User login
The role of immunotherapy in NSCLC to expand
SAN DIEGO – Expect an expanded role of immunotherapy in patients with non–small cell lung cancer, Dr. Roy S. Herbst predicted at the Joint Conference on the Molecular Origins of Lung Cancer sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
Dr. Herbst, who is a professor of medical oncology at Yale University, New Haven, Conn., characterized immunotherapy as "probably the most exciting new and specific therapies we have for NSCLC. The extent in its response is impressive, and this is a therapy that has memory. The adaptability of immunotherapy is important as well."
He advised researchers and clinicians to consider using immunotherapy that includes CTLA-4 antibodies, PD-1 antibodies, and PD-L1 antibodies alone or together in patients with earlier stages of lung disease. Clinical studies of immunotherapy in NSCLC patients suggest that some patients don’t get better with immunotherapy, "but a lot of patients do," said Dr. Herbst.
"We want to figure out who those patients are. When we see activity like this, we think, can we bring this therapy to earlier disease? These agents might have a role in maintenance therapy and adjuvant/neoadjuvant therapy. Of course, we worry about side effects such as pneumonitis, which occurs rarely, but we still hope these agents will have a benefit in the adjuvant setting. The biology speaks to that. But what about using these agents as maintenance therapy? I think that needs to be explored."
Using immunotherapy as frontline treatment in patients with stage IV lung cancer is also feasible, he said. "I’d feel much better about it if we had a marker, but we should think about some single-agent trials," he said.
"Other possibilities in stage IV disease include using immunotherapy with chemotherapy and with tyrosine kinase inhibitors."
Immunotherapy-related adverse events are "not overwhelming, but they’re different than what we see with chemotherapy," Dr. Herbst continued. "For example, some of the endocrine events are not something we often see. We are working on ways to manage this."
Use of biomarkers and immune monitoring can also help clinicians gauge the efficacy of immunotherapy in their NSCLC patients.
Dr. Herbst and his associates at Yale Cancer Center follow these patients with biopsies at baseline, during therapy, and at the end of therapy, "because after their therapy at 1 year or more, you wonder: Is this active tumor? Or is this necrotic tissue?" he said. "We now have ways to figure out who is responding and why they’re responding."
Another trend being seen in the future of immunotherapy involves combining with other agents that address key mechanisms in positive and negative regulation of the immune system.
Dr. Herbst explained that the biological goal of combinations with a checkpoint inhibitor include the ability to induce antigen-specific T cells, provide more antigen-presenting cells (APCs), activation/modulation of APCs, drive T-cell expansion to expand the pool of antigen-specific cells, and remove other regulatory checkpoints/suppressive factors for T-cell activation/expansion in periphery.
"The current challenge is to identify the critical deficiencies in individual patients," he said.
"We have to continue to investigate the biologic significance of all potential ligand-receptor interactions in the tumor microenvironment."
Dr. Herbst disclosed that he is on the scientific advisory boards of Biothera, Diatech, Kolltan, N of 1, Novarx, and Quintiles. He also has done consulting for Ariad, Astellas, and other companies.
SAN DIEGO – Expect an expanded role of immunotherapy in patients with non–small cell lung cancer, Dr. Roy S. Herbst predicted at the Joint Conference on the Molecular Origins of Lung Cancer sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
Dr. Herbst, who is a professor of medical oncology at Yale University, New Haven, Conn., characterized immunotherapy as "probably the most exciting new and specific therapies we have for NSCLC. The extent in its response is impressive, and this is a therapy that has memory. The adaptability of immunotherapy is important as well."
He advised researchers and clinicians to consider using immunotherapy that includes CTLA-4 antibodies, PD-1 antibodies, and PD-L1 antibodies alone or together in patients with earlier stages of lung disease. Clinical studies of immunotherapy in NSCLC patients suggest that some patients don’t get better with immunotherapy, "but a lot of patients do," said Dr. Herbst.
"We want to figure out who those patients are. When we see activity like this, we think, can we bring this therapy to earlier disease? These agents might have a role in maintenance therapy and adjuvant/neoadjuvant therapy. Of course, we worry about side effects such as pneumonitis, which occurs rarely, but we still hope these agents will have a benefit in the adjuvant setting. The biology speaks to that. But what about using these agents as maintenance therapy? I think that needs to be explored."
Using immunotherapy as frontline treatment in patients with stage IV lung cancer is also feasible, he said. "I’d feel much better about it if we had a marker, but we should think about some single-agent trials," he said.
"Other possibilities in stage IV disease include using immunotherapy with chemotherapy and with tyrosine kinase inhibitors."
Immunotherapy-related adverse events are "not overwhelming, but they’re different than what we see with chemotherapy," Dr. Herbst continued. "For example, some of the endocrine events are not something we often see. We are working on ways to manage this."
Use of biomarkers and immune monitoring can also help clinicians gauge the efficacy of immunotherapy in their NSCLC patients.
Dr. Herbst and his associates at Yale Cancer Center follow these patients with biopsies at baseline, during therapy, and at the end of therapy, "because after their therapy at 1 year or more, you wonder: Is this active tumor? Or is this necrotic tissue?" he said. "We now have ways to figure out who is responding and why they’re responding."
Another trend being seen in the future of immunotherapy involves combining with other agents that address key mechanisms in positive and negative regulation of the immune system.
Dr. Herbst explained that the biological goal of combinations with a checkpoint inhibitor include the ability to induce antigen-specific T cells, provide more antigen-presenting cells (APCs), activation/modulation of APCs, drive T-cell expansion to expand the pool of antigen-specific cells, and remove other regulatory checkpoints/suppressive factors for T-cell activation/expansion in periphery.
"The current challenge is to identify the critical deficiencies in individual patients," he said.
"We have to continue to investigate the biologic significance of all potential ligand-receptor interactions in the tumor microenvironment."
Dr. Herbst disclosed that he is on the scientific advisory boards of Biothera, Diatech, Kolltan, N of 1, Novarx, and Quintiles. He also has done consulting for Ariad, Astellas, and other companies.
SAN DIEGO – Expect an expanded role of immunotherapy in patients with non–small cell lung cancer, Dr. Roy S. Herbst predicted at the Joint Conference on the Molecular Origins of Lung Cancer sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
Dr. Herbst, who is a professor of medical oncology at Yale University, New Haven, Conn., characterized immunotherapy as "probably the most exciting new and specific therapies we have for NSCLC. The extent in its response is impressive, and this is a therapy that has memory. The adaptability of immunotherapy is important as well."
He advised researchers and clinicians to consider using immunotherapy that includes CTLA-4 antibodies, PD-1 antibodies, and PD-L1 antibodies alone or together in patients with earlier stages of lung disease. Clinical studies of immunotherapy in NSCLC patients suggest that some patients don’t get better with immunotherapy, "but a lot of patients do," said Dr. Herbst.
"We want to figure out who those patients are. When we see activity like this, we think, can we bring this therapy to earlier disease? These agents might have a role in maintenance therapy and adjuvant/neoadjuvant therapy. Of course, we worry about side effects such as pneumonitis, which occurs rarely, but we still hope these agents will have a benefit in the adjuvant setting. The biology speaks to that. But what about using these agents as maintenance therapy? I think that needs to be explored."
Using immunotherapy as frontline treatment in patients with stage IV lung cancer is also feasible, he said. "I’d feel much better about it if we had a marker, but we should think about some single-agent trials," he said.
"Other possibilities in stage IV disease include using immunotherapy with chemotherapy and with tyrosine kinase inhibitors."
Immunotherapy-related adverse events are "not overwhelming, but they’re different than what we see with chemotherapy," Dr. Herbst continued. "For example, some of the endocrine events are not something we often see. We are working on ways to manage this."
Use of biomarkers and immune monitoring can also help clinicians gauge the efficacy of immunotherapy in their NSCLC patients.
Dr. Herbst and his associates at Yale Cancer Center follow these patients with biopsies at baseline, during therapy, and at the end of therapy, "because after their therapy at 1 year or more, you wonder: Is this active tumor? Or is this necrotic tissue?" he said. "We now have ways to figure out who is responding and why they’re responding."
Another trend being seen in the future of immunotherapy involves combining with other agents that address key mechanisms in positive and negative regulation of the immune system.
Dr. Herbst explained that the biological goal of combinations with a checkpoint inhibitor include the ability to induce antigen-specific T cells, provide more antigen-presenting cells (APCs), activation/modulation of APCs, drive T-cell expansion to expand the pool of antigen-specific cells, and remove other regulatory checkpoints/suppressive factors for T-cell activation/expansion in periphery.
"The current challenge is to identify the critical deficiencies in individual patients," he said.
"We have to continue to investigate the biologic significance of all potential ligand-receptor interactions in the tumor microenvironment."
Dr. Herbst disclosed that he is on the scientific advisory boards of Biothera, Diatech, Kolltan, N of 1, Novarx, and Quintiles. He also has done consulting for Ariad, Astellas, and other companies.
Endoscopic resection for adenocarcinoma
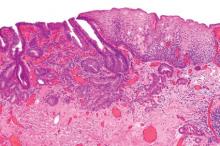
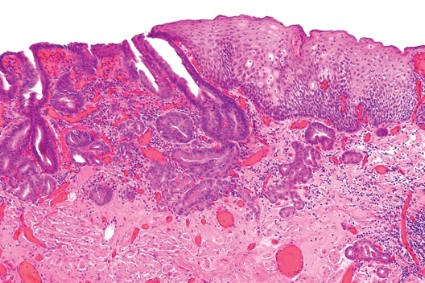
Endoscopic resection for mucosal esophageal adenocarcinoma is safe and highly effective, and should be the new standard of care. That's according to Dr. Oliver Pech, whose study in the March issue of Gastroenterology showed a complete remission rate of 93.8% over nearly 5 years of follow-up (doi: 10.1053/j.gasro.2013.11.006).
Dr. Pech of the University of Regensburg (Germany) and his colleagues looked at 1,000 consecutive patients (mean age, 69 years; 861 men) with mucosal adenocarcinoma of the esophagus, referred to a single center from October 1996 to September 2010.
All patients had mucosal Barrett's carcinoma; lesions judged resectable were subjected to diagnostic endoscopic resection for staging, even when the macroscopic appearance suggested submucosal disease. Patients with low-grade dysplasia, high-grade dysplasia, and submucosal or more advanced cancer (T1 or greater) were excluded.
In total, 481 patients had short-segment Barrett's esophagus, and the remainder had long-segment Barrett's. The majority (n = 493) had intraepithelial adenocarcinoma, according to staging by endoscopic resection, while 240 patients had adenocarcinoma invading the tunica propria, 124 had invasion of the first layer of the muscularis mucosae, and the remaining 143 had disease of the second layer of the muscularis mucosae. En bloc resection was performed in 508 patients; piecemeal resection in the rest.
Complete remission, defined as an R0 resection plus one normal surveillance endoscopy, was achieved in 963 (96.3%) of 1,000 patients in the study.
Among these, recurrence of neoplasia (high-grade dysplasia or adenocarcinoma) was detected in 14.5% of the patients (140 out of the 963) after a median 26.5 months; 115 were successfully retreated with additional endoscopic resection. That translated to a long-term complete remission rate of 93.8% (mean, 56.6 months) and a 5-year survival rate of 91.5%.
Looking at safety, Dr. Pech reported that 15 patients experienced major complications, including bleeding with a corresponding drop in hemoglobin of at least 2 g/dL (in 14 cases) and perforation (in 1). He added that the relatively minor complication of stenosis requiring dilation occurred in 13 cases, all of which were managed endoscopically. Finally, in an analysis of which patients were more likely to have successful endoscopic treatment, they determined that long-segment Barrett's as well as poorly differentiated mucosal adenocarcinoma had a significantly higher risk for failure (P less than .0001 for both).
The authors conceded that referral bias cannot be excluded in this cohort. Additionally, over the long course of the study, best practices for Barrett's esophagus and high-grade dysplasia have evolved considerably.
The authors had no disclosures.
Endoscopic resection for mucosal esophageal adenocarcinoma is safe and highly effective, and should be the new standard of care. That's according to Dr. Oliver Pech, whose study in the March issue of Gastroenterology showed a complete remission rate of 93.8% over nearly 5 years of follow-up (doi: 10.1053/j.gasro.2013.11.006).
Dr. Pech of the University of Regensburg (Germany) and his colleagues looked at 1,000 consecutive patients (mean age, 69 years; 861 men) with mucosal adenocarcinoma of the esophagus, referred to a single center from October 1996 to September 2010.
All patients had mucosal Barrett's carcinoma; lesions judged resectable were subjected to diagnostic endoscopic resection for staging, even when the macroscopic appearance suggested submucosal disease. Patients with low-grade dysplasia, high-grade dysplasia, and submucosal or more advanced cancer (T1 or greater) were excluded.
In total, 481 patients had short-segment Barrett's esophagus, and the remainder had long-segment Barrett's. The majority (n = 493) had intraepithelial adenocarcinoma, according to staging by endoscopic resection, while 240 patients had adenocarcinoma invading the tunica propria, 124 had invasion of the first layer of the muscularis mucosae, and the remaining 143 had disease of the second layer of the muscularis mucosae. En bloc resection was performed in 508 patients; piecemeal resection in the rest.
Complete remission, defined as an R0 resection plus one normal surveillance endoscopy, was achieved in 963 (96.3%) of 1,000 patients in the study.
