User login
What Patients Undergoing Gastrointestinal Endoscopic Procedures Should Receive Antibiotic Prophylaxis?
Case
You are asked to admit two patients. The first is a 75-year-old male with a prosthetic aortic valve on warfarin who presents with bright red blood per rectum and is scheduled for colonoscopy. The second patient is a 35-year-old female with biliary obstruction due to choledocholithiasis; she is afebrile with normal vital signs and no leukocytosis. She underwent endoscopic retrograde cholangiopancreatography (ERCP), which did not resolve her biliary obstruction. Should you prescribe prophylactic antibiotics for either patient?
Overview
Providers are often confused regarding which patients undergoing gastrointestinal (GI) endoscopic procedures should receive antibiotic prophylaxis. To answer this question, it is important to understand the goal of prophylactic antibiotics. Are we trying to prevent infective endocarditis or a localized infection?
There are few large, prospective, randomized controlled trials that have examined the need for antibiotic prophylaxis with GI endoscopic procedures. Guidelines from professional societies are mainly based on expert opinion, evidence from retrospective case studies, and meta-analysis reviews.
Review of the Data
Infective endocarditis resulting from GI endoscopy has been a concern of physicians for decades. The American Heart Association (AHA) first published its recommendations for antibiotic prophylaxis of GI tract procedures in 1965. The most recent antibacterial prophylaxis guidelines, published in 2007, have simplified recommendations and greatly scaled back the indications for antibiotics. The new guidelines conclude that frequent bacteremia from daily activities is more likely to precipitate endocarditis than a single dental, GI, or genitourinary tract procedure.1
The American Society for Gastrointestinal Endoscopy (ASGE) reports that 14.2 million colonoscopies, 2.8 million flexible sigmoidoscopies, and nearly as many upper endoscopies are performed in the U.S. each year, but only 15 cases of endocarditis have been reported with a temporal association to a procedure.2
The British Society of Gastroenterology (BSG) found, after reviewing the histories of patients with infective endocarditis from 1983 through 2006, that there is not enough evidence to warrant antibiotic prophylaxis prior to endoscopy. They noted less than one case of endocarditis after GI endoscopy per year as well as significant variation in the time interval between the procedure and symptoms. The BSG also recognized that antibiotic prophylaxis does not always protect against infection and that clinical factors unrelated to the endoscopy may play a significant role in the development of endocarditis.3
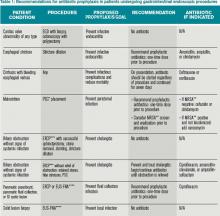
Upper GI Endoscopy, Colonoscopy with Biopsy, and Esophageal Dilatation. Administering antibiotics to prevent infective endocarditis is not recommended for patients undergoing routine procedures such as endoscopy with biopsy and colonoscopy with polypectomy. Likewise, patients with a history of prosthetic heart valves, valve repair with prosthetic material, endocarditis, congenital heart disease, or cardiac transplant with valvulopathy do not need prophylactic antibiotics before GI endoscopic procedures. However, for patients who are being treated for an active GI infection, antibiotic coverage for enterococcus may be warranted given the increased risk of developing endocarditis. The AHA acknowledges there are no published studies to support the efficacy of antibiotics to prevent enterococcal endocarditis in patients in this clinical setting.1
Unlike routine endoscopy, esophageal dilation is associated with an increased rate of bacteremia (12%-100%).4 Streptococcus viridans has been found in blood cultures up to 79% of the time after esophageal dilation.5 Patients with malignant strictures have higher rates of bacteremia than those with benign strictures (52.9% versus 15.7%). Patients treated with multiple passes with the esophageal dilator compared to those treated with a single dilation have a higher risk of bacteremia.6 All patients undergoing esophageal stricture dilation should receive pre-procedural prophylactic antibiotics.7
Patients with bleeding esophageal varices also have high rates of bacteremia. Up to 20% of patients with cirrhosis and GI bleeding on admission develop an infection within 48 hours of presentation.8 There is evidence that the bacteremia may actually be related to the variceal bleeding rather than the procedure.9 Patients with bleeding esophageal varices treated with antibiotics have improved outcomes, including a decrease in mortality.10 Therefore, all patients with bleeding esophageal varices should be placed on antibiotic therapy regardless of whether an endoscopic intervention is planned.
Percutaneous Endoscopic Gastrostomy (PEG) Placement. Prophylactic antibiotics are recommended before placement of a PEG. The indication for prophylactic antibiotics is to prevent a gastrostomy site infection, not infective endocarditis. Gastrostomy site infection is unfortunately a fairly common infection, affecting 4% to 30% of patients who undergo PEG tube placement. There is significant evidence that antibiotics are beneficial in preventing peristomal infections. A meta-analysis showed that only eight patients need to be treated with prophylactic antibiotics to prevent a single peristomal infection.11 Since these infections are believed to be caused by contamination from the oropharynx, physicians should consider prophylaxis against pathogens from the oral flora.12
More recently, it has been noted that methicillin-resistant Staphylococcus aureus (MRSA) is increasingly cultured from infection sites.13 In centers with endemic MRSA, patients should be screened and then undergo decontamination prior to the PEG placement in positive cases.
Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA). Antibiotic prophylaxis before EUS-FNA of a solid lesion in an organ is generally thought to be unnecessary because the risk of bacteremia with this procedure is low, comparable to routine GI endoscopy with biopsy. The recommendation for prophylactic antibiotics before biopsy of a cystic lesion is different. There is concern that puncturing cystic lesions may create a new infected fluid collection.2 A systematic review of more than 10,000 patients undergoing EUS-FNA with a full range of target organs revealed that, overall, 11.2% of patients experienced a fever and 4.7% of patients had a peri-procedural infection. While it was not possible in this study to determine which patients received prophylactic antibiotics, 93.7% of patients with pancreatic cystic lesions were reported to have been treated with antibiotics.14
A separate, single-center, retrospective trial produced different results. This study examined a population of 253 patients who underwent 266 EUS-FNA of pancreatic cysts and found that prophylactic antibiotics were associated with more adverse events and were not protective for the 3% of the patients with infectious symptoms.15 Despite the conflicting data, guidelines at this time recommend prophylactic antibiotics before drainage of a sterile pancreatic fluid collection that communicates with the pancreatic duct and also for aspiration of cystic lesions along the GI tract and the mediastinum.2
Endoscopic Retrograde Cholangiopancreatography (ERCP). In patients undergoing ERCP, the routine use of prophylactic antibiotics has not been found to be effective in decreasing the risk of post-procedure cholangitis.16 Guidelines recommend the use of prophylactic antibiotics only in those patients in which the ERCP may not completely resolve the biliary obstruction.2 In these patients, the thought is that ERCP can precipitate infection by disturbing bacteria already present in the biliary tree, especially with increased intrabiliary pressure at the time of contrast dye injection.17
Patients with incomplete biliary drainage, including those with primary sclerosing cholangitis (PSC), hilar cholangiocarcinoma, persistent biliary that were not extracted, and strictures that continue to obstruct despite attempted intervention, are thought to be at elevated risk of developing cholangitis post-ERCP. These patients should be placed on prophylactic antibiotics at the time of the procedure to cover biliary flora such as enteric gram negatives and enterococci. Antibiotics should be continued until the biliary obstruction is resolved.2
Additional Populations to Consider. Previously, the International Society for Peritoneal Dialysis recommended that patients on peritoneal dialysis receive prophylactic antibiotics and empty their abdomen of dialysate prior to colonoscopy. This recommendation has been removed from the 2010 guidelines.18 There is also no indication that patients with synthetic vascular grafts or cardiac devices should receive prophylactic antibiotics prior to routine GI endoscopy.19 The American Academy of Orthopaedic Surgeons no longer recommends that patients with joint replacements receive antibiotic prophylaxis prior to GI endoscopy.20
Back to the Case
The older gentleman with a prosthetic valve undergoing colonoscopy should not receive prophylactic antibiotics, because even in the setting of valvulopathy, colonoscopy does not pose a significant risk for infective endocarditis. The young patient with severe choledocholithiasis should be placed on prophylactic antibiotics because she has continued biliary obstruction, which could result in a cholangitis after ERCP.
Bottom Line
Prophylactic antibiotics are not recommended for any patient undergoing routine endoscopy or colonoscopy. They are indicated for patients with bleeding esophageal varices and for patients who undergo esophageal stricture dilation, PEG placement, or pseudocyst or cyst drainage, and those with continued biliary obstruction undergoing ERCP as summarized in Table 1.
Drs. Ritter, Jupiter, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center and Harvard Medical School faculty in Boston.
References
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754.
- Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791-798.
- Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58(6):869-880.
- Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57(4):546-556.
- Zuccaro G Jr., Richter JE, Rice TW, et al. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest Endosc. 1998;48(6):568-573.
- Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest Endosc.1998;48(6):563-567.
- Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58(4):475-482.
- Ho H, Zuckerman MJ, Wassem C. A prospective controlled study of the risk of bacteremia in emergency sclerotherapy of esophageal varices. Gastroenterology. 1991;101(6):1642-1648.
- Rolando N, Gimson A, Philpott-Howard J, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: Role of antibiotic prophylaxis. J Hepatol. 1993;18(3):290-294.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-938.
- Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: Antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25(6):647-656.
- Chuang CH, Hung KH, Chen JR, et al. Airway infection predisposes to peristomal infection after percutaneous endoscopic gastrostomy with high concordance between sputum and wound isolates. J Gastrointest Surg. 2010;14(1):45-51.
- Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. PEG site infections: The emergence of methicillin resistant Staphylococcus aureus as a major pathogen. Am J Gastroenterol. 2002;97(7):1713-1716.
- Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73(2):283-290.
- Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: A retrospective, comparative analysis. Gastrointest Endosc. 2011;74(1):81-86.
- Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: A meta-analysis. Pancreas. 2009;38(2):126-130.
- Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: A sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67(3):471-475.
- Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393-423.
- Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015-2031.
- Rethman MP, Watters W III, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745-747.
Case
You are asked to admit two patients. The first is a 75-year-old male with a prosthetic aortic valve on warfarin who presents with bright red blood per rectum and is scheduled for colonoscopy. The second patient is a 35-year-old female with biliary obstruction due to choledocholithiasis; she is afebrile with normal vital signs and no leukocytosis. She underwent endoscopic retrograde cholangiopancreatography (ERCP), which did not resolve her biliary obstruction. Should you prescribe prophylactic antibiotics for either patient?
Overview
Providers are often confused regarding which patients undergoing gastrointestinal (GI) endoscopic procedures should receive antibiotic prophylaxis. To answer this question, it is important to understand the goal of prophylactic antibiotics. Are we trying to prevent infective endocarditis or a localized infection?
There are few large, prospective, randomized controlled trials that have examined the need for antibiotic prophylaxis with GI endoscopic procedures. Guidelines from professional societies are mainly based on expert opinion, evidence from retrospective case studies, and meta-analysis reviews.
Review of the Data
Infective endocarditis resulting from GI endoscopy has been a concern of physicians for decades. The American Heart Association (AHA) first published its recommendations for antibiotic prophylaxis of GI tract procedures in 1965. The most recent antibacterial prophylaxis guidelines, published in 2007, have simplified recommendations and greatly scaled back the indications for antibiotics. The new guidelines conclude that frequent bacteremia from daily activities is more likely to precipitate endocarditis than a single dental, GI, or genitourinary tract procedure.1
The American Society for Gastrointestinal Endoscopy (ASGE) reports that 14.2 million colonoscopies, 2.8 million flexible sigmoidoscopies, and nearly as many upper endoscopies are performed in the U.S. each year, but only 15 cases of endocarditis have been reported with a temporal association to a procedure.2
The British Society of Gastroenterology (BSG) found, after reviewing the histories of patients with infective endocarditis from 1983 through 2006, that there is not enough evidence to warrant antibiotic prophylaxis prior to endoscopy. They noted less than one case of endocarditis after GI endoscopy per year as well as significant variation in the time interval between the procedure and symptoms. The BSG also recognized that antibiotic prophylaxis does not always protect against infection and that clinical factors unrelated to the endoscopy may play a significant role in the development of endocarditis.3
Upper GI Endoscopy, Colonoscopy with Biopsy, and Esophageal Dilatation. Administering antibiotics to prevent infective endocarditis is not recommended for patients undergoing routine procedures such as endoscopy with biopsy and colonoscopy with polypectomy. Likewise, patients with a history of prosthetic heart valves, valve repair with prosthetic material, endocarditis, congenital heart disease, or cardiac transplant with valvulopathy do not need prophylactic antibiotics before GI endoscopic procedures. However, for patients who are being treated for an active GI infection, antibiotic coverage for enterococcus may be warranted given the increased risk of developing endocarditis. The AHA acknowledges there are no published studies to support the efficacy of antibiotics to prevent enterococcal endocarditis in patients in this clinical setting.1
Unlike routine endoscopy, esophageal dilation is associated with an increased rate of bacteremia (12%-100%).4 Streptococcus viridans has been found in blood cultures up to 79% of the time after esophageal dilation.5 Patients with malignant strictures have higher rates of bacteremia than those with benign strictures (52.9% versus 15.7%). Patients treated with multiple passes with the esophageal dilator compared to those treated with a single dilation have a higher risk of bacteremia.6 All patients undergoing esophageal stricture dilation should receive pre-procedural prophylactic antibiotics.7
Patients with bleeding esophageal varices also have high rates of bacteremia. Up to 20% of patients with cirrhosis and GI bleeding on admission develop an infection within 48 hours of presentation.8 There is evidence that the bacteremia may actually be related to the variceal bleeding rather than the procedure.9 Patients with bleeding esophageal varices treated with antibiotics have improved outcomes, including a decrease in mortality.10 Therefore, all patients with bleeding esophageal varices should be placed on antibiotic therapy regardless of whether an endoscopic intervention is planned.
Percutaneous Endoscopic Gastrostomy (PEG) Placement. Prophylactic antibiotics are recommended before placement of a PEG. The indication for prophylactic antibiotics is to prevent a gastrostomy site infection, not infective endocarditis. Gastrostomy site infection is unfortunately a fairly common infection, affecting 4% to 30% of patients who undergo PEG tube placement. There is significant evidence that antibiotics are beneficial in preventing peristomal infections. A meta-analysis showed that only eight patients need to be treated with prophylactic antibiotics to prevent a single peristomal infection.11 Since these infections are believed to be caused by contamination from the oropharynx, physicians should consider prophylaxis against pathogens from the oral flora.12
More recently, it has been noted that methicillin-resistant Staphylococcus aureus (MRSA) is increasingly cultured from infection sites.13 In centers with endemic MRSA, patients should be screened and then undergo decontamination prior to the PEG placement in positive cases.
Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA). Antibiotic prophylaxis before EUS-FNA of a solid lesion in an organ is generally thought to be unnecessary because the risk of bacteremia with this procedure is low, comparable to routine GI endoscopy with biopsy. The recommendation for prophylactic antibiotics before biopsy of a cystic lesion is different. There is concern that puncturing cystic lesions may create a new infected fluid collection.2 A systematic review of more than 10,000 patients undergoing EUS-FNA with a full range of target organs revealed that, overall, 11.2% of patients experienced a fever and 4.7% of patients had a peri-procedural infection. While it was not possible in this study to determine which patients received prophylactic antibiotics, 93.7% of patients with pancreatic cystic lesions were reported to have been treated with antibiotics.14
A separate, single-center, retrospective trial produced different results. This study examined a population of 253 patients who underwent 266 EUS-FNA of pancreatic cysts and found that prophylactic antibiotics were associated with more adverse events and were not protective for the 3% of the patients with infectious symptoms.15 Despite the conflicting data, guidelines at this time recommend prophylactic antibiotics before drainage of a sterile pancreatic fluid collection that communicates with the pancreatic duct and also for aspiration of cystic lesions along the GI tract and the mediastinum.2
Endoscopic Retrograde Cholangiopancreatography (ERCP). In patients undergoing ERCP, the routine use of prophylactic antibiotics has not been found to be effective in decreasing the risk of post-procedure cholangitis.16 Guidelines recommend the use of prophylactic antibiotics only in those patients in which the ERCP may not completely resolve the biliary obstruction.2 In these patients, the thought is that ERCP can precipitate infection by disturbing bacteria already present in the biliary tree, especially with increased intrabiliary pressure at the time of contrast dye injection.17
Patients with incomplete biliary drainage, including those with primary sclerosing cholangitis (PSC), hilar cholangiocarcinoma, persistent biliary that were not extracted, and strictures that continue to obstruct despite attempted intervention, are thought to be at elevated risk of developing cholangitis post-ERCP. These patients should be placed on prophylactic antibiotics at the time of the procedure to cover biliary flora such as enteric gram negatives and enterococci. Antibiotics should be continued until the biliary obstruction is resolved.2
Additional Populations to Consider. Previously, the International Society for Peritoneal Dialysis recommended that patients on peritoneal dialysis receive prophylactic antibiotics and empty their abdomen of dialysate prior to colonoscopy. This recommendation has been removed from the 2010 guidelines.18 There is also no indication that patients with synthetic vascular grafts or cardiac devices should receive prophylactic antibiotics prior to routine GI endoscopy.19 The American Academy of Orthopaedic Surgeons no longer recommends that patients with joint replacements receive antibiotic prophylaxis prior to GI endoscopy.20
Back to the Case
The older gentleman with a prosthetic valve undergoing colonoscopy should not receive prophylactic antibiotics, because even in the setting of valvulopathy, colonoscopy does not pose a significant risk for infective endocarditis. The young patient with severe choledocholithiasis should be placed on prophylactic antibiotics because she has continued biliary obstruction, which could result in a cholangitis after ERCP.
