User login
Antidepressants for patients who are breastfeeding: What to consider
Ms. D, age 32, recently gave birth to her second child. Her psychiatric history includes major depressive disorder. She had been stable on mirtazapine 30 mg at bedtime for 3 years. Based on clinical stability and patient preference, Ms. D elected to taper off mirtazapine 1 month prior to delivery. Now at 1 month postdelivery, Ms. D notes the reemergence of her depressive symptoms; during her child’s latest pediatrician visit, she scores 15 on the Edinburgh Postnatal Depression Scale (EPDS). She breastfeeds her baby and wants more information on the safety of taking an antidepressant while breastfeeding.
Ms. D discusses her previous use of mirtazapine with her treatment team. The team reviews the available resources with Ms. D and together they plan to make a shared decision regarding treatment of her depression at her next appointment.
The American Academy of Pediatrics1 and World Health Organization2 recommend exclusive breastfeeding of infants for their first 6 months of life and support it as a complement to other foods through and beyond age 2. Untreated conditions such as postpartum depression impact maternal well-being and may interfere with parenting and child development. In fact, untreated maternal mental health leads to an increased risk of suicide, reduced maternal economic productivity, and worsened health for both mother and child.3
Because many women experience psychiatric symptoms before they become pregnant as well as during the perinatal period, questions often arise regardingthe use of psychiatric medications—specifically antidepressants—and their safety in patients who are breastfeeding. Key considerations regarding medication management should include the patient’s previous response to medications, the risks of untreated maternal mental illness, and evidence regarding risks and benefits in lactation. This article summarizes where to find evidence-based lactation information, how to interpret that information, and what information is available for select antidepressants.
Locating lactation information
Start by checking the manufacturer’s medication labeling (“prescribing information”) and medication information resources such as Micromedex (www.micromedexsolutions.com) and Lexicomp (www.wolterskluwer.com/en/solutions/lexicomp). The updated labeling includes a risk/benefit assessment of available data on the risk for continued use of a medication during pregnancy compared to the risk if a medication is discontinued and the disorder goes untreated.4 The “breastfeeding considerations” section of medication labeling include details regarding the presence of the medication and the amount of it in breastmilk, adverse events in infants exposed to the medication through breastmilk, and additional pertinent data as applicable. Lexicomp includes information regarding breastfeeding considerations, and a subscription may also include access to Briggs Drugs in Pregnancy and Lactation’s information pages. Micromedex includes its own lactation safety rating scale score.
Several other resources can help guide clinicians toward patient-specific recommendations. From the National Library of Medicine, LactMed (https://www.ncbi.nlm.nih.gov/books/NBK501922/) allows clinicians to search for specific medications to see what information exists pertaining to medication levels in breastmilk and infant blood as well as potential adverse effects in the nursing infant and/or on lactation and breastmilk.5 LactMed provides information regarding alternative medications to consider and references from which the information was gathered.
Another helpful resource is the InfantRisk Center from Texas Tech University Health Sciences Center, which includes a free call center for parents and clinicians who have questions about medications and breastfeeding (806-352-2519; Monday through Friday, 8
Continue to: How to interpret the information
How to interpret the information
Medication levels in breastmilk are affected by several properties, such as the medication’s molecular weight, protein binding, pKa, and volume of distribution. A few commonly used terms in lactation literature for medications include the relative infant dose (RID) and milk/plasma (M/P) ratio.
RID provides information about relative medication exposure for the infant. It is calculated by dividing the infant’s dose of a medication via breastmilk (mg/kg/d) by the mother’s dose (mg/kg/d).7 Most consider an RID <10% to be safe.7
M/P is the ratio of medication concentration in the mother’s milk divided by the medication concentration in the mother’s plasma. A ratio <1 is preferable and generally indicates that a low level of medication has been transferred to human milk.7
Another factor that can be evaluated is protein binding. Medications that are highly protein-bound do not tend to pass as easily into breastmilk and can minimize infant exposure.
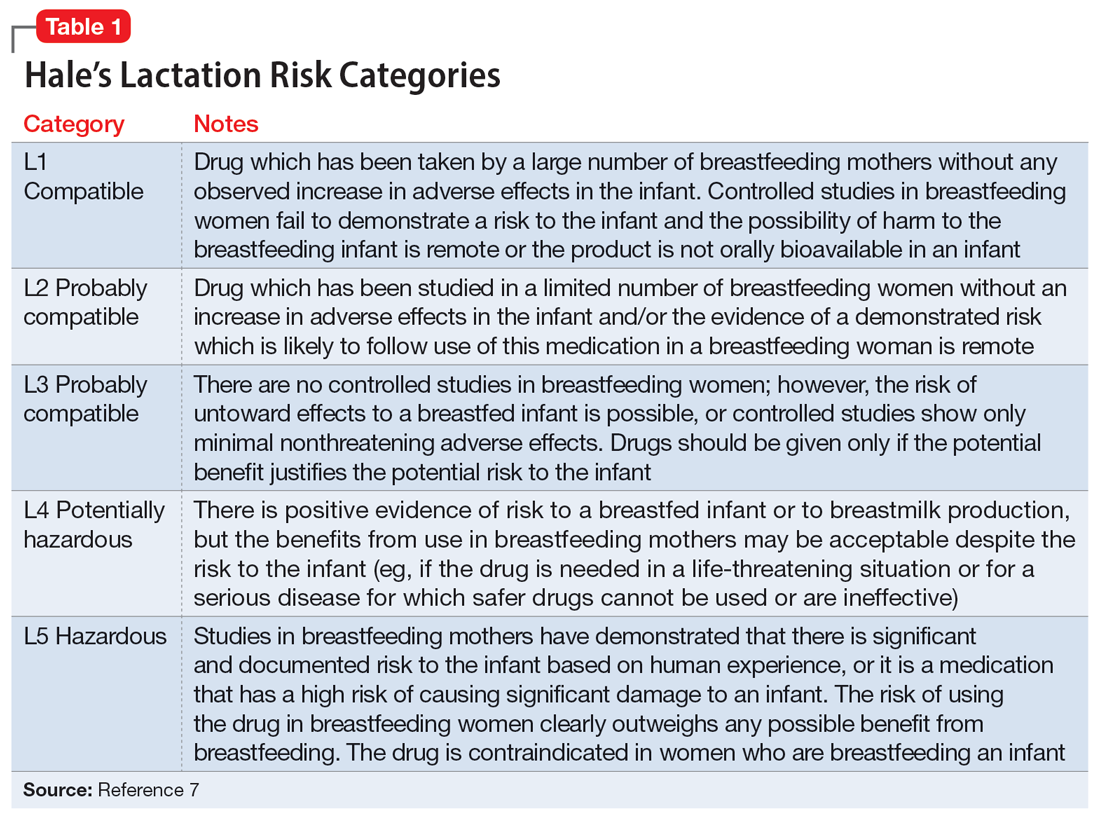
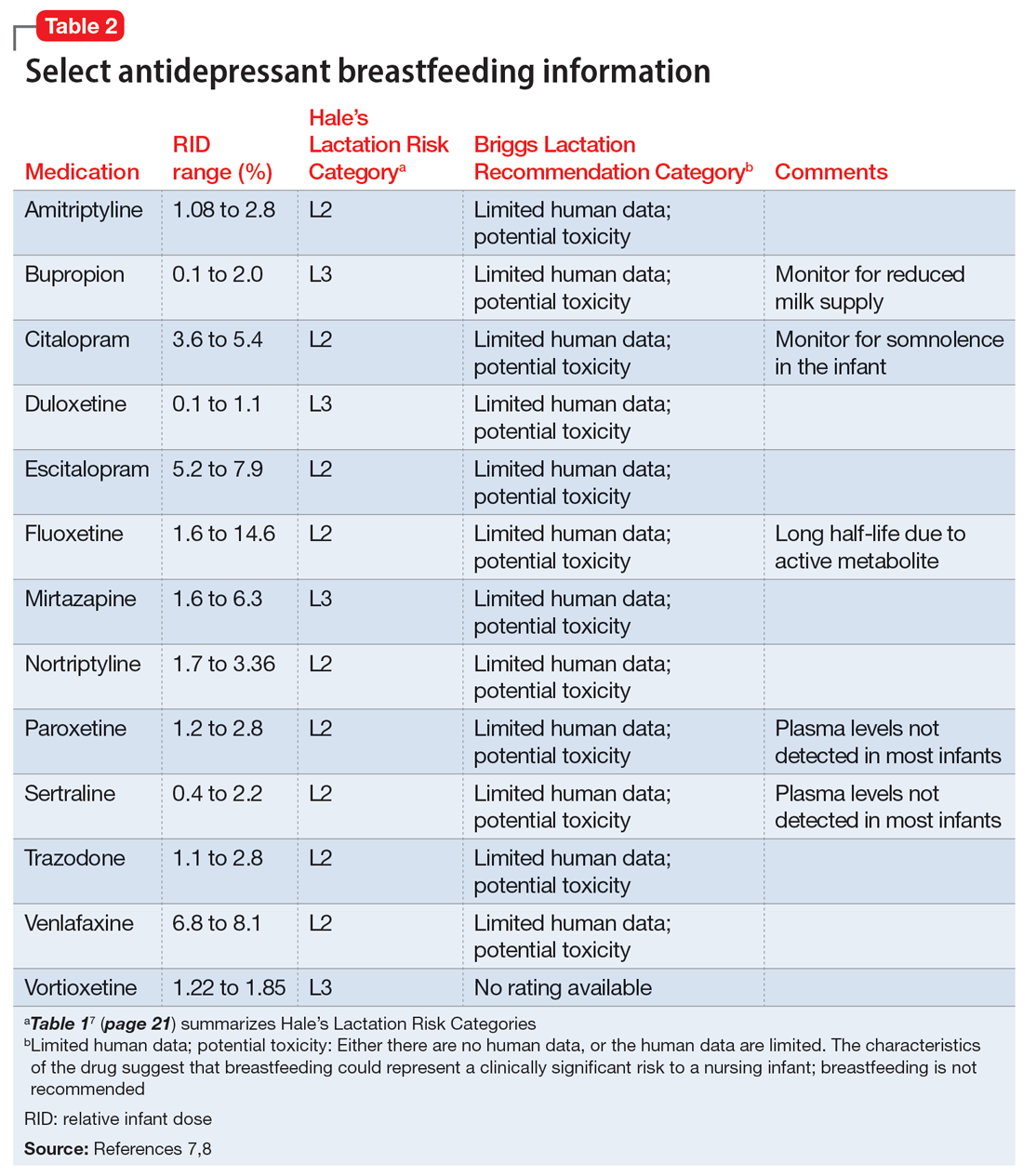
Several risk categorization systems are available, depending upon the resource used to obtain lactation information. One common system is Hale’s Lactation Risk Categories, with 5 safety levels ranging from L1 (breastfeeding compatible) to L5 (hazardous) (Table 17). Briggs et al8 utilize 7 categories to summarize recommendations ranging from breastfeeding-compatible to contraindicated; however, it is important to read the full medication monograph in the context of the rating provided. Table 27,8 provides breastfeeding information from Hale’s7 and from Briggs et al8 for some commonly used antidepressants.
In addition to interpreting available literature, it is also important to consider patient-specific factors, including (but not limited to) the severity of the patient’s psychiatric disorder and their previous response to medication. If a mother achieved remission on a particular antidepressant in the past, it may be preferable to restart that agent rather than trial a new medication.
CASE CONTINUED
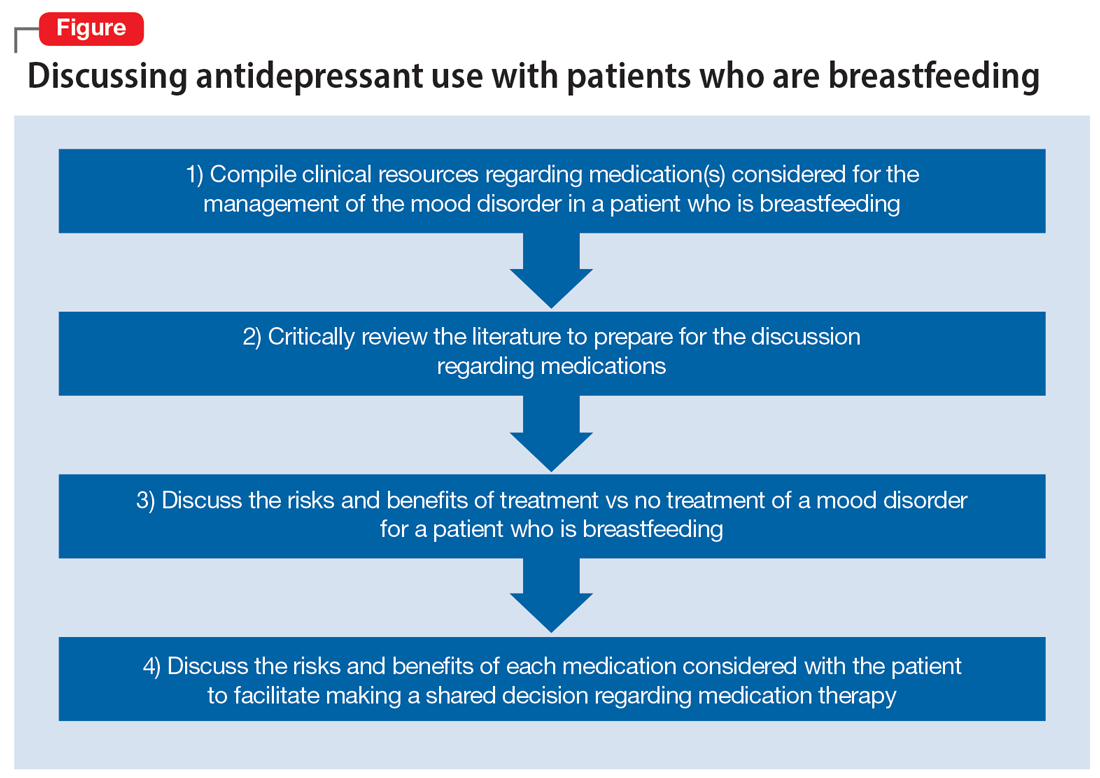
Two weeks later and following the use of a variety of resources, Ms. D’s treatment team finds that mirtazapine is rated Probably Compatible (L3 in Hale’s Lactation Risk Categories), with an M/P ratio of 0.76.7 The RID of mirtazapine ranges from 1.6% to 6.3%, and limited data from infants exposed to maternal use of mirtazapine during breastfeeding have not shown adverse effects.5 The treatment team administers the EDPS to Ms. D again and she scores 18. Given Ms. D’s previous remission with mirtazapine, current severity of depressive symptoms, and the risk/benefit assessment from lactation resources, the decision is made to restart mirtazapine 15 mg/d at bedtime with the option to titrate up if indicated. Ms. D plans to continue breastfeeding and will monitor for signs of any adverse effects in her infant. The Figure provides a summary of navigating this individualized decision with patients.
Related Resources
- MotherToBaby. Medication fact sheets, option to contact for no-charge consultation, free patient education information materials. www.mothertobaby.org
- Reprotox. Summaries on effects of medications on pregnancy, reproduction, and development (subscription required). www.reprotox.org
Drug Brand Names
Bupropion • Wellbutrin
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Trazodone • Oleptro
Venlafaxine • Effexor
Vortioxetine • Trintellix
1. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed April 7, 2023. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
2. World Health Organization. Breastfeeding recommendations. Accessed April 7, 2023. https://www.who.int/health-topics/breastfeeding#tab=tab_2
3. Margiotta C, Gao J, O’Neil S, et al. The economic impact of untreated maternal mental health conditions in Texas. BMC Pregnancy Childbirth. 2022;22(1):700. doi:10.1186/s12884-022-05001-6
4. Freeman MP, Farchione T, Yao L, et al. Psychiatric medications and reproductive safety: scientific and clinical perspectives pertaining to the US FDA pregnancy and lactation labeling rule. J Clin Psychiatry. 2018;79(4):18ah38120.
5. Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2011. Updated April 18, 2016. Accessed September 29, 2022. https://www.ncbi.nlm.nih.gov/books/NBK501922/
6. InfantRisk Center Resources. InfantRisk Center at Texas Tech University Health Sciences Center. Accessed September 29, 2022. https://www.infantrisk.com/infantrisk-center-resources
7. Hale TW, Krutsch K. Hale’s Medications and Mother’s Milk 2023: A Manual of Lactational Pharmacology. Springer Publishing; 2023.
8. Briggs GG, Freeman RK, Towers CV, et al. Briggs Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 12th ed. Lippincott Williams & Wilkins; 2021.
Ms. D, age 32, recently gave birth to her second child. Her psychiatric history includes major depressive disorder. She had been stable on mirtazapine 30 mg at bedtime for 3 years. Based on clinical stability and patient preference, Ms. D elected to taper off mirtazapine 1 month prior to delivery. Now at 1 month postdelivery, Ms. D notes the reemergence of her depressive symptoms; during her child’s latest pediatrician visit, she scores 15 on the Edinburgh Postnatal Depression Scale (EPDS). She breastfeeds her baby and wants more information on the safety of taking an antidepressant while breastfeeding.
Ms. D discusses her previous use of mirtazapine with her treatment team. The team reviews the available resources with Ms. D and together they plan to make a shared decision regarding treatment of her depression at her next appointment.
The American Academy of Pediatrics1 and World Health Organization2 recommend exclusive breastfeeding of infants for their first 6 months of life and support it as a complement to other foods through and beyond age 2. Untreated conditions such as postpartum depression impact maternal well-being and may interfere with parenting and child development. In fact, untreated maternal mental health leads to an increased risk of suicide, reduced maternal economic productivity, and worsened health for both mother and child.3
Because many women experience psychiatric symptoms before they become pregnant as well as during the perinatal period, questions often arise regardingthe use of psychiatric medications—specifically antidepressants—and their safety in patients who are breastfeeding. Key considerations regarding medication management should include the patient’s previous response to medications, the risks of untreated maternal mental illness, and evidence regarding risks and benefits in lactation. This article summarizes where to find evidence-based lactation information, how to interpret that information, and what information is available for select antidepressants.
Locating lactation information
Start by checking the manufacturer’s medication labeling (“prescribing information”) and medication information resources such as Micromedex (www.micromedexsolutions.com) and Lexicomp (www.wolterskluwer.com/en/solutions/lexicomp). The updated labeling includes a risk/benefit assessment of available data on the risk for continued use of a medication during pregnancy compared to the risk if a medication is discontinued and the disorder goes untreated.4 The “breastfeeding considerations” section of medication labeling include details regarding the presence of the medication and the amount of it in breastmilk, adverse events in infants exposed to the medication through breastmilk, and additional pertinent data as applicable. Lexicomp includes information regarding breastfeeding considerations, and a subscription may also include access to Briggs Drugs in Pregnancy and Lactation’s information pages. Micromedex includes its own lactation safety rating scale score.
Several other resources can help guide clinicians toward patient-specific recommendations. From the National Library of Medicine, LactMed (https://www.ncbi.nlm.nih.gov/books/NBK501922/) allows clinicians to search for specific medications to see what information exists pertaining to medication levels in breastmilk and infant blood as well as potential adverse effects in the nursing infant and/or on lactation and breastmilk.5 LactMed provides information regarding alternative medications to consider and references from which the information was gathered.
Another helpful resource is the InfantRisk Center from Texas Tech University Health Sciences Center, which includes a free call center for parents and clinicians who have questions about medications and breastfeeding (806-352-2519; Monday through Friday, 8
Continue to: How to interpret the information
How to interpret the information
Medication levels in breastmilk are affected by several properties, such as the medication’s molecular weight, protein binding, pKa, and volume of distribution. A few commonly used terms in lactation literature for medications include the relative infant dose (RID) and milk/plasma (M/P) ratio.
RID provides information about relative medication exposure for the infant. It is calculated by dividing the infant’s dose of a medication via breastmilk (mg/kg/d) by the mother’s dose (mg/kg/d).7 Most consider an RID <10% to be safe.7
M/P is the ratio of medication concentration in the mother’s milk divided by the medication concentration in the mother’s plasma. A ratio <1 is preferable and generally indicates that a low level of medication has been transferred to human milk.7
Another factor that can be evaluated is protein binding. Medications that are highly protein-bound do not tend to pass as easily into breastmilk and can minimize infant exposure.
Several risk categorization systems are available, depending upon the resource used to obtain lactation information. One common system is Hale’s Lactation Risk Categories, with 5 safety levels ranging from L1 (breastfeeding compatible) to L5 (hazardous) (Table 17). Briggs et al8 utilize 7 categories to summarize recommendations ranging from breastfeeding-compatible to contraindicated; however, it is important to read the full medication monograph in the context of the rating provided. Table 27,8 provides breastfeeding information from Hale’s7 and from Briggs et al8 for some commonly used antidepressants.

