User login
Race and spirometry
The European Respiratory Society (ERS) and American Thoracic Society (ATS) just published an update to their guidelines on lung function interpretation (Stanojevic S, et al. Eur Respir J. 2022; 60: 2101499). As with any update, the document builds on past work and integrates new advances the field has seen since 2005.
The current iteration comes at a time when academics, clinicians, and epidemiologists are re-analyzing what we think we know about the complex ways race and ethnicity intersect with the practice of medicine. Several experts on lung function testing, many if not most of whom are authors on the ERS/ATS guideline, have written letters or published reviews commenting on the way accounting for race or ethnicity affects lung function interpretation.
Race/ethnicity and lung function was also the topic of an excellent session at the recent CHEST 2022 Annual Meeting in Nashville, Tennessee. Here, we’ll provide a brief review and direct the reader to relevant sources for a more detailed analysis.
Spirometry is an integral part of the diagnosis and management of a wide range of pulmonary conditions. Dr. Aaron Baugh from the University of California San Francisco (UCSF) lectured on the spirometer’s history at CHEST 2022 and detailed its interactions with race over the past 2 centuries. Other authors have chronicled this history, as well (Braun L, et al. Can J Respir Ther. 2015;51[4]:99-101). The short version is that since the British surgeon John Hutchinson created the first spirometer in 1846, race has been a part of the discussion of lung function interpretation.
In 2022, we know far more about the factors that determine lung function than we did in the 19th century. Age, height, and sex assigned at birth all explain a high percentage of the variability seen in FEV1 and FVC. When modeled, race also explains a portion of the variability, and the NHANES III investigators found its inclusion in regression equations, along with age, height, and sex, improved their precision. Case closed, right? Modern medicine is defined by phenotyping, precision, and individualized care, so why shouldn’t race be a part of lung function interpretation?
Well, it’s complicated. As clinicians and academics, we must analyze the root cause of differences in health outcomes between racial groups.
Publications on pulse oximetry (Gottlieb ER, et al. JAMA Intern Med. 2022; 182:849-858) and glomerular filtration rate (Williams WW, et al. N Engl J Med. 2021;385:1804-1806) have revealed some of the ways our use of instruments and equations may exacerbate or perpetuate current disparities. Even small differences in a measure like pulse oximetry could have a profound impact on clinical decisions at the individual and population levels.
The 2022 ERS/ATS lung function interpretation guidelines have abandoned the use of NHANES III as a reference set. They now recommend the equations developed by the Global Lung Initiative (GLI) for referencing to normal for spirometry, diffusion capacity, and lung volumes. For spirometry the GLI was able to integrate data from countries around the world. This allowed ethnicity to be included in their regression equations and, similar to NHANES III, they found ethnicity improved the precision of their equations. They also published an equation that did not account for country of origin that could be applied to individuals of any race/ethnicity (Quanjer PH, et al. Eur Respir J. 2014;43:505-512). This allowed for applying the GLI equations to external data sets with or without ethnicity included as a co-variate.
Given well-established discrepancies in spirometry, it should come as no surprise that applying the race/ethnicity-neutral GLI equations to non-White populations increases the percentage of patients with pulmonary defects (Moffett AT, et al. Am J Respir Crit Care Med. 2021; A1030). Other data suggest that elimination of race/ethnicity as a co-variate improves the association between percent predicted lung function and important outcomes like mortality (McCormack MC, et al. Am J Respir Crit Care Med. 2022;205:723-724). The first analysis implies that by adjusting for race/ethnicity we may be missing abnormalities, and the second suggests accuracy for outcomes is lost. So case closed, right? Let’s abandon race/ethnicity as a co- variate for our spirometry reference equations.
Perhaps, but a few caveats are in order. It’s important to note that doing so would result in a dramatic increase in abnormal findings in otherwise healthy and asymptomatic non-White individuals. This could negatively affect eligibility for employment and military service (Townsend MC, et al. Am J Respir Crit Care Med. 2022;789-790). We’ve also yet to fully explain the factors driving differences in lung function between races. If socioeconomic factors explained the entirety of the difference, it would be easier to argue for elimination of using race/ethnicity in our equations. Currently, the etiology is thought to be multifactorial and is yet to be fully explained (Braun L, et al. Eur Respir J. 2013;41:1362-1370).
The more we look for institutional racism, the more we will find it. As we realize that attaining health and wellness is more difficult for the disenfranchised, we need to ensure our current practices are part of the solution.
The ERS/ATS guidelines suggest eliminating fixed correction factors for race but do not require elimination of race/ethnicity as a co-variate in the equations selected for use. This seems very reasonable given what we know now. As pulmonary medicine academics and researchers, we need to continue to study the impact integrating race/ethnicity has on precision, accuracy, and clinical outcomes. As pulmonary medicine clinicians, we need to be aware of the reference equations being used in our lab, understand how inclusion of race/ethnicity affects findings, and act accordingly, depending on the clinical situation.
Dr. Ghionni is a Pulmonary/Critical Care Fellow, and Dr. Woods is Program Director – PCCM Fellowship and Associate Program Director – IM Residency, Medstar Washington Hospital Center; Dr. Woods is Associate Professor of Medicine, Georgetown University School of Medicine, Washington, DC.
The European Respiratory Society (ERS) and American Thoracic Society (ATS) just published an update to their guidelines on lung function interpretation (Stanojevic S, et al. Eur Respir J. 2022; 60: 2101499). As with any update, the document builds on past work and integrates new advances the field has seen since 2005.
The current iteration comes at a time when academics, clinicians, and epidemiologists are re-analyzing what we think we know about the complex ways race and ethnicity intersect with the practice of medicine. Several experts on lung function testing, many if not most of whom are authors on the ERS/ATS guideline, have written letters or published reviews commenting on the way accounting for race or ethnicity affects lung function interpretation.
Race/ethnicity and lung function was also the topic of an excellent session at the recent CHEST 2022 Annual Meeting in Nashville, Tennessee. Here, we’ll provide a brief review and direct the reader to relevant sources for a more detailed analysis.
Spirometry is an integral part of the diagnosis and management of a wide range of pulmonary conditions. Dr. Aaron Baugh from the University of California San Francisco (UCSF) lectured on the spirometer’s history at CHEST 2022 and detailed its interactions with race over the past 2 centuries. Other authors have chronicled this history, as well (Braun L, et al. Can J Respir Ther. 2015;51[4]:99-101). The short version is that since the British surgeon John Hutchinson created the first spirometer in 1846, race has been a part of the discussion of lung function interpretation.
In 2022, we know far more about the factors that determine lung function than we did in the 19th century. Age, height, and sex assigned at birth all explain a high percentage of the variability seen in FEV1 and FVC. When modeled, race also explains a portion of the variability, and the NHANES III investigators found its inclusion in regression equations, along with age, height, and sex, improved their precision. Case closed, right? Modern medicine is defined by phenotyping, precision, and individualized care, so why shouldn’t race be a part of lung function interpretation?
Well, it’s complicated. As clinicians and academics, we must analyze the root cause of differences in health outcomes between racial groups.
Publications on pulse oximetry (Gottlieb ER, et al. JAMA Intern Med. 2022; 182:849-858) and glomerular filtration rate (Williams WW, et al. N Engl J Med. 2021;385:1804-1806) have revealed some of the ways our use of instruments and equations may exacerbate or perpetuate current disparities. Even small differences in a measure like pulse oximetry could have a profound impact on clinical decisions at the individual and population levels.
The 2022 ERS/ATS lung function interpretation guidelines have abandoned the use of NHANES III as a reference set. They now recommend the equations developed by the Global Lung Initiative (GLI) for referencing to normal for spirometry, diffusion capacity, and lung volumes. For spirometry the GLI was able to integrate data from countries around the world. This allowed ethnicity to be included in their regression equations and, similar to NHANES III, they found ethnicity improved the precision of their equations. They also published an equation that did not account for country of origin that could be applied to individuals of any race/ethnicity (Quanjer PH, et al. Eur Respir J. 2014;43:505-512). This allowed for applying the GLI equations to external data sets with or without ethnicity included as a co-variate.
Given well-established discrepancies in spirometry, it should come as no surprise that applying the race/ethnicity-neutral GLI equations to non-White populations increases the percentage of patients with pulmonary defects (Moffett AT, et al. Am J Respir Crit Care Med. 2021; A1030). Other data suggest that elimination of race/ethnicity as a co-variate improves the association between percent predicted lung function and important outcomes like mortality (McCormack MC, et al. Am J Respir Crit Care Med. 2022;205:723-724). The first analysis implies that by adjusting for race/ethnicity we may be missing abnormalities, and the second suggests accuracy for outcomes is lost. So case closed, right? Let’s abandon race/ethnicity as a co- variate for our spirometry reference equations.
Perhaps, but a few caveats are in order. It’s important to note that doing so would result in a dramatic increase in abnormal findings in otherwise healthy and asymptomatic non-White individuals. This could negatively affect eligibility for employment and military service (Townsend MC, et al. Am J Respir Crit Care Med. 2022;789-790). We’ve also yet to fully explain the factors driving differences in lung function between races. If socioeconomic factors explained the entirety of the difference, it would be easier to argue for elimination of using race/ethnicity in our equations. Currently, the etiology is thought to be multifactorial and is yet to be fully explained (Braun L, et al. Eur Respir J. 2013;41:1362-1370).
The more we look for institutional racism, the more we will find it. As we realize that attaining health and wellness is more difficult for the disenfranchised, we need to ensure our current practices are part of the solution.
The ERS/ATS guidelines suggest eliminating fixed correction factors for race but do not require elimination of race/ethnicity as a co-variate in the equations selected for use. This seems very reasonable given what we know now. As pulmonary medicine academics and researchers, we need to continue to study the impact integrating race/ethnicity has on precision, accuracy, and clinical outcomes. As pulmonary medicine clinicians, we need to be aware of the reference equations being used in our lab, understand how inclusion of race/ethnicity affects findings, and act accordingly, depending on the clinical situation.
Dr. Ghionni is a Pulmonary/Critical Care Fellow, and Dr. Woods is Program Director – PCCM Fellowship and Associate Program Director – IM Residency, Medstar Washington Hospital Center; Dr. Woods is Associate Professor of Medicine, Georgetown University School of Medicine, Washington, DC.
The European Respiratory Society (ERS) and American Thoracic Society (ATS) just published an update to their guidelines on lung function interpretation (Stanojevic S, et al. Eur Respir J. 2022; 60: 2101499). As with any update, the document builds on past work and integrates new advances the field has seen since 2005.
The current iteration comes at a time when academics, clinicians, and epidemiologists are re-analyzing what we think we know about the complex ways race and ethnicity intersect with the practice of medicine. Several experts on lung function testing, many if not most of whom are authors on the ERS/ATS guideline, have written letters or published reviews commenting on the way accounting for race or ethnicity affects lung function interpretation.
Race/ethnicity and lung function was also the topic of an excellent session at the recent CHEST 2022 Annual Meeting in Nashville, Tennessee. Here, we’ll provide a brief review and direct the reader to relevant sources for a more detailed analysis.
Spirometry is an integral part of the diagnosis and management of a wide range of pulmonary conditions. Dr. Aaron Baugh from the University of California San Francisco (UCSF) lectured on the spirometer’s history at CHEST 2022 and detailed its interactions with race over the past 2 centuries. Other authors have chronicled this history, as well (Braun L, et al. Can J Respir Ther. 2015;51[4]:99-101). The short version is that since the British surgeon John Hutchinson created the first spirometer in 1846, race has been a part of the discussion of lung function interpretation.
In 2022, we know far more about the factors that determine lung function than we did in the 19th century. Age, height, and sex assigned at birth all explain a high percentage of the variability seen in FEV1 and FVC. When modeled, race also explains a portion of the variability, and the NHANES III investigators found its inclusion in regression equations, along with age, height, and sex, improved their precision. Case closed, right? Modern medicine is defined by phenotyping, precision, and individualized care, so why shouldn’t race be a part of lung function interpretation?
Well, it’s complicated. As clinicians and academics, we must analyze the root cause of differences in health outcomes between racial groups.
Publications on pulse oximetry (Gottlieb ER, et al. JAMA Intern Med. 2022; 182:849-858) and glomerular filtration rate (Williams WW, et al. N Engl J Med. 2021;385:1804-1806) have revealed some of the ways our use of instruments and equations may exacerbate or perpetuate current disparities. Even small differences in a measure like pulse oximetry could have a profound impact on clinical decisions at the individual and population levels.
The 2022 ERS/ATS lung function interpretation guidelines have abandoned the use of NHANES III as a reference set. They now recommend the equations developed by the Global Lung Initiative (GLI) for referencing to normal for spirometry, diffusion capacity, and lung volumes. For spirometry the GLI was able to integrate data from countries around the world. This allowed ethnicity to be included in their regression equations and, similar to NHANES III, they found ethnicity improved the precision of their equations. They also published an equation that did not account for country of origin that could be applied to individuals of any race/ethnicity (Quanjer PH, et al. Eur Respir J. 2014;43:505-512). This allowed for applying the GLI equations to external data sets with or without ethnicity included as a co-variate.
Given well-established discrepancies in spirometry, it should come as no surprise that applying the race/ethnicity-neutral GLI equations to non-White populations increases the percentage of patients with pulmonary defects (Moffett AT, et al. Am J Respir Crit Care Med. 2021; A1030). Other data suggest that elimination of race/ethnicity as a co-variate improves the association between percent predicted lung function and important outcomes like mortality (McCormack MC, et al. Am J Respir Crit Care Med. 2022;205:723-724). The first analysis implies that by adjusting for race/ethnicity we may be missing abnormalities, and the second suggests accuracy for outcomes is lost. So case closed, right? Let’s abandon race/ethnicity as a co- variate for our spirometry reference equations.
Perhaps, but a few caveats are in order. It’s important to note that doing so would result in a dramatic increase in abnormal findings in otherwise healthy and asymptomatic non-White individuals. This could negatively affect eligibility for employment and military service (Townsend MC, et al. Am J Respir Crit Care Med. 2022;789-790). We’ve also yet to fully explain the factors driving differences in lung function between races. If socioeconomic factors explained the entirety of the difference, it would be easier to argue for elimination of using race/ethnicity in our equations. Currently, the etiology is thought to be multifactorial and is yet to be fully explained (Braun L, et al. Eur Respir J. 2013;41:1362-1370).
The more we look for institutional racism, the more we will find it. As we realize that attaining health and wellness is more difficult for the disenfranchised, we need to ensure our current practices are part of the solution.
The ERS/ATS guidelines suggest eliminating fixed correction factors for race but do not require elimination of race/ethnicity as a co-variate in the equations selected for use. This seems very reasonable given what we know now. As pulmonary medicine academics and researchers, we need to continue to study the impact integrating race/ethnicity has on precision, accuracy, and clinical outcomes. As pulmonary medicine clinicians, we need to be aware of the reference equations being used in our lab, understand how inclusion of race/ethnicity affects findings, and act accordingly, depending on the clinical situation.
Dr. Ghionni is a Pulmonary/Critical Care Fellow, and Dr. Woods is Program Director – PCCM Fellowship and Associate Program Director – IM Residency, Medstar Washington Hospital Center; Dr. Woods is Associate Professor of Medicine, Georgetown University School of Medicine, Washington, DC.
Inpatient sleep medicine: An invaluable service for hospital medicine
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.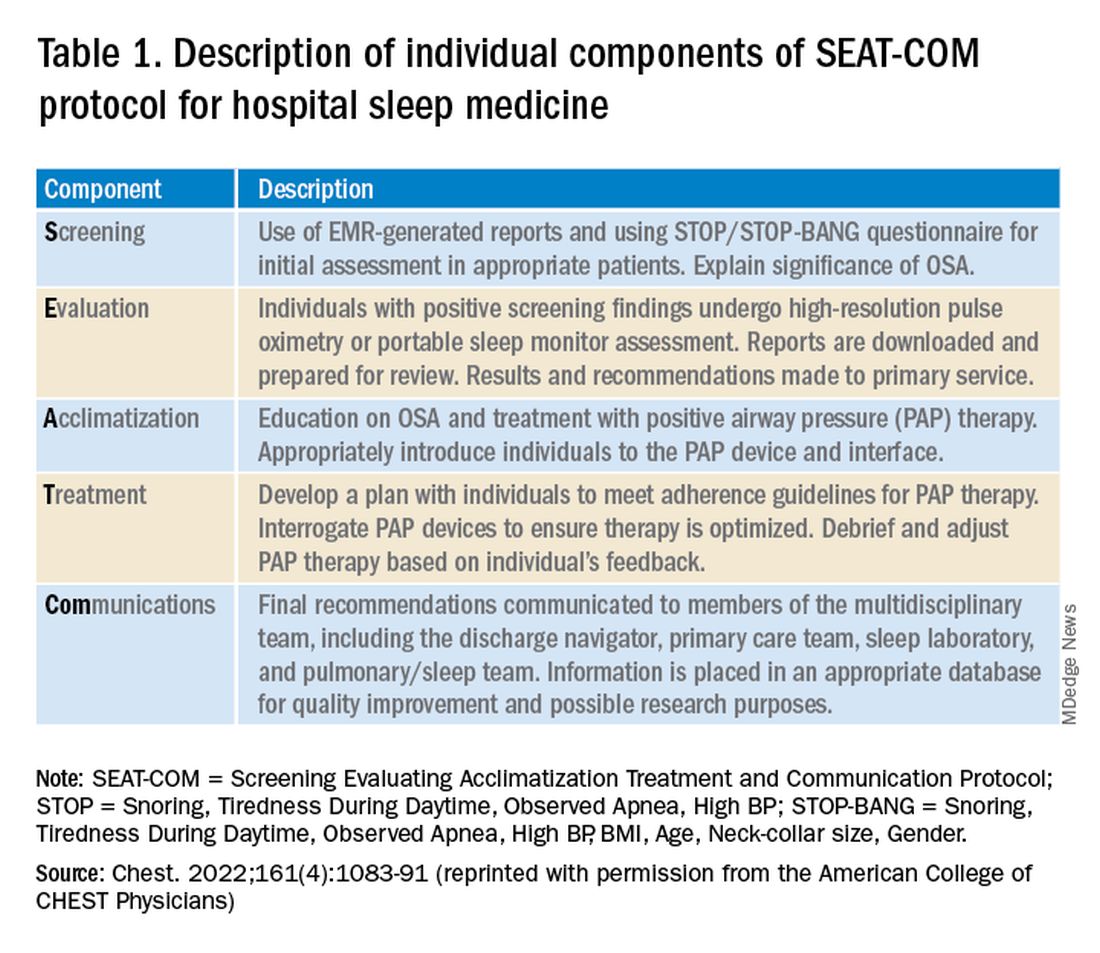
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
ICU telemedicine turns 40
connected with intensivists at the University Hospitals of Cleveland (Grundy, et al. Crit Care Med. 1982;10[7]:471). After this proof-of-concept report, however, ICU telemedicine gained little traction for nearly 20 years, until Johns Hopkins Hospital established a continuously monitored ICU telemedicine service in a nonintensivist staffed surgical ICU. Their pre/post analysis suggested a 64% decrease in severity-adjusted ICU mortality and greater than 30% decrease in ICU length of stay, ICU complications, and costs (Rosenfeld, et al. Crit Care Med. 2000;28[12]:3925).
Along with better and less costly telemedicine technology, rapid adoption of electronic medical records, and a nationwide intensivist shortage, this and other evidence for the service’s clinical and cost effectiveness has spurred explosive growth in ICU telemedicine in the succeeding 2 decades, with at least 18% of hospitals and 28% of ICU beds supported by ICU telemedicine by 2018 (Ofoma, et al. Crit Care Explor. 2021;4[3]:e0468).
Importantly, what “ICU telemedicine” represents varies substantially across hospitals and even across ICUs within systems. Two-way audiovisual technology is the defining feature, and at a minimum, programs provide intensivists and/or nurses who respond to consultation requests. Commonly, telemedicine clinicians directly connect with patients; monitor labs, hemodynamics, and alarms; and proactively contact on-site clinicians with recommendations or place orders directly into the electronic health record depending on whether the clinician acts as the patients’ primary, co-managing, or consultant provider. A centralized hub and spoke model with telemedicine personnel located at a single, remote center is the most common and best studied ICU telemedicine design. Additional staffing may include respiratory therapists, pharmacists, and advanced practice clinicians in coverage models that range from 24/7 to nocturnal and can also differ in whether patients are monitored continuously or on an as needed basis, triggered by alarms or clinician/nursing concerns.
On-demand services may extend to support for teams responding to medical emergencies inside and sometimes outside the ICU. Another equally important role that ICU telemedicine can provide is helping ensure facilities adhere to ICU quality metrics, such as ventilator bundles, DVT prophylaxis, and daily SAT/SBT.
Unsurprisingly, integrating ICU telemedicine into an existing system is very costly and complex, requiring substantial and thoughtful process redesign to maximize fiscal and clinical return on investment. One vendor of proprietary telemedicine technology, Philips eICU, estimates an implementation cost of $50,000 to $100,000 per bed with annual overhead, software maintenance, and IT staffing of ~20% of implementation costs in addition to clinician staffing of $1-2 million per 100 beds. However, some (but not all) evidence suggests that ICU telemedicine programs pay for themselves over time. An influential report from Sentara Healthcare, an early adopter of ICU telemedicine, described equipment costs of more than $1 million for a total of 103 critical care beds but attributed savings of $460,000 per month to decreased length of stay (Coustasse, et al. The Permanente Journal. 2014;18[4]:76).
