User login
Thyroid surgery access and acceptance varies along racial lines
BOSTON – Access to and acceptance of thyroid cancer surgery varies by race, with black patients in particular appearing to be disadvantaged, compared with whites, investigators reported.
A review of data on nearly 138,000 patients diagnosed with thyroid cancer showed that blacks were significantly less likely than were whites to be offered surgery – despite its generally excellent outcomes and low rates of morbidity and mortality, reported Dr. Herbert Castillo Valladares and his colleagues from the department of surgery at the Yale University in New Haven, Conn.
American Indians/Alaskan natives and Asian/Pacific Islanders were significantly more likely to refuse surgery than were whites, the investigators also reported in a poster session at the Society of Surgical Oncology annual cancer symposium.
“In this project, we wanted to focus on the provider-level factors that might be perpetuating these racial disparities, and it appears that we need to educate some providers about the recommendation of surgery or how to educate patients who refuse thyroid cancer surgery,” Dr. Valladares said in an interview.
The investigators noted that although incidence and prevalence rates of thyroid cancer are similar among various racial groups, survival differs by race, and they wanted to find out why. To do so, they polled the Surveillance, Epidemiology, and End Results (SEER) registry to identify 137,483 patients diagnosed with thyroid cancer during 1988-2012. Results were stratified by thyroid cancer type, either papillary, medullary, follicular, or anaplastic.
In all, 82% of the sample were white, 75% were female, 87% had a diagnosis of papillary thyroid cancer, and 95% underwent thyroid cancer surgery.
In logistic regression analysis that controlled for race, the investigators found that blacks, Asian/Pacific Islanders, and persons of unknown race were significantly less likely than whites were to have thyroid cancer surgery (odds ratios, 0.7, 0.82, and 0.34, respectively; P for each less than .0001).
Similarly, surgery was more frequently not recommended for blacks (OR, 1.34; P less than .0001), Asian/Pacific Islanders (OR, 1.2; P = .004) and those of unknown race (OR, 3.06; P less than .0001).
American Indians/Alaskan natives and Asian/Pacific Islanders were also significantly more likely than were whites to refuse surgery (OR, 4.45; P = .0001; OR, 2.96; P less than .0001, respectively).
Compared with whites, blacks – but not other races – had significantly worse 5-year survival (hazard ratio, 1.14; P = .0002).
In an analysis by cancer type, the investigators saw that race was not a predictor for surgery recommendation or refusal of surgery by patients with medullary or anaplastic cancer. However, among patients with papillary thyroid cancer, the most common type, surgery was recommended less often for blacks (OR, 1.2), Asian/Pacific Islanders (OR, 1.3), and patients of unknown race (OR, 3.1; all comparisons significant by 95% confidence interval).
Among patients with follicular histology, patients of unknown race were significantly less likely than were whites to have the surgery recommended (OR, 2.7; significant by 95% CI).
Dr. Valladares explained that the SEER data set does not include information about provider type, such as those in community based versus academic settings, so the next step will be to find a method for analyzing factors at both the patient level and the provider level that might influence recommendations for surgery or patient refusals to accept surgery.
The study was supported by the Paul H. Lavietes, M.D., Summer Research Fellowship of Yale University. The investigators reported no relevant conflicts of interest.
BOSTON – Access to and acceptance of thyroid cancer surgery varies by race, with black patients in particular appearing to be disadvantaged, compared with whites, investigators reported.
A review of data on nearly 138,000 patients diagnosed with thyroid cancer showed that blacks were significantly less likely than were whites to be offered surgery – despite its generally excellent outcomes and low rates of morbidity and mortality, reported Dr. Herbert Castillo Valladares and his colleagues from the department of surgery at the Yale University in New Haven, Conn.
American Indians/Alaskan natives and Asian/Pacific Islanders were significantly more likely to refuse surgery than were whites, the investigators also reported in a poster session at the Society of Surgical Oncology annual cancer symposium.
“In this project, we wanted to focus on the provider-level factors that might be perpetuating these racial disparities, and it appears that we need to educate some providers about the recommendation of surgery or how to educate patients who refuse thyroid cancer surgery,” Dr. Valladares said in an interview.
The investigators noted that although incidence and prevalence rates of thyroid cancer are similar among various racial groups, survival differs by race, and they wanted to find out why. To do so, they polled the Surveillance, Epidemiology, and End Results (SEER) registry to identify 137,483 patients diagnosed with thyroid cancer during 1988-2012. Results were stratified by thyroid cancer type, either papillary, medullary, follicular, or anaplastic.
In all, 82% of the sample were white, 75% were female, 87% had a diagnosis of papillary thyroid cancer, and 95% underwent thyroid cancer surgery.
In logistic regression analysis that controlled for race, the investigators found that blacks, Asian/Pacific Islanders, and persons of unknown race were significantly less likely than whites were to have thyroid cancer surgery (odds ratios, 0.7, 0.82, and 0.34, respectively; P for each less than .0001).
Similarly, surgery was more frequently not recommended for blacks (OR, 1.34; P less than .0001), Asian/Pacific Islanders (OR, 1.2; P = .004) and those of unknown race (OR, 3.06; P less than .0001).
American Indians/Alaskan natives and Asian/Pacific Islanders were also significantly more likely than were whites to refuse surgery (OR, 4.45; P = .0001; OR, 2.96; P less than .0001, respectively).
Compared with whites, blacks – but not other races – had significantly worse 5-year survival (hazard ratio, 1.14; P = .0002).
In an analysis by cancer type, the investigators saw that race was not a predictor for surgery recommendation or refusal of surgery by patients with medullary or anaplastic cancer. However, among patients with papillary thyroid cancer, the most common type, surgery was recommended less often for blacks (OR, 1.2), Asian/Pacific Islanders (OR, 1.3), and patients of unknown race (OR, 3.1; all comparisons significant by 95% confidence interval).
Among patients with follicular histology, patients of unknown race were significantly less likely than were whites to have the surgery recommended (OR, 2.7; significant by 95% CI).
Dr. Valladares explained that the SEER data set does not include information about provider type, such as those in community based versus academic settings, so the next step will be to find a method for analyzing factors at both the patient level and the provider level that might influence recommendations for surgery or patient refusals to accept surgery.
The study was supported by the Paul H. Lavietes, M.D., Summer Research Fellowship of Yale University. The investigators reported no relevant conflicts of interest.
BOSTON – Access to and acceptance of thyroid cancer surgery varies by race, with black patients in particular appearing to be disadvantaged, compared with whites, investigators reported.
A review of data on nearly 138,000 patients diagnosed with thyroid cancer showed that blacks were significantly less likely than were whites to be offered surgery – despite its generally excellent outcomes and low rates of morbidity and mortality, reported Dr. Herbert Castillo Valladares and his colleagues from the department of surgery at the Yale University in New Haven, Conn.
American Indians/Alaskan natives and Asian/Pacific Islanders were significantly more likely to refuse surgery than were whites, the investigators also reported in a poster session at the Society of Surgical Oncology annual cancer symposium.
“In this project, we wanted to focus on the provider-level factors that might be perpetuating these racial disparities, and it appears that we need to educate some providers about the recommendation of surgery or how to educate patients who refuse thyroid cancer surgery,” Dr. Valladares said in an interview.
The investigators noted that although incidence and prevalence rates of thyroid cancer are similar among various racial groups, survival differs by race, and they wanted to find out why. To do so, they polled the Surveillance, Epidemiology, and End Results (SEER) registry to identify 137,483 patients diagnosed with thyroid cancer during 1988-2012. Results were stratified by thyroid cancer type, either papillary, medullary, follicular, or anaplastic.
In all, 82% of the sample were white, 75% were female, 87% had a diagnosis of papillary thyroid cancer, and 95% underwent thyroid cancer surgery.
In logistic regression analysis that controlled for race, the investigators found that blacks, Asian/Pacific Islanders, and persons of unknown race were significantly less likely than whites were to have thyroid cancer surgery (odds ratios, 0.7, 0.82, and 0.34, respectively; P for each less than .0001).
Similarly, surgery was more frequently not recommended for blacks (OR, 1.34; P less than .0001), Asian/Pacific Islanders (OR, 1.2; P = .004) and those of unknown race (OR, 3.06; P less than .0001).
American Indians/Alaskan natives and Asian/Pacific Islanders were also significantly more likely than were whites to refuse surgery (OR, 4.45; P = .0001; OR, 2.96; P less than .0001, respectively).
Compared with whites, blacks – but not other races – had significantly worse 5-year survival (hazard ratio, 1.14; P = .0002).
In an analysis by cancer type, the investigators saw that race was not a predictor for surgery recommendation or refusal of surgery by patients with medullary or anaplastic cancer. However, among patients with papillary thyroid cancer, the most common type, surgery was recommended less often for blacks (OR, 1.2), Asian/Pacific Islanders (OR, 1.3), and patients of unknown race (OR, 3.1; all comparisons significant by 95% confidence interval).
Among patients with follicular histology, patients of unknown race were significantly less likely than were whites to have the surgery recommended (OR, 2.7; significant by 95% CI).
Dr. Valladares explained that the SEER data set does not include information about provider type, such as those in community based versus academic settings, so the next step will be to find a method for analyzing factors at both the patient level and the provider level that might influence recommendations for surgery or patient refusals to accept surgery.
The study was supported by the Paul H. Lavietes, M.D., Summer Research Fellowship of Yale University. The investigators reported no relevant conflicts of interest.
FROM SSO 2016
Key clinical point: Compared with whites, blacks, Asian/Pacific Islanders and persons of unknown race were significantly less likely than were whites to have thyroid cancer surgery.
Major finding: Asian/Pacific Islanders and persons of unknown race were significantly less likely than were whites to have thyroid cancer surgery (OR, 0.7, 0.82, and 0.34, respectively; P for each less than .0001).
Data source: SEER data on 137,483 patients with thyroid cancer during 1988-2012.
Disclosures: The study was supported by a Paul H. Lavietes, M.D., Summer Research Fellowship at Yale University. The investigators reported no relevant conflicts of interest.
Serious complications after cancer surgery linked to worse long-term survival
BOSTON – The operation was a success, but the patient died.
It’s an old chestnut for sure, but there is a painful kernel of truth in it, say investigators who found that patients who undergo complex cancer surgery and have serious complications are at significantly increased risk for death for at least 6 months after surgery, compared with patients who undergo the same procedure with few or no complications.
“Our work has important implications for quality assessment. I think in cancer surgery in particular we have to get away from the short-term metrics of survival, and we have to think about the implications of complications for long-term survival, even if at a very high-quality hospital we’re good at salvaging those patients who do experience those complications,” said Dr. Hari Nathan of the University of Michigan, Ann Arbor.
In a retrospective study, results of which were presented at the annual Society of Surgical Oncology Cancer Symposium, Dr. Nathan and colleagues showed that patients who underwent surgery for cancers of the esophagus and lung who had serious complications but survived at least 30 days after surgery had a more than twofold greater risk for death than did patients who had no complications, and patients with serious complications following surgery for cancer of the pancreas had a nearly twofold greater risk.
The effects of serious complications on survival persisted out to at least 180 days after surgery for each of the three procedures.
The findings suggest that just getting the patient through the operation and keeping him or her alive in the ICU is not sufficient cause for celebration by surgeons, Dr. Nathan said.
The investigators conducted the study to examine the incidence of complications following cancer surgery in older patients, the relationship between surgical complications and long-term survival, and whether the effects of complications would diminish or “wash out” over time. They reviewed Surveillance, Epidemiology and End Results–Medicare data on patients aged 65 years and older who underwent surgery with curative intent for esophageal cancer, non–small cell lung cancer, or pancreatic adenocarcinoma from 2005 through 2009.
