User login
Early Warning Systems: The Neglected Importance of Timing
Automated early warning systems (EWSs) use data inputs to recognize clinical states requiring time-sensitive intervention and then generate notifications through different modalities to clinicians. EWSs serve as common tools for improving the recognition and treatment of important clinical states such as sepsis. However, despite the early enthusiasm, these warning systems have often yielded disappointing outcomes. In sepsis, for example, EWSs have shown mixed results in clinical trials, and concerns regarding the overuse of EWSs in diagnosing sepsis have grown.1-4 We argue that inattention to the importance of timing in EWS training and evaluation provides one reason that EWSs have underperformed. Thus, to improve care, a warning system must not only identify the clinical state accurately, but it must also do so in a sufficiently timely manner to implement the associated interventions, such as administration of antibiotics for sepsis. Although the literature has occasionally highlighted the importance of timing in electronic surveillance systems, no one has linked the temporal dependence of performance metrics and intervention feasibility to the failure of such warning systems and explained how to operationalize timing in their development.5-8 Using sepsis as an example, we explain why timing is important and propose new metrics and strategies for training and evaluating EWS models. EWSs are divided into two types: detection systems that recognize critical illnesses at a particular moment and prediction systems that estimate risk of deterioration over varying time frames.9 We focus primarily on detection systems, but our analysis is also important for prediction systems, which we will discuss in the last section.
CLINICAL TIME ZERO AND POSITIVE PREDICTIVE VALUE
EWS metrics have evolved from focusing on crude measures of discrimination to more clinically relevant metrics, such as the positive predictive value (PPV). The common performance metrics, including the c-statistic, evaluate the performance of EWSs in distinguishing events from nonevents, such as the presence or absence of sepsis in hospitalized patients. However, the c-statistic does not account for disease prevalence. A given c-statistic is compatible with a wide range of PPVs; a low PPV may limit an EWS’s usefulness to promote interventions and generate increased alert fatigue.10
However, the PPV, although important, provides no information on the timing of state recognition in relation to clinical time zero. Time zero is the first moment at which a critical state can be recognized based on available data and current medical science. Different approaches, including laboratory values, clinical assessments, retrospective chart reviews, triage times, and others, have been used to measure time zero.8,11-13 All these approaches feature advantages and disadvantages; the evaluation of timing will exhibit sensitivity to the approach used.14 Further work is needed to gain additional insights into the measurement of time zero.
Just as the same c-statistic is consistent with varying PPVs, so too is the same PPV consistent with different timing in relation to clinical time zero (Figure). An alert-level PPV of 50% indicates that 50% of the alerts signify true cases of sepsis. However, such a value could also indicate any of the following:
a) 50% true cases of sepsis, with a mean time of 35 minutes after clinical time zero;
b) 50% true cases, with a mean time of 60 minutes before clinical time zero (prediction EWS);
c) 50% true cases of sepsis, with a mean time of 1.3 days since clinical time zero, but with 70% of these cases undiagnosed at the time of EWS detection;
d) 50% true cases of cases, with mean time of 1.3 days since clinical time zero, that is, all cases among those promptly detected and treated through routine clinician oversight.
Each of these situations features differing clinical utility to help meet the hospital objective of increasing early administration of antibiotics. More generally, three dimensions of timing are important for detection systems. The first dimension is the timing of detection relative to time zero. The second is the timing relative to ”real-world” clinician detection. The third is timing with respect to the associated clinical objective. For a given PPV, an EWS performs better when detecting a state (1) at, near, or in advance of time zero, (2) prior to clinician detection, and (3) sufficiently in advance of an operational objective to promote change. On the other hand, when an EWS consistently sends alerts after clinician action, it serves a lesser purpose and risks causing alert fatigue; such cases have been described in studies.15
OPERATIONALIZING TIMING IN EWS TRAINING AND EVALUATION
Acknowledging the importance of timing features implications for researchers and health system leaders. Researchers who develop EWS should include how these systems perform relative to both time zero and critical milestones in the clinical course. Operational leadership should understand the trade-offs that occur between alert fatigue (through lower PPV at the margin with earlier detection) and lead time to implement an intervention. Navigating these trade-offs involves a complex organizational decision. The “number needed to evaluate” is one way to quantify this fatigue factor.16 Such a measure gives a sense of the number of cases a clinician will need to evaluate per event. Collaborations between clinical leadership, operational leadership, and data scientists are needed to determine how to evaluate individual systems.
A good metric should capture the three important dimensions of timing while retaining intuitiveness to clinicians and leadership. One graphical option involves plotting the PPVs over time and relative to the clinical state evolution (Figure). This PPV-over-time curve shows when true positives occur relative to the time course of sepsis, including the three major dimensions of timing. This curve can also show a “clinically important window (CIW)”, which is bounded on the right by the latest point in time when recognition could still meet the clinical objective. For sepsis, the curve might be bounded at 2.5 hours to meet an objective of antibiotics within three hours, with the assumption that 0.5 hour is needed for a response. For detection systems, the window would be bounded on the left by clinical time zero. The graph can also designate the point when most cases of sepsis have been recognized clinically with historical data. The Figure depicts an example curve for a detection model.
The metrics derived from this curve may be used alongside the PPV for training and evaluation. Often, adjusting the PPV for its relationship to time zero and the CIW will aid in recognizing the existence of a time beyond which detection fails to help achieve the intended intervention. Detection beyond the window should not credited as a true positive if it fails to facilitate the objective. One option is to credit detection at or before time zero as one and discount later detection by the delay from time zero. More specifically, a true positive could be discounted by the difference between the end of the CIW and the moment of detection divided by the CIW length. This discounted PPV could be displayed alongside the PPV to gauge the temporal dimension of performance and be used for training.
The use of timing places additional demands on validation owing to the need for a time-based gold standard. In such a case, the unit of analysis in system development might not be the patient encounter but rather the patient-hour or patient-15-minute epoch, depending on how frequently the EWS updates risk information and may alert. By contrast, the sepsis detection models used in administrative databases rely on an encounter-level PPV, which provides more limited information compared with real-time EWSs.17 When time zero cannot be measured, alternatives may be used to capture several dimensions of timing; these alternatives include measurement of the percentage of cases that recognize the event prior to clinicians.15
MOVING TOWARD PREDICTION
Detection systems face the limitation that they lack the capability to identify a state before its occurrence. Prediction systems are more likely to be actionable, as they provide more lead time for intervention, but accurate prediction models are also more difficult to develop. With a predictive system, an additional dimension of timing becomes important: the time horizon for prediction. Prediction models may be trained to recognize a state within a specific time frame (eg, 6, 12, or 24 hours), and test characteristics, including PPV, may vary with the window.18 A given PPV (of eventual development of sepsis) is compatible with varying time windows and thus again lacks important information on performance.
The timing relative to clinical time zero remains important for prediction. For a predictive EWS, the graph in the figure may be expected to shift to the left. Models with good performance will occasionally send an alert after time zero. For a prediction system with a time horizon of six hours, it is more useful to have alerts occur a mean time of four hours prior to time zero than four minutes prior.
CONCLUSION
Improving the clinical utility of EWSs requires better measurement of timing. Researchers should incorporate timing into system development, and operational leaders should be cognizant of timing during implementation. Specific steps should include devising better strategies to estimate the relationship of state recognition to clinical time zero and developing methods to discount recognition when it occurs too late to be actionable.
Disclosures
Dr. Rolnick is a consultant to Tuple Health, Inc. and was previously a part-time employee of Acumen, LLC. Dr. Weissman has nothing to disclose.
1. The Lancet Respiratory Medicine. Crying wolf: the growing fatigue around sepsis alerts. Lancet Respir Med. 2018;6(3):161. doi: 10.1016/S2213-2600(18)30072-9.
2. Hooper MH, Weavind L, Wheeler AP, et al. Randomized trial of automated, electronic monitoring to facilitate early detection of sepsis in the intensive care unit. Crit Care Med. 2012;40(7):2096-2101. doi: 10.1097/CCM.0b013e318250a887. PubMed
3. Nelson JL, Smith BL, Jared JD, et al. Prospective trial of real-time electronic surveillance to expedite early care of severe sepsis. Ann Emerg Med. 2011;57(5):500-504. doi: 10.1016/j.annemergmed.2010.12.008. PubMed
4. Umscheid CA, Betesh J, VanZandbergen C, et al. Development, implementation, and impact of an automated early warning and response system for sepsis. J Hosp Med. 2015;10(1):26-31. doi: 10.1002/jhm.2259. PubMed
5. Kleinman KP, Abrams AM. Assessing surveillance using sensitivity, specificity and timeliness. Stat Methods Med Res. 2006;15(5):445-464. doi: 10.1177/0962280206071641. PubMed
6. Jiang X, Cooper GF, Neill DB. Generalized AMOC curves for evaluation and improvement of event surveillance. AMIA Annu Symp Proc. 2009;281-285. PubMed
7. Futoma J, Hariharan S, Sendak M, et al. An improved multi-output Gaussian process RNN with real-time validation for early sepsis detection. In Proceedings of the 2nd Machine Learning for Healthcare Conference (MLHC), Boston, MA, Aug 2017.
8. Rolnick J, Downing N, Shepard J, et al. Validation of test performance and clinical time zero for an electronic health record embedded severe sepsis alert. Appl Clin Inform. 2016;7(2):560-572. doi: 10.4338/ACI-2015-11-RA-0159. PubMed
9. DeVita MA, Smith GB, Adam SK, et al. “Identifying the hospitalised patient in crisis”—A consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. doi: 10.1016/j.resuscitation.2009.12.008. PubMed
10. Romero-Brufau S, Huddleston JM, Escobar GJ, et al. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):284-290. doi: 10.1186/s13054-015-0999-1. PubMed
11. Evans IVR, Phillips GS, Alpern ER, et al. Association between the New York sepsis care mandate and in-hospital mortality for pediatric sepsis. JAMA. 2018;320(4):358-367. doi: 10.1001/jama.2018.9071. PubMed
12. Daniels R, Nutbeam T, McNamara G, et al. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J. 2011;28(6):507-512. doi: 10.1136/emj.2010.095067. PubMed
13. Paul R, Melendez E, Wathen B, et al. A quality improvement collaborative for pediatric sepsis: lessons learned. Pediatr Qual Saf. 2018;3(1):1-8. doi: 10.1097/pq9.0000000000000051. PubMed
14. Rhee C, Brown SR, Jones TM, et al. Variability in determining sepsis time zero and bundle compliance rates for the centers for medicare and medicaid services SEP-1 measure. Infect Control Hosp Epidemiol. 2018;39(9):994-996. doi: 10.1017/ice.2018.134. PubMed
15. Winter MC, Kubis S, Bonafide CP. Beyond reporting early warning score sensitivity: the temporal relationship and clinical relevance of “true positive” alerts that precede critical deterioration. J Hosp Med. 2019;14(3):138-143. doi: 10.12788/jhm.3066. PubMed
1 6. Dummett BA, Adams C, Scruth E, et al. Incorporating an early detection system into routine clinical practice in two community hospitals: Incorporating an EWS into practice. J Hosp Med. 2016;11(51):S25-S31. doi: 10.1002/jhm.2661. PubMed
17. Jolley RJ, Quan H, Jetté N, et al. Validation and optimisation of an ICD-10-coded case definition for sepsis using administrative health data. BMJ Open. 2015;5(12):e009487. doi: 10.1136/bmjopen-2015-009487. PubMed
18. Wellner B, Grand J, Canzone E, et al. Predicting unplanned transfers to the intensive care unit: a machine learning approach leveraging diverse clinical elements. JMIR Med Inform. 2017;5(4):e45. doi: 10.2196/medinform.8680. PubMed
Automated early warning systems (EWSs) use data inputs to recognize clinical states requiring time-sensitive intervention and then generate notifications through different modalities to clinicians. EWSs serve as common tools for improving the recognition and treatment of important clinical states such as sepsis. However, despite the early enthusiasm, these warning systems have often yielded disappointing outcomes. In sepsis, for example, EWSs have shown mixed results in clinical trials, and concerns regarding the overuse of EWSs in diagnosing sepsis have grown.1-4 We argue that inattention to the importance of timing in EWS training and evaluation provides one reason that EWSs have underperformed. Thus, to improve care, a warning system must not only identify the clinical state accurately, but it must also do so in a sufficiently timely manner to implement the associated interventions, such as administration of antibiotics for sepsis. Although the literature has occasionally highlighted the importance of timing in electronic surveillance systems, no one has linked the temporal dependence of performance metrics and intervention feasibility to the failure of such warning systems and explained how to operationalize timing in their development.5-8 Using sepsis as an example, we explain why timing is important and propose new metrics and strategies for training and evaluating EWS models. EWSs are divided into two types: detection systems that recognize critical illnesses at a particular moment and prediction systems that estimate risk of deterioration over varying time frames.9 We focus primarily on detection systems, but our analysis is also important for prediction systems, which we will discuss in the last section.
CLINICAL TIME ZERO AND POSITIVE PREDICTIVE VALUE
EWS metrics have evolved from focusing on crude measures of discrimination to more clinically relevant metrics, such as the positive predictive value (PPV). The common performance metrics, including the c-statistic, evaluate the performance of EWSs in distinguishing events from nonevents, such as the presence or absence of sepsis in hospitalized patients. However, the c-statistic does not account for disease prevalence. A given c-statistic is compatible with a wide range of PPVs; a low PPV may limit an EWS’s usefulness to promote interventions and generate increased alert fatigue.10
However, the PPV, although important, provides no information on the timing of state recognition in relation to clinical time zero. Time zero is the first moment at which a critical state can be recognized based on available data and current medical science. Different approaches, including laboratory values, clinical assessments, retrospective chart reviews, triage times, and others, have been used to measure time zero.8,11-13 All these approaches feature advantages and disadvantages; the evaluation of timing will exhibit sensitivity to the approach used.14 Further work is needed to gain additional insights into the measurement of time zero.
Just as the same c-statistic is consistent with varying PPVs, so too is the same PPV consistent with different timing in relation to clinical time zero (Figure). An alert-level PPV of 50% indicates that 50% of the alerts signify true cases of sepsis. However, such a value could also indicate any of the following:
a) 50% true cases of sepsis, with a mean time of 35 minutes after clinical time zero;
b) 50% true cases, with a mean time of 60 minutes before clinical time zero (prediction EWS);
c) 50% true cases of sepsis, with a mean time of 1.3 days since clinical time zero, but with 70% of these cases undiagnosed at the time of EWS detection;
d) 50% true cases of cases, with mean time of 1.3 days since clinical time zero, that is, all cases among those promptly detected and treated through routine clinician oversight.
Each of these situations features differing clinical utility to help meet the hospital objective of increasing early administration of antibiotics. More generally, three dimensions of timing are important for detection systems. The first dimension is the timing of detection relative to time zero. The second is the timing relative to ”real-world” clinician detection. The third is timing with respect to the associated clinical objective. For a given PPV, an EWS performs better when detecting a state (1) at, near, or in advance of time zero, (2) prior to clinician detection, and (3) sufficiently in advance of an operational objective to promote change. On the other hand, when an EWS consistently sends alerts after clinician action, it serves a lesser purpose and risks causing alert fatigue; such cases have been described in studies.15
OPERATIONALIZING TIMING IN EWS TRAINING AND EVALUATION
Acknowledging the importance of timing features implications for researchers and health system leaders. Researchers who develop EWS should include how these systems perform relative to both time zero and critical milestones in the clinical course. Operational leadership should understand the trade-offs that occur between alert fatigue (through lower PPV at the margin with earlier detection) and lead time to implement an intervention. Navigating these trade-offs involves a complex organizational decision. The “number needed to evaluate” is one way to quantify this fatigue factor.16 Such a measure gives a sense of the number of cases a clinician will need to evaluate per event. Collaborations between clinical leadership, operational leadership, and data scientists are needed to determine how to evaluate individual systems.
A good metric should capture the three important dimensions of timing while retaining intuitiveness to clinicians and leadership. One graphical option involves plotting the PPVs over time and relative to the clinical state evolution (Figure). This PPV-over-time curve shows when true positives occur relative to the time course of sepsis, including the three major dimensions of timing. This curve can also show a “clinically important window (CIW)”, which is bounded on the right by the latest point in time when recognition could still meet the clinical objective. For sepsis, the curve might be bounded at 2.5 hours to meet an objective of antibiotics within three hours, with the assumption that 0.5 hour is needed for a response. For detection systems, the window would be bounded on the left by clinical time zero. The graph can also designate the point when most cases of sepsis have been recognized clinically with historical data. The Figure depicts an example curve for a detection model.
The metrics derived from this curve may be used alongside the PPV for training and evaluation. Often, adjusting the PPV for its relationship to time zero and the CIW will aid in recognizing the existence of a time beyond which detection fails to help achieve the intended intervention. Detection beyond the window should not credited as a true positive if it fails to facilitate the objective. One option is to credit detection at or before time zero as one and discount later detection by the delay from time zero. More specifically, a true positive could be discounted by the difference between the end of the CIW and the moment of detection divided by the CIW length. This discounted PPV could be displayed alongside the PPV to gauge the temporal dimension of performance and be used for training.
The use of timing places additional demands on validation owing to the need for a time-based gold standard. In such a case, the unit of analysis in system development might not be the patient encounter but rather the patient-hour or patient-15-minute epoch, depending on how frequently the EWS updates risk information and may alert. By contrast, the sepsis detection models used in administrative databases rely on an encounter-level PPV, which provides more limited information compared with real-time EWSs.17 When time zero cannot be measured, alternatives may be used to capture several dimensions of timing; these alternatives include measurement of the percentage of cases that recognize the event prior to clinicians.15
MOVING TOWARD PREDICTION
Detection systems face the limitation that they lack the capability to identify a state before its occurrence. Prediction systems are more likely to be actionable, as they provide more lead time for intervention, but accurate prediction models are also more difficult to develop. With a predictive system, an additional dimension of timing becomes important: the time horizon for prediction. Prediction models may be trained to recognize a state within a specific time frame (eg, 6, 12, or 24 hours), and test characteristics, including PPV, may vary with the window.18 A given PPV (of eventual development of sepsis) is compatible with varying time windows and thus again lacks important information on performance.
The timing relative to clinical time zero remains important for prediction. For a predictive EWS, the graph in the figure may be expected to shift to the left. Models with good performance will occasionally send an alert after time zero. For a prediction system with a time horizon of six hours, it is more useful to have alerts occur a mean time of four hours prior to time zero than four minutes prior.
CONCLUSION
Improving the clinical utility of EWSs requires better measurement of timing. Researchers should incorporate timing into system development, and operational leaders should be cognizant of timing during implementation. Specific steps should include devising better strategies to estimate the relationship of state recognition to clinical time zero and developing methods to discount recognition when it occurs too late to be actionable.
Disclosures
Dr. Rolnick is a consultant to Tuple Health, Inc. and was previously a part-time employee of Acumen, LLC. Dr. Weissman has nothing to disclose.
Automated early warning systems (EWSs) use data inputs to recognize clinical states requiring time-sensitive intervention and then generate notifications through different modalities to clinicians. EWSs serve as common tools for improving the recognition and treatment of important clinical states such as sepsis. However, despite the early enthusiasm, these warning systems have often yielded disappointing outcomes. In sepsis, for example, EWSs have shown mixed results in clinical trials, and concerns regarding the overuse of EWSs in diagnosing sepsis have grown.1-4 We argue that inattention to the importance of timing in EWS training and evaluation provides one reason that EWSs have underperformed. Thus, to improve care, a warning system must not only identify the clinical state accurately, but it must also do so in a sufficiently timely manner to implement the associated interventions, such as administration of antibiotics for sepsis. Although the literature has occasionally highlighted the importance of timing in electronic surveillance systems, no one has linked the temporal dependence of performance metrics and intervention feasibility to the failure of such warning systems and explained how to operationalize timing in their development.5-8 Using sepsis as an example, we explain why timing is important and propose new metrics and strategies for training and evaluating EWS models. EWSs are divided into two types: detection systems that recognize critical illnesses at a particular moment and prediction systems that estimate risk of deterioration over varying time frames.9 We focus primarily on detection systems, but our analysis is also important for prediction systems, which we will discuss in the last section.
CLINICAL TIME ZERO AND POSITIVE PREDICTIVE VALUE
EWS metrics have evolved from focusing on crude measures of discrimination to more clinically relevant metrics, such as the positive predictive value (PPV). The common performance metrics, including the c-statistic, evaluate the performance of EWSs in distinguishing events from nonevents, such as the presence or absence of sepsis in hospitalized patients. However, the c-statistic does not account for disease prevalence. A given c-statistic is compatible with a wide range of PPVs; a low PPV may limit an EWS’s usefulness to promote interventions and generate increased alert fatigue.10
However, the PPV, although important, provides no information on the timing of state recognition in relation to clinical time zero. Time zero is the first moment at which a critical state can be recognized based on available data and current medical science. Different approaches, including laboratory values, clinical assessments, retrospective chart reviews, triage times, and others, have been used to measure time zero.8,11-13 All these approaches feature advantages and disadvantages; the evaluation of timing will exhibit sensitivity to the approach used.14 Further work is needed to gain additional insights into the measurement of time zero.
Just as the same c-statistic is consistent with varying PPVs, so too is the same PPV consistent with different timing in relation to clinical time zero (Figure). An alert-level PPV of 50% indicates that 50% of the alerts signify true cases of sepsis. However, such a value could also indicate any of the following:
a) 50% true cases of sepsis, with a mean time of 35 minutes after clinical time zero;
b) 50% true cases, with a mean time of 60 minutes before clinical time zero (prediction EWS);
c) 50% true cases of sepsis, with a mean time of 1.3 days since clinical time zero, but with 70% of these cases undiagnosed at the time of EWS detection;
d) 50% true cases of cases, with mean time of 1.3 days since clinical time zero, that is, all cases among those promptly detected and treated through routine clinician oversight.
Each of these situations features differing clinical utility to help meet the hospital objective of increasing early administration of antibiotics. More generally, three dimensions of timing are important for detection systems. The first dimension is the timing of detection relative to time zero. The second is the timing relative to ”real-world” clinician detection. The third is timing with respect to the associated clinical objective. For a given PPV, an EWS performs better when detecting a state (1) at, near, or in advance of time zero, (2) prior to clinician detection, and (3) sufficiently in advance of an operational objective to promote change. On the other hand, when an EWS consistently sends alerts after clinician action, it serves a lesser purpose and risks causing alert fatigue; such cases have been described in studies.15
OPERATIONALIZING TIMING IN EWS TRAINING AND EVALUATION
Acknowledging the importance of timing features implications for researchers and health system leaders. Researchers who develop EWS should include how these systems perform relative to both time zero and critical milestones in the clinical course. Operational leadership should understand the trade-offs that occur between alert fatigue (through lower PPV at the margin with earlier detection) and lead time to implement an intervention. Navigating these trade-offs involves a complex organizational decision. The “number needed to evaluate” is one way to quantify this fatigue factor.16 Such a measure gives a sense of the number of cases a clinician will need to evaluate per event. Collaborations between clinical leadership, operational leadership, and data scientists are needed to determine how to evaluate individual systems.
A good metric should capture the three important dimensions of timing while retaining intuitiveness to clinicians and leadership. One graphical option involves plotting the PPVs over time and relative to the clinical state evolution (Figure). This PPV-over-time curve shows when true positives occur relative to the time course of sepsis, including the three major dimensions of timing. This curve can also show a “clinically important window (CIW)”, which is bounded on the right by the latest point in time when recognition could still meet the clinical objective. For sepsis, the curve might be bounded at 2.5 hours to meet an objective of antibiotics within three hours, with the assumption that 0.5 hour is needed for a response. For detection systems, the window would be bounded on the left by clinical time zero. The graph can also designate the point when most cases of sepsis have been recognized clinically with historical data. The Figure depicts an example curve for a detection model.
The metrics derived from this curve may be used alongside the PPV for training and evaluation. Often, adjusting the PPV for its relationship to time zero and the CIW will aid in recognizing the existence of a time beyond which detection fails to help achieve the intended intervention. Detection beyond the window should not credited as a true positive if it fails to facilitate the objective. One option is to credit detection at or before time zero as one and discount later detection by the delay from time zero. More specifically, a true positive could be discounted by the difference between the end of the CIW and the moment of detection divided by the CIW length. This discounted PPV could be displayed alongside the PPV to gauge the temporal dimension of performance and be used for training.
The use of timing places additional demands on validation owing to the need for a time-based gold standard. In such a case, the unit of analysis in system development might not be the patient encounter but rather the patient-hour or patient-15-minute epoch, depending on how frequently the EWS updates risk information and may alert. By contrast, the sepsis detection models used in administrative databases rely on an encounter-level PPV, which provides more limited information compared with real-time EWSs.17 When time zero cannot be measured, alternatives may be used to capture several dimensions of timing; these alternatives include measurement of the percentage of cases that recognize the event prior to clinicians.15
MOVING TOWARD PREDICTION
Detection systems face the limitation that they lack the capability to identify a state before its occurrence. Prediction systems are more likely to be actionable, as they provide more lead time for intervention, but accurate prediction models are also more difficult to develop. With a predictive system, an additional dimension of timing becomes important: the time horizon for prediction. Prediction models may be trained to recognize a state within a specific time frame (eg, 6, 12, or 24 hours), and test characteristics, including PPV, may vary with the window.18 A given PPV (of eventual development of sepsis) is compatible with varying time windows and thus again lacks important information on performance.
The timing relative to clinical time zero remains important for prediction. For a predictive EWS, the graph in the figure may be expected to shift to the left. Models with good performance will occasionally send an alert after time zero. For a prediction system with a time horizon of six hours, it is more useful to have alerts occur a mean time of four hours prior to time zero than four minutes prior.
CONCLUSION
Improving the clinical utility of EWSs requires better measurement of timing. Researchers should incorporate timing into system development, and operational leaders should be cognizant of timing during implementation. Specific steps should include devising better strategies to estimate the relationship of state recognition to clinical time zero and developing methods to discount recognition when it occurs too late to be actionable.
Disclosures
Dr. Rolnick is a consultant to Tuple Health, Inc. and was previously a part-time employee of Acumen, LLC. Dr. Weissman has nothing to disclose.
1. The Lancet Respiratory Medicine. Crying wolf: the growing fatigue around sepsis alerts. Lancet Respir Med. 2018;6(3):161. doi: 10.1016/S2213-2600(18)30072-9.
2. Hooper MH, Weavind L, Wheeler AP, et al. Randomized trial of automated, electronic monitoring to facilitate early detection of sepsis in the intensive care unit. Crit Care Med. 2012;40(7):2096-2101. doi: 10.1097/CCM.0b013e318250a887. PubMed
3. Nelson JL, Smith BL, Jared JD, et al. Prospective trial of real-time electronic surveillance to expedite early care of severe sepsis. Ann Emerg Med. 2011;57(5):500-504. doi: 10.1016/j.annemergmed.2010.12.008. PubMed
4. Umscheid CA, Betesh J, VanZandbergen C, et al. Development, implementation, and impact of an automated early warning and response system for sepsis. J Hosp Med. 2015;10(1):26-31. doi: 10.1002/jhm.2259. PubMed
5. Kleinman KP, Abrams AM. Assessing surveillance using sensitivity, specificity and timeliness. Stat Methods Med Res. 2006;15(5):445-464. doi: 10.1177/0962280206071641. PubMed
6. Jiang X, Cooper GF, Neill DB. Generalized AMOC curves for evaluation and improvement of event surveillance. AMIA Annu Symp Proc. 2009;281-285. PubMed
7. Futoma J, Hariharan S, Sendak M, et al. An improved multi-output Gaussian process RNN with real-time validation for early sepsis detection. In Proceedings of the 2nd Machine Learning for Healthcare Conference (MLHC), Boston, MA, Aug 2017.
