPY 2 (Investigation of Serial Studies to Predict Your Therapeutic Response With Imaging And moLecular Analysis 2) if they have tumors measuring at least 2.5 cm. Those whose tumors have all of three favorable characteristics (hormone receptor positive, HER2 negative, and Low MammaPrint result) are excluded.
Eight biomarker signatures are created according to hormone receptor status, HER2 status, and MammaPrint result (High or Ultra High).
The trial uses adaptive randomization, whereby each patient’s MRI and pCR data are fed into an algorithm, affecting how the next patient is randomized. "This allows us to learn and adapt from each patient as we go along, and each new patient benefits from information obtained from the prior patient. In addition, this allows us to add and drop agents as we go along based on success of each agent or regimen," explained Dr. Rugo, who disclosed no conflicts of interest related to the trial.
Patients are randomized to receive weekly paclitaxel plus a novel agent/regimen or weekly paclitaxel alone—each followed by standard doxorubicin and cyclophosphamide (AC) chemotherapy and then surgery. The endpoint of pCR is defined as no residual cancer in the breast or lymph nodes at the time of surgery.
The results are used to predict the probability of superiority in a phase 3 neoadjuvant trial having just 300 patients and using pCR as its endpoint.
The findings that Dr. Rugo presented were based on 115 patients with HER2-negative disease: 71 randomized to veliparib-carboplatin plus chemotherapy and 44 randomized to chemotherapy alone.
Among patients with triple-negative disease, the estimated rate of pCR was 52% with veliparib-carboplatin and 26% without it. There was a 90% predictive probability that the combination with chemotherapy would be superior to chemotherapy alone in phase 3 testing in this subset.
In contrast, the difference in estimated pCR rates with addition of veliparib-carboplatin was much smaller among all patients with HER2-negative disease (33% vs. 22%) and in the opposite direction among the subset with hormone receptor–positive, HER2-negative disease (14% vs. 19%). The predictive probabilities that the combination with chemotherapy would be superior in phase 3 testing in these groups were just 55% and 9%, respectively.
"The toxicity was moderately increased as expected, but it was well managed with dose reduction and delay," Dr. Rugo reported.
Dr. Rugo disclosed no relevant conflicts of interest.
AT SABCS 2013
Major finding: Among patients with triple-negative disease, there was a 90% predictive probability that veliparib-carboplatin added to chemotherapy would be superior to chemotherapy alone in a phase 3 neoadjuvant trial.
Data source: An analysis of 115 women with HER2-negative disease enrolled in an adaptive randomized phase 2 trial (I-SPY 2 trial).
Disclosures: Dr. Rugo disclosed no relevant conflicts of interest.
New Mega-review Underscores Mammography’s Benefits
SAN ANTONIO – The much-publicized wide disparities in the estimated value of mammographic screening for breast cancer reported in recent major reviews are overblown and largely an artifact of methodologic differences, according to a new examination of the evidence.
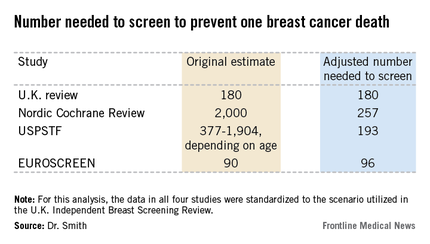
The four recent major reviews of the data regarding the absolute benefits of mammography came up with estimates ranging from 90-2,000 of the number of women who need to be screened (NNS) in order to prevent one death from breast cancer. That greater than 20-fold difference in estimated magnitude of benefit has done little to inspire public and physician confidence that mammography is a key tool in reducing cancer deaths.
But the two analyses with the least supportive outcomes – the Nordic Cochrane and U.S. Preventive Services Task Force (USPSTF-) analyses – used follow-up periods of 10 and 15 years, respectively. That follow-up is too short a time to assess the full value of mammographic screening, Robert A. Smith, Ph.D., asserted at the San Antonio Breast Cancer Symposium.
To illustrate: In a European mammographic screening study with a 30-year follow-up, the NNS after 10 years was 922 women. By 29 years of follow-up, the NNS had fallen to 414.
"At 10 years of follow-up, you haven’t even observed half of the deaths prevented. So follow-up of 20 years at a minimum is really critical to begin to see the full benefit of screening," according to Dr. Smith, senior director of cancer screening at the American Cancer Society in Atlanta.
Also, several of the major reviews estimated the absolute mortality benefit of screening by means of an intent-to-treat analysis based upon the number of women invited to screening in randomized trials. That approach, too, is highly problematic because commonly 30%-40% of women invited to breast cancer screening in randomized trials never actually present for mammography, he said.
"The difference between the number-needed-to-invite and number-needed-to-screen is quite a critical difference in these estimates of absolute benefit. If you want to measure the effectiveness, you have to appreciate that a letter of invitation doesn’t do anyone any good. You have to show up to get mammography in order to benefit from it," Dr. Smith observed.
All of the four recent major reviews – the Nordic Cochrane (Cochrane Database Syst. Rev. 2013;6:CD001877), the USPSTF (Ann. Intern. Med. 2009;151:727-37), the U.K. Independent Breast Screening Review (Br. J. Cancer 2013;108:2205-40), and the European Screening Network (EUROSCREEN) Review (J. Med. Screen. 2012;19 Suppl1:14-25) – painted different pictures of the benefits of mammographic screening because they focused on different age groups, with different screening and follow-up durations, and were inconsistent as to whether the appropriate yardstick was NNS or number-needed-to-invite.
Dr. Smith and his coinvestigators sought to level the playing field by reanalyzing each review, standardizing the data to the scenario utilized in the U.K. independent review. They picked the U.K. review as the reference because it was most recently published and it was led by renowned statistical experts who aren’t part of the debate over mammography’s value. The U.K. review scenario entailed screening every 3 years for 20 years starting at age 50 years, with a 20-year follow-up period and the endpoint being breast cancer mortality at ages 55-79 years. When the data were reanalyzed in this way, the magnitude of the difference between the high and low estimates of absolute benefit among the four major reviews dropped from more than 20-fold to less than 3-fold.
"The so-called controversy over the benefit of mammography screening as estimated from the trials is largely contrived," he declared. "In short, once you standardize the evidence to the same population, the same screening scenario, and the same duration of follow-up, then the differences in absolute benefit over 20 years in the reviews become really not so significant or important at all. They are hardly worth discussing, and are certainly not enough to question the value of mammography over a lifetime of screening."
The flip side of estimating the benefit of mammographic screening in terms of breast cancer deaths avoided is the harm from overdiagnosis of cancers that never would have been symptomatic during a woman’s lifetime and wouldn’t have been detected had screening mammography not been performed. Here again, the estimates reported in the four reviews differed widely because of the divergent analytic methods employed. The U.K. review concluded that for every death from breast cancer avoided via mammography, three people would be overdiagnosed, for an overdiagnosis rate of 19%. The Nordic Cochrane analysis estimated 10 cases of overdiagnosis for every breast cancer death avoided, for a 30% overdiagnosis rate. The USPSTF didn’t give an overdiagnosis estimate. The EUROSCREEN group calculated that for every two breast cancer deaths avoided there would be one case of overdiagnosis, for a 6.5% overdiagnosis rate.
Dr. Smith said the EUROSCREEN estimate of overdiagnosis is the one that rings true. The EUROSCREEN investigators have demonstrated that in estimating mammography overdiagnosis rates, it’s essential to adjust for trends over time in breast cancer incidence and for lead time bias. When that’s not done, estimated overdiagnosis rates run in the 30%-50% range. When adjustments are made, the overdiagnosis rates are in the 0%-10% range, with the EUROSCREEN estimate of 6.5% being representative (J. Med. Screen. 2012;19 Suppl 1:42-56).
The full details of the mega-review were recently published (Breast Cancer Management 2013;2:519-28 [doi:10.2217/bmt.13.53]). The mega-review was funded by the Center for Cancer Prevention, the Wolfson Institute of Preventive Medicine, Barts and the London School of Medicine and Dentistry, and Queen Mary University of London. Dr. Smith declared having no financial conflicts of interest.
I’m not a statistician, but the analysis presented by Dr. Smith makes common sense to me.
Basing benefit solely on those women invited to screen will clearly result in an underestimate of mammography’s true benefit if you’re going to count women who weren’t screened as being in the screened group. More and more, we are learning that breast cancer is a more indolent disease than we thought. Particularly in those with estrogen receptor–positive breast cancer, the most common subtype, many patients don’t recur and die for a decade or more. If you’re not following patients long enough to capture all of the recurrences and deaths from the disease, then you don’t get a full assessment of the value of screening earlier in the course of disease. Long-term follow-up is extremely important.
You’d think that point would be universally accepted in the world of breast cancer screening research, but it isn’t. Mammography is controversial. It takes on some of the same features as debates over global warming. Quite often we see that scientists are just as vulnerable to motivated reasoning as anybody else.
Dr. C. Kent Osborne is the SABCS codirector and director of the Dan L. Duncan Cancer Center and the Lester & Sue Smith Breast Center at Baylor College of Medicine, Houston. He made his comments during the post presentation discussion of the research.
I’m not a statistician, but the analysis presented by Dr. Smith makes common sense to me.
Basing benefit solely on those women invited to screen will clearly result in an underestimate of mammography’s true benefit if you’re going to count women who weren’t screened as being in the screened group. More and more, we are learning that breast cancer is a more indolent disease than we thought. Particularly in those with estrogen receptor–positive breast cancer, the most common subtype, many patients don’t recur and die for a decade or more. If you’re not following patients long enough to capture all of the recurrences and deaths from the disease, then you don’t get a full assessment of the value of screening earlier in the course of disease. Long-term follow-up is extremely important.
You’d think that point would be universally accepted in the world of breast cancer screening research, but it isn’t. Mammography is controversial. It takes on some of the same features as debates over global warming. Quite often we see that scientists are just as vulnerable to motivated reasoning as anybody else.
Dr. C. Kent Osborne is the SABCS codirector and director of the Dan L. Duncan Cancer Center and the Lester & Sue Smith Breast Center at Baylor College of Medicine, Houston. He made his comments during the post presentation discussion of the research.
I’m not a statistician, but the analysis presented by Dr. Smith makes common sense to me.
Basing benefit solely on those women invited to screen will clearly result in an underestimate of mammography’s true benefit if you’re going to count women who weren’t screened as being in the screened group. More and more, we are learning that breast cancer is a more indolent disease than we thought. Particularly in those with estrogen receptor–positive breast cancer, the most common subtype, many patients don’t recur and die for a decade or more. If you’re not following patients long enough to capture all of the recurrences and deaths from the disease, then you don’t get a full assessment of the value of screening earlier in the course of disease. Long-term follow-up is extremely important.
You’d think that point would be universally accepted in the world of breast cancer screening research, but it isn’t. Mammography is controversial. It takes on some of the same features as debates over global warming. Quite often we see that scientists are just as vulnerable to motivated reasoning as anybody else.
Dr. C. Kent Osborne is the SABCS codirector and director of the Dan L. Duncan Cancer Center and the Lester & Sue Smith Breast Center at Baylor College of Medicine, Houston. He made his comments during the post presentation discussion of the research.
SAN ANTONIO – The much-publicized wide disparities in the estimated value of mammographic screening for breast cancer reported in recent major reviews are overblown and largely an artifact of methodologic differences, according to a new examination of the evidence.
The four recent major reviews of the data regarding the absolute benefits of mammography came up with estimates ranging from 90-2,000 of the number of women who need to be screened (NNS) in order to prevent one death from breast cancer. That greater than 20-fold difference in estimated magnitude of benefit has done little to inspire public and physician confidence that mammography is a key tool in reducing cancer deaths.

But the two analyses with the least supportive outcomes – the Nordic Cochrane and U.S. Preventive Services Task Force (USPSTF-) analyses – used follow-up periods of 10 and 15 years, respectively. That follow-up is too short a time to assess the full value of mammographic screening, Robert A. Smith, Ph.D., asserted at the San Antonio Breast Cancer Symposium.
To illustrate: In a European mammographic screening study with a 30-year follow-up, the NNS after 10 years was 922 women. By 29 years of follow-up, the NNS had fallen to 414.
"At 10 years of follow-up, you haven’t even observed half of the deaths prevented. So follow-up of 20 years at a minimum is really critical to begin to see the full benefit of screening," according to Dr. Smith, senior director of cancer screening at the American Cancer Society in Atlanta.
Also, several of the major reviews estimated the absolute mortality benefit of screening by means of an intent-to-treat analysis based upon the number of women invited to screening in randomized trials. That approach, too, is highly problematic because commonly 30%-40% of women invited to breast cancer screening in randomized trials never actually present for mammography, he said.
"The difference between the number-needed-to-invite and number-needed-to-screen is quite a critical difference in these estimates of absolute benefit. If you want to measure the effectiveness, you have to appreciate that a letter of invitation doesn’t do anyone any good. You have to show up to get mammography in order to benefit from it," Dr. Smith observed.
All of the four recent major reviews – the Nordic Cochrane (Cochrane Database Syst. Rev. 2013;6:CD001877), the USPSTF (Ann. Intern. Med. 2009;151:727-37), the U.K. Independent Breast Screening Review (Br. J. Cancer 2013;108:2205-40), and the European Screening Network (EUROSCREEN) Review (J. Med. Screen. 2012;19 Suppl1:14-25) – painted different pictures of the benefits of mammographic screening because they focused on different age groups, with different screening and follow-up durations, and were inconsistent as to whether the appropriate yardstick was NNS or number-needed-to-invite.
Dr. Smith and his coinvestigators sought to level the playing field by reanalyzing each review, standardizing the data to the scenario utilized in the U.K. independent review. They picked the U.K. review as the reference because it was most recently published and it was led by renowned statistical experts who aren’t part of the debate over mammography’s value. The U.K. review scenario entailed screening every 3 years for 20 years starting at age 50 years, with a 20-year follow-up period and the endpoint being breast cancer mortality at ages 55-79 years. When the data were reanalyzed in this way, the magnitude of the difference between the high and low estimates of absolute benefit among the four major reviews dropped from more than 20-fold to less than 3-fold.
"The so-called controversy over the benefit of mammography screening as estimated from the trials is largely contrived," he declared. "In short, once you standardize the evidence to the same population, the same screening scenario, and the same duration of follow-up, then the differences in absolute benefit over 20 years in the reviews become really not so significant or important at all. They are hardly worth discussing, and are certainly not enough to question the value of mammography over a lifetime of screening."
The flip side of estimating the benefit of mammographic screening in terms of breast cancer deaths avoided is the harm from overdiagnosis of cancers that never would have been symptomatic during a woman’s lifetime and wouldn’t have been detected had screening mammography not been performed. Here again, the estimates reported in the four reviews differed widely because of the divergent analytic methods employed. The U.K. review concluded that for every death from breast cancer avoided via mammography, three people would be overdiagnosed, for an overdiagnosis rate of 19%. The Nordic Cochrane analysis estimated 10 cases of overdiagnosis for every breast cancer death avoided, for a 30% overdiagnosis rate. The USPSTF didn’t give an overdiagnosis estimate. The EUROSCREEN group calculated that for every two breast cancer deaths avoided there would be one case of overdiagnosis, for a 6.5% overdiagnosis rate.
Dr. Smith said the EUROSCREEN estimate of overdiagnosis is the one that rings true. The EUROSCREEN investigators have demonstrated that in estimating mammography overdiagnosis rates, it’s essential to adjust for trends over time in breast cancer incidence and for lead time bias. When that’s not done, estimated overdiagnosis rates run in the 30%-50% range. When adjustments are made, the overdiagnosis rates are in the 0%-10% range, with the EUROSCREEN estimate of 6.5% being representative (J. Med. Screen. 2012;19 Suppl 1:42-56).
