User login
Addressing an unmet need in IBD patients: Treatment of acute abdominal pain
In the acute care setting, providers of care for inflammatory bowel disease (IBD) patients are often faced with the dilemma of providing effective abdominal pain management in a population that has worse outcomes with both opioid and NSAID therapy. There is increased mortality associated with opioid use and risk of disease relapse with NSAID use in IBD patients.1,2 Due to this, patients often feel that their pain is inadequately addressed.3,4 There are multiple sources of abdominal pain in IBD, and understanding the mechanisms and presentations can help identify effective treatments. We will review pharmacologic and supportive therapies to optimize pain management in IBD.
Common pain presentations in IBD
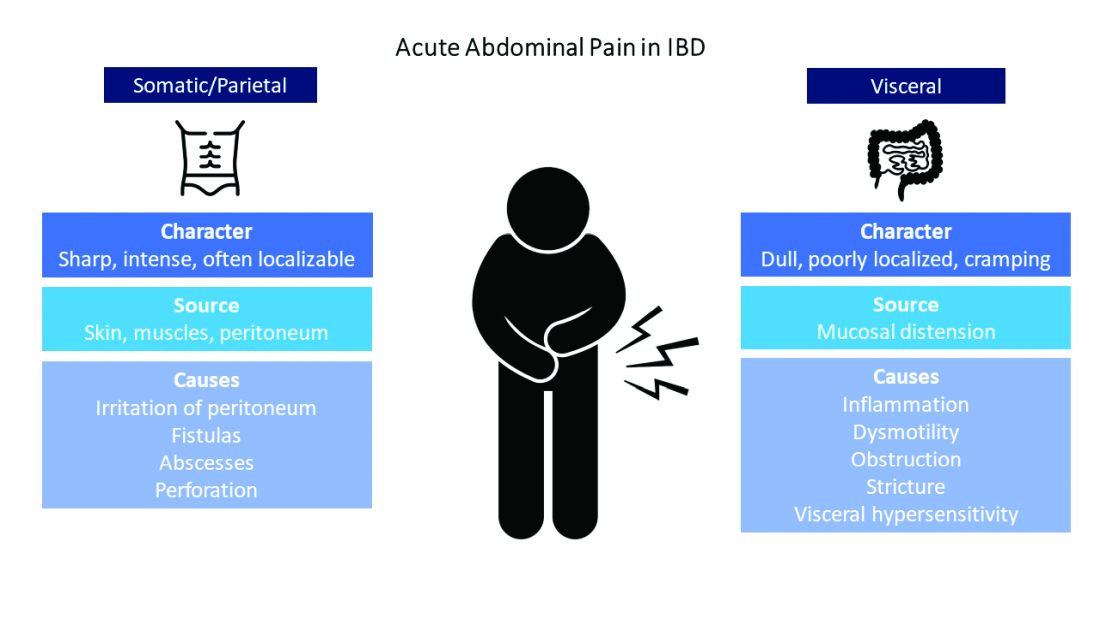
Visceral pain is a dull, poorly localized, cramping pain from intestinal distension. It is associated with inflammation, dysmotility, obstruction, and visceral hypersensitivity. Somatic and parietal pain is sharp, intense, and often localizable. Somatic pain originates from surrounding skin or muscles, and parietal pain arises from irritation of the peritoneum.5 We will review two common pain presentations in IBD.
Case 1: Mr. A is a 32-year-old male with stricturing small bowel Crohn’s disease s/p small bowel resection, who presents to the ED with 3 days of abdominal pain, nausea, and vomiting. C-reactive protein is elevated to 6.8 mg/dL (normal 0.0 – 0.6 mg/dL), and CT is consistent with active small bowel inflammation, intraabdominal abscess at the anastomosis, and associated partial small bowel obstruction. He describes a sharp, intense abdominal pain with cramping. His exam is significant for diffuse abdominal tenderness and distension.
Case 2: Ms. B is a 28-year-old female with ulcerative colitis on mesalamine monotherapy who presents to the hospital for rectal bleeding and cramping abdominal pain. After 3 days of IV steroids her rectal bleeding has resolved, and CRP has normalized. However, she continues to have dull, cramping abdominal pain. Ibuprofen has improved this pain in the past.
Mr. A is having somatic pain from inflammation, abscess, and partial bowel obstruction. He also has visceral pain from luminal distension proximal to the obstruction. Ms. B is having visceral pain despite resolution of inflammation, which may be from postinflammatory visceral hypersensitivity.
Etiologies of pain
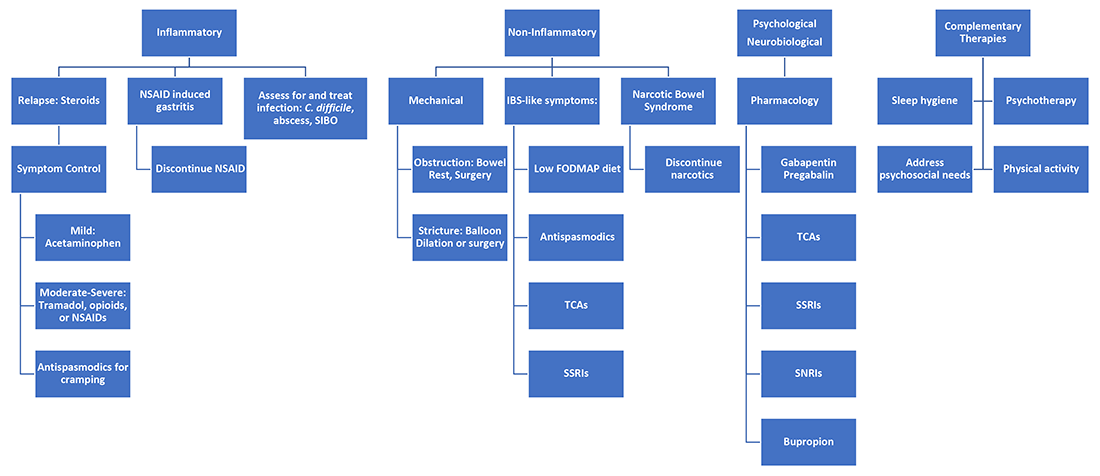
It’s best to group pain etiologies into inflammatory and noninflammatory causes. Inflammatory pain can be secondary to infection, such as abscess or enteric infection, active bowel inflammation, or disease complications (that is, enteric fistula). It is important to recognize that patients with active inflammation may also have noninflammatory pain. These include small bowel obstruction, strictures, adhesions, narcotic bowel syndrome, bacterial overgrowth, and visceral hypersensitivity. See figure 1.
The brain-gut connection matters
Abdominal pain in IBD patients starts from painful stimuli in the gut. In addition to direct pain pathways, multiple areas of the brain modulate perception of pain.6 Patients with psychiatric comorbidities have increased perception of abdominal pain.7 In fact, high perceived stress is associated with disease relapse.8 Treatment of psychiatric disorders improves these symptoms with lasting effects.9 Addressing psychological and psychosocial needs is essential to successful pain management with long-term effect on quality of life and pain perception in IBD patients.