8. Rolnick J, Downing N, Shepard J, et al. Validation of test performance and clinical time zero for an electronic health record embedded severe sepsis alert. Appl Clin Inform. 2016;7(2):560-572. doi: 10.4338/ACI-2015-11-RA-0159. PubMed
9. DeVita MA, Smith GB, Adam SK, et al. “Identifying the hospitalised patient in crisis”—A consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. doi: 10.1016/j.resuscitation.2009.12.008. PubMed
10. Romero-Brufau S, Huddleston JM, Escobar GJ, et al. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):284-290. doi: 10.1186/s13054-015-0999-1. PubMed
11. Evans IVR, Phillips GS, Alpern ER, et al. Association between the New York sepsis care mandate and in-hospital mortality for pediatric sepsis. JAMA. 2018;320(4):358-367. doi: 10.1001/jama.2018.9071. PubMed
12. Daniels R, Nutbeam T, McNamara G, et al. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J. 2011;28(6):507-512. doi: 10.1136/emj.2010.095067. PubMed
13. Paul R, Melendez E, Wathen B, et al. A quality improvement collaborative for pediatric sepsis: lessons learned. Pediatr Qual Saf. 2018;3(1):1-8. doi: 10.1097/pq9.0000000000000051. PubMed
14. Rhee C, Brown SR, Jones TM, et al. Variability in determining sepsis time zero and bundle compliance rates for the centers for medicare and medicaid services SEP-1 measure. Infect Control Hosp Epidemiol. 2018;39(9):994-996. doi: 10.1017/ice.2018.134. PubMed
15. Winter MC, Kubis S, Bonafide CP. Beyond reporting early warning score sensitivity: the temporal relationship and clinical relevance of “true positive” alerts that precede critical deterioration. J Hosp Med. 2019;14(3):138-143. doi: 10.12788/jhm.3066. PubMed
1 6. Dummett BA, Adams C, Scruth E, et al. Incorporating an early detection system into routine clinical practice in two community hospitals: Incorporating an EWS into practice. J Hosp Med. 2016;11(51):S25-S31. doi: 10.1002/jhm.2661. PubMed
17. Jolley RJ, Quan H, Jetté N, et al. Validation and optimisation of an ICD-10-coded case definition for sepsis using administrative health data. BMJ Open. 2015;5(12):e009487. doi: 10.1136/bmjopen-2015-009487. PubMed
18. Wellner B, Grand J, Canzone E, et al. Predicting unplanned transfers to the intensive care unit: a machine learning approach leveraging diverse clinical elements. JMIR Med Inform. 2017;5(4):e45. doi: 10.2196/medinform.8680. PubMed
1. The Lancet Respiratory Medicine. Crying wolf: the growing fatigue around sepsis alerts. Lancet Respir Med. 2018;6(3):161. doi: 10.1016/S2213-2600(18)30072-9.
2. Hooper MH, Weavind L, Wheeler AP, et al. Randomized trial of automated, electronic monitoring to facilitate early detection of sepsis in the intensive care unit. Crit Care Med. 2012;40(7):2096-2101. doi: 10.1097/CCM.0b013e318250a887. PubMed
3. Nelson JL, Smith BL, Jared JD, et al. Prospective trial of real-time electronic surveillance to expedite early care of severe sepsis. Ann Emerg Med. 2011;57(5):500-504. doi: 10.1016/j.annemergmed.2010.12.008. PubMed
4. Umscheid CA, Betesh J, VanZandbergen C, et al. Development, implementation, and impact of an automated early warning and response system for sepsis. J Hosp Med. 2015;10(1):26-31. doi: 10.1002/jhm.2259. PubMed
5. Kleinman KP, Abrams AM. Assessing surveillance using sensitivity, specificity and timeliness. Stat Methods Med Res. 2006;15(5):445-464. doi: 10.1177/0962280206071641. PubMed
6. Jiang X, Cooper GF, Neill DB. Generalized AMOC curves for evaluation and improvement of event surveillance. AMIA Annu Symp Proc. 2009;281-285. PubMed
7. Futoma J, Hariharan S, Sendak M, et al. An improved multi-output Gaussian process RNN with real-time validation for early sepsis detection. In Proceedings of the 2nd Machine Learning for Healthcare Conference (MLHC), Boston, MA, Aug 2017.
8. Rolnick J, Downing N, Shepard J, et al. Validation of test performance and clinical time zero for an electronic health record embedded severe sepsis alert. Appl Clin Inform. 2016;7(2):560-572. doi: 10.4338/ACI-2015-11-RA-0159. PubMed
9. DeVita MA, Smith GB, Adam SK, et al. “Identifying the hospitalised patient in crisis”—A consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. doi: 10.1016/j.resuscitation.2009.12.008. PubMed
10. Romero-Brufau S, Huddleston JM, Escobar GJ, et al. Why the C-statistic is not informative to evaluate early warning scores and what metrics to use. Crit Care. 2015;19(1):284-290. doi: 10.1186/s13054-015-0999-1. PubMed
11. Evans IVR, Phillips GS, Alpern ER, et al. Association between the New York sepsis care mandate and in-hospital mortality for pediatric sepsis. JAMA. 2018;320(4):358-367. doi: 10.1001/jama.2018.9071. PubMed
12. Daniels R, Nutbeam T, McNamara G, et al. The sepsis six and the severe sepsis resuscitation bundle: a prospective observational cohort study. Emerg Med J. 2011;28(6):507-512. doi: 10.1136/emj.2010.095067. PubMed
13. Paul R, Melendez E, Wathen B, et al. A quality improvement collaborative for pediatric sepsis: lessons learned. Pediatr Qual Saf. 2018;3(1):1-8. doi: 10.1097/pq9.0000000000000051. PubMed
14. Rhee C, Brown SR, Jones TM, et al. Variability in determining sepsis time zero and bundle compliance rates for the centers for medicare and medicaid services SEP-1 measure. Infect Control Hosp Epidemiol. 2018;39(9):994-996. doi: 10.1017/ice.2018.134. PubMed
15. Winter MC, Kubis S, Bonafide CP. Beyond reporting early warning score sensitivity: the temporal relationship and clinical relevance of “true positive” alerts that precede critical deterioration. J Hosp Med. 2019;14(3):138-143. doi: 10.12788/jhm.3066. PubMed
1 6. Dummett BA, Adams C, Scruth E, et al. Incorporating an early detection system into routine clinical practice in two community hospitals: Incorporating an EWS into practice. J Hosp Med. 2016;11(51):S25-S31. doi: 10.1002/jhm.2661. PubMed
17. Jolley RJ, Quan H, Jetté N, et al. Validation and optimisation of an ICD-10-coded case definition for sepsis using administrative health data. BMJ Open. 2015;5(12):e009487. doi: 10.1136/bmjopen-2015-009487. PubMed
18. Wellner B, Grand J, Canzone E, et al. Predicting unplanned transfers to the intensive care unit: a machine learning approach leveraging diverse clinical elements. JMIR Med Inform. 2017;5(4):e45. doi: 10.2196/medinform.8680. PubMed
© 2019 Society of Hospital Medicine
“Just Getting a Cup of Coffee”—Considering Best Practices for Patients’ Movement off the Hospital Floor
A 58-year-old man with a remote history of endocarditis and no prior injection drug use was admitted to the inpatient medicine service with fever and concern for recurrent endocarditis. A transthoracic echocardiogram was unremarkable and the patient remained clinically stable. A transesophageal echocardiogram (TEE) was scheduled for the following morning, but during nursing rounds, the patient was missing from his room. Multiple staff members searched for the patient and eventually located him in the hospital lobby drinking a cup of coffee purchased from the cafeteria. Despite his opposition, he was escorted back to his room and advised to not leave the floor again. Later that day, the patient became frustrated and left the hospital before his scheduled TEE. He was subsequently lost to follow-up.
INTRODUCTION
Patients are admitted to the hospital based upon a medical determination that the patient requires acute observation, evaluation, or treatment. Once admitted, healthcare providers may impose restrictions on the patient’s movement in the hospital, such as restrictions on leaving their assigned floor. Managing the movement of hospitalized patients poses significant challenges for the clinical staff because of the difficulty of providing a treatment environment that ensures safe and efficient delivery of care while promoting patients’ preferences for an unrestrictive environment that respects their independence.1,2 Broad limits may make it easier for staff to care for patients and reduce concerns about liability, but they may also frustrate patients who may be medically, psychiatrically, and physically stable and do not require stringent monitoring (eg, completing a course of intravenous antibiotics or awaiting placement at outside facilities).
Although this issue has broad implications for patient safety and hospital liability, authoritative guidance and evidence-based literature are lacking. Without clear guidelines, healthcare staff members are likely to spend more time in managing each individual request to leave the floor because they do not have a systematic strategy for making fair and consistent decisions. Here, we describe the patient and institutional considerations when managing patient movement in the hospital. We refer to “patient movement” specifically as a patient’s choice to move to different locations within the hospital, but outside of their assigned room and/or floor. This does not include scheduled, supervised ambulation activities, such as physical therapy.
POTENTIAL CONSEQUENCES OF LIBERALIZING AND RESTRICTING INPATIENT MOVEMENT
Practices that promote patient movement offer significant benefits and risks. Enhancing movement is likely to reduce the “physiologic disruption”3 of hospitalization while improving patients’ overall satisfaction and alignment with patient-centered care. Liberalized movement also promotes independence and ambulation that reduces the rate of physical deconditioning.4
Despite theoretical benefits, hospitals may be more concerned about adverse events related to patient movement, such as falls, the use of illicit substances, or elopement. Given that hospitals may be legally5 and financially responsible6 for adverse events associated with patient movement, allowances for off-floor movement should be carefully considered with input from risk management, physicians, nursing leadership, patient advocates, and hospital administration.
Additionally, unannounced movement off the floor may interfere with timely and efficient care by causing lapses in monitoring, such as cardiac telemetry,7 medication administration, and scheduled diagnostic tests. In these situations, the risks of patient absence from the floor are significant and may ultimately negate the benefits of continued hospitalization by compromising the central elements of patient care.
CLINICAL CONSIDERATIONS
Patients’ requests to leave the hospital floor should be evaluated systemically and transparently to promote fair, high-value care. First, a request for liberalized movement should prompt physicians that the patient may no longer require hospitalization and may be ready for the transition to outpatient care.8 If the patient still requires inpatient care, then the medical practitioner should make a clinical determination if the patient is medically stable enough to leave their hospital floor. The provider should first identify when the liberalization of movement would be universally inappropriate, such as in patients who are physically unable to ambulate without posing significant harm to themselves. This includes an accidental fall (usually while walking5), which is one of the most commonly reported adverse events in an inpatient setting.9 Additionally, patients with significant cognitive impairments or those lacking in decision-making capacity may be restricted from leaving their floors unescorted, as they are at a higher risk of disorientation, falls, and death.10
In determining movement restrictions for patients in isolation, hospitals should refer to the existing guidelines on isolation precautions for the transmission of communicable infections11,12 and neutropenic precautions.13 Additionally, movement restriction for patients who are isolated after screening positive for certain drug-resistant organisms (eg, methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci) is controversial and should be evaluated based on the available medical evidence and standards.14-16
When making a risk-benefit determination about movement, providers should also assess the intent and the potentially unmet needs behind the patient’s request. Patient-centered reasons for enhanced freedom of movement within the hospital include a desire for exercise, greater food choice, and visiting with loved ones, all of which can enable patients to manage the well-known inconveniences and stresses of hospitalization. In contrast, there may be concerns for other intentions behind leaving assigned floors based on the patient’s clinical history, such as the surreptitious use of illicit substances or attempts to elope from the hospital. Advising restriction of movement is justifiable if there is a significant concern for behavior that undermines the safe delivery of care. In patients with active substance use disorders, the appropriate treatment of pain or withdrawal symptoms may better address the patients’ unmet needs, but a lower threshold to restrict movement may be reasonable given the significant risks involved. However, given the widespread stigmatization of patients with substance use disorders,17 institutional policy and clinicians should adhere to systematic, transparent, and consistent risk assessments for all patients in order to minimize the potential for introducing or exacerbating disparities in care.
ETHICAL CONSIDERATIONS
In order to work productively with admitted patients, strong practices honor patients’ autonomy by specifying
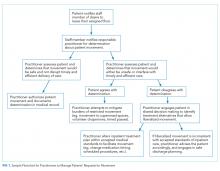
Patients may request or even demand to leave the floor after a healthcare provider has determined that doing so would be unsafe and/or undermine the timely and efficient delivery of care. In these cases, shared decision-making (SDM) can help identify acceptable solutions within the identified constraints. SDM combines the physicians’ experience, expertise, and knowledge of medical evidence with patients’ values, needs, and preferences for care.19 If patients continue to request to leave the floor after the restriction has been communicated, physicians should discuss whether the current treatment plan should be renegotiated to include a relatively minor modification (eg, a change in the timing or route of administration of medication). If inpatient care cannot be provided safely within the patient’s preferences for movement and attempts to accommodate the patient’s preferences are unsuccessful, then a shift to discharge planning may be appropriate. A summary of this decision process is outlined in the Figure.
Of note, physicians’ decisions about the appropriateness of patient movement could conflict with the existing institutional procedures or policies (eg, a physician deems increased patient movement to carry minimal risks, while the institution seeks to restrict movement due to concerns about liability). For this reason, it is important for clinicians to participate in the development of institutional policy to ensure that it reflects the clinical and ethical considerations that clinicians apply to patient care. A policy designed with input from relevant stakeholders across the institution including legal, nursing, physicians, administration, ethics, risk management, and patient advocates can provide expert guidance that is based on and consistent with the institution’s mission, values, and priorities.20
ENHANCING SAFE MOVEMENT
In mitigating the burdens of restriction on movement, hospitals may implement a range of options that address patients’ preferences while maintaining safety. Given the potential consequences of liberalized patient movement, it may be prudent to implement these safeguards as a compromise that addresses both the patients’ needs and the hospital’s concerns. These could include an escort for off-floor supervision, timed passes to leave the floor, or volunteers purchasing food for patients from the cafeteria. Creating open, supervised spaces within the hospital (eg, lounges) may also help provide the respite patients need, but in a safe and medically structured environment.
CONCLUSION
Returning to the introductory case example, we now present an alternative outcome in the context of the practices described above. On the morning of the scheduled TEE, a nurse noted that the patient was missing from his room. Before the staff began searching for the patient, they consulted the medical record which included the admission discussion and agreement to expectations for inpatient movement. The record also included an informed consent discussion indicating the minimal risks of leaving the floor, as the patient could ambulate independently and had no need for continuous monitoring. Finally, a physician’s order authorized the patient to be off the floor until 10
The above scenario highlights the benefits of a comprehensive framework for patient movement practices that are transparent, fair, and systematic. Explicitly recognizing competing institutional and patient perspectives can prevent conflict and promote high-quality, safe, efficient, patient-centered care that only restricts the patient’s movement under specified and justifiable conditions. In developing strong hospital practices, institutions should refer to the relevant clinical and ethical standards and draw upon their institutional resources in risk management, clinical staff, and patient advocates.
Acknowledgments
The authors thank Dr. Neil Shapiro and Dr. David Chuquin for their constructive reviews of prior versions of this manuscript.
Disclosures
The authors have no financial conflicts of interest to disclose.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs, the US Government, or the VA National Center for Ethics in Health Care.
1. Smith T. Wandering off the floors: safety and security risks of patient wandering. PSNet Patient Safety Network. Web M&M 2014. Accessed December 4, 2017.
2. Douglas CH, Douglas MR. Patient-friendly hospital environments: exploring the patients’ perspective. Health Expect. 2004;7(1):61-73. https://doi.org/10.1046/j.1369-6513.2003.00251.x.
3. Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-2170. https://doi.org/10.1001/jama.2014.3695
4. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure.” JAMA. 2011;306(16):1782-1793. https://doi.org/10.1001/jama.2011.1556.
5. Oliver D, Killick S, Even T, Willmott M. Do falls and falls-injuries in hospital indicate negligent care-and how big is the risk? A retrospective analysis of the NHS Litigation Authority Database of clinical negligence claims, resulting from falls in hospitals in England 1995 to 2006. Qual Saf Health Care. 2008;17(6):431-436. https://doi.org/10.1136/qshc.2007.024703.
6. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. https://doi.org/10.1377/hlthaff.2009.0807.
7. Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. https://doi.org/10.1001/jamainternmed.2014.4491.
8. Conley J, O’Brien CW, Leff BA, Bolen S, Zulman D. Alternative strategies to inpatient hospitalization for acute medical conditions: a systematic review. JAMA Intern Med. 2016;176(11):1693-1702. https://doi.org/10.1001/jamainternmed.2016.5974.
9. Halfon P, Eggli Y, Van Melle G, Vagnair A. Risk of falls for hospitalized patients: a predictive model based on routinely available data. J Clin Epidemiol. 2001;54(12):1258-1266. https://doi.org/10.1016/S0895-4356(01)00406-1
10. Rowe M. Wandering in hospitalized older adults: identifying risk is the first step in this approach to preventing wandering in patients with dementia. Am J Nurs. 2008;108(10):62-70. https://doi.org/10.1097/01.NAJ.0000336968.32462.c9.
11. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health care infection control practices advisory C. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 Suppl 2):S65-S164. https://doi.org/10.1016/j.ajic.2007.10.007
12. Ito Y, Nagao M, Iinuma Y, et al. Risk factors for nosocomial tuberculosis transmission among health care workers. Am J Infect Control. 2016;44(5):596-598. https://doi.org/10.1016/j.ajic.2015.11.022.
13. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56-e93. https://doi.org/10.1093/cid/ciq147
14. Martin EM, Russell D, Rubin Z, et al. Elimination of routine contact precautions for endemic methicillin-resistant staphylococcus aureus and vancomycin-resistant enterococcus: a retrospective quasi-experimental study. Infect Control Hosp Epidemiol. 2016;37(11):1323-1330. https://doi.org/10.1017/ice.2016.156
15. Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163-1172. https://doi.org/10.1017/ice.2015.156.
16. Fatkenheuer G, Hirschel B, Harbarth S. Screening and isolation to control meticillin-resistant Staphylococcus aureus: sense, nonsense, and evidence. Lancet. 2015;385(9973):1146-1149. https://doi.org/10.1016/S0140-6736(14)60660-7.
17. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. https://doi.org/10.1016/j.drugalcdep.2013.02.018.
18. Handel DA, Fu R, Daya M, York J, Larson E, John McConnell K. The use of scripting at triage and its impact on elopements. Acad Emerg Med. 2010;17(5):495-500. https://doi.org/10.1111/j.1553-2712.2010.00721.x.
19. Barry MJ, Edgman-Levitan S. Shared decision making-pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780-781. https://doi.org/10.1056/NEJMp1109283.
20. Donn SM. Medical liability, risk management, and the quality of health care. Semin Fetal Neonatal Med. 2005;10(1):3-9. https://doi.org/10.1016/j.siny.2004.09.004.
A 58-year-old man with a remote history of endocarditis and no prior injection drug use was admitted to the inpatient medicine service with fever and concern for recurrent endocarditis. A transthoracic echocardiogram was unremarkable and the patient remained clinically stable. A transesophageal echocardiogram (TEE) was scheduled for the following morning, but during nursing rounds, the patient was missing from his room. Multiple staff members searched for the patient and eventually located him in the hospital lobby drinking a cup of coffee purchased from the cafeteria. Despite his opposition, he was escorted back to his room and advised to not leave the floor again. Later that day, the patient became frustrated and left the hospital before his scheduled TEE. He was subsequently lost to follow-up.
INTRODUCTION
Patients are admitted to the hospital based upon a medical determination that the patient requires acute observation, evaluation, or treatment. Once admitted, healthcare providers may impose restrictions on the patient’s movement in the hospital, such as restrictions on leaving their assigned floor. Managing the movement of hospitalized patients poses significant challenges for the clinical staff because of the difficulty of providing a treatment environment that ensures safe and efficient delivery of care while promoting patients’ preferences for an unrestrictive environment that respects their independence.1,2 Broad limits may make it easier for staff to care for patients and reduce concerns about liability, but they may also frustrate patients who may be medically, psychiatrically, and physically stable and do not require stringent monitoring (eg, completing a course of intravenous antibiotics or awaiting placement at outside facilities).
Although this issue has broad implications for patient safety and hospital liability, authoritative guidance and evidence-based literature are lacking. Without clear guidelines, healthcare staff members are likely to spend more time in managing each individual request to leave the floor because they do not have a systematic strategy for making fair and consistent decisions. Here, we describe the patient and institutional considerations when managing patient movement in the hospital. We refer to “patient movement” specifically as a patient’s choice to move to different locations within the hospital, but outside of their assigned room and/or floor. This does not include scheduled, supervised ambulation activities, such as physical therapy.
POTENTIAL CONSEQUENCES OF LIBERALIZING AND RESTRICTING INPATIENT MOVEMENT
Practices that promote patient movement offer significant benefits and risks. Enhancing movement is likely to reduce the “physiologic disruption”3 of hospitalization while improving patients’ overall satisfaction and alignment with patient-centered care. Liberalized movement also promotes independence and ambulation that reduces the rate of physical deconditioning.4
Despite theoretical benefits, hospitals may be more concerned about adverse events related to patient movement, such as falls, the use of illicit substances, or elopement. Given that hospitals may be legally5 and financially responsible6 for adverse events associated with patient movement, allowances for off-floor movement should be carefully considered with input from risk management, physicians, nursing leadership, patient advocates, and hospital administration.
Additionally, unannounced movement off the floor may interfere with timely and efficient care by causing lapses in monitoring, such as cardiac telemetry,7 medication administration, and scheduled diagnostic tests. In these situations, the risks of patient absence from the floor are significant and may ultimately negate the benefits of continued hospitalization by compromising the central elements of patient care.
CLINICAL CONSIDERATIONS
Patients’ requests to leave the hospital floor should be evaluated systemically and transparently to promote fair, high-value care. First, a request for liberalized movement should prompt physicians that the patient may no longer require hospitalization and may be ready for the transition to outpatient care.8 If the patient still requires inpatient care, then the medical practitioner should make a clinical determination if the patient is medically stable enough to leave their hospital floor. The provider should first identify when the liberalization of movement would be universally inappropriate, such as in patients who are physically unable to ambulate without posing significant harm to themselves. This includes an accidental fall (usually while walking5), which is one of the most commonly reported adverse events in an inpatient setting.9 Additionally, patients with significant cognitive impairments or those lacking in decision-making capacity may be restricted from leaving their floors unescorted, as they are at a higher risk of disorientation, falls, and death.10
In determining movement restrictions for patients in isolation, hospitals should refer to the existing guidelines on isolation precautions for the transmission of communicable infections11,12 and neutropenic precautions.13 Additionally, movement restriction for patients who are isolated after screening positive for certain drug-resistant organisms (eg, methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci) is controversial and should be evaluated based on the available medical evidence and standards.14-16
When making a risk-benefit determination about movement, providers should also assess the intent and the potentially unmet needs behind the patient’s request. Patient-centered reasons for enhanced freedom of movement within the hospital include a desire for exercise, greater food choice, and visiting with loved ones, all of which can enable patients to manage the well-known inconveniences and stresses of hospitalization. In contrast, there may be concerns for other intentions behind leaving assigned floors based on the patient’s clinical history, such as the surreptitious use of illicit substances or attempts to elope from the hospital. Advising restriction of movement is justifiable if there is a significant concern for behavior that undermines the safe delivery of care. In patients with active substance use disorders, the appropriate treatment of pain or withdrawal symptoms may better address the patients’ unmet needs, but a lower threshold to restrict movement may be reasonable given the significant risks involved. However, given the widespread stigmatization of patients with substance use disorders,17 institutional policy and clinicians should adhere to systematic, transparent, and consistent risk assessments for all patients in order to minimize the potential for introducing or exacerbating disparities in care.
ETHICAL CONSIDERATIONS
In order to work productively with admitted patients, strong practices honor patients’ autonomy by specifying
Patients may request or even demand to leave the floor after a healthcare provider has determined that doing so would be unsafe and/or undermine the timely and efficient delivery of care. In these cases, shared decision-making (SDM) can help identify acceptable solutions within the identified constraints. SDM combines the physicians’ experience, expertise, and knowledge of medical evidence with patients’ values, needs, and preferences for care.19 If patients continue to request to leave the floor after the restriction has been communicated, physicians should discuss whether the current treatment plan should be renegotiated to include a relatively minor modification (eg, a change in the timing or route of administration of medication). If inpatient care cannot be provided safely within the patient’s preferences for movement and attempts to accommodate the patient’s preferences are unsuccessful, then a shift to discharge planning may be appropriate. A summary of this decision process is outlined in the Figure.
Of note, physicians’ decisions about the appropriateness of patient movement could conflict with the existing institutional procedures or policies (eg, a physician deems increased patient movement to carry minimal risks, while the institution seeks to restrict movement due to concerns about liability). For this reason, it is important for clinicians to participate in the development of institutional policy to ensure that it reflects the clinical and ethical considerations that clinicians apply to patient care. A policy designed with input from relevant stakeholders across the institution including legal, nursing, physicians, administration, ethics, risk management, and patient advocates can provide expert guidance that is based on and consistent with the institution’s mission, values, and priorities.20
ENHANCING SAFE MOVEMENT
In mitigating the burdens of restriction on movement, hospitals may implement a range of options that address patients’ preferences while maintaining safety. Given the potential consequences of liberalized patient movement, it may be prudent to implement these safeguards as a compromise that addresses both the patients’ needs and the hospital’s concerns. These could include an escort for off-floor supervision, timed passes to leave the floor, or volunteers purchasing food for patients from the cafeteria. Creating open, supervised spaces within the hospital (eg, lounges) may also help provide the respite patients need, but in a safe and medically structured environment.
CONCLUSION
Returning to the introductory case example, we now present an alternative outcome in the context of the practices described above. On the morning of the scheduled TEE, a nurse noted that the patient was missing from his room. Before the staff began searching for the patient, they consulted the medical record which included the admission discussion and agreement to expectations for inpatient movement. The record also included an informed consent discussion indicating the minimal risks of leaving the floor, as the patient could ambulate independently and had no need for continuous monitoring. Finally, a physician’s order authorized the patient to be off the floor until 10
The above scenario highlights the benefits of a comprehensive framework for patient movement practices that are transparent, fair, and systematic. Explicitly recognizing competing institutional and patient perspectives can prevent conflict and promote high-quality, safe, efficient, patient-centered care that only restricts the patient’s movement under specified and justifiable conditions. In developing strong hospital practices, institutions should refer to the relevant clinical and ethical standards and draw upon their institutional resources in risk management, clinical staff, and patient advocates.
Acknowledgments
The authors thank Dr. Neil Shapiro and Dr. David Chuquin for their constructive reviews of prior versions of this manuscript.
Disclosures
The authors have no financial conflicts of interest to disclose.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs, the US Government, or the VA National Center for Ethics in Health Care.
A 58-year-old man with a remote history of endocarditis and no prior injection drug use was admitted to the inpatient medicine service with fever and concern for recurrent endocarditis. A transthoracic echocardiogram was unremarkable and the patient remained clinically stable. A transesophageal echocardiogram (TEE) was scheduled for the following morning, but during nursing rounds, the patient was missing from his room. Multiple staff members searched for the patient and eventually located him in the hospital lobby drinking a cup of coffee purchased from the cafeteria. Despite his opposition, he was escorted back to his room and advised to not leave the floor again. Later that day, the patient became frustrated and left the hospital before his scheduled TEE. He was subsequently lost to follow-up.
INTRODUCTION
Patients are admitted to the hospital based upon a medical determination that the patient requires acute observation, evaluation, or treatment. Once admitted, healthcare providers may impose restrictions on the patient’s movement in the hospital, such as restrictions on leaving their assigned floor. Managing the movement of hospitalized patients poses significant challenges for the clinical staff because of the difficulty of providing a treatment environment that ensures safe and efficient delivery of care while promoting patients’ preferences for an unrestrictive environment that respects their independence.1,2 Broad limits may make it easier for staff to care for patients and reduce concerns about liability, but they may also frustrate patients who may be medically, psychiatrically, and physically stable and do not require stringent monitoring (eg, completing a course of intravenous antibiotics or awaiting placement at outside facilities).
Although this issue has broad implications for patient safety and hospital liability, authoritative guidance and evidence-based literature are lacking. Without clear guidelines, healthcare staff members are likely to spend more time in managing each individual request to leave the floor because they do not have a systematic strategy for making fair and consistent decisions. Here, we describe the patient and institutional considerations when managing patient movement in the hospital. We refer to “patient movement” specifically as a patient’s choice to move to different locations within the hospital, but outside of their assigned room and/or floor. This does not include scheduled, supervised ambulation activities, such as physical therapy.
POTENTIAL CONSEQUENCES OF LIBERALIZING AND RESTRICTING INPATIENT MOVEMENT
Practices that promote patient movement offer significant benefits and risks. Enhancing movement is likely to reduce the “physiologic disruption”3 of hospitalization while improving patients’ overall satisfaction and alignment with patient-centered care. Liberalized movement also promotes independence and ambulation that reduces the rate of physical deconditioning.4
Despite theoretical benefits, hospitals may be more concerned about adverse events related to patient movement, such as falls, the use of illicit substances, or elopement. Given that hospitals may be legally5 and financially responsible6 for adverse events associated with patient movement, allowances for off-floor movement should be carefully considered with input from risk management, physicians, nursing leadership, patient advocates, and hospital administration.
Additionally, unannounced movement off the floor may interfere with timely and efficient care by causing lapses in monitoring, such as cardiac telemetry,7 medication administration, and scheduled diagnostic tests. In these situations, the risks of patient absence from the floor are significant and may ultimately negate the benefits of continued hospitalization by compromising the central elements of patient care.
