User login
Wireless endocardial left ventricular stimulation for CRT shows promise
SAN FRANCISCO – Wireless endocardial left ventricular pacing provides an alternative approach to cardiac resynchronization pacing in heart failure patients, according to preliminary results from an ongoing multicenter trial.
At the annual scientific sessions of the Heart Rhythm Society, Dr. Vivek Y. Reddy presented preliminary results from 19 patients enrolled in the SELECT-LV (Safety and Performance of Electrodes Implanted in the Left Ventricle) study. The purpose of the open-label trial is to evaluate the safety and feasibility of leadless, ultrasound-based pacing using a wireless cardiac stimulation system (WiCS-LV) developed by EBR Systems.
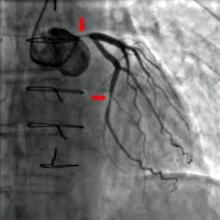
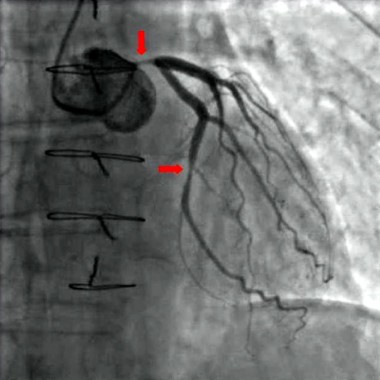
"The idea is to use an existing standard ICD [implantable cardioverter defibrillator] or pacemaker and place this device, which has a transmitter as well as a battery, in a subcutaneous location, and then place a receiver-electrode ‘pellet’ on the left ventricle endocardial wall," explained Dr. Reddy, professor of medicine at Mount Sinai Hospital, New York. "The idea is that the transmitter emits ultrasound impulses detected by the pellet. In turn, the pellet transduces the ultrasound impulse into an electrical pacing pulse to stimulate the heart."
The battery, which is 42 cc in size, is placed subcutaneously in the subaxillary region. The transmitter, which is 13 cc in size, is placed between the ribs "in an optimal position so that there is an echo window which allows you to see the left ventricle," Dr. Reddy said. "Then, on the same day or the next day, the pellet (which is 0.05 cc in size) is placed on the left ventricle via a transfemoral catheter approach."
The SELECT-LV investigators intend to enroll 40 patients at seven centers to evaluate the performance and efficacy of the approach. The primary endpoints are device- and procedure-related complications perioperatively and at 1 month, and biventricular pacing capture on 12-lead ECG at 1 month. Secondary endpoints are device-related or major complications up to 6 months and LV pacing capture at 1, 2, and 6 months, as well as biventricular capture at 6 months on 12-lead ECG. Preliminary efficacy is measured by a composite score of all-cause mortality, heart failure hospitalization, New York Heart Association (NYHA) class, and patient global assessment at 6 months, as well as LV end systolic/diastolic volumes and LV ejection fraction at 6 months.
Patients are eligible for the trial if they have a failed implant of a coronary sinus lead or a chronic issue with their CS lead; if they have no clinical status improvement in 6 months of cardiac resynchronization pacing (CRT); or if they have a previously implanted pacemaker or ICD with a new indication for biventricular pacing but are at risk for a CRT upgrade due to venous occlusion or other factors.
Of the 19 patients who have been implanted to date, 16 (84%) were male, their mean age was 68 years, and they were relatively evenly split between ischemic and nonischemic substrates. Nearly half (47%) had a failed CS lead implant and their mean LV ejection fraction at baseline was 26%.
Primary and secondary endpoint data were available for 15 of the 19 patients, and 6-month data were available for 8 patients. At 1 month, all 15 patients demonstrated Bi-V pacing on 12-lead ECG. The QRS narrowed by 46 ms at 1 month and by 23 ms at 6 months. NYHA class also significantly improved between baseline and 6 months (from II to I; decline of 0.63 points). All patients experienced improvements on their clinical composite score.
No periprocedural adverse events and no device-related serious adverse events occurred within the first month of implant, but 10 serious adverse events occurred in six patients within the first 6 months, including one patient with a hematoma at the transmitter pocket and one patient who had a stroke 3 days after the procedure. "This is a patient who had atrial fibrillation," Dr. Reddy noted. "At the time of the procedure the [warfarin] was stopped. The patient was implanted, did well, but the [warfarin] hadn’t been restarted at the 3-day time point and the patient suffered a stroke. After this experience, we altered the protocol so that patients on anticoagulation for any indication need to continue active coagulation [with no interruption]. We’ll see how that fares."
The study was funded by EBR Systems. Dr. Reddy is a consultant to the company.
On Twitter @dougbrunk
SAN FRANCISCO – Wireless endocardial left ventricular pacing provides an alternative approach to cardiac resynchronization pacing in heart failure patients, according to preliminary results from an ongoing multicenter trial.
At the annual scientific sessions of the Heart Rhythm Society, Dr. Vivek Y. Reddy presented preliminary results from 19 patients enrolled in the SELECT-LV (Safety and Performance of Electrodes Implanted in the Left Ventricle) study. The purpose of the open-label trial is to evaluate the safety and feasibility of leadless, ultrasound-based pacing using a wireless cardiac stimulation system (WiCS-LV) developed by EBR Systems.
"The idea is to use an existing standard ICD [implantable cardioverter defibrillator] or pacemaker and place this device, which has a transmitter as well as a battery, in a subcutaneous location, and then place a receiver-electrode ‘pellet’ on the left ventricle endocardial wall," explained Dr. Reddy, professor of medicine at Mount Sinai Hospital, New York. "The idea is that the transmitter emits ultrasound impulses detected by the pellet. In turn, the pellet transduces the ultrasound impulse into an electrical pacing pulse to stimulate the heart."
The battery, which is 42 cc in size, is placed subcutaneously in the subaxillary region. The transmitter, which is 13 cc in size, is placed between the ribs "in an optimal position so that there is an echo window which allows you to see the left ventricle," Dr. Reddy said. "Then, on the same day or the next day, the pellet (which is 0.05 cc in size) is placed on the left ventricle via a transfemoral catheter approach."
The SELECT-LV investigators intend to enroll 40 patients at seven centers to evaluate the performance and efficacy of the approach. The primary endpoints are device- and procedure-related complications perioperatively and at 1 month, and biventricular pacing capture on 12-lead ECG at 1 month. Secondary endpoints are device-related or major complications up to 6 months and LV pacing capture at 1, 2, and 6 months, as well as biventricular capture at 6 months on 12-lead ECG. Preliminary efficacy is measured by a composite score of all-cause mortality, heart failure hospitalization, New York Heart Association (NYHA) class, and patient global assessment at 6 months, as well as LV end systolic/diastolic volumes and LV ejection fraction at 6 months.
Patients are eligible for the trial if they have a failed implant of a coronary sinus lead or a chronic issue with their CS lead; if they have no clinical status improvement in 6 months of cardiac resynchronization pacing (CRT); or if they have a previously implanted pacemaker or ICD with a new indication for biventricular pacing but are at risk for a CRT upgrade due to venous occlusion or other factors.
Of the 19 patients who have been implanted to date, 16 (84%) were male, their mean age was 68 years, and they were relatively evenly split between ischemic and nonischemic substrates. Nearly half (47%) had a failed CS lead implant and their mean LV ejection fraction at baseline was 26%.
Primary and secondary endpoint data were available for 15 of the 19 patients, and 6-month data were available for 8 patients. At 1 month, all 15 patients demonstrated Bi-V pacing on 12-lead ECG. The QRS narrowed by 46 ms at 1 month and by 23 ms at 6 months. NYHA class also significantly improved between baseline and 6 months (from II to I; decline of 0.63 points). All patients experienced improvements on their clinical composite score.
No periprocedural adverse events and no device-related serious adverse events occurred within the first month of implant, but 10 serious adverse events occurred in six patients within the first 6 months, including one patient with a hematoma at the transmitter pocket and one patient who had a stroke 3 days after the procedure. "This is a patient who had atrial fibrillation," Dr. Reddy noted. "At the time of the procedure the [warfarin] was stopped. The patient was implanted, did well, but the [warfarin] hadn’t been restarted at the 3-day time point and the patient suffered a stroke. After this experience, we altered the protocol so that patients on anticoagulation for any indication need to continue active coagulation [with no interruption]. We’ll see how that fares."
The study was funded by EBR Systems. Dr. Reddy is a consultant to the company.
On Twitter @dougbrunk
SAN FRANCISCO – Wireless endocardial left ventricular pacing provides an alternative approach to cardiac resynchronization pacing in heart failure patients, according to preliminary results from an ongoing multicenter trial.
At the annual scientific sessions of the Heart Rhythm Society, Dr. Vivek Y. Reddy presented preliminary results from 19 patients enrolled in the SELECT-LV (Safety and Performance of Electrodes Implanted in the Left Ventricle) study. The purpose of the open-label trial is to evaluate the safety and feasibility of leadless, ultrasound-based pacing using a wireless cardiac stimulation system (WiCS-LV) developed by EBR Systems.
"The idea is to use an existing standard ICD [implantable cardioverter defibrillator] or pacemaker and place this device, which has a transmitter as well as a battery, in a subcutaneous location, and then place a receiver-electrode ‘pellet’ on the left ventricle endocardial wall," explained Dr. Reddy, professor of medicine at Mount Sinai Hospital, New York. "The idea is that the transmitter emits ultrasound impulses detected by the pellet. In turn, the pellet transduces the ultrasound impulse into an electrical pacing pulse to stimulate the heart."
The battery, which is 42 cc in size, is placed subcutaneously in the subaxillary region. The transmitter, which is 13 cc in size, is placed between the ribs "in an optimal position so that there is an echo window which allows you to see the left ventricle," Dr. Reddy said. "Then, on the same day or the next day, the pellet (which is 0.05 cc in size) is placed on the left ventricle via a transfemoral catheter approach."
The SELECT-LV investigators intend to enroll 40 patients at seven centers to evaluate the performance and efficacy of the approach. The primary endpoints are device- and procedure-related complications perioperatively and at 1 month, and biventricular pacing capture on 12-lead ECG at 1 month. Secondary endpoints are device-related or major complications up to 6 months and LV pacing capture at 1, 2, and 6 months, as well as biventricular capture at 6 months on 12-lead ECG. Preliminary efficacy is measured by a composite score of all-cause mortality, heart failure hospitalization, New York Heart Association (NYHA) class, and patient global assessment at 6 months, as well as LV end systolic/diastolic volumes and LV ejection fraction at 6 months.
Patients are eligible for the trial if they have a failed implant of a coronary sinus lead or a chronic issue with their CS lead; if they have no clinical status improvement in 6 months of cardiac resynchronization pacing (CRT); or if they have a previously implanted pacemaker or ICD with a new indication for biventricular pacing but are at risk for a CRT upgrade due to venous occlusion or other factors.
Of the 19 patients who have been implanted to date, 16 (84%) were male, their mean age was 68 years, and they were relatively evenly split between ischemic and nonischemic substrates. Nearly half (47%) had a failed CS lead implant and their mean LV ejection fraction at baseline was 26%.
Primary and secondary endpoint data were available for 15 of the 19 patients, and 6-month data were available for 8 patients. At 1 month, all 15 patients demonstrated Bi-V pacing on 12-lead ECG. The QRS narrowed by 46 ms at 1 month and by 23 ms at 6 months. NYHA class also significantly improved between baseline and 6 months (from II to I; decline of 0.63 points). All patients experienced improvements on their clinical composite score.
No periprocedural adverse events and no device-related serious adverse events occurred within the first month of implant, but 10 serious adverse events occurred in six patients within the first 6 months, including one patient with a hematoma at the transmitter pocket and one patient who had a stroke 3 days after the procedure. "This is a patient who had atrial fibrillation," Dr. Reddy noted. "At the time of the procedure the [warfarin] was stopped. The patient was implanted, did well, but the [warfarin] hadn’t been restarted at the 3-day time point and the patient suffered a stroke. After this experience, we altered the protocol so that patients on anticoagulation for any indication need to continue active coagulation [with no interruption]. We’ll see how that fares."
The study was funded by EBR Systems. Dr. Reddy is a consultant to the company.
On Twitter @dougbrunk
AT HEART RHYTHM 2014
Key clinical point: Wireless endocardial left ventricular pacing provided an alternative approach to cardiac resynchronization pacing.
Major finding: At 1 month, all patients demonstrated biventricular pacing on 12-lead ECG. The QRS narrowed by 46 ms at 1 month and by 23 ms at 6 months. NYHA class also significantly improved between baseline and 6 months (from II to I).
Data source: Preliminary results from 19 patients enrolled in the SELECT-LV trial, which intends to enroll a total of 40 patients at seven clinical centers.
Disclosures: The study was funded by EBR Systems. Dr. Reddy disclosed that he is a consultant to the company.
