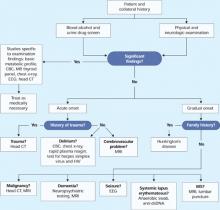
User login
A young man’s affair of the heart
Case: You’re a ‘freak’
A local mental health agency refers Mr. Z, age 23, to our inpatient psychiatry service because of increasing suicidality and psychosis. He began receiving care from the mental health agency 3 years ago, after a psychiatrist diagnosed paranoid schizophrenia.
At presentation, Mr. Z is delusionally preoccupied with a brief relationship he had with a young woman at college 2 years ago. He feels embarrassed about his conduct toward her during a psychotic episode and her subsequent response. He believes strangers are ridiculing him, and he hears voices calling him a “freak” and making crude references to the encounter. He is also contemplating suicide and endorses a suicide plan.
Mr. Z was hospitalized for 1 month last year with schizophrenia symptoms. He is medically healthy and does not abuse alcohol or drugs.
We admit Mr. Z because of his suicidality. Four weeks later, he remains suicidal and hears voices telling him to “rape” and “kill.” Successive 2-week trials of risperidone, 1 mg/d titrated to 5 mg/d, and quetiapine, 200 mg/d titrated to 700 mg/d, cause intolerable akathisia. We try adding propranolol, 20 mg every 8 hours, to alleviate akathisia, but to no avail. Previous trials of olanzapine, 30 mg/d, and haloperidol, dosage unknown, were unsuccessful or caused akathisia.
The authors’ observations
Substantial evidence supports clozapine’s efficacy in treatment-resistant schizophrenia, and this second-generation antipsychotic (SGA) also might reduce suicidality.1,2 Clinicians often combine antipsychotics, switch to an antidepressant, or add a mood stabilizer for treatment-resistant schizophrenia,3 but little evidence supports these options.
Mr. Z had failed at least 4 antipsychotic trials. We consider clozapine for patients with severe psychosis who have failed 2 or 3 antipsychotic trials or cannot tolerate these medications. Severity of psychosis and presence of suicidality warrant use of clozapine in treatment-resistant cases.
If Mr. Z had tolerated risperidone or quetiapine, we would have waited as long as 8 weeks before switching to clozapine. In inpatients, improvement should be seen 2 to 4 weeks after starting an antipsychotic.
Perform blood tests weekly during the first 6 months of clozapine therapy and bi-weekly thereafter to check for abnormally low white blood cell counts that might suggest agranulocytosis.
Myocarditis is a potentially fatal inflammation of the myocardium that can result from a viral infection, toxins, medications, or hypersensitive immune reactions.
Data on myocarditis prevalence are scarce because no relatively noninvasive assessment tools exist. Among 2,200 patients with unexplained heart failure occurring over 5
An FDA-mandated “black box” in clozapine’s package insert describes an “increased risk of fatal myocarditis, especially during—but not limited to—the first month of therapy.”6 Proposed explanations of how clozapine causes myocarditis include:
- direct toxic effect on cardiac myocytes related to impaired clozapine metabolism in some patients7,8
- myocardial damage mediated by clozapine blockade of a muscarinic M2 receptor subtype9
- selenium deficiency or presence of reactive clozapine nitrenium metabolites contributing to myocardial toxicity.10,11
The common presence of peripheral eosinophilia on autopsy—including diffuse eosinophilic infiltrates in myocardial and perivascular areas—might suggest a hypereosinophilic syndrome or a type II hypersensitive immune reaction mediated by clozapine.7,12 Similar immune-mediated conditions of acute, progressive myocarditis have been noted after exposure to other medications such as penicillin or sulfonamides.13
Noting that clozapine increases inflammatory cytokines, some authors believe TNF-alpha and other inflammatory cytokines contribute to myocarditis.14
TREATMENT: New regimen
After discussing clozapine’s risks and benefits with Mr. Z and his parents, we start the medication at 25 mg/d to gauge tolerability, then titrate to 300 mg/d over 10 days. Mr. Z tolerates clozapine well, with some sedation and sialorrhea. A blood test taken 7 days after we start clozapine shows a normal white blood cell count.
After 10 days on clozapine, Mr. Z’s delusions and hallucinations are considerably less intense. He is no longer suicidal and visits his former college with his parents without thinking about his past acquaintance. We discharge him on clozapine, 300 mg/d, and refer him to the local mental health agency.
Two days later, Mr. Z’s parents report that since discharge their son has had extreme fatigue, shortness of breath, leg edema, and chest pain. We advise them to immediately take their son to the ER for cardiac workup.
The authors’ observations
Mr. Z’s sudden-onset physical symptoms suggest myocarditis, a rare but potentially fatal side effect of clozapine whose specific cause is unclear (Box 1).5-14 Myocarditis has been reported in 0.02% to 0.18% of patients exposed to clozapine,15-18 with incidence as high as 1.3% per 235 patients.19
Affected patients typically have been taking clozapine at therapeutic dosages (100 to 450 mg/d).7 Clozapine use is most prevalent among men ages 20 to 40, who tend to have more severe schizophrenia and lower cardiac risk than other populations. Correspondingly, clozapine-induced myocarditis is most prevalent in younger men,20 although what specifically causes this susceptibility is unknown.
Nonspecific symptoms such as dyspnea, tachycardia, chest pain, or fever can signal myocarditis (Table)7,21 and can surface within 4 to 8 weeks of starting clozapine.22 Haas et al20 reported other symptoms—such as leukocytosis—in young (median age 30), predominantly male patients with clozapine-induced myocarditis. Symptoms that typically occur during clozapine titration—such as fever and tachycardia—can mask “subclinical” myocarditis.22
Mr. Z’s nonspecific symptoms could signal clozapine-induced agranulocytosis or a viral syndrome, or could be delusional. The patient’s acute, sudden symptom onset strongly suggests a cardiac cause. Also, his delusions subsided, and normal blood readings helped us rule out agranulocytosis.
Coulter et al23 associated myocarditis and cardiomyopathy, a noninflammatory heart muscle disease, with several antipsychotics—including clozapine, chlorpromazine, fluphenazine, haloperidol, and risperidone—as well as lithium. More research is needed to confirm this association.
Order a cardiology consult and workup including:
- serum electrolytes
- complete blood count
- ECG21
- tests for myocardial damage including creatine kinase with MB fractionation (CK-MB) and testing for serum troponin I,25 lactic dehydrogenase, and aspartate transaminase (SGOT)21
- assessment for immune activation and peripheral eosinophilia.25
Table
Symptoms that could signal myocarditis in patients taking clozapine
|
| Source: Reference 7 |
TESTING: ‘Is this necessary?’
We contact the ER physician to request the above-mentioned tests, but he questions the need for such extensive and costly testing in a psychiatric patient with nonspecific symptoms.
After several phone conversations to review our recommendations, the emergency physician suggests sending Mr. Z home on a watch-and-wait protocol. We politely but firmly emphasize that Mr. Z needs a full cardiac workup, after which the physician consents to the tests (Box 2).
FINDINGS: suspicious readings
Mr. Z’s cardiac imaging results suggest a cardiopathy:
- echocardiogram shows mild ventricular enlargement with a decreased ejection fraction of 45% (normal reading, 55% to 60%)
- ECG shows normal sinus rhythm with low-voltage diffuse T-wave flattening throughout all leads without ST elevation
- creatine phosphokinase (CPK) and CKMB are within normal ranges
- troponin I is 0.33 ng/mL, a high-normal reading.
Based on these readings, the cardiology service admits Mr. Z with a presumptive diagnosis of clozapine-induced cardiomyopathy. The attending cardiologist stops clozapine and starts the angiotensin-converting enzyme inhibitor enalapril, 2.5 mg bid, for ventricular remodeling. Medical workup includes cytologic testing to rule out immunologic or viral disease.
Five days later, Mr. Z’s cardiac symptoms have resolved. The cardiology unit discharges him on enalapril, 2.5 mg bid, and schedules a cardiac ultrasound for 2 weeks after discharge to confirm progress.
The authors’ observations
Maintain high clinical suspicion while using clozapine. Similar to other patients with a clozapine-induced cardiopathy,16 Mr. Z showed rapid symptomatic changes after a benign initial course and experienced fairly vague symptoms that raised limited clinical concern at first.
Before starting clozapine therapy, screen all patients for pre-existing cardiac disease, which contraindicates this medication. Alert patients and caregivers to the risks and symptoms that require close monitoring early in treatment.
Many researchers suggest monitoring for myocarditis during the first month of therapy and ordering ECG at baseline and 2 and 4 weeks after starting clozapine.21,22 Berk et al26 suggest more aggressive monitoring, including:
- baseline ECG
- transthoracic echocardiogram
- baseline troponin/CK-MB
- ECG and troponin/CK-MB at 7 and 14 days
- echocardiogram at 6 and 12 months and then annually.
RELAPSE: Return of the ‘freak’
Immediately after Mr. Z’s discharge from the cardiology unit, we readmit him to inpatient psychiatry. His parents and case manager say he is again becoming preoccupied with his brief college relationship. He has been off clozapine for 5 days.
The authors’ observations
The American Psychiatric Association27 (see http://www.psych.org/psych_pract/treatg/pg/SchizPG-Complete-Feb04.pdf) recommends maximizing 1 medication for at least 2 to 4 weeks to assess schizophrenia symptom response and urges clinicians to consider adverse effects, medical comorbidities, and patient preference before continuing the medication.
These recommendations highlight the challenges of treating medication-resistant schizophrenia. Relapse is common after a serious reaction to clozapine, and combining 2 or more other antipsychotics could lead to significantly greater side effects. A time-limited trial with an antipsychotic and an adjunctive agent might be attempted while carefully weighing the combination’s risks and benefits.27
Clozapine reduced Mr. Z’s psychosis, but rechallenge would likely cause his potentially fatal cardiomyopathy to re-emerge. His sensitivity to adverse antipsychotic effects discourages polypharmacy and further complicates our decision.
How to convince other specialists
Many physicians are reluctant to pursue additional tests or procedures—and risk a confrontation with a consultant, insurer, or ER physician—especially when the risk of abnormality is extremely low. Advocating for cardiac workup in patients with vague symptoms is challenging, particularly if the suspected side effect is rare.
Taking the path of least resistance can increase the risk of a serious—albeit rare—adverse event. Failure to test could prolong a potentially harmful treatment, and the test results—even if negative—could be critical to planning care.
Calmly but firmly spell out the risks of missing a suspected cardiac problem (death, proceeding with potentially harmful treatment). Tell the ER manager or consultant, “I realize this is a very rare side effect, but not catching it could be life-threatening.”
Be circumspect when pleading your case—an overaggressive approach might cause the ER doctor to “dig in his heels” and reject your request. Use a medically focused response such as, “This is a known complication of this medicine with this common time course and presentation.”
TREATMENT: Another trial
We start olanzapine, 5 mg/d, and titrate to 20 mg/d over 1 week. We add sustained-release bupropion, 200 mg bid, for associated dysphoria.
Mr. Z’s symptoms and paranoia gradually decline, and he tolerates off-unit passes with friends and family before discharge. Staff works closely with him to develop cognitive-behavioral strategies to manage residual paranoia and hallucinations, such as assessing evidence for his delusional beliefs and developing tools to distract him from remaining “voices.” He reports no cardiac symptoms and continues taking enalapril, 2.5 mg bid.
We discharge Mr. Z after 1 week, at which point he shows no suicidal or homicidal thoughts. Follow-up echocardiogram 2 weeks later shows ejection fraction has improved to 60%, suggesting absence of cardiomyopathy.
Related resource
- Clozapine safety information.
www.clozaril.com/.
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Enalapril • Vasotec
- Fluphenazine • Prolixin, Permitil
- Haloperidol • Haldol
- Lithium • Eskalith, others
- Olanzapine • Zyprexa
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988;45:789-96.
2. Meltzer HY, Alphs L, Green AI, et al. International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003;60:82-91.
3. Stahl SM. Antipsychotic polypharmacy, part 1: therapeutic option or dirty little secret? J Clin Psychiatry 1999;60:425-6.
4. Clozaril monograph. Novartis Phamaceuticals Corp.; April 12, 2006. Available at http://www.novartis.ca/downloads/en/products/clozaril_scrip_e.pdf. Accessed August 13, 2007.
5. Mason JW, O’Connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med 1995;333:269.-
6. Physicians’ desk reference. 61st ed. Montvale, NJ: Thomson PDR; 2007.
7. Merrill DB, Dec GW, Goff DC. Adverse cardiac effects associated with clozapine. J Clin Psychopharmacol 2005;25:32-41.
8. Jenie LE. Cardiovascular toxicity with clozapine therapy. Riverview Hospital Pharmacy Newsletter 2002;22:1-3.
9. Devarajan S, Kutcher SP, Dursun SM. Clozapine and sudden death. Lancet 2000;355:841.-
10. Vaddadi KS, Soosai E, Vaddadi G. Low blood selenium concentrations in schizophrenic patients on clozapine. Br J Clin Pharmacol 2003;55:307-9.
11. Williams DP, O’Donnell CJ, Maggs JL, et al. Bioactivation of clozapine by murine cardiac tissue in vivo and in vitro. Chem Res Toxicol 2003;16:1359-64.
12. Fineschi V, Neri M, Riezzo I, Turillazzi E. Sudden cardiac death due to hypersensitivity myocarditis during clozapine treatment. Int J Legal Med 2004;118:307-9.
13. Kendell KR, Day JD, Hruban RH, et al. Intimate association of eosinophils to collagen bundles in eosinophilic myocarditis and ranitidine induced hypersensitivity myocarditis. Arch Pathol Lab Med 1995;119:1154-60.
14. Pollmacher T, Schuld A, Kraus T, et al. On the clinical relevance of clozapine-triggered release of cytokines and soluble cytokine-receptors [in German]. Fortschr Neurol Psychiatr 2001;69(suppl 2):S65-S74.
15. Killian JG, Kerr K, Lawrence C, Celermajer DS. Myocarditis and cardiomyopathy associated with clozapine. Lancet 1999;354:1841-5.
16. Committee on Safety of Medicines Myocarditis with antipsychotics: recent cases with clozapine (Clozaril). Curr Probl Pharmacovigilance 1993;19:9.-
17. Degner D, Bleich S, Grohmann R, et al. Myocarditis associated with clozapine treatment. Aust NZ J Psychiatry 2000;34:880.-
18. La Grenade L, Graham D, Trontell A. Myocarditis and cardiomyopathy associated with clozapine use in the United States (letter). N Engl J Med 2001;345:224-5.
19. Reinders J, Parsonage W, Lange D, et al. Clozapinerelated myocarditis and cardiomyopathy in an Australian metropolitan psychiatric service. Aust NZ J Psychiatry 2004;38:915-22.
20. Haas SJ, Hill R, Krum H, et al. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993-2003. Drug Saf 2007;30:47-57.
21. Wehmeier PM, Heiser P, Remschmidt H. Myocarditis, pericarditis and cardiomyopathy in patients treated with clozapine. J Clin Pharm Ther 2005;30:91-6.
22. Merrill DB, Ahmari SE, Bradford JM, Lieberman JA. Myocarditis during clozapine treatment. Am J Psychiatry 2006;163:204-8. Erratum in Am J Psychiatry 2006;163:556.-
23. Coulter DM, Bate A, Meyboom RH, et al. Antipsychotic drugs and heart muscle disorder in international pharmacovigilance: data mining study. BMJ 2001;322:1207-9.
24. Wooltorton E. Antipsychotic clozapine (Clozaril): myocarditis and cardiovascular toxicity. CMAJ 2002;166:1185-6.
25. Kay SE, Doery J, Sholl D. Clozapine associated pericarditis and elevated troponin I. Aust NZ J Psychiatry 2002;36:143-4.
26. Berk M, Fitzsimons J, Lambert T, et al. Monitoring the safe use of clozapine: a consensus view from Victoria, Australia. CNS Drugs 2007;21:117-27.
27. American Psychiatric Association Work Group on Schizophrenia, Lehman AF, chair. Practice guideline for the treatment of patients with schizophrenia, 2nd ed, 2004. Available at: http://www.psych.org/psych_pract/treatg/pg/SchizPG-Complete-Feb04.pdf. Accessed August 15, 2007.
Case: You’re a ‘freak’
A local mental health agency refers Mr. Z, age 23, to our inpatient psychiatry service because of increasing suicidality and psychosis. He began receiving care from the mental health agency 3 years ago, after a psychiatrist diagnosed paranoid schizophrenia.
At presentation, Mr. Z is delusionally preoccupied with a brief relationship he had with a young woman at college 2 years ago. He feels embarrassed about his conduct toward her during a psychotic episode and her subsequent response. He believes strangers are ridiculing him, and he hears voices calling him a “freak” and making crude references to the encounter. He is also contemplating suicide and endorses a suicide plan.
Mr. Z was hospitalized for 1 month last year with schizophrenia symptoms. He is medically healthy and does not abuse alcohol or drugs.
We admit Mr. Z because of his suicidality. Four weeks later, he remains suicidal and hears voices telling him to “rape” and “kill.” Successive 2-week trials of risperidone, 1 mg/d titrated to 5 mg/d, and quetiapine, 200 mg/d titrated to 700 mg/d, cause intolerable akathisia. We try adding propranolol, 20 mg every 8 hours, to alleviate akathisia, but to no avail. Previous trials of olanzapine, 30 mg/d, and haloperidol, dosage unknown, were unsuccessful or caused akathisia.
The authors’ observations
Substantial evidence supports clozapine’s efficacy in treatment-resistant schizophrenia, and this second-generation antipsychotic (SGA) also might reduce suicidality.1,2 Clinicians often combine antipsychotics, switch to an antidepressant, or add a mood stabilizer for treatment-resistant schizophrenia,3 but little evidence supports these options.
Mr. Z had failed at least 4 antipsychotic trials. We consider clozapine for patients with severe psychosis who have failed 2 or 3 antipsychotic trials or cannot tolerate these medications. Severity of psychosis and presence of suicidality warrant use of clozapine in treatment-resistant cases.
If Mr. Z had tolerated risperidone or quetiapine, we would have waited as long as 8 weeks before switching to clozapine. In inpatients, improvement should be seen 2 to 4 weeks after starting an antipsychotic.
Perform blood tests weekly during the first 6 months of clozapine therapy and bi-weekly thereafter to check for abnormally low white blood cell counts that might suggest agranulocytosis.
Myocarditis is a potentially fatal inflammation of the myocardium that can result from a viral infection, toxins, medications, or hypersensitive immune reactions.
Data on myocarditis prevalence are scarce because no relatively noninvasive assessment tools exist. Among 2,200 patients with unexplained heart failure occurring over 5
An FDA-mandated “black box” in clozapine’s package insert describes an “increased risk of fatal myocarditis, especially during—but not limited to—the first month of therapy.”6 Proposed explanations of how clozapine causes myocarditis include:
- direct toxic effect on cardiac myocytes related to impaired clozapine metabolism in some patients7,8
- myocardial damage mediated by clozapine blockade of a muscarinic M2 receptor subtype9
- selenium deficiency or presence of reactive clozapine nitrenium metabolites contributing to myocardial toxicity.10,11
The common presence of peripheral eosinophilia on autopsy—including diffuse eosinophilic infiltrates in myocardial and perivascular areas—might suggest a hypereosinophilic syndrome or a type II hypersensitive immune reaction mediated by clozapine.7,12 Similar immune-mediated conditions of acute, progressive myocarditis have been noted after exposure to other medications such as penicillin or sulfonamides.13
Noting that clozapine increases inflammatory cytokines, some authors believe TNF-alpha and other inflammatory cytokines contribute to myocarditis.14
TREATMENT: New regimen
After discussing clozapine’s risks and benefits with Mr. Z and his parents, we start the medication at 25 mg/d to gauge tolerability, then titrate to 300 mg/d over 10 days. Mr. Z tolerates clozapine well, with some sedation and sialorrhea. A blood test taken 7 days after we start clozapine shows a normal white blood cell count.
After 10 days on clozapine, Mr. Z’s delusions and hallucinations are considerably less intense. He is no longer suicidal and visits his former college with his parents without thinking about his past acquaintance. We discharge him on clozapine, 300 mg/d, and refer him to the local mental health agency.
Two days later, Mr. Z’s parents report that since discharge their son has had extreme fatigue, shortness of breath, leg edema, and chest pain. We advise them to immediately take their son to the ER for cardiac workup.
The authors’ observations
Mr. Z’s sudden-onset physical symptoms suggest myocarditis, a rare but potentially fatal side effect of clozapine whose specific cause is unclear (Box 1).5-14 Myocarditis has been reported in 0.02% to 0.18% of patients exposed to clozapine,15-18 with incidence as high as 1.3% per 235 patients.19
Affected patients typically have been taking clozapine at therapeutic dosages (100 to 450 mg/d).7 Clozapine use is most prevalent among men ages 20 to 40, who tend to have more severe schizophrenia and lower cardiac risk than other populations. Correspondingly, clozapine-induced myocarditis is most prevalent in younger men,20 although what specifically causes this susceptibility is unknown.
Nonspecific symptoms such as dyspnea, tachycardia, chest pain, or fever can signal myocarditis (Table)7,21 and can surface within 4 to 8 weeks of starting clozapine.22 Haas et al20 reported other symptoms—such as leukocytosis—in young (median age 30), predominantly male patients with clozapine-induced myocarditis. Symptoms that typically occur during clozapine titration—such as fever and tachycardia—can mask “subclinical” myocarditis.22
Mr. Z’s nonspecific symptoms could signal clozapine-induced agranulocytosis or a viral syndrome, or could be delusional. The patient’s acute, sudden symptom onset strongly suggests a cardiac cause. Also, his delusions subsided, and normal blood readings helped us rule out agranulocytosis.
Coulter et al23 associated myocarditis and cardiomyopathy, a noninflammatory heart muscle disease, with several antipsychotics—including clozapine, chlorpromazine, fluphenazine, haloperidol, and risperidone—as well as lithium. More research is needed to confirm this association.
Order a cardiology consult and workup including:
- serum electrolytes
- complete blood count
- ECG21
- tests for myocardial damage including creatine kinase with MB fractionation (CK-MB) and testing for serum troponin I,25 lactic dehydrogenase, and aspartate transaminase (SGOT)21
- assessment for immune activation and peripheral eosinophilia.25
Table
Symptoms that could signal myocarditis in patients taking clozapine
|
| Source: Reference 7 |
TESTING: ‘Is this necessary?’
We contact the ER physician to request the above-mentioned tests, but he questions the need for such extensive and costly testing in a psychiatric patient with nonspecific symptoms.
After several phone conversations to review our recommendations, the emergency physician suggests sending Mr. Z home on a watch-and-wait protocol. We politely but firmly emphasize that Mr. Z needs a full cardiac workup, after which the physician consents to the tests (Box 2).
FINDINGS: suspicious readings
Mr. Z’s cardiac imaging results suggest a cardiopathy:
- echocardiogram shows mild ventricular enlargement with a decreased ejection fraction of 45% (normal reading, 55% to 60%)
- ECG shows normal sinus rhythm with low-voltage diffuse T-wave flattening throughout all leads without ST elevation
- creatine phosphokinase (CPK) and CKMB are within normal ranges
- troponin I is 0.33 ng/mL, a high-normal reading.
Based on these readings, the cardiology service admits Mr. Z with a presumptive diagnosis of clozapine-induced cardiomyopathy. The attending cardiologist stops clozapine and starts the angiotensin-converting enzyme inhibitor enalapril, 2.5 mg bid, for ventricular remodeling. Medical workup includes cytologic testing to rule out immunologic or viral disease.
Five days later, Mr. Z’s cardiac symptoms have resolved. The cardiology unit discharges him on enalapril, 2.5 mg bid, and schedules a cardiac ultrasound for 2 weeks after discharge to confirm progress.
The authors’ observations
Maintain high clinical suspicion while using clozapine. Similar to other patients with a clozapine-induced cardiopathy,16 Mr. Z showed rapid symptomatic changes after a benign initial course and experienced fairly vague symptoms that raised limited clinical concern at first.
Before starting clozapine therapy, screen all patients for pre-existing cardiac disease, which contraindicates this medication. Alert patients and caregivers to the risks and symptoms that require close monitoring early in treatment.
Many researchers suggest monitoring for myocarditis during the first month of therapy and ordering ECG at baseline and 2 and 4 weeks after starting clozapine.21,22 Berk et al26 suggest more aggressive monitoring, including:
- baseline ECG
- transthoracic echocardiogram
- baseline troponin/CK-MB
- ECG and troponin/CK-MB at 7 and 14 days
- echocardiogram at 6 and 12 months and then annually.
RELAPSE: Return of the ‘freak’
Immediately after Mr. Z’s discharge from the cardiology unit, we readmit him to inpatient psychiatry. His parents and case manager say he is again becoming preoccupied with his brief college relationship. He has been off clozapine for 5 days.
The authors’ observations
The American Psychiatric Association27 (see http://www.psych.org/psych_pract/treatg/pg/SchizPG-Complete-Feb04.pdf) recommends maximizing 1 medication for at least 2 to 4 weeks to assess schizophrenia symptom response and urges clinicians to consider adverse effects, medical comorbidities, and patient preference before continuing the medication.
These recommendations highlight the challenges of treating medication-resistant schizophrenia. Relapse is common after a serious reaction to clozapine, and combining 2 or more other antipsychotics could lead to significantly greater side effects. A time-limited trial with an antipsychotic and an adjunctive agent might be attempted while carefully weighing the combination’s risks and benefits.27
Clozapine reduced Mr. Z’s psychosis, but rechallenge would likely cause his potentially fatal cardiomyopathy to re-emerge. His sensitivity to adverse antipsychotic effects discourages polypharmacy and further complicates our decision.
How to convince other specialists
Many physicians are reluctant to pursue additional tests or procedures—and risk a confrontation with a consultant, insurer, or ER physician—especially when the risk of abnormality is extremely low. Advocating for cardiac workup in patients with vague symptoms is challenging, particularly if the suspected side effect is rare.
Taking the path of least resistance can increase the risk of a serious—albeit rare—adverse event. Failure to test could prolong a potentially harmful treatment, and the test results—even if negative—could be critical to planning care.
Calmly but firmly spell out the risks of missing a suspected cardiac problem (death, proceeding with potentially harmful treatment). Tell the ER manager or consultant, “I realize this is a very rare side effect, but not catching it could be life-threatening.”
Be circumspect when pleading your case—an overaggressive approach might cause the ER doctor to “dig in his heels” and reject your request. Use a medically focused response such as, “This is a known complication of this medicine with this common time course and presentation.”
TREATMENT: Another trial
We start olanzapine, 5 mg/d, and titrate to 20 mg/d over 1 week. We add sustained-release bupropion, 200 mg bid, for associated dysphoria.
Mr. Z’s symptoms and paranoia gradually decline, and he tolerates off-unit passes with friends and family before discharge. Staff works closely with him to develop cognitive-behavioral strategies to manage residual paranoia and hallucinations, such as assessing evidence for his delusional beliefs and developing tools to distract him from remaining “voices.” He reports no cardiac symptoms and continues taking enalapril, 2.5 mg bid.
We discharge Mr. Z after 1 week, at which point he shows no suicidal or homicidal thoughts. Follow-up echocardiogram 2 weeks later shows ejection fraction has improved to 60%, suggesting absence of cardiomyopathy.
Related resource
- Clozapine safety information.
www.clozaril.com/.
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Enalapril • Vasotec
- Fluphenazine • Prolixin, Permitil
- Haloperidol • Haldol
- Lithium • Eskalith, others
- Olanzapine • Zyprexa
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Case: You’re a ‘freak’
A local mental health agency refers Mr. Z, age 23, to our inpatient psychiatry service because of increasing suicidality and psychosis. He began receiving care from the mental health agency 3 years ago, after a psychiatrist diagnosed paranoid schizophrenia.
At presentation, Mr. Z is delusionally preoccupied with a brief relationship he had with a young woman at college 2 years ago. He feels embarrassed about his conduct toward her during a psychotic episode and her subsequent response. He believes strangers are ridiculing him, and he hears voices calling him a “freak” and making crude references to the encounter. He is also contemplating suicide and endorses a suicide plan.
Mr. Z was hospitalized for 1 month last year with schizophrenia symptoms. He is medically healthy and does not abuse alcohol or drugs.
We admit Mr. Z because of his suicidality. Four weeks later, he remains suicidal and hears voices telling him to “rape” and “kill.” Successive 2-week trials of risperidone, 1 mg/d titrated to 5 mg/d, and quetiapine, 200 mg/d titrated to 700 mg/d, cause intolerable akathisia. We try adding propranolol, 20 mg every 8 hours, to alleviate akathisia, but to no avail. Previous trials of olanzapine, 30 mg/d, and haloperidol, dosage unknown, were unsuccessful or caused akathisia.
The authors’ observations
Substantial evidence supports clozapine’s efficacy in treatment-resistant schizophrenia, and this second-generation antipsychotic (SGA) also might reduce suicidality.1,2 Clinicians often combine antipsychotics, switch to an antidepressant, or add a mood stabilizer for treatment-resistant schizophrenia,3 but little evidence supports these options.
Mr. Z had failed at least 4 antipsychotic trials. We consider clozapine for patients with severe psychosis who have failed 2 or 3 antipsychotic trials or cannot tolerate these medications. Severity of psychosis and presence of suicidality warrant use of clozapine in treatment-resistant cases.
If Mr. Z had tolerated risperidone or quetiapine, we would have waited as long as 8 weeks before switching to clozapine. In inpatients, improvement should be seen 2 to 4 weeks after starting an antipsychotic.
Perform blood tests weekly during the first 6 months of clozapine therapy and bi-weekly thereafter to check for abnormally low white blood cell counts that might suggest agranulocytosis.
Myocarditis is a potentially fatal inflammation of the myocardium that can result from a viral infection, toxins, medications, or hypersensitive immune reactions.
Data on myocarditis prevalence are scarce because no relatively noninvasive assessment tools exist. Among 2,200 patients with unexplained heart failure occurring over 5
An FDA-mandated “black box” in clozapine’s package insert describes an “increased risk of fatal myocarditis, especially during—but not limited to—the first month of therapy.”6 Proposed explanations of how clozapine causes myocarditis include:
- direct toxic effect on cardiac myocytes related to impaired clozapine metabolism in some patients7,8
- myocardial damage mediated by clozapine blockade of a muscarinic M2 receptor subtype9
- selenium deficiency or presence of reactive clozapine nitrenium metabolites contributing to myocardial toxicity.10,11
The common presence of peripheral eosinophilia on autopsy—including diffuse eosinophilic infiltrates in myocardial and perivascular areas—might suggest a hypereosinophilic syndrome or a type II hypersensitive immune reaction mediated by clozapine.7,12 Similar immune-mediated conditions of acute, progressive myocarditis have been noted after exposure to other medications such as penicillin or sulfonamides.13
Noting that clozapine increases inflammatory cytokines, some authors believe TNF-alpha and other inflammatory cytokines contribute to myocarditis.14
TREATMENT: New regimen
After discussing clozapine’s risks and benefits with Mr. Z and his parents, we start the medication at 25 mg/d to gauge tolerability, then titrate to 300 mg/d over 10 days. Mr. Z tolerates clozapine well, with some sedation and sialorrhea. A blood test taken 7 days after we start clozapine shows a normal white blood cell count.
After 10 days on clozapine, Mr. Z’s delusions and hallucinations are considerably less intense. He is no longer suicidal and visits his former college with his parents without thinking about his past acquaintance. We discharge him on clozapine, 300 mg/d, and refer him to the local mental health agency.
Two days later, Mr. Z’s parents report that since discharge their son has had extreme fatigue, shortness of breath, leg edema, and chest pain. We advise them to immediately take their son to the ER for cardiac workup.
The authors’ observations
Mr. Z’s sudden-onset physical symptoms suggest myocarditis, a rare but potentially fatal side effect of clozapine whose specific cause is unclear (Box 1).5-14 Myocarditis has been reported in 0.02% to 0.18% of patients exposed to clozapine,15-18 with incidence as high as 1.3% per 235 patients.19
Affected patients typically have been taking clozapine at therapeutic dosages (100 to 450 mg/d).7 Clozapine use is most prevalent among men ages 20 to 40, who tend to have more severe schizophrenia and lower cardiac risk than other populations. Correspondingly, clozapine-induced myocarditis is most prevalent in younger men,20 although what specifically causes this susceptibility is unknown.
Nonspecific symptoms such as dyspnea, tachycardia, chest pain, or fever can signal myocarditis (Table)7,21 and can surface within 4 to 8 weeks of starting clozapine.22 Haas et al20 reported other symptoms—such as leukocytosis—in young (median age 30), predominantly male patients with clozapine-induced myocarditis. Symptoms that typically occur during clozapine titration—such as fever and tachycardia—can mask “subclinical” myocarditis.22
Mr. Z’s nonspecific symptoms could signal clozapine-induced agranulocytosis or a viral syndrome, or could be delusional. The patient’s acute, sudden symptom onset strongly suggests a cardiac cause. Also, his delusions subsided, and normal blood readings helped us rule out agranulocytosis.
Coulter et al23 associated myocarditis and cardiomyopathy, a noninflammatory heart muscle disease, with several antipsychotics—including clozapine, chlorpromazine, fluphenazine, haloperidol, and risperidone—as well as lithium. More research is needed to confirm this association.
Order a cardiology consult and workup including:
- serum electrolytes
- complete blood count
- ECG21
- tests for myocardial damage including creatine kinase with MB fractionation (CK-MB) and testing for serum troponin I,25 lactic dehydrogenase, and aspartate transaminase (SGOT)21
- assessment for immune activation and peripheral eosinophilia.25
Table
Symptoms that could signal myocarditis in patients taking clozapine
|
| Source: Reference 7 |
TESTING: ‘Is this necessary?’
We contact the ER physician to request the above-mentioned tests, but he questions the need for such extensive and costly testing in a psychiatric patient with nonspecific symptoms.
After several phone conversations to review our recommendations, the emergency physician suggests sending Mr. Z home on a watch-and-wait protocol. We politely but firmly emphasize that Mr. Z needs a full cardiac workup, after which the physician consents to the tests (Box 2).
FINDINGS: suspicious readings
Mr. Z’s cardiac imaging results suggest a cardiopathy:
- echocardiogram shows mild ventricular enlargement with a decreased ejection fraction of 45% (normal reading, 55% to 60%)
- ECG shows normal sinus rhythm with low-voltage diffuse T-wave flattening throughout all leads without ST elevation
- creatine phosphokinase (CPK) and CKMB are within normal ranges
- troponin I is 0.33 ng/mL, a high-normal reading.
Based on these readings, the cardiology service admits Mr. Z with a presumptive diagnosis of clozapine-induced cardiomyopathy. The attending cardiologist stops clozapine and starts the angiotensin-converting enzyme inhibitor enalapril, 2.5 mg bid, for ventricular remodeling. Medical workup includes cytologic testing to rule out immunologic or viral disease.
Five days later, Mr. Z’s cardiac symptoms have resolved. The cardiology unit discharges him on enalapril, 2.5 mg bid, and schedules a cardiac ultrasound for 2 weeks after discharge to confirm progress.
The authors’ observations
Maintain high clinical suspicion while using clozapine. Similar to other patients with a clozapine-induced cardiopathy,16 Mr. Z showed rapid symptomatic changes after a benign initial course and experienced fairly vague symptoms that raised limited clinical concern at first.
Before starting clozapine therapy, screen all patients for pre-existing cardiac disease, which contraindicates this medication. Alert patients and caregivers to the risks and symptoms that require close monitoring early in treatment.
Many researchers suggest monitoring for myocarditis during the first month of therapy and ordering ECG at baseline and 2 and 4 weeks after starting clozapine.21,22 Berk et al26 suggest more aggressive monitoring, including:
- baseline ECG
- transthoracic echocardiogram
- baseline troponin/CK-MB
- ECG and troponin/CK-MB at 7 and 14 days
- echocardiogram at 6 and 12 months and then annually.
RELAPSE: Return of the ‘freak’
Immediately after Mr. Z’s discharge from the cardiology unit, we readmit him to inpatient psychiatry. His parents and case manager say he is again becoming preoccupied with his brief college relationship. He has been off clozapine for 5 days.
The authors’ observations
The American Psychiatric Association27 (see http://www.psych.org/psych_pract/treatg/pg/SchizPG-Complete-Feb04.pdf) recommends maximizing 1 medication for at least 2 to 4 weeks to assess schizophrenia symptom response and urges clinicians to consider adverse effects, medical comorbidities, and patient preference before continuing the medication.
These recommendations highlight the challenges of treating medication-resistant schizophrenia. Relapse is common after a serious reaction to clozapine, and combining 2 or more other antipsychotics could lead to significantly greater side effects. A time-limited trial with an antipsychotic and an adjunctive agent might be attempted while carefully weighing the combination’s risks and benefits.27
Clozapine reduced Mr. Z’s psychosis, but rechallenge would likely cause his potentially fatal cardiomyopathy to re-emerge. His sensitivity to adverse antipsychotic effects discourages polypharmacy and further complicates our decision.
How to convince other specialists
Many physicians are reluctant to pursue additional tests or procedures—and risk a confrontation with a consultant, insurer, or ER physician—especially when the risk of abnormality is extremely low. Advocating for cardiac workup in patients with vague symptoms is challenging, particularly if the suspected side effect is rare.
Taking the path of least resistance can increase the risk of a serious—albeit rare—adverse event. Failure to test could prolong a potentially harmful treatment, and the test results—even if negative—could be critical to planning care.
Calmly but firmly spell out the risks of missing a suspected cardiac problem (death, proceeding with potentially harmful treatment). Tell the ER manager or consultant, “I realize this is a very rare side effect, but not catching it could be life-threatening.”
Be circumspect when pleading your case—an overaggressive approach might cause the ER doctor to “dig in his heels” and reject your request. Use a medically focused response such as, “This is a known complication of this medicine with this common time course and presentation.”
TREATMENT: Another trial
We start olanzapine, 5 mg/d, and titrate to 20 mg/d over 1 week. We add sustained-release bupropion, 200 mg bid, for associated dysphoria.
Mr. Z’s symptoms and paranoia gradually decline, and he tolerates off-unit passes with friends and family before discharge. Staff works closely with him to develop cognitive-behavioral strategies to manage residual paranoia and hallucinations, such as assessing evidence for his delusional beliefs and developing tools to distract him from remaining “voices.” He reports no cardiac symptoms and continues taking enalapril, 2.5 mg bid.
We discharge Mr. Z after 1 week, at which point he shows no suicidal or homicidal thoughts. Follow-up echocardiogram 2 weeks later shows ejection fraction has improved to 60%, suggesting absence of cardiomyopathy.
Related resource
- Clozapine safety information.
www.clozaril.com/.
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Enalapril • Vasotec
- Fluphenazine • Prolixin, Permitil
- Haloperidol • Haldol
- Lithium • Eskalith, others
- Olanzapine • Zyprexa
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988;45:789-96.
2. Meltzer HY, Alphs L, Green AI, et al. International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003;60:82-91.
3. Stahl SM. Antipsychotic polypharmacy, part 1: therapeutic option or dirty little secret? J Clin Psychiatry 1999;60:425-6.
4. Clozaril monograph. Novartis Phamaceuticals Corp.; April 12, 2006. Available at http://www.novartis.ca/downloads/en/products/clozaril_scrip_e.pdf. Accessed August 13, 2007.
5. Mason JW, O’Connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med 1995;333:269.-
6. Physicians’ desk reference. 61st ed. Montvale, NJ: Thomson PDR; 2007.
7. Merrill DB, Dec GW, Goff DC. Adverse cardiac effects associated with clozapine. J Clin Psychopharmacol 2005;25:32-41.
8. Jenie LE. Cardiovascular toxicity with clozapine therapy. Riverview Hospital Pharmacy Newsletter 2002;22:1-3.
9. Devarajan S, Kutcher SP, Dursun SM. Clozapine and sudden death. Lancet 2000;355:841.-
10. Vaddadi KS, Soosai E, Vaddadi G. Low blood selenium concentrations in schizophrenic patients on clozapine. Br J Clin Pharmacol 2003;55:307-9.
11. Williams DP, O’Donnell CJ, Maggs JL, et al. Bioactivation of clozapine by murine cardiac tissue in vivo and in vitro. Chem Res Toxicol 2003;16:1359-64.
12. Fineschi V, Neri M, Riezzo I, Turillazzi E. Sudden cardiac death due to hypersensitivity myocarditis during clozapine treatment. Int J Legal Med 2004;118:307-9.
13. Kendell KR, Day JD, Hruban RH, et al. Intimate association of eosinophils to collagen bundles in eosinophilic myocarditis and ranitidine induced hypersensitivity myocarditis. Arch Pathol Lab Med 1995;119:1154-60.
14. Pollmacher T, Schuld A, Kraus T, et al. On the clinical relevance of clozapine-triggered release of cytokines and soluble cytokine-receptors [in German]. Fortschr Neurol Psychiatr 2001;69(suppl 2):S65-S74.
15. Killian JG, Kerr K, Lawrence C, Celermajer DS. Myocarditis and cardiomyopathy associated with clozapine. Lancet 1999;354:1841-5.
16. Committee on Safety of Medicines Myocarditis with antipsychotics: recent cases with clozapine (Clozaril). Curr Probl Pharmacovigilance 1993;19:9.-
17. Degner D, Bleich S, Grohmann R, et al. Myocarditis associated with clozapine treatment. Aust NZ J Psychiatry 2000;34:880.-
18. La Grenade L, Graham D, Trontell A. Myocarditis and cardiomyopathy associated with clozapine use in the United States (letter). N Engl J Med 2001;345:224-5.
19. Reinders J, Parsonage W, Lange D, et al. Clozapinerelated myocarditis and cardiomyopathy in an Australian metropolitan psychiatric service. Aust NZ J Psychiatry 2004;38:915-22.
20. Haas SJ, Hill R, Krum H, et al. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993-2003. Drug Saf 2007;30:47-57.
21. Wehmeier PM, Heiser P, Remschmidt H. Myocarditis, pericarditis and cardiomyopathy in patients treated with clozapine. J Clin Pharm Ther 2005;30:91-6.
22. Merrill DB, Ahmari SE, Bradford JM, Lieberman JA. Myocarditis during clozapine treatment. Am J Psychiatry 2006;163:204-8. Erratum in Am J Psychiatry 2006;163:556.-
23. Coulter DM, Bate A, Meyboom RH, et al. Antipsychotic drugs and heart muscle disorder in international pharmacovigilance: data mining study. BMJ 2001;322:1207-9.
24. Wooltorton E. Antipsychotic clozapine (Clozaril): myocarditis and cardiovascular toxicity. CMAJ 2002;166:1185-6.
25. Kay SE, Doery J, Sholl D. Clozapine associated pericarditis and elevated troponin I. Aust NZ J Psychiatry 2002;36:143-4.
26. Berk M, Fitzsimons J, Lambert T, et al. Monitoring the safe use of clozapine: a consensus view from Victoria, Australia. CNS Drugs 2007;21:117-27.
27. American Psychiatric Association Work Group on Schizophrenia, Lehman AF, chair. Practice guideline for the treatment of patients with schizophrenia, 2nd ed, 2004. Available at: http://www.psych.org/psych_pract/treatg/pg/SchizPG-Complete-Feb04.pdf. Accessed August 15, 2007.
1. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988;45:789-96.
2. Meltzer HY, Alphs L, Green AI, et al. International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003;60:82-91.
3. Stahl SM. Antipsychotic polypharmacy, part 1: therapeutic option or dirty little secret? J Clin Psychiatry 1999;60:425-6.
4. Clozaril monograph. Novartis Phamaceuticals Corp.; April 12, 2006. Available at http://www.novartis.ca/downloads/en/products/clozaril_scrip_e.pdf. Accessed August 13, 2007.
5. Mason JW, O’Connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med 1995;333:269.-
6. Physicians’ desk reference. 61st ed. Montvale, NJ: Thomson PDR; 2007.
7. Merrill DB, Dec GW, Goff DC. Adverse cardiac effects associated with clozapine. J Clin Psychopharmacol 2005;25:32-41.
8. Jenie LE. Cardiovascular toxicity with clozapine therapy. Riverview Hospital Pharmacy Newsletter 2002;22:1-3.
9. Devarajan S, Kutcher SP, Dursun SM. Clozapine and sudden death. Lancet 2000;355:841.-
10. Vaddadi KS, Soosai E, Vaddadi G. Low blood selenium concentrations in schizophrenic patients on clozapine. Br J Clin Pharmacol 2003;55:307-9.
11. Williams DP, O’Donnell CJ, Maggs JL, et al. Bioactivation of clozapine by murine cardiac tissue in vivo and in vitro. Chem Res Toxicol 2003;16:1359-64.
12. Fineschi V, Neri M, Riezzo I, Turillazzi E. Sudden cardiac death due to hypersensitivity myocarditis during clozapine treatment. Int J Legal Med 2004;118:307-9.
13. Kendell KR, Day JD, Hruban RH, et al. Intimate association of eosinophils to collagen bundles in eosinophilic myocarditis and ranitidine induced hypersensitivity myocarditis. Arch Pathol Lab Med 1995;119:1154-60.
14. Pollmacher T, Schuld A, Kraus T, et al. On the clinical relevance of clozapine-triggered release of cytokines and soluble cytokine-receptors [in German]. Fortschr Neurol Psychiatr 2001;69(suppl 2):S65-S74.
15. Killian JG, Kerr K, Lawrence C, Celermajer DS. Myocarditis and cardiomyopathy associated with clozapine. Lancet 1999;354:1841-5.
16. Committee on Safety of Medicines Myocarditis with antipsychotics: recent cases with clozapine (Clozaril). Curr Probl Pharmacovigilance 1993;19:9.-
17. Degner D, Bleich S, Grohmann R, et al. Myocarditis associated with clozapine treatment. Aust NZ J Psychiatry 2000;34:880.-
18. La Grenade L, Graham D, Trontell A. Myocarditis and cardiomyopathy associated with clozapine use in the United States (letter). N Engl J Med 2001;345:224-5.
19. Reinders J, Parsonage W, Lange D, et al. Clozapinerelated myocarditis and cardiomyopathy in an Australian metropolitan psychiatric service. Aust NZ J Psychiatry 2004;38:915-22.
20. Haas SJ, Hill R, Krum H, et al. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993-2003. Drug Saf 2007;30:47-57.
21. Wehmeier PM, Heiser P, Remschmidt H. Myocarditis, pericarditis and cardiomyopathy in patients treated with clozapine. J Clin Pharm Ther 2005;30:91-6.
22. Merrill DB, Ahmari SE, Bradford JM, Lieberman JA. Myocarditis during clozapine treatment. Am J Psychiatry 2006;163:204-8. Erratum in Am J Psychiatry 2006;163:556.-
23. Coulter DM, Bate A, Meyboom RH, et al. Antipsychotic drugs and heart muscle disorder in international pharmacovigilance: data mining study. BMJ 2001;322:1207-9.
24. Wooltorton E. Antipsychotic clozapine (Clozaril): myocarditis and cardiovascular toxicity. CMAJ 2002;166:1185-6.
25. Kay SE, Doery J, Sholl D. Clozapine associated pericarditis and elevated troponin I. Aust NZ J Psychiatry 2002;36:143-4.
26. Berk M, Fitzsimons J, Lambert T, et al. Monitoring the safe use of clozapine: a consensus view from Victoria, Australia. CNS Drugs 2007;21:117-27.
27. American Psychiatric Association Work Group on Schizophrenia, Lehman AF, chair. Practice guideline for the treatment of patients with schizophrenia, 2nd ed, 2004. Available at: http://www.psych.org/psych_pract/treatg/pg/SchizPG-Complete-Feb04.pdf. Accessed August 15, 2007.
A life of drugs and ‘downtime’
CASE: Near-fatal combination
Inpatient psychiatry refers Mr. B, age 50, to our outpatient psychiatry clinic. Two weeks earlier, he tried to kill himself by sitting on a stepladder, tying a noose around his neck, and consuming large amounts of quetiapine, trazodone, and vodka. His wife found him unconscious on the floor with facial abrasions, empty pill bottles, and the noose lying next to him.
Emergency medical personnel brought Mr. B to the ER. His total Glasgow Coma Scale score of 3 indicated he was comatose. Pulse was 65 bpm (low-normal), and blood alcohol level was 106 mg/dL, suggesting he had ingested hazardous amounts of vodka. Quetiapine and trazodone blood levels were not measured.
Gastric lavage was unsuccessful because the orogastric tube became curled in the distal esophagus. Mr. B was successfully intubated and admitted to the intensive care unit. After 2 days, he was medically stable and regained consciousness, though he was delirious. He was transferred to inpatient psychiatry, where the attending psychiatrist diagnosed major depression and alcohol abuse disorder.
Before presentation, Mr. B had been taking venlafaxine, 75 mg/d, and mirtazapine, 30 mg at bedtime. His previous outpatient psychiatrist had added methylphenidate, 40 mg/d, to augment the antidepressants—which were not alleviating his depression—and the attending continued all 3 medications. Prior trials of sertraline, bupropion, trazodone, quetiapine, and aripiprazole were ineffective.
By the time Mr. B is transferred to us, his suicidal thoughts have remitted but he is still notably depressed. He is anergic, feels hopeless about the future, has markedly diminished self-worth, feels excessively guilty over past actions, is socially withdrawn, and shows a blunted, depressed affect. He also complains of insomnia despite taking mirtazapine at bedtime.
HISTORY: Depression and drugs
Mr. B says he has felt depressed on and off since his teens, and his current episode has been continuously severe for 1½ years. He began abusing alcohol and benzodiazepines during this episode but says he has been clean and sober for 2 weeks. He tried to kill himself 2 other times over 6 months by overdosing on alprazolam and was hospitalized after both attempts. He has no history of mania or psychosis.
Mr. B also abused opioids. In college, he was prescribed codeine for back pain after a sports injury. He experienced profound relief from depression after his first dose and soon began abusing codeine and other opioids for mood effects, including diphenoxylate/atropine and “cough syrup.” He says he has never used heroin.
Twenty years of illicit opioid use destroyed Mr. B’s occupational and social functioning, leaving him unable to work in his chosen field. During that period, he was frequently unemployed, socially isolated, and unable to sustain romantic relationships.
At age 40, Mr. B entered a methadone program, began working steadily, and got married. Five years later, he tapered off methadone and to our knowledge remained continuously opioid-free until presentation. Mr. B’s depression persisted while using opioids and worsened after stopping methadone. He also completed an 8-week residential substance abuse treatment program several months before presentation.
HISTORY: Family problems
Mr. B says he was emotionally abused as a child and described his father as excessively rageful. He says he entered a highly skilled profession to please his father but did not enjoy it and has not worked in the field since his early 30s. He has been unemployed for 1 year because his depression makes him feel “unworthy” to work.
The patient’s marriage of 10 years has been riddled with conflict. His depression, substance abuse, suicidality, and unemployment have fueled his wife’s resentment and anger.
The authors’ observations
Mr. B’s depression is challenging because of its severity and many possible causes and perpetuating factors. In addition to acute psychological stress and recent alcohol and benzodiazepine abuse, he has endured long-term opioid addiction. Although he had stayed opioid-free for 5 years, his past addiction contributed to his depression.
Whether Mr. B’s depression or opioid dependence came first is unclear. Either way, past opioid dependence can worsen depression prognosis.1 Opioid dependence might cause a withdrawal state that lasts years after acute withdrawal has subsided, although some researchers dispute this concept.2 According to Gold et al,3 long-term opioid use can cause endogenous opioid system derangements and depression after exogenous opioid use has ceased.
Depression is difficult to diagnose unambiguously in patients who have been using alcohol or anxiolytics because these CNS depressants’ effects might mimic depression. Patients whose symptoms suggest dual disorders commonly alternate between traditional psychiatric interventions and chemical dependence treatment.
As with Mr. B, a patient who abstains from 1 substance might start abusing another. This “replacement” is part of an “addiction interaction” theory that recognizes multiple substance and/or behavioral addictions in a patient.4 “Replacement” addiction indicates that substance abuse therapy is not adequately addressing some issues.
Coordinating concurrent depression and substance abuse treatment is critical. Although Mr. B’s ongoing psychosocial stress was addressed to varying degrees, endogenous opioid system derangements and/or prolonged opioid withdrawal may have been missed.
TREATMENT: Medication change
We discontinue methylphenidate because it is causing anxiety while leaving Mr. B’s depression unabated. Also, methylphenidate can be addictive.
Over several weeks, we titrate venlafaxine to 300 mg/d and continue mirtazapine, 30 mg at bedtime. We start weekly individual psychotherapy and encourage Mr. B to regularly attend Alcoholics Anonymous (AA) meetings, which he had been attending intermittently for years.
After 1 month, Mr. B’s depression improves marginally, but his depressed mood, anergia, and flat affect persist. He has not relapsed into alcohol or benzodiazepine dependence but reports occasional cravings for opioids and longs for the profound antidepressant effect they once gave him.
The authors’ observations
Sublingual buprenorphine is not FDA-approved to treat depression, although several small studies have described its antidepressant efficacy.5-7 How exogenous opioids reduce depressive symptoms is unknown, although some researchers believe that endogenous opioids:
- work with the mesolimbic dopaminergic system to mediate pleasure and reward
- modulate the mesolimbic system
- or have the same attenuating effect on both psychic and physical pain.
Buprenorphine also is a kappa receptor antagonist, which might explain its antidepressant efficacy.11 Whereas full mu agonism mediates euphoria, kappa receptor agonism results in dysphoria. By contrast, kappa receptor antagonism might cause a more stable, noneuphoric antidepressant effect.
Based on Mr. B’s clinical status, we ask him to consider sublingual buprenorphine/naloxone to treat depression and prevent relapse to opioid addiction.
The authors’ observations
Mr. B’s opioid addiction history and type of depression support buprenorphine augmentation. Whereas switching antidepressants or starting ECT would address only his persistent depression, buprenorphine also would target his opioid craving.
Numerous conventional psychotropics have not alleviated Mr. B’s depression, and changing antidepressants might nullify his small gains over the past month. We might consider ECT if buprenorphine does not reduce his depression.
Doctors need to obtain a waiver from the Drug Enforcement Administration (DEA) before using buprenorphine to treat opioid dependence—its approved indication (Box 1). This waiver is not necessary for off-label buprenorphine use. We needed the DEA waiver for Mr. B because we were using buprenorphine to treat opioid relapse prevention as well as depression. To prescribe buprenorphine without a DEA waiver, document that you are using the drug only for the off-label purpose.
The Drug Enforcement Administration (DEA) requires physicians to obtain a waiver to use buprenorphine to treat opioid dependence in outpatients. This waiver exempts outpatient practitioners from the DEA requirement that only specially licensed opioid treatment programs—such as methadone clinics—can dispense opioid medications.
To obtain the waiver, a physician must:
- show competency to use buprenorphine—usually by completing an 8-hour training course
- certify that he/she can conveniently refer patients for psychosocial treatment.
