User login
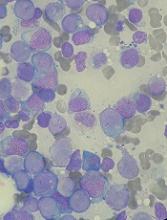
RELAZA2: MRD-guided azacitidine reduces relapse risk in MDS and AML
ATLANTA – in patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) who are at high risk for relapse, according to findings from the open-label, interventional RELAZA2 trial.
Of 205 patients screened between 2011 and 2015 at 11 centers in Germany, 53 became minimal residual disease (MRD) positive while remaining in hematological remission. All 53 started azacitidine-based preemptive treatment, and 6 months after the initiation of the MRD-guided therapy, 31 (58%) were still in complete remission, while 22 (42%) relapsed after a median of three treatment cycles, Uwe Platzbecker, MD, reported at the annual meeting of the American Society of Hematology.
Of those still in complete remission, 21 patients responded with a decline of MRD below a predefined threshold, and 10 achieved stabilization in the absence of relapse, said Dr. Platzbecker of the University Hospital Carl Gustav Carus Dresden, Germany.
The overall response rate was greater in those who underwent allogeneic hematopoietic stem cell transplantation (71% vs. 48%), he noted.
“After 6 months, 24 patients continued to receive a median of nine subsequent azacitidine cycles. Seven patients completed 24 months of treatment according to protocol. Eventually, hematologic relapse occurred in eight of those patients (33%) but was delayed until a median of 397 days after initial MRD detection,” he said in an interview, adding that, overall, 26 of the 53 patients in the study (49%) experienced hematologic relapse, which was delayed until a median of 422 days after initial MRD detection.
Study subjects were adults with a median age of 59 years with measurable MRD suggestive of imminent relapse but who were still in CR. Most (48) had AML, and 5 had MDS. They were treated preemptively with six cycles of 75 mg/m2 of azacitidine given subcutaneously on days 1-7 of each 1-month cycle. Those who continued treatment beyond the initial 6 months were treated with risk-adapted azacitidine-based therapy for up to 18 additional months.
Treatment was well tolerated. Grade 3 or 4 thrombocytopenia occurred in three patients, and grade 3 or 4 neutropenia occurred in 45 patients. Infections and pneumonia, which occurred in four and three patients, respectively, were the main serious side effects during the first 6 cycles.
“With a median follow-up of 13 months after the start of MRD-guided preemptive treatment, the actual overall and progression free survival rate was 76% and 42%, respectively,” Dr. Platzbecker said.
Chemotherapy frequently results in complete remission in patients with MDS or AML, but a substantial proportion of patients relapse even after allogeneic stem cell transplantation, he said, noting that treatment options in these patients are limited.
In the prospective RELAZA 1 trial, short-term preemptive azacitidine therapy was associated with sustained responses. RELAZA2 was designed to assess the ability of early nonintensive azacitidine treatment, directed by MRD monitoring after allogeneic stem cell transplantation and chemotherapy, prior to avert relapse.
The findings suggest that this approach is effective in patients at higher risk of relapse, but the success of treatment seems to be context dependent, Dr. Platzbecker said, explaining that this finding emphasizes the potential immunomodulatory role of hypomethylating agents.
“The study supports the prognostic importance of MRD in AML and may serve as a platform for future studies in combining hypomethylating agents and novel targeted therapies,” he concluded.
The RELAZA2 trial is sponsored by Technische Universität Dresden. Dr. Platzbecker reported serving as a consultant for, and receiving honoraria and research funding from Celgene, Janssen, Novartis, and Acceleron.
SOURCE: Platzbecker U et al. ASH 2017 Abstract #565.
ATLANTA – in patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) who are at high risk for relapse, according to findings from the open-label, interventional RELAZA2 trial.
Of 205 patients screened between 2011 and 2015 at 11 centers in Germany, 53 became minimal residual disease (MRD) positive while remaining in hematological remission. All 53 started azacitidine-based preemptive treatment, and 6 months after the initiation of the MRD-guided therapy, 31 (58%) were still in complete remission, while 22 (42%) relapsed after a median of three treatment cycles, Uwe Platzbecker, MD, reported at the annual meeting of the American Society of Hematology.
Of those still in complete remission, 21 patients responded with a decline of MRD below a predefined threshold, and 10 achieved stabilization in the absence of relapse, said Dr. Platzbecker of the University Hospital Carl Gustav Carus Dresden, Germany.
The overall response rate was greater in those who underwent allogeneic hematopoietic stem cell transplantation (71% vs. 48%), he noted.
“After 6 months, 24 patients continued to receive a median of nine subsequent azacitidine cycles. Seven patients completed 24 months of treatment according to protocol. Eventually, hematologic relapse occurred in eight of those patients (33%) but was delayed until a median of 397 days after initial MRD detection,” he said in an interview, adding that, overall, 26 of the 53 patients in the study (49%) experienced hematologic relapse, which was delayed until a median of 422 days after initial MRD detection.
Study subjects were adults with a median age of 59 years with measurable MRD suggestive of imminent relapse but who were still in CR. Most (48) had AML, and 5 had MDS. They were treated preemptively with six cycles of 75 mg/m2 of azacitidine given subcutaneously on days 1-7 of each 1-month cycle. Those who continued treatment beyond the initial 6 months were treated with risk-adapted azacitidine-based therapy for up to 18 additional months.
Treatment was well tolerated. Grade 3 or 4 thrombocytopenia occurred in three patients, and grade 3 or 4 neutropenia occurred in 45 patients. Infections and pneumonia, which occurred in four and three patients, respectively, were the main serious side effects during the first 6 cycles.
“With a median follow-up of 13 months after the start of MRD-guided preemptive treatment, the actual overall and progression free survival rate was 76% and 42%, respectively,” Dr. Platzbecker said.
Chemotherapy frequently results in complete remission in patients with MDS or AML, but a substantial proportion of patients relapse even after allogeneic stem cell transplantation, he said, noting that treatment options in these patients are limited.
In the prospective RELAZA 1 trial, short-term preemptive azacitidine therapy was associated with sustained responses. RELAZA2 was designed to assess the ability of early nonintensive azacitidine treatment, directed by MRD monitoring after allogeneic stem cell transplantation and chemotherapy, prior to avert relapse.
The findings suggest that this approach is effective in patients at higher risk of relapse, but the success of treatment seems to be context dependent, Dr. Platzbecker said, explaining that this finding emphasizes the potential immunomodulatory role of hypomethylating agents.
“The study supports the prognostic importance of MRD in AML and may serve as a platform for future studies in combining hypomethylating agents and novel targeted therapies,” he concluded.
The RELAZA2 trial is sponsored by Technische Universität Dresden. Dr. Platzbecker reported serving as a consultant for, and receiving honoraria and research funding from Celgene, Janssen, Novartis, and Acceleron.
SOURCE: Platzbecker U et al. ASH 2017 Abstract #565.
ATLANTA – in patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) who are at high risk for relapse, according to findings from the open-label, interventional RELAZA2 trial.
Of 205 patients screened between 2011 and 2015 at 11 centers in Germany, 53 became minimal residual disease (MRD) positive while remaining in hematological remission. All 53 started azacitidine-based preemptive treatment, and 6 months after the initiation of the MRD-guided therapy, 31 (58%) were still in complete remission, while 22 (42%) relapsed after a median of three treatment cycles, Uwe Platzbecker, MD, reported at the annual meeting of the American Society of Hematology.
Of those still in complete remission, 21 patients responded with a decline of MRD below a predefined threshold, and 10 achieved stabilization in the absence of relapse, said Dr. Platzbecker of the University Hospital Carl Gustav Carus Dresden, Germany.
The overall response rate was greater in those who underwent allogeneic hematopoietic stem cell transplantation (71% vs. 48%), he noted.
“After 6 months, 24 patients continued to receive a median of nine subsequent azacitidine cycles. Seven patients completed 24 months of treatment according to protocol. Eventually, hematologic relapse occurred in eight of those patients (33%) but was delayed until a median of 397 days after initial MRD detection,” he said in an interview, adding that, overall, 26 of the 53 patients in the study (49%) experienced hematologic relapse, which was delayed until a median of 422 days after initial MRD detection.
Study subjects were adults with a median age of 59 years with measurable MRD suggestive of imminent relapse but who were still in CR. Most (48) had AML, and 5 had MDS. They were treated preemptively with six cycles of 75 mg/m2 of azacitidine given subcutaneously on days 1-7 of each 1-month cycle. Those who continued treatment beyond the initial 6 months were treated with risk-adapted azacitidine-based therapy for up to 18 additional months.
Treatment was well tolerated. Grade 3 or 4 thrombocytopenia occurred in three patients, and grade 3 or 4 neutropenia occurred in 45 patients. Infections and pneumonia, which occurred in four and three patients, respectively, were the main serious side effects during the first 6 cycles.
“With a median follow-up of 13 months after the start of MRD-guided preemptive treatment, the actual overall and progression free survival rate was 76% and 42%, respectively,” Dr. Platzbecker said.
Chemotherapy frequently results in complete remission in patients with MDS or AML, but a substantial proportion of patients relapse even after allogeneic stem cell transplantation, he said, noting that treatment options in these patients are limited.
In the prospective RELAZA 1 trial, short-term preemptive azacitidine therapy was associated with sustained responses. RELAZA2 was designed to assess the ability of early nonintensive azacitidine treatment, directed by MRD monitoring after allogeneic stem cell transplantation and chemotherapy, prior to avert relapse.
The findings suggest that this approach is effective in patients at higher risk of relapse, but the success of treatment seems to be context dependent, Dr. Platzbecker said, explaining that this finding emphasizes the potential immunomodulatory role of hypomethylating agents.
“The study supports the prognostic importance of MRD in AML and may serve as a platform for future studies in combining hypomethylating agents and novel targeted therapies,” he concluded.
The RELAZA2 trial is sponsored by Technische Universität Dresden. Dr. Platzbecker reported serving as a consultant for, and receiving honoraria and research funding from Celgene, Janssen, Novartis, and Acceleron.
SOURCE: Platzbecker U et al. ASH 2017 Abstract #565.
REPORTING FROM ASH 2017
Key clinical point: MRD-guided azacitidine therapy reduces hematological relapse in high-risk MDS/AML.
Major finding: The relapse-free survival rate at 6 months was 58%.
Study details: An analysis of 53 patients from the open-label RELAZA2 trial.
Disclosures: The RELAZA2 trial is sponsored by Technische Universität Dresden, Germany. Dr. Platzbecker reported serving as a consultant for and receiving honoraria and research funding from Celgene, Janssen, Novartis, and Acceleron Pharma.
Source: Platzbecker U et al. ASH 2017 Abstract #565.
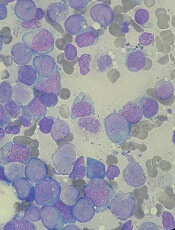
Novel JAK1 inhibitor shows promise for myeloid malignancies
ATLANTA – The novel Janus kinase 1 (JAK1) inhibitor INCB052793 showed encouraging activity, particularly in combination with azacitidine, in certain patients with advanced myeloid malignancies in a phase 1/2 trial.
