User login
Drugs appear comparable for delaying SREs in MM
In a phase 3 trial, denosumab proved non-inferior to zoledronic acid for delaying skeletal-related events (SREs) in patients with multiple myeloma (MM).
The median time to first on-study SRE was 23 months in the denosumab arm and 24 months in the zoledronic acid arm.
There were fewer renal adverse events (AEs) but more hypocalcemia AEs in the denosumab arm.
“Until recently, treatment options for the prevention of skeletal-related events in multiple myeloma were limited to bisphosphonates, which are cleared through the kidneys and can be associated with increased renal impairment,” said Noopur Raje, MD, of Massachusetts General Hospital Cancer Center in Boston.
“Denosumab, which is not cleared through the kidneys, provides a new treatment option for the prevention of skeletal-related events in patients with multiple myeloma.”
Dr Raje and her colleagues conducted this phase 3 trial of denosumab and reported the results in The Lancet Oncology. The trial was sponsored by Amgen, the company developing denosumab.
Denosumab is the first fully human monoclonal antibody that binds to and neutralizes RANK ligand—a protein essential for the formation, function, and survival of osteoclasts—thereby inhibiting osteoclast-mediated bone destruction.
In this trial, researchers compared denosumab to zoledronic acid for the prevention of SREs in adults with newly diagnosed MM and bone disease.
The team randomized 1718 patients to receive subcutaneous denosumab at 120 mg and intravenous placebo every 4 weeks (n=859) or intravenous zoledronic acid at 4 mg (adjusted for renal function at baseline) and subcutaneous placebo every 4 weeks (n=859). All patients also received investigators’ choice of first-line MM therapy.
Skeletal surveys using conventional radiography were obtained every 12 to 24 weeks per protocol. The primary endpoint of the study was non-inferiority of denosumab to zoledronic acid for time to first on-study SRE (pathologic fracture, radiation to bone, surgery to bone, or spinal cord compression).
The primary endpoint was met. The median time to first on-study SRE was 22.8 months for patients in the denosumab arm and 24 months for those in the zoledronic acid arm (hazard ratio [HR]=0.98; 95% confidence interval [CI]: 0.85-1.14; P non-inferiority=0.010).
Approximately 60% of all first SREs occurred within the first 3 months, and 81% occurred within the first 6 months.
Overall survival, a secondary endpoint, was similar between the denosumab and zoledronic acid arms (HR=0.90; 95% CI: 0.70-1.16; P=0.41).
There were fewer renal treatment-emergent AEs in the denosumab arm than the zoledronic acid arm—10% and 17%, respectively. There were more hypocalcemia AEs in the denosumab arm than the zoledronic acid arm—17% and 12%, respectively.
The incidence of osteonecrosis of the jaw was 4% in the denosumab arm and 3% in the zoledronic acid arm.
The most common grade 3 or higher treatment-emergent AEs (in the denosumab and zoledronic acid arms, respectively) were neutropenia (15% in both arms), thrombocytopenia (14% and 12%), anemia (12% and 10%), febrile neutropenia (11% and 10%), and pneumonia (8% in both arms).
The most common serious AE was pneumonia (8% in both arms).
Treatment-emergent AEs led to study drug discontinuation in 13% of patients in the denosumab arm and 12% in the zoledronic acid arm.
One patient in the zoledronic acid arm died of cardiac arrest that was deemed treatment-related. No other deaths were considered treatment-related. ![]()
In a phase 3 trial, denosumab proved non-inferior to zoledronic acid for delaying skeletal-related events (SREs) in patients with multiple myeloma (MM).
The median time to first on-study SRE was 23 months in the denosumab arm and 24 months in the zoledronic acid arm.
There were fewer renal adverse events (AEs) but more hypocalcemia AEs in the denosumab arm.
“Until recently, treatment options for the prevention of skeletal-related events in multiple myeloma were limited to bisphosphonates, which are cleared through the kidneys and can be associated with increased renal impairment,” said Noopur Raje, MD, of Massachusetts General Hospital Cancer Center in Boston.
“Denosumab, which is not cleared through the kidneys, provides a new treatment option for the prevention of skeletal-related events in patients with multiple myeloma.”
Dr Raje and her colleagues conducted this phase 3 trial of denosumab and reported the results in The Lancet Oncology. The trial was sponsored by Amgen, the company developing denosumab.
Denosumab is the first fully human monoclonal antibody that binds to and neutralizes RANK ligand—a protein essential for the formation, function, and survival of osteoclasts—thereby inhibiting osteoclast-mediated bone destruction.
In this trial, researchers compared denosumab to zoledronic acid for the prevention of SREs in adults with newly diagnosed MM and bone disease.
The team randomized 1718 patients to receive subcutaneous denosumab at 120 mg and intravenous placebo every 4 weeks (n=859) or intravenous zoledronic acid at 4 mg (adjusted for renal function at baseline) and subcutaneous placebo every 4 weeks (n=859). All patients also received investigators’ choice of first-line MM therapy.
Skeletal surveys using conventional radiography were obtained every 12 to 24 weeks per protocol. The primary endpoint of the study was non-inferiority of denosumab to zoledronic acid for time to first on-study SRE (pathologic fracture, radiation to bone, surgery to bone, or spinal cord compression).
The primary endpoint was met. The median time to first on-study SRE was 22.8 months for patients in the denosumab arm and 24 months for those in the zoledronic acid arm (hazard ratio [HR]=0.98; 95% confidence interval [CI]: 0.85-1.14; P non-inferiority=0.010).
Approximately 60% of all first SREs occurred within the first 3 months, and 81% occurred within the first 6 months.
Overall survival, a secondary endpoint, was similar between the denosumab and zoledronic acid arms (HR=0.90; 95% CI: 0.70-1.16; P=0.41).
There were fewer renal treatment-emergent AEs in the denosumab arm than the zoledronic acid arm—10% and 17%, respectively. There were more hypocalcemia AEs in the denosumab arm than the zoledronic acid arm—17% and 12%, respectively.
The incidence of osteonecrosis of the jaw was 4% in the denosumab arm and 3% in the zoledronic acid arm.
The most common grade 3 or higher treatment-emergent AEs (in the denosumab and zoledronic acid arms, respectively) were neutropenia (15% in both arms), thrombocytopenia (14% and 12%), anemia (12% and 10%), febrile neutropenia (11% and 10%), and pneumonia (8% in both arms).
The most common serious AE was pneumonia (8% in both arms).
Treatment-emergent AEs led to study drug discontinuation in 13% of patients in the denosumab arm and 12% in the zoledronic acid arm.
One patient in the zoledronic acid arm died of cardiac arrest that was deemed treatment-related. No other deaths were considered treatment-related. ![]()
In a phase 3 trial, denosumab proved non-inferior to zoledronic acid for delaying skeletal-related events (SREs) in patients with multiple myeloma (MM).
The median time to first on-study SRE was 23 months in the denosumab arm and 24 months in the zoledronic acid arm.
There were fewer renal adverse events (AEs) but more hypocalcemia AEs in the denosumab arm.
“Until recently, treatment options for the prevention of skeletal-related events in multiple myeloma were limited to bisphosphonates, which are cleared through the kidneys and can be associated with increased renal impairment,” said Noopur Raje, MD, of Massachusetts General Hospital Cancer Center in Boston.
“Denosumab, which is not cleared through the kidneys, provides a new treatment option for the prevention of skeletal-related events in patients with multiple myeloma.”