Among these, recurrence of neoplasia (high-grade dysplasia or adenocarcinoma) was detected in 14.5% of the patients (140 out of the 963) after a median 26.5 months; 115 were successfully retreated with additional endoscopic resection. That translated to a long-term complete remission rate of 93.8% (mean, 56.6 months) and a 5-year survival rate of 91.5%.
Looking at safety, Dr. Pech reported that 15 patients experienced major complications, including bleeding with a corresponding drop in hemoglobin of at least 2 g/dL (in 14 cases) and perforation (in 1). He added that the relatively minor complication of stenosis requiring dilation occurred in 13 cases, all of which were managed endoscopically. Finally, in an analysis of which patients were more likely to have successful endoscopic treatment, they determined that long-segment Barrett's as well as poorly differentiated mucosal adenocarcinoma had a significantly higher risk for failure (P less than .0001 for both).
The authors conceded that referral bias cannot be excluded in this cohort. Additionally, over the long course of the study, best practices for Barrett's esophagus and high-grade dysplasia have evolved considerably.
The authors had no disclosures.
Endoscopic resection for mucosal esophageal adenocarcinoma is safe and highly effective, and should be the new standard of care. That's according to Dr. Oliver Pech, whose study in the March issue of Gastroenterology showed a complete remission rate of 93.8% over nearly 5 years of follow-up (doi: 10.1053/j.gasro.2013.11.006).
Dr. Pech of the University of Regensburg (Germany) and his colleagues looked at 1,000 consecutive patients (mean age, 69 years; 861 men) with mucosal adenocarcinoma of the esophagus, referred to a single center from October 1996 to September 2010.
All patients had mucosal Barrett's carcinoma; lesions judged resectable were subjected to diagnostic endoscopic resection for staging, even when the macroscopic appearance suggested submucosal disease. Patients with low-grade dysplasia, high-grade dysplasia, and submucosal or more advanced cancer (T1 or greater) were excluded.
In total, 481 patients had short-segment Barrett's esophagus, and the remainder had long-segment Barrett's. The majority (n = 493) had intraepithelial adenocarcinoma, according to staging by endoscopic resection, while 240 patients had adenocarcinoma invading the tunica propria, 124 had invasion of the first layer of the muscularis mucosae, and the remaining 143 had disease of the second layer of the muscularis mucosae. En bloc resection was performed in 508 patients; piecemeal resection in the rest.
Complete remission, defined as an R0 resection plus one normal surveillance endoscopy, was achieved in 963 (96.3%) of 1,000 patients in the study.
Among these, recurrence of neoplasia (high-grade dysplasia or adenocarcinoma) was detected in 14.5% of the patients (140 out of the 963) after a median 26.5 months; 115 were successfully retreated with additional endoscopic resection. That translated to a long-term complete remission rate of 93.8% (mean, 56.6 months) and a 5-year survival rate of 91.5%.
Looking at safety, Dr. Pech reported that 15 patients experienced major complications, including bleeding with a corresponding drop in hemoglobin of at least 2 g/dL (in 14 cases) and perforation (in 1). He added that the relatively minor complication of stenosis requiring dilation occurred in 13 cases, all of which were managed endoscopically. Finally, in an analysis of which patients were more likely to have successful endoscopic treatment, they determined that long-segment Barrett's as well as poorly differentiated mucosal adenocarcinoma had a significantly higher risk for failure (P less than .0001 for both).
The authors conceded that referral bias cannot be excluded in this cohort. Additionally, over the long course of the study, best practices for Barrett's esophagus and high-grade dysplasia have evolved considerably.
The authors had no disclosures.
Major finding: Endoscopic resection of esophageal adenocarcinoma resulted in a long-term complete remission rate of 93.8%.
Data source: Data from 1,000 consecutive patients with mucosal adenocarcinoma of the esophagus.
Disclosures: The authors stated that they had no conflicts of interest to disclose. They disclosed no funding.
Anti-PD-L1 therapy shows promise in early NSCLC trials
SAN DIEGO – Results from studies conducted to date indicate that anti-PD-L1 therapy is well tolerated in patients with non–small cell lung cancer, with rapid response rates.
Responses are "not only very rapid, they’re also very durable," Dr. Leora Horn said at the Joint Conference on the Molecular Origins of Lung Cancer sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
"We’re seeing continued responses even when treatment is discontinued. The response rates appear to be somewhat higher in tumors that express PD-L1, and we see no pneumonitis or treatment-related grade 5 adverse events to date."
The four anti-PD-L1 agents that have been investigated or are currently being investigated in patients with non–small cell lung cancer (NSCLC) are BMS-936559, MPDL3280A, MEDI-4736, and MSB0010718C, said Dr. Horn, clinical director of the thoracic oncology research program at Vanderbilt University, Nashville, Tenn.
BMS-936559, developed by Bristol-Myers Squibb, was part of a phase IA dose study at 0.3, 1, 3, and 10 mg/kg in patients with multiple tumor types including NSCLC. Of the 207 patients enrolled, 75 had NSCLC (N. Engl. J. Med 2012;366:2455-65).
More than half of all patients (61%) had treatment-related adverse events, with fatigue the most common (16%), followed by infusion reaction (10%) and diarrhea (9%). Grade 3 and 4 adverse events occurred in 9% of patients. "One of the themes with anti-PD-L1 therapy is that we’re not seeing grade 3-5 pneumonitis in these like we have seen with anti-PD-1 agents," said Dr. Horn. "This may have to do with different expression of PD-1 and PD-2 within the body and the targets of PD-1 compared to anti-PD-L1 antibodies."
The response rate among NSCLC patients treated in the trial reached about 10%, and responses were seen in squamous and nonsquamous NSCLC. "This agent is not being further developed in NSCLC patients," Dr. Horn said. "Nivolumab, a PD-1- blocking antibody, has really taken over as far as development for Bristol-Myers Squibb."
The next agent she discussed, MPDL3280A, is being developed by Roche and Genentech. A phase I trial presented at the 2013 European Society for Medical Oncology (ESMO) Congress examined different doses in 85 patients with NSCLC ranging from 10 to 20 mg/kg once every week for just under 1 year. Key eligibility criteria were measurable disease per Response Evaluation Criteria in Solid Tumors v1.1 and Eastern Cooperative Oncology Group Performance Status 0 or 1. Dr. Horn said that the majority of adverse events were grade 1-2 and did not require intervention. "There was also no maximum tolerated dose or dose-limiting toxicities, and no grade 3-5 pneumonitis was observed," she said.
In a study of MPDL3280A presented by Dr. Horn and her associates at the 2013 World Conference on Lung Cancer, the clinical impact was assessed in 53 patients with NSCLC. Patients first dosed at 1-20 mg/kg by Oct. 1, 2012, with data cutoff on April 30, 2013. The response rate was 83% among patients who had an immunohistochemistry (IHC) score of 3, 46% among those who were IHC 2 and 3, and 31% among those who were IHC 1, 2, and 3. "In all-comers the response rate was 23%," Dr. Horn said. "The responses in these patients are very rapid. We see them by their first or second CT [computed tomography] scan. The responses are also very durable regardless of their IHC status and in patients who are PD-L1 negative. They’ve also been responding even after treatment has been discontinued."
She and her associates examined the response rate to MPDL3280A based on smoking status and mutational status. The response rate was higher in patients who were current or former smokers compared with never smokers (26% vs. 10%, respectively). By molecular status, the response rate in EGFR [epidermal growth factor receptor] wild-type patients was 26%, compared with 17% in EGFR mutant patients. In addition, there was a 30% response rate in patients who were KRAS wild-type and a 10% response rate in KRAS mutant patients, although the numbers are very small, she noted.
Dr. Horn said there are three separate, ongoing studies looking at MPDL3280A in NSCLC patients: one in patients with PD-L1-positive NSCLC, one in combination with bevacizumab and/or chemotherapy, and one randomized phase III trial comparing the agent with docetaxel in patients after platinum failure.
Data on the next agent Dr. Horn discussed, MEDI-4736 from MedImmune, are limited. A study was presented at the 2013 ESMO Congress and included 11 patients who received doses of 0.1, 0.3, and 1.0 mg/kg. The most common adverse events were diarrhea, vomiting, and dizziness (18% each), and no grade 3 or 4 adverse events or pneumonitis have been reported to date.
Pharmacokinetic studies of MEDI-4736 indicate a dose-dependent increase in target engagement, consistent with binding of the agent to PD-L1. Initial clinical data on eight patients demonstrated response at all different dose levels, ranging from 42% to 80%. "This involves small subsets of patients, so hopefully we’ll see more data on this agent in the next couple of years," Dr. Horn said.
The final anti-PD-L1 in clinical development is MSB0010718C from EMD Serono, but no data are available yet. A three-dose escalation study up to 10 mg/kg is underway. "Hopefully, we’ll see some data on this later this year," Dr. Horn said.
Dr. Horn disclosed that she is a speaker for Bristol-Myers Squibb, Theradex, and other companies.
The PD-L1/PD-1 ligand complex is a natural suppressive pathway used by cells to inhibit IL-2 production and T-cell proliferation so that inflammation is kept under control. However, some remarkably clever cancers including renal cell, ovarian, and non–small cell lung cancer exploit this pathway by up-regulating PD-L1 to evade and hide from the host’s immune system by suppressing inflammation. Four different monoclonal antibodies, such as MPDL3280A, that block PD-L1 have been developed. By blocking this anti-inflammatory pathway, these agents expose the cancer to the host’s activated immune system – the activated "killer" (cytotoxic) T cells.
| Dr. Lary Robinson |
Early results in a number of phase I clinical trials of anti-PD-L1 agents have shown remarkable and exciting responses using these minimally toxic agents in patients with highly chemoresistant stage IV lung cancer. Somewhat higher responses rates are seen with these agents, and the results are rapid and durable even when treatment is discontinued. This novel immunotherapy approach to systemic treatment of lung cancer is regarded by thoracic oncologists as a potential breakthrough in treatment, and it may soon become the preferred first-line, well-tolerated therapy for this very large group of metastatic lung cancer patients who express high levels of PD-L1, as well as PD-L1–negative tumors. Numerous trials with these agents are ongoing in order to ascertain the most appropriate dosages, potential use with other chemotherapy drugs, and most suitable patient population.
Dr. Lary A. Robinson is professor of thoracic surgery and interdisciplinary oncology at the University of South Florida, Tampa.
The PD-L1/PD-1 ligand complex is a natural suppressive pathway used by cells to inhibit IL-2 production and T-cell proliferation so that inflammation is kept under control. However, some remarkably clever cancers including renal cell, ovarian, and non–small cell lung cancer exploit this pathway by up-regulating PD-L1 to evade and hide from the host’s immune system by suppressing inflammation. Four different monoclonal antibodies, such as MPDL3280A, that block PD-L1 have been developed. By blocking this anti-inflammatory pathway, these agents expose the cancer to the host’s activated immune system – the activated "killer" (cytotoxic) T cells.
| Dr. Lary Robinson |
Early results in a number of phase I clinical trials of anti-PD-L1 agents have shown remarkable and exciting responses using these minimally toxic agents in patients with highly chemoresistant stage IV lung cancer. Somewhat higher responses rates are seen with these agents, and the results are rapid and durable even when treatment is discontinued. This novel immunotherapy approach to systemic treatment of lung cancer is regarded by thoracic oncologists as a potential breakthrough in treatment, and it may soon become the preferred first-line, well-tolerated therapy for this very large group of metastatic lung cancer patients who express high levels of PD-L1, as well as PD-L1–negative tumors. Numerous trials with these agents are ongoing in order to ascertain the most appropriate dosages, potential use with other chemotherapy drugs, and most suitable patient population.
Dr. Lary A. Robinson is professor of thoracic surgery and interdisciplinary oncology at the University of South Florida, Tampa.
The PD-L1/PD-1 ligand complex is a natural suppressive pathway used by cells to inhibit IL-2 production and T-cell proliferation so that inflammation is kept under control. However, some remarkably clever cancers including renal cell, ovarian, and non–small cell lung cancer exploit this pathway by up-regulating PD-L1 to evade and hide from the host’s immune system by suppressing inflammation. Four different monoclonal antibodies, such as MPDL3280A, that block PD-L1 have been developed. By blocking this anti-inflammatory pathway, these agents expose the cancer to the host’s activated immune system – the activated "killer" (cytotoxic) T cells.
| Dr. Lary Robinson |
Early results in a number of phase I clinical trials of anti-PD-L1 agents have shown remarkable and exciting responses using these minimally toxic agents in patients with highly chemoresistant stage IV lung cancer. Somewhat higher responses rates are seen with these agents, and the results are rapid and durable even when treatment is discontinued. This novel immunotherapy approach to systemic treatment of lung cancer is regarded by thoracic oncologists as a potential breakthrough in treatment, and it may soon become the preferred first-line, well-tolerated therapy for this very large group of metastatic lung cancer patients who express high levels of PD-L1, as well as PD-L1–negative tumors. Numerous trials with these agents are ongoing in order to ascertain the most appropriate dosages, potential use with other chemotherapy drugs, and most suitable patient population.
Dr. Lary A. Robinson is professor of thoracic surgery and interdisciplinary oncology at the University of South Florida, Tampa.
SAN DIEGO – Results from studies conducted to date indicate that anti-PD-L1 therapy is well tolerated in patients with non–small cell lung cancer, with rapid response rates.
