User login
Diffuse alveolar hemorrhage can complicate a large number of clinical conditions. It may present in different ways and may be life-threatening, and it poses an important challenge for the clinician.1
Diffuse alveolar hemorrhage is an uncommon condition in which blood floods the alveoli, usually at multiple sites. It is also known as intrapulmonary hemorrhage, diffuse pulmonary hemorrhage, pulmonary alveolar hemorrhage, pulmonary capillary hemorrhage, alveolar bleeding, or microvascular pulmonary hemorrhage.
In this article we review the causes, clinical features, diagnostic criteria, treatment, and prognosis of diffuse alveolar hemorrhage.
CAUSES OF DIFFUSE ALVEOLAR HEMORRHAGE
THREE CHARACTERISTIC PATTERNS
In general, diffuse alveolar hemorrhage can occur in three characteristic patterns, which reflect the nature of the underlying vascular injury1:
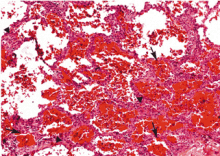
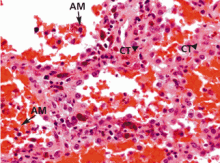
Diffuse alveolar hemorrhage associated with vasculitis or capillaritis. As described by Spencer4 50 years ago, pulmonary capillaritis is the most frequent underlying histologic lesion described in diffuse alveolar hemorrhage. Neutrophils infiltrate the interalveolar and peri-bronchiolar septal vessels (pulmonary interstitium),5 leading to anatomic disruption of the capillaries (ie, impairment of the alveolocapillary barrier) and to extravasation of red blood cells into the alveoli and interstitium. Neutrophil apoptosis and fragmentation, with subsequent release of the intracellular proteolytic enzymes and reactive oxygen species, beget more inflammation, intra-alveolar neutrophilic nuclear dust, fibrin and inflammatory exudate, and fibrinoid necrosis of the interstitium.6,7
‘Bland’ pulmonary hemorrhage (ie, without capillaritis or vasculitis). In this pattern, red blood cells leak into the alveoli without any evidence of inflammation or destruction of the alveolar capillaries, venules, and arterioles. The epithelial lesions are usually microscopic and are scattered geographically.
Diffuse alveolar hemorrhage associated with another process or condition (eg, diffuse alveolar damage, lymphangioleiomyomatosis, drug-induced lung injury, metastatic tumor to the lungs, mitral stenosis). Diffuse alveolar damage is the main underlying lesion of the acute respiratory distress syndrome and is characterized by formation of an intra-alveolar hyaline membrane, by interstitial edema with minimal inflammation, and, at times, by “secondary” diffuse alveolar hemorrhage. In this third category of diffuse alveolar hemorrhage, the underlying process causes alveolar hemorrhage by processes other than pulmonary vascular inflammation or direct extravasation of red cells.
THE CLINICAL PRESENTATION
The clinical presentation of diffuse alveolar hemorrhage may reflect either alveolar bleeding alone or features of the underlying cause (eg, hematuria in Wegener granulomatosis, arthritis in systemic lupus erythematosus). Hence, its recognition requires a high degree of suspicion.
Some patients present with severe acute respiratory distress requiring mechanical ventilation. However, dyspnea, cough, and fever are the common initial symptoms and are most often acute or subacute (ie, present for less than a week). The fever is usually due to the underlying cause, such as lupus.
Hemoptysis may be absent at the time of presentation in up to a third of patients because the total alveolar volume is large and can absorb large amounts of blood, without extending more proximally into the airways. Apparent hemoptysis, if present, must be differentiated from hematemesis or pseudohemoptysis (alveolar flooding with fluid that resembles blood, as in Serratia marcescens pneumonia, in which the reddish hue of the infecting organism can create the impression of alveolar bleeding).
DIAGNOSTIC EVALUATION
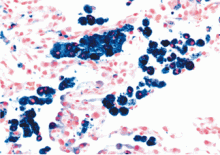
Generally speaking, dyspnea, cough, hemoptysis, and new alveolar infiltrates in conjunction with bloody bronchoalveolar lavage specimens (with numerous erythrocytes and siderophages) establish the diagnosis of diffuse alveolar hemorrhage. Surgical biopsy from the lung or another organ involved by an underlying condition is often necessary.
Physical examination
The physical findings are nonspecific and may reflect the underlying systemic vasculitis or collagen vascular disorder (eg, with accompanying rash, purpura, eye lesions, hepatosplenomegaly, or clubbing).
Imaging studies
Radiography may show new or old or both new and old patchy or diffuse alveolar opacities. Recurrent episodes of hemorrhage may lead to reticular interstitial opacities due to pulmonary fibrosis, usually with minimal (if any) honeycombing. Kerley B lines suggest mitral valve disease or pulmonary veno-occlusive disease as the cause of the hemorrhage.
Computed tomography may show areas of consolidation interspersed with areas of ground-glass attenuation and preserved, normal areas.
Currently, nuclear imaging such as gallium or tagged red blood cell studies have little role in evaluating diffuse alveolar hemorrhage. Other nuclear studies, geared to reveal breakdown of the microcirculatory integrity and extravasation of red blood cells out of the vessels, have also not been proven useful.
Evaluating pulmonary function
Diffuse alveolar hemorrhage may cause impairment of oxygen transfer and hypoxemia. In addition, it can cause several other abnormalities of pulmonary function.
Increased diffusing capacity. Because blood in the lungs can absorb inhaled carbon monoxide, the diffusing capacity for carbon monoxide (DLCO) may be distinctively increased. Serial increases in the DLCO may indicate progressive alveolar hemorrhage. However, the clinical instability of patients experiencing active alveolar bleeding precludes performing the DLCO measurement maneuvers, rendering the DLCO test relatively impractical.
Restrictive changes. Because recurrent episodes of diffuse alveolar hemorrhage can lead to interstitial fibrosis, restrictive changes—ie, decreased total lung capacity, decreased forced vital capacity (FVC), and preserved ratio of the forced expiratory volume in 1 second (FEV1) to the FVC—may characterize diffuse alveolar hemorrhage.
Obstructive changes (less common). Less commonly, patients with diffuse alveolar hemorrhage may have spirometric changes indicating airflow obstruction—ie, decreased FEV1 and decreased ratio of FEV1 to FVC—possibly because neutrophilic infiltration from blood extravasation into the alveolar sacs causes release of reactive oxygen species and proteolytic enzymes, which in turn may cause small airway and parenchymal damage such as bronchiolitis and emphysema. A pattern of obstructive lung disease associated with recurrent diffuse alveolar hemorrhage should prompt consideration of an underlying condition that can cause airflow obstruction, such as sarcoidosis, microscopic polyangiitis, or Wegener granulomatosis, or, less commonly, lymphangioleiomyomatosis, histiocytosis X, pulmonary capillaritis, or sometimes idiopathic pulmonary hemosiderosis.
As an example of an unusual circumstance, we have described elsewhere a case of a woman with idiopathic pulmonary hemosiderosis with multiple episodes of diffuse alveolar hemorrhage and resultant emphysema.8 Radiographic images showed several very large cysts, one of which herniated through the incision site of an open lung biopsy.
Decreased exhaled nitric oxide. Though currently unavailable in most clinical pulmonary function laboratories, evaluation of exhaled gas or condensate may have value in diagnosing diffuse alveolar hemorrhage.9 Specifically, because increased intra-alveolar hemoglobin binds nitric oxide, as it does carbon monoxide, levels of exhaled nitric oxide may be decreased in diffuse alveolar hemorrhage. In contrast to the difficulty of measuring DLCO in patients with active alveolar bleeding or hemoptysis, analysis of exhaled gas is clinically feasible, making this a promising diagnostic test.
Laboratory evaluation
Hematologic assessment in patients with diffuse alveolar hemorrhage generally reveals:
- Acute or chronic anemia
- Leukocytosis
- Elevated erythrocyte sedimentation rate
- Elevated C-reactive protein level (particularly in patients whose alveolar hemorrhage is due to systemic disease or vasculitis, or both).
Renal abnormalities such as elevated blood urea nitrogen and serum creatinine or abnormal findings on urinalysis (with hematuria, proteinuria, and red blood cell casts indicating glomerulonephritis) can also occur, as diffuse alveolar hemorrhage may complicate several pulmonary-renal syndromes such as Goodpasture syndrome and Wegener granulomatosis.
Bronchoscopy
The diagnostic evaluation in diffuse alveolar hemorrhage usually includes bronchoscopic examination,10 which serves two purposes:
- To document alveolar hemorrhage by bronchoalveolar lavage and to exclude airway sources of bleeding by visual inspection
- To exclude an associated infection.
Based on experience with nonmassive hemoptysis of all causes (but not exclusively diffuse alveolar hemorrhage), the diagnostic yield of bronchoscopy is higher if the procedure is performed within the first 48 hours of symptoms rather than later. Evidence supporting diffuse alveolar hemorrhage is persistent (or even increasing) blood on three sequential lavage aliquots from a single affected area of the lung.
FINDING THE UNDERLYING CAUSE
Once the diagnosis of diffuse alveolar hemorrhage is established, the clinician must ascertain whether an underlying cause is present. Serologic studies may prove important, although the results are generally not available in a manner timely enough to guide immediate management.
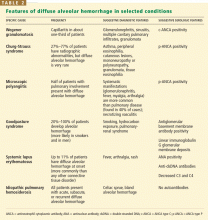
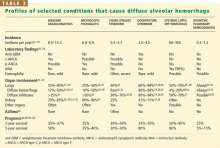
When a pulmonary-renal syndrome is suggested by accompanying hematuria or renal dysfunction, antiglomerular basement membrane antibody and antineutrophil cytoplasmic antibody (ANCA) levels should be checked. Tests for complement fractions C3 and C4, anti-double-stranded DNA, and antiphospholipid antibodies should be ordered if an underlying condition such as lupus or antiphospholipid antibody syndrome is suspected (Table 2).11
If the underlying cause remains elusive after a thorough clinical evaluation that includes imaging studies, serologic studies, and bronchoscopy, then surgical biopsy should be considered.1 Which organ to biopsy (eg, lung, sinus, kidney) depends on the level of suspicion for a specific cause. For example, suspicion of Wegener granulomato-sis with hematuria or renal dysfunction might prompt renal biopsy. However, lung biopsy often needs to be performed with video-assisted thoracoscopy, especially when disease is confined to the lung (as in idiopathic pulmonary hemosiderosis or pauci-immune pulmonary capillaritis). Renal biopsy specimens should also undergo immunofluores-cence staining, which may reveal linear deposition of immunoglobulins and immune complexes along the basement membrane in patients with Goodpasture syndrome, or of granular deposits in patients with systemic lupus erythematosus.