They defined serious complications as “the appearance of a complication associated with a hospital length of stay greater than the 75th percentile for that procedure.”
The cohort included 965 patients who underwent esophageal surgery, 12,395 who had lung surgery, and 1,966 who underwent pancreatic resection. The proportion of patients over 80 years who underwent the procedures, respectively, were 12%, 18%, and 19%.
Serious complications occurred in 17% of patients with esophageal cancer, 10% of those with lung cancer, and 12% of those with cancer of the pancreas. The respective 30-day mortality rates were 6.%, 3.3%, and 3.9%.
Looking only at those patients with lung cancer who survived at least 30 days after surgery, the investigators found that median survival among those who had no complications was 79 months, compared with 60 months for those who had mild complications, and 33 months for patients who had serious complications (P less than .001)
“And indeed, when we performed adjusted survival analyses looking at all three disease sites, we saw a very consistent story: that those patients who had serious complications had decreased long-term survival for all three malignancies we looked at,” Dr. Nathan said.
Specifically, in survival analyses adjusted for sex, age, and procedure code, hazard ratios for patients with serious complications compared with those who had no complications were 2.55 for esophageal cancer patients, 2.13 for lung cancer patients, and 1.57 for pancreatic cancer patients (all comparisons significant as shown by 95% confidence intervals).
The investigators questioned whether the differences in mortality were due to the late effects of perioperative complications.
“In modern ICUs, we can keep virtually anybody alive for 30 days, and there has been a lot interest in longer-term metrics for perioperative mortality, for example, at 30 or 90 days, so we thought maybe that’s what we were seeing here,” he said. To test this idea, the investigators looked at the effects of complications on patient who survived lung cancer surgery for at least 90 days, and those who lived for at least 180 days after surgery, and they saw that the survival curves were similar to those seen with the 30-day survivors, showing significantly and persistently worse survival for patients with serious complications (P less than .001).
For each of the disease states, patients with serious complications were also significantly less likely than were those with no or mild complications to receive adjuvant chemotherapy, even after adjustment for patient age and cancer stage, two significant determinants of the likelihood of receiving chemotherapy.
And even when the effect of chemotherapy for those who did receive it was added into the survival models, patients with serious complications still had significantly worse overall survival, Dr. Nathan noted.
“Serious complications after these three cancer resections are common and they are associated with dramatically inferior long-term survival. Thirty, 60, 90, and even 180-day measures of mortality do not capture the full impact of complications on long-term survival,” he said.
Asked whether it may be possible to identify those patients at higher risk for serious complications due to comorbidities or other factors, and perhaps suggest withholding surgery from such patients, Dr. Nathan agreed, but added that “the best chance for survival for all of these patients is a high-quality surgical resection, so it’s hard to deny a patient that chance unless you think they have a really high risk of perioperative death.”
The study was internally funded. Dr. Nathan reported no significant disclosures.
BOSTON – The operation was a success, but the patient died.
It’s an old chestnut for sure, but there is a painful kernel of truth in it, say investigators who found that patients who undergo complex cancer surgery and have serious complications are at significantly increased risk for death for at least 6 months after surgery, compared with patients who undergo the same procedure with few or no complications.
“Our work has important implications for quality assessment. I think in cancer surgery in particular we have to get away from the short-term metrics of survival, and we have to think about the implications of complications for long-term survival, even if at a very high-quality hospital we’re good at salvaging those patients who do experience those complications,” said Dr. Hari Nathan of the University of Michigan, Ann Arbor.
In a retrospective study, results of which were presented at the annual Society of Surgical Oncology Cancer Symposium, Dr. Nathan and colleagues showed that patients who underwent surgery for cancers of the esophagus and lung who had serious complications but survived at least 30 days after surgery had a more than twofold greater risk for death than did patients who had no complications, and patients with serious complications following surgery for cancer of the pancreas had a nearly twofold greater risk.
The effects of serious complications on survival persisted out to at least 180 days after surgery for each of the three procedures.
The findings suggest that just getting the patient through the operation and keeping him or her alive in the ICU is not sufficient cause for celebration by surgeons, Dr. Nathan said.
The investigators conducted the study to examine the incidence of complications following cancer surgery in older patients, the relationship between surgical complications and long-term survival, and whether the effects of complications would diminish or “wash out” over time. They reviewed Surveillance, Epidemiology and End Results–Medicare data on patients aged 65 years and older who underwent surgery with curative intent for esophageal cancer, non–small cell lung cancer, or pancreatic adenocarcinoma from 2005 through 2009.
They defined serious complications as “the appearance of a complication associated with a hospital length of stay greater than the 75th percentile for that procedure.”
The cohort included 965 patients who underwent esophageal surgery, 12,395 who had lung surgery, and 1,966 who underwent pancreatic resection. The proportion of patients over 80 years who underwent the procedures, respectively, were 12%, 18%, and 19%.
Serious complications occurred in 17% of patients with esophageal cancer, 10% of those with lung cancer, and 12% of those with cancer of the pancreas. The respective 30-day mortality rates were 6.%, 3.3%, and 3.9%.
Looking only at those patients with lung cancer who survived at least 30 days after surgery, the investigators found that median survival among those who had no complications was 79 months, compared with 60 months for those who had mild complications, and 33 months for patients who had serious complications (P less than .001)
“And indeed, when we performed adjusted survival analyses looking at all three disease sites, we saw a very consistent story: that those patients who had serious complications had decreased long-term survival for all three malignancies we looked at,” Dr. Nathan said.
Specifically, in survival analyses adjusted for sex, age, and procedure code, hazard ratios for patients with serious complications compared with those who had no complications were 2.55 for esophageal cancer patients, 2.13 for lung cancer patients, and 1.57 for pancreatic cancer patients (all comparisons significant as shown by 95% confidence intervals).
The investigators questioned whether the differences in mortality were due to the late effects of perioperative complications.
“In modern ICUs, we can keep virtually anybody alive for 30 days, and there has been a lot interest in longer-term metrics for perioperative mortality, for example, at 30 or 90 days, so we thought maybe that’s what we were seeing here,” he said. To test this idea, the investigators looked at the effects of complications on patient who survived lung cancer surgery for at least 90 days, and those who lived for at least 180 days after surgery, and they saw that the survival curves were similar to those seen with the 30-day survivors, showing significantly and persistently worse survival for patients with serious complications (P less than .001).
For each of the disease states, patients with serious complications were also significantly less likely than were those with no or mild complications to receive adjuvant chemotherapy, even after adjustment for patient age and cancer stage, two significant determinants of the likelihood of receiving chemotherapy.
And even when the effect of chemotherapy for those who did receive it was added into the survival models, patients with serious complications still had significantly worse overall survival, Dr. Nathan noted.
“Serious complications after these three cancer resections are common and they are associated with dramatically inferior long-term survival. Thirty, 60, 90, and even 180-day measures of mortality do not capture the full impact of complications on long-term survival,” he said.
Asked whether it may be possible to identify those patients at higher risk for serious complications due to comorbidities or other factors, and perhaps suggest withholding surgery from such patients, Dr. Nathan agreed, but added that “the best chance for survival for all of these patients is a high-quality surgical resection, so it’s hard to deny a patient that chance unless you think they have a really high risk of perioperative death.”
The study was internally funded. Dr. Nathan reported no significant disclosures.
BOSTON – The operation was a success, but the patient died.
It’s an old chestnut for sure, but there is a painful kernel of truth in it, say investigators who found that patients who undergo complex cancer surgery and have serious complications are at significantly increased risk for death for at least 6 months after surgery, compared with patients who undergo the same procedure with few or no complications.
“Our work has important implications for quality assessment. I think in cancer surgery in particular we have to get away from the short-term metrics of survival, and we have to think about the implications of complications for long-term survival, even if at a very high-quality hospital we’re good at salvaging those patients who do experience those complications,” said Dr. Hari Nathan of the University of Michigan, Ann Arbor.
In a retrospective study, results of which were presented at the annual Society of Surgical Oncology Cancer Symposium, Dr. Nathan and colleagues showed that patients who underwent surgery for cancers of the esophagus and lung who had serious complications but survived at least 30 days after surgery had a more than twofold greater risk for death than did patients who had no complications, and patients with serious complications following surgery for cancer of the pancreas had a nearly twofold greater risk.
The effects of serious complications on survival persisted out to at least 180 days after surgery for each of the three procedures.
The findings suggest that just getting the patient through the operation and keeping him or her alive in the ICU is not sufficient cause for celebration by surgeons, Dr. Nathan said.
The investigators conducted the study to examine the incidence of complications following cancer surgery in older patients, the relationship between surgical complications and long-term survival, and whether the effects of complications would diminish or “wash out” over time. They reviewed Surveillance, Epidemiology and End Results–Medicare data on patients aged 65 years and older who underwent surgery with curative intent for esophageal cancer, non–small cell lung cancer, or pancreatic adenocarcinoma from 2005 through 2009.
They defined serious complications as “the appearance of a complication associated with a hospital length of stay greater than the 75th percentile for that procedure.”
The cohort included 965 patients who underwent esophageal surgery, 12,395 who had lung surgery, and 1,966 who underwent pancreatic resection. The proportion of patients over 80 years who underwent the procedures, respectively, were 12%, 18%, and 19%.
Serious complications occurred in 17% of patients with esophageal cancer, 10% of those with lung cancer, and 12% of those with cancer of the pancreas. The respective 30-day mortality rates were 6.%, 3.3%, and 3.9%.
Looking only at those patients with lung cancer who survived at least 30 days after surgery, the investigators found that median survival among those who had no complications was 79 months, compared with 60 months for those who had mild complications, and 33 months for patients who had serious complications (P less than .001)
“And indeed, when we performed adjusted survival analyses looking at all three disease sites, we saw a very consistent story: that those patients who had serious complications had decreased long-term survival for all three malignancies we looked at,” Dr. Nathan said.
Specifically, in survival analyses adjusted for sex, age, and procedure code, hazard ratios for patients with serious complications compared with those who had no complications were 2.55 for esophageal cancer patients, 2.13 for lung cancer patients, and 1.57 for pancreatic cancer patients (all comparisons significant as shown by 95% confidence intervals).
The investigators questioned whether the differences in mortality were due to the late effects of perioperative complications.
“In modern ICUs, we can keep virtually anybody alive for 30 days, and there has been a lot interest in longer-term metrics for perioperative mortality, for example, at 30 or 90 days, so we thought maybe that’s what we were seeing here,” he said. To test this idea, the investigators looked at the effects of complications on patient who survived lung cancer surgery for at least 90 days, and those who lived for at least 180 days after surgery, and they saw that the survival curves were similar to those seen with the 30-day survivors, showing significantly and persistently worse survival for patients with serious complications (P less than .001).
For each of the disease states, patients with serious complications were also significantly less likely than were those with no or mild complications to receive adjuvant chemotherapy, even after adjustment for patient age and cancer stage, two significant determinants of the likelihood of receiving chemotherapy.
And even when the effect of chemotherapy for those who did receive it was added into the survival models, patients with serious complications still had significantly worse overall survival, Dr. Nathan noted.
“Serious complications after these three cancer resections are common and they are associated with dramatically inferior long-term survival. Thirty, 60, 90, and even 180-day measures of mortality do not capture the full impact of complications on long-term survival,” he said.
