User login
In this Update, I outline important findings from several studies published in the past year. First and foremost, what are best practices for performing colposcopy in the United States? The American Society for Colposcopy and Cervical Pathology (ASCCP) released guidelines addressing such practices. Second, what are the implications of repeated negative screening and patients’ acceptance of extended screening intervals? A recent observational cohort study and a large study of Kaiser Permanente’s practices since 2003 shed light on these questions. Last, where do we stand with HPV vaccination? Two studies shed light on the efficacy of vaccination against human papillomavirus (HPV), and subsequent cervical intraepithelial neoplasia (CIN) and cervical cancer.
ASCCP releases updated quality guidelines for performing colposcopy
Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223-229.
Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235-241.
Wentzensen N, Schiffman M, Silver MI, et al. ASCCP colposcopy standards: Risk-based colposcopy practice. J Low Genit Tract Dis. 2017;21(4):230-234.
In October 2017, the ASCCP released a set of standards on the role and performance of colposcopy that represents best practices in women's health care in the United States. The work of these groups comprised a literature search, a national survey of ASCCP members, public comment, and expert consensus, and addressed:
- establishment of a common understanding of 1) the benefits of colposcopy in health maintenance and risk prevention, 2) risks presented by the procedure, and 3) terminology and criteria for reporting results that reduce subjectivity in reporting
- the rationale for, approach to, and recommendations regarding assessment of cervical precancer at colposcopy
- both minimum and comprehensive guidelines for the colposcopic examination, from preprocedure evaluation to follow-up.
Each Working Group performed the analysis and produced its own report and recommendations, published sequentially in a 2017 issue of the Journal of Lower Urinary Tract Disease. The findings and standards that they produced 1) offer essential insight for high- and low-volume coloposcopists and 2) are intended to improve the quality of colposcopy, reduce subjectivity in reporting findings, and improve the sensitivity of the procedure. Aware of the concerns and objectives of payers and hospital credentialing committees, the ASCCP found it important to establish what would be considered US-based minimum quality standards and to present goals that providers and systems could strive to achieve.
Selected details of the 3 guideline reports
The past 6 years have brought us through a great deal of transition in the prevention of cervical precancer, with regard to screening intervals and types of screening (for example, see "HPV−cytology co-testing every 3 years lowers population rates of cervical precancer and cancer," in the 2017 "Cervical Disease Update," OBG Management, May 2017). The most significant change was in 2012, when American Cancer Society/ASCCP guidelines were revised to abandon screening with annual Pap testing on most patients--an effort to strike a balance between the lifesaving value of identifying precancer and the potential harm of excessive colposcopy.
If, as the US Preventive Services Task Force (USPSTF) has declared, excessive colposcopy is a harm of screening, then we should be adapting our practices, especially in terms of the frequency of screening, to 1) reduce the risk of unnecessarily screening and potentially triaging patients to colposcopy and 2) bring the highest standards of performance and reporting to colposcopic practice (see "Why aren't you doing a Pap on me?"). In other words, "This is the way I've always done it" shouldn't characterize efforts to detect disease, when the data are clear that doing less might be more beneficial for our patients. Adherence to extended screening intervals is not yet good enough to balance benefit and risk of harm, as Rendle and colleagues showed in an article this year in Preventive Medicine (discussed in the next section of this "Update"). We need to do better.
Adherence to extended screening intervals means fewer colposcopies and less exposure to risk of attendant harm. But adherence is not purely mechanical: It can be intertwined with how patients feel about the care we provide and about their safety. When a patient moves from years of annual Pap testing to less frequent screening, she might express her concern by challenging your expertise.
In my practice, I have a simple, 1-minute conversation with the patient that is important to wedge into our discussion of her care. I explain that increasing the frequency of screening is only going to increase the chance that I will perform a colposcopy but not increase the chance that I will identify cancer. I conclude by reassuring her that I do not want to harm her, or to cause her anxiety, pain, cramping, or bleeding--or require her to spend time away from work or show her family that she is suffering. Patients are reassured and happy after that, I find. This is a patient-centered discussion that providers need to have if they hope to establish and maintain adherence to recommended screening intervals.
-- Mark H. Einstein, MD, MS
Here is a limited encapsulation of the 3 wide-ranging reports on the ASCCP colposcopy recommendations:
Role of colposcopy; benefits, potential harms, terminology (Khan et al; Working Group 1). The authors provide reinforcement: The strategic benefit of colposcopy is clear--a "drastic" reduction in excisional procedures by limiting them to patients in whom cervical cancer precursors have been confirmed or who present a high risk of occult invasive cervical cancer. Furthermore, the rate of adverse events for colposcopy−including significant bleeding and infection−is low.
Nevertheless, the potential for harm exists when an unskilled provider performs colposcopy; the Working Group emphasizes that proficiency comes with training and experience. Even in skilled hands, however, anxiety and the discomfort of a speculum examination and from acetic acid, as well as cramping and pain, might dissuade some women from receiving regular cervical screening subsequently. The authors cite research showing that educational interventions can help soothe anxiety about colposcopy and potential findings,1,2 although consensus is lacking on the value of such interventions.
The Working Group 1) developed recommended terminology for reporting findings in colposcopy practice in the United States and 2) defined the comprehensive documentation of the procedure as comprising cervix and squamocolumnar junction visibility; acetowhitening; presence of a lesion; lesion visibility, size and location of lesion(s); vascular changes; other features; and colposcopic impression (TABLE 1).3 Minimum criteria for reporting colposcopy results were also proposed, extracted from the comprehensive standards.

Risk-based colposcopy practice (Wentzensen et al). Women referred to colposcopy present with a range of underlying risk of precancer. Assessing that risk at the colposcopy visit allows the provider to modify and individualize the procedure. Risk can be estimated by referral screening tests (eg, cytology, HPV testing) performed in conjunction with the colposcopic impression. As opposed to a lack of standards for a minimum number of biopsies, the Working Group recommends that, as a standard, multiple targeted biopsies (≥2, as many as 4) are needed to improve detection of prevalent precancers. Colposcopic impression alone is not enough to diagnose precancerous cells. Let's face it: Our eyes with a colposcopic magnification of 15X do not make a microscope.
Implementing the Working Group's recommendations is expected to lead to improved detection of cervical precancers at colposcopy and to provide stronger reassurance of negative colposcopy results. Regarding biopsy of lesions, ASCCP did not find added benefit to taking random (nondirected) biopsies for women at low risk for precancer. The sensitivity of biopsy is increased by taking multiple biopsies of suspicious lesions, based on a risk-based approach detailed in the ASCCP guidelines. So, depending on underlying risk (estimated from screening and triage tests), colposcopy practice can be adapted in a useful manner to account for differences in risk:
- When risk of precancer is very high, for example, immediate treatment might reduce cost and prevent the patient from being lost to follow-up. When risk is very low, consider expectant management (serial cytology and HPV testing) with limited need for biopsy. In a setting of intermediate risk, the Working Group proposes, "multiple biopsies of acetowhite lesions lead to increased detection of precancer."
- Perform multiple biopsies that target all areas characterized by 1) acetowhitening, 2) metaplasia, and 3) higher abnormalities.
- Do not perform nontargeted biopsies on patients at the lowest end of risk who have been referred to colposcopy−ie, those with cytology that is less than HSIL; no evidence of HPV types 16/18; and a normal colposcopic impression (ie, no acetowhitening or metaplasia, or other visible abnormality).
- Immediate excision without biopsy confirmation or colposcopy with multiple targeted biopsies is acceptable in nonpregnant women 25 years and older whose risk of precancer is very high (≥2 of the following: HSIL cytology, HPV 16- or HPV 18-positive(or both), and high-grade colposcopy impression). Endocervical sampling should be conducted according to ASCCP's 2012 management guidelines. If biopsies do not show precancer, manage the patient using ASCCP's 2012 management guidelines, the Working Group recommends.
How do we perform colposcopy? Implications for establishing standards (Waxman et al; Working Group 3). To serve as a guide to standardizing colposcopy across the United States, the authors defined and delineated 6 major components (and their constituent parts) of a comprehensive colposcopy:
- precolposcopy evaluation
- the examination
- use of colposcopy adjuncts
- documentation
- biopsy sampling
- postcolposcopy procedures.