What are my options?
When IBD patients present with acute abdominal pain, it is important to directly address their pain as one of your primary concerns and provide them with a management plan. While this seems obvious, it is not routinely done.3-4
Next, it is important to identify the cause, whether it be infection, obstruction, active inflammation, or functional abdominal pain. In the case of active disease, in addition to steroids and optimization of IBD therapies, acetaminophen and antispasmodics can be used for initial pain management. Supportive therapies include sleep hygiene, physical activity, and psychotherapy. If initial treatments are unsuccessful in the acute setting, and presentation is consistent with somatic pain, it may be necessary to escalate to tramadol, opioid, or NSAID therapy. For visceral pain, a neuromodulator, such as a tricyclic antidepressant or gabapentin, may have greater effect. Bupropion, SNRIs, and SSRIs are options; however, they may not be effective in the acute setting. More recent focus in the IBD community has questioned the role of cannabinoids on pain in IBD patients. Cannabis has been shown in a few small studies to provide pain relief in IBD patients with active inflammation.10-11 In patients with mechanical causes for pain, management of obstruction is an important part of the treatment plan.
Let’s talk about opioids in IBD patients
Chronic narcotic use in IBD is associated with worse outcomes. So when is it okay to use opioid therapies in IBD patients? Postoperative patients, patients with severe perianal disease, or those who fail alternative pain management strategies may require opioid medications. The association with mortality and opioids in IBD is with patients who require moderate to heavy use, which is defined as being prescribed opioids more than once a year. Opioid use in IBD patients is also associated with increased risk of readmissions and poor surgical outcomes.12-13 Tramadol does not have increased mortality risk.1 If selecting opioid therapy in managing pain in IBD, it is important to define the course of therapy, with a clear goal of discontinuation after the acute episode. Opioids should be used in tandem with alternative strategies. Patients should be counseled on the synergistic effect of acetaminophen with opioids, which may allow lower effective doses of opioids.
What about NSAID use in IBD patients?
NSAIDs have negative effects in the gastrointestinal tract due to inhibition of protective prostaglandins. They also alter the gut microbiome, although clinical implications of this are unknown.14 A small study showed that IBD patients who used NSAIDs had increased risk of disease relapse.2 Symptoms of relapse would present within 2-9 days of exposure; however, most had resolution of symptoms within 2-11 days of discontinuation.2 Follow-up studies have not reliably found that NSAIDs are associated with disease relapse.8 and thus NSAIDs may be used sparingly if needed in the acute setting.
Case Review: How do we approach Mr. A and Ms. B?
Mr. A presented with a partial small bowel obstruction and abscess. His pain presentation was consistent with both visceral and somatic pain etiologies. In addition to treating active inflammation and infection, bowel rest, acetaminophen, and antispasmodics can be initiated for pain control. Concomitantly, gabapentin, TCA, or SNRI can be initiated for neurobiological pain but may have limited benefit in the acute hospitalized setting. Social work may identify needs that affect pain perception and assist in addressing those needs. If abdominal pain persists, tramadol or hydrocodone-acetaminophen can be considered.
Ms. B presented with disease relapse, but despite improving inflammatory markers she had continued cramping abdominal pain, which can be consistent with visceral hypersensitivity. Antispasmodic and neuromodulating agents, such as a TCA, could be effective. We can recommend discontinuation of chronic ibuprofen due to risk of intestinal inflammation. Patients may inquire about adjuvant cannabis in pain management. While cannabis can be considered, further research is needed to recommend its regular use.
Conclusion
Acute abdominal pain management in IBD can be challenging for providers when typical options are limited in this population. Addressing inflammatory, mechanical, neurobiological, and psychological influences is vital to appropriately address pain. Having a structured plan for pain management in IBD can improve outcomes by decreasing recurrent hospitalizations and use of opioids.15 Figure 2 presents an overview.
Dr. Ahmed is a second-year internal medicine resident at the University of Michigan, Ann Arbor. Dr. Kinnucan is with the department of internal medicine and the division of gastroenterology and hepatology and is an assistant professor of medicine in the division of gastroenterology, both at the University of Michigan. They have no conflicts of interest.
References
1. Burr NE et al. Clin Gastroenterol Hepatol. 2018 Apr;16(4):534-41.e6.
2. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
3. Bernhofer EI et al. Gastroenterol Nurs. 2017 May/Jun;40(3):200-7.
4. Zeitz J et al. PLoS One. 2016 Jun 22;11(6):e0156666.
5. Srinath A et al. Inflamm Bowel Dis. 2014 Dec;20(12):2433-49.
6. Docherty MJ et al. Gastroenterol Hepatol (N Y). 2011 Sep;7(9):592-601.
7. Elsenbruch S et al. Gut. 2010 Apr;59(4):489-95.
8. Bernstein CN et al. Am J Gastroenterol. 2010 Sep;105(9):1994-2002.
9. Palsson OS and Whitehead WE. Clin Gastroenterol Hepatol. 2013 Mar;11(3):208-16; quiz e22-3.
10. Swaminath A et al. Inflamm Bowel Dis. 2019 Mar; 25(3):427-35.
11. Naftali T et al. Clin Gastroenterol Hepatol. 2013 Oct;11(10):1276-80.e1.
12. Sultan K and Swaminath A. J Crohns Colitis. 2020 Sep 16;14(9):1188-89.
13. Hirsch A et al. J Gastrointest Surg. 2015 Oct;19(10):1852-61.
14. Rogers MAM and Aronoff DM. Clin Microbiol Infect. 2016;22(2):178.e1-178.e9.
15. Kaimakliotis P et al. Int J Colorectal Dis. 2021 Jun;36(6):1193-200.
In the acute care setting, providers of care for inflammatory bowel disease (IBD) patients are often faced with the dilemma of providing effective abdominal pain management in a population that has worse outcomes with both opioid and NSAID therapy. There is increased mortality associated with opioid use and risk of disease relapse with NSAID use in IBD patients.1,2 Due to this, patients often feel that their pain is inadequately addressed.3,4 There are multiple sources of abdominal pain in IBD, and understanding the mechanisms and presentations can help identify effective treatments. We will review pharmacologic and supportive therapies to optimize pain management in IBD.
Common pain presentations in IBD
Visceral pain is a dull, poorly localized, cramping pain from intestinal distension. It is associated with inflammation, dysmotility, obstruction, and visceral hypersensitivity. Somatic and parietal pain is sharp, intense, and often localizable. Somatic pain originates from surrounding skin or muscles, and parietal pain arises from irritation of the peritoneum.5 We will review two common pain presentations in IBD.
Case 1: Mr. A is a 32-year-old male with stricturing small bowel Crohn’s disease s/p small bowel resection, who presents to the ED with 3 days of abdominal pain, nausea, and vomiting. C-reactive protein is elevated to 6.8 mg/dL (normal 0.0 – 0.6 mg/dL), and CT is consistent with active small bowel inflammation, intraabdominal abscess at the anastomosis, and associated partial small bowel obstruction. He describes a sharp, intense abdominal pain with cramping. His exam is significant for diffuse abdominal tenderness and distension.
