User login
Normal gut colonizer induces serotonergic system, appears to regulate behavior
Together, Bifidobacterium dentium and its acetate metabolite regulate key parts of the serotonergic system and are associated with “a functional change in adult behavior,” according to a report published in Cellular and Molecular Gastroenterology and Hepatology.
Human gut microbiota had been known to regulate serotonin (5-hydroxytryptamine) production by gut cells, but underlying mechanisms had been unclear. This study showed that a common bacterial colonizer of the healthy adult gut stimulates serotonin (5-hydroxytryptamine, or 5-HT) release from enterochromaffin cells in both mice (in vivo) and humans (in vitro), wrote Melinda A. Engevik, PhD, of Baylor College of Medicine, Houston, and associates. “B. dentium modulates the serotonergic system in both the intestine and the brain [, which] likely influences behavior, and suggests that supplementation with a single, carefully selected, bacterial strain may be able to partially rescue behavioral deficits induced by shifts in the intestinal microbiota,” they added.
In a prior study, B. dentium modulated sensory neurons in rats with visceral hypersensitivity. In mammals, serotonin is primarily produced and released by enterochromaffin cells in the gut. To discover whether acetate – a short-chain fatty acid metabolite of B. dentium and some other microbiota – induces this pathway, the researchers first confirmed that B. dentium itself lacks the gene pathway for 5-HT production, and that growth media inoculated with B. dentium do not subsequently contain 5-HT. Next, they treated adult germ-free mice with either sterile media, live B. dentium, heat-killed B. dentium, or live Bacteroides ovatus (another commensal gut microbe). Gram staining and fluorescence in situ hybridization (FISH) confirmed that live B. dentium colonized mouse ileum and colon. Mass spectrometry, immunostaining, and quantitative PCR showed that mice treated with live B. dentium, but not B. ovatus, had greater intestinal concentrations of acetate, 5-HT, 5-HT receptors (2a and 4), serotonin transporter, and the gene that encodes free fatty acid receptor 2 (FFAR2), through which acetate signals. Furthermore, “[i]ncreases in 5-HT were observed in enteroendocrine cells directly above enteric neurons,” the researchers said.
They also performed RNA in situ hybridization of mouse brain tissue, which showed significantly increased expression of 5-HT-receptor 2a in the B. dentium–treated compared with germ-free controls. Mice were caged with specified numbers of marbles so the researchers could find out if these changes also modified behavior. Those with complete gut microbiota buried an average of 25% of the marbles, B. dentium–monocolonized mice buried 15%, and germ-free mice buried fewer marbles. Hence, even short-term monocolonization by a bacterium that acts on the serotonergic system might help normalize behavior, even later in life, the researchers said. They noted that B. dentium–treated and germ-free mice performed similarly on both balance beam and footprint tests, suggesting that treatment with B. dentium does not affect motor coordination.
In humans, enterochromaffin cells released more 5-HT when exposed to B. dentium or acetate. Taken together, the findings “highlight the importance of Bifidobacterium species, and specifically B. dentium, in the adult microbiome-gut-brain axis,” the researchers wrote. Probiotic strains such as Lactobacillus and Bifidobacterium species are thought to improve health by means of signaling pathways, including the serotonergic system, they noted. “Our findings support the modulation of the serotonergic system by a model gut microbe, B. dentium, and provide a potential mechanism by which select microbes and their metabolites can promote endogenous, localized 5-HT biosynthesis. We speculate this may be an important bridging signal in the microbiome-gut-brain axis.”
The National Institutes of Health, BioGaia AB, and the RNA In Situ Hybridization Core facility supported the work. Two coinvestigators disclosed ties to BioGaia AB, Seed, Biomica, Plexus Worldwide, Tenza, Mikrovia, Probiotech, and Takeda. Dr. Engevik and the other investigators reported having no conflicts of interest.
SOURCE: Engevik MA et al. Cell Molec Gastro Hepatol. 2021;11:221-48. doi: 10.1016/j.jcmgh.2020.08.002.
“Gut-brain axis” is a widely used term that refers to the idea that the functions of these two organs are linked by bidirectional communication. The gut plays host to a large community of microbes and increasing data suggest that metabolites generated by these microbes can alter nervous system function. Such findings raise the exciting possibility that microbes and/or their metabolites could be used to treat a variety of disorders that involve gut-brain axis dysfunction, from irritable bowel syndrome (IBS) to Parkinson’s disease. To realize this possibility, it will be essential to establish clear mechanistic links between microbes, their products, and effects on host physiology. This study by Engevik and colleagues represents an important advance, demonstrating how a single microbe that commonly colonizes the healthy human intestine, Bifidobacterium dentium, is sufficient to stimulate the gut to make serotonin, a powerful signaling molecule known to influence visceral sensitivity, gut motility, and mood.
One key approach to understanding the effects of microbes on host function is to study germ-free mice, which are raised such that they are never exposed to microbes. Germ-free mice have a wide range of immune and neurologic deficits, highlighting how essential microbes are to host function. Previous work has shown that germ-free mice have diminished serotonin levels and abnormal behavior. Exposure to human microbiota could rescue some of these impairments but it was unclear which microbes or signals were essential. This study shows that supplementing germ-free mice with B. dentium is sufficient to stimulate the gut to ramp up serotonin production, alter gene expression in the brain, and rescue some behavioral deficits. Acetate, a short-chain fatty acid produced by B. dentium, was crucial for this phenomenon. This work not only identifies B. dentium as a promising candidate for therapeutic development, it also emphasizes the value of rigorous studies that probe functional interactions between microbes and the nervous system.
Meenakshi Rao, MD, PhD, is a principal investigator at Boston Children’s Hospital, division of gastroenterology, hepatology and nutrition, and assistant professor of pediatrics at Harvard Medical School. She has no conflicts relevant to this study. She receives research support from Boston Pharmaceuticals for unrelated work and has participated on a scientific advisory board for Takeda Pharmaceuticals.
“Gut-brain axis” is a widely used term that refers to the idea that the functions of these two organs are linked by bidirectional communication. The gut plays host to a large community of microbes and increasing data suggest that metabolites generated by these microbes can alter nervous system function. Such findings raise the exciting possibility that microbes and/or their metabolites could be used to treat a variety of disorders that involve gut-brain axis dysfunction, from irritable bowel syndrome (IBS) to Parkinson’s disease. To realize this possibility, it will be essential to establish clear mechanistic links between microbes, their products, and effects on host physiology. This study by Engevik and colleagues represents an important advance, demonstrating how a single microbe that commonly colonizes the healthy human intestine, Bifidobacterium dentium, is sufficient to stimulate the gut to make serotonin, a powerful signaling molecule known to influence visceral sensitivity, gut motility, and mood.
One key approach to understanding the effects of microbes on host function is to study germ-free mice, which are raised such that they are never exposed to microbes. Germ-free mice have a wide range of immune and neurologic deficits, highlighting how essential microbes are to host function. Previous work has shown that germ-free mice have diminished serotonin levels and abnormal behavior. Exposure to human microbiota could rescue some of these impairments but it was unclear which microbes or signals were essential. This study shows that supplementing germ-free mice with B. dentium is sufficient to stimulate the gut to ramp up serotonin production, alter gene expression in the brain, and rescue some behavioral deficits. Acetate, a short-chain fatty acid produced by B. dentium, was crucial for this phenomenon. This work not only identifies B. dentium as a promising candidate for therapeutic development, it also emphasizes the value of rigorous studies that probe functional interactions between microbes and the nervous system.
Meenakshi Rao, MD, PhD, is a principal investigator at Boston Children’s Hospital, division of gastroenterology, hepatology and nutrition, and assistant professor of pediatrics at Harvard Medical School. She has no conflicts relevant to this study. She receives research support from Boston Pharmaceuticals for unrelated work and has participated on a scientific advisory board for Takeda Pharmaceuticals.
“Gut-brain axis” is a widely used term that refers to the idea that the functions of these two organs are linked by bidirectional communication. The gut plays host to a large community of microbes and increasing data suggest that metabolites generated by these microbes can alter nervous system function. Such findings raise the exciting possibility that microbes and/or their metabolites could be used to treat a variety of disorders that involve gut-brain axis dysfunction, from irritable bowel syndrome (IBS) to Parkinson’s disease. To realize this possibility, it will be essential to establish clear mechanistic links between microbes, their products, and effects on host physiology. This study by Engevik and colleagues represents an important advance, demonstrating how a single microbe that commonly colonizes the healthy human intestine, Bifidobacterium dentium, is sufficient to stimulate the gut to make serotonin, a powerful signaling molecule known to influence visceral sensitivity, gut motility, and mood.
One key approach to understanding the effects of microbes on host function is to study germ-free mice, which are raised such that they are never exposed to microbes. Germ-free mice have a wide range of immune and neurologic deficits, highlighting how essential microbes are to host function. Previous work has shown that germ-free mice have diminished serotonin levels and abnormal behavior. Exposure to human microbiota could rescue some of these impairments but it was unclear which microbes or signals were essential. This study shows that supplementing germ-free mice with B. dentium is sufficient to stimulate the gut to ramp up serotonin production, alter gene expression in the brain, and rescue some behavioral deficits. Acetate, a short-chain fatty acid produced by B. dentium, was crucial for this phenomenon. This work not only identifies B. dentium as a promising candidate for therapeutic development, it also emphasizes the value of rigorous studies that probe functional interactions between microbes and the nervous system.
Meenakshi Rao, MD, PhD, is a principal investigator at Boston Children’s Hospital, division of gastroenterology, hepatology and nutrition, and assistant professor of pediatrics at Harvard Medical School. She has no conflicts relevant to this study. She receives research support from Boston Pharmaceuticals for unrelated work and has participated on a scientific advisory board for Takeda Pharmaceuticals.
Together, Bifidobacterium dentium and its acetate metabolite regulate key parts of the serotonergic system and are associated with “a functional change in adult behavior,” according to a report published in Cellular and Molecular Gastroenterology and Hepatology.
Human gut microbiota had been known to regulate serotonin (5-hydroxytryptamine) production by gut cells, but underlying mechanisms had been unclear. This study showed that a common bacterial colonizer of the healthy adult gut stimulates serotonin (5-hydroxytryptamine, or 5-HT) release from enterochromaffin cells in both mice (in vivo) and humans (in vitro), wrote Melinda A. Engevik, PhD, of Baylor College of Medicine, Houston, and associates. “B. dentium modulates the serotonergic system in both the intestine and the brain [, which] likely influences behavior, and suggests that supplementation with a single, carefully selected, bacterial strain may be able to partially rescue behavioral deficits induced by shifts in the intestinal microbiota,” they added.
In a prior study, B. dentium modulated sensory neurons in rats with visceral hypersensitivity. In mammals, serotonin is primarily produced and released by enterochromaffin cells in the gut. To discover whether acetate – a short-chain fatty acid metabolite of B. dentium and some other microbiota – induces this pathway, the researchers first confirmed that B. dentium itself lacks the gene pathway for 5-HT production, and that growth media inoculated with B. dentium do not subsequently contain 5-HT. Next, they treated adult germ-free mice with either sterile media, live B. dentium, heat-killed B. dentium, or live Bacteroides ovatus (another commensal gut microbe). Gram staining and fluorescence in situ hybridization (FISH) confirmed that live B. dentium colonized mouse ileum and colon. Mass spectrometry, immunostaining, and quantitative PCR showed that mice treated with live B. dentium, but not B. ovatus, had greater intestinal concentrations of acetate, 5-HT, 5-HT receptors (2a and 4), serotonin transporter, and the gene that encodes free fatty acid receptor 2 (FFAR2), through which acetate signals. Furthermore, “[i]ncreases in 5-HT were observed in enteroendocrine cells directly above enteric neurons,” the researchers said.
They also performed RNA in situ hybridization of mouse brain tissue, which showed significantly increased expression of 5-HT-receptor 2a in the B. dentium–treated compared with germ-free controls. Mice were caged with specified numbers of marbles so the researchers could find out if these changes also modified behavior. Those with complete gut microbiota buried an average of 25% of the marbles, B. dentium–monocolonized mice buried 15%, and germ-free mice buried fewer marbles. Hence, even short-term monocolonization by a bacterium that acts on the serotonergic system might help normalize behavior, even later in life, the researchers said. They noted that B. dentium–treated and germ-free mice performed similarly on both balance beam and footprint tests, suggesting that treatment with B. dentium does not affect motor coordination.
In humans, enterochromaffin cells released more 5-HT when exposed to B. dentium or acetate. Taken together, the findings “highlight the importance of Bifidobacterium species, and specifically B. dentium, in the adult microbiome-gut-brain axis,” the researchers wrote. Probiotic strains such as Lactobacillus and Bifidobacterium species are thought to improve health by means of signaling pathways, including the serotonergic system, they noted. “Our findings support the modulation of the serotonergic system by a model gut microbe, B. dentium, and provide a potential mechanism by which select microbes and their metabolites can promote endogenous, localized 5-HT biosynthesis. We speculate this may be an important bridging signal in the microbiome-gut-brain axis.”
The National Institutes of Health, BioGaia AB, and the RNA In Situ Hybridization Core facility supported the work. Two coinvestigators disclosed ties to BioGaia AB, Seed, Biomica, Plexus Worldwide, Tenza, Mikrovia, Probiotech, and Takeda. Dr. Engevik and the other investigators reported having no conflicts of interest.
SOURCE: Engevik MA et al. Cell Molec Gastro Hepatol. 2021;11:221-48. doi: 10.1016/j.jcmgh.2020.08.002.
Together, Bifidobacterium dentium and its acetate metabolite regulate key parts of the serotonergic system and are associated with “a functional change in adult behavior,” according to a report published in Cellular and Molecular Gastroenterology and Hepatology.
Human gut microbiota had been known to regulate serotonin (5-hydroxytryptamine) production by gut cells, but underlying mechanisms had been unclear. This study showed that a common bacterial colonizer of the healthy adult gut stimulates serotonin (5-hydroxytryptamine, or 5-HT) release from enterochromaffin cells in both mice (in vivo) and humans (in vitro), wrote Melinda A. Engevik, PhD, of Baylor College of Medicine, Houston, and associates. “B. dentium modulates the serotonergic system in both the intestine and the brain [, which] likely influences behavior, and suggests that supplementation with a single, carefully selected, bacterial strain may be able to partially rescue behavioral deficits induced by shifts in the intestinal microbiota,” they added.
In a prior study, B. dentium modulated sensory neurons in rats with visceral hypersensitivity. In mammals, serotonin is primarily produced and released by enterochromaffin cells in the gut. To discover whether acetate – a short-chain fatty acid metabolite of B. dentium and some other microbiota – induces this pathway, the researchers first confirmed that B. dentium itself lacks the gene pathway for 5-HT production, and that growth media inoculated with B. dentium do not subsequently contain 5-HT. Next, they treated adult germ-free mice with either sterile media, live B. dentium, heat-killed B. dentium, or live Bacteroides ovatus (another commensal gut microbe). Gram staining and fluorescence in situ hybridization (FISH) confirmed that live B. dentium colonized mouse ileum and colon. Mass spectrometry, immunostaining, and quantitative PCR showed that mice treated with live B. dentium, but not B. ovatus, had greater intestinal concentrations of acetate, 5-HT, 5-HT receptors (2a and 4), serotonin transporter, and the gene that encodes free fatty acid receptor 2 (FFAR2), through which acetate signals. Furthermore, “[i]ncreases in 5-HT were observed in enteroendocrine cells directly above enteric neurons,” the researchers said.