The activity was seen even in patients who previously failed treatment with hypomethylating agents, Amer M. Zeidan, MD, reported at the annual meeting of the American Society of Hematology.
In the combination therapy dose escalation phase (phase 1b), seven patients with MM received INCB052793 at doses of 25 mg or 35 mg daily plus dexamethasone, and nine patients with acute myeloid leukemia (AML) or MDS received INCB052793 plus azacitidine. During the dose expansion, 12 patients received a daily dose of 35 mg for 28-day cycles plus azacitidine (in AML and MDS patients), according to Dr. Zeidan of Yale University, New Haven, Conn.
The study employed a 3+3 dose-escalation design until dose-limiting toxicities occurred. Patients were treated in continuous cycles until study termination, consent withdrawal, disease progression, or unacceptable toxicity.
Phase 2 of the study is evaluating INCB052793 in combination with azacitidine in nine patients with AML or high-risk MDS who failed prior therapy with hypomethylating agents. The 35-mg daily dose was selected for this phase based on pharmacodynamic effect and the presence of thrombocytopenia in solid tumor patients at higher doses, he said.
At the data cutoff for this preliminary assessment, 1 of the 11 patients who received INCB052793 monotherapy – a patient with MDS/MPN – experienced complete response (CR) and remained on study at the data cutoff. Two monotherapy patients with MDS/MPN experienced partial remission (PR).
Of seven patients with MM in the INCB052793-plus-dexamethasone group, two had a minimal response with a reduction in M protein.
In the INCB052793-plus-azacitidine group, overall response rates were 67% in 12 patients with AML and 56% in patients with MDS or MDS/MPN.
In the AML group, there was one CR, one morphologic leukemia-free state, and two PRs. In the MDS group, three of seven patients had a CR. Among the two patients in the MDS/MPN group, one had a CR and one had a PR.
Of note, none of the seven patients in the INCB052793-plus-dexamethasone group had received prior treatment with hypomethylating agents, while 10 of 21 patients in the INCB052793-plus-azacitidine phase 1b group had, as well as all of the nine phase 2 patients. The results were as of Nov. 3, 2017, Dr. Zeidan said.
The JAK/STAT pathway plays an important role in cytokine and growth factor signal transduction. Dysregulation of the JAK/STAT pathway is associated with the pathogenesis of various hematologic malignancies, Dr. Zeidan explained, noting that blocking JAK signaling can inhibit AML cell proliferation through STAT3/5 inhibition and induction of caspase-dependent apoptosis.
INCB052793 is a small molecule JAK1 inhibitor with potential as monotherapy or in combination with standard therapies for treating advanced hematologic malignancies. It could be of particular benefit for high-risk MDS patients who have failed prior therapy with hypomethylating agents, as these patients have no available standard of care and their overall survival is often less than 6 months, he said.
These preliminary data show that treatment is associated with a number of nonhematologic and hematologic adverse events. Grade 3 or greater adverse events were observed in 45% of patients receiving INCB052793 monotherapy, 86% of patients receiving INCB052793 plus dexamethasone, and 95% of those receiving INCB052793 plus azacitidine.
The most common adverse events with INCB052793 plus dexamethasone were anemia, hypercalcemia, hypophosphatemia, pneumonia, sepsis, and thrombocytopenia. With INCB052793 plus azacitidine, the most common events were febrile neutropenia, anemia, neutropenia, and thrombocytopenia.
Most patients included in the current analysis discontinued treatment, including 91% of INCB052793 monotherapy patients, 100% of INCB052793-plus-dexamethasone patients, and 90% of INCB052793-plus-azacitidine patients. The primary reasons for discontinuation were disease progression or adverse events.
Despite these events, the findings suggest that combination therapy with INCB052793 and azacitidine is promising for patients with advanced myeloid malignancies, Dr. Zeidan said. However, signals of activity were lacking in multiple myeloma or lymphoid malignancies.
The findings of encouraging activity in patients who previously failed on hypomethylating agents are of particular interest, and suggest that INCB052793 might resensitize refractory/relapsed patients to the effects of these agents, Dr. Zeidan noted, concluding that these preliminary safety and efficacy data support further evaluation of INCB052793 in this setting. Enrollment is ongoing in phase 2 of the trial.
This study is sponsored by Incyte. Dr. Zeidan reported serving as a consultant for Incyte and Otsuka and as a member of the speakers bureau for Takeda. He also reported financial relationships with AbbVie, Pfizer, Gilead, Celgene, and Ariad.
SOURCE: Zeidan A et al. ASH 2017 Abstract 640.
ATLANTA – The novel Janus kinase 1 (JAK1) inhibitor INCB052793 showed encouraging activity, particularly in combination with azacitidine, in certain patients with advanced myeloid malignancies in a phase 1/2 trial.
The activity was seen even in patients who previously failed treatment with hypomethylating agents, Amer M. Zeidan, MD, reported at the annual meeting of the American Society of Hematology.
In the combination therapy dose escalation phase (phase 1b), seven patients with MM received INCB052793 at doses of 25 mg or 35 mg daily plus dexamethasone, and nine patients with acute myeloid leukemia (AML) or MDS received INCB052793 plus azacitidine. During the dose expansion, 12 patients received a daily dose of 35 mg for 28-day cycles plus azacitidine (in AML and MDS patients), according to Dr. Zeidan of Yale University, New Haven, Conn.
The study employed a 3+3 dose-escalation design until dose-limiting toxicities occurred. Patients were treated in continuous cycles until study termination, consent withdrawal, disease progression, or unacceptable toxicity.
Phase 2 of the study is evaluating INCB052793 in combination with azacitidine in nine patients with AML or high-risk MDS who failed prior therapy with hypomethylating agents. The 35-mg daily dose was selected for this phase based on pharmacodynamic effect and the presence of thrombocytopenia in solid tumor patients at higher doses, he said.
At the data cutoff for this preliminary assessment, 1 of the 11 patients who received INCB052793 monotherapy – a patient with MDS/MPN – experienced complete response (CR) and remained on study at the data cutoff. Two monotherapy patients with MDS/MPN experienced partial remission (PR).
Of seven patients with MM in the INCB052793-plus-dexamethasone group, two had a minimal response with a reduction in M protein.
In the INCB052793-plus-azacitidine group, overall response rates were 67% in 12 patients with AML and 56% in patients with MDS or MDS/MPN.
In the AML group, there was one CR, one morphologic leukemia-free state, and two PRs. In the MDS group, three of seven patients had a CR. Among the two patients in the MDS/MPN group, one had a CR and one had a PR.
Of note, none of the seven patients in the INCB052793-plus-dexamethasone group had received prior treatment with hypomethylating agents, while 10 of 21 patients in the INCB052793-plus-azacitidine phase 1b group had, as well as all of the nine phase 2 patients. The results were as of Nov. 3, 2017, Dr. Zeidan said.
The JAK/STAT pathway plays an important role in cytokine and growth factor signal transduction. Dysregulation of the JAK/STAT pathway is associated with the pathogenesis of various hematologic malignancies, Dr. Zeidan explained, noting that blocking JAK signaling can inhibit AML cell proliferation through STAT3/5 inhibition and induction of caspase-dependent apoptosis.
INCB052793 is a small molecule JAK1 inhibitor with potential as monotherapy or in combination with standard therapies for treating advanced hematologic malignancies. It could be of particular benefit for high-risk MDS patients who have failed prior therapy with hypomethylating agents, as these patients have no available standard of care and their overall survival is often less than 6 months, he said.
These preliminary data show that treatment is associated with a number of nonhematologic and hematologic adverse events. Grade 3 or greater adverse events were observed in 45% of patients receiving INCB052793 monotherapy, 86% of patients receiving INCB052793 plus dexamethasone, and 95% of those receiving INCB052793 plus azacitidine.
The most common adverse events with INCB052793 plus dexamethasone were anemia, hypercalcemia, hypophosphatemia, pneumonia, sepsis, and thrombocytopenia. With INCB052793 plus azacitidine, the most common events were febrile neutropenia, anemia, neutropenia, and thrombocytopenia.
Most patients included in the current analysis discontinued treatment, including 91% of INCB052793 monotherapy patients, 100% of INCB052793-plus-dexamethasone patients, and 90% of INCB052793-plus-azacitidine patients. The primary reasons for discontinuation were disease progression or adverse events.
Despite these events, the findings suggest that combination therapy with INCB052793 and azacitidine is promising for patients with advanced myeloid malignancies, Dr. Zeidan said. However, signals of activity were lacking in multiple myeloma or lymphoid malignancies.
The findings of encouraging activity in patients who previously failed on hypomethylating agents are of particular interest, and suggest that INCB052793 might resensitize refractory/relapsed patients to the effects of these agents, Dr. Zeidan noted, concluding that these preliminary safety and efficacy data support further evaluation of INCB052793 in this setting. Enrollment is ongoing in phase 2 of the trial.
This study is sponsored by Incyte. Dr. Zeidan reported serving as a consultant for Incyte and Otsuka and as a member of the speakers bureau for Takeda. He also reported financial relationships with AbbVie, Pfizer, Gilead, Celgene, and Ariad.
SOURCE: Zeidan A et al. ASH 2017 Abstract 640.
ATLANTA – The novel Janus kinase 1 (JAK1) inhibitor INCB052793 showed encouraging activity, particularly in combination with azacitidine, in certain patients with advanced myeloid malignancies in a phase 1/2 trial.
The activity was seen even in patients who previously failed treatment with hypomethylating agents, Amer M. Zeidan, MD, reported at the annual meeting of the American Society of Hematology.
In the combination therapy dose escalation phase (phase 1b), seven patients with MM received INCB052793 at doses of 25 mg or 35 mg daily plus dexamethasone, and nine patients with acute myeloid leukemia (AML) or MDS received INCB052793 plus azacitidine. During the dose expansion, 12 patients received a daily dose of 35 mg for 28-day cycles plus azacitidine (in AML and MDS patients), according to Dr. Zeidan of Yale University, New Haven, Conn.
The study employed a 3+3 dose-escalation design until dose-limiting toxicities occurred. Patients were treated in continuous cycles until study termination, consent withdrawal, disease progression, or unacceptable toxicity.
Phase 2 of the study is evaluating INCB052793 in combination with azacitidine in nine patients with AML or high-risk MDS who failed prior therapy with hypomethylating agents. The 35-mg daily dose was selected for this phase based on pharmacodynamic effect and the presence of thrombocytopenia in solid tumor patients at higher doses, he said.
At the data cutoff for this preliminary assessment, 1 of the 11 patients who received INCB052793 monotherapy – a patient with MDS/MPN – experienced complete response (CR) and remained on study at the data cutoff. Two monotherapy patients with MDS/MPN experienced partial remission (PR).
Of seven patients with MM in the INCB052793-plus-dexamethasone group, two had a minimal response with a reduction in M protein.
In the INCB052793-plus-azacitidine group, overall response rates were 67% in 12 patients with AML and 56% in patients with MDS or MDS/MPN.