Case 2: Ms. B is a 28-year-old female with ulcerative colitis on mesalamine monotherapy who presents to the hospital for rectal bleeding and cramping abdominal pain. After 3 days of IV steroids her rectal bleeding has resolved, and CRP has normalized. However, she continues to have dull, cramping abdominal pain. Ibuprofen has improved this pain in the past.
Mr. A is having somatic pain from inflammation, abscess, and partial bowel obstruction. He also has visceral pain from luminal distension proximal to the obstruction. Ms. B is having visceral pain despite resolution of inflammation, which may be from postinflammatory visceral hypersensitivity.
Etiologies of pain
It’s best to group pain etiologies into inflammatory and noninflammatory causes. Inflammatory pain can be secondary to infection, such as abscess or enteric infection, active bowel inflammation, or disease complications (that is, enteric fistula). It is important to recognize that patients with active inflammation may also have noninflammatory pain. These include small bowel obstruction, strictures, adhesions, narcotic bowel syndrome, bacterial overgrowth, and visceral hypersensitivity. See figure 1.

The brain-gut connection matters
Abdominal pain in IBD patients starts from painful stimuli in the gut. In addition to direct pain pathways, multiple areas of the brain modulate perception of pain.6 Patients with psychiatric comorbidities have increased perception of abdominal pain.7 In fact, high perceived stress is associated with disease relapse.8 Treatment of psychiatric disorders improves these symptoms with lasting effects.9 Addressing psychological and psychosocial needs is essential to successful pain management with long-term effect on quality of life and pain perception in IBD patients.
What are my options?
When IBD patients present with acute abdominal pain, it is important to directly address their pain as one of your primary concerns and provide them with a management plan. While this seems obvious, it is not routinely done.3-4
Next, it is important to identify the cause, whether it be infection, obstruction, active inflammation, or functional abdominal pain. In the case of active disease, in addition to steroids and optimization of IBD therapies, acetaminophen and antispasmodics can be used for initial pain management. Supportive therapies include sleep hygiene, physical activity, and psychotherapy. If initial treatments are unsuccessful in the acute setting, and presentation is consistent with somatic pain, it may be necessary to escalate to tramadol, opioid, or NSAID therapy. For visceral pain, a neuromodulator, such as a tricyclic antidepressant or gabapentin, may have greater effect. Bupropion, SNRIs, and SSRIs are options; however, they may not be effective in the acute setting. More recent focus in the IBD community has questioned the role of cannabinoids on pain in IBD patients. Cannabis has been shown in a few small studies to provide pain relief in IBD patients with active inflammation.10-11 In patients with mechanical causes for pain, management of obstruction is an important part of the treatment plan.
Let’s talk about opioids in IBD patients
Chronic narcotic use in IBD is associated with worse outcomes. So when is it okay to use opioid therapies in IBD patients? Postoperative patients, patients with severe perianal disease, or those who fail alternative pain management strategies may require opioid medications. The association with mortality and opioids in IBD is with patients who require moderate to heavy use, which is defined as being prescribed opioids more than once a year. Opioid use in IBD patients is also associated with increased risk of readmissions and poor surgical outcomes.12-13 Tramadol does not have increased mortality risk.1 If selecting opioid therapy in managing pain in IBD, it is important to define the course of therapy, with a clear goal of discontinuation after the acute episode. Opioids should be used in tandem with alternative strategies. Patients should be counseled on the synergistic effect of acetaminophen with opioids, which may allow lower effective doses of opioids.
What about NSAID use in IBD patients?
NSAIDs have negative effects in the gastrointestinal tract due to inhibition of protective prostaglandins. They also alter the gut microbiome, although clinical implications of this are unknown.14 A small study showed that IBD patients who used NSAIDs had increased risk of disease relapse.2 Symptoms of relapse would present within 2-9 days of exposure; however, most had resolution of symptoms within 2-11 days of discontinuation.2 Follow-up studies have not reliably found that NSAIDs are associated with disease relapse.8 and thus NSAIDs may be used sparingly if needed in the acute setting.
Case Review: How do we approach Mr. A and Ms. B?
Mr. A presented with a partial small bowel obstruction and abscess. His pain presentation was consistent with both visceral and somatic pain etiologies. In addition to treating active inflammation and infection, bowel rest, acetaminophen, and antispasmodics can be initiated for pain control. Concomitantly, gabapentin, TCA, or SNRI can be initiated for neurobiological pain but may have limited benefit in the acute hospitalized setting. Social work may identify needs that affect pain perception and assist in addressing those needs. If abdominal pain persists, tramadol or hydrocodone-acetaminophen can be considered.
Ms. B presented with disease relapse, but despite improving inflammatory markers she had continued cramping abdominal pain, which can be consistent with visceral hypersensitivity. Antispasmodic and neuromodulating agents, such as a TCA, could be effective. We can recommend discontinuation of chronic ibuprofen due to risk of intestinal inflammation. Patients may inquire about adjuvant cannabis in pain management. While cannabis can be considered, further research is needed to recommend its regular use.
Conclusion
Acute abdominal pain management in IBD can be challenging for providers when typical options are limited in this population. Addressing inflammatory, mechanical, neurobiological, and psychological influences is vital to appropriately address pain. Having a structured plan for pain management in IBD can improve outcomes by decreasing recurrent hospitalizations and use of opioids.15 Figure 2 presents an overview.

Dr. Ahmed is a second-year internal medicine resident at the University of Michigan, Ann Arbor. Dr. Kinnucan is with the department of internal medicine and the division of gastroenterology and hepatology and is an assistant professor of medicine in the division of gastroenterology, both at the University of Michigan. They have no conflicts of interest.
References
1. Burr NE et al. Clin Gastroenterol Hepatol. 2018 Apr;16(4):534-41.e6.
2. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
3. Bernhofer EI et al. Gastroenterol Nurs. 2017 May/Jun;40(3):200-7.
4. Zeitz J et al. PLoS One. 2016 Jun 22;11(6):e0156666.
5. Srinath A et al. Inflamm Bowel Dis. 2014 Dec;20(12):2433-49.
6. Docherty MJ et al. Gastroenterol Hepatol (N Y). 2011 Sep;7(9):592-601.
7. Elsenbruch S et al. Gut. 2010 Apr;59(4):489-95.
8. Bernstein CN et al. Am J Gastroenterol. 2010 Sep;105(9):1994-2002.
9. Palsson OS and Whitehead WE. Clin Gastroenterol Hepatol. 2013 Mar;11(3):208-16; quiz e22-3.
10. Swaminath A et al. Inflamm Bowel Dis. 2019 Mar; 25(3):427-35.
11. Naftali T et al. Clin Gastroenterol Hepatol. 2013 Oct;11(10):1276-80.e1.
12. Sultan K and Swaminath A. J Crohns Colitis. 2020 Sep 16;14(9):1188-89.
13. Hirsch A et al. J Gastrointest Surg. 2015 Oct;19(10):1852-61.
14. Rogers MAM and Aronoff DM. Clin Microbiol Infect. 2016;22(2):178.e1-178.e9.
15. Kaimakliotis P et al. Int J Colorectal Dis. 2021 Jun;36(6):1193-200.
In the acute care setting, providers of care for inflammatory bowel disease (IBD) patients are often faced with the dilemma of providing effective abdominal pain management in a population that has worse outcomes with both opioid and NSAID therapy. There is increased mortality associated with opioid use and risk of disease relapse with NSAID use in IBD patients.1,2 Due to this, patients often feel that their pain is inadequately addressed.3,4 There are multiple sources of abdominal pain in IBD, and understanding the mechanisms and presentations can help identify effective treatments. We will review pharmacologic and supportive therapies to optimize pain management in IBD.