Cost savings are great, of course, but ICU telemedicine’s potential to improve clinical outcomes is the real priority. While Sentara’s early report included a 27% decrease in ICU mortality after telemedicine adoption, a 2011 meta-analysis of 13 studies, including 35 ICUs and over 40,000 patients, suggested decreased ICU mortality and LOS with a statistically significant effect on overall hospital mortality and LOS (Young, et al. Arch Intern Med. 2011;171[6]:498). This highlights the Achilles heel of ICU telemedicine evidence: the pretest/posttest studies that dominate this field and likely contribute substantially to the inconsistencies in the evidence base.
In the absence of risk adjustment and control groups, many studies observed postimplementation changes that may reflect trends in patient mix or the effects of unrelated practice changes rather than the causal influence of ICU telemedicine. In fact, in studies using more robust methods, ICU telemedicine’s effect size has been smaller or nonexistent. For example, in 2016, Kahn and colleagues used CMS data to evaluate 132 ICU telemedicine programs using 389 matched controlled hospitals. There was a slight reduction in 90-day mortality (OR=0.96, CI 0.94-0.98) with only 12% showing a statistically significant reduction in mortality. Interestingly, hospitals in urban areas demonstrated greater benefit than rural facilities (Kahn, et al. Medical Care. 2016;54[3]:319).
The heterogeneity of the studied programs (e.g., primary vs consultative role, on-demand vs proactive involvement) and recipient ICUs (e.g., rural vs tertiary care facility, presence of bedside intensivists) further hinders a clear answer to the key question: Would ICU telemedicine benefit my hospital? Fortunately, some recent, well-designed studies have attempted to understand which attributes of ICU telemedicine programs provide results and which ICUs will see the most benefit. In a cohort of 118,990 patients across 56 ICUs, four interventions were associated with lower mortality and reduced LOS: (1) evaluation of patients within 1 hour of ICU admission, (2) frequent leadership review of performance data, (3) ICU best practice compliance, and (4) prompt response to alerts (Lilly, et al. Chest. 2014;145[3]:500). Kahn and colleagues have also investigated this issue, conducting an in-depth ethnographic evaluation of 10 hospitals identified in their 2016 study to have positive, neutral, or negative outcomes after ICU telemedicine implementation (Kahn, et al. Am J Respir Crit Care Med. 2019;199[8]:970). They found that successful programs:
(1) provided consistent services matched to recipient needs;
(2) provided services both proactively and reactively without being obtrusive;
(3) embedded routine engagements unobtrusively into usual routines;
(4) had engaged leadership who set clear expectations and mediated conflicts; and
(5) had bedside clinicians who valued and sought out telemedicine participation in care.
The authors concluded that, “the true value of ICU telemedicine lies not in whether the technology exists but in how it is applied.” However, another recent analysis also suggested that, rather than telemedicine or recipient ICU design, targeting underperforming recipient ICU performance may be the key determinant of whether ICU telemedicine implementation improves outcomes (Fusaro, et al. Crit Care Med. 2019; 47[4]:501). While the finding may reflect regression to the mean, the idea that ICUs with above-expected mortality derive greater benefit from ICU telemedicine support than already well-performing ICUs is certainly logical.
As COVID-19 strained health care systems across the country, we and others found ways to use ICU telemedicine to preserve optimal care delivery for critically ill patients. Our program at Intermountain Healthcare – already supporting 17 ICUs within our 24-hospital health system, as well as 10 external ICUs with experienced critical care physicians, nurses, respiratory therapists, and pharmacists – took on increased responsibility for ICU load balancing and interhospital transfers.
Leveraging telemedicine services also helped community ICUs care for sicker, more complex patients than usual and aided nonintensivist physicians called upon to manage critically ill patients in ad hoc ICUs at referral hospitals. While the pandemic certainly stressed ICU staff, we suspect that telemedicine’s ability to balance caseloads and distribute clinical tasks helped mitigate these stresses. At age 40, ICU telemedicine is both mature and still growing, with continued expansion of bed coverage and the range of services available. Looking ahead, as we confront a national shortage of intensivists, ICU telemedicine likely represents a cost effective and efficient strategy to maintain critical care capacity with the potential to ensure low-cost, high-quality care for all, regardless of location.
Dr. Graham and Dr. Peltan are with the Division of Pulmonary & Critical Care Medicine, Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, Utah; and Dr. Peltan is also with the Division of Pulmonary & Critical Care Medicine, Department of Medicine, Intermountain Medical Center, Murray, Utah.
connected with intensivists at the University Hospitals of Cleveland (Grundy, et al. Crit Care Med. 1982;10[7]:471). After this proof-of-concept report, however, ICU telemedicine gained little traction for nearly 20 years, until Johns Hopkins Hospital established a continuously monitored ICU telemedicine service in a nonintensivist staffed surgical ICU. Their pre/post analysis suggested a 64% decrease in severity-adjusted ICU mortality and greater than 30% decrease in ICU length of stay, ICU complications, and costs (Rosenfeld, et al. Crit Care Med. 2000;28[12]:3925).
Along with better and less costly telemedicine technology, rapid adoption of electronic medical records, and a nationwide intensivist shortage, this and other evidence for the service’s clinical and cost effectiveness has spurred explosive growth in ICU telemedicine in the succeeding 2 decades, with at least 18% of hospitals and 28% of ICU beds supported by ICU telemedicine by 2018 (Ofoma, et al. Crit Care Explor. 2021;4[3]:e0468).
Importantly, what “ICU telemedicine” represents varies substantially across hospitals and even across ICUs within systems. Two-way audiovisual technology is the defining feature, and at a minimum, programs provide intensivists and/or nurses who respond to consultation requests. Commonly, telemedicine clinicians directly connect with patients; monitor labs, hemodynamics, and alarms; and proactively contact on-site clinicians with recommendations or place orders directly into the electronic health record depending on whether the clinician acts as the patients’ primary, co-managing, or consultant provider. A centralized hub and spoke model with telemedicine personnel located at a single, remote center is the most common and best studied ICU telemedicine design. Additional staffing may include respiratory therapists, pharmacists, and advanced practice clinicians in coverage models that range from 24/7 to nocturnal and can also differ in whether patients are monitored continuously or on an as needed basis, triggered by alarms or clinician/nursing concerns.
On-demand services may extend to support for teams responding to medical emergencies inside and sometimes outside the ICU. Another equally important role that ICU telemedicine can provide is helping ensure facilities adhere to ICU quality metrics, such as ventilator bundles, DVT prophylaxis, and daily SAT/SBT.
Unsurprisingly, integrating ICU telemedicine into an existing system is very costly and complex, requiring substantial and thoughtful process redesign to maximize fiscal and clinical return on investment. One vendor of proprietary telemedicine technology, Philips eICU, estimates an implementation cost of $50,000 to $100,000 per bed with annual overhead, software maintenance, and IT staffing of ~20% of implementation costs in addition to clinician staffing of $1-2 million per 100 beds. However, some (but not all) evidence suggests that ICU telemedicine programs pay for themselves over time. An influential report from Sentara Healthcare, an early adopter of ICU telemedicine, described equipment costs of more than $1 million for a total of 103 critical care beds but attributed savings of $460,000 per month to decreased length of stay (Coustasse, et al. The Permanente Journal. 2014;18[4]:76).
Cost savings are great, of course, but ICU telemedicine’s potential to improve clinical outcomes is the real priority. While Sentara’s early report included a 27% decrease in ICU mortality after telemedicine adoption, a 2011 meta-analysis of 13 studies, including 35 ICUs and over 40,000 patients, suggested decreased ICU mortality and LOS with a statistically significant effect on overall hospital mortality and LOS (Young, et al. Arch Intern Med. 2011;171[6]:498). This highlights the Achilles heel of ICU telemedicine evidence: the pretest/posttest studies that dominate this field and likely contribute substantially to the inconsistencies in the evidence base.
In the absence of risk adjustment and control groups, many studies observed postimplementation changes that may reflect trends in patient mix or the effects of unrelated practice changes rather than the causal influence of ICU telemedicine. In fact, in studies using more robust methods, ICU telemedicine’s effect size has been smaller or nonexistent. For example, in 2016, Kahn and colleagues used CMS data to evaluate 132 ICU telemedicine programs using 389 matched controlled hospitals. There was a slight reduction in 90-day mortality (OR=0.96, CI 0.94-0.98) with only 12% showing a statistically significant reduction in mortality. Interestingly, hospitals in urban areas demonstrated greater benefit than rural facilities (Kahn, et al. Medical Care. 2016;54[3]:319).
The heterogeneity of the studied programs (e.g., primary vs consultative role, on-demand vs proactive involvement) and recipient ICUs (e.g., rural vs tertiary care facility, presence of bedside intensivists) further hinders a clear answer to the key question: Would ICU telemedicine benefit my hospital? Fortunately, some recent, well-designed studies have attempted to understand which attributes of ICU telemedicine programs provide results and which ICUs will see the most benefit. In a cohort of 118,990 patients across 56 ICUs, four interventions were associated with lower mortality and reduced LOS: (1) evaluation of patients within 1 hour of ICU admission, (2) frequent leadership review of performance data, (3) ICU best practice compliance, and (4) prompt response to alerts (Lilly, et al. Chest. 2014;145[3]:500). Kahn and colleagues have also investigated this issue, conducting an in-depth ethnographic evaluation of 10 hospitals identified in their 2016 study to have positive, neutral, or negative outcomes after ICU telemedicine implementation (Kahn, et al. Am J Respir Crit Care Med. 2019;199[8]:970). They found that successful programs:
(1) provided consistent services matched to recipient needs;
(2) provided services both proactively and reactively without being obtrusive;
(3) embedded routine engagements unobtrusively into usual routines;
(4) had engaged leadership who set clear expectations and mediated conflicts; and
(5) had bedside clinicians who valued and sought out telemedicine participation in care.
The authors concluded that, “the true value of ICU telemedicine lies not in whether the technology exists but in how it is applied.” However, another recent analysis also suggested that, rather than telemedicine or recipient ICU design, targeting underperforming recipient ICU performance may be the key determinant of whether ICU telemedicine implementation improves outcomes (Fusaro, et al. Crit Care Med. 2019; 47[4]:501). While the finding may reflect regression to the mean, the idea that ICUs with above-expected mortality derive greater benefit from ICU telemedicine support than already well-performing ICUs is certainly logical.
As COVID-19 strained health care systems across the country, we and others found ways to use ICU telemedicine to preserve optimal care delivery for critically ill patients. Our program at Intermountain Healthcare – already supporting 17 ICUs within our 24-hospital health system, as well as 10 external ICUs with experienced critical care physicians, nurses, respiratory therapists, and pharmacists – took on increased responsibility for ICU load balancing and interhospital transfers.
Leveraging telemedicine services also helped community ICUs care for sicker, more complex patients than usual and aided nonintensivist physicians called upon to manage critically ill patients in ad hoc ICUs at referral hospitals. While the pandemic certainly stressed ICU staff, we suspect that telemedicine’s ability to balance caseloads and distribute clinical tasks helped mitigate these stresses. At age 40, ICU telemedicine is both mature and still growing, with continued expansion of bed coverage and the range of services available. Looking ahead, as we confront a national shortage of intensivists, ICU telemedicine likely represents a cost effective and efficient strategy to maintain critical care capacity with the potential to ensure low-cost, high-quality care for all, regardless of location.
Dr. Graham and Dr. Peltan are with the Division of Pulmonary & Critical Care Medicine, Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, Utah; and Dr. Peltan is also with the Division of Pulmonary & Critical Care Medicine, Department of Medicine, Intermountain Medical Center, Murray, Utah.
connected with intensivists at the University Hospitals of Cleveland (Grundy, et al. Crit Care Med. 1982;10[7]:471). After this proof-of-concept report, however, ICU telemedicine gained little traction for nearly 20 years, until Johns Hopkins Hospital established a continuously monitored ICU telemedicine service in a nonintensivist staffed surgical ICU. Their pre/post analysis suggested a 64% decrease in severity-adjusted ICU mortality and greater than 30% decrease in ICU length of stay, ICU complications, and costs (Rosenfeld, et al. Crit Care Med. 2000;28[12]:3925).
Along with better and less costly telemedicine technology, rapid adoption of electronic medical records, and a nationwide intensivist shortage, this and other evidence for the service’s clinical and cost effectiveness has spurred explosive growth in ICU telemedicine in the succeeding 2 decades, with at least 18% of hospitals and 28% of ICU beds supported by ICU telemedicine by 2018 (Ofoma, et al. Crit Care Explor. 2021;4[3]:e0468).
Importantly, what “ICU telemedicine” represents varies substantially across hospitals and even across ICUs within systems. Two-way audiovisual technology is the defining feature, and at a minimum, programs provide intensivists and/or nurses who respond to consultation requests. Commonly, telemedicine clinicians directly connect with patients; monitor labs, hemodynamics, and alarms; and proactively contact on-site clinicians with recommendations or place orders directly into the electronic health record depending on whether the clinician acts as the patients’ primary, co-managing, or consultant provider. A centralized hub and spoke model with telemedicine personnel located at a single, remote center is the most common and best studied ICU telemedicine design. Additional staffing may include respiratory therapists, pharmacists, and advanced practice clinicians in coverage models that range from 24/7 to nocturnal and can also differ in whether patients are monitored continuously or on an as needed basis, triggered by alarms or clinician/nursing concerns.
On-demand services may extend to support for teams responding to medical emergencies inside and sometimes outside the ICU. Another equally important role that ICU telemedicine can provide is helping ensure facilities adhere to ICU quality metrics, such as ventilator bundles, DVT prophylaxis, and daily SAT/SBT.
Unsurprisingly, integrating ICU telemedicine into an existing system is very costly and complex, requiring substantial and thoughtful process redesign to maximize fiscal and clinical return on investment. One vendor of proprietary telemedicine technology, Philips eICU, estimates an implementation cost of $50,000 to $100,000 per bed with annual overhead, software maintenance, and IT staffing of ~20% of implementation costs in addition to clinician staffing of $1-2 million per 100 beds. However, some (but not all) evidence suggests that ICU telemedicine programs pay for themselves over time. An influential report from Sentara Healthcare, an early adopter of ICU telemedicine, described equipment costs of more than $1 million for a total of 103 critical care beds but attributed savings of $460,000 per month to decreased length of stay (Coustasse, et al. The Permanente Journal. 2014;18[4]:76).
Cost savings are great, of course, but ICU telemedicine’s potential to improve clinical outcomes is the real priority. While Sentara’s early report included a 27% decrease in ICU mortality after telemedicine adoption, a 2011 meta-analysis of 13 studies, including 35 ICUs and over 40,000 patients, suggested decreased ICU mortality and LOS with a statistically significant effect on overall hospital mortality and LOS (Young, et al. Arch Intern Med. 2011;171[6]:498). This highlights the Achilles heel of ICU telemedicine evidence: the pretest/posttest studies that dominate this field and likely contribute substantially to the inconsistencies in the evidence base.
In the absence of risk adjustment and control groups, many studies observed postimplementation changes that may reflect trends in patient mix or the effects of unrelated practice changes rather than the causal influence of ICU telemedicine. In fact, in studies using more robust methods, ICU telemedicine’s effect size has been smaller or nonexistent. For example, in 2016, Kahn and colleagues used CMS data to evaluate 132 ICU telemedicine programs using 389 matched controlled hospitals. There was a slight reduction in 90-day mortality (OR=0.96, CI 0.94-0.98) with only 12% showing a statistically significant reduction in mortality. Interestingly, hospitals in urban areas demonstrated greater benefit than rural facilities (Kahn, et al. Medical Care. 2016;54[3]:319).
The heterogeneity of the studied programs (e.g., primary vs consultative role, on-demand vs proactive involvement) and recipient ICUs (e.g., rural vs tertiary care facility, presence of bedside intensivists) further hinders a clear answer to the key question: Would ICU telemedicine benefit my hospital? Fortunately, some recent, well-designed studies have attempted to understand which attributes of ICU telemedicine programs provide results and which ICUs will see the most benefit. In a cohort of 118,990 patients across 56 ICUs, four interventions were associated with lower mortality and reduced LOS: (1) evaluation of patients within 1 hour of ICU admission, (2) frequent leadership review of performance data, (3) ICU best practice compliance, and (4) prompt response to alerts (Lilly, et al. Chest. 2014;145[3]:500). Kahn and colleagues have also investigated this issue, conducting an in-depth ethnographic evaluation of 10 hospitals identified in their 2016 study to have positive, neutral, or negative outcomes after ICU telemedicine implementation (Kahn, et al. Am J Respir Crit Care Med. 2019;199[8]:970). They found that successful programs:
(1) provided consistent services matched to recipient needs;
(2) provided services both proactively and reactively without being obtrusive;
(3) embedded routine engagements unobtrusively into usual routines;
(4) had engaged leadership who set clear expectations and mediated conflicts; and
(5) had bedside clinicians who valued and sought out telemedicine participation in care.
The authors concluded that, “the true value of ICU telemedicine lies not in whether the technology exists but in how it is applied.” However, another recent analysis also suggested that, rather than telemedicine or recipient ICU design, targeting underperforming recipient ICU performance may be the key determinant of whether ICU telemedicine implementation improves outcomes (Fusaro, et al. Crit Care Med. 2019; 47[4]:501). While the finding may reflect regression to the mean, the idea that ICUs with above-expected mortality derive greater benefit from ICU telemedicine support than already well-performing ICUs is certainly logical.
As COVID-19 strained health care systems across the country, we and others found ways to use ICU telemedicine to preserve optimal care delivery for critically ill patients. Our program at Intermountain Healthcare – already supporting 17 ICUs within our 24-hospital health system, as well as 10 external ICUs with experienced critical care physicians, nurses, respiratory therapists, and pharmacists – took on increased responsibility for ICU load balancing and interhospital transfers.
Leveraging telemedicine services also helped community ICUs care for sicker, more complex patients than usual and aided nonintensivist physicians called upon to manage critically ill patients in ad hoc ICUs at referral hospitals. While the pandemic certainly stressed ICU staff, we suspect that telemedicine’s ability to balance caseloads and distribute clinical tasks helped mitigate these stresses. At age 40, ICU telemedicine is both mature and still growing, with continued expansion of bed coverage and the range of services available. Looking ahead, as we confront a national shortage of intensivists, ICU telemedicine likely represents a cost effective and efficient strategy to maintain critical care capacity with the potential to ensure low-cost, high-quality care for all, regardless of location.
Dr. Graham and Dr. Peltan are with the Division of Pulmonary & Critical Care Medicine, Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, Utah; and Dr. Peltan is also with the Division of Pulmonary & Critical Care Medicine, Department of Medicine, Intermountain Medical Center, Murray, Utah.
Advanced POCUS for us all?
Point-of-care ultrasound (POCUS) is a useful, practice-changing bedside tool that spans all medical and surgical specialties. While the definition of POCUS varies, most would agree it is an abbreviated exam that helps to answer a specific clinical question. With the expansion of POCUS training, the clinical questions being asked and answered have increased in scope and volume. The types of exams being utilized in “point of care ultrasound” have also increased and include transthoracic echocardiography; trans-esophageal echocardiography; and lung, gastric, abdominal, and ocular ultrasound. POCUS is used across multiple specialties, including critical care, anesthesiology, emergency medicine, and primary care.
Not only has POCUS become increasingly important clinically, but specialties now test these skills on their respective board examinations. Anesthesia is one of many such examples. The content outline for the American Board of Anesthesiology includes POCUS as a tested item on both the written and applied components of the exam. POCUS training must be directed toward both optimizing patient management and preparing learners for their board examination. A method for teaching this has yet to be defined (Naji A, et al. Cureus. 2021;13[5]:e15217).
One question – how should different specialties approach this educational challenge and should specialties train together? The answer is complicated. Many POCUS courses and certifications exist, and all vary in their content, didactics, and length. No true gold standard exists for POCUS certification for radiology or noncardiology providers. Additionally, there are no defined expectations or testing processes that certify a provider is “certified” to perform POCUS. While waiting for medical society guidelines to address these issues, many in graduate medical education (GME) are coming up with their own ways to incorporate POCUS into their respective training programs (Atkinson P, et al. CJEM. 2015 Mar;17[2]:161).
Who’s training whom?
Over the past decade, several expert committees, including those in critical care, have developed recommendations and consensus statements urging training facilities to independently create POCUS curriculums. The threshold for many programs to enter this realm of expertise is high and oftentimes unobtainable. We’ve seen emergency medicine and anesthesia raise the bar for ultrasound education in their residencies, but it’s unclear whether all fellowship-trained physicians can and should be tasked with obtaining official POCUS certification.
While specific specialties may require tailored certifications, there’s a considerable overlap in POCUS exam content across specialties. One approach to POCUS training could be developing and implementing a multidisciplinary curriculum. This would allow for pooling of resources (equipment, staff) and harnessing knowledge from providers familiar with different phases of patient care (ICU, perioperative, ED, outpatient clinics). By approaching POCUS from a multidisciplinary perspective, the quality of education may be enhanced (Mayo PH, et al. Intensive Care Med. 2014;40[5]:654). Is it then prudent for providers and trainees alike to share in didactics across all areas of the hospital and clinic? Would this close the knowledge gap between specialties who are facile with ultrasound and those not?