Responses are "not only very rapid, they’re also very durable," Dr. Leora Horn said at the Joint Conference on the Molecular Origins of Lung Cancer sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
"We’re seeing continued responses even when treatment is discontinued. The response rates appear to be somewhat higher in tumors that express PD-L1, and we see no pneumonitis or treatment-related grade 5 adverse events to date."
The four anti-PD-L1 agents that have been investigated or are currently being investigated in patients with non–small cell lung cancer (NSCLC) are BMS-936559, MPDL3280A, MEDI-4736, and MSB0010718C, said Dr. Horn, clinical director of the thoracic oncology research program at Vanderbilt University, Nashville, Tenn.
BMS-936559, developed by Bristol-Myers Squibb, was part of a phase IA dose study at 0.3, 1, 3, and 10 mg/kg in patients with multiple tumor types including NSCLC. Of the 207 patients enrolled, 75 had NSCLC (N. Engl. J. Med 2012;366:2455-65).
More than half of all patients (61%) had treatment-related adverse events, with fatigue the most common (16%), followed by infusion reaction (10%) and diarrhea (9%). Grade 3 and 4 adverse events occurred in 9% of patients. "One of the themes with anti-PD-L1 therapy is that we’re not seeing grade 3-5 pneumonitis in these like we have seen with anti-PD-1 agents," said Dr. Horn. "This may have to do with different expression of PD-1 and PD-2 within the body and the targets of PD-1 compared to anti-PD-L1 antibodies."
The response rate among NSCLC patients treated in the trial reached about 10%, and responses were seen in squamous and nonsquamous NSCLC. "This agent is not being further developed in NSCLC patients," Dr. Horn said. "Nivolumab, a PD-1- blocking antibody, has really taken over as far as development for Bristol-Myers Squibb."
The next agent she discussed, MPDL3280A, is being developed by Roche and Genentech. A phase I trial presented at the 2013 European Society for Medical Oncology (ESMO) Congress examined different doses in 85 patients with NSCLC ranging from 10 to 20 mg/kg once every week for just under 1 year. Key eligibility criteria were measurable disease per Response Evaluation Criteria in Solid Tumors v1.1 and Eastern Cooperative Oncology Group Performance Status 0 or 1. Dr. Horn said that the majority of adverse events were grade 1-2 and did not require intervention. "There was also no maximum tolerated dose or dose-limiting toxicities, and no grade 3-5 pneumonitis was observed," she said.
In a study of MPDL3280A presented by Dr. Horn and her associates at the 2013 World Conference on Lung Cancer, the clinical impact was assessed in 53 patients with NSCLC. Patients first dosed at 1-20 mg/kg by Oct. 1, 2012, with data cutoff on April 30, 2013. The response rate was 83% among patients who had an immunohistochemistry (IHC) score of 3, 46% among those who were IHC 2 and 3, and 31% among those who were IHC 1, 2, and 3. "In all-comers the response rate was 23%," Dr. Horn said. "The responses in these patients are very rapid. We see them by their first or second CT [computed tomography] scan. The responses are also very durable regardless of their IHC status and in patients who are PD-L1 negative. They’ve also been responding even after treatment has been discontinued."
She and her associates examined the response rate to MPDL3280A based on smoking status and mutational status. The response rate was higher in patients who were current or former smokers compared with never smokers (26% vs. 10%, respectively). By molecular status, the response rate in EGFR [epidermal growth factor receptor] wild-type patients was 26%, compared with 17% in EGFR mutant patients. In addition, there was a 30% response rate in patients who were KRAS wild-type and a 10% response rate in KRAS mutant patients, although the numbers are very small, she noted.
Dr. Horn said there are three separate, ongoing studies looking at MPDL3280A in NSCLC patients: one in patients with PD-L1-positive NSCLC, one in combination with bevacizumab and/or chemotherapy, and one randomized phase III trial comparing the agent with docetaxel in patients after platinum failure.
Data on the next agent Dr. Horn discussed, MEDI-4736 from MedImmune, are limited. A study was presented at the 2013 ESMO Congress and included 11 patients who received doses of 0.1, 0.3, and 1.0 mg/kg. The most common adverse events were diarrhea, vomiting, and dizziness (18% each), and no grade 3 or 4 adverse events or pneumonitis have been reported to date.
Pharmacokinetic studies of MEDI-4736 indicate a dose-dependent increase in target engagement, consistent with binding of the agent to PD-L1. Initial clinical data on eight patients demonstrated response at all different dose levels, ranging from 42% to 80%. "This involves small subsets of patients, so hopefully we’ll see more data on this agent in the next couple of years," Dr. Horn said.
The final anti-PD-L1 in clinical development is MSB0010718C from EMD Serono, but no data are available yet. A three-dose escalation study up to 10 mg/kg is underway. "Hopefully, we’ll see some data on this later this year," Dr. Horn said.
Dr. Horn disclosed that she is a speaker for Bristol-Myers Squibb, Theradex, and other companies.
SAN DIEGO – Results from studies conducted to date indicate that anti-PD-L1 therapy is well tolerated in patients with non–small cell lung cancer, with rapid response rates.
Responses are "not only very rapid, they’re also very durable," Dr. Leora Horn said at the Joint Conference on the Molecular Origins of Lung Cancer sponsored by the American Association for Cancer Research and the International Association for the Study of Lung Cancer.
"We’re seeing continued responses even when treatment is discontinued. The response rates appear to be somewhat higher in tumors that express PD-L1, and we see no pneumonitis or treatment-related grade 5 adverse events to date."
The four anti-PD-L1 agents that have been investigated or are currently being investigated in patients with non–small cell lung cancer (NSCLC) are BMS-936559, MPDL3280A, MEDI-4736, and MSB0010718C, said Dr. Horn, clinical director of the thoracic oncology research program at Vanderbilt University, Nashville, Tenn.
BMS-936559, developed by Bristol-Myers Squibb, was part of a phase IA dose study at 0.3, 1, 3, and 10 mg/kg in patients with multiple tumor types including NSCLC. Of the 207 patients enrolled, 75 had NSCLC (N. Engl. J. Med 2012;366:2455-65).
More than half of all patients (61%) had treatment-related adverse events, with fatigue the most common (16%), followed by infusion reaction (10%) and diarrhea (9%). Grade 3 and 4 adverse events occurred in 9% of patients. "One of the themes with anti-PD-L1 therapy is that we’re not seeing grade 3-5 pneumonitis in these like we have seen with anti-PD-1 agents," said Dr. Horn. "This may have to do with different expression of PD-1 and PD-2 within the body and the targets of PD-1 compared to anti-PD-L1 antibodies."
The response rate among NSCLC patients treated in the trial reached about 10%, and responses were seen in squamous and nonsquamous NSCLC. "This agent is not being further developed in NSCLC patients," Dr. Horn said. "Nivolumab, a PD-1- blocking antibody, has really taken over as far as development for Bristol-Myers Squibb."
The next agent she discussed, MPDL3280A, is being developed by Roche and Genentech. A phase I trial presented at the 2013 European Society for Medical Oncology (ESMO) Congress examined different doses in 85 patients with NSCLC ranging from 10 to 20 mg/kg once every week for just under 1 year. Key eligibility criteria were measurable disease per Response Evaluation Criteria in Solid Tumors v1.1 and Eastern Cooperative Oncology Group Performance Status 0 or 1. Dr. Horn said that the majority of adverse events were grade 1-2 and did not require intervention. "There was also no maximum tolerated dose or dose-limiting toxicities, and no grade 3-5 pneumonitis was observed," she said.
In a study of MPDL3280A presented by Dr. Horn and her associates at the 2013 World Conference on Lung Cancer, the clinical impact was assessed in 53 patients with NSCLC. Patients first dosed at 1-20 mg/kg by Oct. 1, 2012, with data cutoff on April 30, 2013. The response rate was 83% among patients who had an immunohistochemistry (IHC) score of 3, 46% among those who were IHC 2 and 3, and 31% among those who were IHC 1, 2, and 3. "In all-comers the response rate was 23%," Dr. Horn said. "The responses in these patients are very rapid. We see them by their first or second CT [computed tomography] scan. The responses are also very durable regardless of their IHC status and in patients who are PD-L1 negative. They’ve also been responding even after treatment has been discontinued."
She and her associates examined the response rate to MPDL3280A based on smoking status and mutational status. The response rate was higher in patients who were current or former smokers compared with never smokers (26% vs. 10%, respectively). By molecular status, the response rate in EGFR [epidermal growth factor receptor] wild-type patients was 26%, compared with 17% in EGFR mutant patients. In addition, there was a 30% response rate in patients who were KRAS wild-type and a 10% response rate in KRAS mutant patients, although the numbers are very small, she noted.
Dr. Horn said there are three separate, ongoing studies looking at MPDL3280A in NSCLC patients: one in patients with PD-L1-positive NSCLC, one in combination with bevacizumab and/or chemotherapy, and one randomized phase III trial comparing the agent with docetaxel in patients after platinum failure.
Data on the next agent Dr. Horn discussed, MEDI-4736 from MedImmune, are limited. A study was presented at the 2013 ESMO Congress and included 11 patients who received doses of 0.1, 0.3, and 1.0 mg/kg. The most common adverse events were diarrhea, vomiting, and dizziness (18% each), and no grade 3 or 4 adverse events or pneumonitis have been reported to date.
Pharmacokinetic studies of MEDI-4736 indicate a dose-dependent increase in target engagement, consistent with binding of the agent to PD-L1. Initial clinical data on eight patients demonstrated response at all different dose levels, ranging from 42% to 80%. "This involves small subsets of patients, so hopefully we’ll see more data on this agent in the next couple of years," Dr. Horn said.
The final anti-PD-L1 in clinical development is MSB0010718C from EMD Serono, but no data are available yet. A three-dose escalation study up to 10 mg/kg is underway. "Hopefully, we’ll see some data on this later this year," Dr. Horn said.
Dr. Horn disclosed that she is a speaker for Bristol-Myers Squibb, Theradex, and other companies.
CT lung screen plagued by 18% overdiagnosis
An estimated 18% of the early lung cancers detected by low-dose CT screening in the National Lung Screening Trial were likely indolent and probably represent overdiagnosis, according to a report published in JAMA Internal Medicine.
The NLST found "an encouraging" 20% relative reduction in lung cancer-specific mortality among high-risk patients who were screened using low-dose CT, compared with chest radiography.
"These findings were met with enthusiasm, but before a widespread public health screening program is implemented, risks of screening also need to be considered," said Dr. Edward F. Patz Jr. of the department of radiology, Duke University Medical Center, Durham, N.C., and his associates in the NLST.
The chief risk in this case is overdiagnosis: identifying an early-stage lesion in an asymptomatic patient that would not progress or affect that patient’s long-term health.
It is likely that some of the tumors detected on low-dose CT were just such indolent cancers, and that those patients unnecessarily underwent invasive diagnostic procedures, surgical resection, and multiple follow-up studies.
To estimate how many of the detected cancers in the NLST were indolent and thus overdiagnosed, Dr. Patz and his colleagues used statistical probability methods to analyze extended follow-up data from the study.
The NLST involved 53,452 men and women aged 55-74 years who were enrolled during 2002-2004 and who had at least a 30-pack-year history of cigarette smoking.
The patients were randomly assigned to undergo lung cancer screening using either three annual low-dose CT exams or three annual single-view chest radiographs.
Mean follow-up was approximately 6 years. "At the end of the entire trial, there were 1,089 total lung cancer cases in the low-dose CT arm (649 detected by low-dose CT screening) and 969 cases in the [radiology] arm, for an excess of 120 cases. This gives [an] excess cancer rate of 18.5%," the investigators said (JAMA Intern. Med. 2013 Dec. 9 [doi: 10.1001/jamainternmed.2013.12738]).
"The data from this study suggest that ... 18% of persons in the low-dose CT arm with screen-detected lung cancer and 22% of those in the low-dose CT arm with screen-detected NSCLC [non-small cell lung cancer] may be cases of overdiagnosis," Dr. Patz and his associates said.
"In other words, if these individuals had not entered the NLST, they would not have received a lung cancer diagnosis or treatment, at least for the next 5 years."
In the future, the study authors noted, "once there are better biomarkers and imaging techniques to predict which individuals with a diagnosis of lung cancer will have more or less aggressive disease, treatment options can be optimized, and a mass screening program can become more valuable."
The National Institutes of Health supported the NSLT. No financial conflicts of interest were reported.
An estimated 18% of the early lung cancers detected by low-dose CT screening in the National Lung Screening Trial were likely indolent and probably represent overdiagnosis, according to a report published in JAMA Internal Medicine.
The NLST found "an encouraging" 20% relative reduction in lung cancer-specific mortality among high-risk patients who were screened using low-dose CT, compared with chest radiography.
"These findings were met with enthusiasm, but before a widespread public health screening program is implemented, risks of screening also need to be considered," said Dr. Edward F. Patz Jr. of the department of radiology, Duke University Medical Center, Durham, N.C., and his associates in the NLST.
The chief risk in this case is overdiagnosis: identifying an early-stage lesion in an asymptomatic patient that would not progress or affect that patient’s long-term health.
It is likely that some of the tumors detected on low-dose CT were just such indolent cancers, and that those patients unnecessarily underwent invasive diagnostic procedures, surgical resection, and multiple follow-up studies.
To estimate how many of the detected cancers in the NLST were indolent and thus overdiagnosed, Dr. Patz and his colleagues used statistical probability methods to analyze extended follow-up data from the study.
