User login
Good or bad, immune responses to cancer are similar

Credit: Aaron Logan
SAN DIEGO—Researchers have found evidence to suggest there may be little difference between an immune response that kills cancer cells and one that stimulates tumor growth.
The team set out to determine whether the immune responses that mediate cancer immunosurveillance and those responsible for inflammatory facilitation are qualitatively or quantitatively distinct.
They tested antibodies in mouse models of a few different cancers, including rituximab in Burkitt lymphoma.
And they found that lower antibody concentrations stimulated tumor growth, while higher concentrations inhibited growth, and the dose range was “surprisingly narrow.”
The researchers reported these findings in a paper published in PNAS and a poster presentation at the AACR Annual Meeting 2014 (abstract 1063).
“We have found that the intensity difference between an immune response that stimulates cancer and one that kills it may not be very much,” said principal investigator Ajit Varki, MD, of the University of California, San Diego School of Medicine.
“This may come as a surprise to researchers exploring two areas typically considered distinct: the role of the immune system in preventing and killing cancers and the role of chronic inflammation in stimulating cancers. As always, it turns out that the immune system is a double-edged sword.”
The concept of naturally occurring immunosurveillance against malignancies is not new, and there is compelling evidence for it. But understanding this process is confounded by the fact that some types of immune reaction promote tumor development.
Dr Varki and his colleagues looked specifically at a non-human sialic acid sugar molecule called Neu5Gc. Previous research showed that Neu5Gc accumulates in human tumors from dietary sources, despite an ongoing antibody response against it.
The researchers deployed antibodies against Neu5Gc in a mouse tumor model to determine whether and to what degree the antibodies altered tumor progression. The team found that low antibody doses stimulated growth, but high doses inhibited it.
The effect occurred over a “linear and remarkably narrow range,” according to Dr Varki, generating an immune response curve or “inverse hormesis.” Moreover, this curve could be shifted to the left or right simply by modifying the quality of the immune response.
The researchers uncovered similar findings in experiments with mouse models of colon and lung cancer, as well as when they used rituximab in a model of Burkitt lymphoma.
Dr Varki said these results could have implications for all aspects of cancer research. The immune response can have multiple roles in the genesis of cancers, in altering the progress of established tumors and in anticancer therapies that use antibodies as drugs.
Dr Varki is a co-founder of the company Sialix, Inc., which has licensed UC San Diego technologies related to anti-Neu5Gc antibodies in cancer. ![]()

Credit: Aaron Logan
SAN DIEGO—Researchers have found evidence to suggest there may be little difference between an immune response that kills cancer cells and one that stimulates tumor growth.
The team set out to determine whether the immune responses that mediate cancer immunosurveillance and those responsible for inflammatory facilitation are qualitatively or quantitatively distinct.
They tested antibodies in mouse models of a few different cancers, including rituximab in Burkitt lymphoma.
And they found that lower antibody concentrations stimulated tumor growth, while higher concentrations inhibited growth, and the dose range was “surprisingly narrow.”
The researchers reported these findings in a paper published in PNAS and a poster presentation at the AACR Annual Meeting 2014 (abstract 1063).
“We have found that the intensity difference between an immune response that stimulates cancer and one that kills it may not be very much,” said principal investigator Ajit Varki, MD, of the University of California, San Diego School of Medicine.
“This may come as a surprise to researchers exploring two areas typically considered distinct: the role of the immune system in preventing and killing cancers and the role of chronic inflammation in stimulating cancers. As always, it turns out that the immune system is a double-edged sword.”
The concept of naturally occurring immunosurveillance against malignancies is not new, and there is compelling evidence for it. But understanding this process is confounded by the fact that some types of immune reaction promote tumor development.
Dr Varki and his colleagues looked specifically at a non-human sialic acid sugar molecule called Neu5Gc. Previous research showed that Neu5Gc accumulates in human tumors from dietary sources, despite an ongoing antibody response against it.
The researchers deployed antibodies against Neu5Gc in a mouse tumor model to determine whether and to what degree the antibodies altered tumor progression. The team found that low antibody doses stimulated growth, but high doses inhibited it.
The effect occurred over a “linear and remarkably narrow range,” according to Dr Varki, generating an immune response curve or “inverse hormesis.” Moreover, this curve could be shifted to the left or right simply by modifying the quality of the immune response.
The researchers uncovered similar findings in experiments with mouse models of colon and lung cancer, as well as when they used rituximab in a model of Burkitt lymphoma.
Dr Varki said these results could have implications for all aspects of cancer research. The immune response can have multiple roles in the genesis of cancers, in altering the progress of established tumors and in anticancer therapies that use antibodies as drugs.
Dr Varki is a co-founder of the company Sialix, Inc., which has licensed UC San Diego technologies related to anti-Neu5Gc antibodies in cancer. ![]()

Credit: Aaron Logan
SAN DIEGO—Researchers have found evidence to suggest there may be little difference between an immune response that kills cancer cells and one that stimulates tumor growth.
The team set out to determine whether the immune responses that mediate cancer immunosurveillance and those responsible for inflammatory facilitation are qualitatively or quantitatively distinct.
They tested antibodies in mouse models of a few different cancers, including rituximab in Burkitt lymphoma.
And they found that lower antibody concentrations stimulated tumor growth, while higher concentrations inhibited growth, and the dose range was “surprisingly narrow.”
The researchers reported these findings in a paper published in PNAS and a poster presentation at the AACR Annual Meeting 2014 (abstract 1063).
“We have found that the intensity difference between an immune response that stimulates cancer and one that kills it may not be very much,” said principal investigator Ajit Varki, MD, of the University of California, San Diego School of Medicine.
“This may come as a surprise to researchers exploring two areas typically considered distinct: the role of the immune system in preventing and killing cancers and the role of chronic inflammation in stimulating cancers. As always, it turns out that the immune system is a double-edged sword.”
The concept of naturally occurring immunosurveillance against malignancies is not new, and there is compelling evidence for it. But understanding this process is confounded by the fact that some types of immune reaction promote tumor development.
Dr Varki and his colleagues looked specifically at a non-human sialic acid sugar molecule called Neu5Gc. Previous research showed that Neu5Gc accumulates in human tumors from dietary sources, despite an ongoing antibody response against it.
The researchers deployed antibodies against Neu5Gc in a mouse tumor model to determine whether and to what degree the antibodies altered tumor progression. The team found that low antibody doses stimulated growth, but high doses inhibited it.
The effect occurred over a “linear and remarkably narrow range,” according to Dr Varki, generating an immune response curve or “inverse hormesis.” Moreover, this curve could be shifted to the left or right simply by modifying the quality of the immune response.
The researchers uncovered similar findings in experiments with mouse models of colon and lung cancer, as well as when they used rituximab in a model of Burkitt lymphoma.
Dr Varki said these results could have implications for all aspects of cancer research. The immune response can have multiple roles in the genesis of cancers, in altering the progress of established tumors and in anticancer therapies that use antibodies as drugs.
Dr Varki is a co-founder of the company Sialix, Inc., which has licensed UC San Diego technologies related to anti-Neu5Gc antibodies in cancer. ![]()
How autophagy helps cancer cells evade death

Credit: Sarah Pfau
SAN DIEGO—New research suggests that autophagy may allow cancer cells to recover and divide, rather than die, when faced with chemotherapy.
“What we showed is that if this mechanism doesn’t work right—for example, if autophagy is too high or if the target regulated by autophagy isn’t around—cancer cells may be able to rescue themselves from death caused by chemotherapies,” said study author Andrew Thorburn, PhD, of the University of Colorado Denver.
He and his colleagues believe this finding has important implications. It demonstrates a mechanism whereby autophagy controls cell death, and it further reinforces the clinical potential of inhibiting autophagy to sensitize cancer cells to chemotherapy.
Dr Thorburn and his colleagues recounted their research in Cell Reports and presented it in an education session at the AACR Annual Meeting 2014.
The researchers had set out to examine how autophagy affects canonical death receptor-induced mitochondrial outer membrane permeabilization (MOMP) and apoptosis. They found that MOMP occurs at variable times in cells, and it’s delayed by autophagy.
Furthermore, autophagy leads to inefficient MOMP. This causes some cells to die via a slower process than typical apoptosis, which allows them to eventually recover and divide.
Specifically, the researchers found that, as a cancer cell begins to die, mitochondrial cell walls break down. And the cell’s mitochondria release proteins via MOMP.
But then, high autophagy allows the cell to encapsulate and “digest” these released proteins before MOMP can keep the cell well and truly dead. The cell recovers and goes on to divide.
“The implication here is that if you inhibit autophagy, you’d make this less likely to happen; ie, when you kill cancer cells, they would stay dead,” Dr Thorburn said.
He and his colleagues also found that autophagy depends on the target PUMA to regulate cell death. When PUMA is absent, it doesn’t matter if autophagy is inhibited. Without the communicating action of PUMA, cancer cells evade apoptosis and continue to survive.
The researchers said this suggests autophagy can control apoptosis via a regulator that makes MOMP faster and more efficient, thus ensuring the rapid completion of apoptosis.
“Autophagy is complex and, as yet, not fully understood,” Dr Thorburn said. “But now that we see a molecular mechanism whereby cell fate can be determined by autophagy, we hope to discover patient populations that could benefit from drugs that inhibit this action.” ![]()

