User login
New antifungals effective with shorter treatment course for tinea pedis
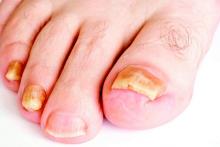
LAS VEGAS – Tinea pedis plagues millions of patients yearly, and treatment is lengthy, cumbersome, and often ineffective.
But two potent new antifungals promise an easier treatment regimen and a higher rate of successful treatment outcomes, according to Dr. David M. Pariser, who shared data about luliconazole and a new formulation of naftifine as topical treatments for tinea infections, at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, pointed out that most antifungals currently approved for tinea pedis require at least daily – and sometimes twice daily – application for at least 4 weeks. Terbinafine and tolnaftate are the exceptions, with treatment periods ranging from 1-6 weeks for the two products, depending on clinical response.
A new formulation of naftifine hydrochloride 2%, (Naftin), a potent prescription topical allylamine antifungal available as a cream or a gel, has shown equivalent efficacy with just two weeks of treatment. Naftifine has lipophilic and keratinophilic properties; further, it has clinically significant anti-inflammatory and antibacterial effects, in addition to its potent fungicidal and fungistatic effects against dermatophytes, Dr. Pariser said at the meeting. The preparations are currently approved for topical treatment of tinea pedis, tinea cruris, and tinea corporis.
Notably, naftifine maintains a “clinically relevant therapeutic reservoir effect after treatment completion, with naftifine detected in the stratum corneum for up to 4 weeks posttreatment,” he said. This reservoir effect permits a significantly easier treatment regimen, with topical application of either formulation daily for just 2 weeks.
The clinical trials of naftifine HCl 2% with daily administration for 2 weeks showed equivalence with the 1% formulation administered for 4 weeks; the higher concentration was well tolerated and was effective in both the moccasin and interdigital distributions of tinea pedis involvement. Trials also showed the mycologic and clinical cure rates of naftifine 2% to be equivalent or superior to those of terbinafine, econazole, clotrimazole, miconazole, and tolnaftate.
Clinical trials showed treatment effectiveness – defined as 90% improvement over baseline and achieving “essentially normal skin” – in 52% of patients receiving naftifine 2%, compared with 20% of patients receiving vehicle only. Overall clinical success – defined as mycologic cure and either clinical cure of effective clinical treatment – was seen in 78% of the naftifine 2% patients, compared with 49% of the vehicle patients.
The second antifungal Dr. Pariser discussed is luliconazole (Luzu), a prescription topical imidazole that is available as a 1% cream. Luliconazole is also a broad-spectrum, potent antifungal with effects that persist several weeks after treatment. The preparation is at least as effective as bifonazole, terbinafine, and lanoconazole, both in vitro and in vivo, Dr. Pariser said.
In two parallel clinical trials comparing luliconazole 1% cream to its vehicle, treatment was effective (at least 90% clearing and with normal-appearing skin) in 48% and 33% of patients receiving luliconazole, compared with 10% and 15% of patients receiving vehicle alone.
An advantage of the topical agents is that there are generally no major systemic side effects, since there is minimal systemic absorption, Dr. Pariser noted. Allergic contact dermatitis may be a local reaction, but tends to be mild and transient, he said.
Clinicians should always be alert for tinea pedis when treating onychomycosis, said Dr. Pariser, and untreated tinea can contribute to recurrence of nail fungus. “If you don’t look for tinea, you might not find it, and you’ve missed a treatment opportunity,” he said.
Dr. Pariser disclosed that he is an investigator and consultant for Valeant and an investigator for Anacor Pharmaceuticals.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Tinea pedis plagues millions of patients yearly, and treatment is lengthy, cumbersome, and often ineffective.
But two potent new antifungals promise an easier treatment regimen and a higher rate of successful treatment outcomes, according to Dr. David M. Pariser, who shared data about luliconazole and a new formulation of naftifine as topical treatments for tinea infections, at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, pointed out that most antifungals currently approved for tinea pedis require at least daily – and sometimes twice daily – application for at least 4 weeks. Terbinafine and tolnaftate are the exceptions, with treatment periods ranging from 1-6 weeks for the two products, depending on clinical response.
A new formulation of naftifine hydrochloride 2%, (Naftin), a potent prescription topical allylamine antifungal available as a cream or a gel, has shown equivalent efficacy with just two weeks of treatment. Naftifine has lipophilic and keratinophilic properties; further, it has clinically significant anti-inflammatory and antibacterial effects, in addition to its potent fungicidal and fungistatic effects against dermatophytes, Dr. Pariser said at the meeting. The preparations are currently approved for topical treatment of tinea pedis, tinea cruris, and tinea corporis.
Notably, naftifine maintains a “clinically relevant therapeutic reservoir effect after treatment completion, with naftifine detected in the stratum corneum for up to 4 weeks posttreatment,” he said. This reservoir effect permits a significantly easier treatment regimen, with topical application of either formulation daily for just 2 weeks.
The clinical trials of naftifine HCl 2% with daily administration for 2 weeks showed equivalence with the 1% formulation administered for 4 weeks; the higher concentration was well tolerated and was effective in both the moccasin and interdigital distributions of tinea pedis involvement. Trials also showed the mycologic and clinical cure rates of naftifine 2% to be equivalent or superior to those of terbinafine, econazole, clotrimazole, miconazole, and tolnaftate.
Clinical trials showed treatment effectiveness – defined as 90% improvement over baseline and achieving “essentially normal skin” – in 52% of patients receiving naftifine 2%, compared with 20% of patients receiving vehicle only. Overall clinical success – defined as mycologic cure and either clinical cure of effective clinical treatment – was seen in 78% of the naftifine 2% patients, compared with 49% of the vehicle patients.
The second antifungal Dr. Pariser discussed is luliconazole (Luzu), a prescription topical imidazole that is available as a 1% cream. Luliconazole is also a broad-spectrum, potent antifungal with effects that persist several weeks after treatment. The preparation is at least as effective as bifonazole, terbinafine, and lanoconazole, both in vitro and in vivo, Dr. Pariser said.
In two parallel clinical trials comparing luliconazole 1% cream to its vehicle, treatment was effective (at least 90% clearing and with normal-appearing skin) in 48% and 33% of patients receiving luliconazole, compared with 10% and 15% of patients receiving vehicle alone.
An advantage of the topical agents is that there are generally no major systemic side effects, since there is minimal systemic absorption, Dr. Pariser noted. Allergic contact dermatitis may be a local reaction, but tends to be mild and transient, he said.
Clinicians should always be alert for tinea pedis when treating onychomycosis, said Dr. Pariser, and untreated tinea can contribute to recurrence of nail fungus. “If you don’t look for tinea, you might not find it, and you’ve missed a treatment opportunity,” he said.
Dr. Pariser disclosed that he is an investigator and consultant for Valeant and an investigator for Anacor Pharmaceuticals.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Tinea pedis plagues millions of patients yearly, and treatment is lengthy, cumbersome, and often ineffective.
But two potent new antifungals promise an easier treatment regimen and a higher rate of successful treatment outcomes, according to Dr. David M. Pariser, who shared data about luliconazole and a new formulation of naftifine as topical treatments for tinea infections, at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, pointed out that most antifungals currently approved for tinea pedis require at least daily – and sometimes twice daily – application for at least 4 weeks. Terbinafine and tolnaftate are the exceptions, with treatment periods ranging from 1-6 weeks for the two products, depending on clinical response.
A new formulation of naftifine hydrochloride 2%, (Naftin), a potent prescription topical allylamine antifungal available as a cream or a gel, has shown equivalent efficacy with just two weeks of treatment. Naftifine has lipophilic and keratinophilic properties; further, it has clinically significant anti-inflammatory and antibacterial effects, in addition to its potent fungicidal and fungistatic effects against dermatophytes, Dr. Pariser said at the meeting. The preparations are currently approved for topical treatment of tinea pedis, tinea cruris, and tinea corporis.
Notably, naftifine maintains a “clinically relevant therapeutic reservoir effect after treatment completion, with naftifine detected in the stratum corneum for up to 4 weeks posttreatment,” he said. This reservoir effect permits a significantly easier treatment regimen, with topical application of either formulation daily for just 2 weeks.
The clinical trials of naftifine HCl 2% with daily administration for 2 weeks showed equivalence with the 1% formulation administered for 4 weeks; the higher concentration was well tolerated and was effective in both the moccasin and interdigital distributions of tinea pedis involvement. Trials also showed the mycologic and clinical cure rates of naftifine 2% to be equivalent or superior to those of terbinafine, econazole, clotrimazole, miconazole, and tolnaftate.
Clinical trials showed treatment effectiveness – defined as 90% improvement over baseline and achieving “essentially normal skin” – in 52% of patients receiving naftifine 2%, compared with 20% of patients receiving vehicle only. Overall clinical success – defined as mycologic cure and either clinical cure of effective clinical treatment – was seen in 78% of the naftifine 2% patients, compared with 49% of the vehicle patients.
The second antifungal Dr. Pariser discussed is luliconazole (Luzu), a prescription topical imidazole that is available as a 1% cream. Luliconazole is also a broad-spectrum, potent antifungal with effects that persist several weeks after treatment. The preparation is at least as effective as bifonazole, terbinafine, and lanoconazole, both in vitro and in vivo, Dr. Pariser said.
In two parallel clinical trials comparing luliconazole 1% cream to its vehicle, treatment was effective (at least 90% clearing and with normal-appearing skin) in 48% and 33% of patients receiving luliconazole, compared with 10% and 15% of patients receiving vehicle alone.
