User login
Hospital Rankings for Stroke Outcomes Must Consider Stroke Severity
Rankings of hospital performance in treating acute ischemic stroke must take into consideration the severity of each case, or the rankings will be extremely inaccurate, researchers say in the July 18 issue of JAMA.
Unfortunately, the rankings that are currently used by accreditation organizations, the Centers for Medicare and Medicaid Services (CMS), and other payers do not incorporate stroke severity. A study that corrected for this oversight found that close to half of the U.S. hospitals ranked in the top or bottom 5%, according to stroke patients’ 30-day mortality, should be reclassified into the middle range of the rankings.
For the 782 hospitals included in this study, the median change in rank position was 79 places when stroke severity was incorporated into the statistical model, said Dr. Gregg C. Fonarow of the Ahmanson-UCLA Cardiomyopathy Center, Los Angeles, and his associates.
When stroke severity is not considered, the rankings systematically favor hospitals that care for patients with less severe illness, regardless of whether the patient care at these hospitals produces better or worse patient outcomes.
If reliance on inaccurate rankings persists, hospitals that want to improve their ranking may consider turning away patients with more severe strokes or transferring them to other hospitals after emergency department assessment, to avoid being classified as low performance, Dr. Fonarow and his associates said.
The researchers used data from the Get With the Guidelines–Stroke Registry and from CMS inpatient claims files to create a risk-adjustment model that incorporated stroke severity, as measured by the NIHSS (National Institutes of Health Stroke Scale), into hospitals’ 30-day mortality profiling. The NIHSS is a 15-item scale that assesses the effect of acute stroke on level of consciousness, language, neglect, visual-field loss, extraocular movement, motor strength, ataxia, dysarthria, and sensory loss.
All types of hospitals in all regions of the United States were represented. The study population included 127,950 patients aged 65 years and older who had acute ischemic stroke and were treated at 782 hospitals participating in the Get With the Guidelines program from April 2003 to December 2009.
The median patient age was 80 years; 57% were women and 86% were white. Patients frequently had serious comorbidities, including hypertension (83%), diabetes (29%), coronary artery disease or prior myocardial infarction (34%), and a history of atrial fibrillation or flutter (27%).
There were 18,186 deaths within 30 days of admission.
The statistical model that incorporated stroke severity into its assessment of hospital performance "demonstrated substantially more accurate classification of hospital 30-day mortality" than did the model currently in use. When hospitals were ranked according to this more accurate profile, their rank position changed by a median of 79 places.
Overall, 206 of the 782 hospitals (26%) ended up in a different performance category once the NIHSS score was incorporated into the model.
Of the 39 hospitals that had been categorized as top performers using the standard model, only 23 remained top performers using the more accurate model. And another 16 hospitals that hadn’t made the grade with the standard model were reclassified as top performers with the more accurate model.
"There was even greater disagreement about the bottom-performing hospitals," Dr. Fonarow and his colleagues said (JAMA 2012;308:257-64).
Of the 40 worst-performing hospitals according to the standard model, nearly half (19) were reclassified as having a middling performance with the more accurate model.
"These findings highlight the importance of including a valid specific measure of stroke severity in hospital risk models for mortality after acute ischemic stroke. ... Furthermore, this study suggests that inclusion of admission stroke severity may be essential for optimal ranking of hospitals with respect to 30-day mortality," they said.
This study was supported by the American Heart Association, American Stroke Association, and Janssen Pharmaceutical Companies of Johnson & Johnson. Dr. Fonarow is an employee of the University of California, which holds a patent on retriever devices for stroke. His associates reported ties to numerous industry sources.
Stroke Severity Is a Key Consideration
In an editorial accompanying Dr. Fonarow’s report, Dr. Tobias Kurth and Dr. Mitchell S.V. Elkind said that the study "clearly highlights the importance of incorporating information on stroke severity when conducting health outcomes research in stroke. Excluding this information will lead to incorrect ranking of hospital performance by failing to consider that hospitals care for different patient populations" (JAMA 2012;308:292-4).
In this study, when considering only the hospitals ranked in the best 20% and worst 20% of the total, close to one-third would have been reclassified if the more accurate statistical model incorporating stroke severity had been used, they noted.
Dr. Kurth is in neuroepidemiology at the University of Bordeaux (France). Dr. Elkind is in the department of neurology in the school of medicine and the department of epidemiology in the school of public health at Columbia University, New York. Dr. Kurth reported ties to Allergan, Merck, and MAP Pharmaceuticals, and Dr. Elkind reported ties to diaDexus, Britol-Myers Squibb/Sanofi Pharmaceuticals Partnership, Novartis, Organon, and GlaxoSmithKline.
Dr. Gregg C. Fonarow
The study by Dr. Fonarow and colleagues "clearly highlights the importance of incorporating information on stroke severity when conducting health outcomes research in stroke. Excluding this information will lead to incorrect ranking of hospital performance by failing to consider that hospitals care for different patient populations," said Dr. Tobias Kurth and Dr. Mitchell S.V. Elkind.
In this study, when considering only the hospitals ranked in the best 20% and worst 20% of the total, close to one-third would have been reclassified if the more accurate statistical model incorporating stroke severity had been used, they noted.
Dr. Kurth is in neuroepidemiology at the University of Bordeaux (France). Dr. Elkind is in the department of neurology in the school of medicine and the department of epidemiology in the school of public health at Columbia University, New York. Dr. Kurth reported ties to Allergan, Merck, and MAP Pharmaceuticals, and Dr. Elkind reported ties to diaDexus, Britol-Myers Squibb/Sanofi Pharmaceuticals Partnership, Novartis, Organon, and GlaxoSmithKline. These remarks were taken from their editorial accompanying Dr. Fonarow’s report (JAMA 2012;308:292-4).
The study by Dr. Fonarow and colleagues "clearly highlights the importance of incorporating information on stroke severity when conducting health outcomes research in stroke. Excluding this information will lead to incorrect ranking of hospital performance by failing to consider that hospitals care for different patient populations," said Dr. Tobias Kurth and Dr. Mitchell S.V. Elkind.
In this study, when considering only the hospitals ranked in the best 20% and worst 20% of the total, close to one-third would have been reclassified if the more accurate statistical model incorporating stroke severity had been used, they noted.
Dr. Kurth is in neuroepidemiology at the University of Bordeaux (France). Dr. Elkind is in the department of neurology in the school of medicine and the department of epidemiology in the school of public health at Columbia University, New York. Dr. Kurth reported ties to Allergan, Merck, and MAP Pharmaceuticals, and Dr. Elkind reported ties to diaDexus, Britol-Myers Squibb/Sanofi Pharmaceuticals Partnership, Novartis, Organon, and GlaxoSmithKline. These remarks were taken from their editorial accompanying Dr. Fonarow’s report (JAMA 2012;308:292-4).
The study by Dr. Fonarow and colleagues "clearly highlights the importance of incorporating information on stroke severity when conducting health outcomes research in stroke. Excluding this information will lead to incorrect ranking of hospital performance by failing to consider that hospitals care for different patient populations," said Dr. Tobias Kurth and Dr. Mitchell S.V. Elkind.
In this study, when considering only the hospitals ranked in the best 20% and worst 20% of the total, close to one-third would have been reclassified if the more accurate statistical model incorporating stroke severity had been used, they noted.
Dr. Kurth is in neuroepidemiology at the University of Bordeaux (France). Dr. Elkind is in the department of neurology in the school of medicine and the department of epidemiology in the school of public health at Columbia University, New York. Dr. Kurth reported ties to Allergan, Merck, and MAP Pharmaceuticals, and Dr. Elkind reported ties to diaDexus, Britol-Myers Squibb/Sanofi Pharmaceuticals Partnership, Novartis, Organon, and GlaxoSmithKline. These remarks were taken from their editorial accompanying Dr. Fonarow’s report (JAMA 2012;308:292-4).
Rankings of hospital performance in treating acute ischemic stroke must take into consideration the severity of each case, or the rankings will be extremely inaccurate, researchers say in the July 18 issue of JAMA.
Unfortunately, the rankings that are currently used by accreditation organizations, the Centers for Medicare and Medicaid Services (CMS), and other payers do not incorporate stroke severity. A study that corrected for this oversight found that close to half of the U.S. hospitals ranked in the top or bottom 5%, according to stroke patients’ 30-day mortality, should be reclassified into the middle range of the rankings.
For the 782 hospitals included in this study, the median change in rank position was 79 places when stroke severity was incorporated into the statistical model, said Dr. Gregg C. Fonarow of the Ahmanson-UCLA Cardiomyopathy Center, Los Angeles, and his associates.
When stroke severity is not considered, the rankings systematically favor hospitals that care for patients with less severe illness, regardless of whether the patient care at these hospitals produces better or worse patient outcomes.
If reliance on inaccurate rankings persists, hospitals that want to improve their ranking may consider turning away patients with more severe strokes or transferring them to other hospitals after emergency department assessment, to avoid being classified as low performance, Dr. Fonarow and his associates said.
The researchers used data from the Get With the Guidelines–Stroke Registry and from CMS inpatient claims files to create a risk-adjustment model that incorporated stroke severity, as measured by the NIHSS (National Institutes of Health Stroke Scale), into hospitals’ 30-day mortality profiling. The NIHSS is a 15-item scale that assesses the effect of acute stroke on level of consciousness, language, neglect, visual-field loss, extraocular movement, motor strength, ataxia, dysarthria, and sensory loss.
All types of hospitals in all regions of the United States were represented. The study population included 127,950 patients aged 65 years and older who had acute ischemic stroke and were treated at 782 hospitals participating in the Get With the Guidelines program from April 2003 to December 2009.
The median patient age was 80 years; 57% were women and 86% were white. Patients frequently had serious comorbidities, including hypertension (83%), diabetes (29%), coronary artery disease or prior myocardial infarction (34%), and a history of atrial fibrillation or flutter (27%).
There were 18,186 deaths within 30 days of admission.
The statistical model that incorporated stroke severity into its assessment of hospital performance "demonstrated substantially more accurate classification of hospital 30-day mortality" than did the model currently in use. When hospitals were ranked according to this more accurate profile, their rank position changed by a median of 79 places.
Overall, 206 of the 782 hospitals (26%) ended up in a different performance category once the NIHSS score was incorporated into the model.
Of the 39 hospitals that had been categorized as top performers using the standard model, only 23 remained top performers using the more accurate model. And another 16 hospitals that hadn’t made the grade with the standard model were reclassified as top performers with the more accurate model.
"There was even greater disagreement about the bottom-performing hospitals," Dr. Fonarow and his colleagues said (JAMA 2012;308:257-64).
Of the 40 worst-performing hospitals according to the standard model, nearly half (19) were reclassified as having a middling performance with the more accurate model.
"These findings highlight the importance of including a valid specific measure of stroke severity in hospital risk models for mortality after acute ischemic stroke. ... Furthermore, this study suggests that inclusion of admission stroke severity may be essential for optimal ranking of hospitals with respect to 30-day mortality," they said.
This study was supported by the American Heart Association, American Stroke Association, and Janssen Pharmaceutical Companies of Johnson & Johnson. Dr. Fonarow is an employee of the University of California, which holds a patent on retriever devices for stroke. His associates reported ties to numerous industry sources.
Stroke Severity Is a Key Consideration
In an editorial accompanying Dr. Fonarow’s report, Dr. Tobias Kurth and Dr. Mitchell S.V. Elkind said that the study "clearly highlights the importance of incorporating information on stroke severity when conducting health outcomes research in stroke. Excluding this information will lead to incorrect ranking of hospital performance by failing to consider that hospitals care for different patient populations" (JAMA 2012;308:292-4).
In this study, when considering only the hospitals ranked in the best 20% and worst 20% of the total, close to one-third would have been reclassified if the more accurate statistical model incorporating stroke severity had been used, they noted.
Dr. Kurth is in neuroepidemiology at the University of Bordeaux (France). Dr. Elkind is in the department of neurology in the school of medicine and the department of epidemiology in the school of public health at Columbia University, New York. Dr. Kurth reported ties to Allergan, Merck, and MAP Pharmaceuticals, and Dr. Elkind reported ties to diaDexus, Britol-Myers Squibb/Sanofi Pharmaceuticals Partnership, Novartis, Organon, and GlaxoSmithKline.
Dr. Gregg C. Fonarow
Rankings of hospital performance in treating acute ischemic stroke must take into consideration the severity of each case, or the rankings will be extremely inaccurate, researchers say in the July 18 issue of JAMA.
Unfortunately, the rankings that are currently used by accreditation organizations, the Centers for Medicare and Medicaid Services (CMS), and other payers do not incorporate stroke severity. A study that corrected for this oversight found that close to half of the U.S. hospitals ranked in the top or bottom 5%, according to stroke patients’ 30-day mortality, should be reclassified into the middle range of the rankings.
For the 782 hospitals included in this study, the median change in rank position was 79 places when stroke severity was incorporated into the statistical model, said Dr. Gregg C. Fonarow of the Ahmanson-UCLA Cardiomyopathy Center, Los Angeles, and his associates.
When stroke severity is not considered, the rankings systematically favor hospitals that care for patients with less severe illness, regardless of whether the patient care at these hospitals produces better or worse patient outcomes.
If reliance on inaccurate rankings persists, hospitals that want to improve their ranking may consider turning away patients with more severe strokes or transferring them to other hospitals after emergency department assessment, to avoid being classified as low performance, Dr. Fonarow and his associates said.
The researchers used data from the Get With the Guidelines–Stroke Registry and from CMS inpatient claims files to create a risk-adjustment model that incorporated stroke severity, as measured by the NIHSS (National Institutes of Health Stroke Scale), into hospitals’ 30-day mortality profiling. The NIHSS is a 15-item scale that assesses the effect of acute stroke on level of consciousness, language, neglect, visual-field loss, extraocular movement, motor strength, ataxia, dysarthria, and sensory loss.
All types of hospitals in all regions of the United States were represented. The study population included 127,950 patients aged 65 years and older who had acute ischemic stroke and were treated at 782 hospitals participating in the Get With the Guidelines program from April 2003 to December 2009.
The median patient age was 80 years; 57% were women and 86% were white. Patients frequently had serious comorbidities, including hypertension (83%), diabetes (29%), coronary artery disease or prior myocardial infarction (34%), and a history of atrial fibrillation or flutter (27%).
There were 18,186 deaths within 30 days of admission.
The statistical model that incorporated stroke severity into its assessment of hospital performance "demonstrated substantially more accurate classification of hospital 30-day mortality" than did the model currently in use. When hospitals were ranked according to this more accurate profile, their rank position changed by a median of 79 places.
Overall, 206 of the 782 hospitals (26%) ended up in a different performance category once the NIHSS score was incorporated into the model.
Of the 39 hospitals that had been categorized as top performers using the standard model, only 23 remained top performers using the more accurate model. And another 16 hospitals that hadn’t made the grade with the standard model were reclassified as top performers with the more accurate model.
"There was even greater disagreement about the bottom-performing hospitals," Dr. Fonarow and his colleagues said (JAMA 2012;308:257-64).
Of the 40 worst-performing hospitals according to the standard model, nearly half (19) were reclassified as having a middling performance with the more accurate model.
"These findings highlight the importance of including a valid specific measure of stroke severity in hospital risk models for mortality after acute ischemic stroke. ... Furthermore, this study suggests that inclusion of admission stroke severity may be essential for optimal ranking of hospitals with respect to 30-day mortality," they said.
This study was supported by the American Heart Association, American Stroke Association, and Janssen Pharmaceutical Companies of Johnson & Johnson. Dr. Fonarow is an employee of the University of California, which holds a patent on retriever devices for stroke. His associates reported ties to numerous industry sources.
Stroke Severity Is a Key Consideration
In an editorial accompanying Dr. Fonarow’s report, Dr. Tobias Kurth and Dr. Mitchell S.V. Elkind said that the study "clearly highlights the importance of incorporating information on stroke severity when conducting health outcomes research in stroke. Excluding this information will lead to incorrect ranking of hospital performance by failing to consider that hospitals care for different patient populations" (JAMA 2012;308:292-4).
In this study, when considering only the hospitals ranked in the best 20% and worst 20% of the total, close to one-third would have been reclassified if the more accurate statistical model incorporating stroke severity had been used, they noted.
Dr. Kurth is in neuroepidemiology at the University of Bordeaux (France). Dr. Elkind is in the department of neurology in the school of medicine and the department of epidemiology in the school of public health at Columbia University, New York. Dr. Kurth reported ties to Allergan, Merck, and MAP Pharmaceuticals, and Dr. Elkind reported ties to diaDexus, Britol-Myers Squibb/Sanofi Pharmaceuticals Partnership, Novartis, Organon, and GlaxoSmithKline.
Dr. Gregg C. Fonarow
FROM JAMA
EMS Prenotification of Hospitals Lags for Incoming Stroke Patients
When emergency medical services personnel alert hospitals of incoming stroke patients, evaluation and treatment are improved, but prenotification occurs in only about two-thirds of cases, according to findings from two new American Heart Association/American Stroke Association (AHA/ASA) studies.
Both of the Get With The Guidelines–Stroke program studies involved the same group of nearly 372,000 patients with acute ischemic stroke, who were transported by emergency medical services to one of 1,585 participating hospitals between April 2003 and April 2011. One of the studies showed that compared with no notification, prenotification of an incoming stroke patient was associated with significantly more rapid evaluation and treatment, and with a significantly greater likelihood of treatment with tissue plasminogen activator (TPA) within 3 hours, Cheryl B. Lin of the Duke–National University of Singapore Graduate Medical School, Singapore, and her colleagues reported online in Circulation: Cardiovascular Quality and Outcomes.
For example, among patients who arrived at the hospital within 3 hours of symptom onset, median door-to-imaging time was 26 minutes, compared with 31 minutes for those without prenotification. Door-to-imaging time was within 25 minutes for 48.8% and 40.5% of those with and without prenotification, respectively. Also, symptom onset–to-door times were lower with prenotification (113 vs. 150 minutes) (Circ. Cardiovasc. Qual. Outcomes 2012 July 10 [doi: 10.1161/circoutcomes.112.965210]).
Prenotification also significantly improved door-to-needle time and symptom onset–to-needle time, and more eligible patients who arrived at the hospital within 2 hours were treated with TPA within 3 hours (71.8% vs. 62.2%).
The problem is that prenotification occurred in only 67% of cases, the authors said.
"Our analysis supports the role [of EMS prenotification] as a potentially important but underused means to improving rapid triage, evaluation, and treatment of patients with acute ischemic stroke," they wrote, noting that although prenotification is recommended in guidelines from both the AHA/ ASA and the National Association of Emergency Medical Services Physicians, it appears many hospitals "still find difficulty in meeting these performance goals."
In the related study published online in the Journal of the American Heart Association, Ms. Lin and her colleagues found that prenotification varied widely by hospital and region, with rates of prenotification ranging from 0% to 100%.
In Washington, D.C., for example, the prenotification rate was 19.7%, compared with 93.4% in Montana, the investigators said (J. Am. Heart Assoc. 2012 July 10 [doi: 10.1161/jaha.112.002345]).
Patient factors associated with increased likelihood of prenotification were younger age, white race, past history of atrial fibrillation, no medical history of previous stroke or transient ischemic attack, diabetes mellitus, and peripheral vascular disease.
"In particular, black patients had decreased odds [of EMS prenotification] when compared to their white counterparts, with adjusted odds ratio of 0.94," the investigators noted.
Hospital factors associated with reduced likelihood of prenotification were academic affiliation, location in the northeastern United States, and lower annual volume of TPA administration.
Rates of prenotification did increase modestly and significantly over time, from 58% to 67% between 2003 and 2011, with a high of 71.1% in 2008, followed by a decline to about 65% in 2009 and 2010, and an increase to 67.3% in 2011, but targeted improvements in rates of EMS prenotification are needed, they said.
"These findings demonstrate gaps in the quality of stroke care provided and support the need for initiatives targeted to improve [EMS prenotification rates] on a national level," they concluded, explaining that a systems approach is needed involving increasing symptom recognition and rapid activation of EMS, adequate training of EMS staff in proper use of stroke-screening instruments and the need for hospital prenotification, and implementation of systems of care in receiving hospitals.
A stroke system-of-care process measure reporting the use of EMS prenotification should be considered, they said.
