User login
Making the difficult diagnosis of bipolar disorder in the school-age child
The diagnosis of bipolar disorder in children can be elusive and is often masked by rapid-cycling mood states or a comorbid disruptive behavior disorder. Bipolar disorder also manifests itself differently in children than it does in adults.
When evaluating a young patient for suspected pediatric bipolar disorder, a careful assessment that satisfies the following four criteria can help lead to an accurate diagnosis:
- Uncovering mood disorders in at least one parent or family member. Because high rates of mood disorders have been reported among family members of youths with bipolar disorders, a meticulous family history—in which the lifetime diagnoses of both biological parents is secured—becomes crucial.
- Finding consistent episodes of elevated mood alternating with episodes of depression or euthymia, with rapid cycling between one mood and the other. While bipolar disorder in adults is generally characterized by long, distinct mood states and periods of recovery between episodes, this condition in children appears with briefer mood states and low rates of recovery between episodes.
- Identifying greater degrees of mood swings that are distinct from episodes of disruptive behavior. Ask about the child's mood states, not necessarily his or her behavior, and scrutinize spontaneous mood swings carefully. During periods of mania and other mood states, children with bipolar disorder may exhibit both irritable and elevated moods, which may mimic symptoms of a behavioral disorder. It is helpful to find out how often these irritable or elevated moods were present during episodes of mania and other mood states.
- Considering a diagnosis of bipolar disorder only after ruling out other diagnoses, including that of an anxiety or disruptive behavior disorder. Affective disorders such as ADHD and OpDD are not by themselves characterized by discrete moods and cycling between mood episodes, but their symptoms may appear in children with bipolar disorder, so it is important to first rule out such conditions, as well as general medical considerations.
Reference
1. Findling RL, Gracious BL, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
Dr. Findling is director of child and adolescent psychiatry at the University Hospitals of Cleveland/Case Western Reserve University School of Medicine, Cleveland, Ohio.
The diagnosis of bipolar disorder in children can be elusive and is often masked by rapid-cycling mood states or a comorbid disruptive behavior disorder. Bipolar disorder also manifests itself differently in children than it does in adults.
When evaluating a young patient for suspected pediatric bipolar disorder, a careful assessment that satisfies the following four criteria can help lead to an accurate diagnosis:
- Uncovering mood disorders in at least one parent or family member. Because high rates of mood disorders have been reported among family members of youths with bipolar disorders, a meticulous family history—in which the lifetime diagnoses of both biological parents is secured—becomes crucial.
- Finding consistent episodes of elevated mood alternating with episodes of depression or euthymia, with rapid cycling between one mood and the other. While bipolar disorder in adults is generally characterized by long, distinct mood states and periods of recovery between episodes, this condition in children appears with briefer mood states and low rates of recovery between episodes.
- Identifying greater degrees of mood swings that are distinct from episodes of disruptive behavior. Ask about the child's mood states, not necessarily his or her behavior, and scrutinize spontaneous mood swings carefully. During periods of mania and other mood states, children with bipolar disorder may exhibit both irritable and elevated moods, which may mimic symptoms of a behavioral disorder. It is helpful to find out how often these irritable or elevated moods were present during episodes of mania and other mood states.
- Considering a diagnosis of bipolar disorder only after ruling out other diagnoses, including that of an anxiety or disruptive behavior disorder. Affective disorders such as ADHD and OpDD are not by themselves characterized by discrete moods and cycling between mood episodes, but their symptoms may appear in children with bipolar disorder, so it is important to first rule out such conditions, as well as general medical considerations.
The diagnosis of bipolar disorder in children can be elusive and is often masked by rapid-cycling mood states or a comorbid disruptive behavior disorder. Bipolar disorder also manifests itself differently in children than it does in adults.
When evaluating a young patient for suspected pediatric bipolar disorder, a careful assessment that satisfies the following four criteria can help lead to an accurate diagnosis:
- Uncovering mood disorders in at least one parent or family member. Because high rates of mood disorders have been reported among family members of youths with bipolar disorders, a meticulous family history—in which the lifetime diagnoses of both biological parents is secured—becomes crucial.
- Finding consistent episodes of elevated mood alternating with episodes of depression or euthymia, with rapid cycling between one mood and the other. While bipolar disorder in adults is generally characterized by long, distinct mood states and periods of recovery between episodes, this condition in children appears with briefer mood states and low rates of recovery between episodes.
- Identifying greater degrees of mood swings that are distinct from episodes of disruptive behavior. Ask about the child's mood states, not necessarily his or her behavior, and scrutinize spontaneous mood swings carefully. During periods of mania and other mood states, children with bipolar disorder may exhibit both irritable and elevated moods, which may mimic symptoms of a behavioral disorder. It is helpful to find out how often these irritable or elevated moods were present during episodes of mania and other mood states.
- Considering a diagnosis of bipolar disorder only after ruling out other diagnoses, including that of an anxiety or disruptive behavior disorder. Affective disorders such as ADHD and OpDD are not by themselves characterized by discrete moods and cycling between mood episodes, but their symptoms may appear in children with bipolar disorder, so it is important to first rule out such conditions, as well as general medical considerations.
Reference
1. Findling RL, Gracious BL, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
Dr. Findling is director of child and adolescent psychiatry at the University Hospitals of Cleveland/Case Western Reserve University School of Medicine, Cleveland, Ohio.
Reference
1. Findling RL, Gracious BL, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
Dr. Findling is director of child and adolescent psychiatry at the University Hospitals of Cleveland/Case Western Reserve University School of Medicine, Cleveland, Ohio.
Making the difficult diagnosis of bipolar disorder in the school-age child
The diagnosis of bipolar disorder in children can be elusive and is often masked by rapid-cycling mood states or a comorbid disruptive behavior disorder. Bipolar disorder also manifests itself differently in children than it does in adults.
When evaluating a young patient for suspected pediatric bipolar disorder, a careful assessment that satisfies the following four criteria can help lead to an accurate diagnosis:
- Uncovering mood disorders in at least one parent or family member. Because high rates of mood disorders have been reported among family members of youths with bipolar disorders, a meticulous family history—in which the lifetime diagnoses of both biological parents is secured—becomes crucial.
- Finding consistent episodes of elevated mood alternating with episodes of depression or euthymia, with rapid cycling between one mood and the other. While bipolar disorder in adults is generally characterized by long, distinct mood states and periods of recovery between episodes, this condition in children appears with briefer mood states and low rates of recovery between episodes.
- Identifying greater degrees of mood swings that are distinct from episodes of disruptive behavior. Ask about the child's mood states, not necessarily his or her behavior, and scrutinize spontaneous mood swings carefully. During periods of mania and other mood states, children with bipolar disorder may exhibit both irritable and elevated moods, which may mimic symptoms of a behavioral disorder. It is helpful to find out how often these irritable or elevated moods were present during episodes of mania and other mood states.
- Considering a diagnosis of bipolar disorder only after ruling out other diagnoses, including that of an anxiety or disruptive behavior disorder. Affective disorders such as ADHD and OpDD are not by themselves characterized by discrete moods and cycling between mood episodes, but their symptoms may appear in children with bipolar disorder, so it is important to first rule out such conditions, as well as general medical considerations.
Reference
1. Findling RL, Gracious BL, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
Dr. Findling is director of child and adolescent psychiatry at the University Hospitals of Cleveland/Case Western Reserve University School of Medicine, Cleveland, Ohio.
The diagnosis of bipolar disorder in children can be elusive and is often masked by rapid-cycling mood states or a comorbid disruptive behavior disorder. Bipolar disorder also manifests itself differently in children than it does in adults.
When evaluating a young patient for suspected pediatric bipolar disorder, a careful assessment that satisfies the following four criteria can help lead to an accurate diagnosis:
- Uncovering mood disorders in at least one parent or family member. Because high rates of mood disorders have been reported among family members of youths with bipolar disorders, a meticulous family history—in which the lifetime diagnoses of both biological parents is secured—becomes crucial.
- Finding consistent episodes of elevated mood alternating with episodes of depression or euthymia, with rapid cycling between one mood and the other. While bipolar disorder in adults is generally characterized by long, distinct mood states and periods of recovery between episodes, this condition in children appears with briefer mood states and low rates of recovery between episodes.
- Identifying greater degrees of mood swings that are distinct from episodes of disruptive behavior. Ask about the child's mood states, not necessarily his or her behavior, and scrutinize spontaneous mood swings carefully. During periods of mania and other mood states, children with bipolar disorder may exhibit both irritable and elevated moods, which may mimic symptoms of a behavioral disorder. It is helpful to find out how often these irritable or elevated moods were present during episodes of mania and other mood states.
- Considering a diagnosis of bipolar disorder only after ruling out other diagnoses, including that of an anxiety or disruptive behavior disorder. Affective disorders such as ADHD and OpDD are not by themselves characterized by discrete moods and cycling between mood episodes, but their symptoms may appear in children with bipolar disorder, so it is important to first rule out such conditions, as well as general medical considerations.
The diagnosis of bipolar disorder in children can be elusive and is often masked by rapid-cycling mood states or a comorbid disruptive behavior disorder. Bipolar disorder also manifests itself differently in children than it does in adults.
When evaluating a young patient for suspected pediatric bipolar disorder, a careful assessment that satisfies the following four criteria can help lead to an accurate diagnosis:
- Uncovering mood disorders in at least one parent or family member. Because high rates of mood disorders have been reported among family members of youths with bipolar disorders, a meticulous family history—in which the lifetime diagnoses of both biological parents is secured—becomes crucial.
- Finding consistent episodes of elevated mood alternating with episodes of depression or euthymia, with rapid cycling between one mood and the other. While bipolar disorder in adults is generally characterized by long, distinct mood states and periods of recovery between episodes, this condition in children appears with briefer mood states and low rates of recovery between episodes.
- Identifying greater degrees of mood swings that are distinct from episodes of disruptive behavior. Ask about the child's mood states, not necessarily his or her behavior, and scrutinize spontaneous mood swings carefully. During periods of mania and other mood states, children with bipolar disorder may exhibit both irritable and elevated moods, which may mimic symptoms of a behavioral disorder. It is helpful to find out how often these irritable or elevated moods were present during episodes of mania and other mood states.
- Considering a diagnosis of bipolar disorder only after ruling out other diagnoses, including that of an anxiety or disruptive behavior disorder. Affective disorders such as ADHD and OpDD are not by themselves characterized by discrete moods and cycling between mood episodes, but their symptoms may appear in children with bipolar disorder, so it is important to first rule out such conditions, as well as general medical considerations.
Reference
1. Findling RL, Gracious BL, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
Dr. Findling is director of child and adolescent psychiatry at the University Hospitals of Cleveland/Case Western Reserve University School of Medicine, Cleveland, Ohio.
Reference
1. Findling RL, Gracious BL, et al. Rapid, continuous cycling and psychiatric co-morbidity in pediatric bipolar I disorder. Bipolar Disord. 2001;3(4):202-210.
Dr. Findling is director of child and adolescent psychiatry at the University Hospitals of Cleveland/Case Western Reserve University School of Medicine, Cleveland, Ohio.
Antipsychotics and mood disorders: A complicated alliance
Major mood disorders are challenging to diagnose and often difficult to treat. They entail unipolar depression; bipolar disorder, which includes manic, depressed, or mixed episodes; and schizoaffective disorder, which includes both depressed and bipolar subtypes. Antidepressants and mood stabilizers are the primary pharmacological treatments. They may be insufficient, however, for patients with more severe episodes, often characterized by psychosis and treatment resistance.
In these patients, antipsychotics have played an important but controversial part in management, primarily as oral or parenteral adjuncts. Literature and clinical experience now support another, unique role for the current generation of novel agents.
Compared to earlier antipsychotics, these agents produce substantially fewer neurological adverse effects, including acute extrapyramidal and tardive syndromes, and can augment antidepressants and mood stabilizers. In addition, they may:
- Possess a better antipsychotic profile, with enhanced therapeutic effects on positive, negative, cognitive, and mood symptoms
- Have a role in the acute and long-term management of these disorders when anticipated parenteral formulations become available (e.g., acute intramuscular olanzapine and ziprasidone—and long-acting intramuscular risperidone)
- Possess inherent thymoleptic properties (see “Unresolved issues with antipsychotics,” below).
- Defining what constitutes a mood stabilizer.1 Proposed definitions suggest that the drug must entail the following:
- Clarifying the mechanisms underlying the apparent mood-regulating effects of novel agents
- Ascertaining both acute and maintenance efficacy
- Clarifying the propensity of some agents to switch depressed patients into mania
- Increasing the number of well-designed studies with sufficient sample sizes, including comparison trials assessing the relative efficacy of different novel agents
- Reducing the tendency to publish only positive reports when new drugs are first available
- Introducing parenteral formulations of novel agents
- Resolving concerns about weight gain, new-onset diabetes, QT c prolongation, and sedation
- Rectifying the current level of substantially greater costs
Management of unipolar depression
Neuroleptics Delusions and hallucinations indicate a more severe form of depressive disorder, with poor short- and long-term outcomes in comparison to those without psychosis. To illustrate, Table 1 lists a summary of response rates in psychotic and nonpsychotic depressed patients given a tricyclic antidepressant (TCA). The data indicate that patients suffering from psychotic depression typically do not benefit from antidepressant monotherapy and usually require a combination of antidepressant and antipsychotic or, alternatively, electroconvulsive therapy (ECT).
There is, however, some limited clinical and neuroimaging evidence that amoxapine can be used as an effective monotherapy in this group. Amoxapine is an antidepressant whose primary active metabolite, 8-hydroxy amoxapine, may have antipsychotic properties.1 With the possible exception of amoxapine, combined antipsychotic-antidepressant treatment is the rule.
Table 1
Psychotic and nonpsychotic depressed patients’ response to monotherapy with a tricyclic antidepressant
| Psychotic | Nonpsychotic | ||||
|---|---|---|---|---|---|
| Responders (%) (n=127) | Nonresponders (%) (n=236) | Responders (%) (n=464) | Nonresponders (%) (n=227) | Difference | |
| 13 studies | 35% | 65% | 67% | 33% | 32% |
| Adapted from Chan CH, Janicak PG, Davis JM, et al. Response of psychotic and nonpsychotic depressed patients to tricyclic antidepressants. J Clin Psychiatry. 1987;48:197-200. | |||||
Historically, studies have also evaluated neuroleptic monotherapy for depressed patients. While some reported superiority over a placebo, none found conventional antipsychotics superior to imipramine. Indeed, patients with schizophrenia who are treated with a neuroleptic often develop symptoms that are difficult to distinguish from depression (e.g., secondary negative symptoms). These often improve when the neuroleptic is discontinued or the patients are switched to a novel antipsychotic such as risperidone, olanzapine, or ziprasidone, all of which have putative antidepressant effects.
When employing an antipsychotic in depressed patients, the dosage and duration of treatment are two critical considerations. To minimize neuromotor adverse effects, use low doses of a neuroleptic (e.g., haloperidol, 1 to 5 mg/d) in conjunction with the primary antidepressant therapy. The neuroleptic should then be tapered gradually after psychotic symptoms have been controlled, usually during the acute phase of treatment. Ideally, patients would then take antidepressant monotherapy through the continuation phase and, if necessary, the maintenance phase of treatment. If psychosis recurs, re-introduce the antipsychotic intermittently.
Novel antipsychotics In contrast to neuroleptics, novel antipsychotics have been reported to improve depression in various psychotic and mood disorders.
For example, ziprasidone has serotonin and noradrenergic reuptake blocking effects comparable to such classic TCAs as imipramine and amitriptyline, as well as high binding affinity at the 5-HT1A, 5-HT1D, and 5-HT2C receptors. This neuroreceptor profile indicates possible antidepressant effects.
While randomized, controlled trials with mood-disordered patients are few, there have been promising preliminary reports of augmentation of antidepressants with risperidone and olanzapine in both psychotic and nonpsychotic depressed patients.
Ostroff and Nelson2 reported the results of an open-label study of eight SSRI-nonresponsive patients (mean treatment 7.3 weeks). These patients had no psychotic features and had a dramatic reduction in depressive symptoms, as well as some improvement in sexual dysfunction, with the addition of 0.5 mg to 1.0 mg risperidone. The clinicians suggested that risperidone’s 5-HT2A antagonism might explain its augmentation of the partial SSRI response.
Olanzapine alone (n=3) or combined with an antidepressant (n=12) has also been reported to improve both depression and psychosis.3 In a double-blind, amitriptyline-controlled trial, Svestka and Synek4 found that olanzapine demonstrated antidepressant efficacy in 33 unipolar and seven bipolar depressed patients. Thirteen of these patients also had psychotic symptoms.
Shelton et al5 reported the results of a two-center, 8-week, double-blind comparison of olanzapine alone, fluoxetine alone, or their combination in 28 patients suffering from treatment-resistant, non-bipolar disorder without psychosis. They found that the combination was superior to either drug alone based on improvement in the Hamilton Depression Rating Scale (HDRS) total score. From their preliminary data, it also appears that the doses required were relatively low, reducing the risk of side effects.