Bottom Line
Prophylactic antibiotics are not recommended for any patient undergoing routine endoscopy or colonoscopy. They are indicated for patients with bleeding esophageal varices and for patients who undergo esophageal stricture dilation, PEG placement, or pseudocyst or cyst drainage, and those with continued biliary obstruction undergoing ERCP as summarized in Table 1.
Drs. Ritter, Jupiter, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center and Harvard Medical School faculty in Boston.
References
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754.
- Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791-798.
- Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58(6):869-880.
- Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57(4):546-556.
- Zuccaro G Jr., Richter JE, Rice TW, et al. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest Endosc. 1998;48(6):568-573.
- Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest Endosc.1998;48(6):563-567.
- Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58(4):475-482.
- Ho H, Zuckerman MJ, Wassem C. A prospective controlled study of the risk of bacteremia in emergency sclerotherapy of esophageal varices. Gastroenterology. 1991;101(6):1642-1648.
- Rolando N, Gimson A, Philpott-Howard J, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: Role of antibiotic prophylaxis. J Hepatol. 1993;18(3):290-294.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-938.
- Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: Antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25(6):647-656.
- Chuang CH, Hung KH, Chen JR, et al. Airway infection predisposes to peristomal infection after percutaneous endoscopic gastrostomy with high concordance between sputum and wound isolates. J Gastrointest Surg. 2010;14(1):45-51.
- Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. PEG site infections: The emergence of methicillin resistant Staphylococcus aureus as a major pathogen. Am J Gastroenterol. 2002;97(7):1713-1716.
- Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73(2):283-290.
- Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: A retrospective, comparative analysis. Gastrointest Endosc. 2011;74(1):81-86.
- Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: A meta-analysis. Pancreas. 2009;38(2):126-130.
- Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: A sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67(3):471-475.
- Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393-423.
- Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015-2031.
- Rethman MP, Watters W III, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745-747.
Case
You are asked to admit two patients. The first is a 75-year-old male with a prosthetic aortic valve on warfarin who presents with bright red blood per rectum and is scheduled for colonoscopy. The second patient is a 35-year-old female with biliary obstruction due to choledocholithiasis; she is afebrile with normal vital signs and no leukocytosis. She underwent endoscopic retrograde cholangiopancreatography (ERCP), which did not resolve her biliary obstruction. Should you prescribe prophylactic antibiotics for either patient?
Overview
Providers are often confused regarding which patients undergoing gastrointestinal (GI) endoscopic procedures should receive antibiotic prophylaxis. To answer this question, it is important to understand the goal of prophylactic antibiotics. Are we trying to prevent infective endocarditis or a localized infection?
There are few large, prospective, randomized controlled trials that have examined the need for antibiotic prophylaxis with GI endoscopic procedures. Guidelines from professional societies are mainly based on expert opinion, evidence from retrospective case studies, and meta-analysis reviews.
Review of the Data
Infective endocarditis resulting from GI endoscopy has been a concern of physicians for decades. The American Heart Association (AHA) first published its recommendations for antibiotic prophylaxis of GI tract procedures in 1965. The most recent antibacterial prophylaxis guidelines, published in 2007, have simplified recommendations and greatly scaled back the indications for antibiotics. The new guidelines conclude that frequent bacteremia from daily activities is more likely to precipitate endocarditis than a single dental, GI, or genitourinary tract procedure.1
The American Society for Gastrointestinal Endoscopy (ASGE) reports that 14.2 million colonoscopies, 2.8 million flexible sigmoidoscopies, and nearly as many upper endoscopies are performed in the U.S. each year, but only 15 cases of endocarditis have been reported with a temporal association to a procedure.2
The British Society of Gastroenterology (BSG) found, after reviewing the histories of patients with infective endocarditis from 1983 through 2006, that there is not enough evidence to warrant antibiotic prophylaxis prior to endoscopy. They noted less than one case of endocarditis after GI endoscopy per year as well as significant variation in the time interval between the procedure and symptoms. The BSG also recognized that antibiotic prophylaxis does not always protect against infection and that clinical factors unrelated to the endoscopy may play a significant role in the development of endocarditis.3
Upper GI Endoscopy, Colonoscopy with Biopsy, and Esophageal Dilatation. Administering antibiotics to prevent infective endocarditis is not recommended for patients undergoing routine procedures such as endoscopy with biopsy and colonoscopy with polypectomy. Likewise, patients with a history of prosthetic heart valves, valve repair with prosthetic material, endocarditis, congenital heart disease, or cardiac transplant with valvulopathy do not need prophylactic antibiotics before GI endoscopic procedures. However, for patients who are being treated for an active GI infection, antibiotic coverage for enterococcus may be warranted given the increased risk of developing endocarditis. The AHA acknowledges there are no published studies to support the efficacy of antibiotics to prevent enterococcal endocarditis in patients in this clinical setting.1
Unlike routine endoscopy, esophageal dilation is associated with an increased rate of bacteremia (12%-100%).4 Streptococcus viridans has been found in blood cultures up to 79% of the time after esophageal dilation.5 Patients with malignant strictures have higher rates of bacteremia than those with benign strictures (52.9% versus 15.7%). Patients treated with multiple passes with the esophageal dilator compared to those treated with a single dilation have a higher risk of bacteremia.6 All patients undergoing esophageal stricture dilation should receive pre-procedural prophylactic antibiotics.7
Patients with bleeding esophageal varices also have high rates of bacteremia. Up to 20% of patients with cirrhosis and GI bleeding on admission develop an infection within 48 hours of presentation.8 There is evidence that the bacteremia may actually be related to the variceal bleeding rather than the procedure.9 Patients with bleeding esophageal varices treated with antibiotics have improved outcomes, including a decrease in mortality.10 Therefore, all patients with bleeding esophageal varices should be placed on antibiotic therapy regardless of whether an endoscopic intervention is planned.
Percutaneous Endoscopic Gastrostomy (PEG) Placement. Prophylactic antibiotics are recommended before placement of a PEG. The indication for prophylactic antibiotics is to prevent a gastrostomy site infection, not infective endocarditis. Gastrostomy site infection is unfortunately a fairly common infection, affecting 4% to 30% of patients who undergo PEG tube placement. There is significant evidence that antibiotics are beneficial in preventing peristomal infections. A meta-analysis showed that only eight patients need to be treated with prophylactic antibiotics to prevent a single peristomal infection.11 Since these infections are believed to be caused by contamination from the oropharynx, physicians should consider prophylaxis against pathogens from the oral flora.12
More recently, it has been noted that methicillin-resistant Staphylococcus aureus (MRSA) is increasingly cultured from infection sites.13 In centers with endemic MRSA, patients should be screened and then undergo decontamination prior to the PEG placement in positive cases.
Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA). Antibiotic prophylaxis before EUS-FNA of a solid lesion in an organ is generally thought to be unnecessary because the risk of bacteremia with this procedure is low, comparable to routine GI endoscopy with biopsy. The recommendation for prophylactic antibiotics before biopsy of a cystic lesion is different. There is concern that puncturing cystic lesions may create a new infected fluid collection.2 A systematic review of more than 10,000 patients undergoing EUS-FNA with a full range of target organs revealed that, overall, 11.2% of patients experienced a fever and 4.7% of patients had a peri-procedural infection. While it was not possible in this study to determine which patients received prophylactic antibiotics, 93.7% of patients with pancreatic cystic lesions were reported to have been treated with antibiotics.14
A separate, single-center, retrospective trial produced different results. This study examined a population of 253 patients who underwent 266 EUS-FNA of pancreatic cysts and found that prophylactic antibiotics were associated with more adverse events and were not protective for the 3% of the patients with infectious symptoms.15 Despite the conflicting data, guidelines at this time recommend prophylactic antibiotics before drainage of a sterile pancreatic fluid collection that communicates with the pancreatic duct and also for aspiration of cystic lesions along the GI tract and the mediastinum.2
Endoscopic Retrograde Cholangiopancreatography (ERCP). In patients undergoing ERCP, the routine use of prophylactic antibiotics has not been found to be effective in decreasing the risk of post-procedure cholangitis.16 Guidelines recommend the use of prophylactic antibiotics only in those patients in which the ERCP may not completely resolve the biliary obstruction.2 In these patients, the thought is that ERCP can precipitate infection by disturbing bacteria already present in the biliary tree, especially with increased intrabiliary pressure at the time of contrast dye injection.17
Patients with incomplete biliary drainage, including those with primary sclerosing cholangitis (PSC), hilar cholangiocarcinoma, persistent biliary that were not extracted, and strictures that continue to obstruct despite attempted intervention, are thought to be at elevated risk of developing cholangitis post-ERCP. These patients should be placed on prophylactic antibiotics at the time of the procedure to cover biliary flora such as enteric gram negatives and enterococci. Antibiotics should be continued until the biliary obstruction is resolved.2
Additional Populations to Consider. Previously, the International Society for Peritoneal Dialysis recommended that patients on peritoneal dialysis receive prophylactic antibiotics and empty their abdomen of dialysate prior to colonoscopy. This recommendation has been removed from the 2010 guidelines.18 There is also no indication that patients with synthetic vascular grafts or cardiac devices should receive prophylactic antibiotics prior to routine GI endoscopy.19 The American Academy of Orthopaedic Surgeons no longer recommends that patients with joint replacements receive antibiotic prophylaxis prior to GI endoscopy.20
Back to the Case
The older gentleman with a prosthetic valve undergoing colonoscopy should not receive prophylactic antibiotics, because even in the setting of valvulopathy, colonoscopy does not pose a significant risk for infective endocarditis. The young patient with severe choledocholithiasis should be placed on prophylactic antibiotics because she has continued biliary obstruction, which could result in a cholangitis after ERCP.
Bottom Line
Prophylactic antibiotics are not recommended for any patient undergoing routine endoscopy or colonoscopy. They are indicated for patients with bleeding esophageal varices and for patients who undergo esophageal stricture dilation, PEG placement, or pseudocyst or cyst drainage, and those with continued biliary obstruction undergoing ERCP as summarized in Table 1.
Drs. Ritter, Jupiter, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center and Harvard Medical School faculty in Boston.
References
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754.
- Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791-798.
- Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58(6):869-880.
- Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57(4):546-556.
- Zuccaro G Jr., Richter JE, Rice TW, et al. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest Endosc. 1998;48(6):568-573.
- Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest Endosc.1998;48(6):563-567.
- Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58(4):475-482.
- Ho H, Zuckerman MJ, Wassem C. A prospective controlled study of the risk of bacteremia in emergency sclerotherapy of esophageal varices. Gastroenterology. 1991;101(6):1642-1648.
- Rolando N, Gimson A, Philpott-Howard J, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: Role of antibiotic prophylaxis. J Hepatol. 1993;18(3):290-294.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-938.
- Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: Antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25(6):647-656.
- Chuang CH, Hung KH, Chen JR, et al. Airway infection predisposes to peristomal infection after percutaneous endoscopic gastrostomy with high concordance between sputum and wound isolates. J Gastrointest Surg. 2010;14(1):45-51.
- Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. PEG site infections: The emergence of methicillin resistant Staphylococcus aureus as a major pathogen. Am J Gastroenterol. 2002;97(7):1713-1716.
- Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73(2):283-290.
- Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: A retrospective, comparative analysis. Gastrointest Endosc. 2011;74(1):81-86.
- Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: A meta-analysis. Pancreas. 2009;38(2):126-130.
- Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: A sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67(3):471-475.
- Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393-423.
- Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015-2031.
- Rethman MP, Watters W III, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745-747.
CDC Identifies Greatest Antibiotic Resistance Threats of Next Decade in U.S.
Clinical question: What antibiotic-resistant bacteria are the greatest threats for the next 10 years?
Background: Two million people suffer antibiotic-resistant infections yearly, and 23,000 die each year as a result. Most of these infections occur in the community, but deaths usually occur in healthcare settings. Cost estimates vary but may be as high as $20 billion in excess direct healthcare costs.
Study design: The CDC used several different surveys and databanks, including the National Antimicrobial Resistance Monitoring System, to collect data. The threat level for antibiotic-resistant bacteria was determined using several factors: clinical impact, economic impact, incidence, 10-year projection of incidence, transmissibility, availability of effective antibiotics, and barriers to prevention.
Setting: United States.
Synopsis: The CDC has three classifications of antibiotic-resistant bacteria: urgent, serious, and concerning. Urgent threats are high-consequence, antibiotic-resistant threats because of significant risks identified across several criteria. These threats might not currently be widespread but have the potential to become so and require urgent public health attention to identify infections and to limit transmission. They include carbapenem-resistant Enterobacteriaceae, drug-resistant Neisseria gonorrhoeae, and Clostridium difficile (does not have true resistance, but is a consequence of antibiotic overuse).
Serious threats are significant antibiotic-resistant threats. These threats will worsen, and might become urgent without ongoing public health monitoring and prevention activities. They include multidrug-resistant Acinetobacter, drug-resistant Campylobacter, fluconazole-resistant Candida (a fungus), extended-spectrum ß-lactamase-producing Enterobacteriaceae, vancomycin-resistant Enterococcus, multidrug-resistant Pseudomonas aeruginosa, drug-resistant non-typhoidal Salmonella, drug-resistant Salmonella Typhimurium, drug-resistant Shigella, methicillin-resistant Staphylococcus aureus, drug-resistant Streptococcus pneumonia, and drug-resistant tuberculosis.
Concerning threats are bacteria for which the threat of antibiotic resistance is low, and/or there are multiple therapeutic options for resistant infections. These bacterial pathogens cause severe illness.
Threats in this category require monitoring and, in some cases, rapid incident or outbreak response. These include vancomycin-resistant Staphylococcus aureus, erythromycin-resistant Group A Streptococcus, and clindamycin-resistant Group B Streptococcus.
Research has shown patients with resistant infections have significantly longer hospital stays, delayed recuperation, long-term disability, and higher mortality. As resistance to current antibiotics occurs, providers are forced to use antibiotics that are more toxic, more expensive, and less effective.
The CDC recommends four core actions to fight antibiotic resistance:
- Preventing infections from occurring and preventing resistant bacteria from spreading (immunization, infection control, screening, treatment, and education);
- Tracking resistant bacteria;
- Improving the use of antibiotics (antibiotic stewardship); and
- Promoting the development of new antibiotics and new diagnostic tests for resistant bacteria.
Bottom line: Antibiotics are a limited resource. The more antibiotics are used today, the less likely they will continue to be effective in the future. The CDC lists 18 antibiotic-resistant organisms as urgent, serious, or concerning and recommends actions to combat the spread of current organisms and emergence of new antibiotic organisms.
Citation: Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. CDC website. September 16, 2013. Available at: www.cdc.gov/drugresistance/threat-report-2013. Accessed Nov. 30, 2013.
Clinical question: What antibiotic-resistant bacteria are the greatest threats for the next 10 years?
Background: Two million people suffer antibiotic-resistant infections yearly, and 23,000 die each year as a result. Most of these infections occur in the community, but deaths usually occur in healthcare settings. Cost estimates vary but may be as high as $20 billion in excess direct healthcare costs.