To receive DEA-approved buprenorphine training, in person or online, contact:
- American Society of Addiction Medicine. (888) 362-6784, www.asam.org/BuprenorphineCME.html
- American Academy of Addiction Psychiatry. (401) 524-3076, www.aaap.org/buprenorphine/buprenorphine.htm
- American Psychiatric Association. (703) 907-7300, www.psych.org/edu/bup_training.cfm
- American Osteopathic Academy of Addiction Medicine. (800) 621-1773, ext. 8163, www.aoaam.org.
For information on obtaining the waiver, visit www.buprenorphine.samhsa.gov.
Buprenorphine risks
Overdose. Buprenorphine can be abused by grinding and dissolving tablets, then injecting them intravenously. Doing this while under the influence of benzodiazepines or other sedatives can cause respiratory depression, leading to coma or death.
Combination buprenorphine/naloxone carries a much lower risk of IV overdose than buprenorphine alone because naloxone blocks mu opioid receptors. This formulation was created specifically to prevent buprenorphine misuse. Because naloxone is metabolized hepatically, it is not pharmacologically active when taken orally and will not block buprenorphine’s effect when buprenorphine/naloxone is taken as prescribed.
Physical dependence and withdrawal. Long-term buprenorphine use can cause physical dependence. Abrupt discontinuation or excessively high doses can precipitate withdrawal. How withdrawal is precipitated is unclear, although some believe the drug displaces itself from mu receptors when doses are too high. Myalgia, headache, abdominal discomfort, rhinorrhea, anxiety, and irritability are common buprenorphine withdrawal symptoms. The dosage at which the drug precipitates withdrawal varies with each patient’s tolerance for opioids.
When stopping buprenorphine therapy, taper the medication gradually to minimize withdrawal discomfort and relapse risk. Start tapering by 2 mg per month, then taper more rapidly or slowly based on the patient’s subjective experience.
TREATMENT: An opioid option
After discussing the risks and benefits with Mr. B and his wife, we add buprenorphine/naloxone, 8 mg/d, then increase it to 16 mg/d the next day. He tolerates the medication, and within 1 week his anergia disappears and he feels more motivated and productive. He reports no euphoria from buprenorphine but says it decreases his craving for alcohol, benzodiazepines, and opioids.
We continue buprenorphine/naloxone, 16 mg/d, and mirtazapine, 30 mg at bedtime, and reduce venlafaxine to 225 mg/d to mitigate sexual side effects. During weekly individual psychotherapy, we target Mr. B’s marital conflict and low self-esteem, and instruct him on overcoming life obstacles such as unemployment. He is looking for work and attends AA approximately 5 times a week.
Remember these 8 steps
- Address depression and substance abuse concurrently
- Communicate regularly with other providers about progress on depression and substance abuse issues
- Recommend and support involvement in 12-step programs such as AA
- Use medications for both depression—such as antidepressants—and relapse prevention—such as naltrexone, acamprosate, or buprenorphine/naloxone
- Explore family history of addiction and how this affected the patient developmentally. Find out if depression and substance abuse had common causes; this helps the patient realize that he/she did not become depressed or addicted by choice
- Ask about and discuss multiple addictions that were not initially reported
- Help the patient express, tolerate, and experience difficult feelings rather than avoid them
- Empathize with the patient; express understanding that factors out of the patient’s control caused depression and addiction
The authors’ observations
Considering the tumultuousness of Mr. B’s life, his willingness to enter psychotherapy and address underlying issues is significant. Adding buprenorphine to his antidepressant regimen helped stabilize his mood and make psychotherapy possible.
Psychotropics have not induced total remission of Mr. B’s depression, which is multifactorial and requires multimodal treatment. Still, we consider buprenorphine therapy at least partially successful—he has gone 6 months without attempting suicide or requiring psychiatric hospitalization.
Some clinicians consider buprenorphine’s potential for physical dependence a drawback to depression therapy. Physical dependence on a psychotropic does not necessarily outweigh its benefit in severe depression. Indeed, patients with depression can experience discontinuation symptoms from selective serotonin reuptake inhibitors and withdrawal from benzodiazepines.2,12
FOLLOW-UP: ‘Bup’ stigma
Mr. B feels stigmatized about buprenorphine use, partly because his wife shames him for his history of addiction and views buprenorphine as a constant reminder of his “failures.”
Mrs. B’s dysfunctional attitude leaves Mr. B too ashamed to tell his fellow AA members that he takes buprenorphine. His inability to share these feelings also diminishes his sense of belonging in the 12-step fellowship. Even so, he feels that buprenorphine has helped him tremendously and wants to continue taking it.
During psychotherapy, we address Mr. B’s buprenorphine-related stigma and pervasive shame stemming from his history of mental illness, addiction, inability to work in his chosen field, and past employment failures. We encourage him to overcome his shame by pointing out his strengths—such as the skills he can offer potential employers—and by emphasizing that he did not choose to become depressed and addicted.
The authors’ observations
Most patients addicted to opiates feel much less stigmatized by buprenorphine therapy than by methadone. Patients who feel shame while taking buprenorphine usually are reacting to past opioid addiction rather than current therapy. Mr. B’s buprenorphine-related shame stems from his personality structure.
Shame, however, could create negative expectations of buprenorphine therapy, and can lower some patients’ self-esteem to the point that they feel they do not deserve to get better. Some patients stop buprenorphine prematurely because they believe they have beaten the addiction, but this often leads to relapse to the previous opioid of choice.
Help patients work through the shame of past addiction and encourage them to view buprenorphine therapy as a positive step toward recovery (Box 2). As mental health professionals, we must not collude with society to shame people with past chemical addiction. Creatively yet responsibly broadening our perspective toward psychiatric intervention can help patients such as Mr. B receive optimal treatment.
Although members of a 12-step group might harbor an idiosyncratic position on medications or treatment, cooperation with professionals is the program’s mainstream stance. Ideally, combination pharmacotherapy, psychotherapy, and guidance for optimal use of support groups can provide a stable foundation for recovery from both psychiatric and addictive disorders.
Related resources
- U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Substance Abuse Treatment Knowledge Application Program, Treatment Improvement Protocol Series. www.kap.samhsa.gov/products/manuals/tips/index.htm.
- Acamprosate • Campral
- Alprazolam • Xanax
- Aripiprazole • Abilify
- Buprenorphine • Subutex
- Buprenorphine/naloxone • Suboxone
- Bupropion • Wellbutrin
- Diphenoxylate/atropine • Lomotil
- Methadone • Dolophine
- Methylphenidate • Ritalin, Concerta
- Mirtazapine • Remeron
- Naltrexone • ReVia, Vivitrol
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Trazodone • Desyrel
- Venlafaxine • Effexor
Dr. Roth is a speaker for Reckitt Benckiser.
Drs. Eiger and Tan report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Nunes EV, Sullivan MA, Levin FR. Treatment of depression in patients with opiate dependence. Biol Psychiatry 2004;56:793-802.
2. Graham AW, Schultz TK, Mayo-Smith MF, et al, eds. Principles of addiction medicine. 3rd ed. Chevy Chase, MD: American Society of Addiction Medicine; 2003.
3. Gold MS, Pottash AL, Extein I, et al. Evidence for an endorphin dysfunction in methadone addicts: lack of ACTH response to naloxone. Drug Alcohol Depend 1981;8:257-62.
4. Carnes PJ, Murray RE, Charpentier L. Addiction interaction disorder. In: Coombs RH, ed. Handbook of addictive disorders: a practical guide to diagnosis and treatment. Hoboken, NJ: John Wiley & Sons 2004:31-59.
5. Kosten TR, Morgan C, Kosten TA. Depressive symptoms during buprenorphine treatment of opioid abusers. J Subst Abuse Treat. 1990;7:51-4.
6. Dean AJ, Bell J, Christie MJ, Mattick RP. Depressive symptoms during buprenorphine vs. methadone maintenance: findings from a randomized, controlled trial in opioid dependence. Eur Psychiatry. 2004;19:510-13.
7. Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15:49-57.
8. Jaffe JH, Jaffe AB. Neurobiology of opioids. In: Galanter M, Kleber HD, eds. Textbook of substance abuse treatment.. 3rd ed. Washington, DC: American Psychiatric Publishing; 2004:17-30.
9. Jones HE. Practical considerations for the clinical use of buprenorphine. NIDA Sci Pract Perspectives. 2004;2:4-20.
10. Geppert CM, Toney GB, Siracusano D, Thorius M. Outpatient buprenorphine treatment for opioid dependence. Fed Practitioner. 2005;22:9-40.
11. Mague SD, Pliakas AM, Todtenkopf MS, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323-30.
12. Van Geffen EC, Hugtenburg JG, Heerdink ER, et al. Discontinuation symptoms in users of selective serotonin reuptake inhibitors in clinical practice: tapering versus abrupt continuation. Eur J Clin Pharmacol. 2005;61:303-7.
CASE: Near-fatal combination
Inpatient psychiatry refers Mr. B, age 50, to our outpatient psychiatry clinic. Two weeks earlier, he tried to kill himself by sitting on a stepladder, tying a noose around his neck, and consuming large amounts of quetiapine, trazodone, and vodka. His wife found him unconscious on the floor with facial abrasions, empty pill bottles, and the noose lying next to him.
Emergency medical personnel brought Mr. B to the ER. His total Glasgow Coma Scale score of 3 indicated he was comatose. Pulse was 65 bpm (low-normal), and blood alcohol level was 106 mg/dL, suggesting he had ingested hazardous amounts of vodka. Quetiapine and trazodone blood levels were not measured.
Gastric lavage was unsuccessful because the orogastric tube became curled in the distal esophagus. Mr. B was successfully intubated and admitted to the intensive care unit. After 2 days, he was medically stable and regained consciousness, though he was delirious. He was transferred to inpatient psychiatry, where the attending psychiatrist diagnosed major depression and alcohol abuse disorder.
Before presentation, Mr. B had been taking venlafaxine, 75 mg/d, and mirtazapine, 30 mg at bedtime. His previous outpatient psychiatrist had added methylphenidate, 40 mg/d, to augment the antidepressants—which were not alleviating his depression—and the attending continued all 3 medications. Prior trials of sertraline, bupropion, trazodone, quetiapine, and aripiprazole were ineffective.
By the time Mr. B is transferred to us, his suicidal thoughts have remitted but he is still notably depressed. He is anergic, feels hopeless about the future, has markedly diminished self-worth, feels excessively guilty over past actions, is socially withdrawn, and shows a blunted, depressed affect. He also complains of insomnia despite taking mirtazapine at bedtime.
HISTORY: Depression and drugs
Mr. B says he has felt depressed on and off since his teens, and his current episode has been continuously severe for 1½ years. He began abusing alcohol and benzodiazepines during this episode but says he has been clean and sober for 2 weeks. He tried to kill himself 2 other times over 6 months by overdosing on alprazolam and was hospitalized after both attempts. He has no history of mania or psychosis.
Mr. B also abused opioids. In college, he was prescribed codeine for back pain after a sports injury. He experienced profound relief from depression after his first dose and soon began abusing codeine and other opioids for mood effects, including diphenoxylate/atropine and “cough syrup.” He says he has never used heroin.
Twenty years of illicit opioid use destroyed Mr. B’s occupational and social functioning, leaving him unable to work in his chosen field. During that period, he was frequently unemployed, socially isolated, and unable to sustain romantic relationships.
At age 40, Mr. B entered a methadone program, began working steadily, and got married. Five years later, he tapered off methadone and to our knowledge remained continuously opioid-free until presentation. Mr. B’s depression persisted while using opioids and worsened after stopping methadone. He also completed an 8-week residential substance abuse treatment program several months before presentation.
HISTORY: Family problems
Mr. B says he was emotionally abused as a child and described his father as excessively rageful. He says he entered a highly skilled profession to please his father but did not enjoy it and has not worked in the field since his early 30s. He has been unemployed for 1 year because his depression makes him feel “unworthy” to work.
The patient’s marriage of 10 years has been riddled with conflict. His depression, substance abuse, suicidality, and unemployment have fueled his wife’s resentment and anger.
The authors’ observations
Mr. B’s depression is challenging because of its severity and many possible causes and perpetuating factors. In addition to acute psychological stress and recent alcohol and benzodiazepine abuse, he has endured long-term opioid addiction. Although he had stayed opioid-free for 5 years, his past addiction contributed to his depression.
Whether Mr. B’s depression or opioid dependence came first is unclear. Either way, past opioid dependence can worsen depression prognosis.1 Opioid dependence might cause a withdrawal state that lasts years after acute withdrawal has subsided, although some researchers dispute this concept.2 According to Gold et al,3 long-term opioid use can cause endogenous opioid system derangements and depression after exogenous opioid use has ceased.
Depression is difficult to diagnose unambiguously in patients who have been using alcohol or anxiolytics because these CNS depressants’ effects might mimic depression. Patients whose symptoms suggest dual disorders commonly alternate between traditional psychiatric interventions and chemical dependence treatment.
As with Mr. B, a patient who abstains from 1 substance might start abusing another. This “replacement” is part of an “addiction interaction” theory that recognizes multiple substance and/or behavioral addictions in a patient.4 “Replacement” addiction indicates that substance abuse therapy is not adequately addressing some issues.
Coordinating concurrent depression and substance abuse treatment is critical. Although Mr. B’s ongoing psychosocial stress was addressed to varying degrees, endogenous opioid system derangements and/or prolonged opioid withdrawal may have been missed.
TREATMENT: Medication change
We discontinue methylphenidate because it is causing anxiety while leaving Mr. B’s depression unabated. Also, methylphenidate can be addictive.
Over several weeks, we titrate venlafaxine to 300 mg/d and continue mirtazapine, 30 mg at bedtime. We start weekly individual psychotherapy and encourage Mr. B to regularly attend Alcoholics Anonymous (AA) meetings, which he had been attending intermittently for years.
After 1 month, Mr. B’s depression improves marginally, but his depressed mood, anergia, and flat affect persist. He has not relapsed into alcohol or benzodiazepine dependence but reports occasional cravings for opioids and longs for the profound antidepressant effect they once gave him.
The authors’ observations
Sublingual buprenorphine is not FDA-approved to treat depression, although several small studies have described its antidepressant efficacy.5-7 How exogenous opioids reduce depressive symptoms is unknown, although some researchers believe that endogenous opioids:
- work with the mesolimbic dopaminergic system to mediate pleasure and reward
- modulate the mesolimbic system
- or have the same attenuating effect on both psychic and physical pain.
Buprenorphine also is a kappa receptor antagonist, which might explain its antidepressant efficacy.11 Whereas full mu agonism mediates euphoria, kappa receptor agonism results in dysphoria. By contrast, kappa receptor antagonism might cause a more stable, noneuphoric antidepressant effect.
Based on Mr. B’s clinical status, we ask him to consider sublingual buprenorphine/naloxone to treat depression and prevent relapse to opioid addiction.
The authors’ observations
Mr. B’s opioid addiction history and type of depression support buprenorphine augmentation. Whereas switching antidepressants or starting ECT would address only his persistent depression, buprenorphine also would target his opioid craving.
Numerous conventional psychotropics have not alleviated Mr. B’s depression, and changing antidepressants might nullify his small gains over the past month. We might consider ECT if buprenorphine does not reduce his depression.
Doctors need to obtain a waiver from the Drug Enforcement Administration (DEA) before using buprenorphine to treat opioid dependence—its approved indication (Box 1). This waiver is not necessary for off-label buprenorphine use. We needed the DEA waiver for Mr. B because we were using buprenorphine to treat opioid relapse prevention as well as depression. To prescribe buprenorphine without a DEA waiver, document that you are using the drug only for the off-label purpose.
The Drug Enforcement Administration (DEA) requires physicians to obtain a waiver to use buprenorphine to treat opioid dependence in outpatients. This waiver exempts outpatient practitioners from the DEA requirement that only specially licensed opioid treatment programs—such as methadone clinics—can dispense opioid medications.
To obtain the waiver, a physician must:
- show competency to use buprenorphine—usually by completing an 8-hour training course
- certify that he/she can conveniently refer patients for psychosocial treatment.
To receive DEA-approved buprenorphine training, in person or online, contact:
- American Society of Addiction Medicine. (888) 362-6784, www.asam.org/BuprenorphineCME.html
- American Academy of Addiction Psychiatry. (401) 524-3076, www.aaap.org/buprenorphine/buprenorphine.htm
- American Psychiatric Association. (703) 907-7300, www.psych.org/edu/bup_training.cfm
- American Osteopathic Academy of Addiction Medicine. (800) 621-1773, ext. 8163, www.aoaam.org.
For information on obtaining the waiver, visit www.buprenorphine.samhsa.gov.
Buprenorphine risks
Overdose. Buprenorphine can be abused by grinding and dissolving tablets, then injecting them intravenously. Doing this while under the influence of benzodiazepines or other sedatives can cause respiratory depression, leading to coma or death.
Combination buprenorphine/naloxone carries a much lower risk of IV overdose than buprenorphine alone because naloxone blocks mu opioid receptors. This formulation was created specifically to prevent buprenorphine misuse. Because naloxone is metabolized hepatically, it is not pharmacologically active when taken orally and will not block buprenorphine’s effect when buprenorphine/naloxone is taken as prescribed.
Physical dependence and withdrawal. Long-term buprenorphine use can cause physical dependence. Abrupt discontinuation or excessively high doses can precipitate withdrawal. How withdrawal is precipitated is unclear, although some believe the drug displaces itself from mu receptors when doses are too high. Myalgia, headache, abdominal discomfort, rhinorrhea, anxiety, and irritability are common buprenorphine withdrawal symptoms. The dosage at which the drug precipitates withdrawal varies with each patient’s tolerance for opioids.
When stopping buprenorphine therapy, taper the medication gradually to minimize withdrawal discomfort and relapse risk. Start tapering by 2 mg per month, then taper more rapidly or slowly based on the patient’s subjective experience.
TREATMENT: An opioid option
After discussing the risks and benefits with Mr. B and his wife, we add buprenorphine/naloxone, 8 mg/d, then increase it to 16 mg/d the next day. He tolerates the medication, and within 1 week his anergia disappears and he feels more motivated and productive. He reports no euphoria from buprenorphine but says it decreases his craving for alcohol, benzodiazepines, and opioids.
We continue buprenorphine/naloxone, 16 mg/d, and mirtazapine, 30 mg at bedtime, and reduce venlafaxine to 225 mg/d to mitigate sexual side effects. During weekly individual psychotherapy, we target Mr. B’s marital conflict and low self-esteem, and instruct him on overcoming life obstacles such as unemployment. He is looking for work and attends AA approximately 5 times a week.
Remember these 8 steps
- Address depression and substance abuse concurrently
- Communicate regularly with other providers about progress on depression and substance abuse issues
- Recommend and support involvement in 12-step programs such as AA
- Use medications for both depression—such as antidepressants—and relapse prevention—such as naltrexone, acamprosate, or buprenorphine/naloxone
- Explore family history of addiction and how this affected the patient developmentally. Find out if depression and substance abuse had common causes; this helps the patient realize that he/she did not become depressed or addicted by choice
- Ask about and discuss multiple addictions that were not initially reported
- Help the patient express, tolerate, and experience difficult feelings rather than avoid them
- Empathize with the patient; express understanding that factors out of the patient’s control caused depression and addiction
The authors’ observations
Considering the tumultuousness of Mr. B’s life, his willingness to enter psychotherapy and address underlying issues is significant. Adding buprenorphine to his antidepressant regimen helped stabilize his mood and make psychotherapy possible.
Psychotropics have not induced total remission of Mr. B’s depression, which is multifactorial and requires multimodal treatment. Still, we consider buprenorphine therapy at least partially successful—he has gone 6 months without attempting suicide or requiring psychiatric hospitalization.
Some clinicians consider buprenorphine’s potential for physical dependence a drawback to depression therapy. Physical dependence on a psychotropic does not necessarily outweigh its benefit in severe depression. Indeed, patients with depression can experience discontinuation symptoms from selective serotonin reuptake inhibitors and withdrawal from benzodiazepines.2,12
FOLLOW-UP: ‘Bup’ stigma
Mr. B feels stigmatized about buprenorphine use, partly because his wife shames him for his history of addiction and views buprenorphine as a constant reminder of his “failures.”
Mrs. B’s dysfunctional attitude leaves Mr. B too ashamed to tell his fellow AA members that he takes buprenorphine. His inability to share these feelings also diminishes his sense of belonging in the 12-step fellowship. Even so, he feels that buprenorphine has helped him tremendously and wants to continue taking it.
During psychotherapy, we address Mr. B’s buprenorphine-related stigma and pervasive shame stemming from his history of mental illness, addiction, inability to work in his chosen field, and past employment failures. We encourage him to overcome his shame by pointing out his strengths—such as the skills he can offer potential employers—and by emphasizing that he did not choose to become depressed and addicted.
The authors’ observations
Most patients addicted to opiates feel much less stigmatized by buprenorphine therapy than by methadone. Patients who feel shame while taking buprenorphine usually are reacting to past opioid addiction rather than current therapy. Mr. B’s buprenorphine-related shame stems from his personality structure.
Shame, however, could create negative expectations of buprenorphine therapy, and can lower some patients’ self-esteem to the point that they feel they do not deserve to get better. Some patients stop buprenorphine prematurely because they believe they have beaten the addiction, but this often leads to relapse to the previous opioid of choice.
Help patients work through the shame of past addiction and encourage them to view buprenorphine therapy as a positive step toward recovery (Box 2). As mental health professionals, we must not collude with society to shame people with past chemical addiction. Creatively yet responsibly broadening our perspective toward psychiatric intervention can help patients such as Mr. B receive optimal treatment.
Although members of a 12-step group might harbor an idiosyncratic position on medications or treatment, cooperation with professionals is the program’s mainstream stance. Ideally, combination pharmacotherapy, psychotherapy, and guidance for optimal use of support groups can provide a stable foundation for recovery from both psychiatric and addictive disorders.
Related resources
- U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Substance Abuse Treatment Knowledge Application Program, Treatment Improvement Protocol Series. www.kap.samhsa.gov/products/manuals/tips/index.htm.
- Acamprosate • Campral
- Alprazolam • Xanax
- Aripiprazole • Abilify
- Buprenorphine • Subutex
- Buprenorphine/naloxone • Suboxone
- Bupropion • Wellbutrin
- Diphenoxylate/atropine • Lomotil
- Methadone • Dolophine
- Methylphenidate • Ritalin, Concerta
- Mirtazapine • Remeron
- Naltrexone • ReVia, Vivitrol
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Trazodone • Desyrel
- Venlafaxine • Effexor
Dr. Roth is a speaker for Reckitt Benckiser.
Drs. Eiger and Tan report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Near-fatal combination
Inpatient psychiatry refers Mr. B, age 50, to our outpatient psychiatry clinic. Two weeks earlier, he tried to kill himself by sitting on a stepladder, tying a noose around his neck, and consuming large amounts of quetiapine, trazodone, and vodka. His wife found him unconscious on the floor with facial abrasions, empty pill bottles, and the noose lying next to him.
Emergency medical personnel brought Mr. B to the ER. His total Glasgow Coma Scale score of 3 indicated he was comatose. Pulse was 65 bpm (low-normal), and blood alcohol level was 106 mg/dL, suggesting he had ingested hazardous amounts of vodka. Quetiapine and trazodone blood levels were not measured.
Gastric lavage was unsuccessful because the orogastric tube became curled in the distal esophagus. Mr. B was successfully intubated and admitted to the intensive care unit. After 2 days, he was medically stable and regained consciousness, though he was delirious. He was transferred to inpatient psychiatry, where the attending psychiatrist diagnosed major depression and alcohol abuse disorder.
Before presentation, Mr. B had been taking venlafaxine, 75 mg/d, and mirtazapine, 30 mg at bedtime. His previous outpatient psychiatrist had added methylphenidate, 40 mg/d, to augment the antidepressants—which were not alleviating his depression—and the attending continued all 3 medications. Prior trials of sertraline, bupropion, trazodone, quetiapine, and aripiprazole were ineffective.
By the time Mr. B is transferred to us, his suicidal thoughts have remitted but he is still notably depressed. He is anergic, feels hopeless about the future, has markedly diminished self-worth, feels excessively guilty over past actions, is socially withdrawn, and shows a blunted, depressed affect. He also complains of insomnia despite taking mirtazapine at bedtime.
HISTORY: Depression and drugs
Mr. B says he has felt depressed on and off since his teens, and his current episode has been continuously severe for 1½ years. He began abusing alcohol and benzodiazepines during this episode but says he has been clean and sober for 2 weeks. He tried to kill himself 2 other times over 6 months by overdosing on alprazolam and was hospitalized after both attempts. He has no history of mania or psychosis.
Mr. B also abused opioids. In college, he was prescribed codeine for back pain after a sports injury. He experienced profound relief from depression after his first dose and soon began abusing codeine and other opioids for mood effects, including diphenoxylate/atropine and “cough syrup.” He says he has never used heroin.
Twenty years of illicit opioid use destroyed Mr. B’s occupational and social functioning, leaving him unable to work in his chosen field. During that period, he was frequently unemployed, socially isolated, and unable to sustain romantic relationships.
At age 40, Mr. B entered a methadone program, began working steadily, and got married. Five years later, he tapered off methadone and to our knowledge remained continuously opioid-free until presentation. Mr. B’s depression persisted while using opioids and worsened after stopping methadone. He also completed an 8-week residential substance abuse treatment program several months before presentation.
HISTORY: Family problems
Mr. B says he was emotionally abused as a child and described his father as excessively rageful. He says he entered a highly skilled profession to please his father but did not enjoy it and has not worked in the field since his early 30s. He has been unemployed for 1 year because his depression makes him feel “unworthy” to work.
The patient’s marriage of 10 years has been riddled with conflict. His depression, substance abuse, suicidality, and unemployment have fueled his wife’s resentment and anger.
The authors’ observations
Mr. B’s depression is challenging because of its severity and many possible causes and perpetuating factors. In addition to acute psychological stress and recent alcohol and benzodiazepine abuse, he has endured long-term opioid addiction. Although he had stayed opioid-free for 5 years, his past addiction contributed to his depression.
Whether Mr. B’s depression or opioid dependence came first is unclear. Either way, past opioid dependence can worsen depression prognosis.1 Opioid dependence might cause a withdrawal state that lasts years after acute withdrawal has subsided, although some researchers dispute this concept.2 According to Gold et al,3 long-term opioid use can cause endogenous opioid system derangements and depression after exogenous opioid use has ceased.
Depression is difficult to diagnose unambiguously in patients who have been using alcohol or anxiolytics because these CNS depressants’ effects might mimic depression. Patients whose symptoms suggest dual disorders commonly alternate between traditional psychiatric interventions and chemical dependence treatment.
As with Mr. B, a patient who abstains from 1 substance might start abusing another. This “replacement” is part of an “addiction interaction” theory that recognizes multiple substance and/or behavioral addictions in a patient.4 “Replacement” addiction indicates that substance abuse therapy is not adequately addressing some issues.
Coordinating concurrent depression and substance abuse treatment is critical. Although Mr. B’s ongoing psychosocial stress was addressed to varying degrees, endogenous opioid system derangements and/or prolonged opioid withdrawal may have been missed.
TREATMENT: Medication change
We discontinue methylphenidate because it is causing anxiety while leaving Mr. B’s depression unabated. Also, methylphenidate can be addictive.
Over several weeks, we titrate venlafaxine to 300 mg/d and continue mirtazapine, 30 mg at bedtime. We start weekly individual psychotherapy and encourage Mr. B to regularly attend Alcoholics Anonymous (AA) meetings, which he had been attending intermittently for years.
After 1 month, Mr. B’s depression improves marginally, but his depressed mood, anergia, and flat affect persist. He has not relapsed into alcohol or benzodiazepine dependence but reports occasional cravings for opioids and longs for the profound antidepressant effect they once gave him.
The authors’ observations
Sublingual buprenorphine is not FDA-approved to treat depression, although several small studies have described its antidepressant efficacy.5-7 How exogenous opioids reduce depressive symptoms is unknown, although some researchers believe that endogenous opioids:
- work with the mesolimbic dopaminergic system to mediate pleasure and reward
- modulate the mesolimbic system
- or have the same attenuating effect on both psychic and physical pain.
Buprenorphine also is a kappa receptor antagonist, which might explain its antidepressant efficacy.11 Whereas full mu agonism mediates euphoria, kappa receptor agonism results in dysphoria. By contrast, kappa receptor antagonism might cause a more stable, noneuphoric antidepressant effect.
Based on Mr. B’s clinical status, we ask him to consider sublingual buprenorphine/naloxone to treat depression and prevent relapse to opioid addiction.
The authors’ observations
Mr. B’s opioid addiction history and type of depression support buprenorphine augmentation. Whereas switching antidepressants or starting ECT would address only his persistent depression, buprenorphine also would target his opioid craving.
Numerous conventional psychotropics have not alleviated Mr. B’s depression, and changing antidepressants might nullify his small gains over the past month. We might consider ECT if buprenorphine does not reduce his depression.
Doctors need to obtain a waiver from the Drug Enforcement Administration (DEA) before using buprenorphine to treat opioid dependence—its approved indication (Box 1). This waiver is not necessary for off-label buprenorphine use. We needed the DEA waiver for Mr. B because we were using buprenorphine to treat opioid relapse prevention as well as depression. To prescribe buprenorphine without a DEA waiver, document that you are using the drug only for the off-label purpose.
The Drug Enforcement Administration (DEA) requires physicians to obtain a waiver to use buprenorphine to treat opioid dependence in outpatients. This waiver exempts outpatient practitioners from the DEA requirement that only specially licensed opioid treatment programs—such as methadone clinics—can dispense opioid medications.
To obtain the waiver, a physician must:
- show competency to use buprenorphine—usually by completing an 8-hour training course
- certify that he/she can conveniently refer patients for psychosocial treatment.
To receive DEA-approved buprenorphine training, in person or online, contact:
- American Society of Addiction Medicine. (888) 362-6784, www.asam.org/BuprenorphineCME.html
- American Academy of Addiction Psychiatry. (401) 524-3076, www.aaap.org/buprenorphine/buprenorphine.htm
- American Psychiatric Association. (703) 907-7300, www.psych.org/edu/bup_training.cfm
- American Osteopathic Academy of Addiction Medicine. (800) 621-1773, ext. 8163, www.aoaam.org.
For information on obtaining the waiver, visit www.buprenorphine.samhsa.gov.
Buprenorphine risks
Overdose. Buprenorphine can be abused by grinding and dissolving tablets, then injecting them intravenously. Doing this while under the influence of benzodiazepines or other sedatives can cause respiratory depression, leading to coma or death.
Combination buprenorphine/naloxone carries a much lower risk of IV overdose than buprenorphine alone because naloxone blocks mu opioid receptors. This formulation was created specifically to prevent buprenorphine misuse. Because naloxone is metabolized hepatically, it is not pharmacologically active when taken orally and will not block buprenorphine’s effect when buprenorphine/naloxone is taken as prescribed.
Physical dependence and withdrawal. Long-term buprenorphine use can cause physical dependence. Abrupt discontinuation or excessively high doses can precipitate withdrawal. How withdrawal is precipitated is unclear, although some believe the drug displaces itself from mu receptors when doses are too high. Myalgia, headache, abdominal discomfort, rhinorrhea, anxiety, and irritability are common buprenorphine withdrawal symptoms. The dosage at which the drug precipitates withdrawal varies with each patient’s tolerance for opioids.
When stopping buprenorphine therapy, taper the medication gradually to minimize withdrawal discomfort and relapse risk. Start tapering by 2 mg per month, then taper more rapidly or slowly based on the patient’s subjective experience.
TREATMENT: An opioid option
After discussing the risks and benefits with Mr. B and his wife, we add buprenorphine/naloxone, 8 mg/d, then increase it to 16 mg/d the next day. He tolerates the medication, and within 1 week his anergia disappears and he feels more motivated and productive. He reports no euphoria from buprenorphine but says it decreases his craving for alcohol, benzodiazepines, and opioids.
We continue buprenorphine/naloxone, 16 mg/d, and mirtazapine, 30 mg at bedtime, and reduce venlafaxine to 225 mg/d to mitigate sexual side effects. During weekly individual psychotherapy, we target Mr. B’s marital conflict and low self-esteem, and instruct him on overcoming life obstacles such as unemployment. He is looking for work and attends AA approximately 5 times a week.
Remember these 8 steps
- Address depression and substance abuse concurrently
- Communicate regularly with other providers about progress on depression and substance abuse issues
- Recommend and support involvement in 12-step programs such as AA
- Use medications for both depression—such as antidepressants—and relapse prevention—such as naltrexone, acamprosate, or buprenorphine/naloxone
- Explore family history of addiction and how this affected the patient developmentally. Find out if depression and substance abuse had common causes; this helps the patient realize that he/she did not become depressed or addicted by choice
- Ask about and discuss multiple addictions that were not initially reported
- Help the patient express, tolerate, and experience difficult feelings rather than avoid them
- Empathize with the patient; express understanding that factors out of the patient’s control caused depression and addiction
The authors’ observations
Considering the tumultuousness of Mr. B’s life, his willingness to enter psychotherapy and address underlying issues is significant. Adding buprenorphine to his antidepressant regimen helped stabilize his mood and make psychotherapy possible.
Psychotropics have not induced total remission of Mr. B’s depression, which is multifactorial and requires multimodal treatment. Still, we consider buprenorphine therapy at least partially successful—he has gone 6 months without attempting suicide or requiring psychiatric hospitalization.
Some clinicians consider buprenorphine’s potential for physical dependence a drawback to depression therapy. Physical dependence on a psychotropic does not necessarily outweigh its benefit in severe depression. Indeed, patients with depression can experience discontinuation symptoms from selective serotonin reuptake inhibitors and withdrawal from benzodiazepines.2,12
FOLLOW-UP: ‘Bup’ stigma
Mr. B feels stigmatized about buprenorphine use, partly because his wife shames him for his history of addiction and views buprenorphine as a constant reminder of his “failures.”
Mrs. B’s dysfunctional attitude leaves Mr. B too ashamed to tell his fellow AA members that he takes buprenorphine. His inability to share these feelings also diminishes his sense of belonging in the 12-step fellowship. Even so, he feels that buprenorphine has helped him tremendously and wants to continue taking it.
During psychotherapy, we address Mr. B’s buprenorphine-related stigma and pervasive shame stemming from his history of mental illness, addiction, inability to work in his chosen field, and past employment failures. We encourage him to overcome his shame by pointing out his strengths—such as the skills he can offer potential employers—and by emphasizing that he did not choose to become depressed and addicted.
The authors’ observations
Most patients addicted to opiates feel much less stigmatized by buprenorphine therapy than by methadone. Patients who feel shame while taking buprenorphine usually are reacting to past opioid addiction rather than current therapy. Mr. B’s buprenorphine-related shame stems from his personality structure.
Shame, however, could create negative expectations of buprenorphine therapy, and can lower some patients’ self-esteem to the point that they feel they do not deserve to get better. Some patients stop buprenorphine prematurely because they believe they have beaten the addiction, but this often leads to relapse to the previous opioid of choice.
Help patients work through the shame of past addiction and encourage them to view buprenorphine therapy as a positive step toward recovery (Box 2). As mental health professionals, we must not collude with society to shame people with past chemical addiction. Creatively yet responsibly broadening our perspective toward psychiatric intervention can help patients such as Mr. B receive optimal treatment.
Although members of a 12-step group might harbor an idiosyncratic position on medications or treatment, cooperation with professionals is the program’s mainstream stance. Ideally, combination pharmacotherapy, psychotherapy, and guidance for optimal use of support groups can provide a stable foundation for recovery from both psychiatric and addictive disorders.
Related resources
- U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration, Center for Substance Abuse Treatment Knowledge Application Program, Treatment Improvement Protocol Series. www.kap.samhsa.gov/products/manuals/tips/index.htm.
- Acamprosate • Campral
- Alprazolam • Xanax
- Aripiprazole • Abilify
- Buprenorphine • Subutex
- Buprenorphine/naloxone • Suboxone
- Bupropion • Wellbutrin
- Diphenoxylate/atropine • Lomotil
- Methadone • Dolophine
- Methylphenidate • Ritalin, Concerta
- Mirtazapine • Remeron
- Naltrexone • ReVia, Vivitrol
- Quetiapine • Seroquel
- Sertraline • Zoloft
- Trazodone • Desyrel
- Venlafaxine • Effexor
Dr. Roth is a speaker for Reckitt Benckiser.
Drs. Eiger and Tan report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Nunes EV, Sullivan MA, Levin FR. Treatment of depression in patients with opiate dependence. Biol Psychiatry 2004;56:793-802.
2. Graham AW, Schultz TK, Mayo-Smith MF, et al, eds. Principles of addiction medicine. 3rd ed. Chevy Chase, MD: American Society of Addiction Medicine; 2003.
3. Gold MS, Pottash AL, Extein I, et al. Evidence for an endorphin dysfunction in methadone addicts: lack of ACTH response to naloxone. Drug Alcohol Depend 1981;8:257-62.
4. Carnes PJ, Murray RE, Charpentier L. Addiction interaction disorder. In: Coombs RH, ed. Handbook of addictive disorders: a practical guide to diagnosis and treatment. Hoboken, NJ: John Wiley & Sons 2004:31-59.
5. Kosten TR, Morgan C, Kosten TA. Depressive symptoms during buprenorphine treatment of opioid abusers. J Subst Abuse Treat. 1990;7:51-4.
6. Dean AJ, Bell J, Christie MJ, Mattick RP. Depressive symptoms during buprenorphine vs. methadone maintenance: findings from a randomized, controlled trial in opioid dependence. Eur Psychiatry. 2004;19:510-13.
7. Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15:49-57.
8. Jaffe JH, Jaffe AB. Neurobiology of opioids. In: Galanter M, Kleber HD, eds. Textbook of substance abuse treatment.. 3rd ed. Washington, DC: American Psychiatric Publishing; 2004:17-30.
9. Jones HE. Practical considerations for the clinical use of buprenorphine. NIDA Sci Pract Perspectives. 2004;2:4-20.
10. Geppert CM, Toney GB, Siracusano D, Thorius M. Outpatient buprenorphine treatment for opioid dependence. Fed Practitioner. 2005;22:9-40.
11. Mague SD, Pliakas AM, Todtenkopf MS, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323-30.
12. Van Geffen EC, Hugtenburg JG, Heerdink ER, et al. Discontinuation symptoms in users of selective serotonin reuptake inhibitors in clinical practice: tapering versus abrupt continuation. Eur J Clin Pharmacol. 2005;61:303-7.
1. Nunes EV, Sullivan MA, Levin FR. Treatment of depression in patients with opiate dependence. Biol Psychiatry 2004;56:793-802.
2. Graham AW, Schultz TK, Mayo-Smith MF, et al, eds. Principles of addiction medicine. 3rd ed. Chevy Chase, MD: American Society of Addiction Medicine; 2003.
3. Gold MS, Pottash AL, Extein I, et al. Evidence for an endorphin dysfunction in methadone addicts: lack of ACTH response to naloxone. Drug Alcohol Depend 1981;8:257-62.
4. Carnes PJ, Murray RE, Charpentier L. Addiction interaction disorder. In: Coombs RH, ed. Handbook of addictive disorders: a practical guide to diagnosis and treatment. Hoboken, NJ: John Wiley & Sons 2004:31-59.
5. Kosten TR, Morgan C, Kosten TA. Depressive symptoms during buprenorphine treatment of opioid abusers. J Subst Abuse Treat. 1990;7:51-4.
6. Dean AJ, Bell J, Christie MJ, Mattick RP. Depressive symptoms during buprenorphine vs. methadone maintenance: findings from a randomized, controlled trial in opioid dependence. Eur Psychiatry. 2004;19:510-13.
7. Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15:49-57.
8. Jaffe JH, Jaffe AB. Neurobiology of opioids. In: Galanter M, Kleber HD, eds. Textbook of substance abuse treatment.. 3rd ed. Washington, DC: American Psychiatric Publishing; 2004:17-30.
9. Jones HE. Practical considerations for the clinical use of buprenorphine. NIDA Sci Pract Perspectives. 2004;2:4-20.
10. Geppert CM, Toney GB, Siracusano D, Thorius M. Outpatient buprenorphine treatment for opioid dependence. Fed Practitioner. 2005;22:9-40.
11. Mague SD, Pliakas AM, Todtenkopf MS, et al. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323-30.
12. Van Geffen EC, Hugtenburg JG, Heerdink ER, et al. Discontinuation symptoms in users of selective serotonin reuptake inhibitors in clinical practice: tapering versus abrupt continuation. Eur J Clin Pharmacol. 2005;61:303-7.
Conquering his fears, one step at a time
CASE: The big freeze
Mr. Q, age 34, is afraid to cross the street. As he steps off the curb, his legs “cramp up.” As the cramping intensifies and his feet stiffen, his heart races, he begins to sweat, and he turns back for fear his legs will buckle in the street. While on the sidewalk, he stays within reach of a building or car in case he falls.
Six months before presentation, Mr. Q walked to church during a blizzard, only to find the church closed because of the storm. He returned home and shoveled snow for 1 hour, during which he repeatedly leaned forward and backward to dump the snow.
The following Sunday, Mr. Q’s legs started to “hurt” as he crossed the street. Thinking he had severely injured himself while shoveling, he began to fear street crossings. At work, he asked coworkers to help him cross over to the subway. By spring, he had become so humiliated by his dependence that he stopped working. His phobia intensified until he presented to us at his family’s urging.
During evaluation, Mr. Q says he can cross only side streets and holds on to his father while crossing. His father, who is retired, spends much of his day helping his son get around.
Complete physical exam by Mr. Q’s primary care physician reveals a possible pulled muscle in his right leg but no other medical problems. Neurologic exam results are normal, ruling out nerve damage.
Later in the evaluation, Mr. Q mentions that at age 10 he was struck by a car. The impact fractured the left side of his skull and left leg, and he temporarily lost consciousness.
Shortly after the accident, Mr. Q developed mild memory and concentration impairments and a moderate stutter. He also experienced nightmares, but they disappeared within days. He says he never received speech therapy or other psychiatric treatment because his family did not have medical insurance.
Mr. Q did not lose function after the accident, but in college his stuttering led to difficulty speaking in class and interacting socially. He suffered panic attacks while on the telephone or during job interviews. He now mostly stays home, where he lives with his parents and a nephew. He interacts only with family members.
During the evaluation, Mr. Q effortlessly walks around the therapist’s office and reports no trouble walking at home. He says the cramps almost never surface at home because he feels “calm” with walls close by. When trying to cross the street, he manages to turn back without falling despite the cramps.
Upon considering this conflict, Mr. Q seems to realize that his fear of street crossings protects him from social situations. His stuttering, however, confounds the evaluation because he has trouble communicating his symptoms.
Mr. Q’s affect is constricted as he describes his anxiety and fear. He says he feels limited and at times depressed by his inability to cross streets, yet shows little dysphoric affect or mourning and seems unusually calm when discussing the problem. He appears relaxed knowing that he can keep avoiding social situations.
The authors’ observations
Mr. Q’s history of fearing interviews and telephone conversations suggests social anxiety, and his fear and avoidance of street crossings suggest a specific phobia. Panic disorder with agoraphobia is not present because the patient never experienced spontaneous panic attacks.
Anxiety is more prevalent among persons who stutter than in fl uent speakers.1,2
Persons who stutter:
- more commonly report speech anxiety3
- are significantly more uneasy in social situations and tend to avoid them4,5
- might not be motivated to eliminate barriers that thwart social interaction.
Worse, his stuttering makes it difficult to ascertain his symptoms or plan treatment because it takes him so long to finish a sentence.
EVALUATION: Flashing back
Later in the evaluation, Mr. Q says that whenever he considers or tries crossing a street, he recalls his childhood vehicular injury and fears he will be struck again. He has nightmares of being run over, and these nightmares and flashbacks have been occurring twice weekly since the snowstorm.
During the mental status examination, Mr. Q is well related with fair to poor eye contact, probably because of his stutter; he looks away from the speaker when his stuttering intensifies. His nightmares and flashbacks suggest comorbid posttraumatic stress disorder (PTSD), although he has no persistent symptoms of increased arousal. He also shows no evidence of acute mood disorder, psychosis, or cognitive disturbance.
The authors’ observations
PTSD symptoms can develop months to years after a precipitating incident,6,7 and repeated trauma can make patients more susceptible.
Interestingly, Mr. Q briefly suffered PTSD symptoms after the childhood accident but had no full-blown symptoms until adulthood. In addition to triggering avoidance behaviors, the muscle pull apparently reignited long-dormant PTSD symptoms (fl ashbacks, nightmares).
Mr. Q suffered no other PTSD symptoms. His stuttering might have signaled a psychogenic anxiety disorder, rather than being an incidental finding that developed after acute brain trauma at age 10.
Stuttering also might have contained Mr. Q’s PTSD symptoms for 24 years, until his snow-shoveling injury shattered that containment. Further, while shoveling in the street amid slippery conditions, he might have subconsciously feared he would have trouble eluding an oncoming vehicle.
The authors’ observations
We must address Mr. Q’s stuttering, phobia, and PTSD simultaneously to restore function. If we were to target his street-crossing phobia alone, we would face considerable resistance while exposing the underlying social phobia.
Supportive psychotherapy and exposure therapy—which would involve taking Mr. Q to an intersection and guiding him across—could help him overcome his fear of being run over. Cognitive-behavioral therapy (CBT) alone or with medications also could help.8,9
Mr. Q’s anxiety, however, is severe enough to keep him from trying exposure therapy. Because staying home is his shield from social contact, he is not motivated to leave his apartment. Although he presented voluntarily, like many patients he is ambivalent toward exposure therapy.
Also, Mr. Q’s stutter makes it difficult to engage him in conversation. His stuttering is so severe that we have trouble doing an adequate CBT case formulation. At times his speech is almost incomprehensible.
Improving Mr. Q’s speech is crucial to completing an assessment, decreasing his social anxiety, and motivating him to conquer his fear of crossing streets. By addressing his stuttering and phobia simultaneously, we can treat his anxiety on 2 fronts:
- the stuttering that stemmed from his car accident at age 10
- the street-crossing phobia that developed after he pulled a leg muscle as an adult.
Week 1—After much coaxing and encouragement, Mr. Q works through a leg cramp and takes 1 step off the curb, first with the therapist and then alone.
Week 2—Mr. Q takes 2 steps into the street—first with the therapist and then alone—after repeated coaxing and despite leg cramping.
Month 1—Patient proceeds 4 steps into the street unaccompanied. When his legs cramp, he intensifies the cramp and releases, then says ‘I can do this.’
Month 2—Patient walks 6 steps into the street, first with the therapist, then alone.
Month 3—Mr. Q walks 8 steps into the street, first with the therapist, then alone.
Month 4—Patient begins crossing 1-way streets alone. After the therapist guides him to the center of a 2-way street, he walks the rest of the way by himself.
Month 5—Patient crosses a 2-way street unassisted.
Month 6—Mr. Q crosses busy intersections near his church, where the cramping began.
TREATMENT: 5-step approach
Negative medical results convince Mr. Q that anxiety is holding him back. This allows us to target his anxiety with CBT, in vivo exposure, deep breathing/relaxation, speech therapy, and pharmacotherapy, all of which we start immediately.
As part of Mr. Q’s psychoeducation, we reiterate his negative physical examination results and point out that his childhood vehicular injuries might be perpetuating his fears. We work on getting him to recognize that leg tightness does not predict falling and getting hit by a car.
- Medications, which help him ‘feel calm’
- Relaxation breathing
- Saying ‘I can do it. I feel calm’ when legs cramp up in the street
- Soaking legs in warm water for 10 minutes twice daily
- Progressive muscle relaxation
- Cognitive intervention: internalizing that anxiety—not a medical problem —is holding him back
- Self empowerment exercise: further cramping his legs, then releasing them when they cramp up
When his legs cramp up while trying to cross, we have him say out loud, “I can do it. I feel calm;” this helps him proceed across the street. We also teach self-empowerment by having him purposely cramp up his legs, then release them to stop the cramping.
Deep breathing/relaxation. We teach Mr. Q progressive muscle relaxation and slow rhythmic breathing exercises, which he does before crossing streets to reduce his anxiety. For homework, he practices these exercises and soaks his legs in warm water for 10 minutes twice daily to relax his muscles and prevent cramping.
Speech therapy. The primary therapist devotes 20 minutes of each session to speech therapy. She employs relaxation training and therapy techniques such as Easy Onset,10 in which the patient stretches each sound, syllable, or word for up to 2 seconds, allowing him to speak at a smooth, slow rate. Mr. Q also practices these speech exercises at home.
After 6 weeks, Mr. Q’s stutter improves slightly but he still has trouble communicating. We refer him to a consulting speech therapist, who sees him twice weekly and leads Easy Onset and relaxation exercises. This gives us more time for supportive psychotherapy.
As his speech becomes more fluent, Mr. Q’s social anxiety and fear of street crossings decreases.
Pharmacotherapy. We instruct Mr. Q to take paroxetine, 20 mg/d, and clonazepam, 0.25 mg bid, 30 minutes before in vivo work to manage his anxiety. We titrate clonazepam to 0.5 mg bid over 1 month. He responds well to this regimen but fears he will become dependent on it.
During therapy, Mr. Q and the therapist rank the above interventions from most to least therapeutic (Box 2) so that we can effectively treat him should he relapse.
The authors’ observations
Although Mr. Q’s case is unusual, we feel our diagnostic and treatment methods can be applied to similar cases. His stutter, however, prevented us from conducting a structured diagnostic interview—which would have uncovered his symptoms more quickly—or performing standard manualized therapy.
Some data11 suggest that combination psychotropics and relaxation therapy can compromise long-term exposure therapy outcomes, as the patient’s fear could return once medication is stopped. Mr. Q’s anxiety was crippling, however, and had to be addressed before we could consider exposure therapy.
More research is needed on overcoming patient communication barriers that can hamper treatment. Rapport with patients often makes or breaks psychiatric treatment, and communication problems can prevent that connection. As clinicians, we must watch for linguistic, cognitive, and cultural impediments to treatment.
FOLLOW-UP: ‘I can cross’
Six months after presentation, Mr. Q crosses all types of streets—from 1-way streets to 6-lane intersections—with minimal anxiety. He has resumed his previous level of functioning and is searching for work. His stutter, though greatly improved, is still audible.
We see Mr. Q monthly. We stop paroxetine after 8 months but continue clonazepam to address his many underlying social anxieties. By November—approximately 1½ years after presentation—we have reduced clonazepam to 0.5 mg each morning. We try reducing the morning dose to 0.25 mg, but Mr. Q’s debilitating anxiety resurfaces.
In December, we increase clonazepam to 0.5 mg bid, then reduce it to 0.5 mg each morning 2 months later. In April, we cut clonazepam to 0.25 mg each morning. So far, Mr. Q is functioning well.
The authors’ observations
Patients who begin antistuttering intervention as adults have a poorer speech improvement prognosis than those who start speech therapy in childhood.12 In leaving his stuttering untreated for 24 years, Mr. Q likely sacrificed quality of life. Speech intervention at an earlier age might have improved his speech and prognosis early on.
Related resources
- Anxiety Disorders Association of America. www.adaa.org.
- Beck AT, Emery G, Greenberg RL. Anxiety disorders and phobias: a cognitive perspective. New York: Basic Books; 1985.
- Leahy RL, Holland SJ. Treatment plans and interventions for depression and anxiety disorders. New York: Guilford Press; 2000.
- Clonazepam • Klonopin
- Paroxetine • Paxil
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Michael Garret, MD, for his assistance in preparing this article.
1. Ezrati-Vinacour R, Levin I. The relationship between anxiety and stuttering: a multidimensional approach. J Fluency Disord 2004;29:135-48.
2. Craig A, Hancock K, Tran Y, Craig M. Anxiety levels in people who stutter: a randomized population study. J Speech Lang Hearing Res 2003;46:1197-206.
3. Cabel RM, Colcord RD, Petrosino L. Self-reported anxiety of adults who do and do not stutter. Perceptual Motor Skills 2002;94:775-84.
4. Kraaimaat FW, Vanryckeghem M, Van Dam-Baggen R. Stuttering and social anxiety. J Fluency Disord 2002;27:319-31.
5. Stein MB, Baird A, Walker JR. Social phobia in adults with stuttering. Am J Psychiatry 1996;153:278-80.
6. Carty J, O’Donnell ML, Creamer M. Delayed-onset PTSD: a prospective study of injury survivors. J Affect Disord 2006;90:257-61.
7. Schnurr PP, Lunney CA, Sengupta A, Waelde LC. A descriptive analysis of PTSD chronicity in Vietnam veterans. J Trauma Stress 2003;6:545-53.
8. Beck AT. Cognitive therapy and the emotional disorders. New York: International University Press; 1976.
9. Beck AT, Emery G, Greenberg RL. Anxiety disorders and phobias: a cognitive perspective. New York: Basic Books; 1985.
10. Hood SB ed. Stuttering words, 3rd ed.. Memphis, TN: Stuttering Foundation of America; 1999.
11. Otto MW, Smits JA, Reese HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. J Clin Psychiatry 2004;65(suppl 5):34-41.
12. Curlee RF, Nielson M, Andrews G. Stuttering and related disorders of fluency. New York: Thieme Medical Publishers; 1993.
Dr. Stein is a fourth-year psychiatric resident, Dr. Friedman is professor of clinical psychiatry, and Dr. Elmouchtari is assistant professor of psychiatry, department of psychiatry, State University of New York Downstate Medical Center, Brooklyn.
CASE: The big freeze
Mr. Q, age 34, is afraid to cross the street. As he steps off the curb, his legs “cramp up.” As the cramping intensifies and his feet stiffen, his heart races, he begins to sweat, and he turns back for fear his legs will buckle in the street. While on the sidewalk, he stays within reach of a building or car in case he falls.
Six months before presentation, Mr. Q walked to church during a blizzard, only to find the church closed because of the storm. He returned home and shoveled snow for 1 hour, during which he repeatedly leaned forward and backward to dump the snow.
The following Sunday, Mr. Q’s legs started to “hurt” as he crossed the street. Thinking he had severely injured himself while shoveling, he began to fear street crossings. At work, he asked coworkers to help him cross over to the subway. By spring, he had become so humiliated by his dependence that he stopped working. His phobia intensified until he presented to us at his family’s urging.
During evaluation, Mr. Q says he can cross only side streets and holds on to his father while crossing. His father, who is retired, spends much of his day helping his son get around.
Complete physical exam by Mr. Q’s primary care physician reveals a possible pulled muscle in his right leg but no other medical problems. Neurologic exam results are normal, ruling out nerve damage.
Later in the evaluation, Mr. Q mentions that at age 10 he was struck by a car. The impact fractured the left side of his skull and left leg, and he temporarily lost consciousness.
Shortly after the accident, Mr. Q developed mild memory and concentration impairments and a moderate stutter. He also experienced nightmares, but they disappeared within days. He says he never received speech therapy or other psychiatric treatment because his family did not have medical insurance.
Mr. Q did not lose function after the accident, but in college his stuttering led to difficulty speaking in class and interacting socially. He suffered panic attacks while on the telephone or during job interviews. He now mostly stays home, where he lives with his parents and a nephew. He interacts only with family members.
