User login
Two Years of Treatment Fails to Resolve Symptoms
ANSWER
The correct choice is iatrogenic (choice “a”)—that is, provider-caused—in this case, the result of prolonged application of a relatively powerful steroid cream. Such use eventuates in what is sometimes termed “steroid addiction.” These patients are often wrongly suspected of being alcoholics (choice “b”) because of their red faces, but there was no reason to suspect that in this case. It is thought that bacteria flourish on steroid-treated skin, which is theorized to worsen rosacea; however, this is not an outright infection (choice “c”). Nor is it the result of using the wrong soap on the face (choice “d”), as irritation from soap would not have been limited to such sharply demarcated areas.
DISCUSSION
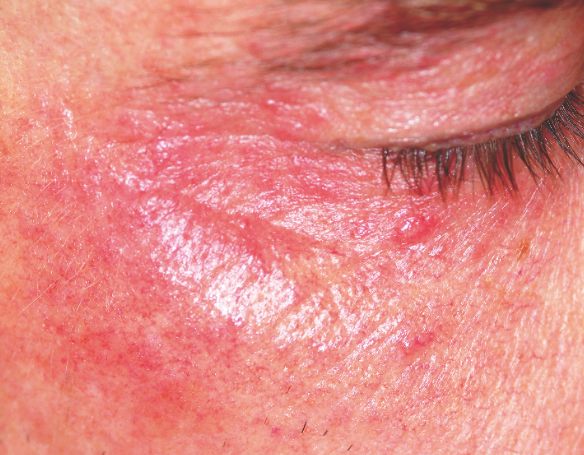
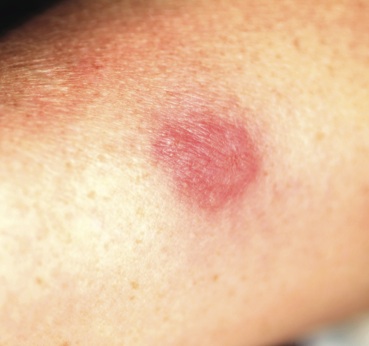
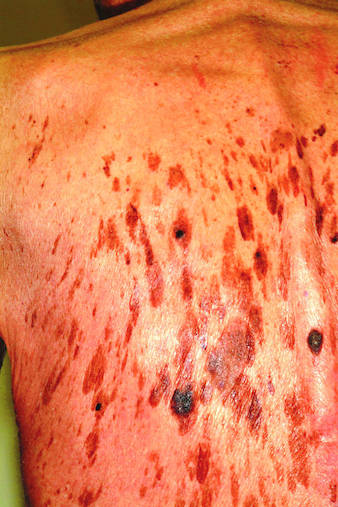
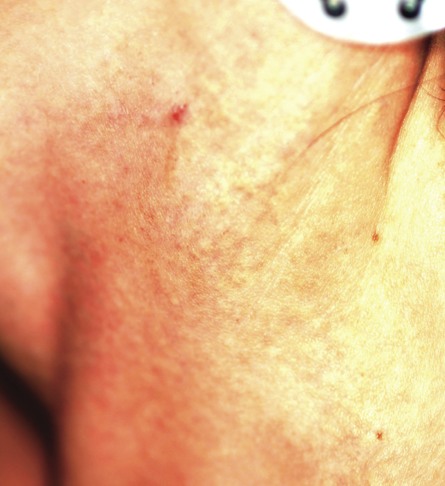
The injudicious application of topical steroids can have a number of negative effects, all demonstrated in this case. It thins the skin, literally uncovering normally hidden vasculature, which manifests as telangiectasia. The epidermis turns atrophic and shiny, and a rosacea-like eruption (so-called “iatrosacea”) often appears.
But the worst adverse effect is the one that forces the patient to keep using the medication long after the treated condition has disappeared: Burning and itching ensue immediately if the application stops or is reduced. This is especially true for atopic patients who are born with thin skin, and even more so for relatively thin facial skin. Eyelids and perioral areas are especially prone to this reaction, and it is often the fault of the prescribing provider who refills the medication over and over, while failing to educate patients about its potentially deleterious effects.
The differential diagnosis for this common condition includes contact versus irritant dermatitis (patients often make rosacea worse by applying numerous OTC products, such as triple-antibiotic ointment), rosacea, granulomatous faciale, and discoid lupus.
TREATMENT
Treatment in such cases begins with cessation of the steroid and a switch either to a steroid preparation of lower potency (such as hydrocortisone 2.5%) or better yet, to a calcineurin inhibitor (such as pimecrolimus or tacrolimus). In addition, oral tetracycline (500 mg bid) may be taken until the condition begins to improve markedly—a process that can take months. The latter treatment should be slowly tapered to one dose per day, then stopped only when the problem has totally cleared. These patients often need frequent return visits initially for reassurance and re-education
ANSWER
The correct choice is iatrogenic (choice “a”)—that is, provider-caused—in this case, the result of prolonged application of a relatively powerful steroid cream. Such use eventuates in what is sometimes termed “steroid addiction.” These patients are often wrongly suspected of being alcoholics (choice “b”) because of their red faces, but there was no reason to suspect that in this case. It is thought that bacteria flourish on steroid-treated skin, which is theorized to worsen rosacea; however, this is not an outright infection (choice “c”). Nor is it the result of using the wrong soap on the face (choice “d”), as irritation from soap would not have been limited to such sharply demarcated areas.
DISCUSSION
The injudicious application of topical steroids can have a number of negative effects, all demonstrated in this case. It thins the skin, literally uncovering normally hidden vasculature, which manifests as telangiectasia. The epidermis turns atrophic and shiny, and a rosacea-like eruption (so-called “iatrosacea”) often appears.
But the worst adverse effect is the one that forces the patient to keep using the medication long after the treated condition has disappeared: Burning and itching ensue immediately if the application stops or is reduced. This is especially true for atopic patients who are born with thin skin, and even more so for relatively thin facial skin. Eyelids and perioral areas are especially prone to this reaction, and it is often the fault of the prescribing provider who refills the medication over and over, while failing to educate patients about its potentially deleterious effects.
The differential diagnosis for this common condition includes contact versus irritant dermatitis (patients often make rosacea worse by applying numerous OTC products, such as triple-antibiotic ointment), rosacea, granulomatous faciale, and discoid lupus.
TREATMENT
Treatment in such cases begins with cessation of the steroid and a switch either to a steroid preparation of lower potency (such as hydrocortisone 2.5%) or better yet, to a calcineurin inhibitor (such as pimecrolimus or tacrolimus). In addition, oral tetracycline (500 mg bid) may be taken until the condition begins to improve markedly—a process that can take months. The latter treatment should be slowly tapered to one dose per day, then stopped only when the problem has totally cleared. These patients often need frequent return visits initially for reassurance and re-education
ANSWER
The correct choice is iatrogenic (choice “a”)—that is, provider-caused—in this case, the result of prolonged application of a relatively powerful steroid cream. Such use eventuates in what is sometimes termed “steroid addiction.” These patients are often wrongly suspected of being alcoholics (choice “b”) because of their red faces, but there was no reason to suspect that in this case. It is thought that bacteria flourish on steroid-treated skin, which is theorized to worsen rosacea; however, this is not an outright infection (choice “c”). Nor is it the result of using the wrong soap on the face (choice “d”), as irritation from soap would not have been limited to such sharply demarcated areas.
DISCUSSION
The injudicious application of topical steroids can have a number of negative effects, all demonstrated in this case. It thins the skin, literally uncovering normally hidden vasculature, which manifests as telangiectasia. The epidermis turns atrophic and shiny, and a rosacea-like eruption (so-called “iatrosacea”) often appears.
But the worst adverse effect is the one that forces the patient to keep using the medication long after the treated condition has disappeared: Burning and itching ensue immediately if the application stops or is reduced. This is especially true for atopic patients who are born with thin skin, and even more so for relatively thin facial skin. Eyelids and perioral areas are especially prone to this reaction, and it is often the fault of the prescribing provider who refills the medication over and over, while failing to educate patients about its potentially deleterious effects.
The differential diagnosis for this common condition includes contact versus irritant dermatitis (patients often make rosacea worse by applying numerous OTC products, such as triple-antibiotic ointment), rosacea, granulomatous faciale, and discoid lupus.
TREATMENT
Treatment in such cases begins with cessation of the steroid and a switch either to a steroid preparation of lower potency (such as hydrocortisone 2.5%) or better yet, to a calcineurin inhibitor (such as pimecrolimus or tacrolimus). In addition, oral tetracycline (500 mg bid) may be taken until the condition begins to improve markedly—a process that can take months. The latter treatment should be slowly tapered to one dose per day, then stopped only when the problem has totally cleared. These patients often need frequent return visits initially for reassurance and re-education
Two years ago, this 45-year-old man developed a faint rash on the bilateral malar areas of his cheeks. His primary care provider diagnosed eczema and prescribed triamcinolone 0.1% cream. The patient has applied this cream to the affected areas twice a day for the entire two-year period. Whenever he tries to stop using the medication, the areas begin to itch and burn, invariably causing him to resume use. Frustrated, the patient seeks and obtains a referral to dermatology. Further history taking reveals that the patient has a number of health problems, including type 2 diabetes, mild renal failure, and a lifelong history of atopic dermatitis. He denies excessive alcohol intake and is employed as administrator of information technology for a hospital. On examination, the patient’s cheeks are both bright red, thin-skinned, and shiny, with many fine telangiectasias covering both malar prominences. Scattered sparsely over these areas are discrete red papules ranging in diameter from 1 to 2 mm. The erythema is highly blanchable and slightly warmer than the surrounding uninvolved skin. The rest of the patient’s facial skin is normal in appearance.
Patient Fears She's "Going Bald"
ANSWER
The correct answer is to perform a biopsy (choice “d”). Although the other choices can be part of the workup for scalp conditions (eg, secondary syphilis, lupus, or fungal infection), the most logical step is to assess the basic histopathologic process. This would provide the clearest direction by revealing characteristic changes in the tissues. Starting an oral antibiotic, such as minocycline, might be a reasonable step if biopsy were not possible.