Their findings, however, need to be replicated in more controlled studies with combinations, addressing possible adverse effects, the potential for clinically relevant drug interactions, decreased compliance rates, and increased cost of treatment. Earlier reports raised concern about the potential of these agents to increase switching to hypomania or mania. But in more recent reports, this has not emerged as a significant problem.7
Finally, several case reports and case series indicate that agents such as clozapine and risperidone may augment ECT in particularly severe, treatment-resistant depressive episodes.7
Management of bipolar and schizoaffective depressed episodes
Neuroleptics Antipsychotics are frequently used to manage more severe, usually psychotic episodes of bipolar and schizoaffective depression. Reports indicate that affectively ill patients receiving neuroleptics may be more prone to develop neuromotor adverse effects than are those suffering from schizophrenia. Thus, their use for such patients must be well justified, limited in dosage and duration, and carefully monitored for the emergence of acute and tardive neurological events.
Novel antipsychotics Novel antipsychotics have demonstrated fewer propensities than have neuroleptics in worsening depression or negative symptoms in schizophrenic patients, and have possible antidepressant effects. In support of this hypothesis, and reminiscent of data from earlier risperidone and olanzapine trials, ziprasidone was observed to improve the Montgomery Asberg Rating Scale (MADRS) and Brief Psychotic Rating Scale (BPRS) depressive cluster scores in three clinical trials with schizophrenic and schizoaffective patients.8,9
Vieta et al reported the efficacy and safety of risperidone add-on therapy for treating various episodes of bipolar (n=358) and schizoaffective (n=183) disorders.6 In this multicenter, open study, 33 patients (6.1%) suffered a depressed episode and received a mean risperidone dose of 1.6 (± 2.3) mg/d added to their ongoing but ineffective drug regimen. Mean HDRS declined significantly over the 6-month course. Further, switch rates were low and in the expected range for spontaneous fluctuations seen in these disorders.
The results of a 6-week, double-blind, controlled trial of risperidone versus haloperidol in 62 patients with schizoaffective disorder, bipolar or depressed subtype, were published.10 Risperidone (average dose of 5.5 mg/d) was comparable to haloperidol (average dose of 10.8 mg/d) in reducing the mean in the Positive and Negative Syndrome Scale and Clinician-Administered Rating Scale for Mania change scores.
In those patients with baseline HDRS scores ≥ 20, risperidone produced a significantly greater reduction in mean change scores than did haloperidol. In addition, patients had no mood switches with risperidone or haloperidol; there was a significantly higher incidence of patients who had extra-pyramidal symptoms with haloperidol than among those taking risperidone; and six patients in the group taking haloperidol dropped out after experiencing adverse effects. None of the patients taking risperidone dropped out.
Table 2
Lithium versus antipsychotics for acute mania
| Lithium | Antipsychotics | ||||
|---|---|---|---|---|---|
| Responders (%) (n=64) | Nonresponders (%) (n=10) | Responders (%) (n=38) | Nonresponders (%) (n=33) | Difference | |
| 5 studies | 89% | 11% | 54% | 46% | 35% |
| Adapted from Janicak PG, Newman RH, Davis JM. Advances in the treatment of mania and related disorders: a reappraisal. Psychiatric Ann. 1992;22(2):94. | |||||
Management of bipolar manic or mixed episodes
Up to 80% of all bipolar patients receive an antipsychotic drug during the acute and/or maintenance phase of their illness, even though loading doses of valproate and benzodiazepines may also be used during an exacerbation and pose much less risk, especially in terms of adverse neurological effects.
Neuroleptics Shortly after their introduction, neuroleptics were found to reduce mortality secondary to dehydration and exhaustion in many highly agitated patients during an acute manic episode such as lethal catatonia.7
While earlier controlled studies found these agents to be effective in the treatment of acute mania, they are clearly less efficacious than lithium for core manic symptoms.11Table 2 demonstrates a meta-analysis of five well-controlled, double-blind studies documenting the statistical superiority of lithium over neuroleptics. These agents, however, offer the advantage of a more rapid onset of action, particularly when given in the acute parenteral formulation, and are superior to lithium in the initial control of agitation. Further, long-acting depot formulations of neuroleptics may be the only viable strategy for chronic, recurrent, noncompliant patients.
As with psychotic depression, dosing and duration of neuroleptic treatment are important concerns. In this context, Rifkin et al demonstrated that 10 mg of haloperidol per day had comparable efficacy but fewer adverse effects than did 30 or 80 mg per day in a group of acutely manic patients.12 Despite such data, high chlorpromazine-equivalent doses are often administered acutely and maintained for sustained periods. This can be a significant problem given the apparent great sensitivity of bipolar patients to the neurological sequelae of these antipsychotic agents.
Novel antipsychotics Early case series reports indicated that clozapine may benefit treatment-refractory bipolar patients. Given the inherent drawbacks of clozapine (e.g., agranulocytosis and seizure induction), attention now focuses on other novel agents with more benign adverse effect profiles than clopazine. Controlled trials with olanzapine and risperidone serve to reinforce the usefulness of these as well as other novel agents.
Tohen et al published the results of a 3-week, double-blind, placebo-controlled trial of olanzapine in 139 patients experiencing an acute bipolar manic or mixed episode.13 Olanzapine produced a statistically greater mean improvement than did the placebo on the Young Mania Rating Scale (YMRS) change scores. Further, 49% of the olanzapine-treated group (n=70) met the a priori criteria for response versus only 24% of the placebo-treated group (n=69). A second study using a higher starting dose of olanzapine, less rescue medication, and longer treatment duration than the first study resulted in a similar outcome.14
Sachs et al reported on the results of a 3-week, double-blind, placebo-controlled trial involving 156 patients with bipolar manic or mixed subtype who received a mood stabilizer (lithium or valproate) plus a placebo, risperidone (1 to 6 mg/d), or haloperidol (2 to 12 mg/d).15 The clinicians concluded that risperidone plus a mood stabilizer was statistically superior to a placebo plus a mood stabilizer, and produced more rapid reduction in manic symptoms, regardless of whether psychosis was present.
Sajatovic et al16 published the results of a prospective, open trial with quetiapine (mean dose = 203 ± 124 mg/d) as add-on therapy in 20 patients (10 bipolar, 10 schizoaffective; 19 male, 1 female) insufficiently responsive to their mood stabilizer or antipsychotic. Pre-post assessments indicated significant improvement in the BPRS, Mania Rating Scale (MRS), and HDRS scores. While the combination was generally well tolerated, there was a mean weight gain of 4.9 kg (10.8 lb). This raises the specter of complications associated with substantial weight gain produced by several of the novel antipsychotics.
A recent report indicates that ziprasidone may also be an effective antimanic agent. In a randomized, double-blind, placebo-controlled, multicenter trial involving 210 bipolar (manic or mixed episodes) patients, ziprasidone (80 to 160 mg/d; n=140) was compared to a placebo (n=70) for 3 weeks.17 By day 2 and all subsequent time points, ziprasidone was superior in terms of mean change scores from the baseline MRS; produced a more rapid and significantly greater improvement in overall psychopathology in both positive and negative symptoms; and did not produce significant adverse effects (including relevant ECG parameter changes) when compared with the placebo. Similar trials are being conducted for risperidone, aripiprazole, and iloperidone.
Finally, Meehan et al18 reported on the results of an acute parenteral formulation of olanzapine used to manage agitation in an acute manic or mixed episode. This was a 24-hour, double-blind, placebo-controlled trial comparing intramuscular olanzapine to intramuscular lorazepam. The following results were indicated:
- Olanzapine (doses of 5 to 10 mg) produced a significantly greater reduction in excitation than did the placebo or lorazepam at 30 minutes post-injection.
- Twice as many patients receiving lorazepam or a placebo versus olanzapine required more than one injection.
- Except for olanzapine-induced tachycardia in one patient, there were no significant changes in vital signs, ECG parameters, or laboratory assays among the three groups.
- Somnolence (13%) and dizziness (9%) were the most frequent side effects in the olanzapine group.
Treatment strategies for depression and mania
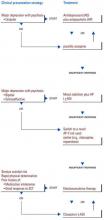
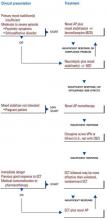
Considering the existing research data, clinical experience, and the risk/benefit ratio, treatment strategies that emphasize the role of antipsychotics in managing severe mood disorders are presented in the algorithms in Figures 1 and 2.
Figure 1 emphasizes the role of antipsychotics in the pharmacological management of patients with major depression. For unipolar depression with psychotic symptoms, options include an antidepressant plus an antipsychotic; amoxapine monotherapy; and possibly monotherapy with a novel agent such as ziprasidone. For bipolar depression with psychosis or schizoaffective disorder with depression, combining a mood stabilizer such as lithium plus an antipsychotic may be sufficient, but often an antidepressant must also be added. If the response is insufficient, consider switching to a novel antipsychotic (e.g., olanzapine or risperidone) plus a mood stabilizer (± antidepressant). In more serious exacerbations (e.g., high suicidality), ECT may be most appropriate. Secondary choices include clozapine with or without an antidepressant or novel antipsychotic such as risperidone combined with ECT.
Figure 2 describes the use of antipsychotics in patients with mania. If response to a primary mood stabilizer such as lithium, valproate, or their combination in the context of a bipolar or schizoaffective disorder is insufficient—or if patients have severe manic or psychotic symptoms—an antipsychotic may be added to the primary mood stabilizer.
Alternatively, when mood stabilizers are not tolerated or a clinical situation such as pregnancy precludes their use, a novel agent such as olanzapine or risperidone may be given as monotherapy. While the safety of these agents in pregnancy is not clearly established, clinical experience thus far indicates they may be safer than agents such as valproate or carbamazepine. These agents would be the first choice given their diminished propensity for extrapyramidal symptoms; absence of clozapine-related adverse effects such as agranulocytosis and seizures; and growing evidence of possible mood stabilizing effects.
Figure 1 ANTIPSYCHOTICS IN THE TREATMENT OF MAJOR DEPRESSION
Figure 2 ANTIPSYCHOTICS IN THE TREATMENT OF MANIA
For patients who remain nonresponsive, clozapine should be considered either as monotherapy or combined with valproate and/or lithium. Combining this agent with carbamazepine is not recommended because of the possibility of an increased risk of hematotoxicity.
Electroconvulsive therapy may be used safely and effectively in patients who are severely ill (e.g., those with manic delirium); pose an immediate danger because of their potential for violence; are in medical crisis; or have medical contraindications to pharmacotherapy. There is preliminary evidence that ECT can be safely administered with novel antipsychotics such as clozapine, risperidone, or olanzapine to produce additional benefit in patients insufficiently responsive to either therapy alone.
Related resources
- International Society for Bipolar Disorders www.isbd.org
Drug brand names
- Amitriptyline • Elavil
- Amoxapine • Asendin
- Aripiprazole • (in development)
- Carbamazepine • Tegretol, Epitol
- Clozapine • Clozaril
- Haloperidol • Haldol
- Iloperidone • (in development)
- Imipramine • Tofranil
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate sodium • Depacon
- Ziprasidone • Geodon
Disclosure
The author reports that he receives research/grant support from, serves as a consultant for, and on the speaker’s bureau of Janssen Pharmaceutica. He also receives research/grant support from Genentech Inc. and Bristol-Myers Squibb Co.; serves as a consultant for Pfizer Inc., Sepracor, and Novartis Pharmaceuticals Corp.; and is on the speaker’s bureau of Abbott Laboratories, Eli Lilly and Co., Pfizer Inc., Forest Pharmaceuticals, Bristol-Myers Squibb Co., and Wyeth-Ayerst Pharmaceuticals.
1. Kapur S, Cho R, Jones C, et al. Is amoxapine an atypical antipsychotic? Positronemission tomography investigation of its dopamine2 and serotonin2 occupancy. Biol Psychiatry. 1999;45:1217-1220.
2. Ostroff RB, Nelson JC. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry. 1999;60:256-259.
3. Rothschild AJ, Bates KS, Boehringer KL, Syed A. Olanzapine response in psychotic depression. J Clin Psychiatry. 1999;60:116-118.
4. Svestka J, Synek O. Does olanzapine have antidepressant effect? A double-blind amitriptyline-controlled study [abstract]. Int J Neuropsychopharmacol. 2000;3(suppl 1):S251.-
5. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry. 2001;158:131-134.
6. Vieta E, Goikolea JM, Corbella B, et al. Risperidone safety and efficacy in the treatment of bipolar and schizoaffective disorders: results from a 5-month, multicenter, open study. J Clin Psychiatry. 2001;62(10):818.-
7. Janicak PG, Davis JM, et al. Principles and Practice of Psychopharmacotherapy. 3rd ed. Philadelphia, Pa: Lippincott-Williams & Wilkins; 2001.
8. Daniel DG, Zimbroff DL, et al. for the Ziprasidone Study Group Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Neuropsychopharmacol. 1999;20(5):491-505.
9. Keck PE, Jr, Buffenstein A, Ferguson J, et al. Ziprasidone 40 and 120 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 4-week placebo-controlled trial. Psychopharmacol. 1998;140:173-184.
10. Janicak PG, Keck PE, Jr, Davis JM, et al. A double-blind, randomized, prospective evaluation of the efficacy and safety of risperidone versus haloperidol in the treatment of schizoaffective disorder. J Clin Psychopharmacol. 2001;21:360-368.
11. Keck PE, Welge JA, McElroy SL, et al. Placebo effect in randomized, controlled studies of acute bipolar mania and depression. Biol Psychiatry. 2000;47(8):756-761.
12. Rifkin A, Doddi S, Karajgi B, et al. Dosage of haloperidol for mania. Br J Psychiatry. 1994;165:113-116.
13. Tohen M, Sanger TM, McElroy SL, et al. Olanzipine versus placebo in the treatment of acute mania. Olanzapine HGEH Study Group. Am J Psychiatry. 1999;156:702-709.
14. Tohen M, Jacobs TG, Grundy SL, et al. Efficacy of olanzapine in acute bipolar mania: a double-blind, placebo-controlled study. The Olanzipine HGGW Study Group. Arch Gen Psychiatry. 2000;57:841-849.
15. Sachs G, Ghaemi N, Grossman F, Bowden C. Risperidone plus mood stabilizer vs. placebo plus mood stabilizer for acute mania of bipolar disorder: a double-blind comparison of efficacy and safety. International Congress on Bipolar Disorders. Pittsburgh, Pa. June 14-16, 2001.
16. Sajatovic M, Briscan DW, Perez DE, et al. Quetiapine alone and added to a mood stabilizer for serious mood disorders. J Clin Psychiatry. 2001;62:728-732.
17. Giller E, Mandel FS, Keck P. Ziprasidone in the acute treatment of mania: a double-blind, placebo-controlled, randomized trial. Schizophr Res. 2001;49(suppl 1-2):229.-
18. Meehan K, Zhang F, David S, Tohen N, Janicak PG, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular (IM) olanzapine versus IM lorazepam and IM placebo in acutely agitated patients diagnosed with mania associated with bipolar disorder. J Clin Psychopharmacol 2001;21:389-397.
Major mood disorders are challenging to diagnose and often difficult to treat. They entail unipolar depression; bipolar disorder, which includes manic, depressed, or mixed episodes; and schizoaffective disorder, which includes both depressed and bipolar subtypes. Antidepressants and mood stabilizers are the primary pharmacological treatments. They may be insufficient, however, for patients with more severe episodes, often characterized by psychosis and treatment resistance.
In these patients, antipsychotics have played an important but controversial part in management, primarily as oral or parenteral adjuncts. Literature and clinical experience now support another, unique role for the current generation of novel agents.
Compared to earlier antipsychotics, these agents produce substantially fewer neurological adverse effects, including acute extrapyramidal and tardive syndromes, and can augment antidepressants and mood stabilizers. In addition, they may:
- Possess a better antipsychotic profile, with enhanced therapeutic effects on positive, negative, cognitive, and mood symptoms
- Have a role in the acute and long-term management of these disorders when anticipated parenteral formulations become available (e.g., acute intramuscular olanzapine and ziprasidone—and long-acting intramuscular risperidone)
- Possess inherent thymoleptic properties (see “Unresolved issues with antipsychotics,” below).
- Defining what constitutes a mood stabilizer.1 Proposed definitions suggest that the drug must entail the following:
- Clarifying the mechanisms underlying the apparent mood-regulating effects of novel agents
- Ascertaining both acute and maintenance efficacy
- Clarifying the propensity of some agents to switch depressed patients into mania
- Increasing the number of well-designed studies with sufficient sample sizes, including comparison trials assessing the relative efficacy of different novel agents
- Reducing the tendency to publish only positive reports when new drugs are first available
- Introducing parenteral formulations of novel agents
- Resolving concerns about weight gain, new-onset diabetes, QT c prolongation, and sedation
- Rectifying the current level of substantially greater costs
Management of unipolar depression
Neuroleptics Delusions and hallucinations indicate a more severe form of depressive disorder, with poor short- and long-term outcomes in comparison to those without psychosis. To illustrate, Table 1 lists a summary of response rates in psychotic and nonpsychotic depressed patients given a tricyclic antidepressant (TCA). The data indicate that patients suffering from psychotic depression typically do not benefit from antidepressant monotherapy and usually require a combination of antidepressant and antipsychotic or, alternatively, electroconvulsive therapy (ECT).
There is, however, some limited clinical and neuroimaging evidence that amoxapine can be used as an effective monotherapy in this group. Amoxapine is an antidepressant whose primary active metabolite, 8-hydroxy amoxapine, may have antipsychotic properties.1 With the possible exception of amoxapine, combined antipsychotic-antidepressant treatment is the rule.