Common pain presentations in IBD
Visceral pain is a dull, poorly localized, cramping pain from intestinal distension. It is associated with inflammation, dysmotility, obstruction, and visceral hypersensitivity. Somatic and parietal pain is sharp, intense, and often localizable. Somatic pain originates from surrounding skin or muscles, and parietal pain arises from irritation of the peritoneum.5 We will review two common pain presentations in IBD.
Case 1: Mr. A is a 32-year-old male with stricturing small bowel Crohn’s disease s/p small bowel resection, who presents to the ED with 3 days of abdominal pain, nausea, and vomiting. C-reactive protein is elevated to 6.8 mg/dL (normal 0.0 – 0.6 mg/dL), and CT is consistent with active small bowel inflammation, intraabdominal abscess at the anastomosis, and associated partial small bowel obstruction. He describes a sharp, intense abdominal pain with cramping. His exam is significant for diffuse abdominal tenderness and distension.
Case 2: Ms. B is a 28-year-old female with ulcerative colitis on mesalamine monotherapy who presents to the hospital for rectal bleeding and cramping abdominal pain. After 3 days of IV steroids her rectal bleeding has resolved, and CRP has normalized. However, she continues to have dull, cramping abdominal pain. Ibuprofen has improved this pain in the past.
Mr. A is having somatic pain from inflammation, abscess, and partial bowel obstruction. He also has visceral pain from luminal distension proximal to the obstruction. Ms. B is having visceral pain despite resolution of inflammation, which may be from postinflammatory visceral hypersensitivity.
Etiologies of pain
It’s best to group pain etiologies into inflammatory and noninflammatory causes. Inflammatory pain can be secondary to infection, such as abscess or enteric infection, active bowel inflammation, or disease complications (that is, enteric fistula). It is important to recognize that patients with active inflammation may also have noninflammatory pain. These include small bowel obstruction, strictures, adhesions, narcotic bowel syndrome, bacterial overgrowth, and visceral hypersensitivity. See figure 1.

The brain-gut connection matters
Abdominal pain in IBD patients starts from painful stimuli in the gut. In addition to direct pain pathways, multiple areas of the brain modulate perception of pain.6 Patients with psychiatric comorbidities have increased perception of abdominal pain.7 In fact, high perceived stress is associated with disease relapse.8 Treatment of psychiatric disorders improves these symptoms with lasting effects.9 Addressing psychological and psychosocial needs is essential to successful pain management with long-term effect on quality of life and pain perception in IBD patients.
What are my options?
When IBD patients present with acute abdominal pain, it is important to directly address their pain as one of your primary concerns and provide them with a management plan. While this seems obvious, it is not routinely done.3-4
Next, it is important to identify the cause, whether it be infection, obstruction, active inflammation, or functional abdominal pain. In the case of active disease, in addition to steroids and optimization of IBD therapies, acetaminophen and antispasmodics can be used for initial pain management. Supportive therapies include sleep hygiene, physical activity, and psychotherapy. If initial treatments are unsuccessful in the acute setting, and presentation is consistent with somatic pain, it may be necessary to escalate to tramadol, opioid, or NSAID therapy. For visceral pain, a neuromodulator, such as a tricyclic antidepressant or gabapentin, may have greater effect. Bupropion, SNRIs, and SSRIs are options; however, they may not be effective in the acute setting. More recent focus in the IBD community has questioned the role of cannabinoids on pain in IBD patients. Cannabis has been shown in a few small studies to provide pain relief in IBD patients with active inflammation.10-11 In patients with mechanical causes for pain, management of obstruction is an important part of the treatment plan.
Let’s talk about opioids in IBD patients
Chronic narcotic use in IBD is associated with worse outcomes. So when is it okay to use opioid therapies in IBD patients? Postoperative patients, patients with severe perianal disease, or those who fail alternative pain management strategies may require opioid medications. The association with mortality and opioids in IBD is with patients who require moderate to heavy use, which is defined as being prescribed opioids more than once a year. Opioid use in IBD patients is also associated with increased risk of readmissions and poor surgical outcomes.12-13 Tramadol does not have increased mortality risk.1 If selecting opioid therapy in managing pain in IBD, it is important to define the course of therapy, with a clear goal of discontinuation after the acute episode. Opioids should be used in tandem with alternative strategies. Patients should be counseled on the synergistic effect of acetaminophen with opioids, which may allow lower effective doses of opioids.
What about NSAID use in IBD patients?
NSAIDs have negative effects in the gastrointestinal tract due to inhibition of protective prostaglandins. They also alter the gut microbiome, although clinical implications of this are unknown.14 A small study showed that IBD patients who used NSAIDs had increased risk of disease relapse.2 Symptoms of relapse would present within 2-9 days of exposure; however, most had resolution of symptoms within 2-11 days of discontinuation.2 Follow-up studies have not reliably found that NSAIDs are associated with disease relapse.8 and thus NSAIDs may be used sparingly if needed in the acute setting.
Case Review: How do we approach Mr. A and Ms. B?
Mr. A presented with a partial small bowel obstruction and abscess. His pain presentation was consistent with both visceral and somatic pain etiologies. In addition to treating active inflammation and infection, bowel rest, acetaminophen, and antispasmodics can be initiated for pain control. Concomitantly, gabapentin, TCA, or SNRI can be initiated for neurobiological pain but may have limited benefit in the acute hospitalized setting. Social work may identify needs that affect pain perception and assist in addressing those needs. If abdominal pain persists, tramadol or hydrocodone-acetaminophen can be considered.
Ms. B presented with disease relapse, but despite improving inflammatory markers she had continued cramping abdominal pain, which can be consistent with visceral hypersensitivity. Antispasmodic and neuromodulating agents, such as a TCA, could be effective. We can recommend discontinuation of chronic ibuprofen due to risk of intestinal inflammation. Patients may inquire about adjuvant cannabis in pain management. While cannabis can be considered, further research is needed to recommend its regular use.
