User login
FDA clears portable hematology analyzer
The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for PixCell Medical’s HemoScreen™.
This portable hematology analyzer is used to perform a complete blood count at the point of care.
HemoScreen requires a single drop of blood and uses disposable cartridges that provide automatic sample preparation.
HemoScreen can analyze 20 standard complete blood count parameters and produces results within 5 minutes.
Study results suggested that HemoScreen provides results comparable to those of another hematology analyzer, Sysmex XE-2100. This study was published in the Journal of Clinical Pathology in 2016.
“The HemoScreen delivers lab-accurate results,” said Avishay Bransky, PhD, chief executive officer of PixCell Medical.
He added that HemoScreen “would be especially useful” in physicians’ offices, emergency rooms, intensive care units, oncology clinics, and remote locations.
HemoScreen makes use of a technology called viscoelastic focusing, which employs microfluidics and machine vision algorithms to analyze cells.
The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for PixCell Medical’s HemoScreen™.
This portable hematology analyzer is used to perform a complete blood count at the point of care.
HemoScreen requires a single drop of blood and uses disposable cartridges that provide automatic sample preparation.
HemoScreen can analyze 20 standard complete blood count parameters and produces results within 5 minutes.
Study results suggested that HemoScreen provides results comparable to those of another hematology analyzer, Sysmex XE-2100. This study was published in the Journal of Clinical Pathology in 2016.
“The HemoScreen delivers lab-accurate results,” said Avishay Bransky, PhD, chief executive officer of PixCell Medical.
He added that HemoScreen “would be especially useful” in physicians’ offices, emergency rooms, intensive care units, oncology clinics, and remote locations.
HemoScreen makes use of a technology called viscoelastic focusing, which employs microfluidics and machine vision algorithms to analyze cells.
The U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for PixCell Medical’s HemoScreen™.
This portable hematology analyzer is used to perform a complete blood count at the point of care.
HemoScreen requires a single drop of blood and uses disposable cartridges that provide automatic sample preparation.
HemoScreen can analyze 20 standard complete blood count parameters and produces results within 5 minutes.
Study results suggested that HemoScreen provides results comparable to those of another hematology analyzer, Sysmex XE-2100. This study was published in the Journal of Clinical Pathology in 2016.
“The HemoScreen delivers lab-accurate results,” said Avishay Bransky, PhD, chief executive officer of PixCell Medical.
He added that HemoScreen “would be especially useful” in physicians’ offices, emergency rooms, intensive care units, oncology clinics, and remote locations.
HemoScreen makes use of a technology called viscoelastic focusing, which employs microfluidics and machine vision algorithms to analyze cells.
Underused PV treatments save lives, doc says
Researchers have found evidence to suggest that phlebotomy and hydroxyurea (HU) provide real-world benefits for older patients with polycythemia vera (PV), but both treatments are underused.
In a study of more than 800 PV patients, phlebotomy and HU treatment were both associated with lower risks of death and thrombosis.
However, 39% of patients didn’t receive HU, and 36% didn’t undergo phlebotomy.
“Our study highlights the value of adhering to PV treatment guidelines,” said study author Nikolai A. Podoltsev, MD, PhD, of Yale Cancer Center in New Haven, Connecticut.
“Use of the two recommended treatments saves lives.”
Dr. Podoltsev and his colleagues described this survival benefit in Blood Advances.
The researchers studied data from the linked Surveillance, Epidemiology, and End Results–Medicare database. They collected information on 820 older adults diagnosed with PV from 2007 to 2013.
The patients’ median age was 77 (range, 71-83), 57% were female, 91.2% were white, 12.7% had a disability, and 13.2% had a prior thrombotic event.
Patients received the following PV treatments:
- Both phlebotomy and HU concurrently or sequentially (41.1%)
- Phlebotomy only (23.0%)
- HU only (19.6%)
- Neither phlebotomy nor HU (16.3%).
Survival
The median follow-up was 2.83 years. During that time, 37.2% of patients (n=305) died.
The median survival was 6.29 years for phlebotomy recipients and 4.50 years for non-recipients (P<0.01). The median survival was 6.02 years for HU recipients and 5.25 years for non-recipients (P<0.01).
In a multivariable analysis, receipt of phlebotomy was associated with decreased mortality. The hazard ratio (HR) for death was 0.65 (P<0.01) for phlebotomy recipients.
Increasing phlebotomy intensity (the number of phlebotomies per year) was also associated with decreased mortality, with an HR of 0.71 (P<0.01).
A higher proportion of days covered (PDC) by HU treatment was associated with decreased mortality as well.
The researchers said every 10% increase of HU PDC was associated with an 8% to 9% lower risk of death. The HR was 0.92 in a model where phlebotomy was a binary variable and 0.91 in a model that included the frequency of phlebotomy (P<0.01 for both).
Thrombosis
In all, 36.1% of patients (n=296) had a thrombotic event, which includes venous and arterial thrombosis.
The incidence of thrombosis was 29.3% (n=142) in phlebotomy recipients and 46.0% (n=154) in non-recipients (P<0.01). The incidence was 27.6% (n=118) in HU recipients and 45.4% (n=178) in non-recipients (P<0.01).
In a multivariable analysis, receipt of phlebotomy was associated with a decreased risk of thrombosis, with an HR of 0.52 (P<0.01).
Increasing phlebotomy intensity was associated with a decreased risk of thrombosis as well, with an HR of 0.46 (P<0.01).
And every 10% increase of HU PDC was associated with an 8% lower risk of thrombosis. The HR was 0.92 in both models (P<0.01 for both).
“All of the patients we studied were high-risk for clot development, and we now know from our findings that guideline-recommended treatments reduce the risk of both thrombosis and death,” Dr. Podoltsev said.
“We hope that our research will raise clinicians’ awareness of and adherence to the guidelines and improve the outcomes of PV patients in the future.”
This research was supported by the Frederick A. DeLuca Foundation. Study authors reported relationships with 24 pharmaceutical companies.
Researchers have found evidence to suggest that phlebotomy and hydroxyurea (HU) provide real-world benefits for older patients with polycythemia vera (PV), but both treatments are underused.
In a study of more than 800 PV patients, phlebotomy and HU treatment were both associated with lower risks of death and thrombosis.
However, 39% of patients didn’t receive HU, and 36% didn’t undergo phlebotomy.
“Our study highlights the value of adhering to PV treatment guidelines,” said study author Nikolai A. Podoltsev, MD, PhD, of Yale Cancer Center in New Haven, Connecticut.
“Use of the two recommended treatments saves lives.”
Dr. Podoltsev and his colleagues described this survival benefit in Blood Advances.
The researchers studied data from the linked Surveillance, Epidemiology, and End Results–Medicare database. They collected information on 820 older adults diagnosed with PV from 2007 to 2013.
The patients’ median age was 77 (range, 71-83), 57% were female, 91.2% were white, 12.7% had a disability, and 13.2% had a prior thrombotic event.
Patients received the following PV treatments:
- Both phlebotomy and HU concurrently or sequentially (41.1%)
- Phlebotomy only (23.0%)
- HU only (19.6%)
- Neither phlebotomy nor HU (16.3%).
Survival
The median follow-up was 2.83 years. During that time, 37.2% of patients (n=305) died.
The median survival was 6.29 years for phlebotomy recipients and 4.50 years for non-recipients (P<0.01). The median survival was 6.02 years for HU recipients and 5.25 years for non-recipients (P<0.01).
In a multivariable analysis, receipt of phlebotomy was associated with decreased mortality. The hazard ratio (HR) for death was 0.65 (P<0.01) for phlebotomy recipients.
Increasing phlebotomy intensity (the number of phlebotomies per year) was also associated with decreased mortality, with an HR of 0.71 (P<0.01).
A higher proportion of days covered (PDC) by HU treatment was associated with decreased mortality as well.
The researchers said every 10% increase of HU PDC was associated with an 8% to 9% lower risk of death. The HR was 0.92 in a model where phlebotomy was a binary variable and 0.91 in a model that included the frequency of phlebotomy (P<0.01 for both).
Thrombosis
In all, 36.1% of patients (n=296) had a thrombotic event, which includes venous and arterial thrombosis.
The incidence of thrombosis was 29.3% (n=142) in phlebotomy recipients and 46.0% (n=154) in non-recipients (P<0.01). The incidence was 27.6% (n=118) in HU recipients and 45.4% (n=178) in non-recipients (P<0.01).
In a multivariable analysis, receipt of phlebotomy was associated with a decreased risk of thrombosis, with an HR of 0.52 (P<0.01).
Increasing phlebotomy intensity was associated with a decreased risk of thrombosis as well, with an HR of 0.46 (P<0.01).
And every 10% increase of HU PDC was associated with an 8% lower risk of thrombosis. The HR was 0.92 in both models (P<0.01 for both).
“All of the patients we studied were high-risk for clot development, and we now know from our findings that guideline-recommended treatments reduce the risk of both thrombosis and death,” Dr. Podoltsev said.
“We hope that our research will raise clinicians’ awareness of and adherence to the guidelines and improve the outcomes of PV patients in the future.”
This research was supported by the Frederick A. DeLuca Foundation. Study authors reported relationships with 24 pharmaceutical companies.
Researchers have found evidence to suggest that phlebotomy and hydroxyurea (HU) provide real-world benefits for older patients with polycythemia vera (PV), but both treatments are underused.
In a study of more than 800 PV patients, phlebotomy and HU treatment were both associated with lower risks of death and thrombosis.
However, 39% of patients didn’t receive HU, and 36% didn’t undergo phlebotomy.
“Our study highlights the value of adhering to PV treatment guidelines,” said study author Nikolai A. Podoltsev, MD, PhD, of Yale Cancer Center in New Haven, Connecticut.
“Use of the two recommended treatments saves lives.”
Dr. Podoltsev and his colleagues described this survival benefit in Blood Advances.
The researchers studied data from the linked Surveillance, Epidemiology, and End Results–Medicare database. They collected information on 820 older adults diagnosed with PV from 2007 to 2013.
The patients’ median age was 77 (range, 71-83), 57% were female, 91.2% were white, 12.7% had a disability, and 13.2% had a prior thrombotic event.
Patients received the following PV treatments:
- Both phlebotomy and HU concurrently or sequentially (41.1%)
- Phlebotomy only (23.0%)
- HU only (19.6%)
- Neither phlebotomy nor HU (16.3%).