Credit: Sarah Pfau
SAN DIEGO—New research suggests that autophagy may allow cancer cells to recover and divide, rather than die, when faced with chemotherapy.
“What we showed is that if this mechanism doesn’t work right—for example, if autophagy is too high or if the target regulated by autophagy isn’t around—cancer cells may be able to rescue themselves from death caused by chemotherapies,” said study author Andrew Thorburn, PhD, of the University of Colorado Denver.
He and his colleagues believe this finding has important implications. It demonstrates a mechanism whereby autophagy controls cell death, and it further reinforces the clinical potential of inhibiting autophagy to sensitize cancer cells to chemotherapy.
Dr Thorburn and his colleagues recounted their research in Cell Reports and presented it in an education session at the AACR Annual Meeting 2014.
The researchers had set out to examine how autophagy affects canonical death receptor-induced mitochondrial outer membrane permeabilization (MOMP) and apoptosis. They found that MOMP occurs at variable times in cells, and it’s delayed by autophagy.
Furthermore, autophagy leads to inefficient MOMP. This causes some cells to die via a slower process than typical apoptosis, which allows them to eventually recover and divide.
Specifically, the researchers found that, as a cancer cell begins to die, mitochondrial cell walls break down. And the cell’s mitochondria release proteins via MOMP.
But then, high autophagy allows the cell to encapsulate and “digest” these released proteins before MOMP can keep the cell well and truly dead. The cell recovers and goes on to divide.
“The implication here is that if you inhibit autophagy, you’d make this less likely to happen; ie, when you kill cancer cells, they would stay dead,” Dr Thorburn said.
He and his colleagues also found that autophagy depends on the target PUMA to regulate cell death. When PUMA is absent, it doesn’t matter if autophagy is inhibited. Without the communicating action of PUMA, cancer cells evade apoptosis and continue to survive.
The researchers said this suggests autophagy can control apoptosis via a regulator that makes MOMP faster and more efficient, thus ensuring the rapid completion of apoptosis.
“Autophagy is complex and, as yet, not fully understood,” Dr Thorburn said. “But now that we see a molecular mechanism whereby cell fate can be determined by autophagy, we hope to discover patient populations that could benefit from drugs that inhibit this action.” ![]()

Credit: Sarah Pfau
SAN DIEGO—New research suggests that autophagy may allow cancer cells to recover and divide, rather than die, when faced with chemotherapy.
“What we showed is that if this mechanism doesn’t work right—for example, if autophagy is too high or if the target regulated by autophagy isn’t around—cancer cells may be able to rescue themselves from death caused by chemotherapies,” said study author Andrew Thorburn, PhD, of the University of Colorado Denver.
He and his colleagues believe this finding has important implications. It demonstrates a mechanism whereby autophagy controls cell death, and it further reinforces the clinical potential of inhibiting autophagy to sensitize cancer cells to chemotherapy.
Dr Thorburn and his colleagues recounted their research in Cell Reports and presented it in an education session at the AACR Annual Meeting 2014.
The researchers had set out to examine how autophagy affects canonical death receptor-induced mitochondrial outer membrane permeabilization (MOMP) and apoptosis. They found that MOMP occurs at variable times in cells, and it’s delayed by autophagy.
Furthermore, autophagy leads to inefficient MOMP. This causes some cells to die via a slower process than typical apoptosis, which allows them to eventually recover and divide.
Specifically, the researchers found that, as a cancer cell begins to die, mitochondrial cell walls break down. And the cell’s mitochondria release proteins via MOMP.
But then, high autophagy allows the cell to encapsulate and “digest” these released proteins before MOMP can keep the cell well and truly dead. The cell recovers and goes on to divide.
“The implication here is that if you inhibit autophagy, you’d make this less likely to happen; ie, when you kill cancer cells, they would stay dead,” Dr Thorburn said.
He and his colleagues also found that autophagy depends on the target PUMA to regulate cell death. When PUMA is absent, it doesn’t matter if autophagy is inhibited. Without the communicating action of PUMA, cancer cells evade apoptosis and continue to survive.
The researchers said this suggests autophagy can control apoptosis via a regulator that makes MOMP faster and more efficient, thus ensuring the rapid completion of apoptosis.
“Autophagy is complex and, as yet, not fully understood,” Dr Thorburn said. “But now that we see a molecular mechanism whereby cell fate can be determined by autophagy, we hope to discover patient populations that could benefit from drugs that inhibit this action.” ![]()
Nanocapsules prevent release of nontargeted radiation

Credit: Rhoda Baer
A novel type of nanocapsule can safely and effectively store isotopes that emit ionizing radiation, according to a paper published in Biochimica et Biophysica Acta.
In experiments, the nanocapsules were taken up by cells, accumulated near the perinuclear region, and persisted without degrading.
They also prevented nontargeted, radioactive daughter ions from escaping. These ions could cause significant damage if released, such as prompting the development of leukemia.
This research suggests the nanocapsules have the potential to advance radiation therapy, according to study author John M. Tomich, PhD, of Kansas State University’s Johnson Cancer Research Center.
Dr Tomich and his colleagues created the nanocapsules, called branched amphiphilic peptide capsules (BAPCs), by combining 2 related sequences of amino acids—bis(FLIVI)-K-KKKK and bis(FLIVIGSII)-K-KKKK.
“We found that the 2 sequences come together to form a thin membrane that assembled into little spheres, which we call capsules,” Dr Tomich said. “While other vesicles have been created from lipids, most are much less stable and break down. Ours are like stones, though. They’re incredibly stable and are not destroyed by cells in the body.”
The capsules’ ability to stay intact with the isotope inside and remain undetected by the body’s clearance systems prompted Dr Tomich to investigate using BAPCs for radiation therapies.
“The problem with current alpha-particle radiation therapies used to treat cancer is that they lead to the release of nontargeted, radioactive daughter ions into the body,” Dr Tomich said. “Radioactive atoms break down to form new atoms, called daughter ions, with the release of some form of energy or energetic particles. Alpha emitters give off an energetic particle that comes off at nearly the speed of light.”
The alpha particle destroys DNA and whatever vital cellular components are in its path. Similarly, the daughter ions recoil with high energy on ejection of the alpha particle. The daughter ions have enough energy to escape the targeting and containment molecules that are currently in use.
“Once freed, the daughter isotopes can end up in places you don’t want them, like bone marrow, which can then lead to leukemia and new challenges,” Dr Tomich said.
To see if the BAPCs could prevent the release of daughter isotopes, the researchers loaded the nanoparticles with 225Actinium. Upon decay, this compound releases 4 alpha particles and numerous daughter ions.
The team found that BAPCs loaded with the compound readily entered cells and migrated to a position alongside the nucleus.
As the alpha-particle-emitting isotopes decayed, the recoiled daughter ions collided with the capsule walls, essentially bouncing off them, and remained trapped inside the BAPCs. This completely blocked the release of the daughter ions, which prevented uptake in nontarget tissues.
Dr Tomich said more studies are needed to add target molecules to the surface of the BAPCs. But he believes the particles could provide a safer option for treating tumors with radiation therapy by reducing the amount of radioisotope required for killing cancer cells and reducing the side effects caused by off-target accumulation of radioisotopes.
“These capsules are easy to make and easy to work with,” Dr Tomich said. “I think we’re just scratching the surface of what we can do with them to improve human health and nanomaterials.” ![]()

Credit: Rhoda Baer
A novel type of nanocapsule can safely and effectively store isotopes that emit ionizing radiation, according to a paper published in Biochimica et Biophysica Acta.
In experiments, the nanocapsules were taken up by cells, accumulated near the perinuclear region, and persisted without degrading.
They also prevented nontargeted, radioactive daughter ions from escaping. These ions could cause significant damage if released, such as prompting the development of leukemia.
This research suggests the nanocapsules have the potential to advance radiation therapy, according to study author John M. Tomich, PhD, of Kansas State University’s Johnson Cancer Research Center.
Dr Tomich and his colleagues created the nanocapsules, called branched amphiphilic peptide capsules (BAPCs), by combining 2 related sequences of amino acids—bis(FLIVI)-K-KKKK and bis(FLIVIGSII)-K-KKKK.
“We found that the 2 sequences come together to form a thin membrane that assembled into little spheres, which we call capsules,” Dr Tomich said. “While other vesicles have been created from lipids, most are much less stable and break down. Ours are like stones, though. They’re incredibly stable and are not destroyed by cells in the body.”
The capsules’ ability to stay intact with the isotope inside and remain undetected by the body’s clearance systems prompted Dr Tomich to investigate using BAPCs for radiation therapies.
“The problem with current alpha-particle radiation therapies used to treat cancer is that they lead to the release of nontargeted, radioactive daughter ions into the body,” Dr Tomich said. “Radioactive atoms break down to form new atoms, called daughter ions, with the release of some form of energy or energetic particles. Alpha emitters give off an energetic particle that comes off at nearly the speed of light.”
The alpha particle destroys DNA and whatever vital cellular components are in its path. Similarly, the daughter ions recoil with high energy on ejection of the alpha particle. The daughter ions have enough energy to escape the targeting and containment molecules that are currently in use.
“Once freed, the daughter isotopes can end up in places you don’t want them, like bone marrow, which can then lead to leukemia and new challenges,” Dr Tomich said.
To see if the BAPCs could prevent the release of daughter isotopes, the researchers loaded the nanoparticles with 225Actinium. Upon decay, this compound releases 4 alpha particles and numerous daughter ions.
The team found that BAPCs loaded with the compound readily entered cells and migrated to a position alongside the nucleus.
As the alpha-particle-emitting isotopes decayed, the recoiled daughter ions collided with the capsule walls, essentially bouncing off them, and remained trapped inside the BAPCs. This completely blocked the release of the daughter ions, which prevented uptake in nontarget tissues.
Dr Tomich said more studies are needed to add target molecules to the surface of the BAPCs. But he believes the particles could provide a safer option for treating tumors with radiation therapy by reducing the amount of radioisotope required for killing cancer cells and reducing the side effects caused by off-target accumulation of radioisotopes.
“These capsules are easy to make and easy to work with,” Dr Tomich said. “I think we’re just scratching the surface of what we can do with them to improve human health and nanomaterials.” ![]()