Conclusion
Acute abdominal pain management in IBD can be challenging for providers when typical options are limited in this population. Addressing inflammatory, mechanical, neurobiological, and psychological influences is vital to appropriately address pain. Having a structured plan for pain management in IBD can improve outcomes by decreasing recurrent hospitalizations and use of opioids.15 Figure 2 presents an overview.

Dr. Ahmed is a second-year internal medicine resident at the University of Michigan, Ann Arbor. Dr. Kinnucan is with the department of internal medicine and the division of gastroenterology and hepatology and is an assistant professor of medicine in the division of gastroenterology, both at the University of Michigan. They have no conflicts of interest.
References
1. Burr NE et al. Clin Gastroenterol Hepatol. 2018 Apr;16(4):534-41.e6.
2. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
3. Bernhofer EI et al. Gastroenterol Nurs. 2017 May/Jun;40(3):200-7.
4. Zeitz J et al. PLoS One. 2016 Jun 22;11(6):e0156666.
5. Srinath A et al. Inflamm Bowel Dis. 2014 Dec;20(12):2433-49.
6. Docherty MJ et al. Gastroenterol Hepatol (N Y). 2011 Sep;7(9):592-601.
7. Elsenbruch S et al. Gut. 2010 Apr;59(4):489-95.
8. Bernstein CN et al. Am J Gastroenterol. 2010 Sep;105(9):1994-2002.
9. Palsson OS and Whitehead WE. Clin Gastroenterol Hepatol. 2013 Mar;11(3):208-16; quiz e22-3.
10. Swaminath A et al. Inflamm Bowel Dis. 2019 Mar; 25(3):427-35.
11. Naftali T et al. Clin Gastroenterol Hepatol. 2013 Oct;11(10):1276-80.e1.
12. Sultan K and Swaminath A. J Crohns Colitis. 2020 Sep 16;14(9):1188-89.
13. Hirsch A et al. J Gastrointest Surg. 2015 Oct;19(10):1852-61.
14. Rogers MAM and Aronoff DM. Clin Microbiol Infect. 2016;22(2):178.e1-178.e9.
15. Kaimakliotis P et al. Int J Colorectal Dis. 2021 Jun;36(6):1193-200.
Ustekinumab effective in treatment-refractory pediatric ulcerative colitis
Key clinical point: Ustekinumab induced and maintained steroid-free clinical remission to 1 year in a significant proportion of children with extensive and treatment-refractory ulcerative colitis (UC).
Major finding: At week 52, 44% of children who received ustekinumab achieved steroid-free remission. This included 69% of those previously treated with antitumor necrosis factor (anti-TNF) only vs. 17% of those who previously failed vedolizumab (P = .008). No adverse events were reported.
Study details: Data come from an open-label prospective cohort study of 25 children with anti-TNF refractory UC who were treated with intravenous ustekinumab. All patients had failed prior infliximab therapy, whereas 12 patients also failed vedolizumab.
Disclosures: The study was funded by the Canadian Institutes of Health Research and Children's Intestinal and Liver Disease Foundation. Some of the authors reported serving as a consultant, speaker, advisory board member for and receiving speaker/consultation fees, honoraria, and/or research support from multiple sources.
Source: Dhaliwal J et al. Aliment Pharmacol Ther. 2021 Apr 28. doi: 10.1111/apt.16388.
Key clinical point: Ustekinumab induced and maintained steroid-free clinical remission to 1 year in a significant proportion of children with extensive and treatment-refractory ulcerative colitis (UC).
Major finding: At week 52, 44% of children who received ustekinumab achieved steroid-free remission. This included 69% of those previously treated with antitumor necrosis factor (anti-TNF) only vs. 17% of those who previously failed vedolizumab (P = .008). No adverse events were reported.
Study details: Data come from an open-label prospective cohort study of 25 children with anti-TNF refractory UC who were treated with intravenous ustekinumab. All patients had failed prior infliximab therapy, whereas 12 patients also failed vedolizumab.
Disclosures: The study was funded by the Canadian Institutes of Health Research and Children's Intestinal and Liver Disease Foundation. Some of the authors reported serving as a consultant, speaker, advisory board member for and receiving speaker/consultation fees, honoraria, and/or research support from multiple sources.
Source: Dhaliwal J et al. Aliment Pharmacol Ther. 2021 Apr 28. doi: 10.1111/apt.16388.
Key clinical point: Ustekinumab induced and maintained steroid-free clinical remission to 1 year in a significant proportion of children with extensive and treatment-refractory ulcerative colitis (UC).
Major finding: At week 52, 44% of children who received ustekinumab achieved steroid-free remission. This included 69% of those previously treated with antitumor necrosis factor (anti-TNF) only vs. 17% of those who previously failed vedolizumab (P = .008). No adverse events were reported.
Study details: Data come from an open-label prospective cohort study of 25 children with anti-TNF refractory UC who were treated with intravenous ustekinumab. All patients had failed prior infliximab therapy, whereas 12 patients also failed vedolizumab.
Disclosures: The study was funded by the Canadian Institutes of Health Research and Children's Intestinal and Liver Disease Foundation. Some of the authors reported serving as a consultant, speaker, advisory board member for and receiving speaker/consultation fees, honoraria, and/or research support from multiple sources.
Source: Dhaliwal J et al. Aliment Pharmacol Ther. 2021 Apr 28. doi: 10.1111/apt.16388.
Sarcopenia predictive of clinical course in acute severe ulcerative colitis
Key clinical point: Sarcopenia is predictive of the clinical course and postoperative outcomes of acute severe ulcerative colitis (ASUC).
Major finding: Sarcopenia was an independent risk factor for intravenous corticosteroid failure (odds ratio [OR], 3.130; P = .001), colectomy after medical rescue therapy failure (OR, 3.401; P = .033), and postoperative complications after colectomy (OR, 4.157; P = .012).
Study details: Findings are from a retrospective cohort study of 233 patients with ASUC.
Disclosures: The work was supported by the National Natural Science Foundation of China and Zhejiang Provincial Natural Science Foundation. The authors declared no conflicts of interest.
Source: Ge X et al. Dig Liver Dis. 2021 Apr 29. doi: 10.1016/j.dld.2021.03.031.
Key clinical point: Sarcopenia is predictive of the clinical course and postoperative outcomes of acute severe ulcerative colitis (ASUC).
Major finding: Sarcopenia was an independent risk factor for intravenous corticosteroid failure (odds ratio [OR], 3.130; P = .001), colectomy after medical rescue therapy failure (OR, 3.401; P = .033), and postoperative complications after colectomy (OR, 4.157; P = .012).
Study details: Findings are from a retrospective cohort study of 233 patients with ASUC.
Disclosures: The work was supported by the National Natural Science Foundation of China and Zhejiang Provincial Natural Science Foundation. The authors declared no conflicts of interest.
Source: Ge X et al. Dig Liver Dis. 2021 Apr 29. doi: 10.1016/j.dld.2021.03.031.
Key clinical point: Sarcopenia is predictive of the clinical course and postoperative outcomes of acute severe ulcerative colitis (ASUC).
