User login
System allows blood group typing at the DNA level

Daniel Gay
A new system allows for accurate blood group typing at the DNA level, on a large scale and at a relatively low cost, according to a paper published in The Journal of Molecular Diagnostics.
Researchers designed this automated genotyping system using 96-well DNA microarrays for blood donation screening and a panel of 8 single-nucleotide polymorphisms (SNPs) to identify 16 alleles in 4 blood group systems—KEL, KIDD, DUFFY, and MNS.
The team said they developed this system because conventional hemagglutination falls short in 2 ways: it’s time consuming and involves a limited range of antigen testing.
“In the French Blood Service, the Etablissement Français du Sang (EFS), blood donation qualification laboratories test all blood donations for A, B, O, Rhesus, and KEL blood groups, but only 5% to 10% of donations are tested for other clinically significant antigens [such as FY1, FY2, JK1, JK2, MNS3, and MNS4],” said study investigator Jean-Charles Brès, PhD, of EFS Pyrénées Méditerranée in Montpellier, France.
So he and his colleagues developed their system—a robotic platform using a 96-well DNA microarray for multiplex blood group genotyping.
They designed an SNP module to allow for simultaneous determination of KEL (KEL*01/KEL*02, KEL*03/KEL*04), KIDD (JK*01/JK*02), DUFFY (FY*01/FY*02, FY*02M.01 or FY*X, and FY*02M.02 or FY*Fy), and MNS (GYPA*01/GYPA*02 or MNS*01/MNS*02, GYPB*03/GYPB*04 or MNS*03/MNS*04) blood group antigens.
The researchers tested the system in a pilot study, using 1132 EDTA-anticoagulated blood samples collected by the EFS. Random donors, mostly Caucasian, were extensively phenotyped using standard serologic hemagglutination techniques.
The team used 172 samples to determine scoring criteria for predicting phenotype and the remaining 960 samples for validation of the 96-well DNA microarray system.
A total of 938 samples were considered valid and assigned genotypes based on the scoring criteria determined for the 8 SNPs. Twenty-two samples were invalid because they were considered “uninterpretable” for all SNPs.
The researchers compared the phenotypes predicted from genotypes with those obtained by serologic typing. And they found the concordance rates between the DNA-based and standard hemagglutination assays were high.
The overall concordance rate was 99.92%. There was 100% concordance for KEL*03/KEL*04; GYPA*01/GYPA*02; and FY*01/FY*02/FY*02M.01/FY*02M.02. And the concordance rate was 99.89% for KEL*01/KEL*02; JK*01/JK*02; and GYPB*03/GYPB*04.
So the researchers said that, overall, this system appears effective. They also noted that the system allows for simultaneous multiplex assay of up to 96 samples in a single reaction run. But other DNA microarray formats with a lower number of wells can be processed as well.
For small batch production, the cost of genotyping, including genomic DNA extraction, labor, and equipment, was less than $2.60 per SNP for a multiplex set of 8 SNPs, which is 4 times lower than the per-antigen cost using serologic methods.
“In addition to providing more fully antigen-matched [red blood cells] and allowing better identification of rare donor blood types, this technology will reduce adverse reactions and decrease the relative cost of analysis,” Dr Brès said.
“High-throughput DNA typing could facilitate support for patients undergoing long-term transfusion who are at high risk of alloantibody production, such as patients with sickle cell disease, thalassemia, or autoimmune hemolytic anemia.” ![]()

Daniel Gay
A new system allows for accurate blood group typing at the DNA level, on a large scale and at a relatively low cost, according to a paper published in The Journal of Molecular Diagnostics.
Researchers designed this automated genotyping system using 96-well DNA microarrays for blood donation screening and a panel of 8 single-nucleotide polymorphisms (SNPs) to identify 16 alleles in 4 blood group systems—KEL, KIDD, DUFFY, and MNS.
The team said they developed this system because conventional hemagglutination falls short in 2 ways: it’s time consuming and involves a limited range of antigen testing.
“In the French Blood Service, the Etablissement Français du Sang (EFS), blood donation qualification laboratories test all blood donations for A, B, O, Rhesus, and KEL blood groups, but only 5% to 10% of donations are tested for other clinically significant antigens [such as FY1, FY2, JK1, JK2, MNS3, and MNS4],” said study investigator Jean-Charles Brès, PhD, of EFS Pyrénées Méditerranée in Montpellier, France.
So he and his colleagues developed their system—a robotic platform using a 96-well DNA microarray for multiplex blood group genotyping.
They designed an SNP module to allow for simultaneous determination of KEL (KEL*01/KEL*02, KEL*03/KEL*04), KIDD (JK*01/JK*02), DUFFY (FY*01/FY*02, FY*02M.01 or FY*X, and FY*02M.02 or FY*Fy), and MNS (GYPA*01/GYPA*02 or MNS*01/MNS*02, GYPB*03/GYPB*04 or MNS*03/MNS*04) blood group antigens.
The researchers tested the system in a pilot study, using 1132 EDTA-anticoagulated blood samples collected by the EFS. Random donors, mostly Caucasian, were extensively phenotyped using standard serologic hemagglutination techniques.
The team used 172 samples to determine scoring criteria for predicting phenotype and the remaining 960 samples for validation of the 96-well DNA microarray system.
A total of 938 samples were considered valid and assigned genotypes based on the scoring criteria determined for the 8 SNPs. Twenty-two samples were invalid because they were considered “uninterpretable” for all SNPs.
The researchers compared the phenotypes predicted from genotypes with those obtained by serologic typing. And they found the concordance rates between the DNA-based and standard hemagglutination assays were high.
The overall concordance rate was 99.92%. There was 100% concordance for KEL*03/KEL*04; GYPA*01/GYPA*02; and FY*01/FY*02/FY*02M.01/FY*02M.02. And the concordance rate was 99.89% for KEL*01/KEL*02; JK*01/JK*02; and GYPB*03/GYPB*04.
So the researchers said that, overall, this system appears effective. They also noted that the system allows for simultaneous multiplex assay of up to 96 samples in a single reaction run. But other DNA microarray formats with a lower number of wells can be processed as well.
For small batch production, the cost of genotyping, including genomic DNA extraction, labor, and equipment, was less than $2.60 per SNP for a multiplex set of 8 SNPs, which is 4 times lower than the per-antigen cost using serologic methods.
“In addition to providing more fully antigen-matched [red blood cells] and allowing better identification of rare donor blood types, this technology will reduce adverse reactions and decrease the relative cost of analysis,” Dr Brès said.
“High-throughput DNA typing could facilitate support for patients undergoing long-term transfusion who are at high risk of alloantibody production, such as patients with sickle cell disease, thalassemia, or autoimmune hemolytic anemia.” ![]()

Daniel Gay
A new system allows for accurate blood group typing at the DNA level, on a large scale and at a relatively low cost, according to a paper published in The Journal of Molecular Diagnostics.
Researchers designed this automated genotyping system using 96-well DNA microarrays for blood donation screening and a panel of 8 single-nucleotide polymorphisms (SNPs) to identify 16 alleles in 4 blood group systems—KEL, KIDD, DUFFY, and MNS.
The team said they developed this system because conventional hemagglutination falls short in 2 ways: it’s time consuming and involves a limited range of antigen testing.
“In the French Blood Service, the Etablissement Français du Sang (EFS), blood donation qualification laboratories test all blood donations for A, B, O, Rhesus, and KEL blood groups, but only 5% to 10% of donations are tested for other clinically significant antigens [such as FY1, FY2, JK1, JK2, MNS3, and MNS4],” said study investigator Jean-Charles Brès, PhD, of EFS Pyrénées Méditerranée in Montpellier, France.
So he and his colleagues developed their system—a robotic platform using a 96-well DNA microarray for multiplex blood group genotyping.
They designed an SNP module to allow for simultaneous determination of KEL (KEL*01/KEL*02, KEL*03/KEL*04), KIDD (JK*01/JK*02), DUFFY (FY*01/FY*02, FY*02M.01 or FY*X, and FY*02M.02 or FY*Fy), and MNS (GYPA*01/GYPA*02 or MNS*01/MNS*02, GYPB*03/GYPB*04 or MNS*03/MNS*04) blood group antigens.
The researchers tested the system in a pilot study, using 1132 EDTA-anticoagulated blood samples collected by the EFS. Random donors, mostly Caucasian, were extensively phenotyped using standard serologic hemagglutination techniques.
The team used 172 samples to determine scoring criteria for predicting phenotype and the remaining 960 samples for validation of the 96-well DNA microarray system.
A total of 938 samples were considered valid and assigned genotypes based on the scoring criteria determined for the 8 SNPs. Twenty-two samples were invalid because they were considered “uninterpretable” for all SNPs.
The researchers compared the phenotypes predicted from genotypes with those obtained by serologic typing. And they found the concordance rates between the DNA-based and standard hemagglutination assays were high.
The overall concordance rate was 99.92%. There was 100% concordance for KEL*03/KEL*04; GYPA*01/GYPA*02; and FY*01/FY*02/FY*02M.01/FY*02M.02. And the concordance rate was 99.89% for KEL*01/KEL*02; JK*01/JK*02; and GYPB*03/GYPB*04.
So the researchers said that, overall, this system appears effective. They also noted that the system allows for simultaneous multiplex assay of up to 96 samples in a single reaction run. But other DNA microarray formats with a lower number of wells can be processed as well.
For small batch production, the cost of genotyping, including genomic DNA extraction, labor, and equipment, was less than $2.60 per SNP for a multiplex set of 8 SNPs, which is 4 times lower than the per-antigen cost using serologic methods.
“In addition to providing more fully antigen-matched [red blood cells] and allowing better identification of rare donor blood types, this technology will reduce adverse reactions and decrease the relative cost of analysis,” Dr Brès said.
“High-throughput DNA typing could facilitate support for patients undergoing long-term transfusion who are at high risk of alloantibody production, such as patients with sickle cell disease, thalassemia, or autoimmune hemolytic anemia.” ![]()
Restrictive transfusion approach may cut risk of HAIs