During the evaluation, Mr. Q effortlessly walks around the therapist’s office and reports no trouble walking at home. He says the cramps almost never surface at home because he feels “calm” with walls close by. When trying to cross the street, he manages to turn back without falling despite the cramps.
Upon considering this conflict, Mr. Q seems to realize that his fear of street crossings protects him from social situations. His stuttering, however, confounds the evaluation because he has trouble communicating his symptoms.
Mr. Q’s affect is constricted as he describes his anxiety and fear. He says he feels limited and at times depressed by his inability to cross streets, yet shows little dysphoric affect or mourning and seems unusually calm when discussing the problem. He appears relaxed knowing that he can keep avoiding social situations.
The authors’ observations
Mr. Q’s history of fearing interviews and telephone conversations suggests social anxiety, and his fear and avoidance of street crossings suggest a specific phobia. Panic disorder with agoraphobia is not present because the patient never experienced spontaneous panic attacks.
Anxiety is more prevalent among persons who stutter than in fl uent speakers.1,2
Persons who stutter:
- more commonly report speech anxiety3
- are significantly more uneasy in social situations and tend to avoid them4,5
- might not be motivated to eliminate barriers that thwart social interaction.
Worse, his stuttering makes it difficult to ascertain his symptoms or plan treatment because it takes him so long to finish a sentence.
EVALUATION: Flashing back
Later in the evaluation, Mr. Q says that whenever he considers or tries crossing a street, he recalls his childhood vehicular injury and fears he will be struck again. He has nightmares of being run over, and these nightmares and flashbacks have been occurring twice weekly since the snowstorm.
During the mental status examination, Mr. Q is well related with fair to poor eye contact, probably because of his stutter; he looks away from the speaker when his stuttering intensifies. His nightmares and flashbacks suggest comorbid posttraumatic stress disorder (PTSD), although he has no persistent symptoms of increased arousal. He also shows no evidence of acute mood disorder, psychosis, or cognitive disturbance.
The authors’ observations
PTSD symptoms can develop months to years after a precipitating incident,6,7 and repeated trauma can make patients more susceptible.
Interestingly, Mr. Q briefly suffered PTSD symptoms after the childhood accident but had no full-blown symptoms until adulthood. In addition to triggering avoidance behaviors, the muscle pull apparently reignited long-dormant PTSD symptoms (fl ashbacks, nightmares).
Mr. Q suffered no other PTSD symptoms. His stuttering might have signaled a psychogenic anxiety disorder, rather than being an incidental finding that developed after acute brain trauma at age 10.
Stuttering also might have contained Mr. Q’s PTSD symptoms for 24 years, until his snow-shoveling injury shattered that containment. Further, while shoveling in the street amid slippery conditions, he might have subconsciously feared he would have trouble eluding an oncoming vehicle.
The authors’ observations
We must address Mr. Q’s stuttering, phobia, and PTSD simultaneously to restore function. If we were to target his street-crossing phobia alone, we would face considerable resistance while exposing the underlying social phobia.
Supportive psychotherapy and exposure therapy—which would involve taking Mr. Q to an intersection and guiding him across—could help him overcome his fear of being run over. Cognitive-behavioral therapy (CBT) alone or with medications also could help.8,9
Mr. Q’s anxiety, however, is severe enough to keep him from trying exposure therapy. Because staying home is his shield from social contact, he is not motivated to leave his apartment. Although he presented voluntarily, like many patients he is ambivalent toward exposure therapy.
Also, Mr. Q’s stutter makes it difficult to engage him in conversation. His stuttering is so severe that we have trouble doing an adequate CBT case formulation. At times his speech is almost incomprehensible.
Improving Mr. Q’s speech is crucial to completing an assessment, decreasing his social anxiety, and motivating him to conquer his fear of crossing streets. By addressing his stuttering and phobia simultaneously, we can treat his anxiety on 2 fronts:
- the stuttering that stemmed from his car accident at age 10
- the street-crossing phobia that developed after he pulled a leg muscle as an adult.
Week 1—After much coaxing and encouragement, Mr. Q works through a leg cramp and takes 1 step off the curb, first with the therapist and then alone.
Week 2—Mr. Q takes 2 steps into the street—first with the therapist and then alone—after repeated coaxing and despite leg cramping.
Month 1—Patient proceeds 4 steps into the street unaccompanied. When his legs cramp, he intensifies the cramp and releases, then says ‘I can do this.’
Month 2—Patient walks 6 steps into the street, first with the therapist, then alone.
Month 3—Mr. Q walks 8 steps into the street, first with the therapist, then alone.
Month 4—Patient begins crossing 1-way streets alone. After the therapist guides him to the center of a 2-way street, he walks the rest of the way by himself.
Month 5—Patient crosses a 2-way street unassisted.
Month 6—Mr. Q crosses busy intersections near his church, where the cramping began.
TREATMENT: 5-step approach
Negative medical results convince Mr. Q that anxiety is holding him back. This allows us to target his anxiety with CBT, in vivo exposure, deep breathing/relaxation, speech therapy, and pharmacotherapy, all of which we start immediately.
As part of Mr. Q’s psychoeducation, we reiterate his negative physical examination results and point out that his childhood vehicular injuries might be perpetuating his fears. We work on getting him to recognize that leg tightness does not predict falling and getting hit by a car.
- Medications, which help him ‘feel calm’
- Relaxation breathing
- Saying ‘I can do it. I feel calm’ when legs cramp up in the street
- Soaking legs in warm water for 10 minutes twice daily
- Progressive muscle relaxation
- Cognitive intervention: internalizing that anxiety—not a medical problem —is holding him back
- Self empowerment exercise: further cramping his legs, then releasing them when they cramp up
When his legs cramp up while trying to cross, we have him say out loud, “I can do it. I feel calm;” this helps him proceed across the street. We also teach self-empowerment by having him purposely cramp up his legs, then release them to stop the cramping.
Deep breathing/relaxation. We teach Mr. Q progressive muscle relaxation and slow rhythmic breathing exercises, which he does before crossing streets to reduce his anxiety. For homework, he practices these exercises and soaks his legs in warm water for 10 minutes twice daily to relax his muscles and prevent cramping.
Speech therapy. The primary therapist devotes 20 minutes of each session to speech therapy. She employs relaxation training and therapy techniques such as Easy Onset,10 in which the patient stretches each sound, syllable, or word for up to 2 seconds, allowing him to speak at a smooth, slow rate. Mr. Q also practices these speech exercises at home.
After 6 weeks, Mr. Q’s stutter improves slightly but he still has trouble communicating. We refer him to a consulting speech therapist, who sees him twice weekly and leads Easy Onset and relaxation exercises. This gives us more time for supportive psychotherapy.
As his speech becomes more fluent, Mr. Q’s social anxiety and fear of street crossings decreases.
Pharmacotherapy. We instruct Mr. Q to take paroxetine, 20 mg/d, and clonazepam, 0.25 mg bid, 30 minutes before in vivo work to manage his anxiety. We titrate clonazepam to 0.5 mg bid over 1 month. He responds well to this regimen but fears he will become dependent on it.
During therapy, Mr. Q and the therapist rank the above interventions from most to least therapeutic (Box 2) so that we can effectively treat him should he relapse.
The authors’ observations
Although Mr. Q’s case is unusual, we feel our diagnostic and treatment methods can be applied to similar cases. His stutter, however, prevented us from conducting a structured diagnostic interview—which would have uncovered his symptoms more quickly—or performing standard manualized therapy.
Some data11 suggest that combination psychotropics and relaxation therapy can compromise long-term exposure therapy outcomes, as the patient’s fear could return once medication is stopped. Mr. Q’s anxiety was crippling, however, and had to be addressed before we could consider exposure therapy.
More research is needed on overcoming patient communication barriers that can hamper treatment. Rapport with patients often makes or breaks psychiatric treatment, and communication problems can prevent that connection. As clinicians, we must watch for linguistic, cognitive, and cultural impediments to treatment.
FOLLOW-UP: ‘I can cross’
Six months after presentation, Mr. Q crosses all types of streets—from 1-way streets to 6-lane intersections—with minimal anxiety. He has resumed his previous level of functioning and is searching for work. His stutter, though greatly improved, is still audible.
We see Mr. Q monthly. We stop paroxetine after 8 months but continue clonazepam to address his many underlying social anxieties. By November—approximately 1½ years after presentation—we have reduced clonazepam to 0.5 mg each morning. We try reducing the morning dose to 0.25 mg, but Mr. Q’s debilitating anxiety resurfaces.
In December, we increase clonazepam to 0.5 mg bid, then reduce it to 0.5 mg each morning 2 months later. In April, we cut clonazepam to 0.25 mg each morning. So far, Mr. Q is functioning well.
The authors’ observations
Patients who begin antistuttering intervention as adults have a poorer speech improvement prognosis than those who start speech therapy in childhood.12 In leaving his stuttering untreated for 24 years, Mr. Q likely sacrificed quality of life. Speech intervention at an earlier age might have improved his speech and prognosis early on.
Related resources
- Anxiety Disorders Association of America. www.adaa.org.
- Beck AT, Emery G, Greenberg RL. Anxiety disorders and phobias: a cognitive perspective. New York: Basic Books; 1985.
- Leahy RL, Holland SJ. Treatment plans and interventions for depression and anxiety disorders. New York: Guilford Press; 2000.
- Clonazepam • Klonopin
- Paroxetine • Paxil
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Michael Garret, MD, for his assistance in preparing this article.
CASE: The big freeze
Mr. Q, age 34, is afraid to cross the street. As he steps off the curb, his legs “cramp up.” As the cramping intensifies and his feet stiffen, his heart races, he begins to sweat, and he turns back for fear his legs will buckle in the street. While on the sidewalk, he stays within reach of a building or car in case he falls.
Six months before presentation, Mr. Q walked to church during a blizzard, only to find the church closed because of the storm. He returned home and shoveled snow for 1 hour, during which he repeatedly leaned forward and backward to dump the snow.
The following Sunday, Mr. Q’s legs started to “hurt” as he crossed the street. Thinking he had severely injured himself while shoveling, he began to fear street crossings. At work, he asked coworkers to help him cross over to the subway. By spring, he had become so humiliated by his dependence that he stopped working. His phobia intensified until he presented to us at his family’s urging.
During evaluation, Mr. Q says he can cross only side streets and holds on to his father while crossing. His father, who is retired, spends much of his day helping his son get around.
Complete physical exam by Mr. Q’s primary care physician reveals a possible pulled muscle in his right leg but no other medical problems. Neurologic exam results are normal, ruling out nerve damage.
Later in the evaluation, Mr. Q mentions that at age 10 he was struck by a car. The impact fractured the left side of his skull and left leg, and he temporarily lost consciousness.
Shortly after the accident, Mr. Q developed mild memory and concentration impairments and a moderate stutter. He also experienced nightmares, but they disappeared within days. He says he never received speech therapy or other psychiatric treatment because his family did not have medical insurance.
Mr. Q did not lose function after the accident, but in college his stuttering led to difficulty speaking in class and interacting socially. He suffered panic attacks while on the telephone or during job interviews. He now mostly stays home, where he lives with his parents and a nephew. He interacts only with family members.
During the evaluation, Mr. Q effortlessly walks around the therapist’s office and reports no trouble walking at home. He says the cramps almost never surface at home because he feels “calm” with walls close by. When trying to cross the street, he manages to turn back without falling despite the cramps.
Upon considering this conflict, Mr. Q seems to realize that his fear of street crossings protects him from social situations. His stuttering, however, confounds the evaluation because he has trouble communicating his symptoms.
Mr. Q’s affect is constricted as he describes his anxiety and fear. He says he feels limited and at times depressed by his inability to cross streets, yet shows little dysphoric affect or mourning and seems unusually calm when discussing the problem. He appears relaxed knowing that he can keep avoiding social situations.
The authors’ observations
Mr. Q’s history of fearing interviews and telephone conversations suggests social anxiety, and his fear and avoidance of street crossings suggest a specific phobia. Panic disorder with agoraphobia is not present because the patient never experienced spontaneous panic attacks.
Anxiety is more prevalent among persons who stutter than in fl uent speakers.1,2
Persons who stutter:
- more commonly report speech anxiety3
- are significantly more uneasy in social situations and tend to avoid them4,5
- might not be motivated to eliminate barriers that thwart social interaction.
Worse, his stuttering makes it difficult to ascertain his symptoms or plan treatment because it takes him so long to finish a sentence.
EVALUATION: Flashing back
Later in the evaluation, Mr. Q says that whenever he considers or tries crossing a street, he recalls his childhood vehicular injury and fears he will be struck again. He has nightmares of being run over, and these nightmares and flashbacks have been occurring twice weekly since the snowstorm.
During the mental status examination, Mr. Q is well related with fair to poor eye contact, probably because of his stutter; he looks away from the speaker when his stuttering intensifies. His nightmares and flashbacks suggest comorbid posttraumatic stress disorder (PTSD), although he has no persistent symptoms of increased arousal. He also shows no evidence of acute mood disorder, psychosis, or cognitive disturbance.
The authors’ observations
PTSD symptoms can develop months to years after a precipitating incident,6,7 and repeated trauma can make patients more susceptible.
Interestingly, Mr. Q briefly suffered PTSD symptoms after the childhood accident but had no full-blown symptoms until adulthood. In addition to triggering avoidance behaviors, the muscle pull apparently reignited long-dormant PTSD symptoms (fl ashbacks, nightmares).
Mr. Q suffered no other PTSD symptoms. His stuttering might have signaled a psychogenic anxiety disorder, rather than being an incidental finding that developed after acute brain trauma at age 10.
Stuttering also might have contained Mr. Q’s PTSD symptoms for 24 years, until his snow-shoveling injury shattered that containment. Further, while shoveling in the street amid slippery conditions, he might have subconsciously feared he would have trouble eluding an oncoming vehicle.
The authors’ observations
We must address Mr. Q’s stuttering, phobia, and PTSD simultaneously to restore function. If we were to target his street-crossing phobia alone, we would face considerable resistance while exposing the underlying social phobia.
Supportive psychotherapy and exposure therapy—which would involve taking Mr. Q to an intersection and guiding him across—could help him overcome his fear of being run over. Cognitive-behavioral therapy (CBT) alone or with medications also could help.8,9
Mr. Q’s anxiety, however, is severe enough to keep him from trying exposure therapy. Because staying home is his shield from social contact, he is not motivated to leave his apartment. Although he presented voluntarily, like many patients he is ambivalent toward exposure therapy.
Also, Mr. Q’s stutter makes it difficult to engage him in conversation. His stuttering is so severe that we have trouble doing an adequate CBT case formulation. At times his speech is almost incomprehensible.
Improving Mr. Q’s speech is crucial to completing an assessment, decreasing his social anxiety, and motivating him to conquer his fear of crossing streets. By addressing his stuttering and phobia simultaneously, we can treat his anxiety on 2 fronts:
- the stuttering that stemmed from his car accident at age 10
- the street-crossing phobia that developed after he pulled a leg muscle as an adult.
Week 1—After much coaxing and encouragement, Mr. Q works through a leg cramp and takes 1 step off the curb, first with the therapist and then alone.
Week 2—Mr. Q takes 2 steps into the street—first with the therapist and then alone—after repeated coaxing and despite leg cramping.
Month 1—Patient proceeds 4 steps into the street unaccompanied. When his legs cramp, he intensifies the cramp and releases, then says ‘I can do this.’
Month 2—Patient walks 6 steps into the street, first with the therapist, then alone.
Month 3—Mr. Q walks 8 steps into the street, first with the therapist, then alone.
Month 4—Patient begins crossing 1-way streets alone. After the therapist guides him to the center of a 2-way street, he walks the rest of the way by himself.
Month 5—Patient crosses a 2-way street unassisted.
Month 6—Mr. Q crosses busy intersections near his church, where the cramping began.
TREATMENT: 5-step approach
Negative medical results convince Mr. Q that anxiety is holding him back. This allows us to target his anxiety with CBT, in vivo exposure, deep breathing/relaxation, speech therapy, and pharmacotherapy, all of which we start immediately.
As part of Mr. Q’s psychoeducation, we reiterate his negative physical examination results and point out that his childhood vehicular injuries might be perpetuating his fears. We work on getting him to recognize that leg tightness does not predict falling and getting hit by a car.
- Medications, which help him ‘feel calm’
- Relaxation breathing
- Saying ‘I can do it. I feel calm’ when legs cramp up in the street
- Soaking legs in warm water for 10 minutes twice daily
- Progressive muscle relaxation
- Cognitive intervention: internalizing that anxiety—not a medical problem —is holding him back
- Self empowerment exercise: further cramping his legs, then releasing them when they cramp up
When his legs cramp up while trying to cross, we have him say out loud, “I can do it. I feel calm;” this helps him proceed across the street. We also teach self-empowerment by having him purposely cramp up his legs, then release them to stop the cramping.
Deep breathing/relaxation. We teach Mr. Q progressive muscle relaxation and slow rhythmic breathing exercises, which he does before crossing streets to reduce his anxiety. For homework, he practices these exercises and soaks his legs in warm water for 10 minutes twice daily to relax his muscles and prevent cramping.
Speech therapy. The primary therapist devotes 20 minutes of each session to speech therapy. She employs relaxation training and therapy techniques such as Easy Onset,10 in which the patient stretches each sound, syllable, or word for up to 2 seconds, allowing him to speak at a smooth, slow rate. Mr. Q also practices these speech exercises at home.
After 6 weeks, Mr. Q’s stutter improves slightly but he still has trouble communicating. We refer him to a consulting speech therapist, who sees him twice weekly and leads Easy Onset and relaxation exercises. This gives us more time for supportive psychotherapy.
As his speech becomes more fluent, Mr. Q’s social anxiety and fear of street crossings decreases.
Pharmacotherapy. We instruct Mr. Q to take paroxetine, 20 mg/d, and clonazepam, 0.25 mg bid, 30 minutes before in vivo work to manage his anxiety. We titrate clonazepam to 0.5 mg bid over 1 month. He responds well to this regimen but fears he will become dependent on it.
During therapy, Mr. Q and the therapist rank the above interventions from most to least therapeutic (Box 2) so that we can effectively treat him should he relapse.
The authors’ observations
Although Mr. Q’s case is unusual, we feel our diagnostic and treatment methods can be applied to similar cases. His stutter, however, prevented us from conducting a structured diagnostic interview—which would have uncovered his symptoms more quickly—or performing standard manualized therapy.
Some data11 suggest that combination psychotropics and relaxation therapy can compromise long-term exposure therapy outcomes, as the patient’s fear could return once medication is stopped. Mr. Q’s anxiety was crippling, however, and had to be addressed before we could consider exposure therapy.
More research is needed on overcoming patient communication barriers that can hamper treatment. Rapport with patients often makes or breaks psychiatric treatment, and communication problems can prevent that connection. As clinicians, we must watch for linguistic, cognitive, and cultural impediments to treatment.
FOLLOW-UP: ‘I can cross’
Six months after presentation, Mr. Q crosses all types of streets—from 1-way streets to 6-lane intersections—with minimal anxiety. He has resumed his previous level of functioning and is searching for work. His stutter, though greatly improved, is still audible.
We see Mr. Q monthly. We stop paroxetine after 8 months but continue clonazepam to address his many underlying social anxieties. By November—approximately 1½ years after presentation—we have reduced clonazepam to 0.5 mg each morning. We try reducing the morning dose to 0.25 mg, but Mr. Q’s debilitating anxiety resurfaces.
In December, we increase clonazepam to 0.5 mg bid, then reduce it to 0.5 mg each morning 2 months later. In April, we cut clonazepam to 0.25 mg each morning. So far, Mr. Q is functioning well.
The authors’ observations
Patients who begin antistuttering intervention as adults have a poorer speech improvement prognosis than those who start speech therapy in childhood.12 In leaving his stuttering untreated for 24 years, Mr. Q likely sacrificed quality of life. Speech intervention at an earlier age might have improved his speech and prognosis early on.
Related resources
- Anxiety Disorders Association of America. www.adaa.org.
- Beck AT, Emery G, Greenberg RL. Anxiety disorders and phobias: a cognitive perspective. New York: Basic Books; 1985.
- Leahy RL, Holland SJ. Treatment plans and interventions for depression and anxiety disorders. New York: Guilford Press; 2000.
- Clonazepam • Klonopin
- Paroxetine • Paxil
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Michael Garret, MD, for his assistance in preparing this article.
1. Ezrati-Vinacour R, Levin I. The relationship between anxiety and stuttering: a multidimensional approach. J Fluency Disord 2004;29:135-48.
2. Craig A, Hancock K, Tran Y, Craig M. Anxiety levels in people who stutter: a randomized population study. J Speech Lang Hearing Res 2003;46:1197-206.
3. Cabel RM, Colcord RD, Petrosino L. Self-reported anxiety of adults who do and do not stutter. Perceptual Motor Skills 2002;94:775-84.
4. Kraaimaat FW, Vanryckeghem M, Van Dam-Baggen R. Stuttering and social anxiety. J Fluency Disord 2002;27:319-31.
5. Stein MB, Baird A, Walker JR. Social phobia in adults with stuttering. Am J Psychiatry 1996;153:278-80.
6. Carty J, O’Donnell ML, Creamer M. Delayed-onset PTSD: a prospective study of injury survivors. J Affect Disord 2006;90:257-61.
7. Schnurr PP, Lunney CA, Sengupta A, Waelde LC. A descriptive analysis of PTSD chronicity in Vietnam veterans. J Trauma Stress 2003;6:545-53.
8. Beck AT. Cognitive therapy and the emotional disorders. New York: International University Press; 1976.
9. Beck AT, Emery G, Greenberg RL. Anxiety disorders and phobias: a cognitive perspective. New York: Basic Books; 1985.
10. Hood SB ed. Stuttering words, 3rd ed.. Memphis, TN: Stuttering Foundation of America; 1999.
11. Otto MW, Smits JA, Reese HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. J Clin Psychiatry 2004;65(suppl 5):34-41.
12. Curlee RF, Nielson M, Andrews G. Stuttering and related disorders of fluency. New York: Thieme Medical Publishers; 1993.
Dr. Stein is a fourth-year psychiatric resident, Dr. Friedman is professor of clinical psychiatry, and Dr. Elmouchtari is assistant professor of psychiatry, department of psychiatry, State University of New York Downstate Medical Center, Brooklyn.
1. Ezrati-Vinacour R, Levin I. The relationship between anxiety and stuttering: a multidimensional approach. J Fluency Disord 2004;29:135-48.
2. Craig A, Hancock K, Tran Y, Craig M. Anxiety levels in people who stutter: a randomized population study. J Speech Lang Hearing Res 2003;46:1197-206.
3. Cabel RM, Colcord RD, Petrosino L. Self-reported anxiety of adults who do and do not stutter. Perceptual Motor Skills 2002;94:775-84.
4. Kraaimaat FW, Vanryckeghem M, Van Dam-Baggen R. Stuttering and social anxiety. J Fluency Disord 2002;27:319-31.
5. Stein MB, Baird A, Walker JR. Social phobia in adults with stuttering. Am J Psychiatry 1996;153:278-80.
6. Carty J, O’Donnell ML, Creamer M. Delayed-onset PTSD: a prospective study of injury survivors. J Affect Disord 2006;90:257-61.
7. Schnurr PP, Lunney CA, Sengupta A, Waelde LC. A descriptive analysis of PTSD chronicity in Vietnam veterans. J Trauma Stress 2003;6:545-53.
8. Beck AT. Cognitive therapy and the emotional disorders. New York: International University Press; 1976.
9. Beck AT, Emery G, Greenberg RL. Anxiety disorders and phobias: a cognitive perspective. New York: Basic Books; 1985.
10. Hood SB ed. Stuttering words, 3rd ed.. Memphis, TN: Stuttering Foundation of America; 1999.
11. Otto MW, Smits JA, Reese HE. Cognitive-behavioral therapy for the treatment of anxiety disorders. J Clin Psychiatry 2004;65(suppl 5):34-41.
12. Curlee RF, Nielson M, Andrews G. Stuttering and related disorders of fluency. New York: Thieme Medical Publishers; 1993.
Dr. Stein is a fourth-year psychiatric resident, Dr. Friedman is professor of clinical psychiatry, and Dr. Elmouchtari is assistant professor of psychiatry, department of psychiatry, State University of New York Downstate Medical Center, Brooklyn.
Getting to the heart of his ‘shocking’ trauma
CASE: ‘Like a sledgehammer’
Mr. J, age 54, is admitted to the cardiac critical care unit after repeated tachycardia episodes over 3 years. He also has depressive symptoms including social isolation, passive suicidal thoughts, lack of interest in sex, weight loss, difficulty sleeping, sadness, and decreased appetite, energy, and ability to concentrate. The psychiatry consult team subsequently evaluates him.
Shortly after retiring as a police officer, Mr. J started having 10-second episodes of loss of consciousness and suffered 30 episodes within 1 year. After diagnosing chronic idiopathic ventricular tachycardia, a cardiologist ablated an aberrant left ventricular pathway and inserted a single-lead implantable cardioverter-defibrillator (ICD). He also prescribed the antiarrhythmic amiodarone, but Mr. J could not tolerate the medication’s side effects.
Mr. J’s tachycardia persisted, and repeated episodes triggered an estimated 13 electrical shocks from the ICD over 5 months. At this point, the cardiologist performed a second ablation, removed the single-lead ICD, and implanted a two-lead ICD, which he hoped would more accurately discern between lethal and nonlethal fast heart rhythms.
In addition, the cardiologist prescribed the antiarrhythmic sotalol—which did not suppress the arrhythmia—before switching to flecainide, 100 mg bid, which did. However, Mr. J still suffered fatigue, exercise intolerance, near-syncope, and chest heaviness.
One week after receiving the first ICD, Mr. J recalls, he felt his first shock while out for a walk. He said the shock lasted 5 to 10 seconds and “felt like somebody took a sledgehammer to my chest.” Another time, he suffered 6 successive shocks that threw him to the ground. Motorists pulled over to assist him, which made him feel ashamed.
Before long, Mr. J became increasingly afraid of repeat discharges. As soon as he began a task, he would feel a “thumping” in the back of his neck and start panicking, fearful that a heart rate increase would trigger another shock.
The stress forced Mr. J to abandon his favorite retirement hobbies—remodeling houses and yard work—and to spend his days lying around watching television. Fearing another discharge in public, he has stopped seeing friends and going to church. He has also stopped driving and depends on his female partner of 14 years for daily visits, grocery shopping, and rides to medical appointments. She feels frustrated by his debility.
The authors’ observations
By delivering electrical shocks when ventricles beat too quickly, an ICD shocks the heart back into a normal rhythm. Based on our observation, Mr. J probably had both anxiety-induced tachycardia and recurrent atrial fibrillation.
Although ICDs have prolonged survival for patients with potentially fatal ventricular arrhythmias,1,2 painful discharges can occur without warning. Patients liken the discharge to an electric shock or to being kicked or punched in the chest.3
Depending on the patient’s activity level, cardiologists routinely program ICDs to discharge at approximately 10 beats per minute above expected heart rates during typical activities. Because ICD leads cannot differentiate between ventricular and supraventricular rhythm disturbances, a rapid supraventricular rhythm might precipitate a discharge intended to treat a more serious ventricular rhythm disturbance.
Frequent ICD discharges could indicate:
- the patient needs a more effective antiarrhythmic
- the device needs to be set at a higher rate to avoid discharge during periods of anxiety/exertion
- or the device is defective.
ICD-induced psychopathology
Depression or tachycardia could have caused Mr. J’s fatigue. Either way, he showed numerous other depressive symptoms.
Fear of implant discharge or malfunction often induces psychiatric disorders, particularly in patients who have experienced discharge. As many as 87% of ICD patients suffer anxiety, depression, or other psychiatric symptoms after implantation,5 and 13% to 38% meet DSM-IV-TR criteria for an anxiety spectrum disorder.6
Multiple psychological theories explain iatrogenic anxiety disorders resulting from ICD firing. Behaviorally, ICD discharge represents an initially unconditioned stimulus that the patient associates with the activity he was engaging in when shocked. The shock discourages the patient from that activity—however benign—for fear it triggered the discharge and could cause future shocks.
ICD recipients often fear the device will malfunction or discharge while they are in public, driving, or operating machinery—leading some to become homebound and cease activities of daily living. The discharge’s unpredictability shatters a patient’s perception of control over his or her life and might induce a learned helplessness7 that can strain relationships, as it did with Mr. J and his partner. The patient also could develop anticipatory anxiety, mistaking benign body symptoms or increasing shock frequency for signs of a potentially fatal heart problem.8
Whether quality of life diminishes as ICD firings become more frequent is uncertain.9 The Canadian Implantable Defibrillator Study (N=317) found greater quality of life improvements with ICD therapy than with amiodarone—200 to 400 mg/d maintenance therapy—but the improvements were lost in patients who experienced ≥5 shocks over 12 months.10 Pauli et al7 found misinterpretation of the reason for increasing shocks to be more emotionally destructive than shock frequency, however.
Detecting ICD maladjustment
Patients with ICD maladjustment typically show anticipatory anxiety and negative cognitive attributions, and many engage in fruitless maneuvers to prevent device firing.5 Nervousness, dizziness, weakness, and fear are common responses to shock by ICD.11
Most patients with new-onset, post-ICD anxiety disorders have no pre-implant psychiatric history.12 Only one trial assessing state and trait anxiety before and after ICD placement reported increased trait anxiety in some patients before implantation.13
HISTORY: Nights in the cornfield
During psychiatric evaluation, Mr. J reveals that his parents physically and emotionally abused him as a child. He says his father frequently beat him with farm tools, and sometimes the beatings were so severe that his parents kept him home from school to prevent teachers from noticing his bruises. He never received medical treatment for his injuries.
For Mr. J, the inescapable threat of painful, unannounced ICD discharges has brought back the anticipatory terror and helplessness of his childhood. Just as he feared his father’s sudden rages, the specter of repeat ICD shocks now haunts him. He says he’d rather have the ICD removed and risk death from tachycardia than live another minute in fear.
The authors’ observations
Mr. J meets DSM-IV-TR criteria for PTSD. He associates ICD discharge with childhood abuse and experiences new-onset flashbacks, hyperarousal, and avoidance behavior.
To our knowledge, ICD shock-induced flashbacks to pre-implant trauma have not been reported, although some data associate ICDs with posttraumatic stress related to heart disease and treatment.14-16 In one case series,14 patients showed:
- cluster B re-experiencing symptoms (cognitive preoccupation with trauma or psychophysiologic reactivity to reminders of the ICD and heart disease)
- cluster C avoidance symptoms (avoiding activities they thought might activate the ICD)
- cluster D hyperarousal symptoms (insomnia, decreased concentration, hypervigilance, and irritability).
The authors’ observations
Treating comorbid anxiety or depression in ICD recipients is critical. A number of psychiatric interventions might alleviate behavioral and psychological effects of body-device interactions.
CBT. In a retrospective study17 of 36 ICD recipients, those who received 9 months of CBT reported decreased depression, anxiety, distress, and sexual problems compared with those who did not. Interestingly, more CBT-group patients (11 of 18) suffered ICD shocks than did controls (6 of 18).
Peer support groups. Out of 58 ICD recipients who answered a post-implant questionnaire, 23 (39%) attended a peer support group.18 Of these, 22 (96%) found the group helpful and were happier, less hostile, and more sociable after participating. Peer group participants also were more likely to return to work than nonparticipants.
How would you handle a patient’s request to deactivate an implantable cardioverter-defibrillator (ICD) or other life-preserving device that is causing debilitating mental anguish? Physicians dealing with such requests will find themselves in an ethical wilderness.
Pinski22 offers guidelines in line with withdrawal of other life-extending technologies in terminally ill patients. “Deactivation of an ICD is appropriate when the device is believed to be prolonging patient suffering,” he writes, adding that preventing ICD shocks induced by frequent or agonal arrhythmias “will not only hasten but also permit a peaceful death.” Disabling the ICD function that responds to bradycardia will prevent agonal pacing and—as a result—shocks.
The literature, however, offers little guidance on responding to patient requests for ICD deactivation and few precedents on which to base such decisions for the terminally ill.
Even less guidance exists when mental illness resulting from ICD complications induces unbearable suffering. The underlying psychiatric condition should be optimally treated before clinicians entertain ICD removal. Mr. J, for example, decided to keep the implant once his crippling anxiety resolved and he was assured that his tachycardia finally was under control.
12 attributes reduction of ICD-induced anxiety to combination individual psychotherapy and unspecified dosages of benzodiazepines. Two patients also received adjunctive fluoxetine or paroxetine, dosages unspecified.
In a double-blind, placebo-controlled crossover study, implantable atrial defibrillator recipients reported decreased pain and anxiety while taking the short-acting benzodiazepine triazolam, 0.375 mg, before patient-activated shock.19
We recommend trying a combination regimen that acts acutely and subacutely. A long-acting benzodiazepine such as clonazepam can calm acute, overwhelming anxiety, and a selective serotonin reuptake inhibitor (SSRI) such as fluoxetine or paroxetine can help manage chronic depressive and generalized anxiety symptoms.
SSRIs are relatively benign but more research on their cardiac safety is needed.20,21 Tricyclic antidepressants, which prolong cardiac conduction, should be avoided.
In addition to psychotropics, concomitant psychotherapy can reduce chronic symptoms.
The authors’ observations
Preparing patients for ICD problems. Anxiety after an ICD shock and the dread of repeat shocks are normal; the goal is to prevent that anxiety from destroying quality of life.
As with Mr. J, many ICD recipients are emotionally unprepared for device-related complications. Most cardiologists do not screen patients for pre-existing anxiety before ICD placement, nor do many adequately address ICD-induced anxiety once the device has been placed.
Psychological screening before implantation can help detect and manage preexisting anxiety disorders. Small-scale evaluations have used anxiety scales to continuously measure anxiety before and after ICD placement.13,23
Increased patient education on how ICDs work can help patients decide whether to proceed with implantation and tolerate discharges should they occur. Psychological screening and brief, routine communication between providers and patients about psychosocial issues can help patients adjust and identify those who need extended psychological services.4
- develop a plan for how a shock would be handled
- perform relaxation exercises immediately after the shock
- resume activities they were involved with when the shock occurred to prevent avoidance.24
TREATMENT: Third attempt
The cardiology team discontinues flecainide and performs a third radioablation, which eradicates ectopic ventricular activity.
Acting on the psychiatry consult team’s advice, Mr. J is transferred to the inpatient mood disorders unit to aggressively treat his PTSD. He undergoes 4 days of intensive CBT designed to explore the connection between his response to the discharges and his father’s abuse. We prescribe clonazepam, 0.5 mg bid, to reduce Mr. J’s agitation and anxiety, and recommend outpatient counseling to help manage his stress—particularly his anxious response to stimuli that remind him of the ICD discharge.
Mr. J is discharged after 12 days in the cardiac and psychiatric units. He has no suicidal thoughts, his sadness has decreased, and his energy, concentration, sleep, and outlook on his future have improved. He also is resolving relationship issues with his partner.
As Mr. J’s anxiety declines and he is increasingly reassured that his arrhythmias are under control, he decides to keep the ICD. His function gradually improves with continued cardiac rehabilitation, although he does not continue psychotherapy.
Related resources
- Stutts LA, Cross NJ, Conti JB, Sears SF. Examination of research trends on patient factors in patients with implantable cardioverter defibrillators. Clin Cardiol 2007;30:64-8.
- Sears SF, Shea JB, Conti JB. How to respond to an implantable cardioverter-defibrillator shock. Circulation 2005;111;380-2. http://circ.ahajournals.org/cgi/reprint/111/23/e380.
- Pauli P, Wiedemann G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76. www.psychosomaticmedicine.org/cgi/reprint/61/1/69.
- Amiodarone • Cordarone
- Clonazepam • Klonopin
- Flecainide • Tambocor
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sotalol • Betapace
- Triazolam • Halcion, others
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Morris PL, Badger J, Chmielewski C, et al. Psychiatric morbidity following implantation of the automatic implantable cardioverter defibrillator. Psychosomatics 1991;32:58-64.
2. Conti JB, Sears SF, Jr. Understanding and managing the psychological impact of the ICD. Card Electrophysiol Rev 2001;5:128-32.
3. Pelletier D, Gallagher R, Mitten-Lewis S, et al. Australian implantable cardiac defibrillator recipients: quality-of-life issues. Int J Nursing Pract 2002;8:68-74.
4. Eads AS, Sears SF, Jr, Sotile WM, Conti JB. Supportive communication with implantable cardioverter defibrillator patients: seven principles to facilitate psychosocial adjustment. J Cardiopulm Rehab 2000;20:109-14.
5. Sola CL, Bostwick JM. Implantable cardioverter-defibrillators, induced anxiety, and quality of life. Mayo Clin Proc 2005;80:232-7.
6. Sears SF, Jr, Todaro JF, Lewis TS, et al. Examining the psychosocial impact of implantable cardioverter defibrillators: a literature review. Clin Cardiol 1999;22:481-9.
7. Goodman M, Hess B. Could implantable cardioverter defibrillators provide a human model supporting the learned helplessness theory of depression? Gen Hosp Psychiatry 1999;21:382-5.
8. Pauli P, Wiedemann G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76.
9. Godemann F, Ahrens B, Behrens S, et al. Classic conditioning and dysfunctional cognitions in patients with panic disorder and agoraphobia treated with an implantable cardioverter/defibrillator. Psychosom Med 2001;63:231-8.
10. Irvine J, Dorian P, Baker B, et al. Quality of life in the Canadian Implantable Defibrillator Study (CIDS). Am Heart J 2002;144:282-9.
11. Dunbar SB, Warner CD, Purcell JA. Internal cardioverter defibrillator device discharge: experiences of patients and family members. Heart Lung 1993;22:494-501.
12. Bourke JP, Turkington D, Thomas G, et al. Florid psychopathology in patients receiving shocks from implanted cardioverter-defibrillators. Heart 1997;78:581-3.
13. Vlay SC, Olson LC, Fricchione GL, Friedman R. Anxiety and anger in patients with ventricular tachyarrhythmias: responses after automatic internal cardioverter defibrillator implantation. Pacing Clin Electrophysiol 1989;12:366-73.
14. Hamner M, Hunt N, Gee J, et al. PTSD and automatic implantable cardioverter defibrillators. Psychosomatics 1998;40:82-5.
15. Friccione GL, Vlay LC, Vlay SC. Cardiac psychiatry and the management of malignant ventricular arrhythmias with the internal cardioverter-defibrillator. Am Heart J 1994;128:1050-9.
16. Friccione GL, Vlay SC. Psychiatric aspects of the implantable cardioverter-defibrillator. In: Estes NAM, Menolis AS, Want PG, eds. Implantable cardioverter-defibrillators. A comprehensive textbook. New York: Marcel Dekker; 1994:405-23.
17. Kohn CS, Petrucci RJ, Baesser C, et al. The effect of psychological intervention on patients’ long-term adjustment to the ICD: a prospective study. Pacing Clin Electrophysiol 2000;23(4 pt 1):450-6.
18. Heller SS, Ormont MA, Lidagoster L, et al. Psychosocial outcome after ICD implantation: a current perspective. Pacing Clin Electrophysiol 1998;21:1207-15.
19. Fabian TJ, Schwartzman DS, Ujhelyi MR, et al. Decreasing pain and anxiety associated with patient-activated atrial shock: a placebo-controlled study of adjunctive sedation with oral triazolam. J Cardivasc Electrophysiol 2006;17:391-5.
20. Sala M, Coppa F, Cappucciati C, et al. Antidepressants: their effects on cardiac channels, QT prolongation and Torsade de Pointes. Curr Opin Investig Drugs 2006;7:256-63.
21. Swenson JR, Doucette S, Fergusson D. Adverse cardiovascular events in antidepressant trials involving high-risk patients: a systematic review of randomized trials. Can J Psychiatry 2006;51:923-9.
22. Pinski SL. Emergencies related to implantable cardioverter-defibrillators. Crit Care Med 2000;28(10 suppl):N174-N180.
23. Kuhl EA, Dixit NK, Walker RL, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-18.
24. Sears SF, Shea JB, Conti JB. How to respond to an implantable cardioverter-defibrillator shock. Circulation 2005;111:380-2.
CASE: ‘Like a sledgehammer’
Mr. J, age 54, is admitted to the cardiac critical care unit after repeated tachycardia episodes over 3 years. He also has depressive symptoms including social isolation, passive suicidal thoughts, lack of interest in sex, weight loss, difficulty sleeping, sadness, and decreased appetite, energy, and ability to concentrate. The psychiatry consult team subsequently evaluates him.
Shortly after retiring as a police officer, Mr. J started having 10-second episodes of loss of consciousness and suffered 30 episodes within 1 year. After diagnosing chronic idiopathic ventricular tachycardia, a cardiologist ablated an aberrant left ventricular pathway and inserted a single-lead implantable cardioverter-defibrillator (ICD). He also prescribed the antiarrhythmic amiodarone, but Mr. J could not tolerate the medication’s side effects.
Mr. J’s tachycardia persisted, and repeated episodes triggered an estimated 13 electrical shocks from the ICD over 5 months. At this point, the cardiologist performed a second ablation, removed the single-lead ICD, and implanted a two-lead ICD, which he hoped would more accurately discern between lethal and nonlethal fast heart rhythms.
In addition, the cardiologist prescribed the antiarrhythmic sotalol—which did not suppress the arrhythmia—before switching to flecainide, 100 mg bid, which did. However, Mr. J still suffered fatigue, exercise intolerance, near-syncope, and chest heaviness.
One week after receiving the first ICD, Mr. J recalls, he felt his first shock while out for a walk. He said the shock lasted 5 to 10 seconds and “felt like somebody took a sledgehammer to my chest.” Another time, he suffered 6 successive shocks that threw him to the ground. Motorists pulled over to assist him, which made him feel ashamed.
Before long, Mr. J became increasingly afraid of repeat discharges. As soon as he began a task, he would feel a “thumping” in the back of his neck and start panicking, fearful that a heart rate increase would trigger another shock.
The stress forced Mr. J to abandon his favorite retirement hobbies—remodeling houses and yard work—and to spend his days lying around watching television. Fearing another discharge in public, he has stopped seeing friends and going to church. He has also stopped driving and depends on his female partner of 14 years for daily visits, grocery shopping, and rides to medical appointments. She feels frustrated by his debility.
The authors’ observations
By delivering electrical shocks when ventricles beat too quickly, an ICD shocks the heart back into a normal rhythm. Based on our observation, Mr. J probably had both anxiety-induced tachycardia and recurrent atrial fibrillation.
Although ICDs have prolonged survival for patients with potentially fatal ventricular arrhythmias,1,2 painful discharges can occur without warning. Patients liken the discharge to an electric shock or to being kicked or punched in the chest.3
Depending on the patient’s activity level, cardiologists routinely program ICDs to discharge at approximately 10 beats per minute above expected heart rates during typical activities. Because ICD leads cannot differentiate between ventricular and supraventricular rhythm disturbances, a rapid supraventricular rhythm might precipitate a discharge intended to treat a more serious ventricular rhythm disturbance.
Frequent ICD discharges could indicate:
- the patient needs a more effective antiarrhythmic
- the device needs to be set at a higher rate to avoid discharge during periods of anxiety/exertion
- or the device is defective.
ICD-induced psychopathology
Depression or tachycardia could have caused Mr. J’s fatigue. Either way, he showed numerous other depressive symptoms.
Fear of implant discharge or malfunction often induces psychiatric disorders, particularly in patients who have experienced discharge. As many as 87% of ICD patients suffer anxiety, depression, or other psychiatric symptoms after implantation,5 and 13% to 38% meet DSM-IV-TR criteria for an anxiety spectrum disorder.6
Multiple psychological theories explain iatrogenic anxiety disorders resulting from ICD firing. Behaviorally, ICD discharge represents an initially unconditioned stimulus that the patient associates with the activity he was engaging in when shocked. The shock discourages the patient from that activity—however benign—for fear it triggered the discharge and could cause future shocks.
ICD recipients often fear the device will malfunction or discharge while they are in public, driving, or operating machinery—leading some to become homebound and cease activities of daily living. The discharge’s unpredictability shatters a patient’s perception of control over his or her life and might induce a learned helplessness7 that can strain relationships, as it did with Mr. J and his partner. The patient also could develop anticipatory anxiety, mistaking benign body symptoms or increasing shock frequency for signs of a potentially fatal heart problem.8
Whether quality of life diminishes as ICD firings become more frequent is uncertain.9 The Canadian Implantable Defibrillator Study (N=317) found greater quality of life improvements with ICD therapy than with amiodarone—200 to 400 mg/d maintenance therapy—but the improvements were lost in patients who experienced ≥5 shocks over 12 months.10 Pauli et al7 found misinterpretation of the reason for increasing shocks to be more emotionally destructive than shock frequency, however.
Detecting ICD maladjustment
Patients with ICD maladjustment typically show anticipatory anxiety and negative cognitive attributions, and many engage in fruitless maneuvers to prevent device firing.5 Nervousness, dizziness, weakness, and fear are common responses to shock by ICD.11
Most patients with new-onset, post-ICD anxiety disorders have no pre-implant psychiatric history.12 Only one trial assessing state and trait anxiety before and after ICD placement reported increased trait anxiety in some patients before implantation.13
HISTORY: Nights in the cornfield
During psychiatric evaluation, Mr. J reveals that his parents physically and emotionally abused him as a child. He says his father frequently beat him with farm tools, and sometimes the beatings were so severe that his parents kept him home from school to prevent teachers from noticing his bruises. He never received medical treatment for his injuries.
For Mr. J, the inescapable threat of painful, unannounced ICD discharges has brought back the anticipatory terror and helplessness of his childhood. Just as he feared his father’s sudden rages, the specter of repeat ICD shocks now haunts him. He says he’d rather have the ICD removed and risk death from tachycardia than live another minute in fear.
The authors’ observations
Mr. J meets DSM-IV-TR criteria for PTSD. He associates ICD discharge with childhood abuse and experiences new-onset flashbacks, hyperarousal, and avoidance behavior.
To our knowledge, ICD shock-induced flashbacks to pre-implant trauma have not been reported, although some data associate ICDs with posttraumatic stress related to heart disease and treatment.14-16 In one case series,14 patients showed:
- cluster B re-experiencing symptoms (cognitive preoccupation with trauma or psychophysiologic reactivity to reminders of the ICD and heart disease)
- cluster C avoidance symptoms (avoiding activities they thought might activate the ICD)
- cluster D hyperarousal symptoms (insomnia, decreased concentration, hypervigilance, and irritability).
The authors’ observations
Treating comorbid anxiety or depression in ICD recipients is critical. A number of psychiatric interventions might alleviate behavioral and psychological effects of body-device interactions.
CBT. In a retrospective study17 of 36 ICD recipients, those who received 9 months of CBT reported decreased depression, anxiety, distress, and sexual problems compared with those who did not. Interestingly, more CBT-group patients (11 of 18) suffered ICD shocks than did controls (6 of 18).
Peer support groups. Out of 58 ICD recipients who answered a post-implant questionnaire, 23 (39%) attended a peer support group.18 Of these, 22 (96%) found the group helpful and were happier, less hostile, and more sociable after participating. Peer group participants also were more likely to return to work than nonparticipants.
How would you handle a patient’s request to deactivate an implantable cardioverter-defibrillator (ICD) or other life-preserving device that is causing debilitating mental anguish? Physicians dealing with such requests will find themselves in an ethical wilderness.
Pinski22 offers guidelines in line with withdrawal of other life-extending technologies in terminally ill patients. “Deactivation of an ICD is appropriate when the device is believed to be prolonging patient suffering,” he writes, adding that preventing ICD shocks induced by frequent or agonal arrhythmias “will not only hasten but also permit a peaceful death.” Disabling the ICD function that responds to bradycardia will prevent agonal pacing and—as a result—shocks.
The literature, however, offers little guidance on responding to patient requests for ICD deactivation and few precedents on which to base such decisions for the terminally ill.
Even less guidance exists when mental illness resulting from ICD complications induces unbearable suffering. The underlying psychiatric condition should be optimally treated before clinicians entertain ICD removal. Mr. J, for example, decided to keep the implant once his crippling anxiety resolved and he was assured that his tachycardia finally was under control.
12 attributes reduction of ICD-induced anxiety to combination individual psychotherapy and unspecified dosages of benzodiazepines. Two patients also received adjunctive fluoxetine or paroxetine, dosages unspecified.
In a double-blind, placebo-controlled crossover study, implantable atrial defibrillator recipients reported decreased pain and anxiety while taking the short-acting benzodiazepine triazolam, 0.375 mg, before patient-activated shock.19
We recommend trying a combination regimen that acts acutely and subacutely. A long-acting benzodiazepine such as clonazepam can calm acute, overwhelming anxiety, and a selective serotonin reuptake inhibitor (SSRI) such as fluoxetine or paroxetine can help manage chronic depressive and generalized anxiety symptoms.
SSRIs are relatively benign but more research on their cardiac safety is needed.20,21 Tricyclic antidepressants, which prolong cardiac conduction, should be avoided.
In addition to psychotropics, concomitant psychotherapy can reduce chronic symptoms.
The authors’ observations
Preparing patients for ICD problems. Anxiety after an ICD shock and the dread of repeat shocks are normal; the goal is to prevent that anxiety from destroying quality of life.
As with Mr. J, many ICD recipients are emotionally unprepared for device-related complications. Most cardiologists do not screen patients for pre-existing anxiety before ICD placement, nor do many adequately address ICD-induced anxiety once the device has been placed.
Psychological screening before implantation can help detect and manage preexisting anxiety disorders. Small-scale evaluations have used anxiety scales to continuously measure anxiety before and after ICD placement.13,23
Increased patient education on how ICDs work can help patients decide whether to proceed with implantation and tolerate discharges should they occur. Psychological screening and brief, routine communication between providers and patients about psychosocial issues can help patients adjust and identify those who need extended psychological services.4
- develop a plan for how a shock would be handled
- perform relaxation exercises immediately after the shock
- resume activities they were involved with when the shock occurred to prevent avoidance.24
TREATMENT: Third attempt
The cardiology team discontinues flecainide and performs a third radioablation, which eradicates ectopic ventricular activity.
Acting on the psychiatry consult team’s advice, Mr. J is transferred to the inpatient mood disorders unit to aggressively treat his PTSD. He undergoes 4 days of intensive CBT designed to explore the connection between his response to the discharges and his father’s abuse. We prescribe clonazepam, 0.5 mg bid, to reduce Mr. J’s agitation and anxiety, and recommend outpatient counseling to help manage his stress—particularly his anxious response to stimuli that remind him of the ICD discharge.
Mr. J is discharged after 12 days in the cardiac and psychiatric units. He has no suicidal thoughts, his sadness has decreased, and his energy, concentration, sleep, and outlook on his future have improved. He also is resolving relationship issues with his partner.
As Mr. J’s anxiety declines and he is increasingly reassured that his arrhythmias are under control, he decides to keep the ICD. His function gradually improves with continued cardiac rehabilitation, although he does not continue psychotherapy.
Related resources
- Stutts LA, Cross NJ, Conti JB, Sears SF. Examination of research trends on patient factors in patients with implantable cardioverter defibrillators. Clin Cardiol 2007;30:64-8.
- Sears SF, Shea JB, Conti JB. How to respond to an implantable cardioverter-defibrillator shock. Circulation 2005;111;380-2. http://circ.ahajournals.org/cgi/reprint/111/23/e380.
- Pauli P, Wiedemann G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76. www.psychosomaticmedicine.org/cgi/reprint/61/1/69.
- Amiodarone • Cordarone
- Clonazepam • Klonopin
- Flecainide • Tambocor
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sotalol • Betapace
- Triazolam • Halcion, others
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: ‘Like a sledgehammer’
Mr. J, age 54, is admitted to the cardiac critical care unit after repeated tachycardia episodes over 3 years. He also has depressive symptoms including social isolation, passive suicidal thoughts, lack of interest in sex, weight loss, difficulty sleeping, sadness, and decreased appetite, energy, and ability to concentrate. The psychiatry consult team subsequently evaluates him.
Shortly after retiring as a police officer, Mr. J started having 10-second episodes of loss of consciousness and suffered 30 episodes within 1 year. After diagnosing chronic idiopathic ventricular tachycardia, a cardiologist ablated an aberrant left ventricular pathway and inserted a single-lead implantable cardioverter-defibrillator (ICD). He also prescribed the antiarrhythmic amiodarone, but Mr. J could not tolerate the medication’s side effects.
Mr. J’s tachycardia persisted, and repeated episodes triggered an estimated 13 electrical shocks from the ICD over 5 months. At this point, the cardiologist performed a second ablation, removed the single-lead ICD, and implanted a two-lead ICD, which he hoped would more accurately discern between lethal and nonlethal fast heart rhythms.
In addition, the cardiologist prescribed the antiarrhythmic sotalol—which did not suppress the arrhythmia—before switching to flecainide, 100 mg bid, which did. However, Mr. J still suffered fatigue, exercise intolerance, near-syncope, and chest heaviness.
One week after receiving the first ICD, Mr. J recalls, he felt his first shock while out for a walk. He said the shock lasted 5 to 10 seconds and “felt like somebody took a sledgehammer to my chest.” Another time, he suffered 6 successive shocks that threw him to the ground. Motorists pulled over to assist him, which made him feel ashamed.
Before long, Mr. J became increasingly afraid of repeat discharges. As soon as he began a task, he would feel a “thumping” in the back of his neck and start panicking, fearful that a heart rate increase would trigger another shock.
The stress forced Mr. J to abandon his favorite retirement hobbies—remodeling houses and yard work—and to spend his days lying around watching television. Fearing another discharge in public, he has stopped seeing friends and going to church. He has also stopped driving and depends on his female partner of 14 years for daily visits, grocery shopping, and rides to medical appointments. She feels frustrated by his debility.
The authors’ observations
By delivering electrical shocks when ventricles beat too quickly, an ICD shocks the heart back into a normal rhythm. Based on our observation, Mr. J probably had both anxiety-induced tachycardia and recurrent atrial fibrillation.
Although ICDs have prolonged survival for patients with potentially fatal ventricular arrhythmias,1,2 painful discharges can occur without warning. Patients liken the discharge to an electric shock or to being kicked or punched in the chest.3
Depending on the patient’s activity level, cardiologists routinely program ICDs to discharge at approximately 10 beats per minute above expected heart rates during typical activities. Because ICD leads cannot differentiate between ventricular and supraventricular rhythm disturbances, a rapid supraventricular rhythm might precipitate a discharge intended to treat a more serious ventricular rhythm disturbance.
Frequent ICD discharges could indicate:
- the patient needs a more effective antiarrhythmic
- the device needs to be set at a higher rate to avoid discharge during periods of anxiety/exertion
- or the device is defective.
ICD-induced psychopathology
Depression or tachycardia could have caused Mr. J’s fatigue. Either way, he showed numerous other depressive symptoms.
