User login
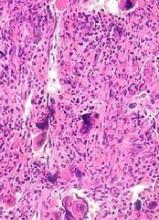
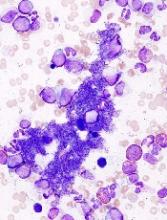
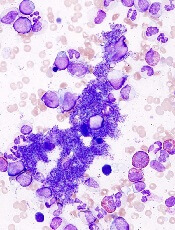
JAK inhibition linked to B-cell lymphoma
New research indicates that JAK inhibitors may increase the risk of lymphoma in patients with myelofibrosis (MF).
The patients studied had a 15- to 25-fold higher risk of developing B-cell lymphoma if they received treatment with JAK inhibitors.
The researchers speculate that screening MF patients for a pre-existing B-cell clone before starting JAK inhibitor therapy may help prevent lymphoma development.
Heinz Gisslinger, MD, of the Medical University of Vienna in Austria, and his colleagues conducted this research and reported the findings in Blood.
“[W]e started noticing sporadic cases of lymphomas developing in patients being treated for myeloproliferative neoplasms and wanted to know if this phenomenon was connected to treatment,” Dr Gisslinger said.
Therefore, he and his colleagues assessed 626 patients receiving treatment for myeloproliferative neoplasms (MPNs) at the Medical University of Vienna.
The incidence of B-cell lymphoma was 5.8% (4/69) in patients treated with JAK inhibitors and 0.36% (2/557) in patients who did not receive JAK inhibitors. That amounts to a 16-fold increased risk of lymphoma in patients receiving JAK inhibitors.
When the researchers analyzed only patients with primary MF (n=216), the increased risk of B-cell lymphoma was even greater. The incidence of lymphoma was 9.68% (3/31) in patients treated with JAK inhibitors and 0.54% (1/185) in patients who did not receive JAK inhibitors.
That corresponds to a 19-fold increased risk of B-cell lymphoma in primary MF patients treated with JAK inhibitors. When the researchers adjusted for age, there was a 21-fold greater risk. When they adjusted for sex, the risk was 25 times higher.
In a second cohort of 929 MPN patients, the incidence of B-cell lymphoma was 3.51% (2/57) in patients who received JAK inhibitors and 0.23% (2/872) in patients who did not. This corresponds to a 15-fold increased risk of lymphoma in the JAK inhibitor recipients.
Lymphoma cases
In all, there were 6 patients who developed lymphoma after JAK inhibitor treatment. Five developed diffuse large B-cell lymphoma, and 1 had high-grade B-cell lymphoma not otherwise specified.
Four of the patients had primary MF, 1 had post-polycythemia vera MF, and 1 had post-essential thrombocythemia (ET) MF. Five patients had a JAK2V617F mutation, and 1 (the post-ET MF patient) had a CALR mutation.
All 6 patients had received treatment with ruxolitinib. One patient also received fedratinib.
B-cell clone
The researchers studied bone marrow samples from 54 of the 69 patients treated with JAK inhibitors in the first cohort. The team found a pre-existing B-cell clone in 3 of the 4 patients who developed lymphoma. Further investigation suggested this was the clone that later transformed into lymphoma.
The researchers also found an association between JAK inhibition and an increased frequency of aggressive B-cell lymphomas in mouse models.
“By replicating this link between this B-cell clone and aggressive lymphoma, we hope to speed the discovery of an alternative therapy for myelofibrosis,” said study author Veronica Sexl, MD, of the University of Veterinary Medicine in Vienna. “These findings are going to be valuable in clinical care.”
“We determined that patients with this pre-existing B-cell clone in their bone marrow are most at risk for developing aggressive lymphoma,” added study author Ulrich Jäger, MD, of the Medical University of Vienna.
“We also know that up to 16% of people with myelofibrosis have immunoglobulin gene rearrangements like this B-cell clone. Therefore, our findings suggest that all patients with myelofibrosis should be tested for such gene rearrangements before prescribing JAK inhibitors to treat their disease.”
New research indicates that JAK inhibitors may increase the risk of lymphoma in patients with myelofibrosis (MF).
The patients studied had a 15- to 25-fold higher risk of developing B-cell lymphoma if they received treatment with JAK inhibitors.
The researchers speculate that screening MF patients for a pre-existing B-cell clone before starting JAK inhibitor therapy may help prevent lymphoma development.
Heinz Gisslinger, MD, of the Medical University of Vienna in Austria, and his colleagues conducted this research and reported the findings in Blood.
“[W]e started noticing sporadic cases of lymphomas developing in patients being treated for myeloproliferative neoplasms and wanted to know if this phenomenon was connected to treatment,” Dr Gisslinger said.
Therefore, he and his colleagues assessed 626 patients receiving treatment for myeloproliferative neoplasms (MPNs) at the Medical University of Vienna.
The incidence of B-cell lymphoma was 5.8% (4/69) in patients treated with JAK inhibitors and 0.36% (2/557) in patients who did not receive JAK inhibitors. That amounts to a 16-fold increased risk of lymphoma in patients receiving JAK inhibitors.
When the researchers analyzed only patients with primary MF (n=216), the increased risk of B-cell lymphoma was even greater. The incidence of lymphoma was 9.68% (3/31) in patients treated with JAK inhibitors and 0.54% (1/185) in patients who did not receive JAK inhibitors.
That corresponds to a 19-fold increased risk of B-cell lymphoma in primary MF patients treated with JAK inhibitors. When the researchers adjusted for age, there was a 21-fold greater risk. When they adjusted for sex, the risk was 25 times higher.
In a second cohort of 929 MPN patients, the incidence of B-cell lymphoma was 3.51% (2/57) in patients who received JAK inhibitors and 0.23% (2/872) in patients who did not. This corresponds to a 15-fold increased risk of lymphoma in the JAK inhibitor recipients.
Lymphoma cases
In all, there were 6 patients who developed lymphoma after JAK inhibitor treatment. Five developed diffuse large B-cell lymphoma, and 1 had high-grade B-cell lymphoma not otherwise specified.
Four of the patients had primary MF, 1 had post-polycythemia vera MF, and 1 had post-essential thrombocythemia (ET) MF. Five patients had a JAK2V617F mutation, and 1 (the post-ET MF patient) had a CALR mutation.
All 6 patients had received treatment with ruxolitinib. One patient also received fedratinib.
B-cell clone
The researchers studied bone marrow samples from 54 of the 69 patients treated with JAK inhibitors in the first cohort. The team found a pre-existing B-cell clone in 3 of the 4 patients who developed lymphoma. Further investigation suggested this was the clone that later transformed into lymphoma.
The researchers also found an association between JAK inhibition and an increased frequency of aggressive B-cell lymphomas in mouse models.
“By replicating this link between this B-cell clone and aggressive lymphoma, we hope to speed the discovery of an alternative therapy for myelofibrosis,” said study author Veronica Sexl, MD, of the University of Veterinary Medicine in Vienna. “These findings are going to be valuable in clinical care.”
“We determined that patients with this pre-existing B-cell clone in their bone marrow are most at risk for developing aggressive lymphoma,” added study author Ulrich Jäger, MD, of the Medical University of Vienna.
“We also know that up to 16% of people with myelofibrosis have immunoglobulin gene rearrangements like this B-cell clone. Therefore, our findings suggest that all patients with myelofibrosis should be tested for such gene rearrangements before prescribing JAK inhibitors to treat their disease.”
New research indicates that JAK inhibitors may increase the risk of lymphoma in patients with myelofibrosis (MF).
The patients studied had a 15- to 25-fold higher risk of developing B-cell lymphoma if they received treatment with JAK inhibitors.
The researchers speculate that screening MF patients for a pre-existing B-cell clone before starting JAK inhibitor therapy may help prevent lymphoma development.
Heinz Gisslinger, MD, of the Medical University of Vienna in Austria, and his colleagues conducted this research and reported the findings in Blood.
“[W]e started noticing sporadic cases of lymphomas developing in patients being treated for myeloproliferative neoplasms and wanted to know if this phenomenon was connected to treatment,” Dr Gisslinger said.
Therefore, he and his colleagues assessed 626 patients receiving treatment for myeloproliferative neoplasms (MPNs) at the Medical University of Vienna.
The incidence of B-cell lymphoma was 5.8% (4/69) in patients treated with JAK inhibitors and 0.36% (2/557) in patients who did not receive JAK inhibitors. That amounts to a 16-fold increased risk of lymphoma in patients receiving JAK inhibitors.
When the researchers analyzed only patients with primary MF (n=216), the increased risk of B-cell lymphoma was even greater. The incidence of lymphoma was 9.68% (3/31) in patients treated with JAK inhibitors and 0.54% (1/185) in patients who did not receive JAK inhibitors.
That corresponds to a 19-fold increased risk of B-cell lymphoma in primary MF patients treated with JAK inhibitors. When the researchers adjusted for age, there was a 21-fold greater risk. When they adjusted for sex, the risk was 25 times higher.
In a second cohort of 929 MPN patients, the incidence of B-cell lymphoma was 3.51% (2/57) in patients who received JAK inhibitors and 0.23% (2/872) in patients who did not. This corresponds to a 15-fold increased risk of lymphoma in the JAK inhibitor recipients.
Lymphoma cases
In all, there were 6 patients who developed lymphoma after JAK inhibitor treatment. Five developed diffuse large B-cell lymphoma, and 1 had high-grade B-cell lymphoma not otherwise specified.
Four of the patients had primary MF, 1 had post-polycythemia vera MF, and 1 had post-essential thrombocythemia (ET) MF. Five patients had a JAK2V617F mutation, and 1 (the post-ET MF patient) had a CALR mutation.
All 6 patients had received treatment with ruxolitinib. One patient also received fedratinib.
B-cell clone
The researchers studied bone marrow samples from 54 of the 69 patients treated with JAK inhibitors in the first cohort. The team found a pre-existing B-cell clone in 3 of the 4 patients who developed lymphoma. Further investigation suggested this was the clone that later transformed into lymphoma.
The researchers also found an association between JAK inhibition and an increased frequency of aggressive B-cell lymphomas in mouse models.
“By replicating this link between this B-cell clone and aggressive lymphoma, we hope to speed the discovery of an alternative therapy for myelofibrosis,” said study author Veronica Sexl, MD, of the University of Veterinary Medicine in Vienna. “These findings are going to be valuable in clinical care.”
“We determined that patients with this pre-existing B-cell clone in their bone marrow are most at risk for developing aggressive lymphoma,” added study author Ulrich Jäger, MD, of the Medical University of Vienna.
“We also know that up to 16% of people with myelofibrosis have immunoglobulin gene rearrangements like this B-cell clone. Therefore, our findings suggest that all patients with myelofibrosis should be tested for such gene rearrangements before prescribing JAK inhibitors to treat their disease.”
Ropeg outperforms HU in PV patients of all ages
STOCKHOLM—Follow-up data suggest that ropeginterferon alfa-2b (ropeg) provides an advantage over hydroxyurea (HU) for patients with polycythemia vera (PV), regardless of their age.
Two-year results from an extension study have shown that, compared to HU, ropeg produces higher rates of complete hematologic response (CHR) and molecular response (MR) in PV patients, including patients age 60 and older.
Additionally, rates of adverse events (AEs) and serious AEs were similar between the ropeg and HU arms.
“In all, I think these data suggest that ropeginterferon alfa-2b provides a valuable, efficacious, and safe new treatment option for PV patients of all ages, including those older than 60 years,” said Jean-Jacques Kiladjian, MD, PhD, of Hôpital Saint-Louis, Université Paris Diderot in Paris, France.
Dr Kiladjian presented these results at the 23rd Congress of the European Hematology Association (EHA) as abstract S132.
The research was sponsored by AOP Orphan Pharmaceuticals AG.
Dr Kiladjian presented data from CONTINUATION-PV, an extension trial of PROUD-PV. Results from PROUD-PV were presented at the 2016 ASH Annual Meeting.
PROUD-PV enrolled 254 patients who were treatment-naive or pretreated with HU. They were randomized to receive ropeg (n=127) or HU (n=127).