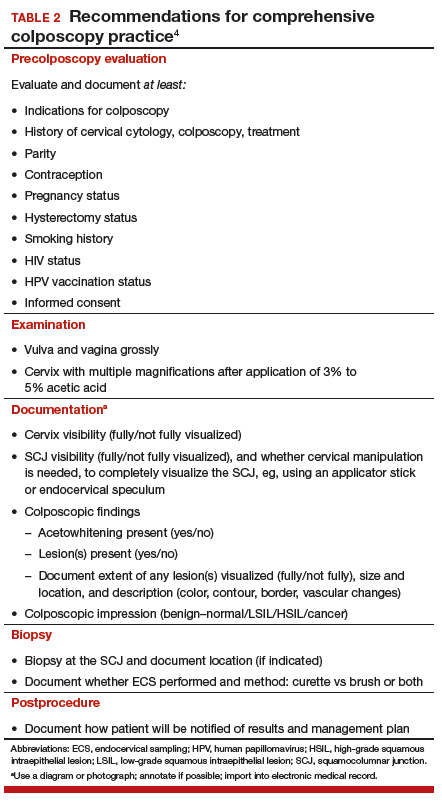
The constituent parts of these components are laid out in TABLE 2.4 A set of components for a minimum colposcopy procedure is drawn mostly from the comprehensive protocol.
The Working Group acknowledges that, in the United States, "the accuracy and reproducibility of colposcopy with biopsy as a diagnostic tool are limited." Why? Three contributing factors, the authors write, might be the absence of practice recommendations for colposcopy-biopsy procedures; of measures of quality assurance; and of standardized terminology.
Standards arrive for practice
Minimum quality standards are becoming part of almost everything US health care providers do−whether it is documentation, billing practices, or good care. Our work in gynecology, including colposcopy, is now being assessed as it is in much of the world, where minimum standards are already in place and guidelines must be followed. (In some countries standards require performing a minimum number of colposcopies per year to be identified as a "certified" colposcopist.)
What should be considered "minimum standards" for colposcopy in the United States? These ASCCP reports ask, and deliver answers to that question, bringing a broad range of concerns about high-quality practice into focus. Physicians and advanced-practice clinicians in this country who perform colposcopies have been trained to do so, but they have never had minimum standards by which to model and assess their performance. A procedure that has the potential to lead to additional testing for either cervical cancer, or to surveillance, should have minimum standards by which it is performed and documented in the United States as it is for much of the world that has widespread cervical cancer screening.
Guidance and recommendations developed by ASCCP offer women's health care providers a set of comprehensive and, alternatively, minimum quality standards that should be incorporated into practice across all aspects of the colposcopic exam, including precolposcopy evaluation, how to perform the procedure, how to document and report findings (TABLE 2), biopsy practice, establish quality control and assurance, as well as postprocedure follow-up. In taking the initiative to draw up these standards, ASCCP encourages providers to exceed the minimum requirements.
Read about adherence to cervical cancer screening.
Cervical screening adherence is relatively low, but safe. Extended intervals are very safe.
Castle PE, Kinney WK, Xue X, et al. Effect of several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer: an observational cohort study. Ann Intern Med. 2018;168(1):20-29.
Rendle KA, Schiffman M, Cheung LC, et al. Adherence patterns to extended cervical screening intervals in women undergoing human papillomavirus (HPV) and cytology cotesting. Prev Med. 2018;109:44-50.
Patients who have been screened for cervical cancer for a long time--decades, even--have a diminishing likelihood that cancer will ever be detected. Furthermore, highest-risk patients already have been triaged into further testing or procedures, such as a loop excision electrosurgical procedure or hysterectomy. Two recent studies examined the implications of repeated negative screening and patients' acceptance of extended screening intervals.
Details of the studies
Several negative rounds of cotesting (HPV and cytology) might justify changes to the screening interval. To determine the rate of detection of CIN3, adenocarcinoma in situ, and cervical cancer (≥CIN3) in routine practice after successive negative screening at 3-year intervals, Castle and colleagues looked at records of more than 990,000 women in an integrated health care system who underwent cotesting (HPV and cytology) between 2003 and 2014. They determined that the risk of invasive cervical cancer and ≥CIN3 declined with each round of cotesting; the absolute risk fell more between first and second rounds than between second and third rounds.
At any given round of cotesting, Castle found that the ability to reassure a patient about cancer and cancer risk was similar when looking at an HPV result alone, whatever the cytology or HPV-cytology cotest result was. The investigators concluded that similar patterns of risk would have been seen had stand-alone HPV testing been used, instead of co-testing, (HPV testing alone might have missed a few cases of CIN3 and adenocarcinoma in situ leading to cancer). A single negative cotest was so effective at ruling out ≥CIN3 and cervical cancer that, after a second round of cotesting, they found that no interval cancer cases were detected among women who had a negative HPV result.
Women aged 50 years or older had a 5- to 6-fold lower risk after their third consecutive negative cotest than women aged 30 to 39 years had after their first negative cotest. These data support the ideas, Castle noted, that 1) assigning screening intervals based on both age and number of previous negative screens and 2) extending the screening interval even further than 3 years after 2--perhaps even after 1--negative cotests or HPV tests are worth entertaining. Screening women of this age becomes inefficient and cost-ineffective, even at 5-year intervals.
Is patients' adherence to an extended interval of cotesting reliable enough to change practice? Rendle and colleagues examined the records of more than 491,000 women (in the same integrated health care system that Castle studied) who had undergone routine cervical cancer screening between 2003 and 2015. Their goal was to determine how high adherence had become to the system's recommendation of an every-3-year screening interval--an interval that mirrors long-standing guidelines elsewhere.
In short, researchers observed increasing and relatively rapid clinical adoption of every-3-year cotesting for routine cervical screening over time; between 2003 and 2009, the cohort grew significantly less likely overall to come in early for screening. In this setting, adoption of an extended screeninginterval appears to run counter to earlier understanding that patients are likely to resist such extension.
Women aged 60 to 64 were most likely to screen early across 2 consecutive intervals. What Rendle termed a "modest" decrease in the percentage of late screeners (but still within a 5-year interval) was also noted during adoption of the 3-year interval.
What next?
Molecular-based testing. Research, mostly outside of the United States, is taking us in the direction of molecular-based technologies as at least a component of cervical cancer screening. Today, we rely mostly on Pap tests and colposcopy, but these are subjective screens, with a human operator. With molecular testing (mostly of components of HPV), results are objective--a "Yes" or "No" finding based on clinically validated thresholds. Methods such as genotyping, P16INK4a/Ki-67 gene product dual-stain cytology, and testing for E6 and E7 HPV mRNA transcripts are in development, and hold promise to allow us to screen safely using almost completely molecular testing, thus eliminating human error and subjectivity and enriching the population that needs further management with very sensitive and potentially specific testing.
We are being presented with the possibility that almost all aspects of screening can be done without a provider, until the patient needs treatment.
Access to screening. Research is also looking at improving access, such as self-sampling for primary screening. That includes home cervical and vaginal sampling, with specimens mailed to the laboratory, from where results and follow-up instructions as communicated to patients. The Netherlands and the United Kingdom are moving to self-sampling primary screens; the United States is not--yet. But that is the direction research is taking us.
Modified guidelines. Eyes are on the work of the USPSTF. Last year, the Task Force issued draft recommendations (https://www.uspre ventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2#clinical), followed by a comment period (now closed), for updating 2012 cervical cancer screening guidelines in a way that would trigger a major change in clinical practice. Those draft recommendations and public comments are under review; final recommendations are possible within this calendar year.
Continue to follow current screening guidelines; they are safe and effective for preventing cervical cancer. This assumes adherence to intervals, which is both the provider's and the patient's responsibility: First, less is more; too much screening ("I've always done it this way") can be harmful. Second, screening at intervals set by the guidelines is extremely safe, despite earlier reports or provider concerns that suggest otherwise.