Device positioning may be culprit behind post-LVAD pump thrombosis
TORONTO – Device positioning may help explain significant increases in pump thrombosis after left ventricular assist device implantation, according to a single-center study presented at the 2014 annual meeting of the American Association for Thoracic Surgery.
Dr. Jay Bhama, associate director of lung and heart transplantation at the University of Pittsburgh, found that more device-positioning issues coincided with more occurrences of pump thrombosis (PT). The adequacy of anticoagulation, major adverse events, and medical noncompliance were not found to be contributing factors, Dr. Bhama said.
His investigation joins recently published data indicating that left ventricular assist device (LVAD) thrombosis nearly quadrupled in less than 2 years in a multicenter study.
The purported mechanisms of PT in patients supported with the HeartMate II LVAD are thought to be multifactorial, but possibly related to design modifications, expansion of use to the destination therapy indication, nonuniform surgical implant technique, and nonuniform anticoagulation strategies across centers and over time.
"Starting in 2010, we started to notice a rapid and sudden increase in the rate of pump thrombosis, which has increased steadily over the last 3 years," said Dr. Bhama, who reported that PT occurred in 10 of 62 patients (16%) treated at the University of Pittsburgh Medical Center, with an overall event rate of 0.281 per patient-year.
In response to the increase, the group at the medical center investigated how potential contributing factors may have changed over time. They retrospectively assessed all primary LVAD implants in patients who survived hospitalization (62 of 74 total implants) between 2004 and 2012, grouping patients according to the era of implant: from June 2004 to December 2009 (era 1; n = 24) and from January 2010 (when FDA approval was given to expand use to destination therapy) to December 2012 (era 2; n = 38).
None of those who died during the index hospitalization experienced PT, Dr. Bhama noted.
PT was defined as either visualized thrombus within the pump at device exchange or significant hemolysis in the setting of heart failure symptoms or pump malfunction.
The actuarial freedom from PT at 24 months was significantly lower in era 2 than in era 1 (57% vs. 100%; P = .016).
Effective anticoagulation (percent of all international normalized ratio [INR] measurements greater than 1.8) was more reliably achieved in era 2 than in era 1 (50% vs. 34%; P less than .001).
To assess device positioning, the researchers looked at the angle of the inflow cannula, defining malposition as either less than the 5th or greater than the 95th percentile of the median of all the inflow cannula angles. Regarding the outflow cannula, they looked at patients who had bend-relief disconnects, either partial or complete, and those who had radiographic evidence of outflow graft malposition or kink.
Device positioning issues were significantly more prevalent during era 2 than during era 1 (29% vs. 4%). Most of this difference was driven by inflow cannula positioning problems, Dr. Bhama noted.
When the patients with concerns related to device positioning were excluded, the freedom from PT at 24 months no longer differed significantly between groups (P = .094).
The groups were demographically similar except for age, which was higher in the era 2 group (57 years, vs. 50 years for era 1; P = .037). More patients in the era 2 group received an LVAD for destination therapy, although this difference actually wasn’t significant (61% vs. 38%; P = .066).
The groups were also similar with regard to early major adverse events (right ventricular failure, bleeding, infection, and stroke) and medical noncompliance.
In the earlier multicenter study, Dr. Randall C. Starling, of the Cleveland Clinic, and his colleagues reported an abrupt increase in LVAD thrombosis: Between March 2011 and Jan. 1, 2013, the occurrence of PT at 3 months after implantation increased from 2.2% to 8.4% (N. Engl. J. Med. 2014;370:33-40).
"Dissecting the root cause of this problem is an extremely difficult task," said Dr. Nader Moazami, the invited discussant for Dr. Bhama’s presentation and the second author on the Starling paper. Dr. Moazami is surgical director of the Kaufman Center for Heart Failure at the Cleveland Clinic.
"While recent advances in LVAD technology with continuous-flow pumps have saved the lives of thousands of dying patients, issues related to adverse events and the associated morbidity and mortality are of immense importance, specifically as we consider the relevance of this technology to the more ambulatory heart failure patients," said Dr. Moazami, commenting on the study.
However, he suggested that the real cause of the recent increase in PT has not yet been discovered, and questioned the validity of assessing inflow cannula angulation based on a chest x-ray. "This to my knowledge has never been validated and was a concern in about half of the patients in the pump thrombosis group," Dr. Moazami said. Patients with "a demonstrable mechanical reason for pump thrombosis" were excluded from the Starling team’s analysis, he added.
In response, Dr. Bhama cited a study by Dr. Abeel Mangi, a cardiac surgeon at Yale University, New Haven, Conn., which found that greater angulation of the HeartMate II inflow cannula, along with the depth of the pump pocket, correlated with the development of PT (Ann. Thorac. Surg. 2013;96:1259-65).
"These aren’t just angles that are slightly off here and there," noted Dr. Bhama. "These are splayed very widely, situations I think where we all would say this is something we should be concerned about."
Dr. Bhama reported no relevant disclosures.
TORONTO – Device positioning may help explain significant increases in pump thrombosis after left ventricular assist device implantation, according to a single-center study presented at the 2014 annual meeting of the American Association for Thoracic Surgery.
Dr. Jay Bhama, associate director of lung and heart transplantation at the University of Pittsburgh, found that more device-positioning issues coincided with more occurrences of pump thrombosis (PT). The adequacy of anticoagulation, major adverse events, and medical noncompliance were not found to be contributing factors, Dr. Bhama said.
His investigation joins recently published data indicating that left ventricular assist device (LVAD) thrombosis nearly quadrupled in less than 2 years in a multicenter study.
The purported mechanisms of PT in patients supported with the HeartMate II LVAD are thought to be multifactorial, but possibly related to design modifications, expansion of use to the destination therapy indication, nonuniform surgical implant technique, and nonuniform anticoagulation strategies across centers and over time.
"Starting in 2010, we started to notice a rapid and sudden increase in the rate of pump thrombosis, which has increased steadily over the last 3 years," said Dr. Bhama, who reported that PT occurred in 10 of 62 patients (16%) treated at the University of Pittsburgh Medical Center, with an overall event rate of 0.281 per patient-year.
In response to the increase, the group at the medical center investigated how potential contributing factors may have changed over time. They retrospectively assessed all primary LVAD implants in patients who survived hospitalization (62 of 74 total implants) between 2004 and 2012, grouping patients according to the era of implant: from June 2004 to December 2009 (era 1; n = 24) and from January 2010 (when FDA approval was given to expand use to destination therapy) to December 2012 (era 2; n = 38).
None of those who died during the index hospitalization experienced PT, Dr. Bhama noted.
PT was defined as either visualized thrombus within the pump at device exchange or significant hemolysis in the setting of heart failure symptoms or pump malfunction.
The actuarial freedom from PT at 24 months was significantly lower in era 2 than in era 1 (57% vs. 100%; P = .016).
Effective anticoagulation (percent of all international normalized ratio [INR] measurements greater than 1.8) was more reliably achieved in era 2 than in era 1 (50% vs. 34%; P less than .001).
To assess device positioning, the researchers looked at the angle of the inflow cannula, defining malposition as either less than the 5th or greater than the 95th percentile of the median of all the inflow cannula angles. Regarding the outflow cannula, they looked at patients who had bend-relief disconnects, either partial or complete, and those who had radiographic evidence of outflow graft malposition or kink.
Device positioning issues were significantly more prevalent during era 2 than during era 1 (29% vs. 4%). Most of this difference was driven by inflow cannula positioning problems, Dr. Bhama noted.
When the patients with concerns related to device positioning were excluded, the freedom from PT at 24 months no longer differed significantly between groups (P = .094).
The groups were demographically similar except for age, which was higher in the era 2 group (57 years, vs. 50 years for era 1; P = .037). More patients in the era 2 group received an LVAD for destination therapy, although this difference actually wasn’t significant (61% vs. 38%; P = .066).
The groups were also similar with regard to early major adverse events (right ventricular failure, bleeding, infection, and stroke) and medical noncompliance.
In the earlier multicenter study, Dr. Randall C. Starling, of the Cleveland Clinic, and his colleagues reported an abrupt increase in LVAD thrombosis: Between March 2011 and Jan. 1, 2013, the occurrence of PT at 3 months after implantation increased from 2.2% to 8.4% (N. Engl. J. Med. 2014;370:33-40).
"Dissecting the root cause of this problem is an extremely difficult task," said Dr. Nader Moazami, the invited discussant for Dr. Bhama’s presentation and the second author on the Starling paper. Dr. Moazami is surgical director of the Kaufman Center for Heart Failure at the Cleveland Clinic.
"While recent advances in LVAD technology with continuous-flow pumps have saved the lives of thousands of dying patients, issues related to adverse events and the associated morbidity and mortality are of immense importance, specifically as we consider the relevance of this technology to the more ambulatory heart failure patients," said Dr. Moazami, commenting on the study.
However, he suggested that the real cause of the recent increase in PT has not yet been discovered, and questioned the validity of assessing inflow cannula angulation based on a chest x-ray. "This to my knowledge has never been validated and was a concern in about half of the patients in the pump thrombosis group," Dr. Moazami said. Patients with "a demonstrable mechanical reason for pump thrombosis" were excluded from the Starling team’s analysis, he added.
In response, Dr. Bhama cited a study by Dr. Abeel Mangi, a cardiac surgeon at Yale University, New Haven, Conn., which found that greater angulation of the HeartMate II inflow cannula, along with the depth of the pump pocket, correlated with the development of PT (Ann. Thorac. Surg. 2013;96:1259-65).
"These aren’t just angles that are slightly off here and there," noted Dr. Bhama. "These are splayed very widely, situations I think where we all would say this is something we should be concerned about."
Dr. Bhama reported no relevant disclosures.
TORONTO – Device positioning may help explain significant increases in pump thrombosis after left ventricular assist device implantation, according to a single-center study presented at the 2014 annual meeting of the American Association for Thoracic Surgery.
Dr. Jay Bhama, associate director of lung and heart transplantation at the University of Pittsburgh, found that more device-positioning issues coincided with more occurrences of pump thrombosis (PT). The adequacy of anticoagulation, major adverse events, and medical noncompliance were not found to be contributing factors, Dr. Bhama said.
His investigation joins recently published data indicating that left ventricular assist device (LVAD) thrombosis nearly quadrupled in less than 2 years in a multicenter study.
The purported mechanisms of PT in patients supported with the HeartMate II LVAD are thought to be multifactorial, but possibly related to design modifications, expansion of use to the destination therapy indication, nonuniform surgical implant technique, and nonuniform anticoagulation strategies across centers and over time.
"Starting in 2010, we started to notice a rapid and sudden increase in the rate of pump thrombosis, which has increased steadily over the last 3 years," said Dr. Bhama, who reported that PT occurred in 10 of 62 patients (16%) treated at the University of Pittsburgh Medical Center, with an overall event rate of 0.281 per patient-year.
In response to the increase, the group at the medical center investigated how potential contributing factors may have changed over time. They retrospectively assessed all primary LVAD implants in patients who survived hospitalization (62 of 74 total implants) between 2004 and 2012, grouping patients according to the era of implant: from June 2004 to December 2009 (era 1; n = 24) and from January 2010 (when FDA approval was given to expand use to destination therapy) to December 2012 (era 2; n = 38).
None of those who died during the index hospitalization experienced PT, Dr. Bhama noted.
PT was defined as either visualized thrombus within the pump at device exchange or significant hemolysis in the setting of heart failure symptoms or pump malfunction.
The actuarial freedom from PT at 24 months was significantly lower in era 2 than in era 1 (57% vs. 100%; P = .016).
Effective anticoagulation (percent of all international normalized ratio [INR] measurements greater than 1.8) was more reliably achieved in era 2 than in era 1 (50% vs. 34%; P less than .001).
To assess device positioning, the researchers looked at the angle of the inflow cannula, defining malposition as either less than the 5th or greater than the 95th percentile of the median of all the inflow cannula angles. Regarding the outflow cannula, they looked at patients who had bend-relief disconnects, either partial or complete, and those who had radiographic evidence of outflow graft malposition or kink.
Device positioning issues were significantly more prevalent during era 2 than during era 1 (29% vs. 4%). Most of this difference was driven by inflow cannula positioning problems, Dr. Bhama noted.
When the patients with concerns related to device positioning were excluded, the freedom from PT at 24 months no longer differed significantly between groups (P = .094).
The groups were demographically similar except for age, which was higher in the era 2 group (57 years, vs. 50 years for era 1; P = .037). More patients in the era 2 group received an LVAD for destination therapy, although this difference actually wasn’t significant (61% vs. 38%; P = .066).
The groups were also similar with regard to early major adverse events (right ventricular failure, bleeding, infection, and stroke) and medical noncompliance.
In the earlier multicenter study, Dr. Randall C. Starling, of the Cleveland Clinic, and his colleagues reported an abrupt increase in LVAD thrombosis: Between March 2011 and Jan. 1, 2013, the occurrence of PT at 3 months after implantation increased from 2.2% to 8.4% (N. Engl. J. Med. 2014;370:33-40).