In addition to interpreting available literature, it is also important to consider patient-specific factors, including (but not limited to) the severity of the patient’s psychiatric disorder and their previous response to medication. If a mother achieved remission on a particular antidepressant in the past, it may be preferable to restart that agent rather than trial a new medication.

CASE CONTINUED
Two weeks later and following the use of a variety of resources, Ms. D’s treatment team finds that mirtazapine is rated Probably Compatible (L3 in Hale’s Lactation Risk Categories), with an M/P ratio of 0.76.7 The RID of mirtazapine ranges from 1.6% to 6.3%, and limited data from infants exposed to maternal use of mirtazapine during breastfeeding have not shown adverse effects.5 The treatment team administers the EDPS to Ms. D again and she scores 18. Given Ms. D’s previous remission with mirtazapine, current severity of depressive symptoms, and the risk/benefit assessment from lactation resources, the decision is made to restart mirtazapine 15 mg/d at bedtime with the option to titrate up if indicated. Ms. D plans to continue breastfeeding and will monitor for signs of any adverse effects in her infant. The Figure provides a summary of navigating this individualized decision with patients.

Related Resources
- MotherToBaby. Medication fact sheets, option to contact for no-charge consultation, free patient education information materials. www.mothertobaby.org
- Reprotox. Summaries on effects of medications on pregnancy, reproduction, and development (subscription required). www.reprotox.org
Drug Brand Names
Bupropion • Wellbutrin
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Trazodone • Oleptro
Venlafaxine • Effexor
Vortioxetine • Trintellix
Ms. D, age 32, recently gave birth to her second child. Her psychiatric history includes major depressive disorder. She had been stable on mirtazapine 30 mg at bedtime for 3 years. Based on clinical stability and patient preference, Ms. D elected to taper off mirtazapine 1 month prior to delivery. Now at 1 month postdelivery, Ms. D notes the reemergence of her depressive symptoms; during her child’s latest pediatrician visit, she scores 15 on the Edinburgh Postnatal Depression Scale (EPDS). She breastfeeds her baby and wants more information on the safety of taking an antidepressant while breastfeeding.
Ms. D discusses her previous use of mirtazapine with her treatment team. The team reviews the available resources with Ms. D and together they plan to make a shared decision regarding treatment of her depression at her next appointment.
The American Academy of Pediatrics1 and World Health Organization2 recommend exclusive breastfeeding of infants for their first 6 months of life and support it as a complement to other foods through and beyond age 2. Untreated conditions such as postpartum depression impact maternal well-being and may interfere with parenting and child development. In fact, untreated maternal mental health leads to an increased risk of suicide, reduced maternal economic productivity, and worsened health for both mother and child.3
Because many women experience psychiatric symptoms before they become pregnant as well as during the perinatal period, questions often arise regardingthe use of psychiatric medications—specifically antidepressants—and their safety in patients who are breastfeeding. Key considerations regarding medication management should include the patient’s previous response to medications, the risks of untreated maternal mental illness, and evidence regarding risks and benefits in lactation. This article summarizes where to find evidence-based lactation information, how to interpret that information, and what information is available for select antidepressants.
Locating lactation information
Start by checking the manufacturer’s medication labeling (“prescribing information”) and medication information resources such as Micromedex (www.micromedexsolutions.com) and Lexicomp (www.wolterskluwer.com/en/solutions/lexicomp). The updated labeling includes a risk/benefit assessment of available data on the risk for continued use of a medication during pregnancy compared to the risk if a medication is discontinued and the disorder goes untreated.4 The “breastfeeding considerations” section of medication labeling include details regarding the presence of the medication and the amount of it in breastmilk, adverse events in infants exposed to the medication through breastmilk, and additional pertinent data as applicable. Lexicomp includes information regarding breastfeeding considerations, and a subscription may also include access to Briggs Drugs in Pregnancy and Lactation’s information pages. Micromedex includes its own lactation safety rating scale score.
Several other resources can help guide clinicians toward patient-specific recommendations. From the National Library of Medicine, LactMed (https://www.ncbi.nlm.nih.gov/books/NBK501922/) allows clinicians to search for specific medications to see what information exists pertaining to medication levels in breastmilk and infant blood as well as potential adverse effects in the nursing infant and/or on lactation and breastmilk.5 LactMed provides information regarding alternative medications to consider and references from which the information was gathered.
Another helpful resource is the InfantRisk Center from Texas Tech University Health Sciences Center, which includes a free call center for parents and clinicians who have questions about medications and breastfeeding (806-352-2519; Monday through Friday, 8
Continue to: How to interpret the information
How to interpret the information
Medication levels in breastmilk are affected by several properties, such as the medication’s molecular weight, protein binding, pKa, and volume of distribution. A few commonly used terms in lactation literature for medications include the relative infant dose (RID) and milk/plasma (M/P) ratio.
RID provides information about relative medication exposure for the infant. It is calculated by dividing the infant’s dose of a medication via breastmilk (mg/kg/d) by the mother’s dose (mg/kg/d).7 Most consider an RID <10% to be safe.7
M/P is the ratio of medication concentration in the mother’s milk divided by the medication concentration in the mother’s plasma. A ratio <1 is preferable and generally indicates that a low level of medication has been transferred to human milk.7
Another factor that can be evaluated is protein binding. Medications that are highly protein-bound do not tend to pass as easily into breastmilk and can minimize infant exposure.
Several risk categorization systems are available, depending upon the resource used to obtain lactation information. One common system is Hale’s Lactation Risk Categories, with 5 safety levels ranging from L1 (breastfeeding compatible) to L5 (hazardous) (Table 17). Briggs et al8 utilize 7 categories to summarize recommendations ranging from breastfeeding-compatible to contraindicated; however, it is important to read the full medication monograph in the context of the rating provided. Table 27,8 provides breastfeeding information from Hale’s7 and from Briggs et al8 for some commonly used antidepressants.

In addition to interpreting available literature, it is also important to consider patient-specific factors, including (but not limited to) the severity of the patient’s psychiatric disorder and their previous response to medication. If a mother achieved remission on a particular antidepressant in the past, it may be preferable to restart that agent rather than trial a new medication.

CASE CONTINUED
Two weeks later and following the use of a variety of resources, Ms. D’s treatment team finds that mirtazapine is rated Probably Compatible (L3 in Hale’s Lactation Risk Categories), with an M/P ratio of 0.76.7 The RID of mirtazapine ranges from 1.6% to 6.3%, and limited data from infants exposed to maternal use of mirtazapine during breastfeeding have not shown adverse effects.5 The treatment team administers the EDPS to Ms. D again and she scores 18. Given Ms. D’s previous remission with mirtazapine, current severity of depressive symptoms, and the risk/benefit assessment from lactation resources, the decision is made to restart mirtazapine 15 mg/d at bedtime with the option to titrate up if indicated. Ms. D plans to continue breastfeeding and will monitor for signs of any adverse effects in her infant. The Figure provides a summary of navigating this individualized decision with patients.