CONTINUATION-PV included 95 of the patients on ropeg and 76 of the patients on HU. Dr Kiladjian noted that baseline characteristics were similar between these groups.
| Patient characteristics | ||||
| Ropeg | HU | |||
| <60 years (n=49) | ≥60 years (n=46) | <60 years (n=39) | ≥60 years (n=37) | |
| Median age (range) | 51 (30-59) | 64 (60-85) | 50 (32-59) | 66 (61-79) |
| Median duration of PV (range) | 1.9 months (0-145.5) | 1.7 months (0.1-102.4) | 1.6 months (0-91.6) | 1.7 months (0-65.6) |
| HU pretreatment | 17 (34.7%) | 14 (30.4%) | 12 (30.8%) | 13 (35.1%) |
| Mean duration of HU treatment | 11.3 months | 14.8 months | 16.0 months | 11.2 months |
| Mean JAK2V617F allele burden | 38.4% | 47.4% | 38.1% | 48.1% |
| Splenomegaly present | 2 (4.1%) | 5 (10.9%) | 4 (10.3%) | 4 (10.8%) |
| Disease-related symptoms present | 5 (10.2%) | 10 (21.7%) | 4 (10.3%) | 13 (35.1%) |
CHR
At 24 months, the rate of CHR was 70.5% (67/95) in the ropeg arm and 49.3% (33/67) in the HU arm (RR=1.42, P<0.05).
In patients younger than 60, the rate of CHR was 77.6% in the ropeg arm and 55.9% in the HU arm. In patients age 60 and older, rates of CHR were 63% and 42.4%, respectively.
Dr Kiladjian noted that CHR rates increased over time in ropeg recipients. In PROUD-PV, CHR rates were similar between the ropeg and HU arms at 12 months. However, at 24 months, the CHR rates were higher in ropeg recipients.
Ropeg recipients were also more likely than HU recipients to maintain their CHR from the first occurrence to 24 months.
In patients younger than 60, 49% of the ropeg arm and 17.9% of the HU arm maintained a CHR (P<0.001). In patients age 60 and older, rates of CHR maintenance were 37% and 18.9%, respectively.
MR and JAK2V617F allele burden
Rates of MR at 24 months were 68.1% (64/94) in the ropeg arm and 34.7% (26/75) in the HU arm (RR=1.85, P<0.01).
In patients younger than 60, the rate of MR was 77.1% in the ropeg arm and 33.3% in the HU arm (P<0.001). In patients age 60 and older, MR rates were 58.7% and 36.1%, respectively.
For patients younger than 60, the reduction in JAK2V617F allele burden at 12 months was 29.9% in the ropeg arm and 42.3% in the HU arm. At 24 months, the reductions were 54.8% and 4.5%, respectively (P<0.001).
For patients 60 and older, the reduction in JAK2V617F allele burden at 12 months was 25.2% in the ropeg arm and 37.5% in the HU arm. At 24 months, the reductions were 35.1% and 18.4%, respectively.
Safety
“I think an important point here is the safety because we assume that [ropeg] is not well tolerated in elderly patients,” Dr Kiladjian said. “So what are the results in this prospective, controlled trial? There was a comparable number of adverse events and serious adverse events in the treatment arms, irrespective of age.”
Dr Kiladjian also pointed out that the number of adverse drug reactions (ADRs) was comparable between the treatment arms for younger patients, and there was a trend toward a lower number of ADRs in the ropeg arm for the patients age 60 and older.
| Safety results at a mean of 2.7 years of treatment (up to 3.6 years) | ||||
| Ropeg | HU | |||
| <60 (n=49) | ≥60 (n=46) | <60 (n=39) | ≥60 (n=37) | |
| Patients with any AE | 44 (89.8%) | 43 (93.5%) | 36 (92.3%) | 34 (91.1%) |
| Serious AE | 3 (6.1%) | 10 (21.7%) | 4 (10.3%) | 9 (24.3%) |
| ADR | 38 (77.6%) | 29 (63%) | 29 (74.4%) | 33 (89.2%) |
| Serious ADR | 0 | 0 | 0 | 4 (10.8%) |
| Grade 3+ AE | 10 (20.4%) | 16 (34.8%) | 10 (25.6%) | 14 (37.8%) |
| Recovered from AE | 43 (87.8%) | 40 (87%) | 35 (89.7%) | 34 (91.9%) |
The 4 serious ADRs in the HU patients age 60 and older were acute leukemia, anemia, leukopenia, and granulocytopenia.
STOCKHOLM—Follow-up data suggest that ropeginterferon alfa-2b (ropeg) provides an advantage over hydroxyurea (HU) for patients with polycythemia vera (PV), regardless of their age.
Two-year results from an extension study have shown that, compared to HU, ropeg produces higher rates of complete hematologic response (CHR) and molecular response (MR) in PV patients, including patients age 60 and older.
Additionally, rates of adverse events (AEs) and serious AEs were similar between the ropeg and HU arms.
“In all, I think these data suggest that ropeginterferon alfa-2b provides a valuable, efficacious, and safe new treatment option for PV patients of all ages, including those older than 60 years,” said Jean-Jacques Kiladjian, MD, PhD, of Hôpital Saint-Louis, Université Paris Diderot in Paris, France.
Dr Kiladjian presented these results at the 23rd Congress of the European Hematology Association (EHA) as abstract S132.
The research was sponsored by AOP Orphan Pharmaceuticals AG.
Dr Kiladjian presented data from CONTINUATION-PV, an extension trial of PROUD-PV. Results from PROUD-PV were presented at the 2016 ASH Annual Meeting.
PROUD-PV enrolled 254 patients who were treatment-naive or pretreated with HU. They were randomized to receive ropeg (n=127) or HU (n=127).
CONTINUATION-PV included 95 of the patients on ropeg and 76 of the patients on HU. Dr Kiladjian noted that baseline characteristics were similar between these groups.
| Patient characteristics | ||||
| Ropeg | HU | |||
| <60 years (n=49) | ≥60 years (n=46) | <60 years (n=39) | ≥60 years (n=37) | |
| Median age (range) | 51 (30-59) | 64 (60-85) | 50 (32-59) | 66 (61-79) |
| Median duration of PV (range) | 1.9 months (0-145.5) | 1.7 months (0.1-102.4) | 1.6 months (0-91.6) | 1.7 months (0-65.6) |
| HU pretreatment | 17 (34.7%) | 14 (30.4%) | 12 (30.8%) | 13 (35.1%) |
| Mean duration of HU treatment | 11.3 months | 14.8 months | 16.0 months | 11.2 months |
| Mean JAK2V617F allele burden | 38.4% | 47.4% | 38.1% | 48.1% |
| Splenomegaly present | 2 (4.1%) | 5 (10.9%) | 4 (10.3%) | 4 (10.8%) |
| Disease-related symptoms present | 5 (10.2%) | 10 (21.7%) | 4 (10.3%) | 13 (35.1%) |
CHR
At 24 months, the rate of CHR was 70.5% (67/95) in the ropeg arm and 49.3% (33/67) in the HU arm (RR=1.42, P<0.05).
In patients younger than 60, the rate of CHR was 77.6% in the ropeg arm and 55.9% in the HU arm. In patients age 60 and older, rates of CHR were 63% and 42.4%, respectively.
Dr Kiladjian noted that CHR rates increased over time in ropeg recipients. In PROUD-PV, CHR rates were similar between the ropeg and HU arms at 12 months. However, at 24 months, the CHR rates were higher in ropeg recipients.
Ropeg recipients were also more likely than HU recipients to maintain their CHR from the first occurrence to 24 months.
In patients younger than 60, 49% of the ropeg arm and 17.9% of the HU arm maintained a CHR (P<0.001). In patients age 60 and older, rates of CHR maintenance were 37% and 18.9%, respectively.
MR and JAK2V617F allele burden
Rates of MR at 24 months were 68.1% (64/94) in the ropeg arm and 34.7% (26/75) in the HU arm (RR=1.85, P<0.01).
In patients younger than 60, the rate of MR was 77.1% in the ropeg arm and 33.3% in the HU arm (P<0.001). In patients age 60 and older, MR rates were 58.7% and 36.1%, respectively.
For patients younger than 60, the reduction in JAK2V617F allele burden at 12 months was 29.9% in the ropeg arm and 42.3% in the HU arm. At 24 months, the reductions were 54.8% and 4.5%, respectively (P<0.001).
For patients 60 and older, the reduction in JAK2V617F allele burden at 12 months was 25.2% in the ropeg arm and 37.5% in the HU arm. At 24 months, the reductions were 35.1% and 18.4%, respectively.
Safety
“I think an important point here is the safety because we assume that [ropeg] is not well tolerated in elderly patients,” Dr Kiladjian said. “So what are the results in this prospective, controlled trial? There was a comparable number of adverse events and serious adverse events in the treatment arms, irrespective of age.”
Dr Kiladjian also pointed out that the number of adverse drug reactions (ADRs) was comparable between the treatment arms for younger patients, and there was a trend toward a lower number of ADRs in the ropeg arm for the patients age 60 and older.
| Safety results at a mean of 2.7 years of treatment (up to 3.6 years) | ||||
| Ropeg | HU | |||
| <60 (n=49) | ≥60 (n=46) | <60 (n=39) | ≥60 (n=37) | |
| Patients with any AE | 44 (89.8%) | 43 (93.5%) | 36 (92.3%) | 34 (91.1%) |
| Serious AE | 3 (6.1%) | 10 (21.7%) | 4 (10.3%) | 9 (24.3%) |
| ADR | 38 (77.6%) | 29 (63%) | 29 (74.4%) | 33 (89.2%) |
| Serious ADR | 0 | 0 | 0 | 4 (10.8%) |
| Grade 3+ AE | 10 (20.4%) | 16 (34.8%) | 10 (25.6%) | 14 (37.8%) |
| Recovered from AE | 43 (87.8%) | 40 (87%) | 35 (89.7%) | 34 (91.9%) |
The 4 serious ADRs in the HU patients age 60 and older were acute leukemia, anemia, leukopenia, and granulocytopenia.
STOCKHOLM—Follow-up data suggest that ropeginterferon alfa-2b (ropeg) provides an advantage over hydroxyurea (HU) for patients with polycythemia vera (PV), regardless of their age.
Two-year results from an extension study have shown that, compared to HU, ropeg produces higher rates of complete hematologic response (CHR) and molecular response (MR) in PV patients, including patients age 60 and older.
Additionally, rates of adverse events (AEs) and serious AEs were similar between the ropeg and HU arms.
“In all, I think these data suggest that ropeginterferon alfa-2b provides a valuable, efficacious, and safe new treatment option for PV patients of all ages, including those older than 60 years,” said Jean-Jacques Kiladjian, MD, PhD, of Hôpital Saint-Louis, Université Paris Diderot in Paris, France.
Dr Kiladjian presented these results at the 23rd Congress of the European Hematology Association (EHA) as abstract S132.
The research was sponsored by AOP Orphan Pharmaceuticals AG.
Dr Kiladjian presented data from CONTINUATION-PV, an extension trial of PROUD-PV. Results from PROUD-PV were presented at the 2016 ASH Annual Meeting.
PROUD-PV enrolled 254 patients who were treatment-naive or pretreated with HU. They were randomized to receive ropeg (n=127) or HU (n=127).
CONTINUATION-PV included 95 of the patients on ropeg and 76 of the patients on HU. Dr Kiladjian noted that baseline characteristics were similar between these groups.
| Patient characteristics | ||||
| Ropeg | HU | |||
| <60 years (n=49) | ≥60 years (n=46) | <60 years (n=39) | ≥60 years (n=37) | |
| Median age (range) | 51 (30-59) | 64 (60-85) | 50 (32-59) | 66 (61-79) |
| Median duration of PV (range) | 1.9 months (0-145.5) | 1.7 months (0.1-102.4) | 1.6 months (0-91.6) | 1.7 months (0-65.6) |
| HU pretreatment | 17 (34.7%) | 14 (30.4%) | 12 (30.8%) | 13 (35.1%) |
| Mean duration of HU treatment | 11.3 months | 14.8 months | 16.0 months | 11.2 months |
| Mean JAK2V617F allele burden | 38.4% | 47.4% | 38.1% | 48.1% |
| Splenomegaly present | 2 (4.1%) | 5 (10.9%) | 4 (10.3%) | 4 (10.8%) |
| Disease-related symptoms present | 5 (10.2%) | 10 (21.7%) | 4 (10.3%) | 13 (35.1%) |
CHR
At 24 months, the rate of CHR was 70.5% (67/95) in the ropeg arm and 49.3% (33/67) in the HU arm (RR=1.42, P<0.05).
