User login
Delay can reduce TEVAR problems with type B dissections
NEW YORK – Patients with acute type B aortic dissections who are treated with thoracic endovascular aortic repair within 48 hours of symptom onset are more than three times as likely to have a severe complication as are those who are treated between 2 and 6 weeks after initial presentation, according to Dr. Nimesh D. Desai.
"There has been a dramatic increase in survival of patients managed with TEVAR for life-threatening complications of type B dissection versus any other therapy. Recently, [FDA] approval of two devices in the U.S. (Gore cTAG and Medtronic Valiant Captiva TEVAR grafts) has really opened up the field of inquiry into the optimal timing of TEVAR in the nonemergent, non–life-threatening complicated type B dissection patient. In the current study, we analyzed the impact of timing of intervention," Dr. Desai said at the meeting sponsored by the American Association for Thoracic Surgery.
Between 2005 and 2012, 317 people were admitted to the Hospital of the University of Pennsylvania, Philadelphia, for acute (less than 6 weeks) type B dissections, said Dr. Desai. He reviewed the outcomes for 132 patients who had undergone TEVAR, dividing them into three groups according to the timing of the procedure: acute/early (TEVAR within 48 hours of symptom onset), n = 70; acute/delayed (TEVAR 48 hours to 14 days following symptom onset), n = 44; and subacute (TEVAR 2-6 weeks following symptom onset), n = 18. Patients in all three groups were generally between 63 and 65 years old. Most were men who had histories of severe hypertension and smoking. About 10% had a previous stroke.
Those in the acute/early group were more likely to have undergone TEVAR for life-threatening conditions. For instance, 43.28% of the acute/early group had a contained rupture vs. 25% of the acute/delayed and 11.11% of the subacute patients, a significant difference. The acute/early group also had higher rates of frank rupture and clinical malperfusion than did the other groups.
In contrast, 56% of the subacute group underwent TEVAR because they manifested "softer indications" of impending rupture or radiographic malperfusion, which were not considered to be as emergent. Three-quarters of the subacute group had already been discharged home and were readmitted for the procedure, reported Dr. Desai, who is at the University of Pennsylvania.
The overall rate of severe postoperative complications was 39% in the acute/early group, 27% in the acute/delayed group, and 11.1% in the subacute group, a significant difference.
In-house mortality was 8.5% in the acute/early group (nine patients), 4.5% in the acute/delayed group (three), and 0 in the subacute group, a nonsignificant difference. Similar, but nonsignificant trends were found in 30-day mortality (11.7%, 6.8%, and 0%) and stroke (5.6%, 4.6%, and 0%).
An opposite trend toward higher rates of paralysis was observed in the subacute group (7.04%, 4.5%, and 9.5%, respectively, in the three groups).
The incidence of retrograde type A aortic dissection was 8.5% in the acute/early, 6.8% in the acute/delayed, and 4.7% in the subacute groups. "Retrograde type A dissections are an iatrogenic disease caused by stenting type B dissections," said Dr. Desai. "They almost never happen with any other type of stent graft category."
He said that in his experience, the risk increases with excessive oversizing of stents and the dissections tend to occur at the interface between the native aorta and stent graft. In addition, he noted, many of the dissections occur a year or more after the original dissection.
"Delayed intervention appears to lower the risk of complications of TEVAR for aortic dissection in patients who are stable enough to wait. Our practice is to wait 10-14 days for remodeling indications," said Dr. Desai. Type B patients who are initially managed medically should be followed closely, he said, because they are at risk for the onset of new indications that might require TEVAR.
Operator experience and adequate case planning are critical when treating patients in the setting of acute or subacute type A dissections, Dr. Desai said, adding that the development of devices specific for dissections, rather than those designed for aneurysm pathologies, may lead to fewer complications.
Dr. Desai is a primary investigator for Food and Drug Administration TEVAR trials for W.L. Gore and Associates, Medtronic, and Cook Medical.
NEW YORK – Patients with acute type B aortic dissections who are treated with thoracic endovascular aortic repair within 48 hours of symptom onset are more than three times as likely to have a severe complication as are those who are treated between 2 and 6 weeks after initial presentation, according to Dr. Nimesh D. Desai.
"There has been a dramatic increase in survival of patients managed with TEVAR for life-threatening complications of type B dissection versus any other therapy. Recently, [FDA] approval of two devices in the U.S. (Gore cTAG and Medtronic Valiant Captiva TEVAR grafts) has really opened up the field of inquiry into the optimal timing of TEVAR in the nonemergent, non–life-threatening complicated type B dissection patient. In the current study, we analyzed the impact of timing of intervention," Dr. Desai said at the meeting sponsored by the American Association for Thoracic Surgery.
Between 2005 and 2012, 317 people were admitted to the Hospital of the University of Pennsylvania, Philadelphia, for acute (less than 6 weeks) type B dissections, said Dr. Desai. He reviewed the outcomes for 132 patients who had undergone TEVAR, dividing them into three groups according to the timing of the procedure: acute/early (TEVAR within 48 hours of symptom onset), n = 70; acute/delayed (TEVAR 48 hours to 14 days following symptom onset), n = 44; and subacute (TEVAR 2-6 weeks following symptom onset), n = 18. Patients in all three groups were generally between 63 and 65 years old. Most were men who had histories of severe hypertension and smoking. About 10% had a previous stroke.
Those in the acute/early group were more likely to have undergone TEVAR for life-threatening conditions. For instance, 43.28% of the acute/early group had a contained rupture vs. 25% of the acute/delayed and 11.11% of the subacute patients, a significant difference. The acute/early group also had higher rates of frank rupture and clinical malperfusion than did the other groups.
In contrast, 56% of the subacute group underwent TEVAR because they manifested "softer indications" of impending rupture or radiographic malperfusion, which were not considered to be as emergent. Three-quarters of the subacute group had already been discharged home and were readmitted for the procedure, reported Dr. Desai, who is at the University of Pennsylvania.
The overall rate of severe postoperative complications was 39% in the acute/early group, 27% in the acute/delayed group, and 11.1% in the subacute group, a significant difference.
In-house mortality was 8.5% in the acute/early group (nine patients), 4.5% in the acute/delayed group (three), and 0 in the subacute group, a nonsignificant difference. Similar, but nonsignificant trends were found in 30-day mortality (11.7%, 6.8%, and 0%) and stroke (5.6%, 4.6%, and 0%).
An opposite trend toward higher rates of paralysis was observed in the subacute group (7.04%, 4.5%, and 9.5%, respectively, in the three groups).
The incidence of retrograde type A aortic dissection was 8.5% in the acute/early, 6.8% in the acute/delayed, and 4.7% in the subacute groups. "Retrograde type A dissections are an iatrogenic disease caused by stenting type B dissections," said Dr. Desai. "They almost never happen with any other type of stent graft category."
He said that in his experience, the risk increases with excessive oversizing of stents and the dissections tend to occur at the interface between the native aorta and stent graft. In addition, he noted, many of the dissections occur a year or more after the original dissection.
"Delayed intervention appears to lower the risk of complications of TEVAR for aortic dissection in patients who are stable enough to wait. Our practice is to wait 10-14 days for remodeling indications," said Dr. Desai. Type B patients who are initially managed medically should be followed closely, he said, because they are at risk for the onset of new indications that might require TEVAR.
Operator experience and adequate case planning are critical when treating patients in the setting of acute or subacute type A dissections, Dr. Desai said, adding that the development of devices specific for dissections, rather than those designed for aneurysm pathologies, may lead to fewer complications.
Dr. Desai is a primary investigator for Food and Drug Administration TEVAR trials for W.L. Gore and Associates, Medtronic, and Cook Medical.
NEW YORK – Patients with acute type B aortic dissections who are treated with thoracic endovascular aortic repair within 48 hours of symptom onset are more than three times as likely to have a severe complication as are those who are treated between 2 and 6 weeks after initial presentation, according to Dr. Nimesh D. Desai.
"There has been a dramatic increase in survival of patients managed with TEVAR for life-threatening complications of type B dissection versus any other therapy. Recently, [FDA] approval of two devices in the U.S. (Gore cTAG and Medtronic Valiant Captiva TEVAR grafts) has really opened up the field of inquiry into the optimal timing of TEVAR in the nonemergent, non–life-threatening complicated type B dissection patient. In the current study, we analyzed the impact of timing of intervention," Dr. Desai said at the meeting sponsored by the American Association for Thoracic Surgery.
Between 2005 and 2012, 317 people were admitted to the Hospital of the University of Pennsylvania, Philadelphia, for acute (less than 6 weeks) type B dissections, said Dr. Desai. He reviewed the outcomes for 132 patients who had undergone TEVAR, dividing them into three groups according to the timing of the procedure: acute/early (TEVAR within 48 hours of symptom onset), n = 70; acute/delayed (TEVAR 48 hours to 14 days following symptom onset), n = 44; and subacute (TEVAR 2-6 weeks following symptom onset), n = 18. Patients in all three groups were generally between 63 and 65 years old. Most were men who had histories of severe hypertension and smoking. About 10% had a previous stroke.
Those in the acute/early group were more likely to have undergone TEVAR for life-threatening conditions. For instance, 43.28% of the acute/early group had a contained rupture vs. 25% of the acute/delayed and 11.11% of the subacute patients, a significant difference. The acute/early group also had higher rates of frank rupture and clinical malperfusion than did the other groups.
In contrast, 56% of the subacute group underwent TEVAR because they manifested "softer indications" of impending rupture or radiographic malperfusion, which were not considered to be as emergent. Three-quarters of the subacute group had already been discharged home and were readmitted for the procedure, reported Dr. Desai, who is at the University of Pennsylvania.
The overall rate of severe postoperative complications was 39% in the acute/early group, 27% in the acute/delayed group, and 11.1% in the subacute group, a significant difference.
In-house mortality was 8.5% in the acute/early group (nine patients), 4.5% in the acute/delayed group (three), and 0 in the subacute group, a nonsignificant difference. Similar, but nonsignificant trends were found in 30-day mortality (11.7%, 6.8%, and 0%) and stroke (5.6%, 4.6%, and 0%).
An opposite trend toward higher rates of paralysis was observed in the subacute group (7.04%, 4.5%, and 9.5%, respectively, in the three groups).
The incidence of retrograde type A aortic dissection was 8.5% in the acute/early, 6.8% in the acute/delayed, and 4.7% in the subacute groups. "Retrograde type A dissections are an iatrogenic disease caused by stenting type B dissections," said Dr. Desai. "They almost never happen with any other type of stent graft category."
He said that in his experience, the risk increases with excessive oversizing of stents and the dissections tend to occur at the interface between the native aorta and stent graft. In addition, he noted, many of the dissections occur a year or more after the original dissection.
"Delayed intervention appears to lower the risk of complications of TEVAR for aortic dissection in patients who are stable enough to wait. Our practice is to wait 10-14 days for remodeling indications," said Dr. Desai. Type B patients who are initially managed medically should be followed closely, he said, because they are at risk for the onset of new indications that might require TEVAR.
Operator experience and adequate case planning are critical when treating patients in the setting of acute or subacute type A dissections, Dr. Desai said, adding that the development of devices specific for dissections, rather than those designed for aneurysm pathologies, may lead to fewer complications.
Dr. Desai is a primary investigator for Food and Drug Administration TEVAR trials for W.L. Gore and Associates, Medtronic, and Cook Medical.
Key clinical point: Delayed intervention appears to lower the risk of complications of TEVAR for aortic dissection in patients who are stable enough to wait.
Major finding: The overall risk of severe complications was more than threefold higher in patients who underwent TEVAR for acute type B aortic dissections within 48 hours of initial presentation, compared with those whose treatment could be delayed until 2-6 weeks after symptom onset.
Data source: A single-center, retrospective registry analysis of 317 patients.
Disclosures: Dr. Desai said he had no relevant financial disclosures.
TAVR beat surgery in high-risk aortic stenosis patients
WASHINGTON - A first in transcatheter aortic valve replacement trials, the CoreValve prosthesis was superior to surgical valve replacement in patients with severe aortic stenosis at increased surgical risk, showing a significantly lower risk of mortality 1 year later.
In the U.S. CoreValve High Risk Study, a prospective randomized controlled study of almost 800 patients, the rate of all-cause mortality at 1 year, the primary endpoint, was 14.2% among those in the transcatheter aortic valve replacement (TAVR) group, compared with 19.1% among those in the surgery group, a statistically significant difference that represented a 26% survival benefit at 1 year for the CoreValve, Dr. David H. Adams reported at the annual meeting of the American College of Cardiology.
This is the first prospective, randomized study to show superiority for transcatheter valve therapy over surgery, and "there's no study or trial that I'm aware of that's suggested that TAVR patients would have a superior survival outcome," Dr. Adams of Mount Sinai Medical Center, New York, said in an interview. Based on these results, he said he expects that TAVR "will increasingly become the alternative of choice for patients" at this level of risk.
The study was the high-risk arm of the U.S. CoreValve pivotal trial. The CoreValve self-expanding prosthesis was approved in January 2014 by the Food and Drug Administration for use in extreme risk patients, based on the results of the extreme risk cohort of patients. The data from the study in the high-risk trial are being reviewed at the FDA, according to the manufacturer, Medtronic.
The study compared the safety and effectiveness of TAVR with the CoreValve device to surgical valve replacement in 795 patients at 45 U.S. centers. The patients had severe aortic stenosis, had New York Heart Association class II heart failure or higher, and were judged to have at least a 15% risk of death within 30 days after surgery and less than a 50% risk of death or irreversible complications within 30 days after surgery. Their mean age was about age 83 years, almost half were females, most had class NYHA class III HF, and cardiac risk factors included coronary artery disease (in about two-thirds), previous coronary artery bypass surgery (about 30%), a previous MI (about 25%), and almost all had heart failure.
At 1 year, a composite of major adverse cardiovascular and cerebrovascular events (death from any cause, MI, any stroke, or reintervention), a secondary endpoint, was significantly lower among those on TAVR (20.4%) vs. the surgical group (27.3%). The rate of any stroke at 30 days was 4.9% in TAVR patients and 6.2% in the surgical group; and at 1 year, those rates were 8.8% and 12.6%, respectively; neither difference was statistically significant. Major vascular complications and permanent pacemaker implantations were significantly higher in the TAVR group (22.3% at 1 year, vs. 11.3% in the surgical group). In the TAVR group, there were five cases of cardiac perforation; there were no perforations in the surgical group.
Patients are being followed through 5 years. The 2-year mortality data are encouraging, with continued separation of the all-cause mortality curves, although the numbers are still small, Dr. Adams said.
More patients in the trial refused surgical valve replacement after randomization and the mortality rate within 30 days after surgery was 4.5%, which was lower than the rate specified for inclusion in the study, which was 15% or higher, so the patients may have been at a lower risk than planned, he said.
During the discussion, the inevitable comparisons to the results of the Placement of Aortic Transcatheter Valves A (PARTNER A) study were raised. In PARTNER A, which compared the safety and effectiveness of the balloon-expandable SAPIEN Transcatheter Heart Valve to aortic valve replacement surgery in high-risk patients with severe symptomatic aortic stenosis, found no difference in mortality between the two arms and an increase in cerebrovascular events in the TAVR arm.
Dr. Adams said that different characteristics of the device in the two trials are possible explanations as to why the TAVR results were superior to surgery in the CoreValve study, and not in PARTNER A. "The size of the catheter as well as perhaps the self-expanding nature of the device both could help explain that," he said.
While patient risk was assessed differently in the studies, and the Society of Thoracic Surgeons scores of the patients were different, "we're confident these were patients at increased risk for surgery," he added.
The study was published simultaneously in the New England Journal of Medicine on March 29 (2014 March 29 [doi:10.1056/NEJMoa1400590]).
The study is funded by the CoreValve manufacturer, Medtronic. Dr. Adams disclosed receiving grant support from Medtronic during the conduct of the study and other support from Medtronic and Edwards Lifesciences outside the submitted work.