Major finding: Sarcopenia was an independent risk factor for intravenous corticosteroid failure (odds ratio [OR], 3.130; P = .001), colectomy after medical rescue therapy failure (OR, 3.401; P = .033), and postoperative complications after colectomy (OR, 4.157; P = .012).
Study details: Findings are from a retrospective cohort study of 233 patients with ASUC.
Disclosures: The work was supported by the National Natural Science Foundation of China and Zhejiang Provincial Natural Science Foundation. The authors declared no conflicts of interest.
Source: Ge X et al. Dig Liver Dis. 2021 Apr 29. doi: 10.1016/j.dld.2021.03.031.
IBD patients at higher risk for stroke
Key clinical point: Inflammatory bowel disease (IBD) may be a risk factor for stroke.
Major finding: IBD was associated with an increased risk for stroke (odds ratio/relative risk [OR/RR], 1.21; P less than .001). Additionally, both Crohn's disease (OR/RR, 1.25; P less than .001) and ulcerative colitis (OR/RR, 1.09; P = .051) were associated with an increased risk for stroke.
Study details: Findings are from a meta-analysis of 9 studies involving 791,010 patients with IBD or stroke.
Disclosures: The study was supported by the General Project of Chongqing Natural Science Foundation and the National Natural Science Foundation of China. All authors declared no conflicts of interest.
Source: Chen Y et al. Brain Behav. 2021 May 7. doi: 10.1002/brb3.2159.
Key clinical point: Inflammatory bowel disease (IBD) may be a risk factor for stroke.
Major finding: IBD was associated with an increased risk for stroke (odds ratio/relative risk [OR/RR], 1.21; P less than .001). Additionally, both Crohn's disease (OR/RR, 1.25; P less than .001) and ulcerative colitis (OR/RR, 1.09; P = .051) were associated with an increased risk for stroke.
Study details: Findings are from a meta-analysis of 9 studies involving 791,010 patients with IBD or stroke.
Disclosures: The study was supported by the General Project of Chongqing Natural Science Foundation and the National Natural Science Foundation of China. All authors declared no conflicts of interest.
Source: Chen Y et al. Brain Behav. 2021 May 7. doi: 10.1002/brb3.2159.
Key clinical point: Inflammatory bowel disease (IBD) may be a risk factor for stroke.
Major finding: IBD was associated with an increased risk for stroke (odds ratio/relative risk [OR/RR], 1.21; P less than .001). Additionally, both Crohn's disease (OR/RR, 1.25; P less than .001) and ulcerative colitis (OR/RR, 1.09; P = .051) were associated with an increased risk for stroke.
Study details: Findings are from a meta-analysis of 9 studies involving 791,010 patients with IBD or stroke.
Disclosures: The study was supported by the General Project of Chongqing Natural Science Foundation and the National Natural Science Foundation of China. All authors declared no conflicts of interest.
Source: Chen Y et al. Brain Behav. 2021 May 7. doi: 10.1002/brb3.2159.
Prenatal exposure to tobacco smoke and antibiotics increases IBD risk in offspring
Key clinical point: Prenatal exposure to tobacco smoke and antibiotics and early life otitis media were risk factors for inflammatory bowel disease (IBD).
Major finding: Prenatal exposure to tobacco smoke (odds ratio [OR], 1.49; 95% confidence interval [CI], 1.17-1.90) and antibiotics (OR, 1.75; 95% CI, 1.22-2.51), and early life otitis media (OR, 2.11; 95% CI, 1.22-3.62) were positively associated with IBD.
Study details: Findings are from a meta-analysis of 39 studies that evaluated the association between early life (prenatal life to 5 years of age) exposures and subsequent risk for IBD.
Disclosures: The study did not receive any funding. Some of the authors reported receiving grants, speaker fees, advisory board fees, personal fees, consultancy, and/or lectures, and/or honoraria from multiple sources. All other authors had no disclosures.
Source: Agrawal M et al. EClinicalMedicine. 2021 May 15. doi: 10.1016/j.eclinm.2021.100884.
Key clinical point: Prenatal exposure to tobacco smoke and antibiotics and early life otitis media were risk factors for inflammatory bowel disease (IBD).
Major finding: Prenatal exposure to tobacco smoke (odds ratio [OR], 1.49; 95% confidence interval [CI], 1.17-1.90) and antibiotics (OR, 1.75; 95% CI, 1.22-2.51), and early life otitis media (OR, 2.11; 95% CI, 1.22-3.62) were positively associated with IBD.
Study details: Findings are from a meta-analysis of 39 studies that evaluated the association between early life (prenatal life to 5 years of age) exposures and subsequent risk for IBD.
Disclosures: The study did not receive any funding. Some of the authors reported receiving grants, speaker fees, advisory board fees, personal fees, consultancy, and/or lectures, and/or honoraria from multiple sources. All other authors had no disclosures.
Source: Agrawal M et al. EClinicalMedicine. 2021 May 15. doi: 10.1016/j.eclinm.2021.100884.
Key clinical point: Prenatal exposure to tobacco smoke and antibiotics and early life otitis media were risk factors for inflammatory bowel disease (IBD).
Major finding: Prenatal exposure to tobacco smoke (odds ratio [OR], 1.49; 95% confidence interval [CI], 1.17-1.90) and antibiotics (OR, 1.75; 95% CI, 1.22-2.51), and early life otitis media (OR, 2.11; 95% CI, 1.22-3.62) were positively associated with IBD.
Study details: Findings are from a meta-analysis of 39 studies that evaluated the association between early life (prenatal life to 5 years of age) exposures and subsequent risk for IBD.
Disclosures: The study did not receive any funding. Some of the authors reported receiving grants, speaker fees, advisory board fees, personal fees, consultancy, and/or lectures, and/or honoraria from multiple sources. All other authors had no disclosures.
Source: Agrawal M et al. EClinicalMedicine. 2021 May 15. doi: 10.1016/j.eclinm.2021.100884.
Crohn's disease: Ustekinumab more effective than vedolizumab in patients refractory to anti-TNF therapy
Key clinical point: Ustekinumab showed higher short- and long-term efficacy than vedolizumab in patients with Crohn's disease with prior antitumor necrosis factor (TNF) therapy failure.
Major finding: Ustekinumab vs. vedolizumab was more effective in achieving corticosteroid-free clinical remission at week 54 (50.6% vs. 40.6%; P = .047) and deep remission at week 14 (17.9% vs. 5.7%; P = .047). Patients treated with ustekinumab vs. vedolizumab had a lower rate of primary nonresponse (6.7% vs. 14.8%, P = .034), a longer time to therapeutic escalation (hazard ratio [HR], 1.35; P = .043), and lower risk for drug discontinuation (HR, 1.53; P = .029).
Study details: This was a retrospective cohort study of 312 patients with Crohn's disease treated with ustekinumab (n=224) or vedolizumab (n=88) after exposure to at least 1 anti-TNF agent.
Disclosures: No information on funding was available. A Buisson reported receiving consulting and lecture fees from multiple sources. The other authors had no disclosures.