"Dissecting the root cause of this problem is an extremely difficult task," said Dr. Nader Moazami, the invited discussant for Dr. Bhama’s presentation and the second author on the Starling paper. Dr. Moazami is surgical director of the Kaufman Center for Heart Failure at the Cleveland Clinic.
"While recent advances in LVAD technology with continuous-flow pumps have saved the lives of thousands of dying patients, issues related to adverse events and the associated morbidity and mortality are of immense importance, specifically as we consider the relevance of this technology to the more ambulatory heart failure patients," said Dr. Moazami, commenting on the study.
However, he suggested that the real cause of the recent increase in PT has not yet been discovered, and questioned the validity of assessing inflow cannula angulation based on a chest x-ray. "This to my knowledge has never been validated and was a concern in about half of the patients in the pump thrombosis group," Dr. Moazami said. Patients with "a demonstrable mechanical reason for pump thrombosis" were excluded from the Starling team’s analysis, he added.
In response, Dr. Bhama cited a study by Dr. Abeel Mangi, a cardiac surgeon at Yale University, New Haven, Conn., which found that greater angulation of the HeartMate II inflow cannula, along with the depth of the pump pocket, correlated with the development of PT (Ann. Thorac. Surg. 2013;96:1259-65).
"These aren’t just angles that are slightly off here and there," noted Dr. Bhama. "These are splayed very widely, situations I think where we all would say this is something we should be concerned about."
Dr. Bhama reported no relevant disclosures.
AT THE AATS ANNUAL MEETING
Key clinical point: Cannula malpositioning may be related to pump thrombosis after LVAD placement.
Major finding: The rate of pump thrombosis after LVAD implantation has rapidly increased since 2010, increasing steadily over the past 3 years. Excluding patients with device positioning concerns eliminated the significant difference seen in pump thrombosis across time.
Data source: Single-center, retrospective study of 63 LVAD implant patient records.
Disclosures: Dr. Bhama reported no relevant disclosures.
Off-label use of novel oral anticoagulants accelerates
WASHINGTON – The off-label use of novel oral anticoagulants for stroke prevention in patients with valvular atrial fibrillation has climbed steeply since the drugs reached the marketplace, mirroring the medications’ rapid adoption for the approved indication of preventing strokes in nonvalvular AF, according to Dr. Sandeep Mahrendra Jani.
An analysis of 190,227 nonvalvular atrial fibrillation (NVAF) patients in 95 practices participating in the American College of Cardiology’s National Cardiovascular Data Registry – PINNACLE Registry – showed that during the first quarter of 2011, just 4.8% were on dabigatran, the sole novel oral anticoagulant then available.
By the fourth quarter of 2012, however, 14.9% of NVAF patients were on a novel oral anticoagulant, either dabigatran or the subsequently approved rivaroxiban, he reported at the annual meeting of the ACC.
Similarly, among 2,142 registry participants with valvular atrial fibrillation (AF), the use of any novel oral anticoagulant shot up from 2.7% in the first quarter of 2011 to 13.8% in the fourth quarter of 2012, noted Dr. Jani of Medstar Washington (D.C.) Hospital Center.
During this time – prior to the arrival of apixiban on the market – the use of warfarin for stroke prevention in patients with NVAF declined from 47.9% to 44.3%. Among patients with valvular atrial fibrillation, the prevalence of warfarin therapy fell from 65.8% in the first quarter of 2011 to 60.1% in fourth quarter 2012.
During the first quarter of 2011, 51.2% of all patients with NVAF and 66.4% with valvular AF were on any oral anticoagulant. By fourth quarter 2012, these rates had increased to 56.9% and 66.8%, respectively.
The use of dabigatran in patients with valvular AF took a hit in late 2012 in response to the premature halt of the RE-ALIGN (Dabigatran Etexilate in Patients With Mechanical Heart Valves) trial, followed by the Food and Drug Administration’s warning against using dabigatran in patients with mechanical heart valves.
Dabigatran was used by 2.7% of valvular AF patients in the first quarter of 2011, rising steadily to 12.1% by the third quarter of 2012, then plunging to just 1.4% in the year’s final quarter.
In light of the rapidly accelerating use of novel oral anticoagulants in patients with valvular AF, despite a lack of evidence of efficacy for stroke prevention in this setting, further studies are a priority, Dr. Jani said.
The PINNACLE registry is funded by the ACC, with founding sponsorship provided by Bristol-Myers Squibb and Pfizer. Dr. Jani reported having no relevant financial conflicts.
WASHINGTON – The off-label use of novel oral anticoagulants for stroke prevention in patients with valvular atrial fibrillation has climbed steeply since the drugs reached the marketplace, mirroring the medications’ rapid adoption for the approved indication of preventing strokes in nonvalvular AF, according to Dr. Sandeep Mahrendra Jani.
An analysis of 190,227 nonvalvular atrial fibrillation (NVAF) patients in 95 practices participating in the American College of Cardiology’s National Cardiovascular Data Registry – PINNACLE Registry – showed that during the first quarter of 2011, just 4.8% were on dabigatran, the sole novel oral anticoagulant then available.
By the fourth quarter of 2012, however, 14.9% of NVAF patients were on a novel oral anticoagulant, either dabigatran or the subsequently approved rivaroxiban, he reported at the annual meeting of the ACC.
Similarly, among 2,142 registry participants with valvular atrial fibrillation (AF), the use of any novel oral anticoagulant shot up from 2.7% in the first quarter of 2011 to 13.8% in the fourth quarter of 2012, noted Dr. Jani of Medstar Washington (D.C.) Hospital Center.
During this time – prior to the arrival of apixiban on the market – the use of warfarin for stroke prevention in patients with NVAF declined from 47.9% to 44.3%. Among patients with valvular atrial fibrillation, the prevalence of warfarin therapy fell from 65.8% in the first quarter of 2011 to 60.1% in fourth quarter 2012.
During the first quarter of 2011, 51.2% of all patients with NVAF and 66.4% with valvular AF were on any oral anticoagulant. By fourth quarter 2012, these rates had increased to 56.9% and 66.8%, respectively.
The use of dabigatran in patients with valvular AF took a hit in late 2012 in response to the premature halt of the RE-ALIGN (Dabigatran Etexilate in Patients With Mechanical Heart Valves) trial, followed by the Food and Drug Administration’s warning against using dabigatran in patients with mechanical heart valves.
Dabigatran was used by 2.7% of valvular AF patients in the first quarter of 2011, rising steadily to 12.1% by the third quarter of 2012, then plunging to just 1.4% in the year’s final quarter.
In light of the rapidly accelerating use of novel oral anticoagulants in patients with valvular AF, despite a lack of evidence of efficacy for stroke prevention in this setting, further studies are a priority, Dr. Jani said.
The PINNACLE registry is funded by the ACC, with founding sponsorship provided by Bristol-Myers Squibb and Pfizer. Dr. Jani reported having no relevant financial conflicts.
WASHINGTON – The off-label use of novel oral anticoagulants for stroke prevention in patients with valvular atrial fibrillation has climbed steeply since the drugs reached the marketplace, mirroring the medications’ rapid adoption for the approved indication of preventing strokes in nonvalvular AF, according to Dr. Sandeep Mahrendra Jani.
An analysis of 190,227 nonvalvular atrial fibrillation (NVAF) patients in 95 practices participating in the American College of Cardiology’s National Cardiovascular Data Registry – PINNACLE Registry – showed that during the first quarter of 2011, just 4.8% were on dabigatran, the sole novel oral anticoagulant then available.
By the fourth quarter of 2012, however, 14.9% of NVAF patients were on a novel oral anticoagulant, either dabigatran or the subsequently approved rivaroxiban, he reported at the annual meeting of the ACC.
Similarly, among 2,142 registry participants with valvular atrial fibrillation (AF), the use of any novel oral anticoagulant shot up from 2.7% in the first quarter of 2011 to 13.8% in the fourth quarter of 2012, noted Dr. Jani of Medstar Washington (D.C.) Hospital Center.
During this time – prior to the arrival of apixiban on the market – the use of warfarin for stroke prevention in patients with NVAF declined from 47.9% to 44.3%. Among patients with valvular atrial fibrillation, the prevalence of warfarin therapy fell from 65.8% in the first quarter of 2011 to 60.1% in fourth quarter 2012.
During the first quarter of 2011, 51.2% of all patients with NVAF and 66.4% with valvular AF were on any oral anticoagulant. By fourth quarter 2012, these rates had increased to 56.9% and 66.8%, respectively.
The use of dabigatran in patients with valvular AF took a hit in late 2012 in response to the premature halt of the RE-ALIGN (Dabigatran Etexilate in Patients With Mechanical Heart Valves) trial, followed by the Food and Drug Administration’s warning against using dabigatran in patients with mechanical heart valves.
Dabigatran was used by 2.7% of valvular AF patients in the first quarter of 2011, rising steadily to 12.1% by the third quarter of 2012, then plunging to just 1.4% in the year’s final quarter.
In light of the rapidly accelerating use of novel oral anticoagulants in patients with valvular AF, despite a lack of evidence of efficacy for stroke prevention in this setting, further studies are a priority, Dr. Jani said.
The PINNACLE registry is funded by the ACC, with founding sponsorship provided by Bristol-Myers Squibb and Pfizer. Dr. Jani reported having no relevant financial conflicts.
Major finding: By the fourth quarter of 2012, 14.9% of patients with nonvalvular AF were on a novel anticoagulant. So were 13.8% of those with valvular AF, even though this is an off-label use of these drugs.
Data source: This study involved more than 190,000 patients with nonvalvular AF and 2,142 with valvular AF in 95 practices participating in the PINNACLE Registry.
Disclosures The PINNACLE Registry is funded by the ACC’s National Cardiovascular Data Registry. The presenter reported having no relevant financial conflicts.
Sternal approach optimal in mitral valve surgeries
NEW YORK – The conventional sternal approach in treating mitral valve disease remains the safest and most flexible, said Dr. Patrick McCarthy, who used his talk at the AATS annual meeting’s adult cardiac surgery symposium, "Becoming a Master Valve Surgeon," to defend its virtues as the "gold standard" for mitral valve operations.
"Patients don’t have much pain, and most are concerned about the risks of open heart surgery, not the cosmetic aspects. Even so, the scar heals to a thin white line," Dr. McCarthy said in an interview. And with a sternal approach, compared with robotic or minimally invasive procedures, "you’re prepared to fix anything," he said.
"So if there’s a technical complication – an aortic dissection, unusual bleeding, a circumflex coronary injury – or you encounter unrecognized aortic valve disease, then you can safely treat it."
Dr. McCarthy said that his own practice has evolved to perform fewer, not more, minimally invasive mitral valve surgeries in recent years.
"Ten years ago about half of my mitral valve operations were minimally invasive, and over time I saw less and less benefit. The length of time on the heart-lung machine and the potential safety issues made me evolve away from that approach."
Many centers and individual surgeons do right thoracotomy or robotic surgery very well and safely, Dr. McCarthy said.
"But the national data would indicate that the perioperative risk of stroke is twice as high with those approaches," he said, and they are not performed as often as they are talked about.
Surgeons who elect not to perform a minimally invasive mitral valve procedure "should not feel that they’re somehow shortchanging the patient. For safety and long-term outcomes we need to focus less on how we approach the mitral valve and more on what operation we do. Can you do a good durable repair, and not a replacement? Can you minimize the risks of open heart surgery?" he said.
"Generations of cardiac surgeons worked hard to minimize those risks and optimize the outcomes of repair, to the point that we now operate with minimal risk on asymptomatic patients with normal ventricles and expect a 95% or greater chance for a durable repair. Don’t compromise the operation for a perceived cosmetic advantage," Dr. McCarthy said.
Also during the course, Dr. Marc Moon of Washington University School of Medicine in St. Louis, Missouri, discussed surgical triggers for patients with aortic stenosis in several nonstandard clinical scenarios. These scenarios include frail patients, patients with severe aortic stenosis, and asymptomatic patients who need major noncardiac surgery.
In addition to drawing from his own center’s experience, Dr. Moon attempted to condense and summarize the most recent guideline and surgical review recommendations for performing – or not performing – aortic valve replacement (AVR) in these and other tricky patient groups.
Patients with aortic stenosis (AS) are initially classed as asymptomatic or symptomatic based on a history and physical exam, and those with symptomatic aortic stenosis should undergo AVR, Dr. Moon said.
Asymptomatic patients can have normal ejection fraction (EF), but generally display left ventricle hypertrophy or diastolic dysfunction once AS becomes severe. In asymptomatic patients, once left ventricle EF falls below 50% (independent of associated coronary artery disease) or pulmonary hypertension appears, AVR should be considered."
However, frailty will make surgical intervention futile in some of these patients. Dr. Moon described new assessment tools to replace the "eyeball test" for frailty that surgeons have been using for years.