Table 1
Psychotic and nonpsychotic depressed patients’ response to monotherapy with a tricyclic antidepressant
| Psychotic | Nonpsychotic | ||||
|---|---|---|---|---|---|
| Responders (%) (n=127) | Nonresponders (%) (n=236) | Responders (%) (n=464) | Nonresponders (%) (n=227) | Difference | |
| 13 studies | 35% | 65% | 67% | 33% | 32% |
| Adapted from Chan CH, Janicak PG, Davis JM, et al. Response of psychotic and nonpsychotic depressed patients to tricyclic antidepressants. J Clin Psychiatry. 1987;48:197-200. | |||||
Historically, studies have also evaluated neuroleptic monotherapy for depressed patients. While some reported superiority over a placebo, none found conventional antipsychotics superior to imipramine. Indeed, patients with schizophrenia who are treated with a neuroleptic often develop symptoms that are difficult to distinguish from depression (e.g., secondary negative symptoms). These often improve when the neuroleptic is discontinued or the patients are switched to a novel antipsychotic such as risperidone, olanzapine, or ziprasidone, all of which have putative antidepressant effects.
When employing an antipsychotic in depressed patients, the dosage and duration of treatment are two critical considerations. To minimize neuromotor adverse effects, use low doses of a neuroleptic (e.g., haloperidol, 1 to 5 mg/d) in conjunction with the primary antidepressant therapy. The neuroleptic should then be tapered gradually after psychotic symptoms have been controlled, usually during the acute phase of treatment. Ideally, patients would then take antidepressant monotherapy through the continuation phase and, if necessary, the maintenance phase of treatment. If psychosis recurs, re-introduce the antipsychotic intermittently.
Novel antipsychotics In contrast to neuroleptics, novel antipsychotics have been reported to improve depression in various psychotic and mood disorders.
For example, ziprasidone has serotonin and noradrenergic reuptake blocking effects comparable to such classic TCAs as imipramine and amitriptyline, as well as high binding affinity at the 5-HT1A, 5-HT1D, and 5-HT2C receptors. This neuroreceptor profile indicates possible antidepressant effects.
While randomized, controlled trials with mood-disordered patients are few, there have been promising preliminary reports of augmentation of antidepressants with risperidone and olanzapine in both psychotic and nonpsychotic depressed patients.
Ostroff and Nelson2 reported the results of an open-label study of eight SSRI-nonresponsive patients (mean treatment 7.3 weeks). These patients had no psychotic features and had a dramatic reduction in depressive symptoms, as well as some improvement in sexual dysfunction, with the addition of 0.5 mg to 1.0 mg risperidone. The clinicians suggested that risperidone’s 5-HT2A antagonism might explain its augmentation of the partial SSRI response.
Olanzapine alone (n=3) or combined with an antidepressant (n=12) has also been reported to improve both depression and psychosis.3 In a double-blind, amitriptyline-controlled trial, Svestka and Synek4 found that olanzapine demonstrated antidepressant efficacy in 33 unipolar and seven bipolar depressed patients. Thirteen of these patients also had psychotic symptoms.
Shelton et al5 reported the results of a two-center, 8-week, double-blind comparison of olanzapine alone, fluoxetine alone, or their combination in 28 patients suffering from treatment-resistant, non-bipolar disorder without psychosis. They found that the combination was superior to either drug alone based on improvement in the Hamilton Depression Rating Scale (HDRS) total score. From their preliminary data, it also appears that the doses required were relatively low, reducing the risk of side effects.
Their findings, however, need to be replicated in more controlled studies with combinations, addressing possible adverse effects, the potential for clinically relevant drug interactions, decreased compliance rates, and increased cost of treatment. Earlier reports raised concern about the potential of these agents to increase switching to hypomania or mania. But in more recent reports, this has not emerged as a significant problem.7
Finally, several case reports and case series indicate that agents such as clozapine and risperidone may augment ECT in particularly severe, treatment-resistant depressive episodes.7
Management of bipolar and schizoaffective depressed episodes
Neuroleptics Antipsychotics are frequently used to manage more severe, usually psychotic episodes of bipolar and schizoaffective depression. Reports indicate that affectively ill patients receiving neuroleptics may be more prone to develop neuromotor adverse effects than are those suffering from schizophrenia. Thus, their use for such patients must be well justified, limited in dosage and duration, and carefully monitored for the emergence of acute and tardive neurological events.
Novel antipsychotics Novel antipsychotics have demonstrated fewer propensities than have neuroleptics in worsening depression or negative symptoms in schizophrenic patients, and have possible antidepressant effects. In support of this hypothesis, and reminiscent of data from earlier risperidone and olanzapine trials, ziprasidone was observed to improve the Montgomery Asberg Rating Scale (MADRS) and Brief Psychotic Rating Scale (BPRS) depressive cluster scores in three clinical trials with schizophrenic and schizoaffective patients.8,9
Vieta et al reported the efficacy and safety of risperidone add-on therapy for treating various episodes of bipolar (n=358) and schizoaffective (n=183) disorders.6 In this multicenter, open study, 33 patients (6.1%) suffered a depressed episode and received a mean risperidone dose of 1.6 (± 2.3) mg/d added to their ongoing but ineffective drug regimen. Mean HDRS declined significantly over the 6-month course. Further, switch rates were low and in the expected range for spontaneous fluctuations seen in these disorders.
The results of a 6-week, double-blind, controlled trial of risperidone versus haloperidol in 62 patients with schizoaffective disorder, bipolar or depressed subtype, were published.10 Risperidone (average dose of 5.5 mg/d) was comparable to haloperidol (average dose of 10.8 mg/d) in reducing the mean in the Positive and Negative Syndrome Scale and Clinician-Administered Rating Scale for Mania change scores.
In those patients with baseline HDRS scores ≥ 20, risperidone produced a significantly greater reduction in mean change scores than did haloperidol. In addition, patients had no mood switches with risperidone or haloperidol; there was a significantly higher incidence of patients who had extra-pyramidal symptoms with haloperidol than among those taking risperidone; and six patients in the group taking haloperidol dropped out after experiencing adverse effects. None of the patients taking risperidone dropped out.
Table 2
Lithium versus antipsychotics for acute mania
| Lithium | Antipsychotics | ||||
|---|---|---|---|---|---|
| Responders (%) (n=64) | Nonresponders (%) (n=10) | Responders (%) (n=38) | Nonresponders (%) (n=33) | Difference | |
| 5 studies | 89% | 11% | 54% | 46% | 35% |
| Adapted from Janicak PG, Newman RH, Davis JM. Advances in the treatment of mania and related disorders: a reappraisal. Psychiatric Ann. 1992;22(2):94. | |||||
Management of bipolar manic or mixed episodes
Up to 80% of all bipolar patients receive an antipsychotic drug during the acute and/or maintenance phase of their illness, even though loading doses of valproate and benzodiazepines may also be used during an exacerbation and pose much less risk, especially in terms of adverse neurological effects.
Neuroleptics Shortly after their introduction, neuroleptics were found to reduce mortality secondary to dehydration and exhaustion in many highly agitated patients during an acute manic episode such as lethal catatonia.7
While earlier controlled studies found these agents to be effective in the treatment of acute mania, they are clearly less efficacious than lithium for core manic symptoms.11Table 2 demonstrates a meta-analysis of five well-controlled, double-blind studies documenting the statistical superiority of lithium over neuroleptics. These agents, however, offer the advantage of a more rapid onset of action, particularly when given in the acute parenteral formulation, and are superior to lithium in the initial control of agitation. Further, long-acting depot formulations of neuroleptics may be the only viable strategy for chronic, recurrent, noncompliant patients.
As with psychotic depression, dosing and duration of neuroleptic treatment are important concerns. In this context, Rifkin et al demonstrated that 10 mg of haloperidol per day had comparable efficacy but fewer adverse effects than did 30 or 80 mg per day in a group of acutely manic patients.12 Despite such data, high chlorpromazine-equivalent doses are often administered acutely and maintained for sustained periods. This can be a significant problem given the apparent great sensitivity of bipolar patients to the neurological sequelae of these antipsychotic agents.
Novel antipsychotics Early case series reports indicated that clozapine may benefit treatment-refractory bipolar patients. Given the inherent drawbacks of clozapine (e.g., agranulocytosis and seizure induction), attention now focuses on other novel agents with more benign adverse effect profiles than clopazine. Controlled trials with olanzapine and risperidone serve to reinforce the usefulness of these as well as other novel agents.
Tohen et al published the results of a 3-week, double-blind, placebo-controlled trial of olanzapine in 139 patients experiencing an acute bipolar manic or mixed episode.13 Olanzapine produced a statistically greater mean improvement than did the placebo on the Young Mania Rating Scale (YMRS) change scores. Further, 49% of the olanzapine-treated group (n=70) met the a priori criteria for response versus only 24% of the placebo-treated group (n=69). A second study using a higher starting dose of olanzapine, less rescue medication, and longer treatment duration than the first study resulted in a similar outcome.14
Sachs et al reported on the results of a 3-week, double-blind, placebo-controlled trial involving 156 patients with bipolar manic or mixed subtype who received a mood stabilizer (lithium or valproate) plus a placebo, risperidone (1 to 6 mg/d), or haloperidol (2 to 12 mg/d).15 The clinicians concluded that risperidone plus a mood stabilizer was statistically superior to a placebo plus a mood stabilizer, and produced more rapid reduction in manic symptoms, regardless of whether psychosis was present.
Sajatovic et al16 published the results of a prospective, open trial with quetiapine (mean dose = 203 ± 124 mg/d) as add-on therapy in 20 patients (10 bipolar, 10 schizoaffective; 19 male, 1 female) insufficiently responsive to their mood stabilizer or antipsychotic. Pre-post assessments indicated significant improvement in the BPRS, Mania Rating Scale (MRS), and HDRS scores. While the combination was generally well tolerated, there was a mean weight gain of 4.9 kg (10.8 lb). This raises the specter of complications associated with substantial weight gain produced by several of the novel antipsychotics.
A recent report indicates that ziprasidone may also be an effective antimanic agent. In a randomized, double-blind, placebo-controlled, multicenter trial involving 210 bipolar (manic or mixed episodes) patients, ziprasidone (80 to 160 mg/d; n=140) was compared to a placebo (n=70) for 3 weeks.17 By day 2 and all subsequent time points, ziprasidone was superior in terms of mean change scores from the baseline MRS; produced a more rapid and significantly greater improvement in overall psychopathology in both positive and negative symptoms; and did not produce significant adverse effects (including relevant ECG parameter changes) when compared with the placebo. Similar trials are being conducted for risperidone, aripiprazole, and iloperidone.
Finally, Meehan et al18 reported on the results of an acute parenteral formulation of olanzapine used to manage agitation in an acute manic or mixed episode. This was a 24-hour, double-blind, placebo-controlled trial comparing intramuscular olanzapine to intramuscular lorazepam. The following results were indicated:
- Olanzapine (doses of 5 to 10 mg) produced a significantly greater reduction in excitation than did the placebo or lorazepam at 30 minutes post-injection.
- Twice as many patients receiving lorazepam or a placebo versus olanzapine required more than one injection.
- Except for olanzapine-induced tachycardia in one patient, there were no significant changes in vital signs, ECG parameters, or laboratory assays among the three groups.
- Somnolence (13%) and dizziness (9%) were the most frequent side effects in the olanzapine group.
Treatment strategies for depression and mania
Considering the existing research data, clinical experience, and the risk/benefit ratio, treatment strategies that emphasize the role of antipsychotics in managing severe mood disorders are presented in the algorithms in Figures 1 and 2.
Figure 1 emphasizes the role of antipsychotics in the pharmacological management of patients with major depression. For unipolar depression with psychotic symptoms, options include an antidepressant plus an antipsychotic; amoxapine monotherapy; and possibly monotherapy with a novel agent such as ziprasidone. For bipolar depression with psychosis or schizoaffective disorder with depression, combining a mood stabilizer such as lithium plus an antipsychotic may be sufficient, but often an antidepressant must also be added. If the response is insufficient, consider switching to a novel antipsychotic (e.g., olanzapine or risperidone) plus a mood stabilizer (± antidepressant). In more serious exacerbations (e.g., high suicidality), ECT may be most appropriate. Secondary choices include clozapine with or without an antidepressant or novel antipsychotic such as risperidone combined with ECT.
Figure 2 describes the use of antipsychotics in patients with mania. If response to a primary mood stabilizer such as lithium, valproate, or their combination in the context of a bipolar or schizoaffective disorder is insufficient—or if patients have severe manic or psychotic symptoms—an antipsychotic may be added to the primary mood stabilizer.
Alternatively, when mood stabilizers are not tolerated or a clinical situation such as pregnancy precludes their use, a novel agent such as olanzapine or risperidone may be given as monotherapy. While the safety of these agents in pregnancy is not clearly established, clinical experience thus far indicates they may be safer than agents such as valproate or carbamazepine. These agents would be the first choice given their diminished propensity for extrapyramidal symptoms; absence of clozapine-related adverse effects such as agranulocytosis and seizures; and growing evidence of possible mood stabilizing effects.
Figure 1 ANTIPSYCHOTICS IN THE TREATMENT OF MAJOR DEPRESSION
Figure 2 ANTIPSYCHOTICS IN THE TREATMENT OF MANIA
For patients who remain nonresponsive, clozapine should be considered either as monotherapy or combined with valproate and/or lithium. Combining this agent with carbamazepine is not recommended because of the possibility of an increased risk of hematotoxicity.
Electroconvulsive therapy may be used safely and effectively in patients who are severely ill (e.g., those with manic delirium); pose an immediate danger because of their potential for violence; are in medical crisis; or have medical contraindications to pharmacotherapy. There is preliminary evidence that ECT can be safely administered with novel antipsychotics such as clozapine, risperidone, or olanzapine to produce additional benefit in patients insufficiently responsive to either therapy alone.
Related resources
- International Society for Bipolar Disorders www.isbd.org
Drug brand names
- Amitriptyline • Elavil
- Amoxapine • Asendin
- Aripiprazole • (in development)
- Carbamazepine • Tegretol, Epitol
- Clozapine • Clozaril
- Haloperidol • Haldol
- Iloperidone • (in development)
- Imipramine • Tofranil
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate sodium • Depacon
- Ziprasidone • Geodon
Disclosure
The author reports that he receives research/grant support from, serves as a consultant for, and on the speaker’s bureau of Janssen Pharmaceutica. He also receives research/grant support from Genentech Inc. and Bristol-Myers Squibb Co.; serves as a consultant for Pfizer Inc., Sepracor, and Novartis Pharmaceuticals Corp.; and is on the speaker’s bureau of Abbott Laboratories, Eli Lilly and Co., Pfizer Inc., Forest Pharmaceuticals, Bristol-Myers Squibb Co., and Wyeth-Ayerst Pharmaceuticals.
Major mood disorders are challenging to diagnose and often difficult to treat. They entail unipolar depression; bipolar disorder, which includes manic, depressed, or mixed episodes; and schizoaffective disorder, which includes both depressed and bipolar subtypes. Antidepressants and mood stabilizers are the primary pharmacological treatments. They may be insufficient, however, for patients with more severe episodes, often characterized by psychosis and treatment resistance.
In these patients, antipsychotics have played an important but controversial part in management, primarily as oral or parenteral adjuncts. Literature and clinical experience now support another, unique role for the current generation of novel agents.
Compared to earlier antipsychotics, these agents produce substantially fewer neurological adverse effects, including acute extrapyramidal and tardive syndromes, and can augment antidepressants and mood stabilizers. In addition, they may:
- Possess a better antipsychotic profile, with enhanced therapeutic effects on positive, negative, cognitive, and mood symptoms
- Have a role in the acute and long-term management of these disorders when anticipated parenteral formulations become available (e.g., acute intramuscular olanzapine and ziprasidone—and long-acting intramuscular risperidone)
- Possess inherent thymoleptic properties (see “Unresolved issues with antipsychotics,” below).
- Defining what constitutes a mood stabilizer.1 Proposed definitions suggest that the drug must entail the following:
- Clarifying the mechanisms underlying the apparent mood-regulating effects of novel agents
- Ascertaining both acute and maintenance efficacy
- Clarifying the propensity of some agents to switch depressed patients into mania
- Increasing the number of well-designed studies with sufficient sample sizes, including comparison trials assessing the relative efficacy of different novel agents
- Reducing the tendency to publish only positive reports when new drugs are first available
- Introducing parenteral formulations of novel agents
- Resolving concerns about weight gain, new-onset diabetes, QT c prolongation, and sedation
- Rectifying the current level of substantially greater costs
Management of unipolar depression
Neuroleptics Delusions and hallucinations indicate a more severe form of depressive disorder, with poor short- and long-term outcomes in comparison to those without psychosis. To illustrate, Table 1 lists a summary of response rates in psychotic and nonpsychotic depressed patients given a tricyclic antidepressant (TCA). The data indicate that patients suffering from psychotic depression typically do not benefit from antidepressant monotherapy and usually require a combination of antidepressant and antipsychotic or, alternatively, electroconvulsive therapy (ECT).
There is, however, some limited clinical and neuroimaging evidence that amoxapine can be used as an effective monotherapy in this group. Amoxapine is an antidepressant whose primary active metabolite, 8-hydroxy amoxapine, may have antipsychotic properties.1 With the possible exception of amoxapine, combined antipsychotic-antidepressant treatment is the rule.