The full details of the mega-review were recently published (Breast Cancer Management 2013;2:519-28 [doi:10.2217/bmt.13.53]). The mega-review was funded by the Center for Cancer Prevention, the Wolfson Institute of Preventive Medicine, Barts and the London School of Medicine and Dentistry, and Queen Mary University of London. Dr. Smith declared having no financial conflicts of interest.
SAN ANTONIO – The much-publicized wide disparities in the estimated value of mammographic screening for breast cancer reported in recent major reviews are overblown and largely an artifact of methodologic differences, according to a new examination of the evidence.
The four recent major reviews of the data regarding the absolute benefits of mammography came up with estimates ranging from 90-2,000 of the number of women who need to be screened (NNS) in order to prevent one death from breast cancer. That greater than 20-fold difference in estimated magnitude of benefit has done little to inspire public and physician confidence that mammography is a key tool in reducing cancer deaths.

But the two analyses with the least supportive outcomes – the Nordic Cochrane and U.S. Preventive Services Task Force (USPSTF-) analyses – used follow-up periods of 10 and 15 years, respectively. That follow-up is too short a time to assess the full value of mammographic screening, Robert A. Smith, Ph.D., asserted at the San Antonio Breast Cancer Symposium.
To illustrate: In a European mammographic screening study with a 30-year follow-up, the NNS after 10 years was 922 women. By 29 years of follow-up, the NNS had fallen to 414.
"At 10 years of follow-up, you haven’t even observed half of the deaths prevented. So follow-up of 20 years at a minimum is really critical to begin to see the full benefit of screening," according to Dr. Smith, senior director of cancer screening at the American Cancer Society in Atlanta.
Also, several of the major reviews estimated the absolute mortality benefit of screening by means of an intent-to-treat analysis based upon the number of women invited to screening in randomized trials. That approach, too, is highly problematic because commonly 30%-40% of women invited to breast cancer screening in randomized trials never actually present for mammography, he said.
"The difference between the number-needed-to-invite and number-needed-to-screen is quite a critical difference in these estimates of absolute benefit. If you want to measure the effectiveness, you have to appreciate that a letter of invitation doesn’t do anyone any good. You have to show up to get mammography in order to benefit from it," Dr. Smith observed.
All of the four recent major reviews – the Nordic Cochrane (Cochrane Database Syst. Rev. 2013;6:CD001877), the USPSTF (Ann. Intern. Med. 2009;151:727-37), the U.K. Independent Breast Screening Review (Br. J. Cancer 2013;108:2205-40), and the European Screening Network (EUROSCREEN) Review (J. Med. Screen. 2012;19 Suppl1:14-25) – painted different pictures of the benefits of mammographic screening because they focused on different age groups, with different screening and follow-up durations, and were inconsistent as to whether the appropriate yardstick was NNS or number-needed-to-invite.
Dr. Smith and his coinvestigators sought to level the playing field by reanalyzing each review, standardizing the data to the scenario utilized in the U.K. independent review. They picked the U.K. review as the reference because it was most recently published and it was led by renowned statistical experts who aren’t part of the debate over mammography’s value. The U.K. review scenario entailed screening every 3 years for 20 years starting at age 50 years, with a 20-year follow-up period and the endpoint being breast cancer mortality at ages 55-79 years. When the data were reanalyzed in this way, the magnitude of the difference between the high and low estimates of absolute benefit among the four major reviews dropped from more than 20-fold to less than 3-fold.
"The so-called controversy over the benefit of mammography screening as estimated from the trials is largely contrived," he declared. "In short, once you standardize the evidence to the same population, the same screening scenario, and the same duration of follow-up, then the differences in absolute benefit over 20 years in the reviews become really not so significant or important at all. They are hardly worth discussing, and are certainly not enough to question the value of mammography over a lifetime of screening."
The flip side of estimating the benefit of mammographic screening in terms of breast cancer deaths avoided is the harm from overdiagnosis of cancers that never would have been symptomatic during a woman’s lifetime and wouldn’t have been detected had screening mammography not been performed. Here again, the estimates reported in the four reviews differed widely because of the divergent analytic methods employed. The U.K. review concluded that for every death from breast cancer avoided via mammography, three people would be overdiagnosed, for an overdiagnosis rate of 19%. The Nordic Cochrane analysis estimated 10 cases of overdiagnosis for every breast cancer death avoided, for a 30% overdiagnosis rate. The USPSTF didn’t give an overdiagnosis estimate. The EUROSCREEN group calculated that for every two breast cancer deaths avoided there would be one case of overdiagnosis, for a 6.5% overdiagnosis rate.
Dr. Smith said the EUROSCREEN estimate of overdiagnosis is the one that rings true. The EUROSCREEN investigators have demonstrated that in estimating mammography overdiagnosis rates, it’s essential to adjust for trends over time in breast cancer incidence and for lead time bias. When that’s not done, estimated overdiagnosis rates run in the 30%-50% range. When adjustments are made, the overdiagnosis rates are in the 0%-10% range, with the EUROSCREEN estimate of 6.5% being representative (J. Med. Screen. 2012;19 Suppl 1:42-56).
The full details of the mega-review were recently published (Breast Cancer Management 2013;2:519-28 [doi:10.2217/bmt.13.53]). The mega-review was funded by the Center for Cancer Prevention, the Wolfson Institute of Preventive Medicine, Barts and the London School of Medicine and Dentistry, and Queen Mary University of London. Dr. Smith declared having no financial conflicts of interest.
EXPERT OPINION FROM SABCS 2013
New mega-review underscores mammography’s benefits
SAN ANTONIO – The much-publicized wide disparities in the estimated value of mammographic screening for breast cancer reported in recent major reviews are overblown and largely an artifact of methodologic differences, according to a new examination of the evidence.
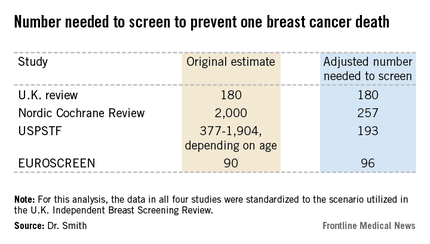
The four recent major reviews of the data regarding the absolute benefits of mammography came up with estimates ranging from 90-2,000 of the number of women who need to be screened (NNS) in order to prevent one death from breast cancer. That greater than 20-fold difference in estimated magnitude of benefit has done little to inspire public and physician confidence that mammography is a key tool in reducing cancer deaths.
But the two analyses with the least supportive outcomes – the Nordic Cochrane and U.S. Preventive Services Task Force (USPSTF-) analyses – used follow-up periods of 10 and 15 years, respectively. That follow-up is too short a time to assess the full value of mammographic screening, Robert A. Smith, Ph.D., asserted at the San Antonio Breast Cancer Symposium.
To illustrate: In a European mammographic screening study with a 30-year follow-up, the NNS after 10 years was 922 women. By 29 years of follow-up, the NNS had fallen to 414.
"At 10 years of follow-up, you haven’t even observed half of the deaths prevented. So follow-up of 20 years at a minimum is really critical to begin to see the full benefit of screening," according to Dr. Smith, senior director of cancer screening at the American Cancer Society in Atlanta.
Also, several of the major reviews estimated the absolute mortality benefit of screening by means of an intent-to-treat analysis based upon the number of women invited to screening in randomized trials. That approach, too, is highly problematic because commonly 30%-40% of women invited to breast cancer screening in randomized trials never actually present for mammography, he said.
"The difference between the number-needed-to-invite and number-needed-to-screen is quite a critical difference in these estimates of absolute benefit. If you want to measure the effectiveness, you have to appreciate that a letter of invitation doesn’t do anyone any good. You have to show up to get mammography in order to benefit from it," Dr. Smith observed.
All of the four recent major reviews – the Nordic Cochrane (Cochrane Database Syst. Rev. 2013;6:CD001877), the USPSTF (Ann. Intern. Med. 2009;151:727-37), the U.K. Independent Breast Screening Review (Br. J. Cancer 2013;108:2205-40), and the European Screening Network (EUROSCREEN) Review (J. Med. Screen. 2012;19 Suppl1:14-25) – painted different pictures of the benefits of mammographic screening because they focused on different age groups, with different screening and follow-up durations, and were inconsistent as to whether the appropriate yardstick was NNS or number-needed-to-invite.
Dr. Smith and his coinvestigators sought to level the playing field by reanalyzing each review, standardizing the data to the scenario utilized in the U.K. independent review. They picked the U.K. review as the reference because it was most recently published and it was led by renowned statistical experts who aren’t part of the debate over mammography’s value. The U.K. review scenario entailed screening every 3 years for 20 years starting at age 50 years, with a 20-year follow-up period and the endpoint being breast cancer mortality at ages 55-79 years. When the data were reanalyzed in this way, the magnitude of the difference between the high and low estimates of absolute benefit among the four major reviews dropped from more than 20-fold to less than 3-fold.
"The so-called controversy over the benefit of mammography screening as estimated from the trials is largely contrived," he declared. "In short, once you standardize the evidence to the same population, the same screening scenario, and the same duration of follow-up, then the differences in absolute benefit over 20 years in the reviews become really not so significant or important at all. They are hardly worth discussing, and are certainly not enough to question the value of mammography over a lifetime of screening."
The flip side of estimating the benefit of mammographic screening in terms of breast cancer deaths avoided is the harm from overdiagnosis of cancers that never would have been symptomatic during a woman’s lifetime and wouldn’t have been detected had screening mammography not been performed. Here again, the estimates reported in the four reviews differed widely because of the divergent analytic methods employed. The U.K. review concluded that for every death from breast cancer avoided via mammography, three people would be overdiagnosed, for an overdiagnosis rate of 19%. The Nordic Cochrane analysis estimated 10 cases of overdiagnosis for every breast cancer death avoided, for a 30% overdiagnosis rate. The USPSTF didn’t give an overdiagnosis estimate. The EUROSCREEN group calculated that for every two breast cancer deaths avoided there would be one case of overdiagnosis, for a 6.5% overdiagnosis rate.
Dr. Smith said the EUROSCREEN estimate of overdiagnosis is the one that rings true. The EUROSCREEN investigators have demonstrated that in estimating mammography overdiagnosis rates, it’s essential to adjust for trends over time in breast cancer incidence and for lead time bias. When that’s not done, estimated overdiagnosis rates run in the 30%-50% range. When adjustments are made, the overdiagnosis rates are in the 0%-10% range, with the EUROSCREEN estimate of 6.5% being representative (J. Med. Screen. 2012;19 Suppl 1:42-56).
The full details of the mega-review were recently published (Breast Cancer Management 2013;2:519-28 [doi:10.2217/bmt.13.53]). The mega-review was funded by the Center for Cancer Prevention, the Wolfson Institute of Preventive Medicine, Barts and the London School of Medicine and Dentistry, and Queen Mary University of London. Dr. Smith declared having no financial conflicts of interest.
I’m not a statistician, but the analysis presented by Dr. Smith makes common sense to me.
Basing benefit solely on those women invited to screen will clearly result in an underestimate of mammography’s true benefit if you’re going to count women who weren’t screened as being in the screened group. More and more, we are learning that breast cancer is a more indolent disease than we thought. Particularly in those with estrogen receptor–positive breast cancer, the most common subtype, many patients don’t recur and die for a decade or more. If you’re not following patients long enough to capture all of the recurrences and deaths from the disease, then you don’t get a full assessment of the value of screening earlier in the course of disease. Long-term follow-up is extremely important.
You’d think that point would be universally accepted in the world of breast cancer screening research, but it isn’t. Mammography is controversial. It takes on some of the same features as debates over global warming. Quite often we see that scientists are just as vulnerable to motivated reasoning as anybody else.
Dr. C. Kent Osborne is the SABCS codirector and director of the Dan L. Duncan Cancer Center and the Lester & Sue Smith Breast Center at Baylor College of Medicine, Houston. He made his comments during the post presentation discussion of the research.
I’m not a statistician, but the analysis presented by Dr. Smith makes common sense to me.
Basing benefit solely on those women invited to screen will clearly result in an underestimate of mammography’s true benefit if you’re going to count women who weren’t screened as being in the screened group. More and more, we are learning that breast cancer is a more indolent disease than we thought. Particularly in those with estrogen receptor–positive breast cancer, the most common subtype, many patients don’t recur and die for a decade or more. If you’re not following patients long enough to capture all of the recurrences and deaths from the disease, then you don’t get a full assessment of the value of screening earlier in the course of disease. Long-term follow-up is extremely important.
You’d think that point would be universally accepted in the world of breast cancer screening research, but it isn’t. Mammography is controversial. It takes on some of the same features as debates over global warming. Quite often we see that scientists are just as vulnerable to motivated reasoning as anybody else.
Dr. C. Kent Osborne is the SABCS codirector and director of the Dan L. Duncan Cancer Center and the Lester & Sue Smith Breast Center at Baylor College of Medicine, Houston. He made his comments during the post presentation discussion of the research.
I’m not a statistician, but the analysis presented by Dr. Smith makes common sense to me.
Basing benefit solely on those women invited to screen will clearly result in an underestimate of mammography’s true benefit if you’re going to count women who weren’t screened as being in the screened group. More and more, we are learning that breast cancer is a more indolent disease than we thought. Particularly in those with estrogen receptor–positive breast cancer, the most common subtype, many patients don’t recur and die for a decade or more. If you’re not following patients long enough to capture all of the recurrences and deaths from the disease, then you don’t get a full assessment of the value of screening earlier in the course of disease. Long-term follow-up is extremely important.
You’d think that point would be universally accepted in the world of breast cancer screening research, but it isn’t. Mammography is controversial. It takes on some of the same features as debates over global warming. Quite often we see that scientists are just as vulnerable to motivated reasoning as anybody else.
Dr. C. Kent Osborne is the SABCS codirector and director of the Dan L. Duncan Cancer Center and the Lester & Sue Smith Breast Center at Baylor College of Medicine, Houston. He made his comments during the post presentation discussion of the research.
SAN ANTONIO – The much-publicized wide disparities in the estimated value of mammographic screening for breast cancer reported in recent major reviews are overblown and largely an artifact of methodologic differences, according to a new examination of the evidence.
The four recent major reviews of the data regarding the absolute benefits of mammography came up with estimates ranging from 90-2,000 of the number of women who need to be screened (NNS) in order to prevent one death from breast cancer. That greater than 20-fold difference in estimated magnitude of benefit has done little to inspire public and physician confidence that mammography is a key tool in reducing cancer deaths.

But the two analyses with the least supportive outcomes – the Nordic Cochrane and U.S. Preventive Services Task Force (USPSTF-) analyses – used follow-up periods of 10 and 15 years, respectively. That follow-up is too short a time to assess the full value of mammographic screening, Robert A. Smith, Ph.D., asserted at the San Antonio Breast Cancer Symposium.
To illustrate: In a European mammographic screening study with a 30-year follow-up, the NNS after 10 years was 922 women. By 29 years of follow-up, the NNS had fallen to 414.
"At 10 years of follow-up, you haven’t even observed half of the deaths prevented. So follow-up of 20 years at a minimum is really critical to begin to see the full benefit of screening," according to Dr. Smith, senior director of cancer screening at the American Cancer Society in Atlanta.
Also, several of the major reviews estimated the absolute mortality benefit of screening by means of an intent-to-treat analysis based upon the number of women invited to screening in randomized trials. That approach, too, is highly problematic because commonly 30%-40% of women invited to breast cancer screening in randomized trials never actually present for mammography, he said.
"The difference between the number-needed-to-invite and number-needed-to-screen is quite a critical difference in these estimates of absolute benefit. If you want to measure the effectiveness, you have to appreciate that a letter of invitation doesn’t do anyone any good. You have to show up to get mammography in order to benefit from it," Dr. Smith observed.
All of the four recent major reviews – the Nordic Cochrane (Cochrane Database Syst. Rev. 2013;6:CD001877), the USPSTF (Ann. Intern. Med. 2009;151:727-37), the U.K. Independent Breast Screening Review (Br. J. Cancer 2013;108:2205-40), and the European Screening Network (EUROSCREEN) Review (J. Med. Screen. 2012;19 Suppl1:14-25) – painted different pictures of the benefits of mammographic screening because they focused on different age groups, with different screening and follow-up durations, and were inconsistent as to whether the appropriate yardstick was NNS or number-needed-to-invite.