"A 6-minute walk test can predict a poor outcome in patients following AVR," he said, so long as the mobility limitations are not mainly due to the AS itself. Slow walkers, who need 6 seconds or more to walk 5 meters, have a significantly increased risk of morbidity or mortality independent of other factors affecting surgical risk.
Other measures of frailty include unintended weight loss of 10 pounds or more over a year, self-reported exhaustion, and weak grip strength.
Patients whose underlying AS is the main contributor to frailty can benefit from AVR, Dr. Moon said, but determining this can be difficult. One approach Dr. Moon and colleagues use is to begin with balloon aortic valvuloplasty in frail patients whose valves are amenable to BAV. For these patients, "we initiate an appropriate heart failure regimen, perform BAV, and reevaluate functional status in 4-6 weeks."
In these difficult cases, BAV is used to determine the contribution of aortic stenosis to the patient’s symptoms associated with underlying chronic lung disease, hepatorenal dysfunction, or poor left ventricular function, he said.
"If there is improvement after BAV, then AS is a contributing, causative factor to the patient’s disability and AVR is recommended. If there is no improvement in functional status, medical therapy is continued or hospice care initiated as appropriate."
NEW YORK – The conventional sternal approach in treating mitral valve disease remains the safest and most flexible, said Dr. Patrick McCarthy, who used his talk at the AATS annual meeting’s adult cardiac surgery symposium, "Becoming a Master Valve Surgeon," to defend its virtues as the "gold standard" for mitral valve operations.
"Patients don’t have much pain, and most are concerned about the risks of open heart surgery, not the cosmetic aspects. Even so, the scar heals to a thin white line," Dr. McCarthy said in an interview. And with a sternal approach, compared with robotic or minimally invasive procedures, "you’re prepared to fix anything," he said.
"So if there’s a technical complication – an aortic dissection, unusual bleeding, a circumflex coronary injury – or you encounter unrecognized aortic valve disease, then you can safely treat it."
Dr. McCarthy said that his own practice has evolved to perform fewer, not more, minimally invasive mitral valve surgeries in recent years.
"Ten years ago about half of my mitral valve operations were minimally invasive, and over time I saw less and less benefit. The length of time on the heart-lung machine and the potential safety issues made me evolve away from that approach."
Many centers and individual surgeons do right thoracotomy or robotic surgery very well and safely, Dr. McCarthy said.
"But the national data would indicate that the perioperative risk of stroke is twice as high with those approaches," he said, and they are not performed as often as they are talked about.
Surgeons who elect not to perform a minimally invasive mitral valve procedure "should not feel that they’re somehow shortchanging the patient. For safety and long-term outcomes we need to focus less on how we approach the mitral valve and more on what operation we do. Can you do a good durable repair, and not a replacement? Can you minimize the risks of open heart surgery?" he said.
"Generations of cardiac surgeons worked hard to minimize those risks and optimize the outcomes of repair, to the point that we now operate with minimal risk on asymptomatic patients with normal ventricles and expect a 95% or greater chance for a durable repair. Don’t compromise the operation for a perceived cosmetic advantage," Dr. McCarthy said.
Also during the course, Dr. Marc Moon of Washington University School of Medicine in St. Louis, Missouri, discussed surgical triggers for patients with aortic stenosis in several nonstandard clinical scenarios. These scenarios include frail patients, patients with severe aortic stenosis, and asymptomatic patients who need major noncardiac surgery.
In addition to drawing from his own center’s experience, Dr. Moon attempted to condense and summarize the most recent guideline and surgical review recommendations for performing – or not performing – aortic valve replacement (AVR) in these and other tricky patient groups.
Patients with aortic stenosis (AS) are initially classed as asymptomatic or symptomatic based on a history and physical exam, and those with symptomatic aortic stenosis should undergo AVR, Dr. Moon said.
Asymptomatic patients can have normal ejection fraction (EF), but generally display left ventricle hypertrophy or diastolic dysfunction once AS becomes severe. In asymptomatic patients, once left ventricle EF falls below 50% (independent of associated coronary artery disease) or pulmonary hypertension appears, AVR should be considered."
However, frailty will make surgical intervention futile in some of these patients. Dr. Moon described new assessment tools to replace the "eyeball test" for frailty that surgeons have been using for years.
"A 6-minute walk test can predict a poor outcome in patients following AVR," he said, so long as the mobility limitations are not mainly due to the AS itself. Slow walkers, who need 6 seconds or more to walk 5 meters, have a significantly increased risk of morbidity or mortality independent of other factors affecting surgical risk.
Other measures of frailty include unintended weight loss of 10 pounds or more over a year, self-reported exhaustion, and weak grip strength.
Patients whose underlying AS is the main contributor to frailty can benefit from AVR, Dr. Moon said, but determining this can be difficult. One approach Dr. Moon and colleagues use is to begin with balloon aortic valvuloplasty in frail patients whose valves are amenable to BAV. For these patients, "we initiate an appropriate heart failure regimen, perform BAV, and reevaluate functional status in 4-6 weeks."
In these difficult cases, BAV is used to determine the contribution of aortic stenosis to the patient’s symptoms associated with underlying chronic lung disease, hepatorenal dysfunction, or poor left ventricular function, he said.
"If there is improvement after BAV, then AS is a contributing, causative factor to the patient’s disability and AVR is recommended. If there is no improvement in functional status, medical therapy is continued or hospice care initiated as appropriate."
NEW YORK – The conventional sternal approach in treating mitral valve disease remains the safest and most flexible, said Dr. Patrick McCarthy, who used his talk at the AATS annual meeting’s adult cardiac surgery symposium, "Becoming a Master Valve Surgeon," to defend its virtues as the "gold standard" for mitral valve operations.
"Patients don’t have much pain, and most are concerned about the risks of open heart surgery, not the cosmetic aspects. Even so, the scar heals to a thin white line," Dr. McCarthy said in an interview. And with a sternal approach, compared with robotic or minimally invasive procedures, "you’re prepared to fix anything," he said.
"So if there’s a technical complication – an aortic dissection, unusual bleeding, a circumflex coronary injury – or you encounter unrecognized aortic valve disease, then you can safely treat it."
Dr. McCarthy said that his own practice has evolved to perform fewer, not more, minimally invasive mitral valve surgeries in recent years.
"Ten years ago about half of my mitral valve operations were minimally invasive, and over time I saw less and less benefit. The length of time on the heart-lung machine and the potential safety issues made me evolve away from that approach."
Many centers and individual surgeons do right thoracotomy or robotic surgery very well and safely, Dr. McCarthy said.
"But the national data would indicate that the perioperative risk of stroke is twice as high with those approaches," he said, and they are not performed as often as they are talked about.
Surgeons who elect not to perform a minimally invasive mitral valve procedure "should not feel that they’re somehow shortchanging the patient. For safety and long-term outcomes we need to focus less on how we approach the mitral valve and more on what operation we do. Can you do a good durable repair, and not a replacement? Can you minimize the risks of open heart surgery?" he said.
"Generations of cardiac surgeons worked hard to minimize those risks and optimize the outcomes of repair, to the point that we now operate with minimal risk on asymptomatic patients with normal ventricles and expect a 95% or greater chance for a durable repair. Don’t compromise the operation for a perceived cosmetic advantage," Dr. McCarthy said.
Also during the course, Dr. Marc Moon of Washington University School of Medicine in St. Louis, Missouri, discussed surgical triggers for patients with aortic stenosis in several nonstandard clinical scenarios. These scenarios include frail patients, patients with severe aortic stenosis, and asymptomatic patients who need major noncardiac surgery.
In addition to drawing from his own center’s experience, Dr. Moon attempted to condense and summarize the most recent guideline and surgical review recommendations for performing – or not performing – aortic valve replacement (AVR) in these and other tricky patient groups.
Patients with aortic stenosis (AS) are initially classed as asymptomatic or symptomatic based on a history and physical exam, and those with symptomatic aortic stenosis should undergo AVR, Dr. Moon said.
Asymptomatic patients can have normal ejection fraction (EF), but generally display left ventricle hypertrophy or diastolic dysfunction once AS becomes severe. In asymptomatic patients, once left ventricle EF falls below 50% (independent of associated coronary artery disease) or pulmonary hypertension appears, AVR should be considered."
However, frailty will make surgical intervention futile in some of these patients. Dr. Moon described new assessment tools to replace the "eyeball test" for frailty that surgeons have been using for years.
"A 6-minute walk test can predict a poor outcome in patients following AVR," he said, so long as the mobility limitations are not mainly due to the AS itself. Slow walkers, who need 6 seconds or more to walk 5 meters, have a significantly increased risk of morbidity or mortality independent of other factors affecting surgical risk.
Other measures of frailty include unintended weight loss of 10 pounds or more over a year, self-reported exhaustion, and weak grip strength.
Patients whose underlying AS is the main contributor to frailty can benefit from AVR, Dr. Moon said, but determining this can be difficult. One approach Dr. Moon and colleagues use is to begin with balloon aortic valvuloplasty in frail patients whose valves are amenable to BAV. For these patients, "we initiate an appropriate heart failure regimen, perform BAV, and reevaluate functional status in 4-6 weeks."
In these difficult cases, BAV is used to determine the contribution of aortic stenosis to the patient’s symptoms associated with underlying chronic lung disease, hepatorenal dysfunction, or poor left ventricular function, he said.
"If there is improvement after BAV, then AS is a contributing, causative factor to the patient’s disability and AVR is recommended. If there is no improvement in functional status, medical therapy is continued or hospice care initiated as appropriate."
Open repair of acute DeBakey type I dissection favorable
TORONTO - Placing a stent-graft in the descending thoracic aorta during surgical repair of the proximal aorta of patients with acute DeBakey type I dissection is becoming an increasingly popular approach. A suggested advantage of this approach is the avoidance of subsequent, high-risk operations to repair the descending and thoracoabdominal aortic segments in those patients whose chronic dissection causes later aneurysm formation.
Dr. Joseph S. Coselli presented a study that he and his colleagues at the Baylor College of Medicine undertook to better define the risks associated with these secondary operations that stenting is suggested to avoid. They found that the use of open repair showed excellent early survival and acceptable morbidity and the use of adjuncts to protect against ischemic complications was associated with improved early outcomes.
"We prospectively examined our contemporary experience with open distal aortic repair in patients with chronic DeBakey type I aortic dissection in 200 consecutive patients with open descending thoracic (n = 29) or thoracoabdominal (n = 171) aortic repairs. Data were collected between January 2005 and June 2013," said Dr. Coselli at the annual meeting of the American Association for Thoracic Surgery.
The median patient age was 57 years, and the median interval between initial proximal aortic repair and the subsequent distal operation was 4.8 years. In 30 patients (15%), repairs were prompted by the onset of acute symptoms; this included 10 patients (5%) who had a new acute DeBakey type III dissection superimposed on the chronic dissection. Forty-three repairs (22%) were emergent or urgent, and hypothermic circulatory arrest was necessary in 17 (9%).
Of the 171 thoracoabdominal aortic repairs, 145 (86%) were Crawford extent I or II; adjuncts used during thoracoabdominal procedures included cerebrospinal fluid drainage in 159 patients (93%), left heart bypass in 128 (75%), and cold renal perfusion in 123 (72%). The researchers used univariate and bivariate analyses to examine associations between potential risk factors and early death.
There were 14 early deaths (7%) and 35 late deaths, yielding an actuarial 6-year survival of 71%. Two patients (1%) developed permanent paraplegia, 4 (2%) had permanent paraparesis, 10 (5%) had strokes, and 9 (5%) had permanent renal failure necessitating dialysis. Greater age and the use of hypothermic circulatory arrest were significantly associated with early death. The use of left heart bypass and the use of cold renal perfusion were each significantly associated with early survival. There were no associations between early death and connective tissue disorders, emergent or urgent surgery, or extent of aortic repair, Dr. Coselli added.
"For our patients who survived DeBakey type I aortic dissection and subsequently develop distal aortic aneurysms, the use of open repair of the descending thoracic or thoracoabdominal aorta results in excellent early survival and acceptable morbidity. We found that the use of adjuncts to protect against ischemic complications is associated with improved early outcomes," Dr. Coselli stated.
In an interview, Dr. Coselli added that it is important to note that the disease process leading to late aneurysm after DeBakey type I dissection is likely much different than it is for the typically older cohort of distal aortic repairs for purely aneurysmal disease. The DeBakey I patients are about a decade or more younger and seem to have much less atherosclerotic disease. "We use the need for visceral endarterectomy as a marker for this," he said.
And, since older age is one of the best predictors of operative mortality and adverse event, the DeBakey I patients tend to do better than the typical older cohort undergoing open distal aortic repair. This is despite the fact that the vast majority of patients had an extensive history of prior aortic repair, including 35 repairs with prior open distal aortic repair or prior TEVAR (and naturally, extensive prior proximal aortic repair).