Table 1
Psychotic and nonpsychotic depressed patients’ response to monotherapy with a tricyclic antidepressant
| Psychotic | Nonpsychotic | ||||
|---|---|---|---|---|---|
| Responders (%) (n=127) | Nonresponders (%) (n=236) | Responders (%) (n=464) | Nonresponders (%) (n=227) | Difference | |
| 13 studies | 35% | 65% | 67% | 33% | 32% |
| Adapted from Chan CH, Janicak PG, Davis JM, et al. Response of psychotic and nonpsychotic depressed patients to tricyclic antidepressants. J Clin Psychiatry. 1987;48:197-200. | |||||
Historically, studies have also evaluated neuroleptic monotherapy for depressed patients. While some reported superiority over a placebo, none found conventional antipsychotics superior to imipramine. Indeed, patients with schizophrenia who are treated with a neuroleptic often develop symptoms that are difficult to distinguish from depression (e.g., secondary negative symptoms). These often improve when the neuroleptic is discontinued or the patients are switched to a novel antipsychotic such as risperidone, olanzapine, or ziprasidone, all of which have putative antidepressant effects.
When employing an antipsychotic in depressed patients, the dosage and duration of treatment are two critical considerations. To minimize neuromotor adverse effects, use low doses of a neuroleptic (e.g., haloperidol, 1 to 5 mg/d) in conjunction with the primary antidepressant therapy. The neuroleptic should then be tapered gradually after psychotic symptoms have been controlled, usually during the acute phase of treatment. Ideally, patients would then take antidepressant monotherapy through the continuation phase and, if necessary, the maintenance phase of treatment. If psychosis recurs, re-introduce the antipsychotic intermittently.
Novel antipsychotics In contrast to neuroleptics, novel antipsychotics have been reported to improve depression in various psychotic and mood disorders.
For example, ziprasidone has serotonin and noradrenergic reuptake blocking effects comparable to such classic TCAs as imipramine and amitriptyline, as well as high binding affinity at the 5-HT1A, 5-HT1D, and 5-HT2C receptors. This neuroreceptor profile indicates possible antidepressant effects.
While randomized, controlled trials with mood-disordered patients are few, there have been promising preliminary reports of augmentation of antidepressants with risperidone and olanzapine in both psychotic and nonpsychotic depressed patients.
Ostroff and Nelson2 reported the results of an open-label study of eight SSRI-nonresponsive patients (mean treatment 7.3 weeks). These patients had no psychotic features and had a dramatic reduction in depressive symptoms, as well as some improvement in sexual dysfunction, with the addition of 0.5 mg to 1.0 mg risperidone. The clinicians suggested that risperidone’s 5-HT2A antagonism might explain its augmentation of the partial SSRI response.
Olanzapine alone (n=3) or combined with an antidepressant (n=12) has also been reported to improve both depression and psychosis.3 In a double-blind, amitriptyline-controlled trial, Svestka and Synek4 found that olanzapine demonstrated antidepressant efficacy in 33 unipolar and seven bipolar depressed patients. Thirteen of these patients also had psychotic symptoms.
Shelton et al5 reported the results of a two-center, 8-week, double-blind comparison of olanzapine alone, fluoxetine alone, or their combination in 28 patients suffering from treatment-resistant, non-bipolar disorder without psychosis. They found that the combination was superior to either drug alone based on improvement in the Hamilton Depression Rating Scale (HDRS) total score. From their preliminary data, it also appears that the doses required were relatively low, reducing the risk of side effects.
Their findings, however, need to be replicated in more controlled studies with combinations, addressing possible adverse effects, the potential for clinically relevant drug interactions, decreased compliance rates, and increased cost of treatment. Earlier reports raised concern about the potential of these agents to increase switching to hypomania or mania. But in more recent reports, this has not emerged as a significant problem.7
Finally, several case reports and case series indicate that agents such as clozapine and risperidone may augment ECT in particularly severe, treatment-resistant depressive episodes.7
Management of bipolar and schizoaffective depressed episodes
Neuroleptics Antipsychotics are frequently used to manage more severe, usually psychotic episodes of bipolar and schizoaffective depression. Reports indicate that affectively ill patients receiving neuroleptics may be more prone to develop neuromotor adverse effects than are those suffering from schizophrenia. Thus, their use for such patients must be well justified, limited in dosage and duration, and carefully monitored for the emergence of acute and tardive neurological events.
Novel antipsychotics Novel antipsychotics have demonstrated fewer propensities than have neuroleptics in worsening depression or negative symptoms in schizophrenic patients, and have possible antidepressant effects. In support of this hypothesis, and reminiscent of data from earlier risperidone and olanzapine trials, ziprasidone was observed to improve the Montgomery Asberg Rating Scale (MADRS) and Brief Psychotic Rating Scale (BPRS) depressive cluster scores in three clinical trials with schizophrenic and schizoaffective patients.8,9
Vieta et al reported the efficacy and safety of risperidone add-on therapy for treating various episodes of bipolar (n=358) and schizoaffective (n=183) disorders.6 In this multicenter, open study, 33 patients (6.1%) suffered a depressed episode and received a mean risperidone dose of 1.6 (± 2.3) mg/d added to their ongoing but ineffective drug regimen. Mean HDRS declined significantly over the 6-month course. Further, switch rates were low and in the expected range for spontaneous fluctuations seen in these disorders.
The results of a 6-week, double-blind, controlled trial of risperidone versus haloperidol in 62 patients with schizoaffective disorder, bipolar or depressed subtype, were published.10 Risperidone (average dose of 5.5 mg/d) was comparable to haloperidol (average dose of 10.8 mg/d) in reducing the mean in the Positive and Negative Syndrome Scale and Clinician-Administered Rating Scale for Mania change scores.
In those patients with baseline HDRS scores ≥ 20, risperidone produced a significantly greater reduction in mean change scores than did haloperidol. In addition, patients had no mood switches with risperidone or haloperidol; there was a significantly higher incidence of patients who had extra-pyramidal symptoms with haloperidol than among those taking risperidone; and six patients in the group taking haloperidol dropped out after experiencing adverse effects. None of the patients taking risperidone dropped out.
Table 2
Lithium versus antipsychotics for acute mania
| Lithium | Antipsychotics | ||||
|---|---|---|---|---|---|
| Responders (%) (n=64) | Nonresponders (%) (n=10) | Responders (%) (n=38) | Nonresponders (%) (n=33) | Difference | |
| 5 studies | 89% | 11% | 54% | 46% | 35% |
| Adapted from Janicak PG, Newman RH, Davis JM. Advances in the treatment of mania and related disorders: a reappraisal. Psychiatric Ann. 1992;22(2):94. | |||||
Management of bipolar manic or mixed episodes
Up to 80% of all bipolar patients receive an antipsychotic drug during the acute and/or maintenance phase of their illness, even though loading doses of valproate and benzodiazepines may also be used during an exacerbation and pose much less risk, especially in terms of adverse neurological effects.
Neuroleptics Shortly after their introduction, neuroleptics were found to reduce mortality secondary to dehydration and exhaustion in many highly agitated patients during an acute manic episode such as lethal catatonia.7
While earlier controlled studies found these agents to be effective in the treatment of acute mania, they are clearly less efficacious than lithium for core manic symptoms.11Table 2 demonstrates a meta-analysis of five well-controlled, double-blind studies documenting the statistical superiority of lithium over neuroleptics. These agents, however, offer the advantage of a more rapid onset of action, particularly when given in the acute parenteral formulation, and are superior to lithium in the initial control of agitation. Further, long-acting depot formulations of neuroleptics may be the only viable strategy for chronic, recurrent, noncompliant patients.
As with psychotic depression, dosing and duration of neuroleptic treatment are important concerns. In this context, Rifkin et al demonstrated that 10 mg of haloperidol per day had comparable efficacy but fewer adverse effects than did 30 or 80 mg per day in a group of acutely manic patients.12 Despite such data, high chlorpromazine-equivalent doses are often administered acutely and maintained for sustained periods. This can be a significant problem given the apparent great sensitivity of bipolar patients to the neurological sequelae of these antipsychotic agents.
Novel antipsychotics Early case series reports indicated that clozapine may benefit treatment-refractory bipolar patients. Given the inherent drawbacks of clozapine (e.g., agranulocytosis and seizure induction), attention now focuses on other novel agents with more benign adverse effect profiles than clopazine. Controlled trials with olanzapine and risperidone serve to reinforce the usefulness of these as well as other novel agents.
Tohen et al published the results of a 3-week, double-blind, placebo-controlled trial of olanzapine in 139 patients experiencing an acute bipolar manic or mixed episode.13 Olanzapine produced a statistically greater mean improvement than did the placebo on the Young Mania Rating Scale (YMRS) change scores. Further, 49% of the olanzapine-treated group (n=70) met the a priori criteria for response versus only 24% of the placebo-treated group (n=69). A second study using a higher starting dose of olanzapine, less rescue medication, and longer treatment duration than the first study resulted in a similar outcome.14
Sachs et al reported on the results of a 3-week, double-blind, placebo-controlled trial involving 156 patients with bipolar manic or mixed subtype who received a mood stabilizer (lithium or valproate) plus a placebo, risperidone (1 to 6 mg/d), or haloperidol (2 to 12 mg/d).15 The clinicians concluded that risperidone plus a mood stabilizer was statistically superior to a placebo plus a mood stabilizer, and produced more rapid reduction in manic symptoms, regardless of whether psychosis was present.
Sajatovic et al16 published the results of a prospective, open trial with quetiapine (mean dose = 203 ± 124 mg/d) as add-on therapy in 20 patients (10 bipolar, 10 schizoaffective; 19 male, 1 female) insufficiently responsive to their mood stabilizer or antipsychotic. Pre-post assessments indicated significant improvement in the BPRS, Mania Rating Scale (MRS), and HDRS scores. While the combination was generally well tolerated, there was a mean weight gain of 4.9 kg (10.8 lb). This raises the specter of complications associated with substantial weight gain produced by several of the novel antipsychotics.
A recent report indicates that ziprasidone may also be an effective antimanic agent. In a randomized, double-blind, placebo-controlled, multicenter trial involving 210 bipolar (manic or mixed episodes) patients, ziprasidone (80 to 160 mg/d; n=140) was compared to a placebo (n=70) for 3 weeks.17 By day 2 and all subsequent time points, ziprasidone was superior in terms of mean change scores from the baseline MRS; produced a more rapid and significantly greater improvement in overall psychopathology in both positive and negative symptoms; and did not produce significant adverse effects (including relevant ECG parameter changes) when compared with the placebo. Similar trials are being conducted for risperidone, aripiprazole, and iloperidone.
Finally, Meehan et al18 reported on the results of an acute parenteral formulation of olanzapine used to manage agitation in an acute manic or mixed episode. This was a 24-hour, double-blind, placebo-controlled trial comparing intramuscular olanzapine to intramuscular lorazepam. The following results were indicated:
- Olanzapine (doses of 5 to 10 mg) produced a significantly greater reduction in excitation than did the placebo or lorazepam at 30 minutes post-injection.
- Twice as many patients receiving lorazepam or a placebo versus olanzapine required more than one injection.
- Except for olanzapine-induced tachycardia in one patient, there were no significant changes in vital signs, ECG parameters, or laboratory assays among the three groups.
- Somnolence (13%) and dizziness (9%) were the most frequent side effects in the olanzapine group.
Treatment strategies for depression and mania
Considering the existing research data, clinical experience, and the risk/benefit ratio, treatment strategies that emphasize the role of antipsychotics in managing severe mood disorders are presented in the algorithms in Figures 1 and 2.
Figure 1 emphasizes the role of antipsychotics in the pharmacological management of patients with major depression. For unipolar depression with psychotic symptoms, options include an antidepressant plus an antipsychotic; amoxapine monotherapy; and possibly monotherapy with a novel agent such as ziprasidone. For bipolar depression with psychosis or schizoaffective disorder with depression, combining a mood stabilizer such as lithium plus an antipsychotic may be sufficient, but often an antidepressant must also be added. If the response is insufficient, consider switching to a novel antipsychotic (e.g., olanzapine or risperidone) plus a mood stabilizer (± antidepressant). In more serious exacerbations (e.g., high suicidality), ECT may be most appropriate. Secondary choices include clozapine with or without an antidepressant or novel antipsychotic such as risperidone combined with ECT.
Figure 2 describes the use of antipsychotics in patients with mania. If response to a primary mood stabilizer such as lithium, valproate, or their combination in the context of a bipolar or schizoaffective disorder is insufficient—or if patients have severe manic or psychotic symptoms—an antipsychotic may be added to the primary mood stabilizer.
Alternatively, when mood stabilizers are not tolerated or a clinical situation such as pregnancy precludes their use, a novel agent such as olanzapine or risperidone may be given as monotherapy. While the safety of these agents in pregnancy is not clearly established, clinical experience thus far indicates they may be safer than agents such as valproate or carbamazepine. These agents would be the first choice given their diminished propensity for extrapyramidal symptoms; absence of clozapine-related adverse effects such as agranulocytosis and seizures; and growing evidence of possible mood stabilizing effects.
Figure 1 ANTIPSYCHOTICS IN THE TREATMENT OF MAJOR DEPRESSION
Figure 2 ANTIPSYCHOTICS IN THE TREATMENT OF MANIA
For patients who remain nonresponsive, clozapine should be considered either as monotherapy or combined with valproate and/or lithium. Combining this agent with carbamazepine is not recommended because of the possibility of an increased risk of hematotoxicity.
Electroconvulsive therapy may be used safely and effectively in patients who are severely ill (e.g., those with manic delirium); pose an immediate danger because of their potential for violence; are in medical crisis; or have medical contraindications to pharmacotherapy. There is preliminary evidence that ECT can be safely administered with novel antipsychotics such as clozapine, risperidone, or olanzapine to produce additional benefit in patients insufficiently responsive to either therapy alone.
Related resources
- International Society for Bipolar Disorders www.isbd.org
Drug brand names
- Amitriptyline • Elavil
- Amoxapine • Asendin
- Aripiprazole • (in development)
- Carbamazepine • Tegretol, Epitol
- Clozapine • Clozaril
- Haloperidol • Haldol
- Iloperidone • (in development)
- Imipramine • Tofranil
- Lorazepam • Ativan
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Valproate sodium • Depacon
- Ziprasidone • Geodon
Disclosure
The author reports that he receives research/grant support from, serves as a consultant for, and on the speaker’s bureau of Janssen Pharmaceutica. He also receives research/grant support from Genentech Inc. and Bristol-Myers Squibb Co.; serves as a consultant for Pfizer Inc., Sepracor, and Novartis Pharmaceuticals Corp.; and is on the speaker’s bureau of Abbott Laboratories, Eli Lilly and Co., Pfizer Inc., Forest Pharmaceuticals, Bristol-Myers Squibb Co., and Wyeth-Ayerst Pharmaceuticals.
1. Kapur S, Cho R, Jones C, et al. Is amoxapine an atypical antipsychotic? Positronemission tomography investigation of its dopamine2 and serotonin2 occupancy. Biol Psychiatry. 1999;45:1217-1220.
2. Ostroff RB, Nelson JC. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry. 1999;60:256-259.
3. Rothschild AJ, Bates KS, Boehringer KL, Syed A. Olanzapine response in psychotic depression. J Clin Psychiatry. 1999;60:116-118.
4. Svestka J, Synek O. Does olanzapine have antidepressant effect? A double-blind amitriptyline-controlled study [abstract]. Int J Neuropsychopharmacol. 2000;3(suppl 1):S251.-
5. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry. 2001;158:131-134.
6. Vieta E, Goikolea JM, Corbella B, et al. Risperidone safety and efficacy in the treatment of bipolar and schizoaffective disorders: results from a 5-month, multicenter, open study. J Clin Psychiatry. 2001;62(10):818.-
7. Janicak PG, Davis JM, et al. Principles and Practice of Psychopharmacotherapy. 3rd ed. Philadelphia, Pa: Lippincott-Williams & Wilkins; 2001.
8. Daniel DG, Zimbroff DL, et al. for the Ziprasidone Study Group Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Neuropsychopharmacol. 1999;20(5):491-505.
9. Keck PE, Jr, Buffenstein A, Ferguson J, et al. Ziprasidone 40 and 120 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 4-week placebo-controlled trial. Psychopharmacol. 1998;140:173-184.
10. Janicak PG, Keck PE, Jr, Davis JM, et al. A double-blind, randomized, prospective evaluation of the efficacy and safety of risperidone versus haloperidol in the treatment of schizoaffective disorder. J Clin Psychopharmacol. 2001;21:360-368.
11. Keck PE, Welge JA, McElroy SL, et al. Placebo effect in randomized, controlled studies of acute bipolar mania and depression. Biol Psychiatry. 2000;47(8):756-761.
12. Rifkin A, Doddi S, Karajgi B, et al. Dosage of haloperidol for mania. Br J Psychiatry. 1994;165:113-116.
13. Tohen M, Sanger TM, McElroy SL, et al. Olanzipine versus placebo in the treatment of acute mania. Olanzapine HGEH Study Group. Am J Psychiatry. 1999;156:702-709.
14. Tohen M, Jacobs TG, Grundy SL, et al. Efficacy of olanzapine in acute bipolar mania: a double-blind, placebo-controlled study. The Olanzipine HGGW Study Group. Arch Gen Psychiatry. 2000;57:841-849.
15. Sachs G, Ghaemi N, Grossman F, Bowden C. Risperidone plus mood stabilizer vs. placebo plus mood stabilizer for acute mania of bipolar disorder: a double-blind comparison of efficacy and safety. International Congress on Bipolar Disorders. Pittsburgh, Pa. June 14-16, 2001.