Dr Raje and her colleagues conducted this phase 3 trial of denosumab and reported the results in The Lancet Oncology. The trial was sponsored by Amgen, the company developing denosumab.
Denosumab is the first fully human monoclonal antibody that binds to and neutralizes RANK ligand—a protein essential for the formation, function, and survival of osteoclasts—thereby inhibiting osteoclast-mediated bone destruction.
In this trial, researchers compared denosumab to zoledronic acid for the prevention of SREs in adults with newly diagnosed MM and bone disease.
The team randomized 1718 patients to receive subcutaneous denosumab at 120 mg and intravenous placebo every 4 weeks (n=859) or intravenous zoledronic acid at 4 mg (adjusted for renal function at baseline) and subcutaneous placebo every 4 weeks (n=859). All patients also received investigators’ choice of first-line MM therapy.
Skeletal surveys using conventional radiography were obtained every 12 to 24 weeks per protocol. The primary endpoint of the study was non-inferiority of denosumab to zoledronic acid for time to first on-study SRE (pathologic fracture, radiation to bone, surgery to bone, or spinal cord compression).
The primary endpoint was met. The median time to first on-study SRE was 22.8 months for patients in the denosumab arm and 24 months for those in the zoledronic acid arm (hazard ratio [HR]=0.98; 95% confidence interval [CI]: 0.85-1.14; P non-inferiority=0.010).
Approximately 60% of all first SREs occurred within the first 3 months, and 81% occurred within the first 6 months.
Overall survival, a secondary endpoint, was similar between the denosumab and zoledronic acid arms (HR=0.90; 95% CI: 0.70-1.16; P=0.41).
There were fewer renal treatment-emergent AEs in the denosumab arm than the zoledronic acid arm—10% and 17%, respectively. There were more hypocalcemia AEs in the denosumab arm than the zoledronic acid arm—17% and 12%, respectively.
The incidence of osteonecrosis of the jaw was 4% in the denosumab arm and 3% in the zoledronic acid arm.
The most common grade 3 or higher treatment-emergent AEs (in the denosumab and zoledronic acid arms, respectively) were neutropenia (15% in both arms), thrombocytopenia (14% and 12%), anemia (12% and 10%), febrile neutropenia (11% and 10%), and pneumonia (8% in both arms).
The most common serious AE was pneumonia (8% in both arms).
Treatment-emergent AEs led to study drug discontinuation in 13% of patients in the denosumab arm and 12% in the zoledronic acid arm.
One patient in the zoledronic acid arm died of cardiac arrest that was deemed treatment-related. No other deaths were considered treatment-related. ![]()
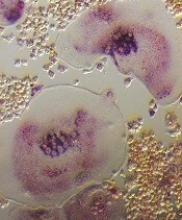
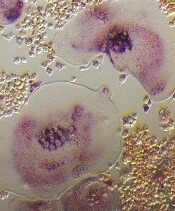
Meningococcal Arthritis Masking as Possible Myeloma
For a group of clinicians in Australia, the diagnosis of meningococcal arthritis was “straightforward” except for abnormal serum total protein, anemia, and immunoglobulin results, which suggested their patient might have a hematological disorder such as myeloma.
The patient came to the hospital after 4 days of worsening knee and arm pain so severe he could not stand. His knees and both wrists showed swelling but no palpable lymphadenopathy or hepatosplenomegaly. The patient’s medical history showed he was taking no regular medications.
Joint aspiration grew Neisseria meningitidis. The patient’s blood tests showed hemoglobin 126 g/dL, white blood cell count 15.3 x 109/L, an unusually high total protein level (100 g/L), and an IgM kappa paraprotein band of 45 g/L on protein electrophoresis. A computed tomography scan showed widespread lymphadenopathy, hepatosplenomegaly, and multilevel thoracic vertebral collapse. A bone marrow biopsy showed evidence of a lymphocytic infiltrate, with lymphoplasmacytoid differentiation.
The histology best fitted a diagnosis of nodal marginal zone lymphoma with plasmacytic differentiation, the clinicians say. Having ruled out other possibilities, they settled on non-Hodgkin lymphoma.
Initially, the suspicion was that the patient had septic arthritis due to Staphylococcus aureus (the most common organism isolated in septic arthritis), and he was given piperacillin/tazobactam. That was changed to flucloxacillin and then to ceftriaxone after the result of N meningitidis. The patient also was treated with rituximab and bendamustine for the lymphoma with a complete remission.
Meningococcal infection presenting as septic arthritis in the case of invasive meningococcemia is rare, the clinicians say, but primary meningococcal arthritis is even rarer. The case highlights the important aspect that “diagnosis of one condition can lead to diagnosis of another”—in this case, the lymphoma-weakened immune system led to the symptoms of polyarthropathy and the diagnosis of primary meningococcal arthritis. The clinicians also cited a case of a patient who presented with meningococcal meningitis and arthritis who was found to have an underlying Waldenström disease, and a patient whose HIV was diagnosed again after the patient presented with meningococcal arthritis symptoms.
The clinicans say such cases underscore the importance of screening for an underlying impaired immune response in patients presenting with rare conditions such as meningococcal arthritis.
For a group of clinicians in Australia, the diagnosis of meningococcal arthritis was “straightforward” except for abnormal serum total protein, anemia, and immunoglobulin results, which suggested their patient might have a hematological disorder such as myeloma.
The patient came to the hospital after 4 days of worsening knee and arm pain so severe he could not stand. His knees and both wrists showed swelling but no palpable lymphadenopathy or hepatosplenomegaly. The patient’s medical history showed he was taking no regular medications.
Joint aspiration grew Neisseria meningitidis. The patient’s blood tests showed hemoglobin 126 g/dL, white blood cell count 15.3 x 109/L, an unusually high total protein level (100 g/L), and an IgM kappa paraprotein band of 45 g/L on protein electrophoresis. A computed tomography scan showed widespread lymphadenopathy, hepatosplenomegaly, and multilevel thoracic vertebral collapse. A bone marrow biopsy showed evidence of a lymphocytic infiltrate, with lymphoplasmacytoid differentiation.
The histology best fitted a diagnosis of nodal marginal zone lymphoma with plasmacytic differentiation, the clinicians say. Having ruled out other possibilities, they settled on non-Hodgkin lymphoma.
Initially, the suspicion was that the patient had septic arthritis due to Staphylococcus aureus (the most common organism isolated in septic arthritis), and he was given piperacillin/tazobactam. That was changed to flucloxacillin and then to ceftriaxone after the result of N meningitidis. The patient also was treated with rituximab and bendamustine for the lymphoma with a complete remission.
Meningococcal infection presenting as septic arthritis in the case of invasive meningococcemia is rare, the clinicians say, but primary meningococcal arthritis is even rarer. The case highlights the important aspect that “diagnosis of one condition can lead to diagnosis of another”—in this case, the lymphoma-weakened immune system led to the symptoms of polyarthropathy and the diagnosis of primary meningococcal arthritis. The clinicians also cited a case of a patient who presented with meningococcal meningitis and arthritis who was found to have an underlying Waldenström disease, and a patient whose HIV was diagnosed again after the patient presented with meningococcal arthritis symptoms.
The clinicans say such cases underscore the importance of screening for an underlying impaired immune response in patients presenting with rare conditions such as meningococcal arthritis.
For a group of clinicians in Australia, the diagnosis of meningococcal arthritis was “straightforward” except for abnormal serum total protein, anemia, and immunoglobulin results, which suggested their patient might have a hematological disorder such as myeloma.
