User login
Team approach best for making purchasing decisions
SAN FRANCISCO – The best decisions about purchasing supplies and equipment for a hospital or medical practice come from administrators and physicians working together as a team, speaker after speaker reiterated during the session on supply chain purchasing decisions at the 2015 AGA Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology.
“In this era of cost constraints, how can physicians and facilities align purchasing decisions so that everybody’s working together to provide the right service for the right patient in the right setting, in the right amount, and at the right time?” asked session moderator Dr. Joel V. Brill, a board-certified internist and gastroenterologist who is medical director of FAIR Health Inc. “Does that mean reducing inventory and suppliers of items? Reducing choice of equipment? Not being able to provide a new technology or procedure to patients? Even of more critical importance is that, if physicians and facilities don’t work collaboratively to address these issues, if you’re not providing a high-quality, value-driven product, you’re in danger of being priced out of the marketplace or deselected from the provider network.”
Kenneth Strople, a health care consultant with more than 25 years of experience as a hospital COO and CEO, advises administrators and physicians to consider a key question: “What is the desired device or equipment going to do for patient care? Is it going to improve the quality of care provided in the facility? Patient care is No. 1; that’s why we do what we do. The latest and greatest is not necessarily the best for your organization and patients.”
He shared three examples of purchasing decisions that can go awry, the first being a surgical robot, which creates increased operating room time and a significant increase in consumables: “$3,500 per case for which there is no additional reimbursement,” said Mr. Strople, who is managing director of California-based Palisades Healthcare Solutions LLC. Buying a surgical robot may enhance marketing efforts and reputation of your hospital or health care group, he acknowledged, but if it’s going to cost your organization additional funds every time the device is used, “my question is, why?,” he said. “Margins in hospitals are quite small. In California, for example, if you’re making 4%-5% on revenue you’re doing great. So when the cost per case increases substantially [hospital administrators] have a problem, because you can’t lose money on every case and make it up in volume.”
The second example he discussed was cardiac stents for balloon angioplasty. When first introduced to the marketplace there was minimal ability for hospitals to receive reimbursement for stents (which cost about $3,000 each), due to the fact that no DRG existed for them. “Commercial contracts didn’t account for the new technology, so hospitals ‘ate’ the cost of stents for a protracted period of time until the contracts could be adjusted,” Mr. Strople said.
Another key purchasing decision involves variation in cost among medical devices on the marketplace. For example, is the $15,000 total knee implant from company A better than the $9,000 implant from company B? “Maybe in some cases, but the sales rep will tell you the one from company A is better, and the rep is in the OR to coordinate things,” Mr. Strople said. “In fact, the rep’s commission may actually be more than the physician fee for the procedure.” He noted that reference pricing and purchaser transparency initiatives are beginning to impact patient behavior, “especially when the additional $6,000 cost is 100% patient responsibility.” The best way to approach such a purchasing decision is to call a meeting with all of the clinicians who use the device or equipment being considered. “Show them what the devices cost, and then you get them to make the responsible decision,” Mr. Strople said. “If you provide them the appropriate data and they believe the data, they’ll make the right decision. They’re not interested in causing the hospital to go out of business.”
Mr. Strople emphasized the importance of putting politics aside when making important purchasing decisions. For example, Dr. Jones may wield more political clout in a hospital than Dr. Smith does, but “a smart hospital administrator isn’t going to go out and just buy that device for Dr. Jones. He’s going to do the analysis, treat everybody the same, and say, ‘bring me the patient-related data and bring me the financial data. I will validate the financial data. I will evaluate cost, revenue, and the feedback I get on the patient care side.”
Another speaker, David A. Pierce, senior vice president and president of the endoscopy division for Boston Scientific in Marlboro, Mass., said that since the passage of the Affordable Care Act, “the future is going to be population health, so [device manufacturing] models and structures are going to have to be built to survive in capitation and bundling situations. For a device company, we’re going to have to demonstrate better outcomes and the ability to manage patients across the entire continuum.” He went on to note that “our whole existence has been in a price mix world, where innovation and technology have always been taken in [and] reimbursed aggressively. But in a population management world, we have to [adopt] a volume-based mindset; we have to make that switch. Our cost structure is probably misaligned with that reality that’s on the horizon. Return on investment for R&D is decreasing all the time.”
Dr. Brill, who is based in Paradise Valley, Ariz., characterized the supply chain purchasing environment as evolutionary. “Traditionally, the hospital was the workshop where the physician did his or her work,” Dr. Brill said. “You came in and performed surgery there, but the hospital still made the purchasing decisions. While the Ambulatory Surgery Center is often more physician-centric, frequently focusing on one specialty such as orthopedics or gastroenterology or ophthalmology, it still has the same issues of staffing and purchasing supplies and capital equipment. As we start thinking about the transition from volume to value, supply chain purchasing is of critical importance, for the margins and overhead will impact what you are willing to contract for with a purchaser or payer when providing an episode or bundled payment for a service, whether an endoscopic procedure or a condition such as reflux or obesity. As we move from silos to collaboration, health care is becoming a team sport: purchasing decisions are a critical component of health care professionals and facilities working together in order to achieve the desired outcomes, demonstrate value, and to drive patient volume.”
On Twitter @dougbrunk
SAN FRANCISCO – The best decisions about purchasing supplies and equipment for a hospital or medical practice come from administrators and physicians working together as a team, speaker after speaker reiterated during the session on supply chain purchasing decisions at the 2015 AGA Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology.
“In this era of cost constraints, how can physicians and facilities align purchasing decisions so that everybody’s working together to provide the right service for the right patient in the right setting, in the right amount, and at the right time?” asked session moderator Dr. Joel V. Brill, a board-certified internist and gastroenterologist who is medical director of FAIR Health Inc. “Does that mean reducing inventory and suppliers of items? Reducing choice of equipment? Not being able to provide a new technology or procedure to patients? Even of more critical importance is that, if physicians and facilities don’t work collaboratively to address these issues, if you’re not providing a high-quality, value-driven product, you’re in danger of being priced out of the marketplace or deselected from the provider network.”
Kenneth Strople, a health care consultant with more than 25 years of experience as a hospital COO and CEO, advises administrators and physicians to consider a key question: “What is the desired device or equipment going to do for patient care? Is it going to improve the quality of care provided in the facility? Patient care is No. 1; that’s why we do what we do. The latest and greatest is not necessarily the best for your organization and patients.”
He shared three examples of purchasing decisions that can go awry, the first being a surgical robot, which creates increased operating room time and a significant increase in consumables: “$3,500 per case for which there is no additional reimbursement,” said Mr. Strople, who is managing director of California-based Palisades Healthcare Solutions LLC. Buying a surgical robot may enhance marketing efforts and reputation of your hospital or health care group, he acknowledged, but if it’s going to cost your organization additional funds every time the device is used, “my question is, why?,” he said. “Margins in hospitals are quite small. In California, for example, if you’re making 4%-5% on revenue you’re doing great. So when the cost per case increases substantially [hospital administrators] have a problem, because you can’t lose money on every case and make it up in volume.”
The second example he discussed was cardiac stents for balloon angioplasty. When first introduced to the marketplace there was minimal ability for hospitals to receive reimbursement for stents (which cost about $3,000 each), due to the fact that no DRG existed for them. “Commercial contracts didn’t account for the new technology, so hospitals ‘ate’ the cost of stents for a protracted period of time until the contracts could be adjusted,” Mr. Strople said.
