User login
Defining Your ‘Success’
Dear Friends,
The prevailing theme of this issue is “Success.” I have learned that “success” is personal and personalized. What “success” looked like 10, or even 5, years ago to me is very different from how I perceive it now; and I know it may be different 5 years from now. My definition of success should not look like another’s — that was the best advice I have gotten over the years and it has kept me constantly redefining what is important to me and placing value on where I want to allocate my time and efforts, at work and at home.
This issue of The New Gastroenterologist highlights topics from successful GIs within their own realms of expertise, offering insights on advancing in academic medicine, navigating financial wellness with a financial adviser, and becoming a future leader in GI.
In this issue’s clinically-focused articles, we spotlight two very nuanced and challenging topics. Dr. Sachin Srinivasan and Dr. Prateek Sharma review Barrett’s esophagus management for our “In Focus” section, with a particular emphasis on Barrett’s endoscopic therapy modalities for dysplasia and early neoplasia. Dr. Brooke Corning and team simplify their approach to pelvic floor dysfunction (PFD) in our “Short Clinical Reviews.” They suggest validated ways to assess patient history, pros and cons of various diagnostic tests, and stepwise management of PFD.
Navigating academic promotion can be overwhelming and may not be at the forefront with our early career GIs’ priorities. In our “Early Career” section, Dr. Vineet Rolston interviews two highly accomplished professors in academic medicine, Dr. Sophie Balzora and Dr. Mark Schattner, for their insights into the promotion process and recommendations for junior faculty.
Dr. Anjuli K. Luthra, a therapeutic endoscopist and founder of The Scope of Finance, emphasizes financial wellness for physicians. She breaks down the search for a financial adviser, including the different types, what to ask when searching for the right fit, and what to expect.
Lastly, this issue highlights an AGA program that invests in the development of leaders for the field — the Future Leaders Program (FLP). Dr. Parakkal Deepak and Dr. Edward L. Barnes, along with their mentor, Dr. Aasma Shaukat, describe their experience as a mentee-mentor triad of FLP and how this program has impacted their careers.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Danielle Kiefer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Dr. C.G. Stockton was the first AGA president in 1897, a Professor of the Principles and Practice of Medicine and Clinical Medicine at the University of Buffalo in New York, and published on the relationship between GI/Hepatology and gout in the Journal of the American Medical Association the same year of his presidency.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
The prevailing theme of this issue is “Success.” I have learned that “success” is personal and personalized. What “success” looked like 10, or even 5, years ago to me is very different from how I perceive it now; and I know it may be different 5 years from now. My definition of success should not look like another’s — that was the best advice I have gotten over the years and it has kept me constantly redefining what is important to me and placing value on where I want to allocate my time and efforts, at work and at home.
This issue of The New Gastroenterologist highlights topics from successful GIs within their own realms of expertise, offering insights on advancing in academic medicine, navigating financial wellness with a financial adviser, and becoming a future leader in GI.
In this issue’s clinically-focused articles, we spotlight two very nuanced and challenging topics. Dr. Sachin Srinivasan and Dr. Prateek Sharma review Barrett’s esophagus management for our “In Focus” section, with a particular emphasis on Barrett’s endoscopic therapy modalities for dysplasia and early neoplasia. Dr. Brooke Corning and team simplify their approach to pelvic floor dysfunction (PFD) in our “Short Clinical Reviews.” They suggest validated ways to assess patient history, pros and cons of various diagnostic tests, and stepwise management of PFD.
Navigating academic promotion can be overwhelming and may not be at the forefront with our early career GIs’ priorities. In our “Early Career” section, Dr. Vineet Rolston interviews two highly accomplished professors in academic medicine, Dr. Sophie Balzora and Dr. Mark Schattner, for their insights into the promotion process and recommendations for junior faculty.
Dr. Anjuli K. Luthra, a therapeutic endoscopist and founder of The Scope of Finance, emphasizes financial wellness for physicians. She breaks down the search for a financial adviser, including the different types, what to ask when searching for the right fit, and what to expect.
Lastly, this issue highlights an AGA program that invests in the development of leaders for the field — the Future Leaders Program (FLP). Dr. Parakkal Deepak and Dr. Edward L. Barnes, along with their mentor, Dr. Aasma Shaukat, describe their experience as a mentee-mentor triad of FLP and how this program has impacted their careers.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Danielle Kiefer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Dr. C.G. Stockton was the first AGA president in 1897, a Professor of the Principles and Practice of Medicine and Clinical Medicine at the University of Buffalo in New York, and published on the relationship between GI/Hepatology and gout in the Journal of the American Medical Association the same year of his presidency.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
The prevailing theme of this issue is “Success.” I have learned that “success” is personal and personalized. What “success” looked like 10, or even 5, years ago to me is very different from how I perceive it now; and I know it may be different 5 years from now. My definition of success should not look like another’s — that was the best advice I have gotten over the years and it has kept me constantly redefining what is important to me and placing value on where I want to allocate my time and efforts, at work and at home.
This issue of The New Gastroenterologist highlights topics from successful GIs within their own realms of expertise, offering insights on advancing in academic medicine, navigating financial wellness with a financial adviser, and becoming a future leader in GI.
In this issue’s clinically-focused articles, we spotlight two very nuanced and challenging topics. Dr. Sachin Srinivasan and Dr. Prateek Sharma review Barrett’s esophagus management for our “In Focus” section, with a particular emphasis on Barrett’s endoscopic therapy modalities for dysplasia and early neoplasia. Dr. Brooke Corning and team simplify their approach to pelvic floor dysfunction (PFD) in our “Short Clinical Reviews.” They suggest validated ways to assess patient history, pros and cons of various diagnostic tests, and stepwise management of PFD.
Navigating academic promotion can be overwhelming and may not be at the forefront with our early career GIs’ priorities. In our “Early Career” section, Dr. Vineet Rolston interviews two highly accomplished professors in academic medicine, Dr. Sophie Balzora and Dr. Mark Schattner, for their insights into the promotion process and recommendations for junior faculty.
Dr. Anjuli K. Luthra, a therapeutic endoscopist and founder of The Scope of Finance, emphasizes financial wellness for physicians. She breaks down the search for a financial adviser, including the different types, what to ask when searching for the right fit, and what to expect.
Lastly, this issue highlights an AGA program that invests in the development of leaders for the field — the Future Leaders Program (FLP). Dr. Parakkal Deepak and Dr. Edward L. Barnes, along with their mentor, Dr. Aasma Shaukat, describe their experience as a mentee-mentor triad of FLP and how this program has impacted their careers.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Danielle Kiefer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Dr. C.G. Stockton was the first AGA president in 1897, a Professor of the Principles and Practice of Medicine and Clinical Medicine at the University of Buffalo in New York, and published on the relationship between GI/Hepatology and gout in the Journal of the American Medical Association the same year of his presidency.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Working together
Dear Friends,
After 6 months in my first faculty position, I have come to appreciate the term “multidisciplinary approach” more than ever. Not only does this facilitate optimal patient care, but I have personally learned so much from experts in other fields. This theme resonates across this issue of The New Gastroenterologist, from treating complex gallbladder disease, to caring for sexual and gender minorities, and collaborating with the tech industry to advance patient care.
Our “In Focus” feature, written by Dr. Andrew Gilman and Dr. Todd Baron, is on endoscopic management of gallbladder disease. They review endoscopic treatment options in patients with benign gallbladder disease, with emphasis on working with surgical and interventional radiology colleagues, as well as relaying endoscopic tips and techniques to achieve success in these complicated procedures.
In the “Short Clinical Reviews” section, Dr. David Chiang and Dr. Victor Chedid highlight the gaps in research and clinical care and competency for sexual and gender minorities, particularly in patients with inflammatory bowel disease. They describe the creation of the Pride in IBD clinic at Mayo Clinic in Rochester, Minn., that creates a culturally sensitive space to care for this community.
As trainees transition to early faculty, becoming a mentor is a new role that can be very rewarding and daunting at the same time. Dr. Anna Lok, recipient of the AGA’s Distinguished Mentor Award, and Dr. Vincent Chen share invaluable experiences and advice on being a mentor from senior and early-career perspectives, respectively. Similarly in the transition to early faculty, Erin Anderson, CPA, answers five common financial questions that arise to better understand and manage a significant increase in salary.
Lastly, Dr. Shifa Umar describes her unique experience as part of the AGA’s annual Tech Summit Fellows Program, a cross-section of medicine, technology, and innovation.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The concept of the clinicopathologic conference (CPC) was introduced by Dr. Walter B. Cannon as a medical student at Harvard Medical School.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
After 6 months in my first faculty position, I have come to appreciate the term “multidisciplinary approach” more than ever. Not only does this facilitate optimal patient care, but I have personally learned so much from experts in other fields. This theme resonates across this issue of The New Gastroenterologist, from treating complex gallbladder disease, to caring for sexual and gender minorities, and collaborating with the tech industry to advance patient care.
Our “In Focus” feature, written by Dr. Andrew Gilman and Dr. Todd Baron, is on endoscopic management of gallbladder disease. They review endoscopic treatment options in patients with benign gallbladder disease, with emphasis on working with surgical and interventional radiology colleagues, as well as relaying endoscopic tips and techniques to achieve success in these complicated procedures.
In the “Short Clinical Reviews” section, Dr. David Chiang and Dr. Victor Chedid highlight the gaps in research and clinical care and competency for sexual and gender minorities, particularly in patients with inflammatory bowel disease. They describe the creation of the Pride in IBD clinic at Mayo Clinic in Rochester, Minn., that creates a culturally sensitive space to care for this community.
As trainees transition to early faculty, becoming a mentor is a new role that can be very rewarding and daunting at the same time. Dr. Anna Lok, recipient of the AGA’s Distinguished Mentor Award, and Dr. Vincent Chen share invaluable experiences and advice on being a mentor from senior and early-career perspectives, respectively. Similarly in the transition to early faculty, Erin Anderson, CPA, answers five common financial questions that arise to better understand and manage a significant increase in salary.
Lastly, Dr. Shifa Umar describes her unique experience as part of the AGA’s annual Tech Summit Fellows Program, a cross-section of medicine, technology, and innovation.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The concept of the clinicopathologic conference (CPC) was introduced by Dr. Walter B. Cannon as a medical student at Harvard Medical School.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
After 6 months in my first faculty position, I have come to appreciate the term “multidisciplinary approach” more than ever. Not only does this facilitate optimal patient care, but I have personally learned so much from experts in other fields. This theme resonates across this issue of The New Gastroenterologist, from treating complex gallbladder disease, to caring for sexual and gender minorities, and collaborating with the tech industry to advance patient care.
Our “In Focus” feature, written by Dr. Andrew Gilman and Dr. Todd Baron, is on endoscopic management of gallbladder disease. They review endoscopic treatment options in patients with benign gallbladder disease, with emphasis on working with surgical and interventional radiology colleagues, as well as relaying endoscopic tips and techniques to achieve success in these complicated procedures.
In the “Short Clinical Reviews” section, Dr. David Chiang and Dr. Victor Chedid highlight the gaps in research and clinical care and competency for sexual and gender minorities, particularly in patients with inflammatory bowel disease. They describe the creation of the Pride in IBD clinic at Mayo Clinic in Rochester, Minn., that creates a culturally sensitive space to care for this community.
As trainees transition to early faculty, becoming a mentor is a new role that can be very rewarding and daunting at the same time. Dr. Anna Lok, recipient of the AGA’s Distinguished Mentor Award, and Dr. Vincent Chen share invaluable experiences and advice on being a mentor from senior and early-career perspectives, respectively. Similarly in the transition to early faculty, Erin Anderson, CPA, answers five common financial questions that arise to better understand and manage a significant increase in salary.
Lastly, Dr. Shifa Umar describes her unique experience as part of the AGA’s annual Tech Summit Fellows Program, a cross-section of medicine, technology, and innovation.
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Jillian Schweitzer ([email protected]), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The concept of the clinicopathologic conference (CPC) was introduced by Dr. Walter B. Cannon as a medical student at Harvard Medical School.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Endoscopic Management of Benign Gallbladder Disease
Introduction
The treatment of benign gallbladder disease has changed substantially in the past decade, but this represents only a snapshot in the evolutionary history of the management of this organ. What began as a problem managed exclusively by open cholecystectomy (CCY) transitioned into a race toward minimally invasive approaches in the 1980s, with advances from gastroenterology, surgery, and radiology.
The opening strides were made in 1980 with the first description of percutaneous cholecystostomy (PC) by Dr. R.W. Radder.1 Shortly thereafter, in 1984, Dr. Richard Kozarek first reported the feasibility of selective cystic duct cannulation during endoscopic retrograde cholangiopancreatography (ERCP).2 Subsequent stenting for the treatment of acute cholecystitis (endoscopic transpapillary gallbladder drainage, ET-GBD) was then reported by Tamada et. al. in 1991.3 Not to be outdone, the first laparoscopic cholecystectomy (LC) was completed by Dr. Med Erich Mühe of Germany in 1985.4 More recently, with the expansion of interventional endoscopic ultrasound (EUS), the first transmural EUS-guided gallbladder drainage (EUS-GBD) was described by Dr. Baron and Dr. Topazian in 2007.5
The subsequent advent of lumen apposing metal stents (LAMS) has cemented EUS-GBD in the toolbox of treatment for benign gallbladder disease. Results of a recent prospective multicenter trial, with a Food and Drug Administration–approved protocol and investigational device exemption, have been published, opening the door for the expansion of FDA approved indications for this device.6
Benign gallbladder disease encompasses both polyps (benign and premalignant) and cholecystitis (acute/chronic, calculous/acalculous), in addition to others. The four management techniques (LC, PC, ET-GBD, and EUS-GBD) have filled integral niches in the management of these patients. Even gallbladder polyps have not been able to escape the reach of endoscopic approaches with the recent description of LAMS-assisted polypectomy as part of a gallbladder preserving strategy.7,8 While EUS-GBD also has been used for biliary decompression in the presence of a patent cystic duct and absence of cholecystitis, .9 Both of these techniques have gained wide recognition and/or guideline support for their use from the American Society for Gastrointestinal Endoscopy (ASGE) and the European Society of Gastrointestinal Endoscopy (ESGE).10,11 In addition, there is now one FDA-approved stent device for treatment of acute cholecystitis in patients unfit for surgery.
Techniques & Tips
ET-GBD
- During ERCP, after successful cannulation of the bile duct, attempted wire cannulation of the cystic duct is performed.
A cholangiogram, which clearly delineates the insertion of the cystic duct into the main bile duct, can enhance cannulation success. Rotatable fluoroscopy can facilitate identification.
- After anatomy is clear, wire access is often best achieved using a sphincterotome or stone retrieval (occlusion) balloon.
The balloon, once inflated, can be pulled downward to establish traction on the main bile duct, which can straighten the approach.
- After superficial wire engagement into the cystic duct, the accessory used can be slowly advanced into the cystic duct to stabilize the catheter and then navigate the valves of Heister to reach the gallbladder lumen.
Use of a sphincterotome, which directs toward the patient’s right (most often direction of cystic duct takeoff), is helpful. Angled guidewires are preferable. We often use a 0.035-inch, 260-cm angled hydrophilic wire (GLIDEWIRE; Terumo, Somerset, NJ) to overcome this challenging portion of ET-GBD.
If despite the above maneuvers the guidewire has failed to enter the cystic duct, cholangioscopy can be used to identify the orifice and/or stabilize deep wire cannulation. This is often cumbersome, time consuming, does not always produce success, and requires additional expertise.
- If a stone is encountered that cannot be extracted or traversed by a guidewire, cholangioscopy with electrohydraulic lithotripsy can be pursued.
- After the guidewire has entered the gallbladder, a 5 French or 7 French plastic double pigtail stent is placed. Typical lengths are 9-15 cm.
Some authors prefer to use two side-by-side plastic stents.12 This has been shown retrospectively to enhance the long term clinical success of ET-GBD but with additional technical difficulty.
- This stent can remain in place indefinitely and need not be exchanged, though it should be removed just prior to CCY if pursued. Alternatively, the surgeon can be alerted to its presence and, if comfortable, it can be removed intraoperatively.
EUS-GBD
- Use of fluoroscopy is optional but can enhance technical success in selected situations.
- Conversion, or internalization, of PC is reasonable and can enhance patient quality of life.13
- If the gallbladder wall is not in close apposition to the duodenal (or gastric) wall, consider measuring the distance.