Source: Manlay L et al. Aliment Pharmacol Ther. 2021 Apr 28. doi: 10.1111/apt.16377.
Key clinical point: Ustekinumab showed higher short- and long-term efficacy than vedolizumab in patients with Crohn's disease with prior antitumor necrosis factor (TNF) therapy failure.
Major finding: Ustekinumab vs. vedolizumab was more effective in achieving corticosteroid-free clinical remission at week 54 (50.6% vs. 40.6%; P = .047) and deep remission at week 14 (17.9% vs. 5.7%; P = .047). Patients treated with ustekinumab vs. vedolizumab had a lower rate of primary nonresponse (6.7% vs. 14.8%, P = .034), a longer time to therapeutic escalation (hazard ratio [HR], 1.35; P = .043), and lower risk for drug discontinuation (HR, 1.53; P = .029).
Study details: This was a retrospective cohort study of 312 patients with Crohn's disease treated with ustekinumab (n=224) or vedolizumab (n=88) after exposure to at least 1 anti-TNF agent.
Disclosures: No information on funding was available. A Buisson reported receiving consulting and lecture fees from multiple sources. The other authors had no disclosures.
Source: Manlay L et al. Aliment Pharmacol Ther. 2021 Apr 28. doi: 10.1111/apt.16377.
Key clinical point: Ustekinumab showed higher short- and long-term efficacy than vedolizumab in patients with Crohn's disease with prior antitumor necrosis factor (TNF) therapy failure.
Major finding: Ustekinumab vs. vedolizumab was more effective in achieving corticosteroid-free clinical remission at week 54 (50.6% vs. 40.6%; P = .047) and deep remission at week 14 (17.9% vs. 5.7%; P = .047). Patients treated with ustekinumab vs. vedolizumab had a lower rate of primary nonresponse (6.7% vs. 14.8%, P = .034), a longer time to therapeutic escalation (hazard ratio [HR], 1.35; P = .043), and lower risk for drug discontinuation (HR, 1.53; P = .029).
Study details: This was a retrospective cohort study of 312 patients with Crohn's disease treated with ustekinumab (n=224) or vedolizumab (n=88) after exposure to at least 1 anti-TNF agent.
Disclosures: No information on funding was available. A Buisson reported receiving consulting and lecture fees from multiple sources. The other authors had no disclosures.
Source: Manlay L et al. Aliment Pharmacol Ther. 2021 Apr 28. doi: 10.1111/apt.16377.
Crohn’s disease: Partial restoration of intestinal microbiome in anti-TNF responders
Key clinical point: Patients with Crohn’s disease (CD) who responded to antitumor necrosis factor (anti-TNF) treatment showed partial restoration of intestinal microbiome characteristics of healthy individuals.
Major finding: Patients with CD vs. healthy cohort showed a decrease in genera of the class Clostridia and an increase in the phylum Proteobacteria (P less than .01). Anti-TNF treatment allowed restoration of bacteria belonging to the class Clostridia in responders. The genus Escherichia/Shigella reduced significantly vs. baseline but did not reach statistical significance in responders vs. healthy control.
Study details: Data come from a prospective multicenter observational study of 27 patients with CD who initiated anti-TNF treatment and 16 healthy individuals. Based on inflammatory activity, patients were classified into responders and nonresponders.
Disclosures: No information on funding was available. The authors declared no conflicts of interest.
Source: Sanchis-Artero L et al. Sci Rep. 2021 May 11. doi: 10.1038/s41598-021-88823-2.
Key clinical point: Patients with Crohn’s disease (CD) who responded to antitumor necrosis factor (anti-TNF) treatment showed partial restoration of intestinal microbiome characteristics of healthy individuals.
Major finding: Patients with CD vs. healthy cohort showed a decrease in genera of the class Clostridia and an increase in the phylum Proteobacteria (P less than .01). Anti-TNF treatment allowed restoration of bacteria belonging to the class Clostridia in responders. The genus Escherichia/Shigella reduced significantly vs. baseline but did not reach statistical significance in responders vs. healthy control.
Study details: Data come from a prospective multicenter observational study of 27 patients with CD who initiated anti-TNF treatment and 16 healthy individuals. Based on inflammatory activity, patients were classified into responders and nonresponders.
Disclosures: No information on funding was available. The authors declared no conflicts of interest.
Source: Sanchis-Artero L et al. Sci Rep. 2021 May 11. doi: 10.1038/s41598-021-88823-2.
Key clinical point: Patients with Crohn’s disease (CD) who responded to antitumor necrosis factor (anti-TNF) treatment showed partial restoration of intestinal microbiome characteristics of healthy individuals.
Major finding: Patients with CD vs. healthy cohort showed a decrease in genera of the class Clostridia and an increase in the phylum Proteobacteria (P less than .01). Anti-TNF treatment allowed restoration of bacteria belonging to the class Clostridia in responders. The genus Escherichia/Shigella reduced significantly vs. baseline but did not reach statistical significance in responders vs. healthy control.
Study details: Data come from a prospective multicenter observational study of 27 patients with CD who initiated anti-TNF treatment and 16 healthy individuals. Based on inflammatory activity, patients were classified into responders and nonresponders.
Disclosures: No information on funding was available. The authors declared no conflicts of interest.
Source: Sanchis-Artero L et al. Sci Rep. 2021 May 11. doi: 10.1038/s41598-021-88823-2.
Crohn’s disease: Ustekinumab safe and effective in a real-world setting
Key clinical point: Ustekinumab was effective and relatively safe in a real-world cohort of patients with Crohn’s disease (CD).
Major finding: After 104 weeks of ustekinumab treatment, 34.0% of patients were in corticosteroid-free clinical remission (Cf-CR). Among patients who were in Cf-CR at week 24, 48.1% remained at Cf-CR at week 104. Lack of response (61.7%) and loss of response (18.3%) were the main reasons for treatment discontinuation. No new safety signals were identified.
Study details: Findings are from a cohort study of 252 patients with CD who initiated ustekinumab and completed at least 2 years of follow-up.
Disclosures: No information on funding was available. The authors declared serving as a speaker, consultant, principal investigator, advisory board member for and/or receiving sponsorship, grants/honoraria, advisory, and/or speaker fees from multiple sources.
Source: Straatmijer T et al. J Crohns Colitis. 2021 Apr 28. doi: 10.1093/ecco-jcc/jjab081.
Key clinical point: Ustekinumab was effective and relatively safe in a real-world cohort of patients with Crohn’s disease (CD).
Major finding: After 104 weeks of ustekinumab treatment, 34.0% of patients were in corticosteroid-free clinical remission (Cf-CR). Among patients who were in Cf-CR at week 24, 48.1% remained at Cf-CR at week 104. Lack of response (61.7%) and loss of response (18.3%) were the main reasons for treatment discontinuation. No new safety signals were identified.
Study details: Findings are from a cohort study of 252 patients with CD who initiated ustekinumab and completed at least 2 years of follow-up.