Credit: Elise Amendola
A review of randomized trials indicates that a restrictive approach to blood transfusion can decrease the risk of healthcare-associated infections (HAIs) for some patients.
Investigators found that, overall, restricting red blood cell (RBC) transfusions to patients with hemoglobin concentrations of 7 g/dL or less was associated with a lower incidence of HAIs such as pneumonia, mediastinitis, and sepsis.
However, when they stratified results by patient type, the researchers found that a restrictive transfusion approach significantly decreased the risk of HAIs only for patients who already had sepsis or were undergoing orthopedic surgery.
Jeffrey M. Rohde, MD, of the University of Michigan in Ann Arbor, and his colleagues reported these findings in JAMA.
The investigators set out to compare restrictive and liberal RBC transfusion strategies using data from 21 randomized trials in 9 countries. Eighteen of the trials (n=7593) contained enough information for a meta-analysis.
The pooled risk of all serious HAIs was 11.8% for patients treated with a restrictive transfusion approach and 16.9% for patients treated with a liberal approach. The risk ratio (RR) for the association between transfusion strategies and serious infection was 0.82.
“The fewer the red blood cell transfusions, the less likely hospitalized patients were to develop infections,” Dr Rohde said. “This is most likely due to the patient’s immune system reacting to donor blood [known as transfusion-associated immunomodulation].”
Even when the transfusions were leukoreduced, the risk of infection remained lower with a restrictive transfusion strategy. The RR was 0.80.
The results suggested that, for every 1000 patients in which RBC transfusion is a consideration, 26 could potentially be spared an HAI if restrictive strategies were used.
On the other hand, the investigators found no significant differences in the incidence of HAIs by RBC threshold for patients with cardiac disease, the critically ill, those with acute upper gastrointestinal bleeding, or for infants with low birth weight.
Yet the risk of infection was significantly lower with a restrictive strategy for patients who already had sepsis or were undergoing orthopedic surgery. The RRs were 0.51 and 0.70, respectively.
Dr Rohde and his colleagues said these results support AABB’s 2012 guidelines for transfusing hospitalized patients. The guidelines recommend a restrictive strategy for all hospitalized patients but also list specific hemoglobin-based recommendations for different patient populations. ![]()

Credit: Elise Amendola
A review of randomized trials indicates that a restrictive approach to blood transfusion can decrease the risk of healthcare-associated infections (HAIs) for some patients.
Investigators found that, overall, restricting red blood cell (RBC) transfusions to patients with hemoglobin concentrations of 7 g/dL or less was associated with a lower incidence of HAIs such as pneumonia, mediastinitis, and sepsis.
However, when they stratified results by patient type, the researchers found that a restrictive transfusion approach significantly decreased the risk of HAIs only for patients who already had sepsis or were undergoing orthopedic surgery.
Jeffrey M. Rohde, MD, of the University of Michigan in Ann Arbor, and his colleagues reported these findings in JAMA.
The investigators set out to compare restrictive and liberal RBC transfusion strategies using data from 21 randomized trials in 9 countries. Eighteen of the trials (n=7593) contained enough information for a meta-analysis.
The pooled risk of all serious HAIs was 11.8% for patients treated with a restrictive transfusion approach and 16.9% for patients treated with a liberal approach. The risk ratio (RR) for the association between transfusion strategies and serious infection was 0.82.
“The fewer the red blood cell transfusions, the less likely hospitalized patients were to develop infections,” Dr Rohde said. “This is most likely due to the patient’s immune system reacting to donor blood [known as transfusion-associated immunomodulation].”
Even when the transfusions were leukoreduced, the risk of infection remained lower with a restrictive transfusion strategy. The RR was 0.80.
The results suggested that, for every 1000 patients in which RBC transfusion is a consideration, 26 could potentially be spared an HAI if restrictive strategies were used.
On the other hand, the investigators found no significant differences in the incidence of HAIs by RBC threshold for patients with cardiac disease, the critically ill, those with acute upper gastrointestinal bleeding, or for infants with low birth weight.
Yet the risk of infection was significantly lower with a restrictive strategy for patients who already had sepsis or were undergoing orthopedic surgery. The RRs were 0.51 and 0.70, respectively.
Dr Rohde and his colleagues said these results support AABB’s 2012 guidelines for transfusing hospitalized patients. The guidelines recommend a restrictive strategy for all hospitalized patients but also list specific hemoglobin-based recommendations for different patient populations. ![]()

Credit: Elise Amendola
A review of randomized trials indicates that a restrictive approach to blood transfusion can decrease the risk of healthcare-associated infections (HAIs) for some patients.
Investigators found that, overall, restricting red blood cell (RBC) transfusions to patients with hemoglobin concentrations of 7 g/dL or less was associated with a lower incidence of HAIs such as pneumonia, mediastinitis, and sepsis.
However, when they stratified results by patient type, the researchers found that a restrictive transfusion approach significantly decreased the risk of HAIs only for patients who already had sepsis or were undergoing orthopedic surgery.
Jeffrey M. Rohde, MD, of the University of Michigan in Ann Arbor, and his colleagues reported these findings in JAMA.
The investigators set out to compare restrictive and liberal RBC transfusion strategies using data from 21 randomized trials in 9 countries. Eighteen of the trials (n=7593) contained enough information for a meta-analysis.
The pooled risk of all serious HAIs was 11.8% for patients treated with a restrictive transfusion approach and 16.9% for patients treated with a liberal approach. The risk ratio (RR) for the association between transfusion strategies and serious infection was 0.82.
“The fewer the red blood cell transfusions, the less likely hospitalized patients were to develop infections,” Dr Rohde said. “This is most likely due to the patient’s immune system reacting to donor blood [known as transfusion-associated immunomodulation].”
Even when the transfusions were leukoreduced, the risk of infection remained lower with a restrictive transfusion strategy. The RR was 0.80.
The results suggested that, for every 1000 patients in which RBC transfusion is a consideration, 26 could potentially be spared an HAI if restrictive strategies were used.
On the other hand, the investigators found no significant differences in the incidence of HAIs by RBC threshold for patients with cardiac disease, the critically ill, those with acute upper gastrointestinal bleeding, or for infants with low birth weight.
Yet the risk of infection was significantly lower with a restrictive strategy for patients who already had sepsis or were undergoing orthopedic surgery. The RRs were 0.51 and 0.70, respectively.
Dr Rohde and his colleagues said these results support AABB’s 2012 guidelines for transfusing hospitalized patients. The guidelines recommend a restrictive strategy for all hospitalized patients but also list specific hemoglobin-based recommendations for different patient populations. ![]()
CDC reports more cases of Heartland virus disease