DISCUSSION
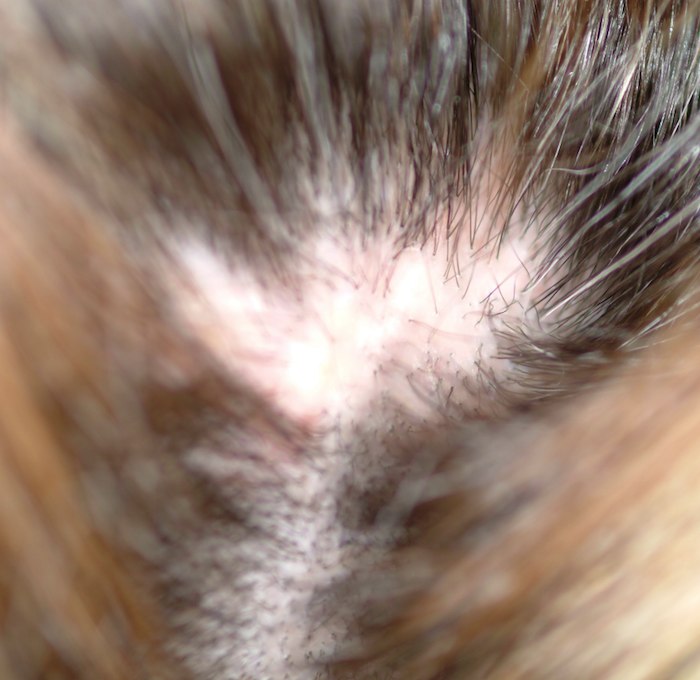
This is a typical case of “scarring alopecia,” a category of hair loss with a wide-ranging differential diagnosis. Included in it would be lymphoma-associated perifollicular mucinosis, discoid lupus, lichen planopilaris (lichen planus affecting the scalp), tinea capitis, secondary syphilis, and pseudopelade. All potentially lead to permanent hair loss; therefore, direct and timely diagnostic information that only a biopsy can provide is essential.
Fungal origin was unlikely for several reasons. Such a diagnosis would be distinctly unusual in a Caucasian woman of this age, particularly since no source (child, spouse, or pet) was identified. The lack of epidermal changes (scale or other broken skin), edema, or adenopathy was a key factor in that regard as well.
In this and most such cases, a 4-mm punch biopsy is taken from the (presumably) active margin of the radially expanding pathologic process; biopsy of the centers would likely only show scar. The biopsy should include at least a few hair shafts, with care taken to penetrate the skin at the same angle from which the hairs emerge. This facilitates collection of the length of the shaft, which could provide valuable diagnostic information.
Done under local anesthesia with lidocaine/epinephrine, the defect is almost always closed with surface sutures, for hemostasis and to speed healing. If one felt strongly about the possibility of infection, an additional sample could be taken and submitted for bacterial and fungal cultures; in this case, neither was suspected.
The results in this case proved the diagnosis of pseudopelade, an inflammatory condition of unknown origin, possibly representing the end-point of either discoid lupus or lichen planopilaris. In any case, the other potential causes of scarring alopecia were ruled out, and appropriate treatment (perilesional injection of 5 mg per cc triamcinolone suspension, oral minocycline 100 mg bid, and topical betamethasone foam) were instituted and follow-up arranged.
ANSWER
The correct answer is to perform a biopsy (choice “d”). Although the other choices can be part of the workup for scalp conditions (eg, secondary syphilis, lupus, or fungal infection), the most logical step is to assess the basic histopathologic process. This would provide the clearest direction by revealing characteristic changes in the tissues. Starting an oral antibiotic, such as minocycline, might be a reasonable step if biopsy were not possible.
DISCUSSION
This is a typical case of “scarring alopecia,” a category of hair loss with a wide-ranging differential diagnosis. Included in it would be lymphoma-associated perifollicular mucinosis, discoid lupus, lichen planopilaris (lichen planus affecting the scalp), tinea capitis, secondary syphilis, and pseudopelade. All potentially lead to permanent hair loss; therefore, direct and timely diagnostic information that only a biopsy can provide is essential.
Fungal origin was unlikely for several reasons. Such a diagnosis would be distinctly unusual in a Caucasian woman of this age, particularly since no source (child, spouse, or pet) was identified. The lack of epidermal changes (scale or other broken skin), edema, or adenopathy was a key factor in that regard as well.
In this and most such cases, a 4-mm punch biopsy is taken from the (presumably) active margin of the radially expanding pathologic process; biopsy of the centers would likely only show scar. The biopsy should include at least a few hair shafts, with care taken to penetrate the skin at the same angle from which the hairs emerge. This facilitates collection of the length of the shaft, which could provide valuable diagnostic information.
Done under local anesthesia with lidocaine/epinephrine, the defect is almost always closed with surface sutures, for hemostasis and to speed healing. If one felt strongly about the possibility of infection, an additional sample could be taken and submitted for bacterial and fungal cultures; in this case, neither was suspected.
The results in this case proved the diagnosis of pseudopelade, an inflammatory condition of unknown origin, possibly representing the end-point of either discoid lupus or lichen planopilaris. In any case, the other potential causes of scarring alopecia were ruled out, and appropriate treatment (perilesional injection of 5 mg per cc triamcinolone suspension, oral minocycline 100 mg bid, and topical betamethasone foam) were instituted and follow-up arranged.
ANSWER
The correct answer is to perform a biopsy (choice “d”). Although the other choices can be part of the workup for scalp conditions (eg, secondary syphilis, lupus, or fungal infection), the most logical step is to assess the basic histopathologic process. This would provide the clearest direction by revealing characteristic changes in the tissues. Starting an oral antibiotic, such as minocycline, might be a reasonable step if biopsy were not possible.
DISCUSSION
This is a typical case of “scarring alopecia,” a category of hair loss with a wide-ranging differential diagnosis. Included in it would be lymphoma-associated perifollicular mucinosis, discoid lupus, lichen planopilaris (lichen planus affecting the scalp), tinea capitis, secondary syphilis, and pseudopelade. All potentially lead to permanent hair loss; therefore, direct and timely diagnostic information that only a biopsy can provide is essential.
Fungal origin was unlikely for several reasons. Such a diagnosis would be distinctly unusual in a Caucasian woman of this age, particularly since no source (child, spouse, or pet) was identified. The lack of epidermal changes (scale or other broken skin), edema, or adenopathy was a key factor in that regard as well.
In this and most such cases, a 4-mm punch biopsy is taken from the (presumably) active margin of the radially expanding pathologic process; biopsy of the centers would likely only show scar. The biopsy should include at least a few hair shafts, with care taken to penetrate the skin at the same angle from which the hairs emerge. This facilitates collection of the length of the shaft, which could provide valuable diagnostic information.
Done under local anesthesia with lidocaine/epinephrine, the defect is almost always closed with surface sutures, for hemostasis and to speed healing. If one felt strongly about the possibility of infection, an additional sample could be taken and submitted for bacterial and fungal cultures; in this case, neither was suspected.
The results in this case proved the diagnosis of pseudopelade, an inflammatory condition of unknown origin, possibly representing the end-point of either discoid lupus or lichen planopilaris. In any case, the other potential causes of scarring alopecia were ruled out, and appropriate treatment (perilesional injection of 5 mg per cc triamcinolone suspension, oral minocycline 100 mg bid, and topical betamethasone foam) were instituted and follow-up arranged.
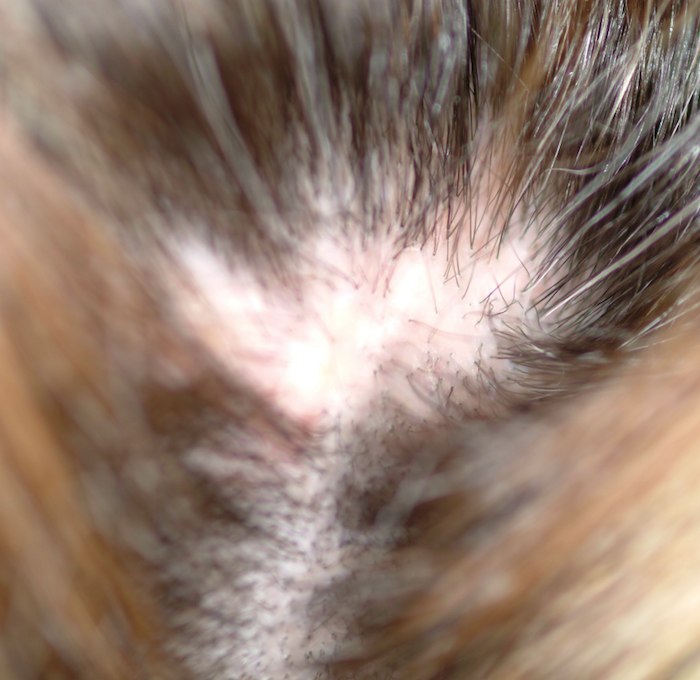
A 48-year-old woman presents to dermatology with a complaint of hair loss. Distraught, she fears she’s “going bald.” This process began several months ago, when she noted a single area of involvement in the frontal scalp. There are now several asymptomatic areas, which have persisted despite the use of several products prescribed by her primary care provider: topical econazole, triamcinolone cream, and most recently, clobetasol foam. None of these has had any beneficial effect; her existing lesions have slowly expanded in size and number. She denies any history of similar problems or of other skin diseases. In general, her health is quite good, with no joint pain, fever, or malaise. Prior to the onset of this condition, she took no prescription or OTC medications. There is no exposure to new pets or other animals, and no one else in her household is affected. She has been married for more than 25 years, in a mutually monogamous relationship. Blood tests (complete blood count, thyroid, chemistry panel, and antinuclear antibody) have been done by her primary care provider. The results were all normal, shedding no light on the condition’s origins. On examination, four distinct areas of hair loss are seen. They range in size from 3 to 6 cm, are roughly fusiform in shape, and are located from frontal to occipital scalp (all in the central quarter of her scalp). In each of these areas, the hair loss is complete, with a scarlike epithelial surface but no other disruption in the skin. Around the periphery of each lesion, there is a faint pinkish tinge. Other than alopecia, no other changes are palpated. There is no tenderness in the lesions and no palpable adenopathy in nodal locations of the head and neck
Patient, Sister Share Skin Problem
ANSWER
The correct answer is pseudoxanthoma elasticum (PXE; choice “d”), details of which are discussed below. Eruptive xanthomata (choice “a”) usually present with widespread eruptive papules and/or nodules and are associated with extreme hypertriglyceridemia. There are several different types of Ehlers-Danlos syndrome, an inherited defect of connective tissues, but the most common involve hypermobile joints (so-called double-jointedness) and/or cutis laxa, neither of which is present in our patient. Moreover, the skin changes seen in our patient are totally unlike those seen in Ehlers-Danlos syndrome.