Another key purchasing decision involves variation in cost among medical devices on the marketplace. For example, is the $15,000 total knee implant from company A better than the $9,000 implant from company B? “Maybe in some cases, but the sales rep will tell you the one from company A is better, and the rep is in the OR to coordinate things,” Mr. Strople said. “In fact, the rep’s commission may actually be more than the physician fee for the procedure.” He noted that reference pricing and purchaser transparency initiatives are beginning to impact patient behavior, “especially when the additional $6,000 cost is 100% patient responsibility.” The best way to approach such a purchasing decision is to call a meeting with all of the clinicians who use the device or equipment being considered. “Show them what the devices cost, and then you get them to make the responsible decision,” Mr. Strople said. “If you provide them the appropriate data and they believe the data, they’ll make the right decision. They’re not interested in causing the hospital to go out of business.”
Mr. Strople emphasized the importance of putting politics aside when making important purchasing decisions. For example, Dr. Jones may wield more political clout in a hospital than Dr. Smith does, but “a smart hospital administrator isn’t going to go out and just buy that device for Dr. Jones. He’s going to do the analysis, treat everybody the same, and say, ‘bring me the patient-related data and bring me the financial data. I will validate the financial data. I will evaluate cost, revenue, and the feedback I get on the patient care side.”
Another speaker, David A. Pierce, senior vice president and president of the endoscopy division for Boston Scientific in Marlboro, Mass., said that since the passage of the Affordable Care Act, “the future is going to be population health, so [device manufacturing] models and structures are going to have to be built to survive in capitation and bundling situations. For a device company, we’re going to have to demonstrate better outcomes and the ability to manage patients across the entire continuum.” He went on to note that “our whole existence has been in a price mix world, where innovation and technology have always been taken in [and] reimbursed aggressively. But in a population management world, we have to [adopt] a volume-based mindset; we have to make that switch. Our cost structure is probably misaligned with that reality that’s on the horizon. Return on investment for R&D is decreasing all the time.”
Dr. Brill, who is based in Paradise Valley, Ariz., characterized the supply chain purchasing environment as evolutionary. “Traditionally, the hospital was the workshop where the physician did his or her work,” Dr. Brill said. “You came in and performed surgery there, but the hospital still made the purchasing decisions. While the Ambulatory Surgery Center is often more physician-centric, frequently focusing on one specialty such as orthopedics or gastroenterology or ophthalmology, it still has the same issues of staffing and purchasing supplies and capital equipment. As we start thinking about the transition from volume to value, supply chain purchasing is of critical importance, for the margins and overhead will impact what you are willing to contract for with a purchaser or payer when providing an episode or bundled payment for a service, whether an endoscopic procedure or a condition such as reflux or obesity. As we move from silos to collaboration, health care is becoming a team sport: purchasing decisions are a critical component of health care professionals and facilities working together in order to achieve the desired outcomes, demonstrate value, and to drive patient volume.”
On Twitter @dougbrunk
SAN FRANCISCO – The best decisions about purchasing supplies and equipment for a hospital or medical practice come from administrators and physicians working together as a team, speaker after speaker reiterated during the session on supply chain purchasing decisions at the 2015 AGA Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology.
“In this era of cost constraints, how can physicians and facilities align purchasing decisions so that everybody’s working together to provide the right service for the right patient in the right setting, in the right amount, and at the right time?” asked session moderator Dr. Joel V. Brill, a board-certified internist and gastroenterologist who is medical director of FAIR Health Inc. “Does that mean reducing inventory and suppliers of items? Reducing choice of equipment? Not being able to provide a new technology or procedure to patients? Even of more critical importance is that, if physicians and facilities don’t work collaboratively to address these issues, if you’re not providing a high-quality, value-driven product, you’re in danger of being priced out of the marketplace or deselected from the provider network.”
Kenneth Strople, a health care consultant with more than 25 years of experience as a hospital COO and CEO, advises administrators and physicians to consider a key question: “What is the desired device or equipment going to do for patient care? Is it going to improve the quality of care provided in the facility? Patient care is No. 1; that’s why we do what we do. The latest and greatest is not necessarily the best for your organization and patients.”
He shared three examples of purchasing decisions that can go awry, the first being a surgical robot, which creates increased operating room time and a significant increase in consumables: “$3,500 per case for which there is no additional reimbursement,” said Mr. Strople, who is managing director of California-based Palisades Healthcare Solutions LLC. Buying a surgical robot may enhance marketing efforts and reputation of your hospital or health care group, he acknowledged, but if it’s going to cost your organization additional funds every time the device is used, “my question is, why?,” he said. “Margins in hospitals are quite small. In California, for example, if you’re making 4%-5% on revenue you’re doing great. So when the cost per case increases substantially [hospital administrators] have a problem, because you can’t lose money on every case and make it up in volume.”
The second example he discussed was cardiac stents for balloon angioplasty. When first introduced to the marketplace there was minimal ability for hospitals to receive reimbursement for stents (which cost about $3,000 each), due to the fact that no DRG existed for them. “Commercial contracts didn’t account for the new technology, so hospitals ‘ate’ the cost of stents for a protracted period of time until the contracts could be adjusted,” Mr. Strople said.
Another key purchasing decision involves variation in cost among medical devices on the marketplace. For example, is the $15,000 total knee implant from company A better than the $9,000 implant from company B? “Maybe in some cases, but the sales rep will tell you the one from company A is better, and the rep is in the OR to coordinate things,” Mr. Strople said. “In fact, the rep’s commission may actually be more than the physician fee for the procedure.” He noted that reference pricing and purchaser transparency initiatives are beginning to impact patient behavior, “especially when the additional $6,000 cost is 100% patient responsibility.” The best way to approach such a purchasing decision is to call a meeting with all of the clinicians who use the device or equipment being considered. “Show them what the devices cost, and then you get them to make the responsible decision,” Mr. Strople said. “If you provide them the appropriate data and they believe the data, they’ll make the right decision. They’re not interested in causing the hospital to go out of business.”
Mr. Strople emphasized the importance of putting politics aside when making important purchasing decisions. For example, Dr. Jones may wield more political clout in a hospital than Dr. Smith does, but “a smart hospital administrator isn’t going to go out and just buy that device for Dr. Jones. He’s going to do the analysis, treat everybody the same, and say, ‘bring me the patient-related data and bring me the financial data. I will validate the financial data. I will evaluate cost, revenue, and the feedback I get on the patient care side.”
Another speaker, David A. Pierce, senior vice president and president of the endoscopy division for Boston Scientific in Marlboro, Mass., said that since the passage of the Affordable Care Act, “the future is going to be population health, so [device manufacturing] models and structures are going to have to be built to survive in capitation and bundling situations. For a device company, we’re going to have to demonstrate better outcomes and the ability to manage patients across the entire continuum.” He went on to note that “our whole existence has been in a price mix world, where innovation and technology have always been taken in [and] reimbursed aggressively. But in a population management world, we have to [adopt] a volume-based mindset; we have to make that switch. Our cost structure is probably misaligned with that reality that’s on the horizon. Return on investment for R&D is decreasing all the time.”