The NLST involved 53,452 men and women aged 55-74 years who were enrolled during 2002-2004 and who had at least a 30-pack-year history of cigarette smoking.
The patients were randomly assigned to undergo lung cancer screening using either three annual low-dose CT exams or three annual single-view chest radiographs.
Mean follow-up was approximately 6 years. "At the end of the entire trial, there were 1,089 total lung cancer cases in the low-dose CT arm (649 detected by low-dose CT screening) and 969 cases in the [radiology] arm, for an excess of 120 cases. This gives [an] excess cancer rate of 18.5%," the investigators said (JAMA Intern. Med. 2013 Dec. 9 [doi: 10.1001/jamainternmed.2013.12738]).
"The data from this study suggest that ... 18% of persons in the low-dose CT arm with screen-detected lung cancer and 22% of those in the low-dose CT arm with screen-detected NSCLC [non-small cell lung cancer] may be cases of overdiagnosis," Dr. Patz and his associates said.
"In other words, if these individuals had not entered the NLST, they would not have received a lung cancer diagnosis or treatment, at least for the next 5 years."
In the future, the study authors noted, "once there are better biomarkers and imaging techniques to predict which individuals with a diagnosis of lung cancer will have more or less aggressive disease, treatment options can be optimized, and a mass screening program can become more valuable."
The National Institutes of Health supported the NSLT. No financial conflicts of interest were reported.
An estimated 18% of the early lung cancers detected by low-dose CT screening in the National Lung Screening Trial were likely indolent and probably represent overdiagnosis, according to a report published in JAMA Internal Medicine.
The NLST found "an encouraging" 20% relative reduction in lung cancer-specific mortality among high-risk patients who were screened using low-dose CT, compared with chest radiography.
"These findings were met with enthusiasm, but before a widespread public health screening program is implemented, risks of screening also need to be considered," said Dr. Edward F. Patz Jr. of the department of radiology, Duke University Medical Center, Durham, N.C., and his associates in the NLST.
The chief risk in this case is overdiagnosis: identifying an early-stage lesion in an asymptomatic patient that would not progress or affect that patient’s long-term health.
It is likely that some of the tumors detected on low-dose CT were just such indolent cancers, and that those patients unnecessarily underwent invasive diagnostic procedures, surgical resection, and multiple follow-up studies.
To estimate how many of the detected cancers in the NLST were indolent and thus overdiagnosed, Dr. Patz and his colleagues used statistical probability methods to analyze extended follow-up data from the study.
The NLST involved 53,452 men and women aged 55-74 years who were enrolled during 2002-2004 and who had at least a 30-pack-year history of cigarette smoking.
The patients were randomly assigned to undergo lung cancer screening using either three annual low-dose CT exams or three annual single-view chest radiographs.
Mean follow-up was approximately 6 years. "At the end of the entire trial, there were 1,089 total lung cancer cases in the low-dose CT arm (649 detected by low-dose CT screening) and 969 cases in the [radiology] arm, for an excess of 120 cases. This gives [an] excess cancer rate of 18.5%," the investigators said (JAMA Intern. Med. 2013 Dec. 9 [doi: 10.1001/jamainternmed.2013.12738]).
"The data from this study suggest that ... 18% of persons in the low-dose CT arm with screen-detected lung cancer and 22% of those in the low-dose CT arm with screen-detected NSCLC [non-small cell lung cancer] may be cases of overdiagnosis," Dr. Patz and his associates said.
"In other words, if these individuals had not entered the NLST, they would not have received a lung cancer diagnosis or treatment, at least for the next 5 years."
In the future, the study authors noted, "once there are better biomarkers and imaging techniques to predict which individuals with a diagnosis of lung cancer will have more or less aggressive disease, treatment options can be optimized, and a mass screening program can become more valuable."
The National Institutes of Health supported the NSLT. No financial conflicts of interest were reported.
Esophagectomy treatment response nodes provide prognostic information
ORLANDO – Tumor response nodes obtained from patients who undergo esophagectomy for early-stage adenocarcinoma provide valuable prognostic information, according to Dr. Dylan Nieman.
Such nodes – lymph nodes with evidence of neoadjuvant treatment effect without residual cancer cells – may mark the prior spread of tumor, and should be counted as positive, Dr. Nieman of the University of Rochester (N.Y.) said at the annual meeting of the Society of Thoracic Surgeons.
The current practice of ignoring these nodes likely results in systematic pathologic understaging, he explained.
In 90 patients who underwent esophagectomy after neoadjuvant therapy for esophageal adenocarcinoma, the median number of nodes found per resection specimen was 18. A total of 100 tumor response nodes without viable malignant cells were identified in 38 (42%) of the patients.
The majority of the patients with treatment response nodes had only one node detected, Dr. Nieman said.
The median survival for the entire cohort was 55.6 months, and the 5-year survival was 35%. The median survival of patients with evidence of treatment response nodes was poorer, but not significantly different from those without treatment response nodes (45.9 vs. 55.6 months), he noted.
"However, for a subset of 62 patients classified as having limited or no nodal involvement – that is, AJCC N0 or N1 – the presence of treatment response nodes was associated with significantly poorer survival. This effect remained when adjusting for patient age and [American Joint Committee on Cancer] stage (hazard ratio, 2.7)," he said.
In a subset of 46 patients with pathologic AJCC stage 2B or less, the presence of treatment response nodes was still associated with poorer survival, even after adjustment for age and AJCC stage.
"To look at this a different way, if tumor response nodes were to be counted as positive, the nodal status of 29 of these patients would be upstaged. This includes 18 of 39 patients, or 46%, who were classified as node negative, and 8 of 23 patients, or 34%, who were classified as N1 by AJCC pathological assessment," he said.
The recategorization of those 29 patients resulted in better survival for the entire group, but particularly for those in the lowest-stage group, he noted.
When investigators modeled stage-adjusted survival, the counting of tumor response nodes as positive offered a better model fit, compared with following the current practice of ignoring tumor response nodes, he explained.
Patients included in the study were identified from a prospectively collected clinical database of esophagectomy patients, and were treated with neoadjuvant therapy for esophageal adenocarcinoma between 2006 and 2011. Most (82 of 90) were men. The patients had a mean age of 62 years, and were followed for a median of 27 months. Forty patients received preoperative chemotherapy, and 50 received preoperative chemoradiation.
In all cases, pathologic resection margins were negative.
On pathologic review, the majority had T3 tumors. More than 40% were staged as node negative, and more than two-thirds were staged with N0 or N1 disease.
"Prior to neoadjuvant therapy, all of these patients were clinically staged stage 2 or stage 3, but at the time of resection after neoadjuvant therapy, by AJCC staging, 24% of the patients were stage 0 or 1, 27% were stage 2, and almost half were stage 3," he said.
The findings are notable, because the current AJCC pathologic staging for esophageal adenocarcinoma is derived from the experience of patients undergoing esophagectomy alone. This approach has unclear relevance in patients who receive multimodality therapy, which has supplanted primary surgery as the standard of care for locoregionally advanced esophageal adenocarcinoma, he said.
"Future efforts at revising the staging system for esophageal adenocarcinoma should consider treatment response lymph nodes. ... We currently have pathological staging models that are of limited usefulness for our neoadjuvantly treated population. Perhaps consideration of these nodes can help improve that," he concluded.
Dr. Nieman reported having no financial disclosures.
ORLANDO – Tumor response nodes obtained from patients who undergo esophagectomy for early-stage adenocarcinoma provide valuable prognostic information, according to Dr. Dylan Nieman.
Such nodes – lymph nodes with evidence of neoadjuvant treatment effect without residual cancer cells – may mark the prior spread of tumor, and should be counted as positive, Dr. Nieman of the University of Rochester (N.Y.) said at the annual meeting of the Society of Thoracic Surgeons.
The current practice of ignoring these nodes likely results in systematic pathologic understaging, he explained.
In 90 patients who underwent esophagectomy after neoadjuvant therapy for esophageal adenocarcinoma, the median number of nodes found per resection specimen was 18. A total of 100 tumor response nodes without viable malignant cells were identified in 38 (42%) of the patients.
The majority of the patients with treatment response nodes had only one node detected, Dr. Nieman said.
The median survival for the entire cohort was 55.6 months, and the 5-year survival was 35%. The median survival of patients with evidence of treatment response nodes was poorer, but not significantly different from those without treatment response nodes (45.9 vs. 55.6 months), he noted.
"However, for a subset of 62 patients classified as having limited or no nodal involvement – that is, AJCC N0 or N1 – the presence of treatment response nodes was associated with significantly poorer survival. This effect remained when adjusting for patient age and [American Joint Committee on Cancer] stage (hazard ratio, 2.7)," he said.
In a subset of 46 patients with pathologic AJCC stage 2B or less, the presence of treatment response nodes was still associated with poorer survival, even after adjustment for age and AJCC stage.
"To look at this a different way, if tumor response nodes were to be counted as positive, the nodal status of 29 of these patients would be upstaged. This includes 18 of 39 patients, or 46%, who were classified as node negative, and 8 of 23 patients, or 34%, who were classified as N1 by AJCC pathological assessment," he said.
The recategorization of those 29 patients resulted in better survival for the entire group, but particularly for those in the lowest-stage group, he noted.
When investigators modeled stage-adjusted survival, the counting of tumor response nodes as positive offered a better model fit, compared with following the current practice of ignoring tumor response nodes, he explained.
Patients included in the study were identified from a prospectively collected clinical database of esophagectomy patients, and were treated with neoadjuvant therapy for esophageal adenocarcinoma between 2006 and 2011. Most (82 of 90) were men. The patients had a mean age of 62 years, and were followed for a median of 27 months. Forty patients received preoperative chemotherapy, and 50 received preoperative chemoradiation.
In all cases, pathologic resection margins were negative.
On pathologic review, the majority had T3 tumors. More than 40% were staged as node negative, and more than two-thirds were staged with N0 or N1 disease.
"Prior to neoadjuvant therapy, all of these patients were clinically staged stage 2 or stage 3, but at the time of resection after neoadjuvant therapy, by AJCC staging, 24% of the patients were stage 0 or 1, 27% were stage 2, and almost half were stage 3," he said.
The findings are notable, because the current AJCC pathologic staging for esophageal adenocarcinoma is derived from the experience of patients undergoing esophagectomy alone. This approach has unclear relevance in patients who receive multimodality therapy, which has supplanted primary surgery as the standard of care for locoregionally advanced esophageal adenocarcinoma, he said.
"Future efforts at revising the staging system for esophageal adenocarcinoma should consider treatment response lymph nodes. ... We currently have pathological staging models that are of limited usefulness for our neoadjuvantly treated population. Perhaps consideration of these nodes can help improve that," he concluded.
Dr. Nieman reported having no financial disclosures.
ORLANDO – Tumor response nodes obtained from patients who undergo esophagectomy for early-stage adenocarcinoma provide valuable prognostic information, according to Dr. Dylan Nieman.
Such nodes – lymph nodes with evidence of neoadjuvant treatment effect without residual cancer cells – may mark the prior spread of tumor, and should be counted as positive, Dr. Nieman of the University of Rochester (N.Y.) said at the annual meeting of the Society of Thoracic Surgeons.
The current practice of ignoring these nodes likely results in systematic pathologic understaging, he explained.
In 90 patients who underwent esophagectomy after neoadjuvant therapy for esophageal adenocarcinoma, the median number of nodes found per resection specimen was 18. A total of 100 tumor response nodes without viable malignant cells were identified in 38 (42%) of the patients.
The majority of the patients with treatment response nodes had only one node detected, Dr. Nieman said.
The median survival for the entire cohort was 55.6 months, and the 5-year survival was 35%. The median survival of patients with evidence of treatment response nodes was poorer, but not significantly different from those without treatment response nodes (45.9 vs. 55.6 months), he noted.
"However, for a subset of 62 patients classified as having limited or no nodal involvement – that is, AJCC N0 or N1 – the presence of treatment response nodes was associated with significantly poorer survival. This effect remained when adjusting for patient age and [American Joint Committee on Cancer] stage (hazard ratio, 2.7)," he said.
In a subset of 46 patients with pathologic AJCC stage 2B or less, the presence of treatment response nodes was still associated with poorer survival, even after adjustment for age and AJCC stage.
"To look at this a different way, if tumor response nodes were to be counted as positive, the nodal status of 29 of these patients would be upstaged. This includes 18 of 39 patients, or 46%, who were classified as node negative, and 8 of 23 patients, or 34%, who were classified as N1 by AJCC pathological assessment," he said.
The recategorization of those 29 patients resulted in better survival for the entire group, but particularly for those in the lowest-stage group, he noted.
When investigators modeled stage-adjusted survival, the counting of tumor response nodes as positive offered a better model fit, compared with following the current practice of ignoring tumor response nodes, he explained.
Patients included in the study were identified from a prospectively collected clinical database of esophagectomy patients, and were treated with neoadjuvant therapy for esophageal adenocarcinoma between 2006 and 2011. Most (82 of 90) were men. The patients had a mean age of 62 years, and were followed for a median of 27 months. Forty patients received preoperative chemotherapy, and 50 received preoperative chemoradiation.
In all cases, pathologic resection margins were negative.
On pathologic review, the majority had T3 tumors. More than 40% were staged as node negative, and more than two-thirds were staged with N0 or N1 disease.