An advantage of the topical agents is that there are generally no major systemic side effects, since there is minimal systemic absorption, Dr. Pariser noted. Allergic contact dermatitis may be a local reaction, but tends to be mild and transient, he said.
Clinicians should always be alert for tinea pedis when treating onychomycosis, said Dr. Pariser, and untreated tinea can contribute to recurrence of nail fungus. “If you don’t look for tinea, you might not find it, and you’ve missed a treatment opportunity,” he said.
Dr. Pariser disclosed that he is an investigator and consultant for Valeant and an investigator for Anacor Pharmaceuticals.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS AT SDEF LAS VEGAS DERMATOLOGY SEMINAR
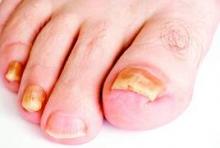
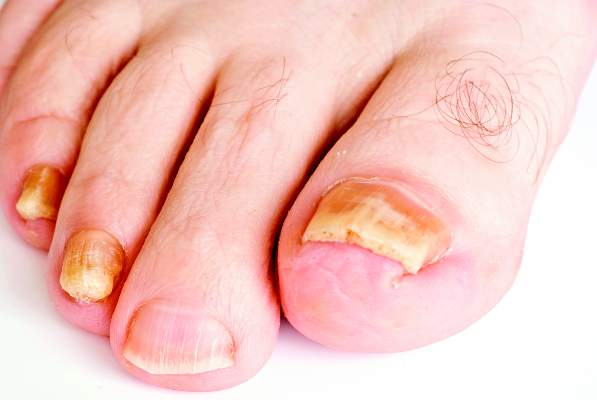
SDEF: Improved responses with newer topical onychomycosis treatments
LAS VEGAS – Better penetration through the nail is a key driver behind the increased efficacy of newer topical treatments for onychomycosis, affording a better chance for a clinical and mycologic cure without the potential for toxicity that comes with systemic treatments, according to Dr. David M. Pariser.
At the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, reviewed clinical trial data for 10% topical efinaconazole solution and 5% tavaborole topical solution, newer treatment options for the old problem of nail fungus. “New topical antifungals have improved the challenge of nail penetration,” which had been a key obstacle to efficacy with earlier topical treatments, he said.
Though topical treatments for onychomycosis avoid the risk of systemic side effects and the need for periodic blood tests to check liver function, historically, these treatments have not been very effective, he commented.
Ciclopirox (Penlac), one of the more efficacious treatments, achieves a cure rate of 5.5%-8.5%, and the buildup of the lacquer vehicle requires frequent nail debridement. Because of real or perceived risks, patients may be reluctant to take oral antifungals for onychomycosis, he said, noting that in addition to the potential for liver injury, use of these medications may also be limited by multiple drug-drug interactions.
The two new topical formulations he reviewed have a low molecular weight to allow better penetration through the dense, keratin-rich nail plate, Dr. Pariser said.
One of the topicals, 10% topical efinaconazole solution (Jublia), uses a formulation with low surface tension for good penetration with no surface buildup of vehicle material, achieving better nail penetration than do lacquer-based products, he noted.
A key efinaconazole study assessed clinical improvement in nail appearance in combination with mycologic cure, defined as negative findings on KOH prep exam and negative fungal culture. A complete cure was defined as a “totally clear target toenail and negative KOH/negative fungal culture,” and an “almost complete cure” was defined as mycologic cure, combined with no more than 5% clinically apparent involvement of the target toenail.
After double-blinded randomization into two parallel studies, patients applied either efinaconazole or the vehicle alone once daily at bedtime to the target toenail for 48 weeks. In the studies, 18% and 15% of those in the efinaconazole arm had achieved complete cure 52 weeks after beginning treatment, compared with 3% and 6% of the vehicle arm patients, respectively. However, for a pooled intent-to-treat population, the treatment arm saw a 28% cured or almost-cured rate, compared with 7% of the pooled vehicle-treated patients (P less than .001). Mycologic cure was achieved by 55% and 53% of the patients in the two efinaconazole arms, compared with 17% of the vehicle-only patients in each arm, according to Dr. Pariser.
Adverse events, similar between treatment arms, were mostly mild to moderate and localized, with dermatitis, vesicles, pain, and ingrown toenails the most commonly reported effects.
Tavaborole topical solution, 5% (Kerydin), is a boron-based compound that is highly water soluble, with broad antifungal activity that persists in the presence of keratin. As with efinaconazole, there is no product buildup, so nail debridement is not needed during treatment, Dr. Pariser said.
Two multicenter tavaborole trials compared the active tavaborole solution with a vehicle-only arm in a randomized, double-blind fashion, with product application daily for 48 weeks. The primary efficacy outcome for the trials was complete cure of the target great toenail at week 52. Secondary endpoints were a completely clear or almost clear (10% or less involvement of the target nail) target great toenail, as well as mycologic cure of the nail. Safety was measured by tracking adverse events, and local tolerability, as well as monitoring labs and ECG parameters.
A complete cure for the tavaborole trials required a completely clear nail on clinical exam, as well as negative mycology (negative KOH and negative fungal culture). At 52 weeks, 6.5% and 9.1% of the tavaborole-treated patients saw a complete cure, compared with 0.5% and 1.5% of vehicle-only patients in the two studies. Of those treated with tavaborole, 31.1% and 35.9% achieved mycologic cure, compared with 7.2% and 12.2% of those in the vehicle arm.
The rates of complete or almost complete clearing of the target great toenail for those in the tavaborole arms were 26.1% and 27.5%, compared with 9.3% and 14.6% in the vehicle arms. Predefined treatment success – a combination of mycologic cure and clear or almost clear target great toenail – was seen in 15.3% and 17.9% of the tavaborole-treated patients, compared with 1.5% and 3.9% of the vehicle-only patients (P less than or equal to .001 for all endpoints in both studies).
Treatment-related adverse events were generally mild and similar between the vehicle and treatment arms, with application site exfoliation, erythema, dermatitis, as well as ingrown toenails, the most commonly reported events for both arms.
Dr. Pariser noted that comparing efficacy of the newer agents directly is difficult, since the pivotal clinical trials for each had different designs, entry criteria, clinical assessments, and endpoints.
He disclosed that he is an investigator and consultant for Valeant, which manufactures the 10% topical efinaconazole solution, and an investigator for Anacor Pharmaceuticals, which markets tavaborole.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Better penetration through the nail is a key driver behind the increased efficacy of newer topical treatments for onychomycosis, affording a better chance for a clinical and mycologic cure without the potential for toxicity that comes with systemic treatments, according to Dr. David M. Pariser.
At the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, reviewed clinical trial data for 10% topical efinaconazole solution and 5% tavaborole topical solution, newer treatment options for the old problem of nail fungus. “New topical antifungals have improved the challenge of nail penetration,” which had been a key obstacle to efficacy with earlier topical treatments, he said.
Though topical treatments for onychomycosis avoid the risk of systemic side effects and the need for periodic blood tests to check liver function, historically, these treatments have not been very effective, he commented.
Ciclopirox (Penlac), one of the more efficacious treatments, achieves a cure rate of 5.5%-8.5%, and the buildup of the lacquer vehicle requires frequent nail debridement. Because of real or perceived risks, patients may be reluctant to take oral antifungals for onychomycosis, he said, noting that in addition to the potential for liver injury, use of these medications may also be limited by multiple drug-drug interactions.
The two new topical formulations he reviewed have a low molecular weight to allow better penetration through the dense, keratin-rich nail plate, Dr. Pariser said.
One of the topicals, 10% topical efinaconazole solution (Jublia), uses a formulation with low surface tension for good penetration with no surface buildup of vehicle material, achieving better nail penetration than do lacquer-based products, he noted.
A key efinaconazole study assessed clinical improvement in nail appearance in combination with mycologic cure, defined as negative findings on KOH prep exam and negative fungal culture. A complete cure was defined as a “totally clear target toenail and negative KOH/negative fungal culture,” and an “almost complete cure” was defined as mycologic cure, combined with no more than 5% clinically apparent involvement of the target toenail.
After double-blinded randomization into two parallel studies, patients applied either efinaconazole or the vehicle alone once daily at bedtime to the target toenail for 48 weeks. In the studies, 18% and 15% of those in the efinaconazole arm had achieved complete cure 52 weeks after beginning treatment, compared with 3% and 6% of the vehicle arm patients, respectively. However, for a pooled intent-to-treat population, the treatment arm saw a 28% cured or almost-cured rate, compared with 7% of the pooled vehicle-treated patients (P less than .001). Mycologic cure was achieved by 55% and 53% of the patients in the two efinaconazole arms, compared with 17% of the vehicle-only patients in each arm, according to Dr. Pariser.
Adverse events, similar between treatment arms, were mostly mild to moderate and localized, with dermatitis, vesicles, pain, and ingrown toenails the most commonly reported effects.
Tavaborole topical solution, 5% (Kerydin), is a boron-based compound that is highly water soluble, with broad antifungal activity that persists in the presence of keratin. As with efinaconazole, there is no product buildup, so nail debridement is not needed during treatment, Dr. Pariser said.
Two multicenter tavaborole trials compared the active tavaborole solution with a vehicle-only arm in a randomized, double-blind fashion, with product application daily for 48 weeks. The primary efficacy outcome for the trials was complete cure of the target great toenail at week 52. Secondary endpoints were a completely clear or almost clear (10% or less involvement of the target nail) target great toenail, as well as mycologic cure of the nail. Safety was measured by tracking adverse events, and local tolerability, as well as monitoring labs and ECG parameters.