Dr. Brill, who is based in Paradise Valley, Ariz., characterized the supply chain purchasing environment as evolutionary. “Traditionally, the hospital was the workshop where the physician did his or her work,” Dr. Brill said. “You came in and performed surgery there, but the hospital still made the purchasing decisions. While the Ambulatory Surgery Center is often more physician-centric, frequently focusing on one specialty such as orthopedics or gastroenterology or ophthalmology, it still has the same issues of staffing and purchasing supplies and capital equipment. As we start thinking about the transition from volume to value, supply chain purchasing is of critical importance, for the margins and overhead will impact what you are willing to contract for with a purchaser or payer when providing an episode or bundled payment for a service, whether an endoscopic procedure or a condition such as reflux or obesity. As we move from silos to collaboration, health care is becoming a team sport: purchasing decisions are a critical component of health care professionals and facilities working together in order to achieve the desired outcomes, demonstrate value, and to drive patient volume.”
On Twitter @dougbrunk
AT THE 2015 AGA TECH SUMMIT
VIDEO: What makes people predictably irrational?
SAN FRANCISCO – Using behavioral economics to help patients identify and overcome psychological barriers to behavioral change has expanded our understanding of what makes people predictably irrational, according to Dr. David A. Asch.
In an interview at the 2015 AGA Tech Summit, Dr. Asch, executive director of the Penn Medicine Center for Health Care Innovation and professor of health care management at the Wharton School at the University of Pennsylvania, both in Philadelphia, discusses how physicians can incorporate the principles of behavioral economics into their practices to improve patient care.
Dr. Asch disclosed that he is a principal at VAL Health, a behavioral consulting firm.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @dougbrunk
SAN FRANCISCO – Using behavioral economics to help patients identify and overcome psychological barriers to behavioral change has expanded our understanding of what makes people predictably irrational, according to Dr. David A. Asch.
In an interview at the 2015 AGA Tech Summit, Dr. Asch, executive director of the Penn Medicine Center for Health Care Innovation and professor of health care management at the Wharton School at the University of Pennsylvania, both in Philadelphia, discusses how physicians can incorporate the principles of behavioral economics into their practices to improve patient care.
Dr. Asch disclosed that he is a principal at VAL Health, a behavioral consulting firm.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @dougbrunk
SAN FRANCISCO – Using behavioral economics to help patients identify and overcome psychological barriers to behavioral change has expanded our understanding of what makes people predictably irrational, according to Dr. David A. Asch.
In an interview at the 2015 AGA Tech Summit, Dr. Asch, executive director of the Penn Medicine Center for Health Care Innovation and professor of health care management at the Wharton School at the University of Pennsylvania, both in Philadelphia, discusses how physicians can incorporate the principles of behavioral economics into their practices to improve patient care.
Dr. Asch disclosed that he is a principal at VAL Health, a behavioral consulting firm.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @dougbrunk
AT THE 2015 AGA TECH SUMMIT
Bioprinted organs – a made-to-order future
SAN FRANCISCO – Gastroenterology might be the proving ground of one of medicine’s boldest new ventures – the creation of fully functional human organs built with a 3-D printer.
Gut is a perfect beginning project, Dr. John Geibel said at the 2015 AGA Tech Summit, which is sponsored by the AGA Center for GI Innovation and Technology. It has a very simple shape – just a long hollow tube. Epithelial cells grow and turn over very quickly, suggesting that a length of artificial intestine could be grown relatively quickly. And although intestine is composed of a number of distinct layers, a 3-D bioprinter would have no trouble laying down concentric circles of each one to recreate their natural morphology.
“It will take time. It will take planning. But this is going to happen,” said Dr. Geibel of Yale University, New Haven, Conn.
To create a length of intestine, the print heads of a bioprinter would be loaded with cells from all of the gut layers – the serosa, the different muscle strata, the mucosa. Each would be laid down in its respective anatomic ring, supported all around by a hydrogel. The print sequence would be repeated over and over until the required length of intestine was created. From then, Dr. Geibel said, it would be only a matter of days before the cells knit themselves together so well that the gel could be dissolved and the new tissue ready for transplant.
Liver would likely be the next organ up for printing, with the ultimate goal of creating fully transplantable organs. The need is enormous, and can’t be overstated. Patients who need a new liver wait an average of 4 years before they receive one. Even then, there is no guarantee that the selected organ will function as it should. Rejection is always a concern. Immunosuppression is a lifelong challenge.
The liver is much more complicated than a length of gut. It is cellularly complex and highly vascularized. But liver-printing is already a reality. Bioprinted “3-D liver-in-a-dish,” created by San Diego–based Organovo, has function, if not form. The cells work together; they grow, divide, and secrete bile acids. However, they exist as a formless, nonvascular blob.
As it stands (or rather, lies) now, bioprinted liver is a perfect preclinical model – perfectly replicating how the liver would respond to drugs without any of the messy adverse events that hurt patients. But it needs some backbone, or more accurately, some matrix, in order to morph again and grow into a complete organ. A liver-shaped collagen matrix could provide the necessary frame for cells to grow in and around; tunnels through it would form pathways for a similarly engineered vasculature.
The logical leap between dish and transplant is likely to be some sort of nonformed “piggyback liver,” which could be used as a bridge to keep liver failure patients alive until a traditional donor organ is found, Dr. Geibel said.
The Holy Grail, of course, would be a transplantable organ, created from the patient’s own cells – a made-to-order liver that’s 100% compatible with the recipient, who is actually also the donor.
“Think of the difference in quality of life these could make for patients who need transplants. The possibility of living without the fear of organ rejection, without the need for lifelong immunosuppression. Without the fear of having no alternative if the organ didn’t function well, or failed. If it did, we could simply grow another and try again.”
SAN FRANCISCO – Gastroenterology might be the proving ground of one of medicine’s boldest new ventures – the creation of fully functional human organs built with a 3-D printer.
Gut is a perfect beginning project, Dr. John Geibel said at the 2015 AGA Tech Summit, which is sponsored by the AGA Center for GI Innovation and Technology. It has a very simple shape – just a long hollow tube. Epithelial cells grow and turn over very quickly, suggesting that a length of artificial intestine could be grown relatively quickly. And although intestine is composed of a number of distinct layers, a 3-D bioprinter would have no trouble laying down concentric circles of each one to recreate their natural morphology.
“It will take time. It will take planning. But this is going to happen,” said Dr. Geibel of Yale University, New Haven, Conn.
To create a length of intestine, the print heads of a bioprinter would be loaded with cells from all of the gut layers – the serosa, the different muscle strata, the mucosa. Each would be laid down in its respective anatomic ring, supported all around by a hydrogel. The print sequence would be repeated over and over until the required length of intestine was created. From then, Dr. Geibel said, it would be only a matter of days before the cells knit themselves together so well that the gel could be dissolved and the new tissue ready for transplant.
Liver would likely be the next organ up for printing, with the ultimate goal of creating fully transplantable organs. The need is enormous, and can’t be overstated. Patients who need a new liver wait an average of 4 years before they receive one. Even then, there is no guarantee that the selected organ will function as it should. Rejection is always a concern. Immunosuppression is a lifelong challenge.
The liver is much more complicated than a length of gut. It is cellularly complex and highly vascularized. But liver-printing is already a reality. Bioprinted “3-D liver-in-a-dish,” created by San Diego–based Organovo, has function, if not form. The cells work together; they grow, divide, and secrete bile acids. However, they exist as a formless, nonvascular blob.
As it stands (or rather, lies) now, bioprinted liver is a perfect preclinical model – perfectly replicating how the liver would respond to drugs without any of the messy adverse events that hurt patients. But it needs some backbone, or more accurately, some matrix, in order to morph again and grow into a complete organ. A liver-shaped collagen matrix could provide the necessary frame for cells to grow in and around; tunnels through it would form pathways for a similarly engineered vasculature.