The patient came to the hospital after 4 days of worsening knee and arm pain so severe he could not stand. His knees and both wrists showed swelling but no palpable lymphadenopathy or hepatosplenomegaly. The patient’s medical history showed he was taking no regular medications.
Joint aspiration grew Neisseria meningitidis. The patient’s blood tests showed hemoglobin 126 g/dL, white blood cell count 15.3 x 109/L, an unusually high total protein level (100 g/L), and an IgM kappa paraprotein band of 45 g/L on protein electrophoresis. A computed tomography scan showed widespread lymphadenopathy, hepatosplenomegaly, and multilevel thoracic vertebral collapse. A bone marrow biopsy showed evidence of a lymphocytic infiltrate, with lymphoplasmacytoid differentiation.
The histology best fitted a diagnosis of nodal marginal zone lymphoma with plasmacytic differentiation, the clinicians say. Having ruled out other possibilities, they settled on non-Hodgkin lymphoma.
Initially, the suspicion was that the patient had septic arthritis due to Staphylococcus aureus (the most common organism isolated in septic arthritis), and he was given piperacillin/tazobactam. That was changed to flucloxacillin and then to ceftriaxone after the result of N meningitidis. The patient also was treated with rituximab and bendamustine for the lymphoma with a complete remission.
Meningococcal infection presenting as septic arthritis in the case of invasive meningococcemia is rare, the clinicians say, but primary meningococcal arthritis is even rarer. The case highlights the important aspect that “diagnosis of one condition can lead to diagnosis of another”—in this case, the lymphoma-weakened immune system led to the symptoms of polyarthropathy and the diagnosis of primary meningococcal arthritis. The clinicians also cited a case of a patient who presented with meningococcal meningitis and arthritis who was found to have an underlying Waldenström disease, and a patient whose HIV was diagnosed again after the patient presented with meningococcal arthritis symptoms.
The clinicans say such cases underscore the importance of screening for an underlying impaired immune response in patients presenting with rare conditions such as meningococcal arthritis.
Three-drug combo delivers PFS for myeloma in OPTIMISMM trial
The addition of pomalidomide to bortezomib and low-dose dexamethasone showed a statistically significant improvement in progression-free survival for patients with relapsed/refractory multiple myeloma, compared with just the two agents, according to Celgene.
Celgene, which markets pomalidomide, announced the results from the phase 3 OPTIMISMM trial (NCT01734928) on Feb. 6. The company expects the results to be presented at future medical meetings, they said.
OPTIMISMM is the first phase 3 trial to examine a triple-drug combination for multiple myeloma patients who have all received prior lenalidomide, Celgene noted.
The pomalidomide/bortezomib/low-dose dexamethasone combination is not currently approved, but pomalidomide plus dexamethasone is approved for multiple myeloma patients who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor, and have shown disease progression within 60 days of last therapy.
The addition of pomalidomide to bortezomib and low-dose dexamethasone showed a statistically significant improvement in progression-free survival for patients with relapsed/refractory multiple myeloma, compared with just the two agents, according to Celgene.
Celgene, which markets pomalidomide, announced the results from the phase 3 OPTIMISMM trial (NCT01734928) on Feb. 6. The company expects the results to be presented at future medical meetings, they said.
OPTIMISMM is the first phase 3 trial to examine a triple-drug combination for multiple myeloma patients who have all received prior lenalidomide, Celgene noted.
The pomalidomide/bortezomib/low-dose dexamethasone combination is not currently approved, but pomalidomide plus dexamethasone is approved for multiple myeloma patients who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor, and have shown disease progression within 60 days of last therapy.
The addition of pomalidomide to bortezomib and low-dose dexamethasone showed a statistically significant improvement in progression-free survival for patients with relapsed/refractory multiple myeloma, compared with just the two agents, according to Celgene.
Celgene, which markets pomalidomide, announced the results from the phase 3 OPTIMISMM trial (NCT01734928) on Feb. 6. The company expects the results to be presented at future medical meetings, they said.
OPTIMISMM is the first phase 3 trial to examine a triple-drug combination for multiple myeloma patients who have all received prior lenalidomide, Celgene noted.
The pomalidomide/bortezomib/low-dose dexamethasone combination is not currently approved, but pomalidomide plus dexamethasone is approved for multiple myeloma patients who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor, and have shown disease progression within 60 days of last therapy.
Analysis reveals potential MM risk variants
Researchers have used high-risk pedigrees (HRPs) to identify gene variants that may cause multiple myeloma (MM).
The team’s analysis revealed shared genomic segments harboring genes with potential MM risk variants.
These single nucleotide variants (SNVs) are in USP45, a gene involved in DNA repair, and ARID1A, a gene in the SWI/SNF chromatin remodeling complex.
Nicola Camp, PhD, of the University of Utah School of Medicine in Salt Lake City, and her colleagues reported these findings in PLOS Genetics.
The researchers developed a new method to analyze HRPs (large, multi-generational families with more affected members than would be expected by chance) to identify shared regions of the genome that likely harbor MM risk variants.
The team applied the method using pedigrees from 11 Utah families at risk of MM as well as whole-exome sequencing of shared genomic segments in 1063 patients with MM or monoclonal gammopathy of undetermined significance (MGUS) and 964 control subjects.
The analysis revealed 2 regions that may contribute to MM. One was a 1.8 Mb segment at 6q16 with SNVs in USP45, and the other was a 1.2 Mb segment at 1p36.11 with SNVs in ARID1A.
One of the SNVs in USP45 was a stop gain—p.Gln691*—which was shared by 3 siblings, 1 with MM and 2 with MGUS. The other was a missense SNV—p.Gln621Glu—which was shared by 2 of 4 siblings in a family, 1 with MM and 1 with MGUS.
One of the missense SNVs in ARID1A—rs752026201, p. Ser90Gly—was shared by 3 MM cases. The other missense SNV—rs140664170, p.Met890Val—was shared by 2 cousins with MM.
The researchers believe these findings show that HRPs can be effective for identifying risk variants in complex diseases.
“We are very encouraged by the new method,” Dr Camp said. “It certainly plays to the strengths of the large Utah pedigrees, revitalizing the family design for complex diseases. As we did in this study, the focused regions can be further investigated in smaller families to find genes and specific mutations. The method can be used for any complex disease.”
“We are already pursuing large pedigrees in several other domains, including other cancers, psychiatric disorders, birth defects, and pre-term birth phenotypes, with several more genome-wide significant regions found. We’re excited about the potential.” ![]()
Researchers have used high-risk pedigrees (HRPs) to identify gene variants that may cause multiple myeloma (MM).
The team’s analysis revealed shared genomic segments harboring genes with potential MM risk variants.
These single nucleotide variants (SNVs) are in USP45, a gene involved in DNA repair, and ARID1A, a gene in the SWI/SNF chromatin remodeling complex.
Nicola Camp, PhD, of the University of Utah School of Medicine in Salt Lake City, and her colleagues reported these findings in PLOS Genetics.
The researchers developed a new method to analyze HRPs (large, multi-generational families with more affected members than would be expected by chance) to identify shared regions of the genome that likely harbor MM risk variants.
The team applied the method using pedigrees from 11 Utah families at risk of MM as well as whole-exome sequencing of shared genomic segments in 1063 patients with MM or monoclonal gammopathy of undetermined significance (MGUS) and 964 control subjects.
The analysis revealed 2 regions that may contribute to MM. One was a 1.8 Mb segment at 6q16 with SNVs in USP45, and the other was a 1.2 Mb segment at 1p36.11 with SNVs in ARID1A.