Study design: The CDC used several different surveys and databanks, including the National Antimicrobial Resistance Monitoring System, to collect data. The threat level for antibiotic-resistant bacteria was determined using several factors: clinical impact, economic impact, incidence, 10-year projection of incidence, transmissibility, availability of effective antibiotics, and barriers to prevention.
Setting: United States.
Synopsis: The CDC has three classifications of antibiotic-resistant bacteria: urgent, serious, and concerning. Urgent threats are high-consequence, antibiotic-resistant threats because of significant risks identified across several criteria. These threats might not currently be widespread but have the potential to become so and require urgent public health attention to identify infections and to limit transmission. They include carbapenem-resistant Enterobacteriaceae, drug-resistant Neisseria gonorrhoeae, and Clostridium difficile (does not have true resistance, but is a consequence of antibiotic overuse).
Serious threats are significant antibiotic-resistant threats. These threats will worsen, and might become urgent without ongoing public health monitoring and prevention activities. They include multidrug-resistant Acinetobacter, drug-resistant Campylobacter, fluconazole-resistant Candida (a fungus), extended-spectrum ß-lactamase-producing Enterobacteriaceae, vancomycin-resistant Enterococcus, multidrug-resistant Pseudomonas aeruginosa, drug-resistant non-typhoidal Salmonella, drug-resistant Salmonella Typhimurium, drug-resistant Shigella, methicillin-resistant Staphylococcus aureus, drug-resistant Streptococcus pneumonia, and drug-resistant tuberculosis.
Concerning threats are bacteria for which the threat of antibiotic resistance is low, and/or there are multiple therapeutic options for resistant infections. These bacterial pathogens cause severe illness.
Threats in this category require monitoring and, in some cases, rapid incident or outbreak response. These include vancomycin-resistant Staphylococcus aureus, erythromycin-resistant Group A Streptococcus, and clindamycin-resistant Group B Streptococcus.
Research has shown patients with resistant infections have significantly longer hospital stays, delayed recuperation, long-term disability, and higher mortality. As resistance to current antibiotics occurs, providers are forced to use antibiotics that are more toxic, more expensive, and less effective.
The CDC recommends four core actions to fight antibiotic resistance:
- Preventing infections from occurring and preventing resistant bacteria from spreading (immunization, infection control, screening, treatment, and education);
- Tracking resistant bacteria;
- Improving the use of antibiotics (antibiotic stewardship); and
- Promoting the development of new antibiotics and new diagnostic tests for resistant bacteria.
Bottom line: Antibiotics are a limited resource. The more antibiotics are used today, the less likely they will continue to be effective in the future. The CDC lists 18 antibiotic-resistant organisms as urgent, serious, or concerning and recommends actions to combat the spread of current organisms and emergence of new antibiotic organisms.
Citation: Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. CDC website. September 16, 2013. Available at: www.cdc.gov/drugresistance/threat-report-2013. Accessed Nov. 30, 2013.
Clinical question: What antibiotic-resistant bacteria are the greatest threats for the next 10 years?
Background: Two million people suffer antibiotic-resistant infections yearly, and 23,000 die each year as a result. Most of these infections occur in the community, but deaths usually occur in healthcare settings. Cost estimates vary but may be as high as $20 billion in excess direct healthcare costs.
Study design: The CDC used several different surveys and databanks, including the National Antimicrobial Resistance Monitoring System, to collect data. The threat level for antibiotic-resistant bacteria was determined using several factors: clinical impact, economic impact, incidence, 10-year projection of incidence, transmissibility, availability of effective antibiotics, and barriers to prevention.
Setting: United States.
Synopsis: The CDC has three classifications of antibiotic-resistant bacteria: urgent, serious, and concerning. Urgent threats are high-consequence, antibiotic-resistant threats because of significant risks identified across several criteria. These threats might not currently be widespread but have the potential to become so and require urgent public health attention to identify infections and to limit transmission. They include carbapenem-resistant Enterobacteriaceae, drug-resistant Neisseria gonorrhoeae, and Clostridium difficile (does not have true resistance, but is a consequence of antibiotic overuse).
Serious threats are significant antibiotic-resistant threats. These threats will worsen, and might become urgent without ongoing public health monitoring and prevention activities. They include multidrug-resistant Acinetobacter, drug-resistant Campylobacter, fluconazole-resistant Candida (a fungus), extended-spectrum ß-lactamase-producing Enterobacteriaceae, vancomycin-resistant Enterococcus, multidrug-resistant Pseudomonas aeruginosa, drug-resistant non-typhoidal Salmonella, drug-resistant Salmonella Typhimurium, drug-resistant Shigella, methicillin-resistant Staphylococcus aureus, drug-resistant Streptococcus pneumonia, and drug-resistant tuberculosis.
Concerning threats are bacteria for which the threat of antibiotic resistance is low, and/or there are multiple therapeutic options for resistant infections. These bacterial pathogens cause severe illness.
Threats in this category require monitoring and, in some cases, rapid incident or outbreak response. These include vancomycin-resistant Staphylococcus aureus, erythromycin-resistant Group A Streptococcus, and clindamycin-resistant Group B Streptococcus.
Research has shown patients with resistant infections have significantly longer hospital stays, delayed recuperation, long-term disability, and higher mortality. As resistance to current antibiotics occurs, providers are forced to use antibiotics that are more toxic, more expensive, and less effective.
The CDC recommends four core actions to fight antibiotic resistance:
- Preventing infections from occurring and preventing resistant bacteria from spreading (immunization, infection control, screening, treatment, and education);
- Tracking resistant bacteria;
- Improving the use of antibiotics (antibiotic stewardship); and
- Promoting the development of new antibiotics and new diagnostic tests for resistant bacteria.
Bottom line: Antibiotics are a limited resource. The more antibiotics are used today, the less likely they will continue to be effective in the future. The CDC lists 18 antibiotic-resistant organisms as urgent, serious, or concerning and recommends actions to combat the spread of current organisms and emergence of new antibiotic organisms.
Citation: Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. CDC website. September 16, 2013. Available at: www.cdc.gov/drugresistance/threat-report-2013. Accessed Nov. 30, 2013.
Hospitalist Reviews of New Research on Antibiotic-Resistant Bacteria, Pressure Ulcers, Severe Alcoholic Hepatitis, and More
In This Edition
Literature At A Glance
A guide to this month’s studies
- Antibiotic resistance threats in the United States
- Turning for ulcer reduction: A multi-site, randomized, clinical trial in nursing homes
- Prednisolone with or without pentoxfylline, and survival of patients with severe alcoholic hepatitis
- Characteristics and impact of a hospitalist-staffed, post-discharge clinic
- Higher continuity of care results in lower rate of preventable hospitalizations
- Variation in surgical readmission rates depends on volume, mortality rates
- Patients prefer inpatient boarding to ED boarding
Antibiotic Resistance Threats in the United States, 2013
Clinical question: What antibiotic-resistant bacteria are the greatest threats for the next 10 years?
Background: Two million people suffer antibiotic-resistant infections yearly, and 23,000 die each year as a result. Most of these infections occur in the community, but deaths usually occur in healthcare settings. Cost estimates vary but may be as high as $20 billion in excess direct healthcare costs.
Study design: The CDC used several different surveys and databanks, including the National Antimicrobial Resistance Monitoring System, to collect data. The threat level for antibiotic-resistant bacteria was determined using several factors: clinical impact, economic impact, incidence, 10-year projection of incidence, transmissibility, availability of effective antibiotics, and barriers to prevention.
Setting: United States.
Synopsis: The CDC has three classifications of antibiotic-resistant bacteria: urgent, serious, and concerning. Urgent threats are high-consequence, antibiotic-resistant threats because of significant risks identified across several criteria. These threats might not currently be widespread but have the potential to become so and require urgent public health attention to identify infections and to limit transmission. They include carbapenem-resistant Enterobacteriaceae, drug-resistant Neisseria gonorrhoeae, and Clostridium difficile (does not have true resistance, but is a consequence of antibiotic overuse).
Serious threats are significant antibiotic-resistant threats. These threats will worsen and might become urgent without ongoing public health monitoring and prevention activities. They include multidrug-resistant Acinetobacter, drug-resistant Campylobacter, fluconazole-resistant Candida (a fungus), extended-spectrum β-lactamase-producing Enterobacteriaceae, vancomycin-resistant Enterococcus, multidrug-resistant Pseudomonas aeruginosa, drug-resistant non-typhoidal Salmonella, drug-resistant Salmonella Typhimurium, drug-resistant Shigella, methicillin-resistant Staphylococcus aureus, drug-resistant Streptococcus pneumonia, and drug-resistant tuberculosis.
Concerning threats are bacteria for which the threat of antibiotic resistance is low, and/ or there are multiple therapeutic options for resistant infections. These bacterial pathogens cause severe illness. Threats in this category require monitoring and, in some cases, rapid incident or outbreak response. These include vancomycin-resistant Staphylococcus aureus, erythromycin-resistant Group A Streptococcus, and clindamycin-resistant Group B Streptococcus. Research has shown patients with resistant infections have significantly longer hospital stays, delayed recuperation, long-term disability, and higher mortality. As resistance to current antibiotics occurs, providers are forced to use antibiotics that are more toxic, more expensive, and less effective.
The CDC recommends four core actions to fight antibiotic resistance:
- Preventing infections from occurring and preventing resistant bacteria from spreading (immunization, infection control, screening, treatment, and education);
- Tracking resistant bacteria;
- Improving the use of antibiotics (antibiotic stewardship); and
- Promoting the development of new antibiotics and new diagnostic tests for resistant bacteria.
Bottom line: Antibiotics are a limited resource. The more antibiotics are used today, the less likely they will continue to be effective in the future. The CDC lists 18 antibiotic-resistant organisms as urgent, serious, or concerning and recommends actions to combat the spread of current organisms and emergence of new antibiotic organisms.
Citation: Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. CDC website. September 16, 2013. Available at: www.cdc.gov/drugresistance/threat-report-2013. Accessed Nov. 30, 2013.
Turning for Ulcer Reduction: A Multi-Site Randomized Clinical Trial in Nursing Homes
Clinical question: Is there a difference between repositioning intervals of two, three, or four hours in pressure ulcer formation in nursing home residents on high-density foam mattresses?
Background: Pressure ulcer formation in nursing home residents is a common problem. Current standard of care requires repositioning every two hours in patients who are at risk for pressure ulcer formation. Few studies have been performed to assess a difference in repositioning interval. This study was conducted to see if there is a difference in pressure ulcer formation among residents on high-density foam mattresses at moderate to high risk (according to the Braden scale).
Study design: Multi-site, randomized, clinical trial.
Setting: Twenty U.S. and seven Canadian nursing homes using high-density foam mattresses.
Synopsis: A multi-site, randomized clinical trial was executed in 20 U.S. and seven Canadian nursing homes. More than 900 residents were randomized to two-, three-, or four-hour intervals for repositioning. All participants were at either moderate (13-14) or high (10-12) risk on the Braden scale for pressure ulcer formation. All facilities used high-density foam mattresses. All participants were monitored for pressure ulcer formation on the sacrum/coccyx, heel, or trochanter for three consecutive weeks.
There was no significant difference in pressure ulcer formation between the two-, three-, or four-hour interval repositioning groups. There was no significant difference in pressure ulcer formation between the moderate or high-risk groups. Only 2% of participants developed a pressure ulcer, all stage I or II.
It is not clear if the outcomes were purely related to the repositioning intervals, as this study group had a much lower rate of pressure ulcer formation compared to national averages and previous studies. The high-density foam mattress might have improved outcomes by evenly redistributing pressure so that less frequent repositioning was required. The level of documentation may have led to earlier recognition of early stage pressure ulcers as well. This study also was limited to nursing home residents at moderate to high risk of pressure ulcer development.
Bottom line: There is no significant difference in pressure ulcer formation between repositioning intervals of two, three, or four hours among moderate and high-risk nursing home residents using high-density foam mattresses.
Citation: Bergstrom N, Horn SD, Rapp MP, Stern A, Barrett R, Watkiss M. Turning for ulcer reduction: a multisite randomized clinical trial in nursing homes. 2013;61(10):1705-1713.
Prednisolone, Pentoxifylline, and Survival of Patients with Severe Alcoholic Hepatitis
Clinical question: Does the addition of pentoxifylline to prednisolone improve six-month mortality compared to prednisolone alone in patients with severe alcoholic hepatitis?
Background: Prednisolone improves liver function and reduces inflammation in patients with alcoholic hepatitis. Pentoxifylline appears to have a protective effect against hepatorenal syndrome in patients with severe alcoholic hepatitis. The medications have different mechanisms of action; therefore, the researchers hypothesized that the combination of medication would improve outcomes.
Study design: Multi-center, randomized, double-blinded clinical trial.
Setting: One Belgian and 23 French hospitals, from December 2007 to October 2010.
Synopsis: This study randomized 270 patients to receive either prednisolone and pentoxifylline or prednisolone and placebo for 28 days. Acute alcoholic hepatitis was defined by a positive biopsy, onset of jaundice three months prior to the study, and a Maddrey’s discriminant function score of >32. All patients were assessed for response to treatment using the Lille model at seven days of treatment, occurrence of hepatorenal syndrome, and survival at six months.
Results showed no significant difference in treatment response, alcohol relapse, death, time to death, or occurrence of hepatorenal syndrome between the two treatment groups; however, there were fewer episodes of hepatorenal syndrome in the pentoxifylline group.
Patients considered responders by the Lille model and those with lower Model for End-Stage Liver Disease scores had improved mortality. Patients treated with pentoxifylline had lower rates of hepatorenal syndrome at one month but no difference by six months. Patients with a lower Lille score had significantly less incidence of hepatorenal syndrome. The study may be underpowered to accurately determine outcomes other than six-month survival.
Bottom line: Adding pentoxifylline to prednisolone does not improve six-month survival in severe alcoholic hepatitis compared to prednisolone alone.
Citation: Mathurin P, Louvet A, Duhamel A, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. 2013;310(10):1033-1041.
Characteristics and Impact of Hospitalist-Staffed, Post-Discharge Clinic
Clinical question: What effect does a hospitalist-staffed, post-discharge clinic have on time to first post-hospitalization visit?
Background: Hospital discharge is a well-recognized care transition that can leave patients vulnerable to morbidity and re-hospitalization. Limited primary care access can hamper complex post-hospital follow-up. Discharge clinic models staffed by hospitalists have been developed to mitigate access issues, but research is lacking to describe their characteristics and benefits.
Study design: Single-center, prospective, observational database review.
Setting: Large, academic primary care practice affiliated with an academic medical center.
Synopsis: Between 2009 and 2011, this hospitalist-staffed, post-discharge clinic saw 596 patients, while the affiliated, large primary care practice saw 10,839 patients. Patients utilizing the hospitalist discharge clinic were more likely to be black (39% vs. 29%, <0.001) and to receive primary care from resident clinics (40% vs. 21%, <0.001). The median duration from hospital discharge to the first clinic visit was shorter for the post-discharge clinic (8.45 ± 0.43 days, <0.001).
The number of radiology and laboratory tests performed at the first post-discharge clinic visit showed similar patterns between the hospitalist discharge clinic and the primary care practice. Study design and size did not permit comparisons of readmission rates or mortality from time of discharge and also precluded evaluation of interventions on discharge-related medication errors or response time to outstanding test results.
Bottom line: A hospitalist-staffed, post-discharge clinic was associated with shorter time to first post-discharge visit, especially for patients who are black and receive primary care from resident clinics.
Citation: Doctoroff L, Nijhawan A, McNally D, Vanka A, Yu R, Mukamal KJ. The characteristics and impact of a hospitalist-staffed post-discharge clinic. 2013;126(11):1016.e9-1016.e15.
Higher Continuity of Care Results in Lower Rate of Preventable Hospitalizations
Clinical question: Is continuity of care related to preventable hospitalizations among older adults?
Background: Preventable hospitalizations cost approximately $25 billion annually in the U.S. The relationship between continuity of care and the risk of preventable hospitalization is unknown.
Study design: Retrospective cohort study.
Setting: Random sample of fee-for-service Medicare beneficiaries, for ambulatory visits and hospital admissions.