Survival
The median follow-up was 2.83 years. During that time, 37.2% of patients (n=305) died.
The median survival was 6.29 years for phlebotomy recipients and 4.50 years for non-recipients (P<0.01). The median survival was 6.02 years for HU recipients and 5.25 years for non-recipients (P<0.01).
In a multivariable analysis, receipt of phlebotomy was associated with decreased mortality. The hazard ratio (HR) for death was 0.65 (P<0.01) for phlebotomy recipients.
Increasing phlebotomy intensity (the number of phlebotomies per year) was also associated with decreased mortality, with an HR of 0.71 (P<0.01).
A higher proportion of days covered (PDC) by HU treatment was associated with decreased mortality as well.
The researchers said every 10% increase of HU PDC was associated with an 8% to 9% lower risk of death. The HR was 0.92 in a model where phlebotomy was a binary variable and 0.91 in a model that included the frequency of phlebotomy (P<0.01 for both).
Thrombosis
In all, 36.1% of patients (n=296) had a thrombotic event, which includes venous and arterial thrombosis.
The incidence of thrombosis was 29.3% (n=142) in phlebotomy recipients and 46.0% (n=154) in non-recipients (P<0.01). The incidence was 27.6% (n=118) in HU recipients and 45.4% (n=178) in non-recipients (P<0.01).
In a multivariable analysis, receipt of phlebotomy was associated with a decreased risk of thrombosis, with an HR of 0.52 (P<0.01).
Increasing phlebotomy intensity was associated with a decreased risk of thrombosis as well, with an HR of 0.46 (P<0.01).
And every 10% increase of HU PDC was associated with an 8% lower risk of thrombosis. The HR was 0.92 in both models (P<0.01 for both).
“All of the patients we studied were high-risk for clot development, and we now know from our findings that guideline-recommended treatments reduce the risk of both thrombosis and death,” Dr. Podoltsev said.
“We hope that our research will raise clinicians’ awareness of and adherence to the guidelines and improve the outcomes of PV patients in the future.”
This research was supported by the Frederick A. DeLuca Foundation. Study authors reported relationships with 24 pharmaceutical companies.
CPI-0610 receives fast track designation for MF
The U.S. Food and Drug Administration (FDA) has granted fast track designation to CPI-0610 for the treatment of myelofibrosis (MF).
CPI-0610 is a BET inhibitor being developed by Constellation Pharmaceuticals, Inc.
The company said results from preclinical studies and translational insights from the first-in-human study of CPI-0610 led to the prioritization of CPI-0610 as a potential treatment for MF.
The company is now enrolling patients in the phase 2 portion of the phase 1/2 MANIFEST trial (NCT02158858).
In this trial, researchers are testing CPI-0610 alone or in combination with ruxolitinib in the following patient groups:
- Transfusion-dependent or -independent MF patients who have previously received a JAK inhibitor and are ineligible for or cannot tolerate additional JAK inhibitor therapy, lost response to a JAK inhibitor, or are resistant or refractory to JAK inhibition
- MF patients who are transfusion-dependent or -independent whose disease is not being adequately controlled by ruxolitinib
- MF patients who are anemic and have not previously received a JAK inhibitor.
According to Constellation Pharmaceuticals, CPI-0610 demonstrated clinical activity in the first four patients treated on this trial, all of whom previously received a JAK inhibitor.
The researchers observed reductions in spleen volume and improvements in hemoglobin levels in patients who received monotherapy (n=2) or CPI-0610 plus ruxolitinib (n=2).
One patient treated with the combination experienced resolution of thrombocytosis and became transfusion-independent.
The company said proof-of-concept data from this trial are expected in mid-2019.
About fast track designation
The FDA’s fast track development program is designed to expedite clinical development and submission of applications for products with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the product’s development plan and written communications about issues such as trial design and use of biomarkers.
Products that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Such products may also be eligible for rolling review, which allows a developer to submit individual sections of a product’s application for review as they are ready, rather than waiting until all sections are complete.
The U.S. Food and Drug Administration (FDA) has granted fast track designation to CPI-0610 for the treatment of myelofibrosis (MF).
CPI-0610 is a BET inhibitor being developed by Constellation Pharmaceuticals, Inc.
The company said results from preclinical studies and translational insights from the first-in-human study of CPI-0610 led to the prioritization of CPI-0610 as a potential treatment for MF.
The company is now enrolling patients in the phase 2 portion of the phase 1/2 MANIFEST trial (NCT02158858).
In this trial, researchers are testing CPI-0610 alone or in combination with ruxolitinib in the following patient groups:
- Transfusion-dependent or -independent MF patients who have previously received a JAK inhibitor and are ineligible for or cannot tolerate additional JAK inhibitor therapy, lost response to a JAK inhibitor, or are resistant or refractory to JAK inhibition
- MF patients who are transfusion-dependent or -independent whose disease is not being adequately controlled by ruxolitinib
- MF patients who are anemic and have not previously received a JAK inhibitor.
According to Constellation Pharmaceuticals, CPI-0610 demonstrated clinical activity in the first four patients treated on this trial, all of whom previously received a JAK inhibitor.
The researchers observed reductions in spleen volume and improvements in hemoglobin levels in patients who received monotherapy (n=2) or CPI-0610 plus ruxolitinib (n=2).
One patient treated with the combination experienced resolution of thrombocytosis and became transfusion-independent.
The company said proof-of-concept data from this trial are expected in mid-2019.
About fast track designation
The FDA’s fast track development program is designed to expedite clinical development and submission of applications for products with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the product’s development plan and written communications about issues such as trial design and use of biomarkers.
Products that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Such products may also be eligible for rolling review, which allows a developer to submit individual sections of a product’s application for review as they are ready, rather than waiting until all sections are complete.
The U.S. Food and Drug Administration (FDA) has granted fast track designation to CPI-0610 for the treatment of myelofibrosis (MF).
CPI-0610 is a BET inhibitor being developed by Constellation Pharmaceuticals, Inc.
The company said results from preclinical studies and translational insights from the first-in-human study of CPI-0610 led to the prioritization of CPI-0610 as a potential treatment for MF.
The company is now enrolling patients in the phase 2 portion of the phase 1/2 MANIFEST trial (NCT02158858).
In this trial, researchers are testing CPI-0610 alone or in combination with ruxolitinib in the following patient groups:
- Transfusion-dependent or -independent MF patients who have previously received a JAK inhibitor and are ineligible for or cannot tolerate additional JAK inhibitor therapy, lost response to a JAK inhibitor, or are resistant or refractory to JAK inhibition
- MF patients who are transfusion-dependent or -independent whose disease is not being adequately controlled by ruxolitinib
- MF patients who are anemic and have not previously received a JAK inhibitor.
According to Constellation Pharmaceuticals, CPI-0610 demonstrated clinical activity in the first four patients treated on this trial, all of whom previously received a JAK inhibitor.
The researchers observed reductions in spleen volume and improvements in hemoglobin levels in patients who received monotherapy (n=2) or CPI-0610 plus ruxolitinib (n=2).
One patient treated with the combination experienced resolution of thrombocytosis and became transfusion-independent.
The company said proof-of-concept data from this trial are expected in mid-2019.
About fast track designation
The FDA’s fast track development program is designed to expedite clinical development and submission of applications for products with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss the product’s development plan and written communications about issues such as trial design and use of biomarkers.
Products that receive fast track designation may be eligible for accelerated approval and priority review if relevant criteria are met. Such products may also be eligible for rolling review, which allows a developer to submit individual sections of a product’s application for review as they are ready, rather than waiting until all sections are complete.
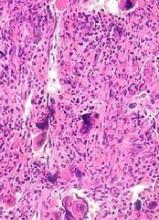
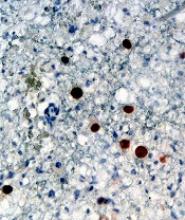
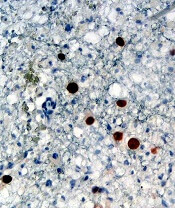
Adoptive T-cell therapy treats PML
Adoptive T-cell therapy has proven effective for treating progressive multifocal leukoencephalopathy (PML), according to research published in The New England Journal of Medicine.
Researchers observed substantial improvements in three PML patients infused with donor T cells targeting the BK virus.
Although one patient ultimately died, two had complete clearance of the JC virus and no clinical signs of PML after treatment.
“The JC and BK viruses are genetically similar and share proteins that can be targeted by the immune system,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Because of these similarities, we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
Dr. Rezvani’s team developed a novel approach for the generation of BK virus-specific T cells from healthy donors and established a bank of viral-specific T cells for immediate clinical use.
The researchers treated three patients with third-party, partially human leukocyte antigen (HLA)-matched, BK virus-specific T cells.
Patient 1 was a 32-year-old female with acute myeloid leukemia (AML) who previously received a double cord blood transplant.
Patient 2 was a 73-year-old female with JAK2-positive polycythemia rubra vera (PV) who had been treated with ruxolitinib.
Patient 3 was a 35-year-old man with AIDS who had discontinued highly active antiretroviral therapy due to side effects and who was no longer able to walk.
Following the first infusion, all three patients had a reduction in JC viral load in their cerebrospinal fluid. Viral loads dropped from:
- 700 to 78 copies in the AML patient
- 230,000 to 5,200 in the PV patient
- 4,300 to 1,300 in the AIDS patient.
“After infusion of viral-specific T cells, patients 1 and 3 had clinical improvement with significant reduction in JC virus in their cerebrospinal fluid,” Dr. Rezvani said.
“Both patients responded despite persistent T-cell immunodeficiency, supporting the concept that the response was mediated by the adoptively infused viral-specific T cells, and there were no infusion-related reactions.”
The AML patient received two additional infusions, which resulted in clearance of the virus in the cerebrospinal fluid and no signs of PML 27 months after the first infusion.
The PV patient received a second infusion that further reduced JC viral load, but no additional improvement was seen. The patient died 8 months after the first infusion.
The AIDS patient received additional infusions, resulting in complete clearance of the JC virus. This patient has regained mobility, and, 9 months after the first infusion, he is able to walk with a cane.
“We are encouraged that off-the-shelf, third-party, partially HLA-matched BK viral-specific T cells may provide a therapy for PML,” Dr. Rezvani said. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events with this treatment.”
This study was supported with funding from the Myelodysplastic Syndromes and Acute Myeloid Leukemia Moon Shot, part of MD Anderson’s Moon Shots Program, as well as the National Institutes of Health.
