User login
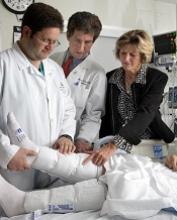
Education can improve adherence to VTE prophylaxis
Education can improve adherence to venous thromboembolism (VTE) prophylaxis among hospitalized patients, according to researchers.
They assessed data from more than 19,000 hospital stays and found that “real-time” educational interventions directed toward patients and nurses significantly reduced nonadministration of prescribed VTE prophylaxis.
However, this did not translate to a significant reduction in VTE incidence.
Elliott Haut, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these results in JAMA Network Open.
For this study, the researchers evaluated patients who were prescribed VTE prophylaxis while admitted to Johns Hopkins Hospital between April 1, 2015, and December 31, 2015.
The researchers evaluated patients in 16 hospital units. Four units were targeted for educational intervention, and the remaining 12 units served as controls.
The educational interventions were given only when patients did not receive prescribed VTE prophylaxis. A bedside nurse would document nonadministration, and an alert built into the hospital’s electronic medical record would email and page a health educator.
If the patient had refused prophylaxis, the patient would receive an educational bundle on VTE, which could consist of any or all of the following (patient’s choice):
- A one-on-one discussion with the health educator
- A two-page paper handout (available in eight languages)
- A 10-minute educational video (on a tablet).
If the nonadministration of prophylaxis was not due to patient refusal or contraindication, the health educator would educate the bedside nurse about the importance of giving all prescribed doses of VTE prophylaxis.
The study included 19,652 patient visits during which VTE prophylaxis was prescribed.
Of these, 726 visits were targeted for educational intervention. In 272 visits, the intervention was administered to a nurse alone (n=45) or the nurse and the patient (n=227).
For the remaining 454 visits, the patient was discharged before the intervention (n=123), there was an order to discontinue prophylaxis (n=111), there was a technical error (n=55), the patient (n=43) or health educator (n=41) was off unit, or “other” reasons (n=81).
Results
The proportion of nonadministered doses of VTE prophylaxis declined significantly in the hospital units targeted with educational intervention—from 9.1% pre-intervention to 5.6% post-intervention (odds ratio [OR]=0.57, P<0.001).
However, there was no significant change in the control units—13.6% and 13.3%, respectively (OR=0.98, P=0.62).
The proportion of nonadministered doses for reasons other than patient refusal decreased significantly in the intervention units—from 2.3% to 1.7% (OR, 0.74, P=0.01)—but not in control units—from 3.4% to 3.3% (OR=0.98, P=0.69).
The proportion of nonadministered doses due to patient refusal decreased significantly in the intervention units—from 5.9% to 3.4% (OR=0.53, P<0.001)—but not in control units—from 8.7% to 8.5% (OR=0.98, P=0.71).
“Our study demonstrates that educating patients quickly, as soon as we learn about a missed dose, is not only possible to implement at a large hospital but is effective in ensuring that patients take the drugs that can save their lives,” Dr. Haut said.
“The educational bundles we created are effective and optimize busy clinicians’ already packed schedules,” added study author Brandyn Lau, of the Johns Hopkins University School of Medicine.
“At the end of the day, we’re here to deliver high quality care and keep patients safe, and this is one method of achieving that mission.”
However, the improved adherence to VTE prophylaxis did not translate to a significant reduction in VTE in this study.
The incidence of VTE decreased from 0.30% to 0.18% (OR=0.60) in intervention units and from 0.24% to 0.20% in control units (OR=0.81).
For all patients, the incidence of VTE was 0.26% pre-intervention and 0.19% post-intervention (P=0.46).
This research was supported by a contract from the Patient-Centered Outcomes Research Institute. The study authors reported support from various government agencies and private organizations.
Education can improve adherence to venous thromboembolism (VTE) prophylaxis among hospitalized patients, according to researchers.
They assessed data from more than 19,000 hospital stays and found that “real-time” educational interventions directed toward patients and nurses significantly reduced nonadministration of prescribed VTE prophylaxis.
However, this did not translate to a significant reduction in VTE incidence.
Elliott Haut, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these results in JAMA Network Open.
For this study, the researchers evaluated patients who were prescribed VTE prophylaxis while admitted to Johns Hopkins Hospital between April 1, 2015, and December 31, 2015.
The researchers evaluated patients in 16 hospital units. Four units were targeted for educational intervention, and the remaining 12 units served as controls.
The educational interventions were given only when patients did not receive prescribed VTE prophylaxis. A bedside nurse would document nonadministration, and an alert built into the hospital’s electronic medical record would email and page a health educator.
If the patient had refused prophylaxis, the patient would receive an educational bundle on VTE, which could consist of any or all of the following (patient’s choice):
- A one-on-one discussion with the health educator
- A two-page paper handout (available in eight languages)
- A 10-minute educational video (on a tablet).
If the nonadministration of prophylaxis was not due to patient refusal or contraindication, the health educator would educate the bedside nurse about the importance of giving all prescribed doses of VTE prophylaxis.
The study included 19,652 patient visits during which VTE prophylaxis was prescribed.
Of these, 726 visits were targeted for educational intervention. In 272 visits, the intervention was administered to a nurse alone (n=45) or the nurse and the patient (n=227).
For the remaining 454 visits, the patient was discharged before the intervention (n=123), there was an order to discontinue prophylaxis (n=111), there was a technical error (n=55), the patient (n=43) or health educator (n=41) was off unit, or “other” reasons (n=81).
Results
The proportion of nonadministered doses of VTE prophylaxis declined significantly in the hospital units targeted with educational intervention—from 9.1% pre-intervention to 5.6% post-intervention (odds ratio [OR]=0.57, P<0.001).
However, there was no significant change in the control units—13.6% and 13.3%, respectively (OR=0.98, P=0.62).
The proportion of nonadministered doses for reasons other than patient refusal decreased significantly in the intervention units—from 2.3% to 1.7% (OR, 0.74, P=0.01)—but not in control units—from 3.4% to 3.3% (OR=0.98, P=0.69).
The proportion of nonadministered doses due to patient refusal decreased significantly in the intervention units—from 5.9% to 3.4% (OR=0.53, P<0.001)—but not in control units—from 8.7% to 8.5% (OR=0.98, P=0.71).
“Our study demonstrates that educating patients quickly, as soon as we learn about a missed dose, is not only possible to implement at a large hospital but is effective in ensuring that patients take the drugs that can save their lives,” Dr. Haut said.
“The educational bundles we created are effective and optimize busy clinicians’ already packed schedules,” added study author Brandyn Lau, of the Johns Hopkins University School of Medicine.
“At the end of the day, we’re here to deliver high quality care and keep patients safe, and this is one method of achieving that mission.”
However, the improved adherence to VTE prophylaxis did not translate to a significant reduction in VTE in this study.
The incidence of VTE decreased from 0.30% to 0.18% (OR=0.60) in intervention units and from 0.24% to 0.20% in control units (OR=0.81).
For all patients, the incidence of VTE was 0.26% pre-intervention and 0.19% post-intervention (P=0.46).
This research was supported by a contract from the Patient-Centered Outcomes Research Institute. The study authors reported support from various government agencies and private organizations.
Education can improve adherence to venous thromboembolism (VTE) prophylaxis among hospitalized patients, according to researchers.
They assessed data from more than 19,000 hospital stays and found that “real-time” educational interventions directed toward patients and nurses significantly reduced nonadministration of prescribed VTE prophylaxis.
However, this did not translate to a significant reduction in VTE incidence.
Elliott Haut, MD, PhD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these results in JAMA Network Open.
For this study, the researchers evaluated patients who were prescribed VTE prophylaxis while admitted to Johns Hopkins Hospital between April 1, 2015, and December 31, 2015.
The researchers evaluated patients in 16 hospital units. Four units were targeted for educational intervention, and the remaining 12 units served as controls.
The educational interventions were given only when patients did not receive prescribed VTE prophylaxis. A bedside nurse would document nonadministration, and an alert built into the hospital’s electronic medical record would email and page a health educator.
If the patient had refused prophylaxis, the patient would receive an educational bundle on VTE, which could consist of any or all of the following (patient’s choice):
- A one-on-one discussion with the health educator
- A two-page paper handout (available in eight languages)
- A 10-minute educational video (on a tablet).
If the nonadministration of prophylaxis was not due to patient refusal or contraindication, the health educator would educate the bedside nurse about the importance of giving all prescribed doses of VTE prophylaxis.
The study included 19,652 patient visits during which VTE prophylaxis was prescribed.
Of these, 726 visits were targeted for educational intervention. In 272 visits, the intervention was administered to a nurse alone (n=45) or the nurse and the patient (n=227).
For the remaining 454 visits, the patient was discharged before the intervention (n=123), there was an order to discontinue prophylaxis (n=111), there was a technical error (n=55), the patient (n=43) or health educator (n=41) was off unit, or “other” reasons (n=81).
Results
The proportion of nonadministered doses of VTE prophylaxis declined significantly in the hospital units targeted with educational intervention—from 9.1% pre-intervention to 5.6% post-intervention (odds ratio [OR]=0.57, P<0.001).
However, there was no significant change in the control units—13.6% and 13.3%, respectively (OR=0.98, P=0.62).
The proportion of nonadministered doses for reasons other than patient refusal decreased significantly in the intervention units—from 2.3% to 1.7% (OR, 0.74, P=0.01)—but not in control units—from 3.4% to 3.3% (OR=0.98, P=0.69).
The proportion of nonadministered doses due to patient refusal decreased significantly in the intervention units—from 5.9% to 3.4% (OR=0.53, P<0.001)—but not in control units—from 8.7% to 8.5% (OR=0.98, P=0.71).
“Our study demonstrates that educating patients quickly, as soon as we learn about a missed dose, is not only possible to implement at a large hospital but is effective in ensuring that patients take the drugs that can save their lives,” Dr. Haut said.
“The educational bundles we created are effective and optimize busy clinicians’ already packed schedules,” added study author Brandyn Lau, of the Johns Hopkins University School of Medicine.
“At the end of the day, we’re here to deliver high quality care and keep patients safe, and this is one method of achieving that mission.”
However, the improved adherence to VTE prophylaxis did not translate to a significant reduction in VTE in this study.