In patients younger than 60, the rate of CHR was 77.6% in the ropeg arm and 55.9% in the HU arm. In patients age 60 and older, rates of CHR were 63% and 42.4%, respectively.
Dr Kiladjian noted that CHR rates increased over time in ropeg recipients. In PROUD-PV, CHR rates were similar between the ropeg and HU arms at 12 months. However, at 24 months, the CHR rates were higher in ropeg recipients.
Ropeg recipients were also more likely than HU recipients to maintain their CHR from the first occurrence to 24 months.
In patients younger than 60, 49% of the ropeg arm and 17.9% of the HU arm maintained a CHR (P<0.001). In patients age 60 and older, rates of CHR maintenance were 37% and 18.9%, respectively.
MR and JAK2V617F allele burden
Rates of MR at 24 months were 68.1% (64/94) in the ropeg arm and 34.7% (26/75) in the HU arm (RR=1.85, P<0.01).
In patients younger than 60, the rate of MR was 77.1% in the ropeg arm and 33.3% in the HU arm (P<0.001). In patients age 60 and older, MR rates were 58.7% and 36.1%, respectively.
For patients younger than 60, the reduction in JAK2V617F allele burden at 12 months was 29.9% in the ropeg arm and 42.3% in the HU arm. At 24 months, the reductions were 54.8% and 4.5%, respectively (P<0.001).
For patients 60 and older, the reduction in JAK2V617F allele burden at 12 months was 25.2% in the ropeg arm and 37.5% in the HU arm. At 24 months, the reductions were 35.1% and 18.4%, respectively.
Safety
“I think an important point here is the safety because we assume that [ropeg] is not well tolerated in elderly patients,” Dr Kiladjian said. “So what are the results in this prospective, controlled trial? There was a comparable number of adverse events and serious adverse events in the treatment arms, irrespective of age.”
Dr Kiladjian also pointed out that the number of adverse drug reactions (ADRs) was comparable between the treatment arms for younger patients, and there was a trend toward a lower number of ADRs in the ropeg arm for the patients age 60 and older.
| Safety results at a mean of 2.7 years of treatment (up to 3.6 years) | ||||
| Ropeg | HU | |||
| <60 (n=49) | ≥60 (n=46) | <60 (n=39) | ≥60 (n=37) | |
| Patients with any AE | 44 (89.8%) | 43 (93.5%) | 36 (92.3%) | 34 (91.1%) |
| Serious AE | 3 (6.1%) | 10 (21.7%) | 4 (10.3%) | 9 (24.3%) |
| ADR | 38 (77.6%) | 29 (63%) | 29 (74.4%) | 33 (89.2%) |
| Serious ADR | 0 | 0 | 0 | 4 (10.8%) |
| Grade 3+ AE | 10 (20.4%) | 16 (34.8%) | 10 (25.6%) | 14 (37.8%) |
| Recovered from AE | 43 (87.8%) | 40 (87%) | 35 (89.7%) | 34 (91.9%) |
The 4 serious ADRs in the HU patients age 60 and older were acute leukemia, anemia, leukopenia, and granulocytopenia.
Umbralisib can revitalize ruxolitinib in MF
STOCKHOLM—The PI3K delta inhibitor umbralisib can “augment or resurrect” responses to ruxolitinib in patients with myelofibrosis (MF), according to a speaker at the 23rd Congress of the European Hematology Association (EHA).
Results of a phase 1 study showed that adding umbralisib to treatment with ruxolitinib could induce responses in MF patients who had a suboptimal or lost response to ruxolitinib.
Of the 23 patients who received the combination, 2 achieved a complete remission (CR), 11 had clinical improvement, and 8 had stable disease.
In addition, umbralisib plus ruxolitinib was considered well-tolerated. The most common adverse event (AE) was anemia.
Tamara K. Moyo, MD, PhD, of Vanderbilt University Medical Center in Nashville, Tennessee, presented these results at the EHA Congress as abstract S133. The research was sponsored by TG Therapeutics.
Patients
Dr Moyo reported results in 23 MF patients who had a suboptimal response, lost a response, or had no response while on a stable dose of ruxolitinib for at least 8 weeks. Their median age was 67 (range, 49-83), and 61% were male.
Patients had primary MF (30%), post-essential thrombocythemia (ET) MF (43%), or post-polycythemia vera (PV) MF (26%). Forty-three percent of patients had JAK2 V617F, 30% had CALR mutations, 17% had MPL mutations, and 13% were triple-negative. One patient had co-occurring CALR and MPL mutations.
Most patients had an ECOG performance score of 0 (39%) or 1 (52%). All had intermediate-1 (35%), intermediate-2 (35%), or high-risk disease (30%) according to DIPSS Plus.
Sixty-one percent of patients had splenomegaly.
Treatment
In stage 1, the patients received stable ruxolitinib and escalating umbralisib. In stage 2, patients received escalating ruxolitinib and umbralisib at the maximum tolerated dose (MTD) established from stage 1.
Patients could then proceed to expansion cohorts in which they would receive any dose of ruxolitinib and umbralisib at the MTD. The expansion cohorts include patients with treatment-naïve MF, PV, chronic myelomonocytic leukemia, and myelodysplastic syndromes/myeloproliferative neoplasms.
However, Dr Moyo reported only on the 23 ruxolitinib-experienced MF patients.
Safety
There were 2 dose-limiting toxicities of asymptomatic, grade 3 amylase/lipase elevations. One occurred in a patient receiving 800 mg of umbralisib daily and 10 mg of ruxolitinib twice daily. The other occurred in a patient receiving 800 mg of umbralisib daily and 15 mg of ruxolitinib twice daily.
Therefore, 600 mg daily was deemed the MTD of umbralisib.
Seventeen patients had at least 1 AE. There were 17 grade 3 or higher AEs in 13 patients.
AEs of any grade included anemia (n=10), neutrophil decrease (n=2), platelet decrease (n=5), AST increase (n=6), ALT increase (n=3), amylase increase (n=3), lipase increase (n=3), diarrhea (n=2), colitis (n=1), dyspnea (n=1), upper respiratory infection (n=2), pneumonia (n=4), other infections (n=6), and sepsis (n=1).
Grade 3 AEs included anemia (n=3), neutrophil decrease (n=2), amylase increase (n=2), lipase increase (n=2), diarrhea (n=2), colitis (n=1), dyspnea (n=1), pneumonia (n=1), and other infections (n=2). The case of sepsis was the only grade 4 AE.
Dr Moyo noted that anemia—the most common AE—was commonly attributed to disease rather than study treatment.
The case of colitis, which was grade 3, was deemed possibly related to treatment, so the patient was removed from the study.
Thirteen patients had discontinued study treatment at the time of analysis. Aside from the patient who discontinued due to colitis, 2 patients went off study due to dose-limiting toxicities, 3 due to progressive disease, 6 due to physician or patient decision, and 1 due to transplant.
Efficacy
Two patients could not be assessed for efficacy, and 8 had stable disease on umbralisib and ruxolitinib.
The combination produced clinical improvement—reduction in spleen volume, increase in hemoglobin, and improvement in MF-related symptoms—in 11 patients (48%).
And 2 patients (9%) achieved a CR. Dr Moyo said there were “few commonalities” between these 2 patients.
Both had intermediate-1-risk disease as well as persistent or progressive MF-related symptoms and thrombocytosis at baseline. However, 1 patient had post-ET MF, and 1 had post-PV MF.
The post-ET MF patient had an MPL driver mutation. She received ruxolitinib at 20 mg twice daily and umbralisib at 400 mg daily. The patient achieved a CR at cycle 15 and remained on study 2 years before proceeding to transplant. The patient is now about 1 year from her transplant with no evidence of disease.
The post-PV patient had a JAK2 V617F driver mutation. She received ruxolitinib at 15 mg twice daily and umbralisib at 600 mg daily. The patient achieved a CR at cycle 5 and remains on study, currently receiving cycle 12 of treatment.
Dr Moyo said these results suggest “the addition of umbralisib to ruxolitinib can augment or resurrect a response in MF patients who have had suboptimal or lost response to ruxolitinib alone, and this treatment combination warrants further investigation.”
STOCKHOLM—The PI3K delta inhibitor umbralisib can “augment or resurrect” responses to ruxolitinib in patients with myelofibrosis (MF), according to a speaker at the 23rd Congress of the European Hematology Association (EHA).
Results of a phase 1 study showed that adding umbralisib to treatment with ruxolitinib could induce responses in MF patients who had a suboptimal or lost response to ruxolitinib.
Of the 23 patients who received the combination, 2 achieved a complete remission (CR), 11 had clinical improvement, and 8 had stable disease.
In addition, umbralisib plus ruxolitinib was considered well-tolerated. The most common adverse event (AE) was anemia.
Tamara K. Moyo, MD, PhD, of Vanderbilt University Medical Center in Nashville, Tennessee, presented these results at the EHA Congress as abstract S133. The research was sponsored by TG Therapeutics.
Patients
Dr Moyo reported results in 23 MF patients who had a suboptimal response, lost a response, or had no response while on a stable dose of ruxolitinib for at least 8 weeks. Their median age was 67 (range, 49-83), and 61% were male.
Patients had primary MF (30%), post-essential thrombocythemia (ET) MF (43%), or post-polycythemia vera (PV) MF (26%). Forty-three percent of patients had JAK2 V617F, 30% had CALR mutations, 17% had MPL mutations, and 13% were triple-negative. One patient had co-occurring CALR and MPL mutations.
Most patients had an ECOG performance score of 0 (39%) or 1 (52%). All had intermediate-1 (35%), intermediate-2 (35%), or high-risk disease (30%) according to DIPSS Plus.
Sixty-one percent of patients had splenomegaly.
Treatment
In stage 1, the patients received stable ruxolitinib and escalating umbralisib. In stage 2, patients received escalating ruxolitinib and umbralisib at the maximum tolerated dose (MTD) established from stage 1.
Patients could then proceed to expansion cohorts in which they would receive any dose of ruxolitinib and umbralisib at the MTD. The expansion cohorts include patients with treatment-naïve MF, PV, chronic myelomonocytic leukemia, and myelodysplastic syndromes/myeloproliferative neoplasms.
However, Dr Moyo reported only on the 23 ruxolitinib-experienced MF patients.
Safety
There were 2 dose-limiting toxicities of asymptomatic, grade 3 amylase/lipase elevations. One occurred in a patient receiving 800 mg of umbralisib daily and 10 mg of ruxolitinib twice daily. The other occurred in a patient receiving 800 mg of umbralisib daily and 15 mg of ruxolitinib twice daily.
Therefore, 600 mg daily was deemed the MTD of umbralisib.
Seventeen patients had at least 1 AE. There were 17 grade 3 or higher AEs in 13 patients.
AEs of any grade included anemia (n=10), neutrophil decrease (n=2), platelet decrease (n=5), AST increase (n=6), ALT increase (n=3), amylase increase (n=3), lipase increase (n=3), diarrhea (n=2), colitis (n=1), dyspnea (n=1), upper respiratory infection (n=2), pneumonia (n=4), other infections (n=6), and sepsis (n=1).
Grade 3 AEs included anemia (n=3), neutrophil decrease (n=2), amylase increase (n=2), lipase increase (n=2), diarrhea (n=2), colitis (n=1), dyspnea (n=1), pneumonia (n=1), and other infections (n=2). The case of sepsis was the only grade 4 AE.