16. Sajatovic M, Briscan DW, Perez DE, et al. Quetiapine alone and added to a mood stabilizer for serious mood disorders. J Clin Psychiatry. 2001;62:728-732.
17. Giller E, Mandel FS, Keck P. Ziprasidone in the acute treatment of mania: a double-blind, placebo-controlled, randomized trial. Schizophr Res. 2001;49(suppl 1-2):229.-
18. Meehan K, Zhang F, David S, Tohen N, Janicak PG, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular (IM) olanzapine versus IM lorazepam and IM placebo in acutely agitated patients diagnosed with mania associated with bipolar disorder. J Clin Psychopharmacol 2001;21:389-397.
1. Kapur S, Cho R, Jones C, et al. Is amoxapine an atypical antipsychotic? Positronemission tomography investigation of its dopamine2 and serotonin2 occupancy. Biol Psychiatry. 1999;45:1217-1220.
2. Ostroff RB, Nelson JC. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry. 1999;60:256-259.
3. Rothschild AJ, Bates KS, Boehringer KL, Syed A. Olanzapine response in psychotic depression. J Clin Psychiatry. 1999;60:116-118.
4. Svestka J, Synek O. Does olanzapine have antidepressant effect? A double-blind amitriptyline-controlled study [abstract]. Int J Neuropsychopharmacol. 2000;3(suppl 1):S251.-
5. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry. 2001;158:131-134.
6. Vieta E, Goikolea JM, Corbella B, et al. Risperidone safety and efficacy in the treatment of bipolar and schizoaffective disorders: results from a 5-month, multicenter, open study. J Clin Psychiatry. 2001;62(10):818.-
7. Janicak PG, Davis JM, et al. Principles and Practice of Psychopharmacotherapy. 3rd ed. Philadelphia, Pa: Lippincott-Williams & Wilkins; 2001.
8. Daniel DG, Zimbroff DL, et al. for the Ziprasidone Study Group Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Neuropsychopharmacol. 1999;20(5):491-505.
9. Keck PE, Jr, Buffenstein A, Ferguson J, et al. Ziprasidone 40 and 120 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 4-week placebo-controlled trial. Psychopharmacol. 1998;140:173-184.
10. Janicak PG, Keck PE, Jr, Davis JM, et al. A double-blind, randomized, prospective evaluation of the efficacy and safety of risperidone versus haloperidol in the treatment of schizoaffective disorder. J Clin Psychopharmacol. 2001;21:360-368.
11. Keck PE, Welge JA, McElroy SL, et al. Placebo effect in randomized, controlled studies of acute bipolar mania and depression. Biol Psychiatry. 2000;47(8):756-761.
12. Rifkin A, Doddi S, Karajgi B, et al. Dosage of haloperidol for mania. Br J Psychiatry. 1994;165:113-116.
13. Tohen M, Sanger TM, McElroy SL, et al. Olanzipine versus placebo in the treatment of acute mania. Olanzapine HGEH Study Group. Am J Psychiatry. 1999;156:702-709.
14. Tohen M, Jacobs TG, Grundy SL, et al. Efficacy of olanzapine in acute bipolar mania: a double-blind, placebo-controlled study. The Olanzipine HGGW Study Group. Arch Gen Psychiatry. 2000;57:841-849.
15. Sachs G, Ghaemi N, Grossman F, Bowden C. Risperidone plus mood stabilizer vs. placebo plus mood stabilizer for acute mania of bipolar disorder: a double-blind comparison of efficacy and safety. International Congress on Bipolar Disorders. Pittsburgh, Pa. June 14-16, 2001.
16. Sajatovic M, Briscan DW, Perez DE, et al. Quetiapine alone and added to a mood stabilizer for serious mood disorders. J Clin Psychiatry. 2001;62:728-732.
17. Giller E, Mandel FS, Keck P. Ziprasidone in the acute treatment of mania: a double-blind, placebo-controlled, randomized trial. Schizophr Res. 2001;49(suppl 1-2):229.-
18. Meehan K, Zhang F, David S, Tohen N, Janicak PG, et al. A double-blind, randomized comparison of the efficacy and safety of intramuscular (IM) olanzapine versus IM lorazepam and IM placebo in acutely agitated patients diagnosed with mania associated with bipolar disorder. J Clin Psychopharmacol 2001;21:389-397.
Antiepileptic drugs for bipolar disorder: Are there any clear winners?
The newer antiepileptic drugs pose a sometimes bewildering range of options for bipolar disorder treatment. Which work best for acute bipolar I mania? Which are best suited for maintenance in patients with mixed episodes, or for those with a history of rapid cycling? What about prevention of depressive episodes? And how do the antiepileptics compare with lithium?
For many patients with bipolar disorder, lithium is still the drug of choice. For others, however, an increasing body of evidence supports the efficacy of some antiepileptics and atypical antipsychotics.
The mood-stabilizing properties of two antiepileptic agents, carbamazepine and valproate, were demonstrated some years ago in randomized controlled trials in patients with bipolar disorder. Since then, there has been considerable interest in the potential thymoleptic properties of the new antiepileptic drugs.1 In recent years gabapentin, lamotrigine, topiramate, oxcarbazepine, zonisamide, tiagabine, and levetiracetam have been approved in the United States for the treatment of various types of epilepsy. These medications have diverse pharmacological properties that distinguish them from earlier agents and from one another.
Do these new agents have anything to offer patients? For the most part, the evidence is not yet in hand, but we will examine what’s available, starting with the most recent trial data regarding the efficacy of valproate and carbamazepine.
Carbamazepine for bipolar mania
Five randomized, controlled trials2 have shown the efficacy of carbamazepine in patients with acute bipolar I mania. Carbamazepine was superior to placebo and comparable to chlorpromazine and lithium. Pooled data reveal an overall response rate (defined as the proportion of patients experiencing > 50% reduction in manic symptoms) of 50% for carbamazepine, 56% for lithium, and 61% for chlorpromazine (differences in overall response rates are not significant).
Until recently, the efficacy of carbamazepine as a maintenance therapy for bipolar disorder was controversial.3 However, two recent large randomized, controlled maintenance studies that compared carbamazepine with lithium validated use of the agent for that purpose.4,5
In the first study, 144 patients received either drug and were followed for up to 2 1/2 years.4 The study showed no significant differences between the two groups in time-to-mood episode recurrence or hospitalization. However, significantly more patients receiving carbamazepine required treatment discontinuation for side effects and additional medications for breakthrough symptoms than did the patients receiving lithium.
Patients without both comorbid disorders and mood-incongruent delusions responded better to lithium, whereas those with mixed episodes and bipolar II disorder or NOS appeared to respond better to carbamazepine.
In the second trial, 52 patients with bipolar I or II disorders received either lithium or carbamazepine for one year, crossed over to the alternate drug the second year, and received both drugs during the third year.5 No significant differences were reported in relapse rates during the first year between lithium (31%) and carbamazepine (37%), or in the percentage of patients who experienced a moderate or better response: 33% on lithium, 31% on carbamazepine, and 55% on the combination. On a variety of other measures, lithium was superior to carbamazepine. But patients with a history of rapid cycling responded significantly better to the combination (56%) than to lithium (28%) or carbamazepine (19%) alone.
As in the previous study, a higher proportion of patients receiving carbamazepine withdrew after experiencing side effects. Both studies found that while carbamazepine is efficacious, lithium is superior overall. But since neither study included a placebo group, carbamazepine’s efficacy in maintenance treatment cannot be determined.
Carbamazepine also was evaluated in three randomized, controlled trials in patients with bipolar depression.2 These small trials suggest that carbamazepine’s antidepressant activity may be less robust than its antimanic effects. Response rates (>50% improvement in depressive symptoms) ranged from 32% to 34%, although many patients who participated had treatment-resistant bipolar depression.
Comparing the known efficacy of antiepileptic agents in bipolar disorder
| Drug | Mania | Depression | Maintenance | Comments |
|---|---|---|---|---|
| Valproate | ◊◊◊◊ | ◊ | ◊◊◊ | New Depakote ER formulation |
| Carbamazepine | ◊◊◊ | ◊◊ | ◊◊◊ | 2 new maintenance studies v. lithium |
| Gabapentin | – | ◊ | ◊ | 2 negative placebo-controlled studies in mania |
| Lamotrigine | × | ◊◊◊ | ◊◊◊ | Antidepressant activity in several controlled trials |
| Topiramate | ◊ | ◊ | ◊ | Dose-related weight loss |
| Oxcarbazepine | ◊◊ | ◊ | ◊ | Improved tolerability & pharmacokinetics |
| Zonisamide | ◊ | ND | ND | May produce weight loss in some patients |
| Tiagabine | × | ND | ND | More data needed regarding tolerability and efficacy |
| Levetiracetam | ND | ND | ND | Data needed regarding efficacy and tolerability |
Key
◊◊◊◊ efficacy demonstrated in ≥2 placebo-controlled trials
◊◊◊ efficacy demonstrated in one placebo-controlled or two large, active comparator trials
◊◊ efficacy in two small or one large active comparator trial
◊ efficacy only in open trials and case series
× conflicting evidence of efficacy in available studies
– lack of efficacy demonstrated in randomized, controlled trials
ND no data presently available
Based on the studies reviewed above, carbamazepine is considered a second-line agent for bipolar mania and maintenance treatment with very limited data regarding its antidepressant effects.6
Valproate: For patients with mixed and mood episodes
The evidence seems to point to valproate as a suitable agent for patients with mixed and mood episodes.
Two earlier randomized, controlled studies7,8 showed that the divalproex sodium formulation of valproate was superior to a placebo, leading to the indication of divalproex for treatment of acute mania in patients with bipolar disorder. Additional analyses of data from the second controlled trial8 indicated that patients with prominent depressive symptoms during mania (mixed episodes) responded better to divalproex than lithium. Further, multiple prior mood episodes were associated with poor lithium response but not with divalproex response.
Two recent randomized, controlled trials compared the antimanic efficacy and tolerability of divalproex and olanzapine.9,10 The design of these two studies differed in two important ways: sample size and starting doses. This may explain the different results of the two trials.
In the first study, 248 patients received starting doses of either divalproex 750 mg/d with upward titration to therapeutic concentrations, or olanzapine 15 mg/d with titration if clinically indicated.9 Olanzapine was found to be superior in the mean reduction of manic symptoms and in the proportion of patients who either responded to treatment or were in remission.
In the second study, the number of patients (N=120) randomized was not sufficient to detect a statistically significant difference between treatment groups.10 Initial dosing consisted of rapid divalproex loading (20 mg/kg/d) versus olanzapine (10 mg/d) with upward titration as clinically indicated. This trial revealed no significant differences in efficacy on any outcome measure and the mean valproic acid plasma concentration was higher than in the first trial.
Differences in side effects between the two agents were similar in both studies. Olanzapine was associated with greater sedation, appetite stimulation, and weight gain, and divalproex with greater gastrointestinal symptoms. Taken together, these two studies indicate that olanzapine is at least as efficacious as divalproex in treating acute mania and that divalproex should be titrated to plasma concentrations well within the therapeutic range consistent with response and tolerability.
The efficacy of divalproex as a maintenance therapy for patients with bipolar disorder has been studied in four trials to date:
- A randomized, open comparison of lithium and divalproex found generally good efficacy for both drugs across 18 months.
- A second naturalistic, pharmacoeconomic, one-year open comparison also found both lithium and divalproex fairly equal in efficacy.
- A large, prospective, randomized one-year maintenance trial showed little difference in relapse rates among patients receiving divalproex (24%), lithium (31%), and a placebo (38%).11
- A recently completed one-year comparison of relapse rates between divalproex and olanzapine showed little difference between the two drugs.12
The overall results suggest that divalproex helps prevent mood episodes in patients with bipolar disorder, but the data are less substantial and conclusive than from placebo-controlled trials of lithium.
The antidepressant effects of divalproex in treating acute bipolar depression have not been studied in randomized controlled trials. Impressions from case reports and case series suggest that divalproex may exert some antidepressant activity, but that this action may be less robust than its antimanic effects.
Divalproex is now available in a once-daily extended release (ER) formulation. This appears to have improved tolerability, especially regarding gastrointestinal side effects. (Clinical experience suggests that the immediate release formulation of divalproex can also be given once daily.) The ER formulation is not bioequivalent to its immediate-release counterpart; it produces plasma concentrations of approximately 80% of those achieved with immediate release. Thus, switching a patient to the ER formulation might require a dose increase.
Lamotrigine for bipolar depression
Several recent randomized controlled trials indicate that lamotrigine has important thymoleptic properties.14-17
Three studies addressed the efficacy of lamotrigine in treating patients with acute bipolar mania. In two small trials, lamotrigine did not display superior efficacy over placebo in reducing manic symptoms.14 A third trial revealed differences between lithium and lamotrigine in reducing manic symptoms, but this study lacked sufficient power to detect potential differences in efficacy.
Two placebo-controlled maintenance studies of lamotrigine were recently reported.15,16 The first found no significant differences in relapse rates in patients with rapid cycling bipolar I and II disorders randomized to lamotrigine or placebo after initial stabilization on lamotrigine.15 However, in a post hoc analysis among bipolar II (but not bipolar I) patients, lamotrigine was significantly more efficacious than placebo in time-to-study dropout and considerably better (P=0.07) in time to need for additional medication.
In the second randomized, placebo-controlled trial, patients with bipolar I disorder who had recently experienced a manic episode were treated with lamotrigine 100-200 mg/d during an open-label phase (8-16 weeks) while other psychotropic agents were tapered and discontinued.16 Patients (N = 171) who remained stable were then randomized to lamotrigine 200-400 mg/d, lithium, or placebo for up to 18 months.
The lamotrigine-treated group showed significantly lower relapse rates than placebo-treated patients in time-to-study dropout, time to intervention for a mood episode, and time to intervention for depressive relapse. Differences between the lamotrigine and placebo groups in time to intervention for manic relapse were insignificant. Lithium was superior to a placebo in time to relapse for any mood episode and time to intervention for a manic episode. Lithium also outperformed lamotrigine in that manic symptoms did not worsen as quickly from baseline to endpoint.
The results here are consistent with those of the rapid cycling study: Lamotrigine helps prevent recurrence of depressive symptoms and episodes. Evidence of prophylaxis against manic recurrences was not compelling, however.
The findings of these two maintenance trials are consistent with the results of a placebo-controlled, randomized, 6-week acute treatment trial of lamotrigine (50 mg/d and 200 mg/d) in patients with bipolar depression.17 Seventeen lamotrigine-treated patients in both dosage groups saw more-significantly reduced depressive symptoms than did placebo-treated patients on the MADRS (but not the HAMD) total score and on the CGI. The 200 mg/d group tended to have greater improvement than the 50 mg/d group. There was little difference among the three treatment groups in the incidence of switching into hypomania or mania.
These studies indicate that lamotrigine has acute and prophylactic efficacy against bipolar depression. In contrast, evidence of the agent’s acute or prophylactic efficacy in mania is lacking.
Other new antiepileptics: More testing needed
Use of the six other newer antiepileptics in patients with bipolar disorder is still being explored. (See “Comparing the known efficacy of antiepileptic agents in bipolar disorder,”) Let’s look at what we know about these agents to this point.
Gabapentin A number of case reports and case series published in recent years suggest that gabapentin may have mood-stabilizing properties. In two randomized, controlled trials, however, gabapentin did not display significantly greater efficacy than a placebo in treating acute mania.13,14 Gabapentin has not been studied in controlled trials as a maintenance treatment or for bipolar depression.
In contrast to these negative studies, gabapentin was superior to placebo in studies of patients with panic disorder, social anxiety disorder, and neuropathic pain. Overall, these studies suggest that gabapentin is not a bona fide mood stabilizer for most patients.
Topiramate More than 10 case reports and case series suggest that topiramate may have mood-stabilizing properties. A number of open trials also have found significant dose-related weight loss in patients with weight gain associated with other psychotropic agents.18 These observations have sparked interest in topiramate’s potential role as an obesity treatment.
Only one randomized, controlled trial of topiramate for bipolar disorder has appeared to date.19 An interim analysis of a placebo-controlled, randomized trial of two doses of topiramate (approximately 250 mg/d and 500 mg/d) in 97 hospitalized patients with acute bipolar I mania revealed strong trends toward reduced manic symptoms for both topiramate groups over placebo. These differences were not significant by the time the study was concluded, however.
Lamotrigine is a novel drug that blocks voltage-sensitive sodium channels, thereby indirectly inhibiting the release of excitatory neurotransmitters, particularly glutamate and aspartate.
Gabapentin was developed to mimic the synaptic effects of gamma-aminobutyric acid (GABA); it does not appear to appreciably interact with GABA receptors, however, and its mechanism of action in epilepsy remains unknown. It has several attractive pharmacokinetic properties, including lack of protein binding, renal clearance rather than hepatic metabolism, and few known drug-drug interactions.
Topiramate is a sulfamate-substituted monosaccharide with a number of possible mechanisms of action, including blockade of voltage-gated sodium channels, antagonism of the kainate/AMPA glutamate receptor subtype, enhancement of GABA activity at the GABA A receptor via interaction with a nonbenzodiazepine receptor site, and carbonic anhydrase inhibition.
Oxcarbazepine is the 10-keto analogue of carbamazepine, a chemical difference that translates into a number of safety advantages over carbamazepine. Oxcarbazepine is converted to an active 10-hydroxy metabolite rather than to the 10,11-epoxide metabolite of carbamazepine. The 10,11-epoxide metabolite of carbamazepine is associated with neurological side effects. Oxcarbazepine is a weak inducer of the CYP450 system, appears to have fewer drug-drug interactions, and offers better overall tolerability.