The incidence of VTE decreased from 0.30% to 0.18% (OR=0.60) in intervention units and from 0.24% to 0.20% in control units (OR=0.81).
For all patients, the incidence of VTE was 0.26% pre-intervention and 0.19% post-intervention (P=0.46).
This research was supported by a contract from the Patient-Centered Outcomes Research Institute. The study authors reported support from various government agencies and private organizations.
Palliative care guidelines relevant for hematologists, doc says
The latest edition of the national palliative care guidelines provides new clinical strategies relevant to hematology practice in the United States, according to a physician-researcher specializing in hematology.
The Clinical Practice Guidelines for Quality Palliative Care, 4th edition, represents a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, a physician-researcher at Duke University School of Medicine in Durham, North Carolina.
However, unlike previous editions, this update to the guidelines emphasizes the importance of palliative care provided by both primary care and specialty care clinicians.
“Part of this report is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” Dr. LeBlanc said.
The latest edition helps establish a foundation for gold standard palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to The National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology.
One key reason for the update, according to NCP, was to acknowledge that today’s healthcare system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on clinicians who don’t practice palliative care to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
That differs from the way palliative care is traditionally practiced, in which specially trained doctors, nurses, and other specialists provide that support.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who really needs us the most and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said.
That’s a major driver behind the emphasis in the latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, Dr. LeBlanc added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
The latest edition of the national palliative care guidelines provides new clinical strategies relevant to hematology practice in the United States, according to a physician-researcher specializing in hematology.
The Clinical Practice Guidelines for Quality Palliative Care, 4th edition, represents a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, a physician-researcher at Duke University School of Medicine in Durham, North Carolina.
However, unlike previous editions, this update to the guidelines emphasizes the importance of palliative care provided by both primary care and specialty care clinicians.
“Part of this report is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” Dr. LeBlanc said.
The latest edition helps establish a foundation for gold standard palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to The National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology.
One key reason for the update, according to NCP, was to acknowledge that today’s healthcare system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on clinicians who don’t practice palliative care to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
That differs from the way palliative care is traditionally practiced, in which specially trained doctors, nurses, and other specialists provide that support.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who really needs us the most and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said.
That’s a major driver behind the emphasis in the latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, Dr. LeBlanc added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
The latest edition of the national palliative care guidelines provides new clinical strategies relevant to hematology practice in the United States, according to a physician-researcher specializing in hematology.
The Clinical Practice Guidelines for Quality Palliative Care, 4th edition, represents a “blueprint for what it looks like to provide high-quality, comprehensive palliative care to people with serious illness,” said Thomas W. LeBlanc, MD, a physician-researcher at Duke University School of Medicine in Durham, North Carolina.
However, unlike previous editions, this update to the guidelines emphasizes the importance of palliative care provided by both primary care and specialty care clinicians.
“Part of this report is about trying to raise the game of everybody in medicine and provide a higher basic level of primary palliative care to all people with serious illness, but then also to figure out who has higher levels of needs where the specialists should be applied, since they are a scarce resource,” Dr. LeBlanc said.
The latest edition helps establish a foundation for gold standard palliative care for people living with serious illness, regardless of diagnosis, prognosis, setting, or age, according to The National Coalition for Hospice and Palliative Care, which published the clinical practice guidelines.
The update was developed by the National Consensus Project for Quality Palliative Care (NCP), which includes 16 national organizations with palliative care and hospice expertise, and is endorsed by more than 80 national organizations, including the American Society of Hematology.
One key reason for the update, according to NCP, was to acknowledge that today’s healthcare system may not be meeting patients’ palliative care needs.
Specifically, the guidelines call on clinicians who don’t practice palliative care to integrate palliative care principles into their routine assessment of seriously ill patients with conditions such as heart failure, lung disease, and cancer.
That differs from the way palliative care is traditionally practiced, in which specially trained doctors, nurses, and other specialists provide that support.
An issue with that traditional model is a shortage of specialized clinicians to meet palliative care needs, said Dr. LeBlanc, whose clinical practice and research focuses on palliative care needs of patients with hematologic malignancies.
“Palliative care has matured as a field such that we are now actually facing workforce shortage issues and really fundamental questions about who really needs us the most and how we increase our reach to improve the lives of more patients and families facing serious illness,” he said.
That’s a major driver behind the emphasis in the latest guidelines on providing palliative care in the community, coordinating care, and dealing with care transitions, Dr. LeBlanc added.
“I hope that this document will help to demonstrate the value and the need for palliative care specialists and for improvements in primary care in the care of patients with hematologic diseases in general,” he said. “To me, this adds increasing legitimacy to this whole field.”
Improving education for pediatric cancer caregivers
A new checklist could improve education for parents and other caregivers of children newly diagnosed with cancer, according to a group of nurses.
The checklist is divided into topics that should be taught according to their level of urgency.
The list includes subjects that should be discussed prior to patients’ initial hospital discharge and topics that can be covered later, either within the first month of the patients’ cancer diagnosis or before patients complete therapy.
Cheryl Rodgers, PhD, RN, and her colleagues provided details on this checklist in the Journal of Pediatric Oncology Nursing.
A team of 19 nurses and 2 parent advocates from the Children’s Oncology Group developed the checklist based on existing education checklists, expert recommendations, and team-based activities and discussions.
The checklist is divided into primary, secondary, and tertiary topics.
Primary topics are those that should be discussed with caregivers before they leave the hospital the first time. Examples include home medication dose and frequency, who and when to call for help, preventing infection, and treatment side effects to know before the next appointment.
Secondary topics are those that can be covered during the first month after a child’s cancer diagnosis. Examples include an explanation of what cancer is, an overview of chemotherapy, and more details on the side effects of cancer treatment.
Tertiary topics can be discussed before the child finishes cancer treatment. Examples include details on tests and procedures, risky behaviors to avoid, coping skills, and insurance issues.
Dr Rodgers and her colleagues said this checklist provides nurses with a clear outline of topics that should be discussed with caregivers immediately and topics that can be safely deferred. This could prevent information overload and help caregivers remember the most important information.
A new checklist could improve education for parents and other caregivers of children newly diagnosed with cancer, according to a group of nurses.
The checklist is divided into topics that should be taught according to their level of urgency.
The list includes subjects that should be discussed prior to patients’ initial hospital discharge and topics that can be covered later, either within the first month of the patients’ cancer diagnosis or before patients complete therapy.
Cheryl Rodgers, PhD, RN, and her colleagues provided details on this checklist in the Journal of Pediatric Oncology Nursing.
A team of 19 nurses and 2 parent advocates from the Children’s Oncology Group developed the checklist based on existing education checklists, expert recommendations, and team-based activities and discussions.
The checklist is divided into primary, secondary, and tertiary topics.
Primary topics are those that should be discussed with caregivers before they leave the hospital the first time. Examples include home medication dose and frequency, who and when to call for help, preventing infection, and treatment side effects to know before the next appointment.
Secondary topics are those that can be covered during the first month after a child’s cancer diagnosis. Examples include an explanation of what cancer is, an overview of chemotherapy, and more details on the side effects of cancer treatment.
Tertiary topics can be discussed before the child finishes cancer treatment. Examples include details on tests and procedures, risky behaviors to avoid, coping skills, and insurance issues.
Dr Rodgers and her colleagues said this checklist provides nurses with a clear outline of topics that should be discussed with caregivers immediately and topics that can be safely deferred. This could prevent information overload and help caregivers remember the most important information.
A new checklist could improve education for parents and other caregivers of children newly diagnosed with cancer, according to a group of nurses.
The checklist is divided into topics that should be taught according to their level of urgency.
The list includes subjects that should be discussed prior to patients’ initial hospital discharge and topics that can be covered later, either within the first month of the patients’ cancer diagnosis or before patients complete therapy.
Cheryl Rodgers, PhD, RN, and her colleagues provided details on this checklist in the Journal of Pediatric Oncology Nursing.
A team of 19 nurses and 2 parent advocates from the Children’s Oncology Group developed the checklist based on existing education checklists, expert recommendations, and team-based activities and discussions.
The checklist is divided into primary, secondary, and tertiary topics.
Primary topics are those that should be discussed with caregivers before they leave the hospital the first time. Examples include home medication dose and frequency, who and when to call for help, preventing infection, and treatment side effects to know before the next appointment.
Secondary topics are those that can be covered during the first month after a child’s cancer diagnosis. Examples include an explanation of what cancer is, an overview of chemotherapy, and more details on the side effects of cancer treatment.
Tertiary topics can be discussed before the child finishes cancer treatment. Examples include details on tests and procedures, risky behaviors to avoid, coping skills, and insurance issues.
Dr Rodgers and her colleagues said this checklist provides nurses with a clear outline of topics that should be discussed with caregivers immediately and topics that can be safely deferred. This could prevent information overload and help caregivers remember the most important information.
Exercise linked to risk of death in cancer patients
CHICAGO—Researchers have identified a link between habitual physical activity (PA) and mortality among cancer patients.
Engaging in regular PA, both pre- and post-diagnosis, was associated with a significantly lower risk of death for the entire population studied and for patients with 8 specific types of cancer.
However, the association was not significant for patients with other cancer types, including hematologic malignancies.
Rikki Cannioto, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, New York, and her colleagues presented these findings at the AACR Annual Meeting 2018 (abstract 5254*).
The researchers examined the association between habitual PA and outcomes in 5807 cancer patients enrolled in the Data Bank and BioRepository at Roswell Park between 2003 and 2016.
The population was 54.8% female and 93% white. The average age at diagnosis was 60.6 years.
The researchers looked at patterns of PA over time, from the decade before the cancer was diagnosed and continuing for up to 1 year after diagnosis.
Patients who engaged in regular, moderate- to vigorous-intensity PA (such as walking, running, aerobics, or other cardiovascular exercise) both before and after their diagnosis were considered habitually active, whereas those who did not exercise regularly were considered habitually inactive.
Overall, 52% of patients reported habitual activity, and 19% reported habitual inactivity. Twenty-three percent of patients said their activity level decreased after diagnosis, and 6% said their activity level increased.
Patients were followed through January 31, 2018. The median time to follow-up was 53 months, and 33.7% of patients (n=1956) died during the follow-up period.