Dr Moyo noted that anemia—the most common AE—was commonly attributed to disease rather than study treatment.
The case of colitis, which was grade 3, was deemed possibly related to treatment, so the patient was removed from the study.
Thirteen patients had discontinued study treatment at the time of analysis. Aside from the patient who discontinued due to colitis, 2 patients went off study due to dose-limiting toxicities, 3 due to progressive disease, 6 due to physician or patient decision, and 1 due to transplant.
Efficacy
Two patients could not be assessed for efficacy, and 8 had stable disease on umbralisib and ruxolitinib.
The combination produced clinical improvement—reduction in spleen volume, increase in hemoglobin, and improvement in MF-related symptoms—in 11 patients (48%).
And 2 patients (9%) achieved a CR. Dr Moyo said there were “few commonalities” between these 2 patients.
Both had intermediate-1-risk disease as well as persistent or progressive MF-related symptoms and thrombocytosis at baseline. However, 1 patient had post-ET MF, and 1 had post-PV MF.
The post-ET MF patient had an MPL driver mutation. She received ruxolitinib at 20 mg twice daily and umbralisib at 400 mg daily. The patient achieved a CR at cycle 15 and remained on study 2 years before proceeding to transplant. The patient is now about 1 year from her transplant with no evidence of disease.
The post-PV patient had a JAK2 V617F driver mutation. She received ruxolitinib at 15 mg twice daily and umbralisib at 600 mg daily. The patient achieved a CR at cycle 5 and remains on study, currently receiving cycle 12 of treatment.
Dr Moyo said these results suggest “the addition of umbralisib to ruxolitinib can augment or resurrect a response in MF patients who have had suboptimal or lost response to ruxolitinib alone, and this treatment combination warrants further investigation.”
STOCKHOLM—The PI3K delta inhibitor umbralisib can “augment or resurrect” responses to ruxolitinib in patients with myelofibrosis (MF), according to a speaker at the 23rd Congress of the European Hematology Association (EHA).
Results of a phase 1 study showed that adding umbralisib to treatment with ruxolitinib could induce responses in MF patients who had a suboptimal or lost response to ruxolitinib.
Of the 23 patients who received the combination, 2 achieved a complete remission (CR), 11 had clinical improvement, and 8 had stable disease.
In addition, umbralisib plus ruxolitinib was considered well-tolerated. The most common adverse event (AE) was anemia.
Tamara K. Moyo, MD, PhD, of Vanderbilt University Medical Center in Nashville, Tennessee, presented these results at the EHA Congress as abstract S133. The research was sponsored by TG Therapeutics.
Patients
Dr Moyo reported results in 23 MF patients who had a suboptimal response, lost a response, or had no response while on a stable dose of ruxolitinib for at least 8 weeks. Their median age was 67 (range, 49-83), and 61% were male.
Patients had primary MF (30%), post-essential thrombocythemia (ET) MF (43%), or post-polycythemia vera (PV) MF (26%). Forty-three percent of patients had JAK2 V617F, 30% had CALR mutations, 17% had MPL mutations, and 13% were triple-negative. One patient had co-occurring CALR and MPL mutations.
Most patients had an ECOG performance score of 0 (39%) or 1 (52%). All had intermediate-1 (35%), intermediate-2 (35%), or high-risk disease (30%) according to DIPSS Plus.
Sixty-one percent of patients had splenomegaly.
Treatment
In stage 1, the patients received stable ruxolitinib and escalating umbralisib. In stage 2, patients received escalating ruxolitinib and umbralisib at the maximum tolerated dose (MTD) established from stage 1.
Patients could then proceed to expansion cohorts in which they would receive any dose of ruxolitinib and umbralisib at the MTD. The expansion cohorts include patients with treatment-naïve MF, PV, chronic myelomonocytic leukemia, and myelodysplastic syndromes/myeloproliferative neoplasms.
However, Dr Moyo reported only on the 23 ruxolitinib-experienced MF patients.
Safety
There were 2 dose-limiting toxicities of asymptomatic, grade 3 amylase/lipase elevations. One occurred in a patient receiving 800 mg of umbralisib daily and 10 mg of ruxolitinib twice daily. The other occurred in a patient receiving 800 mg of umbralisib daily and 15 mg of ruxolitinib twice daily.
Therefore, 600 mg daily was deemed the MTD of umbralisib.
Seventeen patients had at least 1 AE. There were 17 grade 3 or higher AEs in 13 patients.
AEs of any grade included anemia (n=10), neutrophil decrease (n=2), platelet decrease (n=5), AST increase (n=6), ALT increase (n=3), amylase increase (n=3), lipase increase (n=3), diarrhea (n=2), colitis (n=1), dyspnea (n=1), upper respiratory infection (n=2), pneumonia (n=4), other infections (n=6), and sepsis (n=1).
Grade 3 AEs included anemia (n=3), neutrophil decrease (n=2), amylase increase (n=2), lipase increase (n=2), diarrhea (n=2), colitis (n=1), dyspnea (n=1), pneumonia (n=1), and other infections (n=2). The case of sepsis was the only grade 4 AE.
Dr Moyo noted that anemia—the most common AE—was commonly attributed to disease rather than study treatment.
The case of colitis, which was grade 3, was deemed possibly related to treatment, so the patient was removed from the study.
Thirteen patients had discontinued study treatment at the time of analysis. Aside from the patient who discontinued due to colitis, 2 patients went off study due to dose-limiting toxicities, 3 due to progressive disease, 6 due to physician or patient decision, and 1 due to transplant.
Efficacy
Two patients could not be assessed for efficacy, and 8 had stable disease on umbralisib and ruxolitinib.
The combination produced clinical improvement—reduction in spleen volume, increase in hemoglobin, and improvement in MF-related symptoms—in 11 patients (48%).
And 2 patients (9%) achieved a CR. Dr Moyo said there were “few commonalities” between these 2 patients.
Both had intermediate-1-risk disease as well as persistent or progressive MF-related symptoms and thrombocytosis at baseline. However, 1 patient had post-ET MF, and 1 had post-PV MF.
The post-ET MF patient had an MPL driver mutation. She received ruxolitinib at 20 mg twice daily and umbralisib at 400 mg daily. The patient achieved a CR at cycle 15 and remained on study 2 years before proceeding to transplant. The patient is now about 1 year from her transplant with no evidence of disease.
The post-PV patient had a JAK2 V617F driver mutation. She received ruxolitinib at 15 mg twice daily and umbralisib at 600 mg daily. The patient achieved a CR at cycle 5 and remains on study, currently receiving cycle 12 of treatment.
Dr Moyo said these results suggest “the addition of umbralisib to ruxolitinib can augment or resurrect a response in MF patients who have had suboptimal or lost response to ruxolitinib alone, and this treatment combination warrants further investigation.”
PU-H71 receives orphan drug designation for myelofibrosis
The US Food and Drug Administration (FDA) has granted orphan drug designation to PU-H71 to treat patients with myelofibrosis.
The drug specifically targets the epichaperome, a network of high-molecular-weight complexes found in multiple diseases, including cancer and neurologic disorders. These complexes enhance cellular survival, irrespective of tissue of origin or genetic background.
According to research published in Nature Reviews Cancer, Pu-H71 interferes with the epichaperome function in diseases and does not affect normal cells.
PU-H71 is being evaluated in a phase 1b trial in myelofibrosis and advanced metastatic breast cancer.
“In myelofibrosis, the epichaperome plays a central role in optimizing the JAK-STAT pathway,” said Srdan Verstovsek, MD, PhD, “allowing JAK2 to form dimers that evade inhibition with a JAK2 inhibitor such as ruxolitinib.”
“By inhibiting epichaperome function and breaking this mechanism, we believe PU-H71 can increase anti-cancer activity of JAK2 inhibitors,” he said. Dr Verstovsek, of the MD Anderson Cancer Center in Houston, Texas, is lead clinical research advisor for the phase 1b myelofibrosis study.
Phase 1b Study (NCT01393509)
This is a multicenter study designed to assess the safety, tolerability, pharmacokinetic and preliminary efficacy of PU-H71 in patients taking concomitant ruxolitinib.
The safety expansion phase of the trial is open for accrual only to patients with myeloproliferative neoplasms (MPNs).
These patients must have been on ruxolitinib for at least 3 months, be on a stable dose for at least 1 month prior to enrollment and be taking at least 5 mg twice daily.
Patients must have persistent disease manifestations, despite ruxolitinib therapy. These include persistent splenomegaly, abnormal blood counts, persistent constitutional symptoms, residual fibrosis in bone marrow (2+ or greater), or measurable allele burden as evidenced by clonal JAK2 or MPL mutation.
Samus Therapeutics, the developer of PU-H71, announced, simultaneously with the orphan drug designation, the dosing of the first patient in the phase 1b myelofibrosis study.
“Targeting the epichaperome offers an exciting new avenue for treating myelofibrosis and related diseases,” Dr Verstovsek said.
“I look forward to seeing how the combination of these therapies can affect outcomes in patients for whom this resistance is associated with poor prognoses.”
The US Food and Drug Administration (FDA) has granted orphan drug designation to PU-H71 to treat patients with myelofibrosis.
The drug specifically targets the epichaperome, a network of high-molecular-weight complexes found in multiple diseases, including cancer and neurologic disorders. These complexes enhance cellular survival, irrespective of tissue of origin or genetic background.
According to research published in Nature Reviews Cancer, Pu-H71 interferes with the epichaperome function in diseases and does not affect normal cells.
PU-H71 is being evaluated in a phase 1b trial in myelofibrosis and advanced metastatic breast cancer.
“In myelofibrosis, the epichaperome plays a central role in optimizing the JAK-STAT pathway,” said Srdan Verstovsek, MD, PhD, “allowing JAK2 to form dimers that evade inhibition with a JAK2 inhibitor such as ruxolitinib.”
“By inhibiting epichaperome function and breaking this mechanism, we believe PU-H71 can increase anti-cancer activity of JAK2 inhibitors,” he said. Dr Verstovsek, of the MD Anderson Cancer Center in Houston, Texas, is lead clinical research advisor for the phase 1b myelofibrosis study.
Phase 1b Study (NCT01393509)
This is a multicenter study designed to assess the safety, tolerability, pharmacokinetic and preliminary efficacy of PU-H71 in patients taking concomitant ruxolitinib.
The safety expansion phase of the trial is open for accrual only to patients with myeloproliferative neoplasms (MPNs).
These patients must have been on ruxolitinib for at least 3 months, be on a stable dose for at least 1 month prior to enrollment and be taking at least 5 mg twice daily.
Patients must have persistent disease manifestations, despite ruxolitinib therapy. These include persistent splenomegaly, abnormal blood counts, persistent constitutional symptoms, residual fibrosis in bone marrow (2+ or greater), or measurable allele burden as evidenced by clonal JAK2 or MPL mutation.
Samus Therapeutics, the developer of PU-H71, announced, simultaneously with the orphan drug designation, the dosing of the first patient in the phase 1b myelofibrosis study.
“Targeting the epichaperome offers an exciting new avenue for treating myelofibrosis and related diseases,” Dr Verstovsek said.
“I look forward to seeing how the combination of these therapies can affect outcomes in patients for whom this resistance is associated with poor prognoses.”
The US Food and Drug Administration (FDA) has granted orphan drug designation to PU-H71 to treat patients with myelofibrosis.
The drug specifically targets the epichaperome, a network of high-molecular-weight complexes found in multiple diseases, including cancer and neurologic disorders. These complexes enhance cellular survival, irrespective of tissue of origin or genetic background.
According to research published in Nature Reviews Cancer, Pu-H71 interferes with the epichaperome function in diseases and does not affect normal cells.
PU-H71 is being evaluated in a phase 1b trial in myelofibrosis and advanced metastatic breast cancer.
“In myelofibrosis, the epichaperome plays a central role in optimizing the JAK-STAT pathway,” said Srdan Verstovsek, MD, PhD, “allowing JAK2 to form dimers that evade inhibition with a JAK2 inhibitor such as ruxolitinib.”
