User login
Whole blood better for cardiac surgery in young children

Photo by Elise Amendola
Using fresh whole blood (FWB) from single donors for cardiac procedures in children younger than 2 years of age is better than using component blood from multiple donors, researchers say.
FWB reduces the risk of getting transfusion-related illnesses by reducing donor exposures.
“Currently, whole blood is not generally made available to hospitals for use in pediatric heart surgery,” said David R. Jobes, MD, of The Children’s Hospital of Philadelphia in Pennsylvania.
“Blood centers separate donated blood into component parts, which are then stored for use in medical transfusions as needed.”
At The Children’s Hospital of Philadelphia, the standard preoperative blood order for elective pediatric heart surgery with cariopulmonary bypass is 2 units of FWB and 2 units of packed red blood cells. The FWB is to be used during and immediately after surgery and the components thereafter, if necessary.
The researchers set out to examine the effectiveness of this protocol. They conducted a retrospective study of patient records over a period of 15 years from a surgical registry and blood bank, comparing the cohort of 4111 patients to published reports.
The team defined donor exposures as transfusion requirements for the day of operation and the next postoperative day. All blood products issued were presumed to have been tranfused, and all aliquots from a single donor were counted as a single donor exposure.
Patients were a median age of 94 days and weighed a median of 4.4 kg.
Most (3836) patients received FWB, and 252 received components exclusively when no FWB was available. Twenty-three patients did not receive any blood products. A median of 2 whole blood units was transfused, for a total of 2 donor exposures for the entire cohort.
The researchers found that the youngest patients having complex procedures were exposed to the highest number of donors, while older patients having simpler procedures were exposed to fewer donors.
For example, 72 patients who were a median of 5 days old and underwent truncus arteriosus repair had a median of 4 donor exposures (range, 1-14). And 136 older patients who were a median of 610 days old and underwent fontan completion had a median of 1 donor exposure (range, 0-8).
The researchers concluded that the protocol resulted in fewer donor exposures compared with component use reported in the literature.
Dr Jobes said the risk for disease transmission in pediatric patients is essentially the same as the risk for adults, but it may be more costly for pediatric patients in the long run because infants and young children may live longer with chronic illness stemming from transfusion.
He added, “We hope that our research helps to re-examine current blood storage practice and make whole blood more readily available for pediatric patients.”
He and his colleagues described this research in The Annals of Thoracic Surgery. ![]()

Photo by Elise Amendola
Using fresh whole blood (FWB) from single donors for cardiac procedures in children younger than 2 years of age is better than using component blood from multiple donors, researchers say.
FWB reduces the risk of getting transfusion-related illnesses by reducing donor exposures.
“Currently, whole blood is not generally made available to hospitals for use in pediatric heart surgery,” said David R. Jobes, MD, of The Children’s Hospital of Philadelphia in Pennsylvania.
“Blood centers separate donated blood into component parts, which are then stored for use in medical transfusions as needed.”
At The Children’s Hospital of Philadelphia, the standard preoperative blood order for elective pediatric heart surgery with cariopulmonary bypass is 2 units of FWB and 2 units of packed red blood cells. The FWB is to be used during and immediately after surgery and the components thereafter, if necessary.
The researchers set out to examine the effectiveness of this protocol. They conducted a retrospective study of patient records over a period of 15 years from a surgical registry and blood bank, comparing the cohort of 4111 patients to published reports.
The team defined donor exposures as transfusion requirements for the day of operation and the next postoperative day. All blood products issued were presumed to have been tranfused, and all aliquots from a single donor were counted as a single donor exposure.
Patients were a median age of 94 days and weighed a median of 4.4 kg.
Most (3836) patients received FWB, and 252 received components exclusively when no FWB was available. Twenty-three patients did not receive any blood products. A median of 2 whole blood units was transfused, for a total of 2 donor exposures for the entire cohort.
The researchers found that the youngest patients having complex procedures were exposed to the highest number of donors, while older patients having simpler procedures were exposed to fewer donors.
For example, 72 patients who were a median of 5 days old and underwent truncus arteriosus repair had a median of 4 donor exposures (range, 1-14). And 136 older patients who were a median of 610 days old and underwent fontan completion had a median of 1 donor exposure (range, 0-8).
The researchers concluded that the protocol resulted in fewer donor exposures compared with component use reported in the literature.
Dr Jobes said the risk for disease transmission in pediatric patients is essentially the same as the risk for adults, but it may be more costly for pediatric patients in the long run because infants and young children may live longer with chronic illness stemming from transfusion.
He added, “We hope that our research helps to re-examine current blood storage practice and make whole blood more readily available for pediatric patients.”
He and his colleagues described this research in The Annals of Thoracic Surgery. ![]()

Photo by Elise Amendola
Using fresh whole blood (FWB) from single donors for cardiac procedures in children younger than 2 years of age is better than using component blood from multiple donors, researchers say.
FWB reduces the risk of getting transfusion-related illnesses by reducing donor exposures.
“Currently, whole blood is not generally made available to hospitals for use in pediatric heart surgery,” said David R. Jobes, MD, of The Children’s Hospital of Philadelphia in Pennsylvania.
“Blood centers separate donated blood into component parts, which are then stored for use in medical transfusions as needed.”
At The Children’s Hospital of Philadelphia, the standard preoperative blood order for elective pediatric heart surgery with cariopulmonary bypass is 2 units of FWB and 2 units of packed red blood cells. The FWB is to be used during and immediately after surgery and the components thereafter, if necessary.
The researchers set out to examine the effectiveness of this protocol. They conducted a retrospective study of patient records over a period of 15 years from a surgical registry and blood bank, comparing the cohort of 4111 patients to published reports.
The team defined donor exposures as transfusion requirements for the day of operation and the next postoperative day. All blood products issued were presumed to have been tranfused, and all aliquots from a single donor were counted as a single donor exposure.
Patients were a median age of 94 days and weighed a median of 4.4 kg.
Most (3836) patients received FWB, and 252 received components exclusively when no FWB was available. Twenty-three patients did not receive any blood products. A median of 2 whole blood units was transfused, for a total of 2 donor exposures for the entire cohort.
The researchers found that the youngest patients having complex procedures were exposed to the highest number of donors, while older patients having simpler procedures were exposed to fewer donors.
For example, 72 patients who were a median of 5 days old and underwent truncus arteriosus repair had a median of 4 donor exposures (range, 1-14). And 136 older patients who were a median of 610 days old and underwent fontan completion had a median of 1 donor exposure (range, 0-8).
The researchers concluded that the protocol resulted in fewer donor exposures compared with component use reported in the literature.
Dr Jobes said the risk for disease transmission in pediatric patients is essentially the same as the risk for adults, but it may be more costly for pediatric patients in the long run because infants and young children may live longer with chronic illness stemming from transfusion.
He added, “We hope that our research helps to re-examine current blood storage practice and make whole blood more readily available for pediatric patients.”
He and his colleagues described this research in The Annals of Thoracic Surgery. ![]()
Enzyme could enable creation of universal blood type

Photo by Elise Amendola
Chemists have generated an enzyme that shows the potential for converting type A or B blood into a universal blood type.
The enzyme works by snipping off the antigens found in blood types A and B, making these blood types more like O, which can be given to patients of all blood types.
The enzyme was able to remove most of the antigens in type A and B blood. Before it can be used in clinical settings, however, all of the antigens would need to be removed.
David Kwan, PhD, of the University of British Columbia in Vancouver, Canada, and his colleagues described their work with this enzyme in the Journal of the American Chemical Society.
“We produced a mutant enzyme that is very efficient at cutting off the sugars in A and B blood and is much more proficient at removing the subtypes of the A antigen that the parent enzyme struggles with,” Dr Kwan said.
To create the enzyme, Dr Kwan and his colleagues used a technology called directed evolution. It involves inserting mutations into the gene that codes for the enzyme and selecting mutants that are more effective at cutting the antigens.
The team started with the family 98 glycoside hydrolase from Streptococcus pneumoniae SP3-BS71 (Sp3GH98), which cleaves the entire terminal trisaccharide antigenic determinants of both A and B antigens from some of the linkages on red blood cell surface glycans.
Through directed evolution, the researchers developed variants of Sp3GH98 that showed improved activity toward some of the linkages that are resistant to cleavage by the wild-type enzyme.
In 5 generations, the enzyme became 170 times more effective. This Sp3GH98 variant could remove the majority of the antigens in type A and B blood.
The researchers said the enzyme must be able to remove all of the antigens before it can be used in the clinic. The immune system is highly sensitive to blood groups, and even small amounts of residual antigens could trigger an immune response.
The concept of using an enzyme to change blood types is not new, said study author Steve Withers, PhD, also from the University of British Columbia.
“But, until now, we needed so much of the enzyme to make it work that it was impractical,” he said. “Now, I’m confident that we can take this a whole lot further.” ![]()