Results
The researchers found that patients who were active before and after diagnosis were 40% more likely to survive than those who were habitually inactive (P<0.001). Habitually inactive patients had a 66% increased risk of mortality compared to active patients.
The habitually active patients had a 37-month mean survival advantage over the inactive patients.
In addition, patients whose activity level increased after diagnosis had a 25% lower risk of death than patients who remained inactive after diagnosis.
The researchers observed a significant (P<0.05) association between habitual PA and decreased mortality in patients with breast, colon, prostate, bladder, endometrial, ovarian, esophageal, and skin cancers.
However, the association between PA and mortality was not significant for patients with hematologic malignancies (P=0.59) or kidney, liver, lung, pancreas, stomach, or “other” cancers.
The researchers said the associations between habitual PA and decreased mortality remained consistent regardless of a patient’s sex, tumor stage, smoking status, or body mass index.
“[W]hen it comes to exercise, something is better than nothing, but regular, weekly exercise seems to really make a difference,” Dr Cannioto said.
“In fact, patients who were physically active 3 or 4 days a week experienced an even greater benefit than those who exercised daily, and patients who had only 1 or 2 days of regular activity per week did nearly as well. This is particularly encouraging, as cancer patients and survivors can be overwhelmed by current physical activity recommendations.”
*Information in the abstract differs from the presentation.
CHICAGO—Researchers have identified a link between habitual physical activity (PA) and mortality among cancer patients.
Engaging in regular PA, both pre- and post-diagnosis, was associated with a significantly lower risk of death for the entire population studied and for patients with 8 specific types of cancer.
However, the association was not significant for patients with other cancer types, including hematologic malignancies.
Rikki Cannioto, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, New York, and her colleagues presented these findings at the AACR Annual Meeting 2018 (abstract 5254*).
The researchers examined the association between habitual PA and outcomes in 5807 cancer patients enrolled in the Data Bank and BioRepository at Roswell Park between 2003 and 2016.
The population was 54.8% female and 93% white. The average age at diagnosis was 60.6 years.
The researchers looked at patterns of PA over time, from the decade before the cancer was diagnosed and continuing for up to 1 year after diagnosis.
Patients who engaged in regular, moderate- to vigorous-intensity PA (such as walking, running, aerobics, or other cardiovascular exercise) both before and after their diagnosis were considered habitually active, whereas those who did not exercise regularly were considered habitually inactive.
Overall, 52% of patients reported habitual activity, and 19% reported habitual inactivity. Twenty-three percent of patients said their activity level decreased after diagnosis, and 6% said their activity level increased.
Patients were followed through January 31, 2018. The median time to follow-up was 53 months, and 33.7% of patients (n=1956) died during the follow-up period.
Results
The researchers found that patients who were active before and after diagnosis were 40% more likely to survive than those who were habitually inactive (P<0.001). Habitually inactive patients had a 66% increased risk of mortality compared to active patients.
The habitually active patients had a 37-month mean survival advantage over the inactive patients.
In addition, patients whose activity level increased after diagnosis had a 25% lower risk of death than patients who remained inactive after diagnosis.
The researchers observed a significant (P<0.05) association between habitual PA and decreased mortality in patients with breast, colon, prostate, bladder, endometrial, ovarian, esophageal, and skin cancers.
However, the association between PA and mortality was not significant for patients with hematologic malignancies (P=0.59) or kidney, liver, lung, pancreas, stomach, or “other” cancers.
The researchers said the associations between habitual PA and decreased mortality remained consistent regardless of a patient’s sex, tumor stage, smoking status, or body mass index.
“[W]hen it comes to exercise, something is better than nothing, but regular, weekly exercise seems to really make a difference,” Dr Cannioto said.
“In fact, patients who were physically active 3 or 4 days a week experienced an even greater benefit than those who exercised daily, and patients who had only 1 or 2 days of regular activity per week did nearly as well. This is particularly encouraging, as cancer patients and survivors can be overwhelmed by current physical activity recommendations.”
*Information in the abstract differs from the presentation.
CHICAGO—Researchers have identified a link between habitual physical activity (PA) and mortality among cancer patients.
Engaging in regular PA, both pre- and post-diagnosis, was associated with a significantly lower risk of death for the entire population studied and for patients with 8 specific types of cancer.
However, the association was not significant for patients with other cancer types, including hematologic malignancies.
Rikki Cannioto, PhD, of Roswell Park Comprehensive Cancer Center in Buffalo, New York, and her colleagues presented these findings at the AACR Annual Meeting 2018 (abstract 5254*).
The researchers examined the association between habitual PA and outcomes in 5807 cancer patients enrolled in the Data Bank and BioRepository at Roswell Park between 2003 and 2016.
The population was 54.8% female and 93% white. The average age at diagnosis was 60.6 years.
The researchers looked at patterns of PA over time, from the decade before the cancer was diagnosed and continuing for up to 1 year after diagnosis.
Patients who engaged in regular, moderate- to vigorous-intensity PA (such as walking, running, aerobics, or other cardiovascular exercise) both before and after their diagnosis were considered habitually active, whereas those who did not exercise regularly were considered habitually inactive.
Overall, 52% of patients reported habitual activity, and 19% reported habitual inactivity. Twenty-three percent of patients said their activity level decreased after diagnosis, and 6% said their activity level increased.
Patients were followed through January 31, 2018. The median time to follow-up was 53 months, and 33.7% of patients (n=1956) died during the follow-up period.
Results
The researchers found that patients who were active before and after diagnosis were 40% more likely to survive than those who were habitually inactive (P<0.001). Habitually inactive patients had a 66% increased risk of mortality compared to active patients.
The habitually active patients had a 37-month mean survival advantage over the inactive patients.
In addition, patients whose activity level increased after diagnosis had a 25% lower risk of death than patients who remained inactive after diagnosis.
The researchers observed a significant (P<0.05) association between habitual PA and decreased mortality in patients with breast, colon, prostate, bladder, endometrial, ovarian, esophageal, and skin cancers.
However, the association between PA and mortality was not significant for patients with hematologic malignancies (P=0.59) or kidney, liver, lung, pancreas, stomach, or “other” cancers.
The researchers said the associations between habitual PA and decreased mortality remained consistent regardless of a patient’s sex, tumor stage, smoking status, or body mass index.
“[W]hen it comes to exercise, something is better than nothing, but regular, weekly exercise seems to really make a difference,” Dr Cannioto said.
“In fact, patients who were physically active 3 or 4 days a week experienced an even greater benefit than those who exercised daily, and patients who had only 1 or 2 days of regular activity per week did nearly as well. This is particularly encouraging, as cancer patients and survivors can be overwhelmed by current physical activity recommendations.”
*Information in the abstract differs from the presentation.
Art education benefits blood cancer patients
New research suggests a bedside visual art intervention (BVAI) can reduce pain and anxiety in inpatients with hematologic malignancies, including those undergoing transplant.
The BVAI involved an educator teaching patients art technique one-on-one for approximately 30 minutes.
After a single session, patients had significant improvements in positive mood and pain scores, as well as decreases in negative mood and anxiety.
Alexandra P. Wolanskyj, MD, of Mayo Clinic in Rochester, Minnesota, and her colleagues reported these results in the European Journal of Cancer Care.
The study included 21 patients, 19 of them female. Their median age was 53.5 (range, 19-75). Six patients were undergoing hematopoietic stem cell transplant.
The patients had multiple myeloma (n=5), acute myeloid leukemia (n=5), non-Hodgkin lymphoma (n=3), Hodgkin lymphoma (n=2), acute lymphoblastic leukemia (n=1), chronic lymphocytic leukemia (n=1), amyloidosis (n=1), Gardner-Diamond syndrome (n=1), myelodysplastic syndrome (n=1), and Waldenstrom’s macroglobulinemia (n=1).
Nearly half of patients had relapsed disease (47.6%), 23.8% had active and new disease, 19.0% had active disease with primary resistance on chemotherapy, and 9.5% of patients were in remission.
Intervention
The researchers recruited an educator from a community art center to teach art at the patients’ bedsides. Sessions were intended to be about 30 minutes. However, patients could stop at any time or continue beyond 30 minutes.
Patients and their families could make art or just observe. Materials used included watercolors, oil pastels, colored pencils, and clay (all non-toxic and odorless). The materials were left with patients so they could continue to use them after the sessions.
Results
The researchers assessed patients’ pain, anxiety, and mood at baseline and after the patients had a session with the art educator.
After the BVAI, patients had a significant decrease in pain, according to the Visual Analog Scale (VAS). The 14 patients who reported any pain at baseline had a mean reduction in VAS score of 1.5, or a 35.1% reduction in pain (P=0.017).
Patients had a 21.6% reduction in anxiety after the BVAI. Among the 20 patients who completed this assessment, there was a mean 9.2-point decrease in State-Trait Anxiety Inventory (STAI) score (P=0.001).
In addition, patients had a significant increase in positive mood and a significant decrease in negative mood after the BVAI. Mood was assessed in 20 patients using the Positive and Negative Affect Schedule (PANAS) scale.
Positive mood increased 14.6% (P=0.003), and negative mood decreased 18.0% (P=0.015) after the BVAI. Patients’ mean PANAS scores increased 4.6 points for positive mood and decreased 3.3 points for negative mood.
All 21 patients completed a questionnaire on the BVAI. All but 1 patient (95%) said the intervention was positive overall, and 85% of patients (n=18) said they would be interested in participating in future art-based interventions.
The researchers said these results suggest experiences provided by artists in the community may be an adjunct to conventional treatments in patients with cancer-related mood symptoms and pain.
New research suggests a bedside visual art intervention (BVAI) can reduce pain and anxiety in inpatients with hematologic malignancies, including those undergoing transplant.
The BVAI involved an educator teaching patients art technique one-on-one for approximately 30 minutes.
After a single session, patients had significant improvements in positive mood and pain scores, as well as decreases in negative mood and anxiety.
Alexandra P. Wolanskyj, MD, of Mayo Clinic in Rochester, Minnesota, and her colleagues reported these results in the European Journal of Cancer Care.
The study included 21 patients, 19 of them female. Their median age was 53.5 (range, 19-75). Six patients were undergoing hematopoietic stem cell transplant.