TWO GENERAL CLINICAL SCENARIOS
In general, the clinician will be confronted by one of two scenarios: a patient with diffuse alveolar hemorrhage and associated systemic findings, or a patient with hemorrhage and no associated systemic findings.
Hemorrhage with associated systemic findings
Certain clues from the history raise suspicion of diffuse alveolar hemorrhage:
- Recent infection suggests Henoch-Schönlein purpura or cryoglobulinemic vasculitis
- Use of a possibly offending drug such as an anticoagulant, D-penicillamine (Cuprimine, Depen), nitrofurantoin (Furadantin, Macrobid, Macrodantin), amiodarone (Cordarone), propylthiouracil, cocaine, or sirolimus (Rapamune, Rapamycin)
- Exposure to toxic agents such as trimellitic anhydride, insecticides, and pesticides
- A known comorbid condition such as vasculitis, connective tissue disease, mitral valve disease, or solid organ or stem cell transplantation.
If asthma, eosinophilia, pulmonary infiltrates, and diffuse alveolar hemorrhage coexist, consideration should be given to Churg-Strauss syndrome. If sinus disease, skin manifestations, pulmonary parenchymal nodules, and cavitary lesions coexist with positivity for antiproteinase 3 c-ANCA and biopsy-proven granulomata, then Wegener granulomatosis should be considered. Similarly, diffuse alveolar hemorrhage with glomerulonephritis and skin manifestations, positivity for p-ANCA, and necrotizing nongranulomatous lesions on end-organ biopsy may lead to a diagnosis of microscopic polyangiitis. In a young smoker with glomeru-lonephritis and diffuse alveolar hemorrhage presenting as either bland alveolar hemorrhage or pulmonary capillaritis, Goodpasture syndrome or antiglomerular basement membrane antibody disease should be considered.
Hemorrhage with no associated systemic findings
When the above conditions have been considered but no suggestive findings are found, the following four conditions should be considered:
- Antiglomerular basement membrane antibody disease in limited pulmonary form or onset: positivity to the antibody with linear deposits in the lungs would be diagnostic in such a case
- Pulmonary-limited microscopic polyangiitis positive for p-ANCA (a positive anti-myeloperoxidase p-ANCA test makes the diagnosis)
- Pauci-immune isolated pulmonary capillaritis, when the biopsy shows evidence of neutrophilic pulmonary capillaritis
- Idiopathic pulmonary hemosiderosis, a diagnosis of exclusion, when the biopsy shows evidence of acute, subacute, and chronic bland diffuse alveolar hemorrhage and no evidence of vasculitis.
TREATMENT OF DIFFUSE ALVEOLAR HEMORRHAGE
Therapy for diffuse alveolar hemorrhage consists of treating both the autoimmune destruction of the alveolar capillary membrane and the underlying condition. Corticosteroids and immunosuppressive agents remain the gold standard for most patients. Recombinant-activated human factor VII seems to be a promising new therapy, although further evaluation is needed.
Immunosuppressive agents are the mainstay of therapy for diffuse alveolar hemorrhage, especially if associated with systemic or pulmonary vasculitis, Goodpasture syndrome, and connective tissue disorders. Most experts recommend intravenous methylprednisolone (Solu-Medrol) (up to 500 mg every 6 hours, although lower doses seem to have similar efficacy) for 4 or 5 days, followed by a gradual taper to maintenance doses of oral steroids.
In patients with pulmonary-renal syndrome, therapy should be started as soon as possible to prevent irreversible renal failure.
Besides corticosteroids, other immunosuppressive drugs such as cyclophosphamide (Cytoxan), azathioprine (Imuran), mycophenolate mofetil (CellCept), and etanercept (Enbrel) may be used in diffuse alveolar hemorrhage, especially when the condition is severe, when first-line therapy with corticosteroids has proven ineffective (generally not advised, unless the condition is mild) or when specific underlying causes are present (eg, Wegener granulomatosis, Goodpasture syndrome, systemic lupus erythematosus). Intravenous cyclophosphamide (2 mg/kg/day, adjusted to renal function) is generally the preferred adjunctive immunosuppressive drug and may be continued for several weeks or until adverse effects occur, such as blood marrow suppression, infection, or hematuria. Thereafter, most clinicians switch to consolidative or maintenance therapy with methotrexate or another agent.
Plasmapheresis is indicated for diffuse alveolar hemorrhage associated with Good-pasture syndrome or with other vasculitic processes in which the titers of pathogenetic immunoglobulins and immune complexes are very high: for example, ANCA-associated vasculitis with overwhelming endothelial injury and a hypercoagulable state. However, the merits of plasmapharesis in diffuse alveolar hemorrhage associated with conditions other than Goodpasture syndrome has not been evaluated in prospective studies.
It remains unclear whether intravenous immunoglobulin therapy adds to the treatment of diffuse alveolar hemorrhage due to vasculitis or other connective tissue disease.
Several case reports have reported successful use of recombinant activated human factor VII in treating alveolar hemorrhage due to allogeneic hematopoietic stem cell transplantation, ANCA-associated vasculitis, systemic lupus erythematosus, or antiphospholipid syndrome. If borne out by larger experience, recombinant activated human factor VII may gain more widespread use in diffuse alveolar hemorrhage.
Other possible management measures include supplemental oxygen, bronchodilators, reversal of any coagulopathy, intubation with bronchial tamponade, protective strategies for the less involved lung, and mechanical ventilation.
PROGNOSIS
The prognosis for diffuse alveolar hemorrhage depends on the underlying cause (Table 3).
Recurrent episodes may lead to various degrees of interstitial fibrosis, especially in patients with underlying Wegener granulo-matosis, mitral stenosis, long-standing and severe mitral regurgitation, and idiopathic pulmonary hemosiderosis. Obstructive lung disease may also complicate microscopic polyangiitis and idiopathic pulmonary hemosiderosis.
Acknowledgment: We acknowledge and appreciate the assistance of Dr. Carol Farver, who provided the pathologic specimens.
- Ioachimescu OCLaurent GL, Shapiro SD. Alveolar hemorrhage. Encyclopedia of Respiratory Medicine. Amsterdam: Academic Press, 2006:92–100.
- Travis WD, Colby TV, Lombard C, Carpenter HA. A clinicopathologic study of 34 cases of diffuse pulmonary hemorrhage with lung biopsy confirmation. Am J Surg Pathol 1990; 14:1112–1125.
- Jennings CA, King TE, Tuder R, Cherniak RM, Schwarz MI. Diffuse alveolar hemorrhage with underlying isolated, pauciimmune pulmonary capillaritis. Am J Respir Crit Care Med 1997; 155:1101–1109.
- Spencer H. Pulmonary lesions in polyarteritis nodosa. Br J Tuberc Dis Chest 1957; 51:123–130.
- Travis WD. Pathology of pulmonary vasculitis. Semin Respir Crit Care Med 2004; 25:475–482.
- Schwarz MI, Brown KK. Small vessel vasculitis of the lung. Thorax 2000; 55:502–510.
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25:583–592.
- Ioachimescu OC, Jennings C. Intercostal lung cyst hernia in idiopathic pulmonary hemosiderosis (cyst necessitans). Mayo Clin Proc 2006; 81:692.
- Rolla G, Heffler E, Guida G, Bergia R, Bucca C. Exhaled NO in diffuse alveolar haemorrhage. Thorax 2005; 60:614–615.
- Dweik RA, Stoller JK. Role of bronchoscopy in massive hemoptysis. Clin Chest Med 1999; 20:89–105.
- Ioachimescu OCLaurent GL, Shapiro SD. Autoantibodies. Encyclopedia of Respiratory Medicine. Amsterdam: Academic Press, 2006:219–227.
- Watts RA, Carruthers DM, Scott DG. Epidemiology of systemic vasculitis: changing incidence or definition? Semin Arthritis Rheum 1995; 25:28–34.
- Watts RA, Lane SE, Bentham G, Scott DG. Epidemiology of systemic vasculitis: a ten-year study in the United Kingdom. Arthritis Rheum 2000; 43:414–419.
- Watts RA, Jolliffe VA, Carruthers DM, Lockwood M, Scott DG. Effect of classification on the incidence of polyarteritis nodosa and microscopic polyangiitis. Arthritis Rheum 1996; 39:1208–1212.
- Ioachimescu OC, Kotch A, Stoller JK. Idiopathic pulmonary hemosiderosis in adults. Clin Pulm Med 2005; 12:16–25.
- Reinhold-Keller E, Herlyn K, Wagner-Bastmeyer R, et al. No difference in the incidences of vasculitides between north and south Germany: first results of the German vasculitis register. Rheumatology (Oxford) 2002; 41:540–549.
- Mahr A, Guillevin L, Poissonnet M, Ayme S. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener’s granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: a capture-recapture estimate. Arthritis Rheum 2004; 51:92–99.
- Koldingsnes W, Nossent H. Epidemiology of Wegener’s granulomatosis in northern Norway. Arthritis Rheum 2000; 43:2481–2487.
- Kelly PT, Haponik EF. Goodpasture syndrome: molecular and clinical advances. Medicine (Baltimore) 1994; 73:171–185.
- Travis WD, Leslie KOLeslie KO, Wick MR. Pulmonary vasculitis and pulmonary hemorhage. Practical Pulmonary Pathology – a Diagnostic Approach. Philadelphia: Churchill Livingstone-Elsevier, 2005;335–378.