The Get With the Guidelines (GWTG)–Stroke program is provided by the AHA/ASA and is supported in part by a charitable contribution from the Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership and the AHA Pharmaceutical Roundtable. Past support has been provided by Boehringer Ingelheim and Merck. Ms. Lin reported having no relevant conflicts of interest. Several coauthors have worked with GWTG committees, and some have received research grant support from pharmaceutical companies. Some researchers are employees of the University of California, which holds a patent on retriever devices for stroke.
When emergency medical services personnel alert hospitals of incoming stroke patients, evaluation and treatment are improved, but prenotification occurs in only about two-thirds of cases, according to findings from two new American Heart Association/American Stroke Association (AHA/ASA) studies.
Both of the Get With The Guidelines–Stroke program studies involved the same group of nearly 372,000 patients with acute ischemic stroke, who were transported by emergency medical services to one of 1,585 participating hospitals between April 2003 and April 2011. One of the studies showed that compared with no notification, prenotification of an incoming stroke patient was associated with significantly more rapid evaluation and treatment, and with a significantly greater likelihood of treatment with tissue plasminogen activator (TPA) within 3 hours, Cheryl B. Lin of the Duke–National University of Singapore Graduate Medical School, Singapore, and her colleagues reported online in Circulation: Cardiovascular Quality and Outcomes.
For example, among patients who arrived at the hospital within 3 hours of symptom onset, median door-to-imaging time was 26 minutes, compared with 31 minutes for those without prenotification. Door-to-imaging time was within 25 minutes for 48.8% and 40.5% of those with and without prenotification, respectively. Also, symptom onset–to-door times were lower with prenotification (113 vs. 150 minutes) (Circ. Cardiovasc. Qual. Outcomes 2012 July 10 [doi: 10.1161/circoutcomes.112.965210]).
Prenotification also significantly improved door-to-needle time and symptom onset–to-needle time, and more eligible patients who arrived at the hospital within 2 hours were treated with TPA within 3 hours (71.8% vs. 62.2%).
The problem is that prenotification occurred in only 67% of cases, the authors said.
"Our analysis supports the role [of EMS prenotification] as a potentially important but underused means to improving rapid triage, evaluation, and treatment of patients with acute ischemic stroke," they wrote, noting that although prenotification is recommended in guidelines from both the AHA/ ASA and the National Association of Emergency Medical Services Physicians, it appears many hospitals "still find difficulty in meeting these performance goals."
In the related study published online in the Journal of the American Heart Association, Ms. Lin and her colleagues found that prenotification varied widely by hospital and region, with rates of prenotification ranging from 0% to 100%.
In Washington, D.C., for example, the prenotification rate was 19.7%, compared with 93.4% in Montana, the investigators said (J. Am. Heart Assoc. 2012 July 10 [doi: 10.1161/jaha.112.002345]).
Patient factors associated with increased likelihood of prenotification were younger age, white race, past history of atrial fibrillation, no medical history of previous stroke or transient ischemic attack, diabetes mellitus, and peripheral vascular disease.
"In particular, black patients had decreased odds [of EMS prenotification] when compared to their white counterparts, with adjusted odds ratio of 0.94," the investigators noted.
Hospital factors associated with reduced likelihood of prenotification were academic affiliation, location in the northeastern United States, and lower annual volume of TPA administration.
Rates of prenotification did increase modestly and significantly over time, from 58% to 67% between 2003 and 2011, with a high of 71.1% in 2008, followed by a decline to about 65% in 2009 and 2010, and an increase to 67.3% in 2011, but targeted improvements in rates of EMS prenotification are needed, they said.
"These findings demonstrate gaps in the quality of stroke care provided and support the need for initiatives targeted to improve [EMS prenotification rates] on a national level," they concluded, explaining that a systems approach is needed involving increasing symptom recognition and rapid activation of EMS, adequate training of EMS staff in proper use of stroke-screening instruments and the need for hospital prenotification, and implementation of systems of care in receiving hospitals.
A stroke system-of-care process measure reporting the use of EMS prenotification should be considered, they said.
The Get With the Guidelines (GWTG)–Stroke program is provided by the AHA/ASA and is supported in part by a charitable contribution from the Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership and the AHA Pharmaceutical Roundtable. Past support has been provided by Boehringer Ingelheim and Merck. Ms. Lin reported having no relevant conflicts of interest. Several coauthors have worked with GWTG committees, and some have received research grant support from pharmaceutical companies. Some researchers are employees of the University of California, which holds a patent on retriever devices for stroke.
When emergency medical services personnel alert hospitals of incoming stroke patients, evaluation and treatment are improved, but prenotification occurs in only about two-thirds of cases, according to findings from two new American Heart Association/American Stroke Association (AHA/ASA) studies.
Both of the Get With The Guidelines–Stroke program studies involved the same group of nearly 372,000 patients with acute ischemic stroke, who were transported by emergency medical services to one of 1,585 participating hospitals between April 2003 and April 2011. One of the studies showed that compared with no notification, prenotification of an incoming stroke patient was associated with significantly more rapid evaluation and treatment, and with a significantly greater likelihood of treatment with tissue plasminogen activator (TPA) within 3 hours, Cheryl B. Lin of the Duke–National University of Singapore Graduate Medical School, Singapore, and her colleagues reported online in Circulation: Cardiovascular Quality and Outcomes.
For example, among patients who arrived at the hospital within 3 hours of symptom onset, median door-to-imaging time was 26 minutes, compared with 31 minutes for those without prenotification. Door-to-imaging time was within 25 minutes for 48.8% and 40.5% of those with and without prenotification, respectively. Also, symptom onset–to-door times were lower with prenotification (113 vs. 150 minutes) (Circ. Cardiovasc. Qual. Outcomes 2012 July 10 [doi: 10.1161/circoutcomes.112.965210]).
Prenotification also significantly improved door-to-needle time and symptom onset–to-needle time, and more eligible patients who arrived at the hospital within 2 hours were treated with TPA within 3 hours (71.8% vs. 62.2%).
The problem is that prenotification occurred in only 67% of cases, the authors said.
"Our analysis supports the role [of EMS prenotification] as a potentially important but underused means to improving rapid triage, evaluation, and treatment of patients with acute ischemic stroke," they wrote, noting that although prenotification is recommended in guidelines from both the AHA/ ASA and the National Association of Emergency Medical Services Physicians, it appears many hospitals "still find difficulty in meeting these performance goals."
In the related study published online in the Journal of the American Heart Association, Ms. Lin and her colleagues found that prenotification varied widely by hospital and region, with rates of prenotification ranging from 0% to 100%.
In Washington, D.C., for example, the prenotification rate was 19.7%, compared with 93.4% in Montana, the investigators said (J. Am. Heart Assoc. 2012 July 10 [doi: 10.1161/jaha.112.002345]).
Patient factors associated with increased likelihood of prenotification were younger age, white race, past history of atrial fibrillation, no medical history of previous stroke or transient ischemic attack, diabetes mellitus, and peripheral vascular disease.
"In particular, black patients had decreased odds [of EMS prenotification] when compared to their white counterparts, with adjusted odds ratio of 0.94," the investigators noted.
Hospital factors associated with reduced likelihood of prenotification were academic affiliation, location in the northeastern United States, and lower annual volume of TPA administration.
Rates of prenotification did increase modestly and significantly over time, from 58% to 67% between 2003 and 2011, with a high of 71.1% in 2008, followed by a decline to about 65% in 2009 and 2010, and an increase to 67.3% in 2011, but targeted improvements in rates of EMS prenotification are needed, they said.
"These findings demonstrate gaps in the quality of stroke care provided and support the need for initiatives targeted to improve [EMS prenotification rates] on a national level," they concluded, explaining that a systems approach is needed involving increasing symptom recognition and rapid activation of EMS, adequate training of EMS staff in proper use of stroke-screening instruments and the need for hospital prenotification, and implementation of systems of care in receiving hospitals.
A stroke system-of-care process measure reporting the use of EMS prenotification should be considered, they said.
The Get With the Guidelines (GWTG)–Stroke program is provided by the AHA/ASA and is supported in part by a charitable contribution from the Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership and the AHA Pharmaceutical Roundtable. Past support has been provided by Boehringer Ingelheim and Merck. Ms. Lin reported having no relevant conflicts of interest. Several coauthors have worked with GWTG committees, and some have received research grant support from pharmaceutical companies. Some researchers are employees of the University of California, which holds a patent on retriever devices for stroke.
FROM CIRCULATION: CARDIOVASCULAR QUALITY AND OUTCOMES, AND THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Angioplasty Improved Venous Flow in MS
NATIONAL HARBOR, MD. -- Percutaneous balloon angioplasty improved flow dynamics in multiple sclerosis patients with chronic cerebrospinal venous insufficiency in a pilot study.
An association has been made recently between multiple sclerosis and chronic cerebrospinal venous insufficiency (CCSVI) that is characterized by stenosis and reflux of the principal extracranial venous drainage, including the internal jugular veins and the azygous veins. But there has been considerable debate about the validity of percutaneous balloon angioplasty in the treatment of this stenosis.
Dr. Manish Mehta of Albany (N.Y.) Medical College and his colleagues conducted the first angiographic study to quantitatively analyze the impact of percutaneous balloon angioplasty on flow dynamics across these lesions. Dr. Mehta shared their results at the Vascular Annual Meeting.
The researchers assessed 50 internal jugular veins (IJVs) from MS patients with CCSVI, as well as 12 IJVs from healthy volunteers, all of whom underwent detailed angiographic evaluation. The technical components of all venograms were standardized.
Quantitative analysis included the contrast time of flight from the mid-IJV to the superior vena cava, and the primary venous emptying time (PVET), quantified as greater than 50% of venous emptying from the IJV. The time of flight and PVET were recorded in patients with CCSVI prior to and subsequent to balloon angioplasty. The same data were recorded in the healthy controls. All data were collected prospectively, and statistical analysis was performed using two-tailed Student's test.
Of the 50 CCSVI-MS patients who had IJV stenosis greater than 70% and reflux and who underwent balloon angioplasty, technical success (defined as less than 20% residual IJV stenosis) was achieved in 44 (78%). CCSVI patients were observed to have a significant improvement in both the time of flight and PVET following balloon angioplasty that paralleled those of healthy subjects without MS.
"The results of this prospective pilot study suggest an association between MS and CCSVI, which results in abnormally elevated time of flight and PVET through the IJV," Dr. Mehta said. "Furthermore, balloon angioplasty of these lesions improves the hemodynamic parameters so that they are comparable to those of healthy non-MS patients."
Dr. Mehta stressed the need for randomized studies to further investigate this issue and said that this patient population is at high risk for a placebo effect with regard to their reported symptoms. He also stated that this treatment is being provided to many patients around the world and that the U.S. Food and Drug Administration has cautioned against its use outside of well-regulated trials due to lack of safety and efficacy data.
Dr. Mehta reported that he had nothing to disclose.
Manish Mehta
NATIONAL HARBOR, MD. -- Percutaneous balloon angioplasty improved flow dynamics in multiple sclerosis patients with chronic cerebrospinal venous insufficiency in a pilot study.
An association has been made recently between multiple sclerosis and chronic cerebrospinal venous insufficiency (CCSVI) that is characterized by stenosis and reflux of the principal extracranial venous drainage, including the internal jugular veins and the azygous veins. But there has been considerable debate about the validity of percutaneous balloon angioplasty in the treatment of this stenosis.
Dr. Manish Mehta of Albany (N.Y.) Medical College and his colleagues conducted the first angiographic study to quantitatively analyze the impact of percutaneous balloon angioplasty on flow dynamics across these lesions. Dr. Mehta shared their results at the Vascular Annual Meeting.
The researchers assessed 50 internal jugular veins (IJVs) from MS patients with CCSVI, as well as 12 IJVs from healthy volunteers, all of whom underwent detailed angiographic evaluation. The technical components of all venograms were standardized.
Quantitative analysis included the contrast time of flight from the mid-IJV to the superior vena cava, and the primary venous emptying time (PVET), quantified as greater than 50% of venous emptying from the IJV. The time of flight and PVET were recorded in patients with CCSVI prior to and subsequent to balloon angioplasty. The same data were recorded in the healthy controls. All data were collected prospectively, and statistical analysis was performed using two-tailed Student's test.
Of the 50 CCSVI-MS patients who had IJV stenosis greater than 70% and reflux and who underwent balloon angioplasty, technical success (defined as less than 20% residual IJV stenosis) was achieved in 44 (78%). CCSVI patients were observed to have a significant improvement in both the time of flight and PVET following balloon angioplasty that paralleled those of healthy subjects without MS.
"The results of this prospective pilot study suggest an association between MS and CCSVI, which results in abnormally elevated time of flight and PVET through the IJV," Dr. Mehta said. "Furthermore, balloon angioplasty of these lesions improves the hemodynamic parameters so that they are comparable to those of healthy non-MS patients."
Dr. Mehta stressed the need for randomized studies to further investigate this issue and said that this patient population is at high risk for a placebo effect with regard to their reported symptoms. He also stated that this treatment is being provided to many patients around the world and that the U.S. Food and Drug Administration has cautioned against its use outside of well-regulated trials due to lack of safety and efficacy data.
Dr. Mehta reported that he had nothing to disclose.
NATIONAL HARBOR, MD. -- Percutaneous balloon angioplasty improved flow dynamics in multiple sclerosis patients with chronic cerebrospinal venous insufficiency in a pilot study.
An association has been made recently between multiple sclerosis and chronic cerebrospinal venous insufficiency (CCSVI) that is characterized by stenosis and reflux of the principal extracranial venous drainage, including the internal jugular veins and the azygous veins. But there has been considerable debate about the validity of percutaneous balloon angioplasty in the treatment of this stenosis.
Dr. Manish Mehta of Albany (N.Y.) Medical College and his colleagues conducted the first angiographic study to quantitatively analyze the impact of percutaneous balloon angioplasty on flow dynamics across these lesions. Dr. Mehta shared their results at the Vascular Annual Meeting.
The researchers assessed 50 internal jugular veins (IJVs) from MS patients with CCSVI, as well as 12 IJVs from healthy volunteers, all of whom underwent detailed angiographic evaluation. The technical components of all venograms were standardized.
Quantitative analysis included the contrast time of flight from the mid-IJV to the superior vena cava, and the primary venous emptying time (PVET), quantified as greater than 50% of venous emptying from the IJV. The time of flight and PVET were recorded in patients with CCSVI prior to and subsequent to balloon angioplasty. The same data were recorded in the healthy controls. All data were collected prospectively, and statistical analysis was performed using two-tailed Student's test.
Of the 50 CCSVI-MS patients who had IJV stenosis greater than 70% and reflux and who underwent balloon angioplasty, technical success (defined as less than 20% residual IJV stenosis) was achieved in 44 (78%). CCSVI patients were observed to have a significant improvement in both the time of flight and PVET following balloon angioplasty that paralleled those of healthy subjects without MS.
"The results of this prospective pilot study suggest an association between MS and CCSVI, which results in abnormally elevated time of flight and PVET through the IJV," Dr. Mehta said. "Furthermore, balloon angioplasty of these lesions improves the hemodynamic parameters so that they are comparable to those of healthy non-MS patients."
Dr. Mehta stressed the need for randomized studies to further investigate this issue and said that this patient population is at high risk for a placebo effect with regard to their reported symptoms. He also stated that this treatment is being provided to many patients around the world and that the U.S. Food and Drug Administration has cautioned against its use outside of well-regulated trials due to lack of safety and efficacy data.
Dr. Mehta reported that he had nothing to disclose.
Manish Mehta
Manish Mehta
AT THE VASCULAR ANNUAL MEETING
Major Finding: Of the 50 CCSVI-MS patients with IJV stenosis greater than 70% and reflux who underwent balloon angioplasty, technical success was achieved in 78%. CCSVI patients were observed to have a significant improvement in venous flow characteristics following balloon angioplasty that paralleled those of healthy subjects.
Data Source: The researchers performed a comparative pilot study of 50 internal jugular veins (IJVs) from MS patients with chronic cerebrospinal venous insufficiency who underwent balloon angioplasty with 12 IJVs from healthy volunteers who underwent detailed angiographic analysis.
Disclosures: Dr. Mehta reported that he had nothing to disclose.
Carotid Artery Interventions Break Three Ways
Optimal management of carotid stenosis to prevent death and strokes is a work in progress right now, with experts groping for the right balance between carotid endarterectomy, carotid artery stenting, and best medical therapy.
The field is bereft of both conclusive, up-to-date data and – more importantly – wide agreement on data interpretation that clearly tips treatment toward one of these options, so much so that in late January an expert panel organized by the Centers for Medicare and Medicaid Services found itself unable to support with high confidence the application of these treatments to any subgroup of carotid disease patients.
Amid this confusion are the following certainties:
• Use of carotid artery stenting (CAS) on U.S. patients grew substantially since its introduction in the late 1990s and since it began receiving limited Medicare coverage in 2004. Data collected through 2007 showed a greater-than-fourfold increase in CAS use among Medicare beneficiaries, compared with 1998 (Circ. Cardiovasc. Qual. Outcomes 2010;3:15-24).
• Other findings documented a shift in CAS use during the 2000s toward patients with asymptomatic carotid stenosis, with a recent estimate that 70%-90% of CAS patients now fall into that category (Arch. Intern. Med. 2010;170:1225-7).
• The main results of the landmark CREST (Carotid Revascularization Endarterectomy vs. Stenting) trial, first reported 2 years ago (N. Engl. J. Med. 2010;363:11-23), appeared on the surface to show very similar safety and efficacy for CAS and carotid endarterectomy (CEA), but some experts caution that deeper drilling into the results show that this is an oversimplification of how the two treatments compare.
• And many experts agree that best medical therapy has improved in recent years, to the point where it deserves a new appraisal in a large trial that would compare it against carotid revascularization with CAS or CEA in asymptomatic patients. The leaders of CREST themselves have designed a new trial, CREST II, aimed at making this comparison, and have begun to vigorously lobby the National Institutes of Health to support this study.
In the meantime, vascular surgeons, endovascularists, cardiologists, and the other types of physicians who treat patients with carotid disease try as best they can to strike the right balance in how they apply the three management options.
CREST Provides New Guidance
One surgeon who has perhaps given the most thought to sorting out treatment options is Dr. Brajesh K. Lal, a co–principal investigator on CREST, a vascular surgeon at the University of Maryland in Baltimore, and a researcher who spent the past couple of years sorting through CREST’s voluminous data to find new clues to guide patient triage.
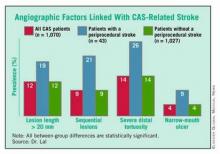
A major, recent guide for matching patients with a specific carotid intervention came from his painstaking analysis of carotid angiograms from 1,070 of the CREST patients who underwent CAS. This effort identified four anatomical features that appear to mark patients with an increased rate of periprocedural stroke when they are treated with CAS. (See box.)
Patients with one or more of these carotid anatomical features "should be treated by endarterectomy," Dr. Lal said in an interview. "If I see any one of these in my clinical practice, I will not stent."
Endovascularists were already routinely assessing carotid anatomy before attempting CAS, but prior to Dr. Lal’s report on these new findings in February at the International Stroke Conference in New Orleans, "I’m not so sure that everyone has been using this [anatomical] information," he said. "When we talk about operator experience in stenting, if we don’t have the facts about what increases the stenting risk, then we won’t improve performance. You’d be surprised at what some interventionalists are willing to do," despite a patient’s challenging carotid anatomy. "These data show that you can work around a single 90-degree bend [of the distal carotid artery], but not around two bends. That is the kind of stuff we’re beginning to find out."
Additional, recent CREST analyses that were also reported at the International Stroke Conference revealed other new, important lessons about CAS and CEA.
First, by 2 years after intervention, both CAS and CEA produced roughly comparable low rates of restenosis: a 6.0% rate in the CAS patients who underwent a prespecified, follow-up duplex ultrasound examination of their carotid artery, and 6.3% in the CEA patients (Stroke 2012;43:abstract A3).
The rates were also similar regardless of whether patients were symptomatic before their carotid intervention, Dr. Lal reported.
This finding appears to lay to rest the concern about a major restenosis risk using bare-metal stents in carotid arteries, in contrast to what happens in coronary arteries, Dr. Lal said.