Synopsis: This study examined 3.2 million Medicare beneficiaries using 2008-2010 claims data to measure continuity and the first preventable hospitalization. The Prevention Quality Indicators definitions and technical specifications from the Agency for Healthcare Research and Quality were used to identify preventable hospitalizations. Both the continuity of care score and usual provider continuity score were used to calculate continuity metrics. Baseline risk of preventable hospitalization included age, sex, race, Medicaid dual-eligible status, and residential zip code.
During a two-year period, 12.6% of patients had a preventable hospitalization. After adjusting for variables, a 0.1 increase in continuity of care was associated with about a 2% lower rate of preventable hospitalization. Interestingly, continuity of care was not related to mortality rates.
This study extends prior research associating continuity of care with reduced rate of hospitalization; however, the associations found cannot assert a causal relationship. This study used coding practices that vary throughout the country, included only older fee-for-service Medicare beneficiaries, and could not verify why some patients had higher continuity of care. The authors suggest that efforts to strengthen physician-patient relationships through high-quality primary care will deter some hospital admissions.
Bottom line: Higher continuity of ambulatory care is associated with lower preventable hospitalizations in Medicare beneficiaries.
Citation: Nyweide DJ, Anthony DL, Bynum JP, et al. Continuity of care and the risk of preventable hospitalization in older adults. 2013;173(20):1879-1885.
Surgical Readmission Rate Variation Dependent on Surgical Volume, Surgical Mortality Rates
Clinical question: What factors determine rates of readmission after major surgery?
Background: Reducing hospital readmission rates has become a national priority. The U.S. patterns for surgical readmissions are unknown, as are the specific structural and quality characteristics of hospitals associated with lower surgical readmission rates.
Study design: Retrospective study of national Medicare data was used to calculate 30-day readmission rates for six major surgical procedures.
Setting: U.S. Hospitals, 2009-2010.
Synopsis: Six major surgical procedures were tracked by Medicare data, with 479,471 discharges from 3,004 hospitals. Structural characteristics included hospital size, teaching status, region, ownership, and proportion of patients living below the federal poverty line. Three well-established measures of surgical quality were used: the HQA surgical score, procedure volume, and 30-day mortality.
Hospitals in the highest quartile for surgical volume had a significantly lower readmission rate. Additionally, hospitals with the lowest surgical mortality rates had significantly lower readmission rates. Interestingly, high adherence to reported surgical process measures was only marginally associated with reduced admission rates. Prior studies have also shown inconsistent relationship between HQA surgical score and mortality.
Limitations to this study include inability to account for factors not captured by billing codes and the focus on a Medicare population.
Bottom line: Surgical readmission rates are associated with measures of surgical quality, specifically procedural volume and mortality.
Citation: Tsai TC, Joynt KE, Orav EJ, Gawande AA, Jha AK. Variation in surgical-readmission rates and quality of hospital care. 2013;369(12):1134-1142.
Patients Overwhelmingly Prefer Inpatient Boarding to ED Boarding
Clinical question: When hallway boarding is required, do patients prefer inpatient units over the ED?
Background: ED crowding is associated with patient dissatisfaction, ambulance diversion, delays in care, medical errors, and higher mortality rates. Strategies to alleviate the problem of boarding admitted patients in the ED can include relocation to inpatient hallways while awaiting a regular hospital bed. Traditional objections to inpatient hallway boarding include concerns regarding patient satisfaction and safety.
Study design: Structured telephone survey.
Setting: Suburban, university-based, teaching hospital.
Synopsis: Patients who required boarding in the ED hallway after hospital admission were eligible for inpatient hallway boarding according to the institutional protocol, which screens for those with only mild to moderate comorbidities. Of 110 consecutive patients contacted who experienced both ED and inpatient hallway boarding, 105 consented to participate in a tested telephone survey instrument.
The overall preferred location was inpatient hallways for 85% (95% CI 75-90) of respondents. Comparing ED boarding to inpatient hallway boarding, respondents preferred inpatient boarding with regard to staff availability (84%), safety (83%), confidentiality (82%), and comfort (79%).
Study results were subject to non-response bias, because working telephone numbers were required for study inclusion, as well as recall bias, because the survey was conducted within several months after discharge. This study’s results are based on actual patient experiences, whereas prior literature relied on patients to hypothesize the preferred environment after experiencing only ED hallway boarding to predict satisfaction.
Bottom line: Boarding in inpatient hallways was associated with higher patient satisfaction compared with ED hallway boarding.
Citation: Viccellio P, Zito JA, Sayage V, et al. Patients overwhelmingly prefer inpatient boarding to emergency department boarding [published online ahead of print September 21, 2013].
In This Edition
Literature At A Glance
A guide to this month’s studies
- Antibiotic resistance threats in the United States
- Turning for ulcer reduction: A multi-site, randomized, clinical trial in nursing homes
- Prednisolone with or without pentoxfylline, and survival of patients with severe alcoholic hepatitis
- Characteristics and impact of a hospitalist-staffed, post-discharge clinic
- Higher continuity of care results in lower rate of preventable hospitalizations
- Variation in surgical readmission rates depends on volume, mortality rates
- Patients prefer inpatient boarding to ED boarding
Antibiotic Resistance Threats in the United States, 2013
Clinical question: What antibiotic-resistant bacteria are the greatest threats for the next 10 years?
Background: Two million people suffer antibiotic-resistant infections yearly, and 23,000 die each year as a result. Most of these infections occur in the community, but deaths usually occur in healthcare settings. Cost estimates vary but may be as high as $20 billion in excess direct healthcare costs.
Study design: The CDC used several different surveys and databanks, including the National Antimicrobial Resistance Monitoring System, to collect data. The threat level for antibiotic-resistant bacteria was determined using several factors: clinical impact, economic impact, incidence, 10-year projection of incidence, transmissibility, availability of effective antibiotics, and barriers to prevention.
Setting: United States.
Synopsis: The CDC has three classifications of antibiotic-resistant bacteria: urgent, serious, and concerning. Urgent threats are high-consequence, antibiotic-resistant threats because of significant risks identified across several criteria. These threats might not currently be widespread but have the potential to become so and require urgent public health attention to identify infections and to limit transmission. They include carbapenem-resistant Enterobacteriaceae, drug-resistant Neisseria gonorrhoeae, and Clostridium difficile (does not have true resistance, but is a consequence of antibiotic overuse).
Serious threats are significant antibiotic-resistant threats. These threats will worsen and might become urgent without ongoing public health monitoring and prevention activities. They include multidrug-resistant Acinetobacter, drug-resistant Campylobacter, fluconazole-resistant Candida (a fungus), extended-spectrum β-lactamase-producing Enterobacteriaceae, vancomycin-resistant Enterococcus, multidrug-resistant Pseudomonas aeruginosa, drug-resistant non-typhoidal Salmonella, drug-resistant Salmonella Typhimurium, drug-resistant Shigella, methicillin-resistant Staphylococcus aureus, drug-resistant Streptococcus pneumonia, and drug-resistant tuberculosis.
Concerning threats are bacteria for which the threat of antibiotic resistance is low, and/ or there are multiple therapeutic options for resistant infections. These bacterial pathogens cause severe illness. Threats in this category require monitoring and, in some cases, rapid incident or outbreak response. These include vancomycin-resistant Staphylococcus aureus, erythromycin-resistant Group A Streptococcus, and clindamycin-resistant Group B Streptococcus. Research has shown patients with resistant infections have significantly longer hospital stays, delayed recuperation, long-term disability, and higher mortality. As resistance to current antibiotics occurs, providers are forced to use antibiotics that are more toxic, more expensive, and less effective.
The CDC recommends four core actions to fight antibiotic resistance:
- Preventing infections from occurring and preventing resistant bacteria from spreading (immunization, infection control, screening, treatment, and education);
- Tracking resistant bacteria;
- Improving the use of antibiotics (antibiotic stewardship); and
- Promoting the development of new antibiotics and new diagnostic tests for resistant bacteria.
Bottom line: Antibiotics are a limited resource. The more antibiotics are used today, the less likely they will continue to be effective in the future. The CDC lists 18 antibiotic-resistant organisms as urgent, serious, or concerning and recommends actions to combat the spread of current organisms and emergence of new antibiotic organisms.
Citation: Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. CDC website. September 16, 2013. Available at: www.cdc.gov/drugresistance/threat-report-2013. Accessed Nov. 30, 2013.
Turning for Ulcer Reduction: A Multi-Site Randomized Clinical Trial in Nursing Homes
Clinical question: Is there a difference between repositioning intervals of two, three, or four hours in pressure ulcer formation in nursing home residents on high-density foam mattresses?
Background: Pressure ulcer formation in nursing home residents is a common problem. Current standard of care requires repositioning every two hours in patients who are at risk for pressure ulcer formation. Few studies have been performed to assess a difference in repositioning interval. This study was conducted to see if there is a difference in pressure ulcer formation among residents on high-density foam mattresses at moderate to high risk (according to the Braden scale).
Study design: Multi-site, randomized, clinical trial.
Setting: Twenty U.S. and seven Canadian nursing homes using high-density foam mattresses.
Synopsis: A multi-site, randomized clinical trial was executed in 20 U.S. and seven Canadian nursing homes. More than 900 residents were randomized to two-, three-, or four-hour intervals for repositioning. All participants were at either moderate (13-14) or high (10-12) risk on the Braden scale for pressure ulcer formation. All facilities used high-density foam mattresses. All participants were monitored for pressure ulcer formation on the sacrum/coccyx, heel, or trochanter for three consecutive weeks.
There was no significant difference in pressure ulcer formation between the two-, three-, or four-hour interval repositioning groups. There was no significant difference in pressure ulcer formation between the moderate or high-risk groups. Only 2% of participants developed a pressure ulcer, all stage I or II.
It is not clear if the outcomes were purely related to the repositioning intervals, as this study group had a much lower rate of pressure ulcer formation compared to national averages and previous studies. The high-density foam mattress might have improved outcomes by evenly redistributing pressure so that less frequent repositioning was required. The level of documentation may have led to earlier recognition of early stage pressure ulcers as well. This study also was limited to nursing home residents at moderate to high risk of pressure ulcer development.
Bottom line: There is no significant difference in pressure ulcer formation between repositioning intervals of two, three, or four hours among moderate and high-risk nursing home residents using high-density foam mattresses.
Citation: Bergstrom N, Horn SD, Rapp MP, Stern A, Barrett R, Watkiss M. Turning for ulcer reduction: a multisite randomized clinical trial in nursing homes. 2013;61(10):1705-1713.
Prednisolone, Pentoxifylline, and Survival of Patients with Severe Alcoholic Hepatitis
Clinical question: Does the addition of pentoxifylline to prednisolone improve six-month mortality compared to prednisolone alone in patients with severe alcoholic hepatitis?
Background: Prednisolone improves liver function and reduces inflammation in patients with alcoholic hepatitis. Pentoxifylline appears to have a protective effect against hepatorenal syndrome in patients with severe alcoholic hepatitis. The medications have different mechanisms of action; therefore, the researchers hypothesized that the combination of medication would improve outcomes.
Study design: Multi-center, randomized, double-blinded clinical trial.
Setting: One Belgian and 23 French hospitals, from December 2007 to October 2010.
Synopsis: This study randomized 270 patients to receive either prednisolone and pentoxifylline or prednisolone and placebo for 28 days. Acute alcoholic hepatitis was defined by a positive biopsy, onset of jaundice three months prior to the study, and a Maddrey’s discriminant function score of >32. All patients were assessed for response to treatment using the Lille model at seven days of treatment, occurrence of hepatorenal syndrome, and survival at six months.
Results showed no significant difference in treatment response, alcohol relapse, death, time to death, or occurrence of hepatorenal syndrome between the two treatment groups; however, there were fewer episodes of hepatorenal syndrome in the pentoxifylline group.
Patients considered responders by the Lille model and those with lower Model for End-Stage Liver Disease scores had improved mortality. Patients treated with pentoxifylline had lower rates of hepatorenal syndrome at one month but no difference by six months. Patients with a lower Lille score had significantly less incidence of hepatorenal syndrome. The study may be underpowered to accurately determine outcomes other than six-month survival.
Bottom line: Adding pentoxifylline to prednisolone does not improve six-month survival in severe alcoholic hepatitis compared to prednisolone alone.
Citation: Mathurin P, Louvet A, Duhamel A, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. 2013;310(10):1033-1041.
Characteristics and Impact of Hospitalist-Staffed, Post-Discharge Clinic
Clinical question: What effect does a hospitalist-staffed, post-discharge clinic have on time to first post-hospitalization visit?
Background: Hospital discharge is a well-recognized care transition that can leave patients vulnerable to morbidity and re-hospitalization. Limited primary care access can hamper complex post-hospital follow-up. Discharge clinic models staffed by hospitalists have been developed to mitigate access issues, but research is lacking to describe their characteristics and benefits.
Study design: Single-center, prospective, observational database review.
Setting: Large, academic primary care practice affiliated with an academic medical center.
Synopsis: Between 2009 and 2011, this hospitalist-staffed, post-discharge clinic saw 596 patients, while the affiliated, large primary care practice saw 10,839 patients. Patients utilizing the hospitalist discharge clinic were more likely to be black (39% vs. 29%, <0.001) and to receive primary care from resident clinics (40% vs. 21%, <0.001). The median duration from hospital discharge to the first clinic visit was shorter for the post-discharge clinic (8.45 ± 0.43 days, <0.001).
The number of radiology and laboratory tests performed at the first post-discharge clinic visit showed similar patterns between the hospitalist discharge clinic and the primary care practice. Study design and size did not permit comparisons of readmission rates or mortality from time of discharge and also precluded evaluation of interventions on discharge-related medication errors or response time to outstanding test results.
Bottom line: A hospitalist-staffed, post-discharge clinic was associated with shorter time to first post-discharge visit, especially for patients who are black and receive primary care from resident clinics.
Citation: Doctoroff L, Nijhawan A, McNally D, Vanka A, Yu R, Mukamal KJ. The characteristics and impact of a hospitalist-staffed post-discharge clinic. 2013;126(11):1016.e9-1016.e15.
Higher Continuity of Care Results in Lower Rate of Preventable Hospitalizations
Clinical question: Is continuity of care related to preventable hospitalizations among older adults?
Background: Preventable hospitalizations cost approximately $25 billion annually in the U.S. The relationship between continuity of care and the risk of preventable hospitalization is unknown.
Study design: Retrospective cohort study.
Setting: Random sample of fee-for-service Medicare beneficiaries, for ambulatory visits and hospital admissions.
Synopsis: This study examined 3.2 million Medicare beneficiaries using 2008-2010 claims data to measure continuity and the first preventable hospitalization. The Prevention Quality Indicators definitions and technical specifications from the Agency for Healthcare Research and Quality were used to identify preventable hospitalizations. Both the continuity of care score and usual provider continuity score were used to calculate continuity metrics. Baseline risk of preventable hospitalization included age, sex, race, Medicaid dual-eligible status, and residential zip code.
During a two-year period, 12.6% of patients had a preventable hospitalization. After adjusting for variables, a 0.1 increase in continuity of care was associated with about a 2% lower rate of preventable hospitalization. Interestingly, continuity of care was not related to mortality rates.
This study extends prior research associating continuity of care with reduced rate of hospitalization; however, the associations found cannot assert a causal relationship. This study used coding practices that vary throughout the country, included only older fee-for-service Medicare beneficiaries, and could not verify why some patients had higher continuity of care. The authors suggest that efforts to strengthen physician-patient relationships through high-quality primary care will deter some hospital admissions.
Bottom line: Higher continuity of ambulatory care is associated with lower preventable hospitalizations in Medicare beneficiaries.
Citation: Nyweide DJ, Anthony DL, Bynum JP, et al. Continuity of care and the risk of preventable hospitalization in older adults. 2013;173(20):1879-1885.
Surgical Readmission Rate Variation Dependent on Surgical Volume, Surgical Mortality Rates
Clinical question: What factors determine rates of readmission after major surgery?
Background: Reducing hospital readmission rates has become a national priority. The U.S. patterns for surgical readmissions are unknown, as are the specific structural and quality characteristics of hospitals associated with lower surgical readmission rates.
Study design: Retrospective study of national Medicare data was used to calculate 30-day readmission rates for six major surgical procedures.
Setting: U.S. Hospitals, 2009-2010.
Synopsis: Six major surgical procedures were tracked by Medicare data, with 479,471 discharges from 3,004 hospitals. Structural characteristics included hospital size, teaching status, region, ownership, and proportion of patients living below the federal poverty line. Three well-established measures of surgical quality were used: the HQA surgical score, procedure volume, and 30-day mortality.