They also performed RNA in situ hybridization of mouse brain tissue, which showed significantly increased expression of 5-HT-receptor 2a in the B. dentium–treated compared with germ-free controls. Mice were caged with specified numbers of marbles so the researchers could find out if these changes also modified behavior. Those with complete gut microbiota buried an average of 25% of the marbles, B. dentium–monocolonized mice buried 15%, and germ-free mice buried fewer marbles. Hence, even short-term monocolonization by a bacterium that acts on the serotonergic system might help normalize behavior, even later in life, the researchers said. They noted that B. dentium–treated and germ-free mice performed similarly on both balance beam and footprint tests, suggesting that treatment with B. dentium does not affect motor coordination.
In humans, enterochromaffin cells released more 5-HT when exposed to B. dentium or acetate. Taken together, the findings “highlight the importance of Bifidobacterium species, and specifically B. dentium, in the adult microbiome-gut-brain axis,” the researchers wrote. Probiotic strains such as Lactobacillus and Bifidobacterium species are thought to improve health by means of signaling pathways, including the serotonergic system, they noted. “Our findings support the modulation of the serotonergic system by a model gut microbe, B. dentium, and provide a potential mechanism by which select microbes and their metabolites can promote endogenous, localized 5-HT biosynthesis. We speculate this may be an important bridging signal in the microbiome-gut-brain axis.”
The National Institutes of Health, BioGaia AB, and the RNA In Situ Hybridization Core facility supported the work. Two coinvestigators disclosed ties to BioGaia AB, Seed, Biomica, Plexus Worldwide, Tenza, Mikrovia, Probiotech, and Takeda. Dr. Engevik and the other investigators reported having no conflicts of interest.
SOURCE: Engevik MA et al. Cell Molec Gastro Hepatol. 2021;11:221-48. doi: 10.1016/j.jcmgh.2020.08.002.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
AGA publishes seronegative enteropathy guidance
The American Gastroenterological Association has published new guidance for the diagnosis and management of seronegative enteropathies.
Seronegative enteropathies are commonly encountered by gastroenterologists, but accurate diagnosis can be complicated by a wide array of etiologies, misinterpreted histologic findings, suboptimal serology testing, and use of immunosuppressive agents that mask serology findings, reported lead author Maureen M. Leonard, MD, of MassGeneral Hospital for Children in Boston, and colleagues.
“Previous work detailing the prevalence of seronegative celiac disease [CeD], diagnosis of seronegative villous atrophy, and management recommendations for seronegative villous atrophy are available,” the investigators wrote in Gastroenterology. “However, there is limited evidence to guide clinicians regarding the minimal serologic tests necessary, the role of the gluten-free diet in diagnosis and management, and the role of an expert pathologist in evaluating the diagnosis of seronegative enteropathy.”
Patients with seronegative enteropathy tend to a have a poorer prognosis than those with classic CeD and other causes of villous atrophy, the investigators noted, but with an accurate diagnosis, distinct therapies can be highly effective.
After a comprehensive literature review, Dr. Leonard and colleagues reached consensus on eight best practice advice statements.
First, the investigators advised clinicians to review histologic findings with an experienced pathologist specializing in gastroenterology, as an expert can ensure proper duodenal orientation, and possibly link a specific finding with an etiology, such as granulomas with Crohn’s disease, or decreased goblet cells with autoimmune enteropathy. Communications with pathologists should also incorporate medical, travel, and medication history.
“Clinicians should pay particular attention to obtaining a thorough medication history to determine whether a patient is taking an angiotensin II receptor antagonist, such as olmesartan, which has been described as causing enteropathy,” the investigators wrote. “In some cases, this has led patients to be incorrectly diagnosis with refractory CeD. Other medications, including azathioprine and mycophenolate mofetil, among others, also have been reported to cause enteropathy, which resolves with discontinuation of medication.”
According to Dr. Leonard and colleagues, histologic findings suggestive of Crohn’s disease should prompt HLA testing, which requires careful attention to detail.
“It is prudent that the gastroenterologist or CeD specialist review all alleles tested and reported (or obtain the alleles if not reported) by the laboratory because commercial and academic laboratories might not report all possible alleles associated with CeD,” they wrote.
If HLA testing is positive, then the patient should begin empiric treatment with a gluten-free diet, followed by clinical and endoscopic reassessment after 1-3 years.
If HLA testing is negative, then a battery of tests may be needed to detect alternative etiologies, such as giardiasis, small intestinal bacterial overgrowth, HIV, and others.
“In cases where an underlying cause was identified, a follow-up esophagogastroduodenoscopy with biopsy might not be indicated, according to the etiology identified, treatment, and clinical status,” the investigators wrote.
Even with a comprehensive work-up, clinicians may be unable to identify an etiology. This outcome may be relatively common, the investigators suggested, citing a study of 200 cases of seronegative villous atrophy, of which 18% had no identifiable etiology. Yet finding an etiology may ultimately be unnecessary, as 72% of idiopathic cases resolved without intervention within 9 months, suggesting transient villous atrophy.
Still, intervention is needed for clinically unstable patients with idiopathic seronegative villous atrophy. Dr. Leonard and colleagues recommended first-line treatment with budesonide, starting at 9 mg daily. Depending on clinical status and response, subsequent therapies may include azathioprine or prednisone.
The clinical practice update was commissioned and approved by the AGA. The investigators disclosed additional relationships with Takeda Pharmaceuticals, HealthMode, Anokion, and others.
SOURCE: Leonard MM et al. Gastroenterology. 2020 Sep 30. doi: 10.1053/j.gastro.2020.08.061.
The American Gastroenterological Association has published new guidance for the diagnosis and management of seronegative enteropathies.
Seronegative enteropathies are commonly encountered by gastroenterologists, but accurate diagnosis can be complicated by a wide array of etiologies, misinterpreted histologic findings, suboptimal serology testing, and use of immunosuppressive agents that mask serology findings, reported lead author Maureen M. Leonard, MD, of MassGeneral Hospital for Children in Boston, and colleagues.
“Previous work detailing the prevalence of seronegative celiac disease [CeD], diagnosis of seronegative villous atrophy, and management recommendations for seronegative villous atrophy are available,” the investigators wrote in Gastroenterology. “However, there is limited evidence to guide clinicians regarding the minimal serologic tests necessary, the role of the gluten-free diet in diagnosis and management, and the role of an expert pathologist in evaluating the diagnosis of seronegative enteropathy.”
Patients with seronegative enteropathy tend to a have a poorer prognosis than those with classic CeD and other causes of villous atrophy, the investigators noted, but with an accurate diagnosis, distinct therapies can be highly effective.
After a comprehensive literature review, Dr. Leonard and colleagues reached consensus on eight best practice advice statements.
First, the investigators advised clinicians to review histologic findings with an experienced pathologist specializing in gastroenterology, as an expert can ensure proper duodenal orientation, and possibly link a specific finding with an etiology, such as granulomas with Crohn’s disease, or decreased goblet cells with autoimmune enteropathy. Communications with pathologists should also incorporate medical, travel, and medication history.
“Clinicians should pay particular attention to obtaining a thorough medication history to determine whether a patient is taking an angiotensin II receptor antagonist, such as olmesartan, which has been described as causing enteropathy,” the investigators wrote. “In some cases, this has led patients to be incorrectly diagnosis with refractory CeD. Other medications, including azathioprine and mycophenolate mofetil, among others, also have been reported to cause enteropathy, which resolves with discontinuation of medication.”
According to Dr. Leonard and colleagues, histologic findings suggestive of Crohn’s disease should prompt HLA testing, which requires careful attention to detail.
“It is prudent that the gastroenterologist or CeD specialist review all alleles tested and reported (or obtain the alleles if not reported) by the laboratory because commercial and academic laboratories might not report all possible alleles associated with CeD,” they wrote.
If HLA testing is positive, then the patient should begin empiric treatment with a gluten-free diet, followed by clinical and endoscopic reassessment after 1-3 years.
If HLA testing is negative, then a battery of tests may be needed to detect alternative etiologies, such as giardiasis, small intestinal bacterial overgrowth, HIV, and others.
“In cases where an underlying cause was identified, a follow-up esophagogastroduodenoscopy with biopsy might not be indicated, according to the etiology identified, treatment, and clinical status,” the investigators wrote.
Even with a comprehensive work-up, clinicians may be unable to identify an etiology. This outcome may be relatively common, the investigators suggested, citing a study of 200 cases of seronegative villous atrophy, of which 18% had no identifiable etiology. Yet finding an etiology may ultimately be unnecessary, as 72% of idiopathic cases resolved without intervention within 9 months, suggesting transient villous atrophy.
Still, intervention is needed for clinically unstable patients with idiopathic seronegative villous atrophy. Dr. Leonard and colleagues recommended first-line treatment with budesonide, starting at 9 mg daily. Depending on clinical status and response, subsequent therapies may include azathioprine or prednisone.
The clinical practice update was commissioned and approved by the AGA. The investigators disclosed additional relationships with Takeda Pharmaceuticals, HealthMode, Anokion, and others.
SOURCE: Leonard MM et al. Gastroenterology. 2020 Sep 30. doi: 10.1053/j.gastro.2020.08.061.
The American Gastroenterological Association has published new guidance for the diagnosis and management of seronegative enteropathies.
Seronegative enteropathies are commonly encountered by gastroenterologists, but accurate diagnosis can be complicated by a wide array of etiologies, misinterpreted histologic findings, suboptimal serology testing, and use of immunosuppressive agents that mask serology findings, reported lead author Maureen M. Leonard, MD, of MassGeneral Hospital for Children in Boston, and colleagues.
“Previous work detailing the prevalence of seronegative celiac disease [CeD], diagnosis of seronegative villous atrophy, and management recommendations for seronegative villous atrophy are available,” the investigators wrote in Gastroenterology. “However, there is limited evidence to guide clinicians regarding the minimal serologic tests necessary, the role of the gluten-free diet in diagnosis and management, and the role of an expert pathologist in evaluating the diagnosis of seronegative enteropathy.”
Patients with seronegative enteropathy tend to a have a poorer prognosis than those with classic CeD and other causes of villous atrophy, the investigators noted, but with an accurate diagnosis, distinct therapies can be highly effective.
After a comprehensive literature review, Dr. Leonard and colleagues reached consensus on eight best practice advice statements.
First, the investigators advised clinicians to review histologic findings with an experienced pathologist specializing in gastroenterology, as an expert can ensure proper duodenal orientation, and possibly link a specific finding with an etiology, such as granulomas with Crohn’s disease, or decreased goblet cells with autoimmune enteropathy. Communications with pathologists should also incorporate medical, travel, and medication history.
“Clinicians should pay particular attention to obtaining a thorough medication history to determine whether a patient is taking an angiotensin II receptor antagonist, such as olmesartan, which has been described as causing enteropathy,” the investigators wrote. “In some cases, this has led patients to be incorrectly diagnosis with refractory CeD. Other medications, including azathioprine and mycophenolate mofetil, among others, also have been reported to cause enteropathy, which resolves with discontinuation of medication.”
According to Dr. Leonard and colleagues, histologic findings suggestive of Crohn’s disease should prompt HLA testing, which requires careful attention to detail.
“It is prudent that the gastroenterologist or CeD specialist review all alleles tested and reported (or obtain the alleles if not reported) by the laboratory because commercial and academic laboratories might not report all possible alleles associated with CeD,” they wrote.
If HLA testing is positive, then the patient should begin empiric treatment with a gluten-free diet, followed by clinical and endoscopic reassessment after 1-3 years.
If HLA testing is negative, then a battery of tests may be needed to detect alternative etiologies, such as giardiasis, small intestinal bacterial overgrowth, HIV, and others.
“In cases where an underlying cause was identified, a follow-up esophagogastroduodenoscopy with biopsy might not be indicated, according to the etiology identified, treatment, and clinical status,” the investigators wrote.
Even with a comprehensive work-up, clinicians may be unable to identify an etiology. This outcome may be relatively common, the investigators suggested, citing a study of 200 cases of seronegative villous atrophy, of which 18% had no identifiable etiology. Yet finding an etiology may ultimately be unnecessary, as 72% of idiopathic cases resolved without intervention within 9 months, suggesting transient villous atrophy.
Still, intervention is needed for clinically unstable patients with idiopathic seronegative villous atrophy. Dr. Leonard and colleagues recommended first-line treatment with budesonide, starting at 9 mg daily. Depending on clinical status and response, subsequent therapies may include azathioprine or prednisone.
The clinical practice update was commissioned and approved by the AGA. The investigators disclosed additional relationships with Takeda Pharmaceuticals, HealthMode, Anokion, and others.
SOURCE: Leonard MM et al. Gastroenterology. 2020 Sep 30. doi: 10.1053/j.gastro.2020.08.061.
FROM GASTROENTEROLOGY
Mortality higher in older adults hospitalized for IBD
Adults older than 65 years with inflammatory bowel diseases (IBD) had significantly higher rates of inpatient mortality, compared with those younger than 65 years, independent of factors including disease severity, based on data from more than 200,000 hospital admissions.
Older adults use a disproportionate share of health care resources, but data on outcomes among hospitalized older adults with gastrointestinal illness are limited, Jeffrey Schwartz, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, and colleagues wrote in the Journal of Clinical Gastroenterology.
“In particular, there remains a significant concern that elderly patients are more susceptible to the development of opportunistic infections and malignancy in the setting of biological therapy, which has evolved into the standard of care for IBD over the past 10 years,” they wrote.
In their study, the researchers identified 162,800 hospital admissions for Crohn’s disease and 96,450 admissions for ulcerative colitis. Of these, 20% and 30%, respectively, were older than 65 years, which the researchers designated as the geriatric group.
In a multivariate analysis, age older than 65 years was significantly associated with increased mortality in both Crohn’s disease (odds ratio, 3.47; 95% confidence interval, 2.72-4.44; P < .001) and ulcerative colitis (OR, 2.75; 95% CI, 2.16-3.49; P < .001). The association was independent of factors included comorbidities, admission type, hospital type, inpatient surgery, and IBD subtype.
The most frequent cause of death in both groups across all ages and disease subtypes was infections (approximately 80% for all groups). The total hospital length of stay was significantly longer for geriatric patients, compared with younger patients with Crohn’s disease, in multivariate analysis (average increase, 0.19 days; P = .009). The total charges also were significantly higher among geriatric Crohn’s disease patients, compared with younger patients (average increase, $2,467; P = .012). No significant differences in hospital stay or total charges appeared between geriatric and younger patients with ulcerative colitis.
The study findings were limited by several factors such as the inclusion of older patients with IBD who were hospitalized for other reasons and by the potential for increased mortality because of comorbidities among elderly patients, the researchers noted. However, the findings support the limited data from similar previous studies and showed greater inpatient mortality for older adults with IBD, compared with hospital inpatients overall.
“Given the high prevalence of IBD patients that require inpatient admission, as well as the rapidly aging nature of the U.S. population, further studies are needed targeting geriatric patients with UC [ulcerative colitis] and CD [Crohn’s disease] to improve their overall management and quality of care to determine if this mortality risk can be reduced,” they concluded.
Tune in to risks in older adults
The study is important because the percentage of the population older than 65 years has been increasing; “at the same time, we are seeing more elderly patients being newly diagnosed with Crohn’s disease and ulcerative colitis,” said Russell D. Cohen, MD, of the University of Chicago, in an interview. “These patients are more vulnerable to complications of the diseases, such as infections, as well as complications from the medications used to treat these diseases.” However, older adults are often excluded from clinical trials and even from many observational studies in IBD, he noted.