Fear of implant discharge or malfunction often induces psychiatric disorders, particularly in patients who have experienced discharge. As many as 87% of ICD patients suffer anxiety, depression, or other psychiatric symptoms after implantation,5 and 13% to 38% meet DSM-IV-TR criteria for an anxiety spectrum disorder.6
Multiple psychological theories explain iatrogenic anxiety disorders resulting from ICD firing. Behaviorally, ICD discharge represents an initially unconditioned stimulus that the patient associates with the activity he was engaging in when shocked. The shock discourages the patient from that activity—however benign—for fear it triggered the discharge and could cause future shocks.
ICD recipients often fear the device will malfunction or discharge while they are in public, driving, or operating machinery—leading some to become homebound and cease activities of daily living. The discharge’s unpredictability shatters a patient’s perception of control over his or her life and might induce a learned helplessness7 that can strain relationships, as it did with Mr. J and his partner. The patient also could develop anticipatory anxiety, mistaking benign body symptoms or increasing shock frequency for signs of a potentially fatal heart problem.8
Whether quality of life diminishes as ICD firings become more frequent is uncertain.9 The Canadian Implantable Defibrillator Study (N=317) found greater quality of life improvements with ICD therapy than with amiodarone—200 to 400 mg/d maintenance therapy—but the improvements were lost in patients who experienced ≥5 shocks over 12 months.10 Pauli et al7 found misinterpretation of the reason for increasing shocks to be more emotionally destructive than shock frequency, however.
Detecting ICD maladjustment
Patients with ICD maladjustment typically show anticipatory anxiety and negative cognitive attributions, and many engage in fruitless maneuvers to prevent device firing.5 Nervousness, dizziness, weakness, and fear are common responses to shock by ICD.11
Most patients with new-onset, post-ICD anxiety disorders have no pre-implant psychiatric history.12 Only one trial assessing state and trait anxiety before and after ICD placement reported increased trait anxiety in some patients before implantation.13
HISTORY: Nights in the cornfield
During psychiatric evaluation, Mr. J reveals that his parents physically and emotionally abused him as a child. He says his father frequently beat him with farm tools, and sometimes the beatings were so severe that his parents kept him home from school to prevent teachers from noticing his bruises. He never received medical treatment for his injuries.
For Mr. J, the inescapable threat of painful, unannounced ICD discharges has brought back the anticipatory terror and helplessness of his childhood. Just as he feared his father’s sudden rages, the specter of repeat ICD shocks now haunts him. He says he’d rather have the ICD removed and risk death from tachycardia than live another minute in fear.
The authors’ observations
Mr. J meets DSM-IV-TR criteria for PTSD. He associates ICD discharge with childhood abuse and experiences new-onset flashbacks, hyperarousal, and avoidance behavior.
To our knowledge, ICD shock-induced flashbacks to pre-implant trauma have not been reported, although some data associate ICDs with posttraumatic stress related to heart disease and treatment.14-16 In one case series,14 patients showed:
- cluster B re-experiencing symptoms (cognitive preoccupation with trauma or psychophysiologic reactivity to reminders of the ICD and heart disease)
- cluster C avoidance symptoms (avoiding activities they thought might activate the ICD)
- cluster D hyperarousal symptoms (insomnia, decreased concentration, hypervigilance, and irritability).
The authors’ observations
Treating comorbid anxiety or depression in ICD recipients is critical. A number of psychiatric interventions might alleviate behavioral and psychological effects of body-device interactions.
CBT. In a retrospective study17 of 36 ICD recipients, those who received 9 months of CBT reported decreased depression, anxiety, distress, and sexual problems compared with those who did not. Interestingly, more CBT-group patients (11 of 18) suffered ICD shocks than did controls (6 of 18).
Peer support groups. Out of 58 ICD recipients who answered a post-implant questionnaire, 23 (39%) attended a peer support group.18 Of these, 22 (96%) found the group helpful and were happier, less hostile, and more sociable after participating. Peer group participants also were more likely to return to work than nonparticipants.
How would you handle a patient’s request to deactivate an implantable cardioverter-defibrillator (ICD) or other life-preserving device that is causing debilitating mental anguish? Physicians dealing with such requests will find themselves in an ethical wilderness.
Pinski22 offers guidelines in line with withdrawal of other life-extending technologies in terminally ill patients. “Deactivation of an ICD is appropriate when the device is believed to be prolonging patient suffering,” he writes, adding that preventing ICD shocks induced by frequent or agonal arrhythmias “will not only hasten but also permit a peaceful death.” Disabling the ICD function that responds to bradycardia will prevent agonal pacing and—as a result—shocks.
The literature, however, offers little guidance on responding to patient requests for ICD deactivation and few precedents on which to base such decisions for the terminally ill.
Even less guidance exists when mental illness resulting from ICD complications induces unbearable suffering. The underlying psychiatric condition should be optimally treated before clinicians entertain ICD removal. Mr. J, for example, decided to keep the implant once his crippling anxiety resolved and he was assured that his tachycardia finally was under control.
12 attributes reduction of ICD-induced anxiety to combination individual psychotherapy and unspecified dosages of benzodiazepines. Two patients also received adjunctive fluoxetine or paroxetine, dosages unspecified.
In a double-blind, placebo-controlled crossover study, implantable atrial defibrillator recipients reported decreased pain and anxiety while taking the short-acting benzodiazepine triazolam, 0.375 mg, before patient-activated shock.19
We recommend trying a combination regimen that acts acutely and subacutely. A long-acting benzodiazepine such as clonazepam can calm acute, overwhelming anxiety, and a selective serotonin reuptake inhibitor (SSRI) such as fluoxetine or paroxetine can help manage chronic depressive and generalized anxiety symptoms.
SSRIs are relatively benign but more research on their cardiac safety is needed.20,21 Tricyclic antidepressants, which prolong cardiac conduction, should be avoided.
In addition to psychotropics, concomitant psychotherapy can reduce chronic symptoms.
The authors’ observations
Preparing patients for ICD problems. Anxiety after an ICD shock and the dread of repeat shocks are normal; the goal is to prevent that anxiety from destroying quality of life.
As with Mr. J, many ICD recipients are emotionally unprepared for device-related complications. Most cardiologists do not screen patients for pre-existing anxiety before ICD placement, nor do many adequately address ICD-induced anxiety once the device has been placed.
Psychological screening before implantation can help detect and manage preexisting anxiety disorders. Small-scale evaluations have used anxiety scales to continuously measure anxiety before and after ICD placement.13,23
Increased patient education on how ICDs work can help patients decide whether to proceed with implantation and tolerate discharges should they occur. Psychological screening and brief, routine communication between providers and patients about psychosocial issues can help patients adjust and identify those who need extended psychological services.4
- develop a plan for how a shock would be handled
- perform relaxation exercises immediately after the shock
- resume activities they were involved with when the shock occurred to prevent avoidance.24
TREATMENT: Third attempt
The cardiology team discontinues flecainide and performs a third radioablation, which eradicates ectopic ventricular activity.
Acting on the psychiatry consult team’s advice, Mr. J is transferred to the inpatient mood disorders unit to aggressively treat his PTSD. He undergoes 4 days of intensive CBT designed to explore the connection between his response to the discharges and his father’s abuse. We prescribe clonazepam, 0.5 mg bid, to reduce Mr. J’s agitation and anxiety, and recommend outpatient counseling to help manage his stress—particularly his anxious response to stimuli that remind him of the ICD discharge.
Mr. J is discharged after 12 days in the cardiac and psychiatric units. He has no suicidal thoughts, his sadness has decreased, and his energy, concentration, sleep, and outlook on his future have improved. He also is resolving relationship issues with his partner.
As Mr. J’s anxiety declines and he is increasingly reassured that his arrhythmias are under control, he decides to keep the ICD. His function gradually improves with continued cardiac rehabilitation, although he does not continue psychotherapy.
Related resources
- Stutts LA, Cross NJ, Conti JB, Sears SF. Examination of research trends on patient factors in patients with implantable cardioverter defibrillators. Clin Cardiol 2007;30:64-8.
- Sears SF, Shea JB, Conti JB. How to respond to an implantable cardioverter-defibrillator shock. Circulation 2005;111;380-2. http://circ.ahajournals.org/cgi/reprint/111/23/e380.
- Pauli P, Wiedemann G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76. www.psychosomaticmedicine.org/cgi/reprint/61/1/69.
- Amiodarone • Cordarone
- Clonazepam • Klonopin
- Flecainide • Tambocor
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sotalol • Betapace
- Triazolam • Halcion, others
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Morris PL, Badger J, Chmielewski C, et al. Psychiatric morbidity following implantation of the automatic implantable cardioverter defibrillator. Psychosomatics 1991;32:58-64.
2. Conti JB, Sears SF, Jr. Understanding and managing the psychological impact of the ICD. Card Electrophysiol Rev 2001;5:128-32.
3. Pelletier D, Gallagher R, Mitten-Lewis S, et al. Australian implantable cardiac defibrillator recipients: quality-of-life issues. Int J Nursing Pract 2002;8:68-74.
4. Eads AS, Sears SF, Jr, Sotile WM, Conti JB. Supportive communication with implantable cardioverter defibrillator patients: seven principles to facilitate psychosocial adjustment. J Cardiopulm Rehab 2000;20:109-14.
5. Sola CL, Bostwick JM. Implantable cardioverter-defibrillators, induced anxiety, and quality of life. Mayo Clin Proc 2005;80:232-7.
6. Sears SF, Jr, Todaro JF, Lewis TS, et al. Examining the psychosocial impact of implantable cardioverter defibrillators: a literature review. Clin Cardiol 1999;22:481-9.
7. Goodman M, Hess B. Could implantable cardioverter defibrillators provide a human model supporting the learned helplessness theory of depression? Gen Hosp Psychiatry 1999;21:382-5.
8. Pauli P, Wiedemann G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76.
9. Godemann F, Ahrens B, Behrens S, et al. Classic conditioning and dysfunctional cognitions in patients with panic disorder and agoraphobia treated with an implantable cardioverter/defibrillator. Psychosom Med 2001;63:231-8.
10. Irvine J, Dorian P, Baker B, et al. Quality of life in the Canadian Implantable Defibrillator Study (CIDS). Am Heart J 2002;144:282-9.
11. Dunbar SB, Warner CD, Purcell JA. Internal cardioverter defibrillator device discharge: experiences of patients and family members. Heart Lung 1993;22:494-501.
12. Bourke JP, Turkington D, Thomas G, et al. Florid psychopathology in patients receiving shocks from implanted cardioverter-defibrillators. Heart 1997;78:581-3.
13. Vlay SC, Olson LC, Fricchione GL, Friedman R. Anxiety and anger in patients with ventricular tachyarrhythmias: responses after automatic internal cardioverter defibrillator implantation. Pacing Clin Electrophysiol 1989;12:366-73.
14. Hamner M, Hunt N, Gee J, et al. PTSD and automatic implantable cardioverter defibrillators. Psychosomatics 1998;40:82-5.
15. Friccione GL, Vlay LC, Vlay SC. Cardiac psychiatry and the management of malignant ventricular arrhythmias with the internal cardioverter-defibrillator. Am Heart J 1994;128:1050-9.
16. Friccione GL, Vlay SC. Psychiatric aspects of the implantable cardioverter-defibrillator. In: Estes NAM, Menolis AS, Want PG, eds. Implantable cardioverter-defibrillators. A comprehensive textbook. New York: Marcel Dekker; 1994:405-23.
17. Kohn CS, Petrucci RJ, Baesser C, et al. The effect of psychological intervention on patients’ long-term adjustment to the ICD: a prospective study. Pacing Clin Electrophysiol 2000;23(4 pt 1):450-6.
18. Heller SS, Ormont MA, Lidagoster L, et al. Psychosocial outcome after ICD implantation: a current perspective. Pacing Clin Electrophysiol 1998;21:1207-15.
19. Fabian TJ, Schwartzman DS, Ujhelyi MR, et al. Decreasing pain and anxiety associated with patient-activated atrial shock: a placebo-controlled study of adjunctive sedation with oral triazolam. J Cardivasc Electrophysiol 2006;17:391-5.
20. Sala M, Coppa F, Cappucciati C, et al. Antidepressants: their effects on cardiac channels, QT prolongation and Torsade de Pointes. Curr Opin Investig Drugs 2006;7:256-63.
21. Swenson JR, Doucette S, Fergusson D. Adverse cardiovascular events in antidepressant trials involving high-risk patients: a systematic review of randomized trials. Can J Psychiatry 2006;51:923-9.
22. Pinski SL. Emergencies related to implantable cardioverter-defibrillators. Crit Care Med 2000;28(10 suppl):N174-N180.
23. Kuhl EA, Dixit NK, Walker RL, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-18.
24. Sears SF, Shea JB, Conti JB. How to respond to an implantable cardioverter-defibrillator shock. Circulation 2005;111:380-2.
1. Morris PL, Badger J, Chmielewski C, et al. Psychiatric morbidity following implantation of the automatic implantable cardioverter defibrillator. Psychosomatics 1991;32:58-64.
2. Conti JB, Sears SF, Jr. Understanding and managing the psychological impact of the ICD. Card Electrophysiol Rev 2001;5:128-32.
3. Pelletier D, Gallagher R, Mitten-Lewis S, et al. Australian implantable cardiac defibrillator recipients: quality-of-life issues. Int J Nursing Pract 2002;8:68-74.
4. Eads AS, Sears SF, Jr, Sotile WM, Conti JB. Supportive communication with implantable cardioverter defibrillator patients: seven principles to facilitate psychosocial adjustment. J Cardiopulm Rehab 2000;20:109-14.
5. Sola CL, Bostwick JM. Implantable cardioverter-defibrillators, induced anxiety, and quality of life. Mayo Clin Proc 2005;80:232-7.
6. Sears SF, Jr, Todaro JF, Lewis TS, et al. Examining the psychosocial impact of implantable cardioverter defibrillators: a literature review. Clin Cardiol 1999;22:481-9.
7. Goodman M, Hess B. Could implantable cardioverter defibrillators provide a human model supporting the learned helplessness theory of depression? Gen Hosp Psychiatry 1999;21:382-5.
8. Pauli P, Wiedemann G, Dengler W, et al. Anxiety in patients with an automatic implantable cardioverter defibrillator: what differentiates them from panic patients? Psychosom Med 1999;61:69-76.
9. Godemann F, Ahrens B, Behrens S, et al. Classic conditioning and dysfunctional cognitions in patients with panic disorder and agoraphobia treated with an implantable cardioverter/defibrillator. Psychosom Med 2001;63:231-8.
10. Irvine J, Dorian P, Baker B, et al. Quality of life in the Canadian Implantable Defibrillator Study (CIDS). Am Heart J 2002;144:282-9.
11. Dunbar SB, Warner CD, Purcell JA. Internal cardioverter defibrillator device discharge: experiences of patients and family members. Heart Lung 1993;22:494-501.
12. Bourke JP, Turkington D, Thomas G, et al. Florid psychopathology in patients receiving shocks from implanted cardioverter-defibrillators. Heart 1997;78:581-3.
13. Vlay SC, Olson LC, Fricchione GL, Friedman R. Anxiety and anger in patients with ventricular tachyarrhythmias: responses after automatic internal cardioverter defibrillator implantation. Pacing Clin Electrophysiol 1989;12:366-73.
14. Hamner M, Hunt N, Gee J, et al. PTSD and automatic implantable cardioverter defibrillators. Psychosomatics 1998;40:82-5.
15. Friccione GL, Vlay LC, Vlay SC. Cardiac psychiatry and the management of malignant ventricular arrhythmias with the internal cardioverter-defibrillator. Am Heart J 1994;128:1050-9.
16. Friccione GL, Vlay SC. Psychiatric aspects of the implantable cardioverter-defibrillator. In: Estes NAM, Menolis AS, Want PG, eds. Implantable cardioverter-defibrillators. A comprehensive textbook. New York: Marcel Dekker; 1994:405-23.
17. Kohn CS, Petrucci RJ, Baesser C, et al. The effect of psychological intervention on patients’ long-term adjustment to the ICD: a prospective study. Pacing Clin Electrophysiol 2000;23(4 pt 1):450-6.
18. Heller SS, Ormont MA, Lidagoster L, et al. Psychosocial outcome after ICD implantation: a current perspective. Pacing Clin Electrophysiol 1998;21:1207-15.
19. Fabian TJ, Schwartzman DS, Ujhelyi MR, et al. Decreasing pain and anxiety associated with patient-activated atrial shock: a placebo-controlled study of adjunctive sedation with oral triazolam. J Cardivasc Electrophysiol 2006;17:391-5.
20. Sala M, Coppa F, Cappucciati C, et al. Antidepressants: their effects on cardiac channels, QT prolongation and Torsade de Pointes. Curr Opin Investig Drugs 2006;7:256-63.
21. Swenson JR, Doucette S, Fergusson D. Adverse cardiovascular events in antidepressant trials involving high-risk patients: a systematic review of randomized trials. Can J Psychiatry 2006;51:923-9.
22. Pinski SL. Emergencies related to implantable cardioverter-defibrillators. Crit Care Med 2000;28(10 suppl):N174-N180.
23. Kuhl EA, Dixit NK, Walker RL, et al. Measurement of patient fears about implantable cardioverter defibrillator shock: an initial evaluation of the Florida Shock Anxiety Scale. Pacing Clin Electrophysiol 2006;29:614-18.
24. Sears SF, Shea JB, Conti JB. How to respond to an implantable cardioverter-defibrillator shock. Circulation 2005;111:380-2.
Depression, medication, and ‘bad blood’
CASE: Sad and suicidal
Mr. G, age 44, has chronic depression with suicidality. At presentation he says he has felt sad and suicidal for 2 weeks. He also has no appetite and trouble sleeping at night.
Mr. G’s depression has left him unable to work and has led to 4 hospitalizations over 10 years. He first attempted suicide in 1984 after his ex-wife took their child and left him. He endorses no suicide plan and has been sober for 7 years after 12-plus years of alcohol abuse, but says he has been tempted lately to resume drinking.
The patient was taking an antidepressant but stopped while at a homeless shelter, where he had been staying for several weeks. For more than 20 years, he also has been taking phenytoin, 300 mg/d, and phenobarbital, 30 mg bid, for a seizure disorder.
Mr. G is admitted with a working diagnosis of recurrent major depressive disorder. White blood cell count (WBC) at admission is 5.12×109/L and neutrophils are 3.6×109/L—both low-normal readings. Other laboratory results are normal.
We continue phenytoin and phenobarbital at the same dosages and start the selective serotonin reuptake inhibitor (SSRI) citalopram, 20 mg/d, which interacts minimally with both anticonvulsants.
After 2 weeks, Mr. G’s seizures are well controlled and he is tolerating citalopram, but his depressive symptoms have not improved. We cross-taper citalopram to prevent SSRI-induced discontinuation syndrome and start the dopamine and norepinephrine reuptake inhibitor bupropion, 75 mg bid. We titrate bupropion over 2 weeks to 150 mg each morning and 300 mg at bedtime, and watch Mr. G closely for seizures. Although his seizure history contraindicates bupropion use, we think he can tolerate the medication because his seizure disorder is well controlled.
Mr. G’s affect, appetite, and energy are improving with bupropion, but a routine complete blood count (CBC) 5 days after the medication is started reveals leukopenia (WBC 3.04×109/L) without neutropenia (neutrophils 1.9×109/L). Repeat blood tests 18 and 32 days after the first blood draw show continued low WBC. The gastrointestinal medicine team tests Mr. G’s liver function but finds no abnormalities.
The author’s observations
A medical cause also is unlikely. Mr. G’s liver function is normal, and he shows no other signs or symptoms of a medical problem. Bone marrow biopsy and immunologic workup could rule out cancer, but the timing of Mr. G’s abnormal blood readings strongly suggests bupropion intolerance.
TREATMENT: Other medications
We immediately stop bupropion, start the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine at 37.5 mg bid, and titrate it over 5 days to 225 mg/d. Blood draws 3 and 5 days after bupropion discontinuation show slight increases in WBC.
Eleven days after venlafaxine is started, Mr. G’s WBC and neutrophils are normal. However, he has become increasingly irritable and volatile, often arguing with a staff nurse and other patients. We cross-taper venlafaxine over 5 days, start the SSRI sertraline at 50 mg/d, and titrate sertraline over 1 week to 150 mg/d. Mr. G’s irritability and depressive symptoms improve at the latter dosage.
Because Mr. G developed neutropenia while taking a medication not associated with this adverse effect, we start watching his WBC counts more closely than usual. WBC is 4.58×109/L 8 days after sertraline is started but falls to 3.4×109/L after another 8 days, with neutrophils at 1.5×109/L for both readings (Table).
We add lithium, 300 mg bid, to increase Mr. G’s neutrophils and augment sertraline’s antidepressant effects. Four days later, WBC is 5.8×109/L with neutrophils at 4.2×109/L.
We stop lithium briefly to see if WBC remains normal. After 3 days, WBC drops to 3.25×109/L with neutrophils at 1.5×109/L. We restart lithium, 300 mg/d, and Mr. G’s WBC increases to 4.18×109/L 4 days later, with neutrophils at 2.1×109/L.
Table
Mr G’s white blood cell (WBC) and neutrophil counts (NC)*
while taking bupropion and sertraline
| Antidepressant | When measurements were taken | WBC | NC |
| None for several weeks | Baseline, first hospital admission | 5.12×109/L | 3.6×109/L |
| Bupropion, 75 mg bid | 5 days after starting bupropion | 3.04×109/L | 1.9×109/L |
| Bupropion, 450 mg/d total | 23 days after starting bupropion | 3.14×109/L | 1.6×109/L |
| Bupropion, 450 mg/d total | 2 weeks after previous test | 2.73×109/L | 1.6×109/L |
| Sertraline, 150 mg/d | 8 days after starting sertraline (titration period) | 4.58×109/L | 1.5×109/L |
| Sertraline, 150 mg/d | 16 days after starting sertraline | 3.4×109/L | 1.5×109/L |
| Sertraline, 150 mg/d, and lithium, 300 mg bid | 4 days after lithium augmentation | 5.8×109/L | 4.2×109/L |
| None for 3 months | Baseline, second hospital admission | 3.7×109/L | 2.1×109/L |
| Sertraline, 150 mg/d | 12 days after restarting sertraline | 2.83×109/L | Not available |
| * Normal WBC values: 4.5 to 11×109/L; normal neutrophil values: 1.5 to 8×109/L | |||
The authors’ observations
For Mr. G, both bupropion and sertraline appear to have caused neutropenia on separate occasions.
To our knowledge, bupropion-induced leukopenia or neutropenia have not been reported in the literature. Neutropenia—a rare adverse effect of antidepressants2—and leukopenia were seen during bupropion’s pre-marketing trials but were not definitely attributed to the drug.1 According to pre- and post-marketing data, leukopenia was “infrequently” reported among 5,100 subjects who received bupropion.3
To our knowledge, sertraline-induced neutropenia has not been reported in nongeriatric patients, although sertraline-induced neutropenia4 and agranulocytosis5 have been reported in patients age >65. The Committee on Safety of Medicine in the United Kingdom has received 2 other reports of neutropenia and 1 report of leukopenia with sertraline.5
In one clinical trial, 2 of 1,304 patients taking unknown dosages of sertraline had low neutrophils (
Medication is the second most common cause of acquired neutropenia, with infection being most common.6 By definition, drug-induced neutropenia occurs within 4 weeks after starting the drug and usually resolves within 30 days after stopping it.
Neutropenia is an idiosyncratic reaction unrelated to pharmacologic action. Although overall neutropenia incidence is unknown, reported incidence of the rare, more severe agranulocytosis ranges from approximately 1 to 10 cases per million people annually, and medications have been implicated in 70% of these cases.6 Conversely, only 2 of 97 incidental neutropenia cases studied by Lima et al7 were medication-induced.
Drug-induced neutropenia can result from immune-mediated destruction of neutrophils by circulating antibodies or from direct toxic effects upon marrow granulocyte precursors. Whereas immune-mediated onset is acute and explosive, toxic effect is insidious (months to years) and asymptomatic.8 Clozapine is thought to deliver a direct toxic effect, whereas the thyroid-regulating drug propylthiouracil generates anti-neutrophil antibodies.9
Mr. G’s acute onset (within 5 to 16 days of starting bupropion or sertraline) and prompt return of neutropenia after stopping lithium suggest acute immune-mediated circulating neutrophil destruction.
Treating leukopenia
After 4 failed or intolerable antidepressant trials, lithium augmentation seemed reasonable and ultimately improved Mr. G’s neutrophil count and his mood.
Lithium has helped resolve clozapine-induced neutropenia in case reports.10-12 Well-controlled studies, however, have followed only patients with antineoplastic, drug-induced neutropenia.1
By acting on cyclic nucleotides, lithium prompts colony-stimulating factor production, which in turn stimulates neutrophil production by pluripotent stem cells. As with Mr. G, patients reach neutrophilia 3 to 7 days after starting lithium.
If the patient cannot tolerate lithium, try switching antidepressants or using growth factors to increase neutrophils.
Switching antidepressants.The SSRIs escitalopram or paroxetine, or the SNRI duloxetine are effective and do not necessarily cause neutropenia. Start at below-normal dosages to gauge tolerability, then titrate to normal dosages. Avoid tricyclics, which pose a higher risk of neutropenia than other antidepressant classes.
Case reports13,14 associate fluoxetine and mirtazapine with neutropenia. The patient who received mirtazapine, 30 mg/d, later responded well to sertraline, 50 mg/d.13
If the new antidepressant is ineffective, consider adding the mood-stabilizing anticonvulsant lamotrigine, 12.5 mg/d. Increase lamotrigine to 25 mg/d after 1 week, then titrate by 25 mg weekly to 100 to 400 mg/d depending on efficacy and tolerability.
Using growth factors.Although their efficacy is not proven, growth factors are minimally toxic and might have helped Mr. G. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor resolved neutropenia in uncontrolled studies, but results of one randomized controlled trial were equivocal.8
TESTING: CT findings
Approximately 2 months after admission—shortly after a blood draw shows normal WBC and neutrophils—Mr. G complains of dizziness. He says he accidentally hit his head against a side table.
We order a full neurologic workup to check for traumatic brain injury or brain damage caused by long-term alcohol abuse:
- Head CT shows evidence of previous cerebrovascular infarcts in the bilateral frontal and cerebellar lobes and basal ganglia.
- MRI shows atrophied mammillary bodies, fornix, and corpus callosum.
- Magnetic resonance angiography reveals small cerebral vessel disease.
FOLLOW-UP: Awaiting discharge
After 3 months of continuous hospitalization, Mr. G has become euthymic and nonsuicidal, though at times oversensitive and combative. We transfer him to an assisted-living center and continue sertraline, 150 mg/d; phenytoin, 300 mg/d; phenobarbital, 30 mg bid; lithium, 300 mg/d; and trazodone, 50 mg at night as needed for insomnia.
We also place Mr. G in a day treatment program for mentally ill chemical abusers. A psychiatrist sees him every 2 weeks, and staff supervise him daily.
The authors’ observations
Mr. G’s extended hospital stay allowed us to closely observe him and offered ready access to laboratory facilities while we cross-tapered medications. In outpatient treatment, however, a serious and life-threatening medication-induced complication could easily be missed.
For medically healthy outpatients, be sure CBC has been checked ≤6 months before presentation. Monitor CBC and urge the patient to see a primary care doctor if infection symptoms emerge. Watch for gingivitis, tooth abscess, and other oral cavity infections—which often are overlooked—and sore throat or fever.
Also check electrolytes and screen for SSRI-induced hyponatremia at baseline for all at-risk patients.
Stop the offending drug when WBC reaches 9/L or with absolute neutrophil count (ANC) 9/L, then take a peripheral smear to confirm neutropenia. If the patient is asymptomatic, check ANC 2 to 3 times weekly, particularly if he or she recently had an infection or started a medication that can cause neutropenia. Neutropenia should resolve within 6 to 8 weeks of stopping the offending drug.
If neutropenia persists, order bone marrow biopsy in collaboration with an internist or hematologist to test for cancer. If the biopsy is negative, test for:
- HIV infection
- antinuclear antibodies to check for collagen vascular disease
- antineutrophil antibody to rule out immune neutropenia
- serum folate and B12 deficiency secondary to low WBC.
FOLLOW-UP: Stressor and relapse
Seven months later, Mr. G is readmitted for depression. Three months earlier, he had stopped all medications and resumed drinking after a family member died. WBC at admission is 3.70×109/L
We refer Mr. G to an outpatient psychiatrist, who sees him monthly. Several months later, the psychiatrist reports a WBC of 4.58×109/L.
Nearly 1 year later, Mr. G still lives at the assisted-living facility. He has not been rehospitalized for depression, is functioning well, and has a girlfriend.
The authors’ observations
Mr. G’s abnormal blood counts after sertraline rechallenge confirms that the SSRI probably was causing leukopenia. If we had restarted bupropion and neutropenia recurred during that regimen, we could have more certainly established a bupropion-leukopenia connection.
- Neutropenia Support Association. www.neutropenia.ca.
- Baehner RL. Overview of neutropenia.UpToDate Online (version 15.1); March 30, 2006. www.uptodate.com.
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Citalopram • Celexa
- Clozapine • Clozaril
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • various
- Mirtazapine • Remeron
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenobarbital • various
- Phenytoin • Dilantin
- Propylthiouracil • various
- Sertraline • Zoloft
- Trazodone • Desyrel
- Valproic acid • Depakene
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McEvoy G, ed. AHFS drug information. Bethesda, MD: American Society of Health-System Pharmacists; 2005.
2. Nelson JC. Safety and tolerability of the new antidepressants. J Clin Psychiatry 1997;60:1101.-
3. Physicians desk reference, 61st ed. Montvale, NJ: Thomson PDR; 2007.
4. Cohn CK, Shrivastava R, Mendels J, et al. Double-blind, multicenter comparison of sertraline and amitriptyline in elderly depressed patients. J Clin Psychiatry 1990;51(suppl B):28-33.
5. Trescoli-Serrano C, Smith NK. Sertraline-induced agranulocytosis. Postgrad Med J 1996;72:446.-
6. Baehner RL. Overview of neutropenia. UpToDate Online (version 15.1); March 30, 2006. Available at: http://www.uptodate.com. Accessed April 16, 2007.
7. Lima CS, Paula EV, Takahashi T, et al. Causes of incidental neutropenia in adulthood. Ann Hematol 2006;85:705-9.
8. Holland SM, Gallin J. Disorders of granulocytes and monocytes. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s principles of internal medicine, 16th ed. New York: McGraw-Hill; 2005.
9. Baehner RL. Drug-induced neutropenia and agranulocytosis. UpToDate Online (version 15.1); June 8, 2005. Available at: http://www.uptodate.com. Accessed April 16, 2007.
10. Sporn A, Gogtay N, Ortiz-Aguayo R, et al. Clozapine-induced neutropenia in children: management with lithium carbonate. J Child Adolesc Psychopharmacol 2003;13:401-4.
11. Blier P, Slater S, Measham T, et al. Lithium and clozapine-induced neutropenia/agranulocytosis. Int Clin Psychopharmacol 1998;13:137-40.
12. Silverstone P. Prevention of clozapine-induced neutropenia by pretreatment with lithium. J Clin Psychopharmacol 1998;18:86-8.
13. Ozcanli T, Unsalver B, Ozdemir S, Ozmen M. Sertraline and mirtazapine-induced severe neutropenia. Am J Psych 2005;162:1386.-
14. Vilinsky FD, Lubin A. Severe neutropenia associated with fluoxetine hydrochloride. Ann Internal Med 1997;127:573-4.
CASE: Sad and suicidal
Mr. G, age 44, has chronic depression with suicidality. At presentation he says he has felt sad and suicidal for 2 weeks. He also has no appetite and trouble sleeping at night.
Mr. G’s depression has left him unable to work and has led to 4 hospitalizations over 10 years. He first attempted suicide in 1984 after his ex-wife took their child and left him. He endorses no suicide plan and has been sober for 7 years after 12-plus years of alcohol abuse, but says he has been tempted lately to resume drinking.
The patient was taking an antidepressant but stopped while at a homeless shelter, where he had been staying for several weeks. For more than 20 years, he also has been taking phenytoin, 300 mg/d, and phenobarbital, 30 mg bid, for a seizure disorder.
Mr. G is admitted with a working diagnosis of recurrent major depressive disorder. White blood cell count (WBC) at admission is 5.12×109/L and neutrophils are 3.6×109/L—both low-normal readings. Other laboratory results are normal.
We continue phenytoin and phenobarbital at the same dosages and start the selective serotonin reuptake inhibitor (SSRI) citalopram, 20 mg/d, which interacts minimally with both anticonvulsants.
After 2 weeks, Mr. G’s seizures are well controlled and he is tolerating citalopram, but his depressive symptoms have not improved. We cross-taper citalopram to prevent SSRI-induced discontinuation syndrome and start the dopamine and norepinephrine reuptake inhibitor bupropion, 75 mg bid. We titrate bupropion over 2 weeks to 150 mg each morning and 300 mg at bedtime, and watch Mr. G closely for seizures. Although his seizure history contraindicates bupropion use, we think he can tolerate the medication because his seizure disorder is well controlled.
Mr. G’s affect, appetite, and energy are improving with bupropion, but a routine complete blood count (CBC) 5 days after the medication is started reveals leukopenia (WBC 3.04×109/L) without neutropenia (neutrophils 1.9×109/L). Repeat blood tests 18 and 32 days after the first blood draw show continued low WBC. The gastrointestinal medicine team tests Mr. G’s liver function but finds no abnormalities.
The author’s observations
A medical cause also is unlikely. Mr. G’s liver function is normal, and he shows no other signs or symptoms of a medical problem. Bone marrow biopsy and immunologic workup could rule out cancer, but the timing of Mr. G’s abnormal blood readings strongly suggests bupropion intolerance.
TREATMENT: Other medications
We immediately stop bupropion, start the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine at 37.5 mg bid, and titrate it over 5 days to 225 mg/d. Blood draws 3 and 5 days after bupropion discontinuation show slight increases in WBC.
Eleven days after venlafaxine is started, Mr. G’s WBC and neutrophils are normal. However, he has become increasingly irritable and volatile, often arguing with a staff nurse and other patients. We cross-taper venlafaxine over 5 days, start the SSRI sertraline at 50 mg/d, and titrate sertraline over 1 week to 150 mg/d. Mr. G’s irritability and depressive symptoms improve at the latter dosage.
Because Mr. G developed neutropenia while taking a medication not associated with this adverse effect, we start watching his WBC counts more closely than usual. WBC is 4.58×109/L 8 days after sertraline is started but falls to 3.4×109/L after another 8 days, with neutrophils at 1.5×109/L for both readings (Table).
We add lithium, 300 mg bid, to increase Mr. G’s neutrophils and augment sertraline’s antidepressant effects. Four days later, WBC is 5.8×109/L with neutrophils at 4.2×109/L.
We stop lithium briefly to see if WBC remains normal. After 3 days, WBC drops to 3.25×109/L with neutrophils at 1.5×109/L. We restart lithium, 300 mg/d, and Mr. G’s WBC increases to 4.18×109/L 4 days later, with neutrophils at 2.1×109/L.
Table
Mr G’s white blood cell (WBC) and neutrophil counts (NC)*
while taking bupropion and sertraline
| Antidepressant | When measurements were taken | WBC | NC |
| None for several weeks | Baseline, first hospital admission | 5.12×109/L | 3.6×109/L |
| Bupropion, 75 mg bid | 5 days after starting bupropion | 3.04×109/L | 1.9×109/L |
| Bupropion, 450 mg/d total | 23 days after starting bupropion | 3.14×109/L | 1.6×109/L |
| Bupropion, 450 mg/d total | 2 weeks after previous test | 2.73×109/L | 1.6×109/L |
| Sertraline, 150 mg/d | 8 days after starting sertraline (titration period) | 4.58×109/L | 1.5×109/L |
| Sertraline, 150 mg/d | 16 days after starting sertraline | 3.4×109/L | 1.5×109/L |
| Sertraline, 150 mg/d, and lithium, 300 mg bid | 4 days after lithium augmentation | 5.8×109/L | 4.2×109/L |
| None for 3 months | Baseline, second hospital admission | 3.7×109/L | 2.1×109/L |
| Sertraline, 150 mg/d | 12 days after restarting sertraline | 2.83×109/L | Not available |
| * Normal WBC values: 4.5 to 11×109/L; normal neutrophil values: 1.5 to 8×109/L | |||
The authors’ observations
For Mr. G, both bupropion and sertraline appear to have caused neutropenia on separate occasions.
To our knowledge, bupropion-induced leukopenia or neutropenia have not been reported in the literature. Neutropenia—a rare adverse effect of antidepressants2—and leukopenia were seen during bupropion’s pre-marketing trials but were not definitely attributed to the drug.1 According to pre- and post-marketing data, leukopenia was “infrequently” reported among 5,100 subjects who received bupropion.3
To our knowledge, sertraline-induced neutropenia has not been reported in nongeriatric patients, although sertraline-induced neutropenia4 and agranulocytosis5 have been reported in patients age >65. The Committee on Safety of Medicine in the United Kingdom has received 2 other reports of neutropenia and 1 report of leukopenia with sertraline.5
In one clinical trial, 2 of 1,304 patients taking unknown dosages of sertraline had low neutrophils (
Medication is the second most common cause of acquired neutropenia, with infection being most common.6 By definition, drug-induced neutropenia occurs within 4 weeks after starting the drug and usually resolves within 30 days after stopping it.
Neutropenia is an idiosyncratic reaction unrelated to pharmacologic action. Although overall neutropenia incidence is unknown, reported incidence of the rare, more severe agranulocytosis ranges from approximately 1 to 10 cases per million people annually, and medications have been implicated in 70% of these cases.6 Conversely, only 2 of 97 incidental neutropenia cases studied by Lima et al7 were medication-induced.
Drug-induced neutropenia can result from immune-mediated destruction of neutrophils by circulating antibodies or from direct toxic effects upon marrow granulocyte precursors. Whereas immune-mediated onset is acute and explosive, toxic effect is insidious (months to years) and asymptomatic.8 Clozapine is thought to deliver a direct toxic effect, whereas the thyroid-regulating drug propylthiouracil generates anti-neutrophil antibodies.9
Mr. G’s acute onset (within 5 to 16 days of starting bupropion or sertraline) and prompt return of neutropenia after stopping lithium suggest acute immune-mediated circulating neutrophil destruction.
Treating leukopenia
After 4 failed or intolerable antidepressant trials, lithium augmentation seemed reasonable and ultimately improved Mr. G’s neutrophil count and his mood.
Lithium has helped resolve clozapine-induced neutropenia in case reports.10-12 Well-controlled studies, however, have followed only patients with antineoplastic, drug-induced neutropenia.1
By acting on cyclic nucleotides, lithium prompts colony-stimulating factor production, which in turn stimulates neutrophil production by pluripotent stem cells. As with Mr. G, patients reach neutrophilia 3 to 7 days after starting lithium.
If the patient cannot tolerate lithium, try switching antidepressants or using growth factors to increase neutrophils.
Switching antidepressants.The SSRIs escitalopram or paroxetine, or the SNRI duloxetine are effective and do not necessarily cause neutropenia. Start at below-normal dosages to gauge tolerability, then titrate to normal dosages. Avoid tricyclics, which pose a higher risk of neutropenia than other antidepressant classes.
Case reports13,14 associate fluoxetine and mirtazapine with neutropenia. The patient who received mirtazapine, 30 mg/d, later responded well to sertraline, 50 mg/d.13
If the new antidepressant is ineffective, consider adding the mood-stabilizing anticonvulsant lamotrigine, 12.5 mg/d. Increase lamotrigine to 25 mg/d after 1 week, then titrate by 25 mg weekly to 100 to 400 mg/d depending on efficacy and tolerability.
Using growth factors.Although their efficacy is not proven, growth factors are minimally toxic and might have helped Mr. G. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor resolved neutropenia in uncontrolled studies, but results of one randomized controlled trial were equivocal.8
TESTING: CT findings
Approximately 2 months after admission—shortly after a blood draw shows normal WBC and neutrophils—Mr. G complains of dizziness. He says he accidentally hit his head against a side table.
We order a full neurologic workup to check for traumatic brain injury or brain damage caused by long-term alcohol abuse:
- Head CT shows evidence of previous cerebrovascular infarcts in the bilateral frontal and cerebellar lobes and basal ganglia.
- MRI shows atrophied mammillary bodies, fornix, and corpus callosum.
- Magnetic resonance angiography reveals small cerebral vessel disease.
FOLLOW-UP: Awaiting discharge
After 3 months of continuous hospitalization, Mr. G has become euthymic and nonsuicidal, though at times oversensitive and combative. We transfer him to an assisted-living center and continue sertraline, 150 mg/d; phenytoin, 300 mg/d; phenobarbital, 30 mg bid; lithium, 300 mg/d; and trazodone, 50 mg at night as needed for insomnia.
We also place Mr. G in a day treatment program for mentally ill chemical abusers. A psychiatrist sees him every 2 weeks, and staff supervise him daily.
The authors’ observations
Mr. G’s extended hospital stay allowed us to closely observe him and offered ready access to laboratory facilities while we cross-tapered medications. In outpatient treatment, however, a serious and life-threatening medication-induced complication could easily be missed.
For medically healthy outpatients, be sure CBC has been checked ≤6 months before presentation. Monitor CBC and urge the patient to see a primary care doctor if infection symptoms emerge. Watch for gingivitis, tooth abscess, and other oral cavity infections—which often are overlooked—and sore throat or fever.
Also check electrolytes and screen for SSRI-induced hyponatremia at baseline for all at-risk patients.
Stop the offending drug when WBC reaches 9/L or with absolute neutrophil count (ANC) 9/L, then take a peripheral smear to confirm neutropenia. If the patient is asymptomatic, check ANC 2 to 3 times weekly, particularly if he or she recently had an infection or started a medication that can cause neutropenia. Neutropenia should resolve within 6 to 8 weeks of stopping the offending drug.
If neutropenia persists, order bone marrow biopsy in collaboration with an internist or hematologist to test for cancer. If the biopsy is negative, test for:
- HIV infection
- antinuclear antibodies to check for collagen vascular disease
- antineutrophil antibody to rule out immune neutropenia
- serum folate and B12 deficiency secondary to low WBC.
FOLLOW-UP: Stressor and relapse
Seven months later, Mr. G is readmitted for depression. Three months earlier, he had stopped all medications and resumed drinking after a family member died. WBC at admission is 3.70×109/L
We refer Mr. G to an outpatient psychiatrist, who sees him monthly. Several months later, the psychiatrist reports a WBC of 4.58×109/L.
Nearly 1 year later, Mr. G still lives at the assisted-living facility. He has not been rehospitalized for depression, is functioning well, and has a girlfriend.
The authors’ observations
Mr. G’s abnormal blood counts after sertraline rechallenge confirms that the SSRI probably was causing leukopenia. If we had restarted bupropion and neutropenia recurred during that regimen, we could have more certainly established a bupropion-leukopenia connection.
- Neutropenia Support Association. www.neutropenia.ca.
- Baehner RL. Overview of neutropenia.UpToDate Online (version 15.1); March 30, 2006. www.uptodate.com.
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Citalopram • Celexa
- Clozapine • Clozaril
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • various
- Mirtazapine • Remeron
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenobarbital • various
- Phenytoin • Dilantin
- Propylthiouracil • various
- Sertraline • Zoloft
- Trazodone • Desyrel
- Valproic acid • Depakene
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Sad and suicidal
Mr. G, age 44, has chronic depression with suicidality. At presentation he says he has felt sad and suicidal for 2 weeks. He also has no appetite and trouble sleeping at night.
Mr. G’s depression has left him unable to work and has led to 4 hospitalizations over 10 years. He first attempted suicide in 1984 after his ex-wife took their child and left him. He endorses no suicide plan and has been sober for 7 years after 12-plus years of alcohol abuse, but says he has been tempted lately to resume drinking.
The patient was taking an antidepressant but stopped while at a homeless shelter, where he had been staying for several weeks. For more than 20 years, he also has been taking phenytoin, 300 mg/d, and phenobarbital, 30 mg bid, for a seizure disorder.
Mr. G is admitted with a working diagnosis of recurrent major depressive disorder. White blood cell count (WBC) at admission is 5.12×109/L and neutrophils are 3.6×109/L—both low-normal readings. Other laboratory results are normal.
We continue phenytoin and phenobarbital at the same dosages and start the selective serotonin reuptake inhibitor (SSRI) citalopram, 20 mg/d, which interacts minimally with both anticonvulsants.
After 2 weeks, Mr. G’s seizures are well controlled and he is tolerating citalopram, but his depressive symptoms have not improved. We cross-taper citalopram to prevent SSRI-induced discontinuation syndrome and start the dopamine and norepinephrine reuptake inhibitor bupropion, 75 mg bid. We titrate bupropion over 2 weeks to 150 mg each morning and 300 mg at bedtime, and watch Mr. G closely for seizures. Although his seizure history contraindicates bupropion use, we think he can tolerate the medication because his seizure disorder is well controlled.
Mr. G’s affect, appetite, and energy are improving with bupropion, but a routine complete blood count (CBC) 5 days after the medication is started reveals leukopenia (WBC 3.04×109/L) without neutropenia (neutrophils 1.9×109/L). Repeat blood tests 18 and 32 days after the first blood draw show continued low WBC. The gastrointestinal medicine team tests Mr. G’s liver function but finds no abnormalities.
The author’s observations
A medical cause also is unlikely. Mr. G’s liver function is normal, and he shows no other signs or symptoms of a medical problem. Bone marrow biopsy and immunologic workup could rule out cancer, but the timing of Mr. G’s abnormal blood readings strongly suggests bupropion intolerance.
TREATMENT: Other medications
We immediately stop bupropion, start the serotonin-norepinephrine reuptake inhibitor (SNRI) venlafaxine at 37.5 mg bid, and titrate it over 5 days to 225 mg/d. Blood draws 3 and 5 days after bupropion discontinuation show slight increases in WBC.
Eleven days after venlafaxine is started, Mr. G’s WBC and neutrophils are normal. However, he has become increasingly irritable and volatile, often arguing with a staff nurse and other patients. We cross-taper venlafaxine over 5 days, start the SSRI sertraline at 50 mg/d, and titrate sertraline over 1 week to 150 mg/d. Mr. G’s irritability and depressive symptoms improve at the latter dosage.
Because Mr. G developed neutropenia while taking a medication not associated with this adverse effect, we start watching his WBC counts more closely than usual. WBC is 4.58×109/L 8 days after sertraline is started but falls to 3.4×109/L after another 8 days, with neutrophils at 1.5×109/L for both readings (Table).
We add lithium, 300 mg bid, to increase Mr. G’s neutrophils and augment sertraline’s antidepressant effects. Four days later, WBC is 5.8×109/L with neutrophils at 4.2×109/L.
We stop lithium briefly to see if WBC remains normal. After 3 days, WBC drops to 3.25×109/L with neutrophils at 1.5×109/L. We restart lithium, 300 mg/d, and Mr. G’s WBC increases to 4.18×109/L 4 days later, with neutrophils at 2.1×109/L.
Table
Mr G’s white blood cell (WBC) and neutrophil counts (NC)*
while taking bupropion and sertraline
| Antidepressant | When measurements were taken | WBC | NC |
| None for several weeks | Baseline, first hospital admission | 5.12×109/L | 3.6×109/L |
| Bupropion, 75 mg bid | 5 days after starting bupropion | 3.04×109/L | 1.9×109/L |
| Bupropion, 450 mg/d total | 23 days after starting bupropion | 3.14×109/L | 1.6×109/L |
| Bupropion, 450 mg/d total | 2 weeks after previous test | 2.73×109/L | 1.6×109/L |
| Sertraline, 150 mg/d | 8 days after starting sertraline (titration period) | 4.58×109/L | 1.5×109/L |
| Sertraline, 150 mg/d | 16 days after starting sertraline | 3.4×109/L | 1.5×109/L |
| Sertraline, 150 mg/d, and lithium, 300 mg bid | 4 days after lithium augmentation | 5.8×109/L | 4.2×109/L |
| None for 3 months | Baseline, second hospital admission | 3.7×109/L | 2.1×109/L |
| Sertraline, 150 mg/d | 12 days after restarting sertraline | 2.83×109/L | Not available |
| * Normal WBC values: 4.5 to 11×109/L; normal neutrophil values: 1.5 to 8×109/L | |||
The authors’ observations
For Mr. G, both bupropion and sertraline appear to have caused neutropenia on separate occasions.
To our knowledge, bupropion-induced leukopenia or neutropenia have not been reported in the literature. Neutropenia—a rare adverse effect of antidepressants2—and leukopenia were seen during bupropion’s pre-marketing trials but were not definitely attributed to the drug.1 According to pre- and post-marketing data, leukopenia was “infrequently” reported among 5,100 subjects who received bupropion.3
To our knowledge, sertraline-induced neutropenia has not been reported in nongeriatric patients, although sertraline-induced neutropenia4 and agranulocytosis5 have been reported in patients age >65. The Committee on Safety of Medicine in the United Kingdom has received 2 other reports of neutropenia and 1 report of leukopenia with sertraline.5
In one clinical trial, 2 of 1,304 patients taking unknown dosages of sertraline had low neutrophils (
Medication is the second most common cause of acquired neutropenia, with infection being most common.6 By definition, drug-induced neutropenia occurs within 4 weeks after starting the drug and usually resolves within 30 days after stopping it.
Neutropenia is an idiosyncratic reaction unrelated to pharmacologic action. Although overall neutropenia incidence is unknown, reported incidence of the rare, more severe agranulocytosis ranges from approximately 1 to 10 cases per million people annually, and medications have been implicated in 70% of these cases.6 Conversely, only 2 of 97 incidental neutropenia cases studied by Lima et al7 were medication-induced.
Drug-induced neutropenia can result from immune-mediated destruction of neutrophils by circulating antibodies or from direct toxic effects upon marrow granulocyte precursors. Whereas immune-mediated onset is acute and explosive, toxic effect is insidious (months to years) and asymptomatic.8 Clozapine is thought to deliver a direct toxic effect, whereas the thyroid-regulating drug propylthiouracil generates anti-neutrophil antibodies.9
Mr. G’s acute onset (within 5 to 16 days of starting bupropion or sertraline) and prompt return of neutropenia after stopping lithium suggest acute immune-mediated circulating neutrophil destruction.
Treating leukopenia
After 4 failed or intolerable antidepressant trials, lithium augmentation seemed reasonable and ultimately improved Mr. G’s neutrophil count and his mood.
Lithium has helped resolve clozapine-induced neutropenia in case reports.10-12 Well-controlled studies, however, have followed only patients with antineoplastic, drug-induced neutropenia.1
By acting on cyclic nucleotides, lithium prompts colony-stimulating factor production, which in turn stimulates neutrophil production by pluripotent stem cells. As with Mr. G, patients reach neutrophilia 3 to 7 days after starting lithium.
If the patient cannot tolerate lithium, try switching antidepressants or using growth factors to increase neutrophils.
Switching antidepressants.The SSRIs escitalopram or paroxetine, or the SNRI duloxetine are effective and do not necessarily cause neutropenia. Start at below-normal dosages to gauge tolerability, then titrate to normal dosages. Avoid tricyclics, which pose a higher risk of neutropenia than other antidepressant classes.
Case reports13,14 associate fluoxetine and mirtazapine with neutropenia. The patient who received mirtazapine, 30 mg/d, later responded well to sertraline, 50 mg/d.13
If the new antidepressant is ineffective, consider adding the mood-stabilizing anticonvulsant lamotrigine, 12.5 mg/d. Increase lamotrigine to 25 mg/d after 1 week, then titrate by 25 mg weekly to 100 to 400 mg/d depending on efficacy and tolerability.
Using growth factors.Although their efficacy is not proven, growth factors are minimally toxic and might have helped Mr. G. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor resolved neutropenia in uncontrolled studies, but results of one randomized controlled trial were equivocal.8
TESTING: CT findings
Approximately 2 months after admission—shortly after a blood draw shows normal WBC and neutrophils—Mr. G complains of dizziness. He says he accidentally hit his head against a side table.
We order a full neurologic workup to check for traumatic brain injury or brain damage caused by long-term alcohol abuse:
- Head CT shows evidence of previous cerebrovascular infarcts in the bilateral frontal and cerebellar lobes and basal ganglia.
- MRI shows atrophied mammillary bodies, fornix, and corpus callosum.
- Magnetic resonance angiography reveals small cerebral vessel disease.
FOLLOW-UP: Awaiting discharge
After 3 months of continuous hospitalization, Mr. G has become euthymic and nonsuicidal, though at times oversensitive and combative. We transfer him to an assisted-living center and continue sertraline, 150 mg/d; phenytoin, 300 mg/d; phenobarbital, 30 mg bid; lithium, 300 mg/d; and trazodone, 50 mg at night as needed for insomnia.
We also place Mr. G in a day treatment program for mentally ill chemical abusers. A psychiatrist sees him every 2 weeks, and staff supervise him daily.
The authors’ observations
Mr. G’s extended hospital stay allowed us to closely observe him and offered ready access to laboratory facilities while we cross-tapered medications. In outpatient treatment, however, a serious and life-threatening medication-induced complication could easily be missed.
For medically healthy outpatients, be sure CBC has been checked ≤6 months before presentation. Monitor CBC and urge the patient to see a primary care doctor if infection symptoms emerge. Watch for gingivitis, tooth abscess, and other oral cavity infections—which often are overlooked—and sore throat or fever.
Also check electrolytes and screen for SSRI-induced hyponatremia at baseline for all at-risk patients.
Stop the offending drug when WBC reaches 9/L or with absolute neutrophil count (ANC) 9/L, then take a peripheral smear to confirm neutropenia. If the patient is asymptomatic, check ANC 2 to 3 times weekly, particularly if he or she recently had an infection or started a medication that can cause neutropenia. Neutropenia should resolve within 6 to 8 weeks of stopping the offending drug.
If neutropenia persists, order bone marrow biopsy in collaboration with an internist or hematologist to test for cancer. If the biopsy is negative, test for:
- HIV infection
- antinuclear antibodies to check for collagen vascular disease
- antineutrophil antibody to rule out immune neutropenia
- serum folate and B12 deficiency secondary to low WBC.
FOLLOW-UP: Stressor and relapse
Seven months later, Mr. G is readmitted for depression. Three months earlier, he had stopped all medications and resumed drinking after a family member died. WBC at admission is 3.70×109/L
We refer Mr. G to an outpatient psychiatrist, who sees him monthly. Several months later, the psychiatrist reports a WBC of 4.58×109/L.
Nearly 1 year later, Mr. G still lives at the assisted-living facility. He has not been rehospitalized for depression, is functioning well, and has a girlfriend.
The authors’ observations
Mr. G’s abnormal blood counts after sertraline rechallenge confirms that the SSRI probably was causing leukopenia. If we had restarted bupropion and neutropenia recurred during that regimen, we could have more certainly established a bupropion-leukopenia connection.
- Neutropenia Support Association. www.neutropenia.ca.