"Prior to neoadjuvant therapy, all of these patients were clinically staged stage 2 or stage 3, but at the time of resection after neoadjuvant therapy, by AJCC staging, 24% of the patients were stage 0 or 1, 27% were stage 2, and almost half were stage 3," he said.
The findings are notable, because the current AJCC pathologic staging for esophageal adenocarcinoma is derived from the experience of patients undergoing esophagectomy alone. This approach has unclear relevance in patients who receive multimodality therapy, which has supplanted primary surgery as the standard of care for locoregionally advanced esophageal adenocarcinoma, he said.
"Future efforts at revising the staging system for esophageal adenocarcinoma should consider treatment response lymph nodes. ... We currently have pathological staging models that are of limited usefulness for our neoadjuvantly treated population. Perhaps consideration of these nodes can help improve that," he concluded.
Dr. Nieman reported having no financial disclosures.
AT THE STS ANNUAL MEETING
Major finding: In 62 patients classified as having limited or no nodal involvement, the presence of treatment response nodes was associated with significantly poorer survival (adjusted hazard ratio, 2.7).
Data source: A review of 90 prospectively collected cases.
Disclosures: Dr. Nieman reported having no financial disclosures.
VTE rate not an accurate measure of hospital quality
Postoperative venous thromboembolism rates may not be an effective way of measuring hospital quality, according to Dr. Karl Y. Bilimoria and his colleagues.
The investigators calculated patient-level rates of venous thromboembolism as well as rates of imaging for VTE using data from the American Hospital Association and Medicare Compare from 2009 to 2010 from nearly 1 million patients discharged from 2,786 hospitals after a major surgery.
They sought to determine the association between hospital adherence to VTE reduction protocols (Surgical Care Improvement Project for VTE or SCIP-VTE-2) and risk-adjusted rates of VTE as measured by Patient Safety Indicator 12 (PSI-12) from the Agency for Healthcare Research and Quality. They also looked at how overall hospital quality scores correlated with VTE prophylaxis and risk-adjusted VTE scores.
They presented their findigns at the annual clinical congress of the American College of Surgeons and in JAMA [doi:10.1001/jama.2013.280048]).
Hospitals that adhered consistently to VTE reduction protocols paradoxically had higher PSI-12 scores, although not significantly so (P = .03). Hospitals with higher overall quality scores also adhered to VTE reduction protocols at a higher rate (93.3% in the lowest quartile vs. 95.5% in the highest) and had significantly higher risk-adjusted VTE event scores (P less than .001).
"Most important, hospital VTE rates were associated with the intensity of detecting VTE with imaging studies," the investigators said. Mean VTE diagnostic imaging rates ranged from 32/1,000 in the lowest quartile to 167/1,000 in the highest. Hospitals with the lowest imaging rates diagnosed 5.0 VTEs per 1,000 discharges, compared with hospitals with the highest imaging rates diagnosing 13.5 VTEs per 1,000 discharges.
In effect, PSI-12 scores the use of VTE imaging by hospitals instead of the quality of care provided, the investigators said. Further, surveillance bias impedes quality performance improvements; thus, decision making becomes more difficult for "patients seeking to identify a high-quality hospital."
In an accompanying editorial, Dr. Edwin H. Livingston, deputy editor of JAMA, noted that hypervigilance of VTEs might further worsen care in that "the very high compliance rate with VTE prophylaxis might result from many patients receiving treatments from which they are not likely to benefit. This is because current process measures were based on older guidelines that overestimated the benefits of VTE prophylaxis" (JAMA [doi:10.1001/jama.2013.280049]).
For that reason, Dr. Livingston recommended that public reporting of VTEs be "reconsidered or curtailed because few hospitals have sufficient numbers of patients to show statistically significant effects of prophylactic measures on VTE rates."
Measuring outcomes in general and safety events in particular is a complex proposition. This is particularly true when using patient safety indicators (PSIs) and hospital-acquired conditions as outcome metrics to compare performance across organizations.
In addition to the usual challenging nuances such as severity of illness adjustment, these indicators rely on accurate documentation and coding and as the Agency for Healthcare Research and Quality states: PSIs identify "potential in-hospital complications and adverse events following surgeries, procedures, and childbirth."
This is well meaning when an analytic team uses these metrics as part of a comprehensive quality and patient safety program to identify potential internal improvement opportunities. However, there are real limitations when using these metrics as outcomes that are tied to public reporting initiatives, payment incentives, and rankings.
The study in JAMA by Dr. Bilimoria and his colleagues highlights another limitation of some of these metrics – that of surveillance bias. Using PSI-12 (postoperative venous thromboembolism), risk-adjusted VTE rates were shown to correlate positively with intensity of imaging use (surveillance) and inversely with other measures of quality such as structure or process.
Thus, those with the highest VTE rates did everything right, but also looked for events more often.
This finding complicates the use of PSI-12 as an indicator to compare outcomes across health care systems. However, when used as an internal driver in the context of other local metrics of quality and safety, the original intent of PSI-12 as an indicator of potential hospital complications does not change.
This highlights the importance of health care systems in understanding the strengths and limitations of quality and safety metrics and in developing the analytic capabilities to turn data points into real opportunities to deliver better care, rather than going down the proverbial rabbit hole. Yet, regulatory agencies should also recognize that using imperfect metrics as a part of payment-reform initiatives needs to be done with extreme caution or there will be unintended consequences that do not lead to our collective goal of exceptional value in health care for our patients.
Dr. Robert Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City.
Measuring outcomes in general and safety events in particular is a complex proposition. This is particularly true when using patient safety indicators (PSIs) and hospital-acquired conditions as outcome metrics to compare performance across organizations.
In addition to the usual challenging nuances such as severity of illness adjustment, these indicators rely on accurate documentation and coding and as the Agency for Healthcare Research and Quality states: PSIs identify "potential in-hospital complications and adverse events following surgeries, procedures, and childbirth."
This is well meaning when an analytic team uses these metrics as part of a comprehensive quality and patient safety program to identify potential internal improvement opportunities. However, there are real limitations when using these metrics as outcomes that are tied to public reporting initiatives, payment incentives, and rankings.
The study in JAMA by Dr. Bilimoria and his colleagues highlights another limitation of some of these metrics – that of surveillance bias. Using PSI-12 (postoperative venous thromboembolism), risk-adjusted VTE rates were shown to correlate positively with intensity of imaging use (surveillance) and inversely with other measures of quality such as structure or process.
Thus, those with the highest VTE rates did everything right, but also looked for events more often.
This finding complicates the use of PSI-12 as an indicator to compare outcomes across health care systems. However, when used as an internal driver in the context of other local metrics of quality and safety, the original intent of PSI-12 as an indicator of potential hospital complications does not change.
This highlights the importance of health care systems in understanding the strengths and limitations of quality and safety metrics and in developing the analytic capabilities to turn data points into real opportunities to deliver better care, rather than going down the proverbial rabbit hole. Yet, regulatory agencies should also recognize that using imperfect metrics as a part of payment-reform initiatives needs to be done with extreme caution or there will be unintended consequences that do not lead to our collective goal of exceptional value in health care for our patients.
Dr. Robert Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City.
Measuring outcomes in general and safety events in particular is a complex proposition. This is particularly true when using patient safety indicators (PSIs) and hospital-acquired conditions as outcome metrics to compare performance across organizations.
In addition to the usual challenging nuances such as severity of illness adjustment, these indicators rely on accurate documentation and coding and as the Agency for Healthcare Research and Quality states: PSIs identify "potential in-hospital complications and adverse events following surgeries, procedures, and childbirth."
This is well meaning when an analytic team uses these metrics as part of a comprehensive quality and patient safety program to identify potential internal improvement opportunities. However, there are real limitations when using these metrics as outcomes that are tied to public reporting initiatives, payment incentives, and rankings.
The study in JAMA by Dr. Bilimoria and his colleagues highlights another limitation of some of these metrics – that of surveillance bias. Using PSI-12 (postoperative venous thromboembolism), risk-adjusted VTE rates were shown to correlate positively with intensity of imaging use (surveillance) and inversely with other measures of quality such as structure or process.
Thus, those with the highest VTE rates did everything right, but also looked for events more often.
This finding complicates the use of PSI-12 as an indicator to compare outcomes across health care systems. However, when used as an internal driver in the context of other local metrics of quality and safety, the original intent of PSI-12 as an indicator of potential hospital complications does not change.
This highlights the importance of health care systems in understanding the strengths and limitations of quality and safety metrics and in developing the analytic capabilities to turn data points into real opportunities to deliver better care, rather than going down the proverbial rabbit hole. Yet, regulatory agencies should also recognize that using imperfect metrics as a part of payment-reform initiatives needs to be done with extreme caution or there will be unintended consequences that do not lead to our collective goal of exceptional value in health care for our patients.
Dr. Robert Pendleton is chief medical quality officer for University of Utah Health Care, Salt Lake City.
Postoperative venous thromboembolism rates may not be an effective way of measuring hospital quality, according to Dr. Karl Y. Bilimoria and his colleagues.
The investigators calculated patient-level rates of venous thromboembolism as well as rates of imaging for VTE using data from the American Hospital Association and Medicare Compare from 2009 to 2010 from nearly 1 million patients discharged from 2,786 hospitals after a major surgery.
They sought to determine the association between hospital adherence to VTE reduction protocols (Surgical Care Improvement Project for VTE or SCIP-VTE-2) and risk-adjusted rates of VTE as measured by Patient Safety Indicator 12 (PSI-12) from the Agency for Healthcare Research and Quality. They also looked at how overall hospital quality scores correlated with VTE prophylaxis and risk-adjusted VTE scores.
They presented their findigns at the annual clinical congress of the American College of Surgeons and in JAMA [doi:10.1001/jama.2013.280048]).
Hospitals that adhered consistently to VTE reduction protocols paradoxically had higher PSI-12 scores, although not significantly so (P = .03). Hospitals with higher overall quality scores also adhered to VTE reduction protocols at a higher rate (93.3% in the lowest quartile vs. 95.5% in the highest) and had significantly higher risk-adjusted VTE event scores (P less than .001).
"Most important, hospital VTE rates were associated with the intensity of detecting VTE with imaging studies," the investigators said. Mean VTE diagnostic imaging rates ranged from 32/1,000 in the lowest quartile to 167/1,000 in the highest. Hospitals with the lowest imaging rates diagnosed 5.0 VTEs per 1,000 discharges, compared with hospitals with the highest imaging rates diagnosing 13.5 VTEs per 1,000 discharges.
In effect, PSI-12 scores the use of VTE imaging by hospitals instead of the quality of care provided, the investigators said. Further, surveillance bias impedes quality performance improvements; thus, decision making becomes more difficult for "patients seeking to identify a high-quality hospital."
In an accompanying editorial, Dr. Edwin H. Livingston, deputy editor of JAMA, noted that hypervigilance of VTEs might further worsen care in that "the very high compliance rate with VTE prophylaxis might result from many patients receiving treatments from which they are not likely to benefit. This is because current process measures were based on older guidelines that overestimated the benefits of VTE prophylaxis" (JAMA [doi:10.1001/jama.2013.280049]).
For that reason, Dr. Livingston recommended that public reporting of VTEs be "reconsidered or curtailed because few hospitals have sufficient numbers of patients to show statistically significant effects of prophylactic measures on VTE rates."
Postoperative venous thromboembolism rates may not be an effective way of measuring hospital quality, according to Dr. Karl Y. Bilimoria and his colleagues.
The investigators calculated patient-level rates of venous thromboembolism as well as rates of imaging for VTE using data from the American Hospital Association and Medicare Compare from 2009 to 2010 from nearly 1 million patients discharged from 2,786 hospitals after a major surgery.
They sought to determine the association between hospital adherence to VTE reduction protocols (Surgical Care Improvement Project for VTE or SCIP-VTE-2) and risk-adjusted rates of VTE as measured by Patient Safety Indicator 12 (PSI-12) from the Agency for Healthcare Research and Quality. They also looked at how overall hospital quality scores correlated with VTE prophylaxis and risk-adjusted VTE scores.
They presented their findigns at the annual clinical congress of the American College of Surgeons and in JAMA [doi:10.1001/jama.2013.280048]).
Hospitals that adhered consistently to VTE reduction protocols paradoxically had higher PSI-12 scores, although not significantly so (P = .03). Hospitals with higher overall quality scores also adhered to VTE reduction protocols at a higher rate (93.3% in the lowest quartile vs. 95.5% in the highest) and had significantly higher risk-adjusted VTE event scores (P less than .001).
"Most important, hospital VTE rates were associated with the intensity of detecting VTE with imaging studies," the investigators said. Mean VTE diagnostic imaging rates ranged from 32/1,000 in the lowest quartile to 167/1,000 in the highest. Hospitals with the lowest imaging rates diagnosed 5.0 VTEs per 1,000 discharges, compared with hospitals with the highest imaging rates diagnosing 13.5 VTEs per 1,000 discharges.
In effect, PSI-12 scores the use of VTE imaging by hospitals instead of the quality of care provided, the investigators said. Further, surveillance bias impedes quality performance improvements; thus, decision making becomes more difficult for "patients seeking to identify a high-quality hospital."
In an accompanying editorial, Dr. Edwin H. Livingston, deputy editor of JAMA, noted that hypervigilance of VTEs might further worsen care in that "the very high compliance rate with VTE prophylaxis might result from many patients receiving treatments from which they are not likely to benefit. This is because current process measures were based on older guidelines that overestimated the benefits of VTE prophylaxis" (JAMA [doi:10.1001/jama.2013.280049]).