The patients had multiple myeloma (n=5), acute myeloid leukemia (n=5), non-Hodgkin lymphoma (n=3), Hodgkin lymphoma (n=2), acute lymphoblastic leukemia (n=1), chronic lymphocytic leukemia (n=1), amyloidosis (n=1), Gardner-Diamond syndrome (n=1), myelodysplastic syndrome (n=1), and Waldenstrom’s macroglobulinemia (n=1).
Nearly half of patients had relapsed disease (47.6%), 23.8% had active and new disease, 19.0% had active disease with primary resistance on chemotherapy, and 9.5% of patients were in remission.
Intervention
The researchers recruited an educator from a community art center to teach art at the patients’ bedsides. Sessions were intended to be about 30 minutes. However, patients could stop at any time or continue beyond 30 minutes.
Patients and their families could make art or just observe. Materials used included watercolors, oil pastels, colored pencils, and clay (all non-toxic and odorless). The materials were left with patients so they could continue to use them after the sessions.
Results
The researchers assessed patients’ pain, anxiety, and mood at baseline and after the patients had a session with the art educator.
After the BVAI, patients had a significant decrease in pain, according to the Visual Analog Scale (VAS). The 14 patients who reported any pain at baseline had a mean reduction in VAS score of 1.5, or a 35.1% reduction in pain (P=0.017).
Patients had a 21.6% reduction in anxiety after the BVAI. Among the 20 patients who completed this assessment, there was a mean 9.2-point decrease in State-Trait Anxiety Inventory (STAI) score (P=0.001).
In addition, patients had a significant increase in positive mood and a significant decrease in negative mood after the BVAI. Mood was assessed in 20 patients using the Positive and Negative Affect Schedule (PANAS) scale.
Positive mood increased 14.6% (P=0.003), and negative mood decreased 18.0% (P=0.015) after the BVAI. Patients’ mean PANAS scores increased 4.6 points for positive mood and decreased 3.3 points for negative mood.
All 21 patients completed a questionnaire on the BVAI. All but 1 patient (95%) said the intervention was positive overall, and 85% of patients (n=18) said they would be interested in participating in future art-based interventions.
The researchers said these results suggest experiences provided by artists in the community may be an adjunct to conventional treatments in patients with cancer-related mood symptoms and pain.
New research suggests a bedside visual art intervention (BVAI) can reduce pain and anxiety in inpatients with hematologic malignancies, including those undergoing transplant.
The BVAI involved an educator teaching patients art technique one-on-one for approximately 30 minutes.
After a single session, patients had significant improvements in positive mood and pain scores, as well as decreases in negative mood and anxiety.
Alexandra P. Wolanskyj, MD, of Mayo Clinic in Rochester, Minnesota, and her colleagues reported these results in the European Journal of Cancer Care.
The study included 21 patients, 19 of them female. Their median age was 53.5 (range, 19-75). Six patients were undergoing hematopoietic stem cell transplant.
The patients had multiple myeloma (n=5), acute myeloid leukemia (n=5), non-Hodgkin lymphoma (n=3), Hodgkin lymphoma (n=2), acute lymphoblastic leukemia (n=1), chronic lymphocytic leukemia (n=1), amyloidosis (n=1), Gardner-Diamond syndrome (n=1), myelodysplastic syndrome (n=1), and Waldenstrom’s macroglobulinemia (n=1).
Nearly half of patients had relapsed disease (47.6%), 23.8% had active and new disease, 19.0% had active disease with primary resistance on chemotherapy, and 9.5% of patients were in remission.
Intervention
The researchers recruited an educator from a community art center to teach art at the patients’ bedsides. Sessions were intended to be about 30 minutes. However, patients could stop at any time or continue beyond 30 minutes.
Patients and their families could make art or just observe. Materials used included watercolors, oil pastels, colored pencils, and clay (all non-toxic and odorless). The materials were left with patients so they could continue to use them after the sessions.
Results
The researchers assessed patients’ pain, anxiety, and mood at baseline and after the patients had a session with the art educator.
After the BVAI, patients had a significant decrease in pain, according to the Visual Analog Scale (VAS). The 14 patients who reported any pain at baseline had a mean reduction in VAS score of 1.5, or a 35.1% reduction in pain (P=0.017).
Patients had a 21.6% reduction in anxiety after the BVAI. Among the 20 patients who completed this assessment, there was a mean 9.2-point decrease in State-Trait Anxiety Inventory (STAI) score (P=0.001).
In addition, patients had a significant increase in positive mood and a significant decrease in negative mood after the BVAI. Mood was assessed in 20 patients using the Positive and Negative Affect Schedule (PANAS) scale.
Positive mood increased 14.6% (P=0.003), and negative mood decreased 18.0% (P=0.015) after the BVAI. Patients’ mean PANAS scores increased 4.6 points for positive mood and decreased 3.3 points for negative mood.
All 21 patients completed a questionnaire on the BVAI. All but 1 patient (95%) said the intervention was positive overall, and 85% of patients (n=18) said they would be interested in participating in future art-based interventions.
The researchers said these results suggest experiences provided by artists in the community may be an adjunct to conventional treatments in patients with cancer-related mood symptoms and pain.
How US healthcare compares to other countries
The US has similar healthcare utilization as other high-income countries but spends more and tends to have worse health outcomes, according to a new study.
In 2016, the US spent 17.8% of its gross domestic product on healthcare. For 10 other high-income countries, spending ranged from 9.6% to 12.4%.
However, sizes of physician and nursing workforces were comparable between the countries, numbers of hospital discharges were similar, and lengths of hospital stay were lower in the US than in most other countries.
Meanwhile, the US had the lowest life expectancy and the highest rate of infant mortality.
This research was published in JAMA.
“There’s been a lot of interest in international comparisons between America and other high-income countries, and there’s been a lot of vagueness about why exactly our [US] spending is so much higher and our health outcomes are not necessarily better and often worse,” said study author Ashish K. Jha, MD, of Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“This study really tries to fill in gaps, I think, across a wide range of issues, from structural capacity to utilization to prices to outcomes.”
For this study, Dr Jha and his colleagues analyzed recent healthcare data, primarily from 2013 to 2016. The team compared differences in healthcare spending, performance, and structural features between the US and 10 high-income countries—UK, Canada, Germany, Australia, Japan, Sweden, France, Netherlands, Switzerland, and Denmark.
Spending
In 2016, healthcare spending, as a percentage of gross domestic product, was as follows:
US—17.8%
Switzerland—12.4%
Sweden—11.9%
Germany—11.3%
France—11%
Japan—10.9%
Denmark—10.8%
Netherlands—10.5%
Canada—10.3%
UK—9.7%
Australia—9.6%.
“The big differences in spending really seem to be driven by prices,” Dr Jha said, noting that salaries for doctors and nurses, administrative expenditures, and pharmaceutical costs are “much higher” in the US.
The total spending on pharmaceuticals per capita was $1443 in the US but ranged from $466 (Netherlands) to $939 (Switzerland) in the other countries.
Administrative costs accounted for 8% of the total national health expenditure in the US but 1% (France, Japan) to 3% (Germany) in the other countries.
Outpatient care expenditures ranged from 22% (Netherlands) to 42% (US). Inpatient care expenditures ranged from 17% (Canada) to 32% (Netherlands), with 19% for the US. And expenditures for medical goods ranged from 10% (Denmark) to 20% (Germany), with the US at 14%.
Physicians’ and nurses’ salaries were higher in the US than other countries. For example, generalist physicians earned $218,173 in the US in 2016 but anywhere from $86,607 (Sweden) to $154,126 (Germany) in the other countries.
Utilization
Although US doctors and nurses earned more than their counterparts in comparator countries, there were no substantial between-country differences in the size of the physician and nursing workforces.
The number of working physicians for every 1000 people ranged from 2.1 (UK) to 4.3 (Switzerland), with the US at 2.6. The number of working nurses for every 1000 people ranged from 8.2 (UK) to 17.4 (Switzerland), with 11.1 for the US.
The US had comparable numbers of hospital beds as some of the other countries. The range was 2.5 (Sweden) to 13.2 (Japan) beds per 1000 people, with the US at 2.8.
The US ranked 6th when it came to hospital discharges for acute myocardial infarction (AMI, 192 per 100,000 people; range, 89 to 287), mental and behavioral issues (679 per 100,000 people; range, 119 to 1719), pneumonia (365 per 100,000 people; range, 187 to 567), and chronic obstructive pulmonary disease (230 per 100,000 people; range, 45 to 352).
The US had greater utilization of computed tomography than the other countries, with 245 examinations per 1000 people (range for other countries, 79 to 231). And US utilization of magnetic resonance imaging was higher than most countries, with 118 examinations per 1000 people (range, 41 to 131).
However, the US ranked on the lower end of the spectrum for length of hospital stay. The length of stay for a “normal delivery” childbirth ranged from a median of 1.5 days (UK) to 5.7 days (Japan), with the US clocking in at 2 days. The median length of stay for AMI ranged from a median of 3.9 days (Netherlands) to 10.3 days (Germany), with the US at 5.4 days.
“So much of the debate about healthcare these days is about over-utilization—that somehow our health system is uniquely bad at avoiding unnecessary services,” Dr Jha said. “I think these data really put that argument to rest. Except for a few pockets, utilization is not really different between us and these high-income Northern European countries, so maybe we need to spend a little less time focusing on that and a little bit more time focusing on prices of our healthcare system.”
Outcomes
The US ranked on the lower end of the spectrum for some mortality outcomes. Thirty-day stroke mortality per 1000 patients ranged from 4.2 in the US to 10 in Canada. Thirty-day AMI mortality per 1000 ranged from 4.1 (Australia) to 8.7 (Germany), with the US at 5.5.
However, infant mortality was highest in the US, at 5.8 deaths per 1000 live births (range for other countries, 2.1 to 5.1). And life expectancy was lowest in the US, at 78.8 years (range for other countries, 80.7 to 83.9).
The researchers noted that the US had the highest percentage of overweight or obese individuals age 15 and older, at 70.1% (range for other countries, 23.8% to 63.4%), but a low percentage of smokers (11.4%; range, 11.2% to 22.4%) and moderate alcohol consumption (8.8 L per capita; range, 7.2 to 11.9).
Limitations of this study include some differences in approaches to collecting and standardizing data across countries, as well as missing data for some countries.