We preferentially use 10-mm diameter by 10-mm saddle length LAMS for EUS-GBD, unless the above distance warrants use of a 15-mm by 15-mm LAMS (AXIOS, Boston Scientific, Marlborough, MA). If the distance is greater than 15 mm, consider searching for an alternative site, using a traditional biliary fully covered self-expandable metal stent (FCSEMS) for longer length, or converting to ET-GBD. Smaller diameter (8 mm) with an 8-mm saddle length can be used as well. The optimal diameter is unknown and also dependent on whether transluminal endoscopic diagnosis or therapy is a consideration.
- If there is difficulty locating the gallbladder, it may be decompressed or small (particularly if PC or a partial CCY has already been performed).
If a cholecystostomy tube is in place, instillation of sterile water via the tube can sometimes improve the target for LAMS placement, though caution should be made to not over-distend the gallbladder. ERCP with placement of a nasobiliary tube into the gallbladder can also serve this purpose and has been previously described.14
The gallbladder can be punctured with a 19-gauge FNA needle to instill sterile water and distend the gallbladder with the added benefit of being able to pass a guidewire, which may enhance procedural safety in difficult cases. However, success of this technique is contingent on fluid remaining within the gallbladder and not transiting out via the cystic duct. Expedient exchange of the FNA needle for the LAMS device may be necessary.
- Attempt to confirm location within the duodenum prior to puncture, as gastric origins can pose unique ramifications (i.e. potential for partial gastric outlet obstruction, obstruction of LAMS with food debris, etc.).
It can be easy to mistake an unintentional pre-pyloric position for a position within the duodenum since the working channel is behind (proximal to) the echoprobe.
- Turning off Doppler flow prior to advancement of the cautery enhanced LAMS can reduce obscurement of views on entry into the gallbladder. Lack of certainty about entry or misdeployment after presumed entry herald the most challenging aspect of EUS-GBD.
Utilization of a previously placed guidewire or advancement of one preloaded into the LAMS can aid in both enhancing confidence in location and assist with salvage maneuvers, if needed.
- After successful deployment of the LAMS we routinely place a double pigtail plastic stent through it (typically 7 French by 4 cm) to maintain patency. This may also prevent bleeding from the LAMS flange abrading the wall of either lumen.
- We routinely exchange the LAMS for two double pigtail plastic stents (typically 7 French by 4 cm) 4 weeks after initial placement especially when there is a more than modest residual stone burden (data in press). These plastic stents can remain in place indefinitely.
This exchange can be deferred if the patient is not expected to survive until the one-year anniversary of LAMS deployment. After one year the LAMS plastic covering may degrade and pose additional problems.15
LAMS Misdeployment Salvage Tips
- Salvage techniques can vary from simple to complex.
- If a wire is in place, it can be used to balloon or catheter dilate the tract and place a FCSEMS traversing the gallbladder and duodenal/gastric lumens. A similar approach can be used if the LAMS deployed on only one side (gallbladder or duodenum/stomach) and the other flange is within the peritoneum.
- The most challenging scenario to salvage is if the LAMS is misdeployed or becomes dislodged and no wire is present. This is why the use of a guidewire, even if preloaded into the LAMS and placement is freehand, is essential for EUS-GBD. A potential technique is to balloon dilate the duodenal/gastric defect and drive the endoscope into the peritoneum to reconnect that lumen to the gallbladder defect or LAMS, depending on the site of misdeployment. Doing so requires a high degree of commitment and skill and should not be done casually.
- If uncertainty remains or if misdeployment has occurred and salvage attempts have failed, consider closure of the duodenal/gastric defect and conversion to ET-GBD.
This may both treat the initial procedural indication and assist with what is essentially a large bile leak, which might also require percutaneous therapy for non-surgical management.
- For endoscopists with limited experience at salvage techniques, it is reasonable for the threshold for conversion to be low, assuming experience with and confidence in ET-GBD is high.
- If salvage is successful but ambiguity remains, consider obtaining a cholangiogram via the LAMS to confirm positioning and absence of leak.
Adverse Events
Both ET-GBD and EUS-GBD should be performed by an endoscopist comfortable with their techniques and the management of their adverse events (AEs). Rates for EUS-GBD AEs in patients at high risk for LC were reported in one international multicenter registry to be 15.3% with a 30-day mortality of 9.2%, with a significant predictor of AE being endoscopist experience less than 25 procedures.16 A meta-analysis also found an overall AE rate of 18.31%, with rates for perforation and stent related AEs (i.e. migration, occlusion, pneumoperitoneum) being 6.71% and 8.16%, respectively.17 For this reason, we recommend that patients with cholecystitis who are deemed to be poor surgical candidates be transferred to a tertiary referral center with expertise in these approaches. Rates of AEs for ET-GBD are similar to that for standard ERCP, with reported ranges of 5%-10.3%.10
Comparisons Between Techniques
The decision on which technique to utilize for endoscopic management of cholecystitis or symptomatic cholelithiasis depends first and foremost on the expertise and comfort level of the endoscopist. Given the additional training that an advanced endoscopist needs to perform EUS-GBD, combined with the perhaps slightly higher AE rate and permanency of endoscopic cholecystostomy, it is reasonable to proceed with a trial of ET-GBD if confidence is insufficient. However, ET-GBD can certainly be more technically challenging and less effective than EUS-GBD, with lower reported technical and clinical success rates (technical 85.3% vs 93.0%, clinical 95.2% vs 97.3%).18 Despite this, the rate of recurrence of cholecystitis is similar between ET-GBD and EUS-GBD (4.6% vs 4.2%).19 As stated above in the Techniques & Tips section, some authors utilize two plastic stents for ET-GBD for this purpose, though with increased technical difficulty. It is important to remember that these numbers, when paired with AE rates, represent the achievements of expert endoscopists.
Discussion with your surgery team is important when deciding modality. If the patient is felt to be a potential candidate for CCY, and EUS-GBD is not being used as a destination therapy, the surgeon may prefer ET-GBD. EUS-GBD may enhance the difficulty of CCY, though at least one study demonstrated that this was no different than PC with similar rates of conversion from LC to open CCY.20 This conversation is most critical for patients who are potential liver transplant candidates. For patients where this is not a consideration there is some evidence to suggest equivalency between LC and EUS-GBD, though certainly EUS-GBD has not yet supplanted LC as the treatment of choice.21
While there may eventually be a shift towards EUS-GBD instead of LC in certain patient groups, what is clearer are the advantages of EUS-GBD over PC. One recent meta-analysis revealed that EUS-GBD has significantly favorable odds of overall adverse events (OR 0.43, 95% CI 0.18-1.00), shorter hospital stay (2.76 less days, 95% CI 0.31-5.20 less days), reinterventions (OR 0.15, 95% CI 0.02-0.98), and unplanned readmissions (OR 0.14, 95% CI 0.03-0.70) compared to PC.22 Beyond the data, though, are the emotional and psychological impacts an external drain can have on a patient.
Conclusion
When expertise is available, endoscopic treatment of benign gallbladder disease has a definite role but should be undertaken only by those with the experience and skill to safely do so. Decision to proceed, especially with EUS-GBD, should be accompanied by conversation and collaboration with surgical teams. If a patient is under consideration for PC instead of LC, it may be worthwhile to seek consultation with a local center with expertise in EUS-GBD or ET-GBD. The adoption of these techniques is part of the paradigm shift, seen broadly throughout medicine, towards minimally invasive interventions, particularly in advanced endoscopy.
Dr. Gilman (X @a_gilman) and Dr. Baron (X @EndoTx) are with the University of North Carolina, Chapel Hill, Division of Gastroenterology & Hepatology. Dr. Gilman has no relevant financial disclosures. Dr. Baron is a consultant and speaker for Ambu, Boston Scientific, Cook Endoscopy, Medtronic, Olympus America, and W.L. Gore.
References
1. Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330-333.
2. Kozarek RA. Selective cannulation of the cystic duct at time of ERCP. J Clin Gastroenterol. 1984;6:37-40.
3. Tamada K et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991;23:2-3.
4. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5:89-94.
5. Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007 Apr;65(4):735-7. doi: 10.1016/j.gie.2006.07.041.
6. Irani SS et al. Endoscopic ultrasound-guided transluminal gallbladder drainage in patients with acute cholecystitis: A prospective multicenter trial. Ann Surg. 2023 Sep 1;278(3):e556-e562. doi: 10.1097/SLA.0000000000005784.
7. Shen Y et al. Endoscopic ultrasound-guided cholecystostomy for resection of gallbladder polyps with lumen-apposing metal stent. Medicine (Baltimore). 2020 Oct 23;99(43):e22903. doi: 10.1097/MD.0000000000022903.
8. Pang H et al. Endoscopic ultrasound-guided gallbladder endoscopic mucosal resection: A pilot porcine study. Minim Invasive Ther Allied Technol. 2023 Feb;32(1):24-32. doi: 10.1080/13645706.2022.2153228.
9. Imai H et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016 Jul;84(1):147-51. doi: 10.1016/j.gie.2015.12.024.
10. Saumoy M et al. Endoscopic therapies for gallbladder drainage. Gastrointest Endosc. 2021 Oct;94(4):671-84. doi: 10.1016/j.gie.2021.05.031.
11. Van der Merwe SW et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Feb;54(2):185-205. doi: 10.1055/a-1717-1391.
12. Storm AC et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest Endosc. 2021 Oct;94(4):742-8 e1. doi: 10.1016/j.gie.2021.03.025.
13. James TW, Baron TH. Converting percutaneous gallbladder drainage to internal drainage using EUS-guided therapy: A review of current practices and procedures. Endosc Ultrasound. 2018 Mar-Apr;7(2):93-6. doi: 10.4103/eus.eus_110_17.
14. James TW, Baron TH. Transpapillary nasocystic tube placement to allow gallbladder distention for EUS-guided cholecystoduodenostomy. VideoGIE. 2019 Dec;4(12):561-2. doi: 10.1016/j.vgie.2019.08.009.
15. Gilman AJ, Baron TH. Delamination of a lumen-apposing metal stent with tissue ingrowth and stent-in-stent removal. Gastrointest Endosc. 2023 Sep;98(3):451-3. doi: 10.1016/j.gie.2023.04.2087.
16. Teoh AY et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 2019 Aug;7(8):E964-E973. doi: 10.1055/a-0915-2098.
17. Kalva NR et al. Efficacy and safety of lumen apposing self-expandable metal stents for EUS guided cholecystostomy: A meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2018;2018:7070961. doi: 10.1155/2018/7070961.
18. Khan MA et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017 Jan;85(1):76-87 e3. doi: 10.1016/j.gie.2016.06.032.
19. Mohan BP et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020 Feb;52(2):96-106. doi: 10.1055/a-1020-3932.
20. Jang JW et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012 Apr;142(4):805-11. doi: 10.1053/j.gastro.2011.12.051.
21. Teoh AYB et al. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis: a propensity score analysis with 1-year follow-up data. Gastrointest Endosc. 2021 Mar;93(3):577-83. doi: 10.1016/j.gie.2020.06.066.
22. Luk SW et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019 Aug;51(8):722-32. doi: 10.1055/a-0929-6603.
Introduction
The treatment of benign gallbladder disease has changed substantially in the past decade, but this represents only a snapshot in the evolutionary history of the management of this organ. What began as a problem managed exclusively by open cholecystectomy (CCY) transitioned into a race toward minimally invasive approaches in the 1980s, with advances from gastroenterology, surgery, and radiology.
The opening strides were made in 1980 with the first description of percutaneous cholecystostomy (PC) by Dr. R.W. Radder.1 Shortly thereafter, in 1984, Dr. Richard Kozarek first reported the feasibility of selective cystic duct cannulation during endoscopic retrograde cholangiopancreatography (ERCP).2 Subsequent stenting for the treatment of acute cholecystitis (endoscopic transpapillary gallbladder drainage, ET-GBD) was then reported by Tamada et. al. in 1991.3 Not to be outdone, the first laparoscopic cholecystectomy (LC) was completed by Dr. Med Erich Mühe of Germany in 1985.4 More recently, with the expansion of interventional endoscopic ultrasound (EUS), the first transmural EUS-guided gallbladder drainage (EUS-GBD) was described by Dr. Baron and Dr. Topazian in 2007.5
The subsequent advent of lumen apposing metal stents (LAMS) has cemented EUS-GBD in the toolbox of treatment for benign gallbladder disease. Results of a recent prospective multicenter trial, with a Food and Drug Administration–approved protocol and investigational device exemption, have been published, opening the door for the expansion of FDA approved indications for this device.6
Benign gallbladder disease encompasses both polyps (benign and premalignant) and cholecystitis (acute/chronic, calculous/acalculous), in addition to others. The four management techniques (LC, PC, ET-GBD, and EUS-GBD) have filled integral niches in the management of these patients. Even gallbladder polyps have not been able to escape the reach of endoscopic approaches with the recent description of LAMS-assisted polypectomy as part of a gallbladder preserving strategy.7,8 While EUS-GBD also has been used for biliary decompression in the presence of a patent cystic duct and absence of cholecystitis, .9 Both of these techniques have gained wide recognition and/or guideline support for their use from the American Society for Gastrointestinal Endoscopy (ASGE) and the European Society of Gastrointestinal Endoscopy (ESGE).10,11 In addition, there is now one FDA-approved stent device for treatment of acute cholecystitis in patients unfit for surgery.
Techniques & Tips
ET-GBD
- During ERCP, after successful cannulation of the bile duct, attempted wire cannulation of the cystic duct is performed.
A cholangiogram, which clearly delineates the insertion of the cystic duct into the main bile duct, can enhance cannulation success. Rotatable fluoroscopy can facilitate identification.
- After anatomy is clear, wire access is often best achieved using a sphincterotome or stone retrieval (occlusion) balloon.
The balloon, once inflated, can be pulled downward to establish traction on the main bile duct, which can straighten the approach.
- After superficial wire engagement into the cystic duct, the accessory used can be slowly advanced into the cystic duct to stabilize the catheter and then navigate the valves of Heister to reach the gallbladder lumen.
Use of a sphincterotome, which directs toward the patient’s right (most often direction of cystic duct takeoff), is helpful. Angled guidewires are preferable. We often use a 0.035-inch, 260-cm angled hydrophilic wire (GLIDEWIRE; Terumo, Somerset, NJ) to overcome this challenging portion of ET-GBD.
If despite the above maneuvers the guidewire has failed to enter the cystic duct, cholangioscopy can be used to identify the orifice and/or stabilize deep wire cannulation. This is often cumbersome, time consuming, does not always produce success, and requires additional expertise.
- If a stone is encountered that cannot be extracted or traversed by a guidewire, cholangioscopy with electrohydraulic lithotripsy can be pursued.
- After the guidewire has entered the gallbladder, a 5 French or 7 French plastic double pigtail stent is placed. Typical lengths are 9-15 cm.
Some authors prefer to use two side-by-side plastic stents.12 This has been shown retrospectively to enhance the long term clinical success of ET-GBD but with additional technical difficulty.
- This stent can remain in place indefinitely and need not be exchanged, though it should be removed just prior to CCY if pursued. Alternatively, the surgeon can be alerted to its presence and, if comfortable, it can be removed intraoperatively.
EUS-GBD
- Use of fluoroscopy is optional but can enhance technical success in selected situations.
- Conversion, or internalization, of PC is reasonable and can enhance patient quality of life.13
- If the gallbladder wall is not in close apposition to the duodenal (or gastric) wall, consider measuring the distance.
We preferentially use 10-mm diameter by 10-mm saddle length LAMS for EUS-GBD, unless the above distance warrants use of a 15-mm by 15-mm LAMS (AXIOS, Boston Scientific, Marlborough, MA). If the distance is greater than 15 mm, consider searching for an alternative site, using a traditional biliary fully covered self-expandable metal stent (FCSEMS) for longer length, or converting to ET-GBD. Smaller diameter (8 mm) with an 8-mm saddle length can be used as well. The optimal diameter is unknown and also dependent on whether transluminal endoscopic diagnosis or therapy is a consideration.
- If there is difficulty locating the gallbladder, it may be decompressed or small (particularly if PC or a partial CCY has already been performed).
If a cholecystostomy tube is in place, instillation of sterile water via the tube can sometimes improve the target for LAMS placement, though caution should be made to not over-distend the gallbladder. ERCP with placement of a nasobiliary tube into the gallbladder can also serve this purpose and has been previously described.14
The gallbladder can be punctured with a 19-gauge FNA needle to instill sterile water and distend the gallbladder with the added benefit of being able to pass a guidewire, which may enhance procedural safety in difficult cases. However, success of this technique is contingent on fluid remaining within the gallbladder and not transiting out via the cystic duct. Expedient exchange of the FNA needle for the LAMS device may be necessary.
- Attempt to confirm location within the duodenum prior to puncture, as gastric origins can pose unique ramifications (i.e. potential for partial gastric outlet obstruction, obstruction of LAMS with food debris, etc.).
