User login
Counting electric sheep: Dreaming of AI in sleep medicine
“Artificial intelligence (AI) in healthcare refers to the use of machine learning (ML), deep learning, natural language processing, and computer vision to process and analyze large amounts of health care data.”
The preceding line is a direct quote from ChatGPT when prompted with the question “What is AI in health care?” (OpenAI, 2022). AI has rapidly infiltrated our lives. From using facial recognition software to unlock our cellphones to scrolling through targeted media suggested by streaming services, our daily existence is interwoven with algorithms. With the recent introduction of GPT-3 (the model that powers ChatGPT) in late 2022 and its even more capable successor, GPT-4, in March 2023, AI will continue to dominate our everyday environment in even more complex and meaningful ways.
For sleep medicine, the initial applications of AI in this field have been innovative and promising. (OSA) (Bandyopadhyay A, et al. Sleep Breath. 2023;27[1]:39). Pépin and colleagues (JAMA Netw Open. 2020;3[1]:e1919657) combined ML with mandibular movement to diagnose OSA with a reasonable agreement to polysomnography as a novel home-based alternative for diagnosis. AI has also been used to predict adherence to positive airway pressure (PAP) therapy in OSA (Scioscia G, et al. Inform Health Soc Care. 2022;47[3]:274) and as a digital intervention tool accessed via a smartphone app for people with insomnia (Philip P, et al, J Med Internet Res. 2020;22[12]:e24268). The data-rich field of sleep medicine is primed for further advancements through AI, albeit with a few hurdles and regulations to overcome before becoming mainstream.
Future promise
Sleep medicine is uniquely positioned to develop robust AI algorithms because of its vast data trove. Using AI, scientists can efficiently analyze the raw data from polysomnography, consumer sleep technology (CST), and nightly remote monitoring (from PAP devices) to substantially improve comprehension and management of sleep disorders.
AI can redefine OSA through analysis of the big data available, rather than solely relying on the apnea-hypopnea index. In addition, novel variables such as facial structure; snoring index; temperature trends; and sleep environment, position, and timing using a camera-based contactless technology may be incorporated to enhance the diagnostic accuracy for OSA or better describe sleep quality. AI algorithms can also be embedded into the electronic health record (EHR) to facilitate screening for sleep disorders using patient characteristics, thus accelerating the recognition and evaluation of possible sleep disorders.
New ways of collecting data may deliver deeper insights into sleep health, as well. CST such as wearables, nearables, and phone applications are improving with each iteration, resulting in more data about sleep for millions of people over thousands of nights.
AI can help achieve precision medicine by integrating multimodal data to establish endotypes and phenotypes of various sleep disorders. Delineating endotypes and phenotypes allows for personalized treatment recommendations, which may improve patient adherence and health outcomes.
Treatment personalization can also be achieved through AI by predicting compliance to various therapies and responses, as well as by discovering alternative forms of delivery to accomplish desired health outcomes. For example, to predict PAP compliance, we can record a patient encounter and use natural language processing to analyze their opinion of their treatment, extracting relevant keywords and combining such processing with other available data, such as environmental factors, sleep schedule, medical history, and other information extracted from the EHR. As another example, AI can determine the optimal time for cancer therapy by predicting a patient’s circadian timing (Hesse J, et al. Cancers (Basel). 2020;12[11]:3103). Circadian timing of drug delivery may be relevant in other specialties including cardiovascular disease, endocrine disorders, and psychiatric conditions due to its associations with sleep. Integration of the various “-omics” (eg, proteomics, genomics, and transcriptomics) with physiologic, behavioral, and environmental data can offer opportunities for drug discovery and possible prediction of sleep disorders and sleep-related morbidity. Although generative pretrained transformers are currently used to predict text (ie, ChatGPT), it is theoretically possible to also apply this technique to identify patients at risk for future sleep disorders from an earlier age.
Challenges to an AI renaissance
Despite making strides in numerous specialties such as radiology, ophthalmology, pathology, oncology, and dermatology, AI has not yet gained mainstream usage. Why isn’t AI as ubiquitous and heavily entrenched in health care as it is in other industries? According to the National Academy of Medicine’s AI in Healthcare: The Hope, The Hype, The Promise, The Peril, there are several realities to address before we fully embrace the AI revolution (Matheny M, et al. 2019).
First, AI algorithms should be trained on quality data that are representative of the population. Interoperability between health care systems and standardization across platforms is required to access large volumes of quality data. The current framework for data gathering is limited due to regulations, patient privacy concerns, and organizational preferences. The challenges to data acquisition and standardization of information will continue to snarl progress unless there are legislative remedies.
Furthermore, datasets should be diverse enough to avoid introducing bias into the AI algorithm. If the dataset is limited and health inequities (eg, societal bias and social determinants of health) are excluded from the training set, then the outcome will perpetuate further explicit and implicit biases.
The Food and Drug Administration (FDA) reviews and authorizes AI/ML-enabled devices. Its current regulatory structure treats AI as a static process and does not allow for exercise of its intrinsic ability to continuously learn from additional data, thereby preventing it from becoming more accurate and evolving with the population over time. A more flexible approach is needed.
Lastly, recent advanced AI algorithms including deep learning and neural network methodology function like a “black box.” The models are not explainable or transparent. Without clear comprehension of its methods, acceptance in clinical practice will be guarded and further risk of inherent biases may ensue.
A path forward
But these challenges, like any, can be overcome. Research in the area of differential privacy and the adoption of recent data-sharing standards (eg, HL7 FHIR) can facilitate access to training data (Saripalle R, et al. J Biomed Inform. 2019;94:103188). Regulators are also open to incorporating feedback from the AI research community and industry in favor of innovation in this frenetic domain. The FDA developed the AI/ML Software as a Medical Device Action Plan in response to stakeholder feedback for oversight (FDA, 2021). Specifically, the “Good Machine Learning Practice” will be developed to describe AI/ML best practices (eg, data management, training, interpretability, evaluation, and documentation) to guide product development and standardization.
Sleep medicine has significantly progressed over the last several decades. Rather than maintain the status quo, AI can help fill the existing knowledge gaps, augment clinical practice, and streamline operations by analyzing and processing data at a volume and efficiency beyond human capacity. Fallibility is inevitable in machines and humans; however, like humans, machines can improve with continued training and exposure.
We asked ChatGPT about the future of AI in sleep medicine. It states that AI could have a “significant impact” on sleep disorders diagnosis, treatment, prevention, and sleep tracking and monitoring. Only time will tell if its claims are accurate.
Dr. Tan is Clinical Associate Professor with the Division of Sleep Medicine at the Stanford University School of Medicine. Dr. Bhargava is Clinical Professor with the Division of Pediatric Pulmonary, Asthma, and Sleep Medicine at the Stanford University School of Medicine.
“Artificial intelligence (AI) in healthcare refers to the use of machine learning (ML), deep learning, natural language processing, and computer vision to process and analyze large amounts of health care data.”
The preceding line is a direct quote from ChatGPT when prompted with the question “What is AI in health care?” (OpenAI, 2022). AI has rapidly infiltrated our lives. From using facial recognition software to unlock our cellphones to scrolling through targeted media suggested by streaming services, our daily existence is interwoven with algorithms. With the recent introduction of GPT-3 (the model that powers ChatGPT) in late 2022 and its even more capable successor, GPT-4, in March 2023, AI will continue to dominate our everyday environment in even more complex and meaningful ways.
For sleep medicine, the initial applications of AI in this field have been innovative and promising. (OSA) (Bandyopadhyay A, et al. Sleep Breath. 2023;27[1]:39). Pépin and colleagues (JAMA Netw Open. 2020;3[1]:e1919657) combined ML with mandibular movement to diagnose OSA with a reasonable agreement to polysomnography as a novel home-based alternative for diagnosis. AI has also been used to predict adherence to positive airway pressure (PAP) therapy in OSA (Scioscia G, et al. Inform Health Soc Care. 2022;47[3]:274) and as a digital intervention tool accessed via a smartphone app for people with insomnia (Philip P, et al, J Med Internet Res. 2020;22[12]:e24268). The data-rich field of sleep medicine is primed for further advancements through AI, albeit with a few hurdles and regulations to overcome before becoming mainstream.
Future promise
Sleep medicine is uniquely positioned to develop robust AI algorithms because of its vast data trove. Using AI, scientists can efficiently analyze the raw data from polysomnography, consumer sleep technology (CST), and nightly remote monitoring (from PAP devices) to substantially improve comprehension and management of sleep disorders.
AI can redefine OSA through analysis of the big data available, rather than solely relying on the apnea-hypopnea index. In addition, novel variables such as facial structure; snoring index; temperature trends; and sleep environment, position, and timing using a camera-based contactless technology may be incorporated to enhance the diagnostic accuracy for OSA or better describe sleep quality. AI algorithms can also be embedded into the electronic health record (EHR) to facilitate screening for sleep disorders using patient characteristics, thus accelerating the recognition and evaluation of possible sleep disorders.
New ways of collecting data may deliver deeper insights into sleep health, as well. CST such as wearables, nearables, and phone applications are improving with each iteration, resulting in more data about sleep for millions of people over thousands of nights.
AI can help achieve precision medicine by integrating multimodal data to establish endotypes and phenotypes of various sleep disorders. Delineating endotypes and phenotypes allows for personalized treatment recommendations, which may improve patient adherence and health outcomes.
Treatment personalization can also be achieved through AI by predicting compliance to various therapies and responses, as well as by discovering alternative forms of delivery to accomplish desired health outcomes. For example, to predict PAP compliance, we can record a patient encounter and use natural language processing to analyze their opinion of their treatment, extracting relevant keywords and combining such processing with other available data, such as environmental factors, sleep schedule, medical history, and other information extracted from the EHR. As another example, AI can determine the optimal time for cancer therapy by predicting a patient’s circadian timing (Hesse J, et al. Cancers (Basel). 2020;12[11]:3103). Circadian timing of drug delivery may be relevant in other specialties including cardiovascular disease, endocrine disorders, and psychiatric conditions due to its associations with sleep. Integration of the various “-omics” (eg, proteomics, genomics, and transcriptomics) with physiologic, behavioral, and environmental data can offer opportunities for drug discovery and possible prediction of sleep disorders and sleep-related morbidity. Although generative pretrained transformers are currently used to predict text (ie, ChatGPT), it is theoretically possible to also apply this technique to identify patients at risk for future sleep disorders from an earlier age.
Challenges to an AI renaissance
Despite making strides in numerous specialties such as radiology, ophthalmology, pathology, oncology, and dermatology, AI has not yet gained mainstream usage. Why isn’t AI as ubiquitous and heavily entrenched in health care as it is in other industries? According to the National Academy of Medicine’s AI in Healthcare: The Hope, The Hype, The Promise, The Peril, there are several realities to address before we fully embrace the AI revolution (Matheny M, et al. 2019).
First, AI algorithms should be trained on quality data that are representative of the population. Interoperability between health care systems and standardization across platforms is required to access large volumes of quality data. The current framework for data gathering is limited due to regulations, patient privacy concerns, and organizational preferences. The challenges to data acquisition and standardization of information will continue to snarl progress unless there are legislative remedies.
Furthermore, datasets should be diverse enough to avoid introducing bias into the AI algorithm. If the dataset is limited and health inequities (eg, societal bias and social determinants of health) are excluded from the training set, then the outcome will perpetuate further explicit and implicit biases.
The Food and Drug Administration (FDA) reviews and authorizes AI/ML-enabled devices. Its current regulatory structure treats AI as a static process and does not allow for exercise of its intrinsic ability to continuously learn from additional data, thereby preventing it from becoming more accurate and evolving with the population over time. A more flexible approach is needed.
Lastly, recent advanced AI algorithms including deep learning and neural network methodology function like a “black box.” The models are not explainable or transparent. Without clear comprehension of its methods, acceptance in clinical practice will be guarded and further risk of inherent biases may ensue.
A path forward
But these challenges, like any, can be overcome. Research in the area of differential privacy and the adoption of recent data-sharing standards (eg, HL7 FHIR) can facilitate access to training data (Saripalle R, et al. J Biomed Inform. 2019;94:103188). Regulators are also open to incorporating feedback from the AI research community and industry in favor of innovation in this frenetic domain. The FDA developed the AI/ML Software as a Medical Device Action Plan in response to stakeholder feedback for oversight (FDA, 2021). Specifically, the “Good Machine Learning Practice” will be developed to describe AI/ML best practices (eg, data management, training, interpretability, evaluation, and documentation) to guide product development and standardization.
Sleep medicine has significantly progressed over the last several decades. Rather than maintain the status quo, AI can help fill the existing knowledge gaps, augment clinical practice, and streamline operations by analyzing and processing data at a volume and efficiency beyond human capacity. Fallibility is inevitable in machines and humans; however, like humans, machines can improve with continued training and exposure.
We asked ChatGPT about the future of AI in sleep medicine. It states that AI could have a “significant impact” on sleep disorders diagnosis, treatment, prevention, and sleep tracking and monitoring. Only time will tell if its claims are accurate.
Dr. Tan is Clinical Associate Professor with the Division of Sleep Medicine at the Stanford University School of Medicine. Dr. Bhargava is Clinical Professor with the Division of Pediatric Pulmonary, Asthma, and Sleep Medicine at the Stanford University School of Medicine.
“Artificial intelligence (AI) in healthcare refers to the use of machine learning (ML), deep learning, natural language processing, and computer vision to process and analyze large amounts of health care data.”
The preceding line is a direct quote from ChatGPT when prompted with the question “What is AI in health care?” (OpenAI, 2022). AI has rapidly infiltrated our lives. From using facial recognition software to unlock our cellphones to scrolling through targeted media suggested by streaming services, our daily existence is interwoven with algorithms. With the recent introduction of GPT-3 (the model that powers ChatGPT) in late 2022 and its even more capable successor, GPT-4, in March 2023, AI will continue to dominate our everyday environment in even more complex and meaningful ways.
For sleep medicine, the initial applications of AI in this field have been innovative and promising. (OSA) (Bandyopadhyay A, et al. Sleep Breath. 2023;27[1]:39). Pépin and colleagues (JAMA Netw Open. 2020;3[1]:e1919657) combined ML with mandibular movement to diagnose OSA with a reasonable agreement to polysomnography as a novel home-based alternative for diagnosis. AI has also been used to predict adherence to positive airway pressure (PAP) therapy in OSA (Scioscia G, et al. Inform Health Soc Care. 2022;47[3]:274) and as a digital intervention tool accessed via a smartphone app for people with insomnia (Philip P, et al, J Med Internet Res. 2020;22[12]:e24268). The data-rich field of sleep medicine is primed for further advancements through AI, albeit with a few hurdles and regulations to overcome before becoming mainstream.
Future promise
Sleep medicine is uniquely positioned to develop robust AI algorithms because of its vast data trove. Using AI, scientists can efficiently analyze the raw data from polysomnography, consumer sleep technology (CST), and nightly remote monitoring (from PAP devices) to substantially improve comprehension and management of sleep disorders.
AI can redefine OSA through analysis of the big data available, rather than solely relying on the apnea-hypopnea index. In addition, novel variables such as facial structure; snoring index; temperature trends; and sleep environment, position, and timing using a camera-based contactless technology may be incorporated to enhance the diagnostic accuracy for OSA or better describe sleep quality. AI algorithms can also be embedded into the electronic health record (EHR) to facilitate screening for sleep disorders using patient characteristics, thus accelerating the recognition and evaluation of possible sleep disorders.
New ways of collecting data may deliver deeper insights into sleep health, as well. CST such as wearables, nearables, and phone applications are improving with each iteration, resulting in more data about sleep for millions of people over thousands of nights.
AI can help achieve precision medicine by integrating multimodal data to establish endotypes and phenotypes of various sleep disorders. Delineating endotypes and phenotypes allows for personalized treatment recommendations, which may improve patient adherence and health outcomes.
Treatment personalization can also be achieved through AI by predicting compliance to various therapies and responses, as well as by discovering alternative forms of delivery to accomplish desired health outcomes. For example, to predict PAP compliance, we can record a patient encounter and use natural language processing to analyze their opinion of their treatment, extracting relevant keywords and combining such processing with other available data, such as environmental factors, sleep schedule, medical history, and other information extracted from the EHR. As another example, AI can determine the optimal time for cancer therapy by predicting a patient’s circadian timing (Hesse J, et al. Cancers (Basel). 2020;12[11]:3103). Circadian timing of drug delivery may be relevant in other specialties including cardiovascular disease, endocrine disorders, and psychiatric conditions due to its associations with sleep. Integration of the various “-omics” (eg, proteomics, genomics, and transcriptomics) with physiologic, behavioral, and environmental data can offer opportunities for drug discovery and possible prediction of sleep disorders and sleep-related morbidity. Although generative pretrained transformers are currently used to predict text (ie, ChatGPT), it is theoretically possible to also apply this technique to identify patients at risk for future sleep disorders from an earlier age.
Challenges to an AI renaissance
Despite making strides in numerous specialties such as radiology, ophthalmology, pathology, oncology, and dermatology, AI has not yet gained mainstream usage. Why isn’t AI as ubiquitous and heavily entrenched in health care as it is in other industries? According to the National Academy of Medicine’s AI in Healthcare: The Hope, The Hype, The Promise, The Peril, there are several realities to address before we fully embrace the AI revolution (Matheny M, et al. 2019).
First, AI algorithms should be trained on quality data that are representative of the population. Interoperability between health care systems and standardization across platforms is required to access large volumes of quality data. The current framework for data gathering is limited due to regulations, patient privacy concerns, and organizational preferences. The challenges to data acquisition and standardization of information will continue to snarl progress unless there are legislative remedies.
Furthermore, datasets should be diverse enough to avoid introducing bias into the AI algorithm. If the dataset is limited and health inequities (eg, societal bias and social determinants of health) are excluded from the training set, then the outcome will perpetuate further explicit and implicit biases.
The Food and Drug Administration (FDA) reviews and authorizes AI/ML-enabled devices. Its current regulatory structure treats AI as a static process and does not allow for exercise of its intrinsic ability to continuously learn from additional data, thereby preventing it from becoming more accurate and evolving with the population over time. A more flexible approach is needed.
Lastly, recent advanced AI algorithms including deep learning and neural network methodology function like a “black box.” The models are not explainable or transparent. Without clear comprehension of its methods, acceptance in clinical practice will be guarded and further risk of inherent biases may ensue.
A path forward
But these challenges, like any, can be overcome. Research in the area of differential privacy and the adoption of recent data-sharing standards (eg, HL7 FHIR) can facilitate access to training data (Saripalle R, et al. J Biomed Inform. 2019;94:103188). Regulators are also open to incorporating feedback from the AI research community and industry in favor of innovation in this frenetic domain. The FDA developed the AI/ML Software as a Medical Device Action Plan in response to stakeholder feedback for oversight (FDA, 2021). Specifically, the “Good Machine Learning Practice” will be developed to describe AI/ML best practices (eg, data management, training, interpretability, evaluation, and documentation) to guide product development and standardization.
Sleep medicine has significantly progressed over the last several decades. Rather than maintain the status quo, AI can help fill the existing knowledge gaps, augment clinical practice, and streamline operations by analyzing and processing data at a volume and efficiency beyond human capacity. Fallibility is inevitable in machines and humans; however, like humans, machines can improve with continued training and exposure.
We asked ChatGPT about the future of AI in sleep medicine. It states that AI could have a “significant impact” on sleep disorders diagnosis, treatment, prevention, and sleep tracking and monitoring. Only time will tell if its claims are accurate.
Dr. Tan is Clinical Associate Professor with the Division of Sleep Medicine at the Stanford University School of Medicine. Dr. Bhargava is Clinical Professor with the Division of Pediatric Pulmonary, Asthma, and Sleep Medicine at the Stanford University School of Medicine.
The triple overlap: COPD-OSA-OHS. Is it time for new definitions?
In our current society, it is likely that the “skinny patient with COPD” who walks into your clinic is less and less your “traditional” patient with COPD. We are seeing in our health care systems more of the “blue bloaters” – patients with COPD and significant obesity. This phenotype is representing what we are seeing worldwide as a consequence of the rising obesity prevalence. In the United States, the prepandemic (2017-2020) estimated percentage of adults over the age of 40 with obesity, defined as a body mass index (BMI) of at least 30 kg/m2, was over 40%. Moreover, the estimated percentage of adults with morbid obesity (BMI at least 40 kg/m2) is close to 10% (Akinbami, LJ et al. Vital Health Stat. 2022:190:1-36) and trending up. These patients with the “triple overlap” of morbid obesity, COPD, and awake daytime hypercapnia are being seen in clinics and in-hospital settings with increasing frequency, often presenting with complicating comorbidities such as acute respiratory failure, acute heart failure, kidney disease, or pulmonary hypertension. We are now faced with managing these patients with complex disease.
The obesity paradox does not seem applicable in the triple overlap phenotype. Patients with COPD who are overweight, defined as “mild obesity,” have lower mortality when compared with normal weight and underweight patients with COPD; however, this effect diminishes when BMI increases beyond 32 kg/m2. With increasing obesity severity and aging, the risk of both obstructive sleep apnea (OSA) and hypoventilation increases. It is well documented that COPD-OSA overlap is linked to worse outcomes and that continuous positive airway pressure (CPAP) as first-line therapy decreases readmission rates and mortality. The pathophysiology of hypoventilation in obesity is complex and multifactorial, and, although significant overlaps likely exist with comorbid COPD, by current definitions, to establish a diagnosis of obesity hypoventilation syndrome (OHS), one must have excluded other causes of hypoventilation, such as COPD.
These patients with the triple overlap of morbid obesity, awake daytime hypercapnia, and COPD are the subset of patients that providers struggle to fit in a diagnosis or in clinical research trials.
The triple overlap is a distinct syndrome
Different labels have been used in the medical literature: hypercapnic OSA-COPD overlap, morbid obesity and OSA-COPD overlap, hypercapnic morbidly obese COPD and OHS-COPD overlap. A better characterization of this distinctive phenotype is much needed. Patients with OSA-COPD overlap, for example, have an increased propensity to develop hypercapnia at higher FEV1 when compared with COPD without OSA – but this is thought to be a consequence of prolonged and frequent apneas and hypopneas compounded with obesity-related central hypoventilation. We found that morbidly obese patients with OSA-COPD overlap have a higher hypoxia burden, more severe OSA, and are frequently prescribed noninvasive ventilation after a failed titration polysomnogram (Htun ZM, et al. Am J Respir Crit Care Med. 2019;199:A1382), perhaps signaling a distinctive phenotype with worse outcomes, but the study had the inherent limitations of a single-center, retrospective design lacking data on awake hypercapnia. On the other side, the term OHS-COPD is contradictory and confusing based on current OHS diagnostic criteria.
In standardizing diagnostic criteria for patients with this triple overlap syndrome, challenges remain: would the patient with a BMI of 70 kg/m2 and fixed chronic airflow obstruction with FEV1 72% fall under the category of hypercapnic COPD vs OHS? Do these patients have worse outcomes regardless of their predominant feature? Would outcomes change if the apnea hypopnea index (AHI) is 10/h vs 65/h? More importantly, do patients with the triple overlap of COPD, morbid obesity, and daytime hypercapnia have worse outcomes when compared with hypercapnic COPD, or OHS with/without OSA? These questions can be better addressed once we agree on a definition. The patients with triple overlap syndrome have been traditionally excluded from clinical trials: the patient with morbid obesity has been excluded from chronic hypercapnic COPD clinical trials, and the patient with COPD has been excluded from OHS trials.
There are no specific clinical guidelines for this triple overlap phenotype. Positive airway pressure is the mainstay of treatment. CPAP is recommended as first-line therapy for patients with OSA-COPD overlap syndrome, while noninvasive ventilation (NIV) with bilevel positive airway pressure (BPAP) is recommended as first-line for the stable ambulatory hypercapnic patient with COPD. It is unclear if NIV is superior to CPAP in patients with triple overlap syndrome, although recently published data showed greater efficacy in reducing carbon dioxide (PaCO2) and improving quality of life in a small group of subjects (Zheng et al. J Clin Sleep Med. 2022;18[1]:99-107). To take a step further, the subtleties of NIV set up, such as rise time and minimum inspiratory time, are contradictory: the goal in ventilating patients with COPD is to shorten inspiratory time, prolonging expiratory time, therefore allowing a shortened inspiratory cycle. In obesity, ventilation strategies aim to prolong and sustain inspiratory time to improve ventilation and dependent atelectasis. Another area of uncertainty is device selection. Should we aim to provide a respiratory assist device (RAD): the traditional, rent to own bilevel PAP without auto-expiratory positive airway pressure (EPAP) capabilities and lower maximum inspiratory pressure delivery capacity, vs a home mechanical ventilator at a higher expense, life-time rental, and one-way only data monitoring, which limits remote prescription adjustments, but allow auto-EPAP settings for patients with comorbid OSA? More importantly, how do we get these patients, who do not fit in any of the specified insurance criteria for PAP therapy approved for treatment?
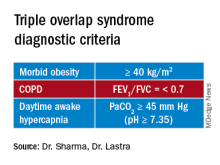
A uniform diagnostic definition and clear taxonomy allows for resource allocation, from government funded grants for clinical trials to a better-informed distribution of health care systems resources and support health care policy changes to improve patient-centric outcomes. Here, we propose that the morbidly obese patient (BMI >40 kg/m2) with chronic airflow obstruction and a forced expiratory ratio (FEV1/FVC) <0.7 with awake daytime hypercapnia (PaCO2 > 45 mm Hg) represents a different entity/phenotype and fits best under the triple overlap syndrome taxonomy.
We suspect that these patients have worse outcomes, including comorbidity burden, quality of life, exacerbation rates, longer hospital length-of-stay, and respiratory and all-cause mortality. Large, multicenter, controlled trials comparing the long-term effectiveness of NIV and CPAP: measurements of respiratory function, gas exchange, blood pressure, and health related quality of life are needed. This is a group of patients that may specifically benefit from volume-targeted pressure support mode ventilation with auto-EPAP capabilities upon discharge from the hospital after an acute exacerbation.
Inpatient (sleep medicine) and outpatient transitions
In patients hospitalized with the triple overlap syndrome, there are certain considerations that are of special interest. Given comorbid hypercapnia and limited data on NIV superiority over CPAP, a sleep study should not be needed for NIV qualification. In addition, the medical team may consider the following (Figure 1):
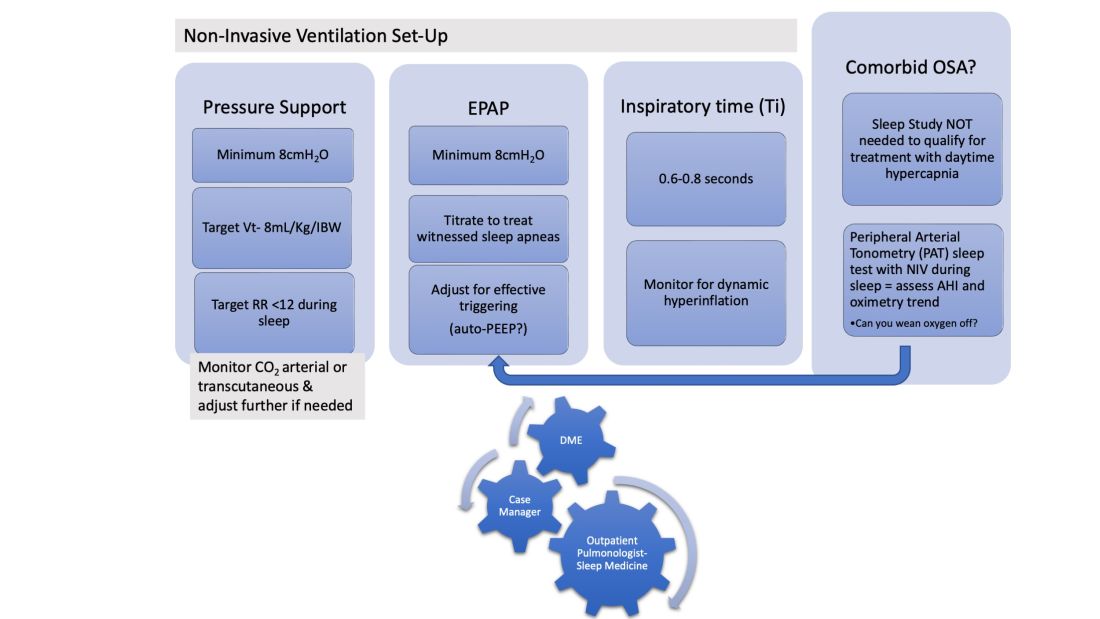
1. Noninvasive Ventilation:
a. Maintaining a high-pressure support differential between inspiratory positive airway pressure (IPAP) and EPAP. This can usually be achieved at 8-10 cm H2O, further adjusting to target a tidal volume (Vt) of 8 mL/kg of ideal body weight (IBW).
b. Higher EPAP: To overcome dependent atelectasis, improve ventilation-perfusion (VQ) matching, and better treat upper airway resistance both during wakefulness and sleep. Also, adjustments of EPAP at bedside should be considered to counteract auto-PEEP-related ineffective triggering if observed.
c. OSA screening and EPAP adjustment: for high residual obstructive apneas or hypopneas if data are available on the NIV device, or with the use of peripheral arterial tonometry sleep testing devices with NIV on overnight before discharge.
d. Does the patient meet criteria for oxygen supplementation at home? Wean oxygen off, if possible.
2. Case-managers can help establish services with a durable medical equipment provider with expertise in advanced PAP devices.3. Obesity management, Consider referral to an obesity management program for lifestyle/dietary modifications along with pharmacotherapy or bariatric surgery interventions.
4. Close follow-up, track exacerbations. Device download data are crucial to monitor adherence/tolerance and treatment effectiveness with particular interest in AHI, oximetry, and CO2 trends monitoring. Some patients may need dedicated titration polysomnograms to adjust ventilation settings, for optimization of residual OSA or for oxygen addition or discontinuation.
Conclusion
Patients with the triple overlap phenotype have not been systematically defined, studied, or included in clinical trials. We anticipate that these patients have worse outcomes: quality of life, symptom and comorbidity burden, exacerbation rates, in-hospital mortality, longer hospital stay and ICU stay, and respiratory and all-cause mortality. This is a group of patients that may specifically benefit from domiciliary NIV set-up upon discharge from the hospital with close follow-up. Properly identifying these patients will help pulmonologists and health care systems direct resources to optimally manage this complex group of patients. Funding of research trials to support clinical guidelines development should be prioritized. Triple overlap syndrome is different from COPD-OSA overlap, OHS with moderate to severe OSA, or OHS without significant OSA.
In our current society, it is likely that the “skinny patient with COPD” who walks into your clinic is less and less your “traditional” patient with COPD. We are seeing in our health care systems more of the “blue bloaters” – patients with COPD and significant obesity. This phenotype is representing what we are seeing worldwide as a consequence of the rising obesity prevalence. In the United States, the prepandemic (2017-2020) estimated percentage of adults over the age of 40 with obesity, defined as a body mass index (BMI) of at least 30 kg/m2, was over 40%. Moreover, the estimated percentage of adults with morbid obesity (BMI at least 40 kg/m2) is close to 10% (Akinbami, LJ et al. Vital Health Stat. 2022:190:1-36) and trending up. These patients with the “triple overlap” of morbid obesity, COPD, and awake daytime hypercapnia are being seen in clinics and in-hospital settings with increasing frequency, often presenting with complicating comorbidities such as acute respiratory failure, acute heart failure, kidney disease, or pulmonary hypertension. We are now faced with managing these patients with complex disease.
The obesity paradox does not seem applicable in the triple overlap phenotype. Patients with COPD who are overweight, defined as “mild obesity,” have lower mortality when compared with normal weight and underweight patients with COPD; however, this effect diminishes when BMI increases beyond 32 kg/m2. With increasing obesity severity and aging, the risk of both obstructive sleep apnea (OSA) and hypoventilation increases. It is well documented that COPD-OSA overlap is linked to worse outcomes and that continuous positive airway pressure (CPAP) as first-line therapy decreases readmission rates and mortality. The pathophysiology of hypoventilation in obesity is complex and multifactorial, and, although significant overlaps likely exist with comorbid COPD, by current definitions, to establish a diagnosis of obesity hypoventilation syndrome (OHS), one must have excluded other causes of hypoventilation, such as COPD.
These patients with the triple overlap of morbid obesity, awake daytime hypercapnia, and COPD are the subset of patients that providers struggle to fit in a diagnosis or in clinical research trials.
The triple overlap is a distinct syndrome
Different labels have been used in the medical literature: hypercapnic OSA-COPD overlap, morbid obesity and OSA-COPD overlap, hypercapnic morbidly obese COPD and OHS-COPD overlap. A better characterization of this distinctive phenotype is much needed. Patients with OSA-COPD overlap, for example, have an increased propensity to develop hypercapnia at higher FEV1 when compared with COPD without OSA – but this is thought to be a consequence of prolonged and frequent apneas and hypopneas compounded with obesity-related central hypoventilation. We found that morbidly obese patients with OSA-COPD overlap have a higher hypoxia burden, more severe OSA, and are frequently prescribed noninvasive ventilation after a failed titration polysomnogram (Htun ZM, et al. Am J Respir Crit Care Med. 2019;199:A1382), perhaps signaling a distinctive phenotype with worse outcomes, but the study had the inherent limitations of a single-center, retrospective design lacking data on awake hypercapnia. On the other side, the term OHS-COPD is contradictory and confusing based on current OHS diagnostic criteria.
In standardizing diagnostic criteria for patients with this triple overlap syndrome, challenges remain: would the patient with a BMI of 70 kg/m2 and fixed chronic airflow obstruction with FEV1 72% fall under the category of hypercapnic COPD vs OHS? Do these patients have worse outcomes regardless of their predominant feature? Would outcomes change if the apnea hypopnea index (AHI) is 10/h vs 65/h? More importantly, do patients with the triple overlap of COPD, morbid obesity, and daytime hypercapnia have worse outcomes when compared with hypercapnic COPD, or OHS with/without OSA? These questions can be better addressed once we agree on a definition. The patients with triple overlap syndrome have been traditionally excluded from clinical trials: the patient with morbid obesity has been excluded from chronic hypercapnic COPD clinical trials, and the patient with COPD has been excluded from OHS trials.
There are no specific clinical guidelines for this triple overlap phenotype. Positive airway pressure is the mainstay of treatment. CPAP is recommended as first-line therapy for patients with OSA-COPD overlap syndrome, while noninvasive ventilation (NIV) with bilevel positive airway pressure (BPAP) is recommended as first-line for the stable ambulatory hypercapnic patient with COPD. It is unclear if NIV is superior to CPAP in patients with triple overlap syndrome, although recently published data showed greater efficacy in reducing carbon dioxide (PaCO2) and improving quality of life in a small group of subjects (Zheng et al. J Clin Sleep Med. 2022;18[1]:99-107). To take a step further, the subtleties of NIV set up, such as rise time and minimum inspiratory time, are contradictory: the goal in ventilating patients with COPD is to shorten inspiratory time, prolonging expiratory time, therefore allowing a shortened inspiratory cycle. In obesity, ventilation strategies aim to prolong and sustain inspiratory time to improve ventilation and dependent atelectasis. Another area of uncertainty is device selection. Should we aim to provide a respiratory assist device (RAD): the traditional, rent to own bilevel PAP without auto-expiratory positive airway pressure (EPAP) capabilities and lower maximum inspiratory pressure delivery capacity, vs a home mechanical ventilator at a higher expense, life-time rental, and one-way only data monitoring, which limits remote prescription adjustments, but allow auto-EPAP settings for patients with comorbid OSA? More importantly, how do we get these patients, who do not fit in any of the specified insurance criteria for PAP therapy approved for treatment?
A uniform diagnostic definition and clear taxonomy allows for resource allocation, from government funded grants for clinical trials to a better-informed distribution of health care systems resources and support health care policy changes to improve patient-centric outcomes. Here, we propose that the morbidly obese patient (BMI >40 kg/m2) with chronic airflow obstruction and a forced expiratory ratio (FEV1/FVC) <0.7 with awake daytime hypercapnia (PaCO2 > 45 mm Hg) represents a different entity/phenotype and fits best under the triple overlap syndrome taxonomy.
We suspect that these patients have worse outcomes, including comorbidity burden, quality of life, exacerbation rates, longer hospital length-of-stay, and respiratory and all-cause mortality. Large, multicenter, controlled trials comparing the long-term effectiveness of NIV and CPAP: measurements of respiratory function, gas exchange, blood pressure, and health related quality of life are needed. This is a group of patients that may specifically benefit from volume-targeted pressure support mode ventilation with auto-EPAP capabilities upon discharge from the hospital after an acute exacerbation.
Inpatient (sleep medicine) and outpatient transitions
In patients hospitalized with the triple overlap syndrome, there are certain considerations that are of special interest. Given comorbid hypercapnia and limited data on NIV superiority over CPAP, a sleep study should not be needed for NIV qualification. In addition, the medical team may consider the following (Figure 1):

1. Noninvasive Ventilation:
a. Maintaining a high-pressure support differential between inspiratory positive airway pressure (IPAP) and EPAP. This can usually be achieved at 8-10 cm H2O, further adjusting to target a tidal volume (Vt) of 8 mL/kg of ideal body weight (IBW).
b. Higher EPAP: To overcome dependent atelectasis, improve ventilation-perfusion (VQ) matching, and better treat upper airway resistance both during wakefulness and sleep. Also, adjustments of EPAP at bedside should be considered to counteract auto-PEEP-related ineffective triggering if observed.
c. OSA screening and EPAP adjustment: for high residual obstructive apneas or hypopneas if data are available on the NIV device, or with the use of peripheral arterial tonometry sleep testing devices with NIV on overnight before discharge.
d. Does the patient meet criteria for oxygen supplementation at home? Wean oxygen off, if possible.
2. Case-managers can help establish services with a durable medical equipment provider with expertise in advanced PAP devices.3. Obesity management, Consider referral to an obesity management program for lifestyle/dietary modifications along with pharmacotherapy or bariatric surgery interventions.
4. Close follow-up, track exacerbations. Device download data are crucial to monitor adherence/tolerance and treatment effectiveness with particular interest in AHI, oximetry, and CO2 trends monitoring. Some patients may need dedicated titration polysomnograms to adjust ventilation settings, for optimization of residual OSA or for oxygen addition or discontinuation.
Conclusion
Patients with the triple overlap phenotype have not been systematically defined, studied, or included in clinical trials. We anticipate that these patients have worse outcomes: quality of life, symptom and comorbidity burden, exacerbation rates, in-hospital mortality, longer hospital stay and ICU stay, and respiratory and all-cause mortality. This is a group of patients that may specifically benefit from domiciliary NIV set-up upon discharge from the hospital with close follow-up. Properly identifying these patients will help pulmonologists and health care systems direct resources to optimally manage this complex group of patients. Funding of research trials to support clinical guidelines development should be prioritized. Triple overlap syndrome is different from COPD-OSA overlap, OHS with moderate to severe OSA, or OHS without significant OSA.
In our current society, it is likely that the “skinny patient with COPD” who walks into your clinic is less and less your “traditional” patient with COPD. We are seeing in our health care systems more of the “blue bloaters” – patients with COPD and significant obesity. This phenotype is representing what we are seeing worldwide as a consequence of the rising obesity prevalence. In the United States, the prepandemic (2017-2020) estimated percentage of adults over the age of 40 with obesity, defined as a body mass index (BMI) of at least 30 kg/m2, was over 40%. Moreover, the estimated percentage of adults with morbid obesity (BMI at least 40 kg/m2) is close to 10% (Akinbami, LJ et al. Vital Health Stat. 2022:190:1-36) and trending up. These patients with the “triple overlap” of morbid obesity, COPD, and awake daytime hypercapnia are being seen in clinics and in-hospital settings with increasing frequency, often presenting with complicating comorbidities such as acute respiratory failure, acute heart failure, kidney disease, or pulmonary hypertension. We are now faced with managing these patients with complex disease.
The obesity paradox does not seem applicable in the triple overlap phenotype. Patients with COPD who are overweight, defined as “mild obesity,” have lower mortality when compared with normal weight and underweight patients with COPD; however, this effect diminishes when BMI increases beyond 32 kg/m2. With increasing obesity severity and aging, the risk of both obstructive sleep apnea (OSA) and hypoventilation increases. It is well documented that COPD-OSA overlap is linked to worse outcomes and that continuous positive airway pressure (CPAP) as first-line therapy decreases readmission rates and mortality. The pathophysiology of hypoventilation in obesity is complex and multifactorial, and, although significant overlaps likely exist with comorbid COPD, by current definitions, to establish a diagnosis of obesity hypoventilation syndrome (OHS), one must have excluded other causes of hypoventilation, such as COPD.
These patients with the triple overlap of morbid obesity, awake daytime hypercapnia, and COPD are the subset of patients that providers struggle to fit in a diagnosis or in clinical research trials.
The triple overlap is a distinct syndrome
Different labels have been used in the medical literature: hypercapnic OSA-COPD overlap, morbid obesity and OSA-COPD overlap, hypercapnic morbidly obese COPD and OHS-COPD overlap. A better characterization of this distinctive phenotype is much needed. Patients with OSA-COPD overlap, for example, have an increased propensity to develop hypercapnia at higher FEV1 when compared with COPD without OSA – but this is thought to be a consequence of prolonged and frequent apneas and hypopneas compounded with obesity-related central hypoventilation. We found that morbidly obese patients with OSA-COPD overlap have a higher hypoxia burden, more severe OSA, and are frequently prescribed noninvasive ventilation after a failed titration polysomnogram (Htun ZM, et al. Am J Respir Crit Care Med. 2019;199:A1382), perhaps signaling a distinctive phenotype with worse outcomes, but the study had the inherent limitations of a single-center, retrospective design lacking data on awake hypercapnia. On the other side, the term OHS-COPD is contradictory and confusing based on current OHS diagnostic criteria.
In standardizing diagnostic criteria for patients with this triple overlap syndrome, challenges remain: would the patient with a BMI of 70 kg/m2 and fixed chronic airflow obstruction with FEV1 72% fall under the category of hypercapnic COPD vs OHS? Do these patients have worse outcomes regardless of their predominant feature? Would outcomes change if the apnea hypopnea index (AHI) is 10/h vs 65/h? More importantly, do patients with the triple overlap of COPD, morbid obesity, and daytime hypercapnia have worse outcomes when compared with hypercapnic COPD, or OHS with/without OSA? These questions can be better addressed once we agree on a definition. The patients with triple overlap syndrome have been traditionally excluded from clinical trials: the patient with morbid obesity has been excluded from chronic hypercapnic COPD clinical trials, and the patient with COPD has been excluded from OHS trials.
There are no specific clinical guidelines for this triple overlap phenotype. Positive airway pressure is the mainstay of treatment. CPAP is recommended as first-line therapy for patients with OSA-COPD overlap syndrome, while noninvasive ventilation (NIV) with bilevel positive airway pressure (BPAP) is recommended as first-line for the stable ambulatory hypercapnic patient with COPD. It is unclear if NIV is superior to CPAP in patients with triple overlap syndrome, although recently published data showed greater efficacy in reducing carbon dioxide (PaCO2) and improving quality of life in a small group of subjects (Zheng et al. J Clin Sleep Med. 2022;18[1]:99-107). To take a step further, the subtleties of NIV set up, such as rise time and minimum inspiratory time, are contradictory: the goal in ventilating patients with COPD is to shorten inspiratory time, prolonging expiratory time, therefore allowing a shortened inspiratory cycle. In obesity, ventilation strategies aim to prolong and sustain inspiratory time to improve ventilation and dependent atelectasis. Another area of uncertainty is device selection. Should we aim to provide a respiratory assist device (RAD): the traditional, rent to own bilevel PAP without auto-expiratory positive airway pressure (EPAP) capabilities and lower maximum inspiratory pressure delivery capacity, vs a home mechanical ventilator at a higher expense, life-time rental, and one-way only data monitoring, which limits remote prescription adjustments, but allow auto-EPAP settings for patients with comorbid OSA? More importantly, how do we get these patients, who do not fit in any of the specified insurance criteria for PAP therapy approved for treatment?
A uniform diagnostic definition and clear taxonomy allows for resource allocation, from government funded grants for clinical trials to a better-informed distribution of health care systems resources and support health care policy changes to improve patient-centric outcomes. Here, we propose that the morbidly obese patient (BMI >40 kg/m2) with chronic airflow obstruction and a forced expiratory ratio (FEV1/FVC) <0.7 with awake daytime hypercapnia (PaCO2 > 45 mm Hg) represents a different entity/phenotype and fits best under the triple overlap syndrome taxonomy.
We suspect that these patients have worse outcomes, including comorbidity burden, quality of life, exacerbation rates, longer hospital length-of-stay, and respiratory and all-cause mortality. Large, multicenter, controlled trials comparing the long-term effectiveness of NIV and CPAP: measurements of respiratory function, gas exchange, blood pressure, and health related quality of life are needed. This is a group of patients that may specifically benefit from volume-targeted pressure support mode ventilation with auto-EPAP capabilities upon discharge from the hospital after an acute exacerbation.
Inpatient (sleep medicine) and outpatient transitions
In patients hospitalized with the triple overlap syndrome, there are certain considerations that are of special interest. Given comorbid hypercapnia and limited data on NIV superiority over CPAP, a sleep study should not be needed for NIV qualification. In addition, the medical team may consider the following (Figure 1):

1. Noninvasive Ventilation:
a. Maintaining a high-pressure support differential between inspiratory positive airway pressure (IPAP) and EPAP. This can usually be achieved at 8-10 cm H2O, further adjusting to target a tidal volume (Vt) of 8 mL/kg of ideal body weight (IBW).
b. Higher EPAP: To overcome dependent atelectasis, improve ventilation-perfusion (VQ) matching, and better treat upper airway resistance both during wakefulness and sleep. Also, adjustments of EPAP at bedside should be considered to counteract auto-PEEP-related ineffective triggering if observed.
c. OSA screening and EPAP adjustment: for high residual obstructive apneas or hypopneas if data are available on the NIV device, or with the use of peripheral arterial tonometry sleep testing devices with NIV on overnight before discharge.
d. Does the patient meet criteria for oxygen supplementation at home? Wean oxygen off, if possible.
2. Case-managers can help establish services with a durable medical equipment provider with expertise in advanced PAP devices.3. Obesity management, Consider referral to an obesity management program for lifestyle/dietary modifications along with pharmacotherapy or bariatric surgery interventions.
4. Close follow-up, track exacerbations. Device download data are crucial to monitor adherence/tolerance and treatment effectiveness with particular interest in AHI, oximetry, and CO2 trends monitoring. Some patients may need dedicated titration polysomnograms to adjust ventilation settings, for optimization of residual OSA or for oxygen addition or discontinuation.
Conclusion
Patients with the triple overlap phenotype have not been systematically defined, studied, or included in clinical trials. We anticipate that these patients have worse outcomes: quality of life, symptom and comorbidity burden, exacerbation rates, in-hospital mortality, longer hospital stay and ICU stay, and respiratory and all-cause mortality. This is a group of patients that may specifically benefit from domiciliary NIV set-up upon discharge from the hospital with close follow-up. Properly identifying these patients will help pulmonologists and health care systems direct resources to optimally manage this complex group of patients. Funding of research trials to support clinical guidelines development should be prioritized. Triple overlap syndrome is different from COPD-OSA overlap, OHS with moderate to severe OSA, or OHS without significant OSA.
Inpatient sleep medicine: An invaluable service for hospital medicine
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.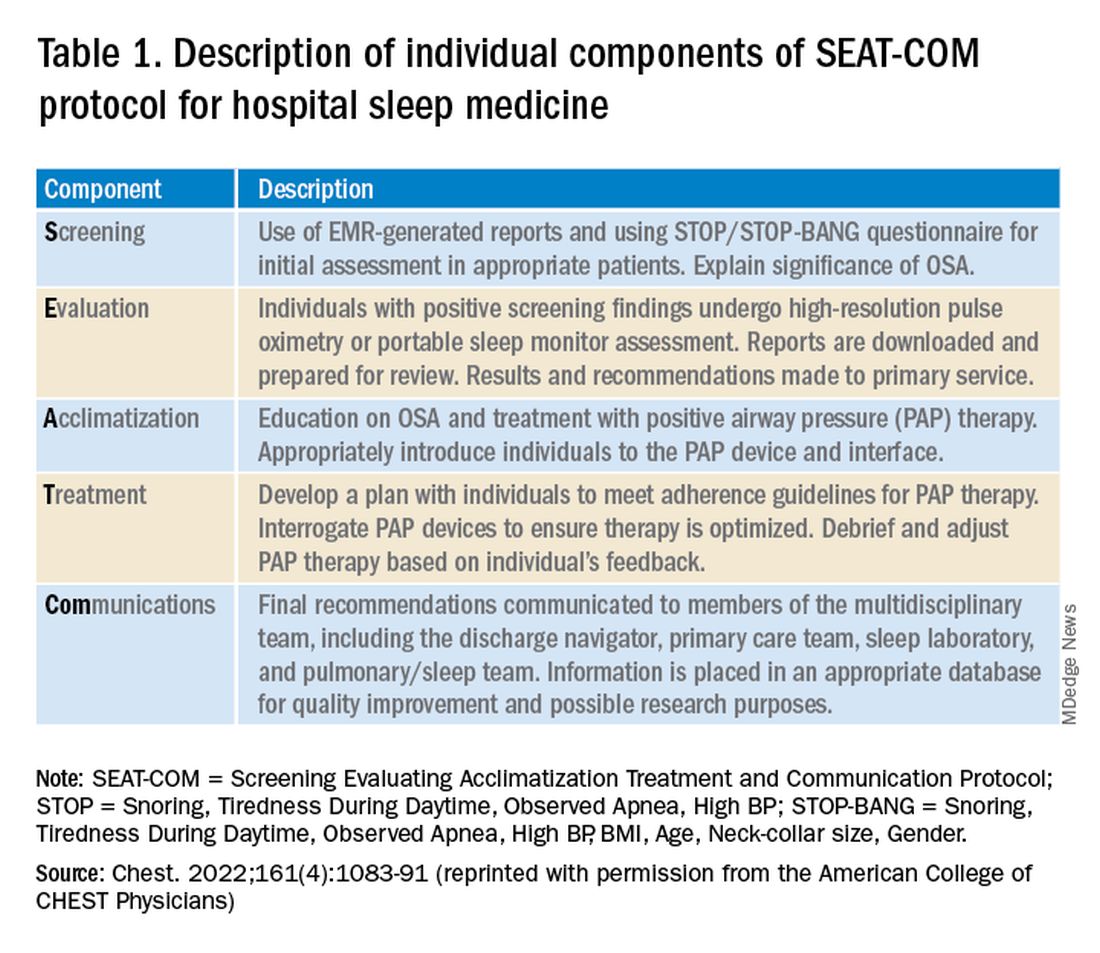
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
What are we missing when it comes to obstructive sleep apnea and atrial fibrillation?
Obstructive sleep apnea is a prevalent and underdiagnosed sleep-related breathing disorder. The estimated prevalence of OSA in the general population of North America ranges from 9% to 38%. This prevalence is higher in men, with a roughly 2:1 male to female ratio, and it also increases with age (Senaratna CV, et al. Sleep Med Rev. 2017;34:70-81). In large epidemiologic studies, the association between OSA and atrial fibrillation (AF) has been well established. The prevalence of OSA in patients with AF is high, with estimates ranging from 21% to 74%. In the OSA population, the Sleep Heart Health Study (Mehra R, et al. Am J Respir Crit Care Med. 2006;173[8]:910-16) and the Multi Ethnic Study of Atherosclerosis (Lin GM, et al. Am J Epidemiol. 2015;182[1]:49-57) found that patients with OSA had a twofold to fourfold increased risk of AF compared with those who did not have OSA. Therefore, the most current American Heart Association guidelines recommend assessing OSA symptoms in all patients with AF and screening for OSA in recurrent patients with AF.
The pathophysiology of OSA involves multiple physiologic stressors that may contribute to an increased propensity for atrial arrhythmias in this population. Among these factors are large changes in intrathoracic pressures that may cause atrial and ventricular wall stretching, recurrent oxidative stress, and a sympathetic surge associated with shortening atrial refractory periods and atrial extrasystoles. By occurring nightly over many years, these physiologic stressors may lead to permanent atrial dilation and structural remodeling, eventually affecting the conduction system and producing a substrate conducive to reentrant circuits. Other common comorbidities in patients with OSA–such as hypertension, obesity, and metabolic syndrome–may also contribute to arrhythmogenicity (Linz D, et al. JAMA Cardiol. 2018;3[6]:532).
Does treating OSA with CPAP prevent the development of AF?
Previous case-control and retrospective observational studies suggested that having OSA makes treating AF more difficult. Patients with OSA had lower response rates to antiarrhythmic drugs, with the lowest in those with more severe OSA. Rhythm control with cardioversion and catheter-based pulmonary vein isolation was also less successful in patients with OSA due to higher rates of AF recurrence. According to one meta-analysis, patients with OSA had a 31% higher rate of AF recurrence after pulmonary vein isolation (Li L, et al. Europace. 2014;16[9]:1309-14).
Prospective studies using CPAP to treat OSA have not demonstrated a reduced risk of adverse cardiovascular outcomes. The SAVE trial is the most well-known of these studies. The primary endpoint was death from cardiovascular causes (myocardial infarction, stroke, or hospitalization for unstable angina, heart failure, or transient ischemic attack). There was no difference in this outcome between the CPAP and usual care groups. A secondary outcome in this study was new-onset AF detected by electrocardiography, and there was no difference between the CPAP and the usual care group. The low amount of CPAP usage in the treatment group was a commonly cited shortcoming of the SAVE trial–mean usage was 4.4 hours per night during the first month of treatment and subsequently decreased to 3.3 hours per night by the 12-month time point (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919-31).
Caples and colleagues screened patients undergoing direct current cardioversion or catheter ablation. They chose those who were also positive for OSA by polysomnography (apnea-hypopnea index – AHI greater than five events per hour). Twenty-five patients were included in the study and were randomly assigned to either CPAP treatment or usual care. Body mass index, blood pressure, ejection fraction, AHI, and nocturnal desaturation levels were comparable between the two groups. The rate of recurrence of AF and the time point following randomization at which the AF recurred did not differ between the two groups (Caples SM, et al. Int J Cardiol. 2019;278:133-6).
A Norwegian trial by Traaen and colleagues included a larger sample of 108 patients with moderate to severe sleep apnea and paroxysmal AF who underwent catheter ablation. Patients were followed for 5 months before and 12 months after ablation. They were randomly assigned to either CPAP therapy plus usual care or usual care alone. The primary goal was to assess AF burden using implanted loop recorders. There was no significant difference in AF burden between the two groups from baseline to the final 3 months of the study (Traaen GM, et al. Am J Respir Crit Care Med. 2021;204[5]:573-82). These two prospective trials, which had AF recurrence or burden as primary outcomes, found no interaction between AF burden and CPAP use, at least within the first year of therapy. Both trials found that their participants used CPAP for more extended periods of time than the SAVE trial, with over 6 hours in the Caples and coworkers’ trial and nearly 5 hours in the Traaen and coworkers’ study.
Is the lack of efficacy due to starting CPAP too late in the course of OSA?
It has been proposed that there may be a critical early period after the onset of OSA when intervention with CPAP (or alternative therapies) will be most effective in preventing adverse cardiovascular outcomes. An answer will almost certainly necessitate a long-term prospective study enrolling people before they develop OSA. Additionally, the AHI is used in most trials to determine the presence and severity of OSA. However, the AHI has been shown to have a poor correlation with sleep-related symptoms, and it may fail to capture key OSA pathophysiologic stressors (e.g., hyperadrenergic drive, cyclical hypoxemia, etc), which may increase the risk of AF. Other disease characteristics and polysomnographic features may better capture disease severity and the cardiovascular risk factors associated with it. The respiratory arousal threshold, arousal index, degree of loop gain, hypoxic burden, heart rate variability, and cardiopulmonary coupling are some examples of such features.
Another possible explanation is that AF is not causally related, and the demonstrated association between the two is because both conditions share risk factors such as age and BMI, among others. Or, if they are causally linked, OSA may be a minor contributor, and the magnitude of that contribution is insufficient to reduce the risk of AF significantly by treating OSA. More research is needed to define the salient intervenable aspects of OSA better and design the optimal timing and duration of intervention.
Dr. Mudrakola is with the Department of Pulmonary & Critical Care Medicine, Summa Health, Akron, Ohio. Dr. Selim is with the Department of Pulmonary & Critical Care Medicine, Mayo Clinic, Rochester, Minnesota.
Obstructive sleep apnea is a prevalent and underdiagnosed sleep-related breathing disorder. The estimated prevalence of OSA in the general population of North America ranges from 9% to 38%. This prevalence is higher in men, with a roughly 2:1 male to female ratio, and it also increases with age (Senaratna CV, et al. Sleep Med Rev. 2017;34:70-81). In large epidemiologic studies, the association between OSA and atrial fibrillation (AF) has been well established. The prevalence of OSA in patients with AF is high, with estimates ranging from 21% to 74%. In the OSA population, the Sleep Heart Health Study (Mehra R, et al. Am J Respir Crit Care Med. 2006;173[8]:910-16) and the Multi Ethnic Study of Atherosclerosis (Lin GM, et al. Am J Epidemiol. 2015;182[1]:49-57) found that patients with OSA had a twofold to fourfold increased risk of AF compared with those who did not have OSA. Therefore, the most current American Heart Association guidelines recommend assessing OSA symptoms in all patients with AF and screening for OSA in recurrent patients with AF.
The pathophysiology of OSA involves multiple physiologic stressors that may contribute to an increased propensity for atrial arrhythmias in this population. Among these factors are large changes in intrathoracic pressures that may cause atrial and ventricular wall stretching, recurrent oxidative stress, and a sympathetic surge associated with shortening atrial refractory periods and atrial extrasystoles. By occurring nightly over many years, these physiologic stressors may lead to permanent atrial dilation and structural remodeling, eventually affecting the conduction system and producing a substrate conducive to reentrant circuits. Other common comorbidities in patients with OSA–such as hypertension, obesity, and metabolic syndrome–may also contribute to arrhythmogenicity (Linz D, et al. JAMA Cardiol. 2018;3[6]:532).
Does treating OSA with CPAP prevent the development of AF?
Previous case-control and retrospective observational studies suggested that having OSA makes treating AF more difficult. Patients with OSA had lower response rates to antiarrhythmic drugs, with the lowest in those with more severe OSA. Rhythm control with cardioversion and catheter-based pulmonary vein isolation was also less successful in patients with OSA due to higher rates of AF recurrence. According to one meta-analysis, patients with OSA had a 31% higher rate of AF recurrence after pulmonary vein isolation (Li L, et al. Europace. 2014;16[9]:1309-14).
Prospective studies using CPAP to treat OSA have not demonstrated a reduced risk of adverse cardiovascular outcomes. The SAVE trial is the most well-known of these studies. The primary endpoint was death from cardiovascular causes (myocardial infarction, stroke, or hospitalization for unstable angina, heart failure, or transient ischemic attack). There was no difference in this outcome between the CPAP and usual care groups. A secondary outcome in this study was new-onset AF detected by electrocardiography, and there was no difference between the CPAP and the usual care group. The low amount of CPAP usage in the treatment group was a commonly cited shortcoming of the SAVE trial–mean usage was 4.4 hours per night during the first month of treatment and subsequently decreased to 3.3 hours per night by the 12-month time point (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919-31).
Caples and colleagues screened patients undergoing direct current cardioversion or catheter ablation. They chose those who were also positive for OSA by polysomnography (apnea-hypopnea index – AHI greater than five events per hour). Twenty-five patients were included in the study and were randomly assigned to either CPAP treatment or usual care. Body mass index, blood pressure, ejection fraction, AHI, and nocturnal desaturation levels were comparable between the two groups. The rate of recurrence of AF and the time point following randomization at which the AF recurred did not differ between the two groups (Caples SM, et al. Int J Cardiol. 2019;278:133-6).
A Norwegian trial by Traaen and colleagues included a larger sample of 108 patients with moderate to severe sleep apnea and paroxysmal AF who underwent catheter ablation. Patients were followed for 5 months before and 12 months after ablation. They were randomly assigned to either CPAP therapy plus usual care or usual care alone. The primary goal was to assess AF burden using implanted loop recorders. There was no significant difference in AF burden between the two groups from baseline to the final 3 months of the study (Traaen GM, et al. Am J Respir Crit Care Med. 2021;204[5]:573-82). These two prospective trials, which had AF recurrence or burden as primary outcomes, found no interaction between AF burden and CPAP use, at least within the first year of therapy. Both trials found that their participants used CPAP for more extended periods of time than the SAVE trial, with over 6 hours in the Caples and coworkers’ trial and nearly 5 hours in the Traaen and coworkers’ study.
Is the lack of efficacy due to starting CPAP too late in the course of OSA?
It has been proposed that there may be a critical early period after the onset of OSA when intervention with CPAP (or alternative therapies) will be most effective in preventing adverse cardiovascular outcomes. An answer will almost certainly necessitate a long-term prospective study enrolling people before they develop OSA. Additionally, the AHI is used in most trials to determine the presence and severity of OSA. However, the AHI has been shown to have a poor correlation with sleep-related symptoms, and it may fail to capture key OSA pathophysiologic stressors (e.g., hyperadrenergic drive, cyclical hypoxemia, etc), which may increase the risk of AF. Other disease characteristics and polysomnographic features may better capture disease severity and the cardiovascular risk factors associated with it. The respiratory arousal threshold, arousal index, degree of loop gain, hypoxic burden, heart rate variability, and cardiopulmonary coupling are some examples of such features.
Another possible explanation is that AF is not causally related, and the demonstrated association between the two is because both conditions share risk factors such as age and BMI, among others. Or, if they are causally linked, OSA may be a minor contributor, and the magnitude of that contribution is insufficient to reduce the risk of AF significantly by treating OSA. More research is needed to define the salient intervenable aspects of OSA better and design the optimal timing and duration of intervention.
Dr. Mudrakola is with the Department of Pulmonary & Critical Care Medicine, Summa Health, Akron, Ohio. Dr. Selim is with the Department of Pulmonary & Critical Care Medicine, Mayo Clinic, Rochester, Minnesota.
Obstructive sleep apnea is a prevalent and underdiagnosed sleep-related breathing disorder. The estimated prevalence of OSA in the general population of North America ranges from 9% to 38%. This prevalence is higher in men, with a roughly 2:1 male to female ratio, and it also increases with age (Senaratna CV, et al. Sleep Med Rev. 2017;34:70-81). In large epidemiologic studies, the association between OSA and atrial fibrillation (AF) has been well established. The prevalence of OSA in patients with AF is high, with estimates ranging from 21% to 74%. In the OSA population, the Sleep Heart Health Study (Mehra R, et al. Am J Respir Crit Care Med. 2006;173[8]:910-16) and the Multi Ethnic Study of Atherosclerosis (Lin GM, et al. Am J Epidemiol. 2015;182[1]:49-57) found that patients with OSA had a twofold to fourfold increased risk of AF compared with those who did not have OSA. Therefore, the most current American Heart Association guidelines recommend assessing OSA symptoms in all patients with AF and screening for OSA in recurrent patients with AF.
The pathophysiology of OSA involves multiple physiologic stressors that may contribute to an increased propensity for atrial arrhythmias in this population. Among these factors are large changes in intrathoracic pressures that may cause atrial and ventricular wall stretching, recurrent oxidative stress, and a sympathetic surge associated with shortening atrial refractory periods and atrial extrasystoles. By occurring nightly over many years, these physiologic stressors may lead to permanent atrial dilation and structural remodeling, eventually affecting the conduction system and producing a substrate conducive to reentrant circuits. Other common comorbidities in patients with OSA–such as hypertension, obesity, and metabolic syndrome–may also contribute to arrhythmogenicity (Linz D, et al. JAMA Cardiol. 2018;3[6]:532).
Does treating OSA with CPAP prevent the development of AF?
Previous case-control and retrospective observational studies suggested that having OSA makes treating AF more difficult. Patients with OSA had lower response rates to antiarrhythmic drugs, with the lowest in those with more severe OSA. Rhythm control with cardioversion and catheter-based pulmonary vein isolation was also less successful in patients with OSA due to higher rates of AF recurrence. According to one meta-analysis, patients with OSA had a 31% higher rate of AF recurrence after pulmonary vein isolation (Li L, et al. Europace. 2014;16[9]:1309-14).
Prospective studies using CPAP to treat OSA have not demonstrated a reduced risk of adverse cardiovascular outcomes. The SAVE trial is the most well-known of these studies. The primary endpoint was death from cardiovascular causes (myocardial infarction, stroke, or hospitalization for unstable angina, heart failure, or transient ischemic attack). There was no difference in this outcome between the CPAP and usual care groups. A secondary outcome in this study was new-onset AF detected by electrocardiography, and there was no difference between the CPAP and the usual care group. The low amount of CPAP usage in the treatment group was a commonly cited shortcoming of the SAVE trial–mean usage was 4.4 hours per night during the first month of treatment and subsequently decreased to 3.3 hours per night by the 12-month time point (McEvoy RD, et al. N Engl J Med. 2016;375[10]:919-31).
Caples and colleagues screened patients undergoing direct current cardioversion or catheter ablation. They chose those who were also positive for OSA by polysomnography (apnea-hypopnea index – AHI greater than five events per hour). Twenty-five patients were included in the study and were randomly assigned to either CPAP treatment or usual care. Body mass index, blood pressure, ejection fraction, AHI, and nocturnal desaturation levels were comparable between the two groups. The rate of recurrence of AF and the time point following randomization at which the AF recurred did not differ between the two groups (Caples SM, et al. Int J Cardiol. 2019;278:133-6).
A Norwegian trial by Traaen and colleagues included a larger sample of 108 patients with moderate to severe sleep apnea and paroxysmal AF who underwent catheter ablation. Patients were followed for 5 months before and 12 months after ablation. They were randomly assigned to either CPAP therapy plus usual care or usual care alone. The primary goal was to assess AF burden using implanted loop recorders. There was no significant difference in AF burden between the two groups from baseline to the final 3 months of the study (Traaen GM, et al. Am J Respir Crit Care Med. 2021;204[5]:573-82). These two prospective trials, which had AF recurrence or burden as primary outcomes, found no interaction between AF burden and CPAP use, at least within the first year of therapy. Both trials found that their participants used CPAP for more extended periods of time than the SAVE trial, with over 6 hours in the Caples and coworkers’ trial and nearly 5 hours in the Traaen and coworkers’ study.
Is the lack of efficacy due to starting CPAP too late in the course of OSA?
It has been proposed that there may be a critical early period after the onset of OSA when intervention with CPAP (or alternative therapies) will be most effective in preventing adverse cardiovascular outcomes. An answer will almost certainly necessitate a long-term prospective study enrolling people before they develop OSA. Additionally, the AHI is used in most trials to determine the presence and severity of OSA. However, the AHI has been shown to have a poor correlation with sleep-related symptoms, and it may fail to capture key OSA pathophysiologic stressors (e.g., hyperadrenergic drive, cyclical hypoxemia, etc), which may increase the risk of AF. Other disease characteristics and polysomnographic features may better capture disease severity and the cardiovascular risk factors associated with it. The respiratory arousal threshold, arousal index, degree of loop gain, hypoxic burden, heart rate variability, and cardiopulmonary coupling are some examples of such features.
Another possible explanation is that AF is not causally related, and the demonstrated association between the two is because both conditions share risk factors such as age and BMI, among others. Or, if they are causally linked, OSA may be a minor contributor, and the magnitude of that contribution is insufficient to reduce the risk of AF significantly by treating OSA. More research is needed to define the salient intervenable aspects of OSA better and design the optimal timing and duration of intervention.
Dr. Mudrakola is with the Department of Pulmonary & Critical Care Medicine, Summa Health, Akron, Ohio. Dr. Selim is with the Department of Pulmonary & Critical Care Medicine, Mayo Clinic, Rochester, Minnesota.
Beyond CPAP: Looking to alternative treatments for obstructive sleep apnea
Overview of the problem
Obstructive sleep apnea (OSA) is an extraordinarily common condition impacting nearly 1 billion individuals globally (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687). For the past 40 years, the mainstay of treatment has been continuous positive airway pressure (CPAP). However, CPAP usage is highly variable, and not all sleep apnea is created the same with respect to underlying mechanism or patient symptoms. Currently, there is a global CPAP shortage, which has expedited the need for alternative therapies in OSA (Owens RL, et al. Am J Respir Crit Care Med. 2021;204[8]:887).
Characterizing OSA
First, it is important to understand that sleep apnea emerges for multiple reasons. Some examples include: an excessively collapsible airway, insufficient upper airway reflexes, low arousal threshold (awakening easily to ventilatory disturbance), as well as an unstable chemoreflex system. This list is not comprehensive. However, we believe that the future of OSA management will be targeted therapy for individual OSA traits.
Notably, the patient experience of OSA is also highly variable. Some individuals are excessively sleepy. Some individuals experience OSA as insomnia. Other patients are asymptomatic, but present to the sleep clinic at the behest of a disgruntled bed partner. These individual factors should all be kept in mind when deciding when and how to treat sleep apnea.
OSA scoring – past, present, and future
The traditional method for scoring sleep apnea severity is the apnea-hypopnea index (AHI), with mild, moderate, and severe OSA being stratified by the number of events per hour. This metric has shaped many of the modern sleep practices and consensus recommendations but is simply not sophisticated enough to capture the nuance of how or why an individual’s sleep is disrupted from flow-limited breathing. As such, there has been a push in recent times to tailor treatment for OSA to an individual’s physiology. Examples of alternative metrics which quantify sleep apnea traits include the apnea-hypopnea event duration, the sleep apnea-specific hypoxic burden (area under the SpO2 curve for flow-limited events), as well as the arousal intensity from sleep in the setting of flow-limited breathing. There are numerous other metrics that have been proposed but are beyond the scope of this review (Malhotra A, et al. Sleep. 2021;44[7]:zsab030).
What therapies are available and how can we individualize them to our patients?
As noted, CPAP has been the gold-standard for OSA treatment for 40 years but is not always accepted or tolerated (Malhotra A, et al. Chest. 2018;153[4]:843). Broad categories of OSA management are presented as follows.
Surgery for OSA
Upper airway surgery is effective for pediatric OSA treatment, where enlarged tonsils are often the culprit for flow-limited breathing in sleep. For adults, however, there is no one best surgery or surgical candidate. For instance, surgery can be used to improve CPAP tolerance or as a primary OSA treatment. Many individuals with sinus disease may require sinus surgery or septoplasty to improve CPAP tolerability by creating more space for airflow through the nasopharynx. Retrognathic individuals, on the other hand, may benefit from maxillomandibular advancement. Others may benefit from genioglossus advancement or hyoid suspension. The characteristics of the soft palate can be predictive of surgical success with respect to uvulopalatopharyngoplasty (UPPP), with longer uvulas and redundant soft palate tissue being attractive surgical targets. Obviously, this list is far from comprehensive, but Friedman tongue position, tonsil size, and body mass index also appear to be important in predicting surgical success (MacKay S, et al. JAMA. 2020;324[12]:1168).
Hypoglossal nerve stimulation is one surgical treatment option for patients with moderate-severe OSA who are unable or unwilling to use CPAP therapy, have a BMI <32-35 kg/m2 (center-dependent), no concentric velopharyngeal collapse on drug-induced sleep endoscopy, and fewer than 25% central/mixed apneas on their sleep study. Areas for further study are whether unilateral or bilateral stimulation are most effective, as well as which of the sleep apnea traits are most predictive of a treatment response (Strohl MM, et al. Curr Sleep Med Rep. 2017;3[3]:133).
Notably, surgical techniques are highly variable, and there are individual patient characteristics, such as lower loop gain (more stable ventilatory control), which may have a greater likelihood of successful upper airway surgery. This is likely because making the upper airway more patent allows for ventilatory overshoots and thereby airway collapse and cyclic, unstable breathing in those with an unstable ventilatory control system. Trials with prespecified surgical techniques based on individual traits are welcome. Additionally, the metrics of a successful surgical treatment for OSA, much like the AHI, are in need of evolution. The Sher criteria, for instance (50% AHI reduction to an AHI < 20/h), are arbitrary, and their clinical utility is unclear.
Oral appliances
Oral appliances fall into two broad categories – tongue-retaining devices and mandibular advancement splints (MAS). Of the two, MAS are much more commonly prescribed. Of the MAS devices, custom made devices by an American Academy of Dental Sleep Medicine (AADSM)–trained dentist are recommended over noncustom MAS in the treatment of primary snoring or OSA for those unwilling or unable to wear CPAP. Notably, the 2015 American Academy of Sleep Medicine (AASM) and AADSM shared guidelines were unable to make OSA treatment recommendations based on severity of disease as stratified by the AHI due to the limited quality of evidence. These devices are broadly thought to work by protruding the mandible/tongue and, in-turn, advancing multiple soft tissue components of the velopharynx. Relatively recent work suggests that the following OSA traits are associated with MAS efficacy: lower loop gain, higher arousal threshold, lower ventilatory response to arousal, moderate pharyngeal collapsibility, and weaker upper airway dilator muscle compensation. However, in order for these devices to be successful, close follow-up for titration with a AADSM-certified dentist, as well as a follow-up efficacy sleep study, are recommended. Adherence for custom device use appears to be about 70% use greater than 4 hours per night, with 35% to 40% of those prescribed a device achieving an AHI less than 5/h. Over the counter devices are not routinely recommended, though some practices do use these devices as a trial to see if patients may tolerate custom made devices (Ramar K, et al. J Clin Sleep Med. 2015;11[7]:773).
Upper airway training
Upper airway training has been shown possibly to be effective in treating OSA, though the ideal endotype is still being established. Upper airway training has taken many forms, from woodwind instrument playing, to nocturnal electrical stimulation of the tongue, and, more recently, daytime awake transoral neuromuscular stimulation. These interventions appear to be effective for mild sleep apnea and snoring, but the best training regimen has yet to be established. Equally, as with other routine exercise, there appears to be a “use it or lose it” component, and the ideal maintenance regimen for each of these therapies is yet to be determined.
Weight loss and bariatric surgery
Obesity is a common, reversible risk factor for OSA. However, not all obese individuals develop OSA (typically those with robust upper airway reflexes). Improvements in weight appear to correlate with reductions in tongue fat, which correlate to AHI reduction. Weight loss also creates lower CPAP requirements for many individuals, conceivably improving tolerability. Ongoing work is seeking to understand whether there are changes in upper airway muscle recruitability as well as other change in endotype traits following weight loss surgery.
Pharmacotherapy for OSA
There is a great deal of promise in tailoring pharmacotherapy to individual sleep traits. Acetazolamide, for instance, results in improvements an AHI for both obstructive and central sleep apnea through changes in chemosensitivity and is generally well-tolerated (Schmickl CN, et al. Physiol Rep. 2021;9[20]:e15071). Eszopiclone has been used to raise the arousal threshold for those who awaken from breathing events too easily. With added time, individuals with a low arousal threshold can more effectively recruit upper airway dilator muscles without waking up. Pharmacotherapy to improve upper airway recruitability with combination noradrenergic stimulation and antimuscarinic activity has limited data thus far but may be a useful part of the sleep armamentarium moving forward.
Summary
OSA is a public health priority, and the current global CPAP shortage emphasizes the need for alternative OSA therapies. The ideal therapy for a given patient requires a careful consideration of their individual traits and will be much more refined when endotyping is available in a routine clinical setting. Individualized sleep apnea treatment is the future of sleep medicine and a one-size fits all approach no longer meets the needs of our patients given the current state of sleep medicine knowledge.
Dr. Nokes, Dr. Schmickl, and Dr. Malhotra are with the University of California, San Diego, Division of Pulmonary, Critical Care, and Sleep Medicine, La, Jolla, CA. Dr. Nokes also is with the Veterans Affairs San Diego Healthcare System, sleep section, San Diego, CA. Dr. Vahabzadeh-Hagh is with the University of California, San Diego, Department of Otolaryngology, San Diego, CA.
Overview of the problem
Obstructive sleep apnea (OSA) is an extraordinarily common condition impacting nearly 1 billion individuals globally (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687). For the past 40 years, the mainstay of treatment has been continuous positive airway pressure (CPAP). However, CPAP usage is highly variable, and not all sleep apnea is created the same with respect to underlying mechanism or patient symptoms. Currently, there is a global CPAP shortage, which has expedited the need for alternative therapies in OSA (Owens RL, et al. Am J Respir Crit Care Med. 2021;204[8]:887).
Characterizing OSA
First, it is important to understand that sleep apnea emerges for multiple reasons. Some examples include: an excessively collapsible airway, insufficient upper airway reflexes, low arousal threshold (awakening easily to ventilatory disturbance), as well as an unstable chemoreflex system. This list is not comprehensive. However, we believe that the future of OSA management will be targeted therapy for individual OSA traits.
Notably, the patient experience of OSA is also highly variable. Some individuals are excessively sleepy. Some individuals experience OSA as insomnia. Other patients are asymptomatic, but present to the sleep clinic at the behest of a disgruntled bed partner. These individual factors should all be kept in mind when deciding when and how to treat sleep apnea.
OSA scoring – past, present, and future
The traditional method for scoring sleep apnea severity is the apnea-hypopnea index (AHI), with mild, moderate, and severe OSA being stratified by the number of events per hour. This metric has shaped many of the modern sleep practices and consensus recommendations but is simply not sophisticated enough to capture the nuance of how or why an individual’s sleep is disrupted from flow-limited breathing. As such, there has been a push in recent times to tailor treatment for OSA to an individual’s physiology. Examples of alternative metrics which quantify sleep apnea traits include the apnea-hypopnea event duration, the sleep apnea-specific hypoxic burden (area under the SpO2 curve for flow-limited events), as well as the arousal intensity from sleep in the setting of flow-limited breathing. There are numerous other metrics that have been proposed but are beyond the scope of this review (Malhotra A, et al. Sleep. 2021;44[7]:zsab030).
What therapies are available and how can we individualize them to our patients?
As noted, CPAP has been the gold-standard for OSA treatment for 40 years but is not always accepted or tolerated (Malhotra A, et al. Chest. 2018;153[4]:843). Broad categories of OSA management are presented as follows.
Surgery for OSA
Upper airway surgery is effective for pediatric OSA treatment, where enlarged tonsils are often the culprit for flow-limited breathing in sleep. For adults, however, there is no one best surgery or surgical candidate. For instance, surgery can be used to improve CPAP tolerance or as a primary OSA treatment. Many individuals with sinus disease may require sinus surgery or septoplasty to improve CPAP tolerability by creating more space for airflow through the nasopharynx. Retrognathic individuals, on the other hand, may benefit from maxillomandibular advancement. Others may benefit from genioglossus advancement or hyoid suspension. The characteristics of the soft palate can be predictive of surgical success with respect to uvulopalatopharyngoplasty (UPPP), with longer uvulas and redundant soft palate tissue being attractive surgical targets. Obviously, this list is far from comprehensive, but Friedman tongue position, tonsil size, and body mass index also appear to be important in predicting surgical success (MacKay S, et al. JAMA. 2020;324[12]:1168).
Hypoglossal nerve stimulation is one surgical treatment option for patients with moderate-severe OSA who are unable or unwilling to use CPAP therapy, have a BMI <32-35 kg/m2 (center-dependent), no concentric velopharyngeal collapse on drug-induced sleep endoscopy, and fewer than 25% central/mixed apneas on their sleep study. Areas for further study are whether unilateral or bilateral stimulation are most effective, as well as which of the sleep apnea traits are most predictive of a treatment response (Strohl MM, et al. Curr Sleep Med Rep. 2017;3[3]:133).
Notably, surgical techniques are highly variable, and there are individual patient characteristics, such as lower loop gain (more stable ventilatory control), which may have a greater likelihood of successful upper airway surgery. This is likely because making the upper airway more patent allows for ventilatory overshoots and thereby airway collapse and cyclic, unstable breathing in those with an unstable ventilatory control system. Trials with prespecified surgical techniques based on individual traits are welcome. Additionally, the metrics of a successful surgical treatment for OSA, much like the AHI, are in need of evolution. The Sher criteria, for instance (50% AHI reduction to an AHI < 20/h), are arbitrary, and their clinical utility is unclear.
Oral appliances
Oral appliances fall into two broad categories – tongue-retaining devices and mandibular advancement splints (MAS). Of the two, MAS are much more commonly prescribed. Of the MAS devices, custom made devices by an American Academy of Dental Sleep Medicine (AADSM)–trained dentist are recommended over noncustom MAS in the treatment of primary snoring or OSA for those unwilling or unable to wear CPAP. Notably, the 2015 American Academy of Sleep Medicine (AASM) and AADSM shared guidelines were unable to make OSA treatment recommendations based on severity of disease as stratified by the AHI due to the limited quality of evidence. These devices are broadly thought to work by protruding the mandible/tongue and, in-turn, advancing multiple soft tissue components of the velopharynx. Relatively recent work suggests that the following OSA traits are associated with MAS efficacy: lower loop gain, higher arousal threshold, lower ventilatory response to arousal, moderate pharyngeal collapsibility, and weaker upper airway dilator muscle compensation. However, in order for these devices to be successful, close follow-up for titration with a AADSM-certified dentist, as well as a follow-up efficacy sleep study, are recommended. Adherence for custom device use appears to be about 70% use greater than 4 hours per night, with 35% to 40% of those prescribed a device achieving an AHI less than 5/h. Over the counter devices are not routinely recommended, though some practices do use these devices as a trial to see if patients may tolerate custom made devices (Ramar K, et al. J Clin Sleep Med. 2015;11[7]:773).
Upper airway training
Upper airway training has been shown possibly to be effective in treating OSA, though the ideal endotype is still being established. Upper airway training has taken many forms, from woodwind instrument playing, to nocturnal electrical stimulation of the tongue, and, more recently, daytime awake transoral neuromuscular stimulation. These interventions appear to be effective for mild sleep apnea and snoring, but the best training regimen has yet to be established. Equally, as with other routine exercise, there appears to be a “use it or lose it” component, and the ideal maintenance regimen for each of these therapies is yet to be determined.
Weight loss and bariatric surgery
Obesity is a common, reversible risk factor for OSA. However, not all obese individuals develop OSA (typically those with robust upper airway reflexes). Improvements in weight appear to correlate with reductions in tongue fat, which correlate to AHI reduction. Weight loss also creates lower CPAP requirements for many individuals, conceivably improving tolerability. Ongoing work is seeking to understand whether there are changes in upper airway muscle recruitability as well as other change in endotype traits following weight loss surgery.
Pharmacotherapy for OSA
There is a great deal of promise in tailoring pharmacotherapy to individual sleep traits. Acetazolamide, for instance, results in improvements an AHI for both obstructive and central sleep apnea through changes in chemosensitivity and is generally well-tolerated (Schmickl CN, et al. Physiol Rep. 2021;9[20]:e15071). Eszopiclone has been used to raise the arousal threshold for those who awaken from breathing events too easily. With added time, individuals with a low arousal threshold can more effectively recruit upper airway dilator muscles without waking up. Pharmacotherapy to improve upper airway recruitability with combination noradrenergic stimulation and antimuscarinic activity has limited data thus far but may be a useful part of the sleep armamentarium moving forward.
Summary
OSA is a public health priority, and the current global CPAP shortage emphasizes the need for alternative OSA therapies. The ideal therapy for a given patient requires a careful consideration of their individual traits and will be much more refined when endotyping is available in a routine clinical setting. Individualized sleep apnea treatment is the future of sleep medicine and a one-size fits all approach no longer meets the needs of our patients given the current state of sleep medicine knowledge.
Dr. Nokes, Dr. Schmickl, and Dr. Malhotra are with the University of California, San Diego, Division of Pulmonary, Critical Care, and Sleep Medicine, La, Jolla, CA. Dr. Nokes also is with the Veterans Affairs San Diego Healthcare System, sleep section, San Diego, CA. Dr. Vahabzadeh-Hagh is with the University of California, San Diego, Department of Otolaryngology, San Diego, CA.
Overview of the problem
Obstructive sleep apnea (OSA) is an extraordinarily common condition impacting nearly 1 billion individuals globally (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687). For the past 40 years, the mainstay of treatment has been continuous positive airway pressure (CPAP). However, CPAP usage is highly variable, and not all sleep apnea is created the same with respect to underlying mechanism or patient symptoms. Currently, there is a global CPAP shortage, which has expedited the need for alternative therapies in OSA (Owens RL, et al. Am J Respir Crit Care Med. 2021;204[8]:887).
Characterizing OSA
First, it is important to understand that sleep apnea emerges for multiple reasons. Some examples include: an excessively collapsible airway, insufficient upper airway reflexes, low arousal threshold (awakening easily to ventilatory disturbance), as well as an unstable chemoreflex system. This list is not comprehensive. However, we believe that the future of OSA management will be targeted therapy for individual OSA traits.
Notably, the patient experience of OSA is also highly variable. Some individuals are excessively sleepy. Some individuals experience OSA as insomnia. Other patients are asymptomatic, but present to the sleep clinic at the behest of a disgruntled bed partner. These individual factors should all be kept in mind when deciding when and how to treat sleep apnea.
OSA scoring – past, present, and future
The traditional method for scoring sleep apnea severity is the apnea-hypopnea index (AHI), with mild, moderate, and severe OSA being stratified by the number of events per hour. This metric has shaped many of the modern sleep practices and consensus recommendations but is simply not sophisticated enough to capture the nuance of how or why an individual’s sleep is disrupted from flow-limited breathing. As such, there has been a push in recent times to tailor treatment for OSA to an individual’s physiology. Examples of alternative metrics which quantify sleep apnea traits include the apnea-hypopnea event duration, the sleep apnea-specific hypoxic burden (area under the SpO2 curve for flow-limited events), as well as the arousal intensity from sleep in the setting of flow-limited breathing. There are numerous other metrics that have been proposed but are beyond the scope of this review (Malhotra A, et al. Sleep. 2021;44[7]:zsab030).
What therapies are available and how can we individualize them to our patients?
As noted, CPAP has been the gold-standard for OSA treatment for 40 years but is not always accepted or tolerated (Malhotra A, et al. Chest. 2018;153[4]:843). Broad categories of OSA management are presented as follows.
Surgery for OSA
Upper airway surgery is effective for pediatric OSA treatment, where enlarged tonsils are often the culprit for flow-limited breathing in sleep. For adults, however, there is no one best surgery or surgical candidate. For instance, surgery can be used to improve CPAP tolerance or as a primary OSA treatment. Many individuals with sinus disease may require sinus surgery or septoplasty to improve CPAP tolerability by creating more space for airflow through the nasopharynx. Retrognathic individuals, on the other hand, may benefit from maxillomandibular advancement. Others may benefit from genioglossus advancement or hyoid suspension. The characteristics of the soft palate can be predictive of surgical success with respect to uvulopalatopharyngoplasty (UPPP), with longer uvulas and redundant soft palate tissue being attractive surgical targets. Obviously, this list is far from comprehensive, but Friedman tongue position, tonsil size, and body mass index also appear to be important in predicting surgical success (MacKay S, et al. JAMA. 2020;324[12]:1168).
Hypoglossal nerve stimulation is one surgical treatment option for patients with moderate-severe OSA who are unable or unwilling to use CPAP therapy, have a BMI <32-35 kg/m2 (center-dependent), no concentric velopharyngeal collapse on drug-induced sleep endoscopy, and fewer than 25% central/mixed apneas on their sleep study. Areas for further study are whether unilateral or bilateral stimulation are most effective, as well as which of the sleep apnea traits are most predictive of a treatment response (Strohl MM, et al. Curr Sleep Med Rep. 2017;3[3]:133).
Notably, surgical techniques are highly variable, and there are individual patient characteristics, such as lower loop gain (more stable ventilatory control), which may have a greater likelihood of successful upper airway surgery. This is likely because making the upper airway more patent allows for ventilatory overshoots and thereby airway collapse and cyclic, unstable breathing in those with an unstable ventilatory control system. Trials with prespecified surgical techniques based on individual traits are welcome. Additionally, the metrics of a successful surgical treatment for OSA, much like the AHI, are in need of evolution. The Sher criteria, for instance (50% AHI reduction to an AHI < 20/h), are arbitrary, and their clinical utility is unclear.
Oral appliances
Oral appliances fall into two broad categories – tongue-retaining devices and mandibular advancement splints (MAS). Of the two, MAS are much more commonly prescribed. Of the MAS devices, custom made devices by an American Academy of Dental Sleep Medicine (AADSM)–trained dentist are recommended over noncustom MAS in the treatment of primary snoring or OSA for those unwilling or unable to wear CPAP. Notably, the 2015 American Academy of Sleep Medicine (AASM) and AADSM shared guidelines were unable to make OSA treatment recommendations based on severity of disease as stratified by the AHI due to the limited quality of evidence. These devices are broadly thought to work by protruding the mandible/tongue and, in-turn, advancing multiple soft tissue components of the velopharynx. Relatively recent work suggests that the following OSA traits are associated with MAS efficacy: lower loop gain, higher arousal threshold, lower ventilatory response to arousal, moderate pharyngeal collapsibility, and weaker upper airway dilator muscle compensation. However, in order for these devices to be successful, close follow-up for titration with a AADSM-certified dentist, as well as a follow-up efficacy sleep study, are recommended. Adherence for custom device use appears to be about 70% use greater than 4 hours per night, with 35% to 40% of those prescribed a device achieving an AHI less than 5/h. Over the counter devices are not routinely recommended, though some practices do use these devices as a trial to see if patients may tolerate custom made devices (Ramar K, et al. J Clin Sleep Med. 2015;11[7]:773).
Upper airway training
Upper airway training has been shown possibly to be effective in treating OSA, though the ideal endotype is still being established. Upper airway training has taken many forms, from woodwind instrument playing, to nocturnal electrical stimulation of the tongue, and, more recently, daytime awake transoral neuromuscular stimulation. These interventions appear to be effective for mild sleep apnea and snoring, but the best training regimen has yet to be established. Equally, as with other routine exercise, there appears to be a “use it or lose it” component, and the ideal maintenance regimen for each of these therapies is yet to be determined.
Weight loss and bariatric surgery
Obesity is a common, reversible risk factor for OSA. However, not all obese individuals develop OSA (typically those with robust upper airway reflexes). Improvements in weight appear to correlate with reductions in tongue fat, which correlate to AHI reduction. Weight loss also creates lower CPAP requirements for many individuals, conceivably improving tolerability. Ongoing work is seeking to understand whether there are changes in upper airway muscle recruitability as well as other change in endotype traits following weight loss surgery.
Pharmacotherapy for OSA
There is a great deal of promise in tailoring pharmacotherapy to individual sleep traits. Acetazolamide, for instance, results in improvements an AHI for both obstructive and central sleep apnea through changes in chemosensitivity and is generally well-tolerated (Schmickl CN, et al. Physiol Rep. 2021;9[20]:e15071). Eszopiclone has been used to raise the arousal threshold for those who awaken from breathing events too easily. With added time, individuals with a low arousal threshold can more effectively recruit upper airway dilator muscles without waking up. Pharmacotherapy to improve upper airway recruitability with combination noradrenergic stimulation and antimuscarinic activity has limited data thus far but may be a useful part of the sleep armamentarium moving forward.
Summary
OSA is a public health priority, and the current global CPAP shortage emphasizes the need for alternative OSA therapies. The ideal therapy for a given patient requires a careful consideration of their individual traits and will be much more refined when endotyping is available in a routine clinical setting. Individualized sleep apnea treatment is the future of sleep medicine and a one-size fits all approach no longer meets the needs of our patients given the current state of sleep medicine knowledge.
Dr. Nokes, Dr. Schmickl, and Dr. Malhotra are with the University of California, San Diego, Division of Pulmonary, Critical Care, and Sleep Medicine, La, Jolla, CA. Dr. Nokes also is with the Veterans Affairs San Diego Healthcare System, sleep section, San Diego, CA. Dr. Vahabzadeh-Hagh is with the University of California, San Diego, Department of Otolaryngology, San Diego, CA.
The quest for a good night’s sleep: An update on pharmacologic therapy for insomnia
Insomnia is one of the most common complaints in medicine, driving millions of clinic visits each year (Table 1). It is estimated that approximately 30% of individuals report at least short-term insomnia symptoms and 10% report chronic insomnia. These rates are even higher in groups that may be more susceptible to insomnia, including women, the elderly, and those of disadvantaged socioeconomic status (Ohayon MM. Sleep Med Rev. 2002;[2]:97-111). While most patients with insomnia find their sleep difficulties self-resolve within 3 months, a substantial number of patients will find their insomnia to persist for longer and require intervention (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
For individuals requiring treatment, cognitive behavioral therapy for insomnia (CBT-I) is considered first-line therapy by the American Academy of Sleep Medicine for both acute and chronic insomnia. Unfortunately, obtaining CBT-I for a patient is often a challenge as the number of trained therapists offering this service is limited, resulting in long wait times or, in some cases, a complete lack of access to this treatment option. Judicious use of sedative-hypnotic medications may be a reasonable alternative for patients with insomnia who are unable to undergo CBT-I, who are still symptomatic despite undergoing CBT-I, or, in some cases, as a temporary treatment (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
Current medications used to treat insomnia are listed in Tables 2 and 3, some of which carry an FDA approval to be used as a hypnotic, while others are used in an off-label manner.
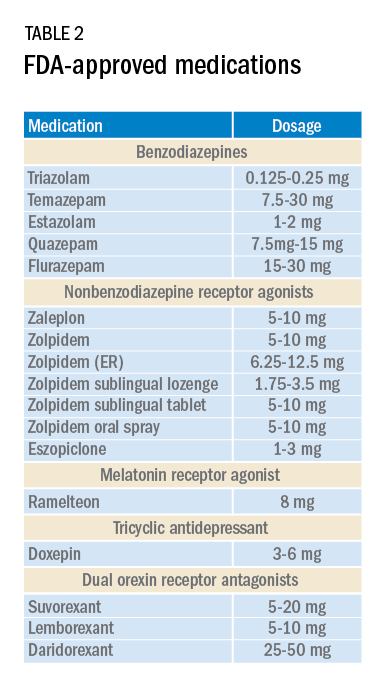
Cautions abound with use of many of these medications. Common concerns include safety, particularly for elderly patients and long-term use, and the potential for developing tolerance and dependence.
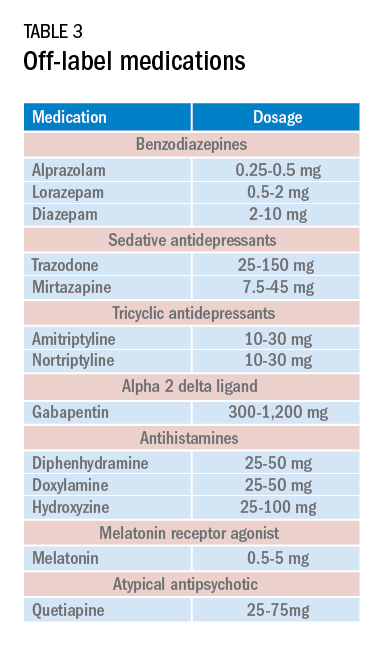
Most medications that have been used for insomnia have been available for decades, but, in recent years, a new class of hypnotics has emerged. Dual orexin receptor antagonists (DORAs) are the newest class of FDA-approved medications (Table 4).
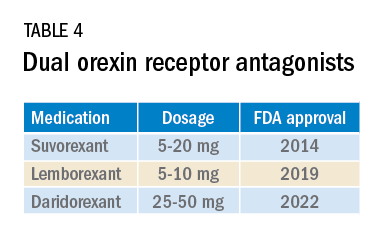
Orexin is a neuropeptide found primarily in the lateral hypothalamus and binds to the orexin 1 and orexin 2 receptors leading to a number of downstream effects, including stimulating wakefulness. Loss of orexin-generating neurons has been implicated as the cause of type 1 narcolepsy, and antagonism of their effects can facilitate sleep by suppressing wakefulness. The first medication in the DORA class to be FDA-approved was suvorexant in 2014, followed by lemborexant’s FDA approval in 2019. These are both indicated for treating sleep onset and sleep maintenance insomnia and have been shown to improve both subjective and objective measures of sleep. The most common side effects reported for both suvorexant and lemborexant are headache and somnolence, with morning-after sleepiness being a frequent complaint.
In January 2022, a new medication in the DORA class named daridorexant was approved by the FDA (Table 5).
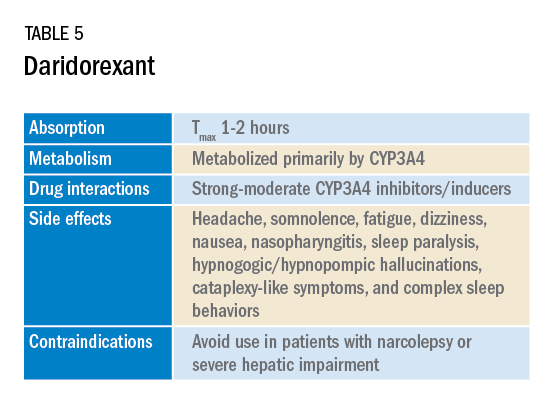
Daridorexant, like its DORA counterparts, has been shown to have efficacy in improving subjective and objective markers of insomnia. This has included polysomnographic measures of wake after sleep onset and latency to persistent sleep, as well as subjective total sleep time. Importantly, in addition to positive sleep outcomes, improvements with daytime function have also been observed with this medication (Mignot E et al. Lancet Neurol. 2022;21[2]:125-39). Daridorexant’s half-life of approximately 8 hours is shorter than that of the other available DORAs, leading to fewer day-after effects. The combination of effectiveness for sleep initiation and maintenance without daytime impairment distinguishes daridorexant from the other DORAs and even other classes of sleep medication.
Safety, especially in patients of age 65 and older, is an important concern with sleep medication, particularly with respect to polypharmacy, over-sedation, increased fall risk, and cognitive impairment, but daridorexant’s available safety data suggest a favorable safety profile (Zammit G et al. Neurology. 2020;94[21]:e2222-32).
Daridorexant at the highest dose available, 50 mg, did not worsen respiratory function, in terms of the apnea-hypopnea index and oxygen saturation in individuals with mild-moderate obstructive sleep apnea regardless of sleep stage (Boof ML et al. Sleep. 2021;44[6]:zsaa275). However, more safety and longitudinal data are needed to have a fuller understanding of any potential limitations of this medication.
While we continue to recommend CBT-I as the first-line treatment whenever possible for patients with insomnia, not all patients have access to this treatment and not all patients will respond satisfactorily to it. Thus, pharmacologic treatment can continue to play an important role in the management of some patients’ insomnia. Each class of medications used for treating insomnia features a unique constellation of advantages and limitations, meaning that the more available options, the greater the chances of finding an option that will be both effective and safe for a particular patient. The growing DORA class, especially its newest available entrant, daridorexant, represents a continued expansion of the armamentarium of options against insomnia.
Dr. Pelekanos and Dr. Sum-Ping are with the Division of Sleep Medicine, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, California.
Insomnia is one of the most common complaints in medicine, driving millions of clinic visits each year (Table 1). It is estimated that approximately 30% of individuals report at least short-term insomnia symptoms and 10% report chronic insomnia. These rates are even higher in groups that may be more susceptible to insomnia, including women, the elderly, and those of disadvantaged socioeconomic status (Ohayon MM. Sleep Med Rev. 2002;[2]:97-111). While most patients with insomnia find their sleep difficulties self-resolve within 3 months, a substantial number of patients will find their insomnia to persist for longer and require intervention (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).

For individuals requiring treatment, cognitive behavioral therapy for insomnia (CBT-I) is considered first-line therapy by the American Academy of Sleep Medicine for both acute and chronic insomnia. Unfortunately, obtaining CBT-I for a patient is often a challenge as the number of trained therapists offering this service is limited, resulting in long wait times or, in some cases, a complete lack of access to this treatment option. Judicious use of sedative-hypnotic medications may be a reasonable alternative for patients with insomnia who are unable to undergo CBT-I, who are still symptomatic despite undergoing CBT-I, or, in some cases, as a temporary treatment (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
Current medications used to treat insomnia are listed in Tables 2 and 3, some of which carry an FDA approval to be used as a hypnotic, while others are used in an off-label manner.

Cautions abound with use of many of these medications. Common concerns include safety, particularly for elderly patients and long-term use, and the potential for developing tolerance and dependence.

Most medications that have been used for insomnia have been available for decades, but, in recent years, a new class of hypnotics has emerged. Dual orexin receptor antagonists (DORAs) are the newest class of FDA-approved medications (Table 4).

Orexin is a neuropeptide found primarily in the lateral hypothalamus and binds to the orexin 1 and orexin 2 receptors leading to a number of downstream effects, including stimulating wakefulness. Loss of orexin-generating neurons has been implicated as the cause of type 1 narcolepsy, and antagonism of their effects can facilitate sleep by suppressing wakefulness. The first medication in the DORA class to be FDA-approved was suvorexant in 2014, followed by lemborexant’s FDA approval in 2019. These are both indicated for treating sleep onset and sleep maintenance insomnia and have been shown to improve both subjective and objective measures of sleep. The most common side effects reported for both suvorexant and lemborexant are headache and somnolence, with morning-after sleepiness being a frequent complaint.
In January 2022, a new medication in the DORA class named daridorexant was approved by the FDA (Table 5).

Daridorexant, like its DORA counterparts, has been shown to have efficacy in improving subjective and objective markers of insomnia. This has included polysomnographic measures of wake after sleep onset and latency to persistent sleep, as well as subjective total sleep time. Importantly, in addition to positive sleep outcomes, improvements with daytime function have also been observed with this medication (Mignot E et al. Lancet Neurol. 2022;21[2]:125-39). Daridorexant’s half-life of approximately 8 hours is shorter than that of the other available DORAs, leading to fewer day-after effects. The combination of effectiveness for sleep initiation and maintenance without daytime impairment distinguishes daridorexant from the other DORAs and even other classes of sleep medication.
Safety, especially in patients of age 65 and older, is an important concern with sleep medication, particularly with respect to polypharmacy, over-sedation, increased fall risk, and cognitive impairment, but daridorexant’s available safety data suggest a favorable safety profile (Zammit G et al. Neurology. 2020;94[21]:e2222-32).
Daridorexant at the highest dose available, 50 mg, did not worsen respiratory function, in terms of the apnea-hypopnea index and oxygen saturation in individuals with mild-moderate obstructive sleep apnea regardless of sleep stage (Boof ML et al. Sleep. 2021;44[6]:zsaa275). However, more safety and longitudinal data are needed to have a fuller understanding of any potential limitations of this medication.
While we continue to recommend CBT-I as the first-line treatment whenever possible for patients with insomnia, not all patients have access to this treatment and not all patients will respond satisfactorily to it. Thus, pharmacologic treatment can continue to play an important role in the management of some patients’ insomnia. Each class of medications used for treating insomnia features a unique constellation of advantages and limitations, meaning that the more available options, the greater the chances of finding an option that will be both effective and safe for a particular patient. The growing DORA class, especially its newest available entrant, daridorexant, represents a continued expansion of the armamentarium of options against insomnia.
Dr. Pelekanos and Dr. Sum-Ping are with the Division of Sleep Medicine, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, California.
Insomnia is one of the most common complaints in medicine, driving millions of clinic visits each year (Table 1). It is estimated that approximately 30% of individuals report at least short-term insomnia symptoms and 10% report chronic insomnia. These rates are even higher in groups that may be more susceptible to insomnia, including women, the elderly, and those of disadvantaged socioeconomic status (Ohayon MM. Sleep Med Rev. 2002;[2]:97-111). While most patients with insomnia find their sleep difficulties self-resolve within 3 months, a substantial number of patients will find their insomnia to persist for longer and require intervention (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).

For individuals requiring treatment, cognitive behavioral therapy for insomnia (CBT-I) is considered first-line therapy by the American Academy of Sleep Medicine for both acute and chronic insomnia. Unfortunately, obtaining CBT-I for a patient is often a challenge as the number of trained therapists offering this service is limited, resulting in long wait times or, in some cases, a complete lack of access to this treatment option. Judicious use of sedative-hypnotic medications may be a reasonable alternative for patients with insomnia who are unable to undergo CBT-I, who are still symptomatic despite undergoing CBT-I, or, in some cases, as a temporary treatment (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
Current medications used to treat insomnia are listed in Tables 2 and 3, some of which carry an FDA approval to be used as a hypnotic, while others are used in an off-label manner.

Cautions abound with use of many of these medications. Common concerns include safety, particularly for elderly patients and long-term use, and the potential for developing tolerance and dependence.

Most medications that have been used for insomnia have been available for decades, but, in recent years, a new class of hypnotics has emerged. Dual orexin receptor antagonists (DORAs) are the newest class of FDA-approved medications (Table 4).

Orexin is a neuropeptide found primarily in the lateral hypothalamus and binds to the orexin 1 and orexin 2 receptors leading to a number of downstream effects, including stimulating wakefulness. Loss of orexin-generating neurons has been implicated as the cause of type 1 narcolepsy, and antagonism of their effects can facilitate sleep by suppressing wakefulness. The first medication in the DORA class to be FDA-approved was suvorexant in 2014, followed by lemborexant’s FDA approval in 2019. These are both indicated for treating sleep onset and sleep maintenance insomnia and have been shown to improve both subjective and objective measures of sleep. The most common side effects reported for both suvorexant and lemborexant are headache and somnolence, with morning-after sleepiness being a frequent complaint.
In January 2022, a new medication in the DORA class named daridorexant was approved by the FDA (Table 5).

Daridorexant, like its DORA counterparts, has been shown to have efficacy in improving subjective and objective markers of insomnia. This has included polysomnographic measures of wake after sleep onset and latency to persistent sleep, as well as subjective total sleep time. Importantly, in addition to positive sleep outcomes, improvements with daytime function have also been observed with this medication (Mignot E et al. Lancet Neurol. 2022;21[2]:125-39). Daridorexant’s half-life of approximately 8 hours is shorter than that of the other available DORAs, leading to fewer day-after effects. The combination of effectiveness for sleep initiation and maintenance without daytime impairment distinguishes daridorexant from the other DORAs and even other classes of sleep medication.
Safety, especially in patients of age 65 and older, is an important concern with sleep medication, particularly with respect to polypharmacy, over-sedation, increased fall risk, and cognitive impairment, but daridorexant’s available safety data suggest a favorable safety profile (Zammit G et al. Neurology. 2020;94[21]:e2222-32).
Daridorexant at the highest dose available, 50 mg, did not worsen respiratory function, in terms of the apnea-hypopnea index and oxygen saturation in individuals with mild-moderate obstructive sleep apnea regardless of sleep stage (Boof ML et al. Sleep. 2021;44[6]:zsaa275). However, more safety and longitudinal data are needed to have a fuller understanding of any potential limitations of this medication.
While we continue to recommend CBT-I as the first-line treatment whenever possible for patients with insomnia, not all patients have access to this treatment and not all patients will respond satisfactorily to it. Thus, pharmacologic treatment can continue to play an important role in the management of some patients’ insomnia. Each class of medications used for treating insomnia features a unique constellation of advantages and limitations, meaning that the more available options, the greater the chances of finding an option that will be both effective and safe for a particular patient. The growing DORA class, especially its newest available entrant, daridorexant, represents a continued expansion of the armamentarium of options against insomnia.
Dr. Pelekanos and Dr. Sum-Ping are with the Division of Sleep Medicine, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, California.
The apnea-hypopnea index: Limitations and future directions
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway collapse resulting in intermittent hypoxemia and hypercapnia, large intrathoracic pressure swings, and cortical arousals. The rate of apneas and hypopneas observed during sleep, the apnea-hypopnea index (AHI), has been used for decades to diagnose OSA and to classify its severity. Despite the wide acceptance of this metric by the sleep medicine community, clinical research has found poor correlations between the AHI- and OSA-related complications or symptoms. We have come to learn that the AHI is an oversimplification of a complex and diverse disease process. (Punjabi. Chest. 2016;149[1]:16-9).
The most important features of a disease metric are reliability, and the ability to predict clinically relevant outcomes. The reliability of the AHI has been in question due to substantial night-to-night variability that can lead to missed diagnosis and disease severity misclassification (Dzierzewski et al. J Clin Sleep Med. 2020;16[4]:539-44). Furthermore, the AHI fails to reflect some important physiologic derangements resulting from respiratory events. Apart from imperfectly set thresholds for scoring, it disregards the depth and the duration of ventilatory disturbances. For example, a hypopnea lasting 30 seconds and resulting in a decrease of 10% in oxyhemoglobin saturation is considered equivalent to a hypopnea lasting 10 seconds and resulting in a decrease of 4% in oxyhemoglobin saturation. The AHI also assumes that apneas and hypopneas are equal in their biological effects regardless of when they occur during sleep (NREM vs REM), despite reports suggesting that the sequalae of OSA are sleep-stage dependent (Varga, Mokhlesi. Sleep Breath. 2019;23[2]:413-23). This is further complicated by the varying hypopnea definitions and the difficulties in differentiating obstructive vs central hypopneas. It is doubtful that these events, which differ in mechanism, would result in similar outcomes.
Over the past decade, our understanding of the different pathophysiological mechanisms leading to OSA has grown substantially, suggesting the need for a phenotype-specific treatment approach (Zinchuk, Yaggi. Chest. 2020;157[2]:403-20). The reliance on a single metric that does not capture this heterogeneity may prove detrimental to our therapeutic efforts. One extremely important dimension that is missed by the AHI is the patient. Individual response to airway obstruction varies with age, genetics, gender, and comorbidities, among other things. This may explain the difference in symptoms and outcomes experienced by patients with the same AHI. During the era of precision medicine, the concept of defining a clinical condition by a single test result, without regard to patient characteristics, is antiquated.
Several studies have attempted to propose complementary metrics that may better characterize OSA and predict outcomes. The hypoxic burden has gained a lot of attention as it is generally felt that hypoxemia is a major factor contributing to the pathogenesis of OSA-related comorbidities. Azarbarzin, et al. reported a hypoxic burden metric by measuring the area under the oxygen desaturation curve during a respiratory event (Azarbarzin et al. Eur Heart J. 2019;40[14]:1149-57). It factors the length and depth of the desaturations into a single value that expresses the average desaturation burden per hour of sleep time. The hypoxic burden was independently predictive of cardiovascular mortality in two large cohorts. Interestingly, the AHI did not have such an association. Similarly, another novel proposed parameter, the oxygen desaturation rate (ODR), outperformed the AHI in predicting cardiovascular outcomes in severe OSA patients (Wang et al. J Clin Sleep Med. 2020;16[7]:1055-62). The ODR measures the speed of an oxygen desaturation during an apnea event. Subjects with a faster ODR were found to have higher blood pressure values and variability. The authors hypothesized that slower desaturations generate hypoxemia-conditioning that may protect from exaggerated hemodynamic changes. These findings of novel hypoxemia metrics, albeit having their own limitations, recapitulate the need to move beyond the AHI to characterize OSA.
The apnea-hypopnea event duration is another overlooked feature that may impact OSA outcomes. Butler, et al. demonstrated that shorter event duration predicted a higher all-cause mortality over and beyond that predicted by AHI (Butler et al. Am J Respir Crit Care Med. 2019;199[7]:903-12). These results contrast views that early arousals in response to respiratory events may improve outcomes as they reflect a protective mechanism to prevent further hypoxemia and sympatho-excitation. For example, Ma, et al. found that higher percentage of total sleep time spent in apnea/hypopnea (AHT%) predicted worse daytime sleepiness to a higher degree than standard AHI (Ma et al. Sci Rep. 2021;11[1]:4702). However, shorter event duration may represent lower arousal thresholds (increased excitability), and ventilatory control instability (higher loop gain), predisposing patients to augmented sympathetic activity. Along similar lines, the intensity of respiratory-related arousals (as measured by EEG wavelet transformation) was found to be independent of preceding respiratory stimulus, with higher arousal intensity levels correlating with higher respiratory and heart rate responses (Amatoury et al. Sleep. 2016;39[12]:2091-100). The contribution of arousals to OSA morbidity is of particular importance for women in whom long-term outcomes of elevated AHI are poorly understood. Bearing in mind the differences in the metrics used, these results underscore the role of event duration and arousability in the pathogenesis of OSA-related morbidity.
The AHI is certainly an important piece of data that is informative and somewhat predictive. However, when used as a sole disease-defining metric, it has yielded disappointing results, especially after OSA treatment trials failed to show cardiovascular benefits despite therapies achieving a low residual AHI. As we aim to achieve a more personalized approach for diagnosing and treating OSA, we need to explore beyond the concept of a single metric to define a heterogenous and complex disorder. Instead of relying on the frequency of respiratory events, it is time to use complementary polysomnographic data that better reflect the origin and systemic effects of these disturbances. Machine-learning methods may offer sophisticated approaches to identifying polysomnographic patterns for future research. Clinical characteristics will also likely need to be considered in OSA severity scales. The identification of symptom subtypes or blood biomarkers may help identify patient groups who may be impacted differently by OSA, and consequently have a different treatment response (Malhotra et al. Sleep. 2021;44[7]:zsab030).
Almost half a century has lapsed since the original descriptions of OSA. Since then, our understanding of the disorder has improved greatly, with much still to be discovered, but our method of disease capture is unwavering. Future research requires a focus on novel measures aimed at identifying OSA endophenotypes, which will transform our understanding of disease traits and propel us into personalized therapies.
Dr. Mansour is Assistant Professor of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Duke University School of Medicine, Durham, North Carolina. Dr. Won is Associate Professor of Medicine, Section of Pulmonary, Critical Care, and Sleep Medicine, Yale University School of Medicine; and VA Connecticut Healthcare System, West Haven, Connecticut.
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway collapse resulting in intermittent hypoxemia and hypercapnia, large intrathoracic pressure swings, and cortical arousals. The rate of apneas and hypopneas observed during sleep, the apnea-hypopnea index (AHI), has been used for decades to diagnose OSA and to classify its severity. Despite the wide acceptance of this metric by the sleep medicine community, clinical research has found poor correlations between the AHI- and OSA-related complications or symptoms. We have come to learn that the AHI is an oversimplification of a complex and diverse disease process. (Punjabi. Chest. 2016;149[1]:16-9).
The most important features of a disease metric are reliability, and the ability to predict clinically relevant outcomes. The reliability of the AHI has been in question due to substantial night-to-night variability that can lead to missed diagnosis and disease severity misclassification (Dzierzewski et al. J Clin Sleep Med. 2020;16[4]:539-44). Furthermore, the AHI fails to reflect some important physiologic derangements resulting from respiratory events. Apart from imperfectly set thresholds for scoring, it disregards the depth and the duration of ventilatory disturbances. For example, a hypopnea lasting 30 seconds and resulting in a decrease of 10% in oxyhemoglobin saturation is considered equivalent to a hypopnea lasting 10 seconds and resulting in a decrease of 4% in oxyhemoglobin saturation. The AHI also assumes that apneas and hypopneas are equal in their biological effects regardless of when they occur during sleep (NREM vs REM), despite reports suggesting that the sequalae of OSA are sleep-stage dependent (Varga, Mokhlesi. Sleep Breath. 2019;23[2]:413-23). This is further complicated by the varying hypopnea definitions and the difficulties in differentiating obstructive vs central hypopneas. It is doubtful that these events, which differ in mechanism, would result in similar outcomes.
Over the past decade, our understanding of the different pathophysiological mechanisms leading to OSA has grown substantially, suggesting the need for a phenotype-specific treatment approach (Zinchuk, Yaggi. Chest. 2020;157[2]:403-20). The reliance on a single metric that does not capture this heterogeneity may prove detrimental to our therapeutic efforts. One extremely important dimension that is missed by the AHI is the patient. Individual response to airway obstruction varies with age, genetics, gender, and comorbidities, among other things. This may explain the difference in symptoms and outcomes experienced by patients with the same AHI. During the era of precision medicine, the concept of defining a clinical condition by a single test result, without regard to patient characteristics, is antiquated.
Several studies have attempted to propose complementary metrics that may better characterize OSA and predict outcomes. The hypoxic burden has gained a lot of attention as it is generally felt that hypoxemia is a major factor contributing to the pathogenesis of OSA-related comorbidities. Azarbarzin, et al. reported a hypoxic burden metric by measuring the area under the oxygen desaturation curve during a respiratory event (Azarbarzin et al. Eur Heart J. 2019;40[14]:1149-57). It factors the length and depth of the desaturations into a single value that expresses the average desaturation burden per hour of sleep time. The hypoxic burden was independently predictive of cardiovascular mortality in two large cohorts. Interestingly, the AHI did not have such an association. Similarly, another novel proposed parameter, the oxygen desaturation rate (ODR), outperformed the AHI in predicting cardiovascular outcomes in severe OSA patients (Wang et al. J Clin Sleep Med. 2020;16[7]:1055-62). The ODR measures the speed of an oxygen desaturation during an apnea event. Subjects with a faster ODR were found to have higher blood pressure values and variability. The authors hypothesized that slower desaturations generate hypoxemia-conditioning that may protect from exaggerated hemodynamic changes. These findings of novel hypoxemia metrics, albeit having their own limitations, recapitulate the need to move beyond the AHI to characterize OSA.
The apnea-hypopnea event duration is another overlooked feature that may impact OSA outcomes. Butler, et al. demonstrated that shorter event duration predicted a higher all-cause mortality over and beyond that predicted by AHI (Butler et al. Am J Respir Crit Care Med. 2019;199[7]:903-12). These results contrast views that early arousals in response to respiratory events may improve outcomes as they reflect a protective mechanism to prevent further hypoxemia and sympatho-excitation. For example, Ma, et al. found that higher percentage of total sleep time spent in apnea/hypopnea (AHT%) predicted worse daytime sleepiness to a higher degree than standard AHI (Ma et al. Sci Rep. 2021;11[1]:4702). However, shorter event duration may represent lower arousal thresholds (increased excitability), and ventilatory control instability (higher loop gain), predisposing patients to augmented sympathetic activity. Along similar lines, the intensity of respiratory-related arousals (as measured by EEG wavelet transformation) was found to be independent of preceding respiratory stimulus, with higher arousal intensity levels correlating with higher respiratory and heart rate responses (Amatoury et al. Sleep. 2016;39[12]:2091-100). The contribution of arousals to OSA morbidity is of particular importance for women in whom long-term outcomes of elevated AHI are poorly understood. Bearing in mind the differences in the metrics used, these results underscore the role of event duration and arousability in the pathogenesis of OSA-related morbidity.
The AHI is certainly an important piece of data that is informative and somewhat predictive. However, when used as a sole disease-defining metric, it has yielded disappointing results, especially after OSA treatment trials failed to show cardiovascular benefits despite therapies achieving a low residual AHI. As we aim to achieve a more personalized approach for diagnosing and treating OSA, we need to explore beyond the concept of a single metric to define a heterogenous and complex disorder. Instead of relying on the frequency of respiratory events, it is time to use complementary polysomnographic data that better reflect the origin and systemic effects of these disturbances. Machine-learning methods may offer sophisticated approaches to identifying polysomnographic patterns for future research. Clinical characteristics will also likely need to be considered in OSA severity scales. The identification of symptom subtypes or blood biomarkers may help identify patient groups who may be impacted differently by OSA, and consequently have a different treatment response (Malhotra et al. Sleep. 2021;44[7]:zsab030).
Almost half a century has lapsed since the original descriptions of OSA. Since then, our understanding of the disorder has improved greatly, with much still to be discovered, but our method of disease capture is unwavering. Future research requires a focus on novel measures aimed at identifying OSA endophenotypes, which will transform our understanding of disease traits and propel us into personalized therapies.
Dr. Mansour is Assistant Professor of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Duke University School of Medicine, Durham, North Carolina. Dr. Won is Associate Professor of Medicine, Section of Pulmonary, Critical Care, and Sleep Medicine, Yale University School of Medicine; and VA Connecticut Healthcare System, West Haven, Connecticut.
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway collapse resulting in intermittent hypoxemia and hypercapnia, large intrathoracic pressure swings, and cortical arousals. The rate of apneas and hypopneas observed during sleep, the apnea-hypopnea index (AHI), has been used for decades to diagnose OSA and to classify its severity. Despite the wide acceptance of this metric by the sleep medicine community, clinical research has found poor correlations between the AHI- and OSA-related complications or symptoms. We have come to learn that the AHI is an oversimplification of a complex and diverse disease process. (Punjabi. Chest. 2016;149[1]:16-9).
The most important features of a disease metric are reliability, and the ability to predict clinically relevant outcomes. The reliability of the AHI has been in question due to substantial night-to-night variability that can lead to missed diagnosis and disease severity misclassification (Dzierzewski et al. J Clin Sleep Med. 2020;16[4]:539-44). Furthermore, the AHI fails to reflect some important physiologic derangements resulting from respiratory events. Apart from imperfectly set thresholds for scoring, it disregards the depth and the duration of ventilatory disturbances. For example, a hypopnea lasting 30 seconds and resulting in a decrease of 10% in oxyhemoglobin saturation is considered equivalent to a hypopnea lasting 10 seconds and resulting in a decrease of 4% in oxyhemoglobin saturation. The AHI also assumes that apneas and hypopneas are equal in their biological effects regardless of when they occur during sleep (NREM vs REM), despite reports suggesting that the sequalae of OSA are sleep-stage dependent (Varga, Mokhlesi. Sleep Breath. 2019;23[2]:413-23). This is further complicated by the varying hypopnea definitions and the difficulties in differentiating obstructive vs central hypopneas. It is doubtful that these events, which differ in mechanism, would result in similar outcomes.
Over the past decade, our understanding of the different pathophysiological mechanisms leading to OSA has grown substantially, suggesting the need for a phenotype-specific treatment approach (Zinchuk, Yaggi. Chest. 2020;157[2]:403-20). The reliance on a single metric that does not capture this heterogeneity may prove detrimental to our therapeutic efforts. One extremely important dimension that is missed by the AHI is the patient. Individual response to airway obstruction varies with age, genetics, gender, and comorbidities, among other things. This may explain the difference in symptoms and outcomes experienced by patients with the same AHI. During the era of precision medicine, the concept of defining a clinical condition by a single test result, without regard to patient characteristics, is antiquated.
Several studies have attempted to propose complementary metrics that may better characterize OSA and predict outcomes. The hypoxic burden has gained a lot of attention as it is generally felt that hypoxemia is a major factor contributing to the pathogenesis of OSA-related comorbidities. Azarbarzin, et al. reported a hypoxic burden metric by measuring the area under the oxygen desaturation curve during a respiratory event (Azarbarzin et al. Eur Heart J. 2019;40[14]:1149-57). It factors the length and depth of the desaturations into a single value that expresses the average desaturation burden per hour of sleep time. The hypoxic burden was independently predictive of cardiovascular mortality in two large cohorts. Interestingly, the AHI did not have such an association. Similarly, another novel proposed parameter, the oxygen desaturation rate (ODR), outperformed the AHI in predicting cardiovascular outcomes in severe OSA patients (Wang et al. J Clin Sleep Med. 2020;16[7]:1055-62). The ODR measures the speed of an oxygen desaturation during an apnea event. Subjects with a faster ODR were found to have higher blood pressure values and variability. The authors hypothesized that slower desaturations generate hypoxemia-conditioning that may protect from exaggerated hemodynamic changes. These findings of novel hypoxemia metrics, albeit having their own limitations, recapitulate the need to move beyond the AHI to characterize OSA.
The apnea-hypopnea event duration is another overlooked feature that may impact OSA outcomes. Butler, et al. demonstrated that shorter event duration predicted a higher all-cause mortality over and beyond that predicted by AHI (Butler et al. Am J Respir Crit Care Med. 2019;199[7]:903-12). These results contrast views that early arousals in response to respiratory events may improve outcomes as they reflect a protective mechanism to prevent further hypoxemia and sympatho-excitation. For example, Ma, et al. found that higher percentage of total sleep time spent in apnea/hypopnea (AHT%) predicted worse daytime sleepiness to a higher degree than standard AHI (Ma et al. Sci Rep. 2021;11[1]:4702). However, shorter event duration may represent lower arousal thresholds (increased excitability), and ventilatory control instability (higher loop gain), predisposing patients to augmented sympathetic activity. Along similar lines, the intensity of respiratory-related arousals (as measured by EEG wavelet transformation) was found to be independent of preceding respiratory stimulus, with higher arousal intensity levels correlating with higher respiratory and heart rate responses (Amatoury et al. Sleep. 2016;39[12]:2091-100). The contribution of arousals to OSA morbidity is of particular importance for women in whom long-term outcomes of elevated AHI are poorly understood. Bearing in mind the differences in the metrics used, these results underscore the role of event duration and arousability in the pathogenesis of OSA-related morbidity.
The AHI is certainly an important piece of data that is informative and somewhat predictive. However, when used as a sole disease-defining metric, it has yielded disappointing results, especially after OSA treatment trials failed to show cardiovascular benefits despite therapies achieving a low residual AHI. As we aim to achieve a more personalized approach for diagnosing and treating OSA, we need to explore beyond the concept of a single metric to define a heterogenous and complex disorder. Instead of relying on the frequency of respiratory events, it is time to use complementary polysomnographic data that better reflect the origin and systemic effects of these disturbances. Machine-learning methods may offer sophisticated approaches to identifying polysomnographic patterns for future research. Clinical characteristics will also likely need to be considered in OSA severity scales. The identification of symptom subtypes or blood biomarkers may help identify patient groups who may be impacted differently by OSA, and consequently have a different treatment response (Malhotra et al. Sleep. 2021;44[7]:zsab030).
Almost half a century has lapsed since the original descriptions of OSA. Since then, our understanding of the disorder has improved greatly, with much still to be discovered, but our method of disease capture is unwavering. Future research requires a focus on novel measures aimed at identifying OSA endophenotypes, which will transform our understanding of disease traits and propel us into personalized therapies.
Dr. Mansour is Assistant Professor of Medicine, Division of Pulmonary, Allergy, and Critical Care Medicine, Duke University School of Medicine, Durham, North Carolina. Dr. Won is Associate Professor of Medicine, Section of Pulmonary, Critical Care, and Sleep Medicine, Yale University School of Medicine; and VA Connecticut Healthcare System, West Haven, Connecticut.
Staying up to date with consumer sleep technology
With Siri and Alexa sitting at our kitchen tables and listening to our conversations, we have all but forgotten about the before times – when we had to use the Yellow Pages to look up a number or address and when we had no idea how many steps we took in a given day. Wearable technology has become ubiquitous and has us watching not only our step count but also our sleep. Did I get enough deep sleep? What does my sleep score of 82 mean? Should I be worried?
As clinicians, we must also navigate how this information impacts our clinical decision-making and consider how our patients are interpreting these data on a daily basis. There is an inherent assumption that we, as sleep clinicians, will understand the nuances of each consumer-facing sleep technology (CST) whether it is a wearable, a nearable (a device that sits near the body but not on the body), or an app. Very little validation data exist, as most of these technologies are marketed as wellness devices and are not intended to render a diagnosis. It therefore falls to us to determine how to utilize this information in an already busy clinic.
One strategy is to use these technologies as patient engagement tools – a way to increase public awareness of the importance of sleep. While this certainly should be beneficial, oftentimes, the data are confusing and can lead to misunderstandings about what normal sleep should look like. Approaching these data as partners to our patients allows us to set expectations around normal sleep cycles and sleep duration. It also allows us to discuss appropriate sleep timing and sleep hygiene.
Many wearable devices have incorporated oximetry into their metrics, and some claim to have accuracy that is better than hospital-grade oximeters. Many of these companies are no longer in business. Others specify higher accuracy in dark-skinned individuals (“CIRCUL Ring Pulse Oximeter in Dark-Pigmented Individuals: Clinical Study Validates Efficacy and Reliability,” Medical Device News Magazine, Feb. 26, 2021).
Despite these claims, they are registered as wellness devices with the FDA and are not diagnostic devices. Logically, if one of these devices demonstrates worrisome data, then it can prompt further clinical queries and, potentially, objective testing for obstructive sleep apnea (OSA). The reverse, however, cannot be claimed. A normal reading by CST does not obviate the need for objective testing if the clinical symptoms warrant it.
There are CSTs that have been created around very specific needs - such as jet lag- and provide guidance for how to quickly acclimate to the destination time zone by providing nudges for light exposure and timed melatonin or dark glasses (https://www.timeshifter.com/).
Others analyze the sleep space for extrinsic sounds (https://www.sleepcycle.com/), while a plethora of apps provides advice for how to optimize your sleep environment and wind-down routine. There is even a sleep robot designed to facilitate sleep onset (https://somnox.com/). This bean-shaped device is designed to “breathe” as you hold it, and the user is meant to emulate those same breathing patterns. It is a take on the 4-7-8 breathing pattern long endorsed by yogis.
Although validation data are lacking for the vast majority of CST, a recent study (www.ncbi.nlm.nih.gov/pmc/articles/PMC8120339/pdf/zsaa291.pdf).demonstrated that CST had high performance when compared with actigraphy in assessing sleep and wakefulness and, as such, may improve the evaluation of sleep and wake opportunities prior to MSLT or improve identification of circadian sleep-wake disorders. Many practices do not currently utilize actigraphy due to its expense and very limited potential for reimbursement. Using a patient’s sleep-tracking device may allow access to these data without financial outlay. While these data demonstrate the ability of CST to potentially differentiate sleep from wakefulness, it is notable that this study also found that the determination of individual sleep stages is less robust. In general, CST cannot identify an underlying sleep disorder, however, may raise awareness that a disorder might be present.
This leads to more reflection on the role of CST in a typical sleep clinic. Many years ago, discussion around this technology was primarily patient-initiated and often times met with skepticism on the part of the clinician. As technology has improved and has become more accessible, there appears to be more acceptance among our colleagues – not, perhaps, in terms of absolute actionable data, but rather as an opportunity to discuss sleep with our patients and to support their own efforts at improving their sleep. Trends in the data in response to CBT-I or medications can be observed. Abnormalities identified via CST often serve as the initial prompt for a clinical visit and, as such, should not be eschewed. Rather, reframing the use of this information while also addressing other sleep issues is likely to be the more appropriate path forward.
Assessing this information can be time-consuming, and best practice suggests establishing expectations around this process (J Clin Sleep Med 2018 May 15. doi: 10.5664/jcsm.7128).
Agreements can be made with patients that the data are reviewed in the context of a clinical visit rather than longitudinally as data are uploaded and then sent via messaging unless such an understanding has already been agreed upon. RPM billing codes may ultimately allow for reimbursement and recognition of this workload. At the present time, RPM billing is limited to FDA-cleared, prescription devices, and CST does not yet qualify.
There also needs to be recognition of potential harm from CST. Inevitably, some patients will develop orthosomnia, a term coined by Dr. Kelly Baron, where patients become so fixated on achieving perfect sleep scores that it contributes to insomnia. In this case, identification of orthosomnia is made via the clinical visit and patients are advised to stop tracking their sleep for a set period of time. This allows the anxiety around achieving “perfect sleep” to dissipate.
Google and the AASM recently announced a partnership. Essentially, the Google Nest Hub will serve to detect sleep concerns (such as timing of sleep, snoring, insufficient sleep, etc.) and will direct the user to educational resources such as www.sleepeducation.org. The idea behind this is that people are often unaware of an underlying sleep disorder such as OSA and don’t know what to search for. The Nest Hub uses information it collects and directs users to appropriate resources, thus obviating the need to know what to Google.
Clearly, big tech has invested heavily in our field. Between the copious wearables, nearables, and apps that are sleep-focused, these industry giants obviously believe that sleep is worthy of such a significant allocation of resources. This has improved the overall awareness of the importance of sleep and of identifying and treating sleep disorders. While these technologies are no replacement for a clinical evaluation, they can serve as patient engagement tools, as well as potentially large-scale OSA screening tools and may help us improve the percentage of patients with undiagnosed OSA, estimated to be 80% (Frost and Sullivan, “Hidden Health Crisis Costing America Billions,” American Academy of Sleep Medicine, 2016).
CST may allow us to better identify circadian sleep-wake disorders and evaluate sleep satiation prior to MLST that no longer requires investment in expensive actigraphy devices. They also allow us to partner with our patients by meeting them where they are and recognizing the efforts they have already made to improve their sleep before we even meet them.
Dr. Khosla is Medical Director, North Dakota Center for Sleep, Fargo, North Dakota.
With Siri and Alexa sitting at our kitchen tables and listening to our conversations, we have all but forgotten about the before times – when we had to use the Yellow Pages to look up a number or address and when we had no idea how many steps we took in a given day. Wearable technology has become ubiquitous and has us watching not only our step count but also our sleep. Did I get enough deep sleep? What does my sleep score of 82 mean? Should I be worried?
As clinicians, we must also navigate how this information impacts our clinical decision-making and consider how our patients are interpreting these data on a daily basis. There is an inherent assumption that we, as sleep clinicians, will understand the nuances of each consumer-facing sleep technology (CST) whether it is a wearable, a nearable (a device that sits near the body but not on the body), or an app. Very little validation data exist, as most of these technologies are marketed as wellness devices and are not intended to render a diagnosis. It therefore falls to us to determine how to utilize this information in an already busy clinic.
One strategy is to use these technologies as patient engagement tools – a way to increase public awareness of the importance of sleep. While this certainly should be beneficial, oftentimes, the data are confusing and can lead to misunderstandings about what normal sleep should look like. Approaching these data as partners to our patients allows us to set expectations around normal sleep cycles and sleep duration. It also allows us to discuss appropriate sleep timing and sleep hygiene.
Many wearable devices have incorporated oximetry into their metrics, and some claim to have accuracy that is better than hospital-grade oximeters. Many of these companies are no longer in business. Others specify higher accuracy in dark-skinned individuals (“CIRCUL Ring Pulse Oximeter in Dark-Pigmented Individuals: Clinical Study Validates Efficacy and Reliability,” Medical Device News Magazine, Feb. 26, 2021).
Despite these claims, they are registered as wellness devices with the FDA and are not diagnostic devices. Logically, if one of these devices demonstrates worrisome data, then it can prompt further clinical queries and, potentially, objective testing for obstructive sleep apnea (OSA). The reverse, however, cannot be claimed. A normal reading by CST does not obviate the need for objective testing if the clinical symptoms warrant it.
There are CSTs that have been created around very specific needs - such as jet lag- and provide guidance for how to quickly acclimate to the destination time zone by providing nudges for light exposure and timed melatonin or dark glasses (https://www.timeshifter.com/).
Others analyze the sleep space for extrinsic sounds (https://www.sleepcycle.com/), while a plethora of apps provides advice for how to optimize your sleep environment and wind-down routine. There is even a sleep robot designed to facilitate sleep onset (https://somnox.com/). This bean-shaped device is designed to “breathe” as you hold it, and the user is meant to emulate those same breathing patterns. It is a take on the 4-7-8 breathing pattern long endorsed by yogis.
Although validation data are lacking for the vast majority of CST, a recent study (www.ncbi.nlm.nih.gov/pmc/articles/PMC8120339/pdf/zsaa291.pdf).demonstrated that CST had high performance when compared with actigraphy in assessing sleep and wakefulness and, as such, may improve the evaluation of sleep and wake opportunities prior to MSLT or improve identification of circadian sleep-wake disorders. Many practices do not currently utilize actigraphy due to its expense and very limited potential for reimbursement. Using a patient’s sleep-tracking device may allow access to these data without financial outlay. While these data demonstrate the ability of CST to potentially differentiate sleep from wakefulness, it is notable that this study also found that the determination of individual sleep stages is less robust. In general, CST cannot identify an underlying sleep disorder, however, may raise awareness that a disorder might be present.
This leads to more reflection on the role of CST in a typical sleep clinic. Many years ago, discussion around this technology was primarily patient-initiated and often times met with skepticism on the part of the clinician. As technology has improved and has become more accessible, there appears to be more acceptance among our colleagues – not, perhaps, in terms of absolute actionable data, but rather as an opportunity to discuss sleep with our patients and to support their own efforts at improving their sleep. Trends in the data in response to CBT-I or medications can be observed. Abnormalities identified via CST often serve as the initial prompt for a clinical visit and, as such, should not be eschewed. Rather, reframing the use of this information while also addressing other sleep issues is likely to be the more appropriate path forward.
Assessing this information can be time-consuming, and best practice suggests establishing expectations around this process (J Clin Sleep Med 2018 May 15. doi: 10.5664/jcsm.7128).
Agreements can be made with patients that the data are reviewed in the context of a clinical visit rather than longitudinally as data are uploaded and then sent via messaging unless such an understanding has already been agreed upon. RPM billing codes may ultimately allow for reimbursement and recognition of this workload. At the present time, RPM billing is limited to FDA-cleared, prescription devices, and CST does not yet qualify.
There also needs to be recognition of potential harm from CST. Inevitably, some patients will develop orthosomnia, a term coined by Dr. Kelly Baron, where patients become so fixated on achieving perfect sleep scores that it contributes to insomnia. In this case, identification of orthosomnia is made via the clinical visit and patients are advised to stop tracking their sleep for a set period of time. This allows the anxiety around achieving “perfect sleep” to dissipate.
Google and the AASM recently announced a partnership. Essentially, the Google Nest Hub will serve to detect sleep concerns (such as timing of sleep, snoring, insufficient sleep, etc.) and will direct the user to educational resources such as www.sleepeducation.org. The idea behind this is that people are often unaware of an underlying sleep disorder such as OSA and don’t know what to search for. The Nest Hub uses information it collects and directs users to appropriate resources, thus obviating the need to know what to Google.
Clearly, big tech has invested heavily in our field. Between the copious wearables, nearables, and apps that are sleep-focused, these industry giants obviously believe that sleep is worthy of such a significant allocation of resources. This has improved the overall awareness of the importance of sleep and of identifying and treating sleep disorders. While these technologies are no replacement for a clinical evaluation, they can serve as patient engagement tools, as well as potentially large-scale OSA screening tools and may help us improve the percentage of patients with undiagnosed OSA, estimated to be 80% (Frost and Sullivan, “Hidden Health Crisis Costing America Billions,” American Academy of Sleep Medicine, 2016).
CST may allow us to better identify circadian sleep-wake disorders and evaluate sleep satiation prior to MLST that no longer requires investment in expensive actigraphy devices. They also allow us to partner with our patients by meeting them where they are and recognizing the efforts they have already made to improve their sleep before we even meet them.
Dr. Khosla is Medical Director, North Dakota Center for Sleep, Fargo, North Dakota.
With Siri and Alexa sitting at our kitchen tables and listening to our conversations, we have all but forgotten about the before times – when we had to use the Yellow Pages to look up a number or address and when we had no idea how many steps we took in a given day. Wearable technology has become ubiquitous and has us watching not only our step count but also our sleep. Did I get enough deep sleep? What does my sleep score of 82 mean? Should I be worried?
As clinicians, we must also navigate how this information impacts our clinical decision-making and consider how our patients are interpreting these data on a daily basis. There is an inherent assumption that we, as sleep clinicians, will understand the nuances of each consumer-facing sleep technology (CST) whether it is a wearable, a nearable (a device that sits near the body but not on the body), or an app. Very little validation data exist, as most of these technologies are marketed as wellness devices and are not intended to render a diagnosis. It therefore falls to us to determine how to utilize this information in an already busy clinic.
One strategy is to use these technologies as patient engagement tools – a way to increase public awareness of the importance of sleep. While this certainly should be beneficial, oftentimes, the data are confusing and can lead to misunderstandings about what normal sleep should look like. Approaching these data as partners to our patients allows us to set expectations around normal sleep cycles and sleep duration. It also allows us to discuss appropriate sleep timing and sleep hygiene.
Many wearable devices have incorporated oximetry into their metrics, and some claim to have accuracy that is better than hospital-grade oximeters. Many of these companies are no longer in business. Others specify higher accuracy in dark-skinned individuals (“CIRCUL Ring Pulse Oximeter in Dark-Pigmented Individuals: Clinical Study Validates Efficacy and Reliability,” Medical Device News Magazine, Feb. 26, 2021).
Despite these claims, they are registered as wellness devices with the FDA and are not diagnostic devices. Logically, if one of these devices demonstrates worrisome data, then it can prompt further clinical queries and, potentially, objective testing for obstructive sleep apnea (OSA). The reverse, however, cannot be claimed. A normal reading by CST does not obviate the need for objective testing if the clinical symptoms warrant it.
There are CSTs that have been created around very specific needs - such as jet lag- and provide guidance for how to quickly acclimate to the destination time zone by providing nudges for light exposure and timed melatonin or dark glasses (https://www.timeshifter.com/).
Others analyze the sleep space for extrinsic sounds (https://www.sleepcycle.com/), while a plethora of apps provides advice for how to optimize your sleep environment and wind-down routine. There is even a sleep robot designed to facilitate sleep onset (https://somnox.com/). This bean-shaped device is designed to “breathe” as you hold it, and the user is meant to emulate those same breathing patterns. It is a take on the 4-7-8 breathing pattern long endorsed by yogis.
Although validation data are lacking for the vast majority of CST, a recent study (www.ncbi.nlm.nih.gov/pmc/articles/PMC8120339/pdf/zsaa291.pdf).demonstrated that CST had high performance when compared with actigraphy in assessing sleep and wakefulness and, as such, may improve the evaluation of sleep and wake opportunities prior to MSLT or improve identification of circadian sleep-wake disorders. Many practices do not currently utilize actigraphy due to its expense and very limited potential for reimbursement. Using a patient’s sleep-tracking device may allow access to these data without financial outlay. While these data demonstrate the ability of CST to potentially differentiate sleep from wakefulness, it is notable that this study also found that the determination of individual sleep stages is less robust. In general, CST cannot identify an underlying sleep disorder, however, may raise awareness that a disorder might be present.
This leads to more reflection on the role of CST in a typical sleep clinic. Many years ago, discussion around this technology was primarily patient-initiated and often times met with skepticism on the part of the clinician. As technology has improved and has become more accessible, there appears to be more acceptance among our colleagues – not, perhaps, in terms of absolute actionable data, but rather as an opportunity to discuss sleep with our patients and to support their own efforts at improving their sleep. Trends in the data in response to CBT-I or medications can be observed. Abnormalities identified via CST often serve as the initial prompt for a clinical visit and, as such, should not be eschewed. Rather, reframing the use of this information while also addressing other sleep issues is likely to be the more appropriate path forward.
Assessing this information can be time-consuming, and best practice suggests establishing expectations around this process (J Clin Sleep Med 2018 May 15. doi: 10.5664/jcsm.7128).
Agreements can be made with patients that the data are reviewed in the context of a clinical visit rather than longitudinally as data are uploaded and then sent via messaging unless such an understanding has already been agreed upon. RPM billing codes may ultimately allow for reimbursement and recognition of this workload. At the present time, RPM billing is limited to FDA-cleared, prescription devices, and CST does not yet qualify.
There also needs to be recognition of potential harm from CST. Inevitably, some patients will develop orthosomnia, a term coined by Dr. Kelly Baron, where patients become so fixated on achieving perfect sleep scores that it contributes to insomnia. In this case, identification of orthosomnia is made via the clinical visit and patients are advised to stop tracking their sleep for a set period of time. This allows the anxiety around achieving “perfect sleep” to dissipate.
Google and the AASM recently announced a partnership. Essentially, the Google Nest Hub will serve to detect sleep concerns (such as timing of sleep, snoring, insufficient sleep, etc.) and will direct the user to educational resources such as www.sleepeducation.org. The idea behind this is that people are often unaware of an underlying sleep disorder such as OSA and don’t know what to search for. The Nest Hub uses information it collects and directs users to appropriate resources, thus obviating the need to know what to Google.
Clearly, big tech has invested heavily in our field. Between the copious wearables, nearables, and apps that are sleep-focused, these industry giants obviously believe that sleep is worthy of such a significant allocation of resources. This has improved the overall awareness of the importance of sleep and of identifying and treating sleep disorders. While these technologies are no replacement for a clinical evaluation, they can serve as patient engagement tools, as well as potentially large-scale OSA screening tools and may help us improve the percentage of patients with undiagnosed OSA, estimated to be 80% (Frost and Sullivan, “Hidden Health Crisis Costing America Billions,” American Academy of Sleep Medicine, 2016).
CST may allow us to better identify circadian sleep-wake disorders and evaluate sleep satiation prior to MLST that no longer requires investment in expensive actigraphy devices. They also allow us to partner with our patients by meeting them where they are and recognizing the efforts they have already made to improve their sleep before we even meet them.
Dr. Khosla is Medical Director, North Dakota Center for Sleep, Fargo, North Dakota.
Updates on COVID-19 guidance for sleep medicine
Background
Well into its second year, the worldwide COVID-19 pandemic continues to pose substantial challenges for health care access and delivery. Regulatory agencies such as the Centers for Disease Control (CDC) do not currently have guidance related to COVID-19 specific to sleep centers and laboratories. In March 2020, within days of the World Health Organization pandemic declaration, the American Academy of Sleep Medicine (AASM) posted detailed guidance on mitigation strategies for sleep medicine practices (COVID-19 Resources).
This initial guidance has been previously reported in this publication (Sullivan S, Gurubhagavatula I. CHEST Physician 2020 May 8), and the guidance has been periodically updated during the pandemic. It was restructured in mid-2020 to include sections summarizing CDC recommendations germane for sleep practices; additional sleep medicine-specific guidance from the AASM COVID-19 Task Force (TF); and a frequently asked questions (FAQ) section. The last major update from the task force occurred on Jan. 18, 2021, though subsequent posts – especially related to recent CDC changes in masking guidelines – were made in May 2021. The purpose of this article is to summarize these updates and to call attention to areas of ongoing interest to sleep medicine. Notably, the AASM Task Force guidance is nonbinding and offered as a framework for considering best practices in this evolving situation, acknowledging the importance of weighing local factors, conditions, and regulations, as well as the interests of and risks to the patient, staff, and providers.
Key updates
Data on exposure and transmission risks specific to sleep medicine
Measures for reducing viral transmission have been central to managing the spread of the virus in clinical settings. In its last major update, the AASM TF noted that no known outbreaks of COVID-19 related to sleep center exposure have been reported. A perspective and data published in the Journal of the American Medical Association concluded that hospital transmission of the virus “in the setting of universal masking is likely rare, even during periods of high community prevalence.” It also concluded that hospital-based outbreaks are more likely to occur in small workrooms and during mealtime when staff are less adherent to masking and physical distancing (Richterman A, et al. JAMA. 2020;324[21]:2155-6). The TF elaborated on considerations to reduce transmission, which include not just telework and foundational infection control practices, but also broader workplace considerations such as optimizing ventilation, taking advantage of outdoor spaces (e.g., for breaks and eating), scheduling to reduce interactions between personnel from different teams, minimizing contact in meeting/break rooms, removing tables and chairs from lounge areas, and following CDC guidance for effective facility operations.
Vaccination
In the January update, the AASM COVID-19 TF stated that, “sleep facility leaders should encourage staff and patients to be vaccinated in accordance with CDC guidance.” The role of the sleep medicine community in encouraging healthy sleep habits before and after vaccination was emphasized, pointing to evidence linking sleep and immunity, specifically between sleep duration and vaccination response (Healthy sleep and immune response to COVID-19 vaccination. 2021 Jan.).
In an FAQ update from March 26, 2021, considering whether continued COVID-19 testing was needed following full vaccination, the AASM advised testing prior to potential aerosol-generating procedures should be made on the basis of a risk-benefit assessment by the sleep clinician. Several considerations were highlighted, including recent COVID-19 infection, vaccination status of contacts, local prevalence of newer variants, and whether individuals are receiving positive airway pressure therapy. The TF focused on the vigilance for residents and staff in long-term care facilities, which have been associated with a number of outbreaks.
Masking in the context of the COVID-19 vaccine
The most significant change in recommendations is the recent relaxation of masking guidance by the CDC in the setting of the approval and distribution of COVID-19 vaccinations. In May, the CDC stated that fully vaccinated individuals can resume activities without masking or physically distancing except in scenarios of travel and where required by laws, regulations, and local businesses, due to the efficacy of the vaccines, increasing evidence of reduced asymptomatic carriage and transmission after vaccination, and anticipated increased uptake of vaccination. However, the CDC also noted that these updates did not apply to health care facilities, where the recommendation remains that patients and visitors should continue to mask throughout their stay. Additionally, fully vaccinated health care workers should continue to practice infection control measures while working with patients. On May 14, the AASM TF provided a detailed FAQ acknowledging the CDC’s new guidance, emphasizing that masking guidance in health care facilities remains unchanged, and encouraging individuals to follow CDC guidance regarding vaccination, noting that emergence of newer variants continues to be monitored, and existing vaccines still appear to induce neutralizing antibodies even if to a somewhat lower degree. The situation for pediatric sleep centers has been highlighted in particular because the potential risk posed by newer variants to children remains under investigation, and children under age 12 are not approved for vaccination.
Important caveats to discussions around vaccination status are the lack of a centralized method to identify vaccinated individuals, the unknown duration of immunity, and reports of the use of fake vaccine cards. At this time, in health care settings, vaccination status should not exempt mask usage for any individual.
Sleep medicine care for those with COVID-19
Regarding the duration of isolation and precautions for adults with COVID-19, the TF highlighted the CDC’s symptom-based strategy, rather than test-based strategy, for ending isolation of these patients, availing them of sleep medicine services in person.
In line with the CDC guidance, this approach indicates that scheduling in-person care such as polysomnography for a COVID-19–positive patient may be appropriate at least 10 days after symptom onset (or after a positive test if the patient never developed symptoms); or at least 20 days after symptom onset if the illness was severe; or if at least 90 days have elapsed since symptom onset, consider preappointment COVID-19 screening. In the context of immunocompromised individuals, involvement from infectious disease specialists may be needed to help guide decisions.
Patient communications
For many, a repercussion of the pandemic has been delaying care or avoiding addressing medical issues, including sleep disorders. The AASM encouraged practices to consider communicating with patients that delaying needed care can increase health risks; COVID-19 transmission to patients in health care settings has been low; effective safety procedures are in place; and whether remote/telehealth services are available.
Disparities in care
In addition to the specific guidance above, there are ongoing concerns regarding disparities in care resulting from a variety of sources and becoming more evident during the pandemic. Complex factors, ranging from economic, geographic, contextual, occupational, and others contribute to disparities that health care systems – and sleep medicine - have not been able to adequately address (Jackson CL and Johnson DA. J Clin Sleep Med. 16[8]:1401-2). More specific differences may include Internet access, reduced access due to socioeconomic barriers, transportation limitations, medical mistrust, and membership in a medically vulnerable group such as children, the elderly, and those with high acuity needs. For example, in pediatric patients there exist few evidence-based alternatives and guidelines to in-lab testing and care, which may have negatively impacted access to needed sleep medicine services (Sullivan S et al. J Clin Sleep Med. 2021 Mar 1;17[3]:361-2).
Economics in the COVID-19 pandemic
The economic effects of COVID-19 on medical institutions and in sleep medicine is a story that continues to unfold. Reductions in patient visits and elective procedures, infection control measures limiting capacity, increased costs to maintain such measures, and variability of responses by payer and region are just a few of the issues. The Centers for Medicare & Medicaid Services has employed waivers to increased flexibility and promote safe and effective care including the use of telemedicine during the public health emergency, but the future of these waivers remains uncertain. Alarmingly, a sizeable portion of sleep practices reported financial solvency concerns related to the pandemic (Ramar K. J Clin Sleep Med. 2020;16[11]:1939-42).
Conclusion
As the COVID-19 pandemic and related public health guidance continues to evolve, sleep medicine practices continue to adapt. Vaccination, new variants, changes in mask guidance, new outbreaks around the globe, financial and staffing uncertainties, as well as addressing disparities in care and outcomes that may be augmented by the pandemic remain salient areas of ongoing development.
Dr. Lee is a Postdoctoral and Pediatric Pulmonary Fellow, Department of Pediatrics, Division of Pulmonary, Asthma, and Sleep Medicine, Stanford University School of Medicine; Dr. Sullivan is Clinical Professor, Department of Pediatrics, Division of Pulmonary, Asthma, and Sleep Medicine, and by courtesy, Division of Sleep Medicine, Department of Psychiatry, Stanford University School of Medicine, Palo Alto, CA.
Background
Well into its second year, the worldwide COVID-19 pandemic continues to pose substantial challenges for health care access and delivery. Regulatory agencies such as the Centers for Disease Control (CDC) do not currently have guidance related to COVID-19 specific to sleep centers and laboratories. In March 2020, within days of the World Health Organization pandemic declaration, the American Academy of Sleep Medicine (AASM) posted detailed guidance on mitigation strategies for sleep medicine practices (COVID-19 Resources).
This initial guidance has been previously reported in this publication (Sullivan S, Gurubhagavatula I. CHEST Physician 2020 May 8), and the guidance has been periodically updated during the pandemic. It was restructured in mid-2020 to include sections summarizing CDC recommendations germane for sleep practices; additional sleep medicine-specific guidance from the AASM COVID-19 Task Force (TF); and a frequently asked questions (FAQ) section. The last major update from the task force occurred on Jan. 18, 2021, though subsequent posts – especially related to recent CDC changes in masking guidelines – were made in May 2021. The purpose of this article is to summarize these updates and to call attention to areas of ongoing interest to sleep medicine. Notably, the AASM Task Force guidance is nonbinding and offered as a framework for considering best practices in this evolving situation, acknowledging the importance of weighing local factors, conditions, and regulations, as well as the interests of and risks to the patient, staff, and providers.
Key updates
Data on exposure and transmission risks specific to sleep medicine
Measures for reducing viral transmission have been central to managing the spread of the virus in clinical settings. In its last major update, the AASM TF noted that no known outbreaks of COVID-19 related to sleep center exposure have been reported. A perspective and data published in the Journal of the American Medical Association concluded that hospital transmission of the virus “in the setting of universal masking is likely rare, even during periods of high community prevalence.” It also concluded that hospital-based outbreaks are more likely to occur in small workrooms and during mealtime when staff are less adherent to masking and physical distancing (Richterman A, et al. JAMA. 2020;324[21]:2155-6). The TF elaborated on considerations to reduce transmission, which include not just telework and foundational infection control practices, but also broader workplace considerations such as optimizing ventilation, taking advantage of outdoor spaces (e.g., for breaks and eating), scheduling to reduce interactions between personnel from different teams, minimizing contact in meeting/break rooms, removing tables and chairs from lounge areas, and following CDC guidance for effective facility operations.
Vaccination
In the January update, the AASM COVID-19 TF stated that, “sleep facility leaders should encourage staff and patients to be vaccinated in accordance with CDC guidance.” The role of the sleep medicine community in encouraging healthy sleep habits before and after vaccination was emphasized, pointing to evidence linking sleep and immunity, specifically between sleep duration and vaccination response (Healthy sleep and immune response to COVID-19 vaccination. 2021 Jan.).
In an FAQ update from March 26, 2021, considering whether continued COVID-19 testing was needed following full vaccination, the AASM advised testing prior to potential aerosol-generating procedures should be made on the basis of a risk-benefit assessment by the sleep clinician. Several considerations were highlighted, including recent COVID-19 infection, vaccination status of contacts, local prevalence of newer variants, and whether individuals are receiving positive airway pressure therapy. The TF focused on the vigilance for residents and staff in long-term care facilities, which have been associated with a number of outbreaks.
Masking in the context of the COVID-19 vaccine
The most significant change in recommendations is the recent relaxation of masking guidance by the CDC in the setting of the approval and distribution of COVID-19 vaccinations. In May, the CDC stated that fully vaccinated individuals can resume activities without masking or physically distancing except in scenarios of travel and where required by laws, regulations, and local businesses, due to the efficacy of the vaccines, increasing evidence of reduced asymptomatic carriage and transmission after vaccination, and anticipated increased uptake of vaccination. However, the CDC also noted that these updates did not apply to health care facilities, where the recommendation remains that patients and visitors should continue to mask throughout their stay. Additionally, fully vaccinated health care workers should continue to practice infection control measures while working with patients. On May 14, the AASM TF provided a detailed FAQ acknowledging the CDC’s new guidance, emphasizing that masking guidance in health care facilities remains unchanged, and encouraging individuals to follow CDC guidance regarding vaccination, noting that emergence of newer variants continues to be monitored, and existing vaccines still appear to induce neutralizing antibodies even if to a somewhat lower degree. The situation for pediatric sleep centers has been highlighted in particular because the potential risk posed by newer variants to children remains under investigation, and children under age 12 are not approved for vaccination.
Important caveats to discussions around vaccination status are the lack of a centralized method to identify vaccinated individuals, the unknown duration of immunity, and reports of the use of fake vaccine cards. At this time, in health care settings, vaccination status should not exempt mask usage for any individual.
Sleep medicine care for those with COVID-19
Regarding the duration of isolation and precautions for adults with COVID-19, the TF highlighted the CDC’s symptom-based strategy, rather than test-based strategy, for ending isolation of these patients, availing them of sleep medicine services in person.
In line with the CDC guidance, this approach indicates that scheduling in-person care such as polysomnography for a COVID-19–positive patient may be appropriate at least 10 days after symptom onset (or after a positive test if the patient never developed symptoms); or at least 20 days after symptom onset if the illness was severe; or if at least 90 days have elapsed since symptom onset, consider preappointment COVID-19 screening. In the context of immunocompromised individuals, involvement from infectious disease specialists may be needed to help guide decisions.
Patient communications
For many, a repercussion of the pandemic has been delaying care or avoiding addressing medical issues, including sleep disorders. The AASM encouraged practices to consider communicating with patients that delaying needed care can increase health risks; COVID-19 transmission to patients in health care settings has been low; effective safety procedures are in place; and whether remote/telehealth services are available.
Disparities in care
In addition to the specific guidance above, there are ongoing concerns regarding disparities in care resulting from a variety of sources and becoming more evident during the pandemic. Complex factors, ranging from economic, geographic, contextual, occupational, and others contribute to disparities that health care systems – and sleep medicine - have not been able to adequately address (Jackson CL and Johnson DA. J Clin Sleep Med. 16[8]:1401-2). More specific differences may include Internet access, reduced access due to socioeconomic barriers, transportation limitations, medical mistrust, and membership in a medically vulnerable group such as children, the elderly, and those with high acuity needs. For example, in pediatric patients there exist few evidence-based alternatives and guidelines to in-lab testing and care, which may have negatively impacted access to needed sleep medicine services (Sullivan S et al. J Clin Sleep Med. 2021 Mar 1;17[3]:361-2).
Economics in the COVID-19 pandemic
The economic effects of COVID-19 on medical institutions and in sleep medicine is a story that continues to unfold. Reductions in patient visits and elective procedures, infection control measures limiting capacity, increased costs to maintain such measures, and variability of responses by payer and region are just a few of the issues. The Centers for Medicare & Medicaid Services has employed waivers to increased flexibility and promote safe and effective care including the use of telemedicine during the public health emergency, but the future of these waivers remains uncertain. Alarmingly, a sizeable portion of sleep practices reported financial solvency concerns related to the pandemic (Ramar K. J Clin Sleep Med. 2020;16[11]:1939-42).
Conclusion
As the COVID-19 pandemic and related public health guidance continues to evolve, sleep medicine practices continue to adapt. Vaccination, new variants, changes in mask guidance, new outbreaks around the globe, financial and staffing uncertainties, as well as addressing disparities in care and outcomes that may be augmented by the pandemic remain salient areas of ongoing development.
Dr. Lee is a Postdoctoral and Pediatric Pulmonary Fellow, Department of Pediatrics, Division of Pulmonary, Asthma, and Sleep Medicine, Stanford University School of Medicine; Dr. Sullivan is Clinical Professor, Department of Pediatrics, Division of Pulmonary, Asthma, and Sleep Medicine, and by courtesy, Division of Sleep Medicine, Department of Psychiatry, Stanford University School of Medicine, Palo Alto, CA.
Background
Well into its second year, the worldwide COVID-19 pandemic continues to pose substantial challenges for health care access and delivery. Regulatory agencies such as the Centers for Disease Control (CDC) do not currently have guidance related to COVID-19 specific to sleep centers and laboratories. In March 2020, within days of the World Health Organization pandemic declaration, the American Academy of Sleep Medicine (AASM) posted detailed guidance on mitigation strategies for sleep medicine practices (COVID-19 Resources).
This initial guidance has been previously reported in this publication (Sullivan S, Gurubhagavatula I. CHEST Physician 2020 May 8), and the guidance has been periodically updated during the pandemic. It was restructured in mid-2020 to include sections summarizing CDC recommendations germane for sleep practices; additional sleep medicine-specific guidance from the AASM COVID-19 Task Force (TF); and a frequently asked questions (FAQ) section. The last major update from the task force occurred on Jan. 18, 2021, though subsequent posts – especially related to recent CDC changes in masking guidelines – were made in May 2021. The purpose of this article is to summarize these updates and to call attention to areas of ongoing interest to sleep medicine. Notably, the AASM Task Force guidance is nonbinding and offered as a framework for considering best practices in this evolving situation, acknowledging the importance of weighing local factors, conditions, and regulations, as well as the interests of and risks to the patient, staff, and providers.
Key updates
Data on exposure and transmission risks specific to sleep medicine
Measures for reducing viral transmission have been central to managing the spread of the virus in clinical settings. In its last major update, the AASM TF noted that no known outbreaks of COVID-19 related to sleep center exposure have been reported. A perspective and data published in the Journal of the American Medical Association concluded that hospital transmission of the virus “in the setting of universal masking is likely rare, even during periods of high community prevalence.” It also concluded that hospital-based outbreaks are more likely to occur in small workrooms and during mealtime when staff are less adherent to masking and physical distancing (Richterman A, et al. JAMA. 2020;324[21]:2155-6). The TF elaborated on considerations to reduce transmission, which include not just telework and foundational infection control practices, but also broader workplace considerations such as optimizing ventilation, taking advantage of outdoor spaces (e.g., for breaks and eating), scheduling to reduce interactions between personnel from different teams, minimizing contact in meeting/break rooms, removing tables and chairs from lounge areas, and following CDC guidance for effective facility operations.
Vaccination
In the January update, the AASM COVID-19 TF stated that, “sleep facility leaders should encourage staff and patients to be vaccinated in accordance with CDC guidance.” The role of the sleep medicine community in encouraging healthy sleep habits before and after vaccination was emphasized, pointing to evidence linking sleep and immunity, specifically between sleep duration and vaccination response (Healthy sleep and immune response to COVID-19 vaccination. 2021 Jan.).
In an FAQ update from March 26, 2021, considering whether continued COVID-19 testing was needed following full vaccination, the AASM advised testing prior to potential aerosol-generating procedures should be made on the basis of a risk-benefit assessment by the sleep clinician. Several considerations were highlighted, including recent COVID-19 infection, vaccination status of contacts, local prevalence of newer variants, and whether individuals are receiving positive airway pressure therapy. The TF focused on the vigilance for residents and staff in long-term care facilities, which have been associated with a number of outbreaks.
Masking in the context of the COVID-19 vaccine
The most significant change in recommendations is the recent relaxation of masking guidance by the CDC in the setting of the approval and distribution of COVID-19 vaccinations. In May, the CDC stated that fully vaccinated individuals can resume activities without masking or physically distancing except in scenarios of travel and where required by laws, regulations, and local businesses, due to the efficacy of the vaccines, increasing evidence of reduced asymptomatic carriage and transmission after vaccination, and anticipated increased uptake of vaccination. However, the CDC also noted that these updates did not apply to health care facilities, where the recommendation remains that patients and visitors should continue to mask throughout their stay. Additionally, fully vaccinated health care workers should continue to practice infection control measures while working with patients. On May 14, the AASM TF provided a detailed FAQ acknowledging the CDC’s new guidance, emphasizing that masking guidance in health care facilities remains unchanged, and encouraging individuals to follow CDC guidance regarding vaccination, noting that emergence of newer variants continues to be monitored, and existing vaccines still appear to induce neutralizing antibodies even if to a somewhat lower degree. The situation for pediatric sleep centers has been highlighted in particular because the potential risk posed by newer variants to children remains under investigation, and children under age 12 are not approved for vaccination.
Important caveats to discussions around vaccination status are the lack of a centralized method to identify vaccinated individuals, the unknown duration of immunity, and reports of the use of fake vaccine cards. At this time, in health care settings, vaccination status should not exempt mask usage for any individual.
Sleep medicine care for those with COVID-19
Regarding the duration of isolation and precautions for adults with COVID-19, the TF highlighted the CDC’s symptom-based strategy, rather than test-based strategy, for ending isolation of these patients, availing them of sleep medicine services in person.
In line with the CDC guidance, this approach indicates that scheduling in-person care such as polysomnography for a COVID-19–positive patient may be appropriate at least 10 days after symptom onset (or after a positive test if the patient never developed symptoms); or at least 20 days after symptom onset if the illness was severe; or if at least 90 days have elapsed since symptom onset, consider preappointment COVID-19 screening. In the context of immunocompromised individuals, involvement from infectious disease specialists may be needed to help guide decisions.
Patient communications
For many, a repercussion of the pandemic has been delaying care or avoiding addressing medical issues, including sleep disorders. The AASM encouraged practices to consider communicating with patients that delaying needed care can increase health risks; COVID-19 transmission to patients in health care settings has been low; effective safety procedures are in place; and whether remote/telehealth services are available.
Disparities in care
In addition to the specific guidance above, there are ongoing concerns regarding disparities in care resulting from a variety of sources and becoming more evident during the pandemic. Complex factors, ranging from economic, geographic, contextual, occupational, and others contribute to disparities that health care systems – and sleep medicine - have not been able to adequately address (Jackson CL and Johnson DA. J Clin Sleep Med. 16[8]:1401-2). More specific differences may include Internet access, reduced access due to socioeconomic barriers, transportation limitations, medical mistrust, and membership in a medically vulnerable group such as children, the elderly, and those with high acuity needs. For example, in pediatric patients there exist few evidence-based alternatives and guidelines to in-lab testing and care, which may have negatively impacted access to needed sleep medicine services (Sullivan S et al. J Clin Sleep Med. 2021 Mar 1;17[3]:361-2).
Economics in the COVID-19 pandemic
The economic effects of COVID-19 on medical institutions and in sleep medicine is a story that continues to unfold. Reductions in patient visits and elective procedures, infection control measures limiting capacity, increased costs to maintain such measures, and variability of responses by payer and region are just a few of the issues. The Centers for Medicare & Medicaid Services has employed waivers to increased flexibility and promote safe and effective care including the use of telemedicine during the public health emergency, but the future of these waivers remains uncertain. Alarmingly, a sizeable portion of sleep practices reported financial solvency concerns related to the pandemic (Ramar K. J Clin Sleep Med. 2020;16[11]:1939-42).
Conclusion
As the COVID-19 pandemic and related public health guidance continues to evolve, sleep medicine practices continue to adapt. Vaccination, new variants, changes in mask guidance, new outbreaks around the globe, financial and staffing uncertainties, as well as addressing disparities in care and outcomes that may be augmented by the pandemic remain salient areas of ongoing development.
Dr. Lee is a Postdoctoral and Pediatric Pulmonary Fellow, Department of Pediatrics, Division of Pulmonary, Asthma, and Sleep Medicine, Stanford University School of Medicine; Dr. Sullivan is Clinical Professor, Department of Pediatrics, Division of Pulmonary, Asthma, and Sleep Medicine, and by courtesy, Division of Sleep Medicine, Department of Psychiatry, Stanford University School of Medicine, Palo Alto, CA.
Obstructive sleep apnea and COVID-19
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused by the novel coronavirus of the year 2019 (COVID-19) has had a major impact on global health and economy. United States reported a total caseload of 28,998,834 patients and total mortality of 525,031 as of March 2021 (NPR.org; worldometer. Accessed March 8, 2021). The beginning of 2021 ushered positivity with the development of multiple highly effective SARS-CoV-2 vaccines. Although the medical world has gained much knowledge about this deadly disease, there are many unknowns and still much to be learned.
Two early landmark studies from Italy (Lombardy) and United States (New York City area) provided initial insight on comorbid conditions associated with increased risk of severe COVID-19 infection (Richardson S, et al. JAMA. 2020;323[20]:2052; Grasselli G, et al. JAMA Intern Med. 2020;180[10]:1345). In the United States cohort, hypertension (HTN), obesity, and diabetes (DM) were independent risk factors for severe disease, while in the Italy cohort, older age, male, COPD, hypercholesterolemia, and diabetes were independent risk factors for increased mortality. Obstructive sleep apnea (OSA) was not mentioned as a comorbid risk factor.
There is much speculation regarding OSA as an independent risk factor for severe COVID-19 infection. OSA is a common sleep-related breathing disorder with increased prevalence in men, older age, and higher body mass index (BMI); and OSA is associated with hypertension, obesity, and diabetes, all of which are risk factors for severe COVID-19. Because of the shared similarities in pathophysiology between OSA and COVID-19 (Tufik S, et al. J Clin Sleep Med. 2020;16[8]:1425), and shared comorbid conditions associated with increased risk of severe COVID-19 disease, OSA has been suggested as an independent risk factor for unfavorable COVID-19-related outcomes.
SARS-CoV-2 triggers a severe inflammatory response involving type-II pneumocytes and angiotensin-converting enzyme 2 pathway. OSA is characterized by intermittent hypoxia and sleep fragmentation, leading to a cascade of systemic inflammatory response involving oxidative stress, pro-inflammatory cytokines, endothelial dysfunction, and consequent cardiovascular injury (Jose RJ, et al. Lancet Respir Med. 2020;8[6]:e46; Saxena K, et al. Sleep Medicine. 2021;79:223). In this regard, OSA may contribute to COVID-19 “cytokine storm” by causing or exacerbating endothelial dysfunction, inflammation, and oxidative stress.
Multiple studies have recently been published on the impact of OSA on COVID-19 outcomes. The Coronavirus SARS-CoV-2 and Diabetes Outcomes (CORONADO) study was one of the initial studies that analyzed the relationship between OSA and COVID-19-related outcomes. This was a multicenter observational study involving diabetic patients hospitalized with COVID-19. The primary outcome was mechanical ventilation and/or death within 7 days of admission. Multivariate adjustment showed that age, BMI, and OSA, among other factors, were independently associated with risk of death on day 7 (Cariou B, et al. Diabetologia. 2020;63[8]:1500). Strausz and colleagues also evaluated OSA as an independent risk factor for severe COVID-19 in a large registry of hospital discharge patients (FinnGen study). The authors reported that although the risk of contracting COVID-19 was the same for patients with or without OSA, after adjusting for age, sex, and BMI, OSA was associated with higher risk of hospitalization (Strausz S, et al. BMJ Open Resp Res. 2021;8:e000845). Similar findings were confirmed by the Maas et al. study, which utilized a large socioeconomically diverse database composed of 10 hospital systems. Diagnoses and outcomes were identified by ICD-10 coding and medical record data. After adjustments for diabetes, HTN, and BMI, OSA conferred an eight-fold risk for COVID-19 infection, was associated with increased risk of hospitalization, and doubled the risk of developing respiratory failure (Maas MB, et al. Sleep Breath. 2020 Sep; 29:1-3. doi: 10.1007/s11325-020-02203-0).
Peker and colleagues conducted a prospective multicenter observational study comparing clinical outcomes of severe COVID-19 infection in patients with low vs high pretest probability of having OSA based on the Berlin questionnaire. The authors reported a clinically significant risk of poorer clinical outcomes in the high pretest probability OSA group after adjustments for age, sex, and comorbidities (Peker Y, et al. Ann Am Thorac Soc. 2021. Feb 17. doi: 10.1513/AnnalsATS.202011-1409OC). A timely meta-analysis including 21 studies (19 with retrospective design) with 54,276 COVID-19 patients and 4,640 OSA patients concluded poor composite outcomes including severe COVID-19, intensive care unit admission, mechanical ventilatory support, and death in association with OSA (OR – 1.72 95% CI 1.55-1.91, P< .00001). In patients with obesity, OSA is a highly prevalent co-morbid condition. BMI, however, was not adjusted in this model (Hariyanto TI, et al. Sleep Med. 2021. doi: 10.1016/j.sleep.2021.03.029).
Other studies have concluded the opposite with OSA not being an independent risk factor for severe COVID-19 infection. Cade and colleagues conducted a retrospective analysis from a comprehensive electronic health dataset using ICD codes to identify OSA patients with severe COVID-19 infection. A significant association between OSA and COVID-19 death was noted after adjustment for demographics (ethnicity, age, sex). However, when fully adjusted for demographics, BMI, asthma, COPD, HTN, or DM, OSA was not an independent risk factor for COVID-19-related mortality and hospitalization (Cade BE, et al. Am J Respir Crit Care Med. 2020;202[10]:1462). The FinnGen study (Strausz et al.) was part of a meta-analysis examining the association between OSA and severe COVID-19 with and without adjustments for BMI. This meta-analysis consisted of 15,835 COVID-19 patients including 1,294 with OSA. The authors found that OSA was a risk factor with a two-fold increased risk of severe COVID-19 infection (OR = 2.37, P = .021). However, after adjustments were made for BMI, this finding lost statistical significance (OR=1.55, P=.13) (Strausz S, et al. BMJ Open Resp Res. 2021;8:e000845).
It is worth noting that a majority of studies identified OSA by indirect and imperfect methods through chart review, ICD codes, and databases. Confirmed OSA based on formal testing with a sleep study in COVID-19 patients remains a challenge. Perhaps well performed screening questionnaires, such as STOP-Bang, Berlin, or NoSAS, can be utilized as was the case in one study. It is also unclear if outcomes of COVID-19 infection differ in patients with treated or untreated OSA, as raised by the CORONADO study. A recent cross-sectional telephone interview survey of patients with confirmed OSA in Iran alluded to higher prevalence of COVID-19 in patients with severe OSA with suggestion of lower prevalence in patients who were currently receiving OSA treatment with positive airway pressure (PAP) therapy (Najafi A, et al. Sleep Health. 2021 Feb;7[1]:14). This is a crucial question as PAP therapy is considered an aerosol-generating procedure (Lance CG. Cleve Clin J Med. 2020 May 5. doi: 10.3949/ccjm.87a.ccc003). Studies have suggested continued use of PAP therapy with additional measures to mitigate the spread of virus, since failure to use PAP could be deleterious to the patient’s quality of life. Interestingly, PAP adherence seemed to have improved during the pandemic as evidenced by a telephonic survey done in New York City that showed 88% of patients with OSA used a PAP device consistently (Attias D, et al. Eur Respir J. 2020 Jul 30;56[1]:2001607. doi: 10.1183/13993003.01607-2020).
In summary, the jury is still out on whether OSA is a facilitator for viral replication, or an independent risk factor for poor prognosis related to COVID-19 infection, or has no clinical relevance to COVID-19. COVID-19 and OSA share comorbidities and pathways leading to a systemic inflammatory cascade. Theoretically, it would make sense that OSA is a risk factor for severe COVID-19 infection; however, it remains to be proven. The recent studies are limited by retrospective and observational nature, imprecise OSA classification/diagnostic criteria, and confounded by difficult to control variables. Further research is needed to expand our understanding of OSA -induced intermittent hypoxemia, inflammation, and endothelial dysfunction that may play a role in COVID-19 morbidity and mortality. Until we have more clarity, close monitoring of OSA patients infected with COVID-19 is recommended along with implementation of safe protocols for continuation of PAP usage during the infectious phase. Identifying underlying comorbid conditions that contribute to worsening of a COVID-19 infectious course is a crucial step in improving clinical outcomes.
Dr. Sahni is Assistant Professor of Clinical Medicine, Division of Pulmonary, Critical Care, Sleep and Allergy, Department of Medicine, University of Illinois at Chicago. Dr. Cao is Clinical Associate Professor, Division of Sleep Medicine and Division of Neuromuscular Medicine, Department of Psychiatry and Department of Neurology, Stanford (Calif.) University.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused by the novel coronavirus of the year 2019 (COVID-19) has had a major impact on global health and economy. United States reported a total caseload of 28,998,834 patients and total mortality of 525,031 as of March 2021 (NPR.org; worldometer. Accessed March 8, 2021). The beginning of 2021 ushered positivity with the development of multiple highly effective SARS-CoV-2 vaccines. Although the medical world has gained much knowledge about this deadly disease, there are many unknowns and still much to be learned.
Two early landmark studies from Italy (Lombardy) and United States (New York City area) provided initial insight on comorbid conditions associated with increased risk of severe COVID-19 infection (Richardson S, et al. JAMA. 2020;323[20]:2052; Grasselli G, et al. JAMA Intern Med. 2020;180[10]:1345). In the United States cohort, hypertension (HTN), obesity, and diabetes (DM) were independent risk factors for severe disease, while in the Italy cohort, older age, male, COPD, hypercholesterolemia, and diabetes were independent risk factors for increased mortality. Obstructive sleep apnea (OSA) was not mentioned as a comorbid risk factor.
There is much speculation regarding OSA as an independent risk factor for severe COVID-19 infection. OSA is a common sleep-related breathing disorder with increased prevalence in men, older age, and higher body mass index (BMI); and OSA is associated with hypertension, obesity, and diabetes, all of which are risk factors for severe COVID-19. Because of the shared similarities in pathophysiology between OSA and COVID-19 (Tufik S, et al. J Clin Sleep Med. 2020;16[8]:1425), and shared comorbid conditions associated with increased risk of severe COVID-19 disease, OSA has been suggested as an independent risk factor for unfavorable COVID-19-related outcomes.
SARS-CoV-2 triggers a severe inflammatory response involving type-II pneumocytes and angiotensin-converting enzyme 2 pathway. OSA is characterized by intermittent hypoxia and sleep fragmentation, leading to a cascade of systemic inflammatory response involving oxidative stress, pro-inflammatory cytokines, endothelial dysfunction, and consequent cardiovascular injury (Jose RJ, et al. Lancet Respir Med. 2020;8[6]:e46; Saxena K, et al. Sleep Medicine. 2021;79:223). In this regard, OSA may contribute to COVID-19 “cytokine storm” by causing or exacerbating endothelial dysfunction, inflammation, and oxidative stress.
Multiple studies have recently been published on the impact of OSA on COVID-19 outcomes. The Coronavirus SARS-CoV-2 and Diabetes Outcomes (CORONADO) study was one of the initial studies that analyzed the relationship between OSA and COVID-19-related outcomes. This was a multicenter observational study involving diabetic patients hospitalized with COVID-19. The primary outcome was mechanical ventilation and/or death within 7 days of admission. Multivariate adjustment showed that age, BMI, and OSA, among other factors, were independently associated with risk of death on day 7 (Cariou B, et al. Diabetologia. 2020;63[8]:1500). Strausz and colleagues also evaluated OSA as an independent risk factor for severe COVID-19 in a large registry of hospital discharge patients (FinnGen study). The authors reported that although the risk of contracting COVID-19 was the same for patients with or without OSA, after adjusting for age, sex, and BMI, OSA was associated with higher risk of hospitalization (Strausz S, et al. BMJ Open Resp Res. 2021;8:e000845). Similar findings were confirmed by the Maas et al. study, which utilized a large socioeconomically diverse database composed of 10 hospital systems. Diagnoses and outcomes were identified by ICD-10 coding and medical record data. After adjustments for diabetes, HTN, and BMI, OSA conferred an eight-fold risk for COVID-19 infection, was associated with increased risk of hospitalization, and doubled the risk of developing respiratory failure (Maas MB, et al. Sleep Breath. 2020 Sep; 29:1-3. doi: 10.1007/s11325-020-02203-0).
Peker and colleagues conducted a prospective multicenter observational study comparing clinical outcomes of severe COVID-19 infection in patients with low vs high pretest probability of having OSA based on the Berlin questionnaire. The authors reported a clinically significant risk of poorer clinical outcomes in the high pretest probability OSA group after adjustments for age, sex, and comorbidities (Peker Y, et al. Ann Am Thorac Soc. 2021. Feb 17. doi: 10.1513/AnnalsATS.202011-1409OC). A timely meta-analysis including 21 studies (19 with retrospective design) with 54,276 COVID-19 patients and 4,640 OSA patients concluded poor composite outcomes including severe COVID-19, intensive care unit admission, mechanical ventilatory support, and death in association with OSA (OR – 1.72 95% CI 1.55-1.91, P< .00001). In patients with obesity, OSA is a highly prevalent co-morbid condition. BMI, however, was not adjusted in this model (Hariyanto TI, et al. Sleep Med. 2021. doi: 10.1016/j.sleep.2021.03.029).
Other studies have concluded the opposite with OSA not being an independent risk factor for severe COVID-19 infection. Cade and colleagues conducted a retrospective analysis from a comprehensive electronic health dataset using ICD codes to identify OSA patients with severe COVID-19 infection. A significant association between OSA and COVID-19 death was noted after adjustment for demographics (ethnicity, age, sex). However, when fully adjusted for demographics, BMI, asthma, COPD, HTN, or DM, OSA was not an independent risk factor for COVID-19-related mortality and hospitalization (Cade BE, et al. Am J Respir Crit Care Med. 2020;202[10]:1462). The FinnGen study (Strausz et al.) was part of a meta-analysis examining the association between OSA and severe COVID-19 with and without adjustments for BMI. This meta-analysis consisted of 15,835 COVID-19 patients including 1,294 with OSA. The authors found that OSA was a risk factor with a two-fold increased risk of severe COVID-19 infection (OR = 2.37, P = .021). However, after adjustments were made for BMI, this finding lost statistical significance (OR=1.55, P=.13) (Strausz S, et al. BMJ Open Resp Res. 2021;8:e000845).
It is worth noting that a majority of studies identified OSA by indirect and imperfect methods through chart review, ICD codes, and databases. Confirmed OSA based on formal testing with a sleep study in COVID-19 patients remains a challenge. Perhaps well performed screening questionnaires, such as STOP-Bang, Berlin, or NoSAS, can be utilized as was the case in one study. It is also unclear if outcomes of COVID-19 infection differ in patients with treated or untreated OSA, as raised by the CORONADO study. A recent cross-sectional telephone interview survey of patients with confirmed OSA in Iran alluded to higher prevalence of COVID-19 in patients with severe OSA with suggestion of lower prevalence in patients who were currently receiving OSA treatment with positive airway pressure (PAP) therapy (Najafi A, et al. Sleep Health. 2021 Feb;7[1]:14). This is a crucial question as PAP therapy is considered an aerosol-generating procedure (Lance CG. Cleve Clin J Med. 2020 May 5. doi: 10.3949/ccjm.87a.ccc003). Studies have suggested continued use of PAP therapy with additional measures to mitigate the spread of virus, since failure to use PAP could be deleterious to the patient’s quality of life. Interestingly, PAP adherence seemed to have improved during the pandemic as evidenced by a telephonic survey done in New York City that showed 88% of patients with OSA used a PAP device consistently (Attias D, et al. Eur Respir J. 2020 Jul 30;56[1]:2001607. doi: 10.1183/13993003.01607-2020).
In summary, the jury is still out on whether OSA is a facilitator for viral replication, or an independent risk factor for poor prognosis related to COVID-19 infection, or has no clinical relevance to COVID-19. COVID-19 and OSA share comorbidities and pathways leading to a systemic inflammatory cascade. Theoretically, it would make sense that OSA is a risk factor for severe COVID-19 infection; however, it remains to be proven. The recent studies are limited by retrospective and observational nature, imprecise OSA classification/diagnostic criteria, and confounded by difficult to control variables. Further research is needed to expand our understanding of OSA -induced intermittent hypoxemia, inflammation, and endothelial dysfunction that may play a role in COVID-19 morbidity and mortality. Until we have more clarity, close monitoring of OSA patients infected with COVID-19 is recommended along with implementation of safe protocols for continuation of PAP usage during the infectious phase. Identifying underlying comorbid conditions that contribute to worsening of a COVID-19 infectious course is a crucial step in improving clinical outcomes.
Dr. Sahni is Assistant Professor of Clinical Medicine, Division of Pulmonary, Critical Care, Sleep and Allergy, Department of Medicine, University of Illinois at Chicago. Dr. Cao is Clinical Associate Professor, Division of Sleep Medicine and Division of Neuromuscular Medicine, Department of Psychiatry and Department of Neurology, Stanford (Calif.) University.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused by the novel coronavirus of the year 2019 (COVID-19) has had a major impact on global health and economy. United States reported a total caseload of 28,998,834 patients and total mortality of 525,031 as of March 2021 (NPR.org; worldometer. Accessed March 8, 2021). The beginning of 2021 ushered positivity with the development of multiple highly effective SARS-CoV-2 vaccines. Although the medical world has gained much knowledge about this deadly disease, there are many unknowns and still much to be learned.
Two early landmark studies from Italy (Lombardy) and United States (New York City area) provided initial insight on comorbid conditions associated with increased risk of severe COVID-19 infection (Richardson S, et al. JAMA. 2020;323[20]:2052; Grasselli G, et al. JAMA Intern Med. 2020;180[10]:1345). In the United States cohort, hypertension (HTN), obesity, and diabetes (DM) were independent risk factors for severe disease, while in the Italy cohort, older age, male, COPD, hypercholesterolemia, and diabetes were independent risk factors for increased mortality. Obstructive sleep apnea (OSA) was not mentioned as a comorbid risk factor.
There is much speculation regarding OSA as an independent risk factor for severe COVID-19 infection. OSA is a common sleep-related breathing disorder with increased prevalence in men, older age, and higher body mass index (BMI); and OSA is associated with hypertension, obesity, and diabetes, all of which are risk factors for severe COVID-19. Because of the shared similarities in pathophysiology between OSA and COVID-19 (Tufik S, et al. J Clin Sleep Med. 2020;16[8]:1425), and shared comorbid conditions associated with increased risk of severe COVID-19 disease, OSA has been suggested as an independent risk factor for unfavorable COVID-19-related outcomes.
SARS-CoV-2 triggers a severe inflammatory response involving type-II pneumocytes and angiotensin-converting enzyme 2 pathway. OSA is characterized by intermittent hypoxia and sleep fragmentation, leading to a cascade of systemic inflammatory response involving oxidative stress, pro-inflammatory cytokines, endothelial dysfunction, and consequent cardiovascular injury (Jose RJ, et al. Lancet Respir Med. 2020;8[6]:e46; Saxena K, et al. Sleep Medicine. 2021;79:223). In this regard, OSA may contribute to COVID-19 “cytokine storm” by causing or exacerbating endothelial dysfunction, inflammation, and oxidative stress.
Multiple studies have recently been published on the impact of OSA on COVID-19 outcomes. The Coronavirus SARS-CoV-2 and Diabetes Outcomes (CORONADO) study was one of the initial studies that analyzed the relationship between OSA and COVID-19-related outcomes. This was a multicenter observational study involving diabetic patients hospitalized with COVID-19. The primary outcome was mechanical ventilation and/or death within 7 days of admission. Multivariate adjustment showed that age, BMI, and OSA, among other factors, were independently associated with risk of death on day 7 (Cariou B, et al. Diabetologia. 2020;63[8]:1500). Strausz and colleagues also evaluated OSA as an independent risk factor for severe COVID-19 in a large registry of hospital discharge patients (FinnGen study). The authors reported that although the risk of contracting COVID-19 was the same for patients with or without OSA, after adjusting for age, sex, and BMI, OSA was associated with higher risk of hospitalization (Strausz S, et al. BMJ Open Resp Res. 2021;8:e000845). Similar findings were confirmed by the Maas et al. study, which utilized a large socioeconomically diverse database composed of 10 hospital systems. Diagnoses and outcomes were identified by ICD-10 coding and medical record data. After adjustments for diabetes, HTN, and BMI, OSA conferred an eight-fold risk for COVID-19 infection, was associated with increased risk of hospitalization, and doubled the risk of developing respiratory failure (Maas MB, et al. Sleep Breath. 2020 Sep; 29:1-3. doi: 10.1007/s11325-020-02203-0).
Peker and colleagues conducted a prospective multicenter observational study comparing clinical outcomes of severe COVID-19 infection in patients with low vs high pretest probability of having OSA based on the Berlin questionnaire. The authors reported a clinically significant risk of poorer clinical outcomes in the high pretest probability OSA group after adjustments for age, sex, and comorbidities (Peker Y, et al. Ann Am Thorac Soc. 2021. Feb 17. doi: 10.1513/AnnalsATS.202011-1409OC). A timely meta-analysis including 21 studies (19 with retrospective design) with 54,276 COVID-19 patients and 4,640 OSA patients concluded poor composite outcomes including severe COVID-19, intensive care unit admission, mechanical ventilatory support, and death in association with OSA (OR – 1.72 95% CI 1.55-1.91, P< .00001). In patients with obesity, OSA is a highly prevalent co-morbid condition. BMI, however, was not adjusted in this model (Hariyanto TI, et al. Sleep Med. 2021. doi: 10.1016/j.sleep.2021.03.029).
Other studies have concluded the opposite with OSA not being an independent risk factor for severe COVID-19 infection. Cade and colleagues conducted a retrospective analysis from a comprehensive electronic health dataset using ICD codes to identify OSA patients with severe COVID-19 infection. A significant association between OSA and COVID-19 death was noted after adjustment for demographics (ethnicity, age, sex). However, when fully adjusted for demographics, BMI, asthma, COPD, HTN, or DM, OSA was not an independent risk factor for COVID-19-related mortality and hospitalization (Cade BE, et al. Am J Respir Crit Care Med. 2020;202[10]:1462). The FinnGen study (Strausz et al.) was part of a meta-analysis examining the association between OSA and severe COVID-19 with and without adjustments for BMI. This meta-analysis consisted of 15,835 COVID-19 patients including 1,294 with OSA. The authors found that OSA was a risk factor with a two-fold increased risk of severe COVID-19 infection (OR = 2.37, P = .021). However, after adjustments were made for BMI, this finding lost statistical significance (OR=1.55, P=.13) (Strausz S, et al. BMJ Open Resp Res. 2021;8:e000845).
It is worth noting that a majority of studies identified OSA by indirect and imperfect methods through chart review, ICD codes, and databases. Confirmed OSA based on formal testing with a sleep study in COVID-19 patients remains a challenge. Perhaps well performed screening questionnaires, such as STOP-Bang, Berlin, or NoSAS, can be utilized as was the case in one study. It is also unclear if outcomes of COVID-19 infection differ in patients with treated or untreated OSA, as raised by the CORONADO study. A recent cross-sectional telephone interview survey of patients with confirmed OSA in Iran alluded to higher prevalence of COVID-19 in patients with severe OSA with suggestion of lower prevalence in patients who were currently receiving OSA treatment with positive airway pressure (PAP) therapy (Najafi A, et al. Sleep Health. 2021 Feb;7[1]:14). This is a crucial question as PAP therapy is considered an aerosol-generating procedure (Lance CG. Cleve Clin J Med. 2020 May 5. doi: 10.3949/ccjm.87a.ccc003). Studies have suggested continued use of PAP therapy with additional measures to mitigate the spread of virus, since failure to use PAP could be deleterious to the patient’s quality of life. Interestingly, PAP adherence seemed to have improved during the pandemic as evidenced by a telephonic survey done in New York City that showed 88% of patients with OSA used a PAP device consistently (Attias D, et al. Eur Respir J. 2020 Jul 30;56[1]:2001607. doi: 10.1183/13993003.01607-2020).
In summary, the jury is still out on whether OSA is a facilitator for viral replication, or an independent risk factor for poor prognosis related to COVID-19 infection, or has no clinical relevance to COVID-19. COVID-19 and OSA share comorbidities and pathways leading to a systemic inflammatory cascade. Theoretically, it would make sense that OSA is a risk factor for severe COVID-19 infection; however, it remains to be proven. The recent studies are limited by retrospective and observational nature, imprecise OSA classification/diagnostic criteria, and confounded by difficult to control variables. Further research is needed to expand our understanding of OSA -induced intermittent hypoxemia, inflammation, and endothelial dysfunction that may play a role in COVID-19 morbidity and mortality. Until we have more clarity, close monitoring of OSA patients infected with COVID-19 is recommended along with implementation of safe protocols for continuation of PAP usage during the infectious phase. Identifying underlying comorbid conditions that contribute to worsening of a COVID-19 infectious course is a crucial step in improving clinical outcomes.
Dr. Sahni is Assistant Professor of Clinical Medicine, Division of Pulmonary, Critical Care, Sleep and Allergy, Department of Medicine, University of Illinois at Chicago. Dr. Cao is Clinical Associate Professor, Division of Sleep Medicine and Division of Neuromuscular Medicine, Department of Psychiatry and Department of Neurology, Stanford (Calif.) University.