Patients who have undergone several rounds of negative screening have a markedly diminished risk of cervical cancer. Serve them best by performing this underutilized gyn procedure: Sit on your hands.
Be aware that winds of change are blowing: What constitutes appropriate screening intervals is up for discussion this year, and molecular-based testing technologies that are under investigation have the potential to someday be a vast improvement over current good, but subjective, interpretations of results.
Last, promote primary prevention of cervical cancer with HPV vaccination in your practice to increase the percentage of protected patients. Doing so will contribute not only to their long-term health but also, at a societal level, to a herd immunity effect.5 Any positive HPV infection in a future of a well-vaccinated population will be significant, and HPV-targeted technologies to identify the highest risk women will be the most efficient screening.
Read about the safety and efficacy of HPV vaccination.
Primary prevention of cervical cancer with vaccination is critical in any cancer prevention program
Benard VB, Castle PE, Jenison SA, et al; New Mexico HPV Pap Registry Steering Committee. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3(6):833-837.
Luostarinen T, Apter D, Dillner J, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer. 2018;142(10):2186-2187.
The success story of HPV vaccination, after more than a decade of use, continued to unfold in important ways over the past year.
Safety. With tens of millions of doses delivered, we know that the vaccine is safe, and we have retreated on some of the precautions that we once took: For example, we no longer perform a routine pregnancy test before vaccination on reproductive-age women.
Efficacy. We have learned, based on what we see in Australia and Western Europe, that vaccination is highly effective. We are also starting to see evidence of efficacy in areas of the United States, even though the vaccine is voluntary and there are no school-based recommendations. And we know that herd vaccination is very effective. The 2 studies described here add to our understanding of how vaccination is having an impact on endpoints.
Findings of the 2 studies
HPV vaccination has a direct impact on the precursor of cancer, CIN. Benard and colleagues examined data from the New Mexico HPV Pap Registry, a mandatory statewide surveillance system of cervical cancer screening that captured estimates of both screening prevalence and CIN since the time HPV vaccination was introduced in 2007 to 2014. The investigators examined registry data to gauge trends in the rate of CIN and to estimate the effect of HPV vaccination on that rate when adjusted for changes in screening for cervical cancer.
The incidence of CIN declined significantly across all grades in 2 groups between 2007 and 2015: females aged 15 to 19 years and females aged 20 to 24 years (but not in females 25 to 29 years of age). During those years, mean uptake of HPV vaccination among females 13 to 17 years of age reached as high as 40% (in 2014).
Although a reduction in CIN2 and CIN3 precancers "are early benchmarks for achieving this aim [of reducing the rate of cancer]," the investigators note, a reduction in CIN1 is "a direct measure of reductions in HPV infections requisite to the development of almost all invasive cervical cancer."
Benard moves on to conclude that a reduction in clinical outcomes of CIN among groups who are partially vaccinated for HPV is going to change clinical practice and reduce the cost-effectiveness of clinical care that supports prevention of cervical cancer. Of greatest importance, modalities and strategies for screening, and management algorithms, are going to need to evolve as HPV vaccination and cervical screening are integrated in a rational manner. Furthermore, it might be feasible to factor in population-level decreases in CIN among cohorts who are partially vaccinated for HPV when reassessing clinical practice guidelines for cervical cancer screening.
What does this mean? As we start to eliminate HPV from the population, any positive screening result will be that much more meaningful because the outcome--cervical cancer--will be much rarer. The onus will be on providers and public health officials to re-strategize how to screen what is going to be a widely-vaccinated population; more and more, we will be looking for needles in a haystack.
How are we going to someday screen women in their 20s who were vaccinated at 11 or 12 years of age? Likely, screening will start at a later age, and screening will be conducted at longer intervals. Any finding of HPV or disease is going to be highly significant, and likely, far less frequent.
HPV vaccination protects against invasive HPV-associated cancer. Luostarinen and colleagues report proof of highly efficacious protection offered by a population-based HPV vaccination program in Finland, in the form of a decrease in the key endpoint: cases of invasive HPV-associated cancer. Examining vaccinated (3,331 females) and unvaccinated (15,665 females) cohorts in the nationwide Finnish Cancer Registry, the investigators identified 10 cases of HPV-caused cancer (8 cervical, 1 oropharyngeal, 1 vulvar) in the unvaccinated females and 0 cases in vaccinated females--a statistically significant difference.
From the evidence gathered in this first intention-to-treat trial, the investigators conclude that vaccination protects against invasive HPV-associated cancer--what they call "an awaited, pivotal corollary" to high vaccine efficacy against HPV infection.
Summing up
This success story continues to unfold, despite well-organized, antivaccine campaigns. The HPV vaccine has been an easy target: It is novel, it involves a sexually transmitted infection, and the endpoint of protecting against invasive HPV-associated cancer is years--decades--away. But antivaccine groups can no longer argue the point that studies have not been designed to yield evidence of the impact of the vaccine on decisive endpoints, including cervical cancer.
The exciting news that the sought-out endpoint of HPV vaccination -- prevention of invasive HPV-associated cervical cancer -- is being realized. This should all the more energize you to:
- urge vaccination for your patients in whom it is indicated
- emphasize vaccine coverage in the young -- especially for the routinely recommended age group of 11 - and 12-year-olds
- strenuously reject and counter arguments made by segments of the public that HPV vaccination is neither safe nor necessary
- prepare for potential changes down the road in practice guidelines regarding screening (eg, raising the age at which screening begins) as the impact of vaccination on the health of women is felt.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Walsh JC, Curtis R, Mylotte M. Anxiety levels in women attending a colposcopy clinic: a randomised trial of an educational intervention using video colposcopy. Patient Educ Couns. 2004;55(2):247–251.
- Tomaino-Brunner C, Freda MC, Damus K, Runowicz CD. Can precolposcopy education increase knowledge and decrease anxiety? J Obstet Gynecol Neonatal Nurs. 1998;27(6):636–645.
- Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223–229.
- Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235–241.
- Wentzensen N, Schiffman M. Accelerating cervical cancer control and prevention. Lancet Public Health. 2018;3(1):e6–e7.
In this Update, I outline important findings from several studies published in the past year. First and foremost, what are best practices for performing colposcopy in the United States? The American Society for Colposcopy and Cervical Pathology (ASCCP) released guidelines addressing such practices. Second, what are the implications of repeated negative screening and patients’ acceptance of extended screening intervals? A recent observational cohort study and a large study of Kaiser Permanente’s practices since 2003 shed light on these questions. Last, where do we stand with HPV vaccination? Two studies shed light on the efficacy of vaccination against human papillomavirus (HPV), and subsequent cervical intraepithelial neoplasia (CIN) and cervical cancer.
ASCCP releases updated quality guidelines for performing colposcopy
Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223-229.
Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235-241.
Wentzensen N, Schiffman M, Silver MI, et al. ASCCP colposcopy standards: Risk-based colposcopy practice. J Low Genit Tract Dis. 2017;21(4):230-234.
In October 2017, the ASCCP released a set of standards on the role and performance of colposcopy that represents best practices in women's health care in the United States. The work of these groups comprised a literature search, a national survey of ASCCP members, public comment, and expert consensus, and addressed:
- establishment of a common understanding of 1) the benefits of colposcopy in health maintenance and risk prevention, 2) risks presented by the procedure, and 3) terminology and criteria for reporting results that reduce subjectivity in reporting
- the rationale for, approach to, and recommendations regarding assessment of cervical precancer at colposcopy
- both minimum and comprehensive guidelines for the colposcopic examination, from preprocedure evaluation to follow-up.
Each Working Group performed the analysis and produced its own report and recommendations, published sequentially in a 2017 issue of the Journal of Lower Urinary Tract Disease. The findings and standards that they produced 1) offer essential insight for high- and low-volume coloposcopists and 2) are intended to improve the quality of colposcopy, reduce subjectivity in reporting findings, and improve the sensitivity of the procedure. Aware of the concerns and objectives of payers and hospital credentialing committees, the ASCCP found it important to establish what would be considered US-based minimum quality standards and to present goals that providers and systems could strive to achieve.