- Baehner RL. Overview of neutropenia.UpToDate Online (version 15.1); March 30, 2006. www.uptodate.com.
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol, others
- Citalopram • Celexa
- Clozapine • Clozaril
- Duloxetine • Cymbalta
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • various
- Mirtazapine • Remeron
- Oxcarbazepine • Trileptal
- Paroxetine • Paxil
- Phenobarbital • various
- Phenytoin • Dilantin
- Propylthiouracil • various
- Sertraline • Zoloft
- Trazodone • Desyrel
- Valproic acid • Depakene
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. McEvoy G, ed. AHFS drug information. Bethesda, MD: American Society of Health-System Pharmacists; 2005.
2. Nelson JC. Safety and tolerability of the new antidepressants. J Clin Psychiatry 1997;60:1101.-
3. Physicians desk reference, 61st ed. Montvale, NJ: Thomson PDR; 2007.
4. Cohn CK, Shrivastava R, Mendels J, et al. Double-blind, multicenter comparison of sertraline and amitriptyline in elderly depressed patients. J Clin Psychiatry 1990;51(suppl B):28-33.
5. Trescoli-Serrano C, Smith NK. Sertraline-induced agranulocytosis. Postgrad Med J 1996;72:446.-
6. Baehner RL. Overview of neutropenia. UpToDate Online (version 15.1); March 30, 2006. Available at: http://www.uptodate.com. Accessed April 16, 2007.
7. Lima CS, Paula EV, Takahashi T, et al. Causes of incidental neutropenia in adulthood. Ann Hematol 2006;85:705-9.
8. Holland SM, Gallin J. Disorders of granulocytes and monocytes. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s principles of internal medicine, 16th ed. New York: McGraw-Hill; 2005.
9. Baehner RL. Drug-induced neutropenia and agranulocytosis. UpToDate Online (version 15.1); June 8, 2005. Available at: http://www.uptodate.com. Accessed April 16, 2007.
10. Sporn A, Gogtay N, Ortiz-Aguayo R, et al. Clozapine-induced neutropenia in children: management with lithium carbonate. J Child Adolesc Psychopharmacol 2003;13:401-4.
11. Blier P, Slater S, Measham T, et al. Lithium and clozapine-induced neutropenia/agranulocytosis. Int Clin Psychopharmacol 1998;13:137-40.
12. Silverstone P. Prevention of clozapine-induced neutropenia by pretreatment with lithium. J Clin Psychopharmacol 1998;18:86-8.
13. Ozcanli T, Unsalver B, Ozdemir S, Ozmen M. Sertraline and mirtazapine-induced severe neutropenia. Am J Psych 2005;162:1386.-
14. Vilinsky FD, Lubin A. Severe neutropenia associated with fluoxetine hydrochloride. Ann Internal Med 1997;127:573-4.
1. McEvoy G, ed. AHFS drug information. Bethesda, MD: American Society of Health-System Pharmacists; 2005.
2. Nelson JC. Safety and tolerability of the new antidepressants. J Clin Psychiatry 1997;60:1101.-
3. Physicians desk reference, 61st ed. Montvale, NJ: Thomson PDR; 2007.
4. Cohn CK, Shrivastava R, Mendels J, et al. Double-blind, multicenter comparison of sertraline and amitriptyline in elderly depressed patients. J Clin Psychiatry 1990;51(suppl B):28-33.
5. Trescoli-Serrano C, Smith NK. Sertraline-induced agranulocytosis. Postgrad Med J 1996;72:446.-
6. Baehner RL. Overview of neutropenia. UpToDate Online (version 15.1); March 30, 2006. Available at: http://www.uptodate.com. Accessed April 16, 2007.
7. Lima CS, Paula EV, Takahashi T, et al. Causes of incidental neutropenia in adulthood. Ann Hematol 2006;85:705-9.
8. Holland SM, Gallin J. Disorders of granulocytes and monocytes. In: Kasper DL, Braunwald E, Fauci AS, et al, eds. Harrison’s principles of internal medicine, 16th ed. New York: McGraw-Hill; 2005.
9. Baehner RL. Drug-induced neutropenia and agranulocytosis. UpToDate Online (version 15.1); June 8, 2005. Available at: http://www.uptodate.com. Accessed April 16, 2007.
10. Sporn A, Gogtay N, Ortiz-Aguayo R, et al. Clozapine-induced neutropenia in children: management with lithium carbonate. J Child Adolesc Psychopharmacol 2003;13:401-4.
11. Blier P, Slater S, Measham T, et al. Lithium and clozapine-induced neutropenia/agranulocytosis. Int Clin Psychopharmacol 1998;13:137-40.
12. Silverstone P. Prevention of clozapine-induced neutropenia by pretreatment with lithium. J Clin Psychopharmacol 1998;18:86-8.
13. Ozcanli T, Unsalver B, Ozdemir S, Ozmen M. Sertraline and mirtazapine-induced severe neutropenia. Am J Psych 2005;162:1386.-
14. Vilinsky FD, Lubin A. Severe neutropenia associated with fluoxetine hydrochloride. Ann Internal Med 1997;127:573-4.
After 3 months, she’s still ‘mad’
History: ‘They want to kill me’
Police and security agents arrest Ms. A, age 64, at a metropolitan airport. She is extremely agitated and behaving bizarrely, yelling that “the Mafia” is trying to kill her. She has spent 3 days hiding in area hotels, fleeing her “assailants.”
Police arrange Ms. A’s return home; under court order, she is hospitalized in a psychiatric facility. She is diagnosed with paranoid schizophrenia and receives IM haloperidol, 2 mg bid, but shows minimal improvement after 2½ weeks. Her psychotic symptoms improve slightly after the psychiatrist switches her to risperidone, 2 mg bid, but she still cannot function normally. Three weeks after admission, she is transferred to a nursing home for long-term care. She continues risperidone but remains paranoid and delusional.
Three months later, Ms. A is rehospitalized. She is anxious, delusional, confused, and hallucinating at admission. The patient is verbally and physically combative, fearful that medical staff will harm her. She is too violent to be examined, but staff notice that her skin appears thickened, her eyes puffy, and her hair coarse. Her voice sounds low and raspy.
I speak with Ms. A’s son, who reports that before his mother’s arrest he found her in the kitchen wielding a knife, exclaiming she wanted to kill herself. He says she heard a “whoosh” or “ringing” in her right ear while a male voice in her left ear told her, “End it, end it.”
Ms. A is severely obese (weight 325 lbs, body mass index 49 kg/m2). Blood pressure is 140/90 mm Hg, and she is taking captopril, 50 mg bid, for hypertension. Pulse rate and temperature are normal.
Dr. Lachover’s observations
Ms. A’s hallucinatory experiences are atypical, and her psychotic symptoms show little response after 2 months of aggressive inpatient treatment. Three months after discharge, she is rehospitalized in a florid paranoid psychotic state.
The patient’s weight poses an additional obstacle. I avoided second-generation antipsychotics (SGAs) that can cause weight gain, such as clozapine or olanzapine. I tried the SGA risperidone after IM haloperidol, a first-generation antipsychotic, produced minimal response.
Ms. A’s physical symptoms (thickened skin, coarse hair, puffiness under her eyes, and vocal raspiness) suggest an underlying organic process that might be causing her psychosis.
TESTING: Telling results
I order laboratory and other tests to check for an underlying organic disorder:
- Brain MRI is normal, as are CBC, renal and liver function, and serum copper, ceruloplasmin, vitamin B12, and heavy metal levels.
- Slit lamp eye exam reveals no Kayser-Fleischer ring, which would have indicated Wilson’s disease.
- EEG shows a diffuse, nonspecific, abnormal pattern of slowing and decreased amplitude, suggesting diffuse cerebral dysfunction.
- ECG shows sinus bradycardia and a significantly prolonged corrected QT (QTc) interval, indicating delayed ventricular repolarization.
- Thyroid panel is abnormal with markedly elevated thyrotropin (31.07 mIU/L).
Across 3 weeks, Ms. A’s delusional perceptions and hallucination intensity decrease, and her reality testing and socialization skills improve. She is discharged, after which the internist and I see her weekly to monitor thyroid function and psychiatric symptoms, respectively. Thyroid function gradually returns to normal over 4 to 6 months, and she is maintained on levothyroxine, 0.025 mg/d. Her weight gradually decreases over 12 months to 229 lbs.
Six months after discharge, Ms. A is notably more adept at activities of daily living. Mental status exam shows progressively improved reality testing and decreased paranoia. She is more active, and her mood and affect have brightened. Risperidone is stopped 10 months after discharge, and she has not been rehospitalized for psychiatric problems.
Table 1
Ms. A’s thyroid panel values
| Component | Ms. A’s readings | Normal values |
| Serum cholesterol | 310 mg/dL | 100 to 199 mg/dL |
| TSH (thyrotropin) | 31.07 mIU/L | 0.25 to 4.30 mIU/L |
| Free T4 | 0.34 ng/dL | 0.80 to 1.80 ng/dL |
| Total T4 (serum thyroxine) | 1.5 µg/dL | 4.6 to 12 µg/dL |
| Total T3 (serum triiodothyronine) | 67 ng/dL | 70 to 180 ng/dL |
Dr. Lachover’s observations
Erroneously diagnosed with paranoid schizophrenia, Ms. A endured 2 extended hospitalizations. Her psychosis and mental state—both of which improved with thyroid replacement therapy—appear to have been a psychiatric manifestation of severe hypothyroidism, or “myxedema madness” (Box).1-3
Myxedema prevalence in the general public has been reported at 0.5% to 18%. It is roughly 10 times more common in women than in men,4 and 5% to 15% of patients with myxedema might develop signs of psychosis.4 Myxedema-induced psychosis usually occurs during middle age but has been reported between ages 18 and 73. Prevalence increases with age.4
Recognizing ‘myxedema madness’
Detecting and treating myxedema in patients with treatment-resistant psychosis can resolve psychiatric and medical symptoms and restore quality of life. Left untreated, it can impair cognitive function and cause lethargy, dysarthria, myopathy, neuropathy, status epilepticus, and coma.5-7
Myxedema can impair perception and intellectual functioning,9 and acute mania has been reported in some cases.10 Increasing delirium reduces integration of perceptual input, leading to misidentification and disorientation. Cognitive functioning may be impaired, and abnormal thyroid hormone levels might delay event-related brain potential.11
Physical signs also can be telling. The patient might show general psychomotor retardation and slowed speech. The tongue might be swollen, the voice hoarse and croaking. Hair is often coarse and brittle, with hair loss along the sides of the eyebrows. Body temperature often dips below normal.4
Dr. Lachover’s observations
Detecting Ms. A’s hypothyroidism early could have prevented needless hospitalizations and failed treatment. Order a baseline thyroid panel for every patient who presents with psychotic symptoms or depression, which is the primary affective disturbance seen in myxedema.
Researchers have proposed many potential causes for the psychotic and depressive symptoms seen in myxedema.
Psychotic symptoms. Tonks1 has attributed psychosis in myxedema to decreases in cerebral oxygenation and glucose metabolism, resulting in a relative cerebral hypoxia. Among patients with myxedema, Sheinberg et al2 reported markedly reduced cardiac output and found that:
- cerebral blood flow was reduced 38%
- oxygen and glucose absorption were decreased approximately 30%
- cerebrovascular resistance was notably increased.
Depressive symptoms. Catecholamine deficiency at the neuronal receptor sites might cause depression in hypothyroidism. Evidence suggests that thyroid hormone influences catecholamine function at the neuronal level.3
Monoamine oxidase, which is increased in myxedema, has also been implicated. This enzyme might lead to depression by helping to break down catecholamines at the neuronal axon-dendrite levels.3
Diffuse slowing of background activity is the most common EEG change found in myxedema.13 ECG might show slow, regular sinus rhythm or bradycardia, low voltage, prolonged QTc interval, and flattened T waves.14 Prolonged QRS complexes on ECG indicate delayed ventricular repolarization.11,15 Torsades de pointes, the potentially fatal ventricular tachycardia, can result from a prolonged QTc interval in rare myxedema cases.16
Table 2
Is it myxedema? Check the lab findings
| Component | Values that suggest myxedema |
| Serum cholesterol | >200 mg/dL |
| Free T4 | |
| Total T4 (serum thyroxine) | |
| Total T3 (serum triiodothyronine) | |
| TSH (thyrotropin) | >4.5 mIU/L |
| EEG | Diffuse slowing |
| EKG | Prolonged QTc interval |
Treating 2 sets of symptoms
Prescribe concomitant dessicated thyroid and low-dose antipsychotics over 4 to 6 months to treat both the thyroid dysfunction and psychosis. Because weight gain is common in myxedema, choose an antipsychotic that carries a relatively low risk of weight gain, such as risperidone, 2 mg bid, or aripiprazole, 5 to 10 mg/d.
Many patients reach euthyroidism and their psychosis improves gradually but notably over weeks or months after starting thyroid hormone replacement. Psychosis could recur if desiccated thyroid is stopped; restarting it will improve the patient’s mental state.17 Recovery takes about 3 months on average.4
Continue the SGA until delusion perception is gone and reality testing improves, then taper the medication until all psychotic symptoms have abated. Monitor thyroid function monthly.
For patients with myxedema-induced depression, supplement thyroid hormone replacement with a selective serotonin reuptake inhibitor such as sertraline at regular starting dosages.
Dr. Lachover’s observations
Consider contributing medical illness in any patient with psychosis, particularly with psychotic symptom onset after age 40 and lack of response to weeks of adequate antipsychotic therapy.
A meticulous search to rule out medical disorders in all patients with psychosis and/or depression is essential to planning treatment. Testing is especially urgent for elderly patients, as multiple medical comorbidities or medication side effects can mask hypothyroidism’s signs and symptoms and delay diagnosis.18
Check complete blood count, electrolytes, thyroid panel, urinalysis, urine drug screen, blood urea nitrogen, and creatinine to rule out an underlying metabolic or endocrinologic cause for psychosis. Watch for signs of anticholinergic syndrome during physical examination.
If any of the above results suggest a medical problem, test for the following as clinical suspicion warrants:
- serum copper/ceruloplasmin and liver function to rule out Wilson’s disease, a genetic disorder that causes copper to accumulate in the liver and brain
- systemic lupus erythematosus
- lead, magnesium, mercury, or manganese to rule out metal poisoning.
- Cronin AJ. The Citadel. Boston: Little, Brown & Co.;1937:399.
- Asher R. Myxoedamatous madness. BMJ 1949;2:555-62.
- Aripiprazole • Abilify
- Captopril • Capoten
- Clozapine • Clozaril
- Haloperidol • Haldol
- Levothyroxine • Synthroid
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tonks CM. Mental illness and hypothyroid patients. Br J Psychiatry 1964;110:706-10.
2. Scheinberg P, et al. Cerebral metabolism and cardiac output in myxedema. J Clin Invest 1950;29:1139-46.
3. Whybrow PC, Prange AJ, Treadway CR. Mental changes accompanying thyroid gland dysfunction. Arch Gen Psychiatry 1969;20:48-63.
4. Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis: myxedema madness revisited. Prim Care Companion J Clin Psychiatry 2003;5:260-6.
5. Jansen HJ, Doebe SR, Louwerse ES, et al. Status epilepticus caused by a myxoedema coma. Neth J Med 2006;64:202-5.
6. Pimental L, Hansen KN. Thyroid disease in the emergency department: a clinical and laboratory review. J Emerg Med 2005;28:201-9.
7. Wartofsky L. Myxedema coma. Endocrinol Metab Clin North Am 2006;35:687-98.
8. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Int Med 2006;145:573-81.
9. Adams CW. Electrocardiographic changes in hypothyroidism. Chest 1964;46:87-8.
10. Stowell CP, Barnhill JW. Acute mania in the setting of severe hypothyroidism. Psychosomatics 2005;46:259-61.
11. Strachan SR, Afolabi O, Brown N, Gray D. Chest pain, enzymes, and hypothyroidism. Postgrad Med J 2000;76:168-9.
12. Lolas F, de la Parra G, Gramegna G. Event-related slow potential (ERSP) correlates of thyroid gland function levels. Psychosom Med 1978;40:226-35.
13. Pinto A, Glick M. Management of patients with thyroid disease: oral health considerations. J Am Dent Assoc 2002;133:849-58.
14. Khedr EM, El Toony LF, Tarkhan MN, Abdella G. Peripheral and central nervous system alterations in hypothyroidism; electrophysiological findings. Neuropsychobiology 2000;41:88-94.
15. Bosch R, Wang Z, Li GR, Nattel S. Electrophysiological mechanisms by which hypothyroidism delays repolarization in guinea pig hearts. Am J Physiol 1999;277(1 Pt 2):H211-20.
16. Schenck JB, Rizvi AA, Lin T. Severe primary hypothyroidism manifesting with torsades de pointes. Am J Med Sci 2006;331:154-6.
17. McGaffee J, Barnes MA, Lippmann S. Psychiatric presentations of hypothyroidism. Am Fam Physicia 1981;23:129-33.
18. Rehman SU, Cope DW, Senseney AD, Brzezinski W. Thyroid disorders in elderly patients. South Med J 2005;98:543-9.
History: ‘They want to kill me’
Police and security agents arrest Ms. A, age 64, at a metropolitan airport. She is extremely agitated and behaving bizarrely, yelling that “the Mafia” is trying to kill her. She has spent 3 days hiding in area hotels, fleeing her “assailants.”
Police arrange Ms. A’s return home; under court order, she is hospitalized in a psychiatric facility. She is diagnosed with paranoid schizophrenia and receives IM haloperidol, 2 mg bid, but shows minimal improvement after 2½ weeks. Her psychotic symptoms improve slightly after the psychiatrist switches her to risperidone, 2 mg bid, but she still cannot function normally. Three weeks after admission, she is transferred to a nursing home for long-term care. She continues risperidone but remains paranoid and delusional.
Three months later, Ms. A is rehospitalized. She is anxious, delusional, confused, and hallucinating at admission. The patient is verbally and physically combative, fearful that medical staff will harm her. She is too violent to be examined, but staff notice that her skin appears thickened, her eyes puffy, and her hair coarse. Her voice sounds low and raspy.
I speak with Ms. A’s son, who reports that before his mother’s arrest he found her in the kitchen wielding a knife, exclaiming she wanted to kill herself. He says she heard a “whoosh” or “ringing” in her right ear while a male voice in her left ear told her, “End it, end it.”
Ms. A is severely obese (weight 325 lbs, body mass index 49 kg/m2). Blood pressure is 140/90 mm Hg, and she is taking captopril, 50 mg bid, for hypertension. Pulse rate and temperature are normal.
Dr. Lachover’s observations
Ms. A’s hallucinatory experiences are atypical, and her psychotic symptoms show little response after 2 months of aggressive inpatient treatment. Three months after discharge, she is rehospitalized in a florid paranoid psychotic state.
The patient’s weight poses an additional obstacle. I avoided second-generation antipsychotics (SGAs) that can cause weight gain, such as clozapine or olanzapine. I tried the SGA risperidone after IM haloperidol, a first-generation antipsychotic, produced minimal response.
Ms. A’s physical symptoms (thickened skin, coarse hair, puffiness under her eyes, and vocal raspiness) suggest an underlying organic process that might be causing her psychosis.
TESTING: Telling results
I order laboratory and other tests to check for an underlying organic disorder:
- Brain MRI is normal, as are CBC, renal and liver function, and serum copper, ceruloplasmin, vitamin B12, and heavy metal levels.
- Slit lamp eye exam reveals no Kayser-Fleischer ring, which would have indicated Wilson’s disease.
- EEG shows a diffuse, nonspecific, abnormal pattern of slowing and decreased amplitude, suggesting diffuse cerebral dysfunction.
- ECG shows sinus bradycardia and a significantly prolonged corrected QT (QTc) interval, indicating delayed ventricular repolarization.
- Thyroid panel is abnormal with markedly elevated thyrotropin (31.07 mIU/L).
Across 3 weeks, Ms. A’s delusional perceptions and hallucination intensity decrease, and her reality testing and socialization skills improve. She is discharged, after which the internist and I see her weekly to monitor thyroid function and psychiatric symptoms, respectively. Thyroid function gradually returns to normal over 4 to 6 months, and she is maintained on levothyroxine, 0.025 mg/d. Her weight gradually decreases over 12 months to 229 lbs.
Six months after discharge, Ms. A is notably more adept at activities of daily living. Mental status exam shows progressively improved reality testing and decreased paranoia. She is more active, and her mood and affect have brightened. Risperidone is stopped 10 months after discharge, and she has not been rehospitalized for psychiatric problems.
Table 1
Ms. A’s thyroid panel values
| Component | Ms. A’s readings | Normal values |
| Serum cholesterol | 310 mg/dL | 100 to 199 mg/dL |
| TSH (thyrotropin) | 31.07 mIU/L | 0.25 to 4.30 mIU/L |
| Free T4 | 0.34 ng/dL | 0.80 to 1.80 ng/dL |
| Total T4 (serum thyroxine) | 1.5 µg/dL | 4.6 to 12 µg/dL |
| Total T3 (serum triiodothyronine) | 67 ng/dL | 70 to 180 ng/dL |
Dr. Lachover’s observations
Erroneously diagnosed with paranoid schizophrenia, Ms. A endured 2 extended hospitalizations. Her psychosis and mental state—both of which improved with thyroid replacement therapy—appear to have been a psychiatric manifestation of severe hypothyroidism, or “myxedema madness” (Box).1-3
Myxedema prevalence in the general public has been reported at 0.5% to 18%. It is roughly 10 times more common in women than in men,4 and 5% to 15% of patients with myxedema might develop signs of psychosis.4 Myxedema-induced psychosis usually occurs during middle age but has been reported between ages 18 and 73. Prevalence increases with age.4
Recognizing ‘myxedema madness’
Detecting and treating myxedema in patients with treatment-resistant psychosis can resolve psychiatric and medical symptoms and restore quality of life. Left untreated, it can impair cognitive function and cause lethargy, dysarthria, myopathy, neuropathy, status epilepticus, and coma.5-7
Myxedema can impair perception and intellectual functioning,9 and acute mania has been reported in some cases.10 Increasing delirium reduces integration of perceptual input, leading to misidentification and disorientation. Cognitive functioning may be impaired, and abnormal thyroid hormone levels might delay event-related brain potential.11
Physical signs also can be telling. The patient might show general psychomotor retardation and slowed speech. The tongue might be swollen, the voice hoarse and croaking. Hair is often coarse and brittle, with hair loss along the sides of the eyebrows. Body temperature often dips below normal.4
Dr. Lachover’s observations
Detecting Ms. A’s hypothyroidism early could have prevented needless hospitalizations and failed treatment. Order a baseline thyroid panel for every patient who presents with psychotic symptoms or depression, which is the primary affective disturbance seen in myxedema.
Researchers have proposed many potential causes for the psychotic and depressive symptoms seen in myxedema.
Psychotic symptoms. Tonks1 has attributed psychosis in myxedema to decreases in cerebral oxygenation and glucose metabolism, resulting in a relative cerebral hypoxia. Among patients with myxedema, Sheinberg et al2 reported markedly reduced cardiac output and found that:
- cerebral blood flow was reduced 38%
- oxygen and glucose absorption were decreased approximately 30%
- cerebrovascular resistance was notably increased.
Depressive symptoms. Catecholamine deficiency at the neuronal receptor sites might cause depression in hypothyroidism. Evidence suggests that thyroid hormone influences catecholamine function at the neuronal level.3
Monoamine oxidase, which is increased in myxedema, has also been implicated. This enzyme might lead to depression by helping to break down catecholamines at the neuronal axon-dendrite levels.3
Diffuse slowing of background activity is the most common EEG change found in myxedema.13 ECG might show slow, regular sinus rhythm or bradycardia, low voltage, prolonged QTc interval, and flattened T waves.14 Prolonged QRS complexes on ECG indicate delayed ventricular repolarization.11,15 Torsades de pointes, the potentially fatal ventricular tachycardia, can result from a prolonged QTc interval in rare myxedema cases.16
Table 2
Is it myxedema? Check the lab findings
| Component | Values that suggest myxedema |
| Serum cholesterol | >200 mg/dL |
| Free T4 | |
| Total T4 (serum thyroxine) | |
| Total T3 (serum triiodothyronine) | |
| TSH (thyrotropin) | >4.5 mIU/L |
| EEG | Diffuse slowing |
| EKG | Prolonged QTc interval |
Treating 2 sets of symptoms
Prescribe concomitant dessicated thyroid and low-dose antipsychotics over 4 to 6 months to treat both the thyroid dysfunction and psychosis. Because weight gain is common in myxedema, choose an antipsychotic that carries a relatively low risk of weight gain, such as risperidone, 2 mg bid, or aripiprazole, 5 to 10 mg/d.
Many patients reach euthyroidism and their psychosis improves gradually but notably over weeks or months after starting thyroid hormone replacement. Psychosis could recur if desiccated thyroid is stopped; restarting it will improve the patient’s mental state.17 Recovery takes about 3 months on average.4
Continue the SGA until delusion perception is gone and reality testing improves, then taper the medication until all psychotic symptoms have abated. Monitor thyroid function monthly.
For patients with myxedema-induced depression, supplement thyroid hormone replacement with a selective serotonin reuptake inhibitor such as sertraline at regular starting dosages.
Dr. Lachover’s observations
Consider contributing medical illness in any patient with psychosis, particularly with psychotic symptom onset after age 40 and lack of response to weeks of adequate antipsychotic therapy.
A meticulous search to rule out medical disorders in all patients with psychosis and/or depression is essential to planning treatment. Testing is especially urgent for elderly patients, as multiple medical comorbidities or medication side effects can mask hypothyroidism’s signs and symptoms and delay diagnosis.18
Check complete blood count, electrolytes, thyroid panel, urinalysis, urine drug screen, blood urea nitrogen, and creatinine to rule out an underlying metabolic or endocrinologic cause for psychosis. Watch for signs of anticholinergic syndrome during physical examination.
If any of the above results suggest a medical problem, test for the following as clinical suspicion warrants:
- serum copper/ceruloplasmin and liver function to rule out Wilson’s disease, a genetic disorder that causes copper to accumulate in the liver and brain
- systemic lupus erythematosus
- lead, magnesium, mercury, or manganese to rule out metal poisoning.
- Cronin AJ. The Citadel. Boston: Little, Brown & Co.;1937:399.
- Asher R. Myxoedamatous madness. BMJ 1949;2:555-62.
- Aripiprazole • Abilify
- Captopril • Capoten
- Clozapine • Clozaril
- Haloperidol • Haldol
- Levothyroxine • Synthroid
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
History: ‘They want to kill me’
Police and security agents arrest Ms. A, age 64, at a metropolitan airport. She is extremely agitated and behaving bizarrely, yelling that “the Mafia” is trying to kill her. She has spent 3 days hiding in area hotels, fleeing her “assailants.”
Police arrange Ms. A’s return home; under court order, she is hospitalized in a psychiatric facility. She is diagnosed with paranoid schizophrenia and receives IM haloperidol, 2 mg bid, but shows minimal improvement after 2½ weeks. Her psychotic symptoms improve slightly after the psychiatrist switches her to risperidone, 2 mg bid, but she still cannot function normally. Three weeks after admission, she is transferred to a nursing home for long-term care. She continues risperidone but remains paranoid and delusional.
Three months later, Ms. A is rehospitalized. She is anxious, delusional, confused, and hallucinating at admission. The patient is verbally and physically combative, fearful that medical staff will harm her. She is too violent to be examined, but staff notice that her skin appears thickened, her eyes puffy, and her hair coarse. Her voice sounds low and raspy.
I speak with Ms. A’s son, who reports that before his mother’s arrest he found her in the kitchen wielding a knife, exclaiming she wanted to kill herself. He says she heard a “whoosh” or “ringing” in her right ear while a male voice in her left ear told her, “End it, end it.”
Ms. A is severely obese (weight 325 lbs, body mass index 49 kg/m2). Blood pressure is 140/90 mm Hg, and she is taking captopril, 50 mg bid, for hypertension. Pulse rate and temperature are normal.
Dr. Lachover’s observations
Ms. A’s hallucinatory experiences are atypical, and her psychotic symptoms show little response after 2 months of aggressive inpatient treatment. Three months after discharge, she is rehospitalized in a florid paranoid psychotic state.
The patient’s weight poses an additional obstacle. I avoided second-generation antipsychotics (SGAs) that can cause weight gain, such as clozapine or olanzapine. I tried the SGA risperidone after IM haloperidol, a first-generation antipsychotic, produced minimal response.
Ms. A’s physical symptoms (thickened skin, coarse hair, puffiness under her eyes, and vocal raspiness) suggest an underlying organic process that might be causing her psychosis.
TESTING: Telling results
I order laboratory and other tests to check for an underlying organic disorder:
- Brain MRI is normal, as are CBC, renal and liver function, and serum copper, ceruloplasmin, vitamin B12, and heavy metal levels.
- Slit lamp eye exam reveals no Kayser-Fleischer ring, which would have indicated Wilson’s disease.
- EEG shows a diffuse, nonspecific, abnormal pattern of slowing and decreased amplitude, suggesting diffuse cerebral dysfunction.
- ECG shows sinus bradycardia and a significantly prolonged corrected QT (QTc) interval, indicating delayed ventricular repolarization.
- Thyroid panel is abnormal with markedly elevated thyrotropin (31.07 mIU/L).
Across 3 weeks, Ms. A’s delusional perceptions and hallucination intensity decrease, and her reality testing and socialization skills improve. She is discharged, after which the internist and I see her weekly to monitor thyroid function and psychiatric symptoms, respectively. Thyroid function gradually returns to normal over 4 to 6 months, and she is maintained on levothyroxine, 0.025 mg/d. Her weight gradually decreases over 12 months to 229 lbs.
Six months after discharge, Ms. A is notably more adept at activities of daily living. Mental status exam shows progressively improved reality testing and decreased paranoia. She is more active, and her mood and affect have brightened. Risperidone is stopped 10 months after discharge, and she has not been rehospitalized for psychiatric problems.
Table 1
Ms. A’s thyroid panel values
| Component | Ms. A’s readings | Normal values |
| Serum cholesterol | 310 mg/dL | 100 to 199 mg/dL |
| TSH (thyrotropin) | 31.07 mIU/L | 0.25 to 4.30 mIU/L |
| Free T4 | 0.34 ng/dL | 0.80 to 1.80 ng/dL |
| Total T4 (serum thyroxine) | 1.5 µg/dL | 4.6 to 12 µg/dL |
| Total T3 (serum triiodothyronine) | 67 ng/dL | 70 to 180 ng/dL |
Dr. Lachover’s observations
Erroneously diagnosed with paranoid schizophrenia, Ms. A endured 2 extended hospitalizations. Her psychosis and mental state—both of which improved with thyroid replacement therapy—appear to have been a psychiatric manifestation of severe hypothyroidism, or “myxedema madness” (Box).1-3
Myxedema prevalence in the general public has been reported at 0.5% to 18%. It is roughly 10 times more common in women than in men,4 and 5% to 15% of patients with myxedema might develop signs of psychosis.4 Myxedema-induced psychosis usually occurs during middle age but has been reported between ages 18 and 73. Prevalence increases with age.4
Recognizing ‘myxedema madness’
Detecting and treating myxedema in patients with treatment-resistant psychosis can resolve psychiatric and medical symptoms and restore quality of life. Left untreated, it can impair cognitive function and cause lethargy, dysarthria, myopathy, neuropathy, status epilepticus, and coma.5-7
Myxedema can impair perception and intellectual functioning,9 and acute mania has been reported in some cases.10 Increasing delirium reduces integration of perceptual input, leading to misidentification and disorientation. Cognitive functioning may be impaired, and abnormal thyroid hormone levels might delay event-related brain potential.11
Physical signs also can be telling. The patient might show general psychomotor retardation and slowed speech. The tongue might be swollen, the voice hoarse and croaking. Hair is often coarse and brittle, with hair loss along the sides of the eyebrows. Body temperature often dips below normal.4
Dr. Lachover’s observations
Detecting Ms. A’s hypothyroidism early could have prevented needless hospitalizations and failed treatment. Order a baseline thyroid panel for every patient who presents with psychotic symptoms or depression, which is the primary affective disturbance seen in myxedema.
Researchers have proposed many potential causes for the psychotic and depressive symptoms seen in myxedema.
Psychotic symptoms. Tonks1 has attributed psychosis in myxedema to decreases in cerebral oxygenation and glucose metabolism, resulting in a relative cerebral hypoxia. Among patients with myxedema, Sheinberg et al2 reported markedly reduced cardiac output and found that:
- cerebral blood flow was reduced 38%
- oxygen and glucose absorption were decreased approximately 30%
- cerebrovascular resistance was notably increased.
Depressive symptoms. Catecholamine deficiency at the neuronal receptor sites might cause depression in hypothyroidism. Evidence suggests that thyroid hormone influences catecholamine function at the neuronal level.3
Monoamine oxidase, which is increased in myxedema, has also been implicated. This enzyme might lead to depression by helping to break down catecholamines at the neuronal axon-dendrite levels.3
Diffuse slowing of background activity is the most common EEG change found in myxedema.13 ECG might show slow, regular sinus rhythm or bradycardia, low voltage, prolonged QTc interval, and flattened T waves.14 Prolonged QRS complexes on ECG indicate delayed ventricular repolarization.11,15 Torsades de pointes, the potentially fatal ventricular tachycardia, can result from a prolonged QTc interval in rare myxedema cases.16
Table 2
Is it myxedema? Check the lab findings
| Component | Values that suggest myxedema |
| Serum cholesterol | >200 mg/dL |
| Free T4 | |
| Total T4 (serum thyroxine) | |
| Total T3 (serum triiodothyronine) | |
| TSH (thyrotropin) | >4.5 mIU/L |
| EEG | Diffuse slowing |
| EKG | Prolonged QTc interval |
Treating 2 sets of symptoms
Prescribe concomitant dessicated thyroid and low-dose antipsychotics over 4 to 6 months to treat both the thyroid dysfunction and psychosis. Because weight gain is common in myxedema, choose an antipsychotic that carries a relatively low risk of weight gain, such as risperidone, 2 mg bid, or aripiprazole, 5 to 10 mg/d.
Many patients reach euthyroidism and their psychosis improves gradually but notably over weeks or months after starting thyroid hormone replacement. Psychosis could recur if desiccated thyroid is stopped; restarting it will improve the patient’s mental state.17 Recovery takes about 3 months on average.4
Continue the SGA until delusion perception is gone and reality testing improves, then taper the medication until all psychotic symptoms have abated. Monitor thyroid function monthly.
For patients with myxedema-induced depression, supplement thyroid hormone replacement with a selective serotonin reuptake inhibitor such as sertraline at regular starting dosages.
Dr. Lachover’s observations
Consider contributing medical illness in any patient with psychosis, particularly with psychotic symptom onset after age 40 and lack of response to weeks of adequate antipsychotic therapy.
A meticulous search to rule out medical disorders in all patients with psychosis and/or depression is essential to planning treatment. Testing is especially urgent for elderly patients, as multiple medical comorbidities or medication side effects can mask hypothyroidism’s signs and symptoms and delay diagnosis.18
Check complete blood count, electrolytes, thyroid panel, urinalysis, urine drug screen, blood urea nitrogen, and creatinine to rule out an underlying metabolic or endocrinologic cause for psychosis. Watch for signs of anticholinergic syndrome during physical examination.
If any of the above results suggest a medical problem, test for the following as clinical suspicion warrants:
- serum copper/ceruloplasmin and liver function to rule out Wilson’s disease, a genetic disorder that causes copper to accumulate in the liver and brain
- systemic lupus erythematosus
- lead, magnesium, mercury, or manganese to rule out metal poisoning.
- Cronin AJ. The Citadel. Boston: Little, Brown & Co.;1937:399.
- Asher R. Myxoedamatous madness. BMJ 1949;2:555-62.
- Aripiprazole • Abilify
- Captopril • Capoten
- Clozapine • Clozaril
- Haloperidol • Haldol
- Levothyroxine • Synthroid
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tonks CM. Mental illness and hypothyroid patients. Br J Psychiatry 1964;110:706-10.
2. Scheinberg P, et al. Cerebral metabolism and cardiac output in myxedema. J Clin Invest 1950;29:1139-46.
3. Whybrow PC, Prange AJ, Treadway CR. Mental changes accompanying thyroid gland dysfunction. Arch Gen Psychiatry 1969;20:48-63.
4. Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis: myxedema madness revisited. Prim Care Companion J Clin Psychiatry 2003;5:260-6.
5. Jansen HJ, Doebe SR, Louwerse ES, et al. Status epilepticus caused by a myxoedema coma. Neth J Med 2006;64:202-5.
6. Pimental L, Hansen KN. Thyroid disease in the emergency department: a clinical and laboratory review. J Emerg Med 2005;28:201-9.
7. Wartofsky L. Myxedema coma. Endocrinol Metab Clin North Am 2006;35:687-98.
8. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Int Med 2006;145:573-81.
9. Adams CW. Electrocardiographic changes in hypothyroidism. Chest 1964;46:87-8.
10. Stowell CP, Barnhill JW. Acute mania in the setting of severe hypothyroidism. Psychosomatics 2005;46:259-61.
11. Strachan SR, Afolabi O, Brown N, Gray D. Chest pain, enzymes, and hypothyroidism. Postgrad Med J 2000;76:168-9.
12. Lolas F, de la Parra G, Gramegna G. Event-related slow potential (ERSP) correlates of thyroid gland function levels. Psychosom Med 1978;40:226-35.
13. Pinto A, Glick M. Management of patients with thyroid disease: oral health considerations. J Am Dent Assoc 2002;133:849-58.
14. Khedr EM, El Toony LF, Tarkhan MN, Abdella G. Peripheral and central nervous system alterations in hypothyroidism; electrophysiological findings. Neuropsychobiology 2000;41:88-94.
15. Bosch R, Wang Z, Li GR, Nattel S. Electrophysiological mechanisms by which hypothyroidism delays repolarization in guinea pig hearts. Am J Physiol 1999;277(1 Pt 2):H211-20.
16. Schenck JB, Rizvi AA, Lin T. Severe primary hypothyroidism manifesting with torsades de pointes. Am J Med Sci 2006;331:154-6.
17. McGaffee J, Barnes MA, Lippmann S. Psychiatric presentations of hypothyroidism. Am Fam Physicia 1981;23:129-33.
18. Rehman SU, Cope DW, Senseney AD, Brzezinski W. Thyroid disorders in elderly patients. South Med J 2005;98:543-9.
1. Tonks CM. Mental illness and hypothyroid patients. Br J Psychiatry 1964;110:706-10.
2. Scheinberg P, et al. Cerebral metabolism and cardiac output in myxedema. J Clin Invest 1950;29:1139-46.
3. Whybrow PC, Prange AJ, Treadway CR. Mental changes accompanying thyroid gland dysfunction. Arch Gen Psychiatry 1969;20:48-63.
4. Heinrich TW, Grahm G. Hypothyroidism presenting as psychosis: myxedema madness revisited. Prim Care Companion J Clin Psychiatry 2003;5:260-6.
5. Jansen HJ, Doebe SR, Louwerse ES, et al. Status epilepticus caused by a myxoedema coma. Neth J Med 2006;64:202-5.
6. Pimental L, Hansen KN. Thyroid disease in the emergency department: a clinical and laboratory review. J Emerg Med 2005;28:201-9.
7. Wartofsky L. Myxedema coma. Endocrinol Metab Clin North Am 2006;35:687-98.
8. Roberts LM, Pattison H, Roalfe A, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Int Med 2006;145:573-81.
9. Adams CW. Electrocardiographic changes in hypothyroidism. Chest 1964;46:87-8.
10. Stowell CP, Barnhill JW. Acute mania in the setting of severe hypothyroidism. Psychosomatics 2005;46:259-61.
11. Strachan SR, Afolabi O, Brown N, Gray D. Chest pain, enzymes, and hypothyroidism. Postgrad Med J 2000;76:168-9.
12. Lolas F, de la Parra G, Gramegna G. Event-related slow potential (ERSP) correlates of thyroid gland function levels. Psychosom Med 1978;40:226-35.
13. Pinto A, Glick M. Management of patients with thyroid disease: oral health considerations. J Am Dent Assoc 2002;133:849-58.
14. Khedr EM, El Toony LF, Tarkhan MN, Abdella G. Peripheral and central nervous system alterations in hypothyroidism; electrophysiological findings. Neuropsychobiology 2000;41:88-94.
15. Bosch R, Wang Z, Li GR, Nattel S. Electrophysiological mechanisms by which hypothyroidism delays repolarization in guinea pig hearts. Am J Physiol 1999;277(1 Pt 2):H211-20.
16. Schenck JB, Rizvi AA, Lin T. Severe primary hypothyroidism manifesting with torsades de pointes. Am J Med Sci 2006;331:154-6.
17. McGaffee J, Barnes MA, Lippmann S. Psychiatric presentations of hypothyroidism. Am Fam Physicia 1981;23:129-33.
18. Rehman SU, Cope DW, Senseney AD, Brzezinski W. Thyroid disorders in elderly patients. South Med J 2005;98:543-9.
‘Killer trolls’: One older man’s battle
History: bipolar for 30 years
Mr. B, age 66, was diagnosed 30 years ago with type I bipolar disorder and has type 2 diabetes, hypertension, alcohol abuse disorder, and cardiac disease. After repeated suicide attempts and hospitalizations in the past, he has been stable for 20 years on lithium, 600 mg bid, and nortriptyline, 50 mg at bedtime. He has had intermittent mania with little evidence of depression.
Two years ago, Mr. B called a local clinic to report that an intruder had him “holed up.” His speech was pressured and garbled, and his thoughts were tangential, irrational, and markedly paranoid. A clinic psychiatrist called Mr. B’s son, who said his father “built a bomb shelter” because “trolls and little people” were out to kill him. A family member called police, and Mr. B was brought to the ER and admitted for treatment.
A hospital psychiatrist stopped lithium in light of Mr. B’s history of cardiac problems and because the psychiatrist considered the medication ineffective, even though serum lithium was only 0.03 mEq/L. The psychiatrist then started:
- divalproex at 500 mg bid, titrated over 1 week to 500 mg each morning and 1,000 mg at bed-time to reach serum valproate of 80 mEq/L
- quetiapine at 200 mg at bedtime, titrated over 1 week to 400 mg at bedtime.
Two weeks later, Mr. B is brought to another psychiatric hospital, where a psychiatrist restarts unknown dosages of lithium, risperidone, and nortriptyline. From there, he is transferred to our in-patient unit. At presentation, he claims he has been drinking and that members of a drug cartel have recruited him. He says he has been skipping medications because he is “unclear which drugs to take.”
We stop lithium and restart divalproex, 500 mg each morning and 1,500 mg at bedtime, to try to treat his mania without causing cognitive problems.
We stop risperidone because of his hypotension and nortriptyline because it was not working, and restart quetiapine, 600 mg at bedtime, for his paranoia. He remains paranoid 1 week later but his mania improves, so we discharge him on the above regimen. We urge him to take his medications and follow up with his outpatient psychiatrist 1 week later.
Divorced, Mr. B lives alone with no family nearby. His son comes in from out of town to help him resettle after discharge, then leaves the next day.
Several months later, Mr. B’s paranoia returns. He is not taking his medications because “the doctors took away my lithium and these new drugs don’t work.” He tells staff he is a martial arts expert and has purchased 7 cars in recent weeks. We restart lithium at 600 mg bid; serum lithium reaches 1.1 mEq/L, but his mania persists. After 5 days, we add aripiprazole, 15 mg/d.
Nearly 2 weeks after admission, a county hearing officer recommends discharging Mr. B despite his severe mania and paranoia. We release him on the above regimen, arrange appointments with his outpatient psychiatrist and primary care physician, and urge medication adherence. We schedule a blood test 3 days after discharge to check serum lithium, but Mr. B does not keep the appointment.
The authors’ observations
Suspect delirium after rapid onset of mania or paranoia in any patient. Also consider dementia and cognitive deficits in older adults, although Mr. B’s symptoms resembled those of previous manic episodes. Although Mr. B’s psychosis was more severe than before, his case underscores the importance of a thorough patient history.
Late-life bipolar disorder. Little is known about diagnosing and treating bipolar disorder (BPD) in older patients. Gaps in empiric knowledge can confound diagnosis, treatment, and outcome. Also, patients age ≥65 with BPD often have severe medical illness and are difficult to treat.1
Keys to detecting late-life BPD include:
- recognizing clinical features of BPD unique to older persons
- differentiating the disorder from late-life schizophrenia (Table).1,2
Secondary cause. When an older patient’s mania has atypical features or doesn’t respond to conventional treatment, look for a nonpsychiatric process such as a general medical condition or substance abuse (see possible medical causes with this article at www.currentpsychiatry.com). Order laboratory and other tests as clinical suspicion warrants.
Cognitive deficits secondary to BPD can occur at any age and be persistent or progressive,4 although Depp et al1 found more-severe impairment in older patients. Cognitive impairment can endure after successful BPD treatment, although acute treatment might improve cognition in older patients.5
Lithium can cause dull affect, cognitive slowing, and depersonalization. Titrating to the lowest effective dosage might minimize these effects.
Dementia. Cognitive deficits that accompany mania in older adults could suggest dementia, which usually develops over years and is preceded by cognitive changes without manic-type symptoms. By contrast, bipolar mania emerges more abruptly and is accompanied by affective symptoms. Agitation and psychosis—both symptoms of late-stage dementia—can be early signs of geriatric BPD.2
Delirium. Restlessness, irritability, aggression, and changes in affect can accompany delirium, especially the hyperactive or hyperalert types. Symptoms of anxiety, depression, fear, and loose or tangential thinking also are common.
Mania shares some of these features but typically presents with an abnormally and persistently elevated or irritable mood lasting ≥1 week, usually without prominent cognitive impairment.6 Mania can also include:
- grandiosity
- decreased need for sleep
- flight of ideas
- distractibility
- pressured or increased rate of speech
- psychomotor agitation
- potentially harmful activities
- increased goal-directed activities.6
Frontal lobe lesions. Decreased prefrontal executive control could underlie mania’s cognitive and emotional symptoms. Decreased right rostral and orbital prefrontal cortex activation has been associated with impaired planning, judgment, and insight, as well as inappropriate conduct.7
Table
Clinical features of geriatric bipolar disorder (BPD)
| Psychotic features (delusions, hallucinations) |
|
| Family history |
|
| Compared with younger adults with BPD, older patients: |
|
| Compared with late-life schizophrenia, late-life BPD patients show: |
|
| Source: References 1,2 |
Continued treatment: depression emerges
Several months later, Mr. B presents with severe depression and continued medication nonadherence. He complains of hypersomnia, poor appetite, anhedonia, amotivation, and a leaden-like paresis in his hands and feet.
We readmit Mr. B to the psychiatric unit. He avoids contact with others, has lost 18 lbs over 6 weeks, and suffers hypotension caused by poor hydration before admission. Three weeks later, he complains that ants are crawling around his room and into his mouth.
Noncontrast brain CT shows no abnormalities. Laboratory tests performed at admission show a subtherapeutic lithium level (0.03 mEq/L), unremarkable thyroid panel, and normal B12 and folate, so we begin to rule out a medical cause for his psychiatric symptoms.
The authors’ observations
Check for these and other possible causes of depressive symptoms in older patients with a history of BPD. Mr. B’s depression likely resulted from multiple causes, including medical disease, functional impairment, loss of social and family contacts, and substance abuse—all late-life predictors of depression. BPD also predisposed him to depression.
Bipolar depression. Despite its profound morbidity and mortality, bipolar depression remains a mystery, especially in the elderly. Mr. B’s depression emerged after he was free of depressive symptoms for more than 20 years.
Some researchers believe that compared with other depressions, bipolar depression has a more acute onset, marked psychomotor retardation, and lessened response to antidepressants.6,8 Kraepelin associated bipolar depression with lethargy, mental slowing, and hypersomnia, whereas agitation and insomnia signal unipolar depression.9
To differentiate bipolar from unipolar or secondary depression in older patients, watch for:
- suicide risk, which is heightened during BPD’s depressive phase9
- secondary manias, for which underlying causes must be determined and treated if possible.
Depression caused by medication might be limited to somatic complaints such as fatigue or tiredness,9 and often lacks features seen with mood disorders such as depressed mood, anhedonia, guilt, and diminished interest in activities. Mr. B’s anhedonia and amotivation suggest his depression was not medication-induced.10
Disease-induced depression. Medical comorbidities are common among older persons with mood disorders and can complicate treatment response and outcome. Physical disease can cause or worsen depression:11
- Endocrine and immunologic diseases might cause depression or mania.
- Cardiovascular and cerebrovascular diseases; CNS disorders such as dementia, Parkinson’s disease, and multiple sclerosis; cancer; and connective tissue disease increase risk for comorbid depression.
11
Vascular depression. Comorbid depressive symptoms and vascular disease—or “vascular depression”—can cause ischemic brain lesions, cognitive impairment, increased apathy and retardation, and impaired fluency and naming.12
What defines vascular depression has been debated. Watch for clinical or laboratory evidence of vascular disease, depression, and neuropsychological impairment.13
Treatment: searching for evidence
Two days after admission, Mr. B is transferred to the ICU after suffering severe hypoglycemia and showing signs of medically induced delirium. Elevated creatinine (1.7 mg/dL) indicates acute renal failure, which could be related to his elevated serum lithium (1.7 mEq/L). Acting on the internist’s advice, the consulting psychiatrist stops lithium and restarts valproate, 500 mg bid.
Mr. B becomes medically stable after 3 days, mostly through acute IV hydration and by withholding oral diabetes medications, which normalizes his blood sugar. He is transferred back to the psychiatry unit. We try lithium again at 300 mg bid, but creatinine and serum lithium quickly rise.
Mr. B remains hospitalized for 3 months with severe, treatment-resistant depression. Trials of nearly every second-generation antipsychotic (SGA) cause symptomatic orthostatic hypotension, leading to several falls. He does not respond to divalproex, up to 2,000 mg/d; citalopram, 60 mg/d; mirtazapine, 30 mg at bedtime; venlafaxine, 100 mg tid; or bupropion, 100 mg tid.
We suggest electroconvulsive therapy (ECT) but Mr. B declines, saying this treatment caused his mother to decompensate. We try lamotrigine, 25 mg/d, and titrate it over 6 weeks to 200 mg bid. After we add haloperidol, 5 mg at bedtime, and bupropion, 300 mg/d, Mr. B becomes mentally stable.
The authors’ observations
Numerous clinical challenges—such as managing complicated/refractory BPD, medical comorbidity, and medication adherence (Box)14,15—complicate treatment of late-life BPD.16 Regular communication with providers and integrating health care services can minimize complication risk.16
Pharmacotherapy, a core element of BPD treatment, is challenging in older patients because of their:
- heightened threat of complications and sensitivity to side effects because of age-related pharmacokinetic changes
- increased risk of drug-drug interactions
- increased potential for age-related psychosocial problems (increased social isolation, financial difficulties, demoralization, increased stress, inability to work).
Between 40% and 60% of patients do not take medications as prescribed.14 That percentage probably is higher among cognitively impaired older adults because cognitive problems can compound other causes of nonadherence.
Few published controlled clinical trials have addressed adherence interventions for older adults. Educational approaches combined with cognitive supports are most likely to succeed. Ownby et al15 hypothesized that effective approaches usually employ multiple components including counseling, information reminders, and family therapy.
Techniques for improving adherence include:
- addressing the patient’s beliefs about his or her illness
- exploring how patient characteristics affect medication adherence
- use of memory aids, such as 7-day pill boxes
- working with caregivers
- prescribing lower-than-normal dosages to minimize side effects.
Consider side effects, medical and neurologic comorbidities, and treatment history before prescribing a mood stabilizer, antipsychotic, or antidepressant to an older patient. Avoid unwarranted discontinuation of a previously effective agent, such as when drug concentrations are elevated or inadequate—as happened with Mr. B.5 Also investigate the patient’s side-effect history before stopping a medication.5
Medications. Mr. B’s inability to tolerate lithium posed a treatment challenge. Adjusting lithium dosages to compensate for age-related pharmacokinetic, pharmacodynamic, and renal clearance changes can prevent toxicity.5 Avoid stopping lithium abruptly, as this can trigger recurrence of manic or depressive episodes.17
Lamotrigine, indicated for BPD maintenance therapy, appears to prevent depressive/mood relapse. Compared with other anticonvulsants, lamotrigine might cause fewer negative effects on cognition and less induction of hepatic enzymes. It is well tolerated by older patients but has not been studied adequately in this age group.16
Antipsychotics are widely used in BPD,16 especially when psychosis is present with mania or depression or the patient is agitated. Most studies of antipsychotics in BPD have followed younger adults, however, and most studies in older patients have followed those with dementia or schizophrenia.
Use of first-generation antipsychotics such as haloperidol is especially challenging in the elderly because these drugs increase risk of cardiovascular effects, extrapyramidal symptoms, and tardive dyskinesia and can cause depression in BPD.16 By comparison, SGAs carry a lower risk of involuntary motion18 but can increase risk of obesity, diabetes, and dyslipidemia. However:
- The need to manage psychosis usually overrides concerns about metabolic sequelae.
- Older patients might be less susceptible to metabolic effects,16 though this has not been confirmed.
SGAs can be used safely in patients with a history of diabetes. Start at lower-than-normal dosages and titrate slowly. Perform baseline and regular checks—including weight, blood glucose, lipid levels, and blood pressure—following American Psychiatric Association and American Diabetes Association consensus guidelines.19 Also check glycosylated hemoglobin every 3 to 6 months in patients with diabetes, and follow up with other providers to ensure proper diabetes management.
As with most aspects of late-life BPD, scant evidence guides SGA use. Avoid low-potency neuroleptics such as chlorpromazine, which can cause severe sedation and orthostatic hypotension. For Mr. B, a more-tolerable SGA such as aripiprazole or ziprasidone might be prudent, given his propensity for orthostatic hypotension and history of diabetes. Olanzapine or clozapine can cause anticholinergic effects and—in Mr. B’s case—lead to weight gain and worsen diabetes.
Antidepressant use in BPD usually is reserved for depressive symptoms that impair occupational or social functioning and exceed DSM-IV-TR diagnostic criteria.8,9 Consider later-generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs), because tricyclics pose a greater risk of triggering a switch into hypomania or mania and can cause sedation and orthostatic, cardiac, anticholinergic, and anti-alpha 1 effects.
Among SSRIs, consider citalopram, escitalopram, or sertraline for older patients taking one or more other medications, as these antidepressants have less potential for drug-drug interactions than fluoxetine and paroxetine.11 In a recent comparison of newer antidepressants,20 venlafaxine showed the highest relative risk of mood polarity switching and bupropion the lowest.
Consider ECT for older patients with refractory mania or depression or who show evidence of suicidality or inadequate nutrition.5
Follow-up: ongoing issues
Three months after his admission, we discharge Mr. B to a board-and-care facility because family members will not take him in. Several weeks later, he again ignores his prescriptions and decompensates with worsening depression.