Asked whether it may be possible to identify those patients at higher risk for serious complications due to comorbidities or other factors, and perhaps suggest withholding surgery from such patients, Dr. Nathan agreed, but added that “the best chance for survival for all of these patients is a high-quality surgical resection, so it’s hard to deny a patient that chance unless you think they have a really high risk of perioperative death.”
The study was internally funded. Dr. Nathan reported no significant disclosures.
AT SSO 2016
Key clinical point: Thirty-day postoperative survival may not be an adequate measure of success of complex cancer surgeries.
Major finding: Patients with serious complications from esophageal, lung, and pancreatic cancer operations had significantly worse survival out to 180 days ,compared with those with mild or no complications.
Data source: Retrospective review of SEER-Medicare data from 2005-2009.
Disclosures: The study was internally funded. Dr. Nathan reported no significant disclosures.
NSQIP calculator shown inadequate to stratify risk in stage I non–small cell lung cancer.
A study performed to validate the National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator for use in patients receiving surgery or stereotactic body radiation therapy (SBRT) for stage I non–small cell lung cancer showed the calculator to be inadequate for both classification and risk stratification. The study was reported in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151;697-705).
Dr. Pamela Samson of Washington University in St. Louis and her colleagues performed a retrospective analysis of 485 patients with clinical stage I NSCLC who underwent either surgery (277) or SBRT (195) from 2009 to 2012. Surgery was either wedge resection (19.3%) or lobectomy (74.5%), with smaller percentages receiving segmentectomy (4.0%), pneumonectomy (1.5%), and bilobectomy (0.7%). A large majority of surgical patients (84.1%) underwent a video-assisted thoracoscopic surgery (VATS) approach.
The researchers calculated NSQIP complication risk estimates for both surgical and SBRT patients using the NSQIP Surgical Risk Calculator. They compared predicted risk with actual adverse events.
Compared with patients undergoing VATS wedge resection, patients receiving SBRT were older, had larger tumors, lower forced expiratory volume (FEV1) and diffusing capacity of the lungs for carbon monoxide (DLCO), higher American Society of Anesthesiologist scores, higher rates of dyspnea and higher NSQIP serious complication risk estimates, all significant at P less than .05. Similar disparities were seen in comparing patients receiving SBRT vs. VATS lobectomy.
The actual serious complication rate for surgical patients was significantly higher than the NSQIP risk calculator prediction (16.6% vs. 8.8%), as was the rate of pneumonia (6.0% vs. 3.2%), both at P less than .05.
Overall, the NSQIP Surgical Risk Calculator provided a fair level of discrimination between VATS lobectomy and SBRT on receiver operating characteristic (ROC) curve analysis, but it was a poor model for differentiating between VATS wedge resection and SBRT. “Unfortunately, it is this latter population of the highest risk surgical patients (for whom a lobectomy is not a surgical option) where risk models and decision aids are needed most,” Dr. Samson and her colleagues stated.
“Counseling the high-risk but operable patient with clinical stage I NSCLC in regard to lobectomy, sublobar resection, or SBRT is challenging for both the clinician and the patient,” according to the researchers. “We believe that a model tailored to patients with clinical stage I needs to serve as both an estimator of operative risks and a patient decision aid for surgery versus SBRT, especially with projected increases in the number of early-stage lung cancers as a result of increased lung cancer screening efforts,” they added.
“Our analysis suggests that the NSQIP Surgical Risk Calculator likely does not profile the risk of a patient with lung cancer closely enough to dichotomize surgical and inoperable SBRT cases (especially when patients are being considered for a wedge resection) or adequately estimate a surgical patient’s risk of serious complications,” Dr. Samson and her colleagues concluded.
The study was supported by grants from National Institutes of Health. The authors had no relevant financial disclosures.
In their reported study, Dr. Samson and her colleagues found that the NSQIP tool underestimated morbidity. They also found that risk predicted by the NSQIP tool was not necessarily aligned with their institution’s actual treatment selection for stage I NSCLC, which they based upon a number of factors. “This study potentially has important clinical implications,” according to Dr. Xiaofei Wang and Dr. Mark F. Berry in their invited commentary (J Thorac Cardiovasc Surg. 2016 Mar;151:706-7). “This present study shows that even a robust, well-managed tool from the NSQIP does not adequately stratify surgical risk... Their analysis implies that the treatment decision made by the institutional clinicians is optimal.”
“The lackluster performance of the NSQIP score is understandable, because it was not designed to optimally differentiate patients who benefited most from surgery or SBRT. Randomized clinical trials or well-controlled prospective observations are needed to develop and validate specific predictive tools for optimal treatment selection. These models must consider not only treatment morbidity, but also the cost of possible recurrence with each therapy,” Dr. Wang and Dr. Berry stated.
“Perhaps the most important conclusion that can be drawn from this present study is that current risk assessment tools can be helpful, but cannot replace evaluation by clinicians for whom all management options are available when therapy is chosen for a specific patient,” they concluded.
Dr. Wang is from the department of biostatistics and bioinformatics at Duke University, Durham, N.C., and Dr. Berry is from the department of cardiothoracic surgery, Stanford University, Stanford, Calif. They had no relevant financial disclosures.
In their reported study, Dr. Samson and her colleagues found that the NSQIP tool underestimated morbidity. They also found that risk predicted by the NSQIP tool was not necessarily aligned with their institution’s actual treatment selection for stage I NSCLC, which they based upon a number of factors. “This study potentially has important clinical implications,” according to Dr. Xiaofei Wang and Dr. Mark F. Berry in their invited commentary (J Thorac Cardiovasc Surg. 2016 Mar;151:706-7). “This present study shows that even a robust, well-managed tool from the NSQIP does not adequately stratify surgical risk... Their analysis implies that the treatment decision made by the institutional clinicians is optimal.”
“The lackluster performance of the NSQIP score is understandable, because it was not designed to optimally differentiate patients who benefited most from surgery or SBRT. Randomized clinical trials or well-controlled prospective observations are needed to develop and validate specific predictive tools for optimal treatment selection. These models must consider not only treatment morbidity, but also the cost of possible recurrence with each therapy,” Dr. Wang and Dr. Berry stated.
“Perhaps the most important conclusion that can be drawn from this present study is that current risk assessment tools can be helpful, but cannot replace evaluation by clinicians for whom all management options are available when therapy is chosen for a specific patient,” they concluded.
Dr. Wang is from the department of biostatistics and bioinformatics at Duke University, Durham, N.C., and Dr. Berry is from the department of cardiothoracic surgery, Stanford University, Stanford, Calif. They had no relevant financial disclosures.
In their reported study, Dr. Samson and her colleagues found that the NSQIP tool underestimated morbidity. They also found that risk predicted by the NSQIP tool was not necessarily aligned with their institution’s actual treatment selection for stage I NSCLC, which they based upon a number of factors. “This study potentially has important clinical implications,” according to Dr. Xiaofei Wang and Dr. Mark F. Berry in their invited commentary (J Thorac Cardiovasc Surg. 2016 Mar;151:706-7). “This present study shows that even a robust, well-managed tool from the NSQIP does not adequately stratify surgical risk... Their analysis implies that the treatment decision made by the institutional clinicians is optimal.”
“The lackluster performance of the NSQIP score is understandable, because it was not designed to optimally differentiate patients who benefited most from surgery or SBRT. Randomized clinical trials or well-controlled prospective observations are needed to develop and validate specific predictive tools for optimal treatment selection. These models must consider not only treatment morbidity, but also the cost of possible recurrence with each therapy,” Dr. Wang and Dr. Berry stated.
“Perhaps the most important conclusion that can be drawn from this present study is that current risk assessment tools can be helpful, but cannot replace evaluation by clinicians for whom all management options are available when therapy is chosen for a specific patient,” they concluded.
Dr. Wang is from the department of biostatistics and bioinformatics at Duke University, Durham, N.C., and Dr. Berry is from the department of cardiothoracic surgery, Stanford University, Stanford, Calif. They had no relevant financial disclosures.
A study performed to validate the National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator for use in patients receiving surgery or stereotactic body radiation therapy (SBRT) for stage I non–small cell lung cancer showed the calculator to be inadequate for both classification and risk stratification. The study was reported in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151;697-705).
Dr. Pamela Samson of Washington University in St. Louis and her colleagues performed a retrospective analysis of 485 patients with clinical stage I NSCLC who underwent either surgery (277) or SBRT (195) from 2009 to 2012. Surgery was either wedge resection (19.3%) or lobectomy (74.5%), with smaller percentages receiving segmentectomy (4.0%), pneumonectomy (1.5%), and bilobectomy (0.7%). A large majority of surgical patients (84.1%) underwent a video-assisted thoracoscopic surgery (VATS) approach.
The researchers calculated NSQIP complication risk estimates for both surgical and SBRT patients using the NSQIP Surgical Risk Calculator. They compared predicted risk with actual adverse events.
Compared with patients undergoing VATS wedge resection, patients receiving SBRT were older, had larger tumors, lower forced expiratory volume (FEV1) and diffusing capacity of the lungs for carbon monoxide (DLCO), higher American Society of Anesthesiologist scores, higher rates of dyspnea and higher NSQIP serious complication risk estimates, all significant at P less than .05. Similar disparities were seen in comparing patients receiving SBRT vs. VATS lobectomy.
The actual serious complication rate for surgical patients was significantly higher than the NSQIP risk calculator prediction (16.6% vs. 8.8%), as was the rate of pneumonia (6.0% vs. 3.2%), both at P less than .05.
Overall, the NSQIP Surgical Risk Calculator provided a fair level of discrimination between VATS lobectomy and SBRT on receiver operating characteristic (ROC) curve analysis, but it was a poor model for differentiating between VATS wedge resection and SBRT. “Unfortunately, it is this latter population of the highest risk surgical patients (for whom a lobectomy is not a surgical option) where risk models and decision aids are needed most,” Dr. Samson and her colleagues stated.
“Counseling the high-risk but operable patient with clinical stage I NSCLC in regard to lobectomy, sublobar resection, or SBRT is challenging for both the clinician and the patient,” according to the researchers. “We believe that a model tailored to patients with clinical stage I needs to serve as both an estimator of operative risks and a patient decision aid for surgery versus SBRT, especially with projected increases in the number of early-stage lung cancers as a result of increased lung cancer screening efforts,” they added.
“Our analysis suggests that the NSQIP Surgical Risk Calculator likely does not profile the risk of a patient with lung cancer closely enough to dichotomize surgical and inoperable SBRT cases (especially when patients are being considered for a wedge resection) or adequately estimate a surgical patient’s risk of serious complications,” Dr. Samson and her colleagues concluded.
The study was supported by grants from National Institutes of Health. The authors had no relevant financial disclosures.
A study performed to validate the National Surgical Quality Improvement Program (NSQIP) Surgical Risk Calculator for use in patients receiving surgery or stereotactic body radiation therapy (SBRT) for stage I non–small cell lung cancer showed the calculator to be inadequate for both classification and risk stratification. The study was reported in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151;697-705).
Dr. Pamela Samson of Washington University in St. Louis and her colleagues performed a retrospective analysis of 485 patients with clinical stage I NSCLC who underwent either surgery (277) or SBRT (195) from 2009 to 2012. Surgery was either wedge resection (19.3%) or lobectomy (74.5%), with smaller percentages receiving segmentectomy (4.0%), pneumonectomy (1.5%), and bilobectomy (0.7%). A large majority of surgical patients (84.1%) underwent a video-assisted thoracoscopic surgery (VATS) approach.
The researchers calculated NSQIP complication risk estimates for both surgical and SBRT patients using the NSQIP Surgical Risk Calculator. They compared predicted risk with actual adverse events.