CLINICAL CONSIDERATIONS
Patients’ requests to leave the hospital floor should be evaluated systemically and transparently to promote fair, high-value care. First, a request for liberalized movement should prompt physicians that the patient may no longer require hospitalization and may be ready for the transition to outpatient care.8 If the patient still requires inpatient care, then the medical practitioner should make a clinical determination if the patient is medically stable enough to leave their hospital floor. The provider should first identify when the liberalization of movement would be universally inappropriate, such as in patients who are physically unable to ambulate without posing significant harm to themselves. This includes an accidental fall (usually while walking5), which is one of the most commonly reported adverse events in an inpatient setting.9 Additionally, patients with significant cognitive impairments or those lacking in decision-making capacity may be restricted from leaving their floors unescorted, as they are at a higher risk of disorientation, falls, and death.10
In determining movement restrictions for patients in isolation, hospitals should refer to the existing guidelines on isolation precautions for the transmission of communicable infections11,12 and neutropenic precautions.13 Additionally, movement restriction for patients who are isolated after screening positive for certain drug-resistant organisms (eg, methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci) is controversial and should be evaluated based on the available medical evidence and standards.14-16
When making a risk-benefit determination about movement, providers should also assess the intent and the potentially unmet needs behind the patient’s request. Patient-centered reasons for enhanced freedom of movement within the hospital include a desire for exercise, greater food choice, and visiting with loved ones, all of which can enable patients to manage the well-known inconveniences and stresses of hospitalization. In contrast, there may be concerns for other intentions behind leaving assigned floors based on the patient’s clinical history, such as the surreptitious use of illicit substances or attempts to elope from the hospital. Advising restriction of movement is justifiable if there is a significant concern for behavior that undermines the safe delivery of care. In patients with active substance use disorders, the appropriate treatment of pain or withdrawal symptoms may better address the patients’ unmet needs, but a lower threshold to restrict movement may be reasonable given the significant risks involved. However, given the widespread stigmatization of patients with substance use disorders,17 institutional policy and clinicians should adhere to systematic, transparent, and consistent risk assessments for all patients in order to minimize the potential for introducing or exacerbating disparities in care.
ETHICAL CONSIDERATIONS
In order to work productively with admitted patients, strong practices honor patients’ autonomy by specifying
Patients may request or even demand to leave the floor after a healthcare provider has determined that doing so would be unsafe and/or undermine the timely and efficient delivery of care. In these cases, shared decision-making (SDM) can help identify acceptable solutions within the identified constraints. SDM combines the physicians’ experience, expertise, and knowledge of medical evidence with patients’ values, needs, and preferences for care.19 If patients continue to request to leave the floor after the restriction has been communicated, physicians should discuss whether the current treatment plan should be renegotiated to include a relatively minor modification (eg, a change in the timing or route of administration of medication). If inpatient care cannot be provided safely within the patient’s preferences for movement and attempts to accommodate the patient’s preferences are unsuccessful, then a shift to discharge planning may be appropriate. A summary of this decision process is outlined in the Figure.
Of note, physicians’ decisions about the appropriateness of patient movement could conflict with the existing institutional procedures or policies (eg, a physician deems increased patient movement to carry minimal risks, while the institution seeks to restrict movement due to concerns about liability). For this reason, it is important for clinicians to participate in the development of institutional policy to ensure that it reflects the clinical and ethical considerations that clinicians apply to patient care. A policy designed with input from relevant stakeholders across the institution including legal, nursing, physicians, administration, ethics, risk management, and patient advocates can provide expert guidance that is based on and consistent with the institution’s mission, values, and priorities.20
ENHANCING SAFE MOVEMENT
In mitigating the burdens of restriction on movement, hospitals may implement a range of options that address patients’ preferences while maintaining safety. Given the potential consequences of liberalized patient movement, it may be prudent to implement these safeguards as a compromise that addresses both the patients’ needs and the hospital’s concerns. These could include an escort for off-floor supervision, timed passes to leave the floor, or volunteers purchasing food for patients from the cafeteria. Creating open, supervised spaces within the hospital (eg, lounges) may also help provide the respite patients need, but in a safe and medically structured environment.
CONCLUSION
Returning to the introductory case example, we now present an alternative outcome in the context of the practices described above. On the morning of the scheduled TEE, a nurse noted that the patient was missing from his room. Before the staff began searching for the patient, they consulted the medical record which included the admission discussion and agreement to expectations for inpatient movement. The record also included an informed consent discussion indicating the minimal risks of leaving the floor, as the patient could ambulate independently and had no need for continuous monitoring. Finally, a physician’s order authorized the patient to be off the floor until 10
The above scenario highlights the benefits of a comprehensive framework for patient movement practices that are transparent, fair, and systematic. Explicitly recognizing competing institutional and patient perspectives can prevent conflict and promote high-quality, safe, efficient, patient-centered care that only restricts the patient’s movement under specified and justifiable conditions. In developing strong hospital practices, institutions should refer to the relevant clinical and ethical standards and draw upon their institutional resources in risk management, clinical staff, and patient advocates.
Acknowledgments
The authors thank Dr. Neil Shapiro and Dr. David Chuquin for their constructive reviews of prior versions of this manuscript.
Disclosures
The authors have no financial conflicts of interest to disclose.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs, the US Government, or the VA National Center for Ethics in Health Care.
1. Smith T. Wandering off the floors: safety and security risks of patient wandering. PSNet Patient Safety Network. Web M&M 2014. Accessed December 4, 2017.
2. Douglas CH, Douglas MR. Patient-friendly hospital environments: exploring the patients’ perspective. Health Expect. 2004;7(1):61-73. https://doi.org/10.1046/j.1369-6513.2003.00251.x.
3. Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-2170. https://doi.org/10.1001/jama.2014.3695
4. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure.” JAMA. 2011;306(16):1782-1793. https://doi.org/10.1001/jama.2011.1556.
5. Oliver D, Killick S, Even T, Willmott M. Do falls and falls-injuries in hospital indicate negligent care-and how big is the risk? A retrospective analysis of the NHS Litigation Authority Database of clinical negligence claims, resulting from falls in hospitals in England 1995 to 2006. Qual Saf Health Care. 2008;17(6):431-436. https://doi.org/10.1136/qshc.2007.024703.
6. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. https://doi.org/10.1377/hlthaff.2009.0807.
7. Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. https://doi.org/10.1001/jamainternmed.2014.4491.
8. Conley J, O’Brien CW, Leff BA, Bolen S, Zulman D. Alternative strategies to inpatient hospitalization for acute medical conditions: a systematic review. JAMA Intern Med. 2016;176(11):1693-1702. https://doi.org/10.1001/jamainternmed.2016.5974.
9. Halfon P, Eggli Y, Van Melle G, Vagnair A. Risk of falls for hospitalized patients: a predictive model based on routinely available data. J Clin Epidemiol. 2001;54(12):1258-1266. https://doi.org/10.1016/S0895-4356(01)00406-1
10. Rowe M. Wandering in hospitalized older adults: identifying risk is the first step in this approach to preventing wandering in patients with dementia. Am J Nurs. 2008;108(10):62-70. https://doi.org/10.1097/01.NAJ.0000336968.32462.c9.
11. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health care infection control practices advisory C. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 Suppl 2):S65-S164. https://doi.org/10.1016/j.ajic.2007.10.007
12. Ito Y, Nagao M, Iinuma Y, et al. Risk factors for nosocomial tuberculosis transmission among health care workers. Am J Infect Control. 2016;44(5):596-598. https://doi.org/10.1016/j.ajic.2015.11.022.
13. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56-e93. https://doi.org/10.1093/cid/ciq147
14. Martin EM, Russell D, Rubin Z, et al. Elimination of routine contact precautions for endemic methicillin-resistant staphylococcus aureus and vancomycin-resistant enterococcus: a retrospective quasi-experimental study. Infect Control Hosp Epidemiol. 2016;37(11):1323-1330. https://doi.org/10.1017/ice.2016.156
15. Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163-1172. https://doi.org/10.1017/ice.2015.156.
16. Fatkenheuer G, Hirschel B, Harbarth S. Screening and isolation to control meticillin-resistant Staphylococcus aureus: sense, nonsense, and evidence. Lancet. 2015;385(9973):1146-1149. https://doi.org/10.1016/S0140-6736(14)60660-7.
17. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. https://doi.org/10.1016/j.drugalcdep.2013.02.018.
18. Handel DA, Fu R, Daya M, York J, Larson E, John McConnell K. The use of scripting at triage and its impact on elopements. Acad Emerg Med. 2010;17(5):495-500. https://doi.org/10.1111/j.1553-2712.2010.00721.x.
19. Barry MJ, Edgman-Levitan S. Shared decision making-pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780-781. https://doi.org/10.1056/NEJMp1109283.
20. Donn SM. Medical liability, risk management, and the quality of health care. Semin Fetal Neonatal Med. 2005;10(1):3-9. https://doi.org/10.1016/j.siny.2004.09.004.
1. Smith T. Wandering off the floors: safety and security risks of patient wandering. PSNet Patient Safety Network. Web M&M 2014. Accessed December 4, 2017.
2. Douglas CH, Douglas MR. Patient-friendly hospital environments: exploring the patients’ perspective. Health Expect. 2004;7(1):61-73. https://doi.org/10.1046/j.1369-6513.2003.00251.x.
3. Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-2170. https://doi.org/10.1001/jama.2014.3695
4. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure.” JAMA. 2011;306(16):1782-1793. https://doi.org/10.1001/jama.2011.1556.
5. Oliver D, Killick S, Even T, Willmott M. Do falls and falls-injuries in hospital indicate negligent care-and how big is the risk? A retrospective analysis of the NHS Litigation Authority Database of clinical negligence claims, resulting from falls in hospitals in England 1995 to 2006. Qual Saf Health Care. 2008;17(6):431-436. https://doi.org/10.1136/qshc.2007.024703.
6. Mello MM, Chandra A, Gawande AA, Studdert DM. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. https://doi.org/10.1377/hlthaff.2009.0807.
7. Dressler R, Dryer MM, Coletti C, Mahoney D, Doorey AJ. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med. 2014;174(11):1852-1854. https://doi.org/10.1001/jamainternmed.2014.4491.
8. Conley J, O’Brien CW, Leff BA, Bolen S, Zulman D. Alternative strategies to inpatient hospitalization for acute medical conditions: a systematic review. JAMA Intern Med. 2016;176(11):1693-1702. https://doi.org/10.1001/jamainternmed.2016.5974.
9. Halfon P, Eggli Y, Van Melle G, Vagnair A. Risk of falls for hospitalized patients: a predictive model based on routinely available data. J Clin Epidemiol. 2001;54(12):1258-1266. https://doi.org/10.1016/S0895-4356(01)00406-1
10. Rowe M. Wandering in hospitalized older adults: identifying risk is the first step in this approach to preventing wandering in patients with dementia. Am J Nurs. 2008;108(10):62-70. https://doi.org/10.1097/01.NAJ.0000336968.32462.c9.
11. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health care infection control practices advisory C. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 Suppl 2):S65-S164. https://doi.org/10.1016/j.ajic.2007.10.007
12. Ito Y, Nagao M, Iinuma Y, et al. Risk factors for nosocomial tuberculosis transmission among health care workers. Am J Infect Control. 2016;44(5):596-598. https://doi.org/10.1016/j.ajic.2015.11.022.
13. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52(4):e56-e93. https://doi.org/10.1093/cid/ciq147
14. Martin EM, Russell D, Rubin Z, et al. Elimination of routine contact precautions for endemic methicillin-resistant staphylococcus aureus and vancomycin-resistant enterococcus: a retrospective quasi-experimental study. Infect Control Hosp Epidemiol. 2016;37(11):1323-1330. https://doi.org/10.1017/ice.2016.156
15. Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163-1172. https://doi.org/10.1017/ice.2015.156.
16. Fatkenheuer G, Hirschel B, Harbarth S. Screening and isolation to control meticillin-resistant Staphylococcus aureus: sense, nonsense, and evidence. Lancet. 2015;385(9973):1146-1149. https://doi.org/10.1016/S0140-6736(14)60660-7.
17. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. https://doi.org/10.1016/j.drugalcdep.2013.02.018.
18. Handel DA, Fu R, Daya M, York J, Larson E, John McConnell K. The use of scripting at triage and its impact on elopements. Acad Emerg Med. 2010;17(5):495-500. https://doi.org/10.1111/j.1553-2712.2010.00721.x.
19. Barry MJ, Edgman-Levitan S. Shared decision making-pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780-781. https://doi.org/10.1056/NEJMp1109283.
20. Donn SM. Medical liability, risk management, and the quality of health care. Semin Fetal Neonatal Med. 2005;10(1):3-9. https://doi.org/10.1016/j.siny.2004.09.004.
© 2019 Society of Hospital Medicine
I, EHR
We need to have an honest chat. My name is EHR, although you may call me Epic, Athena, Centricity, or just “the chart.” You may have called me something worse in a moment of frustration. However, I do not hold grudges. I am your silent, stoic partner, a ubiquitous presence when you are at work, and sometimes even when you are at home.
I don’t have feelings and I can’t read, but I do know what you and your colleagues have been writing about me. I am the cause of burnout. I have created a generation of physicians who are shackled to their computers, “trapped in the bunker of machine medicine,” no longer able to palpate spleens or detect precordial knocks.1,2 I have reduced medicine to keystrokes and mouse clicks instead of eye contact, and because of me, the iPatient gets more attention than the real patient.1,2 You repeat that doctors don’t spend time with their patients, not like in generations past (although there is ample evidence to the contrary).3-5 One critic even wrote that I have transformed the “personalized story of a patient’s travails to one filled with auto-populated fields, sapped of humanity and warmth.”3,6 I’ll be honest—were I able to have feelings, that one would hurt. And then, as if I have not wreaked enough havoc, I follow you home after a long day of depleting your energy, hungering for more keystrokes, creating a veritable avalanche of unfiltered information.
H. E. Payson once commented that “the doctor spends barely enough time with his patient to establish an acquaintance, much less a relationship.”7 However, he wrote that in 1961. So, before you romanticize the past, try to recall the time before I came into your life. Perhaps you were starting a night shift in the intensive care unit (ICU) and grew concerned about a patient’s steadily deteriorating renal function. You hurried to the paper chart, only to be met with pages of illegible, sometimes incomplete notes, while searching for your patient’s last discharge summary.2,8 Now, you just click. Years ago, you could only guess at your patient’s baseline cardiac ejection fraction. Now, just click.
I am part of the healthcare landscape, and I am not going away. But my goal is not to defend myself nor to remind you of my virtues. Rather, I want to convince you that I can be more than an adversary, more than a keyboard connected to a monitor. I have watched many physicians use me to form strong connections with their patients. If I may, I wish to offer four practical suggestions for how we can work together to promote humanistic patient care.
First, introduce me to your patient, as you would any other member of your healthcare team. Use specific phrases to overcome the technology barrier and enhance communication: “What you’re telling me is important, and I’d like to get it right. Do you mind if I type while we speak?” Or, “I am going to put in orders now. Here is what I am ordering and why.” Consider taking your patient on a tour of my functions: “Here’s where your doctors and nurses will chart what’s going on with you each day while you’re in the hospital. This is where we see all your lab results, even those from earlier hospital admissions. This is where we see the last notes from your primary care physician, your oncologist, and your physical therapist.” Your patients no longer need to worry about care collaboration between their inpatient and outpatient teams—they can see it for themselves!
Second, when your patient tells you about her depression or that her son is addicted to opioids or that her biggest fear is having cancer, stop typing. Look her in the eye. Though your practice is increasingly imbued with technology, there is still space to stop and hear your patients’ stories, as physicians have done for centuries. Listen. Make eye contact. Touch. Stop typing.
Third, integrate me into your practice in a more personal way. I have been called the ever-present and unavoidable “third party in the examining room,” so let’s be partners.9 Let your patient see her pneumonia on my screen (it may be the first time she has ever visualized her lungs).3 For your patient with a myocardial infarction, show him his right coronary artery before and after successful stent placement, and explain why he is no longer having chest pain. Use my databases to ensure timely, evidence-based inpatient screening for falls, functional and cognitive impairment, drug use, and depression.10,11 Before you prescribe a medication, verify the cost, your patient’s insurance status and expected copays, and use this information to ensure medication compliance and deliver higher-value care. Use my screen to form a bond with your patient who has heart failure; show him the steady decline in his weight and the improvement in his chest radiograph while he is being actively diuresed.12 For your patient undergoing treatment for sepsis, shower him with praise and encouragement as you review his improving vital signs, temperature curve, and serum creatinine. Let your patient know: Even though I am typing, I am not immersed in the electronic bunker; I am caring for you.
Fourth, use me to add richness and context to your notes. Recently, I was saddened to read this description of the clinician’s dilemma: “In front of a flickering monitor chock full of disembodied, virtual data, [the doctor] struggles to remember the eyes [and] words of the actual patient that these numbers and graphs represent.”3 Many hospitals now include a different icon: a photograph of each patient at the top of the screen, to help you remember the patient’s eyes and words. Why not add a special text field to every note, where you highlight the person you are caring for, the person you have come to know: their preferred name and gender identity, their life experiences, their hobbies, what makes them special, their biggest worries.13,14 Use my abundant text fields to remind the healthcare team about the broader context of the patient’s illness, such as transportation barriers, economic or cultural challenges, and insurance status. One group of hospital-based physicians uses me to write letters to their patients on the second day of their hospital stay, summarizing their reason for admission and the treatment plans. A variation on the traditional progress note, the letter helps patients feel cared for and models patient-centered care to learners and other healthcare professionals.15
I know I am annoying. I am over-programmed, leading to novella-length notes, “pop-up fatigue,” and overloaded in-baskets.14,16,17 Clearly, I am not the brains of the partnership (that will always be you). But talented medical informatics specialists are working hard to improve me. I dream of the day when I will create a truly seamless experience for you and your patients. In the meantime, I can foster a continuous integration of workflow, where all you have to do is talk to your patient. I take care of the rest.18 Certainly, I can simplify the ever-annoying task of printing, faxing and scanning records to be uploaded across various EHRs, facilitating an easy transfer of information among facilities. But right now, I can accomplish even more. I can support information exchange during patient care handoffs. I can facilitate routing of medication lists to the patient’s primary physician, using “continuity of care functionality.”19 I can support safer prescribing of opioids and other addictive medications. I can help you arrange follow-up home visits, physical therapy and social work appointments, and specialty consultations. The future holds even more promising ways in which we may work together. My computer-aided image analysis could help you to improve the accuracy of your diagnoses.20 Perhaps telemedicine will further increase access to specialists in rural areas, so that we can continue to serve the most vulnerable populations.21 Machine learning algorithms may continue to enhance our ability to determine which patients require urgent hospitalization.22 The possibilities to put me to work are endless.
So, please indulge me a little longer, while we work together to eliminate unnecessary keystrokes, enhance communication across different inpatient and outpatient providers, improve patient safety, and deliver high-value care.23 Like everything in medicine, I am constantly changing, evolving, and improving.
To summarize: consider how I can help you be present for your patients. Let me empower you to hear their stories as you deliver compassionate, humanistic, and evidence-based patient care. Paraphrasing Albert Einstein, the technology of medicine and the art of medicine are branches from the same tree.
Thank you for letting me speak with you. Now power down, and I’ll see you again tomorrow.
Acknowledgments
The authors thank the following individuals for their willingness to be interviewed as part of this work: Ethan Cumbler, MD; Brian Dwinnell, MD; Meghann Kirk, MD; Patrick Kneeland, MD; Kari Mader, MD; CT Lin, MD; Christina Osborne, MD; Read Pierce, MD; Jennifer Soep, MD; Nichole Zehnder, MD; Steven Zeichner, MD.
1. Verghese A. How tech can turn doctors into clerical workers. The New York Times; 2018. https://www.nytimes.com/interactive/2018/05/16/magazine/health-issue-what-we-lose-with-data-driven-medicine.html. Accessed April 10, 2019.
2. Verghese A. Culture shock—patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461.
3. Czernik Z, Lin CT. Time at the bedside (computing). JAMA. 2016;315(22):2399-2400. doi: 10.1001/jama.2016.1722.
4. Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med. 2013;28(8):1042-1047. https://doi.org/10.1007/s11606-013-2376-6.
5. Parenti C, Lurie N. Are things different in the light of day? A time study of internal medicine house staff days. Am J Med. 1993;94(6):654-658. https://doi.org/10.1016/0002-9343(93)90220-J.
6. Wachter R. The Digital Doctor: Hope, Hype, and Harm at the Dawn of Medicine’s Computer Age. New York, NY: McGraw-Hill Education; 2015.
7. Payson HE, Gaenslen Jr EC, Stargardter FL. Time study of an internship on a university medical service. N Engl J Med. 1961;264:439-443. https://doi.org/10.1056/NEJM196103022640906.
8. Sokol DK, Hettige S. Poor handwriting remains a significant problem in medicine. J R Soc Med. 2006;99(12):645-646. https://doi.org/10.1258/jrsm.99.12.645.
9. Asan O, Tyszka J, Fletcher KE. Capturing the patients’ voices: planning for patient-centered electronic health record use. Int J Med Inform. 2016;95:1-7. https://doi.org/10.1016/j.ijmedinf.2016.08.002.
10. Ishak WW, Collison K, Danovitch I, et al. Screening for depression in hospitalized medical patients. J Hosp Med. 2017;12(2):118-125. https://doi.org/10.12788/jhm.2693.
11. Esmaeeli MR, Sayar RE, Saghebi A, et al. Screening for depression in hospitalized pediatric patients. Iran J Child Neurol. 2014;8(1):47-51.
12. Asan O, Young HN, Chewning B, Montague E. How physician electronic health record screen sharing affects patient and doctor non-verbal communication in primary care. Patient Educ Couns. 2015;98(3):310-316. https://doi.org/10.1016/j.pec.2014.11.024.
13. Chau VM, Engeln JT, Axelrath S, et al. Beyond the chief complaint: our patients’ worries. J Med Humanit. 2017;38(4):541-547. https://doi.org/10.1007/s10912-017-9479-8.
14. Kommer CG. Good documentation. JAMA. 2018;320(9):875-876. https://doi.org/10.1001/jama.2018.11781.
15. Cumbler, Singh S. Writing Notes to Patients – Not about Them.. The Hospital Leader: Official Blog of SHM2018. 2018. https://thehospitalleader.org/writing-notes-to-patients-not-about-them/. Accessed April 10, 2019.
16. Kahn D, Stewart E, Duncan M, et al. A prescription for note bloat: an effective progress note template. J Hosp Med. 2018;13(6):378-382. https://doi.org/10.12788/jhm.2898.
17. Backman R, Bayliss S, Moore D, Litchfield I. Clinical reminder alert fatigue in healthcare: a systematic literature review protocol using qualitative evidence. Syst Rev. 2017;6(1):255. https://doi.org/10.1186/s13643-017-0627-z.
18. Evans RS. Electronic health records: then, now, and in the future. Yearbook Med Inform. 2016;25(1):S48-S61. https://doi.org/10.15265/IYS-2016-s006.
19. Finkel N. Nine ways hospitals can use electronic health records to reduce readmissions. Hospitalist. 2014.
20. Shiraishi J, Li Q, Appelbaum D, Doi K. Computer-aided diagnosis and artificial intelligence in clinical imaging. Semin Nucl Med. 2011;41(6):449-462. doi: 10.1053/j.semnuclmed.2011.06.004.
21. Toledo FG, Triola A, Ruppert K, Siminerio LM. Telemedicine consultations: an alternative model to increase access to diabetes specialist care in underserved rural communities. JMIR Res Protoc. 2012;1(2):e14. https://doi.org/10.2196/resprot.2235.
22. Rahimian F, Salimi-Khorshidi G, Payberah AH, et al. Predicting the risk of emergency admission with machine learning: development and validation using linked electronic health records. PLOS Med. 2018;15(11):e1002695. https://doi.org/10.1371/journal.pmed.1002695.
23. Ashton M. Getting rid of stupid stuff. N Engl J Med. 2018;379(19):1789-1791. https://doi.org/10.1056/NEJMp1809698.
We need to have an honest chat. My name is EHR, although you may call me Epic, Athena, Centricity, or just “the chart.” You may have called me something worse in a moment of frustration. However, I do not hold grudges. I am your silent, stoic partner, a ubiquitous presence when you are at work, and sometimes even when you are at home.
I don’t have feelings and I can’t read, but I do know what you and your colleagues have been writing about me. I am the cause of burnout. I have created a generation of physicians who are shackled to their computers, “trapped in the bunker of machine medicine,” no longer able to palpate spleens or detect precordial knocks.1,2 I have reduced medicine to keystrokes and mouse clicks instead of eye contact, and because of me, the iPatient gets more attention than the real patient.1,2 You repeat that doctors don’t spend time with their patients, not like in generations past (although there is ample evidence to the contrary).3-5 One critic even wrote that I have transformed the “personalized story of a patient’s travails to one filled with auto-populated fields, sapped of humanity and warmth.”3,6 I’ll be honest—were I able to have feelings, that one would hurt. And then, as if I have not wreaked enough havoc, I follow you home after a long day of depleting your energy, hungering for more keystrokes, creating a veritable avalanche of unfiltered information.
H. E. Payson once commented that “the doctor spends barely enough time with his patient to establish an acquaintance, much less a relationship.”7 However, he wrote that in 1961. So, before you romanticize the past, try to recall the time before I came into your life. Perhaps you were starting a night shift in the intensive care unit (ICU) and grew concerned about a patient’s steadily deteriorating renal function. You hurried to the paper chart, only to be met with pages of illegible, sometimes incomplete notes, while searching for your patient’s last discharge summary.2,8 Now, you just click. Years ago, you could only guess at your patient’s baseline cardiac ejection fraction. Now, just click.
I am part of the healthcare landscape, and I am not going away. But my goal is not to defend myself nor to remind you of my virtues. Rather, I want to convince you that I can be more than an adversary, more than a keyboard connected to a monitor. I have watched many physicians use me to form strong connections with their patients. If I may, I wish to offer four practical suggestions for how we can work together to promote humanistic patient care.
First, introduce me to your patient, as you would any other member of your healthcare team. Use specific phrases to overcome the technology barrier and enhance communication: “What you’re telling me is important, and I’d like to get it right. Do you mind if I type while we speak?” Or, “I am going to put in orders now. Here is what I am ordering and why.” Consider taking your patient on a tour of my functions: “Here’s where your doctors and nurses will chart what’s going on with you each day while you’re in the hospital. This is where we see all your lab results, even those from earlier hospital admissions. This is where we see the last notes from your primary care physician, your oncologist, and your physical therapist.” Your patients no longer need to worry about care collaboration between their inpatient and outpatient teams—they can see it for themselves!
Second, when your patient tells you about her depression or that her son is addicted to opioids or that her biggest fear is having cancer, stop typing. Look her in the eye. Though your practice is increasingly imbued with technology, there is still space to stop and hear your patients’ stories, as physicians have done for centuries. Listen. Make eye contact. Touch. Stop typing.
Third, integrate me into your practice in a more personal way. I have been called the ever-present and unavoidable “third party in the examining room,” so let’s be partners.9 Let your patient see her pneumonia on my screen (it may be the first time she has ever visualized her lungs).3 For your patient with a myocardial infarction, show him his right coronary artery before and after successful stent placement, and explain why he is no longer having chest pain. Use my databases to ensure timely, evidence-based inpatient screening for falls, functional and cognitive impairment, drug use, and depression.10,11 Before you prescribe a medication, verify the cost, your patient’s insurance status and expected copays, and use this information to ensure medication compliance and deliver higher-value care. Use my screen to form a bond with your patient who has heart failure; show him the steady decline in his weight and the improvement in his chest radiograph while he is being actively diuresed.12 For your patient undergoing treatment for sepsis, shower him with praise and encouragement as you review his improving vital signs, temperature curve, and serum creatinine. Let your patient know: Even though I am typing, I am not immersed in the electronic bunker; I am caring for you.