"The rate of permanent paraplegia was low, we speculated that the progressive nature of late distal aortic dilatation contributes to this low rate. Often DTA repair is followed by TAAA repair or vice versa. This is similar to the 'staged model' of distal aortic repair that Dr. Randall Griepp and Dr. C.D. Etz have published [Eur. J. Cardiothorac. Surg. 2008 34(3):605-14], possibly allowing for collateral circulation. This combined with our aggressive reattachment strategy for intercostal/lumbar arteries, may have contributed to this low rate," he added.
"Lastly, the reintervention rates for antegrade TEVAR and frozen elephant trunk tend to be high, and there is concern for an enhanced risk of paraplegia during these types of repairs. And with standard TEVAR, the rates of reintervention in patients with chronic dissection appear greater than those with only an aneurysm," Dr. Coselli concluded.
The authors of the study reported having no conflicts.
TORONTO - Placing a stent-graft in the descending thoracic aorta during surgical repair of the proximal aorta of patients with acute DeBakey type I dissection is becoming an increasingly popular approach. A suggested advantage of this approach is the avoidance of subsequent, high-risk operations to repair the descending and thoracoabdominal aortic segments in those patients whose chronic dissection causes later aneurysm formation.
Dr. Joseph S. Coselli presented a study that he and his colleagues at the Baylor College of Medicine undertook to better define the risks associated with these secondary operations that stenting is suggested to avoid. They found that the use of open repair showed excellent early survival and acceptable morbidity and the use of adjuncts to protect against ischemic complications was associated with improved early outcomes.
"We prospectively examined our contemporary experience with open distal aortic repair in patients with chronic DeBakey type I aortic dissection in 200 consecutive patients with open descending thoracic (n = 29) or thoracoabdominal (n = 171) aortic repairs. Data were collected between January 2005 and June 2013," said Dr. Coselli at the annual meeting of the American Association for Thoracic Surgery.
The median patient age was 57 years, and the median interval between initial proximal aortic repair and the subsequent distal operation was 4.8 years. In 30 patients (15%), repairs were prompted by the onset of acute symptoms; this included 10 patients (5%) who had a new acute DeBakey type III dissection superimposed on the chronic dissection. Forty-three repairs (22%) were emergent or urgent, and hypothermic circulatory arrest was necessary in 17 (9%).
Of the 171 thoracoabdominal aortic repairs, 145 (86%) were Crawford extent I or II; adjuncts used during thoracoabdominal procedures included cerebrospinal fluid drainage in 159 patients (93%), left heart bypass in 128 (75%), and cold renal perfusion in 123 (72%). The researchers used univariate and bivariate analyses to examine associations between potential risk factors and early death.
There were 14 early deaths (7%) and 35 late deaths, yielding an actuarial 6-year survival of 71%. Two patients (1%) developed permanent paraplegia, 4 (2%) had permanent paraparesis, 10 (5%) had strokes, and 9 (5%) had permanent renal failure necessitating dialysis. Greater age and the use of hypothermic circulatory arrest were significantly associated with early death. The use of left heart bypass and the use of cold renal perfusion were each significantly associated with early survival. There were no associations between early death and connective tissue disorders, emergent or urgent surgery, or extent of aortic repair, Dr. Coselli added.
"For our patients who survived DeBakey type I aortic dissection and subsequently develop distal aortic aneurysms, the use of open repair of the descending thoracic or thoracoabdominal aorta results in excellent early survival and acceptable morbidity. We found that the use of adjuncts to protect against ischemic complications is associated with improved early outcomes," Dr. Coselli stated.
In an interview, Dr. Coselli added that it is important to note that the disease process leading to late aneurysm after DeBakey type I dissection is likely much different than it is for the typically older cohort of distal aortic repairs for purely aneurysmal disease. The DeBakey I patients are about a decade or more younger and seem to have much less atherosclerotic disease. "We use the need for visceral endarterectomy as a marker for this," he said.
And, since older age is one of the best predictors of operative mortality and adverse event, the DeBakey I patients tend to do better than the typical older cohort undergoing open distal aortic repair. This is despite the fact that the vast majority of patients had an extensive history of prior aortic repair, including 35 repairs with prior open distal aortic repair or prior TEVAR (and naturally, extensive prior proximal aortic repair).
"The rate of permanent paraplegia was low, we speculated that the progressive nature of late distal aortic dilatation contributes to this low rate. Often DTA repair is followed by TAAA repair or vice versa. This is similar to the 'staged model' of distal aortic repair that Dr. Randall Griepp and Dr. C.D. Etz have published [Eur. J. Cardiothorac. Surg. 2008 34(3):605-14], possibly allowing for collateral circulation. This combined with our aggressive reattachment strategy for intercostal/lumbar arteries, may have contributed to this low rate," he added.
"Lastly, the reintervention rates for antegrade TEVAR and frozen elephant trunk tend to be high, and there is concern for an enhanced risk of paraplegia during these types of repairs. And with standard TEVAR, the rates of reintervention in patients with chronic dissection appear greater than those with only an aneurysm," Dr. Coselli concluded.
The authors of the study reported having no conflicts.
TORONTO - Placing a stent-graft in the descending thoracic aorta during surgical repair of the proximal aorta of patients with acute DeBakey type I dissection is becoming an increasingly popular approach. A suggested advantage of this approach is the avoidance of subsequent, high-risk operations to repair the descending and thoracoabdominal aortic segments in those patients whose chronic dissection causes later aneurysm formation.
Dr. Joseph S. Coselli presented a study that he and his colleagues at the Baylor College of Medicine undertook to better define the risks associated with these secondary operations that stenting is suggested to avoid. They found that the use of open repair showed excellent early survival and acceptable morbidity and the use of adjuncts to protect against ischemic complications was associated with improved early outcomes.
"We prospectively examined our contemporary experience with open distal aortic repair in patients with chronic DeBakey type I aortic dissection in 200 consecutive patients with open descending thoracic (n = 29) or thoracoabdominal (n = 171) aortic repairs. Data were collected between January 2005 and June 2013," said Dr. Coselli at the annual meeting of the American Association for Thoracic Surgery.
The median patient age was 57 years, and the median interval between initial proximal aortic repair and the subsequent distal operation was 4.8 years. In 30 patients (15%), repairs were prompted by the onset of acute symptoms; this included 10 patients (5%) who had a new acute DeBakey type III dissection superimposed on the chronic dissection. Forty-three repairs (22%) were emergent or urgent, and hypothermic circulatory arrest was necessary in 17 (9%).
Of the 171 thoracoabdominal aortic repairs, 145 (86%) were Crawford extent I or II; adjuncts used during thoracoabdominal procedures included cerebrospinal fluid drainage in 159 patients (93%), left heart bypass in 128 (75%), and cold renal perfusion in 123 (72%). The researchers used univariate and bivariate analyses to examine associations between potential risk factors and early death.
There were 14 early deaths (7%) and 35 late deaths, yielding an actuarial 6-year survival of 71%. Two patients (1%) developed permanent paraplegia, 4 (2%) had permanent paraparesis, 10 (5%) had strokes, and 9 (5%) had permanent renal failure necessitating dialysis. Greater age and the use of hypothermic circulatory arrest were significantly associated with early death. The use of left heart bypass and the use of cold renal perfusion were each significantly associated with early survival. There were no associations between early death and connective tissue disorders, emergent or urgent surgery, or extent of aortic repair, Dr. Coselli added.
"For our patients who survived DeBakey type I aortic dissection and subsequently develop distal aortic aneurysms, the use of open repair of the descending thoracic or thoracoabdominal aorta results in excellent early survival and acceptable morbidity. We found that the use of adjuncts to protect against ischemic complications is associated with improved early outcomes," Dr. Coselli stated.
In an interview, Dr. Coselli added that it is important to note that the disease process leading to late aneurysm after DeBakey type I dissection is likely much different than it is for the typically older cohort of distal aortic repairs for purely aneurysmal disease. The DeBakey I patients are about a decade or more younger and seem to have much less atherosclerotic disease. "We use the need for visceral endarterectomy as a marker for this," he said.
And, since older age is one of the best predictors of operative mortality and adverse event, the DeBakey I patients tend to do better than the typical older cohort undergoing open distal aortic repair. This is despite the fact that the vast majority of patients had an extensive history of prior aortic repair, including 35 repairs with prior open distal aortic repair or prior TEVAR (and naturally, extensive prior proximal aortic repair).
"The rate of permanent paraplegia was low, we speculated that the progressive nature of late distal aortic dilatation contributes to this low rate. Often DTA repair is followed by TAAA repair or vice versa. This is similar to the 'staged model' of distal aortic repair that Dr. Randall Griepp and Dr. C.D. Etz have published [Eur. J. Cardiothorac. Surg. 2008 34(3):605-14], possibly allowing for collateral circulation. This combined with our aggressive reattachment strategy for intercostal/lumbar arteries, may have contributed to this low rate," he added.
"Lastly, the reintervention rates for antegrade TEVAR and frozen elephant trunk tend to be high, and there is concern for an enhanced risk of paraplegia during these types of repairs. And with standard TEVAR, the rates of reintervention in patients with chronic dissection appear greater than those with only an aneurysm," Dr. Coselli concluded.
The authors of the study reported having no conflicts.
BITA improves CABG in diabetic patients
Diabetes has historically been associated with greater mortality and more and worse postoperative complications after coronary artery bypass grafting (CABG). Researchers continue to search for improved surgical techniques and other options to improve CABG outcomes in diabetic patients.
The use of bilateral internal thoracic artery (ITA) grafting and complete revascularization was found to improve the results of CABG in patients with diabetes, according to the results of an assessment of nearly 12,000 diabetic patients who underwent surgical revascularization, according to Dr. Sajjad Raza, a research fellow in cardiac surgery, who presented the results of a study that he and his colleagues performed at the Cleveland Clinic.
Patients who were operated upon from January 1972 to January 2011 were included in the study.
"Our research was designed to identify surgical revascularization techniques that can improve early and late results in patients with diabetes undergoing CABG," said Dr. Raza at the annual meeting of the American Association for Thoracic Surgery.
Surgical revascularization techniques investigated comprised single (71%) and bilateral (7.9%) ITA with or without other grafts vs. the use of saphenous vein grafting alone (SVG, 21%), as well as incomplete (18%) vs. complete revascularization, and the use of off- (5.0%) vs. on-pump CABG. The median follow-up was nearly 8 years, with total follow-up of more than 100,000 patient-years, according to Dr. Raza.
Multivariable analysis was performed to assess the effect of surgical techniques on early and late mortality.
After adjustment for patient characteristics, single ITA grafting with or without other grafts vs. SVG alone was associated with a 43% decrease in early mortality and 17% decrease in late mortality. The use of bilateral ITA grafting with or without other grafts led to a 60% decrease in early mortality and a 33% decrease in late mortality, with all hazard ratios being within their confidence intervals, and hence significant, said Dr.Raza.
In addition, bilateral ITA grafting was found to be significantly more effective than single ITA grafting in decreasing late mortality in patients with multisystem disease.
Incomplete revascularization was not found to be associated with increased early mortality, but was significantly associated with a 10% increase in late mortality The use of off-pump vs. on-pump CABG had no statistically significant effect on early and late mortality, Dr. Raza added.
"Overall, bilateral ITA grafting and complete revascularization improved results of CABG in diabetics. These surgical techniques should be used in diabetics undergoing CABG," Dr. Raza stated.
In an interview, commenting on the significance of the research, Dr. Raza added: "Diabetes is an emerging epidemic affecting nearly 382 million people worldwide. Because it is a risk factor for development of coronary artery disease, the number of diabetics undergoing coronary surgery has increased to nearly 50% of all patients undergoing surgical revascularization today.
"We know from the BARI2D and FREEDOM studies that for diabetics with multivessel coronary artery disease, CABG results in better outcomes than medical therapy or percutaneous coronary intervention. However, what remains unclear is which surgical revascularization techniques improve the outcomes of CABG in patients with diabetes. Our study helps to answer this important question," he concluded.
Dr. Raza reported having no relevant disclosures.
Diabetes has historically been associated with greater mortality and more and worse postoperative complications after coronary artery bypass grafting (CABG). Researchers continue to search for improved surgical techniques and other options to improve CABG outcomes in diabetic patients.
The use of bilateral internal thoracic artery (ITA) grafting and complete revascularization was found to improve the results of CABG in patients with diabetes, according to the results of an assessment of nearly 12,000 diabetic patients who underwent surgical revascularization, according to Dr. Sajjad Raza, a research fellow in cardiac surgery, who presented the results of a study that he and his colleagues performed at the Cleveland Clinic.
Patients who were operated upon from January 1972 to January 2011 were included in the study.
"Our research was designed to identify surgical revascularization techniques that can improve early and late results in patients with diabetes undergoing CABG," said Dr. Raza at the annual meeting of the American Association for Thoracic Surgery.