For that reason, Dr. Livingston recommended that public reporting of VTEs be "reconsidered or curtailed because few hospitals have sufficient numbers of patients to show statistically significant effects of prophylactic measures on VTE rates."
Major finding: Hospitals that adhered consistently to VTE reduction protocols had higher rates of VTE, although not significantly so (P = .03).
Data source: Study of hospital risk-adjusted VTE prophylaxis adherence rates to postoperative VTE event rates in 2,786 hospitals.
Disclosures: The study was funded by the AHRQ and Northwestern University. Dr. Bilimoria has received honoraria from hospitals, professional societies, and continuing medical education companies for presentation on quality improvement.
SBRT in marginally operable NSCLC
ATLANTA – For older patients with marginally operable stage 1 non–small cell lung cancer, stereotactic body radiation therapy is significantly more cost effective than surgery.
For patients with clearly operable non–small cell lung cancer (NSCLS) tumors, however, lobectomy is the most cost-effective option, reported Dr. Anand Shah, a radiation oncology resident at Columbia University Medical Center in New York.
The findings, based on cost-effectiveness modeling, were robust over a wide range of assumptions, including various scenarios about treatment efficacies, toxicities, costs, and health state utilities.
"The rationale behind our study was that the traditional treatment for clearly operable patients with stage 1 lung cancer is lobectomy, whereas wedge resection and SBRT [stereotactic body radiation therapy] serve as alternatives in marginally operable patients.
Given an aging population and an increased prevalence of screening, it is likely more people will be diagnosed with stage 1 lung cancer, and thus we felt it was critical to compare the cost-effectiveness of these treatments," he said at the annual meeting of the American Society for Radiation Oncology.
The researchers created a Markov model in which hypothetical patient cohorts transition from one discrete, mutually exclusive health state to another at fixed time increments and at defined probabilities.
For a cohort with marginally operable disease, they compared SBRT with wedge resection, and for a cohort with clearly operable disease, they compared SBRT with lobectomy. Patients in the model were older than age 65
The model assumes that in both cohorts, SBRT will be similarly efficacious, but with higher toxicity for marginally operable patients, who are more likely to experience treatment-related morbidities.
The authors considered both open and less-invasive visually assisted surgical procedures for patients undergoing lobectomy and wedge resection. They considered costs from a Medicare perspective using 2012 dollars.
For the base case, SBRT for the marginally operable cohort cost a mean of $42,084, and the mean quality-adjusted life-year (QALY) gain was 8.03 years. In contrast, wedge resection cost a mean of $51,487, for a QALY gain of 7.93 years. In statistical parlance, SBRT for this cohort was the less costly and most effective strategy.
For clearly operable patients, however, SBRT was less costly than surgery. The mean cost was $40,107 vs. $49,083, but with less efficacy at 8.21 QALY compared with 8.89 for lobectomy. The investigators calculated an incremental cost-effectiveness ratio favoring lobectomy in this cohort, at a cost of $13,200 per QALY gained.
"We conducted a number of sensitivity analyses in which we varied the cost, efficacy, utility, and toxicity data, and in the marginally operable cohort SBRT was nearly always the dominant and thus cost-effective strategy. For patients who were considered clearly operable, lobectomy was the cost-effective treatment in nearly every sensitivity analysis," Dr. Shah said.
Dr. James B. Yu, the invited discussant, said that given current data, the findings of the study generally support current practice.
"However, even if you don’t agree that lobectomy is more cost effective for the clearly operable patient, at the very least this study will illuminate what we disagree about and where better data and clearer goals are needed," he said.
Dr. Yu is a therapeutic radiologist and cancer outcomes researcher at Yale School of Medicine in New Haven, Conn.
The funding source for the study was not disclosed. Dr. Shah and Dr. Yu reported having no relevant financial disclosures.
ATLANTA – For older patients with marginally operable stage 1 non–small cell lung cancer, stereotactic body radiation therapy is significantly more cost effective than surgery.
For patients with clearly operable non–small cell lung cancer (NSCLS) tumors, however, lobectomy is the most cost-effective option, reported Dr. Anand Shah, a radiation oncology resident at Columbia University Medical Center in New York.
The findings, based on cost-effectiveness modeling, were robust over a wide range of assumptions, including various scenarios about treatment efficacies, toxicities, costs, and health state utilities.
"The rationale behind our study was that the traditional treatment for clearly operable patients with stage 1 lung cancer is lobectomy, whereas wedge resection and SBRT [stereotactic body radiation therapy] serve as alternatives in marginally operable patients.
Given an aging population and an increased prevalence of screening, it is likely more people will be diagnosed with stage 1 lung cancer, and thus we felt it was critical to compare the cost-effectiveness of these treatments," he said at the annual meeting of the American Society for Radiation Oncology.
The researchers created a Markov model in which hypothetical patient cohorts transition from one discrete, mutually exclusive health state to another at fixed time increments and at defined probabilities.
For a cohort with marginally operable disease, they compared SBRT with wedge resection, and for a cohort with clearly operable disease, they compared SBRT with lobectomy. Patients in the model were older than age 65
The model assumes that in both cohorts, SBRT will be similarly efficacious, but with higher toxicity for marginally operable patients, who are more likely to experience treatment-related morbidities.
The authors considered both open and less-invasive visually assisted surgical procedures for patients undergoing lobectomy and wedge resection. They considered costs from a Medicare perspective using 2012 dollars.
For the base case, SBRT for the marginally operable cohort cost a mean of $42,084, and the mean quality-adjusted life-year (QALY) gain was 8.03 years. In contrast, wedge resection cost a mean of $51,487, for a QALY gain of 7.93 years. In statistical parlance, SBRT for this cohort was the less costly and most effective strategy.
For clearly operable patients, however, SBRT was less costly than surgery. The mean cost was $40,107 vs. $49,083, but with less efficacy at 8.21 QALY compared with 8.89 for lobectomy. The investigators calculated an incremental cost-effectiveness ratio favoring lobectomy in this cohort, at a cost of $13,200 per QALY gained.
"We conducted a number of sensitivity analyses in which we varied the cost, efficacy, utility, and toxicity data, and in the marginally operable cohort SBRT was nearly always the dominant and thus cost-effective strategy. For patients who were considered clearly operable, lobectomy was the cost-effective treatment in nearly every sensitivity analysis," Dr. Shah said.
Dr. James B. Yu, the invited discussant, said that given current data, the findings of the study generally support current practice.
"However, even if you don’t agree that lobectomy is more cost effective for the clearly operable patient, at the very least this study will illuminate what we disagree about and where better data and clearer goals are needed," he said.
Dr. Yu is a therapeutic radiologist and cancer outcomes researcher at Yale School of Medicine in New Haven, Conn.
The funding source for the study was not disclosed. Dr. Shah and Dr. Yu reported having no relevant financial disclosures.
ATLANTA – For older patients with marginally operable stage 1 non–small cell lung cancer, stereotactic body radiation therapy is significantly more cost effective than surgery.
For patients with clearly operable non–small cell lung cancer (NSCLS) tumors, however, lobectomy is the most cost-effective option, reported Dr. Anand Shah, a radiation oncology resident at Columbia University Medical Center in New York.
The findings, based on cost-effectiveness modeling, were robust over a wide range of assumptions, including various scenarios about treatment efficacies, toxicities, costs, and health state utilities.
"The rationale behind our study was that the traditional treatment for clearly operable patients with stage 1 lung cancer is lobectomy, whereas wedge resection and SBRT [stereotactic body radiation therapy] serve as alternatives in marginally operable patients.
Given an aging population and an increased prevalence of screening, it is likely more people will be diagnosed with stage 1 lung cancer, and thus we felt it was critical to compare the cost-effectiveness of these treatments," he said at the annual meeting of the American Society for Radiation Oncology.
The researchers created a Markov model in which hypothetical patient cohorts transition from one discrete, mutually exclusive health state to another at fixed time increments and at defined probabilities.
For a cohort with marginally operable disease, they compared SBRT with wedge resection, and for a cohort with clearly operable disease, they compared SBRT with lobectomy. Patients in the model were older than age 65
The model assumes that in both cohorts, SBRT will be similarly efficacious, but with higher toxicity for marginally operable patients, who are more likely to experience treatment-related morbidities.
The authors considered both open and less-invasive visually assisted surgical procedures for patients undergoing lobectomy and wedge resection. They considered costs from a Medicare perspective using 2012 dollars.
For the base case, SBRT for the marginally operable cohort cost a mean of $42,084, and the mean quality-adjusted life-year (QALY) gain was 8.03 years. In contrast, wedge resection cost a mean of $51,487, for a QALY gain of 7.93 years. In statistical parlance, SBRT for this cohort was the less costly and most effective strategy.
For clearly operable patients, however, SBRT was less costly than surgery. The mean cost was $40,107 vs. $49,083, but with less efficacy at 8.21 QALY compared with 8.89 for lobectomy. The investigators calculated an incremental cost-effectiveness ratio favoring lobectomy in this cohort, at a cost of $13,200 per QALY gained.
"We conducted a number of sensitivity analyses in which we varied the cost, efficacy, utility, and toxicity data, and in the marginally operable cohort SBRT was nearly always the dominant and thus cost-effective strategy. For patients who were considered clearly operable, lobectomy was the cost-effective treatment in nearly every sensitivity analysis," Dr. Shah said.
Dr. James B. Yu, the invited discussant, said that given current data, the findings of the study generally support current practice.
"However, even if you don’t agree that lobectomy is more cost effective for the clearly operable patient, at the very least this study will illuminate what we disagree about and where better data and clearer goals are needed," he said.
Dr. Yu is a therapeutic radiologist and cancer outcomes researcher at Yale School of Medicine in New Haven, Conn.
The funding source for the study was not disclosed. Dr. Shah and Dr. Yu reported having no relevant financial disclosures.
USPSTF supports CT screening for lung cancer
Low-dose computed tomography reduces lung cancer mortality and all-cause mortality when used as a screening tool in asymptomatic adults at high risk for the disease, according to the results of a systematic review conducted for the U.S. Preventive Services Task Force.
In 2004, the USPSTF deemed the evidence insufficient for recommending for or against low-dose computed tomography (LDCT) for lung cancer screening in asymptomatic individuals, but the current review suggests screening has a definite benefit for most patients, Dr. Linda L. Humphrey, of Oregon Health and Science University and the Portland Veterans Affairs Medical Center, and her colleagues reported.
A draft recommendation based on the findings, published online in the Annals of Internal Medicine (2013 July 29 [doi: 10.7326/0003-4819-159-6-201309170-00690]), is available on the USPSTF website for comment.
The researchers reviewed the literature published between 2000 and May 2013 and identified four trials that reported findings on the efficacy of LDCT screening in patients with smoking exposure for both intervention and control groups. Three small trials showed varying degrees of benefit with screening, but were underpowered; one large trial – the National Lung Screening Trial (NLST) – showed a significant 20% reduction in lung cancer mortality among those screened, as well as a 6.7% reduction in all-cause mortality.
The randomized multicenter NLST compared annual LDCT scans with annual single-view posterior-anterior chest radiographs for 3 years in more than 53,000 current or former smokers aged 55-74 years with at least a 30–pack-year history of smoking (N. Engl. J. Med. 2013;368:1980-91). One cancer death was prevented for every 320 patients who completed one screening, and one death from any cause was prevented for every 219 patients screened in that study; the trial was stopped early after 6.5 years of follow-up based on the findings.
The benefits of LDCT for lung cancer screening in this population outweighed the risks, they noted.
Harms associated with LDCT, according to findings from 7 trials and 13 cohort studies that reported on such outcomes, included radiation exposure, overdiagnosis, and a high rate of false-positive findings that were resolved by further imaging in most cases. False negatives were reported in six studies, and the rates ranged from 0% to 20%, but none of the studies evaluated the harm of false reassurance, the investigators noted. Screening benefits must be weighed against these potential harms.
Lung cancer is the leading cause of cancer-related deaths, accounting for nearly 27%. Furthermore, about 85% of U.S. lung cancer cases are attributable to smoking, and since about 20% of Americans currently smoke – and many more are former smokers who remain at increased risk because of their smoking history – "lung cancer will remain a major public health problem in this country for decades," the investigators wrote.
The studies included in this review were conducted in patients at high risk for lung cancer based on current or former smoking. However, patients at an increased risk for lung cancer, including older adults and those with a family history of lung cancer, chronic obstructive pulmonary disease, pulmonary fibrosis, and certain environmental and occupational exposures, may also benefit from LDCT screening.
"Future research to identify methods for focusing LDCT screening on persons at highest risk for disease, to improve discrimination between benign and malignant pulmonary nodules, and to find early indicators of aggressive disease is warranted," the investigators noted.
"If LDCT screening becomes routine, the risk for harms should be measured and methods to limit them identified."
The review was funded by grants from the Agency for Healthcare Research and Quality and the Portland Veterans Affairs Medical Center.
Low-dose computed tomography reduces lung cancer mortality and all-cause mortality when used as a screening tool in asymptomatic adults at high risk for the disease, according to the results of a systematic review conducted for the U.S. Preventive Services Task Force.
In 2004, the USPSTF deemed the evidence insufficient for recommending for or against low-dose computed tomography (LDCT) for lung cancer screening in asymptomatic individuals, but the current review suggests screening has a definite benefit for most patients, Dr. Linda L. Humphrey, of Oregon Health and Science University and the Portland Veterans Affairs Medical Center, and her colleagues reported.