Fourth, use me to add richness and context to your notes. Recently, I was saddened to read this description of the clinician’s dilemma: “In front of a flickering monitor chock full of disembodied, virtual data, [the doctor] struggles to remember the eyes [and] words of the actual patient that these numbers and graphs represent.”3 Many hospitals now include a different icon: a photograph of each patient at the top of the screen, to help you remember the patient’s eyes and words. Why not add a special text field to every note, where you highlight the person you are caring for, the person you have come to know: their preferred name and gender identity, their life experiences, their hobbies, what makes them special, their biggest worries.13,14 Use my abundant text fields to remind the healthcare team about the broader context of the patient’s illness, such as transportation barriers, economic or cultural challenges, and insurance status. One group of hospital-based physicians uses me to write letters to their patients on the second day of their hospital stay, summarizing their reason for admission and the treatment plans. A variation on the traditional progress note, the letter helps patients feel cared for and models patient-centered care to learners and other healthcare professionals.15
I know I am annoying. I am over-programmed, leading to novella-length notes, “pop-up fatigue,” and overloaded in-baskets.14,16,17 Clearly, I am not the brains of the partnership (that will always be you). But talented medical informatics specialists are working hard to improve me. I dream of the day when I will create a truly seamless experience for you and your patients. In the meantime, I can foster a continuous integration of workflow, where all you have to do is talk to your patient. I take care of the rest.18 Certainly, I can simplify the ever-annoying task of printing, faxing and scanning records to be uploaded across various EHRs, facilitating an easy transfer of information among facilities. But right now, I can accomplish even more. I can support information exchange during patient care handoffs. I can facilitate routing of medication lists to the patient’s primary physician, using “continuity of care functionality.”19 I can support safer prescribing of opioids and other addictive medications. I can help you arrange follow-up home visits, physical therapy and social work appointments, and specialty consultations. The future holds even more promising ways in which we may work together. My computer-aided image analysis could help you to improve the accuracy of your diagnoses.20 Perhaps telemedicine will further increase access to specialists in rural areas, so that we can continue to serve the most vulnerable populations.21 Machine learning algorithms may continue to enhance our ability to determine which patients require urgent hospitalization.22 The possibilities to put me to work are endless.
So, please indulge me a little longer, while we work together to eliminate unnecessary keystrokes, enhance communication across different inpatient and outpatient providers, improve patient safety, and deliver high-value care.23 Like everything in medicine, I am constantly changing, evolving, and improving.
To summarize: consider how I can help you be present for your patients. Let me empower you to hear their stories as you deliver compassionate, humanistic, and evidence-based patient care. Paraphrasing Albert Einstein, the technology of medicine and the art of medicine are branches from the same tree.
Thank you for letting me speak with you. Now power down, and I’ll see you again tomorrow.
Acknowledgments
The authors thank the following individuals for their willingness to be interviewed as part of this work: Ethan Cumbler, MD; Brian Dwinnell, MD; Meghann Kirk, MD; Patrick Kneeland, MD; Kari Mader, MD; CT Lin, MD; Christina Osborne, MD; Read Pierce, MD; Jennifer Soep, MD; Nichole Zehnder, MD; Steven Zeichner, MD.
We need to have an honest chat. My name is EHR, although you may call me Epic, Athena, Centricity, or just “the chart.” You may have called me something worse in a moment of frustration. However, I do not hold grudges. I am your silent, stoic partner, a ubiquitous presence when you are at work, and sometimes even when you are at home.
I don’t have feelings and I can’t read, but I do know what you and your colleagues have been writing about me. I am the cause of burnout. I have created a generation of physicians who are shackled to their computers, “trapped in the bunker of machine medicine,” no longer able to palpate spleens or detect precordial knocks.1,2 I have reduced medicine to keystrokes and mouse clicks instead of eye contact, and because of me, the iPatient gets more attention than the real patient.1,2 You repeat that doctors don’t spend time with their patients, not like in generations past (although there is ample evidence to the contrary).3-5 One critic even wrote that I have transformed the “personalized story of a patient’s travails to one filled with auto-populated fields, sapped of humanity and warmth.”3,6 I’ll be honest—were I able to have feelings, that one would hurt. And then, as if I have not wreaked enough havoc, I follow you home after a long day of depleting your energy, hungering for more keystrokes, creating a veritable avalanche of unfiltered information.
H. E. Payson once commented that “the doctor spends barely enough time with his patient to establish an acquaintance, much less a relationship.”7 However, he wrote that in 1961. So, before you romanticize the past, try to recall the time before I came into your life. Perhaps you were starting a night shift in the intensive care unit (ICU) and grew concerned about a patient’s steadily deteriorating renal function. You hurried to the paper chart, only to be met with pages of illegible, sometimes incomplete notes, while searching for your patient’s last discharge summary.2,8 Now, you just click. Years ago, you could only guess at your patient’s baseline cardiac ejection fraction. Now, just click.
I am part of the healthcare landscape, and I am not going away. But my goal is not to defend myself nor to remind you of my virtues. Rather, I want to convince you that I can be more than an adversary, more than a keyboard connected to a monitor. I have watched many physicians use me to form strong connections with their patients. If I may, I wish to offer four practical suggestions for how we can work together to promote humanistic patient care.
First, introduce me to your patient, as you would any other member of your healthcare team. Use specific phrases to overcome the technology barrier and enhance communication: “What you’re telling me is important, and I’d like to get it right. Do you mind if I type while we speak?” Or, “I am going to put in orders now. Here is what I am ordering and why.” Consider taking your patient on a tour of my functions: “Here’s where your doctors and nurses will chart what’s going on with you each day while you’re in the hospital. This is where we see all your lab results, even those from earlier hospital admissions. This is where we see the last notes from your primary care physician, your oncologist, and your physical therapist.” Your patients no longer need to worry about care collaboration between their inpatient and outpatient teams—they can see it for themselves!
Second, when your patient tells you about her depression or that her son is addicted to opioids or that her biggest fear is having cancer, stop typing. Look her in the eye. Though your practice is increasingly imbued with technology, there is still space to stop and hear your patients’ stories, as physicians have done for centuries. Listen. Make eye contact. Touch. Stop typing.
Third, integrate me into your practice in a more personal way. I have been called the ever-present and unavoidable “third party in the examining room,” so let’s be partners.9 Let your patient see her pneumonia on my screen (it may be the first time she has ever visualized her lungs).3 For your patient with a myocardial infarction, show him his right coronary artery before and after successful stent placement, and explain why he is no longer having chest pain. Use my databases to ensure timely, evidence-based inpatient screening for falls, functional and cognitive impairment, drug use, and depression.10,11 Before you prescribe a medication, verify the cost, your patient’s insurance status and expected copays, and use this information to ensure medication compliance and deliver higher-value care. Use my screen to form a bond with your patient who has heart failure; show him the steady decline in his weight and the improvement in his chest radiograph while he is being actively diuresed.12 For your patient undergoing treatment for sepsis, shower him with praise and encouragement as you review his improving vital signs, temperature curve, and serum creatinine. Let your patient know: Even though I am typing, I am not immersed in the electronic bunker; I am caring for you.
Fourth, use me to add richness and context to your notes. Recently, I was saddened to read this description of the clinician’s dilemma: “In front of a flickering monitor chock full of disembodied, virtual data, [the doctor] struggles to remember the eyes [and] words of the actual patient that these numbers and graphs represent.”3 Many hospitals now include a different icon: a photograph of each patient at the top of the screen, to help you remember the patient’s eyes and words. Why not add a special text field to every note, where you highlight the person you are caring for, the person you have come to know: their preferred name and gender identity, their life experiences, their hobbies, what makes them special, their biggest worries.13,14 Use my abundant text fields to remind the healthcare team about the broader context of the patient’s illness, such as transportation barriers, economic or cultural challenges, and insurance status. One group of hospital-based physicians uses me to write letters to their patients on the second day of their hospital stay, summarizing their reason for admission and the treatment plans. A variation on the traditional progress note, the letter helps patients feel cared for and models patient-centered care to learners and other healthcare professionals.15
I know I am annoying. I am over-programmed, leading to novella-length notes, “pop-up fatigue,” and overloaded in-baskets.14,16,17 Clearly, I am not the brains of the partnership (that will always be you). But talented medical informatics specialists are working hard to improve me. I dream of the day when I will create a truly seamless experience for you and your patients. In the meantime, I can foster a continuous integration of workflow, where all you have to do is talk to your patient. I take care of the rest.18 Certainly, I can simplify the ever-annoying task of printing, faxing and scanning records to be uploaded across various EHRs, facilitating an easy transfer of information among facilities. But right now, I can accomplish even more. I can support information exchange during patient care handoffs. I can facilitate routing of medication lists to the patient’s primary physician, using “continuity of care functionality.”19 I can support safer prescribing of opioids and other addictive medications. I can help you arrange follow-up home visits, physical therapy and social work appointments, and specialty consultations. The future holds even more promising ways in which we may work together. My computer-aided image analysis could help you to improve the accuracy of your diagnoses.20 Perhaps telemedicine will further increase access to specialists in rural areas, so that we can continue to serve the most vulnerable populations.21 Machine learning algorithms may continue to enhance our ability to determine which patients require urgent hospitalization.22 The possibilities to put me to work are endless.
So, please indulge me a little longer, while we work together to eliminate unnecessary keystrokes, enhance communication across different inpatient and outpatient providers, improve patient safety, and deliver high-value care.23 Like everything in medicine, I am constantly changing, evolving, and improving.
To summarize: consider how I can help you be present for your patients. Let me empower you to hear their stories as you deliver compassionate, humanistic, and evidence-based patient care. Paraphrasing Albert Einstein, the technology of medicine and the art of medicine are branches from the same tree.
Thank you for letting me speak with you. Now power down, and I’ll see you again tomorrow.
Acknowledgments
The authors thank the following individuals for their willingness to be interviewed as part of this work: Ethan Cumbler, MD; Brian Dwinnell, MD; Meghann Kirk, MD; Patrick Kneeland, MD; Kari Mader, MD; CT Lin, MD; Christina Osborne, MD; Read Pierce, MD; Jennifer Soep, MD; Nichole Zehnder, MD; Steven Zeichner, MD.
1. Verghese A. How tech can turn doctors into clerical workers. The New York Times; 2018. https://www.nytimes.com/interactive/2018/05/16/magazine/health-issue-what-we-lose-with-data-driven-medicine.html. Accessed April 10, 2019.
2. Verghese A. Culture shock—patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461.
3. Czernik Z, Lin CT. Time at the bedside (computing). JAMA. 2016;315(22):2399-2400. doi: 10.1001/jama.2016.1722.
4. Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med. 2013;28(8):1042-1047. https://doi.org/10.1007/s11606-013-2376-6.
5. Parenti C, Lurie N. Are things different in the light of day? A time study of internal medicine house staff days. Am J Med. 1993;94(6):654-658. https://doi.org/10.1016/0002-9343(93)90220-J.
6. Wachter R. The Digital Doctor: Hope, Hype, and Harm at the Dawn of Medicine’s Computer Age. New York, NY: McGraw-Hill Education; 2015.
7. Payson HE, Gaenslen Jr EC, Stargardter FL. Time study of an internship on a university medical service. N Engl J Med. 1961;264:439-443. https://doi.org/10.1056/NEJM196103022640906.
8. Sokol DK, Hettige S. Poor handwriting remains a significant problem in medicine. J R Soc Med. 2006;99(12):645-646. https://doi.org/10.1258/jrsm.99.12.645.
9. Asan O, Tyszka J, Fletcher KE. Capturing the patients’ voices: planning for patient-centered electronic health record use. Int J Med Inform. 2016;95:1-7. https://doi.org/10.1016/j.ijmedinf.2016.08.002.
10. Ishak WW, Collison K, Danovitch I, et al. Screening for depression in hospitalized medical patients. J Hosp Med. 2017;12(2):118-125. https://doi.org/10.12788/jhm.2693.
11. Esmaeeli MR, Sayar RE, Saghebi A, et al. Screening for depression in hospitalized pediatric patients. Iran J Child Neurol. 2014;8(1):47-51.
12. Asan O, Young HN, Chewning B, Montague E. How physician electronic health record screen sharing affects patient and doctor non-verbal communication in primary care. Patient Educ Couns. 2015;98(3):310-316. https://doi.org/10.1016/j.pec.2014.11.024.
13. Chau VM, Engeln JT, Axelrath S, et al. Beyond the chief complaint: our patients’ worries. J Med Humanit. 2017;38(4):541-547. https://doi.org/10.1007/s10912-017-9479-8.
14. Kommer CG. Good documentation. JAMA. 2018;320(9):875-876. https://doi.org/10.1001/jama.2018.11781.
15. Cumbler, Singh S. Writing Notes to Patients – Not about Them.. The Hospital Leader: Official Blog of SHM2018. 2018. https://thehospitalleader.org/writing-notes-to-patients-not-about-them/. Accessed April 10, 2019.
16. Kahn D, Stewart E, Duncan M, et al. A prescription for note bloat: an effective progress note template. J Hosp Med. 2018;13(6):378-382. https://doi.org/10.12788/jhm.2898.
17. Backman R, Bayliss S, Moore D, Litchfield I. Clinical reminder alert fatigue in healthcare: a systematic literature review protocol using qualitative evidence. Syst Rev. 2017;6(1):255. https://doi.org/10.1186/s13643-017-0627-z.
18. Evans RS. Electronic health records: then, now, and in the future. Yearbook Med Inform. 2016;25(1):S48-S61. https://doi.org/10.15265/IYS-2016-s006.
19. Finkel N. Nine ways hospitals can use electronic health records to reduce readmissions. Hospitalist. 2014.
20. Shiraishi J, Li Q, Appelbaum D, Doi K. Computer-aided diagnosis and artificial intelligence in clinical imaging. Semin Nucl Med. 2011;41(6):449-462. doi: 10.1053/j.semnuclmed.2011.06.004.
21. Toledo FG, Triola A, Ruppert K, Siminerio LM. Telemedicine consultations: an alternative model to increase access to diabetes specialist care in underserved rural communities. JMIR Res Protoc. 2012;1(2):e14. https://doi.org/10.2196/resprot.2235.
22. Rahimian F, Salimi-Khorshidi G, Payberah AH, et al. Predicting the risk of emergency admission with machine learning: development and validation using linked electronic health records. PLOS Med. 2018;15(11):e1002695. https://doi.org/10.1371/journal.pmed.1002695.
23. Ashton M. Getting rid of stupid stuff. N Engl J Med. 2018;379(19):1789-1791. https://doi.org/10.1056/NEJMp1809698.
1. Verghese A. How tech can turn doctors into clerical workers. The New York Times; 2018. https://www.nytimes.com/interactive/2018/05/16/magazine/health-issue-what-we-lose-with-data-driven-medicine.html. Accessed April 10, 2019.
2. Verghese A. Culture shock—patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461.
3. Czernik Z, Lin CT. Time at the bedside (computing). JAMA. 2016;315(22):2399-2400. doi: 10.1001/jama.2016.1722.
4. Block L, Habicht R, Wu AW, et al. In the wake of the 2003 and 2011 duty hours regulations, how do internal medicine interns spend their time? J Gen Intern Med. 2013;28(8):1042-1047. https://doi.org/10.1007/s11606-013-2376-6.
5. Parenti C, Lurie N. Are things different in the light of day? A time study of internal medicine house staff days. Am J Med. 1993;94(6):654-658. https://doi.org/10.1016/0002-9343(93)90220-J.
6. Wachter R. The Digital Doctor: Hope, Hype, and Harm at the Dawn of Medicine’s Computer Age. New York, NY: McGraw-Hill Education; 2015.
7. Payson HE, Gaenslen Jr EC, Stargardter FL. Time study of an internship on a university medical service. N Engl J Med. 1961;264:439-443. https://doi.org/10.1056/NEJM196103022640906.
8. Sokol DK, Hettige S. Poor handwriting remains a significant problem in medicine. J R Soc Med. 2006;99(12):645-646. https://doi.org/10.1258/jrsm.99.12.645.
9. Asan O, Tyszka J, Fletcher KE. Capturing the patients’ voices: planning for patient-centered electronic health record use. Int J Med Inform. 2016;95:1-7. https://doi.org/10.1016/j.ijmedinf.2016.08.002.
10. Ishak WW, Collison K, Danovitch I, et al. Screening for depression in hospitalized medical patients. J Hosp Med. 2017;12(2):118-125. https://doi.org/10.12788/jhm.2693.
11. Esmaeeli MR, Sayar RE, Saghebi A, et al. Screening for depression in hospitalized pediatric patients. Iran J Child Neurol. 2014;8(1):47-51.
12. Asan O, Young HN, Chewning B, Montague E. How physician electronic health record screen sharing affects patient and doctor non-verbal communication in primary care. Patient Educ Couns. 2015;98(3):310-316. https://doi.org/10.1016/j.pec.2014.11.024.
13. Chau VM, Engeln JT, Axelrath S, et al. Beyond the chief complaint: our patients’ worries. J Med Humanit. 2017;38(4):541-547. https://doi.org/10.1007/s10912-017-9479-8.
14. Kommer CG. Good documentation. JAMA. 2018;320(9):875-876. https://doi.org/10.1001/jama.2018.11781.
15. Cumbler, Singh S. Writing Notes to Patients – Not about Them.. The Hospital Leader: Official Blog of SHM2018. 2018. https://thehospitalleader.org/writing-notes-to-patients-not-about-them/. Accessed April 10, 2019.
16. Kahn D, Stewart E, Duncan M, et al. A prescription for note bloat: an effective progress note template. J Hosp Med. 2018;13(6):378-382. https://doi.org/10.12788/jhm.2898.
17. Backman R, Bayliss S, Moore D, Litchfield I. Clinical reminder alert fatigue in healthcare: a systematic literature review protocol using qualitative evidence. Syst Rev. 2017;6(1):255. https://doi.org/10.1186/s13643-017-0627-z.
18. Evans RS. Electronic health records: then, now, and in the future. Yearbook Med Inform. 2016;25(1):S48-S61. https://doi.org/10.15265/IYS-2016-s006.
19. Finkel N. Nine ways hospitals can use electronic health records to reduce readmissions. Hospitalist. 2014.
20. Shiraishi J, Li Q, Appelbaum D, Doi K. Computer-aided diagnosis and artificial intelligence in clinical imaging. Semin Nucl Med. 2011;41(6):449-462. doi: 10.1053/j.semnuclmed.2011.06.004.
21. Toledo FG, Triola A, Ruppert K, Siminerio LM. Telemedicine consultations: an alternative model to increase access to diabetes specialist care in underserved rural communities. JMIR Res Protoc. 2012;1(2):e14. https://doi.org/10.2196/resprot.2235.
22. Rahimian F, Salimi-Khorshidi G, Payberah AH, et al. Predicting the risk of emergency admission with machine learning: development and validation using linked electronic health records. PLOS Med. 2018;15(11):e1002695. https://doi.org/10.1371/journal.pmed.1002695.
23. Ashton M. Getting rid of stupid stuff. N Engl J Med. 2018;379(19):1789-1791. https://doi.org/10.1056/NEJMp1809698.
© 2020 Society of Hospital Medicine
Tackling the Minimizers Hiding Behind High-Value Care
With the escalating need for academic health centers to control costs, high-value care initiatives targeted at residents have exploded. Recent estimates suggest that more than two-thirds of internal medicine residency programs have high-value care curricula.1 This growth has been catalyzed, in part, by compelling evidence suggesting that where the residents undergo training is strongly associated with their future utilization.2 Although we encourage, support, and participate in high-value care education, as hospitalists, there are potential consequences of the high-value care movement in medical training.
Minimizers – physicians who underestimate the signs and symptoms of a patient, hastily concluding that they have the most benign condition possible – have always existed within residency training. The ethos of “doing nothing” has been around since at least the days of the widely read medical satire House of God.3 However, the increasing focus on high-value care creates a socially acceptable banner for minimizers to hide behind when defending inappropriately doing less. For an inpatient with unexplained localized abdominal pain not responding to conservative therapy, a minimizing resident may report to the attending, “They’re fine. I am trying to practice high-value care and avoid getting a CT scan.”
In their 2011 book, Your Medical Mind, Groopman and Hartzband described how people naturally fall on a scale between medical maximizing and minimizing and how this influences their approach toward healthcare.4 Researchers have expanded this construct to create a “Maximizer-Minimizer Scale,” which has been used for studying patients and how these traits affect the degree of medical care they receive.5 Similar approaches could be used for identifying physicians and trainees at risk of too much minimizer behavior. Although the vast majority of trainees are not minimizers, and overuse continues to be the bigger problem in the majority of academic settings, it is important to understand how the high-value care movement could facilitate minimalist behavior in some residents. Although this article focuses on the educational system, the potential for minimization exists at all levels of clinical practice, including faculty and practicing physicians. Tackling this problem requires understanding the factors that promote the creation of minimizers, how patients and trainees are affected, and the solutions for preventing the spread of minimizers.
FACTORS THAT PROMOTE THE CREATION OF MINIMIZERS
Several factors may predispose a resident physician to become a minimizer. For example, resident burnout and overwhelming caseloads can contribute to the desire to decrease work by any means necessary. There are several ways a minimizer can accomplish this goal on inpatient rounds. First, a minimizer may present an important or acute problem as an “outpatient issue” that does not require inpatient workup. Second, minimizers may avoid requesting necessary consults, particularly those associated with intensive workups such as neurology, infectious disease, and rheumatology. Minimizers would claim that this is because of a concern of an unnecessary “costly workup,” when in reality they fear discovery of new problems, more tests to follow-up, and a potentially prolonged length of stay. Ironically, an institutional focus on hospital throughput can reinforce minimizers since the attending physicians or the hospital administrators may applaud them for avoiding “extra nights” in the hospital.
In addition to high workloads, inadequate clinical expertise favors the creation of minimizers. Although resident physicians may be aware that the probability of a rare disease is low, they may not recognize when ruling it out is appropriate. Thus, they could dismiss subtle cues or patterns that point to the need for further workup. Although attending physicians serve as a safety net, it could take time for them to recognize a resident minimizer who may be presenting biased information that influences their clinical decisions. Moreover, attending physicians may avoid further probing so that they are not perceived as promoting overuse and waste.
DANGERS OF MINIMIZERS
There are several dangers posed by minimizers, but the most concerning is the impact on patients. Missed diagnoses are a common source of patient maltreatment and contribute to avoidable deaths.6 Patients treated by minimizers may continue to experience their acute problem or have to be readmitted because of inadequate treatment. These patients may also lose faith or their trust in the medical system because of inattention to their problems. In fact, minimizing behaviors could have the greatest negative impact on the most vulnerable patients, who often cannot advocate for themselves or who may face conscious and unconscious biases, such as assumptions that they are “pain medication-seeking.”
In addition to harming patients, minimizers can jeopardize learning opportunities. A minimizer resident squanders the chance to recognize and contribute toward caring for a patient with a rare disease, diminishing their overall clinical development. Other trainees lose the opportunity to learn due to consultations or procedures never obtained. Lastly, as inappropriate attitudes and practices of minimizers spread through the hidden curriculum, particularly to medical students beginning their training, the overall clinical learning environment suffers.
SOLUTIONS FOR PREVENTING THE CREATION OF MINIMIZERS
There are specific techniques that academic hospitalists and teaching attending physicians can use to help curb the creation of minimizers and promote a clinical learning environment that counters these behaviors. First, instead of focusing on financial costs, it is important for educators to teach the true concept of healthcare value and the primary importance of improving patient outcomes. Embedding appropriateness criteria, such as those from the American College of Radiology, into daily workflows can enable residents to consider not just the cost of imaging but rather the appropriateness given a specific indication.7 Training programs can provide residents with a closed-loop feedback on patient outcomes so that they can recognize whether a diagnosis was missed or a necessary test was not ordered. Additionally, it is critical for residents to understand that improving healthcare value requires taking a big picture view of costs, particularly from the perspective of patients.8 A patient readmitted after receiving a minimalist workup is more costly to both the patient and the healthcare system.
Second, it is important for the hospitalist faculty to emphasize when a patient has failed a conservative approach and a more specialized, and sometimes intensive, workup or management strategy is appropriate. The classic example is a patient transferred from a community hospital to a tertiary center for further evaluation. Such patients are outside the scope of well-established guidelines. It is precisely these patients that Choosing Wisely or “Less is More” recommendations often do not apply. In contrast, transfer patients often do not end up receiving the specialty procedures that they were originally referred for9; it is important that all remain vigilant and committed to high-value care to avoid overuse in these situations.
Exposing residents to cognitive biases is equally important. For example, anchoring can lead to early closure, an easy path for a minimizer to follow. Given the recent focus on the harms related to diagnostic errors, more training in these biases can help promote better patient outcomes.10
Lastly, it is critical that hospitalists emphasize the importance of prioritizing a patient’s overall health to learners. Although it is tempting for trainees to focus only on acute episodes of a hospital stay, a holistic approach to patients and their quality of life can avoid the minimizer trap. The recent proposal to use home-to-home days in lieu of the routine length of hospital stay is a wonderful example of “measuring what matters to patients” and removing incentives for inappropriately shifting care to other clinicians or venues.11 Likewise, alternative payment models for emphasizing patient outcomes over time can create systems that reinforce holistic views of patient health.
CONCLUSION
The increasing focus on delivering high-value care has created a socially acceptable excuse for minimizers, who could thrive relatively unchecked in the clinical learning environment. To counter this unintended consequence, hospitalists must learn to identify minimizing behavior and actively guard against these tendencies by highlighting the value of appropriate care, not just doing less, and always striving to provide the best care for patients.
Disclosures
Dr. Arora reports personal fees from the American Board of Internal Medicine and personal fees from McGraw Hill, outside the submitted work. Dr. Moriates reports personal fees from McGraw Hill, outside the submitted work.
1. 2014 APDIM Program Directors Survey- Summary File. http://www.im.org/d/do/6030. Accessed on July 18, 2017.
2. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. doi: 10.1001/jama.2014.15973 PubMed
3. Shem S. The House of God. London, UK: Bodley Head; 1979.
4. Groopman J, Hartzband P. Your Medical Mind: How to Decide What Is Right for You. Reprint edition. New York, NY: Penguin Books; 2012.
5. Scherer LD, Caverly TJ, Burke J, et al. Development of the Medical Maximizer-Minimizer Scale. Health Psychol. 2016;35(11):1276-1287. doi: 10.1037/hea0000417 PubMed
6. National Academies of Sciences E. Improving Diagnosis in Health Care.; 2015. https://www.nap.edu/catalog/21794/improving-diagnosis-in-health-care. Accessed September 13, 2018.
7. American College of Radiology Appropriateness Criteria. https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed on July 28, 2018.
8. Parikh RB, Milstein A, Jain SH. Getting real about health care costs — a broader approach to cost stewardship in medical education. N Engl J Med.2017;376(10):913-915. doi: 10.1056/NEJMp1612517 PubMed
9. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Interhospital transfer and receipt of specialty procedures. J Hosp Med. 2018;13(6):383-387. doi: 10.12788/jhm.2875 PubMed
10. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(2 Suppl):ii28-ii32. PubMed
11. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time - measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. PubMed
With the escalating need for academic health centers to control costs, high-value care initiatives targeted at residents have exploded. Recent estimates suggest that more than two-thirds of internal medicine residency programs have high-value care curricula.1 This growth has been catalyzed, in part, by compelling evidence suggesting that where the residents undergo training is strongly associated with their future utilization.2 Although we encourage, support, and participate in high-value care education, as hospitalists, there are potential consequences of the high-value care movement in medical training.
Minimizers – physicians who underestimate the signs and symptoms of a patient, hastily concluding that they have the most benign condition possible – have always existed within residency training. The ethos of “doing nothing” has been around since at least the days of the widely read medical satire House of God.3 However, the increasing focus on high-value care creates a socially acceptable banner for minimizers to hide behind when defending inappropriately doing less. For an inpatient with unexplained localized abdominal pain not responding to conservative therapy, a minimizing resident may report to the attending, “They’re fine. I am trying to practice high-value care and avoid getting a CT scan.”
In their 2011 book, Your Medical Mind, Groopman and Hartzband described how people naturally fall on a scale between medical maximizing and minimizing and how this influences their approach toward healthcare.4 Researchers have expanded this construct to create a “Maximizer-Minimizer Scale,” which has been used for studying patients and how these traits affect the degree of medical care they receive.5 Similar approaches could be used for identifying physicians and trainees at risk of too much minimizer behavior. Although the vast majority of trainees are not minimizers, and overuse continues to be the bigger problem in the majority of academic settings, it is important to understand how the high-value care movement could facilitate minimalist behavior in some residents. Although this article focuses on the educational system, the potential for minimization exists at all levels of clinical practice, including faculty and practicing physicians. Tackling this problem requires understanding the factors that promote the creation of minimizers, how patients and trainees are affected, and the solutions for preventing the spread of minimizers.
FACTORS THAT PROMOTE THE CREATION OF MINIMIZERS
Several factors may predispose a resident physician to become a minimizer. For example, resident burnout and overwhelming caseloads can contribute to the desire to decrease work by any means necessary. There are several ways a minimizer can accomplish this goal on inpatient rounds. First, a minimizer may present an important or acute problem as an “outpatient issue” that does not require inpatient workup. Second, minimizers may avoid requesting necessary consults, particularly those associated with intensive workups such as neurology, infectious disease, and rheumatology. Minimizers would claim that this is because of a concern of an unnecessary “costly workup,” when in reality they fear discovery of new problems, more tests to follow-up, and a potentially prolonged length of stay. Ironically, an institutional focus on hospital throughput can reinforce minimizers since the attending physicians or the hospital administrators may applaud them for avoiding “extra nights” in the hospital.