“We have known from past studies that infections such as sepsis are a leading cause of death in our IBD patients,” said Dr. Cohen. “It is also understandable that those patients who have had complicated courses and those with other comorbidities have a higher mortality rate. However, what was surprising in the current study is that, even when the authors controlled for these factors, the geriatric patients still had two and three-quarters to three and a half times the mortality than those who were younger.”
The take-home message for clinicians is that “the geriatric patient with IBD is at a much higher rate for inpatient mortality, most commonly from infectious complications, than younger patients,” Dr. Cohen emphasized. “Quicker attention to what may seem minor but could become a potentially life-threatening infection is imperative. Caution with the use of multiple immune suppressing medications in older patients is paramount, as is timely surgical intervention in IBD patients in whom medications simply are not working.”
Focus research on infection prevention, cost burden
“More research should be directed at finding out whether these deadly infections could be prevented, perhaps by preventative ‘prophylactic’ antibiotics in the elderly patients, especially those on multiple immunosuppressive agents,” said Dr. Cohen. “In addition, research into the undue cost burden that these patients place on our health care system and counter that with better access to the newer, safer biological therapies [most of which Medicare does not cover] rather than corticosteroids.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Cohen disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb/Celgene, Eli Lilly, Gilead Sciences, Janssen, Pfizer, Takeda, and UCB Pharma.
SOURCE: Schwartz J et al. J Clin Gastroenterol. 2020 Nov 23. doi: 10.1097/MCG.0000000000001458.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
Adults older than 65 years with inflammatory bowel diseases (IBD) had significantly higher rates of inpatient mortality, compared with those younger than 65 years, independent of factors including disease severity, based on data from more than 200,000 hospital admissions.
Older adults use a disproportionate share of health care resources, but data on outcomes among hospitalized older adults with gastrointestinal illness are limited, Jeffrey Schwartz, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, and colleagues wrote in the Journal of Clinical Gastroenterology.
“In particular, there remains a significant concern that elderly patients are more susceptible to the development of opportunistic infections and malignancy in the setting of biological therapy, which has evolved into the standard of care for IBD over the past 10 years,” they wrote.
In their study, the researchers identified 162,800 hospital admissions for Crohn’s disease and 96,450 admissions for ulcerative colitis. Of these, 20% and 30%, respectively, were older than 65 years, which the researchers designated as the geriatric group.
In a multivariate analysis, age older than 65 years was significantly associated with increased mortality in both Crohn’s disease (odds ratio, 3.47; 95% confidence interval, 2.72-4.44; P < .001) and ulcerative colitis (OR, 2.75; 95% CI, 2.16-3.49; P < .001). The association was independent of factors included comorbidities, admission type, hospital type, inpatient surgery, and IBD subtype.
The most frequent cause of death in both groups across all ages and disease subtypes was infections (approximately 80% for all groups). The total hospital length of stay was significantly longer for geriatric patients, compared with younger patients with Crohn’s disease, in multivariate analysis (average increase, 0.19 days; P = .009). The total charges also were significantly higher among geriatric Crohn’s disease patients, compared with younger patients (average increase, $2,467; P = .012). No significant differences in hospital stay or total charges appeared between geriatric and younger patients with ulcerative colitis.
The study findings were limited by several factors such as the inclusion of older patients with IBD who were hospitalized for other reasons and by the potential for increased mortality because of comorbidities among elderly patients, the researchers noted. However, the findings support the limited data from similar previous studies and showed greater inpatient mortality for older adults with IBD, compared with hospital inpatients overall.
“Given the high prevalence of IBD patients that require inpatient admission, as well as the rapidly aging nature of the U.S. population, further studies are needed targeting geriatric patients with UC [ulcerative colitis] and CD [Crohn’s disease] to improve their overall management and quality of care to determine if this mortality risk can be reduced,” they concluded.
Tune in to risks in older adults
The study is important because the percentage of the population older than 65 years has been increasing; “at the same time, we are seeing more elderly patients being newly diagnosed with Crohn’s disease and ulcerative colitis,” said Russell D. Cohen, MD, of the University of Chicago, in an interview. “These patients are more vulnerable to complications of the diseases, such as infections, as well as complications from the medications used to treat these diseases.” However, older adults are often excluded from clinical trials and even from many observational studies in IBD, he noted.
“We have known from past studies that infections such as sepsis are a leading cause of death in our IBD patients,” said Dr. Cohen. “It is also understandable that those patients who have had complicated courses and those with other comorbidities have a higher mortality rate. However, what was surprising in the current study is that, even when the authors controlled for these factors, the geriatric patients still had two and three-quarters to three and a half times the mortality than those who were younger.”
The take-home message for clinicians is that “the geriatric patient with IBD is at a much higher rate for inpatient mortality, most commonly from infectious complications, than younger patients,” Dr. Cohen emphasized. “Quicker attention to what may seem minor but could become a potentially life-threatening infection is imperative. Caution with the use of multiple immune suppressing medications in older patients is paramount, as is timely surgical intervention in IBD patients in whom medications simply are not working.”
Focus research on infection prevention, cost burden
“More research should be directed at finding out whether these deadly infections could be prevented, perhaps by preventative ‘prophylactic’ antibiotics in the elderly patients, especially those on multiple immunosuppressive agents,” said Dr. Cohen. “In addition, research into the undue cost burden that these patients place on our health care system and counter that with better access to the newer, safer biological therapies [most of which Medicare does not cover] rather than corticosteroids.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Cohen disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb/Celgene, Eli Lilly, Gilead Sciences, Janssen, Pfizer, Takeda, and UCB Pharma.
SOURCE: Schwartz J et al. J Clin Gastroenterol. 2020 Nov 23. doi: 10.1097/MCG.0000000000001458.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
Adults older than 65 years with inflammatory bowel diseases (IBD) had significantly higher rates of inpatient mortality, compared with those younger than 65 years, independent of factors including disease severity, based on data from more than 200,000 hospital admissions.
Older adults use a disproportionate share of health care resources, but data on outcomes among hospitalized older adults with gastrointestinal illness are limited, Jeffrey Schwartz, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, both in Boston, and colleagues wrote in the Journal of Clinical Gastroenterology.
“In particular, there remains a significant concern that elderly patients are more susceptible to the development of opportunistic infections and malignancy in the setting of biological therapy, which has evolved into the standard of care for IBD over the past 10 years,” they wrote.
In their study, the researchers identified 162,800 hospital admissions for Crohn’s disease and 96,450 admissions for ulcerative colitis. Of these, 20% and 30%, respectively, were older than 65 years, which the researchers designated as the geriatric group.
In a multivariate analysis, age older than 65 years was significantly associated with increased mortality in both Crohn’s disease (odds ratio, 3.47; 95% confidence interval, 2.72-4.44; P < .001) and ulcerative colitis (OR, 2.75; 95% CI, 2.16-3.49; P < .001). The association was independent of factors included comorbidities, admission type, hospital type, inpatient surgery, and IBD subtype.
The most frequent cause of death in both groups across all ages and disease subtypes was infections (approximately 80% for all groups). The total hospital length of stay was significantly longer for geriatric patients, compared with younger patients with Crohn’s disease, in multivariate analysis (average increase, 0.19 days; P = .009). The total charges also were significantly higher among geriatric Crohn’s disease patients, compared with younger patients (average increase, $2,467; P = .012). No significant differences in hospital stay or total charges appeared between geriatric and younger patients with ulcerative colitis.
The study findings were limited by several factors such as the inclusion of older patients with IBD who were hospitalized for other reasons and by the potential for increased mortality because of comorbidities among elderly patients, the researchers noted. However, the findings support the limited data from similar previous studies and showed greater inpatient mortality for older adults with IBD, compared with hospital inpatients overall.
“Given the high prevalence of IBD patients that require inpatient admission, as well as the rapidly aging nature of the U.S. population, further studies are needed targeting geriatric patients with UC [ulcerative colitis] and CD [Crohn’s disease] to improve their overall management and quality of care to determine if this mortality risk can be reduced,” they concluded.
Tune in to risks in older adults
The study is important because the percentage of the population older than 65 years has been increasing; “at the same time, we are seeing more elderly patients being newly diagnosed with Crohn’s disease and ulcerative colitis,” said Russell D. Cohen, MD, of the University of Chicago, in an interview. “These patients are more vulnerable to complications of the diseases, such as infections, as well as complications from the medications used to treat these diseases.” However, older adults are often excluded from clinical trials and even from many observational studies in IBD, he noted.
“We have known from past studies that infections such as sepsis are a leading cause of death in our IBD patients,” said Dr. Cohen. “It is also understandable that those patients who have had complicated courses and those with other comorbidities have a higher mortality rate. However, what was surprising in the current study is that, even when the authors controlled for these factors, the geriatric patients still had two and three-quarters to three and a half times the mortality than those who were younger.”
The take-home message for clinicians is that “the geriatric patient with IBD is at a much higher rate for inpatient mortality, most commonly from infectious complications, than younger patients,” Dr. Cohen emphasized. “Quicker attention to what may seem minor but could become a potentially life-threatening infection is imperative. Caution with the use of multiple immune suppressing medications in older patients is paramount, as is timely surgical intervention in IBD patients in whom medications simply are not working.”
Focus research on infection prevention, cost burden
“More research should be directed at finding out whether these deadly infections could be prevented, perhaps by preventative ‘prophylactic’ antibiotics in the elderly patients, especially those on multiple immunosuppressive agents,” said Dr. Cohen. “In addition, research into the undue cost burden that these patients place on our health care system and counter that with better access to the newer, safer biological therapies [most of which Medicare does not cover] rather than corticosteroids.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Cohen disclosed relationships with multiple companies including AbbVie, Bristol-Myers Squibb/Celgene, Eli Lilly, Gilead Sciences, Janssen, Pfizer, Takeda, and UCB Pharma.
SOURCE: Schwartz J et al. J Clin Gastroenterol. 2020 Nov 23. doi: 10.1097/MCG.0000000000001458.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
AGA publishes recommendations for managing IBD in elderly patients
The American Gastroenterological Association has published a Clinical Practice Update for management of inflammatory bowel disease (IBD) in elderly patients, including 15 best practice advice statements.
According to lead author Ashwin N. Ananthakrishnan, MD, MPH, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues, this topic is becoming increasingly relevant, as the population is aging, and prevalence of IBD among elderly is rising approximately 5% per year.
“Up to 15% of IBD in North America and Asia is diagnosed after the age of 60 years,” the investigators wrote in Gastroenterology.
Dr. Ananthakrishnan and colleagues noted that “care of elderly IBD patients poses unique challenges with respect to diagnosis and therapeutic decision-making.”
Challenges include greater frequency of comorbidities, increased risk of infection with anti–tumor necrosis factor therapy, increased risk of lymphoma with thiopurine therapy, greater likelihood of surgical complications, and, for Crohn’s disease, an elevated mortality rate, according to the update.
Another challenge is a lack of data.
“It should be noted that most clinical data to inform these practices are based on observational data or indirect evidence as elderly IBD patients comprise a very small proportion of subjects enrolled in IBD clinical trials or long-term pharmacovigilance initiatives,” the investigators wrote.
With this in mind, the update offers guidance for diagnosis, treatment, and ongoing health maintenance.
Diagnosis
Dr. Ananthakrishnan and colleagues first suggested that clinicians remain vigilant for IBD in elderly people, in consideration of the 15% prevalence rate in this subpopulation.
For elderly individuals with a low probability of IBD, the investigators recommended fecal calprotectin or lactoferrin to determine if endoscopy is needed. For elderly patients with chronic diarrhea or hematochezia, plus moderate to high suspicion of IBD, colorectal neoplasia, or microscopic colitis, they recommended colonoscopy.
Lastly, the expert panel suggested that elderly patients presenting with segmental left-sided colitis and diverticulosis may also have Crohn’s disease or IBD unclassified.
Treatment
The clinical practice update offers 10 best practice statements for treating elderly patients with IBD. There is a recurring emphasis on treatment personalization, which should be informed by patient goals and priorities, risk/presence of severe disease, chronological age, functional status, independence, comorbidities, frailty, and several other age-associated risk factors (e.g., venous thromboembolism).
Concerning specific therapies, the investigators cautioned against systemic corticosteroids for maintenance therapy; instead, nonsystemic corticosteroids (e.g., budesonide) are favored, or possibly early biological therapy if budesonide is not indicated. When selecting a biologic, Dr. Ananthakrishnan and colleagues recommended those associated with a lower risk of malignancy and infection (e.g., ustekinumab or vedolizumab).
The advantages of thiopurine monotherapy being oral, relatively inexpensive compared to biologicals and having a long track record of success in maintenance of remission must be balanced against the need for ongoing serological monitoring, and increased risk of some malignancies.
Finally, the expert panel recommended that all elderly patients receive multidisciplinary care, which may include primary care providers, mental health professionals, nutritionists, and other specialists. It may also be productive to consult with family and caregivers during treatment planning.
Health maintenance
The last two best practice advice statements concern health maintenance.
First, the investigators recommended that elderly patients with IBD adhere to vaccination schedules, including herpes zoster, pneumococcus, and influenza vaccines, ideally, before starting immunosuppression.
Second, Dr. Ananthakrishnan and colleagues advised that cessation of colorectal cancer surveillance may be considered in elderly patients with IBD; however, this decision should take into account a variety of factors, including comorbidities, age, life expectancy, likelihood of endoscopic resection, and surgical candidacy.
The review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed relationships with Gilead, Sun Pharma, Kyn Therapeutics, and others.
SOURCE: Ananthakrishnan AN et al. Gastroenterology. 2020 Sep 30. doi: 10.1053/j.gastro.2020.08.060.
This story was updated on 12/4/2020.
The American Gastroenterological Association has published a Clinical Practice Update for management of inflammatory bowel disease (IBD) in elderly patients, including 15 best practice advice statements.
According to lead author Ashwin N. Ananthakrishnan, MD, MPH, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues, this topic is becoming increasingly relevant, as the population is aging, and prevalence of IBD among elderly is rising approximately 5% per year.
“Up to 15% of IBD in North America and Asia is diagnosed after the age of 60 years,” the investigators wrote in Gastroenterology.
Dr. Ananthakrishnan and colleagues noted that “care of elderly IBD patients poses unique challenges with respect to diagnosis and therapeutic decision-making.”
Challenges include greater frequency of comorbidities, increased risk of infection with anti–tumor necrosis factor therapy, increased risk of lymphoma with thiopurine therapy, greater likelihood of surgical complications, and, for Crohn’s disease, an elevated mortality rate, according to the update.
Another challenge is a lack of data.
“It should be noted that most clinical data to inform these practices are based on observational data or indirect evidence as elderly IBD patients comprise a very small proportion of subjects enrolled in IBD clinical trials or long-term pharmacovigilance initiatives,” the investigators wrote.
With this in mind, the update offers guidance for diagnosis, treatment, and ongoing health maintenance.
Diagnosis
Dr. Ananthakrishnan and colleagues first suggested that clinicians remain vigilant for IBD in elderly people, in consideration of the 15% prevalence rate in this subpopulation.
For elderly individuals with a low probability of IBD, the investigators recommended fecal calprotectin or lactoferrin to determine if endoscopy is needed. For elderly patients with chronic diarrhea or hematochezia, plus moderate to high suspicion of IBD, colorectal neoplasia, or microscopic colitis, they recommended colonoscopy.
Lastly, the expert panel suggested that elderly patients presenting with segmental left-sided colitis and diverticulosis may also have Crohn’s disease or IBD unclassified.
Treatment
The clinical practice update offers 10 best practice statements for treating elderly patients with IBD. There is a recurring emphasis on treatment personalization, which should be informed by patient goals and priorities, risk/presence of severe disease, chronological age, functional status, independence, comorbidities, frailty, and several other age-associated risk factors (e.g., venous thromboembolism).