One of the SNVs in USP45 was a stop gain—p.Gln691*—which was shared by 3 siblings, 1 with MM and 2 with MGUS. The other was a missense SNV—p.Gln621Glu—which was shared by 2 of 4 siblings in a family, 1 with MM and 1 with MGUS.
One of the missense SNVs in ARID1A—rs752026201, p. Ser90Gly—was shared by 3 MM cases. The other missense SNV—rs140664170, p.Met890Val—was shared by 2 cousins with MM.
The researchers believe these findings show that HRPs can be effective for identifying risk variants in complex diseases.
“We are very encouraged by the new method,” Dr Camp said. “It certainly plays to the strengths of the large Utah pedigrees, revitalizing the family design for complex diseases. As we did in this study, the focused regions can be further investigated in smaller families to find genes and specific mutations. The method can be used for any complex disease.”
“We are already pursuing large pedigrees in several other domains, including other cancers, psychiatric disorders, birth defects, and pre-term birth phenotypes, with several more genome-wide significant regions found. We’re excited about the potential.” ![]()
Researchers have used high-risk pedigrees (HRPs) to identify gene variants that may cause multiple myeloma (MM).
The team’s analysis revealed shared genomic segments harboring genes with potential MM risk variants.
These single nucleotide variants (SNVs) are in USP45, a gene involved in DNA repair, and ARID1A, a gene in the SWI/SNF chromatin remodeling complex.
Nicola Camp, PhD, of the University of Utah School of Medicine in Salt Lake City, and her colleagues reported these findings in PLOS Genetics.
The researchers developed a new method to analyze HRPs (large, multi-generational families with more affected members than would be expected by chance) to identify shared regions of the genome that likely harbor MM risk variants.
The team applied the method using pedigrees from 11 Utah families at risk of MM as well as whole-exome sequencing of shared genomic segments in 1063 patients with MM or monoclonal gammopathy of undetermined significance (MGUS) and 964 control subjects.
The analysis revealed 2 regions that may contribute to MM. One was a 1.8 Mb segment at 6q16 with SNVs in USP45, and the other was a 1.2 Mb segment at 1p36.11 with SNVs in ARID1A.
One of the SNVs in USP45 was a stop gain—p.Gln691*—which was shared by 3 siblings, 1 with MM and 2 with MGUS. The other was a missense SNV—p.Gln621Glu—which was shared by 2 of 4 siblings in a family, 1 with MM and 1 with MGUS.
One of the missense SNVs in ARID1A—rs752026201, p. Ser90Gly—was shared by 3 MM cases. The other missense SNV—rs140664170, p.Met890Val—was shared by 2 cousins with MM.
The researchers believe these findings show that HRPs can be effective for identifying risk variants in complex diseases.
“We are very encouraged by the new method,” Dr Camp said. “It certainly plays to the strengths of the large Utah pedigrees, revitalizing the family design for complex diseases. As we did in this study, the focused regions can be further investigated in smaller families to find genes and specific mutations. The method can be used for any complex disease.”
“We are already pursuing large pedigrees in several other domains, including other cancers, psychiatric disorders, birth defects, and pre-term birth phenotypes, with several more genome-wide significant regions found. We’re excited about the potential.” ![]()
ASCO expands recommendations on bone-modifying agents in myeloma
Bisphosphonates should be prescribed for any patient receiving treatment for active multiple myeloma, regardless of whether or not there is evidence of lytic bone destruction or spinal compression fracture, according to updated guidelines from the American Society of Clinical Oncology.
Previous guidelines from the society, last updated in 2007, recommended the use of intravenous bisphosphonates for patients with myeloma with evidence of bone disease, according to the expert panel that drafted the update.
“Fewer adverse events related to renal toxicity have been noted with denosumab, compared with zoledronic acid,” and “this may be preferred in patients with compromised renal function,” wrote the expert panel, led by cochairs Kenneth C. Anderson, MD, of Dana-Farber Cancer Institute, Boston, and Robert A. Kyle, MD, of Mayo Clinic, Rochester, Minn.
ASCO guidelines on bisphosphonates in myeloma were first drafted in 2002 and then updated in 2007. The new recommendations on bone-modifying therapy in myeloma are based on review of an additional 35 publications. The new guidelines are “consistent with the previous recommendations” while updating indications for therapy and information on denosumab, according to the expert panel.
Evidence that myeloma patients without lytic bone disease will benefit from intravenous bisphosphonates comes from the randomized MRC IX trial, in which patients who received zoledronic acid had reduced skeletal-related events at relapse and improved progression-free survival.
Denosumab, a receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor, was noninferior to zoledronic acid for prevention of skeletal-related events in a randomized phase 3 clinical trial; however, it is “more expensive than zoledronic acid or pamidronate and must be considered in treatment decisions,” the guidelines authors wrote.
The total price in the United States for a 1-year treatment cycle of denosumab is just under $26,000, according to data included in the ASCO guideline. By comparison, the 1-year treatment cycle price for the bisphosphonates ranges from $214 to $697, depending on the regimen.
When intravenous bisphosphonate therapy is warranted, the guideline-recommended schedule is infusion of zoledronic acid 4 mg over at least 15 minutes, or pamidronate 90 mg over 2 hours, every 3-4 weeks.
The guidelines also address osteonecrosis of the jaw (ONJ), a major complication observed not only with the potent bisphosphonates pamidronate and zoledronic acid, but also with denosumab.
The panel said they were in agreement with revised labels from the Food and Drug Administration for zoledronic acid and pamidronate, among other papers or statements addressing ONJ and noted that patients need a comprehensive dental exam and preventive dentistry as appropriate before starting bone-modifying therapy.
“The risk of ONJ has prompted the use of less-frequent dosing of zoledronic acid, which may be an option for patients,” they said in their report.
Guideline authors reported ties to Amgen, Celgene, Millennium Pharmaceuticals, Gilead Sciences, Bristol-Myers Squibb, Novartis, Pfizer, and others.
SOURCE: Anderson K et al. J Clin Oncol. 2018 Jan 17:JCO2017766402. doi: 10.1200/JCO.2017.76.6402.
Bisphosphonates should be prescribed for any patient receiving treatment for active multiple myeloma, regardless of whether or not there is evidence of lytic bone destruction or spinal compression fracture, according to updated guidelines from the American Society of Clinical Oncology.
Previous guidelines from the society, last updated in 2007, recommended the use of intravenous bisphosphonates for patients with myeloma with evidence of bone disease, according to the expert panel that drafted the update.
“Fewer adverse events related to renal toxicity have been noted with denosumab, compared with zoledronic acid,” and “this may be preferred in patients with compromised renal function,” wrote the expert panel, led by cochairs Kenneth C. Anderson, MD, of Dana-Farber Cancer Institute, Boston, and Robert A. Kyle, MD, of Mayo Clinic, Rochester, Minn.
ASCO guidelines on bisphosphonates in myeloma were first drafted in 2002 and then updated in 2007. The new recommendations on bone-modifying therapy in myeloma are based on review of an additional 35 publications. The new guidelines are “consistent with the previous recommendations” while updating indications for therapy and information on denosumab, according to the expert panel.
Evidence that myeloma patients without lytic bone disease will benefit from intravenous bisphosphonates comes from the randomized MRC IX trial, in which patients who received zoledronic acid had reduced skeletal-related events at relapse and improved progression-free survival.