Dr. Smith and his coinvestigators sought to level the playing field by reanalyzing each review, standardizing the data to the scenario utilized in the U.K. independent review. They picked the U.K. review as the reference because it was most recently published and it was led by renowned statistical experts who aren’t part of the debate over mammography’s value. The U.K. review scenario entailed screening every 3 years for 20 years starting at age 50 years, with a 20-year follow-up period and the endpoint being breast cancer mortality at ages 55-79 years. When the data were reanalyzed in this way, the magnitude of the difference between the high and low estimates of absolute benefit among the four major reviews dropped from more than 20-fold to less than 3-fold.
"The so-called controversy over the benefit of mammography screening as estimated from the trials is largely contrived," he declared. "In short, once you standardize the evidence to the same population, the same screening scenario, and the same duration of follow-up, then the differences in absolute benefit over 20 years in the reviews become really not so significant or important at all. They are hardly worth discussing, and are certainly not enough to question the value of mammography over a lifetime of screening."
The flip side of estimating the benefit of mammographic screening in terms of breast cancer deaths avoided is the harm from overdiagnosis of cancers that never would have been symptomatic during a woman’s lifetime and wouldn’t have been detected had screening mammography not been performed. Here again, the estimates reported in the four reviews differed widely because of the divergent analytic methods employed. The U.K. review concluded that for every death from breast cancer avoided via mammography, three people would be overdiagnosed, for an overdiagnosis rate of 19%. The Nordic Cochrane analysis estimated 10 cases of overdiagnosis for every breast cancer death avoided, for a 30% overdiagnosis rate. The USPSTF didn’t give an overdiagnosis estimate. The EUROSCREEN group calculated that for every two breast cancer deaths avoided there would be one case of overdiagnosis, for a 6.5% overdiagnosis rate.
Dr. Smith said the EUROSCREEN estimate of overdiagnosis is the one that rings true. The EUROSCREEN investigators have demonstrated that in estimating mammography overdiagnosis rates, it’s essential to adjust for trends over time in breast cancer incidence and for lead time bias. When that’s not done, estimated overdiagnosis rates run in the 30%-50% range. When adjustments are made, the overdiagnosis rates are in the 0%-10% range, with the EUROSCREEN estimate of 6.5% being representative (J. Med. Screen. 2012;19 Suppl 1:42-56).
The full details of the mega-review were recently published (Breast Cancer Management 2013;2:519-28 [doi:10.2217/bmt.13.53]). The mega-review was funded by the Center for Cancer Prevention, the Wolfson Institute of Preventive Medicine, Barts and the London School of Medicine and Dentistry, and Queen Mary University of London. Dr. Smith declared having no financial conflicts of interest.
SAN ANTONIO – The much-publicized wide disparities in the estimated value of mammographic screening for breast cancer reported in recent major reviews are overblown and largely an artifact of methodologic differences, according to a new examination of the evidence.
The four recent major reviews of the data regarding the absolute benefits of mammography came up with estimates ranging from 90-2,000 of the number of women who need to be screened (NNS) in order to prevent one death from breast cancer. That greater than 20-fold difference in estimated magnitude of benefit has done little to inspire public and physician confidence that mammography is a key tool in reducing cancer deaths.

But the two analyses with the least supportive outcomes – the Nordic Cochrane and U.S. Preventive Services Task Force (USPSTF-) analyses – used follow-up periods of 10 and 15 years, respectively. That follow-up is too short a time to assess the full value of mammographic screening, Robert A. Smith, Ph.D., asserted at the San Antonio Breast Cancer Symposium.
To illustrate: In a European mammographic screening study with a 30-year follow-up, the NNS after 10 years was 922 women. By 29 years of follow-up, the NNS had fallen to 414.
"At 10 years of follow-up, you haven’t even observed half of the deaths prevented. So follow-up of 20 years at a minimum is really critical to begin to see the full benefit of screening," according to Dr. Smith, senior director of cancer screening at the American Cancer Society in Atlanta.
Also, several of the major reviews estimated the absolute mortality benefit of screening by means of an intent-to-treat analysis based upon the number of women invited to screening in randomized trials. That approach, too, is highly problematic because commonly 30%-40% of women invited to breast cancer screening in randomized trials never actually present for mammography, he said.
"The difference between the number-needed-to-invite and number-needed-to-screen is quite a critical difference in these estimates of absolute benefit. If you want to measure the effectiveness, you have to appreciate that a letter of invitation doesn’t do anyone any good. You have to show up to get mammography in order to benefit from it," Dr. Smith observed.
All of the four recent major reviews – the Nordic Cochrane (Cochrane Database Syst. Rev. 2013;6:CD001877), the USPSTF (Ann. Intern. Med. 2009;151:727-37), the U.K. Independent Breast Screening Review (Br. J. Cancer 2013;108:2205-40), and the European Screening Network (EUROSCREEN) Review (J. Med. Screen. 2012;19 Suppl1:14-25) – painted different pictures of the benefits of mammographic screening because they focused on different age groups, with different screening and follow-up durations, and were inconsistent as to whether the appropriate yardstick was NNS or number-needed-to-invite.
Dr. Smith and his coinvestigators sought to level the playing field by reanalyzing each review, standardizing the data to the scenario utilized in the U.K. independent review. They picked the U.K. review as the reference because it was most recently published and it was led by renowned statistical experts who aren’t part of the debate over mammography’s value. The U.K. review scenario entailed screening every 3 years for 20 years starting at age 50 years, with a 20-year follow-up period and the endpoint being breast cancer mortality at ages 55-79 years. When the data were reanalyzed in this way, the magnitude of the difference between the high and low estimates of absolute benefit among the four major reviews dropped from more than 20-fold to less than 3-fold.
"The so-called controversy over the benefit of mammography screening as estimated from the trials is largely contrived," he declared. "In short, once you standardize the evidence to the same population, the same screening scenario, and the same duration of follow-up, then the differences in absolute benefit over 20 years in the reviews become really not so significant or important at all. They are hardly worth discussing, and are certainly not enough to question the value of mammography over a lifetime of screening."
The flip side of estimating the benefit of mammographic screening in terms of breast cancer deaths avoided is the harm from overdiagnosis of cancers that never would have been symptomatic during a woman’s lifetime and wouldn’t have been detected had screening mammography not been performed. Here again, the estimates reported in the four reviews differed widely because of the divergent analytic methods employed. The U.K. review concluded that for every death from breast cancer avoided via mammography, three people would be overdiagnosed, for an overdiagnosis rate of 19%. The Nordic Cochrane analysis estimated 10 cases of overdiagnosis for every breast cancer death avoided, for a 30% overdiagnosis rate. The USPSTF didn’t give an overdiagnosis estimate. The EUROSCREEN group calculated that for every two breast cancer deaths avoided there would be one case of overdiagnosis, for a 6.5% overdiagnosis rate.
Dr. Smith said the EUROSCREEN estimate of overdiagnosis is the one that rings true. The EUROSCREEN investigators have demonstrated that in estimating mammography overdiagnosis rates, it’s essential to adjust for trends over time in breast cancer incidence and for lead time bias. When that’s not done, estimated overdiagnosis rates run in the 30%-50% range. When adjustments are made, the overdiagnosis rates are in the 0%-10% range, with the EUROSCREEN estimate of 6.5% being representative (J. Med. Screen. 2012;19 Suppl 1:42-56).
The full details of the mega-review were recently published (Breast Cancer Management 2013;2:519-28 [doi:10.2217/bmt.13.53]). The mega-review was funded by the Center for Cancer Prevention, the Wolfson Institute of Preventive Medicine, Barts and the London School of Medicine and Dentistry, and Queen Mary University of London. Dr. Smith declared having no financial conflicts of interest.
EXPERT OPINION FROM SABCS 2013
Exercise dampens aromatase inhibitor–related joint pain
SAN ANTONIO – Adopting a standard exercise program resulted in a clinically meaningful 30% reduction in aromatase inhibitor-associated joint pain in breast cancer patients who participated in a year-long randomized trial.
The exercise prescription utilized in the HOPE (Hormones and Physical Exercise) trial was what’s recommended in national guidelines both for cancer survivors and healthy adults: 150 minutes per week of at least moderate-intensity aerobic activity, such as brisk walking, along with two strength-training sessions per week, Melinda L. Irwin, Ph.D., explained at the San Antonio Breast Cancer Symposium.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The HOPE studies enrolled 121 postmenopausal women who had stage 1-3, hormone receptor–positive breast cancers and were physically inactive and overweight yet physically able to exercise. At enrollment, they were experiencing moderate aromatase inhibitor (AI)–associated joint pain, defined as a score of 5-7 on the 0-10 Brief Pain Inventory (BPI), after about 18 months on the medication. Roughly two-thirds of participants had no history of joint pain prior to starting AI therapy; the rest reported the AI exacerbated their preexisting joint pain. Subjects were randomized to the exercise program or to usual care, which included written information about the importance of exercise.
The primary study endpoint was the 12-month change in BPI worst pain score, which dropped by an average of 30% among the exercise group. This translated to an improvement in pain level from moderate at baseline to mild at follow-up. In addition, BPI scores rating pain severity and pain interference improved by about 20%. In contrast, patients in the usual care control group experienced a slight increase in BPI scores in all three domains over time, added Dr. Irwin, co-leader of the cancer prevention and control research program at Yale University Cancer Center, New Haven, Conn.
This degree of improvement in joint pain is greater than reported in studies of glucosamine, acupuncture, or vitamin D supplementation, she noted.
The improvement in pain scores in the exercise group was greater at 12 months than at 3 or 6, suggesting that a year-long exercise program is probably necessary to see sustained reduction in joint pain.
At 12 months of follow-up, women in the exercise group averaged 159 minutes of physical activity per week, 110 minutes more than controls. Compliance with the supervised exercise program was notably good, with women attending an average of 70% of the twice-weekly small-group strength-training sessions.
In addition to the improvement in AI-related arthralgias, the exercise group experienced ancillary benefits: a mean 6.5% improvement in peak oxygen consumption, or VO2 max, compared with baseline, along with a 3% reduction in body weight.
HOPE was the first randomized trial to examine the effects of exercise on AI side effects in breast cancer patients. The impetus for the study was the recognition that arthralgias are the most common reason for poor adherence to and discontinuation of AI therapy. Up to 20% of breast cancer patients discontinue their AI within the first year. And both early discontinuation and poor adherence have been shown to be predictive of increased mortality risk.
The HOPE results received an enthusiastic audience reception. Physicians were particularly impressed with the 70% exercise adherence rate over the course of a year. They asked how they can keep their previously sedentary patients’ commitment to regular exercise from waning after an initial burst of enthusiasm, as so often happens.
Dr. Irwin replied that adherence to lifestyle change is always a challenge. Social support is quite helpful. The exercise group in HOPE received a paid gym membership and met in small groups with a personal trainer twice weekly.
"The women really bonded with each other. And there are now a growing number of free programs throughout the country, which give cancer survivors a start on an exercise program with a free gym membership for several months. For example, the Livestrong Foundation has partnered with the YMCA to offer free exercise programs for cancer survivors at local Ys," she said.
The HOPE trial was funded by the National Cancer Institute. Dr. Irwin reported having no financial conflicts of interest.
SAN ANTONIO – Adopting a standard exercise program resulted in a clinically meaningful 30% reduction in aromatase inhibitor-associated joint pain in breast cancer patients who participated in a year-long randomized trial.
The exercise prescription utilized in the HOPE (Hormones and Physical Exercise) trial was what’s recommended in national guidelines both for cancer survivors and healthy adults: 150 minutes per week of at least moderate-intensity aerobic activity, such as brisk walking, along with two strength-training sessions per week, Melinda L. Irwin, Ph.D., explained at the San Antonio Breast Cancer Symposium.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The HOPE studies enrolled 121 postmenopausal women who had stage 1-3, hormone receptor–positive breast cancers and were physically inactive and overweight yet physically able to exercise. At enrollment, they were experiencing moderate aromatase inhibitor (AI)–associated joint pain, defined as a score of 5-7 on the 0-10 Brief Pain Inventory (BPI), after about 18 months on the medication. Roughly two-thirds of participants had no history of joint pain prior to starting AI therapy; the rest reported the AI exacerbated their preexisting joint pain. Subjects were randomized to the exercise program or to usual care, which included written information about the importance of exercise.
The primary study endpoint was the 12-month change in BPI worst pain score, which dropped by an average of 30% among the exercise group. This translated to an improvement in pain level from moderate at baseline to mild at follow-up. In addition, BPI scores rating pain severity and pain interference improved by about 20%. In contrast, patients in the usual care control group experienced a slight increase in BPI scores in all three domains over time, added Dr. Irwin, co-leader of the cancer prevention and control research program at Yale University Cancer Center, New Haven, Conn.
This degree of improvement in joint pain is greater than reported in studies of glucosamine, acupuncture, or vitamin D supplementation, she noted.
The improvement in pain scores in the exercise group was greater at 12 months than at 3 or 6, suggesting that a year-long exercise program is probably necessary to see sustained reduction in joint pain.
At 12 months of follow-up, women in the exercise group averaged 159 minutes of physical activity per week, 110 minutes more than controls. Compliance with the supervised exercise program was notably good, with women attending an average of 70% of the twice-weekly small-group strength-training sessions.
In addition to the improvement in AI-related arthralgias, the exercise group experienced ancillary benefits: a mean 6.5% improvement in peak oxygen consumption, or VO2 max, compared with baseline, along with a 3% reduction in body weight.
HOPE was the first randomized trial to examine the effects of exercise on AI side effects in breast cancer patients. The impetus for the study was the recognition that arthralgias are the most common reason for poor adherence to and discontinuation of AI therapy. Up to 20% of breast cancer patients discontinue their AI within the first year. And both early discontinuation and poor adherence have been shown to be predictive of increased mortality risk.
The HOPE results received an enthusiastic audience reception. Physicians were particularly impressed with the 70% exercise adherence rate over the course of a year. They asked how they can keep their previously sedentary patients’ commitment to regular exercise from waning after an initial burst of enthusiasm, as so often happens.
Dr. Irwin replied that adherence to lifestyle change is always a challenge. Social support is quite helpful. The exercise group in HOPE received a paid gym membership and met in small groups with a personal trainer twice weekly.
"The women really bonded with each other. And there are now a growing number of free programs throughout the country, which give cancer survivors a start on an exercise program with a free gym membership for several months. For example, the Livestrong Foundation has partnered with the YMCA to offer free exercise programs for cancer survivors at local Ys," she said.
The HOPE trial was funded by the National Cancer Institute. Dr. Irwin reported having no financial conflicts of interest.
SAN ANTONIO – Adopting a standard exercise program resulted in a clinically meaningful 30% reduction in aromatase inhibitor-associated joint pain in breast cancer patients who participated in a year-long randomized trial.
The exercise prescription utilized in the HOPE (Hormones and Physical Exercise) trial was what’s recommended in national guidelines both for cancer survivors and healthy adults: 150 minutes per week of at least moderate-intensity aerobic activity, such as brisk walking, along with two strength-training sessions per week, Melinda L. Irwin, Ph.D., explained at the San Antonio Breast Cancer Symposium.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The HOPE studies enrolled 121 postmenopausal women who had stage 1-3, hormone receptor–positive breast cancers and were physically inactive and overweight yet physically able to exercise. At enrollment, they were experiencing moderate aromatase inhibitor (AI)–associated joint pain, defined as a score of 5-7 on the 0-10 Brief Pain Inventory (BPI), after about 18 months on the medication. Roughly two-thirds of participants had no history of joint pain prior to starting AI therapy; the rest reported the AI exacerbated their preexisting joint pain. Subjects were randomized to the exercise program or to usual care, which included written information about the importance of exercise.