Concerning specific therapies, the investigators cautioned against systemic corticosteroids for maintenance therapy; instead, nonsystemic corticosteroids (e.g., budesonide) are favored, or possibly early biological therapy if budesonide is not indicated. When selecting a biologic, Dr. Ananthakrishnan and colleagues recommended those associated with a lower risk of malignancy and infection (e.g., ustekinumab or vedolizumab).
The advantages of thiopurine monotherapy being oral, relatively inexpensive compared to biologicals and having a long track record of success in maintenance of remission must be balanced against the need for ongoing serological monitoring, and increased risk of some malignancies.
Finally, the expert panel recommended that all elderly patients receive multidisciplinary care, which may include primary care providers, mental health professionals, nutritionists, and other specialists. It may also be productive to consult with family and caregivers during treatment planning.
Health maintenance
The last two best practice advice statements concern health maintenance.
First, the investigators recommended that elderly patients with IBD adhere to vaccination schedules, including herpes zoster, pneumococcus, and influenza vaccines, ideally, before starting immunosuppression.
Second, Dr. Ananthakrishnan and colleagues advised that cessation of colorectal cancer surveillance may be considered in elderly patients with IBD; however, this decision should take into account a variety of factors, including comorbidities, age, life expectancy, likelihood of endoscopic resection, and surgical candidacy.
The review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed relationships with Gilead, Sun Pharma, Kyn Therapeutics, and others.
SOURCE: Ananthakrishnan AN et al. Gastroenterology. 2020 Sep 30. doi: 10.1053/j.gastro.2020.08.060.
This story was updated on 12/4/2020.
The American Gastroenterological Association has published a Clinical Practice Update for management of inflammatory bowel disease (IBD) in elderly patients, including 15 best practice advice statements.
According to lead author Ashwin N. Ananthakrishnan, MD, MPH, of Massachusetts General Hospital and Harvard Medical School, both in Boston, and colleagues, this topic is becoming increasingly relevant, as the population is aging, and prevalence of IBD among elderly is rising approximately 5% per year.
“Up to 15% of IBD in North America and Asia is diagnosed after the age of 60 years,” the investigators wrote in Gastroenterology.
Dr. Ananthakrishnan and colleagues noted that “care of elderly IBD patients poses unique challenges with respect to diagnosis and therapeutic decision-making.”
Challenges include greater frequency of comorbidities, increased risk of infection with anti–tumor necrosis factor therapy, increased risk of lymphoma with thiopurine therapy, greater likelihood of surgical complications, and, for Crohn’s disease, an elevated mortality rate, according to the update.
Another challenge is a lack of data.
“It should be noted that most clinical data to inform these practices are based on observational data or indirect evidence as elderly IBD patients comprise a very small proportion of subjects enrolled in IBD clinical trials or long-term pharmacovigilance initiatives,” the investigators wrote.
With this in mind, the update offers guidance for diagnosis, treatment, and ongoing health maintenance.
Diagnosis
Dr. Ananthakrishnan and colleagues first suggested that clinicians remain vigilant for IBD in elderly people, in consideration of the 15% prevalence rate in this subpopulation.
For elderly individuals with a low probability of IBD, the investigators recommended fecal calprotectin or lactoferrin to determine if endoscopy is needed. For elderly patients with chronic diarrhea or hematochezia, plus moderate to high suspicion of IBD, colorectal neoplasia, or microscopic colitis, they recommended colonoscopy.
Lastly, the expert panel suggested that elderly patients presenting with segmental left-sided colitis and diverticulosis may also have Crohn’s disease or IBD unclassified.
Treatment
The clinical practice update offers 10 best practice statements for treating elderly patients with IBD. There is a recurring emphasis on treatment personalization, which should be informed by patient goals and priorities, risk/presence of severe disease, chronological age, functional status, independence, comorbidities, frailty, and several other age-associated risk factors (e.g., venous thromboembolism).
Concerning specific therapies, the investigators cautioned against systemic corticosteroids for maintenance therapy; instead, nonsystemic corticosteroids (e.g., budesonide) are favored, or possibly early biological therapy if budesonide is not indicated. When selecting a biologic, Dr. Ananthakrishnan and colleagues recommended those associated with a lower risk of malignancy and infection (e.g., ustekinumab or vedolizumab).
The advantages of thiopurine monotherapy being oral, relatively inexpensive compared to biologicals and having a long track record of success in maintenance of remission must be balanced against the need for ongoing serological monitoring, and increased risk of some malignancies.
Finally, the expert panel recommended that all elderly patients receive multidisciplinary care, which may include primary care providers, mental health professionals, nutritionists, and other specialists. It may also be productive to consult with family and caregivers during treatment planning.
Health maintenance
The last two best practice advice statements concern health maintenance.
First, the investigators recommended that elderly patients with IBD adhere to vaccination schedules, including herpes zoster, pneumococcus, and influenza vaccines, ideally, before starting immunosuppression.
Second, Dr. Ananthakrishnan and colleagues advised that cessation of colorectal cancer surveillance may be considered in elderly patients with IBD; however, this decision should take into account a variety of factors, including comorbidities, age, life expectancy, likelihood of endoscopic resection, and surgical candidacy.
The review was commissioned and approved by the AGA Institute Clinical Practice Updates Committee and the AGA Governing Board. The investigators disclosed relationships with Gilead, Sun Pharma, Kyn Therapeutics, and others.
SOURCE: Ananthakrishnan AN et al. Gastroenterology. 2020 Sep 30. doi: 10.1053/j.gastro.2020.08.060.
This story was updated on 12/4/2020.
FROM GASTROENTEROLOGY
Histologic remission fails to be related to UC relapse
Relapse in ulcerative colitis patients with endoscopic remission was unaffected by histologic remission status, based on data from a retrospective study of 269 adults.
Data from previous studies suggest that histologic remission may be the strongest predictor of prognosis of disease course, wrote Neeraj Narula, MD, of McMaster University, Hamilton, Ont., and colleagues.
“However, it is unclear if UC patients who have achieved endoscopic healing have additional benefit in clinical outcomes if they have achieved histologic remission as well compared to those with ongoing histology activity,” they said.
In a study published in Alimentary Pharmacology and Therapeutics, the researchers identified 269 adults with ulcerative colitis who had endoscopic remission. Of these, 53 had normal histology, 138 had histologically inactive colitis, and 78 had histologically active colitis.
Overall, clinical relapse occurred in 64 patients, including 12 with normal histology (22.6%), 32 with inactive colitis (23.2%), and 29 with active colitis (25.6%).
No significant difference occurred in the time to relapse in patients with inactive vs. active colitis (adjusted hazard ratio 1.17, P = .67) or in patients with normal histology vs. inactive histology (AHR 0.67, P = .39). The median time to relapse was 2.92 years, 3.0 years, and 4.0 years in the normal, inactive, and active groups, respectively. Factors associated with a shorter time to relapse included older age at colonoscopy, use of 5-aminosalicylic acid, and disease extent in cases of pancolitis and left-sided colitis.
The study findings were limited by several factors including the possibility of bias in histologic scoring, lack of objective measures of disease activity, and the lack of uniformity is histologic assessment, the researchers noted. However, the results were strengthened by the large size compared with previous studies and by the adjustments for known confounding factors, they said.
“While clinical and endoscopic remission [is the target] of therapy for patients with UC, our study does not support targeting histologic remission in patients who have already achieved endoscopic remission,” they concluded.
More research may support clinical applications
“I was rather surprised by the findings, as a majority of studies have shown that histologic healing more accurately predicts clinical relapse than endoscopic remission in UC,” Atsushi Sakuraba, MD, of the University of Chicago, said in an interview.
“Although of a good sample size, this was a retrospective study, so no firm conclusion can be made,” said Dr. Sakuraba. “Using histologic healing as a therapeutic goal is still an evolving field, and it is too early to draw a conclusion as to whether (or not) to introduce histologic healing in clinical decision making,” he emphasized.
Going forward, prospective studies are needed that match for confounders such as postendoscopy medication use, age, and disease extent, Dr. Sakuraba said.
The study received no outside funding. Lead author Dr. Narula disclosed honoraria from Janssen, AbbVie, Takeda, Pfizer, Merck, and Ferring. Dr. Sakuraba had no financial conflicts to disclose.
SOURCE: Narula N et al. Aliment Pharmacol Ther. 2020 Nov 1. doi: 10.1111/apt.16147.
Relapse in ulcerative colitis patients with endoscopic remission was unaffected by histologic remission status, based on data from a retrospective study of 269 adults.
Data from previous studies suggest that histologic remission may be the strongest predictor of prognosis of disease course, wrote Neeraj Narula, MD, of McMaster University, Hamilton, Ont., and colleagues.
“However, it is unclear if UC patients who have achieved endoscopic healing have additional benefit in clinical outcomes if they have achieved histologic remission as well compared to those with ongoing histology activity,” they said.
In a study published in Alimentary Pharmacology and Therapeutics, the researchers identified 269 adults with ulcerative colitis who had endoscopic remission. Of these, 53 had normal histology, 138 had histologically inactive colitis, and 78 had histologically active colitis.
Overall, clinical relapse occurred in 64 patients, including 12 with normal histology (22.6%), 32 with inactive colitis (23.2%), and 29 with active colitis (25.6%).
No significant difference occurred in the time to relapse in patients with inactive vs. active colitis (adjusted hazard ratio 1.17, P = .67) or in patients with normal histology vs. inactive histology (AHR 0.67, P = .39). The median time to relapse was 2.92 years, 3.0 years, and 4.0 years in the normal, inactive, and active groups, respectively. Factors associated with a shorter time to relapse included older age at colonoscopy, use of 5-aminosalicylic acid, and disease extent in cases of pancolitis and left-sided colitis.
The study findings were limited by several factors including the possibility of bias in histologic scoring, lack of objective measures of disease activity, and the lack of uniformity is histologic assessment, the researchers noted. However, the results were strengthened by the large size compared with previous studies and by the adjustments for known confounding factors, they said.
“While clinical and endoscopic remission [is the target] of therapy for patients with UC, our study does not support targeting histologic remission in patients who have already achieved endoscopic remission,” they concluded.
More research may support clinical applications
“I was rather surprised by the findings, as a majority of studies have shown that histologic healing more accurately predicts clinical relapse than endoscopic remission in UC,” Atsushi Sakuraba, MD, of the University of Chicago, said in an interview.
“Although of a good sample size, this was a retrospective study, so no firm conclusion can be made,” said Dr. Sakuraba. “Using histologic healing as a therapeutic goal is still an evolving field, and it is too early to draw a conclusion as to whether (or not) to introduce histologic healing in clinical decision making,” he emphasized.
Going forward, prospective studies are needed that match for confounders such as postendoscopy medication use, age, and disease extent, Dr. Sakuraba said.
The study received no outside funding. Lead author Dr. Narula disclosed honoraria from Janssen, AbbVie, Takeda, Pfizer, Merck, and Ferring. Dr. Sakuraba had no financial conflicts to disclose.
SOURCE: Narula N et al. Aliment Pharmacol Ther. 2020 Nov 1. doi: 10.1111/apt.16147.
Relapse in ulcerative colitis patients with endoscopic remission was unaffected by histologic remission status, based on data from a retrospective study of 269 adults.
Data from previous studies suggest that histologic remission may be the strongest predictor of prognosis of disease course, wrote Neeraj Narula, MD, of McMaster University, Hamilton, Ont., and colleagues.
“However, it is unclear if UC patients who have achieved endoscopic healing have additional benefit in clinical outcomes if they have achieved histologic remission as well compared to those with ongoing histology activity,” they said.
In a study published in Alimentary Pharmacology and Therapeutics, the researchers identified 269 adults with ulcerative colitis who had endoscopic remission. Of these, 53 had normal histology, 138 had histologically inactive colitis, and 78 had histologically active colitis.
Overall, clinical relapse occurred in 64 patients, including 12 with normal histology (22.6%), 32 with inactive colitis (23.2%), and 29 with active colitis (25.6%).
No significant difference occurred in the time to relapse in patients with inactive vs. active colitis (adjusted hazard ratio 1.17, P = .67) or in patients with normal histology vs. inactive histology (AHR 0.67, P = .39). The median time to relapse was 2.92 years, 3.0 years, and 4.0 years in the normal, inactive, and active groups, respectively. Factors associated with a shorter time to relapse included older age at colonoscopy, use of 5-aminosalicylic acid, and disease extent in cases of pancolitis and left-sided colitis.
The study findings were limited by several factors including the possibility of bias in histologic scoring, lack of objective measures of disease activity, and the lack of uniformity is histologic assessment, the researchers noted. However, the results were strengthened by the large size compared with previous studies and by the adjustments for known confounding factors, they said.
“While clinical and endoscopic remission [is the target] of therapy for patients with UC, our study does not support targeting histologic remission in patients who have already achieved endoscopic remission,” they concluded.
More research may support clinical applications
“I was rather surprised by the findings, as a majority of studies have shown that histologic healing more accurately predicts clinical relapse than endoscopic remission in UC,” Atsushi Sakuraba, MD, of the University of Chicago, said in an interview.
“Although of a good sample size, this was a retrospective study, so no firm conclusion can be made,” said Dr. Sakuraba. “Using histologic healing as a therapeutic goal is still an evolving field, and it is too early to draw a conclusion as to whether (or not) to introduce histologic healing in clinical decision making,” he emphasized.
Going forward, prospective studies are needed that match for confounders such as postendoscopy medication use, age, and disease extent, Dr. Sakuraba said.
The study received no outside funding. Lead author Dr. Narula disclosed honoraria from Janssen, AbbVie, Takeda, Pfizer, Merck, and Ferring. Dr. Sakuraba had no financial conflicts to disclose.
SOURCE: Narula N et al. Aliment Pharmacol Ther. 2020 Nov 1. doi: 10.1111/apt.16147.
FROM ALIMENTARY PHARMACOLOGY AND THERAPEUTICS
Rapid relief of opioid-induced constipation with MNTX
Subcutaneously administered methylnaltrexone (MNTX) (Relistor), a peripherally acting mu-opioid receptor antagonist, relieves opioid-induced constipation (OID) in both chronic, noncancer-related illness and cancer-related illness, a new analysis concludes.
“While these are two very different patient groups, the ability to have something to treat OIC in noncancer patients who stay on opioids for whatever reason helps, because [otherwise] these patients are not doing well,” said lead author Eric Shah, MD, motility director for the Dartmouth program at Dartmouth Hitchcock Health, Lebanon, N.H.
Importantly, peripherally acting mu-opioid receptor antagonists such as MNTX do not affect overall pain control to any significant extent, which is “reassuring,” he said in an interview.
These drugs decrease the constipating effects of opioids without reversing CNS-mediated opioid effects, he explained.
“Methylnaltrexone has already been approved for the treatment of OIC in adults with chronic noncancer pain as well as for OIC in adults with advanced illness who are receiving palliative care, which is often the case in patients with cancer-related pain,” he noted.
Dr. Shah discussed the new analysis during PAINWeek 2020, the American Society of Regional Anesthesia and Pain Medicine 19th Annual Pain Medicine Meeting.
The analysis was based on a review of data collected in two previously reported randomized, placebo-controlled studies (study 302 and 4000), which were used to gain approval.
The new analysis shows that “the drug works up front, and the effect is able to be maintained. I think the studies are clinically relevant in that patients are able to have a bowel movement quickly after you give them an injectable formulation when they are vomiting or otherwise can’t tolerate a pill and they are feeling miserable,” Dr. Shah commented. Many patients with OIC are constipated for reasons other than from opioid use. They often have other side effects from opioids, including bloating, nausea, and vomiting.