Related Resources
- MotherToBaby. Medication fact sheets, option to contact for no-charge consultation, free patient education information materials. www.mothertobaby.org
- Reprotox. Summaries on effects of medications on pregnancy, reproduction, and development (subscription required). www.reprotox.org
Drug Brand Names
Bupropion • Wellbutrin
Citalopram • Celexa
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Sertraline • Zoloft
Trazodone • Oleptro
Venlafaxine • Effexor
Vortioxetine • Trintellix
1. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed April 7, 2023. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
2. World Health Organization. Breastfeeding recommendations. Accessed April 7, 2023. https://www.who.int/health-topics/breastfeeding#tab=tab_2
3. Margiotta C, Gao J, O’Neil S, et al. The economic impact of untreated maternal mental health conditions in Texas. BMC Pregnancy Childbirth. 2022;22(1):700. doi:10.1186/s12884-022-05001-6
4. Freeman MP, Farchione T, Yao L, et al. Psychiatric medications and reproductive safety: scientific and clinical perspectives pertaining to the US FDA pregnancy and lactation labeling rule. J Clin Psychiatry. 2018;79(4):18ah38120.
5. Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2011. Updated April 18, 2016. Accessed September 29, 2022. https://www.ncbi.nlm.nih.gov/books/NBK501922/
6. InfantRisk Center Resources. InfantRisk Center at Texas Tech University Health Sciences Center. Accessed September 29, 2022. https://www.infantrisk.com/infantrisk-center-resources
7. Hale TW, Krutsch K. Hale’s Medications and Mother’s Milk 2023: A Manual of Lactational Pharmacology. Springer Publishing; 2023.
8. Briggs GG, Freeman RK, Towers CV, et al. Briggs Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 12th ed. Lippincott Williams & Wilkins; 2021.
1. American Academy of Pediatrics. American Academy of Pediatrics calls for more support for breastfeeding mothers within updated policy recommendations. June 27, 2022. Accessed April 7, 2023. https://www.aap.org/en/news-room/news-releases/aap/2022/american-academy-of-pediatrics-calls-for-more-support-for-breastfeeding-mothers-within-updated-policy-recommendations
2. World Health Organization. Breastfeeding recommendations. Accessed April 7, 2023. https://www.who.int/health-topics/breastfeeding#tab=tab_2
3. Margiotta C, Gao J, O’Neil S, et al. The economic impact of untreated maternal mental health conditions in Texas. BMC Pregnancy Childbirth. 2022;22(1):700. doi:10.1186/s12884-022-05001-6
4. Freeman MP, Farchione T, Yao L, et al. Psychiatric medications and reproductive safety: scientific and clinical perspectives pertaining to the US FDA pregnancy and lactation labeling rule. J Clin Psychiatry. 2018;79(4):18ah38120.
5. Drugs and Lactation Database (LactMed). National Library of Medicine (US); 2011. Updated April 18, 2016. Accessed September 29, 2022. https://www.ncbi.nlm.nih.gov/books/NBK501922/
6. InfantRisk Center Resources. InfantRisk Center at Texas Tech University Health Sciences Center. Accessed September 29, 2022. https://www.infantrisk.com/infantrisk-center-resources
7. Hale TW, Krutsch K. Hale’s Medications and Mother’s Milk 2023: A Manual of Lactational Pharmacology. Springer Publishing; 2023.
8. Briggs GG, Freeman RK, Towers CV, et al. Briggs Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. 12th ed. Lippincott Williams & Wilkins; 2021.
Lithium-induced diabetes insipidus: Pathophysiology and treatment
Ms. V, age 58, presents to the emergency department after falling in the middle of the night while walking to the bathroom. Her medical history includes bipolar I disorder (BDI). According to her granddaughter, Ms. V has been stable on lithium 600 mg twice daily for 1 to 2 years. Her laboratory workup shows a serum creatinine level of 0.93 mg/dL (reference range 0.6 to 1.2 mg/dL), high sodium (154 mEq/L; reference range 135 to 145 mEq/L), and a lithium level of 0.9 mEq/L (therapeutic range 0.6 to 1.2 mEq/L). On Day 2 of admission, Ms. V’s sodium level remains high (152 mEq/L), her urine output is 5 L/d (normal output <2 L/d), and her serum osmolality is high (326 mmol/kg; reference range 275 to 295 mmol/kg).
After additional questioning, Ms. V says for the past 3 weeks she has been urinating approximately 4 times per night and experiencing excessive thirst. Given her laboratory values and physical presentation, a desmopressin challenge test is performed and confirms a diagnosis of lithium-induced nephrogenic diabetes insipidus (Li-NDI). Nephrogenic diabetes insipidus (NDI) occurs when the kidneys become unresponsive to the action of antidiuretic hormone (ADH; also known as vasopressin).1 The most common cause of NDI is lithium. The prevalence varies from 50% to 73% with long-term lithium use.1,2 It is important to recognize the homeostatic regulation of water prior to understanding Li-NDI. The excretion of water is regulated by ADH. ADH binds to the vasopressin receptors on the basolateral membrane of the collecting duct cells. This stimulates Gs protein and adenylate cyclase, which subsequently increase intracellular cyclic adenosine monophosphate (cAMP).1 Eventually, this leads to the activation of protein kinase A and phosphorylation of aquaporin 2 (AQP2) water channels. The AQP2 channels redistribute from storage vesicles to the apical membrane and the membrane becomes permeable to water, allowing for reabsorption.1,3
In Li-NDI, lithium enters the cells of the collecting duct through the epithelial sodium channel (ENaC).1,4 There, lithium inhibits the action of ADH, glycogen synthase kinase-3 (GSK-3) activity, and the generation of cAMP.1,4 It also induces cyclooxygenase-2 expression in renal interstitial cells and the production of prostaglandin E2 (PGE2).1,5-8 Lithium may also reduce the amount of AQP2 water channels in the apical membrane of the collecting duct. 1,3 Additionally, polymorphisms of the GSK-3 beta gene can occur, which may be related to differences in the extent of the lithium-induced renal concentrating defect among patients who take lithium.9
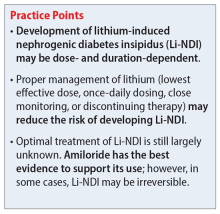
Symptoms of Li-NDI include polyuria (ie, urine production >3 L/day) and polydipsia.1 More than 40% of patients with symptomatic Li-NDI experience a significant interference with their daily routine and occupational activities, and may be at risk for severe dehydration with concurrent electrolyte disturbances, resulting in lithium toxicity.1,2 This could especially impact older adults, who may have a diminished thirst sensation and insufficient fluid intake (ie, psychological decompensation, decreased mobility).1,2
Li-NDI is reversible early in treatment; however, it may become irreversible over time.1 The degree of reversibility depends on the stage of kidney damage (ie, functional vs morphological) and/or duration of lithium treatment.7 Even with the discontinuation of lithium, symptoms may persist. Imaging can be used to identify the extent of kidney damage, but given the inconsistent data regarding the reversibility of Li-NDI, it would be difficult to predict if symptoms will resolve.8
Establishing the diagnosis
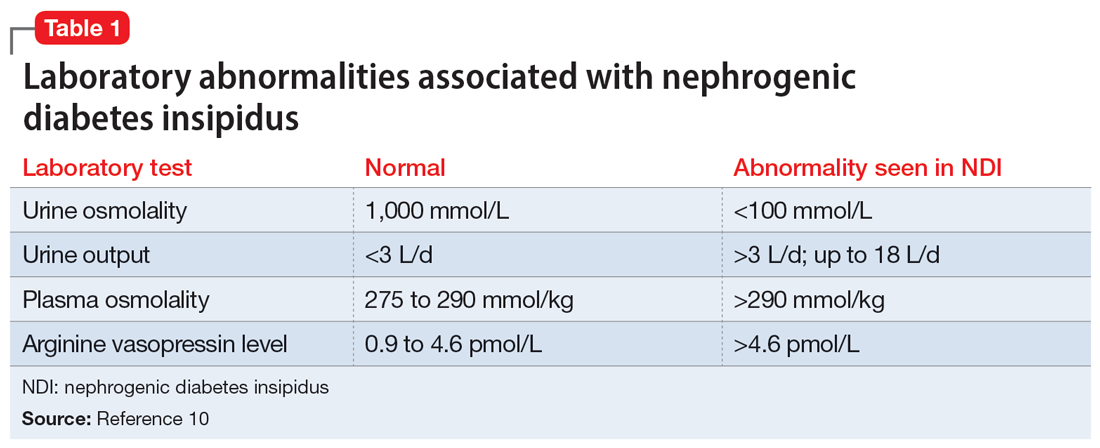
A physical examination and laboratory workup are the first steps in diagnosing and determining the underlying cause of NDI. Table 110 outlines common laboratory abnormalities associated with NDI. Additionally, serum sodium levels can be used to determine water balance; hypernatremia is often seen in cases of NDI.10 Water deprivation tests are useful for diagnosing diabetes insipidus and allow for differentiation of nephrogenic vs central diabetes insipidus.10 Once the patient is water-deprived for ≥4 hours, a single 5-unit dose of subcutaneous desmopressin may be administered. In Li-NDI, the urine often remains dilute with urine osmolality levels <200 mmol/kg, even after administration of exogenous arginine vasopressin.10
Several treatment options
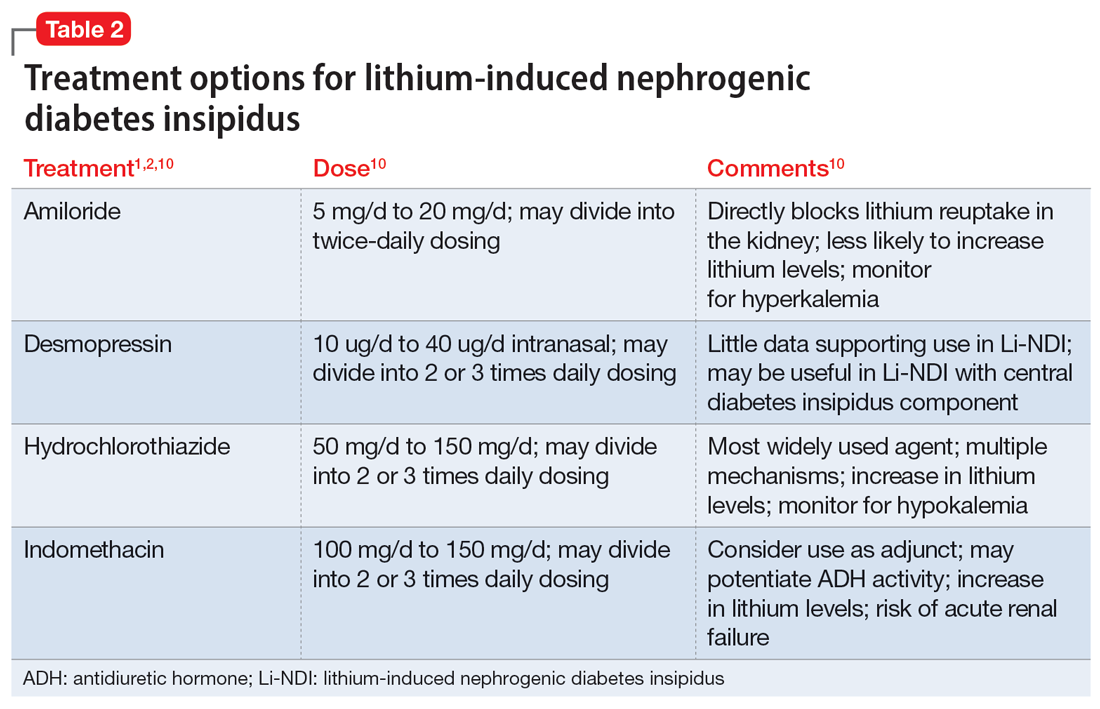
In many cases, Li-NDI symptoms can be reduced by using the lowest effective dose of lithium, switching to a once-daily formulation, or discontinuing therapy. Some patients may find relief from certain diuretics, such as amiloride. Thiazide diuretics can also be used but may require a ≥50% reduction in lithium dose. Nonsteroid anti-inflammatory drugs, such as indomethacin, in combination with diuretics, have been found to be effective by increasing the concentration of urine.1,2 Table 21,2,10 summarizes potential treatment options.
Continue to: Amiloride has the most...
Amiloride has the most supporting evidence in the treatment of Li-NDI. A potassium-sparing diuretic, amiloride works by blocking the ENaC in the distal and collecting duct. Blocking the ENaC inhibits uptake of lithium into the principal cells of the collecting duct within the kidney. Research has shown that amiloride can be effective in treating existing Li-NDI, but there is a lack of evidence supporting its preventative effects.1
Thiazide diuretics work by blocking the sodium-chloride cotransporter in the distal tubules of the kidney. They also upregulate the AQP2 water channels.1 Research has shown that sodium replacement counteracts the antidiuretic effect of thiazide diuretics; limitations in dietary sodium intake may be necessary for treatment efficacy.1
Within the kidneys, PGE2 inhibits adenyl cyclase and diminishes water permeability.10 This causes water to be excreted in urine rather than be reabsorbed.10 Indomethacin blocks PGE2 activity and increases water reabsorption in the collecting ducts, and sodium reabsorption in the thick ascending loop of Henle.10 This mechanism can lead to increased lithium reabsorption, which may precipitate toxicity. Research has shown increases in lithium levels by as much as 59% in addition to the risk of causing acute renal failure, especially in older adults.10 Due to these risks, indomethacin should not be considered a first-line treatment for Li-NDI.
Overall, several medications have shown benefits in the treatment of Li-NDI, with amiloride having the most data. There are currently no medications with sufficient evidence to support prophylactic use.
CASE CONTINUED
Ms. V’s treatment team initiates amiloride 5 mg/d. They increase the dose to 10 mg/d after 2 days, and Ms. V’s hypernatremia resolves as her serum sodium normalizes to 142 mEq/L. Her urinary output also decreases to <3 L/d. Throughout treatment, Ms. V continues taking lithium carbonate to prevent destabilization of her BDI. The team subsequently discharges her, and she has been stable for the past 6 months.
Related Resources
- Andreasen A, Ellingrod V. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
- Zhang P, Gandhi H, Kassis N. Lithium-induced nephropathy; one medication with multiple side effects: a case report. BMC Nephrol. 2022;23(1):309. doi:10.1186/s12882-022-02934-0
Drug Brand Names
Amiloride • Midamor
Desmopressin • DDAVP
Hydrochlorothiazide • Microzide
Indomethacin • Indocin, Tivorbex
Lithium • Eskalith, Lithobid
1. Schoot TS, Molmans THJ, Grootens KP, et al. Systematic review and practical guideline from the prevention and management of renal side effects of lithium therapy. Eur Neuropsychopharmacol. 2020;31:16-32.
2. Lithium induced diabetes insipidus. DiabetesInsipidus.org. Accessed June 7, 2022. https://diabetesinsipidus.org/lithium-induced-diabetes-insipidus
3. Rej S, Segal M, Low NC, et al. The McGill geriatric lithium-induced diabetes insipidus clinical study (McGLIDICS). Can J Psychiatry. 2014;59(6):327-334.
4. Christensen BM, Zuber AM, Loffing J, et al. alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol. 2011;22(2):253-261.
5. Bendz H, Aurell M, Balldin J, et al. Kidney damage in long-term lithium patients: a cross sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant. 1994;9(9):1250-1254.
6. Bendz H. Kidney function in a selected lithium population. A prospective, controlled, lithium-withdrawal study. Acta Psychiatr Scand. 1985;72(5):451-463.
7. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28.
8. Garofeanu CG, Weir M, Rosas-Arellano MP, et al. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45(4):626-637.
9. Bucht G, Whalin A. Renal concentrating capacity in long-term lithium treatment and after withdrawal of lithium. Acta Med Scand. 1980;207(4):309-314.
10. Finch CK, Brooks TWA, Yam P, et al. Management and treatment of lithium-induced nephrogenic diabetes insipidus. Therapy. 2005;2(4):669-675. doi:10.1586/14750708.2.4.669
Ms. V, age 58, presents to the emergency department after falling in the middle of the night while walking to the bathroom. Her medical history includes bipolar I disorder (BDI). According to her granddaughter, Ms. V has been stable on lithium 600 mg twice daily for 1 to 2 years. Her laboratory workup shows a serum creatinine level of 0.93 mg/dL (reference range 0.6 to 1.2 mg/dL), high sodium (154 mEq/L; reference range 135 to 145 mEq/L), and a lithium level of 0.9 mEq/L (therapeutic range 0.6 to 1.2 mEq/L). On Day 2 of admission, Ms. V’s sodium level remains high (152 mEq/L), her urine output is 5 L/d (normal output <2 L/d), and her serum osmolality is high (326 mmol/kg; reference range 275 to 295 mmol/kg).
After additional questioning, Ms. V says for the past 3 weeks she has been urinating approximately 4 times per night and experiencing excessive thirst. Given her laboratory values and physical presentation, a desmopressin challenge test is performed and confirms a diagnosis of lithium-induced nephrogenic diabetes insipidus (Li-NDI). Nephrogenic diabetes insipidus (NDI) occurs when the kidneys become unresponsive to the action of antidiuretic hormone (ADH; also known as vasopressin).1 The most common cause of NDI is lithium. The prevalence varies from 50% to 73% with long-term lithium use.1,2 It is important to recognize the homeostatic regulation of water prior to understanding Li-NDI. The excretion of water is regulated by ADH. ADH binds to the vasopressin receptors on the basolateral membrane of the collecting duct cells. This stimulates Gs protein and adenylate cyclase, which subsequently increase intracellular cyclic adenosine monophosphate (cAMP).1 Eventually, this leads to the activation of protein kinase A and phosphorylation of aquaporin 2 (AQP2) water channels. The AQP2 channels redistribute from storage vesicles to the apical membrane and the membrane becomes permeable to water, allowing for reabsorption.1,3
In Li-NDI, lithium enters the cells of the collecting duct through the epithelial sodium channel (ENaC).1,4 There, lithium inhibits the action of ADH, glycogen synthase kinase-3 (GSK-3) activity, and the generation of cAMP.1,4 It also induces cyclooxygenase-2 expression in renal interstitial cells and the production of prostaglandin E2 (PGE2).1,5-8 Lithium may also reduce the amount of AQP2 water channels in the apical membrane of the collecting duct. 1,3 Additionally, polymorphisms of the GSK-3 beta gene can occur, which may be related to differences in the extent of the lithium-induced renal concentrating defect among patients who take lithium.9
Symptoms of Li-NDI include polyuria (ie, urine production >3 L/day) and polydipsia.1 More than 40% of patients with symptomatic Li-NDI experience a significant interference with their daily routine and occupational activities, and may be at risk for severe dehydration with concurrent electrolyte disturbances, resulting in lithium toxicity.1,2 This could especially impact older adults, who may have a diminished thirst sensation and insufficient fluid intake (ie, psychological decompensation, decreased mobility).1,2
Li-NDI is reversible early in treatment; however, it may become irreversible over time.1 The degree of reversibility depends on the stage of kidney damage (ie, functional vs morphological) and/or duration of lithium treatment.7 Even with the discontinuation of lithium, symptoms may persist. Imaging can be used to identify the extent of kidney damage, but given the inconsistent data regarding the reversibility of Li-NDI, it would be difficult to predict if symptoms will resolve.8
Establishing the diagnosis
A physical examination and laboratory workup are the first steps in diagnosing and determining the underlying cause of NDI. Table 110 outlines common laboratory abnormalities associated with NDI. Additionally, serum sodium levels can be used to determine water balance; hypernatremia is often seen in cases of NDI.10 Water deprivation tests are useful for diagnosing diabetes insipidus and allow for differentiation of nephrogenic vs central diabetes insipidus.10 Once the patient is water-deprived for ≥4 hours, a single 5-unit dose of subcutaneous desmopressin may be administered. In Li-NDI, the urine often remains dilute with urine osmolality levels <200 mmol/kg, even after administration of exogenous arginine vasopressin.10