A draft recommendation based on the findings, published online in the Annals of Internal Medicine (2013 July 29 [doi: 10.7326/0003-4819-159-6-201309170-00690]), is available on the USPSTF website for comment.
The researchers reviewed the literature published between 2000 and May 2013 and identified four trials that reported findings on the efficacy of LDCT screening in patients with smoking exposure for both intervention and control groups. Three small trials showed varying degrees of benefit with screening, but were underpowered; one large trial – the National Lung Screening Trial (NLST) – showed a significant 20% reduction in lung cancer mortality among those screened, as well as a 6.7% reduction in all-cause mortality.
The randomized multicenter NLST compared annual LDCT scans with annual single-view posterior-anterior chest radiographs for 3 years in more than 53,000 current or former smokers aged 55-74 years with at least a 30–pack-year history of smoking (N. Engl. J. Med. 2013;368:1980-91). One cancer death was prevented for every 320 patients who completed one screening, and one death from any cause was prevented for every 219 patients screened in that study; the trial was stopped early after 6.5 years of follow-up based on the findings.
The benefits of LDCT for lung cancer screening in this population outweighed the risks, they noted.
Harms associated with LDCT, according to findings from 7 trials and 13 cohort studies that reported on such outcomes, included radiation exposure, overdiagnosis, and a high rate of false-positive findings that were resolved by further imaging in most cases. False negatives were reported in six studies, and the rates ranged from 0% to 20%, but none of the studies evaluated the harm of false reassurance, the investigators noted. Screening benefits must be weighed against these potential harms.
Lung cancer is the leading cause of cancer-related deaths, accounting for nearly 27%. Furthermore, about 85% of U.S. lung cancer cases are attributable to smoking, and since about 20% of Americans currently smoke – and many more are former smokers who remain at increased risk because of their smoking history – "lung cancer will remain a major public health problem in this country for decades," the investigators wrote.
The studies included in this review were conducted in patients at high risk for lung cancer based on current or former smoking. However, patients at an increased risk for lung cancer, including older adults and those with a family history of lung cancer, chronic obstructive pulmonary disease, pulmonary fibrosis, and certain environmental and occupational exposures, may also benefit from LDCT screening.
"Future research to identify methods for focusing LDCT screening on persons at highest risk for disease, to improve discrimination between benign and malignant pulmonary nodules, and to find early indicators of aggressive disease is warranted," the investigators noted.
"If LDCT screening becomes routine, the risk for harms should be measured and methods to limit them identified."
The review was funded by grants from the Agency for Healthcare Research and Quality and the Portland Veterans Affairs Medical Center.
Low-dose computed tomography reduces lung cancer mortality and all-cause mortality when used as a screening tool in asymptomatic adults at high risk for the disease, according to the results of a systematic review conducted for the U.S. Preventive Services Task Force.
In 2004, the USPSTF deemed the evidence insufficient for recommending for or against low-dose computed tomography (LDCT) for lung cancer screening in asymptomatic individuals, but the current review suggests screening has a definite benefit for most patients, Dr. Linda L. Humphrey, of Oregon Health and Science University and the Portland Veterans Affairs Medical Center, and her colleagues reported.
A draft recommendation based on the findings, published online in the Annals of Internal Medicine (2013 July 29 [doi: 10.7326/0003-4819-159-6-201309170-00690]), is available on the USPSTF website for comment.
The researchers reviewed the literature published between 2000 and May 2013 and identified four trials that reported findings on the efficacy of LDCT screening in patients with smoking exposure for both intervention and control groups. Three small trials showed varying degrees of benefit with screening, but were underpowered; one large trial – the National Lung Screening Trial (NLST) – showed a significant 20% reduction in lung cancer mortality among those screened, as well as a 6.7% reduction in all-cause mortality.
The randomized multicenter NLST compared annual LDCT scans with annual single-view posterior-anterior chest radiographs for 3 years in more than 53,000 current or former smokers aged 55-74 years with at least a 30–pack-year history of smoking (N. Engl. J. Med. 2013;368:1980-91). One cancer death was prevented for every 320 patients who completed one screening, and one death from any cause was prevented for every 219 patients screened in that study; the trial was stopped early after 6.5 years of follow-up based on the findings.
The benefits of LDCT for lung cancer screening in this population outweighed the risks, they noted.
Harms associated with LDCT, according to findings from 7 trials and 13 cohort studies that reported on such outcomes, included radiation exposure, overdiagnosis, and a high rate of false-positive findings that were resolved by further imaging in most cases. False negatives were reported in six studies, and the rates ranged from 0% to 20%, but none of the studies evaluated the harm of false reassurance, the investigators noted. Screening benefits must be weighed against these potential harms.
Lung cancer is the leading cause of cancer-related deaths, accounting for nearly 27%. Furthermore, about 85% of U.S. lung cancer cases are attributable to smoking, and since about 20% of Americans currently smoke – and many more are former smokers who remain at increased risk because of their smoking history – "lung cancer will remain a major public health problem in this country for decades," the investigators wrote.
The studies included in this review were conducted in patients at high risk for lung cancer based on current or former smoking. However, patients at an increased risk for lung cancer, including older adults and those with a family history of lung cancer, chronic obstructive pulmonary disease, pulmonary fibrosis, and certain environmental and occupational exposures, may also benefit from LDCT screening.
"Future research to identify methods for focusing LDCT screening on persons at highest risk for disease, to improve discrimination between benign and malignant pulmonary nodules, and to find early indicators of aggressive disease is warranted," the investigators noted.
"If LDCT screening becomes routine, the risk for harms should be measured and methods to limit them identified."
The review was funded by grants from the Agency for Healthcare Research and Quality and the Portland Veterans Affairs Medical Center.
Similar cancer-free survival seen with EET, esophagectomy
ORLANDO – Mid- and long-term esophageal cancer–free survival rates are similar in patients with early esophageal adenocarcinoma who undergo endoscopic eradication therapy and those who undergo surgical resection, according to findings from a large population-based study.
Of 1,087 patients with early esophageal adenocarcinoma (EAC) who were included in the Surveillance, Epidemiology and End Results (SEER) database, 283 underwent endoscopic eradication therapy (EET), and 804 underwent surgical resection. No significant differences were seen between the groups with respect to 2-year esophageal cancer–free survival (93.5% and 89.6% in the EET and surgery groups, respectively) or 5-year survival (69.3% and 75.8%, respectively), Dr. Sachin Wani reported during a late-breaking abstract session at the annual Digestive Disease Week.
However, the EET group had higher mortality than the surgery group due to non-EAC causes (12.8% vs. 5.7% at 2 years, and 34.8% vs. 12.9% at 5 years), he said. Cardiovascular disease was the most common cause of non-EAC mortality.
Variables significantly associated with mortality were older age (hazard ratio, 1.02), stage T1a disease (compared with T0 disease; HR, 2.71), year of diagnosis (HR, 0.93), and radiation therapy (HR, 5.29), said Dr. Wani of the University of Colorado, Aurora.
Treatment arm was not a predictor of overall survival.
A time-trend analysis showed a significant increase in the proportion of patients with T0 disease undergoing endoscopic eradication therapy. A similar significant increase was noted in patients with stage T1a disease, as well, he said.
Notably, patients undergoing EET were significantly older than those undergoing surgery (70 vs. 63 years), and more likely to be diagnosed with T0 disease (32.5% vs. 23.1% of patients) with well-differentiated histology (33% vs. 24%). They also were less likely to be men, and less likely to receive radiation therapy.
"However, the overall follow-up in the endoscopy arm was shorter than for their surgical counterparts," Dr. Wani noted.
Regional variations were observed in the proportions of patients undergoing EET and surgery, he said.
The differences between the groups, along with the significant differences in non–EAC-related mortality between the treatment groups, highlight selection bias with respect to the therapies offered to patients with EAC, he said.
Patients included in this analysis were adults who had EAC between 1998 and 2009. EAC was defined as carcinoma in situ (T0 disease), or invasive tumor confined to the mucosa, lamina propria, and muscularis mucosae (T1a disease).
The vast majority of patients in the endoscopy arm underwent endoscopic mucosal resection alone; the vast majority in the surgery arm underwent esophagectomy plus partial or total gastrectomy, Dr. Wani said.
Though limited by the use of population-based data that lacked details on recurrences, pathology, staging modalities, and complications and morbidity, this study analyzed one of the largest cohorts of patients with EAC undergoing endoscopic therapy.
The findings are important, because EETs for EAC have gained wide acceptance, and have been endorsed by society guidelines despite a paucity of long-term data examining the differences in outcomes between EET and the gold standard of surgical resection, he said.
Indeed, esophagectomy has traditionally been considered the treatment of choice for EAC, but it is also associated with high morbidity and mortality, even in expert centers, he noted.
"The implications of our study? It really provides a greater degree of confidence in what we do on a daily basis and this whole concept of endoscopic eradication therapy for patients with early esophageal cancer. However we need long-term data – i.e., 5-year data, at least – with newer ablative therapies, such as radiofrequency ablation in combination with endoscopy mucosal resection," he said. Future studies should focus on identifying patient and provider determinants of optimal outcomes, he added.
Dr. Wani reported having no disclosures.
ORLANDO – Mid- and long-term esophageal cancer–free survival rates are similar in patients with early esophageal adenocarcinoma who undergo endoscopic eradication therapy and those who undergo surgical resection, according to findings from a large population-based study.
Of 1,087 patients with early esophageal adenocarcinoma (EAC) who were included in the Surveillance, Epidemiology and End Results (SEER) database, 283 underwent endoscopic eradication therapy (EET), and 804 underwent surgical resection. No significant differences were seen between the groups with respect to 2-year esophageal cancer–free survival (93.5% and 89.6% in the EET and surgery groups, respectively) or 5-year survival (69.3% and 75.8%, respectively), Dr. Sachin Wani reported during a late-breaking abstract session at the annual Digestive Disease Week.
However, the EET group had higher mortality than the surgery group due to non-EAC causes (12.8% vs. 5.7% at 2 years, and 34.8% vs. 12.9% at 5 years), he said. Cardiovascular disease was the most common cause of non-EAC mortality.
Variables significantly associated with mortality were older age (hazard ratio, 1.02), stage T1a disease (compared with T0 disease; HR, 2.71), year of diagnosis (HR, 0.93), and radiation therapy (HR, 5.29), said Dr. Wani of the University of Colorado, Aurora.
Treatment arm was not a predictor of overall survival.
A time-trend analysis showed a significant increase in the proportion of patients with T0 disease undergoing endoscopic eradication therapy. A similar significant increase was noted in patients with stage T1a disease, as well, he said.
Notably, patients undergoing EET were significantly older than those undergoing surgery (70 vs. 63 years), and more likely to be diagnosed with T0 disease (32.5% vs. 23.1% of patients) with well-differentiated histology (33% vs. 24%). They also were less likely to be men, and less likely to receive radiation therapy.
"However, the overall follow-up in the endoscopy arm was shorter than for their surgical counterparts," Dr. Wani noted.
Regional variations were observed in the proportions of patients undergoing EET and surgery, he said.
The differences between the groups, along with the significant differences in non–EAC-related mortality between the treatment groups, highlight selection bias with respect to the therapies offered to patients with EAC, he said.
Patients included in this analysis were adults who had EAC between 1998 and 2009. EAC was defined as carcinoma in situ (T0 disease), or invasive tumor confined to the mucosa, lamina propria, and muscularis mucosae (T1a disease).
The vast majority of patients in the endoscopy arm underwent endoscopic mucosal resection alone; the vast majority in the surgery arm underwent esophagectomy plus partial or total gastrectomy, Dr. Wani said.
Though limited by the use of population-based data that lacked details on recurrences, pathology, staging modalities, and complications and morbidity, this study analyzed one of the largest cohorts of patients with EAC undergoing endoscopic therapy.
The findings are important, because EETs for EAC have gained wide acceptance, and have been endorsed by society guidelines despite a paucity of long-term data examining the differences in outcomes between EET and the gold standard of surgical resection, he said.
Indeed, esophagectomy has traditionally been considered the treatment of choice for EAC, but it is also associated with high morbidity and mortality, even in expert centers, he noted.
"The implications of our study? It really provides a greater degree of confidence in what we do on a daily basis and this whole concept of endoscopic eradication therapy for patients with early esophageal cancer. However we need long-term data – i.e., 5-year data, at least – with newer ablative therapies, such as radiofrequency ablation in combination with endoscopy mucosal resection," he said. Future studies should focus on identifying patient and provider determinants of optimal outcomes, he added.
Dr. Wani reported having no disclosures.
ORLANDO – Mid- and long-term esophageal cancer–free survival rates are similar in patients with early esophageal adenocarcinoma who undergo endoscopic eradication therapy and those who undergo surgical resection, according to findings from a large population-based study.
Of 1,087 patients with early esophageal adenocarcinoma (EAC) who were included in the Surveillance, Epidemiology and End Results (SEER) database, 283 underwent endoscopic eradication therapy (EET), and 804 underwent surgical resection. No significant differences were seen between the groups with respect to 2-year esophageal cancer–free survival (93.5% and 89.6% in the EET and surgery groups, respectively) or 5-year survival (69.3% and 75.8%, respectively), Dr. Sachin Wani reported during a late-breaking abstract session at the annual Digestive Disease Week.