“When patients go to the emergency room, it’s not just that they are not able to have a bowel movement; they are often also vomiting, so it’s important to have agents that can be given in a manner that avoids the need for oral medication,” Dr. Shah said. MNTX is the only peripherally acting opioid antagonist available in a subcutaneous formulation.
Moreover, if patients are able to control these symptoms at home with an injectable formulation, they may not need to go to the ED for treatment of their gastrointestinal distress, he added.
Viable product
In a comment, Darren Brenner, MD, associate professor of medicine and surgery, Northwestern University, Chicago, who has worked with this subcutaneous formulation, said it is “definitely a viable product.
“The data presented here were in patients with advanced illness receiving palliative care when other laxatives have failed, and the difference and the potential benefit for MNTX is that it is the only peripherally acting mu-opioid receptor antagonist that is approved for advanced cancer,” he added. The other products that are currently approved, naloxegol (Movantik) and naldemedine (Symproic), are both indicated for chronic, noncancer pain.
The other potential benefit of subcutaneous MNTX is that it can work very rapidly for the patients who respond to it. “One of the things investigators did not mention in these two trials but which has been shown in previous studies is that almost half of patients who respond to this drug respond within the first 30 minutes of receiving the injection,” Dr. Brenner said in an interview.
This can be very beneficial in an emergency setting, because it may avoid having patients admitted to hospital. They can be discharged and sent home with enough drug to use on demand, Dr. Brenner suggested.
New analysis of data from studies 302 and 4000
Both studies were carried out in adults with advanced illness and OIC whose conditions were refractory to laxative use. Both of the studies were placebo controlled.
Study 302 involved 78 patients with cancer and 56 patients with noncancer-related OIC. MNTX was given at a dose of 0.15 mg/kg subcutaneously every other day for 2 weeks.
Study 4000 included 152 patients with cancer and OIC and 78 patients with noncancer-related OIC. In this study, the dose of MNTX was based on body weight. Seven or fewer doses of either 8 mg or 12 mg were given subcutaneously for 2 weeks.
The main endpoints of both studies was the proportion of patients who achieved a rescue-free laxation (RFL) response within 4 hours after the first dose and the proportion of patients with an RFL response within 4 hours for two or more of the first four doses within 24 hours.
Dr. Shah explained that RFL is a meaningful clinical endpoint. Patients could achieve a bowel movement with the two prespecified time endpoints in both studies.
Not all patients were hospitalized for OIC, Dr. Shah noted. Entry criteria were strict and included having fewer than three bowel movements during the previous week and no clinically significant laxation (defecation) within 48 hours of receiving the first dose of study drug.
“In both studies, a significantly greater proportion of patients treated with MNTX versus placebo achieved an RFL within 4 hours after the first dose among both cancer and noncancer patients,” the investigators reported.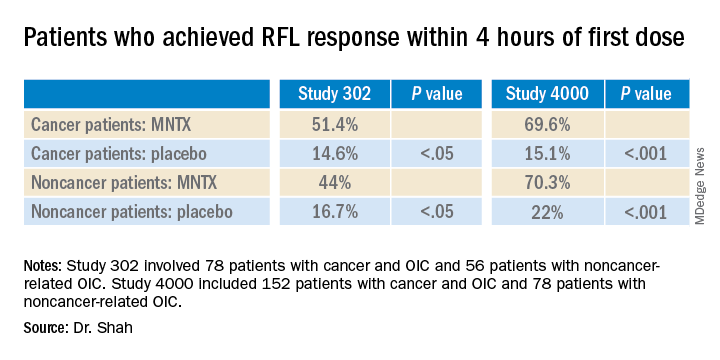
Results were relatively comparable between cancer and noncancer patients who were treated for OIC in study 4000, the investigators noted.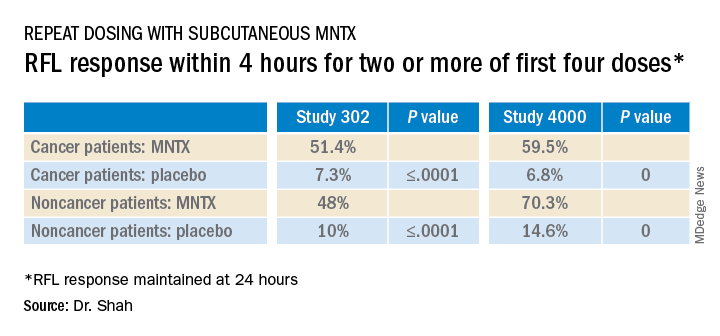
Both studies were sponsored by Salix Pharmaceuticals. Dr. Shah has received travel fees from Salix Pharmaceuticals. Dr. Brenner has served as a consultant for Salix Pharmaceuticals, AstraZeneca, and Purdue Pharma. AstraZeneca developed naloxegol.
This article first appeared on Medscape.com.
Subcutaneously administered methylnaltrexone (MNTX) (Relistor), a peripherally acting mu-opioid receptor antagonist, relieves opioid-induced constipation (OID) in both chronic, noncancer-related illness and cancer-related illness, a new analysis concludes.
“While these are two very different patient groups, the ability to have something to treat OIC in noncancer patients who stay on opioids for whatever reason helps, because [otherwise] these patients are not doing well,” said lead author Eric Shah, MD, motility director for the Dartmouth program at Dartmouth Hitchcock Health, Lebanon, N.H.
Importantly, peripherally acting mu-opioid receptor antagonists such as MNTX do not affect overall pain control to any significant extent, which is “reassuring,” he said in an interview.
These drugs decrease the constipating effects of opioids without reversing CNS-mediated opioid effects, he explained.
“Methylnaltrexone has already been approved for the treatment of OIC in adults with chronic noncancer pain as well as for OIC in adults with advanced illness who are receiving palliative care, which is often the case in patients with cancer-related pain,” he noted.
Dr. Shah discussed the new analysis during PAINWeek 2020, the American Society of Regional Anesthesia and Pain Medicine 19th Annual Pain Medicine Meeting.
The analysis was based on a review of data collected in two previously reported randomized, placebo-controlled studies (study 302 and 4000), which were used to gain approval.
The new analysis shows that “the drug works up front, and the effect is able to be maintained. I think the studies are clinically relevant in that patients are able to have a bowel movement quickly after you give them an injectable formulation when they are vomiting or otherwise can’t tolerate a pill and they are feeling miserable,” Dr. Shah commented. Many patients with OIC are constipated for reasons other than from opioid use. They often have other side effects from opioids, including bloating, nausea, and vomiting.
“When patients go to the emergency room, it’s not just that they are not able to have a bowel movement; they are often also vomiting, so it’s important to have agents that can be given in a manner that avoids the need for oral medication,” Dr. Shah said. MNTX is the only peripherally acting opioid antagonist available in a subcutaneous formulation.
Moreover, if patients are able to control these symptoms at home with an injectable formulation, they may not need to go to the ED for treatment of their gastrointestinal distress, he added.
Viable product
In a comment, Darren Brenner, MD, associate professor of medicine and surgery, Northwestern University, Chicago, who has worked with this subcutaneous formulation, said it is “definitely a viable product.
“The data presented here were in patients with advanced illness receiving palliative care when other laxatives have failed, and the difference and the potential benefit for MNTX is that it is the only peripherally acting mu-opioid receptor antagonist that is approved for advanced cancer,” he added. The other products that are currently approved, naloxegol (Movantik) and naldemedine (Symproic), are both indicated for chronic, noncancer pain.
The other potential benefit of subcutaneous MNTX is that it can work very rapidly for the patients who respond to it. “One of the things investigators did not mention in these two trials but which has been shown in previous studies is that almost half of patients who respond to this drug respond within the first 30 minutes of receiving the injection,” Dr. Brenner said in an interview.
This can be very beneficial in an emergency setting, because it may avoid having patients admitted to hospital. They can be discharged and sent home with enough drug to use on demand, Dr. Brenner suggested.
New analysis of data from studies 302 and 4000
Both studies were carried out in adults with advanced illness and OIC whose conditions were refractory to laxative use. Both of the studies were placebo controlled.
Study 302 involved 78 patients with cancer and 56 patients with noncancer-related OIC. MNTX was given at a dose of 0.15 mg/kg subcutaneously every other day for 2 weeks.
Study 4000 included 152 patients with cancer and OIC and 78 patients with noncancer-related OIC. In this study, the dose of MNTX was based on body weight. Seven or fewer doses of either 8 mg or 12 mg were given subcutaneously for 2 weeks.
The main endpoints of both studies was the proportion of patients who achieved a rescue-free laxation (RFL) response within 4 hours after the first dose and the proportion of patients with an RFL response within 4 hours for two or more of the first four doses within 24 hours.
Dr. Shah explained that RFL is a meaningful clinical endpoint. Patients could achieve a bowel movement with the two prespecified time endpoints in both studies.
Not all patients were hospitalized for OIC, Dr. Shah noted. Entry criteria were strict and included having fewer than three bowel movements during the previous week and no clinically significant laxation (defecation) within 48 hours of receiving the first dose of study drug.
“In both studies, a significantly greater proportion of patients treated with MNTX versus placebo achieved an RFL within 4 hours after the first dose among both cancer and noncancer patients,” the investigators reported.
Results were relatively comparable between cancer and noncancer patients who were treated for OIC in study 4000, the investigators noted.
Both studies were sponsored by Salix Pharmaceuticals. Dr. Shah has received travel fees from Salix Pharmaceuticals. Dr. Brenner has served as a consultant for Salix Pharmaceuticals, AstraZeneca, and Purdue Pharma. AstraZeneca developed naloxegol.
This article first appeared on Medscape.com.
Subcutaneously administered methylnaltrexone (MNTX) (Relistor), a peripherally acting mu-opioid receptor antagonist, relieves opioid-induced constipation (OID) in both chronic, noncancer-related illness and cancer-related illness, a new analysis concludes.
“While these are two very different patient groups, the ability to have something to treat OIC in noncancer patients who stay on opioids for whatever reason helps, because [otherwise] these patients are not doing well,” said lead author Eric Shah, MD, motility director for the Dartmouth program at Dartmouth Hitchcock Health, Lebanon, N.H.
Importantly, peripherally acting mu-opioid receptor antagonists such as MNTX do not affect overall pain control to any significant extent, which is “reassuring,” he said in an interview.
These drugs decrease the constipating effects of opioids without reversing CNS-mediated opioid effects, he explained.
“Methylnaltrexone has already been approved for the treatment of OIC in adults with chronic noncancer pain as well as for OIC in adults with advanced illness who are receiving palliative care, which is often the case in patients with cancer-related pain,” he noted.
Dr. Shah discussed the new analysis during PAINWeek 2020, the American Society of Regional Anesthesia and Pain Medicine 19th Annual Pain Medicine Meeting.
The analysis was based on a review of data collected in two previously reported randomized, placebo-controlled studies (study 302 and 4000), which were used to gain approval.
The new analysis shows that “the drug works up front, and the effect is able to be maintained. I think the studies are clinically relevant in that patients are able to have a bowel movement quickly after you give them an injectable formulation when they are vomiting or otherwise can’t tolerate a pill and they are feeling miserable,” Dr. Shah commented. Many patients with OIC are constipated for reasons other than from opioid use. They often have other side effects from opioids, including bloating, nausea, and vomiting.
“When patients go to the emergency room, it’s not just that they are not able to have a bowel movement; they are often also vomiting, so it’s important to have agents that can be given in a manner that avoids the need for oral medication,” Dr. Shah said. MNTX is the only peripherally acting opioid antagonist available in a subcutaneous formulation.
Moreover, if patients are able to control these symptoms at home with an injectable formulation, they may not need to go to the ED for treatment of their gastrointestinal distress, he added.
Viable product
In a comment, Darren Brenner, MD, associate professor of medicine and surgery, Northwestern University, Chicago, who has worked with this subcutaneous formulation, said it is “definitely a viable product.
“The data presented here were in patients with advanced illness receiving palliative care when other laxatives have failed, and the difference and the potential benefit for MNTX is that it is the only peripherally acting mu-opioid receptor antagonist that is approved for advanced cancer,” he added. The other products that are currently approved, naloxegol (Movantik) and naldemedine (Symproic), are both indicated for chronic, noncancer pain.
The other potential benefit of subcutaneous MNTX is that it can work very rapidly for the patients who respond to it. “One of the things investigators did not mention in these two trials but which has been shown in previous studies is that almost half of patients who respond to this drug respond within the first 30 minutes of receiving the injection,” Dr. Brenner said in an interview.
This can be very beneficial in an emergency setting, because it may avoid having patients admitted to hospital. They can be discharged and sent home with enough drug to use on demand, Dr. Brenner suggested.
New analysis of data from studies 302 and 4000
Both studies were carried out in adults with advanced illness and OIC whose conditions were refractory to laxative use. Both of the studies were placebo controlled.
Study 302 involved 78 patients with cancer and 56 patients with noncancer-related OIC. MNTX was given at a dose of 0.15 mg/kg subcutaneously every other day for 2 weeks.
Study 4000 included 152 patients with cancer and OIC and 78 patients with noncancer-related OIC. In this study, the dose of MNTX was based on body weight. Seven or fewer doses of either 8 mg or 12 mg were given subcutaneously for 2 weeks.
The main endpoints of both studies was the proportion of patients who achieved a rescue-free laxation (RFL) response within 4 hours after the first dose and the proportion of patients with an RFL response within 4 hours for two or more of the first four doses within 24 hours.
Dr. Shah explained that RFL is a meaningful clinical endpoint. Patients could achieve a bowel movement with the two prespecified time endpoints in both studies.
Not all patients were hospitalized for OIC, Dr. Shah noted. Entry criteria were strict and included having fewer than three bowel movements during the previous week and no clinically significant laxation (defecation) within 48 hours of receiving the first dose of study drug.
“In both studies, a significantly greater proportion of patients treated with MNTX versus placebo achieved an RFL within 4 hours after the first dose among both cancer and noncancer patients,” the investigators reported.
Results were relatively comparable between cancer and noncancer patients who were treated for OIC in study 4000, the investigators noted.
Both studies were sponsored by Salix Pharmaceuticals. Dr. Shah has received travel fees from Salix Pharmaceuticals. Dr. Brenner has served as a consultant for Salix Pharmaceuticals, AstraZeneca, and Purdue Pharma. AstraZeneca developed naloxegol.
This article first appeared on Medscape.com.
Rising IBD rates in minorities heighten need for awareness, strategies to close treatment gaps
Inflammatory bowel disease (IBD) is rapidly increasing among racial and ethnic minorities, which makes it important to consider for patients with compatible symptoms, experts wrote in Gastroenterology.
Crohn’s disease and ulcerative colitis are “chronic diseases with intermittent periods of flare and remission, so access to specialists, appropriate therapies, and frequent follow-up visits are vital to good outcomes,” wrote Edward L. Barnes, MD, MPH, of University of North Carolina at Chapel Hill, with his associates. However, Blacks with IBD tend to be diagnosed later than Whites, are less likely to receive recommended biologics and immunomodulators, and are more likely to receive care at an emergency department, to experience delays in colectomy, and to miss regular visits to IBD specialists because of financial and transportation barriers, they added.
These disparities are known to worsen outcomes. Compared with Whites, for example, Black patients with Crohn’s disease have higher rates of stricture and penetrating lesions and are at greater risk for postsurgical complications and death, even after potential confounders such age, sex, smoking status, time to operation, and obesity are controlled for. To help close these gaps, Dr. Barnes and his associates recommended enhanced recovery after surgery (ERAS) protocols, which “streamline [the] multidisciplinary management of patients with IBD before surgery, incorporating evidence-based practices focused on nutrition, prevention of postoperative ileus, and use of nonopioid analgesia and goal-directed fluid therapy.”