Credit: Rhoda Baer
A novel type of nanocapsule can safely and effectively store isotopes that emit ionizing radiation, according to a paper published in Biochimica et Biophysica Acta.
In experiments, the nanocapsules were taken up by cells, accumulated near the perinuclear region, and persisted without degrading.
They also prevented nontargeted, radioactive daughter ions from escaping. These ions could cause significant damage if released, such as prompting the development of leukemia.
This research suggests the nanocapsules have the potential to advance radiation therapy, according to study author John M. Tomich, PhD, of Kansas State University’s Johnson Cancer Research Center.
Dr Tomich and his colleagues created the nanocapsules, called branched amphiphilic peptide capsules (BAPCs), by combining 2 related sequences of amino acids—bis(FLIVI)-K-KKKK and bis(FLIVIGSII)-K-KKKK.
“We found that the 2 sequences come together to form a thin membrane that assembled into little spheres, which we call capsules,” Dr Tomich said. “While other vesicles have been created from lipids, most are much less stable and break down. Ours are like stones, though. They’re incredibly stable and are not destroyed by cells in the body.”
The capsules’ ability to stay intact with the isotope inside and remain undetected by the body’s clearance systems prompted Dr Tomich to investigate using BAPCs for radiation therapies.
“The problem with current alpha-particle radiation therapies used to treat cancer is that they lead to the release of nontargeted, radioactive daughter ions into the body,” Dr Tomich said. “Radioactive atoms break down to form new atoms, called daughter ions, with the release of some form of energy or energetic particles. Alpha emitters give off an energetic particle that comes off at nearly the speed of light.”
The alpha particle destroys DNA and whatever vital cellular components are in its path. Similarly, the daughter ions recoil with high energy on ejection of the alpha particle. The daughter ions have enough energy to escape the targeting and containment molecules that are currently in use.
“Once freed, the daughter isotopes can end up in places you don’t want them, like bone marrow, which can then lead to leukemia and new challenges,” Dr Tomich said.
To see if the BAPCs could prevent the release of daughter isotopes, the researchers loaded the nanoparticles with 225Actinium. Upon decay, this compound releases 4 alpha particles and numerous daughter ions.
The team found that BAPCs loaded with the compound readily entered cells and migrated to a position alongside the nucleus.
As the alpha-particle-emitting isotopes decayed, the recoiled daughter ions collided with the capsule walls, essentially bouncing off them, and remained trapped inside the BAPCs. This completely blocked the release of the daughter ions, which prevented uptake in nontarget tissues.
Dr Tomich said more studies are needed to add target molecules to the surface of the BAPCs. But he believes the particles could provide a safer option for treating tumors with radiation therapy by reducing the amount of radioisotope required for killing cancer cells and reducing the side effects caused by off-target accumulation of radioisotopes.
“These capsules are easy to make and easy to work with,” Dr Tomich said. “I think we’re just scratching the surface of what we can do with them to improve human health and nanomaterials.” ![]()
How STAT3 blocks an antitumor mechanism

Credit: Ed Uthman
Researchers say they’ve discovered how the protein STAT3 interferes with an antitumor mechanism in cells, thereby promoting the growth of lymphoma and other cancers.
The group made their discovery using the Epstein-Barr virus (EBV) as a tool to investigate cancer development.
“Our findings add to the short list of known mechanisms by which a key cellular antitumor barrier is breached by STAT3 prior to cancer development,” said Sumita Bhaduri-McIntosh, MD, PhD, of Stony Brook University School of Medicine in New York.
“Because STAT3 interferes with this innate antitumor mechanism in cells, the opposite occurs when blood cells are infected in the lab with the cancer-causing virus EBV, and the cells continue to divide—a necessary step in cancer development.”
Dr Bhaduri-McIntosh and her colleagues described their research in PNAS.
The team explained that STAT3 inhibits a cancer-suppressing cellular activity called the DNA damage response (DDR). Normally, this response pauses cell division, allowing for the repair of damaged DNA.
But this study showed that EBV not only causes DNA damage when it infects and replicates in cells; it also activates and increases STAT3 expression. This starts a chain reaction that leads to an “un-pause” in cell division, thereby promoting cell proliferation. This, in combination with other pro-proliferative effects of the virus, can lead to cancers.
The researchers found that DDR does detect replication stress-associated DNA damage resulting from EBV infection. But signaling downstream of ATR proteins is impaired by STAT3. And this leads to relaxation of the intra-S phase checkpoint of the cell cycle.
STAT3 interrupts signaling from ATR to the protein Chk1 by promoting the loss of Claspin, a protein that assists ATR to phosphorylate Chk1. The loss of Claspin, which facilitates cell proliferation, is mediated by caspase 7.
Previous research suggested that STAT3 and Chk1 are potential targets for cancer therapies. Dr Bhaduri-McIntosh’s team said their results provide new insight into anticancer drug development because they reveal a mechanistic link between these 2 proteins.
Dr Bhaduri-McIntosh emphasized that, because STAT3 is involved in most cancers, her group’s findings could potentially impact the prevention or treatment of several types of cancer—something her lab is investigating. ![]()

Credit: Ed Uthman
Researchers say they’ve discovered how the protein STAT3 interferes with an antitumor mechanism in cells, thereby promoting the growth of lymphoma and other cancers.
The group made their discovery using the Epstein-Barr virus (EBV) as a tool to investigate cancer development.
“Our findings add to the short list of known mechanisms by which a key cellular antitumor barrier is breached by STAT3 prior to cancer development,” said Sumita Bhaduri-McIntosh, MD, PhD, of Stony Brook University School of Medicine in New York.
“Because STAT3 interferes with this innate antitumor mechanism in cells, the opposite occurs when blood cells are infected in the lab with the cancer-causing virus EBV, and the cells continue to divide—a necessary step in cancer development.”
Dr Bhaduri-McIntosh and her colleagues described their research in PNAS.
The team explained that STAT3 inhibits a cancer-suppressing cellular activity called the DNA damage response (DDR). Normally, this response pauses cell division, allowing for the repair of damaged DNA.
But this study showed that EBV not only causes DNA damage when it infects and replicates in cells; it also activates and increases STAT3 expression. This starts a chain reaction that leads to an “un-pause” in cell division, thereby promoting cell proliferation. This, in combination with other pro-proliferative effects of the virus, can lead to cancers.
The researchers found that DDR does detect replication stress-associated DNA damage resulting from EBV infection. But signaling downstream of ATR proteins is impaired by STAT3. And this leads to relaxation of the intra-S phase checkpoint of the cell cycle.
STAT3 interrupts signaling from ATR to the protein Chk1 by promoting the loss of Claspin, a protein that assists ATR to phosphorylate Chk1. The loss of Claspin, which facilitates cell proliferation, is mediated by caspase 7.
Previous research suggested that STAT3 and Chk1 are potential targets for cancer therapies. Dr Bhaduri-McIntosh’s team said their results provide new insight into anticancer drug development because they reveal a mechanistic link between these 2 proteins.
Dr Bhaduri-McIntosh emphasized that, because STAT3 is involved in most cancers, her group’s findings could potentially impact the prevention or treatment of several types of cancer—something her lab is investigating. ![]()

Credit: Ed Uthman
Researchers say they’ve discovered how the protein STAT3 interferes with an antitumor mechanism in cells, thereby promoting the growth of lymphoma and other cancers.
The group made their discovery using the Epstein-Barr virus (EBV) as a tool to investigate cancer development.
“Our findings add to the short list of known mechanisms by which a key cellular antitumor barrier is breached by STAT3 prior to cancer development,” said Sumita Bhaduri-McIntosh, MD, PhD, of Stony Brook University School of Medicine in New York.
“Because STAT3 interferes with this innate antitumor mechanism in cells, the opposite occurs when blood cells are infected in the lab with the cancer-causing virus EBV, and the cells continue to divide—a necessary step in cancer development.”
Dr Bhaduri-McIntosh and her colleagues described their research in PNAS.
The team explained that STAT3 inhibits a cancer-suppressing cellular activity called the DNA damage response (DDR). Normally, this response pauses cell division, allowing for the repair of damaged DNA.
But this study showed that EBV not only causes DNA damage when it infects and replicates in cells; it also activates and increases STAT3 expression. This starts a chain reaction that leads to an “un-pause” in cell division, thereby promoting cell proliferation. This, in combination with other pro-proliferative effects of the virus, can lead to cancers.
The researchers found that DDR does detect replication stress-associated DNA damage resulting from EBV infection. But signaling downstream of ATR proteins is impaired by STAT3. And this leads to relaxation of the intra-S phase checkpoint of the cell cycle.
STAT3 interrupts signaling from ATR to the protein Chk1 by promoting the loss of Claspin, a protein that assists ATR to phosphorylate Chk1. The loss of Claspin, which facilitates cell proliferation, is mediated by caspase 7.
Previous research suggested that STAT3 and Chk1 are potential targets for cancer therapies. Dr Bhaduri-McIntosh’s team said their results provide new insight into anticancer drug development because they reveal a mechanistic link between these 2 proteins.
Dr Bhaduri-McIntosh emphasized that, because STAT3 is involved in most cancers, her group’s findings could potentially impact the prevention or treatment of several types of cancer—something her lab is investigating. ![]()
EC approves SC formulation of rituximab