The primary study endpoint was the 12-month change in BPI worst pain score, which dropped by an average of 30% among the exercise group. This translated to an improvement in pain level from moderate at baseline to mild at follow-up. In addition, BPI scores rating pain severity and pain interference improved by about 20%. In contrast, patients in the usual care control group experienced a slight increase in BPI scores in all three domains over time, added Dr. Irwin, co-leader of the cancer prevention and control research program at Yale University Cancer Center, New Haven, Conn.
This degree of improvement in joint pain is greater than reported in studies of glucosamine, acupuncture, or vitamin D supplementation, she noted.
The improvement in pain scores in the exercise group was greater at 12 months than at 3 or 6, suggesting that a year-long exercise program is probably necessary to see sustained reduction in joint pain.
At 12 months of follow-up, women in the exercise group averaged 159 minutes of physical activity per week, 110 minutes more than controls. Compliance with the supervised exercise program was notably good, with women attending an average of 70% of the twice-weekly small-group strength-training sessions.
In addition to the improvement in AI-related arthralgias, the exercise group experienced ancillary benefits: a mean 6.5% improvement in peak oxygen consumption, or VO2 max, compared with baseline, along with a 3% reduction in body weight.
HOPE was the first randomized trial to examine the effects of exercise on AI side effects in breast cancer patients. The impetus for the study was the recognition that arthralgias are the most common reason for poor adherence to and discontinuation of AI therapy. Up to 20% of breast cancer patients discontinue their AI within the first year. And both early discontinuation and poor adherence have been shown to be predictive of increased mortality risk.
The HOPE results received an enthusiastic audience reception. Physicians were particularly impressed with the 70% exercise adherence rate over the course of a year. They asked how they can keep their previously sedentary patients’ commitment to regular exercise from waning after an initial burst of enthusiasm, as so often happens.
Dr. Irwin replied that adherence to lifestyle change is always a challenge. Social support is quite helpful. The exercise group in HOPE received a paid gym membership and met in small groups with a personal trainer twice weekly.
"The women really bonded with each other. And there are now a growing number of free programs throughout the country, which give cancer survivors a start on an exercise program with a free gym membership for several months. For example, the Livestrong Foundation has partnered with the YMCA to offer free exercise programs for cancer survivors at local Ys," she said.
The HOPE trial was funded by the National Cancer Institute. Dr. Irwin reported having no financial conflicts of interest.
AT SABCS 20123
Major finding: Breast cancer patients experienced a 30% reduction in aromatase inhibitor–related joint pain, with pain scores going from moderate to mild, in response to a year-long exercise intervention.
Data source: The HOPE study was a 1-year randomized trial in which 121 postmenopausal breast cancer patients with significant pain from aromatase inhibitor–related arthralgias were randomized to a structured exercise program or to usual care.
Disclosures: The trial was funded by the National Cancer Institute. The presenter reported having no financial conflicts.
No gain seen from adjuvant bevacizumab in HER2-positive disease
SAN ANTONIO – Adjuvant bevacizumab does not further improve the already very good outcomes being achieved with contemporary therapy in high-risk HER2-positive breast cancer, according to results of the randomized, phase III BETH trial.
There was little room for improvement on chemotherapy and HER2-targeted therapy with trastuzumab as the standard of care, as an estimated 92% of patients were still alive and free of disease after a median follow-up of about 3 years, lead investigator Dr. Dennis J. Slamon reported at the San Antonio Breast Cancer Symposium.
"The addition of bevacizumab didn’t add any efficacy but certainly added some safety concerns," Dr. Slamon commented in a press briefing. Increased rates of grade 3/4 adverse events, such as hypertension and bowel perforation, were associated with use of bevacizumab.
BETH thus joins a host of trials with negative results for antiangiogenic therapy in breast cancer, he said. "Unless there is a new drug or strategy where we can find the subgroup [that benefits], I think this is not going anywhere. We have gotten a pretty good effect with what we have. We have very little room at the top. We have to think about new strategies for those patients who aren’t getting the benefit that we hope they will with targeted therapy."
In other trial findings, there were consistently high rates of invasive disease–free survival regardless of whether the chemotherapy/trastuzumab regimen used (left up to the treating centers) did not contain an anthracycline (docetaxel, carboplatin, and trastuzumab [Herceptin] – TCH) or did contain an anthracycline (5-fluorouracil, epirubicin, and cyclophosphamide – FEC).
Given the known toxicity of anthracyclines and the availability of an equally effective and safer alternative, their use in HER2-positive breast cancer is no longer justified, according to Dr. Slamon, director of clinical/translational research at the University of California, Los Angeles (UCLA) Jonsson Comprehensive Cancer Center and professor of medicine and chief of the division of hematology/oncology at UCLA.
Acknowledging that the topic is intensely debated, Dr. Slamon noted that "there is no reason to take that risk now that we have more than 4,000 patients treated [with TCH] between BCIRG-006 [which first rigorously tested this regimen] and BETH that show that this gives a pretty favorable outcome. We at our institution do not use these drugs [anthracyclines]. It doesn’t mean they are not effective; they just don’t provide any incremental benefit over other drugs that would give you the same effect without the safety concerns."
While praising the trial as "exceptionally well run" and agreeing on interpretation of the bevacizumab results, press briefing moderator Dr. Jennifer Litton expressed an opposing view.
"I’m totally on the other side of the aisle, that we shouldn’t say that all anthracyclines should go away," maintained Dr. Litton, associate professor in the department of breast medical oncology at the University of Texas MD Anderson Cancer Center in Houston. "The BETH trial specifically asked the question: Does bevacizumab add anything? And the answer quite clearly is no." It did it compare an anthracycline- and a non–anthracycline-containing arm. "That was not the design or intent," she said.
Additionally, in contrast to other similar trials, almost half of the patients who participated in BETH had stage I disease, raising the possibility of selection bias for who received TCH.
"But I do feel that the Herceptin story and the combination stories to come" with lapatinib, pertuzumab, and TDM1 "are really going to also be the landscape changers in HER2 disease," Dr. Litton concluded. "And we are going to be continuing to find specific strategies and not a one-therapy-fits-all for all HER2 disease because we are learning more and more about the molecular subsets of cancer. How we have broken them up into triple receptors is really going to go by the wayside."
Bevacizumab (Avastin) is an antibody to vascular endothelial growth factor (VEGF). It is approved for use by the Food and Drug Administration to treat colorectal cancer, glioblastoma, non–small cell lung cancer, and renal cell cancer.
The 3,509 women enrolled in BETH (Bevacizumab and Trastuzumab Adjuvant Therapy in HER2-Positive Breast Cancer) had HER2-positive, node-positive, or high-risk node-negative breast cancer; 92% received TCH as their chemotherapy.
With a median follow-up of 38 months in the trial population overall, there was no significant difference between patients randomized to receive bevacizumab and those who did not in terms of the 3-year rate of invasive disease–free survival (92% vs. 92%) and overall survival (97% vs. 96%).
The findings were consistent across subgroups stratified by a variety of patient and disease characteristics, Dr. Slamon noted.
Additionally, the rate of invasive disease–free survival was statistically indistinguishable with or without bevacizumab in both the cohort given TCH (92% vs. 92%) and the cohort given FEC (91% vs. 89%).
In the trial population overall, addition of bevacizumab to chemotherapy tripled the rate of all grade 3/4 adverse events of special interest (27% vs. 8%, P less than .0001), such as hypertension, congestive heart failure, and gastrointestinal perforation.
Dr. Slamon disclosed that he serves as an adviser to Roche/Genentech, which supported the trial.
SAN ANTONIO – Adjuvant bevacizumab does not further improve the already very good outcomes being achieved with contemporary therapy in high-risk HER2-positive breast cancer, according to results of the randomized, phase III BETH trial.
There was little room for improvement on chemotherapy and HER2-targeted therapy with trastuzumab as the standard of care, as an estimated 92% of patients were still alive and free of disease after a median follow-up of about 3 years, lead investigator Dr. Dennis J. Slamon reported at the San Antonio Breast Cancer Symposium.
"The addition of bevacizumab didn’t add any efficacy but certainly added some safety concerns," Dr. Slamon commented in a press briefing. Increased rates of grade 3/4 adverse events, such as hypertension and bowel perforation, were associated with use of bevacizumab.
BETH thus joins a host of trials with negative results for antiangiogenic therapy in breast cancer, he said. "Unless there is a new drug or strategy where we can find the subgroup [that benefits], I think this is not going anywhere. We have gotten a pretty good effect with what we have. We have very little room at the top. We have to think about new strategies for those patients who aren’t getting the benefit that we hope they will with targeted therapy."
In other trial findings, there were consistently high rates of invasive disease–free survival regardless of whether the chemotherapy/trastuzumab regimen used (left up to the treating centers) did not contain an anthracycline (docetaxel, carboplatin, and trastuzumab [Herceptin] – TCH) or did contain an anthracycline (5-fluorouracil, epirubicin, and cyclophosphamide – FEC).
Given the known toxicity of anthracyclines and the availability of an equally effective and safer alternative, their use in HER2-positive breast cancer is no longer justified, according to Dr. Slamon, director of clinical/translational research at the University of California, Los Angeles (UCLA) Jonsson Comprehensive Cancer Center and professor of medicine and chief of the division of hematology/oncology at UCLA.
Acknowledging that the topic is intensely debated, Dr. Slamon noted that "there is no reason to take that risk now that we have more than 4,000 patients treated [with TCH] between BCIRG-006 [which first rigorously tested this regimen] and BETH that show that this gives a pretty favorable outcome. We at our institution do not use these drugs [anthracyclines]. It doesn’t mean they are not effective; they just don’t provide any incremental benefit over other drugs that would give you the same effect without the safety concerns."
While praising the trial as "exceptionally well run" and agreeing on interpretation of the bevacizumab results, press briefing moderator Dr. Jennifer Litton expressed an opposing view.
"I’m totally on the other side of the aisle, that we shouldn’t say that all anthracyclines should go away," maintained Dr. Litton, associate professor in the department of breast medical oncology at the University of Texas MD Anderson Cancer Center in Houston. "The BETH trial specifically asked the question: Does bevacizumab add anything? And the answer quite clearly is no." It did it compare an anthracycline- and a non–anthracycline-containing arm. "That was not the design or intent," she said.
Additionally, in contrast to other similar trials, almost half of the patients who participated in BETH had stage I disease, raising the possibility of selection bias for who received TCH.
"But I do feel that the Herceptin story and the combination stories to come" with lapatinib, pertuzumab, and TDM1 "are really going to also be the landscape changers in HER2 disease," Dr. Litton concluded. "And we are going to be continuing to find specific strategies and not a one-therapy-fits-all for all HER2 disease because we are learning more and more about the molecular subsets of cancer. How we have broken them up into triple receptors is really going to go by the wayside."
Bevacizumab (Avastin) is an antibody to vascular endothelial growth factor (VEGF). It is approved for use by the Food and Drug Administration to treat colorectal cancer, glioblastoma, non–small cell lung cancer, and renal cell cancer.
The 3,509 women enrolled in BETH (Bevacizumab and Trastuzumab Adjuvant Therapy in HER2-Positive Breast Cancer) had HER2-positive, node-positive, or high-risk node-negative breast cancer; 92% received TCH as their chemotherapy.
With a median follow-up of 38 months in the trial population overall, there was no significant difference between patients randomized to receive bevacizumab and those who did not in terms of the 3-year rate of invasive disease–free survival (92% vs. 92%) and overall survival (97% vs. 96%).
The findings were consistent across subgroups stratified by a variety of patient and disease characteristics, Dr. Slamon noted.
Additionally, the rate of invasive disease–free survival was statistically indistinguishable with or without bevacizumab in both the cohort given TCH (92% vs. 92%) and the cohort given FEC (91% vs. 89%).
In the trial population overall, addition of bevacizumab to chemotherapy tripled the rate of all grade 3/4 adverse events of special interest (27% vs. 8%, P less than .0001), such as hypertension, congestive heart failure, and gastrointestinal perforation.
Dr. Slamon disclosed that he serves as an adviser to Roche/Genentech, which supported the trial.
SAN ANTONIO – Adjuvant bevacizumab does not further improve the already very good outcomes being achieved with contemporary therapy in high-risk HER2-positive breast cancer, according to results of the randomized, phase III BETH trial.
There was little room for improvement on chemotherapy and HER2-targeted therapy with trastuzumab as the standard of care, as an estimated 92% of patients were still alive and free of disease after a median follow-up of about 3 years, lead investigator Dr. Dennis J. Slamon reported at the San Antonio Breast Cancer Symposium.
"The addition of bevacizumab didn’t add any efficacy but certainly added some safety concerns," Dr. Slamon commented in a press briefing. Increased rates of grade 3/4 adverse events, such as hypertension and bowel perforation, were associated with use of bevacizumab.
BETH thus joins a host of trials with negative results for antiangiogenic therapy in breast cancer, he said. "Unless there is a new drug or strategy where we can find the subgroup [that benefits], I think this is not going anywhere. We have gotten a pretty good effect with what we have. We have very little room at the top. We have to think about new strategies for those patients who aren’t getting the benefit that we hope they will with targeted therapy."
In other trial findings, there were consistently high rates of invasive disease–free survival regardless of whether the chemotherapy/trastuzumab regimen used (left up to the treating centers) did not contain an anthracycline (docetaxel, carboplatin, and trastuzumab [Herceptin] – TCH) or did contain an anthracycline (5-fluorouracil, epirubicin, and cyclophosphamide – FEC).
Given the known toxicity of anthracyclines and the availability of an equally effective and safer alternative, their use in HER2-positive breast cancer is no longer justified, according to Dr. Slamon, director of clinical/translational research at the University of California, Los Angeles (UCLA) Jonsson Comprehensive Cancer Center and professor of medicine and chief of the division of hematology/oncology at UCLA.
Acknowledging that the topic is intensely debated, Dr. Slamon noted that "there is no reason to take that risk now that we have more than 4,000 patients treated [with TCH] between BCIRG-006 [which first rigorously tested this regimen] and BETH that show that this gives a pretty favorable outcome. We at our institution do not use these drugs [anthracyclines]. It doesn’t mean they are not effective; they just don’t provide any incremental benefit over other drugs that would give you the same effect without the safety concerns."
While praising the trial as "exceptionally well run" and agreeing on interpretation of the bevacizumab results, press briefing moderator Dr. Jennifer Litton expressed an opposing view.
"I’m totally on the other side of the aisle, that we shouldn’t say that all anthracyclines should go away," maintained Dr. Litton, associate professor in the department of breast medical oncology at the University of Texas MD Anderson Cancer Center in Houston. "The BETH trial specifically asked the question: Does bevacizumab add anything? And the answer quite clearly is no." It did it compare an anthracycline- and a non–anthracycline-containing arm. "That was not the design or intent," she said.
Additionally, in contrast to other similar trials, almost half of the patients who participated in BETH had stage I disease, raising the possibility of selection bias for who received TCH.
"But I do feel that the Herceptin story and the combination stories to come" with lapatinib, pertuzumab, and TDM1 "are really going to also be the landscape changers in HER2 disease," Dr. Litton concluded. "And we are going to be continuing to find specific strategies and not a one-therapy-fits-all for all HER2 disease because we are learning more and more about the molecular subsets of cancer. How we have broken them up into triple receptors is really going to go by the wayside."
Bevacizumab (Avastin) is an antibody to vascular endothelial growth factor (VEGF). It is approved for use by the Food and Drug Administration to treat colorectal cancer, glioblastoma, non–small cell lung cancer, and renal cell cancer.
The 3,509 women enrolled in BETH (Bevacizumab and Trastuzumab Adjuvant Therapy in HER2-Positive Breast Cancer) had HER2-positive, node-positive, or high-risk node-negative breast cancer; 92% received TCH as their chemotherapy.