Credit: CDC
Health officials have reported 6 new cases of Heartland virus disease—5 in Missouri and 1 in Tennessee.
These cases, discovered in 2012 and 2013, add to the 2 cases discovered in 2009 and are described in the Centers for Disease Control and Prevention (CDC) Morbidity and Mortality Weekly Report.
The Heartland virus was first reported in 2 farmers in northwestern Missouri who were hospitalized in 2009 with what was thought to be ehrlichiosis, a tick-borne disease.
However, the patients failed to improve with treatment, and testing failed to confirm ehlrlichiosis.
Working with state and local partners, the CDC eventually identified the cause of the men’s illness: a previously unknown phlebovirus, now dubbed the Heartland virus.
Although we do not know for certain how patients are infected with the virus, research has suggested that ticks, namely lone star ticks, transmit it.
Ongoing investigations have uncovered 6 more cases of Heartland virus disease. All of the patients were white men older than 50 years of age. Five of them reported tick bites in the days or weeks before they fell ill.
Their symptoms started in May to September and included fever, fatigue, loss of appetite, headache, nausea, and muscle pain. The patients also had leukopenia and thrombocytopenia.
Four of the 6 patients were hospitalized. And 1 man, who suffered from other health conditions, died. It is not known if the Heartland virus was the cause of death or how much it contributed to his death.
The CDC has been working with the Missouri and Tennessee state health departments and other federal agencies to advance our understanding of Heartland virus disease by learning more about the patients who were infected, their illness, and their exposure to ticks.
The CDC aims to determine the symptoms and severity of the disease, where it is found, how people are being infected, and how to prevent infections.
CDC studies to date have shown that the Heartland virus is carried by lone star ticks, which are primarily found in the southeastern and eastern US.
Researchers hope additional studies can confirm whether ticks can spread the virus and reveal which other insects or animals may be involved in the transmission cycle. The CDC is also looking for the Heartland virus in other parts of the country to understand how widely it may be distributed.
“During the past 2 years, CDC has worked closely with state health departments, hospitals, and many experts from universities and other federal agencies to learn more about Heartland virus,” said Roger Nasci, PhD, chief of the CDC’s Arboviral Diseases Branch.
“By gathering information about the disease Heartland virus causes, and about how it’s spread to people, we hope to better understand the potential impact on the public’s health and how we can help protect people from this virus.”
The CDC developed the blood tests used to confirm the new cases of Heartland virus disease. CDC teams are working to further validate these tests and develop additional tests. The researchers hope to develop a diagnostic test that public health laboratories could use to test for the virus.
There is no specific treatment or vaccine for Heartland virus disease. However, supportive therapies such as intravenous fluids and fever reducers can relieve some symptoms of Heartland disease.
To reduce the risk of Heartland and other vector-borne diseases, the CDC recommends:
- Avoiding wooded and bushy areas with high grass and leaf litter
- Using insect repellent when outdoors
- Using products that contain permethrin on clothing
- Conducting a full-body tick check after spending time outdoors
- Bathing as soon as possible after coming indoors to wash off and more easily find any ticks
- Examining gear and pets, as ticks can “ride” into the home and attach to a person later.
For more information on the Heartland virus, visit the CDC website. ![]()

Credit: CDC
Health officials have reported 6 new cases of Heartland virus disease—5 in Missouri and 1 in Tennessee.
These cases, discovered in 2012 and 2013, add to the 2 cases discovered in 2009 and are described in the Centers for Disease Control and Prevention (CDC) Morbidity and Mortality Weekly Report.
The Heartland virus was first reported in 2 farmers in northwestern Missouri who were hospitalized in 2009 with what was thought to be ehrlichiosis, a tick-borne disease.
However, the patients failed to improve with treatment, and testing failed to confirm ehlrlichiosis.
Working with state and local partners, the CDC eventually identified the cause of the men’s illness: a previously unknown phlebovirus, now dubbed the Heartland virus.
Although we do not know for certain how patients are infected with the virus, research has suggested that ticks, namely lone star ticks, transmit it.
Ongoing investigations have uncovered 6 more cases of Heartland virus disease. All of the patients were white men older than 50 years of age. Five of them reported tick bites in the days or weeks before they fell ill.
Their symptoms started in May to September and included fever, fatigue, loss of appetite, headache, nausea, and muscle pain. The patients also had leukopenia and thrombocytopenia.
Four of the 6 patients were hospitalized. And 1 man, who suffered from other health conditions, died. It is not known if the Heartland virus was the cause of death or how much it contributed to his death.
The CDC has been working with the Missouri and Tennessee state health departments and other federal agencies to advance our understanding of Heartland virus disease by learning more about the patients who were infected, their illness, and their exposure to ticks.
The CDC aims to determine the symptoms and severity of the disease, where it is found, how people are being infected, and how to prevent infections.
CDC studies to date have shown that the Heartland virus is carried by lone star ticks, which are primarily found in the southeastern and eastern US.
Researchers hope additional studies can confirm whether ticks can spread the virus and reveal which other insects or animals may be involved in the transmission cycle. The CDC is also looking for the Heartland virus in other parts of the country to understand how widely it may be distributed.
“During the past 2 years, CDC has worked closely with state health departments, hospitals, and many experts from universities and other federal agencies to learn more about Heartland virus,” said Roger Nasci, PhD, chief of the CDC’s Arboviral Diseases Branch.
“By gathering information about the disease Heartland virus causes, and about how it’s spread to people, we hope to better understand the potential impact on the public’s health and how we can help protect people from this virus.”
The CDC developed the blood tests used to confirm the new cases of Heartland virus disease. CDC teams are working to further validate these tests and develop additional tests. The researchers hope to develop a diagnostic test that public health laboratories could use to test for the virus.
There is no specific treatment or vaccine for Heartland virus disease. However, supportive therapies such as intravenous fluids and fever reducers can relieve some symptoms of Heartland disease.
To reduce the risk of Heartland and other vector-borne diseases, the CDC recommends:
- Avoiding wooded and bushy areas with high grass and leaf litter
- Using insect repellent when outdoors
- Using products that contain permethrin on clothing
- Conducting a full-body tick check after spending time outdoors
- Bathing as soon as possible after coming indoors to wash off and more easily find any ticks
- Examining gear and pets, as ticks can “ride” into the home and attach to a person later.
For more information on the Heartland virus, visit the CDC website. ![]()

Credit: CDC
Health officials have reported 6 new cases of Heartland virus disease—5 in Missouri and 1 in Tennessee.
These cases, discovered in 2012 and 2013, add to the 2 cases discovered in 2009 and are described in the Centers for Disease Control and Prevention (CDC) Morbidity and Mortality Weekly Report.
The Heartland virus was first reported in 2 farmers in northwestern Missouri who were hospitalized in 2009 with what was thought to be ehrlichiosis, a tick-borne disease.
However, the patients failed to improve with treatment, and testing failed to confirm ehlrlichiosis.
Working with state and local partners, the CDC eventually identified the cause of the men’s illness: a previously unknown phlebovirus, now dubbed the Heartland virus.
Although we do not know for certain how patients are infected with the virus, research has suggested that ticks, namely lone star ticks, transmit it.
Ongoing investigations have uncovered 6 more cases of Heartland virus disease. All of the patients were white men older than 50 years of age. Five of them reported tick bites in the days or weeks before they fell ill.
Their symptoms started in May to September and included fever, fatigue, loss of appetite, headache, nausea, and muscle pain. The patients also had leukopenia and thrombocytopenia.
Four of the 6 patients were hospitalized. And 1 man, who suffered from other health conditions, died. It is not known if the Heartland virus was the cause of death or how much it contributed to his death.
The CDC has been working with the Missouri and Tennessee state health departments and other federal agencies to advance our understanding of Heartland virus disease by learning more about the patients who were infected, their illness, and their exposure to ticks.
The CDC aims to determine the symptoms and severity of the disease, where it is found, how people are being infected, and how to prevent infections.
CDC studies to date have shown that the Heartland virus is carried by lone star ticks, which are primarily found in the southeastern and eastern US.
Researchers hope additional studies can confirm whether ticks can spread the virus and reveal which other insects or animals may be involved in the transmission cycle. The CDC is also looking for the Heartland virus in other parts of the country to understand how widely it may be distributed.
“During the past 2 years, CDC has worked closely with state health departments, hospitals, and many experts from universities and other federal agencies to learn more about Heartland virus,” said Roger Nasci, PhD, chief of the CDC’s Arboviral Diseases Branch.
“By gathering information about the disease Heartland virus causes, and about how it’s spread to people, we hope to better understand the potential impact on the public’s health and how we can help protect people from this virus.”
The CDC developed the blood tests used to confirm the new cases of Heartland virus disease. CDC teams are working to further validate these tests and develop additional tests. The researchers hope to develop a diagnostic test that public health laboratories could use to test for the virus.
There is no specific treatment or vaccine for Heartland virus disease. However, supportive therapies such as intravenous fluids and fever reducers can relieve some symptoms of Heartland disease.
To reduce the risk of Heartland and other vector-borne diseases, the CDC recommends:
- Avoiding wooded and bushy areas with high grass and leaf litter
- Using insect repellent when outdoors
- Using products that contain permethrin on clothing
- Conducting a full-body tick check after spending time outdoors
- Bathing as soon as possible after coming indoors to wash off and more easily find any ticks
- Examining gear and pets, as ticks can “ride” into the home and attach to a person later.
For more information on the Heartland virus, visit the CDC website. ![]()
Discovery could aid treatment of hemolysis