Zonisamide, a sulfonamide derivative, blocks voltage-sensitive sodium channels and T-type calcium currents, modulates GABAergic and dopaminergic neurotransmission, and is a free-radical scavenger.
Tiagabine is a selective GABA reuptake inhibitor.
Levetiracetam is the S-enantiomer of the ethyl analogue of the nootropic agent piracetam. Its mechanism of action in treating epilepsy is unknown. It does not appear to interact significantly with voltage-sensitive sodium channels or T-type calcium channels, nor does it significantly alter levels of GABA, glutamate, or glutamine in the central nervous system.
When patients with mania associated with recent antidepressant use were excluded from post hocanalysis, topiramate’s superiority over placebo was re-established. These tantalizing results require further study. Similarly, studies of topiramate’s potential efficacy in acute bipolar depression and as a maintenance treatment are needed.
Oxcarbazepine Oxcarbazepine is a relative newcomer to the United States, but has been available abroad for some time.
While numerous studies have tested carbamazepine in treating various aspects of bipolar disorder, few controlled trials have tested oxcarbazepine for this use.20
Oxcarbazepine as a treatment for acute bipolar mania was comparable to haloperidol in two small randomized, controlled trials and to lithium in one small trial. None of these studies had adequate power to detect potential differences in efficacy. Similarly, two very small maintenance studies (total N=25) found no differences in efficacy between oxcarbazepine and lithium.20
Additionally, to our knowledge oxcarbazepine has not been formally tested as a treatment for acute bipolar depression. So while oxcarbazepine is an attractive alternative to carbamazepine for pharmacokinetic and pharmacodynamic reasons, data supporting oxcarbazepine’s efficacy in bipolar disorder are far less substantial.
Zonisamide The only report of zonisamide as a bipolar disorder treatment so far is a case series of 15 patients who received the drug adjunctively for manic symptoms. Of these patients, 80% showed at least moderate improvement and 33% were rated as markedly improved. These preliminary observations require further study. Like topiramate, zonisamide may also be associated with weight loss in some patients.
Tiagabine A few case reports and case series describe tiagabine’s effects in patients with bipolar disorder. Conflicting results have been reported to date and some reports of poor tolerability have emerged. Fundamental safety and efficacy data for this agent in bipolar disorder are needed.
Levetiracetam Studies of levetiracetam for treatment of bipolar disorder are just under way. The Stanley Foundation Bipolar Treatment Network, a program of the National Alliance for the Mentally Ill Research Institute, is investigating its potential thymoleptic properties.
Related resources
- Joffe RT, Calabrese JR. Anticonvulsants in Mood Disorders. New York: Marcel Dekker Inc., 1994.
- Modigh K, Robak OH, Vestergaard P. Anticonvulsants in Psychiatry. Bristol, Pa: Wrightson Biomedical Publishing, Ltd., 1994.
- Schatzberg AF, Nemeroff CB. Textbook of Psychopharmacology, 2 nd ed. Washington, DC: American Psychiatric Press, 1998.
- Nathan P, Gorman J. A Guide to Treatments that Work, 2 nd ed. New York: Oxford University Press, 2001.
- Janicak PG, Davis JM, Preskorn SH, Ayd FJ, Jr. Principles and Practice of Psychopharmacotherapy, 2 nd ed. Baltimore, MD: Williams & Wilkins, 1997.
Drug brand names
- Carbamazepine • Tegretol, Epitol, Atretol
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Oxcarbazepine • Trileptal
- Tiagabine • Gabatril
- Topiramate • Topamax
- Valproate-divalproex sodium • Depakote, Depakote ER
- Zonisamide • Zonegran
Disclosure
Dr. Keck reports that he receives grant/research support from and serves as a consultant to Abbott Laboratories, AstraZeneca, Pfizer Inc., and Eli Lilly and Co. He also receives grant/research support from Merck and Co. and Otsuka America Pharmaceutical, and serves as a consultant to Bristol-Myers Squibb Co., GlaxoSmithKline, and Janssen Pharmaceutica.
Dr. McElroy reports that she receives grant/research support from and serves as a consultant to Abbott Laboratories, Elan Pharmaceuticals, Cephalon Inc. GlaxoSmithKline, and Eli Lilly and Co. She also receives grant/research support from Forest Pharmaceuticals and Solvay Pharmaceuticals, and serves as a consultant to Bristol-Myers Squibb Co., Ortho-McNeil Pharmaceutical, and Janssen Pharmaceutica.
1. Keck PE, Jr, McElroy SL, Strakowski SM. Anticonvulsants and antipsychotics in the treatment of bipolar disorder. J Clin Psychiatry. 1998;59(Suppl 6):74-81.
2. McElroy SL, Keck PE, Jr. Pharmacologic agents for the treatment of acute bipolar mania. Biol Psychiatry. 2000;48:539-557.
3. Post RM, Denicoff KD, Frye MA, Leverich GS. Re-evaluating carbamazepine prophylaxis in bipolar disorder. Br J Psychiatry. 1997;170:202-204.
4. Greil W, Ludwig-Mayerhofer W, Erazon-Scheichlin C, Schmidt S, Engel RR, et al. Lithium versus carbamazepine in the maintenance treatment of bipolar disorders–a randomized study. J Affect Disorders. 1997;43:151-161.
5. Denicoff KD, Smith-Jackson EE, Disney ER, Ali SO, Leverich GS, Post RM. Comparative prophylactic efficacy of lithium, carbamazepine and the combination in bipolar disorder. J Clin Psychiatry. 1997;58:470-478.
6. Suppes T, Swann AC, Dennehy EB, Habermacher ED, Mason M, Crismon ML, Toprac MG, Rush AJ, Shon SP, Altshuler KZ. Texas Medication Algorithm Project: development and feasibility testing of a treatment algorithm for patients with bipolar disorder. J Clin Psychiatry. 2001;62:439-447.
7. Pope HG, Jr, McElroy SL, Keck PE, Jr, Hudson JI. Valproate in the treatment of acute mania: a placebo-controlled study. Arch Gen Psychiatry. 1991;48:62-68.
8. Bowden CL, Brugger AM, Swann AC, Calabrese JR, Janicak PG, Petty F, et al. Efficacy of divalproex sodium vs. lithium and placebo in the treatment of mania. JAMA. 1994;271:915-924.
9. Tohen M, Baker RW, Milton DR, Risser RC, Gilmore JA, Davis AR, et al. Olanzapine versus divalproex sodium for the treatment of acute mania. Bipolar Disorders. 2001;3(Suppl 1):60-61.
10. Zajecka J. Divalproex versus olanzapine in the treatment of acute mania. Presented at the 39 th Annual Meeting of the American College of Neuropsychopharmacology. San Juan, Puerto Rico, Dec. 10-14, 2000.
11. Bowden CL, Calabrese JR, McElroy SL, Hirschfeld RMA, Petty F, Gyulai LL, Pope HG, Jr, et al. Efficacy of divalproex versus lithium and placebo in maintenance treatment of bipolar disorder. Arch Gen Psychiatry. 2000;57:481-489.
12. Tohen M, Baker RW, Altshuler LL, Zarate CA, Suppes T, Ketter T, et al. Olanzapine versus divalproex for bipolar mania: a 47-week study. Presented at the European College of Neuropsychopharmacology Meeting, Istanbul, Turkey, Oct. 15, 2001.
13. Pande AD, Crockatt JG, Janney CA, Werth JL, Tsaaroucha G. and the Gabapentin Bipolar Disorder Study Group. Bipolar Disorders. 2000;2:249-255.
14. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;24:205-212.
15. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled study of lamotrigine monotherapy in rapid-cycling bipolar disorder. J Clin Psychiatry. 2000;61:841-850.
16. Bowden CL, Calabrese JR, Akthar S, Olajossy M, Montgomery S, Ascher J, et al. Lamotrigine demonstrates long-term mood stabilization in manic patients. Bipolar Disorders. 2001;3(Suppl 1):27-28.
17. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind, placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry. 1999;60:79-88.
18. McElroy SL, Suppes T, Keck PE, Jr, Frye MA, Denicoff KD, Altshuler LL, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biol Psychiatry. 2000;47:1025-1033.
19. Calabrese JR. A double-blind, placebo-controlled trial of topiramate monotherapy in the treatment of acute mania. Presented at the Annual Meeting of the European College of Neuropsychopharmacology. Munich, Germany, Sept. 9-13, 2000.
20. Emrich H, Schiwy W, Silverstone T. eds. Carbamazepine and Oxcarbazepine in Psychiatry. London: CNS Publishers, 1990.
The newer antiepileptic drugs pose a sometimes bewildering range of options for bipolar disorder treatment. Which work best for acute bipolar I mania? Which are best suited for maintenance in patients with mixed episodes, or for those with a history of rapid cycling? What about prevention of depressive episodes? And how do the antiepileptics compare with lithium?
For many patients with bipolar disorder, lithium is still the drug of choice. For others, however, an increasing body of evidence supports the efficacy of some antiepileptics and atypical antipsychotics.
The mood-stabilizing properties of two antiepileptic agents, carbamazepine and valproate, were demonstrated some years ago in randomized controlled trials in patients with bipolar disorder. Since then, there has been considerable interest in the potential thymoleptic properties of the new antiepileptic drugs.1 In recent years gabapentin, lamotrigine, topiramate, oxcarbazepine, zonisamide, tiagabine, and levetiracetam have been approved in the United States for the treatment of various types of epilepsy. These medications have diverse pharmacological properties that distinguish them from earlier agents and from one another.
Do these new agents have anything to offer patients? For the most part, the evidence is not yet in hand, but we will examine what’s available, starting with the most recent trial data regarding the efficacy of valproate and carbamazepine.
Carbamazepine for bipolar mania
Five randomized, controlled trials2 have shown the efficacy of carbamazepine in patients with acute bipolar I mania. Carbamazepine was superior to placebo and comparable to chlorpromazine and lithium. Pooled data reveal an overall response rate (defined as the proportion of patients experiencing > 50% reduction in manic symptoms) of 50% for carbamazepine, 56% for lithium, and 61% for chlorpromazine (differences in overall response rates are not significant).
Until recently, the efficacy of carbamazepine as a maintenance therapy for bipolar disorder was controversial.3 However, two recent large randomized, controlled maintenance studies that compared carbamazepine with lithium validated use of the agent for that purpose.4,5
In the first study, 144 patients received either drug and were followed for up to 2 1/2 years.4 The study showed no significant differences between the two groups in time-to-mood episode recurrence or hospitalization. However, significantly more patients receiving carbamazepine required treatment discontinuation for side effects and additional medications for breakthrough symptoms than did the patients receiving lithium.
Patients without both comorbid disorders and mood-incongruent delusions responded better to lithium, whereas those with mixed episodes and bipolar II disorder or NOS appeared to respond better to carbamazepine.
In the second trial, 52 patients with bipolar I or II disorders received either lithium or carbamazepine for one year, crossed over to the alternate drug the second year, and received both drugs during the third year.5 No significant differences were reported in relapse rates during the first year between lithium (31%) and carbamazepine (37%), or in the percentage of patients who experienced a moderate or better response: 33% on lithium, 31% on carbamazepine, and 55% on the combination. On a variety of other measures, lithium was superior to carbamazepine. But patients with a history of rapid cycling responded significantly better to the combination (56%) than to lithium (28%) or carbamazepine (19%) alone.
As in the previous study, a higher proportion of patients receiving carbamazepine withdrew after experiencing side effects. Both studies found that while carbamazepine is efficacious, lithium is superior overall. But since neither study included a placebo group, carbamazepine’s efficacy in maintenance treatment cannot be determined.
Carbamazepine also was evaluated in three randomized, controlled trials in patients with bipolar depression.2 These small trials suggest that carbamazepine’s antidepressant activity may be less robust than its antimanic effects. Response rates (>50% improvement in depressive symptoms) ranged from 32% to 34%, although many patients who participated had treatment-resistant bipolar depression.
Comparing the known efficacy of antiepileptic agents in bipolar disorder
| Drug | Mania | Depression | Maintenance | Comments |
|---|---|---|---|---|
| Valproate | ◊◊◊◊ | ◊ | ◊◊◊ | New Depakote ER formulation |
| Carbamazepine | ◊◊◊ | ◊◊ | ◊◊◊ | 2 new maintenance studies v. lithium |
| Gabapentin | – | ◊ | ◊ | 2 negative placebo-controlled studies in mania |
| Lamotrigine | × | ◊◊◊ | ◊◊◊ | Antidepressant activity in several controlled trials |
| Topiramate | ◊ | ◊ | ◊ | Dose-related weight loss |
| Oxcarbazepine | ◊◊ | ◊ | ◊ | Improved tolerability & pharmacokinetics |
| Zonisamide | ◊ | ND | ND | May produce weight loss in some patients |
| Tiagabine | × | ND | ND | More data needed regarding tolerability and efficacy |
| Levetiracetam | ND | ND | ND | Data needed regarding efficacy and tolerability |
Key
◊◊◊◊ efficacy demonstrated in ≥2 placebo-controlled trials
◊◊◊ efficacy demonstrated in one placebo-controlled or two large, active comparator trials
◊◊ efficacy in two small or one large active comparator trial
◊ efficacy only in open trials and case series
× conflicting evidence of efficacy in available studies
– lack of efficacy demonstrated in randomized, controlled trials
ND no data presently available
Based on the studies reviewed above, carbamazepine is considered a second-line agent for bipolar mania and maintenance treatment with very limited data regarding its antidepressant effects.6
Valproate: For patients with mixed and mood episodes
The evidence seems to point to valproate as a suitable agent for patients with mixed and mood episodes.
Two earlier randomized, controlled studies7,8 showed that the divalproex sodium formulation of valproate was superior to a placebo, leading to the indication of divalproex for treatment of acute mania in patients with bipolar disorder. Additional analyses of data from the second controlled trial8 indicated that patients with prominent depressive symptoms during mania (mixed episodes) responded better to divalproex than lithium. Further, multiple prior mood episodes were associated with poor lithium response but not with divalproex response.
Two recent randomized, controlled trials compared the antimanic efficacy and tolerability of divalproex and olanzapine.9,10 The design of these two studies differed in two important ways: sample size and starting doses. This may explain the different results of the two trials.
In the first study, 248 patients received starting doses of either divalproex 750 mg/d with upward titration to therapeutic concentrations, or olanzapine 15 mg/d with titration if clinically indicated.9 Olanzapine was found to be superior in the mean reduction of manic symptoms and in the proportion of patients who either responded to treatment or were in remission.
In the second study, the number of patients (N=120) randomized was not sufficient to detect a statistically significant difference between treatment groups.10 Initial dosing consisted of rapid divalproex loading (20 mg/kg/d) versus olanzapine (10 mg/d) with upward titration as clinically indicated. This trial revealed no significant differences in efficacy on any outcome measure and the mean valproic acid plasma concentration was higher than in the first trial.
Differences in side effects between the two agents were similar in both studies. Olanzapine was associated with greater sedation, appetite stimulation, and weight gain, and divalproex with greater gastrointestinal symptoms. Taken together, these two studies indicate that olanzapine is at least as efficacious as divalproex in treating acute mania and that divalproex should be titrated to plasma concentrations well within the therapeutic range consistent with response and tolerability.
The efficacy of divalproex as a maintenance therapy for patients with bipolar disorder has been studied in four trials to date:
- A randomized, open comparison of lithium and divalproex found generally good efficacy for both drugs across 18 months.
- A second naturalistic, pharmacoeconomic, one-year open comparison also found both lithium and divalproex fairly equal in efficacy.
- A large, prospective, randomized one-year maintenance trial showed little difference in relapse rates among patients receiving divalproex (24%), lithium (31%), and a placebo (38%).11
- A recently completed one-year comparison of relapse rates between divalproex and olanzapine showed little difference between the two drugs.12
The overall results suggest that divalproex helps prevent mood episodes in patients with bipolar disorder, but the data are less substantial and conclusive than from placebo-controlled trials of lithium.
The antidepressant effects of divalproex in treating acute bipolar depression have not been studied in randomized controlled trials. Impressions from case reports and case series suggest that divalproex may exert some antidepressant activity, but that this action may be less robust than its antimanic effects.
Divalproex is now available in a once-daily extended release (ER) formulation. This appears to have improved tolerability, especially regarding gastrointestinal side effects. (Clinical experience suggests that the immediate release formulation of divalproex can also be given once daily.) The ER formulation is not bioequivalent to its immediate-release counterpart; it produces plasma concentrations of approximately 80% of those achieved with immediate release. Thus, switching a patient to the ER formulation might require a dose increase.
Lamotrigine for bipolar depression
Several recent randomized controlled trials indicate that lamotrigine has important thymoleptic properties.14-17
Three studies addressed the efficacy of lamotrigine in treating patients with acute bipolar mania. In two small trials, lamotrigine did not display superior efficacy over placebo in reducing manic symptoms.14 A third trial revealed differences between lithium and lamotrigine in reducing manic symptoms, but this study lacked sufficient power to detect potential differences in efficacy.
Two placebo-controlled maintenance studies of lamotrigine were recently reported.15,16 The first found no significant differences in relapse rates in patients with rapid cycling bipolar I and II disorders randomized to lamotrigine or placebo after initial stabilization on lamotrigine.15 However, in a post hoc analysis among bipolar II (but not bipolar I) patients, lamotrigine was significantly more efficacious than placebo in time-to-study dropout and considerably better (P=0.07) in time to need for additional medication.