The US has similar healthcare utilization as other high-income countries but spends more and tends to have worse health outcomes, according to a new study.
In 2016, the US spent 17.8% of its gross domestic product on healthcare. For 10 other high-income countries, spending ranged from 9.6% to 12.4%.
However, sizes of physician and nursing workforces were comparable between the countries, numbers of hospital discharges were similar, and lengths of hospital stay were lower in the US than in most other countries.
Meanwhile, the US had the lowest life expectancy and the highest rate of infant mortality.
This research was published in JAMA.
“There’s been a lot of interest in international comparisons between America and other high-income countries, and there’s been a lot of vagueness about why exactly our [US] spending is so much higher and our health outcomes are not necessarily better and often worse,” said study author Ashish K. Jha, MD, of Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“This study really tries to fill in gaps, I think, across a wide range of issues, from structural capacity to utilization to prices to outcomes.”
For this study, Dr Jha and his colleagues analyzed recent healthcare data, primarily from 2013 to 2016. The team compared differences in healthcare spending, performance, and structural features between the US and 10 high-income countries—UK, Canada, Germany, Australia, Japan, Sweden, France, Netherlands, Switzerland, and Denmark.
Spending
In 2016, healthcare spending, as a percentage of gross domestic product, was as follows:
US—17.8%
Switzerland—12.4%
Sweden—11.9%
Germany—11.3%
France—11%
Japan—10.9%
Denmark—10.8%
Netherlands—10.5%
Canada—10.3%
UK—9.7%
Australia—9.6%.
“The big differences in spending really seem to be driven by prices,” Dr Jha said, noting that salaries for doctors and nurses, administrative expenditures, and pharmaceutical costs are “much higher” in the US.
The total spending on pharmaceuticals per capita was $1443 in the US but ranged from $466 (Netherlands) to $939 (Switzerland) in the other countries.
Administrative costs accounted for 8% of the total national health expenditure in the US but 1% (France, Japan) to 3% (Germany) in the other countries.
Outpatient care expenditures ranged from 22% (Netherlands) to 42% (US). Inpatient care expenditures ranged from 17% (Canada) to 32% (Netherlands), with 19% for the US. And expenditures for medical goods ranged from 10% (Denmark) to 20% (Germany), with the US at 14%.
Physicians’ and nurses’ salaries were higher in the US than other countries. For example, generalist physicians earned $218,173 in the US in 2016 but anywhere from $86,607 (Sweden) to $154,126 (Germany) in the other countries.
Utilization
Although US doctors and nurses earned more than their counterparts in comparator countries, there were no substantial between-country differences in the size of the physician and nursing workforces.
The number of working physicians for every 1000 people ranged from 2.1 (UK) to 4.3 (Switzerland), with the US at 2.6. The number of working nurses for every 1000 people ranged from 8.2 (UK) to 17.4 (Switzerland), with 11.1 for the US.
The US had comparable numbers of hospital beds as some of the other countries. The range was 2.5 (Sweden) to 13.2 (Japan) beds per 1000 people, with the US at 2.8.
The US ranked 6th when it came to hospital discharges for acute myocardial infarction (AMI, 192 per 100,000 people; range, 89 to 287), mental and behavioral issues (679 per 100,000 people; range, 119 to 1719), pneumonia (365 per 100,000 people; range, 187 to 567), and chronic obstructive pulmonary disease (230 per 100,000 people; range, 45 to 352).
The US had greater utilization of computed tomography than the other countries, with 245 examinations per 1000 people (range for other countries, 79 to 231). And US utilization of magnetic resonance imaging was higher than most countries, with 118 examinations per 1000 people (range, 41 to 131).
However, the US ranked on the lower end of the spectrum for length of hospital stay. The length of stay for a “normal delivery” childbirth ranged from a median of 1.5 days (UK) to 5.7 days (Japan), with the US clocking in at 2 days. The median length of stay for AMI ranged from a median of 3.9 days (Netherlands) to 10.3 days (Germany), with the US at 5.4 days.
“So much of the debate about healthcare these days is about over-utilization—that somehow our health system is uniquely bad at avoiding unnecessary services,” Dr Jha said. “I think these data really put that argument to rest. Except for a few pockets, utilization is not really different between us and these high-income Northern European countries, so maybe we need to spend a little less time focusing on that and a little bit more time focusing on prices of our healthcare system.”
Outcomes
The US ranked on the lower end of the spectrum for some mortality outcomes. Thirty-day stroke mortality per 1000 patients ranged from 4.2 in the US to 10 in Canada. Thirty-day AMI mortality per 1000 ranged from 4.1 (Australia) to 8.7 (Germany), with the US at 5.5.
However, infant mortality was highest in the US, at 5.8 deaths per 1000 live births (range for other countries, 2.1 to 5.1). And life expectancy was lowest in the US, at 78.8 years (range for other countries, 80.7 to 83.9).
The researchers noted that the US had the highest percentage of overweight or obese individuals age 15 and older, at 70.1% (range for other countries, 23.8% to 63.4%), but a low percentage of smokers (11.4%; range, 11.2% to 22.4%) and moderate alcohol consumption (8.8 L per capita; range, 7.2 to 11.9).
Limitations of this study include some differences in approaches to collecting and standardizing data across countries, as well as missing data for some countries.
The US has similar healthcare utilization as other high-income countries but spends more and tends to have worse health outcomes, according to a new study.
In 2016, the US spent 17.8% of its gross domestic product on healthcare. For 10 other high-income countries, spending ranged from 9.6% to 12.4%.
However, sizes of physician and nursing workforces were comparable between the countries, numbers of hospital discharges were similar, and lengths of hospital stay were lower in the US than in most other countries.
Meanwhile, the US had the lowest life expectancy and the highest rate of infant mortality.
This research was published in JAMA.
“There’s been a lot of interest in international comparisons between America and other high-income countries, and there’s been a lot of vagueness about why exactly our [US] spending is so much higher and our health outcomes are not necessarily better and often worse,” said study author Ashish K. Jha, MD, of Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“This study really tries to fill in gaps, I think, across a wide range of issues, from structural capacity to utilization to prices to outcomes.”
For this study, Dr Jha and his colleagues analyzed recent healthcare data, primarily from 2013 to 2016. The team compared differences in healthcare spending, performance, and structural features between the US and 10 high-income countries—UK, Canada, Germany, Australia, Japan, Sweden, France, Netherlands, Switzerland, and Denmark.
Spending
In 2016, healthcare spending, as a percentage of gross domestic product, was as follows:
US—17.8%
Switzerland—12.4%
Sweden—11.9%
Germany—11.3%
France—11%
Japan—10.9%
Denmark—10.8%
Netherlands—10.5%
Canada—10.3%
UK—9.7%
Australia—9.6%.
“The big differences in spending really seem to be driven by prices,” Dr Jha said, noting that salaries for doctors and nurses, administrative expenditures, and pharmaceutical costs are “much higher” in the US.
The total spending on pharmaceuticals per capita was $1443 in the US but ranged from $466 (Netherlands) to $939 (Switzerland) in the other countries.
Administrative costs accounted for 8% of the total national health expenditure in the US but 1% (France, Japan) to 3% (Germany) in the other countries.
Outpatient care expenditures ranged from 22% (Netherlands) to 42% (US). Inpatient care expenditures ranged from 17% (Canada) to 32% (Netherlands), with 19% for the US. And expenditures for medical goods ranged from 10% (Denmark) to 20% (Germany), with the US at 14%.
Physicians’ and nurses’ salaries were higher in the US than other countries. For example, generalist physicians earned $218,173 in the US in 2016 but anywhere from $86,607 (Sweden) to $154,126 (Germany) in the other countries.
Utilization
Although US doctors and nurses earned more than their counterparts in comparator countries, there were no substantial between-country differences in the size of the physician and nursing workforces.
The number of working physicians for every 1000 people ranged from 2.1 (UK) to 4.3 (Switzerland), with the US at 2.6. The number of working nurses for every 1000 people ranged from 8.2 (UK) to 17.4 (Switzerland), with 11.1 for the US.
The US had comparable numbers of hospital beds as some of the other countries. The range was 2.5 (Sweden) to 13.2 (Japan) beds per 1000 people, with the US at 2.8.
The US ranked 6th when it came to hospital discharges for acute myocardial infarction (AMI, 192 per 100,000 people; range, 89 to 287), mental and behavioral issues (679 per 100,000 people; range, 119 to 1719), pneumonia (365 per 100,000 people; range, 187 to 567), and chronic obstructive pulmonary disease (230 per 100,000 people; range, 45 to 352).
The US had greater utilization of computed tomography than the other countries, with 245 examinations per 1000 people (range for other countries, 79 to 231). And US utilization of magnetic resonance imaging was higher than most countries, with 118 examinations per 1000 people (range, 41 to 131).
However, the US ranked on the lower end of the spectrum for length of hospital stay. The length of stay for a “normal delivery” childbirth ranged from a median of 1.5 days (UK) to 5.7 days (Japan), with the US clocking in at 2 days. The median length of stay for AMI ranged from a median of 3.9 days (Netherlands) to 10.3 days (Germany), with the US at 5.4 days.
“So much of the debate about healthcare these days is about over-utilization—that somehow our health system is uniquely bad at avoiding unnecessary services,” Dr Jha said. “I think these data really put that argument to rest. Except for a few pockets, utilization is not really different between us and these high-income Northern European countries, so maybe we need to spend a little less time focusing on that and a little bit more time focusing on prices of our healthcare system.”
Outcomes
The US ranked on the lower end of the spectrum for some mortality outcomes. Thirty-day stroke mortality per 1000 patients ranged from 4.2 in the US to 10 in Canada. Thirty-day AMI mortality per 1000 ranged from 4.1 (Australia) to 8.7 (Germany), with the US at 5.5.
However, infant mortality was highest in the US, at 5.8 deaths per 1000 live births (range for other countries, 2.1 to 5.1). And life expectancy was lowest in the US, at 78.8 years (range for other countries, 80.7 to 83.9).
The researchers noted that the US had the highest percentage of overweight or obese individuals age 15 and older, at 70.1% (range for other countries, 23.8% to 63.4%), but a low percentage of smokers (11.4%; range, 11.2% to 22.4%) and moderate alcohol consumption (8.8 L per capita; range, 7.2 to 11.9).