A complete cure for the tavaborole trials required a completely clear nail on clinical exam, as well as negative mycology (negative KOH and negative fungal culture). At 52 weeks, 6.5% and 9.1% of the tavaborole-treated patients saw a complete cure, compared with 0.5% and 1.5% of vehicle-only patients in the two studies. Of those treated with tavaborole, 31.1% and 35.9% achieved mycologic cure, compared with 7.2% and 12.2% of those in the vehicle arm.
The rates of complete or almost complete clearing of the target great toenail for those in the tavaborole arms were 26.1% and 27.5%, compared with 9.3% and 14.6% in the vehicle arms. Predefined treatment success – a combination of mycologic cure and clear or almost clear target great toenail – was seen in 15.3% and 17.9% of the tavaborole-treated patients, compared with 1.5% and 3.9% of the vehicle-only patients (P less than or equal to .001 for all endpoints in both studies).
Treatment-related adverse events were generally mild and similar between the vehicle and treatment arms, with application site exfoliation, erythema, dermatitis, as well as ingrown toenails, the most commonly reported events for both arms.
Dr. Pariser noted that comparing efficacy of the newer agents directly is difficult, since the pivotal clinical trials for each had different designs, entry criteria, clinical assessments, and endpoints.
He disclosed that he is an investigator and consultant for Valeant, which manufactures the 10% topical efinaconazole solution, and an investigator for Anacor Pharmaceuticals, which markets tavaborole.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Better penetration through the nail is a key driver behind the increased efficacy of newer topical treatments for onychomycosis, affording a better chance for a clinical and mycologic cure without the potential for toxicity that comes with systemic treatments, according to Dr. David M. Pariser.
At the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, reviewed clinical trial data for 10% topical efinaconazole solution and 5% tavaborole topical solution, newer treatment options for the old problem of nail fungus. “New topical antifungals have improved the challenge of nail penetration,” which had been a key obstacle to efficacy with earlier topical treatments, he said.
Though topical treatments for onychomycosis avoid the risk of systemic side effects and the need for periodic blood tests to check liver function, historically, these treatments have not been very effective, he commented.
Ciclopirox (Penlac), one of the more efficacious treatments, achieves a cure rate of 5.5%-8.5%, and the buildup of the lacquer vehicle requires frequent nail debridement. Because of real or perceived risks, patients may be reluctant to take oral antifungals for onychomycosis, he said, noting that in addition to the potential for liver injury, use of these medications may also be limited by multiple drug-drug interactions.
The two new topical formulations he reviewed have a low molecular weight to allow better penetration through the dense, keratin-rich nail plate, Dr. Pariser said.
One of the topicals, 10% topical efinaconazole solution (Jublia), uses a formulation with low surface tension for good penetration with no surface buildup of vehicle material, achieving better nail penetration than do lacquer-based products, he noted.
A key efinaconazole study assessed clinical improvement in nail appearance in combination with mycologic cure, defined as negative findings on KOH prep exam and negative fungal culture. A complete cure was defined as a “totally clear target toenail and negative KOH/negative fungal culture,” and an “almost complete cure” was defined as mycologic cure, combined with no more than 5% clinically apparent involvement of the target toenail.
After double-blinded randomization into two parallel studies, patients applied either efinaconazole or the vehicle alone once daily at bedtime to the target toenail for 48 weeks. In the studies, 18% and 15% of those in the efinaconazole arm had achieved complete cure 52 weeks after beginning treatment, compared with 3% and 6% of the vehicle arm patients, respectively. However, for a pooled intent-to-treat population, the treatment arm saw a 28% cured or almost-cured rate, compared with 7% of the pooled vehicle-treated patients (P less than .001). Mycologic cure was achieved by 55% and 53% of the patients in the two efinaconazole arms, compared with 17% of the vehicle-only patients in each arm, according to Dr. Pariser.
Adverse events, similar between treatment arms, were mostly mild to moderate and localized, with dermatitis, vesicles, pain, and ingrown toenails the most commonly reported effects.
Tavaborole topical solution, 5% (Kerydin), is a boron-based compound that is highly water soluble, with broad antifungal activity that persists in the presence of keratin. As with efinaconazole, there is no product buildup, so nail debridement is not needed during treatment, Dr. Pariser said.
Two multicenter tavaborole trials compared the active tavaborole solution with a vehicle-only arm in a randomized, double-blind fashion, with product application daily for 48 weeks. The primary efficacy outcome for the trials was complete cure of the target great toenail at week 52. Secondary endpoints were a completely clear or almost clear (10% or less involvement of the target nail) target great toenail, as well as mycologic cure of the nail. Safety was measured by tracking adverse events, and local tolerability, as well as monitoring labs and ECG parameters.
A complete cure for the tavaborole trials required a completely clear nail on clinical exam, as well as negative mycology (negative KOH and negative fungal culture). At 52 weeks, 6.5% and 9.1% of the tavaborole-treated patients saw a complete cure, compared with 0.5% and 1.5% of vehicle-only patients in the two studies. Of those treated with tavaborole, 31.1% and 35.9% achieved mycologic cure, compared with 7.2% and 12.2% of those in the vehicle arm.
The rates of complete or almost complete clearing of the target great toenail for those in the tavaborole arms were 26.1% and 27.5%, compared with 9.3% and 14.6% in the vehicle arms. Predefined treatment success – a combination of mycologic cure and clear or almost clear target great toenail – was seen in 15.3% and 17.9% of the tavaborole-treated patients, compared with 1.5% and 3.9% of the vehicle-only patients (P less than or equal to .001 for all endpoints in both studies).
Treatment-related adverse events were generally mild and similar between the vehicle and treatment arms, with application site exfoliation, erythema, dermatitis, as well as ingrown toenails, the most commonly reported events for both arms.
Dr. Pariser noted that comparing efficacy of the newer agents directly is difficult, since the pivotal clinical trials for each had different designs, entry criteria, clinical assessments, and endpoints.
He disclosed that he is an investigator and consultant for Valeant, which manufactures the 10% topical efinaconazole solution, and an investigator for Anacor Pharmaceuticals, which markets tavaborole.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Infections from endemic fungi, mycobacteria rare in patients on TNFIs
The development of infections from mycobacteria and fungi endemic to U.S. regions in patients taking tumor necrosis factor–alpha inhibitors (TNFIs) is rare and is not influenced by prescreening of targeted infections, research suggests.
A case-control study of 30,772 patients taking TNFIs showed that only 158 (0.51%) patients developed the fungal and/or mycobacterial infections targeted in this study, with tuberculosis and histoplasmosis being the most common infections.
Targeted infections were nontuberculous mycobacterial infection, blastomycosis, coccidioidomyocosis, cryptococcal infection, histoplasmosis, pneumocystosis, tuberculosis disease, and unspecified fungal infection.
Prednisone was the only predictive factor for infection and was associated with a twofold increase in the likelihood of patients seeking medical attention for a fungal or mycobacterial infection, which the authors said was supported by previous research, according to a paper published online in Arthritis & Rheumatology.
“Thus, the question remains if the increased infection rates are related solely to the use of the glucocorticoids or the active disease for which the medication is being prescribed,” wrote Elizabeth Salt, Ph.D., of the University of Kentucky, Lexington, and coauthors (Arthritis Rheumatol. 2015 Oct 16 doi: 10.1002/art.39462).
Researchers also noted that sulfamethoxazole-trimethoprim was associated with a nonsignificant 45% increase in the likelihood of requiring medical care, compared with controls.
“It is possible that providers recognized the infectious risk of this population and made attempts at controlling infectious processes among those most vulnerable.”
The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
The development of infections from mycobacteria and fungi endemic to U.S. regions in patients taking tumor necrosis factor–alpha inhibitors (TNFIs) is rare and is not influenced by prescreening of targeted infections, research suggests.
A case-control study of 30,772 patients taking TNFIs showed that only 158 (0.51%) patients developed the fungal and/or mycobacterial infections targeted in this study, with tuberculosis and histoplasmosis being the most common infections.
Targeted infections were nontuberculous mycobacterial infection, blastomycosis, coccidioidomyocosis, cryptococcal infection, histoplasmosis, pneumocystosis, tuberculosis disease, and unspecified fungal infection.
Prednisone was the only predictive factor for infection and was associated with a twofold increase in the likelihood of patients seeking medical attention for a fungal or mycobacterial infection, which the authors said was supported by previous research, according to a paper published online in Arthritis & Rheumatology.
“Thus, the question remains if the increased infection rates are related solely to the use of the glucocorticoids or the active disease for which the medication is being prescribed,” wrote Elizabeth Salt, Ph.D., of the University of Kentucky, Lexington, and coauthors (Arthritis Rheumatol. 2015 Oct 16 doi: 10.1002/art.39462).
Researchers also noted that sulfamethoxazole-trimethoprim was associated with a nonsignificant 45% increase in the likelihood of requiring medical care, compared with controls.
“It is possible that providers recognized the infectious risk of this population and made attempts at controlling infectious processes among those most vulnerable.”
The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
The development of infections from mycobacteria and fungi endemic to U.S. regions in patients taking tumor necrosis factor–alpha inhibitors (TNFIs) is rare and is not influenced by prescreening of targeted infections, research suggests.
A case-control study of 30,772 patients taking TNFIs showed that only 158 (0.51%) patients developed the fungal and/or mycobacterial infections targeted in this study, with tuberculosis and histoplasmosis being the most common infections.
Targeted infections were nontuberculous mycobacterial infection, blastomycosis, coccidioidomyocosis, cryptococcal infection, histoplasmosis, pneumocystosis, tuberculosis disease, and unspecified fungal infection.
Prednisone was the only predictive factor for infection and was associated with a twofold increase in the likelihood of patients seeking medical attention for a fungal or mycobacterial infection, which the authors said was supported by previous research, according to a paper published online in Arthritis & Rheumatology.