Hospitals in the highest quartile for surgical volume had a significantly lower readmission rate. Additionally, hospitals with the lowest surgical mortality rates had significantly lower readmission rates. Interestingly, high adherence to reported surgical process measures was only marginally associated with reduced admission rates. Prior studies have also shown inconsistent relationship between HQA surgical score and mortality.
Limitations to this study include inability to account for factors not captured by billing codes and the focus on a Medicare population.
Bottom line: Surgical readmission rates are associated with measures of surgical quality, specifically procedural volume and mortality.
Citation: Tsai TC, Joynt KE, Orav EJ, Gawande AA, Jha AK. Variation in surgical-readmission rates and quality of hospital care. 2013;369(12):1134-1142.
Patients Overwhelmingly Prefer Inpatient Boarding to ED Boarding
Clinical question: When hallway boarding is required, do patients prefer inpatient units over the ED?
Background: ED crowding is associated with patient dissatisfaction, ambulance diversion, delays in care, medical errors, and higher mortality rates. Strategies to alleviate the problem of boarding admitted patients in the ED can include relocation to inpatient hallways while awaiting a regular hospital bed. Traditional objections to inpatient hallway boarding include concerns regarding patient satisfaction and safety.
Study design: Structured telephone survey.
Setting: Suburban, university-based, teaching hospital.
Synopsis: Patients who required boarding in the ED hallway after hospital admission were eligible for inpatient hallway boarding according to the institutional protocol, which screens for those with only mild to moderate comorbidities. Of 110 consecutive patients contacted who experienced both ED and inpatient hallway boarding, 105 consented to participate in a tested telephone survey instrument.
The overall preferred location was inpatient hallways for 85% (95% CI 75-90) of respondents. Comparing ED boarding to inpatient hallway boarding, respondents preferred inpatient boarding with regard to staff availability (84%), safety (83%), confidentiality (82%), and comfort (79%).
Study results were subject to non-response bias, because working telephone numbers were required for study inclusion, as well as recall bias, because the survey was conducted within several months after discharge. This study’s results are based on actual patient experiences, whereas prior literature relied on patients to hypothesize the preferred environment after experiencing only ED hallway boarding to predict satisfaction.
Bottom line: Boarding in inpatient hallways was associated with higher patient satisfaction compared with ED hallway boarding.
Citation: Viccellio P, Zito JA, Sayage V, et al. Patients overwhelmingly prefer inpatient boarding to emergency department boarding [published online ahead of print September 21, 2013].
In This Edition
Literature At A Glance
A guide to this month’s studies
- Antibiotic resistance threats in the United States
- Turning for ulcer reduction: A multi-site, randomized, clinical trial in nursing homes
- Prednisolone with or without pentoxfylline, and survival of patients with severe alcoholic hepatitis
- Characteristics and impact of a hospitalist-staffed, post-discharge clinic
- Higher continuity of care results in lower rate of preventable hospitalizations
- Variation in surgical readmission rates depends on volume, mortality rates
- Patients prefer inpatient boarding to ED boarding
Antibiotic Resistance Threats in the United States, 2013
Clinical question: What antibiotic-resistant bacteria are the greatest threats for the next 10 years?
Background: Two million people suffer antibiotic-resistant infections yearly, and 23,000 die each year as a result. Most of these infections occur in the community, but deaths usually occur in healthcare settings. Cost estimates vary but may be as high as $20 billion in excess direct healthcare costs.
Study design: The CDC used several different surveys and databanks, including the National Antimicrobial Resistance Monitoring System, to collect data. The threat level for antibiotic-resistant bacteria was determined using several factors: clinical impact, economic impact, incidence, 10-year projection of incidence, transmissibility, availability of effective antibiotics, and barriers to prevention.
Setting: United States.
Synopsis: The CDC has three classifications of antibiotic-resistant bacteria: urgent, serious, and concerning. Urgent threats are high-consequence, antibiotic-resistant threats because of significant risks identified across several criteria. These threats might not currently be widespread but have the potential to become so and require urgent public health attention to identify infections and to limit transmission. They include carbapenem-resistant Enterobacteriaceae, drug-resistant Neisseria gonorrhoeae, and Clostridium difficile (does not have true resistance, but is a consequence of antibiotic overuse).
Serious threats are significant antibiotic-resistant threats. These threats will worsen and might become urgent without ongoing public health monitoring and prevention activities. They include multidrug-resistant Acinetobacter, drug-resistant Campylobacter, fluconazole-resistant Candida (a fungus), extended-spectrum β-lactamase-producing Enterobacteriaceae, vancomycin-resistant Enterococcus, multidrug-resistant Pseudomonas aeruginosa, drug-resistant non-typhoidal Salmonella, drug-resistant Salmonella Typhimurium, drug-resistant Shigella, methicillin-resistant Staphylococcus aureus, drug-resistant Streptococcus pneumonia, and drug-resistant tuberculosis.
Concerning threats are bacteria for which the threat of antibiotic resistance is low, and/ or there are multiple therapeutic options for resistant infections. These bacterial pathogens cause severe illness. Threats in this category require monitoring and, in some cases, rapid incident or outbreak response. These include vancomycin-resistant Staphylococcus aureus, erythromycin-resistant Group A Streptococcus, and clindamycin-resistant Group B Streptococcus. Research has shown patients with resistant infections have significantly longer hospital stays, delayed recuperation, long-term disability, and higher mortality. As resistance to current antibiotics occurs, providers are forced to use antibiotics that are more toxic, more expensive, and less effective.
The CDC recommends four core actions to fight antibiotic resistance:
- Preventing infections from occurring and preventing resistant bacteria from spreading (immunization, infection control, screening, treatment, and education);
- Tracking resistant bacteria;
- Improving the use of antibiotics (antibiotic stewardship); and
- Promoting the development of new antibiotics and new diagnostic tests for resistant bacteria.
Bottom line: Antibiotics are a limited resource. The more antibiotics are used today, the less likely they will continue to be effective in the future. The CDC lists 18 antibiotic-resistant organisms as urgent, serious, or concerning and recommends actions to combat the spread of current organisms and emergence of new antibiotic organisms.
Citation: Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. CDC website. September 16, 2013. Available at: www.cdc.gov/drugresistance/threat-report-2013. Accessed Nov. 30, 2013.
Turning for Ulcer Reduction: A Multi-Site Randomized Clinical Trial in Nursing Homes
Clinical question: Is there a difference between repositioning intervals of two, three, or four hours in pressure ulcer formation in nursing home residents on high-density foam mattresses?
Background: Pressure ulcer formation in nursing home residents is a common problem. Current standard of care requires repositioning every two hours in patients who are at risk for pressure ulcer formation. Few studies have been performed to assess a difference in repositioning interval. This study was conducted to see if there is a difference in pressure ulcer formation among residents on high-density foam mattresses at moderate to high risk (according to the Braden scale).
Study design: Multi-site, randomized, clinical trial.
Setting: Twenty U.S. and seven Canadian nursing homes using high-density foam mattresses.
Synopsis: A multi-site, randomized clinical trial was executed in 20 U.S. and seven Canadian nursing homes. More than 900 residents were randomized to two-, three-, or four-hour intervals for repositioning. All participants were at either moderate (13-14) or high (10-12) risk on the Braden scale for pressure ulcer formation. All facilities used high-density foam mattresses. All participants were monitored for pressure ulcer formation on the sacrum/coccyx, heel, or trochanter for three consecutive weeks.
There was no significant difference in pressure ulcer formation between the two-, three-, or four-hour interval repositioning groups. There was no significant difference in pressure ulcer formation between the moderate or high-risk groups. Only 2% of participants developed a pressure ulcer, all stage I or II.
It is not clear if the outcomes were purely related to the repositioning intervals, as this study group had a much lower rate of pressure ulcer formation compared to national averages and previous studies. The high-density foam mattress might have improved outcomes by evenly redistributing pressure so that less frequent repositioning was required. The level of documentation may have led to earlier recognition of early stage pressure ulcers as well. This study also was limited to nursing home residents at moderate to high risk of pressure ulcer development.
Bottom line: There is no significant difference in pressure ulcer formation between repositioning intervals of two, three, or four hours among moderate and high-risk nursing home residents using high-density foam mattresses.
Citation: Bergstrom N, Horn SD, Rapp MP, Stern A, Barrett R, Watkiss M. Turning for ulcer reduction: a multisite randomized clinical trial in nursing homes. 2013;61(10):1705-1713.
Prednisolone, Pentoxifylline, and Survival of Patients with Severe Alcoholic Hepatitis
Clinical question: Does the addition of pentoxifylline to prednisolone improve six-month mortality compared to prednisolone alone in patients with severe alcoholic hepatitis?
Background: Prednisolone improves liver function and reduces inflammation in patients with alcoholic hepatitis. Pentoxifylline appears to have a protective effect against hepatorenal syndrome in patients with severe alcoholic hepatitis. The medications have different mechanisms of action; therefore, the researchers hypothesized that the combination of medication would improve outcomes.
Study design: Multi-center, randomized, double-blinded clinical trial.
Setting: One Belgian and 23 French hospitals, from December 2007 to October 2010.
Synopsis: This study randomized 270 patients to receive either prednisolone and pentoxifylline or prednisolone and placebo for 28 days. Acute alcoholic hepatitis was defined by a positive biopsy, onset of jaundice three months prior to the study, and a Maddrey’s discriminant function score of >32. All patients were assessed for response to treatment using the Lille model at seven days of treatment, occurrence of hepatorenal syndrome, and survival at six months.
Results showed no significant difference in treatment response, alcohol relapse, death, time to death, or occurrence of hepatorenal syndrome between the two treatment groups; however, there were fewer episodes of hepatorenal syndrome in the pentoxifylline group.
Patients considered responders by the Lille model and those with lower Model for End-Stage Liver Disease scores had improved mortality. Patients treated with pentoxifylline had lower rates of hepatorenal syndrome at one month but no difference by six months. Patients with a lower Lille score had significantly less incidence of hepatorenal syndrome. The study may be underpowered to accurately determine outcomes other than six-month survival.
Bottom line: Adding pentoxifylline to prednisolone does not improve six-month survival in severe alcoholic hepatitis compared to prednisolone alone.
Citation: Mathurin P, Louvet A, Duhamel A, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomized clinical trial. 2013;310(10):1033-1041.
Characteristics and Impact of Hospitalist-Staffed, Post-Discharge Clinic
Clinical question: What effect does a hospitalist-staffed, post-discharge clinic have on time to first post-hospitalization visit?
Background: Hospital discharge is a well-recognized care transition that can leave patients vulnerable to morbidity and re-hospitalization. Limited primary care access can hamper complex post-hospital follow-up. Discharge clinic models staffed by hospitalists have been developed to mitigate access issues, but research is lacking to describe their characteristics and benefits.
Study design: Single-center, prospective, observational database review.
Setting: Large, academic primary care practice affiliated with an academic medical center.
Synopsis: Between 2009 and 2011, this hospitalist-staffed, post-discharge clinic saw 596 patients, while the affiliated, large primary care practice saw 10,839 patients. Patients utilizing the hospitalist discharge clinic were more likely to be black (39% vs. 29%, <0.001) and to receive primary care from resident clinics (40% vs. 21%, <0.001). The median duration from hospital discharge to the first clinic visit was shorter for the post-discharge clinic (8.45 ± 0.43 days, <0.001).
The number of radiology and laboratory tests performed at the first post-discharge clinic visit showed similar patterns between the hospitalist discharge clinic and the primary care practice. Study design and size did not permit comparisons of readmission rates or mortality from time of discharge and also precluded evaluation of interventions on discharge-related medication errors or response time to outstanding test results.
Bottom line: A hospitalist-staffed, post-discharge clinic was associated with shorter time to first post-discharge visit, especially for patients who are black and receive primary care from resident clinics.
Citation: Doctoroff L, Nijhawan A, McNally D, Vanka A, Yu R, Mukamal KJ. The characteristics and impact of a hospitalist-staffed post-discharge clinic. 2013;126(11):1016.e9-1016.e15.
Higher Continuity of Care Results in Lower Rate of Preventable Hospitalizations
Clinical question: Is continuity of care related to preventable hospitalizations among older adults?
Background: Preventable hospitalizations cost approximately $25 billion annually in the U.S. The relationship between continuity of care and the risk of preventable hospitalization is unknown.
Study design: Retrospective cohort study.
Setting: Random sample of fee-for-service Medicare beneficiaries, for ambulatory visits and hospital admissions.
Synopsis: This study examined 3.2 million Medicare beneficiaries using 2008-2010 claims data to measure continuity and the first preventable hospitalization. The Prevention Quality Indicators definitions and technical specifications from the Agency for Healthcare Research and Quality were used to identify preventable hospitalizations. Both the continuity of care score and usual provider continuity score were used to calculate continuity metrics. Baseline risk of preventable hospitalization included age, sex, race, Medicaid dual-eligible status, and residential zip code.
During a two-year period, 12.6% of patients had a preventable hospitalization. After adjusting for variables, a 0.1 increase in continuity of care was associated with about a 2% lower rate of preventable hospitalization. Interestingly, continuity of care was not related to mortality rates.
This study extends prior research associating continuity of care with reduced rate of hospitalization; however, the associations found cannot assert a causal relationship. This study used coding practices that vary throughout the country, included only older fee-for-service Medicare beneficiaries, and could not verify why some patients had higher continuity of care. The authors suggest that efforts to strengthen physician-patient relationships through high-quality primary care will deter some hospital admissions.
Bottom line: Higher continuity of ambulatory care is associated with lower preventable hospitalizations in Medicare beneficiaries.
Citation: Nyweide DJ, Anthony DL, Bynum JP, et al. Continuity of care and the risk of preventable hospitalization in older adults. 2013;173(20):1879-1885.
Surgical Readmission Rate Variation Dependent on Surgical Volume, Surgical Mortality Rates
Clinical question: What factors determine rates of readmission after major surgery?
Background: Reducing hospital readmission rates has become a national priority. The U.S. patterns for surgical readmissions are unknown, as are the specific structural and quality characteristics of hospitals associated with lower surgical readmission rates.
Study design: Retrospective study of national Medicare data was used to calculate 30-day readmission rates for six major surgical procedures.
Setting: U.S. Hospitals, 2009-2010.
Synopsis: Six major surgical procedures were tracked by Medicare data, with 479,471 discharges from 3,004 hospitals. Structural characteristics included hospital size, teaching status, region, ownership, and proportion of patients living below the federal poverty line. Three well-established measures of surgical quality were used: the HQA surgical score, procedure volume, and 30-day mortality.
Hospitals in the highest quartile for surgical volume had a significantly lower readmission rate. Additionally, hospitals with the lowest surgical mortality rates had significantly lower readmission rates. Interestingly, high adherence to reported surgical process measures was only marginally associated with reduced admission rates. Prior studies have also shown inconsistent relationship between HQA surgical score and mortality.
Limitations to this study include inability to account for factors not captured by billing codes and the focus on a Medicare population.
Bottom line: Surgical readmission rates are associated with measures of surgical quality, specifically procedural volume and mortality.
Citation: Tsai TC, Joynt KE, Orav EJ, Gawande AA, Jha AK. Variation in surgical-readmission rates and quality of hospital care. 2013;369(12):1134-1142.
Patients Overwhelmingly Prefer Inpatient Boarding to ED Boarding
Clinical question: When hallway boarding is required, do patients prefer inpatient units over the ED?
Background: ED crowding is associated with patient dissatisfaction, ambulance diversion, delays in care, medical errors, and higher mortality rates. Strategies to alleviate the problem of boarding admitted patients in the ED can include relocation to inpatient hallways while awaiting a regular hospital bed. Traditional objections to inpatient hallway boarding include concerns regarding patient satisfaction and safety.
Study design: Structured telephone survey.
Setting: Suburban, university-based, teaching hospital.
Synopsis: Patients who required boarding in the ED hallway after hospital admission were eligible for inpatient hallway boarding according to the institutional protocol, which screens for those with only mild to moderate comorbidities. Of 110 consecutive patients contacted who experienced both ED and inpatient hallway boarding, 105 consented to participate in a tested telephone survey instrument.