Disclosures: No information on funding was available. The authors declared serving as a speaker, consultant, principal investigator, advisory board member for and/or receiving sponsorship, grants/honoraria, advisory, and/or speaker fees from multiple sources.
Source: Straatmijer T et al. J Crohns Colitis. 2021 Apr 28. doi: 10.1093/ecco-jcc/jjab081.
Key clinical point: Ustekinumab was effective and relatively safe in a real-world cohort of patients with Crohn’s disease (CD).
Major finding: After 104 weeks of ustekinumab treatment, 34.0% of patients were in corticosteroid-free clinical remission (Cf-CR). Among patients who were in Cf-CR at week 24, 48.1% remained at Cf-CR at week 104. Lack of response (61.7%) and loss of response (18.3%) were the main reasons for treatment discontinuation. No new safety signals were identified.
Study details: Findings are from a cohort study of 252 patients with CD who initiated ustekinumab and completed at least 2 years of follow-up.
Disclosures: No information on funding was available. The authors declared serving as a speaker, consultant, principal investigator, advisory board member for and/or receiving sponsorship, grants/honoraria, advisory, and/or speaker fees from multiple sources.
Source: Straatmijer T et al. J Crohns Colitis. 2021 Apr 28. doi: 10.1093/ecco-jcc/jjab081.
Intravenous steroids for acute inflammatory bowel disease
Key clinical point: Patients with an acute flare of inflammatory bowel disease (IBD) treated with intravenous methylprednisolone (IVMP) required significantly more rescue biologics or cyclosporine. Additionally, IVMP significantly reduced rates of hypokalemia compared with intravenous hydrocortisone (IVHC).
Major finding: IVMP was associated with a greater requirement for rescue biologics or cyclosporine (odds ratio [OR], 2.79; P less than .001) and lower rates of hypokalemia (OR, 0.49; P = .005) than IVHC.
Study details: This was a multicenter cohort study of 359 patients hospitalized with an acute flare of IBD and treated with either IVMP 60 mg daily (n=129) or IVHC 100 mg 4 times daily (n=230).
Disclosures: The study did not receive any funding. All the authors declared no conflicts of interest.
Source: Schauer C et al. J Gastroenterol Hepatol. 2021 May 3. doi: 10.1111/jgh.15535.
Key clinical point: Patients with an acute flare of inflammatory bowel disease (IBD) treated with intravenous methylprednisolone (IVMP) required significantly more rescue biologics or cyclosporine. Additionally, IVMP significantly reduced rates of hypokalemia compared with intravenous hydrocortisone (IVHC).
Major finding: IVMP was associated with a greater requirement for rescue biologics or cyclosporine (odds ratio [OR], 2.79; P less than .001) and lower rates of hypokalemia (OR, 0.49; P = .005) than IVHC.
Study details: This was a multicenter cohort study of 359 patients hospitalized with an acute flare of IBD and treated with either IVMP 60 mg daily (n=129) or IVHC 100 mg 4 times daily (n=230).
Disclosures: The study did not receive any funding. All the authors declared no conflicts of interest.
Source: Schauer C et al. J Gastroenterol Hepatol. 2021 May 3. doi: 10.1111/jgh.15535.
Key clinical point: Patients with an acute flare of inflammatory bowel disease (IBD) treated with intravenous methylprednisolone (IVMP) required significantly more rescue biologics or cyclosporine. Additionally, IVMP significantly reduced rates of hypokalemia compared with intravenous hydrocortisone (IVHC).
Major finding: IVMP was associated with a greater requirement for rescue biologics or cyclosporine (odds ratio [OR], 2.79; P less than .001) and lower rates of hypokalemia (OR, 0.49; P = .005) than IVHC.
Study details: This was a multicenter cohort study of 359 patients hospitalized with an acute flare of IBD and treated with either IVMP 60 mg daily (n=129) or IVHC 100 mg 4 times daily (n=230).
Disclosures: The study did not receive any funding. All the authors declared no conflicts of interest.
Source: Schauer C et al. J Gastroenterol Hepatol. 2021 May 3. doi: 10.1111/jgh.15535.
IBD: Significant proportion of patients remain in remission after anti-TNF discontinuation
Key clinical point: A significant proportion of patients with inflammatory bowel disease (IBD) remain in remission after discontinuation of antitumor necrosis factor-alpha (anti-TNF) post clinical remission.
Major finding: After a median follow-up of 34 months, incidence rate of relapse was 12% per patient-year (95% confidence interval [CI], 11-14) and the cumulative incidence of relapse was 50% (95% CI, 47-53) with 19%, 31%, 38%, 44%, and 48% at 1, 2, 3, 4, and 5 years of follow-up.
Study details: Data come from an extension of the EVODIS study that included 1,055 patients with Crohn's disease or ulcerative colitis who discontinued anti-TNF therapy after achieving clinical remission.
Disclosures: The study did not receive any funding. Some of the authors reported serving as a speaker, consultant, advisory member, and receiving research and/or education funding from multiple sources.
Source: Casanova MJ et al. Aliment Pharmacol Ther. 2021 May 7. doi: 10.1111/apt.16361.
Key clinical point: A significant proportion of patients with inflammatory bowel disease (IBD) remain in remission after discontinuation of antitumor necrosis factor-alpha (anti-TNF) post clinical remission.
Major finding: After a median follow-up of 34 months, incidence rate of relapse was 12% per patient-year (95% confidence interval [CI], 11-14) and the cumulative incidence of relapse was 50% (95% CI, 47-53) with 19%, 31%, 38%, 44%, and 48% at 1, 2, 3, 4, and 5 years of follow-up.
Study details: Data come from an extension of the EVODIS study that included 1,055 patients with Crohn's disease or ulcerative colitis who discontinued anti-TNF therapy after achieving clinical remission.
Disclosures: The study did not receive any funding. Some of the authors reported serving as a speaker, consultant, advisory member, and receiving research and/or education funding from multiple sources.
Source: Casanova MJ et al. Aliment Pharmacol Ther. 2021 May 7. doi: 10.1111/apt.16361.
Key clinical point: A significant proportion of patients with inflammatory bowel disease (IBD) remain in remission after discontinuation of antitumor necrosis factor-alpha (anti-TNF) post clinical remission.
Major finding: After a median follow-up of 34 months, incidence rate of relapse was 12% per patient-year (95% confidence interval [CI], 11-14) and the cumulative incidence of relapse was 50% (95% CI, 47-53) with 19%, 31%, 38%, 44%, and 48% at 1, 2, 3, 4, and 5 years of follow-up.
Study details: Data come from an extension of the EVODIS study that included 1,055 patients with Crohn's disease or ulcerative colitis who discontinued anti-TNF therapy after achieving clinical remission.
Disclosures: The study did not receive any funding. Some of the authors reported serving as a speaker, consultant, advisory member, and receiving research and/or education funding from multiple sources.
Source: Casanova MJ et al. Aliment Pharmacol Ther. 2021 May 7. doi: 10.1111/apt.16361.