Determining the role of transesophageal echocardiography in a POCUS curriculum
This modality of imaging has been, until recently, reserved for cardiologists and anesthesiologists. More recently transesophageal echocardiography (TEE) has been utilized by emergency and critical care medicine physicians. TEE is part of recommended training for these specialties as a tool for diagnostic and rescue measures, including ventilator management, emergency procedures, and medication titration. Rescue TEE can also be utilized perioperatively where the transthoracic exam is limited by poor windows or the operative procedure precludes access to the chest. While transthoracic echocardiography (TTE) is often used in a point of care fashion, TEE is utilized less often. This may stem from the invasive nature of the procedure but likely also results from lack of equipment and training. Like POCUS overall, TEE POCUS will require incorporation into training programs to achieve widespread use and acceptance.
A deluge of research on TEE for the noncardiologist shows this modality is minimally invasive, safe, and effective. As it becomes more readily available and technology improves, there is no reason why an esophageal probe can’t be used in a patient with a secured airway (Wray TC, et al. J Intensive Care Med. 2021;36[1]:123).
Ultrasound for hemodynamic monitoring
There are many methods employed for hemodynamic monitoring in the ICU. Although echocardiographic and vascular parameters have been validated in the cardiac and perioperative fields, their application in the ICU setting for resuscitation and volume management remain somewhat controversial. The use of TEE and more advanced understanding of spectral doppler and pulmonary ultrasonography using TEE has revolutionized the way providers are managing critically ill patients. (Garcia YA, et al. Chest. 2017;152[4]:736).
In our opinion, physiology and imaging training for residents and fellows should be required for critical care medicine trainees. Delving into the nuances of frank-starling curves, stroke work, and diastolic function will enrich their understanding and highlight the applicability of ultrasonography. Furthermore, all clinicians caring for patients with critical illness should be privy to the nuances of physiologic derangement, and to that end, advanced echocardiographic principles and image acquisition. The heart-lung interactions are demonstrated in real time using POCUS and can clearly delineate treatment goals (Vieillard-Baron A, et al. Intensive Care Med. 2019;45[6]:770).
Documentation and billing
If clinicians are making medical decisions based off imaging gathered at the bedside and interpreted in real-time, documentation should reflect that. That documentation will invariably lead to billing and possibly audit or quality review by colleagues or other healthcare staff. Radiology and cardiology have perfected the billing process for image interpretation, but their form of documentation and interpretation may not easily be implemented in the perioperative or critical care settings. An abbreviated document with focused information should take the place of the formal study. With that, the credentialing and board certification process will allow providers to feel empowered to make clinical decisions based off these focused examinations.
Dr. Goertzen is Chief Fellow, Pulmonary/Critical Care; Dr. Knuf is Program Director, Department of Anesthesia; and Dr. Villalobos is Director of Medical ICU, Department of Internal Medicine, San Antonio Military Medical Center, San Antonio, Texas.
Point-of-care ultrasound (POCUS) is a useful, practice-changing bedside tool that spans all medical and surgical specialties. While the definition of POCUS varies, most would agree it is an abbreviated exam that helps to answer a specific clinical question. With the expansion of POCUS training, the clinical questions being asked and answered have increased in scope and volume. The types of exams being utilized in “point of care ultrasound” have also increased and include transthoracic echocardiography; trans-esophageal echocardiography; and lung, gastric, abdominal, and ocular ultrasound. POCUS is used across multiple specialties, including critical care, anesthesiology, emergency medicine, and primary care.
Not only has POCUS become increasingly important clinically, but specialties now test these skills on their respective board examinations. Anesthesia is one of many such examples. The content outline for the American Board of Anesthesiology includes POCUS as a tested item on both the written and applied components of the exam. POCUS training must be directed toward both optimizing patient management and preparing learners for their board examination. A method for teaching this has yet to be defined (Naji A, et al. Cureus. 2021;13[5]:e15217).
One question – how should different specialties approach this educational challenge and should specialties train together? The answer is complicated. Many POCUS courses and certifications exist, and all vary in their content, didactics, and length. No true gold standard exists for POCUS certification for radiology or noncardiology providers. Additionally, there are no defined expectations or testing processes that certify a provider is “certified” to perform POCUS. While waiting for medical society guidelines to address these issues, many in graduate medical education (GME) are coming up with their own ways to incorporate POCUS into their respective training programs (Atkinson P, et al. CJEM. 2015 Mar;17[2]:161).
Who’s training whom?
Over the past decade, several expert committees, including those in critical care, have developed recommendations and consensus statements urging training facilities to independently create POCUS curriculums. The threshold for many programs to enter this realm of expertise is high and oftentimes unobtainable. We’ve seen emergency medicine and anesthesia raise the bar for ultrasound education in their residencies, but it’s unclear whether all fellowship-trained physicians can and should be tasked with obtaining official POCUS certification.
While specific specialties may require tailored certifications, there’s a considerable overlap in POCUS exam content across specialties. One approach to POCUS training could be developing and implementing a multidisciplinary curriculum. This would allow for pooling of resources (equipment, staff) and harnessing knowledge from providers familiar with different phases of patient care (ICU, perioperative, ED, outpatient clinics). By approaching POCUS from a multidisciplinary perspective, the quality of education may be enhanced (Mayo PH, et al. Intensive Care Med. 2014;40[5]:654). Is it then prudent for providers and trainees alike to share in didactics across all areas of the hospital and clinic? Would this close the knowledge gap between specialties who are facile with ultrasound and those not?
Determining the role of transesophageal echocardiography in a POCUS curriculum
This modality of imaging has been, until recently, reserved for cardiologists and anesthesiologists. More recently transesophageal echocardiography (TEE) has been utilized by emergency and critical care medicine physicians. TEE is part of recommended training for these specialties as a tool for diagnostic and rescue measures, including ventilator management, emergency procedures, and medication titration. Rescue TEE can also be utilized perioperatively where the transthoracic exam is limited by poor windows or the operative procedure precludes access to the chest. While transthoracic echocardiography (TTE) is often used in a point of care fashion, TEE is utilized less often. This may stem from the invasive nature of the procedure but likely also results from lack of equipment and training. Like POCUS overall, TEE POCUS will require incorporation into training programs to achieve widespread use and acceptance.
A deluge of research on TEE for the noncardiologist shows this modality is minimally invasive, safe, and effective. As it becomes more readily available and technology improves, there is no reason why an esophageal probe can’t be used in a patient with a secured airway (Wray TC, et al. J Intensive Care Med. 2021;36[1]:123).
Ultrasound for hemodynamic monitoring
There are many methods employed for hemodynamic monitoring in the ICU. Although echocardiographic and vascular parameters have been validated in the cardiac and perioperative fields, their application in the ICU setting for resuscitation and volume management remain somewhat controversial. The use of TEE and more advanced understanding of spectral doppler and pulmonary ultrasonography using TEE has revolutionized the way providers are managing critically ill patients. (Garcia YA, et al. Chest. 2017;152[4]:736).
In our opinion, physiology and imaging training for residents and fellows should be required for critical care medicine trainees. Delving into the nuances of frank-starling curves, stroke work, and diastolic function will enrich their understanding and highlight the applicability of ultrasonography. Furthermore, all clinicians caring for patients with critical illness should be privy to the nuances of physiologic derangement, and to that end, advanced echocardiographic principles and image acquisition. The heart-lung interactions are demonstrated in real time using POCUS and can clearly delineate treatment goals (Vieillard-Baron A, et al. Intensive Care Med. 2019;45[6]:770).
Documentation and billing
If clinicians are making medical decisions based off imaging gathered at the bedside and interpreted in real-time, documentation should reflect that. That documentation will invariably lead to billing and possibly audit or quality review by colleagues or other healthcare staff. Radiology and cardiology have perfected the billing process for image interpretation, but their form of documentation and interpretation may not easily be implemented in the perioperative or critical care settings. An abbreviated document with focused information should take the place of the formal study. With that, the credentialing and board certification process will allow providers to feel empowered to make clinical decisions based off these focused examinations.
Dr. Goertzen is Chief Fellow, Pulmonary/Critical Care; Dr. Knuf is Program Director, Department of Anesthesia; and Dr. Villalobos is Director of Medical ICU, Department of Internal Medicine, San Antonio Military Medical Center, San Antonio, Texas.
Point-of-care ultrasound (POCUS) is a useful, practice-changing bedside tool that spans all medical and surgical specialties. While the definition of POCUS varies, most would agree it is an abbreviated exam that helps to answer a specific clinical question. With the expansion of POCUS training, the clinical questions being asked and answered have increased in scope and volume. The types of exams being utilized in “point of care ultrasound” have also increased and include transthoracic echocardiography; trans-esophageal echocardiography; and lung, gastric, abdominal, and ocular ultrasound. POCUS is used across multiple specialties, including critical care, anesthesiology, emergency medicine, and primary care.
Not only has POCUS become increasingly important clinically, but specialties now test these skills on their respective board examinations. Anesthesia is one of many such examples. The content outline for the American Board of Anesthesiology includes POCUS as a tested item on both the written and applied components of the exam. POCUS training must be directed toward both optimizing patient management and preparing learners for their board examination. A method for teaching this has yet to be defined (Naji A, et al. Cureus. 2021;13[5]:e15217).
One question – how should different specialties approach this educational challenge and should specialties train together? The answer is complicated. Many POCUS courses and certifications exist, and all vary in their content, didactics, and length. No true gold standard exists for POCUS certification for radiology or noncardiology providers. Additionally, there are no defined expectations or testing processes that certify a provider is “certified” to perform POCUS. While waiting for medical society guidelines to address these issues, many in graduate medical education (GME) are coming up with their own ways to incorporate POCUS into their respective training programs (Atkinson P, et al. CJEM. 2015 Mar;17[2]:161).
Who’s training whom?
Over the past decade, several expert committees, including those in critical care, have developed recommendations and consensus statements urging training facilities to independently create POCUS curriculums. The threshold for many programs to enter this realm of expertise is high and oftentimes unobtainable. We’ve seen emergency medicine and anesthesia raise the bar for ultrasound education in their residencies, but it’s unclear whether all fellowship-trained physicians can and should be tasked with obtaining official POCUS certification.
While specific specialties may require tailored certifications, there’s a considerable overlap in POCUS exam content across specialties. One approach to POCUS training could be developing and implementing a multidisciplinary curriculum. This would allow for pooling of resources (equipment, staff) and harnessing knowledge from providers familiar with different phases of patient care (ICU, perioperative, ED, outpatient clinics). By approaching POCUS from a multidisciplinary perspective, the quality of education may be enhanced (Mayo PH, et al. Intensive Care Med. 2014;40[5]:654). Is it then prudent for providers and trainees alike to share in didactics across all areas of the hospital and clinic? Would this close the knowledge gap between specialties who are facile with ultrasound and those not?
Determining the role of transesophageal echocardiography in a POCUS curriculum
This modality of imaging has been, until recently, reserved for cardiologists and anesthesiologists. More recently transesophageal echocardiography (TEE) has been utilized by emergency and critical care medicine physicians. TEE is part of recommended training for these specialties as a tool for diagnostic and rescue measures, including ventilator management, emergency procedures, and medication titration. Rescue TEE can also be utilized perioperatively where the transthoracic exam is limited by poor windows or the operative procedure precludes access to the chest. While transthoracic echocardiography (TTE) is often used in a point of care fashion, TEE is utilized less often. This may stem from the invasive nature of the procedure but likely also results from lack of equipment and training. Like POCUS overall, TEE POCUS will require incorporation into training programs to achieve widespread use and acceptance.
A deluge of research on TEE for the noncardiologist shows this modality is minimally invasive, safe, and effective. As it becomes more readily available and technology improves, there is no reason why an esophageal probe can’t be used in a patient with a secured airway (Wray TC, et al. J Intensive Care Med. 2021;36[1]:123).
Ultrasound for hemodynamic monitoring
There are many methods employed for hemodynamic monitoring in the ICU. Although echocardiographic and vascular parameters have been validated in the cardiac and perioperative fields, their application in the ICU setting for resuscitation and volume management remain somewhat controversial. The use of TEE and more advanced understanding of spectral doppler and pulmonary ultrasonography using TEE has revolutionized the way providers are managing critically ill patients. (Garcia YA, et al. Chest. 2017;152[4]:736).
In our opinion, physiology and imaging training for residents and fellows should be required for critical care medicine trainees. Delving into the nuances of frank-starling curves, stroke work, and diastolic function will enrich their understanding and highlight the applicability of ultrasonography. Furthermore, all clinicians caring for patients with critical illness should be privy to the nuances of physiologic derangement, and to that end, advanced echocardiographic principles and image acquisition. The heart-lung interactions are demonstrated in real time using POCUS and can clearly delineate treatment goals (Vieillard-Baron A, et al. Intensive Care Med. 2019;45[6]:770).
Documentation and billing
If clinicians are making medical decisions based off imaging gathered at the bedside and interpreted in real-time, documentation should reflect that. That documentation will invariably lead to billing and possibly audit or quality review by colleagues or other healthcare staff. Radiology and cardiology have perfected the billing process for image interpretation, but their form of documentation and interpretation may not easily be implemented in the perioperative or critical care settings. An abbreviated document with focused information should take the place of the formal study. With that, the credentialing and board certification process will allow providers to feel empowered to make clinical decisions based off these focused examinations.
Dr. Goertzen is Chief Fellow, Pulmonary/Critical Care; Dr. Knuf is Program Director, Department of Anesthesia; and Dr. Villalobos is Director of Medical ICU, Department of Internal Medicine, San Antonio Military Medical Center, San Antonio, Texas.
Pneumothorax, pneumomediastinum, and subcutaneous emphysema: The many faces of COVID-19 ARDS
I recall early in the pandemic being called to the bedside to examine an acutely decompensating patient with COVID-19. This was a 33-year-old, previously healthy woman, admitted to the medical ICU with hypoxemic respiratory failure requiring mechanical ventilation and undergoing treatment for severe acute respiratory distress syndrome (ARDS). I quickly realized she was seconds away from an arrest. As I examined her, one thing caught my eye. Her airway pressures had skyrocketed over the past few minutes. Could it be? I thought to myself as I reached for the ultrasound that confirmed my suspicions, tension pneumothorax. One emergent needle decompression and chest tube later and she survives, only to die a week later from overwhelming hypoxemia.
As we reflect on these past 26 months, we recall that caring for the critically ill patient with COVID-19 has posed numerous challenges. One challenge was the overwhelming incidence of the so-called “barotrauma-related complications.” However, we also recall seeing many patients develop such complications while receiving supplemental noninvasive forms of respiratory support. Perhaps, this is in agreement with prior literature that specifically discusses the presence of air outside the tracheobronchial tree and how it does not always correlate with high airway pressure and high tidal volumes, refuting the argument that these complications always fall under the umbrella of barotrauma. We will discuss these complications and attempt to shed light on the potential variables associated with their development.
The development of pneumothorax is a well-recognized complication associated with ventilator-dependent ARDS thought to be a form of barotrauma, with some reports indicating an incidence of 48.8% (Gattinoni L et al. JAMA. 1994;271[2]):1772-9) and a significantly increased mortality rate compared with postprocedural pneumothorax in the ICU (Chen K et al. Chest. 2002;122[2]:678-83). The incidence of such complication in COVID-19-related ARDS is significantly higher than in ARDS from other causes (Belletti A et al. Crit Care Med. 2022;50[3]:491-500), with a mortality rate approaching 100% (Chong WH et al. Heart Lung. 2021;50[5]:599-608).
So why are patients with COVID-19 developing these complications at a higher rate? When we examine the literature, we note that Leisman and colleagues (Am J Respir Crit Care Med. 2022;205[5]:507-19) describe higher baseline markers of alveolar damage, including RAGE (receptor for advanced glycation end-products) in mechanically ventilated patients with COVID-19 vs patients requiring mechanical ventilation for other causes. This poses a question that perhaps one of the main reasons patients with COVID-19 ARDS are at an increased risk for developing certain complications, such as pneumothorax, is inherent to the unique type of alveolar injury sustained with the infection. The authors also note that alveolar markers of injury had moderate to poor discrimination for invasive ventilation early in the disease and diminished over time in both ventilated patients receiving lung protective ventilation strategy and those spontaneously breathing. Likewise, this important finding suggests that the development of pneumothorax in patients with COVID-19 may not be entirely related to barotrauma.
Another phenomenon worth investigating is the development of pneumomediastinum and subcutaneous emphysema, with a reported seven-fold increased risk of development in patients with COVID-19. Lemmers and colleagues (ERJ Open Res. 2020;6[4]:00385-2020) found no statistically significant difference in PEEP, plateau pressure, ratio of tidal volume to ideal body weight, or compliance between patients who developed this complication and those who did not, again, signifying that perhaps there is more to the story here.
Belletti and colleagues (J Cardiothorac Vasc Anesth. 2021;35[12]:3642-51) published an article examining the predictors of pneumothorax and pneumomediastinum in patients with COVID-19. The authors found that the time from symptom onset to intubation and the total bilirubin level were the only two significant predictors for the development of these complications. They explain that longer time from symptom onset to intubation likely increased the risk for self-induced lung injury, inflammation, and fibrosis, contributing to the development of such complications. It is important to note that the authors did not find a significant difference in the ventilation parameters between patients who developed pneumothorax/pneumomediastinum and those who did not.
In our institute, we examined a total of 102 patients admitted to the ICU with COVID-19 ARDS over a 3-month period from March 2020 to May 2020. We identified a total of 36 patients who developed pneumothorax, pneumomediastinum, and/or subcutaneous emphysema. We compared these subjects to age- and gender-matched control subjects. Higher age was associated with an increased risk of development of these complications, whereas the presence of diabetes mellitus, hypertension, and chronic kidney disease at baseline was associated with lower risk. This translated into lower mSOFA scores in our subjects as opposed to the control subjects mainly due to higher creatinine levels at baseline in the control group, skewing our data and indicating that some predictive criteria may not reflect the underlying disease severity and risk for development of such complications. In analyzing our ventilator data and comparing the subjects to the control group, we found no differences in mode of ventilation, set tidal volumes, or PEEP levels between the two. The subjects had significantly higher peak airway pressures, lower compliance, and longer ventilator days. Intubation was needed significantly earlier in the subjects compared with the control group with a median of 2 days vs 6 days from admission. Our data are in concordance with prior published reports and are set to be presented in abstract form this May.
COVID-19 remains a challenging disease with the potential for morbid outcomes. As we phase out of the pandemic and move into an epidemic, future research direction will likely focus on some of the more unusually common complications, such as the ones presented here.
Dr. Abdullah is with the Henry Ford Health System, Detroit, Michigan.
I recall early in the pandemic being called to the bedside to examine an acutely decompensating patient with COVID-19. This was a 33-year-old, previously healthy woman, admitted to the medical ICU with hypoxemic respiratory failure requiring mechanical ventilation and undergoing treatment for severe acute respiratory distress syndrome (ARDS). I quickly realized she was seconds away from an arrest. As I examined her, one thing caught my eye. Her airway pressures had skyrocketed over the past few minutes. Could it be? I thought to myself as I reached for the ultrasound that confirmed my suspicions, tension pneumothorax. One emergent needle decompression and chest tube later and she survives, only to die a week later from overwhelming hypoxemia.
As we reflect on these past 26 months, we recall that caring for the critically ill patient with COVID-19 has posed numerous challenges. One challenge was the overwhelming incidence of the so-called “barotrauma-related complications.” However, we also recall seeing many patients develop such complications while receiving supplemental noninvasive forms of respiratory support. Perhaps, this is in agreement with prior literature that specifically discusses the presence of air outside the tracheobronchial tree and how it does not always correlate with high airway pressure and high tidal volumes, refuting the argument that these complications always fall under the umbrella of barotrauma. We will discuss these complications and attempt to shed light on the potential variables associated with their development.
The development of pneumothorax is a well-recognized complication associated with ventilator-dependent ARDS thought to be a form of barotrauma, with some reports indicating an incidence of 48.8% (Gattinoni L et al. JAMA. 1994;271[2]):1772-9) and a significantly increased mortality rate compared with postprocedural pneumothorax in the ICU (Chen K et al. Chest. 2002;122[2]:678-83). The incidence of such complication in COVID-19-related ARDS is significantly higher than in ARDS from other causes (Belletti A et al. Crit Care Med. 2022;50[3]:491-500), with a mortality rate approaching 100% (Chong WH et al. Heart Lung. 2021;50[5]:599-608).
So why are patients with COVID-19 developing these complications at a higher rate? When we examine the literature, we note that Leisman and colleagues (Am J Respir Crit Care Med. 2022;205[5]:507-19) describe higher baseline markers of alveolar damage, including RAGE (receptor for advanced glycation end-products) in mechanically ventilated patients with COVID-19 vs patients requiring mechanical ventilation for other causes. This poses a question that perhaps one of the main reasons patients with COVID-19 ARDS are at an increased risk for developing certain complications, such as pneumothorax, is inherent to the unique type of alveolar injury sustained with the infection. The authors also note that alveolar markers of injury had moderate to poor discrimination for invasive ventilation early in the disease and diminished over time in both ventilated patients receiving lung protective ventilation strategy and those spontaneously breathing. Likewise, this important finding suggests that the development of pneumothorax in patients with COVID-19 may not be entirely related to barotrauma.