Surgical revascularization techniques investigated comprised single (71%) and bilateral (7.9%) ITA with or without other grafts vs. the use of saphenous vein grafting alone (SVG, 21%), as well as incomplete (18%) vs. complete revascularization, and the use of off- (5.0%) vs. on-pump CABG. The median follow-up was nearly 8 years, with total follow-up of more than 100,000 patient-years, according to Dr. Raza.
Multivariable analysis was performed to assess the effect of surgical techniques on early and late mortality.
After adjustment for patient characteristics, single ITA grafting with or without other grafts vs. SVG alone was associated with a 43% decrease in early mortality and 17% decrease in late mortality. The use of bilateral ITA grafting with or without other grafts led to a 60% decrease in early mortality and a 33% decrease in late mortality, with all hazard ratios being within their confidence intervals, and hence significant, said Dr.Raza.
In addition, bilateral ITA grafting was found to be significantly more effective than single ITA grafting in decreasing late mortality in patients with multisystem disease.
Incomplete revascularization was not found to be associated with increased early mortality, but was significantly associated with a 10% increase in late mortality The use of off-pump vs. on-pump CABG had no statistically significant effect on early and late mortality, Dr. Raza added.
"Overall, bilateral ITA grafting and complete revascularization improved results of CABG in diabetics. These surgical techniques should be used in diabetics undergoing CABG," Dr. Raza stated.
In an interview, commenting on the significance of the research, Dr. Raza added: "Diabetes is an emerging epidemic affecting nearly 382 million people worldwide. Because it is a risk factor for development of coronary artery disease, the number of diabetics undergoing coronary surgery has increased to nearly 50% of all patients undergoing surgical revascularization today.
"We know from the BARI2D and FREEDOM studies that for diabetics with multivessel coronary artery disease, CABG results in better outcomes than medical therapy or percutaneous coronary intervention. However, what remains unclear is which surgical revascularization techniques improve the outcomes of CABG in patients with diabetes. Our study helps to answer this important question," he concluded.
Dr. Raza reported having no relevant disclosures.
Diabetes has historically been associated with greater mortality and more and worse postoperative complications after coronary artery bypass grafting (CABG). Researchers continue to search for improved surgical techniques and other options to improve CABG outcomes in diabetic patients.
The use of bilateral internal thoracic artery (ITA) grafting and complete revascularization was found to improve the results of CABG in patients with diabetes, according to the results of an assessment of nearly 12,000 diabetic patients who underwent surgical revascularization, according to Dr. Sajjad Raza, a research fellow in cardiac surgery, who presented the results of a study that he and his colleagues performed at the Cleveland Clinic.
Patients who were operated upon from January 1972 to January 2011 were included in the study.
"Our research was designed to identify surgical revascularization techniques that can improve early and late results in patients with diabetes undergoing CABG," said Dr. Raza at the annual meeting of the American Association for Thoracic Surgery.
Surgical revascularization techniques investigated comprised single (71%) and bilateral (7.9%) ITA with or without other grafts vs. the use of saphenous vein grafting alone (SVG, 21%), as well as incomplete (18%) vs. complete revascularization, and the use of off- (5.0%) vs. on-pump CABG. The median follow-up was nearly 8 years, with total follow-up of more than 100,000 patient-years, according to Dr. Raza.
Multivariable analysis was performed to assess the effect of surgical techniques on early and late mortality.
After adjustment for patient characteristics, single ITA grafting with or without other grafts vs. SVG alone was associated with a 43% decrease in early mortality and 17% decrease in late mortality. The use of bilateral ITA grafting with or without other grafts led to a 60% decrease in early mortality and a 33% decrease in late mortality, with all hazard ratios being within their confidence intervals, and hence significant, said Dr.Raza.
In addition, bilateral ITA grafting was found to be significantly more effective than single ITA grafting in decreasing late mortality in patients with multisystem disease.
Incomplete revascularization was not found to be associated with increased early mortality, but was significantly associated with a 10% increase in late mortality The use of off-pump vs. on-pump CABG had no statistically significant effect on early and late mortality, Dr. Raza added.
"Overall, bilateral ITA grafting and complete revascularization improved results of CABG in diabetics. These surgical techniques should be used in diabetics undergoing CABG," Dr. Raza stated.
In an interview, commenting on the significance of the research, Dr. Raza added: "Diabetes is an emerging epidemic affecting nearly 382 million people worldwide. Because it is a risk factor for development of coronary artery disease, the number of diabetics undergoing coronary surgery has increased to nearly 50% of all patients undergoing surgical revascularization today.
"We know from the BARI2D and FREEDOM studies that for diabetics with multivessel coronary artery disease, CABG results in better outcomes than medical therapy or percutaneous coronary intervention. However, what remains unclear is which surgical revascularization techniques improve the outcomes of CABG in patients with diabetes. Our study helps to answer this important question," he concluded.
Dr. Raza reported having no relevant disclosures.
TEVAR slows stable type B dissection
NEW YORK – Five-year results from the INSTEAD trial showed that patients with stable type B aortic dissections who were randomized to receive thoracic endovascular aneurysm repair plus optimal medical treatment showed significantly less aorta-specific mortality and delayed disease progression than did those who received OMT alone, according to Rachel Clough, Ph.D., MRCS, who presented the findings at the AATS 2014 Aortic Symposium.
The results indicate that delayed adverse events, such as sudden fatalities, expansion of the false lumen, rupture, and other late complications that occur in patients treated medically in the years subsequent to dissection, are prevented or attenuated with stent grafts.
"Uncomplicated patients with type B aortic dissection have traditionally been treated with antihypertensive medication but what we see is, even in patients with very good blood pressure control, there is a continued attrition rate looking out toward 5 years. In some series, more than 50% of patients are dead [at that time point]," said Dr. Clough of King’s College London.
INSTEAD (Investigation of Stent Grafts in Aortic Dissection) is the first randomized trial comparing thoracic endovascular aneurysm repair (TEVAR) with a stent graft. When 2-year results were published in 2009 (Circulation 2009;120:2519-28), there was criticism that the study was underpowered as no differences were seen in all-cause deaths, aorta-related deaths due to aortic rupture, or dissection progression plus rupture. There was even a trend toward better cumulative survival in the OMT group.
Sixty-eight patients were randomized to receive OMT and 72 patients received TEVAR plus OMT. Eighty-three percent had one stent placed, 11% had two stents, and 6% had three stents. The proximal thoracic aorta was covered in all cases, and the range of coverage was 15-35 cm.
No significant differences between groups were found in the comorbidity profile, presence of risk factors, or dissection morphology. With patients followed for 5 years, differences in outcomes between patients who had undergone TEVAR plus OMT and those who received only OMT began to emerge.
Analysis of these long-term data shows that patients with stable type B aortic dissections who were randomized to receive TEVAR plus OMT showed less aorta-specific mortality (7 vs. 19%, P = .04) and delayed disease progression (27% vs. 46%, P = .04) than did those who received OMT alone. Landmark analysis suggested a benefit of TEVAR for all endpoints between 2 and 5 years, including all-cause mortality (0 vs. 17%, P = .0003).
Both improved survival and less progression over 5 years after TEVAR were associated with stent graft–induced false lumen thrombosis in 91% of cases (P less than .0001). Use of the stent graft resulted in a significant reduction in false lumen diameter (P less than .0001) and significant increase in true lumen diameter (P less than .001). In contrast, a significant increase in aortic diameter was noted in those who received only OMT (P less than .0001).
"In conclusion, endovascular therapy in addition to medical therapy is associated with improved 5-year aortic-specific survival and delayed disease progression. In uncomplicated type B dissection with suitable anatomy, preemptive TEVAR should be considered to improve late outcomes," said Dr. Clough.
Dr. Clough had no disclosures. INSTEAD was sponsored by the Medtronic Bakken Research Center.
NEW YORK – Five-year results from the INSTEAD trial showed that patients with stable type B aortic dissections who were randomized to receive thoracic endovascular aneurysm repair plus optimal medical treatment showed significantly less aorta-specific mortality and delayed disease progression than did those who received OMT alone, according to Rachel Clough, Ph.D., MRCS, who presented the findings at the AATS 2014 Aortic Symposium.
The results indicate that delayed adverse events, such as sudden fatalities, expansion of the false lumen, rupture, and other late complications that occur in patients treated medically in the years subsequent to dissection, are prevented or attenuated with stent grafts.
"Uncomplicated patients with type B aortic dissection have traditionally been treated with antihypertensive medication but what we see is, even in patients with very good blood pressure control, there is a continued attrition rate looking out toward 5 years. In some series, more than 50% of patients are dead [at that time point]," said Dr. Clough of King’s College London.
INSTEAD (Investigation of Stent Grafts in Aortic Dissection) is the first randomized trial comparing thoracic endovascular aneurysm repair (TEVAR) with a stent graft. When 2-year results were published in 2009 (Circulation 2009;120:2519-28), there was criticism that the study was underpowered as no differences were seen in all-cause deaths, aorta-related deaths due to aortic rupture, or dissection progression plus rupture. There was even a trend toward better cumulative survival in the OMT group.
Sixty-eight patients were randomized to receive OMT and 72 patients received TEVAR plus OMT. Eighty-three percent had one stent placed, 11% had two stents, and 6% had three stents. The proximal thoracic aorta was covered in all cases, and the range of coverage was 15-35 cm.
No significant differences between groups were found in the comorbidity profile, presence of risk factors, or dissection morphology. With patients followed for 5 years, differences in outcomes between patients who had undergone TEVAR plus OMT and those who received only OMT began to emerge.
Analysis of these long-term data shows that patients with stable type B aortic dissections who were randomized to receive TEVAR plus OMT showed less aorta-specific mortality (7 vs. 19%, P = .04) and delayed disease progression (27% vs. 46%, P = .04) than did those who received OMT alone. Landmark analysis suggested a benefit of TEVAR for all endpoints between 2 and 5 years, including all-cause mortality (0 vs. 17%, P = .0003).
Both improved survival and less progression over 5 years after TEVAR were associated with stent graft–induced false lumen thrombosis in 91% of cases (P less than .0001). Use of the stent graft resulted in a significant reduction in false lumen diameter (P less than .0001) and significant increase in true lumen diameter (P less than .001). In contrast, a significant increase in aortic diameter was noted in those who received only OMT (P less than .0001).
"In conclusion, endovascular therapy in addition to medical therapy is associated with improved 5-year aortic-specific survival and delayed disease progression. In uncomplicated type B dissection with suitable anatomy, preemptive TEVAR should be considered to improve late outcomes," said Dr. Clough.
Dr. Clough had no disclosures. INSTEAD was sponsored by the Medtronic Bakken Research Center.
NEW YORK – Five-year results from the INSTEAD trial showed that patients with stable type B aortic dissections who were randomized to receive thoracic endovascular aneurysm repair plus optimal medical treatment showed significantly less aorta-specific mortality and delayed disease progression than did those who received OMT alone, according to Rachel Clough, Ph.D., MRCS, who presented the findings at the AATS 2014 Aortic Symposium.
The results indicate that delayed adverse events, such as sudden fatalities, expansion of the false lumen, rupture, and other late complications that occur in patients treated medically in the years subsequent to dissection, are prevented or attenuated with stent grafts.
"Uncomplicated patients with type B aortic dissection have traditionally been treated with antihypertensive medication but what we see is, even in patients with very good blood pressure control, there is a continued attrition rate looking out toward 5 years. In some series, more than 50% of patients are dead [at that time point]," said Dr. Clough of King’s College London.
INSTEAD (Investigation of Stent Grafts in Aortic Dissection) is the first randomized trial comparing thoracic endovascular aneurysm repair (TEVAR) with a stent graft. When 2-year results were published in 2009 (Circulation 2009;120:2519-28), there was criticism that the study was underpowered as no differences were seen in all-cause deaths, aorta-related deaths due to aortic rupture, or dissection progression plus rupture. There was even a trend toward better cumulative survival in the OMT group.
Sixty-eight patients were randomized to receive OMT and 72 patients received TEVAR plus OMT. Eighty-three percent had one stent placed, 11% had two stents, and 6% had three stents. The proximal thoracic aorta was covered in all cases, and the range of coverage was 15-35 cm.
No significant differences between groups were found in the comorbidity profile, presence of risk factors, or dissection morphology. With patients followed for 5 years, differences in outcomes between patients who had undergone TEVAR plus OMT and those who received only OMT began to emerge.
Analysis of these long-term data shows that patients with stable type B aortic dissections who were randomized to receive TEVAR plus OMT showed less aorta-specific mortality (7 vs. 19%, P = .04) and delayed disease progression (27% vs. 46%, P = .04) than did those who received OMT alone. Landmark analysis suggested a benefit of TEVAR for all endpoints between 2 and 5 years, including all-cause mortality (0 vs. 17%, P = .0003).