Photo by Elise Amendola
Chemists have generated an enzyme that shows the potential for converting type A or B blood into a universal blood type.
The enzyme works by snipping off the antigens found in blood types A and B, making these blood types more like O, which can be given to patients of all blood types.
The enzyme was able to remove most of the antigens in type A and B blood. Before it can be used in clinical settings, however, all of the antigens would need to be removed.
David Kwan, PhD, of the University of British Columbia in Vancouver, Canada, and his colleagues described their work with this enzyme in the Journal of the American Chemical Society.
“We produced a mutant enzyme that is very efficient at cutting off the sugars in A and B blood and is much more proficient at removing the subtypes of the A antigen that the parent enzyme struggles with,” Dr Kwan said.
To create the enzyme, Dr Kwan and his colleagues used a technology called directed evolution. It involves inserting mutations into the gene that codes for the enzyme and selecting mutants that are more effective at cutting the antigens.
The team started with the family 98 glycoside hydrolase from Streptococcus pneumoniae SP3-BS71 (Sp3GH98), which cleaves the entire terminal trisaccharide antigenic determinants of both A and B antigens from some of the linkages on red blood cell surface glycans.
Through directed evolution, the researchers developed variants of Sp3GH98 that showed improved activity toward some of the linkages that are resistant to cleavage by the wild-type enzyme.
In 5 generations, the enzyme became 170 times more effective. This Sp3GH98 variant could remove the majority of the antigens in type A and B blood.
The researchers said the enzyme must be able to remove all of the antigens before it can be used in the clinic. The immune system is highly sensitive to blood groups, and even small amounts of residual antigens could trigger an immune response.
The concept of using an enzyme to change blood types is not new, said study author Steve Withers, PhD, also from the University of British Columbia.
“But, until now, we needed so much of the enzyme to make it work that it was impractical,” he said. “Now, I’m confident that we can take this a whole lot further.” ![]()

Photo by Elise Amendola
Chemists have generated an enzyme that shows the potential for converting type A or B blood into a universal blood type.
The enzyme works by snipping off the antigens found in blood types A and B, making these blood types more like O, which can be given to patients of all blood types.
The enzyme was able to remove most of the antigens in type A and B blood. Before it can be used in clinical settings, however, all of the antigens would need to be removed.
David Kwan, PhD, of the University of British Columbia in Vancouver, Canada, and his colleagues described their work with this enzyme in the Journal of the American Chemical Society.
“We produced a mutant enzyme that is very efficient at cutting off the sugars in A and B blood and is much more proficient at removing the subtypes of the A antigen that the parent enzyme struggles with,” Dr Kwan said.
To create the enzyme, Dr Kwan and his colleagues used a technology called directed evolution. It involves inserting mutations into the gene that codes for the enzyme and selecting mutants that are more effective at cutting the antigens.
The team started with the family 98 glycoside hydrolase from Streptococcus pneumoniae SP3-BS71 (Sp3GH98), which cleaves the entire terminal trisaccharide antigenic determinants of both A and B antigens from some of the linkages on red blood cell surface glycans.
Through directed evolution, the researchers developed variants of Sp3GH98 that showed improved activity toward some of the linkages that are resistant to cleavage by the wild-type enzyme.
In 5 generations, the enzyme became 170 times more effective. This Sp3GH98 variant could remove the majority of the antigens in type A and B blood.
The researchers said the enzyme must be able to remove all of the antigens before it can be used in the clinic. The immune system is highly sensitive to blood groups, and even small amounts of residual antigens could trigger an immune response.
The concept of using an enzyme to change blood types is not new, said study author Steve Withers, PhD, also from the University of British Columbia.
“But, until now, we needed so much of the enzyme to make it work that it was impractical,” he said. “Now, I’m confident that we can take this a whole lot further.” ![]()
Meeting plasma transfusion guideline is feasible

Photo by Cristina Granados
High-volume trauma centers can provide consistent, rapid delivery of universal-donor plasma to massively hemorrhaging patients without excessive wastage, results of the PROPPR trial suggest.
For this study, researchers assessed the feasibility of the 2013 guidelines issued by the American College of Surgeons, which recommend that universal-donor products be immediately available upon the arrival of severely injured patients.
This recommendation may be outside the capabilities of many facilities, but it is likely to become the expected standard in the near future, the researchers said.
So Deborah Novak, MD, of the University of Arizona in Tucson, and her colleagues tested the feasibility of following the guidelines and reported their findings in Transfusion.
PROPPR was a randomized trial in which the researchers compared survival after the transfusion of 2 different blood component ratios in patients with traumatic massive hemorrhage. Transfusion services supporting the study were expected to provide thawed plasma, platelets, and red blood cells within 10 minutes of a request.
Twelve Level 1 trauma centers were involved in the trial. Participants collected data on the blood components transfused and the amount of time it took to deliver those products, but they focused primarily on plasma.
The researchers evaluated the adequacy of site plans by comparing the blood availability times to study goals and the American College of Surgeons guidelines.
The 680 patients in this trial received about 4700 units of plasma. Eleven of the sites consistently delivered 6 units of thawed, universal-donor plasma to their trauma-receiving unit within the required 10 minutes. The sites were able to deliver 12 units of plasma within 20 minutes.
Three sites used blood group A plasma instead of AB for massive transfusion and did not see any complications. None of the sites experienced shortages of AB plasma that limited enrollment. Two of the sites reported wasting nearly 25% of the AB plasma prepared.
“We hope the descriptions of the various ways in which centers fulfilled the requirement of delivering blood components to the bedside within 10 minutes inspire other facilities to devise the most effective way for their own circumstances,” Dr Novak said. ![]()

Photo by Cristina Granados
High-volume trauma centers can provide consistent, rapid delivery of universal-donor plasma to massively hemorrhaging patients without excessive wastage, results of the PROPPR trial suggest.
For this study, researchers assessed the feasibility of the 2013 guidelines issued by the American College of Surgeons, which recommend that universal-donor products be immediately available upon the arrival of severely injured patients.
This recommendation may be outside the capabilities of many facilities, but it is likely to become the expected standard in the near future, the researchers said.
So Deborah Novak, MD, of the University of Arizona in Tucson, and her colleagues tested the feasibility of following the guidelines and reported their findings in Transfusion.
PROPPR was a randomized trial in which the researchers compared survival after the transfusion of 2 different blood component ratios in patients with traumatic massive hemorrhage. Transfusion services supporting the study were expected to provide thawed plasma, platelets, and red blood cells within 10 minutes of a request.
Twelve Level 1 trauma centers were involved in the trial. Participants collected data on the blood components transfused and the amount of time it took to deliver those products, but they focused primarily on plasma.
The researchers evaluated the adequacy of site plans by comparing the blood availability times to study goals and the American College of Surgeons guidelines.
The 680 patients in this trial received about 4700 units of plasma. Eleven of the sites consistently delivered 6 units of thawed, universal-donor plasma to their trauma-receiving unit within the required 10 minutes. The sites were able to deliver 12 units of plasma within 20 minutes.
Three sites used blood group A plasma instead of AB for massive transfusion and did not see any complications. None of the sites experienced shortages of AB plasma that limited enrollment. Two of the sites reported wasting nearly 25% of the AB plasma prepared.
“We hope the descriptions of the various ways in which centers fulfilled the requirement of delivering blood components to the bedside within 10 minutes inspire other facilities to devise the most effective way for their own circumstances,” Dr Novak said. ![]()