Denosumab, a receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor, was noninferior to zoledronic acid for prevention of skeletal-related events in a randomized phase 3 clinical trial; however, it is “more expensive than zoledronic acid or pamidronate and must be considered in treatment decisions,” the guidelines authors wrote.
The total price in the United States for a 1-year treatment cycle of denosumab is just under $26,000, according to data included in the ASCO guideline. By comparison, the 1-year treatment cycle price for the bisphosphonates ranges from $214 to $697, depending on the regimen.
When intravenous bisphosphonate therapy is warranted, the guideline-recommended schedule is infusion of zoledronic acid 4 mg over at least 15 minutes, or pamidronate 90 mg over 2 hours, every 3-4 weeks.
The guidelines also address osteonecrosis of the jaw (ONJ), a major complication observed not only with the potent bisphosphonates pamidronate and zoledronic acid, but also with denosumab.
The panel said they were in agreement with revised labels from the Food and Drug Administration for zoledronic acid and pamidronate, among other papers or statements addressing ONJ and noted that patients need a comprehensive dental exam and preventive dentistry as appropriate before starting bone-modifying therapy.
“The risk of ONJ has prompted the use of less-frequent dosing of zoledronic acid, which may be an option for patients,” they said in their report.
Guideline authors reported ties to Amgen, Celgene, Millennium Pharmaceuticals, Gilead Sciences, Bristol-Myers Squibb, Novartis, Pfizer, and others.
SOURCE: Anderson K et al. J Clin Oncol. 2018 Jan 17:JCO2017766402. doi: 10.1200/JCO.2017.76.6402.
Bisphosphonates should be prescribed for any patient receiving treatment for active multiple myeloma, regardless of whether or not there is evidence of lytic bone destruction or spinal compression fracture, according to updated guidelines from the American Society of Clinical Oncology.
Previous guidelines from the society, last updated in 2007, recommended the use of intravenous bisphosphonates for patients with myeloma with evidence of bone disease, according to the expert panel that drafted the update.
“Fewer adverse events related to renal toxicity have been noted with denosumab, compared with zoledronic acid,” and “this may be preferred in patients with compromised renal function,” wrote the expert panel, led by cochairs Kenneth C. Anderson, MD, of Dana-Farber Cancer Institute, Boston, and Robert A. Kyle, MD, of Mayo Clinic, Rochester, Minn.
ASCO guidelines on bisphosphonates in myeloma were first drafted in 2002 and then updated in 2007. The new recommendations on bone-modifying therapy in myeloma are based on review of an additional 35 publications. The new guidelines are “consistent with the previous recommendations” while updating indications for therapy and information on denosumab, according to the expert panel.
Evidence that myeloma patients without lytic bone disease will benefit from intravenous bisphosphonates comes from the randomized MRC IX trial, in which patients who received zoledronic acid had reduced skeletal-related events at relapse and improved progression-free survival.
Denosumab, a receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor, was noninferior to zoledronic acid for prevention of skeletal-related events in a randomized phase 3 clinical trial; however, it is “more expensive than zoledronic acid or pamidronate and must be considered in treatment decisions,” the guidelines authors wrote.
The total price in the United States for a 1-year treatment cycle of denosumab is just under $26,000, according to data included in the ASCO guideline. By comparison, the 1-year treatment cycle price for the bisphosphonates ranges from $214 to $697, depending on the regimen.
When intravenous bisphosphonate therapy is warranted, the guideline-recommended schedule is infusion of zoledronic acid 4 mg over at least 15 minutes, or pamidronate 90 mg over 2 hours, every 3-4 weeks.
The guidelines also address osteonecrosis of the jaw (ONJ), a major complication observed not only with the potent bisphosphonates pamidronate and zoledronic acid, but also with denosumab.
The panel said they were in agreement with revised labels from the Food and Drug Administration for zoledronic acid and pamidronate, among other papers or statements addressing ONJ and noted that patients need a comprehensive dental exam and preventive dentistry as appropriate before starting bone-modifying therapy.
“The risk of ONJ has prompted the use of less-frequent dosing of zoledronic acid, which may be an option for patients,” they said in their report.
Guideline authors reported ties to Amgen, Celgene, Millennium Pharmaceuticals, Gilead Sciences, Bristol-Myers Squibb, Novartis, Pfizer, and others.
SOURCE: Anderson K et al. J Clin Oncol. 2018 Jan 17:JCO2017766402. doi: 10.1200/JCO.2017.76.6402.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Real-world study makes case for continuous treatment in multiple myeloma
Longer duration of second-line therapy is strongly linked to improved 1-year overall survival in patients with relapsed and refractory multiple myeloma, according to findings from a real-world analysis.
The retrospective, observational study evaluated outcomes in 628 patients with multiple myeloma who relapsed after initial treatment. Parameswaran Hari, MD, of the Medical College of Wisconsin in Milwaukee and his colleagues sought to test the growing consensus that prolonged therapy is the best approach in a cohort of patients who received care in a routine setting and were older and had more comorbidities than patients in clinical trials.
Median overall survival was 41 months (95% confidence interval, 32.1-59.5) among the 628 relapsed/refractory patients who started second-line therapy. Each extra month of second-line therapy was associated with a reduced risk of death at 1 year (adjusted odds ratio, 0.78; 95% CI, 0.77-0.83; P less than .001). the median duration of second-line therapy for patients in the study was 6.9 months. When researchers extended the duration to 11 months, it was correlated with a 12.7% higher 1-year overall survival probability.
Age was an important factor in survival. The 1-year mortality was significantly lower in patients under 75 years (OR, 0.37; 95% CI, 0.20-0.68).
“Despite substantial heterogeneity in patient and disease characteristics and treatment patterns, the clinical benefit of continued longer-term therapy at relapse appears to be generalizable to patients receiving care in the routine care settings,” the researchers wrote.
Takeda funded the study and four of the authors are Takeda employees. Dr. Hari reported fees from Takeda and several other pharmaceutical companies.
SOURCE: Hari P et al. Clin Lymphoma Myeloma Leuk. 2018 Jan 5. doi: 10.1016/j.clml.2017.12.012.
Longer duration of second-line therapy is strongly linked to improved 1-year overall survival in patients with relapsed and refractory multiple myeloma, according to findings from a real-world analysis.
The retrospective, observational study evaluated outcomes in 628 patients with multiple myeloma who relapsed after initial treatment. Parameswaran Hari, MD, of the Medical College of Wisconsin in Milwaukee and his colleagues sought to test the growing consensus that prolonged therapy is the best approach in a cohort of patients who received care in a routine setting and were older and had more comorbidities than patients in clinical trials.
Median overall survival was 41 months (95% confidence interval, 32.1-59.5) among the 628 relapsed/refractory patients who started second-line therapy. Each extra month of second-line therapy was associated with a reduced risk of death at 1 year (adjusted odds ratio, 0.78; 95% CI, 0.77-0.83; P less than .001). the median duration of second-line therapy for patients in the study was 6.9 months. When researchers extended the duration to 11 months, it was correlated with a 12.7% higher 1-year overall survival probability.
Age was an important factor in survival. The 1-year mortality was significantly lower in patients under 75 years (OR, 0.37; 95% CI, 0.20-0.68).
“Despite substantial heterogeneity in patient and disease characteristics and treatment patterns, the clinical benefit of continued longer-term therapy at relapse appears to be generalizable to patients receiving care in the routine care settings,” the researchers wrote.