"To my mind, these data are fairly definitive. We followed more than 2,000 patients, and every ultrasound was reviewed and adjudicated. I’m fairly comfortable with these results."
A third CREST analysis that was also reported in February showed that, after adjustment for baseline differences, patients had no significant changes in their rates of periprocedural events during and after CAS throughout the study, from 2000 to 2008 (Stroke 2012;43:abstract A1). "We hoped to show that CAS was getting better [during the 9 years of CREST], but we saw no evidence it got better with time," said George Howard, Dr.P.H., professor and chairman of the department of biostatistics at the University of Alabama at Birmingham. "It would make sense if CAS got better; it’s a new procedure that people learn." But the CREST results showed no evidence of a learning curve, perhaps because the 224 interventionalists who performed CAS in CREST went through a rigorous credentialing process and also performed their first several CAS procedures in CREST during a lead-in phase that was not included in the main outcomes.
Choosing Among the Options
Dr. Lal said that he draws several other lessons from the CREST results to guide his treatment decisions: Patients who are physiologically elderly, those with a large amount of calcified atheroma in their aortic arch, and patients with significant peripheral arterial disease that impedes endovascular access are all poor CAS candidates, he said, adding that he focuses on a patient’s physiological age rather than chronological age.
Patients who are well suited to CAS are those with a younger physiological age, those with a history of radiation treatment, and patients with restenotic carotid lesions.
Although CREST showed worse performance of CAS in women compared with men, "I don’t know why, so it’s hard for me to exclude or offer a particular treatment" based on the patient’s sex, he said. "I treat women just like men, and work through all the other things that I know about to help me decide."
Then there is a third subgroup, patients with carotid disease who he feels "benefit the most" from medical management only. This category primarily includes asymptomatic patients with moderate stenosis (that is, less than 80% carotid occlusion).
His prescription for medical management includes 325 mg of aspirin daily, treatment with a statin, good blood pressure and blood sugar control, smoking cessation, and lifestyle modification. If, despite this, an asymptomatic patient has continued stenotic progression that advances beyond 80%, he will then recommend revascularization.
Dr. Lal said that in his practice today, roughly one-third of carotid-disease patients are on medical management, a third undergo CEA, and a third receive CAS. "We are at a stage of equipoise," for these three options, which is why he helped design and is working to get funding for CREST II.
Dr. Lal personally applies all three treatment options – CAS, CEA, or medical management. Having physicians involved in the decision-making process who are comfortable using each of these options is critical for making a balanced management decision, said Dr. L. Nelson Hopkins, another CREST collaborator and professor and chairman of neurosurgery at the State University of New York at Buffalo.
"The single most important thing we do is have a weekly, multidisciplinary conference to discuss each case, with input from everyone, to decide the best treatment for each [carotid disease] patient," he said when he spoke at ISET 2012, an International Symposium on Endovascular Therapy in Miami Beach in January.
"The most important factors" guiding the decision of whether or not to perform CAS are, does a symptomatic patient have "hot" lesions, what is the arterial tortuosity, and what is the calcification, he said. Other important features include whether the patient has a contralateral carotid occlusion, whether the carotid has a high bifurcation, and whether there are ostial or tandem lesions. "If the anatomy is favorable, CAS is fine even in a 95-year-old," Dr. Hopkins said.
What About Insurance Coverage?
Carotid specialists concede that another, nonmedical issue also plays a big role in deciding how to manage a patient: What will the patient’s health insurance cover?
In its most recent decision on the issue, effective in April 2010, the Centers for Medicare and Medicaid Services (CMS) said that Medicare covers CAS performed on a routine basis for patients with symptomatic carotid disease who are at high risk for CEA and have at least 70% carotid stenosis. CMS also said that Medicare will cover FDA-approved investigations of CAS in symptomatic patients who are at high risk for CEA and have at least 50% stenosis, as well as CAS investigations in asymptomatic patients at high risk for CEA with at least 80% carotid stenosis.
As a result, patients with other forms of carotid disease often do not have insurance coverage for CAS.
"The issue for us is primarily reimbursement. If we can do [a procedure] and be reimbursed, we often stent the patient," said Dr. Carlos H. Timaran, a vascular surgeon and chief of endovascular surgery at the University of Texas Southwestern Medical Center in Dallas.
"For my private practice patients, we assess their risk based on their
[anatomical] and medical criteria. If they are high risk, we usually stent. We have a bias toward stenting because it is faster and easier. Some people say they can do endarterectomy in an hour, but I don’t believe that. I do CEA with residents and fellows, and it takes 2 hours. But with stenting, I can do it in 40 minutes, even with a fellow. If I have three patients with carotid disease to treat [and] if I do CEA on all three, it will take all day. If I do CAS, it will take the morning, or less," he said in an interview.
In addition, "CEA is a nice procedure, but you need anesthesia. CAS is easier, I can do it myself, and I don’t need anesthesia. I am in more control of the case," Dr. Timaran said.
For patients who are eligible for both CAS and CEA, "it also comes down to anatomy," he grants. But if the patient has anatomy favorable to either method, "I offer patients both, and they will probably go for a stent," because patients usually prefer the quicker recovery that stenting offers, compared with CEA.
An interesting footnote to the issue of how to best manage patients who are eligible for CAS under CMS rules is that the CREST results offer no direct guidance on the relative safety and efficacy of CAS and CEA in patients with Medicare coverage. That’s because "the CREST cohort was a conventional-risk cohort; most criteria that would make someone a high surgical risk would have been excluded from CREST," Dr. Thomas G. Brott, professor of neurology at the Mayo Clinic in Jacksonville, Fla., and lead investigator for CREST. "The high-surgical-risk patients who are currently covered by Medicare would not have even been enrolled in CREST," Dr. Brott said in an interview.
Where Does Medical Therapy Fit In?
Although Dr. Timaran sees the potential value of medical therapy only for asymptomatic patients, he also urges caution.
"I don’t think [the efficacy of medical treatment] has been proven, and until it’s proven I don’t think physicians should deny revascularization treatment to these patients," he said. "The standard of care for a patient with severe carotid stenosis is revascularization, even if the patient is asymptomatic, unless there is some other consideration." But, he admitted, "there is little downside to medical treatment in asymptomatic patients, even if they have severe stenosis." That’s because their stroke risk is low regardless of which option a patient chooses.
"I tell asymptomatic patients that ‘based on the data, your risk of stroke is 2% per year, and that risk may be even lower with best medical therapy.’ With a carotid intervention, we can lower their risk to 1% per year." In other words, the patient’s stroke risk is low regardless of which option is chosen. Plus, medical therapy only is the best choice for patients with special risk factors, such as radiation-induced stenosis, Dr. Timaran added.
Other experts see medical treatment alone as an excellent option for many carotid-stenosis patients.
"The majority of patients I see don’t get an intervention; we use medical treatment only," said Dr. Jon S. Matsumura in an interview. "I think that’s true for most vascular surgery practices, and in cardiology and neurology practices. A lot of these patients just need reassurance," said Dr. Matsumura, professor and chairman of vascular surgery at the University of Wisconsin in Madison.
"Most asymptomatic patients [with carotid stenosis] should be treated by best medical therapy, with very few – fewer than 5% – treated by CAS or CEA," said Dr. Frank J. Veith at the International Symposium on Endovascular Therapy (ISET 12) in January. He acknowledged that well-controlled trials done during the 1990s showed that CEA led to superior outcomes compared with medical therapy, but since the early 2000s, "best medical therapy to prevent strokes leapt forward," he said, especially with improved statin therapy. He cited findings from a 2010 prospective study that showed asymptomatic Canadian patients managed starting in 2003 with best medical therapy had an annual stroke rate of 0.7% (Arch. Neurol. 2010;67:180-6).
He singled out four small groups of asymptomatic patients who warrant revascularization: patients with CT or MRI evidence for a history of silent strokes, patients with very-high-grade carotid stenosis that leaves just 1% of the carotid lumen open, those with a contralateral occlusion, and patients who serially experience transient occlusions detected by transcranial Doppler ultrasound examination, said Dr. Veith, professor of surgery at New York
University. Aside from this small fraction of patients with asymptomatic disease, performing interventions on anyone else will "cause more strokes than you’ll prevent," he warned in an interview.
What Will CMS Do?
Dr. Veith’s take on limiting CAS in asymptomatic patients received substantial support early this year in the days leading up to the Jan. 25 meeting of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) that heard evidence and opinions for and against expanding Medicare coverage for CAS. In early January, Dr. Veith joined with 40 other vascular surgeons and stroke specialists to urge CMS not to broaden CAS coverage (Eur. J. Vasc. Endovasc. Surg. 2012 Jan. 5 [doi:10.1016/j.ejvs.2011.12.006]). The Society for Vascular Surgeons has also been on the forefront of this issue, urging CMS to maintain appropriate coverage restrictions.
But, as MEDCAC heard during this meeting, the proponents of expanded coverage remain staunch.. "CMS continues to restrict access to CAS to about 10% of patients" with significant carotid stenosis, said Dr. William A. Gray, director of endovascular services at Columbia University in New York, speaking at ISET 2012 in January. "Expanded CAS coverage would allow greater patient access and device development, and therefore create a safer option for patients." Dr. Gray presented his positive views on CAS to MEDCAC in January as well.
At press time, CMS had not announced whether or not the January MEDCAC hearing will result in a change to its CAS coverage policy. But the votes cast by MEDCAC’s membership in January suggested that the issues surrounding management of carotid stenosis remain too unresolved to trigger immediate changes.
Dr. Lal, Dr. Timaran, and Dr. Veith said that they had no disclosures. Dr. Howard said that he has received grant support from Abbott. Dr. Hopkins said that he has financial relationships with Bard, Boston Scientific, Cordis, Abbott Vascular, Toshiba, W.L. Gore, Medtronic, Micrus, St. Jude, Access Closure, and other device companies. Dr. Brott said that he has been a consultant to Edwards Lifesciences. Dr. Matsumura said that he has received research grants from Abbott, Cook, Covidien, Endologix, and W.L. Gore. Dr. Gray said that he has had financial relationships with Boston Scientific, Coherex, Contego, Cordis, and a number of other device companies.
Optimal management of carotid stenosis to prevent death and strokes is a work in progress right now, with experts groping for the right balance between carotid endarterectomy, carotid artery stenting, and best medical therapy.
The field is bereft of both conclusive, up-to-date data and – more importantly – wide agreement on data interpretation that clearly tips treatment toward one of these options, so much so that in late January an expert panel organized by the Centers for Medicare and Medicaid Services found itself unable to support with high confidence the application of these treatments to any subgroup of carotid disease patients.
Amid this confusion are the following certainties:
• Use of carotid artery stenting (CAS) on U.S. patients grew substantially since its introduction in the late 1990s and since it began receiving limited Medicare coverage in 2004. Data collected through 2007 showed a greater-than-fourfold increase in CAS use among Medicare beneficiaries, compared with 1998 (Circ. Cardiovasc. Qual. Outcomes 2010;3:15-24).
• Other findings documented a shift in CAS use during the 2000s toward patients with asymptomatic carotid stenosis, with a recent estimate that 70%-90% of CAS patients now fall into that category (Arch. Intern. Med. 2010;170:1225-7).
• The main results of the landmark CREST (Carotid Revascularization Endarterectomy vs. Stenting) trial, first reported 2 years ago (N. Engl. J. Med. 2010;363:11-23), appeared on the surface to show very similar safety and efficacy for CAS and carotid endarterectomy (CEA), but some experts caution that deeper drilling into the results show that this is an oversimplification of how the two treatments compare.
• And many experts agree that best medical therapy has improved in recent years, to the point where it deserves a new appraisal in a large trial that would compare it against carotid revascularization with CAS or CEA in asymptomatic patients. The leaders of CREST themselves have designed a new trial, CREST II, aimed at making this comparison, and have begun to vigorously lobby the National Institutes of Health to support this study.
In the meantime, vascular surgeons, endovascularists, cardiologists, and the other types of physicians who treat patients with carotid disease try as best they can to strike the right balance in how they apply the three management options.
CREST Provides New Guidance
One surgeon who has perhaps given the most thought to sorting out treatment options is Dr. Brajesh K. Lal, a co–principal investigator on CREST, a vascular surgeon at the University of Maryland in Baltimore, and a researcher who spent the past couple of years sorting through CREST’s voluminous data to find new clues to guide patient triage.
A major, recent guide for matching patients with a specific carotid intervention came from his painstaking analysis of carotid angiograms from 1,070 of the CREST patients who underwent CAS. This effort identified four anatomical features that appear to mark patients with an increased rate of periprocedural stroke when they are treated with CAS. (See box.)
Patients with one or more of these carotid anatomical features "should be treated by endarterectomy," Dr. Lal said in an interview. "If I see any one of these in my clinical practice, I will not stent."
Endovascularists were already routinely assessing carotid anatomy before attempting CAS, but prior to Dr. Lal’s report on these new findings in February at the International Stroke Conference in New Orleans, "I’m not so sure that everyone has been using this [anatomical] information," he said. "When we talk about operator experience in stenting, if we don’t have the facts about what increases the stenting risk, then we won’t improve performance. You’d be surprised at what some interventionalists are willing to do," despite a patient’s challenging carotid anatomy. "These data show that you can work around a single 90-degree bend [of the distal carotid artery], but not around two bends. That is the kind of stuff we’re beginning to find out."
Additional, recent CREST analyses that were also reported at the International Stroke Conference revealed other new, important lessons about CAS and CEA.
First, by 2 years after intervention, both CAS and CEA produced roughly comparable low rates of restenosis: a 6.0% rate in the CAS patients who underwent a prespecified, follow-up duplex ultrasound examination of their carotid artery, and 6.3% in the CEA patients (Stroke 2012;43:abstract A3).
The rates were also similar regardless of whether patients were symptomatic before their carotid intervention, Dr. Lal reported.
This finding appears to lay to rest the concern about a major restenosis risk using bare-metal stents in carotid arteries, in contrast to what happens in coronary arteries, Dr. Lal said.
"To my mind, these data are fairly definitive. We followed more than 2,000 patients, and every ultrasound was reviewed and adjudicated. I’m fairly comfortable with these results."
A third CREST analysis that was also reported in February showed that, after adjustment for baseline differences, patients had no significant changes in their rates of periprocedural events during and after CAS throughout the study, from 2000 to 2008 (Stroke 2012;43:abstract A1). "We hoped to show that CAS was getting better [during the 9 years of CREST], but we saw no evidence it got better with time," said George Howard, Dr.P.H., professor and chairman of the department of biostatistics at the University of Alabama at Birmingham. "It would make sense if CAS got better; it’s a new procedure that people learn." But the CREST results showed no evidence of a learning curve, perhaps because the 224 interventionalists who performed CAS in CREST went through a rigorous credentialing process and also performed their first several CAS procedures in CREST during a lead-in phase that was not included in the main outcomes.
Choosing Among the Options
Dr. Lal said that he draws several other lessons from the CREST results to guide his treatment decisions: Patients who are physiologically elderly, those with a large amount of calcified atheroma in their aortic arch, and patients with significant peripheral arterial disease that impedes endovascular access are all poor CAS candidates, he said, adding that he focuses on a patient’s physiological age rather than chronological age.
Patients who are well suited to CAS are those with a younger physiological age, those with a history of radiation treatment, and patients with restenotic carotid lesions.
Although CREST showed worse performance of CAS in women compared with men, "I don’t know why, so it’s hard for me to exclude or offer a particular treatment" based on the patient’s sex, he said. "I treat women just like men, and work through all the other things that I know about to help me decide."
Then there is a third subgroup, patients with carotid disease who he feels "benefit the most" from medical management only. This category primarily includes asymptomatic patients with moderate stenosis (that is, less than 80% carotid occlusion).
His prescription for medical management includes 325 mg of aspirin daily, treatment with a statin, good blood pressure and blood sugar control, smoking cessation, and lifestyle modification. If, despite this, an asymptomatic patient has continued stenotic progression that advances beyond 80%, he will then recommend revascularization.
Dr. Lal said that in his practice today, roughly one-third of carotid-disease patients are on medical management, a third undergo CEA, and a third receive CAS. "We are at a stage of equipoise," for these three options, which is why he helped design and is working to get funding for CREST II.
Dr. Lal personally applies all three treatment options – CAS, CEA, or medical management. Having physicians involved in the decision-making process who are comfortable using each of these options is critical for making a balanced management decision, said Dr. L. Nelson Hopkins, another CREST collaborator and professor and chairman of neurosurgery at the State University of New York at Buffalo.
"The single most important thing we do is have a weekly, multidisciplinary conference to discuss each case, with input from everyone, to decide the best treatment for each [carotid disease] patient," he said when he spoke at ISET 2012, an International Symposium on Endovascular Therapy in Miami Beach in January.
"The most important factors" guiding the decision of whether or not to perform CAS are, does a symptomatic patient have "hot" lesions, what is the arterial tortuosity, and what is the calcification, he said. Other important features include whether the patient has a contralateral carotid occlusion, whether the carotid has a high bifurcation, and whether there are ostial or tandem lesions. "If the anatomy is favorable, CAS is fine even in a 95-year-old," Dr. Hopkins said.
What About Insurance Coverage?
Carotid specialists concede that another, nonmedical issue also plays a big role in deciding how to manage a patient: What will the patient’s health insurance cover?
In its most recent decision on the issue, effective in April 2010, the Centers for Medicare and Medicaid Services (CMS) said that Medicare covers CAS performed on a routine basis for patients with symptomatic carotid disease who are at high risk for CEA and have at least 70% carotid stenosis. CMS also said that Medicare will cover FDA-approved investigations of CAS in symptomatic patients who are at high risk for CEA and have at least 50% stenosis, as well as CAS investigations in asymptomatic patients at high risk for CEA with at least 80% carotid stenosis.
As a result, patients with other forms of carotid disease often do not have insurance coverage for CAS.
"The issue for us is primarily reimbursement. If we can do [a procedure] and be reimbursed, we often stent the patient," said Dr. Carlos H. Timaran, a vascular surgeon and chief of endovascular surgery at the University of Texas Southwestern Medical Center in Dallas.
"For my private practice patients, we assess their risk based on their
[anatomical] and medical criteria. If they are high risk, we usually stent. We have a bias toward stenting because it is faster and easier. Some people say they can do endarterectomy in an hour, but I don’t believe that. I do CEA with residents and fellows, and it takes 2 hours. But with stenting, I can do it in 40 minutes, even with a fellow. If I have three patients with carotid disease to treat [and] if I do CEA on all three, it will take all day. If I do CAS, it will take the morning, or less," he said in an interview.
In addition, "CEA is a nice procedure, but you need anesthesia. CAS is easier, I can do it myself, and I don’t need anesthesia. I am in more control of the case," Dr. Timaran said.
For patients who are eligible for both CAS and CEA, "it also comes down to anatomy," he grants. But if the patient has anatomy favorable to either method, "I offer patients both, and they will probably go for a stent," because patients usually prefer the quicker recovery that stenting offers, compared with CEA.
An interesting footnote to the issue of how to best manage patients who are eligible for CAS under CMS rules is that the CREST results offer no direct guidance on the relative safety and efficacy of CAS and CEA in patients with Medicare coverage. That’s because "the CREST cohort was a conventional-risk cohort; most criteria that would make someone a high surgical risk would have been excluded from CREST," Dr. Thomas G. Brott, professor of neurology at the Mayo Clinic in Jacksonville, Fla., and lead investigator for CREST. "The high-surgical-risk patients who are currently covered by Medicare would not have even been enrolled in CREST," Dr. Brott said in an interview.
Where Does Medical Therapy Fit In?
Although Dr. Timaran sees the potential value of medical therapy only for asymptomatic patients, he also urges caution.
"I don’t think [the efficacy of medical treatment] has been proven, and until it’s proven I don’t think physicians should deny revascularization treatment to these patients," he said. "The standard of care for a patient with severe carotid stenosis is revascularization, even if the patient is asymptomatic, unless there is some other consideration." But, he admitted, "there is little downside to medical treatment in asymptomatic patients, even if they have severe stenosis." That’s because their stroke risk is low regardless of which option a patient chooses.
"I tell asymptomatic patients that ‘based on the data, your risk of stroke is 2% per year, and that risk may be even lower with best medical therapy.’ With a carotid intervention, we can lower their risk to 1% per year." In other words, the patient’s stroke risk is low regardless of which option is chosen. Plus, medical therapy only is the best choice for patients with special risk factors, such as radiation-induced stenosis, Dr. Timaran added.
Other experts see medical treatment alone as an excellent option for many carotid-stenosis patients.
"The majority of patients I see don’t get an intervention; we use medical treatment only," said Dr. Jon S. Matsumura in an interview. "I think that’s true for most vascular surgery practices, and in cardiology and neurology practices. A lot of these patients just need reassurance," said Dr. Matsumura, professor and chairman of vascular surgery at the University of Wisconsin in Madison.
"Most asymptomatic patients [with carotid stenosis] should be treated by best medical therapy, with very few – fewer than 5% – treated by CAS or CEA," said Dr. Frank J. Veith at the International Symposium on Endovascular Therapy (ISET 12) in January. He acknowledged that well-controlled trials done during the 1990s showed that CEA led to superior outcomes compared with medical therapy, but since the early 2000s, "best medical therapy to prevent strokes leapt forward," he said, especially with improved statin therapy. He cited findings from a 2010 prospective study that showed asymptomatic Canadian patients managed starting in 2003 with best medical therapy had an annual stroke rate of 0.7% (Arch. Neurol. 2010;67:180-6).
He singled out four small groups of asymptomatic patients who warrant revascularization: patients with CT or MRI evidence for a history of silent strokes, patients with very-high-grade carotid stenosis that leaves just 1% of the carotid lumen open, those with a contralateral occlusion, and patients who serially experience transient occlusions detected by transcranial Doppler ultrasound examination, said Dr. Veith, professor of surgery at New York
University. Aside from this small fraction of patients with asymptomatic disease, performing interventions on anyone else will "cause more strokes than you’ll prevent," he warned in an interview.
What Will CMS Do?
Dr. Veith’s take on limiting CAS in asymptomatic patients received substantial support early this year in the days leading up to the Jan. 25 meeting of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) that heard evidence and opinions for and against expanding Medicare coverage for CAS. In early January, Dr. Veith joined with 40 other vascular surgeons and stroke specialists to urge CMS not to broaden CAS coverage (Eur. J. Vasc. Endovasc. Surg. 2012 Jan. 5 [doi:10.1016/j.ejvs.2011.12.006]). The Society for Vascular Surgeons has also been on the forefront of this issue, urging CMS to maintain appropriate coverage restrictions.
But, as MEDCAC heard during this meeting, the proponents of expanded coverage remain staunch.. "CMS continues to restrict access to CAS to about 10% of patients" with significant carotid stenosis, said Dr. William A. Gray, director of endovascular services at Columbia University in New York, speaking at ISET 2012 in January. "Expanded CAS coverage would allow greater patient access and device development, and therefore create a safer option for patients." Dr. Gray presented his positive views on CAS to MEDCAC in January as well.
At press time, CMS had not announced whether or not the January MEDCAC hearing will result in a change to its CAS coverage policy. But the votes cast by MEDCAC’s membership in January suggested that the issues surrounding management of carotid stenosis remain too unresolved to trigger immediate changes.
Dr. Lal, Dr. Timaran, and Dr. Veith said that they had no disclosures. Dr. Howard said that he has received grant support from Abbott. Dr. Hopkins said that he has financial relationships with Bard, Boston Scientific, Cordis, Abbott Vascular, Toshiba, W.L. Gore, Medtronic, Micrus, St. Jude, Access Closure, and other device companies. Dr. Brott said that he has been a consultant to Edwards Lifesciences. Dr. Matsumura said that he has received research grants from Abbott, Cook, Covidien, Endologix, and W.L. Gore. Dr. Gray said that he has had financial relationships with Boston Scientific, Coherex, Contego, Cordis, and a number of other device companies.
Optimal management of carotid stenosis to prevent death and strokes is a work in progress right now, with experts groping for the right balance between carotid endarterectomy, carotid artery stenting, and best medical therapy.
The field is bereft of both conclusive, up-to-date data and – more importantly – wide agreement on data interpretation that clearly tips treatment toward one of these options, so much so that in late January an expert panel organized by the Centers for Medicare and Medicaid Services found itself unable to support with high confidence the application of these treatments to any subgroup of carotid disease patients.
Amid this confusion are the following certainties:
• Use of carotid artery stenting (CAS) on U.S. patients grew substantially since its introduction in the late 1990s and since it began receiving limited Medicare coverage in 2004. Data collected through 2007 showed a greater-than-fourfold increase in CAS use among Medicare beneficiaries, compared with 1998 (Circ. Cardiovasc. Qual. Outcomes 2010;3:15-24).
• Other findings documented a shift in CAS use during the 2000s toward patients with asymptomatic carotid stenosis, with a recent estimate that 70%-90% of CAS patients now fall into that category (Arch. Intern. Med. 2010;170:1225-7).
• The main results of the landmark CREST (Carotid Revascularization Endarterectomy vs. Stenting) trial, first reported 2 years ago (N. Engl. J. Med. 2010;363:11-23), appeared on the surface to show very similar safety and efficacy for CAS and carotid endarterectomy (CEA), but some experts caution that deeper drilling into the results show that this is an oversimplification of how the two treatments compare.
• And many experts agree that best medical therapy has improved in recent years, to the point where it deserves a new appraisal in a large trial that would compare it against carotid revascularization with CAS or CEA in asymptomatic patients. The leaders of CREST themselves have designed a new trial, CREST II, aimed at making this comparison, and have begun to vigorously lobby the National Institutes of Health to support this study.
In the meantime, vascular surgeons, endovascularists, cardiologists, and the other types of physicians who treat patients with carotid disease try as best they can to strike the right balance in how they apply the three management options.
CREST Provides New Guidance
One surgeon who has perhaps given the most thought to sorting out treatment options is Dr. Brajesh K. Lal, a co–principal investigator on CREST, a vascular surgeon at the University of Maryland in Baltimore, and a researcher who spent the past couple of years sorting through CREST’s voluminous data to find new clues to guide patient triage.
A major, recent guide for matching patients with a specific carotid intervention came from his painstaking analysis of carotid angiograms from 1,070 of the CREST patients who underwent CAS. This effort identified four anatomical features that appear to mark patients with an increased rate of periprocedural stroke when they are treated with CAS. (See box.)
Patients with one or more of these carotid anatomical features "should be treated by endarterectomy," Dr. Lal said in an interview. "If I see any one of these in my clinical practice, I will not stent."
Endovascularists were already routinely assessing carotid anatomy before attempting CAS, but prior to Dr. Lal’s report on these new findings in February at the International Stroke Conference in New Orleans, "I’m not so sure that everyone has been using this [anatomical] information," he said. "When we talk about operator experience in stenting, if we don’t have the facts about what increases the stenting risk, then we won’t improve performance. You’d be surprised at what some interventionalists are willing to do," despite a patient’s challenging carotid anatomy. "These data show that you can work around a single 90-degree bend [of the distal carotid artery], but not around two bends. That is the kind of stuff we’re beginning to find out."
Additional, recent CREST analyses that were also reported at the International Stroke Conference revealed other new, important lessons about CAS and CEA.
First, by 2 years after intervention, both CAS and CEA produced roughly comparable low rates of restenosis: a 6.0% rate in the CAS patients who underwent a prespecified, follow-up duplex ultrasound examination of their carotid artery, and 6.3% in the CEA patients (Stroke 2012;43:abstract A3).
The rates were also similar regardless of whether patients were symptomatic before their carotid intervention, Dr. Lal reported.
This finding appears to lay to rest the concern about a major restenosis risk using bare-metal stents in carotid arteries, in contrast to what happens in coronary arteries, Dr. Lal said.
"To my mind, these data are fairly definitive. We followed more than 2,000 patients, and every ultrasound was reviewed and adjudicated. I’m fairly comfortable with these results."
A third CREST analysis that was also reported in February showed that, after adjustment for baseline differences, patients had no significant changes in their rates of periprocedural events during and after CAS throughout the study, from 2000 to 2008 (Stroke 2012;43:abstract A1). "We hoped to show that CAS was getting better [during the 9 years of CREST], but we saw no evidence it got better with time," said George Howard, Dr.P.H., professor and chairman of the department of biostatistics at the University of Alabama at Birmingham. "It would make sense if CAS got better; it’s a new procedure that people learn." But the CREST results showed no evidence of a learning curve, perhaps because the 224 interventionalists who performed CAS in CREST went through a rigorous credentialing process and also performed their first several CAS procedures in CREST during a lead-in phase that was not included in the main outcomes.
Choosing Among the Options
Dr. Lal said that he draws several other lessons from the CREST results to guide his treatment decisions: Patients who are physiologically elderly, those with a large amount of calcified atheroma in their aortic arch, and patients with significant peripheral arterial disease that impedes endovascular access are all poor CAS candidates, he said, adding that he focuses on a patient’s physiological age rather than chronological age.
Patients who are well suited to CAS are those with a younger physiological age, those with a history of radiation treatment, and patients with restenotic carotid lesions.
Although CREST showed worse performance of CAS in women compared with men, "I don’t know why, so it’s hard for me to exclude or offer a particular treatment" based on the patient’s sex, he said. "I treat women just like men, and work through all the other things that I know about to help me decide."
Then there is a third subgroup, patients with carotid disease who he feels "benefit the most" from medical management only. This category primarily includes asymptomatic patients with moderate stenosis (that is, less than 80% carotid occlusion).
His prescription for medical management includes 325 mg of aspirin daily, treatment with a statin, good blood pressure and blood sugar control, smoking cessation, and lifestyle modification. If, despite this, an asymptomatic patient has continued stenotic progression that advances beyond 80%, he will then recommend revascularization.
Dr. Lal said that in his practice today, roughly one-third of carotid-disease patients are on medical management, a third undergo CEA, and a third receive CAS. "We are at a stage of equipoise," for these three options, which is why he helped design and is working to get funding for CREST II.
Dr. Lal personally applies all three treatment options – CAS, CEA, or medical management. Having physicians involved in the decision-making process who are comfortable using each of these options is critical for making a balanced management decision, said Dr. L. Nelson Hopkins, another CREST collaborator and professor and chairman of neurosurgery at the State University of New York at Buffalo.
"The single most important thing we do is have a weekly, multidisciplinary conference to discuss each case, with input from everyone, to decide the best treatment for each [carotid disease] patient," he said when he spoke at ISET 2012, an International Symposium on Endovascular Therapy in Miami Beach in January.
"The most important factors" guiding the decision of whether or not to perform CAS are, does a symptomatic patient have "hot" lesions, what is the arterial tortuosity, and what is the calcification, he said. Other important features include whether the patient has a contralateral carotid occlusion, whether the carotid has a high bifurcation, and whether there are ostial or tandem lesions. "If the anatomy is favorable, CAS is fine even in a 95-year-old," Dr. Hopkins said.
What About Insurance Coverage?
Carotid specialists concede that another, nonmedical issue also plays a big role in deciding how to manage a patient: What will the patient’s health insurance cover?
In its most recent decision on the issue, effective in April 2010, the Centers for Medicare and Medicaid Services (CMS) said that Medicare covers CAS performed on a routine basis for patients with symptomatic carotid disease who are at high risk for CEA and have at least 70% carotid stenosis. CMS also said that Medicare will cover FDA-approved investigations of CAS in symptomatic patients who are at high risk for CEA and have at least 50% stenosis, as well as CAS investigations in asymptomatic patients at high risk for CEA with at least 80% carotid stenosis.
As a result, patients with other forms of carotid disease often do not have insurance coverage for CAS.
"The issue for us is primarily reimbursement. If we can do [a procedure] and be reimbursed, we often stent the patient," said Dr. Carlos H. Timaran, a vascular surgeon and chief of endovascular surgery at the University of Texas Southwestern Medical Center in Dallas.
"For my private practice patients, we assess their risk based on their
[anatomical] and medical criteria. If they are high risk, we usually stent. We have a bias toward stenting because it is faster and easier. Some people say they can do endarterectomy in an hour, but I don’t believe that. I do CEA with residents and fellows, and it takes 2 hours. But with stenting, I can do it in 40 minutes, even with a fellow. If I have three patients with carotid disease to treat [and] if I do CEA on all three, it will take all day. If I do CAS, it will take the morning, or less," he said in an interview.
In addition, "CEA is a nice procedure, but you need anesthesia. CAS is easier, I can do it myself, and I don’t need anesthesia. I am in more control of the case," Dr. Timaran said.
For patients who are eligible for both CAS and CEA, "it also comes down to anatomy," he grants. But if the patient has anatomy favorable to either method, "I offer patients both, and they will probably go for a stent," because patients usually prefer the quicker recovery that stenting offers, compared with CEA.
An interesting footnote to the issue of how to best manage patients who are eligible for CAS under CMS rules is that the CREST results offer no direct guidance on the relative safety and efficacy of CAS and CEA in patients with Medicare coverage. That’s because "the CREST cohort was a conventional-risk cohort; most criteria that would make someone a high surgical risk would have been excluded from CREST," Dr. Thomas G. Brott, professor of neurology at the Mayo Clinic in Jacksonville, Fla., and lead investigator for CREST. "The high-surgical-risk patients who are currently covered by Medicare would not have even been enrolled in CREST," Dr. Brott said in an interview.
Where Does Medical Therapy Fit In?
Although Dr. Timaran sees the potential value of medical therapy only for asymptomatic patients, he also urges caution.
"I don’t think [the efficacy of medical treatment] has been proven, and until it’s proven I don’t think physicians should deny revascularization treatment to these patients," he said. "The standard of care for a patient with severe carotid stenosis is revascularization, even if the patient is asymptomatic, unless there is some other consideration." But, he admitted, "there is little downside to medical treatment in asymptomatic patients, even if they have severe stenosis." That’s because their stroke risk is low regardless of which option a patient chooses.
"I tell asymptomatic patients that ‘based on the data, your risk of stroke is 2% per year, and that risk may be even lower with best medical therapy.’ With a carotid intervention, we can lower their risk to 1% per year." In other words, the patient’s stroke risk is low regardless of which option is chosen. Plus, medical therapy only is the best choice for patients with special risk factors, such as radiation-induced stenosis, Dr. Timaran added.
Other experts see medical treatment alone as an excellent option for many carotid-stenosis patients.
"The majority of patients I see don’t get an intervention; we use medical treatment only," said Dr. Jon S. Matsumura in an interview. "I think that’s true for most vascular surgery practices, and in cardiology and neurology practices. A lot of these patients just need reassurance," said Dr. Matsumura, professor and chairman of vascular surgery at the University of Wisconsin in Madison.
"Most asymptomatic patients [with carotid stenosis] should be treated by best medical therapy, with very few – fewer than 5% – treated by CAS or CEA," said Dr. Frank J. Veith at the International Symposium on Endovascular Therapy (ISET 12) in January. He acknowledged that well-controlled trials done during the 1990s showed that CEA led to superior outcomes compared with medical therapy, but since the early 2000s, "best medical therapy to prevent strokes leapt forward," he said, especially with improved statin therapy. He cited findings from a 2010 prospective study that showed asymptomatic Canadian patients managed starting in 2003 with best medical therapy had an annual stroke rate of 0.7% (Arch. Neurol. 2010;67:180-6).
He singled out four small groups of asymptomatic patients who warrant revascularization: patients with CT or MRI evidence for a history of silent strokes, patients with very-high-grade carotid stenosis that leaves just 1% of the carotid lumen open, those with a contralateral occlusion, and patients who serially experience transient occlusions detected by transcranial Doppler ultrasound examination, said Dr. Veith, professor of surgery at New York
University. Aside from this small fraction of patients with asymptomatic disease, performing interventions on anyone else will "cause more strokes than you’ll prevent," he warned in an interview.
What Will CMS Do?
Dr. Veith’s take on limiting CAS in asymptomatic patients received substantial support early this year in the days leading up to the Jan. 25 meeting of the Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) that heard evidence and opinions for and against expanding Medicare coverage for CAS. In early January, Dr. Veith joined with 40 other vascular surgeons and stroke specialists to urge CMS not to broaden CAS coverage (Eur. J. Vasc. Endovasc. Surg. 2012 Jan. 5 [doi:10.1016/j.ejvs.2011.12.006]). The Society for Vascular Surgeons has also been on the forefront of this issue, urging CMS to maintain appropriate coverage restrictions.
But, as MEDCAC heard during this meeting, the proponents of expanded coverage remain staunch.. "CMS continues to restrict access to CAS to about 10% of patients" with significant carotid stenosis, said Dr. William A. Gray, director of endovascular services at Columbia University in New York, speaking at ISET 2012 in January. "Expanded CAS coverage would allow greater patient access and device development, and therefore create a safer option for patients." Dr. Gray presented his positive views on CAS to MEDCAC in January as well.
At press time, CMS had not announced whether or not the January MEDCAC hearing will result in a change to its CAS coverage policy. But the votes cast by MEDCAC’s membership in January suggested that the issues surrounding management of carotid stenosis remain too unresolved to trigger immediate changes.
Dr. Lal, Dr. Timaran, and Dr. Veith said that they had no disclosures. Dr. Howard said that he has received grant support from Abbott. Dr. Hopkins said that he has financial relationships with Bard, Boston Scientific, Cordis, Abbott Vascular, Toshiba, W.L. Gore, Medtronic, Micrus, St. Jude, Access Closure, and other device companies. Dr. Brott said that he has been a consultant to Edwards Lifesciences. Dr. Matsumura said that he has received research grants from Abbott, Cook, Covidien, Endologix, and W.L. Gore. Dr. Gray said that he has had financial relationships with Boston Scientific, Coherex, Contego, Cordis, and a number of other device companies.
Experts Can't Pin Down Best Carotid Atherosclerosis Treatments
There are still no clear answers about the best treatments to use to prevent stroke in patients with carotid artery stenosis, even with the input of a Medicare advisory panel of experts.
The Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) voted yesterday that they are only moderately confident that there is adequate evidence to determine which interventions – carotid artery endarterectomy (CEA), carotid artery stenting (CAS), or best medical treatment (BMT) – to use for different patient populations within the overall Medicare population. There’s unlikely to be any consensus in the near future as patient populations, detection, and treatments evolve.
"If there was ever a moving target problem in assessing health care interventions, this was one. The epidemiology is changing, the patient population is changing accordingly, [and] all of the interventions continue to change. We’ve not been keeping up in our data collection for the safety and effectiveness of these interventions as they continue to evolve," commented committee chair, Clifford Goodman, Ph.D. "This means that to the extent that CMS is going to revisit its coverage decision making over time, this is an ongoing data collection issue. You’re not done collecting these data; you may not ever be done collecting these data as long as the patient population continues to change and as long as we have very innovative people improving these interventions."
The panel was asked to vote on several questions regarding their confidence level that there is adequate evidence to identify specific patient populations and in some cases to select the best treatment option. Most scenarios garnered an "intermediate" confidence level. Confidence ran high only for the lowest-risk patients.
The panel voted that they had moderate confidence that the evidence is adequate to determine whether:
• Patients who are asymptomatic for carotid atherosclerosis can be identified as being at high risk for stroke in either cerebral hemisphere.
• Patients who are considering carotid revascularization can be identified as being at high risk for adverse events from CEA.
• Either CAS or CEA is the favored treatment strategy compared to BMT alone in patients with symptomatic carotid atherosclerosis and narrowing (at least 50% by angiography or at least 70% by ultrasound) who are not generally at high risk for adverse events from CEA.
• CAS, CEA, or BMT is the favored treatment strategy to decrease stroke or death for patients with asymptomatic carotid atherosclerosis.
In addition, for patients with symptomatic carotid atherosclerosis, who are not generally at high risk for adverse events from CEA, the panel had intermediate confidence that CEA is the favored treatment strategy to decrease stroke and death. For patients with asymptomatic carotid atherosclerosis, the panel had intermediate to high confidence that BMT is the favored treatment strategy to decrease stroke and death.
However, the panel was less confident that for patients with asymptomatic carotid atherosclerosis but with carotid narrowing – who are not generally considered at high risk for adverse events from CEA – that there is adequate evidence to determine whether or not either CAS or CEA is the favored strategy compared to BMT alone to decrease stroke or death in the Medicare population. The panel had very low confidence that there is adequate evidence to determine whether carotid artery screening of asymptomatic people decreases stroke or death.
The panel primarily based its decisions on the results of a handful of randomized, controlled trials with different patient populations, at different time periods, using different criteria to assess efficacy and safety. The results of the most recent of these – the CREST trial (Carotid Revascularization Endarterectomy vs. Stenting Trial) – were announced in 2010. The overall safety and efficacy of the CAS and CEA at 4 years were considered equivocal in terms of benefits for both men and for women, for patients who had previously had a stroke, and for those who had not (N. Engl. J. Med. 2010;363:11-23).
However, differences were seen between the two procedures for heart attacks and strokes. More heart attacks occurred with CEA – 2.3% vs. 1.1% – than for CAS, while more strokes occurred in the stenting group – 4.1% vs. 2.3% –than for CEA in the weeks following the procedure. There was also a difference in outcomes based on patient age. At approximately age 69 and younger, stenting results were slightly better, with CAS benefits increasing for decreasing patient age. Conversely, for patients older than 70, CEA results were slightly superior to stenting, with larger benefits for surgery with older patient age.
Instead of answering the question of which technique has better outcomes and safety, the trial left proponents of each technique still arguing.
In addition, BMT has not been assessed in clinical trials for many years and has evolved substantially since that time with the development and growing use of statins, antiplatelet medications, beta-blockers, ACE inhibitors, calcium channel blockers, and others.
What is lacking is a randomized, controlled trial pitting all three treatments against one another in a head-to-head comparison of the most current techniques, the panel noted repeatedly.
"I think that drawing conclusions from indirect comparisons of things that were done in the past is incredibly hazardous and we need to have a contemporary direct comparison to know what the right thing to do is," argued panel member Dr. Larry B. Goldstein, who is a professor of neurology and director of the Duke Stroke Center in Chapel Hill, N.C.
Both surgery and CAS received a class I recommendation in guidelines published by the American Heart Association and other organizations in 2011 (J. Am. Coll. Cardiol. 2011;57:1002-44), but the level of evidence was stronger for CEA (level A) than for CAS (level B).
Planning is in the works for a possible CREST II trial in asymptomatic patients intended to compare intervention (with either CEA or CAS) vs. medical treatment alone. Many panel members expressed the need for this trial to proceed.
Twelve members of the panel reported that they did not have any relevant financial relationships. Dr. Spence reported receiving speaking fees from several pharmaceutical companies, Dr. Curtis reported that he owns stock in Medtronic and receives grant support from Boston Scientific, industry representative Dr. Peter Juhn works for Medco Health Solutions, and committee chair Dr. Clifford Goodman is the senior vice president at the Lewin Group, which has ties to both government and industry.
There are still no clear answers about the best treatments to use to prevent stroke in patients with carotid artery stenosis, even with the input of a Medicare advisory panel of experts.
The Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) voted yesterday that they are only moderately confident that there is adequate evidence to determine which interventions – carotid artery endarterectomy (CEA), carotid artery stenting (CAS), or best medical treatment (BMT) – to use for different patient populations within the overall Medicare population. There’s unlikely to be any consensus in the near future as patient populations, detection, and treatments evolve.
"If there was ever a moving target problem in assessing health care interventions, this was one. The epidemiology is changing, the patient population is changing accordingly, [and] all of the interventions continue to change. We’ve not been keeping up in our data collection for the safety and effectiveness of these interventions as they continue to evolve," commented committee chair, Clifford Goodman, Ph.D. "This means that to the extent that CMS is going to revisit its coverage decision making over time, this is an ongoing data collection issue. You’re not done collecting these data; you may not ever be done collecting these data as long as the patient population continues to change and as long as we have very innovative people improving these interventions."
The panel was asked to vote on several questions regarding their confidence level that there is adequate evidence to identify specific patient populations and in some cases to select the best treatment option. Most scenarios garnered an "intermediate" confidence level. Confidence ran high only for the lowest-risk patients.
The panel voted that they had moderate confidence that the evidence is adequate to determine whether:
• Patients who are asymptomatic for carotid atherosclerosis can be identified as being at high risk for stroke in either cerebral hemisphere.
• Patients who are considering carotid revascularization can be identified as being at high risk for adverse events from CEA.
• Either CAS or CEA is the favored treatment strategy compared to BMT alone in patients with symptomatic carotid atherosclerosis and narrowing (at least 50% by angiography or at least 70% by ultrasound) who are not generally at high risk for adverse events from CEA.
• CAS, CEA, or BMT is the favored treatment strategy to decrease stroke or death for patients with asymptomatic carotid atherosclerosis.
In addition, for patients with symptomatic carotid atherosclerosis, who are not generally at high risk for adverse events from CEA, the panel had intermediate confidence that CEA is the favored treatment strategy to decrease stroke and death. For patients with asymptomatic carotid atherosclerosis, the panel had intermediate to high confidence that BMT is the favored treatment strategy to decrease stroke and death.
However, the panel was less confident that for patients with asymptomatic carotid atherosclerosis but with carotid narrowing – who are not generally considered at high risk for adverse events from CEA – that there is adequate evidence to determine whether or not either CAS or CEA is the favored strategy compared to BMT alone to decrease stroke or death in the Medicare population. The panel had very low confidence that there is adequate evidence to determine whether carotid artery screening of asymptomatic people decreases stroke or death.
The panel primarily based its decisions on the results of a handful of randomized, controlled trials with different patient populations, at different time periods, using different criteria to assess efficacy and safety. The results of the most recent of these – the CREST trial (Carotid Revascularization Endarterectomy vs. Stenting Trial) – were announced in 2010. The overall safety and efficacy of the CAS and CEA at 4 years were considered equivocal in terms of benefits for both men and for women, for patients who had previously had a stroke, and for those who had not (N. Engl. J. Med. 2010;363:11-23).
However, differences were seen between the two procedures for heart attacks and strokes. More heart attacks occurred with CEA – 2.3% vs. 1.1% – than for CAS, while more strokes occurred in the stenting group – 4.1% vs. 2.3% –than for CEA in the weeks following the procedure. There was also a difference in outcomes based on patient age. At approximately age 69 and younger, stenting results were slightly better, with CAS benefits increasing for decreasing patient age. Conversely, for patients older than 70, CEA results were slightly superior to stenting, with larger benefits for surgery with older patient age.
Instead of answering the question of which technique has better outcomes and safety, the trial left proponents of each technique still arguing.
In addition, BMT has not been assessed in clinical trials for many years and has evolved substantially since that time with the development and growing use of statins, antiplatelet medications, beta-blockers, ACE inhibitors, calcium channel blockers, and others.
What is lacking is a randomized, controlled trial pitting all three treatments against one another in a head-to-head comparison of the most current techniques, the panel noted repeatedly.
"I think that drawing conclusions from indirect comparisons of things that were done in the past is incredibly hazardous and we need to have a contemporary direct comparison to know what the right thing to do is," argued panel member Dr. Larry B. Goldstein, who is a professor of neurology and director of the Duke Stroke Center in Chapel Hill, N.C.
Both surgery and CAS received a class I recommendation in guidelines published by the American Heart Association and other organizations in 2011 (J. Am. Coll. Cardiol. 2011;57:1002-44), but the level of evidence was stronger for CEA (level A) than for CAS (level B).
Planning is in the works for a possible CREST II trial in asymptomatic patients intended to compare intervention (with either CEA or CAS) vs. medical treatment alone. Many panel members expressed the need for this trial to proceed.
Twelve members of the panel reported that they did not have any relevant financial relationships. Dr. Spence reported receiving speaking fees from several pharmaceutical companies, Dr. Curtis reported that he owns stock in Medtronic and receives grant support from Boston Scientific, industry representative Dr. Peter Juhn works for Medco Health Solutions, and committee chair Dr. Clifford Goodman is the senior vice president at the Lewin Group, which has ties to both government and industry.
There are still no clear answers about the best treatments to use to prevent stroke in patients with carotid artery stenosis, even with the input of a Medicare advisory panel of experts.
The Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) voted yesterday that they are only moderately confident that there is adequate evidence to determine which interventions – carotid artery endarterectomy (CEA), carotid artery stenting (CAS), or best medical treatment (BMT) – to use for different patient populations within the overall Medicare population. There’s unlikely to be any consensus in the near future as patient populations, detection, and treatments evolve.
"If there was ever a moving target problem in assessing health care interventions, this was one. The epidemiology is changing, the patient population is changing accordingly, [and] all of the interventions continue to change. We’ve not been keeping up in our data collection for the safety and effectiveness of these interventions as they continue to evolve," commented committee chair, Clifford Goodman, Ph.D. "This means that to the extent that CMS is going to revisit its coverage decision making over time, this is an ongoing data collection issue. You’re not done collecting these data; you may not ever be done collecting these data as long as the patient population continues to change and as long as we have very innovative people improving these interventions."
The panel was asked to vote on several questions regarding their confidence level that there is adequate evidence to identify specific patient populations and in some cases to select the best treatment option. Most scenarios garnered an "intermediate" confidence level. Confidence ran high only for the lowest-risk patients.
The panel voted that they had moderate confidence that the evidence is adequate to determine whether:
• Patients who are asymptomatic for carotid atherosclerosis can be identified as being at high risk for stroke in either cerebral hemisphere.
• Patients who are considering carotid revascularization can be identified as being at high risk for adverse events from CEA.
• Either CAS or CEA is the favored treatment strategy compared to BMT alone in patients with symptomatic carotid atherosclerosis and narrowing (at least 50% by angiography or at least 70% by ultrasound) who are not generally at high risk for adverse events from CEA.
• CAS, CEA, or BMT is the favored treatment strategy to decrease stroke or death for patients with asymptomatic carotid atherosclerosis.
In addition, for patients with symptomatic carotid atherosclerosis, who are not generally at high risk for adverse events from CEA, the panel had intermediate confidence that CEA is the favored treatment strategy to decrease stroke and death. For patients with asymptomatic carotid atherosclerosis, the panel had intermediate to high confidence that BMT is the favored treatment strategy to decrease stroke and death.
However, the panel was less confident that for patients with asymptomatic carotid atherosclerosis but with carotid narrowing – who are not generally considered at high risk for adverse events from CEA – that there is adequate evidence to determine whether or not either CAS or CEA is the favored strategy compared to BMT alone to decrease stroke or death in the Medicare population. The panel had very low confidence that there is adequate evidence to determine whether carotid artery screening of asymptomatic people decreases stroke or death.
The panel primarily based its decisions on the results of a handful of randomized, controlled trials with different patient populations, at different time periods, using different criteria to assess efficacy and safety. The results of the most recent of these – the CREST trial (Carotid Revascularization Endarterectomy vs. Stenting Trial) – were announced in 2010. The overall safety and efficacy of the CAS and CEA at 4 years were considered equivocal in terms of benefits for both men and for women, for patients who had previously had a stroke, and for those who had not (N. Engl. J. Med. 2010;363:11-23).
However, differences were seen between the two procedures for heart attacks and strokes. More heart attacks occurred with CEA – 2.3% vs. 1.1% – than for CAS, while more strokes occurred in the stenting group – 4.1% vs. 2.3% –than for CEA in the weeks following the procedure. There was also a difference in outcomes based on patient age. At approximately age 69 and younger, stenting results were slightly better, with CAS benefits increasing for decreasing patient age. Conversely, for patients older than 70, CEA results were slightly superior to stenting, with larger benefits for surgery with older patient age.
Instead of answering the question of which technique has better outcomes and safety, the trial left proponents of each technique still arguing.
In addition, BMT has not been assessed in clinical trials for many years and has evolved substantially since that time with the development and growing use of statins, antiplatelet medications, beta-blockers, ACE inhibitors, calcium channel blockers, and others.
What is lacking is a randomized, controlled trial pitting all three treatments against one another in a head-to-head comparison of the most current techniques, the panel noted repeatedly.
"I think that drawing conclusions from indirect comparisons of things that were done in the past is incredibly hazardous and we need to have a contemporary direct comparison to know what the right thing to do is," argued panel member Dr. Larry B. Goldstein, who is a professor of neurology and director of the Duke Stroke Center in Chapel Hill, N.C.
Both surgery and CAS received a class I recommendation in guidelines published by the American Heart Association and other organizations in 2011 (J. Am. Coll. Cardiol. 2011;57:1002-44), but the level of evidence was stronger for CEA (level A) than for CAS (level B).
Planning is in the works for a possible CREST II trial in asymptomatic patients intended to compare intervention (with either CEA or CAS) vs. medical treatment alone. Many panel members expressed the need for this trial to proceed.
Twelve members of the panel reported that they did not have any relevant financial relationships. Dr. Spence reported receiving speaking fees from several pharmaceutical companies, Dr. Curtis reported that he owns stock in Medtronic and receives grant support from Boston Scientific, industry representative Dr. Peter Juhn works for Medco Health Solutions, and committee chair Dr. Clifford Goodman is the senior vice president at the Lewin Group, which has ties to both government and industry.
FROM A MEETING OF THE MEDICARE EVIDENCE DEVELOPMENT AND COVERAGE ADVISORY COMMITTEE
Do EPDs Make Carotid Stenting Safer?
The potential for filter-type embolic protection devices to reduce a patient’s risk of macroemboli during carotid angioplasty and stenting makes them ideal for preventing embolic stroke. Or does it? Based on the literature available, there is not enough evidence to suggest that embolic protection devices (EPDs) should be mandatory when treating carotid artery stenosis (CAS), said Dr. Jos C. van den Berg of Ospedale Regionale di Lugano, Switzerland. And some studies suggest that the use of EPDs when treating carotid artery stenosis increases the risk of complications, including stroke and death.
Dr. van den Berg discussed his literature review and how widely study results varied at the VEITH Symposium.
For example, the World Carotid Artery Stent Registry shows that the stroke and death rate was 5.29% without protection devices versus 2.23% with protection devices.
The Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial revealed that at 30 days after surgery the stroke rate was 3.9 times higher in unprotected carotid angioplasty and stenting (CAS) (4/15 vs. 5/58).
Other studies show the opposite to be true. For example, in 5,341 patients in the Prospective Registry of Carotid Angioplasty and Stenting (Pro-CAS), periprocedural stroke and death rates with and without protective devices were 3.4% and 3.2%, respectively – an indicator that EPD use is not an independent predictor of adverse outcomes.
In another study, researchers assigned 30 symptomatic patients to filter-protected or unprotected CAS. Magnetic resonance imaging revealed new lesions in 29% of patients treated with EPDs versus 18% in the unprotected group. This difference was sustained at 30 days (26% vs. 12%, respectively).
Meanwhile, secondary analysis of the SPACE (Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy) study, which used an EPD in 25% of procedures (145 vs. 418), showed a 30-day ipsilateral stroke and death rate of 6.2% in the unprotected group and 8.3% in the protected group.
Adverse events were significantly lower in patients treated with a closed cell–design stent. In the open cell–design stent group, the use of EPDs showed some benefit. In all, 50% of all adverse events occurred peri-interventionally, 40% after removal of the endovascular device, and 10% during catheter manipulation in the aortic arch.
Other studies show equal outcomes of unprotected stenting and procedures using protection devices. For example, pooled analysis of the SPACE and EVA-3S studies showed no difference in adverse outcomes between the unprotected and protected groups (7.3% for unprotected, 8.1% for protected group).
"Results of filter-protected versus unprotected stenting are similar," Dr. van den Berg said. "Part of the adverse events occur postprocedure. Hyperperfusion will not be prevented, and protection will not prevent contralateral and vertebrobasilar stroke (arch manipulation). A substantial part of strokes do not occur during the procedure." Another consideration is that "protective devices are not free of complications," he said.
Disadvantages to EPDs include additional time needed to perform a procedure, an additional learning curve, and increased costs. In protected CAS (using filter-type devices), predilatation is sometimes necessary and large emboli (1,000 micrometers) may occur when the surgeon passes a distal EPD through the stenosis. Potential EPD complications include vasospasm and dissection, intimal damage, and microemboli during deployment and retrieval.
"There is no level 1 evidence for a beneficial effect of distal filter protection devices," Dr. van den Berg said. "Several studies indicate that carotid artery stenting can be performed at least as safely without (distal) EPD. Carotid stenting with filter-type EPDs leads to more (micro) embolic events. A large part of the procedure remains unprotected anyway, and adverse events related to EPDs do occur." Given these contradictory findings, more studies are needed to decide whether EPDs should become mandatory with CAS, he added.
Cerebral protection devices (CPDs) have been generally considered to play a critical role in reducing neurological event rates associated with carotid stenting. Dr. van den Berg has found that the data for the benefits of protection devices is equivocal. However, the manipulation required to place and retrieve the device may result in enough events to reduce the protective value of CPDs. Further study into this issue is warranted. It may be that technical aspects of the arch, neck vessels, and lesion may influence the net benefit or harm of using protection devices.
Professor Robert A Fitridge, M.S., is one of two new international associate medical editors for Vascular Specialist. He is located at the University of Adelaide, Discipline of Surgery, The Queen Elizabeth Hospital, Adelaide, Australia.
Cerebral protection devices (CPDs) have been generally considered to play a critical role in reducing neurological event rates associated with carotid stenting. Dr. van den Berg has found that the data for the benefits of protection devices is equivocal. However, the manipulation required to place and retrieve the device may result in enough events to reduce the protective value of CPDs. Further study into this issue is warranted. It may be that technical aspects of the arch, neck vessels, and lesion may influence the net benefit or harm of using protection devices.
Professor Robert A Fitridge, M.S., is one of two new international associate medical editors for Vascular Specialist. He is located at the University of Adelaide, Discipline of Surgery, The Queen Elizabeth Hospital, Adelaide, Australia.
Cerebral protection devices (CPDs) have been generally considered to play a critical role in reducing neurological event rates associated with carotid stenting. Dr. van den Berg has found that the data for the benefits of protection devices is equivocal. However, the manipulation required to place and retrieve the device may result in enough events to reduce the protective value of CPDs. Further study into this issue is warranted. It may be that technical aspects of the arch, neck vessels, and lesion may influence the net benefit or harm of using protection devices.
Professor Robert A Fitridge, M.S., is one of two new international associate medical editors for Vascular Specialist. He is located at the University of Adelaide, Discipline of Surgery, The Queen Elizabeth Hospital, Adelaide, Australia.
The potential for filter-type embolic protection devices to reduce a patient’s risk of macroemboli during carotid angioplasty and stenting makes them ideal for preventing embolic stroke. Or does it? Based on the literature available, there is not enough evidence to suggest that embolic protection devices (EPDs) should be mandatory when treating carotid artery stenosis (CAS), said Dr. Jos C. van den Berg of Ospedale Regionale di Lugano, Switzerland. And some studies suggest that the use of EPDs when treating carotid artery stenosis increases the risk of complications, including stroke and death.
Dr. van den Berg discussed his literature review and how widely study results varied at the VEITH Symposium.
For example, the World Carotid Artery Stent Registry shows that the stroke and death rate was 5.29% without protection devices versus 2.23% with protection devices.
The Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial revealed that at 30 days after surgery the stroke rate was 3.9 times higher in unprotected carotid angioplasty and stenting (CAS) (4/15 vs. 5/58).
Other studies show the opposite to be true. For example, in 5,341 patients in the Prospective Registry of Carotid Angioplasty and Stenting (Pro-CAS), periprocedural stroke and death rates with and without protective devices were 3.4% and 3.2%, respectively – an indicator that EPD use is not an independent predictor of adverse outcomes.
In another study, researchers assigned 30 symptomatic patients to filter-protected or unprotected CAS. Magnetic resonance imaging revealed new lesions in 29% of patients treated with EPDs versus 18% in the unprotected group. This difference was sustained at 30 days (26% vs. 12%, respectively).
Meanwhile, secondary analysis of the SPACE (Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy) study, which used an EPD in 25% of procedures (145 vs. 418), showed a 30-day ipsilateral stroke and death rate of 6.2% in the unprotected group and 8.3% in the protected group.
Adverse events were significantly lower in patients treated with a closed cell–design stent. In the open cell–design stent group, the use of EPDs showed some benefit. In all, 50% of all adverse events occurred peri-interventionally, 40% after removal of the endovascular device, and 10% during catheter manipulation in the aortic arch.
Other studies show equal outcomes of unprotected stenting and procedures using protection devices. For example, pooled analysis of the SPACE and EVA-3S studies showed no difference in adverse outcomes between the unprotected and protected groups (7.3% for unprotected, 8.1% for protected group).
"Results of filter-protected versus unprotected stenting are similar," Dr. van den Berg said. "Part of the adverse events occur postprocedure. Hyperperfusion will not be prevented, and protection will not prevent contralateral and vertebrobasilar stroke (arch manipulation). A substantial part of strokes do not occur during the procedure." Another consideration is that "protective devices are not free of complications," he said.
Disadvantages to EPDs include additional time needed to perform a procedure, an additional learning curve, and increased costs. In protected CAS (using filter-type devices), predilatation is sometimes necessary and large emboli (1,000 micrometers) may occur when the surgeon passes a distal EPD through the stenosis. Potential EPD complications include vasospasm and dissection, intimal damage, and microemboli during deployment and retrieval.
"There is no level 1 evidence for a beneficial effect of distal filter protection devices," Dr. van den Berg said. "Several studies indicate that carotid artery stenting can be performed at least as safely without (distal) EPD. Carotid stenting with filter-type EPDs leads to more (micro) embolic events. A large part of the procedure remains unprotected anyway, and adverse events related to EPDs do occur." Given these contradictory findings, more studies are needed to decide whether EPDs should become mandatory with CAS, he added.
The potential for filter-type embolic protection devices to reduce a patient’s risk of macroemboli during carotid angioplasty and stenting makes them ideal for preventing embolic stroke. Or does it? Based on the literature available, there is not enough evidence to suggest that embolic protection devices (EPDs) should be mandatory when treating carotid artery stenosis (CAS), said Dr. Jos C. van den Berg of Ospedale Regionale di Lugano, Switzerland. And some studies suggest that the use of EPDs when treating carotid artery stenosis increases the risk of complications, including stroke and death.
Dr. van den Berg discussed his literature review and how widely study results varied at the VEITH Symposium.
For example, the World Carotid Artery Stent Registry shows that the stroke and death rate was 5.29% without protection devices versus 2.23% with protection devices.
The Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial revealed that at 30 days after surgery the stroke rate was 3.9 times higher in unprotected carotid angioplasty and stenting (CAS) (4/15 vs. 5/58).
Other studies show the opposite to be true. For example, in 5,341 patients in the Prospective Registry of Carotid Angioplasty and Stenting (Pro-CAS), periprocedural stroke and death rates with and without protective devices were 3.4% and 3.2%, respectively – an indicator that EPD use is not an independent predictor of adverse outcomes.
In another study, researchers assigned 30 symptomatic patients to filter-protected or unprotected CAS. Magnetic resonance imaging revealed new lesions in 29% of patients treated with EPDs versus 18% in the unprotected group. This difference was sustained at 30 days (26% vs. 12%, respectively).
Meanwhile, secondary analysis of the SPACE (Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy) study, which used an EPD in 25% of procedures (145 vs. 418), showed a 30-day ipsilateral stroke and death rate of 6.2% in the unprotected group and 8.3% in the protected group.
Adverse events were significantly lower in patients treated with a closed cell–design stent. In the open cell–design stent group, the use of EPDs showed some benefit. In all, 50% of all adverse events occurred peri-interventionally, 40% after removal of the endovascular device, and 10% during catheter manipulation in the aortic arch.
Other studies show equal outcomes of unprotected stenting and procedures using protection devices. For example, pooled analysis of the SPACE and EVA-3S studies showed no difference in adverse outcomes between the unprotected and protected groups (7.3% for unprotected, 8.1% for protected group).
"Results of filter-protected versus unprotected stenting are similar," Dr. van den Berg said. "Part of the adverse events occur postprocedure. Hyperperfusion will not be prevented, and protection will not prevent contralateral and vertebrobasilar stroke (arch manipulation). A substantial part of strokes do not occur during the procedure." Another consideration is that "protective devices are not free of complications," he said.
Disadvantages to EPDs include additional time needed to perform a procedure, an additional learning curve, and increased costs. In protected CAS (using filter-type devices), predilatation is sometimes necessary and large emboli (1,000 micrometers) may occur when the surgeon passes a distal EPD through the stenosis. Potential EPD complications include vasospasm and dissection, intimal damage, and microemboli during deployment and retrieval.
"There is no level 1 evidence for a beneficial effect of distal filter protection devices," Dr. van den Berg said. "Several studies indicate that carotid artery stenting can be performed at least as safely without (distal) EPD. Carotid stenting with filter-type EPDs leads to more (micro) embolic events. A large part of the procedure remains unprotected anyway, and adverse events related to EPDs do occur." Given these contradictory findings, more studies are needed to decide whether EPDs should become mandatory with CAS, he added.
Urgent Intervention After TIA Reduced Risk
Urgent intervention after a transient ischemic attack by an acute care team followed by nurse-conducted health counseling can substantially reduce the risk of new vascular events within the first year, according to results of the Aarhus TIA study.
Careful evaluation and use of preventive measures helped the team lower the cumulative risk of stroke below predicted levels 7 days and 90 days after TIA.
Immediate intervention is important because TIA can increase patients’ risk of ischemic stroke by more than 30% within the first 3 months after the attack. Studies have shown that urgent intervention can substantially reduce this risk, but little is known about stroke risk beyond 90 days. Additional studies have shown poor treatment and compliance rates for preventive measures such as cholesterol and blood pressure reduction and smoking cessation.
Dr. Paul von Weitzel-Mudersbach and his colleagues at Aarhus (Denmark) University Hospital established an acute TIA team that served patients with TIA on the stroke unit and the TIA clinic, and examined all patients with TIA who were referred to the hospital between March 1, 2007, and Feb. 28, 2008. A total of 306 patients (56.2% men) with a median age of 65.8 years met the study’s inclusion criteria. These patients underwent a detailed diagnostic work-up and received 150 mg aspirin immediately after CT/MRI. Patients with symptomatic carotid or intracerebral stenosis also received 300 mg clopidogrel unless carotid endarterectomy was planned within 1 week. A trained nurse discussed lifestyle changes, including smoking cessation, healthy diet, and physical exercise at baseline and during follow-up telephone interviews at 7 days and 90 days (Eur. J. Neurol. 2011;18:1285-90).
Urgent treatment was associated with a reduced risk of adverse clinical outcomes, the researchers found. Within 1 year, 16 (5%) of the 306 patients had a stroke, non-fatal MI, or vascular death. The cumulative stroke risk was 1.3% after 2 days. The intervention resulted in a significantly lower cumulative stroke risk than what was predicted with ABCD2 criteria after both 7 days (1.6% vs. 4.5%) and 90 days (2% vs. 7.5%).
At the end of 1 year, the cumulated stroke risk was 4.2% (13 patients, all had ischemic stroke), and the cumulated mortality was 2.9%, or 9 patients; 5 of these deaths were due to vascular causes, and the remainder were caused by cancer and sepsis. TIA recurred in the first year in 10.2% of patients. There were no non-fatal MIs.
The investigators found that 8.1% of patients underwent carotid endarterectomy, with a median time to operation of 11 days after the first contact with the acute TIA team, 12.5 days after the call for attention, and 17.5 days after the index TIA.
The number of patients who achieved the secondary prevention target increased from 34% of patients at baseline to 48% after 1 year. The secondary prevention target was considered attained if a patient fulfilled at least three of the following four criteria: systolic blood pressure 130 mm Hg or less and diastolic 80 mm Hg or less; total cholesterol less than 4.5 mmol/L; no smoking; and self-reported adherence to antithrombotic treatment. At least 95% of patients fulfilled at least one prevention measure after 1 year.
Patients with large artery disease (LAD) had the best compliance: 55% reached the secondary prevention target and 100% were adherent to antithrombotic treatment. LAD was not a predictor for vascular events within the first year, possibly due to good adherence to secondary prevention.
The study was supported by the Danish Heart Foundation and the Research Council in the former Aarhus County. The authors had no disclosures.☐
Although it should have been self-evident for many years, performing carotid intervention in a vacuum, meaning without attention to addressing atherogenic risk factors (smoking, hypertension, hyperlipidemia, etc), is unacceptable practice. As vascular interventionalists who perform both carotid endarterectomy and carotid stenting, aggressive and comprehensive medical management is integral to the short and long-tem success of our interventions. This has been repeatedly emphasized and integrated into our treatment algorithms, while this study and the results provide strong support for this approach. As we strive for durability following carotid interventions, having a dialogue with our patients about these issues at the outset is clearly the preferred approach.
Dr. Ron Fairman is professor of surgery and chief of the division of Vascular Surgery and Endovascular Therapy at the Hospital of the University of Pennsylvania in Philadelphia. He is an associate med-ical editor for Vascular Specialist.
Although it should have been self-evident for many years, performing carotid intervention in a vacuum, meaning without attention to addressing atherogenic risk factors (smoking, hypertension, hyperlipidemia, etc), is unacceptable practice. As vascular interventionalists who perform both carotid endarterectomy and carotid stenting, aggressive and comprehensive medical management is integral to the short and long-tem success of our interventions. This has been repeatedly emphasized and integrated into our treatment algorithms, while this study and the results provide strong support for this approach. As we strive for durability following carotid interventions, having a dialogue with our patients about these issues at the outset is clearly the preferred approach.
Dr. Ron Fairman is professor of surgery and chief of the division of Vascular Surgery and Endovascular Therapy at the Hospital of the University of Pennsylvania in Philadelphia. He is an associate med-ical editor for Vascular Specialist.
Although it should have been self-evident for many years, performing carotid intervention in a vacuum, meaning without attention to addressing atherogenic risk factors (smoking, hypertension, hyperlipidemia, etc), is unacceptable practice. As vascular interventionalists who perform both carotid endarterectomy and carotid stenting, aggressive and comprehensive medical management is integral to the short and long-tem success of our interventions. This has been repeatedly emphasized and integrated into our treatment algorithms, while this study and the results provide strong support for this approach. As we strive for durability following carotid interventions, having a dialogue with our patients about these issues at the outset is clearly the preferred approach.
Dr. Ron Fairman is professor of surgery and chief of the division of Vascular Surgery and Endovascular Therapy at the Hospital of the University of Pennsylvania in Philadelphia. He is an associate med-ical editor for Vascular Specialist.
Urgent intervention after a transient ischemic attack by an acute care team followed by nurse-conducted health counseling can substantially reduce the risk of new vascular events within the first year, according to results of the Aarhus TIA study.
Careful evaluation and use of preventive measures helped the team lower the cumulative risk of stroke below predicted levels 7 days and 90 days after TIA.
Immediate intervention is important because TIA can increase patients’ risk of ischemic stroke by more than 30% within the first 3 months after the attack. Studies have shown that urgent intervention can substantially reduce this risk, but little is known about stroke risk beyond 90 days. Additional studies have shown poor treatment and compliance rates for preventive measures such as cholesterol and blood pressure reduction and smoking cessation.
Dr. Paul von Weitzel-Mudersbach and his colleagues at Aarhus (Denmark) University Hospital established an acute TIA team that served patients with TIA on the stroke unit and the TIA clinic, and examined all patients with TIA who were referred to the hospital between March 1, 2007, and Feb. 28, 2008. A total of 306 patients (56.2% men) with a median age of 65.8 years met the study’s inclusion criteria. These patients underwent a detailed diagnostic work-up and received 150 mg aspirin immediately after CT/MRI. Patients with symptomatic carotid or intracerebral stenosis also received 300 mg clopidogrel unless carotid endarterectomy was planned within 1 week. A trained nurse discussed lifestyle changes, including smoking cessation, healthy diet, and physical exercise at baseline and during follow-up telephone interviews at 7 days and 90 days (Eur. J. Neurol. 2011;18:1285-90).
Urgent treatment was associated with a reduced risk of adverse clinical outcomes, the researchers found. Within 1 year, 16 (5%) of the 306 patients had a stroke, non-fatal MI, or vascular death. The cumulative stroke risk was 1.3% after 2 days. The intervention resulted in a significantly lower cumulative stroke risk than what was predicted with ABCD2 criteria after both 7 days (1.6% vs. 4.5%) and 90 days (2% vs. 7.5%).
At the end of 1 year, the cumulated stroke risk was 4.2% (13 patients, all had ischemic stroke), and the cumulated mortality was 2.9%, or 9 patients; 5 of these deaths were due to vascular causes, and the remainder were caused by cancer and sepsis. TIA recurred in the first year in 10.2% of patients. There were no non-fatal MIs.
The investigators found that 8.1% of patients underwent carotid endarterectomy, with a median time to operation of 11 days after the first contact with the acute TIA team, 12.5 days after the call for attention, and 17.5 days after the index TIA.
The number of patients who achieved the secondary prevention target increased from 34% of patients at baseline to 48% after 1 year. The secondary prevention target was considered attained if a patient fulfilled at least three of the following four criteria: systolic blood pressure 130 mm Hg or less and diastolic 80 mm Hg or less; total cholesterol less than 4.5 mmol/L; no smoking; and self-reported adherence to antithrombotic treatment. At least 95% of patients fulfilled at least one prevention measure after 1 year.
Patients with large artery disease (LAD) had the best compliance: 55% reached the secondary prevention target and 100% were adherent to antithrombotic treatment. LAD was not a predictor for vascular events within the first year, possibly due to good adherence to secondary prevention.
The study was supported by the Danish Heart Foundation and the Research Council in the former Aarhus County. The authors had no disclosures.☐
Urgent intervention after a transient ischemic attack by an acute care team followed by nurse-conducted health counseling can substantially reduce the risk of new vascular events within the first year, according to results of the Aarhus TIA study.
Careful evaluation and use of preventive measures helped the team lower the cumulative risk of stroke below predicted levels 7 days and 90 days after TIA.
Immediate intervention is important because TIA can increase patients’ risk of ischemic stroke by more than 30% within the first 3 months after the attack. Studies have shown that urgent intervention can substantially reduce this risk, but little is known about stroke risk beyond 90 days. Additional studies have shown poor treatment and compliance rates for preventive measures such as cholesterol and blood pressure reduction and smoking cessation.
Dr. Paul von Weitzel-Mudersbach and his colleagues at Aarhus (Denmark) University Hospital established an acute TIA team that served patients with TIA on the stroke unit and the TIA clinic, and examined all patients with TIA who were referred to the hospital between March 1, 2007, and Feb. 28, 2008. A total of 306 patients (56.2% men) with a median age of 65.8 years met the study’s inclusion criteria. These patients underwent a detailed diagnostic work-up and received 150 mg aspirin immediately after CT/MRI. Patients with symptomatic carotid or intracerebral stenosis also received 300 mg clopidogrel unless carotid endarterectomy was planned within 1 week. A trained nurse discussed lifestyle changes, including smoking cessation, healthy diet, and physical exercise at baseline and during follow-up telephone interviews at 7 days and 90 days (Eur. J. Neurol. 2011;18:1285-90).
Urgent treatment was associated with a reduced risk of adverse clinical outcomes, the researchers found. Within 1 year, 16 (5%) of the 306 patients had a stroke, non-fatal MI, or vascular death. The cumulative stroke risk was 1.3% after 2 days. The intervention resulted in a significantly lower cumulative stroke risk than what was predicted with ABCD2 criteria after both 7 days (1.6% vs. 4.5%) and 90 days (2% vs. 7.5%).
At the end of 1 year, the cumulated stroke risk was 4.2% (13 patients, all had ischemic stroke), and the cumulated mortality was 2.9%, or 9 patients; 5 of these deaths were due to vascular causes, and the remainder were caused by cancer and sepsis. TIA recurred in the first year in 10.2% of patients. There were no non-fatal MIs.
The investigators found that 8.1% of patients underwent carotid endarterectomy, with a median time to operation of 11 days after the first contact with the acute TIA team, 12.5 days after the call for attention, and 17.5 days after the index TIA.
The number of patients who achieved the secondary prevention target increased from 34% of patients at baseline to 48% after 1 year. The secondary prevention target was considered attained if a patient fulfilled at least three of the following four criteria: systolic blood pressure 130 mm Hg or less and diastolic 80 mm Hg or less; total cholesterol less than 4.5 mmol/L; no smoking; and self-reported adherence to antithrombotic treatment. At least 95% of patients fulfilled at least one prevention measure after 1 year.
Patients with large artery disease (LAD) had the best compliance: 55% reached the secondary prevention target and 100% were adherent to antithrombotic treatment. LAD was not a predictor for vascular events within the first year, possibly due to good adherence to secondary prevention.
The study was supported by the Danish Heart Foundation and the Research Council in the former Aarhus County. The authors had no disclosures.☐
Major Finding: Urgent intervention after TIA resulted in a significantly lower cumulative stroke risk than what was predicted with ABCD2 criteria after both 7 days (1.6% vs. 4.5%) and 90 days (2% vs. 7.5%).
Data Source: Evaluation of 306 patients with TIA referred to Aarhus University Hospital between March 1, 2007, and Feb. 28, 2008.
Disclosures: The study was supported by the Danish Heart Foundation and the Research Council in the former Aarhus County. The authors had no disclosures to report.
New Trial Proposed for Asymptomatic Stenosis
SAN DIEGO – Citing vastly improved management of carotid stenosis, the principal investigator of the Carotid Revascularization Endarterectomy vs. Stenting Trial has called for yet another trial, this one to clarify risks and benefits of carotid revascularization with either endarterectomy or stenting vs. aggressive medical management to prevent stroke in asymptomatic patients.
Every contemporary intervention to prevent strokes – endarterectomy (CEA), carotid stenting (CAS), and aggressive medical management of risk factors – is becoming safer and more efficacious, said Dr. Thomas G. Brott of the Mayo Clinic, Jacksonville, Fla., at the annual meeting of the American Neurological Association. The dilemma, according to Dr. Brott: "We don’t know how they stack up."
Current clinical practice was shaped by results of the ACAS (Asymptomatic Carotid Atherosclerosis Study) and the ACST (Asymptomatic Carotid Surgery Trial), in which CEA trumped medical management for prevention of stroke.
CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial), which began enrolling only symptomatic patients, added asymptomatic subjects after publication of ACST results in 2004. In the end, it concluded that perioperative stroke and death rates were "low and similar" for CAS (2.5%) and CEA (1.4%).
Revascularization with CAS has remained somewhat controversial, with the current body of evidence suggesting the need for better control of rare, but real, complications and mortality, particularly in Medicare-aged patients.
Meanwhile, recent epidemiologic studies demonstrate profoundly lowered stroke rates without surgery or stenting, via intensive medical therapy to control risk factors such as hypertension, hyperlipidemia, and insulin resistance. For example, a population-based study in the United Kingdom found that the rate of ipsilateral stroke in medically treated patients who had carotid stenosis of 50% or greater was 0.3%.
Two new, randomized trials suggest that intensive medical therapy can indeed produce far more impressive results than anticipated in a prospective study of patients with asymptomatic carotid stenosis (Arch. Neurol. 2010;67:180-6), and even in patients with severe intracranial artery stenosis in the SAMMPRIS (Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) trial (N. Engl. J. Med. 2011;365:993-1003).
Various groups, including CMS, the American Heart Association, and the American Stroke Association, have weighed in with opinions on the management of asymptomatic patients in the hopes of preventing an estimated 5%-10% of all strokes.
The problem, Dr. Brott said, is not a lack of guidance for treatment decisions, but the lack of a direct comparison of carotid revascularization (either CEA or CAS) vs. contemporary medical therapy.
"We have evolving opinion without evolving data," he asserted.
Much is at stake, with CEA selected for 80,000-90,000 asymptomatic patients each year in the United States, and CAS for another 40,000 patients.
Dr. Brott proposed a trial to enroll 950 patients at 70 centers, with the CREST team providing the interventional arm (either endarterectomy or stenting) and the SAMMPRIS team providing the medical management arm in asymptomatic patients exhibiting at least 70% carotid stenosis by angiography or ultrasound.
The effect size of 1.2% would be equal to the absolute difference in the primary end point (periprocedural stroke and death or subsequent ipsilateral stroke) in the ACAS trial, a difference substantial enough to alter clinical practice.
Dr. Brot said he had no disclosures.
There is building consensus among vascular surgeons and other providers who manage patients with asymptomatic carotid stenosis that the results from the medical arm of ACAS are obsolete considering current medical therapy. For instance, since ACAS was published, statin use has become very prevalent. I agree that a modern trial comparing carotid intervention with medical therapy is necessary but the design of such a trial should be carefully considered.
It would be a huge mistake to fail to stratify for high-grade(around 80%) vs. moderate (around 50%-79%) stenosis with enough power in each group to draw separate conclusions. Similarly, it would be a mistake not to include morphological studies of plaque composition (calcification, hemorrhage, fibrous cap thickness, necrotic core size) which are now possible with sophisticated MRI protocols. Whether these important study design elements can be satisfied with a trial of 950 patients is unclear from this report of Dr Brott’s comments. In particular, a trial that fails to discriminate between high- and moderate-risk patients by lumping them all together will have little credibility upon completion and is likely to be money wasted.
Dr. Larry W. Kraiss is chief of Vascular Surgery at the University of Utah, Salt Lake City. and an associate medical editor of Vascular Specialist.
There is building consensus among vascular surgeons and other providers who manage patients with asymptomatic carotid stenosis that the results from the medical arm of ACAS are obsolete considering current medical therapy. For instance, since ACAS was published, statin use has become very prevalent. I agree that a modern trial comparing carotid intervention with medical therapy is necessary but the design of such a trial should be carefully considered.
It would be a huge mistake to fail to stratify for high-grade(around 80%) vs. moderate (around 50%-79%) stenosis with enough power in each group to draw separate conclusions. Similarly, it would be a mistake not to include morphological studies of plaque composition (calcification, hemorrhage, fibrous cap thickness, necrotic core size) which are now possible with sophisticated MRI protocols. Whether these important study design elements can be satisfied with a trial of 950 patients is unclear from this report of Dr Brott’s comments. In particular, a trial that fails to discriminate between high- and moderate-risk patients by lumping them all together will have little credibility upon completion and is likely to be money wasted.
Dr. Larry W. Kraiss is chief of Vascular Surgery at the University of Utah, Salt Lake City. and an associate medical editor of Vascular Specialist.
There is building consensus among vascular surgeons and other providers who manage patients with asymptomatic carotid stenosis that the results from the medical arm of ACAS are obsolete considering current medical therapy. For instance, since ACAS was published, statin use has become very prevalent. I agree that a modern trial comparing carotid intervention with medical therapy is necessary but the design of such a trial should be carefully considered.
It would be a huge mistake to fail to stratify for high-grade(around 80%) vs. moderate (around 50%-79%) stenosis with enough power in each group to draw separate conclusions. Similarly, it would be a mistake not to include morphological studies of plaque composition (calcification, hemorrhage, fibrous cap thickness, necrotic core size) which are now possible with sophisticated MRI protocols. Whether these important study design elements can be satisfied with a trial of 950 patients is unclear from this report of Dr Brott’s comments. In particular, a trial that fails to discriminate between high- and moderate-risk patients by lumping them all together will have little credibility upon completion and is likely to be money wasted.
Dr. Larry W. Kraiss is chief of Vascular Surgery at the University of Utah, Salt Lake City. and an associate medical editor of Vascular Specialist.
SAN DIEGO – Citing vastly improved management of carotid stenosis, the principal investigator of the Carotid Revascularization Endarterectomy vs. Stenting Trial has called for yet another trial, this one to clarify risks and benefits of carotid revascularization with either endarterectomy or stenting vs. aggressive medical management to prevent stroke in asymptomatic patients.
Every contemporary intervention to prevent strokes – endarterectomy (CEA), carotid stenting (CAS), and aggressive medical management of risk factors – is becoming safer and more efficacious, said Dr. Thomas G. Brott of the Mayo Clinic, Jacksonville, Fla., at the annual meeting of the American Neurological Association. The dilemma, according to Dr. Brott: "We don’t know how they stack up."
Current clinical practice was shaped by results of the ACAS (Asymptomatic Carotid Atherosclerosis Study) and the ACST (Asymptomatic Carotid Surgery Trial), in which CEA trumped medical management for prevention of stroke.
CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial), which began enrolling only symptomatic patients, added asymptomatic subjects after publication of ACST results in 2004. In the end, it concluded that perioperative stroke and death rates were "low and similar" for CAS (2.5%) and CEA (1.4%).
Revascularization with CAS has remained somewhat controversial, with the current body of evidence suggesting the need for better control of rare, but real, complications and mortality, particularly in Medicare-aged patients.
Meanwhile, recent epidemiologic studies demonstrate profoundly lowered stroke rates without surgery or stenting, via intensive medical therapy to control risk factors such as hypertension, hyperlipidemia, and insulin resistance. For example, a population-based study in the United Kingdom found that the rate of ipsilateral stroke in medically treated patients who had carotid stenosis of 50% or greater was 0.3%.
Two new, randomized trials suggest that intensive medical therapy can indeed produce far more impressive results than anticipated in a prospective study of patients with asymptomatic carotid stenosis (Arch. Neurol. 2010;67:180-6), and even in patients with severe intracranial artery stenosis in the SAMMPRIS (Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) trial (N. Engl. J. Med. 2011;365:993-1003).
Various groups, including CMS, the American Heart Association, and the American Stroke Association, have weighed in with opinions on the management of asymptomatic patients in the hopes of preventing an estimated 5%-10% of all strokes.
The problem, Dr. Brott said, is not a lack of guidance for treatment decisions, but the lack of a direct comparison of carotid revascularization (either CEA or CAS) vs. contemporary medical therapy.
"We have evolving opinion without evolving data," he asserted.
Much is at stake, with CEA selected for 80,000-90,000 asymptomatic patients each year in the United States, and CAS for another 40,000 patients.
Dr. Brott proposed a trial to enroll 950 patients at 70 centers, with the CREST team providing the interventional arm (either endarterectomy or stenting) and the SAMMPRIS team providing the medical management arm in asymptomatic patients exhibiting at least 70% carotid stenosis by angiography or ultrasound.
The effect size of 1.2% would be equal to the absolute difference in the primary end point (periprocedural stroke and death or subsequent ipsilateral stroke) in the ACAS trial, a difference substantial enough to alter clinical practice.
Dr. Brot said he had no disclosures.
SAN DIEGO – Citing vastly improved management of carotid stenosis, the principal investigator of the Carotid Revascularization Endarterectomy vs. Stenting Trial has called for yet another trial, this one to clarify risks and benefits of carotid revascularization with either endarterectomy or stenting vs. aggressive medical management to prevent stroke in asymptomatic patients.
Every contemporary intervention to prevent strokes – endarterectomy (CEA), carotid stenting (CAS), and aggressive medical management of risk factors – is becoming safer and more efficacious, said Dr. Thomas G. Brott of the Mayo Clinic, Jacksonville, Fla., at the annual meeting of the American Neurological Association. The dilemma, according to Dr. Brott: "We don’t know how they stack up."
Current clinical practice was shaped by results of the ACAS (Asymptomatic Carotid Atherosclerosis Study) and the ACST (Asymptomatic Carotid Surgery Trial), in which CEA trumped medical management for prevention of stroke.
CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial), which began enrolling only symptomatic patients, added asymptomatic subjects after publication of ACST results in 2004. In the end, it concluded that perioperative stroke and death rates were "low and similar" for CAS (2.5%) and CEA (1.4%).
Revascularization with CAS has remained somewhat controversial, with the current body of evidence suggesting the need for better control of rare, but real, complications and mortality, particularly in Medicare-aged patients.
Meanwhile, recent epidemiologic studies demonstrate profoundly lowered stroke rates without surgery or stenting, via intensive medical therapy to control risk factors such as hypertension, hyperlipidemia, and insulin resistance. For example, a population-based study in the United Kingdom found that the rate of ipsilateral stroke in medically treated patients who had carotid stenosis of 50% or greater was 0.3%.
Two new, randomized trials suggest that intensive medical therapy can indeed produce far more impressive results than anticipated in a prospective study of patients with asymptomatic carotid stenosis (Arch. Neurol. 2010;67:180-6), and even in patients with severe intracranial artery stenosis in the SAMMPRIS (Stenting vs. Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) trial (N. Engl. J. Med. 2011;365:993-1003).
Various groups, including CMS, the American Heart Association, and the American Stroke Association, have weighed in with opinions on the management of asymptomatic patients in the hopes of preventing an estimated 5%-10% of all strokes.
The problem, Dr. Brott said, is not a lack of guidance for treatment decisions, but the lack of a direct comparison of carotid revascularization (either CEA or CAS) vs. contemporary medical therapy.
"We have evolving opinion without evolving data," he asserted.
Much is at stake, with CEA selected for 80,000-90,000 asymptomatic patients each year in the United States, and CAS for another 40,000 patients.
Dr. Brott proposed a trial to enroll 950 patients at 70 centers, with the CREST team providing the interventional arm (either endarterectomy or stenting) and the SAMMPRIS team providing the medical management arm in asymptomatic patients exhibiting at least 70% carotid stenosis by angiography or ultrasound.
The effect size of 1.2% would be equal to the absolute difference in the primary end point (periprocedural stroke and death or subsequent ipsilateral stroke) in the ACAS trial, a difference substantial enough to alter clinical practice.
Dr. Brot said he had no disclosures.
Major Finding:
Data Source:
Disclosures:
Octogenarian Stroke Rate Lower With Carotid Endarterectomy vs. Stenting
NAPLES, FLA. – When it comes to cerebral revascularization of octogenarian patients, carotid endarterectomy may be the treatment of choice unless compelling evidence points to carotid artery angioplasty and stenting, according to Dr. Caron B. Rockman.
She and her colleagues compared in-hospital perioperative stroke and death rates between these procedures among older and younger patients in a study of 54,658 patients who underwent carotid revascularization in 2004 and 2005 in the Healthcare Cost and Utilization Project National Inpatient Sample database.
Octogenarians have been excluded from many previous trials of carotid endarterectomy (CEA), said Dr. Rockman, who is on the surgery faculty and is director of clinical research in the division of vascular surgery at New York University. However, patients aged 80 years and older accounted for 10,820 (or nearly 20%) of the patients.
"Advanced age is relevant when treatment of asymptomatic severe cerebrovascular disease is considered," Dr. Rockman said.
The prevalence of preoperative symptoms was essentially the same in the octogenarian (5.4%) and younger (5.2%) groups.
"I am floored that 95% of octogenarians who get intervention are asymptomatic," said study discussant Dr. Samuel R. Money, chair of the department of surgery at the Mayo Clinic in Scottsdale, Ariz., at the meeting. Because the Nationwide Inpatient Sample data represent about 20% of U.S. hospital discharges, he estimated that approximately 50,000 people aged 80 years and older undergo these procedures each year in the United States. Dr. Money asked if such high utilization is justified in an era with medical management options such as statins. He estimated that these interventions cost $75 million to $1 billion annually.
"I agree with you," Dr. Rockman replied. "This is astounding."
She theorized that people who enter the ICD-9 procedural codes might incorrectly classify some patients as asymptomatic. "I hope people coding these are missing some of the symptomatology. It can be difficult to pick up [symptoms] from patient records, so I hope it’s not as alarming as it seems."
"However, I do think there is a role for endarterectomy or CAS in an elderly patient with severe disease," Dr. Rockman added.
The results of the study showed that "octogenarians had essentially equivalent rates of periprocedural stroke [1.1% in both groups], but slightly higher rates of in-hospital mortality than their younger counterparts," she said.
A meeting attendee asked why stroke outcomes were not significantly worse among octogenarians.
"I don’t know why I did not see it," said Dr. Rockman, noting that a separate meta-analysis of CAS studies found that the relative risk for periprocedural stroke in octogenarians fell above an acceptable rate of 3% in seven of eight studies (J. Am. Coll. Surg. 2009;208:1124-31). "It could be [because] this database is in-hospital only, so even if stroke occurs the day after discharge, it’s not included in this national database."
In a separate analysis of only octogenarian patients, the periprocedural stroke rate was significantly higher among those who underwent CAS than had CEA (2.2% vs. 1.1%). The rate also was consistently higher for both asymptomatic (1.9% vs. 0.9%) and symptomatic (5.2% vs. 2.3%) patients.
Because asymptomatic patients account for the largest proportion of the octogenarians, "this is arguably the most clinically important group," Dr. Rockman said.
Previous research has raised concerns about higher stroke risks associated with CAS vs. CEA in older patients (J. Vasc. Surg. 2004;40:1106-11; Catheter Cardiovasc. Interv. 2007;70:1025-33). Yet in the current study, a significantly greater percentage of asymptomatic octogenarians (10.1%) underwent CAS than did asymptomatic younger patients (5.7%).
Even though the in-hospital death rate was statistically significantly higher among octogenarians than among younger patients (1% vs. 0.6%), Dr. Rockman said that the small difference was, in her mind, "not clinically significant."
However, the story was different among symptomatic patients. The in-hospital death rate was 4.1% in the older group vs. 2.6% in younger patients. "I think this is concerning in both age categories," Dr. Rockman said. The investigators did not have access to cause-of-death information in the database.
Dr. Money asked about the causes of death in the study. "The cause of death is not in the database," Dr. Rockman responded, adding that only "in-hospital mortality" is noted.
"There are limits and good points about using a national database," Dr. Rockman said. She noted that procedures in the discharge administrative database "are really coded for the purpose of hospital reimbursement," but the large number of cases it contains may reflect "real world" practice better than do other types of studies.
Dr. Rockman said that she had no relevant disclosures.
Body text goes here
Doctor’s Bio
Body text goes here
Doctor’s Bio
Body text goes here
Doctor’s Bio
NAPLES, FLA. – When it comes to cerebral revascularization of octogenarian patients, carotid endarterectomy may be the treatment of choice unless compelling evidence points to carotid artery angioplasty and stenting, according to Dr. Caron B. Rockman.
She and her colleagues compared in-hospital perioperative stroke and death rates between these procedures among older and younger patients in a study of 54,658 patients who underwent carotid revascularization in 2004 and 2005 in the Healthcare Cost and Utilization Project National Inpatient Sample database.
Octogenarians have been excluded from many previous trials of carotid endarterectomy (CEA), said Dr. Rockman, who is on the surgery faculty and is director of clinical research in the division of vascular surgery at New York University. However, patients aged 80 years and older accounted for 10,820 (or nearly 20%) of the patients.
"Advanced age is relevant when treatment of asymptomatic severe cerebrovascular disease is considered," Dr. Rockman said.
The prevalence of preoperative symptoms was essentially the same in the octogenarian (5.4%) and younger (5.2%) groups.
"I am floored that 95% of octogenarians who get intervention are asymptomatic," said study discussant Dr. Samuel R. Money, chair of the department of surgery at the Mayo Clinic in Scottsdale, Ariz., at the meeting. Because the Nationwide Inpatient Sample data represent about 20% of U.S. hospital discharges, he estimated that approximately 50,000 people aged 80 years and older undergo these procedures each year in the United States. Dr. Money asked if such high utilization is justified in an era with medical management options such as statins. He estimated that these interventions cost $75 million to $1 billion annually.
"I agree with you," Dr. Rockman replied. "This is astounding."
She theorized that people who enter the ICD-9 procedural codes might incorrectly classify some patients as asymptomatic. "I hope people coding these are missing some of the symptomatology. It can be difficult to pick up [symptoms] from patient records, so I hope it’s not as alarming as it seems."
"However, I do think there is a role for endarterectomy or CAS in an elderly patient with severe disease," Dr. Rockman added.
The results of the study showed that "octogenarians had essentially equivalent rates of periprocedural stroke [1.1% in both groups], but slightly higher rates of in-hospital mortality than their younger counterparts," she said.
A meeting attendee asked why stroke outcomes were not significantly worse among octogenarians.
"I don’t know why I did not see it," said Dr. Rockman, noting that a separate meta-analysis of CAS studies found that the relative risk for periprocedural stroke in octogenarians fell above an acceptable rate of 3% in seven of eight studies (J. Am. Coll. Surg. 2009;208:1124-31). "It could be [because] this database is in-hospital only, so even if stroke occurs the day after discharge, it’s not included in this national database."
In a separate analysis of only octogenarian patients, the periprocedural stroke rate was significantly higher among those who underwent CAS than had CEA (2.2% vs. 1.1%). The rate also was consistently higher for both asymptomatic (1.9% vs. 0.9%) and symptomatic (5.2% vs. 2.3%) patients.
Because asymptomatic patients account for the largest proportion of the octogenarians, "this is arguably the most clinically important group," Dr. Rockman said.
Previous research has raised concerns about higher stroke risks associated with CAS vs. CEA in older patients (J. Vasc. Surg. 2004;40:1106-11; Catheter Cardiovasc. Interv. 2007;70:1025-33). Yet in the current study, a significantly greater percentage of asymptomatic octogenarians (10.1%) underwent CAS than did asymptomatic younger patients (5.7%).
Even though the in-hospital death rate was statistically significantly higher among octogenarians than among younger patients (1% vs. 0.6%), Dr. Rockman said that the small difference was, in her mind, "not clinically significant."
However, the story was different among symptomatic patients. The in-hospital death rate was 4.1% in the older group vs. 2.6% in younger patients. "I think this is concerning in both age categories," Dr. Rockman said. The investigators did not have access to cause-of-death information in the database.
Dr. Money asked about the causes of death in the study. "The cause of death is not in the database," Dr. Rockman responded, adding that only "in-hospital mortality" is noted.
"There are limits and good points about using a national database," Dr. Rockman said. She noted that procedures in the discharge administrative database "are really coded for the purpose of hospital reimbursement," but the large number of cases it contains may reflect "real world" practice better than do other types of studies.
Dr. Rockman said that she had no relevant disclosures.
NAPLES, FLA. – When it comes to cerebral revascularization of octogenarian patients, carotid endarterectomy may be the treatment of choice unless compelling evidence points to carotid artery angioplasty and stenting, according to Dr. Caron B. Rockman.
She and her colleagues compared in-hospital perioperative stroke and death rates between these procedures among older and younger patients in a study of 54,658 patients who underwent carotid revascularization in 2004 and 2005 in the Healthcare Cost and Utilization Project National Inpatient Sample database.
Octogenarians have been excluded from many previous trials of carotid endarterectomy (CEA), said Dr. Rockman, who is on the surgery faculty and is director of clinical research in the division of vascular surgery at New York University. However, patients aged 80 years and older accounted for 10,820 (or nearly 20%) of the patients.
"Advanced age is relevant when treatment of asymptomatic severe cerebrovascular disease is considered," Dr. Rockman said.
The prevalence of preoperative symptoms was essentially the same in the octogenarian (5.4%) and younger (5.2%) groups.
"I am floored that 95% of octogenarians who get intervention are asymptomatic," said study discussant Dr. Samuel R. Money, chair of the department of surgery at the Mayo Clinic in Scottsdale, Ariz., at the meeting. Because the Nationwide Inpatient Sample data represent about 20% of U.S. hospital discharges, he estimated that approximately 50,000 people aged 80 years and older undergo these procedures each year in the United States. Dr. Money asked if such high utilization is justified in an era with medical management options such as statins. He estimated that these interventions cost $75 million to $1 billion annually.
"I agree with you," Dr. Rockman replied. "This is astounding."
She theorized that people who enter the ICD-9 procedural codes might incorrectly classify some patients as asymptomatic. "I hope people coding these are missing some of the symptomatology. It can be difficult to pick up [symptoms] from patient records, so I hope it’s not as alarming as it seems."
"However, I do think there is a role for endarterectomy or CAS in an elderly patient with severe disease," Dr. Rockman added.
The results of the study showed that "octogenarians had essentially equivalent rates of periprocedural stroke [1.1% in both groups], but slightly higher rates of in-hospital mortality than their younger counterparts," she said.
A meeting attendee asked why stroke outcomes were not significantly worse among octogenarians.
"I don’t know why I did not see it," said Dr. Rockman, noting that a separate meta-analysis of CAS studies found that the relative risk for periprocedural stroke in octogenarians fell above an acceptable rate of 3% in seven of eight studies (J. Am. Coll. Surg. 2009;208:1124-31). "It could be [because] this database is in-hospital only, so even if stroke occurs the day after discharge, it’s not included in this national database."
In a separate analysis of only octogenarian patients, the periprocedural stroke rate was significantly higher among those who underwent CAS than had CEA (2.2% vs. 1.1%). The rate also was consistently higher for both asymptomatic (1.9% vs. 0.9%) and symptomatic (5.2% vs. 2.3%) patients.