“By inhibiting epichaperome function and breaking this mechanism, we believe PU-H71 can increase anti-cancer activity of JAK2 inhibitors,” he said. Dr Verstovsek, of the MD Anderson Cancer Center in Houston, Texas, is lead clinical research advisor for the phase 1b myelofibrosis study.
Phase 1b Study (NCT01393509)
This is a multicenter study designed to assess the safety, tolerability, pharmacokinetic and preliminary efficacy of PU-H71 in patients taking concomitant ruxolitinib.
The safety expansion phase of the trial is open for accrual only to patients with myeloproliferative neoplasms (MPNs).
These patients must have been on ruxolitinib for at least 3 months, be on a stable dose for at least 1 month prior to enrollment and be taking at least 5 mg twice daily.
Patients must have persistent disease manifestations, despite ruxolitinib therapy. These include persistent splenomegaly, abnormal blood counts, persistent constitutional symptoms, residual fibrosis in bone marrow (2+ or greater), or measurable allele burden as evidenced by clonal JAK2 or MPL mutation.
Samus Therapeutics, the developer of PU-H71, announced, simultaneously with the orphan drug designation, the dosing of the first patient in the phase 1b myelofibrosis study.
“Targeting the epichaperome offers an exciting new avenue for treating myelofibrosis and related diseases,” Dr Verstovsek said.
“I look forward to seeing how the combination of these therapies can affect outcomes in patients for whom this resistance is associated with poor prognoses.”
How ruxolitinib reduces thrombosis in MPNs
Preclinical research helps explain how the JAK1/2 inhibitor ruxolitinib can reduce thrombosis in patients with myeloproliferative neoplasms (MPNs).
Experiments revealed a link between JAK2V617F and the formation of neutrophil extracellular traps (NETs), which have been implicated in thrombosis.
Researchers found that ruxolitinib reduced NET formation and decreased thrombosis in JAK2-mutant mice.
Benjamin L. Ebert, MD, PhD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues reported these findings in Science Translational Medicine.
The researchers noted that patients with MPNs have an increased risk of thrombosis, and previous research linked thrombosis to the formation of NETs. NETs are structures that help trap pathogens but may also promote autoimmune responses and excessive blood clotting.
With the current study, Dr Ebert and his colleagues examined whether NET formation could promote thrombosis in patients with MPNs.
The team found that neutrophils from MPN patients were more likely to form NETs than neutrophils from healthy individuals. However, incubating neutrophils with ruxolitinib reduced NET formation.
The researchers also found that mice with JAK2V617F were more likely to form NETs and develop thrombosis when compared to wild-type mice. However, treatment with ruxolitinib reduced rates of thrombosis and prevented NET formation in the mice.
The researchers noted that expression of PAD4, a protein required for NET formation, is increased in JAK2V617F-expressing neutrophils. And the team found that PAD4 is required for JAK2V617F-driven NET formation and thrombosis in mouse models of MPNs.
Lastly, the researchers looked at data from 10,893 human subjects. None of these subjects had MPNs, but some had JAK2V617F.
Subjects who were JAK2V617F-positive had a significantly higher risk of thrombotic events than subjects without the mutation (P=0.0003).
Taken together, these findings suggest that JAK2 inhibition can reduce NET formation and ameliorate thrombosis in patients with MPNs or the JAK2V617F mutation.
The researchers noted that, in the phase 3 RESPONSE trial, patients with polycythemia vera had a lower rate of thromboembolic events if they received ruxolitinib rather than standard therapy.
Preclinical research helps explain how the JAK1/2 inhibitor ruxolitinib can reduce thrombosis in patients with myeloproliferative neoplasms (MPNs).
Experiments revealed a link between JAK2V617F and the formation of neutrophil extracellular traps (NETs), which have been implicated in thrombosis.
Researchers found that ruxolitinib reduced NET formation and decreased thrombosis in JAK2-mutant mice.
Benjamin L. Ebert, MD, PhD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues reported these findings in Science Translational Medicine.
The researchers noted that patients with MPNs have an increased risk of thrombosis, and previous research linked thrombosis to the formation of NETs. NETs are structures that help trap pathogens but may also promote autoimmune responses and excessive blood clotting.
With the current study, Dr Ebert and his colleagues examined whether NET formation could promote thrombosis in patients with MPNs.
The team found that neutrophils from MPN patients were more likely to form NETs than neutrophils from healthy individuals. However, incubating neutrophils with ruxolitinib reduced NET formation.
The researchers also found that mice with JAK2V617F were more likely to form NETs and develop thrombosis when compared to wild-type mice. However, treatment with ruxolitinib reduced rates of thrombosis and prevented NET formation in the mice.
The researchers noted that expression of PAD4, a protein required for NET formation, is increased in JAK2V617F-expressing neutrophils. And the team found that PAD4 is required for JAK2V617F-driven NET formation and thrombosis in mouse models of MPNs.
Lastly, the researchers looked at data from 10,893 human subjects. None of these subjects had MPNs, but some had JAK2V617F.
Subjects who were JAK2V617F-positive had a significantly higher risk of thrombotic events than subjects without the mutation (P=0.0003).
Taken together, these findings suggest that JAK2 inhibition can reduce NET formation and ameliorate thrombosis in patients with MPNs or the JAK2V617F mutation.
The researchers noted that, in the phase 3 RESPONSE trial, patients with polycythemia vera had a lower rate of thromboembolic events if they received ruxolitinib rather than standard therapy.
Preclinical research helps explain how the JAK1/2 inhibitor ruxolitinib can reduce thrombosis in patients with myeloproliferative neoplasms (MPNs).
Experiments revealed a link between JAK2V617F and the formation of neutrophil extracellular traps (NETs), which have been implicated in thrombosis.
Researchers found that ruxolitinib reduced NET formation and decreased thrombosis in JAK2-mutant mice.
Benjamin L. Ebert, MD, PhD, of Brigham and Women’s Hospital in Boston, Massachusetts, and his colleagues reported these findings in Science Translational Medicine.
The researchers noted that patients with MPNs have an increased risk of thrombosis, and previous research linked thrombosis to the formation of NETs. NETs are structures that help trap pathogens but may also promote autoimmune responses and excessive blood clotting.
With the current study, Dr Ebert and his colleagues examined whether NET formation could promote thrombosis in patients with MPNs.
The team found that neutrophils from MPN patients were more likely to form NETs than neutrophils from healthy individuals. However, incubating neutrophils with ruxolitinib reduced NET formation.
The researchers also found that mice with JAK2V617F were more likely to form NETs and develop thrombosis when compared to wild-type mice. However, treatment with ruxolitinib reduced rates of thrombosis and prevented NET formation in the mice.
The researchers noted that expression of PAD4, a protein required for NET formation, is increased in JAK2V617F-expressing neutrophils. And the team found that PAD4 is required for JAK2V617F-driven NET formation and thrombosis in mouse models of MPNs.
Lastly, the researchers looked at data from 10,893 human subjects. None of these subjects had MPNs, but some had JAK2V617F.
Subjects who were JAK2V617F-positive had a significantly higher risk of thrombotic events than subjects without the mutation (P=0.0003).
Taken together, these findings suggest that JAK2 inhibition can reduce NET formation and ameliorate thrombosis in patients with MPNs or the JAK2V617F mutation.
The researchers noted that, in the phase 3 RESPONSE trial, patients with polycythemia vera had a lower rate of thromboembolic events if they received ruxolitinib rather than standard therapy.
Pacritinib bests BAT, doesn’t seem to affect survival
Final results from the PERSIST-2 trial suggest pacritinib can be more effective than best available therapy (BAT) for patients with myelofibrosis and thrombocytopenia, and the drug has no significant effect on survival.
Patients who received pacritinib were more likely to experience at least a 35% reduction in spleen volume and a 50% reduction in total symptom score (TSS).
In addition, there was no significant difference in survival between patients who received pacritinib and those who received BAT.
Interim results from PERSIST-2 had indicated that pacritinib negatively impacted survival, which was consistent with results from PERSIST-1. Because of this, the US Food and Drug Administration placed pacritinib trials on clinical hold in February 2016. The hold was lifted in January 2017.
The final results from PERSIST-2 were published in JAMA Oncology. The study was sponsored by CTI BioPharma Corp.
In this phase 3 study, researchers compared 2 dosing schedules of pacritinib to BAT. The study enrolled patients with previously treated or untreated myelofibrosis (intermediate-1/2 or high-risk) and thrombocytopenia (platelet counts ≤ 100 x 109/L).
There were 311 patients randomized to receive pacritinib once daily (n=104), pacritinib twice daily (n=107), or BAT (n=100). Patients in the BAT arm received ruxolitinib (n=44), hydroxyurea (n=19), prednisone and/or prednisolone (n=13), as well as “watchful waiting” (n=19).
Patients could crossover from BAT to pacritinib after week 24 or for progression of splenomegaly. Fifty patients in the BAT arm did cross over.
All patients had discontinued treatment at a median of 23 weeks (pacritinib once daily), 25 weeks (twice daily), and 21 weeks (BAT) from the start of treatment.
Common reasons for discontinuation (in the once daily, twice daily, and BAT arms, respectively) were the clinical hold (59%, 71%, and 27%), adverse events (14%, 9%, and 4%), physician decision (5%, 3%, and 41%), progressive disease (5%, 7%, and 11%), and death (5%, 2%, and 5%).
Efficacy
The intention-to-treat efficacy population included 75 patients in the once-daily arm, 74 in the twice-daily arm, and 72 in the BAT arm. The researchers said baseline characteristics were balanced across the arms.
The co-primary endpoints were the rate of patients achieving a spleen volume reduction (SVR) of 35% or more and a 50% or more reduction in TSS at week 24.
Eighteen percent of patients in the combined pacritinib arms and 3% of patients in the BAT arm achieved an SVR of 35% or more (P=0.001). Fifteen percent of patients in the pacritinib once-daily arm and 22% of patients in the twice-daily arm achieved this endpoint (P values of 0.02 and 0.001, respectively, for comparison with BAT).
Twenty-five percent of patients in the combined pacritinib arms and 14% in the BAT arm had at least a 50% reduction in TSS (P=0.079). Seventeen percent of patients in the pacritinib once-daily arm and 32% of patients in the twice-daily arm achieved this endpoint (P values of 0.65 and 0.01, respectively, for comparison with BAT).
“Pacritinib was shown to reduce both spleen volume and total symptom score, 2 very important clinical measures in myelofibrosis patients with thrombocytopenia, including those patients who received prior treatment with ruxolitinib,” said study author John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York.
Survival
When the clinical hold was placed, there was no significant difference in overall survival between the 3 treatment arms.
The rates of death were 14% (n=14) in the pacritinib once-daily arm, 9% (n=10) in the twice-daily arm, and 14% (n=14) in the BAT arm. For patients in the BAT arm, the death rate was lower for those who crossed over to pacritinib (8%, n=4) than for those who did not (20%, n=10).
The hazard ratios for death were 1.18 in the once-daily pacritinib arm and 0.68 in the twice-daily pacritinib arm.
Safety
Dr Mascarenhas said pacritinib had “a generally manageable safety profile.”
Common adverse events—in the once daily, twice daily, and BAT arms, respectively—included:
- Diarrhea—67%, 48%, and 15%
- Nausea—38%, 32%, and 11%
- Thrombocytopenia—33%, 34%, and 23%
- Anemia—28%, 24%, and 15%
- Vomiting—21%, 19%, and 5%
- Fatigue—17%, 17%, and 16%
- Peripheral edema—13%, 2%, and 15%
- Dizziness—14%, 15%, and 5%
- Abdominal pain—19%, 9%, and 19%
- Pyrexia—11%, 15%, and 3%.
Grade 3/4 events—in the once daily, twice daily, and BAT arms, respectively—included:
- Thrombocytopenia—31%, 32%, and 18%
- Anemia—27%, 22%, and 14%
- Neutropenia—9%, 7%, and 5%
- Pneumonia—4%, 7%, and 3%
- Fatigue—7%, 3%, and 5%
- Diarrhea—5%, 4%, and 0%
- Epistaxis—2%, 5%, and 1%.