Credit: NHLBI
Results of preclinical research could aid the development of new treatments for hemolysis, which may have implications for patients with sickle cell anemia and those who receive blood transfusions.
The researchers were investigating the possibility of using haptoglobin to prevent the chemical reactions triggered by hemoglobin after hemolysis.
Haptoglobin is known to bind acellular adult hemoglobin dimers and facilitate their clearance after hemolysis.
But haptoglobin exists in different forms. The 3 main phenotypes—Hp1-1, Hp2-1, and Hp2-2—have diverse structural configurations, and previous research suggested they have different biological activities.
With the current study, however, the researchers showed the different forms of haptoglobin actually exhibit similar activity.
Todd L. Mollan, PhD, of the Center for Biologics Evaluation and Research at the Food and Drug Administration in Bethesda, Maryland, and his colleagues presented these findings in Free Radical Biology and Medicine.
The researchers studied hemoglobin dimers in complex with unfractionated haptoglobin (a mixture of Hp1-1, Hp2-1, and Hp2-2); fractionated, dimeric haptoglobin (Hp1-1); and fractionated, polymeric haptoglobin (predominantly Hp2-2, with minor amounts of Hp2-1).
The team also complexed ferrous and ferric hemoglobins with unfractionated haptoglobin and its fractionated forms.
Experiments revealed no significant differences among the different complexes with regard to hemoglobin-haptoglobin binding kinetics, hydrogen-peroxide-driven oxidative transitions of the heme iron, radical formation, heme loss, or intrinsic redox potential.
The researchers said these results should be taken into account when designing phenotype-specific haptoglobin therapies. ![]()

Credit: NHLBI
Results of preclinical research could aid the development of new treatments for hemolysis, which may have implications for patients with sickle cell anemia and those who receive blood transfusions.
The researchers were investigating the possibility of using haptoglobin to prevent the chemical reactions triggered by hemoglobin after hemolysis.
Haptoglobin is known to bind acellular adult hemoglobin dimers and facilitate their clearance after hemolysis.
But haptoglobin exists in different forms. The 3 main phenotypes—Hp1-1, Hp2-1, and Hp2-2—have diverse structural configurations, and previous research suggested they have different biological activities.
With the current study, however, the researchers showed the different forms of haptoglobin actually exhibit similar activity.
Todd L. Mollan, PhD, of the Center for Biologics Evaluation and Research at the Food and Drug Administration in Bethesda, Maryland, and his colleagues presented these findings in Free Radical Biology and Medicine.
The researchers studied hemoglobin dimers in complex with unfractionated haptoglobin (a mixture of Hp1-1, Hp2-1, and Hp2-2); fractionated, dimeric haptoglobin (Hp1-1); and fractionated, polymeric haptoglobin (predominantly Hp2-2, with minor amounts of Hp2-1).
The team also complexed ferrous and ferric hemoglobins with unfractionated haptoglobin and its fractionated forms.
Experiments revealed no significant differences among the different complexes with regard to hemoglobin-haptoglobin binding kinetics, hydrogen-peroxide-driven oxidative transitions of the heme iron, radical formation, heme loss, or intrinsic redox potential.
The researchers said these results should be taken into account when designing phenotype-specific haptoglobin therapies. ![]()

Credit: NHLBI
Results of preclinical research could aid the development of new treatments for hemolysis, which may have implications for patients with sickle cell anemia and those who receive blood transfusions.
The researchers were investigating the possibility of using haptoglobin to prevent the chemical reactions triggered by hemoglobin after hemolysis.
Haptoglobin is known to bind acellular adult hemoglobin dimers and facilitate their clearance after hemolysis.
But haptoglobin exists in different forms. The 3 main phenotypes—Hp1-1, Hp2-1, and Hp2-2—have diverse structural configurations, and previous research suggested they have different biological activities.
With the current study, however, the researchers showed the different forms of haptoglobin actually exhibit similar activity.
Todd L. Mollan, PhD, of the Center for Biologics Evaluation and Research at the Food and Drug Administration in Bethesda, Maryland, and his colleagues presented these findings in Free Radical Biology and Medicine.
The researchers studied hemoglobin dimers in complex with unfractionated haptoglobin (a mixture of Hp1-1, Hp2-1, and Hp2-2); fractionated, dimeric haptoglobin (Hp1-1); and fractionated, polymeric haptoglobin (predominantly Hp2-2, with minor amounts of Hp2-1).
The team also complexed ferrous and ferric hemoglobins with unfractionated haptoglobin and its fractionated forms.
Experiments revealed no significant differences among the different complexes with regard to hemoglobin-haptoglobin binding kinetics, hydrogen-peroxide-driven oxidative transitions of the heme iron, radical formation, heme loss, or intrinsic redox potential.
The researchers said these results should be taken into account when designing phenotype-specific haptoglobin therapies. ![]()
Group aims to make African blood supply safer

Credit: UAB Hospital
Three organizations have joined together to adapt a pathogen inactivation system so that it works in whole blood and can be used in sub-Saharan Africa.
The INTERCEPT Blood System is currently used to inactivate bacteria, viruses, parasites, and leukocytes in donated platelets and plasma.
However, as the common practice in many African countries is to transfuse whole blood, the organizations want to adapt the system so it can be used with whole blood.
They also want to ensure the system can function in regions that may not have the infrastructure to support complex devices or have access to controlled temperature storage. Ideally, the system will not require electricity to inactivate pathogens or leukocytes.
For this endeavor, the company that makes the INTERCEPT system, Cerus Corporation, has partnered with SRTS Geneva and Swiss Transfusion SRC.
The Humanitarian Foundation Swiss Red Cross has granted funds to Swiss Transfusion SRC for the project. The initial funding of 1.5 million Swiss Francs will support the feasibility phase of the project and the completion of in vitro studies to support clinical trials.
“We believe pathogen inactivation for whole blood has the potential to improve the safety of transfusions in sub-Saharan Africa, where diminished blood availability due to severe anemia from malaria, HIV, and obstetric bleeding is common,” said Rudolf Schwabe, chief executive officer of the Swiss Red Cross.
“Based on our experience over the past 3 years with the INTERCEPT system, we have seen first-hand the substantial impact that pathogen inactivation has had in reducing transfusion-transmitted infectious risk in platelets and plasma.”
“This technology should be made available to developing countries such as those in sub-Saharan Africa, where the risk of bacterial contamination is about 2500 times greater than in Switzerland, and 10% to 15% of HIV infections are caused by contaminated transfusions.”
Cerus currently sells the INTERCEPT Blood System for both platelets and plasma in Europe, the Commonwealth of Independent States, the Middle East, and select countries in other regions around the world.
In the US, Cerus is seeking regulatory approval of the INTERCEPT Blood System for plasma and platelets.
The INTERCEPT red blood cell system is in clinical development. For more information, see the Cerus website. ![]()

Credit: UAB Hospital
Three organizations have joined together to adapt a pathogen inactivation system so that it works in whole blood and can be used in sub-Saharan Africa.
The INTERCEPT Blood System is currently used to inactivate bacteria, viruses, parasites, and leukocytes in donated platelets and plasma.
However, as the common practice in many African countries is to transfuse whole blood, the organizations want to adapt the system so it can be used with whole blood.
They also want to ensure the system can function in regions that may not have the infrastructure to support complex devices or have access to controlled temperature storage. Ideally, the system will not require electricity to inactivate pathogens or leukocytes.
For this endeavor, the company that makes the INTERCEPT system, Cerus Corporation, has partnered with SRTS Geneva and Swiss Transfusion SRC.
The Humanitarian Foundation Swiss Red Cross has granted funds to Swiss Transfusion SRC for the project. The initial funding of 1.5 million Swiss Francs will support the feasibility phase of the project and the completion of in vitro studies to support clinical trials.
“We believe pathogen inactivation for whole blood has the potential to improve the safety of transfusions in sub-Saharan Africa, where diminished blood availability due to severe anemia from malaria, HIV, and obstetric bleeding is common,” said Rudolf Schwabe, chief executive officer of the Swiss Red Cross.
“Based on our experience over the past 3 years with the INTERCEPT system, we have seen first-hand the substantial impact that pathogen inactivation has had in reducing transfusion-transmitted infectious risk in platelets and plasma.”
“This technology should be made available to developing countries such as those in sub-Saharan Africa, where the risk of bacterial contamination is about 2500 times greater than in Switzerland, and 10% to 15% of HIV infections are caused by contaminated transfusions.”
Cerus currently sells the INTERCEPT Blood System for both platelets and plasma in Europe, the Commonwealth of Independent States, the Middle East, and select countries in other regions around the world.
In the US, Cerus is seeking regulatory approval of the INTERCEPT Blood System for plasma and platelets.
The INTERCEPT red blood cell system is in clinical development. For more information, see the Cerus website. ![]()