Compared with patients undergoing VATS wedge resection, patients receiving SBRT were older, had larger tumors, lower forced expiratory volume (FEV1) and diffusing capacity of the lungs for carbon monoxide (DLCO), higher American Society of Anesthesiologist scores, higher rates of dyspnea and higher NSQIP serious complication risk estimates, all significant at P less than .05. Similar disparities were seen in comparing patients receiving SBRT vs. VATS lobectomy.
The actual serious complication rate for surgical patients was significantly higher than the NSQIP risk calculator prediction (16.6% vs. 8.8%), as was the rate of pneumonia (6.0% vs. 3.2%), both at P less than .05.
Overall, the NSQIP Surgical Risk Calculator provided a fair level of discrimination between VATS lobectomy and SBRT on receiver operating characteristic (ROC) curve analysis, but it was a poor model for differentiating between VATS wedge resection and SBRT. “Unfortunately, it is this latter population of the highest risk surgical patients (for whom a lobectomy is not a surgical option) where risk models and decision aids are needed most,” Dr. Samson and her colleagues stated.
“Counseling the high-risk but operable patient with clinical stage I NSCLC in regard to lobectomy, sublobar resection, or SBRT is challenging for both the clinician and the patient,” according to the researchers. “We believe that a model tailored to patients with clinical stage I needs to serve as both an estimator of operative risks and a patient decision aid for surgery versus SBRT, especially with projected increases in the number of early-stage lung cancers as a result of increased lung cancer screening efforts,” they added.
“Our analysis suggests that the NSQIP Surgical Risk Calculator likely does not profile the risk of a patient with lung cancer closely enough to dichotomize surgical and inoperable SBRT cases (especially when patients are being considered for a wedge resection) or adequately estimate a surgical patient’s risk of serious complications,” Dr. Samson and her colleagues concluded.
The study was supported by grants from National Institutes of Health. The authors had no relevant financial disclosures.
FROM JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: The current NSQIP Surgical Risk Calculator does not adequately estimate risk among patients with clinical stage I non–small cell lung cancer.
Major finding: The NSQIP risk calculator significantly underestimated serious complication risk in operative patients (16.6% actual risk vs. 8.8% predicted) and did not adequately stratify risk between surgical and stereotactic body radiation therapy (SBRT) patients.
Data source: Researchers retrospectively assessed 279 NSCLC stage I lung cancer patients who underwent surgery vs. 206 patients who underwent SBRT from 2009 to 2012.
Disclosures: The study was supported by grants from the National Institutes of Health. The authors had no relevant financial disclosures.
Study evaluates which prior cancers pose a risk for developing NSCLC
PHOENIX – Patients with a history of head and neck, lung, bladder, and hematologic malignancies had increased rates of subsequent non–small cell lung cancer (NSCLC), a large analysis of national data found.
“It is unclear to what extent the higher rate of primary NSCLC in these patients may be attributed to smoking, previous cancer history, or other lung cancer risk factors,” researchers led by Dr. Geena Wu wrote in an abstract presented during a poster session at the annual meeting of the Society of Thoracic Surgeons. “Further research using individual smoking data may better delineate who is at increased risk of NSCLC based on prior cancer site and smoking history.”
In a study that Dr. Wu led during a research fellowship at the City of Hope National Medical Center, Duarte, Calif., she and her associates used the Surveillance, Epidemiology, and End Results (SEER) 1992-2007 dataset to identify 32,058 patients with a prior malignancy who subsequently developed primary lung cancer at 6 months or more after their initial cancer. They calculated standardized incidence ratios (SIRs) for NSCLC as a rate of observed to expected NSCLC cases adjusted by person-years at risk, age, gender, and time of diagnosis.
The researchers found that patients with a history of the following cancers had higher rates of second primary NSCLC than expected: head and neck (SIR, 4.00), colon and rectum (SIR, 1.16), pancreas (SIR, 1.44), lung (SIR, 4.88), bladder (SIR, 1.97), kidney (SIR, 1.21), breast (SIR, 1.09), and leukemia or lymphoma (SIR, 1.40).
At the same time, patients with a history of pancreatic or breast cancer who were treated with radiation had a higher incidence of second primary NSCLC (SIR of 2.54 and SIR of 1.14, respectively), while those who were not treated with radiation did not.
Although the SEER database does not contain information about patient smoking history, the researchers evaluated adult smoking rates by state by using a national map from the Centers for Disease Control and Prevention’s 2013 Behavioral Risk Factor Surveillance System. Smoking rates were low (defined as 13.7% or less) in California, Hawaii, and Utah, and were higher in all other states, especially in Southeastern states. Dr. Wu and her associates found that patients from high smoking areas who had previous cancers of the colon and rectum, pancreas, kidney, thyroid, and breast had higher rates of a primary NSCLC, while those from low smoking areas did not. Interestingly, patients from high smoking areas who previously had uterine cancer, prostate, or melanoma had did not have higher rates of a primary NSCLC.
“Just because someone has a previous history of cancer, they’re not necessarily at increased risk of a second lung cancer,” Dr. Wu, who is now a fourth-year general surgery resident at Maricopa County Hospital in Phoenix said in an interview at the meeting. “You have to look at what kind of cancer they had and what their smoking history is – whether or not they continue to smoke, because smoking is such an important risk factor.”
Another limitation of the SEER database is that it lacks details about the type of chemotherapy patients receive, “so whether or not chemotherapy plays an impact in the elevated risk of lung cancer we can’t say.”
Dr. Wu reported having no financial disclosures.
PHOENIX – Patients with a history of head and neck, lung, bladder, and hematologic malignancies had increased rates of subsequent non–small cell lung cancer (NSCLC), a large analysis of national data found.
“It is unclear to what extent the higher rate of primary NSCLC in these patients may be attributed to smoking, previous cancer history, or other lung cancer risk factors,” researchers led by Dr. Geena Wu wrote in an abstract presented during a poster session at the annual meeting of the Society of Thoracic Surgeons. “Further research using individual smoking data may better delineate who is at increased risk of NSCLC based on prior cancer site and smoking history.”
In a study that Dr. Wu led during a research fellowship at the City of Hope National Medical Center, Duarte, Calif., she and her associates used the Surveillance, Epidemiology, and End Results (SEER) 1992-2007 dataset to identify 32,058 patients with a prior malignancy who subsequently developed primary lung cancer at 6 months or more after their initial cancer. They calculated standardized incidence ratios (SIRs) for NSCLC as a rate of observed to expected NSCLC cases adjusted by person-years at risk, age, gender, and time of diagnosis.
The researchers found that patients with a history of the following cancers had higher rates of second primary NSCLC than expected: head and neck (SIR, 4.00), colon and rectum (SIR, 1.16), pancreas (SIR, 1.44), lung (SIR, 4.88), bladder (SIR, 1.97), kidney (SIR, 1.21), breast (SIR, 1.09), and leukemia or lymphoma (SIR, 1.40).
At the same time, patients with a history of pancreatic or breast cancer who were treated with radiation had a higher incidence of second primary NSCLC (SIR of 2.54 and SIR of 1.14, respectively), while those who were not treated with radiation did not.
Although the SEER database does not contain information about patient smoking history, the researchers evaluated adult smoking rates by state by using a national map from the Centers for Disease Control and Prevention’s 2013 Behavioral Risk Factor Surveillance System. Smoking rates were low (defined as 13.7% or less) in California, Hawaii, and Utah, and were higher in all other states, especially in Southeastern states. Dr. Wu and her associates found that patients from high smoking areas who had previous cancers of the colon and rectum, pancreas, kidney, thyroid, and breast had higher rates of a primary NSCLC, while those from low smoking areas did not. Interestingly, patients from high smoking areas who previously had uterine cancer, prostate, or melanoma had did not have higher rates of a primary NSCLC.
“Just because someone has a previous history of cancer, they’re not necessarily at increased risk of a second lung cancer,” Dr. Wu, who is now a fourth-year general surgery resident at Maricopa County Hospital in Phoenix said in an interview at the meeting. “You have to look at what kind of cancer they had and what their smoking history is – whether or not they continue to smoke, because smoking is such an important risk factor.”
Another limitation of the SEER database is that it lacks details about the type of chemotherapy patients receive, “so whether or not chemotherapy plays an impact in the elevated risk of lung cancer we can’t say.”
Dr. Wu reported having no financial disclosures.
PHOENIX – Patients with a history of head and neck, lung, bladder, and hematologic malignancies had increased rates of subsequent non–small cell lung cancer (NSCLC), a large analysis of national data found.
“It is unclear to what extent the higher rate of primary NSCLC in these patients may be attributed to smoking, previous cancer history, or other lung cancer risk factors,” researchers led by Dr. Geena Wu wrote in an abstract presented during a poster session at the annual meeting of the Society of Thoracic Surgeons. “Further research using individual smoking data may better delineate who is at increased risk of NSCLC based on prior cancer site and smoking history.”
In a study that Dr. Wu led during a research fellowship at the City of Hope National Medical Center, Duarte, Calif., she and her associates used the Surveillance, Epidemiology, and End Results (SEER) 1992-2007 dataset to identify 32,058 patients with a prior malignancy who subsequently developed primary lung cancer at 6 months or more after their initial cancer. They calculated standardized incidence ratios (SIRs) for NSCLC as a rate of observed to expected NSCLC cases adjusted by person-years at risk, age, gender, and time of diagnosis.
The researchers found that patients with a history of the following cancers had higher rates of second primary NSCLC than expected: head and neck (SIR, 4.00), colon and rectum (SIR, 1.16), pancreas (SIR, 1.44), lung (SIR, 4.88), bladder (SIR, 1.97), kidney (SIR, 1.21), breast (SIR, 1.09), and leukemia or lymphoma (SIR, 1.40).
At the same time, patients with a history of pancreatic or breast cancer who were treated with radiation had a higher incidence of second primary NSCLC (SIR of 2.54 and SIR of 1.14, respectively), while those who were not treated with radiation did not.
Although the SEER database does not contain information about patient smoking history, the researchers evaluated adult smoking rates by state by using a national map from the Centers for Disease Control and Prevention’s 2013 Behavioral Risk Factor Surveillance System. Smoking rates were low (defined as 13.7% or less) in California, Hawaii, and Utah, and were higher in all other states, especially in Southeastern states. Dr. Wu and her associates found that patients from high smoking areas who had previous cancers of the colon and rectum, pancreas, kidney, thyroid, and breast had higher rates of a primary NSCLC, while those from low smoking areas did not. Interestingly, patients from high smoking areas who previously had uterine cancer, prostate, or melanoma had did not have higher rates of a primary NSCLC.
“Just because someone has a previous history of cancer, they’re not necessarily at increased risk of a second lung cancer,” Dr. Wu, who is now a fourth-year general surgery resident at Maricopa County Hospital in Phoenix said in an interview at the meeting. “You have to look at what kind of cancer they had and what their smoking history is – whether or not they continue to smoke, because smoking is such an important risk factor.”
Another limitation of the SEER database is that it lacks details about the type of chemotherapy patients receive, “so whether or not chemotherapy plays an impact in the elevated risk of lung cancer we can’t say.”
Dr. Wu reported having no financial disclosures.
AT THE STS ANNUAL MEETING
Key clinical point: Not all patients with history of cancer face an increased risk of developing subsequent NSCLC.
Major finding: Patients with a history of the following cancers had higher rates of second primary NSCLC than expected: head and neck (SIR, 4.00), colon and rectum (SIR, 1.16), pancreas (SIR, 1.44), lung (SIR, 4.88), bladder (SIR, 1.97), kidney (SIR, 1.21), breast (SIR, 1.09), and leukemia or lymphoma (SIR, 1.40).