WASHINGTON - A first in transcatheter aortic valve replacement trials, the CoreValve prosthesis was superior to surgical valve replacement in patients with severe aortic stenosis at increased surgical risk, showing a significantly lower risk of mortality 1 year later.
In the U.S. CoreValve High Risk Study, a prospective randomized controlled study of almost 800 patients, the rate of all-cause mortality at 1 year, the primary endpoint, was 14.2% among those in the transcatheter aortic valve replacement (TAVR) group, compared with 19.1% among those in the surgery group, a statistically significant difference that represented a 26% survival benefit at 1 year for the CoreValve, Dr. David H. Adams reported at the annual meeting of the American College of Cardiology.
This is the first prospective, randomized study to show superiority for transcatheter valve therapy over surgery, and "there's no study or trial that I'm aware of that's suggested that TAVR patients would have a superior survival outcome," Dr. Adams of Mount Sinai Medical Center, New York, said in an interview. Based on these results, he said he expects that TAVR "will increasingly become the alternative of choice for patients" at this level of risk.
The study was the high-risk arm of the U.S. CoreValve pivotal trial. The CoreValve self-expanding prosthesis was approved in January 2014 by the Food and Drug Administration for use in extreme risk patients, based on the results of the extreme risk cohort of patients. The data from the study in the high-risk trial are being reviewed at the FDA, according to the manufacturer, Medtronic.
The study compared the safety and effectiveness of TAVR with the CoreValve device to surgical valve replacement in 795 patients at 45 U.S. centers. The patients had severe aortic stenosis, had New York Heart Association class II heart failure or higher, and were judged to have at least a 15% risk of death within 30 days after surgery and less than a 50% risk of death or irreversible complications within 30 days after surgery. Their mean age was about age 83 years, almost half were females, most had class NYHA class III HF, and cardiac risk factors included coronary artery disease (in about two-thirds), previous coronary artery bypass surgery (about 30%), a previous MI (about 25%), and almost all had heart failure.
At 1 year, a composite of major adverse cardiovascular and cerebrovascular events (death from any cause, MI, any stroke, or reintervention), a secondary endpoint, was significantly lower among those on TAVR (20.4%) vs. the surgical group (27.3%). The rate of any stroke at 30 days was 4.9% in TAVR patients and 6.2% in the surgical group; and at 1 year, those rates were 8.8% and 12.6%, respectively; neither difference was statistically significant. Major vascular complications and permanent pacemaker implantations were significantly higher in the TAVR group (22.3% at 1 year, vs. 11.3% in the surgical group). In the TAVR group, there were five cases of cardiac perforation; there were no perforations in the surgical group.
Patients are being followed through 5 years. The 2-year mortality data are encouraging, with continued separation of the all-cause mortality curves, although the numbers are still small, Dr. Adams said.
More patients in the trial refused surgical valve replacement after randomization and the mortality rate within 30 days after surgery was 4.5%, which was lower than the rate specified for inclusion in the study, which was 15% or higher, so the patients may have been at a lower risk than planned, he said.
During the discussion, the inevitable comparisons to the results of the Placement of Aortic Transcatheter Valves A (PARTNER A) study were raised. In PARTNER A, which compared the safety and effectiveness of the balloon-expandable SAPIEN Transcatheter Heart Valve to aortic valve replacement surgery in high-risk patients with severe symptomatic aortic stenosis, found no difference in mortality between the two arms and an increase in cerebrovascular events in the TAVR arm.
Dr. Adams said that different characteristics of the device in the two trials are possible explanations as to why the TAVR results were superior to surgery in the CoreValve study, and not in PARTNER A. "The size of the catheter as well as perhaps the self-expanding nature of the device both could help explain that," he said.
While patient risk was assessed differently in the studies, and the Society of Thoracic Surgeons scores of the patients were different, "we're confident these were patients at increased risk for surgery," he added.
The study was published simultaneously in the New England Journal of Medicine on March 29 (2014 March 29 [doi:10.1056/NEJMoa1400590]).
The study is funded by the CoreValve manufacturer, Medtronic. Dr. Adams disclosed receiving grant support from Medtronic during the conduct of the study and other support from Medtronic and Edwards Lifesciences outside the submitted work.
WASHINGTON - A first in transcatheter aortic valve replacement trials, the CoreValve prosthesis was superior to surgical valve replacement in patients with severe aortic stenosis at increased surgical risk, showing a significantly lower risk of mortality 1 year later.
In the U.S. CoreValve High Risk Study, a prospective randomized controlled study of almost 800 patients, the rate of all-cause mortality at 1 year, the primary endpoint, was 14.2% among those in the transcatheter aortic valve replacement (TAVR) group, compared with 19.1% among those in the surgery group, a statistically significant difference that represented a 26% survival benefit at 1 year for the CoreValve, Dr. David H. Adams reported at the annual meeting of the American College of Cardiology.
This is the first prospective, randomized study to show superiority for transcatheter valve therapy over surgery, and "there's no study or trial that I'm aware of that's suggested that TAVR patients would have a superior survival outcome," Dr. Adams of Mount Sinai Medical Center, New York, said in an interview. Based on these results, he said he expects that TAVR "will increasingly become the alternative of choice for patients" at this level of risk.
The study was the high-risk arm of the U.S. CoreValve pivotal trial. The CoreValve self-expanding prosthesis was approved in January 2014 by the Food and Drug Administration for use in extreme risk patients, based on the results of the extreme risk cohort of patients. The data from the study in the high-risk trial are being reviewed at the FDA, according to the manufacturer, Medtronic.
The study compared the safety and effectiveness of TAVR with the CoreValve device to surgical valve replacement in 795 patients at 45 U.S. centers. The patients had severe aortic stenosis, had New York Heart Association class II heart failure or higher, and were judged to have at least a 15% risk of death within 30 days after surgery and less than a 50% risk of death or irreversible complications within 30 days after surgery. Their mean age was about age 83 years, almost half were females, most had class NYHA class III HF, and cardiac risk factors included coronary artery disease (in about two-thirds), previous coronary artery bypass surgery (about 30%), a previous MI (about 25%), and almost all had heart failure.
At 1 year, a composite of major adverse cardiovascular and cerebrovascular events (death from any cause, MI, any stroke, or reintervention), a secondary endpoint, was significantly lower among those on TAVR (20.4%) vs. the surgical group (27.3%). The rate of any stroke at 30 days was 4.9% in TAVR patients and 6.2% in the surgical group; and at 1 year, those rates were 8.8% and 12.6%, respectively; neither difference was statistically significant. Major vascular complications and permanent pacemaker implantations were significantly higher in the TAVR group (22.3% at 1 year, vs. 11.3% in the surgical group). In the TAVR group, there were five cases of cardiac perforation; there were no perforations in the surgical group.
Patients are being followed through 5 years. The 2-year mortality data are encouraging, with continued separation of the all-cause mortality curves, although the numbers are still small, Dr. Adams said.
More patients in the trial refused surgical valve replacement after randomization and the mortality rate within 30 days after surgery was 4.5%, which was lower than the rate specified for inclusion in the study, which was 15% or higher, so the patients may have been at a lower risk than planned, he said.
During the discussion, the inevitable comparisons to the results of the Placement of Aortic Transcatheter Valves A (PARTNER A) study were raised. In PARTNER A, which compared the safety and effectiveness of the balloon-expandable SAPIEN Transcatheter Heart Valve to aortic valve replacement surgery in high-risk patients with severe symptomatic aortic stenosis, found no difference in mortality between the two arms and an increase in cerebrovascular events in the TAVR arm.
Dr. Adams said that different characteristics of the device in the two trials are possible explanations as to why the TAVR results were superior to surgery in the CoreValve study, and not in PARTNER A. "The size of the catheter as well as perhaps the self-expanding nature of the device both could help explain that," he said.
While patient risk was assessed differently in the studies, and the Society of Thoracic Surgeons scores of the patients were different, "we're confident these were patients at increased risk for surgery," he added.
The study was published simultaneously in the New England Journal of Medicine on March 29 (2014 March 29 [doi:10.1056/NEJMoa1400590]).
The study is funded by the CoreValve manufacturer, Medtronic. Dr. Adams disclosed receiving grant support from Medtronic during the conduct of the study and other support from Medtronic and Edwards Lifesciences outside the submitted work.
Major finding: All-cause mortality was 14.2% among high-risk patients with severe aortic stenosis 1 year after TAVR with a self-expanding aortic valve bioprosthesis, vs. 19.1% among those who had surgical aortic valve replacement, a highly statistically significant difference.
Data source: The multicenter prospective U.S. study compared survival at 1 year in 795 patients at high risk for surgery who were randomized to TAVR with the CoreValve device or surgical aortic valve replacement.
Disclosures: The study is funded by the CoreValve manufacturer, Medtronic. Dr. Adams disclosed receiving grant support from Medtronic during the conduct of the study and other support from Medtronic and Edwards Lifesciences outside the study.
DECAAF: Assess extent of fibrosis before AF ablation
For patients scheduled to undergo AF catheter ablation, estimating the extent of atrial fibrosis using delayed enhancement MRI can help distinguish those likely to respond from patients likely to have recurrent arrhythmia, according to a report published online Feb. 4 in JAMA.
In the prospective, observational DECAAF (Delayed-Enhancement MRI Determinant of Successful Radiofrequency Ablation of Atrial Fibrillation) study, 260 such patients (mean age, 59 years) underwent quantification of left atrial fibrosis via delayed enhancement MRI with gadolinium at 15 medical centers in six countries, before undergoing AF ablation. These centers had varying levels of experience with cardiac imaging and used different ablation procedures, said Dr. Nassir F. Marrouche, director of the comprehensive arrhythmia and research management center, University of Utah, Salt Lake City, and his associates.
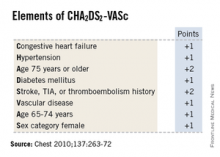
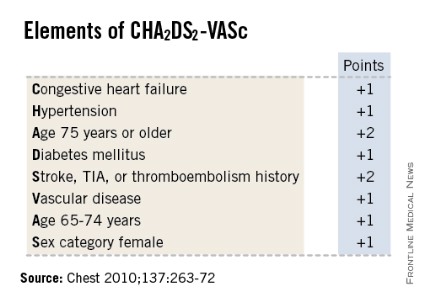
Four categories of fibrosis were used: involvement of less than 10% of the atrial wall (stage 1, 49 patients), 10%-19% of the atrial wall (stage 2, 107 patients), 20%-29% of the atrial wall (stage 3, 80 patients), and 30% or more of the atrial wall (stage 4, 24 patients). The estimated percentage of atrial fibrosis strongly correlated with arrhythmia recurrence at 1 year, even after the data were adjusted to account for variables such as patient age and sex; the presence of hypertension, heart failure, mitral valve disease, or diabetes; and the type of AF (paroxysmal vs persistent).
The hazard ratio for recurrent arrhythmia was 1.06 for every 1% increase in the extent of atrial fibrosis (JAMA 2014 Feb. 4 [doi:10.1001/jama.2014.3]).
This is the first multicenter study to demonstrate the feasibility and potential clinical value of quantifying the degree of AF fibrosis using delayed enhancement MRI before performing ablation, offering a noninvasive, effective method for determining which patients are likely to benefit and which should avoid the procedure, Dr. Marrouche and his associates said.
The JAMA report expands on results presented at the annual meeting of the Heart Rhythm Society last year.
The study was funded by the Comprehensive Arrhythmia and Research Management Center at the University of Utah and the George S. and Dolores Dore Eccles Foundation. Dr. Marrouche reported owning stock and being named in two patents licensed to Marrek; his associates reported numerous ties to industry sources.
For patients scheduled to undergo AF catheter ablation, estimating the extent of atrial fibrosis using delayed enhancement MRI can help distinguish those likely to respond from patients likely to have recurrent arrhythmia, according to a report published online Feb. 4 in JAMA.
In the prospective, observational DECAAF (Delayed-Enhancement MRI Determinant of Successful Radiofrequency Ablation of Atrial Fibrillation) study, 260 such patients (mean age, 59 years) underwent quantification of left atrial fibrosis via delayed enhancement MRI with gadolinium at 15 medical centers in six countries, before undergoing AF ablation. These centers had varying levels of experience with cardiac imaging and used different ablation procedures, said Dr. Nassir F. Marrouche, director of the comprehensive arrhythmia and research management center, University of Utah, Salt Lake City, and his associates.
Four categories of fibrosis were used: involvement of less than 10% of the atrial wall (stage 1, 49 patients), 10%-19% of the atrial wall (stage 2, 107 patients), 20%-29% of the atrial wall (stage 3, 80 patients), and 30% or more of the atrial wall (stage 4, 24 patients). The estimated percentage of atrial fibrosis strongly correlated with arrhythmia recurrence at 1 year, even after the data were adjusted to account for variables such as patient age and sex; the presence of hypertension, heart failure, mitral valve disease, or diabetes; and the type of AF (paroxysmal vs persistent).
The hazard ratio for recurrent arrhythmia was 1.06 for every 1% increase in the extent of atrial fibrosis (JAMA 2014 Feb. 4 [doi:10.1001/jama.2014.3]).
This is the first multicenter study to demonstrate the feasibility and potential clinical value of quantifying the degree of AF fibrosis using delayed enhancement MRI before performing ablation, offering a noninvasive, effective method for determining which patients are likely to benefit and which should avoid the procedure, Dr. Marrouche and his associates said.
The JAMA report expands on results presented at the annual meeting of the Heart Rhythm Society last year.
The study was funded by the Comprehensive Arrhythmia and Research Management Center at the University of Utah and the George S. and Dolores Dore Eccles Foundation. Dr. Marrouche reported owning stock and being named in two patents licensed to Marrek; his associates reported numerous ties to industry sources.
For patients scheduled to undergo AF catheter ablation, estimating the extent of atrial fibrosis using delayed enhancement MRI can help distinguish those likely to respond from patients likely to have recurrent arrhythmia, according to a report published online Feb. 4 in JAMA.
In the prospective, observational DECAAF (Delayed-Enhancement MRI Determinant of Successful Radiofrequency Ablation of Atrial Fibrillation) study, 260 such patients (mean age, 59 years) underwent quantification of left atrial fibrosis via delayed enhancement MRI with gadolinium at 15 medical centers in six countries, before undergoing AF ablation. These centers had varying levels of experience with cardiac imaging and used different ablation procedures, said Dr. Nassir F. Marrouche, director of the comprehensive arrhythmia and research management center, University of Utah, Salt Lake City, and his associates.
Four categories of fibrosis were used: involvement of less than 10% of the atrial wall (stage 1, 49 patients), 10%-19% of the atrial wall (stage 2, 107 patients), 20%-29% of the atrial wall (stage 3, 80 patients), and 30% or more of the atrial wall (stage 4, 24 patients). The estimated percentage of atrial fibrosis strongly correlated with arrhythmia recurrence at 1 year, even after the data were adjusted to account for variables such as patient age and sex; the presence of hypertension, heart failure, mitral valve disease, or diabetes; and the type of AF (paroxysmal vs persistent).
The hazard ratio for recurrent arrhythmia was 1.06 for every 1% increase in the extent of atrial fibrosis (JAMA 2014 Feb. 4 [doi:10.1001/jama.2014.3]).
This is the first multicenter study to demonstrate the feasibility and potential clinical value of quantifying the degree of AF fibrosis using delayed enhancement MRI before performing ablation, offering a noninvasive, effective method for determining which patients are likely to benefit and which should avoid the procedure, Dr. Marrouche and his associates said.
The JAMA report expands on results presented at the annual meeting of the Heart Rhythm Society last year.
The study was funded by the Comprehensive Arrhythmia and Research Management Center at the University of Utah and the George S. and Dolores Dore Eccles Foundation. Dr. Marrouche reported owning stock and being named in two patents licensed to Marrek; his associates reported numerous ties to industry sources.
Frailty assessment central to TAVR decision
SNOWMASS, COLO.– Transcatheter aortic valve replacement in nonsurgical candidates with severe symptomatic aortic stenosis carries a hefty price tag of $116,500 per quality-adjusted life-year gained over medical management.