However, the EET group had higher mortality than the surgery group due to non-EAC causes (12.8% vs. 5.7% at 2 years, and 34.8% vs. 12.9% at 5 years), he said. Cardiovascular disease was the most common cause of non-EAC mortality.
Variables significantly associated with mortality were older age (hazard ratio, 1.02), stage T1a disease (compared with T0 disease; HR, 2.71), year of diagnosis (HR, 0.93), and radiation therapy (HR, 5.29), said Dr. Wani of the University of Colorado, Aurora.
Treatment arm was not a predictor of overall survival.
A time-trend analysis showed a significant increase in the proportion of patients with T0 disease undergoing endoscopic eradication therapy. A similar significant increase was noted in patients with stage T1a disease, as well, he said.
Notably, patients undergoing EET were significantly older than those undergoing surgery (70 vs. 63 years), and more likely to be diagnosed with T0 disease (32.5% vs. 23.1% of patients) with well-differentiated histology (33% vs. 24%). They also were less likely to be men, and less likely to receive radiation therapy.
"However, the overall follow-up in the endoscopy arm was shorter than for their surgical counterparts," Dr. Wani noted.
Regional variations were observed in the proportions of patients undergoing EET and surgery, he said.
The differences between the groups, along with the significant differences in non–EAC-related mortality between the treatment groups, highlight selection bias with respect to the therapies offered to patients with EAC, he said.
Patients included in this analysis were adults who had EAC between 1998 and 2009. EAC was defined as carcinoma in situ (T0 disease), or invasive tumor confined to the mucosa, lamina propria, and muscularis mucosae (T1a disease).
The vast majority of patients in the endoscopy arm underwent endoscopic mucosal resection alone; the vast majority in the surgery arm underwent esophagectomy plus partial or total gastrectomy, Dr. Wani said.
Though limited by the use of population-based data that lacked details on recurrences, pathology, staging modalities, and complications and morbidity, this study analyzed one of the largest cohorts of patients with EAC undergoing endoscopic therapy.
The findings are important, because EETs for EAC have gained wide acceptance, and have been endorsed by society guidelines despite a paucity of long-term data examining the differences in outcomes between EET and the gold standard of surgical resection, he said.
Indeed, esophagectomy has traditionally been considered the treatment of choice for EAC, but it is also associated with high morbidity and mortality, even in expert centers, he noted.
"The implications of our study? It really provides a greater degree of confidence in what we do on a daily basis and this whole concept of endoscopic eradication therapy for patients with early esophageal cancer. However we need long-term data – i.e., 5-year data, at least – with newer ablative therapies, such as radiofrequency ablation in combination with endoscopy mucosal resection," he said. Future studies should focus on identifying patient and provider determinants of optimal outcomes, he added.
Dr. Wani reported having no disclosures.
Barrett's recurs in about one-third within 2 years after RFA
About one-third of patients with Barrett’s esophagus can expect recurrence of intestinal metaplasia by 2 years after obtaining complete remission with radiofrequency ablation.
The finding highlights the importance of frequent endoscopic surveillance in these patients, even after remission is achieved, wrote Dr. Milli Gupta and colleagues in the July issue of Gastroenterology.
In the study, Dr. Gupta, of the Mayo Clinic, Rochester, Minn., and her associates looked at 448 patients with Barrett’s treated with radiofrequency ablation (RFA) at three different tertiary referral centers (doi: .1053/j.gastro.2013.03.008).
The data were collected from patients treated and followed up in these centers from January 2003 to November 2011 as part of the Barrett’s Esophagus Translational Research Network consortium.
The patients’ mean age was 64 years, 85% were men, and the mean length of Barrett’s esophagus that was treated was 4.1 cm, the authors reported.
Looking at the indication for RFA, 11% had intramucosal esophageal adenocarcinoma, 60% had high-grade dysplasia, 15% had low-grade dysplasia, and 14% had nondysplastic BE.
Slightly more than half (55%) of patients also underwent endoscopic mucosal resection before RFA, and 1% had received cryotherapy.
After RFA, the authors found that by 1 year, roughly 26% of patients achieved complete remission of intestinal metaplasia (CRIM), defined as two negative endoscopies and histology clear of intestinal metaplasia.
That figure climbed to 56% by 2 years, and to 71% by 3 years, with 29% of patients receiving one RFA treatment session, 35% receiving two sessions, and the remaining percentage of patients undergoing 3-10 sessions before remission.
Adverse events occurred in 6.5% of patients, with stricture formation being the most common, followed by bleeding, mucosal tears, and hospitalizations for dysrhythmias.
However, using a Kaplan-Meier survival curve, they found that after CRIM was attained, 20% of patients would have recurrence of intestinal metaplasia by 1 year. Similarly, by 2 years after attainment of CRIM, the rate of recurrence was 33%.
"By 3 years, recurrences continued to occur, but precise estimates were difficult to establish because of the small number of at-risk subjects (n = 11), which made estimates of recurrence less precise," Dr. Gupta and her colleagues wrote.
According to the authors, the most common type of recurrence was intestinal metaplasia without dysplasia, which was seen in 78% of patients who recurred after CRIM.
The remaining patients who recurred (22%) developed dysplastic Barrett’s esophagus, with 11% showing high-grade dysplasia, 8% showing low-grade dysplasia, and 3% developing cancer.
There were no discernable endoscopic or patient factors associated with time to recurrence on univariate and multivariate analyses, the authors added.
"Despite the ability of RFA to achieve CRIM, recurrence of intestinal metaplasia and dysplasia appears to occur in a substantial proportion of subjects," wrote Dr. Gupta.
She pointed out that current recommendations call for intense surveillance at 3- to 6-month intervals, and if no recurrence is found, "surveillance generally is decreased to 6- to 12-month intervals."
However, "until predictive biomarkers are identified and available for clinical practice, endoscopic surveillance directed at the gastroesophageal junction and original BE [Barrett’s esophagus] segment should be continued."
Dr. Gupta had no conflicts of interest. Several of her coauthors disclosed financial relationships with drug makers, including the makers of therapies for Barrett’s esophagus. The study was funded by a grant from the National Institutes of Health. The Barrett’s Esophagus Translational Research Network consortium is funded by the National Cancer Institute.
About one-third of patients with Barrett’s esophagus can expect recurrence of intestinal metaplasia by 2 years after obtaining complete remission with radiofrequency ablation.
The finding highlights the importance of frequent endoscopic surveillance in these patients, even after remission is achieved, wrote Dr. Milli Gupta and colleagues in the July issue of Gastroenterology.
In the study, Dr. Gupta, of the Mayo Clinic, Rochester, Minn., and her associates looked at 448 patients with Barrett’s treated with radiofrequency ablation (RFA) at three different tertiary referral centers (doi: .1053/j.gastro.2013.03.008).
The data were collected from patients treated and followed up in these centers from January 2003 to November 2011 as part of the Barrett’s Esophagus Translational Research Network consortium.
The patients’ mean age was 64 years, 85% were men, and the mean length of Barrett’s esophagus that was treated was 4.1 cm, the authors reported.
Looking at the indication for RFA, 11% had intramucosal esophageal adenocarcinoma, 60% had high-grade dysplasia, 15% had low-grade dysplasia, and 14% had nondysplastic BE.
Slightly more than half (55%) of patients also underwent endoscopic mucosal resection before RFA, and 1% had received cryotherapy.
After RFA, the authors found that by 1 year, roughly 26% of patients achieved complete remission of intestinal metaplasia (CRIM), defined as two negative endoscopies and histology clear of intestinal metaplasia.
That figure climbed to 56% by 2 years, and to 71% by 3 years, with 29% of patients receiving one RFA treatment session, 35% receiving two sessions, and the remaining percentage of patients undergoing 3-10 sessions before remission.
Adverse events occurred in 6.5% of patients, with stricture formation being the most common, followed by bleeding, mucosal tears, and hospitalizations for dysrhythmias.
However, using a Kaplan-Meier survival curve, they found that after CRIM was attained, 20% of patients would have recurrence of intestinal metaplasia by 1 year. Similarly, by 2 years after attainment of CRIM, the rate of recurrence was 33%.
"By 3 years, recurrences continued to occur, but precise estimates were difficult to establish because of the small number of at-risk subjects (n = 11), which made estimates of recurrence less precise," Dr. Gupta and her colleagues wrote.
According to the authors, the most common type of recurrence was intestinal metaplasia without dysplasia, which was seen in 78% of patients who recurred after CRIM.
The remaining patients who recurred (22%) developed dysplastic Barrett’s esophagus, with 11% showing high-grade dysplasia, 8% showing low-grade dysplasia, and 3% developing cancer.
There were no discernable endoscopic or patient factors associated with time to recurrence on univariate and multivariate analyses, the authors added.
"Despite the ability of RFA to achieve CRIM, recurrence of intestinal metaplasia and dysplasia appears to occur in a substantial proportion of subjects," wrote Dr. Gupta.
She pointed out that current recommendations call for intense surveillance at 3- to 6-month intervals, and if no recurrence is found, "surveillance generally is decreased to 6- to 12-month intervals."
However, "until predictive biomarkers are identified and available for clinical practice, endoscopic surveillance directed at the gastroesophageal junction and original BE [Barrett’s esophagus] segment should be continued."
Dr. Gupta had no conflicts of interest. Several of her coauthors disclosed financial relationships with drug makers, including the makers of therapies for Barrett’s esophagus. The study was funded by a grant from the National Institutes of Health. The Barrett’s Esophagus Translational Research Network consortium is funded by the National Cancer Institute.
About one-third of patients with Barrett’s esophagus can expect recurrence of intestinal metaplasia by 2 years after obtaining complete remission with radiofrequency ablation.
The finding highlights the importance of frequent endoscopic surveillance in these patients, even after remission is achieved, wrote Dr. Milli Gupta and colleagues in the July issue of Gastroenterology.
In the study, Dr. Gupta, of the Mayo Clinic, Rochester, Minn., and her associates looked at 448 patients with Barrett’s treated with radiofrequency ablation (RFA) at three different tertiary referral centers (doi: .1053/j.gastro.2013.03.008).
The data were collected from patients treated and followed up in these centers from January 2003 to November 2011 as part of the Barrett’s Esophagus Translational Research Network consortium.
The patients’ mean age was 64 years, 85% were men, and the mean length of Barrett’s esophagus that was treated was 4.1 cm, the authors reported.
Looking at the indication for RFA, 11% had intramucosal esophageal adenocarcinoma, 60% had high-grade dysplasia, 15% had low-grade dysplasia, and 14% had nondysplastic BE.
Slightly more than half (55%) of patients also underwent endoscopic mucosal resection before RFA, and 1% had received cryotherapy.
After RFA, the authors found that by 1 year, roughly 26% of patients achieved complete remission of intestinal metaplasia (CRIM), defined as two negative endoscopies and histology clear of intestinal metaplasia.
That figure climbed to 56% by 2 years, and to 71% by 3 years, with 29% of patients receiving one RFA treatment session, 35% receiving two sessions, and the remaining percentage of patients undergoing 3-10 sessions before remission.
Adverse events occurred in 6.5% of patients, with stricture formation being the most common, followed by bleeding, mucosal tears, and hospitalizations for dysrhythmias.
However, using a Kaplan-Meier survival curve, they found that after CRIM was attained, 20% of patients would have recurrence of intestinal metaplasia by 1 year. Similarly, by 2 years after attainment of CRIM, the rate of recurrence was 33%.
"By 3 years, recurrences continued to occur, but precise estimates were difficult to establish because of the small number of at-risk subjects (n = 11), which made estimates of recurrence less precise," Dr. Gupta and her colleagues wrote.
According to the authors, the most common type of recurrence was intestinal metaplasia without dysplasia, which was seen in 78% of patients who recurred after CRIM.
The remaining patients who recurred (22%) developed dysplastic Barrett’s esophagus, with 11% showing high-grade dysplasia, 8% showing low-grade dysplasia, and 3% developing cancer.
There were no discernable endoscopic or patient factors associated with time to recurrence on univariate and multivariate analyses, the authors added.
"Despite the ability of RFA to achieve CRIM, recurrence of intestinal metaplasia and dysplasia appears to occur in a substantial proportion of subjects," wrote Dr. Gupta.
She pointed out that current recommendations call for intense surveillance at 3- to 6-month intervals, and if no recurrence is found, "surveillance generally is decreased to 6- to 12-month intervals."
However, "until predictive biomarkers are identified and available for clinical practice, endoscopic surveillance directed at the gastroesophageal junction and original BE [Barrett’s esophagus] segment should be continued."
Dr. Gupta had no conflicts of interest. Several of her coauthors disclosed financial relationships with drug makers, including the makers of therapies for Barrett’s esophagus. The study was funded by a grant from the National Institutes of Health. The Barrett’s Esophagus Translational Research Network consortium is funded by the National Cancer Institute.
Major finding: At 2 years after attainment of complete remission of intestinal metaplasia in Barrett’s esophagus treated with radiofrequency ablation, 33% of patients experienced a recurrence of dysplasia.
Data source: A total of 448 patients from the Barrett’s Esophagus Translational Research Network consortium.
Disclosures: Dr. Gupta had no conflicts of interest. Several of her coauthors disclosed financial relationships with drug makers, including the makers of therapies for Barrett’s esophagus. The study was funded by a grant from the National Institutes of Health. The Barrett’s Esophagus Translational Research Network consortium is funded by the National Cancer Institute.