Data source: An analysis of 32,058 patients with a prior malignancy that subsequently developed primary lung cancer at 6 months or more after their initial cancer.
Disclosures: Dr. Wu reported having no financial disclosures.
Cadaveric allograft system used to reconstruct anterior chest wall
PHOENIX – Cadaveric allograft sternal replacement has proven to be safe, providing optimal stability to the chest wall and protection of surrounding organs, an analysis of 18 cases demonstrated.
“The allograft was biologically well tolerated, allowing a perfect integration into the host,” Dr. Giuseppe Marulli said at the annual meeting of the Society of Thoracic Surgeons. “Donor cryopreserved sternochondral allograft may become the ideal way for anterior chest wall reconstruction, particularly for wide resections.”
Dr. Marulli, a thoracic surgeon at the University of Padova, Italy, noted that prior experimental studies have demonstrated that cryopreserved bone allografts preserve osteoconduction and osteoinduction capacity (Eur Spine J. 2001 Oct;10:S96-101). “Therefore, they form the basis for new bone tissue formation, allowing for the capillary and perivascular blood supply,” he said.
Limitations of current materials used for sternal reconstruction include “excessive rigidity with risk of erosion and insufficient support for large chest wall defects,” he said. Perceived advantages of using cadaveric bone allograft include easy incorporation, no risk of rejection, and a low risk of infection. For each procedure used in the current analysis, cadaveric allograft sternums with costal cartilages were harvested with an aseptic method and treated with an antibiotic solution for 72 hours. Next, they were cryopreserved at –80º C and underwent microbiologic testing for at least 1 month to ensure sterility and absence of immunogenic capacity.
Dr. Marulli reported results from 18 patients who underwent the procedure between January 2009 and January 2015, 13 of whom were female. Their median age was 59 years, their median tumor diameter was 4.75 cm, most (88%) had undergone preoperative needle biopsy, and 50% had undergone induction therapy. The main indication for sternectomy was a single-site sternal metastasis (nine patients), primary chondrosarcoma (four cases), sternal dehiscence after cardiac surgery (two cases), malignant fibrous tumor (one case), radioinduced soft-tissue sarcoma (one case), and a thymic carcinoma invading the sternum (one case).
All patients were extubated in the OR, and one patient died in the hospital from a pulmonary embolism. Two patients (11%) developed postoperative complications: one case of Candida urinary infection and one case of bleeding at the site of the muscle flap. The median postoperative length of stay was 11 days.
To date, no infections or rejections of the grafts have occurred, Dr. Marulli said. After a median of 36 months, 13 patients are alive and 4 are dead (3 from a metastatic recurrence and 1 from an unrelated cause). One patient required removal of a clavicular screw for dislocation 4 months after the operation.
Dr. Marulli reported having no financial disclosures.
PHOENIX – Cadaveric allograft sternal replacement has proven to be safe, providing optimal stability to the chest wall and protection of surrounding organs, an analysis of 18 cases demonstrated.
“The allograft was biologically well tolerated, allowing a perfect integration into the host,” Dr. Giuseppe Marulli said at the annual meeting of the Society of Thoracic Surgeons. “Donor cryopreserved sternochondral allograft may become the ideal way for anterior chest wall reconstruction, particularly for wide resections.”
Dr. Marulli, a thoracic surgeon at the University of Padova, Italy, noted that prior experimental studies have demonstrated that cryopreserved bone allografts preserve osteoconduction and osteoinduction capacity (Eur Spine J. 2001 Oct;10:S96-101). “Therefore, they form the basis for new bone tissue formation, allowing for the capillary and perivascular blood supply,” he said.
Limitations of current materials used for sternal reconstruction include “excessive rigidity with risk of erosion and insufficient support for large chest wall defects,” he said. Perceived advantages of using cadaveric bone allograft include easy incorporation, no risk of rejection, and a low risk of infection. For each procedure used in the current analysis, cadaveric allograft sternums with costal cartilages were harvested with an aseptic method and treated with an antibiotic solution for 72 hours. Next, they were cryopreserved at –80º C and underwent microbiologic testing for at least 1 month to ensure sterility and absence of immunogenic capacity.
Dr. Marulli reported results from 18 patients who underwent the procedure between January 2009 and January 2015, 13 of whom were female. Their median age was 59 years, their median tumor diameter was 4.75 cm, most (88%) had undergone preoperative needle biopsy, and 50% had undergone induction therapy. The main indication for sternectomy was a single-site sternal metastasis (nine patients), primary chondrosarcoma (four cases), sternal dehiscence after cardiac surgery (two cases), malignant fibrous tumor (one case), radioinduced soft-tissue sarcoma (one case), and a thymic carcinoma invading the sternum (one case).
All patients were extubated in the OR, and one patient died in the hospital from a pulmonary embolism. Two patients (11%) developed postoperative complications: one case of Candida urinary infection and one case of bleeding at the site of the muscle flap. The median postoperative length of stay was 11 days.
To date, no infections or rejections of the grafts have occurred, Dr. Marulli said. After a median of 36 months, 13 patients are alive and 4 are dead (3 from a metastatic recurrence and 1 from an unrelated cause). One patient required removal of a clavicular screw for dislocation 4 months after the operation.
Dr. Marulli reported having no financial disclosures.
PHOENIX – Cadaveric allograft sternal replacement has proven to be safe, providing optimal stability to the chest wall and protection of surrounding organs, an analysis of 18 cases demonstrated.
“The allograft was biologically well tolerated, allowing a perfect integration into the host,” Dr. Giuseppe Marulli said at the annual meeting of the Society of Thoracic Surgeons. “Donor cryopreserved sternochondral allograft may become the ideal way for anterior chest wall reconstruction, particularly for wide resections.”
Dr. Marulli, a thoracic surgeon at the University of Padova, Italy, noted that prior experimental studies have demonstrated that cryopreserved bone allografts preserve osteoconduction and osteoinduction capacity (Eur Spine J. 2001 Oct;10:S96-101). “Therefore, they form the basis for new bone tissue formation, allowing for the capillary and perivascular blood supply,” he said.
Limitations of current materials used for sternal reconstruction include “excessive rigidity with risk of erosion and insufficient support for large chest wall defects,” he said. Perceived advantages of using cadaveric bone allograft include easy incorporation, no risk of rejection, and a low risk of infection. For each procedure used in the current analysis, cadaveric allograft sternums with costal cartilages were harvested with an aseptic method and treated with an antibiotic solution for 72 hours. Next, they were cryopreserved at –80º C and underwent microbiologic testing for at least 1 month to ensure sterility and absence of immunogenic capacity.
Dr. Marulli reported results from 18 patients who underwent the procedure between January 2009 and January 2015, 13 of whom were female. Their median age was 59 years, their median tumor diameter was 4.75 cm, most (88%) had undergone preoperative needle biopsy, and 50% had undergone induction therapy. The main indication for sternectomy was a single-site sternal metastasis (nine patients), primary chondrosarcoma (four cases), sternal dehiscence after cardiac surgery (two cases), malignant fibrous tumor (one case), radioinduced soft-tissue sarcoma (one case), and a thymic carcinoma invading the sternum (one case).
All patients were extubated in the OR, and one patient died in the hospital from a pulmonary embolism. Two patients (11%) developed postoperative complications: one case of Candida urinary infection and one case of bleeding at the site of the muscle flap. The median postoperative length of stay was 11 days.
To date, no infections or rejections of the grafts have occurred, Dr. Marulli said. After a median of 36 months, 13 patients are alive and 4 are dead (3 from a metastatic recurrence and 1 from an unrelated cause). One patient required removal of a clavicular screw for dislocation 4 months after the operation.
Dr. Marulli reported having no financial disclosures.
AT THE STS ANNUAL MEETING
Key clinical point: Cadaveric allograft sternal replacement appears to be an effective option for reconstructing the anterior chest wall.
Major finding: To date, no infections or rejections of the grafts have occurred in patients who underwent cadaveric allograft sternal replacement.
Data source: An analysis of 18 patients who underwent the procedure between January 2009 and January 2015.
Disclosures: Dr. Marulli reported having no financial disclosures.
Fewer general surgery residents doing thoracic surgery cases
PHOENIX – Over the past 11 years, fewer general surgery residents have participated in important types of general thoracic surgery cases, a retrospective review found.
“These findings may be the result of the work-hours reduction causing less exposure to general thoracic surgery and/or a reluctance to allow general surgery residents to perform the increasingly common minimally invasive procedures,” researchers led by Dr. William S. Ragalie wrote in an abstract presented during a poster session at the annual meeting of the Society of Thoracic Surgeons.
Dr. Ragalie of the Medical College of Wisconsin, Milwaukee, and his associates retrospectively reviewed the Accreditation Council for Graduate Medical Education resident case log database for the most recent 11 years in an effort to quantify and trend the operative experience among general surgery residents. They categorized cases by year, level of resident participation, and level of complexity. Major general thoracic cases were defined as esophagectomy, pneumonectomy, and lobectomy, while cases that did not involve hilar dissection were classified as “other thoracic.”
The researchers found that the 90th percentile of first assist thoracic surgery cases decreased significantly over the study period by an average of 1.46 cases per year (P = .0012). Decreased case volumes in pneumonectomy were also noted at the junior level (–0.012 cases per year; P less than .0001) and at the chief resident level (–0.31 cases per year; P less than .001). This was also true of open lobectomy cases (–0.14 cases per year at the junior level; P less than .001, and –3.41 cases per year at the chief resident level; P less than .0001).
As for video-assisted thoracoscopic surgery (VATS) lobectomy, the researchers observed an increase in average case volume at the junior surgeon level of .13 cases per year, but a decrease at the chief resident level of one case per year (P less than .001 for both).
Dr. Ragalie and his associates also observed a decrease in the following procedures performed by chief residents: open exploratory thoracoscopy (–3.17 cases per year; P less than .001), VATS exploratory thoracoscopy (–2.95 cases per year; P less than .0001), open wedge resection (–1.52 cases per year; P less than .0227), VATS wedge resection (–2.72 cases per year; P less than .0002), “other thoracic” (–6.3 cases per year; P = .0001), and thoracoscopic pleurodesis (–2.09 cases per year; P less than .0001).
At the same time, a significant trend of decreased case volume at the junior surgeon level was noted for open exploratory thoracoscopy (–0.10 cases per year; P less than .0001) and open wedge resection (–0.22 cases per year; P = . 0115).
The researchers reported having no financial disclosures.
PHOENIX – Over the past 11 years, fewer general surgery residents have participated in important types of general thoracic surgery cases, a retrospective review found.
“These findings may be the result of the work-hours reduction causing less exposure to general thoracic surgery and/or a reluctance to allow general surgery residents to perform the increasingly common minimally invasive procedures,” researchers led by Dr. William S. Ragalie wrote in an abstract presented during a poster session at the annual meeting of the Society of Thoracic Surgeons.
Dr. Ragalie of the Medical College of Wisconsin, Milwaukee, and his associates retrospectively reviewed the Accreditation Council for Graduate Medical Education resident case log database for the most recent 11 years in an effort to quantify and trend the operative experience among general surgery residents. They categorized cases by year, level of resident participation, and level of complexity. Major general thoracic cases were defined as esophagectomy, pneumonectomy, and lobectomy, while cases that did not involve hilar dissection were classified as “other thoracic.”