Takeda funded the study and four of the authors are Takeda employees. Dr. Hari reported fees from Takeda and several other pharmaceutical companies.
SOURCE: Hari P et al. Clin Lymphoma Myeloma Leuk. 2018 Jan 5. doi: 10.1016/j.clml.2017.12.012.
Longer duration of second-line therapy is strongly linked to improved 1-year overall survival in patients with relapsed and refractory multiple myeloma, according to findings from a real-world analysis.
The retrospective, observational study evaluated outcomes in 628 patients with multiple myeloma who relapsed after initial treatment. Parameswaran Hari, MD, of the Medical College of Wisconsin in Milwaukee and his colleagues sought to test the growing consensus that prolonged therapy is the best approach in a cohort of patients who received care in a routine setting and were older and had more comorbidities than patients in clinical trials.
Median overall survival was 41 months (95% confidence interval, 32.1-59.5) among the 628 relapsed/refractory patients who started second-line therapy. Each extra month of second-line therapy was associated with a reduced risk of death at 1 year (adjusted odds ratio, 0.78; 95% CI, 0.77-0.83; P less than .001). the median duration of second-line therapy for patients in the study was 6.9 months. When researchers extended the duration to 11 months, it was correlated with a 12.7% higher 1-year overall survival probability.
Age was an important factor in survival. The 1-year mortality was significantly lower in patients under 75 years (OR, 0.37; 95% CI, 0.20-0.68).
“Despite substantial heterogeneity in patient and disease characteristics and treatment patterns, the clinical benefit of continued longer-term therapy at relapse appears to be generalizable to patients receiving care in the routine care settings,” the researchers wrote.
Takeda funded the study and four of the authors are Takeda employees. Dr. Hari reported fees from Takeda and several other pharmaceutical companies.
SOURCE: Hari P et al. Clin Lymphoma Myeloma Leuk. 2018 Jan 5. doi: 10.1016/j.clml.2017.12.012.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: Each additional month of second-line therapy was associated with a reduced risk of death at 1 year (aOR, 0.78).
Study details: A retrospective study of 628 newly diagnosed multiple myeloma patients between January 2008 and June 2015.
Disclosures: Takeda funded the study and four of the authors are Takeda employees. Dr. Hari reported fees from Takeda and several other pharmaceutical companies.
Source: Hari P et al. Clin Lymphoma Myeloma Leuk. 2018 Jan 5. doi: 10.1016/j.clml.2017.12.012.
Phototherapeutic technology could fight MM, other cancers
Preclinical research suggests that light-triggered, chemotherapy-loaded nanoparticles could treat multiple myeloma (MM) and other malignancies.
Researchers showed that light emitted as part of traditional cancer-imaging techniques could also trigger a light-sensitive chemotherapy drug.
When this drug was packaged into nanoparticles that target lit-up cancer cells, the drug produced toxic free radicals that killed the cancer cells.
Researchers found this technique to be effective in mice with MM and aggressive, metastatic breast cancer.
“Our study shows that this phototherapeutic technology is particularly suited to attacking small tumors that spread to different parts of the body, including deep in the bone marrow,” said Samuel Achilefu, PhD, of Washington University in St. Louis, Missouri.
Dr Achilefu and his colleagues described the technology in Nature Communications.
The technology harnesses the chemotherapy drug titanocene. When used alone, titanocene did not work well in clinical trials, even at relatively high doses. However, when it is exposed to the radiation emitted by visible light, titanocene produces reactive particles that are toxic to cells, even at low doses.
Dr Achilefu and his colleagues packaged low doses of titanocene inside nanoparticles targeted to proteins on the surface of cancer cells. Specifically, the team used nanomicelles targeting VLA-4, “an attractive target for precision imaging and therapy” in MM, according to the researchers.
When these nanomicelles made contact with MM cells, their membranes fused together, releasing titanocene into the cells.
The researchers then delivered the imaging agent fluorodeoxyglucose (FDG). MM cells took up the FDG at high rates, causing the cells to glow in a positron emission tomography scan. This glow also triggered the titanocene, releasing free radicals and killing the MM cells.
This treatment strategy was used on mice with MM once a week for 4 weeks. In the weeks following, the treated mice had significantly smaller tumors and survived longer than control mice. Fifty percent of treated mice survived at least 90 days, and 50% of control mice survived 62 days.
This strategy also produced an anti-tumor effect in mice with breast cancer, although, in these experiments, the researchers used human serum albumin nanoparticles.
The effect in breast cancer was less pronounced than in MM. The researchers said this was likely due to the extreme aggressiveness of the breast cancer cell line used.
The team also found that certain MM cells were resistant to this treatment technique.
“This is an opportunity to learn because it’s similar to what is seen in patients—some of the cells become dormant but don’t die after treatment,” Dr Achilefu said. “When we looked closer at the cells that were resistant to our phototherapy, we saw that the surface protein we are targeting was not there.”
Specifically, the resistant cells had downregulated expression of CD49d, and the researchers believe this may have impaired the binding of nanomicelles to the MM cells.
“So next, we want to find out if we can pinpoint another surface protein to target and kill these resistant cells along with the myeloma cells that did respond to the original therapy, which could lead to complete remission,” Dr Achilefu said.
Furthermore, Dr Achilefu envisions that, one day, doctors might be able to use this technology to prevent cancer from recurring.
“We are interested in exploring whether this is something a patient in remission could take once a year for prevention,” Dr Achilefu said. “The toxicity appears to be low, so we imagine an outpatient procedure that could involve zapping any cancerous cells, making cancer a chronic condition that could be controlled long-term.”
Dr Achilefu and his colleagues believe this phototherapeutic technology is less toxic than standard radiation and chemotherapy because the titanocene and FDG are targeted to the same place at the same time only in cancer cells.
The body rids itself of titanocene through the liver, while FDG is cleared through the kidneys. The fact that these components are disposed of separately minimizes damage to other organs. When separated, the components are not toxic, according to the researchers. ![]()
Preclinical research suggests that light-triggered, chemotherapy-loaded nanoparticles could treat multiple myeloma (MM) and other malignancies.
Researchers showed that light emitted as part of traditional cancer-imaging techniques could also trigger a light-sensitive chemotherapy drug.
When this drug was packaged into nanoparticles that target lit-up cancer cells, the drug produced toxic free radicals that killed the cancer cells.
Researchers found this technique to be effective in mice with MM and aggressive, metastatic breast cancer.
“Our study shows that this phototherapeutic technology is particularly suited to attacking small tumors that spread to different parts of the body, including deep in the bone marrow,” said Samuel Achilefu, PhD, of Washington University in St. Louis, Missouri.
Dr Achilefu and his colleagues described the technology in Nature Communications.
The technology harnesses the chemotherapy drug titanocene. When used alone, titanocene did not work well in clinical trials, even at relatively high doses. However, when it is exposed to the radiation emitted by visible light, titanocene produces reactive particles that are toxic to cells, even at low doses.
Dr Achilefu and his colleagues packaged low doses of titanocene inside nanoparticles targeted to proteins on the surface of cancer cells. Specifically, the team used nanomicelles targeting VLA-4, “an attractive target for precision imaging and therapy” in MM, according to the researchers.
When these nanomicelles made contact with MM cells, their membranes fused together, releasing titanocene into the cells.
The researchers then delivered the imaging agent fluorodeoxyglucose (FDG). MM cells took up the FDG at high rates, causing the cells to glow in a positron emission tomography scan. This glow also triggered the titanocene, releasing free radicals and killing the MM cells.