In the AML group, there was one CR, one morphologic leukemia-free state, and two PRs. In the MDS group, three of seven patients had a CR. Among the two patients in the MDS/MPN group, one had a CR and one had a PR.
Of note, none of the seven patients in the INCB052793-plus-dexamethasone group had received prior treatment with hypomethylating agents, while 10 of 21 patients in the INCB052793-plus-azacitidine phase 1b group had, as well as all of the nine phase 2 patients. The results were as of Nov. 3, 2017, Dr. Zeidan said.
The JAK/STAT pathway plays an important role in cytokine and growth factor signal transduction. Dysregulation of the JAK/STAT pathway is associated with the pathogenesis of various hematologic malignancies, Dr. Zeidan explained, noting that blocking JAK signaling can inhibit AML cell proliferation through STAT3/5 inhibition and induction of caspase-dependent apoptosis.
INCB052793 is a small molecule JAK1 inhibitor with potential as monotherapy or in combination with standard therapies for treating advanced hematologic malignancies. It could be of particular benefit for high-risk MDS patients who have failed prior therapy with hypomethylating agents, as these patients have no available standard of care and their overall survival is often less than 6 months, he said.
These preliminary data show that treatment is associated with a number of nonhematologic and hematologic adverse events. Grade 3 or greater adverse events were observed in 45% of patients receiving INCB052793 monotherapy, 86% of patients receiving INCB052793 plus dexamethasone, and 95% of those receiving INCB052793 plus azacitidine.
The most common adverse events with INCB052793 plus dexamethasone were anemia, hypercalcemia, hypophosphatemia, pneumonia, sepsis, and thrombocytopenia. With INCB052793 plus azacitidine, the most common events were febrile neutropenia, anemia, neutropenia, and thrombocytopenia.
Most patients included in the current analysis discontinued treatment, including 91% of INCB052793 monotherapy patients, 100% of INCB052793-plus-dexamethasone patients, and 90% of INCB052793-plus-azacitidine patients. The primary reasons for discontinuation were disease progression or adverse events.
Despite these events, the findings suggest that combination therapy with INCB052793 and azacitidine is promising for patients with advanced myeloid malignancies, Dr. Zeidan said. However, signals of activity were lacking in multiple myeloma or lymphoid malignancies.
The findings of encouraging activity in patients who previously failed on hypomethylating agents are of particular interest, and suggest that INCB052793 might resensitize refractory/relapsed patients to the effects of these agents, Dr. Zeidan noted, concluding that these preliminary safety and efficacy data support further evaluation of INCB052793 in this setting. Enrollment is ongoing in phase 2 of the trial.
This study is sponsored by Incyte. Dr. Zeidan reported serving as a consultant for Incyte and Otsuka and as a member of the speakers bureau for Takeda. He also reported financial relationships with AbbVie, Pfizer, Gilead, Celgene, and Ariad.
SOURCE: Zeidan A et al. ASH 2017 Abstract 640.
REPORTING FROM ASH 2017
Key clinical point:
Major finding: Overall response rates with INCB052793 plus azacitidine were 67% in AML and 56% in MDS or MDS/MPN.
Study details: A phase 1/2 study involving 58 initial patients.
Disclosures: This study is sponsored by Incyte. Dr. Zeidan reported serving as a consultant for Incyte and Otsuka and as a member of the speakers bureau for Takeda. He also reported financial relationships with AbbVie, Pfizer, Gilead, Celgene, and Ariad.
Source: Zeidan A et al. ASH 2017 Abstract 640.
FDA approves injection treatment for low-risk APL
The Food and Drug Administration announced the approval of arsenic trioxide injection (Trisenox) in combination with tretinoin for the treatment of adults with newly diagnosed, low-risk acute promyelocytic leukemia (APL) characterized by t(15;17) translocation or PML/RAR-alpha gene expression.
The expanded indication was granted by the FDA on Jan. 12 after priority review. It is based on published studies and a review of Teva’s global safety database for arsenic trioxide.
A recent randomized, phase 3 trial compared tretinoin plus arsenic trioxide with tretinoin plus chemotherapy as first-line treatment for APL (J Clin Oncol. 2017 Feb 20;35[6]:605-12). It found that 100% of 127 patients in the tretinoin plus arsenic trioxide arm achieved complete remission, compared with 97% of 136 patients in the tretinoin plus chemotherapy arm. After a median follow-up of 40.6 months, the event-free survival at 50 months for patients in the tretinoin/arsenic trioxide arm was 97.3% vs. 80% for tretinoin/chemotherapy (P = .001).
The arsenic trioxide injection carries a boxed warning for differentiation syndrome and cardiac conduction abnormalities.
The Food and Drug Administration announced the approval of arsenic trioxide injection (Trisenox) in combination with tretinoin for the treatment of adults with newly diagnosed, low-risk acute promyelocytic leukemia (APL) characterized by t(15;17) translocation or PML/RAR-alpha gene expression.
The expanded indication was granted by the FDA on Jan. 12 after priority review. It is based on published studies and a review of Teva’s global safety database for arsenic trioxide.
A recent randomized, phase 3 trial compared tretinoin plus arsenic trioxide with tretinoin plus chemotherapy as first-line treatment for APL (J Clin Oncol. 2017 Feb 20;35[6]:605-12). It found that 100% of 127 patients in the tretinoin plus arsenic trioxide arm achieved complete remission, compared with 97% of 136 patients in the tretinoin plus chemotherapy arm. After a median follow-up of 40.6 months, the event-free survival at 50 months for patients in the tretinoin/arsenic trioxide arm was 97.3% vs. 80% for tretinoin/chemotherapy (P = .001).
The arsenic trioxide injection carries a boxed warning for differentiation syndrome and cardiac conduction abnormalities.
The Food and Drug Administration announced the approval of arsenic trioxide injection (Trisenox) in combination with tretinoin for the treatment of adults with newly diagnosed, low-risk acute promyelocytic leukemia (APL) characterized by t(15;17) translocation or PML/RAR-alpha gene expression.
The expanded indication was granted by the FDA on Jan. 12 after priority review. It is based on published studies and a review of Teva’s global safety database for arsenic trioxide.
A recent randomized, phase 3 trial compared tretinoin plus arsenic trioxide with tretinoin plus chemotherapy as first-line treatment for APL (J Clin Oncol. 2017 Feb 20;35[6]:605-12). It found that 100% of 127 patients in the tretinoin plus arsenic trioxide arm achieved complete remission, compared with 97% of 136 patients in the tretinoin plus chemotherapy arm. After a median follow-up of 40.6 months, the event-free survival at 50 months for patients in the tretinoin/arsenic trioxide arm was 97.3% vs. 80% for tretinoin/chemotherapy (P = .001).
The arsenic trioxide injection carries a boxed warning for differentiation syndrome and cardiac conduction abnormalities.
FDA expands approval for arsenic trioxide
The US Food and Drug Administration (FDA) has expanded the approved use of arsenic trioxide (TRISENOX®) injection.
The drug is now approved for use in combination with all-trans retinoic acid (ATRA) for the treatment of adults with newly diagnosed, low-risk acute promyelocytic leukemia (APL) whose disease is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
Arsenic trioxide is also FDA-approved for induction of remission and consolidation in patients with APL who are refractory to, or have relapsed after, retinoid and anthracycline chemotherapy and whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
“This label expansion represents an important benefit, as TRISENOX is now an FDA-approved, first-line treatment option for patients with acute promyelocytic leukemia,” said Paul Rittman, senior vice-president and general manager of Teva Oncology.
The expanded approval for arsenic trioxide was based on a priority review by the FDA of data from the scientific literature and a review of Teva’s global safety database for arsenic trioxide.
Data from this database were presented at the 2016 ASH Annual Meeting.
According to the presentation, the most common adverse events observed in patients receiving arsenic trioxide were QT prolongation, decrease in white blood cells, APL differentiation syndrome, febrile neutropenia, neutropenia, pyrexia, alanine aminotransferase increase, neutrophil decrease, platelet count decrease, aspartate aminotransferase increase, leukocytosis, and pancytopenia.
The combination of arsenic trioxide and ATRA was evaluated in a phase 3 trial of patients with APL. Results from this trial were published in the Journal of Clinical Oncology in February 2017.
The study included 276 adults (ages 18 to 71) with newly diagnosed, low- or intermediate-risk APL. Patients were randomized to receive ATRA plus arsenic trioxide or ATRA plus chemotherapy.
A total of 263 patients were evaluable for response to induction. One hundred percent of patients in the arsenic trioxide arm (127/127) achieved a complete response (CR), as did 97% (132/136) of patients in the chemotherapy arm (P=0.12).
After a median follow-up of 40.6 months, the event-free survival was 97.3% in the arsenic trioxide arm and 80% in the chemotherapy arm (P<0.001). The cumulative incidence of relapse was 1.9% and 13.9%, respectively (P=0.0013).
At 50 months, the overall survival was 99.2% in the arsenic trioxide arm and 92.6% in the chemotherapy arm (P=0.0073).
After induction, there were 2 relapses and 1 death in CR in the arsenic trioxide arm.
In the chemotherapy arm, there were 2 instances of molecular resistance after third consolidation, 15 relapses, 5 deaths in CR, and 2 patients who developed a therapy-related myeloid neoplasm. ![]()
The US Food and Drug Administration (FDA) has expanded the approved use of arsenic trioxide (TRISENOX®) injection.
The drug is now approved for use in combination with all-trans retinoic acid (ATRA) for the treatment of adults with newly diagnosed, low-risk acute promyelocytic leukemia (APL) whose disease is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
Arsenic trioxide is also FDA-approved for induction of remission and consolidation in patients with APL who are refractory to, or have relapsed after, retinoid and anthracycline chemotherapy and whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
“This label expansion represents an important benefit, as TRISENOX is now an FDA-approved, first-line treatment option for patients with acute promyelocytic leukemia,” said Paul Rittman, senior vice-president and general manager of Teva Oncology.
The expanded approval for arsenic trioxide was based on a priority review by the FDA of data from the scientific literature and a review of Teva’s global safety database for arsenic trioxide.
Data from this database were presented at the 2016 ASH Annual Meeting.
According to the presentation, the most common adverse events observed in patients receiving arsenic trioxide were QT prolongation, decrease in white blood cells, APL differentiation syndrome, febrile neutropenia, neutropenia, pyrexia, alanine aminotransferase increase, neutrophil decrease, platelet count decrease, aspartate aminotransferase increase, leukocytosis, and pancytopenia.
The combination of arsenic trioxide and ATRA was evaluated in a phase 3 trial of patients with APL. Results from this trial were published in the Journal of Clinical Oncology in February 2017.
The study included 276 adults (ages 18 to 71) with newly diagnosed, low- or intermediate-risk APL. Patients were randomized to receive ATRA plus arsenic trioxide or ATRA plus chemotherapy.
A total of 263 patients were evaluable for response to induction. One hundred percent of patients in the arsenic trioxide arm (127/127) achieved a complete response (CR), as did 97% (132/136) of patients in the chemotherapy arm (P=0.12).