Adoptive T-cell therapy has proven effective for treating progressive multifocal leukoencephalopathy (PML), according to research published in The New England Journal of Medicine.
Researchers observed substantial improvements in three PML patients infused with donor T cells targeting the BK virus.
Although one patient ultimately died, two had complete clearance of the JC virus and no clinical signs of PML after treatment.
“The JC and BK viruses are genetically similar and share proteins that can be targeted by the immune system,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Because of these similarities, we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
Dr. Rezvani’s team developed a novel approach for the generation of BK virus-specific T cells from healthy donors and established a bank of viral-specific T cells for immediate clinical use.
The researchers treated three patients with third-party, partially human leukocyte antigen (HLA)-matched, BK virus-specific T cells.
Patient 1 was a 32-year-old female with acute myeloid leukemia (AML) who previously received a double cord blood transplant.
Patient 2 was a 73-year-old female with JAK2-positive polycythemia rubra vera (PV) who had been treated with ruxolitinib.
Patient 3 was a 35-year-old man with AIDS who had discontinued highly active antiretroviral therapy due to side effects and who was no longer able to walk.
Following the first infusion, all three patients had a reduction in JC viral load in their cerebrospinal fluid. Viral loads dropped from:
- 700 to 78 copies in the AML patient
- 230,000 to 5,200 in the PV patient
- 4,300 to 1,300 in the AIDS patient.
“After infusion of viral-specific T cells, patients 1 and 3 had clinical improvement with significant reduction in JC virus in their cerebrospinal fluid,” Dr. Rezvani said.
“Both patients responded despite persistent T-cell immunodeficiency, supporting the concept that the response was mediated by the adoptively infused viral-specific T cells, and there were no infusion-related reactions.”
The AML patient received two additional infusions, which resulted in clearance of the virus in the cerebrospinal fluid and no signs of PML 27 months after the first infusion.
The PV patient received a second infusion that further reduced JC viral load, but no additional improvement was seen. The patient died 8 months after the first infusion.
The AIDS patient received additional infusions, resulting in complete clearance of the JC virus. This patient has regained mobility, and, 9 months after the first infusion, he is able to walk with a cane.
“We are encouraged that off-the-shelf, third-party, partially HLA-matched BK viral-specific T cells may provide a therapy for PML,” Dr. Rezvani said. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events with this treatment.”
This study was supported with funding from the Myelodysplastic Syndromes and Acute Myeloid Leukemia Moon Shot, part of MD Anderson’s Moon Shots Program, as well as the National Institutes of Health.
Adoptive T-cell therapy has proven effective for treating progressive multifocal leukoencephalopathy (PML), according to research published in The New England Journal of Medicine.
Researchers observed substantial improvements in three PML patients infused with donor T cells targeting the BK virus.
Although one patient ultimately died, two had complete clearance of the JC virus and no clinical signs of PML after treatment.
“The JC and BK viruses are genetically similar and share proteins that can be targeted by the immune system,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Because of these similarities, we hypothesized that T cells developed against BK virus may also be effective against JC virus infection.”
Dr. Rezvani’s team developed a novel approach for the generation of BK virus-specific T cells from healthy donors and established a bank of viral-specific T cells for immediate clinical use.
The researchers treated three patients with third-party, partially human leukocyte antigen (HLA)-matched, BK virus-specific T cells.
Patient 1 was a 32-year-old female with acute myeloid leukemia (AML) who previously received a double cord blood transplant.
Patient 2 was a 73-year-old female with JAK2-positive polycythemia rubra vera (PV) who had been treated with ruxolitinib.
Patient 3 was a 35-year-old man with AIDS who had discontinued highly active antiretroviral therapy due to side effects and who was no longer able to walk.
Following the first infusion, all three patients had a reduction in JC viral load in their cerebrospinal fluid. Viral loads dropped from:
- 700 to 78 copies in the AML patient
- 230,000 to 5,200 in the PV patient
- 4,300 to 1,300 in the AIDS patient.
“After infusion of viral-specific T cells, patients 1 and 3 had clinical improvement with significant reduction in JC virus in their cerebrospinal fluid,” Dr. Rezvani said.
“Both patients responded despite persistent T-cell immunodeficiency, supporting the concept that the response was mediated by the adoptively infused viral-specific T cells, and there were no infusion-related reactions.”
The AML patient received two additional infusions, which resulted in clearance of the virus in the cerebrospinal fluid and no signs of PML 27 months after the first infusion.
The PV patient received a second infusion that further reduced JC viral load, but no additional improvement was seen. The patient died 8 months after the first infusion.
The AIDS patient received additional infusions, resulting in complete clearance of the JC virus. This patient has regained mobility, and, 9 months after the first infusion, he is able to walk with a cane.
“We are encouraged that off-the-shelf, third-party, partially HLA-matched BK viral-specific T cells may provide a therapy for PML,” Dr. Rezvani said. “Further study in a larger group of patients is required to determine the success rate, durability, and longer-term adverse events with this treatment.”
This study was supported with funding from the Myelodysplastic Syndromes and Acute Myeloid Leukemia Moon Shot, part of MD Anderson’s Moon Shots Program, as well as the National Institutes of Health.
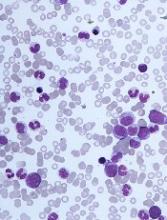
The challenges of diagnosing CMML
DUBROVNIK, CROATIA—Diagnosing chronic myelomonocytic leukemia (CMML) remains a challenge in 2018, according to a presentation at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
Even with updated World Health Organization (WHO) criteria, karyotyping, and genetic analyses, it can be difficult to distinguish CMML from other conditions, according to Nadira Duraković, MD, PhD, of the University Hospital Zagreb in Croatia.
However, Dr. Duraković said there are characteristics that differentiate CMML from myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), and atypical chronic myeloid leukemia (CML).
Furthermore, studies have suggested that monocyte subset distribution analysis can be useful for diagnosing CMML.
Dr. Duraković began her presentation with an overview of the 2016 WHO classification of CMML (Blood 2016 127:2391-2405).
According to the WHO, patients have CMML if:
- They have persistent peripheral blood monocytosis (1×109/L) with monocytes accounting for 10% of the white blood cell count
- They do not meet WHO criteria for BCR-ABL1-positive CML, primary myelofibrosis, polycythemia vera, or essential thrombocythemia
- There is no evidence of PCM1-JAK2 or PDGFRA, PDGFRB, or FGFR1 rearrangement
- They have fewer than 20% blasts in the blood and bone marrow
- They have dysplasia in one or more myeloid lineages
- If myelodysplasia is absent or minimal, an acquired clonal cytogenetic or molecular genetic abnormality must be present.
Alternatively, if patients have monocytosis that has persisted for at least 3 months, and all other causes of monocytosis have been excluded, “you can say that your patient has CMML,” Dr. Duraković said.
Other causes of monocytosis include infections, malignancies, medications, inflammatory conditions, and other conditions such as pregnancy.
However, Dr. Duraković pointed out that the cause of monocytosis cannot always be determined, and, in some cases, CMML patients may not meet the WHO criteria.
“[T]here are cases where there just aren’t enough monocytes to fulfill the WHO criteria,” Dr. Duraković said. “You can have a patient with peripheral blood cytopenia and monocytosis who does not have 1,000 monocytes. Patients can have progressive dysplasia, can have splenomegaly, be really sick, but fail to meet WHO criteria.”
Distinguishing CMML from other conditions
“Differentiating CMML from myelodysplastic syndromes can be tough,” Dr. Duraković said. “There are dysplastic features that are present in CMML . . . but, in CMML, they are more subtle, and they are more difficult to appreciate than in myelodysplastic syndromes.”
The ratio of myeloid to erythroid cells is elevated in CMML, and patients may have atypical monocytes (paramyeloid cells) that are unique to CMML.
Dr. Duraković noted that megakaryocyte dysplasia in CMML can be characterized by “myeloproliferative megakaryocytes,” which are large cells that cluster and have hyperlobulated nuclei, or “MDS megakaryocytes,” which are small, solitary cells with hypolobulated nuclei.
She went on to explain that “MPN phenotype” CMML is characterized by leukocytosis, monocytosis, hepatomegaly, splenomegaly, and clinical features of myeloproliferation (fatigue, night sweats, bone pain, weight loss, etc.).
Thirty percent of cases are associated with splenomegaly, and 30% of patients can have an increase in bone marrow reticulin fibrosis.
Dr. Duraković also noted that a prior MPN diagnosis excludes CMML. The presence of common MPN mutations, such as JAK2, CALR, or MPL, suggests a patient has an MPN with monocytosis rather than CMML.
Patients who have unclassified MPNs or MDS, rather than CMML, either do not have 1,000 monocytes or the monocytes do not represent more than 10% of the differential, Dr. Duraković said.
She also noted that it can be difficult to differentiate CMML from atypical CML.
“Atypical CML is characterized by profound dysgranulopoiesis, absence of the BCR-ABL1 fusion gene, and neutrophilia,” Dr. Duraković explained. “Those patients [commonly] have monocytosis, but, here, that 10% rule is valuable because their monocytes comprise less than 10% of the entire white blood cell count.”
Karyotyping, genotyping, and immunophenotyping
“There is no disease-defining karyotype abnormality [in CMML],” Dr. Duraković noted.
She said 30% of patients have abnormal karyotype, and the most common abnormality is trisomy 8. Unlike in patients with MDS, del(5q) and monosomal karyotypes are infrequent in patients with CMML.
Similarly, there are no “disease-defining” mutations or genetic changes in CMML, although CMML is genetically distinct from MDS, Dr. Duraković said.
For instance, SRSF2 encodes a component of the spliceosome that is mutated in almost half of CMML patients and less than 10% of MDS patients. Likewise, ASLX1 and TET2 are “much more frequently involved” in CMML than in MDS, Dr. Duraković said.
In a 2012 study of 275 CMML patients, researchers found that 93% of patients had at least one somatic mutation in nine recurrently mutated genes—SRFS2, ASXL1, CBL, EZH2, JAK2V617F, KRAS, NRAS, RUNX1, and TET2 (Blood 2012 120:3080-3088).
However, Dr. Duraković noted that these mutations are found in other disorders as well, so this information may not be helpful in differentiating CMML from other disorders.
A 2015 study revealed a technique that does appear useful for identifying CMML—monocyte subset distribution analysis (Blood 2015 125(23): 3618–3626).