It can be easy to mistake an unintentional pre-pyloric position for a position within the duodenum since the working channel is behind (proximal to) the echoprobe.
- Turning off Doppler flow prior to advancement of the cautery enhanced LAMS can reduce obscurement of views on entry into the gallbladder. Lack of certainty about entry or misdeployment after presumed entry herald the most challenging aspect of EUS-GBD.
Utilization of a previously placed guidewire or advancement of one preloaded into the LAMS can aid in both enhancing confidence in location and assist with salvage maneuvers, if needed.
- After successful deployment of the LAMS we routinely place a double pigtail plastic stent through it (typically 7 French by 4 cm) to maintain patency. This may also prevent bleeding from the LAMS flange abrading the wall of either lumen.
- We routinely exchange the LAMS for two double pigtail plastic stents (typically 7 French by 4 cm) 4 weeks after initial placement especially when there is a more than modest residual stone burden (data in press). These plastic stents can remain in place indefinitely.
This exchange can be deferred if the patient is not expected to survive until the one-year anniversary of LAMS deployment. After one year the LAMS plastic covering may degrade and pose additional problems.15
LAMS Misdeployment Salvage Tips
- Salvage techniques can vary from simple to complex.
- If a wire is in place, it can be used to balloon or catheter dilate the tract and place a FCSEMS traversing the gallbladder and duodenal/gastric lumens. A similar approach can be used if the LAMS deployed on only one side (gallbladder or duodenum/stomach) and the other flange is within the peritoneum.
- The most challenging scenario to salvage is if the LAMS is misdeployed or becomes dislodged and no wire is present. This is why the use of a guidewire, even if preloaded into the LAMS and placement is freehand, is essential for EUS-GBD. A potential technique is to balloon dilate the duodenal/gastric defect and drive the endoscope into the peritoneum to reconnect that lumen to the gallbladder defect or LAMS, depending on the site of misdeployment. Doing so requires a high degree of commitment and skill and should not be done casually.
- If uncertainty remains or if misdeployment has occurred and salvage attempts have failed, consider closure of the duodenal/gastric defect and conversion to ET-GBD.
This may both treat the initial procedural indication and assist with what is essentially a large bile leak, which might also require percutaneous therapy for non-surgical management.
- For endoscopists with limited experience at salvage techniques, it is reasonable for the threshold for conversion to be low, assuming experience with and confidence in ET-GBD is high.
- If salvage is successful but ambiguity remains, consider obtaining a cholangiogram via the LAMS to confirm positioning and absence of leak.
Adverse Events
Both ET-GBD and EUS-GBD should be performed by an endoscopist comfortable with their techniques and the management of their adverse events (AEs). Rates for EUS-GBD AEs in patients at high risk for LC were reported in one international multicenter registry to be 15.3% with a 30-day mortality of 9.2%, with a significant predictor of AE being endoscopist experience less than 25 procedures.16 A meta-analysis also found an overall AE rate of 18.31%, with rates for perforation and stent related AEs (i.e. migration, occlusion, pneumoperitoneum) being 6.71% and 8.16%, respectively.17 For this reason, we recommend that patients with cholecystitis who are deemed to be poor surgical candidates be transferred to a tertiary referral center with expertise in these approaches. Rates of AEs for ET-GBD are similar to that for standard ERCP, with reported ranges of 5%-10.3%.10
Comparisons Between Techniques
The decision on which technique to utilize for endoscopic management of cholecystitis or symptomatic cholelithiasis depends first and foremost on the expertise and comfort level of the endoscopist. Given the additional training that an advanced endoscopist needs to perform EUS-GBD, combined with the perhaps slightly higher AE rate and permanency of endoscopic cholecystostomy, it is reasonable to proceed with a trial of ET-GBD if confidence is insufficient. However, ET-GBD can certainly be more technically challenging and less effective than EUS-GBD, with lower reported technical and clinical success rates (technical 85.3% vs 93.0%, clinical 95.2% vs 97.3%).18 Despite this, the rate of recurrence of cholecystitis is similar between ET-GBD and EUS-GBD (4.6% vs 4.2%).19 As stated above in the Techniques & Tips section, some authors utilize two plastic stents for ET-GBD for this purpose, though with increased technical difficulty. It is important to remember that these numbers, when paired with AE rates, represent the achievements of expert endoscopists.
Discussion with your surgery team is important when deciding modality. If the patient is felt to be a potential candidate for CCY, and EUS-GBD is not being used as a destination therapy, the surgeon may prefer ET-GBD. EUS-GBD may enhance the difficulty of CCY, though at least one study demonstrated that this was no different than PC with similar rates of conversion from LC to open CCY.20 This conversation is most critical for patients who are potential liver transplant candidates. For patients where this is not a consideration there is some evidence to suggest equivalency between LC and EUS-GBD, though certainly EUS-GBD has not yet supplanted LC as the treatment of choice.21
While there may eventually be a shift towards EUS-GBD instead of LC in certain patient groups, what is clearer are the advantages of EUS-GBD over PC. One recent meta-analysis revealed that EUS-GBD has significantly favorable odds of overall adverse events (OR 0.43, 95% CI 0.18-1.00), shorter hospital stay (2.76 less days, 95% CI 0.31-5.20 less days), reinterventions (OR 0.15, 95% CI 0.02-0.98), and unplanned readmissions (OR 0.14, 95% CI 0.03-0.70) compared to PC.22 Beyond the data, though, are the emotional and psychological impacts an external drain can have on a patient.
Conclusion
When expertise is available, endoscopic treatment of benign gallbladder disease has a definite role but should be undertaken only by those with the experience and skill to safely do so. Decision to proceed, especially with EUS-GBD, should be accompanied by conversation and collaboration with surgical teams. If a patient is under consideration for PC instead of LC, it may be worthwhile to seek consultation with a local center with expertise in EUS-GBD or ET-GBD. The adoption of these techniques is part of the paradigm shift, seen broadly throughout medicine, towards minimally invasive interventions, particularly in advanced endoscopy.
Dr. Gilman (X @a_gilman) and Dr. Baron (X @EndoTx) are with the University of North Carolina, Chapel Hill, Division of Gastroenterology & Hepatology. Dr. Gilman has no relevant financial disclosures. Dr. Baron is a consultant and speaker for Ambu, Boston Scientific, Cook Endoscopy, Medtronic, Olympus America, and W.L. Gore.
References
1. Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330-333.
2. Kozarek RA. Selective cannulation of the cystic duct at time of ERCP. J Clin Gastroenterol. 1984;6:37-40.
3. Tamada K et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991;23:2-3.
4. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5:89-94.
5. Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007 Apr;65(4):735-7. doi: 10.1016/j.gie.2006.07.041.
6. Irani SS et al. Endoscopic ultrasound-guided transluminal gallbladder drainage in patients with acute cholecystitis: A prospective multicenter trial. Ann Surg. 2023 Sep 1;278(3):e556-e562. doi: 10.1097/SLA.0000000000005784.
7. Shen Y et al. Endoscopic ultrasound-guided cholecystostomy for resection of gallbladder polyps with lumen-apposing metal stent. Medicine (Baltimore). 2020 Oct 23;99(43):e22903. doi: 10.1097/MD.0000000000022903.
8. Pang H et al. Endoscopic ultrasound-guided gallbladder endoscopic mucosal resection: A pilot porcine study. Minim Invasive Ther Allied Technol. 2023 Feb;32(1):24-32. doi: 10.1080/13645706.2022.2153228.
9. Imai H et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016 Jul;84(1):147-51. doi: 10.1016/j.gie.2015.12.024.
10. Saumoy M et al. Endoscopic therapies for gallbladder drainage. Gastrointest Endosc. 2021 Oct;94(4):671-84. doi: 10.1016/j.gie.2021.05.031.
11. Van der Merwe SW et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Feb;54(2):185-205. doi: 10.1055/a-1717-1391.
12. Storm AC et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest Endosc. 2021 Oct;94(4):742-8 e1. doi: 10.1016/j.gie.2021.03.025.
13. James TW, Baron TH. Converting percutaneous gallbladder drainage to internal drainage using EUS-guided therapy: A review of current practices and procedures. Endosc Ultrasound. 2018 Mar-Apr;7(2):93-6. doi: 10.4103/eus.eus_110_17.
14. James TW, Baron TH. Transpapillary nasocystic tube placement to allow gallbladder distention for EUS-guided cholecystoduodenostomy. VideoGIE. 2019 Dec;4(12):561-2. doi: 10.1016/j.vgie.2019.08.009.
15. Gilman AJ, Baron TH. Delamination of a lumen-apposing metal stent with tissue ingrowth and stent-in-stent removal. Gastrointest Endosc. 2023 Sep;98(3):451-3. doi: 10.1016/j.gie.2023.04.2087.
16. Teoh AY et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 2019 Aug;7(8):E964-E973. doi: 10.1055/a-0915-2098.
17. Kalva NR et al. Efficacy and safety of lumen apposing self-expandable metal stents for EUS guided cholecystostomy: A meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2018;2018:7070961. doi: 10.1155/2018/7070961.
18. Khan MA et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017 Jan;85(1):76-87 e3. doi: 10.1016/j.gie.2016.06.032.
19. Mohan BP et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020 Feb;52(2):96-106. doi: 10.1055/a-1020-3932.
20. Jang JW et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012 Apr;142(4):805-11. doi: 10.1053/j.gastro.2011.12.051.
21. Teoh AYB et al. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis: a propensity score analysis with 1-year follow-up data. Gastrointest Endosc. 2021 Mar;93(3):577-83. doi: 10.1016/j.gie.2020.06.066.
22. Luk SW et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019 Aug;51(8):722-32. doi: 10.1055/a-0929-6603.
Introduction
The treatment of benign gallbladder disease has changed substantially in the past decade, but this represents only a snapshot in the evolutionary history of the management of this organ. What began as a problem managed exclusively by open cholecystectomy (CCY) transitioned into a race toward minimally invasive approaches in the 1980s, with advances from gastroenterology, surgery, and radiology.
The opening strides were made in 1980 with the first description of percutaneous cholecystostomy (PC) by Dr. R.W. Radder.1 Shortly thereafter, in 1984, Dr. Richard Kozarek first reported the feasibility of selective cystic duct cannulation during endoscopic retrograde cholangiopancreatography (ERCP).2 Subsequent stenting for the treatment of acute cholecystitis (endoscopic transpapillary gallbladder drainage, ET-GBD) was then reported by Tamada et. al. in 1991.3 Not to be outdone, the first laparoscopic cholecystectomy (LC) was completed by Dr. Med Erich Mühe of Germany in 1985.4 More recently, with the expansion of interventional endoscopic ultrasound (EUS), the first transmural EUS-guided gallbladder drainage (EUS-GBD) was described by Dr. Baron and Dr. Topazian in 2007.5
The subsequent advent of lumen apposing metal stents (LAMS) has cemented EUS-GBD in the toolbox of treatment for benign gallbladder disease. Results of a recent prospective multicenter trial, with a Food and Drug Administration–approved protocol and investigational device exemption, have been published, opening the door for the expansion of FDA approved indications for this device.6
Benign gallbladder disease encompasses both polyps (benign and premalignant) and cholecystitis (acute/chronic, calculous/acalculous), in addition to others. The four management techniques (LC, PC, ET-GBD, and EUS-GBD) have filled integral niches in the management of these patients. Even gallbladder polyps have not been able to escape the reach of endoscopic approaches with the recent description of LAMS-assisted polypectomy as part of a gallbladder preserving strategy.7,8 While EUS-GBD also has been used for biliary decompression in the presence of a patent cystic duct and absence of cholecystitis, .9 Both of these techniques have gained wide recognition and/or guideline support for their use from the American Society for Gastrointestinal Endoscopy (ASGE) and the European Society of Gastrointestinal Endoscopy (ESGE).10,11 In addition, there is now one FDA-approved stent device for treatment of acute cholecystitis in patients unfit for surgery.
Techniques & Tips
ET-GBD
- During ERCP, after successful cannulation of the bile duct, attempted wire cannulation of the cystic duct is performed.
A cholangiogram, which clearly delineates the insertion of the cystic duct into the main bile duct, can enhance cannulation success. Rotatable fluoroscopy can facilitate identification.
- After anatomy is clear, wire access is often best achieved using a sphincterotome or stone retrieval (occlusion) balloon.
The balloon, once inflated, can be pulled downward to establish traction on the main bile duct, which can straighten the approach.
- After superficial wire engagement into the cystic duct, the accessory used can be slowly advanced into the cystic duct to stabilize the catheter and then navigate the valves of Heister to reach the gallbladder lumen.
Use of a sphincterotome, which directs toward the patient’s right (most often direction of cystic duct takeoff), is helpful. Angled guidewires are preferable. We often use a 0.035-inch, 260-cm angled hydrophilic wire (GLIDEWIRE; Terumo, Somerset, NJ) to overcome this challenging portion of ET-GBD.
If despite the above maneuvers the guidewire has failed to enter the cystic duct, cholangioscopy can be used to identify the orifice and/or stabilize deep wire cannulation. This is often cumbersome, time consuming, does not always produce success, and requires additional expertise.
- If a stone is encountered that cannot be extracted or traversed by a guidewire, cholangioscopy with electrohydraulic lithotripsy can be pursued.
- After the guidewire has entered the gallbladder, a 5 French or 7 French plastic double pigtail stent is placed. Typical lengths are 9-15 cm.
Some authors prefer to use two side-by-side plastic stents.12 This has been shown retrospectively to enhance the long term clinical success of ET-GBD but with additional technical difficulty.
- This stent can remain in place indefinitely and need not be exchanged, though it should be removed just prior to CCY if pursued. Alternatively, the surgeon can be alerted to its presence and, if comfortable, it can be removed intraoperatively.
EUS-GBD
- Use of fluoroscopy is optional but can enhance technical success in selected situations.
- Conversion, or internalization, of PC is reasonable and can enhance patient quality of life.13
- If the gallbladder wall is not in close apposition to the duodenal (or gastric) wall, consider measuring the distance.
We preferentially use 10-mm diameter by 10-mm saddle length LAMS for EUS-GBD, unless the above distance warrants use of a 15-mm by 15-mm LAMS (AXIOS, Boston Scientific, Marlborough, MA). If the distance is greater than 15 mm, consider searching for an alternative site, using a traditional biliary fully covered self-expandable metal stent (FCSEMS) for longer length, or converting to ET-GBD. Smaller diameter (8 mm) with an 8-mm saddle length can be used as well. The optimal diameter is unknown and also dependent on whether transluminal endoscopic diagnosis or therapy is a consideration.
- If there is difficulty locating the gallbladder, it may be decompressed or small (particularly if PC or a partial CCY has already been performed).
If a cholecystostomy tube is in place, instillation of sterile water via the tube can sometimes improve the target for LAMS placement, though caution should be made to not over-distend the gallbladder. ERCP with placement of a nasobiliary tube into the gallbladder can also serve this purpose and has been previously described.14
The gallbladder can be punctured with a 19-gauge FNA needle to instill sterile water and distend the gallbladder with the added benefit of being able to pass a guidewire, which may enhance procedural safety in difficult cases. However, success of this technique is contingent on fluid remaining within the gallbladder and not transiting out via the cystic duct. Expedient exchange of the FNA needle for the LAMS device may be necessary.
- Attempt to confirm location within the duodenum prior to puncture, as gastric origins can pose unique ramifications (i.e. potential for partial gastric outlet obstruction, obstruction of LAMS with food debris, etc.).
It can be easy to mistake an unintentional pre-pyloric position for a position within the duodenum since the working channel is behind (proximal to) the echoprobe.
- Turning off Doppler flow prior to advancement of the cautery enhanced LAMS can reduce obscurement of views on entry into the gallbladder. Lack of certainty about entry or misdeployment after presumed entry herald the most challenging aspect of EUS-GBD.
Utilization of a previously placed guidewire or advancement of one preloaded into the LAMS can aid in both enhancing confidence in location and assist with salvage maneuvers, if needed.
- After successful deployment of the LAMS we routinely place a double pigtail plastic stent through it (typically 7 French by 4 cm) to maintain patency. This may also prevent bleeding from the LAMS flange abrading the wall of either lumen.
- We routinely exchange the LAMS for two double pigtail plastic stents (typically 7 French by 4 cm) 4 weeks after initial placement especially when there is a more than modest residual stone burden (data in press). These plastic stents can remain in place indefinitely.
This exchange can be deferred if the patient is not expected to survive until the one-year anniversary of LAMS deployment. After one year the LAMS plastic covering may degrade and pose additional problems.15
LAMS Misdeployment Salvage Tips
- Salvage techniques can vary from simple to complex.
- If a wire is in place, it can be used to balloon or catheter dilate the tract and place a FCSEMS traversing the gallbladder and duodenal/gastric lumens. A similar approach can be used if the LAMS deployed on only one side (gallbladder or duodenum/stomach) and the other flange is within the peritoneum.
- The most challenging scenario to salvage is if the LAMS is misdeployed or becomes dislodged and no wire is present. This is why the use of a guidewire, even if preloaded into the LAMS and placement is freehand, is essential for EUS-GBD. A potential technique is to balloon dilate the duodenal/gastric defect and drive the endoscope into the peritoneum to reconnect that lumen to the gallbladder defect or LAMS, depending on the site of misdeployment. Doing so requires a high degree of commitment and skill and should not be done casually.
- If uncertainty remains or if misdeployment has occurred and salvage attempts have failed, consider closure of the duodenal/gastric defect and conversion to ET-GBD.
This may both treat the initial procedural indication and assist with what is essentially a large bile leak, which might also require percutaneous therapy for non-surgical management.
- For endoscopists with limited experience at salvage techniques, it is reasonable for the threshold for conversion to be low, assuming experience with and confidence in ET-GBD is high.
- If salvage is successful but ambiguity remains, consider obtaining a cholangiogram via the LAMS to confirm positioning and absence of leak.
Adverse Events
Both ET-GBD and EUS-GBD should be performed by an endoscopist comfortable with their techniques and the management of their adverse events (AEs). Rates for EUS-GBD AEs in patients at high risk for LC were reported in one international multicenter registry to be 15.3% with a 30-day mortality of 9.2%, with a significant predictor of AE being endoscopist experience less than 25 procedures.16 A meta-analysis also found an overall AE rate of 18.31%, with rates for perforation and stent related AEs (i.e. migration, occlusion, pneumoperitoneum) being 6.71% and 8.16%, respectively.17 For this reason, we recommend that patients with cholecystitis who are deemed to be poor surgical candidates be transferred to a tertiary referral center with expertise in these approaches. Rates of AEs for ET-GBD are similar to that for standard ERCP, with reported ranges of 5%-10.3%.10
Comparisons Between Techniques
The decision on which technique to utilize for endoscopic management of cholecystitis or symptomatic cholelithiasis depends first and foremost on the expertise and comfort level of the endoscopist. Given the additional training that an advanced endoscopist needs to perform EUS-GBD, combined with the perhaps slightly higher AE rate and permanency of endoscopic cholecystostomy, it is reasonable to proceed with a trial of ET-GBD if confidence is insufficient. However, ET-GBD can certainly be more technically challenging and less effective than EUS-GBD, with lower reported technical and clinical success rates (technical 85.3% vs 93.0%, clinical 95.2% vs 97.3%).18 Despite this, the rate of recurrence of cholecystitis is similar between ET-GBD and EUS-GBD (4.6% vs 4.2%).19 As stated above in the Techniques & Tips section, some authors utilize two plastic stents for ET-GBD for this purpose, though with increased technical difficulty. It is important to remember that these numbers, when paired with AE rates, represent the achievements of expert endoscopists.
Discussion with your surgery team is important when deciding modality. If the patient is felt to be a potential candidate for CCY, and EUS-GBD is not being used as a destination therapy, the surgeon may prefer ET-GBD. EUS-GBD may enhance the difficulty of CCY, though at least one study demonstrated that this was no different than PC with similar rates of conversion from LC to open CCY.20 This conversation is most critical for patients who are potential liver transplant candidates. For patients where this is not a consideration there is some evidence to suggest equivalency between LC and EUS-GBD, though certainly EUS-GBD has not yet supplanted LC as the treatment of choice.21
While there may eventually be a shift towards EUS-GBD instead of LC in certain patient groups, what is clearer are the advantages of EUS-GBD over PC. One recent meta-analysis revealed that EUS-GBD has significantly favorable odds of overall adverse events (OR 0.43, 95% CI 0.18-1.00), shorter hospital stay (2.76 less days, 95% CI 0.31-5.20 less days), reinterventions (OR 0.15, 95% CI 0.02-0.98), and unplanned readmissions (OR 0.14, 95% CI 0.03-0.70) compared to PC.22 Beyond the data, though, are the emotional and psychological impacts an external drain can have on a patient.
Conclusion
When expertise is available, endoscopic treatment of benign gallbladder disease has a definite role but should be undertaken only by those with the experience and skill to safely do so. Decision to proceed, especially with EUS-GBD, should be accompanied by conversation and collaboration with surgical teams. If a patient is under consideration for PC instead of LC, it may be worthwhile to seek consultation with a local center with expertise in EUS-GBD or ET-GBD. The adoption of these techniques is part of the paradigm shift, seen broadly throughout medicine, towards minimally invasive interventions, particularly in advanced endoscopy.
Dr. Gilman (X @a_gilman) and Dr. Baron (X @EndoTx) are with the University of North Carolina, Chapel Hill, Division of Gastroenterology & Hepatology. Dr. Gilman has no relevant financial disclosures. Dr. Baron is a consultant and speaker for Ambu, Boston Scientific, Cook Endoscopy, Medtronic, Olympus America, and W.L. Gore.
References
1. Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330-333.
2. Kozarek RA. Selective cannulation of the cystic duct at time of ERCP. J Clin Gastroenterol. 1984;6:37-40.
3. Tamada K et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991;23:2-3.
4. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5:89-94.
5. Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007 Apr;65(4):735-7. doi: 10.1016/j.gie.2006.07.041.
6. Irani SS et al. Endoscopic ultrasound-guided transluminal gallbladder drainage in patients with acute cholecystitis: A prospective multicenter trial. Ann Surg. 2023 Sep 1;278(3):e556-e562. doi: 10.1097/SLA.0000000000005784.
7. Shen Y et al. Endoscopic ultrasound-guided cholecystostomy for resection of gallbladder polyps with lumen-apposing metal stent. Medicine (Baltimore). 2020 Oct 23;99(43):e22903. doi: 10.1097/MD.0000000000022903.
8. Pang H et al. Endoscopic ultrasound-guided gallbladder endoscopic mucosal resection: A pilot porcine study. Minim Invasive Ther Allied Technol. 2023 Feb;32(1):24-32. doi: 10.1080/13645706.2022.2153228.
9. Imai H et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016 Jul;84(1):147-51. doi: 10.1016/j.gie.2015.12.024.
10. Saumoy M et al. Endoscopic therapies for gallbladder drainage. Gastrointest Endosc. 2021 Oct;94(4):671-84. doi: 10.1016/j.gie.2021.05.031.
11. Van der Merwe SW et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Feb;54(2):185-205. doi: 10.1055/a-1717-1391.
12. Storm AC et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest Endosc. 2021 Oct;94(4):742-8 e1. doi: 10.1016/j.gie.2021.03.025.
13. James TW, Baron TH. Converting percutaneous gallbladder drainage to internal drainage using EUS-guided therapy: A review of current practices and procedures. Endosc Ultrasound. 2018 Mar-Apr;7(2):93-6. doi: 10.4103/eus.eus_110_17.
14. James TW, Baron TH. Transpapillary nasocystic tube placement to allow gallbladder distention for EUS-guided cholecystoduodenostomy. VideoGIE. 2019 Dec;4(12):561-2. doi: 10.1016/j.vgie.2019.08.009.
15. Gilman AJ, Baron TH. Delamination of a lumen-apposing metal stent with tissue ingrowth and stent-in-stent removal. Gastrointest Endosc. 2023 Sep;98(3):451-3. doi: 10.1016/j.gie.2023.04.2087.
16. Teoh AY et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 2019 Aug;7(8):E964-E973. doi: 10.1055/a-0915-2098.
17. Kalva NR et al. Efficacy and safety of lumen apposing self-expandable metal stents for EUS guided cholecystostomy: A meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2018;2018:7070961. doi: 10.1155/2018/7070961.
18. Khan MA et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017 Jan;85(1):76-87 e3. doi: 10.1016/j.gie.2016.06.032.
19. Mohan BP et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020 Feb;52(2):96-106. doi: 10.1055/a-1020-3932.
20. Jang JW et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012 Apr;142(4):805-11. doi: 10.1053/j.gastro.2011.12.051.
21. Teoh AYB et al. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis: a propensity score analysis with 1-year follow-up data. Gastrointest Endosc. 2021 Mar;93(3):577-83. doi: 10.1016/j.gie.2020.06.066.
22. Luk SW et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019 Aug;51(8):722-32. doi: 10.1055/a-0929-6603.
February 2024 – ICYMI
Gastroenterology
October 2023
El-Salhy M et al. Efficacy of Fecal Microbiota Transplantation for Patients With Irritable Bowel Syndrome at 3 Years After Transplantation. Gastroenterology. 2022 Oct;163(4):982-994.e14. doi: 10.1053/j.gastro.2022.06.020. Epub 2022 Jun 14. PMID: 35709830.
Bajaj JS and Nagy LE. Natural History of Alcohol-Associated Liver Disease: Understanding the Changing Landscape of Pathophysiology and Patient Care. Gastroenterology. 2022 Oct;163(4):840-851. doi: 10.1053/j.gastro.2022.05.031. Epub 2022 May 19. PMID: 35598629; PMCID: PMC9509416.
Lo CH et al. Association of Proton Pump Inhibitor Use With All-Cause and Cause-Specific Mortality. Gastroenterology. 2022 Oct;163(4):852-861.e2. doi: 10.1053/j.gastro.2022.06.067. Epub 2022 Jul 1. PMID: 35788344; PMCID: PMC9509450.
November 2023
Khoshiwal AM et al. The Tissue Systems Pathology Test Outperforms Pathology Review in Risk Stratifying Patients With Low-Grade Dysplasia. Gastroenterology. 2023 Nov;165(5):1168-1179.e6. doi: 10.1053/j.gastro.2023.07.029. Epub 2023 Aug 30. PMID: 37657759.
Chen YI et al. Endoscopic Ultrasound-Guided Biliary Drainage of First Intent With a Lumen-Apposing Metal Stent vs Endoscopic Retrograde Cholangiopancreatography in Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Study (ELEMENT Trial). Gastroenterology. 2023 Nov;165(5):1249-1261.e5. doi: 10.1053/j.gastro.2023.07.024. Epub 2023 Aug 6. PMID: 37549753.
December 2023
Almario CV et al. Prevalence and Burden of Illness of Rome IV Irritable Bowel Syndrome in the United States: Results From a Nationwide Cross-Sectional Study. Gastroenterology. 2023 Dec;165(6):1475-1487. doi: 10.1053/j.gastro.2023.08.010. Epub 2023 Aug 16. PMID: 37595647.
Koopmann BDM et al. The Natural Disease Course of Pancreatic Cyst-Associated Neoplasia, Dysplasia, and Ductal Adenocarcinoma: Results of a Microsimulation Model. Gastroenterology. 2023 Dec;165(6):1522-1532. doi: 10.1053/j.gastro.2023.08.027. Epub 2023 Aug 24. PMID: 37633497.
Clinical Gastroenterology and Hepatology
October 2023
Jung DH et al. Comparison of a Polysaccharide Hemostatic Powder and Conventional Therapy for Peptic Ulcer Bleeding. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2844-2253.e5. doi: 10.1016/j.cgh.2023.02.031. Epub 2023 Mar 10. PMID: 36906081.
Liang PS et al. Blood Test Increases Colorectal Cancer Screening in Persons Who Declined Colonoscopy and Fecal Immunochemical Test: A Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2951-2957.e2. doi: 10.1016/j.cgh.2023.03.036. Epub 2023 Apr 8. PMID: 37037262; PMCID: PMC10523873.
November 2023
Li YK et al. Risk of Postcolonoscopy Thromboembolic Events: A Real-World Cohort Study. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3051-3059.e4. doi: 10.1016/j.cgh.2022.09.021. Epub 2022 Sep 24. PMID: 36167228.
Tome J et al. Bile Acid Sequestrants in Microscopic Colitis: Clinical Outcomes and Utility of Bile Acid Testing. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3125-3131.e2. doi: 10.1016/j.cgh.2023.04.031. Epub 2023 May 10. PMID: 37172800.
Berry SK et al. A Randomized Parallel-group Study of Digital Gut-directed Hypnotherapy vs Muscle Relaxation for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3152-3159.e2. doi: 10.1016/j.cgh.2023.06.015. Epub 2023 Jun 28. PMID: 37391055.
December 2023
Kanwal F et al. Risk Stratification Model for Hepatocellular Cancer in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3296-3304.e3. doi: 10.1016/j.cgh.2023.04.019. Epub 2023 Apr 30. PMID: 37390101; PMCID: PMC10661677.
Forss A et al. Patients With Microscopic Colitis Are at Higher Risk of Major Adverse Cardiovascular Events: A Matched Cohort Study. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3356-3364.e9. doi: 10.1016/j.cgh.2023.05.014. Epub 2023 May 26. PMID: 37245713.
Zheng T et al. A Randomized, Controlled Trial of Efficacy and Safety of Cannabidiol in Idiopathic and Diabetic Gastroparesis. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3405-3414.e4. doi: 10.1016/j.cgh.2023.07.008. Epub 2023 Jul 22. PMID: 37482172.
Techniques and Innovations in Gastrointestinal Endoscopy
Rengarajan A and Aadam A. Peroral Endoscopic Myotomy (POEM) and Its Use in Esophageal Dysmotility. Tech Innov Gastrointest Endosc. 2023 Dec 16. doi: 10.1016/j.tige.2023.12.004.
Wang D et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2023 Nov 7. doi: 10.1016/j.tige.2023.10.003
Gastro Hep Advances
Gregory MH et al. Short Bowel Syndrome: Transition of Pediatric Patients to Adult Gastroenterology Care. Gastro Hep Advances. 2023 Sep 8. doi: 10.1016/j.gastha.2023.09.006.
Viser AC et al. Inflammatory Bowel Disease Patients in the Ambulatory Setting Commonly Screen Positive for Malnutrition. Gastro Hep Advances. 2023 Nov 16. doi: 10.1016/j.gastha.2023.11.007.
Gastroenterology
October 2023
El-Salhy M et al. Efficacy of Fecal Microbiota Transplantation for Patients With Irritable Bowel Syndrome at 3 Years After Transplantation. Gastroenterology. 2022 Oct;163(4):982-994.e14. doi: 10.1053/j.gastro.2022.06.020. Epub 2022 Jun 14. PMID: 35709830.
Bajaj JS and Nagy LE. Natural History of Alcohol-Associated Liver Disease: Understanding the Changing Landscape of Pathophysiology and Patient Care. Gastroenterology. 2022 Oct;163(4):840-851. doi: 10.1053/j.gastro.2022.05.031. Epub 2022 May 19. PMID: 35598629; PMCID: PMC9509416.
Lo CH et al. Association of Proton Pump Inhibitor Use With All-Cause and Cause-Specific Mortality. Gastroenterology. 2022 Oct;163(4):852-861.e2. doi: 10.1053/j.gastro.2022.06.067. Epub 2022 Jul 1. PMID: 35788344; PMCID: PMC9509450.
November 2023
Khoshiwal AM et al. The Tissue Systems Pathology Test Outperforms Pathology Review in Risk Stratifying Patients With Low-Grade Dysplasia. Gastroenterology. 2023 Nov;165(5):1168-1179.e6. doi: 10.1053/j.gastro.2023.07.029. Epub 2023 Aug 30. PMID: 37657759.
Chen YI et al. Endoscopic Ultrasound-Guided Biliary Drainage of First Intent With a Lumen-Apposing Metal Stent vs Endoscopic Retrograde Cholangiopancreatography in Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Study (ELEMENT Trial). Gastroenterology. 2023 Nov;165(5):1249-1261.e5. doi: 10.1053/j.gastro.2023.07.024. Epub 2023 Aug 6. PMID: 37549753.
December 2023
Almario CV et al. Prevalence and Burden of Illness of Rome IV Irritable Bowel Syndrome in the United States: Results From a Nationwide Cross-Sectional Study. Gastroenterology. 2023 Dec;165(6):1475-1487. doi: 10.1053/j.gastro.2023.08.010. Epub 2023 Aug 16. PMID: 37595647.
Koopmann BDM et al. The Natural Disease Course of Pancreatic Cyst-Associated Neoplasia, Dysplasia, and Ductal Adenocarcinoma: Results of a Microsimulation Model. Gastroenterology. 2023 Dec;165(6):1522-1532. doi: 10.1053/j.gastro.2023.08.027. Epub 2023 Aug 24. PMID: 37633497.
Clinical Gastroenterology and Hepatology
October 2023
Jung DH et al. Comparison of a Polysaccharide Hemostatic Powder and Conventional Therapy for Peptic Ulcer Bleeding. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2844-2253.e5. doi: 10.1016/j.cgh.2023.02.031. Epub 2023 Mar 10. PMID: 36906081.
Liang PS et al. Blood Test Increases Colorectal Cancer Screening in Persons Who Declined Colonoscopy and Fecal Immunochemical Test: A Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2951-2957.e2. doi: 10.1016/j.cgh.2023.03.036. Epub 2023 Apr 8. PMID: 37037262; PMCID: PMC10523873.
November 2023
Li YK et al. Risk of Postcolonoscopy Thromboembolic Events: A Real-World Cohort Study. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3051-3059.e4. doi: 10.1016/j.cgh.2022.09.021. Epub 2022 Sep 24. PMID: 36167228.
Tome J et al. Bile Acid Sequestrants in Microscopic Colitis: Clinical Outcomes and Utility of Bile Acid Testing. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3125-3131.e2. doi: 10.1016/j.cgh.2023.04.031. Epub 2023 May 10. PMID: 37172800.
Berry SK et al. A Randomized Parallel-group Study of Digital Gut-directed Hypnotherapy vs Muscle Relaxation for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3152-3159.e2. doi: 10.1016/j.cgh.2023.06.015. Epub 2023 Jun 28. PMID: 37391055.
December 2023
Kanwal F et al. Risk Stratification Model for Hepatocellular Cancer in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3296-3304.e3. doi: 10.1016/j.cgh.2023.04.019. Epub 2023 Apr 30. PMID: 37390101; PMCID: PMC10661677.
Forss A et al. Patients With Microscopic Colitis Are at Higher Risk of Major Adverse Cardiovascular Events: A Matched Cohort Study. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3356-3364.e9. doi: 10.1016/j.cgh.2023.05.014. Epub 2023 May 26. PMID: 37245713.
Zheng T et al. A Randomized, Controlled Trial of Efficacy and Safety of Cannabidiol in Idiopathic and Diabetic Gastroparesis. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3405-3414.e4. doi: 10.1016/j.cgh.2023.07.008. Epub 2023 Jul 22. PMID: 37482172.
Techniques and Innovations in Gastrointestinal Endoscopy
Rengarajan A and Aadam A. Peroral Endoscopic Myotomy (POEM) and Its Use in Esophageal Dysmotility. Tech Innov Gastrointest Endosc. 2023 Dec 16. doi: 10.1016/j.tige.2023.12.004.
Wang D et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2023 Nov 7. doi: 10.1016/j.tige.2023.10.003
Gastro Hep Advances
Gregory MH et al. Short Bowel Syndrome: Transition of Pediatric Patients to Adult Gastroenterology Care. Gastro Hep Advances. 2023 Sep 8. doi: 10.1016/j.gastha.2023.09.006.
Viser AC et al. Inflammatory Bowel Disease Patients in the Ambulatory Setting Commonly Screen Positive for Malnutrition. Gastro Hep Advances. 2023 Nov 16. doi: 10.1016/j.gastha.2023.11.007.
Gastroenterology
October 2023
El-Salhy M et al. Efficacy of Fecal Microbiota Transplantation for Patients With Irritable Bowel Syndrome at 3 Years After Transplantation. Gastroenterology. 2022 Oct;163(4):982-994.e14. doi: 10.1053/j.gastro.2022.06.020. Epub 2022 Jun 14. PMID: 35709830.
Bajaj JS and Nagy LE. Natural History of Alcohol-Associated Liver Disease: Understanding the Changing Landscape of Pathophysiology and Patient Care. Gastroenterology. 2022 Oct;163(4):840-851. doi: 10.1053/j.gastro.2022.05.031. Epub 2022 May 19. PMID: 35598629; PMCID: PMC9509416.
Lo CH et al. Association of Proton Pump Inhibitor Use With All-Cause and Cause-Specific Mortality. Gastroenterology. 2022 Oct;163(4):852-861.e2. doi: 10.1053/j.gastro.2022.06.067. Epub 2022 Jul 1. PMID: 35788344; PMCID: PMC9509450.
November 2023
Khoshiwal AM et al. The Tissue Systems Pathology Test Outperforms Pathology Review in Risk Stratifying Patients With Low-Grade Dysplasia. Gastroenterology. 2023 Nov;165(5):1168-1179.e6. doi: 10.1053/j.gastro.2023.07.029. Epub 2023 Aug 30. PMID: 37657759.
Chen YI et al. Endoscopic Ultrasound-Guided Biliary Drainage of First Intent With a Lumen-Apposing Metal Stent vs Endoscopic Retrograde Cholangiopancreatography in Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Study (ELEMENT Trial). Gastroenterology. 2023 Nov;165(5):1249-1261.e5. doi: 10.1053/j.gastro.2023.07.024. Epub 2023 Aug 6. PMID: 37549753.
December 2023
Almario CV et al. Prevalence and Burden of Illness of Rome IV Irritable Bowel Syndrome in the United States: Results From a Nationwide Cross-Sectional Study. Gastroenterology. 2023 Dec;165(6):1475-1487. doi: 10.1053/j.gastro.2023.08.010. Epub 2023 Aug 16. PMID: 37595647.
Koopmann BDM et al. The Natural Disease Course of Pancreatic Cyst-Associated Neoplasia, Dysplasia, and Ductal Adenocarcinoma: Results of a Microsimulation Model. Gastroenterology. 2023 Dec;165(6):1522-1532. doi: 10.1053/j.gastro.2023.08.027. Epub 2023 Aug 24. PMID: 37633497.
Clinical Gastroenterology and Hepatology
October 2023
Jung DH et al. Comparison of a Polysaccharide Hemostatic Powder and Conventional Therapy for Peptic Ulcer Bleeding. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2844-2253.e5. doi: 10.1016/j.cgh.2023.02.031. Epub 2023 Mar 10. PMID: 36906081.
Liang PS et al. Blood Test Increases Colorectal Cancer Screening in Persons Who Declined Colonoscopy and Fecal Immunochemical Test: A Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2951-2957.e2. doi: 10.1016/j.cgh.2023.03.036. Epub 2023 Apr 8. PMID: 37037262; PMCID: PMC10523873.
November 2023
Li YK et al. Risk of Postcolonoscopy Thromboembolic Events: A Real-World Cohort Study. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3051-3059.e4. doi: 10.1016/j.cgh.2022.09.021. Epub 2022 Sep 24. PMID: 36167228.
Tome J et al. Bile Acid Sequestrants in Microscopic Colitis: Clinical Outcomes and Utility of Bile Acid Testing. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3125-3131.e2. doi: 10.1016/j.cgh.2023.04.031. Epub 2023 May 10. PMID: 37172800.
Berry SK et al. A Randomized Parallel-group Study of Digital Gut-directed Hypnotherapy vs Muscle Relaxation for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3152-3159.e2. doi: 10.1016/j.cgh.2023.06.015. Epub 2023 Jun 28. PMID: 37391055.
December 2023
Kanwal F et al. Risk Stratification Model for Hepatocellular Cancer in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3296-3304.e3. doi: 10.1016/j.cgh.2023.04.019. Epub 2023 Apr 30. PMID: 37390101; PMCID: PMC10661677.
Forss A et al. Patients With Microscopic Colitis Are at Higher Risk of Major Adverse Cardiovascular Events: A Matched Cohort Study. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3356-3364.e9. doi: 10.1016/j.cgh.2023.05.014. Epub 2023 May 26. PMID: 37245713.
Zheng T et al. A Randomized, Controlled Trial of Efficacy and Safety of Cannabidiol in Idiopathic and Diabetic Gastroparesis. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3405-3414.e4. doi: 10.1016/j.cgh.2023.07.008. Epub 2023 Jul 22. PMID: 37482172.
Techniques and Innovations in Gastrointestinal Endoscopy
Rengarajan A and Aadam A. Peroral Endoscopic Myotomy (POEM) and Its Use in Esophageal Dysmotility. Tech Innov Gastrointest Endosc. 2023 Dec 16. doi: 10.1016/j.tige.2023.12.004.
Wang D et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2023 Nov 7. doi: 10.1016/j.tige.2023.10.003
Gastro Hep Advances
Gregory MH et al. Short Bowel Syndrome: Transition of Pediatric Patients to Adult Gastroenterology Care. Gastro Hep Advances. 2023 Sep 8. doi: 10.1016/j.gastha.2023.09.006.
Viser AC et al. Inflammatory Bowel Disease Patients in the Ambulatory Setting Commonly Screen Positive for Malnutrition. Gastro Hep Advances. 2023 Nov 16. doi: 10.1016/j.gastha.2023.11.007.
Elevate Your Career: AGA Women in GI Regional Workshops Await
As a woman in a dynamic and ever-changing profession, balancing life as a powerhouse physician or scientist is no easy feat.
Expanded to six workshops in 2024, AGA is pleased to offer regionally curated workshops with distinguished speakers at all experience levels to fuel your professional and personal growth. Participate in candid discussions regarding the distinct challenges you face as a woman navigating the 21st century healthcare environment. Derive inspiration from your community and cultivate meaningful connections that will carry you beyond the workshop.
Join us in-person or virtually, whatever fits into your busy schedule. We are also pleased to offer travel grants of up to $300 (per workshop) to help offset the costs of attending this program for one selected individual per region. The travel grant is to support travel and registration fees for early-career women. Additional details for the Maria Leo-Lieber Travel Award may be found in your confirmation email.
Ready to thrive? Register today to attend one of our first workshops or stay tuned for an additional workshop coming near you.
This program is supported by Janssen.
Midwest Regional Workshop
Saturday, Feb. 24, 2024
8 a.m.-3 p.m. CT
University of Chicago, Gleacher Center, Chicago, IL
Deadline to apply for a travel grant: Feb. 9, 2024 Deadline to register: Feb. 16, 2024
Click here to register.
Western Regional Workshop
Saturday, April 27, 2024
8 a.m.-3 p.m. PT
UCLA Luskin Conference Center, Los Angeles, CA
Meet fellow attendees at our pre-workshop networking event on Friday, Apr. 26 from 8 p.m. to 10:30 p.m.
Deadline to apply for a travel grant: April 12, 2024 Deadline to register: April 19, 2024
Click here to register.
As a woman in a dynamic and ever-changing profession, balancing life as a powerhouse physician or scientist is no easy feat.
Expanded to six workshops in 2024, AGA is pleased to offer regionally curated workshops with distinguished speakers at all experience levels to fuel your professional and personal growth. Participate in candid discussions regarding the distinct challenges you face as a woman navigating the 21st century healthcare environment. Derive inspiration from your community and cultivate meaningful connections that will carry you beyond the workshop.
Join us in-person or virtually, whatever fits into your busy schedule. We are also pleased to offer travel grants of up to $300 (per workshop) to help offset the costs of attending this program for one selected individual per region. The travel grant is to support travel and registration fees for early-career women. Additional details for the Maria Leo-Lieber Travel Award may be found in your confirmation email.
Ready to thrive? Register today to attend one of our first workshops or stay tuned for an additional workshop coming near you.
This program is supported by Janssen.
Midwest Regional Workshop
Saturday, Feb. 24, 2024
8 a.m.-3 p.m. CT
University of Chicago, Gleacher Center, Chicago, IL
Deadline to apply for a travel grant: Feb. 9, 2024 Deadline to register: Feb. 16, 2024
Click here to register.
Western Regional Workshop
Saturday, April 27, 2024
8 a.m.-3 p.m. PT
UCLA Luskin Conference Center, Los Angeles, CA
Meet fellow attendees at our pre-workshop networking event on Friday, Apr. 26 from 8 p.m. to 10:30 p.m.
Deadline to apply for a travel grant: April 12, 2024 Deadline to register: April 19, 2024
Click here to register.
As a woman in a dynamic and ever-changing profession, balancing life as a powerhouse physician or scientist is no easy feat.
Expanded to six workshops in 2024, AGA is pleased to offer regionally curated workshops with distinguished speakers at all experience levels to fuel your professional and personal growth. Participate in candid discussions regarding the distinct challenges you face as a woman navigating the 21st century healthcare environment. Derive inspiration from your community and cultivate meaningful connections that will carry you beyond the workshop.
Join us in-person or virtually, whatever fits into your busy schedule. We are also pleased to offer travel grants of up to $300 (per workshop) to help offset the costs of attending this program for one selected individual per region. The travel grant is to support travel and registration fees for early-career women. Additional details for the Maria Leo-Lieber Travel Award may be found in your confirmation email.
Ready to thrive? Register today to attend one of our first workshops or stay tuned for an additional workshop coming near you.
This program is supported by Janssen.
Midwest Regional Workshop
Saturday, Feb. 24, 2024
8 a.m.-3 p.m. CT
University of Chicago, Gleacher Center, Chicago, IL
Deadline to apply for a travel grant: Feb. 9, 2024 Deadline to register: Feb. 16, 2024
Click here to register.
Western Regional Workshop
Saturday, April 27, 2024
8 a.m.-3 p.m. PT
UCLA Luskin Conference Center, Los Angeles, CA
Meet fellow attendees at our pre-workshop networking event on Friday, Apr. 26 from 8 p.m. to 10:30 p.m.
Deadline to apply for a travel grant: April 12, 2024 Deadline to register: April 19, 2024
Click here to register.
AGA Tech Summit: Bridging the Gap Between Innovation, Industry, and Gastroenterologists
Medicine is transforming at a remarkable pace. It is therefore imperative for the future of the field that physicians understand innovation and collaborate with industry partners. Innovation can be defined as invention, adoption, and diffusion.1 During my training in gastroenterology and advanced fellowships, I learned about multiple endoscopic tools and techniques and became familiar with industry names that I frequently encountered in the endoscopy unit or clinic.
I was nominated to attend the AGA Tech Summit Fellows Program by my advanced endoscopy fellowship program director. A total of 22 fellows from around the United States at various stages of their training and interests in the field of gastroenterology and hepatology were selected for the program through an application process. The program included registration, travel, and accommodations to attend the AGA Tech Summit and Fellows Immersion Day at Medtronic.
The first event in the program was a visit to the Medtronic Santa Clara office, where our initial stop was at the research and development lab. We were introduced to design and biomedical engineers who reviewed with us the extensive testing that devices and endoscopy equipment undergo before coming to the market. These labs have a heavy focus on prototyping and experimentation and exist to promote in-house innovation and inventions.

During the day, we met physicians who shared their journeys on how they developed and advanced their careers in partnership with industry. Our visit also included a session with the business development and strategy manager at Medtronic, who discussed strategy and steps involved in product development — from the inception of an idea, institutional policies, and patents, to industry collaboration, and finally to successful commercialization. During medical school and training, we are focused on appropriately learning and applying medical knowledge to clinical care. The Medtronic Fellows Immersion Day experience offered a different perspective and showed other ways by which clinical knowledge and experience can be used to make an impact, in collaboration with industry and stakeholders. It also highlighted alternative career paths for medical professionals. The evening concluded with a meet and greet with the AGA Center for GI Innovation & Technology (CGIT) members and leadership.

The AGA Tech Summit was unlike any conference I have been to in my 13 years of training in medicine (which included mostly clinically focused scientific meetings). Sessions involved ergonomics, applications of artificial intelligence, advances in imaging, environmental endoscopy, the role of the FDA, and innovations around the world. The audience included but was not limited to industry executives, AGA CGIT leadership, physician innovators, gastroenterologists, venture capitalists, and others. Attendees represented the diversity of our field in terms of organizational structures and backgrounds. This resulted in an opportunity to hear and learn different perspectives about products, emerging technology, and the costs involved for physicians, industry, and patients.
The final session of the summit, the AGA Shark Tank, was perhaps the most intriguing one of all. The session showcased landscape-changing technology to AGA investors and venture capitalists. The participants presented their own pitches and faced the sharks (judges). The winner received additional funding, tailored guidance from the AGA CGIT committee, partnering opportunities with interested parties, and the opportunity to represent AGA Shark Tank at the Digestive Disease Week (DDW).
The AGA Tech Summit Fellows Program is a learning platform that not only helps you find your niche in the world of GI innovation but also equips you with resources and connections to make an impact. It is also a great way to infuse new ideas into your practice or research. As healthcare professionals, we must create a culture where innovation can flourish, and where staff and patients feel empowered to contribute to the innovation process and help make change happen — to me, the AGA Tech Summit is one such avenue.
Reference
1. Kelly CJ and Young AJ. Promoting innovation in healthcare. Future Healthc J. 2017 Jun. doi: 10.7861/futurehosp.4-2-121.
Dr. Umar is Assistant Professor of Medicine, Section of Gastroenterology and Hepatology, Baylor College of Medicine, Houston, Texas, and a staff physician at Michael E. DeBakey VA Medical Center, Houston. Dr. Umar has no relevant financial conflicts and is on X, formerly Twitter, @shifaumarMD.
Medicine is transforming at a remarkable pace. It is therefore imperative for the future of the field that physicians understand innovation and collaborate with industry partners. Innovation can be defined as invention, adoption, and diffusion.1 During my training in gastroenterology and advanced fellowships, I learned about multiple endoscopic tools and techniques and became familiar with industry names that I frequently encountered in the endoscopy unit or clinic.
I was nominated to attend the AGA Tech Summit Fellows Program by my advanced endoscopy fellowship program director. A total of 22 fellows from around the United States at various stages of their training and interests in the field of gastroenterology and hepatology were selected for the program through an application process. The program included registration, travel, and accommodations to attend the AGA Tech Summit and Fellows Immersion Day at Medtronic.
The first event in the program was a visit to the Medtronic Santa Clara office, where our initial stop was at the research and development lab. We were introduced to design and biomedical engineers who reviewed with us the extensive testing that devices and endoscopy equipment undergo before coming to the market. These labs have a heavy focus on prototyping and experimentation and exist to promote in-house innovation and inventions.

During the day, we met physicians who shared their journeys on how they developed and advanced their careers in partnership with industry. Our visit also included a session with the business development and strategy manager at Medtronic, who discussed strategy and steps involved in product development — from the inception of an idea, institutional policies, and patents, to industry collaboration, and finally to successful commercialization. During medical school and training, we are focused on appropriately learning and applying medical knowledge to clinical care. The Medtronic Fellows Immersion Day experience offered a different perspective and showed other ways by which clinical knowledge and experience can be used to make an impact, in collaboration with industry and stakeholders. It also highlighted alternative career paths for medical professionals. The evening concluded with a meet and greet with the AGA Center for GI Innovation & Technology (CGIT) members and leadership.

The AGA Tech Summit was unlike any conference I have been to in my 13 years of training in medicine (which included mostly clinically focused scientific meetings). Sessions involved ergonomics, applications of artificial intelligence, advances in imaging, environmental endoscopy, the role of the FDA, and innovations around the world. The audience included but was not limited to industry executives, AGA CGIT leadership, physician innovators, gastroenterologists, venture capitalists, and others. Attendees represented the diversity of our field in terms of organizational structures and backgrounds. This resulted in an opportunity to hear and learn different perspectives about products, emerging technology, and the costs involved for physicians, industry, and patients.
The final session of the summit, the AGA Shark Tank, was perhaps the most intriguing one of all. The session showcased landscape-changing technology to AGA investors and venture capitalists. The participants presented their own pitches and faced the sharks (judges). The winner received additional funding, tailored guidance from the AGA CGIT committee, partnering opportunities with interested parties, and the opportunity to represent AGA Shark Tank at the Digestive Disease Week (DDW).
The AGA Tech Summit Fellows Program is a learning platform that not only helps you find your niche in the world of GI innovation but also equips you with resources and connections to make an impact. It is also a great way to infuse new ideas into your practice or research. As healthcare professionals, we must create a culture where innovation can flourish, and where staff and patients feel empowered to contribute to the innovation process and help make change happen — to me, the AGA Tech Summit is one such avenue.
Reference
1. Kelly CJ and Young AJ. Promoting innovation in healthcare. Future Healthc J. 2017 Jun. doi: 10.7861/futurehosp.4-2-121.
Dr. Umar is Assistant Professor of Medicine, Section of Gastroenterology and Hepatology, Baylor College of Medicine, Houston, Texas, and a staff physician at Michael E. DeBakey VA Medical Center, Houston. Dr. Umar has no relevant financial conflicts and is on X, formerly Twitter, @shifaumarMD.
Medicine is transforming at a remarkable pace. It is therefore imperative for the future of the field that physicians understand innovation and collaborate with industry partners. Innovation can be defined as invention, adoption, and diffusion.1 During my training in gastroenterology and advanced fellowships, I learned about multiple endoscopic tools and techniques and became familiar with industry names that I frequently encountered in the endoscopy unit or clinic.
I was nominated to attend the AGA Tech Summit Fellows Program by my advanced endoscopy fellowship program director. A total of 22 fellows from around the United States at various stages of their training and interests in the field of gastroenterology and hepatology were selected for the program through an application process. The program included registration, travel, and accommodations to attend the AGA Tech Summit and Fellows Immersion Day at Medtronic.
The first event in the program was a visit to the Medtronic Santa Clara office, where our initial stop was at the research and development lab. We were introduced to design and biomedical engineers who reviewed with us the extensive testing that devices and endoscopy equipment undergo before coming to the market. These labs have a heavy focus on prototyping and experimentation and exist to promote in-house innovation and inventions.

During the day, we met physicians who shared their journeys on how they developed and advanced their careers in partnership with industry. Our visit also included a session with the business development and strategy manager at Medtronic, who discussed strategy and steps involved in product development — from the inception of an idea, institutional policies, and patents, to industry collaboration, and finally to successful commercialization. During medical school and training, we are focused on appropriately learning and applying medical knowledge to clinical care. The Medtronic Fellows Immersion Day experience offered a different perspective and showed other ways by which clinical knowledge and experience can be used to make an impact, in collaboration with industry and stakeholders. It also highlighted alternative career paths for medical professionals. The evening concluded with a meet and greet with the AGA Center for GI Innovation & Technology (CGIT) members and leadership.

The AGA Tech Summit was unlike any conference I have been to in my 13 years of training in medicine (which included mostly clinically focused scientific meetings). Sessions involved ergonomics, applications of artificial intelligence, advances in imaging, environmental endoscopy, the role of the FDA, and innovations around the world. The audience included but was not limited to industry executives, AGA CGIT leadership, physician innovators, gastroenterologists, venture capitalists, and others. Attendees represented the diversity of our field in terms of organizational structures and backgrounds. This resulted in an opportunity to hear and learn different perspectives about products, emerging technology, and the costs involved for physicians, industry, and patients.
The final session of the summit, the AGA Shark Tank, was perhaps the most intriguing one of all. The session showcased landscape-changing technology to AGA investors and venture capitalists. The participants presented their own pitches and faced the sharks (judges). The winner received additional funding, tailored guidance from the AGA CGIT committee, partnering opportunities with interested parties, and the opportunity to represent AGA Shark Tank at the Digestive Disease Week (DDW).
The AGA Tech Summit Fellows Program is a learning platform that not only helps you find your niche in the world of GI innovation but also equips you with resources and connections to make an impact. It is also a great way to infuse new ideas into your practice or research. As healthcare professionals, we must create a culture where innovation can flourish, and where staff and patients feel empowered to contribute to the innovation process and help make change happen — to me, the AGA Tech Summit is one such avenue.
Reference
1. Kelly CJ and Young AJ. Promoting innovation in healthcare. Future Healthc J. 2017 Jun. doi: 10.7861/futurehosp.4-2-121.
Dr. Umar is Assistant Professor of Medicine, Section of Gastroenterology and Hepatology, Baylor College of Medicine, Houston, Texas, and a staff physician at Michael E. DeBakey VA Medical Center, Houston. Dr. Umar has no relevant financial conflicts and is on X, formerly Twitter, @shifaumarMD.
2024 Gut Microbiota for Health World Summit Explores the Clinical Impacts of the Microbiome
Join global experts in-person or online as they gather for the 2024 Gut Microbiota for Health World Summit (GMFH) on March 23-24, 2024, in Washington, DC.
This meeting brings together an international and multidisciplinary community of GI clinicians, dietitians, and researchers to discuss personalized approaches to modifying the gut microbiome to improve health and treat disease.
This year’s program will explore:
- Better health through the gut microbiome.
- Big data and the gut microbiome.
- Human-derived to synthetic communities.
- Bringing new microbiome-based products to market.
Early-career faculty and trainees are encouraged to submit abstracts for presentation during the reception. Five $1,000 abstract prizes are available for top-scoring submissions.
Register here.
Join global experts in-person or online as they gather for the 2024 Gut Microbiota for Health World Summit (GMFH) on March 23-24, 2024, in Washington, DC.
This meeting brings together an international and multidisciplinary community of GI clinicians, dietitians, and researchers to discuss personalized approaches to modifying the gut microbiome to improve health and treat disease.
This year’s program will explore:
- Better health through the gut microbiome.
- Big data and the gut microbiome.
- Human-derived to synthetic communities.
- Bringing new microbiome-based products to market.
Early-career faculty and trainees are encouraged to submit abstracts for presentation during the reception. Five $1,000 abstract prizes are available for top-scoring submissions.
Register here.
Join global experts in-person or online as they gather for the 2024 Gut Microbiota for Health World Summit (GMFH) on March 23-24, 2024, in Washington, DC.
This meeting brings together an international and multidisciplinary community of GI clinicians, dietitians, and researchers to discuss personalized approaches to modifying the gut microbiome to improve health and treat disease.
This year’s program will explore:
- Better health through the gut microbiome.
- Big data and the gut microbiome.
- Human-derived to synthetic communities.
- Bringing new microbiome-based products to market.
Early-career faculty and trainees are encouraged to submit abstracts for presentation during the reception. Five $1,000 abstract prizes are available for top-scoring submissions.
Register here.
Announcing AGA Journal Social Media Editors
AGA journals have welcomed new social media editors for Clinical Gastroenterology and Hepatology (CGH), Cellular and Molecular Gastroenterology and Hepatology (CMGH), Techniques and Innovations in Gastrointestinal Endoscopy (TIGE) and Gastro Hep Advances (GHA).
Clinical Gastroenterology and Hepatology (CGH)
Joseph Sleiman, MD
University of Pittsburgh Medical Center
Dr. Sleiman’s research interests include inflammatory bowel disease (IBD), immunotherapy-induced colitis, Lynch Syndrome surveillance strategies and machine learning for GI research purposes.
Follow Dr. Sleiman
Cellular and Molecular Gastroenterology and Hepatology (CMGH)
Lindsey Kennedy, PhD
Indiana University School of Medicine
Dr. Kennedy’s research interests include the cellular crosstalk and pathological mechanisms regulating biliary and liver damage in cholestatic disorders, such as primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC).
Follow Dr. Kennedy
Techniques and Innovations in Gastrointestinal Endoscopy (TIGE)
Judy Trieu, MD, MPH
Washington University Physicians
Dr. Trieu specializes in interventional endoscopy and general gastroenterology.
Follow Dr. Trieu
Gastro Hep Advances (GHA)
Shida Haghighat, MD, MPH
University of Miami
Dr. Haghighat’s research interests center around the prevention and screening of gastrointestinal cancers.
Follow Dr. Haghihat
AGA journals have welcomed new social media editors for Clinical Gastroenterology and Hepatology (CGH), Cellular and Molecular Gastroenterology and Hepatology (CMGH), Techniques and Innovations in Gastrointestinal Endoscopy (TIGE) and Gastro Hep Advances (GHA).
Clinical Gastroenterology and Hepatology (CGH)
Joseph Sleiman, MD
University of Pittsburgh Medical Center
Dr. Sleiman’s research interests include inflammatory bowel disease (IBD), immunotherapy-induced colitis, Lynch Syndrome surveillance strategies and machine learning for GI research purposes.
Follow Dr. Sleiman
Cellular and Molecular Gastroenterology and Hepatology (CMGH)
Lindsey Kennedy, PhD
Indiana University School of Medicine
Dr. Kennedy’s research interests include the cellular crosstalk and pathological mechanisms regulating biliary and liver damage in cholestatic disorders, such as primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC).
Follow Dr. Kennedy
Techniques and Innovations in Gastrointestinal Endoscopy (TIGE)
Judy Trieu, MD, MPH
Washington University Physicians
Dr. Trieu specializes in interventional endoscopy and general gastroenterology.
Follow Dr. Trieu
Gastro Hep Advances (GHA)
Shida Haghighat, MD, MPH
University of Miami
Dr. Haghighat’s research interests center around the prevention and screening of gastrointestinal cancers.
Follow Dr. Haghihat
AGA journals have welcomed new social media editors for Clinical Gastroenterology and Hepatology (CGH), Cellular and Molecular Gastroenterology and Hepatology (CMGH), Techniques and Innovations in Gastrointestinal Endoscopy (TIGE) and Gastro Hep Advances (GHA).
Clinical Gastroenterology and Hepatology (CGH)
Joseph Sleiman, MD
University of Pittsburgh Medical Center
Dr. Sleiman’s research interests include inflammatory bowel disease (IBD), immunotherapy-induced colitis, Lynch Syndrome surveillance strategies and machine learning for GI research purposes.
Follow Dr. Sleiman
Cellular and Molecular Gastroenterology and Hepatology (CMGH)
Lindsey Kennedy, PhD
Indiana University School of Medicine
Dr. Kennedy’s research interests include the cellular crosstalk and pathological mechanisms regulating biliary and liver damage in cholestatic disorders, such as primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC).
Follow Dr. Kennedy
Techniques and Innovations in Gastrointestinal Endoscopy (TIGE)
Judy Trieu, MD, MPH
Washington University Physicians
Dr. Trieu specializes in interventional endoscopy and general gastroenterology.
Follow Dr. Trieu
Gastro Hep Advances (GHA)
Shida Haghighat, MD, MPH
University of Miami
Dr. Haghighat’s research interests center around the prevention and screening of gastrointestinal cancers.
Follow Dr. Haghihat
From Mentee to Mentor
Mentoring is universally recognized as a key contributor to a successful career in academic medicine. Most of those who recently transitioned from fellow to faculty got to their current positions with the help of one or more mentors. While many will still need mentoring, coaching, and sponsoring, many are also eager to give back and wonder when and how to make that transition from mentee to mentor.
Dr. Lok: Senior Mentor’s Perspective
I (ASL) completed my hepatology fellowship training in London under Professor Dame Sheila Sherlock. I did not realize how fortunate I was until Dame Sheila’s retirement celebration (2 months before the end of my fellowship) when more than 200 former mentees flew in from all over the world to express their appreciation. Dame Sheila had always embraced all of us as part of the Sherlock family. I benefited tremendously not only from clinical and research training with Dame Sheila and her motherly love that continued well after I completed my fellowship but also the connections and support from my “siblings” who were the Who’s Who in Hepatology.
My transition from mentee to mentor occurred insidiously after my return to Hong Kong, coaching and collaborating with residents, fellows, and early career faculty in their research projects. A key tip I shared with them was the importance of establishing a robust database and sample repository — a vital element to success as a clinical investigator. Working in a busy clinical environment with no protected time and limited resources, we began by identifying clinical dilemmas that we faced in clinics each day and determined which ones were “solvable” if we dove deep. Through keen observations, protocolized clinical care, and robust data recording, we published in Gastroenterology one of the first prospective studies of hepatitis B reactivation in patients receiving chemotherapy, and it continues to be cited. Many principles in mentoring apply universally. Indeed, one of my most accomplished mentees in Hong Kong is a nephrologist with whom I continue to coauthor topics in UpToDate. This is an example of how mentee-mentor relationship can evolve and last, and how each can learn from the other to provide guidance on multi-disciplinary care of complex medical problems.
I became more involved in mentoring after I moved to the United States. I was first hired as Hepatology Program Director at Tulane University and then at the University of Michigan. These roles gave me a sense of responsibility not just to mentor one resident, fellow, or faculty on a research project but to have a holistic approach, providing the necessary guidance and support to help mentees make the best of their potentials and build successful careers, which in turn allows me to build a world-class program.
Over the years, I have mentored more than 60 trainees from all over the world, some of whom have now become division chiefs, department chairs, and chief medical officers of hospitals. Every mentor has a different style, and I had been criticized for being a “Tiger Mom.” I have mellowed over the years, and I hope I am no longer perceived as a “tiger,” though tough love is crucial in mentoring. I hope I am still considered a “mom,” because I see the role of a mentor as that of a parent, providing unconditional love and support with the only expectation that the mentees try to do their best to maximize their potentials and reach their goals. Mentoring is a time investment. It can be exhausting, frustrating, and heart-breaking. It is rarely recognized, and the time and effort rarely compensated. Thus, one should take on mentoring as a calling, a desire to pay it forward, and an understanding that problems can be solved only when generations of physicians and researchers continue to work on them.
A mentor, just like a parent, helps mentees recognize their potentials — passion, strengths, and weaknesses — and to set ambitious yet realistic goals. A very important role of a mentor is to help mentees determine their short- and long-term goals by guiding them to leverage their strengths and passion toward areas and niches that are important and attainable.