Credit: UAB Hospital
Three organizations have joined together to adapt a pathogen inactivation system so that it works in whole blood and can be used in sub-Saharan Africa.
The INTERCEPT Blood System is currently used to inactivate bacteria, viruses, parasites, and leukocytes in donated platelets and plasma.
However, as the common practice in many African countries is to transfuse whole blood, the organizations want to adapt the system so it can be used with whole blood.
They also want to ensure the system can function in regions that may not have the infrastructure to support complex devices or have access to controlled temperature storage. Ideally, the system will not require electricity to inactivate pathogens or leukocytes.
For this endeavor, the company that makes the INTERCEPT system, Cerus Corporation, has partnered with SRTS Geneva and Swiss Transfusion SRC.
The Humanitarian Foundation Swiss Red Cross has granted funds to Swiss Transfusion SRC for the project. The initial funding of 1.5 million Swiss Francs will support the feasibility phase of the project and the completion of in vitro studies to support clinical trials.
“We believe pathogen inactivation for whole blood has the potential to improve the safety of transfusions in sub-Saharan Africa, where diminished blood availability due to severe anemia from malaria, HIV, and obstetric bleeding is common,” said Rudolf Schwabe, chief executive officer of the Swiss Red Cross.
“Based on our experience over the past 3 years with the INTERCEPT system, we have seen first-hand the substantial impact that pathogen inactivation has had in reducing transfusion-transmitted infectious risk in platelets and plasma.”
“This technology should be made available to developing countries such as those in sub-Saharan Africa, where the risk of bacterial contamination is about 2500 times greater than in Switzerland, and 10% to 15% of HIV infections are caused by contaminated transfusions.”
Cerus currently sells the INTERCEPT Blood System for both platelets and plasma in Europe, the Commonwealth of Independent States, the Middle East, and select countries in other regions around the world.
In the US, Cerus is seeking regulatory approval of the INTERCEPT Blood System for plasma and platelets.
The INTERCEPT red blood cell system is in clinical development. For more information, see the Cerus website. ![]()
Company issues nationwide recall of blood sets

Credit: Elise Amendola
Hospira, Inc. has announced a nationwide recall of 2 lots of Hemoset Dual Channel Plum Sets, which are used to administer blood products.
The affected lots—28005-5H and 34100-5H (list number 11241-03)—contain an incorrect component.
Using these sets, which were distributed across the US, could result in the over-delivery of blood products.
However, Hospira has not received any reports of adverse events associated with the sets. The recall is a precautionary measure.
Possible risk associated with the sets
The Hemostat Dual Channel Plum Set is designed to administer blood and blood products via the Plum infusion pump. If the Plum infusion pump is used with one of the sets being recalled, the blood product will be delivered at its intended dosage.
However, if one of the affected sets is removed from the Plum infusion pump and used in a gravity infusion, there is a risk of over-delivering blood products, due to the incorrect component—a lower lid.
In a gravity delivery, the correct lower lid dispenses 15 drops per mL. But the incorrect lower lid dispenses 10 drops per mL. If a caregiver does not realize that each drop contains more volume, over-delivery could occur.
Over-delivery of blood products in the populations at greatest risk (eg, neonates and patients with heart and/or kidney failure) may result in injuries that require medical intervention. These injuries are expected to fully resolve with medical intervention.
Steps to take
The sets impacted by the recall were distributed to US healthcare and veterinary facilities from May 2013 through December 2013.
Customers should check their inventory and immediately quarantine any affected sets. They should also inform individuals who might use the sets about the recall.
The affected sets should be returned to Stericycle. To do so, call 1-888-240-4282, Monday through Friday between 8 am and 5 pm Eastern Time.
For medical inquiries, contact Hospira Medical Communications at 1-800-615-0187.
Adverse reactions or quality problems associated with the use of these sets can be reported to the US Food and Drug Administration’s MedWatch Adverse Event Reporting Program. ![]()

Credit: Elise Amendola
Hospira, Inc. has announced a nationwide recall of 2 lots of Hemoset Dual Channel Plum Sets, which are used to administer blood products.
The affected lots—28005-5H and 34100-5H (list number 11241-03)—contain an incorrect component.
Using these sets, which were distributed across the US, could result in the over-delivery of blood products.
However, Hospira has not received any reports of adverse events associated with the sets. The recall is a precautionary measure.
Possible risk associated with the sets
The Hemostat Dual Channel Plum Set is designed to administer blood and blood products via the Plum infusion pump. If the Plum infusion pump is used with one of the sets being recalled, the blood product will be delivered at its intended dosage.
However, if one of the affected sets is removed from the Plum infusion pump and used in a gravity infusion, there is a risk of over-delivering blood products, due to the incorrect component—a lower lid.
In a gravity delivery, the correct lower lid dispenses 15 drops per mL. But the incorrect lower lid dispenses 10 drops per mL. If a caregiver does not realize that each drop contains more volume, over-delivery could occur.
Over-delivery of blood products in the populations at greatest risk (eg, neonates and patients with heart and/or kidney failure) may result in injuries that require medical intervention. These injuries are expected to fully resolve with medical intervention.
Steps to take
The sets impacted by the recall were distributed to US healthcare and veterinary facilities from May 2013 through December 2013.
Customers should check their inventory and immediately quarantine any affected sets. They should also inform individuals who might use the sets about the recall.
The affected sets should be returned to Stericycle. To do so, call 1-888-240-4282, Monday through Friday between 8 am and 5 pm Eastern Time.
For medical inquiries, contact Hospira Medical Communications at 1-800-615-0187.
Adverse reactions or quality problems associated with the use of these sets can be reported to the US Food and Drug Administration’s MedWatch Adverse Event Reporting Program. ![]()

Credit: Elise Amendola
Hospira, Inc. has announced a nationwide recall of 2 lots of Hemoset Dual Channel Plum Sets, which are used to administer blood products.
The affected lots—28005-5H and 34100-5H (list number 11241-03)—contain an incorrect component.
Using these sets, which were distributed across the US, could result in the over-delivery of blood products.
However, Hospira has not received any reports of adverse events associated with the sets. The recall is a precautionary measure.
Possible risk associated with the sets
The Hemostat Dual Channel Plum Set is designed to administer blood and blood products via the Plum infusion pump. If the Plum infusion pump is used with one of the sets being recalled, the blood product will be delivered at its intended dosage.
However, if one of the affected sets is removed from the Plum infusion pump and used in a gravity infusion, there is a risk of over-delivering blood products, due to the incorrect component—a lower lid.
In a gravity delivery, the correct lower lid dispenses 15 drops per mL. But the incorrect lower lid dispenses 10 drops per mL. If a caregiver does not realize that each drop contains more volume, over-delivery could occur.
Over-delivery of blood products in the populations at greatest risk (eg, neonates and patients with heart and/or kidney failure) may result in injuries that require medical intervention. These injuries are expected to fully resolve with medical intervention.
Steps to take
The sets impacted by the recall were distributed to US healthcare and veterinary facilities from May 2013 through December 2013.
Customers should check their inventory and immediately quarantine any affected sets. They should also inform individuals who might use the sets about the recall.
The affected sets should be returned to Stericycle. To do so, call 1-888-240-4282, Monday through Friday between 8 am and 5 pm Eastern Time.
For medical inquiries, contact Hospira Medical Communications at 1-800-615-0187.
Adverse reactions or quality problems associated with the use of these sets can be reported to the US Food and Drug Administration’s MedWatch Adverse Event Reporting Program. ![]()
New approach for treating PNH

Investigators have identified a novel strategy for treating paroxysmal nocturnal hemoglobinuria (PNH), according to a paper published in Blood.
In patients with PNH, defective expression of regulatory proteins on the surface of red blood cells leaves the cells vulnerable to attack by the complement immune system.
This can lead to hemolysis, which results in severe anemia and contributes to a high risk of thrombosis.
Eculizumab is the only approved therapeutic for PNH. The drug reduces hemolysis and can provide patients with relief from blood transfusions.
However, eculizumab is costly (currently more than $400,000 per year per patient), and one third of PNH patients who receive eculizumab continue to require blood transfusions to manage their anemia.
Investigators previously discovered that this non-response is due to fragments of complement C3 proteins on the surface of red blood cells, which are eventually attacked by immune cells.
Therefore, John Lambris, PhD, of the University of Pennsylvania, and his colleagues hypothesized that using small molecules to inhibit the complement cascade at the level of C3 proteins might be an effective strategy for treating PNH.
The team thought this method would prevent both hemolysis and immune cell recognition, and it might be more cost-effective than the current antibody-based treatment.
So they investigated the effect of a C3 inhibitor called Cp40 and its long-acting form, PEG-Cp40, on self-attack and resulting hemolysis using human PNH cells. Both compounds effectively inhibited hemolysis and efficiently prevented deposition of C3 fragments on PNH red blood cells.
In non-human primates, a single injection of PEG-Cp40 had an elimination half-life of more than 5 days. However, the investigators found evidence to suggest the drug may affect plasma levels of C3.
“We think these 2 compounds are excellent and potentially cost-effective candidates for further clinical investigation,” Dr Lambris said.
He hopes the compounds will be tested in clinical trials by 2015. Dr Lambris and his colleague, Daniel Ricklin, PhD, are the inventors of patents and patent applications owned by the University of Pennsylvania that describe the use of complement inhibitors for therapeutic purposes.
And Dr Lambris is a founder and equity holder of Amyndas Pharmaceuticals, which has exclusively licensed the Cp40 and PEG-Cp40 technologies from the university and is developing complement inhibitors for clinical applications. ![]()