The logical leap between dish and transplant is likely to be some sort of nonformed “piggyback liver,” which could be used as a bridge to keep liver failure patients alive until a traditional donor organ is found, Dr. Geibel said.
The Holy Grail, of course, would be a transplantable organ, created from the patient’s own cells – a made-to-order liver that’s 100% compatible with the recipient, who is actually also the donor.
“Think of the difference in quality of life these could make for patients who need transplants. The possibility of living without the fear of organ rejection, without the need for lifelong immunosuppression. Without the fear of having no alternative if the organ didn’t function well, or failed. If it did, we could simply grow another and try again.”
SAN FRANCISCO – Gastroenterology might be the proving ground of one of medicine’s boldest new ventures – the creation of fully functional human organs built with a 3-D printer.
Gut is a perfect beginning project, Dr. John Geibel said at the 2015 AGA Tech Summit, which is sponsored by the AGA Center for GI Innovation and Technology. It has a very simple shape – just a long hollow tube. Epithelial cells grow and turn over very quickly, suggesting that a length of artificial intestine could be grown relatively quickly. And although intestine is composed of a number of distinct layers, a 3-D bioprinter would have no trouble laying down concentric circles of each one to recreate their natural morphology.
“It will take time. It will take planning. But this is going to happen,” said Dr. Geibel of Yale University, New Haven, Conn.
To create a length of intestine, the print heads of a bioprinter would be loaded with cells from all of the gut layers – the serosa, the different muscle strata, the mucosa. Each would be laid down in its respective anatomic ring, supported all around by a hydrogel. The print sequence would be repeated over and over until the required length of intestine was created. From then, Dr. Geibel said, it would be only a matter of days before the cells knit themselves together so well that the gel could be dissolved and the new tissue ready for transplant.
Liver would likely be the next organ up for printing, with the ultimate goal of creating fully transplantable organs. The need is enormous, and can’t be overstated. Patients who need a new liver wait an average of 4 years before they receive one. Even then, there is no guarantee that the selected organ will function as it should. Rejection is always a concern. Immunosuppression is a lifelong challenge.
The liver is much more complicated than a length of gut. It is cellularly complex and highly vascularized. But liver-printing is already a reality. Bioprinted “3-D liver-in-a-dish,” created by San Diego–based Organovo, has function, if not form. The cells work together; they grow, divide, and secrete bile acids. However, they exist as a formless, nonvascular blob.
As it stands (or rather, lies) now, bioprinted liver is a perfect preclinical model – perfectly replicating how the liver would respond to drugs without any of the messy adverse events that hurt patients. But it needs some backbone, or more accurately, some matrix, in order to morph again and grow into a complete organ. A liver-shaped collagen matrix could provide the necessary frame for cells to grow in and around; tunnels through it would form pathways for a similarly engineered vasculature.
The logical leap between dish and transplant is likely to be some sort of nonformed “piggyback liver,” which could be used as a bridge to keep liver failure patients alive until a traditional donor organ is found, Dr. Geibel said.
The Holy Grail, of course, would be a transplantable organ, created from the patient’s own cells – a made-to-order liver that’s 100% compatible with the recipient, who is actually also the donor.
“Think of the difference in quality of life these could make for patients who need transplants. The possibility of living without the fear of organ rejection, without the need for lifelong immunosuppression. Without the fear of having no alternative if the organ didn’t function well, or failed. If it did, we could simply grow another and try again.”
AT THE 2015 AGA TECH SUMMIT
2014 – For GI tech interventions, it was a very good year
SAN FRANCISCO– The past 18 months may well have been a watershed moment for medical technology in the gastroenterology field, with bullish venture investment in GI med/biotech, Dr. Jay Pasricha said at the 2015 AGA Tech Summit, which is sponsored by the AGA Center for GI Innovation and Technology.
Both small and large companies did well, raising millions in private capital, said Dr. Pasricha of Johns Hopkins University, Baltimore. Several devices moved forward with Food and Drug Administration approval as well.
“I think we have crossed the Rubicon,” he said. “It’s really exciting after years of watching this momentum build. I think GI technology is really going to take off finally, and there is the promise of much more to come.”
The SmartPill food chain perfectly illustrates Dr. Pasricha’s optimistic view. In 2013, Covidien purchased Israel-based Given Imaging for $860 million, acquiring all seven of the company’s product lines, including PillCam, a minimally invasive, nonsedation, optical endoscopy technology that is swallowed to visualize the small bowel, esophagus, and colon; the ManoScan high-resolution manometry technology; Bravo capsule-based pH monitoring; and SmartPill motility monitoring systems.
It didn’t take long for an even bigger fish to join the frenzy. Just this January, Medtronic purchased Covidien for about $50 billion – the largest purchase in medical device history. “The question now, however, is ‘How will it impact us in the GI field in general?’” Dr. Pasricha said.
He outlined three possible scenarios.
“The first, which I fervently hope will happen, is that we finally realize a validation of this space” of GI technologic interventions. “This really does look like the event that will put this field on the map.”
He expressed some hope that the Medtronic purchase will alert big-name surgical device companies to the possibilities inherent in minimally invasive GI interventions. “I hope it will attract key surgical players to the field ,,, shake things up and lead to more research and development, and a rapid advance of technology.”
It took an event like this to set the field’s stars in motion. But dawn hasn’t broken yet, he cautioned.
“It’s also possible that Medtronic will look at the field and decide it really doesn’t like everything in GI, and that it will focus on just a couple of fields, like neuromodulation, at the cost of others.”
The third scenario is the gloomiest, Dr. Pasricha said – although he said it’s probably the least likely.
“It is possible that GI will just turn out to be a speck of dust in Medtronic’s big picture. We’ll just have to wait and see what happens in the next couple of years.”
Overall, 2014 was series of good-news stories for GI med/biotech. The year saw an overall 61% increase in venture capital investments in the arena – nearly $50 billion among 4,356 deals. Nearly $9 billion of this went directly to life science investments, in a 29% jump over 2013. Almost $3 billion was directed to medical device investments – up 27%. Devices in both early- and late-stage development felt the financial love (according to a recent PricewaterhouseCoopers report).
Less invasive GERD interventions fared particularly well last year. A landmark paper demonstrated that Endogastric Solutions’ (San Mateo, Calif.) transoral incisionless fundoplication significantly outperformed medical therapy with proton pump inhibition. EndoGastric Solutions is currently working with the AGA Center for GI Innovation and Technology to collect real-world data evaluating safety, efficacy, and comparative outcomes for this procedure compared with laparoscopic surgery, through the STAR Registry.
EndoStim (Amsterdam) is running a hot race as well – ready to launch a pivotal trial sometime this year. Its implantable device stimulates the lower esophageal sphincter to control reflux. The device is not yet approved for reimbursement in any country. But on March 11, the German Institute for the Hospital Remuneration System granted it a status 1 level, which entitles hospitals to negotiate with payers for at least partial coverage.
All of this activity bodes well for the future, Dr. Pasricha said.
“We have seen GERD procedures come and go now for 15 years. Now the data are really starting to support endoscopic treatment here. I think we are past the inflection point and will see this approach really take over the field.”
The American Medical Association is keeping abreast of all the progress. On March 10, the AMA created a CPT code for transoral incisionless fundoplication. “This will really legitimize this entire field. In 5 years, these techniques may become serious contenders for first-line therapy instead of chronic PPI use. And third-generation therapies will likely emerge to tackle the more difficult cases that are now excluded, like patients with a large hiatal hernia. There’s no question but that transoral is going to be the preferred way of treating GERD.”