That’s the bottom line in a cost-effectiveness study led by cardiologist Dr. Mark A. Hlatky. The investigators applied data on the costs and benefits of transfemoral transcatheter aortic valve replacement (TAVR) as documented in the landmark PARTNER (Placement of Aortic Transcatheter Valves) trial in their Markov model involving a hypothetical patient cohort. The estimated incremental cost-effectiveness of $116,500 per quality-adjusted life-year gained is well in excess of the $50,000 figure widely accepted by health policy makers as defining a cutoff for cost-effective therapy.
In this cost-effectiveness analysis (Circ. Cardiovasc. Qual. Outcomes 2013;6:419-28), TAVR boosted life expectancy by roughly 11 months, from 2.08 years with medical therapy to 2.93 years. Quality-adjusted life expectancy rose from 1.19 to 1.93 years. TAVR also resulted in 1.4 fewer hospitalizations than with medical management. However, undergoing TAVR rather than medical management raised the lifetime stroke risk from 1% to 11% and increased lifetime health care costs from $83,600 to $169,100, reported the group led by Dr. Hlatky, professor of health research and policy and also professor of medicine at Stanford (Calif.) University.
"This is a fascinating study," Dr. Karen P. Alexander said at the Annual Cardiovascular Conference at Snowmass. "I think the lesson here is that futility from a cost perspective is also something that should be in the discussion" regarding TAVR vs. medical therapy in patients with inoperable aortic stenosis.
She highlighted the Hlatky study in discussing the key role frailty assessment plays in considering TAVR. The study showed that the cost-effectiveness of TAVR is greater in patients with a lower burden of noncardiac disease, which is another way saying "those who are less frail."
This conclusion underscores a statement in the 2012 American College of Cardiology/American Association for Thoracic Surgery/Society for Cardiovascular Angiography and Interventions/Society of Thoracic Surgeons expert consensus document on TAVR paraphrased by Dr. Alexander: Frailty will assume central importance in patient selection for TAVR by virtue of the extensive comorbidities in this population. Existing models do not have predictive variables of interest in high-risk patients. (J. Am. Coll. Cardiol. 2012;59:1200-54).
Dr. Alexander of Duke University in Durham, N.C., said frailty is important when considering TAVR because it has been shown to be associated with increased rates of post-TAVR 30-day morbidity and mortality, prolonged hospital length of stay, and 1-year mortality.
She defined frailty as a multisystem impairment resulting in reduced physiologic reserve and increased vulnerability to stress. Frailty is a physiologic phenotype associated with slow gait, weakness, weight loss, exhaustion, and a low daily activity level.
While the degree of a patient’s frailty is an important consideration in deciding on TAVR vs. medical management, frailty per se is no contraindication to the procedure. Indeed, the prevalence of frailty as defined simply by a baseline 5-meter walk time in excess of 6 seconds was 72% among the 7,710 TAVR patients, mean age 84 years, included in the recent first report of the comprehensive national STS/ACC Transcatheter Valve Therapy Registry (JAMA 2013;310:2069-77). That’s nearly twice the 38% prevalence among community-dwelling 85-year-olds, Dr. Alexander noted, citing data from the Canadian Study of Health and Aging (CMAJ 2011;183:e487-94).
More than 20 different frailty risk scores are now in circulation. Dr. Alexander is particularly enthusiastic about the frailty risk tool developed as part of the ACC’s new Championing Care for the Patient With Aortic Stenosis Initiative. It efficiently assesses five domains of frailty – slowness, weakness, malnutrition, inactivity with loss of independence, and malnutrition – and generates a clinically useful qualitative frailty rating. A patient with a high frailty score may not have sufficient life expectancy to obtain the benefits of TAVR.
With regard to treatment futility, Dr. Alexander observed that it can be defined as either lack of medical efficacy as judged by physicians or as lack of meaningful survival as judged by a patient’s personal values. Yet one in four Americans aged 75 years or older has given little or no thought to their own wishes for end-of-life medical therapy, according to a recent Pew Research Center survey.
The telephone survey, conducted last spring, included a representative sample of 1,994 U.S. adults. While 47% of respondents aged 75 years or older indicated they had given their own wishes for end-of-life medical care a great deal of thought, 25% said they had given the matter "not much or none." Reflection on those personal wishes needs to be part of the physician/patient discussion about TAVR, according to Dr. Alexander.
With regard to general views on end-of-life therapy, 74% of surveyed individuals age 75 and up declared there should be circumstances in which a patient should be allowed to die. Another 22% said medical staff should do everything possible to save a patient’s life under all circumstances.
Dr. Alexander reported serving as a consultant to Gilead and Pozen.
SNOWMASS, COLO.– Transcatheter aortic valve replacement in nonsurgical candidates with severe symptomatic aortic stenosis carries a hefty price tag of $116,500 per quality-adjusted life-year gained over medical management.
That’s the bottom line in a cost-effectiveness study led by cardiologist Dr. Mark A. Hlatky. The investigators applied data on the costs and benefits of transfemoral transcatheter aortic valve replacement (TAVR) as documented in the landmark PARTNER (Placement of Aortic Transcatheter Valves) trial in their Markov model involving a hypothetical patient cohort. The estimated incremental cost-effectiveness of $116,500 per quality-adjusted life-year gained is well in excess of the $50,000 figure widely accepted by health policy makers as defining a cutoff for cost-effective therapy.
In this cost-effectiveness analysis (Circ. Cardiovasc. Qual. Outcomes 2013;6:419-28), TAVR boosted life expectancy by roughly 11 months, from 2.08 years with medical therapy to 2.93 years. Quality-adjusted life expectancy rose from 1.19 to 1.93 years. TAVR also resulted in 1.4 fewer hospitalizations than with medical management. However, undergoing TAVR rather than medical management raised the lifetime stroke risk from 1% to 11% and increased lifetime health care costs from $83,600 to $169,100, reported the group led by Dr. Hlatky, professor of health research and policy and also professor of medicine at Stanford (Calif.) University.
"This is a fascinating study," Dr. Karen P. Alexander said at the Annual Cardiovascular Conference at Snowmass. "I think the lesson here is that futility from a cost perspective is also something that should be in the discussion" regarding TAVR vs. medical therapy in patients with inoperable aortic stenosis.
She highlighted the Hlatky study in discussing the key role frailty assessment plays in considering TAVR. The study showed that the cost-effectiveness of TAVR is greater in patients with a lower burden of noncardiac disease, which is another way saying "those who are less frail."
This conclusion underscores a statement in the 2012 American College of Cardiology/American Association for Thoracic Surgery/Society for Cardiovascular Angiography and Interventions/Society of Thoracic Surgeons expert consensus document on TAVR paraphrased by Dr. Alexander: Frailty will assume central importance in patient selection for TAVR by virtue of the extensive comorbidities in this population. Existing models do not have predictive variables of interest in high-risk patients. (J. Am. Coll. Cardiol. 2012;59:1200-54).
Dr. Alexander of Duke University in Durham, N.C., said frailty is important when considering TAVR because it has been shown to be associated with increased rates of post-TAVR 30-day morbidity and mortality, prolonged hospital length of stay, and 1-year mortality.
She defined frailty as a multisystem impairment resulting in reduced physiologic reserve and increased vulnerability to stress. Frailty is a physiologic phenotype associated with slow gait, weakness, weight loss, exhaustion, and a low daily activity level.
While the degree of a patient’s frailty is an important consideration in deciding on TAVR vs. medical management, frailty per se is no contraindication to the procedure. Indeed, the prevalence of frailty as defined simply by a baseline 5-meter walk time in excess of 6 seconds was 72% among the 7,710 TAVR patients, mean age 84 years, included in the recent first report of the comprehensive national STS/ACC Transcatheter Valve Therapy Registry (JAMA 2013;310:2069-77). That’s nearly twice the 38% prevalence among community-dwelling 85-year-olds, Dr. Alexander noted, citing data from the Canadian Study of Health and Aging (CMAJ 2011;183:e487-94).
More than 20 different frailty risk scores are now in circulation. Dr. Alexander is particularly enthusiastic about the frailty risk tool developed as part of the ACC’s new Championing Care for the Patient With Aortic Stenosis Initiative. It efficiently assesses five domains of frailty – slowness, weakness, malnutrition, inactivity with loss of independence, and malnutrition – and generates a clinically useful qualitative frailty rating. A patient with a high frailty score may not have sufficient life expectancy to obtain the benefits of TAVR.
With regard to treatment futility, Dr. Alexander observed that it can be defined as either lack of medical efficacy as judged by physicians or as lack of meaningful survival as judged by a patient’s personal values. Yet one in four Americans aged 75 years or older has given little or no thought to their own wishes for end-of-life medical therapy, according to a recent Pew Research Center survey.
The telephone survey, conducted last spring, included a representative sample of 1,994 U.S. adults. While 47% of respondents aged 75 years or older indicated they had given their own wishes for end-of-life medical care a great deal of thought, 25% said they had given the matter "not much or none." Reflection on those personal wishes needs to be part of the physician/patient discussion about TAVR, according to Dr. Alexander.
With regard to general views on end-of-life therapy, 74% of surveyed individuals age 75 and up declared there should be circumstances in which a patient should be allowed to die. Another 22% said medical staff should do everything possible to save a patient’s life under all circumstances.
Dr. Alexander reported serving as a consultant to Gilead and Pozen.
SNOWMASS, COLO.– Transcatheter aortic valve replacement in nonsurgical candidates with severe symptomatic aortic stenosis carries a hefty price tag of $116,500 per quality-adjusted life-year gained over medical management.
That’s the bottom line in a cost-effectiveness study led by cardiologist Dr. Mark A. Hlatky. The investigators applied data on the costs and benefits of transfemoral transcatheter aortic valve replacement (TAVR) as documented in the landmark PARTNER (Placement of Aortic Transcatheter Valves) trial in their Markov model involving a hypothetical patient cohort. The estimated incremental cost-effectiveness of $116,500 per quality-adjusted life-year gained is well in excess of the $50,000 figure widely accepted by health policy makers as defining a cutoff for cost-effective therapy.
In this cost-effectiveness analysis (Circ. Cardiovasc. Qual. Outcomes 2013;6:419-28), TAVR boosted life expectancy by roughly 11 months, from 2.08 years with medical therapy to 2.93 years. Quality-adjusted life expectancy rose from 1.19 to 1.93 years. TAVR also resulted in 1.4 fewer hospitalizations than with medical management. However, undergoing TAVR rather than medical management raised the lifetime stroke risk from 1% to 11% and increased lifetime health care costs from $83,600 to $169,100, reported the group led by Dr. Hlatky, professor of health research and policy and also professor of medicine at Stanford (Calif.) University.
"This is a fascinating study," Dr. Karen P. Alexander said at the Annual Cardiovascular Conference at Snowmass. "I think the lesson here is that futility from a cost perspective is also something that should be in the discussion" regarding TAVR vs. medical therapy in patients with inoperable aortic stenosis.
She highlighted the Hlatky study in discussing the key role frailty assessment plays in considering TAVR. The study showed that the cost-effectiveness of TAVR is greater in patients with a lower burden of noncardiac disease, which is another way saying "those who are less frail."
This conclusion underscores a statement in the 2012 American College of Cardiology/American Association for Thoracic Surgery/Society for Cardiovascular Angiography and Interventions/Society of Thoracic Surgeons expert consensus document on TAVR paraphrased by Dr. Alexander: Frailty will assume central importance in patient selection for TAVR by virtue of the extensive comorbidities in this population. Existing models do not have predictive variables of interest in high-risk patients. (J. Am. Coll. Cardiol. 2012;59:1200-54).
Dr. Alexander of Duke University in Durham, N.C., said frailty is important when considering TAVR because it has been shown to be associated with increased rates of post-TAVR 30-day morbidity and mortality, prolonged hospital length of stay, and 1-year mortality.
She defined frailty as a multisystem impairment resulting in reduced physiologic reserve and increased vulnerability to stress. Frailty is a physiologic phenotype associated with slow gait, weakness, weight loss, exhaustion, and a low daily activity level.
While the degree of a patient’s frailty is an important consideration in deciding on TAVR vs. medical management, frailty per se is no contraindication to the procedure. Indeed, the prevalence of frailty as defined simply by a baseline 5-meter walk time in excess of 6 seconds was 72% among the 7,710 TAVR patients, mean age 84 years, included in the recent first report of the comprehensive national STS/ACC Transcatheter Valve Therapy Registry (JAMA 2013;310:2069-77). That’s nearly twice the 38% prevalence among community-dwelling 85-year-olds, Dr. Alexander noted, citing data from the Canadian Study of Health and Aging (CMAJ 2011;183:e487-94).
More than 20 different frailty risk scores are now in circulation. Dr. Alexander is particularly enthusiastic about the frailty risk tool developed as part of the ACC’s new Championing Care for the Patient With Aortic Stenosis Initiative. It efficiently assesses five domains of frailty – slowness, weakness, malnutrition, inactivity with loss of independence, and malnutrition – and generates a clinically useful qualitative frailty rating. A patient with a high frailty score may not have sufficient life expectancy to obtain the benefits of TAVR.
With regard to treatment futility, Dr. Alexander observed that it can be defined as either lack of medical efficacy as judged by physicians or as lack of meaningful survival as judged by a patient’s personal values. Yet one in four Americans aged 75 years or older has given little or no thought to their own wishes for end-of-life medical therapy, according to a recent Pew Research Center survey.
The telephone survey, conducted last spring, included a representative sample of 1,994 U.S. adults. While 47% of respondents aged 75 years or older indicated they had given their own wishes for end-of-life medical care a great deal of thought, 25% said they had given the matter "not much or none." Reflection on those personal wishes needs to be part of the physician/patient discussion about TAVR, according to Dr. Alexander.
With regard to general views on end-of-life therapy, 74% of surveyed individuals age 75 and up declared there should be circumstances in which a patient should be allowed to die. Another 22% said medical staff should do everything possible to save a patient’s life under all circumstances.
Dr. Alexander reported serving as a consultant to Gilead and Pozen.
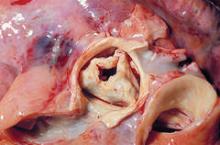
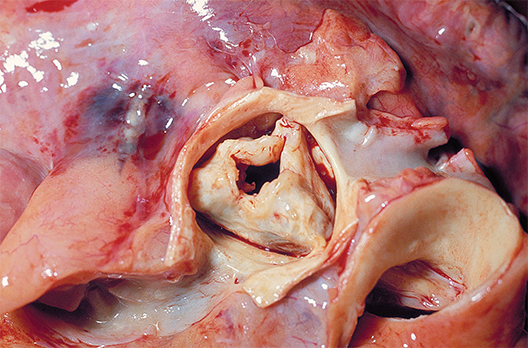
New valve guideline promotes early surgery
The updated practice guideline for managing adults with valvular heart disease has a new, "modular" format to facilitate clinicians' access to "concise, relevant bytes of information at the point of care, when clinical knowledge is needed most," according to reports published online simultaneously March 3 in Circulation and the Journal of the American College of Cardiology.
The guideline, compiled by a committee of cardiologists, interventionalists, surgeons, and anesthesiologists under the aegis of the American Heart Association and the American College of Cardiology, was last updated in 2008.
"Some recommendations from the earlier valvular heart disease guideline have been updated as warranted by new evidence or a better understanding of earlier evidence, whereas others that were inaccurate, irrelevant, or overlapping were deleted or modified," said writing committee cochairs Dr. Rick A. Nishimura of the division of cardiovascular diseases, Mayo Clinic, Rochester, Minn.; and Dr. Catherine M. Otto, director of the University of Washington Medical Center's Heart Valve Clinic, Seattle.
The narrative text of the guideline is limited, and instead it uses decision pathway diagrams and numerous summary tables of current evidence and recommendations. These include links to relevant references. It is hoped that clinicians can more easily use the new guideline as a quick reference. This format also will enable individual sections to be updated or amended as new evidence comes to light. The PDF of the guideline is available for free.
"This novel approach to evidence-based guideline development will revolutionize the clinical impact of guideline recommendations, ensuring they are always current and allowing seamless integration with electronic medical record systems," Dr. Otto said in a press statement accompanying the reports.