Selected details of the 3 guideline reports
The past 6 years have brought us through a great deal of transition in the prevention of cervical precancer, with regard to screening intervals and types of screening (for example, see "HPV−cytology co-testing every 3 years lowers population rates of cervical precancer and cancer," in the 2017 "Cervical Disease Update," OBG Management, May 2017). The most significant change was in 2012, when American Cancer Society/ASCCP guidelines were revised to abandon screening with annual Pap testing on most patients--an effort to strike a balance between the lifesaving value of identifying precancer and the potential harm of excessive colposcopy.
If, as the US Preventive Services Task Force (USPSTF) has declared, excessive colposcopy is a harm of screening, then we should be adapting our practices, especially in terms of the frequency of screening, to 1) reduce the risk of unnecessarily screening and potentially triaging patients to colposcopy and 2) bring the highest standards of performance and reporting to colposcopic practice (see "Why aren't you doing a Pap on me?"). In other words, "This is the way I've always done it" shouldn't characterize efforts to detect disease, when the data are clear that doing less might be more beneficial for our patients. Adherence to extended screening intervals is not yet good enough to balance benefit and risk of harm, as Rendle and colleagues showed in an article this year in Preventive Medicine (discussed in the next section of this "Update"). We need to do better.
Adherence to extended screening intervals means fewer colposcopies and less exposure to risk of attendant harm. But adherence is not purely mechanical: It can be intertwined with how patients feel about the care we provide and about their safety. When a patient moves from years of annual Pap testing to less frequent screening, she might express her concern by challenging your expertise.
In my practice, I have a simple, 1-minute conversation with the patient that is important to wedge into our discussion of her care. I explain that increasing the frequency of screening is only going to increase the chance that I will perform a colposcopy but not increase the chance that I will identify cancer. I conclude by reassuring her that I do not want to harm her, or to cause her anxiety, pain, cramping, or bleeding--or require her to spend time away from work or show her family that she is suffering. Patients are reassured and happy after that, I find. This is a patient-centered discussion that providers need to have if they hope to establish and maintain adherence to recommended screening intervals.
-- Mark H. Einstein, MD, MS
Here is a limited encapsulation of the 3 wide-ranging reports on the ASCCP colposcopy recommendations:
Role of colposcopy; benefits, potential harms, terminology (Khan et al; Working Group 1). The authors provide reinforcement: The strategic benefit of colposcopy is clear--a "drastic" reduction in excisional procedures by limiting them to patients in whom cervical cancer precursors have been confirmed or who present a high risk of occult invasive cervical cancer. Furthermore, the rate of adverse events for colposcopy−including significant bleeding and infection−is low.
Nevertheless, the potential for harm exists when an unskilled provider performs colposcopy; the Working Group emphasizes that proficiency comes with training and experience. Even in skilled hands, however, anxiety and the discomfort of a speculum examination and from acetic acid, as well as cramping and pain, might dissuade some women from receiving regular cervical screening subsequently. The authors cite research showing that educational interventions can help soothe anxiety about colposcopy and potential findings,1,2 although consensus is lacking on the value of such interventions.
The Working Group 1) developed recommended terminology for reporting findings in colposcopy practice in the United States and 2) defined the comprehensive documentation of the procedure as comprising cervix and squamocolumnar junction visibility; acetowhitening; presence of a lesion; lesion visibility, size and location of lesion(s); vascular changes; other features; and colposcopic impression (TABLE 1).3 Minimum criteria for reporting colposcopy results were also proposed, extracted from the comprehensive standards.

Risk-based colposcopy practice (Wentzensen et al). Women referred to colposcopy present with a range of underlying risk of precancer. Assessing that risk at the colposcopy visit allows the provider to modify and individualize the procedure. Risk can be estimated by referral screening tests (eg, cytology, HPV testing) performed in conjunction with the colposcopic impression. As opposed to a lack of standards for a minimum number of biopsies, the Working Group recommends that, as a standard, multiple targeted biopsies (≥2, as many as 4) are needed to improve detection of prevalent precancers. Colposcopic impression alone is not enough to diagnose precancerous cells. Let's face it: Our eyes with a colposcopic magnification of 15X do not make a microscope.
Implementing the Working Group's recommendations is expected to lead to improved detection of cervical precancers at colposcopy and to provide stronger reassurance of negative colposcopy results. Regarding biopsy of lesions, ASCCP did not find added benefit to taking random (nondirected) biopsies for women at low risk for precancer. The sensitivity of biopsy is increased by taking multiple biopsies of suspicious lesions, based on a risk-based approach detailed in the ASCCP guidelines. So, depending on underlying risk (estimated from screening and triage tests), colposcopy practice can be adapted in a useful manner to account for differences in risk:
- When risk of precancer is very high, for example, immediate treatment might reduce cost and prevent the patient from being lost to follow-up. When risk is very low, consider expectant management (serial cytology and HPV testing) with limited need for biopsy. In a setting of intermediate risk, the Working Group proposes, "multiple biopsies of acetowhite lesions lead to increased detection of precancer."
- Perform multiple biopsies that target all areas characterized by 1) acetowhitening, 2) metaplasia, and 3) higher abnormalities.
- Do not perform nontargeted biopsies on patients at the lowest end of risk who have been referred to colposcopy−ie, those with cytology that is less than HSIL; no evidence of HPV types 16/18; and a normal colposcopic impression (ie, no acetowhitening or metaplasia, or other visible abnormality).
- Immediate excision without biopsy confirmation or colposcopy with multiple targeted biopsies is acceptable in nonpregnant women 25 years and older whose risk of precancer is very high (≥2 of the following: HSIL cytology, HPV 16- or HPV 18-positive(or both), and high-grade colposcopy impression). Endocervical sampling should be conducted according to ASCCP's 2012 management guidelines. If biopsies do not show precancer, manage the patient using ASCCP's 2012 management guidelines, the Working Group recommends.
How do we perform colposcopy? Implications for establishing standards (Waxman et al; Working Group 3). To serve as a guide to standardizing colposcopy across the United States, the authors defined and delineated 6 major components (and their constituent parts) of a comprehensive colposcopy:
- precolposcopy evaluation
- the examination
- use of colposcopy adjuncts
- documentation
- biopsy sampling
- postcolposcopy procedures.
The constituent parts of these components are laid out in TABLE 2.4 A set of components for a minimum colposcopy procedure is drawn mostly from the comprehensive protocol.

The Working Group acknowledges that, in the United States, "the accuracy and reproducibility of colposcopy with biopsy as a diagnostic tool are limited." Why? Three contributing factors, the authors write, might be the absence of practice recommendations for colposcopy-biopsy procedures; of measures of quality assurance; and of standardized terminology.
Standards arrive for practice
Minimum quality standards are becoming part of almost everything US health care providers do−whether it is documentation, billing practices, or good care. Our work in gynecology, including colposcopy, is now being assessed as it is in much of the world, where minimum standards are already in place and guidelines must be followed. (In some countries standards require performing a minimum number of colposcopies per year to be identified as a "certified" colposcopist.)
What should be considered "minimum standards" for colposcopy in the United States? These ASCCP reports ask, and deliver answers to that question, bringing a broad range of concerns about high-quality practice into focus. Physicians and advanced-practice clinicians in this country who perform colposcopies have been trained to do so, but they have never had minimum standards by which to model and assess their performance. A procedure that has the potential to lead to additional testing for either cervical cancer, or to surveillance, should have minimum standards by which it is performed and documented in the United States as it is for much of the world that has widespread cervical cancer screening.
Guidance and recommendations developed by ASCCP offer women's health care providers a set of comprehensive and, alternatively, minimum quality standards that should be incorporated into practice across all aspects of the colposcopic exam, including precolposcopy evaluation, how to perform the procedure, how to document and report findings (TABLE 2), biopsy practice, establish quality control and assurance, as well as postprocedure follow-up. In taking the initiative to draw up these standards, ASCCP encourages providers to exceed the minimum requirements.
Read about adherence to cervical cancer screening.
Cervical screening adherence is relatively low, but safe. Extended intervals are very safe.
Castle PE, Kinney WK, Xue X, et al. Effect of several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer: an observational cohort study. Ann Intern Med. 2018;168(1):20-29.