Limitations of this study include some differences in approaches to collecting and standardizing data across countries, as well as missing data for some countries.
Researchers question validity of NCCN guidelines
New research suggests guidelines from the National Comprehensive Cancer Network (NCCN) may sometimes be supported by low-quality evidence or no evidence at all.
Researchers compared NCCN recommendations for cancer drugs to US cancer drug approvals over a 5-year period.
Thirty-nine percent of NCCN’s treatment recommendations did not coincide with uses approved by the US Food and Drug Administration (FDA).
For most of these recommendations (84%), NCCN did not provide supporting data from randomized, phase 3 trials.
For 36% of the recommendations, NCCN gave no supporting evidence.
Vinay Prasad, MD, of Oregon Health & Science University in Portland, Oregon, and his colleagues reported these findings in The BMJ.
Dr Prasad and his colleagues compared FDA approvals of cancer drugs between 2011 and 2015 with NCCN recommendations as of March 25, 2016.
When NCCN made recommendations beyond FDA approvals, the researchers evaluated the evidence used to support those recommendations.
Forty-seven new cancer drugs were approved by the FDA for 69 indications between 2011 and 2015. NCCN recommended the 47 drugs for 113 indications, including the 69 FDA-approved indications.
So 39% (n=44) of NCCN’s recommendations were not approved by the FDA, and NCCN gave the following evidence to support these recommendations:
- No evidence—36% (n=16)
- Phase 2 trial without randomization—30% (n=13)
- Randomized, phase 3 trial—16% (n=7)
- Phase 2 trial with randomization—7% (n=3)
- Case report or series of less than 5 patients—5% (n=2)
- Book chapter or review article—2% (n=1)
- Phase 1 trial—2% (n=1)
- Ongoing trial—2% (n=1).
Dr Prasad and his colleagues did point out that not all FDA approvals are supported by randomized, phase 3 trials.
And when the team followed-up 21 months after their initial analysis, they found that 6 of the 44 (14%) additional recommendations by NCCN had received FDA approval.
The researchers also noted that they did not search for independent evidence to support NCCN recommendations beyond the references NCCN provided. So some of the recommendations may have had more or better supporting evidence than what was provided.
Still, the team said these results suggest NCCN “frequently” makes recommendations that go beyond FDA approvals and “often fails to cite evidence or relies on low levels of evidence.” Therefore, NCCN should cite all evidence used to formulate its recommendations.
NCCN argues that it does provide ample evidence to support the recommendations in its guidelines.
“The NCCN guidelines contain more than 24,500 references to inform users of the evidence used in making its decisions,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“These data are supplemented by the analysis of the available evidence by expert clinician researchers and patient advocates who evaluate each recommendation and come to consensus. Each recommendation is labeled with a Category of Evidence, and the vast majority of those for systemic therapies are accompanied by Evidence Blocks, which outline, on 1-5 scales, the efficacy, safety, quality of the evidence, consistency of the evidence, and affordability of the treatment.”
New research suggests guidelines from the National Comprehensive Cancer Network (NCCN) may sometimes be supported by low-quality evidence or no evidence at all.
Researchers compared NCCN recommendations for cancer drugs to US cancer drug approvals over a 5-year period.
Thirty-nine percent of NCCN’s treatment recommendations did not coincide with uses approved by the US Food and Drug Administration (FDA).
For most of these recommendations (84%), NCCN did not provide supporting data from randomized, phase 3 trials.
For 36% of the recommendations, NCCN gave no supporting evidence.
Vinay Prasad, MD, of Oregon Health & Science University in Portland, Oregon, and his colleagues reported these findings in The BMJ.
Dr Prasad and his colleagues compared FDA approvals of cancer drugs between 2011 and 2015 with NCCN recommendations as of March 25, 2016.
When NCCN made recommendations beyond FDA approvals, the researchers evaluated the evidence used to support those recommendations.
Forty-seven new cancer drugs were approved by the FDA for 69 indications between 2011 and 2015. NCCN recommended the 47 drugs for 113 indications, including the 69 FDA-approved indications.
So 39% (n=44) of NCCN’s recommendations were not approved by the FDA, and NCCN gave the following evidence to support these recommendations:
- No evidence—36% (n=16)
- Phase 2 trial without randomization—30% (n=13)
- Randomized, phase 3 trial—16% (n=7)
- Phase 2 trial with randomization—7% (n=3)
- Case report or series of less than 5 patients—5% (n=2)
- Book chapter or review article—2% (n=1)
- Phase 1 trial—2% (n=1)
- Ongoing trial—2% (n=1).
Dr Prasad and his colleagues did point out that not all FDA approvals are supported by randomized, phase 3 trials.
And when the team followed-up 21 months after their initial analysis, they found that 6 of the 44 (14%) additional recommendations by NCCN had received FDA approval.
The researchers also noted that they did not search for independent evidence to support NCCN recommendations beyond the references NCCN provided. So some of the recommendations may have had more or better supporting evidence than what was provided.
Still, the team said these results suggest NCCN “frequently” makes recommendations that go beyond FDA approvals and “often fails to cite evidence or relies on low levels of evidence.” Therefore, NCCN should cite all evidence used to formulate its recommendations.
NCCN argues that it does provide ample evidence to support the recommendations in its guidelines.
“The NCCN guidelines contain more than 24,500 references to inform users of the evidence used in making its decisions,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“These data are supplemented by the analysis of the available evidence by expert clinician researchers and patient advocates who evaluate each recommendation and come to consensus. Each recommendation is labeled with a Category of Evidence, and the vast majority of those for systemic therapies are accompanied by Evidence Blocks, which outline, on 1-5 scales, the efficacy, safety, quality of the evidence, consistency of the evidence, and affordability of the treatment.”
New research suggests guidelines from the National Comprehensive Cancer Network (NCCN) may sometimes be supported by low-quality evidence or no evidence at all.
Researchers compared NCCN recommendations for cancer drugs to US cancer drug approvals over a 5-year period.
Thirty-nine percent of NCCN’s treatment recommendations did not coincide with uses approved by the US Food and Drug Administration (FDA).
For most of these recommendations (84%), NCCN did not provide supporting data from randomized, phase 3 trials.
For 36% of the recommendations, NCCN gave no supporting evidence.
Vinay Prasad, MD, of Oregon Health & Science University in Portland, Oregon, and his colleagues reported these findings in The BMJ.
Dr Prasad and his colleagues compared FDA approvals of cancer drugs between 2011 and 2015 with NCCN recommendations as of March 25, 2016.
When NCCN made recommendations beyond FDA approvals, the researchers evaluated the evidence used to support those recommendations.
Forty-seven new cancer drugs were approved by the FDA for 69 indications between 2011 and 2015. NCCN recommended the 47 drugs for 113 indications, including the 69 FDA-approved indications.
So 39% (n=44) of NCCN’s recommendations were not approved by the FDA, and NCCN gave the following evidence to support these recommendations:
- No evidence—36% (n=16)
- Phase 2 trial without randomization—30% (n=13)
- Randomized, phase 3 trial—16% (n=7)
- Phase 2 trial with randomization—7% (n=3)
- Case report or series of less than 5 patients—5% (n=2)
- Book chapter or review article—2% (n=1)
- Phase 1 trial—2% (n=1)
- Ongoing trial—2% (n=1).
Dr Prasad and his colleagues did point out that not all FDA approvals are supported by randomized, phase 3 trials.
And when the team followed-up 21 months after their initial analysis, they found that 6 of the 44 (14%) additional recommendations by NCCN had received FDA approval.
The researchers also noted that they did not search for independent evidence to support NCCN recommendations beyond the references NCCN provided. So some of the recommendations may have had more or better supporting evidence than what was provided.
Still, the team said these results suggest NCCN “frequently” makes recommendations that go beyond FDA approvals and “often fails to cite evidence or relies on low levels of evidence.” Therefore, NCCN should cite all evidence used to formulate its recommendations.
NCCN argues that it does provide ample evidence to support the recommendations in its guidelines.
“The NCCN guidelines contain more than 24,500 references to inform users of the evidence used in making its decisions,” said Robert W. Carlson, MD, chief executive officer of NCCN.
“These data are supplemented by the analysis of the available evidence by expert clinician researchers and patient advocates who evaluate each recommendation and come to consensus. Each recommendation is labeled with a Category of Evidence, and the vast majority of those for systemic therapies are accompanied by Evidence Blocks, which outline, on 1-5 scales, the efficacy, safety, quality of the evidence, consistency of the evidence, and affordability of the treatment.”
Intervention helps kids stay active after cancer treatment
ORLANDO—Results of a pilot study suggest a web-based, reward-driven intervention can motivate adolescent cancer survivors to stay physically active.
Time spent performing moderate-to-vigorous physical activity (MVPA) increased by an average of 5 minutes a week for subjects who were randomized to the intervention.
For control subjects, MVPA decreased by an average of 24 minutes a week.
These findings were presented at the 2018 Cancer Survivorship Symposium (abstract 102).
“Compared to the general population, childhood cancer survivors have an increased risk for obesity and metabolic syndrome, conditions that can lead to heart disease, stroke, and diabetes, so it is really important that they are physically active,” said study investigator Carrie R. Howell, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“By intervening in this young age group, we hope to help kids develop healthy exercise habits for life.”
Dr Howell and her colleagues studied cancer survivors, ages 11 to 15, who were no longer receiving cancer treatment and were physically active less than 60 minutes a day.
The subjects were randomized to the intervention or to a control group. Controls received a wearable activity monitor and an educational handout with information about the importance of physical activity and examples of activities.
The intervention group received the handout and activity monitor but also had access to an interactive website. On at least a weekly basis, subjects would connect their monitor to a computer and log their activity through the website. Upon achieving certain thresholds of activity, they received rewards, such as T-shirts and gift cards by mail.
At the beginning and end of the study, participants visited St. Jude for an assessment of their physical fitness (strength, flexibility, and endurance) and neurocognitive measures (attention and memory), as well as health-related quality of life (assessed using the Pediatric Quality of Life Inventory questionnaire).
Results
Seventy-eight cancer survivors completed the 24-week study, 53 of them in the intervention group and 25 in the control group.