“Thus, the question remains if the increased infection rates are related solely to the use of the glucocorticoids or the active disease for which the medication is being prescribed,” wrote Elizabeth Salt, Ph.D., of the University of Kentucky, Lexington, and coauthors (Arthritis Rheumatol. 2015 Oct 16 doi: 10.1002/art.39462).
Researchers also noted that sulfamethoxazole-trimethoprim was associated with a nonsignificant 45% increase in the likelihood of requiring medical care, compared with controls.
“It is possible that providers recognized the infectious risk of this population and made attempts at controlling infectious processes among those most vulnerable.”
The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: The incidence of select mycobacterial and fungal infections in patients taking TNFIs is low.
Major finding: Only 0.51% of patients taking TNFIs developed the mycobacterial and fungal infections targeted in this study.
Data source: A case-control study of 30,772 patients taking TNFIs.
Disclosures: The study was supported by the National Institutes of Health. There were no conflicts of interest declared.
IDWeek: Rifapentine had best completion rates for health care workers with latent TB
SAN DIEGO – Health care workers with latent tuberculosis infection (LTBI) were significantly more likely to continue a shorter course of weekly rifapentine plus isoniazid (INH) than daily INH monotherapy, researchers reported at an annual scientific meeting on infectious diseases.
“Consideration should be given to no longer routinely recommending INH for the treatment of LTBI among health care workers,” said Dr. Esther Arguello Perez of Memorial Sloan Kettering Cancer Center, New York.
Health care workers face a greater risk of TB infection than the general population, regardless of the income level in the country where they live; patients with undiagnosed laryngeal or pulmonary TB usually pose the greatest risk, especially during procedures that cause coughing, such as sputum induction and bronchoscopy (Int J Tuberc Lung Dis. 2007;11[6]:593-605).
Although occupational TB testing is routine in U.S. health care organizations, more than half of health care workers who start treatment for LTBI historically have failed to finish (Chest. 2010;137[2]:401-9. doi: 10.1378/chest.09-0394). The standard LTBI regimen – 300 mg INH daily for 9 months – has been linked to potentially intolerable adverse effects such as hepatotoxicity, persistent gastrointestinal symptoms, rash, and neuropsychiatric problems (Drug Healthc Patient Saf. 2014;6:145-9. doi: 10.2147/DHPS.S68837).
In a 2011 multicenter trial, investigators reported a significantly higher completion rate for weekly rifapentine plus INH (900 mg each; 82% vs. 69% for daily INH; P < .001). Rates of adverse effects were significantly lower with weekly rifapentine plus INH, although grade 3-4 events and risk of death did not differ between the groups (N Engl J Med. 2011;365:2155-66. doi: 10.1056/NEJMoa1104875). The results of that trial quickly transformed recommendations for LTBI treatment (MMWR. 2011:60(48);1650-53).
Memorial Sloan Kettering implemented weekly rifapentine plus INH for its LTBI personnel in 2011. By 2014, about three-quarters of personnel with LTBI received rifapentine plus INH, while the rest were evenly split between rifampin and INH monotherapy, Dr. Arguello Perez reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
To understand how health care workers’ attitudes and treatment acceptance shifted along with practice, the investigators reviewed records from all health care workers at Memorial Sloan Kettering who were diagnosed with LTBI for 2005-2014. Among 930 patients, only 357 (38%) accepted treatment, although 76% of these individuals finished the regimen they started, she noted. Rifapentine plus INH had the highest completion rate (88%), significantly exceeding rates for a 4-month course of daily rifampin (84%) and for 9 months of INH monotherapy (70%; P < .01 for both differences). In contrast, completion rates for rifampin and INH did not significantly differ, Dr. Arguello Perez said.
Notably, LTBI treatment completion rates among health care workers rose by 26% between 2013, when most prescriptions were for rifampin or INH monotherapy, and 2014, when most were for rifapentine plus INH. “Health care workers might be more likely to accept treatment for LTBI if they know about alternatives to INH,” she concluded.
Dr. Arguello Perez and her associates reported no funding sources and had no financial disclosures.
SAN DIEGO – Health care workers with latent tuberculosis infection (LTBI) were significantly more likely to continue a shorter course of weekly rifapentine plus isoniazid (INH) than daily INH monotherapy, researchers reported at an annual scientific meeting on infectious diseases.
“Consideration should be given to no longer routinely recommending INH for the treatment of LTBI among health care workers,” said Dr. Esther Arguello Perez of Memorial Sloan Kettering Cancer Center, New York.
Health care workers face a greater risk of TB infection than the general population, regardless of the income level in the country where they live; patients with undiagnosed laryngeal or pulmonary TB usually pose the greatest risk, especially during procedures that cause coughing, such as sputum induction and bronchoscopy (Int J Tuberc Lung Dis. 2007;11[6]:593-605).
Although occupational TB testing is routine in U.S. health care organizations, more than half of health care workers who start treatment for LTBI historically have failed to finish (Chest. 2010;137[2]:401-9. doi: 10.1378/chest.09-0394). The standard LTBI regimen – 300 mg INH daily for 9 months – has been linked to potentially intolerable adverse effects such as hepatotoxicity, persistent gastrointestinal symptoms, rash, and neuropsychiatric problems (Drug Healthc Patient Saf. 2014;6:145-9. doi: 10.2147/DHPS.S68837).
In a 2011 multicenter trial, investigators reported a significantly higher completion rate for weekly rifapentine plus INH (900 mg each; 82% vs. 69% for daily INH; P < .001). Rates of adverse effects were significantly lower with weekly rifapentine plus INH, although grade 3-4 events and risk of death did not differ between the groups (N Engl J Med. 2011;365:2155-66. doi: 10.1056/NEJMoa1104875). The results of that trial quickly transformed recommendations for LTBI treatment (MMWR. 2011:60(48);1650-53).
Memorial Sloan Kettering implemented weekly rifapentine plus INH for its LTBI personnel in 2011. By 2014, about three-quarters of personnel with LTBI received rifapentine plus INH, while the rest were evenly split between rifampin and INH monotherapy, Dr. Arguello Perez reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
To understand how health care workers’ attitudes and treatment acceptance shifted along with practice, the investigators reviewed records from all health care workers at Memorial Sloan Kettering who were diagnosed with LTBI for 2005-2014. Among 930 patients, only 357 (38%) accepted treatment, although 76% of these individuals finished the regimen they started, she noted. Rifapentine plus INH had the highest completion rate (88%), significantly exceeding rates for a 4-month course of daily rifampin (84%) and for 9 months of INH monotherapy (70%; P < .01 for both differences). In contrast, completion rates for rifampin and INH did not significantly differ, Dr. Arguello Perez said.
Notably, LTBI treatment completion rates among health care workers rose by 26% between 2013, when most prescriptions were for rifampin or INH monotherapy, and 2014, when most were for rifapentine plus INH. “Health care workers might be more likely to accept treatment for LTBI if they know about alternatives to INH,” she concluded.
Dr. Arguello Perez and her associates reported no funding sources and had no financial disclosures.
SAN DIEGO – Health care workers with latent tuberculosis infection (LTBI) were significantly more likely to continue a shorter course of weekly rifapentine plus isoniazid (INH) than daily INH monotherapy, researchers reported at an annual scientific meeting on infectious diseases.
“Consideration should be given to no longer routinely recommending INH for the treatment of LTBI among health care workers,” said Dr. Esther Arguello Perez of Memorial Sloan Kettering Cancer Center, New York.
Health care workers face a greater risk of TB infection than the general population, regardless of the income level in the country where they live; patients with undiagnosed laryngeal or pulmonary TB usually pose the greatest risk, especially during procedures that cause coughing, such as sputum induction and bronchoscopy (Int J Tuberc Lung Dis. 2007;11[6]:593-605).
Although occupational TB testing is routine in U.S. health care organizations, more than half of health care workers who start treatment for LTBI historically have failed to finish (Chest. 2010;137[2]:401-9. doi: 10.1378/chest.09-0394). The standard LTBI regimen – 300 mg INH daily for 9 months – has been linked to potentially intolerable adverse effects such as hepatotoxicity, persistent gastrointestinal symptoms, rash, and neuropsychiatric problems (Drug Healthc Patient Saf. 2014;6:145-9. doi: 10.2147/DHPS.S68837).
In a 2011 multicenter trial, investigators reported a significantly higher completion rate for weekly rifapentine plus INH (900 mg each; 82% vs. 69% for daily INH; P < .001). Rates of adverse effects were significantly lower with weekly rifapentine plus INH, although grade 3-4 events and risk of death did not differ between the groups (N Engl J Med. 2011;365:2155-66. doi: 10.1056/NEJMoa1104875). The results of that trial quickly transformed recommendations for LTBI treatment (MMWR. 2011:60(48);1650-53).
Memorial Sloan Kettering implemented weekly rifapentine plus INH for its LTBI personnel in 2011. By 2014, about three-quarters of personnel with LTBI received rifapentine plus INH, while the rest were evenly split between rifampin and INH monotherapy, Dr. Arguello Perez reported at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
To understand how health care workers’ attitudes and treatment acceptance shifted along with practice, the investigators reviewed records from all health care workers at Memorial Sloan Kettering who were diagnosed with LTBI for 2005-2014. Among 930 patients, only 357 (38%) accepted treatment, although 76% of these individuals finished the regimen they started, she noted. Rifapentine plus INH had the highest completion rate (88%), significantly exceeding rates for a 4-month course of daily rifampin (84%) and for 9 months of INH monotherapy (70%; P < .01 for both differences). In contrast, completion rates for rifampin and INH did not significantly differ, Dr. Arguello Perez said.