Rendle KA, Schiffman M, Cheung LC, et al. Adherence patterns to extended cervical screening intervals in women undergoing human papillomavirus (HPV) and cytology cotesting. Prev Med. 2018;109:44-50.
Patients who have been screened for cervical cancer for a long time--decades, even--have a diminishing likelihood that cancer will ever be detected. Furthermore, highest-risk patients already have been triaged into further testing or procedures, such as a loop excision electrosurgical procedure or hysterectomy. Two recent studies examined the implications of repeated negative screening and patients' acceptance of extended screening intervals.
Details of the studies
Several negative rounds of cotesting (HPV and cytology) might justify changes to the screening interval. To determine the rate of detection of CIN3, adenocarcinoma in situ, and cervical cancer (≥CIN3) in routine practice after successive negative screening at 3-year intervals, Castle and colleagues looked at records of more than 990,000 women in an integrated health care system who underwent cotesting (HPV and cytology) between 2003 and 2014. They determined that the risk of invasive cervical cancer and ≥CIN3 declined with each round of cotesting; the absolute risk fell more between first and second rounds than between second and third rounds.
At any given round of cotesting, Castle found that the ability to reassure a patient about cancer and cancer risk was similar when looking at an HPV result alone, whatever the cytology or HPV-cytology cotest result was. The investigators concluded that similar patterns of risk would have been seen had stand-alone HPV testing been used, instead of co-testing, (HPV testing alone might have missed a few cases of CIN3 and adenocarcinoma in situ leading to cancer). A single negative cotest was so effective at ruling out ≥CIN3 and cervical cancer that, after a second round of cotesting, they found that no interval cancer cases were detected among women who had a negative HPV result.
Women aged 50 years or older had a 5- to 6-fold lower risk after their third consecutive negative cotest than women aged 30 to 39 years had after their first negative cotest. These data support the ideas, Castle noted, that 1) assigning screening intervals based on both age and number of previous negative screens and 2) extending the screening interval even further than 3 years after 2--perhaps even after 1--negative cotests or HPV tests are worth entertaining. Screening women of this age becomes inefficient and cost-ineffective, even at 5-year intervals.
Is patients' adherence to an extended interval of cotesting reliable enough to change practice? Rendle and colleagues examined the records of more than 491,000 women (in the same integrated health care system that Castle studied) who had undergone routine cervical cancer screening between 2003 and 2015. Their goal was to determine how high adherence had become to the system's recommendation of an every-3-year screening interval--an interval that mirrors long-standing guidelines elsewhere.
In short, researchers observed increasing and relatively rapid clinical adoption of every-3-year cotesting for routine cervical screening over time; between 2003 and 2009, the cohort grew significantly less likely overall to come in early for screening. In this setting, adoption of an extended screeninginterval appears to run counter to earlier understanding that patients are likely to resist such extension.
Women aged 60 to 64 were most likely to screen early across 2 consecutive intervals. What Rendle termed a "modest" decrease in the percentage of late screeners (but still within a 5-year interval) was also noted during adoption of the 3-year interval.
What next?
Molecular-based testing. Research, mostly outside of the United States, is taking us in the direction of molecular-based technologies as at least a component of cervical cancer screening. Today, we rely mostly on Pap tests and colposcopy, but these are subjective screens, with a human operator. With molecular testing (mostly of components of HPV), results are objective--a "Yes" or "No" finding based on clinically validated thresholds. Methods such as genotyping, P16INK4a/Ki-67 gene product dual-stain cytology, and testing for E6 and E7 HPV mRNA transcripts are in development, and hold promise to allow us to screen safely using almost completely molecular testing, thus eliminating human error and subjectivity and enriching the population that needs further management with very sensitive and potentially specific testing.
We are being presented with the possibility that almost all aspects of screening can be done without a provider, until the patient needs treatment.
Access to screening. Research is also looking at improving access, such as self-sampling for primary screening. That includes home cervical and vaginal sampling, with specimens mailed to the laboratory, from where results and follow-up instructions as communicated to patients. The Netherlands and the United Kingdom are moving to self-sampling primary screens; the United States is not--yet. But that is the direction research is taking us.
Modified guidelines. Eyes are on the work of the USPSTF. Last year, the Task Force issued draft recommendations (https://www.uspre ventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2#clinical), followed by a comment period (now closed), for updating 2012 cervical cancer screening guidelines in a way that would trigger a major change in clinical practice. Those draft recommendations and public comments are under review; final recommendations are possible within this calendar year.
Continue to follow current screening guidelines; they are safe and effective for preventing cervical cancer. This assumes adherence to intervals, which is both the provider's and the patient's responsibility: First, less is more; too much screening ("I've always done it this way") can be harmful. Second, screening at intervals set by the guidelines is extremely safe, despite earlier reports or provider concerns that suggest otherwise.
Patients who have undergone several rounds of negative screening have a markedly diminished risk of cervical cancer. Serve them best by performing this underutilized gyn procedure: Sit on your hands.
Be aware that winds of change are blowing: What constitutes appropriate screening intervals is up for discussion this year, and molecular-based testing technologies that are under investigation have the potential to someday be a vast improvement over current good, but subjective, interpretations of results.
Last, promote primary prevention of cervical cancer with HPV vaccination in your practice to increase the percentage of protected patients. Doing so will contribute not only to their long-term health but also, at a societal level, to a herd immunity effect.5 Any positive HPV infection in a future of a well-vaccinated population will be significant, and HPV-targeted technologies to identify the highest risk women will be the most efficient screening.
Read about the safety and efficacy of HPV vaccination.
Primary prevention of cervical cancer with vaccination is critical in any cancer prevention program
Benard VB, Castle PE, Jenison SA, et al; New Mexico HPV Pap Registry Steering Committee. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3(6):833-837.
Luostarinen T, Apter D, Dillner J, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer. 2018;142(10):2186-2187.
The success story of HPV vaccination, after more than a decade of use, continued to unfold in important ways over the past year.
Safety. With tens of millions of doses delivered, we know that the vaccine is safe, and we have retreated on some of the precautions that we once took: For example, we no longer perform a routine pregnancy test before vaccination on reproductive-age women.
Efficacy. We have learned, based on what we see in Australia and Western Europe, that vaccination is highly effective. We are also starting to see evidence of efficacy in areas of the United States, even though the vaccine is voluntary and there are no school-based recommendations. And we know that herd vaccination is very effective. The 2 studies described here add to our understanding of how vaccination is having an impact on endpoints.
Findings of the 2 studies
HPV vaccination has a direct impact on the precursor of cancer, CIN. Benard and colleagues examined data from the New Mexico HPV Pap Registry, a mandatory statewide surveillance system of cervical cancer screening that captured estimates of both screening prevalence and CIN since the time HPV vaccination was introduced in 2007 to 2014. The investigators examined registry data to gauge trends in the rate of CIN and to estimate the effect of HPV vaccination on that rate when adjusted for changes in screening for cervical cancer.
The incidence of CIN declined significantly across all grades in 2 groups between 2007 and 2015: females aged 15 to 19 years and females aged 20 to 24 years (but not in females 25 to 29 years of age). During those years, mean uptake of HPV vaccination among females 13 to 17 years of age reached as high as 40% (in 2014).
Although a reduction in CIN2 and CIN3 precancers "are early benchmarks for achieving this aim [of reducing the rate of cancer]," the investigators note, a reduction in CIN1 is "a direct measure of reductions in HPV infections requisite to the development of almost all invasive cervical cancer."
Benard moves on to conclude that a reduction in clinical outcomes of CIN among groups who are partially vaccinated for HPV is going to change clinical practice and reduce the cost-effectiveness of clinical care that supports prevention of cervical cancer. Of greatest importance, modalities and strategies for screening, and management algorithms, are going to need to evolve as HPV vaccination and cervical screening are integrated in a rational manner. Furthermore, it might be feasible to factor in population-level decreases in CIN among cohorts who are partially vaccinated for HPV when reassessing clinical practice guidelines for cervical cancer screening.