The guideline now includes gradations of disease severity, to help clinicians determine the optimal timing of intervention. Whether or not intervention is indicated depends on five factors: the presence or absence of symptoms, the severity of valvular heart disease, the response of the left and/or right ventricle to the volume or pressure overload caused by the valvular disease, the effect on the pulmonary or systemic circulation, and any change in heart rhythm.
Disease severity ranges from stage A, "at risk," which denotes patients who have risk factors for developing valvular heart disease; through stage B, "progressive," which indicates patients who are asymptomatic but have mildly to moderately severe disease; through stage C, "asymptomatic severe," which includes patients with severe yet still asymptomatic valvular disease in which the left or right ventricle remains compensated or in which the left or right ventricle has decompensated; to stage D, "symptomatic severe," which indicates patients whose severe valvular disease has produced symptoms.
"In patients with stenotic lesions, there is an additional category of 'very severe' stenosis based on studies of the natural history showing that prognosis becomes poorer as the severity of stenosis increases," the guideline states.
Information is provided for assessing the various disease states associated with the aortic, mitral, and tricuspid valves, and addresses the issues of valve repair, replacement, and the use of prosthetic valves.
Compared with the previous guideline, the new one suggests surgical intervention at an earlier stage for certain patients. "Due to more knowledge regarding the natural history of untreated patients with severe valvular heart disease and better outcomes from surgery, we've lowered the threshold for operation to include more patients with asymptomatic severe disease. Now, select patients with severe asymptomatic aortic stenosis and severe asymptomatic mitral regurgitation can be considered for intervention, depending on certain other factors such as operative mortality and … the ability to achieve a durable valve repair," Dr. Nishimura said in the press statement.
The new guideline also proposes a new approach to risk assessment, to be applied to all patients for whom intervention is being considered. Previous risk scoring systems were "useful but limited"; the new approach takes into consideration "procedure-specific impediments, major organ system compromise, comorbidities, patient frailty, and the Society of Thoracic Surgeons predicted risk of mortality model."
For the first time, the guideline discusses transcatheter aortic valve replacement and other catheter-based treatments, new technologies that have improved patient care but also have complicated risk assessment. Separate recommendations are now offered regarding the choice and the timing of these interventions.
In addition to the AHA and the ACC, this guideline was developed in collaboration with the American Association for Thoracic Surgery, American Society for Echocardiography, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons.
The complete 2014 Guideline for the Management of Patients With Valvular Heart Disease is available from the American College of Cardiology and the American Heart Association.
Dr. Nishimura and Dr. Otto reported no financial conflicts of interest; their associates on the ACC/AHA Task Force's writing committee reported ties to Edwards Scientific, Medtronic, and St. Jude Medical.
The updated practice guideline for managing adults with valvular heart disease has a new, "modular" format to facilitate clinicians' access to "concise, relevant bytes of information at the point of care, when clinical knowledge is needed most," according to reports published online simultaneously March 3 in Circulation and the Journal of the American College of Cardiology.
The guideline, compiled by a committee of cardiologists, interventionalists, surgeons, and anesthesiologists under the aegis of the American Heart Association and the American College of Cardiology, was last updated in 2008.
"Some recommendations from the earlier valvular heart disease guideline have been updated as warranted by new evidence or a better understanding of earlier evidence, whereas others that were inaccurate, irrelevant, or overlapping were deleted or modified," said writing committee cochairs Dr. Rick A. Nishimura of the division of cardiovascular diseases, Mayo Clinic, Rochester, Minn.; and Dr. Catherine M. Otto, director of the University of Washington Medical Center's Heart Valve Clinic, Seattle.
The narrative text of the guideline is limited, and instead it uses decision pathway diagrams and numerous summary tables of current evidence and recommendations. These include links to relevant references. It is hoped that clinicians can more easily use the new guideline as a quick reference. This format also will enable individual sections to be updated or amended as new evidence comes to light. The PDF of the guideline is available for free.
"This novel approach to evidence-based guideline development will revolutionize the clinical impact of guideline recommendations, ensuring they are always current and allowing seamless integration with electronic medical record systems," Dr. Otto said in a press statement accompanying the reports.
The guideline now includes gradations of disease severity, to help clinicians determine the optimal timing of intervention. Whether or not intervention is indicated depends on five factors: the presence or absence of symptoms, the severity of valvular heart disease, the response of the left and/or right ventricle to the volume or pressure overload caused by the valvular disease, the effect on the pulmonary or systemic circulation, and any change in heart rhythm.
Disease severity ranges from stage A, "at risk," which denotes patients who have risk factors for developing valvular heart disease; through stage B, "progressive," which indicates patients who are asymptomatic but have mildly to moderately severe disease; through stage C, "asymptomatic severe," which includes patients with severe yet still asymptomatic valvular disease in which the left or right ventricle remains compensated or in which the left or right ventricle has decompensated; to stage D, "symptomatic severe," which indicates patients whose severe valvular disease has produced symptoms.
"In patients with stenotic lesions, there is an additional category of 'very severe' stenosis based on studies of the natural history showing that prognosis becomes poorer as the severity of stenosis increases," the guideline states.
Information is provided for assessing the various disease states associated with the aortic, mitral, and tricuspid valves, and addresses the issues of valve repair, replacement, and the use of prosthetic valves.
Compared with the previous guideline, the new one suggests surgical intervention at an earlier stage for certain patients. "Due to more knowledge regarding the natural history of untreated patients with severe valvular heart disease and better outcomes from surgery, we've lowered the threshold for operation to include more patients with asymptomatic severe disease. Now, select patients with severe asymptomatic aortic stenosis and severe asymptomatic mitral regurgitation can be considered for intervention, depending on certain other factors such as operative mortality and … the ability to achieve a durable valve repair," Dr. Nishimura said in the press statement.
The new guideline also proposes a new approach to risk assessment, to be applied to all patients for whom intervention is being considered. Previous risk scoring systems were "useful but limited"; the new approach takes into consideration "procedure-specific impediments, major organ system compromise, comorbidities, patient frailty, and the Society of Thoracic Surgeons predicted risk of mortality model."
For the first time, the guideline discusses transcatheter aortic valve replacement and other catheter-based treatments, new technologies that have improved patient care but also have complicated risk assessment. Separate recommendations are now offered regarding the choice and the timing of these interventions.
In addition to the AHA and the ACC, this guideline was developed in collaboration with the American Association for Thoracic Surgery, American Society for Echocardiography, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons.
The complete 2014 Guideline for the Management of Patients With Valvular Heart Disease is available from the American College of Cardiology and the American Heart Association.
Dr. Nishimura and Dr. Otto reported no financial conflicts of interest; their associates on the ACC/AHA Task Force's writing committee reported ties to Edwards Scientific, Medtronic, and St. Jude Medical.
The updated practice guideline for managing adults with valvular heart disease has a new, "modular" format to facilitate clinicians' access to "concise, relevant bytes of information at the point of care, when clinical knowledge is needed most," according to reports published online simultaneously March 3 in Circulation and the Journal of the American College of Cardiology.
The guideline, compiled by a committee of cardiologists, interventionalists, surgeons, and anesthesiologists under the aegis of the American Heart Association and the American College of Cardiology, was last updated in 2008.
"Some recommendations from the earlier valvular heart disease guideline have been updated as warranted by new evidence or a better understanding of earlier evidence, whereas others that were inaccurate, irrelevant, or overlapping were deleted or modified," said writing committee cochairs Dr. Rick A. Nishimura of the division of cardiovascular diseases, Mayo Clinic, Rochester, Minn.; and Dr. Catherine M. Otto, director of the University of Washington Medical Center's Heart Valve Clinic, Seattle.
The narrative text of the guideline is limited, and instead it uses decision pathway diagrams and numerous summary tables of current evidence and recommendations. These include links to relevant references. It is hoped that clinicians can more easily use the new guideline as a quick reference. This format also will enable individual sections to be updated or amended as new evidence comes to light. The PDF of the guideline is available for free.
"This novel approach to evidence-based guideline development will revolutionize the clinical impact of guideline recommendations, ensuring they are always current and allowing seamless integration with electronic medical record systems," Dr. Otto said in a press statement accompanying the reports.
The guideline now includes gradations of disease severity, to help clinicians determine the optimal timing of intervention. Whether or not intervention is indicated depends on five factors: the presence or absence of symptoms, the severity of valvular heart disease, the response of the left and/or right ventricle to the volume or pressure overload caused by the valvular disease, the effect on the pulmonary or systemic circulation, and any change in heart rhythm.
Disease severity ranges from stage A, "at risk," which denotes patients who have risk factors for developing valvular heart disease; through stage B, "progressive," which indicates patients who are asymptomatic but have mildly to moderately severe disease; through stage C, "asymptomatic severe," which includes patients with severe yet still asymptomatic valvular disease in which the left or right ventricle remains compensated or in which the left or right ventricle has decompensated; to stage D, "symptomatic severe," which indicates patients whose severe valvular disease has produced symptoms.
"In patients with stenotic lesions, there is an additional category of 'very severe' stenosis based on studies of the natural history showing that prognosis becomes poorer as the severity of stenosis increases," the guideline states.
Information is provided for assessing the various disease states associated with the aortic, mitral, and tricuspid valves, and addresses the issues of valve repair, replacement, and the use of prosthetic valves.
Compared with the previous guideline, the new one suggests surgical intervention at an earlier stage for certain patients. "Due to more knowledge regarding the natural history of untreated patients with severe valvular heart disease and better outcomes from surgery, we've lowered the threshold for operation to include more patients with asymptomatic severe disease. Now, select patients with severe asymptomatic aortic stenosis and severe asymptomatic mitral regurgitation can be considered for intervention, depending on certain other factors such as operative mortality and … the ability to achieve a durable valve repair," Dr. Nishimura said in the press statement.
The new guideline also proposes a new approach to risk assessment, to be applied to all patients for whom intervention is being considered. Previous risk scoring systems were "useful but limited"; the new approach takes into consideration "procedure-specific impediments, major organ system compromise, comorbidities, patient frailty, and the Society of Thoracic Surgeons predicted risk of mortality model."
For the first time, the guideline discusses transcatheter aortic valve replacement and other catheter-based treatments, new technologies that have improved patient care but also have complicated risk assessment. Separate recommendations are now offered regarding the choice and the timing of these interventions.
In addition to the AHA and the ACC, this guideline was developed in collaboration with the American Association for Thoracic Surgery, American Society for Echocardiography, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons.
The complete 2014 Guideline for the Management of Patients With Valvular Heart Disease is available from the American College of Cardiology and the American Heart Association.
Dr. Nishimura and Dr. Otto reported no financial conflicts of interest; their associates on the ACC/AHA Task Force's writing committee reported ties to Edwards Scientific, Medtronic, and St. Jude Medical.
Preoperative organ dysfunction worsens SAVR outcomes
SNOWMASS, COLO. – The presence of preoperative dysfunction in more than any one of four key organ systems profoundly reduces survival in patients undergoing surgical aortic valve replacement, a study showed.
"If you have two or more dysfunctional organ systems, you really need to think about what you’re doing for this patient. At 5 years, only about 40% of these patients are alive. It makes a lot of sense to me to say that if you have a patient with severe COPD [chronic obstructive pulmonary disease] and renal dysfunction, that patient should probably never get a surgical valve," Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
In a retrospective analysis of a registry with prospectively entered data, 29% of 1,759 patients who underwent surgical aortic valve replacement (SAVR) with or without coronary artery bypass grafting at Emory University during 2002-2010 had preoperative dysfunction of one or more of four organ systems under scrutiny. Eighty-five patients had severe COPD, as defined by a forced expiratory volume in 1 second (FEV1) that was less than 50% of predicted, 140 had chronic renal failure, 149 had a prior stroke, and 241 had heart failure with a left ventricular ejection less than 35%.
Patients with chronic renal failure had far and away the worst 30-day and long-term outcomes. Half were dead within 3 years. The 7-year survival rate was just 11.7%.
The second-worst outcomes were seen in patients with severe COPD preoperatively. Their 7-year survival rate was 30.8%.
"Anyone with an FEV1 below about 40% becomes a higher-risk surgical candidate; think instead of TAVR [transcatheter aortic valve replacement],"advised Dr. Thourani of the division of cardiothoracic surgery at Emory University, Atlanta.
In contrast, outcomes in patients with either heart failure or prior stroke "were not that bad," he said, pointing to 7-year survival rates of 55.9% and 48.6%, respectively.
Ninety-five patients (5.4%) in this recently published study (Ann. Thorac. Surg. 2013;95:838-45) had more than one dysfunctional organ system prior to SAVR. Median survival in patients without dysfunction in any of the four organ systems was 8.2 years and counting. With one dysfunctional organ, it was still good at 7.2 years. However, with two dysfunctional organ systems, the median survival dropped precipitously to 4.1 years. With three dysfunctional organ systems, it was 5.9 years.
Dr. Thourin serves as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
SNOWMASS, COLO. – The presence of preoperative dysfunction in more than any one of four key organ systems profoundly reduces survival in patients undergoing surgical aortic valve replacement, a study showed.
"If you have two or more dysfunctional organ systems, you really need to think about what you’re doing for this patient. At 5 years, only about 40% of these patients are alive. It makes a lot of sense to me to say that if you have a patient with severe COPD [chronic obstructive pulmonary disease] and renal dysfunction, that patient should probably never get a surgical valve," Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
In a retrospective analysis of a registry with prospectively entered data, 29% of 1,759 patients who underwent surgical aortic valve replacement (SAVR) with or without coronary artery bypass grafting at Emory University during 2002-2010 had preoperative dysfunction of one or more of four organ systems under scrutiny. Eighty-five patients had severe COPD, as defined by a forced expiratory volume in 1 second (FEV1) that was less than 50% of predicted, 140 had chronic renal failure, 149 had a prior stroke, and 241 had heart failure with a left ventricular ejection less than 35%.
Patients with chronic renal failure had far and away the worst 30-day and long-term outcomes. Half were dead within 3 years. The 7-year survival rate was just 11.7%.
The second-worst outcomes were seen in patients with severe COPD preoperatively. Their 7-year survival rate was 30.8%.
"Anyone with an FEV1 below about 40% becomes a higher-risk surgical candidate; think instead of TAVR [transcatheter aortic valve replacement],"advised Dr. Thourani of the division of cardiothoracic surgery at Emory University, Atlanta.
In contrast, outcomes in patients with either heart failure or prior stroke "were not that bad," he said, pointing to 7-year survival rates of 55.9% and 48.6%, respectively.
Ninety-five patients (5.4%) in this recently published study (Ann. Thorac. Surg. 2013;95:838-45) had more than one dysfunctional organ system prior to SAVR. Median survival in patients without dysfunction in any of the four organ systems was 8.2 years and counting. With one dysfunctional organ, it was still good at 7.2 years. However, with two dysfunctional organ systems, the median survival dropped precipitously to 4.1 years. With three dysfunctional organ systems, it was 5.9 years.
Dr. Thourin serves as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
SNOWMASS, COLO. – The presence of preoperative dysfunction in more than any one of four key organ systems profoundly reduces survival in patients undergoing surgical aortic valve replacement, a study showed.
"If you have two or more dysfunctional organ systems, you really need to think about what you’re doing for this patient. At 5 years, only about 40% of these patients are alive. It makes a lot of sense to me to say that if you have a patient with severe COPD [chronic obstructive pulmonary disease] and renal dysfunction, that patient should probably never get a surgical valve," Dr. Vinod H. Thourani said at the Annual Cardiovascular Conference at Snowmass.
In a retrospective analysis of a registry with prospectively entered data, 29% of 1,759 patients who underwent surgical aortic valve replacement (SAVR) with or without coronary artery bypass grafting at Emory University during 2002-2010 had preoperative dysfunction of one or more of four organ systems under scrutiny. Eighty-five patients had severe COPD, as defined by a forced expiratory volume in 1 second (FEV1) that was less than 50% of predicted, 140 had chronic renal failure, 149 had a prior stroke, and 241 had heart failure with a left ventricular ejection less than 35%.