The overall preferred location was inpatient hallways for 85% (95% CI 75-90) of respondents. Comparing ED boarding to inpatient hallway boarding, respondents preferred inpatient boarding with regard to staff availability (84%), safety (83%), confidentiality (82%), and comfort (79%).
Study results were subject to non-response bias, because working telephone numbers were required for study inclusion, as well as recall bias, because the survey was conducted within several months after discharge. This study’s results are based on actual patient experiences, whereas prior literature relied on patients to hypothesize the preferred environment after experiencing only ED hallway boarding to predict satisfaction.
Bottom line: Boarding in inpatient hallways was associated with higher patient satisfaction compared with ED hallway boarding.
Citation: Viccellio P, Zito JA, Sayage V, et al. Patients overwhelmingly prefer inpatient boarding to emergency department boarding [published online ahead of print September 21, 2013].
CDC Expert Discusses MRSA Infections and Monitoring for Anti-Microbial Resistance
Click here to listen to more of our interview with Dr. Patel
Click here to listen to more of our interview with Dr. Patel
Click here to listen to more of our interview with Dr. Patel
Hospitalists Poised to Prevent, Combat Antibiotic-Resistant Pathogens
Describing formally for the first time the enormity of the problem of antibiotic resistance and warning of the “potentially catastrophic consequences of inaction,” the Centers for Disease Control and Prevention (CDC) announced in September that more than two million people a year are sickened by infections that are resistant to treatment with antibiotics.
Moreover, the CDC says 23,000 people die as a result.
And because those numbers are based only on the data available—and the agency assumes that many infections are not captured—the CDC says its estimate is a conservative one and the real number is probably higher.
The report is a call to action for hospitalists, who are in an almost ideal position to participate in efforts to prevent infections and control their spread once they’re discovered, says Jean Patel, PhD, deputy director of the office of antimicrobial resistance at the CDC.
“I think it’s a sobering number, and it indicates how far we have to go in combating this problem of antimicrobial resistance,” Dr. Patel says.
The medical community, she adds, cannot expect that new treatments will become available to fight all of these new infections.
“All of the drugs also are going to have some gaps in their range of activity, so there’s no drug coming that’s going to be effective against all the antimicrobial-resistant drugs that we face today,” Dr. Patel explains. “For that reason, we’re sounding the alarm that it’s important to pay attention to infection control and antibiotic stewardship practices.”
The report, “Antibiotic Resistance Threats to the United States, 2013,” creates three categories of antibiotic-resistant pathogens. In the “urgent” tier are Clostridium difficile, which the CDC estimates is responsible for 250,000 infections a year and 14,000 deaths; carbapenem-resistant Enterobacteriaceae, estimated to be responsible for 9,000 drug-resistant infections a year and 600 deaths; and drug-resistant Neisseria gonorrhoeae, at 246,000 drug-resistant infections.
These bacteria are considered an “immediate public health threat that requires urgent and aggressive action.”
There are 12 pathogens in the second category, described as “a serious concern” requiring “prompt and sustained action to ensure the problem does not grow.”
Of particular interest to hospitalists in this group, Dr. Patel says, is methicillin-resistant Staphylococcus aureus (MRSA). The CDC estimates that more than 80,000 severe MRSA infections and more than 11,000 deaths occur in the U.S. every year.
MRSA was not ranked as an “urgent” threat only because the number of infections is actually decreasing, especially in healthcare institutions, and because there are antibiotics that still work on MRSA.
“If either of those things were to change—for example, if the rate of infections were to increase, or if these isolates were to become more resistant—then we would have to think about changing this from a serious threat to an urgent threat,” Dr. Patel says.
Another infection in the serious category that should be on hospitalists’ radar is drug-resistant Streptococcus pneumoniae. A new vaccine is helping to decrease the number of these infections, but hospitalists should be vigilant about infections that could escape the vaccine and become resistant, Dr. Patel says.
The report estimates as much as $20 billion in excess healthcare costs due to antimicrobial-resistant infections, with $35 billion in lost productivity in 2008 dollars.1
Ketino Kobaidze, MD, assistant professor at the Emory University School of Medicine in Atlanta and a member of the antimicrobial stewardship and infectious disease control committees at Emory University Hospital Midtown, says the sheer numbers are sure to get people to take notice.
“Two million is lots of patients,” she says. “It’s eye-opening, really, for many doctors and patients and society.”
The silver lining, she says, is that the field is moving toward diagnostic tools that will provide quick feedback on the type of infection at work.
It may be that hospitalists have no choice but to give an antibiotic to a patient because of the risk involved in not giving one; however, providers should quickly tailor that treatment to target the specific pathogen when more information is available.
—Ketino Kobaidze, MD, assistant professor, Emory University School of Medicine, Atlanta, member, antimicrobial stewardship and infectious disease control committees, Emory University Hospital Midtown
“The most important thing, I think, for hospital medicine and medicine anywhere, is to follow up with whatever you’re ordering and notice right away what happens with these tests. If it’s positive or negative, redirect your care,” Dr. Kobaidze says. “Time is really an important issue here.
“As hospitalists, we need to be extremely cautious not to give them something they don’t need.”
Dr. Kobaidze was particularly struck by gonorrhea being listed in the “urgent” threat category.
“It was so easy to treat before,” she says. “It was nothing, piece of cake. This makes me a little bit concerned.”
Robert Orenstein, DO, an infectious disease expert at Mayo Clinic, praises the report and says hospitalists have a key role to play.
“I think this has a clear impact on hospitalists, who are the primary caregivers of many of these ill patients,” he says. “We need to educate them and build systems that target antimicrobials to the infecting agents and limit their use. Hospitalists are also the people who can help protect patients from the spread of these in the hospital by following appropriate infection prevention guidelines and educating their colleagues of the importance of this.”
He also stresses the importance of being aware of threats within your specific region.
“Many of these MDROs [multi-drug resistant organisms] have regional prevalence,” he says. “And it’s important to know which bugs are in your region so you can work with your institution and public health to tackle these.”
Tom Collins is a freelance writer in South Florida.
Reference
Describing formally for the first time the enormity of the problem of antibiotic resistance and warning of the “potentially catastrophic consequences of inaction,” the Centers for Disease Control and Prevention (CDC) announced in September that more than two million people a year are sickened by infections that are resistant to treatment with antibiotics.
Moreover, the CDC says 23,000 people die as a result.
And because those numbers are based only on the data available—and the agency assumes that many infections are not captured—the CDC says its estimate is a conservative one and the real number is probably higher.
The report is a call to action for hospitalists, who are in an almost ideal position to participate in efforts to prevent infections and control their spread once they’re discovered, says Jean Patel, PhD, deputy director of the office of antimicrobial resistance at the CDC.
“I think it’s a sobering number, and it indicates how far we have to go in combating this problem of antimicrobial resistance,” Dr. Patel says.
The medical community, she adds, cannot expect that new treatments will become available to fight all of these new infections.
“All of the drugs also are going to have some gaps in their range of activity, so there’s no drug coming that’s going to be effective against all the antimicrobial-resistant drugs that we face today,” Dr. Patel explains. “For that reason, we’re sounding the alarm that it’s important to pay attention to infection control and antibiotic stewardship practices.”
The report, “Antibiotic Resistance Threats to the United States, 2013,” creates three categories of antibiotic-resistant pathogens. In the “urgent” tier are Clostridium difficile, which the CDC estimates is responsible for 250,000 infections a year and 14,000 deaths; carbapenem-resistant Enterobacteriaceae, estimated to be responsible for 9,000 drug-resistant infections a year and 600 deaths; and drug-resistant Neisseria gonorrhoeae, at 246,000 drug-resistant infections.
These bacteria are considered an “immediate public health threat that requires urgent and aggressive action.”
There are 12 pathogens in the second category, described as “a serious concern” requiring “prompt and sustained action to ensure the problem does not grow.”
Of particular interest to hospitalists in this group, Dr. Patel says, is methicillin-resistant Staphylococcus aureus (MRSA). The CDC estimates that more than 80,000 severe MRSA infections and more than 11,000 deaths occur in the U.S. every year.
MRSA was not ranked as an “urgent” threat only because the number of infections is actually decreasing, especially in healthcare institutions, and because there are antibiotics that still work on MRSA.
“If either of those things were to change—for example, if the rate of infections were to increase, or if these isolates were to become more resistant—then we would have to think about changing this from a serious threat to an urgent threat,” Dr. Patel says.
Another infection in the serious category that should be on hospitalists’ radar is drug-resistant Streptococcus pneumoniae. A new vaccine is helping to decrease the number of these infections, but hospitalists should be vigilant about infections that could escape the vaccine and become resistant, Dr. Patel says.
The report estimates as much as $20 billion in excess healthcare costs due to antimicrobial-resistant infections, with $35 billion in lost productivity in 2008 dollars.1
Ketino Kobaidze, MD, assistant professor at the Emory University School of Medicine in Atlanta and a member of the antimicrobial stewardship and infectious disease control committees at Emory University Hospital Midtown, says the sheer numbers are sure to get people to take notice.
“Two million is lots of patients,” she says. “It’s eye-opening, really, for many doctors and patients and society.”
The silver lining, she says, is that the field is moving toward diagnostic tools that will provide quick feedback on the type of infection at work.
It may be that hospitalists have no choice but to give an antibiotic to a patient because of the risk involved in not giving one; however, providers should quickly tailor that treatment to target the specific pathogen when more information is available.
—Ketino Kobaidze, MD, assistant professor, Emory University School of Medicine, Atlanta, member, antimicrobial stewardship and infectious disease control committees, Emory University Hospital Midtown
“The most important thing, I think, for hospital medicine and medicine anywhere, is to follow up with whatever you’re ordering and notice right away what happens with these tests. If it’s positive or negative, redirect your care,” Dr. Kobaidze says. “Time is really an important issue here.
“As hospitalists, we need to be extremely cautious not to give them something they don’t need.”
Dr. Kobaidze was particularly struck by gonorrhea being listed in the “urgent” threat category.
“It was so easy to treat before,” she says. “It was nothing, piece of cake. This makes me a little bit concerned.”
Robert Orenstein, DO, an infectious disease expert at Mayo Clinic, praises the report and says hospitalists have a key role to play.
“I think this has a clear impact on hospitalists, who are the primary caregivers of many of these ill patients,” he says. “We need to educate them and build systems that target antimicrobials to the infecting agents and limit their use. Hospitalists are also the people who can help protect patients from the spread of these in the hospital by following appropriate infection prevention guidelines and educating their colleagues of the importance of this.”
He also stresses the importance of being aware of threats within your specific region.
“Many of these MDROs [multi-drug resistant organisms] have regional prevalence,” he says. “And it’s important to know which bugs are in your region so you can work with your institution and public health to tackle these.”
Tom Collins is a freelance writer in South Florida.
Reference
Describing formally for the first time the enormity of the problem of antibiotic resistance and warning of the “potentially catastrophic consequences of inaction,” the Centers for Disease Control and Prevention (CDC) announced in September that more than two million people a year are sickened by infections that are resistant to treatment with antibiotics.
Moreover, the CDC says 23,000 people die as a result.
And because those numbers are based only on the data available—and the agency assumes that many infections are not captured—the CDC says its estimate is a conservative one and the real number is probably higher.
The report is a call to action for hospitalists, who are in an almost ideal position to participate in efforts to prevent infections and control their spread once they’re discovered, says Jean Patel, PhD, deputy director of the office of antimicrobial resistance at the CDC.
“I think it’s a sobering number, and it indicates how far we have to go in combating this problem of antimicrobial resistance,” Dr. Patel says.
The medical community, she adds, cannot expect that new treatments will become available to fight all of these new infections.
“All of the drugs also are going to have some gaps in their range of activity, so there’s no drug coming that’s going to be effective against all the antimicrobial-resistant drugs that we face today,” Dr. Patel explains. “For that reason, we’re sounding the alarm that it’s important to pay attention to infection control and antibiotic stewardship practices.”
The report, “Antibiotic Resistance Threats to the United States, 2013,” creates three categories of antibiotic-resistant pathogens. In the “urgent” tier are Clostridium difficile, which the CDC estimates is responsible for 250,000 infections a year and 14,000 deaths; carbapenem-resistant Enterobacteriaceae, estimated to be responsible for 9,000 drug-resistant infections a year and 600 deaths; and drug-resistant Neisseria gonorrhoeae, at 246,000 drug-resistant infections.
These bacteria are considered an “immediate public health threat that requires urgent and aggressive action.”
There are 12 pathogens in the second category, described as “a serious concern” requiring “prompt and sustained action to ensure the problem does not grow.”
Of particular interest to hospitalists in this group, Dr. Patel says, is methicillin-resistant Staphylococcus aureus (MRSA). The CDC estimates that more than 80,000 severe MRSA infections and more than 11,000 deaths occur in the U.S. every year.
MRSA was not ranked as an “urgent” threat only because the number of infections is actually decreasing, especially in healthcare institutions, and because there are antibiotics that still work on MRSA.
“If either of those things were to change—for example, if the rate of infections were to increase, or if these isolates were to become more resistant—then we would have to think about changing this from a serious threat to an urgent threat,” Dr. Patel says.
Another infection in the serious category that should be on hospitalists’ radar is drug-resistant Streptococcus pneumoniae. A new vaccine is helping to decrease the number of these infections, but hospitalists should be vigilant about infections that could escape the vaccine and become resistant, Dr. Patel says.
The report estimates as much as $20 billion in excess healthcare costs due to antimicrobial-resistant infections, with $35 billion in lost productivity in 2008 dollars.1
Ketino Kobaidze, MD, assistant professor at the Emory University School of Medicine in Atlanta and a member of the antimicrobial stewardship and infectious disease control committees at Emory University Hospital Midtown, says the sheer numbers are sure to get people to take notice.
“Two million is lots of patients,” she says. “It’s eye-opening, really, for many doctors and patients and society.”
The silver lining, she says, is that the field is moving toward diagnostic tools that will provide quick feedback on the type of infection at work.
It may be that hospitalists have no choice but to give an antibiotic to a patient because of the risk involved in not giving one; however, providers should quickly tailor that treatment to target the specific pathogen when more information is available.
—Ketino Kobaidze, MD, assistant professor, Emory University School of Medicine, Atlanta, member, antimicrobial stewardship and infectious disease control committees, Emory University Hospital Midtown
“The most important thing, I think, for hospital medicine and medicine anywhere, is to follow up with whatever you’re ordering and notice right away what happens with these tests. If it’s positive or negative, redirect your care,” Dr. Kobaidze says. “Time is really an important issue here.
“As hospitalists, we need to be extremely cautious not to give them something they don’t need.”
Dr. Kobaidze was particularly struck by gonorrhea being listed in the “urgent” threat category.
“It was so easy to treat before,” she says. “It was nothing, piece of cake. This makes me a little bit concerned.”
Robert Orenstein, DO, an infectious disease expert at Mayo Clinic, praises the report and says hospitalists have a key role to play.
“I think this has a clear impact on hospitalists, who are the primary caregivers of many of these ill patients,” he says. “We need to educate them and build systems that target antimicrobials to the infecting agents and limit their use. Hospitalists are also the people who can help protect patients from the spread of these in the hospital by following appropriate infection prevention guidelines and educating their colleagues of the importance of this.”
He also stresses the importance of being aware of threats within your specific region.
“Many of these MDROs [multi-drug resistant organisms] have regional prevalence,” he says. “And it’s important to know which bugs are in your region so you can work with your institution and public health to tackle these.”
Tom Collins is a freelance writer in South Florida.
Reference
CDC Report Confirms Hospitalists’ Role in Fight against Antibiotic-Resistant Pathogens
Describing the enormity of the problem of antibiotic resistance and warning of the “potentially catastrophic consequences of inaction,” the Centers for Disease Control and Prevention (CDC) announced in September that more than 2 million people a year are sickened by infections that are resistant to treatment with antibiotics.
Moreover, the CDC says 23,000 people die as a result.
The report is a call to action for hospitalists, who are in a position to participate in efforts to prevent infections and control their spread once they’re discovered, says Jean Patel, PhD, deputy director of the office of antimicrobial resistance at the CDC. She also says the medical community cannot expect that new treatments will become available to fight all of these new infections.

—Robert Orenstein, DO, infectious disease expert, Mayo Clinic, Rochester, Minn.
“All of the drugs also are going to have some gaps in their range of activity” Dr. Patel explains. “For that reason, we’re sounding the alarm that it’s important to pay attention to infection control and antibiotic stewardship practices.”