Several treatment options
In many cases, Li-NDI symptoms can be reduced by using the lowest effective dose of lithium, switching to a once-daily formulation, or discontinuing therapy. Some patients may find relief from certain diuretics, such as amiloride. Thiazide diuretics can also be used but may require a ≥50% reduction in lithium dose. Nonsteroid anti-inflammatory drugs, such as indomethacin, in combination with diuretics, have been found to be effective by increasing the concentration of urine.1,2 Table 21,2,10 summarizes potential treatment options.

Continue to: Amiloride has the most...
Amiloride has the most supporting evidence in the treatment of Li-NDI. A potassium-sparing diuretic, amiloride works by blocking the ENaC in the distal and collecting duct. Blocking the ENaC inhibits uptake of lithium into the principal cells of the collecting duct within the kidney. Research has shown that amiloride can be effective in treating existing Li-NDI, but there is a lack of evidence supporting its preventative effects.1
Thiazide diuretics work by blocking the sodium-chloride cotransporter in the distal tubules of the kidney. They also upregulate the AQP2 water channels.1 Research has shown that sodium replacement counteracts the antidiuretic effect of thiazide diuretics; limitations in dietary sodium intake may be necessary for treatment efficacy.1
Within the kidneys, PGE2 inhibits adenyl cyclase and diminishes water permeability.10 This causes water to be excreted in urine rather than be reabsorbed.10 Indomethacin blocks PGE2 activity and increases water reabsorption in the collecting ducts, and sodium reabsorption in the thick ascending loop of Henle.10 This mechanism can lead to increased lithium reabsorption, which may precipitate toxicity. Research has shown increases in lithium levels by as much as 59% in addition to the risk of causing acute renal failure, especially in older adults.10 Due to these risks, indomethacin should not be considered a first-line treatment for Li-NDI.
Overall, several medications have shown benefits in the treatment of Li-NDI, with amiloride having the most data. There are currently no medications with sufficient evidence to support prophylactic use.
CASE CONTINUED
Ms. V’s treatment team initiates amiloride 5 mg/d. They increase the dose to 10 mg/d after 2 days, and Ms. V’s hypernatremia resolves as her serum sodium normalizes to 142 mEq/L. Her urinary output also decreases to <3 L/d. Throughout treatment, Ms. V continues taking lithium carbonate to prevent destabilization of her BDI. The team subsequently discharges her, and she has been stable for the past 6 months.
Related Resources
- Andreasen A, Ellingrod V. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
- Zhang P, Gandhi H, Kassis N. Lithium-induced nephropathy; one medication with multiple side effects: a case report. BMC Nephrol. 2022;23(1):309. doi:10.1186/s12882-022-02934-0
Drug Brand Names
Amiloride • Midamor
Desmopressin • DDAVP
Hydrochlorothiazide • Microzide
Indomethacin • Indocin, Tivorbex
Lithium • Eskalith, Lithobid
Ms. V, age 58, presents to the emergency department after falling in the middle of the night while walking to the bathroom. Her medical history includes bipolar I disorder (BDI). According to her granddaughter, Ms. V has been stable on lithium 600 mg twice daily for 1 to 2 years. Her laboratory workup shows a serum creatinine level of 0.93 mg/dL (reference range 0.6 to 1.2 mg/dL), high sodium (154 mEq/L; reference range 135 to 145 mEq/L), and a lithium level of 0.9 mEq/L (therapeutic range 0.6 to 1.2 mEq/L). On Day 2 of admission, Ms. V’s sodium level remains high (152 mEq/L), her urine output is 5 L/d (normal output <2 L/d), and her serum osmolality is high (326 mmol/kg; reference range 275 to 295 mmol/kg).
After additional questioning, Ms. V says for the past 3 weeks she has been urinating approximately 4 times per night and experiencing excessive thirst. Given her laboratory values and physical presentation, a desmopressin challenge test is performed and confirms a diagnosis of lithium-induced nephrogenic diabetes insipidus (Li-NDI). Nephrogenic diabetes insipidus (NDI) occurs when the kidneys become unresponsive to the action of antidiuretic hormone (ADH; also known as vasopressin).1 The most common cause of NDI is lithium. The prevalence varies from 50% to 73% with long-term lithium use.1,2 It is important to recognize the homeostatic regulation of water prior to understanding Li-NDI. The excretion of water is regulated by ADH. ADH binds to the vasopressin receptors on the basolateral membrane of the collecting duct cells. This stimulates Gs protein and adenylate cyclase, which subsequently increase intracellular cyclic adenosine monophosphate (cAMP).1 Eventually, this leads to the activation of protein kinase A and phosphorylation of aquaporin 2 (AQP2) water channels. The AQP2 channels redistribute from storage vesicles to the apical membrane and the membrane becomes permeable to water, allowing for reabsorption.1,3
In Li-NDI, lithium enters the cells of the collecting duct through the epithelial sodium channel (ENaC).1,4 There, lithium inhibits the action of ADH, glycogen synthase kinase-3 (GSK-3) activity, and the generation of cAMP.1,4 It also induces cyclooxygenase-2 expression in renal interstitial cells and the production of prostaglandin E2 (PGE2).1,5-8 Lithium may also reduce the amount of AQP2 water channels in the apical membrane of the collecting duct. 1,3 Additionally, polymorphisms of the GSK-3 beta gene can occur, which may be related to differences in the extent of the lithium-induced renal concentrating defect among patients who take lithium.9
Symptoms of Li-NDI include polyuria (ie, urine production >3 L/day) and polydipsia.1 More than 40% of patients with symptomatic Li-NDI experience a significant interference with their daily routine and occupational activities, and may be at risk for severe dehydration with concurrent electrolyte disturbances, resulting in lithium toxicity.1,2 This could especially impact older adults, who may have a diminished thirst sensation and insufficient fluid intake (ie, psychological decompensation, decreased mobility).1,2
Li-NDI is reversible early in treatment; however, it may become irreversible over time.1 The degree of reversibility depends on the stage of kidney damage (ie, functional vs morphological) and/or duration of lithium treatment.7 Even with the discontinuation of lithium, symptoms may persist. Imaging can be used to identify the extent of kidney damage, but given the inconsistent data regarding the reversibility of Li-NDI, it would be difficult to predict if symptoms will resolve.8
Establishing the diagnosis
A physical examination and laboratory workup are the first steps in diagnosing and determining the underlying cause of NDI. Table 110 outlines common laboratory abnormalities associated with NDI. Additionally, serum sodium levels can be used to determine water balance; hypernatremia is often seen in cases of NDI.10 Water deprivation tests are useful for diagnosing diabetes insipidus and allow for differentiation of nephrogenic vs central diabetes insipidus.10 Once the patient is water-deprived for ≥4 hours, a single 5-unit dose of subcutaneous desmopressin may be administered. In Li-NDI, the urine often remains dilute with urine osmolality levels <200 mmol/kg, even after administration of exogenous arginine vasopressin.10

Several treatment options
In many cases, Li-NDI symptoms can be reduced by using the lowest effective dose of lithium, switching to a once-daily formulation, or discontinuing therapy. Some patients may find relief from certain diuretics, such as amiloride. Thiazide diuretics can also be used but may require a ≥50% reduction in lithium dose. Nonsteroid anti-inflammatory drugs, such as indomethacin, in combination with diuretics, have been found to be effective by increasing the concentration of urine.1,2 Table 21,2,10 summarizes potential treatment options.