Photo by Cristina Granados
High-volume trauma centers can provide consistent, rapid delivery of universal-donor plasma to massively hemorrhaging patients without excessive wastage, results of the PROPPR trial suggest.
For this study, researchers assessed the feasibility of the 2013 guidelines issued by the American College of Surgeons, which recommend that universal-donor products be immediately available upon the arrival of severely injured patients.
This recommendation may be outside the capabilities of many facilities, but it is likely to become the expected standard in the near future, the researchers said.
So Deborah Novak, MD, of the University of Arizona in Tucson, and her colleagues tested the feasibility of following the guidelines and reported their findings in Transfusion.
PROPPR was a randomized trial in which the researchers compared survival after the transfusion of 2 different blood component ratios in patients with traumatic massive hemorrhage. Transfusion services supporting the study were expected to provide thawed plasma, platelets, and red blood cells within 10 minutes of a request.
Twelve Level 1 trauma centers were involved in the trial. Participants collected data on the blood components transfused and the amount of time it took to deliver those products, but they focused primarily on plasma.
The researchers evaluated the adequacy of site plans by comparing the blood availability times to study goals and the American College of Surgeons guidelines.
The 680 patients in this trial received about 4700 units of plasma. Eleven of the sites consistently delivered 6 units of thawed, universal-donor plasma to their trauma-receiving unit within the required 10 minutes. The sites were able to deliver 12 units of plasma within 20 minutes.
Three sites used blood group A plasma instead of AB for massive transfusion and did not see any complications. None of the sites experienced shortages of AB plasma that limited enrollment. Two of the sites reported wasting nearly 25% of the AB plasma prepared.
“We hope the descriptions of the various ways in which centers fulfilled the requirement of delivering blood components to the bedside within 10 minutes inspire other facilities to devise the most effective way for their own circumstances,” Dr Novak said. ![]()
RBC age doesn’t affect outcomes, trial suggests

Photo by Elise Amendola
Results of the RECESS trial suggest the duration of red blood cell (RBC) storage does not affect clinical outcomes in patients undergoing cardiac surgery.
Patients who received older RBCs (stored for 21 days or more) did not have significantly higher multi-organ dysfunction scores, mortality rates, or rates of serious adverse events, when compared to patients who received newer RBCs (stored for 10 days or fewer).
Marie E. Stein, MD, of the University of Minnesota in Minneapolis, and her colleagues reported these results in NEJM. Dr Stein presented the same data last October at the AABB Annual Meeting 2014. ![]()

Photo by Elise Amendola
Results of the RECESS trial suggest the duration of red blood cell (RBC) storage does not affect clinical outcomes in patients undergoing cardiac surgery.
Patients who received older RBCs (stored for 21 days or more) did not have significantly higher multi-organ dysfunction scores, mortality rates, or rates of serious adverse events, when compared to patients who received newer RBCs (stored for 10 days or fewer).
Marie E. Stein, MD, of the University of Minnesota in Minneapolis, and her colleagues reported these results in NEJM. Dr Stein presented the same data last October at the AABB Annual Meeting 2014. ![]()

Photo by Elise Amendola
Results of the RECESS trial suggest the duration of red blood cell (RBC) storage does not affect clinical outcomes in patients undergoing cardiac surgery.
Patients who received older RBCs (stored for 21 days or more) did not have significantly higher multi-organ dysfunction scores, mortality rates, or rates of serious adverse events, when compared to patients who received newer RBCs (stored for 10 days or fewer).
Marie E. Stein, MD, of the University of Minnesota in Minneapolis, and her colleagues reported these results in NEJM. Dr Stein presented the same data last October at the AABB Annual Meeting 2014. ![]()
Blood products can transmit food allergies
In rare cases, children can develop allergies to previously tolerated foods after receiving blood products via transfusion, according to a case study published in Canadian Medical Association Journal.
“It is very unusual to identify someone who experienced passive transfer of allergy from blood products,” said study author Julia Upton, MD, of The Hospital for Sick Children in Toronto, Ontario, Canada.
“Importantly, this condition has an excellent prognosis and typically resolves within a few months.”
Dr Upton and her colleagues found that blood donors who have food allergies can transfer immunoglobulin E, an antibody that reacts against allergens, from blood products such as platelets, although this is rare.
The researchers said it is important for parents and physicians to be aware of this event in case children have anaphylactic reactions after receiving blood products, particularly after eating peanuts, tree nuts, and fish, foods they could previously consume without reaction.
These reactions—with symptoms such as facial swelling, throat discomfort, or sudden fatigue—should be treated immediately at an emergency department.
When there is passive transfer of allergies after blood transfusion, physicians should follow up with the family after a few months to decide the timing of careful reintroduction of the temporary allergens into a child’s diet.
Physicians should report suspected cases of passive transfer of allergies to the hospital’s transfusion service to investigate the cause and ensure the safety of the country’s blood supply. ![]()

In rare cases, children can develop allergies to previously tolerated foods after receiving blood products via transfusion, according to a case study published in Canadian Medical Association Journal.
“It is very unusual to identify someone who experienced passive transfer of allergy from blood products,” said study author Julia Upton, MD, of The Hospital for Sick Children in Toronto, Ontario, Canada.
“Importantly, this condition has an excellent prognosis and typically resolves within a few months.”
Dr Upton and her colleagues found that blood donors who have food allergies can transfer immunoglobulin E, an antibody that reacts against allergens, from blood products such as platelets, although this is rare.
The researchers said it is important for parents and physicians to be aware of this event in case children have anaphylactic reactions after receiving blood products, particularly after eating peanuts, tree nuts, and fish, foods they could previously consume without reaction.
These reactions—with symptoms such as facial swelling, throat discomfort, or sudden fatigue—should be treated immediately at an emergency department.
When there is passive transfer of allergies after blood transfusion, physicians should follow up with the family after a few months to decide the timing of careful reintroduction of the temporary allergens into a child’s diet.
Physicians should report suspected cases of passive transfer of allergies to the hospital’s transfusion service to investigate the cause and ensure the safety of the country’s blood supply. ![]()

In rare cases, children can develop allergies to previously tolerated foods after receiving blood products via transfusion, according to a case study published in Canadian Medical Association Journal.
“It is very unusual to identify someone who experienced passive transfer of allergy from blood products,” said study author Julia Upton, MD, of The Hospital for Sick Children in Toronto, Ontario, Canada.
“Importantly, this condition has an excellent prognosis and typically resolves within a few months.”
Dr Upton and her colleagues found that blood donors who have food allergies can transfer immunoglobulin E, an antibody that reacts against allergens, from blood products such as platelets, although this is rare.
The researchers said it is important for parents and physicians to be aware of this event in case children have anaphylactic reactions after receiving blood products, particularly after eating peanuts, tree nuts, and fish, foods they could previously consume without reaction.
These reactions—with symptoms such as facial swelling, throat discomfort, or sudden fatigue—should be treated immediately at an emergency department.
When there is passive transfer of allergies after blood transfusion, physicians should follow up with the family after a few months to decide the timing of careful reintroduction of the temporary allergens into a child’s diet.
Physicians should report suspected cases of passive transfer of allergies to the hospital’s transfusion service to investigate the cause and ensure the safety of the country’s blood supply. ![]()
Topical TXA decreases use of blood transfusions