MVPA increased by an average of 4.7 minutes per week in the intervention group and decreased by an average of 24.3 minutes per week in the control group.
“In this age group, it is common to see a decrease in physical activity over time, even among healthy kids,” Dr Howell said. “Therefore, we are encouraged that our intervention was successful at maintaining physical activity levels, but a longer program may be needed to create lasting exercise habits.”
In addition to increases in MVPA, the intervention group had the following improvements in fitness:
- Increase in hand grip strength from an average of 19.9 kg to 21.0 kg
- Increase in number of push-ups from an average of 15 to 18
- Increase in number of sit-ups from an average of 11 to 14.
Furthermore, subjects in the intervention group saw their verbal fluency z-score increase by an average of 0.13 points and their general cognition z-score increase by an average of 0.23 points.
Their quality of life scores increased as well. Both overall quality of life and physical-function-related quality of life scores increased from an average of 74.2 to 78.0.
Control subjects had no significant changes in fitness, neurocognitive measures, or quality of life.
This study was supported by the National Cancer Institute, the American Lebanese Syrian Associated Charities, and HopeLab.
Based on the results of this study, the investigators have designed a larger trial (ALTE1631) to test a web-based physical activity intervention. They hope to enroll 384 survivors of childhood acute lymphoblastic leukemia at institutions across the US. The intervention will last a year, with follow-up at 18 months.
Further down the line, the investigators plan to explore the relationship between physical activity and cognition.
ORLANDO—Results of a pilot study suggest a web-based, reward-driven intervention can motivate adolescent cancer survivors to stay physically active.
Time spent performing moderate-to-vigorous physical activity (MVPA) increased by an average of 5 minutes a week for subjects who were randomized to the intervention.
For control subjects, MVPA decreased by an average of 24 minutes a week.
These findings were presented at the 2018 Cancer Survivorship Symposium (abstract 102).
“Compared to the general population, childhood cancer survivors have an increased risk for obesity and metabolic syndrome, conditions that can lead to heart disease, stroke, and diabetes, so it is really important that they are physically active,” said study investigator Carrie R. Howell, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“By intervening in this young age group, we hope to help kids develop healthy exercise habits for life.”
Dr Howell and her colleagues studied cancer survivors, ages 11 to 15, who were no longer receiving cancer treatment and were physically active less than 60 minutes a day.
The subjects were randomized to the intervention or to a control group. Controls received a wearable activity monitor and an educational handout with information about the importance of physical activity and examples of activities.
The intervention group received the handout and activity monitor but also had access to an interactive website. On at least a weekly basis, subjects would connect their monitor to a computer and log their activity through the website. Upon achieving certain thresholds of activity, they received rewards, such as T-shirts and gift cards by mail.
At the beginning and end of the study, participants visited St. Jude for an assessment of their physical fitness (strength, flexibility, and endurance) and neurocognitive measures (attention and memory), as well as health-related quality of life (assessed using the Pediatric Quality of Life Inventory questionnaire).
Results
Seventy-eight cancer survivors completed the 24-week study, 53 of them in the intervention group and 25 in the control group.
MVPA increased by an average of 4.7 minutes per week in the intervention group and decreased by an average of 24.3 minutes per week in the control group.
“In this age group, it is common to see a decrease in physical activity over time, even among healthy kids,” Dr Howell said. “Therefore, we are encouraged that our intervention was successful at maintaining physical activity levels, but a longer program may be needed to create lasting exercise habits.”
In addition to increases in MVPA, the intervention group had the following improvements in fitness:
- Increase in hand grip strength from an average of 19.9 kg to 21.0 kg
- Increase in number of push-ups from an average of 15 to 18
- Increase in number of sit-ups from an average of 11 to 14.
Furthermore, subjects in the intervention group saw their verbal fluency z-score increase by an average of 0.13 points and their general cognition z-score increase by an average of 0.23 points.
Their quality of life scores increased as well. Both overall quality of life and physical-function-related quality of life scores increased from an average of 74.2 to 78.0.
Control subjects had no significant changes in fitness, neurocognitive measures, or quality of life.
This study was supported by the National Cancer Institute, the American Lebanese Syrian Associated Charities, and HopeLab.
Based on the results of this study, the investigators have designed a larger trial (ALTE1631) to test a web-based physical activity intervention. They hope to enroll 384 survivors of childhood acute lymphoblastic leukemia at institutions across the US. The intervention will last a year, with follow-up at 18 months.
Further down the line, the investigators plan to explore the relationship between physical activity and cognition.
ORLANDO—Results of a pilot study suggest a web-based, reward-driven intervention can motivate adolescent cancer survivors to stay physically active.
Time spent performing moderate-to-vigorous physical activity (MVPA) increased by an average of 5 minutes a week for subjects who were randomized to the intervention.
For control subjects, MVPA decreased by an average of 24 minutes a week.
These findings were presented at the 2018 Cancer Survivorship Symposium (abstract 102).
“Compared to the general population, childhood cancer survivors have an increased risk for obesity and metabolic syndrome, conditions that can lead to heart disease, stroke, and diabetes, so it is really important that they are physically active,” said study investigator Carrie R. Howell, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“By intervening in this young age group, we hope to help kids develop healthy exercise habits for life.”
Dr Howell and her colleagues studied cancer survivors, ages 11 to 15, who were no longer receiving cancer treatment and were physically active less than 60 minutes a day.
The subjects were randomized to the intervention or to a control group. Controls received a wearable activity monitor and an educational handout with information about the importance of physical activity and examples of activities.
The intervention group received the handout and activity monitor but also had access to an interactive website. On at least a weekly basis, subjects would connect their monitor to a computer and log their activity through the website. Upon achieving certain thresholds of activity, they received rewards, such as T-shirts and gift cards by mail.
At the beginning and end of the study, participants visited St. Jude for an assessment of their physical fitness (strength, flexibility, and endurance) and neurocognitive measures (attention and memory), as well as health-related quality of life (assessed using the Pediatric Quality of Life Inventory questionnaire).
Results
Seventy-eight cancer survivors completed the 24-week study, 53 of them in the intervention group and 25 in the control group.
MVPA increased by an average of 4.7 minutes per week in the intervention group and decreased by an average of 24.3 minutes per week in the control group.
“In this age group, it is common to see a decrease in physical activity over time, even among healthy kids,” Dr Howell said. “Therefore, we are encouraged that our intervention was successful at maintaining physical activity levels, but a longer program may be needed to create lasting exercise habits.”
In addition to increases in MVPA, the intervention group had the following improvements in fitness:
- Increase in hand grip strength from an average of 19.9 kg to 21.0 kg
- Increase in number of push-ups from an average of 15 to 18
- Increase in number of sit-ups from an average of 11 to 14.
Furthermore, subjects in the intervention group saw their verbal fluency z-score increase by an average of 0.13 points and their general cognition z-score increase by an average of 0.23 points.
Their quality of life scores increased as well. Both overall quality of life and physical-function-related quality of life scores increased from an average of 74.2 to 78.0.
Control subjects had no significant changes in fitness, neurocognitive measures, or quality of life.
This study was supported by the National Cancer Institute, the American Lebanese Syrian Associated Charities, and HopeLab.
Based on the results of this study, the investigators have designed a larger trial (ALTE1631) to test a web-based physical activity intervention. They hope to enroll 384 survivors of childhood acute lymphoblastic leukemia at institutions across the US. The intervention will last a year, with follow-up at 18 months.
Further down the line, the investigators plan to explore the relationship between physical activity and cognition.
Study reveals lack of sexual aids for cancer survivors
ORLANDO—A new study suggests many US cancer centers do not have therapeutic aids for patients who experience sexual dysfunction after cancer treatment.
Of 25 cancer centers polled, 80% said they had no sexual aids available on site for men, and 64% said they had no such aids for women.
Sharon Bober, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and her colleagues presented this research at the 2018 Cancer Survivorship Symposium (abstract 134*).
“[P]roviding sexual aids is one step toward treating sexual health like any other aspect of survivorship care,” Dr Bober said.
“It should be no different than providing wigs and head coverings to women who have lost their hair due to chemotherapy. It’s important to give patients the message that regaining sexual health is a perfectly valid and life-affirming aspect of regaining overall quality of life.”
Dr Bober and her colleagues conducted this study to determine the availability of sexual aids at 25 National Cancer Institute-designated cancer centers.
The researchers called these centers posing as a spouse, adult child, or sibling of a patient. The team made separate calls to ask about sexual aids for women and those for men.
Women’s sexual aids
Twenty-four percent of cancer centers (n=6) said they had sexual aids for women, 64% (n=16) did not, and 12% of centers were unreachable (n=3).
The most common aids were personal lubrication, vaginal moisturizer, and vaginal dilators—all of which were available at 5 centers.
Three centers had vibrators, 2 had books/pamphlets, 2 had pelvic floor exercisers, and 2 had product lists.
Men’s sexual aids
Twelve percent of cancer centers (n=3) said they had sexual aids for men, 80% (n=20) did not, and 8% (n=2) were unreachable.
Two centers said they had personal lubrication available for men, 2 had penile support rings, 1 had vacuum erection devices, and 1 had books/pamphlets.
Next steps
Now, Dr Bober and her colleagues hope to query the other 44 National Cancer Institute-designated cancer centers to see what products they are selling and perhaps conduct patient surveys to find out what types of resources are most useful for cancer survivors.
“What we really need to do is go to the centers that are successfully providing sexual health products and find out how they promote and provide resources to their patients,” Dr Bober said.
“We can’t keep the conversation at the 10,000-foot level. We need to talk concretely about how to partner with providers to make sexual health resources, including sexual health aids, available so cancer survivors can get the help that they need.”
*Information presented differs from the abstract.
ORLANDO—A new study suggests many US cancer centers do not have therapeutic aids for patients who experience sexual dysfunction after cancer treatment.
Of 25 cancer centers polled, 80% said they had no sexual aids available on site for men, and 64% said they had no such aids for women.
Sharon Bober, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and her colleagues presented this research at the 2018 Cancer Survivorship Symposium (abstract 134*).
“[P]roviding sexual aids is one step toward treating sexual health like any other aspect of survivorship care,” Dr Bober said.
“It should be no different than providing wigs and head coverings to women who have lost their hair due to chemotherapy. It’s important to give patients the message that regaining sexual health is a perfectly valid and life-affirming aspect of regaining overall quality of life.”