For this analysis, monocytes are divided into the following categories:
- Classical/MO1 (CD14bright/CD16−)
- Intermediate/MO2 (CD14bright/CD16+)
- Non-classical/MO3 (CD14dim/CD16+).
The researchers found that CMML patients had an increase in the fraction of classical monocytes (with a cutoff value of 94.0%), as compared to healthy control subjects, patients with another hematologic disorder, and patients with reactive monocytosis.
A 2018 study confirmed that monocyte subset distribution analysis could differentiate CMML from other hematologic disorders, with the exception of atypical CML (Am J Clin Pathol 2018 150(4):293-302).
This study also suggested that a decreased percentage of non-classical monocytes was more sensitive than an increased percentage of classical monocytes.
Despite the differences between these studies, “monocyte subset distribution analysis is showing promise as a method of identifying hard-to-identify CMML patients with ease and affordability,” Dr. Duraković said.
She added that the technique can be implemented in clinical practice using the HematoflowTM solution (Cytometry B Clin Cytom 2018 94(5):658-661).
Dr. Duraković did not report any conflicts of interest.
DUBROVNIK, CROATIA—Diagnosing chronic myelomonocytic leukemia (CMML) remains a challenge in 2018, according to a presentation at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
Even with updated World Health Organization (WHO) criteria, karyotyping, and genetic analyses, it can be difficult to distinguish CMML from other conditions, according to Nadira Duraković, MD, PhD, of the University Hospital Zagreb in Croatia.
However, Dr. Duraković said there are characteristics that differentiate CMML from myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), and atypical chronic myeloid leukemia (CML).
Furthermore, studies have suggested that monocyte subset distribution analysis can be useful for diagnosing CMML.
Dr. Duraković began her presentation with an overview of the 2016 WHO classification of CMML (Blood 2016 127:2391-2405).
According to the WHO, patients have CMML if:
- They have persistent peripheral blood monocytosis (1×109/L) with monocytes accounting for 10% of the white blood cell count
- They do not meet WHO criteria for BCR-ABL1-positive CML, primary myelofibrosis, polycythemia vera, or essential thrombocythemia
- There is no evidence of PCM1-JAK2 or PDGFRA, PDGFRB, or FGFR1 rearrangement
- They have fewer than 20% blasts in the blood and bone marrow
- They have dysplasia in one or more myeloid lineages
- If myelodysplasia is absent or minimal, an acquired clonal cytogenetic or molecular genetic abnormality must be present.
Alternatively, if patients have monocytosis that has persisted for at least 3 months, and all other causes of monocytosis have been excluded, “you can say that your patient has CMML,” Dr. Duraković said.
Other causes of monocytosis include infections, malignancies, medications, inflammatory conditions, and other conditions such as pregnancy.
However, Dr. Duraković pointed out that the cause of monocytosis cannot always be determined, and, in some cases, CMML patients may not meet the WHO criteria.
“[T]here are cases where there just aren’t enough monocytes to fulfill the WHO criteria,” Dr. Duraković said. “You can have a patient with peripheral blood cytopenia and monocytosis who does not have 1,000 monocytes. Patients can have progressive dysplasia, can have splenomegaly, be really sick, but fail to meet WHO criteria.”
Distinguishing CMML from other conditions
“Differentiating CMML from myelodysplastic syndromes can be tough,” Dr. Duraković said. “There are dysplastic features that are present in CMML . . . but, in CMML, they are more subtle, and they are more difficult to appreciate than in myelodysplastic syndromes.”
The ratio of myeloid to erythroid cells is elevated in CMML, and patients may have atypical monocytes (paramyeloid cells) that are unique to CMML.
Dr. Duraković noted that megakaryocyte dysplasia in CMML can be characterized by “myeloproliferative megakaryocytes,” which are large cells that cluster and have hyperlobulated nuclei, or “MDS megakaryocytes,” which are small, solitary cells with hypolobulated nuclei.
She went on to explain that “MPN phenotype” CMML is characterized by leukocytosis, monocytosis, hepatomegaly, splenomegaly, and clinical features of myeloproliferation (fatigue, night sweats, bone pain, weight loss, etc.).
Thirty percent of cases are associated with splenomegaly, and 30% of patients can have an increase in bone marrow reticulin fibrosis.
Dr. Duraković also noted that a prior MPN diagnosis excludes CMML. The presence of common MPN mutations, such as JAK2, CALR, or MPL, suggests a patient has an MPN with monocytosis rather than CMML.
Patients who have unclassified MPNs or MDS, rather than CMML, either do not have 1,000 monocytes or the monocytes do not represent more than 10% of the differential, Dr. Duraković said.
She also noted that it can be difficult to differentiate CMML from atypical CML.
“Atypical CML is characterized by profound dysgranulopoiesis, absence of the BCR-ABL1 fusion gene, and neutrophilia,” Dr. Duraković explained. “Those patients [commonly] have monocytosis, but, here, that 10% rule is valuable because their monocytes comprise less than 10% of the entire white blood cell count.”
Karyotyping, genotyping, and immunophenotyping
“There is no disease-defining karyotype abnormality [in CMML],” Dr. Duraković noted.
She said 30% of patients have abnormal karyotype, and the most common abnormality is trisomy 8. Unlike in patients with MDS, del(5q) and monosomal karyotypes are infrequent in patients with CMML.
Similarly, there are no “disease-defining” mutations or genetic changes in CMML, although CMML is genetically distinct from MDS, Dr. Duraković said.
For instance, SRSF2 encodes a component of the spliceosome that is mutated in almost half of CMML patients and less than 10% of MDS patients. Likewise, ASLX1 and TET2 are “much more frequently involved” in CMML than in MDS, Dr. Duraković said.
In a 2012 study of 275 CMML patients, researchers found that 93% of patients had at least one somatic mutation in nine recurrently mutated genes—SRFS2, ASXL1, CBL, EZH2, JAK2V617F, KRAS, NRAS, RUNX1, and TET2 (Blood 2012 120:3080-3088).
However, Dr. Duraković noted that these mutations are found in other disorders as well, so this information may not be helpful in differentiating CMML from other disorders.
A 2015 study revealed a technique that does appear useful for identifying CMML—monocyte subset distribution analysis (Blood 2015 125(23): 3618–3626).
For this analysis, monocytes are divided into the following categories:
- Classical/MO1 (CD14bright/CD16−)
- Intermediate/MO2 (CD14bright/CD16+)
- Non-classical/MO3 (CD14dim/CD16+).
The researchers found that CMML patients had an increase in the fraction of classical monocytes (with a cutoff value of 94.0%), as compared to healthy control subjects, patients with another hematologic disorder, and patients with reactive monocytosis.
A 2018 study confirmed that monocyte subset distribution analysis could differentiate CMML from other hematologic disorders, with the exception of atypical CML (Am J Clin Pathol 2018 150(4):293-302).
This study also suggested that a decreased percentage of non-classical monocytes was more sensitive than an increased percentage of classical monocytes.
Despite the differences between these studies, “monocyte subset distribution analysis is showing promise as a method of identifying hard-to-identify CMML patients with ease and affordability,” Dr. Duraković said.
She added that the technique can be implemented in clinical practice using the HematoflowTM solution (Cytometry B Clin Cytom 2018 94(5):658-661).
Dr. Duraković did not report any conflicts of interest.
DUBROVNIK, CROATIA—Diagnosing chronic myelomonocytic leukemia (CMML) remains a challenge in 2018, according to a presentation at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
Even with updated World Health Organization (WHO) criteria, karyotyping, and genetic analyses, it can be difficult to distinguish CMML from other conditions, according to Nadira Duraković, MD, PhD, of the University Hospital Zagreb in Croatia.
However, Dr. Duraković said there are characteristics that differentiate CMML from myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), and atypical chronic myeloid leukemia (CML).
Furthermore, studies have suggested that monocyte subset distribution analysis can be useful for diagnosing CMML.
Dr. Duraković began her presentation with an overview of the 2016 WHO classification of CMML (Blood 2016 127:2391-2405).
According to the WHO, patients have CMML if:
- They have persistent peripheral blood monocytosis (1×109/L) with monocytes accounting for 10% of the white blood cell count
- They do not meet WHO criteria for BCR-ABL1-positive CML, primary myelofibrosis, polycythemia vera, or essential thrombocythemia
- There is no evidence of PCM1-JAK2 or PDGFRA, PDGFRB, or FGFR1 rearrangement
- They have fewer than 20% blasts in the blood and bone marrow
- They have dysplasia in one or more myeloid lineages
- If myelodysplasia is absent or minimal, an acquired clonal cytogenetic or molecular genetic abnormality must be present.
Alternatively, if patients have monocytosis that has persisted for at least 3 months, and all other causes of monocytosis have been excluded, “you can say that your patient has CMML,” Dr. Duraković said.
Other causes of monocytosis include infections, malignancies, medications, inflammatory conditions, and other conditions such as pregnancy.
However, Dr. Duraković pointed out that the cause of monocytosis cannot always be determined, and, in some cases, CMML patients may not meet the WHO criteria.
“[T]here are cases where there just aren’t enough monocytes to fulfill the WHO criteria,” Dr. Duraković said. “You can have a patient with peripheral blood cytopenia and monocytosis who does not have 1,000 monocytes. Patients can have progressive dysplasia, can have splenomegaly, be really sick, but fail to meet WHO criteria.”
Distinguishing CMML from other conditions
“Differentiating CMML from myelodysplastic syndromes can be tough,” Dr. Duraković said. “There are dysplastic features that are present in CMML . . . but, in CMML, they are more subtle, and they are more difficult to appreciate than in myelodysplastic syndromes.”
The ratio of myeloid to erythroid cells is elevated in CMML, and patients may have atypical monocytes (paramyeloid cells) that are unique to CMML.
Dr. Duraković noted that megakaryocyte dysplasia in CMML can be characterized by “myeloproliferative megakaryocytes,” which are large cells that cluster and have hyperlobulated nuclei, or “MDS megakaryocytes,” which are small, solitary cells with hypolobulated nuclei.
She went on to explain that “MPN phenotype” CMML is characterized by leukocytosis, monocytosis, hepatomegaly, splenomegaly, and clinical features of myeloproliferation (fatigue, night sweats, bone pain, weight loss, etc.).
Thirty percent of cases are associated with splenomegaly, and 30% of patients can have an increase in bone marrow reticulin fibrosis.