Each goal must be accompanied by a plan on how to get there based on resources available. Here is where tough love comes into play. Because there are so many distractions in life, mentees can veer off and be lost. Research projects (and life) never go exactly as planned, and it is difficult to keep going when projects hit a roadblock and papers and grants are rejected. A mentor must help mentees accept and learn from failures and persevere with renewed commitment or find an alternative path (when it is clear the original path is doomed). The most important role of the mentor is to continue to believe in the mentee. Project failure must not be equated to mentee failure though there are times when it is clear some mentees have their interests and talents in other areas. Helping mentees find an alternative path to success and fulfillment can be a blessing. Indeed, two of my mentees who were successful researchers during their early careers have now become successful chief medical officers of major hospitals. They are happy, and I am very proud of them. Times have changed, so my coauthor, who has been faculty for 3.5 years, will share his journey from mentee to mentor.
Dr. Chen: Early Mentor’s Perspective
I (VLC) completed training in 2020 and have mentored only people who are early in their careers, i.e., medical students, residents, and fellows. My transition from mentee to mentor was primarily motivated by gratitude to my past mentors. Watching my own former trainees move on to the next stages of their careers has been hugely fulfilling. It is important that mentee-mentor relationships are mutually beneficial, and I offer a few points to junior faculty considering taking on trainees as mentees.
Taking on a mentee is a commitment. Take it seriously. While a mentee’s success is ultimately their responsibility, mentors are implicitly agreeing to give them opportunities commensurate to their skills and motivation. If you are not in a position to offer such opportunities, do not accept mentees.
Mentorship takes time. Explaining and reviewing research protocols, reading abstract or manuscript drafts, and meeting with mentees to plan for next steps take more time than one might expect.
Understand what potential mentees want. Most trainees are looking for help making it to the next stage of their career (college to medical school, residency to fellowship, etc.) and need abstracts and/or publications to get there. When I work with residents applying to GI fellowship, the goal is that by the time fellowship applications are submitted (early in third year of residency), they have at a minimum presented an abstract at Digestive Diseases Week (DDW) in their second year and submitted an abstract to the American College of Gastroenterology and/or American Association for the Study of Liver Diseases meetings in their third year. This requires planning to ensure they start working early enough to meet conference abstract deadlines. In my opinion, it is reasonable to give the trainee a less ambitious project or a piece of a larger project (i.e., middle authorship on a paper).
By contrast, for trainees who are seriously interested in a research career, the goal is not superfluous abstracts. Rather it is crucial to ensure that the trainee leads a meaningful project that will be a steppingstone to their future career and/or provide preliminary data to support grant applications. Similarly, training in research methodology should be more rigorous for these mentees.
Recognize the limitations of your circumstances. Early-stage faculty often operate on a shoestring budget and little protected time. Even those with 50% or more protected research time and excellent nursing support will find that the time they spend on patient care extends far beyond the time spent in endoscopy units and clinics. Time management and discipline — including not getting bogged down on low-impact research studies — are essential skills.
Be (slightly) selfish. Make sure that you get something out of the mentee as well. Ask yourself:
Do I have work they can help me with? Avoid creating projects simply to give a trainee something to do. It is much better to have them work on a project that you want to do anyway.
How do the trainee’s skills fit in with the type of work that I do? A trainee with no background in statistics may not be able to conduct analyses but may be able to do chart reviews.
Consider “testing” a potential mentee by assigning a limited, straightforward task. If the mentee completes this quickly and to a high standard, then move on to progressively more important or high-stakes projects.
Set concrete and realistic expectations, keeping in mind that trainees have other commitments such as classes and clinical rotations.
Serving as a mentor to the next generation of gastroenterologists is a privilege that junior faculty should not take lightly, and an opportunity for a symbiotic relationship.
Dr. Chen and Dr. Lok are with the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan. They have no financial conflicts related to this article.
Mentoring is universally recognized as a key contributor to a successful career in academic medicine. Most of those who recently transitioned from fellow to faculty got to their current positions with the help of one or more mentors. While many will still need mentoring, coaching, and sponsoring, many are also eager to give back and wonder when and how to make that transition from mentee to mentor.
Dr. Lok: Senior Mentor’s Perspective
I (ASL) completed my hepatology fellowship training in London under Professor Dame Sheila Sherlock. I did not realize how fortunate I was until Dame Sheila’s retirement celebration (2 months before the end of my fellowship) when more than 200 former mentees flew in from all over the world to express their appreciation. Dame Sheila had always embraced all of us as part of the Sherlock family. I benefited tremendously not only from clinical and research training with Dame Sheila and her motherly love that continued well after I completed my fellowship but also the connections and support from my “siblings” who were the Who’s Who in Hepatology.
My transition from mentee to mentor occurred insidiously after my return to Hong Kong, coaching and collaborating with residents, fellows, and early career faculty in their research projects. A key tip I shared with them was the importance of establishing a robust database and sample repository — a vital element to success as a clinical investigator. Working in a busy clinical environment with no protected time and limited resources, we began by identifying clinical dilemmas that we faced in clinics each day and determined which ones were “solvable” if we dove deep. Through keen observations, protocolized clinical care, and robust data recording, we published in Gastroenterology one of the first prospective studies of hepatitis B reactivation in patients receiving chemotherapy, and it continues to be cited. Many principles in mentoring apply universally. Indeed, one of my most accomplished mentees in Hong Kong is a nephrologist with whom I continue to coauthor topics in UpToDate. This is an example of how mentee-mentor relationship can evolve and last, and how each can learn from the other to provide guidance on multi-disciplinary care of complex medical problems.
I became more involved in mentoring after I moved to the United States. I was first hired as Hepatology Program Director at Tulane University and then at the University of Michigan. These roles gave me a sense of responsibility not just to mentor one resident, fellow, or faculty on a research project but to have a holistic approach, providing the necessary guidance and support to help mentees make the best of their potentials and build successful careers, which in turn allows me to build a world-class program.
Over the years, I have mentored more than 60 trainees from all over the world, some of whom have now become division chiefs, department chairs, and chief medical officers of hospitals. Every mentor has a different style, and I had been criticized for being a “Tiger Mom.” I have mellowed over the years, and I hope I am no longer perceived as a “tiger,” though tough love is crucial in mentoring. I hope I am still considered a “mom,” because I see the role of a mentor as that of a parent, providing unconditional love and support with the only expectation that the mentees try to do their best to maximize their potentials and reach their goals. Mentoring is a time investment. It can be exhausting, frustrating, and heart-breaking. It is rarely recognized, and the time and effort rarely compensated. Thus, one should take on mentoring as a calling, a desire to pay it forward, and an understanding that problems can be solved only when generations of physicians and researchers continue to work on them.
A mentor, just like a parent, helps mentees recognize their potentials — passion, strengths, and weaknesses — and to set ambitious yet realistic goals. A very important role of a mentor is to help mentees determine their short- and long-term goals by guiding them to leverage their strengths and passion toward areas and niches that are important and attainable.
Each goal must be accompanied by a plan on how to get there based on resources available. Here is where tough love comes into play. Because there are so many distractions in life, mentees can veer off and be lost. Research projects (and life) never go exactly as planned, and it is difficult to keep going when projects hit a roadblock and papers and grants are rejected. A mentor must help mentees accept and learn from failures and persevere with renewed commitment or find an alternative path (when it is clear the original path is doomed). The most important role of the mentor is to continue to believe in the mentee. Project failure must not be equated to mentee failure though there are times when it is clear some mentees have their interests and talents in other areas. Helping mentees find an alternative path to success and fulfillment can be a blessing. Indeed, two of my mentees who were successful researchers during their early careers have now become successful chief medical officers of major hospitals. They are happy, and I am very proud of them. Times have changed, so my coauthor, who has been faculty for 3.5 years, will share his journey from mentee to mentor.
Dr. Chen: Early Mentor’s Perspective
I (VLC) completed training in 2020 and have mentored only people who are early in their careers, i.e., medical students, residents, and fellows. My transition from mentee to mentor was primarily motivated by gratitude to my past mentors. Watching my own former trainees move on to the next stages of their careers has been hugely fulfilling. It is important that mentee-mentor relationships are mutually beneficial, and I offer a few points to junior faculty considering taking on trainees as mentees.
Taking on a mentee is a commitment. Take it seriously. While a mentee’s success is ultimately their responsibility, mentors are implicitly agreeing to give them opportunities commensurate to their skills and motivation. If you are not in a position to offer such opportunities, do not accept mentees.
Mentorship takes time. Explaining and reviewing research protocols, reading abstract or manuscript drafts, and meeting with mentees to plan for next steps take more time than one might expect.
Understand what potential mentees want. Most trainees are looking for help making it to the next stage of their career (college to medical school, residency to fellowship, etc.) and need abstracts and/or publications to get there. When I work with residents applying to GI fellowship, the goal is that by the time fellowship applications are submitted (early in third year of residency), they have at a minimum presented an abstract at Digestive Diseases Week (DDW) in their second year and submitted an abstract to the American College of Gastroenterology and/or American Association for the Study of Liver Diseases meetings in their third year. This requires planning to ensure they start working early enough to meet conference abstract deadlines. In my opinion, it is reasonable to give the trainee a less ambitious project or a piece of a larger project (i.e., middle authorship on a paper).
By contrast, for trainees who are seriously interested in a research career, the goal is not superfluous abstracts. Rather it is crucial to ensure that the trainee leads a meaningful project that will be a steppingstone to their future career and/or provide preliminary data to support grant applications. Similarly, training in research methodology should be more rigorous for these mentees.
Recognize the limitations of your circumstances. Early-stage faculty often operate on a shoestring budget and little protected time. Even those with 50% or more protected research time and excellent nursing support will find that the time they spend on patient care extends far beyond the time spent in endoscopy units and clinics. Time management and discipline — including not getting bogged down on low-impact research studies — are essential skills.
Be (slightly) selfish. Make sure that you get something out of the mentee as well. Ask yourself:
Do I have work they can help me with? Avoid creating projects simply to give a trainee something to do. It is much better to have them work on a project that you want to do anyway.
How do the trainee’s skills fit in with the type of work that I do? A trainee with no background in statistics may not be able to conduct analyses but may be able to do chart reviews.
Consider “testing” a potential mentee by assigning a limited, straightforward task. If the mentee completes this quickly and to a high standard, then move on to progressively more important or high-stakes projects.
Set concrete and realistic expectations, keeping in mind that trainees have other commitments such as classes and clinical rotations.
Serving as a mentor to the next generation of gastroenterologists is a privilege that junior faculty should not take lightly, and an opportunity for a symbiotic relationship.
Dr. Chen and Dr. Lok are with the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan. They have no financial conflicts related to this article.
Mentoring is universally recognized as a key contributor to a successful career in academic medicine. Most of those who recently transitioned from fellow to faculty got to their current positions with the help of one or more mentors. While many will still need mentoring, coaching, and sponsoring, many are also eager to give back and wonder when and how to make that transition from mentee to mentor.
Dr. Lok: Senior Mentor’s Perspective
I (ASL) completed my hepatology fellowship training in London under Professor Dame Sheila Sherlock. I did not realize how fortunate I was until Dame Sheila’s retirement celebration (2 months before the end of my fellowship) when more than 200 former mentees flew in from all over the world to express their appreciation. Dame Sheila had always embraced all of us as part of the Sherlock family. I benefited tremendously not only from clinical and research training with Dame Sheila and her motherly love that continued well after I completed my fellowship but also the connections and support from my “siblings” who were the Who’s Who in Hepatology.
My transition from mentee to mentor occurred insidiously after my return to Hong Kong, coaching and collaborating with residents, fellows, and early career faculty in their research projects. A key tip I shared with them was the importance of establishing a robust database and sample repository — a vital element to success as a clinical investigator. Working in a busy clinical environment with no protected time and limited resources, we began by identifying clinical dilemmas that we faced in clinics each day and determined which ones were “solvable” if we dove deep. Through keen observations, protocolized clinical care, and robust data recording, we published in Gastroenterology one of the first prospective studies of hepatitis B reactivation in patients receiving chemotherapy, and it continues to be cited. Many principles in mentoring apply universally. Indeed, one of my most accomplished mentees in Hong Kong is a nephrologist with whom I continue to coauthor topics in UpToDate. This is an example of how mentee-mentor relationship can evolve and last, and how each can learn from the other to provide guidance on multi-disciplinary care of complex medical problems.
I became more involved in mentoring after I moved to the United States. I was first hired as Hepatology Program Director at Tulane University and then at the University of Michigan. These roles gave me a sense of responsibility not just to mentor one resident, fellow, or faculty on a research project but to have a holistic approach, providing the necessary guidance and support to help mentees make the best of their potentials and build successful careers, which in turn allows me to build a world-class program.
Over the years, I have mentored more than 60 trainees from all over the world, some of whom have now become division chiefs, department chairs, and chief medical officers of hospitals. Every mentor has a different style, and I had been criticized for being a “Tiger Mom.” I have mellowed over the years, and I hope I am no longer perceived as a “tiger,” though tough love is crucial in mentoring. I hope I am still considered a “mom,” because I see the role of a mentor as that of a parent, providing unconditional love and support with the only expectation that the mentees try to do their best to maximize their potentials and reach their goals. Mentoring is a time investment. It can be exhausting, frustrating, and heart-breaking. It is rarely recognized, and the time and effort rarely compensated. Thus, one should take on mentoring as a calling, a desire to pay it forward, and an understanding that problems can be solved only when generations of physicians and researchers continue to work on them.
A mentor, just like a parent, helps mentees recognize their potentials — passion, strengths, and weaknesses — and to set ambitious yet realistic goals. A very important role of a mentor is to help mentees determine their short- and long-term goals by guiding them to leverage their strengths and passion toward areas and niches that are important and attainable.
Each goal must be accompanied by a plan on how to get there based on resources available. Here is where tough love comes into play. Because there are so many distractions in life, mentees can veer off and be lost. Research projects (and life) never go exactly as planned, and it is difficult to keep going when projects hit a roadblock and papers and grants are rejected. A mentor must help mentees accept and learn from failures and persevere with renewed commitment or find an alternative path (when it is clear the original path is doomed). The most important role of the mentor is to continue to believe in the mentee. Project failure must not be equated to mentee failure though there are times when it is clear some mentees have their interests and talents in other areas. Helping mentees find an alternative path to success and fulfillment can be a blessing. Indeed, two of my mentees who were successful researchers during their early careers have now become successful chief medical officers of major hospitals. They are happy, and I am very proud of them. Times have changed, so my coauthor, who has been faculty for 3.5 years, will share his journey from mentee to mentor.
Dr. Chen: Early Mentor’s Perspective
I (VLC) completed training in 2020 and have mentored only people who are early in their careers, i.e., medical students, residents, and fellows. My transition from mentee to mentor was primarily motivated by gratitude to my past mentors. Watching my own former trainees move on to the next stages of their careers has been hugely fulfilling. It is important that mentee-mentor relationships are mutually beneficial, and I offer a few points to junior faculty considering taking on trainees as mentees.
Taking on a mentee is a commitment. Take it seriously. While a mentee’s success is ultimately their responsibility, mentors are implicitly agreeing to give them opportunities commensurate to their skills and motivation. If you are not in a position to offer such opportunities, do not accept mentees.
Mentorship takes time. Explaining and reviewing research protocols, reading abstract or manuscript drafts, and meeting with mentees to plan for next steps take more time than one might expect.
Understand what potential mentees want. Most trainees are looking for help making it to the next stage of their career (college to medical school, residency to fellowship, etc.) and need abstracts and/or publications to get there. When I work with residents applying to GI fellowship, the goal is that by the time fellowship applications are submitted (early in third year of residency), they have at a minimum presented an abstract at Digestive Diseases Week (DDW) in their second year and submitted an abstract to the American College of Gastroenterology and/or American Association for the Study of Liver Diseases meetings in their third year. This requires planning to ensure they start working early enough to meet conference abstract deadlines. In my opinion, it is reasonable to give the trainee a less ambitious project or a piece of a larger project (i.e., middle authorship on a paper).
By contrast, for trainees who are seriously interested in a research career, the goal is not superfluous abstracts. Rather it is crucial to ensure that the trainee leads a meaningful project that will be a steppingstone to their future career and/or provide preliminary data to support grant applications. Similarly, training in research methodology should be more rigorous for these mentees.
Recognize the limitations of your circumstances. Early-stage faculty often operate on a shoestring budget and little protected time. Even those with 50% or more protected research time and excellent nursing support will find that the time they spend on patient care extends far beyond the time spent in endoscopy units and clinics. Time management and discipline — including not getting bogged down on low-impact research studies — are essential skills.
Be (slightly) selfish. Make sure that you get something out of the mentee as well. Ask yourself:
Do I have work they can help me with? Avoid creating projects simply to give a trainee something to do. It is much better to have them work on a project that you want to do anyway.
How do the trainee’s skills fit in with the type of work that I do? A trainee with no background in statistics may not be able to conduct analyses but may be able to do chart reviews.