The report, “Antibiotic Resistance Threats to the United States, 2013” creates three categories of antibiotic-resistant pathogens. In the “urgent” tier are Clostridium difficile, which the CDC estimates is responsible for 250,000 infections a year and 14,000 deaths; carbapenem-resistant Enterobacteriaceae, estimated to be responsible for 9,000 infections a year and 600 deaths; and Neisseria gonorrhoeae, at 246,000 infections.
These bacteria are considered an “immediate public health threat that requires urgent and aggressive action.”
Twelve pathogens in the second category, described as “a serious concern,” require “prompt and sustained action to ensure the problem does not grow.” Of particular interest to hospitalists in this group, Dr. Patel says, is methicillin-resistant Staphylococcus aureus (MRSA). The CDC estimates that more than 80,000 severe MRSA infections and more than 11,000 deaths occur in the U.S. every year.
MRSA was not ranked as an “urgent” threat only because the number of infections is decreasing, and because there are antibiotics that still work on MRSA.
Another infection that should be on hospitalists’ radar is Streptococcus pneumoniae. A new vaccine is helping to decrease the number of these infections, but hospitalists should be vigilant about infections that could escape the vaccine and become resistant, Dr. Patel says.
Ketino Kobaidze, MD, assistant professor at the Emory University School of Medicine in Atlanta and a member of the antimicrobial stewardship and infectious disease control committees at Emory University Hospital Midtown, says the most important thing for hospitalists “is to follow up with whatever you’re ordering and notice right away what happens with these tests. If it’s positive or negative, redirect your care.”
Robert Orenstein, DO, an infectious disease expert at Mayo Clinic, praises the report and says hospitalists have a key role to play. “We need to educate them and build systems that target antimicrobials to the infecting agents and limit their use,” he says. TH
Tom Collins is a freelance writer in South Florida.
Describing the enormity of the problem of antibiotic resistance and warning of the “potentially catastrophic consequences of inaction,” the Centers for Disease Control and Prevention (CDC) announced in September that more than 2 million people a year are sickened by infections that are resistant to treatment with antibiotics.
Moreover, the CDC says 23,000 people die as a result.
The report is a call to action for hospitalists, who are in a position to participate in efforts to prevent infections and control their spread once they’re discovered, says Jean Patel, PhD, deputy director of the office of antimicrobial resistance at the CDC. She also says the medical community cannot expect that new treatments will become available to fight all of these new infections.

—Robert Orenstein, DO, infectious disease expert, Mayo Clinic, Rochester, Minn.
“All of the drugs also are going to have some gaps in their range of activity” Dr. Patel explains. “For that reason, we’re sounding the alarm that it’s important to pay attention to infection control and antibiotic stewardship practices.”
The report, “Antibiotic Resistance Threats to the United States, 2013” creates three categories of antibiotic-resistant pathogens. In the “urgent” tier are Clostridium difficile, which the CDC estimates is responsible for 250,000 infections a year and 14,000 deaths; carbapenem-resistant Enterobacteriaceae, estimated to be responsible for 9,000 infections a year and 600 deaths; and Neisseria gonorrhoeae, at 246,000 infections.
These bacteria are considered an “immediate public health threat that requires urgent and aggressive action.”
Twelve pathogens in the second category, described as “a serious concern,” require “prompt and sustained action to ensure the problem does not grow.” Of particular interest to hospitalists in this group, Dr. Patel says, is methicillin-resistant Staphylococcus aureus (MRSA). The CDC estimates that more than 80,000 severe MRSA infections and more than 11,000 deaths occur in the U.S. every year.
MRSA was not ranked as an “urgent” threat only because the number of infections is decreasing, and because there are antibiotics that still work on MRSA.
Another infection that should be on hospitalists’ radar is Streptococcus pneumoniae. A new vaccine is helping to decrease the number of these infections, but hospitalists should be vigilant about infections that could escape the vaccine and become resistant, Dr. Patel says.
Ketino Kobaidze, MD, assistant professor at the Emory University School of Medicine in Atlanta and a member of the antimicrobial stewardship and infectious disease control committees at Emory University Hospital Midtown, says the most important thing for hospitalists “is to follow up with whatever you’re ordering and notice right away what happens with these tests. If it’s positive or negative, redirect your care.”
Robert Orenstein, DO, an infectious disease expert at Mayo Clinic, praises the report and says hospitalists have a key role to play. “We need to educate them and build systems that target antimicrobials to the infecting agents and limit their use,” he says. TH
Tom Collins is a freelance writer in South Florida.
Describing the enormity of the problem of antibiotic resistance and warning of the “potentially catastrophic consequences of inaction,” the Centers for Disease Control and Prevention (CDC) announced in September that more than 2 million people a year are sickened by infections that are resistant to treatment with antibiotics.
Moreover, the CDC says 23,000 people die as a result.
The report is a call to action for hospitalists, who are in a position to participate in efforts to prevent infections and control their spread once they’re discovered, says Jean Patel, PhD, deputy director of the office of antimicrobial resistance at the CDC. She also says the medical community cannot expect that new treatments will become available to fight all of these new infections.

—Robert Orenstein, DO, infectious disease expert, Mayo Clinic, Rochester, Minn.
“All of the drugs also are going to have some gaps in their range of activity” Dr. Patel explains. “For that reason, we’re sounding the alarm that it’s important to pay attention to infection control and antibiotic stewardship practices.”
The report, “Antibiotic Resistance Threats to the United States, 2013” creates three categories of antibiotic-resistant pathogens. In the “urgent” tier are Clostridium difficile, which the CDC estimates is responsible for 250,000 infections a year and 14,000 deaths; carbapenem-resistant Enterobacteriaceae, estimated to be responsible for 9,000 infections a year and 600 deaths; and Neisseria gonorrhoeae, at 246,000 infections.
These bacteria are considered an “immediate public health threat that requires urgent and aggressive action.”
Twelve pathogens in the second category, described as “a serious concern,” require “prompt and sustained action to ensure the problem does not grow.” Of particular interest to hospitalists in this group, Dr. Patel says, is methicillin-resistant Staphylococcus aureus (MRSA). The CDC estimates that more than 80,000 severe MRSA infections and more than 11,000 deaths occur in the U.S. every year.
MRSA was not ranked as an “urgent” threat only because the number of infections is decreasing, and because there are antibiotics that still work on MRSA.
Another infection that should be on hospitalists’ radar is Streptococcus pneumoniae. A new vaccine is helping to decrease the number of these infections, but hospitalists should be vigilant about infections that could escape the vaccine and become resistant, Dr. Patel says.
Ketino Kobaidze, MD, assistant professor at the Emory University School of Medicine in Atlanta and a member of the antimicrobial stewardship and infectious disease control committees at Emory University Hospital Midtown, says the most important thing for hospitalists “is to follow up with whatever you’re ordering and notice right away what happens with these tests. If it’s positive or negative, redirect your care.”
Robert Orenstein, DO, an infectious disease expert at Mayo Clinic, praises the report and says hospitalists have a key role to play. “We need to educate them and build systems that target antimicrobials to the infecting agents and limit their use,” he says. TH
Tom Collins is a freelance writer in South Florida.
Universal MRSA Decolonization in ICU Leads to Fewer Bloodstream Infections
Clinical question
Does universal decolonization for methicillin-resistant Staphylococcus aureus (MRSA) in patients in the intensive care unit decrease the rate of MRSA-positive clinical cultures?
Bottom line
As compared with no decolonization or a targeted decolonization, a universal decolonization strategy for MRSA using intranasal mupirocin and chlorhexidine bathing cloths for all patients admitted to the intensive care unit (ICU) is most effective at decreasing MRSA-positive clinical cultures and ICU-acquired bloodstream infections. Overall, you would need to treat 54 patients with universal decolonization to prevent one bloodstream infection. The cost effectiveness of this strategy as well as the concern of emerging resistance was not addressed in this study. (LOE = 1b-)
Reference
Study design
Randomized controlled trial (nonblinded)
Funding source
Government
Allocation
Uncertain
Setting
Inpatient (ICU only)
Synopsis
Prior research has shown that daily bathing with chlorhexidine lowers the rate of MRSA acquisition and decreases the overall number of hospital-acquired bloodstream infections in the ICU (Daily POEM 4/26/13). The current study's goal was to identify whether targeted or universal MRSA decolonization is the most effective at reducing MRSA infections in the ICU. Investigators randomized 43 hospitals to use 1 of 3 strategies within all their adult ICUs: (1) MRSA screening and contact isolation only; (2) screening, isolation, and decolonization of MRSA carriers; (3) decolonization of all patients without any screening procedures. Screening for MRSA was performed via swabs of bilateral nares upon ICU admission in the first 2 groups. Contact precautions were implemented for those with a positive MRSA screening result in groups 1 and 2 and for those with history of MRSA colonization or infection in all groups. Decolonization in groups 2 and 3 consisted of 5 days of twice-daily intranasal mupirocin, as well as daily bathing with chlorhexidine cloths during the entire ICU stay. Baseline characteristics of the patient populations in each group were similar. Patients in all adult ICUs of a participating hospital were assigned to the same study group. Although both universal and targeted decolonization resulted in a significant reduction in the primary outcome of MRSA-positive clinical cultures, the universal strategy was found to be most effective (hazard ratio [HR] = 0.63 for the universal strategy; HR = 0.75 for the targeted strategy; and HR = 0.92 for screening and isolation; P = .01). Additionally, universal decolonization led to the greatest reduction of overall bloodstream infections (HR = 0.56 for universal; HR = 0.78 for targeted; HR = 0.99 for screening and isolation; P < .001). Of note, the universal decolonization group contained 3 of the 4 hospitals that performed bone marrow and solid-organ transplantations, resulting in a higher baseline risk of infection than the other groups, but this difference was not statistically significant. Overall, only severe adverse events were noted in this study and all were classified as mild pruritus or rash due to chlorhexidine bathing. Investigators did not evaluate the cost-effectiveness of the different strategies nor did they examine the emergence of resistance with widespread use of chlorhexidine and mupirocin.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does universal decolonization for methicillin-resistant Staphylococcus aureus (MRSA) in patients in the intensive care unit decrease the rate of MRSA-positive clinical cultures?
Bottom line
As compared with no decolonization or a targeted decolonization, a universal decolonization strategy for MRSA using intranasal mupirocin and chlorhexidine bathing cloths for all patients admitted to the intensive care unit (ICU) is most effective at decreasing MRSA-positive clinical cultures and ICU-acquired bloodstream infections. Overall, you would need to treat 54 patients with universal decolonization to prevent one bloodstream infection. The cost effectiveness of this strategy as well as the concern of emerging resistance was not addressed in this study. (LOE = 1b-)
Reference
Study design
Randomized controlled trial (nonblinded)
Funding source
Government
Allocation
Uncertain
Setting
Inpatient (ICU only)
Synopsis
Prior research has shown that daily bathing with chlorhexidine lowers the rate of MRSA acquisition and decreases the overall number of hospital-acquired bloodstream infections in the ICU (Daily POEM 4/26/13). The current study's goal was to identify whether targeted or universal MRSA decolonization is the most effective at reducing MRSA infections in the ICU. Investigators randomized 43 hospitals to use 1 of 3 strategies within all their adult ICUs: (1) MRSA screening and contact isolation only; (2) screening, isolation, and decolonization of MRSA carriers; (3) decolonization of all patients without any screening procedures. Screening for MRSA was performed via swabs of bilateral nares upon ICU admission in the first 2 groups. Contact precautions were implemented for those with a positive MRSA screening result in groups 1 and 2 and for those with history of MRSA colonization or infection in all groups. Decolonization in groups 2 and 3 consisted of 5 days of twice-daily intranasal mupirocin, as well as daily bathing with chlorhexidine cloths during the entire ICU stay. Baseline characteristics of the patient populations in each group were similar. Patients in all adult ICUs of a participating hospital were assigned to the same study group. Although both universal and targeted decolonization resulted in a significant reduction in the primary outcome of MRSA-positive clinical cultures, the universal strategy was found to be most effective (hazard ratio [HR] = 0.63 for the universal strategy; HR = 0.75 for the targeted strategy; and HR = 0.92 for screening and isolation; P = .01). Additionally, universal decolonization led to the greatest reduction of overall bloodstream infections (HR = 0.56 for universal; HR = 0.78 for targeted; HR = 0.99 for screening and isolation; P < .001). Of note, the universal decolonization group contained 3 of the 4 hospitals that performed bone marrow and solid-organ transplantations, resulting in a higher baseline risk of infection than the other groups, but this difference was not statistically significant. Overall, only severe adverse events were noted in this study and all were classified as mild pruritus or rash due to chlorhexidine bathing. Investigators did not evaluate the cost-effectiveness of the different strategies nor did they examine the emergence of resistance with widespread use of chlorhexidine and mupirocin.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does universal decolonization for methicillin-resistant Staphylococcus aureus (MRSA) in patients in the intensive care unit decrease the rate of MRSA-positive clinical cultures?
Bottom line
As compared with no decolonization or a targeted decolonization, a universal decolonization strategy for MRSA using intranasal mupirocin and chlorhexidine bathing cloths for all patients admitted to the intensive care unit (ICU) is most effective at decreasing MRSA-positive clinical cultures and ICU-acquired bloodstream infections. Overall, you would need to treat 54 patients with universal decolonization to prevent one bloodstream infection. The cost effectiveness of this strategy as well as the concern of emerging resistance was not addressed in this study. (LOE = 1b-)
Reference
Study design
Randomized controlled trial (nonblinded)
Funding source
Government
Allocation
Uncertain
Setting
Inpatient (ICU only)
Synopsis
Prior research has shown that daily bathing with chlorhexidine lowers the rate of MRSA acquisition and decreases the overall number of hospital-acquired bloodstream infections in the ICU (Daily POEM 4/26/13). The current study's goal was to identify whether targeted or universal MRSA decolonization is the most effective at reducing MRSA infections in the ICU. Investigators randomized 43 hospitals to use 1 of 3 strategies within all their adult ICUs: (1) MRSA screening and contact isolation only; (2) screening, isolation, and decolonization of MRSA carriers; (3) decolonization of all patients without any screening procedures. Screening for MRSA was performed via swabs of bilateral nares upon ICU admission in the first 2 groups. Contact precautions were implemented for those with a positive MRSA screening result in groups 1 and 2 and for those with history of MRSA colonization or infection in all groups. Decolonization in groups 2 and 3 consisted of 5 days of twice-daily intranasal mupirocin, as well as daily bathing with chlorhexidine cloths during the entire ICU stay. Baseline characteristics of the patient populations in each group were similar. Patients in all adult ICUs of a participating hospital were assigned to the same study group. Although both universal and targeted decolonization resulted in a significant reduction in the primary outcome of MRSA-positive clinical cultures, the universal strategy was found to be most effective (hazard ratio [HR] = 0.63 for the universal strategy; HR = 0.75 for the targeted strategy; and HR = 0.92 for screening and isolation; P = .01). Additionally, universal decolonization led to the greatest reduction of overall bloodstream infections (HR = 0.56 for universal; HR = 0.78 for targeted; HR = 0.99 for screening and isolation; P < .001). Of note, the universal decolonization group contained 3 of the 4 hospitals that performed bone marrow and solid-organ transplantations, resulting in a higher baseline risk of infection than the other groups, but this difference was not statistically significant. Overall, only severe adverse events were noted in this study and all were classified as mild pruritus or rash due to chlorhexidine bathing. Investigators did not evaluate the cost-effectiveness of the different strategies nor did they examine the emergence of resistance with widespread use of chlorhexidine and mupirocin.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Surgical-Site-Infection Risk Not Associated with Prophylactic Antibiotic Timing
Clinical question: How does timing of surgical antibiotic prophylaxis affect risk of postoperative surgical-site infections (SSIs)?
Background: Antibiotic prophylaxis for major surgical procedures has been proven in clinical trials to reduce rates of SSI. The Centers for Medicare & Medicaid Services’ (CMS) Surgical Care Improvement Project (SCIP) has implemented quality metrics to ensure antibiotics are administered within 60 minutes of incision; however, studies have failed to show that a 60-minute pre-incision window is advantageous.
Study design: Retrospective cohort.
Setting: Veterans Affairs hospitals.