- Jennette JC, Thomas DB, Falk RJ. Microscopic polyangiitis (microscopic polyarteritis). Semin Diagn Pathol 2001; 18:3–13.
- Katzenstein AKatzenstein A, Askin F. Alveolar hemorrhage syndromes. Surgical Pathology of Non-neoplastic Lung Disease. Philadelphia: WB Saunders, 1997:153–159.
- Schwarz MI, Cherniack RM, King TEMurray JF, Nadel J. Diffuse alveolar hemorrhage and other rare infiltrative disorders. Textbook of Respiratory Medicine. Philadelphia: WB Saunders, 2000:1733–1755.
- Lynch JP, Leatherman JWFishman A. Alveolar hemorrhage syndromes. Fishman’s Pulmonary Diseases and Disorders. New York: McGraw-Hill, 1998:1193–1210.
- Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest 1990; 97:906–912.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Fauci AS, Haynes BF, Katz P, Wolff SM. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 1983; 98:76–85.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Langford CA, Hoffman GS. Rare diseases 3: Wegener’s granulomatosis. Thorax 1999; 54:629–637.
- Mark EJ, Matsubara O, Tan-Liu NS, Fienberg R. The pulmonary biopsy in the early diagnosis of Wegener’s (pathergic) granulomatosis: a study based on 35 open lung biopsies. Hum Pathol 1988; 19:1065–1071.
- Sheehan RE, Flint JD, Muller NL. Computed tomography features of the thoracic manifestations of Wegener granulomatosis. J Thorac Imaging 2003: 18:34–41.
- Specks USchwarz MI, King TE. Pulmonary vasculitis. Interstitial Lung Disease. Decker BC. Hamilton, Ontario, Canada: Decker, 2003:599–631.
- Ten Berge IJ, Wilmink JM, Meyer CJ, et al. Clinical and immunological follow-up of patients with severe renal disease in Wegener’s granulo-matosis. Am J Nephrol 1985; 5:21–29.
- Brandwein S, Esdaile J, Danoff D, Tannenbaum H. Wegener’s granulo-matosis. Clinical features and outcome in 13 patients. Arch Intern Med 1983; 143:476–479.
- Pinching AJ, Lockwood CM, Pussell BA, et al. Wegener’s granulomatosis: observations on 18 patients with severe renal disease. Q J Med 1983; 52:435–460.
- Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med 1997; 337:1512–1523.
- Lauque D, Cadranel J, Lazor R, et al. Microscopic polyangiitis with alveolar hemorrhage. A study of 29 cases and review of the literature. Groupe d’Études et de Recherche sur les Maladies “Orphelines” Pulmonaires. Medicine (Baltimore) 2000; 79:222–233.
- Johnson JP, Moore J, Austin HA, Balow JE, Antonovych TT, Wilson CB. Therapy of anti-glomerular basement membrane antibody disease: analysis of prognostic significance of clinical, pathologic and treatment factors. Medicine (Baltimore) 1985; 64:219–227.
- Savage CO, Winearls CG, Evans DJ, Rees AJ, Lockwood CM. Microscopic polyarteritis: presentation, pathology, and prognosis. Q J Med 1985; 56:467–483.
- Haworth SJ, Savage CO, Carr D. Pulmonary hemorrhage complicating Wegener’s granulomatosis and microscopic polyarteritis. Br Med J 1985; 290:1175–1178.
- Smyth L, Gaskin G, Pusey CD. Microscopic polyangiitis. Semin Respir Crit Care Med 2004; 25:523–533.
- Lanham JG, Elkon KB, Pusey CD, Hughes GR. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine (Baltimore) 1984; 63:65–81.
- Leatherman JW. Autoimmune diffuse alveolar hemorrhage. Clin Pulm Med 1994; 1:356–364.
- Boyce NW, Holdsworth SR. Pulmonary manifestations of the clinical syndrome of acute glomerulonephritis and lung hemorrhage. Am J Kidney Dis 1986; 8:31–36.
- Emlen W. Systemic lupus erythematosus and mixed connective tissue disease. Immunol Allergy Clin North Am 1979; 105:291–311.
- Hunninghake GW, Fauci AS. Pulmonary involvement in the collagen vascular diseases. Am Rev Respir Dis 1979; 119:471–503.
- Keane MP, Lynch JP. Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax 2000; 55:159–166.
- Zamora MR, Warner ML, Tuder R, Schwarz MI. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltimore) 1997; 76:192–202.
- Lee CK, Koh JH, Cha HS, et al. Pulmonary alveolar hemorrhage in patients with rheumatic diseases in Korea. Scand J Rheumatol 2000; 29:288–294.
- Vazquez-Del Mercado M, Mendoza-Topete A, Best-Aguilera CR, Garcia-De La Torre I. Diffuse alveolar hemorrhage in limited cutaneous systemic sclerosis with positive perinuclear antineutrophil cytoplasmic antibodies. J Rheumatol 1996; 23:1821–1823.
- Fenlon HM, Doran M, Sant SM, Breatnach E. High-resolution chest CT in systemic lupus erythematosus. AJR Am J Roentgenol 1996; 166:301–307.
- Ioachimescu OC. Idiopathic pulmonary hemosiderosis in adults. Pneumologia 2003; 52:38–43.
- Ioachimescu OC, Sieber S, Kotch A. Idiopathic pulmonary haemosiderosis revisited. Eur Respir J 2004; 24:162–170.
- Franks TJ, Koss MN. Pulmonary capillaritis. Curr Opin Pulm Med 2000; 6:430–435.
- Travis WD, Hoffman GS, Leavitt RY, Pass HI, Fauci AS. Surgical pathology of the lung in Wegener’s granulomatosis. Review of 87 open lung biopsies from 67 patients. Am J Surg Pathol 1991; 15:315–333.
- Zashin S, Fattor R, Fortin D. Microscopic polyarteritis: a forgotten aetiology of haemoptysis and rapidly progressive glomerulonephritis. Ann Rheum Dis 1990; 49:53–56.
- Yoshikawa Y, Watanabe T. Pulmonary lesions in Wegener’s granulo-matosis: a clinicopathologic study of 22 autopsy cases. Hum Pathol 1986; 17:401–410.
- Teague CA, Doak PB, Simpson IJ, Rainer SP, Herdson PB. Goodpasture’s syndrome: an analysis of 29 cases. Kidney Int 1978; 13:492–504.
- Abu-Shakra M, Smythe H, Lewtas J, Badley E, Weber D, Keystone E. Outcome of polyarteritis nodosa and Churg-Strauss syndrome. An analysis of twenty-five patients. Arthritis Rheum 1994; 37:1798–1803.
- Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine (Baltimore) 1999; 78:26–37.
- Schwab EP, Schumacher HR, Freundlich B, Callegari PE. Pulmonary alveolar hemorrhage in systemic lupus erythematosus. Semin Arthritis Rheum 1993; 23:8–15.
- Koh WH, Thumboo J, Boey ML. Pulmonary haemorrhage in Oriental patients with systemic lupus erythematosus. Lupus 1997; 6:713–716.
Diffuse alveolar hemorrhage can complicate a large number of clinical conditions. It may present in different ways and may be life-threatening, and it poses an important challenge for the clinician.1
Diffuse alveolar hemorrhage is an uncommon condition in which blood floods the alveoli, usually at multiple sites. It is also known as intrapulmonary hemorrhage, diffuse pulmonary hemorrhage, pulmonary alveolar hemorrhage, pulmonary capillary hemorrhage, alveolar bleeding, or microvascular pulmonary hemorrhage.
In this article we review the causes, clinical features, diagnostic criteria, treatment, and prognosis of diffuse alveolar hemorrhage.
CAUSES OF DIFFUSE ALVEOLAR HEMORRHAGE
THREE CHARACTERISTIC PATTERNS
In general, diffuse alveolar hemorrhage can occur in three characteristic patterns, which reflect the nature of the underlying vascular injury1:
Diffuse alveolar hemorrhage associated with vasculitis or capillaritis. As described by Spencer4 50 years ago, pulmonary capillaritis is the most frequent underlying histologic lesion described in diffuse alveolar hemorrhage. Neutrophils infiltrate the interalveolar and peri-bronchiolar septal vessels (pulmonary interstitium),5 leading to anatomic disruption of the capillaries (ie, impairment of the alveolocapillary barrier) and to extravasation of red blood cells into the alveoli and interstitium. Neutrophil apoptosis and fragmentation, with subsequent release of the intracellular proteolytic enzymes and reactive oxygen species, beget more inflammation, intra-alveolar neutrophilic nuclear dust, fibrin and inflammatory exudate, and fibrinoid necrosis of the interstitium.6,7
‘Bland’ pulmonary hemorrhage (ie, without capillaritis or vasculitis). In this pattern, red blood cells leak into the alveoli without any evidence of inflammation or destruction of the alveolar capillaries, venules, and arterioles. The epithelial lesions are usually microscopic and are scattered geographically.
Diffuse alveolar hemorrhage associated with another process or condition (eg, diffuse alveolar damage, lymphangioleiomyomatosis, drug-induced lung injury, metastatic tumor to the lungs, mitral stenosis). Diffuse alveolar damage is the main underlying lesion of the acute respiratory distress syndrome and is characterized by formation of an intra-alveolar hyaline membrane, by interstitial edema with minimal inflammation, and, at times, by “secondary” diffuse alveolar hemorrhage. In this third category of diffuse alveolar hemorrhage, the underlying process causes alveolar hemorrhage by processes other than pulmonary vascular inflammation or direct extravasation of red cells.
THE CLINICAL PRESENTATION
The clinical presentation of diffuse alveolar hemorrhage may reflect either alveolar bleeding alone or features of the underlying cause (eg, hematuria in Wegener granulomatosis, arthritis in systemic lupus erythematosus). Hence, its recognition requires a high degree of suspicion.
Some patients present with severe acute respiratory distress requiring mechanical ventilation. However, dyspnea, cough, and fever are the common initial symptoms and are most often acute or subacute (ie, present for less than a week). The fever is usually due to the underlying cause, such as lupus.
Hemoptysis may be absent at the time of presentation in up to a third of patients because the total alveolar volume is large and can absorb large amounts of blood, without extending more proximally into the airways. Apparent hemoptysis, if present, must be differentiated from hematemesis or pseudohemoptysis (alveolar flooding with fluid that resembles blood, as in Serratia marcescens pneumonia, in which the reddish hue of the infecting organism can create the impression of alveolar bleeding).
DIAGNOSTIC EVALUATION
Generally speaking, dyspnea, cough, hemoptysis, and new alveolar infiltrates in conjunction with bloody bronchoalveolar lavage specimens (with numerous erythrocytes and siderophages) establish the diagnosis of diffuse alveolar hemorrhage. Surgical biopsy from the lung or another organ involved by an underlying condition is often necessary.
Physical examination
The physical findings are nonspecific and may reflect the underlying systemic vasculitis or collagen vascular disorder (eg, with accompanying rash, purpura, eye lesions, hepatosplenomegaly, or clubbing).
Imaging studies
Radiography may show new or old or both new and old patchy or diffuse alveolar opacities. Recurrent episodes of hemorrhage may lead to reticular interstitial opacities due to pulmonary fibrosis, usually with minimal (if any) honeycombing. Kerley B lines suggest mitral valve disease or pulmonary veno-occlusive disease as the cause of the hemorrhage.
Computed tomography may show areas of consolidation interspersed with areas of ground-glass attenuation and preserved, normal areas.
Currently, nuclear imaging such as gallium or tagged red blood cell studies have little role in evaluating diffuse alveolar hemorrhage. Other nuclear studies, geared to reveal breakdown of the microcirculatory integrity and extravasation of red blood cells out of the vessels, have also not been proven useful.
Evaluating pulmonary function
Diffuse alveolar hemorrhage may cause impairment of oxygen transfer and hypoxemia. In addition, it can cause several other abnormalities of pulmonary function.
Increased diffusing capacity. Because blood in the lungs can absorb inhaled carbon monoxide, the diffusing capacity for carbon monoxide (DLCO) may be distinctively increased. Serial increases in the DLCO may indicate progressive alveolar hemorrhage. However, the clinical instability of patients experiencing active alveolar bleeding precludes performing the DLCO measurement maneuvers, rendering the DLCO test relatively impractical.
Restrictive changes. Because recurrent episodes of diffuse alveolar hemorrhage can lead to interstitial fibrosis, restrictive changes—ie, decreased total lung capacity, decreased forced vital capacity (FVC), and preserved ratio of the forced expiratory volume in 1 second (FEV1) to the FVC—may characterize diffuse alveolar hemorrhage.
Obstructive changes (less common). Less commonly, patients with diffuse alveolar hemorrhage may have spirometric changes indicating airflow obstruction—ie, decreased FEV1 and decreased ratio of FEV1 to FVC—possibly because neutrophilic infiltration from blood extravasation into the alveolar sacs causes release of reactive oxygen species and proteolytic enzymes, which in turn may cause small airway and parenchymal damage such as bronchiolitis and emphysema. A pattern of obstructive lung disease associated with recurrent diffuse alveolar hemorrhage should prompt consideration of an underlying condition that can cause airflow obstruction, such as sarcoidosis, microscopic polyangiitis, or Wegener granulomatosis, or, less commonly, lymphangioleiomyomatosis, histiocytosis X, pulmonary capillaritis, or sometimes idiopathic pulmonary hemosiderosis.
As an example of an unusual circumstance, we have described elsewhere a case of a woman with idiopathic pulmonary hemosiderosis with multiple episodes of diffuse alveolar hemorrhage and resultant emphysema.8 Radiographic images showed several very large cysts, one of which herniated through the incision site of an open lung biopsy.
Decreased exhaled nitric oxide. Though currently unavailable in most clinical pulmonary function laboratories, evaluation of exhaled gas or condensate may have value in diagnosing diffuse alveolar hemorrhage.9 Specifically, because increased intra-alveolar hemoglobin binds nitric oxide, as it does carbon monoxide, levels of exhaled nitric oxide may be decreased in diffuse alveolar hemorrhage. In contrast to the difficulty of measuring DLCO in patients with active alveolar bleeding or hemoptysis, analysis of exhaled gas is clinically feasible, making this a promising diagnostic test.
Laboratory evaluation
Hematologic assessment in patients with diffuse alveolar hemorrhage generally reveals:
- Acute or chronic anemia
- Leukocytosis
- Elevated erythrocyte sedimentation rate
- Elevated C-reactive protein level (particularly in patients whose alveolar hemorrhage is due to systemic disease or vasculitis, or both).
Renal abnormalities such as elevated blood urea nitrogen and serum creatinine or abnormal findings on urinalysis (with hematuria, proteinuria, and red blood cell casts indicating glomerulonephritis) can also occur, as diffuse alveolar hemorrhage may complicate several pulmonary-renal syndromes such as Goodpasture syndrome and Wegener granulomatosis.
Bronchoscopy
The diagnostic evaluation in diffuse alveolar hemorrhage usually includes bronchoscopic examination,10 which serves two purposes:
- To document alveolar hemorrhage by bronchoalveolar lavage and to exclude airway sources of bleeding by visual inspection
- To exclude an associated infection.
Based on experience with nonmassive hemoptysis of all causes (but not exclusively diffuse alveolar hemorrhage), the diagnostic yield of bronchoscopy is higher if the procedure is performed within the first 48 hours of symptoms rather than later. Evidence supporting diffuse alveolar hemorrhage is persistent (or even increasing) blood on three sequential lavage aliquots from a single affected area of the lung.
FINDING THE UNDERLYING CAUSE
Once the diagnosis of diffuse alveolar hemorrhage is established, the clinician must ascertain whether an underlying cause is present. Serologic studies may prove important, although the results are generally not available in a manner timely enough to guide immediate management.
When a pulmonary-renal syndrome is suggested by accompanying hematuria or renal dysfunction, antiglomerular basement membrane antibody and antineutrophil cytoplasmic antibody (ANCA) levels should be checked. Tests for complement fractions C3 and C4, anti-double-stranded DNA, and antiphospholipid antibodies should be ordered if an underlying condition such as lupus or antiphospholipid antibody syndrome is suspected (Table 2).11
If the underlying cause remains elusive after a thorough clinical evaluation that includes imaging studies, serologic studies, and bronchoscopy, then surgical biopsy should be considered.1 Which organ to biopsy (eg, lung, sinus, kidney) depends on the level of suspicion for a specific cause. For example, suspicion of Wegener granulomato-sis with hematuria or renal dysfunction might prompt renal biopsy. However, lung biopsy often needs to be performed with video-assisted thoracoscopy, especially when disease is confined to the lung (as in idiopathic pulmonary hemosiderosis or pauci-immune pulmonary capillaritis). Renal biopsy specimens should also undergo immunofluores-cence staining, which may reveal linear deposition of immunoglobulins and immune complexes along the basement membrane in patients with Goodpasture syndrome, or of granular deposits in patients with systemic lupus erythematosus.
TWO GENERAL CLINICAL SCENARIOS
In general, the clinician will be confronted by one of two scenarios: a patient with diffuse alveolar hemorrhage and associated systemic findings, or a patient with hemorrhage and no associated systemic findings.
Hemorrhage with associated systemic findings
Certain clues from the history raise suspicion of diffuse alveolar hemorrhage:
- Recent infection suggests Henoch-Schönlein purpura or cryoglobulinemic vasculitis
- Use of a possibly offending drug such as an anticoagulant, D-penicillamine (Cuprimine, Depen), nitrofurantoin (Furadantin, Macrobid, Macrodantin), amiodarone (Cordarone), propylthiouracil, cocaine, or sirolimus (Rapamune, Rapamycin)
- Exposure to toxic agents such as trimellitic anhydride, insecticides, and pesticides
- A known comorbid condition such as vasculitis, connective tissue disease, mitral valve disease, or solid organ or stem cell transplantation.
If asthma, eosinophilia, pulmonary infiltrates, and diffuse alveolar hemorrhage coexist, consideration should be given to Churg-Strauss syndrome. If sinus disease, skin manifestations, pulmonary parenchymal nodules, and cavitary lesions coexist with positivity for antiproteinase 3 c-ANCA and biopsy-proven granulomata, then Wegener granulomatosis should be considered. Similarly, diffuse alveolar hemorrhage with glomerulonephritis and skin manifestations, positivity for p-ANCA, and necrotizing nongranulomatous lesions on end-organ biopsy may lead to a diagnosis of microscopic polyangiitis. In a young smoker with glomeru-lonephritis and diffuse alveolar hemorrhage presenting as either bland alveolar hemorrhage or pulmonary capillaritis, Goodpasture syndrome or antiglomerular basement membrane antibody disease should be considered.
Hemorrhage with no associated systemic findings
When the above conditions have been considered but no suggestive findings are found, the following four conditions should be considered:
- Antiglomerular basement membrane antibody disease in limited pulmonary form or onset: positivity to the antibody with linear deposits in the lungs would be diagnostic in such a case
- Pulmonary-limited microscopic polyangiitis positive for p-ANCA (a positive anti-myeloperoxidase p-ANCA test makes the diagnosis)
- Pauci-immune isolated pulmonary capillaritis, when the biopsy shows evidence of neutrophilic pulmonary capillaritis
- Idiopathic pulmonary hemosiderosis, a diagnosis of exclusion, when the biopsy shows evidence of acute, subacute, and chronic bland diffuse alveolar hemorrhage and no evidence of vasculitis.
TREATMENT OF DIFFUSE ALVEOLAR HEMORRHAGE
Therapy for diffuse alveolar hemorrhage consists of treating both the autoimmune destruction of the alveolar capillary membrane and the underlying condition. Corticosteroids and immunosuppressive agents remain the gold standard for most patients. Recombinant-activated human factor VII seems to be a promising new therapy, although further evaluation is needed.
Immunosuppressive agents are the mainstay of therapy for diffuse alveolar hemorrhage, especially if associated with systemic or pulmonary vasculitis, Goodpasture syndrome, and connective tissue disorders. Most experts recommend intravenous methylprednisolone (Solu-Medrol) (up to 500 mg every 6 hours, although lower doses seem to have similar efficacy) for 4 or 5 days, followed by a gradual taper to maintenance doses of oral steroids.
In patients with pulmonary-renal syndrome, therapy should be started as soon as possible to prevent irreversible renal failure.
Besides corticosteroids, other immunosuppressive drugs such as cyclophosphamide (Cytoxan), azathioprine (Imuran), mycophenolate mofetil (CellCept), and etanercept (Enbrel) may be used in diffuse alveolar hemorrhage, especially when the condition is severe, when first-line therapy with corticosteroids has proven ineffective (generally not advised, unless the condition is mild) or when specific underlying causes are present (eg, Wegener granulomatosis, Goodpasture syndrome, systemic lupus erythematosus). Intravenous cyclophosphamide (2 mg/kg/day, adjusted to renal function) is generally the preferred adjunctive immunosuppressive drug and may be continued for several weeks or until adverse effects occur, such as blood marrow suppression, infection, or hematuria. Thereafter, most clinicians switch to consolidative or maintenance therapy with methotrexate or another agent.
Plasmapheresis is indicated for diffuse alveolar hemorrhage associated with Good-pasture syndrome or with other vasculitic processes in which the titers of pathogenetic immunoglobulins and immune complexes are very high: for example, ANCA-associated vasculitis with overwhelming endothelial injury and a hypercoagulable state. However, the merits of plasmapharesis in diffuse alveolar hemorrhage associated with conditions other than Goodpasture syndrome has not been evaluated in prospective studies.
It remains unclear whether intravenous immunoglobulin therapy adds to the treatment of diffuse alveolar hemorrhage due to vasculitis or other connective tissue disease.
Several case reports have reported successful use of recombinant activated human factor VII in treating alveolar hemorrhage due to allogeneic hematopoietic stem cell transplantation, ANCA-associated vasculitis, systemic lupus erythematosus, or antiphospholipid syndrome. If borne out by larger experience, recombinant activated human factor VII may gain more widespread use in diffuse alveolar hemorrhage.
Other possible management measures include supplemental oxygen, bronchodilators, reversal of any coagulopathy, intubation with bronchial tamponade, protective strategies for the less involved lung, and mechanical ventilation.
PROGNOSIS
The prognosis for diffuse alveolar hemorrhage depends on the underlying cause (Table 3).
Recurrent episodes may lead to various degrees of interstitial fibrosis, especially in patients with underlying Wegener granulo-matosis, mitral stenosis, long-standing and severe mitral regurgitation, and idiopathic pulmonary hemosiderosis. Obstructive lung disease may also complicate microscopic polyangiitis and idiopathic pulmonary hemosiderosis.
Acknowledgment: We acknowledge and appreciate the assistance of Dr. Carol Farver, who provided the pathologic specimens.
Diffuse alveolar hemorrhage can complicate a large number of clinical conditions. It may present in different ways and may be life-threatening, and it poses an important challenge for the clinician.1
Diffuse alveolar hemorrhage is an uncommon condition in which blood floods the alveoli, usually at multiple sites. It is also known as intrapulmonary hemorrhage, diffuse pulmonary hemorrhage, pulmonary alveolar hemorrhage, pulmonary capillary hemorrhage, alveolar bleeding, or microvascular pulmonary hemorrhage.
In this article we review the causes, clinical features, diagnostic criteria, treatment, and prognosis of diffuse alveolar hemorrhage.
CAUSES OF DIFFUSE ALVEOLAR HEMORRHAGE
THREE CHARACTERISTIC PATTERNS
In general, diffuse alveolar hemorrhage can occur in three characteristic patterns, which reflect the nature of the underlying vascular injury1:
Diffuse alveolar hemorrhage associated with vasculitis or capillaritis. As described by Spencer4 50 years ago, pulmonary capillaritis is the most frequent underlying histologic lesion described in diffuse alveolar hemorrhage. Neutrophils infiltrate the interalveolar and peri-bronchiolar septal vessels (pulmonary interstitium),5 leading to anatomic disruption of the capillaries (ie, impairment of the alveolocapillary barrier) and to extravasation of red blood cells into the alveoli and interstitium. Neutrophil apoptosis and fragmentation, with subsequent release of the intracellular proteolytic enzymes and reactive oxygen species, beget more inflammation, intra-alveolar neutrophilic nuclear dust, fibrin and inflammatory exudate, and fibrinoid necrosis of the interstitium.6,7
‘Bland’ pulmonary hemorrhage (ie, without capillaritis or vasculitis). In this pattern, red blood cells leak into the alveoli without any evidence of inflammation or destruction of the alveolar capillaries, venules, and arterioles. The epithelial lesions are usually microscopic and are scattered geographically.
Diffuse alveolar hemorrhage associated with another process or condition (eg, diffuse alveolar damage, lymphangioleiomyomatosis, drug-induced lung injury, metastatic tumor to the lungs, mitral stenosis). Diffuse alveolar damage is the main underlying lesion of the acute respiratory distress syndrome and is characterized by formation of an intra-alveolar hyaline membrane, by interstitial edema with minimal inflammation, and, at times, by “secondary” diffuse alveolar hemorrhage. In this third category of diffuse alveolar hemorrhage, the underlying process causes alveolar hemorrhage by processes other than pulmonary vascular inflammation or direct extravasation of red cells.
THE CLINICAL PRESENTATION
The clinical presentation of diffuse alveolar hemorrhage may reflect either alveolar bleeding alone or features of the underlying cause (eg, hematuria in Wegener granulomatosis, arthritis in systemic lupus erythematosus). Hence, its recognition requires a high degree of suspicion.
Some patients present with severe acute respiratory distress requiring mechanical ventilation. However, dyspnea, cough, and fever are the common initial symptoms and are most often acute or subacute (ie, present for less than a week). The fever is usually due to the underlying cause, such as lupus.
Hemoptysis may be absent at the time of presentation in up to a third of patients because the total alveolar volume is large and can absorb large amounts of blood, without extending more proximally into the airways. Apparent hemoptysis, if present, must be differentiated from hematemesis or pseudohemoptysis (alveolar flooding with fluid that resembles blood, as in Serratia marcescens pneumonia, in which the reddish hue of the infecting organism can create the impression of alveolar bleeding).
DIAGNOSTIC EVALUATION
Generally speaking, dyspnea, cough, hemoptysis, and new alveolar infiltrates in conjunction with bloody bronchoalveolar lavage specimens (with numerous erythrocytes and siderophages) establish the diagnosis of diffuse alveolar hemorrhage. Surgical biopsy from the lung or another organ involved by an underlying condition is often necessary.
Physical examination
The physical findings are nonspecific and may reflect the underlying systemic vasculitis or collagen vascular disorder (eg, with accompanying rash, purpura, eye lesions, hepatosplenomegaly, or clubbing).
Imaging studies
Radiography may show new or old or both new and old patchy or diffuse alveolar opacities. Recurrent episodes of hemorrhage may lead to reticular interstitial opacities due to pulmonary fibrosis, usually with minimal (if any) honeycombing. Kerley B lines suggest mitral valve disease or pulmonary veno-occlusive disease as the cause of the hemorrhage.
Computed tomography may show areas of consolidation interspersed with areas of ground-glass attenuation and preserved, normal areas.
Currently, nuclear imaging such as gallium or tagged red blood cell studies have little role in evaluating diffuse alveolar hemorrhage. Other nuclear studies, geared to reveal breakdown of the microcirculatory integrity and extravasation of red blood cells out of the vessels, have also not been proven useful.
Evaluating pulmonary function
Diffuse alveolar hemorrhage may cause impairment of oxygen transfer and hypoxemia. In addition, it can cause several other abnormalities of pulmonary function.
Increased diffusing capacity. Because blood in the lungs can absorb inhaled carbon monoxide, the diffusing capacity for carbon monoxide (DLCO) may be distinctively increased. Serial increases in the DLCO may indicate progressive alveolar hemorrhage. However, the clinical instability of patients experiencing active alveolar bleeding precludes performing the DLCO measurement maneuvers, rendering the DLCO test relatively impractical.
Restrictive changes. Because recurrent episodes of diffuse alveolar hemorrhage can lead to interstitial fibrosis, restrictive changes—ie, decreased total lung capacity, decreased forced vital capacity (FVC), and preserved ratio of the forced expiratory volume in 1 second (FEV1) to the FVC—may characterize diffuse alveolar hemorrhage.
Obstructive changes (less common). Less commonly, patients with diffuse alveolar hemorrhage may have spirometric changes indicating airflow obstruction—ie, decreased FEV1 and decreased ratio of FEV1 to FVC—possibly because neutrophilic infiltration from blood extravasation into the alveolar sacs causes release of reactive oxygen species and proteolytic enzymes, which in turn may cause small airway and parenchymal damage such as bronchiolitis and emphysema. A pattern of obstructive lung disease associated with recurrent diffuse alveolar hemorrhage should prompt consideration of an underlying condition that can cause airflow obstruction, such as sarcoidosis, microscopic polyangiitis, or Wegener granulomatosis, or, less commonly, lymphangioleiomyomatosis, histiocytosis X, pulmonary capillaritis, or sometimes idiopathic pulmonary hemosiderosis.
As an example of an unusual circumstance, we have described elsewhere a case of a woman with idiopathic pulmonary hemosiderosis with multiple episodes of diffuse alveolar hemorrhage and resultant emphysema.8 Radiographic images showed several very large cysts, one of which herniated through the incision site of an open lung biopsy.
Decreased exhaled nitric oxide. Though currently unavailable in most clinical pulmonary function laboratories, evaluation of exhaled gas or condensate may have value in diagnosing diffuse alveolar hemorrhage.9 Specifically, because increased intra-alveolar hemoglobin binds nitric oxide, as it does carbon monoxide, levels of exhaled nitric oxide may be decreased in diffuse alveolar hemorrhage. In contrast to the difficulty of measuring DLCO in patients with active alveolar bleeding or hemoptysis, analysis of exhaled gas is clinically feasible, making this a promising diagnostic test.
Laboratory evaluation
Hematologic assessment in patients with diffuse alveolar hemorrhage generally reveals:
- Acute or chronic anemia
- Leukocytosis
- Elevated erythrocyte sedimentation rate
- Elevated C-reactive protein level (particularly in patients whose alveolar hemorrhage is due to systemic disease or vasculitis, or both).
Renal abnormalities such as elevated blood urea nitrogen and serum creatinine or abnormal findings on urinalysis (with hematuria, proteinuria, and red blood cell casts indicating glomerulonephritis) can also occur, as diffuse alveolar hemorrhage may complicate several pulmonary-renal syndromes such as Goodpasture syndrome and Wegener granulomatosis.
Bronchoscopy
The diagnostic evaluation in diffuse alveolar hemorrhage usually includes bronchoscopic examination,10 which serves two purposes:
- To document alveolar hemorrhage by bronchoalveolar lavage and to exclude airway sources of bleeding by visual inspection
- To exclude an associated infection.
Based on experience with nonmassive hemoptysis of all causes (but not exclusively diffuse alveolar hemorrhage), the diagnostic yield of bronchoscopy is higher if the procedure is performed within the first 48 hours of symptoms rather than later. Evidence supporting diffuse alveolar hemorrhage is persistent (or even increasing) blood on three sequential lavage aliquots from a single affected area of the lung.
FINDING THE UNDERLYING CAUSE
Once the diagnosis of diffuse alveolar hemorrhage is established, the clinician must ascertain whether an underlying cause is present. Serologic studies may prove important, although the results are generally not available in a manner timely enough to guide immediate management.
When a pulmonary-renal syndrome is suggested by accompanying hematuria or renal dysfunction, antiglomerular basement membrane antibody and antineutrophil cytoplasmic antibody (ANCA) levels should be checked. Tests for complement fractions C3 and C4, anti-double-stranded DNA, and antiphospholipid antibodies should be ordered if an underlying condition such as lupus or antiphospholipid antibody syndrome is suspected (Table 2).11
If the underlying cause remains elusive after a thorough clinical evaluation that includes imaging studies, serologic studies, and bronchoscopy, then surgical biopsy should be considered.1 Which organ to biopsy (eg, lung, sinus, kidney) depends on the level of suspicion for a specific cause. For example, suspicion of Wegener granulomato-sis with hematuria or renal dysfunction might prompt renal biopsy. However, lung biopsy often needs to be performed with video-assisted thoracoscopy, especially when disease is confined to the lung (as in idiopathic pulmonary hemosiderosis or pauci-immune pulmonary capillaritis). Renal biopsy specimens should also undergo immunofluores-cence staining, which may reveal linear deposition of immunoglobulins and immune complexes along the basement membrane in patients with Goodpasture syndrome, or of granular deposits in patients with systemic lupus erythematosus.
TWO GENERAL CLINICAL SCENARIOS
In general, the clinician will be confronted by one of two scenarios: a patient with diffuse alveolar hemorrhage and associated systemic findings, or a patient with hemorrhage and no associated systemic findings.
Hemorrhage with associated systemic findings
Certain clues from the history raise suspicion of diffuse alveolar hemorrhage:
- Recent infection suggests Henoch-Schönlein purpura or cryoglobulinemic vasculitis
- Use of a possibly offending drug such as an anticoagulant, D-penicillamine (Cuprimine, Depen), nitrofurantoin (Furadantin, Macrobid, Macrodantin), amiodarone (Cordarone), propylthiouracil, cocaine, or sirolimus (Rapamune, Rapamycin)
- Exposure to toxic agents such as trimellitic anhydride, insecticides, and pesticides
- A known comorbid condition such as vasculitis, connective tissue disease, mitral valve disease, or solid organ or stem cell transplantation.
If asthma, eosinophilia, pulmonary infiltrates, and diffuse alveolar hemorrhage coexist, consideration should be given to Churg-Strauss syndrome. If sinus disease, skin manifestations, pulmonary parenchymal nodules, and cavitary lesions coexist with positivity for antiproteinase 3 c-ANCA and biopsy-proven granulomata, then Wegener granulomatosis should be considered. Similarly, diffuse alveolar hemorrhage with glomerulonephritis and skin manifestations, positivity for p-ANCA, and necrotizing nongranulomatous lesions on end-organ biopsy may lead to a diagnosis of microscopic polyangiitis. In a young smoker with glomeru-lonephritis and diffuse alveolar hemorrhage presenting as either bland alveolar hemorrhage or pulmonary capillaritis, Goodpasture syndrome or antiglomerular basement membrane antibody disease should be considered.
Hemorrhage with no associated systemic findings
When the above conditions have been considered but no suggestive findings are found, the following four conditions should be considered:
- Antiglomerular basement membrane antibody disease in limited pulmonary form or onset: positivity to the antibody with linear deposits in the lungs would be diagnostic in such a case
- Pulmonary-limited microscopic polyangiitis positive for p-ANCA (a positive anti-myeloperoxidase p-ANCA test makes the diagnosis)
- Pauci-immune isolated pulmonary capillaritis, when the biopsy shows evidence of neutrophilic pulmonary capillaritis
- Idiopathic pulmonary hemosiderosis, a diagnosis of exclusion, when the biopsy shows evidence of acute, subacute, and chronic bland diffuse alveolar hemorrhage and no evidence of vasculitis.
TREATMENT OF DIFFUSE ALVEOLAR HEMORRHAGE
Therapy for diffuse alveolar hemorrhage consists of treating both the autoimmune destruction of the alveolar capillary membrane and the underlying condition. Corticosteroids and immunosuppressive agents remain the gold standard for most patients. Recombinant-activated human factor VII seems to be a promising new therapy, although further evaluation is needed.
Immunosuppressive agents are the mainstay of therapy for diffuse alveolar hemorrhage, especially if associated with systemic or pulmonary vasculitis, Goodpasture syndrome, and connective tissue disorders. Most experts recommend intravenous methylprednisolone (Solu-Medrol) (up to 500 mg every 6 hours, although lower doses seem to have similar efficacy) for 4 or 5 days, followed by a gradual taper to maintenance doses of oral steroids.
In patients with pulmonary-renal syndrome, therapy should be started as soon as possible to prevent irreversible renal failure.
Besides corticosteroids, other immunosuppressive drugs such as cyclophosphamide (Cytoxan), azathioprine (Imuran), mycophenolate mofetil (CellCept), and etanercept (Enbrel) may be used in diffuse alveolar hemorrhage, especially when the condition is severe, when first-line therapy with corticosteroids has proven ineffective (generally not advised, unless the condition is mild) or when specific underlying causes are present (eg, Wegener granulomatosis, Goodpasture syndrome, systemic lupus erythematosus). Intravenous cyclophosphamide (2 mg/kg/day, adjusted to renal function) is generally the preferred adjunctive immunosuppressive drug and may be continued for several weeks or until adverse effects occur, such as blood marrow suppression, infection, or hematuria. Thereafter, most clinicians switch to consolidative or maintenance therapy with methotrexate or another agent.
Plasmapheresis is indicated for diffuse alveolar hemorrhage associated with Good-pasture syndrome or with other vasculitic processes in which the titers of pathogenetic immunoglobulins and immune complexes are very high: for example, ANCA-associated vasculitis with overwhelming endothelial injury and a hypercoagulable state. However, the merits of plasmapharesis in diffuse alveolar hemorrhage associated with conditions other than Goodpasture syndrome has not been evaluated in prospective studies.
It remains unclear whether intravenous immunoglobulin therapy adds to the treatment of diffuse alveolar hemorrhage due to vasculitis or other connective tissue disease.
Several case reports have reported successful use of recombinant activated human factor VII in treating alveolar hemorrhage due to allogeneic hematopoietic stem cell transplantation, ANCA-associated vasculitis, systemic lupus erythematosus, or antiphospholipid syndrome. If borne out by larger experience, recombinant activated human factor VII may gain more widespread use in diffuse alveolar hemorrhage.
Other possible management measures include supplemental oxygen, bronchodilators, reversal of any coagulopathy, intubation with bronchial tamponade, protective strategies for the less involved lung, and mechanical ventilation.
PROGNOSIS
The prognosis for diffuse alveolar hemorrhage depends on the underlying cause (Table 3).
Recurrent episodes may lead to various degrees of interstitial fibrosis, especially in patients with underlying Wegener granulo-matosis, mitral stenosis, long-standing and severe mitral regurgitation, and idiopathic pulmonary hemosiderosis. Obstructive lung disease may also complicate microscopic polyangiitis and idiopathic pulmonary hemosiderosis.
Acknowledgment: We acknowledge and appreciate the assistance of Dr. Carol Farver, who provided the pathologic specimens.
- Ioachimescu OCLaurent GL, Shapiro SD. Alveolar hemorrhage. Encyclopedia of Respiratory Medicine. Amsterdam: Academic Press, 2006:92–100.
- Travis WD, Colby TV, Lombard C, Carpenter HA. A clinicopathologic study of 34 cases of diffuse pulmonary hemorrhage with lung biopsy confirmation. Am J Surg Pathol 1990; 14:1112–1125.
- Jennings CA, King TE, Tuder R, Cherniak RM, Schwarz MI. Diffuse alveolar hemorrhage with underlying isolated, pauciimmune pulmonary capillaritis. Am J Respir Crit Care Med 1997; 155:1101–1109.
- Spencer H. Pulmonary lesions in polyarteritis nodosa. Br J Tuberc Dis Chest 1957; 51:123–130.
- Travis WD. Pathology of pulmonary vasculitis. Semin Respir Crit Care Med 2004; 25:475–482.
- Schwarz MI, Brown KK. Small vessel vasculitis of the lung. Thorax 2000; 55:502–510.
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25:583–592.
- Ioachimescu OC, Jennings C. Intercostal lung cyst hernia in idiopathic pulmonary hemosiderosis (cyst necessitans). Mayo Clin Proc 2006; 81:692.
- Rolla G, Heffler E, Guida G, Bergia R, Bucca C. Exhaled NO in diffuse alveolar haemorrhage. Thorax 2005; 60:614–615.
- Dweik RA, Stoller JK. Role of bronchoscopy in massive hemoptysis. Clin Chest Med 1999; 20:89–105.
- Ioachimescu OCLaurent GL, Shapiro SD. Autoantibodies. Encyclopedia of Respiratory Medicine. Amsterdam: Academic Press, 2006:219–227.
- Watts RA, Carruthers DM, Scott DG. Epidemiology of systemic vasculitis: changing incidence or definition? Semin Arthritis Rheum 1995; 25:28–34.
- Watts RA, Lane SE, Bentham G, Scott DG. Epidemiology of systemic vasculitis: a ten-year study in the United Kingdom. Arthritis Rheum 2000; 43:414–419.
- Watts RA, Jolliffe VA, Carruthers DM, Lockwood M, Scott DG. Effect of classification on the incidence of polyarteritis nodosa and microscopic polyangiitis. Arthritis Rheum 1996; 39:1208–1212.
- Ioachimescu OC, Kotch A, Stoller JK. Idiopathic pulmonary hemosiderosis in adults. Clin Pulm Med 2005; 12:16–25.
- Reinhold-Keller E, Herlyn K, Wagner-Bastmeyer R, et al. No difference in the incidences of vasculitides between north and south Germany: first results of the German vasculitis register. Rheumatology (Oxford) 2002; 41:540–549.
- Mahr A, Guillevin L, Poissonnet M, Ayme S. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener’s granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: a capture-recapture estimate. Arthritis Rheum 2004; 51:92–99.
- Koldingsnes W, Nossent H. Epidemiology of Wegener’s granulomatosis in northern Norway. Arthritis Rheum 2000; 43:2481–2487.
- Kelly PT, Haponik EF. Goodpasture syndrome: molecular and clinical advances. Medicine (Baltimore) 1994; 73:171–185.
- Travis WD, Leslie KOLeslie KO, Wick MR. Pulmonary vasculitis and pulmonary hemorhage. Practical Pulmonary Pathology – a Diagnostic Approach. Philadelphia: Churchill Livingstone-Elsevier, 2005;335–378.
- Jennette JC, Thomas DB, Falk RJ. Microscopic polyangiitis (microscopic polyarteritis). Semin Diagn Pathol 2001; 18:3–13.
- Katzenstein AKatzenstein A, Askin F. Alveolar hemorrhage syndromes. Surgical Pathology of Non-neoplastic Lung Disease. Philadelphia: WB Saunders, 1997:153–159.
- Schwarz MI, Cherniack RM, King TEMurray JF, Nadel J. Diffuse alveolar hemorrhage and other rare infiltrative disorders. Textbook of Respiratory Medicine. Philadelphia: WB Saunders, 2000:1733–1755.
- Lynch JP, Leatherman JWFishman A. Alveolar hemorrhage syndromes. Fishman’s Pulmonary Diseases and Disorders. New York: McGraw-Hill, 1998:1193–1210.
- Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest 1990; 97:906–912.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Fauci AS, Haynes BF, Katz P, Wolff SM. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 1983; 98:76–85.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Langford CA, Hoffman GS. Rare diseases 3: Wegener’s granulomatosis. Thorax 1999; 54:629–637.
- Mark EJ, Matsubara O, Tan-Liu NS, Fienberg R. The pulmonary biopsy in the early diagnosis of Wegener’s (pathergic) granulomatosis: a study based on 35 open lung biopsies. Hum Pathol 1988; 19:1065–1071.
- Sheehan RE, Flint JD, Muller NL. Computed tomography features of the thoracic manifestations of Wegener granulomatosis. J Thorac Imaging 2003: 18:34–41.
- Specks USchwarz MI, King TE. Pulmonary vasculitis. Interstitial Lung Disease. Decker BC. Hamilton, Ontario, Canada: Decker, 2003:599–631.
- Ten Berge IJ, Wilmink JM, Meyer CJ, et al. Clinical and immunological follow-up of patients with severe renal disease in Wegener’s granulo-matosis. Am J Nephrol 1985; 5:21–29.
- Brandwein S, Esdaile J, Danoff D, Tannenbaum H. Wegener’s granulo-matosis. Clinical features and outcome in 13 patients. Arch Intern Med 1983; 143:476–479.
- Pinching AJ, Lockwood CM, Pussell BA, et al. Wegener’s granulomatosis: observations on 18 patients with severe renal disease. Q J Med 1983; 52:435–460.
- Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med 1997; 337:1512–1523.
- Lauque D, Cadranel J, Lazor R, et al. Microscopic polyangiitis with alveolar hemorrhage. A study of 29 cases and review of the literature. Groupe d’Études et de Recherche sur les Maladies “Orphelines” Pulmonaires. Medicine (Baltimore) 2000; 79:222–233.
- Johnson JP, Moore J, Austin HA, Balow JE, Antonovych TT, Wilson CB. Therapy of anti-glomerular basement membrane antibody disease: analysis of prognostic significance of clinical, pathologic and treatment factors. Medicine (Baltimore) 1985; 64:219–227.
- Savage CO, Winearls CG, Evans DJ, Rees AJ, Lockwood CM. Microscopic polyarteritis: presentation, pathology, and prognosis. Q J Med 1985; 56:467–483.
- Haworth SJ, Savage CO, Carr D. Pulmonary hemorrhage complicating Wegener’s granulomatosis and microscopic polyarteritis. Br Med J 1985; 290:1175–1178.
- Smyth L, Gaskin G, Pusey CD. Microscopic polyangiitis. Semin Respir Crit Care Med 2004; 25:523–533.
- Lanham JG, Elkon KB, Pusey CD, Hughes GR. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine (Baltimore) 1984; 63:65–81.
- Leatherman JW. Autoimmune diffuse alveolar hemorrhage. Clin Pulm Med 1994; 1:356–364.
- Boyce NW, Holdsworth SR. Pulmonary manifestations of the clinical syndrome of acute glomerulonephritis and lung hemorrhage. Am J Kidney Dis 1986; 8:31–36.
- Emlen W. Systemic lupus erythematosus and mixed connective tissue disease. Immunol Allergy Clin North Am 1979; 105:291–311.
- Hunninghake GW, Fauci AS. Pulmonary involvement in the collagen vascular diseases. Am Rev Respir Dis 1979; 119:471–503.
- Keane MP, Lynch JP. Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax 2000; 55:159–166.
- Zamora MR, Warner ML, Tuder R, Schwarz MI. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltimore) 1997; 76:192–202.
- Lee CK, Koh JH, Cha HS, et al. Pulmonary alveolar hemorrhage in patients with rheumatic diseases in Korea. Scand J Rheumatol 2000; 29:288–294.
- Vazquez-Del Mercado M, Mendoza-Topete A, Best-Aguilera CR, Garcia-De La Torre I. Diffuse alveolar hemorrhage in limited cutaneous systemic sclerosis with positive perinuclear antineutrophil cytoplasmic antibodies. J Rheumatol 1996; 23:1821–1823.
- Fenlon HM, Doran M, Sant SM, Breatnach E. High-resolution chest CT in systemic lupus erythematosus. AJR Am J Roentgenol 1996; 166:301–307.
- Ioachimescu OC. Idiopathic pulmonary hemosiderosis in adults. Pneumologia 2003; 52:38–43.
- Ioachimescu OC, Sieber S, Kotch A. Idiopathic pulmonary haemosiderosis revisited. Eur Respir J 2004; 24:162–170.
- Franks TJ, Koss MN. Pulmonary capillaritis. Curr Opin Pulm Med 2000; 6:430–435.
- Travis WD, Hoffman GS, Leavitt RY, Pass HI, Fauci AS. Surgical pathology of the lung in Wegener’s granulomatosis. Review of 87 open lung biopsies from 67 patients. Am J Surg Pathol 1991; 15:315–333.
- Zashin S, Fattor R, Fortin D. Microscopic polyarteritis: a forgotten aetiology of haemoptysis and rapidly progressive glomerulonephritis. Ann Rheum Dis 1990; 49:53–56.
- Yoshikawa Y, Watanabe T. Pulmonary lesions in Wegener’s granulo-matosis: a clinicopathologic study of 22 autopsy cases. Hum Pathol 1986; 17:401–410.
- Teague CA, Doak PB, Simpson IJ, Rainer SP, Herdson PB. Goodpasture’s syndrome: an analysis of 29 cases. Kidney Int 1978; 13:492–504.
- Abu-Shakra M, Smythe H, Lewtas J, Badley E, Weber D, Keystone E. Outcome of polyarteritis nodosa and Churg-Strauss syndrome. An analysis of twenty-five patients. Arthritis Rheum 1994; 37:1798–1803.
- Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine (Baltimore) 1999; 78:26–37.
- Schwab EP, Schumacher HR, Freundlich B, Callegari PE. Pulmonary alveolar hemorrhage in systemic lupus erythematosus. Semin Arthritis Rheum 1993; 23:8–15.
- Koh WH, Thumboo J, Boey ML. Pulmonary haemorrhage in Oriental patients with systemic lupus erythematosus. Lupus 1997; 6:713–716.
- Ioachimescu OCLaurent GL, Shapiro SD. Alveolar hemorrhage. Encyclopedia of Respiratory Medicine. Amsterdam: Academic Press, 2006:92–100.
- Travis WD, Colby TV, Lombard C, Carpenter HA. A clinicopathologic study of 34 cases of diffuse pulmonary hemorrhage with lung biopsy confirmation. Am J Surg Pathol 1990; 14:1112–1125.
- Jennings CA, King TE, Tuder R, Cherniak RM, Schwarz MI. Diffuse alveolar hemorrhage with underlying isolated, pauciimmune pulmonary capillaritis. Am J Respir Crit Care Med 1997; 155:1101–1109.
- Spencer H. Pulmonary lesions in polyarteritis nodosa. Br J Tuberc Dis Chest 1957; 51:123–130.
- Travis WD. Pathology of pulmonary vasculitis. Semin Respir Crit Care Med 2004; 25:475–482.
- Schwarz MI, Brown KK. Small vessel vasculitis of the lung. Thorax 2000; 55:502–510.
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004; 25:583–592.
- Ioachimescu OC, Jennings C. Intercostal lung cyst hernia in idiopathic pulmonary hemosiderosis (cyst necessitans). Mayo Clin Proc 2006; 81:692.
- Rolla G, Heffler E, Guida G, Bergia R, Bucca C. Exhaled NO in diffuse alveolar haemorrhage. Thorax 2005; 60:614–615.
- Dweik RA, Stoller JK. Role of bronchoscopy in massive hemoptysis. Clin Chest Med 1999; 20:89–105.
- Ioachimescu OCLaurent GL, Shapiro SD. Autoantibodies. Encyclopedia of Respiratory Medicine. Amsterdam: Academic Press, 2006:219–227.
- Watts RA, Carruthers DM, Scott DG. Epidemiology of systemic vasculitis: changing incidence or definition? Semin Arthritis Rheum 1995; 25:28–34.
- Watts RA, Lane SE, Bentham G, Scott DG. Epidemiology of systemic vasculitis: a ten-year study in the United Kingdom. Arthritis Rheum 2000; 43:414–419.
- Watts RA, Jolliffe VA, Carruthers DM, Lockwood M, Scott DG. Effect of classification on the incidence of polyarteritis nodosa and microscopic polyangiitis. Arthritis Rheum 1996; 39:1208–1212.
- Ioachimescu OC, Kotch A, Stoller JK. Idiopathic pulmonary hemosiderosis in adults. Clin Pulm Med 2005; 12:16–25.
- Reinhold-Keller E, Herlyn K, Wagner-Bastmeyer R, et al. No difference in the incidences of vasculitides between north and south Germany: first results of the German vasculitis register. Rheumatology (Oxford) 2002; 41:540–549.
- Mahr A, Guillevin L, Poissonnet M, Ayme S. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener’s granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: a capture-recapture estimate. Arthritis Rheum 2004; 51:92–99.
- Koldingsnes W, Nossent H. Epidemiology of Wegener’s granulomatosis in northern Norway. Arthritis Rheum 2000; 43:2481–2487.
- Kelly PT, Haponik EF. Goodpasture syndrome: molecular and clinical advances. Medicine (Baltimore) 1994; 73:171–185.
- Travis WD, Leslie KOLeslie KO, Wick MR. Pulmonary vasculitis and pulmonary hemorhage. Practical Pulmonary Pathology – a Diagnostic Approach. Philadelphia: Churchill Livingstone-Elsevier, 2005;335–378.
- Jennette JC, Thomas DB, Falk RJ. Microscopic polyangiitis (microscopic polyarteritis). Semin Diagn Pathol 2001; 18:3–13.
- Katzenstein AKatzenstein A, Askin F. Alveolar hemorrhage syndromes. Surgical Pathology of Non-neoplastic Lung Disease. Philadelphia: WB Saunders, 1997:153–159.
- Schwarz MI, Cherniack RM, King TEMurray JF, Nadel J. Diffuse alveolar hemorrhage and other rare infiltrative disorders. Textbook of Respiratory Medicine. Philadelphia: WB Saunders, 2000:1733–1755.
- Lynch JP, Leatherman JWFishman A. Alveolar hemorrhage syndromes. Fishman’s Pulmonary Diseases and Disorders. New York: McGraw-Hill, 1998:1193–1210.
- Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest 1990; 97:906–912.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Fauci AS, Haynes BF, Katz P, Wolff SM. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med 1983; 98:76–85.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Langford CA, Hoffman GS. Rare diseases 3: Wegener’s granulomatosis. Thorax 1999; 54:629–637.
- Mark EJ, Matsubara O, Tan-Liu NS, Fienberg R. The pulmonary biopsy in the early diagnosis of Wegener’s (pathergic) granulomatosis: a study based on 35 open lung biopsies. Hum Pathol 1988; 19:1065–1071.
- Sheehan RE, Flint JD, Muller NL. Computed tomography features of the thoracic manifestations of Wegener granulomatosis. J Thorac Imaging 2003: 18:34–41.
- Specks USchwarz MI, King TE. Pulmonary vasculitis. Interstitial Lung Disease. Decker BC. Hamilton, Ontario, Canada: Decker, 2003:599–631.
- Ten Berge IJ, Wilmink JM, Meyer CJ, et al. Clinical and immunological follow-up of patients with severe renal disease in Wegener’s granulo-matosis. Am J Nephrol 1985; 5:21–29.
- Brandwein S, Esdaile J, Danoff D, Tannenbaum H. Wegener’s granulo-matosis. Clinical features and outcome in 13 patients. Arch Intern Med 1983; 143:476–479.
- Pinching AJ, Lockwood CM, Pussell BA, et al. Wegener’s granulomatosis: observations on 18 patients with severe renal disease. Q J Med 1983; 52:435–460.
- Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med 1997; 337:1512–1523.
- Lauque D, Cadranel J, Lazor R, et al. Microscopic polyangiitis with alveolar hemorrhage. A study of 29 cases and review of the literature. Groupe d’Études et de Recherche sur les Maladies “Orphelines” Pulmonaires. Medicine (Baltimore) 2000; 79:222–233.
- Johnson JP, Moore J, Austin HA, Balow JE, Antonovych TT, Wilson CB. Therapy of anti-glomerular basement membrane antibody disease: analysis of prognostic significance of clinical, pathologic and treatment factors. Medicine (Baltimore) 1985; 64:219–227.
- Savage CO, Winearls CG, Evans DJ, Rees AJ, Lockwood CM. Microscopic polyarteritis: presentation, pathology, and prognosis. Q J Med 1985; 56:467–483.
- Haworth SJ, Savage CO, Carr D. Pulmonary hemorrhage complicating Wegener’s granulomatosis and microscopic polyarteritis. Br Med J 1985; 290:1175–1178.
- Smyth L, Gaskin G, Pusey CD. Microscopic polyangiitis. Semin Respir Crit Care Med 2004; 25:523–533.
- Lanham JG, Elkon KB, Pusey CD, Hughes GR. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine (Baltimore) 1984; 63:65–81.
- Leatherman JW. Autoimmune diffuse alveolar hemorrhage. Clin Pulm Med 1994; 1:356–364.
- Boyce NW, Holdsworth SR. Pulmonary manifestations of the clinical syndrome of acute glomerulonephritis and lung hemorrhage. Am J Kidney Dis 1986; 8:31–36.
- Emlen W. Systemic lupus erythematosus and mixed connective tissue disease. Immunol Allergy Clin North Am 1979; 105:291–311.
- Hunninghake GW, Fauci AS. Pulmonary involvement in the collagen vascular diseases. Am Rev Respir Dis 1979; 119:471–503.
- Keane MP, Lynch JP. Pleuropulmonary manifestations of systemic lupus erythematosus. Thorax 2000; 55:159–166.
- Zamora MR, Warner ML, Tuder R, Schwarz MI. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltimore) 1997; 76:192–202.
- Lee CK, Koh JH, Cha HS, et al. Pulmonary alveolar hemorrhage in patients with rheumatic diseases in Korea. Scand J Rheumatol 2000; 29:288–294.
- Vazquez-Del Mercado M, Mendoza-Topete A, Best-Aguilera CR, Garcia-De La Torre I. Diffuse alveolar hemorrhage in limited cutaneous systemic sclerosis with positive perinuclear antineutrophil cytoplasmic antibodies. J Rheumatol 1996; 23:1821–1823.
- Fenlon HM, Doran M, Sant SM, Breatnach E. High-resolution chest CT in systemic lupus erythematosus. AJR Am J Roentgenol 1996; 166:301–307.
- Ioachimescu OC. Idiopathic pulmonary hemosiderosis in adults. Pneumologia 2003; 52:38–43.
- Ioachimescu OC, Sieber S, Kotch A. Idiopathic pulmonary haemosiderosis revisited. Eur Respir J 2004; 24:162–170.
- Franks TJ, Koss MN. Pulmonary capillaritis. Curr Opin Pulm Med 2000; 6:430–435.
- Travis WD, Hoffman GS, Leavitt RY, Pass HI, Fauci AS. Surgical pathology of the lung in Wegener’s granulomatosis. Review of 87 open lung biopsies from 67 patients. Am J Surg Pathol 1991; 15:315–333.
- Zashin S, Fattor R, Fortin D. Microscopic polyarteritis: a forgotten aetiology of haemoptysis and rapidly progressive glomerulonephritis. Ann Rheum Dis 1990; 49:53–56.
- Yoshikawa Y, Watanabe T. Pulmonary lesions in Wegener’s granulo-matosis: a clinicopathologic study of 22 autopsy cases. Hum Pathol 1986; 17:401–410.
- Teague CA, Doak PB, Simpson IJ, Rainer SP, Herdson PB. Goodpasture’s syndrome: an analysis of 29 cases. Kidney Int 1978; 13:492–504.
- Abu-Shakra M, Smythe H, Lewtas J, Badley E, Weber D, Keystone E. Outcome of polyarteritis nodosa and Churg-Strauss syndrome. An analysis of twenty-five patients. Arthritis Rheum 1994; 37:1798–1803.
- Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome. Clinical study and long-term follow-up of 96 patients. Medicine (Baltimore) 1999; 78:26–37.
- Schwab EP, Schumacher HR, Freundlich B, Callegari PE. Pulmonary alveolar hemorrhage in systemic lupus erythematosus. Semin Arthritis Rheum 1993; 23:8–15.
- Koh WH, Thumboo J, Boey ML. Pulmonary haemorrhage in Oriental patients with systemic lupus erythematosus. Lupus 1997; 6:713–716.
KEY POINTS
- Most patients present with dyspnea, cough, hemoptysis, and new alveolar infiltrates. Early bronchoscopy with bronchoalveolar lavage is generally required to confirm the diagnosis; blood in the lavage specimens (with numerous erythrocytes and siderophages) establishes the diagnosis.
- Therapy targets both the autoimmune destruction of the alveolar capillary membrane and the underlying condition. Corticosteroids and immunosuppressive agents remain the gold standard.
- In patients with diffuse alveolar hemorrhage and renal impairment (pulmonary-renal syndrome), kidney biopsy can be considered to identify the cause and to direct therapy.