Dr Bober and her colleagues conducted this study to determine the availability of sexual aids at 25 National Cancer Institute-designated cancer centers.
The researchers called these centers posing as a spouse, adult child, or sibling of a patient. The team made separate calls to ask about sexual aids for women and those for men.
Women’s sexual aids
Twenty-four percent of cancer centers (n=6) said they had sexual aids for women, 64% (n=16) did not, and 12% of centers were unreachable (n=3).
The most common aids were personal lubrication, vaginal moisturizer, and vaginal dilators—all of which were available at 5 centers.
Three centers had vibrators, 2 had books/pamphlets, 2 had pelvic floor exercisers, and 2 had product lists.
Men’s sexual aids
Twelve percent of cancer centers (n=3) said they had sexual aids for men, 80% (n=20) did not, and 8% (n=2) were unreachable.
Two centers said they had personal lubrication available for men, 2 had penile support rings, 1 had vacuum erection devices, and 1 had books/pamphlets.
Next steps
Now, Dr Bober and her colleagues hope to query the other 44 National Cancer Institute-designated cancer centers to see what products they are selling and perhaps conduct patient surveys to find out what types of resources are most useful for cancer survivors.
“What we really need to do is go to the centers that are successfully providing sexual health products and find out how they promote and provide resources to their patients,” Dr Bober said.
“We can’t keep the conversation at the 10,000-foot level. We need to talk concretely about how to partner with providers to make sexual health resources, including sexual health aids, available so cancer survivors can get the help that they need.”
*Information presented differs from the abstract.
ORLANDO—A new study suggests many US cancer centers do not have therapeutic aids for patients who experience sexual dysfunction after cancer treatment.
Of 25 cancer centers polled, 80% said they had no sexual aids available on site for men, and 64% said they had no such aids for women.
Sharon Bober, PhD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and her colleagues presented this research at the 2018 Cancer Survivorship Symposium (abstract 134*).
“[P]roviding sexual aids is one step toward treating sexual health like any other aspect of survivorship care,” Dr Bober said.
“It should be no different than providing wigs and head coverings to women who have lost their hair due to chemotherapy. It’s important to give patients the message that regaining sexual health is a perfectly valid and life-affirming aspect of regaining overall quality of life.”
Dr Bober and her colleagues conducted this study to determine the availability of sexual aids at 25 National Cancer Institute-designated cancer centers.
The researchers called these centers posing as a spouse, adult child, or sibling of a patient. The team made separate calls to ask about sexual aids for women and those for men.
Women’s sexual aids
Twenty-four percent of cancer centers (n=6) said they had sexual aids for women, 64% (n=16) did not, and 12% of centers were unreachable (n=3).
The most common aids were personal lubrication, vaginal moisturizer, and vaginal dilators—all of which were available at 5 centers.
Three centers had vibrators, 2 had books/pamphlets, 2 had pelvic floor exercisers, and 2 had product lists.
Men’s sexual aids
Twelve percent of cancer centers (n=3) said they had sexual aids for men, 80% (n=20) did not, and 8% (n=2) were unreachable.
Two centers said they had personal lubrication available for men, 2 had penile support rings, 1 had vacuum erection devices, and 1 had books/pamphlets.
Next steps
Now, Dr Bober and her colleagues hope to query the other 44 National Cancer Institute-designated cancer centers to see what products they are selling and perhaps conduct patient surveys to find out what types of resources are most useful for cancer survivors.
“What we really need to do is go to the centers that are successfully providing sexual health products and find out how they promote and provide resources to their patients,” Dr Bober said.
“We can’t keep the conversation at the 10,000-foot level. We need to talk concretely about how to partner with providers to make sexual health resources, including sexual health aids, available so cancer survivors can get the help that they need.”
*Information presented differs from the abstract.
ASCO, NCCN release guidelines on checkpoint inhibitors
Two cancer organizations have released guidelines for managing the side effects of immune checkpoint inhibitors.
The American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network® (NCCN) developed these guidelines because patients who receive immune checkpoint inhibitors experience unique side effects that can be severe, irreversible, and life-threatening.
Given that checkpoint inhibitors have entered the clinic fairly recently, clinicians may need guidance in recognizing and treating these side effects.
“With rapidly increasing use of immune checkpoint inhibitors, it is imperative that clinicians are knowledgeable about their unique toxicity profiles,” said ASCO Chief Executive Officer Clifford A. Hudis, MD.
“These new guidelines from ASCO and NCCN will help our community continue to provide the highest quality of care to all patients as they incorporate these agents into routine care.”
To the develop their guidelines, ASCO and NCCN convened multidisciplinary panels with representation from hematology, oncology, dermatology, gastroenterology, rheumatology, pulmonology, endocrinology, urology, neurology, emergency medicine, and nursing, as well as patient advocacy experts.
The clinical recommendations are based on a systematic review of the literature and an informal consensus process. The recommendations pertain only to checkpoint inhibitors currently approved in the US—pembrolizumab, nivolumab, atezolizumab, avelumab, ipilimumab, and durvalumab.
Key recommendations from the guidelines include:
- In general, checkpoint inhibitors can be continued with close monitoring if patients experience grade 1 toxicities, with the exception of some neurologic, cardiac, and hematologic toxicities.
- For grade 2 toxicities, checkpoint inhibitors should be held until symptoms and/or lab values revert to grade 1 levels or lower. Corticosteroids may be offered.
- For grade 3 toxicities, patients should receive high-dose corticosteroids for at least 6 weeks. Extreme caution is recommended when restarting immunotherapy after grade 3 toxicity, if it is restarted at all.
- In general, grade 4 toxicities necessitate stopping checkpoint inhibitor therapy permanently.
Consult the guidelines for specific recommendations.
ASCO’s guidelines, “Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline,” have been published in the Journal of Clinical Oncology.
NCCN’s guidelines, “Management of Immunotherapy-Related Toxicities (Immune Checkpoint Inhibitor-Related Toxicities),” are available on the NCCN website.
Two cancer organizations have released guidelines for managing the side effects of immune checkpoint inhibitors.
The American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network® (NCCN) developed these guidelines because patients who receive immune checkpoint inhibitors experience unique side effects that can be severe, irreversible, and life-threatening.
Given that checkpoint inhibitors have entered the clinic fairly recently, clinicians may need guidance in recognizing and treating these side effects.
“With rapidly increasing use of immune checkpoint inhibitors, it is imperative that clinicians are knowledgeable about their unique toxicity profiles,” said ASCO Chief Executive Officer Clifford A. Hudis, MD.
“These new guidelines from ASCO and NCCN will help our community continue to provide the highest quality of care to all patients as they incorporate these agents into routine care.”
To the develop their guidelines, ASCO and NCCN convened multidisciplinary panels with representation from hematology, oncology, dermatology, gastroenterology, rheumatology, pulmonology, endocrinology, urology, neurology, emergency medicine, and nursing, as well as patient advocacy experts.
The clinical recommendations are based on a systematic review of the literature and an informal consensus process. The recommendations pertain only to checkpoint inhibitors currently approved in the US—pembrolizumab, nivolumab, atezolizumab, avelumab, ipilimumab, and durvalumab.
Key recommendations from the guidelines include:
- In general, checkpoint inhibitors can be continued with close monitoring if patients experience grade 1 toxicities, with the exception of some neurologic, cardiac, and hematologic toxicities.
- For grade 2 toxicities, checkpoint inhibitors should be held until symptoms and/or lab values revert to grade 1 levels or lower. Corticosteroids may be offered.
- For grade 3 toxicities, patients should receive high-dose corticosteroids for at least 6 weeks. Extreme caution is recommended when restarting immunotherapy after grade 3 toxicity, if it is restarted at all.
- In general, grade 4 toxicities necessitate stopping checkpoint inhibitor therapy permanently.
Consult the guidelines for specific recommendations.
ASCO’s guidelines, “Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline,” have been published in the Journal of Clinical Oncology.
NCCN’s guidelines, “Management of Immunotherapy-Related Toxicities (Immune Checkpoint Inhibitor-Related Toxicities),” are available on the NCCN website.
Two cancer organizations have released guidelines for managing the side effects of immune checkpoint inhibitors.
The American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network® (NCCN) developed these guidelines because patients who receive immune checkpoint inhibitors experience unique side effects that can be severe, irreversible, and life-threatening.
Given that checkpoint inhibitors have entered the clinic fairly recently, clinicians may need guidance in recognizing and treating these side effects.
“With rapidly increasing use of immune checkpoint inhibitors, it is imperative that clinicians are knowledgeable about their unique toxicity profiles,” said ASCO Chief Executive Officer Clifford A. Hudis, MD.
“These new guidelines from ASCO and NCCN will help our community continue to provide the highest quality of care to all patients as they incorporate these agents into routine care.”
To the develop their guidelines, ASCO and NCCN convened multidisciplinary panels with representation from hematology, oncology, dermatology, gastroenterology, rheumatology, pulmonology, endocrinology, urology, neurology, emergency medicine, and nursing, as well as patient advocacy experts.
The clinical recommendations are based on a systematic review of the literature and an informal consensus process. The recommendations pertain only to checkpoint inhibitors currently approved in the US—pembrolizumab, nivolumab, atezolizumab, avelumab, ipilimumab, and durvalumab.
Key recommendations from the guidelines include:
- In general, checkpoint inhibitors can be continued with close monitoring if patients experience grade 1 toxicities, with the exception of some neurologic, cardiac, and hematologic toxicities.
- For grade 2 toxicities, checkpoint inhibitors should be held until symptoms and/or lab values revert to grade 1 levels or lower. Corticosteroids may be offered.
- For grade 3 toxicities, patients should receive high-dose corticosteroids for at least 6 weeks. Extreme caution is recommended when restarting immunotherapy after grade 3 toxicity, if it is restarted at all.
- In general, grade 4 toxicities necessitate stopping checkpoint inhibitor therapy permanently.
Consult the guidelines for specific recommendations.
ASCO’s guidelines, “Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline,” have been published in the Journal of Clinical Oncology.
NCCN’s guidelines, “Management of Immunotherapy-Related Toxicities (Immune Checkpoint Inhibitor-Related Toxicities),” are available on the NCCN website.