Notably, LTBI treatment completion rates among health care workers rose by 26% between 2013, when most prescriptions were for rifampin or INH monotherapy, and 2014, when most were for rifapentine plus INH. “Health care workers might be more likely to accept treatment for LTBI if they know about alternatives to INH,” she concluded.
Dr. Arguello Perez and her associates reported no funding sources and had no financial disclosures.
AT IDweek 2015
Key clinical point: Isoniazid monotherapy should not be recommended routinely for health care workers with latent tuberculosis infections.
Major finding: Rates of treatment completion were significantly higher for weekly rifapentine plus isoniazid than for daily isoniazid monotherapy (P < .01).
Data source: Retrospective analysis of all health care workers with LTBI at Memorial Sloan Kettering Cancer Center during 2005-2014.
Disclosures: The researchers reported no funding sources and made no financial disclosures.
Coccidioidomycosis a respiratory threat to construction workers in Southwest
The expansion of the solar energy industry in Coccidioides-endemic areas of the southwestern United States is exposing more workers to the infection, say the authors of a study that found an attack rate of 1.2 cases per 100 workers.
A study among 3,572 workers at two solar power–generating facilities in California identified 44 individuals with the infection between October 2011 and April 2014, 9 of whom were hospitalized, according to a paper published in the Oct. 14 edition of Emerging Infectious Diseases.
The disease is acquired through inhalation of the soil-dwelling Coccidioides fungus spores and while the majority of the patients said they had received safety training about the risk of coccidioidomycosis, only six of those who regularly performed soil-disruptive work reported regularly using respiratory protection (Emerg Infect Dis. 2015 Oct 14; doi: ).
“Large-scale construction, including solar farm construction, might involve substantial soil disturbance for months, and many employees, particularly from non–Coccidioides-endemic areas, probably lack immunity to Coccidioides,” wrote Jason A. Wilken, Ph.D., of the Centers for Disease Control and Prevention, and his coauthors.
“Medical providers should consider work-related coccidioidomycosis when evaluating construction workers with prolonged febrile respiratory illness, particularly after work in Central or Southern California or in Arizona, and medical providers should follow all statutory requirements for documenting and reporting occupational illness,” Dr. Wilken concluded.
The study was supported by the Centers for Disease Control and Prevention. No conflicts of interest were declared.
The expansion of the solar energy industry in Coccidioides-endemic areas of the southwestern United States is exposing more workers to the infection, say the authors of a study that found an attack rate of 1.2 cases per 100 workers.
A study among 3,572 workers at two solar power–generating facilities in California identified 44 individuals with the infection between October 2011 and April 2014, 9 of whom were hospitalized, according to a paper published in the Oct. 14 edition of Emerging Infectious Diseases.
The disease is acquired through inhalation of the soil-dwelling Coccidioides fungus spores and while the majority of the patients said they had received safety training about the risk of coccidioidomycosis, only six of those who regularly performed soil-disruptive work reported regularly using respiratory protection (Emerg Infect Dis. 2015 Oct 14; doi: ).
“Large-scale construction, including solar farm construction, might involve substantial soil disturbance for months, and many employees, particularly from non–Coccidioides-endemic areas, probably lack immunity to Coccidioides,” wrote Jason A. Wilken, Ph.D., of the Centers for Disease Control and Prevention, and his coauthors.
“Medical providers should consider work-related coccidioidomycosis when evaluating construction workers with prolonged febrile respiratory illness, particularly after work in Central or Southern California or in Arizona, and medical providers should follow all statutory requirements for documenting and reporting occupational illness,” Dr. Wilken concluded.
The study was supported by the Centers for Disease Control and Prevention. No conflicts of interest were declared.
The expansion of the solar energy industry in Coccidioides-endemic areas of the southwestern United States is exposing more workers to the infection, say the authors of a study that found an attack rate of 1.2 cases per 100 workers.
A study among 3,572 workers at two solar power–generating facilities in California identified 44 individuals with the infection between October 2011 and April 2014, 9 of whom were hospitalized, according to a paper published in the Oct. 14 edition of Emerging Infectious Diseases.
The disease is acquired through inhalation of the soil-dwelling Coccidioides fungus spores and while the majority of the patients said they had received safety training about the risk of coccidioidomycosis, only six of those who regularly performed soil-disruptive work reported regularly using respiratory protection (Emerg Infect Dis. 2015 Oct 14; doi: ).
“Large-scale construction, including solar farm construction, might involve substantial soil disturbance for months, and many employees, particularly from non–Coccidioides-endemic areas, probably lack immunity to Coccidioides,” wrote Jason A. Wilken, Ph.D., of the Centers for Disease Control and Prevention, and his coauthors.
“Medical providers should consider work-related coccidioidomycosis when evaluating construction workers with prolonged febrile respiratory illness, particularly after work in Central or Southern California or in Arizona, and medical providers should follow all statutory requirements for documenting and reporting occupational illness,” Dr. Wilken concluded.
The study was supported by the Centers for Disease Control and Prevention. No conflicts of interest were declared.
FROM EMERGING INFECTIOUS DISEASES
Key clinical point:Coccidioidomycosis is a significant risk in workers on solar power–generating facilities in Coccidioides-endemic areas of the Southwestern United States.
Major finding: The attack rate of Coccidioides could be as high 1.2 cases per 100 workers involved in constructing solar power–generating facilities.
Data source: A study among 3,572 workers at two solar power–generating facilities in California.
Disclosures: The study was supported by the Centers for Disease Control and Prevention. No conflicts of interest were declared.
Fungal foot infections risk secondary infection in diabetic patients
VANCOUVER – Fungal foot infections in diabetic patients are often ignored and are far more than a cosmetic problem.
In patients with diabetes, tinea pedis and onychomycosis triple the likelihood of secondary bacterial infections including gram-negative intertrigo, cellulitis, and osteomyelitis. Further, they boost up to fivefold the risk of life- and limb-threatening gangrene, Dr. Manuela Papini said at the World Congress of Dermatology.
Her own work (G Ital Dermatol Venereol. 2013 Dec;148(6):603-8), as well as that of others, indicates tinea pedis and onychomycosis in diabetic patients often goes undiagnosed, ignored, or inadequately treated.
In her own experience, four out of five diabetic patients with a fungal foot infection are unaware of it, she said. Moreover, half of those with a diagnosed fungal foot infection remain untreated or insufficiently treated, added Dr. Papini, a dermatologist at the University of Perugia (Italy).
Fungal infections of the foot are three times more common among diabetic individuals than the general population. The reasons for this disparity included impaired peripheral circulation, an immunocompromised state, autonomic neuropathy, and the inability to maintain good foot hygiene because of obesity, impaired vision, or advanced age.
The causative organisms of fungal infections in diabetic patients are the same as those seen in the general population. So are the recommended first-line treatments. But treatment response is generally poor – much worse than in nondiabetics. Adherence to antifungal medication also is a real problem in diabetic patients, due in large part to the high prevalence of comorbid conditions and resultant polypharmacy.
“Most diabetic patients say their large pill burden is an issue, and they think onychomycosis is the least important of their problems,” she explained.
Photodynamic therapy and laser treatments show promise, but the supporting data aren’t yet sufficient to warrant their introduction into clinical practice, according to Dr. Papini.
As for contemporary therapy, she noted that the British Association of Dermatologists, in its current onychomycosis treatment guidelines, reserves its A-strength recommendations for two oral drugs given daily for 12 weeks: terbinafine and itraconazole, although itraconazole can alternatively be used as pulse therapy for 3-6 months. Topical therapies are advised only for superficial white onychomycosis and early distal lateral subungual onychomycosis (Br J Dermatol. 2014 Nov;171(5):937-58).
A systematic review of the published literature on treatment of diabetic fungal foot infections concluded that there is good evidence that oral terbinafine is as safe and effective as itraconazole for treating onychomycosis. The authors, however, found that there is no evidence to guide treatment of tinea pedis in the diabetic population (J Foot Ankle Res. 2011 Dec 4;4:26).
In the nondiabetic population, the first-line treatment for tinea pedis is typically a topical antifungal. A good option in diabetic patients is luliconazole (Luzu), which is active against Trichophyton rubrum – the most common causative organism – and has the advantage of simplicity: the regimen is once-daily treatment for 2 weeks, much shorter than for many other topical antifungals, Dr. Papini observed.
Until new and better treatments come along, she continued, the key to preventing relapse of fungal foot infections in diabetic patients is to choose the simplest and most effective therapy, stress to patients the importance of completing the treatment course, and provide instruction in self-inspection and disinfection of shoes and socks.
Dr. Papini reported having no financial conflicts of interest.
VANCOUVER – Fungal foot infections in diabetic patients are often ignored and are far more than a cosmetic problem.
In patients with diabetes, tinea pedis and onychomycosis triple the likelihood of secondary bacterial infections including gram-negative intertrigo, cellulitis, and osteomyelitis. Further, they boost up to fivefold the risk of life- and limb-threatening gangrene, Dr. Manuela Papini said at the World Congress of Dermatology.
Her own work (G Ital Dermatol Venereol. 2013 Dec;148(6):603-8), as well as that of others, indicates tinea pedis and onychomycosis in diabetic patients often goes undiagnosed, ignored, or inadequately treated.
In her own experience, four out of five diabetic patients with a fungal foot infection are unaware of it, she said. Moreover, half of those with a diagnosed fungal foot infection remain untreated or insufficiently treated, added Dr. Papini, a dermatologist at the University of Perugia (Italy).