Family members have Mr. B admitted to an inpatient psychiatric facility closer to their home. He remains depressed, stays at the facility on and off for almost 1 year, and is eventually conserved by the county. Adverse side effects—mostly constipation and orthostatic hypotension—continue to complicate treatment.
Before Mr. B’s most recent discharge, another psychiatrist restarts lithium, 300 mg bid, and nortriptyline, 100 mg at bedtime—the combination that kept Mr. B relatively stable for more than 2 decades.
- National Institute of Mental Health—depression information. www.nimh.nih.gov/healthinformation/depressionmenu.cfm.
- Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). www.stepbd.org.
- Medicinenet.com. Medicines that cause depression. www.medicinenet.com/depression/index.htm. Click on “Medicines that cause depression.”
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Various
- Mirtazapine • Remeron
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Ziprasidone • Geodon
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Depp C, Jeste D. Bipolar disorder in older adults: a critical review. Bipolar Disord 2004;6:343-67.
2. Sajatovic M, Blow FC, Ignacio RV, Kales HC. New-onset bipolar disorder in later life. Am J Geriatr Psychiatry 2005;13:282-9.
3. Arciniegas DB. New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry 2006;163:198-203.
4. Young RC. Bipolar disorder in older persons: perspectives and new findings. Am J Geriatr Psychiatry 2005;13:265-7.
5. Young RC. Evidence-based pharmacological treatment of geriatric bipolar disorder. Psychiatr Clin North Am 2005;28:837-69.
6. Diagnostic and statistical manual of mental disorders, 4 ed, text rev. Washington, DC; American Psychiatric Association; 2000.
7. Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999;156:1986-8.
8. Gitlin M. Treatment-resistant bipolar disorder. Mol Psychiatry 2006;11:227-40.
9. Dubovsky SL. Treatment of bipolar depression. Psychiatr Clin N Am 2005;28:349-70.
10. Kroenke K. A 75-year-old man with depression. JAMA 2002;287:1568-76.
11. Shanmugham B, Karp J, Drayer R, et al. Evidence-based pharmacological interventions for geriatric depression. Psychiatr Clin N Am 2005;28:821-35.
12. Alexopoulos GS. In: Sadavoy J, Jarvik LF, Grossberg GT, Meyers BS, eds. Comprehensive textbook of geriatric psychiatry, 3 ed. New York: WW Norton and Co.; 2002:609-53.
13. Sneed JR, Roose SP, Sackeim HA. Vascular depression: a distinct diagnostic subtype? Biol Psychiatry 2006;60:1295-8.
14. Higgins N, Regan C. A systematic review of the effectiveness of interventions to help older people adhere to medication regimes. Age Ageing 2004;33:224-9.
15. Ownby RL, Hertzog C, Crocco E, Duara R. Factors related to medication adherence in memory disorder clinic patients. Aging & Mental Health 2006;10:378-85.
16. Sajatovic M, Madhusoodanan S, Coconcea N. Managing bipolar disorder in the elderly: defining the role of the newer agents. Drugs Aging 2005;22:39-54.
17. Cavanagh J, Smyth R, Goodwin GM. Relapse into mania or depression following lithium discontinuation: a 7-year follow up. Acta Psychiatr Scand 2004;109:91-5.
18. Dunner DL. Atypical antipsychotics: efficacy across bipolar disorder subpopulations. J Clin Psychiatry 2005;66(suppl 3):20-7.
19. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
20. Leverich GS, Altshuler LL, Frye MA, et al. Risk of switch in mood polarity to hypomania or mania in patient with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry 2006;163:232-9.
History: bipolar for 30 years
Mr. B, age 66, was diagnosed 30 years ago with type I bipolar disorder and has type 2 diabetes, hypertension, alcohol abuse disorder, and cardiac disease. After repeated suicide attempts and hospitalizations in the past, he has been stable for 20 years on lithium, 600 mg bid, and nortriptyline, 50 mg at bedtime. He has had intermittent mania with little evidence of depression.
Two years ago, Mr. B called a local clinic to report that an intruder had him “holed up.” His speech was pressured and garbled, and his thoughts were tangential, irrational, and markedly paranoid. A clinic psychiatrist called Mr. B’s son, who said his father “built a bomb shelter” because “trolls and little people” were out to kill him. A family member called police, and Mr. B was brought to the ER and admitted for treatment.
A hospital psychiatrist stopped lithium in light of Mr. B’s history of cardiac problems and because the psychiatrist considered the medication ineffective, even though serum lithium was only 0.03 mEq/L. The psychiatrist then started:
- divalproex at 500 mg bid, titrated over 1 week to 500 mg each morning and 1,000 mg at bed-time to reach serum valproate of 80 mEq/L
- quetiapine at 200 mg at bedtime, titrated over 1 week to 400 mg at bedtime.
Two weeks later, Mr. B is brought to another psychiatric hospital, where a psychiatrist restarts unknown dosages of lithium, risperidone, and nortriptyline. From there, he is transferred to our in-patient unit. At presentation, he claims he has been drinking and that members of a drug cartel have recruited him. He says he has been skipping medications because he is “unclear which drugs to take.”
We stop lithium and restart divalproex, 500 mg each morning and 1,500 mg at bedtime, to try to treat his mania without causing cognitive problems.
We stop risperidone because of his hypotension and nortriptyline because it was not working, and restart quetiapine, 600 mg at bedtime, for his paranoia. He remains paranoid 1 week later but his mania improves, so we discharge him on the above regimen. We urge him to take his medications and follow up with his outpatient psychiatrist 1 week later.
Divorced, Mr. B lives alone with no family nearby. His son comes in from out of town to help him resettle after discharge, then leaves the next day.
Several months later, Mr. B’s paranoia returns. He is not taking his medications because “the doctors took away my lithium and these new drugs don’t work.” He tells staff he is a martial arts expert and has purchased 7 cars in recent weeks. We restart lithium at 600 mg bid; serum lithium reaches 1.1 mEq/L, but his mania persists. After 5 days, we add aripiprazole, 15 mg/d.
Nearly 2 weeks after admission, a county hearing officer recommends discharging Mr. B despite his severe mania and paranoia. We release him on the above regimen, arrange appointments with his outpatient psychiatrist and primary care physician, and urge medication adherence. We schedule a blood test 3 days after discharge to check serum lithium, but Mr. B does not keep the appointment.
The authors’ observations
Suspect delirium after rapid onset of mania or paranoia in any patient. Also consider dementia and cognitive deficits in older adults, although Mr. B’s symptoms resembled those of previous manic episodes. Although Mr. B’s psychosis was more severe than before, his case underscores the importance of a thorough patient history.
Late-life bipolar disorder. Little is known about diagnosing and treating bipolar disorder (BPD) in older patients. Gaps in empiric knowledge can confound diagnosis, treatment, and outcome. Also, patients age ≥65 with BPD often have severe medical illness and are difficult to treat.1
Keys to detecting late-life BPD include:
- recognizing clinical features of BPD unique to older persons
- differentiating the disorder from late-life schizophrenia (Table).1,2
Secondary cause. When an older patient’s mania has atypical features or doesn’t respond to conventional treatment, look for a nonpsychiatric process such as a general medical condition or substance abuse (see possible medical causes with this article at www.currentpsychiatry.com). Order laboratory and other tests as clinical suspicion warrants.
Cognitive deficits secondary to BPD can occur at any age and be persistent or progressive,4 although Depp et al1 found more-severe impairment in older patients. Cognitive impairment can endure after successful BPD treatment, although acute treatment might improve cognition in older patients.5
Lithium can cause dull affect, cognitive slowing, and depersonalization. Titrating to the lowest effective dosage might minimize these effects.
Dementia. Cognitive deficits that accompany mania in older adults could suggest dementia, which usually develops over years and is preceded by cognitive changes without manic-type symptoms. By contrast, bipolar mania emerges more abruptly and is accompanied by affective symptoms. Agitation and psychosis—both symptoms of late-stage dementia—can be early signs of geriatric BPD.2
Delirium. Restlessness, irritability, aggression, and changes in affect can accompany delirium, especially the hyperactive or hyperalert types. Symptoms of anxiety, depression, fear, and loose or tangential thinking also are common.
Mania shares some of these features but typically presents with an abnormally and persistently elevated or irritable mood lasting ≥1 week, usually without prominent cognitive impairment.6 Mania can also include:
- grandiosity
- decreased need for sleep
- flight of ideas
- distractibility
- pressured or increased rate of speech
- psychomotor agitation
- potentially harmful activities
- increased goal-directed activities.6
Frontal lobe lesions. Decreased prefrontal executive control could underlie mania’s cognitive and emotional symptoms. Decreased right rostral and orbital prefrontal cortex activation has been associated with impaired planning, judgment, and insight, as well as inappropriate conduct.7
Table
Clinical features of geriatric bipolar disorder (BPD)
| Psychotic features (delusions, hallucinations) |
|
| Family history |
|
| Compared with younger adults with BPD, older patients: |
|
| Compared with late-life schizophrenia, late-life BPD patients show: |
|
| Source: References 1,2 |
Continued treatment: depression emerges
Several months later, Mr. B presents with severe depression and continued medication nonadherence. He complains of hypersomnia, poor appetite, anhedonia, amotivation, and a leaden-like paresis in his hands and feet.
We readmit Mr. B to the psychiatric unit. He avoids contact with others, has lost 18 lbs over 6 weeks, and suffers hypotension caused by poor hydration before admission. Three weeks later, he complains that ants are crawling around his room and into his mouth.
Noncontrast brain CT shows no abnormalities. Laboratory tests performed at admission show a subtherapeutic lithium level (0.03 mEq/L), unremarkable thyroid panel, and normal B12 and folate, so we begin to rule out a medical cause for his psychiatric symptoms.
The authors’ observations
Check for these and other possible causes of depressive symptoms in older patients with a history of BPD. Mr. B’s depression likely resulted from multiple causes, including medical disease, functional impairment, loss of social and family contacts, and substance abuse—all late-life predictors of depression. BPD also predisposed him to depression.
Bipolar depression. Despite its profound morbidity and mortality, bipolar depression remains a mystery, especially in the elderly. Mr. B’s depression emerged after he was free of depressive symptoms for more than 20 years.
Some researchers believe that compared with other depressions, bipolar depression has a more acute onset, marked psychomotor retardation, and lessened response to antidepressants.6,8 Kraepelin associated bipolar depression with lethargy, mental slowing, and hypersomnia, whereas agitation and insomnia signal unipolar depression.9
To differentiate bipolar from unipolar or secondary depression in older patients, watch for:
- suicide risk, which is heightened during BPD’s depressive phase9
- secondary manias, for which underlying causes must be determined and treated if possible.
Depression caused by medication might be limited to somatic complaints such as fatigue or tiredness,9 and often lacks features seen with mood disorders such as depressed mood, anhedonia, guilt, and diminished interest in activities. Mr. B’s anhedonia and amotivation suggest his depression was not medication-induced.10
Disease-induced depression. Medical comorbidities are common among older persons with mood disorders and can complicate treatment response and outcome. Physical disease can cause or worsen depression:11
- Endocrine and immunologic diseases might cause depression or mania.
- Cardiovascular and cerebrovascular diseases; CNS disorders such as dementia, Parkinson’s disease, and multiple sclerosis; cancer; and connective tissue disease increase risk for comorbid depression.
11
Vascular depression. Comorbid depressive symptoms and vascular disease—or “vascular depression”—can cause ischemic brain lesions, cognitive impairment, increased apathy and retardation, and impaired fluency and naming.12
What defines vascular depression has been debated. Watch for clinical or laboratory evidence of vascular disease, depression, and neuropsychological impairment.13
Treatment: searching for evidence
Two days after admission, Mr. B is transferred to the ICU after suffering severe hypoglycemia and showing signs of medically induced delirium. Elevated creatinine (1.7 mg/dL) indicates acute renal failure, which could be related to his elevated serum lithium (1.7 mEq/L). Acting on the internist’s advice, the consulting psychiatrist stops lithium and restarts valproate, 500 mg bid.
Mr. B becomes medically stable after 3 days, mostly through acute IV hydration and by withholding oral diabetes medications, which normalizes his blood sugar. He is transferred back to the psychiatry unit. We try lithium again at 300 mg bid, but creatinine and serum lithium quickly rise.
Mr. B remains hospitalized for 3 months with severe, treatment-resistant depression. Trials of nearly every second-generation antipsychotic (SGA) cause symptomatic orthostatic hypotension, leading to several falls. He does not respond to divalproex, up to 2,000 mg/d; citalopram, 60 mg/d; mirtazapine, 30 mg at bedtime; venlafaxine, 100 mg tid; or bupropion, 100 mg tid.
We suggest electroconvulsive therapy (ECT) but Mr. B declines, saying this treatment caused his mother to decompensate. We try lamotrigine, 25 mg/d, and titrate it over 6 weeks to 200 mg bid. After we add haloperidol, 5 mg at bedtime, and bupropion, 300 mg/d, Mr. B becomes mentally stable.
The authors’ observations
Numerous clinical challenges—such as managing complicated/refractory BPD, medical comorbidity, and medication adherence (Box)14,15—complicate treatment of late-life BPD.16 Regular communication with providers and integrating health care services can minimize complication risk.16
Pharmacotherapy, a core element of BPD treatment, is challenging in older patients because of their:
- heightened threat of complications and sensitivity to side effects because of age-related pharmacokinetic changes
- increased risk of drug-drug interactions
- increased potential for age-related psychosocial problems (increased social isolation, financial difficulties, demoralization, increased stress, inability to work).
Between 40% and 60% of patients do not take medications as prescribed.14 That percentage probably is higher among cognitively impaired older adults because cognitive problems can compound other causes of nonadherence.
Few published controlled clinical trials have addressed adherence interventions for older adults. Educational approaches combined with cognitive supports are most likely to succeed. Ownby et al15 hypothesized that effective approaches usually employ multiple components including counseling, information reminders, and family therapy.
Techniques for improving adherence include:
- addressing the patient’s beliefs about his or her illness
- exploring how patient characteristics affect medication adherence
- use of memory aids, such as 7-day pill boxes
- working with caregivers
- prescribing lower-than-normal dosages to minimize side effects.
Consider side effects, medical and neurologic comorbidities, and treatment history before prescribing a mood stabilizer, antipsychotic, or antidepressant to an older patient. Avoid unwarranted discontinuation of a previously effective agent, such as when drug concentrations are elevated or inadequate—as happened with Mr. B.5 Also investigate the patient’s side-effect history before stopping a medication.5
Medications. Mr. B’s inability to tolerate lithium posed a treatment challenge. Adjusting lithium dosages to compensate for age-related pharmacokinetic, pharmacodynamic, and renal clearance changes can prevent toxicity.5 Avoid stopping lithium abruptly, as this can trigger recurrence of manic or depressive episodes.17
Lamotrigine, indicated for BPD maintenance therapy, appears to prevent depressive/mood relapse. Compared with other anticonvulsants, lamotrigine might cause fewer negative effects on cognition and less induction of hepatic enzymes. It is well tolerated by older patients but has not been studied adequately in this age group.16
Antipsychotics are widely used in BPD,16 especially when psychosis is present with mania or depression or the patient is agitated. Most studies of antipsychotics in BPD have followed younger adults, however, and most studies in older patients have followed those with dementia or schizophrenia.
Use of first-generation antipsychotics such as haloperidol is especially challenging in the elderly because these drugs increase risk of cardiovascular effects, extrapyramidal symptoms, and tardive dyskinesia and can cause depression in BPD.16 By comparison, SGAs carry a lower risk of involuntary motion18 but can increase risk of obesity, diabetes, and dyslipidemia. However:
- The need to manage psychosis usually overrides concerns about metabolic sequelae.
- Older patients might be less susceptible to metabolic effects,16 though this has not been confirmed.
SGAs can be used safely in patients with a history of diabetes. Start at lower-than-normal dosages and titrate slowly. Perform baseline and regular checks—including weight, blood glucose, lipid levels, and blood pressure—following American Psychiatric Association and American Diabetes Association consensus guidelines.19 Also check glycosylated hemoglobin every 3 to 6 months in patients with diabetes, and follow up with other providers to ensure proper diabetes management.
As with most aspects of late-life BPD, scant evidence guides SGA use. Avoid low-potency neuroleptics such as chlorpromazine, which can cause severe sedation and orthostatic hypotension. For Mr. B, a more-tolerable SGA such as aripiprazole or ziprasidone might be prudent, given his propensity for orthostatic hypotension and history of diabetes. Olanzapine or clozapine can cause anticholinergic effects and—in Mr. B’s case—lead to weight gain and worsen diabetes.
Antidepressant use in BPD usually is reserved for depressive symptoms that impair occupational or social functioning and exceed DSM-IV-TR diagnostic criteria.8,9 Consider later-generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs), because tricyclics pose a greater risk of triggering a switch into hypomania or mania and can cause sedation and orthostatic, cardiac, anticholinergic, and anti-alpha 1 effects.
Among SSRIs, consider citalopram, escitalopram, or sertraline for older patients taking one or more other medications, as these antidepressants have less potential for drug-drug interactions than fluoxetine and paroxetine.11 In a recent comparison of newer antidepressants,20 venlafaxine showed the highest relative risk of mood polarity switching and bupropion the lowest.
Consider ECT for older patients with refractory mania or depression or who show evidence of suicidality or inadequate nutrition.5
Follow-up: ongoing issues
Three months after his admission, we discharge Mr. B to a board-and-care facility because family members will not take him in. Several weeks later, he again ignores his prescriptions and decompensates with worsening depression.
Family members have Mr. B admitted to an inpatient psychiatric facility closer to their home. He remains depressed, stays at the facility on and off for almost 1 year, and is eventually conserved by the county. Adverse side effects—mostly constipation and orthostatic hypotension—continue to complicate treatment.
Before Mr. B’s most recent discharge, another psychiatrist restarts lithium, 300 mg bid, and nortriptyline, 100 mg at bedtime—the combination that kept Mr. B relatively stable for more than 2 decades.
- National Institute of Mental Health—depression information. www.nimh.nih.gov/healthinformation/depressionmenu.cfm.
- Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). www.stepbd.org.
- Medicinenet.com. Medicines that cause depression. www.medicinenet.com/depression/index.htm. Click on “Medicines that cause depression.”
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Various
- Mirtazapine • Remeron
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Ziprasidone • Geodon
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
History: bipolar for 30 years
Mr. B, age 66, was diagnosed 30 years ago with type I bipolar disorder and has type 2 diabetes, hypertension, alcohol abuse disorder, and cardiac disease. After repeated suicide attempts and hospitalizations in the past, he has been stable for 20 years on lithium, 600 mg bid, and nortriptyline, 50 mg at bedtime. He has had intermittent mania with little evidence of depression.
Two years ago, Mr. B called a local clinic to report that an intruder had him “holed up.” His speech was pressured and garbled, and his thoughts were tangential, irrational, and markedly paranoid. A clinic psychiatrist called Mr. B’s son, who said his father “built a bomb shelter” because “trolls and little people” were out to kill him. A family member called police, and Mr. B was brought to the ER and admitted for treatment.
A hospital psychiatrist stopped lithium in light of Mr. B’s history of cardiac problems and because the psychiatrist considered the medication ineffective, even though serum lithium was only 0.03 mEq/L. The psychiatrist then started:
- divalproex at 500 mg bid, titrated over 1 week to 500 mg each morning and 1,000 mg at bed-time to reach serum valproate of 80 mEq/L
- quetiapine at 200 mg at bedtime, titrated over 1 week to 400 mg at bedtime.
Two weeks later, Mr. B is brought to another psychiatric hospital, where a psychiatrist restarts unknown dosages of lithium, risperidone, and nortriptyline. From there, he is transferred to our in-patient unit. At presentation, he claims he has been drinking and that members of a drug cartel have recruited him. He says he has been skipping medications because he is “unclear which drugs to take.”
We stop lithium and restart divalproex, 500 mg each morning and 1,500 mg at bedtime, to try to treat his mania without causing cognitive problems.
We stop risperidone because of his hypotension and nortriptyline because it was not working, and restart quetiapine, 600 mg at bedtime, for his paranoia. He remains paranoid 1 week later but his mania improves, so we discharge him on the above regimen. We urge him to take his medications and follow up with his outpatient psychiatrist 1 week later.
Divorced, Mr. B lives alone with no family nearby. His son comes in from out of town to help him resettle after discharge, then leaves the next day.
Several months later, Mr. B’s paranoia returns. He is not taking his medications because “the doctors took away my lithium and these new drugs don’t work.” He tells staff he is a martial arts expert and has purchased 7 cars in recent weeks. We restart lithium at 600 mg bid; serum lithium reaches 1.1 mEq/L, but his mania persists. After 5 days, we add aripiprazole, 15 mg/d.
Nearly 2 weeks after admission, a county hearing officer recommends discharging Mr. B despite his severe mania and paranoia. We release him on the above regimen, arrange appointments with his outpatient psychiatrist and primary care physician, and urge medication adherence. We schedule a blood test 3 days after discharge to check serum lithium, but Mr. B does not keep the appointment.
The authors’ observations
Suspect delirium after rapid onset of mania or paranoia in any patient. Also consider dementia and cognitive deficits in older adults, although Mr. B’s symptoms resembled those of previous manic episodes. Although Mr. B’s psychosis was more severe than before, his case underscores the importance of a thorough patient history.
Late-life bipolar disorder. Little is known about diagnosing and treating bipolar disorder (BPD) in older patients. Gaps in empiric knowledge can confound diagnosis, treatment, and outcome. Also, patients age ≥65 with BPD often have severe medical illness and are difficult to treat.1
Keys to detecting late-life BPD include:
- recognizing clinical features of BPD unique to older persons
- differentiating the disorder from late-life schizophrenia (Table).1,2
Secondary cause. When an older patient’s mania has atypical features or doesn’t respond to conventional treatment, look for a nonpsychiatric process such as a general medical condition or substance abuse (see possible medical causes with this article at www.currentpsychiatry.com). Order laboratory and other tests as clinical suspicion warrants.
Cognitive deficits secondary to BPD can occur at any age and be persistent or progressive,4 although Depp et al1 found more-severe impairment in older patients. Cognitive impairment can endure after successful BPD treatment, although acute treatment might improve cognition in older patients.5
Lithium can cause dull affect, cognitive slowing, and depersonalization. Titrating to the lowest effective dosage might minimize these effects.
Dementia. Cognitive deficits that accompany mania in older adults could suggest dementia, which usually develops over years and is preceded by cognitive changes without manic-type symptoms. By contrast, bipolar mania emerges more abruptly and is accompanied by affective symptoms. Agitation and psychosis—both symptoms of late-stage dementia—can be early signs of geriatric BPD.2
Delirium. Restlessness, irritability, aggression, and changes in affect can accompany delirium, especially the hyperactive or hyperalert types. Symptoms of anxiety, depression, fear, and loose or tangential thinking also are common.
Mania shares some of these features but typically presents with an abnormally and persistently elevated or irritable mood lasting ≥1 week, usually without prominent cognitive impairment.6 Mania can also include:
- grandiosity
- decreased need for sleep
- flight of ideas
- distractibility
- pressured or increased rate of speech
- psychomotor agitation
- potentially harmful activities
- increased goal-directed activities.6
Frontal lobe lesions. Decreased prefrontal executive control could underlie mania’s cognitive and emotional symptoms. Decreased right rostral and orbital prefrontal cortex activation has been associated with impaired planning, judgment, and insight, as well as inappropriate conduct.7
Table
Clinical features of geriatric bipolar disorder (BPD)
| Psychotic features (delusions, hallucinations) |
|
| Family history |
|
| Compared with younger adults with BPD, older patients: |
|
| Compared with late-life schizophrenia, late-life BPD patients show: |
|
| Source: References 1,2 |
Continued treatment: depression emerges
Several months later, Mr. B presents with severe depression and continued medication nonadherence. He complains of hypersomnia, poor appetite, anhedonia, amotivation, and a leaden-like paresis in his hands and feet.
We readmit Mr. B to the psychiatric unit. He avoids contact with others, has lost 18 lbs over 6 weeks, and suffers hypotension caused by poor hydration before admission. Three weeks later, he complains that ants are crawling around his room and into his mouth.
Noncontrast brain CT shows no abnormalities. Laboratory tests performed at admission show a subtherapeutic lithium level (0.03 mEq/L), unremarkable thyroid panel, and normal B12 and folate, so we begin to rule out a medical cause for his psychiatric symptoms.
The authors’ observations
Check for these and other possible causes of depressive symptoms in older patients with a history of BPD. Mr. B’s depression likely resulted from multiple causes, including medical disease, functional impairment, loss of social and family contacts, and substance abuse—all late-life predictors of depression. BPD also predisposed him to depression.
Bipolar depression. Despite its profound morbidity and mortality, bipolar depression remains a mystery, especially in the elderly. Mr. B’s depression emerged after he was free of depressive symptoms for more than 20 years.
Some researchers believe that compared with other depressions, bipolar depression has a more acute onset, marked psychomotor retardation, and lessened response to antidepressants.6,8 Kraepelin associated bipolar depression with lethargy, mental slowing, and hypersomnia, whereas agitation and insomnia signal unipolar depression.9
To differentiate bipolar from unipolar or secondary depression in older patients, watch for:
- suicide risk, which is heightened during BPD’s depressive phase9
- secondary manias, for which underlying causes must be determined and treated if possible.
Depression caused by medication might be limited to somatic complaints such as fatigue or tiredness,9 and often lacks features seen with mood disorders such as depressed mood, anhedonia, guilt, and diminished interest in activities. Mr. B’s anhedonia and amotivation suggest his depression was not medication-induced.10
Disease-induced depression. Medical comorbidities are common among older persons with mood disorders and can complicate treatment response and outcome. Physical disease can cause or worsen depression:11
- Endocrine and immunologic diseases might cause depression or mania.
- Cardiovascular and cerebrovascular diseases; CNS disorders such as dementia, Parkinson’s disease, and multiple sclerosis; cancer; and connective tissue disease increase risk for comorbid depression.
11
Vascular depression. Comorbid depressive symptoms and vascular disease—or “vascular depression”—can cause ischemic brain lesions, cognitive impairment, increased apathy and retardation, and impaired fluency and naming.12
What defines vascular depression has been debated. Watch for clinical or laboratory evidence of vascular disease, depression, and neuropsychological impairment.13
Treatment: searching for evidence
Two days after admission, Mr. B is transferred to the ICU after suffering severe hypoglycemia and showing signs of medically induced delirium. Elevated creatinine (1.7 mg/dL) indicates acute renal failure, which could be related to his elevated serum lithium (1.7 mEq/L). Acting on the internist’s advice, the consulting psychiatrist stops lithium and restarts valproate, 500 mg bid.
Mr. B becomes medically stable after 3 days, mostly through acute IV hydration and by withholding oral diabetes medications, which normalizes his blood sugar. He is transferred back to the psychiatry unit. We try lithium again at 300 mg bid, but creatinine and serum lithium quickly rise.
Mr. B remains hospitalized for 3 months with severe, treatment-resistant depression. Trials of nearly every second-generation antipsychotic (SGA) cause symptomatic orthostatic hypotension, leading to several falls. He does not respond to divalproex, up to 2,000 mg/d; citalopram, 60 mg/d; mirtazapine, 30 mg at bedtime; venlafaxine, 100 mg tid; or bupropion, 100 mg tid.
We suggest electroconvulsive therapy (ECT) but Mr. B declines, saying this treatment caused his mother to decompensate. We try lamotrigine, 25 mg/d, and titrate it over 6 weeks to 200 mg bid. After we add haloperidol, 5 mg at bedtime, and bupropion, 300 mg/d, Mr. B becomes mentally stable.
The authors’ observations
Numerous clinical challenges—such as managing complicated/refractory BPD, medical comorbidity, and medication adherence (Box)14,15—complicate treatment of late-life BPD.16 Regular communication with providers and integrating health care services can minimize complication risk.16
Pharmacotherapy, a core element of BPD treatment, is challenging in older patients because of their:
- heightened threat of complications and sensitivity to side effects because of age-related pharmacokinetic changes
- increased risk of drug-drug interactions
- increased potential for age-related psychosocial problems (increased social isolation, financial difficulties, demoralization, increased stress, inability to work).
Between 40% and 60% of patients do not take medications as prescribed.14 That percentage probably is higher among cognitively impaired older adults because cognitive problems can compound other causes of nonadherence.
Few published controlled clinical trials have addressed adherence interventions for older adults. Educational approaches combined with cognitive supports are most likely to succeed. Ownby et al15 hypothesized that effective approaches usually employ multiple components including counseling, information reminders, and family therapy.
Techniques for improving adherence include:
- addressing the patient’s beliefs about his or her illness
- exploring how patient characteristics affect medication adherence
- use of memory aids, such as 7-day pill boxes
- working with caregivers
- prescribing lower-than-normal dosages to minimize side effects.
Consider side effects, medical and neurologic comorbidities, and treatment history before prescribing a mood stabilizer, antipsychotic, or antidepressant to an older patient. Avoid unwarranted discontinuation of a previously effective agent, such as when drug concentrations are elevated or inadequate—as happened with Mr. B.5 Also investigate the patient’s side-effect history before stopping a medication.5
Medications. Mr. B’s inability to tolerate lithium posed a treatment challenge. Adjusting lithium dosages to compensate for age-related pharmacokinetic, pharmacodynamic, and renal clearance changes can prevent toxicity.5 Avoid stopping lithium abruptly, as this can trigger recurrence of manic or depressive episodes.17
Lamotrigine, indicated for BPD maintenance therapy, appears to prevent depressive/mood relapse. Compared with other anticonvulsants, lamotrigine might cause fewer negative effects on cognition and less induction of hepatic enzymes. It is well tolerated by older patients but has not been studied adequately in this age group.16
Antipsychotics are widely used in BPD,16 especially when psychosis is present with mania or depression or the patient is agitated. Most studies of antipsychotics in BPD have followed younger adults, however, and most studies in older patients have followed those with dementia or schizophrenia.
Use of first-generation antipsychotics such as haloperidol is especially challenging in the elderly because these drugs increase risk of cardiovascular effects, extrapyramidal symptoms, and tardive dyskinesia and can cause depression in BPD.16 By comparison, SGAs carry a lower risk of involuntary motion18 but can increase risk of obesity, diabetes, and dyslipidemia. However:
- The need to manage psychosis usually overrides concerns about metabolic sequelae.
- Older patients might be less susceptible to metabolic effects,16 though this has not been confirmed.
SGAs can be used safely in patients with a history of diabetes. Start at lower-than-normal dosages and titrate slowly. Perform baseline and regular checks—including weight, blood glucose, lipid levels, and blood pressure—following American Psychiatric Association and American Diabetes Association consensus guidelines.19 Also check glycosylated hemoglobin every 3 to 6 months in patients with diabetes, and follow up with other providers to ensure proper diabetes management.
As with most aspects of late-life BPD, scant evidence guides SGA use. Avoid low-potency neuroleptics such as chlorpromazine, which can cause severe sedation and orthostatic hypotension. For Mr. B, a more-tolerable SGA such as aripiprazole or ziprasidone might be prudent, given his propensity for orthostatic hypotension and history of diabetes. Olanzapine or clozapine can cause anticholinergic effects and—in Mr. B’s case—lead to weight gain and worsen diabetes.
Antidepressant use in BPD usually is reserved for depressive symptoms that impair occupational or social functioning and exceed DSM-IV-TR diagnostic criteria.8,9 Consider later-generation antidepressants such as selective serotonin reuptake inhibitors (SSRIs), because tricyclics pose a greater risk of triggering a switch into hypomania or mania and can cause sedation and orthostatic, cardiac, anticholinergic, and anti-alpha 1 effects.
Among SSRIs, consider citalopram, escitalopram, or sertraline for older patients taking one or more other medications, as these antidepressants have less potential for drug-drug interactions than fluoxetine and paroxetine.11 In a recent comparison of newer antidepressants,20 venlafaxine showed the highest relative risk of mood polarity switching and bupropion the lowest.
Consider ECT for older patients with refractory mania or depression or who show evidence of suicidality or inadequate nutrition.5
Follow-up: ongoing issues
Three months after his admission, we discharge Mr. B to a board-and-care facility because family members will not take him in. Several weeks later, he again ignores his prescriptions and decompensates with worsening depression.
Family members have Mr. B admitted to an inpatient psychiatric facility closer to their home. He remains depressed, stays at the facility on and off for almost 1 year, and is eventually conserved by the county. Adverse side effects—mostly constipation and orthostatic hypotension—continue to complicate treatment.
Before Mr. B’s most recent discharge, another psychiatrist restarts lithium, 300 mg bid, and nortriptyline, 100 mg at bedtime—the combination that kept Mr. B relatively stable for more than 2 decades.
- National Institute of Mental Health—depression information. www.nimh.nih.gov/healthinformation/depressionmenu.cfm.
- Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). www.stepbd.org.
- Medicinenet.com. Medicines that cause depression. www.medicinenet.com/depression/index.htm. Click on “Medicines that cause depression.”
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clozapine • Clozaril
- Divalproex • Depakote
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Various
- Mirtazapine • Remeron
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Ziprasidone • Geodon
The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Depp C, Jeste D. Bipolar disorder in older adults: a critical review. Bipolar Disord 2004;6:343-67.
2. Sajatovic M, Blow FC, Ignacio RV, Kales HC. New-onset bipolar disorder in later life. Am J Geriatr Psychiatry 2005;13:282-9.
3. Arciniegas DB. New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry 2006;163:198-203.
4. Young RC. Bipolar disorder in older persons: perspectives and new findings. Am J Geriatr Psychiatry 2005;13:265-7.
5. Young RC. Evidence-based pharmacological treatment of geriatric bipolar disorder. Psychiatr Clin North Am 2005;28:837-69.
6. Diagnostic and statistical manual of mental disorders, 4 ed, text rev. Washington, DC; American Psychiatric Association; 2000.
7. Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999;156:1986-8.
8. Gitlin M. Treatment-resistant bipolar disorder. Mol Psychiatry 2006;11:227-40.
9. Dubovsky SL. Treatment of bipolar depression. Psychiatr Clin N Am 2005;28:349-70.
10. Kroenke K. A 75-year-old man with depression. JAMA 2002;287:1568-76.
11. Shanmugham B, Karp J, Drayer R, et al. Evidence-based pharmacological interventions for geriatric depression. Psychiatr Clin N Am 2005;28:821-35.
12. Alexopoulos GS. In: Sadavoy J, Jarvik LF, Grossberg GT, Meyers BS, eds. Comprehensive textbook of geriatric psychiatry, 3 ed. New York: WW Norton and Co.; 2002:609-53.
13. Sneed JR, Roose SP, Sackeim HA. Vascular depression: a distinct diagnostic subtype? Biol Psychiatry 2006;60:1295-8.
14. Higgins N, Regan C. A systematic review of the effectiveness of interventions to help older people adhere to medication regimes. Age Ageing 2004;33:224-9.
15. Ownby RL, Hertzog C, Crocco E, Duara R. Factors related to medication adherence in memory disorder clinic patients. Aging & Mental Health 2006;10:378-85.
16. Sajatovic M, Madhusoodanan S, Coconcea N. Managing bipolar disorder in the elderly: defining the role of the newer agents. Drugs Aging 2005;22:39-54.
17. Cavanagh J, Smyth R, Goodwin GM. Relapse into mania or depression following lithium discontinuation: a 7-year follow up. Acta Psychiatr Scand 2004;109:91-5.
18. Dunner DL. Atypical antipsychotics: efficacy across bipolar disorder subpopulations. J Clin Psychiatry 2005;66(suppl 3):20-7.
19. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
20. Leverich GS, Altshuler LL, Frye MA, et al. Risk of switch in mood polarity to hypomania or mania in patient with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry 2006;163:232-9.
1. Depp C, Jeste D. Bipolar disorder in older adults: a critical review. Bipolar Disord 2004;6:343-67.
2. Sajatovic M, Blow FC, Ignacio RV, Kales HC. New-onset bipolar disorder in later life. Am J Geriatr Psychiatry 2005;13:282-9.
3. Arciniegas DB. New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry 2006;163:198-203.
4. Young RC. Bipolar disorder in older persons: perspectives and new findings. Am J Geriatr Psychiatry 2005;13:265-7.
5. Young RC. Evidence-based pharmacological treatment of geriatric bipolar disorder. Psychiatr Clin North Am 2005;28:837-69.
6. Diagnostic and statistical manual of mental disorders, 4 ed, text rev. Washington, DC; American Psychiatric Association; 2000.
7. Blumberg HP, Stern E, Ricketts S, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999;156:1986-8.
8. Gitlin M. Treatment-resistant bipolar disorder. Mol Psychiatry 2006;11:227-40.
9. Dubovsky SL. Treatment of bipolar depression. Psychiatr Clin N Am 2005;28:349-70.
10. Kroenke K. A 75-year-old man with depression. JAMA 2002;287:1568-76.
11. Shanmugham B, Karp J, Drayer R, et al. Evidence-based pharmacological interventions for geriatric depression. Psychiatr Clin N Am 2005;28:821-35.
12. Alexopoulos GS. In: Sadavoy J, Jarvik LF, Grossberg GT, Meyers BS, eds. Comprehensive textbook of geriatric psychiatry, 3 ed. New York: WW Norton and Co.; 2002:609-53.
13. Sneed JR, Roose SP, Sackeim HA. Vascular depression: a distinct diagnostic subtype? Biol Psychiatry 2006;60:1295-8.
14. Higgins N, Regan C. A systematic review of the effectiveness of interventions to help older people adhere to medication regimes. Age Ageing 2004;33:224-9.
15. Ownby RL, Hertzog C, Crocco E, Duara R. Factors related to medication adherence in memory disorder clinic patients. Aging & Mental Health 2006;10:378-85.
16. Sajatovic M, Madhusoodanan S, Coconcea N. Managing bipolar disorder in the elderly: defining the role of the newer agents. Drugs Aging 2005;22:39-54.
17. Cavanagh J, Smyth R, Goodwin GM. Relapse into mania or depression following lithium discontinuation: a 7-year follow up. Acta Psychiatr Scand 2004;109:91-5.
18. Dunner DL. Atypical antipsychotics: efficacy across bipolar disorder subpopulations. J Clin Psychiatry 2005;66(suppl 3):20-7.
19. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
20. Leverich GS, Altshuler LL, Frye MA, et al. Risk of switch in mood polarity to hypomania or mania in patient with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry 2006;163:232-9.
Did antismoking therapy make him sick?
Presentation: unconscious on the street
Emergency medical personnel bring Mr. M, age 66, to the ER after passers-by find him supine on the sidewalk. On arrival, he is comatose as confirmed by a Glasgow Coma Scale score of 8 (eye opening 3, verbal response 2, motor response 3). Systolic blood pressure is 108 mm Hg on palpation, pulse is 135 beats per minute, and temperature is 105 °F. Minor abrasions cover his face and arms, and his hands and feet are rigid.
Mr. M has lived at a board-and-care facility for 30 years. The facility’s operator tells us that Mr. M has had schizophrenia for 40 years and has been taking:
- olanzapine, 7.5 mg each morning and 10 mg at bedtime
- chlorpromazine, 50 mg nightly
- lithium carbonate, 300 mg tid
- and benztropine, 2 mg bid.
Three weeks ago, Mr. M was hospitalized for 6 days with pneumonia. In 3 months, he will undergo surgery for prostate cancer. He is taking no medication for the prostate cancer.
Creatine phosphokinase (CPK) is 2,939 IU/L, indicating neuroleptic malignant syndrome (NMS). Other laboratory test results suggest diabetes or renal failure (Table 1). Lumbar puncture shows protein at 91 mg/dL, glucose at 74 mg/dL, and red- and white-blood-cell counts at 0 and 1, respectively. CSF Gram’s stain and brain CT are unremarkable. ECG is normal except for sinus tachycardia. Serum lithium is normal (1.1 mmol/L).
Mr. M undergoes tracheal intubation and receives ceftazidime, dose unknown, because chest radiograph shows lower lung opacities, suggesting aspiration. He receives morphine, 2 to 4 mg hourly as needed, to calm him during intubation. He is then transferred to the intensive care unit.
Table 1
Diabetes, renal failure, or NMS? The story behind Mr. M’s laboratory values
| Mr. M’s reading | Normal range | Might suggest | |
|---|---|---|---|
| CPK | 2,939 IU/L | 8-150 IU/L | NMS |
| Serum creatinine | 1.9 mg/dL | 0.6-1.5 mg/dL | Renal failure, a complication from elevated CPK |
| Serum glucose | 143 mg/dL | 66-99 mg/dL | Diabetes mellitus |
| NMS: Neuroleptic malignant syndrome | |||
| CPK: Creatine phosphokinase | |||
The authors’ observations
NMS, a potentially fatal side effect of antipsychotics, is characterized by rigidity, hyperthermia, and autonomic instability1—as seen with Mr. M.
The patient’s rigidity, elevated creatine kinase, and face and arm abrasions could suggest a seizure. Mr. M’s EEG is negative, however, and he has no history of seizures or head trauma, so seizure is ruled out.
Researchers have associated bupropion with a small risk of developing seizures. Richmond and Zwar2 reported a 0.1% risk with bupropion, ≥300 mg/d, but Mr. M was taking 150 mg/d. Dunner et al3 estimated the risk of developing seizure while taking standard-release bupropion—the form Mr. M used—at 0.06%, but patients in this study who developed seizures typically had a past seizure disorder or head trauma.
The combination of hyperthermia, tachycardia, altered mental status, and positive chest X-ray suggest pneumonia, which was addressed with antibiotics. Pneumonia, however, does not solely account for Mr. M’s fever, rigidity, and profoundly increased CPK. These findings suggest NMS.
The Glasgow Coma Scale (GCS) is used to quantitatively rate degree of responsiveness in critically ill or injured patients (Table 2). Total scores range from 3 to 15 based on the patient’s best eye, motor, and verbal responses. Total score ≤8 indicates a probable coma. Serial GCS scores can measure clinical course in comatose patients.
Table 2
Using Glasgow Coma Scale to determine level of consciousness
| Component | Response | Score |
|---|---|---|
| Best eye response | No eye opening | 1 |
| Eye opening to pain | 2 | |
| Eye opening to verbal command | 3 | |
| Eyes open spontaneously | 4 | |
| Best verbal response | No verbal response | 1 |
| Incomprehensible sounds | 2 | |
| Inappropriate words | 3 | |
| Confused | 4 | |
| Oriented | 5 | |
| Best motor response | No motor response | 1 |
| Extension to pain | 2 | |
| Flexion to pain | 3 | |
| Withdrawal from pain | 4 | |
| Localizing pain | 5 | |
| Obeys commands | 6 | |
| Total score ≤8 is severe, and 90% of patients with scores ≤8 are in a coma). Coma is defined as not opening eyes, not obeying commands, and not saying understandable words. Composite scores listing eye, verbal, and motor responses (such as E3V3M5) are clinically more useful than totals. | ||
| Source: Reprinted from Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;304(7872):81-4, with permission from Elsevier. | ||
Treatment: slow progress
In the ICU, we diagnose NMS and stop all psychotropics, fearing that interactions between any of them might be causing NMS. We give midazolam, 1 to 2 mg hourly as needed for agitation, and continue morphine, 2 to 4 mg hourly as needed for pain. We stop ceftazidime after ruling out aspiration risk.
On day 2 of hospitalization, we call the neurology and consultation-liaison (C-L) psychiatry services. The C-L psychiatrist attempts a mental status examination, but Mr. M is too frail and sedated to communicate. Neurologic exam shows increased foot rigidity, and follow-up studies show negative EEG, normal head and neck MRIs and MRAs, a peak in CPK at 5,487 IU/L, and normal chest films.
We taper and discontinue midazolam and morphine, and Mr. M’s consciousness improves as the dosages decrease. We add lorazepam, 1 mg tid, to address Mr. M’s agitation. He also starts physical therapy to address potential movement problems caused by laying static for 3 days. By day 7, he is extubated and transferred to the general medical unit.
On day 9, Mr. M’s recall and concentration are diminished, and he cannot follow a 3-step command. His Mini-Mental State Examination (MMSE) score of 17 points to a cognitive impairment.
By day 12, residual psychosis is increasing Mr. M’s confusion, paranoia, and agitation. Despite this complication, he is able to work with his occupational and physical therapists.
By day 20, Mr. M becomes more paranoid, with tangential and loose associations. To address these symptoms, we stop lorazepam and start aripiprazole, 15 mg each morning. Because aripiprazole is a partial dopamine agonist and antagonist, it is less likely than other antipsychotics to cause recurrence of NMS symptoms.
Four days later, Mr. M is medically cleared for transfer to the county psychiatric hospital. Creatinine and CPK elevations, metabolic acidosis, and anemia have resolved.
Treatment: new facility, new drugs
On initial evaluation at the psychiatric hospital, Mr. M is cooperative and aware of person, place, and time. His thought processes range from tangential to disorganized, and his paranoia persists.
The attending psychiatrist stops aripiprazole and starts risperidone, 1 mg bid, possibly because he is less familiar with aripiprazole—a newer antipsychotic— than with risperidone. Laboratory results within 3 days of starting risperidone show normal serum levels, blood counts, liver enzymes, and CPK.
On day 2 at the psychiatric hospital, Mr. M’s behavior worsens; he frequently disrobes in front of others, yells at staff, and requires verbal redirection. His MMSE score has fallen to 15. The attending psychiatrist modifies risperidone to 2 mg nightly and adds donepezil, 10 mg each morning, to try to reverse his cognitive decline.
By day 8, Mr. M is more cooperative and his behavior improves. He is transferred back to his board-and-care facility on risperidone and donepezil at the above dosages.
The following month, Mr. M presents to his outpatient psychiatrist with improved cognitive function, but he is still delusional. The psychiatrist stops risperidone and donepezil and resumes olanzapine, 7.5 mg each morning and 10 mg nightly, and chlorpromazine, 50 mg nightly, to try to restore the patient’s pre-NMS function.
Mr. M undergoes successful prostate cancer surgery before his 3-month psychiatry follow-up, at which the psychiatrist adds lithium carbonate, 300 mg tid, for residual irritability. Serum lithium levels are normal; bupropion is not restarted.
One year after presentation, Mr. M is minimally delusional but functioning well. No symptoms suggesting NMS recurrence have been reported.
The authors’ observations
Though the precise mechanism is unknown, NMS has been linked with use of FGAs such as chlorpromazine, which can trigger excessive dopamine blockade.4 Studies increasingly associate SGAs such as olanzapine, risperidone, and aripiprazole with NMS onset.4-6 Mood stabilizers such as lithium carbonate also have been implicated, especially when used with antipsychotics.6-9 No association between antibiotics and NMS has been found.
For years, Mr. M has been taking FGAs and concomitant olanzapine and lithium carbonate without developing NMS symptoms until now. Since discharge, he has been free of NMS symptoms despite taking two SGAs (aripiprazole and risperidone) at different times and later resuming chlorpromazine, olanzapine, and lithium carbonate.
Of note, bupropion—the last psychotropic added before NMS onset—has not been restarted. The literature does not link bupropion to NMS, although one case report10 suggests an association between fluoxetine and NMS after the patient had taken several antipsychotic/antidepressant combinations.
As a dopamine agonist, bupropion should protect against NMS. Case reports,11,12 however, have described patients who developed NMS after antipsychotics were discontinued, and stopping an antipsychotic essentially mimics bupropion’s action by eliminating the dopamine blockade. Additionally, bupropion’s norepinephrine modulation could have precipitated NMS by dysregulating the sympathetic nervous system.13
Mr. M’s board-and-care operator indicated that the patient’s tobacco consumption decreased—from about a pack to a half-pack of cigarettes daily—after bupropion was added. Alternatively, the effects of pneumonia could have curtailed Mr. M’s smoking. Because nicotine increases metabolism of neuroleptics,14,15 decreased nicotine consumption might have increased dopamine blockade to the point of causing NMS.
Other possibilities. Mr. M’s pneumonia might have caused dehydration, which can also lead to NMS.
Bupropion also reportedly alters metabolism of chlorpromazine and other phenothiazine antipsychotics by inhibiting the cytochrome P-450 2D6 isoenzyme. This pharmacokinetic interaction could have precipitated Mr. M’s NMS episode independent of an antipsychotic dosage increase.16
Because this case is so complex, pinpointing a specific cause for Mr. M’s apparent NMS symptoms is difficult. Be aware that combining psychotropics can lead to NMS. Patients who present with mental status changes, hyperthermia, rigidity, and/or increased creatine kinase while taking psychotropics should be promptly evaluated and managed.
Treating NMS
A review of NMS treatment by Davis et al17 suggests that you:
- consider NMS in the differential diagnosis of an acutely delirious patient who has used antipsychotics, no matter how long he or she has been taking the medication(s) or how stable the dosage
- check for other signs of NMS—such as rigidity or autonomic instability—during the physical examination.
- consider NMS as a possible cause of dysarthria, diaphoresis, dysphagia, sialorrhea, and myoclonus, although these are less common signs of the disorder
- include CPK levels, chemistry panel, CBC, and liver enzyme assessment in the early evaluation of laboratory results. Consider performing a urine drug screen to check for illicit substance use. Head CT results might also help confirm NMS diagnosis.
If sedation becomes necessary, use benzodiazepines cautiously. Serial CPKs and daily reassessment of clinical degree of rigidity are essential; continued rigidity may indicate use of dopamine agonists and dantrolene.17
Related resources
- Neuroleptic Malignant Syndrome Information Service. Archive of articles addressing NMS diagnosis and treatment, and listing of psychotropics associated with NMS. www.nmsis.org.
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bupropion SR • Wellbutrin, Zyban
- Ceftazidime • various
- Chlorpromazine • Thorazine
- Dantrolene • Dantrium
- Donepezil • Aricept
- Lithium carbonate • various
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association, 2000.
2. Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev 2003;22:203-20.
3. Dunner DL, Zisook S, Billow A, et al. A prospective safety study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry 1998;59:366-73.
4. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatr Ann 2000;30:314-21.
5. Berry N, Pradhan S, Sagar R, Gupta SK. Neuroleptic malignant syndrome in an adolescent receiving olanzapine-lithium combination therapy. Pharmacotherapy 2003;23:255-9
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65:464-70.
7. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
8. Bourgeois JA, Kahn DR. Neuroleptic malignant syndrome following administration of risperidone and lithium. J Clin Psychopharmacol 2003;23:315-6.
9. Gill J, Singh H, Nugent K. Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy 2003;23:811-15.
10. Halman M, Goldbloom DS. Fluoxetine and neuroleptic malignant syndrome. Biol Psychiatry 1990;28:518-21.
11. Spivak B, Gonen N, Mester R, et al. Neuroleptic malignant syndrome associated with abrupt withdrawal of anticholinergic agents. Int Clin Psychopharmacol 1996;11:207-9.
12. Rosse R, Ciolino C. Dopamine agonists and neuroleptic malignant syndrome. Am J Psychiatry 1985;142:270-1.
13. Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry 1999;156:169-80.
14. Ereshefsky L, Jann MW, Saklad SR, et al. Effects of smoking on fluphenazine clearance in psychiatric inpatients. Biol Psychiatry 1985;20:329-32.
15. Jann MW, Saklad SR, Ereshefsky L, et al. Effects of smoking on haloperidol and reduced haloperidol plasma concentrations and haloperidol clearance. Psychopharmacology (Berl) 1986;90:468-70.
16. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics 2005;46:464-94.
17. Davis JM, Caroff SN, Mann SC. Treatment of neuroleptic malignant syndrome. Psychiatr Ann 2000;30:325-31.
Presentation: unconscious on the street
Emergency medical personnel bring Mr. M, age 66, to the ER after passers-by find him supine on the sidewalk. On arrival, he is comatose as confirmed by a Glasgow Coma Scale score of 8 (eye opening 3, verbal response 2, motor response 3). Systolic blood pressure is 108 mm Hg on palpation, pulse is 135 beats per minute, and temperature is 105 °F. Minor abrasions cover his face and arms, and his hands and feet are rigid.
Mr. M has lived at a board-and-care facility for 30 years. The facility’s operator tells us that Mr. M has had schizophrenia for 40 years and has been taking:
- olanzapine, 7.5 mg each morning and 10 mg at bedtime
- chlorpromazine, 50 mg nightly
- lithium carbonate, 300 mg tid
- and benztropine, 2 mg bid.
Three weeks ago, Mr. M was hospitalized for 6 days with pneumonia. In 3 months, he will undergo surgery for prostate cancer. He is taking no medication for the prostate cancer.
Creatine phosphokinase (CPK) is 2,939 IU/L, indicating neuroleptic malignant syndrome (NMS). Other laboratory test results suggest diabetes or renal failure (Table 1). Lumbar puncture shows protein at 91 mg/dL, glucose at 74 mg/dL, and red- and white-blood-cell counts at 0 and 1, respectively. CSF Gram’s stain and brain CT are unremarkable. ECG is normal except for sinus tachycardia. Serum lithium is normal (1.1 mmol/L).
Mr. M undergoes tracheal intubation and receives ceftazidime, dose unknown, because chest radiograph shows lower lung opacities, suggesting aspiration. He receives morphine, 2 to 4 mg hourly as needed, to calm him during intubation. He is then transferred to the intensive care unit.
Table 1
Diabetes, renal failure, or NMS? The story behind Mr. M’s laboratory values
| Mr. M’s reading | Normal range | Might suggest | |
|---|---|---|---|
| CPK | 2,939 IU/L | 8-150 IU/L | NMS |
| Serum creatinine | 1.9 mg/dL | 0.6-1.5 mg/dL | Renal failure, a complication from elevated CPK |
| Serum glucose | 143 mg/dL | 66-99 mg/dL | Diabetes mellitus |
| NMS: Neuroleptic malignant syndrome | |||
| CPK: Creatine phosphokinase | |||
The authors’ observations
NMS, a potentially fatal side effect of antipsychotics, is characterized by rigidity, hyperthermia, and autonomic instability1—as seen with Mr. M.
The patient’s rigidity, elevated creatine kinase, and face and arm abrasions could suggest a seizure. Mr. M’s EEG is negative, however, and he has no history of seizures or head trauma, so seizure is ruled out.
Researchers have associated bupropion with a small risk of developing seizures. Richmond and Zwar2 reported a 0.1% risk with bupropion, ≥300 mg/d, but Mr. M was taking 150 mg/d. Dunner et al3 estimated the risk of developing seizure while taking standard-release bupropion—the form Mr. M used—at 0.06%, but patients in this study who developed seizures typically had a past seizure disorder or head trauma.
The combination of hyperthermia, tachycardia, altered mental status, and positive chest X-ray suggest pneumonia, which was addressed with antibiotics. Pneumonia, however, does not solely account for Mr. M’s fever, rigidity, and profoundly increased CPK. These findings suggest NMS.
The Glasgow Coma Scale (GCS) is used to quantitatively rate degree of responsiveness in critically ill or injured patients (Table 2). Total scores range from 3 to 15 based on the patient’s best eye, motor, and verbal responses. Total score ≤8 indicates a probable coma. Serial GCS scores can measure clinical course in comatose patients.