The researchers found that the 90th percentile of first assist thoracic surgery cases decreased significantly over the study period by an average of 1.46 cases per year (P = .0012). Decreased case volumes in pneumonectomy were also noted at the junior level (–0.012 cases per year; P less than .0001) and at the chief resident level (–0.31 cases per year; P less than .001). This was also true of open lobectomy cases (–0.14 cases per year at the junior level; P less than .001, and –3.41 cases per year at the chief resident level; P less than .0001).
As for video-assisted thoracoscopic surgery (VATS) lobectomy, the researchers observed an increase in average case volume at the junior surgeon level of .13 cases per year, but a decrease at the chief resident level of one case per year (P less than .001 for both).
Dr. Ragalie and his associates also observed a decrease in the following procedures performed by chief residents: open exploratory thoracoscopy (–3.17 cases per year; P less than .001), VATS exploratory thoracoscopy (–2.95 cases per year; P less than .0001), open wedge resection (–1.52 cases per year; P less than .0227), VATS wedge resection (–2.72 cases per year; P less than .0002), “other thoracic” (–6.3 cases per year; P = .0001), and thoracoscopic pleurodesis (–2.09 cases per year; P less than .0001).
At the same time, a significant trend of decreased case volume at the junior surgeon level was noted for open exploratory thoracoscopy (–0.10 cases per year; P less than .0001) and open wedge resection (–0.22 cases per year; P = . 0115).
The researchers reported having no financial disclosures.
PHOENIX – Over the past 11 years, fewer general surgery residents have participated in important types of general thoracic surgery cases, a retrospective review found.
“These findings may be the result of the work-hours reduction causing less exposure to general thoracic surgery and/or a reluctance to allow general surgery residents to perform the increasingly common minimally invasive procedures,” researchers led by Dr. William S. Ragalie wrote in an abstract presented during a poster session at the annual meeting of the Society of Thoracic Surgeons.
Dr. Ragalie of the Medical College of Wisconsin, Milwaukee, and his associates retrospectively reviewed the Accreditation Council for Graduate Medical Education resident case log database for the most recent 11 years in an effort to quantify and trend the operative experience among general surgery residents. They categorized cases by year, level of resident participation, and level of complexity. Major general thoracic cases were defined as esophagectomy, pneumonectomy, and lobectomy, while cases that did not involve hilar dissection were classified as “other thoracic.”
The researchers found that the 90th percentile of first assist thoracic surgery cases decreased significantly over the study period by an average of 1.46 cases per year (P = .0012). Decreased case volumes in pneumonectomy were also noted at the junior level (–0.012 cases per year; P less than .0001) and at the chief resident level (–0.31 cases per year; P less than .001). This was also true of open lobectomy cases (–0.14 cases per year at the junior level; P less than .001, and –3.41 cases per year at the chief resident level; P less than .0001).
As for video-assisted thoracoscopic surgery (VATS) lobectomy, the researchers observed an increase in average case volume at the junior surgeon level of .13 cases per year, but a decrease at the chief resident level of one case per year (P less than .001 for both).
Dr. Ragalie and his associates also observed a decrease in the following procedures performed by chief residents: open exploratory thoracoscopy (–3.17 cases per year; P less than .001), VATS exploratory thoracoscopy (–2.95 cases per year; P less than .0001), open wedge resection (–1.52 cases per year; P less than .0227), VATS wedge resection (–2.72 cases per year; P less than .0002), “other thoracic” (–6.3 cases per year; P = .0001), and thoracoscopic pleurodesis (–2.09 cases per year; P less than .0001).
At the same time, a significant trend of decreased case volume at the junior surgeon level was noted for open exploratory thoracoscopy (–0.10 cases per year; P less than .0001) and open wedge resection (–0.22 cases per year; P = . 0115).
The researchers reported having no financial disclosures.
AT THE STS ANNUAL MEETING
Key clinical point: Fewer general surgery residents are participating in important types of general thoracic surgery cases during their residency.
Major finding: The 90th percentile of first-assist thoracic surgery cases decreased significantly over the study period by an average of 1.46 cases per year (P = .0012).
Data source: A retrospective analysis of the Accreditation Council for Graduate Medical Education resident case log database for the most recent 11 years.
Disclosures: The researchers reported having no financial disclosures.
A better way to relieve rib fracture pain in the ICU
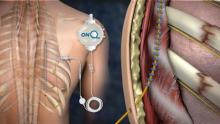
SAN ANTONIO – A new pain relief option for multiple rib fractures means that you might not have to wait around anymore for anesthesiology to place thoracic epidurals.
It’s called posterior paramedian subrhomboidal (PoPS) analgesia. A skin incision is made below the lowest fractured rib just paramedian to the spinus processes; a tunneling device is then used to work a catheter upwards under the rhomboids just past the highest fractured rib. The catheter has multiple openings along its length – like a sprinkler hose – so analgesic bathes the intercostal nerves as it runs down from a reservoir into the patient. The reservoir can be set to a desired flow rate or for on-demand use (ON-Q Pain Relief System – Halyard).
A pilot study at the University of Kansas, Kansas City, found that pain control from PoPS was at least equivalent to standard thoracic epidural analgesia (TEA), and that the system can be placed by a variety of hospital staff, not just anesthesiologists.
The 11 PoPS patients also used fewer rescue narcotics than the 19 TEA patients and had less hypotension. Because they weren’t at risk for epidural hematomas, they started venous thromboembolism prophylaxis without delay and at full dose.
“Our results are very promising. PoPS provides pain control similar to that of TEA,” with several “other benefits. You are not relying on one specialty for pain control,” so patients probably get faster relief. “PoPS can also be placed in patients whose injuries prohibit TEA, such as those with spinal cord injuries or increased intracranial pressure,” said investigator Dr. Casey Shelley, a University of Kansas general surgery resident.
PoPS was placed in the study either by anesthesiologists or by a trauma surgeon who practiced placement beforehand in the cadaver lab. The do-it-yourself potential for surgeons “is key. Most of us trauma surgeons are sick of begging anesthesiologists to come place thoracic epidurals,” said an audience member after Dr. Shelley’s presentation at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Ropivacaine 0.2% was used in both PoPS and TEA patients, all of whom had at least three broken ribs.
Median pain scores dropped from 8.5 to 2.5 on a 10-point scale an hour after PoPS placement, versus a median drop from 8 to 5 points an hour after TEA (P = .03). Although not statistically significant, median pain scores were about 1.5 points better with PoPS over the next several days, hovering around 3.5 versus around 5 points with TEA. Anesthesiology “usually won’t place high thoracic epidurals. With PoPS, you can tunnel up as far as you need to go to get to higher ribs,” which might explain the better pain control, Dr. Shelley said.
PoPS patients used about 70 mg/day oral morphine equivalents versus about 90 mg/day with TEA through day 6, but again the difference was not statistically significant. Even so, it might explain why six TEA patients (32%) were hypotensive over that time, compared with two PoPS patients (18%).
PoPS patients were a little older on average (mean 63 versus 55 years), with more fractured ribs (mean eight versus seven), and higher Injury Severity Scale scores (mean 20 versus 16). They were also more likely to have bilateral fractures, longer ICU stays (mean 4.9 versus 3.1 days), and longer overall lengths of stay (mean 14.8 versus 9.8 days), but none of those trends were statistically significant.
Both groups had mean chest Abbreviated Injury Scale scores of 3, and there were no statistical differences in daily spirometry readings. The majority of patients in both groups were men.
Favorable results were also reported in 2010 for ON-Q rib pain control, but the investigators did not compare the system to TEA (World J Surg. 2010 Oct;34:2359-62).
Dr. Shelley said Halyard was not involved in the study, and that she has no disclosures.
SAN ANTONIO – A new pain relief option for multiple rib fractures means that you might not have to wait around anymore for anesthesiology to place thoracic epidurals.
It’s called posterior paramedian subrhomboidal (PoPS) analgesia. A skin incision is made below the lowest fractured rib just paramedian to the spinus processes; a tunneling device is then used to work a catheter upwards under the rhomboids just past the highest fractured rib. The catheter has multiple openings along its length – like a sprinkler hose – so analgesic bathes the intercostal nerves as it runs down from a reservoir into the patient. The reservoir can be set to a desired flow rate or for on-demand use (ON-Q Pain Relief System – Halyard).
A pilot study at the University of Kansas, Kansas City, found that pain control from PoPS was at least equivalent to standard thoracic epidural analgesia (TEA), and that the system can be placed by a variety of hospital staff, not just anesthesiologists.
The 11 PoPS patients also used fewer rescue narcotics than the 19 TEA patients and had less hypotension. Because they weren’t at risk for epidural hematomas, they started venous thromboembolism prophylaxis without delay and at full dose.
“Our results are very promising. PoPS provides pain control similar to that of TEA,” with several “other benefits. You are not relying on one specialty for pain control,” so patients probably get faster relief. “PoPS can also be placed in patients whose injuries prohibit TEA, such as those with spinal cord injuries or increased intracranial pressure,” said investigator Dr. Casey Shelley, a University of Kansas general surgery resident.
PoPS was placed in the study either by anesthesiologists or by a trauma surgeon who practiced placement beforehand in the cadaver lab. The do-it-yourself potential for surgeons “is key. Most of us trauma surgeons are sick of begging anesthesiologists to come place thoracic epidurals,” said an audience member after Dr. Shelley’s presentation at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Ropivacaine 0.2% was used in both PoPS and TEA patients, all of whom had at least three broken ribs.
Median pain scores dropped from 8.5 to 2.5 on a 10-point scale an hour after PoPS placement, versus a median drop from 8 to 5 points an hour after TEA (P = .03). Although not statistically significant, median pain scores were about 1.5 points better with PoPS over the next several days, hovering around 3.5 versus around 5 points with TEA. Anesthesiology “usually won’t place high thoracic epidurals. With PoPS, you can tunnel up as far as you need to go to get to higher ribs,” which might explain the better pain control, Dr. Shelley said.
PoPS patients used about 70 mg/day oral morphine equivalents versus about 90 mg/day with TEA through day 6, but again the difference was not statistically significant. Even so, it might explain why six TEA patients (32%) were hypotensive over that time, compared with two PoPS patients (18%).
PoPS patients were a little older on average (mean 63 versus 55 years), with more fractured ribs (mean eight versus seven), and higher Injury Severity Scale scores (mean 20 versus 16). They were also more likely to have bilateral fractures, longer ICU stays (mean 4.9 versus 3.1 days), and longer overall lengths of stay (mean 14.8 versus 9.8 days), but none of those trends were statistically significant.
Both groups had mean chest Abbreviated Injury Scale scores of 3, and there were no statistical differences in daily spirometry readings. The majority of patients in both groups were men.
Favorable results were also reported in 2010 for ON-Q rib pain control, but the investigators did not compare the system to TEA (World J Surg. 2010 Oct;34:2359-62).
Dr. Shelley said Halyard was not involved in the study, and that she has no disclosures.
SAN ANTONIO – A new pain relief option for multiple rib fractures means that you might not have to wait around anymore for anesthesiology to place thoracic epidurals.
It’s called posterior paramedian subrhomboidal (PoPS) analgesia. A skin incision is made below the lowest fractured rib just paramedian to the spinus processes; a tunneling device is then used to work a catheter upwards under the rhomboids just past the highest fractured rib. The catheter has multiple openings along its length – like a sprinkler hose – so analgesic bathes the intercostal nerves as it runs down from a reservoir into the patient. The reservoir can be set to a desired flow rate or for on-demand use (ON-Q Pain Relief System – Halyard).
A pilot study at the University of Kansas, Kansas City, found that pain control from PoPS was at least equivalent to standard thoracic epidural analgesia (TEA), and that the system can be placed by a variety of hospital staff, not just anesthesiologists.