What does this mean? As we start to eliminate HPV from the population, any positive screening result will be that much more meaningful because the outcome--cervical cancer--will be much rarer. The onus will be on providers and public health officials to re-strategize how to screen what is going to be a widely-vaccinated population; more and more, we will be looking for needles in a haystack.
How are we going to someday screen women in their 20s who were vaccinated at 11 or 12 years of age? Likely, screening will start at a later age, and screening will be conducted at longer intervals. Any finding of HPV or disease is going to be highly significant, and likely, far less frequent.
HPV vaccination protects against invasive HPV-associated cancer. Luostarinen and colleagues report proof of highly efficacious protection offered by a population-based HPV vaccination program in Finland, in the form of a decrease in the key endpoint: cases of invasive HPV-associated cancer. Examining vaccinated (3,331 females) and unvaccinated (15,665 females) cohorts in the nationwide Finnish Cancer Registry, the investigators identified 10 cases of HPV-caused cancer (8 cervical, 1 oropharyngeal, 1 vulvar) in the unvaccinated females and 0 cases in vaccinated females--a statistically significant difference.
From the evidence gathered in this first intention-to-treat trial, the investigators conclude that vaccination protects against invasive HPV-associated cancer--what they call "an awaited, pivotal corollary" to high vaccine efficacy against HPV infection.
Summing up
This success story continues to unfold, despite well-organized, antivaccine campaigns. The HPV vaccine has been an easy target: It is novel, it involves a sexually transmitted infection, and the endpoint of protecting against invasive HPV-associated cancer is years--decades--away. But antivaccine groups can no longer argue the point that studies have not been designed to yield evidence of the impact of the vaccine on decisive endpoints, including cervical cancer.
The exciting news that the sought-out endpoint of HPV vaccination -- prevention of invasive HPV-associated cervical cancer -- is being realized. This should all the more energize you to:
- urge vaccination for your patients in whom it is indicated
- emphasize vaccine coverage in the young -- especially for the routinely recommended age group of 11 - and 12-year-olds
- strenuously reject and counter arguments made by segments of the public that HPV vaccination is neither safe nor necessary
- prepare for potential changes down the road in practice guidelines regarding screening (eg, raising the age at which screening begins) as the impact of vaccination on the health of women is felt.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Update, I outline important findings from several studies published in the past year. First and foremost, what are best practices for performing colposcopy in the United States? The American Society for Colposcopy and Cervical Pathology (ASCCP) released guidelines addressing such practices. Second, what are the implications of repeated negative screening and patients’ acceptance of extended screening intervals? A recent observational cohort study and a large study of Kaiser Permanente’s practices since 2003 shed light on these questions. Last, where do we stand with HPV vaccination? Two studies shed light on the efficacy of vaccination against human papillomavirus (HPV), and subsequent cervical intraepithelial neoplasia (CIN) and cervical cancer.
ASCCP releases updated quality guidelines for performing colposcopy
Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223-229.
Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235-241.
Wentzensen N, Schiffman M, Silver MI, et al. ASCCP colposcopy standards: Risk-based colposcopy practice. J Low Genit Tract Dis. 2017;21(4):230-234.
In October 2017, the ASCCP released a set of standards on the role and performance of colposcopy that represents best practices in women's health care in the United States. The work of these groups comprised a literature search, a national survey of ASCCP members, public comment, and expert consensus, and addressed:
- establishment of a common understanding of 1) the benefits of colposcopy in health maintenance and risk prevention, 2) risks presented by the procedure, and 3) terminology and criteria for reporting results that reduce subjectivity in reporting
- the rationale for, approach to, and recommendations regarding assessment of cervical precancer at colposcopy
- both minimum and comprehensive guidelines for the colposcopic examination, from preprocedure evaluation to follow-up.
Each Working Group performed the analysis and produced its own report and recommendations, published sequentially in a 2017 issue of the Journal of Lower Urinary Tract Disease. The findings and standards that they produced 1) offer essential insight for high- and low-volume coloposcopists and 2) are intended to improve the quality of colposcopy, reduce subjectivity in reporting findings, and improve the sensitivity of the procedure. Aware of the concerns and objectives of payers and hospital credentialing committees, the ASCCP found it important to establish what would be considered US-based minimum quality standards and to present goals that providers and systems could strive to achieve.
Selected details of the 3 guideline reports
The past 6 years have brought us through a great deal of transition in the prevention of cervical precancer, with regard to screening intervals and types of screening (for example, see "HPV−cytology co-testing every 3 years lowers population rates of cervical precancer and cancer," in the 2017 "Cervical Disease Update," OBG Management, May 2017). The most significant change was in 2012, when American Cancer Society/ASCCP guidelines were revised to abandon screening with annual Pap testing on most patients--an effort to strike a balance between the lifesaving value of identifying precancer and the potential harm of excessive colposcopy.
If, as the US Preventive Services Task Force (USPSTF) has declared, excessive colposcopy is a harm of screening, then we should be adapting our practices, especially in terms of the frequency of screening, to 1) reduce the risk of unnecessarily screening and potentially triaging patients to colposcopy and 2) bring the highest standards of performance and reporting to colposcopic practice (see "Why aren't you doing a Pap on me?"). In other words, "This is the way I've always done it" shouldn't characterize efforts to detect disease, when the data are clear that doing less might be more beneficial for our patients. Adherence to extended screening intervals is not yet good enough to balance benefit and risk of harm, as Rendle and colleagues showed in an article this year in Preventive Medicine (discussed in the next section of this "Update"). We need to do better.
Adherence to extended screening intervals means fewer colposcopies and less exposure to risk of attendant harm. But adherence is not purely mechanical: It can be intertwined with how patients feel about the care we provide and about their safety. When a patient moves from years of annual Pap testing to less frequent screening, she might express her concern by challenging your expertise.
In my practice, I have a simple, 1-minute conversation with the patient that is important to wedge into our discussion of her care. I explain that increasing the frequency of screening is only going to increase the chance that I will perform a colposcopy but not increase the chance that I will identify cancer. I conclude by reassuring her that I do not want to harm her, or to cause her anxiety, pain, cramping, or bleeding--or require her to spend time away from work or show her family that she is suffering. Patients are reassured and happy after that, I find. This is a patient-centered discussion that providers need to have if they hope to establish and maintain adherence to recommended screening intervals.
-- Mark H. Einstein, MD, MS
Here is a limited encapsulation of the 3 wide-ranging reports on the ASCCP colposcopy recommendations:
Role of colposcopy; benefits, potential harms, terminology (Khan et al; Working Group 1). The authors provide reinforcement: The strategic benefit of colposcopy is clear--a "drastic" reduction in excisional procedures by limiting them to patients in whom cervical cancer precursors have been confirmed or who present a high risk of occult invasive cervical cancer. Furthermore, the rate of adverse events for colposcopy−including significant bleeding and infection−is low.
Nevertheless, the potential for harm exists when an unskilled provider performs colposcopy; the Working Group emphasizes that proficiency comes with training and experience. Even in skilled hands, however, anxiety and the discomfort of a speculum examination and from acetic acid, as well as cramping and pain, might dissuade some women from receiving regular cervical screening subsequently. The authors cite research showing that educational interventions can help soothe anxiety about colposcopy and potential findings,1,2 although consensus is lacking on the value of such interventions.
The Working Group 1) developed recommended terminology for reporting findings in colposcopy practice in the United States and 2) defined the comprehensive documentation of the procedure as comprising cervix and squamocolumnar junction visibility; acetowhitening; presence of a lesion; lesion visibility, size and location of lesion(s); vascular changes; other features; and colposcopic impression (TABLE 1).3 Minimum criteria for reporting colposcopy results were also proposed, extracted from the comprehensive standards.

Risk-based colposcopy practice (Wentzensen et al). Women referred to colposcopy present with a range of underlying risk of precancer. Assessing that risk at the colposcopy visit allows the provider to modify and individualize the procedure. Risk can be estimated by referral screening tests (eg, cytology, HPV testing) performed in conjunction with the colposcopic impression. As opposed to a lack of standards for a minimum number of biopsies, the Working Group recommends that, as a standard, multiple targeted biopsies (≥2, as many as 4) are needed to improve detection of prevalent precancers. Colposcopic impression alone is not enough to diagnose precancerous cells. Let's face it: Our eyes with a colposcopic magnification of 15X do not make a microscope.