The European Commission (EC) has approved a subcutaneous (SC) formulation of rituximab (MabThera) to treat patients with follicular lymphoma or diffuse large B-cell lymphoma.
This formulation allows for 5-minute administration, a significant decrease over the 2.5-hour infusion time required to administer intravenous (IV) rituximab.
The drug’s maker, Roche, plans to begin launching SC rituximab in a number of European markets this year.
The EC’s approval of this formulation was primarily based on data from the SABRINA trial, which was recently published in The Lancet Oncology and funded by Roche.
In this phase 3 trial, researchers compared 3-week cycles of fixed-dose, SC rituximab to IV rituximab. They enrolled 127 patients with previously untreated, grade 1-3a, CD20-positive follicular lymphoma.
Patients were randomized to receive IV rituximab (375 mg/m2) or SC rituximab (1400 mg). After randomization, they received 1 induction dose of IV rituximab in cycle 1 and then their allocated treatment for cycles 2 through 8. Patients with a complete or partial response continued their treatment as maintenance every 8 weeks.
The study’s primary endpoint was the ratio of observed rituximab serum trough concentrations (Ctrough) between the 2 groups at cycle 7.
Pharmacokinetic data were available for 75% of patients (48/64) in the IV arm and 86% of the patients (54/63) in the SC arm.
An analysis of these data suggested SC rituximab was non-inferior to the IV formulation. The geometric mean Ctrough was 83.13 μg/mL in the IV arm and 134.58 μg/mL in the SC arm (ratio, 1.62).
The rate of adverse events was similar between the 2 arms, occurring in 88% (57/65) of patients in the IV arm and 92% (57/62) of patients in the SC arm. Grade 3 or higher adverse events occurred in 46% (n=30) and 47% (n=29) of patients, respectively.
The most common grade 3 or higher adverse event in both arms was neutropenia. It occurred in 22% (n=14) of patients in the IV arm and 26% (n=16) in the SC arm.
Adverse events related to administration were mostly grade 1-2. And they occurred more often in the SC arm than in the IV arm, in 50% (n=31) and 32% (n=21) of patients, respectively.
The researchers said these results suggest the SC formulation of rituximab is non-inferior to the IV formulation and poses no new safety concerns. ![]()

The European Commission (EC) has approved a subcutaneous (SC) formulation of rituximab (MabThera) to treat patients with follicular lymphoma or diffuse large B-cell lymphoma.
This formulation allows for 5-minute administration, a significant decrease over the 2.5-hour infusion time required to administer intravenous (IV) rituximab.
The drug’s maker, Roche, plans to begin launching SC rituximab in a number of European markets this year.
The EC’s approval of this formulation was primarily based on data from the SABRINA trial, which was recently published in The Lancet Oncology and funded by Roche.
In this phase 3 trial, researchers compared 3-week cycles of fixed-dose, SC rituximab to IV rituximab. They enrolled 127 patients with previously untreated, grade 1-3a, CD20-positive follicular lymphoma.
Patients were randomized to receive IV rituximab (375 mg/m2) or SC rituximab (1400 mg). After randomization, they received 1 induction dose of IV rituximab in cycle 1 and then their allocated treatment for cycles 2 through 8. Patients with a complete or partial response continued their treatment as maintenance every 8 weeks.
The study’s primary endpoint was the ratio of observed rituximab serum trough concentrations (Ctrough) between the 2 groups at cycle 7.
Pharmacokinetic data were available for 75% of patients (48/64) in the IV arm and 86% of the patients (54/63) in the SC arm.
An analysis of these data suggested SC rituximab was non-inferior to the IV formulation. The geometric mean Ctrough was 83.13 μg/mL in the IV arm and 134.58 μg/mL in the SC arm (ratio, 1.62).
The rate of adverse events was similar between the 2 arms, occurring in 88% (57/65) of patients in the IV arm and 92% (57/62) of patients in the SC arm. Grade 3 or higher adverse events occurred in 46% (n=30) and 47% (n=29) of patients, respectively.
The most common grade 3 or higher adverse event in both arms was neutropenia. It occurred in 22% (n=14) of patients in the IV arm and 26% (n=16) in the SC arm.
Adverse events related to administration were mostly grade 1-2. And they occurred more often in the SC arm than in the IV arm, in 50% (n=31) and 32% (n=21) of patients, respectively.
The researchers said these results suggest the SC formulation of rituximab is non-inferior to the IV formulation and poses no new safety concerns. ![]()

The European Commission (EC) has approved a subcutaneous (SC) formulation of rituximab (MabThera) to treat patients with follicular lymphoma or diffuse large B-cell lymphoma.
This formulation allows for 5-minute administration, a significant decrease over the 2.5-hour infusion time required to administer intravenous (IV) rituximab.
The drug’s maker, Roche, plans to begin launching SC rituximab in a number of European markets this year.
The EC’s approval of this formulation was primarily based on data from the SABRINA trial, which was recently published in The Lancet Oncology and funded by Roche.
In this phase 3 trial, researchers compared 3-week cycles of fixed-dose, SC rituximab to IV rituximab. They enrolled 127 patients with previously untreated, grade 1-3a, CD20-positive follicular lymphoma.
Patients were randomized to receive IV rituximab (375 mg/m2) or SC rituximab (1400 mg). After randomization, they received 1 induction dose of IV rituximab in cycle 1 and then their allocated treatment for cycles 2 through 8. Patients with a complete or partial response continued their treatment as maintenance every 8 weeks.
The study’s primary endpoint was the ratio of observed rituximab serum trough concentrations (Ctrough) between the 2 groups at cycle 7.
Pharmacokinetic data were available for 75% of patients (48/64) in the IV arm and 86% of the patients (54/63) in the SC arm.
An analysis of these data suggested SC rituximab was non-inferior to the IV formulation. The geometric mean Ctrough was 83.13 μg/mL in the IV arm and 134.58 μg/mL in the SC arm (ratio, 1.62).
The rate of adverse events was similar between the 2 arms, occurring in 88% (57/65) of patients in the IV arm and 92% (57/62) of patients in the SC arm. Grade 3 or higher adverse events occurred in 46% (n=30) and 47% (n=29) of patients, respectively.
The most common grade 3 or higher adverse event in both arms was neutropenia. It occurred in 22% (n=14) of patients in the IV arm and 26% (n=16) in the SC arm.
Adverse events related to administration were mostly grade 1-2. And they occurred more often in the SC arm than in the IV arm, in 50% (n=31) and 32% (n=21) of patients, respectively.
The researchers said these results suggest the SC formulation of rituximab is non-inferior to the IV formulation and poses no new safety concerns. ![]()
NHL among top 10 most common cancers in US

Credit: Rhoda Baer
A new report shows the rate of invasive cancer among US men and women dropped slightly from 2009 to 2010, and the most common cancers were solid tumor malignancies.
However, non-Hodgkin lymphoma (NHL) consistently rated among the top 10 most common cancers, regardless of patient gender or race.
The report was prepared by the Centers for Disease Control and Prevention (CDC) and appears in the current Morbidity and Mortality Weekly Report.
Researchers analyzed new cases of invasive cancers diagnosed in 2010 and reported to the CDC’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology and Results Program.
Data from all states (except Arkansas and Minnesota) and the District of Columbia were included in the analysis, which covered 97% of the US population.
The researchers found the rates of invasive cancer cases dropped from 459 per 100,000 people in 2009 to 446 per 100,000 in 2010.
Cancer rates were higher among men (503 per 100,000) than women (405 per 100,000). In all, there were 745,383 cases reported among men and 711,113 among women in 2010.
The highest rates were for cancers of the prostate (126 per 100,000), female breast (119 per 100,000), lung and bronchus (62 per 100,000), and colon and rectum (40 per 100,000). Together, these accounted for half of all cancer cases in the US.
However, hematologic malignancies were fairly common as well. NHL was the 6th most common cancer for men of all races/ethnicities except Hispanic. For this group, NHL was the 4th most common cancer.
NHL was the 7th most common cancer for black and white women and 6th for the remaining groups, which included American Indian/Alaskan native, Asian/Pacific Islander, and Hispanic women.
Leukemia and myeloma were also among the top 10 most common invasive cancers for certain patients.
Leukemia was the 9th most common cancer for Hispanic and white men and the 10th for American Indian/Alaskan Native women. Myeloma was the 8th most common cancer for black women.
Overall, cancer rates were highest among black patients (455 per 100,000), followed by whites (445 per 100,000), Hispanics (344 per 100,000), Asian/Pacific Islanders (289 per 100,000), and American Indians/Alaskan Natives (270 per 100,000). ![]()