Synopsis: Using SCIP and VA Surgical Quality Improvement Program data from 112 VA hospitals, 32,459 cases of hip or knee arthroplasty, colorectal surgery, arterial vascular surgery, and hysterectomy from 2005 to 2009 were reviewed. A post-operative SSI occurred in 1,497 cases (4.6%). Using several statistical methods, the relationship between timing of prophylactic antibiotic administration and postoperative SSI within 30 days was evaluated.
In unadjusted models, higher SSI rates were observed with antibiotic administration more than 60 minutes prior to incision (OR 1.34, 95% CI 1.08-1.66) but not after incision (OR 1.26, 95% CI 0.92-1.72), compared with procedures with antibiotics administered within 60 minutes pre-incision. However, after adjustment for patient, procedure, and antibiotic variables, no significant relationship between timing and SSI was observed (P=0.50 for all specialties).
The study sample was comprised primarily of older men and did not include patients who underwent cardiac procedures, limiting the generalizability of the findings. Nonetheless, the study is the largest of its kind and confirms previous studies that suggest there is no significant relationship between timing of antibiotics and SSI. Prophylactic antibiotics should still be used when indicated; however, using timing of prophylactic antibiotics as a quality measure is unlikely to improve outcomes.
Bottom line: Adherence to the empiric 60-minute window metric for timing of prophylactic antibiotics is not significantly associated with risk of SSI.
Citation: Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013 March 20:1-8. doi: 10.1001/jamasurg.2013.134 [Epub ahead of print].
Visit our website for more physician reviews of recent HM-relevant literature.
Clinical question: How does timing of surgical antibiotic prophylaxis affect risk of postoperative surgical-site infections (SSIs)?
Background: Antibiotic prophylaxis for major surgical procedures has been proven in clinical trials to reduce rates of SSI. The Centers for Medicare & Medicaid Services’ (CMS) Surgical Care Improvement Project (SCIP) has implemented quality metrics to ensure antibiotics are administered within 60 minutes of incision; however, studies have failed to show that a 60-minute pre-incision window is advantageous.
Study design: Retrospective cohort.
Setting: Veterans Affairs hospitals.
Synopsis: Using SCIP and VA Surgical Quality Improvement Program data from 112 VA hospitals, 32,459 cases of hip or knee arthroplasty, colorectal surgery, arterial vascular surgery, and hysterectomy from 2005 to 2009 were reviewed. A post-operative SSI occurred in 1,497 cases (4.6%). Using several statistical methods, the relationship between timing of prophylactic antibiotic administration and postoperative SSI within 30 days was evaluated.
In unadjusted models, higher SSI rates were observed with antibiotic administration more than 60 minutes prior to incision (OR 1.34, 95% CI 1.08-1.66) but not after incision (OR 1.26, 95% CI 0.92-1.72), compared with procedures with antibiotics administered within 60 minutes pre-incision. However, after adjustment for patient, procedure, and antibiotic variables, no significant relationship between timing and SSI was observed (P=0.50 for all specialties).
The study sample was comprised primarily of older men and did not include patients who underwent cardiac procedures, limiting the generalizability of the findings. Nonetheless, the study is the largest of its kind and confirms previous studies that suggest there is no significant relationship between timing of antibiotics and SSI. Prophylactic antibiotics should still be used when indicated; however, using timing of prophylactic antibiotics as a quality measure is unlikely to improve outcomes.
Bottom line: Adherence to the empiric 60-minute window metric for timing of prophylactic antibiotics is not significantly associated with risk of SSI.
Citation: Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013 March 20:1-8. doi: 10.1001/jamasurg.2013.134 [Epub ahead of print].
Visit our website for more physician reviews of recent HM-relevant literature.
Clinical question: How does timing of surgical antibiotic prophylaxis affect risk of postoperative surgical-site infections (SSIs)?
Background: Antibiotic prophylaxis for major surgical procedures has been proven in clinical trials to reduce rates of SSI. The Centers for Medicare & Medicaid Services’ (CMS) Surgical Care Improvement Project (SCIP) has implemented quality metrics to ensure antibiotics are administered within 60 minutes of incision; however, studies have failed to show that a 60-minute pre-incision window is advantageous.
Study design: Retrospective cohort.
Setting: Veterans Affairs hospitals.
Synopsis: Using SCIP and VA Surgical Quality Improvement Program data from 112 VA hospitals, 32,459 cases of hip or knee arthroplasty, colorectal surgery, arterial vascular surgery, and hysterectomy from 2005 to 2009 were reviewed. A post-operative SSI occurred in 1,497 cases (4.6%). Using several statistical methods, the relationship between timing of prophylactic antibiotic administration and postoperative SSI within 30 days was evaluated.
In unadjusted models, higher SSI rates were observed with antibiotic administration more than 60 minutes prior to incision (OR 1.34, 95% CI 1.08-1.66) but not after incision (OR 1.26, 95% CI 0.92-1.72), compared with procedures with antibiotics administered within 60 minutes pre-incision. However, after adjustment for patient, procedure, and antibiotic variables, no significant relationship between timing and SSI was observed (P=0.50 for all specialties).
The study sample was comprised primarily of older men and did not include patients who underwent cardiac procedures, limiting the generalizability of the findings. Nonetheless, the study is the largest of its kind and confirms previous studies that suggest there is no significant relationship between timing of antibiotics and SSI. Prophylactic antibiotics should still be used when indicated; however, using timing of prophylactic antibiotics as a quality measure is unlikely to improve outcomes.
Bottom line: Adherence to the empiric 60-minute window metric for timing of prophylactic antibiotics is not significantly associated with risk of SSI.
Citation: Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013 March 20:1-8. doi: 10.1001/jamasurg.2013.134 [Epub ahead of print].
Visit our website for more physician reviews of recent HM-relevant literature.
Surgical-Site Infection Risk Not Associated with Prophylactic Antibiotic Timing
Clinical question: How does timing of surgical antibiotic prophylaxis affect risk of postoperative surgical-site infections (SSIs)?
Background: Antibiotic prophylaxis for major surgical procedures has been proven in clinical trials to reduce rates of SSI. The Centers for Medicare & Medicaid Services’ (CMS) Surgical Care Improvement Project (SCIP) has implemented quality metrics to ensure antibiotics are administered within 60 minutes of incision; however, studies have failed to show that a 60-minute pre-incision window is advantageous.
Study design: Retrospective cohort.
Setting: Veterans Affairs hospitals.
Synopsis: Using SCIP and VA Surgical Quality Improvement Program data from 112 VA hospitals, 32,459 cases of hip or knee arthroplasty, colorectal surgery, arterial vascular surgery, and hysterectomy from 2005-2009 were reviewed. A postoperative SSI occurred in 1,497 cases (4.6%). Using several statistical methods, the relationship between timing of prophylactic antibiotic administration and postoperative SSI within 30 days was evaluated. In unadjusted models, higher SSI rates were observed with antibiotic administration more than 60 minutes prior to incision (OR 1.34, 95% CI 1.08-1.66) but not after incision (OR 1.26, 95% CI 0.92-1.72), compared with procedures with antibiotics administered within 60 minutes pre-incision. However, after adjustment for patient, procedure, and antibiotic variables, no significant relationship between timing and SSI was observed (P=0.50 for all specialties).
The study sample was comprised primarily of older men and did not include patients who underwent cardiac procedures, limiting the generalizability of the findings. Nonetheless, the study is the largest of its kind and confirms previous studies that suggest there is no significant relationship between timing of antibiotics and SSI. Prophylactic antibiotics should still be used when indicated; however, using timing of prophylactic antibiotics as a quality measure is unlikely to improve outcomes.
Bottom line: Adherence to the empiric 60-minute window metric for timing of prophylactic antibiotics is not significantly associated with risk of SSI.
Citation: Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013 March 20:1-8. doi: 10.1001/jamasurg.2013.134 [Epub ahead of print].
Clinical question: How does timing of surgical antibiotic prophylaxis affect risk of postoperative surgical-site infections (SSIs)?
Background: Antibiotic prophylaxis for major surgical procedures has been proven in clinical trials to reduce rates of SSI. The Centers for Medicare & Medicaid Services’ (CMS) Surgical Care Improvement Project (SCIP) has implemented quality metrics to ensure antibiotics are administered within 60 minutes of incision; however, studies have failed to show that a 60-minute pre-incision window is advantageous.
Study design: Retrospective cohort.
Setting: Veterans Affairs hospitals.
Synopsis: Using SCIP and VA Surgical Quality Improvement Program data from 112 VA hospitals, 32,459 cases of hip or knee arthroplasty, colorectal surgery, arterial vascular surgery, and hysterectomy from 2005-2009 were reviewed. A postoperative SSI occurred in 1,497 cases (4.6%). Using several statistical methods, the relationship between timing of prophylactic antibiotic administration and postoperative SSI within 30 days was evaluated. In unadjusted models, higher SSI rates were observed with antibiotic administration more than 60 minutes prior to incision (OR 1.34, 95% CI 1.08-1.66) but not after incision (OR 1.26, 95% CI 0.92-1.72), compared with procedures with antibiotics administered within 60 minutes pre-incision. However, after adjustment for patient, procedure, and antibiotic variables, no significant relationship between timing and SSI was observed (P=0.50 for all specialties).
The study sample was comprised primarily of older men and did not include patients who underwent cardiac procedures, limiting the generalizability of the findings. Nonetheless, the study is the largest of its kind and confirms previous studies that suggest there is no significant relationship between timing of antibiotics and SSI. Prophylactic antibiotics should still be used when indicated; however, using timing of prophylactic antibiotics as a quality measure is unlikely to improve outcomes.
Bottom line: Adherence to the empiric 60-minute window metric for timing of prophylactic antibiotics is not significantly associated with risk of SSI.
Citation: Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013 March 20:1-8. doi: 10.1001/jamasurg.2013.134 [Epub ahead of print].
Clinical question: How does timing of surgical antibiotic prophylaxis affect risk of postoperative surgical-site infections (SSIs)?
Background: Antibiotic prophylaxis for major surgical procedures has been proven in clinical trials to reduce rates of SSI. The Centers for Medicare & Medicaid Services’ (CMS) Surgical Care Improvement Project (SCIP) has implemented quality metrics to ensure antibiotics are administered within 60 minutes of incision; however, studies have failed to show that a 60-minute pre-incision window is advantageous.
Study design: Retrospective cohort.
Setting: Veterans Affairs hospitals.
Synopsis: Using SCIP and VA Surgical Quality Improvement Program data from 112 VA hospitals, 32,459 cases of hip or knee arthroplasty, colorectal surgery, arterial vascular surgery, and hysterectomy from 2005-2009 were reviewed. A postoperative SSI occurred in 1,497 cases (4.6%). Using several statistical methods, the relationship between timing of prophylactic antibiotic administration and postoperative SSI within 30 days was evaluated. In unadjusted models, higher SSI rates were observed with antibiotic administration more than 60 minutes prior to incision (OR 1.34, 95% CI 1.08-1.66) but not after incision (OR 1.26, 95% CI 0.92-1.72), compared with procedures with antibiotics administered within 60 minutes pre-incision. However, after adjustment for patient, procedure, and antibiotic variables, no significant relationship between timing and SSI was observed (P=0.50 for all specialties).
The study sample was comprised primarily of older men and did not include patients who underwent cardiac procedures, limiting the generalizability of the findings. Nonetheless, the study is the largest of its kind and confirms previous studies that suggest there is no significant relationship between timing of antibiotics and SSI. Prophylactic antibiotics should still be used when indicated; however, using timing of prophylactic antibiotics as a quality measure is unlikely to improve outcomes.
Bottom line: Adherence to the empiric 60-minute window metric for timing of prophylactic antibiotics is not significantly associated with risk of SSI.
Citation: Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013 March 20:1-8. doi: 10.1001/jamasurg.2013.134 [Epub ahead of print].
Probiotics prevent C. diff-associated diarrhea in patients taking antibiotics
Clinical question
Does the use of probiotics prevent Clostridium difficile-associated diarrhea in patients taking antibiotics?
Bottom line
Moderate-quality evidence suggests that probiotic administration reduces the incidence of C. difficile-associated diarrhea (CDAD) in patients who are taking antibiotics. LOE = 1a-
Reference
Study Design
Meta-analysis (other)
Funding Source
None
Setting
Various (meta-analysis)
Synopsis
These investigators searched multiple databases, including the Cochrane Register, MEDLINE, EMBASE, as well as reviewed bibliographies of relevant articles and spoke to experts in the field, to find randomized controlled trials that compared probiotics with placebo in reducing the incidence of CDAD in patients taking antibiotics. Two reviewers independently selected the articles, extracted data, and assessed study quality. Half of the 20 studies selected had either an unclear or high risk of bias; 7 studies had an overall low risk of bias. Patients included in the individual studies (N = 3818) varied in age and baseline risk of CDAD. Meta-analysis of the data showed that probiotics, as compared with placebo, reduced the incidence of CDAD in patients taking antibiotics (relative risk = 0.34; 95% CI, 0.24-0.49). Subgroup analyses showed similar results in adults and children, with lower and higher doses of probiotics, and with different probiotic species. There was no evidence of an increased risk of adverse events in the probiotics group. The majority of the studies excluded immunocompromised patients, thus limiting the generalizability of the results. Addtionally, the authors downrated the level of evidence to moderate quality because the overall sample size was smaller than what would be required for an optimally powered single study, which decreases the precision of the results.
Clinical question
Does the use of probiotics prevent Clostridium difficile-associated diarrhea in patients taking antibiotics?
Bottom line
Moderate-quality evidence suggests that probiotic administration reduces the incidence of C. difficile-associated diarrhea (CDAD) in patients who are taking antibiotics. LOE = 1a-
Reference
Study Design
Meta-analysis (other)
Funding Source
None
Setting
Various (meta-analysis)
Synopsis
These investigators searched multiple databases, including the Cochrane Register, MEDLINE, EMBASE, as well as reviewed bibliographies of relevant articles and spoke to experts in the field, to find randomized controlled trials that compared probiotics with placebo in reducing the incidence of CDAD in patients taking antibiotics. Two reviewers independently selected the articles, extracted data, and assessed study quality. Half of the 20 studies selected had either an unclear or high risk of bias; 7 studies had an overall low risk of bias. Patients included in the individual studies (N = 3818) varied in age and baseline risk of CDAD. Meta-analysis of the data showed that probiotics, as compared with placebo, reduced the incidence of CDAD in patients taking antibiotics (relative risk = 0.34; 95% CI, 0.24-0.49). Subgroup analyses showed similar results in adults and children, with lower and higher doses of probiotics, and with different probiotic species. There was no evidence of an increased risk of adverse events in the probiotics group. The majority of the studies excluded immunocompromised patients, thus limiting the generalizability of the results. Addtionally, the authors downrated the level of evidence to moderate quality because the overall sample size was smaller than what would be required for an optimally powered single study, which decreases the precision of the results.
Clinical question
Does the use of probiotics prevent Clostridium difficile-associated diarrhea in patients taking antibiotics?
Bottom line
Moderate-quality evidence suggests that probiotic administration reduces the incidence of C. difficile-associated diarrhea (CDAD) in patients who are taking antibiotics. LOE = 1a-
Reference
Study Design
Meta-analysis (other)
Funding Source
None
Setting
Various (meta-analysis)
Synopsis
These investigators searched multiple databases, including the Cochrane Register, MEDLINE, EMBASE, as well as reviewed bibliographies of relevant articles and spoke to experts in the field, to find randomized controlled trials that compared probiotics with placebo in reducing the incidence of CDAD in patients taking antibiotics. Two reviewers independently selected the articles, extracted data, and assessed study quality. Half of the 20 studies selected had either an unclear or high risk of bias; 7 studies had an overall low risk of bias. Patients included in the individual studies (N = 3818) varied in age and baseline risk of CDAD. Meta-analysis of the data showed that probiotics, as compared with placebo, reduced the incidence of CDAD in patients taking antibiotics (relative risk = 0.34; 95% CI, 0.24-0.49). Subgroup analyses showed similar results in adults and children, with lower and higher doses of probiotics, and with different probiotic species. There was no evidence of an increased risk of adverse events in the probiotics group. The majority of the studies excluded immunocompromised patients, thus limiting the generalizability of the results. Addtionally, the authors downrated the level of evidence to moderate quality because the overall sample size was smaller than what would be required for an optimally powered single study, which decreases the precision of the results.