Investigators have identified a novel strategy for treating paroxysmal nocturnal hemoglobinuria (PNH), according to a paper published in Blood.
In patients with PNH, defective expression of regulatory proteins on the surface of red blood cells leaves the cells vulnerable to attack by the complement immune system.
This can lead to hemolysis, which results in severe anemia and contributes to a high risk of thrombosis.
Eculizumab is the only approved therapeutic for PNH. The drug reduces hemolysis and can provide patients with relief from blood transfusions.
However, eculizumab is costly (currently more than $400,000 per year per patient), and one third of PNH patients who receive eculizumab continue to require blood transfusions to manage their anemia.
Investigators previously discovered that this non-response is due to fragments of complement C3 proteins on the surface of red blood cells, which are eventually attacked by immune cells.
Therefore, John Lambris, PhD, of the University of Pennsylvania, and his colleagues hypothesized that using small molecules to inhibit the complement cascade at the level of C3 proteins might be an effective strategy for treating PNH.
The team thought this method would prevent both hemolysis and immune cell recognition, and it might be more cost-effective than the current antibody-based treatment.
So they investigated the effect of a C3 inhibitor called Cp40 and its long-acting form, PEG-Cp40, on self-attack and resulting hemolysis using human PNH cells. Both compounds effectively inhibited hemolysis and efficiently prevented deposition of C3 fragments on PNH red blood cells.
In non-human primates, a single injection of PEG-Cp40 had an elimination half-life of more than 5 days. However, the investigators found evidence to suggest the drug may affect plasma levels of C3.
“We think these 2 compounds are excellent and potentially cost-effective candidates for further clinical investigation,” Dr Lambris said.
He hopes the compounds will be tested in clinical trials by 2015. Dr Lambris and his colleague, Daniel Ricklin, PhD, are the inventors of patents and patent applications owned by the University of Pennsylvania that describe the use of complement inhibitors for therapeutic purposes.
And Dr Lambris is a founder and equity holder of Amyndas Pharmaceuticals, which has exclusively licensed the Cp40 and PEG-Cp40 technologies from the university and is developing complement inhibitors for clinical applications. ![]()

Investigators have identified a novel strategy for treating paroxysmal nocturnal hemoglobinuria (PNH), according to a paper published in Blood.
In patients with PNH, defective expression of regulatory proteins on the surface of red blood cells leaves the cells vulnerable to attack by the complement immune system.
This can lead to hemolysis, which results in severe anemia and contributes to a high risk of thrombosis.
Eculizumab is the only approved therapeutic for PNH. The drug reduces hemolysis and can provide patients with relief from blood transfusions.
However, eculizumab is costly (currently more than $400,000 per year per patient), and one third of PNH patients who receive eculizumab continue to require blood transfusions to manage their anemia.
Investigators previously discovered that this non-response is due to fragments of complement C3 proteins on the surface of red blood cells, which are eventually attacked by immune cells.
Therefore, John Lambris, PhD, of the University of Pennsylvania, and his colleagues hypothesized that using small molecules to inhibit the complement cascade at the level of C3 proteins might be an effective strategy for treating PNH.
The team thought this method would prevent both hemolysis and immune cell recognition, and it might be more cost-effective than the current antibody-based treatment.
So they investigated the effect of a C3 inhibitor called Cp40 and its long-acting form, PEG-Cp40, on self-attack and resulting hemolysis using human PNH cells. Both compounds effectively inhibited hemolysis and efficiently prevented deposition of C3 fragments on PNH red blood cells.
In non-human primates, a single injection of PEG-Cp40 had an elimination half-life of more than 5 days. However, the investigators found evidence to suggest the drug may affect plasma levels of C3.
“We think these 2 compounds are excellent and potentially cost-effective candidates for further clinical investigation,” Dr Lambris said.
He hopes the compounds will be tested in clinical trials by 2015. Dr Lambris and his colleague, Daniel Ricklin, PhD, are the inventors of patents and patent applications owned by the University of Pennsylvania that describe the use of complement inhibitors for therapeutic purposes.
And Dr Lambris is a founder and equity holder of Amyndas Pharmaceuticals, which has exclusively licensed the Cp40 and PEG-Cp40 technologies from the university and is developing complement inhibitors for clinical applications.
Team uses light to measure coagulation
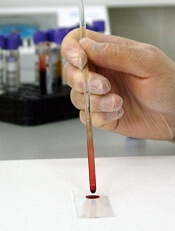
Credit: Максим Кукушкин
Researchers have developed an optical device that requires only a few drops of blood and a few minutes to measure coagulation parameters that can guide blood transfusions and anticoagulant therapy.
The team described their device in Biomedical Optics Express.
“Currently, the most comprehensive measures of coagulation are a battery of lab tests that are expensive and can take hours to perform,” said study author Seemantini Nadkarni, PhD, of Massachusetts General Hospital in Boston.
She noted that other systems have been developed that provide clotting measurements at the point of care, but the systems can be big and expensive or have other limitations, such as requiring significant amounts of blood or only measuring clotting time.
“Our goal is to provide as much information as a lab test, but to provide it quickly and cheaply at a patient’s bedside,” Dr Nadkarni said.
To reach this goal, she and her colleagues turned to an optical technique they pioneered called laser speckle rheology. The technique involves shining a laser into a sample and monitoring the patterns of light that bounce back.
The researchers had previously used the technique to measure the mechanical properties of a range of different tissue types and found that it was extremely sensitive to the coagulation of blood.
When light hits a blood sample, blood cells and platelets scatter the light. In unclotted blood, these light-scattering particles move easily about, making the pattern of scattered light, a speckle pattern, fluctuate rapidly.
“It’s almost like looking at a starry night sky, with twinkling stars,” Dr Nadkarni said. “But as the blood starts to coagulate, blood cells and platelets come together within a fibrin network to form a clot. The motion is restricted as the sample gets stiffer, and the ‘twinkling’ of the speckle pattern is reduced significantly.”
Dr Nadkarni and her colleagues used a miniature high-speed camera to record the fluctuating speckle pattern and then correlated the intensity of changes in the pattern with 2 blood sample measurements: clotting time and fibrinogen concentration.
The team noted that physicians could use the measurements to make decisions about how much blood to give a bleeding patient and what type of blood product is needed most.
“The timely detection of clotting defects followed by the appropriate blood product transfusion is critical in managing bleeding patients,” Dr Nadkarni said. “If you transfuse too much, there could be further coagulation defects that occur, but if you don’t transfuse enough, bleeding continues.”
On the other end of the spectrum, the device could help patients on anticoagulant therapy. Having a small device that can analyze their blood in a doctor’s office or at home could reduce the cost and inconvenience of blood tests, while increasing the safety of anticoagulation treatment, Dr Nadkarni said.
At present, her team’s device is about the size of a tissue box and is connected to a computer. The researchers are working to further miniaturize the system and aim to perform clinical studies with a version smaller than a cell phone within the next year.

Credit: Максим Кукушкин
Researchers have developed an optical device that requires only a few drops of blood and a few minutes to measure coagulation parameters that can guide blood transfusions and anticoagulant therapy.
The team described their device in Biomedical Optics Express.
“Currently, the most comprehensive measures of coagulation are a battery of lab tests that are expensive and can take hours to perform,” said study author Seemantini Nadkarni, PhD, of Massachusetts General Hospital in Boston.
She noted that other systems have been developed that provide clotting measurements at the point of care, but the systems can be big and expensive or have other limitations, such as requiring significant amounts of blood or only measuring clotting time.
“Our goal is to provide as much information as a lab test, but to provide it quickly and cheaply at a patient’s bedside,” Dr Nadkarni said.
To reach this goal, she and her colleagues turned to an optical technique they pioneered called laser speckle rheology. The technique involves shining a laser into a sample and monitoring the patterns of light that bounce back.
The researchers had previously used the technique to measure the mechanical properties of a range of different tissue types and found that it was extremely sensitive to the coagulation of blood.
When light hits a blood sample, blood cells and platelets scatter the light. In unclotted blood, these light-scattering particles move easily about, making the pattern of scattered light, a speckle pattern, fluctuate rapidly.
“It’s almost like looking at a starry night sky, with twinkling stars,” Dr Nadkarni said. “But as the blood starts to coagulate, blood cells and platelets come together within a fibrin network to form a clot. The motion is restricted as the sample gets stiffer, and the ‘twinkling’ of the speckle pattern is reduced significantly.”
Dr Nadkarni and her colleagues used a miniature high-speed camera to record the fluctuating speckle pattern and then correlated the intensity of changes in the pattern with 2 blood sample measurements: clotting time and fibrinogen concentration.
The team noted that physicians could use the measurements to make decisions about how much blood to give a bleeding patient and what type of blood product is needed most.
“The timely detection of clotting defects followed by the appropriate blood product transfusion is critical in managing bleeding patients,” Dr Nadkarni said. “If you transfuse too much, there could be further coagulation defects that occur, but if you don’t transfuse enough, bleeding continues.”
On the other end of the spectrum, the device could help patients on anticoagulant therapy. Having a small device that can analyze their blood in a doctor’s office or at home could reduce the cost and inconvenience of blood tests, while increasing the safety of anticoagulation treatment, Dr Nadkarni said.
At present, her team’s device is about the size of a tissue box and is connected to a computer. The researchers are working to further miniaturize the system and aim to perform clinical studies with a version smaller than a cell phone within the next year.