Another phenomenon worth investigating is the development of pneumomediastinum and subcutaneous emphysema, with a reported seven-fold increased risk of development in patients with COVID-19. Lemmers and colleagues (ERJ Open Res. 2020;6[4]:00385-2020) found no statistically significant difference in PEEP, plateau pressure, ratio of tidal volume to ideal body weight, or compliance between patients who developed this complication and those who did not, again, signifying that perhaps there is more to the story here.
Belletti and colleagues (J Cardiothorac Vasc Anesth. 2021;35[12]:3642-51) published an article examining the predictors of pneumothorax and pneumomediastinum in patients with COVID-19. The authors found that the time from symptom onset to intubation and the total bilirubin level were the only two significant predictors for the development of these complications. They explain that longer time from symptom onset to intubation likely increased the risk for self-induced lung injury, inflammation, and fibrosis, contributing to the development of such complications. It is important to note that the authors did not find a significant difference in the ventilation parameters between patients who developed pneumothorax/pneumomediastinum and those who did not.
In our institute, we examined a total of 102 patients admitted to the ICU with COVID-19 ARDS over a 3-month period from March 2020 to May 2020. We identified a total of 36 patients who developed pneumothorax, pneumomediastinum, and/or subcutaneous emphysema. We compared these subjects to age- and gender-matched control subjects. Higher age was associated with an increased risk of development of these complications, whereas the presence of diabetes mellitus, hypertension, and chronic kidney disease at baseline was associated with lower risk. This translated into lower mSOFA scores in our subjects as opposed to the control subjects mainly due to higher creatinine levels at baseline in the control group, skewing our data and indicating that some predictive criteria may not reflect the underlying disease severity and risk for development of such complications. In analyzing our ventilator data and comparing the subjects to the control group, we found no differences in mode of ventilation, set tidal volumes, or PEEP levels between the two. The subjects had significantly higher peak airway pressures, lower compliance, and longer ventilator days. Intubation was needed significantly earlier in the subjects compared with the control group with a median of 2 days vs 6 days from admission. Our data are in concordance with prior published reports and are set to be presented in abstract form this May.
COVID-19 remains a challenging disease with the potential for morbid outcomes. As we phase out of the pandemic and move into an epidemic, future research direction will likely focus on some of the more unusually common complications, such as the ones presented here.
Dr. Abdullah is with the Henry Ford Health System, Detroit, Michigan.
I recall early in the pandemic being called to the bedside to examine an acutely decompensating patient with COVID-19. This was a 33-year-old, previously healthy woman, admitted to the medical ICU with hypoxemic respiratory failure requiring mechanical ventilation and undergoing treatment for severe acute respiratory distress syndrome (ARDS). I quickly realized she was seconds away from an arrest. As I examined her, one thing caught my eye. Her airway pressures had skyrocketed over the past few minutes. Could it be? I thought to myself as I reached for the ultrasound that confirmed my suspicions, tension pneumothorax. One emergent needle decompression and chest tube later and she survives, only to die a week later from overwhelming hypoxemia.
As we reflect on these past 26 months, we recall that caring for the critically ill patient with COVID-19 has posed numerous challenges. One challenge was the overwhelming incidence of the so-called “barotrauma-related complications.” However, we also recall seeing many patients develop such complications while receiving supplemental noninvasive forms of respiratory support. Perhaps, this is in agreement with prior literature that specifically discusses the presence of air outside the tracheobronchial tree and how it does not always correlate with high airway pressure and high tidal volumes, refuting the argument that these complications always fall under the umbrella of barotrauma. We will discuss these complications and attempt to shed light on the potential variables associated with their development.
The development of pneumothorax is a well-recognized complication associated with ventilator-dependent ARDS thought to be a form of barotrauma, with some reports indicating an incidence of 48.8% (Gattinoni L et al. JAMA. 1994;271[2]):1772-9) and a significantly increased mortality rate compared with postprocedural pneumothorax in the ICU (Chen K et al. Chest. 2002;122[2]:678-83). The incidence of such complication in COVID-19-related ARDS is significantly higher than in ARDS from other causes (Belletti A et al. Crit Care Med. 2022;50[3]:491-500), with a mortality rate approaching 100% (Chong WH et al. Heart Lung. 2021;50[5]:599-608).
So why are patients with COVID-19 developing these complications at a higher rate? When we examine the literature, we note that Leisman and colleagues (Am J Respir Crit Care Med. 2022;205[5]:507-19) describe higher baseline markers of alveolar damage, including RAGE (receptor for advanced glycation end-products) in mechanically ventilated patients with COVID-19 vs patients requiring mechanical ventilation for other causes. This poses a question that perhaps one of the main reasons patients with COVID-19 ARDS are at an increased risk for developing certain complications, such as pneumothorax, is inherent to the unique type of alveolar injury sustained with the infection. The authors also note that alveolar markers of injury had moderate to poor discrimination for invasive ventilation early in the disease and diminished over time in both ventilated patients receiving lung protective ventilation strategy and those spontaneously breathing. Likewise, this important finding suggests that the development of pneumothorax in patients with COVID-19 may not be entirely related to barotrauma.
Another phenomenon worth investigating is the development of pneumomediastinum and subcutaneous emphysema, with a reported seven-fold increased risk of development in patients with COVID-19. Lemmers and colleagues (ERJ Open Res. 2020;6[4]:00385-2020) found no statistically significant difference in PEEP, plateau pressure, ratio of tidal volume to ideal body weight, or compliance between patients who developed this complication and those who did not, again, signifying that perhaps there is more to the story here.
Belletti and colleagues (J Cardiothorac Vasc Anesth. 2021;35[12]:3642-51) published an article examining the predictors of pneumothorax and pneumomediastinum in patients with COVID-19. The authors found that the time from symptom onset to intubation and the total bilirubin level were the only two significant predictors for the development of these complications. They explain that longer time from symptom onset to intubation likely increased the risk for self-induced lung injury, inflammation, and fibrosis, contributing to the development of such complications. It is important to note that the authors did not find a significant difference in the ventilation parameters between patients who developed pneumothorax/pneumomediastinum and those who did not.
In our institute, we examined a total of 102 patients admitted to the ICU with COVID-19 ARDS over a 3-month period from March 2020 to May 2020. We identified a total of 36 patients who developed pneumothorax, pneumomediastinum, and/or subcutaneous emphysema. We compared these subjects to age- and gender-matched control subjects. Higher age was associated with an increased risk of development of these complications, whereas the presence of diabetes mellitus, hypertension, and chronic kidney disease at baseline was associated with lower risk. This translated into lower mSOFA scores in our subjects as opposed to the control subjects mainly due to higher creatinine levels at baseline in the control group, skewing our data and indicating that some predictive criteria may not reflect the underlying disease severity and risk for development of such complications. In analyzing our ventilator data and comparing the subjects to the control group, we found no differences in mode of ventilation, set tidal volumes, or PEEP levels between the two. The subjects had significantly higher peak airway pressures, lower compliance, and longer ventilator days. Intubation was needed significantly earlier in the subjects compared with the control group with a median of 2 days vs 6 days from admission. Our data are in concordance with prior published reports and are set to be presented in abstract form this May.
COVID-19 remains a challenging disease with the potential for morbid outcomes. As we phase out of the pandemic and move into an epidemic, future research direction will likely focus on some of the more unusually common complications, such as the ones presented here.
Dr. Abdullah is with the Henry Ford Health System, Detroit, Michigan.
Updates on eosinophilia in asthma
Our understanding of asthma endotypes and phenotypes has grown substantially in the last decade. Endotype-targeted therapy has become a foundation of management, and classification of patients during initial assessment is extremely important. The use of history, laboratory data, and pulmonary function testing together help to categorize our patients and help guide therapy. One lab test, that of sputum or blood eosinophils, facilitates categorization and has been evaluated for its ability to determine response to medications and predict exacerbations.
In particular, eosinophilia has been extensively studied in severe asthma and is associated with type 2 inflammation. The 2021 GINA guidelines describe type 2 inflammation as characterized by cytokines (especially IL-4, IL-5, and IL-13). “T2-high patients” tend to have elevated blood or sputum eosinophil counts and elevated fractional concentration of exhaled nitric oxide (FENO) and are more likely to respond to biologic therapy. (Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2021).
However, what about patients with more mild-to-moderate asthma? Two recent studies have asked this question. In 2020, Pavord and colleagues performed a prespecified secondary subgroup analysis on an open-label randomized control trial comparing prn salbutamol alone to budesonide and as needed salbutamol to as needed budesonide-formoterol. The population was 675 adults with mild asthma receiving only as needed short acting beta-agonists (SABA) at baseline. The primary outcome was annual rate of asthma exacerbation, and whether it was different based on blood eosinophil count, FENO or a composite of both. They had several interesting findings. First, for patients only on an as needed SABA, the proportion having a severe exacerbation increased progressively with increasing blood eosinophil count. Second, inhaled corticosteroids (ICS) plus as needed SABA were more effective than SABA alone in patients with a blood eosinophil count of ≥300 cells/μL, both in terms of total exacerbations and severe exacerbations. The effects of budesonide-formoterol on exacerbations, however, was not associated with blood eosinophil count or FENO. This last point is particularly interesting in light of GINA guidelines that prioritize this combination (Pavord ID et al. Lancet Respir Med. 2020;8[7]:671-80).
More recently, a prespecified secondary analysis of the SIENA trial looked at 295 subjects with mild persistent asthma (237 adults aged 18+, and 58 adolescents aged 12-17). The primary outcome was a composite of asthma control (treatment failure, asthma control days, and FEV1). They found that sputum eosinophil levels, blood eosinophil levels, and FENO all predicted response to ICS in adults; however, the area under the receiver operative characteristic curve (AUC) was less than 0.7 for each of these findings, which was below the threshold for acceptability. A blood eosinophil count of ≥100 cells/μL offered 87% sensitivity and 17% specificity for response to ICS (Krishnan JA et al. Ann Am Thorac Soc. 2022;19[3]:372-80).
What does this tell us? Blood eosinophil count may help determine who will respond to ICS, and there remains utility in assessing blood eosinophil count in severe asthma for determining candidacy for biologic therapies. However, the overall utility of blood eosinophils in mild to moderate asthma is not as clear.
But, are we asking the right questions? Many studies look at a single blood eosinophil level, either at a single point in time, a baseline level, or a highest level over a specific time period. But do eosinophil counts vary over time?
A 2018 single-center study initially asked this question. The authors evaluated blood eosinophil levels in 219 adult patients at the NYU/Bellevue Hospital Asthma Clinic over a 5-year period. They found that individual patients had variable eosinophil levels. For example, only 6% (n=13) of patients had levels consistently above 300 cells/μL, but nearly 50% (n=104) had at least one level above 300. The degree of variability was then assessed by K-mean clustering yielding three clusters. Cluster 2 had the largest variability in blood eosinophil counts and a slightly higher absolute eosinophil level. While not significant, there was a suggestion of worse asthma control with more hospitalizations and more prescriptions for multiple controllers in this cluster with more variability. Clearly, this warranted further study (Rakowski E et al. Clin Exp Allergy. 2019;49[2]:163-70).
Variability was re-examined more recently in 2021. A post hoc analysis of two phase III clinical trials from the reslizumab BREATH program looked at eosinophil counts in the 476 patients randomized to receive placebo during the 52-week study. These patients did have eosinophilic asthma by definition and had to have an elevated eosinophil count >400 cells/μL over the 4-week enrollment period to enter the study. However, 124 patients (26.1%) had an eosinophil level <400 cells/μL immediately before the first dose of placebo. The primary outcome was variability in blood eosinophil count. Of patients who started with serum eosinophils <400, 27% to 56% of patients shifted to the ≥400 cells/μL category during the treatment period (this wide range is across three categories of low “baseline” blood eosinophil count; <150, 150 to 300, and 300 to 400). On the contrary, patients who started with eosinophils ≥400 cells/μL tended to stay at that level. The variability is reduced by taking two to three repeat measurements at baseline (Corren et al. J Allergy Clin Immunol Pract. 2021;9[3]:1224-31).
Does this variability have clinical significance? A recent retrospective cohort study looked at 10,059 stable adult patients with asthma from the MAJORICA cohort in Spain, compared with 8,557 control subjects. The primary outcome was total blood eosinophil count and an “eosinophil variability index” (EVI) where EVI=(Eosmax – Eosmin / Eosmax) x 100%. They found that an elevated EVI was associated with hospitalization, more so than maximum eosinophil count or any other eosinophil count variable, with an odds ratio of 3.18 by univariate regression (2.51 by multivariate). They also found that patients with an EVI ≥50% were twice as likely to be hospitalized or visit the ED than those with a lower EVI (Toledo-Pons N et al. Ann Am Thorac Soc. 2022;19[3]:407-14). These results are very interesting and merit further research.
So, what to do with this information? We know that patients with peripheral eosinophilia and severe asthma symptoms are candidates for biologic therapy. They are also more likely to respond to steroids, although the utility of this assessment alone in mild to moderate asthma is less clear. It does seem that more variability in eosinophils over time may be linked to more difficult-to-treat asthma.
Should you check eosinophils in your patients with asthma? GINA 2021 guidelines say to consider it, and list blood eosinophilia as a risk factor for future exacerbation, even if patients have few asthma symptoms. They also say to repeat blood eosinophils in patients with severe asthma, if the level is low at first assessment, based on the studies discussed above. We would agree. We also see the blood eosinophil count as one part of a clinical assessment of a patient’s overall asthma control – even if the patient has mild symptoms. More study on variability is welcome.
Dr. Haber and Dr. Jamieson are with Medstar Georgetown University Hospital, Washington, D.C.
Our understanding of asthma endotypes and phenotypes has grown substantially in the last decade. Endotype-targeted therapy has become a foundation of management, and classification of patients during initial assessment is extremely important. The use of history, laboratory data, and pulmonary function testing together help to categorize our patients and help guide therapy. One lab test, that of sputum or blood eosinophils, facilitates categorization and has been evaluated for its ability to determine response to medications and predict exacerbations.
In particular, eosinophilia has been extensively studied in severe asthma and is associated with type 2 inflammation. The 2021 GINA guidelines describe type 2 inflammation as characterized by cytokines (especially IL-4, IL-5, and IL-13). “T2-high patients” tend to have elevated blood or sputum eosinophil counts and elevated fractional concentration of exhaled nitric oxide (FENO) and are more likely to respond to biologic therapy. (Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2021).
However, what about patients with more mild-to-moderate asthma? Two recent studies have asked this question. In 2020, Pavord and colleagues performed a prespecified secondary subgroup analysis on an open-label randomized control trial comparing prn salbutamol alone to budesonide and as needed salbutamol to as needed budesonide-formoterol. The population was 675 adults with mild asthma receiving only as needed short acting beta-agonists (SABA) at baseline. The primary outcome was annual rate of asthma exacerbation, and whether it was different based on blood eosinophil count, FENO or a composite of both. They had several interesting findings. First, for patients only on an as needed SABA, the proportion having a severe exacerbation increased progressively with increasing blood eosinophil count. Second, inhaled corticosteroids (ICS) plus as needed SABA were more effective than SABA alone in patients with a blood eosinophil count of ≥300 cells/μL, both in terms of total exacerbations and severe exacerbations. The effects of budesonide-formoterol on exacerbations, however, was not associated with blood eosinophil count or FENO. This last point is particularly interesting in light of GINA guidelines that prioritize this combination (Pavord ID et al. Lancet Respir Med. 2020;8[7]:671-80).
More recently, a prespecified secondary analysis of the SIENA trial looked at 295 subjects with mild persistent asthma (237 adults aged 18+, and 58 adolescents aged 12-17). The primary outcome was a composite of asthma control (treatment failure, asthma control days, and FEV1). They found that sputum eosinophil levels, blood eosinophil levels, and FENO all predicted response to ICS in adults; however, the area under the receiver operative characteristic curve (AUC) was less than 0.7 for each of these findings, which was below the threshold for acceptability. A blood eosinophil count of ≥100 cells/μL offered 87% sensitivity and 17% specificity for response to ICS (Krishnan JA et al. Ann Am Thorac Soc. 2022;19[3]:372-80).
What does this tell us? Blood eosinophil count may help determine who will respond to ICS, and there remains utility in assessing blood eosinophil count in severe asthma for determining candidacy for biologic therapies. However, the overall utility of blood eosinophils in mild to moderate asthma is not as clear.
But, are we asking the right questions? Many studies look at a single blood eosinophil level, either at a single point in time, a baseline level, or a highest level over a specific time period. But do eosinophil counts vary over time?
A 2018 single-center study initially asked this question. The authors evaluated blood eosinophil levels in 219 adult patients at the NYU/Bellevue Hospital Asthma Clinic over a 5-year period. They found that individual patients had variable eosinophil levels. For example, only 6% (n=13) of patients had levels consistently above 300 cells/μL, but nearly 50% (n=104) had at least one level above 300. The degree of variability was then assessed by K-mean clustering yielding three clusters. Cluster 2 had the largest variability in blood eosinophil counts and a slightly higher absolute eosinophil level. While not significant, there was a suggestion of worse asthma control with more hospitalizations and more prescriptions for multiple controllers in this cluster with more variability. Clearly, this warranted further study (Rakowski E et al. Clin Exp Allergy. 2019;49[2]:163-70).
Variability was re-examined more recently in 2021. A post hoc analysis of two phase III clinical trials from the reslizumab BREATH program looked at eosinophil counts in the 476 patients randomized to receive placebo during the 52-week study. These patients did have eosinophilic asthma by definition and had to have an elevated eosinophil count >400 cells/μL over the 4-week enrollment period to enter the study. However, 124 patients (26.1%) had an eosinophil level <400 cells/μL immediately before the first dose of placebo. The primary outcome was variability in blood eosinophil count. Of patients who started with serum eosinophils <400, 27% to 56% of patients shifted to the ≥400 cells/μL category during the treatment period (this wide range is across three categories of low “baseline” blood eosinophil count; <150, 150 to 300, and 300 to 400). On the contrary, patients who started with eosinophils ≥400 cells/μL tended to stay at that level. The variability is reduced by taking two to three repeat measurements at baseline (Corren et al. J Allergy Clin Immunol Pract. 2021;9[3]:1224-31).
Does this variability have clinical significance? A recent retrospective cohort study looked at 10,059 stable adult patients with asthma from the MAJORICA cohort in Spain, compared with 8,557 control subjects. The primary outcome was total blood eosinophil count and an “eosinophil variability index” (EVI) where EVI=(Eosmax – Eosmin / Eosmax) x 100%. They found that an elevated EVI was associated with hospitalization, more so than maximum eosinophil count or any other eosinophil count variable, with an odds ratio of 3.18 by univariate regression (2.51 by multivariate). They also found that patients with an EVI ≥50% were twice as likely to be hospitalized or visit the ED than those with a lower EVI (Toledo-Pons N et al. Ann Am Thorac Soc. 2022;19[3]:407-14). These results are very interesting and merit further research.
So, what to do with this information? We know that patients with peripheral eosinophilia and severe asthma symptoms are candidates for biologic therapy. They are also more likely to respond to steroids, although the utility of this assessment alone in mild to moderate asthma is less clear. It does seem that more variability in eosinophils over time may be linked to more difficult-to-treat asthma.
Should you check eosinophils in your patients with asthma? GINA 2021 guidelines say to consider it, and list blood eosinophilia as a risk factor for future exacerbation, even if patients have few asthma symptoms. They also say to repeat blood eosinophils in patients with severe asthma, if the level is low at first assessment, based on the studies discussed above. We would agree. We also see the blood eosinophil count as one part of a clinical assessment of a patient’s overall asthma control – even if the patient has mild symptoms. More study on variability is welcome.
Dr. Haber and Dr. Jamieson are with Medstar Georgetown University Hospital, Washington, D.C.
Our understanding of asthma endotypes and phenotypes has grown substantially in the last decade. Endotype-targeted therapy has become a foundation of management, and classification of patients during initial assessment is extremely important. The use of history, laboratory data, and pulmonary function testing together help to categorize our patients and help guide therapy. One lab test, that of sputum or blood eosinophils, facilitates categorization and has been evaluated for its ability to determine response to medications and predict exacerbations.
In particular, eosinophilia has been extensively studied in severe asthma and is associated with type 2 inflammation. The 2021 GINA guidelines describe type 2 inflammation as characterized by cytokines (especially IL-4, IL-5, and IL-13). “T2-high patients” tend to have elevated blood or sputum eosinophil counts and elevated fractional concentration of exhaled nitric oxide (FENO) and are more likely to respond to biologic therapy. (Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2021).
However, what about patients with more mild-to-moderate asthma? Two recent studies have asked this question. In 2020, Pavord and colleagues performed a prespecified secondary subgroup analysis on an open-label randomized control trial comparing prn salbutamol alone to budesonide and as needed salbutamol to as needed budesonide-formoterol. The population was 675 adults with mild asthma receiving only as needed short acting beta-agonists (SABA) at baseline. The primary outcome was annual rate of asthma exacerbation, and whether it was different based on blood eosinophil count, FENO or a composite of both. They had several interesting findings. First, for patients only on an as needed SABA, the proportion having a severe exacerbation increased progressively with increasing blood eosinophil count. Second, inhaled corticosteroids (ICS) plus as needed SABA were more effective than SABA alone in patients with a blood eosinophil count of ≥300 cells/μL, both in terms of total exacerbations and severe exacerbations. The effects of budesonide-formoterol on exacerbations, however, was not associated with blood eosinophil count or FENO. This last point is particularly interesting in light of GINA guidelines that prioritize this combination (Pavord ID et al. Lancet Respir Med. 2020;8[7]:671-80).