Credit: Rhoda Baer
A new report shows the rate of invasive cancer among US men and women dropped slightly from 2009 to 2010, and the most common cancers were solid tumor malignancies.
However, non-Hodgkin lymphoma (NHL) consistently rated among the top 10 most common cancers, regardless of patient gender or race.
The report was prepared by the Centers for Disease Control and Prevention (CDC) and appears in the current Morbidity and Mortality Weekly Report.
Researchers analyzed new cases of invasive cancers diagnosed in 2010 and reported to the CDC’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology and Results Program.
Data from all states (except Arkansas and Minnesota) and the District of Columbia were included in the analysis, which covered 97% of the US population.
The researchers found the rates of invasive cancer cases dropped from 459 per 100,000 people in 2009 to 446 per 100,000 in 2010.
Cancer rates were higher among men (503 per 100,000) than women (405 per 100,000). In all, there were 745,383 cases reported among men and 711,113 among women in 2010.
The highest rates were for cancers of the prostate (126 per 100,000), female breast (119 per 100,000), lung and bronchus (62 per 100,000), and colon and rectum (40 per 100,000). Together, these accounted for half of all cancer cases in the US.
However, hematologic malignancies were fairly common as well. NHL was the 6th most common cancer for men of all races/ethnicities except Hispanic. For this group, NHL was the 4th most common cancer.
NHL was the 7th most common cancer for black and white women and 6th for the remaining groups, which included American Indian/Alaskan native, Asian/Pacific Islander, and Hispanic women.
Leukemia and myeloma were also among the top 10 most common invasive cancers for certain patients.
Leukemia was the 9th most common cancer for Hispanic and white men and the 10th for American Indian/Alaskan Native women. Myeloma was the 8th most common cancer for black women.
Overall, cancer rates were highest among black patients (455 per 100,000), followed by whites (445 per 100,000), Hispanics (344 per 100,000), Asian/Pacific Islanders (289 per 100,000), and American Indians/Alaskan Natives (270 per 100,000). ![]()

Credit: Rhoda Baer
A new report shows the rate of invasive cancer among US men and women dropped slightly from 2009 to 2010, and the most common cancers were solid tumor malignancies.
However, non-Hodgkin lymphoma (NHL) consistently rated among the top 10 most common cancers, regardless of patient gender or race.
The report was prepared by the Centers for Disease Control and Prevention (CDC) and appears in the current Morbidity and Mortality Weekly Report.
Researchers analyzed new cases of invasive cancers diagnosed in 2010 and reported to the CDC’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology and Results Program.
Data from all states (except Arkansas and Minnesota) and the District of Columbia were included in the analysis, which covered 97% of the US population.
The researchers found the rates of invasive cancer cases dropped from 459 per 100,000 people in 2009 to 446 per 100,000 in 2010.
Cancer rates were higher among men (503 per 100,000) than women (405 per 100,000). In all, there were 745,383 cases reported among men and 711,113 among women in 2010.
The highest rates were for cancers of the prostate (126 per 100,000), female breast (119 per 100,000), lung and bronchus (62 per 100,000), and colon and rectum (40 per 100,000). Together, these accounted for half of all cancer cases in the US.
However, hematologic malignancies were fairly common as well. NHL was the 6th most common cancer for men of all races/ethnicities except Hispanic. For this group, NHL was the 4th most common cancer.
NHL was the 7th most common cancer for black and white women and 6th for the remaining groups, which included American Indian/Alaskan native, Asian/Pacific Islander, and Hispanic women.
Leukemia and myeloma were also among the top 10 most common invasive cancers for certain patients.
Leukemia was the 9th most common cancer for Hispanic and white men and the 10th for American Indian/Alaskan Native women. Myeloma was the 8th most common cancer for black women.
Overall, cancer rates were highest among black patients (455 per 100,000), followed by whites (445 per 100,000), Hispanics (344 per 100,000), Asian/Pacific Islanders (289 per 100,000), and American Indians/Alaskan Natives (270 per 100,000). ![]()
Study provides insights on gene linked to lymphoma
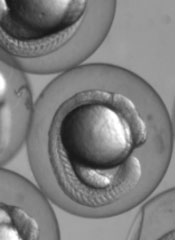
Research in zebrafish embryos may help explain the link between Max’s giant-associated protein (MGA) and Richter’s syndrome.
Previous studies showed that mutations in MGA are associated with a high risk of Richter’s syndrome, a rare lymphoma that can occur in patients with chronic lymphocytic leukemia.
Now, a team of biologists has discovered how MGA controls developmental processes. They described their discoveries in Developmental Cell.
“The same genes that are involved in building a person during embryonic development can mutate and cause cancer later in life,” said study author Scott Dougan, PhD, of the University of Georgia in Athens.
“No one has done a systematic study of MGA, but now that some studies connect it to cancer, there is tremendous interest.”
Preliminary tests have suggested that Richter’s syndrome might develop when MGA does not successfully control the activities of the MYC oncogene.
Dr Dougan and his colleagues decided to alter the levels of MGA in zebrafish embryos to see if they could discover any other roles for MGA.
They found that MGA also helps control the expression of bone morphogenetic proteins (BMPs). Specifically, a transcription factor complex consisting of MGA, Max, and Smad4 controls the expression of bmp2b/swirl in the zebrafish yolk syncytial layer. And this controls BMP signaling throughout the embryo.
BMPs are responsible for bone development in the embryo, but, in adults, changes in BMP activity can result in tumor development. This research suggests MGA may be part of this transformation.
“Scientists are only beginning to understand the roles this MGA protein plays, but our tests show that MGA may control many more processes than first imagined,” Dr Dougan said. “MGA may be involved in a number of other cancers, but we need to do more research before we’re sure.”
In the coming months, Dr Dougan and his colleagues plan to further examine the roles of MGA to determine when it controls MYC, when it controls BMP, and how it is involved in tumor formation.
“[W]e need investigations like these to understand the fundamentals of our biology,” Dr Dougan said. “Once we have this understanding, we can begin to develop new therapies to treat diseases in new, more effective ways.” ![]()

Research in zebrafish embryos may help explain the link between Max’s giant-associated protein (MGA) and Richter’s syndrome.
Previous studies showed that mutations in MGA are associated with a high risk of Richter’s syndrome, a rare lymphoma that can occur in patients with chronic lymphocytic leukemia.
Now, a team of biologists has discovered how MGA controls developmental processes. They described their discoveries in Developmental Cell.
“The same genes that are involved in building a person during embryonic development can mutate and cause cancer later in life,” said study author Scott Dougan, PhD, of the University of Georgia in Athens.
“No one has done a systematic study of MGA, but now that some studies connect it to cancer, there is tremendous interest.”
Preliminary tests have suggested that Richter’s syndrome might develop when MGA does not successfully control the activities of the MYC oncogene.
Dr Dougan and his colleagues decided to alter the levels of MGA in zebrafish embryos to see if they could discover any other roles for MGA.
They found that MGA also helps control the expression of bone morphogenetic proteins (BMPs). Specifically, a transcription factor complex consisting of MGA, Max, and Smad4 controls the expression of bmp2b/swirl in the zebrafish yolk syncytial layer. And this controls BMP signaling throughout the embryo.
BMPs are responsible for bone development in the embryo, but, in adults, changes in BMP activity can result in tumor development. This research suggests MGA may be part of this transformation.
“Scientists are only beginning to understand the roles this MGA protein plays, but our tests show that MGA may control many more processes than first imagined,” Dr Dougan said. “MGA may be involved in a number of other cancers, but we need to do more research before we’re sure.”
In the coming months, Dr Dougan and his colleagues plan to further examine the roles of MGA to determine when it controls MYC, when it controls BMP, and how it is involved in tumor formation.
“[W]e need investigations like these to understand the fundamentals of our biology,” Dr Dougan said. “Once we have this understanding, we can begin to develop new therapies to treat diseases in new, more effective ways.” ![]()

Research in zebrafish embryos may help explain the link between Max’s giant-associated protein (MGA) and Richter’s syndrome.
Previous studies showed that mutations in MGA are associated with a high risk of Richter’s syndrome, a rare lymphoma that can occur in patients with chronic lymphocytic leukemia.
Now, a team of biologists has discovered how MGA controls developmental processes. They described their discoveries in Developmental Cell.
“The same genes that are involved in building a person during embryonic development can mutate and cause cancer later in life,” said study author Scott Dougan, PhD, of the University of Georgia in Athens.
“No one has done a systematic study of MGA, but now that some studies connect it to cancer, there is tremendous interest.”
Preliminary tests have suggested that Richter’s syndrome might develop when MGA does not successfully control the activities of the MYC oncogene.
Dr Dougan and his colleagues decided to alter the levels of MGA in zebrafish embryos to see if they could discover any other roles for MGA.
They found that MGA also helps control the expression of bone morphogenetic proteins (BMPs). Specifically, a transcription factor complex consisting of MGA, Max, and Smad4 controls the expression of bmp2b/swirl in the zebrafish yolk syncytial layer. And this controls BMP signaling throughout the embryo.
BMPs are responsible for bone development in the embryo, but, in adults, changes in BMP activity can result in tumor development. This research suggests MGA may be part of this transformation.
“Scientists are only beginning to understand the roles this MGA protein plays, but our tests show that MGA may control many more processes than first imagined,” Dr Dougan said. “MGA may be involved in a number of other cancers, but we need to do more research before we’re sure.”
In the coming months, Dr Dougan and his colleagues plan to further examine the roles of MGA to determine when it controls MYC, when it controls BMP, and how it is involved in tumor formation.
“[W]e need investigations like these to understand the fundamentals of our biology,” Dr Dougan said. “Once we have this understanding, we can begin to develop new therapies to treat diseases in new, more effective ways.”
Vorinostat demonstrates antitumor activity in FL

Single-agent treatment with the HDAC inhibitor vorinostat can be effective in certain patients with indolent non-Hodgkin lymphoma (NHL), according to research published in the British Journal of Haematology.
In the phase 2 study, vorinostat prompted a 49% overall response rate among 39 patients with relapsed or refractory follicular lymphoma (FL).
However, none of the 4 patients with previously treated mantle cell lymphoma (MCL) achieved a response.
Michinori Ogura, MD, PhD, of Nagoya Daini Red Cross Hospital in Japan, and his colleagues set out to analyze the effects of vorinostat in 56 patients with indolent NHL. Six patients were excluded from the final analysis, as their diseases could not be classified.
Thirty-nine patients had FL, 4 had extranodal marginal zone lymphoma (MZL) of MALT type, 4 had MCL, 2 had small B-cell lymphoma not otherwise specified (NOS), and 1 had small lymphocytic lymphoma.
The median age was 60 years (range, 33-75), and the median number of prior therapies was 2 (range, 1-4). These therapies included rituximab (n=40), alkylating agents (n=7), purine analogs (n=5), and radioimmunotherapy (n=3).
The patients received vorinostat for a median of 8 months. The planned dosage was 200 mg twice daily for 14 consecutive days in a 21-day cycle.
At the first data cutoff point (1 year from the last patient’s enrollment), 18 patients remained on treatment. Thirty-eight had discontinued due to disease progression (n=25), drug-related adverse events (n=9), or withdrawn consent (n=4).
The overall response rate was 49% among FL patients. Ten percent (n=4) achieved a complete response, 8% (n=3) achieved an unconfirmed complete response, and 31% (n=12) achieved a partial response.
None of the patients with MCL responded, but 3 of the 7 (43%) patients with other indolent NHLs achieved a response.
That included 2 patients with small B-cell lymphoma NOS and 1 with extranodal MZL of MALT type. One of the patients with small B-cell lymphoma NOS achieved a complete response.
Approximately 81% of all 56 patients remained alive at 2 years after the last patients had enrolled (the second data cutoff point).
At that point, the median overall survival had not been reached. And the median progression-free survival was 26 months among the FL patients who responded.
There were no treatment-related deaths. The most common drug-related events (in all 56 patients) were thrombocytopenia (93%), diarrhea (68%), neutropenia (68%), decreased appetite (63%), nausea (61%), leukopenia (55%), and fatigue (52%).
Eighty percent of patients (n=45) experienced grade 3/4 adverse events, the most common of which were thrombocytopenia (23% grade 3; 25% grade 4) and neutropenia (36% grade 3; 5% grade 4).
However, all of the patients with thrombocytopenia or neutropenia recovered after they received adequate supportive care and their vorinostat dose was reduced or treatment was interrupted or discontinued.
Taking these results together, the researchers concluded that vorinostat offers sustained antitumor activity and has an acceptable safety profile for patients with relapsed or refractory FL. The team noted, however, that because this was a single-arm study with limited data, a comparative study is needed.

Single-agent treatment with the HDAC inhibitor vorinostat can be effective in certain patients with indolent non-Hodgkin lymphoma (NHL), according to research published in the British Journal of Haematology.
In the phase 2 study, vorinostat prompted a 49% overall response rate among 39 patients with relapsed or refractory follicular lymphoma (FL).
However, none of the 4 patients with previously treated mantle cell lymphoma (MCL) achieved a response.
Michinori Ogura, MD, PhD, of Nagoya Daini Red Cross Hospital in Japan, and his colleagues set out to analyze the effects of vorinostat in 56 patients with indolent NHL. Six patients were excluded from the final analysis, as their diseases could not be classified.
Thirty-nine patients had FL, 4 had extranodal marginal zone lymphoma (MZL) of MALT type, 4 had MCL, 2 had small B-cell lymphoma not otherwise specified (NOS), and 1 had small lymphocytic lymphoma.
The median age was 60 years (range, 33-75), and the median number of prior therapies was 2 (range, 1-4). These therapies included rituximab (n=40), alkylating agents (n=7), purine analogs (n=5), and radioimmunotherapy (n=3).
The patients received vorinostat for a median of 8 months. The planned dosage was 200 mg twice daily for 14 consecutive days in a 21-day cycle.
At the first data cutoff point (1 year from the last patient’s enrollment), 18 patients remained on treatment. Thirty-eight had discontinued due to disease progression (n=25), drug-related adverse events (n=9), or withdrawn consent (n=4).
The overall response rate was 49% among FL patients. Ten percent (n=4) achieved a complete response, 8% (n=3) achieved an unconfirmed complete response, and 31% (n=12) achieved a partial response.
None of the patients with MCL responded, but 3 of the 7 (43%) patients with other indolent NHLs achieved a response.
That included 2 patients with small B-cell lymphoma NOS and 1 with extranodal MZL of MALT type. One of the patients with small B-cell lymphoma NOS achieved a complete response.
Approximately 81% of all 56 patients remained alive at 2 years after the last patients had enrolled (the second data cutoff point).
At that point, the median overall survival had not been reached. And the median progression-free survival was 26 months among the FL patients who responded.
There were no treatment-related deaths. The most common drug-related events (in all 56 patients) were thrombocytopenia (93%), diarrhea (68%), neutropenia (68%), decreased appetite (63%), nausea (61%), leukopenia (55%), and fatigue (52%).
Eighty percent of patients (n=45) experienced grade 3/4 adverse events, the most common of which were thrombocytopenia (23% grade 3; 25% grade 4) and neutropenia (36% grade 3; 5% grade 4).
However, all of the patients with thrombocytopenia or neutropenia recovered after they received adequate supportive care and their vorinostat dose was reduced or treatment was interrupted or discontinued.
Taking these results together, the researchers concluded that vorinostat offers sustained antitumor activity and has an acceptable safety profile for patients with relapsed or refractory FL. The team noted, however, that because this was a single-arm study with limited data, a comparative study is needed.

Single-agent treatment with the HDAC inhibitor vorinostat can be effective in certain patients with indolent non-Hodgkin lymphoma (NHL), according to research published in the British Journal of Haematology.
In the phase 2 study, vorinostat prompted a 49% overall response rate among 39 patients with relapsed or refractory follicular lymphoma (FL).
However, none of the 4 patients with previously treated mantle cell lymphoma (MCL) achieved a response.
Michinori Ogura, MD, PhD, of Nagoya Daini Red Cross Hospital in Japan, and his colleagues set out to analyze the effects of vorinostat in 56 patients with indolent NHL. Six patients were excluded from the final analysis, as their diseases could not be classified.
Thirty-nine patients had FL, 4 had extranodal marginal zone lymphoma (MZL) of MALT type, 4 had MCL, 2 had small B-cell lymphoma not otherwise specified (NOS), and 1 had small lymphocytic lymphoma.
The median age was 60 years (range, 33-75), and the median number of prior therapies was 2 (range, 1-4). These therapies included rituximab (n=40), alkylating agents (n=7), purine analogs (n=5), and radioimmunotherapy (n=3).
The patients received vorinostat for a median of 8 months. The planned dosage was 200 mg twice daily for 14 consecutive days in a 21-day cycle.
At the first data cutoff point (1 year from the last patient’s enrollment), 18 patients remained on treatment. Thirty-eight had discontinued due to disease progression (n=25), drug-related adverse events (n=9), or withdrawn consent (n=4).
The overall response rate was 49% among FL patients. Ten percent (n=4) achieved a complete response, 8% (n=3) achieved an unconfirmed complete response, and 31% (n=12) achieved a partial response.
None of the patients with MCL responded, but 3 of the 7 (43%) patients with other indolent NHLs achieved a response.
That included 2 patients with small B-cell lymphoma NOS and 1 with extranodal MZL of MALT type. One of the patients with small B-cell lymphoma NOS achieved a complete response.
Approximately 81% of all 56 patients remained alive at 2 years after the last patients had enrolled (the second data cutoff point).
At that point, the median overall survival had not been reached. And the median progression-free survival was 26 months among the FL patients who responded.
There were no treatment-related deaths. The most common drug-related events (in all 56 patients) were thrombocytopenia (93%), diarrhea (68%), neutropenia (68%), decreased appetite (63%), nausea (61%), leukopenia (55%), and fatigue (52%).
Eighty percent of patients (n=45) experienced grade 3/4 adverse events, the most common of which were thrombocytopenia (23% grade 3; 25% grade 4) and neutropenia (36% grade 3; 5% grade 4).
However, all of the patients with thrombocytopenia or neutropenia recovered after they received adequate supportive care and their vorinostat dose was reduced or treatment was interrupted or discontinued.
Taking these results together, the researchers concluded that vorinostat offers sustained antitumor activity and has an acceptable safety profile for patients with relapsed or refractory FL. The team noted, however, that because this was a single-arm study with limited data, a comparative study is needed.
Immunotherapy shows promise in CBCL

diffuse large B-cell lymphoma
Credit: Leszek Woźniak
& Krzysztof W. Zieliński
An immunotherapeutic agent can confer clinical benefit in patients with relapsed cutaneous B-cell lymphoma (CBCL), results of a small phase 2 study suggest.
The therapy, TG1042, is human adenovirus type 5 engineered to express interferon-gamma.
Repeat injections of TG1042 elicited responses in 11 of 12 evaluable patients, with complete responses in 7.
All 13 of the patients enrolled on the study experienced an adverse event that may have been related to TG1042, but most were grade 1 or 2 in severity.
“Intralesional TG1042 therapy is well-tolerated and results in lasting tumor regressions,” said study author Reinhard Dummer, MD, of the University of Zurich in Switzerland.
He and his colleagues reported these results in PLOS ONE. The study was sponsored by Transgene SA, makers of TG1042.
The trial consisted of 13 patients with primary CBCL, including primary cutaneous marginal zone B-cell lymphoma, primary cutaneous follicle center B-cell lymphoma, primary cutaneous diffuse large B-cell lymphoma other than leg type, and T-cell/histiocyte-rich B-cell lymphoma.
Patients were required to have either relapsed or active disease after at least 1 first-line treatment.
The patients received intralesional injections of TG1042 at 5 x 1010 viral particles per lesion. They could receive injections in up to 6 lesions, which were treated simultaneously on days 1, 8, and 15 of a 4-week cycle.
Patients did not receive treatment during the fourth week. At the end of the cycle, the researchers evaluated tumors for response.
If patients’ disease did not progress, they could receive an additional cycle, up to a maximum of 4. If patients responded to treatment and their lesions disappeared, they were eligible to receive a second series of injections in untreated lesions.
Of the 13 patients treated, 12 were evaluable for response. Eleven of the patients (85%) achieved an objective response—7 complete responses and 4 partial responses. One patient had stable disease.
All reviewed skin biopsies showed that lesions improved after treatment, with a decrease of the lymphoid infiltrate.
The median time to first objective response was 3.2 months (rage, 1-17.5 months). Among complete responders, the median time to response was 4.3 months (range, 1.4-17.5 months).
The median time to progression was 23.5 months (range, 6.5-26.4+ months).
All 13 patients were included in the safety evaluation, and all experienced 1 or more adverse events that were considered possibly or probably related to the treatment.
One patient discontinued treatment due to influenza-like illness, pyrexia, headache, and skin blisters that were possibly related to TG1042.
Another patient had grade 3 increased lipase, but this was thought to be unrelated to TG1042. And it resolved without treatment.
All other adverse events were grade 1 or 2 in nature. They included fatigue, headache, pyrexia, chills, influenza-like illness, injection site irritation, injection site erythema, and injection site pain.
All of these reactions resolved after treatment discontinuation.

diffuse large B-cell lymphoma
Credit: Leszek Woźniak
& Krzysztof W. Zieliński
An immunotherapeutic agent can confer clinical benefit in patients with relapsed cutaneous B-cell lymphoma (CBCL), results of a small phase 2 study suggest.
The therapy, TG1042, is human adenovirus type 5 engineered to express interferon-gamma.
Repeat injections of TG1042 elicited responses in 11 of 12 evaluable patients, with complete responses in 7.
All 13 of the patients enrolled on the study experienced an adverse event that may have been related to TG1042, but most were grade 1 or 2 in severity.
“Intralesional TG1042 therapy is well-tolerated and results in lasting tumor regressions,” said study author Reinhard Dummer, MD, of the University of Zurich in Switzerland.
He and his colleagues reported these results in PLOS ONE. The study was sponsored by Transgene SA, makers of TG1042.
The trial consisted of 13 patients with primary CBCL, including primary cutaneous marginal zone B-cell lymphoma, primary cutaneous follicle center B-cell lymphoma, primary cutaneous diffuse large B-cell lymphoma other than leg type, and T-cell/histiocyte-rich B-cell lymphoma.
Patients were required to have either relapsed or active disease after at least 1 first-line treatment.
The patients received intralesional injections of TG1042 at 5 x 1010 viral particles per lesion. They could receive injections in up to 6 lesions, which were treated simultaneously on days 1, 8, and 15 of a 4-week cycle.
Patients did not receive treatment during the fourth week. At the end of the cycle, the researchers evaluated tumors for response.
If patients’ disease did not progress, they could receive an additional cycle, up to a maximum of 4. If patients responded to treatment and their lesions disappeared, they were eligible to receive a second series of injections in untreated lesions.
Of the 13 patients treated, 12 were evaluable for response. Eleven of the patients (85%) achieved an objective response—7 complete responses and 4 partial responses. One patient had stable disease.
All reviewed skin biopsies showed that lesions improved after treatment, with a decrease of the lymphoid infiltrate.
The median time to first objective response was 3.2 months (rage, 1-17.5 months). Among complete responders, the median time to response was 4.3 months (range, 1.4-17.5 months).
The median time to progression was 23.5 months (range, 6.5-26.4+ months).
All 13 patients were included in the safety evaluation, and all experienced 1 or more adverse events that were considered possibly or probably related to the treatment.
One patient discontinued treatment due to influenza-like illness, pyrexia, headache, and skin blisters that were possibly related to TG1042.
Another patient had grade 3 increased lipase, but this was thought to be unrelated to TG1042. And it resolved without treatment.
All other adverse events were grade 1 or 2 in nature. They included fatigue, headache, pyrexia, chills, influenza-like illness, injection site irritation, injection site erythema, and injection site pain.
All of these reactions resolved after treatment discontinuation.

diffuse large B-cell lymphoma
Credit: Leszek Woźniak
& Krzysztof W. Zieliński
An immunotherapeutic agent can confer clinical benefit in patients with relapsed cutaneous B-cell lymphoma (CBCL), results of a small phase 2 study suggest.
The therapy, TG1042, is human adenovirus type 5 engineered to express interferon-gamma.
Repeat injections of TG1042 elicited responses in 11 of 12 evaluable patients, with complete responses in 7.
All 13 of the patients enrolled on the study experienced an adverse event that may have been related to TG1042, but most were grade 1 or 2 in severity.
“Intralesional TG1042 therapy is well-tolerated and results in lasting tumor regressions,” said study author Reinhard Dummer, MD, of the University of Zurich in Switzerland.
He and his colleagues reported these results in PLOS ONE. The study was sponsored by Transgene SA, makers of TG1042.
The trial consisted of 13 patients with primary CBCL, including primary cutaneous marginal zone B-cell lymphoma, primary cutaneous follicle center B-cell lymphoma, primary cutaneous diffuse large B-cell lymphoma other than leg type, and T-cell/histiocyte-rich B-cell lymphoma.
Patients were required to have either relapsed or active disease after at least 1 first-line treatment.
The patients received intralesional injections of TG1042 at 5 x 1010 viral particles per lesion. They could receive injections in up to 6 lesions, which were treated simultaneously on days 1, 8, and 15 of a 4-week cycle.
Patients did not receive treatment during the fourth week. At the end of the cycle, the researchers evaluated tumors for response.
If patients’ disease did not progress, they could receive an additional cycle, up to a maximum of 4. If patients responded to treatment and their lesions disappeared, they were eligible to receive a second series of injections in untreated lesions.
Of the 13 patients treated, 12 were evaluable for response. Eleven of the patients (85%) achieved an objective response—7 complete responses and 4 partial responses. One patient had stable disease.
All reviewed skin biopsies showed that lesions improved after treatment, with a decrease of the lymphoid infiltrate.
The median time to first objective response was 3.2 months (rage, 1-17.5 months). Among complete responders, the median time to response was 4.3 months (range, 1.4-17.5 months).
The median time to progression was 23.5 months (range, 6.5-26.4+ months).
All 13 patients were included in the safety evaluation, and all experienced 1 or more adverse events that were considered possibly or probably related to the treatment.
One patient discontinued treatment due to influenza-like illness, pyrexia, headache, and skin blisters that were possibly related to TG1042.
Another patient had grade 3 increased lipase, but this was thought to be unrelated to TG1042. And it resolved without treatment.
All other adverse events were grade 1 or 2 in nature. They included fatigue, headache, pyrexia, chills, influenza-like illness, injection site irritation, injection site erythema, and injection site pain.
All of these reactions resolved after treatment discontinuation.
Endothelial cells seem to support lymphoma growth

Credit: NIH
Researchers have found evidence to suggest that endothelial cells produce proteins that nurture lymphoma, thereby turning a slow-growing malignancy into an aggressive, treatment-resistant disease.
Their findings, published in Cancer Cell, challenge previous theories about cancer growth and development.
The research suggests it is not simply the number of genetic mutations in cancer cells that determines the aggressiveness of the disease.
Rather, lethality occurs when the cancer hijacks the reparative function of blood vessels, a step that ensures tumor cells’ ability to spread and resist treatment.
The researchers also found the crucial nurturing molecules that cancer co-opts from tumor blood vessels to promote invasiveness and resistance to chemotherapy. Experiments in mice showed that shutting down these previously unrecognized biological signals makes lymphoma less aggressive and improves survival.
“The endothelial cells that line the vessels orchestrate a wide variety of biological processes, good and bad,” said study author Shahin Rafii, MD, of Weill Cornell Medical College in New York.
“The understanding and control of blood vessel function and how this changes the malignant behaviors of cancer cells is a transformative concept and will pave the way for designing innovative treatments that disrupt signals from the local environment housing the tumor cells—a strategy that has been unappreciated.”
Dr Rafii and his colleagues studied human B-cell lymphoma cells in vitro and in mice. The team found that although the lymphoma cells harbor the same mutations, it is their interaction with and support from endothelial cells that dictates the fate and features of the disease.
Specifically, when slow-growing tumor cells come into contact with endothelial cells expressing the protein Jagged1 (Jag1), they become more aggressive and resistant to chemotherapy. However, when Jag1 is not available from surrounding blood vessels, the lethal features of the tumor cells are absent.
The researchers also found that when Jag1 binds to and activates the receptor Notch2 on tumor cells, the lymphoma becomes more tolerant of chemotherapy.
“We think signals from these abnormally stimulated tumor endothelial cells modulate the malignant features of lymphoma cells,” said Joseph Scandura, MD, PhD, of Weill Cornell. “This is a reversible process dictated by the location of the tumor cells rather than their genetics.”
“This is a critical finding because it suggests that targeting the endothelial cells with agents that disrupt their specific pro-tumorigenic signals can transform aggressive cancers into slow-growing cancers that are more sensitive to chemotherapy.”
The researchers found, for example, that blocking the Notch2 receptor in lymphoma cells or Jag1 on blood vessels made the lymphoma cells significantly more vulnerable to chemotherapy.
“This new approach to treatment would interfere with the nurturing proteins produced by tumor blood vessels,” said Bi-Sen Ding, PhD, of Weill Cornell. “It is different from traditional anti-angiogenic therapy that aims to eradicate all blood vessels in the tumor and prevent them from bringing oxygen and nutrients to the cancer.”
Dr Ding noted that conventional anti-angiogenic therapy can sometimes increase tumor cell aggressiveness by enhancing the expansion of tumor blood vessels.
But blocking specific proteins produced by the tumor blood vessels, such as Jag1, without altering oxygen and nutrient delivery, can circumvent this problem. And this approach could be translated to the clinical setting.
“[W]e can target tumor blood vessels by delivering biological cruise missiles loaded with inhibitory agents for specific cancer-promoting proteins,” Dr Ding said. This could halt tumor growth and increase sensitivity to chemotherapy.
The researchers also believe this study suggests that screening for anticancer drugs may be more effective if tumor cells are assayed in the context of signals derived from the subverted blood vessels.

Credit: NIH
Researchers have found evidence to suggest that endothelial cells produce proteins that nurture lymphoma, thereby turning a slow-growing malignancy into an aggressive, treatment-resistant disease.
Their findings, published in Cancer Cell, challenge previous theories about cancer growth and development.
The research suggests it is not simply the number of genetic mutations in cancer cells that determines the aggressiveness of the disease.
Rather, lethality occurs when the cancer hijacks the reparative function of blood vessels, a step that ensures tumor cells’ ability to spread and resist treatment.
The researchers also found the crucial nurturing molecules that cancer co-opts from tumor blood vessels to promote invasiveness and resistance to chemotherapy. Experiments in mice showed that shutting down these previously unrecognized biological signals makes lymphoma less aggressive and improves survival.
“The endothelial cells that line the vessels orchestrate a wide variety of biological processes, good and bad,” said study author Shahin Rafii, MD, of Weill Cornell Medical College in New York.
“The understanding and control of blood vessel function and how this changes the malignant behaviors of cancer cells is a transformative concept and will pave the way for designing innovative treatments that disrupt signals from the local environment housing the tumor cells—a strategy that has been unappreciated.”
Dr Rafii and his colleagues studied human B-cell lymphoma cells in vitro and in mice. The team found that although the lymphoma cells harbor the same mutations, it is their interaction with and support from endothelial cells that dictates the fate and features of the disease.
Specifically, when slow-growing tumor cells come into contact with endothelial cells expressing the protein Jagged1 (Jag1), they become more aggressive and resistant to chemotherapy. However, when Jag1 is not available from surrounding blood vessels, the lethal features of the tumor cells are absent.
The researchers also found that when Jag1 binds to and activates the receptor Notch2 on tumor cells, the lymphoma becomes more tolerant of chemotherapy.
“We think signals from these abnormally stimulated tumor endothelial cells modulate the malignant features of lymphoma cells,” said Joseph Scandura, MD, PhD, of Weill Cornell. “This is a reversible process dictated by the location of the tumor cells rather than their genetics.”
“This is a critical finding because it suggests that targeting the endothelial cells with agents that disrupt their specific pro-tumorigenic signals can transform aggressive cancers into slow-growing cancers that are more sensitive to chemotherapy.”
The researchers found, for example, that blocking the Notch2 receptor in lymphoma cells or Jag1 on blood vessels made the lymphoma cells significantly more vulnerable to chemotherapy.
“This new approach to treatment would interfere with the nurturing proteins produced by tumor blood vessels,” said Bi-Sen Ding, PhD, of Weill Cornell. “It is different from traditional anti-angiogenic therapy that aims to eradicate all blood vessels in the tumor and prevent them from bringing oxygen and nutrients to the cancer.”
Dr Ding noted that conventional anti-angiogenic therapy can sometimes increase tumor cell aggressiveness by enhancing the expansion of tumor blood vessels.
But blocking specific proteins produced by the tumor blood vessels, such as Jag1, without altering oxygen and nutrient delivery, can circumvent this problem. And this approach could be translated to the clinical setting.
“[W]e can target tumor blood vessels by delivering biological cruise missiles loaded with inhibitory agents for specific cancer-promoting proteins,” Dr Ding said. This could halt tumor growth and increase sensitivity to chemotherapy.
The researchers also believe this study suggests that screening for anticancer drugs may be more effective if tumor cells are assayed in the context of signals derived from the subverted blood vessels.

Credit: NIH
Researchers have found evidence to suggest that endothelial cells produce proteins that nurture lymphoma, thereby turning a slow-growing malignancy into an aggressive, treatment-resistant disease.
Their findings, published in Cancer Cell, challenge previous theories about cancer growth and development.
The research suggests it is not simply the number of genetic mutations in cancer cells that determines the aggressiveness of the disease.
Rather, lethality occurs when the cancer hijacks the reparative function of blood vessels, a step that ensures tumor cells’ ability to spread and resist treatment.
The researchers also found the crucial nurturing molecules that cancer co-opts from tumor blood vessels to promote invasiveness and resistance to chemotherapy. Experiments in mice showed that shutting down these previously unrecognized biological signals makes lymphoma less aggressive and improves survival.
“The endothelial cells that line the vessels orchestrate a wide variety of biological processes, good and bad,” said study author Shahin Rafii, MD, of Weill Cornell Medical College in New York.
“The understanding and control of blood vessel function and how this changes the malignant behaviors of cancer cells is a transformative concept and will pave the way for designing innovative treatments that disrupt signals from the local environment housing the tumor cells—a strategy that has been unappreciated.”
Dr Rafii and his colleagues studied human B-cell lymphoma cells in vitro and in mice. The team found that although the lymphoma cells harbor the same mutations, it is their interaction with and support from endothelial cells that dictates the fate and features of the disease.
Specifically, when slow-growing tumor cells come into contact with endothelial cells expressing the protein Jagged1 (Jag1), they become more aggressive and resistant to chemotherapy. However, when Jag1 is not available from surrounding blood vessels, the lethal features of the tumor cells are absent.
The researchers also found that when Jag1 binds to and activates the receptor Notch2 on tumor cells, the lymphoma becomes more tolerant of chemotherapy.
“We think signals from these abnormally stimulated tumor endothelial cells modulate the malignant features of lymphoma cells,” said Joseph Scandura, MD, PhD, of Weill Cornell. “This is a reversible process dictated by the location of the tumor cells rather than their genetics.”
“This is a critical finding because it suggests that targeting the endothelial cells with agents that disrupt their specific pro-tumorigenic signals can transform aggressive cancers into slow-growing cancers that are more sensitive to chemotherapy.”
The researchers found, for example, that blocking the Notch2 receptor in lymphoma cells or Jag1 on blood vessels made the lymphoma cells significantly more vulnerable to chemotherapy.
“This new approach to treatment would interfere with the nurturing proteins produced by tumor blood vessels,” said Bi-Sen Ding, PhD, of Weill Cornell. “It is different from traditional anti-angiogenic therapy that aims to eradicate all blood vessels in the tumor and prevent them from bringing oxygen and nutrients to the cancer.”
Dr Ding noted that conventional anti-angiogenic therapy can sometimes increase tumor cell aggressiveness by enhancing the expansion of tumor blood vessels.
But blocking specific proteins produced by the tumor blood vessels, such as Jag1, without altering oxygen and nutrient delivery, can circumvent this problem. And this approach could be translated to the clinical setting.
“[W]e can target tumor blood vessels by delivering biological cruise missiles loaded with inhibitory agents for specific cancer-promoting proteins,” Dr Ding said. This could halt tumor growth and increase sensitivity to chemotherapy.
The researchers also believe this study suggests that screening for anticancer drugs may be more effective if tumor cells are assayed in the context of signals derived from the subverted blood vessels.