Fungal infections of the foot are three times more common among diabetic individuals than the general population. The reasons for this disparity included impaired peripheral circulation, an immunocompromised state, autonomic neuropathy, and the inability to maintain good foot hygiene because of obesity, impaired vision, or advanced age.
The causative organisms of fungal infections in diabetic patients are the same as those seen in the general population. So are the recommended first-line treatments. But treatment response is generally poor – much worse than in nondiabetics. Adherence to antifungal medication also is a real problem in diabetic patients, due in large part to the high prevalence of comorbid conditions and resultant polypharmacy.
“Most diabetic patients say their large pill burden is an issue, and they think onychomycosis is the least important of their problems,” she explained.
Photodynamic therapy and laser treatments show promise, but the supporting data aren’t yet sufficient to warrant their introduction into clinical practice, according to Dr. Papini.
As for contemporary therapy, she noted that the British Association of Dermatologists, in its current onychomycosis treatment guidelines, reserves its A-strength recommendations for two oral drugs given daily for 12 weeks: terbinafine and itraconazole, although itraconazole can alternatively be used as pulse therapy for 3-6 months. Topical therapies are advised only for superficial white onychomycosis and early distal lateral subungual onychomycosis (Br J Dermatol. 2014 Nov;171(5):937-58).
A systematic review of the published literature on treatment of diabetic fungal foot infections concluded that there is good evidence that oral terbinafine is as safe and effective as itraconazole for treating onychomycosis. The authors, however, found that there is no evidence to guide treatment of tinea pedis in the diabetic population (J Foot Ankle Res. 2011 Dec 4;4:26).
In the nondiabetic population, the first-line treatment for tinea pedis is typically a topical antifungal. A good option in diabetic patients is luliconazole (Luzu), which is active against Trichophyton rubrum – the most common causative organism – and has the advantage of simplicity: the regimen is once-daily treatment for 2 weeks, much shorter than for many other topical antifungals, Dr. Papini observed.
Until new and better treatments come along, she continued, the key to preventing relapse of fungal foot infections in diabetic patients is to choose the simplest and most effective therapy, stress to patients the importance of completing the treatment course, and provide instruction in self-inspection and disinfection of shoes and socks.
Dr. Papini reported having no financial conflicts of interest.
VANCOUVER – Fungal foot infections in diabetic patients are often ignored and are far more than a cosmetic problem.
In patients with diabetes, tinea pedis and onychomycosis triple the likelihood of secondary bacterial infections including gram-negative intertrigo, cellulitis, and osteomyelitis. Further, they boost up to fivefold the risk of life- and limb-threatening gangrene, Dr. Manuela Papini said at the World Congress of Dermatology.
Her own work (G Ital Dermatol Venereol. 2013 Dec;148(6):603-8), as well as that of others, indicates tinea pedis and onychomycosis in diabetic patients often goes undiagnosed, ignored, or inadequately treated.
In her own experience, four out of five diabetic patients with a fungal foot infection are unaware of it, she said. Moreover, half of those with a diagnosed fungal foot infection remain untreated or insufficiently treated, added Dr. Papini, a dermatologist at the University of Perugia (Italy).
Fungal infections of the foot are three times more common among diabetic individuals than the general population. The reasons for this disparity included impaired peripheral circulation, an immunocompromised state, autonomic neuropathy, and the inability to maintain good foot hygiene because of obesity, impaired vision, or advanced age.
The causative organisms of fungal infections in diabetic patients are the same as those seen in the general population. So are the recommended first-line treatments. But treatment response is generally poor – much worse than in nondiabetics. Adherence to antifungal medication also is a real problem in diabetic patients, due in large part to the high prevalence of comorbid conditions and resultant polypharmacy.
“Most diabetic patients say their large pill burden is an issue, and they think onychomycosis is the least important of their problems,” she explained.
Photodynamic therapy and laser treatments show promise, but the supporting data aren’t yet sufficient to warrant their introduction into clinical practice, according to Dr. Papini.
As for contemporary therapy, she noted that the British Association of Dermatologists, in its current onychomycosis treatment guidelines, reserves its A-strength recommendations for two oral drugs given daily for 12 weeks: terbinafine and itraconazole, although itraconazole can alternatively be used as pulse therapy for 3-6 months. Topical therapies are advised only for superficial white onychomycosis and early distal lateral subungual onychomycosis (Br J Dermatol. 2014 Nov;171(5):937-58).
A systematic review of the published literature on treatment of diabetic fungal foot infections concluded that there is good evidence that oral terbinafine is as safe and effective as itraconazole for treating onychomycosis. The authors, however, found that there is no evidence to guide treatment of tinea pedis in the diabetic population (J Foot Ankle Res. 2011 Dec 4;4:26).
In the nondiabetic population, the first-line treatment for tinea pedis is typically a topical antifungal. A good option in diabetic patients is luliconazole (Luzu), which is active against Trichophyton rubrum – the most common causative organism – and has the advantage of simplicity: the regimen is once-daily treatment for 2 weeks, much shorter than for many other topical antifungals, Dr. Papini observed.
Until new and better treatments come along, she continued, the key to preventing relapse of fungal foot infections in diabetic patients is to choose the simplest and most effective therapy, stress to patients the importance of completing the treatment course, and provide instruction in self-inspection and disinfection of shoes and socks.
Dr. Papini reported having no financial conflicts of interest.
EXPERT ANALYSIS FROM WCD 2015
Newer antifungals shorten tinea pedis treatment duration, promote adherence
MIAMI BEACH – Two new antifungal agents on the market – luliconazole and naftifine – each have something unique to offer when it comes to treating tinea pedis, according to Dr. Boni E. Elewski.
Luliconazole is an azole drug, meaning it is broad spectrum and kills dermatophytes, yeast, and molds. Also, like all azoles, it has some antibacterial activity, she said at the South Beach Symposium.
Naftifine is an allylamine drug, and is mainly an antidermatophyte agent – albeit a “very, very potent antidermatophyte” – with no antibacterial activity, she said.
Both are approved for once-daily use for 2 weeks, and that’s good because the short treatment duration improves adherence to the regimen, especially compared with other drugs that require 4-6 weeks of treatment to eradicate the problem, noted Dr. Elewski, professor of dermatology and director of clinical trials research at the University of Alabama at Birmingham.
Both drugs also stay in the skin and continue working after treatment stops.
Making the choice regarding which drug or class of drugs to use depends on the patient’s symptoms.
“First of all, tinea pedis may not be obvious. People don’t often tell you, ‘This is what I have – it’s tinea pedis,’ ” she said.
Keep in mind that tinea pedis and onychomycosis are related. If you have a patient who you think has onychomycosis, look at the bottom of their foot, she advised.
“If they don’t have tinea pedis, they probably don’t have onychomycosis unless they’ve had tinea pedis recently and got rid of it,” she said.
Also, look for collarettes of scale, which may be very subtle and may look like “tiny little circular pieces of scale on the medial or lateral foot.”
“If you are not sure, just keep looking harder because you might see it,” Dr. Elewski said.
Interdigital tinea pedis will be a little more obvious, with scaling and crusting between the toes, as well as maceration and oozing in many cases.
When the toe web is oozing, you’re likely dealing with intertrigo, she said.
In such cases, an azole cream is the better treatment choice, because azoles will kill Candida, bacteria, and dermatophytes that are there, she said.
“So when I have a moist macerated space, I like an azole. If you have a dry scaly process – with or without the collarettes – you’re probably better with an allylamine, particularly if you use a keratolytic with it, something that has urea or a lactic acid,” she said.
Dr. Elewski is a consultant for Valeant Pharmaceuticals International and a contracted researcher for Anacor Pharmaceuticals.
MIAMI BEACH – Two new antifungal agents on the market – luliconazole and naftifine – each have something unique to offer when it comes to treating tinea pedis, according to Dr. Boni E. Elewski.
Luliconazole is an azole drug, meaning it is broad spectrum and kills dermatophytes, yeast, and molds. Also, like all azoles, it has some antibacterial activity, she said at the South Beach Symposium.
Naftifine is an allylamine drug, and is mainly an antidermatophyte agent – albeit a “very, very potent antidermatophyte” – with no antibacterial activity, she said.
Both are approved for once-daily use for 2 weeks, and that’s good because the short treatment duration improves adherence to the regimen, especially compared with other drugs that require 4-6 weeks of treatment to eradicate the problem, noted Dr. Elewski, professor of dermatology and director of clinical trials research at the University of Alabama at Birmingham.
Both drugs also stay in the skin and continue working after treatment stops.
Making the choice regarding which drug or class of drugs to use depends on the patient’s symptoms.
“First of all, tinea pedis may not be obvious. People don’t often tell you, ‘This is what I have – it’s tinea pedis,’ ” she said.
Keep in mind that tinea pedis and onychomycosis are related. If you have a patient who you think has onychomycosis, look at the bottom of their foot, she advised.
“If they don’t have tinea pedis, they probably don’t have onychomycosis unless they’ve had tinea pedis recently and got rid of it,” she said.
Also, look for collarettes of scale, which may be very subtle and may look like “tiny little circular pieces of scale on the medial or lateral foot.”
“If you are not sure, just keep looking harder because you might see it,” Dr. Elewski said.
Interdigital tinea pedis will be a little more obvious, with scaling and crusting between the toes, as well as maceration and oozing in many cases.
When the toe web is oozing, you’re likely dealing with intertrigo, she said.
In such cases, an azole cream is the better treatment choice, because azoles will kill Candida, bacteria, and dermatophytes that are there, she said.
“So when I have a moist macerated space, I like an azole. If you have a dry scaly process – with or without the collarettes – you’re probably better with an allylamine, particularly if you use a keratolytic with it, something that has urea or a lactic acid,” she said.