In the second randomized, placebo-controlled trial, patients with bipolar I disorder who had recently experienced a manic episode were treated with lamotrigine 100-200 mg/d during an open-label phase (8-16 weeks) while other psychotropic agents were tapered and discontinued.16 Patients (N = 171) who remained stable were then randomized to lamotrigine 200-400 mg/d, lithium, or placebo for up to 18 months.
The lamotrigine-treated group showed significantly lower relapse rates than placebo-treated patients in time-to-study dropout, time to intervention for a mood episode, and time to intervention for depressive relapse. Differences between the lamotrigine and placebo groups in time to intervention for manic relapse were insignificant. Lithium was superior to a placebo in time to relapse for any mood episode and time to intervention for a manic episode. Lithium also outperformed lamotrigine in that manic symptoms did not worsen as quickly from baseline to endpoint.
The results here are consistent with those of the rapid cycling study: Lamotrigine helps prevent recurrence of depressive symptoms and episodes. Evidence of prophylaxis against manic recurrences was not compelling, however.
The findings of these two maintenance trials are consistent with the results of a placebo-controlled, randomized, 6-week acute treatment trial of lamotrigine (50 mg/d and 200 mg/d) in patients with bipolar depression.17 Seventeen lamotrigine-treated patients in both dosage groups saw more-significantly reduced depressive symptoms than did placebo-treated patients on the MADRS (but not the HAMD) total score and on the CGI. The 200 mg/d group tended to have greater improvement than the 50 mg/d group. There was little difference among the three treatment groups in the incidence of switching into hypomania or mania.
These studies indicate that lamotrigine has acute and prophylactic efficacy against bipolar depression. In contrast, evidence of the agent’s acute or prophylactic efficacy in mania is lacking.
Other new antiepileptics: More testing needed
Use of the six other newer antiepileptics in patients with bipolar disorder is still being explored. (See “Comparing the known efficacy of antiepileptic agents in bipolar disorder,”) Let’s look at what we know about these agents to this point.
Gabapentin A number of case reports and case series published in recent years suggest that gabapentin may have mood-stabilizing properties. In two randomized, controlled trials, however, gabapentin did not display significantly greater efficacy than a placebo in treating acute mania.13,14 Gabapentin has not been studied in controlled trials as a maintenance treatment or for bipolar depression.
In contrast to these negative studies, gabapentin was superior to placebo in studies of patients with panic disorder, social anxiety disorder, and neuropathic pain. Overall, these studies suggest that gabapentin is not a bona fide mood stabilizer for most patients.
Topiramate More than 10 case reports and case series suggest that topiramate may have mood-stabilizing properties. A number of open trials also have found significant dose-related weight loss in patients with weight gain associated with other psychotropic agents.18 These observations have sparked interest in topiramate’s potential role as an obesity treatment.
Only one randomized, controlled trial of topiramate for bipolar disorder has appeared to date.19 An interim analysis of a placebo-controlled, randomized trial of two doses of topiramate (approximately 250 mg/d and 500 mg/d) in 97 hospitalized patients with acute bipolar I mania revealed strong trends toward reduced manic symptoms for both topiramate groups over placebo. These differences were not significant by the time the study was concluded, however.
Lamotrigine is a novel drug that blocks voltage-sensitive sodium channels, thereby indirectly inhibiting the release of excitatory neurotransmitters, particularly glutamate and aspartate.
Gabapentin was developed to mimic the synaptic effects of gamma-aminobutyric acid (GABA); it does not appear to appreciably interact with GABA receptors, however, and its mechanism of action in epilepsy remains unknown. It has several attractive pharmacokinetic properties, including lack of protein binding, renal clearance rather than hepatic metabolism, and few known drug-drug interactions.
Topiramate is a sulfamate-substituted monosaccharide with a number of possible mechanisms of action, including blockade of voltage-gated sodium channels, antagonism of the kainate/AMPA glutamate receptor subtype, enhancement of GABA activity at the GABA A receptor via interaction with a nonbenzodiazepine receptor site, and carbonic anhydrase inhibition.
Oxcarbazepine is the 10-keto analogue of carbamazepine, a chemical difference that translates into a number of safety advantages over carbamazepine. Oxcarbazepine is converted to an active 10-hydroxy metabolite rather than to the 10,11-epoxide metabolite of carbamazepine. The 10,11-epoxide metabolite of carbamazepine is associated with neurological side effects. Oxcarbazepine is a weak inducer of the CYP450 system, appears to have fewer drug-drug interactions, and offers better overall tolerability.
Zonisamide, a sulfonamide derivative, blocks voltage-sensitive sodium channels and T-type calcium currents, modulates GABAergic and dopaminergic neurotransmission, and is a free-radical scavenger.
Tiagabine is a selective GABA reuptake inhibitor.
Levetiracetam is the S-enantiomer of the ethyl analogue of the nootropic agent piracetam. Its mechanism of action in treating epilepsy is unknown. It does not appear to interact significantly with voltage-sensitive sodium channels or T-type calcium channels, nor does it significantly alter levels of GABA, glutamate, or glutamine in the central nervous system.
When patients with mania associated with recent antidepressant use were excluded from post hocanalysis, topiramate’s superiority over placebo was re-established. These tantalizing results require further study. Similarly, studies of topiramate’s potential efficacy in acute bipolar depression and as a maintenance treatment are needed.
Oxcarbazepine Oxcarbazepine is a relative newcomer to the United States, but has been available abroad for some time.
While numerous studies have tested carbamazepine in treating various aspects of bipolar disorder, few controlled trials have tested oxcarbazepine for this use.20
Oxcarbazepine as a treatment for acute bipolar mania was comparable to haloperidol in two small randomized, controlled trials and to lithium in one small trial. None of these studies had adequate power to detect potential differences in efficacy. Similarly, two very small maintenance studies (total N=25) found no differences in efficacy between oxcarbazepine and lithium.20
Additionally, to our knowledge oxcarbazepine has not been formally tested as a treatment for acute bipolar depression. So while oxcarbazepine is an attractive alternative to carbamazepine for pharmacokinetic and pharmacodynamic reasons, data supporting oxcarbazepine’s efficacy in bipolar disorder are far less substantial.
Zonisamide The only report of zonisamide as a bipolar disorder treatment so far is a case series of 15 patients who received the drug adjunctively for manic symptoms. Of these patients, 80% showed at least moderate improvement and 33% were rated as markedly improved. These preliminary observations require further study. Like topiramate, zonisamide may also be associated with weight loss in some patients.
Tiagabine A few case reports and case series describe tiagabine’s effects in patients with bipolar disorder. Conflicting results have been reported to date and some reports of poor tolerability have emerged. Fundamental safety and efficacy data for this agent in bipolar disorder are needed.
Levetiracetam Studies of levetiracetam for treatment of bipolar disorder are just under way. The Stanley Foundation Bipolar Treatment Network, a program of the National Alliance for the Mentally Ill Research Institute, is investigating its potential thymoleptic properties.
Related resources
- Joffe RT, Calabrese JR. Anticonvulsants in Mood Disorders. New York: Marcel Dekker Inc., 1994.
- Modigh K, Robak OH, Vestergaard P. Anticonvulsants in Psychiatry. Bristol, Pa: Wrightson Biomedical Publishing, Ltd., 1994.
- Schatzberg AF, Nemeroff CB. Textbook of Psychopharmacology, 2 nd ed. Washington, DC: American Psychiatric Press, 1998.
- Nathan P, Gorman J. A Guide to Treatments that Work, 2 nd ed. New York: Oxford University Press, 2001.
- Janicak PG, Davis JM, Preskorn SH, Ayd FJ, Jr. Principles and Practice of Psychopharmacotherapy, 2 nd ed. Baltimore, MD: Williams & Wilkins, 1997.
Drug brand names
- Carbamazepine • Tegretol, Epitol, Atretol
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Oxcarbazepine • Trileptal
- Tiagabine • Gabatril
- Topiramate • Topamax
- Valproate-divalproex sodium • Depakote, Depakote ER
- Zonisamide • Zonegran
Disclosure
Dr. Keck reports that he receives grant/research support from and serves as a consultant to Abbott Laboratories, AstraZeneca, Pfizer Inc., and Eli Lilly and Co. He also receives grant/research support from Merck and Co. and Otsuka America Pharmaceutical, and serves as a consultant to Bristol-Myers Squibb Co., GlaxoSmithKline, and Janssen Pharmaceutica.
Dr. McElroy reports that she receives grant/research support from and serves as a consultant to Abbott Laboratories, Elan Pharmaceuticals, Cephalon Inc. GlaxoSmithKline, and Eli Lilly and Co. She also receives grant/research support from Forest Pharmaceuticals and Solvay Pharmaceuticals, and serves as a consultant to Bristol-Myers Squibb Co., Ortho-McNeil Pharmaceutical, and Janssen Pharmaceutica.
The newer antiepileptic drugs pose a sometimes bewildering range of options for bipolar disorder treatment. Which work best for acute bipolar I mania? Which are best suited for maintenance in patients with mixed episodes, or for those with a history of rapid cycling? What about prevention of depressive episodes? And how do the antiepileptics compare with lithium?
For many patients with bipolar disorder, lithium is still the drug of choice. For others, however, an increasing body of evidence supports the efficacy of some antiepileptics and atypical antipsychotics.
The mood-stabilizing properties of two antiepileptic agents, carbamazepine and valproate, were demonstrated some years ago in randomized controlled trials in patients with bipolar disorder. Since then, there has been considerable interest in the potential thymoleptic properties of the new antiepileptic drugs.1 In recent years gabapentin, lamotrigine, topiramate, oxcarbazepine, zonisamide, tiagabine, and levetiracetam have been approved in the United States for the treatment of various types of epilepsy. These medications have diverse pharmacological properties that distinguish them from earlier agents and from one another.
Do these new agents have anything to offer patients? For the most part, the evidence is not yet in hand, but we will examine what’s available, starting with the most recent trial data regarding the efficacy of valproate and carbamazepine.
Carbamazepine for bipolar mania
Five randomized, controlled trials2 have shown the efficacy of carbamazepine in patients with acute bipolar I mania. Carbamazepine was superior to placebo and comparable to chlorpromazine and lithium. Pooled data reveal an overall response rate (defined as the proportion of patients experiencing > 50% reduction in manic symptoms) of 50% for carbamazepine, 56% for lithium, and 61% for chlorpromazine (differences in overall response rates are not significant).
Until recently, the efficacy of carbamazepine as a maintenance therapy for bipolar disorder was controversial.3 However, two recent large randomized, controlled maintenance studies that compared carbamazepine with lithium validated use of the agent for that purpose.4,5
In the first study, 144 patients received either drug and were followed for up to 2 1/2 years.4 The study showed no significant differences between the two groups in time-to-mood episode recurrence or hospitalization. However, significantly more patients receiving carbamazepine required treatment discontinuation for side effects and additional medications for breakthrough symptoms than did the patients receiving lithium.
Patients without both comorbid disorders and mood-incongruent delusions responded better to lithium, whereas those with mixed episodes and bipolar II disorder or NOS appeared to respond better to carbamazepine.
In the second trial, 52 patients with bipolar I or II disorders received either lithium or carbamazepine for one year, crossed over to the alternate drug the second year, and received both drugs during the third year.5 No significant differences were reported in relapse rates during the first year between lithium (31%) and carbamazepine (37%), or in the percentage of patients who experienced a moderate or better response: 33% on lithium, 31% on carbamazepine, and 55% on the combination. On a variety of other measures, lithium was superior to carbamazepine. But patients with a history of rapid cycling responded significantly better to the combination (56%) than to lithium (28%) or carbamazepine (19%) alone.
As in the previous study, a higher proportion of patients receiving carbamazepine withdrew after experiencing side effects. Both studies found that while carbamazepine is efficacious, lithium is superior overall. But since neither study included a placebo group, carbamazepine’s efficacy in maintenance treatment cannot be determined.
Carbamazepine also was evaluated in three randomized, controlled trials in patients with bipolar depression.2 These small trials suggest that carbamazepine’s antidepressant activity may be less robust than its antimanic effects. Response rates (>50% improvement in depressive symptoms) ranged from 32% to 34%, although many patients who participated had treatment-resistant bipolar depression.
Comparing the known efficacy of antiepileptic agents in bipolar disorder
| Drug | Mania | Depression | Maintenance | Comments |
|---|---|---|---|---|
| Valproate | ◊◊◊◊ | ◊ | ◊◊◊ | New Depakote ER formulation |
| Carbamazepine | ◊◊◊ | ◊◊ | ◊◊◊ | 2 new maintenance studies v. lithium |
| Gabapentin | – | ◊ | ◊ | 2 negative placebo-controlled studies in mania |
| Lamotrigine | × | ◊◊◊ | ◊◊◊ | Antidepressant activity in several controlled trials |
| Topiramate | ◊ | ◊ | ◊ | Dose-related weight loss |
| Oxcarbazepine | ◊◊ | ◊ | ◊ | Improved tolerability & pharmacokinetics |
| Zonisamide | ◊ | ND | ND | May produce weight loss in some patients |
| Tiagabine | × | ND | ND | More data needed regarding tolerability and efficacy |
| Levetiracetam | ND | ND | ND | Data needed regarding efficacy and tolerability |
Key
◊◊◊◊ efficacy demonstrated in ≥2 placebo-controlled trials
◊◊◊ efficacy demonstrated in one placebo-controlled or two large, active comparator trials
◊◊ efficacy in two small or one large active comparator trial
◊ efficacy only in open trials and case series
× conflicting evidence of efficacy in available studies
– lack of efficacy demonstrated in randomized, controlled trials
ND no data presently available
Based on the studies reviewed above, carbamazepine is considered a second-line agent for bipolar mania and maintenance treatment with very limited data regarding its antidepressant effects.6
Valproate: For patients with mixed and mood episodes
The evidence seems to point to valproate as a suitable agent for patients with mixed and mood episodes.
Two earlier randomized, controlled studies7,8 showed that the divalproex sodium formulation of valproate was superior to a placebo, leading to the indication of divalproex for treatment of acute mania in patients with bipolar disorder. Additional analyses of data from the second controlled trial8 indicated that patients with prominent depressive symptoms during mania (mixed episodes) responded better to divalproex than lithium. Further, multiple prior mood episodes were associated with poor lithium response but not with divalproex response.
Two recent randomized, controlled trials compared the antimanic efficacy and tolerability of divalproex and olanzapine.9,10 The design of these two studies differed in two important ways: sample size and starting doses. This may explain the different results of the two trials.
In the first study, 248 patients received starting doses of either divalproex 750 mg/d with upward titration to therapeutic concentrations, or olanzapine 15 mg/d with titration if clinically indicated.9 Olanzapine was found to be superior in the mean reduction of manic symptoms and in the proportion of patients who either responded to treatment or were in remission.
In the second study, the number of patients (N=120) randomized was not sufficient to detect a statistically significant difference between treatment groups.10 Initial dosing consisted of rapid divalproex loading (20 mg/kg/d) versus olanzapine (10 mg/d) with upward titration as clinically indicated. This trial revealed no significant differences in efficacy on any outcome measure and the mean valproic acid plasma concentration was higher than in the first trial.
Differences in side effects between the two agents were similar in both studies. Olanzapine was associated with greater sedation, appetite stimulation, and weight gain, and divalproex with greater gastrointestinal symptoms. Taken together, these two studies indicate that olanzapine is at least as efficacious as divalproex in treating acute mania and that divalproex should be titrated to plasma concentrations well within the therapeutic range consistent with response and tolerability.
The efficacy of divalproex as a maintenance therapy for patients with bipolar disorder has been studied in four trials to date:
- A randomized, open comparison of lithium and divalproex found generally good efficacy for both drugs across 18 months.
- A second naturalistic, pharmacoeconomic, one-year open comparison also found both lithium and divalproex fairly equal in efficacy.
- A large, prospective, randomized one-year maintenance trial showed little difference in relapse rates among patients receiving divalproex (24%), lithium (31%), and a placebo (38%).11
- A recently completed one-year comparison of relapse rates between divalproex and olanzapine showed little difference between the two drugs.12
The overall results suggest that divalproex helps prevent mood episodes in patients with bipolar disorder, but the data are less substantial and conclusive than from placebo-controlled trials of lithium.
The antidepressant effects of divalproex in treating acute bipolar depression have not been studied in randomized controlled trials. Impressions from case reports and case series suggest that divalproex may exert some antidepressant activity, but that this action may be less robust than its antimanic effects.
Divalproex is now available in a once-daily extended release (ER) formulation. This appears to have improved tolerability, especially regarding gastrointestinal side effects. (Clinical experience suggests that the immediate release formulation of divalproex can also be given once daily.) The ER formulation is not bioequivalent to its immediate-release counterpart; it produces plasma concentrations of approximately 80% of those achieved with immediate release. Thus, switching a patient to the ER formulation might require a dose increase.
Lamotrigine for bipolar depression
Several recent randomized controlled trials indicate that lamotrigine has important thymoleptic properties.14-17
Three studies addressed the efficacy of lamotrigine in treating patients with acute bipolar mania. In two small trials, lamotrigine did not display superior efficacy over placebo in reducing manic symptoms.14 A third trial revealed differences between lithium and lamotrigine in reducing manic symptoms, but this study lacked sufficient power to detect potential differences in efficacy.