Dr. Duraković also noted that a prior MPN diagnosis excludes CMML. The presence of common MPN mutations, such as JAK2, CALR, or MPL, suggests a patient has an MPN with monocytosis rather than CMML.
Patients who have unclassified MPNs or MDS, rather than CMML, either do not have 1,000 monocytes or the monocytes do not represent more than 10% of the differential, Dr. Duraković said.
She also noted that it can be difficult to differentiate CMML from atypical CML.
“Atypical CML is characterized by profound dysgranulopoiesis, absence of the BCR-ABL1 fusion gene, and neutrophilia,” Dr. Duraković explained. “Those patients [commonly] have monocytosis, but, here, that 10% rule is valuable because their monocytes comprise less than 10% of the entire white blood cell count.”
Karyotyping, genotyping, and immunophenotyping
“There is no disease-defining karyotype abnormality [in CMML],” Dr. Duraković noted.
She said 30% of patients have abnormal karyotype, and the most common abnormality is trisomy 8. Unlike in patients with MDS, del(5q) and monosomal karyotypes are infrequent in patients with CMML.
Similarly, there are no “disease-defining” mutations or genetic changes in CMML, although CMML is genetically distinct from MDS, Dr. Duraković said.
For instance, SRSF2 encodes a component of the spliceosome that is mutated in almost half of CMML patients and less than 10% of MDS patients. Likewise, ASLX1 and TET2 are “much more frequently involved” in CMML than in MDS, Dr. Duraković said.
In a 2012 study of 275 CMML patients, researchers found that 93% of patients had at least one somatic mutation in nine recurrently mutated genes—SRFS2, ASXL1, CBL, EZH2, JAK2V617F, KRAS, NRAS, RUNX1, and TET2 (Blood 2012 120:3080-3088).
However, Dr. Duraković noted that these mutations are found in other disorders as well, so this information may not be helpful in differentiating CMML from other disorders.
A 2015 study revealed a technique that does appear useful for identifying CMML—monocyte subset distribution analysis (Blood 2015 125(23): 3618–3626).
For this analysis, monocytes are divided into the following categories:
- Classical/MO1 (CD14bright/CD16−)
- Intermediate/MO2 (CD14bright/CD16+)
- Non-classical/MO3 (CD14dim/CD16+).
The researchers found that CMML patients had an increase in the fraction of classical monocytes (with a cutoff value of 94.0%), as compared to healthy control subjects, patients with another hematologic disorder, and patients with reactive monocytosis.
A 2018 study confirmed that monocyte subset distribution analysis could differentiate CMML from other hematologic disorders, with the exception of atypical CML (Am J Clin Pathol 2018 150(4):293-302).
This study also suggested that a decreased percentage of non-classical monocytes was more sensitive than an increased percentage of classical monocytes.
Despite the differences between these studies, “monocyte subset distribution analysis is showing promise as a method of identifying hard-to-identify CMML patients with ease and affordability,” Dr. Duraković said.
She added that the technique can be implemented in clinical practice using the HematoflowTM solution (Cytometry B Clin Cytom 2018 94(5):658-661).
Dr. Duraković did not report any conflicts of interest.
Genomic findings may predict outcomes in MPN patients
New research suggests genomic characteristics of patients with myeloproliferative neoplasms (MPNs) can predict clinical outcomes.
Investigators defined eight genomic subgroups of MPNs, each with distinct clinical features, including event-free survival, risk of leukemic transformation, and blood counts.
Jacob Grinfeld, MD, of the University of Cambridge in the U.K., and his colleagues described these findings in The New England Journal of Medicine.
This study included 2,035 patients with MPNs, including essential thrombocythemia, polycythemia vera, myelofibrosis, and other diagnoses.
The investigators performed targeted sequencing for the full coding sequence of 69 genes and genome-wide copy number information in 1,887 patients. Whole-exome sequencing was performed in another 148 patients.
By sequencing coding exons from 69 myeloid cancer genes, the investigators were able to survey the diversity of mutations across MPN patients and identify mutation-associated clinical outcomes.
The results showed that slightly less than half (45%) of the patients had a solitary abnormality in CALR, MPL, or JAK2, while the remaining patients had additional driver mutations.
In some instances, additional mutations were numerous, particularly in older patients with advanced disease. In at least five cases, 33 genes had driver mutations.
Further analysis revealed eight genomic subgroups that could predict clinical outcomes based on shared chromosomal abnormalities and mutations.
For example, one subgroup included patients with TP53 mutations. These individuals had a “dismal prognosis” and were 15.5 times more likely to transform to acute myeloid leukemia, compared with the JAK2-heterozygous subgroup (P<0.001).
Because prognosis is “a key determinant” of MPN treatment, genomic subgrouping may one day guide clinical decision-making, the investigators concluded.
To further this cause, they have made available an online calculator of individualized patient outcomes, which can be accessed at https://cancer.sanger.ac.uk/mpn-multistage/.
This study was funded by the Wellcome Trust, the National Institute for Health Research Cambridge Biomedical Research Centre, Cancer Research UK, and others. Some study authors reported fees from Celgene, Novartis, Gilead, Shire, and others outside of the study.
New research suggests genomic characteristics of patients with myeloproliferative neoplasms (MPNs) can predict clinical outcomes.
Investigators defined eight genomic subgroups of MPNs, each with distinct clinical features, including event-free survival, risk of leukemic transformation, and blood counts.
Jacob Grinfeld, MD, of the University of Cambridge in the U.K., and his colleagues described these findings in The New England Journal of Medicine.
This study included 2,035 patients with MPNs, including essential thrombocythemia, polycythemia vera, myelofibrosis, and other diagnoses.
The investigators performed targeted sequencing for the full coding sequence of 69 genes and genome-wide copy number information in 1,887 patients. Whole-exome sequencing was performed in another 148 patients.
By sequencing coding exons from 69 myeloid cancer genes, the investigators were able to survey the diversity of mutations across MPN patients and identify mutation-associated clinical outcomes.
The results showed that slightly less than half (45%) of the patients had a solitary abnormality in CALR, MPL, or JAK2, while the remaining patients had additional driver mutations.
In some instances, additional mutations were numerous, particularly in older patients with advanced disease. In at least five cases, 33 genes had driver mutations.
Further analysis revealed eight genomic subgroups that could predict clinical outcomes based on shared chromosomal abnormalities and mutations.
For example, one subgroup included patients with TP53 mutations. These individuals had a “dismal prognosis” and were 15.5 times more likely to transform to acute myeloid leukemia, compared with the JAK2-heterozygous subgroup (P<0.001).
Because prognosis is “a key determinant” of MPN treatment, genomic subgrouping may one day guide clinical decision-making, the investigators concluded.
To further this cause, they have made available an online calculator of individualized patient outcomes, which can be accessed at https://cancer.sanger.ac.uk/mpn-multistage/.
This study was funded by the Wellcome Trust, the National Institute for Health Research Cambridge Biomedical Research Centre, Cancer Research UK, and others. Some study authors reported fees from Celgene, Novartis, Gilead, Shire, and others outside of the study.
New research suggests genomic characteristics of patients with myeloproliferative neoplasms (MPNs) can predict clinical outcomes.
Investigators defined eight genomic subgroups of MPNs, each with distinct clinical features, including event-free survival, risk of leukemic transformation, and blood counts.
Jacob Grinfeld, MD, of the University of Cambridge in the U.K., and his colleagues described these findings in The New England Journal of Medicine.
This study included 2,035 patients with MPNs, including essential thrombocythemia, polycythemia vera, myelofibrosis, and other diagnoses.
The investigators performed targeted sequencing for the full coding sequence of 69 genes and genome-wide copy number information in 1,887 patients. Whole-exome sequencing was performed in another 148 patients.
By sequencing coding exons from 69 myeloid cancer genes, the investigators were able to survey the diversity of mutations across MPN patients and identify mutation-associated clinical outcomes.
The results showed that slightly less than half (45%) of the patients had a solitary abnormality in CALR, MPL, or JAK2, while the remaining patients had additional driver mutations.
In some instances, additional mutations were numerous, particularly in older patients with advanced disease. In at least five cases, 33 genes had driver mutations.
Further analysis revealed eight genomic subgroups that could predict clinical outcomes based on shared chromosomal abnormalities and mutations.
For example, one subgroup included patients with TP53 mutations. These individuals had a “dismal prognosis” and were 15.5 times more likely to transform to acute myeloid leukemia, compared with the JAK2-heterozygous subgroup (P<0.001).
Because prognosis is “a key determinant” of MPN treatment, genomic subgrouping may one day guide clinical decision-making, the investigators concluded.
To further this cause, they have made available an online calculator of individualized patient outcomes, which can be accessed at https://cancer.sanger.ac.uk/mpn-multistage/.
This study was funded by the Wellcome Trust, the National Institute for Health Research Cambridge Biomedical Research Centre, Cancer Research UK, and others. Some study authors reported fees from Celgene, Novartis, Gilead, Shire, and others outside of the study.
GPS appears to predict survival in myelofibrosis
DUBROVNIK, CROATIA—The Glasgow Prognostic Score (GPS) may predict survival in patients with myelofibrosis (MF), according to research presented at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
In a retrospective study, MF patients who were considered intermediate-risk according to the GPS had roughly twice the risk of death as good-risk patients.
High-risk patients had a nearly 24-fold greater risk of death than good-risk patients.
Marko Lucijanić, MD, PhD, of the University Hospital Dubrava in Zagreb, Croatia, presented these findings at the meeting. Results were also published in a recent issue of Blood Cells, Molecules, and Diseases.1
Dr. Lucijanić and his colleagues analyzed 88 patients—67 with primary MF and 21 with secondary MF—who were treated at the University Hospital Dubrava from 2004 to 2018.
Patients were divided into GPS risk categories:
- Good-risk patients had C reactive protein (CRP) ≤10 mg/L and albumin ≥35 g/L
- Intermediate-risk patients had either CRP >10 mg/L or albumin <35 g/L
- Poor-risk patients had both CRP >10 mg/L and albumin <35 g/L.
Results
The researchers found that CRP and albumin were independent predictors of overall survival (OS) in MF.
“What we saw is that both CRP and albumin were univariately associated with inferior overall survival when the patients were divided at the proposed cutoff levels,” Dr. Lucijanić said.
“And both CRP and albumin remained statistically significant when analyzed together in a Cox regression model additionally adjusted for DIPPS [Dynamic International Prognostic Scoring System].”
In the univariate analysis, the hazard ratio (HR) for death was 3.42 (P<0.001) for patients with CRP >10 mg/L and 4.68 (P<0.001) for patients with albumin <35 g/L.
In a multivariate analysis, the HR for death was 2.49 (P=0.013) for patients with CRP >10 mg/L and 2.74 (P=0.031) for patients with albumin <35 g/L.
“So no surprise that, when [CRP and albumin were] combined into the Glasgow Prognostic Score, the GPS could identify three subgroups of patients with distinct prognosis,” Dr. Lucijanić said.
When the researchers assessed OS according to GPS, they found the HR for death was:
- 2.77 for intermediate-risk patients compared to good-risk patients (P<0.001).
- 15.78 for poor-risk patients compared to good-risk patients (P<0.001).
- 5.82 for poor-risk patients compared to intermediate-risk patients (P<0.001).
In a Cox-regression model for OS that was adjusted for DIPPS, age, and gender, the HR was:
- 2.08 for intermediate-risk patients compared to good-risk patients (P=0.040)
- 23.52 for poor-risk patients compared to good-risk patients (P<0.001).
Dr. Lucijanić noted that patients who were intermediate- or poor-risk according to the GPS were more likely to have symptoms of aggressive disease that are associated with non-response to JAK inhibitors.
The intermediate- or poor-risk patients were more likely to have constitutional symptoms, massive splenomegaly, blast phase disease, circulatory blasts, higher absolute monocyte counts, lower hemoglobin, lower platelets, higher lactate dehydrogenase, higher red cell distribution width, transfusion dependency, and higher ferritin. The patients also had higher DIPSS scores.
“So, to summarize, higher GPS recognizes patients with more aggressive disease features at a higher risk of death,” Dr. Lucijanić said. “And not only does it discriminate survival of myelofibrosis patients, it seems to do so in a DIPPS-independent manner. However, you should be aware that this is a small, retrospective study from a single center with a very long follow-up period during which patients were exposed to different types of therapies.”
Dr. Lucijanić did not declare any conflicts of interest.
1. Lucijanić M et al. Blood Cells Mol Dis. 2018 Sep;72:14-16. doi: 10.1016/j.bcmd.2018.06.001.
DUBROVNIK, CROATIA—The Glasgow Prognostic Score (GPS) may predict survival in patients with myelofibrosis (MF), according to research presented at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
In a retrospective study, MF patients who were considered intermediate-risk according to the GPS had roughly twice the risk of death as good-risk patients.
High-risk patients had a nearly 24-fold greater risk of death than good-risk patients.
Marko Lucijanić, MD, PhD, of the University Hospital Dubrava in Zagreb, Croatia, presented these findings at the meeting. Results were also published in a recent issue of Blood Cells, Molecules, and Diseases.1
Dr. Lucijanić and his colleagues analyzed 88 patients—67 with primary MF and 21 with secondary MF—who were treated at the University Hospital Dubrava from 2004 to 2018.
Patients were divided into GPS risk categories:
- Good-risk patients had C reactive protein (CRP) ≤10 mg/L and albumin ≥35 g/L
- Intermediate-risk patients had either CRP >10 mg/L or albumin <35 g/L
- Poor-risk patients had both CRP >10 mg/L and albumin <35 g/L.
Results
The researchers found that CRP and albumin were independent predictors of overall survival (OS) in MF.
“What we saw is that both CRP and albumin were univariately associated with inferior overall survival when the patients were divided at the proposed cutoff levels,” Dr. Lucijanić said.
“And both CRP and albumin remained statistically significant when analyzed together in a Cox regression model additionally adjusted for DIPPS [Dynamic International Prognostic Scoring System].”
In the univariate analysis, the hazard ratio (HR) for death was 3.42 (P<0.001) for patients with CRP >10 mg/L and 4.68 (P<0.001) for patients with albumin <35 g/L.
In a multivariate analysis, the HR for death was 2.49 (P=0.013) for patients with CRP >10 mg/L and 2.74 (P=0.031) for patients with albumin <35 g/L.
“So no surprise that, when [CRP and albumin were] combined into the Glasgow Prognostic Score, the GPS could identify three subgroups of patients with distinct prognosis,” Dr. Lucijanić said.
When the researchers assessed OS according to GPS, they found the HR for death was:
- 2.77 for intermediate-risk patients compared to good-risk patients (P<0.001).
- 15.78 for poor-risk patients compared to good-risk patients (P<0.001).
- 5.82 for poor-risk patients compared to intermediate-risk patients (P<0.001).
In a Cox-regression model for OS that was adjusted for DIPPS, age, and gender, the HR was:
- 2.08 for intermediate-risk patients compared to good-risk patients (P=0.040)
- 23.52 for poor-risk patients compared to good-risk patients (P<0.001).
Dr. Lucijanić noted that patients who were intermediate- or poor-risk according to the GPS were more likely to have symptoms of aggressive disease that are associated with non-response to JAK inhibitors.
The intermediate- or poor-risk patients were more likely to have constitutional symptoms, massive splenomegaly, blast phase disease, circulatory blasts, higher absolute monocyte counts, lower hemoglobin, lower platelets, higher lactate dehydrogenase, higher red cell distribution width, transfusion dependency, and higher ferritin. The patients also had higher DIPSS scores.
“So, to summarize, higher GPS recognizes patients with more aggressive disease features at a higher risk of death,” Dr. Lucijanić said. “And not only does it discriminate survival of myelofibrosis patients, it seems to do so in a DIPPS-independent manner. However, you should be aware that this is a small, retrospective study from a single center with a very long follow-up period during which patients were exposed to different types of therapies.”
Dr. Lucijanić did not declare any conflicts of interest.
1. Lucijanić M et al. Blood Cells Mol Dis. 2018 Sep;72:14-16. doi: 10.1016/j.bcmd.2018.06.001.
DUBROVNIK, CROATIA—The Glasgow Prognostic Score (GPS) may predict survival in patients with myelofibrosis (MF), according to research presented at Leukemia and Lymphoma: Europe and the USA, Linking Knowledge and Practice.
In a retrospective study, MF patients who were considered intermediate-risk according to the GPS had roughly twice the risk of death as good-risk patients.
High-risk patients had a nearly 24-fold greater risk of death than good-risk patients.
Marko Lucijanić, MD, PhD, of the University Hospital Dubrava in Zagreb, Croatia, presented these findings at the meeting. Results were also published in a recent issue of Blood Cells, Molecules, and Diseases.1
Dr. Lucijanić and his colleagues analyzed 88 patients—67 with primary MF and 21 with secondary MF—who were treated at the University Hospital Dubrava from 2004 to 2018.
Patients were divided into GPS risk categories:
- Good-risk patients had C reactive protein (CRP) ≤10 mg/L and albumin ≥35 g/L
- Intermediate-risk patients had either CRP >10 mg/L or albumin <35 g/L
- Poor-risk patients had both CRP >10 mg/L and albumin <35 g/L.
Results
The researchers found that CRP and albumin were independent predictors of overall survival (OS) in MF.
“What we saw is that both CRP and albumin were univariately associated with inferior overall survival when the patients were divided at the proposed cutoff levels,” Dr. Lucijanić said.
“And both CRP and albumin remained statistically significant when analyzed together in a Cox regression model additionally adjusted for DIPPS [Dynamic International Prognostic Scoring System].”
In the univariate analysis, the hazard ratio (HR) for death was 3.42 (P<0.001) for patients with CRP >10 mg/L and 4.68 (P<0.001) for patients with albumin <35 g/L.
In a multivariate analysis, the HR for death was 2.49 (P=0.013) for patients with CRP >10 mg/L and 2.74 (P=0.031) for patients with albumin <35 g/L.
“So no surprise that, when [CRP and albumin were] combined into the Glasgow Prognostic Score, the GPS could identify three subgroups of patients with distinct prognosis,” Dr. Lucijanić said.
When the researchers assessed OS according to GPS, they found the HR for death was:
- 2.77 for intermediate-risk patients compared to good-risk patients (P<0.001).
- 15.78 for poor-risk patients compared to good-risk patients (P<0.001).
- 5.82 for poor-risk patients compared to intermediate-risk patients (P<0.001).
In a Cox-regression model for OS that was adjusted for DIPPS, age, and gender, the HR was:
- 2.08 for intermediate-risk patients compared to good-risk patients (P=0.040)
- 23.52 for poor-risk patients compared to good-risk patients (P<0.001).
Dr. Lucijanić noted that patients who were intermediate- or poor-risk according to the GPS were more likely to have symptoms of aggressive disease that are associated with non-response to JAK inhibitors.
The intermediate- or poor-risk patients were more likely to have constitutional symptoms, massive splenomegaly, blast phase disease, circulatory blasts, higher absolute monocyte counts, lower hemoglobin, lower platelets, higher lactate dehydrogenase, higher red cell distribution width, transfusion dependency, and higher ferritin. The patients also had higher DIPSS scores.
“So, to summarize, higher GPS recognizes patients with more aggressive disease features at a higher risk of death,” Dr. Lucijanić said. “And not only does it discriminate survival of myelofibrosis patients, it seems to do so in a DIPPS-independent manner. However, you should be aware that this is a small, retrospective study from a single center with a very long follow-up period during which patients were exposed to different types of therapies.”
Dr. Lucijanić did not declare any conflicts of interest.
1. Lucijanić M et al. Blood Cells Mol Dis. 2018 Sep;72:14-16. doi: 10.1016/j.bcmd.2018.06.001.
AMP publishes report on DNA variants in CMNs
A new report addresses the clinical relevance of DNA variants in chronic myeloid neoplasms (CMNs).
The report is intended to aid clinical laboratory professionals with the management of most CMNs and the development of high-throughput pan-myeloid sequencing testing panels.
The authors list 34 genes they consider “critical” for sequencing tests to help standardize clinical practice and improve care of patients with CMNs.
The Association for Molecular Pathology (AMP) established a CMN Working Group to generate the report, which was published in The Journal of Molecular Diagnostics.
“The molecular pathology community has witnessed a recent explosion of scientific literature highlighting the clinical significance of small DNA variants in CMNs,” said Rebecca F. McClure, MD, a member of the AMP CMN Working Group and an associate professor at Health Sciences North/Horizon Santé-Nord in Sudbury, Ontario, Canada.
“AMP’s working group recognized a clear, unmet need for evidence-based recommendations to assist in the development of the high-quality pan-myeloid gene panels that provide relevant diagnostic and prognostic information and enable monitoring of clonal architecture.”
The increasing availability of targeted, high-throughput, next-generation sequencing panels has enabled scientists to explore the genetic heterogeneity and clinical relevance of the small DNA variants in CMNs.
However, the biological complexity and multiple forms of CMNs have led to variability in the genes included on the available panels that are used to make an accurate diagnosis, provide reliable prognostic information, and select an appropriate therapy based on DNA variant profiles present at various time points.
AMP established its CMN Working Group to review the published literature on CMNs, summarize key findings that support clinical utility, and define a set of critical gene inclusions for all high-throughput pan-myeloid sequencing testing panels.
The group proposed the following 34 genes as a minimum recommended testing list: ASXL1, BCOR, BCORL1, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NF1, NPM1, NRAS, PHF6, PPM1D, PTPN11, RAD21, RUNX1, SETBP1, SF3B1, SMC3, SRSF2, STAG2, TET2, TP53, U2AF1, and ZRSR2.
“While the goal of the study was to distill the literature for molecular pathologists, in doing so, we also revealed recurrent mutational patterns of clonal evolution that will [help] hematologist/oncologists, researchers, and pathologists understand how to interpret the results of these panels as they reveal critical biology of the neoplasms,” said Annette S. Kim, MD, PhD, CMN Working Group Chair and an associate professor at Harvard Medical School and Brigham and Women’s Hospital in Boston, Massachusetts.
A new report addresses the clinical relevance of DNA variants in chronic myeloid neoplasms (CMNs).
The report is intended to aid clinical laboratory professionals with the management of most CMNs and the development of high-throughput pan-myeloid sequencing testing panels.
The authors list 34 genes they consider “critical” for sequencing tests to help standardize clinical practice and improve care of patients with CMNs.
The Association for Molecular Pathology (AMP) established a CMN Working Group to generate the report, which was published in The Journal of Molecular Diagnostics.
“The molecular pathology community has witnessed a recent explosion of scientific literature highlighting the clinical significance of small DNA variants in CMNs,” said Rebecca F. McClure, MD, a member of the AMP CMN Working Group and an associate professor at Health Sciences North/Horizon Santé-Nord in Sudbury, Ontario, Canada.
“AMP’s working group recognized a clear, unmet need for evidence-based recommendations to assist in the development of the high-quality pan-myeloid gene panels that provide relevant diagnostic and prognostic information and enable monitoring of clonal architecture.”
The increasing availability of targeted, high-throughput, next-generation sequencing panels has enabled scientists to explore the genetic heterogeneity and clinical relevance of the small DNA variants in CMNs.
However, the biological complexity and multiple forms of CMNs have led to variability in the genes included on the available panels that are used to make an accurate diagnosis, provide reliable prognostic information, and select an appropriate therapy based on DNA variant profiles present at various time points.
AMP established its CMN Working Group to review the published literature on CMNs, summarize key findings that support clinical utility, and define a set of critical gene inclusions for all high-throughput pan-myeloid sequencing testing panels.
The group proposed the following 34 genes as a minimum recommended testing list: ASXL1, BCOR, BCORL1, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NF1, NPM1, NRAS, PHF6, PPM1D, PTPN11, RAD21, RUNX1, SETBP1, SF3B1, SMC3, SRSF2, STAG2, TET2, TP53, U2AF1, and ZRSR2.
“While the goal of the study was to distill the literature for molecular pathologists, in doing so, we also revealed recurrent mutational patterns of clonal evolution that will [help] hematologist/oncologists, researchers, and pathologists understand how to interpret the results of these panels as they reveal critical biology of the neoplasms,” said Annette S. Kim, MD, PhD, CMN Working Group Chair and an associate professor at Harvard Medical School and Brigham and Women’s Hospital in Boston, Massachusetts.
A new report addresses the clinical relevance of DNA variants in chronic myeloid neoplasms (CMNs).
The report is intended to aid clinical laboratory professionals with the management of most CMNs and the development of high-throughput pan-myeloid sequencing testing panels.
The authors list 34 genes they consider “critical” for sequencing tests to help standardize clinical practice and improve care of patients with CMNs.
The Association for Molecular Pathology (AMP) established a CMN Working Group to generate the report, which was published in The Journal of Molecular Diagnostics.
“The molecular pathology community has witnessed a recent explosion of scientific literature highlighting the clinical significance of small DNA variants in CMNs,” said Rebecca F. McClure, MD, a member of the AMP CMN Working Group and an associate professor at Health Sciences North/Horizon Santé-Nord in Sudbury, Ontario, Canada.
“AMP’s working group recognized a clear, unmet need for evidence-based recommendations to assist in the development of the high-quality pan-myeloid gene panels that provide relevant diagnostic and prognostic information and enable monitoring of clonal architecture.”
The increasing availability of targeted, high-throughput, next-generation sequencing panels has enabled scientists to explore the genetic heterogeneity and clinical relevance of the small DNA variants in CMNs.
However, the biological complexity and multiple forms of CMNs have led to variability in the genes included on the available panels that are used to make an accurate diagnosis, provide reliable prognostic information, and select an appropriate therapy based on DNA variant profiles present at various time points.
AMP established its CMN Working Group to review the published literature on CMNs, summarize key findings that support clinical utility, and define a set of critical gene inclusions for all high-throughput pan-myeloid sequencing testing panels.
The group proposed the following 34 genes as a minimum recommended testing list: ASXL1, BCOR, BCORL1, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NF1, NPM1, NRAS, PHF6, PPM1D, PTPN11, RAD21, RUNX1, SETBP1, SF3B1, SMC3, SRSF2, STAG2, TET2, TP53, U2AF1, and ZRSR2.
“While the goal of the study was to distill the literature for molecular pathologists, in doing so, we also revealed recurrent mutational patterns of clonal evolution that will [help] hematologist/oncologists, researchers, and pathologists understand how to interpret the results of these panels as they reveal critical biology of the neoplasms,” said Annette S. Kim, MD, PhD, CMN Working Group Chair and an associate professor at Harvard Medical School and Brigham and Women’s Hospital in Boston, Massachusetts.
Familial risk of myeloid malignancies
A large study has revealed “the strongest evidence yet” supporting genetic susceptibility to myeloid malignancies, according to a researcher.
The study showed that first-degree relatives of patients with myeloid malignancies had double the risk of developing a myeloid malignancy themselves, when compared to the general population.
The researchers observed significant risks for developing acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), essential thrombocythemia (ET), and polycythemia vera (PV).
“Our study provides the strongest evidence yet for inherited risk for these diseases—evidence that has proved evasive before, in part, because these cancers are relatively uncommon, and our ability to characterize these diseases has, until recently, been limited,” said Amit Sud, MBChB, PhD, of The Institute of Cancer Research in London, UK.
Dr Sud and his colleagues described their research in a letter to Blood.
The researchers analyzed data from the Swedish Family-Cancer Database, which included 93,199 first-degree relatives of 35,037 patients with myeloid malignancies. The patients had been diagnosed between 1958 and 2015.
First-degree relatives of the patients had an increased risk of all myeloid malignancies, with a standardized incidence ratio (SIR) of 1.99 (95% CI 1.12-2.04).
For individual diseases, there was a significant association between family history and increased risk for:
- AML—SIR=1.53 (95% CI 1.21-2.17)
- ET—SIR=6.30 (95% CI 3.95-9.54)
- MDS—SIR=6.87 (95% CI 4.07-10.86)
- PV—SIR=7.66 (95% CI 5.74-10.02).
Dr Sud and his colleagues noted that the strongest familial relative risks tended to occur for the same disease, but there were significant associations between different myeloid malignancies as well.
Risk by age group
The researchers also looked at familial relative risk for the same disease by patients’ age at diagnosis and observed a significantly increased risk for younger cases for all myeloproliferative neoplasms (MPNs) combined, PV, and MDS.
The SIRs for MPNs were 6.46 (95% CI 5.12-8.04) for patients age 59 or younger and 4.15 (95% CI 3.38-5.04) for patients older than 59.
The SIRs for PV were 10.90 (95% CI 7.12-15.97) for patients age 59 or younger and 5.96 (95% CI 3.93-8.67) for patients older than 59.
The SIRs for MDS were 11.95 (95% CI 6.36-20.43) for patients age 68 or younger and 3.27 (95% CI 1.06-7.63) for patients older than 68.
Risk by number of relatives
Dr Sud and his colleagues also discovered that familial relative risks of all myeloid malignancies and MPNs were significantly associated with the number of first-degree relatives affected by myeloid malignancies or MPNs.
The SIRs for first-degree relatives with 2 or more affected relatives were 4.55 (95% CI 2.08-8.64) for all myeloid malignancies and 17.82 (95% CI 5.79-24.89) for MPNs.
The SIRs for first-degree relatives with 1 affected relative were 1.96 (95% CI 1.79-2.15) for all myeloid malignancies and 4.83 (95% CI 4.14-5.60) for MPNs.
The researchers said these results suggest inherited genetic changes increase the risk of myeloid malignancies, although environmental factors shared in families could also play a role.
“In the future, our findings could help identify people at higher risk than normal because of their family background who could be prioritized for medical help like screening to catch the disease earlier if it arises,” Dr Sud said.
This study was funded by German Cancer Aid, the Swedish Research Council, ALF funding from Region Skåne, DKFZ, and Bloodwise.
A large study has revealed “the strongest evidence yet” supporting genetic susceptibility to myeloid malignancies, according to a researcher.
The study showed that first-degree relatives of patients with myeloid malignancies had double the risk of developing a myeloid malignancy themselves, when compared to the general population.
The researchers observed significant risks for developing acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), essential thrombocythemia (ET), and polycythemia vera (PV).
“Our study provides the strongest evidence yet for inherited risk for these diseases—evidence that has proved evasive before, in part, because these cancers are relatively uncommon, and our ability to characterize these diseases has, until recently, been limited,” said Amit Sud, MBChB, PhD, of The Institute of Cancer Research in London, UK.
Dr Sud and his colleagues described their research in a letter to Blood.
The researchers analyzed data from the Swedish Family-Cancer Database, which included 93,199 first-degree relatives of 35,037 patients with myeloid malignancies. The patients had been diagnosed between 1958 and 2015.
First-degree relatives of the patients had an increased risk of all myeloid malignancies, with a standardized incidence ratio (SIR) of 1.99 (95% CI 1.12-2.04).
For individual diseases, there was a significant association between family history and increased risk for:
- AML—SIR=1.53 (95% CI 1.21-2.17)
- ET—SIR=6.30 (95% CI 3.95-9.54)
- MDS—SIR=6.87 (95% CI 4.07-10.86)
- PV—SIR=7.66 (95% CI 5.74-10.02).
Dr Sud and his colleagues noted that the strongest familial relative risks tended to occur for the same disease, but there were significant associations between different myeloid malignancies as well.
Risk by age group
The researchers also looked at familial relative risk for the same disease by patients’ age at diagnosis and observed a significantly increased risk for younger cases for all myeloproliferative neoplasms (MPNs) combined, PV, and MDS.
The SIRs for MPNs were 6.46 (95% CI 5.12-8.04) for patients age 59 or younger and 4.15 (95% CI 3.38-5.04) for patients older than 59.
The SIRs for PV were 10.90 (95% CI 7.12-15.97) for patients age 59 or younger and 5.96 (95% CI 3.93-8.67) for patients older than 59.
The SIRs for MDS were 11.95 (95% CI 6.36-20.43) for patients age 68 or younger and 3.27 (95% CI 1.06-7.63) for patients older than 68.
Risk by number of relatives
Dr Sud and his colleagues also discovered that familial relative risks of all myeloid malignancies and MPNs were significantly associated with the number of first-degree relatives affected by myeloid malignancies or MPNs.
The SIRs for first-degree relatives with 2 or more affected relatives were 4.55 (95% CI 2.08-8.64) for all myeloid malignancies and 17.82 (95% CI 5.79-24.89) for MPNs.
The SIRs for first-degree relatives with 1 affected relative were 1.96 (95% CI 1.79-2.15) for all myeloid malignancies and 4.83 (95% CI 4.14-5.60) for MPNs.
The researchers said these results suggest inherited genetic changes increase the risk of myeloid malignancies, although environmental factors shared in families could also play a role.
“In the future, our findings could help identify people at higher risk than normal because of their family background who could be prioritized for medical help like screening to catch the disease earlier if it arises,” Dr Sud said.
This study was funded by German Cancer Aid, the Swedish Research Council, ALF funding from Region Skåne, DKFZ, and Bloodwise.
A large study has revealed “the strongest evidence yet” supporting genetic susceptibility to myeloid malignancies, according to a researcher.
The study showed that first-degree relatives of patients with myeloid malignancies had double the risk of developing a myeloid malignancy themselves, when compared to the general population.
The researchers observed significant risks for developing acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), essential thrombocythemia (ET), and polycythemia vera (PV).
“Our study provides the strongest evidence yet for inherited risk for these diseases—evidence that has proved evasive before, in part, because these cancers are relatively uncommon, and our ability to characterize these diseases has, until recently, been limited,” said Amit Sud, MBChB, PhD, of The Institute of Cancer Research in London, UK.
Dr Sud and his colleagues described their research in a letter to Blood.
The researchers analyzed data from the Swedish Family-Cancer Database, which included 93,199 first-degree relatives of 35,037 patients with myeloid malignancies. The patients had been diagnosed between 1958 and 2015.
First-degree relatives of the patients had an increased risk of all myeloid malignancies, with a standardized incidence ratio (SIR) of 1.99 (95% CI 1.12-2.04).
For individual diseases, there was a significant association between family history and increased risk for:
- AML—SIR=1.53 (95% CI 1.21-2.17)
- ET—SIR=6.30 (95% CI 3.95-9.54)
- MDS—SIR=6.87 (95% CI 4.07-10.86)
- PV—SIR=7.66 (95% CI 5.74-10.02).
Dr Sud and his colleagues noted that the strongest familial relative risks tended to occur for the same disease, but there were significant associations between different myeloid malignancies as well.
Risk by age group
The researchers also looked at familial relative risk for the same disease by patients’ age at diagnosis and observed a significantly increased risk for younger cases for all myeloproliferative neoplasms (MPNs) combined, PV, and MDS.
The SIRs for MPNs were 6.46 (95% CI 5.12-8.04) for patients age 59 or younger and 4.15 (95% CI 3.38-5.04) for patients older than 59.
The SIRs for PV were 10.90 (95% CI 7.12-15.97) for patients age 59 or younger and 5.96 (95% CI 3.93-8.67) for patients older than 59.
The SIRs for MDS were 11.95 (95% CI 6.36-20.43) for patients age 68 or younger and 3.27 (95% CI 1.06-7.63) for patients older than 68.
Risk by number of relatives
Dr Sud and his colleagues also discovered that familial relative risks of all myeloid malignancies and MPNs were significantly associated with the number of first-degree relatives affected by myeloid malignancies or MPNs.
The SIRs for first-degree relatives with 2 or more affected relatives were 4.55 (95% CI 2.08-8.64) for all myeloid malignancies and 17.82 (95% CI 5.79-24.89) for MPNs.
The SIRs for first-degree relatives with 1 affected relative were 1.96 (95% CI 1.79-2.15) for all myeloid malignancies and 4.83 (95% CI 4.14-5.60) for MPNs.
The researchers said these results suggest inherited genetic changes increase the risk of myeloid malignancies, although environmental factors shared in families could also play a role.
“In the future, our findings could help identify people at higher risk than normal because of their family background who could be prioritized for medical help like screening to catch the disease earlier if it arises,” Dr Sud said.
This study was funded by German Cancer Aid, the Swedish Research Council, ALF funding from Region Skåne, DKFZ, and Bloodwise.
Tool may reveal optimal time for SCT in MF
A new tool can help patients with myelofibrosis (MF) decide when to pursue stem cell transplant (SCT), according to the MPN Research Foundation.
The SCT Spectrum Transplant Timing tool (SSTT) is an online MF risk calculator.
It is designed to inform patients of their risk status in a timely manner so they can be proactive in speaking to their doctors about SCT.
“There is an optimal time to start SCT, simply because the odds of success soar in our favor when we start early rather than late in the game,” said Zhenya Senyak, editor of MPN Forum, an MF patient, and creator of the SSTT.
“The SCT Spectrum Timing Tool can help inform the timing of that decision.”
The SSTT incorporates the Dynamic International Prognostic Scoring System (DIPSS), asking patients about their age, white blood cell count, hemoglobin level, peripheral blood blast percentage, and constitutional symptoms.
Patients’ answers are used to create a color signal that indicates their current MF risk level. Accompanying information explains what the risk level means, provides median survival times for that level, and alerts patients of the relative urgency of SCT.
Patients can review these results with their hematologist to ensure accuracy and incorporate risk factors not measured by the SSTT.
The SSTT also includes resources to support SCT discussions between patients and hematologists.
Senyak created SSTT with help from the MPN SCT Transplantation Timing Taskforce, a group of 18 MPN and transplant specialists, patient advocates, and SCT survivors. The work was sponsored by the MPN Research Foundation.
“The decision of whether or not to pursue a stem cell transplant is incredibly fraught for any person,” said Ruben Mesa, MD, a member of the MPN SCT Transplantation Timing Taskforce.
“Our hope in assisting with this effort is to lend our knowledge and experience to create a guide to assist with patient-doctor communication around this very complicated issue.”
A new tool can help patients with myelofibrosis (MF) decide when to pursue stem cell transplant (SCT), according to the MPN Research Foundation.
The SCT Spectrum Transplant Timing tool (SSTT) is an online MF risk calculator.
It is designed to inform patients of their risk status in a timely manner so they can be proactive in speaking to their doctors about SCT.
“There is an optimal time to start SCT, simply because the odds of success soar in our favor when we start early rather than late in the game,” said Zhenya Senyak, editor of MPN Forum, an MF patient, and creator of the SSTT.
“The SCT Spectrum Timing Tool can help inform the timing of that decision.”
The SSTT incorporates the Dynamic International Prognostic Scoring System (DIPSS), asking patients about their age, white blood cell count, hemoglobin level, peripheral blood blast percentage, and constitutional symptoms.
Patients’ answers are used to create a color signal that indicates their current MF risk level. Accompanying information explains what the risk level means, provides median survival times for that level, and alerts patients of the relative urgency of SCT.
Patients can review these results with their hematologist to ensure accuracy and incorporate risk factors not measured by the SSTT.
The SSTT also includes resources to support SCT discussions between patients and hematologists.
Senyak created SSTT with help from the MPN SCT Transplantation Timing Taskforce, a group of 18 MPN and transplant specialists, patient advocates, and SCT survivors. The work was sponsored by the MPN Research Foundation.
“The decision of whether or not to pursue a stem cell transplant is incredibly fraught for any person,” said Ruben Mesa, MD, a member of the MPN SCT Transplantation Timing Taskforce.
“Our hope in assisting with this effort is to lend our knowledge and experience to create a guide to assist with patient-doctor communication around this very complicated issue.”
A new tool can help patients with myelofibrosis (MF) decide when to pursue stem cell transplant (SCT), according to the MPN Research Foundation.
The SCT Spectrum Transplant Timing tool (SSTT) is an online MF risk calculator.
It is designed to inform patients of their risk status in a timely manner so they can be proactive in speaking to their doctors about SCT.
“There is an optimal time to start SCT, simply because the odds of success soar in our favor when we start early rather than late in the game,” said Zhenya Senyak, editor of MPN Forum, an MF patient, and creator of the SSTT.
“The SCT Spectrum Timing Tool can help inform the timing of that decision.”
The SSTT incorporates the Dynamic International Prognostic Scoring System (DIPSS), asking patients about their age, white blood cell count, hemoglobin level, peripheral blood blast percentage, and constitutional symptoms.
Patients’ answers are used to create a color signal that indicates their current MF risk level. Accompanying information explains what the risk level means, provides median survival times for that level, and alerts patients of the relative urgency of SCT.
Patients can review these results with their hematologist to ensure accuracy and incorporate risk factors not measured by the SSTT.
The SSTT also includes resources to support SCT discussions between patients and hematologists.
Senyak created SSTT with help from the MPN SCT Transplantation Timing Taskforce, a group of 18 MPN and transplant specialists, patient advocates, and SCT survivors. The work was sponsored by the MPN Research Foundation.
“The decision of whether or not to pursue a stem cell transplant is incredibly fraught for any person,” said Ruben Mesa, MD, a member of the MPN SCT Transplantation Timing Taskforce.
“Our hope in assisting with this effort is to lend our knowledge and experience to create a guide to assist with patient-doctor communication around this very complicated issue.”