Similar approaches also might improve nonsurgical outcomes in minorities with IBD, the experts said. In the Sinai-Helmsley Alliance for Research Excellence (SHARE) study, Black patients had more complicated IBD at baseline but similar clinical outcomes and patterns of medication use as Whites when they were treated at academic IBD centers. In other studies, race and ethnicity did not affect patterns of medication use, surgery, or surgical outcomes if patients had similar access to care. Such findings “indicate that when patients of minority races and ethnicities have access to appropriate specialty care and IBD-related therapy, many previously identified disparities are resolved or reduced,” the experts said.
However, race and ethnicity do affect some aspects of IBD disease activity, genetics, and treatment safety and efficacy. Since White patients have made up the vast majority of research participants, studies of racial and ethnic minorities are needed to improve their IBD diagnosis, prevention, and treatment. Such research is particularly vital because IBD incidence is rising three times faster rates in racial and ethnic minorities than Whites, said Aline Charabaty, MD, AGAF, clinical director of the gastroenterology division at Johns Hopkins University in Baltimore, and director of the IBD Center at Sibley Memorial Hospital in Washington.
She explained that, when immigrants from countries where IBD is rare adopt the United States’ sedentary lifestyle and Western diet (low in fruits and vegetables; high in proinflammatory saturated fats, sugars, and processed foods), their gut microbiome shifts and their IBD risk increases markedly. Studies in other countries have produced similar findings, said Dr. Charabaty, who did not help author the review article.
She also noted that patients from communities with a historically low prevalence of IBD may not understand its chronicity or the need for long-term treatment. However, treatment adherence is a common issue for patients of all backgrounds with IBD, she said. “What is unique is barriers to continuity of care – not being able to get to the treatment center, not being able to afford treatment or take time off work if you live paycheck to paycheck, not being able to pay someone to care for your kids while you see the doctor.”
Other potential barriers to seeking IBD treatment include cultural taboos against discussing lower GI symptoms or concerns that chronic disease will harm marriage prospects, Dr. Charabaty said. Such challenges only heighten the need to ascertain IBD symptoms: “Studies show that minorities have less follow-up care and their symptoms tend to be minimized. There is a lot of unconscious bias among providers that factors into this. The barriers are multiple, and it is important to define them and find strategies to overcome them at the level of the patient, the clinician, and the health system.”
The Crohn’s and Colitis Foundation supported the work. Dr. Barnes disclosed ties to AbbVie, Gilead, Takeda, and Target Pharmasolutions. Two coauthors also disclosed relevant ties to pharmaceutical companies. Dr. Charabaty disclosed relationships with AbbVie, Takeda, Pfizer, Janssen, and UCB.
AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
SOURCE: Barnes EL et al. Gastroenterology. 2020 Oct 20. doi: 10.1053/j.gastro.2020.08.064.
Inflammatory bowel disease (IBD) is rapidly increasing among racial and ethnic minorities, which makes it important to consider for patients with compatible symptoms, experts wrote in Gastroenterology.
Crohn’s disease and ulcerative colitis are “chronic diseases with intermittent periods of flare and remission, so access to specialists, appropriate therapies, and frequent follow-up visits are vital to good outcomes,” wrote Edward L. Barnes, MD, MPH, of University of North Carolina at Chapel Hill, with his associates. However, Blacks with IBD tend to be diagnosed later than Whites, are less likely to receive recommended biologics and immunomodulators, and are more likely to receive care at an emergency department, to experience delays in colectomy, and to miss regular visits to IBD specialists because of financial and transportation barriers, they added.
These disparities are known to worsen outcomes. Compared with Whites, for example, Black patients with Crohn’s disease have higher rates of stricture and penetrating lesions and are at greater risk for postsurgical complications and death, even after potential confounders such age, sex, smoking status, time to operation, and obesity are controlled for. To help close these gaps, Dr. Barnes and his associates recommended enhanced recovery after surgery (ERAS) protocols, which “streamline [the] multidisciplinary management of patients with IBD before surgery, incorporating evidence-based practices focused on nutrition, prevention of postoperative ileus, and use of nonopioid analgesia and goal-directed fluid therapy.”
Similar approaches also might improve nonsurgical outcomes in minorities with IBD, the experts said. In the Sinai-Helmsley Alliance for Research Excellence (SHARE) study, Black patients had more complicated IBD at baseline but similar clinical outcomes and patterns of medication use as Whites when they were treated at academic IBD centers. In other studies, race and ethnicity did not affect patterns of medication use, surgery, or surgical outcomes if patients had similar access to care. Such findings “indicate that when patients of minority races and ethnicities have access to appropriate specialty care and IBD-related therapy, many previously identified disparities are resolved or reduced,” the experts said.
However, race and ethnicity do affect some aspects of IBD disease activity, genetics, and treatment safety and efficacy. Since White patients have made up the vast majority of research participants, studies of racial and ethnic minorities are needed to improve their IBD diagnosis, prevention, and treatment. Such research is particularly vital because IBD incidence is rising three times faster rates in racial and ethnic minorities than Whites, said Aline Charabaty, MD, AGAF, clinical director of the gastroenterology division at Johns Hopkins University in Baltimore, and director of the IBD Center at Sibley Memorial Hospital in Washington.
She explained that, when immigrants from countries where IBD is rare adopt the United States’ sedentary lifestyle and Western diet (low in fruits and vegetables; high in proinflammatory saturated fats, sugars, and processed foods), their gut microbiome shifts and their IBD risk increases markedly. Studies in other countries have produced similar findings, said Dr. Charabaty, who did not help author the review article.
She also noted that patients from communities with a historically low prevalence of IBD may not understand its chronicity or the need for long-term treatment. However, treatment adherence is a common issue for patients of all backgrounds with IBD, she said. “What is unique is barriers to continuity of care – not being able to get to the treatment center, not being able to afford treatment or take time off work if you live paycheck to paycheck, not being able to pay someone to care for your kids while you see the doctor.”
Other potential barriers to seeking IBD treatment include cultural taboos against discussing lower GI symptoms or concerns that chronic disease will harm marriage prospects, Dr. Charabaty said. Such challenges only heighten the need to ascertain IBD symptoms: “Studies show that minorities have less follow-up care and their symptoms tend to be minimized. There is a lot of unconscious bias among providers that factors into this. The barriers are multiple, and it is important to define them and find strategies to overcome them at the level of the patient, the clinician, and the health system.”
The Crohn’s and Colitis Foundation supported the work. Dr. Barnes disclosed ties to AbbVie, Gilead, Takeda, and Target Pharmasolutions. Two coauthors also disclosed relevant ties to pharmaceutical companies. Dr. Charabaty disclosed relationships with AbbVie, Takeda, Pfizer, Janssen, and UCB.
AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
SOURCE: Barnes EL et al. Gastroenterology. 2020 Oct 20. doi: 10.1053/j.gastro.2020.08.064.
Inflammatory bowel disease (IBD) is rapidly increasing among racial and ethnic minorities, which makes it important to consider for patients with compatible symptoms, experts wrote in Gastroenterology.
Crohn’s disease and ulcerative colitis are “chronic diseases with intermittent periods of flare and remission, so access to specialists, appropriate therapies, and frequent follow-up visits are vital to good outcomes,” wrote Edward L. Barnes, MD, MPH, of University of North Carolina at Chapel Hill, with his associates. However, Blacks with IBD tend to be diagnosed later than Whites, are less likely to receive recommended biologics and immunomodulators, and are more likely to receive care at an emergency department, to experience delays in colectomy, and to miss regular visits to IBD specialists because of financial and transportation barriers, they added.
These disparities are known to worsen outcomes. Compared with Whites, for example, Black patients with Crohn’s disease have higher rates of stricture and penetrating lesions and are at greater risk for postsurgical complications and death, even after potential confounders such age, sex, smoking status, time to operation, and obesity are controlled for. To help close these gaps, Dr. Barnes and his associates recommended enhanced recovery after surgery (ERAS) protocols, which “streamline [the] multidisciplinary management of patients with IBD before surgery, incorporating evidence-based practices focused on nutrition, prevention of postoperative ileus, and use of nonopioid analgesia and goal-directed fluid therapy.”
Similar approaches also might improve nonsurgical outcomes in minorities with IBD, the experts said. In the Sinai-Helmsley Alliance for Research Excellence (SHARE) study, Black patients had more complicated IBD at baseline but similar clinical outcomes and patterns of medication use as Whites when they were treated at academic IBD centers. In other studies, race and ethnicity did not affect patterns of medication use, surgery, or surgical outcomes if patients had similar access to care. Such findings “indicate that when patients of minority races and ethnicities have access to appropriate specialty care and IBD-related therapy, many previously identified disparities are resolved or reduced,” the experts said.
However, race and ethnicity do affect some aspects of IBD disease activity, genetics, and treatment safety and efficacy. Since White patients have made up the vast majority of research participants, studies of racial and ethnic minorities are needed to improve their IBD diagnosis, prevention, and treatment. Such research is particularly vital because IBD incidence is rising three times faster rates in racial and ethnic minorities than Whites, said Aline Charabaty, MD, AGAF, clinical director of the gastroenterology division at Johns Hopkins University in Baltimore, and director of the IBD Center at Sibley Memorial Hospital in Washington.
She explained that, when immigrants from countries where IBD is rare adopt the United States’ sedentary lifestyle and Western diet (low in fruits and vegetables; high in proinflammatory saturated fats, sugars, and processed foods), their gut microbiome shifts and their IBD risk increases markedly. Studies in other countries have produced similar findings, said Dr. Charabaty, who did not help author the review article.
She also noted that patients from communities with a historically low prevalence of IBD may not understand its chronicity or the need for long-term treatment. However, treatment adherence is a common issue for patients of all backgrounds with IBD, she said. “What is unique is barriers to continuity of care – not being able to get to the treatment center, not being able to afford treatment or take time off work if you live paycheck to paycheck, not being able to pay someone to care for your kids while you see the doctor.”
Other potential barriers to seeking IBD treatment include cultural taboos against discussing lower GI symptoms or concerns that chronic disease will harm marriage prospects, Dr. Charabaty said. Such challenges only heighten the need to ascertain IBD symptoms: “Studies show that minorities have less follow-up care and their symptoms tend to be minimized. There is a lot of unconscious bias among providers that factors into this. The barriers are multiple, and it is important to define them and find strategies to overcome them at the level of the patient, the clinician, and the health system.”
The Crohn’s and Colitis Foundation supported the work. Dr. Barnes disclosed ties to AbbVie, Gilead, Takeda, and Target Pharmasolutions. Two coauthors also disclosed relevant ties to pharmaceutical companies. Dr. Charabaty disclosed relationships with AbbVie, Takeda, Pfizer, Janssen, and UCB.
AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
SOURCE: Barnes EL et al. Gastroenterology. 2020 Oct 20. doi: 10.1053/j.gastro.2020.08.064.
FROM GASTROENTEROLOGY
Tool predicted vedolizumab nonresponse in routine practice
Among patients with ulcerative colitis who were treated in routine practice, a point-based clinical scoring tool predicted nonresponse to vedolizumab therapy, according to study findings published online in Clinical Gastroenterology and Hepatology.
A cutoff of 26 points or less was 93% sensitive (95% confidence interval, 79%-98%) for identifying patients who did not reach corticosteroid-free remission during 26 weeks of treatment and was 88% sensitive (95% CI, 83%-92%) for identifying patients who required colectomy, reported Parambir S. Dulai, MBBS, of the University of California, San Diego, and his associates. The tool was less reliable for predicting response to tumor necrosis factor (TNF) antagonists, indicating that it is treatment specific, they noted.
Vedolizumab, an alpha-4-beta-7 anti-integrin that restricts the migration of proinflammatory lymphocytes to the gut, can induce corticosteroid-free remissions and mucosal healing in patients with ulcerative colitis. In clinical practice, 22-week rates of clinical response and remission were approximately 51% and 30%, respectively, in a recent study. Noting the lack of real-world data on predictors of response, the researchers modeled data from 620 patients who received induction and maintenance vedolizumab during the blinded phase 3 GEMINI 1 trial. They used this model to create the clinical scoring tool, which they validated in a cohort of 322 patients with ulcerative colitis who had received vedolizumab (199 patients) or TNF antagonists (123 patients) in routine practice during the Vedolizumab for Health Outcomes in Inflammatory Bowel Diseases (VICTORY) study.
In the final multivariable model, predictors of steroid-free remission were TNF antagonist naivety, at least a 2-year history of ulcerative colitis, moderate rather than severe endoscopy activity, and baseline albumin concentration. The resulting clinical scoring tool included these four variables and multiplied them by factors ranging from 0.0647 (for baseline albumin concentration) to 0.2820 (for no prior TNF antagonist exposure). In the validation cohort, patients were categorized as “high probability” (of response) if they scored 33 points or more on the tool and “low probability” if they scored 26 points or fewer. Rates of corticosteroid-free remissions were substantially different at 32% and 12%, respectively. The tool also predicted responses to vedolizumab more accurately than it did predicted responses to TNF antagonists, indicating that it was drug specific.
In the validation cohort, 46% (10 of 22) of low- or intermediate-probability patients showed least a 50% decrease in symptom activity after their vedolizumab infusion interval was shortened to address an insufficient initial response. “However, none of the high-probability patients showed a clinical response to interval shortening,” probably because they had higher vedolizumab trough concentrations to begin with, the researchers said. They called for prospective validation of this finding, “ideally in a randomized, controlled trial setting.”
The derivation and validation cohorts differed in several important ways. The validation cohort included significantly more females, smokers, patients with moderate endoscopic disease, and patients who had failed prior TNF antagonist therapy. These patients also had a significantly higher median albumin level and a longer history of disease. “The lower bound of the confidence interval for the [model’s] performance reached 0.5, suggesting that model discrimination may not be ideal,” the researchers said. “Further validation will therefore be needed to understand external validity on additional cohorts.”
An American Gastroenterological Association Research Scholar Award supported the work. Dr. Dulai reported holding a provisional patent for the prediction model and consulting relationships and other ties to Takeda, Janssen, Pfizer, and AbbVie. His coinvestigators reported ties to numerous pharmaceutical companies.
SOURCE: Dulai PS et al. Clin Gastroenterol Hepatol. 2020 Feb 13. doi: 10.1016/j.cgh.2020.02.010.
The management of moderate to severe ulcerative colitis has become more complex because of the greater number of Food and Drug–approved biologic and small-molecule agents presently available. With more options, practitioners are faced with the challenge of choosing the most appropriate agent based on disease- and patient-specific risk factors. The goals of early intervention are to achieve steroid-free remission with mucosal healing and the associated improvements in quality of life, reduced colectomy, and lower colon cancer risks.
The value in these types of tools is to assist in early biologic decision-making by providing a numeric cutoff that can be used to recommend one agent versus the other. Another noted feature of this tool is the potential to identify which patients may benefit from dose optimization because lower or intermediate scores tended to respond to dose escalation in vedolizumab partial responders. However, because this tool predominantly assists with the choice of anti-TNF vs. vedolizumab, one may not be able to extrapolate these results to ustekinumab and tofacitinib positioning in ulcerative colitis. Further studies are needed to determine if these variables similarly affect steroid-free remission for these agents.
Christina Ha, MD, FACG, AGAF, is an associate professor of medicine, Inflammatory Bowel Disease Center, Cedars-Sinai, Los Angeles. She is on the advisory board of AbbVie, Janssen, Takeda, Pfizer, Salix, and InDex Pharmaceuticals; has received grant support from Pfizer; and has received research support from Pfizer and Lilly.
The management of moderate to severe ulcerative colitis has become more complex because of the greater number of Food and Drug–approved biologic and small-molecule agents presently available. With more options, practitioners are faced with the challenge of choosing the most appropriate agent based on disease- and patient-specific risk factors. The goals of early intervention are to achieve steroid-free remission with mucosal healing and the associated improvements in quality of life, reduced colectomy, and lower colon cancer risks.
The value in these types of tools is to assist in early biologic decision-making by providing a numeric cutoff that can be used to recommend one agent versus the other. Another noted feature of this tool is the potential to identify which patients may benefit from dose optimization because lower or intermediate scores tended to respond to dose escalation in vedolizumab partial responders. However, because this tool predominantly assists with the choice of anti-TNF vs. vedolizumab, one may not be able to extrapolate these results to ustekinumab and tofacitinib positioning in ulcerative colitis. Further studies are needed to determine if these variables similarly affect steroid-free remission for these agents.
Christina Ha, MD, FACG, AGAF, is an associate professor of medicine, Inflammatory Bowel Disease Center, Cedars-Sinai, Los Angeles. She is on the advisory board of AbbVie, Janssen, Takeda, Pfizer, Salix, and InDex Pharmaceuticals; has received grant support from Pfizer; and has received research support from Pfizer and Lilly.
The management of moderate to severe ulcerative colitis has become more complex because of the greater number of Food and Drug–approved biologic and small-molecule agents presently available. With more options, practitioners are faced with the challenge of choosing the most appropriate agent based on disease- and patient-specific risk factors. The goals of early intervention are to achieve steroid-free remission with mucosal healing and the associated improvements in quality of life, reduced colectomy, and lower colon cancer risks.
The value in these types of tools is to assist in early biologic decision-making by providing a numeric cutoff that can be used to recommend one agent versus the other. Another noted feature of this tool is the potential to identify which patients may benefit from dose optimization because lower or intermediate scores tended to respond to dose escalation in vedolizumab partial responders. However, because this tool predominantly assists with the choice of anti-TNF vs. vedolizumab, one may not be able to extrapolate these results to ustekinumab and tofacitinib positioning in ulcerative colitis. Further studies are needed to determine if these variables similarly affect steroid-free remission for these agents.
Christina Ha, MD, FACG, AGAF, is an associate professor of medicine, Inflammatory Bowel Disease Center, Cedars-Sinai, Los Angeles. She is on the advisory board of AbbVie, Janssen, Takeda, Pfizer, Salix, and InDex Pharmaceuticals; has received grant support from Pfizer; and has received research support from Pfizer and Lilly.
Among patients with ulcerative colitis who were treated in routine practice, a point-based clinical scoring tool predicted nonresponse to vedolizumab therapy, according to study findings published online in Clinical Gastroenterology and Hepatology.
A cutoff of 26 points or less was 93% sensitive (95% confidence interval, 79%-98%) for identifying patients who did not reach corticosteroid-free remission during 26 weeks of treatment and was 88% sensitive (95% CI, 83%-92%) for identifying patients who required colectomy, reported Parambir S. Dulai, MBBS, of the University of California, San Diego, and his associates. The tool was less reliable for predicting response to tumor necrosis factor (TNF) antagonists, indicating that it is treatment specific, they noted.
Vedolizumab, an alpha-4-beta-7 anti-integrin that restricts the migration of proinflammatory lymphocytes to the gut, can induce corticosteroid-free remissions and mucosal healing in patients with ulcerative colitis. In clinical practice, 22-week rates of clinical response and remission were approximately 51% and 30%, respectively, in a recent study. Noting the lack of real-world data on predictors of response, the researchers modeled data from 620 patients who received induction and maintenance vedolizumab during the blinded phase 3 GEMINI 1 trial. They used this model to create the clinical scoring tool, which they validated in a cohort of 322 patients with ulcerative colitis who had received vedolizumab (199 patients) or TNF antagonists (123 patients) in routine practice during the Vedolizumab for Health Outcomes in Inflammatory Bowel Diseases (VICTORY) study.
In the final multivariable model, predictors of steroid-free remission were TNF antagonist naivety, at least a 2-year history of ulcerative colitis, moderate rather than severe endoscopy activity, and baseline albumin concentration. The resulting clinical scoring tool included these four variables and multiplied them by factors ranging from 0.0647 (for baseline albumin concentration) to 0.2820 (for no prior TNF antagonist exposure). In the validation cohort, patients were categorized as “high probability” (of response) if they scored 33 points or more on the tool and “low probability” if they scored 26 points or fewer. Rates of corticosteroid-free remissions were substantially different at 32% and 12%, respectively. The tool also predicted responses to vedolizumab more accurately than it did predicted responses to TNF antagonists, indicating that it was drug specific.
In the validation cohort, 46% (10 of 22) of low- or intermediate-probability patients showed least a 50% decrease in symptom activity after their vedolizumab infusion interval was shortened to address an insufficient initial response. “However, none of the high-probability patients showed a clinical response to interval shortening,” probably because they had higher vedolizumab trough concentrations to begin with, the researchers said. They called for prospective validation of this finding, “ideally in a randomized, controlled trial setting.”
The derivation and validation cohorts differed in several important ways. The validation cohort included significantly more females, smokers, patients with moderate endoscopic disease, and patients who had failed prior TNF antagonist therapy. These patients also had a significantly higher median albumin level and a longer history of disease. “The lower bound of the confidence interval for the [model’s] performance reached 0.5, suggesting that model discrimination may not be ideal,” the researchers said. “Further validation will therefore be needed to understand external validity on additional cohorts.”
An American Gastroenterological Association Research Scholar Award supported the work. Dr. Dulai reported holding a provisional patent for the prediction model and consulting relationships and other ties to Takeda, Janssen, Pfizer, and AbbVie. His coinvestigators reported ties to numerous pharmaceutical companies.
SOURCE: Dulai PS et al. Clin Gastroenterol Hepatol. 2020 Feb 13. doi: 10.1016/j.cgh.2020.02.010.
Among patients with ulcerative colitis who were treated in routine practice, a point-based clinical scoring tool predicted nonresponse to vedolizumab therapy, according to study findings published online in Clinical Gastroenterology and Hepatology.
A cutoff of 26 points or less was 93% sensitive (95% confidence interval, 79%-98%) for identifying patients who did not reach corticosteroid-free remission during 26 weeks of treatment and was 88% sensitive (95% CI, 83%-92%) for identifying patients who required colectomy, reported Parambir S. Dulai, MBBS, of the University of California, San Diego, and his associates. The tool was less reliable for predicting response to tumor necrosis factor (TNF) antagonists, indicating that it is treatment specific, they noted.
Vedolizumab, an alpha-4-beta-7 anti-integrin that restricts the migration of proinflammatory lymphocytes to the gut, can induce corticosteroid-free remissions and mucosal healing in patients with ulcerative colitis. In clinical practice, 22-week rates of clinical response and remission were approximately 51% and 30%, respectively, in a recent study. Noting the lack of real-world data on predictors of response, the researchers modeled data from 620 patients who received induction and maintenance vedolizumab during the blinded phase 3 GEMINI 1 trial. They used this model to create the clinical scoring tool, which they validated in a cohort of 322 patients with ulcerative colitis who had received vedolizumab (199 patients) or TNF antagonists (123 patients) in routine practice during the Vedolizumab for Health Outcomes in Inflammatory Bowel Diseases (VICTORY) study.
In the final multivariable model, predictors of steroid-free remission were TNF antagonist naivety, at least a 2-year history of ulcerative colitis, moderate rather than severe endoscopy activity, and baseline albumin concentration. The resulting clinical scoring tool included these four variables and multiplied them by factors ranging from 0.0647 (for baseline albumin concentration) to 0.2820 (for no prior TNF antagonist exposure). In the validation cohort, patients were categorized as “high probability” (of response) if they scored 33 points or more on the tool and “low probability” if they scored 26 points or fewer. Rates of corticosteroid-free remissions were substantially different at 32% and 12%, respectively. The tool also predicted responses to vedolizumab more accurately than it did predicted responses to TNF antagonists, indicating that it was drug specific.
In the validation cohort, 46% (10 of 22) of low- or intermediate-probability patients showed least a 50% decrease in symptom activity after their vedolizumab infusion interval was shortened to address an insufficient initial response. “However, none of the high-probability patients showed a clinical response to interval shortening,” probably because they had higher vedolizumab trough concentrations to begin with, the researchers said. They called for prospective validation of this finding, “ideally in a randomized, controlled trial setting.”
The derivation and validation cohorts differed in several important ways. The validation cohort included significantly more females, smokers, patients with moderate endoscopic disease, and patients who had failed prior TNF antagonist therapy. These patients also had a significantly higher median albumin level and a longer history of disease. “The lower bound of the confidence interval for the [model’s] performance reached 0.5, suggesting that model discrimination may not be ideal,” the researchers said. “Further validation will therefore be needed to understand external validity on additional cohorts.”
An American Gastroenterological Association Research Scholar Award supported the work. Dr. Dulai reported holding a provisional patent for the prediction model and consulting relationships and other ties to Takeda, Janssen, Pfizer, and AbbVie. His coinvestigators reported ties to numerous pharmaceutical companies.
SOURCE: Dulai PS et al. Clin Gastroenterol Hepatol. 2020 Feb 13. doi: 10.1016/j.cgh.2020.02.010.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Life expectancy gap persists for IBD patients
Life expectancy increased for adults with inflammatory bowel disease (IBD) over recent decades, but still remained lower than life expectancy for individuals without IBD, according to data from a retrospective cohort study using Canadian health databases.
“Management of IBD has improved through increased access to specialist care, biologic therapies, and a treat-to-target approach,” wrote M. Ellen Kuenzig, PhD, of the Children’s Hospital of Eastern Ontario and colleagues. However, “Most studies evaluating mortality were conducted before the biologic era, and none evaluated life expectancy or health-adjusted life expectancy,” they said.
In a study published in the Canadian Medical Association Journal, the researchers used Canadian databases to identify a study population of 32,818 people with IBD matched to 163,284 people without IBD in 1996 that increased to 83,672 people with IBD matched to 418,360 people without IBD in 2011.
Life expectancy increases, but with caveats
Overall, life expectancy for IBD patients increased from 75.5 years to 78.4 years for women and from 72.2 years to 75.5 years for men between 1996 and 2011.
However, health-adjusted life expectancy, defined as the number of years a person is expected to live in full health, decreased by 3.9 years for men with IBD between 1996 and 2008, but did not change significantly for women with IBD, although the gap in health-adjusted life expectancy increased between women with IBD and women without IBD.
Both life expectancy and health-adjusted life expectancy remained consistently lower for individuals with IBD compared to those without IBD. Differences in life expectancy for women with and without IBD ranged from 6.6 to 8.1 years and from 5.0 to 6.1 years for men with and without IBD, depending on the year.
Differences in health-adjusted life expectancy ranged from 9.5 to 13.5 years in women with and without IBD, and from 2.6 years to 6.7 years in men with and without IBD depending on the year, the researchers said.
In addition, the researchers used the pain-attributed utility of the Health Utility Index (HUI) to calculate health-adjusted life expectancy with the effect of pain on health status. The health-adjusted life expectancy using HUI for pain was stable in women with IBD, but increased over time for women without IBD. This pattern was similar for individuals with Crohn’s disease but stable for women with and without ulcerative colitis. In men, health-adjusted life expectancy using the pain-attributed utility of the HUI was stable for those with IBD, decreased in those with Crohn’s disease, and increased among those with ulcerative colitis.
The study findings were limited by several factors including potential confounding by disease phenotype and severity, as well as lack of data on medication use for individuals younger than 65 years and limited data on confounders including smoking, ethnicity, and environmental factors, the researchers said.
In addition, “mortality may increase with disease duration, which would violate the assumption that age- and sex-specific mortality rates remain constant over a person’s lifetime that is required when using lifetables,” they said.
Future research should pursue effect of pain
The results support the persistence of a gap in life expectancy between individuals with and without IBD and suggest the need for improving pain management in IBD patients, given the contribution of pain to reduced health-adjusted life expectancy, they concluded. Additional research is needed to determine the effect of comorbid conditions and medication use on the difference in life expectancy for those with and without IBD, they added.
The study was supported by the Institute for Clinical Evaluative Services (Canada). Lead author Dr. Kuenzig received a Post-Doctoral Fellowship Award from the Canadian Institutes of Health Research, Canadian Association of Gastroenterology, and Crohn’s and Colitis Canada. The researchers had no financial conflicts to disclose.
SOURCE: Kuenzig ME et al. CMAJ. 2020 Nov 9. doi: 10.1503/cmaj.190976.
Life expectancy increased for adults with inflammatory bowel disease (IBD) over recent decades, but still remained lower than life expectancy for individuals without IBD, according to data from a retrospective cohort study using Canadian health databases.
“Management of IBD has improved through increased access to specialist care, biologic therapies, and a treat-to-target approach,” wrote M. Ellen Kuenzig, PhD, of the Children’s Hospital of Eastern Ontario and colleagues. However, “Most studies evaluating mortality were conducted before the biologic era, and none evaluated life expectancy or health-adjusted life expectancy,” they said.
In a study published in the Canadian Medical Association Journal, the researchers used Canadian databases to identify a study population of 32,818 people with IBD matched to 163,284 people without IBD in 1996 that increased to 83,672 people with IBD matched to 418,360 people without IBD in 2011.
Life expectancy increases, but with caveats
Overall, life expectancy for IBD patients increased from 75.5 years to 78.4 years for women and from 72.2 years to 75.5 years for men between 1996 and 2011.
However, health-adjusted life expectancy, defined as the number of years a person is expected to live in full health, decreased by 3.9 years for men with IBD between 1996 and 2008, but did not change significantly for women with IBD, although the gap in health-adjusted life expectancy increased between women with IBD and women without IBD.
Both life expectancy and health-adjusted life expectancy remained consistently lower for individuals with IBD compared to those without IBD. Differences in life expectancy for women with and without IBD ranged from 6.6 to 8.1 years and from 5.0 to 6.1 years for men with and without IBD, depending on the year.
Differences in health-adjusted life expectancy ranged from 9.5 to 13.5 years in women with and without IBD, and from 2.6 years to 6.7 years in men with and without IBD depending on the year, the researchers said.
In addition, the researchers used the pain-attributed utility of the Health Utility Index (HUI) to calculate health-adjusted life expectancy with the effect of pain on health status. The health-adjusted life expectancy using HUI for pain was stable in women with IBD, but increased over time for women without IBD. This pattern was similar for individuals with Crohn’s disease but stable for women with and without ulcerative colitis. In men, health-adjusted life expectancy using the pain-attributed utility of the HUI was stable for those with IBD, decreased in those with Crohn’s disease, and increased among those with ulcerative colitis.
The study findings were limited by several factors including potential confounding by disease phenotype and severity, as well as lack of data on medication use for individuals younger than 65 years and limited data on confounders including smoking, ethnicity, and environmental factors, the researchers said.
In addition, “mortality may increase with disease duration, which would violate the assumption that age- and sex-specific mortality rates remain constant over a person’s lifetime that is required when using lifetables,” they said.
Future research should pursue effect of pain
The results support the persistence of a gap in life expectancy between individuals with and without IBD and suggest the need for improving pain management in IBD patients, given the contribution of pain to reduced health-adjusted life expectancy, they concluded. Additional research is needed to determine the effect of comorbid conditions and medication use on the difference in life expectancy for those with and without IBD, they added.