More recently, a prespecified secondary analysis of the SIENA trial looked at 295 subjects with mild persistent asthma (237 adults aged 18+, and 58 adolescents aged 12-17). The primary outcome was a composite of asthma control (treatment failure, asthma control days, and FEV1). They found that sputum eosinophil levels, blood eosinophil levels, and FENO all predicted response to ICS in adults; however, the area under the receiver operative characteristic curve (AUC) was less than 0.7 for each of these findings, which was below the threshold for acceptability. A blood eosinophil count of ≥100 cells/μL offered 87% sensitivity and 17% specificity for response to ICS (Krishnan JA et al. Ann Am Thorac Soc. 2022;19[3]:372-80).
What does this tell us? Blood eosinophil count may help determine who will respond to ICS, and there remains utility in assessing blood eosinophil count in severe asthma for determining candidacy for biologic therapies. However, the overall utility of blood eosinophils in mild to moderate asthma is not as clear.
But, are we asking the right questions? Many studies look at a single blood eosinophil level, either at a single point in time, a baseline level, or a highest level over a specific time period. But do eosinophil counts vary over time?
A 2018 single-center study initially asked this question. The authors evaluated blood eosinophil levels in 219 adult patients at the NYU/Bellevue Hospital Asthma Clinic over a 5-year period. They found that individual patients had variable eosinophil levels. For example, only 6% (n=13) of patients had levels consistently above 300 cells/μL, but nearly 50% (n=104) had at least one level above 300. The degree of variability was then assessed by K-mean clustering yielding three clusters. Cluster 2 had the largest variability in blood eosinophil counts and a slightly higher absolute eosinophil level. While not significant, there was a suggestion of worse asthma control with more hospitalizations and more prescriptions for multiple controllers in this cluster with more variability. Clearly, this warranted further study (Rakowski E et al. Clin Exp Allergy. 2019;49[2]:163-70).
Variability was re-examined more recently in 2021. A post hoc analysis of two phase III clinical trials from the reslizumab BREATH program looked at eosinophil counts in the 476 patients randomized to receive placebo during the 52-week study. These patients did have eosinophilic asthma by definition and had to have an elevated eosinophil count >400 cells/μL over the 4-week enrollment period to enter the study. However, 124 patients (26.1%) had an eosinophil level <400 cells/μL immediately before the first dose of placebo. The primary outcome was variability in blood eosinophil count. Of patients who started with serum eosinophils <400, 27% to 56% of patients shifted to the ≥400 cells/μL category during the treatment period (this wide range is across three categories of low “baseline” blood eosinophil count; <150, 150 to 300, and 300 to 400). On the contrary, patients who started with eosinophils ≥400 cells/μL tended to stay at that level. The variability is reduced by taking two to three repeat measurements at baseline (Corren et al. J Allergy Clin Immunol Pract. 2021;9[3]:1224-31).
Does this variability have clinical significance? A recent retrospective cohort study looked at 10,059 stable adult patients with asthma from the MAJORICA cohort in Spain, compared with 8,557 control subjects. The primary outcome was total blood eosinophil count and an “eosinophil variability index” (EVI) where EVI=(Eosmax – Eosmin / Eosmax) x 100%. They found that an elevated EVI was associated with hospitalization, more so than maximum eosinophil count or any other eosinophil count variable, with an odds ratio of 3.18 by univariate regression (2.51 by multivariate). They also found that patients with an EVI ≥50% were twice as likely to be hospitalized or visit the ED than those with a lower EVI (Toledo-Pons N et al. Ann Am Thorac Soc. 2022;19[3]:407-14). These results are very interesting and merit further research.
So, what to do with this information? We know that patients with peripheral eosinophilia and severe asthma symptoms are candidates for biologic therapy. They are also more likely to respond to steroids, although the utility of this assessment alone in mild to moderate asthma is less clear. It does seem that more variability in eosinophils over time may be linked to more difficult-to-treat asthma.
Should you check eosinophils in your patients with asthma? GINA 2021 guidelines say to consider it, and list blood eosinophilia as a risk factor for future exacerbation, even if patients have few asthma symptoms. They also say to repeat blood eosinophils in patients with severe asthma, if the level is low at first assessment, based on the studies discussed above. We would agree. We also see the blood eosinophil count as one part of a clinical assessment of a patient’s overall asthma control – even if the patient has mild symptoms. More study on variability is welcome.
Dr. Haber and Dr. Jamieson are with Medstar Georgetown University Hospital, Washington, D.C.
The quest for a good night’s sleep: An update on pharmacologic therapy for insomnia
Insomnia is one of the most common complaints in medicine, driving millions of clinic visits each year (Table 1). It is estimated that approximately 30% of individuals report at least short-term insomnia symptoms and 10% report chronic insomnia. These rates are even higher in groups that may be more susceptible to insomnia, including women, the elderly, and those of disadvantaged socioeconomic status (Ohayon MM. Sleep Med Rev. 2002;[2]:97-111). While most patients with insomnia find their sleep difficulties self-resolve within 3 months, a substantial number of patients will find their insomnia to persist for longer and require intervention (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
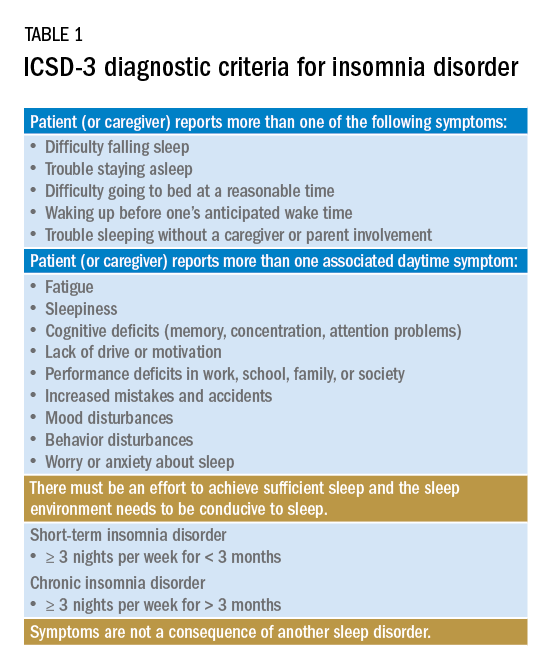
For individuals requiring treatment, cognitive behavioral therapy for insomnia (CBT-I) is considered first-line therapy by the American Academy of Sleep Medicine for both acute and chronic insomnia. Unfortunately, obtaining CBT-I for a patient is often a challenge as the number of trained therapists offering this service is limited, resulting in long wait times or, in some cases, a complete lack of access to this treatment option. Judicious use of sedative-hypnotic medications may be a reasonable alternative for patients with insomnia who are unable to undergo CBT-I, who are still symptomatic despite undergoing CBT-I, or, in some cases, as a temporary treatment (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
Current medications used to treat insomnia are listed in Tables 2 and 3, some of which carry an FDA approval to be used as a hypnotic, while others are used in an off-label manner.
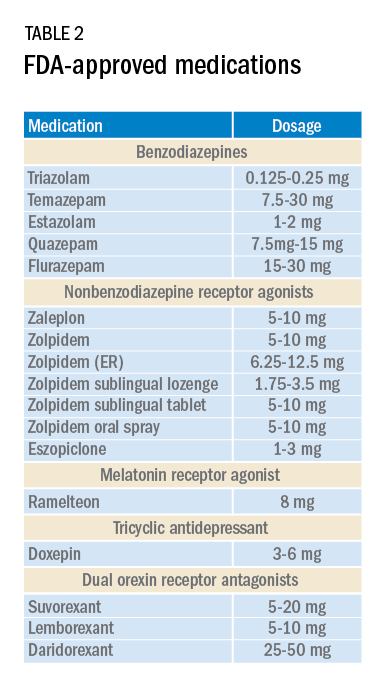
Cautions abound with use of many of these medications. Common concerns include safety, particularly for elderly patients and long-term use, and the potential for developing tolerance and dependence.
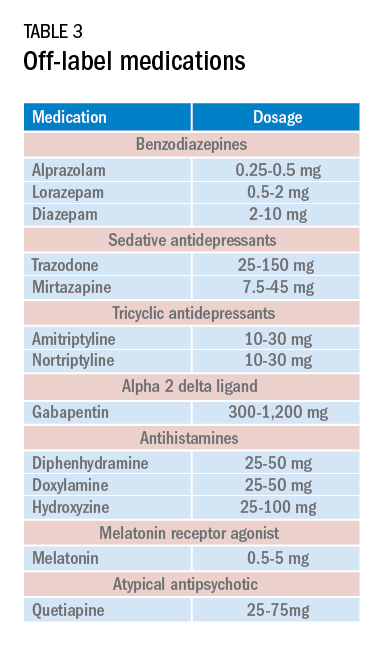
Most medications that have been used for insomnia have been available for decades, but, in recent years, a new class of hypnotics has emerged. Dual orexin receptor antagonists (DORAs) are the newest class of FDA-approved medications (Table 4).
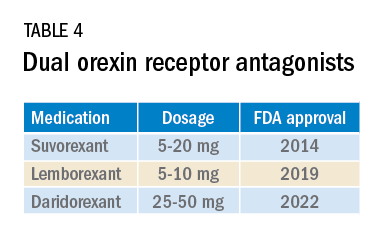
Orexin is a neuropeptide found primarily in the lateral hypothalamus and binds to the orexin 1 and orexin 2 receptors leading to a number of downstream effects, including stimulating wakefulness. Loss of orexin-generating neurons has been implicated as the cause of type 1 narcolepsy, and antagonism of their effects can facilitate sleep by suppressing wakefulness. The first medication in the DORA class to be FDA-approved was suvorexant in 2014, followed by lemborexant’s FDA approval in 2019. These are both indicated for treating sleep onset and sleep maintenance insomnia and have been shown to improve both subjective and objective measures of sleep. The most common side effects reported for both suvorexant and lemborexant are headache and somnolence, with morning-after sleepiness being a frequent complaint.
In January 2022, a new medication in the DORA class named daridorexant was approved by the FDA (Table 5).
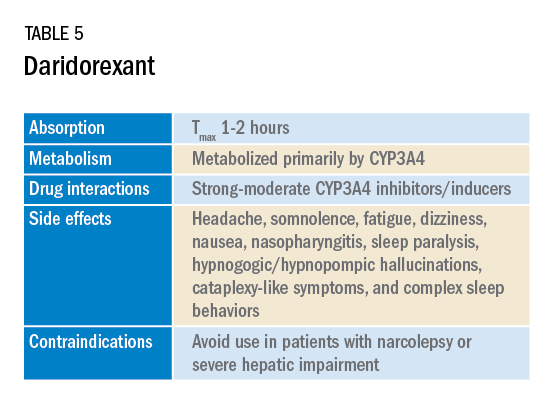
Daridorexant, like its DORA counterparts, has been shown to have efficacy in improving subjective and objective markers of insomnia. This has included polysomnographic measures of wake after sleep onset and latency to persistent sleep, as well as subjective total sleep time. Importantly, in addition to positive sleep outcomes, improvements with daytime function have also been observed with this medication (Mignot E et al. Lancet Neurol. 2022;21[2]:125-39). Daridorexant’s half-life of approximately 8 hours is shorter than that of the other available DORAs, leading to fewer day-after effects. The combination of effectiveness for sleep initiation and maintenance without daytime impairment distinguishes daridorexant from the other DORAs and even other classes of sleep medication.
Safety, especially in patients of age 65 and older, is an important concern with sleep medication, particularly with respect to polypharmacy, over-sedation, increased fall risk, and cognitive impairment, but daridorexant’s available safety data suggest a favorable safety profile (Zammit G et al. Neurology. 2020;94[21]:e2222-32).
Daridorexant at the highest dose available, 50 mg, did not worsen respiratory function, in terms of the apnea-hypopnea index and oxygen saturation in individuals with mild-moderate obstructive sleep apnea regardless of sleep stage (Boof ML et al. Sleep. 2021;44[6]:zsaa275). However, more safety and longitudinal data are needed to have a fuller understanding of any potential limitations of this medication.
While we continue to recommend CBT-I as the first-line treatment whenever possible for patients with insomnia, not all patients have access to this treatment and not all patients will respond satisfactorily to it. Thus, pharmacologic treatment can continue to play an important role in the management of some patients’ insomnia. Each class of medications used for treating insomnia features a unique constellation of advantages and limitations, meaning that the more available options, the greater the chances of finding an option that will be both effective and safe for a particular patient. The growing DORA class, especially its newest available entrant, daridorexant, represents a continued expansion of the armamentarium of options against insomnia.
Dr. Pelekanos and Dr. Sum-Ping are with the Division of Sleep Medicine, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, California.
Insomnia is one of the most common complaints in medicine, driving millions of clinic visits each year (Table 1). It is estimated that approximately 30% of individuals report at least short-term insomnia symptoms and 10% report chronic insomnia. These rates are even higher in groups that may be more susceptible to insomnia, including women, the elderly, and those of disadvantaged socioeconomic status (Ohayon MM. Sleep Med Rev. 2002;[2]:97-111). While most patients with insomnia find their sleep difficulties self-resolve within 3 months, a substantial number of patients will find their insomnia to persist for longer and require intervention (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).

For individuals requiring treatment, cognitive behavioral therapy for insomnia (CBT-I) is considered first-line therapy by the American Academy of Sleep Medicine for both acute and chronic insomnia. Unfortunately, obtaining CBT-I for a patient is often a challenge as the number of trained therapists offering this service is limited, resulting in long wait times or, in some cases, a complete lack of access to this treatment option. Judicious use of sedative-hypnotic medications may be a reasonable alternative for patients with insomnia who are unable to undergo CBT-I, who are still symptomatic despite undergoing CBT-I, or, in some cases, as a temporary treatment (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
Current medications used to treat insomnia are listed in Tables 2 and 3, some of which carry an FDA approval to be used as a hypnotic, while others are used in an off-label manner.

Cautions abound with use of many of these medications. Common concerns include safety, particularly for elderly patients and long-term use, and the potential for developing tolerance and dependence.

Most medications that have been used for insomnia have been available for decades, but, in recent years, a new class of hypnotics has emerged. Dual orexin receptor antagonists (DORAs) are the newest class of FDA-approved medications (Table 4).

Orexin is a neuropeptide found primarily in the lateral hypothalamus and binds to the orexin 1 and orexin 2 receptors leading to a number of downstream effects, including stimulating wakefulness. Loss of orexin-generating neurons has been implicated as the cause of type 1 narcolepsy, and antagonism of their effects can facilitate sleep by suppressing wakefulness. The first medication in the DORA class to be FDA-approved was suvorexant in 2014, followed by lemborexant’s FDA approval in 2019. These are both indicated for treating sleep onset and sleep maintenance insomnia and have been shown to improve both subjective and objective measures of sleep. The most common side effects reported for both suvorexant and lemborexant are headache and somnolence, with morning-after sleepiness being a frequent complaint.
In January 2022, a new medication in the DORA class named daridorexant was approved by the FDA (Table 5).

Daridorexant, like its DORA counterparts, has been shown to have efficacy in improving subjective and objective markers of insomnia. This has included polysomnographic measures of wake after sleep onset and latency to persistent sleep, as well as subjective total sleep time. Importantly, in addition to positive sleep outcomes, improvements with daytime function have also been observed with this medication (Mignot E et al. Lancet Neurol. 2022;21[2]:125-39). Daridorexant’s half-life of approximately 8 hours is shorter than that of the other available DORAs, leading to fewer day-after effects. The combination of effectiveness for sleep initiation and maintenance without daytime impairment distinguishes daridorexant from the other DORAs and even other classes of sleep medication.
Safety, especially in patients of age 65 and older, is an important concern with sleep medication, particularly with respect to polypharmacy, over-sedation, increased fall risk, and cognitive impairment, but daridorexant’s available safety data suggest a favorable safety profile (Zammit G et al. Neurology. 2020;94[21]:e2222-32).
Daridorexant at the highest dose available, 50 mg, did not worsen respiratory function, in terms of the apnea-hypopnea index and oxygen saturation in individuals with mild-moderate obstructive sleep apnea regardless of sleep stage (Boof ML et al. Sleep. 2021;44[6]:zsaa275). However, more safety and longitudinal data are needed to have a fuller understanding of any potential limitations of this medication.
While we continue to recommend CBT-I as the first-line treatment whenever possible for patients with insomnia, not all patients have access to this treatment and not all patients will respond satisfactorily to it. Thus, pharmacologic treatment can continue to play an important role in the management of some patients’ insomnia. Each class of medications used for treating insomnia features a unique constellation of advantages and limitations, meaning that the more available options, the greater the chances of finding an option that will be both effective and safe for a particular patient. The growing DORA class, especially its newest available entrant, daridorexant, represents a continued expansion of the armamentarium of options against insomnia.
Dr. Pelekanos and Dr. Sum-Ping are with the Division of Sleep Medicine, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, California.
Insomnia is one of the most common complaints in medicine, driving millions of clinic visits each year (Table 1). It is estimated that approximately 30% of individuals report at least short-term insomnia symptoms and 10% report chronic insomnia. These rates are even higher in groups that may be more susceptible to insomnia, including women, the elderly, and those of disadvantaged socioeconomic status (Ohayon MM. Sleep Med Rev. 2002;[2]:97-111). While most patients with insomnia find their sleep difficulties self-resolve within 3 months, a substantial number of patients will find their insomnia to persist for longer and require intervention (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).

For individuals requiring treatment, cognitive behavioral therapy for insomnia (CBT-I) is considered first-line therapy by the American Academy of Sleep Medicine for both acute and chronic insomnia. Unfortunately, obtaining CBT-I for a patient is often a challenge as the number of trained therapists offering this service is limited, resulting in long wait times or, in some cases, a complete lack of access to this treatment option. Judicious use of sedative-hypnotic medications may be a reasonable alternative for patients with insomnia who are unable to undergo CBT-I, who are still symptomatic despite undergoing CBT-I, or, in some cases, as a temporary treatment (Sateia MJ et al. J Clin Sleep Med. 2017;13[2]:307-49).
Current medications used to treat insomnia are listed in Tables 2 and 3, some of which carry an FDA approval to be used as a hypnotic, while others are used in an off-label manner.

Cautions abound with use of many of these medications. Common concerns include safety, particularly for elderly patients and long-term use, and the potential for developing tolerance and dependence.

Most medications that have been used for insomnia have been available for decades, but, in recent years, a new class of hypnotics has emerged. Dual orexin receptor antagonists (DORAs) are the newest class of FDA-approved medications (Table 4).

Orexin is a neuropeptide found primarily in the lateral hypothalamus and binds to the orexin 1 and orexin 2 receptors leading to a number of downstream effects, including stimulating wakefulness. Loss of orexin-generating neurons has been implicated as the cause of type 1 narcolepsy, and antagonism of their effects can facilitate sleep by suppressing wakefulness. The first medication in the DORA class to be FDA-approved was suvorexant in 2014, followed by lemborexant’s FDA approval in 2019. These are both indicated for treating sleep onset and sleep maintenance insomnia and have been shown to improve both subjective and objective measures of sleep. The most common side effects reported for both suvorexant and lemborexant are headache and somnolence, with morning-after sleepiness being a frequent complaint.
In January 2022, a new medication in the DORA class named daridorexant was approved by the FDA (Table 5).

Daridorexant, like its DORA counterparts, has been shown to have efficacy in improving subjective and objective markers of insomnia. This has included polysomnographic measures of wake after sleep onset and latency to persistent sleep, as well as subjective total sleep time. Importantly, in addition to positive sleep outcomes, improvements with daytime function have also been observed with this medication (Mignot E et al. Lancet Neurol. 2022;21[2]:125-39). Daridorexant’s half-life of approximately 8 hours is shorter than that of the other available DORAs, leading to fewer day-after effects. The combination of effectiveness for sleep initiation and maintenance without daytime impairment distinguishes daridorexant from the other DORAs and even other classes of sleep medication.
Safety, especially in patients of age 65 and older, is an important concern with sleep medication, particularly with respect to polypharmacy, over-sedation, increased fall risk, and cognitive impairment, but daridorexant’s available safety data suggest a favorable safety profile (Zammit G et al. Neurology. 2020;94[21]:e2222-32).
Daridorexant at the highest dose available, 50 mg, did not worsen respiratory function, in terms of the apnea-hypopnea index and oxygen saturation in individuals with mild-moderate obstructive sleep apnea regardless of sleep stage (Boof ML et al. Sleep. 2021;44[6]:zsaa275). However, more safety and longitudinal data are needed to have a fuller understanding of any potential limitations of this medication.
While we continue to recommend CBT-I as the first-line treatment whenever possible for patients with insomnia, not all patients have access to this treatment and not all patients will respond satisfactorily to it. Thus, pharmacologic treatment can continue to play an important role in the management of some patients’ insomnia. Each class of medications used for treating insomnia features a unique constellation of advantages and limitations, meaning that the more available options, the greater the chances of finding an option that will be both effective and safe for a particular patient. The growing DORA class, especially its newest available entrant, daridorexant, represents a continued expansion of the armamentarium of options against insomnia.