Both improved survival and less progression over 5 years after TEVAR were associated with stent graft–induced false lumen thrombosis in 91% of cases (P less than .0001). Use of the stent graft resulted in a significant reduction in false lumen diameter (P less than .0001) and significant increase in true lumen diameter (P less than .001). In contrast, a significant increase in aortic diameter was noted in those who received only OMT (P less than .0001).
"In conclusion, endovascular therapy in addition to medical therapy is associated with improved 5-year aortic-specific survival and delayed disease progression. In uncomplicated type B dissection with suitable anatomy, preemptive TEVAR should be considered to improve late outcomes," said Dr. Clough.
Dr. Clough had no disclosures. INSTEAD was sponsored by the Medtronic Bakken Research Center.
Major finding: Patients with stable type B aortic dissections who were randomized to receive TEVAR plus optimal medical treatment (OMT) showed significantly less aorta-specific mortality and delayed disease progression than did those who received OMT alone.
Data source: The study assessed the 5-year results of the INSTEAD trial of 68 patients randomized to receive OMT and 72 patients received TEVAR plus OMT
Disclosures: Dr. Clough had nothing to disclose. INSTEAD was sponsored by the Medtronic Bakken Research Center. Medtronic manufactures the Medtronic Talent Stent-Graft-System used as the endograft.
Risks of two types of polymer stents comparable
Biodegradable biolimus-eluting stents are as safe and effective as durable everolimus-eluting stents at 2 years’ follow-up, with no significant differences seen in rates of target lesion revascularization, mortality, or myocardial infarction.
The findings come from NEXT (NOBORI Biolimus-Eluting vs. XIENCE/PROMUS Everolimus-Eluting Stent Trial), a 3-year randomized trial led by Dr. Masahiro Natsuaki of Saiseikai Fukuoka (Japan) General Hospital. They were presented at the annual meeting of the American College of Cardiology and published in JAMA (doi:10.1001/jama.2014.3584).
NEXT compared the biodegradable polymer biolimus-eluting stent (BP-BES) to the durable polymer everolimus-eluting stent (DP-EES), measuring target lesion revascularization and whether BP-BES carries a risk for excess mortality or MI, compared with DP-EES, as shorter studies and meta-analyses have suggested.
Dr. Natsuaki and colleagues randomized 3,235 patients from nearly 100 treatment centers to BP-BES (n = 1,617) or DP-EES (n = 1,618), with 98% of all patients completing follow-up. Mortality and MI were comparable for both stents (7.8% for BP-BES vs. 7.7% for DP-EES; noninferiority, P = .003), and the need for target lesion revascularization was also comparable for both stents (6.2% vs. 6%; noninferiority, P less than .001). The researchers noted that "2 years is not long enough to confirm the long-term safety of BP-BES, and the study was underpowered for the interim analysis. Follow-up at 3 years will be important."
NEXT was sponsored by Terumo Japan, maker of the biodegradable stents. Two investigators disclosed they are advisers for Terumo Japan and Abbott Vascular Japan, maker of the durable polymer stents used.
Biodegradable biolimus-eluting stents are as safe and effective as durable everolimus-eluting stents at 2 years’ follow-up, with no significant differences seen in rates of target lesion revascularization, mortality, or myocardial infarction.
The findings come from NEXT (NOBORI Biolimus-Eluting vs. XIENCE/PROMUS Everolimus-Eluting Stent Trial), a 3-year randomized trial led by Dr. Masahiro Natsuaki of Saiseikai Fukuoka (Japan) General Hospital. They were presented at the annual meeting of the American College of Cardiology and published in JAMA (doi:10.1001/jama.2014.3584).
NEXT compared the biodegradable polymer biolimus-eluting stent (BP-BES) to the durable polymer everolimus-eluting stent (DP-EES), measuring target lesion revascularization and whether BP-BES carries a risk for excess mortality or MI, compared with DP-EES, as shorter studies and meta-analyses have suggested.
Dr. Natsuaki and colleagues randomized 3,235 patients from nearly 100 treatment centers to BP-BES (n = 1,617) or DP-EES (n = 1,618), with 98% of all patients completing follow-up. Mortality and MI were comparable for both stents (7.8% for BP-BES vs. 7.7% for DP-EES; noninferiority, P = .003), and the need for target lesion revascularization was also comparable for both stents (6.2% vs. 6%; noninferiority, P less than .001). The researchers noted that "2 years is not long enough to confirm the long-term safety of BP-BES, and the study was underpowered for the interim analysis. Follow-up at 3 years will be important."
NEXT was sponsored by Terumo Japan, maker of the biodegradable stents. Two investigators disclosed they are advisers for Terumo Japan and Abbott Vascular Japan, maker of the durable polymer stents used.
Biodegradable biolimus-eluting stents are as safe and effective as durable everolimus-eluting stents at 2 years’ follow-up, with no significant differences seen in rates of target lesion revascularization, mortality, or myocardial infarction.
The findings come from NEXT (NOBORI Biolimus-Eluting vs. XIENCE/PROMUS Everolimus-Eluting Stent Trial), a 3-year randomized trial led by Dr. Masahiro Natsuaki of Saiseikai Fukuoka (Japan) General Hospital. They were presented at the annual meeting of the American College of Cardiology and published in JAMA (doi:10.1001/jama.2014.3584).
NEXT compared the biodegradable polymer biolimus-eluting stent (BP-BES) to the durable polymer everolimus-eluting stent (DP-EES), measuring target lesion revascularization and whether BP-BES carries a risk for excess mortality or MI, compared with DP-EES, as shorter studies and meta-analyses have suggested.
Dr. Natsuaki and colleagues randomized 3,235 patients from nearly 100 treatment centers to BP-BES (n = 1,617) or DP-EES (n = 1,618), with 98% of all patients completing follow-up. Mortality and MI were comparable for both stents (7.8% for BP-BES vs. 7.7% for DP-EES; noninferiority, P = .003), and the need for target lesion revascularization was also comparable for both stents (6.2% vs. 6%; noninferiority, P less than .001). The researchers noted that "2 years is not long enough to confirm the long-term safety of BP-BES, and the study was underpowered for the interim analysis. Follow-up at 3 years will be important."
NEXT was sponsored by Terumo Japan, maker of the biodegradable stents. Two investigators disclosed they are advisers for Terumo Japan and Abbott Vascular Japan, maker of the durable polymer stents used.
Major finding: Mortality and myocardial infarction were comparable for BP-BES (7.8%) vs. DP-EES (7.7%); noninferiority, P = .003), as was the need for target lesion revascularization (6.2% vs. 6%; noninferiority, P less than .001).
Data source: The NOBORI Biolimus-Eluting vs. XIENCE/PROMUS Everolimus-Eluting Stent Trial, a randomized trial of 3,235 patients.
Disclosures: NEXT was sponsored by Terumo Japan, the maker of BP-BES. Two investigators disclosed that they serve as advisers for Terumo Japan and Abbott Vascular Japan, maker of DP-EES.
Angiography timing not a risk for acute kidney injury
Acute kidney injury is a serious adverse effect of cardiac surgery, and contrast-induced nephropathy from coronary angiography has been suggested as a potentially important component. However, the results of a retrospective study of more than 2,500 patients showed that acute kidney injury is significantly higher only in those patients who have combined cardiac surgery within 24 hours of catheterization.
The study by Dr. Giovanni Mariscalco of the Varese (Italy) University Hospital, and his colleagues assessed all consecutive patients undergoing cardiac surgery at the hospital between Jan. 1, 2005, and Dec. 31, 2011. The operations performed were isolated coronary artery bypass grafting (CABG), valve surgery with or without concomitant CABG, and proximal aortic procedures. After exclusion of patients who did not undergo cardiopulmonary bypass, a known major cause of acute kidney injury (AKI), and those who died during the procedure, a total of 2,504 patients remained. These patients had a mean age of 68.4 years; 67.3% were men, according to the report.
The primary endpoint was the effect of timing between cardiac catheterization and surgery on the development of AKI. Postoperative AKI was defined by the consensus RIFLE criteria (risk, injury, failure, loss of function, and end-stage renal disease), using the maximal change in serum creatinine and the estimated glomerular filtration rate during the first 7 days after surgery, compared with baseline.
The overall incidence of AKI after surgery was 9.2% (230/2,504 patients). A breakdown by procedure showed that AKI occurred in 7.7% of isolated CABG patients, 12.2% of isolated valve patients, 9.5% of combined-procedure patients, and 9.5% of the proximal aorta surgery patients (Int. J. Cardiol. 2014;173:46-54).
As has been seen in previous studies, AKI was associated with patient-specific pre- and perioperative variables, including increased patient age, added comorbidities, longer cardiopulmonary bypass (CPB) times, higher rates of combined procedures, and the use of intra-aortic balloon pumps.
Unadjusted analysis of the total cohort showed AKI was significantly associated with contrast exposure within 1 day of surgery. However, in multivariable analysis, the time interval between catheterization and surgery was not an independent predictor of postoperative AKI for the total cohort. In subgroup analysis, only the combined valve and CABG group of patients showed an independent association of contrast exposure within 1 day before surgery and AKI in both the prematched (odds ratio, 2.69; P = .004) and the postmatched (OR, 3.68; P = .014) groups.
"Avoiding surgery within 1 day after contrast exposure should be recommended for patients undergoing valve surgery with concomitant CABG only. For other types of cardiac operations, delaying cardiac surgery after contrast exposure seems not to be justified," the researchers concluded.
The study was supported by the Fondazione Cesare Bartorelli. The authors reported having no disclosures.
Acute kidney injury is a serious adverse effect of cardiac surgery, and contrast-induced nephropathy from coronary angiography has been suggested as a potentially important component. However, the results of a retrospective study of more than 2,500 patients showed that acute kidney injury is significantly higher only in those patients who have combined cardiac surgery within 24 hours of catheterization.
The study by Dr. Giovanni Mariscalco of the Varese (Italy) University Hospital, and his colleagues assessed all consecutive patients undergoing cardiac surgery at the hospital between Jan. 1, 2005, and Dec. 31, 2011. The operations performed were isolated coronary artery bypass grafting (CABG), valve surgery with or without concomitant CABG, and proximal aortic procedures. After exclusion of patients who did not undergo cardiopulmonary bypass, a known major cause of acute kidney injury (AKI), and those who died during the procedure, a total of 2,504 patients remained. These patients had a mean age of 68.4 years; 67.3% were men, according to the report.
The primary endpoint was the effect of timing between cardiac catheterization and surgery on the development of AKI. Postoperative AKI was defined by the consensus RIFLE criteria (risk, injury, failure, loss of function, and end-stage renal disease), using the maximal change in serum creatinine and the estimated glomerular filtration rate during the first 7 days after surgery, compared with baseline.
The overall incidence of AKI after surgery was 9.2% (230/2,504 patients). A breakdown by procedure showed that AKI occurred in 7.7% of isolated CABG patients, 12.2% of isolated valve patients, 9.5% of combined-procedure patients, and 9.5% of the proximal aorta surgery patients (Int. J. Cardiol. 2014;173:46-54).
As has been seen in previous studies, AKI was associated with patient-specific pre- and perioperative variables, including increased patient age, added comorbidities, longer cardiopulmonary bypass (CPB) times, higher rates of combined procedures, and the use of intra-aortic balloon pumps.
Unadjusted analysis of the total cohort showed AKI was significantly associated with contrast exposure within 1 day of surgery. However, in multivariable analysis, the time interval between catheterization and surgery was not an independent predictor of postoperative AKI for the total cohort. In subgroup analysis, only the combined valve and CABG group of patients showed an independent association of contrast exposure within 1 day before surgery and AKI in both the prematched (odds ratio, 2.69; P = .004) and the postmatched (OR, 3.68; P = .014) groups.
"Avoiding surgery within 1 day after contrast exposure should be recommended for patients undergoing valve surgery with concomitant CABG only. For other types of cardiac operations, delaying cardiac surgery after contrast exposure seems not to be justified," the researchers concluded.
The study was supported by the Fondazione Cesare Bartorelli. The authors reported having no disclosures.
Acute kidney injury is a serious adverse effect of cardiac surgery, and contrast-induced nephropathy from coronary angiography has been suggested as a potentially important component. However, the results of a retrospective study of more than 2,500 patients showed that acute kidney injury is significantly higher only in those patients who have combined cardiac surgery within 24 hours of catheterization.
The study by Dr. Giovanni Mariscalco of the Varese (Italy) University Hospital, and his colleagues assessed all consecutive patients undergoing cardiac surgery at the hospital between Jan. 1, 2005, and Dec. 31, 2011. The operations performed were isolated coronary artery bypass grafting (CABG), valve surgery with or without concomitant CABG, and proximal aortic procedures. After exclusion of patients who did not undergo cardiopulmonary bypass, a known major cause of acute kidney injury (AKI), and those who died during the procedure, a total of 2,504 patients remained. These patients had a mean age of 68.4 years; 67.3% were men, according to the report.