Solar elastosis (choice “c”) represents sun-caused basophilic degeneration of the deep dermis and presents with a whitish “chicken skin” look. Most commonly seen on the upper forehead of older men with an extensive history of overexposure to ultraviolet light, it almost never appears in intertriginous or other areas unexposed to sunlight.
DISCUSSION
PXE is an autosomal recessive disease with prevalence estimates ranging from 1:25,000 to 1:100,000. It does not demonstrate racial or geographic predilections, although it appears to have a slight preponderance in females. It typically begins to manifest in the second to third decade of life. This genetic defect results in the improper function of elastin and elastic fibers in the deep dermis, the intima of midsized arteries, and the Bruch membrane in the eye, where characteristic clinical and histopathologic changes occur. Thus, three main organ systems are affected: the skin, the eyes, and the cardiovascular system. However, the manifestations can vary a great deal—even among siblings.
Almost 100% of patients older than 30 eventually show signs of the disease in the eyes, with angioid streaks often becoming symptomatic only after trauma to the eye. The end point is reparative neovascularization, leakage from which can eventuate in hemorrhage, scarring, and loss of vision.
PXE also affects midsized arteries, especially of the extremities, where pathologic changes in the vessel walls lead to the premature formation of atheromatous plaques and eventual claudication, loss of peripheral pulses, hypertension, angina, and myocardial infarction that occur at a much younger age than in the unaffected population. Cerebral ischemic attacks, with the exception of aneurysms, are common with PXE. Gastrointestinal bleeds are common, but PXE does not affect the liver, lung, or kidneys.
“Treatment” for the appearance of PXE-affected skin is nearly nonexistent, but there are steps that should be taken. For example: (1) genetic counseling to assess the risk for future generations, as well as presymptomatic testing; (2) regular, periodic eye examination, including funduscopic examination, as well as advice to avoid heavy lifting, trauma, smoking, or other counterproductive activities; and (3) emphasis on preventive lifestyle choices to avoid cardiovascular complications, with early detection a more attainable goal with continued surveillance.
PXE is only one of several so-called “genodermatoses,” that is, inheritable conditions with dermatologic manifestations. Other examples include neurofibromatosis type 1, the aforementioned Ehlers-Danlos syndrome, and epidermolysis bullosa—among many, many others.
ANSWER
The correct answer is pseudoxanthoma elasticum (PXE; choice “d”), details of which are discussed below. Eruptive xanthomata (choice “a”) usually present with widespread eruptive papules and/or nodules and are associated with extreme hypertriglyceridemia. There are several different types of Ehlers-Danlos syndrome, an inherited defect of connective tissues, but the most common involve hypermobile joints (so-called double-jointedness) and/or cutis laxa, neither of which is present in our patient. Moreover, the skin changes seen in our patient are totally unlike those seen in Ehlers-Danlos syndrome.
Solar elastosis (choice “c”) represents sun-caused basophilic degeneration of the deep dermis and presents with a whitish “chicken skin” look. Most commonly seen on the upper forehead of older men with an extensive history of overexposure to ultraviolet light, it almost never appears in intertriginous or other areas unexposed to sunlight.
DISCUSSION
PXE is an autosomal recessive disease with prevalence estimates ranging from 1:25,000 to 1:100,000. It does not demonstrate racial or geographic predilections, although it appears to have a slight preponderance in females. It typically begins to manifest in the second to third decade of life. This genetic defect results in the improper function of elastin and elastic fibers in the deep dermis, the intima of midsized arteries, and the Bruch membrane in the eye, where characteristic clinical and histopathologic changes occur. Thus, three main organ systems are affected: the skin, the eyes, and the cardiovascular system. However, the manifestations can vary a great deal—even among siblings.
Almost 100% of patients older than 30 eventually show signs of the disease in the eyes, with angioid streaks often becoming symptomatic only after trauma to the eye. The end point is reparative neovascularization, leakage from which can eventuate in hemorrhage, scarring, and loss of vision.
PXE also affects midsized arteries, especially of the extremities, where pathologic changes in the vessel walls lead to the premature formation of atheromatous plaques and eventual claudication, loss of peripheral pulses, hypertension, angina, and myocardial infarction that occur at a much younger age than in the unaffected population. Cerebral ischemic attacks, with the exception of aneurysms, are common with PXE. Gastrointestinal bleeds are common, but PXE does not affect the liver, lung, or kidneys.
“Treatment” for the appearance of PXE-affected skin is nearly nonexistent, but there are steps that should be taken. For example: (1) genetic counseling to assess the risk for future generations, as well as presymptomatic testing; (2) regular, periodic eye examination, including funduscopic examination, as well as advice to avoid heavy lifting, trauma, smoking, or other counterproductive activities; and (3) emphasis on preventive lifestyle choices to avoid cardiovascular complications, with early detection a more attainable goal with continued surveillance.
PXE is only one of several so-called “genodermatoses,” that is, inheritable conditions with dermatologic manifestations. Other examples include neurofibromatosis type 1, the aforementioned Ehlers-Danlos syndrome, and epidermolysis bullosa—among many, many others.
ANSWER
The correct answer is pseudoxanthoma elasticum (PXE; choice “d”), details of which are discussed below. Eruptive xanthomata (choice “a”) usually present with widespread eruptive papules and/or nodules and are associated with extreme hypertriglyceridemia. There are several different types of Ehlers-Danlos syndrome, an inherited defect of connective tissues, but the most common involve hypermobile joints (so-called double-jointedness) and/or cutis laxa, neither of which is present in our patient. Moreover, the skin changes seen in our patient are totally unlike those seen in Ehlers-Danlos syndrome.
Solar elastosis (choice “c”) represents sun-caused basophilic degeneration of the deep dermis and presents with a whitish “chicken skin” look. Most commonly seen on the upper forehead of older men with an extensive history of overexposure to ultraviolet light, it almost never appears in intertriginous or other areas unexposed to sunlight.
DISCUSSION
PXE is an autosomal recessive disease with prevalence estimates ranging from 1:25,000 to 1:100,000. It does not demonstrate racial or geographic predilections, although it appears to have a slight preponderance in females. It typically begins to manifest in the second to third decade of life. This genetic defect results in the improper function of elastin and elastic fibers in the deep dermis, the intima of midsized arteries, and the Bruch membrane in the eye, where characteristic clinical and histopathologic changes occur. Thus, three main organ systems are affected: the skin, the eyes, and the cardiovascular system. However, the manifestations can vary a great deal—even among siblings.
Almost 100% of patients older than 30 eventually show signs of the disease in the eyes, with angioid streaks often becoming symptomatic only after trauma to the eye. The end point is reparative neovascularization, leakage from which can eventuate in hemorrhage, scarring, and loss of vision.
PXE also affects midsized arteries, especially of the extremities, where pathologic changes in the vessel walls lead to the premature formation of atheromatous plaques and eventual claudication, loss of peripheral pulses, hypertension, angina, and myocardial infarction that occur at a much younger age than in the unaffected population. Cerebral ischemic attacks, with the exception of aneurysms, are common with PXE. Gastrointestinal bleeds are common, but PXE does not affect the liver, lung, or kidneys.
“Treatment” for the appearance of PXE-affected skin is nearly nonexistent, but there are steps that should be taken. For example: (1) genetic counseling to assess the risk for future generations, as well as presymptomatic testing; (2) regular, periodic eye examination, including funduscopic examination, as well as advice to avoid heavy lifting, trauma, smoking, or other counterproductive activities; and (3) emphasis on preventive lifestyle choices to avoid cardiovascular complications, with early detection a more attainable goal with continued surveillance.
PXE is only one of several so-called “genodermatoses,” that is, inheritable conditions with dermatologic manifestations. Other examples include neurofibromatosis type 1, the aforementioned Ehlers-Danlos syndrome, and epidermolysis bullosa—among many, many others.