Photo by Daniel Gay
Using topical tranexamic acid (TXA) in patients undergoing primary total hip and knee arthroplasty can reduce the need for blood transfusions, according to a study published in The Journal of Arthroplasty.
Topical TXA reduced the transfusion rate by 12%, thereby reducing transfusion costs.
Topical TXA also enabled about 9% more patients to be discharged to their homes rather than a skilled nursing facility, and it did not affect the rate of complications.
“Historically, with hip or knee replacement, there was a 25% to 30% chance of a blood transfusion,” said study author John Froehlich, MD, of The Miriam Hospital in Providence, Rhode Island.
“We realized that this high frequency of transfusions was associated with longer hospital stays and a higher risk of infections, which we are always working to avoid. Tranexamic acid has been around for 30 years, but because there was concern about the danger of administering it intravenously, we opted to inject it in the joints. We found it to be effective in reducing ongoing blood loss and the subsequent need for transfusion, and we have now standardized the practice.”
TXA is a synthetic derivative of the amino acid lysine that produces antifibrinolytic activity by competitively inhibiting lysine binding sites on plasminogen molecules. TXA helps the body stabilize blood clot formation, thereby reducing bleeding at surgical sites.
Most protocols of TXA in total joint arthroplasty have involved intravenous delivery. However, studies have indicated that topical injection may provide advantages, such as potentially reduced costs with a single injection, surgeon control, and localization and concentration of the drug more precisely at the surgical site.
“As the evidence for topical TXA grew, our arthroplasty surgeons started adopting topical TXA for total joint arthroplasty,” Dr Froehlich said.
He and his colleagues studied topical TXA in patients undergoing primary hip or knee arthroplasty by 5 surgeons from March 2012 to March 2013. Of the 591 consecutive patients, 311 received topical TXA, and 280 served as controls.
The researchers found that topical TXA reduced the proportion of red blood cell units transfused by 18%, from 28.6% to 10.6% (P<0.001). The drug also reduce the number of patients who required transfusions by 12%, from 17.5% to 5.5% (P<0.001).
On, the other hand, there was no significant difference between the TXA and control groups with regard to tourniquet time, operative time, time in the operating room, or the length of hospital stay.
Still, more patients in the TXA arm than in the control arm were able to go home rather than to a subacute nursing facility—71.4% and 62.1%, respectively (P<0.02).
And TXA conferred a cost benefit based solely on the rate of transfusion reduction. The researchers’ cost analysis revealed a net savings of $8372.66 per 100 patients treated, which amounted to $83.73 per patient.
“[Topical TXA] reduces transfusion rates, increases home disposition, and reduces cost in primary hip and knee arthroplasty,” said study author Lee Rubin, MD, of The Miriam Hospital.
“[W]e have now developed a simple, standardized, and cost-effective protocol for the use of topical TXA during total joint replacement that can be immediately used by any surgeon around the world to improve patient care.” ![]()

Photo by Daniel Gay
Using topical tranexamic acid (TXA) in patients undergoing primary total hip and knee arthroplasty can reduce the need for blood transfusions, according to a study published in The Journal of Arthroplasty.
Topical TXA reduced the transfusion rate by 12%, thereby reducing transfusion costs.
Topical TXA also enabled about 9% more patients to be discharged to their homes rather than a skilled nursing facility, and it did not affect the rate of complications.
“Historically, with hip or knee replacement, there was a 25% to 30% chance of a blood transfusion,” said study author John Froehlich, MD, of The Miriam Hospital in Providence, Rhode Island.
“We realized that this high frequency of transfusions was associated with longer hospital stays and a higher risk of infections, which we are always working to avoid. Tranexamic acid has been around for 30 years, but because there was concern about the danger of administering it intravenously, we opted to inject it in the joints. We found it to be effective in reducing ongoing blood loss and the subsequent need for transfusion, and we have now standardized the practice.”
TXA is a synthetic derivative of the amino acid lysine that produces antifibrinolytic activity by competitively inhibiting lysine binding sites on plasminogen molecules. TXA helps the body stabilize blood clot formation, thereby reducing bleeding at surgical sites.
Most protocols of TXA in total joint arthroplasty have involved intravenous delivery. However, studies have indicated that topical injection may provide advantages, such as potentially reduced costs with a single injection, surgeon control, and localization and concentration of the drug more precisely at the surgical site.
“As the evidence for topical TXA grew, our arthroplasty surgeons started adopting topical TXA for total joint arthroplasty,” Dr Froehlich said.
He and his colleagues studied topical TXA in patients undergoing primary hip or knee arthroplasty by 5 surgeons from March 2012 to March 2013. Of the 591 consecutive patients, 311 received topical TXA, and 280 served as controls.
The researchers found that topical TXA reduced the proportion of red blood cell units transfused by 18%, from 28.6% to 10.6% (P<0.001). The drug also reduce the number of patients who required transfusions by 12%, from 17.5% to 5.5% (P<0.001).
On, the other hand, there was no significant difference between the TXA and control groups with regard to tourniquet time, operative time, time in the operating room, or the length of hospital stay.
Still, more patients in the TXA arm than in the control arm were able to go home rather than to a subacute nursing facility—71.4% and 62.1%, respectively (P<0.02).
And TXA conferred a cost benefit based solely on the rate of transfusion reduction. The researchers’ cost analysis revealed a net savings of $8372.66 per 100 patients treated, which amounted to $83.73 per patient.
“[Topical TXA] reduces transfusion rates, increases home disposition, and reduces cost in primary hip and knee arthroplasty,” said study author Lee Rubin, MD, of The Miriam Hospital.
“[W]e have now developed a simple, standardized, and cost-effective protocol for the use of topical TXA during total joint replacement that can be immediately used by any surgeon around the world to improve patient care.” ![]()

Photo by Daniel Gay
Using topical tranexamic acid (TXA) in patients undergoing primary total hip and knee arthroplasty can reduce the need for blood transfusions, according to a study published in The Journal of Arthroplasty.
Topical TXA reduced the transfusion rate by 12%, thereby reducing transfusion costs.
Topical TXA also enabled about 9% more patients to be discharged to their homes rather than a skilled nursing facility, and it did not affect the rate of complications.
“Historically, with hip or knee replacement, there was a 25% to 30% chance of a blood transfusion,” said study author John Froehlich, MD, of The Miriam Hospital in Providence, Rhode Island.
“We realized that this high frequency of transfusions was associated with longer hospital stays and a higher risk of infections, which we are always working to avoid. Tranexamic acid has been around for 30 years, but because there was concern about the danger of administering it intravenously, we opted to inject it in the joints. We found it to be effective in reducing ongoing blood loss and the subsequent need for transfusion, and we have now standardized the practice.”
TXA is a synthetic derivative of the amino acid lysine that produces antifibrinolytic activity by competitively inhibiting lysine binding sites on plasminogen molecules. TXA helps the body stabilize blood clot formation, thereby reducing bleeding at surgical sites.
Most protocols of TXA in total joint arthroplasty have involved intravenous delivery. However, studies have indicated that topical injection may provide advantages, such as potentially reduced costs with a single injection, surgeon control, and localization and concentration of the drug more precisely at the surgical site.
“As the evidence for topical TXA grew, our arthroplasty surgeons started adopting topical TXA for total joint arthroplasty,” Dr Froehlich said.
He and his colleagues studied topical TXA in patients undergoing primary hip or knee arthroplasty by 5 surgeons from March 2012 to March 2013. Of the 591 consecutive patients, 311 received topical TXA, and 280 served as controls.
The researchers found that topical TXA reduced the proportion of red blood cell units transfused by 18%, from 28.6% to 10.6% (P<0.001). The drug also reduce the number of patients who required transfusions by 12%, from 17.5% to 5.5% (P<0.001).
On, the other hand, there was no significant difference between the TXA and control groups with regard to tourniquet time, operative time, time in the operating room, or the length of hospital stay.
Still, more patients in the TXA arm than in the control arm were able to go home rather than to a subacute nursing facility—71.4% and 62.1%, respectively (P<0.02).
And TXA conferred a cost benefit based solely on the rate of transfusion reduction. The researchers’ cost analysis revealed a net savings of $8372.66 per 100 patients treated, which amounted to $83.73 per patient.
“[Topical TXA] reduces transfusion rates, increases home disposition, and reduces cost in primary hip and knee arthroplasty,” said study author Lee Rubin, MD, of The Miriam Hospital.
“[W]e have now developed a simple, standardized, and cost-effective protocol for the use of topical TXA during total joint replacement that can be immediately used by any surgeon around the world to improve patient care.” ![]()
Storage time doesn’t affect blood quality, study shows