Dr. Elewski is a consultant for Valeant Pharmaceuticals International and a contracted researcher for Anacor Pharmaceuticals.
MIAMI BEACH – Two new antifungal agents on the market – luliconazole and naftifine – each have something unique to offer when it comes to treating tinea pedis, according to Dr. Boni E. Elewski.
Luliconazole is an azole drug, meaning it is broad spectrum and kills dermatophytes, yeast, and molds. Also, like all azoles, it has some antibacterial activity, she said at the South Beach Symposium.
Naftifine is an allylamine drug, and is mainly an antidermatophyte agent – albeit a “very, very potent antidermatophyte” – with no antibacterial activity, she said.
Both are approved for once-daily use for 2 weeks, and that’s good because the short treatment duration improves adherence to the regimen, especially compared with other drugs that require 4-6 weeks of treatment to eradicate the problem, noted Dr. Elewski, professor of dermatology and director of clinical trials research at the University of Alabama at Birmingham.
Both drugs also stay in the skin and continue working after treatment stops.
Making the choice regarding which drug or class of drugs to use depends on the patient’s symptoms.
“First of all, tinea pedis may not be obvious. People don’t often tell you, ‘This is what I have – it’s tinea pedis,’ ” she said.
Keep in mind that tinea pedis and onychomycosis are related. If you have a patient who you think has onychomycosis, look at the bottom of their foot, she advised.
“If they don’t have tinea pedis, they probably don’t have onychomycosis unless they’ve had tinea pedis recently and got rid of it,” she said.
Also, look for collarettes of scale, which may be very subtle and may look like “tiny little circular pieces of scale on the medial or lateral foot.”
“If you are not sure, just keep looking harder because you might see it,” Dr. Elewski said.
Interdigital tinea pedis will be a little more obvious, with scaling and crusting between the toes, as well as maceration and oozing in many cases.
When the toe web is oozing, you’re likely dealing with intertrigo, she said.
In such cases, an azole cream is the better treatment choice, because azoles will kill Candida, bacteria, and dermatophytes that are there, she said.
“So when I have a moist macerated space, I like an azole. If you have a dry scaly process – with or without the collarettes – you’re probably better with an allylamine, particularly if you use a keratolytic with it, something that has urea or a lactic acid,” she said.
Dr. Elewski is a consultant for Valeant Pharmaceuticals International and a contracted researcher for Anacor Pharmaceuticals.
AT THE SOUTH BEACH SYMPOSIUM
Antifungal treatment may cause DNA strain type switching in onychomycosis
Although DNA strain type switches are known to be a natural occurrence in patients with onychomycosis, increases in strain type switching that follow treatment failure could be an antifungal-induced response, according to the results of a study published in the British Journal of Dermatology.
“The dermatophyte Trichophyton rubrum is responsible for the majority (~80%) of [onychomycosis] cases, many of which frequently relapse after successful antifungal treatment,” noted the study authors, led by Dr. Aditya K. Gupta of the University of Toronto. Despite several previous studies of various facets related to onychomycosis, “data outlining onychomycosis infections of T. rubrum with DNA strain type, treatments, outcome and geographical location are still warranted,” they added (Br. J. Dermatol. 2015;172:74-80).
Dr. Gupta and his associates examined 50 adults infected with T. rubrum, determined via analysis of toenail specimens from onychomycosis patients in southwest Ontario. The patients were divided into cohorts based on the treatment they received: oral terbinafine, laser, or placebo (no terbinafine and no laser). Typing of DNA strains was done only in culture-positive samples before and after treatment, leaving a study population of six in the terbinafine group, nine in the laser group, and eight in the placebo group.
Half of the terbinafine subjects were prescribed oral terbinafine 250 mg/day for 12 weeks, while the other three received oral terbinafine 250 mg/day pulse therapy at on/off intervals of 2 weeks up to 12 weeks.
The investigators also used three DNA strains known to be common in Europe for comparison and found that six distinct strains, labeled A-F, accounted for 94% of the T. rubrum strains – these strains corresponded to the European ones. However, three other strains (6% of strains) were found that investigators concluded were native to North America.
Strain type switching occurred in five (83%) of the terbinafine subjects, five (56%) of the laser cohort subjects, and two (25%) of those in the placebo cohort. Roughly half of the type switches noted in the terbinafine cohort were associated with mycological cures and were followed by relapse shortly thereafter. Dr. Gupta and his associates also found that all DNA strains in this cohort were susceptible to terbinafine while in vitro. Strain types in the laser and placebo cohorts did not show any signs of intermittent cures.
The patients were sampled at intervals of 0, 12, 24, 36, 48, 60, and 72 weeks of treatment, and T. rubrum DNA strain types were determined at week 0 (n = 6) and week 48 (n = 1) or 72 (n = 5). Patients in the laser cohort were treated at weeks 0, 8, and 16 and sampled at weeks 0, 8, 16, 24, and 48, with T. rubrum DNA strain types determined at week 0 (n = 9) and week 24 (n = 5) or 48 (n = 4). Finally, placebo patients were sampled at the same regularity as those in the laser cohort, with T. rubrum DNA strain types determined at week 0 (n = 8) and week 24 (n = 1) or 48 (n = 7), they reported.
“The T. rubrum DNA strain type switches observed in ongoing infections among all treatment groups could be attributed to microevolution or coinfections of DNA strains,” the researchers noted. “The presence of coinfecting T. rubrum DNA strains that flux with environmental conditions or local niches could account for the DNA strain type switches observed in all treatment groups, where only the relatively stable types are able to propagate in culture,” they added.
Dr. Gupta and his associates did not disclose any source of funding or any relevant conflicts of interest.
Although DNA strain type switches are known to be a natural occurrence in patients with onychomycosis, increases in strain type switching that follow treatment failure could be an antifungal-induced response, according to the results of a study published in the British Journal of Dermatology.
“The dermatophyte Trichophyton rubrum is responsible for the majority (~80%) of [onychomycosis] cases, many of which frequently relapse after successful antifungal treatment,” noted the study authors, led by Dr. Aditya K. Gupta of the University of Toronto. Despite several previous studies of various facets related to onychomycosis, “data outlining onychomycosis infections of T. rubrum with DNA strain type, treatments, outcome and geographical location are still warranted,” they added (Br. J. Dermatol. 2015;172:74-80).
Dr. Gupta and his associates examined 50 adults infected with T. rubrum, determined via analysis of toenail specimens from onychomycosis patients in southwest Ontario. The patients were divided into cohorts based on the treatment they received: oral terbinafine, laser, or placebo (no terbinafine and no laser). Typing of DNA strains was done only in culture-positive samples before and after treatment, leaving a study population of six in the terbinafine group, nine in the laser group, and eight in the placebo group.
Half of the terbinafine subjects were prescribed oral terbinafine 250 mg/day for 12 weeks, while the other three received oral terbinafine 250 mg/day pulse therapy at on/off intervals of 2 weeks up to 12 weeks.
The investigators also used three DNA strains known to be common in Europe for comparison and found that six distinct strains, labeled A-F, accounted for 94% of the T. rubrum strains – these strains corresponded to the European ones. However, three other strains (6% of strains) were found that investigators concluded were native to North America.
Strain type switching occurred in five (83%) of the terbinafine subjects, five (56%) of the laser cohort subjects, and two (25%) of those in the placebo cohort. Roughly half of the type switches noted in the terbinafine cohort were associated with mycological cures and were followed by relapse shortly thereafter. Dr. Gupta and his associates also found that all DNA strains in this cohort were susceptible to terbinafine while in vitro. Strain types in the laser and placebo cohorts did not show any signs of intermittent cures.
The patients were sampled at intervals of 0, 12, 24, 36, 48, 60, and 72 weeks of treatment, and T. rubrum DNA strain types were determined at week 0 (n = 6) and week 48 (n = 1) or 72 (n = 5). Patients in the laser cohort were treated at weeks 0, 8, and 16 and sampled at weeks 0, 8, 16, 24, and 48, with T. rubrum DNA strain types determined at week 0 (n = 9) and week 24 (n = 5) or 48 (n = 4). Finally, placebo patients were sampled at the same regularity as those in the laser cohort, with T. rubrum DNA strain types determined at week 0 (n = 8) and week 24 (n = 1) or 48 (n = 7), they reported.
“The T. rubrum DNA strain type switches observed in ongoing infections among all treatment groups could be attributed to microevolution or coinfections of DNA strains,” the researchers noted. “The presence of coinfecting T. rubrum DNA strains that flux with environmental conditions or local niches could account for the DNA strain type switches observed in all treatment groups, where only the relatively stable types are able to propagate in culture,” they added.
Dr. Gupta and his associates did not disclose any source of funding or any relevant conflicts of interest.
Although DNA strain type switches are known to be a natural occurrence in patients with onychomycosis, increases in strain type switching that follow treatment failure could be an antifungal-induced response, according to the results of a study published in the British Journal of Dermatology.
“The dermatophyte Trichophyton rubrum is responsible for the majority (~80%) of [onychomycosis] cases, many of which frequently relapse after successful antifungal treatment,” noted the study authors, led by Dr. Aditya K. Gupta of the University of Toronto. Despite several previous studies of various facets related to onychomycosis, “data outlining onychomycosis infections of T. rubrum with DNA strain type, treatments, outcome and geographical location are still warranted,” they added (Br. J. Dermatol. 2015;172:74-80).
Dr. Gupta and his associates examined 50 adults infected with T. rubrum, determined via analysis of toenail specimens from onychomycosis patients in southwest Ontario. The patients were divided into cohorts based on the treatment they received: oral terbinafine, laser, or placebo (no terbinafine and no laser). Typing of DNA strains was done only in culture-positive samples before and after treatment, leaving a study population of six in the terbinafine group, nine in the laser group, and eight in the placebo group.