Credit: Максим Кукушкин
Researchers have developed an optical device that requires only a few drops of blood and a few minutes to measure coagulation parameters that can guide blood transfusions and anticoagulant therapy.
The team described their device in Biomedical Optics Express.
“Currently, the most comprehensive measures of coagulation are a battery of lab tests that are expensive and can take hours to perform,” said study author Seemantini Nadkarni, PhD, of Massachusetts General Hospital in Boston.
She noted that other systems have been developed that provide clotting measurements at the point of care, but the systems can be big and expensive or have other limitations, such as requiring significant amounts of blood or only measuring clotting time.
“Our goal is to provide as much information as a lab test, but to provide it quickly and cheaply at a patient’s bedside,” Dr Nadkarni said.
To reach this goal, she and her colleagues turned to an optical technique they pioneered called laser speckle rheology. The technique involves shining a laser into a sample and monitoring the patterns of light that bounce back.
The researchers had previously used the technique to measure the mechanical properties of a range of different tissue types and found that it was extremely sensitive to the coagulation of blood.
When light hits a blood sample, blood cells and platelets scatter the light. In unclotted blood, these light-scattering particles move easily about, making the pattern of scattered light, a speckle pattern, fluctuate rapidly.
“It’s almost like looking at a starry night sky, with twinkling stars,” Dr Nadkarni said. “But as the blood starts to coagulate, blood cells and platelets come together within a fibrin network to form a clot. The motion is restricted as the sample gets stiffer, and the ‘twinkling’ of the speckle pattern is reduced significantly.”
Dr Nadkarni and her colleagues used a miniature high-speed camera to record the fluctuating speckle pattern and then correlated the intensity of changes in the pattern with 2 blood sample measurements: clotting time and fibrinogen concentration.
The team noted that physicians could use the measurements to make decisions about how much blood to give a bleeding patient and what type of blood product is needed most.
“The timely detection of clotting defects followed by the appropriate blood product transfusion is critical in managing bleeding patients,” Dr Nadkarni said. “If you transfuse too much, there could be further coagulation defects that occur, but if you don’t transfuse enough, bleeding continues.”
On the other end of the spectrum, the device could help patients on anticoagulant therapy. Having a small device that can analyze their blood in a doctor’s office or at home could reduce the cost and inconvenience of blood tests, while increasing the safety of anticoagulation treatment, Dr Nadkarni said.
At present, her team’s device is about the size of a tissue box and is connected to a computer. The researchers are working to further miniaturize the system and aim to perform clinical studies with a version smaller than a cell phone within the next year.
Transfusion increases risks in PCI patients, study shows

Credit: UAB Hospital
In a large study, patients who received red blood cell (RBC) transfusions after percutaneous coronary intervention (PCI) had a higher risk of in-hospital heart attack, stroke, and death than their non-transfused peers.
The retrospective study included data on nearly 2 million patients who underwent a PCI at hospitals across the US.
The research revealed considerable variation in transfusion practices for this patient population, although the overall rate of transfusion was low.
This makes sense, as giving RBC transfusions to patients with coronary artery disease is controversial, according to the study authors.
They said there is a growing body of evidence suggesting that transfusion in the setting of acute coronary syndromes (ACS) and in hospitalized patients with a history of coronary artery disease may be associated with an increased risk of heart attack and death.
Furthermore, current guideline statements are cautious about recommending transfusion in hospitalized patients with a history of coronary artery disease and make no recommendation on transfusion in the setting of ACS, citing an absence of definitive evidence.
With this in mind, Matthew W. Sherwood, MD, of Duke Clinical Research Institute in Durham, North Carolina, and his colleagues examined transfusion practice patterns and outcomes in 1,967,218 patients (2,258,711 visits) who underwent PCI from July 2009 to March 2013 at 1431 US hospitals.
The team reported their findings in JAMA.
Overall, 2.1% of patients had a transfusion. However, transfusion practices varied among the hospitals. The unadjusted transfusion rates ranged from 0% to 13%. Overall, 96.3% of hospitals transfused less than 5% of patients, and 3.7% of hospitals transfused 5% of patients or more.
Risk-standardized rates of transfusion by hospital ranged from 0.3% to 9.3%. The risk was adjusted for factors such as age, sex, body mass index, ACS presentation, PCI status, history of congestive heart failure, etc.
Compared to no transfusion, receiving an RBC transfusion was associated with a greater risk of heart attack (4.5% vs 1.8%), stroke (2.0% vs 0.2%), and in-hospital death (12.5% vs 1.2%), irrespective of bleeding complications.
Patients were more likely to receive a transfusion if they were older, female, and had hypertension, diabetes, advanced renal dysfunction, and prior heart attack or heart failure.
The researchers speculated that the variation in transfusion practice patterns observed in this study may be related to several factors, including previously held beliefs about the benefit of transfusion and recently published data indicating the lack of benefit and potential hazard associated with transfusion.
The team said these data highlight the need for randomized trials of transfusion strategies to guide practice in patients undergoing PCI. And until these trials provide more definitive answers, clinicians should try to reduce the risk of bleeding and, therefore, the need for transfusion in patients undergoing PCI.

Credit: UAB Hospital
In a large study, patients who received red blood cell (RBC) transfusions after percutaneous coronary intervention (PCI) had a higher risk of in-hospital heart attack, stroke, and death than their non-transfused peers.
The retrospective study included data on nearly 2 million patients who underwent a PCI at hospitals across the US.
The research revealed considerable variation in transfusion practices for this patient population, although the overall rate of transfusion was low.
This makes sense, as giving RBC transfusions to patients with coronary artery disease is controversial, according to the study authors.
They said there is a growing body of evidence suggesting that transfusion in the setting of acute coronary syndromes (ACS) and in hospitalized patients with a history of coronary artery disease may be associated with an increased risk of heart attack and death.
Furthermore, current guideline statements are cautious about recommending transfusion in hospitalized patients with a history of coronary artery disease and make no recommendation on transfusion in the setting of ACS, citing an absence of definitive evidence.
With this in mind, Matthew W. Sherwood, MD, of Duke Clinical Research Institute in Durham, North Carolina, and his colleagues examined transfusion practice patterns and outcomes in 1,967,218 patients (2,258,711 visits) who underwent PCI from July 2009 to March 2013 at 1431 US hospitals.
The team reported their findings in JAMA.
Overall, 2.1% of patients had a transfusion. However, transfusion practices varied among the hospitals. The unadjusted transfusion rates ranged from 0% to 13%. Overall, 96.3% of hospitals transfused less than 5% of patients, and 3.7% of hospitals transfused 5% of patients or more.
Risk-standardized rates of transfusion by hospital ranged from 0.3% to 9.3%. The risk was adjusted for factors such as age, sex, body mass index, ACS presentation, PCI status, history of congestive heart failure, etc.
Compared to no transfusion, receiving an RBC transfusion was associated with a greater risk of heart attack (4.5% vs 1.8%), stroke (2.0% vs 0.2%), and in-hospital death (12.5% vs 1.2%), irrespective of bleeding complications.
Patients were more likely to receive a transfusion if they were older, female, and had hypertension, diabetes, advanced renal dysfunction, and prior heart attack or heart failure.
The researchers speculated that the variation in transfusion practice patterns observed in this study may be related to several factors, including previously held beliefs about the benefit of transfusion and recently published data indicating the lack of benefit and potential hazard associated with transfusion.
The team said these data highlight the need for randomized trials of transfusion strategies to guide practice in patients undergoing PCI. And until these trials provide more definitive answers, clinicians should try to reduce the risk of bleeding and, therefore, the need for transfusion in patients undergoing PCI.