Table 2
Using Glasgow Coma Scale to determine level of consciousness
| Component | Response | Score |
|---|---|---|
| Best eye response | No eye opening | 1 |
| Eye opening to pain | 2 | |
| Eye opening to verbal command | 3 | |
| Eyes open spontaneously | 4 | |
| Best verbal response | No verbal response | 1 |
| Incomprehensible sounds | 2 | |
| Inappropriate words | 3 | |
| Confused | 4 | |
| Oriented | 5 | |
| Best motor response | No motor response | 1 |
| Extension to pain | 2 | |
| Flexion to pain | 3 | |
| Withdrawal from pain | 4 | |
| Localizing pain | 5 | |
| Obeys commands | 6 | |
| Total score ≤8 is severe, and 90% of patients with scores ≤8 are in a coma). Coma is defined as not opening eyes, not obeying commands, and not saying understandable words. Composite scores listing eye, verbal, and motor responses (such as E3V3M5) are clinically more useful than totals. | ||
| Source: Reprinted from Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;304(7872):81-4, with permission from Elsevier. | ||
Treatment: slow progress
In the ICU, we diagnose NMS and stop all psychotropics, fearing that interactions between any of them might be causing NMS. We give midazolam, 1 to 2 mg hourly as needed for agitation, and continue morphine, 2 to 4 mg hourly as needed for pain. We stop ceftazidime after ruling out aspiration risk.
On day 2 of hospitalization, we call the neurology and consultation-liaison (C-L) psychiatry services. The C-L psychiatrist attempts a mental status examination, but Mr. M is too frail and sedated to communicate. Neurologic exam shows increased foot rigidity, and follow-up studies show negative EEG, normal head and neck MRIs and MRAs, a peak in CPK at 5,487 IU/L, and normal chest films.
We taper and discontinue midazolam and morphine, and Mr. M’s consciousness improves as the dosages decrease. We add lorazepam, 1 mg tid, to address Mr. M’s agitation. He also starts physical therapy to address potential movement problems caused by laying static for 3 days. By day 7, he is extubated and transferred to the general medical unit.
On day 9, Mr. M’s recall and concentration are diminished, and he cannot follow a 3-step command. His Mini-Mental State Examination (MMSE) score of 17 points to a cognitive impairment.
By day 12, residual psychosis is increasing Mr. M’s confusion, paranoia, and agitation. Despite this complication, he is able to work with his occupational and physical therapists.
By day 20, Mr. M becomes more paranoid, with tangential and loose associations. To address these symptoms, we stop lorazepam and start aripiprazole, 15 mg each morning. Because aripiprazole is a partial dopamine agonist and antagonist, it is less likely than other antipsychotics to cause recurrence of NMS symptoms.
Four days later, Mr. M is medically cleared for transfer to the county psychiatric hospital. Creatinine and CPK elevations, metabolic acidosis, and anemia have resolved.
Treatment: new facility, new drugs
On initial evaluation at the psychiatric hospital, Mr. M is cooperative and aware of person, place, and time. His thought processes range from tangential to disorganized, and his paranoia persists.
The attending psychiatrist stops aripiprazole and starts risperidone, 1 mg bid, possibly because he is less familiar with aripiprazole—a newer antipsychotic— than with risperidone. Laboratory results within 3 days of starting risperidone show normal serum levels, blood counts, liver enzymes, and CPK.
On day 2 at the psychiatric hospital, Mr. M’s behavior worsens; he frequently disrobes in front of others, yells at staff, and requires verbal redirection. His MMSE score has fallen to 15. The attending psychiatrist modifies risperidone to 2 mg nightly and adds donepezil, 10 mg each morning, to try to reverse his cognitive decline.
By day 8, Mr. M is more cooperative and his behavior improves. He is transferred back to his board-and-care facility on risperidone and donepezil at the above dosages.
The following month, Mr. M presents to his outpatient psychiatrist with improved cognitive function, but he is still delusional. The psychiatrist stops risperidone and donepezil and resumes olanzapine, 7.5 mg each morning and 10 mg nightly, and chlorpromazine, 50 mg nightly, to try to restore the patient’s pre-NMS function.
Mr. M undergoes successful prostate cancer surgery before his 3-month psychiatry follow-up, at which the psychiatrist adds lithium carbonate, 300 mg tid, for residual irritability. Serum lithium levels are normal; bupropion is not restarted.
One year after presentation, Mr. M is minimally delusional but functioning well. No symptoms suggesting NMS recurrence have been reported.
The authors’ observations
Though the precise mechanism is unknown, NMS has been linked with use of FGAs such as chlorpromazine, which can trigger excessive dopamine blockade.4 Studies increasingly associate SGAs such as olanzapine, risperidone, and aripiprazole with NMS onset.4-6 Mood stabilizers such as lithium carbonate also have been implicated, especially when used with antipsychotics.6-9 No association between antibiotics and NMS has been found.
For years, Mr. M has been taking FGAs and concomitant olanzapine and lithium carbonate without developing NMS symptoms until now. Since discharge, he has been free of NMS symptoms despite taking two SGAs (aripiprazole and risperidone) at different times and later resuming chlorpromazine, olanzapine, and lithium carbonate.
Of note, bupropion—the last psychotropic added before NMS onset—has not been restarted. The literature does not link bupropion to NMS, although one case report10 suggests an association between fluoxetine and NMS after the patient had taken several antipsychotic/antidepressant combinations.
As a dopamine agonist, bupropion should protect against NMS. Case reports,11,12 however, have described patients who developed NMS after antipsychotics were discontinued, and stopping an antipsychotic essentially mimics bupropion’s action by eliminating the dopamine blockade. Additionally, bupropion’s norepinephrine modulation could have precipitated NMS by dysregulating the sympathetic nervous system.13
Mr. M’s board-and-care operator indicated that the patient’s tobacco consumption decreased—from about a pack to a half-pack of cigarettes daily—after bupropion was added. Alternatively, the effects of pneumonia could have curtailed Mr. M’s smoking. Because nicotine increases metabolism of neuroleptics,14,15 decreased nicotine consumption might have increased dopamine blockade to the point of causing NMS.
Other possibilities. Mr. M’s pneumonia might have caused dehydration, which can also lead to NMS.
Bupropion also reportedly alters metabolism of chlorpromazine and other phenothiazine antipsychotics by inhibiting the cytochrome P-450 2D6 isoenzyme. This pharmacokinetic interaction could have precipitated Mr. M’s NMS episode independent of an antipsychotic dosage increase.16
Because this case is so complex, pinpointing a specific cause for Mr. M’s apparent NMS symptoms is difficult. Be aware that combining psychotropics can lead to NMS. Patients who present with mental status changes, hyperthermia, rigidity, and/or increased creatine kinase while taking psychotropics should be promptly evaluated and managed.
Treating NMS
A review of NMS treatment by Davis et al17 suggests that you:
- consider NMS in the differential diagnosis of an acutely delirious patient who has used antipsychotics, no matter how long he or she has been taking the medication(s) or how stable the dosage
- check for other signs of NMS—such as rigidity or autonomic instability—during the physical examination.
- consider NMS as a possible cause of dysarthria, diaphoresis, dysphagia, sialorrhea, and myoclonus, although these are less common signs of the disorder
- include CPK levels, chemistry panel, CBC, and liver enzyme assessment in the early evaluation of laboratory results. Consider performing a urine drug screen to check for illicit substance use. Head CT results might also help confirm NMS diagnosis.
If sedation becomes necessary, use benzodiazepines cautiously. Serial CPKs and daily reassessment of clinical degree of rigidity are essential; continued rigidity may indicate use of dopamine agonists and dantrolene.17
Related resources
- Neuroleptic Malignant Syndrome Information Service. Archive of articles addressing NMS diagnosis and treatment, and listing of psychotropics associated with NMS. www.nmsis.org.
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bupropion SR • Wellbutrin, Zyban
- Ceftazidime • various
- Chlorpromazine • Thorazine
- Dantrolene • Dantrium
- Donepezil • Aricept
- Lithium carbonate • various
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Presentation: unconscious on the street
Emergency medical personnel bring Mr. M, age 66, to the ER after passers-by find him supine on the sidewalk. On arrival, he is comatose as confirmed by a Glasgow Coma Scale score of 8 (eye opening 3, verbal response 2, motor response 3). Systolic blood pressure is 108 mm Hg on palpation, pulse is 135 beats per minute, and temperature is 105 °F. Minor abrasions cover his face and arms, and his hands and feet are rigid.
Mr. M has lived at a board-and-care facility for 30 years. The facility’s operator tells us that Mr. M has had schizophrenia for 40 years and has been taking:
- olanzapine, 7.5 mg each morning and 10 mg at bedtime
- chlorpromazine, 50 mg nightly
- lithium carbonate, 300 mg tid
- and benztropine, 2 mg bid.
Three weeks ago, Mr. M was hospitalized for 6 days with pneumonia. In 3 months, he will undergo surgery for prostate cancer. He is taking no medication for the prostate cancer.
Creatine phosphokinase (CPK) is 2,939 IU/L, indicating neuroleptic malignant syndrome (NMS). Other laboratory test results suggest diabetes or renal failure (Table 1). Lumbar puncture shows protein at 91 mg/dL, glucose at 74 mg/dL, and red- and white-blood-cell counts at 0 and 1, respectively. CSF Gram’s stain and brain CT are unremarkable. ECG is normal except for sinus tachycardia. Serum lithium is normal (1.1 mmol/L).
Mr. M undergoes tracheal intubation and receives ceftazidime, dose unknown, because chest radiograph shows lower lung opacities, suggesting aspiration. He receives morphine, 2 to 4 mg hourly as needed, to calm him during intubation. He is then transferred to the intensive care unit.
Table 1
Diabetes, renal failure, or NMS? The story behind Mr. M’s laboratory values
| Mr. M’s reading | Normal range | Might suggest | |
|---|---|---|---|
| CPK | 2,939 IU/L | 8-150 IU/L | NMS |
| Serum creatinine | 1.9 mg/dL | 0.6-1.5 mg/dL | Renal failure, a complication from elevated CPK |
| Serum glucose | 143 mg/dL | 66-99 mg/dL | Diabetes mellitus |
| NMS: Neuroleptic malignant syndrome | |||
| CPK: Creatine phosphokinase | |||
The authors’ observations
NMS, a potentially fatal side effect of antipsychotics, is characterized by rigidity, hyperthermia, and autonomic instability1—as seen with Mr. M.
The patient’s rigidity, elevated creatine kinase, and face and arm abrasions could suggest a seizure. Mr. M’s EEG is negative, however, and he has no history of seizures or head trauma, so seizure is ruled out.
Researchers have associated bupropion with a small risk of developing seizures. Richmond and Zwar2 reported a 0.1% risk with bupropion, ≥300 mg/d, but Mr. M was taking 150 mg/d. Dunner et al3 estimated the risk of developing seizure while taking standard-release bupropion—the form Mr. M used—at 0.06%, but patients in this study who developed seizures typically had a past seizure disorder or head trauma.
The combination of hyperthermia, tachycardia, altered mental status, and positive chest X-ray suggest pneumonia, which was addressed with antibiotics. Pneumonia, however, does not solely account for Mr. M’s fever, rigidity, and profoundly increased CPK. These findings suggest NMS.
The Glasgow Coma Scale (GCS) is used to quantitatively rate degree of responsiveness in critically ill or injured patients (Table 2). Total scores range from 3 to 15 based on the patient’s best eye, motor, and verbal responses. Total score ≤8 indicates a probable coma. Serial GCS scores can measure clinical course in comatose patients.
Table 2
Using Glasgow Coma Scale to determine level of consciousness
| Component | Response | Score |
|---|---|---|
| Best eye response | No eye opening | 1 |
| Eye opening to pain | 2 | |
| Eye opening to verbal command | 3 | |
| Eyes open spontaneously | 4 | |
| Best verbal response | No verbal response | 1 |
| Incomprehensible sounds | 2 | |
| Inappropriate words | 3 | |
| Confused | 4 | |
| Oriented | 5 | |
| Best motor response | No motor response | 1 |
| Extension to pain | 2 | |
| Flexion to pain | 3 | |
| Withdrawal from pain | 4 | |
| Localizing pain | 5 | |
| Obeys commands | 6 | |
| Total score ≤8 is severe, and 90% of patients with scores ≤8 are in a coma). Coma is defined as not opening eyes, not obeying commands, and not saying understandable words. Composite scores listing eye, verbal, and motor responses (such as E3V3M5) are clinically more useful than totals. | ||
| Source: Reprinted from Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;304(7872):81-4, with permission from Elsevier. | ||
Treatment: slow progress
In the ICU, we diagnose NMS and stop all psychotropics, fearing that interactions between any of them might be causing NMS. We give midazolam, 1 to 2 mg hourly as needed for agitation, and continue morphine, 2 to 4 mg hourly as needed for pain. We stop ceftazidime after ruling out aspiration risk.
On day 2 of hospitalization, we call the neurology and consultation-liaison (C-L) psychiatry services. The C-L psychiatrist attempts a mental status examination, but Mr. M is too frail and sedated to communicate. Neurologic exam shows increased foot rigidity, and follow-up studies show negative EEG, normal head and neck MRIs and MRAs, a peak in CPK at 5,487 IU/L, and normal chest films.
We taper and discontinue midazolam and morphine, and Mr. M’s consciousness improves as the dosages decrease. We add lorazepam, 1 mg tid, to address Mr. M’s agitation. He also starts physical therapy to address potential movement problems caused by laying static for 3 days. By day 7, he is extubated and transferred to the general medical unit.
On day 9, Mr. M’s recall and concentration are diminished, and he cannot follow a 3-step command. His Mini-Mental State Examination (MMSE) score of 17 points to a cognitive impairment.
By day 12, residual psychosis is increasing Mr. M’s confusion, paranoia, and agitation. Despite this complication, he is able to work with his occupational and physical therapists.
By day 20, Mr. M becomes more paranoid, with tangential and loose associations. To address these symptoms, we stop lorazepam and start aripiprazole, 15 mg each morning. Because aripiprazole is a partial dopamine agonist and antagonist, it is less likely than other antipsychotics to cause recurrence of NMS symptoms.
Four days later, Mr. M is medically cleared for transfer to the county psychiatric hospital. Creatinine and CPK elevations, metabolic acidosis, and anemia have resolved.
Treatment: new facility, new drugs
On initial evaluation at the psychiatric hospital, Mr. M is cooperative and aware of person, place, and time. His thought processes range from tangential to disorganized, and his paranoia persists.
The attending psychiatrist stops aripiprazole and starts risperidone, 1 mg bid, possibly because he is less familiar with aripiprazole—a newer antipsychotic— than with risperidone. Laboratory results within 3 days of starting risperidone show normal serum levels, blood counts, liver enzymes, and CPK.
On day 2 at the psychiatric hospital, Mr. M’s behavior worsens; he frequently disrobes in front of others, yells at staff, and requires verbal redirection. His MMSE score has fallen to 15. The attending psychiatrist modifies risperidone to 2 mg nightly and adds donepezil, 10 mg each morning, to try to reverse his cognitive decline.
By day 8, Mr. M is more cooperative and his behavior improves. He is transferred back to his board-and-care facility on risperidone and donepezil at the above dosages.
The following month, Mr. M presents to his outpatient psychiatrist with improved cognitive function, but he is still delusional. The psychiatrist stops risperidone and donepezil and resumes olanzapine, 7.5 mg each morning and 10 mg nightly, and chlorpromazine, 50 mg nightly, to try to restore the patient’s pre-NMS function.
Mr. M undergoes successful prostate cancer surgery before his 3-month psychiatry follow-up, at which the psychiatrist adds lithium carbonate, 300 mg tid, for residual irritability. Serum lithium levels are normal; bupropion is not restarted.
One year after presentation, Mr. M is minimally delusional but functioning well. No symptoms suggesting NMS recurrence have been reported.
The authors’ observations
Though the precise mechanism is unknown, NMS has been linked with use of FGAs such as chlorpromazine, which can trigger excessive dopamine blockade.4 Studies increasingly associate SGAs such as olanzapine, risperidone, and aripiprazole with NMS onset.4-6 Mood stabilizers such as lithium carbonate also have been implicated, especially when used with antipsychotics.6-9 No association between antibiotics and NMS has been found.
For years, Mr. M has been taking FGAs and concomitant olanzapine and lithium carbonate without developing NMS symptoms until now. Since discharge, he has been free of NMS symptoms despite taking two SGAs (aripiprazole and risperidone) at different times and later resuming chlorpromazine, olanzapine, and lithium carbonate.
Of note, bupropion—the last psychotropic added before NMS onset—has not been restarted. The literature does not link bupropion to NMS, although one case report10 suggests an association between fluoxetine and NMS after the patient had taken several antipsychotic/antidepressant combinations.
As a dopamine agonist, bupropion should protect against NMS. Case reports,11,12 however, have described patients who developed NMS after antipsychotics were discontinued, and stopping an antipsychotic essentially mimics bupropion’s action by eliminating the dopamine blockade. Additionally, bupropion’s norepinephrine modulation could have precipitated NMS by dysregulating the sympathetic nervous system.13
Mr. M’s board-and-care operator indicated that the patient’s tobacco consumption decreased—from about a pack to a half-pack of cigarettes daily—after bupropion was added. Alternatively, the effects of pneumonia could have curtailed Mr. M’s smoking. Because nicotine increases metabolism of neuroleptics,14,15 decreased nicotine consumption might have increased dopamine blockade to the point of causing NMS.
Other possibilities. Mr. M’s pneumonia might have caused dehydration, which can also lead to NMS.
Bupropion also reportedly alters metabolism of chlorpromazine and other phenothiazine antipsychotics by inhibiting the cytochrome P-450 2D6 isoenzyme. This pharmacokinetic interaction could have precipitated Mr. M’s NMS episode independent of an antipsychotic dosage increase.16
Because this case is so complex, pinpointing a specific cause for Mr. M’s apparent NMS symptoms is difficult. Be aware that combining psychotropics can lead to NMS. Patients who present with mental status changes, hyperthermia, rigidity, and/or increased creatine kinase while taking psychotropics should be promptly evaluated and managed.
Treating NMS
A review of NMS treatment by Davis et al17 suggests that you:
- consider NMS in the differential diagnosis of an acutely delirious patient who has used antipsychotics, no matter how long he or she has been taking the medication(s) or how stable the dosage
- check for other signs of NMS—such as rigidity or autonomic instability—during the physical examination.
- consider NMS as a possible cause of dysarthria, diaphoresis, dysphagia, sialorrhea, and myoclonus, although these are less common signs of the disorder
- include CPK levels, chemistry panel, CBC, and liver enzyme assessment in the early evaluation of laboratory results. Consider performing a urine drug screen to check for illicit substance use. Head CT results might also help confirm NMS diagnosis.
If sedation becomes necessary, use benzodiazepines cautiously. Serial CPKs and daily reassessment of clinical degree of rigidity are essential; continued rigidity may indicate use of dopamine agonists and dantrolene.17
Related resources
- Neuroleptic Malignant Syndrome Information Service. Archive of articles addressing NMS diagnosis and treatment, and listing of psychotropics associated with NMS. www.nmsis.org.
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Bupropion SR • Wellbutrin, Zyban
- Ceftazidime • various
- Chlorpromazine • Thorazine
- Dantrolene • Dantrium
- Donepezil • Aricept
- Lithium carbonate • various
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Risperidone • Risperdal
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association, 2000.
2. Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev 2003;22:203-20.
3. Dunner DL, Zisook S, Billow A, et al. A prospective safety study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry 1998;59:366-73.
4. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatr Ann 2000;30:314-21.
5. Berry N, Pradhan S, Sagar R, Gupta SK. Neuroleptic malignant syndrome in an adolescent receiving olanzapine-lithium combination therapy. Pharmacotherapy 2003;23:255-9
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65:464-70.
7. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
8. Bourgeois JA, Kahn DR. Neuroleptic malignant syndrome following administration of risperidone and lithium. J Clin Psychopharmacol 2003;23:315-6.
9. Gill J, Singh H, Nugent K. Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy 2003;23:811-15.
10. Halman M, Goldbloom DS. Fluoxetine and neuroleptic malignant syndrome. Biol Psychiatry 1990;28:518-21.
11. Spivak B, Gonen N, Mester R, et al. Neuroleptic malignant syndrome associated with abrupt withdrawal of anticholinergic agents. Int Clin Psychopharmacol 1996;11:207-9.
12. Rosse R, Ciolino C. Dopamine agonists and neuroleptic malignant syndrome. Am J Psychiatry 1985;142:270-1.
13. Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry 1999;156:169-80.
14. Ereshefsky L, Jann MW, Saklad SR, et al. Effects of smoking on fluphenazine clearance in psychiatric inpatients. Biol Psychiatry 1985;20:329-32.
15. Jann MW, Saklad SR, Ereshefsky L, et al. Effects of smoking on haloperidol and reduced haloperidol plasma concentrations and haloperidol clearance. Psychopharmacology (Berl) 1986;90:468-70.
16. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics 2005;46:464-94.
17. Davis JM, Caroff SN, Mann SC. Treatment of neuroleptic malignant syndrome. Psychiatr Ann 2000;30:325-31.
1. Diagnostic and statistical manual of mental disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association, 2000.
2. Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev 2003;22:203-20.
3. Dunner DL, Zisook S, Billow A, et al. A prospective safety study for bupropion sustained-release in the treatment of depression. J Clin Psychiatry 1998;59:366-73.
4. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatr Ann 2000;30:314-21.
5. Berry N, Pradhan S, Sagar R, Gupta SK. Neuroleptic malignant syndrome in an adolescent receiving olanzapine-lithium combination therapy. Pharmacotherapy 2003;23:255-9
6. Ananth J, Parameswaran S, Gunatilake S, et al. Neuroleptic malignant syndrome and atypical antipsychotic drugs. J Clin Psychiatry 2004;65:464-70.
7. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77:185-202.
8. Bourgeois JA, Kahn DR. Neuroleptic malignant syndrome following administration of risperidone and lithium. J Clin Psychopharmacol 2003;23:315-6.
9. Gill J, Singh H, Nugent K. Acute lithium intoxication and neuroleptic malignant syndrome. Pharmacotherapy 2003;23:811-15.
10. Halman M, Goldbloom DS. Fluoxetine and neuroleptic malignant syndrome. Biol Psychiatry 1990;28:518-21.
11. Spivak B, Gonen N, Mester R, et al. Neuroleptic malignant syndrome associated with abrupt withdrawal of anticholinergic agents. Int Clin Psychopharmacol 1996;11:207-9.
12. Rosse R, Ciolino C. Dopamine agonists and neuroleptic malignant syndrome. Am J Psychiatry 1985;142:270-1.
13. Gurrera RJ. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry 1999;156:169-80.
14. Ereshefsky L, Jann MW, Saklad SR, et al. Effects of smoking on fluphenazine clearance in psychiatric inpatients. Biol Psychiatry 1985;20:329-32.
15. Jann MW, Saklad SR, Ereshefsky L, et al. Effects of smoking on haloperidol and reduced haloperidol plasma concentrations and haloperidol clearance. Psychopharmacology (Berl) 1986;90:468-70.
16. Sandson NB, Armstrong SC, Cozza KL. An overview of psychotropic drug-drug interactions. Psychosomatics 2005;46:464-94.
17. Davis JM, Caroff SN, Mann SC. Treatment of neuroleptic malignant syndrome. Psychiatr Ann 2000;30:325-31.
Psychosis: Is it a medical problem?
History: shop talk
Ms. B, age 46, presents to the ER at her brother’s insistence. For about 6 months, she says, she has been “hearing voices”—including that of her boss—talking to each other about work.
Ms. B has no personal or family psychiatric history but notes that her sister died 6 months ago, and her father died the following month. At work, she is having trouble getting along with her boss. She adds that she has been skipping church lately because she believes her church is under investigation and the inquiry might be targeting her.
Ms. B has been a company manager for 20 years. She is divorced, has no children, and lives alone. She says she does not smoke or use illicit drugs and seldom drinks alcohol. She denies suicidal or homicidal thoughts, depressed mood, or visual hallucinations. She says she is sleeping only 3 to 4 hours nightly and feels fatigued in the afternoon. She denies loss of concentration or functioning.
Mental status. Ms. B is well groomed, maintains good eye contact, and is superficially cooperative but increasingly guarded with further questioning. She describes her mood as “OK,” but her affect is blunted. Thought process is logical but circumstantial at times, and her thoughts consist of auditory hallucinations, paranoid thinking, persecutory delusions, and ideas of reference. She has poor insight into her symptoms and does not want to be admitted.
Physical examination and laboratory tests are unremarkable. Negative ethanol and urine drug screens rule out substance abuse, and preliminary noncontrast head CT shows no acute changes.
The author’s observations
In women, schizophrenia typically emerges between ages 17 and 37;1 onset after age 45 is unusual.2 Ms. B’s age, family history, and lack of a formal thought disorder or negative symptoms make late-onset schizophrenia unlikely, though it cannot be ruled out.
Ms. B denies mood symptoms, but significant stressors—such as the recent deaths of her sister and father and difficulties at work—could precipitate a mood disorder. Of the possible diagnoses, major depressive disorder is most likely at this time.1,3 Because Ms. B’s symptoms do not clearly match any diagnosis, we speak with her brother and sister-in-law to seek collateral information.
Collateral history: beware of spies
Ms. B’s brother says his sister began behaving strangely about 8 months ago and has worsened lately. He says she suspects that her boss hired spies to watch her house, car, and her parent’s house. After work, she often parks in paid garages rather than at home to avoid being “followed.” When visiting, he says, she leaves her keys outside because she fears they contain a tracking device. Family members say Ms. B sometimes drops by at night—as late as 5 AM—complaining that she cannot sleep because she is being “watched.”
Ms. B’s family hired a private investigator 3 or 4 months ago to examine her house and car. Although no tracking devices were found, her brother says, Ms. B remains convinced she is being followed. He says she often speaks in “code” and whispers to herself.
According to her brother, Ms. B often hears voices while trying to sleep, saying such things as “Why won’t she turn over?” She reportedly wears a towel while showering because the “spies” are watching. During a conference she attended last week, she told her brother that a group of government investigators followed her there and arrested her boss.
Ms. B’s sister-in-law says the patient’s functioning has declined sharply, and that she has been helping Ms. B complete routine work. Neither she nor Ms. B’s brother have noticed a change in the patient’s energy, productivity, or speech production or speed, thus ruling out bipolar disorder. Ms. B’s brother confirms that there is no family history of mental illness.
The author’s observations
Collateral information about Ms. B points to psychosis rather than a mood disorder with psychotic features, but she lacks the formal thought disorder and negative symptoms common in primary psychotic disorders.
Because Ms. B’s presentation is atypical, we order brain MRI to check for a general medical condition (Figure 1). If brain MRI suggests a medical problem, we will follow with EEG, lumbar puncture, or other tests.
Figure 1 Clinical steps to rule out medical causes of late-onset psychosis
treatment, testing: what mri suggests
We admit Ms. B to the locked inpatient psychiatric unit—where she remains paranoid and guarded—and prescribe risperidone, 1 mg/d, to address her paranoia. She refuses medication at first because she feels she does not need psychiatric care, but we give her lorazepam, 0.5 mg/d for her anxiety, along with psychoeducation and family support. After 3 days, we stop lorazepam and Ms. B agrees to take risperidone.
Within 4 days of starting risperidone, Ms. B’s auditory hallucinations and paranoia have lessened and her insight is improved. We recommend increasing the dosage to 2 mg/d because we feel that 1 mg/d will not sufficiently control her symptoms. She remains paranoid but is reluctant to increase the dosage for fear of adverse effects, though she has reported none so far.
Brain MRI taken the night Ms. B was admitted shows:
- multiple focal, well-defined hyperintense periventricular lesions on fluid-attenuated inversion recovery (FLAIR)- and T2-weighted images (Figure 2). Some lesions are flame-shaped.
- a 1.5-cm lesion adjacent to the right frontal horn showing a hyperintense signal on T2-weighted images and a hypointense signal on T1-weighted images without contrast enhancement. White-matter edema surrounds this lesion.
- no gadolinium-enhancing lesions.
Two radiologists confirm possible demyelination, suggesting multiple sclerosis (MS). Final report of initial brain CT shows lowdensity, periventricular white matter changes consistent with the MRI findings.
Results of subsequent laboratory tests are normal. Erythrocyte sedimentation rate is slightly elevated at 35 mm/hr, suggesting a possible autoimmune disorder. ECG shows sinus bradycardia, and chest x-ray and MR angiogram are unremarkable, as are EEG and visual evoked potential results.
Lumbar puncture and CSF studies show increased immunoglobulin G to albumin ratio. CSF fluid is clear, blood counts and protein are normal, Gram’s stain and culture are negative, and cytologic findings show a marked increase in mature lymphocytes. These results suggest inflammation, but follow-up neurologic exam is unremarkable.
Figure 2 FLAIR-weighted image after Ms. B’s brain MRI
Right 1.5-cm lesion adjacent to right frontal horn and multiple left hyperintense lesions on fluid-attenuated inversion recovery (FLAIR)-weighted image.
The authors’ observations
Determining disease dissemination in time and space is key to diagnosing MS. Clinical presentation or MRI can determine both criteria (Table 1). Ms. B’s lesions and CSF results suggest that MS has disseminated throughout her body, but neurologic examination shows no objective clinical evidence of lesions.
Neuropsychological testing might help evaluate Ms. B’s cognition and executive functioning, but these deficits do not specifically suggest MS. The cortex, particularly the prefrontal cortex, is believed to coordinate organization, planning, and socially appropriate behavior. MS typically involves white matter rather than the cortex, but researchers have suggested that MS-related demyelination might disrupt the axonal circuits that connect the cortex to other brain areas.18
Increased lesion load has been correlated with decreased cognitive function. Neuropsychological testing could indirectly point to a lesion load increase by recording decreased cognitive function, but this decline cannot be attributed to MS without an MRI.
Ms. B’s psychotic symptoms could be clinical evidence of MS, but we cannot solidify the diagnosis until we establish dissemination in time. To do that, we need a second MRI 3 months after the first one. Concurrent late-onset paraphrenia and MS is possible but rare.
Table 1
Findings needed to determine MS diagnosis based on clinical presentation
| Clinical presentation | Findings needed for MS diagnosis |
|---|---|
| >2 clinical attacks* Objective clinical evidence of >2 lesions | None |
| >2 clinical attacks Objective clinical evidence of 1 lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| or | |
| Await further attack implicating a different site | |
| 1 clinical attack >2 objective clinical lesions | Dissemination in time by MRI |
| or | |
| Second clinical attack | |
| 1 clinical attack 1 objective clinical lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| and | |
| Dissemination in time by MRI | |
| or | |
| Second clinical attack | |
| * Clinical attack: neurologic disturbance defined by subjective report or objective observation lasting at least 24 hours. | |
| Source: Reference 5 | |
Follow-up: where is she?
Ms. B is discharged after 10 days. She denies hallucinations, and staff notices decreased paranoia, brighter affect, and improved insight. We tell her to continue taking risperidone, 1 mg/d.
Three weeks later, Ms. B sees an outpatient psychiatrist. She is paranoid, guarded, and has not been taking risperidone.
Because Ms. B’s previous MRI results are suspect, we ask the hospital’s neurology service to examine her. Findings are unremarkable, but the neurologist recommends a followup brain MRI in 3 months or sooner if symptoms emerge. More than 2 years later, she has not completed a second MRI or contacted her psychiatrist or neurologist.
The authors’ observations
Ms. B’s case highlights the importance of:
- recognizing an atypical presentation of a primary psychotic disorder
- checking for a medical cause of psychosis (Table 2)
- knowing which psychiatric symptoms are common in MS.
Despite absence of neurologic symptoms, Ms. B’s psychosis could have been the initial presentation of MS, which is more prevalent among psychiatric inpatients than in the general population.6,7 In a prospective study,8 95% of patients with MS had neuropsychiatric symptoms, and 79% had depressive symptoms. Hallucinations and delusions were reported in 10% and 7% of MS patients, respectively. These findings suggest that mood disturbances are considerably more common than psychosis among patients with MS.
Diagnosis of MSrelated psychosis has been addressed only in case reports or small studies, most of which have not clearly defined psychosis or adequately described the symptoms or confounding factors such as medications. Findings on prevalence of psychosis as the initial presentation in MS are more limited and confounded by instances in which neurologic symptoms might have been overlooked.9,11
Few studies have investigated whether lesion location correlates with specific neuropsychiatric symptoms. In one study,8 brain MRI taken within 9 months of presenting symptoms showed that MS was not significantly more severe among patients with psychosis compared with nonpsychotic MS patients. These data support psychosis as a possible early finding in MS.
At least two studies12,13 suggest a correlation between temporal lobe lesions and psychosis, but both study samples were small (8 and 10 patients) and used a combination of diagnoses. One case report also supports this correlation.14
Table 2
Medical conditions that can cause psychotic symptoms
| Cerebral malignancy (primary and metastases) | |
| Cerebral trauma | |
| Cerebral vascular accident | |
| Creutzfeldt-Jakob disease | |
| Delirium | |
| Dementia | |
| Epilepsy | |
| Huntington's disease | |
| Infection | |
| Multiple sclerosis | |
| Parkinson's disease | |
| Systemic lupus erythematosus | |
| Source: Reference 4 | |
Treating ms-related psychosis
MS-related psychosis should abate with MS treatment, but no systematized studies have verified this or determined which antipsychotics would be suitable. Single case reports suggest successful treatment with risperidone,13 haloperidol,15 clozapine,16 or ziprasidone.17 Ms. B showed initial improvement with risperidone, but because she was lost to follow-up we cannot say if this medication would work long-term.
Related resources
- Feinstein A. The clinical neuropsychiatry of multiple sclerosis. New York: Cambridge University Press; 1999.
- National Multiple Sclerosis Society. www.nationalmssociety.org.
Drug brand name
- Clozapine • Clozaril
- Risperidone • Risperdal
- Haloperidol • Haldol
- Ziprasidone • Geodon
- Lorazepam • Ativan
Disclosures
Dr. Higgins reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rafeyan is a speaker for AstraZeneca, BristolMyers Squibb Co., Eli Lilly and Co., GlaxoSmithKline, Pfizer, and Wyeth. He is also an advisor to Abbott Laboratories and Forest Pharmaceuticals.
1. Kaplan B, Sadock V, eds. Comprehensive textbook of psychiatry, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2000:1107,1299.
2. Diagnostic and statistical manual of mental disorders 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Howard R, Rabins P, Seeman M, Jeste D. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. Am J Psychiatry 2000;157:172-8.
4. Lautenschlager NT, Forstl H. Organic psychosis: insight into the biology of psychosis. Curr Psychiatry Rep 2001;3:319-25.
5. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121-7.
6. Pine D, Douglas C, Charles E, et al. Patients with multiple sclerosis presenting to psychiatric hospitals. J Clin Psychiatry 1995;56:297-306.
7. Lyoo IK, Seol HY, Byun HS, Renshaw PF. Unsuspected multiple sclerosis in patients with psychiatric disorders: a magnetic resonance imaging study. J Neuropsychiatry Clin Neurosci 1996;8:54-9.
8. Diaz-Olavarrieta C, Cummings JL, Velazquez J, Garcia de la Cadena C. Neuropsychiatric manifestations of multiple sclerosis. J Neuropsychiatry Clin Neurosci 1999;11:51-7.
9. Felgenhauer K. Psychiatric disorders in the encephalitic form of multiple sclerosis. J Neurol 1990;237:11-8.
10. Skegg K, Corwin P, Skegg D. How often is multiple sclerosis mistaken for a psychiatric disorder? Psychol Med 1988;18:733-6.
11. Kohler J, Heilmeyer H, Volk B. Multiple sclerosis presenting as chronic atypical psychosis. J Neurol Neurosurg Psychiatry 1988;51:281-4.
12. Honer G, Hurwitz T, Li D, et al. Temporal lobe involvement in multiple sclerosis patients with psychiatric disorders. Arch Neurol 1987;44:187-90.
13. Feinstein A, du Boulay G, Ron M. Psychotic illness in multiple sclerosis: a clinical and magnetic resonance imaging study. Br J Psychiatry 1992;161:680-5.
14. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry 2003;25:27-33.
15. Drake ME. Acute paranoid psychosis in multiple sclerosis. Psychosomatics 1984;25:60-3.
16. Chong SA, Ko SM. Clozapine treatment of psychosis associated with multiple sclerosis. Can J Psychiatry 1997;42:90-1.
17. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:743-4.
18. Asghar-Ali A, Taber K, Hurley R, Hayman L. Pure neuropsychiatric presentation of multiple sclerosis. Am J Psychiatry 2004;161:226-31.
History: shop talk
Ms. B, age 46, presents to the ER at her brother’s insistence. For about 6 months, she says, she has been “hearing voices”—including that of her boss—talking to each other about work.
Ms. B has no personal or family psychiatric history but notes that her sister died 6 months ago, and her father died the following month. At work, she is having trouble getting along with her boss. She adds that she has been skipping church lately because she believes her church is under investigation and the inquiry might be targeting her.
Ms. B has been a company manager for 20 years. She is divorced, has no children, and lives alone. She says she does not smoke or use illicit drugs and seldom drinks alcohol. She denies suicidal or homicidal thoughts, depressed mood, or visual hallucinations. She says she is sleeping only 3 to 4 hours nightly and feels fatigued in the afternoon. She denies loss of concentration or functioning.
Mental status. Ms. B is well groomed, maintains good eye contact, and is superficially cooperative but increasingly guarded with further questioning. She describes her mood as “OK,” but her affect is blunted. Thought process is logical but circumstantial at times, and her thoughts consist of auditory hallucinations, paranoid thinking, persecutory delusions, and ideas of reference. She has poor insight into her symptoms and does not want to be admitted.
Physical examination and laboratory tests are unremarkable. Negative ethanol and urine drug screens rule out substance abuse, and preliminary noncontrast head CT shows no acute changes.
The author’s observations
In women, schizophrenia typically emerges between ages 17 and 37;1 onset after age 45 is unusual.2 Ms. B’s age, family history, and lack of a formal thought disorder or negative symptoms make late-onset schizophrenia unlikely, though it cannot be ruled out.
Ms. B denies mood symptoms, but significant stressors—such as the recent deaths of her sister and father and difficulties at work—could precipitate a mood disorder. Of the possible diagnoses, major depressive disorder is most likely at this time.1,3 Because Ms. B’s symptoms do not clearly match any diagnosis, we speak with her brother and sister-in-law to seek collateral information.
Collateral history: beware of spies
Ms. B’s brother says his sister began behaving strangely about 8 months ago and has worsened lately. He says she suspects that her boss hired spies to watch her house, car, and her parent’s house. After work, she often parks in paid garages rather than at home to avoid being “followed.” When visiting, he says, she leaves her keys outside because she fears they contain a tracking device. Family members say Ms. B sometimes drops by at night—as late as 5 AM—complaining that she cannot sleep because she is being “watched.”
Ms. B’s family hired a private investigator 3 or 4 months ago to examine her house and car. Although no tracking devices were found, her brother says, Ms. B remains convinced she is being followed. He says she often speaks in “code” and whispers to herself.
According to her brother, Ms. B often hears voices while trying to sleep, saying such things as “Why won’t she turn over?” She reportedly wears a towel while showering because the “spies” are watching. During a conference she attended last week, she told her brother that a group of government investigators followed her there and arrested her boss.
Ms. B’s sister-in-law says the patient’s functioning has declined sharply, and that she has been helping Ms. B complete routine work. Neither she nor Ms. B’s brother have noticed a change in the patient’s energy, productivity, or speech production or speed, thus ruling out bipolar disorder. Ms. B’s brother confirms that there is no family history of mental illness.
The author’s observations
Collateral information about Ms. B points to psychosis rather than a mood disorder with psychotic features, but she lacks the formal thought disorder and negative symptoms common in primary psychotic disorders.
Because Ms. B’s presentation is atypical, we order brain MRI to check for a general medical condition (Figure 1). If brain MRI suggests a medical problem, we will follow with EEG, lumbar puncture, or other tests.
Figure 1 Clinical steps to rule out medical causes of late-onset psychosis
treatment, testing: what mri suggests
We admit Ms. B to the locked inpatient psychiatric unit—where she remains paranoid and guarded—and prescribe risperidone, 1 mg/d, to address her paranoia. She refuses medication at first because she feels she does not need psychiatric care, but we give her lorazepam, 0.5 mg/d for her anxiety, along with psychoeducation and family support. After 3 days, we stop lorazepam and Ms. B agrees to take risperidone.
Within 4 days of starting risperidone, Ms. B’s auditory hallucinations and paranoia have lessened and her insight is improved. We recommend increasing the dosage to 2 mg/d because we feel that 1 mg/d will not sufficiently control her symptoms. She remains paranoid but is reluctant to increase the dosage for fear of adverse effects, though she has reported none so far.
Brain MRI taken the night Ms. B was admitted shows:
- multiple focal, well-defined hyperintense periventricular lesions on fluid-attenuated inversion recovery (FLAIR)- and T2-weighted images (Figure 2). Some lesions are flame-shaped.
- a 1.5-cm lesion adjacent to the right frontal horn showing a hyperintense signal on T2-weighted images and a hypointense signal on T1-weighted images without contrast enhancement. White-matter edema surrounds this lesion.
- no gadolinium-enhancing lesions.
Two radiologists confirm possible demyelination, suggesting multiple sclerosis (MS). Final report of initial brain CT shows lowdensity, periventricular white matter changes consistent with the MRI findings.
Results of subsequent laboratory tests are normal. Erythrocyte sedimentation rate is slightly elevated at 35 mm/hr, suggesting a possible autoimmune disorder. ECG shows sinus bradycardia, and chest x-ray and MR angiogram are unremarkable, as are EEG and visual evoked potential results.
Lumbar puncture and CSF studies show increased immunoglobulin G to albumin ratio. CSF fluid is clear, blood counts and protein are normal, Gram’s stain and culture are negative, and cytologic findings show a marked increase in mature lymphocytes. These results suggest inflammation, but follow-up neurologic exam is unremarkable.
Figure 2 FLAIR-weighted image after Ms. B’s brain MRI
Right 1.5-cm lesion adjacent to right frontal horn and multiple left hyperintense lesions on fluid-attenuated inversion recovery (FLAIR)-weighted image.
The authors’ observations
Determining disease dissemination in time and space is key to diagnosing MS. Clinical presentation or MRI can determine both criteria (Table 1). Ms. B’s lesions and CSF results suggest that MS has disseminated throughout her body, but neurologic examination shows no objective clinical evidence of lesions.
Neuropsychological testing might help evaluate Ms. B’s cognition and executive functioning, but these deficits do not specifically suggest MS. The cortex, particularly the prefrontal cortex, is believed to coordinate organization, planning, and socially appropriate behavior. MS typically involves white matter rather than the cortex, but researchers have suggested that MS-related demyelination might disrupt the axonal circuits that connect the cortex to other brain areas.18
Increased lesion load has been correlated with decreased cognitive function. Neuropsychological testing could indirectly point to a lesion load increase by recording decreased cognitive function, but this decline cannot be attributed to MS without an MRI.
Ms. B’s psychotic symptoms could be clinical evidence of MS, but we cannot solidify the diagnosis until we establish dissemination in time. To do that, we need a second MRI 3 months after the first one. Concurrent late-onset paraphrenia and MS is possible but rare.
Table 1
Findings needed to determine MS diagnosis based on clinical presentation
| Clinical presentation | Findings needed for MS diagnosis |
|---|---|
| >2 clinical attacks* Objective clinical evidence of >2 lesions | None |
| >2 clinical attacks Objective clinical evidence of 1 lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| or | |
| Await further attack implicating a different site | |
| 1 clinical attack >2 objective clinical lesions | Dissemination in time by MRI |
| or | |
| Second clinical attack | |
| 1 clinical attack 1 objective clinical lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| and | |
| Dissemination in time by MRI | |
| or | |
| Second clinical attack | |
| * Clinical attack: neurologic disturbance defined by subjective report or objective observation lasting at least 24 hours. | |
| Source: Reference 5 | |
Follow-up: where is she?
Ms. B is discharged after 10 days. She denies hallucinations, and staff notices decreased paranoia, brighter affect, and improved insight. We tell her to continue taking risperidone, 1 mg/d.
Three weeks later, Ms. B sees an outpatient psychiatrist. She is paranoid, guarded, and has not been taking risperidone.
Because Ms. B’s previous MRI results are suspect, we ask the hospital’s neurology service to examine her. Findings are unremarkable, but the neurologist recommends a followup brain MRI in 3 months or sooner if symptoms emerge. More than 2 years later, she has not completed a second MRI or contacted her psychiatrist or neurologist.
The authors’ observations
Ms. B’s case highlights the importance of:
- recognizing an atypical presentation of a primary psychotic disorder
- checking for a medical cause of psychosis (Table 2)
- knowing which psychiatric symptoms are common in MS.
Despite absence of neurologic symptoms, Ms. B’s psychosis could have been the initial presentation of MS, which is more prevalent among psychiatric inpatients than in the general population.6,7 In a prospective study,8 95% of patients with MS had neuropsychiatric symptoms, and 79% had depressive symptoms. Hallucinations and delusions were reported in 10% and 7% of MS patients, respectively. These findings suggest that mood disturbances are considerably more common than psychosis among patients with MS.
Diagnosis of MSrelated psychosis has been addressed only in case reports or small studies, most of which have not clearly defined psychosis or adequately described the symptoms or confounding factors such as medications. Findings on prevalence of psychosis as the initial presentation in MS are more limited and confounded by instances in which neurologic symptoms might have been overlooked.9,11
Few studies have investigated whether lesion location correlates with specific neuropsychiatric symptoms. In one study,8 brain MRI taken within 9 months of presenting symptoms showed that MS was not significantly more severe among patients with psychosis compared with nonpsychotic MS patients. These data support psychosis as a possible early finding in MS.
At least two studies12,13 suggest a correlation between temporal lobe lesions and psychosis, but both study samples were small (8 and 10 patients) and used a combination of diagnoses. One case report also supports this correlation.14
Table 2
Medical conditions that can cause psychotic symptoms
| Cerebral malignancy (primary and metastases) | |
| Cerebral trauma | |
| Cerebral vascular accident | |
| Creutzfeldt-Jakob disease | |
| Delirium | |
| Dementia | |
| Epilepsy | |
| Huntington's disease | |
| Infection | |
| Multiple sclerosis | |
| Parkinson's disease | |
| Systemic lupus erythematosus | |
| Source: Reference 4 | |
Treating ms-related psychosis
MS-related psychosis should abate with MS treatment, but no systematized studies have verified this or determined which antipsychotics would be suitable. Single case reports suggest successful treatment with risperidone,13 haloperidol,15 clozapine,16 or ziprasidone.17 Ms. B showed initial improvement with risperidone, but because she was lost to follow-up we cannot say if this medication would work long-term.
Related resources
- Feinstein A. The clinical neuropsychiatry of multiple sclerosis. New York: Cambridge University Press; 1999.
- National Multiple Sclerosis Society. www.nationalmssociety.org.
Drug brand name
- Clozapine • Clozaril
- Risperidone • Risperdal
- Haloperidol • Haldol
- Ziprasidone • Geodon
- Lorazepam • Ativan
Disclosures
Dr. Higgins reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rafeyan is a speaker for AstraZeneca, BristolMyers Squibb Co., Eli Lilly and Co., GlaxoSmithKline, Pfizer, and Wyeth. He is also an advisor to Abbott Laboratories and Forest Pharmaceuticals.
History: shop talk
Ms. B, age 46, presents to the ER at her brother’s insistence. For about 6 months, she says, she has been “hearing voices”—including that of her boss—talking to each other about work.
Ms. B has no personal or family psychiatric history but notes that her sister died 6 months ago, and her father died the following month. At work, she is having trouble getting along with her boss. She adds that she has been skipping church lately because she believes her church is under investigation and the inquiry might be targeting her.
Ms. B has been a company manager for 20 years. She is divorced, has no children, and lives alone. She says she does not smoke or use illicit drugs and seldom drinks alcohol. She denies suicidal or homicidal thoughts, depressed mood, or visual hallucinations. She says she is sleeping only 3 to 4 hours nightly and feels fatigued in the afternoon. She denies loss of concentration or functioning.
Mental status. Ms. B is well groomed, maintains good eye contact, and is superficially cooperative but increasingly guarded with further questioning. She describes her mood as “OK,” but her affect is blunted. Thought process is logical but circumstantial at times, and her thoughts consist of auditory hallucinations, paranoid thinking, persecutory delusions, and ideas of reference. She has poor insight into her symptoms and does not want to be admitted.
Physical examination and laboratory tests are unremarkable. Negative ethanol and urine drug screens rule out substance abuse, and preliminary noncontrast head CT shows no acute changes.
The author’s observations
In women, schizophrenia typically emerges between ages 17 and 37;1 onset after age 45 is unusual.2 Ms. B’s age, family history, and lack of a formal thought disorder or negative symptoms make late-onset schizophrenia unlikely, though it cannot be ruled out.
Ms. B denies mood symptoms, but significant stressors—such as the recent deaths of her sister and father and difficulties at work—could precipitate a mood disorder. Of the possible diagnoses, major depressive disorder is most likely at this time.1,3 Because Ms. B’s symptoms do not clearly match any diagnosis, we speak with her brother and sister-in-law to seek collateral information.
Collateral history: beware of spies
Ms. B’s brother says his sister began behaving strangely about 8 months ago and has worsened lately. He says she suspects that her boss hired spies to watch her house, car, and her parent’s house. After work, she often parks in paid garages rather than at home to avoid being “followed.” When visiting, he says, she leaves her keys outside because she fears they contain a tracking device. Family members say Ms. B sometimes drops by at night—as late as 5 AM—complaining that she cannot sleep because she is being “watched.”
Ms. B’s family hired a private investigator 3 or 4 months ago to examine her house and car. Although no tracking devices were found, her brother says, Ms. B remains convinced she is being followed. He says she often speaks in “code” and whispers to herself.
According to her brother, Ms. B often hears voices while trying to sleep, saying such things as “Why won’t she turn over?” She reportedly wears a towel while showering because the “spies” are watching. During a conference she attended last week, she told her brother that a group of government investigators followed her there and arrested her boss.
Ms. B’s sister-in-law says the patient’s functioning has declined sharply, and that she has been helping Ms. B complete routine work. Neither she nor Ms. B’s brother have noticed a change in the patient’s energy, productivity, or speech production or speed, thus ruling out bipolar disorder. Ms. B’s brother confirms that there is no family history of mental illness.
The author’s observations
Collateral information about Ms. B points to psychosis rather than a mood disorder with psychotic features, but she lacks the formal thought disorder and negative symptoms common in primary psychotic disorders.
Because Ms. B’s presentation is atypical, we order brain MRI to check for a general medical condition (Figure 1). If brain MRI suggests a medical problem, we will follow with EEG, lumbar puncture, or other tests.
Figure 1 Clinical steps to rule out medical causes of late-onset psychosis
treatment, testing: what mri suggests
We admit Ms. B to the locked inpatient psychiatric unit—where she remains paranoid and guarded—and prescribe risperidone, 1 mg/d, to address her paranoia. She refuses medication at first because she feels she does not need psychiatric care, but we give her lorazepam, 0.5 mg/d for her anxiety, along with psychoeducation and family support. After 3 days, we stop lorazepam and Ms. B agrees to take risperidone.
Within 4 days of starting risperidone, Ms. B’s auditory hallucinations and paranoia have lessened and her insight is improved. We recommend increasing the dosage to 2 mg/d because we feel that 1 mg/d will not sufficiently control her symptoms. She remains paranoid but is reluctant to increase the dosage for fear of adverse effects, though she has reported none so far.
Brain MRI taken the night Ms. B was admitted shows:
- multiple focal, well-defined hyperintense periventricular lesions on fluid-attenuated inversion recovery (FLAIR)- and T2-weighted images (Figure 2). Some lesions are flame-shaped.
- a 1.5-cm lesion adjacent to the right frontal horn showing a hyperintense signal on T2-weighted images and a hypointense signal on T1-weighted images without contrast enhancement. White-matter edema surrounds this lesion.
- no gadolinium-enhancing lesions.
Two radiologists confirm possible demyelination, suggesting multiple sclerosis (MS). Final report of initial brain CT shows lowdensity, periventricular white matter changes consistent with the MRI findings.
Results of subsequent laboratory tests are normal. Erythrocyte sedimentation rate is slightly elevated at 35 mm/hr, suggesting a possible autoimmune disorder. ECG shows sinus bradycardia, and chest x-ray and MR angiogram are unremarkable, as are EEG and visual evoked potential results.
Lumbar puncture and CSF studies show increased immunoglobulin G to albumin ratio. CSF fluid is clear, blood counts and protein are normal, Gram’s stain and culture are negative, and cytologic findings show a marked increase in mature lymphocytes. These results suggest inflammation, but follow-up neurologic exam is unremarkable.
Figure 2 FLAIR-weighted image after Ms. B’s brain MRI
Right 1.5-cm lesion adjacent to right frontal horn and multiple left hyperintense lesions on fluid-attenuated inversion recovery (FLAIR)-weighted image.
The authors’ observations
Determining disease dissemination in time and space is key to diagnosing MS. Clinical presentation or MRI can determine both criteria (Table 1). Ms. B’s lesions and CSF results suggest that MS has disseminated throughout her body, but neurologic examination shows no objective clinical evidence of lesions.
Neuropsychological testing might help evaluate Ms. B’s cognition and executive functioning, but these deficits do not specifically suggest MS. The cortex, particularly the prefrontal cortex, is believed to coordinate organization, planning, and socially appropriate behavior. MS typically involves white matter rather than the cortex, but researchers have suggested that MS-related demyelination might disrupt the axonal circuits that connect the cortex to other brain areas.18
Increased lesion load has been correlated with decreased cognitive function. Neuropsychological testing could indirectly point to a lesion load increase by recording decreased cognitive function, but this decline cannot be attributed to MS without an MRI.
Ms. B’s psychotic symptoms could be clinical evidence of MS, but we cannot solidify the diagnosis until we establish dissemination in time. To do that, we need a second MRI 3 months after the first one. Concurrent late-onset paraphrenia and MS is possible but rare.
Table 1
Findings needed to determine MS diagnosis based on clinical presentation
| Clinical presentation | Findings needed for MS diagnosis |
|---|---|
| >2 clinical attacks* Objective clinical evidence of >2 lesions | None |
| >2 clinical attacks Objective clinical evidence of 1 lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| or | |
| Await further attack implicating a different site | |
| 1 clinical attack >2 objective clinical lesions | Dissemination in time by MRI |
| or | |
| Second clinical attack | |
| 1 clinical attack 1 objective clinical lesion | Dissemination in space by MRI |
| or | |
| >2 MRI-detected lesions consistent with MS plus positive CSF | |
| and | |
| Dissemination in time by MRI | |
| or | |
| Second clinical attack | |
| * Clinical attack: neurologic disturbance defined by subjective report or objective observation lasting at least 24 hours. | |
| Source: Reference 5 | |
Follow-up: where is she?
Ms. B is discharged after 10 days. She denies hallucinations, and staff notices decreased paranoia, brighter affect, and improved insight. We tell her to continue taking risperidone, 1 mg/d.
Three weeks later, Ms. B sees an outpatient psychiatrist. She is paranoid, guarded, and has not been taking risperidone.
Because Ms. B’s previous MRI results are suspect, we ask the hospital’s neurology service to examine her. Findings are unremarkable, but the neurologist recommends a followup brain MRI in 3 months or sooner if symptoms emerge. More than 2 years later, she has not completed a second MRI or contacted her psychiatrist or neurologist.
The authors’ observations
Ms. B’s case highlights the importance of:
- recognizing an atypical presentation of a primary psychotic disorder
- checking for a medical cause of psychosis (Table 2)
- knowing which psychiatric symptoms are common in MS.
Despite absence of neurologic symptoms, Ms. B’s psychosis could have been the initial presentation of MS, which is more prevalent among psychiatric inpatients than in the general population.6,7 In a prospective study,8 95% of patients with MS had neuropsychiatric symptoms, and 79% had depressive symptoms. Hallucinations and delusions were reported in 10% and 7% of MS patients, respectively. These findings suggest that mood disturbances are considerably more common than psychosis among patients with MS.
Diagnosis of MSrelated psychosis has been addressed only in case reports or small studies, most of which have not clearly defined psychosis or adequately described the symptoms or confounding factors such as medications. Findings on prevalence of psychosis as the initial presentation in MS are more limited and confounded by instances in which neurologic symptoms might have been overlooked.9,11
Few studies have investigated whether lesion location correlates with specific neuropsychiatric symptoms. In one study,8 brain MRI taken within 9 months of presenting symptoms showed that MS was not significantly more severe among patients with psychosis compared with nonpsychotic MS patients. These data support psychosis as a possible early finding in MS.
At least two studies12,13 suggest a correlation between temporal lobe lesions and psychosis, but both study samples were small (8 and 10 patients) and used a combination of diagnoses. One case report also supports this correlation.14
Table 2
Medical conditions that can cause psychotic symptoms
| Cerebral malignancy (primary and metastases) | |
| Cerebral trauma | |
| Cerebral vascular accident | |
| Creutzfeldt-Jakob disease | |
| Delirium | |
| Dementia | |
| Epilepsy | |
| Huntington's disease | |
| Infection | |
| Multiple sclerosis | |
| Parkinson's disease | |
| Systemic lupus erythematosus | |
| Source: Reference 4 | |
Treating ms-related psychosis
MS-related psychosis should abate with MS treatment, but no systematized studies have verified this or determined which antipsychotics would be suitable. Single case reports suggest successful treatment with risperidone,13 haloperidol,15 clozapine,16 or ziprasidone.17 Ms. B showed initial improvement with risperidone, but because she was lost to follow-up we cannot say if this medication would work long-term.
Related resources
- Feinstein A. The clinical neuropsychiatry of multiple sclerosis. New York: Cambridge University Press; 1999.
- National Multiple Sclerosis Society. www.nationalmssociety.org.
Drug brand name
- Clozapine • Clozaril
- Risperidone • Risperdal
- Haloperidol • Haldol
- Ziprasidone • Geodon
- Lorazepam • Ativan
Disclosures
Dr. Higgins reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rafeyan is a speaker for AstraZeneca, BristolMyers Squibb Co., Eli Lilly and Co., GlaxoSmithKline, Pfizer, and Wyeth. He is also an advisor to Abbott Laboratories and Forest Pharmaceuticals.
1. Kaplan B, Sadock V, eds. Comprehensive textbook of psychiatry, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2000:1107,1299.
2. Diagnostic and statistical manual of mental disorders 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Howard R, Rabins P, Seeman M, Jeste D. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. Am J Psychiatry 2000;157:172-8.
4. Lautenschlager NT, Forstl H. Organic psychosis: insight into the biology of psychosis. Curr Psychiatry Rep 2001;3:319-25.
5. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121-7.
6. Pine D, Douglas C, Charles E, et al. Patients with multiple sclerosis presenting to psychiatric hospitals. J Clin Psychiatry 1995;56:297-306.
7. Lyoo IK, Seol HY, Byun HS, Renshaw PF. Unsuspected multiple sclerosis in patients with psychiatric disorders: a magnetic resonance imaging study. J Neuropsychiatry Clin Neurosci 1996;8:54-9.
8. Diaz-Olavarrieta C, Cummings JL, Velazquez J, Garcia de la Cadena C. Neuropsychiatric manifestations of multiple sclerosis. J Neuropsychiatry Clin Neurosci 1999;11:51-7.
9. Felgenhauer K. Psychiatric disorders in the encephalitic form of multiple sclerosis. J Neurol 1990;237:11-8.
10. Skegg K, Corwin P, Skegg D. How often is multiple sclerosis mistaken for a psychiatric disorder? Psychol Med 1988;18:733-6.
11. Kohler J, Heilmeyer H, Volk B. Multiple sclerosis presenting as chronic atypical psychosis. J Neurol Neurosurg Psychiatry 1988;51:281-4.
12. Honer G, Hurwitz T, Li D, et al. Temporal lobe involvement in multiple sclerosis patients with psychiatric disorders. Arch Neurol 1987;44:187-90.
13. Feinstein A, du Boulay G, Ron M. Psychotic illness in multiple sclerosis: a clinical and magnetic resonance imaging study. Br J Psychiatry 1992;161:680-5.
14. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry 2003;25:27-33.
15. Drake ME. Acute paranoid psychosis in multiple sclerosis. Psychosomatics 1984;25:60-3.
16. Chong SA, Ko SM. Clozapine treatment of psychosis associated with multiple sclerosis. Can J Psychiatry 1997;42:90-1.
17. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:743-4.
18. Asghar-Ali A, Taber K, Hurley R, Hayman L. Pure neuropsychiatric presentation of multiple sclerosis. Am J Psychiatry 2004;161:226-31.
1. Kaplan B, Sadock V, eds. Comprehensive textbook of psychiatry, 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2000:1107,1299.
2. Diagnostic and statistical manual of mental disorders 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
3. Howard R, Rabins P, Seeman M, Jeste D. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: an international consensus. Am J Psychiatry 2000;157:172-8.
4. Lautenschlager NT, Forstl H. Organic psychosis: insight into the biology of psychosis. Curr Psychiatry Rep 2001;3:319-25.
5. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001;50:121-7.
6. Pine D, Douglas C, Charles E, et al. Patients with multiple sclerosis presenting to psychiatric hospitals. J Clin Psychiatry 1995;56:297-306.
7. Lyoo IK, Seol HY, Byun HS, Renshaw PF. Unsuspected multiple sclerosis in patients with psychiatric disorders: a magnetic resonance imaging study. J Neuropsychiatry Clin Neurosci 1996;8:54-9.
8. Diaz-Olavarrieta C, Cummings JL, Velazquez J, Garcia de la Cadena C. Neuropsychiatric manifestations of multiple sclerosis. J Neuropsychiatry Clin Neurosci 1999;11:51-7.
9. Felgenhauer K. Psychiatric disorders in the encephalitic form of multiple sclerosis. J Neurol 1990;237:11-8.
10. Skegg K, Corwin P, Skegg D. How often is multiple sclerosis mistaken for a psychiatric disorder? Psychol Med 1988;18:733-6.
11. Kohler J, Heilmeyer H, Volk B. Multiple sclerosis presenting as chronic atypical psychosis. J Neurol Neurosurg Psychiatry 1988;51:281-4.
12. Honer G, Hurwitz T, Li D, et al. Temporal lobe involvement in multiple sclerosis patients with psychiatric disorders. Arch Neurol 1987;44:187-90.
13. Feinstein A, du Boulay G, Ron M. Psychotic illness in multiple sclerosis: a clinical and magnetic resonance imaging study. Br J Psychiatry 1992;161:680-5.
14. Sirois F. Steroid psychosis: a review. Gen Hosp Psychiatry 2003;25:27-33.
15. Drake ME. Acute paranoid psychosis in multiple sclerosis. Psychosomatics 1984;25:60-3.
16. Chong SA, Ko SM. Clozapine treatment of psychosis associated with multiple sclerosis. Can J Psychiatry 1997;42:90-1.
17. Davids E, Hartwig U, Gastpar M. Antipsychotic treatment of psychosis associated with multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry 2004;28:743-4.
18. Asghar-Ali A, Taber K, Hurley R, Hayman L. Pure neuropsychiatric presentation of multiple sclerosis. Am J Psychiatry 2004;161:226-31.
Gender dysphoria: ‘I’m a man, but…’
History: normal on paper
Mr. C, age 65, presents to an endocrinologist complaining of hot flashes and low libido. Initial testing shows low male testosterone, but a repeat test shows normal levels. No medical cause is found for his symptoms.
“My testosterone might be normal on paper,” Mr. C tells the endocrinologist, “but I’m not. I think I’m a woman.”
Mr. C requests referral to a female psychiatrist because he feels more comfortable discussing sexual issues with a woman. The endocrinologist refers him to me for evaluation.
Over 7 years, Mr. C’s other psychiatrist—a man—has been treating him for obsessive-compulsive disorder (OCD), anxiety disorder, and bipolar disorder type II. Mr. C takes paroxetine, 60 mg/d, for depressive symptoms and was taking divalproex, 1,500 mg/d, to stabilize his mood. He recently stopped divalproex because it was causing nausea and sedation.
During our initial visit, Mr. C says he’s “through pretending to be a man.” He says he first questioned his sexual identity in early childhood, when he sometimes dressed in his mother’s clothes for play. As an adult, he mostly cross-dresses in lingerie; he wears a woman’s tank top in public once or twice weekly underneath his polo dress shirt. Fifteen years ago, he suffered anorexia and bulimia while trying to look as svelte as a woman.
At 6 feet, 2 inches with good muscle tone and short, wavy black hair, Mr. C looks strikingly masculine. Now retired, he served in the Air Force and later worked as a commercial pilot and in construction. In private, however, he prefers gardening and cooking over sports and cars.
Mr. C is married but seldom has sexual intercourse with women. He gains sexual fulfillment by visualizing himself as a woman having sex with other women or with himself as a man. He denies interest in male-male sex.
The patient has been masturbating since age 5, mostly by rubbing his scrotum against a swing set pole he still keeps in his utility shed. He often tucks his penis to mimic female genitalia and makes believe his rectum is a vagina.
- Transsexualism
- Pure transvestism (having a firm gender identity but becoming sexually aroused by cross-dressing)
- Dual-role transvestism (cross-dressing solely to experience temporary membership in the opposite sex)
- Stress-related cross-dressing
- Men who desire penectomy or castration but no other gender-reassignment interventions
- Congenital intersex conditions, such as hermaphrodism
Mr. C’s Mini-Mental State Examination score of 30 indicates no underlying dementia. He shows stable affect with no evidence of derailment, paranoia, thought blocking, or auditory hallucinations.
Medical examination results are normal. Negative urine toxicology screen rules out substance abuse, and negative rapid plasma reagin rules out syphilis. Testosterone is not rechecked because levels were normal 2 days before.
The author’s observations
I suspect gender dysphoria, which describes a heterogeneous group of persons who express varying degrees of distress with their anatomic sex and sometimes desire secondary opposite-gender sexual characteristics (Box).
Sexual identity in gender dysphoria is often fluid. Symptoms might suggest transvestism, then evolve to transsexualism. Recognizing this heterogeneity and fluidity is crucial to diagnosis and treatment.
Primary transsexualism. The term “transsexualism” describes persons who want to live and be accepted within the opposite sex.1 The transsexual identity persists for ≥ 2 years and is not caused by another mental disorder or intersexed condition. Fetishism is classically absent and cross-dressing is not sexually gratifying. Most transsexuals want surgical and hormone treatment to make their bodies as congruent as possible with the preferred sex.
In 1994, DSM-IV recognized that some late-onset transsexuals showed features of comorbid transvestism and were sexually aroused by female dress and behaviors. Gender identity disorder (GID) replaced the term “transsexualism” and includes these individuals. A secondary diagnosis of transvestism is applied.
Secondary transsexualism. Case reports2 describe psychosis-induced transsexual desires in patients with schizophrenia. Gender dysphoria improved as their schizophrenia symptoms lessened.
The relationship between transsexualism and schizophrenia has been debated. Hyde and Kenna3 view transsexualism as a schizophrenia spectrum disorder, whereas sexologists consider transsexualism and schizophrenia distinct syndromes that can occur simultaneously.
Affective disorders might also alter contentment with gender role, but the relationship is unclear. Case reports of patients with bipolar disorder suggest that gender dysphoria intensity fluctuates with affective excursions.4 O’Gorman,5 however, described a bipolar patient whose gender dysphoria was mitigated during manic episodes.
Paraphilias are sexual disorders with recurrent intense urges and fantasies that do not follow normative arousal patterns and can diminish capacity for sexual intimacy.6 Manifestations include exhibitionism, fetishism, frotteurism, pedophilia, masochism/sadism, voyeurism, and transvestic fetishism.
Dividing transsexualism and pure transvestism paraphilia into discrete categories is simplistic, as transvestites can develop secondary components of transsexualism. Hoenig and Kenna7 assert that transsexualism—though not an anomalous erotic preference—is almost always preceded by transvestism or accompanied by cross-gender fetishism.
Nonparaphilic sexual addiction—included in DSM-IV-TR as sexual disorder not otherwise specified—describes culturally acceptable sexual interests and behaviors that are frequent or intense enough to reduce capacity for sexual intimacy. Such behaviors include compulsive masturbation, repetitive promiscuity, and dependence on anonymous sexual encounters.
An addiction model conceptualizes paraphilia as a form of pleasure seeking that has become habitual and self-destructive. Treatment involves directing patients to 12-step groups patterned after Alcoholics Anonymous.
Other models place paraphilias and related disorders within the OCD spectrum.8-13 Persons with OCD often are obsessed with sexual content and might grapple with religious and moral concerns about sexual issues. They typically consider their symptoms intrusive or senseless. Selective serotonin reuptake inhibitors—the standard medication for OCD—might alleviate paraphilia, but results are mixed.14
Mr. C’s symptoms. Mr. C shows features of GID and transvestism. His strong, persistent cross-gender identification and sense of inappropriateness with being a man indicate GID. His recurrent sexual urges and fantasies and impaired capacity for sexual intimacy suggest a paraphilia or transvestism.
The significance of Mr. C’s comorbid bipolar disorder and OCD is unclear. Both appeared controlled, but the potential for mania-induced hypersexuality cannot be ignored.
Diagnosing gender dysphoria
A thorough medical, psychiatric, and sexual history can reveal sexual identity symptoms’ source.
Consider a medical cause. Your medical workup may include a genital exam to check for irregularities such as hermaphrodism that can compound questions of sexual identity, and karyotyping to probe chromosomal anomalies, such as mosaicism or chimerism.
Consider schizophrenia or bipolar disorder, as mania or psychosis can cause aberrant sexual behavior. In gender-dysphoric patients with either disorder, treating the psychiatric comorbidity might alleviate the dysphoria. Watch for fluctuations in gender dysphoria intensity when you treat other psychopathologies.
Take a thorough sexual history. Being matter-of-fact while discussing unusual sexual acts will help the patient “open up” about his sexual problems. Ask him if he:
- showed gender-atypical behavior as a child, which can predict transsexualism or homosexuality has engaged in heterosexual, homosexual, or abnormal sexual acts; ask about frequency and preference
- is married or has a girlfriend. If so, are they getting along? How often do they have sex?
- cross-dresses. Does his partner cross-dress as well and, if so, do they cross-dress for sexual gratification or to identify with the opposite gender? Has this response changed over time? Where and how often do they cross-dress?
- is achieving sexual gratification. If so, how?
- has sexual fantasies involving breast-feeding, giving birth, or forced feminization through gender-changing surgeries or other means
- “tucks” his penis, urinates sitting down, or mimics other stereotypical feminine behavior.
The answers will uncover a motivation behind these behaviors, which is key to diagnosis. Sexual gratification as a motive suggests paraphilia, whereas a desire to live as a woman points to transsexualism. Because of the myriad presentations, multiple patient visits are necessary for a specific diagnosis.
Diagnosis: ‘i enjoy womanhood, but…’
I diagnose gender dysphoria, but because Mr. C’s mood is euthymic, I cannot discern how his mood instability might affect his dysphoria. His sexual fantasies are mood-congruent and evoke no shame.
Mr. C then states that he adamantly opposes living outwardly as a woman, and fears that an overt sex change would destroy his marriage and other relationships. Even so, he desires hormone therapy and surgical breast implants so he can more closely mimic physical womanhood and make masturbation more pleasurable. He says he would flatten his breasts with gauze while in public so he can continue to look like a man.
Though comfortable with his sexual fantasies, Mr. C laments that presenting himself as an “alpha male” drains his psychic energy.
The author’s observations
Mr. C meets criteria for GID and transvestism. Some transvestites also meet criteria for autogynephilia and report erotic arousal upon seeing oneself as a woman. Character pathology, specifically sexual fantasies associated with schizoid personality, might also contribute to unusual gender presentation. Sexologists also propose fluidity in gender identification across populations and over a person’s life span.
Autogynephilia—by which a man becomes sexually aroused by imagining or seeing himself as a woman15—usually is associated with transvestism. Autogynephiles often have sexual fantasies of possessing female anatomical structures, engaging in feminine behaviors, or performing female bodily functions such as lactation, menstruation, or childbirth.
Autogynephilia may be a misdirected heterosexuality and is more prevalent among male-to-female transsexuals who are attracted to women, both sexes, or neither sex than among those attracted only to men.16
Gender identity fluidity. Clinicians have recorded fluidity in gender identity (sense of masculinity or femininity) and sexual orientation (the sex to which one is attracted). Sexologists have tried to create scales that gauge these changes.
The Kinsey Heterosexual-Homosexual Rating Scale17 is based on Kinsey’s theory that men are not strictly heterosexual or homosexual. Scores between 0 and 6 indicate some degree of both (Table).
The Benjamin Gender Disorientation Scale, which measures gender identity variations, recognizes variability of gender dysphoria expression and underscores the difficulty of classifying patients who—like Mr. C—present with varied symptoms. The scale is available at www.wpath.org.
I did not administer the Kinsey or Benjamin scales to Mr. C. Although his case shows how innate sense of masculinity or femininity can vary among patients with gender dysphoria, his presentation has been stable, albeit unusual.
Mr. C’s symptoms. Mr. C shows autogynephilic features. He lacks the schizoid’s emotional inertness and his gender presentation is static, though dramatic. He appears to meet criteria for GID and transvestism, autogynephilic variant.
Schizoid and other personality disorders are associated with unusual sexual fantasies. Mr. C lacks primary schizoid features, such as flattened affectivity and indifference to close relationships.
Table
Kinsey Heterosexual-Homosexual Rating Scale
| Score | Indicates… |
|---|---|
| 0 | Exclusively heterosexual |
| 1 | Predominantly heterosexual, incidentally homosexual |
| 2 | Predominantly heterosexual, more than incidentally homosexual |
| 3 | Equally heterosexual and homosexual |
| 4 | Predominantly homosexual, more than incidentally heterosexual |
| 5 | Predominantly homosexual, incidentally heterosexual |
| 6 | Exclusively homosexual |
| Source: Reference 17. Reprinted by permission of The Kinsey Institute for Research in Sex, Gender, and Reproduction, Inc., Indiana University, Bloomington. | |
The author’s observations
Mr. C is a poor candidate for hormone therapy or gender reassignment surgery because of his circumscribed desire to live as a woman at home. Also, sexual gratification is his primary motivation for wanting to develop breasts.
Treating gender dysphoria
Serotonergic agents such as fluoxetine have shown effectiveness for treating paraphilias and nonparaphilic sexual addiction in case reports.18,19
Behavioral techniques, however, might have a more definite impact on gender dysphoria. Marks19 reported a 4-year remission of transsexualism in a patient after comorbid OCD improved with self-exposure therapy.
Psychotherapy will not resolve gender identity disorder but can promote a stable lifestyle and improve the patient’s chances for success in relationships, education, work, and gender identity expression.20 Psychotherapy can also help determine patients’ readiness for sexual reassignment surgery.
Treatment: learning to accept
I refer Mr. C back to his primary psychiatrist, who adds aripiprazole, 5 mg/d, to address grandiosity and hypomania that emerged months after my initial evaluation.
I also refer Mr. C to a gender disorder specialist for psychotherapy directed at examining his history, understanding his dilemmas, and identifying unrealistic ideas and maladaptive behaviors. The therapist has been teaching Mr. C coping skills and educating him on gender disorders and normal gender variations in activities and interests. He has been attending weekly sessions for 4 months.
To address his resentment over trying to look manly, I assure him that he doesn’t need to assume additional “masculine” behaviors and attitudes, and that his height and features make him appear masculine.
- World Professional Association for Transgender Health, formerly the Harry Benjamin International Gender Dysphoria Association (offers links to transgender resources, gender programs, and sexologists). www.hbigda.org.
- Divalproex sodium • Depakote
- Fluoxetine • Prozac
- Paroxetine • Paxil
Dr. Martin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mental and behavioral disorders, diagnostic criteria for research. In: The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva: World Health Organization; 1993.
2. Caldwell C, Keshavan MS. Schizophrenia with secondary transsexualism. Can J Psychiatry 1991;36:300-1.
3. Hyde C, Kenna JC. A male MZ twin pair, concordant for transsexualism, discordant for schizophrenia. Acta Psychiatr Scand 1977;56:265-75.
4. Habermeyer E, Kamps I, Kawohl W. A case of bipolar psychosis and transsexualism. Psychopathology 2003;36:168-70.
5. O’Gorman EC. The effect of psychosis on gender identity. Br J Psychiatry 1980;136:314-5.
6. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000:566-76.
7. Hoenig J, Kenna JC. The nosolgical position of transsexualism. Arch Sex Behav 1974;3:273-87.
8. Jenike MA. Obsessive-compulsive and related disorders: a hidden epidemic. N Engl J Med 1989;24;321:539-41.
9. Stein DJ, Hollander E. The spectrum of obsessive-compulsive related disorders. In: Hollander E, ed. The obsessive-compulsive related disorders. Washington, DC: American Psychiatric Publishing. In press.
10. Hollander E. Serotonergic drugs and the treatment of disorders related to obsessive-compulsive disorder. In: Pato MT, Zohar J, eds. Current treatments of obsessive-compulsive disorder. Washington, DC: American Psychiatric Publishing; 1991:173-92.
11. Quadland MC. Compulsive sexual behavior: definition of a problem and an approach to treatment. J Sex Marital Ther 1985;11:121-32.
12. Coleman E. Sexual compulsivity: definition, etiology, and treatment considerations. In: Coleman E, ed. Chemical dependency and intimacy dysfunction. New York: Haworth Press; 1988.
13. Anthony DT, Hollander E. Sexual compulsions. In: Hollander E, ed. The obsessive-compulsive related disorders. Washington, DC: American Psychiatric Publishing. In press.
14. Perilstein RD, Lipper S, Friedman LJ. Three cases of paraphilias responsive to fluoxetine treatment. J Clin Psychiatry 1991;52:169-70.
15. Blanchard R. Nonmonotonic relation of autogynephilia and heterosexual attraction. J Abnorm Psychol 1992;101:271-6.
16. Hirschfeld M. Sexual anomalies. New York: Emerson Books; 1948.
17. Kinsey AC, Pomeroy WB, Martin CE. Sexual behavior in the human male. Philadelphia: WB Saunders; 1948;636-59.
18. Kafka MP. Successful antidepressant treatment of nonparaphilic sexual addictions and paraphilias in men. J Clin Psychiatry 1991;52:60-5.
19. Marks IM, Mataix-Cols D. Four-year remission of transsexualism after comorbid obsessive-compulsive disorder improved with self-exposure therapy. Case report. Br J Psychiatry 1997;171:389-90.
20. Harry Benjamin International Gender Dysphoria Association’s Standards of Care for Gender Identity Disorders. Int J Transgenderism 2001; 5(1). Available at: http://www.symposion.com/ijt/index.htm. Accessed November 3, 2006.
History: normal on paper
Mr. C, age 65, presents to an endocrinologist complaining of hot flashes and low libido. Initial testing shows low male testosterone, but a repeat test shows normal levels. No medical cause is found for his symptoms.
“My testosterone might be normal on paper,” Mr. C tells the endocrinologist, “but I’m not. I think I’m a woman.”
Mr. C requests referral to a female psychiatrist because he feels more comfortable discussing sexual issues with a woman. The endocrinologist refers him to me for evaluation.
Over 7 years, Mr. C’s other psychiatrist—a man—has been treating him for obsessive-compulsive disorder (OCD), anxiety disorder, and bipolar disorder type II. Mr. C takes paroxetine, 60 mg/d, for depressive symptoms and was taking divalproex, 1,500 mg/d, to stabilize his mood. He recently stopped divalproex because it was causing nausea and sedation.
During our initial visit, Mr. C says he’s “through pretending to be a man.” He says he first questioned his sexual identity in early childhood, when he sometimes dressed in his mother’s clothes for play. As an adult, he mostly cross-dresses in lingerie; he wears a woman’s tank top in public once or twice weekly underneath his polo dress shirt. Fifteen years ago, he suffered anorexia and bulimia while trying to look as svelte as a woman.
At 6 feet, 2 inches with good muscle tone and short, wavy black hair, Mr. C looks strikingly masculine. Now retired, he served in the Air Force and later worked as a commercial pilot and in construction. In private, however, he prefers gardening and cooking over sports and cars.
Mr. C is married but seldom has sexual intercourse with women. He gains sexual fulfillment by visualizing himself as a woman having sex with other women or with himself as a man. He denies interest in male-male sex.
The patient has been masturbating since age 5, mostly by rubbing his scrotum against a swing set pole he still keeps in his utility shed. He often tucks his penis to mimic female genitalia and makes believe his rectum is a vagina.
- Transsexualism
- Pure transvestism (having a firm gender identity but becoming sexually aroused by cross-dressing)
- Dual-role transvestism (cross-dressing solely to experience temporary membership in the opposite sex)
- Stress-related cross-dressing
- Men who desire penectomy or castration but no other gender-reassignment interventions
- Congenital intersex conditions, such as hermaphrodism
Mr. C’s Mini-Mental State Examination score of 30 indicates no underlying dementia. He shows stable affect with no evidence of derailment, paranoia, thought blocking, or auditory hallucinations.
Medical examination results are normal. Negative urine toxicology screen rules out substance abuse, and negative rapid plasma reagin rules out syphilis. Testosterone is not rechecked because levels were normal 2 days before.
The author’s observations
I suspect gender dysphoria, which describes a heterogeneous group of persons who express varying degrees of distress with their anatomic sex and sometimes desire secondary opposite-gender sexual characteristics (Box).
Sexual identity in gender dysphoria is often fluid. Symptoms might suggest transvestism, then evolve to transsexualism. Recognizing this heterogeneity and fluidity is crucial to diagnosis and treatment.
Primary transsexualism. The term “transsexualism” describes persons who want to live and be accepted within the opposite sex.1 The transsexual identity persists for ≥ 2 years and is not caused by another mental disorder or intersexed condition. Fetishism is classically absent and cross-dressing is not sexually gratifying. Most transsexuals want surgical and hormone treatment to make their bodies as congruent as possible with the preferred sex.
In 1994, DSM-IV recognized that some late-onset transsexuals showed features of comorbid transvestism and were sexually aroused by female dress and behaviors. Gender identity disorder (GID) replaced the term “transsexualism” and includes these individuals. A secondary diagnosis of transvestism is applied.
Secondary transsexualism. Case reports2 describe psychosis-induced transsexual desires in patients with schizophrenia. Gender dysphoria improved as their schizophrenia symptoms lessened.
The relationship between transsexualism and schizophrenia has been debated. Hyde and Kenna3 view transsexualism as a schizophrenia spectrum disorder, whereas sexologists consider transsexualism and schizophrenia distinct syndromes that can occur simultaneously.
Affective disorders might also alter contentment with gender role, but the relationship is unclear. Case reports of patients with bipolar disorder suggest that gender dysphoria intensity fluctuates with affective excursions.4 O’Gorman,5 however, described a bipolar patient whose gender dysphoria was mitigated during manic episodes.
Paraphilias are sexual disorders with recurrent intense urges and fantasies that do not follow normative arousal patterns and can diminish capacity for sexual intimacy.6 Manifestations include exhibitionism, fetishism, frotteurism, pedophilia, masochism/sadism, voyeurism, and transvestic fetishism.
Dividing transsexualism and pure transvestism paraphilia into discrete categories is simplistic, as transvestites can develop secondary components of transsexualism. Hoenig and Kenna7 assert that transsexualism—though not an anomalous erotic preference—is almost always preceded by transvestism or accompanied by cross-gender fetishism.
Nonparaphilic sexual addiction—included in DSM-IV-TR as sexual disorder not otherwise specified—describes culturally acceptable sexual interests and behaviors that are frequent or intense enough to reduce capacity for sexual intimacy. Such behaviors include compulsive masturbation, repetitive promiscuity, and dependence on anonymous sexual encounters.
An addiction model conceptualizes paraphilia as a form of pleasure seeking that has become habitual and self-destructive. Treatment involves directing patients to 12-step groups patterned after Alcoholics Anonymous.
Other models place paraphilias and related disorders within the OCD spectrum.8-13 Persons with OCD often are obsessed with sexual content and might grapple with religious and moral concerns about sexual issues. They typically consider their symptoms intrusive or senseless. Selective serotonin reuptake inhibitors—the standard medication for OCD—might alleviate paraphilia, but results are mixed.14
Mr. C’s symptoms. Mr. C shows features of GID and transvestism. His strong, persistent cross-gender identification and sense of inappropriateness with being a man indicate GID. His recurrent sexual urges and fantasies and impaired capacity for sexual intimacy suggest a paraphilia or transvestism.
The significance of Mr. C’s comorbid bipolar disorder and OCD is unclear. Both appeared controlled, but the potential for mania-induced hypersexuality cannot be ignored.
Diagnosing gender dysphoria
A thorough medical, psychiatric, and sexual history can reveal sexual identity symptoms’ source.
Consider a medical cause. Your medical workup may include a genital exam to check for irregularities such as hermaphrodism that can compound questions of sexual identity, and karyotyping to probe chromosomal anomalies, such as mosaicism or chimerism.
Consider schizophrenia or bipolar disorder, as mania or psychosis can cause aberrant sexual behavior. In gender-dysphoric patients with either disorder, treating the psychiatric comorbidity might alleviate the dysphoria. Watch for fluctuations in gender dysphoria intensity when you treat other psychopathologies.
Take a thorough sexual history. Being matter-of-fact while discussing unusual sexual acts will help the patient “open up” about his sexual problems. Ask him if he:
- showed gender-atypical behavior as a child, which can predict transsexualism or homosexuality has engaged in heterosexual, homosexual, or abnormal sexual acts; ask about frequency and preference
- is married or has a girlfriend. If so, are they getting along? How often do they have sex?
- cross-dresses. Does his partner cross-dress as well and, if so, do they cross-dress for sexual gratification or to identify with the opposite gender? Has this response changed over time? Where and how often do they cross-dress?
- is achieving sexual gratification. If so, how?
- has sexual fantasies involving breast-feeding, giving birth, or forced feminization through gender-changing surgeries or other means
- “tucks” his penis, urinates sitting down, or mimics other stereotypical feminine behavior.
The answers will uncover a motivation behind these behaviors, which is key to diagnosis. Sexual gratification as a motive suggests paraphilia, whereas a desire to live as a woman points to transsexualism. Because of the myriad presentations, multiple patient visits are necessary for a specific diagnosis.
Diagnosis: ‘i enjoy womanhood, but…’
I diagnose gender dysphoria, but because Mr. C’s mood is euthymic, I cannot discern how his mood instability might affect his dysphoria. His sexual fantasies are mood-congruent and evoke no shame.
Mr. C then states that he adamantly opposes living outwardly as a woman, and fears that an overt sex change would destroy his marriage and other relationships. Even so, he desires hormone therapy and surgical breast implants so he can more closely mimic physical womanhood and make masturbation more pleasurable. He says he would flatten his breasts with gauze while in public so he can continue to look like a man.
Though comfortable with his sexual fantasies, Mr. C laments that presenting himself as an “alpha male” drains his psychic energy.
The author’s observations
Mr. C meets criteria for GID and transvestism. Some transvestites also meet criteria for autogynephilia and report erotic arousal upon seeing oneself as a woman. Character pathology, specifically sexual fantasies associated with schizoid personality, might also contribute to unusual gender presentation. Sexologists also propose fluidity in gender identification across populations and over a person’s life span.
Autogynephilia—by which a man becomes sexually aroused by imagining or seeing himself as a woman15—usually is associated with transvestism. Autogynephiles often have sexual fantasies of possessing female anatomical structures, engaging in feminine behaviors, or performing female bodily functions such as lactation, menstruation, or childbirth.
Autogynephilia may be a misdirected heterosexuality and is more prevalent among male-to-female transsexuals who are attracted to women, both sexes, or neither sex than among those attracted only to men.16
Gender identity fluidity. Clinicians have recorded fluidity in gender identity (sense of masculinity or femininity) and sexual orientation (the sex to which one is attracted). Sexologists have tried to create scales that gauge these changes.
The Kinsey Heterosexual-Homosexual Rating Scale17 is based on Kinsey’s theory that men are not strictly heterosexual or homosexual. Scores between 0 and 6 indicate some degree of both (Table).
The Benjamin Gender Disorientation Scale, which measures gender identity variations, recognizes variability of gender dysphoria expression and underscores the difficulty of classifying patients who—like Mr. C—present with varied symptoms. The scale is available at www.wpath.org.
I did not administer the Kinsey or Benjamin scales to Mr. C. Although his case shows how innate sense of masculinity or femininity can vary among patients with gender dysphoria, his presentation has been stable, albeit unusual.
Mr. C’s symptoms. Mr. C shows autogynephilic features. He lacks the schizoid’s emotional inertness and his gender presentation is static, though dramatic. He appears to meet criteria for GID and transvestism, autogynephilic variant.
Schizoid and other personality disorders are associated with unusual sexual fantasies. Mr. C lacks primary schizoid features, such as flattened affectivity and indifference to close relationships.
Table
Kinsey Heterosexual-Homosexual Rating Scale
| Score | Indicates… |
|---|---|
| 0 | Exclusively heterosexual |
| 1 | Predominantly heterosexual, incidentally homosexual |
| 2 | Predominantly heterosexual, more than incidentally homosexual |
| 3 | Equally heterosexual and homosexual |
| 4 | Predominantly homosexual, more than incidentally heterosexual |
| 5 | Predominantly homosexual, incidentally heterosexual |
| 6 | Exclusively homosexual |
| Source: Reference 17. Reprinted by permission of The Kinsey Institute for Research in Sex, Gender, and Reproduction, Inc., Indiana University, Bloomington. | |
The author’s observations
Mr. C is a poor candidate for hormone therapy or gender reassignment surgery because of his circumscribed desire to live as a woman at home. Also, sexual gratification is his primary motivation for wanting to develop breasts.
Treating gender dysphoria
Serotonergic agents such as fluoxetine have shown effectiveness for treating paraphilias and nonparaphilic sexual addiction in case reports.18,19
Behavioral techniques, however, might have a more definite impact on gender dysphoria. Marks19 reported a 4-year remission of transsexualism in a patient after comorbid OCD improved with self-exposure therapy.
Psychotherapy will not resolve gender identity disorder but can promote a stable lifestyle and improve the patient’s chances for success in relationships, education, work, and gender identity expression.20 Psychotherapy can also help determine patients’ readiness for sexual reassignment surgery.
Treatment: learning to accept
I refer Mr. C back to his primary psychiatrist, who adds aripiprazole, 5 mg/d, to address grandiosity and hypomania that emerged months after my initial evaluation.
I also refer Mr. C to a gender disorder specialist for psychotherapy directed at examining his history, understanding his dilemmas, and identifying unrealistic ideas and maladaptive behaviors. The therapist has been teaching Mr. C coping skills and educating him on gender disorders and normal gender variations in activities and interests. He has been attending weekly sessions for 4 months.
To address his resentment over trying to look manly, I assure him that he doesn’t need to assume additional “masculine” behaviors and attitudes, and that his height and features make him appear masculine.
- World Professional Association for Transgender Health, formerly the Harry Benjamin International Gender Dysphoria Association (offers links to transgender resources, gender programs, and sexologists). www.hbigda.org.
- Divalproex sodium • Depakote
- Fluoxetine • Prozac
- Paroxetine • Paxil
Dr. Martin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
History: normal on paper
Mr. C, age 65, presents to an endocrinologist complaining of hot flashes and low libido. Initial testing shows low male testosterone, but a repeat test shows normal levels. No medical cause is found for his symptoms.
“My testosterone might be normal on paper,” Mr. C tells the endocrinologist, “but I’m not. I think I’m a woman.”
Mr. C requests referral to a female psychiatrist because he feels more comfortable discussing sexual issues with a woman. The endocrinologist refers him to me for evaluation.
Over 7 years, Mr. C’s other psychiatrist—a man—has been treating him for obsessive-compulsive disorder (OCD), anxiety disorder, and bipolar disorder type II. Mr. C takes paroxetine, 60 mg/d, for depressive symptoms and was taking divalproex, 1,500 mg/d, to stabilize his mood. He recently stopped divalproex because it was causing nausea and sedation.
During our initial visit, Mr. C says he’s “through pretending to be a man.” He says he first questioned his sexual identity in early childhood, when he sometimes dressed in his mother’s clothes for play. As an adult, he mostly cross-dresses in lingerie; he wears a woman’s tank top in public once or twice weekly underneath his polo dress shirt. Fifteen years ago, he suffered anorexia and bulimia while trying to look as svelte as a woman.
At 6 feet, 2 inches with good muscle tone and short, wavy black hair, Mr. C looks strikingly masculine. Now retired, he served in the Air Force and later worked as a commercial pilot and in construction. In private, however, he prefers gardening and cooking over sports and cars.
Mr. C is married but seldom has sexual intercourse with women. He gains sexual fulfillment by visualizing himself as a woman having sex with other women or with himself as a man. He denies interest in male-male sex.
The patient has been masturbating since age 5, mostly by rubbing his scrotum against a swing set pole he still keeps in his utility shed. He often tucks his penis to mimic female genitalia and makes believe his rectum is a vagina.
- Transsexualism
- Pure transvestism (having a firm gender identity but becoming sexually aroused by cross-dressing)
- Dual-role transvestism (cross-dressing solely to experience temporary membership in the opposite sex)
- Stress-related cross-dressing
- Men who desire penectomy or castration but no other gender-reassignment interventions
- Congenital intersex conditions, such as hermaphrodism
Mr. C’s Mini-Mental State Examination score of 30 indicates no underlying dementia. He shows stable affect with no evidence of derailment, paranoia, thought blocking, or auditory hallucinations.
Medical examination results are normal. Negative urine toxicology screen rules out substance abuse, and negative rapid plasma reagin rules out syphilis. Testosterone is not rechecked because levels were normal 2 days before.
The author’s observations
I suspect gender dysphoria, which describes a heterogeneous group of persons who express varying degrees of distress with their anatomic sex and sometimes desire secondary opposite-gender sexual characteristics (Box).
Sexual identity in gender dysphoria is often fluid. Symptoms might suggest transvestism, then evolve to transsexualism. Recognizing this heterogeneity and fluidity is crucial to diagnosis and treatment.
Primary transsexualism. The term “transsexualism” describes persons who want to live and be accepted within the opposite sex.1 The transsexual identity persists for ≥ 2 years and is not caused by another mental disorder or intersexed condition. Fetishism is classically absent and cross-dressing is not sexually gratifying. Most transsexuals want surgical and hormone treatment to make their bodies as congruent as possible with the preferred sex.
In 1994, DSM-IV recognized that some late-onset transsexuals showed features of comorbid transvestism and were sexually aroused by female dress and behaviors. Gender identity disorder (GID) replaced the term “transsexualism” and includes these individuals. A secondary diagnosis of transvestism is applied.
Secondary transsexualism. Case reports2 describe psychosis-induced transsexual desires in patients with schizophrenia. Gender dysphoria improved as their schizophrenia symptoms lessened.
The relationship between transsexualism and schizophrenia has been debated. Hyde and Kenna3 view transsexualism as a schizophrenia spectrum disorder, whereas sexologists consider transsexualism and schizophrenia distinct syndromes that can occur simultaneously.
Affective disorders might also alter contentment with gender role, but the relationship is unclear. Case reports of patients with bipolar disorder suggest that gender dysphoria intensity fluctuates with affective excursions.4 O’Gorman,5 however, described a bipolar patient whose gender dysphoria was mitigated during manic episodes.
Paraphilias are sexual disorders with recurrent intense urges and fantasies that do not follow normative arousal patterns and can diminish capacity for sexual intimacy.6 Manifestations include exhibitionism, fetishism, frotteurism, pedophilia, masochism/sadism, voyeurism, and transvestic fetishism.
Dividing transsexualism and pure transvestism paraphilia into discrete categories is simplistic, as transvestites can develop secondary components of transsexualism. Hoenig and Kenna7 assert that transsexualism—though not an anomalous erotic preference—is almost always preceded by transvestism or accompanied by cross-gender fetishism.
Nonparaphilic sexual addiction—included in DSM-IV-TR as sexual disorder not otherwise specified—describes culturally acceptable sexual interests and behaviors that are frequent or intense enough to reduce capacity for sexual intimacy. Such behaviors include compulsive masturbation, repetitive promiscuity, and dependence on anonymous sexual encounters.
An addiction model conceptualizes paraphilia as a form of pleasure seeking that has become habitual and self-destructive. Treatment involves directing patients to 12-step groups patterned after Alcoholics Anonymous.
Other models place paraphilias and related disorders within the OCD spectrum.8-13 Persons with OCD often are obsessed with sexual content and might grapple with religious and moral concerns about sexual issues. They typically consider their symptoms intrusive or senseless. Selective serotonin reuptake inhibitors—the standard medication for OCD—might alleviate paraphilia, but results are mixed.14
Mr. C’s symptoms. Mr. C shows features of GID and transvestism. His strong, persistent cross-gender identification and sense of inappropriateness with being a man indicate GID. His recurrent sexual urges and fantasies and impaired capacity for sexual intimacy suggest a paraphilia or transvestism.
The significance of Mr. C’s comorbid bipolar disorder and OCD is unclear. Both appeared controlled, but the potential for mania-induced hypersexuality cannot be ignored.
Diagnosing gender dysphoria
A thorough medical, psychiatric, and sexual history can reveal sexual identity symptoms’ source.
Consider a medical cause. Your medical workup may include a genital exam to check for irregularities such as hermaphrodism that can compound questions of sexual identity, and karyotyping to probe chromosomal anomalies, such as mosaicism or chimerism.
Consider schizophrenia or bipolar disorder, as mania or psychosis can cause aberrant sexual behavior. In gender-dysphoric patients with either disorder, treating the psychiatric comorbidity might alleviate the dysphoria. Watch for fluctuations in gender dysphoria intensity when you treat other psychopathologies.
Take a thorough sexual history. Being matter-of-fact while discussing unusual sexual acts will help the patient “open up” about his sexual problems. Ask him if he:
- showed gender-atypical behavior as a child, which can predict transsexualism or homosexuality has engaged in heterosexual, homosexual, or abnormal sexual acts; ask about frequency and preference
- is married or has a girlfriend. If so, are they getting along? How often do they have sex?
- cross-dresses. Does his partner cross-dress as well and, if so, do they cross-dress for sexual gratification or to identify with the opposite gender? Has this response changed over time? Where and how often do they cross-dress?
- is achieving sexual gratification. If so, how?
- has sexual fantasies involving breast-feeding, giving birth, or forced feminization through gender-changing surgeries or other means
- “tucks” his penis, urinates sitting down, or mimics other stereotypical feminine behavior.
The answers will uncover a motivation behind these behaviors, which is key to diagnosis. Sexual gratification as a motive suggests paraphilia, whereas a desire to live as a woman points to transsexualism. Because of the myriad presentations, multiple patient visits are necessary for a specific diagnosis.
Diagnosis: ‘i enjoy womanhood, but…’
I diagnose gender dysphoria, but because Mr. C’s mood is euthymic, I cannot discern how his mood instability might affect his dysphoria. His sexual fantasies are mood-congruent and evoke no shame.
Mr. C then states that he adamantly opposes living outwardly as a woman, and fears that an overt sex change would destroy his marriage and other relationships. Even so, he desires hormone therapy and surgical breast implants so he can more closely mimic physical womanhood and make masturbation more pleasurable. He says he would flatten his breasts with gauze while in public so he can continue to look like a man.
Though comfortable with his sexual fantasies, Mr. C laments that presenting himself as an “alpha male” drains his psychic energy.
The author’s observations
Mr. C meets criteria for GID and transvestism. Some transvestites also meet criteria for autogynephilia and report erotic arousal upon seeing oneself as a woman. Character pathology, specifically sexual fantasies associated with schizoid personality, might also contribute to unusual gender presentation. Sexologists also propose fluidity in gender identification across populations and over a person’s life span.
Autogynephilia—by which a man becomes sexually aroused by imagining or seeing himself as a woman15—usually is associated with transvestism. Autogynephiles often have sexual fantasies of possessing female anatomical structures, engaging in feminine behaviors, or performing female bodily functions such as lactation, menstruation, or childbirth.
Autogynephilia may be a misdirected heterosexuality and is more prevalent among male-to-female transsexuals who are attracted to women, both sexes, or neither sex than among those attracted only to men.16
Gender identity fluidity. Clinicians have recorded fluidity in gender identity (sense of masculinity or femininity) and sexual orientation (the sex to which one is attracted). Sexologists have tried to create scales that gauge these changes.
The Kinsey Heterosexual-Homosexual Rating Scale17 is based on Kinsey’s theory that men are not strictly heterosexual or homosexual. Scores between 0 and 6 indicate some degree of both (Table).
The Benjamin Gender Disorientation Scale, which measures gender identity variations, recognizes variability of gender dysphoria expression and underscores the difficulty of classifying patients who—like Mr. C—present with varied symptoms. The scale is available at www.wpath.org.
I did not administer the Kinsey or Benjamin scales to Mr. C. Although his case shows how innate sense of masculinity or femininity can vary among patients with gender dysphoria, his presentation has been stable, albeit unusual.
Mr. C’s symptoms. Mr. C shows autogynephilic features. He lacks the schizoid’s emotional inertness and his gender presentation is static, though dramatic. He appears to meet criteria for GID and transvestism, autogynephilic variant.
Schizoid and other personality disorders are associated with unusual sexual fantasies. Mr. C lacks primary schizoid features, such as flattened affectivity and indifference to close relationships.
Table
Kinsey Heterosexual-Homosexual Rating Scale
| Score | Indicates… |
|---|---|
| 0 | Exclusively heterosexual |
| 1 | Predominantly heterosexual, incidentally homosexual |
| 2 | Predominantly heterosexual, more than incidentally homosexual |
| 3 | Equally heterosexual and homosexual |
| 4 | Predominantly homosexual, more than incidentally heterosexual |
| 5 | Predominantly homosexual, incidentally heterosexual |
| 6 | Exclusively homosexual |
| Source: Reference 17. Reprinted by permission of The Kinsey Institute for Research in Sex, Gender, and Reproduction, Inc., Indiana University, Bloomington. | |
The author’s observations
Mr. C is a poor candidate for hormone therapy or gender reassignment surgery because of his circumscribed desire to live as a woman at home. Also, sexual gratification is his primary motivation for wanting to develop breasts.
Treating gender dysphoria
Serotonergic agents such as fluoxetine have shown effectiveness for treating paraphilias and nonparaphilic sexual addiction in case reports.18,19
Behavioral techniques, however, might have a more definite impact on gender dysphoria. Marks19 reported a 4-year remission of transsexualism in a patient after comorbid OCD improved with self-exposure therapy.
Psychotherapy will not resolve gender identity disorder but can promote a stable lifestyle and improve the patient’s chances for success in relationships, education, work, and gender identity expression.20 Psychotherapy can also help determine patients’ readiness for sexual reassignment surgery.
Treatment: learning to accept
I refer Mr. C back to his primary psychiatrist, who adds aripiprazole, 5 mg/d, to address grandiosity and hypomania that emerged months after my initial evaluation.
I also refer Mr. C to a gender disorder specialist for psychotherapy directed at examining his history, understanding his dilemmas, and identifying unrealistic ideas and maladaptive behaviors. The therapist has been teaching Mr. C coping skills and educating him on gender disorders and normal gender variations in activities and interests. He has been attending weekly sessions for 4 months.
To address his resentment over trying to look manly, I assure him that he doesn’t need to assume additional “masculine” behaviors and attitudes, and that his height and features make him appear masculine.
- World Professional Association for Transgender Health, formerly the Harry Benjamin International Gender Dysphoria Association (offers links to transgender resources, gender programs, and sexologists). www.hbigda.org.
- Divalproex sodium • Depakote
- Fluoxetine • Prozac
- Paroxetine • Paxil
Dr. Martin reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mental and behavioral disorders, diagnostic criteria for research. In: The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva: World Health Organization; 1993.
2. Caldwell C, Keshavan MS. Schizophrenia with secondary transsexualism. Can J Psychiatry 1991;36:300-1.
3. Hyde C, Kenna JC. A male MZ twin pair, concordant for transsexualism, discordant for schizophrenia. Acta Psychiatr Scand 1977;56:265-75.
4. Habermeyer E, Kamps I, Kawohl W. A case of bipolar psychosis and transsexualism. Psychopathology 2003;36:168-70.
5. O’Gorman EC. The effect of psychosis on gender identity. Br J Psychiatry 1980;136:314-5.
6. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000:566-76.
7. Hoenig J, Kenna JC. The nosolgical position of transsexualism. Arch Sex Behav 1974;3:273-87.
8. Jenike MA. Obsessive-compulsive and related disorders: a hidden epidemic. N Engl J Med 1989;24;321:539-41.
9. Stein DJ, Hollander E. The spectrum of obsessive-compulsive related disorders. In: Hollander E, ed. The obsessive-compulsive related disorders. Washington, DC: American Psychiatric Publishing. In press.
10. Hollander E. Serotonergic drugs and the treatment of disorders related to obsessive-compulsive disorder. In: Pato MT, Zohar J, eds. Current treatments of obsessive-compulsive disorder. Washington, DC: American Psychiatric Publishing; 1991:173-92.
11. Quadland MC. Compulsive sexual behavior: definition of a problem and an approach to treatment. J Sex Marital Ther 1985;11:121-32.
12. Coleman E. Sexual compulsivity: definition, etiology, and treatment considerations. In: Coleman E, ed. Chemical dependency and intimacy dysfunction. New York: Haworth Press; 1988.
13. Anthony DT, Hollander E. Sexual compulsions. In: Hollander E, ed. The obsessive-compulsive related disorders. Washington, DC: American Psychiatric Publishing. In press.
14. Perilstein RD, Lipper S, Friedman LJ. Three cases of paraphilias responsive to fluoxetine treatment. J Clin Psychiatry 1991;52:169-70.
15. Blanchard R. Nonmonotonic relation of autogynephilia and heterosexual attraction. J Abnorm Psychol 1992;101:271-6.
16. Hirschfeld M. Sexual anomalies. New York: Emerson Books; 1948.
17. Kinsey AC, Pomeroy WB, Martin CE. Sexual behavior in the human male. Philadelphia: WB Saunders; 1948;636-59.
18. Kafka MP. Successful antidepressant treatment of nonparaphilic sexual addictions and paraphilias in men. J Clin Psychiatry 1991;52:60-5.
19. Marks IM, Mataix-Cols D. Four-year remission of transsexualism after comorbid obsessive-compulsive disorder improved with self-exposure therapy. Case report. Br J Psychiatry 1997;171:389-90.
20. Harry Benjamin International Gender Dysphoria Association’s Standards of Care for Gender Identity Disorders. Int J Transgenderism 2001; 5(1). Available at: http://www.symposion.com/ijt/index.htm. Accessed November 3, 2006.
1. Mental and behavioral disorders, diagnostic criteria for research. In: The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research. Geneva: World Health Organization; 1993.
2. Caldwell C, Keshavan MS. Schizophrenia with secondary transsexualism. Can J Psychiatry 1991;36:300-1.
3. Hyde C, Kenna JC. A male MZ twin pair, concordant for transsexualism, discordant for schizophrenia. Acta Psychiatr Scand 1977;56:265-75.
4. Habermeyer E, Kamps I, Kawohl W. A case of bipolar psychosis and transsexualism. Psychopathology 2003;36:168-70.
5. O’Gorman EC. The effect of psychosis on gender identity. Br J Psychiatry 1980;136:314-5.
6. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000:566-76.
7. Hoenig J, Kenna JC. The nosolgical position of transsexualism. Arch Sex Behav 1974;3:273-87.
8. Jenike MA. Obsessive-compulsive and related disorders: a hidden epidemic. N Engl J Med 1989;24;321:539-41.
9. Stein DJ, Hollander E. The spectrum of obsessive-compulsive related disorders. In: Hollander E, ed. The obsessive-compulsive related disorders. Washington, DC: American Psychiatric Publishing. In press.
10. Hollander E. Serotonergic drugs and the treatment of disorders related to obsessive-compulsive disorder. In: Pato MT, Zohar J, eds. Current treatments of obsessive-compulsive disorder. Washington, DC: American Psychiatric Publishing; 1991:173-92.
11. Quadland MC. Compulsive sexual behavior: definition of a problem and an approach to treatment. J Sex Marital Ther 1985;11:121-32.
12. Coleman E. Sexual compulsivity: definition, etiology, and treatment considerations. In: Coleman E, ed. Chemical dependency and intimacy dysfunction. New York: Haworth Press; 1988.
13. Anthony DT, Hollander E. Sexual compulsions. In: Hollander E, ed. The obsessive-compulsive related disorders. Washington, DC: American Psychiatric Publishing. In press.
14. Perilstein RD, Lipper S, Friedman LJ. Three cases of paraphilias responsive to fluoxetine treatment. J Clin Psychiatry 1991;52:169-70.
15. Blanchard R. Nonmonotonic relation of autogynephilia and heterosexual attraction. J Abnorm Psychol 1992;101:271-6.
16. Hirschfeld M. Sexual anomalies. New York: Emerson Books; 1948.
17. Kinsey AC, Pomeroy WB, Martin CE. Sexual behavior in the human male. Philadelphia: WB Saunders; 1948;636-59.
18. Kafka MP. Successful antidepressant treatment of nonparaphilic sexual addictions and paraphilias in men. J Clin Psychiatry 1991;52:60-5.
19. Marks IM, Mataix-Cols D. Four-year remission of transsexualism after comorbid obsessive-compulsive disorder improved with self-exposure therapy. Case report. Br J Psychiatry 1997;171:389-90.
20. Harry Benjamin International Gender Dysphoria Association’s Standards of Care for Gender Identity Disorders. Int J Transgenderism 2001; 5(1). Available at: http://www.symposion.com/ijt/index.htm. Accessed November 3, 2006.