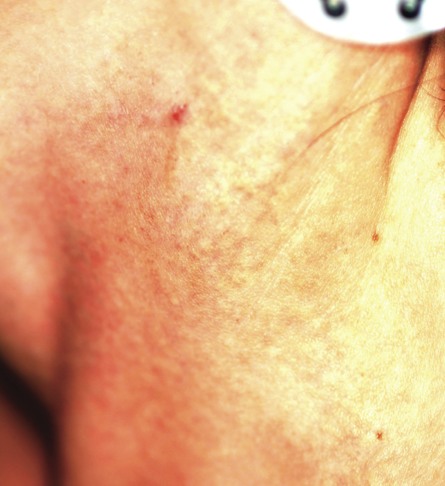
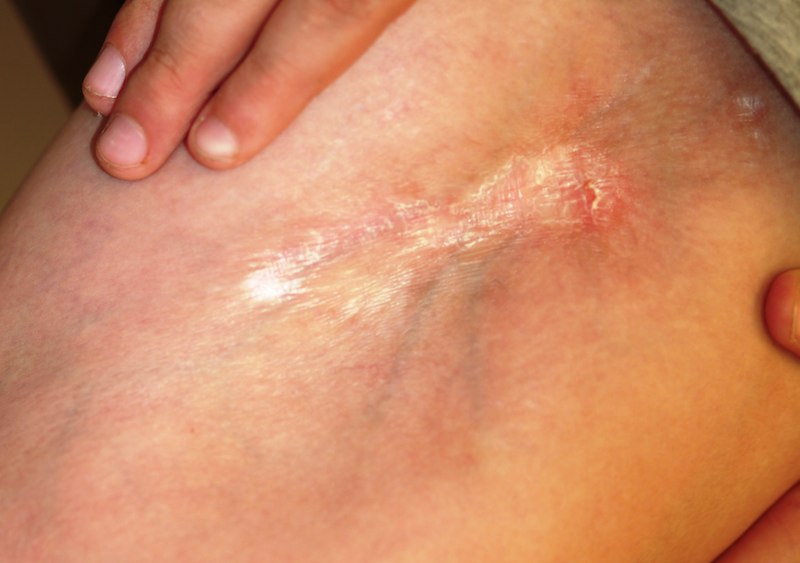
A 29-year-old woman presents for evaluation of changes in her skin, which she first noticed during her late teens. The changes have been asymptomatic and slowly progressive, and various providers have given different explanations for them at different times. However, she has never been seen by dermatology prior to this visit, which is ostensibly for treatment of hand warts and eyelid dermatitis. Aside from being somewhat atopic, the patient is in otherwise excellent health. Laboratory work done as part of a recent physical showed her lipid levels to be well within normal limits. A discussion of family history reveals she has a sister with the same type of skin changes; however, she too has never received a definitive diagnosis from any of the many providers who have evaluated her over the years. The patient points to changes in several intertriginous locations, including bilateral neck skin, both antecubital areas, axillae, and crural folds. The skin in those areas has an odd cobblestone-like papularity, appears atrophic, and is light yellow. The changes are barely palpable. The midline posterior neck is spared, but the same changes can be seen on oral mucosal surfaces. Elsewhere, there are no signs of hypermobile joints or of cutis laxa. No evidence of excessive sun exposure is observed. Several punch biopsies show distorted, fragmented elastic fibers in the mid to deep reticular dermis.
Common "Lesion" Leads to Rare Diagnosis
ANSWER
The one incorrect statement is choice “a,” since morphea does not progress into systemic sclerosis (SS), although the local histologic process is almost identical in each. Morphea—especially in children, and especially with linear types that course over crucial structures such as joints or faces—can interfere with normal function and growth, making choice “b” correct. Several theories have arisen to explain the genesis of this disease, including an autoimmune basis; to date, none has been proven, so both choice “c” and choice “d” are technically correct.
DISCUSSION
This case is a perfect example of an established maxim of dermatology: One seldom diagnoses what one has never heard of. The reported incidence of morphea is 25 cases per one million Americans, but it is a common enough complaint in dermatology practices.
Although the precise cause is unknown, there is general agreement that morphea represents localized dysregulation of collagen synthesis and deposition in the dermis. While that process is identical to that seen in SS, the two are otherwise not part of the same clinical continuum—good news for the morphea patient, since SS is a far more serious disease. Morphea patients do not, for example, experience the components of SS such as Raynaud’s phenomenon, sclerodactyly, or involvement of internal organs, nor do they share the potentially dire prognosis of SS patients.
Morphea takes several clinical forms; the most common is the plaque type, which typically presents as an annular lesion on the trunk or extremity, with the potential to grow to considerable size. Most will stop growing in time, but the discolored “burnt-out” lesion remains, leaving a purplish brown atrophic area.
Another form of morphea is a linear configuration. This is seen particularly in children, in whom it can interfere with normal growth and function. Examples include the morphea variants Parry-Romberg syndrome and en coup de sabre; both appear on the face, and deeper structures such as soft tissue and bone can be affected.
TREATMENT
This patient has advanced disease, for which calcipotriene cream (to be applied bid for two months) was prescribed, with follow-up to monitor his disease and its effect on the joint as well as the skin. Other medications have been tried for morphea, including minocycline and methotrexate, with decidedly mixed results.
ANSWER
The one incorrect statement is choice “a,” since morphea does not progress into systemic sclerosis (SS), although the local histologic process is almost identical in each. Morphea—especially in children, and especially with linear types that course over crucial structures such as joints or faces—can interfere with normal function and growth, making choice “b” correct. Several theories have arisen to explain the genesis of this disease, including an autoimmune basis; to date, none has been proven, so both choice “c” and choice “d” are technically correct.
DISCUSSION
This case is a perfect example of an established maxim of dermatology: One seldom diagnoses what one has never heard of. The reported incidence of morphea is 25 cases per one million Americans, but it is a common enough complaint in dermatology practices.
Although the precise cause is unknown, there is general agreement that morphea represents localized dysregulation of collagen synthesis and deposition in the dermis. While that process is identical to that seen in SS, the two are otherwise not part of the same clinical continuum—good news for the morphea patient, since SS is a far more serious disease. Morphea patients do not, for example, experience the components of SS such as Raynaud’s phenomenon, sclerodactyly, or involvement of internal organs, nor do they share the potentially dire prognosis of SS patients.
Morphea takes several clinical forms; the most common is the plaque type, which typically presents as an annular lesion on the trunk or extremity, with the potential to grow to considerable size. Most will stop growing in time, but the discolored “burnt-out” lesion remains, leaving a purplish brown atrophic area.
Another form of morphea is a linear configuration. This is seen particularly in children, in whom it can interfere with normal growth and function. Examples include the morphea variants Parry-Romberg syndrome and en coup de sabre; both appear on the face, and deeper structures such as soft tissue and bone can be affected.
TREATMENT
This patient has advanced disease, for which calcipotriene cream (to be applied bid for two months) was prescribed, with follow-up to monitor his disease and its effect on the joint as well as the skin. Other medications have been tried for morphea, including minocycline and methotrexate, with decidedly mixed results.
ANSWER
The one incorrect statement is choice “a,” since morphea does not progress into systemic sclerosis (SS), although the local histologic process is almost identical in each. Morphea—especially in children, and especially with linear types that course over crucial structures such as joints or faces—can interfere with normal function and growth, making choice “b” correct. Several theories have arisen to explain the genesis of this disease, including an autoimmune basis; to date, none has been proven, so both choice “c” and choice “d” are technically correct.
DISCUSSION
This case is a perfect example of an established maxim of dermatology: One seldom diagnoses what one has never heard of. The reported incidence of morphea is 25 cases per one million Americans, but it is a common enough complaint in dermatology practices.
Although the precise cause is unknown, there is general agreement that morphea represents localized dysregulation of collagen synthesis and deposition in the dermis. While that process is identical to that seen in SS, the two are otherwise not part of the same clinical continuum—good news for the morphea patient, since SS is a far more serious disease. Morphea patients do not, for example, experience the components of SS such as Raynaud’s phenomenon, sclerodactyly, or involvement of internal organs, nor do they share the potentially dire prognosis of SS patients.
Morphea takes several clinical forms; the most common is the plaque type, which typically presents as an annular lesion on the trunk or extremity, with the potential to grow to considerable size. Most will stop growing in time, but the discolored “burnt-out” lesion remains, leaving a purplish brown atrophic area.
Another form of morphea is a linear configuration. This is seen particularly in children, in whom it can interfere with normal growth and function. Examples include the morphea variants Parry-Romberg syndrome and en coup de sabre; both appear on the face, and deeper structures such as soft tissue and bone can be affected.
TREATMENT
This patient has advanced disease, for which calcipotriene cream (to be applied bid for two months) was prescribed, with follow-up to monitor his disease and its effect on the joint as well as the skin. Other medications have been tried for morphea, including minocycline and methotrexate, with decidedly mixed results.
For five years, a 12-year-old boy has had an expanding, asymptomatic lesion on his left thigh and knee. There was no known precipitating event—no trauma or insect bite. No other areas are involved. The boy’s health is otherwise good. On examination, a 20 x 10–cm “lesion” is noted on the left medial thigh and knee. The entire area is surprisingly firm to the touch and is brownish blue, with a sclerotic look and feel. There is a central area of depression that, at its deepest, is at least 2 cm lower than the surrounding skin. The area is covered by a cicatricial fusiform plaque measuring 8 x 3 cm. The overlying skin is remarkably smooth. The courses of the subcutaneous venous plexus can be easily traced through the atrophic skin. The pathologic process covers the medial thigh, crosses the joint, and is (based on the history) extending inferiorly in a somewhat linear configuration. No other areas of involvement are appreciated, and the patient’s skin is otherwise normal. In an effort to avoid the creation of a nonhealing wound, a 5-mm punch biopsy is performed on the wall of the depressed area, avoiding the central cicatricial portion. The results are as follows: thickening and homogenization of collagen bundles concentrated in the lower reticular dermis. It therefore seems clear that the diagnosis is morphea. Which of the following statements about this condition is false?
An Unexpected Effect of Crohn's
ANSWER
The correct answer is Sweet’s syndrome (choice “a”), also known as acute febrile neutrophilic dermatosis. Read on for a discussion of this condition.
Mastocytosis (choice “b”) can present with solitary lesions (“mastocytoma”), but biopsy would have shown a marked increase in mast cells, not the mature polymorphonuclear leukocytes seen in this biopsy. Pyoderma gangrenosum (choice “c”) lesions eventually ulcerate, and biopsy would have revealed a mixed infiltrate instead of the purely neutrophilic one seen. Cellulitis (choice “d”) would have manifested acutely, quickly becoming suppurative, in contrast to the persistence seen with this lesion.