Serious adverse events—in the once daily, twice daily, and BAT arms, respectively—included:
- Anemia—5%, 8%, and 3%
- Thrombocytopenia—2%, 6%, and 2%
- Pneumonia—5%, 6%, and 4%
- Acute renal failure—5%, 2%, and 2%
- Congestive heart failure—1%, 4%, and 2%
- Atrial fibrillation—3%, 0%, and 3%
- Cardiac arrest—2%, 0%, and 0%
- Epistaxis—2%, 2%, and 1%
- Subdural hematoma—2%, 0%, and 0%.
Final results from the PERSIST-2 trial suggest pacritinib can be more effective than best available therapy (BAT) for patients with myelofibrosis and thrombocytopenia, and the drug has no significant effect on survival.
Patients who received pacritinib were more likely to experience at least a 35% reduction in spleen volume and a 50% reduction in total symptom score (TSS).
In addition, there was no significant difference in survival between patients who received pacritinib and those who received BAT.
Interim results from PERSIST-2 had indicated that pacritinib negatively impacted survival, which was consistent with results from PERSIST-1. Because of this, the US Food and Drug Administration placed pacritinib trials on clinical hold in February 2016. The hold was lifted in January 2017.
The final results from PERSIST-2 were published in JAMA Oncology. The study was sponsored by CTI BioPharma Corp.
In this phase 3 study, researchers compared 2 dosing schedules of pacritinib to BAT. The study enrolled patients with previously treated or untreated myelofibrosis (intermediate-1/2 or high-risk) and thrombocytopenia (platelet counts ≤ 100 x 109/L).
There were 311 patients randomized to receive pacritinib once daily (n=104), pacritinib twice daily (n=107), or BAT (n=100). Patients in the BAT arm received ruxolitinib (n=44), hydroxyurea (n=19), prednisone and/or prednisolone (n=13), as well as “watchful waiting” (n=19).
Patients could crossover from BAT to pacritinib after week 24 or for progression of splenomegaly. Fifty patients in the BAT arm did cross over.
All patients had discontinued treatment at a median of 23 weeks (pacritinib once daily), 25 weeks (twice daily), and 21 weeks (BAT) from the start of treatment.
Common reasons for discontinuation (in the once daily, twice daily, and BAT arms, respectively) were the clinical hold (59%, 71%, and 27%), adverse events (14%, 9%, and 4%), physician decision (5%, 3%, and 41%), progressive disease (5%, 7%, and 11%), and death (5%, 2%, and 5%).
Efficacy
The intention-to-treat efficacy population included 75 patients in the once-daily arm, 74 in the twice-daily arm, and 72 in the BAT arm. The researchers said baseline characteristics were balanced across the arms.
The co-primary endpoints were the rate of patients achieving a spleen volume reduction (SVR) of 35% or more and a 50% or more reduction in TSS at week 24.
Eighteen percent of patients in the combined pacritinib arms and 3% of patients in the BAT arm achieved an SVR of 35% or more (P=0.001). Fifteen percent of patients in the pacritinib once-daily arm and 22% of patients in the twice-daily arm achieved this endpoint (P values of 0.02 and 0.001, respectively, for comparison with BAT).
Twenty-five percent of patients in the combined pacritinib arms and 14% in the BAT arm had at least a 50% reduction in TSS (P=0.079). Seventeen percent of patients in the pacritinib once-daily arm and 32% of patients in the twice-daily arm achieved this endpoint (P values of 0.65 and 0.01, respectively, for comparison with BAT).
“Pacritinib was shown to reduce both spleen volume and total symptom score, 2 very important clinical measures in myelofibrosis patients with thrombocytopenia, including those patients who received prior treatment with ruxolitinib,” said study author John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York.
Survival
When the clinical hold was placed, there was no significant difference in overall survival between the 3 treatment arms.
The rates of death were 14% (n=14) in the pacritinib once-daily arm, 9% (n=10) in the twice-daily arm, and 14% (n=14) in the BAT arm. For patients in the BAT arm, the death rate was lower for those who crossed over to pacritinib (8%, n=4) than for those who did not (20%, n=10).
The hazard ratios for death were 1.18 in the once-daily pacritinib arm and 0.68 in the twice-daily pacritinib arm.
Safety
Dr Mascarenhas said pacritinib had “a generally manageable safety profile.”
Common adverse events—in the once daily, twice daily, and BAT arms, respectively—included:
- Diarrhea—67%, 48%, and 15%
- Nausea—38%, 32%, and 11%
- Thrombocytopenia—33%, 34%, and 23%
- Anemia—28%, 24%, and 15%
- Vomiting—21%, 19%, and 5%
- Fatigue—17%, 17%, and 16%
- Peripheral edema—13%, 2%, and 15%
- Dizziness—14%, 15%, and 5%
- Abdominal pain—19%, 9%, and 19%
- Pyrexia—11%, 15%, and 3%.
Grade 3/4 events—in the once daily, twice daily, and BAT arms, respectively—included:
- Thrombocytopenia—31%, 32%, and 18%
- Anemia—27%, 22%, and 14%
- Neutropenia—9%, 7%, and 5%
- Pneumonia—4%, 7%, and 3%
- Fatigue—7%, 3%, and 5%
- Diarrhea—5%, 4%, and 0%
- Epistaxis—2%, 5%, and 1%.
Serious adverse events—in the once daily, twice daily, and BAT arms, respectively—included:
- Anemia—5%, 8%, and 3%
- Thrombocytopenia—2%, 6%, and 2%
- Pneumonia—5%, 6%, and 4%
- Acute renal failure—5%, 2%, and 2%
- Congestive heart failure—1%, 4%, and 2%
- Atrial fibrillation—3%, 0%, and 3%
- Cardiac arrest—2%, 0%, and 0%
- Epistaxis—2%, 2%, and 1%
- Subdural hematoma—2%, 0%, and 0%.
Final results from the PERSIST-2 trial suggest pacritinib can be more effective than best available therapy (BAT) for patients with myelofibrosis and thrombocytopenia, and the drug has no significant effect on survival.
Patients who received pacritinib were more likely to experience at least a 35% reduction in spleen volume and a 50% reduction in total symptom score (TSS).
In addition, there was no significant difference in survival between patients who received pacritinib and those who received BAT.
Interim results from PERSIST-2 had indicated that pacritinib negatively impacted survival, which was consistent with results from PERSIST-1. Because of this, the US Food and Drug Administration placed pacritinib trials on clinical hold in February 2016. The hold was lifted in January 2017.
The final results from PERSIST-2 were published in JAMA Oncology. The study was sponsored by CTI BioPharma Corp.
In this phase 3 study, researchers compared 2 dosing schedules of pacritinib to BAT. The study enrolled patients with previously treated or untreated myelofibrosis (intermediate-1/2 or high-risk) and thrombocytopenia (platelet counts ≤ 100 x 109/L).
There were 311 patients randomized to receive pacritinib once daily (n=104), pacritinib twice daily (n=107), or BAT (n=100). Patients in the BAT arm received ruxolitinib (n=44), hydroxyurea (n=19), prednisone and/or prednisolone (n=13), as well as “watchful waiting” (n=19).
Patients could crossover from BAT to pacritinib after week 24 or for progression of splenomegaly. Fifty patients in the BAT arm did cross over.
All patients had discontinued treatment at a median of 23 weeks (pacritinib once daily), 25 weeks (twice daily), and 21 weeks (BAT) from the start of treatment.
Common reasons for discontinuation (in the once daily, twice daily, and BAT arms, respectively) were the clinical hold (59%, 71%, and 27%), adverse events (14%, 9%, and 4%), physician decision (5%, 3%, and 41%), progressive disease (5%, 7%, and 11%), and death (5%, 2%, and 5%).
Efficacy
The intention-to-treat efficacy population included 75 patients in the once-daily arm, 74 in the twice-daily arm, and 72 in the BAT arm. The researchers said baseline characteristics were balanced across the arms.
The co-primary endpoints were the rate of patients achieving a spleen volume reduction (SVR) of 35% or more and a 50% or more reduction in TSS at week 24.
Eighteen percent of patients in the combined pacritinib arms and 3% of patients in the BAT arm achieved an SVR of 35% or more (P=0.001). Fifteen percent of patients in the pacritinib once-daily arm and 22% of patients in the twice-daily arm achieved this endpoint (P values of 0.02 and 0.001, respectively, for comparison with BAT).
Twenty-five percent of patients in the combined pacritinib arms and 14% in the BAT arm had at least a 50% reduction in TSS (P=0.079). Seventeen percent of patients in the pacritinib once-daily arm and 32% of patients in the twice-daily arm achieved this endpoint (P values of 0.65 and 0.01, respectively, for comparison with BAT).
“Pacritinib was shown to reduce both spleen volume and total symptom score, 2 very important clinical measures in myelofibrosis patients with thrombocytopenia, including those patients who received prior treatment with ruxolitinib,” said study author John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York.
Survival
When the clinical hold was placed, there was no significant difference in overall survival between the 3 treatment arms.
The rates of death were 14% (n=14) in the pacritinib once-daily arm, 9% (n=10) in the twice-daily arm, and 14% (n=14) in the BAT arm. For patients in the BAT arm, the death rate was lower for those who crossed over to pacritinib (8%, n=4) than for those who did not (20%, n=10).
The hazard ratios for death were 1.18 in the once-daily pacritinib arm and 0.68 in the twice-daily pacritinib arm.
Safety
Dr Mascarenhas said pacritinib had “a generally manageable safety profile.”
Common adverse events—in the once daily, twice daily, and BAT arms, respectively—included:
- Diarrhea—67%, 48%, and 15%
- Nausea—38%, 32%, and 11%
- Thrombocytopenia—33%, 34%, and 23%
- Anemia—28%, 24%, and 15%
- Vomiting—21%, 19%, and 5%
- Fatigue—17%, 17%, and 16%
- Peripheral edema—13%, 2%, and 15%
- Dizziness—14%, 15%, and 5%
- Abdominal pain—19%, 9%, and 19%
- Pyrexia—11%, 15%, and 3%.
Grade 3/4 events—in the once daily, twice daily, and BAT arms, respectively—included:
- Thrombocytopenia—31%, 32%, and 18%
- Anemia—27%, 22%, and 14%
- Neutropenia—9%, 7%, and 5%
- Pneumonia—4%, 7%, and 3%
- Fatigue—7%, 3%, and 5%
- Diarrhea—5%, 4%, and 0%
- Epistaxis—2%, 5%, and 1%.
Serious adverse events—in the once daily, twice daily, and BAT arms, respectively—included:
- Anemia—5%, 8%, and 3%
- Thrombocytopenia—2%, 6%, and 2%
- Pneumonia—5%, 6%, and 4%
- Acute renal failure—5%, 2%, and 2%
- Congestive heart failure—1%, 4%, and 2%
- Atrial fibrillation—3%, 0%, and 3%
- Cardiac arrest—2%, 0%, and 0%
- Epistaxis—2%, 2%, and 1%
- Subdural hematoma—2%, 0%, and 0%.
New mutation linked to familial erythrocytosis
Researchers say they have discovered a mutation associated with hereditary erythrocytosis.
The mutation causes a messenger RNA (mRNA) that is not normally involved in the formation of proteins to be reprogrammed so that it produces erythropoietin (EPO), thereby abnormally increasing red blood cell production.
Radek Skoda, MD, of the University of Basel in Switzerland, and his colleagues described this discovery in NEJM.
The team found the mutation in a family with hereditary erythrocytosis. The researchers studied 10 affected family members spanning 4 generations.
Genome-wide linkage analysis and gene sequencing revealed a heterozygous single-base deletion in exon 2 of EPO (chromosome 7: 100,319,199 GG→G) in all 10 affected family members.
However, the researchers were initially puzzled. This c.32delG mutation should actually lead to a loss of function of the EPO gene because the absence of the base shifts the reading frame of the genetic code, meaning that no more EPO protein can be formed.
Despite this, the concentration of EPO hormone in the patients’ blood measurably increased rather than decreased.