This treatment strategy was used on mice with MM once a week for 4 weeks. In the weeks following, the treated mice had significantly smaller tumors and survived longer than control mice. Fifty percent of treated mice survived at least 90 days, and 50% of control mice survived 62 days.
This strategy also produced an anti-tumor effect in mice with breast cancer, although, in these experiments, the researchers used human serum albumin nanoparticles.
The effect in breast cancer was less pronounced than in MM. The researchers said this was likely due to the extreme aggressiveness of the breast cancer cell line used.
The team also found that certain MM cells were resistant to this treatment technique.
“This is an opportunity to learn because it’s similar to what is seen in patients—some of the cells become dormant but don’t die after treatment,” Dr Achilefu said. “When we looked closer at the cells that were resistant to our phototherapy, we saw that the surface protein we are targeting was not there.”
Specifically, the resistant cells had downregulated expression of CD49d, and the researchers believe this may have impaired the binding of nanomicelles to the MM cells.
“So next, we want to find out if we can pinpoint another surface protein to target and kill these resistant cells along with the myeloma cells that did respond to the original therapy, which could lead to complete remission,” Dr Achilefu said.
Furthermore, Dr Achilefu envisions that, one day, doctors might be able to use this technology to prevent cancer from recurring.
“We are interested in exploring whether this is something a patient in remission could take once a year for prevention,” Dr Achilefu said. “The toxicity appears to be low, so we imagine an outpatient procedure that could involve zapping any cancerous cells, making cancer a chronic condition that could be controlled long-term.”
Dr Achilefu and his colleagues believe this phototherapeutic technology is less toxic than standard radiation and chemotherapy because the titanocene and FDG are targeted to the same place at the same time only in cancer cells.
The body rids itself of titanocene through the liver, while FDG is cleared through the kidneys. The fact that these components are disposed of separately minimizes damage to other organs. When separated, the components are not toxic, according to the researchers. ![]()
Preclinical research suggests that light-triggered, chemotherapy-loaded nanoparticles could treat multiple myeloma (MM) and other malignancies.
Researchers showed that light emitted as part of traditional cancer-imaging techniques could also trigger a light-sensitive chemotherapy drug.
When this drug was packaged into nanoparticles that target lit-up cancer cells, the drug produced toxic free radicals that killed the cancer cells.
Researchers found this technique to be effective in mice with MM and aggressive, metastatic breast cancer.
“Our study shows that this phototherapeutic technology is particularly suited to attacking small tumors that spread to different parts of the body, including deep in the bone marrow,” said Samuel Achilefu, PhD, of Washington University in St. Louis, Missouri.
Dr Achilefu and his colleagues described the technology in Nature Communications.
The technology harnesses the chemotherapy drug titanocene. When used alone, titanocene did not work well in clinical trials, even at relatively high doses. However, when it is exposed to the radiation emitted by visible light, titanocene produces reactive particles that are toxic to cells, even at low doses.
Dr Achilefu and his colleagues packaged low doses of titanocene inside nanoparticles targeted to proteins on the surface of cancer cells. Specifically, the team used nanomicelles targeting VLA-4, “an attractive target for precision imaging and therapy” in MM, according to the researchers.
When these nanomicelles made contact with MM cells, their membranes fused together, releasing titanocene into the cells.
The researchers then delivered the imaging agent fluorodeoxyglucose (FDG). MM cells took up the FDG at high rates, causing the cells to glow in a positron emission tomography scan. This glow also triggered the titanocene, releasing free radicals and killing the MM cells.
This treatment strategy was used on mice with MM once a week for 4 weeks. In the weeks following, the treated mice had significantly smaller tumors and survived longer than control mice. Fifty percent of treated mice survived at least 90 days, and 50% of control mice survived 62 days.
This strategy also produced an anti-tumor effect in mice with breast cancer, although, in these experiments, the researchers used human serum albumin nanoparticles.
The effect in breast cancer was less pronounced than in MM. The researchers said this was likely due to the extreme aggressiveness of the breast cancer cell line used.
The team also found that certain MM cells were resistant to this treatment technique.
“This is an opportunity to learn because it’s similar to what is seen in patients—some of the cells become dormant but don’t die after treatment,” Dr Achilefu said. “When we looked closer at the cells that were resistant to our phototherapy, we saw that the surface protein we are targeting was not there.”
Specifically, the resistant cells had downregulated expression of CD49d, and the researchers believe this may have impaired the binding of nanomicelles to the MM cells.
“So next, we want to find out if we can pinpoint another surface protein to target and kill these resistant cells along with the myeloma cells that did respond to the original therapy, which could lead to complete remission,” Dr Achilefu said.
Furthermore, Dr Achilefu envisions that, one day, doctors might be able to use this technology to prevent cancer from recurring.
“We are interested in exploring whether this is something a patient in remission could take once a year for prevention,” Dr Achilefu said. “The toxicity appears to be low, so we imagine an outpatient procedure that could involve zapping any cancerous cells, making cancer a chronic condition that could be controlled long-term.”
Dr Achilefu and his colleagues believe this phototherapeutic technology is less toxic than standard radiation and chemotherapy because the titanocene and FDG are targeted to the same place at the same time only in cancer cells.
The body rids itself of titanocene through the liver, while FDG is cleared through the kidneys. The fact that these components are disposed of separately minimizes damage to other organs. When separated, the components are not toxic, according to the researchers. ![]()
FDA clears assay for myeloma patients
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Sebia’s Hydrashift 2/4 daratumumab immunofixation assay.
This in vitro diagnostic test allows for assessment of response in patients with multiple myeloma by mitigating potential interference caused by the anti-CD38 antibody daratumumab (Darzalex®).
Daratumumab can interfere with the visualization of M-proteins in immunofixation electrophoresis.
The Hydrashift 2/4 daratumumab assay is intended to be used with Sebia’s Hydragel IF kit to provide qualitative detection of monoclonal proteins in human serum by immunofixation electrophoresis.
The assay is performed on Sebia’s Hydrasys 2 agarose gel platform.
The Hydrashift 2/4 daratumumab assay is the result of a collaboration between Sebia and Janssen Biotech, Inc. Sebia received development rights from Janssen and is the worldwide supplier of this assay.
The Hydrashift 2/4 daratumumab assay received the CE mark in November 2016. ![]()
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Sebia’s Hydrashift 2/4 daratumumab immunofixation assay.
This in vitro diagnostic test allows for assessment of response in patients with multiple myeloma by mitigating potential interference caused by the anti-CD38 antibody daratumumab (Darzalex®).
Daratumumab can interfere with the visualization of M-proteins in immunofixation electrophoresis.
The Hydrashift 2/4 daratumumab assay is intended to be used with Sebia’s Hydragel IF kit to provide qualitative detection of monoclonal proteins in human serum by immunofixation electrophoresis.
The assay is performed on Sebia’s Hydrasys 2 agarose gel platform.
The Hydrashift 2/4 daratumumab assay is the result of a collaboration between Sebia and Janssen Biotech, Inc. Sebia received development rights from Janssen and is the worldwide supplier of this assay.
The Hydrashift 2/4 daratumumab assay received the CE mark in November 2016. ![]()
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Sebia’s Hydrashift 2/4 daratumumab immunofixation assay.
This in vitro diagnostic test allows for assessment of response in patients with multiple myeloma by mitigating potential interference caused by the anti-CD38 antibody daratumumab (Darzalex®).
Daratumumab can interfere with the visualization of M-proteins in immunofixation electrophoresis.