DISCUSSION/TREATMENT
Sweet’s syndrome was first described in 1964 and has since been organized into three basic categories: the classic type, often idiopathic since most cases are of unknown origin; malignancy-associated, typically hematologic (eg, myelogenous leukemia); and drug induced, which was first described in association with sulfa-trimethoprim.
The classic presentation can be triggered by, among other things, upper respiratory infections, pregnancy, and inflammatory bowel disease—as in our patient, whose lesion is quite typical. Arguably the most significant association is with a malignancy, a search for which is undertaken when other possible triggers are absent.
The gold standard for treatment of Sweet’s syndrome is systemic corticosteroids, but other drugs have been used with success, including colchicine and dapsone. This patient is being successfully treated with topical clobetasol cream under occlusion, selected because her disease is very limited. Complete blood count and manual differential diagnosis failed to show any evidence for leukemia.
ANSWER
The correct answer is Sweet’s syndrome (choice “a”), also known as acute febrile neutrophilic dermatosis. Read on for a discussion of this condition.
Mastocytosis (choice “b”) can present with solitary lesions (“mastocytoma”), but biopsy would have shown a marked increase in mast cells, not the mature polymorphonuclear leukocytes seen in this biopsy. Pyoderma gangrenosum (choice “c”) lesions eventually ulcerate, and biopsy would have revealed a mixed infiltrate instead of the purely neutrophilic one seen. Cellulitis (choice “d”) would have manifested acutely, quickly becoming suppurative, in contrast to the persistence seen with this lesion.
DISCUSSION/TREATMENT
Sweet’s syndrome was first described in 1964 and has since been organized into three basic categories: the classic type, often idiopathic since most cases are of unknown origin; malignancy-associated, typically hematologic (eg, myelogenous leukemia); and drug induced, which was first described in association with sulfa-trimethoprim.
The classic presentation can be triggered by, among other things, upper respiratory infections, pregnancy, and inflammatory bowel disease—as in our patient, whose lesion is quite typical. Arguably the most significant association is with a malignancy, a search for which is undertaken when other possible triggers are absent.
The gold standard for treatment of Sweet’s syndrome is systemic corticosteroids, but other drugs have been used with success, including colchicine and dapsone. This patient is being successfully treated with topical clobetasol cream under occlusion, selected because her disease is very limited. Complete blood count and manual differential diagnosis failed to show any evidence for leukemia.
ANSWER
The correct answer is Sweet’s syndrome (choice “a”), also known as acute febrile neutrophilic dermatosis. Read on for a discussion of this condition.
Mastocytosis (choice “b”) can present with solitary lesions (“mastocytoma”), but biopsy would have shown a marked increase in mast cells, not the mature polymorphonuclear leukocytes seen in this biopsy. Pyoderma gangrenosum (choice “c”) lesions eventually ulcerate, and biopsy would have revealed a mixed infiltrate instead of the purely neutrophilic one seen. Cellulitis (choice “d”) would have manifested acutely, quickly becoming suppurative, in contrast to the persistence seen with this lesion.
DISCUSSION/TREATMENT
Sweet’s syndrome was first described in 1964 and has since been organized into three basic categories: the classic type, often idiopathic since most cases are of unknown origin; malignancy-associated, typically hematologic (eg, myelogenous leukemia); and drug induced, which was first described in association with sulfa-trimethoprim.
The classic presentation can be triggered by, among other things, upper respiratory infections, pregnancy, and inflammatory bowel disease—as in our patient, whose lesion is quite typical. Arguably the most significant association is with a malignancy, a search for which is undertaken when other possible triggers are absent.
The gold standard for treatment of Sweet’s syndrome is systemic corticosteroids, but other drugs have been used with success, including colchicine and dapsone. This patient is being successfully treated with topical clobetasol cream under occlusion, selected because her disease is very limited. Complete blood count and manual differential diagnosis failed to show any evidence for leukemia.

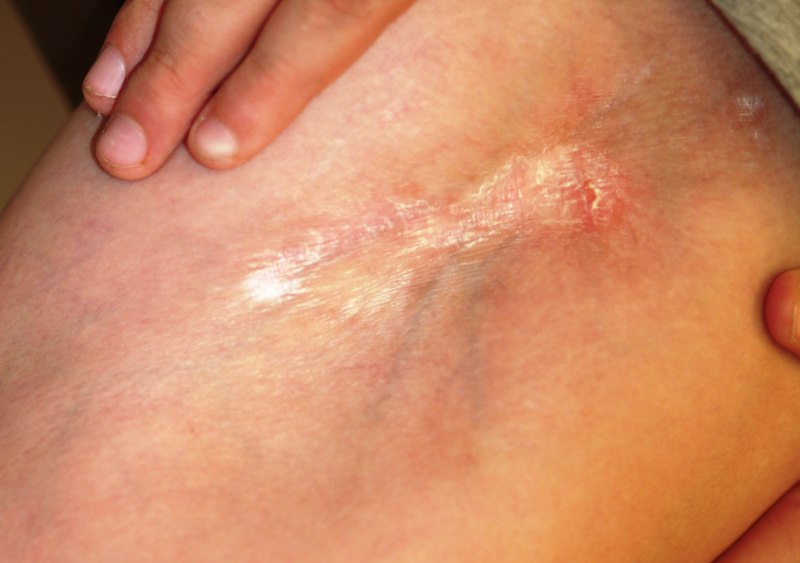
A 47-year-old woman presents with a lesion that first appeared on her arm two months ago. The lesion has grown increasingly tender and large and has persisted despite a course of antibiotics (cephalexin 500 mg qid for 10 days). She denies any history of similar problems but does relate a 20-year history of Crohn’s disease. She was recently hospitalized for care of her Crohn’s-related pyoderma gangrenosum, which occured on her legs. Despite all this, the patient otherwise feels well, reporting no fever or malaise. Examination reveals a woman who looks her stated age, with a 2.5-cm brownish red round plaque located on the dorsal right forearm. Little if any erythema extends beyond the sharply defined border. The surface of the plaque looks slightly blistery, but on palpation the lesion feels solid and not fluid filled. It is moderately tender to touch and much warmer than the surrounding skin. No lymph nodes can be palpated in either epitrochlear or axillary areas, and no similar lesions are seen elsewhere. A 5-mm punch biopsy is performed on the lesion. The results show a dense neutrophilic infiltrate, accentuated on the dermal papillae.
Lesion Has Doubled in Size in Two Weeks
ANSWER
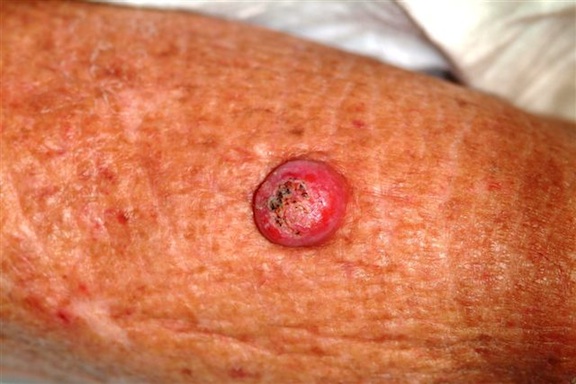
The one incorrect statement is choice “a,” since the history and appearance of the lesion are quite consistent with keratoacanthoma—considered by most to be a low-grade form of squamous cell carcinoma (SCC). All the other statements are true.
DISCUSSION/TREATMENT
Keratoacanthomas (KAs) are quite common, especially in older patients with fair, sun-damaged skin. They appear on areas of the skin that have been directly exposed to the sun. The dorsal forearm is especially typical, but KAs can also appear on the face, hands, shoulders, and back.
The relatively rapid growth is in marked contrast to most other skin cancers and has been linked to the human papillomavirus DNA, which has been found in these lesions. However, by no means is this connection universally accepted as the cause, even though immune suppression, in the susceptible patient, does appear to play a role.
Microscopically, KAs bear a marked resemblance to SCCs. Indeed, they have been known to metastasize in rare instances, although most will involute (albeit with minor scarring) on their own, without treatment. The standard of treatment in this country is to remove KAs surgically and to submit the tissue for pathologic examination, which would officially show “SCC, KA type,” or “well-differentiated SCC.”
The differential diagnosis includes wart, invasive SCC, Merkel cell carcinoma, and melanoma. KAs have nothing to do with bacterial infection.
ANSWER
The one incorrect statement is choice “a,” since the history and appearance of the lesion are quite consistent with keratoacanthoma—considered by most to be a low-grade form of squamous cell carcinoma (SCC). All the other statements are true.
DISCUSSION/TREATMENT
Keratoacanthomas (KAs) are quite common, especially in older patients with fair, sun-damaged skin. They appear on areas of the skin that have been directly exposed to the sun. The dorsal forearm is especially typical, but KAs can also appear on the face, hands, shoulders, and back.
The relatively rapid growth is in marked contrast to most other skin cancers and has been linked to the human papillomavirus DNA, which has been found in these lesions. However, by no means is this connection universally accepted as the cause, even though immune suppression, in the susceptible patient, does appear to play a role.
Microscopically, KAs bear a marked resemblance to SCCs. Indeed, they have been known to metastasize in rare instances, although most will involute (albeit with minor scarring) on their own, without treatment. The standard of treatment in this country is to remove KAs surgically and to submit the tissue for pathologic examination, which would officially show “SCC, KA type,” or “well-differentiated SCC.”
The differential diagnosis includes wart, invasive SCC, Merkel cell carcinoma, and melanoma. KAs have nothing to do with bacterial infection.
ANSWER
The one incorrect statement is choice “a,” since the history and appearance of the lesion are quite consistent with keratoacanthoma—considered by most to be a low-grade form of squamous cell carcinoma (SCC). All the other statements are true.
DISCUSSION/TREATMENT
Keratoacanthomas (KAs) are quite common, especially in older patients with fair, sun-damaged skin. They appear on areas of the skin that have been directly exposed to the sun. The dorsal forearm is especially typical, but KAs can also appear on the face, hands, shoulders, and back.