Photo by Elise Amendola
The length of time red blood cells (RBCs) are stored does not affect transfusion outcomes in critically ill patients, results of the ABLE study suggest.
Researchers compared critically ill patients who received RBCs stored for an average of about 3 weeks to those who received RBCs stored for less than a week.
And there were no significant differences between the groups with regard to mortality, transfusion reactions, and other outcomes.
“Previous observational and laboratory studies have suggested that fresh blood may be better because of the breakdown of red blood cells and accumulation of toxins during storage,” said Alan Tinmouth, MD, of the University of Ottawa in Ontario, Canada.
“But this definitive clinical trial clearly shows that these changes do not affect the quality of blood.”
Dr Tinmouth and his colleagues reported these results in NEJM.
The researchers enrolled 2430 adult intensive care patients in the trial, comparing patients who received fresh RBCs (n=1211) to those who received older RBCs (n=1219). Fresh RBCs were stored for a median of 6.1±4.9 days, and older RBCs were stored for a median of 22.0±8.4 days.
There was no significant difference between the arms with regard to the primary outcome, 90-day mortality. This endpoint was met by 37% of patients who received fresh blood and 35.3% of those who received older blood.
There were no significant differences between the fresh and older RBC arms for other mortality outcomes, either. This included death in the intensive care unit (26.7% vs 24.2%), in-hospital death (33.3% vs 31.9%), and death by day 28 (30.6% vs 28.8%).
Similarly, there were no significant differences between the fresh and older blood arms with regard to major illnesses, including multiple organ dysfunction syndrome (13.4% vs 13%), acute respiratory distress syndrome (5.7% vs 6.6%), cardiovascular failure (5.1% vs 4.2%), cardiac ischemia or infarction (4.5% vs 3.6%), venous thromboembolism (3.6% for both), nosocomial infection (34.1% vs 31.3%), and acute transfusion reaction (0.3% vs 0.5%).
In addition, there was no significant difference between the fresh RBC arm and the older RBC arm in the length of time patients required mechanical ventilation (15.0±18.0 days vs 14.7±14.9 days), cardiac or vasoactive drugs (7.1±10.2 days vs 7.5±11.2 days), or extrarenal epuration (2.5±10.1 days vs 2.5±8.3 days).
And there was no significant difference between the fresh and older blood arms in patients’ length of stay in the hospital (34.4±39.5 days vs 33.9±38.8 days) or the intensive care unit (15.3±15.4 days vs 15.3±14.8 days).
Based on these results, the researchers said there is no need to worry about the age of blood routinely used in hospitals. The team is now conducting a trial to determine if the same can be said for transfusions in pediatric patients. ![]()

Photo by Elise Amendola
The length of time red blood cells (RBCs) are stored does not affect transfusion outcomes in critically ill patients, results of the ABLE study suggest.
Researchers compared critically ill patients who received RBCs stored for an average of about 3 weeks to those who received RBCs stored for less than a week.
And there were no significant differences between the groups with regard to mortality, transfusion reactions, and other outcomes.
“Previous observational and laboratory studies have suggested that fresh blood may be better because of the breakdown of red blood cells and accumulation of toxins during storage,” said Alan Tinmouth, MD, of the University of Ottawa in Ontario, Canada.
“But this definitive clinical trial clearly shows that these changes do not affect the quality of blood.”
Dr Tinmouth and his colleagues reported these results in NEJM.
The researchers enrolled 2430 adult intensive care patients in the trial, comparing patients who received fresh RBCs (n=1211) to those who received older RBCs (n=1219). Fresh RBCs were stored for a median of 6.1±4.9 days, and older RBCs were stored for a median of 22.0±8.4 days.
There was no significant difference between the arms with regard to the primary outcome, 90-day mortality. This endpoint was met by 37% of patients who received fresh blood and 35.3% of those who received older blood.
There were no significant differences between the fresh and older RBC arms for other mortality outcomes, either. This included death in the intensive care unit (26.7% vs 24.2%), in-hospital death (33.3% vs 31.9%), and death by day 28 (30.6% vs 28.8%).
Similarly, there were no significant differences between the fresh and older blood arms with regard to major illnesses, including multiple organ dysfunction syndrome (13.4% vs 13%), acute respiratory distress syndrome (5.7% vs 6.6%), cardiovascular failure (5.1% vs 4.2%), cardiac ischemia or infarction (4.5% vs 3.6%), venous thromboembolism (3.6% for both), nosocomial infection (34.1% vs 31.3%), and acute transfusion reaction (0.3% vs 0.5%).
In addition, there was no significant difference between the fresh RBC arm and the older RBC arm in the length of time patients required mechanical ventilation (15.0±18.0 days vs 14.7±14.9 days), cardiac or vasoactive drugs (7.1±10.2 days vs 7.5±11.2 days), or extrarenal epuration (2.5±10.1 days vs 2.5±8.3 days).
And there was no significant difference between the fresh and older blood arms in patients’ length of stay in the hospital (34.4±39.5 days vs 33.9±38.8 days) or the intensive care unit (15.3±15.4 days vs 15.3±14.8 days).
Based on these results, the researchers said there is no need to worry about the age of blood routinely used in hospitals. The team is now conducting a trial to determine if the same can be said for transfusions in pediatric patients. ![]()

Photo by Elise Amendola
The length of time red blood cells (RBCs) are stored does not affect transfusion outcomes in critically ill patients, results of the ABLE study suggest.
Researchers compared critically ill patients who received RBCs stored for an average of about 3 weeks to those who received RBCs stored for less than a week.
And there were no significant differences between the groups with regard to mortality, transfusion reactions, and other outcomes.
“Previous observational and laboratory studies have suggested that fresh blood may be better because of the breakdown of red blood cells and accumulation of toxins during storage,” said Alan Tinmouth, MD, of the University of Ottawa in Ontario, Canada.
“But this definitive clinical trial clearly shows that these changes do not affect the quality of blood.”
Dr Tinmouth and his colleagues reported these results in NEJM.
The researchers enrolled 2430 adult intensive care patients in the trial, comparing patients who received fresh RBCs (n=1211) to those who received older RBCs (n=1219). Fresh RBCs were stored for a median of 6.1±4.9 days, and older RBCs were stored for a median of 22.0±8.4 days.
There was no significant difference between the arms with regard to the primary outcome, 90-day mortality. This endpoint was met by 37% of patients who received fresh blood and 35.3% of those who received older blood.
There were no significant differences between the fresh and older RBC arms for other mortality outcomes, either. This included death in the intensive care unit (26.7% vs 24.2%), in-hospital death (33.3% vs 31.9%), and death by day 28 (30.6% vs 28.8%).
Similarly, there were no significant differences between the fresh and older blood arms with regard to major illnesses, including multiple organ dysfunction syndrome (13.4% vs 13%), acute respiratory distress syndrome (5.7% vs 6.6%), cardiovascular failure (5.1% vs 4.2%), cardiac ischemia or infarction (4.5% vs 3.6%), venous thromboembolism (3.6% for both), nosocomial infection (34.1% vs 31.3%), and acute transfusion reaction (0.3% vs 0.5%).
In addition, there was no significant difference between the fresh RBC arm and the older RBC arm in the length of time patients required mechanical ventilation (15.0±18.0 days vs 14.7±14.9 days), cardiac or vasoactive drugs (7.1±10.2 days vs 7.5±11.2 days), or extrarenal epuration (2.5±10.1 days vs 2.5±8.3 days).
And there was no significant difference between the fresh and older blood arms in patients’ length of stay in the hospital (34.4±39.5 days vs 33.9±38.8 days) or the intensive care unit (15.3±15.4 days vs 15.3±14.8 days).
Based on these results, the researchers said there is no need to worry about the age of blood routinely used in hospitals. The team is now conducting a trial to determine if the same can be said for transfusions in pediatric patients.
Study seems to support liberal transfusion strategy