The study was supported by the Institute for Clinical Evaluative Services (Canada). Lead author Dr. Kuenzig received a Post-Doctoral Fellowship Award from the Canadian Institutes of Health Research, Canadian Association of Gastroenterology, and Crohn’s and Colitis Canada. The researchers had no financial conflicts to disclose.
SOURCE: Kuenzig ME et al. CMAJ. 2020 Nov 9. doi: 10.1503/cmaj.190976.
Life expectancy increased for adults with inflammatory bowel disease (IBD) over recent decades, but still remained lower than life expectancy for individuals without IBD, according to data from a retrospective cohort study using Canadian health databases.
“Management of IBD has improved through increased access to specialist care, biologic therapies, and a treat-to-target approach,” wrote M. Ellen Kuenzig, PhD, of the Children’s Hospital of Eastern Ontario and colleagues. However, “Most studies evaluating mortality were conducted before the biologic era, and none evaluated life expectancy or health-adjusted life expectancy,” they said.
In a study published in the Canadian Medical Association Journal, the researchers used Canadian databases to identify a study population of 32,818 people with IBD matched to 163,284 people without IBD in 1996 that increased to 83,672 people with IBD matched to 418,360 people without IBD in 2011.
Life expectancy increases, but with caveats
Overall, life expectancy for IBD patients increased from 75.5 years to 78.4 years for women and from 72.2 years to 75.5 years for men between 1996 and 2011.
However, health-adjusted life expectancy, defined as the number of years a person is expected to live in full health, decreased by 3.9 years for men with IBD between 1996 and 2008, but did not change significantly for women with IBD, although the gap in health-adjusted life expectancy increased between women with IBD and women without IBD.
Both life expectancy and health-adjusted life expectancy remained consistently lower for individuals with IBD compared to those without IBD. Differences in life expectancy for women with and without IBD ranged from 6.6 to 8.1 years and from 5.0 to 6.1 years for men with and without IBD, depending on the year.
Differences in health-adjusted life expectancy ranged from 9.5 to 13.5 years in women with and without IBD, and from 2.6 years to 6.7 years in men with and without IBD depending on the year, the researchers said.
In addition, the researchers used the pain-attributed utility of the Health Utility Index (HUI) to calculate health-adjusted life expectancy with the effect of pain on health status. The health-adjusted life expectancy using HUI for pain was stable in women with IBD, but increased over time for women without IBD. This pattern was similar for individuals with Crohn’s disease but stable for women with and without ulcerative colitis. In men, health-adjusted life expectancy using the pain-attributed utility of the HUI was stable for those with IBD, decreased in those with Crohn’s disease, and increased among those with ulcerative colitis.
The study findings were limited by several factors including potential confounding by disease phenotype and severity, as well as lack of data on medication use for individuals younger than 65 years and limited data on confounders including smoking, ethnicity, and environmental factors, the researchers said.
In addition, “mortality may increase with disease duration, which would violate the assumption that age- and sex-specific mortality rates remain constant over a person’s lifetime that is required when using lifetables,” they said.
Future research should pursue effect of pain
The results support the persistence of a gap in life expectancy between individuals with and without IBD and suggest the need for improving pain management in IBD patients, given the contribution of pain to reduced health-adjusted life expectancy, they concluded. Additional research is needed to determine the effect of comorbid conditions and medication use on the difference in life expectancy for those with and without IBD, they added.
The study was supported by the Institute for Clinical Evaluative Services (Canada). Lead author Dr. Kuenzig received a Post-Doctoral Fellowship Award from the Canadian Institutes of Health Research, Canadian Association of Gastroenterology, and Crohn’s and Colitis Canada. The researchers had no financial conflicts to disclose.
SOURCE: Kuenzig ME et al. CMAJ. 2020 Nov 9. doi: 10.1503/cmaj.190976.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Study explores reasons for link between gastroparesis symptoms, constipation
Severe constipation affected 34% of adults with gastroparesis symptoms and showed a significant positive correlation with symptom severity in a multicenter prospective study.
Henry P. Parkman, MD, of Temple University in Philadelphia and his associates used a modified GI symptoms questionnaire, gastric-emptying scintigraphy, and wireless motility capsule studies of 338 participants in the National Institutes of Health Gastroparesis Registry, which enrolls individuals with gastroparesis symptoms (whether or not they have delayed gastric emptying). In the multivariable analysis, severe constipation (a score of 4 or 5 on a 5-point scale) correlated significantly with a higher score on the Gastroparesis Cardinal Symptoms Index (GCSI), with an odds ratio of 1.85 (95% confidence interval, 1.30-2.67). In addition, patients with gastroparesis symptoms were significantly more likely to report pain in the lower abdomen (OR, 1.34; 95% CI, 1.06-1.69) and to use medications to manage constipation (OR, 5.09; 95% CI, 2.75-9.41). The findings were published online in Clinical Gastroenterology and Hepatology.
Constipation was not significantly linked with the use of individual drug classes, including opiates, tricyclic antidepressants, 5HT3 receptor antagonists, or cannabinoids. However, many patients were taking combinations of medications, and it is unclear if these induced constipation or if patients had primary disorders, such as abnormal colonic motility or anorectal dysfunction, said Adil E. Bharucha, MBBS, MD, a professor of medicine in the gastroenterology and hepatology division and a medical director in the office of clinical trials at Mayo Clinic, Rochester, Minn., who was not involved in the study. For patients with gastroparesis and constipation, clinicians should consider withdrawing constipating medications, performing anorectal testing, and referring patients for pelvic floor biofeedback therapy if anorectal tests are positive, he said while acknowledging the need for more data on these approaches. For patients without evidence of anorectal disorders, he recommended “simple laxatives or, if necessary, prescription medications, some of which may also benefit upper gastrointestinal symptoms.”
In this study, constipation also did not correlate with gastric emptying, which suggests that “motility disturbances in the foregut are separable from those in the hindgut,” said David Levinthal, MD, PhD, director of the neurogastroenterology and motility center at the University of Pittsburgh Medical Center, who also was not involved in the work. Constipation was only marginally linked with colonic transit time (OR, 1.04; 95% CI, 1.00-1.07), and delayed gastric emptying did not predict the severity of dyspepsia, he noted. “These observations highlight that sensory mechanisms are very important factors that are not interrogated by physiological motility tests, but that nonetheless may have an outsized impact on how patients feel.”
Despite “fairly good phenotyping of patients [based on] physiological measures, medication use, and detailed symptom questionnaires,” the study’s method of grouping patients based on continuous variables could mask relevant clinical nuances, Dr. Levinthal said. He emphasized that individual physiological tests do not reliably predict the presence or severity of GI symptoms: “What would you make of a 50-hour colonic transit time [CTT]? Or a 60-hour CTT? One could have either no constipation or severe constipation with those values. In clinical practice, it is less certain how useful it is to know a specific CTT result [when] formulating a treatment plan.”
Therefore, future studies of patients with gastroparesis and constipation should forgo grouping patients based on GI motor patterns and instead validate patient-reported symptom measures by using novel sensory tests with stimuli such as eating, drinking, and balloon distension, Dr. Levinthal said. He also recommended studying cognitive and emotional functioning in this patients, given that conditions such as depression and anxiety are known to affect GI sensation.
The National Institute of Diabetes and Digestive and Kidney Diseases provided funding. The investigators reported having no conflicts of interest. Dr. Bharucha reported having filed patents for anorectal devices jointly with Minnesota Medical Technologies, Medspira, and Medtronic and receiving royalties from Medspira. Dr. Levinthal reported having served on advisory boards for Takeda Pharmaceuticals and Alexza Pharmaceuticals.
SOURCE: Parkman HP et al. Clin Gastroenterol Hepatol. 2020 Oct 28. doi: 10.1016/j.cgh.2020.10.045.
Severe constipation affected 34% of adults with gastroparesis symptoms and showed a significant positive correlation with symptom severity in a multicenter prospective study.
Henry P. Parkman, MD, of Temple University in Philadelphia and his associates used a modified GI symptoms questionnaire, gastric-emptying scintigraphy, and wireless motility capsule studies of 338 participants in the National Institutes of Health Gastroparesis Registry, which enrolls individuals with gastroparesis symptoms (whether or not they have delayed gastric emptying). In the multivariable analysis, severe constipation (a score of 4 or 5 on a 5-point scale) correlated significantly with a higher score on the Gastroparesis Cardinal Symptoms Index (GCSI), with an odds ratio of 1.85 (95% confidence interval, 1.30-2.67). In addition, patients with gastroparesis symptoms were significantly more likely to report pain in the lower abdomen (OR, 1.34; 95% CI, 1.06-1.69) and to use medications to manage constipation (OR, 5.09; 95% CI, 2.75-9.41). The findings were published online in Clinical Gastroenterology and Hepatology.
Constipation was not significantly linked with the use of individual drug classes, including opiates, tricyclic antidepressants, 5HT3 receptor antagonists, or cannabinoids. However, many patients were taking combinations of medications, and it is unclear if these induced constipation or if patients had primary disorders, such as abnormal colonic motility or anorectal dysfunction, said Adil E. Bharucha, MBBS, MD, a professor of medicine in the gastroenterology and hepatology division and a medical director in the office of clinical trials at Mayo Clinic, Rochester, Minn., who was not involved in the study. For patients with gastroparesis and constipation, clinicians should consider withdrawing constipating medications, performing anorectal testing, and referring patients for pelvic floor biofeedback therapy if anorectal tests are positive, he said while acknowledging the need for more data on these approaches. For patients without evidence of anorectal disorders, he recommended “simple laxatives or, if necessary, prescription medications, some of which may also benefit upper gastrointestinal symptoms.”
In this study, constipation also did not correlate with gastric emptying, which suggests that “motility disturbances in the foregut are separable from those in the hindgut,” said David Levinthal, MD, PhD, director of the neurogastroenterology and motility center at the University of Pittsburgh Medical Center, who also was not involved in the work. Constipation was only marginally linked with colonic transit time (OR, 1.04; 95% CI, 1.00-1.07), and delayed gastric emptying did not predict the severity of dyspepsia, he noted. “These observations highlight that sensory mechanisms are very important factors that are not interrogated by physiological motility tests, but that nonetheless may have an outsized impact on how patients feel.”
Despite “fairly good phenotyping of patients [based on] physiological measures, medication use, and detailed symptom questionnaires,” the study’s method of grouping patients based on continuous variables could mask relevant clinical nuances, Dr. Levinthal said. He emphasized that individual physiological tests do not reliably predict the presence or severity of GI symptoms: “What would you make of a 50-hour colonic transit time [CTT]? Or a 60-hour CTT? One could have either no constipation or severe constipation with those values. In clinical practice, it is less certain how useful it is to know a specific CTT result [when] formulating a treatment plan.”
Therefore, future studies of patients with gastroparesis and constipation should forgo grouping patients based on GI motor patterns and instead validate patient-reported symptom measures by using novel sensory tests with stimuli such as eating, drinking, and balloon distension, Dr. Levinthal said. He also recommended studying cognitive and emotional functioning in this patients, given that conditions such as depression and anxiety are known to affect GI sensation.
The National Institute of Diabetes and Digestive and Kidney Diseases provided funding. The investigators reported having no conflicts of interest. Dr. Bharucha reported having filed patents for anorectal devices jointly with Minnesota Medical Technologies, Medspira, and Medtronic and receiving royalties from Medspira. Dr. Levinthal reported having served on advisory boards for Takeda Pharmaceuticals and Alexza Pharmaceuticals.
SOURCE: Parkman HP et al. Clin Gastroenterol Hepatol. 2020 Oct 28. doi: 10.1016/j.cgh.2020.10.045.
Severe constipation affected 34% of adults with gastroparesis symptoms and showed a significant positive correlation with symptom severity in a multicenter prospective study.
Henry P. Parkman, MD, of Temple University in Philadelphia and his associates used a modified GI symptoms questionnaire, gastric-emptying scintigraphy, and wireless motility capsule studies of 338 participants in the National Institutes of Health Gastroparesis Registry, which enrolls individuals with gastroparesis symptoms (whether or not they have delayed gastric emptying). In the multivariable analysis, severe constipation (a score of 4 or 5 on a 5-point scale) correlated significantly with a higher score on the Gastroparesis Cardinal Symptoms Index (GCSI), with an odds ratio of 1.85 (95% confidence interval, 1.30-2.67). In addition, patients with gastroparesis symptoms were significantly more likely to report pain in the lower abdomen (OR, 1.34; 95% CI, 1.06-1.69) and to use medications to manage constipation (OR, 5.09; 95% CI, 2.75-9.41). The findings were published online in Clinical Gastroenterology and Hepatology.
Constipation was not significantly linked with the use of individual drug classes, including opiates, tricyclic antidepressants, 5HT3 receptor antagonists, or cannabinoids. However, many patients were taking combinations of medications, and it is unclear if these induced constipation or if patients had primary disorders, such as abnormal colonic motility or anorectal dysfunction, said Adil E. Bharucha, MBBS, MD, a professor of medicine in the gastroenterology and hepatology division and a medical director in the office of clinical trials at Mayo Clinic, Rochester, Minn., who was not involved in the study. For patients with gastroparesis and constipation, clinicians should consider withdrawing constipating medications, performing anorectal testing, and referring patients for pelvic floor biofeedback therapy if anorectal tests are positive, he said while acknowledging the need for more data on these approaches. For patients without evidence of anorectal disorders, he recommended “simple laxatives or, if necessary, prescription medications, some of which may also benefit upper gastrointestinal symptoms.”
In this study, constipation also did not correlate with gastric emptying, which suggests that “motility disturbances in the foregut are separable from those in the hindgut,” said David Levinthal, MD, PhD, director of the neurogastroenterology and motility center at the University of Pittsburgh Medical Center, who also was not involved in the work. Constipation was only marginally linked with colonic transit time (OR, 1.04; 95% CI, 1.00-1.07), and delayed gastric emptying did not predict the severity of dyspepsia, he noted. “These observations highlight that sensory mechanisms are very important factors that are not interrogated by physiological motility tests, but that nonetheless may have an outsized impact on how patients feel.”
Despite “fairly good phenotyping of patients [based on] physiological measures, medication use, and detailed symptom questionnaires,” the study’s method of grouping patients based on continuous variables could mask relevant clinical nuances, Dr. Levinthal said. He emphasized that individual physiological tests do not reliably predict the presence or severity of GI symptoms: “What would you make of a 50-hour colonic transit time [CTT]? Or a 60-hour CTT? One could have either no constipation or severe constipation with those values. In clinical practice, it is less certain how useful it is to know a specific CTT result [when] formulating a treatment plan.”
Therefore, future studies of patients with gastroparesis and constipation should forgo grouping patients based on GI motor patterns and instead validate patient-reported symptom measures by using novel sensory tests with stimuli such as eating, drinking, and balloon distension, Dr. Levinthal said. He also recommended studying cognitive and emotional functioning in this patients, given that conditions such as depression and anxiety are known to affect GI sensation.
The National Institute of Diabetes and Digestive and Kidney Diseases provided funding. The investigators reported having no conflicts of interest. Dr. Bharucha reported having filed patents for anorectal devices jointly with Minnesota Medical Technologies, Medspira, and Medtronic and receiving royalties from Medspira. Dr. Levinthal reported having served on advisory boards for Takeda Pharmaceuticals and Alexza Pharmaceuticals.
SOURCE: Parkman HP et al. Clin Gastroenterol Hepatol. 2020 Oct 28. doi: 10.1016/j.cgh.2020.10.045.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY