User login
Ruxolitinib receives priority review for acute GVHD
The Food and Drug Administration has accepted the JAK1/JAK2 inhibitor ruxolitinib (Jakafi) for priority review.
Incyte is seeking approval for ruxolitinib as a treatment for patients with acute graft-versus-host disease (GVHD) who have had an inadequate response to corticosteroids.
“If approved, ruxolitinib will be the first and only treatment available in the U.S. for patients with acute GVHD who have not responded adequately to corticosteroid therapy,” Steven Stein, MD, chief medical officer at Incyte, said in a statement.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions. The designation generally means that the agency will act on the application within 6 months, rather than 10 months.
In addition to priority review, the FDA previously granted ruxolitinib breakthrough therapy and orphan drug designations.
The application is based on data from the ongoing, phase 2 REACH1 trial (NCT02953678), which is evaluating ruxolitinib in combination with corticosteroids in patients who have steroid-refractory acute GVHD.
Incyte announced top-line results from REACH1 in June, reporting on outcomes in 71 patients.
The study’s primary endpoint – overall response rate at day 28 – was met. Ruxolitinib produced an overall response rate of 55% at that time. However, 73% of patients responded to ruxolitinib at some point during the trial. Incyte said the most common treatment-emergent adverse events were anemia, thrombocytopenia, and neutropenia.
The Food and Drug Administration has accepted the JAK1/JAK2 inhibitor ruxolitinib (Jakafi) for priority review.
Incyte is seeking approval for ruxolitinib as a treatment for patients with acute graft-versus-host disease (GVHD) who have had an inadequate response to corticosteroids.
“If approved, ruxolitinib will be the first and only treatment available in the U.S. for patients with acute GVHD who have not responded adequately to corticosteroid therapy,” Steven Stein, MD, chief medical officer at Incyte, said in a statement.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions. The designation generally means that the agency will act on the application within 6 months, rather than 10 months.
In addition to priority review, the FDA previously granted ruxolitinib breakthrough therapy and orphan drug designations.
The application is based on data from the ongoing, phase 2 REACH1 trial (NCT02953678), which is evaluating ruxolitinib in combination with corticosteroids in patients who have steroid-refractory acute GVHD.
Incyte announced top-line results from REACH1 in June, reporting on outcomes in 71 patients.
The study’s primary endpoint – overall response rate at day 28 – was met. Ruxolitinib produced an overall response rate of 55% at that time. However, 73% of patients responded to ruxolitinib at some point during the trial. Incyte said the most common treatment-emergent adverse events were anemia, thrombocytopenia, and neutropenia.
The Food and Drug Administration has accepted the JAK1/JAK2 inhibitor ruxolitinib (Jakafi) for priority review.
Incyte is seeking approval for ruxolitinib as a treatment for patients with acute graft-versus-host disease (GVHD) who have had an inadequate response to corticosteroids.
“If approved, ruxolitinib will be the first and only treatment available in the U.S. for patients with acute GVHD who have not responded adequately to corticosteroid therapy,” Steven Stein, MD, chief medical officer at Incyte, said in a statement.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions. The designation generally means that the agency will act on the application within 6 months, rather than 10 months.
In addition to priority review, the FDA previously granted ruxolitinib breakthrough therapy and orphan drug designations.
The application is based on data from the ongoing, phase 2 REACH1 trial (NCT02953678), which is evaluating ruxolitinib in combination with corticosteroids in patients who have steroid-refractory acute GVHD.
Incyte announced top-line results from REACH1 in June, reporting on outcomes in 71 patients.
The study’s primary endpoint – overall response rate at day 28 – was met. Ruxolitinib produced an overall response rate of 55% at that time. However, 73% of patients responded to ruxolitinib at some point during the trial. Incyte said the most common treatment-emergent adverse events were anemia, thrombocytopenia, and neutropenia.
Older age predicts mortality after alloHCT in NHL, but not relapse
Elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within 1 year of allogeneic hematopoietic cell transplantation (alloHCT), compared with younger or middle-age patients, according to investigators.
Comorbidities also increased risks of nonrelapse mortality (NRM) at 1 year, but to a lesser extent than that of elderly status, reported lead author Charalampia Kyriakou, MD, PhD, of the department of haematology at University College London Hospital and London North West University Healthcare NHS Trust, and her colleagues.
“Although alloHCT is feasible and effective in very old patients, the increased NRM risk must be taken into account when assessing the indication for alloHCT for NHL in this age group,” the investigators wrote in Biology of Blood and Marrow Transplantation.
This decision is becoming more common, they noted. “With the advent of reduced-intensity conditioning (RIC) strategies and other improvements in transplantation technology, alloHCT is being increasingly considered in elderly patients with [relapsed and refractory] NHL.”
The retrospective study analyzed 3,919 patients with NHL who underwent alloHCT between 2003 and 2013. Patients were sorted into three age groups: young (18-50 years), middle age (51-65 years), or elderly (66-77 years).
Disease types also were reported: 1,461 patients had follicular lymphoma (FL; 37%), 1,192 had diffuse large B cell lymphoma (DLBCL; 30%), 823 had mantle cell lymphoma (MCL; 21%), and 443 had peripheral T cell lymphoma (PTCL; 11%).
At the time of alloHCT, about 85% of patients were chemosensitive, with the remainder being chemorefractory. The age groups had similar patient characteristics, with exceptions noted for unrelated donors, MCL, and RIC, which became increasingly overrepresented with age.
The results showed that NRM at 1 year was 13% for young patients, 20% for middle-age patients, and 33% for elderly patients (P less than .001). Overall survival at 3 years followed an inverse trend, decreasing with age from 60% in young patients to 54% in middle-age patients, before dropping more dramatically to 38% in the elderly (P less than .001).
In contrast to these significant associations between age and survival, relapse risk at 3 years remained relatively consistent, with young patients at 30%, middle-age patients at 31%, and elderly patients at 28% (P = .355).
The investigators noted that the risk of NRM increased most dramatically between middle age and old age, with less significant differences between the middle-age and young groups. They suggested that “age per se should have a limited impact on the indication for alloHCT for NHL in patients up to age 65 years.”
The increased risk with elderly status could not be fully explained by comorbidities, although these were more common in elderly patients. After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.” Therefore, age remains an independent risk factor.
“The information provided in this cohort of patients with NHL, the largest reported to date, is useful and relevant, especially in the era of evolving therapies,” the investigators wrote. They added that the information is “even more relevant now with the availability of treatment with ... chimeric antigen receptor (CAR) T cells ... after relapse post-alloHCT.”
The investigators reported having no financial disclosures.
SOURCE: Kyriakou C et al. Biol Blood Marrow Transplant. 2018 Sep 13. doi: 10.1016/j.bbmt.2018.08.025.
Elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within 1 year of allogeneic hematopoietic cell transplantation (alloHCT), compared with younger or middle-age patients, according to investigators.
Comorbidities also increased risks of nonrelapse mortality (NRM) at 1 year, but to a lesser extent than that of elderly status, reported lead author Charalampia Kyriakou, MD, PhD, of the department of haematology at University College London Hospital and London North West University Healthcare NHS Trust, and her colleagues.
“Although alloHCT is feasible and effective in very old patients, the increased NRM risk must be taken into account when assessing the indication for alloHCT for NHL in this age group,” the investigators wrote in Biology of Blood and Marrow Transplantation.
This decision is becoming more common, they noted. “With the advent of reduced-intensity conditioning (RIC) strategies and other improvements in transplantation technology, alloHCT is being increasingly considered in elderly patients with [relapsed and refractory] NHL.”
The retrospective study analyzed 3,919 patients with NHL who underwent alloHCT between 2003 and 2013. Patients were sorted into three age groups: young (18-50 years), middle age (51-65 years), or elderly (66-77 years).
Disease types also were reported: 1,461 patients had follicular lymphoma (FL; 37%), 1,192 had diffuse large B cell lymphoma (DLBCL; 30%), 823 had mantle cell lymphoma (MCL; 21%), and 443 had peripheral T cell lymphoma (PTCL; 11%).
At the time of alloHCT, about 85% of patients were chemosensitive, with the remainder being chemorefractory. The age groups had similar patient characteristics, with exceptions noted for unrelated donors, MCL, and RIC, which became increasingly overrepresented with age.
The results showed that NRM at 1 year was 13% for young patients, 20% for middle-age patients, and 33% for elderly patients (P less than .001). Overall survival at 3 years followed an inverse trend, decreasing with age from 60% in young patients to 54% in middle-age patients, before dropping more dramatically to 38% in the elderly (P less than .001).
In contrast to these significant associations between age and survival, relapse risk at 3 years remained relatively consistent, with young patients at 30%, middle-age patients at 31%, and elderly patients at 28% (P = .355).
The investigators noted that the risk of NRM increased most dramatically between middle age and old age, with less significant differences between the middle-age and young groups. They suggested that “age per se should have a limited impact on the indication for alloHCT for NHL in patients up to age 65 years.”
The increased risk with elderly status could not be fully explained by comorbidities, although these were more common in elderly patients. After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.” Therefore, age remains an independent risk factor.
“The information provided in this cohort of patients with NHL, the largest reported to date, is useful and relevant, especially in the era of evolving therapies,” the investigators wrote. They added that the information is “even more relevant now with the availability of treatment with ... chimeric antigen receptor (CAR) T cells ... after relapse post-alloHCT.”
The investigators reported having no financial disclosures.
SOURCE: Kyriakou C et al. Biol Blood Marrow Transplant. 2018 Sep 13. doi: 10.1016/j.bbmt.2018.08.025.
Elderly patients with non-Hodgkin lymphoma (NHL) are more likely to die, but not relapse, within 1 year of allogeneic hematopoietic cell transplantation (alloHCT), compared with younger or middle-age patients, according to investigators.
Comorbidities also increased risks of nonrelapse mortality (NRM) at 1 year, but to a lesser extent than that of elderly status, reported lead author Charalampia Kyriakou, MD, PhD, of the department of haematology at University College London Hospital and London North West University Healthcare NHS Trust, and her colleagues.
“Although alloHCT is feasible and effective in very old patients, the increased NRM risk must be taken into account when assessing the indication for alloHCT for NHL in this age group,” the investigators wrote in Biology of Blood and Marrow Transplantation.
This decision is becoming more common, they noted. “With the advent of reduced-intensity conditioning (RIC) strategies and other improvements in transplantation technology, alloHCT is being increasingly considered in elderly patients with [relapsed and refractory] NHL.”
The retrospective study analyzed 3,919 patients with NHL who underwent alloHCT between 2003 and 2013. Patients were sorted into three age groups: young (18-50 years), middle age (51-65 years), or elderly (66-77 years).
Disease types also were reported: 1,461 patients had follicular lymphoma (FL; 37%), 1,192 had diffuse large B cell lymphoma (DLBCL; 30%), 823 had mantle cell lymphoma (MCL; 21%), and 443 had peripheral T cell lymphoma (PTCL; 11%).
At the time of alloHCT, about 85% of patients were chemosensitive, with the remainder being chemorefractory. The age groups had similar patient characteristics, with exceptions noted for unrelated donors, MCL, and RIC, which became increasingly overrepresented with age.
The results showed that NRM at 1 year was 13% for young patients, 20% for middle-age patients, and 33% for elderly patients (P less than .001). Overall survival at 3 years followed an inverse trend, decreasing with age from 60% in young patients to 54% in middle-age patients, before dropping more dramatically to 38% in the elderly (P less than .001).
In contrast to these significant associations between age and survival, relapse risk at 3 years remained relatively consistent, with young patients at 30%, middle-age patients at 31%, and elderly patients at 28% (P = .355).
The investigators noted that the risk of NRM increased most dramatically between middle age and old age, with less significant differences between the middle-age and young groups. They suggested that “age per se should have a limited impact on the indication for alloHCT for NHL in patients up to age 65 years.”
The increased risk with elderly status could not be fully explained by comorbidities, although these were more common in elderly patients. After analyzing information from a subset of patients, the investigators concluded that “the presence of comorbidities is a significant risk factor for NRM and survival, but this does not fully explain the outcome disadvantages in our [elderly] group.” Therefore, age remains an independent risk factor.
“The information provided in this cohort of patients with NHL, the largest reported to date, is useful and relevant, especially in the era of evolving therapies,” the investigators wrote. They added that the information is “even more relevant now with the availability of treatment with ... chimeric antigen receptor (CAR) T cells ... after relapse post-alloHCT.”
The investigators reported having no financial disclosures.
SOURCE: Kyriakou C et al. Biol Blood Marrow Transplant. 2018 Sep 13. doi: 10.1016/j.bbmt.2018.08.025.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Key clinical point:
Major finding: One-year nonrelapse mortality (NRM) was 13% for young patients, 20% for middle-age patients, and 33% for elderly patients (P less than .001).
Study details: A retrospective analysis of 3,919 patients with NHL who underwent alloHCT between 2003 and 2013.
Disclosures: The researchers reported having no financial disclosures.
Source: Kyriakou C et al. Biol Blood Marrow Transplant. 2018 Sep 13. doi: 10.1016/j.bbmt.2018.08.025.
Ablation plus transplant for severe scleroderma shows 11-year benefits
CHICAGO – Follow-up out to as long as 11 years from treatment confirmed the long-term efficacy and safety of myeloablative autologous stem cell transplantation for patients with severe scleroderma.
This extended follow-up comprised 43 survivors from the 75 patients originally randomized in a controlled, 6-year trial. Follow-up showed that, among the patients who underwent myeloablation and autologous transplant with hematopoietic stem cells, there were no long-term deaths or cancers, there was an 88% survival rate, and 92% remained off disease-modifying treatment, Keith M. Sullivan, MD, said at the annual meeting of the American College of Rheumatology.
Long-term survival among patients randomized to the study’s control arm, who received treatment with cyclophosphamide, was 53%.
Patients with severe scleroderma with significant internal organ damage who “are improved and off of disease-modifying antirheumatic drugs after 10 or more years from treatment is something new in autoimmune disease,” said Dr. Sullivan, a professor of medicine at Duke University, Durham, N.C.
Based on accumulated data from this randomized trial and other studies, the American Society for Blood and Marrow Transplantation issued a position statement in June 2018 that endorsed autologous hematopoietic stem cell transplantation as “standard of care” for systemic sclerosis (Biol Blood Marrow Transplant. 2018 June 25. doi: 10.1016/j.bbmt.2018.06.025), Dr. Sullivan noted in a video interview.
The SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial randomized 75 patients with severe scleroderma and substantial internal organ involvement to receive treatment with either cyclophosphamide or myeloablative radiation followed by immune reconstitution with an autologous hematopoietic stem cell transplant. The trial’s primary endpoint, the global rank composite score at 54 months, showed the superiority of transplantation over standard treatment (N Engl J Med. 2018 Jan 4;378[1]:35-47).
Dr. Sullivan and his associates ran their long-term follow-up study on 43 of these 75 patients (25 from the transplanted group and 18 controls), excluding 21 patients who died during the original study, 4 additional patients from the control arm who died following the end of the original SCOT protocol, and 7 patients either lost to follow-up or who refused to participate in follow-up. Among the 25 transplanted patients, none died during the extended follow-up, 2 experienced cardiac failure, and 23 remained off of any disease-modifying antirheumatic drugs. Among the 18 survivors in the control arm, 3 had cardiac failure, 3 had respiratory failure, and 7 were on treatment with disease-modifying drugs, Dr. Sullivan reported.
In addition, 23 of the 25 (92%) transplanted patients had normal performance status by the Eastern Cooperative Oncology Group criteria, compared with 11 of the 18 controls (61%). A total of 14 (56%) transplant patients were employed, compared with 6 of the 18 controls (33%).
Patients who were transplanted “have their life back, are doing well, and are off treatment,” Dr. Sullivan noted.
Myeloablation and transplant is appropriate for scleroderma patients with significant internal organ involvement, about half of all patients with this disease. The best gauge of severe organ involvement is a pulmonary function test, with a forced vital capacity of 70% or less of predicted as a flag for patients who should consider transplantation, Dr. Sullivan said. He recommended monitoring lung function every 3 months in scleroderma patients because it can deteriorate very suddenly and quickly.
SCOT received no commercial funding. Dr. Sullivan had no disclosures to report.
SOURCE: Sullivan KM et al. ACR Annual Meeting, Abstract 1820.
CHICAGO – Follow-up out to as long as 11 years from treatment confirmed the long-term efficacy and safety of myeloablative autologous stem cell transplantation for patients with severe scleroderma.
This extended follow-up comprised 43 survivors from the 75 patients originally randomized in a controlled, 6-year trial. Follow-up showed that, among the patients who underwent myeloablation and autologous transplant with hematopoietic stem cells, there were no long-term deaths or cancers, there was an 88% survival rate, and 92% remained off disease-modifying treatment, Keith M. Sullivan, MD, said at the annual meeting of the American College of Rheumatology.
Long-term survival among patients randomized to the study’s control arm, who received treatment with cyclophosphamide, was 53%.
Patients with severe scleroderma with significant internal organ damage who “are improved and off of disease-modifying antirheumatic drugs after 10 or more years from treatment is something new in autoimmune disease,” said Dr. Sullivan, a professor of medicine at Duke University, Durham, N.C.
Based on accumulated data from this randomized trial and other studies, the American Society for Blood and Marrow Transplantation issued a position statement in June 2018 that endorsed autologous hematopoietic stem cell transplantation as “standard of care” for systemic sclerosis (Biol Blood Marrow Transplant. 2018 June 25. doi: 10.1016/j.bbmt.2018.06.025), Dr. Sullivan noted in a video interview.
The SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial randomized 75 patients with severe scleroderma and substantial internal organ involvement to receive treatment with either cyclophosphamide or myeloablative radiation followed by immune reconstitution with an autologous hematopoietic stem cell transplant. The trial’s primary endpoint, the global rank composite score at 54 months, showed the superiority of transplantation over standard treatment (N Engl J Med. 2018 Jan 4;378[1]:35-47).
Dr. Sullivan and his associates ran their long-term follow-up study on 43 of these 75 patients (25 from the transplanted group and 18 controls), excluding 21 patients who died during the original study, 4 additional patients from the control arm who died following the end of the original SCOT protocol, and 7 patients either lost to follow-up or who refused to participate in follow-up. Among the 25 transplanted patients, none died during the extended follow-up, 2 experienced cardiac failure, and 23 remained off of any disease-modifying antirheumatic drugs. Among the 18 survivors in the control arm, 3 had cardiac failure, 3 had respiratory failure, and 7 were on treatment with disease-modifying drugs, Dr. Sullivan reported.
In addition, 23 of the 25 (92%) transplanted patients had normal performance status by the Eastern Cooperative Oncology Group criteria, compared with 11 of the 18 controls (61%). A total of 14 (56%) transplant patients were employed, compared with 6 of the 18 controls (33%).
Patients who were transplanted “have their life back, are doing well, and are off treatment,” Dr. Sullivan noted.
Myeloablation and transplant is appropriate for scleroderma patients with significant internal organ involvement, about half of all patients with this disease. The best gauge of severe organ involvement is a pulmonary function test, with a forced vital capacity of 70% or less of predicted as a flag for patients who should consider transplantation, Dr. Sullivan said. He recommended monitoring lung function every 3 months in scleroderma patients because it can deteriorate very suddenly and quickly.
SCOT received no commercial funding. Dr. Sullivan had no disclosures to report.
SOURCE: Sullivan KM et al. ACR Annual Meeting, Abstract 1820.
CHICAGO – Follow-up out to as long as 11 years from treatment confirmed the long-term efficacy and safety of myeloablative autologous stem cell transplantation for patients with severe scleroderma.
This extended follow-up comprised 43 survivors from the 75 patients originally randomized in a controlled, 6-year trial. Follow-up showed that, among the patients who underwent myeloablation and autologous transplant with hematopoietic stem cells, there were no long-term deaths or cancers, there was an 88% survival rate, and 92% remained off disease-modifying treatment, Keith M. Sullivan, MD, said at the annual meeting of the American College of Rheumatology.
Long-term survival among patients randomized to the study’s control arm, who received treatment with cyclophosphamide, was 53%.
Patients with severe scleroderma with significant internal organ damage who “are improved and off of disease-modifying antirheumatic drugs after 10 or more years from treatment is something new in autoimmune disease,” said Dr. Sullivan, a professor of medicine at Duke University, Durham, N.C.
Based on accumulated data from this randomized trial and other studies, the American Society for Blood and Marrow Transplantation issued a position statement in June 2018 that endorsed autologous hematopoietic stem cell transplantation as “standard of care” for systemic sclerosis (Biol Blood Marrow Transplant. 2018 June 25. doi: 10.1016/j.bbmt.2018.06.025), Dr. Sullivan noted in a video interview.
The SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial randomized 75 patients with severe scleroderma and substantial internal organ involvement to receive treatment with either cyclophosphamide or myeloablative radiation followed by immune reconstitution with an autologous hematopoietic stem cell transplant. The trial’s primary endpoint, the global rank composite score at 54 months, showed the superiority of transplantation over standard treatment (N Engl J Med. 2018 Jan 4;378[1]:35-47).
Dr. Sullivan and his associates ran their long-term follow-up study on 43 of these 75 patients (25 from the transplanted group and 18 controls), excluding 21 patients who died during the original study, 4 additional patients from the control arm who died following the end of the original SCOT protocol, and 7 patients either lost to follow-up or who refused to participate in follow-up. Among the 25 transplanted patients, none died during the extended follow-up, 2 experienced cardiac failure, and 23 remained off of any disease-modifying antirheumatic drugs. Among the 18 survivors in the control arm, 3 had cardiac failure, 3 had respiratory failure, and 7 were on treatment with disease-modifying drugs, Dr. Sullivan reported.
In addition, 23 of the 25 (92%) transplanted patients had normal performance status by the Eastern Cooperative Oncology Group criteria, compared with 11 of the 18 controls (61%). A total of 14 (56%) transplant patients were employed, compared with 6 of the 18 controls (33%).
Patients who were transplanted “have their life back, are doing well, and are off treatment,” Dr. Sullivan noted.
Myeloablation and transplant is appropriate for scleroderma patients with significant internal organ involvement, about half of all patients with this disease. The best gauge of severe organ involvement is a pulmonary function test, with a forced vital capacity of 70% or less of predicted as a flag for patients who should consider transplantation, Dr. Sullivan said. He recommended monitoring lung function every 3 months in scleroderma patients because it can deteriorate very suddenly and quickly.
SCOT received no commercial funding. Dr. Sullivan had no disclosures to report.
SOURCE: Sullivan KM et al. ACR Annual Meeting, Abstract 1820.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Survival after 11 years was 88% among transplanted patients and 53% among control patients treated with cyclophosphamide.
Study details: A long-term follow-up of 43 of the 75 patients enrolled in the SCOT trial.
Disclosures: SCOT received no commercial funding. Dr. Sullivan had no disclosures to report.
Source: Sullivan KM et al. ACR Annual Meeting, Abstract 1820.
First reported case of induced resistance to tisagenlecleucel
Unintentional transduction of a single leukemic B cell appears to have induced resistance to CTL019 (tisagenlecleucel, Kymriah) therapy, a recent case study suggests.
A total of 9 months after receiving a seemingly successful CD19-targeted chimeric antigen receptor (CAR) T-cell (CTL019; tisagenlecleucel) infusion, a 20-year-old man with B-cell acute lymphoblastic leukemia (B-ALL) had a frank relapse, with more than 90% bone marrow infiltration of CAR-transduced B-cell leukemia cells. Further investigation showed that the CAR gene had unintentionally been added to a solitary leukemic B cell during the CAR T-cell manufacturing process, reported Marco Ruella, MD, of the University of Pennsylvania, Philadelphia.
“The transduction of a single leukemic cell with an anti-CD19 CAR lentivirus during CTL019 manufacturing is sufficient to mediate resistance through masking of the CD19 epitope. This is a rare event, as this is the only case out of 369 patients reported worldwide at the time of publication. ... These findings illustrate the need for improved manufacturing technologies that can purge residual contaminating tumor cells from engineered T cells,” the authors wrote in Nature Medicine.
The findings also confirm the cancer stem cell hypothesis in humans, “given that clonal analysis indicated that the relapse and subsequent death of the patient were attributed to the progeny of a single leukemic blast cell with extensive replicative capacity, both in culture and in vivo,” they wrote.
Initially, “the infused CTL019 cells displayed the typical pattern of in vivo engraftment and expansion by CAR19-specific flow cytometry, followed by decline to an undetectable level in the peripheral blood” of the affected patient, the authors wrote. “The expansion and contraction phases and long-term persistence of CAR T cells were confirmed via qPCR using CAR-specific primers.” The patient was in complete remission at day 28.
However, they added, routine peripheral blood monitoring with quantitative polymerase chain reaction for CAR-specific sequences identified “the emergence of a second expansion phase of CAR cells starting at day 252, which did not correlate with re-expansion of CAR + T cells by flow cytometry.” Frank relapse soon followed.
Analysis confirmed “that the lack of detection of CD19 by flow cytometry was due to CAR19 binding in cis to CD19 on the surface of leukemic blasts, thus masking the epitope from detection by standard flow cytometry,” the authors wrote.
Study funding was provided by Bristol-Myers Squibb, Novartis, the National Institutes of Health, and others. Dr. Ruella and several of his colleagues work under a research collaboration involving the University of Pennsylvania and the Novartis Institutes of Biomedical Research and are inventors of intellectual property licensed by the University of Pennsylvania to Novartis.
SOURCE: Ruella M et al. Nat Med. 2018 Oct 1. doi: 10.1038/s41591-018-0201-9.
Unintentional transduction of a single leukemic B cell appears to have induced resistance to CTL019 (tisagenlecleucel, Kymriah) therapy, a recent case study suggests.
A total of 9 months after receiving a seemingly successful CD19-targeted chimeric antigen receptor (CAR) T-cell (CTL019; tisagenlecleucel) infusion, a 20-year-old man with B-cell acute lymphoblastic leukemia (B-ALL) had a frank relapse, with more than 90% bone marrow infiltration of CAR-transduced B-cell leukemia cells. Further investigation showed that the CAR gene had unintentionally been added to a solitary leukemic B cell during the CAR T-cell manufacturing process, reported Marco Ruella, MD, of the University of Pennsylvania, Philadelphia.
“The transduction of a single leukemic cell with an anti-CD19 CAR lentivirus during CTL019 manufacturing is sufficient to mediate resistance through masking of the CD19 epitope. This is a rare event, as this is the only case out of 369 patients reported worldwide at the time of publication. ... These findings illustrate the need for improved manufacturing technologies that can purge residual contaminating tumor cells from engineered T cells,” the authors wrote in Nature Medicine.
The findings also confirm the cancer stem cell hypothesis in humans, “given that clonal analysis indicated that the relapse and subsequent death of the patient were attributed to the progeny of a single leukemic blast cell with extensive replicative capacity, both in culture and in vivo,” they wrote.
Initially, “the infused CTL019 cells displayed the typical pattern of in vivo engraftment and expansion by CAR19-specific flow cytometry, followed by decline to an undetectable level in the peripheral blood” of the affected patient, the authors wrote. “The expansion and contraction phases and long-term persistence of CAR T cells were confirmed via qPCR using CAR-specific primers.” The patient was in complete remission at day 28.
However, they added, routine peripheral blood monitoring with quantitative polymerase chain reaction for CAR-specific sequences identified “the emergence of a second expansion phase of CAR cells starting at day 252, which did not correlate with re-expansion of CAR + T cells by flow cytometry.” Frank relapse soon followed.
Analysis confirmed “that the lack of detection of CD19 by flow cytometry was due to CAR19 binding in cis to CD19 on the surface of leukemic blasts, thus masking the epitope from detection by standard flow cytometry,” the authors wrote.
Study funding was provided by Bristol-Myers Squibb, Novartis, the National Institutes of Health, and others. Dr. Ruella and several of his colleagues work under a research collaboration involving the University of Pennsylvania and the Novartis Institutes of Biomedical Research and are inventors of intellectual property licensed by the University of Pennsylvania to Novartis.
SOURCE: Ruella M et al. Nat Med. 2018 Oct 1. doi: 10.1038/s41591-018-0201-9.
Unintentional transduction of a single leukemic B cell appears to have induced resistance to CTL019 (tisagenlecleucel, Kymriah) therapy, a recent case study suggests.
A total of 9 months after receiving a seemingly successful CD19-targeted chimeric antigen receptor (CAR) T-cell (CTL019; tisagenlecleucel) infusion, a 20-year-old man with B-cell acute lymphoblastic leukemia (B-ALL) had a frank relapse, with more than 90% bone marrow infiltration of CAR-transduced B-cell leukemia cells. Further investigation showed that the CAR gene had unintentionally been added to a solitary leukemic B cell during the CAR T-cell manufacturing process, reported Marco Ruella, MD, of the University of Pennsylvania, Philadelphia.
“The transduction of a single leukemic cell with an anti-CD19 CAR lentivirus during CTL019 manufacturing is sufficient to mediate resistance through masking of the CD19 epitope. This is a rare event, as this is the only case out of 369 patients reported worldwide at the time of publication. ... These findings illustrate the need for improved manufacturing technologies that can purge residual contaminating tumor cells from engineered T cells,” the authors wrote in Nature Medicine.
The findings also confirm the cancer stem cell hypothesis in humans, “given that clonal analysis indicated that the relapse and subsequent death of the patient were attributed to the progeny of a single leukemic blast cell with extensive replicative capacity, both in culture and in vivo,” they wrote.
Initially, “the infused CTL019 cells displayed the typical pattern of in vivo engraftment and expansion by CAR19-specific flow cytometry, followed by decline to an undetectable level in the peripheral blood” of the affected patient, the authors wrote. “The expansion and contraction phases and long-term persistence of CAR T cells were confirmed via qPCR using CAR-specific primers.” The patient was in complete remission at day 28.
However, they added, routine peripheral blood monitoring with quantitative polymerase chain reaction for CAR-specific sequences identified “the emergence of a second expansion phase of CAR cells starting at day 252, which did not correlate with re-expansion of CAR + T cells by flow cytometry.” Frank relapse soon followed.
Analysis confirmed “that the lack of detection of CD19 by flow cytometry was due to CAR19 binding in cis to CD19 on the surface of leukemic blasts, thus masking the epitope from detection by standard flow cytometry,” the authors wrote.
Study funding was provided by Bristol-Myers Squibb, Novartis, the National Institutes of Health, and others. Dr. Ruella and several of his colleagues work under a research collaboration involving the University of Pennsylvania and the Novartis Institutes of Biomedical Research and are inventors of intellectual property licensed by the University of Pennsylvania to Novartis.
SOURCE: Ruella M et al. Nat Med. 2018 Oct 1. doi: 10.1038/s41591-018-0201-9.
FROM NATURE MEDICINE
Key clinical point: Unintentional transduction of a single leukemic B cell induced resistance to CTL019 (tisagenlecleucel) therapy.
Major finding: A patient with B-cell acute lymphoblastic leukemia (B-ALL) had frank relapse 9 months after a CTL019 infusion, with more than 90% bone marrow infiltration of chimeric antigen receptor–transduced B-cell leukemia cells.
Study details: A case study of a 20-year-old male with B-ALL undergoing CTL019 therapy.
Disclosures: Study funding was provided by Bristol-Myers Squibb, Novartis, the National Institutes of Health, and others. Dr. Ruella and several of his colleagues work under a research collaboration involving the University of Pennsylvania and the Novartis Institutes of Biomedical Research and are inventors of intellectual property licensed by the University of Pennsylvania to Novartis.
Source: Ruella M et al. Nat Med. 2018 Oct 1. doi: 10.1038/s41591-018-0201-9.
Autologous fecal transplant restores microbiota after allo-HSCT
For patients who undergo allogeneic hematopoietic stem cell transplant (allo-HSCT) with intensive antibiotics, a subsequent autologous fecal transplant (auto-FMT) can restore intestinal microbiota, a recent study found.
Loss of normal gut bacteria after allo-HSCT and antibiotics is a common occurrence and known risk factor for graft-versus-host disease (GVHD) and intestinal infection.
“Overall, patients who lose gut microbiota diversity at the time of hematopoietic stem cell engraftment have higher rates of transplant-related death,” reported Ying Taur, MD, of the Memorial Sloan Kettering Cancer Center in New York, and his colleagues. “We explored whether the microbiota could be restored in allo-HSCT patients through the use of auto-FMT.”
Allo-HSCT patients are immune suppressed for months after engraftment, so safety concerns led the investigators to use auto-FMT rather than a fecal transplant from another individual. The complex population of viruses, fungi, archaea, bacteria, and protozoa that inhabit the human gut remains poorly understood, as does the infectious potential of a heterologous fecal donor.
“Despite remarkable advances in recent years, current technologies are incapable of comprehensively determining fecal composition,” the authors wrote in Science Translational Medicine.
The study involved 25 patients undergoing allo-HSCT with intensive antibiotic therapy. Prior to engraftment, fecal samples were collected from all patients and analyzed for composition and diversity, measured by inverse Simpson index.
Samples were then frozen and stored. Fecal analysis also was performed after engraftment, and again after the auto-FMT time point. Auto-FMT was performed in 14 patients; 11 patients served as controls and did not receive treatment. Patients were followed for 1 year.
The investigators found that all of the patients who underwent auto-FMT recovered their pre–allo-HSCT microbiota composition and diversity, compared with none of the controls (P less than .0001). Further analysis showed that auto-FMT increased diversity (inverse Simpson index) by 64%, compared with 38% in controls.
“We have demonstrated the potential of auto-FMT as a clinical intervention to restore intestinal microbiota diversity to levels deemed safe in patients, thereby reversing the disruptive effects of broad-spectrum antibiotic treatment for patients undergoing allo-HSCT transplant,” the investigators concluded.
Study funding was provided by the Leonard Tow Foundation and the Memorial Sloan Kettering’s Center for Microbes, Inflammation, and Cancer. The authors reported financial relationships with Merck, AbbVie, Nektar Therapeutics, Novartis, and others.
SOURCE: Taur Y et al. Sci Transl Med. 2018 Sep 26. doi: 10.1126/scitranslmed.aap9489.
For patients who undergo allogeneic hematopoietic stem cell transplant (allo-HSCT) with intensive antibiotics, a subsequent autologous fecal transplant (auto-FMT) can restore intestinal microbiota, a recent study found.
Loss of normal gut bacteria after allo-HSCT and antibiotics is a common occurrence and known risk factor for graft-versus-host disease (GVHD) and intestinal infection.
“Overall, patients who lose gut microbiota diversity at the time of hematopoietic stem cell engraftment have higher rates of transplant-related death,” reported Ying Taur, MD, of the Memorial Sloan Kettering Cancer Center in New York, and his colleagues. “We explored whether the microbiota could be restored in allo-HSCT patients through the use of auto-FMT.”
Allo-HSCT patients are immune suppressed for months after engraftment, so safety concerns led the investigators to use auto-FMT rather than a fecal transplant from another individual. The complex population of viruses, fungi, archaea, bacteria, and protozoa that inhabit the human gut remains poorly understood, as does the infectious potential of a heterologous fecal donor.
“Despite remarkable advances in recent years, current technologies are incapable of comprehensively determining fecal composition,” the authors wrote in Science Translational Medicine.
The study involved 25 patients undergoing allo-HSCT with intensive antibiotic therapy. Prior to engraftment, fecal samples were collected from all patients and analyzed for composition and diversity, measured by inverse Simpson index.
Samples were then frozen and stored. Fecal analysis also was performed after engraftment, and again after the auto-FMT time point. Auto-FMT was performed in 14 patients; 11 patients served as controls and did not receive treatment. Patients were followed for 1 year.
The investigators found that all of the patients who underwent auto-FMT recovered their pre–allo-HSCT microbiota composition and diversity, compared with none of the controls (P less than .0001). Further analysis showed that auto-FMT increased diversity (inverse Simpson index) by 64%, compared with 38% in controls.
“We have demonstrated the potential of auto-FMT as a clinical intervention to restore intestinal microbiota diversity to levels deemed safe in patients, thereby reversing the disruptive effects of broad-spectrum antibiotic treatment for patients undergoing allo-HSCT transplant,” the investigators concluded.
Study funding was provided by the Leonard Tow Foundation and the Memorial Sloan Kettering’s Center for Microbes, Inflammation, and Cancer. The authors reported financial relationships with Merck, AbbVie, Nektar Therapeutics, Novartis, and others.
SOURCE: Taur Y et al. Sci Transl Med. 2018 Sep 26. doi: 10.1126/scitranslmed.aap9489.
For patients who undergo allogeneic hematopoietic stem cell transplant (allo-HSCT) with intensive antibiotics, a subsequent autologous fecal transplant (auto-FMT) can restore intestinal microbiota, a recent study found.
Loss of normal gut bacteria after allo-HSCT and antibiotics is a common occurrence and known risk factor for graft-versus-host disease (GVHD) and intestinal infection.
“Overall, patients who lose gut microbiota diversity at the time of hematopoietic stem cell engraftment have higher rates of transplant-related death,” reported Ying Taur, MD, of the Memorial Sloan Kettering Cancer Center in New York, and his colleagues. “We explored whether the microbiota could be restored in allo-HSCT patients through the use of auto-FMT.”
Allo-HSCT patients are immune suppressed for months after engraftment, so safety concerns led the investigators to use auto-FMT rather than a fecal transplant from another individual. The complex population of viruses, fungi, archaea, bacteria, and protozoa that inhabit the human gut remains poorly understood, as does the infectious potential of a heterologous fecal donor.
“Despite remarkable advances in recent years, current technologies are incapable of comprehensively determining fecal composition,” the authors wrote in Science Translational Medicine.
The study involved 25 patients undergoing allo-HSCT with intensive antibiotic therapy. Prior to engraftment, fecal samples were collected from all patients and analyzed for composition and diversity, measured by inverse Simpson index.
Samples were then frozen and stored. Fecal analysis also was performed after engraftment, and again after the auto-FMT time point. Auto-FMT was performed in 14 patients; 11 patients served as controls and did not receive treatment. Patients were followed for 1 year.
The investigators found that all of the patients who underwent auto-FMT recovered their pre–allo-HSCT microbiota composition and diversity, compared with none of the controls (P less than .0001). Further analysis showed that auto-FMT increased diversity (inverse Simpson index) by 64%, compared with 38% in controls.
“We have demonstrated the potential of auto-FMT as a clinical intervention to restore intestinal microbiota diversity to levels deemed safe in patients, thereby reversing the disruptive effects of broad-spectrum antibiotic treatment for patients undergoing allo-HSCT transplant,” the investigators concluded.
Study funding was provided by the Leonard Tow Foundation and the Memorial Sloan Kettering’s Center for Microbes, Inflammation, and Cancer. The authors reported financial relationships with Merck, AbbVie, Nektar Therapeutics, Novartis, and others.
SOURCE: Taur Y et al. Sci Transl Med. 2018 Sep 26. doi: 10.1126/scitranslmed.aap9489.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point:
Major finding: All patients who received auto-FMT regained pre–allo-HSCT microbiota composition and diversity (P less than .0001).
Study details: An open-label study involving 25 allo-HSCT patients that compared auto-FMT with no treatment.
Disclosures: Study funding was provided by the Leonard Tow Foundation and the Memorial Sloan Kettering’s Center for Microbes, Inflammation, and Cancer. The authors reported disclosures related Merck, AbbVie, Nektar Therapeutics, Novartis, and others.
Source: Taur Y et al. Sci Transl Med. 2018 Sep 26. doi: 10.1126/scitranslmed.aap9489.
CAR T-cell studies dominate ongoing cellular therapy trials
NEW YORK – The cell therapy landscape increasingly involves strategies beyond chimeric antigen receptor (CAR) T-cell therapy, but those studies still predominate among investigational trials, according to Frederick L. Locke, MD, of Moffitt Cancer Center in Tampa.
Researchers are looking at CAR T-cell therapy for earlier lines of treatment, especially in patients with aggressive lymphomas, Dr. Locke said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
Of 753 trials examining cell therapies and listed at ClinicalTrials.gov as of March 30, 2018, about half (404) were CAR T-cell therapies. The others included T-cell receptor therapies, tumor infiltrating lymphocyte therapies, dendritic cell vaccines, and natural killer cell–based therapies, according to an article in Nature Reviews.
“The development isn’t just here in the United States,” Dr. Locke said. “It’s really global. We see a lot of activity in Europe, but also in China. We’re seeing medical advances across the world through molecular biology and gene engineering of T cells and other immune cells which can be adoptively transferred into patients.”
That activity includes studies seeking to move CAR T-cell therapy earlier in the treatment paradigm for some diseases, he added. “CAR T-cell therapy in non-Hodgkin lymphoma is really beginning a paradigm shift, at least in my mind.”
Several large, randomized trials that are now comparing CD19 CAR T-cell therapy with second-line standard-of-care therapies for patients with aggressive B-cell lymphomas. Among those trials is ZUMA-7, a phase 3, randomized trial comparing axicabtagene ciloleucel with standard-of-care treatment in patients with relapsed or refractory diffuse large B-cell lymphoma.
While prognosis remains poor for relapsed or progressing aggressive B-cell lymphomas treated with chemotherapy, data to date suggest CAR T-cell therapy produces durable, long-term remissions in about 40% of patients at “a year out and counting,” Dr. Locke said.
He presented a proposed treatment algorithm that included R-CHOP chemotherapy up front and CAR T-cell therapy in later lines of treatment, an approach that Dr. Locke speculated could result in a cure rate of perhaps 80% in large-cell lymphomas.
Encouraging longer-term data is emerging, with some patients with aggressive T-cell lymphomas now without recurrence for 5 years or more following a single infusion of CAR T-cell therapy, he said.
Dr. Locke reported a financial disclosure related to Cellular Biomedicine Group.
NEW YORK – The cell therapy landscape increasingly involves strategies beyond chimeric antigen receptor (CAR) T-cell therapy, but those studies still predominate among investigational trials, according to Frederick L. Locke, MD, of Moffitt Cancer Center in Tampa.
Researchers are looking at CAR T-cell therapy for earlier lines of treatment, especially in patients with aggressive lymphomas, Dr. Locke said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
Of 753 trials examining cell therapies and listed at ClinicalTrials.gov as of March 30, 2018, about half (404) were CAR T-cell therapies. The others included T-cell receptor therapies, tumor infiltrating lymphocyte therapies, dendritic cell vaccines, and natural killer cell–based therapies, according to an article in Nature Reviews.
“The development isn’t just here in the United States,” Dr. Locke said. “It’s really global. We see a lot of activity in Europe, but also in China. We’re seeing medical advances across the world through molecular biology and gene engineering of T cells and other immune cells which can be adoptively transferred into patients.”
That activity includes studies seeking to move CAR T-cell therapy earlier in the treatment paradigm for some diseases, he added. “CAR T-cell therapy in non-Hodgkin lymphoma is really beginning a paradigm shift, at least in my mind.”
Several large, randomized trials that are now comparing CD19 CAR T-cell therapy with second-line standard-of-care therapies for patients with aggressive B-cell lymphomas. Among those trials is ZUMA-7, a phase 3, randomized trial comparing axicabtagene ciloleucel with standard-of-care treatment in patients with relapsed or refractory diffuse large B-cell lymphoma.
While prognosis remains poor for relapsed or progressing aggressive B-cell lymphomas treated with chemotherapy, data to date suggest CAR T-cell therapy produces durable, long-term remissions in about 40% of patients at “a year out and counting,” Dr. Locke said.
He presented a proposed treatment algorithm that included R-CHOP chemotherapy up front and CAR T-cell therapy in later lines of treatment, an approach that Dr. Locke speculated could result in a cure rate of perhaps 80% in large-cell lymphomas.
Encouraging longer-term data is emerging, with some patients with aggressive T-cell lymphomas now without recurrence for 5 years or more following a single infusion of CAR T-cell therapy, he said.
Dr. Locke reported a financial disclosure related to Cellular Biomedicine Group.
NEW YORK – The cell therapy landscape increasingly involves strategies beyond chimeric antigen receptor (CAR) T-cell therapy, but those studies still predominate among investigational trials, according to Frederick L. Locke, MD, of Moffitt Cancer Center in Tampa.
Researchers are looking at CAR T-cell therapy for earlier lines of treatment, especially in patients with aggressive lymphomas, Dr. Locke said at the annual congress on Hematologic Malignancies held by the National Comprehensive Cancer Network.
Of 753 trials examining cell therapies and listed at ClinicalTrials.gov as of March 30, 2018, about half (404) were CAR T-cell therapies. The others included T-cell receptor therapies, tumor infiltrating lymphocyte therapies, dendritic cell vaccines, and natural killer cell–based therapies, according to an article in Nature Reviews.
“The development isn’t just here in the United States,” Dr. Locke said. “It’s really global. We see a lot of activity in Europe, but also in China. We’re seeing medical advances across the world through molecular biology and gene engineering of T cells and other immune cells which can be adoptively transferred into patients.”
That activity includes studies seeking to move CAR T-cell therapy earlier in the treatment paradigm for some diseases, he added. “CAR T-cell therapy in non-Hodgkin lymphoma is really beginning a paradigm shift, at least in my mind.”
Several large, randomized trials that are now comparing CD19 CAR T-cell therapy with second-line standard-of-care therapies for patients with aggressive B-cell lymphomas. Among those trials is ZUMA-7, a phase 3, randomized trial comparing axicabtagene ciloleucel with standard-of-care treatment in patients with relapsed or refractory diffuse large B-cell lymphoma.
While prognosis remains poor for relapsed or progressing aggressive B-cell lymphomas treated with chemotherapy, data to date suggest CAR T-cell therapy produces durable, long-term remissions in about 40% of patients at “a year out and counting,” Dr. Locke said.
He presented a proposed treatment algorithm that included R-CHOP chemotherapy up front and CAR T-cell therapy in later lines of treatment, an approach that Dr. Locke speculated could result in a cure rate of perhaps 80% in large-cell lymphomas.
Encouraging longer-term data is emerging, with some patients with aggressive T-cell lymphomas now without recurrence for 5 years or more following a single infusion of CAR T-cell therapy, he said.
Dr. Locke reported a financial disclosure related to Cellular Biomedicine Group.
EXPERT ANALYSIS FROM THE NCCN HEMATOLOGIC MALIGNANCIES CONGRESS
NICE looks likely to reject use of Kymriah for DLBCL
The National Institute for Health and Care Excellence (NICE) has issued draft guidance recommending against tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).
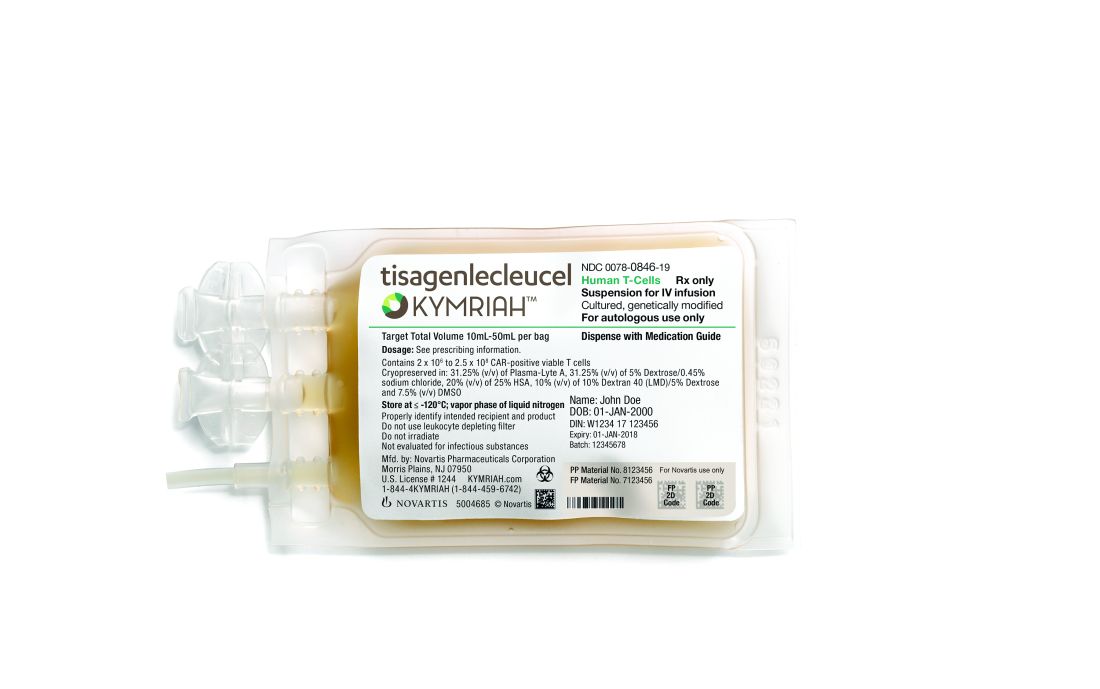
Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also European Commission–approved to treat patients up to age 25 years who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse posttransplant, or in second or later relapse.
In September 2018, the National Health Service (NHS) in England announced tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, in who have received two or more lines of systemic therapy. NICE noted that there is no standard treatment for this patient group, and that salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy. Additionally, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
All cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is 282,000 pounds. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
In August, NICE issued a similar draft guidance document recommending against use of another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta). Axicabtagene ciloleucel is approved in Europe for the treatment of patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
The National Institute for Health and Care Excellence (NICE) has issued draft guidance recommending against tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).

Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also European Commission–approved to treat patients up to age 25 years who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse posttransplant, or in second or later relapse.
In September 2018, the National Health Service (NHS) in England announced tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, in who have received two or more lines of systemic therapy. NICE noted that there is no standard treatment for this patient group, and that salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy. Additionally, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
All cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is 282,000 pounds. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
In August, NICE issued a similar draft guidance document recommending against use of another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta). Axicabtagene ciloleucel is approved in Europe for the treatment of patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
The National Institute for Health and Care Excellence (NICE) has issued draft guidance recommending against tisagenlecleucel (Kymriah) as a treatment for adults with diffuse large B-cell lymphoma (DLBCL).

Tisagenlecleucel is a chimeric antigen receptor (CAR) T-cell therapy that was recently approved by the European Commission to treat adults with relapsed or refractory DLBCL who have received two or more lines of systemic therapy.
Tisagenlecleucel is also European Commission–approved to treat patients up to age 25 years who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse posttransplant, or in second or later relapse.
In September 2018, the National Health Service (NHS) in England announced tisagenlecleucel will be made available for these ALL patients through the Cancer Drugs Fund.
However, in who have received two or more lines of systemic therapy. NICE noted that there is no standard treatment for this patient group, and that salvage chemotherapy is the most common treatment option.
Although the latest results from the JULIET trial suggest tisagenlecleucel can produce responses in patients with relapsed/refractory DLBCL, there are no data comparing tisagenlecleucel with salvage chemotherapy. Additionally, tisagenlecleucel cannot be considered a life-extending treatment at the end of life, according to NICE criteria.
All cost-effectiveness estimates for tisagenlecleucel are above the range NICE normally considers acceptable, and tisagenlecleucel does not meet criteria for inclusion in the Cancer Drugs Fund.
The list price for tisagenlecleucel is 282,000 pounds. However, Novartis, the company developing tisagenlecleucel, has a confidential commercial arrangement with the NHS that lowers the price of tisagenlecleucel for the ALL indication. This arrangement would apply if tisagenlecleucel were recommended for the DLBCL indication.
In August, NICE issued a similar draft guidance document recommending against use of another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta). Axicabtagene ciloleucel is approved in Europe for the treatment of patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy.
Kymriah cost effectiveness depends on long-term outcomes
The cost-effectiveness of tisagenlecleucel (Kymriah) depends on long-term clinical outcomes, which are presently unknown, according to investigators.
If the long-term outcomes are more modest than clinical trials suggest, then payers may be unwilling to cover the costly therapy, reported John K. Lin, MD, of the Center for Primary Care and Outcomes Research at Stanford (Calif.) University, and his colleagues. Lowering the price or setting up an outcomes-based pricing structure may be necessary to get insurers to cover the therapy.
Tisagenlecleucel is an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy that was approved by the Food and Drug Administration in August 2017 for relapsed or refractory pediatric B-cell acute lymphoblastic leukemia (ALL). In 2018, the FDA expanded the indication for tisagenlecleucel to include adults with relapsed or refractory large B-cell lymphoma, though outcomes from lymphoma trials are not analyzed in the current study.
At a wholesale acquisition cost of $475,000 per infusion, it is the most expensive existing oncology therapy to date, and can be accompanied by expensive, potentially fatal adverse effects. However, clinical trials suggest that tisagenlecleucel can offer years of relapse-free remission, thereby allowing patients to forgo other expensive therapies such as hematopoietic stem cell transplantation (HSCT).
“Although tisagenlecleucel-induced remission rates are promising, compared with those of established therapies (greater than 80% vs. less than 50%), only short-term follow-up data currently exist,” the investigators wrote in the Journal of Clinical Oncology. “Given the high cost and broad applicability in other malignancies of tisagenlecleucel, a pressing question for policy makers, payers, patients, and clinicians is whether the cost of therapy represents reasonable value.”
The study used a Markov model to assess various long-term clinical outcome rates and cost thresholds of tisagenlecleucel. The lifetime cost of therapy was assessed and compared with costs of existing therapies.
The results showed that a 5-year relapse free survival rate of 40% would make the present cost ($475,000) of tisagenlecleucel economically reasonable. In this scenario, the increased life expectancy would be 12.1 years and would result in an additional 5.07 quality-adjusted life years (QALY) gained at a cost of $61,000 per QALY, compared with blinatumomab.
But if long-term outcomes are less favorable, tisagenlecleucel becomes much less cost effective. A 5-year relapse-free survival rate of 20% would drop increased life expectancy to 3.8 years, resulting in 1.80 QALYs gained and raising the cost to $151,000 per QALY.
“Our results suggest that at tisagenlecleucel’s current price and payment structure, its economic value is uncertain,” the investigators wrote.
They suggested a price drop to $200,000 or $350,000, which would allow the drug to remain cost effective even in a worse-case scenario, in which patients relapse and tisagenlecleucel is a bridge to transplant. Another option is to move to outcomes-based pricing. Making payment conditional on 7 months of remission would make the treatment cost effective, according to the analysis.
“Price reductions of tisagenlecleucel or payment only for longer-term remissions would favorably influence cost-effectiveness, even if long-term clinical outcomes are modest,” the investigators wrote.
The study was funded by a Veterans Affairs Office of Academic Affiliations advanced fellowship in health service and research development, and a National Center for Advancing Translational Science Clinical and Translational Science Award. One of the study coauthors reported consulting and research funding from Novartis.
SOURCE: Lin et al. J Clin Oncol. 2018 Sep 13. doi: 10.1200/JCO.2018.79.0642.
The cost-effectiveness of tisagenlecleucel (Kymriah) depends on long-term clinical outcomes, which are presently unknown, according to investigators.
If the long-term outcomes are more modest than clinical trials suggest, then payers may be unwilling to cover the costly therapy, reported John K. Lin, MD, of the Center for Primary Care and Outcomes Research at Stanford (Calif.) University, and his colleagues. Lowering the price or setting up an outcomes-based pricing structure may be necessary to get insurers to cover the therapy.
Tisagenlecleucel is an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy that was approved by the Food and Drug Administration in August 2017 for relapsed or refractory pediatric B-cell acute lymphoblastic leukemia (ALL). In 2018, the FDA expanded the indication for tisagenlecleucel to include adults with relapsed or refractory large B-cell lymphoma, though outcomes from lymphoma trials are not analyzed in the current study.
At a wholesale acquisition cost of $475,000 per infusion, it is the most expensive existing oncology therapy to date, and can be accompanied by expensive, potentially fatal adverse effects. However, clinical trials suggest that tisagenlecleucel can offer years of relapse-free remission, thereby allowing patients to forgo other expensive therapies such as hematopoietic stem cell transplantation (HSCT).
“Although tisagenlecleucel-induced remission rates are promising, compared with those of established therapies (greater than 80% vs. less than 50%), only short-term follow-up data currently exist,” the investigators wrote in the Journal of Clinical Oncology. “Given the high cost and broad applicability in other malignancies of tisagenlecleucel, a pressing question for policy makers, payers, patients, and clinicians is whether the cost of therapy represents reasonable value.”
The study used a Markov model to assess various long-term clinical outcome rates and cost thresholds of tisagenlecleucel. The lifetime cost of therapy was assessed and compared with costs of existing therapies.
The results showed that a 5-year relapse free survival rate of 40% would make the present cost ($475,000) of tisagenlecleucel economically reasonable. In this scenario, the increased life expectancy would be 12.1 years and would result in an additional 5.07 quality-adjusted life years (QALY) gained at a cost of $61,000 per QALY, compared with blinatumomab.
But if long-term outcomes are less favorable, tisagenlecleucel becomes much less cost effective. A 5-year relapse-free survival rate of 20% would drop increased life expectancy to 3.8 years, resulting in 1.80 QALYs gained and raising the cost to $151,000 per QALY.
“Our results suggest that at tisagenlecleucel’s current price and payment structure, its economic value is uncertain,” the investigators wrote.
They suggested a price drop to $200,000 or $350,000, which would allow the drug to remain cost effective even in a worse-case scenario, in which patients relapse and tisagenlecleucel is a bridge to transplant. Another option is to move to outcomes-based pricing. Making payment conditional on 7 months of remission would make the treatment cost effective, according to the analysis.
“Price reductions of tisagenlecleucel or payment only for longer-term remissions would favorably influence cost-effectiveness, even if long-term clinical outcomes are modest,” the investigators wrote.
The study was funded by a Veterans Affairs Office of Academic Affiliations advanced fellowship in health service and research development, and a National Center for Advancing Translational Science Clinical and Translational Science Award. One of the study coauthors reported consulting and research funding from Novartis.
SOURCE: Lin et al. J Clin Oncol. 2018 Sep 13. doi: 10.1200/JCO.2018.79.0642.
The cost-effectiveness of tisagenlecleucel (Kymriah) depends on long-term clinical outcomes, which are presently unknown, according to investigators.
If the long-term outcomes are more modest than clinical trials suggest, then payers may be unwilling to cover the costly therapy, reported John K. Lin, MD, of the Center for Primary Care and Outcomes Research at Stanford (Calif.) University, and his colleagues. Lowering the price or setting up an outcomes-based pricing structure may be necessary to get insurers to cover the therapy.
Tisagenlecleucel is an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy that was approved by the Food and Drug Administration in August 2017 for relapsed or refractory pediatric B-cell acute lymphoblastic leukemia (ALL). In 2018, the FDA expanded the indication for tisagenlecleucel to include adults with relapsed or refractory large B-cell lymphoma, though outcomes from lymphoma trials are not analyzed in the current study.
At a wholesale acquisition cost of $475,000 per infusion, it is the most expensive existing oncology therapy to date, and can be accompanied by expensive, potentially fatal adverse effects. However, clinical trials suggest that tisagenlecleucel can offer years of relapse-free remission, thereby allowing patients to forgo other expensive therapies such as hematopoietic stem cell transplantation (HSCT).
“Although tisagenlecleucel-induced remission rates are promising, compared with those of established therapies (greater than 80% vs. less than 50%), only short-term follow-up data currently exist,” the investigators wrote in the Journal of Clinical Oncology. “Given the high cost and broad applicability in other malignancies of tisagenlecleucel, a pressing question for policy makers, payers, patients, and clinicians is whether the cost of therapy represents reasonable value.”
The study used a Markov model to assess various long-term clinical outcome rates and cost thresholds of tisagenlecleucel. The lifetime cost of therapy was assessed and compared with costs of existing therapies.
The results showed that a 5-year relapse free survival rate of 40% would make the present cost ($475,000) of tisagenlecleucel economically reasonable. In this scenario, the increased life expectancy would be 12.1 years and would result in an additional 5.07 quality-adjusted life years (QALY) gained at a cost of $61,000 per QALY, compared with blinatumomab.
But if long-term outcomes are less favorable, tisagenlecleucel becomes much less cost effective. A 5-year relapse-free survival rate of 20% would drop increased life expectancy to 3.8 years, resulting in 1.80 QALYs gained and raising the cost to $151,000 per QALY.
“Our results suggest that at tisagenlecleucel’s current price and payment structure, its economic value is uncertain,” the investigators wrote.
They suggested a price drop to $200,000 or $350,000, which would allow the drug to remain cost effective even in a worse-case scenario, in which patients relapse and tisagenlecleucel is a bridge to transplant. Another option is to move to outcomes-based pricing. Making payment conditional on 7 months of remission would make the treatment cost effective, according to the analysis.
“Price reductions of tisagenlecleucel or payment only for longer-term remissions would favorably influence cost-effectiveness, even if long-term clinical outcomes are modest,” the investigators wrote.
The study was funded by a Veterans Affairs Office of Academic Affiliations advanced fellowship in health service and research development, and a National Center for Advancing Translational Science Clinical and Translational Science Award. One of the study coauthors reported consulting and research funding from Novartis.
SOURCE: Lin et al. J Clin Oncol. 2018 Sep 13. doi: 10.1200/JCO.2018.79.0642.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: If 40% of patients achieve 5-year remission without relapse, then tisagenlecleucel would cost $61,000 per quality-adjusted life year.
Study details: An economic analysis involving tisagenlecleucel costs and clinical trial outcomes.
Disclosures: The study was funded by a Veterans Affairs Office of Academic Affiliations advanced fellowship in health service and research development, and a National Center for Advancing Translational Science Clinical and Translational Science Award. One study coauthor reported consulting and research funding from Novartis.