Photo courtesy of UAB Hospital
Results of a large, randomized trial suggest a liberal transfusion strategy may benefit patients undergoing cardiac surgery.
Patients who received blood transfusions when their hemoglobin (Hb) levels were below 9 g/dL fared better than patients who only received transfusions once their Hb levels were below 7.5 g/dL.
The “low” Hb group had a slightly higher incidence of serious complications and a significantly higher rate of 90-day mortality than the “high” Hb group.
However, the researchers noted that the latter finding, while important, is difficult to interpret because the trial was not primarily designed to compare the difference in the number of deaths.
“Although only a hypothesis, the suggestion that it might be better rather than worse to transfuse patients who are only mildly anemic goes against the evidence about when to transfuse in non-cardiac surgery settings,” said Barnaby Reeves, DPhil, of the University of Bristol in the UK.
“Transfusing more rather than fewer patients would create a challenge for hospitals. With an aging population and possibly an increase in heart disease, obesity, and diabetes, it can only become more difficult in the future to maintain the national blood supply in the UK and in other developed countries around the world. Our findings emphasize the importance of interventions to reduce blood loss in the first place.”
Dr Reeves and his colleagues reported their findings in NEJM.
The team conducted their randomized, controlled trial to determine whether transfusing cardiac surgery patients at a lower Hb level would be safer or more cost-effective, as has been shown in other patient groups.
Individuals older than 16 who were undergoing non-emergency cardiac surgery were recruited to the trial at 17 UK hospitals. Patients with an Hb level of less than 9 g/dL after their operations were randomized to have a transfusion either when they became substantially anemic—with an Hb level of less than 7.5 g/dL—or right away, when they were mildly anemic—with an Hb level of less than 9 g/dL.
To compare the two transfusion strategies, the researchers assessed the incidence of serious infection, ischemic event, heart attack, infarction of the gut, and acute kidney injury in the first 3 months after the operation.
The team analyzed data for 2003 patients. Nearly all of the patients in the high Hb group received a transfusion (92.2%), compared to just over half of patients in the low Hb group (53.4%).
Slightly more patients in the low Hb group than the high Hb group had one or more of the aforementioned serious complications—35.1% and 33%, respectively (P=0.30). And significantly more patients had died at 90 days in the low Hb group than the high group—4.2% and 2.6%, respectively (P=0.045).
The researchers found no significant differences between the high and low Hb groups with respect to other information measured to assess recovery, but some of the other findings in the trial showed a trend in the same direction.
In addition, healthcare costs up to 3 months after surgery were similar in the high and low Hb groups.
“Even though the high group were given more blood, it was interesting that this did not lead to them costing more once the costs of treating complications were added to the analysis,” said Sarah Wordsworth, PhD, of the University of Oxford in the UK.
Based on the overall pattern of findings, the researchers proposed that a high or liberal transfusion threshold may be better after cardiac surgery. This challenges most prevailing guidelines and current health policy.
“Existing national and international transfusion guidelines recommend that blood transfusions only be given to patients who develop very low hemoglobin concentrations,” said Gavin Murphy, FRCS, of the University of Leicester in the UK.
“We have shown that this strategy may increase the number of deaths in cardiac surgery. This was the largest randomized trial ever conducted in the UK in a surgical or cardiac surgery population. It was the largest trial ever conducted that has considered indications for transfusion in cardiac surgery, and recruited over twice the number of patients recruited in all the previous trials put together. It . . . recruited patients from the majority of [National Health Service] cardiac surgery centers in the UK and therefore reflects current UK practice and is relevant to UK patients.”

Photo courtesy of UAB Hospital
Results of a large, randomized trial suggest a liberal transfusion strategy may benefit patients undergoing cardiac surgery.
Patients who received blood transfusions when their hemoglobin (Hb) levels were below 9 g/dL fared better than patients who only received transfusions once their Hb levels were below 7.5 g/dL.
The “low” Hb group had a slightly higher incidence of serious complications and a significantly higher rate of 90-day mortality than the “high” Hb group.
However, the researchers noted that the latter finding, while important, is difficult to interpret because the trial was not primarily designed to compare the difference in the number of deaths.
“Although only a hypothesis, the suggestion that it might be better rather than worse to transfuse patients who are only mildly anemic goes against the evidence about when to transfuse in non-cardiac surgery settings,” said Barnaby Reeves, DPhil, of the University of Bristol in the UK.
“Transfusing more rather than fewer patients would create a challenge for hospitals. With an aging population and possibly an increase in heart disease, obesity, and diabetes, it can only become more difficult in the future to maintain the national blood supply in the UK and in other developed countries around the world. Our findings emphasize the importance of interventions to reduce blood loss in the first place.”
Dr Reeves and his colleagues reported their findings in NEJM.
The team conducted their randomized, controlled trial to determine whether transfusing cardiac surgery patients at a lower Hb level would be safer or more cost-effective, as has been shown in other patient groups.
Individuals older than 16 who were undergoing non-emergency cardiac surgery were recruited to the trial at 17 UK hospitals. Patients with an Hb level of less than 9 g/dL after their operations were randomized to have a transfusion either when they became substantially anemic—with an Hb level of less than 7.5 g/dL—or right away, when they were mildly anemic—with an Hb level of less than 9 g/dL.
To compare the two transfusion strategies, the researchers assessed the incidence of serious infection, ischemic event, heart attack, infarction of the gut, and acute kidney injury in the first 3 months after the operation.
The team analyzed data for 2003 patients. Nearly all of the patients in the high Hb group received a transfusion (92.2%), compared to just over half of patients in the low Hb group (53.4%).
Slightly more patients in the low Hb group than the high Hb group had one or more of the aforementioned serious complications—35.1% and 33%, respectively (P=0.30). And significantly more patients had died at 90 days in the low Hb group than the high group—4.2% and 2.6%, respectively (P=0.045).
The researchers found no significant differences between the high and low Hb groups with respect to other information measured to assess recovery, but some of the other findings in the trial showed a trend in the same direction.
In addition, healthcare costs up to 3 months after surgery were similar in the high and low Hb groups.
“Even though the high group were given more blood, it was interesting that this did not lead to them costing more once the costs of treating complications were added to the analysis,” said Sarah Wordsworth, PhD, of the University of Oxford in the UK.
Based on the overall pattern of findings, the researchers proposed that a high or liberal transfusion threshold may be better after cardiac surgery. This challenges most prevailing guidelines and current health policy.
“Existing national and international transfusion guidelines recommend that blood transfusions only be given to patients who develop very low hemoglobin concentrations,” said Gavin Murphy, FRCS, of the University of Leicester in the UK.
“We have shown that this strategy may increase the number of deaths in cardiac surgery. This was the largest randomized trial ever conducted in the UK in a surgical or cardiac surgery population. It was the largest trial ever conducted that has considered indications for transfusion in cardiac surgery, and recruited over twice the number of patients recruited in all the previous trials put together. It . . . recruited patients from the majority of [National Health Service] cardiac surgery centers in the UK and therefore reflects current UK practice and is relevant to UK patients.”