With a median follow-up of 38 months in the trial population overall, there was no significant difference between patients randomized to receive bevacizumab and those who did not in terms of the 3-year rate of invasive disease–free survival (92% vs. 92%) and overall survival (97% vs. 96%).
The findings were consistent across subgroups stratified by a variety of patient and disease characteristics, Dr. Slamon noted.
Additionally, the rate of invasive disease–free survival was statistically indistinguishable with or without bevacizumab in both the cohort given TCH (92% vs. 92%) and the cohort given FEC (91% vs. 89%).
In the trial population overall, addition of bevacizumab to chemotherapy tripled the rate of all grade 3/4 adverse events of special interest (27% vs. 8%, P less than .0001), such as hypertension, congestive heart failure, and gastrointestinal perforation.
Dr. Slamon disclosed that he serves as an adviser to Roche/Genentech, which supported the trial.
AT SABCS 2013
Major Finding: The rate of invasive disease–free survival did not differ significantly between patients on bevacizumab and those who were not (92% vs. 92%).
Data Source: A randomized, phase III trial of adjuvant chemotherapy plus trastuzumab, with or without bevacizumab, in 3,509 patients with HER2-positive, node-positive, or high-risk node-negative breast cancer (BETH trial).
Disclosures: Dr. Slamon disclosed that he serves as an adviser to Roche/Genentech, which supported the trial.
IBIS-II: Anastrozole highly effective in preventing breast cancer
SAN ANTONIO – The aromatase inhibitor anastrozole has emerged as a major new agent for the primary prevention of breast cancer in high-risk postmenopausal women on the strength of a 53% reduction relative to placebo in the IBIS-II trial.
"Our results provide substantial support for the use of anastrozole as the treatment of first choice for prevention of breast cancer in high-risk postmenopausal women," Jack Cuzick, Ph.D., declared in presenting the study results at the San Antonio Breast Cancer Symposium.
IBIS-II (the International Breast Cancer Intervention Study II) was an 16-country, double-blind, randomized, placebo-controlled trial in which 3,864 postmenopausal women aged 40-70 years at high risk for breast cancer were randomized to 1 mg of oral anastrozole (Arimidex) or placebo daily for 5 years.
At 7 years of follow-up, the cumulative incidence of all breast cancers in an intent-to-treat analysis was 5.6% with placebo and 2.8% in the anastrozole group, for a highly significant 53% reduction in risk. This translates into a number needed to treat of 36; that is, it’s estimated that treating 36 women for 5 years will prevent one breast cancer in 7 years.
The incidence of estrogen receptor–positive invasive breast cancer was 3.3% in controls, compared with 1.4% with anastrozole, for a 58% relative risk reduction, added Dr. Cuzick, head of the Cancer Research U.K. Center for Cancer Prevention and director of the Wolfson Institute of Preventive Medicine at Queen Mary University of London.
The incidence of high-grade tumors was 35% lower in the anastrozole group. With additional years of follow-up, this will probably translate into a reduction in deaths from breast cancer in the anastrozole group. The plan is for at least 10 years of follow-up in IBIS-II.
Among women on placebo, 72% completed 5 years, as did 68% assigned to anastrozole.
"One of the most important findings of this trial is that the drug was very well tolerated, with only an absolute 4% difference in compliance. So drug side effects didn’t influence compliance in any major way," Dr. Cuzick observed.
The incidence of other cancers was 3.6% with placebo and 2.1% with anastrozole, for a significant 42% relative risk reduction. This was mostly driven by markedly fewer cases of skin cancer and colorectal cancer in patients on the aromatase inhibitor.
"We don’t really understand this. It’s another exciting possibility – that this drug may have an effect in reducing the risk of other cancers – which we will continue to explore," he continued.
Going into the trial, the anastrozole side effect of chief concern was fractures, but under the study protocol all participants had a baseline bone mineral density scan and were placed on a bisphosphonate if indicated. That’s presumably the explanation for the finding that the incidence of fractures didn’t differ significantly between the two study arms.
Musculoskeletal pain was reported by 64% of anastrozole-treated patients and 58% on placebo. The incidence of moderate musculoskeletal pain was 22% with anastrozole and 19% with placebo, while severe pain was reported by 8% of women in the anastrozole group, compared with 6% on placebo.
Pointing to the 58% incidence of musculoskeletal side effects in the placebo group, Dr. Cuzick said that "there’s a general perception that the aromatase inhibitors are very toxic and cause a lot of aches and pains. But 90% of the aches and pains in this study had nothing to do with the drug. They’re just a reflection of the fact that in the postmenopausal years women do get aches and pains."
Vasomotor symptoms occurred in 49% of the placebo group and 57% of women on anastrozole, a statistically significant difference.
Dr. Cuzick said that he believes the sharp reduction in breast cancer seen with anastrozole in IBIS-II is probably a class effect common to the other aromatase inhibitors. In the MAP.3 trial, an earlier study with shorter follow-up (N. Engl. J. Med. 2011;364:2381-91), exemestane showed results similar to those seen in IBIS-II. He added that the greater size and duration of IBIS-II, coupled with the fact that contralateral breast cancer rates continue to be lower at 10 years of follow-up in the anastrozole breast cancer treatment trials, makes him "quite confident" in declaring anastrozole to be the drug of first choice for prevention.
Audience member Dr. Pamela J. Goodwin rose to take issue with that assertion. She noted that there are two approved agents for this indication – tamoxifen and raloxifene – yet placebo was used as the comparator in IBIS-II.
"The evidence is indirect evidence," Dr. Cuzick replied. "Anastrozole and exemestane have shown very, very similar results. The aromatase inhibitors show larger risk reductions than tamoxifen or raloxifene, and overall serious side effects are substantially less. For that reason we think they are the appropriate first choice."
"Those are cross-trial comparisons. I’m not sure I would go that far," retorted Dr. Goodwin, professor of medicine at the University of Toronto.
In a press conference announcing the IBIS-II results, Dr. Cuzick noted that neither tamoxifen nor raloxifene is widely utilized for primary prevention of breast cancer. More public education is in order, he added.
"I think there’s a lot to be done to model what the cardiologists have done: They’ve convinced people that high cholesterol and high blood pressure are actually diseases. In fact, they’re only risk factors, but by taking drugs to reduce those risk factors there’s been a major effect. We need to make people aware there are effective ways of reducing the risk of breast cancer by more than 50%. The toxicities are limited, and if you have toxicity you simply stop treatment," he said.
The IBIS-II study was funded by Cancer Research U.K., the National Health and Medical Research Council of Australia, AstraZeneca, and Sanofi-Aventis. Dr. Cuzick is on the speaker’s bureau for AstraZeneca.
Simultaneous with Dr. Cuzick’s presentation in San Antonio, the IBIS-II results were published online in the Lancet (2013 Dec. 12 [doi:10.1016/S0140-6736(13)62292-8]).
SAN ANTONIO – The aromatase inhibitor anastrozole has emerged as a major new agent for the primary prevention of breast cancer in high-risk postmenopausal women on the strength of a 53% reduction relative to placebo in the IBIS-II trial.
"Our results provide substantial support for the use of anastrozole as the treatment of first choice for prevention of breast cancer in high-risk postmenopausal women," Jack Cuzick, Ph.D., declared in presenting the study results at the San Antonio Breast Cancer Symposium.
IBIS-II (the International Breast Cancer Intervention Study II) was an 16-country, double-blind, randomized, placebo-controlled trial in which 3,864 postmenopausal women aged 40-70 years at high risk for breast cancer were randomized to 1 mg of oral anastrozole (Arimidex) or placebo daily for 5 years.
At 7 years of follow-up, the cumulative incidence of all breast cancers in an intent-to-treat analysis was 5.6% with placebo and 2.8% in the anastrozole group, for a highly significant 53% reduction in risk. This translates into a number needed to treat of 36; that is, it’s estimated that treating 36 women for 5 years will prevent one breast cancer in 7 years.
The incidence of estrogen receptor–positive invasive breast cancer was 3.3% in controls, compared with 1.4% with anastrozole, for a 58% relative risk reduction, added Dr. Cuzick, head of the Cancer Research U.K. Center for Cancer Prevention and director of the Wolfson Institute of Preventive Medicine at Queen Mary University of London.
The incidence of high-grade tumors was 35% lower in the anastrozole group. With additional years of follow-up, this will probably translate into a reduction in deaths from breast cancer in the anastrozole group. The plan is for at least 10 years of follow-up in IBIS-II.
Among women on placebo, 72% completed 5 years, as did 68% assigned to anastrozole.
"One of the most important findings of this trial is that the drug was very well tolerated, with only an absolute 4% difference in compliance. So drug side effects didn’t influence compliance in any major way," Dr. Cuzick observed.
The incidence of other cancers was 3.6% with placebo and 2.1% with anastrozole, for a significant 42% relative risk reduction. This was mostly driven by markedly fewer cases of skin cancer and colorectal cancer in patients on the aromatase inhibitor.
"We don’t really understand this. It’s another exciting possibility – that this drug may have an effect in reducing the risk of other cancers – which we will continue to explore," he continued.
Going into the trial, the anastrozole side effect of chief concern was fractures, but under the study protocol all participants had a baseline bone mineral density scan and were placed on a bisphosphonate if indicated. That’s presumably the explanation for the finding that the incidence of fractures didn’t differ significantly between the two study arms.
Musculoskeletal pain was reported by 64% of anastrozole-treated patients and 58% on placebo. The incidence of moderate musculoskeletal pain was 22% with anastrozole and 19% with placebo, while severe pain was reported by 8% of women in the anastrozole group, compared with 6% on placebo.
Pointing to the 58% incidence of musculoskeletal side effects in the placebo group, Dr. Cuzick said that "there’s a general perception that the aromatase inhibitors are very toxic and cause a lot of aches and pains. But 90% of the aches and pains in this study had nothing to do with the drug. They’re just a reflection of the fact that in the postmenopausal years women do get aches and pains."
Vasomotor symptoms occurred in 49% of the placebo group and 57% of women on anastrozole, a statistically significant difference.
Dr. Cuzick said that he believes the sharp reduction in breast cancer seen with anastrozole in IBIS-II is probably a class effect common to the other aromatase inhibitors. In the MAP.3 trial, an earlier study with shorter follow-up (N. Engl. J. Med. 2011;364:2381-91), exemestane showed results similar to those seen in IBIS-II. He added that the greater size and duration of IBIS-II, coupled with the fact that contralateral breast cancer rates continue to be lower at 10 years of follow-up in the anastrozole breast cancer treatment trials, makes him "quite confident" in declaring anastrozole to be the drug of first choice for prevention.
Audience member Dr. Pamela J. Goodwin rose to take issue with that assertion. She noted that there are two approved agents for this indication – tamoxifen and raloxifene – yet placebo was used as the comparator in IBIS-II.
"The evidence is indirect evidence," Dr. Cuzick replied. "Anastrozole and exemestane have shown very, very similar results. The aromatase inhibitors show larger risk reductions than tamoxifen or raloxifene, and overall serious side effects are substantially less. For that reason we think they are the appropriate first choice."
"Those are cross-trial comparisons. I’m not sure I would go that far," retorted Dr. Goodwin, professor of medicine at the University of Toronto.
In a press conference announcing the IBIS-II results, Dr. Cuzick noted that neither tamoxifen nor raloxifene is widely utilized for primary prevention of breast cancer. More public education is in order, he added.
"I think there’s a lot to be done to model what the cardiologists have done: They’ve convinced people that high cholesterol and high blood pressure are actually diseases. In fact, they’re only risk factors, but by taking drugs to reduce those risk factors there’s been a major effect. We need to make people aware there are effective ways of reducing the risk of breast cancer by more than 50%. The toxicities are limited, and if you have toxicity you simply stop treatment," he said.
The IBIS-II study was funded by Cancer Research U.K., the National Health and Medical Research Council of Australia, AstraZeneca, and Sanofi-Aventis. Dr. Cuzick is on the speaker’s bureau for AstraZeneca.
Simultaneous with Dr. Cuzick’s presentation in San Antonio, the IBIS-II results were published online in the Lancet (2013 Dec. 12 [doi:10.1016/S0140-6736(13)62292-8]).
SAN ANTONIO – The aromatase inhibitor anastrozole has emerged as a major new agent for the primary prevention of breast cancer in high-risk postmenopausal women on the strength of a 53% reduction relative to placebo in the IBIS-II trial.
"Our results provide substantial support for the use of anastrozole as the treatment of first choice for prevention of breast cancer in high-risk postmenopausal women," Jack Cuzick, Ph.D., declared in presenting the study results at the San Antonio Breast Cancer Symposium.
IBIS-II (the International Breast Cancer Intervention Study II) was an 16-country, double-blind, randomized, placebo-controlled trial in which 3,864 postmenopausal women aged 40-70 years at high risk for breast cancer were randomized to 1 mg of oral anastrozole (Arimidex) or placebo daily for 5 years.
At 7 years of follow-up, the cumulative incidence of all breast cancers in an intent-to-treat analysis was 5.6% with placebo and 2.8% in the anastrozole group, for a highly significant 53% reduction in risk. This translates into a number needed to treat of 36; that is, it’s estimated that treating 36 women for 5 years will prevent one breast cancer in 7 years.
The incidence of estrogen receptor–positive invasive breast cancer was 3.3% in controls, compared with 1.4% with anastrozole, for a 58% relative risk reduction, added Dr. Cuzick, head of the Cancer Research U.K. Center for Cancer Prevention and director of the Wolfson Institute of Preventive Medicine at Queen Mary University of London.
The incidence of high-grade tumors was 35% lower in the anastrozole group. With additional years of follow-up, this will probably translate into a reduction in deaths from breast cancer in the anastrozole group. The plan is for at least 10 years of follow-up in IBIS-II.
Among women on placebo, 72% completed 5 years, as did 68% assigned to anastrozole.
"One of the most important findings of this trial is that the drug was very well tolerated, with only an absolute 4% difference in compliance. So drug side effects didn’t influence compliance in any major way," Dr. Cuzick observed.
The incidence of other cancers was 3.6% with placebo and 2.1% with anastrozole, for a significant 42% relative risk reduction. This was mostly driven by markedly fewer cases of skin cancer and colorectal cancer in patients on the aromatase inhibitor.
"We don’t really understand this. It’s another exciting possibility – that this drug may have an effect in reducing the risk of other cancers – which we will continue to explore," he continued.
Going into the trial, the anastrozole side effect of chief concern was fractures, but under the study protocol all participants had a baseline bone mineral density scan and were placed on a bisphosphonate if indicated. That’s presumably the explanation for the finding that the incidence of fractures didn’t differ significantly between the two study arms.
Musculoskeletal pain was reported by 64% of anastrozole-treated patients and 58% on placebo. The incidence of moderate musculoskeletal pain was 22% with anastrozole and 19% with placebo, while severe pain was reported by 8% of women in the anastrozole group, compared with 6% on placebo.
Pointing to the 58% incidence of musculoskeletal side effects in the placebo group, Dr. Cuzick said that "there’s a general perception that the aromatase inhibitors are very toxic and cause a lot of aches and pains. But 90% of the aches and pains in this study had nothing to do with the drug. They’re just a reflection of the fact that in the postmenopausal years women do get aches and pains."
Vasomotor symptoms occurred in 49% of the placebo group and 57% of women on anastrozole, a statistically significant difference.
Dr. Cuzick said that he believes the sharp reduction in breast cancer seen with anastrozole in IBIS-II is probably a class effect common to the other aromatase inhibitors. In the MAP.3 trial, an earlier study with shorter follow-up (N. Engl. J. Med. 2011;364:2381-91), exemestane showed results similar to those seen in IBIS-II. He added that the greater size and duration of IBIS-II, coupled with the fact that contralateral breast cancer rates continue to be lower at 10 years of follow-up in the anastrozole breast cancer treatment trials, makes him "quite confident" in declaring anastrozole to be the drug of first choice for prevention.