The primary endpoint was the effect of timing between cardiac catheterization and surgery on the development of AKI. Postoperative AKI was defined by the consensus RIFLE criteria (risk, injury, failure, loss of function, and end-stage renal disease), using the maximal change in serum creatinine and the estimated glomerular filtration rate during the first 7 days after surgery, compared with baseline.
The overall incidence of AKI after surgery was 9.2% (230/2,504 patients). A breakdown by procedure showed that AKI occurred in 7.7% of isolated CABG patients, 12.2% of isolated valve patients, 9.5% of combined-procedure patients, and 9.5% of the proximal aorta surgery patients (Int. J. Cardiol. 2014;173:46-54).
As has been seen in previous studies, AKI was associated with patient-specific pre- and perioperative variables, including increased patient age, added comorbidities, longer cardiopulmonary bypass (CPB) times, higher rates of combined procedures, and the use of intra-aortic balloon pumps.
Unadjusted analysis of the total cohort showed AKI was significantly associated with contrast exposure within 1 day of surgery. However, in multivariable analysis, the time interval between catheterization and surgery was not an independent predictor of postoperative AKI for the total cohort. In subgroup analysis, only the combined valve and CABG group of patients showed an independent association of contrast exposure within 1 day before surgery and AKI in both the prematched (odds ratio, 2.69; P = .004) and the postmatched (OR, 3.68; P = .014) groups.
"Avoiding surgery within 1 day after contrast exposure should be recommended for patients undergoing valve surgery with concomitant CABG only. For other types of cardiac operations, delaying cardiac surgery after contrast exposure seems not to be justified," the researchers concluded.
The study was supported by the Fondazione Cesare Bartorelli. The authors reported having no disclosures.
Major finding: Only combined surgery plus contrast within 1 day before surgery was significantly associated with AKI in the prematched (OR, 2.69) and postmatched (OR, 3.68) groups.
Data source: A single-institute, retrospective study of 2,504 cardiac surgery patients.
Disclosures: The study was supported by the Fondazione Cesare Bartorelli. The authors reported having no financial disclosures.
Open TAAA repair in octogenarians: Exhibit caution
NEW YORK – Outcomes of thoracoabdominal aortic aneurysm (TAAA) repair in octogenarians vary considerably with the extent of repair. Those who undergo Extent II TAAA repair have significantly higher risks of morbidity and mortality, while Extent I, III, and IV repairs can be performed with relatively good outcomes, according to Dr. Muhammad Aftab, who presented the findings at the 2014 AATSAortic Symposium
"Extensive TAAA repair should be performed with caution in octogenarians," says Dr. Aftab, a Fellow in CT surgery at the Baylor College of Medicine–Texas Heart Institute, Houston. He recommends that a thorough preoperative discussion to assess the risks and benefits with the patient and his family is necessary before proceeding with surgery.
In this retrospective review of patients seen between January 2005 and September 2013, octogenarians with TAAAs (n = 88) were compared with a younger cohort (n = 1,179 patients, aged 70 years). Dr. Aftab found that octogenarians were threefold more likely to present with aneurysm rupture (13.6% vs. 4.6%; P less than .001) but less likely to present with aortic dissections (12.5% vs. 43.9%; P less than .001) than did the younger patients.
The use of adjunctive interventions, such as left heart bypass, cerebrospinal fluid drainage, cold renal perfusion, and visceral perfusion differed significantly among the octogenarians based on the extent of repair and clinical condition (all P less than .001). Because the octogenarians had a greater atherosclerotic burden and higher incidence of renal and mesenteric occlusive disease, they were also more likely to require renal/visceral endarterectomy, stenting, or both (57.9% vs. 33.6%; P less than.001).
Octogenarians had higher rates of operative mortality (26.1% vs. 6.9%), in-hospital deaths (25% vs. 6.4%), 30-day deaths (13.6% vs. 4.8%), and adverse outcomes (36.4% vs. 15.7%; P less than .001) than did the younger group, all significant differences.
The outcomes included significantly higher rates of permanent renal failure, cardiac complications, and pulmonary complications. The octogenarians had longer recovery times, as suggested by longer postoperative ICU and hospital stays. Poor outcomes differed according to the extent of surgery, and seemed to be exacerbated for those who underwent repair of Extent II aneurysms (according to the Crawford Classification, these involve the subclavian artery and extend to the bifurcation of the aorta in the pelvis). For instance, the Extent II group had the highest risk of operative mortality (61.5%) vs. Extent I (31.6%), III (21.4%), and IV (10.7%), a significant difference. The Extent II group also had much higher rates of in-hospital and 30-day death rates. The most common causes of deaths for the Extent II octogenarians were multisystem organ failure and cardiac problems.
Adverse outcomes were also significantly much higher for the Extent II group (76.9%) than for the other groups (42.1%, 28.6%, and 21.4%). Similar patterns were found for permanent paraplegia, renal failure requiring permanent dialysis, stroke, and days spent in the ICU. Almost 85% of those who required Extent II repair needed renal/visceral endarterectomy, stenting, or both as a part of the surgical procedure.
Extent II TAAA repair was an independent predictor of perioperative mortality by multivariate analysis, conferring an 11-fold increased risk of death. Aneurysm rupture and dissection were also identified as predictors of perioperative mortality while only Extent II TAAA and dissection were independent predictors of adverse outcomes.
While these problems were exacerbated in those with Extent II repairs, Extent I, III, and IV TAAA repairs may be performed with relatively low risk, according to Dr. Aftab.
Dr. Aftab had no disclosures.
NEW YORK – Outcomes of thoracoabdominal aortic aneurysm (TAAA) repair in octogenarians vary considerably with the extent of repair. Those who undergo Extent II TAAA repair have significantly higher risks of morbidity and mortality, while Extent I, III, and IV repairs can be performed with relatively good outcomes, according to Dr. Muhammad Aftab, who presented the findings at the 2014 AATSAortic Symposium
"Extensive TAAA repair should be performed with caution in octogenarians," says Dr. Aftab, a Fellow in CT surgery at the Baylor College of Medicine–Texas Heart Institute, Houston. He recommends that a thorough preoperative discussion to assess the risks and benefits with the patient and his family is necessary before proceeding with surgery.
In this retrospective review of patients seen between January 2005 and September 2013, octogenarians with TAAAs (n = 88) were compared with a younger cohort (n = 1,179 patients, aged 70 years). Dr. Aftab found that octogenarians were threefold more likely to present with aneurysm rupture (13.6% vs. 4.6%; P less than .001) but less likely to present with aortic dissections (12.5% vs. 43.9%; P less than .001) than did the younger patients.
The use of adjunctive interventions, such as left heart bypass, cerebrospinal fluid drainage, cold renal perfusion, and visceral perfusion differed significantly among the octogenarians based on the extent of repair and clinical condition (all P less than .001). Because the octogenarians had a greater atherosclerotic burden and higher incidence of renal and mesenteric occlusive disease, they were also more likely to require renal/visceral endarterectomy, stenting, or both (57.9% vs. 33.6%; P less than.001).
Octogenarians had higher rates of operative mortality (26.1% vs. 6.9%), in-hospital deaths (25% vs. 6.4%), 30-day deaths (13.6% vs. 4.8%), and adverse outcomes (36.4% vs. 15.7%; P less than .001) than did the younger group, all significant differences.
The outcomes included significantly higher rates of permanent renal failure, cardiac complications, and pulmonary complications. The octogenarians had longer recovery times, as suggested by longer postoperative ICU and hospital stays. Poor outcomes differed according to the extent of surgery, and seemed to be exacerbated for those who underwent repair of Extent II aneurysms (according to the Crawford Classification, these involve the subclavian artery and extend to the bifurcation of the aorta in the pelvis). For instance, the Extent II group had the highest risk of operative mortality (61.5%) vs. Extent I (31.6%), III (21.4%), and IV (10.7%), a significant difference. The Extent II group also had much higher rates of in-hospital and 30-day death rates. The most common causes of deaths for the Extent II octogenarians were multisystem organ failure and cardiac problems.
Adverse outcomes were also significantly much higher for the Extent II group (76.9%) than for the other groups (42.1%, 28.6%, and 21.4%). Similar patterns were found for permanent paraplegia, renal failure requiring permanent dialysis, stroke, and days spent in the ICU. Almost 85% of those who required Extent II repair needed renal/visceral endarterectomy, stenting, or both as a part of the surgical procedure.
Extent II TAAA repair was an independent predictor of perioperative mortality by multivariate analysis, conferring an 11-fold increased risk of death. Aneurysm rupture and dissection were also identified as predictors of perioperative mortality while only Extent II TAAA and dissection were independent predictors of adverse outcomes.
While these problems were exacerbated in those with Extent II repairs, Extent I, III, and IV TAAA repairs may be performed with relatively low risk, according to Dr. Aftab.
Dr. Aftab had no disclosures.
NEW YORK – Outcomes of thoracoabdominal aortic aneurysm (TAAA) repair in octogenarians vary considerably with the extent of repair. Those who undergo Extent II TAAA repair have significantly higher risks of morbidity and mortality, while Extent I, III, and IV repairs can be performed with relatively good outcomes, according to Dr. Muhammad Aftab, who presented the findings at the 2014 AATSAortic Symposium
"Extensive TAAA repair should be performed with caution in octogenarians," says Dr. Aftab, a Fellow in CT surgery at the Baylor College of Medicine–Texas Heart Institute, Houston. He recommends that a thorough preoperative discussion to assess the risks and benefits with the patient and his family is necessary before proceeding with surgery.
In this retrospective review of patients seen between January 2005 and September 2013, octogenarians with TAAAs (n = 88) were compared with a younger cohort (n = 1,179 patients, aged 70 years). Dr. Aftab found that octogenarians were threefold more likely to present with aneurysm rupture (13.6% vs. 4.6%; P less than .001) but less likely to present with aortic dissections (12.5% vs. 43.9%; P less than .001) than did the younger patients.
The use of adjunctive interventions, such as left heart bypass, cerebrospinal fluid drainage, cold renal perfusion, and visceral perfusion differed significantly among the octogenarians based on the extent of repair and clinical condition (all P less than .001). Because the octogenarians had a greater atherosclerotic burden and higher incidence of renal and mesenteric occlusive disease, they were also more likely to require renal/visceral endarterectomy, stenting, or both (57.9% vs. 33.6%; P less than.001).
Octogenarians had higher rates of operative mortality (26.1% vs. 6.9%), in-hospital deaths (25% vs. 6.4%), 30-day deaths (13.6% vs. 4.8%), and adverse outcomes (36.4% vs. 15.7%; P less than .001) than did the younger group, all significant differences.
The outcomes included significantly higher rates of permanent renal failure, cardiac complications, and pulmonary complications. The octogenarians had longer recovery times, as suggested by longer postoperative ICU and hospital stays. Poor outcomes differed according to the extent of surgery, and seemed to be exacerbated for those who underwent repair of Extent II aneurysms (according to the Crawford Classification, these involve the subclavian artery and extend to the bifurcation of the aorta in the pelvis). For instance, the Extent II group had the highest risk of operative mortality (61.5%) vs. Extent I (31.6%), III (21.4%), and IV (10.7%), a significant difference. The Extent II group also had much higher rates of in-hospital and 30-day death rates. The most common causes of deaths for the Extent II octogenarians were multisystem organ failure and cardiac problems.
Adverse outcomes were also significantly much higher for the Extent II group (76.9%) than for the other groups (42.1%, 28.6%, and 21.4%). Similar patterns were found for permanent paraplegia, renal failure requiring permanent dialysis, stroke, and days spent in the ICU. Almost 85% of those who required Extent II repair needed renal/visceral endarterectomy, stenting, or both as a part of the surgical procedure.
Extent II TAAA repair was an independent predictor of perioperative mortality by multivariate analysis, conferring an 11-fold increased risk of death. Aneurysm rupture and dissection were also identified as predictors of perioperative mortality while only Extent II TAAA and dissection were independent predictors of adverse outcomes.
While these problems were exacerbated in those with Extent II repairs, Extent I, III, and IV TAAA repairs may be performed with relatively low risk, according to Dr. Aftab.
Dr. Aftab had no disclosures.
Key clinical point: Octogenarians with TAAAs present more challenges than younger individuals and their outcomes vary greatly according to the type of aneurysm repair.
Major finding: A study that compared octogenarians with thoracoabdominal aortic aneurysms (TAAAs) to a younger cohort found that octogenarians were more at risk for aneurysm rupture, were more likely to need visceral-branch endarterectomy/stenting, had more adverse postoperative outcomes, and higher rates of operative mortality and longer postoperative ICU and hospital stays. While these problems were exacerbated in those with Extent II repairs, Extent I, III, and IV TAAA repairs can be performed with relatively low risk. Younger patients were more likely than octogenarians to present with aortic dissections.
Data source: Retrospective review.
Disclosures: Dr. Aftab had no relevant disclosures.