The relatively rapid growth is in marked contrast to most other skin cancers and has been linked to the human papillomavirus DNA, which has been found in these lesions. However, by no means is this connection universally accepted as the cause, even though immune suppression, in the susceptible patient, does appear to play a role.
Microscopically, KAs bear a marked resemblance to SCCs. Indeed, they have been known to metastasize in rare instances, although most will involute (albeit with minor scarring) on their own, without treatment. The standard of treatment in this country is to remove KAs surgically and to submit the tissue for pathologic examination, which would officially show “SCC, KA type,” or “well-differentiated SCC.”
The differential diagnosis includes wart, invasive SCC, Merkel cell carcinoma, and melanoma. KAs have nothing to do with bacterial infection.
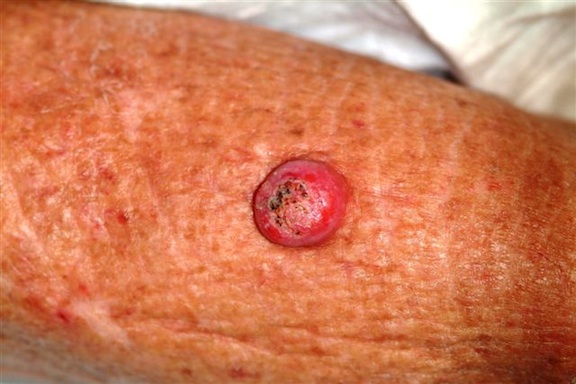
Six weeks ago, a new lesion began to appear on the forearm of this 63-year-old woman; it has grown rapidly in the ensuing time. She first went to her primary care provider, who diagnosed a staph infection and prescribed an antibiotic that hasn’t helped. The lesion, while asymptomatic, is nonetheless alarming; it has doubled in size in the past two weeks, which is why the patient now presents to the dermatology clinic. Her medical history includes well-controlled hypertension and a remote incidence of several basal cell carcinomas. On examination, the patient’s skin, in general, is severely sun-damaged, as evidenced by a weathered, leathery look to all exposed skin, as well as by multiple telangiectasias and solar lentigines. The lesion in question is a striking 1.8-cm dome-like nodule with a central keratotic core, located on the mid-dorsal forearm. Smooth and shiny, the surface of this firm nodule is also covered with tiny telangiectasias. Careful examination of the rest of the patient’s skin shows no other worrisome lesions.
Eyelids Are Irritated (But Eyes Are Fine)
ANSWER
The one incorrect answer is yeast infection (choice “c”). All the others should be included in the list of possible explanations. Given the inherent dryness and relatively low temperature of the affected area, as well as the nonresponse to imidazole cream, candida is quite unlikely.
DISCUSSION
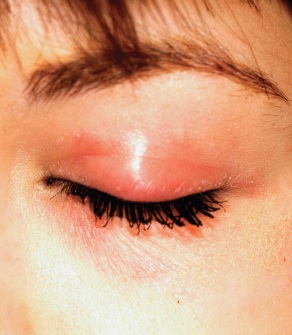
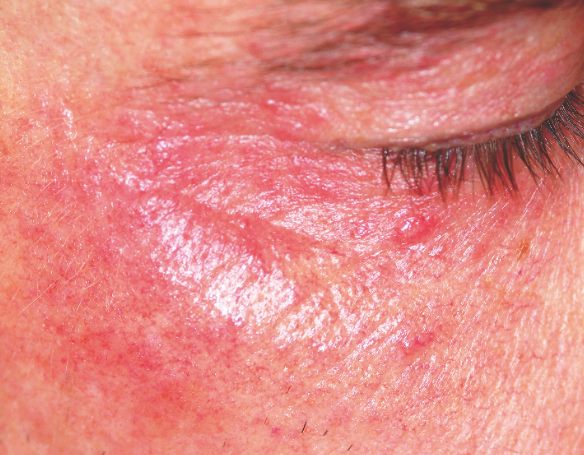
The only way this patient could acquire a yeast infection in this area is if she were immunosuppressed. Eyelid dermatitis is seen daily in derm practices; patients are often referred by frustrated primary care providers who are unable to help.
This problem is almost never seen in male patients, which suggests the involvement of makeup, eye shadow, or skin care products, especially cleansers. But even when these are changed or eliminated, the problem often continues.
Here’s why: Women—especially those with sensitive skin in general—will often manifest that sensitivity in areas where skin is the thinnest and most accessible, and therefore the most easily damaged. Once the problem starts, it is difficult for these women to leave it alone. They scratch it, rub it, and often apply multiple products to it, all of which only serves to worsen it.
In contrast, most men use no products at all on their faces, let alone “eye creams” or special cleansers. The male version of this problem is the equally ubiquitous chronic anterior scrotal rash—which develops for many of the same reasons.
Many of these women also happen to have a history of atopic dermatitis, which not only means extraordinarily sensitive skin all over the body but also tends to involve unusually dry skin (xerosis). These women have multiple allergies to contactants, such as nickel and nail polish.
TREATMENT
As the cycle of applying different (unhelpful) products to the problem area continues, women are increasingly likely to use products with irritating ingredients. If they are to get any relief, this cycle must be broken.
I usually prescribe hydrocortisone 2.5% ointment (not the cream, which has a drying effect) or tacrolimus 0.1% ointment (either treatment to be used twice a day), plus once-a-day application of the greasiest moisturizer they can stand (eg, petroleum jelly) over the affected area.
They can use this same approach with future episodes, although there must be strict limits on the duration (two weeks) and frequency of the application. Overuse of the steroid can lead to glaucoma and permanent thinning of already thin skin.
Obviously, it would be of great utility if the patient were in fact found to be allergic to nail polish, but in my experience, most women have eliminated that as a potential cause before they get to a dermatology provider. The same goes for makeup.
The eyelid dermatitis patient certainly could be suffering from seborrhea or psoriasis; however, in such cases, the same itch-scratch-itch cycle often results, and the treatment would be identical.
ANSWER
The one incorrect answer is yeast infection (choice “c”). All the others should be included in the list of possible explanations. Given the inherent dryness and relatively low temperature of the affected area, as well as the nonresponse to imidazole cream, candida is quite unlikely.
DISCUSSION
The only way this patient could acquire a yeast infection in this area is if she were immunosuppressed. Eyelid dermatitis is seen daily in derm practices; patients are often referred by frustrated primary care providers who are unable to help.
This problem is almost never seen in male patients, which suggests the involvement of makeup, eye shadow, or skin care products, especially cleansers. But even when these are changed or eliminated, the problem often continues.
Here’s why: Women—especially those with sensitive skin in general—will often manifest that sensitivity in areas where skin is the thinnest and most accessible, and therefore the most easily damaged. Once the problem starts, it is difficult for these women to leave it alone. They scratch it, rub it, and often apply multiple products to it, all of which only serves to worsen it.
In contrast, most men use no products at all on their faces, let alone “eye creams” or special cleansers. The male version of this problem is the equally ubiquitous chronic anterior scrotal rash—which develops for many of the same reasons.
Many of these women also happen to have a history of atopic dermatitis, which not only means extraordinarily sensitive skin all over the body but also tends to involve unusually dry skin (xerosis). These women have multiple allergies to contactants, such as nickel and nail polish.
TREATMENT
As the cycle of applying different (unhelpful) products to the problem area continues, women are increasingly likely to use products with irritating ingredients. If they are to get any relief, this cycle must be broken.
I usually prescribe hydrocortisone 2.5% ointment (not the cream, which has a drying effect) or tacrolimus 0.1% ointment (either treatment to be used twice a day), plus once-a-day application of the greasiest moisturizer they can stand (eg, petroleum jelly) over the affected area.
They can use this same approach with future episodes, although there must be strict limits on the duration (two weeks) and frequency of the application. Overuse of the steroid can lead to glaucoma and permanent thinning of already thin skin.
Obviously, it would be of great utility if the patient were in fact found to be allergic to nail polish, but in my experience, most women have eliminated that as a potential cause before they get to a dermatology provider. The same goes for makeup.
The eyelid dermatitis patient certainly could be suffering from seborrhea or psoriasis; however, in such cases, the same itch-scratch-itch cycle often results, and the treatment would be identical.
ANSWER
The one incorrect answer is yeast infection (choice “c”). All the others should be included in the list of possible explanations. Given the inherent dryness and relatively low temperature of the affected area, as well as the nonresponse to imidazole cream, candida is quite unlikely.
DISCUSSION
The only way this patient could acquire a yeast infection in this area is if she were immunosuppressed. Eyelid dermatitis is seen daily in derm practices; patients are often referred by frustrated primary care providers who are unable to help.
This problem is almost never seen in male patients, which suggests the involvement of makeup, eye shadow, or skin care products, especially cleansers. But even when these are changed or eliminated, the problem often continues.
Here’s why: Women—especially those with sensitive skin in general—will often manifest that sensitivity in areas where skin is the thinnest and most accessible, and therefore the most easily damaged. Once the problem starts, it is difficult for these women to leave it alone. They scratch it, rub it, and often apply multiple products to it, all of which only serves to worsen it.
In contrast, most men use no products at all on their faces, let alone “eye creams” or special cleansers. The male version of this problem is the equally ubiquitous chronic anterior scrotal rash—which develops for many of the same reasons.
Many of these women also happen to have a history of atopic dermatitis, which not only means extraordinarily sensitive skin all over the body but also tends to involve unusually dry skin (xerosis). These women have multiple allergies to contactants, such as nickel and nail polish.
TREATMENT
As the cycle of applying different (unhelpful) products to the problem area continues, women are increasingly likely to use products with irritating ingredients. If they are to get any relief, this cycle must be broken.
I usually prescribe hydrocortisone 2.5% ointment (not the cream, which has a drying effect) or tacrolimus 0.1% ointment (either treatment to be used twice a day), plus once-a-day application of the greasiest moisturizer they can stand (eg, petroleum jelly) over the affected area.