The 11 PoPS patients also used fewer rescue narcotics than the 19 TEA patients and had less hypotension. Because they weren’t at risk for epidural hematomas, they started venous thromboembolism prophylaxis without delay and at full dose.
“Our results are very promising. PoPS provides pain control similar to that of TEA,” with several “other benefits. You are not relying on one specialty for pain control,” so patients probably get faster relief. “PoPS can also be placed in patients whose injuries prohibit TEA, such as those with spinal cord injuries or increased intracranial pressure,” said investigator Dr. Casey Shelley, a University of Kansas general surgery resident.
PoPS was placed in the study either by anesthesiologists or by a trauma surgeon who practiced placement beforehand in the cadaver lab. The do-it-yourself potential for surgeons “is key. Most of us trauma surgeons are sick of begging anesthesiologists to come place thoracic epidurals,” said an audience member after Dr. Shelley’s presentation at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
Ropivacaine 0.2% was used in both PoPS and TEA patients, all of whom had at least three broken ribs.
Median pain scores dropped from 8.5 to 2.5 on a 10-point scale an hour after PoPS placement, versus a median drop from 8 to 5 points an hour after TEA (P = .03). Although not statistically significant, median pain scores were about 1.5 points better with PoPS over the next several days, hovering around 3.5 versus around 5 points with TEA. Anesthesiology “usually won’t place high thoracic epidurals. With PoPS, you can tunnel up as far as you need to go to get to higher ribs,” which might explain the better pain control, Dr. Shelley said.
PoPS patients used about 70 mg/day oral morphine equivalents versus about 90 mg/day with TEA through day 6, but again the difference was not statistically significant. Even so, it might explain why six TEA patients (32%) were hypotensive over that time, compared with two PoPS patients (18%).
PoPS patients were a little older on average (mean 63 versus 55 years), with more fractured ribs (mean eight versus seven), and higher Injury Severity Scale scores (mean 20 versus 16). They were also more likely to have bilateral fractures, longer ICU stays (mean 4.9 versus 3.1 days), and longer overall lengths of stay (mean 14.8 versus 9.8 days), but none of those trends were statistically significant.
Both groups had mean chest Abbreviated Injury Scale scores of 3, and there were no statistical differences in daily spirometry readings. The majority of patients in both groups were men.
Favorable results were also reported in 2010 for ON-Q rib pain control, but the investigators did not compare the system to TEA (World J Surg. 2010 Oct;34:2359-62).
Dr. Shelley said Halyard was not involved in the study, and that she has no disclosures.
AT THE EAST SCIENTIFIC ASSEMBLY
Key clinical point: You might not have to wait around anymore for anesthesiology to place thoracic epidurals.
Major finding: Median pain scores dropped from 8.5 to 2.5 on a 10-point scale an hour after posterior PoPS placement, versus a median drop from 8 to 5 points an hour after thoracic epidural analgesia (P = .03).
Data source: Pilot study of 30 adults with at least three fractured ribs.
Disclosures: The maker of the PoPS system was not involved in the study, and the presenter had no disclosures.
Poor adherence to quality indicators found for NSCLC surgery
PHOENIX – National adherence to quality indicators for surgery in stage I non–small cell lung cancer is suboptimal, results from a large analysis of national data suggest.
“Compliance with such guidelines is a strong predictor of long-term survival, and vigorous efforts should be instituted at the level of national societies to improve such adherence,” researchers led by Dr. Pamela P. Samson wrote in an abstract presented at the annual meeting of the Society of Thoracic Surgeons. “National organizations, including American College of Chest Physicians, the National Comprehensive Cancer Network, and the American College of Surgeons Commission on Cancer, have recommended quality standards for surgery in early-stage non–small cell lung cancer (NSCLC). The determinants and outcomes of adherence to these guidelines for early-stage lung cancer patients are largely unknown.”
Dr. Samson, a general surgery resident at Washington University in St. Louis, and her associates used the National Cancer Data Base to evaluate data from 146,908 patients undergoing surgery for clinical stage I NSCLC between 2004 and 2013. They selected the following four quality measures for evaluation: performing an anatomical pulmonary resection, surgery within 8 weeks of diagnosis, R0 resection, and evaluation of 10 or more lymph nodes. Next, the researchers fitted multivariate models to identify variables independently associated with adherence to quality measures, and created a Cox multivariate model to evaluate long-term overall survival.
Dr. Varun Puri, senior author of the study, presented the findings at the STS meeting on behalf of Dr. Samson, and discussed the findings in a video interview. The researchers found that between 2004 and 2013, nearly 100% of patients met at least one of the four recommended criteria, 95% met two, 69% met three, and 22% met all four. Sampling of 10 or more lymph nodes was the least frequently met measure, occurring in only 31% of surgical patients. Patient factors associated with a greater likelihood of receiving all four quality measures included average income in ZIP code of at least $38,000 (odds ratio, 1.20), private insurance (OR, 1.22), or having Medicare (OR, 1.16). Institutional factors associated with a greater likelihood of meeting all four quality measures included higher-volume centers, defined as treating at least 38 cases per year (OR, 1.18), or being an academic institution (OR, 1.31).
At the same time, factors associated with a lower likelihood of recommended surgical care included increasing age (per year increase, OR, 0.99) and a higher Charlson/Deyo comorbidity score (OR, 0.90 for a score of 1 and OR, 0.82 for a score of 2 or more). The strongest determinant of long-term overall survival included pathologic upstaging (HR 1.84) and meeting all four quality indicators (HR 0.39). Every additional quality met was associated with a significant reduction in overall mortality.
“We believe this study can be a starting point to draw attention to institution- and surgeon-specific practice patterns that may vary widely,” Dr. Samson said in an interview prior to the meeting. “At our own institution, we are working to decrease time to surgery, as well as implementing quality improvement measures to increase nodal sampling rates. Improving these trends nationally must start at the local level, with a tailored approach.”
Dr. Samson is currently supported by a T32 NIH training grant for research fellows in cardiothoracic surgery. Study coauthor Dr. Bryan Meyers, has received honoraria from Varian Medical Systems and is a consultant/advisory board member of Ethicon. Senior author Dr. Varun Puri is supported by NIH career awards.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – National adherence to quality indicators for surgery in stage I non–small cell lung cancer is suboptimal, results from a large analysis of national data suggest.
“Compliance with such guidelines is a strong predictor of long-term survival, and vigorous efforts should be instituted at the level of national societies to improve such adherence,” researchers led by Dr. Pamela P. Samson wrote in an abstract presented at the annual meeting of the Society of Thoracic Surgeons. “National organizations, including American College of Chest Physicians, the National Comprehensive Cancer Network, and the American College of Surgeons Commission on Cancer, have recommended quality standards for surgery in early-stage non–small cell lung cancer (NSCLC). The determinants and outcomes of adherence to these guidelines for early-stage lung cancer patients are largely unknown.”
Dr. Samson, a general surgery resident at Washington University in St. Louis, and her associates used the National Cancer Data Base to evaluate data from 146,908 patients undergoing surgery for clinical stage I NSCLC between 2004 and 2013. They selected the following four quality measures for evaluation: performing an anatomical pulmonary resection, surgery within 8 weeks of diagnosis, R0 resection, and evaluation of 10 or more lymph nodes. Next, the researchers fitted multivariate models to identify variables independently associated with adherence to quality measures, and created a Cox multivariate model to evaluate long-term overall survival.
Dr. Varun Puri, senior author of the study, presented the findings at the STS meeting on behalf of Dr. Samson, and discussed the findings in a video interview. The researchers found that between 2004 and 2013, nearly 100% of patients met at least one of the four recommended criteria, 95% met two, 69% met three, and 22% met all four. Sampling of 10 or more lymph nodes was the least frequently met measure, occurring in only 31% of surgical patients. Patient factors associated with a greater likelihood of receiving all four quality measures included average income in ZIP code of at least $38,000 (odds ratio, 1.20), private insurance (OR, 1.22), or having Medicare (OR, 1.16). Institutional factors associated with a greater likelihood of meeting all four quality measures included higher-volume centers, defined as treating at least 38 cases per year (OR, 1.18), or being an academic institution (OR, 1.31).
At the same time, factors associated with a lower likelihood of recommended surgical care included increasing age (per year increase, OR, 0.99) and a higher Charlson/Deyo comorbidity score (OR, 0.90 for a score of 1 and OR, 0.82 for a score of 2 or more). The strongest determinant of long-term overall survival included pathologic upstaging (HR 1.84) and meeting all four quality indicators (HR 0.39). Every additional quality met was associated with a significant reduction in overall mortality.
“We believe this study can be a starting point to draw attention to institution- and surgeon-specific practice patterns that may vary widely,” Dr. Samson said in an interview prior to the meeting. “At our own institution, we are working to decrease time to surgery, as well as implementing quality improvement measures to increase nodal sampling rates. Improving these trends nationally must start at the local level, with a tailored approach.”
Dr. Samson is currently supported by a T32 NIH training grant for research fellows in cardiothoracic surgery. Study coauthor Dr. Bryan Meyers, has received honoraria from Varian Medical Systems and is a consultant/advisory board member of Ethicon. Senior author Dr. Varun Puri is supported by NIH career awards.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – National adherence to quality indicators for surgery in stage I non–small cell lung cancer is suboptimal, results from a large analysis of national data suggest.
“Compliance with such guidelines is a strong predictor of long-term survival, and vigorous efforts should be instituted at the level of national societies to improve such adherence,” researchers led by Dr. Pamela P. Samson wrote in an abstract presented at the annual meeting of the Society of Thoracic Surgeons. “National organizations, including American College of Chest Physicians, the National Comprehensive Cancer Network, and the American College of Surgeons Commission on Cancer, have recommended quality standards for surgery in early-stage non–small cell lung cancer (NSCLC). The determinants and outcomes of adherence to these guidelines for early-stage lung cancer patients are largely unknown.”
Dr. Samson, a general surgery resident at Washington University in St. Louis, and her associates used the National Cancer Data Base to evaluate data from 146,908 patients undergoing surgery for clinical stage I NSCLC between 2004 and 2013. They selected the following four quality measures for evaluation: performing an anatomical pulmonary resection, surgery within 8 weeks of diagnosis, R0 resection, and evaluation of 10 or more lymph nodes. Next, the researchers fitted multivariate models to identify variables independently associated with adherence to quality measures, and created a Cox multivariate model to evaluate long-term overall survival.
Dr. Varun Puri, senior author of the study, presented the findings at the STS meeting on behalf of Dr. Samson, and discussed the findings in a video interview. The researchers found that between 2004 and 2013, nearly 100% of patients met at least one of the four recommended criteria, 95% met two, 69% met three, and 22% met all four. Sampling of 10 or more lymph nodes was the least frequently met measure, occurring in only 31% of surgical patients. Patient factors associated with a greater likelihood of receiving all four quality measures included average income in ZIP code of at least $38,000 (odds ratio, 1.20), private insurance (OR, 1.22), or having Medicare (OR, 1.16). Institutional factors associated with a greater likelihood of meeting all four quality measures included higher-volume centers, defined as treating at least 38 cases per year (OR, 1.18), or being an academic institution (OR, 1.31).
At the same time, factors associated with a lower likelihood of recommended surgical care included increasing age (per year increase, OR, 0.99) and a higher Charlson/Deyo comorbidity score (OR, 0.90 for a score of 1 and OR, 0.82 for a score of 2 or more). The strongest determinant of long-term overall survival included pathologic upstaging (HR 1.84) and meeting all four quality indicators (HR 0.39). Every additional quality met was associated with a significant reduction in overall mortality.