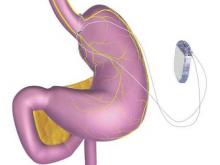
Endoscopy wasn’t the only subspecialty to bask in this new sunny clime. In January, the FDA approved St. Paul–based EnteroMedics’ Maestro system – a neuromodulatory approach to weight loss for obese adults. Maestro is an implanted vagus nerve stimulator that modulates neural signaling, controlling gastric emptying. It also seems to affect hunger signaling, Dr. Pasricha said.
Despite Maestro’s unique method of action – and the fact that it’s the first new weight-loss device approved since 2007 – EnteroMedics’ bottom line hasn’t surged, yet. But it may be too early to look for the impact and in general the outlook for nonsurgical approaches to obesity remains bullish.
Dr. Pasricha reminded the audience about some very basic core principles for successful innovation. “To succeed, you have to have two of three key things. You have to fill an unmet need. The intervention should be simple. And it should be effective.”
Since most GERD patients do fine on medical therapy, those interventions won’t succeed based on need alone. But endoscopic GERD treatments are simple and quite effective. Obesity, on the other hand, presents an enormous unmet need. But the current interventions – including Maestro – are far from simple. And efficacy hasn’t been completely confirmed yet, Dr. Pasricha said.
A couple of other obesity devices are in the works. Apollo Endosurgery (Austin) is focusing on the ORBERA single intragastric balloon. ReShape Medical (San Clemente, Calif.) reported positive data from its pivotal phase III trial of its own intragastric balloon. The device consists of two linked balloons, which the company maintains fit the stomach contours better than does a single balloon.
Over 6 months, patients treated with the dual balloon experienced a mean total body weight loss of 16% (about 17 kg). It seemed to impart some lasting benefit, as those who had continued nutritional counseling after removal maintained about 94% of their weight loss over 7 months of follow-up.
On the basis of these data, ReShape was able to secure close to $7 million in private funding to continue work on the device.
“The great potential for balloon therapy is to make it easier for patients to enter the area of bariatric surgery,” Dr. Pasricha said. “They are not naturally drawn into surgery – they usually have to be pushed into it. But with balloon treatment, they are able to enter, and the threshold for more interventions is then much lower.”
SAN FRANCISCO– The past 18 months may well have been a watershed moment for medical technology in the gastroenterology field, with bullish venture investment in GI med/biotech, Dr. Jay Pasricha said at the 2015 AGA Tech Summit, which is sponsored by the AGA Center for GI Innovation and Technology.
Both small and large companies did well, raising millions in private capital, said Dr. Pasricha of Johns Hopkins University, Baltimore. Several devices moved forward with Food and Drug Administration approval as well.
“I think we have crossed the Rubicon,” he said. “It’s really exciting after years of watching this momentum build. I think GI technology is really going to take off finally, and there is the promise of much more to come.”
The SmartPill food chain perfectly illustrates Dr. Pasricha’s optimistic view. In 2013, Covidien purchased Israel-based Given Imaging for $860 million, acquiring all seven of the company’s product lines, including PillCam, a minimally invasive, nonsedation, optical endoscopy technology that is swallowed to visualize the small bowel, esophagus, and colon; the ManoScan high-resolution manometry technology; Bravo capsule-based pH monitoring; and SmartPill motility monitoring systems.
It didn’t take long for an even bigger fish to join the frenzy. Just this January, Medtronic purchased Covidien for about $50 billion – the largest purchase in medical device history. “The question now, however, is ‘How will it impact us in the GI field in general?’” Dr. Pasricha said.
He outlined three possible scenarios.
“The first, which I fervently hope will happen, is that we finally realize a validation of this space” of GI technologic interventions. “This really does look like the event that will put this field on the map.”
He expressed some hope that the Medtronic purchase will alert big-name surgical device companies to the possibilities inherent in minimally invasive GI interventions. “I hope it will attract key surgical players to the field ,,, shake things up and lead to more research and development, and a rapid advance of technology.”
It took an event like this to set the field’s stars in motion. But dawn hasn’t broken yet, he cautioned.
“It’s also possible that Medtronic will look at the field and decide it really doesn’t like everything in GI, and that it will focus on just a couple of fields, like neuromodulation, at the cost of others.”
The third scenario is the gloomiest, Dr. Pasricha said – although he said it’s probably the least likely.
“It is possible that GI will just turn out to be a speck of dust in Medtronic’s big picture. We’ll just have to wait and see what happens in the next couple of years.”
Overall, 2014 was series of good-news stories for GI med/biotech. The year saw an overall 61% increase in venture capital investments in the arena – nearly $50 billion among 4,356 deals. Nearly $9 billion of this went directly to life science investments, in a 29% jump over 2013. Almost $3 billion was directed to medical device investments – up 27%. Devices in both early- and late-stage development felt the financial love (according to a recent PricewaterhouseCoopers report).
Less invasive GERD interventions fared particularly well last year. A landmark paper demonstrated that Endogastric Solutions’ (San Mateo, Calif.) transoral incisionless fundoplication significantly outperformed medical therapy with proton pump inhibition. EndoGastric Solutions is currently working with the AGA Center for GI Innovation and Technology to collect real-world data evaluating safety, efficacy, and comparative outcomes for this procedure compared with laparoscopic surgery, through the STAR Registry.
EndoStim (Amsterdam) is running a hot race as well – ready to launch a pivotal trial sometime this year. Its implantable device stimulates the lower esophageal sphincter to control reflux. The device is not yet approved for reimbursement in any country. But on March 11, the German Institute for the Hospital Remuneration System granted it a status 1 level, which entitles hospitals to negotiate with payers for at least partial coverage.
All of this activity bodes well for the future, Dr. Pasricha said.
“We have seen GERD procedures come and go now for 15 years. Now the data are really starting to support endoscopic treatment here. I think we are past the inflection point and will see this approach really take over the field.”
The American Medical Association is keeping abreast of all the progress. On March 10, the AMA created a CPT code for transoral incisionless fundoplication. “This will really legitimize this entire field. In 5 years, these techniques may become serious contenders for first-line therapy instead of chronic PPI use. And third-generation therapies will likely emerge to tackle the more difficult cases that are now excluded, like patients with a large hiatal hernia. There’s no question but that transoral is going to be the preferred way of treating GERD.”
Endoscopy wasn’t the only subspecialty to bask in this new sunny clime. In January, the FDA approved St. Paul–based EnteroMedics’ Maestro system – a neuromodulatory approach to weight loss for obese adults. Maestro is an implanted vagus nerve stimulator that modulates neural signaling, controlling gastric emptying. It also seems to affect hunger signaling, Dr. Pasricha said.
Despite Maestro’s unique method of action – and the fact that it’s the first new weight-loss device approved since 2007 – EnteroMedics’ bottom line hasn’t surged, yet. But it may be too early to look for the impact and in general the outlook for nonsurgical approaches to obesity remains bullish.
Dr. Pasricha reminded the audience about some very basic core principles for successful innovation. “To succeed, you have to have two of three key things. You have to fill an unmet need. The intervention should be simple. And it should be effective.”
Since most GERD patients do fine on medical therapy, those interventions won’t succeed based on need alone. But endoscopic GERD treatments are simple and quite effective. Obesity, on the other hand, presents an enormous unmet need. But the current interventions – including Maestro – are far from simple. And efficacy hasn’t been completely confirmed yet, Dr. Pasricha said.