Implementing the Working Group's recommendations is expected to lead to improved detection of cervical precancers at colposcopy and to provide stronger reassurance of negative colposcopy results. Regarding biopsy of lesions, ASCCP did not find added benefit to taking random (nondirected) biopsies for women at low risk for precancer. The sensitivity of biopsy is increased by taking multiple biopsies of suspicious lesions, based on a risk-based approach detailed in the ASCCP guidelines. So, depending on underlying risk (estimated from screening and triage tests), colposcopy practice can be adapted in a useful manner to account for differences in risk:
- When risk of precancer is very high, for example, immediate treatment might reduce cost and prevent the patient from being lost to follow-up. When risk is very low, consider expectant management (serial cytology and HPV testing) with limited need for biopsy. In a setting of intermediate risk, the Working Group proposes, "multiple biopsies of acetowhite lesions lead to increased detection of precancer."
- Perform multiple biopsies that target all areas characterized by 1) acetowhitening, 2) metaplasia, and 3) higher abnormalities.
- Do not perform nontargeted biopsies on patients at the lowest end of risk who have been referred to colposcopy−ie, those with cytology that is less than HSIL; no evidence of HPV types 16/18; and a normal colposcopic impression (ie, no acetowhitening or metaplasia, or other visible abnormality).
- Immediate excision without biopsy confirmation or colposcopy with multiple targeted biopsies is acceptable in nonpregnant women 25 years and older whose risk of precancer is very high (≥2 of the following: HSIL cytology, HPV 16- or HPV 18-positive(or both), and high-grade colposcopy impression). Endocervical sampling should be conducted according to ASCCP's 2012 management guidelines. If biopsies do not show precancer, manage the patient using ASCCP's 2012 management guidelines, the Working Group recommends.
How do we perform colposcopy? Implications for establishing standards (Waxman et al; Working Group 3). To serve as a guide to standardizing colposcopy across the United States, the authors defined and delineated 6 major components (and their constituent parts) of a comprehensive colposcopy:
- precolposcopy evaluation
- the examination
- use of colposcopy adjuncts
- documentation
- biopsy sampling
- postcolposcopy procedures.
The constituent parts of these components are laid out in TABLE 2.4 A set of components for a minimum colposcopy procedure is drawn mostly from the comprehensive protocol.

The Working Group acknowledges that, in the United States, "the accuracy and reproducibility of colposcopy with biopsy as a diagnostic tool are limited." Why? Three contributing factors, the authors write, might be the absence of practice recommendations for colposcopy-biopsy procedures; of measures of quality assurance; and of standardized terminology.
Standards arrive for practice
Minimum quality standards are becoming part of almost everything US health care providers do−whether it is documentation, billing practices, or good care. Our work in gynecology, including colposcopy, is now being assessed as it is in much of the world, where minimum standards are already in place and guidelines must be followed. (In some countries standards require performing a minimum number of colposcopies per year to be identified as a "certified" colposcopist.)
What should be considered "minimum standards" for colposcopy in the United States? These ASCCP reports ask, and deliver answers to that question, bringing a broad range of concerns about high-quality practice into focus. Physicians and advanced-practice clinicians in this country who perform colposcopies have been trained to do so, but they have never had minimum standards by which to model and assess their performance. A procedure that has the potential to lead to additional testing for either cervical cancer, or to surveillance, should have minimum standards by which it is performed and documented in the United States as it is for much of the world that has widespread cervical cancer screening.
Guidance and recommendations developed by ASCCP offer women's health care providers a set of comprehensive and, alternatively, minimum quality standards that should be incorporated into practice across all aspects of the colposcopic exam, including precolposcopy evaluation, how to perform the procedure, how to document and report findings (TABLE 2), biopsy practice, establish quality control and assurance, as well as postprocedure follow-up. In taking the initiative to draw up these standards, ASCCP encourages providers to exceed the minimum requirements.
Read about adherence to cervical cancer screening.
Cervical screening adherence is relatively low, but safe. Extended intervals are very safe.
Castle PE, Kinney WK, Xue X, et al. Effect of several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer: an observational cohort study. Ann Intern Med. 2018;168(1):20-29.
Rendle KA, Schiffman M, Cheung LC, et al. Adherence patterns to extended cervical screening intervals in women undergoing human papillomavirus (HPV) and cytology cotesting. Prev Med. 2018;109:44-50.
Patients who have been screened for cervical cancer for a long time--decades, even--have a diminishing likelihood that cancer will ever be detected. Furthermore, highest-risk patients already have been triaged into further testing or procedures, such as a loop excision electrosurgical procedure or hysterectomy. Two recent studies examined the implications of repeated negative screening and patients' acceptance of extended screening intervals.
Details of the studies
Several negative rounds of cotesting (HPV and cytology) might justify changes to the screening interval. To determine the rate of detection of CIN3, adenocarcinoma in situ, and cervical cancer (≥CIN3) in routine practice after successive negative screening at 3-year intervals, Castle and colleagues looked at records of more than 990,000 women in an integrated health care system who underwent cotesting (HPV and cytology) between 2003 and 2014. They determined that the risk of invasive cervical cancer and ≥CIN3 declined with each round of cotesting; the absolute risk fell more between first and second rounds than between second and third rounds.
At any given round of cotesting, Castle found that the ability to reassure a patient about cancer and cancer risk was similar when looking at an HPV result alone, whatever the cytology or HPV-cytology cotest result was. The investigators concluded that similar patterns of risk would have been seen had stand-alone HPV testing been used, instead of co-testing, (HPV testing alone might have missed a few cases of CIN3 and adenocarcinoma in situ leading to cancer). A single negative cotest was so effective at ruling out ≥CIN3 and cervical cancer that, after a second round of cotesting, they found that no interval cancer cases were detected among women who had a negative HPV result.
Women aged 50 years or older had a 5- to 6-fold lower risk after their third consecutive negative cotest than women aged 30 to 39 years had after their first negative cotest. These data support the ideas, Castle noted, that 1) assigning screening intervals based on both age and number of previous negative screens and 2) extending the screening interval even further than 3 years after 2--perhaps even after 1--negative cotests or HPV tests are worth entertaining. Screening women of this age becomes inefficient and cost-ineffective, even at 5-year intervals.
Is patients' adherence to an extended interval of cotesting reliable enough to change practice? Rendle and colleagues examined the records of more than 491,000 women (in the same integrated health care system that Castle studied) who had undergone routine cervical cancer screening between 2003 and 2015. Their goal was to determine how high adherence had become to the system's recommendation of an every-3-year screening interval--an interval that mirrors long-standing guidelines elsewhere.
In short, researchers observed increasing and relatively rapid clinical adoption of every-3-year cotesting for routine cervical screening over time; between 2003 and 2009, the cohort grew significantly less likely overall to come in early for screening. In this setting, adoption of an extended screeninginterval appears to run counter to earlier understanding that patients are likely to resist such extension.
Women aged 60 to 64 were most likely to screen early across 2 consecutive intervals. What Rendle termed a "modest" decrease in the percentage of late screeners (but still within a 5-year interval) was also noted during adoption of the 3-year interval.
What next?
Molecular-based testing. Research, mostly outside of the United States, is taking us in the direction of molecular-based technologies as at least a component of cervical cancer screening. Today, we rely mostly on Pap tests and colposcopy, but these are subjective screens, with a human operator. With molecular testing (mostly of components of HPV), results are objective--a "Yes" or "No" finding based on clinically validated thresholds. Methods such as genotyping, P16INK4a/Ki-67 gene product dual-stain cytology, and testing for E6 and E7 HPV mRNA transcripts are in development, and hold promise to allow us to screen safely using almost completely molecular testing, thus eliminating human error and subjectivity and enriching the population that needs further management with very sensitive and potentially specific testing.
We are being presented with the possibility that almost all aspects of screening can be done without a provider, until the patient needs treatment.