Audience member Dr. Pamela J. Goodwin rose to take issue with that assertion. She noted that there are two approved agents for this indication – tamoxifen and raloxifene – yet placebo was used as the comparator in IBIS-II.
"The evidence is indirect evidence," Dr. Cuzick replied. "Anastrozole and exemestane have shown very, very similar results. The aromatase inhibitors show larger risk reductions than tamoxifen or raloxifene, and overall serious side effects are substantially less. For that reason we think they are the appropriate first choice."
"Those are cross-trial comparisons. I’m not sure I would go that far," retorted Dr. Goodwin, professor of medicine at the University of Toronto.
In a press conference announcing the IBIS-II results, Dr. Cuzick noted that neither tamoxifen nor raloxifene is widely utilized for primary prevention of breast cancer. More public education is in order, he added.
"I think there’s a lot to be done to model what the cardiologists have done: They’ve convinced people that high cholesterol and high blood pressure are actually diseases. In fact, they’re only risk factors, but by taking drugs to reduce those risk factors there’s been a major effect. We need to make people aware there are effective ways of reducing the risk of breast cancer by more than 50%. The toxicities are limited, and if you have toxicity you simply stop treatment," he said.
The IBIS-II study was funded by Cancer Research U.K., the National Health and Medical Research Council of Australia, AstraZeneca, and Sanofi-Aventis. Dr. Cuzick is on the speaker’s bureau for AstraZeneca.
Simultaneous with Dr. Cuzick’s presentation in San Antonio, the IBIS-II results were published online in the Lancet (2013 Dec. 12 [doi:10.1016/S0140-6736(13)62292-8]).
AT SABCS 2013
Major finding: The 7-year cumulative incidence of all breast cancers in high-risk postmenopausal women who took anastrozole once daily for 5 years was 2.8%, representing a 53% reduction in risk compared with the 5.6% rate in controls. The number needed to treat for 5 years to prevent one breast cancer in 7 years was 36 women.
Data source: The IBIS-II trial was a randomized, double-blind, placebo-controlled study in which 3,864 postmenopausal women at high risk for breast cancer were assigned to 1 mg/day of anastrozole or placebo for 5 years.
Disclosures: The study was funded by Cancer Research U.K., the National Health and Medical Research Council of Australia, AstraZeneca, and Sanofi-Aventis. The presenter is on the speaker’s bureau for AstraZeneca.
Dasatinib gives letrozole a boost in HR-positive advanced breast cancer
SAN ANTONIO – The oral tyrosine kinase inhibitor dasatinib appears to increase the efficacy of first-line aromatase inhibitor therapy in women with advanced breast cancer, suggests a randomized phase 2 trial presented at the San Antonio Breast Cancer Symposium.
Adding dasatinib (SPRYCEL) to letrozole (Femara) did not improve the clinical benefit rate – the trial’s primary endpoint – among the 120 postmenopausal women with hormone receptor–positive, HER2-negative locally recurrent or metastatic breast cancer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
But the combination doubled patients’ median progression-free survival from 10 months to 20 months in an intent-to-treat analysis, Dr. Dev Paul of U.S. Oncology and the Rocky Mountain Cancer Centers in Denver reported in a session and a related press briefing at the meeting.
"Dasatinib may be inhibiting the emergence of resistance to aromatase inhibitor therapy," he said.
The researchers were unable to identify factors that explained why the clinical benefit rate was the same, but the progression-free survival was different for the two groups. The study was small, did not use a placebo, and permitted crossover; "all of these things could affect the results, and this is a preliminary exploratory study," Dr. Paul said.
Combining dasatinib with other aromatase inhibitors – fulvestrant or exemestane – has not improved progression-free survival benefit in women with metastatic breast cancer previously treated with a nonsteroidal aromatase inhibitor, Dr. Paul noted; thus, it may be important to give dasatinib the first time that patients are given an aromatase inhibitor. "Putative predictive biomarkers for benefit from dasatinib will be assessed in patients’ archival breast cancer tissues to help inform patient selection for future studies."
In additional findings from the trial, adding dasatinib appeared to attenuate the adverse impact of letrozole on bone health. At the end of the study, patients given the combination therapy were half as likely as their counterparts given letrozole alone to have osteopenia.
The findings on bone density suggest "an on-target effect of the Src inhibitor [dasatinib]," commented Dr. Carlos L. Arteaga, who moderated the press briefing. Dr. Arteaga is president-elect of the American Association for Cancer Research and associate director for translational/clinical research and director of the breast cancer program at Vanderbilt-Ingram Cancer Center in Nashville, Tenn., and a codirector of SABCS.
Dr. Paul noted that the observations occurred in an intent-to-treat analysis that included all patients according to their initial treatment assignment.
During a discussion after the presentation, one session attendee questioned the interpretation that dasatinib was inhibiting the emergence of resistance to letrozole and pointed out that 23% of patients who had progression on letrozole monotherapy had clinical benefit after crossing over to the combination. "You could also interpret the crossover data [as saying] that [dasatinib] simply treats endocrine resistance. So my question is, have you looked at the time to total progression? Have you looked at that 23% clinical benefit in the cross-over group? Because [dasatinib] may not stop resistance, it may just treat it."
Looking at the total time to progression for sequential vs. combined therapy, it may be that the time to progression curves really don’t separate quite so much, the discussant maintained.
Dr. Paul acknowledged that this alternate interpretation was possible and said that such analyses have not yet been done.
"Src is a pleiotropic nonreceptor tyrosine kinase involved in breast cancer invasion, proliferation, and survival. Membrane estrogen receptor-alpha complexes with Src and PI3 kinase to drive breast cancer growth and endocrine therapy resistance," Dr. Paul said, giving some background to the trial. "Src also regulates osteoclast-mediated bone turnover."
Dasatinib inhibits Src tyrosine kinase and is currently approved by the Food and Drug Administration for treatment of Philadelphia chromosome–positive acute lymphoblastic leukemia and chronic myelogenous leukemia.
Women enrolled in the trial were allowed to have had prior non–aromatase inhibitor endocrine therapy for metastatic disease; prior adjuvant aromatase inhibitor therapy if completed at least 1 year before study entry; and one prior chemotherapy regimen for metastatic disease.
After stratification by disease-free interval and prior receipt of tamoxifen, patients were randomized to receive letrozole plus dasatinib or letrozole alone on 28-day cycles.
Patients on letrozole alone were allowed to cross over if they experienced progression, and 56% ultimately did so, Dr. Paul reported.
About half of the women in each treatment arm had not received any prior chemotherapy; 60% of those randomized to the combination and 49% of those randomized to letrozole alone had not received any prior endocrine therapy.
The clinical benefit rate – consisting of complete responses, partial responses, and stable disease for at least 6 months – was 71% with dasatinib plus letrozole and 66% with letrozole alone, a nonsignificant difference.
However, in intent-to-treat analyses, median progression-free survival was more than twice as long with the combination (20.1 months vs. 9.9 months; exploratory hazard ratio, 0.69).
In each treatment arm, about one-third of patients had a bone density T score below –1.5 at baseline, and about one-third received bisphosphonates during the study. But, by the end of the study, the proportion of patients with a T score below –1.5 was smaller in those on combination therapy (14% vs. 32%).
"There were no unexpected toxicities associated with dasatinib," Dr. Paul commented. The addition of this agent was associated with higher rates of grade 3 rash, edema, fatigue, anemia, neutropenia, arthralgia, and pleural effusion, but there were no grade 4 toxicities. Overall, one-quarter of patients needed a dasatinib dose reduction.
Dr. Paul disclosed no relevant conflicts of interest. The trial was sponsored by Bristol-Myers Squibb, the makers of SPRYCEL (dasatinib).
SAN ANTONIO – The oral tyrosine kinase inhibitor dasatinib appears to increase the efficacy of first-line aromatase inhibitor therapy in women with advanced breast cancer, suggests a randomized phase 2 trial presented at the San Antonio Breast Cancer Symposium.
Adding dasatinib (SPRYCEL) to letrozole (Femara) did not improve the clinical benefit rate – the trial’s primary endpoint – among the 120 postmenopausal women with hormone receptor–positive, HER2-negative locally recurrent or metastatic breast cancer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
But the combination doubled patients’ median progression-free survival from 10 months to 20 months in an intent-to-treat analysis, Dr. Dev Paul of U.S. Oncology and the Rocky Mountain Cancer Centers in Denver reported in a session and a related press briefing at the meeting.
"Dasatinib may be inhibiting the emergence of resistance to aromatase inhibitor therapy," he said.
The researchers were unable to identify factors that explained why the clinical benefit rate was the same, but the progression-free survival was different for the two groups. The study was small, did not use a placebo, and permitted crossover; "all of these things could affect the results, and this is a preliminary exploratory study," Dr. Paul said.
Combining dasatinib with other aromatase inhibitors – fulvestrant or exemestane – has not improved progression-free survival benefit in women with metastatic breast cancer previously treated with a nonsteroidal aromatase inhibitor, Dr. Paul noted; thus, it may be important to give dasatinib the first time that patients are given an aromatase inhibitor. "Putative predictive biomarkers for benefit from dasatinib will be assessed in patients’ archival breast cancer tissues to help inform patient selection for future studies."
In additional findings from the trial, adding dasatinib appeared to attenuate the adverse impact of letrozole on bone health. At the end of the study, patients given the combination therapy were half as likely as their counterparts given letrozole alone to have osteopenia.
The findings on bone density suggest "an on-target effect of the Src inhibitor [dasatinib]," commented Dr. Carlos L. Arteaga, who moderated the press briefing. Dr. Arteaga is president-elect of the American Association for Cancer Research and associate director for translational/clinical research and director of the breast cancer program at Vanderbilt-Ingram Cancer Center in Nashville, Tenn., and a codirector of SABCS.
Dr. Paul noted that the observations occurred in an intent-to-treat analysis that included all patients according to their initial treatment assignment.
During a discussion after the presentation, one session attendee questioned the interpretation that dasatinib was inhibiting the emergence of resistance to letrozole and pointed out that 23% of patients who had progression on letrozole monotherapy had clinical benefit after crossing over to the combination. "You could also interpret the crossover data [as saying] that [dasatinib] simply treats endocrine resistance. So my question is, have you looked at the time to total progression? Have you looked at that 23% clinical benefit in the cross-over group? Because [dasatinib] may not stop resistance, it may just treat it."
Looking at the total time to progression for sequential vs. combined therapy, it may be that the time to progression curves really don’t separate quite so much, the discussant maintained.
Dr. Paul acknowledged that this alternate interpretation was possible and said that such analyses have not yet been done.
"Src is a pleiotropic nonreceptor tyrosine kinase involved in breast cancer invasion, proliferation, and survival. Membrane estrogen receptor-alpha complexes with Src and PI3 kinase to drive breast cancer growth and endocrine therapy resistance," Dr. Paul said, giving some background to the trial. "Src also regulates osteoclast-mediated bone turnover."
Dasatinib inhibits Src tyrosine kinase and is currently approved by the Food and Drug Administration for treatment of Philadelphia chromosome–positive acute lymphoblastic leukemia and chronic myelogenous leukemia.
Women enrolled in the trial were allowed to have had prior non–aromatase inhibitor endocrine therapy for metastatic disease; prior adjuvant aromatase inhibitor therapy if completed at least 1 year before study entry; and one prior chemotherapy regimen for metastatic disease.
After stratification by disease-free interval and prior receipt of tamoxifen, patients were randomized to receive letrozole plus dasatinib or letrozole alone on 28-day cycles.
Patients on letrozole alone were allowed to cross over if they experienced progression, and 56% ultimately did so, Dr. Paul reported.
About half of the women in each treatment arm had not received any prior chemotherapy; 60% of those randomized to the combination and 49% of those randomized to letrozole alone had not received any prior endocrine therapy.
The clinical benefit rate – consisting of complete responses, partial responses, and stable disease for at least 6 months – was 71% with dasatinib plus letrozole and 66% with letrozole alone, a nonsignificant difference.
However, in intent-to-treat analyses, median progression-free survival was more than twice as long with the combination (20.1 months vs. 9.9 months; exploratory hazard ratio, 0.69).
In each treatment arm, about one-third of patients had a bone density T score below –1.5 at baseline, and about one-third received bisphosphonates during the study. But, by the end of the study, the proportion of patients with a T score below –1.5 was smaller in those on combination therapy (14% vs. 32%).
"There were no unexpected toxicities associated with dasatinib," Dr. Paul commented. The addition of this agent was associated with higher rates of grade 3 rash, edema, fatigue, anemia, neutropenia, arthralgia, and pleural effusion, but there were no grade 4 toxicities. Overall, one-quarter of patients needed a dasatinib dose reduction.
Dr. Paul disclosed no relevant conflicts of interest. The trial was sponsored by Bristol-Myers Squibb, the makers of SPRYCEL (dasatinib).
SAN ANTONIO – The oral tyrosine kinase inhibitor dasatinib appears to increase the efficacy of first-line aromatase inhibitor therapy in women with advanced breast cancer, suggests a randomized phase 2 trial presented at the San Antonio Breast Cancer Symposium.
Adding dasatinib (SPRYCEL) to letrozole (Femara) did not improve the clinical benefit rate – the trial’s primary endpoint – among the 120 postmenopausal women with hormone receptor–positive, HER2-negative locally recurrent or metastatic breast cancer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
But the combination doubled patients’ median progression-free survival from 10 months to 20 months in an intent-to-treat analysis, Dr. Dev Paul of U.S. Oncology and the Rocky Mountain Cancer Centers in Denver reported in a session and a related press briefing at the meeting.
"Dasatinib may be inhibiting the emergence of resistance to aromatase inhibitor therapy," he said.
The researchers were unable to identify factors that explained why the clinical benefit rate was the same, but the progression-free survival was different for the two groups. The study was small, did not use a placebo, and permitted crossover; "all of these things could affect the results, and this is a preliminary exploratory study," Dr. Paul said.
Combining dasatinib with other aromatase inhibitors – fulvestrant or exemestane – has not improved progression-free survival benefit in women with metastatic breast cancer previously treated with a nonsteroidal aromatase inhibitor, Dr. Paul noted; thus, it may be important to give dasatinib the first time that patients are given an aromatase inhibitor. "Putative predictive biomarkers for benefit from dasatinib will be assessed in patients’ archival breast cancer tissues to help inform patient selection for future studies."
In additional findings from the trial, adding dasatinib appeared to attenuate the adverse impact of letrozole on bone health. At the end of the study, patients given the combination therapy were half as likely as their counterparts given letrozole alone to have osteopenia.
The findings on bone density suggest "an on-target effect of the Src inhibitor [dasatinib]," commented Dr. Carlos L. Arteaga, who moderated the press briefing. Dr. Arteaga is president-elect of the American Association for Cancer Research and associate director for translational/clinical research and director of the breast cancer program at Vanderbilt-Ingram Cancer Center in Nashville, Tenn., and a codirector of SABCS.
Dr. Paul noted that the observations occurred in an intent-to-treat analysis that included all patients according to their initial treatment assignment.
During a discussion after the presentation, one session attendee questioned the interpretation that dasatinib was inhibiting the emergence of resistance to letrozole and pointed out that 23% of patients who had progression on letrozole monotherapy had clinical benefit after crossing over to the combination. "You could also interpret the crossover data [as saying] that [dasatinib] simply treats endocrine resistance. So my question is, have you looked at the time to total progression? Have you looked at that 23% clinical benefit in the cross-over group? Because [dasatinib] may not stop resistance, it may just treat it."
Looking at the total time to progression for sequential vs. combined therapy, it may be that the time to progression curves really don’t separate quite so much, the discussant maintained.
Dr. Paul acknowledged that this alternate interpretation was possible and said that such analyses have not yet been done.
"Src is a pleiotropic nonreceptor tyrosine kinase involved in breast cancer invasion, proliferation, and survival. Membrane estrogen receptor-alpha complexes with Src and PI3 kinase to drive breast cancer growth and endocrine therapy resistance," Dr. Paul said, giving some background to the trial. "Src also regulates osteoclast-mediated bone turnover."
Dasatinib inhibits Src tyrosine kinase and is currently approved by the Food and Drug Administration for treatment of Philadelphia chromosome–positive acute lymphoblastic leukemia and chronic myelogenous leukemia.
Women enrolled in the trial were allowed to have had prior non–aromatase inhibitor endocrine therapy for metastatic disease; prior adjuvant aromatase inhibitor therapy if completed at least 1 year before study entry; and one prior chemotherapy regimen for metastatic disease.
After stratification by disease-free interval and prior receipt of tamoxifen, patients were randomized to receive letrozole plus dasatinib or letrozole alone on 28-day cycles.
Patients on letrozole alone were allowed to cross over if they experienced progression, and 56% ultimately did so, Dr. Paul reported.
About half of the women in each treatment arm had not received any prior chemotherapy; 60% of those randomized to the combination and 49% of those randomized to letrozole alone had not received any prior endocrine therapy.
The clinical benefit rate – consisting of complete responses, partial responses, and stable disease for at least 6 months – was 71% with dasatinib plus letrozole and 66% with letrozole alone, a nonsignificant difference.
However, in intent-to-treat analyses, median progression-free survival was more than twice as long with the combination (20.1 months vs. 9.9 months; exploratory hazard ratio, 0.69).
In each treatment arm, about one-third of patients had a bone density T score below –1.5 at baseline, and about one-third received bisphosphonates during the study. But, by the end of the study, the proportion of patients with a T score below –1.5 was smaller in those on combination therapy (14% vs. 32%).
"There were no unexpected toxicities associated with dasatinib," Dr. Paul commented. The addition of this agent was associated with higher rates of grade 3 rash, edema, fatigue, anemia, neutropenia, arthralgia, and pleural effusion, but there were no grade 4 toxicities. Overall, one-quarter of patients needed a dasatinib dose reduction.
Dr. Paul disclosed no relevant conflicts of interest. The trial was sponsored by Bristol-Myers Squibb, the makers of SPRYCEL (dasatinib).
AT SABCS 2013
Major finding: Adding dasatinib to letrozole did not improve the clinical benefit rate, but it more than doubled median progression-free survival (20.1 vs. 9.9 months).
Data source: A randomized phase II trial of 120 postmenopausal women with hormone receptor–positive, HER2-negative locally recurrent or metastatic breast cancer
Disclosures: Dr. Paul disclosed no relevant conflicts of interest. The trial was sponsored by Bristol-Myers Squibb, the makers of SPRYCEL (dasatinib).
VIDEO: Tumor-infiltrating lymphocytes may predict trastuzumab success
SAN ANTONIO – High levels of tumor-infiltrating lymphocytes in primary breast cancer are a good biomarker for favorable clinical response to trastuzumab, according to research presented at the San Antonio Breast Cancer Symposium.
In an exclusive interview with Frontline Medical News, Dr. Sherene Loi discusses the findings' clinical implications, including the potential that breast cancers could be amenable to immunotherapeutic approaches.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – High levels of tumor-infiltrating lymphocytes in primary breast cancer are a good biomarker for favorable clinical response to trastuzumab, according to research presented at the San Antonio Breast Cancer Symposium.
In an exclusive interview with Frontline Medical News, Dr. Sherene Loi discusses the findings' clinical implications, including the potential that breast cancers could be amenable to immunotherapeutic approaches.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – High levels of tumor-infiltrating lymphocytes in primary breast cancer are a good biomarker for favorable clinical response to trastuzumab, according to research presented at the San Antonio Breast Cancer Symposium.
In an exclusive interview with Frontline Medical News, Dr. Sherene Loi discusses the findings' clinical implications, including the potential that breast cancers could be amenable to immunotherapeutic approaches.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Radiotherapy can be omitted for many older breast cancer patients
SAN ANTONIO – Avoiding whole-breast radiation therapy is a reasonable – and even attractive – option for many older women with early-stage breast cancer, according to the results of the Postoperative Radiotherapy in Minimum-Risk Elderly (PRIME II) trial.
The patient population identified in PRIME II as being suitable for omission of postoperative radiotherapy on the basis of a relatively benign natural history consists of women aged 65 or older who are on adjuvant hormone therapy after undergoing lumpectomy with clear margins for estrogen receptor–rich, axillary node–negative breast cancer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PRIME II was a six-country trial in which 1,326 patients 65 or older with hormone receptor–positive early breast cancer were randomized to radiotherapy or no radiotherapy following breast-conserving surgery and endocrine therapy. The 5-year actuarial rate of ipsilateral breast cancer recurrence – the primary study endpoint – was 1.3% in those who received radiotherapy and 4.1% in those who did not, Dr. Ian H. Kunkler reported at the San Antonio Breast Cancer Symposium.
The 5-year actuarial rate of overall survival was 94.2% in patients randomized to radiotherapy and closely similar at 93.8% in the no-radiotherapy group, added Dr. Kunkler, professor of clinical oncology at the University of Edinburgh.
The relative benefit of radiotherapy was even smaller in the 91% of subjects who had estrogen-rich tumors as defined by an ER score of at least 7. They had a local recurrence rate of 3.2% with radiotherapy and 0.8% without. While that absolute 2.4% difference was statistically significant, it is arguably not clinically meaningful. For every 100 women who fit the description carefully defined in PRIME II and who undergo radiotherapy, three will have a recurrence prevented, one will have a recurrence anyway, and 96 will have had treatment that was not beneficial, he said.
"I think we’re really at the cusp of overtreatment here. I think it’s a matter for discussion between the physician and patient as to whether that very modest benefit is worth the potential complications of radiotherapy and the burdens of ongoing treatment, as well as the costs to the health service. Older patients find radiotherapy very burdensome, the relative benefits are very small, and there is no compromise in terms of overall survival with its omission," Dr. Kunkler said.
An important caveat: Among the 9% of patients with low estrogen receptor status, the local recurrence rate was 11.1% with no radiotherapy compared to zero with radiation.
"This is a group for whom radiotherapy should not be omitted," Dr. Kunkler declared.
More than one-half of all early breast cancers present in women aged 65 or older. While postoperative radiotherapy after lumpectomy has been the standard of care regardless of age and other risk factors, there has been only sparse high-quality supporting evidence for this practice in older patients.
Dr. Kunkler estimated that the PRIME II findings are generalizable to 60%-70% of all breast cancer patients over age 65. He predicted that the PRIME II study will "very likely" alter practice in the United Kingdom, and symposium codirector Dr. C. Kent Osborne predicted that the study will be practice changing in the United States as well.
"When I was in training, everybody thought that more was better: more drug treatment, more radiation, more surgery, high-dose chemotherapy, and bone marrow transplant. As we’ve evolved over the last 3 decades, that’s turning out not to be the case. I think we’re gradually doing less and less treatment, either with radiotherapy or with surgery, to control the local disease in appropriate patients. And I think more and more people will begin to accept it," said Dr. Osborne, director of the Dan L. Duncan Cancer Center and the Lester and Sue Smith Breast Center at Baylor College of Medicine, Houston.
PRIME II was funded by the Chief Scientist Office for Scotland. Dr. Kunkler declared having no conflicts of interest.
SAN ANTONIO – Avoiding whole-breast radiation therapy is a reasonable – and even attractive – option for many older women with early-stage breast cancer, according to the results of the Postoperative Radiotherapy in Minimum-Risk Elderly (PRIME II) trial.
The patient population identified in PRIME II as being suitable for omission of postoperative radiotherapy on the basis of a relatively benign natural history consists of women aged 65 or older who are on adjuvant hormone therapy after undergoing lumpectomy with clear margins for estrogen receptor–rich, axillary node–negative breast cancer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PRIME II was a six-country trial in which 1,326 patients 65 or older with hormone receptor–positive early breast cancer were randomized to radiotherapy or no radiotherapy following breast-conserving surgery and endocrine therapy. The 5-year actuarial rate of ipsilateral breast cancer recurrence – the primary study endpoint – was 1.3% in those who received radiotherapy and 4.1% in those who did not, Dr. Ian H. Kunkler reported at the San Antonio Breast Cancer Symposium.
The 5-year actuarial rate of overall survival was 94.2% in patients randomized to radiotherapy and closely similar at 93.8% in the no-radiotherapy group, added Dr. Kunkler, professor of clinical oncology at the University of Edinburgh.
The relative benefit of radiotherapy was even smaller in the 91% of subjects who had estrogen-rich tumors as defined by an ER score of at least 7. They had a local recurrence rate of 3.2% with radiotherapy and 0.8% without. While that absolute 2.4% difference was statistically significant, it is arguably not clinically meaningful. For every 100 women who fit the description carefully defined in PRIME II and who undergo radiotherapy, three will have a recurrence prevented, one will have a recurrence anyway, and 96 will have had treatment that was not beneficial, he said.
"I think we’re really at the cusp of overtreatment here. I think it’s a matter for discussion between the physician and patient as to whether that very modest benefit is worth the potential complications of radiotherapy and the burdens of ongoing treatment, as well as the costs to the health service. Older patients find radiotherapy very burdensome, the relative benefits are very small, and there is no compromise in terms of overall survival with its omission," Dr. Kunkler said.
An important caveat: Among the 9% of patients with low estrogen receptor status, the local recurrence rate was 11.1% with no radiotherapy compared to zero with radiation.
"This is a group for whom radiotherapy should not be omitted," Dr. Kunkler declared.
More than one-half of all early breast cancers present in women aged 65 or older. While postoperative radiotherapy after lumpectomy has been the standard of care regardless of age and other risk factors, there has been only sparse high-quality supporting evidence for this practice in older patients.
Dr. Kunkler estimated that the PRIME II findings are generalizable to 60%-70% of all breast cancer patients over age 65. He predicted that the PRIME II study will "very likely" alter practice in the United Kingdom, and symposium codirector Dr. C. Kent Osborne predicted that the study will be practice changing in the United States as well.
"When I was in training, everybody thought that more was better: more drug treatment, more radiation, more surgery, high-dose chemotherapy, and bone marrow transplant. As we’ve evolved over the last 3 decades, that’s turning out not to be the case. I think we’re gradually doing less and less treatment, either with radiotherapy or with surgery, to control the local disease in appropriate patients. And I think more and more people will begin to accept it," said Dr. Osborne, director of the Dan L. Duncan Cancer Center and the Lester and Sue Smith Breast Center at Baylor College of Medicine, Houston.
PRIME II was funded by the Chief Scientist Office for Scotland. Dr. Kunkler declared having no conflicts of interest.
SAN ANTONIO – Avoiding whole-breast radiation therapy is a reasonable – and even attractive – option for many older women with early-stage breast cancer, according to the results of the Postoperative Radiotherapy in Minimum-Risk Elderly (PRIME II) trial.
The patient population identified in PRIME II as being suitable for omission of postoperative radiotherapy on the basis of a relatively benign natural history consists of women aged 65 or older who are on adjuvant hormone therapy after undergoing lumpectomy with clear margins for estrogen receptor–rich, axillary node–negative breast cancer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PRIME II was a six-country trial in which 1,326 patients 65 or older with hormone receptor–positive early breast cancer were randomized to radiotherapy or no radiotherapy following breast-conserving surgery and endocrine therapy. The 5-year actuarial rate of ipsilateral breast cancer recurrence – the primary study endpoint – was 1.3% in those who received radiotherapy and 4.1% in those who did not, Dr. Ian H. Kunkler reported at the San Antonio Breast Cancer Symposium.
The 5-year actuarial rate of overall survival was 94.2% in patients randomized to radiotherapy and closely similar at 93.8% in the no-radiotherapy group, added Dr. Kunkler, professor of clinical oncology at the University of Edinburgh.
The relative benefit of radiotherapy was even smaller in the 91% of subjects who had estrogen-rich tumors as defined by an ER score of at least 7. They had a local recurrence rate of 3.2% with radiotherapy and 0.8% without. While that absolute 2.4% difference was statistically significant, it is arguably not clinically meaningful. For every 100 women who fit the description carefully defined in PRIME II and who undergo radiotherapy, three will have a recurrence prevented, one will have a recurrence anyway, and 96 will have had treatment that was not beneficial, he said.
"I think we’re really at the cusp of overtreatment here. I think it’s a matter for discussion between the physician and patient as to whether that very modest benefit is worth the potential complications of radiotherapy and the burdens of ongoing treatment, as well as the costs to the health service. Older patients find radiotherapy very burdensome, the relative benefits are very small, and there is no compromise in terms of overall survival with its omission," Dr. Kunkler said.
An important caveat: Among the 9% of patients with low estrogen receptor status, the local recurrence rate was 11.1% with no radiotherapy compared to zero with radiation.
"This is a group for whom radiotherapy should not be omitted," Dr. Kunkler declared.
More than one-half of all early breast cancers present in women aged 65 or older. While postoperative radiotherapy after lumpectomy has been the standard of care regardless of age and other risk factors, there has been only sparse high-quality supporting evidence for this practice in older patients.
Dr. Kunkler estimated that the PRIME II findings are generalizable to 60%-70% of all breast cancer patients over age 65. He predicted that the PRIME II study will "very likely" alter practice in the United Kingdom, and symposium codirector Dr. C. Kent Osborne predicted that the study will be practice changing in the United States as well.
"When I was in training, everybody thought that more was better: more drug treatment, more radiation, more surgery, high-dose chemotherapy, and bone marrow transplant. As we’ve evolved over the last 3 decades, that’s turning out not to be the case. I think we’re gradually doing less and less treatment, either with radiotherapy or with surgery, to control the local disease in appropriate patients. And I think more and more people will begin to accept it," said Dr. Osborne, director of the Dan L. Duncan Cancer Center and the Lester and Sue Smith Breast Center at Baylor College of Medicine, Houston.
PRIME II was funded by the Chief Scientist Office for Scotland. Dr. Kunkler declared having no conflicts of interest.
AT SABCS 2013
Major finding: The 5-year ipsilateral breast cancer recurrence rate in a selected population of older women undergoing breast-conserving surgery and adjuvant hormone therapy was 1.3% with postoperative radiotherapy and 4.1% without it, a modest difference that did not impact overall survival.
Data source: A prospective randomized trial in six countries, involving 1,326 patients aged 65 or older who underwent lumpectomy with clear margins for hormone receptor–positive, axillary node–negative breast cancer and were on adjuvant endocrine therapy. They were randomized to postoperative radiotherapy or no radiotherapy.
Disclosures: The PRIME II study was funded by the Chief Scientist Office for Scotland. The presenter reported having no financial conflicts.
Pathologic complete response to HER2 therapy portends better outcomes
SAN ANTONIO – Pathologic complete response is a strong prognostic factor in women with HER2-positive early breast cancer who receive neoadjuvant HER2-targeted therapy, investigators reported at the annual San Antonio Breast Cancer Symposium.
A team led by Dr. Martine Piccart-Gebhart, chair of the Breast International Group (BIG) in Brussels, analyzed data from 455 patients in the randomized NeoALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) trial, who received one or both HER2 therapies, along with chemotherapy.
With a median follow-up of almost 4 years, those who experienced a pathologic complete response (pCR) – meaning no detectable invasive disease in either the breast or axilla at surgery – were 62% less likely to have had events such as recurrences or second primaries, and 65% less likely to have died, she reported in a session and related press briefing.
The benefit of pCR was more pronounced among patients whose tumors were negative for hormone receptors than among their counterparts whose tumors were positive.
The new data should not change the standard of care, trastuzumab and chemotherapy, she cautioned. "But what this trial indicates to us is that it is possible that for this particular subgroup – hormone receptor–negative, HER2-positive – we could potentially use a neoadjuvant model to speed up approval of new agents. And that’s important because we know that adjuvant trials in breast cancer take forever and are very time- and money-consuming, and require thousands of patients."
Thus, the findings would lend support to the Food and Drug Administration’s use of pCR as the bar for accelerated approval of new drugs for this patient subgroup, she said.
Event-free and overall survival did not differ significantly according to the specific HER2 therapy received, although dual therapy tended to have an edge in patients with hormone receptor–negative tumors. However, the trial was not powered to detect moderate differences in these outcomes, according to Dr. Piccart-Gebhart.
"It is the very large ALTTO trial, with the recruitment of 8,300 women, that will provide a robust answer on the effect of dual HER2 blockade on long-term outcomes, and we expect to report these results at ASCO [American Society of Clinical Oncology] next year," she commented.
Analyses of NeoALTTO will be repeated after another 2.5 years, Dr. Piccart-Gebhart added. "It is possible that with longer follow-up, we will also see a bigger treatment effect in the hormone receptor–positive subgroup. But clearly, this trial provides further evidence that HER2-positive, hormone receptor–negative and HER2-positive, hormone receptor–positive groups are two different diseases."
Press briefing moderator Dr. Jennifer Litton of the department of breast medical oncology at the University of Texas MD Anderson Cancer Center, Houston, noted that pCR was used to gain initial approval of pertuzumab (Perjeta), another targeted therapy, in the neoadjuvant setting.
The reported data "in my opinion, show that it continues to be a very valid surrogate endpoint, and continues to [address the issue] of how we can develop new drugs, get them to patients quicker, with a smaller number of patients involved and less cost," she said.
Session attendee Dr. Steven E. Vogl, an oncologist in New York, asked whether pCR should be abandoned as a surrogate marker for the hormone receptor–positive subset. "It looks like the benefit of pCR was, at best, tiny in your study for those women, and probably we should think twice about using pCR in deciding which therapy is better – we should wait for event-free and overall survival. What do you think?"
Dr. Piccart-Gebhart replied that a meta-analysis of HER2 neoadjuvant therapy performed by the FDA with a much longer follow-up did find a benefit of pCR in the hormone receptor–positive subset. "It was less striking, but it was there. So I think that we have to be cautious because NeoALTTO is still a relatively small study.
"But I would tend to agree with you that the data look a lot stronger in the hormone receptor–negative subgroup, and there, based on the results I showed today, I feel confident that you could use the neoadjuvant model for accelerated approval of new drugs," she added. "For the hormone receptor–positive patients, I’m a little bit more cautious. I think these are slow actors. The events come later; you need a longer follow-up. And then of course the endocrine treatment is important and you give it after surgery in all these trials, which could be a mistake."
In the NeoALTTO study (also known as BIG 1-06), women with HER2-positive early breast cancer measuring at least 2 cm were randomly assigned to 18 weeks of neoadjuvant therapy with dual HER2 blockade using both lapatinib (Tykerb) and trastuzumab (Herceptin), or single HER2 blockade with one of the agents alone – each given along with paclitaxel.
After surgery, they received combination chemotherapy and then more of the same HER2 therapy out to 52 weeks, as well as hormonal therapy if indicated.
Initial results, previously reported, showed that the rate of pCR – the trial’s primary endpoint – was 24.7% with lapatinib alone, 29.5% with trastuzumab alone, and 51.3% with the combination, Dr. Piccart-Gebhart reported.
The new results showed that there were no significant differences between the treatment arms in the adjusted 3-year rates of event-free survival (78%, 76%, and 84%, respectively) or overall survival (93%, 90%, and 95%, respectively). But there was greater separation of the curves in the hormone receptor–negative subset, suggesting a possible treatment effect, according to Dr. Piccart-Gebhart.
In an analysis of all trial arms combined that used a starting point of 30 weeks after randomization, relative to patients who did not have a pCR, those who did were significantly less likely to have events (postoperative recurrence, second primary, or death, or progression in those who did not have surgery) (hazard ratio, 0.38; P = .0003) and to die (HR, 0.35; P = .005).
"There were no surprises" in terms of adverse events, she reported. "The adverse events were consistent with the known safety profiles of lapatinib and trastuzumab."
Over the entire study period, compared with trastuzumab alone, the combination of lapatinib plus trastuzumab led to higher rates of severe diarrhea, hepatobiliary adverse events, and rash, as well as a higher rate of cardiac events.
Dr. Piccart-Gebhart disclosed that she has received consulting fees from Roche, and her institution has received research funding from GlaxoSmithKline PLC and Roche. The trial was sponsored by GlaxoSmithKline.
SAN ANTONIO – Pathologic complete response is a strong prognostic factor in women with HER2-positive early breast cancer who receive neoadjuvant HER2-targeted therapy, investigators reported at the annual San Antonio Breast Cancer Symposium.
A team led by Dr. Martine Piccart-Gebhart, chair of the Breast International Group (BIG) in Brussels, analyzed data from 455 patients in the randomized NeoALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) trial, who received one or both HER2 therapies, along with chemotherapy.
With a median follow-up of almost 4 years, those who experienced a pathologic complete response (pCR) – meaning no detectable invasive disease in either the breast or axilla at surgery – were 62% less likely to have had events such as recurrences or second primaries, and 65% less likely to have died, she reported in a session and related press briefing.
The benefit of pCR was more pronounced among patients whose tumors were negative for hormone receptors than among their counterparts whose tumors were positive.
The new data should not change the standard of care, trastuzumab and chemotherapy, she cautioned. "But what this trial indicates to us is that it is possible that for this particular subgroup – hormone receptor–negative, HER2-positive – we could potentially use a neoadjuvant model to speed up approval of new agents. And that’s important because we know that adjuvant trials in breast cancer take forever and are very time- and money-consuming, and require thousands of patients."
Thus, the findings would lend support to the Food and Drug Administration’s use of pCR as the bar for accelerated approval of new drugs for this patient subgroup, she said.
Event-free and overall survival did not differ significantly according to the specific HER2 therapy received, although dual therapy tended to have an edge in patients with hormone receptor–negative tumors. However, the trial was not powered to detect moderate differences in these outcomes, according to Dr. Piccart-Gebhart.
"It is the very large ALTTO trial, with the recruitment of 8,300 women, that will provide a robust answer on the effect of dual HER2 blockade on long-term outcomes, and we expect to report these results at ASCO [American Society of Clinical Oncology] next year," she commented.
Analyses of NeoALTTO will be repeated after another 2.5 years, Dr. Piccart-Gebhart added. "It is possible that with longer follow-up, we will also see a bigger treatment effect in the hormone receptor–positive subgroup. But clearly, this trial provides further evidence that HER2-positive, hormone receptor–negative and HER2-positive, hormone receptor–positive groups are two different diseases."
Press briefing moderator Dr. Jennifer Litton of the department of breast medical oncology at the University of Texas MD Anderson Cancer Center, Houston, noted that pCR was used to gain initial approval of pertuzumab (Perjeta), another targeted therapy, in the neoadjuvant setting.
The reported data "in my opinion, show that it continues to be a very valid surrogate endpoint, and continues to [address the issue] of how we can develop new drugs, get them to patients quicker, with a smaller number of patients involved and less cost," she said.
Session attendee Dr. Steven E. Vogl, an oncologist in New York, asked whether pCR should be abandoned as a surrogate marker for the hormone receptor–positive subset. "It looks like the benefit of pCR was, at best, tiny in your study for those women, and probably we should think twice about using pCR in deciding which therapy is better – we should wait for event-free and overall survival. What do you think?"
Dr. Piccart-Gebhart replied that a meta-analysis of HER2 neoadjuvant therapy performed by the FDA with a much longer follow-up did find a benefit of pCR in the hormone receptor–positive subset. "It was less striking, but it was there. So I think that we have to be cautious because NeoALTTO is still a relatively small study.
"But I would tend to agree with you that the data look a lot stronger in the hormone receptor–negative subgroup, and there, based on the results I showed today, I feel confident that you could use the neoadjuvant model for accelerated approval of new drugs," she added. "For the hormone receptor–positive patients, I’m a little bit more cautious. I think these are slow actors. The events come later; you need a longer follow-up. And then of course the endocrine treatment is important and you give it after surgery in all these trials, which could be a mistake."
In the NeoALTTO study (also known as BIG 1-06), women with HER2-positive early breast cancer measuring at least 2 cm were randomly assigned to 18 weeks of neoadjuvant therapy with dual HER2 blockade using both lapatinib (Tykerb) and trastuzumab (Herceptin), or single HER2 blockade with one of the agents alone – each given along with paclitaxel.
After surgery, they received combination chemotherapy and then more of the same HER2 therapy out to 52 weeks, as well as hormonal therapy if indicated.
Initial results, previously reported, showed that the rate of pCR – the trial’s primary endpoint – was 24.7% with lapatinib alone, 29.5% with trastuzumab alone, and 51.3% with the combination, Dr. Piccart-Gebhart reported.
The new results showed that there were no significant differences between the treatment arms in the adjusted 3-year rates of event-free survival (78%, 76%, and 84%, respectively) or overall survival (93%, 90%, and 95%, respectively). But there was greater separation of the curves in the hormone receptor–negative subset, suggesting a possible treatment effect, according to Dr. Piccart-Gebhart.
In an analysis of all trial arms combined that used a starting point of 30 weeks after randomization, relative to patients who did not have a pCR, those who did were significantly less likely to have events (postoperative recurrence, second primary, or death, or progression in those who did not have surgery) (hazard ratio, 0.38; P = .0003) and to die (HR, 0.35; P = .005).
"There were no surprises" in terms of adverse events, she reported. "The adverse events were consistent with the known safety profiles of lapatinib and trastuzumab."
Over the entire study period, compared with trastuzumab alone, the combination of lapatinib plus trastuzumab led to higher rates of severe diarrhea, hepatobiliary adverse events, and rash, as well as a higher rate of cardiac events.
Dr. Piccart-Gebhart disclosed that she has received consulting fees from Roche, and her institution has received research funding from GlaxoSmithKline PLC and Roche. The trial was sponsored by GlaxoSmithKline.
SAN ANTONIO – Pathologic complete response is a strong prognostic factor in women with HER2-positive early breast cancer who receive neoadjuvant HER2-targeted therapy, investigators reported at the annual San Antonio Breast Cancer Symposium.
A team led by Dr. Martine Piccart-Gebhart, chair of the Breast International Group (BIG) in Brussels, analyzed data from 455 patients in the randomized NeoALTTO (Neoadjuvant Lapatinib and/or Trastuzumab Treatment Optimisation) trial, who received one or both HER2 therapies, along with chemotherapy.
With a median follow-up of almost 4 years, those who experienced a pathologic complete response (pCR) – meaning no detectable invasive disease in either the breast or axilla at surgery – were 62% less likely to have had events such as recurrences or second primaries, and 65% less likely to have died, she reported in a session and related press briefing.
The benefit of pCR was more pronounced among patients whose tumors were negative for hormone receptors than among their counterparts whose tumors were positive.
The new data should not change the standard of care, trastuzumab and chemotherapy, she cautioned. "But what this trial indicates to us is that it is possible that for this particular subgroup – hormone receptor–negative, HER2-positive – we could potentially use a neoadjuvant model to speed up approval of new agents. And that’s important because we know that adjuvant trials in breast cancer take forever and are very time- and money-consuming, and require thousands of patients."
Thus, the findings would lend support to the Food and Drug Administration’s use of pCR as the bar for accelerated approval of new drugs for this patient subgroup, she said.
Event-free and overall survival did not differ significantly according to the specific HER2 therapy received, although dual therapy tended to have an edge in patients with hormone receptor–negative tumors. However, the trial was not powered to detect moderate differences in these outcomes, according to Dr. Piccart-Gebhart.
"It is the very large ALTTO trial, with the recruitment of 8,300 women, that will provide a robust answer on the effect of dual HER2 blockade on long-term outcomes, and we expect to report these results at ASCO [American Society of Clinical Oncology] next year," she commented.
Analyses of NeoALTTO will be repeated after another 2.5 years, Dr. Piccart-Gebhart added. "It is possible that with longer follow-up, we will also see a bigger treatment effect in the hormone receptor–positive subgroup. But clearly, this trial provides further evidence that HER2-positive, hormone receptor–negative and HER2-positive, hormone receptor–positive groups are two different diseases."
Press briefing moderator Dr. Jennifer Litton of the department of breast medical oncology at the University of Texas MD Anderson Cancer Center, Houston, noted that pCR was used to gain initial approval of pertuzumab (Perjeta), another targeted therapy, in the neoadjuvant setting.
The reported data "in my opinion, show that it continues to be a very valid surrogate endpoint, and continues to [address the issue] of how we can develop new drugs, get them to patients quicker, with a smaller number of patients involved and less cost," she said.
Session attendee Dr. Steven E. Vogl, an oncologist in New York, asked whether pCR should be abandoned as a surrogate marker for the hormone receptor–positive subset. "It looks like the benefit of pCR was, at best, tiny in your study for those women, and probably we should think twice about using pCR in deciding which therapy is better – we should wait for event-free and overall survival. What do you think?"
Dr. Piccart-Gebhart replied that a meta-analysis of HER2 neoadjuvant therapy performed by the FDA with a much longer follow-up did find a benefit of pCR in the hormone receptor–positive subset. "It was less striking, but it was there. So I think that we have to be cautious because NeoALTTO is still a relatively small study.
"But I would tend to agree with you that the data look a lot stronger in the hormone receptor–negative subgroup, and there, based on the results I showed today, I feel confident that you could use the neoadjuvant model for accelerated approval of new drugs," she added. "For the hormone receptor–positive patients, I’m a little bit more cautious. I think these are slow actors. The events come later; you need a longer follow-up. And then of course the endocrine treatment is important and you give it after surgery in all these trials, which could be a mistake."
In the NeoALTTO study (also known as BIG 1-06), women with HER2-positive early breast cancer measuring at least 2 cm were randomly assigned to 18 weeks of neoadjuvant therapy with dual HER2 blockade using both lapatinib (Tykerb) and trastuzumab (Herceptin), or single HER2 blockade with one of the agents alone – each given along with paclitaxel.
After surgery, they received combination chemotherapy and then more of the same HER2 therapy out to 52 weeks, as well as hormonal therapy if indicated.
Initial results, previously reported, showed that the rate of pCR – the trial’s primary endpoint – was 24.7% with lapatinib alone, 29.5% with trastuzumab alone, and 51.3% with the combination, Dr. Piccart-Gebhart reported.
The new results showed that there were no significant differences between the treatment arms in the adjusted 3-year rates of event-free survival (78%, 76%, and 84%, respectively) or overall survival (93%, 90%, and 95%, respectively). But there was greater separation of the curves in the hormone receptor–negative subset, suggesting a possible treatment effect, according to Dr. Piccart-Gebhart.
In an analysis of all trial arms combined that used a starting point of 30 weeks after randomization, relative to patients who did not have a pCR, those who did were significantly less likely to have events (postoperative recurrence, second primary, or death, or progression in those who did not have surgery) (hazard ratio, 0.38; P = .0003) and to die (HR, 0.35; P = .005).
"There were no surprises" in terms of adverse events, she reported. "The adverse events were consistent with the known safety profiles of lapatinib and trastuzumab."
Over the entire study period, compared with trastuzumab alone, the combination of lapatinib plus trastuzumab led to higher rates of severe diarrhea, hepatobiliary adverse events, and rash, as well as a higher rate of cardiac events.
Dr. Piccart-Gebhart disclosed that she has received consulting fees from Roche, and her institution has received research funding from GlaxoSmithKline PLC and Roche. The trial was sponsored by GlaxoSmithKline.
AT SABCS 2013
Major Finding: Women who experienced a pCR were 62% less likely to have events such as recurrences and second primaries, and 65% less likely to die.
Data Source: A randomized trial of neoadjuvant HER2 blockade in 455 women with early HER2-positive breast cancer (the NeoALTTO/BIG 1-06 study).
Disclosures: Dr. Piccart-Gebhart disclosed that she has received consulting fees from Roche, and her institution has received research funding from GlaxoSmithKline PLC and Roche. The trial was sponsored by GlaxoSmithKline.