Continue to: Amiloride has the most...
Amiloride has the most supporting evidence in the treatment of Li-NDI. A potassium-sparing diuretic, amiloride works by blocking the ENaC in the distal and collecting duct. Blocking the ENaC inhibits uptake of lithium into the principal cells of the collecting duct within the kidney. Research has shown that amiloride can be effective in treating existing Li-NDI, but there is a lack of evidence supporting its preventative effects.1
Thiazide diuretics work by blocking the sodium-chloride cotransporter in the distal tubules of the kidney. They also upregulate the AQP2 water channels.1 Research has shown that sodium replacement counteracts the antidiuretic effect of thiazide diuretics; limitations in dietary sodium intake may be necessary for treatment efficacy.1
Within the kidneys, PGE2 inhibits adenyl cyclase and diminishes water permeability.10 This causes water to be excreted in urine rather than be reabsorbed.10 Indomethacin blocks PGE2 activity and increases water reabsorption in the collecting ducts, and sodium reabsorption in the thick ascending loop of Henle.10 This mechanism can lead to increased lithium reabsorption, which may precipitate toxicity. Research has shown increases in lithium levels by as much as 59% in addition to the risk of causing acute renal failure, especially in older adults.10 Due to these risks, indomethacin should not be considered a first-line treatment for Li-NDI.
Overall, several medications have shown benefits in the treatment of Li-NDI, with amiloride having the most data. There are currently no medications with sufficient evidence to support prophylactic use.
CASE CONTINUED
Ms. V’s treatment team initiates amiloride 5 mg/d. They increase the dose to 10 mg/d after 2 days, and Ms. V’s hypernatremia resolves as her serum sodium normalizes to 142 mEq/L. Her urinary output also decreases to <3 L/d. Throughout treatment, Ms. V continues taking lithium carbonate to prevent destabilization of her BDI. The team subsequently discharges her, and she has been stable for the past 6 months.
Related Resources
- Andreasen A, Ellingrod V. Lithium-induced diabetes insipidus: prevention and management. Current Psychiatry. 2013;12(7):42-45.
- Zhang P, Gandhi H, Kassis N. Lithium-induced nephropathy; one medication with multiple side effects: a case report. BMC Nephrol. 2022;23(1):309. doi:10.1186/s12882-022-02934-0
Drug Brand Names
Amiloride • Midamor
Desmopressin • DDAVP
Hydrochlorothiazide • Microzide
Indomethacin • Indocin, Tivorbex
Lithium • Eskalith, Lithobid
1. Schoot TS, Molmans THJ, Grootens KP, et al. Systematic review and practical guideline from the prevention and management of renal side effects of lithium therapy. Eur Neuropsychopharmacol. 2020;31:16-32.
2. Lithium induced diabetes insipidus. DiabetesInsipidus.org. Accessed June 7, 2022. https://diabetesinsipidus.org/lithium-induced-diabetes-insipidus
3. Rej S, Segal M, Low NC, et al. The McGill geriatric lithium-induced diabetes insipidus clinical study (McGLIDICS). Can J Psychiatry. 2014;59(6):327-334.
4. Christensen BM, Zuber AM, Loffing J, et al. alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol. 2011;22(2):253-261.
5. Bendz H, Aurell M, Balldin J, et al. Kidney damage in long-term lithium patients: a cross sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant. 1994;9(9):1250-1254.
6. Bendz H. Kidney function in a selected lithium population. A prospective, controlled, lithium-withdrawal study. Acta Psychiatr Scand. 1985;72(5):451-463.
7. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28.
8. Garofeanu CG, Weir M, Rosas-Arellano MP, et al. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45(4):626-637.
9. Bucht G, Whalin A. Renal concentrating capacity in long-term lithium treatment and after withdrawal of lithium. Acta Med Scand. 1980;207(4):309-314.
10. Finch CK, Brooks TWA, Yam P, et al. Management and treatment of lithium-induced nephrogenic diabetes insipidus. Therapy. 2005;2(4):669-675. doi:10.1586/14750708.2.4.669
1. Schoot TS, Molmans THJ, Grootens KP, et al. Systematic review and practical guideline from the prevention and management of renal side effects of lithium therapy. Eur Neuropsychopharmacol. 2020;31:16-32.
2. Lithium induced diabetes insipidus. DiabetesInsipidus.org. Accessed June 7, 2022. https://diabetesinsipidus.org/lithium-induced-diabetes-insipidus
3. Rej S, Segal M, Low NC, et al. The McGill geriatric lithium-induced diabetes insipidus clinical study (McGLIDICS). Can J Psychiatry. 2014;59(6):327-334.
4. Christensen BM, Zuber AM, Loffing J, et al. alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol. 2011;22(2):253-261.
5. Bendz H, Aurell M, Balldin J, et al. Kidney damage in long-term lithium patients: a cross sectional study of patients with 15 years or more on lithium. Nephrol Dial Transplant. 1994;9(9):1250-1254.
6. Bendz H. Kidney function in a selected lithium population. A prospective, controlled, lithium-withdrawal study. Acta Psychiatr Scand. 1985;72(5):451-463.
7. Azab AN, Shnaider A, Osher Y, et al. Lithium nephrotoxicity. Int J Bipolar Disord. 2015;3(1):28.
8. Garofeanu CG, Weir M, Rosas-Arellano MP, et al. Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis. 2005;45(4):626-637.
9. Bucht G, Whalin A. Renal concentrating capacity in long-term lithium treatment and after withdrawal of lithium. Acta Med Scand. 1980;207(4):309-314.
10. Finch CK, Brooks TWA, Yam P, et al. Management and treatment of lithium-induced nephrogenic diabetes insipidus. Therapy. 2005;2(4):669-675. doi:10.1586/14750708.2.4.669
Psychoactive supplements: What to tell patients
Mr. D, age 41, presents to the emergency department (ED) with altered mental status and suspected intoxication. His medical history includes alcohol use disorder and spinal injury. Upon initial examination, he is confused, disorganized, and agitated. He receives IM lorazepam 4 mg to manage his agitation. His laboratory workup includes a negative screening for blood alcohol, slightly elevated creatine kinase, and urine toxicology positive for barbiturates and opioids. During re-evaluation by the consulting psychiatrist the following morning, Mr. D is alert, oriented, and calm with an organized thought process. He does not appear to be in withdrawal from any substances and tells the psychiatrist that he takes butalbital/acetaminophen/caffeine/codeine as needed for migraines. Mr. D says that 3 days before he came to the ED, he also began taking a supplement called phenibut that he purchased online for “well-being and sleep.”
Natural substances have been used throughout history as medicinal agents, sacred substances in religious rituals, and for recreational purposes.1 Supplement use in the United States is prevalent, with 57.6% of adults age ≥20 reporting supplement use in the past 30 days.2 Between 2000 and 2017, US poison control centers recorded a 74.1% increase in calls involving exposure to natural psychoactive substances, mostly driven by cases involving marijuana in adults and adolescents.3 Like synthetic drugs, herbal supplements may have psychoactive properties, including sedative, stimulant, psychedelic, euphoric, or anticholinergic effects. The variety and unregulated nature of supplements makes managing patients who use supplements particularly challenging.
Why patients use supplements
People may use supplements to treat or prevent vitamin deficiencies (eg, vitamin D, iron, calcium). Other reasons may include for promoting wellness in various disease states, for weight loss, for recreational use or misuse, or for overall well-being. In the mental health realm, patients report using supplements to treat depression, anxiety, insomnia, memory, or for vague indications such as “mood support.”4,5
Patients may view supplements as appealing alternatives to prescription medications because they are widely accessible, may be purchased over-the-counter, are inexpensive, and represent a “natural” treatment option.6 For these reasons, they may also falsely perceive supplements as categorically safe.1 People with psychiatric diagnoses may choose such alternative treatments due to a history of adverse effects or treatment failure with traditional psychiatric medications, mistrust of the health care or pharmaceutical industry, or based on the recommendations of others.7
Regulation, safety, and efficacy of dietary supplements
In the US, dietary supplements are regulated more like food products than medications. Under the Dietary Supplement Health and Education Act of 1994, the FDA regulates the quality, safety, and labeling of supplements using Current Good Manufacturing Practice regulations.8 The Federal Trade Commission monitors advertisements and marketing. Despite some regulations, dietary supplements may be adulterated or contaminated, contain unknown or toxic ingredients, have inconsistent potencies, or be sold at toxic doses.9 Importantly, supplements are not required to be evaluated for clinical efficacy. As a result, it is not known if most supplements are effective in treating the conditions for which they are promoted, mainly due to a lack of financial incentive for manufacturers to conduct large, high-quality trials.5
Further complicating matters is the inconsistent labeling of supplements or similar products that are easily obtainable via the internet. These products might be marketed as nutritional supplements or nootropics, which often are referred to as “cognitive enhancers” or “smart drugs.” New psychoactive substances (NPS) are drugs of misuse or abuse developed to imitate illicit drugs or controlled drug substances.10 They are sometimes referred to as “herbal highs” or “legal highs.”11 Supplements may also be labeled as performance- or image-enhancing agents and may include medications marketed to promote weight loss. This includes herbal substances (Table12-19) and medications associated with neuropsychiatric adverse effects that may be easily accessible online without a prescription.12,20
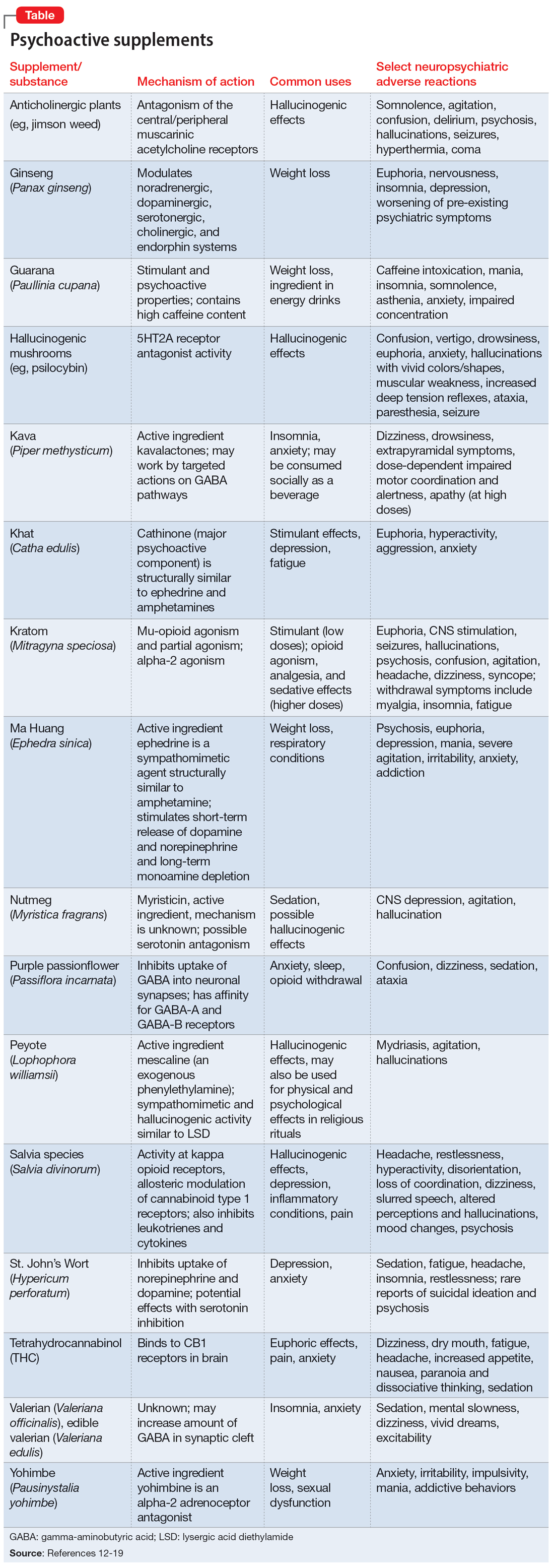
The growing popularity of the internet and social media plays an important role in the availability of supplements and nonregulated substances and may contribute to misleading claims of efficacy and safety. While many herbal supplements are available in pharmacies or supplement stores, NPS are usually sold through anonymous, low-risk means either via traditional online vendors or the deep web (parts of the internet that are not indexed via search engines). Strategies to circumvent regulation and legislative control include labeling NPS as research chemicals, fertilizers, incense, bath salts, or other identifiers and marketing them as “not for human consumption.”21 Manufacturers frequently change the chemical structures of NPS, which allows these products to exist within a legal gray area due to the lag time between when a new compound hits the market and when it is categorized as a regulated substance.10
Continue to: Another category of "supplements"...
Another category of “supplements” includes medications that are not FDA-approved but are approved for therapeutic use in other countries and readily available in the US via online sources. Such medications include phenibut, a glutamic acid derivative that functions as a gamma-aminobutyric acid-B receptor agonist in the brain, spinal cord, and autonomic nervous system. Phenibut was developed in the Soviet Union in the 1960s, and outside of the US it is prescribed for anxiolysis and other psychiatric indications.22 In the US, phenibut may be used as a nootropic or as a dietary supplement to treat anxiety, sleep problems, and other psychiatric disorders.22 It may also be used recreationally to induce euphoria. Chronic phenibut use results in tolerance and abrupt discontinuation may mimic benzodiazepine withdrawal symptoms.13,22
Educating patients about supplements
One of the most critical steps in assessing a patient’s supplement use is to directly ask them about their use of herbal or over-the-counter products. Research has consistently shown that patients are unlikely to disclose supplement use unless they are specifically asked.23,24
Additional strategies include25,26:
- Approach patients without judgment; ask open-ended questions to determine their motivations for using supplements.
- Explain the difference between supplements medically necessary to treat vitamin deficiencies (eg, vitamin D, calcium, magnesium) and those without robust clinical evidence.
- Counsel patients that many supplements with psychoactive properties, if indicated, are generally meant to be used short-term and not as substitutes for prescription medications.
- Educate patients that supplements have limited evidence regarding their safety and efficacy, but like prescription medications, supplements may cause organ damage, adverse effects, and drug-drug interactions.
- Remind patients that commonly used nutritional supplements/dietary aids, including protein or workout supplements, may contain potentially harmful ingredients.
- Utilize evidence-based resources such as the Natural Medicines Comprehensive Database14 or the National Center for Complementary and Integrative Health (https://www.nccih.nih.gov) to review levels of evidence and educate patients.
- When toxicity or withdrawal is suspected, reach out to local poison control centers for guidance.
- For a patient with a potential supplement-related substance use disorder, urine drug screens may be of limited utility and evidence is often sparse; clinicians may need to rely on primary literature such as case reports to guide management.
- If patients wish to continue taking a supplement, recommend they purchase supplements from manufacturers that have achieved the US Pharmacopeia (USP) verification mark. Products with the USP mark undergo quality assurance measures to ensure the product contains the ingredients listed on the label in the declared potency and amounts, does not contain harmful levels of contaminants, will be metabolized in the body within a specified amount of time, and has been produced in keeping with FDA Current Good Manufacturing Practice regulations.
CASE CONTINUED
In the ED, the consulting psychiatry team discusses Mr. D’s use of phenibut with him, and asks if he uses any additional supplements or nonprescription medications. Mr. D discloses he has been anxious and having trouble sleeping, and a friend recommended phenibut as a safe, natural alternative to medication. The team explains to Mr. D that phenibut’s efficacy has not been studied in the US and that based on available evidence, it is likely unsafe. It may have serious adverse effects, drug-drug interactions, and is potentially addictive.
Mr. D says he was unaware of these risks and agrees to stop taking phenibut. The treatment team discharges him from the ED with a referral for outpatient psychiatric services to address his anxiety and insomnia.
Related Resources
- Tillman B. The hidden dangers of supplements: a case of substance-induced psychosis. Current Psychiatry. 2020; 19(7):e7-e8. doi:10.12788/cp.0018
- McQueen CE. Herb–drug interactions: caution patients when changing supplements. Current Psychiatry. 2017; 16(6):38-41.
Drug Brand Names
Butalbital/acetaminophen/caffeine/codeine • Fioricet with Codeine
1. Graziano S, Orsolini L, Rotolo MC, et al. Herbal highs: review on psychoactive effects and neuropharmacology. Curr Neuropharmacol. 2017;15(5):750-761.
2. Mishra S, Stierman B, Gahche JJ, et al. Dietary supplement use among adults: United States, 2017-2018. NCHS Data Brief. 2021;(399):1-8.
3. O’Neill-Dee C, Spiller HA, Casavant MJ, et al. Natural psychoactive substance-related exposures reported to United States poison control centers, 2000-2017. Clin Toxicol (Phila). 2020;58(8):813-820.
4. Gray DC, Rutledge CM. Herbal supplements in primary care: patient perceptions, motivations, and effects on use. Holist Nurs Pract. 2013;27(1):6-12.
5. Wu K, Messamore E. Reimagining roles of dietary supplements in psychiatric care. AMA J Ethics. 2022;24(5):E437-E442.
6. Snyder FJ, Dundas ML, Kirkpatrick C, et al. Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. J Nutr Elder. 2009;28(1):81-95.
7. Schulz P, Hede V. Alternative and complementary approaches in psychiatry: beliefs versus evidence. Dialogues Clin Neurosci. 2018;20(3):207-214.
8. Dietary Supplement Health and Education Act of 1994, Pub L 103-417, 103rd Cong (1993-1994).
9. Starr RR. Too little, too late: ineffective regulation of dietary supplements in the United States. Am J Public Health. 2015;105(3):478-485.
10. New psychoactive substances. Alcohol and Drug Foundation. November 10, 2021. Updated November 28, 2022. Accessed January 25, 2023. https://adf.org.au/drug-facts/new-psychoactive-substances/
11. Shafi A, Berry AJ, Sumnall H, et al. New psychoactive substances: a review and updates. Ther Adv Psychopharmacol. 2020;10:2045125320967197.
12. Bersani FS, Coviello M, Imperatori C, et al. Adverse psychiatric effects associated with herbal weight-loss products. Biomed Res Int. 2015;2015:120679.
13. IBM Micromedex POISINDEX® System. IBM Watson Health. Accessed October 3, 2022. https://www.micromedexsolutions.com
14. Natural Medicines Comprehensive Database. Therapeutic Research Center. Accessed October 3, 2022. https://naturalmedicines.therapeuticresearch.com
15. Savage KM, Stough CK, Byrne GJ, et al. Kava for the treatment of generalised anxiety disorder (K-GAD): study protocol for a randomised controlled trial. Trials. 2015;16:493.
16. Swogger MT, Smith KE, Garcia-Romeu A, et al. Understanding kratom use: a guide for healthcare providers. Front Pharmacol. 2022;13:801855.
17. Modabbernia A, Akhondzadeh S. Saffron, passionflower, valerian and sage for mental health. Psychiatr Clin North Am. 2013;36(1):85-91.
18. Coffeen U, Pellicer F. Salvia divinorum: from recreational hallucinogenic use to analgesic and anti-inflammatory action. J Pain Res. 2019;12:1069-1076.
19. National Institutes of Health, Office of Dietary Supplements. Valerian Fact Sheet for Health Professionals. Updated March 15, 2013. Accessed January 25, 2023. https://ods.od.nih.gov/factsheets/Valerian-HealthProfessional
20. An H, Sohn H, Chung S. Phentermine, sibutramine and affective disorders. Clin Psychopharmacol Neurosci. 2013;11(1):7-12.
21. Miliano C, Margiani G, Fattore L, et al. Sales and advertising channels of new psychoactive substances (NPS): internet, social networks, and smartphone apps. Brain Sci. 2018;8(7):123.
22. Hardman MI, Sprung J, Weingarten TN. Acute phenibut withdrawal: a comprehensive literature review and illustrative case report. Bosn J Basic Med Sci. 2019;19(2):125-129.
23. Guzman JR, Paterniti DA, Liu Y, et al. Factors related to disclosure and nondisclosure of dietary supplements in primary care, integrative medicine, and naturopathic medicine. J Fam Med Dis Prev. 2019;5(4):10.23937/2469-5793/1510109.
24. Foley H, Steel A, Cramer H, et al. Disclosure of complementary medicine use to medical providers: a systematic review and meta-analysis. Sci Rep. 2019;9(1):1573.
25. Aldridge Young C. ‘No miracle cures’: counseling patients about dietary supplements. Pharmacy Today. 2014;February:35.
26. United States Pharmacopeia. USP Verified Mark. Accessed January 25, 2023. https://www.usp.org/verification-services/verified-mark
Mr. D, age 41, presents to the emergency department (ED) with altered mental status and suspected intoxication. His medical history includes alcohol use disorder and spinal injury. Upon initial examination, he is confused, disorganized, and agitated. He receives IM lorazepam 4 mg to manage his agitation. His laboratory workup includes a negative screening for blood alcohol, slightly elevated creatine kinase, and urine toxicology positive for barbiturates and opioids. During re-evaluation by the consulting psychiatrist the following morning, Mr. D is alert, oriented, and calm with an organized thought process. He does not appear to be in withdrawal from any substances and tells the psychiatrist that he takes butalbital/acetaminophen/caffeine/codeine as needed for migraines. Mr. D says that 3 days before he came to the ED, he also began taking a supplement called phenibut that he purchased online for “well-being and sleep.”
Natural substances have been used throughout history as medicinal agents, sacred substances in religious rituals, and for recreational purposes.1 Supplement use in the United States is prevalent, with 57.6% of adults age ≥20 reporting supplement use in the past 30 days.2 Between 2000 and 2017, US poison control centers recorded a 74.1% increase in calls involving exposure to natural psychoactive substances, mostly driven by cases involving marijuana in adults and adolescents.3 Like synthetic drugs, herbal supplements may have psychoactive properties, including sedative, stimulant, psychedelic, euphoric, or anticholinergic effects. The variety and unregulated nature of supplements makes managing patients who use supplements particularly challenging.
Why patients use supplements
People may use supplements to treat or prevent vitamin deficiencies (eg, vitamin D, iron, calcium). Other reasons may include for promoting wellness in various disease states, for weight loss, for recreational use or misuse, or for overall well-being. In the mental health realm, patients report using supplements to treat depression, anxiety, insomnia, memory, or for vague indications such as “mood support.”4,5
Patients may view supplements as appealing alternatives to prescription medications because they are widely accessible, may be purchased over-the-counter, are inexpensive, and represent a “natural” treatment option.6 For these reasons, they may also falsely perceive supplements as categorically safe.1 People with psychiatric diagnoses may choose such alternative treatments due to a history of adverse effects or treatment failure with traditional psychiatric medications, mistrust of the health care or pharmaceutical industry, or based on the recommendations of others.7
Regulation, safety, and efficacy of dietary supplements
In the US, dietary supplements are regulated more like food products than medications. Under the Dietary Supplement Health and Education Act of 1994, the FDA regulates the quality, safety, and labeling of supplements using Current Good Manufacturing Practice regulations.8 The Federal Trade Commission monitors advertisements and marketing. Despite some regulations, dietary supplements may be adulterated or contaminated, contain unknown or toxic ingredients, have inconsistent potencies, or be sold at toxic doses.9 Importantly, supplements are not required to be evaluated for clinical efficacy. As a result, it is not known if most supplements are effective in treating the conditions for which they are promoted, mainly due to a lack of financial incentive for manufacturers to conduct large, high-quality trials.5
Further complicating matters is the inconsistent labeling of supplements or similar products that are easily obtainable via the internet. These products might be marketed as nutritional supplements or nootropics, which often are referred to as “cognitive enhancers” or “smart drugs.” New psychoactive substances (NPS) are drugs of misuse or abuse developed to imitate illicit drugs or controlled drug substances.10 They are sometimes referred to as “herbal highs” or “legal highs.”11 Supplements may also be labeled as performance- or image-enhancing agents and may include medications marketed to promote weight loss. This includes herbal substances (Table12-19) and medications associated with neuropsychiatric adverse effects that may be easily accessible online without a prescription.12,20

The growing popularity of the internet and social media plays an important role in the availability of supplements and nonregulated substances and may contribute to misleading claims of efficacy and safety. While many herbal supplements are available in pharmacies or supplement stores, NPS are usually sold through anonymous, low-risk means either via traditional online vendors or the deep web (parts of the internet that are not indexed via search engines). Strategies to circumvent regulation and legislative control include labeling NPS as research chemicals, fertilizers, incense, bath salts, or other identifiers and marketing them as “not for human consumption.”21 Manufacturers frequently change the chemical structures of NPS, which allows these products to exist within a legal gray area due to the lag time between when a new compound hits the market and when it is categorized as a regulated substance.10
Continue to: Another category of "supplements"...
Another category of “supplements” includes medications that are not FDA-approved but are approved for therapeutic use in other countries and readily available in the US via online sources. Such medications include phenibut, a glutamic acid derivative that functions as a gamma-aminobutyric acid-B receptor agonist in the brain, spinal cord, and autonomic nervous system. Phenibut was developed in the Soviet Union in the 1960s, and outside of the US it is prescribed for anxiolysis and other psychiatric indications.22 In the US, phenibut may be used as a nootropic or as a dietary supplement to treat anxiety, sleep problems, and other psychiatric disorders.22 It may also be used recreationally to induce euphoria. Chronic phenibut use results in tolerance and abrupt discontinuation may mimic benzodiazepine withdrawal symptoms.13,22
Educating patients about supplements
One of the most critical steps in assessing a patient’s supplement use is to directly ask them about their use of herbal or over-the-counter products. Research has consistently shown that patients are unlikely to disclose supplement use unless they are specifically asked.23,24
Additional strategies include25,26:
- Approach patients without judgment; ask open-ended questions to determine their motivations for using supplements.
- Explain the difference between supplements medically necessary to treat vitamin deficiencies (eg, vitamin D, calcium, magnesium) and those without robust clinical evidence.
- Counsel patients that many supplements with psychoactive properties, if indicated, are generally meant to be used short-term and not as substitutes for prescription medications.
- Educate patients that supplements have limited evidence regarding their safety and efficacy, but like prescription medications, supplements may cause organ damage, adverse effects, and drug-drug interactions.
- Remind patients that commonly used nutritional supplements/dietary aids, including protein or workout supplements, may contain potentially harmful ingredients.
- Utilize evidence-based resources such as the Natural Medicines Comprehensive Database14 or the National Center for Complementary and Integrative Health (https://www.nccih.nih.gov) to review levels of evidence and educate patients.
- When toxicity or withdrawal is suspected, reach out to local poison control centers for guidance.
- For a patient with a potential supplement-related substance use disorder, urine drug screens may be of limited utility and evidence is often sparse; clinicians may need to rely on primary literature such as case reports to guide management.
- If patients wish to continue taking a supplement, recommend they purchase supplements from manufacturers that have achieved the US Pharmacopeia (USP) verification mark. Products with the USP mark undergo quality assurance measures to ensure the product contains the ingredients listed on the label in the declared potency and amounts, does not contain harmful levels of contaminants, will be metabolized in the body within a specified amount of time, and has been produced in keeping with FDA Current Good Manufacturing Practice regulations.
CASE CONTINUED
In the ED, the consulting psychiatry team discusses Mr. D’s use of phenibut with him, and asks if he uses any additional supplements or nonprescription medications. Mr. D discloses he has been anxious and having trouble sleeping, and a friend recommended phenibut as a safe, natural alternative to medication. The team explains to Mr. D that phenibut’s efficacy has not been studied in the US and that based on available evidence, it is likely unsafe. It may have serious adverse effects, drug-drug interactions, and is potentially addictive.
Mr. D says he was unaware of these risks and agrees to stop taking phenibut. The treatment team discharges him from the ED with a referral for outpatient psychiatric services to address his anxiety and insomnia.
Related Resources
- Tillman B. The hidden dangers of supplements: a case of substance-induced psychosis. Current Psychiatry. 2020; 19(7):e7-e8. doi:10.12788/cp.0018
- McQueen CE. Herb–drug interactions: caution patients when changing supplements. Current Psychiatry. 2017; 16(6):38-41.
Drug Brand Names
Butalbital/acetaminophen/caffeine/codeine • Fioricet with Codeine
Mr. D, age 41, presents to the emergency department (ED) with altered mental status and suspected intoxication. His medical history includes alcohol use disorder and spinal injury. Upon initial examination, he is confused, disorganized, and agitated. He receives IM lorazepam 4 mg to manage his agitation. His laboratory workup includes a negative screening for blood alcohol, slightly elevated creatine kinase, and urine toxicology positive for barbiturates and opioids. During re-evaluation by the consulting psychiatrist the following morning, Mr. D is alert, oriented, and calm with an organized thought process. He does not appear to be in withdrawal from any substances and tells the psychiatrist that he takes butalbital/acetaminophen/caffeine/codeine as needed for migraines. Mr. D says that 3 days before he came to the ED, he also began taking a supplement called phenibut that he purchased online for “well-being and sleep.”
Natural substances have been used throughout history as medicinal agents, sacred substances in religious rituals, and for recreational purposes.1 Supplement use in the United States is prevalent, with 57.6% of adults age ≥20 reporting supplement use in the past 30 days.2 Between 2000 and 2017, US poison control centers recorded a 74.1% increase in calls involving exposure to natural psychoactive substances, mostly driven by cases involving marijuana in adults and adolescents.3 Like synthetic drugs, herbal supplements may have psychoactive properties, including sedative, stimulant, psychedelic, euphoric, or anticholinergic effects. The variety and unregulated nature of supplements makes managing patients who use supplements particularly challenging.
Why patients use supplements
People may use supplements to treat or prevent vitamin deficiencies (eg, vitamin D, iron, calcium). Other reasons may include for promoting wellness in various disease states, for weight loss, for recreational use or misuse, or for overall well-being. In the mental health realm, patients report using supplements to treat depression, anxiety, insomnia, memory, or for vague indications such as “mood support.”4,5
Patients may view supplements as appealing alternatives to prescription medications because they are widely accessible, may be purchased over-the-counter, are inexpensive, and represent a “natural” treatment option.6 For these reasons, they may also falsely perceive supplements as categorically safe.1 People with psychiatric diagnoses may choose such alternative treatments due to a history of adverse effects or treatment failure with traditional psychiatric medications, mistrust of the health care or pharmaceutical industry, or based on the recommendations of others.7
Regulation, safety, and efficacy of dietary supplements
In the US, dietary supplements are regulated more like food products than medications. Under the Dietary Supplement Health and Education Act of 1994, the FDA regulates the quality, safety, and labeling of supplements using Current Good Manufacturing Practice regulations.8 The Federal Trade Commission monitors advertisements and marketing. Despite some regulations, dietary supplements may be adulterated or contaminated, contain unknown or toxic ingredients, have inconsistent potencies, or be sold at toxic doses.9 Importantly, supplements are not required to be evaluated for clinical efficacy. As a result, it is not known if most supplements are effective in treating the conditions for which they are promoted, mainly due to a lack of financial incentive for manufacturers to conduct large, high-quality trials.5
Further complicating matters is the inconsistent labeling of supplements or similar products that are easily obtainable via the internet. These products might be marketed as nutritional supplements or nootropics, which often are referred to as “cognitive enhancers” or “smart drugs.” New psychoactive substances (NPS) are drugs of misuse or abuse developed to imitate illicit drugs or controlled drug substances.10 They are sometimes referred to as “herbal highs” or “legal highs.”11 Supplements may also be labeled as performance- or image-enhancing agents and may include medications marketed to promote weight loss. This includes herbal substances (Table12-19) and medications associated with neuropsychiatric adverse effects that may be easily accessible online without a prescription.12,20

The growing popularity of the internet and social media plays an important role in the availability of supplements and nonregulated substances and may contribute to misleading claims of efficacy and safety. While many herbal supplements are available in pharmacies or supplement stores, NPS are usually sold through anonymous, low-risk means either via traditional online vendors or the deep web (parts of the internet that are not indexed via search engines). Strategies to circumvent regulation and legislative control include labeling NPS as research chemicals, fertilizers, incense, bath salts, or other identifiers and marketing them as “not for human consumption.”21 Manufacturers frequently change the chemical structures of NPS, which allows these products to exist within a legal gray area due to the lag time between when a new compound hits the market and when it is categorized as a regulated substance.10
Continue to: Another category of "supplements"...
Another category of “supplements” includes medications that are not FDA-approved but are approved for therapeutic use in other countries and readily available in the US via online sources. Such medications include phenibut, a glutamic acid derivative that functions as a gamma-aminobutyric acid-B receptor agonist in the brain, spinal cord, and autonomic nervous system. Phenibut was developed in the Soviet Union in the 1960s, and outside of the US it is prescribed for anxiolysis and other psychiatric indications.22 In the US, phenibut may be used as a nootropic or as a dietary supplement to treat anxiety, sleep problems, and other psychiatric disorders.22 It may also be used recreationally to induce euphoria. Chronic phenibut use results in tolerance and abrupt discontinuation may mimic benzodiazepine withdrawal symptoms.13,22
Educating patients about supplements
One of the most critical steps in assessing a patient’s supplement use is to directly ask them about their use of herbal or over-the-counter products. Research has consistently shown that patients are unlikely to disclose supplement use unless they are specifically asked.23,24
Additional strategies include25,26:
- Approach patients without judgment; ask open-ended questions to determine their motivations for using supplements.
- Explain the difference between supplements medically necessary to treat vitamin deficiencies (eg, vitamin D, calcium, magnesium) and those without robust clinical evidence.
- Counsel patients that many supplements with psychoactive properties, if indicated, are generally meant to be used short-term and not as substitutes for prescription medications.
- Educate patients that supplements have limited evidence regarding their safety and efficacy, but like prescription medications, supplements may cause organ damage, adverse effects, and drug-drug interactions.
- Remind patients that commonly used nutritional supplements/dietary aids, including protein or workout supplements, may contain potentially harmful ingredients.
- Utilize evidence-based resources such as the Natural Medicines Comprehensive Database14 or the National Center for Complementary and Integrative Health (https://www.nccih.nih.gov) to review levels of evidence and educate patients.
- When toxicity or withdrawal is suspected, reach out to local poison control centers for guidance.
- For a patient with a potential supplement-related substance use disorder, urine drug screens may be of limited utility and evidence is often sparse; clinicians may need to rely on primary literature such as case reports to guide management.
- If patients wish to continue taking a supplement, recommend they purchase supplements from manufacturers that have achieved the US Pharmacopeia (USP) verification mark. Products with the USP mark undergo quality assurance measures to ensure the product contains the ingredients listed on the label in the declared potency and amounts, does not contain harmful levels of contaminants, will be metabolized in the body within a specified amount of time, and has been produced in keeping with FDA Current Good Manufacturing Practice regulations.
CASE CONTINUED
In the ED, the consulting psychiatry team discusses Mr. D’s use of phenibut with him, and asks if he uses any additional supplements or nonprescription medications. Mr. D discloses he has been anxious and having trouble sleeping, and a friend recommended phenibut as a safe, natural alternative to medication. The team explains to Mr. D that phenibut’s efficacy has not been studied in the US and that based on available evidence, it is likely unsafe. It may have serious adverse effects, drug-drug interactions, and is potentially addictive.
Mr. D says he was unaware of these risks and agrees to stop taking phenibut. The treatment team discharges him from the ED with a referral for outpatient psychiatric services to address his anxiety and insomnia.
Related Resources
- Tillman B. The hidden dangers of supplements: a case of substance-induced psychosis. Current Psychiatry. 2020; 19(7):e7-e8. doi:10.12788/cp.0018
- McQueen CE. Herb–drug interactions: caution patients when changing supplements. Current Psychiatry. 2017; 16(6):38-41.
Drug Brand Names
Butalbital/acetaminophen/caffeine/codeine • Fioricet with Codeine
1. Graziano S, Orsolini L, Rotolo MC, et al. Herbal highs: review on psychoactive effects and neuropharmacology. Curr Neuropharmacol. 2017;15(5):750-761.
2. Mishra S, Stierman B, Gahche JJ, et al. Dietary supplement use among adults: United States, 2017-2018. NCHS Data Brief. 2021;(399):1-8.
3. O’Neill-Dee C, Spiller HA, Casavant MJ, et al. Natural psychoactive substance-related exposures reported to United States poison control centers, 2000-2017. Clin Toxicol (Phila). 2020;58(8):813-820.
4. Gray DC, Rutledge CM. Herbal supplements in primary care: patient perceptions, motivations, and effects on use. Holist Nurs Pract. 2013;27(1):6-12.
5. Wu K, Messamore E. Reimagining roles of dietary supplements in psychiatric care. AMA J Ethics. 2022;24(5):E437-E442.
6. Snyder FJ, Dundas ML, Kirkpatrick C, et al. Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. J Nutr Elder. 2009;28(1):81-95.
7. Schulz P, Hede V. Alternative and complementary approaches in psychiatry: beliefs versus evidence. Dialogues Clin Neurosci. 2018;20(3):207-214.
8. Dietary Supplement Health and Education Act of 1994, Pub L 103-417, 103rd Cong (1993-1994).
9. Starr RR. Too little, too late: ineffective regulation of dietary supplements in the United States. Am J Public Health. 2015;105(3):478-485.
10. New psychoactive substances. Alcohol and Drug Foundation. November 10, 2021. Updated November 28, 2022. Accessed January 25, 2023. https://adf.org.au/drug-facts/new-psychoactive-substances/
11. Shafi A, Berry AJ, Sumnall H, et al. New psychoactive substances: a review and updates. Ther Adv Psychopharmacol. 2020;10:2045125320967197.
12. Bersani FS, Coviello M, Imperatori C, et al. Adverse psychiatric effects associated with herbal weight-loss products. Biomed Res Int. 2015;2015:120679.
13. IBM Micromedex POISINDEX® System. IBM Watson Health. Accessed October 3, 2022. https://www.micromedexsolutions.com
14. Natural Medicines Comprehensive Database. Therapeutic Research Center. Accessed October 3, 2022. https://naturalmedicines.therapeuticresearch.com
15. Savage KM, Stough CK, Byrne GJ, et al. Kava for the treatment of generalised anxiety disorder (K-GAD): study protocol for a randomised controlled trial. Trials. 2015;16:493.
16. Swogger MT, Smith KE, Garcia-Romeu A, et al. Understanding kratom use: a guide for healthcare providers. Front Pharmacol. 2022;13:801855.
17. Modabbernia A, Akhondzadeh S. Saffron, passionflower, valerian and sage for mental health. Psychiatr Clin North Am. 2013;36(1):85-91.
18. Coffeen U, Pellicer F. Salvia divinorum: from recreational hallucinogenic use to analgesic and anti-inflammatory action. J Pain Res. 2019;12:1069-1076.
19. National Institutes of Health, Office of Dietary Supplements. Valerian Fact Sheet for Health Professionals. Updated March 15, 2013. Accessed January 25, 2023. https://ods.od.nih.gov/factsheets/Valerian-HealthProfessional
20. An H, Sohn H, Chung S. Phentermine, sibutramine and affective disorders. Clin Psychopharmacol Neurosci. 2013;11(1):7-12.
21. Miliano C, Margiani G, Fattore L, et al. Sales and advertising channels of new psychoactive substances (NPS): internet, social networks, and smartphone apps. Brain Sci. 2018;8(7):123.
22. Hardman MI, Sprung J, Weingarten TN. Acute phenibut withdrawal: a comprehensive literature review and illustrative case report. Bosn J Basic Med Sci. 2019;19(2):125-129.
23. Guzman JR, Paterniti DA, Liu Y, et al. Factors related to disclosure and nondisclosure of dietary supplements in primary care, integrative medicine, and naturopathic medicine. J Fam Med Dis Prev. 2019;5(4):10.23937/2469-5793/1510109.
24. Foley H, Steel A, Cramer H, et al. Disclosure of complementary medicine use to medical providers: a systematic review and meta-analysis. Sci Rep. 2019;9(1):1573.
25. Aldridge Young C. ‘No miracle cures’: counseling patients about dietary supplements. Pharmacy Today. 2014;February:35.
26. United States Pharmacopeia. USP Verified Mark. Accessed January 25, 2023. https://www.usp.org/verification-services/verified-mark
1. Graziano S, Orsolini L, Rotolo MC, et al. Herbal highs: review on psychoactive effects and neuropharmacology. Curr Neuropharmacol. 2017;15(5):750-761.
2. Mishra S, Stierman B, Gahche JJ, et al. Dietary supplement use among adults: United States, 2017-2018. NCHS Data Brief. 2021;(399):1-8.
3. O’Neill-Dee C, Spiller HA, Casavant MJ, et al. Natural psychoactive substance-related exposures reported to United States poison control centers, 2000-2017. Clin Toxicol (Phila). 2020;58(8):813-820.
4. Gray DC, Rutledge CM. Herbal supplements in primary care: patient perceptions, motivations, and effects on use. Holist Nurs Pract. 2013;27(1):6-12.
5. Wu K, Messamore E. Reimagining roles of dietary supplements in psychiatric care. AMA J Ethics. 2022;24(5):E437-E442.
6. Snyder FJ, Dundas ML, Kirkpatrick C, et al. Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. J Nutr Elder. 2009;28(1):81-95.
7. Schulz P, Hede V. Alternative and complementary approaches in psychiatry: beliefs versus evidence. Dialogues Clin Neurosci. 2018;20(3):207-214.
8. Dietary Supplement Health and Education Act of 1994, Pub L 103-417, 103rd Cong (1993-1994).
9. Starr RR. Too little, too late: ineffective regulation of dietary supplements in the United States. Am J Public Health. 2015;105(3):478-485.
10. New psychoactive substances. Alcohol and Drug Foundation. November 10, 2021. Updated November 28, 2022. Accessed January 25, 2023. https://adf.org.au/drug-facts/new-psychoactive-substances/
11. Shafi A, Berry AJ, Sumnall H, et al. New psychoactive substances: a review and updates. Ther Adv Psychopharmacol. 2020;10:2045125320967197.
12. Bersani FS, Coviello M, Imperatori C, et al. Adverse psychiatric effects associated with herbal weight-loss products. Biomed Res Int. 2015;2015:120679.
13. IBM Micromedex POISINDEX® System. IBM Watson Health. Accessed October 3, 2022. https://www.micromedexsolutions.com
14. Natural Medicines Comprehensive Database. Therapeutic Research Center. Accessed October 3, 2022. https://naturalmedicines.therapeuticresearch.com
15. Savage KM, Stough CK, Byrne GJ, et al. Kava for the treatment of generalised anxiety disorder (K-GAD): study protocol for a randomised controlled trial. Trials. 2015;16:493.
16. Swogger MT, Smith KE, Garcia-Romeu A, et al. Understanding kratom use: a guide for healthcare providers. Front Pharmacol. 2022;13:801855.
17. Modabbernia A, Akhondzadeh S. Saffron, passionflower, valerian and sage for mental health. Psychiatr Clin North Am. 2013;36(1):85-91.
18. Coffeen U, Pellicer F. Salvia divinorum: from recreational hallucinogenic use to analgesic and anti-inflammatory action. J Pain Res. 2019;12:1069-1076.
19. National Institutes of Health, Office of Dietary Supplements. Valerian Fact Sheet for Health Professionals. Updated March 15, 2013. Accessed January 25, 2023. https://ods.od.nih.gov/factsheets/Valerian-HealthProfessional
20. An H, Sohn H, Chung S. Phentermine, sibutramine and affective disorders. Clin Psychopharmacol Neurosci. 2013;11(1):7-12.
21. Miliano C, Margiani G, Fattore L, et al. Sales and advertising channels of new psychoactive substances (NPS): internet, social networks, and smartphone apps. Brain Sci. 2018;8(7):123.
22. Hardman MI, Sprung J, Weingarten TN. Acute phenibut withdrawal: a comprehensive literature review and illustrative case report. Bosn J Basic Med Sci. 2019;19(2):125-129.
23. Guzman JR, Paterniti DA, Liu Y, et al. Factors related to disclosure and nondisclosure of dietary supplements in primary care, integrative medicine, and naturopathic medicine. J Fam Med Dis Prev. 2019;5(4):10.23937/2469-5793/1510109.
24. Foley H, Steel A, Cramer H, et al. Disclosure of complementary medicine use to medical providers: a systematic review and meta-analysis. Sci Rep. 2019;9(1):1573.
25. Aldridge Young C. ‘No miracle cures’: counseling patients about dietary supplements. Pharmacy Today. 2014;February:35.
26. United States Pharmacopeia. USP Verified Mark. Accessed January 25, 2023. https://www.usp.org/verification-services/verified-mark
Discontinuing a long-acting injectable antipsychotic: What to consider
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.
Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.
No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.

Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.

No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
Mr. R, age 29, was diagnosed with schizophrenia 6 years ago. To manage his disorder, he has been receiving paliperidone palmitate long-acting injectable (LAI) 156 mg once a month for 2 years. Prior to maintenance with paliperidone palmitate, Mr. R was stabilized on oral paliperidone 9 mg/d. Though he was originally initiated on paliperidone palmitate due to nonadherence concerns, Mr. R has been adherent with each injection for 1 year.
At a recent visit, Mr. R says he wants to discontinue the injection because he is not interested in receiving an ongoing injectable medication and is not able to continue monthly clinic visits. He wants to take a daily oral antipsychotic again, despite the availability of longer-acting products.
A paucity of evidence exists regarding the discontinuation of LAI antipsychotics and the next steps that follow in treatment. There is neither a consensus nor recognized guidelines advising how and when to discontinue an LAI and restart an oral antipsychotic. A recent systematic review and meta-analysis evaluated different maintenance treatment strategies; however, switching from an LAI antipsychotic to an oral medication was not a focus.1 In this article, we outline a possible approach to discontinuing an LAI antipsychotic and restarting an oral formulation. Before discontinuing an LAI antipsychotic, clinicians should review with the patient the risks and benefits of switching medications, including the risk of decompensation and potential adverse effects.
Switching to an oral antipsychotic
The first step in the discontinuation process is to determine whether the patient will continue the same oral medication as the LAI antipsychotic or if a different oral antipsychotic will be initiated. Next, determining when to initiate the oral medication requires several pieces of information, including the oral dose equivalent of the patient’s current LAI, the half-life of the LAI, and the release mechanism of the LAI (Table 1).2-5 To determine the appropriate time frame for restarting oral treatment, it is also vital to know the date of the last injection.

Based on the date of the next injection, the clinician will utilize the LAI’s half-life and its release mechanism to determine the appropriate time to start a new oral antipsychotic. Research demonstrates that in patients who have achieved steady state with a first-generation antipsychotic, plasma concentrations stay relatively consistent for 6 to 7 weeks after the last injection, which suggests oral medications may not need to be initiated until that time.6-9
For many second-generation LAI antipsychotics, oral medications may be initiated at the date of the next injection. Initiation of an oral antipsychotic may require more time between the last injection dose and the date of administration for oral medication due to the pharmacokinetic profile of risperidone microspheres. Once a patient is at steady state with risperidone microspheres, trough levels are not observed until 3 to 4 weeks after discontinuation.10
Previous pharmacokinetic model–based stimulations of active moiety plasma concentrations of risperidone microspheres demonstrate that 2 weeks after an injection of risperidone microspheres, the concentration of active moiety continued to approximate the steady-state concentration for 3 to 5 weeks.11 This is likely due to the product’s delay in release being 3 weeks from the time of injection to the last release phase. Of note, there was a rapid decline in the active moiety concentration; it reached nearly 0 by Week 5.11 The same pharmacokinetic model–based stimulation demonstrated a steady and slow decline of the concentration of active moiety of paliperidone palmitate after discontinuation of the LAI.11
Continue to: No guidance exists for...
No guidance exists for aripiprazole LAI medications; however, based on the pharmacokinetic data, administration of oral medications should be initiated at the date of next injection. Given the long half-life of aripiprazole, a cross-titration of the LAI with oral medication is reasonable.
Monitoring drug levels
In addition to utilizing the pharmacokinetic data from LAI antipsychotics, therapeutic drug levels can be instrumental in determining the dose of oral medication to use and when to begin titration (Table 2).12-14 Obtaining a drug level on the date of the next injection can provide the clinician with data regarding the release of the medication specific to the patient. Based on the level and the current symptomatology, the clinician could choose to start the oral medication at a lower dose and titrate back to the LAI equivalent oral dose, or initiate the oral dose at the LAI equivalent oral dose. Continued therapeutic drug levels can aid in this determination.

No guidance exists on the appropriate discontinuation of LAI antipsychotics. Utilizing a medication’s half-life and release mechanism, as well as the patient’s previous medication history, date of last injection, and therapeutic drug levels, should be considered when determining the schedule for restarting an oral antipsychotic.
CASE CONTINUED
Based on the current dosing of paliperidone palmitate of 156 mg once a month, Mr. R likely requires 9 mg/d of oral paliperidone upon discontinuation of the LAI. On the date of the next injection, the clinician could decide to initiate a lower dose of paliperidone, such as to 3 mg/d or 6 mg/d, and increase the dose as tolerated over the next 10 to 14 days as the paliperidone palmitate is further metabolized. Additionally, the clinician may consider obtaining a therapeutic drug level to determine the current paliperidone level prior to initiating the oral medication. Each treatment option offers individual risks and benefits. The decision on when and how to initiate the oral medication will be based on the individual patient’s situation and history, as well as the comfort and discretion of the clinician. The clinician should arrange appropriate monitoring for potential increased symptomatology during the transition, and adverse effects should be assessed regularly until steady state is achieved with the targeted oral dose of medication.
Related Resources
- Parmentier BL. Second-generation long-acting injectable antipsychotics: a practical guide. Current Psychiatry. 2020;19(3):24-32.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Current Psychiatry. 2021;20(4):13-14. doi:10.12788/cp.0103
Drug Brand Names
Aripiprazole lauroxil • Aristada
Aripiprazole monohydrate • Maintena
Haloperidol injection • Haldol decanoate
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate once monthly • Invega Sustenna
Paliperidone palmitate every 3 months • Invega Trinza
Paliperidone palmitate every 6 months • Invega Hafyera
Risperidone microspheres • Risperdal Consta
Risperidone polymer • Perseris
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
1. Ostuzzi G, Vita G, Bertolini F, et al. Continuing, reducing, switching, or stopping antipsychotics in individuals with schizophrenia-spectrum disorders who are clinically stable: a systematic review and network meta-analysis. Lancet Psychiatry. 2022;9(8):614-624.
2. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39-59.
3. Spanarello S, La Ferla T. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9(3):310-317.
4. Meyer JM. Understanding depot antipsychotics: an illustrated guide to kinetics. CNS Spectr. 2013;18(Suppl 1):58-68.
5. Invega Hafyera [package insert]. Janssen Pharmaceuticals, Inc; 2021.
6. Gitlin MJ, Midha KK, Fogelson D, et al. Persistence of fluphenazine in plasma after decanoate withdrawal. J Clin Psychopharmacol. 1988;8(1):53-56.
7. Wistedt B, Jørgensen A, Wiles D. A depot neuroleptic withdrawal study. Plasma concentration of fluphenazine and flupenthixol and relapse frequency. Psychopharmacology. 1982;78(4):301-304.
8. Chang WH, Lin SK, Juang DJ, et al. Prolonged haloperidol and reduced haloperidol plasma concentrations after decanoate withdrawal. Schizophr Res. 1993;9(1):35-40.
9. Eklund K, Forsman A. Minimal effective dose and relapse—double-blind trial: haloperidol decanoate vs. placebo. Clin Neuropharmacol. 1991;1(Suppl 2):S7-S15.
10. Wilson WH. A visual guide to expected blood levels of long-acting injectable risperidone in clinical practice. J Psychiatry Pract. 2004;10(6):393-401.
11. Samtani MN, Sheehan JJ, Fu DJ, et al. Management of antipsychotic treatment discontinuation and interruptions using model-based simulations. Clin Pharmacol. 2012;4:25-40.
12. Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
13. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-2):9-62.
14. Meyer JM, Stahl SM. The Clinical Use of Antipsychotic Plasma Levels. Cambridge University Press; 2021.
Transitioning patients with opioid use disorder from methadone to buprenorphine
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
Mr. M, age 46, has opioid use disorder (OUD). He is currently stabilized on methadone 80 mg/d but presents to your hospital with uncontrolled atrial fibrillation. After Mr. M is admitted, the care team looks to start amiodarone; however, they receive notice of a drug-drug interaction that may cause QTc prolongation. Mr. M agrees to switch to another medication to treat his OUD because he is tired of the regulated process required to receive methadone. The care team would like to taper him to a different OUD medication but would like Mr. M to avoid cravings, symptoms of withdrawal, and potential relapse.
The opioid epidemic has devastated the United States, causing approximately 130 deaths per day.1 The economic burden of this epidemic on medical, social welfare, and correctional services is approximately $1 trillion annually.2 Research supports opioid replacement therapy for treating OUD.1 Multiple types of opioid replacement therapies are available in multiple dosage forms; all act on the mu-opioid receptor. These include full agonist treatment (eg, methadone) and partial agonist treatment (eg, buprenorphine).3 Alternatively, opioid antagonist therapies (eg, naltrexone) have also been found to be effective for treating OUD.1,2,4 This article focuses on partial agonist treatment for OUD, specifically using a buprenorphine microdosing strategy to transition a patient from methadone to buprenorphine.
Buprenorphine for OUD
Buprenorphine binds with high affinity to the mu-opioid receptor, resulting in partial agonism of the receptor.1,2 Buprenorphine has a higher therapeutic index and lower intrinsic agonist activity than other opioids and a low incidence of adverse effects. Due to the partial agonism at the mu receptor, its analgesic effects plateau at higher doses and exhibit antagonist properties.1,2 This distinct “ceiling” effect, combined with a lower risk of respiratory depression, makes buprenorphine significantly safer than methadone.4 Additionally, it has a lower potential for misuse when used with an abuse deterrent such as naloxone.
Common reasons for transitioning a patient from methadone to buprenorphine include intolerable adverse effects of methadone, variable duration of efficacy, drug-drug interactions, or limited access to an opioid treatment program. Traditional buprenorphine induction requires moderate withdrawal before initiating therapy. Due to buprenorphine’s high affinity and partial agonism at the mu receptor, it competes with other opioids (eg, heroin, methadone) and will abruptly displace the receptor’s full agonist with a lower affinity, resulting in precipitated withdrawal.1,3,5 To avoid precipitated withdrawal, it is recommended to leave a sufficient amount of time between full opioid agonist treatment and buprenorphine treatment, a process called “opioid washout.”1,5 Depending on the duration, amount, and specific opioid used, the amount of time between ending opioid agonist treatment and initiating buprenorphine treatment may vary. As a result, many patients who attempt to transition from methadone to buprenorphine remain on methadone due to their inability to tolerate withdrawal. Additionally, given the risk of precipitating withdrawal, initiating buprenorphine may negatively impact pain control.1
Recently, buprenorphine “microdosing” inductions, which do not require patients to be in opioid withdrawal, have been used to overcome some of the challenges of transitioning patients from methadone to buprenorphine.2
Buprenorphine microdosing techniques
Multiple methods of microdosing buprenorphine have been used in both inpatient and outpatient settings.
Bernese method. In 1997, Mendelson et al6 completed a trial with 5 patients maintained on methadone. They found that IV buprenorphine 0.2 mg every 24 hours did not produce a withdrawal effect and was comparable to placebo.6 Haamig et al5 hypothesized that repetitive administration of buprenorphine at minute doses in adequate dosing intervals would not cause withdrawal. Additionally, because of its high receptor binding affinity, buprenorphine will accumulate over time at the mu receptor. Thus, eventually the full mu agonist (eg, methadone) will be replaced by buprenorphine at the mu receptor as the receptor becomes saturated.4,5
Continue to: The goal is to taper...
The goal is to taper the opioid agonist therapy while titrating buprenorphine. This taper method is not described in current treatment guidelines, and as a result, there are differences in doses used in each taper because the amount of opioid agonist and type of opioid agonist therapy can vary. In most cases, buprenorphine is initiated at 0.25 mg/d to 0.5 mg/d and increased by 0.25 mg/d to 1 mg/d as tolerated.4,5 The dose of the full opioid agonist is slowly decreased as the buprenorphine dose increases. The Bernese method does not require frequent dosing, so it is a favorable option for outpatient therapy.4 One limitation to this method is that it is necessary to divide tablets into small doses.4 Additionally, adherence issues may disrupt the tapering method; therefore, some patients may not be appropriate candidates.4
Transdermal patch method. This method aims to provide a consistent amount of buprenorphine—similar to dividing tablets into smaller doses as seen in the Bernese method—but with the goal of avoiding inconsistencies in dosing. Hess et al7 examined 22 patients with OUD who were maintained on methadone 60 mg/d to 100 mg/d. In the buprenorphine transdermal patch method, a 35 mcg/h buprenorphine patch was applied 12 hours after the patient’s final methadone dose.1,7 This was intended to provide continuous delivery over 96 hours.1 Additionally, small, incremental doses of sublingual buprenorphine (SL-BUP) were administered throughout the course of 5 days.1 A potential strength of this method is that like the Bernese method, it may be completed in outpatient therapy.4 Potential limitations include time to initiation, off-label use, and related costs.
Rapid microdosing induction method. Contrary to typical microdosing, rapid microdosing induction requires buprenorphine to be administered every 3 to 4 hours.4 As with most buprenorphine microinduction protocols, this does not require a period of withdrawal prior to initiation and may be performed because of the 1-hour time to peak effect of buprenorphine.4 Due to the frequent dosing schedule, it is recommended to use this method in an inpatient setting.4 With rapid microdosing, an individual may receive SL-BUP 0.5 mg every 3 hours on Day 1, then 1 mg SL-BUP every 3 hours on Day 2. On Day 3, the individual may receive 12 mg SL-BUP with 2 mg as needed. A limitation of this method is that it must be performed in an inpatient setting.4
CASE CONTINUED
To ensure patient-inclusive care, clinicians should conduct a risk-benefit discussion with the patient regarding microdosing buprenorphine. Because Mr. M would like to be managed as an outpatient, rapid microdosing is not an option. Mr. M works with his care team to design a microdosing approach with the Bernese method. They initiate buprenorphine 0.5 mg/d and increase the dose by 0.5 mg to 1 mg from Day 2 to Day 8. The variance in buprenorphine titration occurs due to Mr. M’s tolerance and symptoms of withdrawal. The team decreases the methadone dose by 5 mg to 10 mg each day, depending on symptoms of withdrawal, and discontinues therapy on Day 8. Throughout the microdosing induction, Mr. M does not experience withdrawal symptoms and is now managed on buprenorphine 12 mg/d.
Related Resources
- Van Hale C, Gluck R, Tang Y. Laboratory monitoring for patients on buprenorphine: 10 questions. Current Psychiatry. 2022;21(9):12-15,20-21,26.
- Moreno JL, Johnson JL, Peckham AM. Sublingual buprenorphine plus buprenorphine XR for opioid use disorder. Current Psychiatry. 2022;21(6):39-42,49.
Drug Brand Names
Amiodarone • Cordarone
Buprenorphine • Subutex, Sublocade
Buprenorphine/naloxone • Suboxone, Zubsolv
Methadone • Dolophine, Methadose
Naltrexone • ReVia, Vivitrol
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.
1. Ahmed S, Bhivandkar S, Lonergan B, et al. Microinduction of buprenorphine/naloxone: a review of the literature. Am J Addict. 2021;30:305-315.
2. De Aquino JP, Fairgrieve C, Klair S, et al. Rapid transition from methadone to buprenorphine utilizing a micro-dosing protocol in the outpatient veteran affairs setting. J Addict Med. 2020;14:e271-e273.
3. Lintzeris N, Monds LA, Rivas C, et al. Transferring patients from methadone to buprenorphine: the feasibility and evaluation of practice guidelines. J Addict Med. 2018;12(3):234-240.
4. Ghosh SM, Klaire S, Tanguay R, et al. A review of novel methods to support the transition from methadone and other full agonist opioids to buprenorphine/naloxone sublingual in both community and acute care settings. Can J Addict. 2019;10:41-50.
5. Haamig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105.
6. Mendelson J, Jones RT, Welm S, et al. Buprenorphine and naloxone interactions in methadone maintenance patients. Biol Psychiatry. 1997;41:1095-1101.
7. Hess M, Boesch L, Leisinger R, et al. Transdermal buprenorphine to switch patients from higher dose methadone to buprenorphine without severe withdrawal symptoms. Am J Addict. 2011;20(5):480‐481.