Patients with chronic renal failure had far and away the worst 30-day and long-term outcomes. Half were dead within 3 years. The 7-year survival rate was just 11.7%.
The second-worst outcomes were seen in patients with severe COPD preoperatively. Their 7-year survival rate was 30.8%.
"Anyone with an FEV1 below about 40% becomes a higher-risk surgical candidate; think instead of TAVR [transcatheter aortic valve replacement],"advised Dr. Thourani of the division of cardiothoracic surgery at Emory University, Atlanta.
In contrast, outcomes in patients with either heart failure or prior stroke "were not that bad," he said, pointing to 7-year survival rates of 55.9% and 48.6%, respectively.
Ninety-five patients (5.4%) in this recently published study (Ann. Thorac. Surg. 2013;95:838-45) had more than one dysfunctional organ system prior to SAVR. Median survival in patients without dysfunction in any of the four organ systems was 8.2 years and counting. With one dysfunctional organ, it was still good at 7.2 years. However, with two dysfunctional organ systems, the median survival dropped precipitously to 4.1 years. With three dysfunctional organ systems, it was 5.9 years.
Dr. Thourin serves as a consultant to Edwards Lifesciences, Sorin, and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
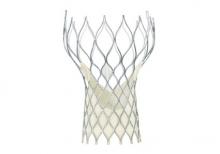
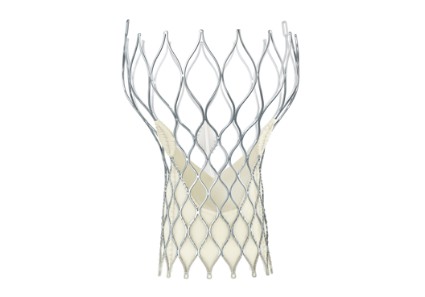
CoreValve holds size advantage for U.S. TAVR
When the Food and Drug Administration in January granted marketing approval to a second transcatheter aortic valve replacement system for inoperable patients with aortic stenosis, the CoreValve marketed by Medtronic, the new valve conceded a greater than 2-year head start to the first system on the U.S. market, Sapien marketed by Edwards.
But cardiologists see that 2-year edge in familiarity eclipsed for at least some patients by two major advantages that CoreValve currently holds over Sapien: delivery via a significantly thinner sheath, and the option of larger-diameter valves that allow replacement in patients with a wider aortic annulus.
The CoreValve delivery sheath is 18 French, compared with a 22F or 24F size for the Sapien transcatheter aortic valve replacement (TAVR) with U.S. approval, and the Sapien valves come in diameters of 23 and 26 mm, compared with options of 23, 26, 29, and 31 mm for the CoreValve.
"CoreValve is the device of choice for patients with smaller vessel sizes. Sapien has been a wonderful device to use, and we have so much experience with it, but the smaller CoreValve size will allow many more patients to be done with a transfemoral approach," said Dr. Peter C. Block, a professor of medicine at Emory University in Atlanta and an interventional cardiologist who performs TAVR.
"More patients will qualify for TAVR and will be treated transfemorally with the larger valve diameters and smaller sheath size," agreed Dr. Mauricio G. Cohen, director of cardiac catheterization at the University of Miami and TAVR interventionalist. Another potential positive of having CoreValve on the U.S. market is that competition between the two options will likely drive down the cost of a TAVR system, which until now has run more than $30,000, Dr. Cohen said in an interview.
CoreValve received FDA approval less than 3 months after researchers first reported data from the company’s U.S. Pivotal Trial Extreme Risk Iliofemoral Study last October at the TCT (Transcatheter Cardiovascular Therapeutics) annual meeting. In that study, 471 inoperable aortic stenosis patients had a 26% 1-year rate of death or major stroke, substantially surpassing the 43% rate that the study set up as the target for superiority, reported Dr. Jeffrey J. Popma, lead investigator on the study.
Dr. Popma warned against comparing CoreValve’s efficacy and safety performance in the trial and the Sapien system’s performance in its pivotal trial in inoperable patients, the PARTNER cohort B trial (N. Engl. J. Med. 2010;363:1597-607). "It’s very difficult to make cross-trial comparison," he said in an interview, a limitation also noted by Dr. Cohen and Dr. Block. But Dr. Popma highlighted the 2.4% 30-day stroke rate in the pivotal trial, and a 1.8% 30-day stroke rate seen with CoreValve in inoperable patients in a continued access program at the trial’s study sites. He also highlighted the 11% rate of moderate paravalvular aortic regurgitation after 30 days that dropped to a 4% rate after 1 year.
Perhaps the biggest downside of CoreValve’s performance in the pivotal trial was that 22% of patients required a permanent pacemaker implant within the first 30 days, increasing to 27% of patients with 1-year follow-up. Increased risk for a pacemaker is an inherent downside of CoreValve because of its longer size compared with the Sapien valve and how the CoreValve sits in the aortic annulus. The CoreValve is designed for supravalvular placement and anchoring in the left ventricular outflow tract near the left bundle branch that can result in mechanical irritation and arrhythmia with the need for pacing, explained Dr. Popma, professor of medicine at Harvard University and an interventional cardiologist at Beth Israel Deaconess Medical Center, Boston.
"I think our pacemaker rate was very acceptable. I don’t think it will ever be as low as with Sapien, but it’s a worthwhile trade-off because the CoreValve functions well and results in a low rate of paravalvular regurgitation," he said.
Dr. Popma also stressed that 1-year mortality was no greater among the patients who required a pacemaker implant in the pivotal trial. A subgroup analysis of results from the trial to try to identify which patients had the greatest risk for needing a pacemaker after a CoreValve implant has not yet finished, he said. It’s possible that certain patients with preexisting conduction abnormalities, such as a right bundle branch block coupled with a left anterior fascicular block, have the greatest vulnerability.
Patients for whom the Sapien system remains ideal are those with a narrow sinus of Valsalva, because the longer CoreValve frame crosses the sinus and may compromise coronary blood flow in patients with a narrow sinus, Dr. Popma said.
The choice between CoreValve and Sapien systems will grow even more complicated for U.S. cardiologists and surgeons when the Sapien XT valve system receives FDA marketing approval, likely later this year. The Sapien XT delivery sheath matches the 18F size of CoreValve and will also come in a 29-mm size, blunting two of CoreValves main advantages.
Medtronic also faces charges of patent infringement by its CoreValve in a court case initiated by Edwards. In mid-January, a jury in a U.S. District Court assessed a penalty of $394 million against Medtronic. Edwards is also seeking a court-ordered halt to U.S. marketing of CoreValve. But Medtronic is appealing the jury verdict and continues to fight the injunction, and a company spokesperson said in an interview that the legal maneuverings will likely take at least another year to fully resolve. In the meantime, Medtronic began U.S. distribution of the CoreValve on Jan. 17.
Dr. Block said that his institution received a research grant to participate in Sapien trials. Dr. Cohen said that he has been a consultant to Medtronic and Edwards. Dr. Popma said that his institution received research support from Medtronic and that he has been a consultant to and received research support from Boston Scientific.
When the Food and Drug Administration in January granted marketing approval to a second transcatheter aortic valve replacement system for inoperable patients with aortic stenosis, the CoreValve marketed by Medtronic, the new valve conceded a greater than 2-year head start to the first system on the U.S. market, Sapien marketed by Edwards.
But cardiologists see that 2-year edge in familiarity eclipsed for at least some patients by two major advantages that CoreValve currently holds over Sapien: delivery via a significantly thinner sheath, and the option of larger-diameter valves that allow replacement in patients with a wider aortic annulus.
The CoreValve delivery sheath is 18 French, compared with a 22F or 24F size for the Sapien transcatheter aortic valve replacement (TAVR) with U.S. approval, and the Sapien valves come in diameters of 23 and 26 mm, compared with options of 23, 26, 29, and 31 mm for the CoreValve.
"CoreValve is the device of choice for patients with smaller vessel sizes. Sapien has been a wonderful device to use, and we have so much experience with it, but the smaller CoreValve size will allow many more patients to be done with a transfemoral approach," said Dr. Peter C. Block, a professor of medicine at Emory University in Atlanta and an interventional cardiologist who performs TAVR.
"More patients will qualify for TAVR and will be treated transfemorally with the larger valve diameters and smaller sheath size," agreed Dr. Mauricio G. Cohen, director of cardiac catheterization at the University of Miami and TAVR interventionalist. Another potential positive of having CoreValve on the U.S. market is that competition between the two options will likely drive down the cost of a TAVR system, which until now has run more than $30,000, Dr. Cohen said in an interview.
CoreValve received FDA approval less than 3 months after researchers first reported data from the company’s U.S. Pivotal Trial Extreme Risk Iliofemoral Study last October at the TCT (Transcatheter Cardiovascular Therapeutics) annual meeting. In that study, 471 inoperable aortic stenosis patients had a 26% 1-year rate of death or major stroke, substantially surpassing the 43% rate that the study set up as the target for superiority, reported Dr. Jeffrey J. Popma, lead investigator on the study.
Dr. Popma warned against comparing CoreValve’s efficacy and safety performance in the trial and the Sapien system’s performance in its pivotal trial in inoperable patients, the PARTNER cohort B trial (N. Engl. J. Med. 2010;363:1597-607). "It’s very difficult to make cross-trial comparison," he said in an interview, a limitation also noted by Dr. Cohen and Dr. Block. But Dr. Popma highlighted the 2.4% 30-day stroke rate in the pivotal trial, and a 1.8% 30-day stroke rate seen with CoreValve in inoperable patients in a continued access program at the trial’s study sites. He also highlighted the 11% rate of moderate paravalvular aortic regurgitation after 30 days that dropped to a 4% rate after 1 year.
Perhaps the biggest downside of CoreValve’s performance in the pivotal trial was that 22% of patients required a permanent pacemaker implant within the first 30 days, increasing to 27% of patients with 1-year follow-up. Increased risk for a pacemaker is an inherent downside of CoreValve because of its longer size compared with the Sapien valve and how the CoreValve sits in the aortic annulus. The CoreValve is designed for supravalvular placement and anchoring in the left ventricular outflow tract near the left bundle branch that can result in mechanical irritation and arrhythmia with the need for pacing, explained Dr. Popma, professor of medicine at Harvard University and an interventional cardiologist at Beth Israel Deaconess Medical Center, Boston.
"I think our pacemaker rate was very acceptable. I don’t think it will ever be as low as with Sapien, but it’s a worthwhile trade-off because the CoreValve functions well and results in a low rate of paravalvular regurgitation," he said.
Dr. Popma also stressed that 1-year mortality was no greater among the patients who required a pacemaker implant in the pivotal trial. A subgroup analysis of results from the trial to try to identify which patients had the greatest risk for needing a pacemaker after a CoreValve implant has not yet finished, he said. It’s possible that certain patients with preexisting conduction abnormalities, such as a right bundle branch block coupled with a left anterior fascicular block, have the greatest vulnerability.
Patients for whom the Sapien system remains ideal are those with a narrow sinus of Valsalva, because the longer CoreValve frame crosses the sinus and may compromise coronary blood flow in patients with a narrow sinus, Dr. Popma said.
The choice between CoreValve and Sapien systems will grow even more complicated for U.S. cardiologists and surgeons when the Sapien XT valve system receives FDA marketing approval, likely later this year. The Sapien XT delivery sheath matches the 18F size of CoreValve and will also come in a 29-mm size, blunting two of CoreValves main advantages.
Medtronic also faces charges of patent infringement by its CoreValve in a court case initiated by Edwards. In mid-January, a jury in a U.S. District Court assessed a penalty of $394 million against Medtronic. Edwards is also seeking a court-ordered halt to U.S. marketing of CoreValve. But Medtronic is appealing the jury verdict and continues to fight the injunction, and a company spokesperson said in an interview that the legal maneuverings will likely take at least another year to fully resolve. In the meantime, Medtronic began U.S. distribution of the CoreValve on Jan. 17.
Dr. Block said that his institution received a research grant to participate in Sapien trials. Dr. Cohen said that he has been a consultant to Medtronic and Edwards. Dr. Popma said that his institution received research support from Medtronic and that he has been a consultant to and received research support from Boston Scientific.
When the Food and Drug Administration in January granted marketing approval to a second transcatheter aortic valve replacement system for inoperable patients with aortic stenosis, the CoreValve marketed by Medtronic, the new valve conceded a greater than 2-year head start to the first system on the U.S. market, Sapien marketed by Edwards.
But cardiologists see that 2-year edge in familiarity eclipsed for at least some patients by two major advantages that CoreValve currently holds over Sapien: delivery via a significantly thinner sheath, and the option of larger-diameter valves that allow replacement in patients with a wider aortic annulus.
The CoreValve delivery sheath is 18 French, compared with a 22F or 24F size for the Sapien transcatheter aortic valve replacement (TAVR) with U.S. approval, and the Sapien valves come in diameters of 23 and 26 mm, compared with options of 23, 26, 29, and 31 mm for the CoreValve.
"CoreValve is the device of choice for patients with smaller vessel sizes. Sapien has been a wonderful device to use, and we have so much experience with it, but the smaller CoreValve size will allow many more patients to be done with a transfemoral approach," said Dr. Peter C. Block, a professor of medicine at Emory University in Atlanta and an interventional cardiologist who performs TAVR.
"More patients will qualify for TAVR and will be treated transfemorally with the larger valve diameters and smaller sheath size," agreed Dr. Mauricio G. Cohen, director of cardiac catheterization at the University of Miami and TAVR interventionalist. Another potential positive of having CoreValve on the U.S. market is that competition between the two options will likely drive down the cost of a TAVR system, which until now has run more than $30,000, Dr. Cohen said in an interview.
CoreValve received FDA approval less than 3 months after researchers first reported data from the company’s U.S. Pivotal Trial Extreme Risk Iliofemoral Study last October at the TCT (Transcatheter Cardiovascular Therapeutics) annual meeting. In that study, 471 inoperable aortic stenosis patients had a 26% 1-year rate of death or major stroke, substantially surpassing the 43% rate that the study set up as the target for superiority, reported Dr. Jeffrey J. Popma, lead investigator on the study.
Dr. Popma warned against comparing CoreValve’s efficacy and safety performance in the trial and the Sapien system’s performance in its pivotal trial in inoperable patients, the PARTNER cohort B trial (N. Engl. J. Med. 2010;363:1597-607). "It’s very difficult to make cross-trial comparison," he said in an interview, a limitation also noted by Dr. Cohen and Dr. Block. But Dr. Popma highlighted the 2.4% 30-day stroke rate in the pivotal trial, and a 1.8% 30-day stroke rate seen with CoreValve in inoperable patients in a continued access program at the trial’s study sites. He also highlighted the 11% rate of moderate paravalvular aortic regurgitation after 30 days that dropped to a 4% rate after 1 year.
Perhaps the biggest downside of CoreValve’s performance in the pivotal trial was that 22% of patients required a permanent pacemaker implant within the first 30 days, increasing to 27% of patients with 1-year follow-up. Increased risk for a pacemaker is an inherent downside of CoreValve because of its longer size compared with the Sapien valve and how the CoreValve sits in the aortic annulus. The CoreValve is designed for supravalvular placement and anchoring in the left ventricular outflow tract near the left bundle branch that can result in mechanical irritation and arrhythmia with the need for pacing, explained Dr. Popma, professor of medicine at Harvard University and an interventional cardiologist at Beth Israel Deaconess Medical Center, Boston.
"I think our pacemaker rate was very acceptable. I don’t think it will ever be as low as with Sapien, but it’s a worthwhile trade-off because the CoreValve functions well and results in a low rate of paravalvular regurgitation," he said.
Dr. Popma also stressed that 1-year mortality was no greater among the patients who required a pacemaker implant in the pivotal trial. A subgroup analysis of results from the trial to try to identify which patients had the greatest risk for needing a pacemaker after a CoreValve implant has not yet finished, he said. It’s possible that certain patients with preexisting conduction abnormalities, such as a right bundle branch block coupled with a left anterior fascicular block, have the greatest vulnerability.
Patients for whom the Sapien system remains ideal are those with a narrow sinus of Valsalva, because the longer CoreValve frame crosses the sinus and may compromise coronary blood flow in patients with a narrow sinus, Dr. Popma said.
The choice between CoreValve and Sapien systems will grow even more complicated for U.S. cardiologists and surgeons when the Sapien XT valve system receives FDA marketing approval, likely later this year. The Sapien XT delivery sheath matches the 18F size of CoreValve and will also come in a 29-mm size, blunting two of CoreValves main advantages.
Medtronic also faces charges of patent infringement by its CoreValve in a court case initiated by Edwards. In mid-January, a jury in a U.S. District Court assessed a penalty of $394 million against Medtronic. Edwards is also seeking a court-ordered halt to U.S. marketing of CoreValve. But Medtronic is appealing the jury verdict and continues to fight the injunction, and a company spokesperson said in an interview that the legal maneuverings will likely take at least another year to fully resolve. In the meantime, Medtronic began U.S. distribution of the CoreValve on Jan. 17.
Dr. Block said that his institution received a research grant to participate in Sapien trials. Dr. Cohen said that he has been a consultant to Medtronic and Edwards. Dr. Popma said that his institution received research support from Medtronic and that he has been a consultant to and received research support from Boston Scientific.
New tools for stroke prediction in atrial fibrillation
SNOWMASS, COLO. – High-sensitivity troponin T and brain natriuretic peptide levels are better predictors of stroke risk in patients with atrial fibrillation than the CHA2DS2-VASc score that will replace the CHADS2 score in the forthcoming revised American College of Cardiology/American Heart Association guidelines.
Recent evidence indicates the biomarkers may be novel tools for improved stroke prediction in atrial fibrillation (AF), with prognostic value above and beyond that provided by the CHA2DS2-VASc scores.
These findings raise important unanswered questions about the relationship between AF and stroke. Conventional wisdom has held that left atrial thrombus is the cause of most strokes in patients with AF. But it’s not that simple, Dr. Bernard J. Gersh asserted at the Annual Cardiovascular Conference at Snowmass.
"What are we measuring with these biomarkers? This is what we really don’t understand. What has high-sensitivity troponin T got to do with left atrial thrombus?" asked Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
It seems increasingly clear that it’s not just the atrial arrhythmia that’s important in stroke risk, it’s also the company AF keeps. In a substantial but still uncertain proportion of patients, AF is a marker of vascular disease burden expressed through atrial and vascular endothelial dysfunction, vascular inflammation, left atrial dilatation and fibrosis, and a hypercoagulable state, the cardiologist continued.
He was a coinvestigator on a couple of recent groundbreaking studies that show the prognostic power of biomarkers in predicting both stroke risk and cardiac death in AF patients.
In one report, the investigators looked at baseline high-sensitivity troponin T (hsTnT) levels, clinical risk factors for stroke, and CHA2DS2-VASc scores in 12,892 patients with AF who were randomized to apixaban or warfarin in the prospective, double-blind ARISTOTLE (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation) trial (N. Engl. J. Med. 2011;365:981-92). During a median 1.9 years of follow-up, patients in the highest quartile for baseline hsTnT had roughly a twofold greater risk of stroke or systemic embolism than did those in the lowest quartile.
Moreover, patients with a low-risk CHA2DS2-VASc score of 0 or 1 but in the top quartile for hsTnT, with a level in excess of 13 ng/L, had a very substantial stroke rate of 2.7% per year despite anticoagulation with apixaban (Eliquis) or warfarin. The relationship was even stronger for cardiac death, where subjects with a low-risk CHA2DS2-VASc score who were in the top quartile for hsTnT had a 6% annual risk. A higher baseline hsTnT was also independently associated with sharply increased risk of major bleeding in a multivariate regression analysis (J. Am. Coll. Cardiol. 2014;63:52-61).
In another recently published study, he and his international coworkers showed in ARISTOTLE participants with baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels that this biomarker also improved stroke prediction in AF, providing added value to CHA2DS2-VASc scores. Subjects in the top quartile for baseline NT-proBNP had an adjusted 2.35-fold greater risk of stroke or systemic embolism than those in the lowest quartile, irrespective of CHA2DS2-VASc score. They also had a 2.5-fold greater risk of cardiac death (J. Am. Coll. Cardiol. 2013;61:2274-84).
A study Dr. Gersh highlighted as "extremely interesting" involved the use of brain natriuretic peptide (BNP) as a marker to rule out delayed AF in stroke patients. The study, led by investigators at the University Hospital Center of Nice (France) and known as TARGET-AF, included 300 consecutive acute stroke patients with no history of AF and no AF on their baseline ECG. During a median 6.8 days of in-hospital Holter monitoring, 17% of the stroke patients developed newly diagnosed AF.
The strongest predictor of delayed AF was baseline plasma BNP. It outperformed the CHA2DS2-VASc score and all the other parameters examined, including anterior circulation location of the stroke, P-wave initial force, gender, National Institutes of Health Stroke Scale score, age, left atrial dilatation, and Score for the Targeting of AF (STAF) score. A BNP level greater than 131 pg/mL had a 98.1% sensitivity, 71.4% specificity, and 99.4% negative predictive value for delayed AF.
"Our data indicate that a BNP level of 131 pg/mL or less might rule out delayed AF in stroke survivors and could be included in algorithms for AF detection," the French investigators concluded (J. Stroke Cerebrovasc. Dis. 2013;22:e103-10).
There is plenty of direct evidence from transesophageal echocardiography studies and other sources that a substantial proportion of thromboemboli are directly the result of AF. However, indirect evidence points to additional causal factors. For example, there is a high incidence of thromboembolic events in AF patients without left atrial appendage thrombus. Plus, in natural history studies patients with AF without additional risk factors have a low incidence of stroke. And CHADS2 and CHA2DS2-VASc scores predict vascular events but don’t correlate with left atrial appendage thrombus, Dr. Gersh noted.
He said the CHA2DS2-VASc score is clearly an improvement over CHADS2, and its adoption in the forthcoming ACC/AHA guidelines is to be welcomed. The CHA2DS2-VASc score increases the number of patients considered at significant risk of stroke and therefore warranting anticoagulation. For example, in a large Danish registry of nearly 48,000 AF patients with a CHADS2 score of 0-1 not on anticoagulation, patients with a CHADS2 score of 1 but a CHA2DS2-VASc score of 2 had twice the stroke risk of patients with a CHA2DS2-VASc of 1 (Thromb. Haemost. 2012;107:1172-9).
That being said, neither risk score is all that impressive. The C-statistic, a measure of a test’s predictive power, is 0.56 for CHADS2 and it was 0.62 for CHA2DS2-VASc in the ARISTOTLE analysis. To put those figures in perspective, a coin toss has a C-statistic of 0.50.
"The individual predictive values are not good. We use CHADS2 and CHA2DS2-VASc in practice and in the guidelines, but we should not pretend they are highly predictive. We need new risk stratification schemes," according to Dr. Gersh.
He reported serving as an adviser to Boston Scientific and St. Jude Medical.
SNOWMASS, COLO. – High-sensitivity troponin T and brain natriuretic peptide levels are better predictors of stroke risk in patients with atrial fibrillation than the CHA2DS2-VASc score that will replace the CHADS2 score in the forthcoming revised American College of Cardiology/American Heart Association guidelines.
Recent evidence indicates the biomarkers may be novel tools for improved stroke prediction in atrial fibrillation (AF), with prognostic value above and beyond that provided by the CHA2DS2-VASc scores.
These findings raise important unanswered questions about the relationship between AF and stroke. Conventional wisdom has held that left atrial thrombus is the cause of most strokes in patients with AF. But it’s not that simple, Dr. Bernard J. Gersh asserted at the Annual Cardiovascular Conference at Snowmass.
"What are we measuring with these biomarkers? This is what we really don’t understand. What has high-sensitivity troponin T got to do with left atrial thrombus?" asked Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
It seems increasingly clear that it’s not just the atrial arrhythmia that’s important in stroke risk, it’s also the company AF keeps. In a substantial but still uncertain proportion of patients, AF is a marker of vascular disease burden expressed through atrial and vascular endothelial dysfunction, vascular inflammation, left atrial dilatation and fibrosis, and a hypercoagulable state, the cardiologist continued.
He was a coinvestigator on a couple of recent groundbreaking studies that show the prognostic power of biomarkers in predicting both stroke risk and cardiac death in AF patients.
In one report, the investigators looked at baseline high-sensitivity troponin T (hsTnT) levels, clinical risk factors for stroke, and CHA2DS2-VASc scores in 12,892 patients with AF who were randomized to apixaban or warfarin in the prospective, double-blind ARISTOTLE (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation) trial (N. Engl. J. Med. 2011;365:981-92). During a median 1.9 years of follow-up, patients in the highest quartile for baseline hsTnT had roughly a twofold greater risk of stroke or systemic embolism than did those in the lowest quartile.
Moreover, patients with a low-risk CHA2DS2-VASc score of 0 or 1 but in the top quartile for hsTnT, with a level in excess of 13 ng/L, had a very substantial stroke rate of 2.7% per year despite anticoagulation with apixaban (Eliquis) or warfarin. The relationship was even stronger for cardiac death, where subjects with a low-risk CHA2DS2-VASc score who were in the top quartile for hsTnT had a 6% annual risk. A higher baseline hsTnT was also independently associated with sharply increased risk of major bleeding in a multivariate regression analysis (J. Am. Coll. Cardiol. 2014;63:52-61).
In another recently published study, he and his international coworkers showed in ARISTOTLE participants with baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels that this biomarker also improved stroke prediction in AF, providing added value to CHA2DS2-VASc scores. Subjects in the top quartile for baseline NT-proBNP had an adjusted 2.35-fold greater risk of stroke or systemic embolism than those in the lowest quartile, irrespective of CHA2DS2-VASc score. They also had a 2.5-fold greater risk of cardiac death (J. Am. Coll. Cardiol. 2013;61:2274-84).
A study Dr. Gersh highlighted as "extremely interesting" involved the use of brain natriuretic peptide (BNP) as a marker to rule out delayed AF in stroke patients. The study, led by investigators at the University Hospital Center of Nice (France) and known as TARGET-AF, included 300 consecutive acute stroke patients with no history of AF and no AF on their baseline ECG. During a median 6.8 days of in-hospital Holter monitoring, 17% of the stroke patients developed newly diagnosed AF.
The strongest predictor of delayed AF was baseline plasma BNP. It outperformed the CHA2DS2-VASc score and all the other parameters examined, including anterior circulation location of the stroke, P-wave initial force, gender, National Institutes of Health Stroke Scale score, age, left atrial dilatation, and Score for the Targeting of AF (STAF) score. A BNP level greater than 131 pg/mL had a 98.1% sensitivity, 71.4% specificity, and 99.4% negative predictive value for delayed AF.
"Our data indicate that a BNP level of 131 pg/mL or less might rule out delayed AF in stroke survivors and could be included in algorithms for AF detection," the French investigators concluded (J. Stroke Cerebrovasc. Dis. 2013;22:e103-10).
There is plenty of direct evidence from transesophageal echocardiography studies and other sources that a substantial proportion of thromboemboli are directly the result of AF. However, indirect evidence points to additional causal factors. For example, there is a high incidence of thromboembolic events in AF patients without left atrial appendage thrombus. Plus, in natural history studies patients with AF without additional risk factors have a low incidence of stroke. And CHADS2 and CHA2DS2-VASc scores predict vascular events but don’t correlate with left atrial appendage thrombus, Dr. Gersh noted.
He said the CHA2DS2-VASc score is clearly an improvement over CHADS2, and its adoption in the forthcoming ACC/AHA guidelines is to be welcomed. The CHA2DS2-VASc score increases the number of patients considered at significant risk of stroke and therefore warranting anticoagulation. For example, in a large Danish registry of nearly 48,000 AF patients with a CHADS2 score of 0-1 not on anticoagulation, patients with a CHADS2 score of 1 but a CHA2DS2-VASc score of 2 had twice the stroke risk of patients with a CHA2DS2-VASc of 1 (Thromb. Haemost. 2012;107:1172-9).
That being said, neither risk score is all that impressive. The C-statistic, a measure of a test’s predictive power, is 0.56 for CHADS2 and it was 0.62 for CHA2DS2-VASc in the ARISTOTLE analysis. To put those figures in perspective, a coin toss has a C-statistic of 0.50.
"The individual predictive values are not good. We use CHADS2 and CHA2DS2-VASc in practice and in the guidelines, but we should not pretend they are highly predictive. We need new risk stratification schemes," according to Dr. Gersh.
He reported serving as an adviser to Boston Scientific and St. Jude Medical.
SNOWMASS, COLO. – High-sensitivity troponin T and brain natriuretic peptide levels are better predictors of stroke risk in patients with atrial fibrillation than the CHA2DS2-VASc score that will replace the CHADS2 score in the forthcoming revised American College of Cardiology/American Heart Association guidelines.
Recent evidence indicates the biomarkers may be novel tools for improved stroke prediction in atrial fibrillation (AF), with prognostic value above and beyond that provided by the CHA2DS2-VASc scores.
These findings raise important unanswered questions about the relationship between AF and stroke. Conventional wisdom has held that left atrial thrombus is the cause of most strokes in patients with AF. But it’s not that simple, Dr. Bernard J. Gersh asserted at the Annual Cardiovascular Conference at Snowmass.
"What are we measuring with these biomarkers? This is what we really don’t understand. What has high-sensitivity troponin T got to do with left atrial thrombus?" asked Dr. Gersh, professor of medicine at the Mayo Clinic in Rochester, Minn.
It seems increasingly clear that it’s not just the atrial arrhythmia that’s important in stroke risk, it’s also the company AF keeps. In a substantial but still uncertain proportion of patients, AF is a marker of vascular disease burden expressed through atrial and vascular endothelial dysfunction, vascular inflammation, left atrial dilatation and fibrosis, and a hypercoagulable state, the cardiologist continued.
He was a coinvestigator on a couple of recent groundbreaking studies that show the prognostic power of biomarkers in predicting both stroke risk and cardiac death in AF patients.
In one report, the investigators looked at baseline high-sensitivity troponin T (hsTnT) levels, clinical risk factors for stroke, and CHA2DS2-VASc scores in 12,892 patients with AF who were randomized to apixaban or warfarin in the prospective, double-blind ARISTOTLE (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation) trial (N. Engl. J. Med. 2011;365:981-92). During a median 1.9 years of follow-up, patients in the highest quartile for baseline hsTnT had roughly a twofold greater risk of stroke or systemic embolism than did those in the lowest quartile.
Moreover, patients with a low-risk CHA2DS2-VASc score of 0 or 1 but in the top quartile for hsTnT, with a level in excess of 13 ng/L, had a very substantial stroke rate of 2.7% per year despite anticoagulation with apixaban (Eliquis) or warfarin. The relationship was even stronger for cardiac death, where subjects with a low-risk CHA2DS2-VASc score who were in the top quartile for hsTnT had a 6% annual risk. A higher baseline hsTnT was also independently associated with sharply increased risk of major bleeding in a multivariate regression analysis (J. Am. Coll. Cardiol. 2014;63:52-61).
In another recently published study, he and his international coworkers showed in ARISTOTLE participants with baseline N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels that this biomarker also improved stroke prediction in AF, providing added value to CHA2DS2-VASc scores. Subjects in the top quartile for baseline NT-proBNP had an adjusted 2.35-fold greater risk of stroke or systemic embolism than those in the lowest quartile, irrespective of CHA2DS2-VASc score. They also had a 2.5-fold greater risk of cardiac death (J. Am. Coll. Cardiol. 2013;61:2274-84).
A study Dr. Gersh highlighted as "extremely interesting" involved the use of brain natriuretic peptide (BNP) as a marker to rule out delayed AF in stroke patients. The study, led by investigators at the University Hospital Center of Nice (France) and known as TARGET-AF, included 300 consecutive acute stroke patients with no history of AF and no AF on their baseline ECG. During a median 6.8 days of in-hospital Holter monitoring, 17% of the stroke patients developed newly diagnosed AF.
The strongest predictor of delayed AF was baseline plasma BNP. It outperformed the CHA2DS2-VASc score and all the other parameters examined, including anterior circulation location of the stroke, P-wave initial force, gender, National Institutes of Health Stroke Scale score, age, left atrial dilatation, and Score for the Targeting of AF (STAF) score. A BNP level greater than 131 pg/mL had a 98.1% sensitivity, 71.4% specificity, and 99.4% negative predictive value for delayed AF.
"Our data indicate that a BNP level of 131 pg/mL or less might rule out delayed AF in stroke survivors and could be included in algorithms for AF detection," the French investigators concluded (J. Stroke Cerebrovasc. Dis. 2013;22:e103-10).
There is plenty of direct evidence from transesophageal echocardiography studies and other sources that a substantial proportion of thromboemboli are directly the result of AF. However, indirect evidence points to additional causal factors. For example, there is a high incidence of thromboembolic events in AF patients without left atrial appendage thrombus. Plus, in natural history studies patients with AF without additional risk factors have a low incidence of stroke. And CHADS2 and CHA2DS2-VASc scores predict vascular events but don’t correlate with left atrial appendage thrombus, Dr. Gersh noted.
He said the CHA2DS2-VASc score is clearly an improvement over CHADS2, and its adoption in the forthcoming ACC/AHA guidelines is to be welcomed. The CHA2DS2-VASc score increases the number of patients considered at significant risk of stroke and therefore warranting anticoagulation. For example, in a large Danish registry of nearly 48,000 AF patients with a CHADS2 score of 0-1 not on anticoagulation, patients with a CHADS2 score of 1 but a CHA2DS2-VASc score of 2 had twice the stroke risk of patients with a CHA2DS2-VASc of 1 (Thromb. Haemost. 2012;107:1172-9).
That being said, neither risk score is all that impressive. The C-statistic, a measure of a test’s predictive power, is 0.56 for CHADS2 and it was 0.62 for CHA2DS2-VASc in the ARISTOTLE analysis. To put those figures in perspective, a coin toss has a C-statistic of 0.50.
"The individual predictive values are not good. We use CHADS2 and CHA2DS2-VASc in practice and in the guidelines, but we should not pretend they are highly predictive. We need new risk stratification schemes," according to Dr. Gersh.
He reported serving as an adviser to Boston Scientific and St. Jude Medical.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
Genotyping adds little to optimized warfarin dosing
DALLAS - Genotyping to guide the starting dosage of warfarin treatment showed no added value above tailoring treatment with a panel of clinical features in a randomized, controlled U.S. trial of more than 1,000 patients.
The resounding null result from adding genotype information should spell the end of this practice, said Dr. Stephen E. Kimmel, lead investigator for the study, and professor and director of cardiovascular epidemiology at the University of Pennsylvania in Philadelphia.
"Based on what we've seen, I don't believe there is sufficient evidence to add genetic information on top of the available clinical algorithms," Dr. Kimmel said at the American Heart Association scientific sessions. "I don't think we'll see another genetics trial in warfarin treatment. I think this is it."
Good outcomes in the comparison group largely accounted for the failure of genotyping to significantly improve the percentage of time that patients' international normalized ratios (INRs) were in the target range of 2.0-3.0. The amount of time in the therapeutic INR range (PTTR) averaged 45% in patients in the comparison arm during the first 4 weeks of warfarin treatment.
"When the comparison group does so well, it's more difficult for genotyping to have an effect," said Dr. Elaine M. Hylek, professor of medicine and an anticoagulant specialist at Boston University.
That limitation, coupled with the shifting anticoagulant landscape, knock genotyping out of the picture, she agreed. "It will be difficult funding [another study of genotyping] with all the anticoagulant alternatives that are now out there" for preventing thrombosis in patients with atrial fibrillation or a recent venous thromboembolism.
The Clarification of Optimal Anticoagulant through Genetics (COAG) trial enrolled 1,015 patients initiating warfarin therapy at 18 U.S. centers during September 2009 to April 2013. The study randomized patients to two different ways to calculate their warfarin dosage during the first 5 days of treatment.
Half had their dosage calculated by a formula that took into account seven clinical and demographic factors, including age, race, smoking status, and body surface area. The others had their dosage calculated with the same formula and factors plus added information on the patient's genotype for two genes that affect warfarin activity, CYP2C9 and VKORC1. Patients' average age was 58 years; just over a quarter were African American.
During the first 4 weeks on treatment, the average PTTR was 45% in both arms of the study, the trial's primary endpoint. Adding genotyping information led to a bigger improvement in the PTTR among the non-African American patients compared with those who were African American, but did not produce a significantly increased PTTR in the non-African American subgroup.
Dr. Munir Pirmohamed reported results from a similar study that enrolled 455 patients starting warfarin therapy at any of five centers in the United Kingdom and Sweden.
The EU-Pharmacogenetics of Anticoagulant Therapy (EU-PACT) Warfarin study mainly differed from the COAG study by the background method used to calculate a starting warfarin dosage over the first 3 days of treatment.
In EU-PACT, the warfarin dosage of all patients was adjusted for age but not for other factors. In the intervention arm, the starting dosages were adjusted for the status of the same two genes, CYP2C9 and VKORC1.
During the first 12 weeks after starting warfarin, the cumulative average PTTR was 67% in the patients whose dose was adjusted by genotype and 60% in patients who were not genotyped, a statistically significant difference for this study's primary endpoint, reported Dr. Pirmohamed, professor and head of molecular and clinical pharmacology at the University of Liverpool, England.
Taken together, the findings of the two studies highlight that patients should not start warfarin treatment on a fixed dosage, said Dr. Patrick T. Ellinor, a cardiologist and arrhythmia specialist at Massachusetts General Hospital, Boston, and designated discussant for both reports at the meeting.
The findings support use of a clinical algorithm that takes into account several clinical factors, he added.
Concurrently with the meeting, the reports were published online for COAG (N. Engl. J. Med. 2013 [doi: 10.1056/NEJMoa1310669]) and for EU-PACT (N. Engl. J. Med. 2013 [doi: 10.1056/NEJMoa1311386]).
Warfarin on top despite competition
Warfarin remains the most widely used anticoagulant in the United States. Among patients with atrial fibrillation taking anticoagulation treatment, 72% used warfarin during the third quarter of 2013, data from a large registry show.
The new anticoagulants on the U.S. market - dabigatran (Pradaxa), apixaban (Eliquis), and rivaroxaban (Xarelto) - are gaining ground and widely acknowledged to be better, safer, and easier to manage. But warfarin clings to the market largely because of familiarity and low price, according to several experts.
"There is no doubt that the new anticoagulants look better compared with warfarin, but the clinical fact is that it's what you know versus what you don't know, and warfarin has been used for 60 years," Dr. Kimmel said at the press conference.
"The new drugs perform better than warfarin does; they get patients to where they need to be more quickly, but warfarin has been around a long time. We know its interactions and risks, there is the ability to reverse its effect, and the cost to patients is a real issue. If the new anticoagulants were the same price as warfarin then I think you'd see a lot more patients get a new drug," Dr. Ellinor said in an interview.
Data on current U.S. uptake of the new oral anticoagulants came from the 148,320 unique patients with atrial fibrillation included during June to September of 2013 in the PINNACLE Registry database run by the American College of Cardiology. Among these patients, about 83,000 (56%) were on some anticoagulant treatment, and within this subgroup, 72% were on warfarin, 27% on a new anticoagulant, and the remainder on different treatment, said Dr. John Gordon Harold, ACC president and a cardiologist at Cedars-Sinai Heart Institute in Los Angeles.
"In my own practice the new anticoagulants are being used with increasing frequency, mainly driven by direct-to-consumer advertising," Dr. Harold said in an interview. "We have patients who are completely stable on warfarin. (They) come in because of a consumer ad and they ask if they should switch drugs. When patients are stable I don't encourage them to switch, but we have a shared decision making conversation and go over the pros and cons, the cost, and the outcomes data. A lot of patients prefer to pay the difference" and switch to a new anticoagulant.
Dr. Harold said he also recommends that patients switch off warfarin if they have problems with compliance and variability in their international normalized ratio (INR).
"If you can keep a patient on warfarin in their INR target range 80% or more of the time then I wouldn't change, but most patients on warfarin have a very hard time maintaining an INR of 2-3," said Dr. Mark S. Link, professor and codirector of the cardiac arrhythmia center at Tufts Medical Center, Boston. But he said cost is a major factor keeping many patients on warfarin.
The new anticoagulants "are better than warfarin, but we are often forced by insurers to start with the cheaper drug," Dr. Link said during the news conference.
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed, Dr. Ellinor, Dr. Harold, and Dr. Link had no relevant disclosures.
On Twitter @mitchelzoler
DALLAS - Genotyping to guide the starting dosage of warfarin treatment showed no added value above tailoring treatment with a panel of clinical features in a randomized, controlled U.S. trial of more than 1,000 patients.
The resounding null result from adding genotype information should spell the end of this practice, said Dr. Stephen E. Kimmel, lead investigator for the study, and professor and director of cardiovascular epidemiology at the University of Pennsylvania in Philadelphia.
"Based on what we've seen, I don't believe there is sufficient evidence to add genetic information on top of the available clinical algorithms," Dr. Kimmel said at the American Heart Association scientific sessions. "I don't think we'll see another genetics trial in warfarin treatment. I think this is it."
Good outcomes in the comparison group largely accounted for the failure of genotyping to significantly improve the percentage of time that patients' international normalized ratios (INRs) were in the target range of 2.0-3.0. The amount of time in the therapeutic INR range (PTTR) averaged 45% in patients in the comparison arm during the first 4 weeks of warfarin treatment.
"When the comparison group does so well, it's more difficult for genotyping to have an effect," said Dr. Elaine M. Hylek, professor of medicine and an anticoagulant specialist at Boston University.
That limitation, coupled with the shifting anticoagulant landscape, knock genotyping out of the picture, she agreed. "It will be difficult funding [another study of genotyping] with all the anticoagulant alternatives that are now out there" for preventing thrombosis in patients with atrial fibrillation or a recent venous thromboembolism.
The Clarification of Optimal Anticoagulant through Genetics (COAG) trial enrolled 1,015 patients initiating warfarin therapy at 18 U.S. centers during September 2009 to April 2013. The study randomized patients to two different ways to calculate their warfarin dosage during the first 5 days of treatment.
Half had their dosage calculated by a formula that took into account seven clinical and demographic factors, including age, race, smoking status, and body surface area. The others had their dosage calculated with the same formula and factors plus added information on the patient's genotype for two genes that affect warfarin activity, CYP2C9 and VKORC1. Patients' average age was 58 years; just over a quarter were African American.
During the first 4 weeks on treatment, the average PTTR was 45% in both arms of the study, the trial's primary endpoint. Adding genotyping information led to a bigger improvement in the PTTR among the non-African American patients compared with those who were African American, but did not produce a significantly increased PTTR in the non-African American subgroup.
Dr. Munir Pirmohamed reported results from a similar study that enrolled 455 patients starting warfarin therapy at any of five centers in the United Kingdom and Sweden.
The EU-Pharmacogenetics of Anticoagulant Therapy (EU-PACT) Warfarin study mainly differed from the COAG study by the background method used to calculate a starting warfarin dosage over the first 3 days of treatment.
In EU-PACT, the warfarin dosage of all patients was adjusted for age but not for other factors. In the intervention arm, the starting dosages were adjusted for the status of the same two genes, CYP2C9 and VKORC1.
During the first 12 weeks after starting warfarin, the cumulative average PTTR was 67% in the patients whose dose was adjusted by genotype and 60% in patients who were not genotyped, a statistically significant difference for this study's primary endpoint, reported Dr. Pirmohamed, professor and head of molecular and clinical pharmacology at the University of Liverpool, England.
Taken together, the findings of the two studies highlight that patients should not start warfarin treatment on a fixed dosage, said Dr. Patrick T. Ellinor, a cardiologist and arrhythmia specialist at Massachusetts General Hospital, Boston, and designated discussant for both reports at the meeting.
The findings support use of a clinical algorithm that takes into account several clinical factors, he added.
Concurrently with the meeting, the reports were published online for COAG (N. Engl. J. Med. 2013 [doi: 10.1056/NEJMoa1310669]) and for EU-PACT (N. Engl. J. Med. 2013 [doi: 10.1056/NEJMoa1311386]).
Warfarin on top despite competition
Warfarin remains the most widely used anticoagulant in the United States. Among patients with atrial fibrillation taking anticoagulation treatment, 72% used warfarin during the third quarter of 2013, data from a large registry show.
The new anticoagulants on the U.S. market - dabigatran (Pradaxa), apixaban (Eliquis), and rivaroxaban (Xarelto) - are gaining ground and widely acknowledged to be better, safer, and easier to manage. But warfarin clings to the market largely because of familiarity and low price, according to several experts.
"There is no doubt that the new anticoagulants look better compared with warfarin, but the clinical fact is that it's what you know versus what you don't know, and warfarin has been used for 60 years," Dr. Kimmel said at the press conference.
"The new drugs perform better than warfarin does; they get patients to where they need to be more quickly, but warfarin has been around a long time. We know its interactions and risks, there is the ability to reverse its effect, and the cost to patients is a real issue. If the new anticoagulants were the same price as warfarin then I think you'd see a lot more patients get a new drug," Dr. Ellinor said in an interview.
Data on current U.S. uptake of the new oral anticoagulants came from the 148,320 unique patients with atrial fibrillation included during June to September of 2013 in the PINNACLE Registry database run by the American College of Cardiology. Among these patients, about 83,000 (56%) were on some anticoagulant treatment, and within this subgroup, 72% were on warfarin, 27% on a new anticoagulant, and the remainder on different treatment, said Dr. John Gordon Harold, ACC president and a cardiologist at Cedars-Sinai Heart Institute in Los Angeles.
"In my own practice the new anticoagulants are being used with increasing frequency, mainly driven by direct-to-consumer advertising," Dr. Harold said in an interview. "We have patients who are completely stable on warfarin. (They) come in because of a consumer ad and they ask if they should switch drugs. When patients are stable I don't encourage them to switch, but we have a shared decision making conversation and go over the pros and cons, the cost, and the outcomes data. A lot of patients prefer to pay the difference" and switch to a new anticoagulant.
Dr. Harold said he also recommends that patients switch off warfarin if they have problems with compliance and variability in their international normalized ratio (INR).
"If you can keep a patient on warfarin in their INR target range 80% or more of the time then I wouldn't change, but most patients on warfarin have a very hard time maintaining an INR of 2-3," said Dr. Mark S. Link, professor and codirector of the cardiac arrhythmia center at Tufts Medical Center, Boston. But he said cost is a major factor keeping many patients on warfarin.
The new anticoagulants "are better than warfarin, but we are often forced by insurers to start with the cheaper drug," Dr. Link said during the news conference.
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed, Dr. Ellinor, Dr. Harold, and Dr. Link had no relevant disclosures.
On Twitter @mitchelzoler
DALLAS - Genotyping to guide the starting dosage of warfarin treatment showed no added value above tailoring treatment with a panel of clinical features in a randomized, controlled U.S. trial of more than 1,000 patients.
The resounding null result from adding genotype information should spell the end of this practice, said Dr. Stephen E. Kimmel, lead investigator for the study, and professor and director of cardiovascular epidemiology at the University of Pennsylvania in Philadelphia.
"Based on what we've seen, I don't believe there is sufficient evidence to add genetic information on top of the available clinical algorithms," Dr. Kimmel said at the American Heart Association scientific sessions. "I don't think we'll see another genetics trial in warfarin treatment. I think this is it."
Good outcomes in the comparison group largely accounted for the failure of genotyping to significantly improve the percentage of time that patients' international normalized ratios (INRs) were in the target range of 2.0-3.0. The amount of time in the therapeutic INR range (PTTR) averaged 45% in patients in the comparison arm during the first 4 weeks of warfarin treatment.