To investigate this, the researchers used CRISPR to engineer cells carrying the c.32delG mutation. In this way, they found a second, hidden mRNA in the EPO gene that is not normally involved in the production of a protein.
The c.32delG mutation also leads to a shift in the reading frame of this second mRNA, with the result that more biologically active EPO hormone is produced.
“The mechanism is intriguing,” Dr Skoda said. “The mutation reprograms the gene product so that it gains a new function and is misused to overproduce EPO.”
Researchers say they have discovered a mutation associated with hereditary erythrocytosis.
The mutation causes a messenger RNA (mRNA) that is not normally involved in the formation of proteins to be reprogrammed so that it produces erythropoietin (EPO), thereby abnormally increasing red blood cell production.
Radek Skoda, MD, of the University of Basel in Switzerland, and his colleagues described this discovery in NEJM.
The team found the mutation in a family with hereditary erythrocytosis. The researchers studied 10 affected family members spanning 4 generations.
Genome-wide linkage analysis and gene sequencing revealed a heterozygous single-base deletion in exon 2 of EPO (chromosome 7: 100,319,199 GG→G) in all 10 affected family members.
However, the researchers were initially puzzled. This c.32delG mutation should actually lead to a loss of function of the EPO gene because the absence of the base shifts the reading frame of the genetic code, meaning that no more EPO protein can be formed.
Despite this, the concentration of EPO hormone in the patients’ blood measurably increased rather than decreased.
To investigate this, the researchers used CRISPR to engineer cells carrying the c.32delG mutation. In this way, they found a second, hidden mRNA in the EPO gene that is not normally involved in the production of a protein.
The c.32delG mutation also leads to a shift in the reading frame of this second mRNA, with the result that more biologically active EPO hormone is produced.
“The mechanism is intriguing,” Dr Skoda said. “The mutation reprograms the gene product so that it gains a new function and is misused to overproduce EPO.”
Researchers say they have discovered a mutation associated with hereditary erythrocytosis.
The mutation causes a messenger RNA (mRNA) that is not normally involved in the formation of proteins to be reprogrammed so that it produces erythropoietin (EPO), thereby abnormally increasing red blood cell production.
Radek Skoda, MD, of the University of Basel in Switzerland, and his colleagues described this discovery in NEJM.
The team found the mutation in a family with hereditary erythrocytosis. The researchers studied 10 affected family members spanning 4 generations.
Genome-wide linkage analysis and gene sequencing revealed a heterozygous single-base deletion in exon 2 of EPO (chromosome 7: 100,319,199 GG→G) in all 10 affected family members.
However, the researchers were initially puzzled. This c.32delG mutation should actually lead to a loss of function of the EPO gene because the absence of the base shifts the reading frame of the genetic code, meaning that no more EPO protein can be formed.
Despite this, the concentration of EPO hormone in the patients’ blood measurably increased rather than decreased.
To investigate this, the researchers used CRISPR to engineer cells carrying the c.32delG mutation. In this way, they found a second, hidden mRNA in the EPO gene that is not normally involved in the production of a protein.
The c.32delG mutation also leads to a shift in the reading frame of this second mRNA, with the result that more biologically active EPO hormone is produced.
“The mechanism is intriguing,” Dr Skoda said. “The mutation reprograms the gene product so that it gains a new function and is misused to overproduce EPO.”
FDA approves test to diagnose MPNs
The US Food and Drug Administration (FDA) has cleared use of QIAGEN’s ipsogen JAK2 RGQ PCR Kit (ipsogen JAK2 assay) for the diagnosis of all myeloproliferative neoplasms (MPNs).
The ipsogen JAK2 assay is a qualitative, in vitro diagnostic test designed to detect the JAK2 V617F/G1849T allele in genomic DNA extracted from EDTA whole blood.
The assay was previously cleared by the FDA for use in conjunction with other clinicopathological factors to aid the diagnosis of polycythemia vera.
Now, the FDA has cleared use of the assay for 2 additional MPNs—essential thrombocythemia and primary myelofibrosis.
“We are eager to expand the use of our ipsogen JAK2 assay, which is already available in Europe and other markets, for use in a wider range of patients in the US,” said Thierry Bernard, senior vice president and head of QIAGEN’s Molecular Diagnostics Business Area.
“Our JAK2 assay makes it easier for hematologists and oncologists to follow recommended diagnostic testing algorithms and international guidelines for their patients suspected of having MPNs.”
The ipsogen JAK2 assay is a real-time PCR test performed on the QIAGEN Rotor-Gene Q MDx instrument.
The test is intended for use as an adjunct to the evaluation of suspected MPNs, in conjunction with other clinicopathological factors.
The ipsogen JAK2 assay does not detect less common JAK2 mutations associated with MPNs, including mutations in exon 12, and is not intended for stand-alone diagnosis of MPNs. ![]()
The US Food and Drug Administration (FDA) has cleared use of QIAGEN’s ipsogen JAK2 RGQ PCR Kit (ipsogen JAK2 assay) for the diagnosis of all myeloproliferative neoplasms (MPNs).
The ipsogen JAK2 assay is a qualitative, in vitro diagnostic test designed to detect the JAK2 V617F/G1849T allele in genomic DNA extracted from EDTA whole blood.
The assay was previously cleared by the FDA for use in conjunction with other clinicopathological factors to aid the diagnosis of polycythemia vera.
Now, the FDA has cleared use of the assay for 2 additional MPNs—essential thrombocythemia and primary myelofibrosis.
“We are eager to expand the use of our ipsogen JAK2 assay, which is already available in Europe and other markets, for use in a wider range of patients in the US,” said Thierry Bernard, senior vice president and head of QIAGEN’s Molecular Diagnostics Business Area.
“Our JAK2 assay makes it easier for hematologists and oncologists to follow recommended diagnostic testing algorithms and international guidelines for their patients suspected of having MPNs.”
The ipsogen JAK2 assay is a real-time PCR test performed on the QIAGEN Rotor-Gene Q MDx instrument.
The test is intended for use as an adjunct to the evaluation of suspected MPNs, in conjunction with other clinicopathological factors.
The ipsogen JAK2 assay does not detect less common JAK2 mutations associated with MPNs, including mutations in exon 12, and is not intended for stand-alone diagnosis of MPNs. ![]()
The US Food and Drug Administration (FDA) has cleared use of QIAGEN’s ipsogen JAK2 RGQ PCR Kit (ipsogen JAK2 assay) for the diagnosis of all myeloproliferative neoplasms (MPNs).
The ipsogen JAK2 assay is a qualitative, in vitro diagnostic test designed to detect the JAK2 V617F/G1849T allele in genomic DNA extracted from EDTA whole blood.
The assay was previously cleared by the FDA for use in conjunction with other clinicopathological factors to aid the diagnosis of polycythemia vera.
Now, the FDA has cleared use of the assay for 2 additional MPNs—essential thrombocythemia and primary myelofibrosis.
“We are eager to expand the use of our ipsogen JAK2 assay, which is already available in Europe and other markets, for use in a wider range of patients in the US,” said Thierry Bernard, senior vice president and head of QIAGEN’s Molecular Diagnostics Business Area.
“Our JAK2 assay makes it easier for hematologists and oncologists to follow recommended diagnostic testing algorithms and international guidelines for their patients suspected of having MPNs.”
The ipsogen JAK2 assay is a real-time PCR test performed on the QIAGEN Rotor-Gene Q MDx instrument.
The test is intended for use as an adjunct to the evaluation of suspected MPNs, in conjunction with other clinicopathological factors.
The ipsogen JAK2 assay does not detect less common JAK2 mutations associated with MPNs, including mutations in exon 12, and is not intended for stand-alone diagnosis of MPNs. ![]()
DNA methylation may predict outcomes in JMML
New research suggests DNA methylation may be used to predict how children with juvenile myelomonocytic leukemia (JMML) will respond to treatment.
Researchers found they could divide patients into risk groups according to their methylation levels.
Patients with the highest methylation levels had the worst event-free survival, while patients who recovered spontaneously had methylation levels similar to those of healthy control subjects.
“This data provides important information that will help clinicians decide how intensively and swiftly to treat their patients,” said Mignon Loh, MD, of Benioff Children’s Hospital, University of California, San Francisco.
She and her colleagues described this research in Nature Communications.
The team studied genome-wide DNA methylation levels in an initial group of 39 patients with JMML and then validated the results in a group of 40 JMML patients.
Statistical analysis revealed that patients fell into 3 clusters with high, intermediate, or low levels of DNA methylation. And patients’ methylation levels were associated with 4-year event-free survival.
In the initial cohort, 6% (1/15) of patients in the lowest methylation group had an event at 4 years, as did 45% (5/11) of patients with intermediate levels of methylation and 61% (8/13) of patients with the highest levels of methylation.
In the validation cohort, 8% (1/12) of patients in the lowest methylation group had an event at 4 years, as did 36% (4/11) of patients with intermediate levels of methylation and 76% (13/17) of patients with the highest levels of methylation.
“For us, this was surprising,” said study author Elliot Stieglitz, MD, also of Benioff Children’s Hospital.
“We are not yet able to say why DNA methylation is different amongst these patients, but for it to be so predictive of outcomes, even more than genetic mutations, was a big surprise.”
The researchers also found that methylation levels might predict spontaneous remission as well.
Thirteen of the 14 patients who had spontaneous remission clustered together. And their DNA methylation levels were closer to those of healthy control subjects than those of other JMML cases.
“Because we have never been able to tell which patients might spontaneously recover, and because the disease can be so aggressive, the standard of care has been to recommend transplants for everybody,” Dr Stieglitz said.
“There are many health risks associated with bone marrow transplants, ranging from infections to long-term short stature and even death in some cases. To be able to avoid this intensive intervention for some patients based on a methylation assay would really be cutting-edge clinical science.” ![]()
New research suggests DNA methylation may be used to predict how children with juvenile myelomonocytic leukemia (JMML) will respond to treatment.
Researchers found they could divide patients into risk groups according to their methylation levels.
Patients with the highest methylation levels had the worst event-free survival, while patients who recovered spontaneously had methylation levels similar to those of healthy control subjects.
“This data provides important information that will help clinicians decide how intensively and swiftly to treat their patients,” said Mignon Loh, MD, of Benioff Children’s Hospital, University of California, San Francisco.
She and her colleagues described this research in Nature Communications.
The team studied genome-wide DNA methylation levels in an initial group of 39 patients with JMML and then validated the results in a group of 40 JMML patients.
Statistical analysis revealed that patients fell into 3 clusters with high, intermediate, or low levels of DNA methylation. And patients’ methylation levels were associated with 4-year event-free survival.
In the initial cohort, 6% (1/15) of patients in the lowest methylation group had an event at 4 years, as did 45% (5/11) of patients with intermediate levels of methylation and 61% (8/13) of patients with the highest levels of methylation.
In the validation cohort, 8% (1/12) of patients in the lowest methylation group had an event at 4 years, as did 36% (4/11) of patients with intermediate levels of methylation and 76% (13/17) of patients with the highest levels of methylation.
“For us, this was surprising,” said study author Elliot Stieglitz, MD, also of Benioff Children’s Hospital.
“We are not yet able to say why DNA methylation is different amongst these patients, but for it to be so predictive of outcomes, even more than genetic mutations, was a big surprise.”
The researchers also found that methylation levels might predict spontaneous remission as well.
Thirteen of the 14 patients who had spontaneous remission clustered together. And their DNA methylation levels were closer to those of healthy control subjects than those of other JMML cases.
“Because we have never been able to tell which patients might spontaneously recover, and because the disease can be so aggressive, the standard of care has been to recommend transplants for everybody,” Dr Stieglitz said.
“There are many health risks associated with bone marrow transplants, ranging from infections to long-term short stature and even death in some cases. To be able to avoid this intensive intervention for some patients based on a methylation assay would really be cutting-edge clinical science.” ![]()
New research suggests DNA methylation may be used to predict how children with juvenile myelomonocytic leukemia (JMML) will respond to treatment.