They can use this same approach with future episodes, although there must be strict limits on the duration (two weeks) and frequency of the application. Overuse of the steroid can lead to glaucoma and permanent thinning of already thin skin.
Obviously, it would be of great utility if the patient were in fact found to be allergic to nail polish, but in my experience, most women have eliminated that as a potential cause before they get to a dermatology provider. The same goes for makeup.
The eyelid dermatitis patient certainly could be suffering from seborrhea or psoriasis; however, in such cases, the same itch-scratch-itch cycle often results, and the treatment would be identical.
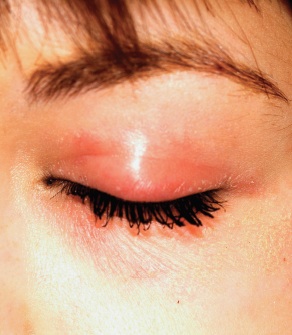
A 22-year-old woman is evaluated for a recurring itchy rash on both the upper and lower bilateral eyelids, which has been present for months. Her eyes themselves are unaffected, but the skin around them is highly irritated. The patient has already seen a number of providers who prescribed or recommended several remedies, including hydrocortisone creams, imidazole creams, and OTC moisturizers. Since none of these has proved helpful, she presents to the dermatology clinic. Her medical history includes seasonal allergies and “sensitive skin”; by the latter, she means frequent rashes or irritation brought on by various products, such as shampoos and conditioners. During this occurrence, as in several previous episodes, she has changed eye shadow and other makeup, to no avail. The patient denies any history of psoriasis, seborrhea, or other skin disease. On examination, a brisk red, dry, wrinkly rash covering the above-mentioned areas is noted. No rash is seen elsewhere, and there are no signs of psoriasis or seborrhea in any of the locations where they typically appear.
Painless Chest Lesion Suddenly Triples in Size
ANSWER
The correct answer is all of the above (choice “d”). All are reasonable diagnoses and important because they drive the decision as to how to proceed.
DISCUSSION
An incisional biopsy would also have been acceptable, but collection of the entire lesion, with modest margins where possible, is almost always the gold standard in establishing a diagnosis in cases such as this one. There are several reasons, one being that the pathologist will then have adequate tissue to examine and will have a good chance of determining whether the lesion began locally or represents spread from a distant site. The only argument against such an approach would be the potential for scarring.
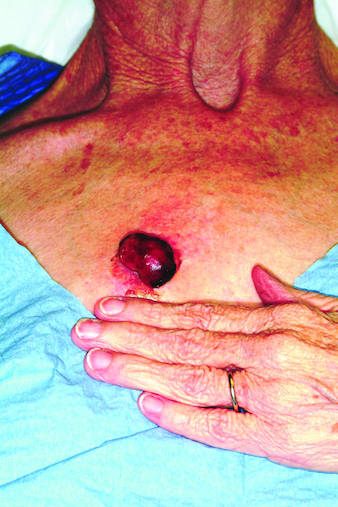
Moreover, in the majority of cases, this procedure can be curative—as in this case, in which the pathology report revealed the lesion to be a well-differentiated squamous cell carcinoma that had been traumatized, resulting in the formation of reparative inappropriate granulation tissue. It was the latter that accounted for the friability and rapid growth. Fortunately, the margins were clear, so no additional surgery or radiation was necessary.
A complete differential diagnosis would also include other types of cancer, including colon cancer and melanoma. Considering the possibilities, the patient was all too happy to have the problem taken care of in one step.
ANSWER
The correct answer is all of the above (choice “d”). All are reasonable diagnoses and important because they drive the decision as to how to proceed.
DISCUSSION
An incisional biopsy would also have been acceptable, but collection of the entire lesion, with modest margins where possible, is almost always the gold standard in establishing a diagnosis in cases such as this one. There are several reasons, one being that the pathologist will then have adequate tissue to examine and will have a good chance of determining whether the lesion began locally or represents spread from a distant site. The only argument against such an approach would be the potential for scarring.
Moreover, in the majority of cases, this procedure can be curative—as in this case, in which the pathology report revealed the lesion to be a well-differentiated squamous cell carcinoma that had been traumatized, resulting in the formation of reparative inappropriate granulation tissue. It was the latter that accounted for the friability and rapid growth. Fortunately, the margins were clear, so no additional surgery or radiation was necessary.
A complete differential diagnosis would also include other types of cancer, including colon cancer and melanoma. Considering the possibilities, the patient was all too happy to have the problem taken care of in one step.
ANSWER
The correct answer is all of the above (choice “d”). All are reasonable diagnoses and important because they drive the decision as to how to proceed.
DISCUSSION
An incisional biopsy would also have been acceptable, but collection of the entire lesion, with modest margins where possible, is almost always the gold standard in establishing a diagnosis in cases such as this one. There are several reasons, one being that the pathologist will then have adequate tissue to examine and will have a good chance of determining whether the lesion began locally or represents spread from a distant site. The only argument against such an approach would be the potential for scarring.
Moreover, in the majority of cases, this procedure can be curative—as in this case, in which the pathology report revealed the lesion to be a well-differentiated squamous cell carcinoma that had been traumatized, resulting in the formation of reparative inappropriate granulation tissue. It was the latter that accounted for the friability and rapid growth. Fortunately, the margins were clear, so no additional surgery or radiation was necessary.
A complete differential diagnosis would also include other types of cancer, including colon cancer and melanoma. Considering the possibilities, the patient was all too happy to have the problem taken care of in one step.
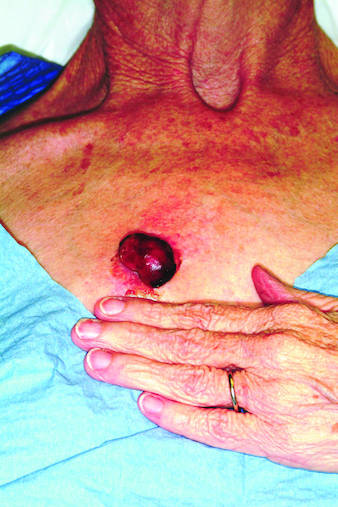
Rapid growth and bleeding are two of the most common reasons that patients seek evaluation for lesions. Both are concerns for this 69-year-old woman who has had a chest lesion for almost two years. Recently, the lesion suddenly tripled in size and began to bleed with minor trauma. Although painless, the lesion is of particular concern since the patient was diagnosed with breast cancer about 18 years ago and underwent a right radical mastectomy at that time. The patient is a smoker with a more than 50–pack-year history. She denies any recent history of shortness of breath, cough, or weight loss. Examination reveals an impressive nodule on the right midchest, measuring 4 cm and demonstrating a smooth, vascular surface and lobular shape. The base of the lesion is pedunculated, and the lesion itself is quite mobile. After consultation with the patient, the decision to excise is quickly reached and the procedure carried out. The excised lesion is sent for pathologic exam.
Dark Lesions Prompt Skin Cancer Scare
ANSWER
The one incorrect statement above is choice “d.” Although most patients call them age spots, age is, in fact, only one factor in the development of such lesions. All the other statements are true and are discussed below.
DISCUSSION
Seborrheic keratoses (SKs) are the most common benign skin lesions seen in older patients—but they’re also commonly seen on the skin of people in their 20s and 30s, so age is only one factor favoring their appearance. Heredity is the main cause in about 50% of cases. One of the most common dermatologic diagnoses, they are responsible for a large percentage of referrals to dermatology. As with this patient, SKs appear to be related to sun exposure in a significant percentage of patients, even though they have no malignant potential themselves.
Becoming thoroughly familiar with the myriad morphologic presentations of SKs is essential in learning dermatology. Their color ranges from gray to pink, tan to brown, and even black. Fortunately, they’re usually distinctly epidermal, with a “stuck-on” look that helps greatly in distinguishing them from malignant lesions. In oilier areas of the body, such as on the face, they can become much less scaly and much darker, making them difficult to distinguish from pigmented basal cell carcinoma or even melanoma in some cases; biopsy is thus necessary. Indeed, the “secret” to learning how to recognize SKs in all their varied presentations is the routine performance of punch biopsies.
SKs, when they’re as numerous as on this patient, can easily obscure a melanoma and can even coexist with the latter in the same location. This is especially true in high-risk patients (ie, those with fair, sun-damaged skin) who tan poorly. Fortunately, this patient had, by history and exam, type IV skin (with type I being the most fair, and type VI being the darkest) and was therefore at relatively low risk for skin cancer.
But on empirical grounds alone, one could correctly surmise that the mere presence of so many similar lesions, often present for years without change, effectively rules out skin cancer. The latter is far more likely to appear as a solitary lesion, as well as to be dynamic in nature (ie, to grow and/or change over time).
ANSWER
The one incorrect statement above is choice “d.” Although most patients call them age spots, age is, in fact, only one factor in the development of such lesions. All the other statements are true and are discussed below.
DISCUSSION
Seborrheic keratoses (SKs) are the most common benign skin lesions seen in older patients—but they’re also commonly seen on the skin of people in their 20s and 30s, so age is only one factor favoring their appearance. Heredity is the main cause in about 50% of cases. One of the most common dermatologic diagnoses, they are responsible for a large percentage of referrals to dermatology. As with this patient, SKs appear to be related to sun exposure in a significant percentage of patients, even though they have no malignant potential themselves.
Becoming thoroughly familiar with the myriad morphologic presentations of SKs is essential in learning dermatology. Their color ranges from gray to pink, tan to brown, and even black. Fortunately, they’re usually distinctly epidermal, with a “stuck-on” look that helps greatly in distinguishing them from malignant lesions. In oilier areas of the body, such as on the face, they can become much less scaly and much darker, making them difficult to distinguish from pigmented basal cell carcinoma or even melanoma in some cases; biopsy is thus necessary. Indeed, the “secret” to learning how to recognize SKs in all their varied presentations is the routine performance of punch biopsies.