Source: Lin JK et al. J Clin Oncol. 2018 Sep 13. doi: 10.1200/JCO.2018.79.0642.
Early CAR T data on P-BCMA-101 in refractory myeloma
Early results from a phase 1 trial suggest the chimeric antigen receptor (CAR) T-cell therapy P-BCMA-101 can produce responses in patients with relapsed/refractory multiple myeloma.
All 11 patients treated have experienced some clinical response, with 8 patients achieving a partial response (PR) or better.
The most common adverse events were neutropenia and thrombocytopenia. One patient was suspected to have cytokine release syndrome (CRS), but the condition resolved without use of tocilizumab or steroids.
These results were presented at the 2018 CAR-TCR Summit by Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics Inc., the company developing P-BCMA-101.
Dr. Ostertag presented data on 11 patients with heavily pretreated multiple myeloma. They had a median of six prior therapies. The median age was 60 years, and most of the patients were considered high risk.
Prior to receiving P-BCMA-101, patients received conditioning with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) for 3 days.
Patients were then treated across three dose groups with average CAR T-cell doses of 51 x 106 (n = 3), 152 x 106 (n = 7), and 430 x 106 (n = 1).
As of Aug. 10, 2018, all 11 patients were still on study.
There were no dose-limiting toxicities. Eight patients developed neutropenia, and five had thrombocytopenia.
Researchers suspected CRS in one patient, but the condition resolved without tocilizumab or steroid treatment. There was no neurotoxicity reported, and none of the patients required admission to an intensive care unit.
All patients showed improvement in biomarkers following treatment.
Ten patients were evaluable for response by International Myeloma Working Group criteria. Seven of these patients achieved at least a PR, including very good partial responses (VGPRs) and stringent complete response (CR).
The eleventh patient also responded to treatment, but this patient has oligosecretory disease and was evaluable only by PET. The patient had a near-CR by PET.
Poseida Therapeutics would not disclose additional details regarding how many patients achieved a PR, VGPR, or CR, but the company plans to release more information on response at an upcoming meeting.
“The latest data results show that P-BCMA-101 induces deep responses in a heavily pretreated population with relapsed/refractory multiple myeloma, with some patients reaching VGPR and even stringent CR at early efficacy assessments,” Dr. Ostertag said.
This study (NCT03288493) is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.
Early results from a phase 1 trial suggest the chimeric antigen receptor (CAR) T-cell therapy P-BCMA-101 can produce responses in patients with relapsed/refractory multiple myeloma.
All 11 patients treated have experienced some clinical response, with 8 patients achieving a partial response (PR) or better.
The most common adverse events were neutropenia and thrombocytopenia. One patient was suspected to have cytokine release syndrome (CRS), but the condition resolved without use of tocilizumab or steroids.
These results were presented at the 2018 CAR-TCR Summit by Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics Inc., the company developing P-BCMA-101.
Dr. Ostertag presented data on 11 patients with heavily pretreated multiple myeloma. They had a median of six prior therapies. The median age was 60 years, and most of the patients were considered high risk.
Prior to receiving P-BCMA-101, patients received conditioning with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) for 3 days.
Patients were then treated across three dose groups with average CAR T-cell doses of 51 x 106 (n = 3), 152 x 106 (n = 7), and 430 x 106 (n = 1).
As of Aug. 10, 2018, all 11 patients were still on study.
There were no dose-limiting toxicities. Eight patients developed neutropenia, and five had thrombocytopenia.
Researchers suspected CRS in one patient, but the condition resolved without tocilizumab or steroid treatment. There was no neurotoxicity reported, and none of the patients required admission to an intensive care unit.
All patients showed improvement in biomarkers following treatment.
Ten patients were evaluable for response by International Myeloma Working Group criteria. Seven of these patients achieved at least a PR, including very good partial responses (VGPRs) and stringent complete response (CR).
The eleventh patient also responded to treatment, but this patient has oligosecretory disease and was evaluable only by PET. The patient had a near-CR by PET.
Poseida Therapeutics would not disclose additional details regarding how many patients achieved a PR, VGPR, or CR, but the company plans to release more information on response at an upcoming meeting.
“The latest data results show that P-BCMA-101 induces deep responses in a heavily pretreated population with relapsed/refractory multiple myeloma, with some patients reaching VGPR and even stringent CR at early efficacy assessments,” Dr. Ostertag said.
This study (NCT03288493) is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.
Early results from a phase 1 trial suggest the chimeric antigen receptor (CAR) T-cell therapy P-BCMA-101 can produce responses in patients with relapsed/refractory multiple myeloma.
All 11 patients treated have experienced some clinical response, with 8 patients achieving a partial response (PR) or better.
The most common adverse events were neutropenia and thrombocytopenia. One patient was suspected to have cytokine release syndrome (CRS), but the condition resolved without use of tocilizumab or steroids.
These results were presented at the 2018 CAR-TCR Summit by Eric Ostertag, MD, PhD, chief executive officer of Poseida Therapeutics Inc., the company developing P-BCMA-101.
Dr. Ostertag presented data on 11 patients with heavily pretreated multiple myeloma. They had a median of six prior therapies. The median age was 60 years, and most of the patients were considered high risk.
Prior to receiving P-BCMA-101, patients received conditioning with fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2) for 3 days.
Patients were then treated across three dose groups with average CAR T-cell doses of 51 x 106 (n = 3), 152 x 106 (n = 7), and 430 x 106 (n = 1).
As of Aug. 10, 2018, all 11 patients were still on study.
There were no dose-limiting toxicities. Eight patients developed neutropenia, and five had thrombocytopenia.
Researchers suspected CRS in one patient, but the condition resolved without tocilizumab or steroid treatment. There was no neurotoxicity reported, and none of the patients required admission to an intensive care unit.
All patients showed improvement in biomarkers following treatment.
Ten patients were evaluable for response by International Myeloma Working Group criteria. Seven of these patients achieved at least a PR, including very good partial responses (VGPRs) and stringent complete response (CR).
The eleventh patient also responded to treatment, but this patient has oligosecretory disease and was evaluable only by PET. The patient had a near-CR by PET.
Poseida Therapeutics would not disclose additional details regarding how many patients achieved a PR, VGPR, or CR, but the company plans to release more information on response at an upcoming meeting.
“The latest data results show that P-BCMA-101 induces deep responses in a heavily pretreated population with relapsed/refractory multiple myeloma, with some patients reaching VGPR and even stringent CR at early efficacy assessments,” Dr. Ostertag said.
This study (NCT03288493) is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.
FROM THE 2018 CAR-TCR SUMMIT
Key clinical point: The chimeric antigen receptor .
Major finding: All 11 patients have shown signs of response, and 8 patients achieved a partial response or better.
Study details: Eleven patients have been treated thus far in this phase 1 trial.
Disclosures: This trial is funded by the California Institute for Regenerative Medicine and Poseida Therapeutics.
England green-lights coverage of one CAR T-cell therapy
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will be available only for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), has not fared as well as in England. The National Institute for Health and Care Excellence (NICE) recently issued draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy. However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and the therapy does not meet the criteria for inclusion in the Cancer Drugs Fund.
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will be available only for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), has not fared as well as in England. The National Institute for Health and Care Excellence (NICE) recently issued draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy. However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and the therapy does not meet the criteria for inclusion in the Cancer Drugs Fund.
The National Health Service (NHS) of England has announced that tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR) T-cell therapy, will soon be available for certain leukemia patients.
, and patients could potentially begin receiving the treatment within weeks.
NHS England struck a deal with Novartis to lower the price of tisagenlecleucel, which costs around £282,000 per patient at its full list price. The discount offered to the NHS is confidential.
Tisagenlecleucel was recently approved by the European Commission (EC) to treat patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
The EC also approved tisagenlecleucel to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
However, tisagenlecleucel will be available only for ALL patients in England, at least initially. A decision has not been made regarding funding for tisagenlecleucel in DLBCL, and Novartis previously decided to launch tisagenlecleucel in ALL first.
“It’s fantastic news for children and young people with this form of leukemia that CAR T-cell therapy will be made available on the NHS, making them the first in Europe to have routine access to this exciting new type of immunotherapy,” said Charles Swanton, Cancer Research UK’s chief clinician.
The first three NHS hospitals to go through the international accreditation process for the provision of tisagenlecleucel are in London, Manchester, and Newcastle. Subject to passing accreditation requirements, the first treatments could begin in a matter of weeks.
Another CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), has not fared as well as in England. The National Institute for Health and Care Excellence (NICE) recently issued draft guidance recommending against the use of axicabtagene ciloleucel in England.
Axicabtagene ciloleucel was approved by the EC to treat patients with relapsed/refractory DLBCL or primary mediastinal B-cell lymphoma who have received two or more lines of systemic therapy. However, NICE said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy. NICE also said the price of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of NHS resources, and the therapy does not meet the criteria for inclusion in the Cancer Drugs Fund.