Researchers found they could divide patients into risk groups according to their methylation levels.
Patients with the highest methylation levels had the worst event-free survival, while patients who recovered spontaneously had methylation levels similar to those of healthy control subjects.
“This data provides important information that will help clinicians decide how intensively and swiftly to treat their patients,” said Mignon Loh, MD, of Benioff Children’s Hospital, University of California, San Francisco.
She and her colleagues described this research in Nature Communications.
The team studied genome-wide DNA methylation levels in an initial group of 39 patients with JMML and then validated the results in a group of 40 JMML patients.
Statistical analysis revealed that patients fell into 3 clusters with high, intermediate, or low levels of DNA methylation. And patients’ methylation levels were associated with 4-year event-free survival.
In the initial cohort, 6% (1/15) of patients in the lowest methylation group had an event at 4 years, as did 45% (5/11) of patients with intermediate levels of methylation and 61% (8/13) of patients with the highest levels of methylation.
In the validation cohort, 8% (1/12) of patients in the lowest methylation group had an event at 4 years, as did 36% (4/11) of patients with intermediate levels of methylation and 76% (13/17) of patients with the highest levels of methylation.
“For us, this was surprising,” said study author Elliot Stieglitz, MD, also of Benioff Children’s Hospital.
“We are not yet able to say why DNA methylation is different amongst these patients, but for it to be so predictive of outcomes, even more than genetic mutations, was a big surprise.”
The researchers also found that methylation levels might predict spontaneous remission as well.
Thirteen of the 14 patients who had spontaneous remission clustered together. And their DNA methylation levels were closer to those of healthy control subjects than those of other JMML cases.
“Because we have never been able to tell which patients might spontaneously recover, and because the disease can be so aggressive, the standard of care has been to recommend transplants for everybody,” Dr Stieglitz said.
“There are many health risks associated with bone marrow transplants, ranging from infections to long-term short stature and even death in some cases. To be able to avoid this intensive intervention for some patients based on a methylation assay would really be cutting-edge clinical science.” ![]()
Team develops new scoring systems for PMF
ATLANTA—Two novel prognostic scoring systems can help clinicians decide how to treat certain patients with primary myelofibrosis (PMF), according to a new study.
The scoring systems, which build upon the International Prognostic Scoring System (IPSS), were developed for use in PMF patients age 70 and younger who are potential candidates for hematopoietic stem cell transplant (HSCT).
One of the scoring systems—MIPSS70—integrates clinical, histologic, and molecular information. The other—MIPSS70-plus—also includes cytogenetic information.
Alessandro M. Vannucchi, MD, of the University of Florence in Italy, presented details on these prognostic scoring systems at the 2017 ASH Annual Meeting (abstract 200*).
The information was published simultaneously in the Journal of Clinical Oncology.
Dr Vannucchi noted that, in PMF, survival is currently predicted by the IPSS, the dynamic IPSS, and the dynamic IPSS-plus.
“The IPSS score is used at the time of diagnosis, while the dynamic IPSS or dynamic IPSS-plus are used to provide survival estimates at the time of patient referral,” he explained. “In clinical practice, these prognostic risk scores are mainly used for [HSCT] decision-making in younger patients.”
Driver mutations and other myeloid neoplasm-associated mutations provide prognostic information that is independent of the IPSS/dynamic IPSS/dynamic IPSS-plus scoring systems.
The degree of bone marrow fibrosis and cytogenetic abnormalities configuring an unfavorable category also contribute prognostic information that is independent of these scoring systems.
With this in mind, Dr Vannucchi and his colleagues set out to develop an updated prognostic score that included molecular information (MIPSS70) and, if possible, cytogenetic information (MIPSS70-plus) for PMF patients age 70 and younger who are potential candidates for HSCT.
The researchers developed 2 prognostic models using a training/validation cohort approach.
For MIPSS70, the training cohort included 490 patients from 6 Italian institutions associated with the Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative project (AGIMM group), and the validation cohort included 211 patients from the Mayo Clinic in Rochester, Minnesota.
For MIPSS70-plus, the training cohort included 315 patients from the Mayo Clinic, and the validation cohort included 261 patients from the AGIMM group.
Using the MIPSS70 risk score in the validation cohort, the 5-year overall survival rate was 96% in low-risk, 67% in intermediate-risk, and 34% in high-risk patients.
“MIPSS70 performed better than IPSS in predicting survival,” Dr Vannucchi said. “About 30% of patients who were high-risk with MIPPS70 were missed by IPSS.”
Using the MIPSS70-plus risk score in the validation cohort, the 5-year overall survival rate was 100% in low-risk, 90% in intermediate-risk, 76% in high-risk, and 46.5% in very high-risk patients.
The MIPSS70-plus risk score also identified patients at very high risk for leukemic transformation, Dr Vannucchi said.
Furthermore, both MIPSS70 and MIPSS70-plus remained predictive of survival when the researchers evaluated patients older than 70 years of age.
“The new MIPSS70 and MIPSS70-plus scores include modern disease-associated risk variables pertinent to both pre-PMF and overt-PMF according to the 2016 WHO classification,” Dr Vannucchi said. “They integrate prognostically relevant clinical, cytogenetic, and mutation data and provide complementary systems of improved risk stratification for transplantation-age patients with PMF.”
Dr Vannucchi disclosed membership in speaker’s bureaus with Gilead, Shire, and Novartis, and research funding and membership on Board of Directors or advisory committees with Novartis. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Two novel prognostic scoring systems can help clinicians decide how to treat certain patients with primary myelofibrosis (PMF), according to a new study.
The scoring systems, which build upon the International Prognostic Scoring System (IPSS), were developed for use in PMF patients age 70 and younger who are potential candidates for hematopoietic stem cell transplant (HSCT).
One of the scoring systems—MIPSS70—integrates clinical, histologic, and molecular information. The other—MIPSS70-plus—also includes cytogenetic information.
Alessandro M. Vannucchi, MD, of the University of Florence in Italy, presented details on these prognostic scoring systems at the 2017 ASH Annual Meeting (abstract 200*).
The information was published simultaneously in the Journal of Clinical Oncology.
Dr Vannucchi noted that, in PMF, survival is currently predicted by the IPSS, the dynamic IPSS, and the dynamic IPSS-plus.
“The IPSS score is used at the time of diagnosis, while the dynamic IPSS or dynamic IPSS-plus are used to provide survival estimates at the time of patient referral,” he explained. “In clinical practice, these prognostic risk scores are mainly used for [HSCT] decision-making in younger patients.”
Driver mutations and other myeloid neoplasm-associated mutations provide prognostic information that is independent of the IPSS/dynamic IPSS/dynamic IPSS-plus scoring systems.
The degree of bone marrow fibrosis and cytogenetic abnormalities configuring an unfavorable category also contribute prognostic information that is independent of these scoring systems.
With this in mind, Dr Vannucchi and his colleagues set out to develop an updated prognostic score that included molecular information (MIPSS70) and, if possible, cytogenetic information (MIPSS70-plus) for PMF patients age 70 and younger who are potential candidates for HSCT.
The researchers developed 2 prognostic models using a training/validation cohort approach.
For MIPSS70, the training cohort included 490 patients from 6 Italian institutions associated with the Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative project (AGIMM group), and the validation cohort included 211 patients from the Mayo Clinic in Rochester, Minnesota.
For MIPSS70-plus, the training cohort included 315 patients from the Mayo Clinic, and the validation cohort included 261 patients from the AGIMM group.
Using the MIPSS70 risk score in the validation cohort, the 5-year overall survival rate was 96% in low-risk, 67% in intermediate-risk, and 34% in high-risk patients.
“MIPSS70 performed better than IPSS in predicting survival,” Dr Vannucchi said. “About 30% of patients who were high-risk with MIPPS70 were missed by IPSS.”
Using the MIPSS70-plus risk score in the validation cohort, the 5-year overall survival rate was 100% in low-risk, 90% in intermediate-risk, 76% in high-risk, and 46.5% in very high-risk patients.
The MIPSS70-plus risk score also identified patients at very high risk for leukemic transformation, Dr Vannucchi said.
Furthermore, both MIPSS70 and MIPSS70-plus remained predictive of survival when the researchers evaluated patients older than 70 years of age.
“The new MIPSS70 and MIPSS70-plus scores include modern disease-associated risk variables pertinent to both pre-PMF and overt-PMF according to the 2016 WHO classification,” Dr Vannucchi said. “They integrate prognostically relevant clinical, cytogenetic, and mutation data and provide complementary systems of improved risk stratification for transplantation-age patients with PMF.”
Dr Vannucchi disclosed membership in speaker’s bureaus with Gilead, Shire, and Novartis, and research funding and membership on Board of Directors or advisory committees with Novartis. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Two novel prognostic scoring systems can help clinicians decide how to treat certain patients with primary myelofibrosis (PMF), according to a new study.
The scoring systems, which build upon the International Prognostic Scoring System (IPSS), were developed for use in PMF patients age 70 and younger who are potential candidates for hematopoietic stem cell transplant (HSCT).
One of the scoring systems—MIPSS70—integrates clinical, histologic, and molecular information. The other—MIPSS70-plus—also includes cytogenetic information.
Alessandro M. Vannucchi, MD, of the University of Florence in Italy, presented details on these prognostic scoring systems at the 2017 ASH Annual Meeting (abstract 200*).
The information was published simultaneously in the Journal of Clinical Oncology.
Dr Vannucchi noted that, in PMF, survival is currently predicted by the IPSS, the dynamic IPSS, and the dynamic IPSS-plus.
“The IPSS score is used at the time of diagnosis, while the dynamic IPSS or dynamic IPSS-plus are used to provide survival estimates at the time of patient referral,” he explained. “In clinical practice, these prognostic risk scores are mainly used for [HSCT] decision-making in younger patients.”
Driver mutations and other myeloid neoplasm-associated mutations provide prognostic information that is independent of the IPSS/dynamic IPSS/dynamic IPSS-plus scoring systems.
The degree of bone marrow fibrosis and cytogenetic abnormalities configuring an unfavorable category also contribute prognostic information that is independent of these scoring systems.
With this in mind, Dr Vannucchi and his colleagues set out to develop an updated prognostic score that included molecular information (MIPSS70) and, if possible, cytogenetic information (MIPSS70-plus) for PMF patients age 70 and younger who are potential candidates for HSCT.
The researchers developed 2 prognostic models using a training/validation cohort approach.
For MIPSS70, the training cohort included 490 patients from 6 Italian institutions associated with the Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative project (AGIMM group), and the validation cohort included 211 patients from the Mayo Clinic in Rochester, Minnesota.
For MIPSS70-plus, the training cohort included 315 patients from the Mayo Clinic, and the validation cohort included 261 patients from the AGIMM group.
Using the MIPSS70 risk score in the validation cohort, the 5-year overall survival rate was 96% in low-risk, 67% in intermediate-risk, and 34% in high-risk patients.
“MIPSS70 performed better than IPSS in predicting survival,” Dr Vannucchi said. “About 30% of patients who were high-risk with MIPPS70 were missed by IPSS.”
Using the MIPSS70-plus risk score in the validation cohort, the 5-year overall survival rate was 100% in low-risk, 90% in intermediate-risk, 76% in high-risk, and 46.5% in very high-risk patients.
The MIPSS70-plus risk score also identified patients at very high risk for leukemic transformation, Dr Vannucchi said.
Furthermore, both MIPSS70 and MIPSS70-plus remained predictive of survival when the researchers evaluated patients older than 70 years of age.
“The new MIPSS70 and MIPSS70-plus scores include modern disease-associated risk variables pertinent to both pre-PMF and overt-PMF according to the 2016 WHO classification,” Dr Vannucchi said. “They integrate prognostically relevant clinical, cytogenetic, and mutation data and provide complementary systems of improved risk stratification for transplantation-age patients with PMF.”
Dr Vannucchi disclosed membership in speaker’s bureaus with Gilead, Shire, and Novartis, and research funding and membership on Board of Directors or advisory committees with Novartis. ![]()
*Data in the presentation differ from the abstract.