“We believe this study can be a starting point to draw attention to institution- and surgeon-specific practice patterns that may vary widely,” Dr. Samson said in an interview prior to the meeting. “At our own institution, we are working to decrease time to surgery, as well as implementing quality improvement measures to increase nodal sampling rates. Improving these trends nationally must start at the local level, with a tailored approach.”
Dr. Samson is currently supported by a T32 NIH training grant for research fellows in cardiothoracic surgery. Study coauthor Dr. Bryan Meyers, has received honoraria from Varian Medical Systems and is a consultant/advisory board member of Ethicon. Senior author Dr. Varun Puri is supported by NIH career awards.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE STS ANNUAL MEETING
Key clinical point: At the national level, compliance with core indicators for surgery in stage I NSCLC is poor.
Major finding: Between 2004 and 2013, nearly 100% of patients met at least one of four recommended criteria for evaluation of stage I NSCLC, 95% met two, 69% met three, and 22% met all four.
Data source: An analysis of 146,908 patients undergoing surgery for clinical stage I NSCLC between 2004 and 2013.
Disclosures: Dr. Samson is currently supported by a T32 NIH training grant for research fellows in cardiothoracic surgery. Study coauthor Dr. Bryan Meyers, has received honoraria from Varian Medical Systems and is a consultant/advisory board member of Ethicon. Senior author Dr. Varun Puri is supported by NIH career awards.
VIDEO: Expert discusses VATS thymectomy for myasthenia gravis
PHOENIX – In the clinical experience of Dr. Joshua R. Sonett, VATS thymectomy for myasthenia gravis is best performed in a bilateral thoracoscopic fashion.
In this approach, surgeons do about 95% of the operation on the left side to form a maximal thymectomy, “and finish taking out the specimen on the right side, making sure we can see both phrenic nerves in their entirety,” Dr. Sonett, chief of general thoracic surgery at Columbia University Medical Center, New York, said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
Although there is no proof to date that thymectomy improves long-term outcomes for patients with myasthenia gravis, results from a large, international trial sponsored by the National Institutes of Health are expected to inform clinical practice about this topic, said Dr. Sonett, who is also director of the university’s high-risk lung assessment program.
Dr. Sonett reported having no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – In the clinical experience of Dr. Joshua R. Sonett, VATS thymectomy for myasthenia gravis is best performed in a bilateral thoracoscopic fashion.
In this approach, surgeons do about 95% of the operation on the left side to form a maximal thymectomy, “and finish taking out the specimen on the right side, making sure we can see both phrenic nerves in their entirety,” Dr. Sonett, chief of general thoracic surgery at Columbia University Medical Center, New York, said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
Although there is no proof to date that thymectomy improves long-term outcomes for patients with myasthenia gravis, results from a large, international trial sponsored by the National Institutes of Health are expected to inform clinical practice about this topic, said Dr. Sonett, who is also director of the university’s high-risk lung assessment program.
Dr. Sonett reported having no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – In the clinical experience of Dr. Joshua R. Sonett, VATS thymectomy for myasthenia gravis is best performed in a bilateral thoracoscopic fashion.
In this approach, surgeons do about 95% of the operation on the left side to form a maximal thymectomy, “and finish taking out the specimen on the right side, making sure we can see both phrenic nerves in their entirety,” Dr. Sonett, chief of general thoracic surgery at Columbia University Medical Center, New York, said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
Although there is no proof to date that thymectomy improves long-term outcomes for patients with myasthenia gravis, results from a large, international trial sponsored by the National Institutes of Health are expected to inform clinical practice about this topic, said Dr. Sonett, who is also director of the university’s high-risk lung assessment program.
Dr. Sonett reported having no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYIS FROM THE STS ANNUAL MEETING
STS: BMI impacts risk for complications after lung resection
PHOENIX – Being underweight is associated with a substantially increased risk of complications following lung resection for cancer, results from a large database study found.
“This is not generally known among surgeons or their patients,” Dr. Trevor Williams said in an interview before the annual meeting of the Society of Thoracic Surgeons. “Studies are conflicting about the relationship of BMI [body mass index] to surgical outcomes. Most of the previous studies simply categorize BMI as overweight or not. We’ve stratified based on World Health Organization categories to get a more precise look at BMI.”
Dr. Williams, a surgeon at the University of Chicago Medical Center, and his associates evaluated 41,446 patients in the STS General Thoracic Surgery Database who underwent elective anatomic lung resection for cancer between 2009 and 2014. Their mean age was 68 years, and 53% were female. The researchers performed multivariable analysis after adjusting for validated STS risk model covariates, including gender and spirometry.
According to WHO criteria for BMI, 3% were underweight (less than 18.5 kg/m2); 33.5% were normal weight (18.5-24.9 kg/m2); 35.4% were overweight (25-29.9 kg/m2); 18.1% were obese I (30-34.9 kg/m2); 6.4% were obese II (35-39.9 kg/m2), and 3.6% were obese III (40 kg/m2 or greater). Dr. Williams and his associates observed that women were more often underweight, compared with men (4.1% vs. 1.8%, respectively; P less than .001), and underweight patients more often had chronic obstructive pulmonary disease (51.7% vs. 35.2%; P less than .001). Pulmonary complication rates were higher among underweight and obese III patients (P less than .001), while being underweight was also associated with higher rates of infections and any surgical complications.
Multivariable analysis revealed that pulmonary and any postoperative complications were more common among underweight patients (odds ratio, 1.44 and OR, 1.41, respectively), while any major complication was more common among obese III patients (OR, 1.18). Overweight and obese I-II patients were less likely to have any postoperative and pulmonary complications, compared with patients who had a normal BMI. “The finding of underweight patients being such a high-risk patient population is suggested in the literature but not demonstrated as clearly as in this study,” Dr. Williams said. “A truly surprising finding was that obese patients actually have a lower risk of pulmonary and overall complications than ‘normal’-BMI patients.”
He concluded that according to the current analysis, “careful risk assessment is appropriate when considering operating on underweight patients. Whether there are interventions that could be instituted to improve an individual’s risk profile has not been determined. Any preconceived notions about not operating on obese patients due to elevated risk appear to be unfounded.”
Dr. Williams reported having no financial disclosures.
PHOENIX – Being underweight is associated with a substantially increased risk of complications following lung resection for cancer, results from a large database study found.
“This is not generally known among surgeons or their patients,” Dr. Trevor Williams said in an interview before the annual meeting of the Society of Thoracic Surgeons. “Studies are conflicting about the relationship of BMI [body mass index] to surgical outcomes. Most of the previous studies simply categorize BMI as overweight or not. We’ve stratified based on World Health Organization categories to get a more precise look at BMI.”
Dr. Williams, a surgeon at the University of Chicago Medical Center, and his associates evaluated 41,446 patients in the STS General Thoracic Surgery Database who underwent elective anatomic lung resection for cancer between 2009 and 2014. Their mean age was 68 years, and 53% were female. The researchers performed multivariable analysis after adjusting for validated STS risk model covariates, including gender and spirometry.
According to WHO criteria for BMI, 3% were underweight (less than 18.5 kg/m2); 33.5% were normal weight (18.5-24.9 kg/m2); 35.4% were overweight (25-29.9 kg/m2); 18.1% were obese I (30-34.9 kg/m2); 6.4% were obese II (35-39.9 kg/m2), and 3.6% were obese III (40 kg/m2 or greater). Dr. Williams and his associates observed that women were more often underweight, compared with men (4.1% vs. 1.8%, respectively; P less than .001), and underweight patients more often had chronic obstructive pulmonary disease (51.7% vs. 35.2%; P less than .001). Pulmonary complication rates were higher among underweight and obese III patients (P less than .001), while being underweight was also associated with higher rates of infections and any surgical complications.
Multivariable analysis revealed that pulmonary and any postoperative complications were more common among underweight patients (odds ratio, 1.44 and OR, 1.41, respectively), while any major complication was more common among obese III patients (OR, 1.18). Overweight and obese I-II patients were less likely to have any postoperative and pulmonary complications, compared with patients who had a normal BMI. “The finding of underweight patients being such a high-risk patient population is suggested in the literature but not demonstrated as clearly as in this study,” Dr. Williams said. “A truly surprising finding was that obese patients actually have a lower risk of pulmonary and overall complications than ‘normal’-BMI patients.”
He concluded that according to the current analysis, “careful risk assessment is appropriate when considering operating on underweight patients. Whether there are interventions that could be instituted to improve an individual’s risk profile has not been determined. Any preconceived notions about not operating on obese patients due to elevated risk appear to be unfounded.”
Dr. Williams reported having no financial disclosures.
PHOENIX – Being underweight is associated with a substantially increased risk of complications following lung resection for cancer, results from a large database study found.
“This is not generally known among surgeons or their patients,” Dr. Trevor Williams said in an interview before the annual meeting of the Society of Thoracic Surgeons. “Studies are conflicting about the relationship of BMI [body mass index] to surgical outcomes. Most of the previous studies simply categorize BMI as overweight or not. We’ve stratified based on World Health Organization categories to get a more precise look at BMI.”
Dr. Williams, a surgeon at the University of Chicago Medical Center, and his associates evaluated 41,446 patients in the STS General Thoracic Surgery Database who underwent elective anatomic lung resection for cancer between 2009 and 2014. Their mean age was 68 years, and 53% were female. The researchers performed multivariable analysis after adjusting for validated STS risk model covariates, including gender and spirometry.
According to WHO criteria for BMI, 3% were underweight (less than 18.5 kg/m2); 33.5% were normal weight (18.5-24.9 kg/m2); 35.4% were overweight (25-29.9 kg/m2); 18.1% were obese I (30-34.9 kg/m2); 6.4% were obese II (35-39.9 kg/m2), and 3.6% were obese III (40 kg/m2 or greater). Dr. Williams and his associates observed that women were more often underweight, compared with men (4.1% vs. 1.8%, respectively; P less than .001), and underweight patients more often had chronic obstructive pulmonary disease (51.7% vs. 35.2%; P less than .001). Pulmonary complication rates were higher among underweight and obese III patients (P less than .001), while being underweight was also associated with higher rates of infections and any surgical complications.
Multivariable analysis revealed that pulmonary and any postoperative complications were more common among underweight patients (odds ratio, 1.44 and OR, 1.41, respectively), while any major complication was more common among obese III patients (OR, 1.18). Overweight and obese I-II patients were less likely to have any postoperative and pulmonary complications, compared with patients who had a normal BMI. “The finding of underweight patients being such a high-risk patient population is suggested in the literature but not demonstrated as clearly as in this study,” Dr. Williams said. “A truly surprising finding was that obese patients actually have a lower risk of pulmonary and overall complications than ‘normal’-BMI patients.”
He concluded that according to the current analysis, “careful risk assessment is appropriate when considering operating on underweight patients. Whether there are interventions that could be instituted to improve an individual’s risk profile has not been determined. Any preconceived notions about not operating on obese patients due to elevated risk appear to be unfounded.”
Dr. Williams reported having no financial disclosures.
AT THE STS ANNUAL MEETING
Key clinical point: Careful risk assessment is appropriate when considering performing lung resection on underweight patients.
Major finding: Multivariable analysis revealed that pulmonary and any postoperative complications were more common among underweight patients (OR, 1.44 and OR, 1.41, respectively), while any major complication was more common among obese III patients (OR, 1.18).
Data source: An analysis of 41,446 patients in the STS General Thoracic Surgery Database who underwent elective lung resection for cancer between 2009 and 2014.
Disclosures: Dr. Williams reported having no financial disclosures.