A couple of other obesity devices are in the works. Apollo Endosurgery (Austin) is focusing on the ORBERA single intragastric balloon. ReShape Medical (San Clemente, Calif.) reported positive data from its pivotal phase III trial of its own intragastric balloon. The device consists of two linked balloons, which the company maintains fit the stomach contours better than does a single balloon.
Over 6 months, patients treated with the dual balloon experienced a mean total body weight loss of 16% (about 17 kg). It seemed to impart some lasting benefit, as those who had continued nutritional counseling after removal maintained about 94% of their weight loss over 7 months of follow-up.
On the basis of these data, ReShape was able to secure close to $7 million in private funding to continue work on the device.
“The great potential for balloon therapy is to make it easier for patients to enter the area of bariatric surgery,” Dr. Pasricha said. “They are not naturally drawn into surgery – they usually have to be pushed into it. But with balloon treatment, they are able to enter, and the threshold for more interventions is then much lower.”
SAN FRANCISCO– The past 18 months may well have been a watershed moment for medical technology in the gastroenterology field, with bullish venture investment in GI med/biotech, Dr. Jay Pasricha said at the 2015 AGA Tech Summit, which is sponsored by the AGA Center for GI Innovation and Technology.
Both small and large companies did well, raising millions in private capital, said Dr. Pasricha of Johns Hopkins University, Baltimore. Several devices moved forward with Food and Drug Administration approval as well.
“I think we have crossed the Rubicon,” he said. “It’s really exciting after years of watching this momentum build. I think GI technology is really going to take off finally, and there is the promise of much more to come.”
The SmartPill food chain perfectly illustrates Dr. Pasricha’s optimistic view. In 2013, Covidien purchased Israel-based Given Imaging for $860 million, acquiring all seven of the company’s product lines, including PillCam, a minimally invasive, nonsedation, optical endoscopy technology that is swallowed to visualize the small bowel, esophagus, and colon; the ManoScan high-resolution manometry technology; Bravo capsule-based pH monitoring; and SmartPill motility monitoring systems.
It didn’t take long for an even bigger fish to join the frenzy. Just this January, Medtronic purchased Covidien for about $50 billion – the largest purchase in medical device history. “The question now, however, is ‘How will it impact us in the GI field in general?’” Dr. Pasricha said.
He outlined three possible scenarios.
“The first, which I fervently hope will happen, is that we finally realize a validation of this space” of GI technologic interventions. “This really does look like the event that will put this field on the map.”
He expressed some hope that the Medtronic purchase will alert big-name surgical device companies to the possibilities inherent in minimally invasive GI interventions. “I hope it will attract key surgical players to the field ,,, shake things up and lead to more research and development, and a rapid advance of technology.”
It took an event like this to set the field’s stars in motion. But dawn hasn’t broken yet, he cautioned.
“It’s also possible that Medtronic will look at the field and decide it really doesn’t like everything in GI, and that it will focus on just a couple of fields, like neuromodulation, at the cost of others.”
The third scenario is the gloomiest, Dr. Pasricha said – although he said it’s probably the least likely.
“It is possible that GI will just turn out to be a speck of dust in Medtronic’s big picture. We’ll just have to wait and see what happens in the next couple of years.”
Overall, 2014 was series of good-news stories for GI med/biotech. The year saw an overall 61% increase in venture capital investments in the arena – nearly $50 billion among 4,356 deals. Nearly $9 billion of this went directly to life science investments, in a 29% jump over 2013. Almost $3 billion was directed to medical device investments – up 27%. Devices in both early- and late-stage development felt the financial love (according to a recent PricewaterhouseCoopers report).
Less invasive GERD interventions fared particularly well last year. A landmark paper demonstrated that Endogastric Solutions’ (San Mateo, Calif.) transoral incisionless fundoplication significantly outperformed medical therapy with proton pump inhibition. EndoGastric Solutions is currently working with the AGA Center for GI Innovation and Technology to collect real-world data evaluating safety, efficacy, and comparative outcomes for this procedure compared with laparoscopic surgery, through the STAR Registry.
EndoStim (Amsterdam) is running a hot race as well – ready to launch a pivotal trial sometime this year. Its implantable device stimulates the lower esophageal sphincter to control reflux. The device is not yet approved for reimbursement in any country. But on March 11, the German Institute for the Hospital Remuneration System granted it a status 1 level, which entitles hospitals to negotiate with payers for at least partial coverage.
All of this activity bodes well for the future, Dr. Pasricha said.
“We have seen GERD procedures come and go now for 15 years. Now the data are really starting to support endoscopic treatment here. I think we are past the inflection point and will see this approach really take over the field.”
The American Medical Association is keeping abreast of all the progress. On March 10, the AMA created a CPT code for transoral incisionless fundoplication. “This will really legitimize this entire field. In 5 years, these techniques may become serious contenders for first-line therapy instead of chronic PPI use. And third-generation therapies will likely emerge to tackle the more difficult cases that are now excluded, like patients with a large hiatal hernia. There’s no question but that transoral is going to be the preferred way of treating GERD.”
Endoscopy wasn’t the only subspecialty to bask in this new sunny clime. In January, the FDA approved St. Paul–based EnteroMedics’ Maestro system – a neuromodulatory approach to weight loss for obese adults. Maestro is an implanted vagus nerve stimulator that modulates neural signaling, controlling gastric emptying. It also seems to affect hunger signaling, Dr. Pasricha said.
Despite Maestro’s unique method of action – and the fact that it’s the first new weight-loss device approved since 2007 – EnteroMedics’ bottom line hasn’t surged, yet. But it may be too early to look for the impact and in general the outlook for nonsurgical approaches to obesity remains bullish.
Dr. Pasricha reminded the audience about some very basic core principles for successful innovation. “To succeed, you have to have two of three key things. You have to fill an unmet need. The intervention should be simple. And it should be effective.”
Since most GERD patients do fine on medical therapy, those interventions won’t succeed based on need alone. But endoscopic GERD treatments are simple and quite effective. Obesity, on the other hand, presents an enormous unmet need. But the current interventions – including Maestro – are far from simple. And efficacy hasn’t been completely confirmed yet, Dr. Pasricha said.
A couple of other obesity devices are in the works. Apollo Endosurgery (Austin) is focusing on the ORBERA single intragastric balloon. ReShape Medical (San Clemente, Calif.) reported positive data from its pivotal phase III trial of its own intragastric balloon. The device consists of two linked balloons, which the company maintains fit the stomach contours better than does a single balloon.
Over 6 months, patients treated with the dual balloon experienced a mean total body weight loss of 16% (about 17 kg). It seemed to impart some lasting benefit, as those who had continued nutritional counseling after removal maintained about 94% of their weight loss over 7 months of follow-up.
On the basis of these data, ReShape was able to secure close to $7 million in private funding to continue work on the device.
“The great potential for balloon therapy is to make it easier for patients to enter the area of bariatric surgery,” Dr. Pasricha said. “They are not naturally drawn into surgery – they usually have to be pushed into it. But with balloon treatment, they are able to enter, and the threshold for more interventions is then much lower.”