Consider “testing” a potential mentee by assigning a limited, straightforward task. If the mentee completes this quickly and to a high standard, then move on to progressively more important or high-stakes projects.
Set concrete and realistic expectations, keeping in mind that trainees have other commitments such as classes and clinical rotations.
Serving as a mentor to the next generation of gastroenterologists is a privilege that junior faculty should not take lightly, and an opportunity for a symbiotic relationship.
Dr. Chen and Dr. Lok are with the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan. They have no financial conflicts related to this article.
Tax Questions Frequently Asked by Physicians
Physicians spend years of their lives in education and training. There are countless hours devoted to studying, researching, and clinical training, not to mention residency and possible fellowships. Then literally overnight, they transition out of a resident salary into a full-time attending pay with little to no education around what to do with this significant increase in salary.
Every job position is unique in terms of benefits, how compensation is earned, job expectations, etc. But they all share one thing in common — taxes. Increased income comes with increased taxes.
FAQ 1. What is the difference between W2 income and 1099 income?
A: If you are a W2 employee, your employer is responsible for paying half of your Social Security and Medicare taxes. You, as the employee, are then responsible only for the remaining half of your Social Security and Medicare taxes. Additionally, your employer will withhold these taxes, along with federal income taxes, from your paycheck each pay period. You are not responsible for remitting any taxes to the IRS or state agencies, as your employer will do this for you. As a W2 employee, you are not able to deduct any employee expenses against your income.
As a 1099 contractor, you are considered self-employed and are responsible for the employer and employee portion of the Social Security and Medicare taxes. You are also responsible for remitting these taxes, as well as quarterly estimated federal withholding, to the IRS and state agencies. You can deduct work-related expenses against your 1099 income.
Both types of income have pros and cons. Either of these can be more beneficial to a specific situation.
FAQ 2. How do I know if I am withholding enough taxes?
A: This is a very common issue I see, especially with physicians who are transitioning out of training into their full-time attending salary. Because this transition happens mid-year, often the first half of the year you are withholding at a rate much lower than what you will be earning as an attending and end up with a tax surprise at filing. One way to remedy this is to look at how much taxes are being withheld from your paycheck and compare this to what tax bracket you anticipate to be in, depending on filing status (Figure 1). If you do this and realize you are not withholding enough taxes, you can submit an amended form W4 to your employer to have additional withholding taken out each pay period.
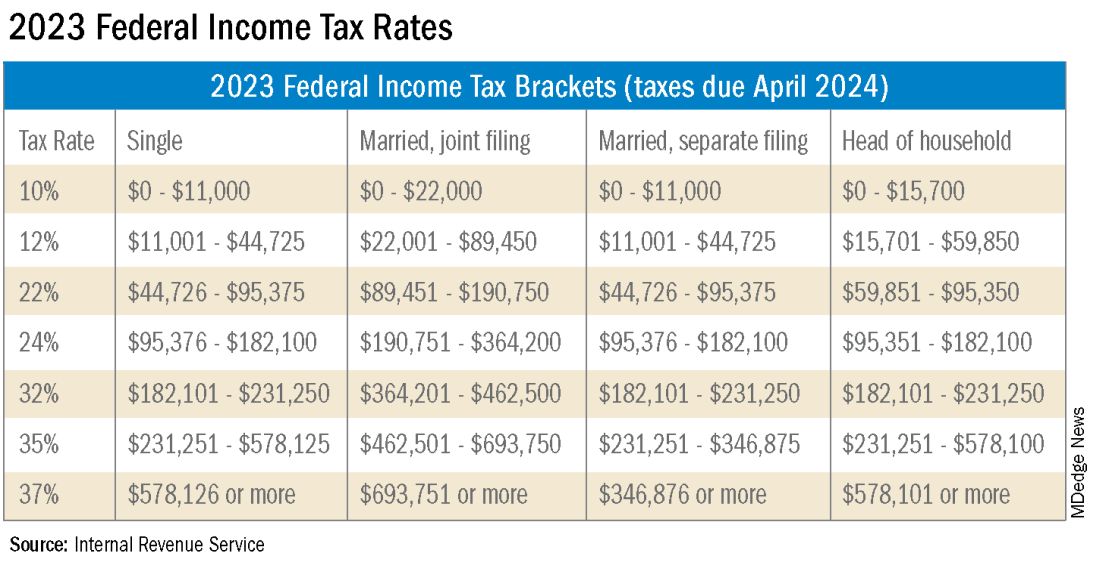
FAQ 3. I am a 1099 contractor; do I need a PLLC, and should I file as an S-Corporation?
A: The term “S-Corp” gets mentioned often related to 1099 contractors and can be extremely beneficial from a tax savings perspective. Often physicians may moonlight — in addition to working in their W2 positions — and would receive this compensation as a 1099 contractor rather than an employee. This is an example of when a Professional Limited Liability Company (PLLC) might be advisable. A PLLC is created at a state level and helps shield owners from potential litigation. The owner of a PLLC pays Social Security and Medicare taxes on all income earned from the entity, and the PLLC is included in the owner’s individual income tax return.
A Small-Corporation (S-Corporation) is a tax classification that passes income through to the owners. The PLLC is now taxed as an S-Corporation, rather than a disregarded entity. The shareholders of the S-Corporation are required to pay a reasonable salary (W2 income). The remaining income passes through to the owner and is not subject to Social Security and Medicare taxes, only federal income tax. This taxation status requires an additional tax return and payroll service. Because there are additional expenses with being taxed as an S-Corporation, a cost-benefit analysis should be done before changing the tax classification to confirm that the tax savings are greater than the additional costs.
FAQ 4. What is the ‘backdoor Roth’ strategy? Should I implement it?
A: A Roth IRA is a specific type of Individual Retirement Account (IRA) that is funded with after-tax dollars. The contributions and growth in a Roth IRA can be withdrawn at retirement, tax free. As physicians who are typically high earners, you are not able to contribute directly to a Roth IRA because of income limitations. This is where the Roth conversion strategy — the backdoor Roth — comes into play. This strategy allows you to make a nondeductible traditional IRA contribution and then convert those dollars into a Roth IRA. In 2023, you can contribute up to $6,500 into this type of account. There are many additional considerations that must be made before implementing this strategy. Discussion with a financial advisor or CPA is recommended.
FAQ 5. I’ve always done my own taxes. Do I need to hire a CPA?
A: For many physicians, especially during training, your tax situation may not warrant the need for a Certified Public Accountant (CPA). However, as your income and tax complexity increase, working with a CPA not only decreases your risk for error, but also helps ensure you are not overpaying in taxes. There are many different types of services that a CPA can offer, the most basic being tax preparation. This is simply compiling your tax return based on the circumstances that occurred in the prior year. Tax planning is an additional level of service that may not be included in tax preparation cost. Tax planning is a proactive approach to taxes and helps maximize tax savings opportunities before return preparation. When interviewing a potential CPA, you can ask what level of services are included in the fees quoted.
These are just a few of the questions I regularly answer related to physicians’ taxation. The tax code is complex and ever changing. Recommendations that are made today might not be applicable or advisable in the future to any given situation. Working with a professional can ensure you have the most up-to-date and accurate information related to your taxes.
Ms. Anderson is with Physician’s Resource Services and is on Instagram @physiciansrs . Dr. Anderson is a CA-1 Resident in Anesthesia at Baylor Scott and White Health. The authors have no conflicts of interest.
Physicians spend years of their lives in education and training. There are countless hours devoted to studying, researching, and clinical training, not to mention residency and possible fellowships. Then literally overnight, they transition out of a resident salary into a full-time attending pay with little to no education around what to do with this significant increase in salary.
Every job position is unique in terms of benefits, how compensation is earned, job expectations, etc. But they all share one thing in common — taxes. Increased income comes with increased taxes.
FAQ 1. What is the difference between W2 income and 1099 income?
A: If you are a W2 employee, your employer is responsible for paying half of your Social Security and Medicare taxes. You, as the employee, are then responsible only for the remaining half of your Social Security and Medicare taxes. Additionally, your employer will withhold these taxes, along with federal income taxes, from your paycheck each pay period. You are not responsible for remitting any taxes to the IRS or state agencies, as your employer will do this for you. As a W2 employee, you are not able to deduct any employee expenses against your income.
As a 1099 contractor, you are considered self-employed and are responsible for the employer and employee portion of the Social Security and Medicare taxes. You are also responsible for remitting these taxes, as well as quarterly estimated federal withholding, to the IRS and state agencies. You can deduct work-related expenses against your 1099 income.
Both types of income have pros and cons. Either of these can be more beneficial to a specific situation.
FAQ 2. How do I know if I am withholding enough taxes?
A: This is a very common issue I see, especially with physicians who are transitioning out of training into their full-time attending salary. Because this transition happens mid-year, often the first half of the year you are withholding at a rate much lower than what you will be earning as an attending and end up with a tax surprise at filing. One way to remedy this is to look at how much taxes are being withheld from your paycheck and compare this to what tax bracket you anticipate to be in, depending on filing status (Figure 1). If you do this and realize you are not withholding enough taxes, you can submit an amended form W4 to your employer to have additional withholding taken out each pay period.

FAQ 3. I am a 1099 contractor; do I need a PLLC, and should I file as an S-Corporation?
A: The term “S-Corp” gets mentioned often related to 1099 contractors and can be extremely beneficial from a tax savings perspective. Often physicians may moonlight — in addition to working in their W2 positions — and would receive this compensation as a 1099 contractor rather than an employee. This is an example of when a Professional Limited Liability Company (PLLC) might be advisable. A PLLC is created at a state level and helps shield owners from potential litigation. The owner of a PLLC pays Social Security and Medicare taxes on all income earned from the entity, and the PLLC is included in the owner’s individual income tax return.
A Small-Corporation (S-Corporation) is a tax classification that passes income through to the owners. The PLLC is now taxed as an S-Corporation, rather than a disregarded entity. The shareholders of the S-Corporation are required to pay a reasonable salary (W2 income). The remaining income passes through to the owner and is not subject to Social Security and Medicare taxes, only federal income tax. This taxation status requires an additional tax return and payroll service. Because there are additional expenses with being taxed as an S-Corporation, a cost-benefit analysis should be done before changing the tax classification to confirm that the tax savings are greater than the additional costs.
FAQ 4. What is the ‘backdoor Roth’ strategy? Should I implement it?
A: A Roth IRA is a specific type of Individual Retirement Account (IRA) that is funded with after-tax dollars. The contributions and growth in a Roth IRA can be withdrawn at retirement, tax free. As physicians who are typically high earners, you are not able to contribute directly to a Roth IRA because of income limitations. This is where the Roth conversion strategy — the backdoor Roth — comes into play. This strategy allows you to make a nondeductible traditional IRA contribution and then convert those dollars into a Roth IRA. In 2023, you can contribute up to $6,500 into this type of account. There are many additional considerations that must be made before implementing this strategy. Discussion with a financial advisor or CPA is recommended.
FAQ 5. I’ve always done my own taxes. Do I need to hire a CPA?
A: For many physicians, especially during training, your tax situation may not warrant the need for a Certified Public Accountant (CPA). However, as your income and tax complexity increase, working with a CPA not only decreases your risk for error, but also helps ensure you are not overpaying in taxes. There are many different types of services that a CPA can offer, the most basic being tax preparation. This is simply compiling your tax return based on the circumstances that occurred in the prior year. Tax planning is an additional level of service that may not be included in tax preparation cost. Tax planning is a proactive approach to taxes and helps maximize tax savings opportunities before return preparation. When interviewing a potential CPA, you can ask what level of services are included in the fees quoted.
These are just a few of the questions I regularly answer related to physicians’ taxation. The tax code is complex and ever changing. Recommendations that are made today might not be applicable or advisable in the future to any given situation. Working with a professional can ensure you have the most up-to-date and accurate information related to your taxes.
Ms. Anderson is with Physician’s Resource Services and is on Instagram @physiciansrs . Dr. Anderson is a CA-1 Resident in Anesthesia at Baylor Scott and White Health. The authors have no conflicts of interest.
Physicians spend years of their lives in education and training. There are countless hours devoted to studying, researching, and clinical training, not to mention residency and possible fellowships. Then literally overnight, they transition out of a resident salary into a full-time attending pay with little to no education around what to do with this significant increase in salary.
Every job position is unique in terms of benefits, how compensation is earned, job expectations, etc. But they all share one thing in common — taxes. Increased income comes with increased taxes.
FAQ 1. What is the difference between W2 income and 1099 income?
A: If you are a W2 employee, your employer is responsible for paying half of your Social Security and Medicare taxes. You, as the employee, are then responsible only for the remaining half of your Social Security and Medicare taxes. Additionally, your employer will withhold these taxes, along with federal income taxes, from your paycheck each pay period. You are not responsible for remitting any taxes to the IRS or state agencies, as your employer will do this for you. As a W2 employee, you are not able to deduct any employee expenses against your income.
As a 1099 contractor, you are considered self-employed and are responsible for the employer and employee portion of the Social Security and Medicare taxes. You are also responsible for remitting these taxes, as well as quarterly estimated federal withholding, to the IRS and state agencies. You can deduct work-related expenses against your 1099 income.
Both types of income have pros and cons. Either of these can be more beneficial to a specific situation.
FAQ 2. How do I know if I am withholding enough taxes?
A: This is a very common issue I see, especially with physicians who are transitioning out of training into their full-time attending salary. Because this transition happens mid-year, often the first half of the year you are withholding at a rate much lower than what you will be earning as an attending and end up with a tax surprise at filing. One way to remedy this is to look at how much taxes are being withheld from your paycheck and compare this to what tax bracket you anticipate to be in, depending on filing status (Figure 1). If you do this and realize you are not withholding enough taxes, you can submit an amended form W4 to your employer to have additional withholding taken out each pay period.

FAQ 3. I am a 1099 contractor; do I need a PLLC, and should I file as an S-Corporation?
A: The term “S-Corp” gets mentioned often related to 1099 contractors and can be extremely beneficial from a tax savings perspective. Often physicians may moonlight — in addition to working in their W2 positions — and would receive this compensation as a 1099 contractor rather than an employee. This is an example of when a Professional Limited Liability Company (PLLC) might be advisable. A PLLC is created at a state level and helps shield owners from potential litigation. The owner of a PLLC pays Social Security and Medicare taxes on all income earned from the entity, and the PLLC is included in the owner’s individual income tax return.
A Small-Corporation (S-Corporation) is a tax classification that passes income through to the owners. The PLLC is now taxed as an S-Corporation, rather than a disregarded entity. The shareholders of the S-Corporation are required to pay a reasonable salary (W2 income). The remaining income passes through to the owner and is not subject to Social Security and Medicare taxes, only federal income tax. This taxation status requires an additional tax return and payroll service. Because there are additional expenses with being taxed as an S-Corporation, a cost-benefit analysis should be done before changing the tax classification to confirm that the tax savings are greater than the additional costs.
FAQ 4. What is the ‘backdoor Roth’ strategy? Should I implement it?
A: A Roth IRA is a specific type of Individual Retirement Account (IRA) that is funded with after-tax dollars. The contributions and growth in a Roth IRA can be withdrawn at retirement, tax free. As physicians who are typically high earners, you are not able to contribute directly to a Roth IRA because of income limitations. This is where the Roth conversion strategy — the backdoor Roth — comes into play. This strategy allows you to make a nondeductible traditional IRA contribution and then convert those dollars into a Roth IRA. In 2023, you can contribute up to $6,500 into this type of account. There are many additional considerations that must be made before implementing this strategy. Discussion with a financial advisor or CPA is recommended.
FAQ 5. I’ve always done my own taxes. Do I need to hire a CPA?
A: For many physicians, especially during training, your tax situation may not warrant the need for a Certified Public Accountant (CPA). However, as your income and tax complexity increase, working with a CPA not only decreases your risk for error, but also helps ensure you are not overpaying in taxes. There are many different types of services that a CPA can offer, the most basic being tax preparation. This is simply compiling your tax return based on the circumstances that occurred in the prior year. Tax planning is an additional level of service that may not be included in tax preparation cost. Tax planning is a proactive approach to taxes and helps maximize tax savings opportunities before return preparation. When interviewing a potential CPA, you can ask what level of services are included in the fees quoted.
These are just a few of the questions I regularly answer related to physicians’ taxation. The tax code is complex and ever changing. Recommendations that are made today might not be applicable or advisable in the future to any given situation. Working with a professional can ensure you have the most up-to-date and accurate information related to your taxes.
Ms. Anderson is with Physician’s Resource Services and is on Instagram @physiciansrs . Dr. Anderson is a CA-1 Resident in Anesthesia at Baylor Scott and White Health. The authors have no conflicts of interest.