Photo courtesy of UAB Hospital
Results of a large, randomized trial suggest a liberal transfusion strategy may benefit patients undergoing cardiac surgery.
Patients who received blood transfusions when their hemoglobin (Hb) levels were below 9 g/dL fared better than patients who only received transfusions once their Hb levels were below 7.5 g/dL.
The “low” Hb group had a slightly higher incidence of serious complications and a significantly higher rate of 90-day mortality than the “high” Hb group.
However, the researchers noted that the latter finding, while important, is difficult to interpret because the trial was not primarily designed to compare the difference in the number of deaths.
“Although only a hypothesis, the suggestion that it might be better rather than worse to transfuse patients who are only mildly anemic goes against the evidence about when to transfuse in non-cardiac surgery settings,” said Barnaby Reeves, DPhil, of the University of Bristol in the UK.
“Transfusing more rather than fewer patients would create a challenge for hospitals. With an aging population and possibly an increase in heart disease, obesity, and diabetes, it can only become more difficult in the future to maintain the national blood supply in the UK and in other developed countries around the world. Our findings emphasize the importance of interventions to reduce blood loss in the first place.”
Dr Reeves and his colleagues reported their findings in NEJM.
The team conducted their randomized, controlled trial to determine whether transfusing cardiac surgery patients at a lower Hb level would be safer or more cost-effective, as has been shown in other patient groups.
Individuals older than 16 who were undergoing non-emergency cardiac surgery were recruited to the trial at 17 UK hospitals. Patients with an Hb level of less than 9 g/dL after their operations were randomized to have a transfusion either when they became substantially anemic—with an Hb level of less than 7.5 g/dL—or right away, when they were mildly anemic—with an Hb level of less than 9 g/dL.
To compare the two transfusion strategies, the researchers assessed the incidence of serious infection, ischemic event, heart attack, infarction of the gut, and acute kidney injury in the first 3 months after the operation.
The team analyzed data for 2003 patients. Nearly all of the patients in the high Hb group received a transfusion (92.2%), compared to just over half of patients in the low Hb group (53.4%).
Slightly more patients in the low Hb group than the high Hb group had one or more of the aforementioned serious complications—35.1% and 33%, respectively (P=0.30). And significantly more patients had died at 90 days in the low Hb group than the high group—4.2% and 2.6%, respectively (P=0.045).
The researchers found no significant differences between the high and low Hb groups with respect to other information measured to assess recovery, but some of the other findings in the trial showed a trend in the same direction.
In addition, healthcare costs up to 3 months after surgery were similar in the high and low Hb groups.
“Even though the high group were given more blood, it was interesting that this did not lead to them costing more once the costs of treating complications were added to the analysis,” said Sarah Wordsworth, PhD, of the University of Oxford in the UK.
Based on the overall pattern of findings, the researchers proposed that a high or liberal transfusion threshold may be better after cardiac surgery. This challenges most prevailing guidelines and current health policy.
“Existing national and international transfusion guidelines recommend that blood transfusions only be given to patients who develop very low hemoglobin concentrations,” said Gavin Murphy, FRCS, of the University of Leicester in the UK.
“We have shown that this strategy may increase the number of deaths in cardiac surgery. This was the largest randomized trial ever conducted in the UK in a surgical or cardiac surgery population. It was the largest trial ever conducted that has considered indications for transfusion in cardiac surgery, and recruited over twice the number of patients recruited in all the previous trials put together. It . . . recruited patients from the majority of [National Health Service] cardiac surgery centers in the UK and therefore reflects current UK practice and is relevant to UK patients.”
Plasma product can be stored longer, FDA says

Photo by Cristina Granados
The US Food and Drug Administration (FDA) has approved a revised label for the pooled plasma product Octaplas, increasing the product’s shelf life.
The new label says Octaplas can now be stored frozen, at or below -18°C (-0.4°F), for 3 years from the date of manufacture.
And thawed Octaplas should be used within 24 hours if refrigerated (between 1°C and 6°C/33.8°F to 42.8°F) or within 8 hours if stored at room temperature (between 20°C and 25°C/68°F to 77°F)
The previous product label said frozen Octaplas could be stored for 2 years, and thawed Octaplas should be used within 12 hours if stored between 2°C and 4°C (35.6°F to 39.2°F) or within 3 hours if stored between 20°C and 25°C (68°F to 77°F).
About Octaplas
Octaplas is a sterile, frozen solution of human plasma from several donors that has been treated with a solvent detergent process to minimize the risk of serious virus transmission. The plasma is collected from US donors who have been screened and tested for diseases transmitted by blood.
Octaplas gained FDA approval in January 2013. The product is indicated for the replacement of multiple coagulation factors in patients with acquired deficiencies due to liver disease or undergoing cardiac surgery or liver transplant. Octaplas can also be used for plasma exchange in patients with thrombotic thrombocytopenic purpura.
Octaplas is contraindicated in patients with immunoglobulin A deficiency, severe deficiency of protein S, history of hypersensitivity to fresh-frozen plasma or to plasma-derived products including any plasma protein, or a history of hypersensitivity reaction to Octaplas.
Serious adverse events observed in clinical trials of Octaplas were anaphylactic shock, citrate toxicity, and severe hypotension. The most common adverse events observed in 1% of patients or more included pruritus, urticaria, nausea, headache, and paresthesia.
Transfusion reactions can occur with ABO blood group mismatches. High infusion rates can induce hypervolemia with consequent pulmonary edema or cardiac failure. Excessive bleeding due to hyperfibrinolysis can occur due to low levels of alpha2-antiplasmin.
Thrombosis can occur due to low levels of protein S. Citrate toxicity can occur with transfusion rates exceeding 1 mL/kg/min of Octaplas. As Octaplas is made from human plasma, it may carry a risk of transmitting infectious agents, such as viruses, the variant Creutzfeldt-Jakob disease agent, and, theoretically, the Creutzfeldt-Jakob disease agent.
For more details on Octaplas, see the complete prescribing information.

Photo by Cristina Granados
The US Food and Drug Administration (FDA) has approved a revised label for the pooled plasma product Octaplas, increasing the product’s shelf life.
The new label says Octaplas can now be stored frozen, at or below -18°C (-0.4°F), for 3 years from the date of manufacture.
And thawed Octaplas should be used within 24 hours if refrigerated (between 1°C and 6°C/33.8°F to 42.8°F) or within 8 hours if stored at room temperature (between 20°C and 25°C/68°F to 77°F)
The previous product label said frozen Octaplas could be stored for 2 years, and thawed Octaplas should be used within 12 hours if stored between 2°C and 4°C (35.6°F to 39.2°F) or within 3 hours if stored between 20°C and 25°C (68°F to 77°F).
About Octaplas
Octaplas is a sterile, frozen solution of human plasma from several donors that has been treated with a solvent detergent process to minimize the risk of serious virus transmission. The plasma is collected from US donors who have been screened and tested for diseases transmitted by blood.
Octaplas gained FDA approval in January 2013. The product is indicated for the replacement of multiple coagulation factors in patients with acquired deficiencies due to liver disease or undergoing cardiac surgery or liver transplant. Octaplas can also be used for plasma exchange in patients with thrombotic thrombocytopenic purpura.
Octaplas is contraindicated in patients with immunoglobulin A deficiency, severe deficiency of protein S, history of hypersensitivity to fresh-frozen plasma or to plasma-derived products including any plasma protein, or a history of hypersensitivity reaction to Octaplas.
Serious adverse events observed in clinical trials of Octaplas were anaphylactic shock, citrate toxicity, and severe hypotension. The most common adverse events observed in 1% of patients or more included pruritus, urticaria, nausea, headache, and paresthesia.
Transfusion reactions can occur with ABO blood group mismatches. High infusion rates can induce hypervolemia with consequent pulmonary edema or cardiac failure. Excessive bleeding due to hyperfibrinolysis can occur due to low levels of alpha2-antiplasmin.
Thrombosis can occur due to low levels of protein S. Citrate toxicity can occur with transfusion rates exceeding 1 mL/kg/min of Octaplas. As Octaplas is made from human plasma, it may carry a risk of transmitting infectious agents, such as viruses, the variant Creutzfeldt-Jakob disease agent, and, theoretically, the Creutzfeldt-Jakob disease agent.
For more details on Octaplas, see the complete prescribing information.