VIDEO: Working with the FDA to get devices to market
SAN FRANCISCO – The AGA Center for GI Innovation and Technology and the Food and Drug Administration are working together in the evaluation of new potential gastroenterological devices and technologies designed to benefit patients. In an interview at the 2015 AGA Tech Summit in San Francisco, Dr. Herbert Lerner, deputy director of the FDA’s Division of Reproductive, Gastro-renal, and Urological Devices, discussed the agency’s willingness to engage openly with experts and innovators with the goal of getting to market new devices and technologies designed to improve patient health.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – The AGA Center for GI Innovation and Technology and the Food and Drug Administration are working together in the evaluation of new potential gastroenterological devices and technologies designed to benefit patients. In an interview at the 2015 AGA Tech Summit in San Francisco, Dr. Herbert Lerner, deputy director of the FDA’s Division of Reproductive, Gastro-renal, and Urological Devices, discussed the agency’s willingness to engage openly with experts and innovators with the goal of getting to market new devices and technologies designed to improve patient health.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN FRANCISCO – The AGA Center for GI Innovation and Technology and the Food and Drug Administration are working together in the evaluation of new potential gastroenterological devices and technologies designed to benefit patients. In an interview at the 2015 AGA Tech Summit in San Francisco, Dr. Herbert Lerner, deputy director of the FDA’s Division of Reproductive, Gastro-renal, and Urological Devices, discussed the agency’s willingness to engage openly with experts and innovators with the goal of getting to market new devices and technologies designed to improve patient health.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE 2015 AGA TECH SUMMIT
Behavioral economics may be the key to improving health-related behaviors
The usual approach to improving health-related behaviors involves education and, in some cases, financial incentives; a better approach involves using “behavioral economics” to help patients identify and overcome psychological barriers to behavioral change, according to Dr. David Asch.
During his keynote presentation titled “Using Behavioral Economics to Improve Individual and Population Health” on Thursday morning at the 2015 AGA Technology Summit, Dr. Asch will describe the concept of behavioral economics and discuss ongoing research demonstrating its value in the health care setting.
“In health care, so many of the outcomes that people achieve are highly determined by their own behaviors,” Dr. Asch, an internist at the University of Pennsylvania, Philadelphia , said in an interview.
This is true regarding diet, exercise, and medication adherence, he added, naming just a few examples.
“Human behavior is really the final common pathway toward a lot of the outcomes we care about in health and health care, and until recently there hasn’t been a lot of insight into how to improve people’s health behavior and to advance their own goals,” he said.
One thing that’s known, however, is that being armed with the knowledge that a certain behavior will derail one’s efforts doesn’t necessarily prevent that behavior. A person may know that it’s safer to use a seat belt, but may avoid using one anyway. A dieter may know that piece of chocolate cake is fattening, but will eat it anyway.
That’s why education – though important – is not typically enough on its own to change behaviors, Dr. Asch said, explaining that the premise of the educational approach is that if you tell dieters that chocolate cake is fattening, they won’t eat it, and therefore they won’t get fat.
It’s clear what a weak argument that is, he said.
Similarly, economic incentives – paying patients to take their medication or quit smoking, or, conversely, charging penalties if they don’t – may have some benefit, but these incentives are expensive and off-putting, and their effectiveness is limited, he noted.
Behavioral economics, on the other hand, has much more to do with psychology than with economics, he said, adding that “the basic principle is that all of us are irrational. We do all sorts of things that are irrational, meaning they’re not consistent with our own best interests.”
The key insight of behavioral economics, however, isn’t that people are irrational – it is that they are irrational in highly predictable ways, Dr. Asch said, explaining that people tend to make the same types of mistakes as other people, which allows for the development of solutions with widespread applicability.
“We can harness these same errors that trip us up and use them to create interventions that improve our health,” he said.
At the summit, which is sponsored by the AGA’s Center for GI Innovation and Technology, Dr. Asch will share his recent research showing how health can be improved by “taking advantage of these psychological foibles.”
One study he will discuss is looking at how behavioral economics can promote medication adherence.
“We have a large study in the field right now for patients who have been discharged from the hospital following a heart attack,” he said explaining that such patients are typically discharged on three or four different types of medications, and despite the seriousness of the condition, adherence is only about 40%.
The study uses a multipronged approach that “game-ifies” medication adherence by monitoring pill use via special pill bottles with a computer chip in the cap. A daily lottery is conducted in which patients are randomly selected for monetary prizes if they have been adherent (the data are transmitted through a cell phone signal), resulting in a doubling of compliance rates, he said.
The approach incorporates powerful psychological forces such as regret, which patients will experience if their number is drawn in the lottery and they lose out on the money because of lack of medication adherence, for example. It also enlists the help of a volunteer support person – such as an adult child of the patient – who is notified if a patient misses 2 days of medication in a row. The latter “turns an unwitnessed event into a witnessed event,” providing further impetus for compliance, Dr. Asch said, explaining that no one wants a witness to their failure.
The study is ongoing, and while it remains to be seen whether the behavioral economics approach reduces admissions and proves cost effective, it’s clear that it works to promote adherence, he said.
“And that’s almost certainly a good thing,” he added.
The usual approach to improving health-related behaviors involves education and, in some cases, financial incentives; a better approach involves using “behavioral economics” to help patients identify and overcome psychological barriers to behavioral change, according to Dr. David Asch.
During his keynote presentation titled “Using Behavioral Economics to Improve Individual and Population Health” on Thursday morning at the 2015 AGA Technology Summit, Dr. Asch will describe the concept of behavioral economics and discuss ongoing research demonstrating its value in the health care setting.
“In health care, so many of the outcomes that people achieve are highly determined by their own behaviors,” Dr. Asch, an internist at the University of Pennsylvania, Philadelphia , said in an interview.
This is true regarding diet, exercise, and medication adherence, he added, naming just a few examples.
“Human behavior is really the final common pathway toward a lot of the outcomes we care about in health and health care, and until recently there hasn’t been a lot of insight into how to improve people’s health behavior and to advance their own goals,” he said.
One thing that’s known, however, is that being armed with the knowledge that a certain behavior will derail one’s efforts doesn’t necessarily prevent that behavior. A person may know that it’s safer to use a seat belt, but may avoid using one anyway. A dieter may know that piece of chocolate cake is fattening, but will eat it anyway.
That’s why education – though important – is not typically enough on its own to change behaviors, Dr. Asch said, explaining that the premise of the educational approach is that if you tell dieters that chocolate cake is fattening, they won’t eat it, and therefore they won’t get fat.
It’s clear what a weak argument that is, he said.
Similarly, economic incentives – paying patients to take their medication or quit smoking, or, conversely, charging penalties if they don’t – may have some benefit, but these incentives are expensive and off-putting, and their effectiveness is limited, he noted.
Behavioral economics, on the other hand, has much more to do with psychology than with economics, he said, adding that “the basic principle is that all of us are irrational. We do all sorts of things that are irrational, meaning they’re not consistent with our own best interests.”
The key insight of behavioral economics, however, isn’t that people are irrational – it is that they are irrational in highly predictable ways, Dr. Asch said, explaining that people tend to make the same types of mistakes as other people, which allows for the development of solutions with widespread applicability.
“We can harness these same errors that trip us up and use them to create interventions that improve our health,” he said.
At the summit, which is sponsored by the AGA’s Center for GI Innovation and Technology, Dr. Asch will share his recent research showing how health can be improved by “taking advantage of these psychological foibles.”
One study he will discuss is looking at how behavioral economics can promote medication adherence.
“We have a large study in the field right now for patients who have been discharged from the hospital following a heart attack,” he said explaining that such patients are typically discharged on three or four different types of medications, and despite the seriousness of the condition, adherence is only about 40%.
The study uses a multipronged approach that “game-ifies” medication adherence by monitoring pill use via special pill bottles with a computer chip in the cap. A daily lottery is conducted in which patients are randomly selected for monetary prizes if they have been adherent (the data are transmitted through a cell phone signal), resulting in a doubling of compliance rates, he said.
The approach incorporates powerful psychological forces such as regret, which patients will experience if their number is drawn in the lottery and they lose out on the money because of lack of medication adherence, for example. It also enlists the help of a volunteer support person – such as an adult child of the patient – who is notified if a patient misses 2 days of medication in a row. The latter “turns an unwitnessed event into a witnessed event,” providing further impetus for compliance, Dr. Asch said, explaining that no one wants a witness to their failure.
The study is ongoing, and while it remains to be seen whether the behavioral economics approach reduces admissions and proves cost effective, it’s clear that it works to promote adherence, he said.
“And that’s almost certainly a good thing,” he added.
The usual approach to improving health-related behaviors involves education and, in some cases, financial incentives; a better approach involves using “behavioral economics” to help patients identify and overcome psychological barriers to behavioral change, according to Dr. David Asch.
During his keynote presentation titled “Using Behavioral Economics to Improve Individual and Population Health” on Thursday morning at the 2015 AGA Technology Summit, Dr. Asch will describe the concept of behavioral economics and discuss ongoing research demonstrating its value in the health care setting.
“In health care, so many of the outcomes that people achieve are highly determined by their own behaviors,” Dr. Asch, an internist at the University of Pennsylvania, Philadelphia , said in an interview.
This is true regarding diet, exercise, and medication adherence, he added, naming just a few examples.
“Human behavior is really the final common pathway toward a lot of the outcomes we care about in health and health care, and until recently there hasn’t been a lot of insight into how to improve people’s health behavior and to advance their own goals,” he said.
One thing that’s known, however, is that being armed with the knowledge that a certain behavior will derail one’s efforts doesn’t necessarily prevent that behavior. A person may know that it’s safer to use a seat belt, but may avoid using one anyway. A dieter may know that piece of chocolate cake is fattening, but will eat it anyway.
That’s why education – though important – is not typically enough on its own to change behaviors, Dr. Asch said, explaining that the premise of the educational approach is that if you tell dieters that chocolate cake is fattening, they won’t eat it, and therefore they won’t get fat.
It’s clear what a weak argument that is, he said.
Similarly, economic incentives – paying patients to take their medication or quit smoking, or, conversely, charging penalties if they don’t – may have some benefit, but these incentives are expensive and off-putting, and their effectiveness is limited, he noted.
Behavioral economics, on the other hand, has much more to do with psychology than with economics, he said, adding that “the basic principle is that all of us are irrational. We do all sorts of things that are irrational, meaning they’re not consistent with our own best interests.”
The key insight of behavioral economics, however, isn’t that people are irrational – it is that they are irrational in highly predictable ways, Dr. Asch said, explaining that people tend to make the same types of mistakes as other people, which allows for the development of solutions with widespread applicability.
“We can harness these same errors that trip us up and use them to create interventions that improve our health,” he said.
At the summit, which is sponsored by the AGA’s Center for GI Innovation and Technology, Dr. Asch will share his recent research showing how health can be improved by “taking advantage of these psychological foibles.”
One study he will discuss is looking at how behavioral economics can promote medication adherence.
“We have a large study in the field right now for patients who have been discharged from the hospital following a heart attack,” he said explaining that such patients are typically discharged on three or four different types of medications, and despite the seriousness of the condition, adherence is only about 40%.
The study uses a multipronged approach that “game-ifies” medication adherence by monitoring pill use via special pill bottles with a computer chip in the cap. A daily lottery is conducted in which patients are randomly selected for monetary prizes if they have been adherent (the data are transmitted through a cell phone signal), resulting in a doubling of compliance rates, he said.
The approach incorporates powerful psychological forces such as regret, which patients will experience if their number is drawn in the lottery and they lose out on the money because of lack of medication adherence, for example. It also enlists the help of a volunteer support person – such as an adult child of the patient – who is notified if a patient misses 2 days of medication in a row. The latter “turns an unwitnessed event into a witnessed event,” providing further impetus for compliance, Dr. Asch said, explaining that no one wants a witness to their failure.
The study is ongoing, and while it remains to be seen whether the behavioral economics approach reduces admissions and proves cost effective, it’s clear that it works to promote adherence, he said.
“And that’s almost certainly a good thing,” he added.
VIDEO: AGA’s technology center helps innovators overcome hurdles
The AGA Center for GI Innovation and Technology is a unique assembly of physicians with different skill sets who work together with the goal of identifying new technologies and methods of care delivery, and then work with the innovators who came up with these ideas to help them find the path forward to routine clinical practice.
This means helping gastroenterology innovators deal with regulatory agencies, payers, and health systems, steps that are critical for getting new technologies adopted and reimbursed, Dr. Michael L. Kochman said in an interview. “The problem for innovators are the regulatory and reimbursement hurdles. The center is very good at helping people learn how to build the evidence base to overcome these hurdles.”
A key facet of the center’s efforts on behalf of innovators is organizing the annual AGA Technology Summit, held this year on March 19 and 20 in San Francisco. The summit brings together representatives of payers, health systems, the Food and Drug Administration, and other regulatory agencies, as well as physicians, corporate leaders, innovators, and leading-edge thinkers, to collectively work through major issues in bringing gastroenterology innovations into practice, said Dr. Kochman, chair of the center and professor of medicine and director of endoscopic education at the University of Pennsylvania, Philadelphia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
The AGA Center for GI Innovation and Technology is a unique assembly of physicians with different skill sets who work together with the goal of identifying new technologies and methods of care delivery, and then work with the innovators who came up with these ideas to help them find the path forward to routine clinical practice.
This means helping gastroenterology innovators deal with regulatory agencies, payers, and health systems, steps that are critical for getting new technologies adopted and reimbursed, Dr. Michael L. Kochman said in an interview. “The problem for innovators are the regulatory and reimbursement hurdles. The center is very good at helping people learn how to build the evidence base to overcome these hurdles.”
A key facet of the center’s efforts on behalf of innovators is organizing the annual AGA Technology Summit, held this year on March 19 and 20 in San Francisco. The summit brings together representatives of payers, health systems, the Food and Drug Administration, and other regulatory agencies, as well as physicians, corporate leaders, innovators, and leading-edge thinkers, to collectively work through major issues in bringing gastroenterology innovations into practice, said Dr. Kochman, chair of the center and professor of medicine and director of endoscopic education at the University of Pennsylvania, Philadelphia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
The AGA Center for GI Innovation and Technology is a unique assembly of physicians with different skill sets who work together with the goal of identifying new technologies and methods of care delivery, and then work with the innovators who came up with these ideas to help them find the path forward to routine clinical practice.
This means helping gastroenterology innovators deal with regulatory agencies, payers, and health systems, steps that are critical for getting new technologies adopted and reimbursed, Dr. Michael L. Kochman said in an interview. “The problem for innovators are the regulatory and reimbursement hurdles. The center is very good at helping people learn how to build the evidence base to overcome these hurdles.”
A key facet of the center’s efforts on behalf of innovators is organizing the annual AGA Technology Summit, held this year on March 19 and 20 in San Francisco. The summit brings together representatives of payers, health systems, the Food and Drug Administration, and other regulatory agencies, as well as physicians, corporate leaders, innovators, and leading-edge thinkers, to collectively work through major issues in bringing gastroenterology innovations into practice, said Dr. Kochman, chair of the center and professor of medicine and director of endoscopic education at the University of Pennsylvania, Philadelphia.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler