User login
FDA approves first gene therapy for hemophilia B
“Gene therapy for hemophilia has been on the horizon for more than 2 decades. Despite advancements in the treatment of hemophilia, the prevention and treatment of bleeding episodes can adversely impact individuals’ quality of life,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research. “Today’s approval provides a new treatment option for patients with hemophilia B and represents important progress in the development of innovative therapies for those experiencing a high burden of disease associated with this form of hemophilia.”
Hemophilia B is caused by a deficiency in clotting factor IX attributable to a faulty gene. The newly approved IV infusion delivers a functional gene to liver cells via an adeno-associated virus that instructs them to make the clotting factor. The genetic instructions remain in the cell but aren’t incorporated into the patient’s own DNA, according to a press release from maker CSL Behring.
The gene therapy will cost $3.5 million, making it the most expensive treatment to date -- more than Bluebird's recently approved gene therapies. A recent analysis from the Institute for Clinical and Economic Review said charging $2.93-$2.96 million would be justified because etranacogene dezaparvovec would offset the need for ongoing factor IX replacement, which can top $20 million over a lifetime.
Approval was based on the single-arm, open-label HOPE-B trial in 54 men who relied on factor IX replacement therapy; most patients with hemophilia B are male.
Over the 18 months after infusion, their adjusted annualized bleeding rate fell 64% compared with baseline (P = .0002), and factor IX–treated bleeds fell 77% (P < .0001); 98% of subjects treated with a full dose of etranacogene dezaparvovec discontinued factor IX prophylaxis.
Durability of the effect remains a concern, but data have been reassuring, with subjects having a mean factor IX activity of 39 IU/dL at 6 months – 39% of normal – and 36.9 IU/dL at 18 months, about 37% of normal. There’s been no sign so far of patients developing inhibitors against the infusion.
Adverse events were common but largely mild and included headache and influenza-like illness, both in 13% of subjects. Nine patients needed steroids for liver enzyme elevations.
The trial was temporarily halted due to a case of liver cancer, but it was ultimately deemed not to be related to treatment, based on molecular tumor characterization and vector integration analysis. A death in the trial was also not considered treatment related.
Other gene therapies are in the pipeline for hemophilia, including valoctocogene roxaparvovec (Roctavian, BioMarin) for hemophilia A. FDA’s approval decision is expected in March 2023.
This article was updated 11/23/22.
Correction, 11/23/22: The brand name Hemgenix was misstated in an earlier version of this article.
“Gene therapy for hemophilia has been on the horizon for more than 2 decades. Despite advancements in the treatment of hemophilia, the prevention and treatment of bleeding episodes can adversely impact individuals’ quality of life,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research. “Today’s approval provides a new treatment option for patients with hemophilia B and represents important progress in the development of innovative therapies for those experiencing a high burden of disease associated with this form of hemophilia.”
Hemophilia B is caused by a deficiency in clotting factor IX attributable to a faulty gene. The newly approved IV infusion delivers a functional gene to liver cells via an adeno-associated virus that instructs them to make the clotting factor. The genetic instructions remain in the cell but aren’t incorporated into the patient’s own DNA, according to a press release from maker CSL Behring.
The gene therapy will cost $3.5 million, making it the most expensive treatment to date -- more than Bluebird's recently approved gene therapies. A recent analysis from the Institute for Clinical and Economic Review said charging $2.93-$2.96 million would be justified because etranacogene dezaparvovec would offset the need for ongoing factor IX replacement, which can top $20 million over a lifetime.
Approval was based on the single-arm, open-label HOPE-B trial in 54 men who relied on factor IX replacement therapy; most patients with hemophilia B are male.
Over the 18 months after infusion, their adjusted annualized bleeding rate fell 64% compared with baseline (P = .0002), and factor IX–treated bleeds fell 77% (P < .0001); 98% of subjects treated with a full dose of etranacogene dezaparvovec discontinued factor IX prophylaxis.
Durability of the effect remains a concern, but data have been reassuring, with subjects having a mean factor IX activity of 39 IU/dL at 6 months – 39% of normal – and 36.9 IU/dL at 18 months, about 37% of normal. There’s been no sign so far of patients developing inhibitors against the infusion.
Adverse events were common but largely mild and included headache and influenza-like illness, both in 13% of subjects. Nine patients needed steroids for liver enzyme elevations.
The trial was temporarily halted due to a case of liver cancer, but it was ultimately deemed not to be related to treatment, based on molecular tumor characterization and vector integration analysis. A death in the trial was also not considered treatment related.
Other gene therapies are in the pipeline for hemophilia, including valoctocogene roxaparvovec (Roctavian, BioMarin) for hemophilia A. FDA’s approval decision is expected in March 2023.
This article was updated 11/23/22.
Correction, 11/23/22: The brand name Hemgenix was misstated in an earlier version of this article.
“Gene therapy for hemophilia has been on the horizon for more than 2 decades. Despite advancements in the treatment of hemophilia, the prevention and treatment of bleeding episodes can adversely impact individuals’ quality of life,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research. “Today’s approval provides a new treatment option for patients with hemophilia B and represents important progress in the development of innovative therapies for those experiencing a high burden of disease associated with this form of hemophilia.”
Hemophilia B is caused by a deficiency in clotting factor IX attributable to a faulty gene. The newly approved IV infusion delivers a functional gene to liver cells via an adeno-associated virus that instructs them to make the clotting factor. The genetic instructions remain in the cell but aren’t incorporated into the patient’s own DNA, according to a press release from maker CSL Behring.
The gene therapy will cost $3.5 million, making it the most expensive treatment to date -- more than Bluebird's recently approved gene therapies. A recent analysis from the Institute for Clinical and Economic Review said charging $2.93-$2.96 million would be justified because etranacogene dezaparvovec would offset the need for ongoing factor IX replacement, which can top $20 million over a lifetime.
Approval was based on the single-arm, open-label HOPE-B trial in 54 men who relied on factor IX replacement therapy; most patients with hemophilia B are male.
Over the 18 months after infusion, their adjusted annualized bleeding rate fell 64% compared with baseline (P = .0002), and factor IX–treated bleeds fell 77% (P < .0001); 98% of subjects treated with a full dose of etranacogene dezaparvovec discontinued factor IX prophylaxis.
Durability of the effect remains a concern, but data have been reassuring, with subjects having a mean factor IX activity of 39 IU/dL at 6 months – 39% of normal – and 36.9 IU/dL at 18 months, about 37% of normal. There’s been no sign so far of patients developing inhibitors against the infusion.
Adverse events were common but largely mild and included headache and influenza-like illness, both in 13% of subjects. Nine patients needed steroids for liver enzyme elevations.
The trial was temporarily halted due to a case of liver cancer, but it was ultimately deemed not to be related to treatment, based on molecular tumor characterization and vector integration analysis. A death in the trial was also not considered treatment related.
Other gene therapies are in the pipeline for hemophilia, including valoctocogene roxaparvovec (Roctavian, BioMarin) for hemophilia A. FDA’s approval decision is expected in March 2023.
This article was updated 11/23/22.
Correction, 11/23/22: The brand name Hemgenix was misstated in an earlier version of this article.
HDL cholesterol not linked to CHD risk in Blacks: REGARDS
High-density lipoprotein cholesterol may not be as effective a biomarker of cardiovascular disease risk as once thought, particularly in Black adults, according to results from a large biracial cohort study that also raised questions about the validity of high HDL cholesterol as a potentially protective factor in White and Black adults alike.
“I think this opens the door to suggest that every biomarker we use might have a race-specific association with disease outcome,” Nathalie Pamir, PhD, an associate professor at Oregon Health & Science University in Portland, said in an interview. “So, something as basic as HDL cholesterol – we’ve known about it since 1970 – has a race signature.”
Dr. Pamir and colleagues reported their findings from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study that recruited 30,239 Black and White individuals aged 45 years and older from the contiguous United States from 2003 to 2007.
The study found that LDL cholesterol “modestly” predicted coronary heart disease (CHD) risk in Black and White adults. However, low HDL cholesterol, while associated with an increased risk in White patients (hazard ratio, 1.22; 95% confidence interval, 1.05-1.43), did not have a similar association in Blacks (HR, 0.94; 95% CI: 0.78-1.14). And high HDL cholesterol wasn’t found to be predictive in either group (HR, 0.96; 95% CI, 0.79-1.16 for White participants: HR, 0.91; 95% CI, 0.74-1.12 for Black participants).
Among 23,901 study participants who were CHD-risk free over a 10-year follow-up, 664 and 951 CHD events occurred in Black and White participants, respectively. The study cohort was 57.8% White and 58.4% women, with a mean age of 65 years.
The study noted that LDL cholesterol and triglycerides conferred similar risks for CHD in both White and Black participants.
Acknowledging that this study focused on Blacks, Dr. Pamir added that “we need to know about Asian Americans; we need to know about Hispanic Americans.”
Change of approach to lipid management called for
Dr. Pamir noted that the current understanding about HDL cholesterol and CHD risk comes from the Framingham heart study in the 1970s, whose population was 100% White.
Care algorithms derived from the Framingham study as well as the Multi-Ethnic Study of Atherosclerosis incorporate that association between HDL cholesterol and CHD risk, she noted, but these findings from REGARDS should change how cardiologists approach lipid management in Black and White patients.
“The conversation would go something like: High HDL cholesterol levels put you in a higher risk [bracket] but HDL cholesterol levels are not something we treat; we have no drugs for that,” Dr. Pamir said.
“The conversation would continue along the lines that: ‘You need to do more exercise, you need to change your diet, incorporate healthy fats, walnuts, and omega 3s.’
“But what might the conversation be for Black patients? ‘We don’t see the association that we see for White patients. Do adopt the good habits to exercise and dietary changes, but don’t get too worried about it.’ ”
The study report raises “caution” about using the Framingham, MESA, and other algorithms for evaluating CHD risk. Dr. Pamir explained what that means. “We might be underestimating risk, because what our study showed was that, when we looked at clinically high HDL cholesterol, about 60 mg/dL, it has no benefit for White and Black patients.”
She added, “So that pat on the back we get for patients that have high HDL-C levels? Maybe that pat on the back shouldn’t be there.”
In an invited commentary, Keith C. Ferdinand, MD, of Tulane University in New Orleans, wrote that using HDL cholesterol in risk calculations could inaccurately assess atherosclerotic cardiovascular risk in Black adults “and become a barrier to optimal care.”
In an interview, he said the REGARDS findings call for consideration of other biomarkers for evaluating CHD risk and point to the importance of socioeconomic factors in health outcomes.
“Physicians and other clinicians need to recognize the powerful impact of the social determinants of health and to also recognize the limits of HDL itself as either protective if it’s high or a definitive predictor of risk if it’s low, and focus on some more modern approaches, including coronary artery calcium scoring.”
He also said risk evaluation should include lipoprotein(a), which, he noted in the editorial, the European Atherosclerosis Society recommends measuring. “One of the reasons it’s underutilized is that we really don’t have a specific treatment for it,” he said of Lp(a) in the United States.
In his editorial comment, Dr. Ferdinand called for future research aimed at eliminating health disparities. “Regardless of the development of better tools for the assessment of risk, newer drugs to treat CVD, the use of coronary artery calcium, if we don’t apply evidence-based medicine equally across the population based on race, ethnicity, sex, gender, socioeconomic status, or geography, then the disparities are going to persist,” he said.
The National Institute of Neurological Disorders and Stroke and the National Institute on Aging provided funding for the study. Dr. Pamir has no relevant relationships to disclose. Dr. Ferdinand disclosed relationships with Boehringer Ingelheim, Novartis, Janssen, and Lilly.
High-density lipoprotein cholesterol may not be as effective a biomarker of cardiovascular disease risk as once thought, particularly in Black adults, according to results from a large biracial cohort study that also raised questions about the validity of high HDL cholesterol as a potentially protective factor in White and Black adults alike.
“I think this opens the door to suggest that every biomarker we use might have a race-specific association with disease outcome,” Nathalie Pamir, PhD, an associate professor at Oregon Health & Science University in Portland, said in an interview. “So, something as basic as HDL cholesterol – we’ve known about it since 1970 – has a race signature.”
Dr. Pamir and colleagues reported their findings from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study that recruited 30,239 Black and White individuals aged 45 years and older from the contiguous United States from 2003 to 2007.
The study found that LDL cholesterol “modestly” predicted coronary heart disease (CHD) risk in Black and White adults. However, low HDL cholesterol, while associated with an increased risk in White patients (hazard ratio, 1.22; 95% confidence interval, 1.05-1.43), did not have a similar association in Blacks (HR, 0.94; 95% CI: 0.78-1.14). And high HDL cholesterol wasn’t found to be predictive in either group (HR, 0.96; 95% CI, 0.79-1.16 for White participants: HR, 0.91; 95% CI, 0.74-1.12 for Black participants).
Among 23,901 study participants who were CHD-risk free over a 10-year follow-up, 664 and 951 CHD events occurred in Black and White participants, respectively. The study cohort was 57.8% White and 58.4% women, with a mean age of 65 years.
The study noted that LDL cholesterol and triglycerides conferred similar risks for CHD in both White and Black participants.
Acknowledging that this study focused on Blacks, Dr. Pamir added that “we need to know about Asian Americans; we need to know about Hispanic Americans.”
Change of approach to lipid management called for
Dr. Pamir noted that the current understanding about HDL cholesterol and CHD risk comes from the Framingham heart study in the 1970s, whose population was 100% White.
Care algorithms derived from the Framingham study as well as the Multi-Ethnic Study of Atherosclerosis incorporate that association between HDL cholesterol and CHD risk, she noted, but these findings from REGARDS should change how cardiologists approach lipid management in Black and White patients.
“The conversation would go something like: High HDL cholesterol levels put you in a higher risk [bracket] but HDL cholesterol levels are not something we treat; we have no drugs for that,” Dr. Pamir said.
“The conversation would continue along the lines that: ‘You need to do more exercise, you need to change your diet, incorporate healthy fats, walnuts, and omega 3s.’
“But what might the conversation be for Black patients? ‘We don’t see the association that we see for White patients. Do adopt the good habits to exercise and dietary changes, but don’t get too worried about it.’ ”
The study report raises “caution” about using the Framingham, MESA, and other algorithms for evaluating CHD risk. Dr. Pamir explained what that means. “We might be underestimating risk, because what our study showed was that, when we looked at clinically high HDL cholesterol, about 60 mg/dL, it has no benefit for White and Black patients.”
She added, “So that pat on the back we get for patients that have high HDL-C levels? Maybe that pat on the back shouldn’t be there.”
In an invited commentary, Keith C. Ferdinand, MD, of Tulane University in New Orleans, wrote that using HDL cholesterol in risk calculations could inaccurately assess atherosclerotic cardiovascular risk in Black adults “and become a barrier to optimal care.”
In an interview, he said the REGARDS findings call for consideration of other biomarkers for evaluating CHD risk and point to the importance of socioeconomic factors in health outcomes.
“Physicians and other clinicians need to recognize the powerful impact of the social determinants of health and to also recognize the limits of HDL itself as either protective if it’s high or a definitive predictor of risk if it’s low, and focus on some more modern approaches, including coronary artery calcium scoring.”
He also said risk evaluation should include lipoprotein(a), which, he noted in the editorial, the European Atherosclerosis Society recommends measuring. “One of the reasons it’s underutilized is that we really don’t have a specific treatment for it,” he said of Lp(a) in the United States.
In his editorial comment, Dr. Ferdinand called for future research aimed at eliminating health disparities. “Regardless of the development of better tools for the assessment of risk, newer drugs to treat CVD, the use of coronary artery calcium, if we don’t apply evidence-based medicine equally across the population based on race, ethnicity, sex, gender, socioeconomic status, or geography, then the disparities are going to persist,” he said.
The National Institute of Neurological Disorders and Stroke and the National Institute on Aging provided funding for the study. Dr. Pamir has no relevant relationships to disclose. Dr. Ferdinand disclosed relationships with Boehringer Ingelheim, Novartis, Janssen, and Lilly.
High-density lipoprotein cholesterol may not be as effective a biomarker of cardiovascular disease risk as once thought, particularly in Black adults, according to results from a large biracial cohort study that also raised questions about the validity of high HDL cholesterol as a potentially protective factor in White and Black adults alike.
“I think this opens the door to suggest that every biomarker we use might have a race-specific association with disease outcome,” Nathalie Pamir, PhD, an associate professor at Oregon Health & Science University in Portland, said in an interview. “So, something as basic as HDL cholesterol – we’ve known about it since 1970 – has a race signature.”
Dr. Pamir and colleagues reported their findings from the REGARDS (Reasons for Geographic and Racial Differences in Stroke) cohort study that recruited 30,239 Black and White individuals aged 45 years and older from the contiguous United States from 2003 to 2007.
The study found that LDL cholesterol “modestly” predicted coronary heart disease (CHD) risk in Black and White adults. However, low HDL cholesterol, while associated with an increased risk in White patients (hazard ratio, 1.22; 95% confidence interval, 1.05-1.43), did not have a similar association in Blacks (HR, 0.94; 95% CI: 0.78-1.14). And high HDL cholesterol wasn’t found to be predictive in either group (HR, 0.96; 95% CI, 0.79-1.16 for White participants: HR, 0.91; 95% CI, 0.74-1.12 for Black participants).
Among 23,901 study participants who were CHD-risk free over a 10-year follow-up, 664 and 951 CHD events occurred in Black and White participants, respectively. The study cohort was 57.8% White and 58.4% women, with a mean age of 65 years.
The study noted that LDL cholesterol and triglycerides conferred similar risks for CHD in both White and Black participants.
Acknowledging that this study focused on Blacks, Dr. Pamir added that “we need to know about Asian Americans; we need to know about Hispanic Americans.”
Change of approach to lipid management called for
Dr. Pamir noted that the current understanding about HDL cholesterol and CHD risk comes from the Framingham heart study in the 1970s, whose population was 100% White.
Care algorithms derived from the Framingham study as well as the Multi-Ethnic Study of Atherosclerosis incorporate that association between HDL cholesterol and CHD risk, she noted, but these findings from REGARDS should change how cardiologists approach lipid management in Black and White patients.
“The conversation would go something like: High HDL cholesterol levels put you in a higher risk [bracket] but HDL cholesterol levels are not something we treat; we have no drugs for that,” Dr. Pamir said.
“The conversation would continue along the lines that: ‘You need to do more exercise, you need to change your diet, incorporate healthy fats, walnuts, and omega 3s.’
“But what might the conversation be for Black patients? ‘We don’t see the association that we see for White patients. Do adopt the good habits to exercise and dietary changes, but don’t get too worried about it.’ ”
The study report raises “caution” about using the Framingham, MESA, and other algorithms for evaluating CHD risk. Dr. Pamir explained what that means. “We might be underestimating risk, because what our study showed was that, when we looked at clinically high HDL cholesterol, about 60 mg/dL, it has no benefit for White and Black patients.”
She added, “So that pat on the back we get for patients that have high HDL-C levels? Maybe that pat on the back shouldn’t be there.”
In an invited commentary, Keith C. Ferdinand, MD, of Tulane University in New Orleans, wrote that using HDL cholesterol in risk calculations could inaccurately assess atherosclerotic cardiovascular risk in Black adults “and become a barrier to optimal care.”
In an interview, he said the REGARDS findings call for consideration of other biomarkers for evaluating CHD risk and point to the importance of socioeconomic factors in health outcomes.
“Physicians and other clinicians need to recognize the powerful impact of the social determinants of health and to also recognize the limits of HDL itself as either protective if it’s high or a definitive predictor of risk if it’s low, and focus on some more modern approaches, including coronary artery calcium scoring.”
He also said risk evaluation should include lipoprotein(a), which, he noted in the editorial, the European Atherosclerosis Society recommends measuring. “One of the reasons it’s underutilized is that we really don’t have a specific treatment for it,” he said of Lp(a) in the United States.
In his editorial comment, Dr. Ferdinand called for future research aimed at eliminating health disparities. “Regardless of the development of better tools for the assessment of risk, newer drugs to treat CVD, the use of coronary artery calcium, if we don’t apply evidence-based medicine equally across the population based on race, ethnicity, sex, gender, socioeconomic status, or geography, then the disparities are going to persist,” he said.
The National Institute of Neurological Disorders and Stroke and the National Institute on Aging provided funding for the study. Dr. Pamir has no relevant relationships to disclose. Dr. Ferdinand disclosed relationships with Boehringer Ingelheim, Novartis, Janssen, and Lilly.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Blenrep for multiple myeloma withdrawn from U.S. market
A drug used in the treatment of relapsed/refractory multiple myeloma (RRMM) is in the process of being pulled off the U.S. market by its manufacturer.
The drug is belantamab mafodotin-blmf (Blenrep), an antibody drug conjugate that targets B-cell maturation antigen (BCMA).
The manufacturer, GSK, announced that it has started the process of withdrawing this drug from the market at the request of the U.S. Food and Drug Administration (FDA).
This request follows disappointing results from a large confirmatory trial, known as DREAMM-3, in which the drug failed to meet the primary endpoint of showing an improvement in progression-free survival (PFS).
The company was obliged to carry out this confirmatory trial after the FDA granted an accelerated approval for the drug in August 2020.
The accelerated approval was based on response data, and it was dependent on later trials’ confirming a clinical benefit. In this case, those trials did not confirm a clinical benefit.
“We respect the Agency’s approach to the accelerated approval regulations and associated process,” commented the GSK Chief Medical Officer Sabine Luik.
The company will continue to “work with the U.S. FDA on a path forward for this important treatment option for patients with multiple myeloma.”
Further clinical trials in the DREAMM program are still underway. Results from the DREAMM-7 and DREAMM-8 trials are expected in early 2023.
The company had high hopes for the drug when it was launched. At that time, belanatamab mafodotin-blmf was the only drug on the market that targeted BCMA, and so it was the first drug in its class.
However, it is no longer unique. In the 2 years that it has been available, several other products that target BCMA have been launched for use in the treatment of multiple myeloma. These include the two chimeric antigen receptor T-cell products, idecabtagene vicleucel (Abecma) and ciltacabtagene autoleucel (Carvykti), as well as the bispecific antibody teclistamab (Tecvayli).
For relapsed/refractory disease
Belantamab mafodotin-blmf was approved for use in patients with RRMM who had already undergone treatment with one of the three major classes of drugs, namely, an immunomodulatory agent, a proteasome inhibitor, and a CD-38 monoclonal antibody.
Patients who are currently taking the drug and would like to continue doing so will have the option to enroll in a compassionate use program to retain their access to treatment, the company said.
“GSK continues to believe, based on the totality of data available from the DREAMM (DRiving Excellence in Approaches to Multiple Myeloma) development program, that the benefit-risk profile of belantamab mafodotin remains favorable in this hard-to-treat RRMM patient population. Patients responding to belantamab mafodotin experienced durable clinical benefit, and safety remains consistent with the known safety profile,” the company said.
Details of DREAMM-3 results
DREAMM-3 was a phase 3 trial that compared single-agent belantamab mafodotin to pomalidomide (Pomalyst) in combination with low-dose dexamethasone (PomDex) for patients with RRMM.
The results for the primary endpoint of PFS did not reach statistical significance: median PFS was 11.2 vs. 7 months with PomDex (hazard ratio, 1.03; 95% confidence interval, 0.72-1.47).
At the time of the primary analysis, the overall survival (OS) data had only achieved 37.5% overall maturity. The median OS was 21.2 vs. 21.1 months with PomDex (HR, 1.14; 95% CI, 0.77-1.68).
A version of this article first appeared on Medscape.com.
A drug used in the treatment of relapsed/refractory multiple myeloma (RRMM) is in the process of being pulled off the U.S. market by its manufacturer.
The drug is belantamab mafodotin-blmf (Blenrep), an antibody drug conjugate that targets B-cell maturation antigen (BCMA).
The manufacturer, GSK, announced that it has started the process of withdrawing this drug from the market at the request of the U.S. Food and Drug Administration (FDA).
This request follows disappointing results from a large confirmatory trial, known as DREAMM-3, in which the drug failed to meet the primary endpoint of showing an improvement in progression-free survival (PFS).
The company was obliged to carry out this confirmatory trial after the FDA granted an accelerated approval for the drug in August 2020.
The accelerated approval was based on response data, and it was dependent on later trials’ confirming a clinical benefit. In this case, those trials did not confirm a clinical benefit.
“We respect the Agency’s approach to the accelerated approval regulations and associated process,” commented the GSK Chief Medical Officer Sabine Luik.
The company will continue to “work with the U.S. FDA on a path forward for this important treatment option for patients with multiple myeloma.”
Further clinical trials in the DREAMM program are still underway. Results from the DREAMM-7 and DREAMM-8 trials are expected in early 2023.
The company had high hopes for the drug when it was launched. At that time, belanatamab mafodotin-blmf was the only drug on the market that targeted BCMA, and so it was the first drug in its class.
However, it is no longer unique. In the 2 years that it has been available, several other products that target BCMA have been launched for use in the treatment of multiple myeloma. These include the two chimeric antigen receptor T-cell products, idecabtagene vicleucel (Abecma) and ciltacabtagene autoleucel (Carvykti), as well as the bispecific antibody teclistamab (Tecvayli).
For relapsed/refractory disease
Belantamab mafodotin-blmf was approved for use in patients with RRMM who had already undergone treatment with one of the three major classes of drugs, namely, an immunomodulatory agent, a proteasome inhibitor, and a CD-38 monoclonal antibody.
Patients who are currently taking the drug and would like to continue doing so will have the option to enroll in a compassionate use program to retain their access to treatment, the company said.
“GSK continues to believe, based on the totality of data available from the DREAMM (DRiving Excellence in Approaches to Multiple Myeloma) development program, that the benefit-risk profile of belantamab mafodotin remains favorable in this hard-to-treat RRMM patient population. Patients responding to belantamab mafodotin experienced durable clinical benefit, and safety remains consistent with the known safety profile,” the company said.
Details of DREAMM-3 results
DREAMM-3 was a phase 3 trial that compared single-agent belantamab mafodotin to pomalidomide (Pomalyst) in combination with low-dose dexamethasone (PomDex) for patients with RRMM.
The results for the primary endpoint of PFS did not reach statistical significance: median PFS was 11.2 vs. 7 months with PomDex (hazard ratio, 1.03; 95% confidence interval, 0.72-1.47).
At the time of the primary analysis, the overall survival (OS) data had only achieved 37.5% overall maturity. The median OS was 21.2 vs. 21.1 months with PomDex (HR, 1.14; 95% CI, 0.77-1.68).
A version of this article first appeared on Medscape.com.
A drug used in the treatment of relapsed/refractory multiple myeloma (RRMM) is in the process of being pulled off the U.S. market by its manufacturer.
The drug is belantamab mafodotin-blmf (Blenrep), an antibody drug conjugate that targets B-cell maturation antigen (BCMA).
The manufacturer, GSK, announced that it has started the process of withdrawing this drug from the market at the request of the U.S. Food and Drug Administration (FDA).
This request follows disappointing results from a large confirmatory trial, known as DREAMM-3, in which the drug failed to meet the primary endpoint of showing an improvement in progression-free survival (PFS).
The company was obliged to carry out this confirmatory trial after the FDA granted an accelerated approval for the drug in August 2020.
The accelerated approval was based on response data, and it was dependent on later trials’ confirming a clinical benefit. In this case, those trials did not confirm a clinical benefit.
“We respect the Agency’s approach to the accelerated approval regulations and associated process,” commented the GSK Chief Medical Officer Sabine Luik.
The company will continue to “work with the U.S. FDA on a path forward for this important treatment option for patients with multiple myeloma.”
Further clinical trials in the DREAMM program are still underway. Results from the DREAMM-7 and DREAMM-8 trials are expected in early 2023.
The company had high hopes for the drug when it was launched. At that time, belanatamab mafodotin-blmf was the only drug on the market that targeted BCMA, and so it was the first drug in its class.
However, it is no longer unique. In the 2 years that it has been available, several other products that target BCMA have been launched for use in the treatment of multiple myeloma. These include the two chimeric antigen receptor T-cell products, idecabtagene vicleucel (Abecma) and ciltacabtagene autoleucel (Carvykti), as well as the bispecific antibody teclistamab (Tecvayli).
For relapsed/refractory disease
Belantamab mafodotin-blmf was approved for use in patients with RRMM who had already undergone treatment with one of the three major classes of drugs, namely, an immunomodulatory agent, a proteasome inhibitor, and a CD-38 monoclonal antibody.
Patients who are currently taking the drug and would like to continue doing so will have the option to enroll in a compassionate use program to retain their access to treatment, the company said.
“GSK continues to believe, based on the totality of data available from the DREAMM (DRiving Excellence in Approaches to Multiple Myeloma) development program, that the benefit-risk profile of belantamab mafodotin remains favorable in this hard-to-treat RRMM patient population. Patients responding to belantamab mafodotin experienced durable clinical benefit, and safety remains consistent with the known safety profile,” the company said.
Details of DREAMM-3 results
DREAMM-3 was a phase 3 trial that compared single-agent belantamab mafodotin to pomalidomide (Pomalyst) in combination with low-dose dexamethasone (PomDex) for patients with RRMM.
The results for the primary endpoint of PFS did not reach statistical significance: median PFS was 11.2 vs. 7 months with PomDex (hazard ratio, 1.03; 95% confidence interval, 0.72-1.47).
At the time of the primary analysis, the overall survival (OS) data had only achieved 37.5% overall maturity. The median OS was 21.2 vs. 21.1 months with PomDex (HR, 1.14; 95% CI, 0.77-1.68).
A version of this article first appeared on Medscape.com.
AAP issues clinical update to cerebral palsy guidelines
Updated clinical guidelines for the early diagnosis and management of cerebral palsy have been issued by the American Academy of Pediatrics.
Coauthored with the American Academy for Cerebral Palsy and Developmental Medicine, the report builds on new evidence for improved care and outcomes since the 2006 consensus guidelines.
Cerebral palsy, the most common neuromotor disorder of childhood, is often accompanied by cognitive impairments, epilepsy, sensory impairments, behavioral problems, communication difficulties, breathing and sleep problems, gastrointestinal and nutritional problems, and bone and orthopedic problems.
In the United States, the estimated prevalence of cerebral palsy ranges from 1.5 to 4 per 1,000 live births.
“Early identification and initiation of evidence-based motor therapies can improve outcomes by taking advantage of the neuroplasticity in the infant brain,” said the guideline authors in an executive summary.
The guideline, published in Pediatrics, is directed to primary care physicians with pediatrics, family practice, or internal medicine training. “It’s a much more comprehensive overview of the important role that primary care providers play in the lifetime care of people with cerebral palsy,” explained Garey Noritz, MD, chair of the 2021-2022 Executive Committee of the Council on Children with Disabilities. Dr. Noritz, a professor of pediatrics at Ohio State University and division chief of the complex health care program at Nationwide Children’s Hospital, both in Columbus, said: “The combined efforts of the primary care physician and specialty providers are needed to achieve the best outcomes.”
The AAP recommends that primary care pediatricians, neonatologists, and other specialists caring for hospitalized newborns recognize those at high risk of cerebral palsy, diagnose them as early as possible, and promptly refer them for therapy. Primary care physicians are advised to identify motor delays early by formalizing standardized developmental surveillance and screening at 9, 18, and 30 months, and to implement family-centered care across multiple specialists.
“If a motor disorder is suspected, primary care physicians should simultaneously begin a medical evaluation, refer to a specialist for definitive diagnosis, and to therapists for treatment,” Dr. Noritz emphasized.
“The earlier any possible movement disorder is recognized and intervention begins, the better a child can develop a gait pattern and work toward living an independent life, said Manish N. Shah, MD, associate professor of pediatric neurosurgery at the University of Texas, Houston, who was not involved in developing the guidelines.
For children in whom physical therapy and medication have not reduced leg spasticity, a minimally invasive spinal procedure can help release contracted tendons and encourage independent walking. The optimal age for selective dorsal rhizotomy is about 4 years, said Dr. Shah, who is director of the Texas Comprehensive Spasticity Center at Children’s Memorial Hermann Hospital in Houston. “You can turn these children into walkers. As adults, they can get jobs, have their own families. It’s life-changing.”
Importantly, the guidelines address the health care disparities leading to a higher prevalence of cerebral palsy in Black children and in those from families with lower socioeconomic status. “Efforts to combat racism and eliminate barriers to culturally sensitive prenatal, perinatal, and later pediatric care may help to improve outcomes for all children with cerebral palsy,” the authors said.
“Every child with cerebral palsy needs an individual plan, but only 30% or 40% are getting interventions,” said Dr. Shah. The updated guidelines could help payers rethink the 15-20 visits a year that are often approved, compared with the 2-3 visits per week that are needed for speech, physical, and occupational therapy, he pointed out.
“Financial issues often compromise the interdisciplinary and coordinated care associated with favorable outcomes in children with cerebral palsy,” said Heidi Feldman, MD, PhD, a developmental and behavioral pediatric specialist at Stanford (Calif.) Medicine Children’s Health’s Johnson Center for Pregnancy and Newborn Services. “With a new guideline, there may be greater willingness to fund these essential services.”
In the meantime, the AAP recommends that pediatricians advise families about available medical, social, and educational services, such as early intervention services, the Title V Maternal and Child Health block grant program, and special education services through the public school system.
Children with cerebral palsy need the same standardized primary care as any child, including the full schedule of recommended vaccinations and vision and hearing testing. They also need to be monitored and treated for the many problems that commonly co-occur, including chronic pain.
When secondary complications arise, the frequency of visits should increase.
Pneumonia, the leading cause of death in children and adolescents with cerebral palsy, can be prevented or minimized through immunization against respiratory diseases and screening for signs and symptoms of aspiration and sleep-disordered breathing.
The AAP also recommends that symptoms or functional declines undergo full investigation into other potential causes.
Since the sedentary lifestyle associated with cerebral palsy is now known to be related to the higher rates of cardiovascular complications in this patient population, the AAP recommends more attention be paid to physical activity and a healthy diet early in life. Pediatricians are advised to help families locate suitable opportunities for adaptive sports and recreation.
Almost 50% of children and adolescents with cerebral palsy have intellectual disability, 60%-80% have difficulty speaking, and about 25% are nonverbal. To address this, pediatricians should maximize the use of augmentative and alternative communication devices and involve experts in speech and language pathology, according to the guidelines.
“Many individuals with cerebral palsy and severe motor limitations have active, creative minds, and may need assistive technology, such as electronic talking devices, to demonstrate that mental life,” said Dr. Feldman. “Primary care clinicians should advocate for assistive technology.”
For challenging behavior, especially in the patient with limited verbal skills, potential nonbehavioral culprits such as constipation, esophageal reflux disease, and musculoskeletal or dental pain must be ruled out.
In the lead-up to adolescence, youth with cerebral palsy must be prepared for puberty, menstruation, and healthy, safe sexual relationships, much like their nonaffected peers. Since a disproportionate number of children with cerebral palsy experience neglect and physical, sexual, and emotional abuse, however, family stressors should be identified and caregivers referred for support services.
For the transition from pediatric to adult health care, the AAP recommends that structured planning begin between 12 and 14 years of age. Before transfer, the pediatrician should prepare a comprehensive medical summary with the input of the patient, parent/guardian, and pediatric subspecialists.
Without a proper handoff, “there is an increased risk of morbidity, medical complications, unnecessary emergency department visits, hospitalizations, and procedures,” the authors warned.
Transitions are likely to run more smoothly when youth are given the opportunity to understand their medical condition and be involved in decisions about their health. With this in mind, the AAP recommends that pediatricians actively discourage overprotective parents from getting in the way of their child developing “maximal independence.”
No potential conflicts of interest were disclosed by the authors, Dr. Shah, or Dr. Feldman.
*This story was updated on Nov. 28, 2022.
Updated clinical guidelines for the early diagnosis and management of cerebral palsy have been issued by the American Academy of Pediatrics.
Coauthored with the American Academy for Cerebral Palsy and Developmental Medicine, the report builds on new evidence for improved care and outcomes since the 2006 consensus guidelines.
Cerebral palsy, the most common neuromotor disorder of childhood, is often accompanied by cognitive impairments, epilepsy, sensory impairments, behavioral problems, communication difficulties, breathing and sleep problems, gastrointestinal and nutritional problems, and bone and orthopedic problems.
In the United States, the estimated prevalence of cerebral palsy ranges from 1.5 to 4 per 1,000 live births.
“Early identification and initiation of evidence-based motor therapies can improve outcomes by taking advantage of the neuroplasticity in the infant brain,” said the guideline authors in an executive summary.
The guideline, published in Pediatrics, is directed to primary care physicians with pediatrics, family practice, or internal medicine training. “It’s a much more comprehensive overview of the important role that primary care providers play in the lifetime care of people with cerebral palsy,” explained Garey Noritz, MD, chair of the 2021-2022 Executive Committee of the Council on Children with Disabilities. Dr. Noritz, a professor of pediatrics at Ohio State University and division chief of the complex health care program at Nationwide Children’s Hospital, both in Columbus, said: “The combined efforts of the primary care physician and specialty providers are needed to achieve the best outcomes.”
The AAP recommends that primary care pediatricians, neonatologists, and other specialists caring for hospitalized newborns recognize those at high risk of cerebral palsy, diagnose them as early as possible, and promptly refer them for therapy. Primary care physicians are advised to identify motor delays early by formalizing standardized developmental surveillance and screening at 9, 18, and 30 months, and to implement family-centered care across multiple specialists.
“If a motor disorder is suspected, primary care physicians should simultaneously begin a medical evaluation, refer to a specialist for definitive diagnosis, and to therapists for treatment,” Dr. Noritz emphasized.
“The earlier any possible movement disorder is recognized and intervention begins, the better a child can develop a gait pattern and work toward living an independent life, said Manish N. Shah, MD, associate professor of pediatric neurosurgery at the University of Texas, Houston, who was not involved in developing the guidelines.
For children in whom physical therapy and medication have not reduced leg spasticity, a minimally invasive spinal procedure can help release contracted tendons and encourage independent walking. The optimal age for selective dorsal rhizotomy is about 4 years, said Dr. Shah, who is director of the Texas Comprehensive Spasticity Center at Children’s Memorial Hermann Hospital in Houston. “You can turn these children into walkers. As adults, they can get jobs, have their own families. It’s life-changing.”
Importantly, the guidelines address the health care disparities leading to a higher prevalence of cerebral palsy in Black children and in those from families with lower socioeconomic status. “Efforts to combat racism and eliminate barriers to culturally sensitive prenatal, perinatal, and later pediatric care may help to improve outcomes for all children with cerebral palsy,” the authors said.
“Every child with cerebral palsy needs an individual plan, but only 30% or 40% are getting interventions,” said Dr. Shah. The updated guidelines could help payers rethink the 15-20 visits a year that are often approved, compared with the 2-3 visits per week that are needed for speech, physical, and occupational therapy, he pointed out.
“Financial issues often compromise the interdisciplinary and coordinated care associated with favorable outcomes in children with cerebral palsy,” said Heidi Feldman, MD, PhD, a developmental and behavioral pediatric specialist at Stanford (Calif.) Medicine Children’s Health’s Johnson Center for Pregnancy and Newborn Services. “With a new guideline, there may be greater willingness to fund these essential services.”
In the meantime, the AAP recommends that pediatricians advise families about available medical, social, and educational services, such as early intervention services, the Title V Maternal and Child Health block grant program, and special education services through the public school system.
Children with cerebral palsy need the same standardized primary care as any child, including the full schedule of recommended vaccinations and vision and hearing testing. They also need to be monitored and treated for the many problems that commonly co-occur, including chronic pain.
When secondary complications arise, the frequency of visits should increase.
Pneumonia, the leading cause of death in children and adolescents with cerebral palsy, can be prevented or minimized through immunization against respiratory diseases and screening for signs and symptoms of aspiration and sleep-disordered breathing.
The AAP also recommends that symptoms or functional declines undergo full investigation into other potential causes.
Since the sedentary lifestyle associated with cerebral palsy is now known to be related to the higher rates of cardiovascular complications in this patient population, the AAP recommends more attention be paid to physical activity and a healthy diet early in life. Pediatricians are advised to help families locate suitable opportunities for adaptive sports and recreation.
Almost 50% of children and adolescents with cerebral palsy have intellectual disability, 60%-80% have difficulty speaking, and about 25% are nonverbal. To address this, pediatricians should maximize the use of augmentative and alternative communication devices and involve experts in speech and language pathology, according to the guidelines.
“Many individuals with cerebral palsy and severe motor limitations have active, creative minds, and may need assistive technology, such as electronic talking devices, to demonstrate that mental life,” said Dr. Feldman. “Primary care clinicians should advocate for assistive technology.”
For challenging behavior, especially in the patient with limited verbal skills, potential nonbehavioral culprits such as constipation, esophageal reflux disease, and musculoskeletal or dental pain must be ruled out.
In the lead-up to adolescence, youth with cerebral palsy must be prepared for puberty, menstruation, and healthy, safe sexual relationships, much like their nonaffected peers. Since a disproportionate number of children with cerebral palsy experience neglect and physical, sexual, and emotional abuse, however, family stressors should be identified and caregivers referred for support services.
For the transition from pediatric to adult health care, the AAP recommends that structured planning begin between 12 and 14 years of age. Before transfer, the pediatrician should prepare a comprehensive medical summary with the input of the patient, parent/guardian, and pediatric subspecialists.
Without a proper handoff, “there is an increased risk of morbidity, medical complications, unnecessary emergency department visits, hospitalizations, and procedures,” the authors warned.
Transitions are likely to run more smoothly when youth are given the opportunity to understand their medical condition and be involved in decisions about their health. With this in mind, the AAP recommends that pediatricians actively discourage overprotective parents from getting in the way of their child developing “maximal independence.”
No potential conflicts of interest were disclosed by the authors, Dr. Shah, or Dr. Feldman.
*This story was updated on Nov. 28, 2022.
Updated clinical guidelines for the early diagnosis and management of cerebral palsy have been issued by the American Academy of Pediatrics.
Coauthored with the American Academy for Cerebral Palsy and Developmental Medicine, the report builds on new evidence for improved care and outcomes since the 2006 consensus guidelines.
Cerebral palsy, the most common neuromotor disorder of childhood, is often accompanied by cognitive impairments, epilepsy, sensory impairments, behavioral problems, communication difficulties, breathing and sleep problems, gastrointestinal and nutritional problems, and bone and orthopedic problems.
In the United States, the estimated prevalence of cerebral palsy ranges from 1.5 to 4 per 1,000 live births.
“Early identification and initiation of evidence-based motor therapies can improve outcomes by taking advantage of the neuroplasticity in the infant brain,” said the guideline authors in an executive summary.
The guideline, published in Pediatrics, is directed to primary care physicians with pediatrics, family practice, or internal medicine training. “It’s a much more comprehensive overview of the important role that primary care providers play in the lifetime care of people with cerebral palsy,” explained Garey Noritz, MD, chair of the 2021-2022 Executive Committee of the Council on Children with Disabilities. Dr. Noritz, a professor of pediatrics at Ohio State University and division chief of the complex health care program at Nationwide Children’s Hospital, both in Columbus, said: “The combined efforts of the primary care physician and specialty providers are needed to achieve the best outcomes.”
The AAP recommends that primary care pediatricians, neonatologists, and other specialists caring for hospitalized newborns recognize those at high risk of cerebral palsy, diagnose them as early as possible, and promptly refer them for therapy. Primary care physicians are advised to identify motor delays early by formalizing standardized developmental surveillance and screening at 9, 18, and 30 months, and to implement family-centered care across multiple specialists.
“If a motor disorder is suspected, primary care physicians should simultaneously begin a medical evaluation, refer to a specialist for definitive diagnosis, and to therapists for treatment,” Dr. Noritz emphasized.
“The earlier any possible movement disorder is recognized and intervention begins, the better a child can develop a gait pattern and work toward living an independent life, said Manish N. Shah, MD, associate professor of pediatric neurosurgery at the University of Texas, Houston, who was not involved in developing the guidelines.
For children in whom physical therapy and medication have not reduced leg spasticity, a minimally invasive spinal procedure can help release contracted tendons and encourage independent walking. The optimal age for selective dorsal rhizotomy is about 4 years, said Dr. Shah, who is director of the Texas Comprehensive Spasticity Center at Children’s Memorial Hermann Hospital in Houston. “You can turn these children into walkers. As adults, they can get jobs, have their own families. It’s life-changing.”
Importantly, the guidelines address the health care disparities leading to a higher prevalence of cerebral palsy in Black children and in those from families with lower socioeconomic status. “Efforts to combat racism and eliminate barriers to culturally sensitive prenatal, perinatal, and later pediatric care may help to improve outcomes for all children with cerebral palsy,” the authors said.
“Every child with cerebral palsy needs an individual plan, but only 30% or 40% are getting interventions,” said Dr. Shah. The updated guidelines could help payers rethink the 15-20 visits a year that are often approved, compared with the 2-3 visits per week that are needed for speech, physical, and occupational therapy, he pointed out.
“Financial issues often compromise the interdisciplinary and coordinated care associated with favorable outcomes in children with cerebral palsy,” said Heidi Feldman, MD, PhD, a developmental and behavioral pediatric specialist at Stanford (Calif.) Medicine Children’s Health’s Johnson Center for Pregnancy and Newborn Services. “With a new guideline, there may be greater willingness to fund these essential services.”
In the meantime, the AAP recommends that pediatricians advise families about available medical, social, and educational services, such as early intervention services, the Title V Maternal and Child Health block grant program, and special education services through the public school system.
Children with cerebral palsy need the same standardized primary care as any child, including the full schedule of recommended vaccinations and vision and hearing testing. They also need to be monitored and treated for the many problems that commonly co-occur, including chronic pain.
When secondary complications arise, the frequency of visits should increase.
Pneumonia, the leading cause of death in children and adolescents with cerebral palsy, can be prevented or minimized through immunization against respiratory diseases and screening for signs and symptoms of aspiration and sleep-disordered breathing.
The AAP also recommends that symptoms or functional declines undergo full investigation into other potential causes.
Since the sedentary lifestyle associated with cerebral palsy is now known to be related to the higher rates of cardiovascular complications in this patient population, the AAP recommends more attention be paid to physical activity and a healthy diet early in life. Pediatricians are advised to help families locate suitable opportunities for adaptive sports and recreation.
Almost 50% of children and adolescents with cerebral palsy have intellectual disability, 60%-80% have difficulty speaking, and about 25% are nonverbal. To address this, pediatricians should maximize the use of augmentative and alternative communication devices and involve experts in speech and language pathology, according to the guidelines.
“Many individuals with cerebral palsy and severe motor limitations have active, creative minds, and may need assistive technology, such as electronic talking devices, to demonstrate that mental life,” said Dr. Feldman. “Primary care clinicians should advocate for assistive technology.”
For challenging behavior, especially in the patient with limited verbal skills, potential nonbehavioral culprits such as constipation, esophageal reflux disease, and musculoskeletal or dental pain must be ruled out.
In the lead-up to adolescence, youth with cerebral palsy must be prepared for puberty, menstruation, and healthy, safe sexual relationships, much like their nonaffected peers. Since a disproportionate number of children with cerebral palsy experience neglect and physical, sexual, and emotional abuse, however, family stressors should be identified and caregivers referred for support services.
For the transition from pediatric to adult health care, the AAP recommends that structured planning begin between 12 and 14 years of age. Before transfer, the pediatrician should prepare a comprehensive medical summary with the input of the patient, parent/guardian, and pediatric subspecialists.
Without a proper handoff, “there is an increased risk of morbidity, medical complications, unnecessary emergency department visits, hospitalizations, and procedures,” the authors warned.
Transitions are likely to run more smoothly when youth are given the opportunity to understand their medical condition and be involved in decisions about their health. With this in mind, the AAP recommends that pediatricians actively discourage overprotective parents from getting in the way of their child developing “maximal independence.”
No potential conflicts of interest were disclosed by the authors, Dr. Shah, or Dr. Feldman.
*This story was updated on Nov. 28, 2022.
FROM PEDIATRICS
Sarilumab effective for polymyalgia rheumatica in phase 3 trial
PHILADELPHIA – Treatment with the interleukin-6 receptor antagonist sarilumab (Kevzara), along with a 14-week taper of glucocorticoids, proved to have significant efficacy in patients with relapsing polymyalgia rheumatica (PMR) who were resistant to glucocorticoids in a phase 3 trial.
No new safety concerns were found with sarilumab in the multicenter, randomized, double-blind, placebo-controlled SAPHYR trial. Sarilumab is approved in the United States for the treatment of moderate to severe active rheumatoid arthritis in adults who have had an inadequate response or intolerance to one or more disease-modifying antirheumatic drugs.
The results, presented at the annual meeting of the American College of Rheumatology by Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York, included clinically meaningful improvement in quality-of-life scores.
The disease, which primarily affects people over age 65, can cause widespread aching and stiffness. It’s one of the most common inflammatory diseases among older adults.
PMR is relatively easy to treat with glucocorticoids, but relapses are common, which means long courses of glucocorticoid therapy and the side effects that come with them.
Need for a steroid-sparing therapy
“We recognize that a steroid-sparing drug in polymyalgia rheumatica seems to be an unmet need,” Dr. Spiera said at the meeting.
The trial, sponsored by Sanofi, included active, refractory PMR patients who flared within 3 months of study entry while on at least 7.5 mg/day of prednisone or the equivalent. They were randomly assigned (1:1) to 52 weeks of treatment with subcutaneous sarilumab 200 mg every 2 weeks plus the rapid 14-week glucocorticoid tapering regimen or were given placebo every 2 weeks plus a more traditional 52-week tapering of glucocorticoids.
COVID hampered recruitment
Recruitment was stopped early because of complications during the COVID-19 pandemic, so between October 2018 and July 2020, 118 of the intended 280 patients were recruited, and 117 were treated (sarilumab = 59, placebo = 58). Median age was 69 years in the treatment group and 70 among those taking placebo.
Of the 117 treated, only 78 patients (67%) completed treatment (sarilumab = 42, placebo = 36). The main reasons for stopping treatment were adverse events – including seven with sarilumab and four with placebo – and lack of efficacy (sarilumab = four, placebo = nine).
The primary outcome was the proportion of patients who reached sustained remission at 52 weeks, defined as disease remission by week 12 and no disease flare, normal C-reactive protein (CRP), and adherence to the glucocorticoid taper during weeks 12-52.
The researchers found that sustained remission was significantly higher in the sarilumab arm versus the control group (28.3% versus 10.3%; P = .0193).
IL-6 inhibitors lower CRP, but if you take CRP out of the definition, Dr. Spiera said, “we still saw this difference: 31.7% of patients treated with sarilumab and 13.8% treated with placebo and a longer taper achieved that endpoint.”
Forty-four percent lower risk of flare with sarilumab
Patients in the sarilumab group also had 44% lower risk of having a flare after achieving clinical remission versus the comparator group (16.7% versus 29.3%; hazard ratio, 0.56; 95% confidence interval, 0.35-0.90; P = .0153).
Patient-reported outcomes, which included physical and mental health scores and disability index results, favored sarilumab.
The incidence of treatment-emergent adverse events (TEAEs) was numerically higher in the sarilumab group, compared with the control group (94.9% versus 84.5%). TEAEs included neutropenia (15.3%) and arthralgia (15.3%) in the sarilumab group and insomnia (15.5%) in the comparator arm.
However, the frequency of serious AEs was higher in the control group, compared with the sarilumab arm (20.7% versus 13.6%). No deaths were reported, and, importantly in this age group treated with concurrent glucocorticoids and an IL-6 inhibitor, Dr. Spiera said, “there were no cases of diverticulitis requiring intervention.”
Dr. Spiera was asked about a seemingly low remission rate. He answered that the bar was very high for remission in this study.
Patients had to achieve remission by week 12 and with the rapid 14-week taper. “That means by week 12 the sarilumab arm patients were only on 2 mg of daily prednisone or its equivalent,” he said.
Patients had to maintain that for another 40 weeks, he noted, adding, “I think especially in the context of quality of life and function indices, these were important results.”
Sebastian E. Sattui, MD, director of the University of Pittsburgh Medical Center vasculitis clinic, told this news organization that prolonged use of glucocorticoids in patients with PMR remains an important concern and the need for other options is critical.
“Around 30% of patients with PMR remain on prednisone 5 years after diagnosis,” he said. “Low-dose glucocorticoids are still associated with significant morbidity. Until recently, there has been a paucity of high-quality data regarding the use of steroid-sparing agents in PMR. “
He noted that the SAPHYR trial data are promising “with sarilumab being successful in achieving remission while minimizing glucocorticoids in patients with relapsing PMR.” The clinically meaningful improvement in patient-reported outcomes was just as important, he added.
The main unanswered question is whether the disease-modifying ability of sarilumab will continue after it is stopped, Dr. Sattui said.
Dr. Spiera is a consultant for Sanofi, which funded the trial. He also disclosed financial relationships with GlaxoSmithKline, Boehringer Ingelheim, Corbus, InflaRx, AbbVie/Abbott, Novartis, Chemocentryx, Roche, and Vera. Dr. Sattui has received research support from AstraZeneca and has done unpaid consulting work for Sanofi.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – Treatment with the interleukin-6 receptor antagonist sarilumab (Kevzara), along with a 14-week taper of glucocorticoids, proved to have significant efficacy in patients with relapsing polymyalgia rheumatica (PMR) who were resistant to glucocorticoids in a phase 3 trial.
No new safety concerns were found with sarilumab in the multicenter, randomized, double-blind, placebo-controlled SAPHYR trial. Sarilumab is approved in the United States for the treatment of moderate to severe active rheumatoid arthritis in adults who have had an inadequate response or intolerance to one or more disease-modifying antirheumatic drugs.
The results, presented at the annual meeting of the American College of Rheumatology by Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York, included clinically meaningful improvement in quality-of-life scores.
The disease, which primarily affects people over age 65, can cause widespread aching and stiffness. It’s one of the most common inflammatory diseases among older adults.
PMR is relatively easy to treat with glucocorticoids, but relapses are common, which means long courses of glucocorticoid therapy and the side effects that come with them.
Need for a steroid-sparing therapy
“We recognize that a steroid-sparing drug in polymyalgia rheumatica seems to be an unmet need,” Dr. Spiera said at the meeting.
The trial, sponsored by Sanofi, included active, refractory PMR patients who flared within 3 months of study entry while on at least 7.5 mg/day of prednisone or the equivalent. They were randomly assigned (1:1) to 52 weeks of treatment with subcutaneous sarilumab 200 mg every 2 weeks plus the rapid 14-week glucocorticoid tapering regimen or were given placebo every 2 weeks plus a more traditional 52-week tapering of glucocorticoids.
COVID hampered recruitment
Recruitment was stopped early because of complications during the COVID-19 pandemic, so between October 2018 and July 2020, 118 of the intended 280 patients were recruited, and 117 were treated (sarilumab = 59, placebo = 58). Median age was 69 years in the treatment group and 70 among those taking placebo.
Of the 117 treated, only 78 patients (67%) completed treatment (sarilumab = 42, placebo = 36). The main reasons for stopping treatment were adverse events – including seven with sarilumab and four with placebo – and lack of efficacy (sarilumab = four, placebo = nine).
The primary outcome was the proportion of patients who reached sustained remission at 52 weeks, defined as disease remission by week 12 and no disease flare, normal C-reactive protein (CRP), and adherence to the glucocorticoid taper during weeks 12-52.
The researchers found that sustained remission was significantly higher in the sarilumab arm versus the control group (28.3% versus 10.3%; P = .0193).
IL-6 inhibitors lower CRP, but if you take CRP out of the definition, Dr. Spiera said, “we still saw this difference: 31.7% of patients treated with sarilumab and 13.8% treated with placebo and a longer taper achieved that endpoint.”
Forty-four percent lower risk of flare with sarilumab
Patients in the sarilumab group also had 44% lower risk of having a flare after achieving clinical remission versus the comparator group (16.7% versus 29.3%; hazard ratio, 0.56; 95% confidence interval, 0.35-0.90; P = .0153).
Patient-reported outcomes, which included physical and mental health scores and disability index results, favored sarilumab.
The incidence of treatment-emergent adverse events (TEAEs) was numerically higher in the sarilumab group, compared with the control group (94.9% versus 84.5%). TEAEs included neutropenia (15.3%) and arthralgia (15.3%) in the sarilumab group and insomnia (15.5%) in the comparator arm.
However, the frequency of serious AEs was higher in the control group, compared with the sarilumab arm (20.7% versus 13.6%). No deaths were reported, and, importantly in this age group treated with concurrent glucocorticoids and an IL-6 inhibitor, Dr. Spiera said, “there were no cases of diverticulitis requiring intervention.”
Dr. Spiera was asked about a seemingly low remission rate. He answered that the bar was very high for remission in this study.
Patients had to achieve remission by week 12 and with the rapid 14-week taper. “That means by week 12 the sarilumab arm patients were only on 2 mg of daily prednisone or its equivalent,” he said.
Patients had to maintain that for another 40 weeks, he noted, adding, “I think especially in the context of quality of life and function indices, these were important results.”
Sebastian E. Sattui, MD, director of the University of Pittsburgh Medical Center vasculitis clinic, told this news organization that prolonged use of glucocorticoids in patients with PMR remains an important concern and the need for other options is critical.
“Around 30% of patients with PMR remain on prednisone 5 years after diagnosis,” he said. “Low-dose glucocorticoids are still associated with significant morbidity. Until recently, there has been a paucity of high-quality data regarding the use of steroid-sparing agents in PMR. “
He noted that the SAPHYR trial data are promising “with sarilumab being successful in achieving remission while minimizing glucocorticoids in patients with relapsing PMR.” The clinically meaningful improvement in patient-reported outcomes was just as important, he added.
The main unanswered question is whether the disease-modifying ability of sarilumab will continue after it is stopped, Dr. Sattui said.
Dr. Spiera is a consultant for Sanofi, which funded the trial. He also disclosed financial relationships with GlaxoSmithKline, Boehringer Ingelheim, Corbus, InflaRx, AbbVie/Abbott, Novartis, Chemocentryx, Roche, and Vera. Dr. Sattui has received research support from AstraZeneca and has done unpaid consulting work for Sanofi.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – Treatment with the interleukin-6 receptor antagonist sarilumab (Kevzara), along with a 14-week taper of glucocorticoids, proved to have significant efficacy in patients with relapsing polymyalgia rheumatica (PMR) who were resistant to glucocorticoids in a phase 3 trial.
No new safety concerns were found with sarilumab in the multicenter, randomized, double-blind, placebo-controlled SAPHYR trial. Sarilumab is approved in the United States for the treatment of moderate to severe active rheumatoid arthritis in adults who have had an inadequate response or intolerance to one or more disease-modifying antirheumatic drugs.
The results, presented at the annual meeting of the American College of Rheumatology by Robert Spiera, MD, director of the Scleroderma, Vasculitis, and Myositis Center at the Hospital for Special Surgery in New York, included clinically meaningful improvement in quality-of-life scores.
The disease, which primarily affects people over age 65, can cause widespread aching and stiffness. It’s one of the most common inflammatory diseases among older adults.
PMR is relatively easy to treat with glucocorticoids, but relapses are common, which means long courses of glucocorticoid therapy and the side effects that come with them.
Need for a steroid-sparing therapy
“We recognize that a steroid-sparing drug in polymyalgia rheumatica seems to be an unmet need,” Dr. Spiera said at the meeting.
The trial, sponsored by Sanofi, included active, refractory PMR patients who flared within 3 months of study entry while on at least 7.5 mg/day of prednisone or the equivalent. They were randomly assigned (1:1) to 52 weeks of treatment with subcutaneous sarilumab 200 mg every 2 weeks plus the rapid 14-week glucocorticoid tapering regimen or were given placebo every 2 weeks plus a more traditional 52-week tapering of glucocorticoids.
COVID hampered recruitment
Recruitment was stopped early because of complications during the COVID-19 pandemic, so between October 2018 and July 2020, 118 of the intended 280 patients were recruited, and 117 were treated (sarilumab = 59, placebo = 58). Median age was 69 years in the treatment group and 70 among those taking placebo.
Of the 117 treated, only 78 patients (67%) completed treatment (sarilumab = 42, placebo = 36). The main reasons for stopping treatment were adverse events – including seven with sarilumab and four with placebo – and lack of efficacy (sarilumab = four, placebo = nine).
The primary outcome was the proportion of patients who reached sustained remission at 52 weeks, defined as disease remission by week 12 and no disease flare, normal C-reactive protein (CRP), and adherence to the glucocorticoid taper during weeks 12-52.
The researchers found that sustained remission was significantly higher in the sarilumab arm versus the control group (28.3% versus 10.3%; P = .0193).
IL-6 inhibitors lower CRP, but if you take CRP out of the definition, Dr. Spiera said, “we still saw this difference: 31.7% of patients treated with sarilumab and 13.8% treated with placebo and a longer taper achieved that endpoint.”
Forty-four percent lower risk of flare with sarilumab
Patients in the sarilumab group also had 44% lower risk of having a flare after achieving clinical remission versus the comparator group (16.7% versus 29.3%; hazard ratio, 0.56; 95% confidence interval, 0.35-0.90; P = .0153).
Patient-reported outcomes, which included physical and mental health scores and disability index results, favored sarilumab.
The incidence of treatment-emergent adverse events (TEAEs) was numerically higher in the sarilumab group, compared with the control group (94.9% versus 84.5%). TEAEs included neutropenia (15.3%) and arthralgia (15.3%) in the sarilumab group and insomnia (15.5%) in the comparator arm.
However, the frequency of serious AEs was higher in the control group, compared with the sarilumab arm (20.7% versus 13.6%). No deaths were reported, and, importantly in this age group treated with concurrent glucocorticoids and an IL-6 inhibitor, Dr. Spiera said, “there were no cases of diverticulitis requiring intervention.”
Dr. Spiera was asked about a seemingly low remission rate. He answered that the bar was very high for remission in this study.
Patients had to achieve remission by week 12 and with the rapid 14-week taper. “That means by week 12 the sarilumab arm patients were only on 2 mg of daily prednisone or its equivalent,” he said.
Patients had to maintain that for another 40 weeks, he noted, adding, “I think especially in the context of quality of life and function indices, these were important results.”
Sebastian E. Sattui, MD, director of the University of Pittsburgh Medical Center vasculitis clinic, told this news organization that prolonged use of glucocorticoids in patients with PMR remains an important concern and the need for other options is critical.
“Around 30% of patients with PMR remain on prednisone 5 years after diagnosis,” he said. “Low-dose glucocorticoids are still associated with significant morbidity. Until recently, there has been a paucity of high-quality data regarding the use of steroid-sparing agents in PMR. “
He noted that the SAPHYR trial data are promising “with sarilumab being successful in achieving remission while minimizing glucocorticoids in patients with relapsing PMR.” The clinically meaningful improvement in patient-reported outcomes was just as important, he added.
The main unanswered question is whether the disease-modifying ability of sarilumab will continue after it is stopped, Dr. Sattui said.
Dr. Spiera is a consultant for Sanofi, which funded the trial. He also disclosed financial relationships with GlaxoSmithKline, Boehringer Ingelheim, Corbus, InflaRx, AbbVie/Abbott, Novartis, Chemocentryx, Roche, and Vera. Dr. Sattui has received research support from AstraZeneca and has done unpaid consulting work for Sanofi.
A version of this article first appeared on Medscape.com.
AT ACR 2022
Hormonal management of gender-diverse patients: SOC8 updates
In September, the World Professional Association for Transgender Health released its much-anticipated standards of care (SOC8). While this update has unfortunately received intense scrutiny for its guidance about gender-diverse adolescents and youth, the SOC8 is their most evidence-based version to date. Recommendations were developed based on data from independent systematic literature reviews, background reviews, and expert opinions.1 These guidelines also recognize knowledge deficits and are intended to be flexible to meet the individual needs of transgender patients. While the scope of this column will not delve into all 258 pages of these new standards, it will highlight pertinent information on hormonal management.
Ever since the original publication of the standards of care in 1979, gender-affirming hormone therapy (GAHT) has been considered medically necessary. The approach to GAHT depends on the patient’s goals and the age at which the patient is seeking to medically transition. Given the complexity of GAHT for transgender youth and adolescents, this article will focus primarily on adult patients.
There are a few pertinent differences in the management and monitoring of GAHT in adults. For patients assigned female at birth, testosterone is the primary modality by which patients can achieve masculinizing features. GAHT for patients assigned male at birth often consists of estrogen and an androgen-lowering medication. Like its predecessor, SOC8 recommends against prescribing ethinyl estradiol because of its marked association with thromboembolic events.
While the formulations of estrogen (oral, injectable, and patches) and hormone blockers (finasteride, spironolactone, gonadotropin-releasing hormone agonists, and bicalutamide) are discussed in prior standards of care, SOC8 further delineates their utilization. It suggests that transdermal estrogen should be considered in transgender women over the age of 45 who are at high risk for developing a venous thromboembolism or have a previous history of thromboembolism. Furthermore, SOC8 establishes spironolactone as the mainstay for androgen blockage and discourages routine usage of bicalutamide and finasteride because of a lack of safety data and questionable efficacy.1 Even though some patients anecdotally report increased breast growth with progesterone supplementation, there is insufficient evidence to regularly prescribe progesterone for breast development.1
Both WPATH and the Endocrine Society recommend checking serum levels of sex hormones every 3 months during the first year until stable levels are achieved, then once or twice a year thereafter.1 Hormone levels should be maintained at physiologic concentrations of the targeted gender. Some patients on feminizing GAHT often request evaluation of estrone/estradiol ratios as there was an assumption that higher ratios were associated with antagonistic effects on breast development. However, recent published evidence refutes this claim and estrone/estradiol ratios need not be measured.1
In addition to monitoring sex hormone levels, providers should check the metabolic effects that can be associated with GAHT. Both testosterone and estrogen can influence lipid panels: Testosterone can increase the red blood cell count, and spironolactone may cause hyperkalemia. While the SOC7 previously encouraged assessment of these laboratory values every 3 months, the new guidelines are more flexible in the frequency of testing of asymptomatic individuals as there is no strong evidence from published studies that supports these 3-month intervals.1
Providers are responsible for informing patients about the possible effects of GAHT on fertility. Estrogen often will cause a reduction in spermatogenesis, which may be irreversible. Patients who plan on taking estrogen should be counseled regarding sperm cryopreservation prior to starting GAHT. Even though testosterone inhibits ovulation and induces menstrual suppression, patients often regain their fertility after cessation of testosterone therapy. However, given the significant knowledge deficit about long-term fertility in transmasculine patients, providers should still offer oocyte or embryo cryopreservation.
Health care providers should collaborate with surgeons regarding preoperative and postoperative GAHT. To mitigate the risk of thromboembolism, many surgeons would stop hormones 1-4 weeks before and after gender-affirming surgery. Recent evidence does not support this practice, as studies indicate no increased risk for venous thromboembolism in individuals on GAHT undergoing surgery. These studies are consistent with other well-established guidelines on preoperative management of cisgender women taking estrogen or progestins. As exogenous sex steroids are necessary for bone health in patients who undergo gonadectomy, surgeons and other health care providers should educate patients on the importance of continuing GAHT.
There are many procedures available for gender-affirming surgery. Many of these surgeries involve three regions: the face, chest/breast, and/or genitalia (both internal and external). Prior to making a surgical referral, providers should be familiar with the surgeon’s scope of practice, performance measures, and surgical outcomes.1 For the first time, the SOC8 also addresses the surgical training of the providers who offer these procedures. While gender-affirming surgery can be performed by a variety of different specialists, training and documented supervision (often by an existing expert in gender-affirming surgery) is essential. Maintaining an active practice in these procedures, tracking surgical outcomes, and continuing education within the field of gender-affirming surgery are additional requirements for surgeons performing these complex operations.1
As their name implies, the SOC8 attempts to create a standardized guide to assist practitioners caring for gender-diverse patients. It’s important for providers to be familiar with updates while also recognizing the evolving nature of this rapidly growing field.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
Reference
1. World Professional Association for Transgender Health. Standards of care for the health of transgender and gender diverse people, Version 8. Int J Transgend Health. 2022 Sep 15. doi: 10.1080/26895269.2022.2100644.
In September, the World Professional Association for Transgender Health released its much-anticipated standards of care (SOC8). While this update has unfortunately received intense scrutiny for its guidance about gender-diverse adolescents and youth, the SOC8 is their most evidence-based version to date. Recommendations were developed based on data from independent systematic literature reviews, background reviews, and expert opinions.1 These guidelines also recognize knowledge deficits and are intended to be flexible to meet the individual needs of transgender patients. While the scope of this column will not delve into all 258 pages of these new standards, it will highlight pertinent information on hormonal management.
Ever since the original publication of the standards of care in 1979, gender-affirming hormone therapy (GAHT) has been considered medically necessary. The approach to GAHT depends on the patient’s goals and the age at which the patient is seeking to medically transition. Given the complexity of GAHT for transgender youth and adolescents, this article will focus primarily on adult patients.
There are a few pertinent differences in the management and monitoring of GAHT in adults. For patients assigned female at birth, testosterone is the primary modality by which patients can achieve masculinizing features. GAHT for patients assigned male at birth often consists of estrogen and an androgen-lowering medication. Like its predecessor, SOC8 recommends against prescribing ethinyl estradiol because of its marked association with thromboembolic events.
While the formulations of estrogen (oral, injectable, and patches) and hormone blockers (finasteride, spironolactone, gonadotropin-releasing hormone agonists, and bicalutamide) are discussed in prior standards of care, SOC8 further delineates their utilization. It suggests that transdermal estrogen should be considered in transgender women over the age of 45 who are at high risk for developing a venous thromboembolism or have a previous history of thromboembolism. Furthermore, SOC8 establishes spironolactone as the mainstay for androgen blockage and discourages routine usage of bicalutamide and finasteride because of a lack of safety data and questionable efficacy.1 Even though some patients anecdotally report increased breast growth with progesterone supplementation, there is insufficient evidence to regularly prescribe progesterone for breast development.1
Both WPATH and the Endocrine Society recommend checking serum levels of sex hormones every 3 months during the first year until stable levels are achieved, then once or twice a year thereafter.1 Hormone levels should be maintained at physiologic concentrations of the targeted gender. Some patients on feminizing GAHT often request evaluation of estrone/estradiol ratios as there was an assumption that higher ratios were associated with antagonistic effects on breast development. However, recent published evidence refutes this claim and estrone/estradiol ratios need not be measured.1
In addition to monitoring sex hormone levels, providers should check the metabolic effects that can be associated with GAHT. Both testosterone and estrogen can influence lipid panels: Testosterone can increase the red blood cell count, and spironolactone may cause hyperkalemia. While the SOC7 previously encouraged assessment of these laboratory values every 3 months, the new guidelines are more flexible in the frequency of testing of asymptomatic individuals as there is no strong evidence from published studies that supports these 3-month intervals.1
Providers are responsible for informing patients about the possible effects of GAHT on fertility. Estrogen often will cause a reduction in spermatogenesis, which may be irreversible. Patients who plan on taking estrogen should be counseled regarding sperm cryopreservation prior to starting GAHT. Even though testosterone inhibits ovulation and induces menstrual suppression, patients often regain their fertility after cessation of testosterone therapy. However, given the significant knowledge deficit about long-term fertility in transmasculine patients, providers should still offer oocyte or embryo cryopreservation.
Health care providers should collaborate with surgeons regarding preoperative and postoperative GAHT. To mitigate the risk of thromboembolism, many surgeons would stop hormones 1-4 weeks before and after gender-affirming surgery. Recent evidence does not support this practice, as studies indicate no increased risk for venous thromboembolism in individuals on GAHT undergoing surgery. These studies are consistent with other well-established guidelines on preoperative management of cisgender women taking estrogen or progestins. As exogenous sex steroids are necessary for bone health in patients who undergo gonadectomy, surgeons and other health care providers should educate patients on the importance of continuing GAHT.
There are many procedures available for gender-affirming surgery. Many of these surgeries involve three regions: the face, chest/breast, and/or genitalia (both internal and external). Prior to making a surgical referral, providers should be familiar with the surgeon’s scope of practice, performance measures, and surgical outcomes.1 For the first time, the SOC8 also addresses the surgical training of the providers who offer these procedures. While gender-affirming surgery can be performed by a variety of different specialists, training and documented supervision (often by an existing expert in gender-affirming surgery) is essential. Maintaining an active practice in these procedures, tracking surgical outcomes, and continuing education within the field of gender-affirming surgery are additional requirements for surgeons performing these complex operations.1
As their name implies, the SOC8 attempts to create a standardized guide to assist practitioners caring for gender-diverse patients. It’s important for providers to be familiar with updates while also recognizing the evolving nature of this rapidly growing field.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
Reference
1. World Professional Association for Transgender Health. Standards of care for the health of transgender and gender diverse people, Version 8. Int J Transgend Health. 2022 Sep 15. doi: 10.1080/26895269.2022.2100644.
In September, the World Professional Association for Transgender Health released its much-anticipated standards of care (SOC8). While this update has unfortunately received intense scrutiny for its guidance about gender-diverse adolescents and youth, the SOC8 is their most evidence-based version to date. Recommendations were developed based on data from independent systematic literature reviews, background reviews, and expert opinions.1 These guidelines also recognize knowledge deficits and are intended to be flexible to meet the individual needs of transgender patients. While the scope of this column will not delve into all 258 pages of these new standards, it will highlight pertinent information on hormonal management.
Ever since the original publication of the standards of care in 1979, gender-affirming hormone therapy (GAHT) has been considered medically necessary. The approach to GAHT depends on the patient’s goals and the age at which the patient is seeking to medically transition. Given the complexity of GAHT for transgender youth and adolescents, this article will focus primarily on adult patients.
There are a few pertinent differences in the management and monitoring of GAHT in adults. For patients assigned female at birth, testosterone is the primary modality by which patients can achieve masculinizing features. GAHT for patients assigned male at birth often consists of estrogen and an androgen-lowering medication. Like its predecessor, SOC8 recommends against prescribing ethinyl estradiol because of its marked association with thromboembolic events.
While the formulations of estrogen (oral, injectable, and patches) and hormone blockers (finasteride, spironolactone, gonadotropin-releasing hormone agonists, and bicalutamide) are discussed in prior standards of care, SOC8 further delineates their utilization. It suggests that transdermal estrogen should be considered in transgender women over the age of 45 who are at high risk for developing a venous thromboembolism or have a previous history of thromboembolism. Furthermore, SOC8 establishes spironolactone as the mainstay for androgen blockage and discourages routine usage of bicalutamide and finasteride because of a lack of safety data and questionable efficacy.1 Even though some patients anecdotally report increased breast growth with progesterone supplementation, there is insufficient evidence to regularly prescribe progesterone for breast development.1
Both WPATH and the Endocrine Society recommend checking serum levels of sex hormones every 3 months during the first year until stable levels are achieved, then once or twice a year thereafter.1 Hormone levels should be maintained at physiologic concentrations of the targeted gender. Some patients on feminizing GAHT often request evaluation of estrone/estradiol ratios as there was an assumption that higher ratios were associated with antagonistic effects on breast development. However, recent published evidence refutes this claim and estrone/estradiol ratios need not be measured.1
In addition to monitoring sex hormone levels, providers should check the metabolic effects that can be associated with GAHT. Both testosterone and estrogen can influence lipid panels: Testosterone can increase the red blood cell count, and spironolactone may cause hyperkalemia. While the SOC7 previously encouraged assessment of these laboratory values every 3 months, the new guidelines are more flexible in the frequency of testing of asymptomatic individuals as there is no strong evidence from published studies that supports these 3-month intervals.1
Providers are responsible for informing patients about the possible effects of GAHT on fertility. Estrogen often will cause a reduction in spermatogenesis, which may be irreversible. Patients who plan on taking estrogen should be counseled regarding sperm cryopreservation prior to starting GAHT. Even though testosterone inhibits ovulation and induces menstrual suppression, patients often regain their fertility after cessation of testosterone therapy. However, given the significant knowledge deficit about long-term fertility in transmasculine patients, providers should still offer oocyte or embryo cryopreservation.
Health care providers should collaborate with surgeons regarding preoperative and postoperative GAHT. To mitigate the risk of thromboembolism, many surgeons would stop hormones 1-4 weeks before and after gender-affirming surgery. Recent evidence does not support this practice, as studies indicate no increased risk for venous thromboembolism in individuals on GAHT undergoing surgery. These studies are consistent with other well-established guidelines on preoperative management of cisgender women taking estrogen or progestins. As exogenous sex steroids are necessary for bone health in patients who undergo gonadectomy, surgeons and other health care providers should educate patients on the importance of continuing GAHT.
There are many procedures available for gender-affirming surgery. Many of these surgeries involve three regions: the face, chest/breast, and/or genitalia (both internal and external). Prior to making a surgical referral, providers should be familiar with the surgeon’s scope of practice, performance measures, and surgical outcomes.1 For the first time, the SOC8 also addresses the surgical training of the providers who offer these procedures. While gender-affirming surgery can be performed by a variety of different specialists, training and documented supervision (often by an existing expert in gender-affirming surgery) is essential. Maintaining an active practice in these procedures, tracking surgical outcomes, and continuing education within the field of gender-affirming surgery are additional requirements for surgeons performing these complex operations.1
As their name implies, the SOC8 attempts to create a standardized guide to assist practitioners caring for gender-diverse patients. It’s important for providers to be familiar with updates while also recognizing the evolving nature of this rapidly growing field.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
Reference
1. World Professional Association for Transgender Health. Standards of care for the health of transgender and gender diverse people, Version 8. Int J Transgend Health. 2022 Sep 15. doi: 10.1080/26895269.2022.2100644.
Primary Malignant Melanoma of the Middle Ear
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
PRACTICE POINTS
- Primary malignant melanoma of the middle ear is rare and has poor prognosis.
- Distant metastasis, including cutaneous metastasis, results from primary middle ear melanoma.
A Trauma-Induced Fatty Mass: The Facts About Posttraumatic Pseudolipomas
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.
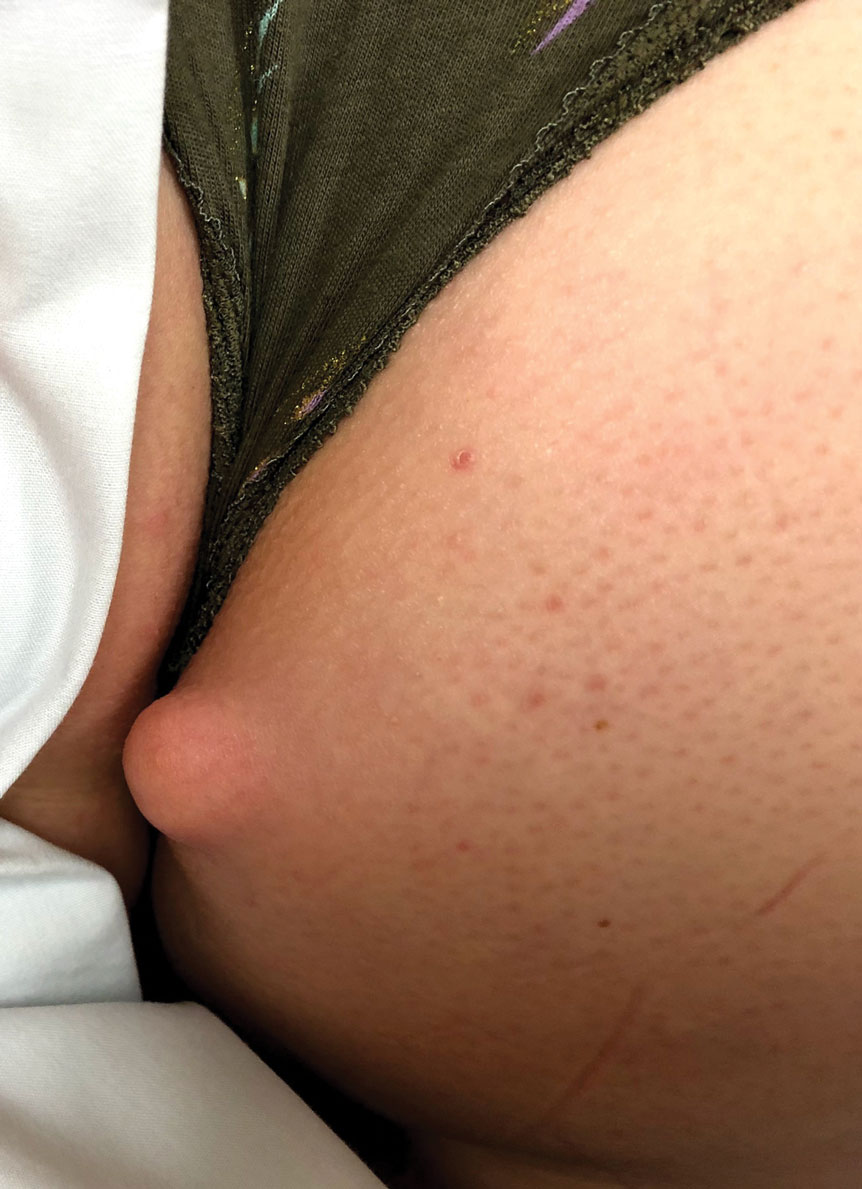
In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5
Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.

In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5

Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.

In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5

Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
Practice Points
- Physicians should include pseudolipoma in the differential diagnosis when evaluating masses that develop in patients at sites of blunt or prolonged trauma.
- A pseudolipoma is an unencapsulated, round, or fusiform fatty mass that differs from a traditional lipoma by the absence of a capsule.
- Further research may elucidate the pathogenesis of these adiposities.
Children and COVID: Weekly cases maintain a low-level plateau
A less-than-1% decrease in weekly COVID-19 cases in children demonstrated continued stability in the pandemic situation as the nation heads into the holiday season.
the American Academy of Pediatrics and the Children’s Hospital Association said in the latest edition of their joint COVID report.
New cases for the week of Nov. 11-17 totaled 27,899, down by 0.9% from the previous week and just 4 weeks removed from the lowest total of the year: 22,719 for Oct. 14-20. There have been just under 15 million cases of COVID-19 in children since the pandemic began, and children represent 18.3% of cases in all ages, the AAP and CHA reported.
Conditions look favorable for that plateau to continue, despite the upcoming holidays, White House COVID-19 coordinator Ashish Jha said recently. “We are in a very different place and we will remain in a different place,” Dr. Jha said, according to STAT News. “We are now at a point where I believe if you’re up to date on your vaccines, you have access to treatments ... there really should be no restrictions on people’s activities.”
One possible spoiler, an apparent spike in COVID-related hospitalizations in children we reported last week, seems to have been a false alarm. The rate of new admissions for Nov. 11, which preliminary data suggested was 0.48 per 100,000 population, has now been revised with more solid data to 0.20 per 100,000, according to the Centers for Disease Control and Prevention.
“We continue to monitor the recent increases in admissions among children. Some of these may be admissions with COVID-19, not because of COVID-19. Co-infections are being noted in our surveillance systems for hospitalizations among children; as much as 10% of admissions or higher have viruses codetected (RSV, influenza, enterovirus/rhinovirus, and other respiratory viruses),” a CDC spokesperson told this news organization.
For children aged 0-17 years, the current 7-day (Nov. 13-19) average number of new admissions with confirmed COVID is 129 per day, down from 147 for the previous 7-day average. Emergency department visits with diagnosed COVID, measured as a percentage of all ED visits, are largely holding steady. The latest 7-day averages available (Nov. 18) – 1.0% for children aged 0-11 years, 0.7% for 12- to 15-year-olds, and 0.8% in 16- to 17-year-olds – are the same or within a tenth of a percent of the rates recorded on Oct. 18, CDC data show.
New vaccinations for the week of Nov. 10-16 were down just slightly for children under age 5 years and for those aged 5-11 years, with a larger drop seen among 12- to 17-year-olds, the AAP said in its weekly vaccination report. So far, 7.9% of all children under age 5 have received at least one dose of COVID vaccine, as have 39.1% of 5 to 11-year-olds and 71.5% of those aged 12-17years, the CDC said on its COVID Data Tracker.
A less-than-1% decrease in weekly COVID-19 cases in children demonstrated continued stability in the pandemic situation as the nation heads into the holiday season.
the American Academy of Pediatrics and the Children’s Hospital Association said in the latest edition of their joint COVID report.
New cases for the week of Nov. 11-17 totaled 27,899, down by 0.9% from the previous week and just 4 weeks removed from the lowest total of the year: 22,719 for Oct. 14-20. There have been just under 15 million cases of COVID-19 in children since the pandemic began, and children represent 18.3% of cases in all ages, the AAP and CHA reported.
Conditions look favorable for that plateau to continue, despite the upcoming holidays, White House COVID-19 coordinator Ashish Jha said recently. “We are in a very different place and we will remain in a different place,” Dr. Jha said, according to STAT News. “We are now at a point where I believe if you’re up to date on your vaccines, you have access to treatments ... there really should be no restrictions on people’s activities.”
One possible spoiler, an apparent spike in COVID-related hospitalizations in children we reported last week, seems to have been a false alarm. The rate of new admissions for Nov. 11, which preliminary data suggested was 0.48 per 100,000 population, has now been revised with more solid data to 0.20 per 100,000, according to the Centers for Disease Control and Prevention.
“We continue to monitor the recent increases in admissions among children. Some of these may be admissions with COVID-19, not because of COVID-19. Co-infections are being noted in our surveillance systems for hospitalizations among children; as much as 10% of admissions or higher have viruses codetected (RSV, influenza, enterovirus/rhinovirus, and other respiratory viruses),” a CDC spokesperson told this news organization.
For children aged 0-17 years, the current 7-day (Nov. 13-19) average number of new admissions with confirmed COVID is 129 per day, down from 147 for the previous 7-day average. Emergency department visits with diagnosed COVID, measured as a percentage of all ED visits, are largely holding steady. The latest 7-day averages available (Nov. 18) – 1.0% for children aged 0-11 years, 0.7% for 12- to 15-year-olds, and 0.8% in 16- to 17-year-olds – are the same or within a tenth of a percent of the rates recorded on Oct. 18, CDC data show.
New vaccinations for the week of Nov. 10-16 were down just slightly for children under age 5 years and for those aged 5-11 years, with a larger drop seen among 12- to 17-year-olds, the AAP said in its weekly vaccination report. So far, 7.9% of all children under age 5 have received at least one dose of COVID vaccine, as have 39.1% of 5 to 11-year-olds and 71.5% of those aged 12-17years, the CDC said on its COVID Data Tracker.
A less-than-1% decrease in weekly COVID-19 cases in children demonstrated continued stability in the pandemic situation as the nation heads into the holiday season.
the American Academy of Pediatrics and the Children’s Hospital Association said in the latest edition of their joint COVID report.
New cases for the week of Nov. 11-17 totaled 27,899, down by 0.9% from the previous week and just 4 weeks removed from the lowest total of the year: 22,719 for Oct. 14-20. There have been just under 15 million cases of COVID-19 in children since the pandemic began, and children represent 18.3% of cases in all ages, the AAP and CHA reported.
Conditions look favorable for that plateau to continue, despite the upcoming holidays, White House COVID-19 coordinator Ashish Jha said recently. “We are in a very different place and we will remain in a different place,” Dr. Jha said, according to STAT News. “We are now at a point where I believe if you’re up to date on your vaccines, you have access to treatments ... there really should be no restrictions on people’s activities.”
One possible spoiler, an apparent spike in COVID-related hospitalizations in children we reported last week, seems to have been a false alarm. The rate of new admissions for Nov. 11, which preliminary data suggested was 0.48 per 100,000 population, has now been revised with more solid data to 0.20 per 100,000, according to the Centers for Disease Control and Prevention.
“We continue to monitor the recent increases in admissions among children. Some of these may be admissions with COVID-19, not because of COVID-19. Co-infections are being noted in our surveillance systems for hospitalizations among children; as much as 10% of admissions or higher have viruses codetected (RSV, influenza, enterovirus/rhinovirus, and other respiratory viruses),” a CDC spokesperson told this news organization.
For children aged 0-17 years, the current 7-day (Nov. 13-19) average number of new admissions with confirmed COVID is 129 per day, down from 147 for the previous 7-day average. Emergency department visits with diagnosed COVID, measured as a percentage of all ED visits, are largely holding steady. The latest 7-day averages available (Nov. 18) – 1.0% for children aged 0-11 years, 0.7% for 12- to 15-year-olds, and 0.8% in 16- to 17-year-olds – are the same or within a tenth of a percent of the rates recorded on Oct. 18, CDC data show.
New vaccinations for the week of Nov. 10-16 were down just slightly for children under age 5 years and for those aged 5-11 years, with a larger drop seen among 12- to 17-year-olds, the AAP said in its weekly vaccination report. So far, 7.9% of all children under age 5 have received at least one dose of COVID vaccine, as have 39.1% of 5 to 11-year-olds and 71.5% of those aged 12-17years, the CDC said on its COVID Data Tracker.
Local-level youth suicides reflect mental health care shortages
Rates of youth suicides at the county level increased as mental health professional shortages increased, based on data from more than 5,000 youth suicides across all counties in the United States.
Suicide remains the second leading cause of death among adolescents in the United States, and shortages of pediatric mental health providers are well known, but the association between mental health workforce shortages and youth suicides at the local level has not been well studied, Jennifer A. Hoffmann, MD, of Northwestern University, Chicago, and colleagues wrote.
Previous studies have shown few or no child psychiatrists or child-focused mental health professionals in most counties across the United States, and shortages are more likely in rural and high-poverty counties, the researchers noted.
In a cross-sectional study published in JAMA Pediatrics, the researchers reviewed all youth suicide data from January 2015 to Dec. 31, 2016 using the Centers for Disease Control and Prevention’s Compressed Mortality File. They used a multivariate binomial regression model to examine the association between youth suicide rates and the presence or absence of mental health care. Mental health care shortages were based on data from the U.S. Health Resources and Services Administration’s assessment of the number of mental health professionals relative to the country population and the availability of nearby services. Areas identified as having shortages were designated as Health Professional Shortage Areas (HPSAs) and scored on a severity level of 0-25, with higher scores indicating greater shortages. Approximately two-thirds (67.6%) of the 3,133 counties included in the study met criteria for mental health workforce shortage areas.
The researchers identified 5,034 suicides in youth aged 5-19 years during the study period, for an annual rate of 3.99 per 100,000 individuals. Of these, 72.8% were male and 68.2% were non-Hispanic White.
Overall, a county designation of mental health care shortage was significantly associated with an increased rate of youth suicide (adjusted incidence rate ratio, 1.16) and also increased rate of youth firearm suicide (aIRR, 1.27) after controlling for county and socioeconomic characteristics including the presence of a children’s mental health hospital, the percentage of children without health insurance, median household income, and racial makeup of the county.
The adjusted youth suicide rate increased by 4% for every 1-point increase in the HPSA score in counties with designated mental health workforce shortages.
The adjusted youth suicide rates were higher in counties with a lower median household income, and youth suicides increased with increases in the percentages of uninsured children, the researchers wrote.
“Reducing poverty, addressing social determinants of health, and improving insurance coverage may be considered as components of a multipronged societal strategy to improve child health and reduce youth suicides,” they said. “Efforts are needed to enhance the mental health professional workforce to match current levels of need.” Possible strategies to increase the pediatric mental health workforce may include improving reimbursement and integrating mental health care into primary care and schools by expanding telehealth services.
The study findings were limited by several factors including the potential misclassification of demographics or cause of death, the researchers noted. Other limitations included the inability to assess actual use of mental health services or firearm ownership in a household, and the possible differences between county-level associations and those of a city, neighborhood, or individual.
However, the results indicate that mental health professional workforce shortages were associated with increased youth suicide rates, and the data may inform local-level suicide prevention efforts, they concluded.
Data support the need for early intervention
“It was very important to conduct this study at this time because mental health problems, to include suicidal ideation, continue to increase in adolescents,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. “This study reinforces the immense import of sufficient mental health workforce to mitigate this increasing risk of suicide in adolescents.”
Dr. Loper said: “I believe that early intervention, or consistent access to mental health services, can go a very long way in preventing suicide in adolescents.
“I think the primary implications of this study are more relevant at the systems level, and reinforce the necessity of clinicians advocating for policies that address mental health workforce shortages in counties that are underserved,” he added.
However, “One primary barrier to increasing the number of mental health professionals at a local level, and specifically the number of child psychiatrists, is that demand is currently outpacing supply,” said Dr. Loper, a pediatrician and psychiatrist who was not involved in the study. “As the study authors cite, increasing telepsychiatry services and increasing mental health workforce specifically in the primary care setting may help offset these deficiencies,” he noted. Looking ahead, primary prevention of mental health problems by grassroots efforts is vital to stopping the trend in increased youth suicides and more mental health professionals are needed to mitigate the phenomenon of isolation and the degradation of community constructs.
As for additional research, Dr. Loper agreed with the study authors comments on the need for “more granular data” to better understand the correlation between mental health workforce and suicide in adolescents. “Data that captures city or neighborhood statistics related to mental health workforce and adolescent suicide could go a long way in our efforts to continue to better understand this very important correlation.”
The study was supported by an Academic Pediatric Association Young Investigator Award. Dr. Hoffmann disclosed research funding from the U.S. Agency for Healthcare Research and Quality unrelated to the current study. Dr. Loper had no financial conflicts to disclose.
Rates of youth suicides at the county level increased as mental health professional shortages increased, based on data from more than 5,000 youth suicides across all counties in the United States.
Suicide remains the second leading cause of death among adolescents in the United States, and shortages of pediatric mental health providers are well known, but the association between mental health workforce shortages and youth suicides at the local level has not been well studied, Jennifer A. Hoffmann, MD, of Northwestern University, Chicago, and colleagues wrote.
Previous studies have shown few or no child psychiatrists or child-focused mental health professionals in most counties across the United States, and shortages are more likely in rural and high-poverty counties, the researchers noted.
In a cross-sectional study published in JAMA Pediatrics, the researchers reviewed all youth suicide data from January 2015 to Dec. 31, 2016 using the Centers for Disease Control and Prevention’s Compressed Mortality File. They used a multivariate binomial regression model to examine the association between youth suicide rates and the presence or absence of mental health care. Mental health care shortages were based on data from the U.S. Health Resources and Services Administration’s assessment of the number of mental health professionals relative to the country population and the availability of nearby services. Areas identified as having shortages were designated as Health Professional Shortage Areas (HPSAs) and scored on a severity level of 0-25, with higher scores indicating greater shortages. Approximately two-thirds (67.6%) of the 3,133 counties included in the study met criteria for mental health workforce shortage areas.
The researchers identified 5,034 suicides in youth aged 5-19 years during the study period, for an annual rate of 3.99 per 100,000 individuals. Of these, 72.8% were male and 68.2% were non-Hispanic White.
Overall, a county designation of mental health care shortage was significantly associated with an increased rate of youth suicide (adjusted incidence rate ratio, 1.16) and also increased rate of youth firearm suicide (aIRR, 1.27) after controlling for county and socioeconomic characteristics including the presence of a children’s mental health hospital, the percentage of children without health insurance, median household income, and racial makeup of the county.
The adjusted youth suicide rate increased by 4% for every 1-point increase in the HPSA score in counties with designated mental health workforce shortages.
The adjusted youth suicide rates were higher in counties with a lower median household income, and youth suicides increased with increases in the percentages of uninsured children, the researchers wrote.
“Reducing poverty, addressing social determinants of health, and improving insurance coverage may be considered as components of a multipronged societal strategy to improve child health and reduce youth suicides,” they said. “Efforts are needed to enhance the mental health professional workforce to match current levels of need.” Possible strategies to increase the pediatric mental health workforce may include improving reimbursement and integrating mental health care into primary care and schools by expanding telehealth services.
The study findings were limited by several factors including the potential misclassification of demographics or cause of death, the researchers noted. Other limitations included the inability to assess actual use of mental health services or firearm ownership in a household, and the possible differences between county-level associations and those of a city, neighborhood, or individual.
However, the results indicate that mental health professional workforce shortages were associated with increased youth suicide rates, and the data may inform local-level suicide prevention efforts, they concluded.
Data support the need for early intervention
“It was very important to conduct this study at this time because mental health problems, to include suicidal ideation, continue to increase in adolescents,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. “This study reinforces the immense import of sufficient mental health workforce to mitigate this increasing risk of suicide in adolescents.”
Dr. Loper said: “I believe that early intervention, or consistent access to mental health services, can go a very long way in preventing suicide in adolescents.
“I think the primary implications of this study are more relevant at the systems level, and reinforce the necessity of clinicians advocating for policies that address mental health workforce shortages in counties that are underserved,” he added.
However, “One primary barrier to increasing the number of mental health professionals at a local level, and specifically the number of child psychiatrists, is that demand is currently outpacing supply,” said Dr. Loper, a pediatrician and psychiatrist who was not involved in the study. “As the study authors cite, increasing telepsychiatry services and increasing mental health workforce specifically in the primary care setting may help offset these deficiencies,” he noted. Looking ahead, primary prevention of mental health problems by grassroots efforts is vital to stopping the trend in increased youth suicides and more mental health professionals are needed to mitigate the phenomenon of isolation and the degradation of community constructs.
As for additional research, Dr. Loper agreed with the study authors comments on the need for “more granular data” to better understand the correlation between mental health workforce and suicide in adolescents. “Data that captures city or neighborhood statistics related to mental health workforce and adolescent suicide could go a long way in our efforts to continue to better understand this very important correlation.”
The study was supported by an Academic Pediatric Association Young Investigator Award. Dr. Hoffmann disclosed research funding from the U.S. Agency for Healthcare Research and Quality unrelated to the current study. Dr. Loper had no financial conflicts to disclose.
Rates of youth suicides at the county level increased as mental health professional shortages increased, based on data from more than 5,000 youth suicides across all counties in the United States.
Suicide remains the second leading cause of death among adolescents in the United States, and shortages of pediatric mental health providers are well known, but the association between mental health workforce shortages and youth suicides at the local level has not been well studied, Jennifer A. Hoffmann, MD, of Northwestern University, Chicago, and colleagues wrote.
Previous studies have shown few or no child psychiatrists or child-focused mental health professionals in most counties across the United States, and shortages are more likely in rural and high-poverty counties, the researchers noted.
In a cross-sectional study published in JAMA Pediatrics, the researchers reviewed all youth suicide data from January 2015 to Dec. 31, 2016 using the Centers for Disease Control and Prevention’s Compressed Mortality File. They used a multivariate binomial regression model to examine the association between youth suicide rates and the presence or absence of mental health care. Mental health care shortages were based on data from the U.S. Health Resources and Services Administration’s assessment of the number of mental health professionals relative to the country population and the availability of nearby services. Areas identified as having shortages were designated as Health Professional Shortage Areas (HPSAs) and scored on a severity level of 0-25, with higher scores indicating greater shortages. Approximately two-thirds (67.6%) of the 3,133 counties included in the study met criteria for mental health workforce shortage areas.
The researchers identified 5,034 suicides in youth aged 5-19 years during the study period, for an annual rate of 3.99 per 100,000 individuals. Of these, 72.8% were male and 68.2% were non-Hispanic White.
Overall, a county designation of mental health care shortage was significantly associated with an increased rate of youth suicide (adjusted incidence rate ratio, 1.16) and also increased rate of youth firearm suicide (aIRR, 1.27) after controlling for county and socioeconomic characteristics including the presence of a children’s mental health hospital, the percentage of children without health insurance, median household income, and racial makeup of the county.
The adjusted youth suicide rate increased by 4% for every 1-point increase in the HPSA score in counties with designated mental health workforce shortages.
The adjusted youth suicide rates were higher in counties with a lower median household income, and youth suicides increased with increases in the percentages of uninsured children, the researchers wrote.
“Reducing poverty, addressing social determinants of health, and improving insurance coverage may be considered as components of a multipronged societal strategy to improve child health and reduce youth suicides,” they said. “Efforts are needed to enhance the mental health professional workforce to match current levels of need.” Possible strategies to increase the pediatric mental health workforce may include improving reimbursement and integrating mental health care into primary care and schools by expanding telehealth services.
The study findings were limited by several factors including the potential misclassification of demographics or cause of death, the researchers noted. Other limitations included the inability to assess actual use of mental health services or firearm ownership in a household, and the possible differences between county-level associations and those of a city, neighborhood, or individual.
However, the results indicate that mental health professional workforce shortages were associated with increased youth suicide rates, and the data may inform local-level suicide prevention efforts, they concluded.
Data support the need for early intervention
“It was very important to conduct this study at this time because mental health problems, to include suicidal ideation, continue to increase in adolescents,” Peter L. Loper Jr., MD, of the University of South Carolina, Columbia, said in an interview. “This study reinforces the immense import of sufficient mental health workforce to mitigate this increasing risk of suicide in adolescents.”
Dr. Loper said: “I believe that early intervention, or consistent access to mental health services, can go a very long way in preventing suicide in adolescents.
“I think the primary implications of this study are more relevant at the systems level, and reinforce the necessity of clinicians advocating for policies that address mental health workforce shortages in counties that are underserved,” he added.
However, “One primary barrier to increasing the number of mental health professionals at a local level, and specifically the number of child psychiatrists, is that demand is currently outpacing supply,” said Dr. Loper, a pediatrician and psychiatrist who was not involved in the study. “As the study authors cite, increasing telepsychiatry services and increasing mental health workforce specifically in the primary care setting may help offset these deficiencies,” he noted. Looking ahead, primary prevention of mental health problems by grassroots efforts is vital to stopping the trend in increased youth suicides and more mental health professionals are needed to mitigate the phenomenon of isolation and the degradation of community constructs.
As for additional research, Dr. Loper agreed with the study authors comments on the need for “more granular data” to better understand the correlation between mental health workforce and suicide in adolescents. “Data that captures city or neighborhood statistics related to mental health workforce and adolescent suicide could go a long way in our efforts to continue to better understand this very important correlation.”
The study was supported by an Academic Pediatric Association Young Investigator Award. Dr. Hoffmann disclosed research funding from the U.S. Agency for Healthcare Research and Quality unrelated to the current study. Dr. Loper had no financial conflicts to disclose.
FROM JAMA PEDIATRICS