Half of the terbinafine subjects were prescribed oral terbinafine 250 mg/day for 12 weeks, while the other three received oral terbinafine 250 mg/day pulse therapy at on/off intervals of 2 weeks up to 12 weeks.
The investigators also used three DNA strains known to be common in Europe for comparison and found that six distinct strains, labeled A-F, accounted for 94% of the T. rubrum strains – these strains corresponded to the European ones. However, three other strains (6% of strains) were found that investigators concluded were native to North America.
Strain type switching occurred in five (83%) of the terbinafine subjects, five (56%) of the laser cohort subjects, and two (25%) of those in the placebo cohort. Roughly half of the type switches noted in the terbinafine cohort were associated with mycological cures and were followed by relapse shortly thereafter. Dr. Gupta and his associates also found that all DNA strains in this cohort were susceptible to terbinafine while in vitro. Strain types in the laser and placebo cohorts did not show any signs of intermittent cures.
The patients were sampled at intervals of 0, 12, 24, 36, 48, 60, and 72 weeks of treatment, and T. rubrum DNA strain types were determined at week 0 (n = 6) and week 48 (n = 1) or 72 (n = 5). Patients in the laser cohort were treated at weeks 0, 8, and 16 and sampled at weeks 0, 8, 16, 24, and 48, with T. rubrum DNA strain types determined at week 0 (n = 9) and week 24 (n = 5) or 48 (n = 4). Finally, placebo patients were sampled at the same regularity as those in the laser cohort, with T. rubrum DNA strain types determined at week 0 (n = 8) and week 24 (n = 1) or 48 (n = 7), they reported.
“The T. rubrum DNA strain type switches observed in ongoing infections among all treatment groups could be attributed to microevolution or coinfections of DNA strains,” the researchers noted. “The presence of coinfecting T. rubrum DNA strains that flux with environmental conditions or local niches could account for the DNA strain type switches observed in all treatment groups, where only the relatively stable types are able to propagate in culture,” they added.
Dr. Gupta and his associates did not disclose any source of funding or any relevant conflicts of interest.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Antifungal treatment of onychomycosis could induce higher rates of DNA strain type switching in certain patients.
Major finding: Strain type switching occurred in 83% of the terbinafine group, 56% of the laser group, and 25% of the placebo group.
Data source: Cohort study of 23 individuals selected from 50 adults with onychomycosis who contributed samples to determine strain types.
Disclosures: The study authors did not disclose any source of funding or any relevant conflicts of interest.
Terbinafine Is Deemed an Attractive Option for Treating Tinea Capitis
MAUI, HAWAII — Oral terbinafine as first-line therapy for tinea capitis offers an unequalled combination of a good cure rate, fast results, minimal adverse events, and a stunningly low cost, according to Dr. Bernard A. Cohen.
“You can get a 30-day supply of terbinafine in my community at Wal-Mart for $4,” Dr. Cohen said at the annual Hawaii dermatology seminar sponsored by Skin Disease Education Foundation.
Compliance is a major issue in treating tinea capitis because the drugs have lengthy treatment durations. However, a course of terbinafine (Lamisil) lasts only about half as long as a course of griseofulvin, and that's an important consideration, noted Dr. Cohen, director of pediatric dermatology at Johns Hopkins Children's Center, Baltimore.
“Terbinafine and griseofulvin are the two drugs I use most often in my practice. Since compliance is an issue, and I think 6 weeks of treatment is a lot easier than 2.5–3 months of treatment, and I have to get proof-of-cure cultures in the kids I treat with griseofulvin, Lamisil simplifies my life. When I can use it as a first-line drug, I will. There are some situations where I'm going to use griseofulvin, though—like in a white kid with a Microsporum canis infection,” Dr. Cohen explained.
Terbinafine, approved by the Food and Drug Administration in 2007 for the treatment of tinea capitis, is more effective for treating Trichophyton tonsurans—the No. 1 cause of the infection—than for treating M. canis, he said. The FDA has approved terbinafine for use in children older than age 4 years, and recommends a pretreatment liver function test.
Dr. Cohen prescribes a single daily 250-mg tablet of terbinafine in children weighing more than 40 kg, half a tablet in those weighing 20–40 kg, and one-quarter tablet in children weighing less than 20 kg. For younger patients, he simply has the family halve or quarter a generic tablet, use a spoon to crush the appropriate portion against a cutting board, and sprinkle the medication in the child's food. Acidic foods interfere with the drug's absorption, though, so terbinafine shouldn't be mixed into applesauce.
Although the approved griseofulvin dosage is 11 mg/kg per day, today most pediatric dermatologists find it necessary to prescribe 15–20 mg/kg per day in order to obtain good efficacy. That may be in part because of the development of increasing resistance to the antifungal during the last several decades, but probably has more to do with compliance considerations. He disclosed having no relevant financial conflicts of interest. SDEF and this newspaper are owned by Elsevier.
MAUI, HAWAII — Oral terbinafine as first-line therapy for tinea capitis offers an unequalled combination of a good cure rate, fast results, minimal adverse events, and a stunningly low cost, according to Dr. Bernard A. Cohen.
“You can get a 30-day supply of terbinafine in my community at Wal-Mart for $4,” Dr. Cohen said at the annual Hawaii dermatology seminar sponsored by Skin Disease Education Foundation.
Compliance is a major issue in treating tinea capitis because the drugs have lengthy treatment durations. However, a course of terbinafine (Lamisil) lasts only about half as long as a course of griseofulvin, and that's an important consideration, noted Dr. Cohen, director of pediatric dermatology at Johns Hopkins Children's Center, Baltimore.
“Terbinafine and griseofulvin are the two drugs I use most often in my practice. Since compliance is an issue, and I think 6 weeks of treatment is a lot easier than 2.5–3 months of treatment, and I have to get proof-of-cure cultures in the kids I treat with griseofulvin, Lamisil simplifies my life. When I can use it as a first-line drug, I will. There are some situations where I'm going to use griseofulvin, though—like in a white kid with a Microsporum canis infection,” Dr. Cohen explained.
Terbinafine, approved by the Food and Drug Administration in 2007 for the treatment of tinea capitis, is more effective for treating Trichophyton tonsurans—the No. 1 cause of the infection—than for treating M. canis, he said. The FDA has approved terbinafine for use in children older than age 4 years, and recommends a pretreatment liver function test.
Dr. Cohen prescribes a single daily 250-mg tablet of terbinafine in children weighing more than 40 kg, half a tablet in those weighing 20–40 kg, and one-quarter tablet in children weighing less than 20 kg. For younger patients, he simply has the family halve or quarter a generic tablet, use a spoon to crush the appropriate portion against a cutting board, and sprinkle the medication in the child's food. Acidic foods interfere with the drug's absorption, though, so terbinafine shouldn't be mixed into applesauce.
Although the approved griseofulvin dosage is 11 mg/kg per day, today most pediatric dermatologists find it necessary to prescribe 15–20 mg/kg per day in order to obtain good efficacy. That may be in part because of the development of increasing resistance to the antifungal during the last several decades, but probably has more to do with compliance considerations. He disclosed having no relevant financial conflicts of interest. SDEF and this newspaper are owned by Elsevier.
MAUI, HAWAII — Oral terbinafine as first-line therapy for tinea capitis offers an unequalled combination of a good cure rate, fast results, minimal adverse events, and a stunningly low cost, according to Dr. Bernard A. Cohen.
“You can get a 30-day supply of terbinafine in my community at Wal-Mart for $4,” Dr. Cohen said at the annual Hawaii dermatology seminar sponsored by Skin Disease Education Foundation.
Compliance is a major issue in treating tinea capitis because the drugs have lengthy treatment durations. However, a course of terbinafine (Lamisil) lasts only about half as long as a course of griseofulvin, and that's an important consideration, noted Dr. Cohen, director of pediatric dermatology at Johns Hopkins Children's Center, Baltimore.
“Terbinafine and griseofulvin are the two drugs I use most often in my practice. Since compliance is an issue, and I think 6 weeks of treatment is a lot easier than 2.5–3 months of treatment, and I have to get proof-of-cure cultures in the kids I treat with griseofulvin, Lamisil simplifies my life. When I can use it as a first-line drug, I will. There are some situations where I'm going to use griseofulvin, though—like in a white kid with a Microsporum canis infection,” Dr. Cohen explained.
Terbinafine, approved by the Food and Drug Administration in 2007 for the treatment of tinea capitis, is more effective for treating Trichophyton tonsurans—the No. 1 cause of the infection—than for treating M. canis, he said. The FDA has approved terbinafine for use in children older than age 4 years, and recommends a pretreatment liver function test.
Dr. Cohen prescribes a single daily 250-mg tablet of terbinafine in children weighing more than 40 kg, half a tablet in those weighing 20–40 kg, and one-quarter tablet in children weighing less than 20 kg. For younger patients, he simply has the family halve or quarter a generic tablet, use a spoon to crush the appropriate portion against a cutting board, and sprinkle the medication in the child's food. Acidic foods interfere with the drug's absorption, though, so terbinafine shouldn't be mixed into applesauce.
Although the approved griseofulvin dosage is 11 mg/kg per day, today most pediatric dermatologists find it necessary to prescribe 15–20 mg/kg per day in order to obtain good efficacy. That may be in part because of the development of increasing resistance to the antifungal during the last several decades, but probably has more to do with compliance considerations. He disclosed having no relevant financial conflicts of interest. SDEF and this newspaper are owned by Elsevier.