In addition to high workloads, inadequate clinical expertise favors the creation of minimizers. Although resident physicians may be aware that the probability of a rare disease is low, they may not recognize when ruling it out is appropriate. Thus, they could dismiss subtle cues or patterns that point to the need for further workup. Although attending physicians serve as a safety net, it could take time for them to recognize a resident minimizer who may be presenting biased information that influences their clinical decisions. Moreover, attending physicians may avoid further probing so that they are not perceived as promoting overuse and waste.
DANGERS OF MINIMIZERS
There are several dangers posed by minimizers, but the most concerning is the impact on patients. Missed diagnoses are a common source of patient maltreatment and contribute to avoidable deaths.6 Patients treated by minimizers may continue to experience their acute problem or have to be readmitted because of inadequate treatment. These patients may also lose faith or their trust in the medical system because of inattention to their problems. In fact, minimizing behaviors could have the greatest negative impact on the most vulnerable patients, who often cannot advocate for themselves or who may face conscious and unconscious biases, such as assumptions that they are “pain medication-seeking.”
In addition to harming patients, minimizers can jeopardize learning opportunities. A minimizer resident squanders the chance to recognize and contribute toward caring for a patient with a rare disease, diminishing their overall clinical development. Other trainees lose the opportunity to learn due to consultations or procedures never obtained. Lastly, as inappropriate attitudes and practices of minimizers spread through the hidden curriculum, particularly to medical students beginning their training, the overall clinical learning environment suffers.
SOLUTIONS FOR PREVENTING THE CREATION OF MINIMIZERS
There are specific techniques that academic hospitalists and teaching attending physicians can use to help curb the creation of minimizers and promote a clinical learning environment that counters these behaviors. First, instead of focusing on financial costs, it is important for educators to teach the true concept of healthcare value and the primary importance of improving patient outcomes. Embedding appropriateness criteria, such as those from the American College of Radiology, into daily workflows can enable residents to consider not just the cost of imaging but rather the appropriateness given a specific indication.7 Training programs can provide residents with a closed-loop feedback on patient outcomes so that they can recognize whether a diagnosis was missed or a necessary test was not ordered. Additionally, it is critical for residents to understand that improving healthcare value requires taking a big picture view of costs, particularly from the perspective of patients.8 A patient readmitted after receiving a minimalist workup is more costly to both the patient and the healthcare system.
Second, it is important for the hospitalist faculty to emphasize when a patient has failed a conservative approach and a more specialized, and sometimes intensive, workup or management strategy is appropriate. The classic example is a patient transferred from a community hospital to a tertiary center for further evaluation. Such patients are outside the scope of well-established guidelines. It is precisely these patients that Choosing Wisely or “Less is More” recommendations often do not apply. In contrast, transfer patients often do not end up receiving the specialty procedures that they were originally referred for9; it is important that all remain vigilant and committed to high-value care to avoid overuse in these situations.
Exposing residents to cognitive biases is equally important. For example, anchoring can lead to early closure, an easy path for a minimizer to follow. Given the recent focus on the harms related to diagnostic errors, more training in these biases can help promote better patient outcomes.10
Lastly, it is critical that hospitalists emphasize the importance of prioritizing a patient’s overall health to learners. Although it is tempting for trainees to focus only on acute episodes of a hospital stay, a holistic approach to patients and their quality of life can avoid the minimizer trap. The recent proposal to use home-to-home days in lieu of the routine length of hospital stay is a wonderful example of “measuring what matters to patients” and removing incentives for inappropriately shifting care to other clinicians or venues.11 Likewise, alternative payment models for emphasizing patient outcomes over time can create systems that reinforce holistic views of patient health.
CONCLUSION
The increasing focus on delivering high-value care has created a socially acceptable excuse for minimizers, who could thrive relatively unchecked in the clinical learning environment. To counter this unintended consequence, hospitalists must learn to identify minimizing behavior and actively guard against these tendencies by highlighting the value of appropriate care, not just doing less, and always striving to provide the best care for patients.
Disclosures
Dr. Arora reports personal fees from the American Board of Internal Medicine and personal fees from McGraw Hill, outside the submitted work. Dr. Moriates reports personal fees from McGraw Hill, outside the submitted work.
With the escalating need for academic health centers to control costs, high-value care initiatives targeted at residents have exploded. Recent estimates suggest that more than two-thirds of internal medicine residency programs have high-value care curricula.1 This growth has been catalyzed, in part, by compelling evidence suggesting that where the residents undergo training is strongly associated with their future utilization.2 Although we encourage, support, and participate in high-value care education, as hospitalists, there are potential consequences of the high-value care movement in medical training.
Minimizers – physicians who underestimate the signs and symptoms of a patient, hastily concluding that they have the most benign condition possible – have always existed within residency training. The ethos of “doing nothing” has been around since at least the days of the widely read medical satire House of God.3 However, the increasing focus on high-value care creates a socially acceptable banner for minimizers to hide behind when defending inappropriately doing less. For an inpatient with unexplained localized abdominal pain not responding to conservative therapy, a minimizing resident may report to the attending, “They’re fine. I am trying to practice high-value care and avoid getting a CT scan.”
In their 2011 book, Your Medical Mind, Groopman and Hartzband described how people naturally fall on a scale between medical maximizing and minimizing and how this influences their approach toward healthcare.4 Researchers have expanded this construct to create a “Maximizer-Minimizer Scale,” which has been used for studying patients and how these traits affect the degree of medical care they receive.5 Similar approaches could be used for identifying physicians and trainees at risk of too much minimizer behavior. Although the vast majority of trainees are not minimizers, and overuse continues to be the bigger problem in the majority of academic settings, it is important to understand how the high-value care movement could facilitate minimalist behavior in some residents. Although this article focuses on the educational system, the potential for minimization exists at all levels of clinical practice, including faculty and practicing physicians. Tackling this problem requires understanding the factors that promote the creation of minimizers, how patients and trainees are affected, and the solutions for preventing the spread of minimizers.
FACTORS THAT PROMOTE THE CREATION OF MINIMIZERS
Several factors may predispose a resident physician to become a minimizer. For example, resident burnout and overwhelming caseloads can contribute to the desire to decrease work by any means necessary. There are several ways a minimizer can accomplish this goal on inpatient rounds. First, a minimizer may present an important or acute problem as an “outpatient issue” that does not require inpatient workup. Second, minimizers may avoid requesting necessary consults, particularly those associated with intensive workups such as neurology, infectious disease, and rheumatology. Minimizers would claim that this is because of a concern of an unnecessary “costly workup,” when in reality they fear discovery of new problems, more tests to follow-up, and a potentially prolonged length of stay. Ironically, an institutional focus on hospital throughput can reinforce minimizers since the attending physicians or the hospital administrators may applaud them for avoiding “extra nights” in the hospital.
In addition to high workloads, inadequate clinical expertise favors the creation of minimizers. Although resident physicians may be aware that the probability of a rare disease is low, they may not recognize when ruling it out is appropriate. Thus, they could dismiss subtle cues or patterns that point to the need for further workup. Although attending physicians serve as a safety net, it could take time for them to recognize a resident minimizer who may be presenting biased information that influences their clinical decisions. Moreover, attending physicians may avoid further probing so that they are not perceived as promoting overuse and waste.
DANGERS OF MINIMIZERS
There are several dangers posed by minimizers, but the most concerning is the impact on patients. Missed diagnoses are a common source of patient maltreatment and contribute to avoidable deaths.6 Patients treated by minimizers may continue to experience their acute problem or have to be readmitted because of inadequate treatment. These patients may also lose faith or their trust in the medical system because of inattention to their problems. In fact, minimizing behaviors could have the greatest negative impact on the most vulnerable patients, who often cannot advocate for themselves or who may face conscious and unconscious biases, such as assumptions that they are “pain medication-seeking.”
In addition to harming patients, minimizers can jeopardize learning opportunities. A minimizer resident squanders the chance to recognize and contribute toward caring for a patient with a rare disease, diminishing their overall clinical development. Other trainees lose the opportunity to learn due to consultations or procedures never obtained. Lastly, as inappropriate attitudes and practices of minimizers spread through the hidden curriculum, particularly to medical students beginning their training, the overall clinical learning environment suffers.
SOLUTIONS FOR PREVENTING THE CREATION OF MINIMIZERS
There are specific techniques that academic hospitalists and teaching attending physicians can use to help curb the creation of minimizers and promote a clinical learning environment that counters these behaviors. First, instead of focusing on financial costs, it is important for educators to teach the true concept of healthcare value and the primary importance of improving patient outcomes. Embedding appropriateness criteria, such as those from the American College of Radiology, into daily workflows can enable residents to consider not just the cost of imaging but rather the appropriateness given a specific indication.7 Training programs can provide residents with a closed-loop feedback on patient outcomes so that they can recognize whether a diagnosis was missed or a necessary test was not ordered. Additionally, it is critical for residents to understand that improving healthcare value requires taking a big picture view of costs, particularly from the perspective of patients.8 A patient readmitted after receiving a minimalist workup is more costly to both the patient and the healthcare system.
Second, it is important for the hospitalist faculty to emphasize when a patient has failed a conservative approach and a more specialized, and sometimes intensive, workup or management strategy is appropriate. The classic example is a patient transferred from a community hospital to a tertiary center for further evaluation. Such patients are outside the scope of well-established guidelines. It is precisely these patients that Choosing Wisely or “Less is More” recommendations often do not apply. In contrast, transfer patients often do not end up receiving the specialty procedures that they were originally referred for9; it is important that all remain vigilant and committed to high-value care to avoid overuse in these situations.
Exposing residents to cognitive biases is equally important. For example, anchoring can lead to early closure, an easy path for a minimizer to follow. Given the recent focus on the harms related to diagnostic errors, more training in these biases can help promote better patient outcomes.10
Lastly, it is critical that hospitalists emphasize the importance of prioritizing a patient’s overall health to learners. Although it is tempting for trainees to focus only on acute episodes of a hospital stay, a holistic approach to patients and their quality of life can avoid the minimizer trap. The recent proposal to use home-to-home days in lieu of the routine length of hospital stay is a wonderful example of “measuring what matters to patients” and removing incentives for inappropriately shifting care to other clinicians or venues.11 Likewise, alternative payment models for emphasizing patient outcomes over time can create systems that reinforce holistic views of patient health.
CONCLUSION
The increasing focus on delivering high-value care has created a socially acceptable excuse for minimizers, who could thrive relatively unchecked in the clinical learning environment. To counter this unintended consequence, hospitalists must learn to identify minimizing behavior and actively guard against these tendencies by highlighting the value of appropriate care, not just doing less, and always striving to provide the best care for patients.
Disclosures
Dr. Arora reports personal fees from the American Board of Internal Medicine and personal fees from McGraw Hill, outside the submitted work. Dr. Moriates reports personal fees from McGraw Hill, outside the submitted work.
1. 2014 APDIM Program Directors Survey- Summary File. http://www.im.org/d/do/6030. Accessed on July 18, 2017.
2. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. doi: 10.1001/jama.2014.15973 PubMed
3. Shem S. The House of God. London, UK: Bodley Head; 1979.
4. Groopman J, Hartzband P. Your Medical Mind: How to Decide What Is Right for You. Reprint edition. New York, NY: Penguin Books; 2012.
5. Scherer LD, Caverly TJ, Burke J, et al. Development of the Medical Maximizer-Minimizer Scale. Health Psychol. 2016;35(11):1276-1287. doi: 10.1037/hea0000417 PubMed
6. National Academies of Sciences E. Improving Diagnosis in Health Care.; 2015. https://www.nap.edu/catalog/21794/improving-diagnosis-in-health-care. Accessed September 13, 2018.
7. American College of Radiology Appropriateness Criteria. https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed on July 28, 2018.
8. Parikh RB, Milstein A, Jain SH. Getting real about health care costs — a broader approach to cost stewardship in medical education. N Engl J Med.2017;376(10):913-915. doi: 10.1056/NEJMp1612517 PubMed
9. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Interhospital transfer and receipt of specialty procedures. J Hosp Med. 2018;13(6):383-387. doi: 10.12788/jhm.2875 PubMed
10. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(2 Suppl):ii28-ii32. PubMed
11. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time - measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. PubMed
1. 2014 APDIM Program Directors Survey- Summary File. http://www.im.org/d/do/6030. Accessed on July 18, 2017.
2. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. 2014;312(22):2385-2393. doi: 10.1001/jama.2014.15973 PubMed
3. Shem S. The House of God. London, UK: Bodley Head; 1979.
4. Groopman J, Hartzband P. Your Medical Mind: How to Decide What Is Right for You. Reprint edition. New York, NY: Penguin Books; 2012.
5. Scherer LD, Caverly TJ, Burke J, et al. Development of the Medical Maximizer-Minimizer Scale. Health Psychol. 2016;35(11):1276-1287. doi: 10.1037/hea0000417 PubMed
6. National Academies of Sciences E. Improving Diagnosis in Health Care.; 2015. https://www.nap.edu/catalog/21794/improving-diagnosis-in-health-care. Accessed September 13, 2018.
7. American College of Radiology Appropriateness Criteria. https://www.acr.org/Clinical-Resources/ACR-Appropriateness-Criteria. Accessed on July 28, 2018.
8. Parikh RB, Milstein A, Jain SH. Getting real about health care costs — a broader approach to cost stewardship in medical education. N Engl J Med.2017;376(10):913-915. doi: 10.1056/NEJMp1612517 PubMed
9. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Interhospital transfer and receipt of specialty procedures. J Hosp Med. 2018;13(6):383-387. doi: 10.12788/jhm.2875 PubMed
10. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(2 Suppl):ii28-ii32. PubMed
11. Barnett ML, Grabowski DC, Mehrotra A. Home-to-home time - measuring what matters to patients and payers. N Engl J Med. 2017;377(1):4-6. PubMed
© 2019 Society of Hospital Medicine
Reimagining Inpatient Care in Canadian Teaching Hospitals: Bold Initiatives or Tinkering at the Margins?
Canada’s 17 medical schools and their affiliated teaching hospitals are instrumental in serving local communities and providing regional and national access to specialized therapies. Akin to many other countries, patients in Canadian teaching hospitals typically receive care from trainees supervised by attending physicians on teams that Canadians refer to as clinical teaching units (CTUs).1 For more than 50 years, the CTU model has served trainees, attendings, and patients well.2 The success of the CTU model has been dependent on several factors including the crucial balance between the number of trainees and volume of patients. However, Canadian teaching hospitals are increasingly challenged by an imbalance in the trainee-to-patient volume equilibrium spurred by increasing patient volumes and declining house staff availability. The challenges we are facing today in Canada are similar to those teaching hospitals in the United States have faced and adapted to over the last 15 years. Can we build a new, sustainable model of inpatient care through attending-directed inpatient services much as has happened in the US?
Canada’s population of 36 million people is growing by approximately 1% per year, largely driven by immigration.3 At the same time, Canada’s population is aging and becoming increasingly medically complex; the percentage of Canadians age 65 years and older is anticipated to rise from approximately 17% today to 25% in 2035.4 Canada’s healthcare system historically functioned with relatively few inpatient beds, encouraging efficiency particularly with respect to which patients require hospital admission and which do not.5 Although data suggest that the number of hospital admissions declined in Canada between 1980 and 1995, recent data documented that General Internal Medicine admissions increased by 32% between 2010 and 2015 and accounted for 24% of total hospital bed days.6,7 The effects of population growth and aging on admission volumes might be mitigated to some extent by innovations in healthcare delivery such as improved access to primary care (largely family physicians in Canada). However, even with these innovations, a growing and aging population is likely to have a disproportionate effect on the types of undifferentiated illnesses that are typically admitted to General Internal Medicine in Canadian teaching hospitals.
Increasing volumes and complexity are occurring at the same time that residency training in Canada is undergoing an extraordinary shift, mirroring trends in other countries.8 CTUs in Canada typically have a census of 20 or more patients and are staffed by an attending, one senior resident, two to three junior residents, and medical students. Recognition that physician fatigue is associated with patient safety events and physician burnout has led to shorter resident shifts, though Canadian hospitals typically operate without concrete work hour limits or “hard” caps on team size.8 To fulfill accreditation standards set by the Royal College of Physicians and Surgeons of Canada, residency programs have required increases in formal teaching sessions during working hours, further reducing resident presence at the bedside. Many specialty training programs (eg, anesthesiology and ophthalmology) that traditionally required trainees to rotate through General Medicine have eliminated this requirement. Moreover, postgraduate training now requires additional time be spent in ambulatory and community hospital settings to better prepare residents for practice.9 There is little enthusiasm for increasing the number of residents, as postgraduate training spots increased by 85% between 2000 and 2013, before stabilizing in recent years.10
These factors are leading to a substantial decline in resident availability on CTUs, shifting increasing amounts of direct patient care to attending physicians in Canadian teaching hospitals across virtually all specialties. Unsurprisingly, increased rates of burnout and decreases in job satisfactio
Canadian teaching hospitals currently find themselves facing a confluence of factors nearly identical to those faced by teaching hospitals in the United States during 2003 when the Accreditation Council for Graduate Medical Education instituted resident duty hour restrictions to address concerns over trainee wellness, shift length, and patient safety.8 Instantly, hundreds of US teaching hospitals faced uncertainty over who would provide patient care when residents were unavailable. Virtually all US teaching hospitals responded with a creativity and speed that we are unaccustomed to in academic medicine. Hospitals reallocated money to finance attending-directed services where patient care was provided directly by attending physicians often working without trainees12 but frequently supported by nurse practitioners or physician assistants.13 Despite the differences between US and Canadian healthcare, 15 years later, we in Canada can and should learn from the US experience.14
Attending-directed services offer several advantages. First, attending-directed services offer patient outcomes including ICU transfer, mortality, readmissions, and satisfaction that are similar, if not modestly improved, when compared with traditional teaching services.15 Results also suggest potential reductions in hospital length of stay and diagnostic testing.16 Attending-directed services can enhance trainee education by insuring attending physician presence and oversight in-hospital 24-hours per day.17 Although not well studied, attending-directed services may reduce variation in CTU patient census so that excess volumes can be absorbed by attending-directed teams even with seasonal surges (eg, influenza). Recognizing that many specialties were experiencing the same challenges as General Medicine in 2003, attending-directed services in the US have been designed to care for a wide spectrum of patients drawn from an array of different specialties with evidence of improved outcomes.12 Building attending-directed services in Canadian teaching hospitals may expand to include patients from multiple specialties and subspecialties (surgery, orthopedics, and cardiology) where patient volumes are increasing and resident coverage is increasingly scarce.
The challenges that accompany the implementation of attending-directed teams must be acknowledged. First, while attending-directed teams solve many problems for teaching hospitals, physician billings may not generate sufficient income to be self-sustaining and require additional financial support.18 Without investment from hospitals or government, attending-directed models cannot flourish in teaching hospitals. US hospitals typically provide substantial financial support ($50,000-$100,000 per physician) to hospitalist programs, but Canadian teaching hospitals have been reluctant to follow suit.
Second, attending-directed services require a sustainable workforce. In Canada, inpatient care is provided predominately by family physician hospitalists in community hospitals, whereas internists typically fulfill these roles in teaching hospitals.19 Family physician hospitalists are commonly represented by the Canadian Society for Hospital Medicine, which is the Canadian branch of the Society of Hospital Medicine. Hospital medicine in Canada is typically organized around physician training (family physician vs internist) rather than clinical focus (outpatient vs inpatient). Collaborative models of care that unite hospitalists from all training streams (family physician, internist, and pediatrics) are only just emerging in Canadian teaching hospitals. How these programs are developed will be critical to the successful growth of attending-directed services. Third, if attending-directed services expand in teaching hospitals, the physicians who staff these services must come from somewhere. Either the “production” of physicians will need to increase or physicians will migrate to attending-directed services from outpatient practice or from community hospitals.20 Canadian teaching hospitals can also explore nurse practitioners and physician assistants, a previously underutilized resource. Though the costs of such programs can be significant,21 the payoff in safety, quality, and efficiency may be worth it—as demonstrated in the US system. Fourth, teaching hospitals and medical schools must create academic homes to support and mentor the physicians working on attending-directed services. Although physicians hired for attending-directed services primarily provide direct patient care, few will join academic medical centers solely for this purpose. Teaching hospitals and medical schools need to carefully consider job descriptions, mentoring, and career advancement opportunities as they build attending-directed services. Finally, the interactions between teaching and attending-directed services are complex. There is an inevitable learning curve as clinical operations and protocols are built and developed. For example, decisions need to be made about how patients are divided between services and whether nocturnists are responsible for teaching overnight residents.17 Successful programs have the potential to benefit hospitals, patients, learners, and faculty alike.
The risks associated with the status quo in Canada must also be addressed. Patient volumes and complexity in Canada are likely to continue to slowly increase, while the number of trainees in Canadian teaching hospitals will remain stable at best. Forcing more patients onto already overtaxed teaching services is likely to worsen hospital efficiency, patient outcomes, and educational experiences.22 Forcing additional patient care onto overstretched faculty will slowly erode the academic work (teaching and research) that has characterized excellence in Canadian medicine.
The changes we propose to overcome the challenges facing Canadian teaching hospitals are neither cheap nor easy (Table). We expect resistance on many fronts. Implementing them will likely require concerted advocacy from a diverse group of champions shining a bright spotlight on the sizable challenges Canadian teaching hospitals are confronting. We believe that each challenge maps to a discrete group of champions with discrete targets within hospital leadership, medical school administration, and government who will need to be engaged. In our opinion, organizing around these challenges offers the best opportunity to overcome the perpetual resistance around costs. Canadian teaching hospitals and their CTUs are under unprecedented pressure. Do we act boldly and embrace attending-directed models of care or continue tinkering at the margins?
Acknowledgments
The authors thank Chaim Bell for his advice and suggestions.
Disclosures
The authors have nothing to disclose.
1. Schrewe B, Pratt DD, McKellin WH. Adapting the forms of yesterday to the functions of today and the needs of tomorrow: a genealogical case study of clinical teaching units in Canada. Adv Health Sci Educ Theory Pract. 2016;21(2):475-499. PubMed
2. Maudsley RF. The clinical teaching unit in transition. CMAJ. 1993;148(9):1564-1566. PubMed
3. Statistics Canada. Recent Changes in Demographic Trends in Canada. Ottawa: Ontario, 2015. https://www150.statcan.gc.ca/n1/pub/75-006-x/2015001/article/14240-eng.htm. Accessed December 9, 2018
4. Statistics Canada. Census, Age and Sex. Ottawa: Ontario, 2016. https://www12.statcan.gc.ca/census-recensement/2016/rt-td/as-eng.cfm. Accessed December 10, 2018.
5. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024-1039. PubMed
6. van Walraven C. Trends in 1-year survival of people admitted to hospital in Ontario, 1994-2009. CMAJ. 2013;185(16):E755-E762. PubMed
7. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: the General Medicine Inpatient Initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5(4):E842-E849. PubMed
8. Pattani R, Wu PE, Dhalla IA. Resident duty hours in Canada: past, present and future. CMAJ. 2014;186(10):761-765. PubMed
9. Royal College of Physicians and Surgeons. Specialty Training Requirements in Internal medicine 2015. http://www.royalcollege.ca/cs/groups/public/documents/document/mdaw/mdg4/~edisp/088402.pdf. Accessed December 12, 2018.
10. Freeman TR, Petterson S, Finnegan S, Bazemore A. Shifting tides in the emigration patterns of Canadian physicians to the United States: a cross-sectional secondary data analysis. BMC Health Serv Res. 2016;16(1):678. PubMed
11. Wong BM, Imrie K. Why resident duty hours regulations must address attending physicians’ workload. Acad Med. 2013;88(9):1209-1211. PubMed
12. Flanders SA, Centor B, Weber V, McGinn T, DeSalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240-246. PubMed
13. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2012;7(3):190-194. PubMed
14. Ivers N, Brown AD, Detsky AS. Lessons from the Canadian experience with single-payer health insurance: just comfortable enough with the status quo. JAMA Intern Med. 2018;178(9):1250-1255. PubMed
15. Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: patient responses to a 30-day postdischarge questionnaire. J Hosp Med. 2016;11(2):99-104. PubMed
16. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. PubMed
17. Farnan JM, Burger A, Boonyasai RT, et al. Survey of overnight academic hospitalist supervision of trainees. J Hosp Med. 2012;7(7):521-523. PubMed
18. Gonzalo JD, Kuperman EF, Chuang CH, Lehman E, Glasser F, Abendroth T. Impact of an overnight internal medicine academic hospitalist program on patient outcomes. J Gen Intern Med. 2015;30(12):1795-1802. PubMed
19. Soong C, Fan E, Howell EE, et al. Characteristics of Hospitalists and Hospitalist Programs in the United States and Canada 2009. J Clin Outcomes Meas. 2009; 16 (2): 69-74.
20. Yousefi V, Maslowski R. Health system drivers of hospital medicine in Canada: systematic review. Can Fam Phys Med Fam Can. 2013;59(7):762-767. PubMed
21. Nuckols TK, Escarce JJ. Cost implications of ACGME’s 2011 changes to resident duty hours and the training environment. J Gen Intern Med. 2012;27(2):241-249. PubMed
22. Elliott DJ, Young RS, Brice J, Aguiar R, Kolm P. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786-793. PubMed
Canada’s 17 medical schools and their affiliated teaching hospitals are instrumental in serving local communities and providing regional and national access to specialized therapies. Akin to many other countries, patients in Canadian teaching hospitals typically receive care from trainees supervised by attending physicians on teams that Canadians refer to as clinical teaching units (CTUs).1 For more than 50 years, the CTU model has served trainees, attendings, and patients well.2 The success of the CTU model has been dependent on several factors including the crucial balance between the number of trainees and volume of patients. However, Canadian teaching hospitals are increasingly challenged by an imbalance in the trainee-to-patient volume equilibrium spurred by increasing patient volumes and declining house staff availability. The challenges we are facing today in Canada are similar to those teaching hospitals in the United States have faced and adapted to over the last 15 years. Can we build a new, sustainable model of inpatient care through attending-directed inpatient services much as has happened in the US?
Canada’s population of 36 million people is growing by approximately 1% per year, largely driven by immigration.3 At the same time, Canada’s population is aging and becoming increasingly medically complex; the percentage of Canadians age 65 years and older is anticipated to rise from approximately 17% today to 25% in 2035.4 Canada’s healthcare system historically functioned with relatively few inpatient beds, encouraging efficiency particularly with respect to which patients require hospital admission and which do not.5 Although data suggest that the number of hospital admissions declined in Canada between 1980 and 1995, recent data documented that General Internal Medicine admissions increased by 32% between 2010 and 2015 and accounted for 24% of total hospital bed days.6,7 The effects of population growth and aging on admission volumes might be mitigated to some extent by innovations in healthcare delivery such as improved access to primary care (largely family physicians in Canada). However, even with these innovations, a growing and aging population is likely to have a disproportionate effect on the types of undifferentiated illnesses that are typically admitted to General Internal Medicine in Canadian teaching hospitals.
Increasing volumes and complexity are occurring at the same time that residency training in Canada is undergoing an extraordinary shift, mirroring trends in other countries.8 CTUs in Canada typically have a census of 20 or more patients and are staffed by an attending, one senior resident, two to three junior residents, and medical students. Recognition that physician fatigue is associated with patient safety events and physician burnout has led to shorter resident shifts, though Canadian hospitals typically operate without concrete work hour limits or “hard” caps on team size.8 To fulfill accreditation standards set by the Royal College of Physicians and Surgeons of Canada, residency programs have required increases in formal teaching sessions during working hours, further reducing resident presence at the bedside. Many specialty training programs (eg, anesthesiology and ophthalmology) that traditionally required trainees to rotate through General Medicine have eliminated this requirement. Moreover, postgraduate training now requires additional time be spent in ambulatory and community hospital settings to better prepare residents for practice.9 There is little enthusiasm for increasing the number of residents, as postgraduate training spots increased by 85% between 2000 and 2013, before stabilizing in recent years.10
These factors are leading to a substantial decline in resident availability on CTUs, shifting increasing amounts of direct patient care to attending physicians in Canadian teaching hospitals across virtually all specialties. Unsurprisingly, increased rates of burnout and decreases in job satisfactio
Canadian teaching hospitals currently find themselves facing a confluence of factors nearly identical to those faced by teaching hospitals in the United States during 2003 when the Accreditation Council for Graduate Medical Education instituted resident duty hour restrictions to address concerns over trainee wellness, shift length, and patient safety.8 Instantly, hundreds of US teaching hospitals faced uncertainty over who would provide patient care when residents were unavailable. Virtually all US teaching hospitals responded with a creativity and speed that we are unaccustomed to in academic medicine. Hospitals reallocated money to finance attending-directed services where patient care was provided directly by attending physicians often working without trainees12 but frequently supported by nurse practitioners or physician assistants.13 Despite the differences between US and Canadian healthcare, 15 years later, we in Canada can and should learn from the US experience.14
Attending-directed services offer several advantages. First, attending-directed services offer patient outcomes including ICU transfer, mortality, readmissions, and satisfaction that are similar, if not modestly improved, when compared with traditional teaching services.15 Results also suggest potential reductions in hospital length of stay and diagnostic testing.16 Attending-directed services can enhance trainee education by insuring attending physician presence and oversight in-hospital 24-hours per day.17 Although not well studied, attending-directed services may reduce variation in CTU patient census so that excess volumes can be absorbed by attending-directed teams even with seasonal surges (eg, influenza). Recognizing that many specialties were experiencing the same challenges as General Medicine in 2003, attending-directed services in the US have been designed to care for a wide spectrum of patients drawn from an array of different specialties with evidence of improved outcomes.12 Building attending-directed services in Canadian teaching hospitals may expand to include patients from multiple specialties and subspecialties (surgery, orthopedics, and cardiology) where patient volumes are increasing and resident coverage is increasingly scarce.