Two placebo-controlled maintenance studies of lamotrigine were recently reported.15,16 The first found no significant differences in relapse rates in patients with rapid cycling bipolar I and II disorders randomized to lamotrigine or placebo after initial stabilization on lamotrigine.15 However, in a post hoc analysis among bipolar II (but not bipolar I) patients, lamotrigine was significantly more efficacious than placebo in time-to-study dropout and considerably better (P=0.07) in time to need for additional medication.
In the second randomized, placebo-controlled trial, patients with bipolar I disorder who had recently experienced a manic episode were treated with lamotrigine 100-200 mg/d during an open-label phase (8-16 weeks) while other psychotropic agents were tapered and discontinued.16 Patients (N = 171) who remained stable were then randomized to lamotrigine 200-400 mg/d, lithium, or placebo for up to 18 months.
The lamotrigine-treated group showed significantly lower relapse rates than placebo-treated patients in time-to-study dropout, time to intervention for a mood episode, and time to intervention for depressive relapse. Differences between the lamotrigine and placebo groups in time to intervention for manic relapse were insignificant. Lithium was superior to a placebo in time to relapse for any mood episode and time to intervention for a manic episode. Lithium also outperformed lamotrigine in that manic symptoms did not worsen as quickly from baseline to endpoint.
The results here are consistent with those of the rapid cycling study: Lamotrigine helps prevent recurrence of depressive symptoms and episodes. Evidence of prophylaxis against manic recurrences was not compelling, however.
The findings of these two maintenance trials are consistent with the results of a placebo-controlled, randomized, 6-week acute treatment trial of lamotrigine (50 mg/d and 200 mg/d) in patients with bipolar depression.17 Seventeen lamotrigine-treated patients in both dosage groups saw more-significantly reduced depressive symptoms than did placebo-treated patients on the MADRS (but not the HAMD) total score and on the CGI. The 200 mg/d group tended to have greater improvement than the 50 mg/d group. There was little difference among the three treatment groups in the incidence of switching into hypomania or mania.
These studies indicate that lamotrigine has acute and prophylactic efficacy against bipolar depression. In contrast, evidence of the agent’s acute or prophylactic efficacy in mania is lacking.
Other new antiepileptics: More testing needed
Use of the six other newer antiepileptics in patients with bipolar disorder is still being explored. (See “Comparing the known efficacy of antiepileptic agents in bipolar disorder,”) Let’s look at what we know about these agents to this point.
Gabapentin A number of case reports and case series published in recent years suggest that gabapentin may have mood-stabilizing properties. In two randomized, controlled trials, however, gabapentin did not display significantly greater efficacy than a placebo in treating acute mania.13,14 Gabapentin has not been studied in controlled trials as a maintenance treatment or for bipolar depression.
In contrast to these negative studies, gabapentin was superior to placebo in studies of patients with panic disorder, social anxiety disorder, and neuropathic pain. Overall, these studies suggest that gabapentin is not a bona fide mood stabilizer for most patients.
Topiramate More than 10 case reports and case series suggest that topiramate may have mood-stabilizing properties. A number of open trials also have found significant dose-related weight loss in patients with weight gain associated with other psychotropic agents.18 These observations have sparked interest in topiramate’s potential role as an obesity treatment.
Only one randomized, controlled trial of topiramate for bipolar disorder has appeared to date.19 An interim analysis of a placebo-controlled, randomized trial of two doses of topiramate (approximately 250 mg/d and 500 mg/d) in 97 hospitalized patients with acute bipolar I mania revealed strong trends toward reduced manic symptoms for both topiramate groups over placebo. These differences were not significant by the time the study was concluded, however.
Lamotrigine is a novel drug that blocks voltage-sensitive sodium channels, thereby indirectly inhibiting the release of excitatory neurotransmitters, particularly glutamate and aspartate.
Gabapentin was developed to mimic the synaptic effects of gamma-aminobutyric acid (GABA); it does not appear to appreciably interact with GABA receptors, however, and its mechanism of action in epilepsy remains unknown. It has several attractive pharmacokinetic properties, including lack of protein binding, renal clearance rather than hepatic metabolism, and few known drug-drug interactions.
Topiramate is a sulfamate-substituted monosaccharide with a number of possible mechanisms of action, including blockade of voltage-gated sodium channels, antagonism of the kainate/AMPA glutamate receptor subtype, enhancement of GABA activity at the GABA A receptor via interaction with a nonbenzodiazepine receptor site, and carbonic anhydrase inhibition.
Oxcarbazepine is the 10-keto analogue of carbamazepine, a chemical difference that translates into a number of safety advantages over carbamazepine. Oxcarbazepine is converted to an active 10-hydroxy metabolite rather than to the 10,11-epoxide metabolite of carbamazepine. The 10,11-epoxide metabolite of carbamazepine is associated with neurological side effects. Oxcarbazepine is a weak inducer of the CYP450 system, appears to have fewer drug-drug interactions, and offers better overall tolerability.
Zonisamide, a sulfonamide derivative, blocks voltage-sensitive sodium channels and T-type calcium currents, modulates GABAergic and dopaminergic neurotransmission, and is a free-radical scavenger.
Tiagabine is a selective GABA reuptake inhibitor.
Levetiracetam is the S-enantiomer of the ethyl analogue of the nootropic agent piracetam. Its mechanism of action in treating epilepsy is unknown. It does not appear to interact significantly with voltage-sensitive sodium channels or T-type calcium channels, nor does it significantly alter levels of GABA, glutamate, or glutamine in the central nervous system.
When patients with mania associated with recent antidepressant use were excluded from post hocanalysis, topiramate’s superiority over placebo was re-established. These tantalizing results require further study. Similarly, studies of topiramate’s potential efficacy in acute bipolar depression and as a maintenance treatment are needed.
Oxcarbazepine Oxcarbazepine is a relative newcomer to the United States, but has been available abroad for some time.
While numerous studies have tested carbamazepine in treating various aspects of bipolar disorder, few controlled trials have tested oxcarbazepine for this use.20
Oxcarbazepine as a treatment for acute bipolar mania was comparable to haloperidol in two small randomized, controlled trials and to lithium in one small trial. None of these studies had adequate power to detect potential differences in efficacy. Similarly, two very small maintenance studies (total N=25) found no differences in efficacy between oxcarbazepine and lithium.20
Additionally, to our knowledge oxcarbazepine has not been formally tested as a treatment for acute bipolar depression. So while oxcarbazepine is an attractive alternative to carbamazepine for pharmacokinetic and pharmacodynamic reasons, data supporting oxcarbazepine’s efficacy in bipolar disorder are far less substantial.
Zonisamide The only report of zonisamide as a bipolar disorder treatment so far is a case series of 15 patients who received the drug adjunctively for manic symptoms. Of these patients, 80% showed at least moderate improvement and 33% were rated as markedly improved. These preliminary observations require further study. Like topiramate, zonisamide may also be associated with weight loss in some patients.
Tiagabine A few case reports and case series describe tiagabine’s effects in patients with bipolar disorder. Conflicting results have been reported to date and some reports of poor tolerability have emerged. Fundamental safety and efficacy data for this agent in bipolar disorder are needed.
Levetiracetam Studies of levetiracetam for treatment of bipolar disorder are just under way. The Stanley Foundation Bipolar Treatment Network, a program of the National Alliance for the Mentally Ill Research Institute, is investigating its potential thymoleptic properties.
Related resources
- Joffe RT, Calabrese JR. Anticonvulsants in Mood Disorders. New York: Marcel Dekker Inc., 1994.
- Modigh K, Robak OH, Vestergaard P. Anticonvulsants in Psychiatry. Bristol, Pa: Wrightson Biomedical Publishing, Ltd., 1994.
- Schatzberg AF, Nemeroff CB. Textbook of Psychopharmacology, 2 nd ed. Washington, DC: American Psychiatric Press, 1998.
- Nathan P, Gorman J. A Guide to Treatments that Work, 2 nd ed. New York: Oxford University Press, 2001.
- Janicak PG, Davis JM, Preskorn SH, Ayd FJ, Jr. Principles and Practice of Psychopharmacotherapy, 2 nd ed. Baltimore, MD: Williams & Wilkins, 1997.
Drug brand names
- Carbamazepine • Tegretol, Epitol, Atretol
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Oxcarbazepine • Trileptal
- Tiagabine • Gabatril
- Topiramate • Topamax
- Valproate-divalproex sodium • Depakote, Depakote ER
- Zonisamide • Zonegran
Disclosure
Dr. Keck reports that he receives grant/research support from and serves as a consultant to Abbott Laboratories, AstraZeneca, Pfizer Inc., and Eli Lilly and Co. He also receives grant/research support from Merck and Co. and Otsuka America Pharmaceutical, and serves as a consultant to Bristol-Myers Squibb Co., GlaxoSmithKline, and Janssen Pharmaceutica.
Dr. McElroy reports that she receives grant/research support from and serves as a consultant to Abbott Laboratories, Elan Pharmaceuticals, Cephalon Inc. GlaxoSmithKline, and Eli Lilly and Co. She also receives grant/research support from Forest Pharmaceuticals and Solvay Pharmaceuticals, and serves as a consultant to Bristol-Myers Squibb Co., Ortho-McNeil Pharmaceutical, and Janssen Pharmaceutica.
1. Keck PE, Jr, McElroy SL, Strakowski SM. Anticonvulsants and antipsychotics in the treatment of bipolar disorder. J Clin Psychiatry. 1998;59(Suppl 6):74-81.
2. McElroy SL, Keck PE, Jr. Pharmacologic agents for the treatment of acute bipolar mania. Biol Psychiatry. 2000;48:539-557.
3. Post RM, Denicoff KD, Frye MA, Leverich GS. Re-evaluating carbamazepine prophylaxis in bipolar disorder. Br J Psychiatry. 1997;170:202-204.
4. Greil W, Ludwig-Mayerhofer W, Erazon-Scheichlin C, Schmidt S, Engel RR, et al. Lithium versus carbamazepine in the maintenance treatment of bipolar disorders–a randomized study. J Affect Disorders. 1997;43:151-161.
5. Denicoff KD, Smith-Jackson EE, Disney ER, Ali SO, Leverich GS, Post RM. Comparative prophylactic efficacy of lithium, carbamazepine and the combination in bipolar disorder. J Clin Psychiatry. 1997;58:470-478.
6. Suppes T, Swann AC, Dennehy EB, Habermacher ED, Mason M, Crismon ML, Toprac MG, Rush AJ, Shon SP, Altshuler KZ. Texas Medication Algorithm Project: development and feasibility testing of a treatment algorithm for patients with bipolar disorder. J Clin Psychiatry. 2001;62:439-447.
7. Pope HG, Jr, McElroy SL, Keck PE, Jr, Hudson JI. Valproate in the treatment of acute mania: a placebo-controlled study. Arch Gen Psychiatry. 1991;48:62-68.
8. Bowden CL, Brugger AM, Swann AC, Calabrese JR, Janicak PG, Petty F, et al. Efficacy of divalproex sodium vs. lithium and placebo in the treatment of mania. JAMA. 1994;271:915-924.
9. Tohen M, Baker RW, Milton DR, Risser RC, Gilmore JA, Davis AR, et al. Olanzapine versus divalproex sodium for the treatment of acute mania. Bipolar Disorders. 2001;3(Suppl 1):60-61.
10. Zajecka J. Divalproex versus olanzapine in the treatment of acute mania. Presented at the 39 th Annual Meeting of the American College of Neuropsychopharmacology. San Juan, Puerto Rico, Dec. 10-14, 2000.
11. Bowden CL, Calabrese JR, McElroy SL, Hirschfeld RMA, Petty F, Gyulai LL, Pope HG, Jr, et al. Efficacy of divalproex versus lithium and placebo in maintenance treatment of bipolar disorder. Arch Gen Psychiatry. 2000;57:481-489.
12. Tohen M, Baker RW, Altshuler LL, Zarate CA, Suppes T, Ketter T, et al. Olanzapine versus divalproex for bipolar mania: a 47-week study. Presented at the European College of Neuropsychopharmacology Meeting, Istanbul, Turkey, Oct. 15, 2001.
13. Pande AD, Crockatt JG, Janney CA, Werth JL, Tsaaroucha G. and the Gabapentin Bipolar Disorder Study Group. Bipolar Disorders. 2000;2:249-255.
14. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;24:205-212.
15. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled study of lamotrigine monotherapy in rapid-cycling bipolar disorder. J Clin Psychiatry. 2000;61:841-850.
16. Bowden CL, Calabrese JR, Akthar S, Olajossy M, Montgomery S, Ascher J, et al. Lamotrigine demonstrates long-term mood stabilization in manic patients. Bipolar Disorders. 2001;3(Suppl 1):27-28.
17. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind, placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry. 1999;60:79-88.
18. McElroy SL, Suppes T, Keck PE, Jr, Frye MA, Denicoff KD, Altshuler LL, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biol Psychiatry. 2000;47:1025-1033.
19. Calabrese JR. A double-blind, placebo-controlled trial of topiramate monotherapy in the treatment of acute mania. Presented at the Annual Meeting of the European College of Neuropsychopharmacology. Munich, Germany, Sept. 9-13, 2000.
20. Emrich H, Schiwy W, Silverstone T. eds. Carbamazepine and Oxcarbazepine in Psychiatry. London: CNS Publishers, 1990.
1. Keck PE, Jr, McElroy SL, Strakowski SM. Anticonvulsants and antipsychotics in the treatment of bipolar disorder. J Clin Psychiatry. 1998;59(Suppl 6):74-81.
2. McElroy SL, Keck PE, Jr. Pharmacologic agents for the treatment of acute bipolar mania. Biol Psychiatry. 2000;48:539-557.
3. Post RM, Denicoff KD, Frye MA, Leverich GS. Re-evaluating carbamazepine prophylaxis in bipolar disorder. Br J Psychiatry. 1997;170:202-204.
4. Greil W, Ludwig-Mayerhofer W, Erazon-Scheichlin C, Schmidt S, Engel RR, et al. Lithium versus carbamazepine in the maintenance treatment of bipolar disorders–a randomized study. J Affect Disorders. 1997;43:151-161.
5. Denicoff KD, Smith-Jackson EE, Disney ER, Ali SO, Leverich GS, Post RM. Comparative prophylactic efficacy of lithium, carbamazepine and the combination in bipolar disorder. J Clin Psychiatry. 1997;58:470-478.
6. Suppes T, Swann AC, Dennehy EB, Habermacher ED, Mason M, Crismon ML, Toprac MG, Rush AJ, Shon SP, Altshuler KZ. Texas Medication Algorithm Project: development and feasibility testing of a treatment algorithm for patients with bipolar disorder. J Clin Psychiatry. 2001;62:439-447.
7. Pope HG, Jr, McElroy SL, Keck PE, Jr, Hudson JI. Valproate in the treatment of acute mania: a placebo-controlled study. Arch Gen Psychiatry. 1991;48:62-68.
8. Bowden CL, Brugger AM, Swann AC, Calabrese JR, Janicak PG, Petty F, et al. Efficacy of divalproex sodium vs. lithium and placebo in the treatment of mania. JAMA. 1994;271:915-924.
9. Tohen M, Baker RW, Milton DR, Risser RC, Gilmore JA, Davis AR, et al. Olanzapine versus divalproex sodium for the treatment of acute mania. Bipolar Disorders. 2001;3(Suppl 1):60-61.
10. Zajecka J. Divalproex versus olanzapine in the treatment of acute mania. Presented at the 39 th Annual Meeting of the American College of Neuropsychopharmacology. San Juan, Puerto Rico, Dec. 10-14, 2000.
11. Bowden CL, Calabrese JR, McElroy SL, Hirschfeld RMA, Petty F, Gyulai LL, Pope HG, Jr, et al. Efficacy of divalproex versus lithium and placebo in maintenance treatment of bipolar disorder. Arch Gen Psychiatry. 2000;57:481-489.
12. Tohen M, Baker RW, Altshuler LL, Zarate CA, Suppes T, Ketter T, et al. Olanzapine versus divalproex for bipolar mania: a 47-week study. Presented at the European College of Neuropsychopharmacology Meeting, Istanbul, Turkey, Oct. 15, 2001.
13. Pande AD, Crockatt JG, Janney CA, Werth JL, Tsaaroucha G. and the Gabapentin Bipolar Disorder Study Group. Bipolar Disorders. 2000;2:249-255.
14. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;24:205-212.
15. Calabrese JR, Suppes T, Bowden CL, et al. A double-blind, placebo-controlled study of lamotrigine monotherapy in rapid-cycling bipolar disorder. J Clin Psychiatry. 2000;61:841-850.
16. Bowden CL, Calabrese JR, Akthar S, Olajossy M, Montgomery S, Ascher J, et al. Lamotrigine demonstrates long-term mood stabilization in manic patients. Bipolar Disorders. 2001;3(Suppl 1):27-28.
17. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind, placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry. 1999;60:79-88.
18. McElroy SL, Suppes T, Keck PE, Jr, Frye MA, Denicoff KD, Altshuler LL, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biol Psychiatry. 2000;47:1025-1033.
19. Calabrese JR. A double-blind, placebo-controlled trial of topiramate monotherapy in the treatment of acute mania. Presented at the Annual Meeting of the European College of Neuropsychopharmacology. Munich, Germany, Sept. 9-13, 2000.
20. Emrich H, Schiwy W, Silverstone T. eds. Carbamazepine and Oxcarbazepine in Psychiatry. London: CNS Publishers, 1990.