SKs, when they’re as numerous as on this patient, can easily obscure a melanoma and can even coexist with the latter in the same location. This is especially true in high-risk patients (ie, those with fair, sun-damaged skin) who tan poorly. Fortunately, this patient had, by history and exam, type IV skin (with type I being the most fair, and type VI being the darkest) and was therefore at relatively low risk for skin cancer.
But on empirical grounds alone, one could correctly surmise that the mere presence of so many similar lesions, often present for years without change, effectively rules out skin cancer. The latter is far more likely to appear as a solitary lesion, as well as to be dynamic in nature (ie, to grow and/or change over time).
ANSWER
The one incorrect statement above is choice “d.” Although most patients call them age spots, age is, in fact, only one factor in the development of such lesions. All the other statements are true and are discussed below.
DISCUSSION
Seborrheic keratoses (SKs) are the most common benign skin lesions seen in older patients—but they’re also commonly seen on the skin of people in their 20s and 30s, so age is only one factor favoring their appearance. Heredity is the main cause in about 50% of cases. One of the most common dermatologic diagnoses, they are responsible for a large percentage of referrals to dermatology. As with this patient, SKs appear to be related to sun exposure in a significant percentage of patients, even though they have no malignant potential themselves.
Becoming thoroughly familiar with the myriad morphologic presentations of SKs is essential in learning dermatology. Their color ranges from gray to pink, tan to brown, and even black. Fortunately, they’re usually distinctly epidermal, with a “stuck-on” look that helps greatly in distinguishing them from malignant lesions. In oilier areas of the body, such as on the face, they can become much less scaly and much darker, making them difficult to distinguish from pigmented basal cell carcinoma or even melanoma in some cases; biopsy is thus necessary. Indeed, the “secret” to learning how to recognize SKs in all their varied presentations is the routine performance of punch biopsies.
SKs, when they’re as numerous as on this patient, can easily obscure a melanoma and can even coexist with the latter in the same location. This is especially true in high-risk patients (ie, those with fair, sun-damaged skin) who tan poorly. Fortunately, this patient had, by history and exam, type IV skin (with type I being the most fair, and type VI being the darkest) and was therefore at relatively low risk for skin cancer.
But on empirical grounds alone, one could correctly surmise that the mere presence of so many similar lesions, often present for years without change, effectively rules out skin cancer. The latter is far more likely to appear as a solitary lesion, as well as to be dynamic in nature (ie, to grow and/or change over time).
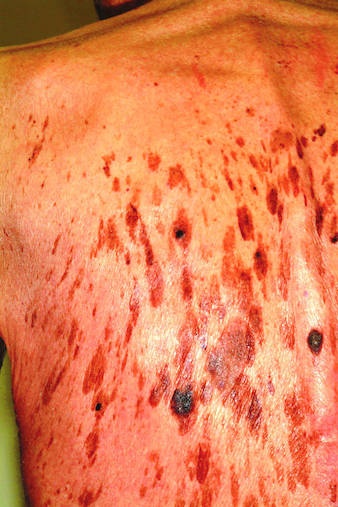
A 71-year-old man is urgently referred to dermatology by his primary care provider at the request of the patient’s children. These adult children recently noticed changes to the skin on their father’s back. On the day of the appointment, two of his children—a son and a daughter—bring the man in and already have his shirt off when you enter the room. Before you can evaluate the patient, his children express their anxiety about the lesions: “They’re so dark, and there are so many of them! We’re really concerned about this being skin cancer.” Examination reveals relatively dark skin with little evidence of sun damage, although the patient reports spending much of his life outdoors. When he is exposed to sun, he says, he tans relatively easily, holding the tan for weeks and very seldom burning. The lesions number easily in the hundreds and are tan to brown, papular, and nodular. Many coalesce into large plaques, with linear and in some cases digitate configuration. They virtually cover his back. On palpation, the surfaces of these lesions are epidermal, dry, and rough, with a warty texture. Lesions are also seen on his face, chest, and arms, though they are far fewer in number. Interestingly, both children have numerous similar lesions that can be easily seen.
Small, Painful Blisters Erupt in Patient's Groin Area
ANSWER
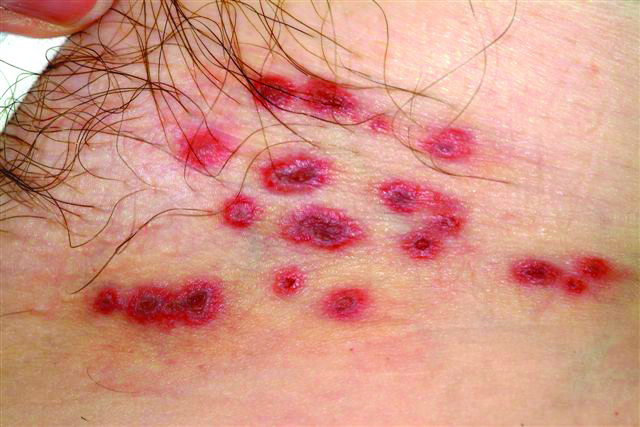
The correct answer is herpes zoster (choice “d”; see discussion). Yeast infections (choice “a”) would be quite unlikely to present with hemorrhagic discrete lesions and far more likely to present with a confluent rash. MRSA (choice “b”) can present in a wide variety of forms but is unlikely to form discrete blisters and would almost certainly involve induration in the area. Without a definitive viral culture, herpes simplex (choice “c”) could not be ruled out in this case, since it presents in a similar manner. But given the patient’s age and the morphology and location of the lesions, zoster was far more likely. See the discussion for further differentiating factors.
DISCUSSION/TREATMENT
The appearance of intralesional hemorrhage in an inflammatory lesion bespeaks a deeper, more vigorous process, since vasculature ends just below the dermoepidermal junction. This occurs infrequently enough with herpes zoster to obscure the diagnosis. Ironically, herpes simplex almost never exhibits this phenomenon, making it useful as a differentiating feature.
In this patient’s case, it seems reasonable to blame the local application of heat as a potential trigger. My experience is that stress is also a factor, though the literature does not bear out that theory.
ANSWER
The correct answer is herpes zoster (choice “d”; see discussion). Yeast infections (choice “a”) would be quite unlikely to present with hemorrhagic discrete lesions and far more likely to present with a confluent rash. MRSA (choice “b”) can present in a wide variety of forms but is unlikely to form discrete blisters and would almost certainly involve induration in the area. Without a definitive viral culture, herpes simplex (choice “c”) could not be ruled out in this case, since it presents in a similar manner. But given the patient’s age and the morphology and location of the lesions, zoster was far more likely. See the discussion for further differentiating factors.
DISCUSSION/TREATMENT
The appearance of intralesional hemorrhage in an inflammatory lesion bespeaks a deeper, more vigorous process, since vasculature ends just below the dermoepidermal junction. This occurs infrequently enough with herpes zoster to obscure the diagnosis. Ironically, herpes simplex almost never exhibits this phenomenon, making it useful as a differentiating feature.
In this patient’s case, it seems reasonable to blame the local application of heat as a potential trigger. My experience is that stress is also a factor, though the literature does not bear out that theory.
ANSWER
The correct answer is herpes zoster (choice “d”; see discussion). Yeast infections (choice “a”) would be quite unlikely to present with hemorrhagic discrete lesions and far more likely to present with a confluent rash. MRSA (choice “b”) can present in a wide variety of forms but is unlikely to form discrete blisters and would almost certainly involve induration in the area. Without a definitive viral culture, herpes simplex (choice “c”) could not be ruled out in this case, since it presents in a similar manner. But given the patient’s age and the morphology and location of the lesions, zoster was far more likely. See the discussion for further differentiating factors.
DISCUSSION/TREATMENT
The appearance of intralesional hemorrhage in an inflammatory lesion bespeaks a deeper, more vigorous process, since vasculature ends just below the dermoepidermal junction. This occurs infrequently enough with herpes zoster to obscure the diagnosis. Ironically, herpes simplex almost never exhibits this phenomenon, making it useful as a differentiating feature.
In this patient’s case, it seems reasonable to blame the local application of heat as a potential trigger. My experience is that stress is also a factor, though the literature does not bear out that theory.
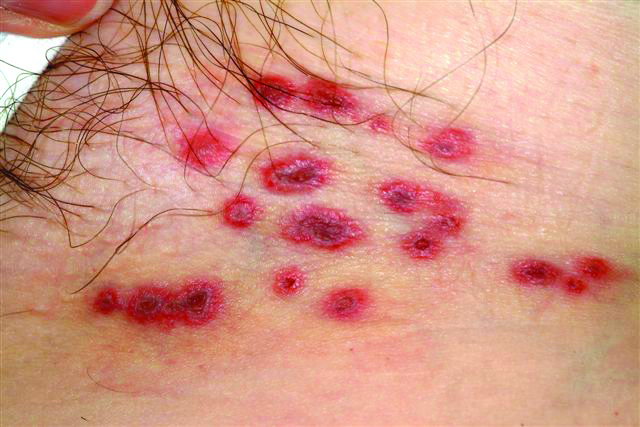
A 60-year-old woman presents with a symptomatic eruption in the left groin that began a week ago, when she first noticed a slight tingling sensation in that area. Within 48 hours, small blisters appeared, clustered in the crural fold. These became slightly painful, with a curious lancinating type of pain. The patient also began to experience fatigue and myalgia. She sought care when her lesions became hemorrhagic and was subsequently referred to dermatology. Additional history reveals that in the past month she has experienced low back pain on the left side, radiating into the groin. In response, she started to use moist hot packs on the area. The onset of her symptoms has coincided with another stressor, her husband’s job loss. She denies the use of any immunosuppressive medications and is well in other respects. Examination shows a grouped collection of targetoid hemorrhagic flaccid low blisters, from 1 to 2 cm in diameter, confined to the left crural fold. No underlying induration is felt, but there is palpable adenopathy confined to that area.