Dr. Pelekanos and Dr. Sum-Ping are with the Division of Sleep Medicine, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, California.
What COVID-19 taught us: The challenge of maintaining contingency level care to proactively forestall crisis care
In 2014, the Task Force for Mass Critical Care (TFMCC) published a CHEST consensus statement on disaster preparedness principles in caring for the critically ill during disasters and pandemics (Christian et al. CHEST. 2014;146[4_suppl]:8s-34s). This publication attempted to guide preparedness for both single-event disasters and more prolonged events, including a feared influenza pandemic.
Despite the foundation of planning and support this guidance provided, the COVID-19 pandemic response revealed substantial gaps in our understanding and preparedness for these more prolonged and widespread events.
In New York City, as the first COVID-19 wave began in March and April of 2020, area hospitals responded with surge plans that prioritized what was felt to be most important (Griffin et al. Am J Respir Crit Care Med. 2020 Jun 1;201[11]:1337-44). Tiered, creative staffing structures were rapidly created with intensivists supervising non-ICU physicians and APPs. Procedure teams were created for intubation, proning, and central line placement. ICU space was created with adaptations to ORs and PACUs, and rooms on med-surg floors and step-down units underwent emergency renovations to allow creation of new “pop-up” ICUs. Triage protocols were altered: patients on high levels of supplemental oxygen, who would under normal circumstances have been admitted to an ICU, were triaged to floors and stepdown units. Equipment was reused, modified, and substituted creatively to optimize care for the maximum number of patients.
In the face of all of these struggles, many around the country and the world felt the efforts, though heroic, resulted in less than standard of care. Two subsequent publications validated this concern (Kadri et al. Ann Int Med. 2021,174;9:1240-51; Bravata DM et al. JAMA Open Network. 2021;4[1]:e2034266), demonstrating during severe surge, COVID-19 patients’ mortality increased significantly beyond that seen in non-surging or less-severe surging times, demonstrating a mortality effect of surge itself. Though these studies observed COVID-19 patients only, there is every reason to believe the findings applied to all critically ill patients cared for during these surges.
These experiences led the TFMCC to report updated strategies for remaining in contingency care levels and avoiding crisis care (Dichter JR et al. CHEST. 2022;161[2]:429-47). Contingency is equivalent to routine care though may require adaptations and employment of otherwise non-traditional resources. The ultimate goal of mass critical care in a public health emergency is to avoid crisis-operating conditions, crisis standards of care, and their associated challenging triage decisions regarding allocation of scarce resources.
The 10 suggestions included in the most recent TFMCC publication include staffing strategies and suggestions based on COVID-19 experiences for graded staff-to-patient ratios, and support processes to preserve the existing health care work force. Strategies also include reduction of redundant documentation, limiting overtime, and most importantly, approaches for improving teamwork and supporting psychological well-being and resilience. Examples include daily unit huddles to update care and share experiences, genuine intra-team recognition and appreciation, and embedding emotional health experts within teams to provide ongoing support.
Consistent communication between incident command and frontline clinicians was also a suggested priority, perhaps with a newly proposed position of physician clinical support supervisor. This would be a formal role within hospital incident command, a liaison between the two groups.
Surge strategies should include empowerment of bedside clinicians and leaders with both planning and real-time assessment of the clinical situation, as being at the front line of care enables the situational awareness to assess ICU strain most effectively. Further, ICU clinicians must recognize when progression deeper into contingency operations occurs and they become perilously close to crisis mode. At this point, decisions are made and scarce resources are modified beyond routine standards of care to preserve life. TFMCC designates this gray area between contingency and crisis as the Critical Clinical Prioritization level (Figure).
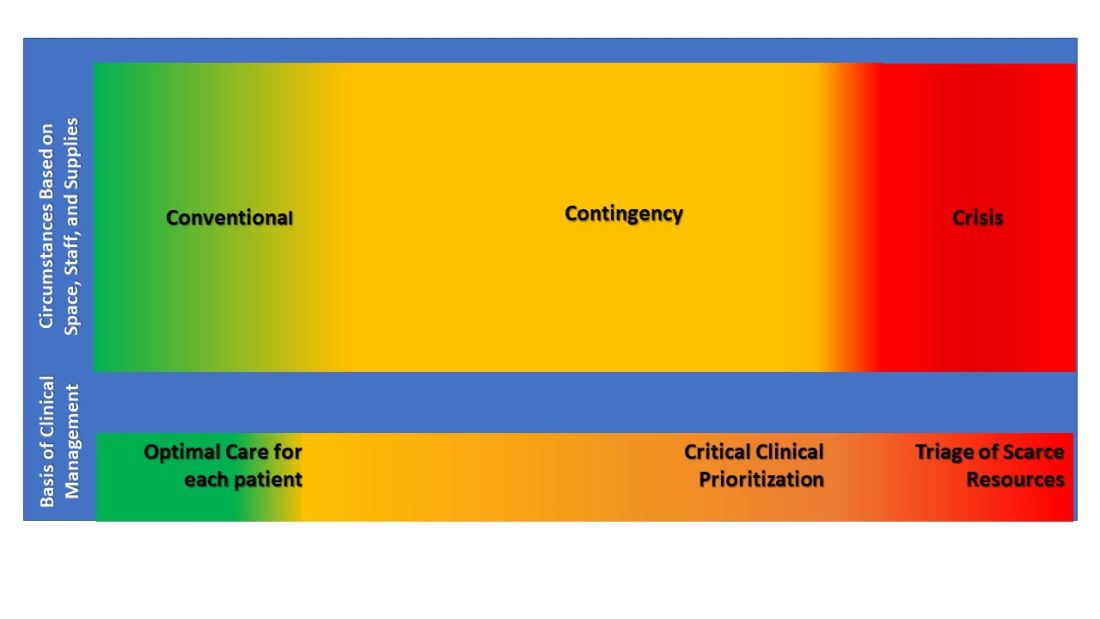
At this point, more resources must be provided, or patients must be transferred to other resourced hospitals.
Critical Clinical Prioritization is an illustration of necessity being the mother of invention, as these are adaptations clinicians devised under duress. Some particularly poignant examples are the spreading of 24 hours of continuous renal replacement therapy (CRRT) resource between two and sometimes three patients to provide life sustainment to all; and when ventilators were in short supply, determining which patients required full ICU ventilator support vs those who could manage with lower functioning ventilators, and trading them between patients when demands changed.
These adaptations can only be done by experienced clinicians proactively managing bedside critical care under duress, further underscoring the importance of our suggestion that Critical Clinical Prioritization and ICU strain be managed by bedside clinicians and leaders.
The response of early transfer of patients – load-balancing - should be considered as soon as any hospital enters contingency conditions. This strategy is commonly implemented within larger health systems, ideally before reaching Critical Clinical Prioritization. Formal, organized state or regional load-balancing coordination, now referred to as medical operations command centers (MOCCs), were highly effective and proved lifesaving for those states that implemented them (including Arizona, Washington, California, Minnesota, and others). Support for establishment of MOCC’s is crucial in prolonging contingency operations and further helps support and protect disadvantaged populations (White et al. N Engl J Med. 2021;385[24]:2211-4).
Establishment of MOCCs has met resistance due to challenges that include interhospital/intersystem competition, logistics of moving critically ill patients sometimes across significant physical distance, and the costs of assuming care of uninsured or underinsured patients. Nevertheless, the benefits to the population as a whole necessitate working through these obstacles as successful MOCCs have done, usually with government and hospital association support.
In their final suggestion of the 2022 updated strategies, TFMCC suggests that hospitals use telemedicine technology both to expand specialists’ ability to provide care and facilitate families virtually visiting their critically ill loved one when safety precludes in-person visits.
These suggestions are pivotal in planning for future public health emergencies that include mass critical care, even during events that are limited in scope and duration.
Lastly, intensivists struggled with legal and ethical concerns when mired in crisis care circumstances and decisions of allocation, and potential reallocation, of scarce resources. These issues were not well addressed during the COVID-19 pandemic, further emphasizing the importance of maintaining contingency level care and requiring further involvement from legal and medical ethics professionals for future planning.
The guiding principle of disaster preparedness is that we must do all the planning we can to ensure that we never need crisis standards of care (National Academies of Sciences, Engineering, and Medicine. 2020 Mar 28. Rapid Expert Consultation on Crisis Standards of Care for the COVID-19 Pandemic. Washington, DC: The National Academies Press.).
We must be prepared. Guidelines and suggestions laid out through decades of experience gained a real-world test in the COVID-19 pandemic. Now we must all reorganize and create new plans or augment old ones with the information we have gained. The time is now. The work must continue.
Dr. Griffin is Assistant Professor of Medicine, New York Presbyterian Hospital – Weill Cornell Medicine. Dr. Dichter is Associate Professor of Medicine, University of Minnesota.
In 2014, the Task Force for Mass Critical Care (TFMCC) published a CHEST consensus statement on disaster preparedness principles in caring for the critically ill during disasters and pandemics (Christian et al. CHEST. 2014;146[4_suppl]:8s-34s). This publication attempted to guide preparedness for both single-event disasters and more prolonged events, including a feared influenza pandemic.
Despite the foundation of planning and support this guidance provided, the COVID-19 pandemic response revealed substantial gaps in our understanding and preparedness for these more prolonged and widespread events.
In New York City, as the first COVID-19 wave began in March and April of 2020, area hospitals responded with surge plans that prioritized what was felt to be most important (Griffin et al. Am J Respir Crit Care Med. 2020 Jun 1;201[11]:1337-44). Tiered, creative staffing structures were rapidly created with intensivists supervising non-ICU physicians and APPs. Procedure teams were created for intubation, proning, and central line placement. ICU space was created with adaptations to ORs and PACUs, and rooms on med-surg floors and step-down units underwent emergency renovations to allow creation of new “pop-up” ICUs. Triage protocols were altered: patients on high levels of supplemental oxygen, who would under normal circumstances have been admitted to an ICU, were triaged to floors and stepdown units. Equipment was reused, modified, and substituted creatively to optimize care for the maximum number of patients.
In the face of all of these struggles, many around the country and the world felt the efforts, though heroic, resulted in less than standard of care. Two subsequent publications validated this concern (Kadri et al. Ann Int Med. 2021,174;9:1240-51; Bravata DM et al. JAMA Open Network. 2021;4[1]:e2034266), demonstrating during severe surge, COVID-19 patients’ mortality increased significantly beyond that seen in non-surging or less-severe surging times, demonstrating a mortality effect of surge itself. Though these studies observed COVID-19 patients only, there is every reason to believe the findings applied to all critically ill patients cared for during these surges.
These experiences led the TFMCC to report updated strategies for remaining in contingency care levels and avoiding crisis care (Dichter JR et al. CHEST. 2022;161[2]:429-47). Contingency is equivalent to routine care though may require adaptations and employment of otherwise non-traditional resources. The ultimate goal of mass critical care in a public health emergency is to avoid crisis-operating conditions, crisis standards of care, and their associated challenging triage decisions regarding allocation of scarce resources.
The 10 suggestions included in the most recent TFMCC publication include staffing strategies and suggestions based on COVID-19 experiences for graded staff-to-patient ratios, and support processes to preserve the existing health care work force. Strategies also include reduction of redundant documentation, limiting overtime, and most importantly, approaches for improving teamwork and supporting psychological well-being and resilience. Examples include daily unit huddles to update care and share experiences, genuine intra-team recognition and appreciation, and embedding emotional health experts within teams to provide ongoing support.
Consistent communication between incident command and frontline clinicians was also a suggested priority, perhaps with a newly proposed position of physician clinical support supervisor. This would be a formal role within hospital incident command, a liaison between the two groups.
Surge strategies should include empowerment of bedside clinicians and leaders with both planning and real-time assessment of the clinical situation, as being at the front line of care enables the situational awareness to assess ICU strain most effectively. Further, ICU clinicians must recognize when progression deeper into contingency operations occurs and they become perilously close to crisis mode. At this point, decisions are made and scarce resources are modified beyond routine standards of care to preserve life. TFMCC designates this gray area between contingency and crisis as the Critical Clinical Prioritization level (Figure).

At this point, more resources must be provided, or patients must be transferred to other resourced hospitals.
Critical Clinical Prioritization is an illustration of necessity being the mother of invention, as these are adaptations clinicians devised under duress. Some particularly poignant examples are the spreading of 24 hours of continuous renal replacement therapy (CRRT) resource between two and sometimes three patients to provide life sustainment to all; and when ventilators were in short supply, determining which patients required full ICU ventilator support vs those who could manage with lower functioning ventilators, and trading them between patients when demands changed.
These adaptations can only be done by experienced clinicians proactively managing bedside critical care under duress, further underscoring the importance of our suggestion that Critical Clinical Prioritization and ICU strain be managed by bedside clinicians and leaders.
The response of early transfer of patients – load-balancing - should be considered as soon as any hospital enters contingency conditions. This strategy is commonly implemented within larger health systems, ideally before reaching Critical Clinical Prioritization. Formal, organized state or regional load-balancing coordination, now referred to as medical operations command centers (MOCCs), were highly effective and proved lifesaving for those states that implemented them (including Arizona, Washington, California, Minnesota, and others). Support for establishment of MOCC’s is crucial in prolonging contingency operations and further helps support and protect disadvantaged populations (White et al. N Engl J Med. 2021;385[24]:2211-4).
Establishment of MOCCs has met resistance due to challenges that include interhospital/intersystem competition, logistics of moving critically ill patients sometimes across significant physical distance, and the costs of assuming care of uninsured or underinsured patients. Nevertheless, the benefits to the population as a whole necessitate working through these obstacles as successful MOCCs have done, usually with government and hospital association support.
In their final suggestion of the 2022 updated strategies, TFMCC suggests that hospitals use telemedicine technology both to expand specialists’ ability to provide care and facilitate families virtually visiting their critically ill loved one when safety precludes in-person visits.
These suggestions are pivotal in planning for future public health emergencies that include mass critical care, even during events that are limited in scope and duration.
Lastly, intensivists struggled with legal and ethical concerns when mired in crisis care circumstances and decisions of allocation, and potential reallocation, of scarce resources. These issues were not well addressed during the COVID-19 pandemic, further emphasizing the importance of maintaining contingency level care and requiring further involvement from legal and medical ethics professionals for future planning.
The guiding principle of disaster preparedness is that we must do all the planning we can to ensure that we never need crisis standards of care (National Academies of Sciences, Engineering, and Medicine. 2020 Mar 28. Rapid Expert Consultation on Crisis Standards of Care for the COVID-19 Pandemic. Washington, DC: The National Academies Press.).
We must be prepared. Guidelines and suggestions laid out through decades of experience gained a real-world test in the COVID-19 pandemic. Now we must all reorganize and create new plans or augment old ones with the information we have gained. The time is now. The work must continue.
Dr. Griffin is Assistant Professor of Medicine, New York Presbyterian Hospital – Weill Cornell Medicine. Dr. Dichter is Associate Professor of Medicine, University of Minnesota.
In 2014, the Task Force for Mass Critical Care (TFMCC) published a CHEST consensus statement on disaster preparedness principles in caring for the critically ill during disasters and pandemics (Christian et al. CHEST. 2014;146[4_suppl]:8s-34s). This publication attempted to guide preparedness for both single-event disasters and more prolonged events, including a feared influenza pandemic.
Despite the foundation of planning and support this guidance provided, the COVID-19 pandemic response revealed substantial gaps in our understanding and preparedness for these more prolonged and widespread events.
In New York City, as the first COVID-19 wave began in March and April of 2020, area hospitals responded with surge plans that prioritized what was felt to be most important (Griffin et al. Am J Respir Crit Care Med. 2020 Jun 1;201[11]:1337-44). Tiered, creative staffing structures were rapidly created with intensivists supervising non-ICU physicians and APPs. Procedure teams were created for intubation, proning, and central line placement. ICU space was created with adaptations to ORs and PACUs, and rooms on med-surg floors and step-down units underwent emergency renovations to allow creation of new “pop-up” ICUs. Triage protocols were altered: patients on high levels of supplemental oxygen, who would under normal circumstances have been admitted to an ICU, were triaged to floors and stepdown units. Equipment was reused, modified, and substituted creatively to optimize care for the maximum number of patients.
In the face of all of these struggles, many around the country and the world felt the efforts, though heroic, resulted in less than standard of care. Two subsequent publications validated this concern (Kadri et al. Ann Int Med. 2021,174;9:1240-51; Bravata DM et al. JAMA Open Network. 2021;4[1]:e2034266), demonstrating during severe surge, COVID-19 patients’ mortality increased significantly beyond that seen in non-surging or less-severe surging times, demonstrating a mortality effect of surge itself. Though these studies observed COVID-19 patients only, there is every reason to believe the findings applied to all critically ill patients cared for during these surges.
These experiences led the TFMCC to report updated strategies for remaining in contingency care levels and avoiding crisis care (Dichter JR et al. CHEST. 2022;161[2]:429-47). Contingency is equivalent to routine care though may require adaptations and employment of otherwise non-traditional resources. The ultimate goal of mass critical care in a public health emergency is to avoid crisis-operating conditions, crisis standards of care, and their associated challenging triage decisions regarding allocation of scarce resources.
The 10 suggestions included in the most recent TFMCC publication include staffing strategies and suggestions based on COVID-19 experiences for graded staff-to-patient ratios, and support processes to preserve the existing health care work force. Strategies also include reduction of redundant documentation, limiting overtime, and most importantly, approaches for improving teamwork and supporting psychological well-being and resilience. Examples include daily unit huddles to update care and share experiences, genuine intra-team recognition and appreciation, and embedding emotional health experts within teams to provide ongoing support.
Consistent communication between incident command and frontline clinicians was also a suggested priority, perhaps with a newly proposed position of physician clinical support supervisor. This would be a formal role within hospital incident command, a liaison between the two groups.
Surge strategies should include empowerment of bedside clinicians and leaders with both planning and real-time assessment of the clinical situation, as being at the front line of care enables the situational awareness to assess ICU strain most effectively. Further, ICU clinicians must recognize when progression deeper into contingency operations occurs and they become perilously close to crisis mode. At this point, decisions are made and scarce resources are modified beyond routine standards of care to preserve life. TFMCC designates this gray area between contingency and crisis as the Critical Clinical Prioritization level (Figure).

At this point, more resources must be provided, or patients must be transferred to other resourced hospitals.
Critical Clinical Prioritization is an illustration of necessity being the mother of invention, as these are adaptations clinicians devised under duress. Some particularly poignant examples are the spreading of 24 hours of continuous renal replacement therapy (CRRT) resource between two and sometimes three patients to provide life sustainment to all; and when ventilators were in short supply, determining which patients required full ICU ventilator support vs those who could manage with lower functioning ventilators, and trading them between patients when demands changed.
These adaptations can only be done by experienced clinicians proactively managing bedside critical care under duress, further underscoring the importance of our suggestion that Critical Clinical Prioritization and ICU strain be managed by bedside clinicians and leaders.
The response of early transfer of patients – load-balancing - should be considered as soon as any hospital enters contingency conditions. This strategy is commonly implemented within larger health systems, ideally before reaching Critical Clinical Prioritization. Formal, organized state or regional load-balancing coordination, now referred to as medical operations command centers (MOCCs), were highly effective and proved lifesaving for those states that implemented them (including Arizona, Washington, California, Minnesota, and others). Support for establishment of MOCC’s is crucial in prolonging contingency operations and further helps support and protect disadvantaged populations (White et al. N Engl J Med. 2021;385[24]:2211-4).
Establishment of MOCCs has met resistance due to challenges that include interhospital/intersystem competition, logistics of moving critically ill patients sometimes across significant physical distance, and the costs of assuming care of uninsured or underinsured patients. Nevertheless, the benefits to the population as a whole necessitate working through these obstacles as successful MOCCs have done, usually with government and hospital association support.
In their final suggestion of the 2022 updated strategies, TFMCC suggests that hospitals use telemedicine technology both to expand specialists’ ability to provide care and facilitate families virtually visiting their critically ill loved one when safety precludes in-person visits.
These suggestions are pivotal in planning for future public health emergencies that include mass critical care, even during events that are limited in scope and duration.
Lastly, intensivists struggled with legal and ethical concerns when mired in crisis care circumstances and decisions of allocation, and potential reallocation, of scarce resources. These issues were not well addressed during the COVID-19 pandemic, further emphasizing the importance of maintaining contingency level care and requiring further involvement from legal and medical ethics professionals for future planning.
The guiding principle of disaster preparedness is that we must do all the planning we can to ensure that we never need crisis standards of care (National Academies of Sciences, Engineering, and Medicine. 2020 Mar 28. Rapid Expert Consultation on Crisis Standards of Care for the COVID-19 Pandemic. Washington, DC: The National Academies Press.).
We must be prepared. Guidelines and suggestions laid out through decades of experience gained a real-world test in the COVID-19 pandemic. Now we must all reorganize and create new plans or augment old ones with the information we have gained. The time is now. The work must continue.
Dr. Griffin is Assistant Professor of Medicine, New York Presbyterian Hospital – Weill Cornell Medicine. Dr. Dichter is Associate Professor of Medicine, University of Minnesota.
Continuous remote patient monitoring
The SARS-CoV-2 pandemic required health care systems around the world to rapidly innovate and adapt to unprecedented operational and clinical strain. Many health care systems leveraged virtual care capabilities as an innovative approach to safely and efficiently manage patients while reducing staff exposure and medical resource constraints (Healthcare [Basel]. 2020 Nov;8[4]:517; JMIR Form Res. 2021 Jan; 5[1]:e23190). With Medicare insurance claims data demonstrating a 30% reduction of in-person health visits, telemedicine has become an essential means to fill the gaps in providing essential medical services (JAMA Intern Med. 2021 Mar;181[3]:388-91). A vast majority of virtual health care visits come via telephonic encounters, which have inherent limitations in the ability to monitor patients with complex or critical medical conditions (Front Public Health. 2020;8:410; N Engl J Med. 2020 Apr;382[18]:1679-81). Remote patient monitoring (RPM) has been established in multiple clinical models as an effective adjunct in telemedicine encounters in order to ensure treatment regimen adherence, make real-time treatment adjustments, and identify patients at risk for early decompensation.
Long-term RPM data has demonstrated cost reduction, reduced burden of in-office visits, expedited management of significant clinical events, and decreased all-cause mortality rates. Previously RPM was limited to the care of patients with chronic conditions, particularly cardiac patients with congestive heart failure and invasive devices, such as pacemakers or implantable cardioverter–defibrillators (JMIR Form Res. 2021 Jan;5[1]:e23190; Front Public Health. 2020; 8:410). In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) added RPM billing codes in 2019 and then included coverage of acute conditions in 2020 that permitted a more extensive role of RPM in telemedicine. This change in financial reimbursement led to a more aggressive expansion of RPM devices to assess physiologic parameters, such as weight, blood pressure, oxygen saturation, and blood glucose levels for clinicians to review.
Currently, RPM devices fall within a low-risk FDA category that do not require clinical trials for validation prior to being cleared for CMS billing in a fee-for-service reimbursement model (N Engl J Med. 2021 Apr;384[15]:1384-6). A shortage of evidence-based publications to guide clinicians in this new landscape creates challenges from underuse, misuse, or abuse of RPM tools. In order to maximize the clinical benefits of RPM, standardized processes and device specifications derived from up-to-date research need to be established in professional society guidelines.
Formalized RPM protocols should play a key role in overcoming the hesitancy of health institutions becoming early adopters of RPM technologies. Some significant challenges leading to reluctance of executing an RPM program were recently highlighted at the REPROGRAM international consortium of telemedicine. These concerns involved building a technological infrastructure, training clinical staff, ensuring remote connectivity with broadband Internet, and working with patients of various technologic literacy (Front Public Health. 2020;8:410). We attempted to address these challenges by using a COVID-19 remote patient monitoring (CRPM) strategy within our Military Health System (MHS). By using the well-established responsible, accountable, consulted, and informed (RACI) matrix process mapping tool, we created a standardized enrollment process of high-risk patients across eight military treatment facilities (MTFs). High risk patients included those with COVID-19 pneumonia and persistent hypoxemia, those recovering from acute exacerbations of congestive heart failure, those with cardiopulmonary instability associated with malignancy, and other conditions that required continuous monitoring outside of the hospital setting.
In our CRPM process, the hospital inpatient unit or ED refer high-risk patients to a primary designated provider at each MTF for enrollment prior to discharge. Enrolled patients are equipped with an FDA-approved home monitoring kit that contains an electronic tablet, a network hub that operates independently of and/or in conjunction with Wi-Fi, and an armband containing a coin-sized monitor. The system has the capability to pair with additional smart-enabled accessories, such as a blood pressure cuff, temperature patch, and digital spirometer. With continuous bio-physiologic and symptom-based monitoring, a team of teleworking critical-care nurses monitor patients continuously. In case of a decompensation necessitating a higher level of care, an emergency action plan (EAP) is activated to ensure patients urgently receive emergency medical services. Once released from the CRPM program, discharged patients use prepaid shipping boxes to facilitate contactless repackaging, sanitization, and pickup for redistribution of devices to the MTF.
Given the increased number of hospital admissions noted during the COVID-19 global pandemic, the CRPM program has allowed us to address overutilization of hospital beds. Furthermore, it has allowed us to address issues of screening and resource utilization as we consider patients for safe implementation of home monitoring. While data concerning the outcome of the CRPM program are pending, we are encouraged about the ability to provide high quality care in a remote setting. To that end, we have addressed technologic difficulties, communication between remote providers and patients in the home environment, and communication between health care providers in various settings, such as the ED, inpatient wards, and the outpatient clinic.
To be sure, there are many challenges in making sure that CRPM adequately addresses the needs of patients, who may have persistent perturbations in cardiopulmonary status, tremendous anxiety about the progress or deterioration in their health status, and lack of understanding about their medical condition. Furthermore, providers face the challenge of making clinical decisions sometimes without the advantage of in-person examinations. Sometimes decisions must be made with incomplete information or when the status of the patient does not follow presupposed algorithms. Nevertheless, like many issues during the COVID-19 pandemic, patients and providers have evolved, pivoted, and made necessary adjustments to address an unprecedented time in recent history.
Ultimately, we believe that a continuous remote patient monitoring program can be designed, implemented, and maintained across a multifacility health care system for safe, effective, and efficient health care delivery. Limitations in implementing such a program might include lack of adequate Internet services, lack of telephonic communication, inadequate home facilities, lack of adequate home support, and, perhaps, lack of available emergency services. However, if the conditions for home monitoring are optimized, CRPM holds the promise of reducing the burden on emergency and inpatient hospital services, particularly when those services are strained in circumstances such as the ongoing global pandemic due to COVID-19. With further study, standardization, and evolution, remote monitoring will likely become a more acceptable and necessary form of health care delivery in the future.
Dr. Salomon is an Internal Medicine Resident (PGY-2); Dr. Muller is an Internal Medicine Resident (PGY-2); Dr. Boster is a Pulmonary and Critical Care Fellow; Dr. Loudermilk is a Pulmonary and Critical Care Fellow; and Dr. Kemp is Pulmonary and Critical Care staff, San Antonio Military Medical Center, Fort Sam Houston, Texas.
The SARS-CoV-2 pandemic required health care systems around the world to rapidly innovate and adapt to unprecedented operational and clinical strain. Many health care systems leveraged virtual care capabilities as an innovative approach to safely and efficiently manage patients while reducing staff exposure and medical resource constraints (Healthcare [Basel]. 2020 Nov;8[4]:517; JMIR Form Res. 2021 Jan; 5[1]:e23190). With Medicare insurance claims data demonstrating a 30% reduction of in-person health visits, telemedicine has become an essential means to fill the gaps in providing essential medical services (JAMA Intern Med. 2021 Mar;181[3]:388-91). A vast majority of virtual health care visits come via telephonic encounters, which have inherent limitations in the ability to monitor patients with complex or critical medical conditions (Front Public Health. 2020;8:410; N Engl J Med. 2020 Apr;382[18]:1679-81). Remote patient monitoring (RPM) has been established in multiple clinical models as an effective adjunct in telemedicine encounters in order to ensure treatment regimen adherence, make real-time treatment adjustments, and identify patients at risk for early decompensation.
Long-term RPM data has demonstrated cost reduction, reduced burden of in-office visits, expedited management of significant clinical events, and decreased all-cause mortality rates. Previously RPM was limited to the care of patients with chronic conditions, particularly cardiac patients with congestive heart failure and invasive devices, such as pacemakers or implantable cardioverter–defibrillators (JMIR Form Res. 2021 Jan;5[1]:e23190; Front Public Health. 2020; 8:410). In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) added RPM billing codes in 2019 and then included coverage of acute conditions in 2020 that permitted a more extensive role of RPM in telemedicine. This change in financial reimbursement led to a more aggressive expansion of RPM devices to assess physiologic parameters, such as weight, blood pressure, oxygen saturation, and blood glucose levels for clinicians to review.
Currently, RPM devices fall within a low-risk FDA category that do not require clinical trials for validation prior to being cleared for CMS billing in a fee-for-service reimbursement model (N Engl J Med. 2021 Apr;384[15]:1384-6). A shortage of evidence-based publications to guide clinicians in this new landscape creates challenges from underuse, misuse, or abuse of RPM tools. In order to maximize the clinical benefits of RPM, standardized processes and device specifications derived from up-to-date research need to be established in professional society guidelines.
Formalized RPM protocols should play a key role in overcoming the hesitancy of health institutions becoming early adopters of RPM technologies. Some significant challenges leading to reluctance of executing an RPM program were recently highlighted at the REPROGRAM international consortium of telemedicine. These concerns involved building a technological infrastructure, training clinical staff, ensuring remote connectivity with broadband Internet, and working with patients of various technologic literacy (Front Public Health. 2020;8:410). We attempted to address these challenges by using a COVID-19 remote patient monitoring (CRPM) strategy within our Military Health System (MHS). By using the well-established responsible, accountable, consulted, and informed (RACI) matrix process mapping tool, we created a standardized enrollment process of high-risk patients across eight military treatment facilities (MTFs). High risk patients included those with COVID-19 pneumonia and persistent hypoxemia, those recovering from acute exacerbations of congestive heart failure, those with cardiopulmonary instability associated with malignancy, and other conditions that required continuous monitoring outside of the hospital setting.
In our CRPM process, the hospital inpatient unit or ED refer high-risk patients to a primary designated provider at each MTF for enrollment prior to discharge. Enrolled patients are equipped with an FDA-approved home monitoring kit that contains an electronic tablet, a network hub that operates independently of and/or in conjunction with Wi-Fi, and an armband containing a coin-sized monitor. The system has the capability to pair with additional smart-enabled accessories, such as a blood pressure cuff, temperature patch, and digital spirometer. With continuous bio-physiologic and symptom-based monitoring, a team of teleworking critical-care nurses monitor patients continuously. In case of a decompensation necessitating a higher level of care, an emergency action plan (EAP) is activated to ensure patients urgently receive emergency medical services. Once released from the CRPM program, discharged patients use prepaid shipping boxes to facilitate contactless repackaging, sanitization, and pickup for redistribution of devices to the MTF.
Given the increased number of hospital admissions noted during the COVID-19 global pandemic, the CRPM program has allowed us to address overutilization of hospital beds. Furthermore, it has allowed us to address issues of screening and resource utilization as we consider patients for safe implementation of home monitoring. While data concerning the outcome of the CRPM program are pending, we are encouraged about the ability to provide high quality care in a remote setting. To that end, we have addressed technologic difficulties, communication between remote providers and patients in the home environment, and communication between health care providers in various settings, such as the ED, inpatient wards, and the outpatient clinic.
To be sure, there are many challenges in making sure that CRPM adequately addresses the needs of patients, who may have persistent perturbations in cardiopulmonary status, tremendous anxiety about the progress or deterioration in their health status, and lack of understanding about their medical condition. Furthermore, providers face the challenge of making clinical decisions sometimes without the advantage of in-person examinations. Sometimes decisions must be made with incomplete information or when the status of the patient does not follow presupposed algorithms. Nevertheless, like many issues during the COVID-19 pandemic, patients and providers have evolved, pivoted, and made necessary adjustments to address an unprecedented time in recent history.
Ultimately, we believe that a continuous remote patient monitoring program can be designed, implemented, and maintained across a multifacility health care system for safe, effective, and efficient health care delivery. Limitations in implementing such a program might include lack of adequate Internet services, lack of telephonic communication, inadequate home facilities, lack of adequate home support, and, perhaps, lack of available emergency services. However, if the conditions for home monitoring are optimized, CRPM holds the promise of reducing the burden on emergency and inpatient hospital services, particularly when those services are strained in circumstances such as the ongoing global pandemic due to COVID-19. With further study, standardization, and evolution, remote monitoring will likely become a more acceptable and necessary form of health care delivery in the future.
Dr. Salomon is an Internal Medicine Resident (PGY-2); Dr. Muller is an Internal Medicine Resident (PGY-2); Dr. Boster is a Pulmonary and Critical Care Fellow; Dr. Loudermilk is a Pulmonary and Critical Care Fellow; and Dr. Kemp is Pulmonary and Critical Care staff, San Antonio Military Medical Center, Fort Sam Houston, Texas.
The SARS-CoV-2 pandemic required health care systems around the world to rapidly innovate and adapt to unprecedented operational and clinical strain. Many health care systems leveraged virtual care capabilities as an innovative approach to safely and efficiently manage patients while reducing staff exposure and medical resource constraints (Healthcare [Basel]. 2020 Nov;8[4]:517; JMIR Form Res. 2021 Jan; 5[1]:e23190). With Medicare insurance claims data demonstrating a 30% reduction of in-person health visits, telemedicine has become an essential means to fill the gaps in providing essential medical services (JAMA Intern Med. 2021 Mar;181[3]:388-91). A vast majority of virtual health care visits come via telephonic encounters, which have inherent limitations in the ability to monitor patients with complex or critical medical conditions (Front Public Health. 2020;8:410; N Engl J Med. 2020 Apr;382[18]:1679-81). Remote patient monitoring (RPM) has been established in multiple clinical models as an effective adjunct in telemedicine encounters in order to ensure treatment regimen adherence, make real-time treatment adjustments, and identify patients at risk for early decompensation.
Long-term RPM data has demonstrated cost reduction, reduced burden of in-office visits, expedited management of significant clinical events, and decreased all-cause mortality rates. Previously RPM was limited to the care of patients with chronic conditions, particularly cardiac patients with congestive heart failure and invasive devices, such as pacemakers or implantable cardioverter–defibrillators (JMIR Form Res. 2021 Jan;5[1]:e23190; Front Public Health. 2020; 8:410). In response to the pandemic, the Centers for Medicare and Medicaid Services (CMS) added RPM billing codes in 2019 and then included coverage of acute conditions in 2020 that permitted a more extensive role of RPM in telemedicine. This change in financial reimbursement led to a more aggressive expansion of RPM devices to assess physiologic parameters, such as weight, blood pressure, oxygen saturation, and blood glucose levels for clinicians to review.
Currently, RPM devices fall within a low-risk FDA category that do not require clinical trials for validation prior to being cleared for CMS billing in a fee-for-service reimbursement model (N Engl J Med. 2021 Apr;384[15]:1384-6). A shortage of evidence-based publications to guide clinicians in this new landscape creates challenges from underuse, misuse, or abuse of RPM tools. In order to maximize the clinical benefits of RPM, standardized processes and device specifications derived from up-to-date research need to be established in professional society guidelines.
Formalized RPM protocols should play a key role in overcoming the hesitancy of health institutions becoming early adopters of RPM technologies. Some significant challenges leading to reluctance of executing an RPM program were recently highlighted at the REPROGRAM international consortium of telemedicine. These concerns involved building a technological infrastructure, training clinical staff, ensuring remote connectivity with broadband Internet, and working with patients of various technologic literacy (Front Public Health. 2020;8:410). We attempted to address these challenges by using a COVID-19 remote patient monitoring (CRPM) strategy within our Military Health System (MHS). By using the well-established responsible, accountable, consulted, and informed (RACI) matrix process mapping tool, we created a standardized enrollment process of high-risk patients across eight military treatment facilities (MTFs). High risk patients included those with COVID-19 pneumonia and persistent hypoxemia, those recovering from acute exacerbations of congestive heart failure, those with cardiopulmonary instability associated with malignancy, and other conditions that required continuous monitoring outside of the hospital setting.
In our CRPM process, the hospital inpatient unit or ED refer high-risk patients to a primary designated provider at each MTF for enrollment prior to discharge. Enrolled patients are equipped with an FDA-approved home monitoring kit that contains an electronic tablet, a network hub that operates independently of and/or in conjunction with Wi-Fi, and an armband containing a coin-sized monitor. The system has the capability to pair with additional smart-enabled accessories, such as a blood pressure cuff, temperature patch, and digital spirometer. With continuous bio-physiologic and symptom-based monitoring, a team of teleworking critical-care nurses monitor patients continuously. In case of a decompensation necessitating a higher level of care, an emergency action plan (EAP) is activated to ensure patients urgently receive emergency medical services. Once released from the CRPM program, discharged patients use prepaid shipping boxes to facilitate contactless repackaging, sanitization, and pickup for redistribution of devices to the MTF.
Given the increased number of hospital admissions noted during the COVID-19 global pandemic, the CRPM program has allowed us to address overutilization of hospital beds. Furthermore, it has allowed us to address issues of screening and resource utilization as we consider patients for safe implementation of home monitoring. While data concerning the outcome of the CRPM program are pending, we are encouraged about the ability to provide high quality care in a remote setting. To that end, we have addressed technologic difficulties, communication between remote providers and patients in the home environment, and communication between health care providers in various settings, such as the ED, inpatient wards, and the outpatient clinic.
To be sure, there are many challenges in making sure that CRPM adequately addresses the needs of patients, who may have persistent perturbations in cardiopulmonary status, tremendous anxiety about the progress or deterioration in their health status, and lack of understanding about their medical condition. Furthermore, providers face the challenge of making clinical decisions sometimes without the advantage of in-person examinations. Sometimes decisions must be made with incomplete information or when the status of the patient does not follow presupposed algorithms. Nevertheless, like many issues during the COVID-19 pandemic, patients and providers have evolved, pivoted, and made necessary adjustments to address an unprecedented time in recent history.
Ultimately, we believe that a continuous remote patient monitoring program can be designed, implemented, and maintained across a multifacility health care system for safe, effective, and efficient health care delivery. Limitations in implementing such a program might include lack of adequate Internet services, lack of telephonic communication, inadequate home facilities, lack of adequate home support, and, perhaps, lack of available emergency services. However, if the conditions for home monitoring are optimized, CRPM holds the promise of reducing the burden on emergency and inpatient hospital services, particularly when those services are strained in circumstances such as the ongoing global pandemic due to COVID-19. With further study, standardization, and evolution, remote monitoring will likely become a more acceptable and necessary form of health care delivery in the future.
Dr. Salomon is an Internal Medicine Resident (PGY-2); Dr. Muller is an Internal Medicine Resident (PGY-2); Dr. Boster is a Pulmonary and Critical Care Fellow; Dr. Loudermilk is a Pulmonary and Critical Care Fellow; and Dr. Kemp is Pulmonary and Critical Care staff, San Antonio Military Medical Center, Fort Sam Houston, Texas.
Decreasing the burden of postacute sequelae of SARS-CoV-2 infection: What we know
On March 11, 2020, the World Health Organization (WHO) declared SARS-CoV-2 a pandemic. As of October 2021, there are over 240 million confirmed COVID-19 cases and over 4 million deaths globally, with the United States having the highest incidence of both cases and deaths (https://covid.cdc.gov/covid-data-tracker/#datatracker-home). As many as 87% of COVID-19 survivors experience persistent symptoms that last beyond the acute phase of illness (Carfi A, et al. JAMA. 2020;324[6]:603-5). In February 2021, the National Institutes of Health (NIH) called for a consensus term to describe this protracted form of COVID-19, and defined it as Post-acute Sequelae of SARS-CoV-2 infection (PASC) (https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid).
What are the PASC manifestations?
PASC has a heterogeneous presentation with a broad spectrum of manifestations and can vary from single to multiorgan system involvement. Commonly, PASC involves pulmonary abnormalities (shortness of breath, exercise intolerance, abnormal pulmonary functional test [PFT] and chest imaging), neurocognitive impairments (difficulty concentrating and memory loss), mental health disorders (anxiety, depression, and post-traumatic stress disorder), functional mobility impairments, as well as general and constitutional symptoms (fatigue and muscle weakness) (Groff D, et al. JAMA Netw Open. 2021;4[10]). The most prevalent pulmonary physiologic impairment is reduced diffusion capacity that has been shown to be associated with the severity of acute illness, while the most common radiologic abnormalities on chest CT scan are ground glass opacities. Some studies have shown a temporal improvement in pulmonary physiology and exercise capacity; however, persistent physiological and radiographic abnormalities persist in some patients up to 12 months after discharge (Wu X, et al. Lancet Respir Med. 2021;9:747-54). An abnormal or persistent hyper-inflammatory state, viral-induced autoimmune reaction, and ongoing viral activity have been proposed as possible biological mechanisms for PASC; however, the pathophysiology remains mostly unknown.
Who does PASC affect?
PASC affects patients irrespective of premorbid condition and severity of symptoms in the acute phase. It spans from those who had mild disease not requiring hospitalization to those who had critical illness requiring intensive care unit (ICU) management. COVID-19 ICU survivors seem to have an overlap of PASC and post-intensive care syndrome (PICS), defined by new or worsening physical, cognitive, and/or psychiatric impairments after critical illness. (Biehl M, et al. Cleve Clin J Med. 2020 Aug 5).
Who do we evaluate for PASC?
Given the complexity and chronicity of the associated symptoms and their impact on several major organ systems, a comprehensive and multidisciplinary approach is essential to assist with diagnosis and management of PASC. Listening empathically to patients and acknowledging their symptoms are key factors. Access to ambulatory care, establishment of rapport, effective collaboration and coordination of care among different disciplines, management of comorbidities, continuity of care, access to rehabilitation programs, and reduction of disease burden are some of the principles that guided the creation of dedicated COVID-19 clinics throughout the world. The most common services offered are primary care, pulmonology, cardiology, mental health, neurology, speech and language pathology, physical and occupational therapy, pharmacy, and case management. The involvement of specialties varies depending on the specific patient’s needs (Parker A, et al. The Lancet Respir Med. 2021;S2213-2600[21]00385-4).
The development of diagnostic and care pathways by different specialties ensures standardization of clinical assessment and management while allowing for individualized care. The commonly used tools to assess the respiratory system are the 6-minute walk test, PFT, chest imaging including radiographs and high-resolution CT scan, ventilation perfusion scan, and echocardiography. Some patients exhibit persistent cardiopulmonary symptoms with no evidence of organ injury. These patients have persistent exertional and functional limitation with normal PFT, resting echocardiography, and chest imaging. Cardiopulmonary exercise testing (CPET) and, more specifically, invasive CPET can be used to further investigate the decreased exercise capacity. CPET studies have identified an augmented exercise hyperventilation, and the causes of exercise limitation varied from anemia and reduced oxygen extraction by peripheral muscles to deconditioning, obesity, and lower ventilatory efficiency. A study looking at invasive CPET showed reduced peak exercise aerobic capacity in post COVID-19 patients compared with control participants and was associated with impaired systemic oxygen extraction and an exaggerated hyperventilatory response (Singh, et al. Chest. 2021;S0012-3692[21]03635). A subset of COVID-19 survivors presents with symptoms of autonomic dysfunction such as orthostatic intolerance and postural orthostatic tachycardia. These symptoms have been reported after other viral infections and could be secondary to gastrointestinal fluid loss, prolonged bed rest, and deconditioning of the cardiovascular system. More research is needed to characterize the dysautonomia in patients post–COVID-19.
What is the treatment?
Therapies depend on symptoms and organ involvement. The duration of pulmonary symptoms in long-haulers is not yet known, with cough and exercise intolerance/dyspnea ranking among the most common complaints in these patients. Exercise therapy plays an essential part in the rehabilitation of long-haulers and several studies are underway to assess different exercise and rehabilitation programs. For most patients with normal laboratory, physiologic, and imaging tests, post–COVID-19 clinics are offering physical therapy, occupational therapy, and neuropsychological rehabilitation. While steroids have been shown to improve mortality in hospitalized patients with COVID-19 requiring mechanical ventilation or supplemental oxygen, their role in outpatient COVID-19 infections and for post–COVID-19 lung disease/organizing pneumonia remains unclear. In a UK study of patients admitted to the hospital with COVID-19 disease of varying severity, interstitial abnormalities were noted in ~5% of patients at 6 weeks postdischarge and in 10.8% of patients with persistent respiratory symptoms (Myall, et al. Ann Am Thorac Soc. 2021;18[5]:799). The most common radiological findings (in > 50% of cases) were consistent with organizing pneumonia. Patients with persistent physiological abnormalities and interstitial findings improved with steroids. However, since the trajectory of the disease is unknown, further studies are required to understand the natural history of the disease and assess treatment strategies in patients with persistent inflammatory lung changes. Several studies looking at systemic or inhaled steroids in different phases of COVID-19 infection and varying disease severity are ongoing (ClinicalTrials.gov). Antifibrotics used to treat idiopathic pulmonary fibrosis and progressive fibrotic ILD are also being investigated in COVID-19 lung disease. The rationale for their use is to treat and prevent severe COVID-19 lung injury and prevent lung fibrosis.
The role of vaccinations
Whether patients who were infected with COVID-19, and, more specifically, patients with long-term symptoms post-COVID-19, should get vaccinated is actively being investigated. Vaccinations are protective at preventing infections and severe illness. Studies showed that patients who had COVID-19 infection and got vaccinated had a significantly higher antibody response than previously uninfected vaccine recipients. A review showed that the protective effect of prior SARS-CoV-2 infection on reinfection is high and similar to that of vaccination. However, a recent study of hospitalized patients revealed higher rates of COVID-19 among unvaccinated adults with previous infection compared with vaccinated adults (http://dx.doi.org/10.15585/mmwr.mm7044e1). On the other hand, the impact of vaccine on long-hauler symptoms has raised interest. A UK survey (not peer reviewed) on more than 800 long-haulers reported about 57% with overall improvement in their symptoms, 24% no change, and 19% with worsening symptoms after their first dose of vaccine, suggesting that the chances of experiencing an overall worsening of symptoms after vaccination is small, with more than half experiencing improvement (go.nature.com/3yfqem2). While awaiting longitudinal trials, the main argument to guide vaccination in long-haulers is that COVID-19 vaccinations provide protection from reinfection and appear to have the potential to improve symptoms.
The availability of a patient’s support system, peer support, and patient advocacy groups assist in providing equitable care and are critical in sustaining the recovery of COVID-19 survivors. Providing social, financial, and cultural support is imperative in decreasing the burden of COVID-19. The dedicated post–COVID-19 clinics will not only offer care to COVID-19 survivors, but will also help our understanding of the determinants and course of PASC, and will provide opportunities for research. Long-term longitudinal observational studies and clinical trials are critical to identify those at high risk for PASC, clarify the extent of health consequences attributable to COVID-19, and define best practices for COVID-19 survivors.
Dr. Biehl is Staff Physician, Pulmonary & Critical Care Medicine, Director, Post-ICU Recovery Clinic Respiratory Institute, Cleveland Clinic; Dr.Farha is with Respiratory and Lerner Institutes, Cleveland Clinic.
On March 11, 2020, the World Health Organization (WHO) declared SARS-CoV-2 a pandemic. As of October 2021, there are over 240 million confirmed COVID-19 cases and over 4 million deaths globally, with the United States having the highest incidence of both cases and deaths (https://covid.cdc.gov/covid-data-tracker/#datatracker-home). As many as 87% of COVID-19 survivors experience persistent symptoms that last beyond the acute phase of illness (Carfi A, et al. JAMA. 2020;324[6]:603-5). In February 2021, the National Institutes of Health (NIH) called for a consensus term to describe this protracted form of COVID-19, and defined it as Post-acute Sequelae of SARS-CoV-2 infection (PASC) (https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid).
What are the PASC manifestations?
PASC has a heterogeneous presentation with a broad spectrum of manifestations and can vary from single to multiorgan system involvement. Commonly, PASC involves pulmonary abnormalities (shortness of breath, exercise intolerance, abnormal pulmonary functional test [PFT] and chest imaging), neurocognitive impairments (difficulty concentrating and memory loss), mental health disorders (anxiety, depression, and post-traumatic stress disorder), functional mobility impairments, as well as general and constitutional symptoms (fatigue and muscle weakness) (Groff D, et al. JAMA Netw Open. 2021;4[10]). The most prevalent pulmonary physiologic impairment is reduced diffusion capacity that has been shown to be associated with the severity of acute illness, while the most common radiologic abnormalities on chest CT scan are ground glass opacities. Some studies have shown a temporal improvement in pulmonary physiology and exercise capacity; however, persistent physiological and radiographic abnormalities persist in some patients up to 12 months after discharge (Wu X, et al. Lancet Respir Med. 2021;9:747-54). An abnormal or persistent hyper-inflammatory state, viral-induced autoimmune reaction, and ongoing viral activity have been proposed as possible biological mechanisms for PASC; however, the pathophysiology remains mostly unknown.
Who does PASC affect?
PASC affects patients irrespective of premorbid condition and severity of symptoms in the acute phase. It spans from those who had mild disease not requiring hospitalization to those who had critical illness requiring intensive care unit (ICU) management. COVID-19 ICU survivors seem to have an overlap of PASC and post-intensive care syndrome (PICS), defined by new or worsening physical, cognitive, and/or psychiatric impairments after critical illness. (Biehl M, et al. Cleve Clin J Med. 2020 Aug 5).
Who do we evaluate for PASC?
Given the complexity and chronicity of the associated symptoms and their impact on several major organ systems, a comprehensive and multidisciplinary approach is essential to assist with diagnosis and management of PASC. Listening empathically to patients and acknowledging their symptoms are key factors. Access to ambulatory care, establishment of rapport, effective collaboration and coordination of care among different disciplines, management of comorbidities, continuity of care, access to rehabilitation programs, and reduction of disease burden are some of the principles that guided the creation of dedicated COVID-19 clinics throughout the world. The most common services offered are primary care, pulmonology, cardiology, mental health, neurology, speech and language pathology, physical and occupational therapy, pharmacy, and case management. The involvement of specialties varies depending on the specific patient’s needs (Parker A, et al. The Lancet Respir Med. 2021;S2213-2600[21]00385-4).
The development of diagnostic and care pathways by different specialties ensures standardization of clinical assessment and management while allowing for individualized care. The commonly used tools to assess the respiratory system are the 6-minute walk test, PFT, chest imaging including radiographs and high-resolution CT scan, ventilation perfusion scan, and echocardiography. Some patients exhibit persistent cardiopulmonary symptoms with no evidence of organ injury. These patients have persistent exertional and functional limitation with normal PFT, resting echocardiography, and chest imaging. Cardiopulmonary exercise testing (CPET) and, more specifically, invasive CPET can be used to further investigate the decreased exercise capacity. CPET studies have identified an augmented exercise hyperventilation, and the causes of exercise limitation varied from anemia and reduced oxygen extraction by peripheral muscles to deconditioning, obesity, and lower ventilatory efficiency. A study looking at invasive CPET showed reduced peak exercise aerobic capacity in post COVID-19 patients compared with control participants and was associated with impaired systemic oxygen extraction and an exaggerated hyperventilatory response (Singh, et al. Chest. 2021;S0012-3692[21]03635). A subset of COVID-19 survivors presents with symptoms of autonomic dysfunction such as orthostatic intolerance and postural orthostatic tachycardia. These symptoms have been reported after other viral infections and could be secondary to gastrointestinal fluid loss, prolonged bed rest, and deconditioning of the cardiovascular system. More research is needed to characterize the dysautonomia in patients post–COVID-19.
What is the treatment?
Therapies depend on symptoms and organ involvement. The duration of pulmonary symptoms in long-haulers is not yet known, with cough and exercise intolerance/dyspnea ranking among the most common complaints in these patients. Exercise therapy plays an essential part in the rehabilitation of long-haulers and several studies are underway to assess different exercise and rehabilitation programs. For most patients with normal laboratory, physiologic, and imaging tests, post–COVID-19 clinics are offering physical therapy, occupational therapy, and neuropsychological rehabilitation. While steroids have been shown to improve mortality in hospitalized patients with COVID-19 requiring mechanical ventilation or supplemental oxygen, their role in outpatient COVID-19 infections and for post–COVID-19 lung disease/organizing pneumonia remains unclear. In a UK study of patients admitted to the hospital with COVID-19 disease of varying severity, interstitial abnormalities were noted in ~5% of patients at 6 weeks postdischarge and in 10.8% of patients with persistent respiratory symptoms (Myall, et al. Ann Am Thorac Soc. 2021;18[5]:799). The most common radiological findings (in > 50% of cases) were consistent with organizing pneumonia. Patients with persistent physiological abnormalities and interstitial findings improved with steroids. However, since the trajectory of the disease is unknown, further studies are required to understand the natural history of the disease and assess treatment strategies in patients with persistent inflammatory lung changes. Several studies looking at systemic or inhaled steroids in different phases of COVID-19 infection and varying disease severity are ongoing (ClinicalTrials.gov). Antifibrotics used to treat idiopathic pulmonary fibrosis and progressive fibrotic ILD are also being investigated in COVID-19 lung disease. The rationale for their use is to treat and prevent severe COVID-19 lung injury and prevent lung fibrosis.
The role of vaccinations
Whether patients who were infected with COVID-19, and, more specifically, patients with long-term symptoms post-COVID-19, should get vaccinated is actively being investigated. Vaccinations are protective at preventing infections and severe illness. Studies showed that patients who had COVID-19 infection and got vaccinated had a significantly higher antibody response than previously uninfected vaccine recipients. A review showed that the protective effect of prior SARS-CoV-2 infection on reinfection is high and similar to that of vaccination. However, a recent study of hospitalized patients revealed higher rates of COVID-19 among unvaccinated adults with previous infection compared with vaccinated adults (http://dx.doi.org/10.15585/mmwr.mm7044e1). On the other hand, the impact of vaccine on long-hauler symptoms has raised interest. A UK survey (not peer reviewed) on more than 800 long-haulers reported about 57% with overall improvement in their symptoms, 24% no change, and 19% with worsening symptoms after their first dose of vaccine, suggesting that the chances of experiencing an overall worsening of symptoms after vaccination is small, with more than half experiencing improvement (go.nature.com/3yfqem2). While awaiting longitudinal trials, the main argument to guide vaccination in long-haulers is that COVID-19 vaccinations provide protection from reinfection and appear to have the potential to improve symptoms.
The availability of a patient’s support system, peer support, and patient advocacy groups assist in providing equitable care and are critical in sustaining the recovery of COVID-19 survivors. Providing social, financial, and cultural support is imperative in decreasing the burden of COVID-19. The dedicated post–COVID-19 clinics will not only offer care to COVID-19 survivors, but will also help our understanding of the determinants and course of PASC, and will provide opportunities for research. Long-term longitudinal observational studies and clinical trials are critical to identify those at high risk for PASC, clarify the extent of health consequences attributable to COVID-19, and define best practices for COVID-19 survivors.
Dr. Biehl is Staff Physician, Pulmonary & Critical Care Medicine, Director, Post-ICU Recovery Clinic Respiratory Institute, Cleveland Clinic; Dr.Farha is with Respiratory and Lerner Institutes, Cleveland Clinic.
On March 11, 2020, the World Health Organization (WHO) declared SARS-CoV-2 a pandemic. As of October 2021, there are over 240 million confirmed COVID-19 cases and over 4 million deaths globally, with the United States having the highest incidence of both cases and deaths (https://covid.cdc.gov/covid-data-tracker/#datatracker-home). As many as 87% of COVID-19 survivors experience persistent symptoms that last beyond the acute phase of illness (Carfi A, et al. JAMA. 2020;324[6]:603-5). In February 2021, the National Institutes of Health (NIH) called for a consensus term to describe this protracted form of COVID-19, and defined it as Post-acute Sequelae of SARS-CoV-2 infection (PASC) (https://www.nih.gov/about-nih/who-we-are/nih-director/statements/nih-launches-new-initiative-study-long-covid).
What are the PASC manifestations?
PASC has a heterogeneous presentation with a broad spectrum of manifestations and can vary from single to multiorgan system involvement. Commonly, PASC involves pulmonary abnormalities (shortness of breath, exercise intolerance, abnormal pulmonary functional test [PFT] and chest imaging), neurocognitive impairments (difficulty concentrating and memory loss), mental health disorders (anxiety, depression, and post-traumatic stress disorder), functional mobility impairments, as well as general and constitutional symptoms (fatigue and muscle weakness) (Groff D, et al. JAMA Netw Open. 2021;4[10]). The most prevalent pulmonary physiologic impairment is reduced diffusion capacity that has been shown to be associated with the severity of acute illness, while the most common radiologic abnormalities on chest CT scan are ground glass opacities. Some studies have shown a temporal improvement in pulmonary physiology and exercise capacity; however, persistent physiological and radiographic abnormalities persist in some patients up to 12 months after discharge (Wu X, et al. Lancet Respir Med. 2021;9:747-54). An abnormal or persistent hyper-inflammatory state, viral-induced autoimmune reaction, and ongoing viral activity have been proposed as possible biological mechanisms for PASC; however, the pathophysiology remains mostly unknown.
Who does PASC affect?
PASC affects patients irrespective of premorbid condition and severity of symptoms in the acute phase. It spans from those who had mild disease not requiring hospitalization to those who had critical illness requiring intensive care unit (ICU) management. COVID-19 ICU survivors seem to have an overlap of PASC and post-intensive care syndrome (PICS), defined by new or worsening physical, cognitive, and/or psychiatric impairments after critical illness. (Biehl M, et al. Cleve Clin J Med. 2020 Aug 5).
Who do we evaluate for PASC?
Given the complexity and chronicity of the associated symptoms and their impact on several major organ systems, a comprehensive and multidisciplinary approach is essential to assist with diagnosis and management of PASC. Listening empathically to patients and acknowledging their symptoms are key factors. Access to ambulatory care, establishment of rapport, effective collaboration and coordination of care among different disciplines, management of comorbidities, continuity of care, access to rehabilitation programs, and reduction of disease burden are some of the principles that guided the creation of dedicated COVID-19 clinics throughout the world. The most common services offered are primary care, pulmonology, cardiology, mental health, neurology, speech and language pathology, physical and occupational therapy, pharmacy, and case management. The involvement of specialties varies depending on the specific patient’s needs (Parker A, et al. The Lancet Respir Med. 2021;S2213-2600[21]00385-4).
The development of diagnostic and care pathways by different specialties ensures standardization of clinical assessment and management while allowing for individualized care. The commonly used tools to assess the respiratory system are the 6-minute walk test, PFT, chest imaging including radiographs and high-resolution CT scan, ventilation perfusion scan, and echocardiography. Some patients exhibit persistent cardiopulmonary symptoms with no evidence of organ injury. These patients have persistent exertional and functional limitation with normal PFT, resting echocardiography, and chest imaging. Cardiopulmonary exercise testing (CPET) and, more specifically, invasive CPET can be used to further investigate the decreased exercise capacity. CPET studies have identified an augmented exercise hyperventilation, and the causes of exercise limitation varied from anemia and reduced oxygen extraction by peripheral muscles to deconditioning, obesity, and lower ventilatory efficiency. A study looking at invasive CPET showed reduced peak exercise aerobic capacity in post COVID-19 patients compared with control participants and was associated with impaired systemic oxygen extraction and an exaggerated hyperventilatory response (Singh, et al. Chest. 2021;S0012-3692[21]03635). A subset of COVID-19 survivors presents with symptoms of autonomic dysfunction such as orthostatic intolerance and postural orthostatic tachycardia. These symptoms have been reported after other viral infections and could be secondary to gastrointestinal fluid loss, prolonged bed rest, and deconditioning of the cardiovascular system. More research is needed to characterize the dysautonomia in patients post–COVID-19.
What is the treatment?
Therapies depend on symptoms and organ involvement. The duration of pulmonary symptoms in long-haulers is not yet known, with cough and exercise intolerance/dyspnea ranking among the most common complaints in these patients. Exercise therapy plays an essential part in the rehabilitation of long-haulers and several studies are underway to assess different exercise and rehabilitation programs. For most patients with normal laboratory, physiologic, and imaging tests, post–COVID-19 clinics are offering physical therapy, occupational therapy, and neuropsychological rehabilitation. While steroids have been shown to improve mortality in hospitalized patients with COVID-19 requiring mechanical ventilation or supplemental oxygen, their role in outpatient COVID-19 infections and for post–COVID-19 lung disease/organizing pneumonia remains unclear. In a UK study of patients admitted to the hospital with COVID-19 disease of varying severity, interstitial abnormalities were noted in ~5% of patients at 6 weeks postdischarge and in 10.8% of patients with persistent respiratory symptoms (Myall, et al. Ann Am Thorac Soc. 2021;18[5]:799). The most common radiological findings (in > 50% of cases) were consistent with organizing pneumonia. Patients with persistent physiological abnormalities and interstitial findings improved with steroids. However, since the trajectory of the disease is unknown, further studies are required to understand the natural history of the disease and assess treatment strategies in patients with persistent inflammatory lung changes. Several studies looking at systemic or inhaled steroids in different phases of COVID-19 infection and varying disease severity are ongoing (ClinicalTrials.gov). Antifibrotics used to treat idiopathic pulmonary fibrosis and progressive fibrotic ILD are also being investigated in COVID-19 lung disease. The rationale for their use is to treat and prevent severe COVID-19 lung injury and prevent lung fibrosis.
The role of vaccinations
Whether patients who were infected with COVID-19, and, more specifically, patients with long-term symptoms post-COVID-19, should get vaccinated is actively being investigated. Vaccinations are protective at preventing infections and severe illness. Studies showed that patients who had COVID-19 infection and got vaccinated had a significantly higher antibody response than previously uninfected vaccine recipients. A review showed that the protective effect of prior SARS-CoV-2 infection on reinfection is high and similar to that of vaccination. However, a recent study of hospitalized patients revealed higher rates of COVID-19 among unvaccinated adults with previous infection compared with vaccinated adults (http://dx.doi.org/10.15585/mmwr.mm7044e1). On the other hand, the impact of vaccine on long-hauler symptoms has raised interest. A UK survey (not peer reviewed) on more than 800 long-haulers reported about 57% with overall improvement in their symptoms, 24% no change, and 19% with worsening symptoms after their first dose of vaccine, suggesting that the chances of experiencing an overall worsening of symptoms after vaccination is small, with more than half experiencing improvement (go.nature.com/3yfqem2). While awaiting longitudinal trials, the main argument to guide vaccination in long-haulers is that COVID-19 vaccinations provide protection from reinfection and appear to have the potential to improve symptoms.
The availability of a patient’s support system, peer support, and patient advocacy groups assist in providing equitable care and are critical in sustaining the recovery of COVID-19 survivors. Providing social, financial, and cultural support is imperative in decreasing the burden of COVID-19. The dedicated post–COVID-19 clinics will not only offer care to COVID-19 survivors, but will also help our understanding of the determinants and course of PASC, and will provide opportunities for research. Long-term longitudinal observational studies and clinical trials are critical to identify those at high risk for PASC, clarify the extent of health consequences attributable to COVID-19, and define best practices for COVID-19 survivors.
Dr. Biehl is Staff Physician, Pulmonary & Critical Care Medicine, Director, Post-ICU Recovery Clinic Respiratory Institute, Cleveland Clinic; Dr.Farha is with Respiratory and Lerner Institutes, Cleveland Clinic.



