Credit: UAB Hospital
In a large study, patients who received red blood cell (RBC) transfusions after percutaneous coronary intervention (PCI) had a higher risk of in-hospital heart attack, stroke, and death than their non-transfused peers.
The retrospective study included data on nearly 2 million patients who underwent a PCI at hospitals across the US.
The research revealed considerable variation in transfusion practices for this patient population, although the overall rate of transfusion was low.
This makes sense, as giving RBC transfusions to patients with coronary artery disease is controversial, according to the study authors.
They said there is a growing body of evidence suggesting that transfusion in the setting of acute coronary syndromes (ACS) and in hospitalized patients with a history of coronary artery disease may be associated with an increased risk of heart attack and death.
Furthermore, current guideline statements are cautious about recommending transfusion in hospitalized patients with a history of coronary artery disease and make no recommendation on transfusion in the setting of ACS, citing an absence of definitive evidence.
With this in mind, Matthew W. Sherwood, MD, of Duke Clinical Research Institute in Durham, North Carolina, and his colleagues examined transfusion practice patterns and outcomes in 1,967,218 patients (2,258,711 visits) who underwent PCI from July 2009 to March 2013 at 1431 US hospitals.
The team reported their findings in JAMA.
Overall, 2.1% of patients had a transfusion. However, transfusion practices varied among the hospitals. The unadjusted transfusion rates ranged from 0% to 13%. Overall, 96.3% of hospitals transfused less than 5% of patients, and 3.7% of hospitals transfused 5% of patients or more.
Risk-standardized rates of transfusion by hospital ranged from 0.3% to 9.3%. The risk was adjusted for factors such as age, sex, body mass index, ACS presentation, PCI status, history of congestive heart failure, etc.
Compared to no transfusion, receiving an RBC transfusion was associated with a greater risk of heart attack (4.5% vs 1.8%), stroke (2.0% vs 0.2%), and in-hospital death (12.5% vs 1.2%), irrespective of bleeding complications.
Patients were more likely to receive a transfusion if they were older, female, and had hypertension, diabetes, advanced renal dysfunction, and prior heart attack or heart failure.
The researchers speculated that the variation in transfusion practice patterns observed in this study may be related to several factors, including previously held beliefs about the benefit of transfusion and recently published data indicating the lack of benefit and potential hazard associated with transfusion.
The team said these data highlight the need for randomized trials of transfusion strategies to guide practice in patients undergoing PCI. And until these trials provide more definitive answers, clinicians should try to reduce the risk of bleeding and, therefore, the need for transfusion in patients undergoing PCI.
Creating an ‘inexhaustible’ supply of platelets

Scientists say they’ve discovered a way to create a potentially inexhaustible supply of functional platelets.
The researchers used human induced pluripotent stem cells (iPSCs) to create immortalized megakaryocyte progenitor cell lines (imMKCLs). And by manipulating the cell lines, the team produced platelets.
These imMKCL-derived platelets were functional, although not as functional as donor-derived platelets.
On the other hand, the imMKCL-derived cells offer an advantage over donated platelets—namely, the imMKCLs can be expanded in culture for up to 5 months, even after cryopreservation.
“[W]e established a method to achieve the long-term self-replication of megakaryocyte progenitors as an immortalized cell line, which could eventually contribute to large-scale cultivation and production of platelets,” said senior study author Koji Eto, MD, PhD, of Kyoto University and the University of Tokyo in Japan.
He and his colleagues believe this work, published in Cell Stem Cell, could eventually help us eliminate platelet shortages. The supply of donated platelets, which have a short shelf-life and must be kept at room temperature, is often insufficient to meet clinical needs.
With that in mind, Dr Eto’s team set out to create large quantities of functional platelets. They first generated stable imMKCLs from iPSC-derived hematopoietic progenitors.
They accomplished this by inducing overexpression of BMI1 and BCL-XL to suppress senescence and apoptosis. They also induced constrained overexpression of c-MYC to promote proliferation, as they found too-high c-MYC expression led to caspase-dependent MKCL apoptosis.
When the researchers turned off expression of c-MYC, BMI1, and BCL-XL, they saw an increase in CD42b+ platelet yield from the imMKCLs and upregulated CD42b expression in CD41a+ platelets. They noted that expression of CD42b is required for clotting initiation and bacterial clearance in vivo.
The team then conducted in vitro and in vivo experiments to test the functionality of their platelets. Most of the in vitro functional parameters indicated that imMKCL-derived platelets produced less robust responses than donor platelets.
But the imMKCL-derived platelets were functional enough to be useful and produced promising results in vivo. In mouse models of thrombocytopenia, the imMKCL-derived platelets contributed to thrombi development better than human endogenous pooled platelets.

Scientists say they’ve discovered a way to create a potentially inexhaustible supply of functional platelets.
The researchers used human induced pluripotent stem cells (iPSCs) to create immortalized megakaryocyte progenitor cell lines (imMKCLs). And by manipulating the cell lines, the team produced platelets.
These imMKCL-derived platelets were functional, although not as functional as donor-derived platelets.
On the other hand, the imMKCL-derived cells offer an advantage over donated platelets—namely, the imMKCLs can be expanded in culture for up to 5 months, even after cryopreservation.
“[W]e established a method to achieve the long-term self-replication of megakaryocyte progenitors as an immortalized cell line, which could eventually contribute to large-scale cultivation and production of platelets,” said senior study author Koji Eto, MD, PhD, of Kyoto University and the University of Tokyo in Japan.
He and his colleagues believe this work, published in Cell Stem Cell, could eventually help us eliminate platelet shortages. The supply of donated platelets, which have a short shelf-life and must be kept at room temperature, is often insufficient to meet clinical needs.
With that in mind, Dr Eto’s team set out to create large quantities of functional platelets. They first generated stable imMKCLs from iPSC-derived hematopoietic progenitors.
They accomplished this by inducing overexpression of BMI1 and BCL-XL to suppress senescence and apoptosis. They also induced constrained overexpression of c-MYC to promote proliferation, as they found too-high c-MYC expression led to caspase-dependent MKCL apoptosis.
When the researchers turned off expression of c-MYC, BMI1, and BCL-XL, they saw an increase in CD42b+ platelet yield from the imMKCLs and upregulated CD42b expression in CD41a+ platelets. They noted that expression of CD42b is required for clotting initiation and bacterial clearance in vivo.
The team then conducted in vitro and in vivo experiments to test the functionality of their platelets. Most of the in vitro functional parameters indicated that imMKCL-derived platelets produced less robust responses than donor platelets.
But the imMKCL-derived platelets were functional enough to be useful and produced promising results in vivo. In mouse models of thrombocytopenia, the imMKCL-derived platelets contributed to thrombi development better than human endogenous pooled platelets.

Scientists say they’ve discovered a way to create a potentially inexhaustible supply of functional platelets.
The researchers used human induced pluripotent stem cells (iPSCs) to create immortalized megakaryocyte progenitor cell lines (imMKCLs). And by manipulating the cell lines, the team produced platelets.
These imMKCL-derived platelets were functional, although not as functional as donor-derived platelets.
On the other hand, the imMKCL-derived cells offer an advantage over donated platelets—namely, the imMKCLs can be expanded in culture for up to 5 months, even after cryopreservation.
“[W]e established a method to achieve the long-term self-replication of megakaryocyte progenitors as an immortalized cell line, which could eventually contribute to large-scale cultivation and production of platelets,” said senior study author Koji Eto, MD, PhD, of Kyoto University and the University of Tokyo in Japan.
He and his colleagues believe this work, published in Cell Stem Cell, could eventually help us eliminate platelet shortages. The supply of donated platelets, which have a short shelf-life and must be kept at room temperature, is often insufficient to meet clinical needs.
With that in mind, Dr Eto’s team set out to create large quantities of functional platelets. They first generated stable imMKCLs from iPSC-derived hematopoietic progenitors.
They accomplished this by inducing overexpression of BMI1 and BCL-XL to suppress senescence and apoptosis. They also induced constrained overexpression of c-MYC to promote proliferation, as they found too-high c-MYC expression led to caspase-dependent MKCL apoptosis.
When the researchers turned off expression of c-MYC, BMI1, and BCL-XL, they saw an increase in CD42b+ platelet yield from the imMKCLs and upregulated CD42b expression in CD41a+ platelets. They noted that expression of CD42b is required for clotting initiation and bacterial clearance in vivo.
The team then conducted in vitro and in vivo experiments to test the functionality of their platelets. Most of the in vitro functional parameters indicated that imMKCL-derived platelets produced less robust responses than donor platelets.
But the imMKCL-derived platelets were functional enough to be useful and produced promising results in vivo. In mouse models of thrombocytopenia, the imMKCL-derived platelets contributed to thrombi development better than human endogenous pooled platelets.