The challenges that accompany the implementation of attending-directed teams must be acknowledged. First, while attending-directed teams solve many problems for teaching hospitals, physician billings may not generate sufficient income to be self-sustaining and require additional financial support.18 Without investment from hospitals or government, attending-directed models cannot flourish in teaching hospitals. US hospitals typically provide substantial financial support ($50,000-$100,000 per physician) to hospitalist programs, but Canadian teaching hospitals have been reluctant to follow suit.
Second, attending-directed services require a sustainable workforce. In Canada, inpatient care is provided predominately by family physician hospitalists in community hospitals, whereas internists typically fulfill these roles in teaching hospitals.19 Family physician hospitalists are commonly represented by the Canadian Society for Hospital Medicine, which is the Canadian branch of the Society of Hospital Medicine. Hospital medicine in Canada is typically organized around physician training (family physician vs internist) rather than clinical focus (outpatient vs inpatient). Collaborative models of care that unite hospitalists from all training streams (family physician, internist, and pediatrics) are only just emerging in Canadian teaching hospitals. How these programs are developed will be critical to the successful growth of attending-directed services. Third, if attending-directed services expand in teaching hospitals, the physicians who staff these services must come from somewhere. Either the “production” of physicians will need to increase or physicians will migrate to attending-directed services from outpatient practice or from community hospitals.20 Canadian teaching hospitals can also explore nurse practitioners and physician assistants, a previously underutilized resource. Though the costs of such programs can be significant,21 the payoff in safety, quality, and efficiency may be worth it—as demonstrated in the US system. Fourth, teaching hospitals and medical schools must create academic homes to support and mentor the physicians working on attending-directed services. Although physicians hired for attending-directed services primarily provide direct patient care, few will join academic medical centers solely for this purpose. Teaching hospitals and medical schools need to carefully consider job descriptions, mentoring, and career advancement opportunities as they build attending-directed services. Finally, the interactions between teaching and attending-directed services are complex. There is an inevitable learning curve as clinical operations and protocols are built and developed. For example, decisions need to be made about how patients are divided between services and whether nocturnists are responsible for teaching overnight residents.17 Successful programs have the potential to benefit hospitals, patients, learners, and faculty alike.
The risks associated with the status quo in Canada must also be addressed. Patient volumes and complexity in Canada are likely to continue to slowly increase, while the number of trainees in Canadian teaching hospitals will remain stable at best. Forcing more patients onto already overtaxed teaching services is likely to worsen hospital efficiency, patient outcomes, and educational experiences.22 Forcing additional patient care onto overstretched faculty will slowly erode the academic work (teaching and research) that has characterized excellence in Canadian medicine.
The changes we propose to overcome the challenges facing Canadian teaching hospitals are neither cheap nor easy (Table). We expect resistance on many fronts. Implementing them will likely require concerted advocacy from a diverse group of champions shining a bright spotlight on the sizable challenges Canadian teaching hospitals are confronting. We believe that each challenge maps to a discrete group of champions with discrete targets within hospital leadership, medical school administration, and government who will need to be engaged. In our opinion, organizing around these challenges offers the best opportunity to overcome the perpetual resistance around costs. Canadian teaching hospitals and their CTUs are under unprecedented pressure. Do we act boldly and embrace attending-directed models of care or continue tinkering at the margins?
Acknowledgments
The authors thank Chaim Bell for his advice and suggestions.
Disclosures
The authors have nothing to disclose.
Canada’s 17 medical schools and their affiliated teaching hospitals are instrumental in serving local communities and providing regional and national access to specialized therapies. Akin to many other countries, patients in Canadian teaching hospitals typically receive care from trainees supervised by attending physicians on teams that Canadians refer to as clinical teaching units (CTUs).1 For more than 50 years, the CTU model has served trainees, attendings, and patients well.2 The success of the CTU model has been dependent on several factors including the crucial balance between the number of trainees and volume of patients. However, Canadian teaching hospitals are increasingly challenged by an imbalance in the trainee-to-patient volume equilibrium spurred by increasing patient volumes and declining house staff availability. The challenges we are facing today in Canada are similar to those teaching hospitals in the United States have faced and adapted to over the last 15 years. Can we build a new, sustainable model of inpatient care through attending-directed inpatient services much as has happened in the US?
Canada’s population of 36 million people is growing by approximately 1% per year, largely driven by immigration.3 At the same time, Canada’s population is aging and becoming increasingly medically complex; the percentage of Canadians age 65 years and older is anticipated to rise from approximately 17% today to 25% in 2035.4 Canada’s healthcare system historically functioned with relatively few inpatient beds, encouraging efficiency particularly with respect to which patients require hospital admission and which do not.5 Although data suggest that the number of hospital admissions declined in Canada between 1980 and 1995, recent data documented that General Internal Medicine admissions increased by 32% between 2010 and 2015 and accounted for 24% of total hospital bed days.6,7 The effects of population growth and aging on admission volumes might be mitigated to some extent by innovations in healthcare delivery such as improved access to primary care (largely family physicians in Canada). However, even with these innovations, a growing and aging population is likely to have a disproportionate effect on the types of undifferentiated illnesses that are typically admitted to General Internal Medicine in Canadian teaching hospitals.
Increasing volumes and complexity are occurring at the same time that residency training in Canada is undergoing an extraordinary shift, mirroring trends in other countries.8 CTUs in Canada typically have a census of 20 or more patients and are staffed by an attending, one senior resident, two to three junior residents, and medical students. Recognition that physician fatigue is associated with patient safety events and physician burnout has led to shorter resident shifts, though Canadian hospitals typically operate without concrete work hour limits or “hard” caps on team size.8 To fulfill accreditation standards set by the Royal College of Physicians and Surgeons of Canada, residency programs have required increases in formal teaching sessions during working hours, further reducing resident presence at the bedside. Many specialty training programs (eg, anesthesiology and ophthalmology) that traditionally required trainees to rotate through General Medicine have eliminated this requirement. Moreover, postgraduate training now requires additional time be spent in ambulatory and community hospital settings to better prepare residents for practice.9 There is little enthusiasm for increasing the number of residents, as postgraduate training spots increased by 85% between 2000 and 2013, before stabilizing in recent years.10
These factors are leading to a substantial decline in resident availability on CTUs, shifting increasing amounts of direct patient care to attending physicians in Canadian teaching hospitals across virtually all specialties. Unsurprisingly, increased rates of burnout and decreases in job satisfactio
Canadian teaching hospitals currently find themselves facing a confluence of factors nearly identical to those faced by teaching hospitals in the United States during 2003 when the Accreditation Council for Graduate Medical Education instituted resident duty hour restrictions to address concerns over trainee wellness, shift length, and patient safety.8 Instantly, hundreds of US teaching hospitals faced uncertainty over who would provide patient care when residents were unavailable. Virtually all US teaching hospitals responded with a creativity and speed that we are unaccustomed to in academic medicine. Hospitals reallocated money to finance attending-directed services where patient care was provided directly by attending physicians often working without trainees12 but frequently supported by nurse practitioners or physician assistants.13 Despite the differences between US and Canadian healthcare, 15 years later, we in Canada can and should learn from the US experience.14
Attending-directed services offer several advantages. First, attending-directed services offer patient outcomes including ICU transfer, mortality, readmissions, and satisfaction that are similar, if not modestly improved, when compared with traditional teaching services.15 Results also suggest potential reductions in hospital length of stay and diagnostic testing.16 Attending-directed services can enhance trainee education by insuring attending physician presence and oversight in-hospital 24-hours per day.17 Although not well studied, attending-directed services may reduce variation in CTU patient census so that excess volumes can be absorbed by attending-directed teams even with seasonal surges (eg, influenza). Recognizing that many specialties were experiencing the same challenges as General Medicine in 2003, attending-directed services in the US have been designed to care for a wide spectrum of patients drawn from an array of different specialties with evidence of improved outcomes.12 Building attending-directed services in Canadian teaching hospitals may expand to include patients from multiple specialties and subspecialties (surgery, orthopedics, and cardiology) where patient volumes are increasing and resident coverage is increasingly scarce.
The challenges that accompany the implementation of attending-directed teams must be acknowledged. First, while attending-directed teams solve many problems for teaching hospitals, physician billings may not generate sufficient income to be self-sustaining and require additional financial support.18 Without investment from hospitals or government, attending-directed models cannot flourish in teaching hospitals. US hospitals typically provide substantial financial support ($50,000-$100,000 per physician) to hospitalist programs, but Canadian teaching hospitals have been reluctant to follow suit.
Second, attending-directed services require a sustainable workforce. In Canada, inpatient care is provided predominately by family physician hospitalists in community hospitals, whereas internists typically fulfill these roles in teaching hospitals.19 Family physician hospitalists are commonly represented by the Canadian Society for Hospital Medicine, which is the Canadian branch of the Society of Hospital Medicine. Hospital medicine in Canada is typically organized around physician training (family physician vs internist) rather than clinical focus (outpatient vs inpatient). Collaborative models of care that unite hospitalists from all training streams (family physician, internist, and pediatrics) are only just emerging in Canadian teaching hospitals. How these programs are developed will be critical to the successful growth of attending-directed services. Third, if attending-directed services expand in teaching hospitals, the physicians who staff these services must come from somewhere. Either the “production” of physicians will need to increase or physicians will migrate to attending-directed services from outpatient practice or from community hospitals.20 Canadian teaching hospitals can also explore nurse practitioners and physician assistants, a previously underutilized resource. Though the costs of such programs can be significant,21 the payoff in safety, quality, and efficiency may be worth it—as demonstrated in the US system. Fourth, teaching hospitals and medical schools must create academic homes to support and mentor the physicians working on attending-directed services. Although physicians hired for attending-directed services primarily provide direct patient care, few will join academic medical centers solely for this purpose. Teaching hospitals and medical schools need to carefully consider job descriptions, mentoring, and career advancement opportunities as they build attending-directed services. Finally, the interactions between teaching and attending-directed services are complex. There is an inevitable learning curve as clinical operations and protocols are built and developed. For example, decisions need to be made about how patients are divided between services and whether nocturnists are responsible for teaching overnight residents.17 Successful programs have the potential to benefit hospitals, patients, learners, and faculty alike.
The risks associated with the status quo in Canada must also be addressed. Patient volumes and complexity in Canada are likely to continue to slowly increase, while the number of trainees in Canadian teaching hospitals will remain stable at best. Forcing more patients onto already overtaxed teaching services is likely to worsen hospital efficiency, patient outcomes, and educational experiences.22 Forcing additional patient care onto overstretched faculty will slowly erode the academic work (teaching and research) that has characterized excellence in Canadian medicine.
The changes we propose to overcome the challenges facing Canadian teaching hospitals are neither cheap nor easy (Table). We expect resistance on many fronts. Implementing them will likely require concerted advocacy from a diverse group of champions shining a bright spotlight on the sizable challenges Canadian teaching hospitals are confronting. We believe that each challenge maps to a discrete group of champions with discrete targets within hospital leadership, medical school administration, and government who will need to be engaged. In our opinion, organizing around these challenges offers the best opportunity to overcome the perpetual resistance around costs. Canadian teaching hospitals and their CTUs are under unprecedented pressure. Do we act boldly and embrace attending-directed models of care or continue tinkering at the margins?
Acknowledgments
The authors thank Chaim Bell for his advice and suggestions.
Disclosures
The authors have nothing to disclose.
1. Schrewe B, Pratt DD, McKellin WH. Adapting the forms of yesterday to the functions of today and the needs of tomorrow: a genealogical case study of clinical teaching units in Canada. Adv Health Sci Educ Theory Pract. 2016;21(2):475-499. PubMed
2. Maudsley RF. The clinical teaching unit in transition. CMAJ. 1993;148(9):1564-1566. PubMed
3. Statistics Canada. Recent Changes in Demographic Trends in Canada. Ottawa: Ontario, 2015. https://www150.statcan.gc.ca/n1/pub/75-006-x/2015001/article/14240-eng.htm. Accessed December 9, 2018
4. Statistics Canada. Census, Age and Sex. Ottawa: Ontario, 2016. https://www12.statcan.gc.ca/census-recensement/2016/rt-td/as-eng.cfm. Accessed December 10, 2018.
5. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024-1039. PubMed
6. van Walraven C. Trends in 1-year survival of people admitted to hospital in Ontario, 1994-2009. CMAJ. 2013;185(16):E755-E762. PubMed
7. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: the General Medicine Inpatient Initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5(4):E842-E849. PubMed
8. Pattani R, Wu PE, Dhalla IA. Resident duty hours in Canada: past, present and future. CMAJ. 2014;186(10):761-765. PubMed
9. Royal College of Physicians and Surgeons. Specialty Training Requirements in Internal medicine 2015. http://www.royalcollege.ca/cs/groups/public/documents/document/mdaw/mdg4/~edisp/088402.pdf. Accessed December 12, 2018.
10. Freeman TR, Petterson S, Finnegan S, Bazemore A. Shifting tides in the emigration patterns of Canadian physicians to the United States: a cross-sectional secondary data analysis. BMC Health Serv Res. 2016;16(1):678. PubMed
11. Wong BM, Imrie K. Why resident duty hours regulations must address attending physicians’ workload. Acad Med. 2013;88(9):1209-1211. PubMed
12. Flanders SA, Centor B, Weber V, McGinn T, DeSalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240-246. PubMed
13. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2012;7(3):190-194. PubMed
14. Ivers N, Brown AD, Detsky AS. Lessons from the Canadian experience with single-payer health insurance: just comfortable enough with the status quo. JAMA Intern Med. 2018;178(9):1250-1255. PubMed
15. Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: patient responses to a 30-day postdischarge questionnaire. J Hosp Med. 2016;11(2):99-104. PubMed
16. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. PubMed
17. Farnan JM, Burger A, Boonyasai RT, et al. Survey of overnight academic hospitalist supervision of trainees. J Hosp Med. 2012;7(7):521-523. PubMed
18. Gonzalo JD, Kuperman EF, Chuang CH, Lehman E, Glasser F, Abendroth T. Impact of an overnight internal medicine academic hospitalist program on patient outcomes. J Gen Intern Med. 2015;30(12):1795-1802. PubMed
19. Soong C, Fan E, Howell EE, et al. Characteristics of Hospitalists and Hospitalist Programs in the United States and Canada 2009. J Clin Outcomes Meas. 2009; 16 (2): 69-74.
20. Yousefi V, Maslowski R. Health system drivers of hospital medicine in Canada: systematic review. Can Fam Phys Med Fam Can. 2013;59(7):762-767. PubMed
21. Nuckols TK, Escarce JJ. Cost implications of ACGME’s 2011 changes to resident duty hours and the training environment. J Gen Intern Med. 2012;27(2):241-249. PubMed
22. Elliott DJ, Young RS, Brice J, Aguiar R, Kolm P. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786-793. PubMed
1. Schrewe B, Pratt DD, McKellin WH. Adapting the forms of yesterday to the functions of today and the needs of tomorrow: a genealogical case study of clinical teaching units in Canada. Adv Health Sci Educ Theory Pract. 2016;21(2):475-499. PubMed
2. Maudsley RF. The clinical teaching unit in transition. CMAJ. 1993;148(9):1564-1566. PubMed
3. Statistics Canada. Recent Changes in Demographic Trends in Canada. Ottawa: Ontario, 2015. https://www150.statcan.gc.ca/n1/pub/75-006-x/2015001/article/14240-eng.htm. Accessed December 9, 2018
4. Statistics Canada. Census, Age and Sex. Ottawa: Ontario, 2016. https://www12.statcan.gc.ca/census-recensement/2016/rt-td/as-eng.cfm. Accessed December 10, 2018.
5. Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024-1039. PubMed
6. van Walraven C. Trends in 1-year survival of people admitted to hospital in Ontario, 1994-2009. CMAJ. 2013;185(16):E755-E762. PubMed
7. Verma AA, Guo Y, Kwan JL, et al. Patient characteristics, resource use and outcomes associated with general internal medicine hospital care: the General Medicine Inpatient Initiative (GEMINI) retrospective cohort study. CMAJ Open. 2017;5(4):E842-E849. PubMed
8. Pattani R, Wu PE, Dhalla IA. Resident duty hours in Canada: past, present and future. CMAJ. 2014;186(10):761-765. PubMed
9. Royal College of Physicians and Surgeons. Specialty Training Requirements in Internal medicine 2015. http://www.royalcollege.ca/cs/groups/public/documents/document/mdaw/mdg4/~edisp/088402.pdf. Accessed December 12, 2018.
10. Freeman TR, Petterson S, Finnegan S, Bazemore A. Shifting tides in the emigration patterns of Canadian physicians to the United States: a cross-sectional secondary data analysis. BMC Health Serv Res. 2016;16(1):678. PubMed
11. Wong BM, Imrie K. Why resident duty hours regulations must address attending physicians’ workload. Acad Med. 2013;88(9):1209-1211. PubMed
12. Flanders SA, Centor B, Weber V, McGinn T, DeSalvo K, Auerbach A. Challenges and opportunities in academic hospital medicine: report from the Academic Hospital Medicine Summit. J Hosp Med. 2009;4(4):240-246. PubMed
13. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2012;7(3):190-194. PubMed
14. Ivers N, Brown AD, Detsky AS. Lessons from the Canadian experience with single-payer health insurance: just comfortable enough with the status quo. JAMA Intern Med. 2018;178(9):1250-1255. PubMed
15. Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: patient responses to a 30-day postdischarge questionnaire. J Hosp Med. 2016;11(2):99-104. PubMed
16. Auerbach AD, Wachter RM, Katz P, Showstack J, Baron RB, Goldman L. Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes. Ann Intern Med. 2002;137(11):859-865. PubMed
17. Farnan JM, Burger A, Boonyasai RT, et al. Survey of overnight academic hospitalist supervision of trainees. J Hosp Med. 2012;7(7):521-523. PubMed
18. Gonzalo JD, Kuperman EF, Chuang CH, Lehman E, Glasser F, Abendroth T. Impact of an overnight internal medicine academic hospitalist program on patient outcomes. J Gen Intern Med. 2015;30(12):1795-1802. PubMed
19. Soong C, Fan E, Howell EE, et al. Characteristics of Hospitalists and Hospitalist Programs in the United States and Canada 2009. J Clin Outcomes Meas. 2009; 16 (2): 69-74.
20. Yousefi V, Maslowski R. Health system drivers of hospital medicine in Canada: systematic review. Can Fam Phys Med Fam Can. 2013;59(7):762-767. PubMed
21. Nuckols TK, Escarce JJ. Cost implications of ACGME’s 2011 changes to resident duty hours and the training environment. J Gen Intern Med. 2012;27(2):241-249. PubMed
22. Elliott DJ, Young RS, Brice J, Aguiar R, Kolm P. Effect of hospitalist workload on the quality and efficiency of care. JAMA Intern Med. 2014;174(5):786-793. PubMed
© 2019 Society of Hospital Medicine
Hospital at Home and Emergence of the Home Hospitalist
Ms. P., an 86-year-old woman with a history of hypertension, hyperlipidemia, coronary artery disease, and transient ischemic attack, presents to the emergency department with a three-day history of cough, fever, purulent sputum, fatigue, and dyspnea on exertion. Her vital signs are notable for a fever of 39.0°C, blood pressure 136/92, pulse 102, respiratory rate 30, and room air oxygen saturation of 91%. She looks ill. She has a white blood cell count of 16,000, lactate 1.9, and a right lower lobe infiltrate on imaging. The emergency department attending physician presents the case to you for admission, and you accept the patient into your inpatient hospitalist service.
Now, let’s imagine a different future in which you are the attending hospitalist on your institution’s Hospital at Home (HaH) service, where you will provide hospital-level care to Ms. P. in the comfort of her own home. Hospitalists should prepare for this paradigm shift.
WHAT IS HOSPITAL AT HOME?
HaH provides hospital-level care in a patient’s home, for those with qualifying acute illnesses and appropriate degrees of acuity, as a substitute for traditional inpatient care.1 This is achieved by bringing the critical elements of hospital care to the home—physician and nursing care, intravenous medications and fluids, oxygen and respiratory therapies, basic radiography and ultrasound, durable medical equipment, skilled therapies, and more.2
All hospitalists have cared for patients like Ms. P., and she and many patients like her will have a straightforward hospital trajectory: initial evaluation in the emergency department, inpatient care provided by a hospitalist inpatient service, a few days of intravenous antibiotics and other hospital services, and finally, discharge to home.
A SHARED RATIONALE FOR HOSPITAL MEDICINE AND HOSPITAL AT HOME
However, not all patients will experience a smooth, or safe, hospital course. Studies that launched the hospital safety movement also provide the rationale for HaH, namely, that hospitals are often dangerous environments for patients.3
A complementary approach to improving outcomes for patients at high risk of iatrogenic illness such as functional decline, falls, delirium, adverse drug events, and hospital-associated disability syndrome,4-6 is to care for patients outside the traditional inpatient hospital environment. Over the past 20 years, many studies—including dozens of randomized controlled trials and several meta-analyses—have shown better outcomes for patients cared for in HaH: decreased length of stay, decreased incidence of adverse events (including substantially lower six-month mortality), better patient and caregiver care experiences, lower caregiver stress, and lower costs.7-9A recent Center for Medicare and Medicaid Innovation (CMMI) Demonstration conducted at the Mount Sinai Health System found similar results.10
GROWING INTEREST IN HOSPITAL AT HOME AND CHALLENGES TO DISSEMINATION
Interest in HaH has increased markedly over the past few years with increased penetration of Medicare and Medicaid managed care, the development and spread of accountable care organizations (ACOs), and a shift in focus among some health systems towards value-based care, population health, and community-based care. Recently, commercial entities have entered the HaH space and have raised substantial capital to fund development. Despite this growing interest in HaH and substantial evidence of its effectiveness, HaH has not been widely implemented or scaled in the United States.
Widespread dissemination and implementation of HaH has been hampered by several barriers. First, despite growing interest in HaH, the culture of healthcare and health system leadership, for the most part, remains focused on facility-based care.11
Second, while HaH makes financial sense in the managed care arena, given the strong evidence for high-quality, lower-cost care, there is currently no standard payment mechanism for HaH in fee-for-service Medicare or in the commercial insurance space. However, there are indications that this may soon change. In the fall of 2017, a proposal for a bundled payment mechanism for acute HaH care plus 30 days of postacute care was unanimously approved by an Advisory Committee to the Secretary of the Department of Health and Human Services (HHS).12,13 The HHS Secretary recently noted that “the Department of Health and Human Services is keenly interested in ideas for home-based, hospital-level care, and agrees … that this proposal holds promise for testing.”14
Third is the need to create the logistics and supply chain to support HaH. There currently exists a well-established supply chain for providing hospital care. A hospitalist orders a dose of intravenous antibiotic or oxygen, and it is supplied in a timely manner. Similarly, the postacute sector of healthcare has a robust supply chain, though it operates on a somewhat different clock from the acute care setting. However, there is currently no easily replicable supply chain to meet the needs of providing acute care in the home. Each HaH has had to create its own system of logistics with the existing healthcare assets in its local environment. Developing this capacity at scale will require significant capital investment.
There are examples where HaH has scaled. Beginning in 1994, in the state of Victoria, Australia (population 6.3 million), the health authority reimbursed HaH care at the same rates as traditional hospital care. At last report, HaH provided approximately 5% of all hospital bed days of care in Victoria. Providing HaH on this scale helped avoid the need to build a new 500-bed hospital to care for those patients.15 The avoided costs of building new hospital beds (and the ongoing need to fill those beds) represents significant societal return on investment attributable to HaH.
EMERGENCE OF THE HOME HOSPITALIST?
A key element in implementing a HaH program is its physician staff in terms of the types of doctors who provide HaH care, how they are organized, and how they interact with patients. To date, HaH physicians have been predominantly geriatricians, but internists and family medicine physicians, employed as full-time members of a dedicated HaH team, also provide care by physically visiting patients in their homes. The reason for significant involvement of geriatricians in HaH may relate to the fact that geriatric fellowship training includes training in home-based medical care, whereas this is less common in family medicine and internal medicine residency training programs.
In order to provide HaH on a nationwide scale, there will be a need for a larger workforce. There is an opportunity here to leverage existing hospital physician staff, such as hospitalists. In addition, while there is significant value in physicians seeing patients in their homes, more scalable versions of HaH are being developed and implemented that leverage biometrically enhanced telemedicine approaches for a dedicated physician component of care, with in-person visits provided by other members of an interdisciplinary team.
We believe that hospitalists can play a key role as HaH physicians as the HaH model continues to evolve and expand. Hospitalists bring valuable expertise relevant to HaH care delivery, including extensive experience with the triage of acutely ill patients, an understanding of the natural course of acute illness and team-based care, and for some, experience with telemedicine care.
While a hospitalist providing HaH care would leverage many of the competencies of the traditional hospitalist, we suggest that such a provider should receive additional training and clinical experience in home-based medical care to help them better understand the unique aspects of providing care in patients’ homes.16 Such training could include experience in making house calls, which can be a transformational experience in helping physicians improve their skills in dealing with social determinants of health, diagnosing and managing geriatric syndromes, and mobilizing community resources in the care of their patients, as well as managing care transitions. Hospitalists delivering care in HaH may also need to upgrade specific clinical skills commonly addressed by home-based medical care providers: wound care, caregiver-related issues, social and ethical issues specific to home-based care, problems with functional status, psychiatric and cognitive issues, management of gastrostomy tubes and bladder catheters, and dermatologic problems, as well as palliative care and end-of-life symptom management. These skills are slightly different from the usual realm of the typical hospitalists’ wheelhouse. However, it is all learnable.17 Similarly, geriatricians can learn from hospitalists as the HaH model evolves; there are HaH programs in existence today that take care of a sicker tranche of patients than earlier versions of HaH, with continuous telemonitoring of patients and the ability to rapidly deploy providers, labs, imaging, and medications. Going forward, as healthcare organizations begin to develop HaH programs staffed by hospitalists, it is probably wise for hospitalists and geriatricians to collaborate on the optimal physician models for HaH.
There may emerge a new specialty. Ticona and Schulman described a “home intensivist” with competencies including informatics of remote monitoring technology, leadership of multidisciplinary care teams, and the interpersonal skills required for compassionate end-of-life care.18 We prefer the term Home Hospitalist. Home Hospitalists would develop an enhanced understanding of the transitions of care and social determinants of health, and they would gain valuable knowledge about the social and environmental challenges many patients face after discharge from the hospital.
When this vision is realized, there will be enormous benefits to both HaH and Hospital Medicine. HaH could tap into a large and competent workforce to enhance its implementation and dissemination. Hospital Medicine would gain a new pathway for its providers and could develop new collaborative efforts with geriatric, internal, and family medicine.
Disclosures
Dr. Danielsson has nothing to disclose. Dr. Leff reports personal fees from Medically Home, other from Dispatch Health, other from Landmark Health, personal fees from Medibank, personal fees from Apple, personal fees from Health Affairs, other from Honor, personal fees from Institute for Healthcare Improvement, outside the submitted work; and American Academy of Home Care Medicine - member board of directors, voluntary.
Funding
Dr. Leff was supported in this work by a grant from The John A. Hartford Foundation.
1. Leff B, Montalto M. Home hospital-toward a tighter definition. J Am Geriatr Soc. 2004;52(12):2141. doi: 10.1111/j.1532-5415.2004.52579_1.x. PubMed
2. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143(11):798-808. doi: 10.7326/0003-4819-143-11-200512060-00008. PubMed
3. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi: 10.1056/NEJM199102073240604. PubMed
4. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. doi: 10.7326/0003-4819-118-3-199302010-00011. PubMed
5. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi: 10.1001/jama.2011.1556. PubMed
6. Wald HL. The Geometry of Patient Safety: Horizontal and Vertical Approaches to the Hazards of Hospitalization. J Am Geriatr Soc. 2017;65(12):2559-2561. doi: 10.1111/jgs.15049. PubMed
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi: 10.1503/cmaj.081491. PubMed
8. Caplan GA, Sulaiman NS, Mangin N, et al. A meta-analysis of “Hospital in the Home”. Med J Aust. 2012;197:512-519. doi: 10.5694/mja12.10480. PubMed
9. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491. doi: 10.1002/14651858.CD007491.pub2. PubMed
10. Federman AD, Soones T, DeCherrie LV, Leff B, Siu AL. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018;178(8):1033-1040. doi: 10.1001/jamainternmed.2018.2562. PubMed
11. Stein PD, Hull RD, Matta F, Willyerd GL. Modest response in translation to home management of deep venous thrombosis. Am J Med. 2010;123(12):1107-1113. doi: 10.1016/j.amjmed.2010.07.016. PubMed
12. Icahn School of Medicine at Mount Sinai. “HaH-Plus” (Hospital at Home Plus) Provider Focused Payment Model. https://aspe.hhs.gov/system/files/pdf/255906/HaHPlusProviderFocusedPaymentModel.pdf. Accessed November 11, 2018.
13. Physician-F ocused Payment Model Technical Advisory Committee. Report to the Secretary of Health and Human Services. Comments and Recommendation on “HaH-Plus (Hospital at Home Plus) Provider-Focused Payment Model. https://aspe.hhs.gov/system/files/pdf/255906/MtSinaiHAHReportSecretary.pdf. Accessed November 11, 2018.
14. The Secretary of Health and Human Services. Response to the Report to the Secretary of Health and Human Services. Comments and Recommendation on “HaH-Plus (Hospital at Home Plus) Provider-Focused Payment Model. https://downloads.cms.gov/files/cmmi/ptac-hhssecresponse-oct17-may18.pdf. Accessed November 11, 2018.
15. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health review of the Hospital in the Home program. Med J Aust. 2010;193(10);598-601. PubMed
16. Hayashi J, Leff B. Geriatric Home-Based Medical Care. New York, NY: Springer Publishers; 2015. PubMed
17. Reckrey JM, Ornstein KA, Wajnberg A, Kopke MV, DeCherrie LV. Teaching home-based primary care. Home Healthc Now. 2017;35(10):561-565. doi: 10.1097/NHH.0000000000000621. PubMed
18. Ticona L, Schulman KA. Extreme home makeover - the role of intensive home health care. N Engl J Med. 2016;375(18):1707-1709. doi: 10.1056/NEJMp1608301. PubMed
Ms. P., an 86-year-old woman with a history of hypertension, hyperlipidemia, coronary artery disease, and transient ischemic attack, presents to the emergency department with a three-day history of cough, fever, purulent sputum, fatigue, and dyspnea on exertion. Her vital signs are notable for a fever of 39.0°C, blood pressure 136/92, pulse 102, respiratory rate 30, and room air oxygen saturation of 91%. She looks ill. She has a white blood cell count of 16,000, lactate 1.9, and a right lower lobe infiltrate on imaging. The emergency department attending physician presents the case to you for admission, and you accept the patient into your inpatient hospitalist service.
Now, let’s imagine a different future in which you are the attending hospitalist on your institution’s Hospital at Home (HaH) service, where you will provide hospital-level care to Ms. P. in the comfort of her own home. Hospitalists should prepare for this paradigm shift.
WHAT IS HOSPITAL AT HOME?
HaH provides hospital-level care in a patient’s home, for those with qualifying acute illnesses and appropriate degrees of acuity, as a substitute for traditional inpatient care.1 This is achieved by bringing the critical elements of hospital care to the home—physician and nursing care, intravenous medications and fluids, oxygen and respiratory therapies, basic radiography and ultrasound, durable medical equipment, skilled therapies, and more.2
All hospitalists have cared for patients like Ms. P., and she and many patients like her will have a straightforward hospital trajectory: initial evaluation in the emergency department, inpatient care provided by a hospitalist inpatient service, a few days of intravenous antibiotics and other hospital services, and finally, discharge to home.
A SHARED RATIONALE FOR HOSPITAL MEDICINE AND HOSPITAL AT HOME
However, not all patients will experience a smooth, or safe, hospital course. Studies that launched the hospital safety movement also provide the rationale for HaH, namely, that hospitals are often dangerous environments for patients.3
A complementary approach to improving outcomes for patients at high risk of iatrogenic illness such as functional decline, falls, delirium, adverse drug events, and hospital-associated disability syndrome,4-6 is to care for patients outside the traditional inpatient hospital environment. Over the past 20 years, many studies—including dozens of randomized controlled trials and several meta-analyses—have shown better outcomes for patients cared for in HaH: decreased length of stay, decreased incidence of adverse events (including substantially lower six-month mortality), better patient and caregiver care experiences, lower caregiver stress, and lower costs.7-9A recent Center for Medicare and Medicaid Innovation (CMMI) Demonstration conducted at the Mount Sinai Health System found similar results.10
GROWING INTEREST IN HOSPITAL AT HOME AND CHALLENGES TO DISSEMINATION
Interest in HaH has increased markedly over the past few years with increased penetration of Medicare and Medicaid managed care, the development and spread of accountable care organizations (ACOs), and a shift in focus among some health systems towards value-based care, population health, and community-based care. Recently, commercial entities have entered the HaH space and have raised substantial capital to fund development. Despite this growing interest in HaH and substantial evidence of its effectiveness, HaH has not been widely implemented or scaled in the United States.
Widespread dissemination and implementation of HaH has been hampered by several barriers. First, despite growing interest in HaH, the culture of healthcare and health system leadership, for the most part, remains focused on facility-based care.11
Second, while HaH makes financial sense in the managed care arena, given the strong evidence for high-quality, lower-cost care, there is currently no standard payment mechanism for HaH in fee-for-service Medicare or in the commercial insurance space. However, there are indications that this may soon change. In the fall of 2017, a proposal for a bundled payment mechanism for acute HaH care plus 30 days of postacute care was unanimously approved by an Advisory Committee to the Secretary of the Department of Health and Human Services (HHS).12,13 The HHS Secretary recently noted that “the Department of Health and Human Services is keenly interested in ideas for home-based, hospital-level care, and agrees … that this proposal holds promise for testing.”14
Third is the need to create the logistics and supply chain to support HaH. There currently exists a well-established supply chain for providing hospital care. A hospitalist orders a dose of intravenous antibiotic or oxygen, and it is supplied in a timely manner. Similarly, the postacute sector of healthcare has a robust supply chain, though it operates on a somewhat different clock from the acute care setting. However, there is currently no easily replicable supply chain to meet the needs of providing acute care in the home. Each HaH has had to create its own system of logistics with the existing healthcare assets in its local environment. Developing this capacity at scale will require significant capital investment.
There are examples where HaH has scaled. Beginning in 1994, in the state of Victoria, Australia (population 6.3 million), the health authority reimbursed HaH care at the same rates as traditional hospital care. At last report, HaH provided approximately 5% of all hospital bed days of care in Victoria. Providing HaH on this scale helped avoid the need to build a new 500-bed hospital to care for those patients.15 The avoided costs of building new hospital beds (and the ongoing need to fill those beds) represents significant societal return on investment attributable to HaH.
EMERGENCE OF THE HOME HOSPITALIST?
A key element in implementing a HaH program is its physician staff in terms of the types of doctors who provide HaH care, how they are organized, and how they interact with patients. To date, HaH physicians have been predominantly geriatricians, but internists and family medicine physicians, employed as full-time members of a dedicated HaH team, also provide care by physically visiting patients in their homes. The reason for significant involvement of geriatricians in HaH may relate to the fact that geriatric fellowship training includes training in home-based medical care, whereas this is less common in family medicine and internal medicine residency training programs.
In order to provide HaH on a nationwide scale, there will be a need for a larger workforce. There is an opportunity here to leverage existing hospital physician staff, such as hospitalists. In addition, while there is significant value in physicians seeing patients in their homes, more scalable versions of HaH are being developed and implemented that leverage biometrically enhanced telemedicine approaches for a dedicated physician component of care, with in-person visits provided by other members of an interdisciplinary team.
We believe that hospitalists can play a key role as HaH physicians as the HaH model continues to evolve and expand. Hospitalists bring valuable expertise relevant to HaH care delivery, including extensive experience with the triage of acutely ill patients, an understanding of the natural course of acute illness and team-based care, and for some, experience with telemedicine care.
While a hospitalist providing HaH care would leverage many of the competencies of the traditional hospitalist, we suggest that such a provider should receive additional training and clinical experience in home-based medical care to help them better understand the unique aspects of providing care in patients’ homes.16 Such training could include experience in making house calls, which can be a transformational experience in helping physicians improve their skills in dealing with social determinants of health, diagnosing and managing geriatric syndromes, and mobilizing community resources in the care of their patients, as well as managing care transitions. Hospitalists delivering care in HaH may also need to upgrade specific clinical skills commonly addressed by home-based medical care providers: wound care, caregiver-related issues, social and ethical issues specific to home-based care, problems with functional status, psychiatric and cognitive issues, management of gastrostomy tubes and bladder catheters, and dermatologic problems, as well as palliative care and end-of-life symptom management. These skills are slightly different from the usual realm of the typical hospitalists’ wheelhouse. However, it is all learnable.17 Similarly, geriatricians can learn from hospitalists as the HaH model evolves; there are HaH programs in existence today that take care of a sicker tranche of patients than earlier versions of HaH, with continuous telemonitoring of patients and the ability to rapidly deploy providers, labs, imaging, and medications. Going forward, as healthcare organizations begin to develop HaH programs staffed by hospitalists, it is probably wise for hospitalists and geriatricians to collaborate on the optimal physician models for HaH.
There may emerge a new specialty. Ticona and Schulman described a “home intensivist” with competencies including informatics of remote monitoring technology, leadership of multidisciplinary care teams, and the interpersonal skills required for compassionate end-of-life care.18 We prefer the term Home Hospitalist. Home Hospitalists would develop an enhanced understanding of the transitions of care and social determinants of health, and they would gain valuable knowledge about the social and environmental challenges many patients face after discharge from the hospital.
When this vision is realized, there will be enormous benefits to both HaH and Hospital Medicine. HaH could tap into a large and competent workforce to enhance its implementation and dissemination. Hospital Medicine would gain a new pathway for its providers and could develop new collaborative efforts with geriatric, internal, and family medicine.
Disclosures
Dr. Danielsson has nothing to disclose. Dr. Leff reports personal fees from Medically Home, other from Dispatch Health, other from Landmark Health, personal fees from Medibank, personal fees from Apple, personal fees from Health Affairs, other from Honor, personal fees from Institute for Healthcare Improvement, outside the submitted work; and American Academy of Home Care Medicine - member board of directors, voluntary.
Funding
Dr. Leff was supported in this work by a grant from The John A. Hartford Foundation.
Ms. P., an 86-year-old woman with a history of hypertension, hyperlipidemia, coronary artery disease, and transient ischemic attack, presents to the emergency department with a three-day history of cough, fever, purulent sputum, fatigue, and dyspnea on exertion. Her vital signs are notable for a fever of 39.0°C, blood pressure 136/92, pulse 102, respiratory rate 30, and room air oxygen saturation of 91%. She looks ill. She has a white blood cell count of 16,000, lactate 1.9, and a right lower lobe infiltrate on imaging. The emergency department attending physician presents the case to you for admission, and you accept the patient into your inpatient hospitalist service.
Now, let’s imagine a different future in which you are the attending hospitalist on your institution’s Hospital at Home (HaH) service, where you will provide hospital-level care to Ms. P. in the comfort of her own home. Hospitalists should prepare for this paradigm shift.
WHAT IS HOSPITAL AT HOME?
HaH provides hospital-level care in a patient’s home, for those with qualifying acute illnesses and appropriate degrees of acuity, as a substitute for traditional inpatient care.1 This is achieved by bringing the critical elements of hospital care to the home—physician and nursing care, intravenous medications and fluids, oxygen and respiratory therapies, basic radiography and ultrasound, durable medical equipment, skilled therapies, and more.2
All hospitalists have cared for patients like Ms. P., and she and many patients like her will have a straightforward hospital trajectory: initial evaluation in the emergency department, inpatient care provided by a hospitalist inpatient service, a few days of intravenous antibiotics and other hospital services, and finally, discharge to home.
A SHARED RATIONALE FOR HOSPITAL MEDICINE AND HOSPITAL AT HOME
However, not all patients will experience a smooth, or safe, hospital course. Studies that launched the hospital safety movement also provide the rationale for HaH, namely, that hospitals are often dangerous environments for patients.3
A complementary approach to improving outcomes for patients at high risk of iatrogenic illness such as functional decline, falls, delirium, adverse drug events, and hospital-associated disability syndrome,4-6 is to care for patients outside the traditional inpatient hospital environment. Over the past 20 years, many studies—including dozens of randomized controlled trials and several meta-analyses—have shown better outcomes for patients cared for in HaH: decreased length of stay, decreased incidence of adverse events (including substantially lower six-month mortality), better patient and caregiver care experiences, lower caregiver stress, and lower costs.7-9A recent Center for Medicare and Medicaid Innovation (CMMI) Demonstration conducted at the Mount Sinai Health System found similar results.10
GROWING INTEREST IN HOSPITAL AT HOME AND CHALLENGES TO DISSEMINATION
Interest in HaH has increased markedly over the past few years with increased penetration of Medicare and Medicaid managed care, the development and spread of accountable care organizations (ACOs), and a shift in focus among some health systems towards value-based care, population health, and community-based care. Recently, commercial entities have entered the HaH space and have raised substantial capital to fund development. Despite this growing interest in HaH and substantial evidence of its effectiveness, HaH has not been widely implemented or scaled in the United States.
Widespread dissemination and implementation of HaH has been hampered by several barriers. First, despite growing interest in HaH, the culture of healthcare and health system leadership, for the most part, remains focused on facility-based care.11
Second, while HaH makes financial sense in the managed care arena, given the strong evidence for high-quality, lower-cost care, there is currently no standard payment mechanism for HaH in fee-for-service Medicare or in the commercial insurance space. However, there are indications that this may soon change. In the fall of 2017, a proposal for a bundled payment mechanism for acute HaH care plus 30 days of postacute care was unanimously approved by an Advisory Committee to the Secretary of the Department of Health and Human Services (HHS).12,13 The HHS Secretary recently noted that “the Department of Health and Human Services is keenly interested in ideas for home-based, hospital-level care, and agrees … that this proposal holds promise for testing.”14
Third is the need to create the logistics and supply chain to support HaH. There currently exists a well-established supply chain for providing hospital care. A hospitalist orders a dose of intravenous antibiotic or oxygen, and it is supplied in a timely manner. Similarly, the postacute sector of healthcare has a robust supply chain, though it operates on a somewhat different clock from the acute care setting. However, there is currently no easily replicable supply chain to meet the needs of providing acute care in the home. Each HaH has had to create its own system of logistics with the existing healthcare assets in its local environment. Developing this capacity at scale will require significant capital investment.
There are examples where HaH has scaled. Beginning in 1994, in the state of Victoria, Australia (population 6.3 million), the health authority reimbursed HaH care at the same rates as traditional hospital care. At last report, HaH provided approximately 5% of all hospital bed days of care in Victoria. Providing HaH on this scale helped avoid the need to build a new 500-bed hospital to care for those patients.15 The avoided costs of building new hospital beds (and the ongoing need to fill those beds) represents significant societal return on investment attributable to HaH.
EMERGENCE OF THE HOME HOSPITALIST?
A key element in implementing a HaH program is its physician staff in terms of the types of doctors who provide HaH care, how they are organized, and how they interact with patients. To date, HaH physicians have been predominantly geriatricians, but internists and family medicine physicians, employed as full-time members of a dedicated HaH team, also provide care by physically visiting patients in their homes. The reason for significant involvement of geriatricians in HaH may relate to the fact that geriatric fellowship training includes training in home-based medical care, whereas this is less common in family medicine and internal medicine residency training programs.
In order to provide HaH on a nationwide scale, there will be a need for a larger workforce. There is an opportunity here to leverage existing hospital physician staff, such as hospitalists. In addition, while there is significant value in physicians seeing patients in their homes, more scalable versions of HaH are being developed and implemented that leverage biometrically enhanced telemedicine approaches for a dedicated physician component of care, with in-person visits provided by other members of an interdisciplinary team.
We believe that hospitalists can play a key role as HaH physicians as the HaH model continues to evolve and expand. Hospitalists bring valuable expertise relevant to HaH care delivery, including extensive experience with the triage of acutely ill patients, an understanding of the natural course of acute illness and team-based care, and for some, experience with telemedicine care.
While a hospitalist providing HaH care would leverage many of the competencies of the traditional hospitalist, we suggest that such a provider should receive additional training and clinical experience in home-based medical care to help them better understand the unique aspects of providing care in patients’ homes.16 Such training could include experience in making house calls, which can be a transformational experience in helping physicians improve their skills in dealing with social determinants of health, diagnosing and managing geriatric syndromes, and mobilizing community resources in the care of their patients, as well as managing care transitions. Hospitalists delivering care in HaH may also need to upgrade specific clinical skills commonly addressed by home-based medical care providers: wound care, caregiver-related issues, social and ethical issues specific to home-based care, problems with functional status, psychiatric and cognitive issues, management of gastrostomy tubes and bladder catheters, and dermatologic problems, as well as palliative care and end-of-life symptom management. These skills are slightly different from the usual realm of the typical hospitalists’ wheelhouse. However, it is all learnable.17 Similarly, geriatricians can learn from hospitalists as the HaH model evolves; there are HaH programs in existence today that take care of a sicker tranche of patients than earlier versions of HaH, with continuous telemonitoring of patients and the ability to rapidly deploy providers, labs, imaging, and medications. Going forward, as healthcare organizations begin to develop HaH programs staffed by hospitalists, it is probably wise for hospitalists and geriatricians to collaborate on the optimal physician models for HaH.
There may emerge a new specialty. Ticona and Schulman described a “home intensivist” with competencies including informatics of remote monitoring technology, leadership of multidisciplinary care teams, and the interpersonal skills required for compassionate end-of-life care.18 We prefer the term Home Hospitalist. Home Hospitalists would develop an enhanced understanding of the transitions of care and social determinants of health, and they would gain valuable knowledge about the social and environmental challenges many patients face after discharge from the hospital.
When this vision is realized, there will be enormous benefits to both HaH and Hospital Medicine. HaH could tap into a large and competent workforce to enhance its implementation and dissemination. Hospital Medicine would gain a new pathway for its providers and could develop new collaborative efforts with geriatric, internal, and family medicine.
Disclosures
Dr. Danielsson has nothing to disclose. Dr. Leff reports personal fees from Medically Home, other from Dispatch Health, other from Landmark Health, personal fees from Medibank, personal fees from Apple, personal fees from Health Affairs, other from Honor, personal fees from Institute for Healthcare Improvement, outside the submitted work; and American Academy of Home Care Medicine - member board of directors, voluntary.
Funding
Dr. Leff was supported in this work by a grant from The John A. Hartford Foundation.
1. Leff B, Montalto M. Home hospital-toward a tighter definition. J Am Geriatr Soc. 2004;52(12):2141. doi: 10.1111/j.1532-5415.2004.52579_1.x. PubMed
2. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143(11):798-808. doi: 10.7326/0003-4819-143-11-200512060-00008. PubMed
3. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi: 10.1056/NEJM199102073240604. PubMed
4. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. doi: 10.7326/0003-4819-118-3-199302010-00011. PubMed
5. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi: 10.1001/jama.2011.1556. PubMed
6. Wald HL. The Geometry of Patient Safety: Horizontal and Vertical Approaches to the Hazards of Hospitalization. J Am Geriatr Soc. 2017;65(12):2559-2561. doi: 10.1111/jgs.15049. PubMed
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi: 10.1503/cmaj.081491. PubMed
8. Caplan GA, Sulaiman NS, Mangin N, et al. A meta-analysis of “Hospital in the Home”. Med J Aust. 2012;197:512-519. doi: 10.5694/mja12.10480. PubMed
9. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491. doi: 10.1002/14651858.CD007491.pub2. PubMed
10. Federman AD, Soones T, DeCherrie LV, Leff B, Siu AL. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018;178(8):1033-1040. doi: 10.1001/jamainternmed.2018.2562. PubMed
11. Stein PD, Hull RD, Matta F, Willyerd GL. Modest response in translation to home management of deep venous thrombosis. Am J Med. 2010;123(12):1107-1113. doi: 10.1016/j.amjmed.2010.07.016. PubMed
12. Icahn School of Medicine at Mount Sinai. “HaH-Plus” (Hospital at Home Plus) Provider Focused Payment Model. https://aspe.hhs.gov/system/files/pdf/255906/HaHPlusProviderFocusedPaymentModel.pdf. Accessed November 11, 2018.
13. Physician-F ocused Payment Model Technical Advisory Committee. Report to the Secretary of Health and Human Services. Comments and Recommendation on “HaH-Plus (Hospital at Home Plus) Provider-Focused Payment Model. https://aspe.hhs.gov/system/files/pdf/255906/MtSinaiHAHReportSecretary.pdf. Accessed November 11, 2018.
14. The Secretary of Health and Human Services. Response to the Report to the Secretary of Health and Human Services. Comments and Recommendation on “HaH-Plus (Hospital at Home Plus) Provider-Focused Payment Model. https://downloads.cms.gov/files/cmmi/ptac-hhssecresponse-oct17-may18.pdf. Accessed November 11, 2018.
15. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health review of the Hospital in the Home program. Med J Aust. 2010;193(10);598-601. PubMed
16. Hayashi J, Leff B. Geriatric Home-Based Medical Care. New York, NY: Springer Publishers; 2015. PubMed
17. Reckrey JM, Ornstein KA, Wajnberg A, Kopke MV, DeCherrie LV. Teaching home-based primary care. Home Healthc Now. 2017;35(10):561-565. doi: 10.1097/NHH.0000000000000621. PubMed
18. Ticona L, Schulman KA. Extreme home makeover - the role of intensive home health care. N Engl J Med. 2016;375(18):1707-1709. doi: 10.1056/NEJMp1608301. PubMed
1. Leff B, Montalto M. Home hospital-toward a tighter definition. J Am Geriatr Soc. 2004;52(12):2141. doi: 10.1111/j.1532-5415.2004.52579_1.x. PubMed
2. Leff B, Burton L, Mader SL, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143(11):798-808. doi: 10.7326/0003-4819-143-11-200512060-00008. PubMed
3. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370-376. doi: 10.1056/NEJM199102073240604. PubMed
4. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. doi: 10.7326/0003-4819-118-3-199302010-00011. PubMed
5. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi: 10.1001/jama.2011.1556. PubMed
6. Wald HL. The Geometry of Patient Safety: Horizontal and Vertical Approaches to the Hazards of Hospitalization. J Am Geriatr Soc. 2017;65(12):2559-2561. doi: 10.1111/jgs.15049. PubMed
7. Shepperd S, Doll H, Angus RM, et al. Avoiding hospital admission through provision of hospital care at home: a systematic review and meta-analysis of individual patient data. CMAJ. 2009;180(2):175-182. doi: 10.1503/cmaj.081491. PubMed
8. Caplan GA, Sulaiman NS, Mangin N, et al. A meta-analysis of “Hospital in the Home”. Med J Aust. 2012;197:512-519. doi: 10.5694/mja12.10480. PubMed
9. Shepperd S, Iliffe S, Doll HA, et al. Admission avoidance hospital at home. Cochrane Database Syst Rev. 2016;9:CD007491. doi: 10.1002/14651858.CD007491.pub2. PubMed
10. Federman AD, Soones T, DeCherrie LV, Leff B, Siu AL. Association of a bundled hospital-at-home and 30-day postacute transitional care program with clinical outcomes and patient experiences. JAMA Intern Med. 2018;178(8):1033-1040. doi: 10.1001/jamainternmed.2018.2562. PubMed
11. Stein PD, Hull RD, Matta F, Willyerd GL. Modest response in translation to home management of deep venous thrombosis. Am J Med. 2010;123(12):1107-1113. doi: 10.1016/j.amjmed.2010.07.016. PubMed
12. Icahn School of Medicine at Mount Sinai. “HaH-Plus” (Hospital at Home Plus) Provider Focused Payment Model. https://aspe.hhs.gov/system/files/pdf/255906/HaHPlusProviderFocusedPaymentModel.pdf. Accessed November 11, 2018.
13. Physician-F ocused Payment Model Technical Advisory Committee. Report to the Secretary of Health and Human Services. Comments and Recommendation on “HaH-Plus (Hospital at Home Plus) Provider-Focused Payment Model. https://aspe.hhs.gov/system/files/pdf/255906/MtSinaiHAHReportSecretary.pdf. Accessed November 11, 2018.
14. The Secretary of Health and Human Services. Response to the Report to the Secretary of Health and Human Services. Comments and Recommendation on “HaH-Plus (Hospital at Home Plus) Provider-Focused Payment Model. https://downloads.cms.gov/files/cmmi/ptac-hhssecresponse-oct17-may18.pdf. Accessed November 11, 2018.
15. Montalto M. The 500-bed hospital that isn’t there: the Victorian Department of Health review of the Hospital in the Home program. Med J Aust. 2010;193(10);598-601. PubMed
16. Hayashi J, Leff B. Geriatric Home-Based Medical Care. New York, NY: Springer Publishers; 2015. PubMed
17. Reckrey JM, Ornstein KA, Wajnberg A, Kopke MV, DeCherrie LV. Teaching home-based primary care. Home Healthc Now. 2017;35(10):561-565. doi: 10.1097/NHH.0000000000000621. PubMed
18. Ticona L, Schulman KA. Extreme home makeover - the role of intensive home health care. N Engl J Med. 2016;375(18):1707-1709. doi: 10.1056/NEJMp1608301. PubMed
© 2019 Society of Hospital Medicine
The Complex Problem of Women Trainees in Academic Medicine
Despite media attention to gender inequality in multiple professions, medicine has only recently begun to identify disparities facing women in academic medicine, focusing primarily on women faculty rather than trainees. Because of the unique and poorly understood juxtaposition of forces affecting their experience, focusing on women medical trainees may provide a representative framework to understand the larger, complex problem of gender equity in medicine. Rather than being a complicated problem with component parts that can be separately addressed, gender equity in medicine is a complex problem—one composed of a myriad of interrelated human and systemic factors. Such a complex problem demands innovative, open-minded, user-centered interventions. Here, we outline some of the factors unique to women trainees, including lack of female role models in leadership, gender bias, sexual harassment, work-life imbalance, and few formal leadership training programs. We propose one potential strategy, leadership programs specifically targeted to women residents and fellows. We recently implemented this strategy at our institution in the form of a day-long symposium of skill-building sessions for women residents and fellows.
Although women have achieved equal representation in several medical training programs, there is still a dearth of women in high-profile leadership positions within academic medicine. Although women comprised 46% of United States medical school applicants and residents in 2015-2016, underrepresentation persists at the level of associate professor (35% women), full professor (22%), department chair (14%), and dean (16%).1 Many potential women leaders may not self-identify as such due to the limited exposure to women role models in positions of power and may in fact be ready for leadership roles earlier but not apply until later in their careers as compared with men.2,3 The lack of role models with a shared background is an even more severe problem for women of color and all of these factors contribute to the “leaky pipeline” phenomenon.4 We aimed to address this mindset and help women see themselves as leaders by overcoming “second-generation gender bias” through our work.2
Due to the intense and inflexible nature of residency and fellowship training programs, many women choose to defer milestones such as childbearing.5 Women trainees are more likely than their male colleagues to avoid having a child during residency due to perceived threat to their career and negative perceptions of colleagues.5,6 Women who are in a domestic partnership often bear the brunt of the household work regardless of the careers of the two partners, a phenomenon termed the “second shift.”7 This work-life imbalance has been shown to correlate with depressive symptoms in women internal medicine trainees.8
Recently, a trainee published on the experience of medical residents being asked whether they were ever called “nurse.” All the women in the room put up their hands; none of their male colleagues did.9 At issue is not the relative importance of the professions of medicine and nursing, but rather the gender stereotypes in medicine that lead to automatic categorization of women into one group. Although the majority of women residents likely have had personal experiences with bias and microaggressions, few are explicitly taught the tools to address these. Beyond microaggressions, women trainees are also subject to more sexual harassment than their male colleagues.10 In addition, women living at the intersections of race, ethnicity, and gender are faced with even higher rates of harassment.11 Reporting sexual assault and harassment can be particularly difficult as a trainee because of the risk of retaliation, fear of poor evaluations from superiors, and lack of confidence in the reporting process.10
Finally, women trainees often receive little training about the skills required for career advancement to achieve parity with their male colleagues. Women are less likely to negotiate due to concerns about backlash or due to general lack of awareness about the importance of negotiation.12 Women are asked to volunteer for “nonpromotable” tasks more often than men by colleagues of both sexes, a barrier to women reaching their full career potential and a difficult workplace scenario to navigate.13 Unlike the fields of business, law, and technology, for example, women in medicine do not routinely have training courses that incorporate skills such as navigating difficult conversations, conflict resolution, curriculum vitae writing, and negotiation. Various solutions have been offered to address some of the barriers facing women in medicine (such as the Association of American Medical Colleges and Executive Leadership in Academic Medicine leadership courses), but typically these focus on faculty rather than trainees. Given that women physicians practicing in the inpatient setting have been shown to have better patient outcomes14 and organizations with female leadership outperform those led by men,15 equipping our women trainees to thrive in the clinical and leadership environments is an essential step in fulfilling our mission as high-quality training programs.