Because asymptomatic patients account for the largest proportion of the octogenarians, "this is arguably the most clinically important group," Dr. Rockman said.
Previous research has raised concerns about higher stroke risks associated with CAS vs. CEA in older patients (J. Vasc. Surg. 2004;40:1106-11; Catheter Cardiovasc. Interv. 2007;70:1025-33). Yet in the current study, a significantly greater percentage of asymptomatic octogenarians (10.1%) underwent CAS than did asymptomatic younger patients (5.7%).
Even though the in-hospital death rate was statistically significantly higher among octogenarians than among younger patients (1% vs. 0.6%), Dr. Rockman said that the small difference was, in her mind, "not clinically significant."
However, the story was different among symptomatic patients. The in-hospital death rate was 4.1% in the older group vs. 2.6% in younger patients. "I think this is concerning in both age categories," Dr. Rockman said. The investigators did not have access to cause-of-death information in the database.
Dr. Money asked about the causes of death in the study. "The cause of death is not in the database," Dr. Rockman responded, adding that only "in-hospital mortality" is noted.
"There are limits and good points about using a national database," Dr. Rockman said. She noted that procedures in the discharge administrative database "are really coded for the purpose of hospital reimbursement," but the large number of cases it contains may reflect "real world" practice better than do other types of studies.
Dr. Rockman said that she had no relevant disclosures.
FROM THE ANNUAL MEETING OF THE SOUTHERN ASSOCIATION FOR VASCULAR SURGERY
Major Finding: Octogenarians had significantly greater rates of periprocedural stroke with CAS than with CEA (2.2% vs. 1.1%).
Data Source: 54,658 patients in the Nationwide Inpatient Sample database who underwent carotid artery revascularization during 2004-2005.
Disclosures: Dr. Rockman said that she had no relevant disclosures.
Carotid Stents: Excess Strokes in Elderly, Women
LOS ANGELES - Women, as well as all patients aged 65 years or older who have substantial carotid artery stenosis that needs revascularization, may prefer endarterectomy, and would want to steer clear of carotid stenting, according to new data from CREST, the largest randomized trial to compare these two carotid interventions.
All patients aged 65 or older who were randomized to treatment by carotid artery stenting had a statistically significant excess of strokes, compared with similar subgroups who were treated with CEA during the periprocedural period and 4-year follow-up, George Howard, Dr.P.H., said at the conference. “Patient age should be an important factor in selecting the treatment option for carotid stenosis," said Dr. Howard, professor and chairman of biostatistics at the University of Alabama at Birmingham.
Analysis of the patients enrolled in CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial) by sex showed that the treatment of carotid stenosis by stenting led to an excess rate of periprocedural strokes among women, but not in men, Virginia J. Howard, Ph.D., said in a separate talk at the conference. Women who underwent an endarterectomy also had no excess risk for myocardial infarctions, compared with women who received a carotid stent, unlike men who had a significantly increased rate of MIs following open surgery, compared with those who got stented, said Dr. Howard, an epidemiologist at the University of Alabama at Birmingham.
The primary results from CREST, first reported last year, showed that all patients who were enrolled in the study had similar rates of stroke, MI, or death regardless of whether they underwent carotid CEA or stenting (N. Engl. J. Med. 2010;363:11-23).
But these new details, which show an excess rate of periprocedural strokes in women undergoing stenting as well as the excess of all strokes in patients aged 65 or older undergoing stenting, may tip the balance away from stenting in these patients.
Going into CREST, which began in 2000, “we thought the results would be the opposite. [At that time,] we preferred to take older patients to stenting," commented Dr. Thomas G. Brott, lead investigator for CREST and a professor of neurology and director or research at the Mayo Clinic in Jacksonville, Fla. “Our interventionalists believe that age is a surrogate marker for patients with calcified and tortuous vessels that might not be suitable for stenting." Regarding the sex-related finding, the implications “depend on how a woman would value [the risk of having] a stroke or a MI. If the woman is more concerned about a periprocedural stroke, then the results suggest there could be a preference for endarterectomy," Dr. Brott said in an interview.
In the sex-based analysis, the rate of periprocedural stroke was 5.5% in stented women and 2.2% in those who underwent CEA, a statistically significant 2.6-fold increased rate with stenting, Dr. Virginia Howard reported.
During the entire follow-up, which added the rate of ipsilateral strokes during 4 years following the intervention, stroke rates were 7.8% in stented women and 5.0% in those who had endarterectomy, a nonsignificant difference. The two treatment options produced no difference in stroke rates in men, either periprocedurally or after 4 years. MI rates were similar in women following either intervention, both periprocedurally and after 4 years. The periprocedural and 4-year MI rates in men were significantly higher with CEA.
In the age-based analyses, a calculation that used age as a continuous variable showed that the number of strokes occurring with either CEA or stenting was similar for patients aged 64 years. For those aged 65 or older, fewer strokes occurred with CEA, a relationship that grew stronger with increasing age. For patients aged 63 or younger, stenting produced fewer strokes, and the relationship grew stronger with decreasing age.
The age analysis also divided patients into three prespecified age groups: younger than 65, 65-74 years, and 75 and older. (See box.) The most striking age effect occurred in patients aged 75 or older: In this subgroup, treatment with stenting more than doubled the total stroke risk, both periprocedural and long-term strokes, compared with patients who were treated with CEA.
The incidence of MI showed a much weaker age effect, and patients who underwent stenting had a reduced rate of MI at all ages, compared with those who had CEA. In addition, in the CEA arm, age had no significant effect on the MI rate, Dr. George Howard said.
Commenting on the study, Christopher J. Moran, M.D., is a professor of radiology and neurological surgery at Washington University in St. Louis, who performs carotid artery stenting but did not participate in CREST, stated, “If you are trying to prevent strokes in patients with carotid artery disease, you would have to think long and hard before treating a woman with carotid stenting. In some cases, CEA may not be an option: The woman may be a poor operative candidate because she has a large neck, or the lesion may be high above the mandible and hard to get above."
In addition, some patients have pulmonary disease and are not good candidates for anesthesia. But if a woman is a good operative candidate, she should be treated with endarterectomy.
Conventional angiography or CT angiography lets an operator assess the size of a woman's arteries, he said.
“The smallest self-expanding stents available for treating carotid disease are 5 mm in diameter, and because these are ideally oversized to the artery, the smallest diameter carotid that should be stented is 4 mm. Many women have carotids that are smaller than 4 mm, and in those cases you should definitely think twice about stenting.
“The same considerations apply to patients who are 70-80 years old. If they are good operative candidates, they should undergo CEA," he concluded.
Recently, carotid angioplasty/stenting (CAS) has been recommended as an alternative to carotid endarterectomy (CEA). Several randomized and non-randomized prospective trials have been conducted over the past several years to evaluate the efficacy of CAS, in comparison to CEA, for the prevention of stroke for both symptomatic and asymptomatic patients. While EVA-3S and the International Carotid Stenting Study showed that CEA was superior to CAS for symptomatic patients, the SPACE trial showed that CAS was not inferior to CEA. However, the CREST trial showed that CAS significantly increased the risk of stroke and decreased the risk of myocardial infarction in comparison to CEA, but the combined stroke, myocardial infarction, and death rates were similar for both. A recent meta-analysis of all randomized trials (including CREST) comparing CEA versus CAS by Murad and published in the Journal of Vascular Surgery showed that CAS significantly increases the risk of stroke, but decreases the risk of MI. This study added another twist of CREST, this time favoring CEA, particularly for women and men who are above 65-70 years of age. The readers must also remember that the real world data as found by the Society for Vascular Surgery registry showed that CEA is safer than CAS in both strokes and strokes/deaths. Therefore, at present, most authorities believe that CAS is a better alternative to CEA in high-risk patients, but its role in normal surgical patients is still unclear
Ali AbuRahma, M.D., is professor and chief of vascular and endovascular surgery and the medical director, Vascular Laboratory, at the Robert C. Byrd Health Sciences Center, West Virginia University, Charleston, Charleston Area Medical Center. He is an associate medical editor for Vascular Specialist.
Recently, carotid angioplasty/stenting (CAS) has been recommended as an alternative to carotid endarterectomy (CEA). Several randomized and non-randomized prospective trials have been conducted over the past several years to evaluate the efficacy of CAS, in comparison to CEA, for the prevention of stroke for both symptomatic and asymptomatic patients. While EVA-3S and the International Carotid Stenting Study showed that CEA was superior to CAS for symptomatic patients, the SPACE trial showed that CAS was not inferior to CEA. However, the CREST trial showed that CAS significantly increased the risk of stroke and decreased the risk of myocardial infarction in comparison to CEA, but the combined stroke, myocardial infarction, and death rates were similar for both. A recent meta-analysis of all randomized trials (including CREST) comparing CEA versus CAS by Murad and published in the Journal of Vascular Surgery showed that CAS significantly increases the risk of stroke, but decreases the risk of MI. This study added another twist of CREST, this time favoring CEA, particularly for women and men who are above 65-70 years of age. The readers must also remember that the real world data as found by the Society for Vascular Surgery registry showed that CEA is safer than CAS in both strokes and strokes/deaths. Therefore, at present, most authorities believe that CAS is a better alternative to CEA in high-risk patients, but its role in normal surgical patients is still unclear
Ali AbuRahma, M.D., is professor and chief of vascular and endovascular surgery and the medical director, Vascular Laboratory, at the Robert C. Byrd Health Sciences Center, West Virginia University, Charleston, Charleston Area Medical Center. He is an associate medical editor for Vascular Specialist.
Recently, carotid angioplasty/stenting (CAS) has been recommended as an alternative to carotid endarterectomy (CEA). Several randomized and non-randomized prospective trials have been conducted over the past several years to evaluate the efficacy of CAS, in comparison to CEA, for the prevention of stroke for both symptomatic and asymptomatic patients. While EVA-3S and the International Carotid Stenting Study showed that CEA was superior to CAS for symptomatic patients, the SPACE trial showed that CAS was not inferior to CEA. However, the CREST trial showed that CAS significantly increased the risk of stroke and decreased the risk of myocardial infarction in comparison to CEA, but the combined stroke, myocardial infarction, and death rates were similar for both. A recent meta-analysis of all randomized trials (including CREST) comparing CEA versus CAS by Murad and published in the Journal of Vascular Surgery showed that CAS significantly increases the risk of stroke, but decreases the risk of MI. This study added another twist of CREST, this time favoring CEA, particularly for women and men who are above 65-70 years of age. The readers must also remember that the real world data as found by the Society for Vascular Surgery registry showed that CEA is safer than CAS in both strokes and strokes/deaths. Therefore, at present, most authorities believe that CAS is a better alternative to CEA in high-risk patients, but its role in normal surgical patients is still unclear
Ali AbuRahma, M.D., is professor and chief of vascular and endovascular surgery and the medical director, Vascular Laboratory, at the Robert C. Byrd Health Sciences Center, West Virginia University, Charleston, Charleston Area Medical Center. He is an associate medical editor for Vascular Specialist.
LOS ANGELES - Women, as well as all patients aged 65 years or older who have substantial carotid artery stenosis that needs revascularization, may prefer endarterectomy, and would want to steer clear of carotid stenting, according to new data from CREST, the largest randomized trial to compare these two carotid interventions.
All patients aged 65 or older who were randomized to treatment by carotid artery stenting had a statistically significant excess of strokes, compared with similar subgroups who were treated with CEA during the periprocedural period and 4-year follow-up, George Howard, Dr.P.H., said at the conference. “Patient age should be an important factor in selecting the treatment option for carotid stenosis," said Dr. Howard, professor and chairman of biostatistics at the University of Alabama at Birmingham.
Analysis of the patients enrolled in CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial) by sex showed that the treatment of carotid stenosis by stenting led to an excess rate of periprocedural strokes among women, but not in men, Virginia J. Howard, Ph.D., said in a separate talk at the conference. Women who underwent an endarterectomy also had no excess risk for myocardial infarctions, compared with women who received a carotid stent, unlike men who had a significantly increased rate of MIs following open surgery, compared with those who got stented, said Dr. Howard, an epidemiologist at the University of Alabama at Birmingham.
The primary results from CREST, first reported last year, showed that all patients who were enrolled in the study had similar rates of stroke, MI, or death regardless of whether they underwent carotid CEA or stenting (N. Engl. J. Med. 2010;363:11-23).
But these new details, which show an excess rate of periprocedural strokes in women undergoing stenting as well as the excess of all strokes in patients aged 65 or older undergoing stenting, may tip the balance away from stenting in these patients.
Going into CREST, which began in 2000, “we thought the results would be the opposite. [At that time,] we preferred to take older patients to stenting," commented Dr. Thomas G. Brott, lead investigator for CREST and a professor of neurology and director or research at the Mayo Clinic in Jacksonville, Fla. “Our interventionalists believe that age is a surrogate marker for patients with calcified and tortuous vessels that might not be suitable for stenting." Regarding the sex-related finding, the implications “depend on how a woman would value [the risk of having] a stroke or a MI. If the woman is more concerned about a periprocedural stroke, then the results suggest there could be a preference for endarterectomy," Dr. Brott said in an interview.
In the sex-based analysis, the rate of periprocedural stroke was 5.5% in stented women and 2.2% in those who underwent CEA, a statistically significant 2.6-fold increased rate with stenting, Dr. Virginia Howard reported.
During the entire follow-up, which added the rate of ipsilateral strokes during 4 years following the intervention, stroke rates were 7.8% in stented women and 5.0% in those who had endarterectomy, a nonsignificant difference. The two treatment options produced no difference in stroke rates in men, either periprocedurally or after 4 years. MI rates were similar in women following either intervention, both periprocedurally and after 4 years. The periprocedural and 4-year MI rates in men were significantly higher with CEA.
In the age-based analyses, a calculation that used age as a continuous variable showed that the number of strokes occurring with either CEA or stenting was similar for patients aged 64 years. For those aged 65 or older, fewer strokes occurred with CEA, a relationship that grew stronger with increasing age. For patients aged 63 or younger, stenting produced fewer strokes, and the relationship grew stronger with decreasing age.
The age analysis also divided patients into three prespecified age groups: younger than 65, 65-74 years, and 75 and older. (See box.) The most striking age effect occurred in patients aged 75 or older: In this subgroup, treatment with stenting more than doubled the total stroke risk, both periprocedural and long-term strokes, compared with patients who were treated with CEA.
The incidence of MI showed a much weaker age effect, and patients who underwent stenting had a reduced rate of MI at all ages, compared with those who had CEA. In addition, in the CEA arm, age had no significant effect on the MI rate, Dr. George Howard said.
Commenting on the study, Christopher J. Moran, M.D., is a professor of radiology and neurological surgery at Washington University in St. Louis, who performs carotid artery stenting but did not participate in CREST, stated, “If you are trying to prevent strokes in patients with carotid artery disease, you would have to think long and hard before treating a woman with carotid stenting. In some cases, CEA may not be an option: The woman may be a poor operative candidate because she has a large neck, or the lesion may be high above the mandible and hard to get above."
In addition, some patients have pulmonary disease and are not good candidates for anesthesia. But if a woman is a good operative candidate, she should be treated with endarterectomy.
Conventional angiography or CT angiography lets an operator assess the size of a woman's arteries, he said.
“The smallest self-expanding stents available for treating carotid disease are 5 mm in diameter, and because these are ideally oversized to the artery, the smallest diameter carotid that should be stented is 4 mm. Many women have carotids that are smaller than 4 mm, and in those cases you should definitely think twice about stenting.
“The same considerations apply to patients who are 70-80 years old. If they are good operative candidates, they should undergo CEA," he concluded.
LOS ANGELES - Women, as well as all patients aged 65 years or older who have substantial carotid artery stenosis that needs revascularization, may prefer endarterectomy, and would want to steer clear of carotid stenting, according to new data from CREST, the largest randomized trial to compare these two carotid interventions.
All patients aged 65 or older who were randomized to treatment by carotid artery stenting had a statistically significant excess of strokes, compared with similar subgroups who were treated with CEA during the periprocedural period and 4-year follow-up, George Howard, Dr.P.H., said at the conference. “Patient age should be an important factor in selecting the treatment option for carotid stenosis," said Dr. Howard, professor and chairman of biostatistics at the University of Alabama at Birmingham.
Analysis of the patients enrolled in CREST (Carotid Revascularization Endarterectomy vs. Stenting Trial) by sex showed that the treatment of carotid stenosis by stenting led to an excess rate of periprocedural strokes among women, but not in men, Virginia J. Howard, Ph.D., said in a separate talk at the conference. Women who underwent an endarterectomy also had no excess risk for myocardial infarctions, compared with women who received a carotid stent, unlike men who had a significantly increased rate of MIs following open surgery, compared with those who got stented, said Dr. Howard, an epidemiologist at the University of Alabama at Birmingham.
The primary results from CREST, first reported last year, showed that all patients who were enrolled in the study had similar rates of stroke, MI, or death regardless of whether they underwent carotid CEA or stenting (N. Engl. J. Med. 2010;363:11-23).
But these new details, which show an excess rate of periprocedural strokes in women undergoing stenting as well as the excess of all strokes in patients aged 65 or older undergoing stenting, may tip the balance away from stenting in these patients.
Going into CREST, which began in 2000, “we thought the results would be the opposite. [At that time,] we preferred to take older patients to stenting," commented Dr. Thomas G. Brott, lead investigator for CREST and a professor of neurology and director or research at the Mayo Clinic in Jacksonville, Fla. “Our interventionalists believe that age is a surrogate marker for patients with calcified and tortuous vessels that might not be suitable for stenting." Regarding the sex-related finding, the implications “depend on how a woman would value [the risk of having] a stroke or a MI. If the woman is more concerned about a periprocedural stroke, then the results suggest there could be a preference for endarterectomy," Dr. Brott said in an interview.
In the sex-based analysis, the rate of periprocedural stroke was 5.5% in stented women and 2.2% in those who underwent CEA, a statistically significant 2.6-fold increased rate with stenting, Dr. Virginia Howard reported.
During the entire follow-up, which added the rate of ipsilateral strokes during 4 years following the intervention, stroke rates were 7.8% in stented women and 5.0% in those who had endarterectomy, a nonsignificant difference. The two treatment options produced no difference in stroke rates in men, either periprocedurally or after 4 years. MI rates were similar in women following either intervention, both periprocedurally and after 4 years. The periprocedural and 4-year MI rates in men were significantly higher with CEA.
In the age-based analyses, a calculation that used age as a continuous variable showed that the number of strokes occurring with either CEA or stenting was similar for patients aged 64 years. For those aged 65 or older, fewer strokes occurred with CEA, a relationship that grew stronger with increasing age. For patients aged 63 or younger, stenting produced fewer strokes, and the relationship grew stronger with decreasing age.
The age analysis also divided patients into three prespecified age groups: younger than 65, 65-74 years, and 75 and older. (See box.) The most striking age effect occurred in patients aged 75 or older: In this subgroup, treatment with stenting more than doubled the total stroke risk, both periprocedural and long-term strokes, compared with patients who were treated with CEA.
The incidence of MI showed a much weaker age effect, and patients who underwent stenting had a reduced rate of MI at all ages, compared with those who had CEA. In addition, in the CEA arm, age had no significant effect on the MI rate, Dr. George Howard said.
Commenting on the study, Christopher J. Moran, M.D., is a professor of radiology and neurological surgery at Washington University in St. Louis, who performs carotid artery stenting but did not participate in CREST, stated, “If you are trying to prevent strokes in patients with carotid artery disease, you would have to think long and hard before treating a woman with carotid stenting. In some cases, CEA may not be an option: The woman may be a poor operative candidate because she has a large neck, or the lesion may be high above the mandible and hard to get above."
In addition, some patients have pulmonary disease and are not good candidates for anesthesia. But if a woman is a good operative candidate, she should be treated with endarterectomy.
Conventional angiography or CT angiography lets an operator assess the size of a woman's arteries, he said.
“The smallest self-expanding stents available for treating carotid disease are 5 mm in diameter, and because these are ideally oversized to the artery, the smallest diameter carotid that should be stented is 4 mm. Many women have carotids that are smaller than 4 mm, and in those cases you should definitely think twice about stenting.
“The same considerations apply to patients who are 70-80 years old. If they are good operative candidates, they should undergo CEA," he concluded.