The Hydrashift 2/4 daratumumab assay is intended to be used with Sebia’s Hydragel IF kit to provide qualitative detection of monoclonal proteins in human serum by immunofixation electrophoresis.
The assay is performed on Sebia’s Hydrasys 2 agarose gel platform.
The Hydrashift 2/4 daratumumab assay is the result of a collaboration between Sebia and Janssen Biotech, Inc. Sebia received development rights from Janssen and is the worldwide supplier of this assay.
The Hydrashift 2/4 daratumumab assay received the CE mark in November 2016. ![]()
Treatment costs threaten cancer program growth
Treatment costs are the greatest threat to the growth of cancer programs, according to a survey of nearly 300 cancer program administrators and providers.
Sixty-eight percent of survey respondents said treatment costs were among the biggest threats to future cancer program growth at their organization.
Other top threats to growth included physician alignment around services and program goals—cited by 47% of respondents—and changes in healthcare coverage—cited by 46%.
This survey—the “2017 Trending Now in Cancer Care Survey”—was conducted by the Association of Community Cancer Centers (ACCC) and Advisory Board’s Oncology Roundtable. It was supported by Pfizer Oncology.
The survey was distributed via email on July 24, 2017. Respondents included 293 cancer program administrators and providers from 209 institutions. They submitted responses over 6 weeks.
Respondents identified the following “biggest threats” to cancer program growth:
- Cost of drugs and/or new treatment modalities—68%
- Physician alignment around services and program goals—47%
- Changes in healthcare coverage—46%
- Cuts to fee-for-service reimbursement—44%
- Shifting reimbursement to value-based care—43%
- Marketplace competition—35%
- Workforce planning (eg, managing staff shortages)—34%
- Network strategy and integration—33%
- Site of care policies issued by private payers—27%
- Access to capital—26%
- Quality reporting requirements—22%
- Health information technology—21%
- Other—6%.
When asked to identify their greatest opportunities for cost savings, respondents overwhelmingly pointed toward clinical standardization (63%) and drugs (62%).
Other opportunities included:
- Supplies—28%
- Capital expenses (eg, imaging technology)—24%
- Non-clinical staff (eg, billing and coding specialists)—22%
- Technology maintenance—20%
- Clinical staff—16%
- Retail pharmacy—14%
- Clinical research—10%
- Support services (eg, acupuncture)—9%
- Other—4%.
Respondents also said the investments most likely to yield a return for their cancer program were:
- Increasing the number of sub-specialists (eg, breast surgeons)—59%
- Marketing—41%
- Specialty pharmacy—36%
- Increasing the number of general oncology physicians—34%
- Screening services (eg, mobile screening unit)—29%
- Capital investments—24%
- Clinical research—16%
- Support services—15%
- Retail pharmacy—14%
- Building upgrades—14%.
More details on the “2017 Trending Now in Cancer Care Survey” can be found on the ACCC website. ![]()
Treatment costs are the greatest threat to the growth of cancer programs, according to a survey of nearly 300 cancer program administrators and providers.
Sixty-eight percent of survey respondents said treatment costs were among the biggest threats to future cancer program growth at their organization.
Other top threats to growth included physician alignment around services and program goals—cited by 47% of respondents—and changes in healthcare coverage—cited by 46%.
This survey—the “2017 Trending Now in Cancer Care Survey”—was conducted by the Association of Community Cancer Centers (ACCC) and Advisory Board’s Oncology Roundtable. It was supported by Pfizer Oncology.
The survey was distributed via email on July 24, 2017. Respondents included 293 cancer program administrators and providers from 209 institutions. They submitted responses over 6 weeks.
Respondents identified the following “biggest threats” to cancer program growth:
- Cost of drugs and/or new treatment modalities—68%
- Physician alignment around services and program goals—47%
- Changes in healthcare coverage—46%
- Cuts to fee-for-service reimbursement—44%
- Shifting reimbursement to value-based care—43%
- Marketplace competition—35%
- Workforce planning (eg, managing staff shortages)—34%
- Network strategy and integration—33%
- Site of care policies issued by private payers—27%
- Access to capital—26%
- Quality reporting requirements—22%
- Health information technology—21%
- Other—6%.
When asked to identify their greatest opportunities for cost savings, respondents overwhelmingly pointed toward clinical standardization (63%) and drugs (62%).
Other opportunities included:
- Supplies—28%
- Capital expenses (eg, imaging technology)—24%
- Non-clinical staff (eg, billing and coding specialists)—22%
- Technology maintenance—20%
- Clinical staff—16%
- Retail pharmacy—14%
- Clinical research—10%
- Support services (eg, acupuncture)—9%
- Other—4%.
Respondents also said the investments most likely to yield a return for their cancer program were:
- Increasing the number of sub-specialists (eg, breast surgeons)—59%
- Marketing—41%
- Specialty pharmacy—36%
- Increasing the number of general oncology physicians—34%
- Screening services (eg, mobile screening unit)—29%
- Capital investments—24%
- Clinical research—16%
- Support services—15%
- Retail pharmacy—14%
- Building upgrades—14%.
More details on the “2017 Trending Now in Cancer Care Survey” can be found on the ACCC website. ![]()
Treatment costs are the greatest threat to the growth of cancer programs, according to a survey of nearly 300 cancer program administrators and providers.
Sixty-eight percent of survey respondents said treatment costs were among the biggest threats to future cancer program growth at their organization.
Other top threats to growth included physician alignment around services and program goals—cited by 47% of respondents—and changes in healthcare coverage—cited by 46%.
This survey—the “2017 Trending Now in Cancer Care Survey”—was conducted by the Association of Community Cancer Centers (ACCC) and Advisory Board’s Oncology Roundtable. It was supported by Pfizer Oncology.
The survey was distributed via email on July 24, 2017. Respondents included 293 cancer program administrators and providers from 209 institutions. They submitted responses over 6 weeks.
Respondents identified the following “biggest threats” to cancer program growth:
- Cost of drugs and/or new treatment modalities—68%
- Physician alignment around services and program goals—47%
- Changes in healthcare coverage—46%
- Cuts to fee-for-service reimbursement—44%
- Shifting reimbursement to value-based care—43%
- Marketplace competition—35%
- Workforce planning (eg, managing staff shortages)—34%
- Network strategy and integration—33%
- Site of care policies issued by private payers—27%
- Access to capital—26%
- Quality reporting requirements—22%
- Health information technology—21%
- Other—6%.
When asked to identify their greatest opportunities for cost savings, respondents overwhelmingly pointed toward clinical standardization (63%) and drugs (62%).
Other opportunities included:
- Supplies—28%
- Capital expenses (eg, imaging technology)—24%
- Non-clinical staff (eg, billing and coding specialists)—22%
- Technology maintenance—20%
- Clinical staff—16%
- Retail pharmacy—14%
- Clinical research—10%
- Support services (eg, acupuncture)—9%
- Other—4%.
Respondents also said the investments most likely to yield a return for their cancer program were:
- Increasing the number of sub-specialists (eg, breast surgeons)—59%
- Marketing—41%
- Specialty pharmacy—36%
- Increasing the number of general oncology physicians—34%
- Screening services (eg, mobile screening unit)—29%
- Capital investments—24%
- Clinical research—16%
- Support services—15%
- Retail pharmacy—14%
- Building upgrades—14%.
More details on the “2017 Trending Now in Cancer Care Survey” can be found on the ACCC website. ![]()
Gene therapy moves from promise to reality
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.