After a median follow-up of 40.6 months, the event-free survival was 97.3% in the arsenic trioxide arm and 80% in the chemotherapy arm (P<0.001). The cumulative incidence of relapse was 1.9% and 13.9%, respectively (P=0.0013).
At 50 months, the overall survival was 99.2% in the arsenic trioxide arm and 92.6% in the chemotherapy arm (P=0.0073).
After induction, there were 2 relapses and 1 death in CR in the arsenic trioxide arm.
In the chemotherapy arm, there were 2 instances of molecular resistance after third consolidation, 15 relapses, 5 deaths in CR, and 2 patients who developed a therapy-related myeloid neoplasm. ![]()
The US Food and Drug Administration (FDA) has expanded the approved use of arsenic trioxide (TRISENOX®) injection.
The drug is now approved for use in combination with all-trans retinoic acid (ATRA) for the treatment of adults with newly diagnosed, low-risk acute promyelocytic leukemia (APL) whose disease is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
Arsenic trioxide is also FDA-approved for induction of remission and consolidation in patients with APL who are refractory to, or have relapsed after, retinoid and anthracycline chemotherapy and whose APL is characterized by the presence of the t(15;17) translocation or PML/RAR-alpha gene expression.
“This label expansion represents an important benefit, as TRISENOX is now an FDA-approved, first-line treatment option for patients with acute promyelocytic leukemia,” said Paul Rittman, senior vice-president and general manager of Teva Oncology.
The expanded approval for arsenic trioxide was based on a priority review by the FDA of data from the scientific literature and a review of Teva’s global safety database for arsenic trioxide.
Data from this database were presented at the 2016 ASH Annual Meeting.
According to the presentation, the most common adverse events observed in patients receiving arsenic trioxide were QT prolongation, decrease in white blood cells, APL differentiation syndrome, febrile neutropenia, neutropenia, pyrexia, alanine aminotransferase increase, neutrophil decrease, platelet count decrease, aspartate aminotransferase increase, leukocytosis, and pancytopenia.
The combination of arsenic trioxide and ATRA was evaluated in a phase 3 trial of patients with APL. Results from this trial were published in the Journal of Clinical Oncology in February 2017.
The study included 276 adults (ages 18 to 71) with newly diagnosed, low- or intermediate-risk APL. Patients were randomized to receive ATRA plus arsenic trioxide or ATRA plus chemotherapy.
A total of 263 patients were evaluable for response to induction. One hundred percent of patients in the arsenic trioxide arm (127/127) achieved a complete response (CR), as did 97% (132/136) of patients in the chemotherapy arm (P=0.12).
After a median follow-up of 40.6 months, the event-free survival was 97.3% in the arsenic trioxide arm and 80% in the chemotherapy arm (P<0.001). The cumulative incidence of relapse was 1.9% and 13.9%, respectively (P=0.0013).
At 50 months, the overall survival was 99.2% in the arsenic trioxide arm and 92.6% in the chemotherapy arm (P=0.0073).
After induction, there were 2 relapses and 1 death in CR in the arsenic trioxide arm.
In the chemotherapy arm, there were 2 instances of molecular resistance after third consolidation, 15 relapses, 5 deaths in CR, and 2 patients who developed a therapy-related myeloid neoplasm. ![]()
EMA recommends orphan designation for pracinostat
The European Medicines Agency (EMA) has recommended that pracinostat receive orphan drug designation.
Pracinostat is an oral histone deacetylase inhibitor currently under investigation in a phase 3 study in combination with azacitidine for the treatment of acute myeloid leukemia (AML) in adult patients unfit to receive induction chemotherapy.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure. The designation also provides a 10-year period of marketing exclusivity if a therapy receives regulatory approval.
The EMA’s Committee for Orphan Medicinal Products adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision.
Phase 2 study
The EMA’s recommendation that pracinostat receive orphan drug designation is based on results of a phase 2 study, which were presented at the 2016 ASH Annual Meeting.
The study included 50 patients who had a median age of 75 (range, 66-84). Sixty-six percent of patients had de novo AML, and 34% had secondary AML.
The patients received pracinostat at 60 mg orally on days 1, 3, and 5 of each week for 21 days of each 28-day cycle. They received azacitidine at 75 mg/m2 subcutaneously or intravenously on days 1-7 or days 1-5 and 8-9 (per site preference) of each 28-day cycle.
As of October 15, 2016, 90% of patients had discontinued treatment, 42% due to progressive disease, 28% due to adverse events (AEs), 14% due to patient decision, and 6% due to investigator decision.
Fifty-two percent of patients (n=26) achieved the primary endpoint of complete response (CR) plus CR with incomplete count recovery (CRi) plus morphologic leukemia-free state (MLFS).
Forty-two percent of patients had a CR, 4% had a CRi, and 6% achieved MLFS. The median duration of CR/CRi/MLFS was 13.2 months. The median duration of CR/CRi was 17.2 months.
The median overall survival was 19.1 months. The 1-year survival rate was 62%, and the 2-year survival rate was 41%.
The most common treatment-emergent AEs were nausea (78%), constipation (70%), fatigue (62%), decreased appetite (56%), diarrhea (50%), vomiting (40%), cough (36%), dyspnea (34%), hypokalemia (34%), peripheral edema (34%), pyrexia (34%), dizziness (32%), back pain (28%), insomnia (28%), febrile neutropenia (48%), thrombocytopenia (46%), anemia (38%), and neutropenia (38%).
Treatment-emergent AEs led to discontinuation in 14 patients. Three of these patients developed sepsis that proved fatal.
The other AEs leading to discontinuation included grade 3 acute axonal neuropathy, grade 3 parainfluenza, grade 3 prolonged QTc/atrial fibrillation, grade 1 acute kidney injury, grade 3 diverticulitis, grade 3 supraglottic ulcer, grade 2 upper respiratory infection, grade 3 fatigue (n=2), and grades 1 and 3 intermittent fatigue (n=2). ![]()
The European Medicines Agency (EMA) has recommended that pracinostat receive orphan drug designation.
Pracinostat is an oral histone deacetylase inhibitor currently under investigation in a phase 3 study in combination with azacitidine for the treatment of acute myeloid leukemia (AML) in adult patients unfit to receive induction chemotherapy.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure. The designation also provides a 10-year period of marketing exclusivity if a therapy receives regulatory approval.
The EMA’s Committee for Orphan Medicinal Products adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision.
Phase 2 study
The EMA’s recommendation that pracinostat receive orphan drug designation is based on results of a phase 2 study, which were presented at the 2016 ASH Annual Meeting.
The study included 50 patients who had a median age of 75 (range, 66-84). Sixty-six percent of patients had de novo AML, and 34% had secondary AML.
The patients received pracinostat at 60 mg orally on days 1, 3, and 5 of each week for 21 days of each 28-day cycle. They received azacitidine at 75 mg/m2 subcutaneously or intravenously on days 1-7 or days 1-5 and 8-9 (per site preference) of each 28-day cycle.
As of October 15, 2016, 90% of patients had discontinued treatment, 42% due to progressive disease, 28% due to adverse events (AEs), 14% due to patient decision, and 6% due to investigator decision.
Fifty-two percent of patients (n=26) achieved the primary endpoint of complete response (CR) plus CR with incomplete count recovery (CRi) plus morphologic leukemia-free state (MLFS).
Forty-two percent of patients had a CR, 4% had a CRi, and 6% achieved MLFS. The median duration of CR/CRi/MLFS was 13.2 months. The median duration of CR/CRi was 17.2 months.
The median overall survival was 19.1 months. The 1-year survival rate was 62%, and the 2-year survival rate was 41%.
The most common treatment-emergent AEs were nausea (78%), constipation (70%), fatigue (62%), decreased appetite (56%), diarrhea (50%), vomiting (40%), cough (36%), dyspnea (34%), hypokalemia (34%), peripheral edema (34%), pyrexia (34%), dizziness (32%), back pain (28%), insomnia (28%), febrile neutropenia (48%), thrombocytopenia (46%), anemia (38%), and neutropenia (38%).
Treatment-emergent AEs led to discontinuation in 14 patients. Three of these patients developed sepsis that proved fatal.
The other AEs leading to discontinuation included grade 3 acute axonal neuropathy, grade 3 parainfluenza, grade 3 prolonged QTc/atrial fibrillation, grade 1 acute kidney injury, grade 3 diverticulitis, grade 3 supraglottic ulcer, grade 2 upper respiratory infection, grade 3 fatigue (n=2), and grades 1 and 3 intermittent fatigue (n=2). ![]()
The European Medicines Agency (EMA) has recommended that pracinostat receive orphan drug designation.
Pracinostat is an oral histone deacetylase inhibitor currently under investigation in a phase 3 study in combination with azacitidine for the treatment of acute myeloid leukemia (AML) in adult patients unfit to receive induction chemotherapy.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure. The designation also provides a 10-year period of marketing exclusivity if a therapy receives regulatory approval.
The EMA’s Committee for Orphan Medicinal Products adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision.
Phase 2 study
The EMA’s recommendation that pracinostat receive orphan drug designation is based on results of a phase 2 study, which were presented at the 2016 ASH Annual Meeting.
The study included 50 patients who had a median age of 75 (range, 66-84). Sixty-six percent of patients had de novo AML, and 34% had secondary AML.
The patients received pracinostat at 60 mg orally on days 1, 3, and 5 of each week for 21 days of each 28-day cycle. They received azacitidine at 75 mg/m2 subcutaneously or intravenously on days 1-7 or days 1-5 and 8-9 (per site preference) of each 28-day cycle.
As of October 15, 2016, 90% of patients had discontinued treatment, 42% due to progressive disease, 28% due to adverse events (AEs), 14% due to patient decision, and 6% due to investigator decision.
Fifty-two percent of patients (n=26) achieved the primary endpoint of complete response (CR) plus CR with incomplete count recovery (CRi) plus morphologic leukemia-free state (MLFS).
Forty-two percent of patients had a CR, 4% had a CRi, and 6% achieved MLFS. The median duration of CR/CRi/MLFS was 13.2 months. The median duration of CR/CRi was 17.2 months.
The median overall survival was 19.1 months. The 1-year survival rate was 62%, and the 2-year survival rate was 41%.
The most common treatment-emergent AEs were nausea (78%), constipation (70%), fatigue (62%), decreased appetite (56%), diarrhea (50%), vomiting (40%), cough (36%), dyspnea (34%), hypokalemia (34%), peripheral edema (34%), pyrexia (34%), dizziness (32%), back pain (28%), insomnia (28%), febrile neutropenia (48%), thrombocytopenia (46%), anemia (38%), and neutropenia (38%).
Treatment-emergent AEs led to discontinuation in 14 patients. Three of these patients developed sepsis that proved fatal.
The other AEs leading to discontinuation included grade 3 acute axonal neuropathy, grade 3 parainfluenza, grade 3 prolonged QTc/atrial fibrillation, grade 1 acute kidney injury, grade 3 diverticulitis, grade 3 supraglottic ulcer, grade 2 upper respiratory infection, grade 3 fatigue (n=2), and grades 1 and 3 intermittent fatigue (n=2). ![]()
Survival differences among AYAs with blood cancers
A new report has revealed differences in survival among adolescents and young adults (AYAs) with hematologic malignancies.
The report includes information on AYAs—ages 15 to 39—living in Los Angeles County who were diagnosed with common cancers between 1988 and 2014.
The data showed differences in 5-year survival rates according to sex, race, age, and socioeconomic status (SES).
For example, lymphoma survival rates were lower for males, African Americans (AAs), older AYAs, and patients with low socioeconomic status (SES).
For AYAs with leukemias, there was no survival difference according to sex, but AAs had worse survival than patients of other races. And the impact of age and SES varied according to leukemia type.
“Cancer survival data are poorly understood for 15- to 39-year-olds,” noted Amie Hwang, PhD, of the University of Southern California Keck School of Medicine in Los Angeles.
That is why she and her colleagues created the report, “Cancer in Los Angeles County: Survival Among Adolescents and Young Adults 1988-2014.”
According to the authors, this is the first report to break down cancer survival rates for AYAs into segments on race/ethnicity, sex, age group, SES, and cancer stage.
Survival data for patients with hematologic malignancies were as follows.
Acute lymphoblastic leukemia
There were 1137 cases of acute lymphoblastic leukemia in the AYA population in Los Angeles County during the period studied. This included 752 males and 385 females.
Five-year survival was similar between males (43%) and females (41%).
Younger AYAs had better survival than older AYAs (48% for ages 15-24, 35% for ages 25-34, and 32% for ages 35-39).
Survival was highest among non-Latino whites (NLWs, 56%), followed by Asian/Pacific Islanders (APIs, 52%), patients of other/unknown races (51%), Latino whites (LWs, 38%), and AAs (29%).
Survival declined with SES (55% for high, 42% for middle, and 36% for low SES).
Acute myeloid leukemia
There were 1195 cases of acute myeloid leukemia—641 males and 554 females.
Five-year survival was similar for males (40%) and females (43%) as well as for the different age groups (45% for ages 15-24 vs 40% for the older age groups).
Survival was highest among NLWs (44%), followed by LWs (43%), APIs (40%), other/unknown (33%), and AAs (25%).
Survival declined somewhat with SES (49% for high, 39% for middle, and 41% for low SES).
Chronic myeloid leukemia
There were 655 cases of chronic myeloid leukemia—408 males and 247 females.
Five-year survival was similar for males (70%) and females (71%), but it was slightly higher for older AYAs (69% for ages 15-24, 68% for ages 25-34, and 76% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (76%), followed by LWs (73%), NLWs/APIs (both 72%), and AAs (57%).
Survival declined somewhat with SES (76% for high, 67% for middle, and 68% for low SES).
Hodgkin lymphoma
There were 2993 AYAs diagnosed with Hodgkin lymphoma—1553 males and 1440 females.
The 5-year survival rate was higher in females (93%) than males (86%) and in younger AYAs (93% for ages 15-24, 89% for ages 25-34, and 85% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (96%), followed by APIs/NLWs (both 91%), LWs (88%), and AAs (83%).
Survival declined with SES (95% for high, 89% for middle, and 83% for low SES).
And survival was lower for patients with advanced-stage disease (93% localized, 94% regional, and 83% distant).
Non-Hodkgin lymphoma
There were 4485 AYAs diagnosed with non-Hodgkin lymphoma during the study period—3064 males and 1421 females.
The 5-year survival rate was higher in females (75%) than males (46%) and in younger AYAs (69% for ages 15-24, 51% for ages 25-34, and 52% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (88%), followed by APIs (68%), LWs/NLWs (both 53%), and AAs (50%).
Survival declined with SES (68% for high, 54% for middle, and 45% for low SES).
And survival was lower for patients with advanced-stage disease (61% localized, 66% regional, and 46% distant).
“Adolescents and young adults go to the doctor less often because they have this superhero mentality, like they’re invincible,” said author Dennis Deapen, DrPH, of the University of Southern California Keck School of Medicine.
“Once they do go to a health professional, their cancer diagnosis can be delayed because cancer isn’t the first concern doctors have for this age group. It comes as no surprise that patients diagnosed with late-stage cancer have reduced survival rates.” ![]()
A new report has revealed differences in survival among adolescents and young adults (AYAs) with hematologic malignancies.
The report includes information on AYAs—ages 15 to 39—living in Los Angeles County who were diagnosed with common cancers between 1988 and 2014.
The data showed differences in 5-year survival rates according to sex, race, age, and socioeconomic status (SES).
For example, lymphoma survival rates were lower for males, African Americans (AAs), older AYAs, and patients with low socioeconomic status (SES).
For AYAs with leukemias, there was no survival difference according to sex, but AAs had worse survival than patients of other races. And the impact of age and SES varied according to leukemia type.
“Cancer survival data are poorly understood for 15- to 39-year-olds,” noted Amie Hwang, PhD, of the University of Southern California Keck School of Medicine in Los Angeles.
That is why she and her colleagues created the report, “Cancer in Los Angeles County: Survival Among Adolescents and Young Adults 1988-2014.”
According to the authors, this is the first report to break down cancer survival rates for AYAs into segments on race/ethnicity, sex, age group, SES, and cancer stage.
Survival data for patients with hematologic malignancies were as follows.
Acute lymphoblastic leukemia
There were 1137 cases of acute lymphoblastic leukemia in the AYA population in Los Angeles County during the period studied. This included 752 males and 385 females.
Five-year survival was similar between males (43%) and females (41%).
Younger AYAs had better survival than older AYAs (48% for ages 15-24, 35% for ages 25-34, and 32% for ages 35-39).
Survival was highest among non-Latino whites (NLWs, 56%), followed by Asian/Pacific Islanders (APIs, 52%), patients of other/unknown races (51%), Latino whites (LWs, 38%), and AAs (29%).
Survival declined with SES (55% for high, 42% for middle, and 36% for low SES).
Acute myeloid leukemia
There were 1195 cases of acute myeloid leukemia—641 males and 554 females.
Five-year survival was similar for males (40%) and females (43%) as well as for the different age groups (45% for ages 15-24 vs 40% for the older age groups).
Survival was highest among NLWs (44%), followed by LWs (43%), APIs (40%), other/unknown (33%), and AAs (25%).
Survival declined somewhat with SES (49% for high, 39% for middle, and 41% for low SES).
Chronic myeloid leukemia
There were 655 cases of chronic myeloid leukemia—408 males and 247 females.
Five-year survival was similar for males (70%) and females (71%), but it was slightly higher for older AYAs (69% for ages 15-24, 68% for ages 25-34, and 76% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (76%), followed by LWs (73%), NLWs/APIs (both 72%), and AAs (57%).
Survival declined somewhat with SES (76% for high, 67% for middle, and 68% for low SES).
Hodgkin lymphoma
There were 2993 AYAs diagnosed with Hodgkin lymphoma—1553 males and 1440 females.
The 5-year survival rate was higher in females (93%) than males (86%) and in younger AYAs (93% for ages 15-24, 89% for ages 25-34, and 85% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (96%), followed by APIs/NLWs (both 91%), LWs (88%), and AAs (83%).
Survival declined with SES (95% for high, 89% for middle, and 83% for low SES).
And survival was lower for patients with advanced-stage disease (93% localized, 94% regional, and 83% distant).
Non-Hodkgin lymphoma
There were 4485 AYAs diagnosed with non-Hodgkin lymphoma during the study period—3064 males and 1421 females.
The 5-year survival rate was higher in females (75%) than males (46%) and in younger AYAs (69% for ages 15-24, 51% for ages 25-34, and 52% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (88%), followed by APIs (68%), LWs/NLWs (both 53%), and AAs (50%).
Survival declined with SES (68% for high, 54% for middle, and 45% for low SES).
And survival was lower for patients with advanced-stage disease (61% localized, 66% regional, and 46% distant).
“Adolescents and young adults go to the doctor less often because they have this superhero mentality, like they’re invincible,” said author Dennis Deapen, DrPH, of the University of Southern California Keck School of Medicine.
“Once they do go to a health professional, their cancer diagnosis can be delayed because cancer isn’t the first concern doctors have for this age group. It comes as no surprise that patients diagnosed with late-stage cancer have reduced survival rates.” ![]()
A new report has revealed differences in survival among adolescents and young adults (AYAs) with hematologic malignancies.
The report includes information on AYAs—ages 15 to 39—living in Los Angeles County who were diagnosed with common cancers between 1988 and 2014.
The data showed differences in 5-year survival rates according to sex, race, age, and socioeconomic status (SES).
For example, lymphoma survival rates were lower for males, African Americans (AAs), older AYAs, and patients with low socioeconomic status (SES).
For AYAs with leukemias, there was no survival difference according to sex, but AAs had worse survival than patients of other races. And the impact of age and SES varied according to leukemia type.
“Cancer survival data are poorly understood for 15- to 39-year-olds,” noted Amie Hwang, PhD, of the University of Southern California Keck School of Medicine in Los Angeles.
That is why she and her colleagues created the report, “Cancer in Los Angeles County: Survival Among Adolescents and Young Adults 1988-2014.”
According to the authors, this is the first report to break down cancer survival rates for AYAs into segments on race/ethnicity, sex, age group, SES, and cancer stage.
Survival data for patients with hematologic malignancies were as follows.
Acute lymphoblastic leukemia
There were 1137 cases of acute lymphoblastic leukemia in the AYA population in Los Angeles County during the period studied. This included 752 males and 385 females.
Five-year survival was similar between males (43%) and females (41%).
Younger AYAs had better survival than older AYAs (48% for ages 15-24, 35% for ages 25-34, and 32% for ages 35-39).
Survival was highest among non-Latino whites (NLWs, 56%), followed by Asian/Pacific Islanders (APIs, 52%), patients of other/unknown races (51%), Latino whites (LWs, 38%), and AAs (29%).
Survival declined with SES (55% for high, 42% for middle, and 36% for low SES).
Acute myeloid leukemia
There were 1195 cases of acute myeloid leukemia—641 males and 554 females.
Five-year survival was similar for males (40%) and females (43%) as well as for the different age groups (45% for ages 15-24 vs 40% for the older age groups).
Survival was highest among NLWs (44%), followed by LWs (43%), APIs (40%), other/unknown (33%), and AAs (25%).
Survival declined somewhat with SES (49% for high, 39% for middle, and 41% for low SES).
Chronic myeloid leukemia
There were 655 cases of chronic myeloid leukemia—408 males and 247 females.
Five-year survival was similar for males (70%) and females (71%), but it was slightly higher for older AYAs (69% for ages 15-24, 68% for ages 25-34, and 76% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (76%), followed by LWs (73%), NLWs/APIs (both 72%), and AAs (57%).
Survival declined somewhat with SES (76% for high, 67% for middle, and 68% for low SES).
Hodgkin lymphoma
There were 2993 AYAs diagnosed with Hodgkin lymphoma—1553 males and 1440 females.
The 5-year survival rate was higher in females (93%) than males (86%) and in younger AYAs (93% for ages 15-24, 89% for ages 25-34, and 85% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (96%), followed by APIs/NLWs (both 91%), LWs (88%), and AAs (83%).
Survival declined with SES (95% for high, 89% for middle, and 83% for low SES).
And survival was lower for patients with advanced-stage disease (93% localized, 94% regional, and 83% distant).
Non-Hodkgin lymphoma
There were 4485 AYAs diagnosed with non-Hodgkin lymphoma during the study period—3064 males and 1421 females.
The 5-year survival rate was higher in females (75%) than males (46%) and in younger AYAs (69% for ages 15-24, 51% for ages 25-34, and 52% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (88%), followed by APIs (68%), LWs/NLWs (both 53%), and AAs (50%).
Survival declined with SES (68% for high, 54% for middle, and 45% for low SES).
And survival was lower for patients with advanced-stage disease (61% localized, 66% regional, and 46% distant).
“Adolescents and young adults go to the doctor less often because they have this superhero mentality, like they’re invincible,” said author Dennis Deapen, DrPH, of the University of Southern California Keck School of Medicine.
“Once they do go to a health professional, their cancer diagnosis can be delayed because cancer isn’t the first concern doctors have for this age group. It comes as no surprise that patients diagnosed with late-stage cancer have reduced survival rates.” ![]()
Marine animals aid development of cytotoxicity assay
Researchers have looked to deep-sea creatures with the goal of creating a better cytotoxicity assay.
The team harnessed the power of enzymes responsible for marine animal bioluminescence to create the “Matador assay,” which can be used to determine whether cellular and immune-therapeutic agents are actually killing target cells.
The researchers said the Matador assay is quick and simple as well as “highly sensitive,” with the ability to detect cytotoxicity induced by several types of therapies.
Preet M. Chaudhary, MD, PhD, of the University of Southern California Keck School of Medicine in Los Angeles, and his colleagues described the assay in Scientific Reports.
“One of the most promising areas in cancer research is immunotherapy. . .,” Dr Chaudhary said. “It is also one of the most difficult because the methods for testing immunotherapies are not ideal.”
“Radioactive chromium release assay is the gold standard for testing whether an immunotherapy kills cancer cells. This method is expensive, complicated, and requires special disposal practices. Other available methods also suffer from limitations and don’t allow scientists to rapidly screen immunotherapeutic agents to find the best candidates.”
Dr Chaudhary and his colleagues set out to develop a simple, precise, and inexpensive cytotoxicity assay based on marine animal luciferases, the enzymes responsible for bioluminescence.
The team used a group of small crustaceans and deep-sea shrimp, which were selected for their bright bioluminescence. Their luciferases became the basis of the Matador assay.
Engineered to get trapped inside cells, the luciferases leak out of cells when they die, causing a visible glow. The level of luminescence can then be measured with a luminometer.
To test the Matador assay’s effectiveness at measuring cell death, the researchers used several types of cancer cells, including chronic myelogenous leukemia, acute myelogenous leukemia, Burkitt lymphoma, and solid tumor cells.
The team treated these cells with a variety of therapies, including chimeric antigen receptor (CAR) T cells, bispecific T-cell engagers, monoclonal antibodies, and natural killer cells.
Results showed the Matador assay could detect the death of a single cell, a level of sensitivity superior to that of existing cytotoxicity assays.
The researchers also pointed out that the Matador assay is fast, inexpensive, and can be performed in a 384-well plate format, saving time and reagents.
“In our hands, the Matador assay can detect cell death in as little as 30 minutes, which can ultimately translate to more expedient treatments for patients getting cellular immunotherapies such as CAR T cells,” Dr Chaudhary said.
In fact, Dr Chaudhary’s lab has developed more than 75 cancer cell lines expressing the marine luciferases and used them with the Matador assay to develop next-generation CAR T cells.
Dr Chaudhary believes the Matador assay has many potential applications in biomedical research and cellular therapy manufacturing.
“It could potentially play a role in screening other types of anticancer agents or even measuring environmental toxins,” he said. ![]()
Researchers have looked to deep-sea creatures with the goal of creating a better cytotoxicity assay.
The team harnessed the power of enzymes responsible for marine animal bioluminescence to create the “Matador assay,” which can be used to determine whether cellular and immune-therapeutic agents are actually killing target cells.
The researchers said the Matador assay is quick and simple as well as “highly sensitive,” with the ability to detect cytotoxicity induced by several types of therapies.
Preet M. Chaudhary, MD, PhD, of the University of Southern California Keck School of Medicine in Los Angeles, and his colleagues described the assay in Scientific Reports.
“One of the most promising areas in cancer research is immunotherapy. . .,” Dr Chaudhary said. “It is also one of the most difficult because the methods for testing immunotherapies are not ideal.”
“Radioactive chromium release assay is the gold standard for testing whether an immunotherapy kills cancer cells. This method is expensive, complicated, and requires special disposal practices. Other available methods also suffer from limitations and don’t allow scientists to rapidly screen immunotherapeutic agents to find the best candidates.”
Dr Chaudhary and his colleagues set out to develop a simple, precise, and inexpensive cytotoxicity assay based on marine animal luciferases, the enzymes responsible for bioluminescence.
The team used a group of small crustaceans and deep-sea shrimp, which were selected for their bright bioluminescence. Their luciferases became the basis of the Matador assay.
Engineered to get trapped inside cells, the luciferases leak out of cells when they die, causing a visible glow. The level of luminescence can then be measured with a luminometer.
To test the Matador assay’s effectiveness at measuring cell death, the researchers used several types of cancer cells, including chronic myelogenous leukemia, acute myelogenous leukemia, Burkitt lymphoma, and solid tumor cells.
The team treated these cells with a variety of therapies, including chimeric antigen receptor (CAR) T cells, bispecific T-cell engagers, monoclonal antibodies, and natural killer cells.
Results showed the Matador assay could detect the death of a single cell, a level of sensitivity superior to that of existing cytotoxicity assays.
The researchers also pointed out that the Matador assay is fast, inexpensive, and can be performed in a 384-well plate format, saving time and reagents.
“In our hands, the Matador assay can detect cell death in as little as 30 minutes, which can ultimately translate to more expedient treatments for patients getting cellular immunotherapies such as CAR T cells,” Dr Chaudhary said.
In fact, Dr Chaudhary’s lab has developed more than 75 cancer cell lines expressing the marine luciferases and used them with the Matador assay to develop next-generation CAR T cells.
Dr Chaudhary believes the Matador assay has many potential applications in biomedical research and cellular therapy manufacturing.
“It could potentially play a role in screening other types of anticancer agents or even measuring environmental toxins,” he said. ![]()
Researchers have looked to deep-sea creatures with the goal of creating a better cytotoxicity assay.
The team harnessed the power of enzymes responsible for marine animal bioluminescence to create the “Matador assay,” which can be used to determine whether cellular and immune-therapeutic agents are actually killing target cells.
The researchers said the Matador assay is quick and simple as well as “highly sensitive,” with the ability to detect cytotoxicity induced by several types of therapies.
Preet M. Chaudhary, MD, PhD, of the University of Southern California Keck School of Medicine in Los Angeles, and his colleagues described the assay in Scientific Reports.
“One of the most promising areas in cancer research is immunotherapy. . .,” Dr Chaudhary said. “It is also one of the most difficult because the methods for testing immunotherapies are not ideal.”
“Radioactive chromium release assay is the gold standard for testing whether an immunotherapy kills cancer cells. This method is expensive, complicated, and requires special disposal practices. Other available methods also suffer from limitations and don’t allow scientists to rapidly screen immunotherapeutic agents to find the best candidates.”
Dr Chaudhary and his colleagues set out to develop a simple, precise, and inexpensive cytotoxicity assay based on marine animal luciferases, the enzymes responsible for bioluminescence.
The team used a group of small crustaceans and deep-sea shrimp, which were selected for their bright bioluminescence. Their luciferases became the basis of the Matador assay.
Engineered to get trapped inside cells, the luciferases leak out of cells when they die, causing a visible glow. The level of luminescence can then be measured with a luminometer.
To test the Matador assay’s effectiveness at measuring cell death, the researchers used several types of cancer cells, including chronic myelogenous leukemia, acute myelogenous leukemia, Burkitt lymphoma, and solid tumor cells.
The team treated these cells with a variety of therapies, including chimeric antigen receptor (CAR) T cells, bispecific T-cell engagers, monoclonal antibodies, and natural killer cells.
Results showed the Matador assay could detect the death of a single cell, a level of sensitivity superior to that of existing cytotoxicity assays.
The researchers also pointed out that the Matador assay is fast, inexpensive, and can be performed in a 384-well plate format, saving time and reagents.
“In our hands, the Matador assay can detect cell death in as little as 30 minutes, which can ultimately translate to more expedient treatments for patients getting cellular immunotherapies such as CAR T cells,” Dr Chaudhary said.
In fact, Dr Chaudhary’s lab has developed more than 75 cancer cell lines expressing the marine luciferases and used them with the Matador assay to develop next-generation CAR T cells.
Dr Chaudhary believes the Matador assay has many potential applications in biomedical research and cellular therapy manufacturing.
“It could potentially play a role in screening other types of anticancer agents or even measuring environmental toxins,” he said. ![]()
Team finds way to target MYB in AML
Researchers say they have found a way to target the oncogenic transcription factor MYB in acute myeloid leukemia (AML).
By inducing the expression of a peptide in mouse models of AML, the researchers were able to prevent MYB from promoting leukemia growth.
In fact, the team observed AML regression with no harmful impact on the function of normal cells.
Christopher Vakoc, MD, PhD, of Cold Spring Harbor Laboratory in Cold Spring Harbor, New York, and his colleagues described these findings in Cancer Cell.
“MYB is a dream target in cancer research because it’s involved in so many cancers,” Dr Vakoc said. “In leukemia, it’s special because we know from previous research that, by targeting MYB, you can get AML not just to stop growing but actually to regress.”
With the current research, Dr Vakoc and his colleagues discovered how to selectively target MYB.
First, the researchers found that MYB activates gene expression by docking at a giant gene-co-activation protein called TFIID.
Specifically, the MYB transactivation domain binds to the TAF12/TAF4 histone-fold domain (HFD) heterodimer. TAF12 and TAF4 are subunits of TFIID.
Based on this finding, the researchers hypothesized that “a minimal HFD fragment of TAF4 would be an ideal probe for blocking MYB function, since this peptide binds with high affinity and specificity to the TAF12 HFD.”
The team thought the peptide would form an ineffective complex with TAF12 and MYB, interfering with the endogenous MYB-TFIID interaction.
In in vitro experiments, TAF4-HFD expression inhibited AML growth and blast colony formation, induced differentiation, and destabilized MYB protein.
For their in vivo experiments, the researchers transduced RN2 cells with a dox-inducible TAF4-HFD retroviral vector and transplanted the cells in mice.
The team observed “marked” AML regression and prolonged survival in these mice compared to controls.
While the peptide is not itself a drug, Dr Vakoc said its action could be replicated by a drug.
“It’s a concept we’re now discussing with the pharmaceutical industry,” he said. “It is going to take lots of work before it can result in a medicine leukemia patients might take. But we’re excited about this new approach because MYB is such an important player in many cancers and, until now, has eluded efforts to selectively target it.” ![]()
Researchers say they have found a way to target the oncogenic transcription factor MYB in acute myeloid leukemia (AML).
By inducing the expression of a peptide in mouse models of AML, the researchers were able to prevent MYB from promoting leukemia growth.
In fact, the team observed AML regression with no harmful impact on the function of normal cells.
Christopher Vakoc, MD, PhD, of Cold Spring Harbor Laboratory in Cold Spring Harbor, New York, and his colleagues described these findings in Cancer Cell.
“MYB is a dream target in cancer research because it’s involved in so many cancers,” Dr Vakoc said. “In leukemia, it’s special because we know from previous research that, by targeting MYB, you can get AML not just to stop growing but actually to regress.”
With the current research, Dr Vakoc and his colleagues discovered how to selectively target MYB.
First, the researchers found that MYB activates gene expression by docking at a giant gene-co-activation protein called TFIID.
Specifically, the MYB transactivation domain binds to the TAF12/TAF4 histone-fold domain (HFD) heterodimer. TAF12 and TAF4 are subunits of TFIID.
Based on this finding, the researchers hypothesized that “a minimal HFD fragment of TAF4 would be an ideal probe for blocking MYB function, since this peptide binds with high affinity and specificity to the TAF12 HFD.”
The team thought the peptide would form an ineffective complex with TAF12 and MYB, interfering with the endogenous MYB-TFIID interaction.
In in vitro experiments, TAF4-HFD expression inhibited AML growth and blast colony formation, induced differentiation, and destabilized MYB protein.
For their in vivo experiments, the researchers transduced RN2 cells with a dox-inducible TAF4-HFD retroviral vector and transplanted the cells in mice.
The team observed “marked” AML regression and prolonged survival in these mice compared to controls.
While the peptide is not itself a drug, Dr Vakoc said its action could be replicated by a drug.
“It’s a concept we’re now discussing with the pharmaceutical industry,” he said. “It is going to take lots of work before it can result in a medicine leukemia patients might take. But we’re excited about this new approach because MYB is such an important player in many cancers and, until now, has eluded efforts to selectively target it.” ![]()
Researchers say they have found a way to target the oncogenic transcription factor MYB in acute myeloid leukemia (AML).
By inducing the expression of a peptide in mouse models of AML, the researchers were able to prevent MYB from promoting leukemia growth.
In fact, the team observed AML regression with no harmful impact on the function of normal cells.
Christopher Vakoc, MD, PhD, of Cold Spring Harbor Laboratory in Cold Spring Harbor, New York, and his colleagues described these findings in Cancer Cell.
“MYB is a dream target in cancer research because it’s involved in so many cancers,” Dr Vakoc said. “In leukemia, it’s special because we know from previous research that, by targeting MYB, you can get AML not just to stop growing but actually to regress.”
With the current research, Dr Vakoc and his colleagues discovered how to selectively target MYB.
First, the researchers found that MYB activates gene expression by docking at a giant gene-co-activation protein called TFIID.
Specifically, the MYB transactivation domain binds to the TAF12/TAF4 histone-fold domain (HFD) heterodimer. TAF12 and TAF4 are subunits of TFIID.
Based on this finding, the researchers hypothesized that “a minimal HFD fragment of TAF4 would be an ideal probe for blocking MYB function, since this peptide binds with high affinity and specificity to the TAF12 HFD.”
The team thought the peptide would form an ineffective complex with TAF12 and MYB, interfering with the endogenous MYB-TFIID interaction.
In in vitro experiments, TAF4-HFD expression inhibited AML growth and blast colony formation, induced differentiation, and destabilized MYB protein.
For their in vivo experiments, the researchers transduced RN2 cells with a dox-inducible TAF4-HFD retroviral vector and transplanted the cells in mice.
The team observed “marked” AML regression and prolonged survival in these mice compared to controls.
While the peptide is not itself a drug, Dr Vakoc said its action could be replicated by a drug.
“It’s a concept we’re now discussing with the pharmaceutical industry,” he said. “It is going to take lots of work before it can result in a medicine leukemia patients might take. But we’re excited about this new approach because MYB is such an important player in many cancers and, until now, has eluded efforts to selectively target it.” ![]()
DNA sequencing could help identify relapse risk in treated AML
ATLANTA – Clinicians may be able to get a jump on identifying risk factors for relapse among adults with acute myeloid leukemia (AML) in first complete remission through the use of next-generation DNA sequencing, investigators reported.
Among 430 adults with AML with somatic driver mutations persistent in bone marrow during morphological complete remission (CR) following induction therapy, the presence of minimal residual disease (MRD) bearing specific disease-related mutations on next-generation sequencing (NGS) was significantly associated with both the cumulative incidence of relapse and with overall survival. Tim Grob, MD, of Erasmus University Medical Center in Rotterdam, the Netherlands, reported the findings during a late-breaking abstract session at the annual meeting of the American Society of Hematology.
By excluding three common AML mutations in genes commonly associated with clonal hematopoiesis – DNMT3A, TET2, and ASXL1 (collectively, DTA) – Dr. Grob and his colleagues at centers in the Netherlands, Belgium, and Switzerland were able to demonstrate that non-DTA mutations present in the marrow of patients in CR are highly predictive for relapse within 5 years and for worse overall survival.
They also showed that mutations associated with clonal hematopoiesis (the presence of small, preleukemic clones) in CR is not significantly associated with risk of relapse.
More than 80% of patients with AML are able to have a CR after induction therapy, but a significant proportion of patients will also experience relapse. Investigators have yet to nail down which leukemia-specific mutations that linger in patients with CR may be responsible for subsequent relapses, Dr. Grob said.
To get a better handle on which residual mutations may signal the need for extra vigilance or additional therapy in patients in CR after two cycles of induction therapy, the investigators used targeted next-generation (high-throughput) sequencing at the time of diagnosis and first CR in 430 patients enrolled in joint Dutch/Swiss clinical trials. The median patient age was 51.
The investigators screened marrow samples using a commercially available gene panel (Illumina) covering 54 genes that are commonly mutated in myeloid malignancies.
They divided the patients into a training cohort (283 patients) and a validation set (147) for confirmation of results.
About half of all patients in the training cohort (51.4%) had persistent mutations in bone marrow that occurred with highly variable variant allele frequencies. The most common mutations were in the DTA group with the most frequently mutated gene being DNMT3A (78.7% variant allele frequency), followed by TET2 (54.2%) and ASXL1 (51.6%).
Mutations in DTA genes in this cohort were not associated with the incidence of relapse at any variant allele frequency cut-off point used, which indicated that these mutations represented a stage of clonal hematopoiesis rather than early relapse signals.
However, among patients who had persistent DTA mutations, there was significant correlation with a risk for relapse when they also had persistence of any other non-DTA mutations. The cumulative 5-year incidence of relapse in patients with both persistent DTA and non-DTA mutations was 76.4%, compared with 39.4% for those without other, non-DTA mutations (P = .002).
Also in the training cohort, persistent non-DTA mutations (NGS MRD) were found to be highly associated with the risk of relapse with a subdistribution hazard ratio (SHR) of 1.85 (P = .001). In the validation set the effect was even stronger, with an SHR or 2.81 (P less than .001).
When data from the training and validation cohort were combined, the 5-year cumulative incidence of relapse was 58.3% for non-DTA mutation, vs. 33.9% (P less than .001).
NGS MRD was also predictive of overall survival, with a hazard ratio in the training cohort of 1.64 (P = .012) and an HR in the validation cohort of 3.08 (P less than .001).
In multivariable analysis of all 430 patients, adjusted for age, white blood cell count, 2017 European LeukemiaNet risk category, and number of induction cycles need to achieve CR, NGS MRD was an independent prognostic factor for both relapse (SHR 1.89, P less than .001) and overall survival (HR 1.64 P = .003).
When the investigators conducted a sensitivity analysis with time-dependent correction for allogeneic stem cell transplantation, they found that NGS MRD was still significantly prognostic for both relapse and survival.
The study was supported by the Dutch Cancer Society, the Haemato-Oncology Foundation for Adults in the Netherlands, the Swiss Group for Clinical Cancer Research, and The Netherlands Organization for Health Research and Development. Dr. Grob reported having no relevant disclosures.
SOURCE: Jongen-Lavrencic M et al. ASH 2017 Abstract LBA 5.
ATLANTA – Clinicians may be able to get a jump on identifying risk factors for relapse among adults with acute myeloid leukemia (AML) in first complete remission through the use of next-generation DNA sequencing, investigators reported.
Among 430 adults with AML with somatic driver mutations persistent in bone marrow during morphological complete remission (CR) following induction therapy, the presence of minimal residual disease (MRD) bearing specific disease-related mutations on next-generation sequencing (NGS) was significantly associated with both the cumulative incidence of relapse and with overall survival. Tim Grob, MD, of Erasmus University Medical Center in Rotterdam, the Netherlands, reported the findings during a late-breaking abstract session at the annual meeting of the American Society of Hematology.
By excluding three common AML mutations in genes commonly associated with clonal hematopoiesis – DNMT3A, TET2, and ASXL1 (collectively, DTA) – Dr. Grob and his colleagues at centers in the Netherlands, Belgium, and Switzerland were able to demonstrate that non-DTA mutations present in the marrow of patients in CR are highly predictive for relapse within 5 years and for worse overall survival.
They also showed that mutations associated with clonal hematopoiesis (the presence of small, preleukemic clones) in CR is not significantly associated with risk of relapse.
More than 80% of patients with AML are able to have a CR after induction therapy, but a significant proportion of patients will also experience relapse. Investigators have yet to nail down which leukemia-specific mutations that linger in patients with CR may be responsible for subsequent relapses, Dr. Grob said.
To get a better handle on which residual mutations may signal the need for extra vigilance or additional therapy in patients in CR after two cycles of induction therapy, the investigators used targeted next-generation (high-throughput) sequencing at the time of diagnosis and first CR in 430 patients enrolled in joint Dutch/Swiss clinical trials. The median patient age was 51.
The investigators screened marrow samples using a commercially available gene panel (Illumina) covering 54 genes that are commonly mutated in myeloid malignancies.
They divided the patients into a training cohort (283 patients) and a validation set (147) for confirmation of results.
About half of all patients in the training cohort (51.4%) had persistent mutations in bone marrow that occurred with highly variable variant allele frequencies. The most common mutations were in the DTA group with the most frequently mutated gene being DNMT3A (78.7% variant allele frequency), followed by TET2 (54.2%) and ASXL1 (51.6%).
Mutations in DTA genes in this cohort were not associated with the incidence of relapse at any variant allele frequency cut-off point used, which indicated that these mutations represented a stage of clonal hematopoiesis rather than early relapse signals.
However, among patients who had persistent DTA mutations, there was significant correlation with a risk for relapse when they also had persistence of any other non-DTA mutations. The cumulative 5-year incidence of relapse in patients with both persistent DTA and non-DTA mutations was 76.4%, compared with 39.4% for those without other, non-DTA mutations (P = .002).
Also in the training cohort, persistent non-DTA mutations (NGS MRD) were found to be highly associated with the risk of relapse with a subdistribution hazard ratio (SHR) of 1.85 (P = .001). In the validation set the effect was even stronger, with an SHR or 2.81 (P less than .001).
When data from the training and validation cohort were combined, the 5-year cumulative incidence of relapse was 58.3% for non-DTA mutation, vs. 33.9% (P less than .001).
NGS MRD was also predictive of overall survival, with a hazard ratio in the training cohort of 1.64 (P = .012) and an HR in the validation cohort of 3.08 (P less than .001).
In multivariable analysis of all 430 patients, adjusted for age, white blood cell count, 2017 European LeukemiaNet risk category, and number of induction cycles need to achieve CR, NGS MRD was an independent prognostic factor for both relapse (SHR 1.89, P less than .001) and overall survival (HR 1.64 P = .003).
When the investigators conducted a sensitivity analysis with time-dependent correction for allogeneic stem cell transplantation, they found that NGS MRD was still significantly prognostic for both relapse and survival.
The study was supported by the Dutch Cancer Society, the Haemato-Oncology Foundation for Adults in the Netherlands, the Swiss Group for Clinical Cancer Research, and The Netherlands Organization for Health Research and Development. Dr. Grob reported having no relevant disclosures.
SOURCE: Jongen-Lavrencic M et al. ASH 2017 Abstract LBA 5.
ATLANTA – Clinicians may be able to get a jump on identifying risk factors for relapse among adults with acute myeloid leukemia (AML) in first complete remission through the use of next-generation DNA sequencing, investigators reported.
Among 430 adults with AML with somatic driver mutations persistent in bone marrow during morphological complete remission (CR) following induction therapy, the presence of minimal residual disease (MRD) bearing specific disease-related mutations on next-generation sequencing (NGS) was significantly associated with both the cumulative incidence of relapse and with overall survival. Tim Grob, MD, of Erasmus University Medical Center in Rotterdam, the Netherlands, reported the findings during a late-breaking abstract session at the annual meeting of the American Society of Hematology.
By excluding three common AML mutations in genes commonly associated with clonal hematopoiesis – DNMT3A, TET2, and ASXL1 (collectively, DTA) – Dr. Grob and his colleagues at centers in the Netherlands, Belgium, and Switzerland were able to demonstrate that non-DTA mutations present in the marrow of patients in CR are highly predictive for relapse within 5 years and for worse overall survival.
They also showed that mutations associated with clonal hematopoiesis (the presence of small, preleukemic clones) in CR is not significantly associated with risk of relapse.
More than 80% of patients with AML are able to have a CR after induction therapy, but a significant proportion of patients will also experience relapse. Investigators have yet to nail down which leukemia-specific mutations that linger in patients with CR may be responsible for subsequent relapses, Dr. Grob said.
To get a better handle on which residual mutations may signal the need for extra vigilance or additional therapy in patients in CR after two cycles of induction therapy, the investigators used targeted next-generation (high-throughput) sequencing at the time of diagnosis and first CR in 430 patients enrolled in joint Dutch/Swiss clinical trials. The median patient age was 51.
The investigators screened marrow samples using a commercially available gene panel (Illumina) covering 54 genes that are commonly mutated in myeloid malignancies.
They divided the patients into a training cohort (283 patients) and a validation set (147) for confirmation of results.
About half of all patients in the training cohort (51.4%) had persistent mutations in bone marrow that occurred with highly variable variant allele frequencies. The most common mutations were in the DTA group with the most frequently mutated gene being DNMT3A (78.7% variant allele frequency), followed by TET2 (54.2%) and ASXL1 (51.6%).
Mutations in DTA genes in this cohort were not associated with the incidence of relapse at any variant allele frequency cut-off point used, which indicated that these mutations represented a stage of clonal hematopoiesis rather than early relapse signals.
However, among patients who had persistent DTA mutations, there was significant correlation with a risk for relapse when they also had persistence of any other non-DTA mutations. The cumulative 5-year incidence of relapse in patients with both persistent DTA and non-DTA mutations was 76.4%, compared with 39.4% for those without other, non-DTA mutations (P = .002).
Also in the training cohort, persistent non-DTA mutations (NGS MRD) were found to be highly associated with the risk of relapse with a subdistribution hazard ratio (SHR) of 1.85 (P = .001). In the validation set the effect was even stronger, with an SHR or 2.81 (P less than .001).
When data from the training and validation cohort were combined, the 5-year cumulative incidence of relapse was 58.3% for non-DTA mutation, vs. 33.9% (P less than .001).
NGS MRD was also predictive of overall survival, with a hazard ratio in the training cohort of 1.64 (P = .012) and an HR in the validation cohort of 3.08 (P less than .001).
In multivariable analysis of all 430 patients, adjusted for age, white blood cell count, 2017 European LeukemiaNet risk category, and number of induction cycles need to achieve CR, NGS MRD was an independent prognostic factor for both relapse (SHR 1.89, P less than .001) and overall survival (HR 1.64 P = .003).
When the investigators conducted a sensitivity analysis with time-dependent correction for allogeneic stem cell transplantation, they found that NGS MRD was still significantly prognostic for both relapse and survival.
The study was supported by the Dutch Cancer Society, the Haemato-Oncology Foundation for Adults in the Netherlands, the Swiss Group for Clinical Cancer Research, and The Netherlands Organization for Health Research and Development. Dr. Grob reported having no relevant disclosures.
SOURCE: Jongen-Lavrencic M et al. ASH 2017 Abstract LBA 5.
REPORTING FROM ASH 2017
Key clinical point:
Major finding: The presence of any non-DTA mutation after CR was an independent prognostic factor for relapse (SHR 1.89) and overall survival (HR 1.64).
Study details: Prospective analysis of bone marrow samples from 430 patients with AML at diagnosis and in first complete remission.
Disclosures: The study was supported by the Dutch Cancer Society, the Haemato-Oncology Foundation for Adults in the Netherlands, the Swiss Group for Clinical Cancer Research, and The Netherlands Organization for Health Research and Development. Dr. Grob reported having no relevant disclosures.
Source: Jongen-Lavrencic M et al. ASH 2017 Abstract LBA 5.
CX-01 receives orphan designation for AML
The US Food and Drug Administration (FDA) has granted orphan drug designation to CX-01 for the treatment of acute myeloid leukemia (AML).
CX-01 is a polysaccharide derived from heparin that is thought to enhance chemotherapy by disrupting the adhesion of leukemia cells in the bone marrow.
CX-01 inhibits the activity of HMGB1, disrupts the CXCL12/CXCR4 axis, and neutralizes the activity of platelet factor 4.
HMGB1 has been implicated in autophagy, a mechanism by which cells withstand the effects of chemotherapy. The CXCL12/CXCR4 axis is thought to be involved in protecting leukemia cells from chemotherapy. And platelet factor 4 inhibits bone marrow recovery after chemotherapy.
Cantex Pharmaceuticals, Inc., is conducting a randomized, phase 2b study to determine whether CX-01 can improve the efficacy of frontline chemotherapy in patients with AML.
This study builds upon results of a pilot study, which were presented at the 2015 ASCO Annual Meeting (abstract 7053).
The study enrolled 12 adults with newly diagnosed AML. They received CX-01 as a 7-day continuous infusion, along with standard induction chemotherapy (cytarabine and idarubicin, 7+3).
Eleven patients (92%), all of whom had de novo AML, had a complete response (CR) with a single induction cycle.
The median time to neutrophil recovery was 23 days, and the median time to platelet recovery was 22 days.
With a median follow-up of 14.2 months, the median event-free survival exceeded 11.6 months, and the median overall survival exceeded 13.6 months.
No adverse events related to CX-01 were reported.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to CX-01 for the treatment of acute myeloid leukemia (AML).
CX-01 is a polysaccharide derived from heparin that is thought to enhance chemotherapy by disrupting the adhesion of leukemia cells in the bone marrow.
CX-01 inhibits the activity of HMGB1, disrupts the CXCL12/CXCR4 axis, and neutralizes the activity of platelet factor 4.
HMGB1 has been implicated in autophagy, a mechanism by which cells withstand the effects of chemotherapy. The CXCL12/CXCR4 axis is thought to be involved in protecting leukemia cells from chemotherapy. And platelet factor 4 inhibits bone marrow recovery after chemotherapy.
Cantex Pharmaceuticals, Inc., is conducting a randomized, phase 2b study to determine whether CX-01 can improve the efficacy of frontline chemotherapy in patients with AML.
This study builds upon results of a pilot study, which were presented at the 2015 ASCO Annual Meeting (abstract 7053).
The study enrolled 12 adults with newly diagnosed AML. They received CX-01 as a 7-day continuous infusion, along with standard induction chemotherapy (cytarabine and idarubicin, 7+3).
Eleven patients (92%), all of whom had de novo AML, had a complete response (CR) with a single induction cycle.
The median time to neutrophil recovery was 23 days, and the median time to platelet recovery was 22 days.
With a median follow-up of 14.2 months, the median event-free survival exceeded 11.6 months, and the median overall survival exceeded 13.6 months.
No adverse events related to CX-01 were reported.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to CX-01 for the treatment of acute myeloid leukemia (AML).
CX-01 is a polysaccharide derived from heparin that is thought to enhance chemotherapy by disrupting the adhesion of leukemia cells in the bone marrow.
CX-01 inhibits the activity of HMGB1, disrupts the CXCL12/CXCR4 axis, and neutralizes the activity of platelet factor 4.
HMGB1 has been implicated in autophagy, a mechanism by which cells withstand the effects of chemotherapy. The CXCL12/CXCR4 axis is thought to be involved in protecting leukemia cells from chemotherapy. And platelet factor 4 inhibits bone marrow recovery after chemotherapy.
Cantex Pharmaceuticals, Inc., is conducting a randomized, phase 2b study to determine whether CX-01 can improve the efficacy of frontline chemotherapy in patients with AML.
This study builds upon results of a pilot study, which were presented at the 2015 ASCO Annual Meeting (abstract 7053).
The study enrolled 12 adults with newly diagnosed AML. They received CX-01 as a 7-day continuous infusion, along with standard induction chemotherapy (cytarabine and idarubicin, 7+3).
Eleven patients (92%), all of whom had de novo AML, had a complete response (CR) with a single induction cycle.
The median time to neutrophil recovery was 23 days, and the median time to platelet recovery was 22 days.
With a median follow-up of 14.2 months, the median event-free survival exceeded 11.6 months, and the median overall survival exceeded 13.6 months.
No adverse events related to CX-01 were reported.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()