Photo by Cristina Granados
The US Food and Drug Administration (FDA) has approved a revised label for the pooled plasma product Octaplas, increasing the product’s shelf life.
The new label says Octaplas can now be stored frozen, at or below -18°C (-0.4°F), for 3 years from the date of manufacture.
And thawed Octaplas should be used within 24 hours if refrigerated (between 1°C and 6°C/33.8°F to 42.8°F) or within 8 hours if stored at room temperature (between 20°C and 25°C/68°F to 77°F)
The previous product label said frozen Octaplas could be stored for 2 years, and thawed Octaplas should be used within 12 hours if stored between 2°C and 4°C (35.6°F to 39.2°F) or within 3 hours if stored between 20°C and 25°C (68°F to 77°F).
About Octaplas
Octaplas is a sterile, frozen solution of human plasma from several donors that has been treated with a solvent detergent process to minimize the risk of serious virus transmission. The plasma is collected from US donors who have been screened and tested for diseases transmitted by blood.
Octaplas gained FDA approval in January 2013. The product is indicated for the replacement of multiple coagulation factors in patients with acquired deficiencies due to liver disease or undergoing cardiac surgery or liver transplant. Octaplas can also be used for plasma exchange in patients with thrombotic thrombocytopenic purpura.
Octaplas is contraindicated in patients with immunoglobulin A deficiency, severe deficiency of protein S, history of hypersensitivity to fresh-frozen plasma or to plasma-derived products including any plasma protein, or a history of hypersensitivity reaction to Octaplas.
Serious adverse events observed in clinical trials of Octaplas were anaphylactic shock, citrate toxicity, and severe hypotension. The most common adverse events observed in 1% of patients or more included pruritus, urticaria, nausea, headache, and paresthesia.
Transfusion reactions can occur with ABO blood group mismatches. High infusion rates can induce hypervolemia with consequent pulmonary edema or cardiac failure. Excessive bleeding due to hyperfibrinolysis can occur due to low levels of alpha2-antiplasmin.
Thrombosis can occur due to low levels of protein S. Citrate toxicity can occur with transfusion rates exceeding 1 mL/kg/min of Octaplas. As Octaplas is made from human plasma, it may carry a risk of transmitting infectious agents, such as viruses, the variant Creutzfeldt-Jakob disease agent, and, theoretically, the Creutzfeldt-Jakob disease agent.
For more details on Octaplas, see the complete prescribing information.
Polymer can stop lethal bleeding in vivo
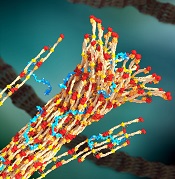
a blood clot, with PolySTAT
(blue) binding strands together
Image by William Walker–
University of Washington
Preclinical research suggests an injectable polymer known as PolySTAT may one day be able to halt life-threatening bleeding in soldiers and trauma patients.
Once injected, this hemostatic polymer circulates in the blood, homes to sites of vascular injury, and promotes the formation of blood clots.
In experiments with rats, 100% of animals injected with PolySTAT survived a typically lethal injury to the femoral artery. In comparison, 0% to 40% of controls survived.
“Most of the patients who die from bleeding die quickly,” said Nathan White, MD, of the University of Washington in Seattle.
“[PolySTAT] is something you could potentially put in a syringe inside a backpack and give right away to reduce blood loss and keep people alive long enough to make it to medical care.”
Dr White and his colleagues described their work with PolySTAT in Science Translational Medicine. A related Focus article addressed the promises and challenges of advancing PolySTAT and other clotting approaches from proof-of-principle to clinical development.
PolySTAT induces hemostasis by cross-linking the fibrin matrix within blood clots, just as factor XIII does. But the researchers said PolySTAT offers greater protection against natural enzymes that dissolve blood clots.
That’s because PolySTAT binds to fibrin monomers and is uniformly integrated into fibrin fibers during polymerization. This produces a fortified, hybrid polymer network that can resist enzymatic degradation.
In vitro experiments showed that PolySTAT accelerated clotting kinetics, increased the strength of blood clots, and delayed clot breakdown.
The researchers also assessed how PolySTAT affected rats following a femoral artery injury, comparing results with PolySTAT to those with volume control (0.9% saline), a nonbinding scrambled control polymer (PolySCRAM), rat albumin, and human FXIIIa.
The team found that PolySTAT conferred superior survival by reducing blood loss and fluid resuscitation requirements.
All of the rats treated with PolySTAT (5/5) survived to the end of the experiment, compared to none of the rats that received albumin, 20% that received PolySCRAM or FXIIIa, and 40% that received volume control.
The researchers said PolySTAT’s initial safety profile looks promising, but they are still planning to test the polymer on larger animals and conduct additional screening to find out if PolySTAT binds to any other unintended substances.
The team also plans to investigate PolySTAT’s potential for treating hemophilia and for integration into bandages.

a blood clot, with PolySTAT
(blue) binding strands together
Image by William Walker–
University of Washington
Preclinical research suggests an injectable polymer known as PolySTAT may one day be able to halt life-threatening bleeding in soldiers and trauma patients.
Once injected, this hemostatic polymer circulates in the blood, homes to sites of vascular injury, and promotes the formation of blood clots.
In experiments with rats, 100% of animals injected with PolySTAT survived a typically lethal injury to the femoral artery. In comparison, 0% to 40% of controls survived.
“Most of the patients who die from bleeding die quickly,” said Nathan White, MD, of the University of Washington in Seattle.
“[PolySTAT] is something you could potentially put in a syringe inside a backpack and give right away to reduce blood loss and keep people alive long enough to make it to medical care.”
Dr White and his colleagues described their work with PolySTAT in Science Translational Medicine. A related Focus article addressed the promises and challenges of advancing PolySTAT and other clotting approaches from proof-of-principle to clinical development.
PolySTAT induces hemostasis by cross-linking the fibrin matrix within blood clots, just as factor XIII does. But the researchers said PolySTAT offers greater protection against natural enzymes that dissolve blood clots.
That’s because PolySTAT binds to fibrin monomers and is uniformly integrated into fibrin fibers during polymerization. This produces a fortified, hybrid polymer network that can resist enzymatic degradation.
In vitro experiments showed that PolySTAT accelerated clotting kinetics, increased the strength of blood clots, and delayed clot breakdown.
The researchers also assessed how PolySTAT affected rats following a femoral artery injury, comparing results with PolySTAT to those with volume control (0.9% saline), a nonbinding scrambled control polymer (PolySCRAM), rat albumin, and human FXIIIa.
The team found that PolySTAT conferred superior survival by reducing blood loss and fluid resuscitation requirements.
All of the rats treated with PolySTAT (5/5) survived to the end of the experiment, compared to none of the rats that received albumin, 20% that received PolySCRAM or FXIIIa, and 40% that received volume control.
The researchers said PolySTAT’s initial safety profile looks promising, but they are still planning to test the polymer on larger animals and conduct additional screening to find out if PolySTAT binds to any other unintended substances.
The team also plans to investigate PolySTAT’s potential for treating hemophilia and for integration into bandages.

a blood clot, with PolySTAT
(blue) binding strands together
Image by William Walker–
University of Washington
Preclinical research suggests an injectable polymer known as PolySTAT may one day be able to halt life-threatening bleeding in soldiers and trauma patients.
Once injected, this hemostatic polymer circulates in the blood, homes to sites of vascular injury, and promotes the formation of blood clots.
In experiments with rats, 100% of animals injected with PolySTAT survived a typically lethal injury to the femoral artery. In comparison, 0% to 40% of controls survived.
“Most of the patients who die from bleeding die quickly,” said Nathan White, MD, of the University of Washington in Seattle.
“[PolySTAT] is something you could potentially put in a syringe inside a backpack and give right away to reduce blood loss and keep people alive long enough to make it to medical care.”
Dr White and his colleagues described their work with PolySTAT in Science Translational Medicine. A related Focus article addressed the promises and challenges of advancing PolySTAT and other clotting approaches from proof-of-principle to clinical development.
PolySTAT induces hemostasis by cross-linking the fibrin matrix within blood clots, just as factor XIII does. But the researchers said PolySTAT offers greater protection against natural enzymes that dissolve blood clots.
That’s because PolySTAT binds to fibrin monomers and is uniformly integrated into fibrin fibers during polymerization. This produces a fortified, hybrid polymer network that can resist enzymatic degradation.
In vitro experiments showed that PolySTAT accelerated clotting kinetics, increased the strength of blood clots, and delayed clot breakdown.
The researchers also assessed how PolySTAT affected rats following a femoral artery injury, comparing results with PolySTAT to those with volume control (0.9% saline), a nonbinding scrambled control polymer (PolySCRAM), rat albumin, and human FXIIIa.
The team found that PolySTAT conferred superior survival by reducing blood loss and fluid resuscitation requirements.
All of the rats treated with PolySTAT (5/5) survived to the end of the experiment, compared to none of the rats that received albumin, 20% that received PolySCRAM or FXIIIa, and 40% that received volume control.
The researchers said PolySTAT’s initial safety profile looks promising, but they are still planning to test the polymer on larger animals and conduct additional screening to find out if PolySTAT binds to any other unintended substances.
The team also plans to investigate PolySTAT’s potential for treating hemophilia and for integration into bandages.