Access to screening. Research is also looking at improving access, such as self-sampling for primary screening. That includes home cervical and vaginal sampling, with specimens mailed to the laboratory, from where results and follow-up instructions as communicated to patients. The Netherlands and the United Kingdom are moving to self-sampling primary screens; the United States is not--yet. But that is the direction research is taking us.
Modified guidelines. Eyes are on the work of the USPSTF. Last year, the Task Force issued draft recommendations (https://www.uspre ventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/cervical-cancer-screening2#clinical), followed by a comment period (now closed), for updating 2012 cervical cancer screening guidelines in a way that would trigger a major change in clinical practice. Those draft recommendations and public comments are under review; final recommendations are possible within this calendar year.
Continue to follow current screening guidelines; they are safe and effective for preventing cervical cancer. This assumes adherence to intervals, which is both the provider's and the patient's responsibility: First, less is more; too much screening ("I've always done it this way") can be harmful. Second, screening at intervals set by the guidelines is extremely safe, despite earlier reports or provider concerns that suggest otherwise.
Patients who have undergone several rounds of negative screening have a markedly diminished risk of cervical cancer. Serve them best by performing this underutilized gyn procedure: Sit on your hands.
Be aware that winds of change are blowing: What constitutes appropriate screening intervals is up for discussion this year, and molecular-based testing technologies that are under investigation have the potential to someday be a vast improvement over current good, but subjective, interpretations of results.
Last, promote primary prevention of cervical cancer with HPV vaccination in your practice to increase the percentage of protected patients. Doing so will contribute not only to their long-term health but also, at a societal level, to a herd immunity effect.5 Any positive HPV infection in a future of a well-vaccinated population will be significant, and HPV-targeted technologies to identify the highest risk women will be the most efficient screening.
Read about the safety and efficacy of HPV vaccination.
Primary prevention of cervical cancer with vaccination is critical in any cancer prevention program
Benard VB, Castle PE, Jenison SA, et al; New Mexico HPV Pap Registry Steering Committee. Population-based incidence rates of cervical intraepithelial neoplasia in the human papillomavirus vaccine era. JAMA Oncol. 2017;3(6):833-837.
Luostarinen T, Apter D, Dillner J, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer. 2018;142(10):2186-2187.
The success story of HPV vaccination, after more than a decade of use, continued to unfold in important ways over the past year.
Safety. With tens of millions of doses delivered, we know that the vaccine is safe, and we have retreated on some of the precautions that we once took: For example, we no longer perform a routine pregnancy test before vaccination on reproductive-age women.
Efficacy. We have learned, based on what we see in Australia and Western Europe, that vaccination is highly effective. We are also starting to see evidence of efficacy in areas of the United States, even though the vaccine is voluntary and there are no school-based recommendations. And we know that herd vaccination is very effective. The 2 studies described here add to our understanding of how vaccination is having an impact on endpoints.
Findings of the 2 studies
HPV vaccination has a direct impact on the precursor of cancer, CIN. Benard and colleagues examined data from the New Mexico HPV Pap Registry, a mandatory statewide surveillance system of cervical cancer screening that captured estimates of both screening prevalence and CIN since the time HPV vaccination was introduced in 2007 to 2014. The investigators examined registry data to gauge trends in the rate of CIN and to estimate the effect of HPV vaccination on that rate when adjusted for changes in screening for cervical cancer.
The incidence of CIN declined significantly across all grades in 2 groups between 2007 and 2015: females aged 15 to 19 years and females aged 20 to 24 years (but not in females 25 to 29 years of age). During those years, mean uptake of HPV vaccination among females 13 to 17 years of age reached as high as 40% (in 2014).
Although a reduction in CIN2 and CIN3 precancers "are early benchmarks for achieving this aim [of reducing the rate of cancer]," the investigators note, a reduction in CIN1 is "a direct measure of reductions in HPV infections requisite to the development of almost all invasive cervical cancer."
Benard moves on to conclude that a reduction in clinical outcomes of CIN among groups who are partially vaccinated for HPV is going to change clinical practice and reduce the cost-effectiveness of clinical care that supports prevention of cervical cancer. Of greatest importance, modalities and strategies for screening, and management algorithms, are going to need to evolve as HPV vaccination and cervical screening are integrated in a rational manner. Furthermore, it might be feasible to factor in population-level decreases in CIN among cohorts who are partially vaccinated for HPV when reassessing clinical practice guidelines for cervical cancer screening.
What does this mean? As we start to eliminate HPV from the population, any positive screening result will be that much more meaningful because the outcome--cervical cancer--will be much rarer. The onus will be on providers and public health officials to re-strategize how to screen what is going to be a widely-vaccinated population; more and more, we will be looking for needles in a haystack.
How are we going to someday screen women in their 20s who were vaccinated at 11 or 12 years of age? Likely, screening will start at a later age, and screening will be conducted at longer intervals. Any finding of HPV or disease is going to be highly significant, and likely, far less frequent.
HPV vaccination protects against invasive HPV-associated cancer. Luostarinen and colleagues report proof of highly efficacious protection offered by a population-based HPV vaccination program in Finland, in the form of a decrease in the key endpoint: cases of invasive HPV-associated cancer. Examining vaccinated (3,331 females) and unvaccinated (15,665 females) cohorts in the nationwide Finnish Cancer Registry, the investigators identified 10 cases of HPV-caused cancer (8 cervical, 1 oropharyngeal, 1 vulvar) in the unvaccinated females and 0 cases in vaccinated females--a statistically significant difference.
From the evidence gathered in this first intention-to-treat trial, the investigators conclude that vaccination protects against invasive HPV-associated cancer--what they call "an awaited, pivotal corollary" to high vaccine efficacy against HPV infection.
Summing up
This success story continues to unfold, despite well-organized, antivaccine campaigns. The HPV vaccine has been an easy target: It is novel, it involves a sexually transmitted infection, and the endpoint of protecting against invasive HPV-associated cancer is years--decades--away. But antivaccine groups can no longer argue the point that studies have not been designed to yield evidence of the impact of the vaccine on decisive endpoints, including cervical cancer.
The exciting news that the sought-out endpoint of HPV vaccination -- prevention of invasive HPV-associated cervical cancer -- is being realized. This should all the more energize you to:
- urge vaccination for your patients in whom it is indicated
- emphasize vaccine coverage in the young -- especially for the routinely recommended age group of 11 - and 12-year-olds
- strenuously reject and counter arguments made by segments of the public that HPV vaccination is neither safe nor necessary
- prepare for potential changes down the road in practice guidelines regarding screening (eg, raising the age at which screening begins) as the impact of vaccination on the health of women is felt.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Walsh JC, Curtis R, Mylotte M. Anxiety levels in women attending a colposcopy clinic: a randomised trial of an educational intervention using video colposcopy. Patient Educ Couns. 2004;55(2):247–251.
- Tomaino-Brunner C, Freda MC, Damus K, Runowicz CD. Can precolposcopy education increase knowledge and decrease anxiety? J Obstet Gynecol Neonatal Nurs. 1998;27(6):636–645.
- Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223–229.
- Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235–241.
- Wentzensen N, Schiffman M. Accelerating cervical cancer control and prevention. Lancet Public Health. 2018;3(1):e6–e7.
- Walsh JC, Curtis R, Mylotte M. Anxiety levels in women attending a colposcopy clinic: a randomised trial of an educational intervention using video colposcopy. Patient Educ Couns. 2004;55(2):247–251.
- Tomaino-Brunner C, Freda MC, Damus K, Runowicz CD. Can precolposcopy education increase knowledge and decrease anxiety? J Obstet Gynecol Neonatal Nurs. 1998;27(6):636–645.
- Khan MJ, Werner CL, Darragh TM, et al. ASCCP colposcopy standards: Role of colposcopy, benefits, potential harms, and terminology for colposcopic practice. J Low Genit Tract Dis. 2017;21(4):223–229.
- Waxman AG, Conageski C, Silver MI, et al. ASCCP colposcopy standards: How do we perform colposcopy? Implications for establishing standards. J Low Genit Tract Dis. 2017;21(4):235–241.
- Wentzensen N, Schiffman M. Accelerating cervical cancer control and prevention. Lancet Public Health. 2018;3(1):e6–e7.