"When the comparison group does so well, it's more difficult for genotyping to have an effect," said Dr. Elaine M. Hylek, professor of medicine and an anticoagulant specialist at Boston University.
That limitation, coupled with the shifting anticoagulant landscape, knock genotyping out of the picture, she agreed. "It will be difficult funding [another study of genotyping] with all the anticoagulant alternatives that are now out there" for preventing thrombosis in patients with atrial fibrillation or a recent venous thromboembolism.
The Clarification of Optimal Anticoagulant through Genetics (COAG) trial enrolled 1,015 patients initiating warfarin therapy at 18 U.S. centers during September 2009 to April 2013. The study randomized patients to two different ways to calculate their warfarin dosage during the first 5 days of treatment.
Half had their dosage calculated by a formula that took into account seven clinical and demographic factors, including age, race, smoking status, and body surface area. The others had their dosage calculated with the same formula and factors plus added information on the patient's genotype for two genes that affect warfarin activity, CYP2C9 and VKORC1. Patients' average age was 58 years; just over a quarter were African American.
During the first 4 weeks on treatment, the average PTTR was 45% in both arms of the study, the trial's primary endpoint. Adding genotyping information led to a bigger improvement in the PTTR among the non-African American patients compared with those who were African American, but did not produce a significantly increased PTTR in the non-African American subgroup.
Dr. Munir Pirmohamed reported results from a similar study that enrolled 455 patients starting warfarin therapy at any of five centers in the United Kingdom and Sweden.
The EU-Pharmacogenetics of Anticoagulant Therapy (EU-PACT) Warfarin study mainly differed from the COAG study by the background method used to calculate a starting warfarin dosage over the first 3 days of treatment.
In EU-PACT, the warfarin dosage of all patients was adjusted for age but not for other factors. In the intervention arm, the starting dosages were adjusted for the status of the same two genes, CYP2C9 and VKORC1.
During the first 12 weeks after starting warfarin, the cumulative average PTTR was 67% in the patients whose dose was adjusted by genotype and 60% in patients who were not genotyped, a statistically significant difference for this study's primary endpoint, reported Dr. Pirmohamed, professor and head of molecular and clinical pharmacology at the University of Liverpool, England.
Taken together, the findings of the two studies highlight that patients should not start warfarin treatment on a fixed dosage, said Dr. Patrick T. Ellinor, a cardiologist and arrhythmia specialist at Massachusetts General Hospital, Boston, and designated discussant for both reports at the meeting.
The findings support use of a clinical algorithm that takes into account several clinical factors, he added.
Concurrently with the meeting, the reports were published online for COAG (N. Engl. J. Med. 2013 [doi: 10.1056/NEJMoa1310669]) and for EU-PACT (N. Engl. J. Med. 2013 [doi: 10.1056/NEJMoa1311386]).
Warfarin on top despite competition
Warfarin remains the most widely used anticoagulant in the United States. Among patients with atrial fibrillation taking anticoagulation treatment, 72% used warfarin during the third quarter of 2013, data from a large registry show.
The new anticoagulants on the U.S. market - dabigatran (Pradaxa), apixaban (Eliquis), and rivaroxaban (Xarelto) - are gaining ground and widely acknowledged to be better, safer, and easier to manage. But warfarin clings to the market largely because of familiarity and low price, according to several experts.
"There is no doubt that the new anticoagulants look better compared with warfarin, but the clinical fact is that it's what you know versus what you don't know, and warfarin has been used for 60 years," Dr. Kimmel said at the press conference.
"The new drugs perform better than warfarin does; they get patients to where they need to be more quickly, but warfarin has been around a long time. We know its interactions and risks, there is the ability to reverse its effect, and the cost to patients is a real issue. If the new anticoagulants were the same price as warfarin then I think you'd see a lot more patients get a new drug," Dr. Ellinor said in an interview.
Data on current U.S. uptake of the new oral anticoagulants came from the 148,320 unique patients with atrial fibrillation included during June to September of 2013 in the PINNACLE Registry database run by the American College of Cardiology. Among these patients, about 83,000 (56%) were on some anticoagulant treatment, and within this subgroup, 72% were on warfarin, 27% on a new anticoagulant, and the remainder on different treatment, said Dr. John Gordon Harold, ACC president and a cardiologist at Cedars-Sinai Heart Institute in Los Angeles.
"In my own practice the new anticoagulants are being used with increasing frequency, mainly driven by direct-to-consumer advertising," Dr. Harold said in an interview. "We have patients who are completely stable on warfarin. (They) come in because of a consumer ad and they ask if they should switch drugs. When patients are stable I don't encourage them to switch, but we have a shared decision making conversation and go over the pros and cons, the cost, and the outcomes data. A lot of patients prefer to pay the difference" and switch to a new anticoagulant.
Dr. Harold said he also recommends that patients switch off warfarin if they have problems with compliance and variability in their international normalized ratio (INR).
"If you can keep a patient on warfarin in their INR target range 80% or more of the time then I wouldn't change, but most patients on warfarin have a very hard time maintaining an INR of 2-3," said Dr. Mark S. Link, professor and codirector of the cardiac arrhythmia center at Tufts Medical Center, Boston. But he said cost is a major factor keeping many patients on warfarin.
The new anticoagulants "are better than warfarin, but we are often forced by insurers to start with the cheaper drug," Dr. Link said during the news conference.
The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed, Dr. Ellinor, Dr. Harold, and Dr. Link had no relevant disclosures.
On Twitter @mitchelzoler
Major finding: Patients starting warfarin with dosing based on a formula that took into account seven clinical and demographic factors averaged 45% of the time in therapeutic range regardless of whether the starting dosage was adjusted based on genotype results.
Data source: COAG, a randomized trial with 1,015 patients starting warfarin therapy at 18 U.S. centers.
Disclosures: The COAG and EU-PACT studies did not receive any direct commercial sponsorship. Dr. Kimmel has been a consultant to Pfizer and Janssen. Dr. Hylek has been a consultant or adviser to Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Johnson & Johnson, and Pfizer. Dr. Pirmohamed and Dr. Ellinor had no disclosures.
Postop atrial fib onset occurs at two distinct times
DALLAS -- New-onset postoperative atrial fibrillation following cardiac surgery occurs in two distinct phases, with different risk factors for each.
That's the key finding in an analysis of the recent experience at the University of Alabama, Birmingham, where new-onset postoperative AF occurred in 27% of 1,583 consecutive patients who underwent coronary artery bypass graft and/or valve surgery.
The first peak in the onset of postoperative AF occurred in the first 3 hours after surgery. This was followed by a sharp decline in incidence over the next 24 hours. The second peak happened at postoperative day 2, followed by a more gradual tail off, Dr. Spencer J. Melby reported at the American Heart Association scientific sessions.
Ninety-six percent of all cases of postoperative AF in this retrospective chart review began within the first 7 days after surgery. Of the 423 patients who developed this common and vexing condition, 16% did so within the first 24 hours, 48% had their onset at 24-72 hours, and 36% did so after 72 hours, according to Dr. Melby, a cardiothoracic surgeon at the university.
In a multivariate analysis, the most potent risk factor for onset of postoperative AF during the first phase was mitral valve repair or replacement, which was associated with a 2.5-fold increased risk. The other significant risk factors for early-onset AF were older age -- with 70-year-olds having a 1.6-fold greater risk than 50-year-olds -- and longer ischemic time, with patients whose cross-clamp time was 70 minutes having a 1.3-fold greater risk compared with those who had a cross-clamp time of 35 minutes.
Advanced age was also a risk factor for late-phase onset of AF, but it was a stronger risk factor there than for early onset AF, with 70-year-olds being at threefold greater risk than 50-year-olds -- nearly twice as great a risk as for early-onset postoperative AF. The other significant risk factors for late-phase postoperative AF in a multivariate analysis were white race, with a 1.9-fold increased risk, and heavier body weight, with patients weighing 100 kg being at 1.6-fold greater risk than those tipping the scales at 70 kg.
"We believe that these different risk factors at each time phase are indicative of change over time in the mechanisms that drive postoperative atrial fibrillation," the surgeon said.
The first peak may be the result of tissue trauma, which is typically greater with more complex operations having longer cross-clamp times. And mitral valve procedures often entail contact with the left atrium.
"We?ve found that in patients with onset in the second peak there?s a polymorphonuclear leukocyte riot going on in the pericardial space after surgery. Inflammation is high, oxidative stress is high. Troponin levels are extremely high in the pericardial fluid, so there are problems in the heart muscle itself. The body is trying to heal and may be overdoing it as reflected by the inflammation in that space," Dr. Melby explained.
Dr. Brendan M. Everett, session cochair, noted that postoperative day 2 is when a host of process-of-care issues potentially relevant to postoperative AF come into play.
"Postop day 2 is when pain control begins to slip a bit. Patients are mobilize; chest tubes are pulled. A lot of factors are going on that are very important overall to getting a patient up and out of the hospital but are serious stimuli -- adrenergic and otherwise -- that may force a patient to go into atrial fibrillation," observed Dr. Everett, director of the general cardiology inpatient service at Brigham and Women's Hospital, Boston.
Audience members praised Dr. Melby's study as having "tremendous implications" for clinical practice, especially with regard to patient management guidelines. Dr. Melby noted that postoperative AF is a costly matter: It increases hospital length of stay by 1-2 days at a cost of roughly $10,000 per episode. Nationally, postoperative AF costs $4 billion per year.
Dr. Melby reported having no financial conflicts regarding his study.
DALLAS -- New-onset postoperative atrial fibrillation following cardiac surgery occurs in two distinct phases, with different risk factors for each.
That's the key finding in an analysis of the recent experience at the University of Alabama, Birmingham, where new-onset postoperative AF occurred in 27% of 1,583 consecutive patients who underwent coronary artery bypass graft and/or valve surgery.
The first peak in the onset of postoperative AF occurred in the first 3 hours after surgery. This was followed by a sharp decline in incidence over the next 24 hours. The second peak happened at postoperative day 2, followed by a more gradual tail off, Dr. Spencer J. Melby reported at the American Heart Association scientific sessions.
Ninety-six percent of all cases of postoperative AF in this retrospective chart review began within the first 7 days after surgery. Of the 423 patients who developed this common and vexing condition, 16% did so within the first 24 hours, 48% had their onset at 24-72 hours, and 36% did so after 72 hours, according to Dr. Melby, a cardiothoracic surgeon at the university.
In a multivariate analysis, the most potent risk factor for onset of postoperative AF during the first phase was mitral valve repair or replacement, which was associated with a 2.5-fold increased risk. The other significant risk factors for early-onset AF were older age -- with 70-year-olds having a 1.6-fold greater risk than 50-year-olds -- and longer ischemic time, with patients whose cross-clamp time was 70 minutes having a 1.3-fold greater risk compared with those who had a cross-clamp time of 35 minutes.
Advanced age was also a risk factor for late-phase onset of AF, but it was a stronger risk factor there than for early onset AF, with 70-year-olds being at threefold greater risk than 50-year-olds -- nearly twice as great a risk as for early-onset postoperative AF. The other significant risk factors for late-phase postoperative AF in a multivariate analysis were white race, with a 1.9-fold increased risk, and heavier body weight, with patients weighing 100 kg being at 1.6-fold greater risk than those tipping the scales at 70 kg.
"We believe that these different risk factors at each time phase are indicative of change over time in the mechanisms that drive postoperative atrial fibrillation," the surgeon said.
The first peak may be the result of tissue trauma, which is typically greater with more complex operations having longer cross-clamp times. And mitral valve procedures often entail contact with the left atrium.
"We?ve found that in patients with onset in the second peak there?s a polymorphonuclear leukocyte riot going on in the pericardial space after surgery. Inflammation is high, oxidative stress is high. Troponin levels are extremely high in the pericardial fluid, so there are problems in the heart muscle itself. The body is trying to heal and may be overdoing it as reflected by the inflammation in that space," Dr. Melby explained.
Dr. Brendan M. Everett, session cochair, noted that postoperative day 2 is when a host of process-of-care issues potentially relevant to postoperative AF come into play.
"Postop day 2 is when pain control begins to slip a bit. Patients are mobilize; chest tubes are pulled. A lot of factors are going on that are very important overall to getting a patient up and out of the hospital but are serious stimuli -- adrenergic and otherwise -- that may force a patient to go into atrial fibrillation," observed Dr. Everett, director of the general cardiology inpatient service at Brigham and Women's Hospital, Boston.
Audience members praised Dr. Melby's study as having "tremendous implications" for clinical practice, especially with regard to patient management guidelines. Dr. Melby noted that postoperative AF is a costly matter: It increases hospital length of stay by 1-2 days at a cost of roughly $10,000 per episode. Nationally, postoperative AF costs $4 billion per year.
Dr. Melby reported having no financial conflicts regarding his study.
DALLAS -- New-onset postoperative atrial fibrillation following cardiac surgery occurs in two distinct phases, with different risk factors for each.
That's the key finding in an analysis of the recent experience at the University of Alabama, Birmingham, where new-onset postoperative AF occurred in 27% of 1,583 consecutive patients who underwent coronary artery bypass graft and/or valve surgery.
The first peak in the onset of postoperative AF occurred in the first 3 hours after surgery. This was followed by a sharp decline in incidence over the next 24 hours. The second peak happened at postoperative day 2, followed by a more gradual tail off, Dr. Spencer J. Melby reported at the American Heart Association scientific sessions.
Ninety-six percent of all cases of postoperative AF in this retrospective chart review began within the first 7 days after surgery. Of the 423 patients who developed this common and vexing condition, 16% did so within the first 24 hours, 48% had their onset at 24-72 hours, and 36% did so after 72 hours, according to Dr. Melby, a cardiothoracic surgeon at the university.
In a multivariate analysis, the most potent risk factor for onset of postoperative AF during the first phase was mitral valve repair or replacement, which was associated with a 2.5-fold increased risk. The other significant risk factors for early-onset AF were older age -- with 70-year-olds having a 1.6-fold greater risk than 50-year-olds -- and longer ischemic time, with patients whose cross-clamp time was 70 minutes having a 1.3-fold greater risk compared with those who had a cross-clamp time of 35 minutes.
Advanced age was also a risk factor for late-phase onset of AF, but it was a stronger risk factor there than for early onset AF, with 70-year-olds being at threefold greater risk than 50-year-olds -- nearly twice as great a risk as for early-onset postoperative AF. The other significant risk factors for late-phase postoperative AF in a multivariate analysis were white race, with a 1.9-fold increased risk, and heavier body weight, with patients weighing 100 kg being at 1.6-fold greater risk than those tipping the scales at 70 kg.
"We believe that these different risk factors at each time phase are indicative of change over time in the mechanisms that drive postoperative atrial fibrillation," the surgeon said.
The first peak may be the result of tissue trauma, which is typically greater with more complex operations having longer cross-clamp times. And mitral valve procedures often entail contact with the left atrium.
"We?ve found that in patients with onset in the second peak there?s a polymorphonuclear leukocyte riot going on in the pericardial space after surgery. Inflammation is high, oxidative stress is high. Troponin levels are extremely high in the pericardial fluid, so there are problems in the heart muscle itself. The body is trying to heal and may be overdoing it as reflected by the inflammation in that space," Dr. Melby explained.
Dr. Brendan M. Everett, session cochair, noted that postoperative day 2 is when a host of process-of-care issues potentially relevant to postoperative AF come into play.
"Postop day 2 is when pain control begins to slip a bit. Patients are mobilize; chest tubes are pulled. A lot of factors are going on that are very important overall to getting a patient up and out of the hospital but are serious stimuli -- adrenergic and otherwise -- that may force a patient to go into atrial fibrillation," observed Dr. Everett, director of the general cardiology inpatient service at Brigham and Women's Hospital, Boston.
Audience members praised Dr. Melby's study as having "tremendous implications" for clinical practice, especially with regard to patient management guidelines. Dr. Melby noted that postoperative AF is a costly matter: It increases hospital length of stay by 1-2 days at a cost of roughly $10,000 per episode. Nationally, postoperative AF costs $4 billion per year.
Dr. Melby reported having no financial conflicts regarding his study.