At our institution, we recognized the need for training in leadership skills for women medical trainees and designed a day-long symposium for internal medicine women residents and fellows. Before developing the curriculum, we conducted a needs assessment to ascertain which skills women wanted to develop; women overwhelmingly wanted to learn about public speaking skills, work-life integration, and mentoring. Based on these responses, a group spanning multiple levels of training (residency, fellowship, and faculty) designed a combination of large-group lectures and small-group workshops termed “Women in Leadership Development” (WILD). The day-long curriculum included sessions on public speaking skills, women as change agents, mentorship, conflict resolution, and addressing microaggressions and concluded with a networking event for women faculty and trainees (Table).
In total, 77 medicine residents and fellows voluntarily participated in the symposium in 2017 and 2018. The public speaking skills session received the highest reviews, with 98% of participants reporting that they identified ways to change public speaking styles to project confidence. This session was facilitated by an outside consultant in public speaking, highlighting the benefit of seeking experts outside of academic medicine. Another novel session focused on responding to microaggressions, defined as subtle and sometimes unintentional actions that express prejudice toward marginalized groups, in the clinical and academic environments. Microaggressions can undermine the recipient’s confidence, feeling of belonging, and effectiveness at work.16 At our institution, trainees in graduate medical education report the largest single source of microaggressions as patients (greater than attendings, fellow trainees, or staff), with gender bias being responsible for the greatest number of microaggressions (Schaeffer, unpublished data). Navigating these situations to ensure good patient care and strong patient-provider relationships, while also establishing a climate of mutual respect, can be challenging for all women physicians, in particular for trainees who are just beginning to experience the clinical environment independently. Our session on microaggressions was purposefully led by a national expert in patient-provider communication and offered an opportunity for women trainees to reflect on their past experiences being the target of microaggressions, to name them as such, and then to brainstorm possible responses as a group. The result was a “toolkit” of resources for responding to microaggressions.17
Of the attendees of WILD 2017 and 2018, 91% strongly agreed that participation in the symposium was a useful experience. One attendee reflected that they “feel more empowered to discuss women-related issues in academics with peers, mentors, mentees” and another stated that as a result of WILD, they would “sponsor peers and mentors, speak out more about gender bias, seek out leadership positions.” Challenges for our symposium included obtaining protected curricular time from busy trainee schedules. Supportive leadership at all levels was critical to our success; carving out dedicated curricular time will be essential for the sustainability of this leadership symposium. Our group has recently received funding to expand to a longitudinal course open to all women residents and fellows across graduate medical education.
Although the complex and unique problems facing women medical trainees are unlikely to be comprehensively addressed by a leadership course, we urge other institutions to adopt and expand on our model for teaching vital leadership skills. In addition to leadership skills, academic medical centers should adopt a multipronged approach to support their female trainees, including clear and confidential reporting practices of sexual harassment without fear of retaliation, training for all staff on harassment and bias, involvement of men as allies, and mentorship programs for women trainees. Further research is needed to better understand this complex problem, its impact on career outcomes, and a path to achieving gender equality in medicine.
Acknowledgments
The authors are indebted to Catherine Lucey, MD, for her framing of the issues for women in medicine as a complex problem and to Sarah Schaeffer, MD, for her unpublished data on microaggressions at our institution. The authors are also grateful to the UCSF Department of Medicine and the UCSF Chancellor’s Advisory Committee on the Status of Women for their financial support of the WILD (Women In Leadership Development) program.
Disclosures
The authors declare no conflict of interest.
Funding
The authors report no external funding source for this study.
1. AAMC [website]. 2018. https://www.aamc.org/. Accessed May 5, 2018.
2. Ibarra H, Ely, Robin J, Kolb D. Women rising: the unseen barriers. Harvard Bus Rev. 2013;91(9):60-66.
3. Stevenson EJ, Orr E. We interviewed 57 female CEOs to find out how more women can get to the top. Harvard Bus Rev. 2017.
4. Mahoney MR, Wilson E, Odom KL, Flowers L, Adler SR. Minority faculty voices on diversity in academic medicine: perspectives from one school. Acad Med. 2008;83(8):781-786. doi: 10.1097/ACM.0b013e31817ec002. PubMed
5. Turner PL, Lumpkins K, Gabre J, Lin MJ, Liu X, Terrin M. Pregnancy among women surgeons: trends over time. Arch Surg. 2012;147(5):474-479. doi: 10.1001/archsurg.2011.1693. PubMed
6. Willett LL, Wellons MF, Hartig JR, et al. Do women residents delay childbearing due to perceived career threats? Acad Med. 2010;85(4):640-646. doi: 10.1097/ACM.0b013e3181d2cb5b. PubMed
7. Jolly S, Griffith KA, DeCastro R, Stewart A, Ubel P, Jagsi R. Gender differences in time spent on parenting and domestic responsibilities by high-achieving young physician-researchers. Ann Intern Med. 2014;160(5):344-353. doi: 10.7326/M13-0974. PubMed
8. Guille C, Frank E, Zhao Z, et al. Work-family conflict and the sex difference in depression among training physicians. JAMA Intern Med. 2017;177(12):1766-1772. doi: 10.1001/jamainternmed.2017.5138. PubMed
9. DeFilippis EM. Putting the “She” in doctor. JAMA Intern Med. 2018;178(3):323-324. doi: 10.1001/jamainternmed.2017.8362. PubMed
10. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. doi: 10.1056/NEJM199302043280507. PubMed
11. Corbie-Smith G, Frank E, Nickens HW, Elon L. Prevalences and correlates of ethnic harassment in the U.S. Women Physicians’ Health Study. Acad Med. 1999;74(6):695-701. doi: 10.1097/00001888-199906000-00018. PubMed
12. Amanatullah ET, Morris MW. Negotiating gender roles: gender differences in assertive negotiating are mediated by women’s fear of backlash and attenuated when negotiating on behalf of others. J Pers Soc Psychol. 2010;98(2):256-267. doi: 10.1037/a0017094. PubMed
13. Babcock L, Maria PR, Vesterlund L. Why women volunteer for tasks that don’t lead to promotions. Harvard Bus Rev. 2018.
14. Tsugawa Y, Jena AB, Figueroa JF, Orav EJ, Blumenthal DM, Jha AK. Comparison of hospital mortality and readmission rates for medicare patients treated by male vs female physicians. JAMA Intern Med. 2017;177(2):206-213. doi: 10.1001/jamainternmed.2016.7875. PubMed
15. Landel M. Why gender balance can’t wait. Harvard Bus Rev. 2016.
16. Wolf TM, Randall HM, von Almen K, Tynes LL. Perceived mistreatment and attitude change by graduating medical students: a retrospective study. Med Educ. 1991;25(3):182-190. doi: 10.1111/j.1365-2923.1991.tb00050.x. PubMed
17. Wheeler DJ, Zapata J, Davis D, Chou C. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2018:1-6. doi: 10.1080/0142159X.2018.1506097. PubMed
Despite media attention to gender inequality in multiple professions, medicine has only recently begun to identify disparities facing women in academic medicine, focusing primarily on women faculty rather than trainees. Because of the unique and poorly understood juxtaposition of forces affecting their experience, focusing on women medical trainees may provide a representative framework to understand the larger, complex problem of gender equity in medicine. Rather than being a complicated problem with component parts that can be separately addressed, gender equity in medicine is a complex problem—one composed of a myriad of interrelated human and systemic factors. Such a complex problem demands innovative, open-minded, user-centered interventions. Here, we outline some of the factors unique to women trainees, including lack of female role models in leadership, gender bias, sexual harassment, work-life imbalance, and few formal leadership training programs. We propose one potential strategy, leadership programs specifically targeted to women residents and fellows. We recently implemented this strategy at our institution in the form of a day-long symposium of skill-building sessions for women residents and fellows.
Although women have achieved equal representation in several medical training programs, there is still a dearth of women in high-profile leadership positions within academic medicine. Although women comprised 46% of United States medical school applicants and residents in 2015-2016, underrepresentation persists at the level of associate professor (35% women), full professor (22%), department chair (14%), and dean (16%).1 Many potential women leaders may not self-identify as such due to the limited exposure to women role models in positions of power and may in fact be ready for leadership roles earlier but not apply until later in their careers as compared with men.2,3 The lack of role models with a shared background is an even more severe problem for women of color and all of these factors contribute to the “leaky pipeline” phenomenon.4 We aimed to address this mindset and help women see themselves as leaders by overcoming “second-generation gender bias” through our work.2
Due to the intense and inflexible nature of residency and fellowship training programs, many women choose to defer milestones such as childbearing.5 Women trainees are more likely than their male colleagues to avoid having a child during residency due to perceived threat to their career and negative perceptions of colleagues.5,6 Women who are in a domestic partnership often bear the brunt of the household work regardless of the careers of the two partners, a phenomenon termed the “second shift.”7 This work-life imbalance has been shown to correlate with depressive symptoms in women internal medicine trainees.8
Recently, a trainee published on the experience of medical residents being asked whether they were ever called “nurse.” All the women in the room put up their hands; none of their male colleagues did.9 At issue is not the relative importance of the professions of medicine and nursing, but rather the gender stereotypes in medicine that lead to automatic categorization of women into one group. Although the majority of women residents likely have had personal experiences with bias and microaggressions, few are explicitly taught the tools to address these. Beyond microaggressions, women trainees are also subject to more sexual harassment than their male colleagues.10 In addition, women living at the intersections of race, ethnicity, and gender are faced with even higher rates of harassment.11 Reporting sexual assault and harassment can be particularly difficult as a trainee because of the risk of retaliation, fear of poor evaluations from superiors, and lack of confidence in the reporting process.10
Finally, women trainees often receive little training about the skills required for career advancement to achieve parity with their male colleagues. Women are less likely to negotiate due to concerns about backlash or due to general lack of awareness about the importance of negotiation.12 Women are asked to volunteer for “nonpromotable” tasks more often than men by colleagues of both sexes, a barrier to women reaching their full career potential and a difficult workplace scenario to navigate.13 Unlike the fields of business, law, and technology, for example, women in medicine do not routinely have training courses that incorporate skills such as navigating difficult conversations, conflict resolution, curriculum vitae writing, and negotiation. Various solutions have been offered to address some of the barriers facing women in medicine (such as the Association of American Medical Colleges and Executive Leadership in Academic Medicine leadership courses), but typically these focus on faculty rather than trainees. Given that women physicians practicing in the inpatient setting have been shown to have better patient outcomes14 and organizations with female leadership outperform those led by men,15 equipping our women trainees to thrive in the clinical and leadership environments is an essential step in fulfilling our mission as high-quality training programs.
At our institution, we recognized the need for training in leadership skills for women medical trainees and designed a day-long symposium for internal medicine women residents and fellows. Before developing the curriculum, we conducted a needs assessment to ascertain which skills women wanted to develop; women overwhelmingly wanted to learn about public speaking skills, work-life integration, and mentoring. Based on these responses, a group spanning multiple levels of training (residency, fellowship, and faculty) designed a combination of large-group lectures and small-group workshops termed “Women in Leadership Development” (WILD). The day-long curriculum included sessions on public speaking skills, women as change agents, mentorship, conflict resolution, and addressing microaggressions and concluded with a networking event for women faculty and trainees (Table).
In total, 77 medicine residents and fellows voluntarily participated in the symposium in 2017 and 2018. The public speaking skills session received the highest reviews, with 98% of participants reporting that they identified ways to change public speaking styles to project confidence. This session was facilitated by an outside consultant in public speaking, highlighting the benefit of seeking experts outside of academic medicine. Another novel session focused on responding to microaggressions, defined as subtle and sometimes unintentional actions that express prejudice toward marginalized groups, in the clinical and academic environments. Microaggressions can undermine the recipient’s confidence, feeling of belonging, and effectiveness at work.16 At our institution, trainees in graduate medical education report the largest single source of microaggressions as patients (greater than attendings, fellow trainees, or staff), with gender bias being responsible for the greatest number of microaggressions (Schaeffer, unpublished data). Navigating these situations to ensure good patient care and strong patient-provider relationships, while also establishing a climate of mutual respect, can be challenging for all women physicians, in particular for trainees who are just beginning to experience the clinical environment independently. Our session on microaggressions was purposefully led by a national expert in patient-provider communication and offered an opportunity for women trainees to reflect on their past experiences being the target of microaggressions, to name them as such, and then to brainstorm possible responses as a group. The result was a “toolkit” of resources for responding to microaggressions.17
Of the attendees of WILD 2017 and 2018, 91% strongly agreed that participation in the symposium was a useful experience. One attendee reflected that they “feel more empowered to discuss women-related issues in academics with peers, mentors, mentees” and another stated that as a result of WILD, they would “sponsor peers and mentors, speak out more about gender bias, seek out leadership positions.” Challenges for our symposium included obtaining protected curricular time from busy trainee schedules. Supportive leadership at all levels was critical to our success; carving out dedicated curricular time will be essential for the sustainability of this leadership symposium. Our group has recently received funding to expand to a longitudinal course open to all women residents and fellows across graduate medical education.
Although the complex and unique problems facing women medical trainees are unlikely to be comprehensively addressed by a leadership course, we urge other institutions to adopt and expand on our model for teaching vital leadership skills. In addition to leadership skills, academic medical centers should adopt a multipronged approach to support their female trainees, including clear and confidential reporting practices of sexual harassment without fear of retaliation, training for all staff on harassment and bias, involvement of men as allies, and mentorship programs for women trainees. Further research is needed to better understand this complex problem, its impact on career outcomes, and a path to achieving gender equality in medicine.
Acknowledgments
The authors are indebted to Catherine Lucey, MD, for her framing of the issues for women in medicine as a complex problem and to Sarah Schaeffer, MD, for her unpublished data on microaggressions at our institution. The authors are also grateful to the UCSF Department of Medicine and the UCSF Chancellor’s Advisory Committee on the Status of Women for their financial support of the WILD (Women In Leadership Development) program.
Disclosures
The authors declare no conflict of interest.
Funding
The authors report no external funding source for this study.
Despite media attention to gender inequality in multiple professions, medicine has only recently begun to identify disparities facing women in academic medicine, focusing primarily on women faculty rather than trainees. Because of the unique and poorly understood juxtaposition of forces affecting their experience, focusing on women medical trainees may provide a representative framework to understand the larger, complex problem of gender equity in medicine. Rather than being a complicated problem with component parts that can be separately addressed, gender equity in medicine is a complex problem—one composed of a myriad of interrelated human and systemic factors. Such a complex problem demands innovative, open-minded, user-centered interventions. Here, we outline some of the factors unique to women trainees, including lack of female role models in leadership, gender bias, sexual harassment, work-life imbalance, and few formal leadership training programs. We propose one potential strategy, leadership programs specifically targeted to women residents and fellows. We recently implemented this strategy at our institution in the form of a day-long symposium of skill-building sessions for women residents and fellows.
Although women have achieved equal representation in several medical training programs, there is still a dearth of women in high-profile leadership positions within academic medicine. Although women comprised 46% of United States medical school applicants and residents in 2015-2016, underrepresentation persists at the level of associate professor (35% women), full professor (22%), department chair (14%), and dean (16%).1 Many potential women leaders may not self-identify as such due to the limited exposure to women role models in positions of power and may in fact be ready for leadership roles earlier but not apply until later in their careers as compared with men.2,3 The lack of role models with a shared background is an even more severe problem for women of color and all of these factors contribute to the “leaky pipeline” phenomenon.4 We aimed to address this mindset and help women see themselves as leaders by overcoming “second-generation gender bias” through our work.2
Due to the intense and inflexible nature of residency and fellowship training programs, many women choose to defer milestones such as childbearing.5 Women trainees are more likely than their male colleagues to avoid having a child during residency due to perceived threat to their career and negative perceptions of colleagues.5,6 Women who are in a domestic partnership often bear the brunt of the household work regardless of the careers of the two partners, a phenomenon termed the “second shift.”7 This work-life imbalance has been shown to correlate with depressive symptoms in women internal medicine trainees.8
Recently, a trainee published on the experience of medical residents being asked whether they were ever called “nurse.” All the women in the room put up their hands; none of their male colleagues did.9 At issue is not the relative importance of the professions of medicine and nursing, but rather the gender stereotypes in medicine that lead to automatic categorization of women into one group. Although the majority of women residents likely have had personal experiences with bias and microaggressions, few are explicitly taught the tools to address these. Beyond microaggressions, women trainees are also subject to more sexual harassment than their male colleagues.10 In addition, women living at the intersections of race, ethnicity, and gender are faced with even higher rates of harassment.11 Reporting sexual assault and harassment can be particularly difficult as a trainee because of the risk of retaliation, fear of poor evaluations from superiors, and lack of confidence in the reporting process.10
Finally, women trainees often receive little training about the skills required for career advancement to achieve parity with their male colleagues. Women are less likely to negotiate due to concerns about backlash or due to general lack of awareness about the importance of negotiation.12 Women are asked to volunteer for “nonpromotable” tasks more often than men by colleagues of both sexes, a barrier to women reaching their full career potential and a difficult workplace scenario to navigate.13 Unlike the fields of business, law, and technology, for example, women in medicine do not routinely have training courses that incorporate skills such as navigating difficult conversations, conflict resolution, curriculum vitae writing, and negotiation. Various solutions have been offered to address some of the barriers facing women in medicine (such as the Association of American Medical Colleges and Executive Leadership in Academic Medicine leadership courses), but typically these focus on faculty rather than trainees. Given that women physicians practicing in the inpatient setting have been shown to have better patient outcomes14 and organizations with female leadership outperform those led by men,15 equipping our women trainees to thrive in the clinical and leadership environments is an essential step in fulfilling our mission as high-quality training programs.
At our institution, we recognized the need for training in leadership skills for women medical trainees and designed a day-long symposium for internal medicine women residents and fellows. Before developing the curriculum, we conducted a needs assessment to ascertain which skills women wanted to develop; women overwhelmingly wanted to learn about public speaking skills, work-life integration, and mentoring. Based on these responses, a group spanning multiple levels of training (residency, fellowship, and faculty) designed a combination of large-group lectures and small-group workshops termed “Women in Leadership Development” (WILD). The day-long curriculum included sessions on public speaking skills, women as change agents, mentorship, conflict resolution, and addressing microaggressions and concluded with a networking event for women faculty and trainees (Table).
In total, 77 medicine residents and fellows voluntarily participated in the symposium in 2017 and 2018. The public speaking skills session received the highest reviews, with 98% of participants reporting that they identified ways to change public speaking styles to project confidence. This session was facilitated by an outside consultant in public speaking, highlighting the benefit of seeking experts outside of academic medicine. Another novel session focused on responding to microaggressions, defined as subtle and sometimes unintentional actions that express prejudice toward marginalized groups, in the clinical and academic environments. Microaggressions can undermine the recipient’s confidence, feeling of belonging, and effectiveness at work.16 At our institution, trainees in graduate medical education report the largest single source of microaggressions as patients (greater than attendings, fellow trainees, or staff), with gender bias being responsible for the greatest number of microaggressions (Schaeffer, unpublished data). Navigating these situations to ensure good patient care and strong patient-provider relationships, while also establishing a climate of mutual respect, can be challenging for all women physicians, in particular for trainees who are just beginning to experience the clinical environment independently. Our session on microaggressions was purposefully led by a national expert in patient-provider communication and offered an opportunity for women trainees to reflect on their past experiences being the target of microaggressions, to name them as such, and then to brainstorm possible responses as a group. The result was a “toolkit” of resources for responding to microaggressions.17
Of the attendees of WILD 2017 and 2018, 91% strongly agreed that participation in the symposium was a useful experience. One attendee reflected that they “feel more empowered to discuss women-related issues in academics with peers, mentors, mentees” and another stated that as a result of WILD, they would “sponsor peers and mentors, speak out more about gender bias, seek out leadership positions.” Challenges for our symposium included obtaining protected curricular time from busy trainee schedules. Supportive leadership at all levels was critical to our success; carving out dedicated curricular time will be essential for the sustainability of this leadership symposium. Our group has recently received funding to expand to a longitudinal course open to all women residents and fellows across graduate medical education.
Although the complex and unique problems facing women medical trainees are unlikely to be comprehensively addressed by a leadership course, we urge other institutions to adopt and expand on our model for teaching vital leadership skills. In addition to leadership skills, academic medical centers should adopt a multipronged approach to support their female trainees, including clear and confidential reporting practices of sexual harassment without fear of retaliation, training for all staff on harassment and bias, involvement of men as allies, and mentorship programs for women trainees. Further research is needed to better understand this complex problem, its impact on career outcomes, and a path to achieving gender equality in medicine.
Acknowledgments
The authors are indebted to Catherine Lucey, MD, for her framing of the issues for women in medicine as a complex problem and to Sarah Schaeffer, MD, for her unpublished data on microaggressions at our institution. The authors are also grateful to the UCSF Department of Medicine and the UCSF Chancellor’s Advisory Committee on the Status of Women for their financial support of the WILD (Women In Leadership Development) program.
Disclosures
The authors declare no conflict of interest.
Funding
The authors report no external funding source for this study.
1. AAMC [website]. 2018. https://www.aamc.org/. Accessed May 5, 2018.
2. Ibarra H, Ely, Robin J, Kolb D. Women rising: the unseen barriers. Harvard Bus Rev. 2013;91(9):60-66.
3. Stevenson EJ, Orr E. We interviewed 57 female CEOs to find out how more women can get to the top. Harvard Bus Rev. 2017.
4. Mahoney MR, Wilson E, Odom KL, Flowers L, Adler SR. Minority faculty voices on diversity in academic medicine: perspectives from one school. Acad Med. 2008;83(8):781-786. doi: 10.1097/ACM.0b013e31817ec002. PubMed
5. Turner PL, Lumpkins K, Gabre J, Lin MJ, Liu X, Terrin M. Pregnancy among women surgeons: trends over time. Arch Surg. 2012;147(5):474-479. doi: 10.1001/archsurg.2011.1693. PubMed
6. Willett LL, Wellons MF, Hartig JR, et al. Do women residents delay childbearing due to perceived career threats? Acad Med. 2010;85(4):640-646. doi: 10.1097/ACM.0b013e3181d2cb5b. PubMed
7. Jolly S, Griffith KA, DeCastro R, Stewart A, Ubel P, Jagsi R. Gender differences in time spent on parenting and domestic responsibilities by high-achieving young physician-researchers. Ann Intern Med. 2014;160(5):344-353. doi: 10.7326/M13-0974. PubMed
8. Guille C, Frank E, Zhao Z, et al. Work-family conflict and the sex difference in depression among training physicians. JAMA Intern Med. 2017;177(12):1766-1772. doi: 10.1001/jamainternmed.2017.5138. PubMed
9. DeFilippis EM. Putting the “She” in doctor. JAMA Intern Med. 2018;178(3):323-324. doi: 10.1001/jamainternmed.2017.8362. PubMed
10. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. doi: 10.1056/NEJM199302043280507. PubMed
11. Corbie-Smith G, Frank E, Nickens HW, Elon L. Prevalences and correlates of ethnic harassment in the U.S. Women Physicians’ Health Study. Acad Med. 1999;74(6):695-701. doi: 10.1097/00001888-199906000-00018. PubMed
12. Amanatullah ET, Morris MW. Negotiating gender roles: gender differences in assertive negotiating are mediated by women’s fear of backlash and attenuated when negotiating on behalf of others. J Pers Soc Psychol. 2010;98(2):256-267. doi: 10.1037/a0017094. PubMed
13. Babcock L, Maria PR, Vesterlund L. Why women volunteer for tasks that don’t lead to promotions. Harvard Bus Rev. 2018.
14. Tsugawa Y, Jena AB, Figueroa JF, Orav EJ, Blumenthal DM, Jha AK. Comparison of hospital mortality and readmission rates for medicare patients treated by male vs female physicians. JAMA Intern Med. 2017;177(2):206-213. doi: 10.1001/jamainternmed.2016.7875. PubMed
15. Landel M. Why gender balance can’t wait. Harvard Bus Rev. 2016.
16. Wolf TM, Randall HM, von Almen K, Tynes LL. Perceived mistreatment and attitude change by graduating medical students: a retrospective study. Med Educ. 1991;25(3):182-190. doi: 10.1111/j.1365-2923.1991.tb00050.x. PubMed
17. Wheeler DJ, Zapata J, Davis D, Chou C. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2018:1-6. doi: 10.1080/0142159X.2018.1506097. PubMed
1. AAMC [website]. 2018. https://www.aamc.org/. Accessed May 5, 2018.
2. Ibarra H, Ely, Robin J, Kolb D. Women rising: the unseen barriers. Harvard Bus Rev. 2013;91(9):60-66.
3. Stevenson EJ, Orr E. We interviewed 57 female CEOs to find out how more women can get to the top. Harvard Bus Rev. 2017.
4. Mahoney MR, Wilson E, Odom KL, Flowers L, Adler SR. Minority faculty voices on diversity in academic medicine: perspectives from one school. Acad Med. 2008;83(8):781-786. doi: 10.1097/ACM.0b013e31817ec002. PubMed
5. Turner PL, Lumpkins K, Gabre J, Lin MJ, Liu X, Terrin M. Pregnancy among women surgeons: trends over time. Arch Surg. 2012;147(5):474-479. doi: 10.1001/archsurg.2011.1693. PubMed
6. Willett LL, Wellons MF, Hartig JR, et al. Do women residents delay childbearing due to perceived career threats? Acad Med. 2010;85(4):640-646. doi: 10.1097/ACM.0b013e3181d2cb5b. PubMed
7. Jolly S, Griffith KA, DeCastro R, Stewart A, Ubel P, Jagsi R. Gender differences in time spent on parenting and domestic responsibilities by high-achieving young physician-researchers. Ann Intern Med. 2014;160(5):344-353. doi: 10.7326/M13-0974. PubMed
8. Guille C, Frank E, Zhao Z, et al. Work-family conflict and the sex difference in depression among training physicians. JAMA Intern Med. 2017;177(12):1766-1772. doi: 10.1001/jamainternmed.2017.5138. PubMed
9. DeFilippis EM. Putting the “She” in doctor. JAMA Intern Med. 2018;178(3):323-324. doi: 10.1001/jamainternmed.2017.8362. PubMed
10. Komaromy M, Bindman AB, Haber RJ, Sande MA. Sexual harassment in medical training. N Engl J Med. 1993;328(5):322-326. doi: 10.1056/NEJM199302043280507. PubMed
11. Corbie-Smith G, Frank E, Nickens HW, Elon L. Prevalences and correlates of ethnic harassment in the U.S. Women Physicians’ Health Study. Acad Med. 1999;74(6):695-701. doi: 10.1097/00001888-199906000-00018. PubMed
12. Amanatullah ET, Morris MW. Negotiating gender roles: gender differences in assertive negotiating are mediated by women’s fear of backlash and attenuated when negotiating on behalf of others. J Pers Soc Psychol. 2010;98(2):256-267. doi: 10.1037/a0017094. PubMed
13. Babcock L, Maria PR, Vesterlund L. Why women volunteer for tasks that don’t lead to promotions. Harvard Bus Rev. 2018.
14. Tsugawa Y, Jena AB, Figueroa JF, Orav EJ, Blumenthal DM, Jha AK. Comparison of hospital mortality and readmission rates for medicare patients treated by male vs female physicians. JAMA Intern Med. 2017;177(2):206-213. doi: 10.1001/jamainternmed.2016.7875. PubMed
15. Landel M. Why gender balance can’t wait. Harvard Bus Rev. 2016.
16. Wolf TM, Randall HM, von Almen K, Tynes LL. Perceived mistreatment and attitude change by graduating medical students: a retrospective study. Med Educ. 1991;25(3):182-190. doi: 10.1111/j.1365-2923.1991.tb00050.x. PubMed
17. Wheeler DJ, Zapata J, Davis D, Chou C. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2018:1-6. doi: 10.1080/0142159X.2018.1506097. PubMed
© 2019 Society of Hospital Medicine